We will keep fighting for all libraries - stand with us!

Internet Archive Audio

- This Just In
- Grateful Dead
- Old Time Radio
- 78 RPMs and Cylinder Recordings
- Audio Books & Poetry
- Computers, Technology and Science
- Music, Arts & Culture
- News & Public Affairs
- Spirituality & Religion
- Radio News Archive

- Flickr Commons
- Occupy Wall Street Flickr
- NASA Images
- Solar System Collection
- Ames Research Center

- All Software
- Old School Emulation
- MS-DOS Games
- Historical Software
- Classic PC Games
- Software Library
- Kodi Archive and Support File
- Vintage Software
- CD-ROM Software
- CD-ROM Software Library
- Software Sites
- Tucows Software Library
- Shareware CD-ROMs
- Software Capsules Compilation
- CD-ROM Images
- ZX Spectrum
- DOOM Level CD

- Smithsonian Libraries
- FEDLINK (US)
- Lincoln Collection
- American Libraries
- Canadian Libraries
- Universal Library
- Project Gutenberg
- Children's Library
- Biodiversity Heritage Library
- Books by Language
- Additional Collections

- Prelinger Archives
- Democracy Now!
- Occupy Wall Street
- TV NSA Clip Library
- Animation & Cartoons
- Arts & Music
- Computers & Technology
- Cultural & Academic Films
- Ephemeral Films
- Sports Videos
- Videogame Videos
- Youth Media
Search the history of over 866 billion web pages on the Internet.
Mobile Apps
- Wayback Machine (iOS)
- Wayback Machine (Android)
Browser Extensions
Archive-it subscription.
- Explore the Collections
- Build Collections
Save Page Now
Capture a web page as it appears now for use as a trusted citation in the future.
Please enter a valid web address
- Donate Donate icon An illustration of a heart shape
1000 Solved Problems in Heat Transfer: Schaum's solved problems series
Bookreader item preview, share or embed this item, flag this item for.
- Graphic Violence
- Explicit Sexual Content
- Hate Speech
- Misinformation/Disinformation
- Marketing/Phishing/Advertising
- Misleading/Inaccurate/Missing Metadata
Cut-off text on some pages due to tight binding
![[WorldCat (this item)] [WorldCat (this item)]](https://archive.org/images/worldcat-small.png)
plus-circle Add Review comment Reviews
2,187 Previews
11 Favorites
DOWNLOAD OPTIONS
No suitable files to display here.
PDF access not available for this item.
IN COLLECTIONS
Uploaded by station24.cebu on July 7, 2021
SIMILAR ITEMS (based on metadata)

- Engineering & Transportation
- Engineering
Fulfillment by Amazon (FBA) is a service we offer sellers that lets them store their products in Amazon's fulfillment centers, and we directly pack, ship, and provide customer service for these products. Something we hope you'll especially enjoy: FBA items qualify for FREE Shipping and Amazon Prime.
If you're a seller, Fulfillment by Amazon can help you grow your business. Learn more about the program.

Download the free Kindle app and start reading Kindle books instantly on your smartphone, tablet, or computer - no Kindle device required .
Read instantly on your browser with Kindle for Web.
Using your mobile phone camera - scan the code below and download the Kindle app.

Image Unavailable

- To view this video download Flash Player
Follow the author

1000 Solved Problems in Heat Transfer (Schaum's Solved Problems Series)
- ISBN-10 0070502048
- ISBN-13 978-0070502048
- Publisher McGraw-Hill
- Publication date January 1, 1990
- Language English
- Dimensions 8.5 x 1 x 11.25 inches
- Print length 750 pages
- See all details

Product details
- Publisher : McGraw-Hill (January 1, 1990)
- Language : English
- Paperback : 750 pages
- ISBN-10 : 0070502048
- ISBN-13 : 978-0070502048
- Item Weight : 1.72 pounds
- Dimensions : 8.5 x 1 x 11.25 inches
- #9,187 in Mechanical Engineering (Books)
- #11,430 in Civil & Environmental Engineering
- #18,645 in Technology (Books)
About the author
Donald r. pitts.
Discover more of the author’s books, see similar authors, read author blogs and more
Customer reviews
Customer Reviews, including Product Star Ratings help customers to learn more about the product and decide whether it is the right product for them.
To calculate the overall star rating and percentage breakdown by star, we don’t use a simple average. Instead, our system considers things like how recent a review is and if the reviewer bought the item on Amazon. It also analyzed reviews to verify trustworthiness.
Solutions manual
Solutions to more than 490 problems are on the following links.
Solutions for Chapter 1 (v1.01, 16 MB, February 2023)
Solutions for Chapter 2 (v1.0, 13 MB, August 2020)
Solutions for Chapter 3 (v1.0, 15 MB, August 2020)
Partial solutions for Chapters 4-11 (v1.05, 24 MB, 24 March 2023) Includes solutions for all problems in Chapters 4, 5, 6, 10 & 11
If additional solutions become available, they will be posted here.
Most of the handwritten solutions were prepared many years ago, and some use property data that don’t precisely match today’s Appendix A. In most instances, the differences are small.
Between them, the authors have spent more than 100 years using various textbook solutions manuals. In our experience, none are without errors. If you happen to find one in our solutions manual, do let us know.
Items related to 1000 Solved Problems in Heat Transfer (Schaum's...
1000 solved problems in heat transfer (schaum's solved problems series) - softcover, pitts, donald r. ; sissom, leighton e..

This specific ISBN edition is currently not available.
- About this edition
"synopsis" may belong to another edition of this title.
- Publisher McGraw-Hill
- Publication date 1990
- ISBN 10 0070502048
- ISBN 13 9780070502048
- Binding Paperback
- Number of pages 750
Convert currency
Shipping: US$ 6.65 Within U.S.A.
Add to Basket
Other Popular Editions of the Same Title
Featured edition.
ISBN 10: ISBN 13: 9780071006538 Publisher: Mcgraw-Hill Book Comp Softcover
Top Search Results from the AbeBooks Marketplace
1000 solved problems in heat tra.
Book Description Condition: New. New. In shrink wrap. Looks like an interesting title! 3.4. Seller Inventory # Q-0070502048
More information about this seller | Contact seller
1000 Solved Problems in Heat Transfer (Schaum's Solved Problems Series)
Book Description Paperback. Condition: new. New. Fast Shipping and good customer service. Seller Inventory # Holz_New_0070502048
Book Description Paperback. Condition: new. New Copy. Customer Service Guaranteed. Seller Inventory # think0070502048
Book Description Paperback. Condition: new. New. Seller Inventory # Wizard0070502048
1000 Solved Problems in Heat Transfer (Schaum's Solved Problems Series) Pitts, Donald R. and Sissom, Leighton E.
Book Description Condition: New. (inventory#Shelf). Seller Inventory # EZ153536
Book Description Condition: New. (inventory#Shelf). Seller Inventory # EZ163765
Book Description Condition: New. (inventory#Shelf). Seller Inventory # EZ167775
Book Description Paperback. Condition: New. Seller Inventory # Abebooks1547

1.6 Mechanisms of Heat Transfer
Learning objectives.
By the end of this section, you will be able to:
- Explain some phenomena that involve conductive, convective, and radiative heat transfer
- Solve problems on the relationships between heat transfer, time, and rate of heat transfer
- Solve problems using the formulas for conduction and radiation
Just as interesting as the effects of heat transfer on a system are the methods by which it occurs. Whenever there is a temperature difference, heat transfer occurs. It may occur rapidly, as through a cooking pan, or slowly, as through the walls of a picnic ice chest. So many processes involve heat transfer that it is hard to imagine a situation where no heat transfer occurs. Yet every heat transfer takes place by only three methods:
- Conduction is heat transfer through stationary matter by physical contact. (The matter is stationary on a macroscopic scale—we know that thermal motion of the atoms and molecules occurs at any temperature above absolute zero.) Heat transferred from the burner of a stove through the bottom of a pan to food in the pan is transferred by conduction .
- Convection is the heat transfer by the macroscopic movement of a fluid. This type of transfer takes place in a forced-air furnace and in weather systems, for example.
- Heat transfer by radiation occurs when microwaves, infrared radiation, visible light, or another form of electromagnetic radiation is emitted or absorbed. An obvious example is the warming of Earth by the Sun. A less obvious example is thermal radiation from the human body.
In the illustration at the beginning of this chapter, the fire warms the snowshoers’ faces largely by radiation. Convection carries some heat to them, but most of the air flow from the fire is upward (creating the familiar shape of flames), carrying heat to the food being cooked and into the sky. The snowshoers wear clothes designed with low conductivity to prevent heat flow out of their bodies.
In this section, we examine these methods in some detail. Each method has unique and interesting characteristics, but all three have two things in common: there is a net transfer of heat solely because of a temperature difference, and the greater the temperature difference, the faster the heat transfer ( Figure 1.19 ).
Check Your Understanding 1.6
Name an example from daily life (different from the text) for each mechanism of heat transfer.
As you walk barefoot across the living room carpet in a cold house and then step onto the kitchen tile floor, your feet feel colder on the tile. This result is intriguing, since the carpet and tile floor are both at the same temperature. The different sensation is explained by the different rates of heat transfer: The heat loss is faster for skin in contact with the tiles than with the carpet, so the sensation of cold is more intense.
Some materials conduct thermal energy faster than others. Figure 1.20 shows a material that conducts heat slowly—it is a good thermal insulator, or poor heat conductor—used to reduce heat flow into and out of a house.
A molecular picture of heat conduction will help justify the equation that describes it. Figure 1.21 shows molecules in two bodies at different temperatures, T h T h and T c , T c , for “hot” and “cold.” The average kinetic energy of a molecule in the hot body is higher than in the colder body. If two molecules collide, energy transfers from the high-energy to the low-energy molecule. In a metal, the picture would also include free valence electrons colliding with each other and with atoms, likewise transferring energy. The cumulative effect of all collisions is a net flux of heat from the hotter body to the colder body. Thus, the rate of heat transfer increases with increasing temperature difference Δ T = T h − T c . Δ T = T h − T c . If the temperatures are the same, the net heat transfer rate is zero. Because the number of collisions increases with increasing area, heat conduction is proportional to the cross-sectional area—a second factor in the equation.
A third quantity that affects the conduction rate is the thickness of the material through which heat transfers. Figure 1.22 shows a slab of material with a higher temperature on the left than on the right. Heat transfers from the left to the right by a series of molecular collisions. The greater the distance between hot and cold, the more time the material takes to transfer the same amount of heat.
All four of these quantities appear in a simple equation deduced from and confirmed by experiments. The rate of conductive heat transfer through a slab of material, such as the one in Figure 1.22 , is given by
where P is the power or rate of heat transfer in watts or in kilocalories per second, A and d are its surface area and thickness, as shown in Figure 1.22 , T h − T c T h − T c is the temperature difference across the slab, and k is the thermal conductivity of the material. Table 1.5 gives representative values of thermal conductivity.
More generally, we can write
where x is the coordinate in the direction of heat flow. Since in Figure 1.22 , the power and area are constant, dT / dx is constant, and the temperature decreases linearly from T h T h to T c . T c .
Example 1.10
Calculating heat transfer through conduction.
k = 0.010 W/m · ° C k = 0.010 W/m · ° C for polystyrene foam; A = 0.950 m 2 ; A = 0.950 m 2 ; d = 2.50 cm = 0.0250 m; d = 2.50 cm = 0.0250 m; ; T c = 0 ° C; T c = 0 ° C; T h = 35.0 ° C T h = 35.0 ° C ; t = 1 day = 24 hours - 86,400 s. t = 1 day = 24 hours - 86,400 s.
Then we identify the unknowns. We need to solve for the mass of the ice, m . We also need to solve for the net heat transferred to melt the ice, Q . The rate of heat transfer by conduction is given by
The heat used to melt the ice is Q = m L f Q = m L f .We insert the known values:
Multiplying the rate of heat transfer by the time we obtain
We set this equal to the heat transferred to melt the ice, Q = m L f , Q = m L f , and solve for the mass m :
Significance
Table 1.5 shows that polystyrene foam is a very poor conductor and thus a good insulator. Other good insulators include fiberglass, wool, and goosedown feathers. Like polystyrene foam, these all contain many small pockets of air, taking advantage of air’s poor thermal conductivity.
In developing insulation , the smaller the conductivity k and the larger the thickness d , the better. Thus, the ratio d/k , called the R factor , is large for a good insulator. The rate of conductive heat transfer is inversely proportional to R . R factors are most commonly quoted for household insulation, refrigerators, and the like. Unfortunately, in the United States, R is still in non-metric units of ft 2 · °F · h/Btu ft 2 · °F · h/Btu , although the unit usually goes unstated [1 British thermal unit (Btu) is the amount of energy needed to change the temperature of 1.0 lb of water by 1.0 ° F 1.0 ° F , which is 1055.1 J]. A couple of representative values are an R factor of 11 for 3.5-inch-thick fiberglass batts (pieces) of insulation and an R factor of 19 for 6.5-inch-thick fiberglass batts ( Figure 1.23 ). In the US, walls are usually insulated with 3.5-inch batts, whereas ceilings are usually insulated with 6.5-inch batts. In cold climates, thicker batts may be used.
Note that in Table 1.5 , most of the best thermal conductors—silver, copper, gold, and aluminum—are also the best electrical conductors, because they contain many free electrons that can transport thermal energy. (Diamond, an electrical insulator, conducts heat by atomic vibrations.) Cooking utensils are typically made from good conductors, but the handles of those used on the stove are made from good insulators (bad conductors).
Example 1.11
Two conductors end to end.
We repeat the calculation with a second method, in which we use the thermal resistance R of the rod, since it simply adds when two rods are joined end to end. (We will use a similar method in the chapter on direct-current circuits.)
- Identify the knowns and convert them to SI units. The length of each rod is L A1 = L steel = 0.25 m, L A1 = L steel = 0.25 m, the cross-sectional area of each rod is A A1 = A steel = 7.85 × 10 −5 m 2 , A A1 = A steel = 7.85 × 10 −5 m 2 , the thermal conductivity of aluminum is k A1 = 220 W/m · ° C k A1 = 220 W/m · ° C , the thermal conductivity of steel is k steel = 80 W/m · ° C k steel = 80 W/m · ° C , the temperature at the hot end is T = 100 ° C T = 100 ° C , and the temperature at the cold end is T = 20 ° C T = 20 ° C .
- Calculate the heat-conduction rate through the steel rod and the heat-conduction rate through the aluminum rod in terms of the unknown temperature T at the joint: P steel = k steel A steel Δ T steel L steel = ( 80 W/m · ° C ) ( 7.85 × 10 −5 m 2 ) ( 100 ° C − T ) 0.25 m = ( 0.0251 W/ ° C ) ( 100 ° C − T ) ; P steel = k steel A steel Δ T steel L steel = ( 80 W/m · ° C ) ( 7.85 × 10 −5 m 2 ) ( 100 ° C − T ) 0.25 m = ( 0.0251 W/ ° C ) ( 100 ° C − T ) ; P A1 = k Al A A1 Δ T Al L A1 = ( 220 W/m · ° C ) ( 7.85 × 10 −5 m 2 ) ( T − 20 ° C ) 0.25 m = ( 0.0691 W/ ° C ) ( T − 20 ° C ) . P A1 = k Al A A1 Δ T Al L A1 = ( 220 W/m · ° C ) ( 7.85 × 10 −5 m 2 ) ( T − 20 ° C ) 0.25 m = ( 0.0691 W/ ° C ) ( T − 20 ° C ) .
- Set the two rates equal and solve for the unknown temperature: ( 0.0691 W/ ° C ) ( T − 20 ° C ) = ( 0.0251 W/ ° C ) ( 100 ° C − T ) T = 41.3 ° C . ( 0.0691 W/ ° C ) ( T − 20 ° C ) = ( 0.0251 W/ ° C ) ( 100 ° C − T ) T = 41.3 ° C .
- Calculate either rate: P steel = ( 0.0251 W/ ° C ) ( 100 ° C − 41.3 ° C ) = 1.47 W . P steel = ( 0.0251 W/ ° C ) ( 100 ° C − 41.3 ° C ) = 1.47 W .
- If desired, check your answer by calculating the other rate.
- Recall that R = L / k R = L / k . Now P = A Δ T / R , or Δ T = P R / A . P = A Δ T / R , or Δ T = P R / A .
- We know that Δ T steel + Δ T Al = 100 ° C − 20 ° C = 80 ° C Δ T steel + Δ T Al = 100 ° C − 20 ° C = 80 ° C . We also know that P steel = P Al , P steel = P Al , and we denote that rate of heat flow by P . Combine the equations: P R steel A + P R Al A = 80 ° C . P R steel A + P R Al A = 80 ° C . Thus, we can simply add R factors. Now, P = ( 80 ° C ) ( A ) R steel + R Al P = ( 80 ° C ) ( A ) R steel + R Al .
- Find the R s R s from the known quantities: R steel = 3.13 × 10 −3 m 2 · ° C/W R steel = 3.13 × 10 −3 m 2 · ° C/W and R Al = 1.14 × 10 −3 m 2 · ° C/W . R Al = 1.14 × 10 −3 m 2 · ° C/W .
- Substitute these values in to find P = 1.47 W P = 1.47 W as before.
- Determine Δ T Δ T for the aluminum rod (or for the steel rod) and use it to find T at the joint. Δ T Al = P R Al A = ( 1.47 W ) ( 1.14 × 10 −3 m 2 · ° C/W ) 7.85 × 10 −5 m 2 = 21.3 ° C, Δ T Al = P R Al A = ( 1.47 W ) ( 1.14 × 10 −3 m 2 · ° C/W ) 7.85 × 10 −5 m 2 = 21.3 ° C, so T = 20 ° C + 21.3 ° C = 41.3 ° C T = 20 ° C + 21.3 ° C = 41.3 ° C , as in Solution 1 1 .
- If desired, check by determining Δ T Δ T for the other rod.
Check Your Understanding 1.7
How does the rate of heat transfer by conduction change when all spatial dimensions are doubled?
Conduction is caused by the random motion of atoms and molecules. As such, it is an ineffective mechanism for heat transport over macroscopic distances and short times. For example, the temperature on Earth would be unbearably cold during the night and extremely hot during the day if heat transport into and through the atmosphere were only through conduction and radiation. Also, car engines would overheat unless there was a more efficient way to remove excess heat from the pistons. The next module discusses the important heat-transfer mechanism in such situations.
In convection , thermal energy is carried by the large-scale flow of matter. It can be divided into two types. In forced convection , the flow is driven by fans, pumps, and the like. A simple example is a fan that blows air past you in hot surroundings and cools you by replacing the air heated by your body with cooler air. A more complicated example is the cooling system of a typical car, in which a pump moves coolant through the radiator and engine to cool the engine and a fan blows air to cool the radiator.
In free or natural convection , the flow is driven by buoyant forces: hot fluid rises and cold fluid sinks because density decreases as temperature increases. The house in Figure 1.24 is kept warm by natural convection, as is the pot of water on the stove in Figure 1.25 . Ocean currents and large-scale atmospheric circulation, which result from the buoyancy of warm air and water, transfer hot air from the tropics toward the poles and cold air from the poles toward the tropics. (Earth’s rotation interacts with those flows, causing the observed eastward flow of air in the temperate zones.)
Interactive
Natural convection like that of Figure 1.24 and Figure 1.25 , but acting on rock in Earth’s mantle, drives plate tectonics that are the motions that have shaped Earth’s surface.
Convection is usually more complicated than conduction. Beyond noting that the convection rate is often approximately proportional to the temperature difference, we will not do any quantitative work comparable to the formula for conduction. However, we can describe convection qualitatively and relate convection rates to heat and time. Air is a poor conductor, so convection dominates heat transfer by air. Therefore, the amount of available space for airflow determines whether air transfers heat rapidly or slowly. There is little heat transfer in a space filled with air with a small amount of other material that prevents flow. The space between the inside and outside walls of a typical American house, for example, is about 9 cm (3.5 in.)—large enough for convection to work effectively. The addition of wall insulation prevents airflow, so heat loss (or gain) is decreased. On the other hand, the gap between the two panes of a double-paned window is about 1 cm, which largely prevents convection and takes advantage of air’s low conductivity reduce heat loss. Fur, cloth, and fiberglass also take advantage of the low conductivity of air by trapping it in spaces too small to support convection ( Figure 1.26 ).
Some interesting phenomena happen when convection is accompanied by a phase change. The combination allows us to cool off by sweating even if the temperature of the surrounding air exceeds body temperature. Heat from the skin is required for sweat to evaporate from the skin, but without air flow, the air becomes saturated and evaporation stops. Air flow caused by convection replaces the saturated air by dry air and evaporation continues.
Example 1.12
Calculating the flow of mass during convection.
We divide both sides of the equation by L v L v to find that the mass evaporated per unit time is
Another important example of the combination of phase change and convection occurs when water evaporates from the oceans. Heat is removed from the ocean when water evaporates. If the water vapor condenses in liquid droplets as clouds form, possibly far from the ocean, heat is released in the atmosphere. Thus, there is an overall transfer of heat from the ocean to the atmosphere. This process is the driving power behind thunderheads, those great cumulus clouds that rise as much as 20.0 km into the stratosphere ( Figure 1.27 ). Water vapor carried in by convection condenses, releasing tremendous amounts of energy. This energy causes the air to expand and rise to colder altitudes. More condensation occurs in these regions, which in turn drives the cloud even higher. This mechanism is an example of positive feedback, since the process reinforces and accelerates itself. It sometimes produces violent storms, with lightning and hail. The same mechanism drives hurricanes.
This time-lapse video shows convection currents in a thunderstorm, including “rolling” motion similar to that of boiling water.
Check Your Understanding 1.8
Explain why using a fan in the summer feels refreshing.
You can feel the heat transfer from the Sun. The space between Earth and the Sun is largely empty, so the Sun warms us without any possibility of heat transfer by convection or conduction. Similarly, you can sometimes tell that the oven is hot without touching its door or looking inside—it may just warm you as you walk by. In these examples, heat is transferred by radiation ( Figure 1.28 ). That is, the hot body emits electromagnetic waves that are absorbed by the skin. No medium is required for electromagnetic waves to propagate. Different names are used for electromagnetic waves of different wavelengths: radio waves, microwaves, infrared radiation, visible light, ultraviolet radiation, X-rays, and gamma rays.
The energy of electromagnetic radiation varies over a wide range, depending on the wavelength: A shorter wavelength (or higher frequency) corresponds to a higher energy. Because more heat is radiated at higher temperatures, higher temperatures produce more intensity at every wavelength but especially at shorter wavelengths. In visible light, wavelength determines color—red has the longest wavelength and violet the shortest—so a temperature change is accompanied by a color change. For example, an electric heating element on a stove glows from red to orange, while the higher-temperature steel in a blast furnace glows from yellow to white. Infrared radiation is the predominant form radiated by objects cooler than the electric element and the steel. The radiated energy as a function of wavelength depends on its intensity, which is represented in Figure 1.29 by the height of the distribution. ( Electromagnetic Waves explains more about the electromagnetic spectrum, and Photons and Matter Waves discusses why the decrease in wavelength corresponds to an increase in energy.)
The rate of heat transfer by radiation also depends on the object’s color. Black is the most effective, and white is the least effective. On a clear summer day, black asphalt in a parking lot is hotter than adjacent gray sidewalk, because black absorbs better than gray ( Figure 1.30 ). The reverse is also true—black radiates better than gray. Thus, on a clear summer night, the asphalt is colder than the gray sidewalk, because black radiates the energy more rapidly than gray. A perfectly black object would be an ideal radiator and an ideal absorber , as it would capture all the radiation that falls on it. In contrast, a perfectly white object or a perfect mirror would reflect all radiation, and a perfectly transparent object would transmit it all ( Figure 1.31 ). Such objects would not emit any radiation. Mathematically, the color is represented by the emissivity e . A “blackbody” radiator would have an e = 1 e = 1 , whereas a perfect reflector or transmitter would have e = 0 e = 0 . For real examples, tungsten light bulb filaments have an e of about 0.5, and carbon black (a material used in printer toner) has an emissivity of about 0.95.
To see that, consider a silver object and a black object that can exchange heat by radiation and are in thermal equilibrium. We know from experience that they will stay in equilibrium (the result of a principle that will be discussed at length in Second Law of Thermodynamics ). For the black object’s temperature to stay constant, it must emit as much radiation as it absorbs, so it must be as good at radiating as absorbing. Similar considerations show that the silver object must radiate as little as it absorbs. Thus, one property, emissivity, controls both radiation and absorption.
Finally, the radiated heat is proportional to the object’s surface area, since every part of the surface radiates. If you knock apart the coals of a fire, the radiation increases noticeably due to an increase in radiating surface area.
The rate of heat transfer by emitted radiation is described by the Stefan-Boltzmann law of radiation :
where σ = 5.67 × 10 −8 J/s · m 2 · K 4 σ = 5.67 × 10 −8 J/s · m 2 · K 4 is the Stefan-Boltzmann constant, a combination of fundamental constants of nature; A is the surface area of the object; and T is its temperature in kelvins.
The proportionality to the fourth power of the absolute temperature is a remarkably strong temperature dependence. It allows the detection of even small temperature variations. Images called thermographs can be used medically to detect regions of abnormally high temperature in the body, perhaps indicative of disease. Similar techniques can be used to detect heat leaks in homes ( Figure 1.32 ), optimize performance of blast furnaces, improve comfort levels in work environments, and even remotely map Earth’s temperature profile.
The Stefan-Boltzmann equation needs only slight refinement to deal with a simple case of an object’s absorption of radiation from its surroundings. Assuming that an object with a temperature T 1 T 1 is surrounded by an environment with uniform temperature T 2 T 2 , the net rate of heat transfer by radiation is
where e is the emissivity of the object alone. In other words, it does not matter whether the surroundings are white, gray, or black: The balance of radiation into and out of the object depends on how well it emits and absorbs radiation. When T 2 > T 1 , T 2 > T 1 , the quantity P net P net is positive, that is, the net heat transfer is from hot to cold.
Before doing an example, we have a complication to discuss: different emissivities at different wavelengths. If the fraction of incident radiation an object reflects is the same at all visible wavelengths, the object is gray; if the fraction depends on the wavelength, the object has some other color. For instance, a red or reddish object reflects red light more strongly than other visible wavelengths. Because it absorbs less red, it radiates less red when hot. Differential reflection and absorption of wavelengths outside the visible range have no effect on what we see, but they may have physically important effects. Skin is a very good absorber and emitter of infrared radiation, having an emissivity of 0.97 in the infrared spectrum. Thus, in spite of the obvious variations in skin color, we are all nearly black in the infrared. This high infrared emissivity is why we can so easily feel radiation on our skin. It is also the basis for the effectiveness of night-vision scopes used by law enforcement and the military to detect human beings.
Example 1.13
Calculating the net heat transfer of a person.
The average temperature of Earth is the subject of much current discussion. Earth is in radiative contact with both the Sun and dark space, so we cannot use the equation for an environment at a uniform temperature. Earth receives almost all its energy from radiation of the Sun and reflects some of it back into outer space. Conversely, dark space is very cold, about 3 K, so that Earth radiates energy into the dark sky. The rate of heat transfer from soil and grasses can be so rapid that frost may occur on clear summer evenings, even in warm latitudes.
The average temperature of Earth is determined by its energy balance. To a first approximation, it is the temperature at which Earth radiates heat to space as fast as it receives energy from the Sun.
An important parameter in calculating the temperature of Earth is its emissivity ( e ). On average, it is about 0.65, but calculation of this value is complicated by the great day-to-day variation in the highly reflective cloud coverage. Because clouds have lower emissivity than either oceans or land masses, they emit some of the radiation back to the surface, greatly reducing heat transfer into dark space, just as they greatly reduce heat transfer into the atmosphere during the day. There is negative feedback (in which a change produces an effect that opposes that change) between clouds and heat transfer; higher temperatures evaporate more water to form more clouds, which reflect more radiation back into space, reducing the temperature.
The often-mentioned greenhouse effect is directly related to the variation of Earth’s emissivity with wavelength ( Figure 1.33 ). The greenhouse effect is a natural phenomenon responsible for providing temperatures suitable for life on Earth and for making Venus unsuitable for human life. Most of the infrared radiation emitted from Earth is absorbed by carbon dioxide ( CO 2 CO 2 ) and water ( H 2 O H 2 O ) in the atmosphere and then re-radiated into outer space or back to Earth. Re-radiation back to Earth maintains its surface temperature about 40 °C 40 °C higher than it would be if there were no atmosphere. (The glass walls and roof of a greenhouse increase the temperature inside by blocking convective heat losses, not radiative losses.)
The greenhouse effect and its causes were first predicted by Eunice Newton Foote after she designed and conducted experiments on heating of different gases. After filling flasks with carbon dioxide, hydrogen, and regular air, then also modifying moisture, she placed them in the sun and carefully measured their heating and, especially, their heat retention. She discovered that the CO 2 flask gained the most temperature and held it the longest. After subsequent research, her paper "Circumstances affecting the Heat of the Sun’s Rays" included conclusions that an atmosphere consisting of more carbon dioxide would be hotter resulting from the gas trapping radiation.
The greenhouse effect is central to the discussion of global warming due to emission of carbon dioxide and methane (and other greenhouse gases) into Earth’s atmosphere from industry, transportation, and farming. Changes in global climate could lead to more intense storms, precipitation changes (affecting agriculture), reduction in rain forest biodiversity, and rising sea levels.
You can explore a simulation of the greenhouse effect that takes the point of view that the atmosphere scatters (redirects) infrared radiation rather than absorbing it and reradiating it. You may want to run the simulation first with no greenhouse gases in the atmosphere and then look at how adding greenhouse gases affects the infrared radiation from the Earth and the Earth’s temperature.
Problem-Solving Strategy
Effects of heat transfer.
- Examine the situation to determine what type of heat transfer is involved.
- Identify the type(s) of heat transfer—conduction, convection, or radiation.
- Identify exactly what needs to be determined in the problem (identify the unknowns). A written list is useful.
- Make a list of what is given or what can be inferred from the problem as stated (identify the knowns).
- Solve the appropriate equation for the quantity to be determined (the unknown).
- For conduction, use the equation P = k A Δ T d P = k A Δ T d . Table 1.5 lists thermal conductivities. For convection, determine the amount of matter moved and the equation Q = m c Δ T Q = m c Δ T , along with Q = m L f Q = m L f or Q = m L V Q = m L V if a substance changes phase. For radiation, the equation P net = σ e A ( T 2 4 − T 1 4 ) P net = σ e A ( T 2 4 − T 1 4 ) gives the net heat transfer rate.
- Substitute the knowns along with their units into the appropriate equation and obtain numerical solutions complete with units.
- Check the answer to see if it is reasonable. Does it make sense?
Check Your Understanding 1.9
How much greater is the rate of heat radiation when a body is at the temperature 40 ° C 40 ° C than when it is at the temperature 20 ° C 20 ° C ?
As an Amazon Associate we earn from qualifying purchases.
This book may not be used in the training of large language models or otherwise be ingested into large language models or generative AI offerings without OpenStax's permission.
Want to cite, share, or modify this book? This book uses the Creative Commons Attribution License and you must attribute OpenStax.
Access for free at https://openstax.org/books/university-physics-volume-2/pages/1-introduction
- Authors: Samuel J. Ling, William Moebs, Jeff Sanny
- Publisher/website: OpenStax
- Book title: University Physics Volume 2
- Publication date: Oct 6, 2016
- Location: Houston, Texas
- Book URL: https://openstax.org/books/university-physics-volume-2/pages/1-introduction
- Section URL: https://openstax.org/books/university-physics-volume-2/pages/1-6-mechanisms-of-heat-transfer
© Jan 19, 2024 OpenStax. Textbook content produced by OpenStax is licensed under a Creative Commons Attribution License . The OpenStax name, OpenStax logo, OpenStax book covers, OpenStax CNX name, and OpenStax CNX logo are not subject to the Creative Commons license and may not be reproduced without the prior and express written consent of Rice University.
- Terminology
- Radiant Heat Transfer
- Heat Exchangers
- Boiling Heat Transfer
- Heat Generation
- Continuity Equation
- Laminar/Turbulent Flow
- Bernoulli's Equation
- Natural Circulation
- Two-Phase Fluid Flow
- Centrifugal Pumps
- Unit Systems
- Force and Motion
- Newton's Laws
- Energy, Work, and Power
- Structure of Metals
- Properties of Metals
- Material Selection
- Thermal Stress
- Brittle Fracture
Check out these structural calculators : • Beam Analysis • Bolted Joints • Lug Analysis • Column Buckling
Conduction Heat Transfer
This page provides the chapter on conduction heat transfer from the "DOE Fundamentals Handbook: Thermodynamics, Heat Transfer, and Fluid Flow," DOE-HDBK-1012/2-92 , U.S. Department of Energy, June 1992.
Other related chapters from the "DOE Fundamentals Handbook: Thermodynamics, Heat Transfer, and Fluid Flow" can be seen to the right.
- Heat Transfer Terminology
- Convection Heat Transfer
Conduction heat transfer is the transfer of thermal energy by interactions between adjacent atoms and molecules of a solid .
Conduction involves the transfer of heat by the interaction between adjacent molecules of a material. Heat transfer by conduction is dependent upon the driving "force" of temperature difference and the resistance to heat transfer. The resistance to heat transfer is dependent upon the nature and dimensions of the heat transfer medium. All heat transfer problems involve the temperature difference, the geometry, and the physical properties of the object being studied.
In conduction heat transfer problems, the object being studied is usually a solid. Convection problems involve a fluid medium. Radiation heat transfer problems involve either solid or fluid surfaces, separated by a gas, vapor, or vacuum. There are several ways to correlate the geometry, physical properties, and temperature difference of an object with the rate of heat transfer through the object. In conduction heat transfer, the most common means of correlation is through Fourier's Law of Conduction. The law, in its equation form, is used most often in its rectangular or cylindrical form (pipes and cylinders), both of which are presented below.
The use of Equations 2-4 and 2-5 in determining the amount of heat transferred by conduction is demonstrated in the following examples.
Conduction-Rectangular Coordinates
1000 Btu/hr is conducted through a section of insulating material shown in Figure 1 that measures 1 ft 2 in cross-sectional area. The thickness is 1 in. and the thermal conductivity is 0.12 Btu/hr-ft-°F. Compute the temperature difference across the material.
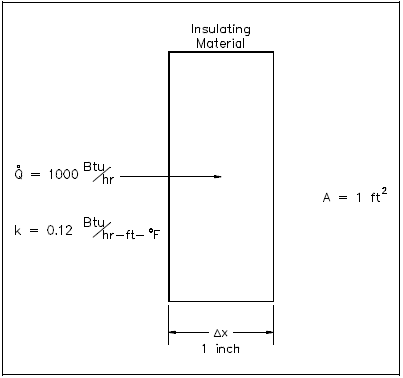
Using Equation 2-4:
Solving for ΔT :
A concrete floor with a conductivity of 0.8 Btu/hr-ft-°F measures 30 ft by 40 ft with a thickness of 4 inches. The floor has a surface temperature of 70°F and the temperature beneath it is 60°F. What is the heat flux and the heat transfer rate through the floor?
Using Equations 2-1 and 2-4:
Using Equation 2-3:
Equivalent Resistance Method
It is possible to compare heat transfer to current flow in electrical circuits. The heat transfer rate may be considered as a current flow and the combination of thermal conductivity , thickness of material, and area as a resistance to this flow. The temperature difference is the potential or driving function for the heat flow, resulting in the Fourier equation being written in a form similar to Ohm's Law of Electrical Circuit Theory. If the thermal resistance term Δx/k is written as a resistance term where the resistance is the reciprocal of the thermal conductivity divided by the thickness of the material, the result is the conduction equation being analogous to electrical systems or networks. The electrical analogy may be used to solve complex problems involving both series and parallel thermal resistances. The student is referred to Figure 2, showing the equivalent resistance circuit. A typical conduction problem in its analogous electrical form is given in the following example, where the "electrical" Fourier equation may be written as follows.
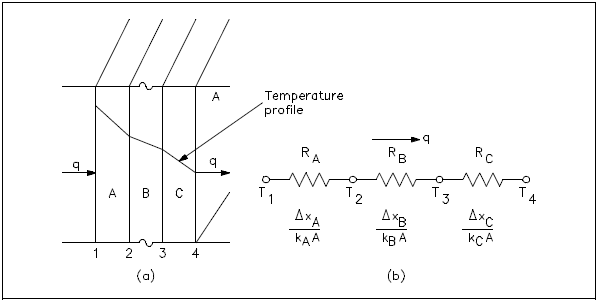
Electrical Analogy
A composite protective wall is formed of a 1 in. copper plate, a 1/8 in. layer of asbestos, and a 2 in. layer of fiberglass. The thermal conductivities of the materials in units of Btu/hr-ft-°F are as follows: k Cu = 240, k asb = 0.048, and k fib = 0.022. The overall temperature difference across the wall is 500°F. Calculate the thermal resistance of each layer of the wall and the heat transfer rate per unit area ( heat flux ) through the composite structure.
Conduction-Cylindrical Coordinates
Heat transfer across a rectangular solid is the most direct application of Fourier's law. Heat transfer across a pipe or heat exchanger tube wall is more complicated to evaluate. Across a cylindrical wall, the heat transfer surface area is continually increasing or decreasing. Figure 3 is a cross-sectional view of a pipe constructed of a homogeneous material.
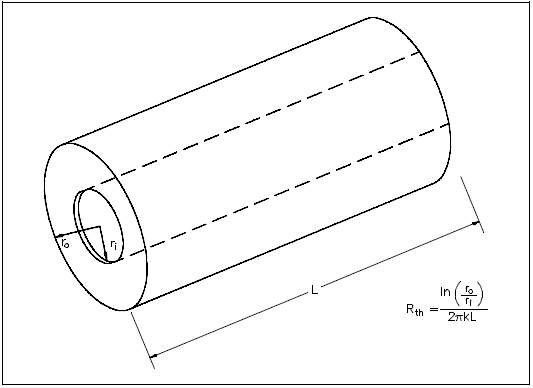
The surface area ( A ) for transferring heat through the pipe (neglecting the pipe ends) is directly proportional to the radius ( r ) of the pipe and the length ( L ) of the pipe.
As the radius increases from the inner wall to the outer wall, the heat transfer area increases.
The development of an equation evaluating heat transfer through an object with cylindrical geometry begins with Fourier's law Equation 2-5.
From the discussion above, it is seen that no simple expression for area is accurate. Neither the area of the inner surface nor the area of the outer surface alone can be used in the equation. For a problem involving cylindrical geometry, it is necessary to define a log mean cross-sectional area ( A lm ).
Substituting the expression 2πrL for area in Equation 2-7 allows the log mean area to be calculated from the inner and outer radius without first calculating the inner and outer area.
This expression for log mean area can be inserted into Equation 2-5, allowing us to calculate the heat transfer rate for cylindrical geometries.
A stainless steel pipe with a length of 35 ft has an inner diameter of 0.92 ft and an outer diameter of 1.08 ft. The temperature of the inner surface of the pipe is 122°F and the temperature of the outer surface is 118°F. The thermal conductivity of the stainless steel is 108 Btu/hr-ft-°F.
Calculate the heat transfer rate through the pipe.
Calculate the heat flux at the outer surface of the pipe.
A 10 ft length of pipe with an inner radius of 1 in and an outer radius of 1.25 in has an outer surface temperature of 250°F. The heat transfer rate is 30,000 Btu/hr. Find the interior surface temperature. Assume k = 25 Btu/hr-ft-°F.
Solving for T h :
The evaluation of heat transfer through a cylindrical wall can be extended to include a composite body composed of several concentric, cylindrical layers, as shown in Figure 4.
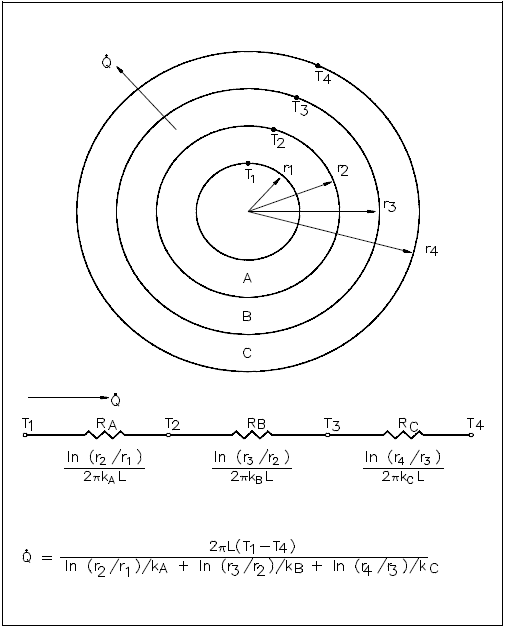
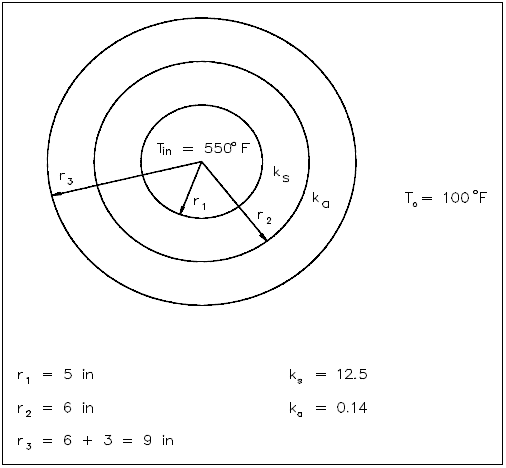
A thick-walled nuclear coolant pipe ( k s = 12.5 Btu/hr-ft-°F) with 10 in. inside diameter ( ID ) and 12 in. outside diameter ( OD ) is covered with a 3 in. layer of asbestos insulation ( k a = 0.14 Btu/hr-ft-°F) as shown in Figure 5. If the inside wall temperature of the pipe is maintained at 550°F, calculate the heat loss per foot of length. The outside temperature is 100°F.
Chapter: Mechanical : Heat and Mass Transfer : Conduction
Solved problems - heat and mass transfer - conduction.
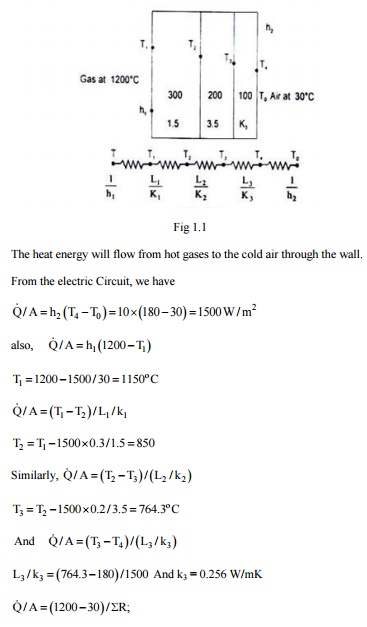
1. A composite wall consists of three layers of thicknesses 300 mm, 200mm and100mm with thermal conductivities 1.5, 3.5 and is W/m K respectively. The inside surface is exposed to gases at 1200°C with convection heat transfer coefficient as 30W/m 2 K. The temperature of air on the other side of the wall is 30°C with convective heat transfer coefficient 10 Wm 2 K. If the temperature at the outside surface of the wall is 180°C, calculate the temperature at other surface of the wall, the rate of heat transfer and the overall heat transfer coefficient.
Solution: The composite wall and its equivalent thermal circuits is shown in the figure.
2.Derivethe General Heat Conduction Equation for an Isotropic Solid with Constant
Thermal Conductivity in Cartesian coordinates.
Any physical phenomenon is generally accompanied by a change in space and time of its physical properties. The heat transfer by conduction in solids can only take place when there is a variation of temperature, in both space and time. Let us consider a small volume of a solid
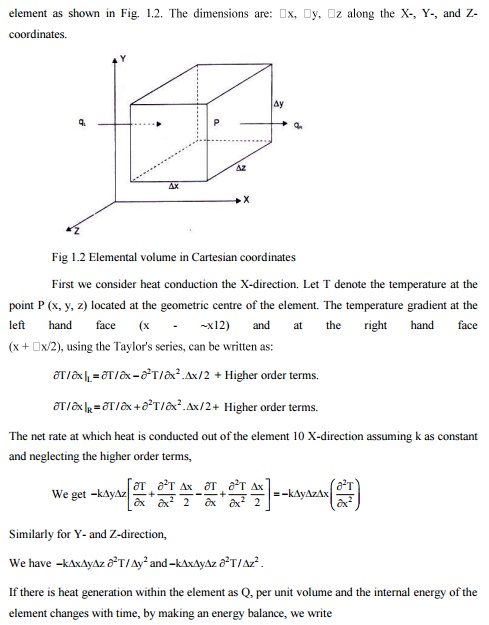
It should be noted that Fourier law can always be used to compute the rate of heat transfer by conduction from the knowledge of temperature distribution even for unsteady condition and with internal heat generation.
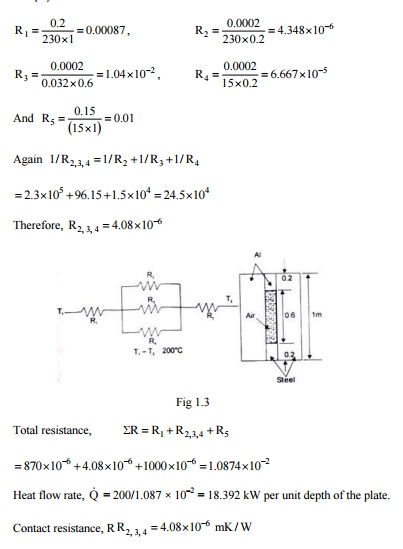
A 20 cm thick slab of aluminums (k = 230 W/mK) is placed in contact with a 15 cm thick stainless steel plate (k = 15 W/mK). Due to roughness, 40 percent of the area is in direct contact and the gap (0.0002 m) is filled with air (k = 0.032 W/mK). The difference in temperature between the two outside surfaces of the plate is 200°C Estimate (i) the heat flow rate, (ii) the contact resistance, and (iii) the drop in temperature at the interface.
Solution: Let us assume that out of 40% area m direct contact, half the surface area is occupied by steel and half is occupied by aluminums.
The physical system and its analogous electric circuits is shown in Fig. 1.3.
4. A steel pipe. Inside diameter 100 mm, outside diameter 120 mm (k 50 W/m K) IS Insulated with a40mm thick hightemperature Insulation(k = 0.09 W/m K) and another Insulation 60 mm thick (k = 0.07 W/m K). The ambient temperature IS 25°C. The heattransfer coefficient for the inside and outside surfaces are 550 and 15 W/m 2 K respectively. The pipe carries steam at 300 o C. Calculate (1) the rate of heat loss by steam per unit length of the pipe (11) the temperature of the outside surface .
Solution: A cross-section of the pipe with two layers of insulation is shown Fig. 1.4with its analogous electrical circuit.
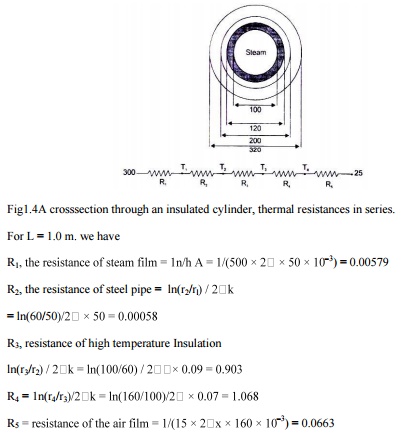
Fig1.4A crosssection through an insulated cylinder, thermal resistances in series.
For L = 1.0 m.

5. Steel balls 10 mm in diameter (k = 48 W/mK), (C = 600 J/kgK) are cooled in air at temperature 35°C from an initial temperature of 750°C. Calculate the time required for the temperature to drop to 150°C when h = 25 W/m2K and density p = 7800 kg/m3. (AU 2012).
Solution: Characteristic length, L = VIA = 4/3 p r 3 /4 p r 2 = r/3 = 5 × 10 -3 /3m Bi = hL/k = 25 × 5 × 10- 3 / (3 × 48) = 8.68 × 10 -4 << 0.1,
Since the internal resistance is negligible, we make use of lumped capacity analysis: Eq. (3.4),
( T - T ¥ ) / ( T s - T ¥ )=exp(-Bi Fo) ; (150 35) / (750 35) = 0.16084
\ Bi × Fo = 1827; Fo = 1.827/ (8. 68 × 10 -4 ) 2.1× 10 3
Or, a t/ L 2 = k/ ( r CL 2 )t = 2100 and t = 568 = 0.158 hour
We can also compute the change in the internal energy of the object as:
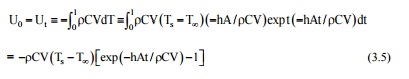
= -7800 × 600 × (4/3) p (5 × 10 -3 ) 3 (750-35) (0.16084 - 1)
= 1.47 × 10 3 J = 1.47 kJ.
If we allow the time't' to go to infinity, we would have a situation that corresponds to steady state in the new environment. The change in internal energy will be U0 - U ¥ = [ r CV( T s - T ¥ ) exp(-
¥ )- 1] = [ r CV( T s - T ¥ ].
We can also compute the instantaneous heal transfer rate at any time.
Or. Q = - r VCdT/dt = - r VCd/dt[ T ¥ + ( T s - T ¥ )exp(-hAt/ r CV) ]
= hA( T s - T ¥ )[exp(-hAt/ r CV)) and for t = 60s,
Q = 25 × 4 × 3.142 (5 × 10 -3 ) 2 (750 35) [exp( -25 × 3 × 60/5 × 10 -3 × 7800 × 600)] = 4.63 W.
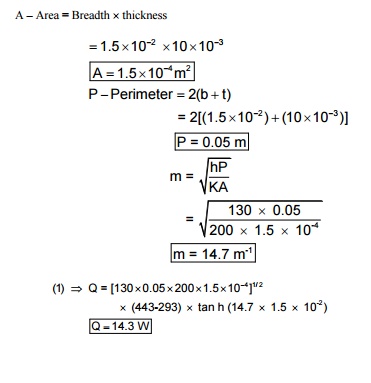
6.Aluminums fins 1.5 cm wide and 10 mm thick are placed on a 2.5 cm diameter tube to dissipate the heat. The tube surface temperature is 170 ° C ambient temperatures is 20 ° C. calculate the heat loss per fin. Take h = 130 W/m 2 C and K = 200 W/m 2 C for aluminums.
Wide of the fin b = 1.5 cm = 1.5 ´ 10 -2 m
Thickness t = 10 mm = 10 ´ 10 -3 m
Diameter of the tube d = 2.5 cm = 2.5 ´ 10 -2 m
Surface temperature T b = 170 ° C + 273 = 443 K
Ambient temperature T ¥ = 20 ° C + 273 = 293 K
Heat transfer co-efficient h = 130 W/m 2 ° C
Thermal conductivity K = 200 W/m ° C
Assume fin end is insulated, so this is short fin end insulated type problem.
Heat transfer [short fin, end insulated]
Q = (hPKA) 1/2 (T b - T ¥ ) tan h(mL) ……..(1) [FromNoHMT.41] data book
A –Area = Breadth ´ thickness
Related Topics
Privacy Policy , Terms and Conditions , DMCA Policy and Compliant
Copyright © 2018-2024 BrainKart.com; All Rights Reserved. Developed by Therithal info, Chennai.
Academia.edu no longer supports Internet Explorer.
To browse Academia.edu and the wider internet faster and more securely, please take a few seconds to upgrade your browser .
Enter the email address you signed up with and we'll email you a reset link.
- We're Hiring!
- Help Center

157927275-solved-problems-in-heat-transfer

Related Papers
Gerardo Mendez
he wall of a refrigerator is constructed of fiberglass insulation sandwiched between two since the e k = 15.1 W/m⋅°C for sheet metal ion can be ° C = 45 Using the thermal resistance network, heat transfer Chapter 3, Problem 35. The wall of a refrigerator is constructed of fiberglass insulation (k = 0.035 W/m ⋅ °C) sandwiched between two layers of 1-mm-thick sheet metal (k = 15.1 W/m ⋅ °C). The refrigerated space is maintained at 3°C, and the average heat transfer coefficients at the inner and outer s of the wall are 4 W/m 2 ⋅ °C and 9 W/m 2 ⋅ °C, respectively. The kitchen temperature avera 25°C. It is observed that condensation occurs on the outer surfaces of the refrigerator when the temperature of the outer surface drops to 20°C. Determine the minimum thickness of fibergla insulation that needs to be used in the wall in order to avoid condensation on the outer surfaces
Samuel Dowal-Asselin
Isabel Siles Guevara
Kelompok 8 Kelas C Labtek II 4.5-5. Cooling and Overall U. Oil flowing at the rate of 7258 kg/h with a c pm 2.01 kJ/kg K is cooled from 394.3 K to 338.9 K in a counterflow heat exchanger by water entering at 294.3 K and leaving at 305.4 K. Calculate the flow rate of the water and the overall Ui if the Ai is 5.11 m 2. Solution Assume c pm water is 4.187 kJ/kg K. Heat balance Q oil = Q water (m c pm ΔT) oil = (m c pm ΔT) water 7258. 2.01. (394.3-338.9) = m. 4.187. (305.4-294.3) m = 17389 kg/h ΔTm = Q = U i A i ΔT m 808207.332. = U i 5.11. 64.22 U i = 684 W/m 2 K 4.5-6. Laminar Flow and Heating of Oil. A hydrocarbon oil having the same physical properties as the oil in Example 4.5-5 enters at 175 0 F inside a pipe having an inside diameter of 0.0303 ft and a length of 15 ft. The inside pipe surface temperature is constant at 325 0 F. The oil is to be heated to 250 0 F in the pipe. How many lb m/h oil can be heated? Solution
Whitaker, Stephen
Abhirup Chakraborty
A set of problems on Heat and thermodynamics for 10+2 level buddies!
Nikhil Kumar
Cristian Esteban Arango
Faishol Mochammad
RELATED PAPERS
Joey Carroll
Joanna R Pieńkowska
celine stuart
Caderno Enic
leda santos
Andrew Dykens
Adriana Pavinatto
Bianka Schulz
Natasja Jensen
ethic@ - An international Journal for Moral Philosophy
Aguinaldo Antonio Cavalheiro Pavao
Laura Isabella Pardo
Circulation Journal
kritika singh
International Journal of Osteopathic Medicine
Narkeesh Arumugam
Biomass and Bioenergy
Nilce Ortiz
Research Gate
JIGNA LAKHANI
Via Atlântica
MARIA DANIELA ROSALES SALGADO
Sibonginkosi Maposa
International Journal of Applied Electromagnetics and Mechanics
Optics Letters
Djamel Benredjem
Profesional De La Informacion
Vanesa Saiz Echezarreta
Arquivos Brasileiros de Cardiologia
Aline LimA Lima
Historia Ambiental Latinoamericana y Caribeña
Márcia Franz Amaral
Jurnal Penelitian Agama Hindu
wayan wirta
Citizen Science: Theory and Practice
Talia Arjona
Astronomy and Astrophysics
Miriam Rengel
Arlinda Cantero Dorsa
See More Documents Like This
RELATED TOPICS
- We're Hiring!
- Help Center
- Find new research papers in:
- Health Sciences
- Earth Sciences
- Cognitive Science
- Mathematics
- Computer Science
- Academia ©2024

IMAGES
VIDEO
COMMENTS
1000 Solved Problems in Heat Transfer: Schaum's solved problems series by Ditts, Donald R. Publication date 1991 Topics Heat -- Transmission Publisher New York: McGraw-Hill Collection printdisabled; internetarchivebooks; inlibrary Contributor Internet Archive Language English. 421 tr Notes.
1000 Solved Problems in Heat Transfer (Schaum's Solved Problems Series) by Donald R. Pitts (Author), Leighton E. Sissom (Author) 5.0 out of 5 stars 1
Books. 1000 Solved Problems in Heat Transfer. Donald R. Pitts, Leighton E. Sissom. McGraw-Hill, 1991 - Heat - 421 pages. A compilation of 1000 problem-solving exercises with solutions on heat transfer, this text for undergraduates aims to provide a range of all possible problems which students may face.
Download site for A Heat Transfer Textbook. Solutions to more than 490 problems are on the following links. Solutions for Chapter 1 (v1.01, 16 MB, February 2023). Solutions for Chapter 2 (v1.0, 13 MB, August 2020). Solutions for Chapter 3 (v1.0, 15 MB, August 2020). Partial solutions for Chapters 4-11 (v1.05, 24 MB, 24 March 2023) Includes solutions for all problems in Chapters 4, 5, 6, 10 & 11
Heat and mass Transfer Unit I November 2008 1. Calculate the rate of heat loss through the vertical walls of a boiler furnace of size 4 m by 3 m by 3 m high. The walls are constructed from an inner fire brick wall 25 cm thick of thermal conductivity 0.4 W/mK, a layer of ceramic blanket insulation of
Authors: Donald R. Pitts, Leighton E. Sissom. Summary: A compilation of 1000 problem-solving exercises with solutions on heat transfer, this text for undergraduates aims to provide a range of all possible problems which students may face. Print Book, English, ©1991. Edition: View all formats and editions. Publisher: McGraw-Hill, New York, ©1991.
1,000 Solved Problems In Heat Transfer (Schaum's Solved Problems Series) Paperback - Import, 30 November 1990 by Donald Pitts (Author) 5.0 5.0 out of 5 stars 1 rating
Buy 1,000 Solved Problems In Heat Transfer (Schaum's Solved Problems Series) by Pitts, Donald (ISBN: 9780070502048) from Amazon's Book Store. Everyday low prices and free delivery on eligible orders.
1000 Solved Problems in Heat Transfer (Schaum's Solved Problems Series) by Pitts, Donald R.; Sissom, Leighton E. - ISBN 10: 0070502048 - ISBN 13: 9780070502048 - McGraw-Hill - 1990 - Softcover
Solve problems involving heat transfer; ... Because the density of water is 1000 kg/m 3 1000 kg/m 3, 1 L of water has a mass of 1 kg, and the mass of 0.250 L of water is m w = 0.250 kg m w = 0.250 kg. Calculate the heat transferred to the water. Use the specific heat of water in Table 1.3:
SCHAUM'S SOLVED PROBLEMS SERIES 1000 SOLVED PROBLEMS IN HEAT TRANSFER by Donald R. Pitts, Ph.D. The University of Tennessee Leighton E. Sissom, Ph.D. Tennessee Technological University McGRAW-HILL, INC. New York St. Louis San Francisco Auckland Bogota Caracas Hamburg Lisbon London Madrid Mexico Milan Montreal New Delhi Paris San Juan Sao ...
Dr. Osama M Elmardi. This book aims to give students of engineering a thorough grounding in the subject of heat transfer. The book is comprehensive in its coverage without sacrificing the necessary theoretical details. The book is designed as a complete course text in heat transfer for degree courses in mechanical and production engineering and ...
Solve problems on the relationships between heat transfer, time, and rate of heat transfer; Solve problems using the formulas for conduction and radiation; Just as interesting as the effects of heat transfer on a system are the methods by which it occurs. Whenever there is a temperature difference, heat transfer occurs.
In conduction heat transfer problems, the object being studied is usually a solid. Convection problems involve a fluid medium. ... 1000 Btu/hr is conducted through a section of insulating material shown in Figure 1 that measures 1 ft 2 in cross-sectional area. ... The electrical analogy may be used to solve complex problems involving both ...
208 solved problems having units and 63 solved problems with no units. The percentage split between SI and English units is approximately 75/25 whereas the first edition had approximately 60/40 English/SI problems. But we have included enough English unit system solved problems to satisfy the needs of students and
The heat transfer coefficient for conduction and convection from the casing to the ambient air is obtained from Nu = 2 + .6Re1/2Pr1/3, with Re = 104 and Pr = 0.69. The temperature of the ambient air is 30 C and the thermal conductivity of air is k = 0.02 W/m.K. Find the heat flux from the surface at steady state.
Heat Transfer {Practical Lecture 5 (Solved Problems) 24.A uid condenses at the temperature of 50 C (h= 3500Wm 2 K 1) when circulating inside a steel pipe (k= 50Wm 1 K 1) with an external diameter of 25mm. The thickness of the tube is 1mm. A fan provides an air ow at 25 C perpendicular to the tube (v 1= 20ms 1), the convection coe cient being ...
Solved Problems - Heat and Mass Transfer - Conduction. 1. A composite wall consists of three layers of thicknesses 300 mm, 200mm and100mm with thermal conductivities 1.5, 3.5 and is W/m K respectively. The inside surface is exposed to gases at 1200°C with convection heat transfer coefficient as 30W/m2K. The temperature of air on the other side ...
Here is the equation: Q = mcΔT. Q is thermal energy in joules (J) m is the mass of the object in grams (g) c is the caloric requirement for the phase of matter the object is in (J/g⋅°C) - for ...
Read reviews from the world's largest community for readers. undefined
The refrigerated space is maintained at 3°C, and the average heat transfer coefficients at the inner and outer s of the wall are 4 W/m 2 ⋅ °C and 9 W/m 2 ⋅ °C, respectively. The kitchen temperature avera 25°C. It is observed that condensation occurs on the outer surfaces of the refrigerator when the temperature of the outer surface ...
Solved Problems of Mass and Heat Conduction . Dr. Mohammad Ali ... Noting That Heat Transfer Is One-Dimensional In The Radial R Direction And Heat Flux Is In The ... 1000. 255.8 . 1200. 264.5 ...
universities. The selected problems display a large variety and conform to syllabi which are currently being used in various countries. The book is divided into 15 chapters. Each chapter begins with basic concepts and a set of formulae used for solving problems for quick reference, followed by a number of problems and their solutions.