
- For Ophthalmologists
- For Practice Management
- For Clinical Teams
- For Public & Patients
Museum of the Eye
- Browse All Education
- Learning Plans
- Interactive
- Focal Points
- Wills Eye Manual
- Disease Reviews
- Clinical Webinars
- Diagnose This
- Self-Assessments
- Glaucoma Education Center
- Pediatric Ophthalmology Education Center
- Global Ophthalmology Guide
- Laser Surgery Education Center
- Redmond Ethics Center
- Ocular Trauma Resources
- Myopia Resources
- Thyroid Eye Disease Resources
- Practice Guidelines
- Drug-Resistant Pseudomonas Outbreak
- Preferred Practice Patterns
- Clinical Statements
- Ophthalmic Technology Assessments
- Patient Safety Statements
- Complementary Therapy Assessments
- Medical Information Technology
- Diagnostic Excellence
- Choosing Wisely
- Eye Care for Older Adults
- Eye Disease Statistics
- About the Hoskins Center
- Artificial Intelligence
- Premium IOLs
- Patient-Reported Outcomes with LASIK Symptoms and Satisfaction
- Multimedia Library
- 1-Minute Videos
- Presentations and Lectures
- Master Class Videos
- Basic Skills Videos
- Clinical and Surgical Videos
- Resident Lectures
- Submit a Video
- YO Video Contest
- Browse Podcast Library
- Experts InSight Podcast
- Ophthalmology Journal Podcast
- Submit an Image
- Browse Clinical News
- Editors' Choice
- Current Insight
- CME Central
- About Continuing Certification
- Claim CME Credit and View Transcript
- CME Planning Resources
- Complete Your Financial Disclosure
- LEO Continuing Education Recognition Award
- Safe ER/LA Opioid Prescribing
- Check Your Industry Payment Records
- Resident Education Home
- Flashcards and Study Presentations
- Interactive Cases and Simulations
- Diversity and Inclusion Education
- News and Advice from YO Info
- Board Prep Resources
- OKAP and Board Review Presentations
- Study Flashcards
- PGY-1 and PGY-2 Resources
- Physician Wellness
- Resident Knowledge Exchange
- Simulation in Resident Education
- Ophthalmology Job Center
- Clinical Education /
- Education /
- Interactive /

Chronic Vision Loss

- Mark Complete
In this interactive case you will learn about evaluating a patient who presents with chronic vision loss, the causes and treatments for chronic vision loss and when to refer a patient to an ophthalmologist.

All content on the Academy’s website is protected by copyright law and the Terms of Service . This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
- About the Academy
- Jobs at the Academy
- Financial Relationships with Industry
- Medical Disclaimer
- Privacy Policy
- Terms of Service
- Statement on Artificial Intelligence
- For Advertisers
FOLLOW THE ACADEMY
Medical Professionals
Public & Patients
Learn how UpToDate can help you.
Select the option that best describes you
- Medical Professional
- Resident, Fellow, or Student
- Hospital or Institution
- Group Practice
- Patient or Caregiver
- Find in topic
RELATED TOPICS
INTRODUCTION AND DEFINITIONS
Transient visual loss (TVL), either monocular or binocular, reflects a heterogeneous group of disorders, some relatively benign and others with grave neurologic or ophthalmologic implications. The task of the clinician is to use the history and examination to localize the problem to a region in the visual pathways, identify potential etiologies, and, when indicated, perform a focused battery of laboratory tests to confirm or exclude certain causes. Therapeutic interventions and prognostic implications are specific to the underlying cause.
This topic discusses TVL. Other ocular and cerebral ischemic syndromes are discussed separately. (See "Central and branch retinal artery occlusion" and "Posterior circulation cerebrovascular syndromes" .)
APPROACH TO TRANSIENT VISUAL LOSS
Few case series of patients with TVL are reported. Details from the Framingham cohort provide some interesting insights into the challenges of evaluating this symptom. Between 1971 and 1989, participants were systematically questioned regarding specific symptoms of transient ischemic attack (TIA) and stroke; 186 of 2110 subjects reported onset of a sudden visual deficit (not necessarily transient) [ 4 ]. Follow-up evaluation determined the underlying cause to be stroke or TIA (24 percent), ocular disease (17 percent), transient monocular blindness (10 percent), and migraine (14 percent). The cause remained unknown in 22 percent, and a miscellany of etiologies comprised the remaining 12 percent.
- See us on twitter
- See us on facebook
- See us on linkedin
Why Giving Matters: Gaining Insight Into Vision Loss
As virtually anyone who has suffered vision loss can attest, the condition can be life-altering and frightening, potentially affecting one’s mobility, independence, security, and quality of life. And when that vision loss occurs suddenly and without warning, it can be downright terrifying.

Just ask Duke Rohlen. Diagnosed with a condition called non-arteritic anterior ischemic optic neuropathy, or NAION, four years ago, the Bay Area industry leader’s medical journey began when he woke one day with sudden and dramatic visual impairment. As he relates in a moving essay , Duke faced the prospect of having to transition from an active, healthy life to one that was irretrievably affected by vision loss and perhaps even blindness.
Luckily, however, Duke was able to quickly meet with a team of specialists at the Byers Eye Institute at Stanford after his diagnosis. Joyce Liao, MD, PhD, professor of ophthalmology and of neurology and director of neuro-ophthalmology, and Shannon Beres, MD, clinical associate professor of neurology and clinical assistant professor of ophthalmology, were able to quickly and accurately assess Duke’s condition and help stop the progression of his disease. Dr. Liao and her lab have been conducting pioneering research into this and other optic nerve conditions for several years, which allowed him to receive the best possible care.
Today, Duke is happy to report that after receiving comprehensive, expert care at Stanford, he has had a positive outcome and is leading a rich, meaningful life. He remains forever grateful to Dr. Liao and her team and has made a generous donation to the Byers Eye Institute to further research into NAION.
“I have had the extraordinarily good fortune to have been cared for at the Byers Eye Institute at Stanford,” Duke says. “The cutting-edge doctors, staff, and technology are collectively pushing the boundaries of advanced eye care. My wife, Kendall, and I are honored to financially support this team and their scientific initiatives.”
Research Into a Rare Disease
NAION affects approximately 10 out of every 100,000 people over the age of 50. The condition is analogous to an optic-nerve stroke—one that affects the information highway that connects the eye and the brain. It is caused by a loss of blood supply, and therefore oxygen delivery, to the highly metabolically active optic nerve. While NAION typically occurs in one eye, the second eye has about a 15 percent chance of developing the same problem.
“The eye acts as the camera that captures the information and the brain processes that information,” Dr. Liao explains. “So, when you lose oxygen in this information highway, you disconnect the two parts of the visual system, thereby leading to vision loss or visual dysfunction.”
There are very few specialists with the knowledge and expertise to treat NAION. In fact, the Byers Eye Institute is the only center west of the Mississippi that is conducting the research vital to studying, and hopefully one day curing, this devastating disease.
“We need to make dramatic progress in escalating our pace of discovery to help patients suffering from this terrible disease, and the way we can do that is through philanthropy. We’re so thankful to donors like Duke.”
— Joyce Liao, MD, PhD
The research Dr. Liao and her team are conducting is wide-ranging and comprehensive. In addition to studying the role of genetics and environment on NAION, they are engaged in advanced eye imaging, as well as analysis of plasma proteins, metabolic processes, and mitochondrial function in skin fibroblasts to gain an extremely detailed profile of factors that may be involved in the disease. They are also testing hyperbaric oxygen therapy in patients with acute NAION, in which oxygen is delivered directly to the injured tissue where the blood vessels are compromised. Patients are assessed before and after treatment to better understand the key retinal changes that occur following treatment.
Since NAION is sometimes observed as a consequence of high-altitude exposure, such as during travel on commercial airlines or skiing in the mountains, Dr. Liao’s team is using an animal model of systemic hypoxia to better understand the key changes in the optic nerve and retina following such activities and how best to promote functional recovery. In addition, to understand why some patients develop NAION in only one eye while others unfortunately progress to optic-nerve stroke in both eyes, Dr. Liao’s team is generating retinal neurons using stem cells taken from the skin of NAION patients and studying these cells in a petri dish in the lab.
Of course, all of this work requires funding. “There is no foundation supporting research into this disease, and funding from the National Institutes of Health can never be enough,” Dr. Liao says. “It’s reasonable to say that without the generous support of our patients and our supporters, this research would not be possible.
“We need to make dramatic progress in escalating our pace of discovery to help patients suffering from this terrible disease,” she adds, “and the way we can do that is through philanthropy. We’re so thankful to donors like Duke.”
NAION Treatment at Stanford
Although there is currently no cure and no standard proven treatment for NAION, Dr. Liao and her team have made important strides in researching the disease and helping to stop its progression, and even achieving some visual recovery in some patients. While treatment is individualized according to a patient’s risk factors, interventions might include a combination of medications (typically drugs that have been approved by the U.S. Food and Drug Administration for use in other conditions), treatment in a hyperbaric chamber, and perhaps surgery to correct risk factors that lead to systemic hypoxia. Patient-derived stem cells can also be developed for use in novel, regenerative therapy—the ultimate precision medicine for the treatment of NAION.
“We do absolutely everything we can for every patient,” Dr. Liao says. “We check off every single box we can. And because of the research we’re conducting, we can check off more boxes than anyone else in the world right now.”
Beyond the clinical side of care, Dr. Liao and her team also focus on the psychological and emotional aspect of treatment. “Obviously it is important for patients to continue to try to lead the lives they want to live,” Dr. Liao says. “As we understand more about this disease, we can better counsel patients about what they can do. But above all, they should continue to do the things they really love.”
That’s just what Duke Rohlen is doing.
You, too, can make a difference by supporting NAION Research. Please click on the button below, then under "Direct your gift," choose "Other Designation" and enter "NAION Research – Ophthalmology Department" in the field. Thank you for your support!
To learn more about NAION research and how you can support this work, contact Melanie Erasmus .
Make a difference. Support us by making an online donation today.

The Clinical Problem Solvers
Cpsolvers x 100. exclusive figures, videos, and case challenges uploaded regularly..

The goal of this website is to teach clinical reasoning with a focus on diagnosis.
Diagnosis is one of the most important clinical skills. You cannot treat a patient or provide a prognosis without the right diagnosis.
Get all the latest news and updates from RLRCPSolvers
You can unsubscribe at any time by clicking the link in the footer of our emails. For information about our privacy practices, please visit our Privacy Policy page.
Get access to the latest Schemas, Scripts and Videos

Figures will cover all topics in medicine, including neurology, through diagnostic schemas and illness scripts.

The videos feature Reza and Rabih teaching you medicine with passion as if you were on the wards with them.
Case Challenges
Reza and Rabih present each other unknown cases in these recordings. We encourage you to pause the recording after each aliquot, reflect on the aliquot, then unpause and compare your thinking to Reza and Rabih. We will upload at least 3 case challenges per month.
With Regular Content Updates!
Recent case challenges.

Case Challenge #93: Rabih and Reza discuss with Uncle Bob, a patient with left knee pain

Case Challenge #92: Recapping the Journey and Supplementary Teaching Material – Syncope and dysphagia

Case Challenge #92: Rabih and Reza discuss a patient with syncope and dysphagia

Case Challenge #91: Rabih and Reza discuss with Aaron, a patient with subacute confusion
Monthly plan.
- Access with no long-term commitments
Quarterly Plan
- Save $30 when paying quarterly!
Annual Plan
- Save $240 when paying annually!

Students! You may be able to claim a 50% discount on an RLRCPSolvers membership. Click here to find out how.
Contact us for institutional rates.
Disclaimer: The CPSolvers provides information for educational purposes only. It is not intended to be medical advice.
Read our Terms and Conditions and Privacy Policy
Member Reviews
Living my md dream through you.
Oh my word! This is my absolute favorite podcast and now website(subscription) in all the world, planets, and galaxies!! My dream was to go to medical school. Unfortunately many things disrupted that. I love my current job as a Teacher/Specialist with a Master’s in Literacy. I did start nursing school in the evenings after teaching school all day. That was very challenging and life happened. So, listening to Rabih and Reza’s deduce and solve these cases fills my hole for my dream deferred. I like to pretend I’m the MD and figure out what is happening and look for the little clues for the not so obvious diagnoses. I love the chemistry between those two. There is just enough humor and side stories to give added interest. I have 3 Dr’s in my family, and I’ve told them all to add this to their playlist. This has even helped me in my teaching of non-medical materials. It would take me pages to say how much I have learned from this! At the end of the day, I’m so excited to turn on my Bluetooth shower speaker and enjoy a case challenge. Thanks for making this once hopeful MD fulfilled. I wish you all the best in your endeavors.
Hard to navigate
It’s really hard to find the extra episodes and the videos and reviews that are mentioned. I paid for the service but haven’t been able to really gain much from it
My favorite medical podcast for many reasons
Is there a tier where I can have you on speed dial? Seriously though, I have followed you through all of your platforms over the years and have learned so much from EVERY EPISODE in terms of problem representation, framework, clinical reasoning, not to mention kindness, respect and humility in medicine! You both embody the type of medical professional I hope to be. Thank you for all you are doing and please, please, please keep up these recordings forever
Cholesytalgia RLR in the wild
First, you guys are amazing. I found your podcast after listening to an episode where you guested on the curbsiders, and I’ve been hooked since! I’m working my way through all of the rlr episodes, and recently listened to the episode about a patient with chf and cholesystalgia. The very next week, I saw a patient in my small community ED with days of RUQ pain. Recent history of infective endocarditis, complicated by significant aortic regurgitation requiring valve replacement. Valve replacement surgery complicated by 3rd degree heart block post operatively requiring pacemaker placement. Vitals were stable in triage, physical exam showed well healing surgical incisions, no signs of infection, regular rate and rhythm, mechanical valve clearly auscultated. Patient was tender in RUQ, +Murphys. No rebound or guarding. laboratory workup was unremarkable with the exception of being slightly supratherapeutic on warfarin (INR 3.3 with a target of 2-3) , ruq US showed some gallbladder wall edema, but no stones or pericholecystic fluid. per radiology report, “in the right clinical setting, this could be consistent with acute cholecystitis”.
Something did not quite feel like an acute chole, so I did a quick POCUS and on subxiphoid view I noted a HUGE effusion.
He ended up being transferred to tertiary center for pericardial window placement. I reviewed his op report, he had a 900 cc hemopericardium! Slow bleed after surgery that didn’t resolve due to anticoagulation.
*learns something new on podcast*
Week passes
*opportunity to cement learning walks into ED*
Thank you guys for everything you do! Your work helps create better clinicians!!
A Medical Student Must Have!
Rabih and Reza are all-star clinicians that teach with such authenticity and humbleness the art of clinical reasoning. Learning their diagnostic schemas through videos and diagrams and reinforcing the knowledge in the context of case discussions will give you unparalleled confidence in being complete with your differential diagnosis in such a straight forward and conceptual manner. I have found so much joy in medicine through their resources and discussions!

- Privacy Overview
- Strictly Necessary Cookies
- 3rd Party Cookies
This website uses cookies so that we can provide you with the best user experience possible. Cookie information is stored in your browser and performs functions such as recognising you when you return to our website and helping our team to understand which sections of the website you find most interesting and useful.
Strictly Necessary Cookie should be enabled at all times so that we can save your preferences for cookie settings.
If you disable this cookie, we will not be able to save your preferences. This means that every time you visit this website you will need to enable or disable cookies again.
This website uses Google Analytics to collect anonymous information such as the number of visitors to the site, and the most popular pages.
Keeping this cookie enabled helps us to improve our website.
Please enable Strictly Necessary Cookies first so that we can save your preferences!

MATTHEW PFLIPSEN, MD, MARIAMA MASSAQUOI, MD, AND SUZANNE WOLF, DO
Am Fam Physician. 2016;93(12):991-998
Related letter : Importance of Careful Corneal Inspection Prior to Fluorescein Examination
Author disclosure: No relevant financial affiliations.
Eye problems constitute 2% to 3% of all primary care and emergency department visits. Common eye conditions that can cause eye pain are conjunctivitis, corneal abrasion, and hordeolum, and some of the most serious eye conditions include acute angle-closure glaucoma, orbital cellulitis, and herpetic keratitis. The history should focus on vision changes, foreign body sensation, photophobia, and associated symptoms, such as headache. The physical examination includes an assessment of visual acuity and systematic evaluation of the conjunctiva, eyelids, sclera, cornea, pupil, anterior chamber, and anterior uvea. Further examination with fluorescein staining and tonometry is often necessary. Because eye pain can be the first sign of an ophthalmologic emergency, the physician should determine if referral is warranted. Specific conditions that require ophthalmology consultation include acute angle-closure glaucoma, optic neuritis, orbital cellulitis, scleritis, anterior uveitis, and infectious keratitis.
Eye problems constitute 2% to 3% of all primary care and emergency department visits. 1 , 2 Conjunctivitis, corneal abrasion, and hordeolum account for more than 50% of eye problems. 1 , 2 Disorders that cause eye pain can be divided by anatomic area, with most affecting the cornea. Because most conditions that cause eye pain are associated with ocular signs and symptoms, familiarity with the differential diagnosis allows clinicians to appropriately tailor the history and physical examination ( Table 1 3 – 20 and Table 2 11 ) .
Initial evaluation should include questions about vision loss or changes. Eye pain with vision loss requires immediate ophthalmology referral.
A foreign body sensation suggests a corneal process, such as a corneal abrasion, retained foreign body, or keratitis. 3 In contrast, a scratchy, gritty, or sandy sensation is more likely to be associated with conjunctivitis. 4
When assessing for keratitis, clinicians should ask about contact lens use and discuss lens care regimens. A contact lens history includes wearing schedule; overnight wear; contact lens hygiene protocol; use of tap water to rinse contact lenses; and swimming, using a hot tub, or showering while wearing contact lenses. Bacterial and Acanthamoeba keratitis are associated with inappropriate contact lens use or care. 12 , 21 , 22
Photophobia can be a sign of corneal involvement. 3 Photophobia with eye pain is associated with most forms of keratitis, but can also occur with anterior uveitis and less commonly with migraine headache. 5 , 23
Headache with associated eye pain can be a sign of ophthalmologic and neurologic conditions, such as acute angle-closure glaucoma, scleritis, cluster headaches, and less commonly migraines. 3 , 5 Cluster headaches present as severe unilateral eye pain, ptosis, ipsilateral conjunctival injection, and headache. 5 , 6
Systemic disease should be considered in patients with certain ocular conditions. For example, one study demonstrated that about 50% of patients with scleritis had associated rheumatologic disease. 20 Another study showed that about 40% of patients with optic neuritis will develop multiple sclerosis over a 10-year period. 24 Although uveitis is idiopathic in 60% of cases, workup for systemic inflammatory disease and infectious etiologies should be considered when uveitis is recurrent or bilateral. 3 , 25
Physical Examination
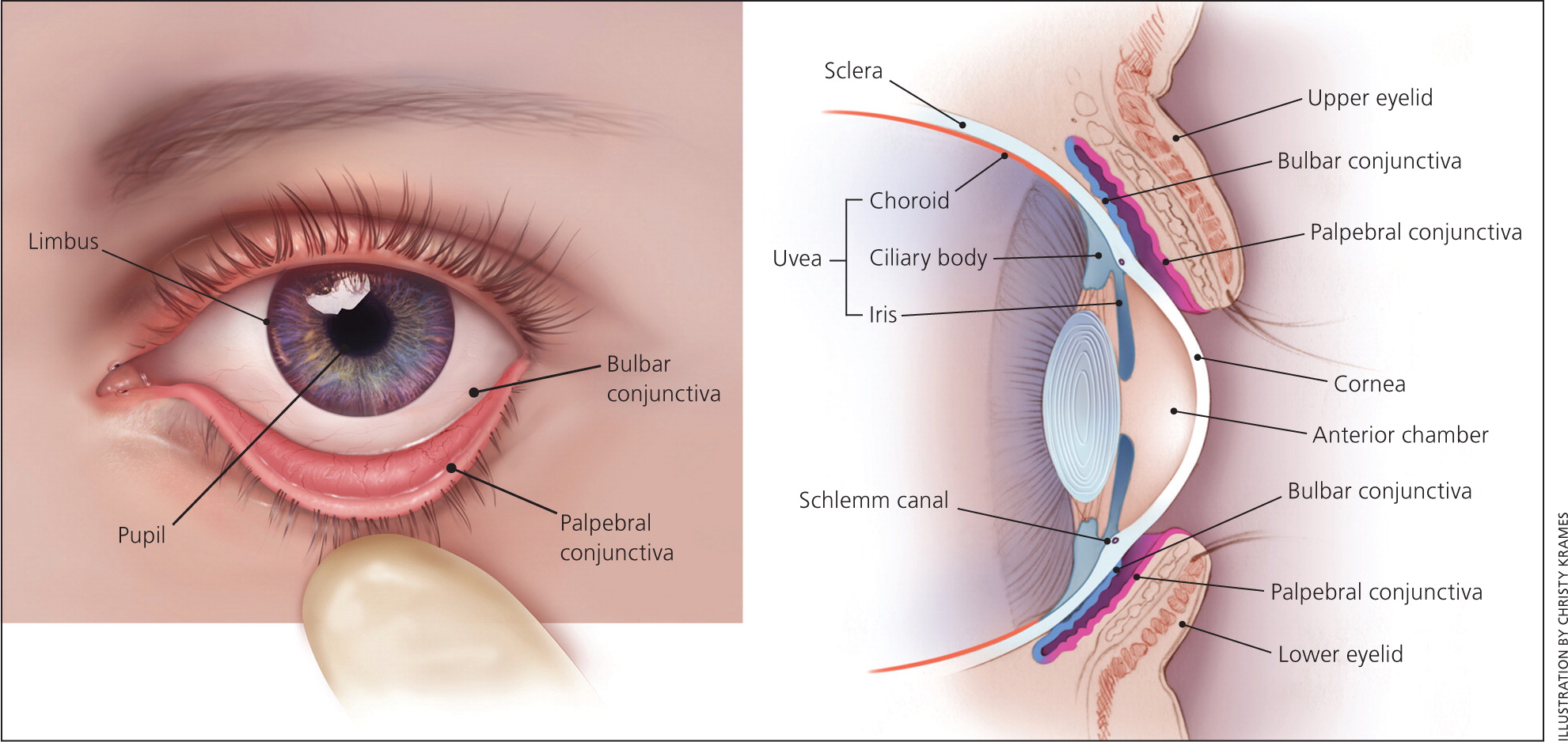
It is important for clinicians to be familiar with the basic anatomy of the eye ( Figure 1 ) so that they can perform an adequate examination. Equipment for assessing eye anatomy and function that is most often available to the primary care physician includes a Snellen chart, tonometer, penlight, fluorescein stain, and Wood lamp. Figure 2 outlines a stepwise approach to the evaluation of eye pain.
Functional Assessment
All patients presenting with eye pain should be assessed for vision loss. Having the patient read a Snellen chart ( https://www.aafp.org/afp/2013/0815/p241.html#afp20130815p241-f1 ) at a distance of 20 ft (6 m) is the standard test to evaluate visual acuity. Gross visual deficits are assessed using confrontational testing. The kinetic red test is performed by taking a 5-mm, red-topped pen and moving it inward from the boundary of each visual quadrant until the patient can see it. This test may be combined with the more common static finger wiggle test to improve sensitivity for detecting visual field loss. This combination is the most sensitive way to assess for visual field deficit in the primary care setting. 26 Determining more subtle differences, such as whether vision loss is diffuse, central, or peripheral, may require ophthalmology referral for more precise testing.
Conditions that cause eye pain and can cause decreased visual acuity include acute angle-closure glaucoma, herpes simplex virus (HSV) keratitis, optic neuritis, and orbital cellulitis. Acute angle-closure glaucoma can cause severe central visual field defects 27 ; similar visual findings may occur in patients with optic neuritis, with diffuse and central loss predominant in the affected eye. 28 Visual acuity of the affected eye is reduced to 20/100 in 10% of recurrent HSV keratitis cases. 7 Most painful eye conditions causing decreased visual acuity require ophthalmology referral.
EXTRAOCULAR MOVEMENT
To test extraocular movements, the patient should be instructed to fixate on a target with both eyes and follow it in at least four different directions. Increased intraocular pressure from acute angle-closure glaucoma may cause disordered eye motility or pain with eye movement. 29 Pain associated with eye movement may also occur with scleritis, optic neuritis, and orbital cellulitis.
Anatomic Assessment
External structures.
Clinicians should look for inflammation and erythema of the eyelids, making note of any lesions or abnormalities. A hordeolum is a tender, inflamed nodule and can be observed with careful inspection of the external or internal eyelid. The upper lid should be everted if a corneal abrasion is suspected to look for a foreign body. Orbital cellulitis presents as unilateral erythema, swelling, and ptosis of the eyelid, with associated pain with eye movement and decreased visual acuity. 8
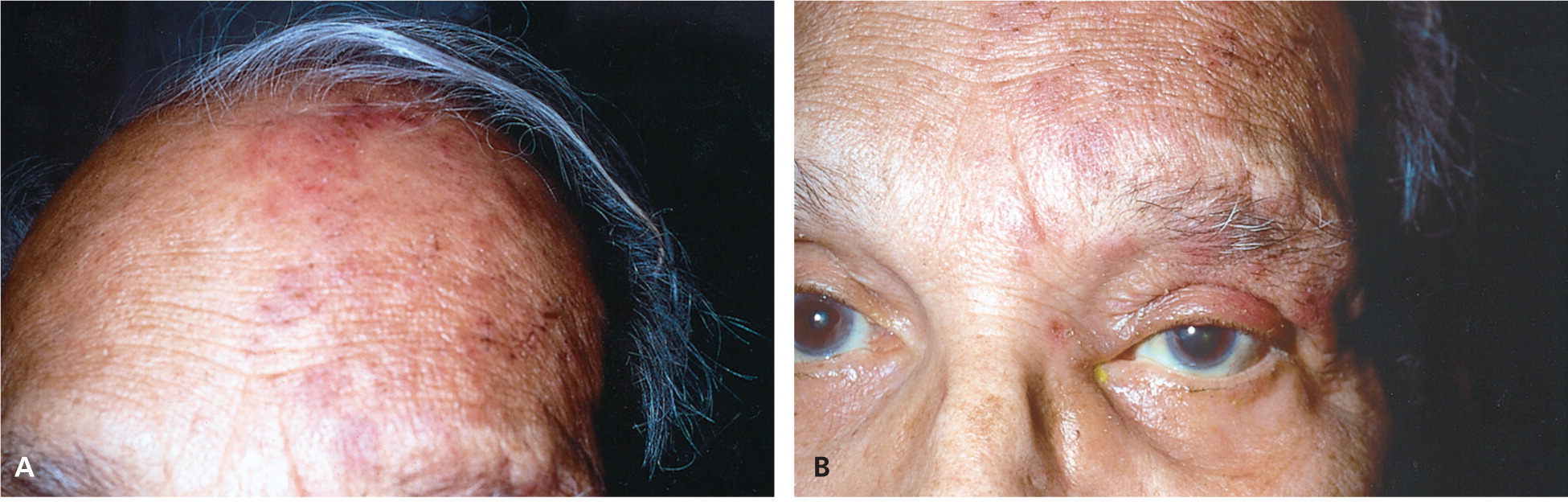
The eyelid and surrounding region should also be inspected for rashes or vesicles. Conjunctival or eyelid vesicles occur in about one-half of patients with HSV keratitis, 30 whereas herpes zoster ophthalmicus leads to associated pain and vesicular lesions appearing in a larger dermatome pattern ( Figure 3 31 ) on the forehead, nose, and upper eyelid (V 1 distribution of the trigeminal nerve). 9 , 29 Figure 4 shows slit lamp findings in a patient with herpes zoster ophthalmicus. 31
CONJUNCTIVA
The conjunctiva is a thin mucous membrane that covers the posterior eyelids (palpebral conjunctiva) and anterior sclera (bulbar conjunctiva). Injection of the conjunctiva is a result of inflammation or infection. Diffuse injection is caused by disease within the conjunctiva itself, whereas a ciliary flush sign (injection radiating outward from the limbus) is more common with a disease process in the uvea or anterior chamber, such as anterior uveitis or acute angle-closure glaucoma. 4 , 32
The sclera is a fibrous, protective coating of the eye. The episclera covers the sclera anteriorly and is continuous with the cornea. The sclera's bluish discoloration helps to distinguish it and differentiate scleritis from episcleritis. Inflammation of the sclera is usually very painful, whereas inflammation of the episclera is not. Scleritis can also impair vision, and vision is unaffected with episcleritis. 20 Episcleritis causes engorgement of the more superficial vessels, which are often sectoral and easily blanched with topical application of phenylephrine. 3
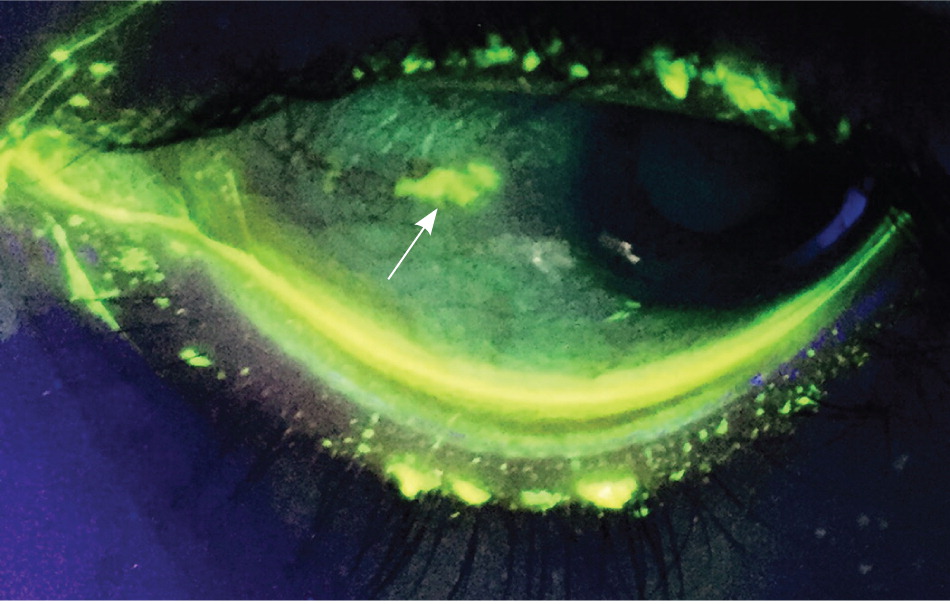
The cornea (transparent structure covering the anterior of the eye) should be evaluated with fluorescein staining. In the primary care setting, a Wood lamp or ophthalmoscope with a cobalt filter is often used for fluorescein visualization. If pain precludes evaluation, proparacaine 0.5% or other topical anesthetic should be applied first.
A healthy cornea is smooth, shiny, and clear. In normal light, corneal lesions appear yellow. Illumination with cobalt light or a Wood lamp causes the lesion to fluoresce green ( Figure 5 ) . An abrasion caused by trauma or a foreign body is typically linear or has a geographic shape. Abrasions from the use of contact lenses often consist of several punctate lesions that coalesce into a round central defect. Herpetic keratitis has a branching, dendritic appearance. 10
Normal pupillary size is 2 to 4 mm. Each pupil should constrict with consensual and direct light. Anisocoria (unequal pupil size) of less than 1 mm occurs in up to 20% of the general population. 33 Anisocoria associated with eye pain can be a sign of anterior uveitis. A fixed dilated pupil at 4 to 6 mm can occur with acute angle-closure glaucoma.
Photophobia using the penlight test can identify patients with uveitis or keratitis. 23 This test is performed by shining a penlight directly into each eye independently from a distance of 6 in (15 cm) for two seconds to determine if there is discomfort with light. A negative result makes uveitis and keratitis unlikely (negative predictive value = 90%). 23 , 34
The swinging flashlight test (see video at https://www.youtube.com/watch?v=soiKbngQxgw ) is used to diagnose an afferent pupillary defect (Marcus Gunn pupil). The defect is present in a pupil that dilates when the light is swung to it from the opposite pupil (constricting more with consensual light than with direct light). A relative afferent pupillary defect in a patient presenting with eye pain can indicate optic neuritis, although a negative result does not rule it out. 35 , 36
ANTERIOR CHAMBER
The anterior chamber between the cornea and iris is filled with aqueous humor. This fluid is absorbed where the cornea and iris meet at the Schlemm canal. The oblique flashlight test (see video at https://www.youtube.com/watch?v=81jEkGmQ4so ) can be used to approximate the depth of the anterior chamber angle. The examiner shines a penlight tangentially across the cornea from the temporal side. Illumination of the entire cornea implies a wide anterior chamber angle, and a shadow over the nasal portion of the cornea implies a narrow angle. 29 Acute angle-closure glaucoma is more common in persons with a narrow angle. If acute angle-closure glaucoma is suspected, tonometry should be performed. Pressures greater than 40 to 50 mm Hg are consistent with the diagnosis.
ANTERIOR UVEA
The iris and ciliary body make up the anterior uvea. Inflammation of one or both of these structures is considered anterior uveitis. Although hypopyon (white blood cells in the anterior chamber) can often be seen without magnification, a slit lamp is necessary for adequate evaluation. 3 The hallmark of acute anterior uveitis is the presence of white blood cells floating in the aqueous humor of the anterior chamber and a cloudy appearance consistent with a proteinaceous flare. Symptoms include achy eye pain, photophobia, and blurred vision in the involved eye. 25 , 32
There are a few indications for imaging when evaluating eye pain. Gadolinium-enhanced magnetic resonance imaging of the brain and orbits is essential in the workup of suspected optic neuritis. In suspected orbital cellulitis, computed tomography of the orbits and paranasal passages helps confirm the diagnosis and evaluate for associated complications, such as an abscess. However, the diagnostic yield of neuroimaging is minimal in patients with unilateral eye or facial pain, normal examination findings, and no history findings suggestive of a specific diagnosis or pain syndrome. 37
Emergent Ophthalmologic Disease
A history of trauma and signs of hyphema or corneal penetration warrant urgent, same-day evaluation by an ophthalmologist. A hyphema is a collection of blood in the anterior chamber between the cornea and the iris. A positive result on the Seidel test indicates a foreign body that has penetrated the full thickness of the cornea. This occurs when the anterior chamber has been punctured and its aqueous humor dilutes the fluorescein dye, causing it to flow across the cornea. 38
In acute angle-closure glaucoma, optic nerve atrophy and permanent loss of vision can occur within hours if not adequately treated. Prompt consultation with an ophthalmologist is recommended for treatment to lower the intraocular pressure. 39
Orbital cellulitis requires hospital admission, broad-spectrum intravenous antibiotics, and ophthalmology consultation. Workup includes contrast-enhanced computed tomography of the orbits and paranasal sinuses, as well as complete blood count and blood cultures. 11
Because scleritis can cause vision loss, the involvement of the more posterior structure, such as the retina, should be determined and managed accordingly.
Immediate referral is important if anterior uveitis is suspected because this disorder can also impair vision. A slit lamp examination looking for inflammatory cells in the anterior chamber is key to the diagnosis.
Optic neuritis warrants neurology and ophthalmology consultation. Acute management of optic neuritis includes administration of high-dose corticosteroids, which improves short-term recovery and expedites resolution of vision loss. 40
Infectious keratitis (caused by bacteria, Acanthamoeba , HSV, and herpes zoster ophthalmicus) necessitates ophthalmologic referral. Recurrent HSV keratitis increases risk of visual loss from corneal damage, 7 and herpes zoster ophthalmicus can cause chronic ocular inflammation, vision loss, and disabling pain. 9
Data Sources : We searched the Cochrane Database of Systematic Reviews, Essential Evidence Plus, Clinical Evidence, the National Guideline Clearinghouse, National Institute for Health and Clinical Excellence guidelines, and PubMed. The search included meta-analyses, randomized controlled trials, clinical trials, and reviews. We used the following key words: eye pain, conjunctivitis, keratitis, corneal abrasion, acute close-angle glaucoma, scleritis, episcleritis, uveitis, orbital cellulitis, optic neuritis, migraine headache, and cluster headache. Search dates: May 3, 2015, and February 16, 2016.
note: This review updates a previous article on this topic by Fiore, et al. 41
The opinions and assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the U.S. Army Medical Department or the U.S. Army Service at large.
Shields T, Sloane PD. A comparison of eye problems in primary care and ophthalmology practices. Fam Med. 1991;23(7):544-546.
Nash EA, Margo CE. Patterns of emergency department visits for disorders of the eye and ocular adnexa. Arch Ophthalmol. 1998;116(9):1222-1226.
Dargin JM, Lowenstein RA. The painful eye. Emerg Med Clin North Am. 2008;26(1):199-216.
Azari AA, Barney NP. Conjunctivitis [published correction appears in JAMA . 2014;311(1):95]. JAMA. 2013;310(16):1721-1729.
National Institute for Health and Care Excellence. Headaches in over 12s. https://www.nice.org.uk/guidance/cg150 . Accessed May 3, 2015.
Weaver-Agostoni J. Cluster headache. Am Fam Physician. 2013;88(2):122-128.
Kaye S, Choudhary A. Herpes simplex keratitis. Prog Retin Eye Res. 2006;25(4):355-380.
Lee S, Yen MT. Management of preseptal and orbital cellulitis. Saudi J Ophthalmol. 2011;25(1):21-29.
Catron T, Hern HG. Herpes zoster ophthalmicus. West J Emerg Med. 2008;9(3):174-176.
Wipperman JL, Dorsch JN. Evaluation and management of corneal abrasions. Am Fam Physician. 2013;87(2):114-120.
Gerstenblith AT, Rabinowitz MP. The Wills Eye Manual: Office and Emergency Room Diagnosis and Treatment of Eye Disease . 6th ed. Philadelphia, Pa.: Lippincott Williams & Wilkins; 2012.
Walochnik J, Scheikl U, Haller-Schober EM. Twenty years of acanthamoeba diagnostics in Austria. J Eukaryot Microbiol. 2015;62(1):3-11.
Sambursky RP, Fram N, Cohen EJ. The prevalence of adenoviral conjunctivitis at the Wills Eye Hospital Emergency Room. Optometry. 2007;78(5):236-239.
Lorenzo-Morales J, Khan NA, Walochnik J. An update on Acanthamoeba keratitis: diagnosis, pathogenesis and treatment. Parasite. 2015;22:10.
Shields SR. Managing eye disease in primary care. Part 3. When to refer for ophthalmologic care. Postgrad Med. 2000;108(5):99-106.
Saw SM, Gazzard G, Friedman DS. Interventions for angle-closure glaucoma. Ophthalmology. 2003;110(10):1869-1878.
Jabs DA, Rosenbaum JT, Foster CS, et al. Guidelines for the use of immunosuppressive drugs in patients with ocular inflammatory disorders. Am J Ophthalmol. 2000;130(4):492-513.
Smith JM, Bratton EM, DeWitt P, Davies BW, Hink EM, Durairaj VD. Predicting the need for surgical intervention in pediatric orbital cellulitis. Am J Ophthalmol. 2014;158(2):387-394.e1.
Pula JH, Macdonald CJ. Current options for the treatment of optic neuritis. Clin Ophthalmol. 2012;6:1211-1223.
Jabs DA, Mudun A, Dunn JP, Marsh MJ. Episcleritis and scleritis. Am J Ophthalmol. 2000;130(4):469-476.
Dart JK. Predisposing factors in microbial keratitis: the significance of contact lens wear. Br J Ophthalmol. 1988;72(12):926-930.
Schein OD. Contact lens abrasions and the nonophthalmologist. Am J Emerg Med. 1993;11(6):606-608.
Yaphe J, Pandher KS. The predictive value of the penlight test for photophobia for serious eye pathology in general practice. Fam Pract. 2003;20(4):425-427.
Beck RW, Trobe JD, Moke PS, et al.; Optic Neuritis Study Group. High- and low-risk profiles for the development of multiple sclerosis within 10 years after optic neuritis. Arch Ophthalmol. 2003;121(7):944-949.
Harman LE, Margo CE, Roetzheim RG. Uveitis: the collaborative diagnostic evaluation. Am Fam Physician. 2014;90(10):711-716.
Kerr NM, Chew SS, Eady EK, et al. Diagnostic accuracy of confrontation visual field tests. Neurology. 2010;74(15):1184-1190.
Han F, Yuan YS. Characteristics of visual field defects in primary angle-closure glaucoma [in Chinese]. Zhonghua Yan Ke Za Zhi. 2009;45(1):14-20.
Keltner JL, Johnson CA, Cello KE, et al. Visual field profile of optic neuritis. Arch Ophthalmol. 2010;128(3):330-337.
Robinett DA, Kahn JH. The physical examination of the eye. Emerg Med Clin North Am. 2008;26(1):1-16.
Liesegang TJ. Epidemiology of ocular herpes simplex. Natural history in Rochester, Minn, 1950 through 1982. Arch Ophthalmol. 1989;107(8):1160-1165.
Shaikh S, Ta CN. Evaluation and management of herpes zoster ophthalmicus. Am Fam Physician. 2002;66(9):1723-1730.
Leibowitz HM. The red eye. N Engl J Med. 2000;343(5):345-351.
Spector RH. The pupils. In: Walker HK, Hall WD, Hurst JW. Clinical Methods: The History, Physical, and Laboratory Examinations . Boston, Mass.: Butterworths; 1990.
Chong NV, Murray PI. Pen torch test in patients with unilateral red eye. Br J Gen Pract. 1993;43(371):259.
Blazek P, Davis SL, Greenberg BM, et al. Objective characterization of the relative afferent pupillary defect in MS. J Neurol Sci. 2012;323(1–2):193-200.
Stanley JA, Baise GR. The swinging flashlight test to detect minimal optic neuropathy. Arch Ophthalmol. 1968;80(6):769-771.
Harooni H, Golnik KC, Geddie B, Eggenberger ER, Lee AG. Diagnostic yield for neuroimaging in patients with unilateral eye or facial pain. Can J Ophthalmol. 2005;40(6):759-763.
Cain W, Sinskey RM. Detection of anterior chamber leakage with Seidel's test. Arch Ophthalmol. 1981;99(11):2013.
Pokhrel PK, Loftus SA. Ocular emergencies [published correction appears in Am Fam Physician . 2008;77(7):920]. Am Fam Physician. 2007;76(6):829-836.
Waldman CW, Waldman SD, Waldman RA. A practical approach to ocular pain for the non-ophthalmologist. Pain Manag. 2014;4(6):413-426.
Fiore DC, Pasternak AV, Radwan RM. Pain in the quiet (not red) eye. Am Fam Physician. 2010;82(1):69-73.
Continue Reading

More in AFP
More in pubmed.
Copyright © 2016 by the American Academy of Family Physicians.
This content is owned by the AAFP. A person viewing it online may make one printout of the material and may use that printout only for his or her personal, non-commercial reference. This material may not otherwise be downloaded, copied, printed, stored, transmitted or reproduced in any medium, whether now known or later invented, except as authorized in writing by the AAFP. See permissions for copyright questions and/or permission requests.
Copyright © 2024 American Academy of Family Physicians. All Rights Reserved.

Open source ophthalmology education for students, residents, fellows, healthcare workers, and clinicians. Produced by the Moran Eye Center in partnership with the Eccles Library

Search Moran CORE
- Support CORE
- International
- About Moran
Red Flag Symptoms of Unilateral Vision Loss
Home / Basic Ophthalmology Review / Visual Acuity and Vision Loss
Title : Red Flag Symptoms of Unilateral Vision Loss
Author : Troy Teeples, 4 th year medical student, University of Utah School of Medicine; Griffin Jardine, MD
Photographer: James Gilman, CRA, FOPS
Date : 8/7/2018
Keywords/Main Subjects: Unilateral vision loss, monocular vision loss, red flags, headache, painful eye, pain with eye movement, floaters, flashes, atherosclerosis, central retinal artery occlusion, central retinal vein occlusion, giant cell arteritis, acute angle closure glaucoma, optic neuritis, keratitis, retinal detachment, vitreous hemorrhage, amaurosis fugax
Introduction
Acute, monocular vision loss is a frightening experience for patients and may have long-term consequences depending on the etiology. The key to providing efficient, effective care is a careful history, a focused physical exam and knowing when to seek help from an ophthalmologist. The goal of this section is to help identify red flag signs and symptoms during a work up of unilateral vision loss in order to able to 1) efficiently narrow a differential diagnosis and 2) know when to urgently consult ophthalmology.
Red Flags from History and Physical Exam
There are key elements that need to be addressed when working up a patient with unilateral vision loss. Providers should look for the following associated symptoms and signs in order to guide the decision-making process.
When a patient over the age of 60 complains of a headache and unilateral vision loss, Giant Cell Arteritis (GCA) should be immediately considered given the potential for permanent vision loss. Ask the patient about a history of polymyalgia rheumatica, scalp tenderness, jaw claudication and other constitutional symptoms such as fever, malaise, weight loss or anorexia. If GCA is suspected, order an ESR, CRP and CBC looking for an elevated platelet count. If there is a high enough suspicion for GCA, don’t wait for the lab results to initiate high-dose systemic corticosteroids. An ophthalmologist should be consulted to evaluate the cause of the vision loss, specifically looking for a central retinal artery occlusion. The patient should then be schedule for a diagnostic temporal artery biopsy within the next week as an outpatient.
Red, Painful Eye
There are several causes of monocular vision loss accompanied by a red, painful eye. After inquiring about recent trauma and ruling out a ruptured globe , check the patient’s intraocular pressure (IOP) with a Tono-pen® to evaluate for Acute Angle Closure Glaucoma , as this may lead to permanent vision loss if not treated appropriately. Patients will present with a red, painful eye as well as a headache , and nausea/vomiting . They may also complain of halos around lights. Physical exam will reveal a steamy (hazy) cornea , a dilated pupil that is not reactive to light , and an IOP greater than 40 typically. Consult an ophthalmologist if suspected.
Keratitis or corneal ulcers may also present with a red, painful eye and unilateral decreased or blurry vision . Patients may complain of excessive tears or discharge , and photophobia. Ask about contact lens wear, autoimmune conditions such as rheumatoid arthritis and look for corneal whitening or loss of corneal clarity and consult an ophthalmologist if concerned.
Pain with eye movement
Optic neuritis will present with acute vision loss, typically over the course of < 1 week. The majority of these patients will have pain with eye movement and decreased color vision. They may have a history of demyelinating symptoms or a known diagnosis of multiple sclerosis. On exam, a relative afferent pupillary defect (APD) will be seen during a swinging flashlight test.
Floaters and flashes
Another combination of concerning symptoms are flashes and floaters in combination with monocular vision loss. Flashes and floaters of acute onset are concerning for a retinal detachment . Patients are commonly myopic (short-sighted) and may additionally complain of vision loss as a “ curtain drawn ” over their vision. A retinal detachment is painless but a surgical emergency and a vitreoretinal specialist should be consulted.
A vitreous hemorrhage may also present as painless monocular vision loss associated with floaters . Patients should be questioned regarding a history of trauma, ocular surgery, diabetes, sickle cell anemia, leukemia and high myopia, all of which may precipitate a vitreous hemorrhage.
Atherosclerosis Risk Factors
If a patient presents with painless, temporary monocular vision loss with subsequent restoration of sight, then amaurosis fugax should be high on the differential. A thorough history should include atherosclerotic risk factors such as diabetes mellitus, smoking, CAD , and HTN . Fundoscopy may reveal Hollenhorst plaques (cholesterol emboli).
Central Retinal Artery Occlusion (CRAO) and Central Retinal Vein Occlusion (CRVO) are nearly impossible to distinguish by history alone. Patients will present with acute, painless monocular vision loss without other associated symptoms. These diagnoses are made with fundoscopy revealing a “ blood and thunder ” appearance in CRVO along with diffuse hemorrhages and cotton wool spots (figure 1). No emergent treatment is particularly effective in reversing the changes, but there are several long-term sequelae and corresponding treatments so these patients should be referred to an ophthalmologist for close follow-up.
CRAO, on the other hand, does have a few emergent treatment options and is an ophthalmologic emergency. It can by recognized on fundoscopy by diffuse ischemic retinal whitening and a cherry red fovea along with boxcar segmentation of blood in the retinal veins (figure 2) . Consult an ophthalmologist immediately if suspected and within the first several hours of the vision loss.
Images or video :

Figure 1: A color fundus photo of the left eye with diffuse retinal hemorrhages in all four quadrants (“blood and thunder”) and optic nerve edema, consistent with a central retinal vein occlusion.

Figure 2: A color fundus photo of the right eye demonstrating diffuse, ischemic retinal whitening; arterial attenuation and a “cherry red spot” at the fovea—pathognomonic in the context of retinal whitening and sudden, painless vision loss for a central retinal artery occlusion.
References :
- Farris W, Waymack JR. Central Retinal Artery Occlusion. [Updated 2017 Dec 5]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2018 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK470354/ . Accessed 6/19/2018.
- Khazaeni B, Khazaeni L. Glaucoma, Acute Closed Angle. [Updated 2017 Apr 9]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2018 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK430857/ . Accessed 6/20/2018.
- Ness T, Bley TA, Schmidt WA, Lamprecht P. The diagnosis and treatment of giant cell arteritis. Dtsch Arztebl Int 110: 376-385, 2013.
- Patel A, Nguyen C, Lu S. Central Retinal Vein Occlusion: A Review of Current Evidence-based Treatment Options. Middle East Afr J Ophthalmol. 2016 Jan-Mar;23(1):44-8. PubMed PMID: 26957838.
Faculty Approval by: Griffin Jardine, MD
Copyright statement: Copyright Troy Teeples, ©2018. For further information regarding the rights to this collection, please visit: http://morancore.utah.edu/terms-of-use/
Filter by Specialty
- BASIC OPHTHALMOLOGY REVIEW
- OPHTHALMIC PATHOLOGY
- NEURO-OPHTHALMOLOGY
- PEDIATRIC OPHTHALMOLOGY AND STRABISMUS
- ORBIT, EYELIDS, AND LACRIMAL SYSTEM
- EXTERNAL DISEASE AND CORNEA
- INTRAOCULAR INFLAMMATION AND UVEITIS
- LENS AND CATARACT
- RETINA AND VITREOUS
- REFRACTIVE SURGERY
- OPHTHALMIC SURGERY
- ETHICS / RESEARCH / STATISTICS / GENETICS
- MORAN OPHTHALMOLOGY LEARNING EXPERIENCE
- ALLIED OPHTHALMIC TRAINING PROGRAM
- QUALITY IMPROVEMENT
Quick Links
Eccles Library NOVEL Webvision
Site Information
Guidelines for Authors Student Assignments Terms of Use Disclaimer
Social Media

An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Population Health and Public Health Practice; Committee on Public Health Approaches to Reduce Vision Impairment and Promote Eye Health; Welp A, Woodbury RB, McCoy MA, et al., editors. Making Eye Health a Population Health Imperative: Vision for Tomorrow. Washington (DC): National Academies Press (US); 2016 Sep 15.

Making Eye Health a Population Health Imperative: Vision for Tomorrow.
- Hardcopy Version at National Academies Press
3 The Impact of Vision Loss
Vision loss has a significant impact on the lives of those who experience it as well as on their families, their friends, and society. The complete loss or the deterioration of existing eyesight can feel frightening and overwhelming, leaving those affected to wonder about their ability to maintain their independence, pay for needed medical care, retain employment, and provide for themselves and their families. The health consequences associated with vision loss extend well beyond the eye and visual system. Vision loss can affect one's quality of life (QOL), independence, and mobility and has been linked to falls, injury, and worsened status in domains spanning mental health, cognition, social function, employment, and educational attainment. Although confounding factors likely contribute to some of the harms that have been associated with vision impairment, testimony from visually impaired persons speaks to the significant role that vision plays in health, vocation, and social well-being.
The economic impact of vision loss is also substantial. One national study commissioned by Prevent Blindness found that direct medical expenses, other direct expenses, loss of productivity, and other indirect costs for visual disorders across all age groups were approximately $139 billion in 2013 dollars ( Wittenborn and Rein, 2013 ), with direct costs for the under-40 population reaching $14.5 billion dollars ( Wittenborn et al., 2013 ). These costs affect not only national health care expenditures, but also related expenses and the resources of individuals and their families. For example, Köberlein and colleagues (2013) found that the time spent by caregivers increases substantially as vision decreases.
This chapter explores the impact of chronic vision loss in the United States—both in terms of its financial costs and its effects on QOL. The first two sections of the chapter details the consequences of vision impairment and the relationship between chronic vision impairment and other chronic conditions. The third section of this chapter provides an overview of the economic impact of vision loss on individuals, insurers, and society, including estimates of direct and indirect costs, and life years lost. The final section discusses the state of cost-effectiveness research for clinical eye and vision care.
- CONSEQUENCES OF VISION IMPAIRMENT
Quality of Life
Vision impairment is associated with a reduced QOL, which is a “complex trait that encompasses vision functioning , symptoms, emotional well-being, social relationships, concerns, and convenience as they are affected by vision” ( Lamoureux and Pesudovs, 2011, p. 195 ). Numerous studies have shown that vision impairment is often associated with various negative health outcomes and poor QOL ( Chia et al., 2006 ; Langelaan et al., 2007 ). A recent study using Behavioral Risk Factor Surveillance System (BRFSS) data from 22 states examined unadjusted health-related QOL among individuals ages 40 to 64 years by visual impairment status and found that the percentage of individuals reporting life dissatisfaction, fair or poor reported health, physical and mental unhealthy days, and days of limited activity increased as the self-reported severity of vision impairment increased ( Crews et al., 2016b ) (see Table 3-1 ). An earlier study found similar results among people ages 65 and older ( Crews et al., 2014 ). Based on a variety of measurement instruments, reduced QOL has been related to the severity of disease in glaucoma, cataract, age-related macular degeneration, and strabismus ( Chai et al., 2009 ; Chatziralli et al., 2012 ; Cheng et al., 2015 ; Freedman et al., 2014 ; Hassell et al., 2006 ; Orta et al., 2015 ). Although greater emphasis is traditionally placed on the better-seeing eye's role in visual function, one study concluded that the worse-seeing eye contributes importantly to patients' estimates of vision-related QOL, particularly when the underlying eye disease affects peripheral vision (e.g., in the case of glaucoma) ( Hirneiss, 2014 ).
Unadjusted Health-Related Quality of Life Among Those Ages 40 to 60 by Visual Impairment Status in 22 States, 2006 to 2010, United States.
A study by Rein and colleagues (2007) found that the QOL begins to slowly decline with the onset of vision loss, and then decreases more precipitously as measures of visual field defects increase. A systematic literature review of studies that reported QOL in patients with central vision loss or peripheral vision loss, and found that both types of vision loss were associated with similar degrees of detriment to QOL, although “different domains were affected” which “might be a function of the pathology of diseases” ( Evans et al., 2009, p. 433 ). A recent Korean study, using the EQ-5D instrument 1 examined QOL scores based on whether participants were visually impaired 2 and whether they had 1 of 14 chronic conditions. The authors found that QOL scores in persons with each of the 14 chronic conditions, excepting coronary artery disease, were lower among individuals with that condition alone than individuals who also had any co-existing vision impairment ( Park et al., 2015 ). The impact of vision impairment on people with chronic conditions is explored further later in this chapter.
Two studies indicated that the QOL impact of vision loss may be perceived differently by health care providers than by the patients themselves. One study administered time-trade-off utilities to Canadian medical students and patients for different levels of vision loss (anchors were death = 0 and perfect vision = 1.0); the study found that medical students tended to underestimate the impact of vision loss ( Chaudry et al., 2015 ). In a similar study in China, utility values for mild glaucoma and severe glaucoma were obtained from glaucoma patients and ophthalmologists; the ophthalmologists' utility ratings for mild glaucoma were significantly higher than the patients', suggesting that physicians may underestimate the impact of mild glaucoma on QOL ( Zhang et al., 2015 ).
Loss of vision affects patients' ability to work or care for themselves (or others), and it affects numerous casual activities such as reading, socializing, and pursuing hobbies ( Brown et al., 2014 ). Vision impairment makes it more difficult to perform the basic self-care activities of daily living such as eating and dressing as well as instrumental activities of daily living such as shopping, financial management, medication management, and driving ( Brown et al., 2014 ; Haymes et al., 2002 ; Whitson et al., 2007 , 2014 ). Most studies have found that vision loss has a greater impact on dependency in instrumental activities of daily living than in basic activities of daily living. The instrumental activities of daily living are critical to one's ability to function in modern society. In particular, the loss of near vision affects one's ability to perform a variety of tasks that involve reading (e.g., getting information from medication labels, balancing bank statements, or following recipes), recognizing faces and images (e.g., socializing, playing cards, using a smartphone), or manipulating small objects (e.g., sewing, replacing batteries). One cross-sectional study found that individuals with visual impairment, defined as a best-corrected binocular presenting visual acuity of 20/30 or worse, had greater disability across functional measures, such as task performance, walking speeds, and driving when compared to people with normal vision and even uncorrected refractive error 3 ( Zebardast et al., 2015 ). Visual field deficits affect one's ability to perform tasks that require ambulation in challenging settings (e.g., moving along crowded city streets, negotiating stairwells) or the use of peripheral vision (e.g., driving).
Due to the challenges that vision impairment imposes for independent living, older adults with vision impairment may be more likely to require long-term care. In the Australian Blue Mountains Eye Study, with each line of reduction in presenting visual acuity at baseline, there was a 7 percent increased risk of subsequent nursing home placement ( Wang et al., 2003 ). For participants in the Beaver Dam Eye Study, the odds ratio for nursing home placement was 4.23 (95% confidence interval [CI] = 2.34, 7.64) for low best-corrected visual acuity in the better eye, 5.00 (95% CI = 2.28, 10.94) for poor near vision, and 2.40 (95% CI = 1.46, 3.92) for poor contrast sensitivity, after adjustment for age, sex, self-rated health, and arthritis ( Klein et al., 2003 ).
For persons with vision loss who desire to be a part of the workforce, vision impairment often poses barriers to employment opportunities ( O'Day, 1999 ). Unfortunately, employment statistics pertaining to Americans with vision loss are lacking because available nationally representative data sources, such as the U.S. Census, group persons with vision impairment with all people who have sensory impairments or with people with sensory or communication impairments ( U.S. Census Bureau, 2014 ).
Mobility and Falls
In a person with intact eyesight, the primary sense used to navigate three-dimensional space is vision. Mobility is therefore greatly affected by vision loss, whether resulting from changes in visual acuity, visual fields, depth perception, or contrast sensitivity ( Bibby et al., 2007 ; Lord and Dayhew, 2001 ; Marron and Bailey, 1982 ). In the Salisbury Eye Evaluation (SEE) project, vision impairment (defined by visual acuity or visual field deficit) was significantly associated with self-reported difficulty with walking or going up or down steps ( Swenor et al., 2013 ). Also in the SEE project, visual field deficits—but not visual acuity or contrast sensitivity deficits—were predictive of a slower-than-usual gait speed while navigating an obstacle course ( Patel et al., 2006 ). A study from the United Kingdom found that 46 percent of frail elderly individuals admitted for hip fracture in two hospital districts had visual impairment, most frequently untreated cataract (49 percent) and macular degeneration (21 percent), but also uncorrected refractive error (17 percent); the visually impaired hip fracture patients were less likely than those without vision impairment to be under an eye provider's care and more likely to live in areas of social deprivation ( Cox et al., 2005 ). In the Low Vision Rehabilitation Outcomes Study, 16.3 percent of participants referred to vision rehabilitation at 28 U.S. centers indicated that one of their chief vision-related problems was mobility ( Brown et al., 2014 ).
Multiple peer-reviewed studies have documented a relationship between vision impairment and falls ( Crews et al., 2016a ; Lord, 2006 ). A 2016 study by Crews and colleagues that used 2014 BRFSS data to analyze the state-specific annual prevalence of falls among persons aged 65 years or older found that 46.7 percent of persons with severe vision impairment (state prevalence range 30.8–59.1 percent) and 27.7 percent of older adults without such impairment (state prevalence range 20.4–32.4 percent) reported having fallen during the previous year ( Crews et al., 2016a ). The visual parameters that have been strongly and consistently associated with falls include poor contrast sensitivity, reduced depth perception, and visual field loss ( de Boer et al., 2004 ; Ivers et al., 1998 ; Klein et al., 2003 ; Lord and Dayhew, 2001 ; Lord et al., 1991 , 1994 ; Nevitt et al., 1989 ). A review of studies that reported the univariate relationship between visual deficits (defined variously) and falls found that the relative risk ratios across studies was 2.5 (CI = 1.6, 3.5) ( Rubenstein and Josephson, 2002 ).
Evidence is limited or conflicting on the need for vision assessment and specific interventions to reduce falls among visually impaired populations. The U.S. Preventive Services Task Force determined that vision correction was among several potential interventions that “lack[ed] sufficient evidence for or against use in prevention of falls in community-dwelling older adults” ( Moyer, 2012, p. 200 ; see also, Schneider et al., 2012 ). Unfortunately, the visual deficits most strongly linked to fall risk (contrast sensitivity, depth perception, and visual field deficits) are generally less amenable to remediation than visual acuity. Other factors such as weakness, other chronic conditions, and the use of medications are also associated with falls, suggesting that successful interventions to reduce falls in visually impaired populations will require a multi-pronged approach ( Steinman et al., 2011 ). Evidence is needed to determine which training aspects, equipment, and environmental modifications are most effective at reducing falls and improving mobility. However, it is the committee's assessment that there remains a role for vision rehabilitation in mitigating fall risk associated with vision loss.
Vision impairment has been shown to be associated with an increased risk of fractures in multiple studies. In the Framingham Eye Study, which included a subset of participants from the Framingham Study Cohort, those participants with visual acuity worse than 20/100 were more than twice as likely to have had hip fractures than participants with visual acuity of 20/25 or better (relative risk [RR] = 2.17; 95% CI = 1.24, 3.80) ( Felson et al., 1989 ). In the EPIDOS Prospective Study, among a prospective cohort of 7,575 French women, those with visual acuity of 2/10 (using the decimal Snellen fraction, thus equivalent to 20/100) or worse had a RR of 4.3 (95% CI = 3.1, 6.1) of hip fracture compared to those with visual acuity better than 7/10 (roughly equivalent to 20/30) (RR = 1.0) ( Dargent-Molina et al., 1996 ). Various other aspects of visual impairment besides poor visual acuity have been shown to be associated with an increased fracture risk. In the Study of Osteoporotic Fractures, white women in the lowest quartile of depth perception measures were estimated to have a 40 percent increased risk of fractures compared with women in the other three quartiles (RR = 1.4; 95% CI = 1.0, 1.9), and the risk of fractures increased by 20 percent for each standard deviation decrease in low-frequency contrast sensitivity (RR = 1.2; 95% CI = 1.0, 1.5) ( Cummings et al., 1995 ). Furthermore, in the same cohort, women with mild, moderate, or severe binocular visual field loss had an increased risk of hip fractures when compared with women without binocular visual field loss, and women with moderate or severe visual field loss had an increased risk of non-hip and non-spine fractures compared with women without binocular visual field loss ( Coleman et al., 2009 ).
Studies have suggested that reversing vision impairment from cataract may be protective against fractures. A randomized controlled trial that evaluated expedited versus routinely scheduled cataract surgery in 306 women found that women with expedited cataract surgery had a 67 percent lower risk of fractures within 1 year after surgery than women with routinely scheduled surgery (RR = 0.33; 95% CI = 0.1, 1.0) ( Harwood et al., 2005 ). A large study of more than 1.1 million men and women with cataract in the national U.S. Medicare database found that compared to patients with cataract who did not undergo surgery, patients with cataract surgery had a 16 percent lower risk of hip fracture (odds ratio [OR] = 0.84; 95% CI = 0.81, 0.87) and a 5 percent lower risk of any fracture (OR = 0.95; 95% CI = 0.93, 0.97). Furthermore, this protective association was modified by the effects of age and systemic disease burden, and the apparent protective relationship between surgery and fracture, based on having a high Charlson Comorbidity Index score, was even stronger among participants who were elderly or ill ( Tseng et al., 2012 ).
The protective association between cataract surgery and fractures may extend beyond a reduction in fracture risk. In a recent study of the same large population of Medicare beneficiaries with cataract, those who had cataract surgery experienced 27 percent decreased risk in long-term mortality compared with those without cataract surgery (hazards ratio [HR] = 0.73; 95% CI = 0.72, 0.74) ( Tseng et al., 2016 ). Similar to what was seen in the study of cataract surgery and fractures, the protective association between cataract surgery and mortality was modified by the effects of age and systemic disease burden, where patients who were elderly or who had a moderate burden of systemic disease experienced even stronger protective effects than the overall population. Although this study did not examine the mechanisms of the protective effect between cataract surgery and mortality and the study design does not permit conclusions about causation, the reduction in the risk of fractures and accidents was proposed as a contributing factor in the reduced risk of death. The protective association between cataract surgery and mortality in this study was supported by additional data from two earlier studies in the Blue Mountains region, west of Sydney, Australia, both of which demonstrated that patients with vision improvement after cataract surgery had decreased mortality risk compared with patients with vision impairment due to cataract who had not undergone surgery or those with persistent vision impairment after cataract surgery ( Fong et al., 2013 , 2014 ).
Subsequent Injury
People with vision loss are at higher risk for several types of injury. Of these, the link between vision loss and fall-related injuries has been most clearly documented. In a population-based cohort of Latinos in California, a greater risk of injurious falls was reported in those with both central vision impairment (OR = 2.76; 95% CI = 1.10, 7.02) and peripheral vision impairment (OR = 1.40; 95% CI = 0.94, 2.05) ( Patino et al., 2010 ). A loss of visual field was associated with fall-related fractures, and a relationship between a recently acquired decline in visual acuity and falls with fracture was observed in the Blue Mountain Eye Study ( Hong et al., 2014 ; Klein et al., 2003 ). Interestingly, both falls and falls with fracture were more likely in participants with a unilateral, rather than bilateral, visual acuity deficit, which is similar to the findings of an earlier study, suggesting that poor depth perception may be a contributor to falls ( Felson et al., 1989 ). Indeed, poor depth perception has been associated with hip fracture in other epidemiological studies ( Cummings et al., 1995 ). Poor contrast sensitivity is also associated with risk of fall-related fractures ( de Boer et al., 2004 ).
In a prospective study of seniors between the ages of 75 and 80 years, lowered vision 4 at baseline was associated with an increased risk of injurious accidents requiring hospitalization over 10 years of follow-up ( Kulmala et al., 2008 ). A visual acuity worse than 0.3 on the Landolt ring chart (roughly equivalent to 20/65) was not associated with a risk of injurious accidents, possibly because persons with more severe visual impairment restricted their activities, resulting in less opportunity for injury. However, in a separate study that used the National Health Interview Survey (NHIS) to follow more than 100,000 adults for up to 7 years, severe bilateral vision impairment was associated with a risk of death due to unintentional injury (HR = 7.4; 95% CI = 3.0, 17.8) ( Lee et al., 2003 ).
Mental Health
Compared to people with normal vision, those with vision impairment are at a higher risk for depression, anxiety, and other psychological problems ( Kempen et al., 2012 ). Among older adults with vision impairment, the rates of depression and anxiety are significantly higher than among both individuals of similar ages without vision impairment and those of similar ages suffering from other chronic conditions, such as asthma or chronic bronchitis, heart conditions, and hypertension ( Kempen et al., 2012 ). Distress related to vision loss is more strongly correlated with depression than other key risk factors such as negative life events or poor health status ( Rees et al., 2010 ). Among visually impaired individuals, those with depressive symptoms report more functional limitations. The reasons for the relationship between depression and poor visual function are unclear and may be bi-directional, but patient-level differences in eye disease and general medical condition did not account for the observed relationship ( Rovner and Casten, 2002 ; Rovner et al., 2006 ). One randomized, controlled trial of an integrated mental health and vision rehabilitation program (compared with vision rehabilitation with non-directed supportive therapy) for patients with macular degeneration and subsyndromal depressive symptoms found significantly reduced rates of depression symptoms and better functional outcomes in the intervention group ( Rovner et al., 2014 ). This work suggests that some of the functional and affective consequences of vision loss are remediable.
As discussed in Chapter 2 , children with uncorrected refractive error are more likely to underperform on some metrics of academic performance ( Kulp et al., 2016 ). Academic problems have been found to be negatively associated with anxiety, with the frequency increasing with age in both children and adolescents ( Mazzone et al., 2007 ). Similarly, among adolescents, vision impairment is associated with an increased prevalence of psychopathological symptoms, including depression and anxiety ( Garaigordobil and Bernarás, 2009 ). An analysis of data from NHIS did not show evidence for a direct relationship between vision impairment and death from suicide (HR = 1.50; 95% CI = 0.90, 2.49); however, the study did indicate an indirect effect of visual impairment on death from suicide due to poorer self-rated health (HR = 1.05; 95% CI = 1.02, 1.08) and the number of non-ocular health conditions (HR = 1.12; 95% CI = 1.01, 1.24). These results suggest that people with vision impairment may be at greater risk of suicide due to vision impairment's association with poor general health ( Lam et al., 2008 ).
Several studies have found that cognitive impairment is more prevalent and progresses more rapidly in older adults with vision impairment than in those without ( Lin et al., 2004 ; Ong et al., 2013 ; Reyes-Ortiz et al., 2005 ; Rogers and Langa, 2010 ; Tay et al., 2006 ; Whitson et al., 2007 ). About 4 percent of community-dwelling persons over age 65 have both cognitive and vision impairments, making the co-occurrence of these problems more prevalent than such well-recognized conditions as Parkinson's disease and emphysema ( Whitson et al., 2007 ). People with age-related macular degeneration (AMD) have higher rates of cognitive impairment than their peers, lower scores on cognitive tests, and a higher risk of incident dementia ( Baker et al., 2009 ; Clemons et al., 2006 ; Klaver et al., 1999 ; Pham et al., 2006 ; Wong et al., 2002 ; Woo et al., 2012 ). Other studies suggest that, even without dementia, AMD patients still perform more poorly on tests of verbal fluency and memory ( Clemons et al., 2006 ; Whitson et al., 2010 , 2015; Wong et al., 2002 ). Research has failed to demonstrate a clear genetic link between AMD and dementia ( Butler et al., 2015 ; Souied et al., 1998 ). These results suggest more research is needed to fully assess the reasons behind the link between vision and cognitive impairment in adults.
In children, uncorrectable vision impairment frequently occurs in the context of comorbid conditions, making it difficult to quantify the direct impact of visual impairment and blindness on cognitive skills, academic performance, and QOL. Many children who have been diagnosed with neurodevelopmental disorders (genetic or acquired) have been found to also have an associated vision problem that has led to visual impairment. Current research focuses on determining the prevalence of these eye health and vision disorders that occur with the underlying neurodevelopmental diagnosis ( Salt and Sargent, 2014 ). For example, children with cerebral palsy have been found to have a higher prevalence of strabismus, visual impairment due to uncorrected refractive error, eye movement disorders, and visual perceptual deficits than normally sighted children of the same age ( Lew et al., 2015 ; Salt and Sargent, 2014 ). A higher rate of vision impairment has also been documented for children with Down syndrome ( Cregg et al., 2003 ). It is difficult to ascertain the influence of the vision loss on cognitive or academic function in diagnoses that are already associated with cognitive impairment. One study demonstrated that children diagnosed with toxoplasmosis who present with reduced vision perform more poorly than children diagnosed with toxoplasmosis without vision impairment on verbal and performance measure of intellectual ability ( Roizen et al., 2006 ). A meta-analysis on children with cerebral palsy found that visual perceptual deficits were prevalent in those children but none of the studies had a control comparison group ( Ego et al., 2015 ). These children often perform below the level expected for their chronological ages, yet they have neither been categorized as visually impaired, nor referred for services ( Flanagan et al., 2003 ).
Although an association exists between vision impairment—as well as some specific eye disorders—and cognition, the mechanisms underlying this relationship are unclear. One possibility is that diseases of the eye have a negative effect on cognitive processes, either directly or indirectly. In people with vision impairment, the loss of cognitively stimulating activities, such as reading, may diminish other cognitive abilities ( Lindenberger and Baltes, 1994 ). Additionally, the brain is known to change in response to decreased visual input, and these changes may affect regions or neuronal pathways that support cognitive processes ( Liu et al., 2007 , 2010 ; Pascual-Leone et al., 2005 ). A second possibility is the “common cause” theory, which holds that genetic, environmental, or medical risk factors cause disease in the brain and eye simultaneously ( Klaver et al., 1999 ; Lindenberger and Baltes, 1994 ). Another possibility is that confounding factors, such as behavior and economic status, contribute to the observed relationship between vision impairment and cognitive impairment.
Hearing Impairment
The prevalence of co-existing impairment in vision and hearing, also referred to as dual sensory impairment (DSI), increases markedly with age. A range from 9 to 21 percent of adults over the age of 70 possess some degree of DSI ( Saunders and Echt, 2007 ). In an Australian cohort, the prevalence of DSI was even higher, reported to be 26.8 percent in participants ages 80 and older ( Schneider et al., 2012 ). In a cross-sectional study of a random sample of 446 older adults (mean age 79.9 years) from Marin County, California, eight measures of visual ability were associated with risk of hearing impairment (defined as moderate bilateral hearing loss, threshold >40 dB) ( Schneck et al., 2012 ). However, the relationship between vision impairment and hearing impairment only achieved statistical significance for three measures of visual acuity in low contrast conditions. Additional research is needed to determine whether vision loss is an independent risk factor for hearing loss and, if so, what factors underlie this relationship.
Several studies report associations between vision impairment and an increased risk for all-cause and injury-related mortality, as compared to controls with normal vision ( Christ et al., 2014 ; Lam et al., 2008 ; Lee et al., 2002 , 2003 ; Zheng et al., 2014 ). One possible cause of the greater mortality in visually impaired people may be their elevated risk of accidents and falls. In the longitudinal study by Christ and colleagues (2014) , the relationship between worse visual acuity and mortality was mediated by disability in instrumental activities of daily living, which suggests that some deaths may result from an impaired ability for self-care and disease management.
The relationship between vision impairment and mortality is certainly confounded by medical conditions (e.g., diabetes, obesity, hypertension, autoimmune disorders), lifestyle factors (e.g., smoking, alcohol use), and socio-demographic factors (e.g., race, age, socioeconomic disadvantage). As detailed in the next section, the complicated interplay between eye health and other medical comorbidities is an important factor in monitoring and reducing the overall public health burden of vision loss.
- MULTIPLE COMORBID CONDITIONS
The Office of the Assistant Secretary for Health defines chronic conditions as, “conditions that last a year or more and require ongoing medical attention and/or limit activities of daily living” ( Goodman et al., 2013, p. 3 ). Chronic conditions are associated with an increased risk of “early mortality, poor functional status, unnecessary hospitalizations, adverse drug events, duplicative tests, and conflicting medical advice” ( HHS, 2010, p. 2 ; see also, Hwang et al., 2001 ; Vogeli et al., 2007 ; Wolff et al., 2002 ). Expenditures related to chronic conditions are substantial, with an estimated 66 percent of total health care spending attributable to care for Americans with multiple chronic conditions ( HHS, 2010 ). Approximately 14 percent of Medicare beneficiaries with six or more chronic conditions accounted for 46 percent of total Medicare spending in 2010, while the 32 percent of beneficiaries with one or fewer chronic conditions accounted for 7 percent of spending ( CMS, 2012 ).
Irreversible vision impairment resulting from eye disease should be considered a chronic condition; it can amplify the adverse effects of other illnesses and injuries, and people with vision loss commonly live with multiple chronic conditions. As of 2012, 117 million people had at least one chronic condition, with one in four adults reporting two or more chronic health conditions ( CDC, 2016 ). Data from the Medical Expenditure Panel Survey show that among Americans over age 65 with eye disease, four out of five also had at least one of the following conditions: hypertension, heart disease, diabetes, or arthritis ( Anderson and Horvath, 2004 ). According to a 2008 NHIS, a substantial number of people with chronic diseases reported trouble seeing: 34.8 percent of those with chronic kidney disease, 30.9 percent of those with stroke, 23.8 percent of those with coronary heart disease, 23.6 percent of those with diabetes, 22.1 percent of those with arthritis, 19.7 percent of those with patients, and 19.4 percent of those with hypertension ( Crews and Chou, 2012 ). Whether or not any causal relationship exists between vision impairment and non-ocular comorbidities, it is clear that any successful efforts to alleviate the burden of vision impairment and loss will need to take comorbidities into account.
Vision Loss Amplifies the Effects of Other Conditions
A study of individuals ages 65 and older found that patients with a visual impairment and any of several other illnesses or conditions were many times more likely to have difficulty performing basic physical and social tasks than individuals in the same age range without visual impairment and without the particular illness or condition ( Crews et al., 2006 ). For example, elderly individuals with severe depression, visual impairment, or both were 10.0, 2.9, and 23.9 times more likely, respectively, to have moderate or severe limitations in their ability to socialize than people without either severe depression or visual impairment. Table 3-2 details the increased odds of encountering difficulty when undertaking these basic physical and social tasks among persons with visual impairment or a given comorbidity, or both. Whether or not comorbid vision impairment directly caused the excess disability (which cannot be inferred from descriptive data), vision impairment may help identify high-risk individuals or individuals with unmet needs who could be targeted for services and interventions across a variety of other clinical specialties.
Adjusted Odds Ratio for the Self-Reported Difficulty Performing Tasks Among U.S. Adults Ages 65 and Older with Vision Impairment and/or Other Condition or Disease.
Both cognitive impairment and vision impairment are disabling in their own right, but the co-occurrence of the two has been associated with even higher rates of disability and low self-rated health ( Whitson et al., 2007 , 2012a ). Dual sensory impairment (concurrent vision and hearing deficits) has been associated with a higher risk of cognitive decline, disability, depression, and mortality ( Gopinath et al., 2013 ; Heine and Browning, 2014 ; Lee et al., 2007 ; Lin et al., 2004 ; Schneider et al., 2011 ). Evidence is inconclusive regarding whether the combined effects of vision impairment and other impairments (cognition or hearing) on outcomes are synergistic or merely additive ( Schneider et al., 2011 ; Whitson et al., 2007 ).
Vision Loss Complicates the Management of Other Conditions
As reviewed above, vision loss creates significant challenges in daily life. The challenge of not being able to see well can affect various vision-reliant tasks that are frequently required for good chronic disease management, including self-care (e.g., foot checks in diabetics, preparing nutritious meals) and transportation (e.g., getting to and from clinic visits). In addition, vision loss may create difficulties in medication adherence and management (e.g., reading pill bottles, ordering refills) so that individuals who develop vision loss associated with chronic conditions, such as diabetes or glaucoma, are at a disadvantage in managing those chronic conditions. For example, vision loss makes it difficult to properly administer medications such as insulin or eye drops. Thus, affected individuals are at risk of entering a “vicious cycle” of worsening health.
Other Conditions Affect the Management of Eye Disease
Comorbidities also affect patients' ability to manage and cope with their vision impairment and eye health. One area of eye care where the impact of comorbid conditions has been studied is vision rehabilitation. Both cognitive impairment and depression have been associated with worse functional outcomes in vision rehabilitation ( Rovner et al., 2002 ; Whitson et al., 2012b ). A qualitative study of 98 older adults and their companions/caregivers in an outpatient vision rehabilitation clinic identified five themes regarding the impact of comorbid medical conditions on the patients' experiences in vision rehabilitation ( Whitson et al., 2011 ). Comorbidities had the following implications for the success of vision rehabilitation: (1) concurrent medical problems resulted in fluctuating health status with “good days and bad days” that were unrelated to eye disease, (2) comorbid conditions (e.g., hearing impairment, cognitive impairment) often amplified communication barriers between patients and providers, (3) participants and caregivers felt “overwhelmed” by competing health care demands, (4) comorbidities tended to delay progress in vision rehabilitation programs because of unexpected health events (e.g., falls, hospitalization, disease flares), and (5) some barriers imposed by comorbid conditions seemed to be reduced by the effective involvement of an informal companion 5 ( Whitson et al., 2011 ). A second qualitative study focused on the impact of comorbid cognitive impairment in vision rehabilitation ( Lawrence et al., 2009 ). This study interviewed 17 individuals with co-existing vision impairment and dementia, 17 family caregivers, and 18 vision or dementia health specialists involved in the patients' care ( Lawrence et al., 2009 ). The study found that vision-related service providers felt ill equipped to manage dementia-related needs, while visual needs were accorded a low priority by those providing dementia services; a lack of collaboration between the two services led to an overcautious approach ( Lawrence et al., 2009 ).
Comorbidities can also affect patients' ability to manage specific aspects of their eye care. In particular, the administration of eye drops can be challenging for patients with a limited range of motion in the neck, with arthritis or neuropathy involving the hands, or with cognitive impairments. The precise impact of these comorbidities on medication adherence and the proper administration of eye drops merits further research, but one multisite study that video-taped glaucoma patients self-administering a single drop reported that individuals with arthritis were significantly less likely to have the drop land in their eye ( Sayner et al., 2015 ).
- OVERVIEW OF EXPENDITURES
Few studies are available that examine the total costs associated with all eye disease and vision impairment on a national level. A 2013 analysis of the economic burden of vision loss and eye disorders that was commissioned by Prevent Blindness estimated prevalence and costs from National Health and Nutrition Examination Survey (NHANES) data, Medical Expenditure Panel Survey (MEPS) data, and data from the Survey of Income and Program Participation, the 2011 U.S. Census, and federal budgets ( Wittenborn and Rein, 2013 ). This analysis estimated the direct and indirect costs attributable to vision loss and eye disease to be $138.9 billion in the United States in 2013 dollars and found that costs for individual states ranged from $250 million in Wyoming to more than $15.6 billion in California ( Wittenborn and Rein, 2013 ). 6 The direct medical costs summed across all age groups attributable to, for example, diagnosed disorders, undiagnosed visual loss, and optometry 7 visits were $48.7 billion, $3.0 billion, and $2.8 billion, respectively ( Wittenborn and Rein, 2013 ). The total direct and indirect costs for eye disorders and vision loss per payer were $47.4 billion for government entities, $22.1 billion for private insurers, and $71.7 billion for patients ( Wittenborn and Rein, 2013 ).
Table 3-3 provides a breakdown of the comprehensive costs by age group for major categories of direct and indirect costs associated with eye care in the United States. Directs costs associated with diagnosed vision impairments along with indirect costs associated with productivity loss account for approximately 70 percent of the comprehensive costs across all age groups. Medical vision aids, which include eyeglasses and contact lenses, are the next largest expense category. Nursing home expenses account for an additional 30 percent of indirect costs but are attributable only to the over-65 population. These data suggest that interventions targeting the prevention and reduction of vision impairment have the potential to reduce overall costs. Although more data are needed for a comprehensive analysis of this assertion, shifting the burden of vision expenditures away from the possible downstream consequences of severe vision impairment toward items and services that promote the earlier diagnosis and treatment of vision-threatening diseases or conditions would extend the productivity and function of populations with vision impairment.
Economic Burden of Eye Disorders and Vision Loss (in millions of dollars).
The costs of eye disorders and subsequent vision loss are shared by the government, private insurance, and individuals, including patients and families. According to a recent analysis, the $47.4 billion that the government spends annually on eye disorders and vision loss is mostly for direct medical costs and long-term care ( Wittenborn and Rein, 2013 ). One systematic review examined the average annual expense per patient in a cohort of Medicare beneficiaries and found per-patient costs in 2011 dollars to range from $12,175 to $14,029 for moderate vision impairment, $13,154 to $16,321 for severe visual impairment, and $14,882 to $24,180 for blindness ( Köberlein et al., 2013 ). In comparison, the authors cited a mean expense of $8,695 for patients with no vision loss as the control, indicating that expenses for blind individuals can sometimes be more than double the control cost at the upper end of the range ( Köberlein et al., 2013 ). The total of all these costs is substantial, considering that Medicare had 52.2 million beneficiaries in 2013 ( CMS, 2014 ).
Private insurers covered approximately one-third of the total, or $22.1 billion ( Wittenborn and Rein, 2013 ). As with public insurance, the majority of these costs ($20.8 billion) were related to direct medical costs and supplies ( Wittenborn and Rein, 2013 ). Costs associated with diagnosed disorders were by far the most substantial costs for private insurers, at more than $17 billion. The relatively small amount spent for medical vision aids ($2.6 billion) reflects the limited available reimbursement coverage and accounts for the high spending burden for such aids by the individual payer ($9.7 billion) ( Wittenborn and Rein, 2013 ). The rest of the costs are attributable to reimbursement for long-term care. The costs associated with diagnosed blindness and vision impairment averaged (across all payers) $6,680 per year ( Wittenborn and Rein, 2013 ). By way of comparison, the annual costs for all different types of diagnosed medical disorders average $3,432 per person ( Wittenborn and Rein, 2013 ). Despite the high costs associated with vision impairment and loss, the per-person costs for vision correction average only $81 per year ( Wittenborn and Rein, 2013 ). One expert suggested that the cost to expand all required pediatric vision-related services under the Patient Protection and Affordable Care Act of 2010 to all beneficiaries covered by private insurance would range from $1 to $2 per member per month ( Spahr, 2015 ).
Individuals paid for slightly more than half—$71.7 billion—of the total cost of eye disorders and vision loss, “largely due to productivity and informal care losses” ( Wittenborn and Rein, 2013, p. 5 ). Of that $71.7 billion covered by individuals, direct costs accounted for approximately $15.5 billion primarily for medical vision aids ($9.7 billion), diagnosed disorders ($4.7 billion), aids and devices ($749 million), and undiagnosed vision impairment ($372 million) ( Wittenborn and Rein, 2013 ). Indirect costs accounted for more than $56 billion of the individual costs. Those indirect costs were due to productivity losses caused by reduced workforce involvement and lower wages, the costs of informal care, and long-term care costs ( Wittenborn and Rein, 2013 ). One national survey of working age adults found that 52 percent of them had less than $1,000 on hand to pay for out-of-pocket expenses associated with the diagnosis of an unexpected serious illness; 28 percent had less than $500 ( Aflac, 2015 ).
Figure 3-1 indicates that the costs attributed to eye and vision health increase with age across all payers and that the over-65 population is responsible for the vast majority of expenses for all payers, except private insurance. This is not surprising given the individual costs attributable to specific age-related eye diseases and conditions and the prevalence of diabetes in older populations. For example, diabetic retinopathy cost the United States $493 million in 2004, with 60 percent of the direct medical costs incurred by 40 to 60 years olds ( Rein et al., 2006 ). Similarly, in 2009, the estimated costs to Medicare from glaucoma reached $748 million ( Quigley et al., 2013 ). Schmier and Levine (2013) estimated the total loss in gross domestic product related to AMD was almost $42 billion in 2012 dollars. The costs attributable to individual cases vary by the severity of the disease or condition. For example, the distribution of AMD-associated costs varies by disease stage, “with greater cost for diagnosis procedures with earlier AMD and more on caregiving and institutional care with wet AMD” ( Schmier and Levine, 2013 ). One study found a four-fold increase in direct ophthalmology-related costs between asymptomatic ocular hypertension/earliest glaucoma ($623 per year) and end-stage glaucoma/blindness ($2,511 per year) ( Varma et al., 2011 ). The authors suggested that “early identification and treatment of patients with glaucoma and those with ocular hypertension at high risk of developing vision loss may reduce the individual burden of disease on [health-related quality of life] and also may minimize personal and societal economic burdens” (p. 5).
Costs by payer by age group for eye and vision health. a “Comprehensive” is the cumulative sum of costs borne by the three payer categories: patient, government, and insurance providers. b Government total includes transfer payment costs (more...)
In addition to incurring direct costs related to vision care, people with vision impairment tend to experience a lower QOL and decreased health status (as discussed in this chapter), and vision loss can complicate and exacerbate other comorbid conditions, driving up costs and worsening outcomes. For example, Bramley and colleagues (2008) demonstrated among Medicare beneficiaries with glaucoma that patients with any vision loss had 46.7 percent higher costs compared with patients without vision loss; the higher costs were the result of the increased risk for nursing home admission, depression, falls, accidents, and injury. These outcomes account for some of the most substantial health expenditures. As such, in order to secure population-level improvements in the field it will be critical to understand that the costs associated with vision impairment and eye disease are borne not only by individuals, but also by their caregivers, taxpayers, and employers. Without dedicated action, society as a whole will increasingly bear the burden of the direct costs from increasing yet avoidable Medicare spending and of the indirect costs from substantial lost productivity and a reduced labor force.
Vision impairment results in significant expenditures, both direct and indirect, and has the potential to affect almost every aspect of a person's life. Vision loss affects more than one's ability to see the world clearly. The consequences of vision impairment often negatively impact QOL, including the number of physical and mental unhealthy days and overall dissatisfaction with life. Individuals with vision impairment are also more likely to experience restrictions in their independence, mobility, and educational achievement, as well as an increased risk of falls, fractures, injuries, poor mental health, cognitive deficits, and social isolation.
Vision loss also amplifies the effects of other chronic conditions and is a chronic condition itself. People with a vision impairment and other illnesses or conditions are more likely to have difficulty performing tasks and reporting poor health. Vision loss can also complicate chronic disease management, including self-care, transportation to and from doctor's appointments, and the proper administration of medicine. Moreover, other conditions may affect the management of eye disease, including vision rehabilitation to improve the functionality and quality of life for those with vision impairments.
No studies are available on the total costs attributable to the promotion of eye and vision health and the economic impact of vision loss in the United States. However, the few studies available that have looked at overall direct and indirect costs found that national costs are in the billions each year and vary substantially by state. Total costs also vary by age and by payer, with substantial costs incurred by individuals, including costs of caring for family members with vision impairment. Population health approaches to improve eye and vision health will need to focus on the direct and indirect costs as objective measures of the impact of vision impairment but also as measures of equity among populations most likely to be affected by vision impairment.
- Aflac. National trends: 2015 fact sheet: Aflac workforces report. 2015. [April 7, 2016]. https://www .aflac.com /docs/awr/pdf/2015-detailed-findings /2015-national-fact-sheet.pdf .
- Anderson G, Horvath J. The growing burden of chronic disease in America. Public Health Reports. 2004; 119 (3):263–270. [ PMC free article : PMC1497638 ] [ PubMed : 15158105 ]
- Baker ML, Wang JJ, Rogers S, Klein R, Kuller LH, Larsen EK, Wong TY. Early age-related macular degeneration, cognitive function, and dementia: The Cardiovascular Health Study. Archives of Ophthalmology. 2009; 127 (5):667–673. [ PMC free article : PMC3001290 ] [ PubMed : 19433718 ]
- Bibby SA, Maslin ER, McIlraith R, Soong GP. Vision and self-reported mobility performance in patients with low vision. Clinical and Experimental Optometry. 2007; 90 (2):115–123. [ PubMed : 17311573 ]
- Bramley T, Peeples P, Walt JG, Juhasz M, Hansen JE. Impact of vision loss on costs and outcomes in Medicare beneficiaries with glaucoma. Archives of Ophthalmology. 2008; 126 (6):849–856. [ PubMed : 18541852 ]
- Brown JC, Goldstein JE, Chan TL, Massof R, Ramulu P., Low Vision Research Network Study Group. Characterizing functional complaints in patients seeking outpatient low-vision services in the United States. Ophthalmology. 2014; 121 (8):1655–1662. e1651. [ PMC free article : PMC6746569 ] [ PubMed : 24768243 ]
- Butler JM, Sharif U, Ali M, McKibbin M, Thompson JP, Gale R, Yang YC, Inglehearn C, Paraoan L. A missense variant in CST3 exerts a recessive effect on susceptibility to age-related macular degeneration resembling its association with Alzheimer’s disease. Human Genetics. 2015; 134 (7):705–715. [ PMC free article : PMC4460273 ] [ PubMed : 25893795 ]
- CDC (Centers for Disease Control and Prevention). Chronic disease overview. 2016. [April 7, 2016]. http://www .cdc.gov/chronicdisease /overview .
- Chai Y, Shao Y, Lin S, Xiong K, Chen W, Li Y, Yi J, Zhang L, Tan G, Tang J. Vision-related quality of life and emotional impact in children with strabismus: A prospective study. Journal of International Medical Research. 2009; 37 (4):1108–1114. [ PubMed : 19761693 ]
- Chatziralli IP, Sergentanis TN, Peponis VG, Papazisis LE, Moschos MM. Risk factors for poor vision-related quality of life among cataract patients. Evaluation of baseline data. Graefe’s Archive for Clinical and Experimental Ophthalmology. 2012; 251 (3):783–789. [ PubMed : 23150044 ]
- Chaudry I, Brown GC, Brown MM. Medical student and patient perceptions of quality of life associated with vision loss. Canadian Journal of Ophthalmology. 2015; 50 (3):217–224. [ PubMed : 26040222 ]
- Cheng HC, Guo CY, Chen MJ, Ko YC, Huang N, Liu CJL. Patient-reported vision-related quality of life differences between superior and inferior hemifield visual field defects in primary open-angle glaucoma. JAMA Ophthalmology. 2015; 133 (3):269–275. [ PubMed : 25429608 ]
- Chia EM, Mitchell P, Ojaimi E, Rochtchina E, Wang JJ. Assessment of vision-related quality of life in an older population subsample: The Blue Mountains Eye Study. Ophthalmic Epidemiology. 2006; 13 (6):371–377. [ PubMed : 17169850 ]
- Christ SL, Zheng DD, Swenor BK, Lam BL, West SK, Tannenbaum SL, Munoz BE, Lee DJ. Longitudinal relationships among visual acuity, daily functional status, and mortality: The Salisbury Eye Evaluation study. JAMA Ophthalmology. 2014; 132 (12):1400–1406. [ PMC free article : PMC7894742 ] [ PubMed : 25144579 ]
- Clemons TE, Rankin MW, McBee WL. Cognitive impairment in the Age-Related Eye Disease Study: AREDS report no. 16. Archives of Ophthalmology. 2006; 124 (4):537–543. [ PMC free article : PMC1472655 ] [ PubMed : 16606880 ]
- CMS (Centers for Medicare & Medicaid Services). Chronic conditions among Medicare beneficiaries. Baltimore, MD: CMS; 2012.
- CMS (Centers for Medicare & Medicaid Services). Medicare enrollment—National trends 1966–2013. Baltimore, MD: CMS; 2014. [March 7, 2016]. https://www .cms.gov/Research-Statistics-Data-and-Systems /Statistics-Trends-andReports /MedicareEnrpts/Downloads/SMI2013 .pdf .
- Coleman AL, Cummings SR, Ensrud KE, Yu F, Gutierrez P, Stone KL, Cauley JA, Pedula KL, Hochberg MC, Mangione CM. Visual field loss and risk of fractures in older women. Journal of the American Geriatric Society. 2009; 57 (10):1825–1832. [ PMC free article : PMC3355977 ] [ PubMed : 19702619 ]
- Cox A, Blaikie A, MacEwen CJ, Jones D, Thompson K, Holding D, Sharma T, Miller S, Dobson S, Sanders R. Visual impairment in elderly patients with hip fracture: Causes and associations. Eye (London). 2005; 19 (6):652–656. [ PubMed : 15332096 ]
- Cregg M, Woodhouse JM, Stewart RE, Pakeman VH, Bromham NR, Gunter HL, Trojanowska L, Parker M, Fraser WI. Development of refractive error and strabismus in children with Down syndrome. Investigative Ophthalmology & Visual Science. 2003; 44 (3):1023–1030. [ PubMed : 12601024 ]
- Crews J, Chou CF. Vision impairment and multiple chronic conditions: Findings from the 2002, 2008 National Health Interview Survey. 2012. [April 7, 2016]. http: //visionproblemsus .org/presentations/Crews.pdf .
- Crews JE, Jones GC, Kim JH. Double jeopardy: The effects of comorbid conditions among older people with vision loss. Journal of Visual Impairment and Blindness. 2006; 100 :824.
- Crews JE, Chou CF, Zhang X, Zack MM, Saaddine JB. Health-related quality of life among people aged ≥65 years with self-reported visual impairment: Findings from the 2006–2010 Behavioral Risk Factor Surveillance System. Ophthalmic Epidemiology. 2014; 21 (5):287–296. [ PMC free article : PMC4924345 ] [ PubMed : 24955821 ]
- Crews JE, Chiu-Fung Chou CF, Stevens JA, Saadine JB. Falls among persons aged > 65 years with and without severe vision impairment—United States, 2014. Morbidity and Mortality Weekly Report. 2016a; 65 (17):433–437. [ PubMed : 27148832 ]
- Crews JE, Chou CF, Zack MM, Zhang X, Bullard KM, Morse AR, Saaddine JB. The association of health-related quality of life with severity of visual impairment among people aged 40–64 years: Findings from the 2006–2010 Behavioral Risk Factor Surveillance System. Ophthalmic Epidemiology. 2016b; 23 (3):145–153. [ PMC free article : PMC4924343 ] [ PubMed : 27159347 ]
- Cummings SR, Nevitt MC, Browner WS, Stone K, Fox KM, Ensrud KE, Cauley J, Black D, Vogt TM. Risk factors for hip fracture in white women. Study of Osteoporotic Fractures Research Group. New England Journal of Medicine. 1995; 332 (12):767–773. [ PubMed : 7862179 ]
- Dargent-Molina P, Favier F, Grandjean H, Baudoin C, Schott A, Hausherr E, Meunier P, Breart G. Fall-related factors and risk of hip fracture: The EPIDOS prospective study. Lancet. 1996; 348 (9021):145–149. [ PubMed : 8684153 ]
- de Boer MR, Pluijm SM, Lips P, Moll AC, Volker-Dieben HJ, Deeg DJ, van Rens GH. Different aspects of visual impairment as risk factors for falls and fractures in older men and women. Journal of Bone and Mineral Research. 2004; 19 (9):1539–1547. [ PubMed : 15312256 ]
- Ego A, Lidzba K, Brovedani P, Belmonti V, Gonzalez-Monge S, Boudia B, Ritz A, Cans C. Visual–perceptual impairment in children with cerebral palsy: A systematic review. Developmental Medicine & Child Neurology. 2015; 57 (s2):46–51. [ PubMed : 25690117 ]
- Evans K, Law SK, Walt J, Buchholz P, Hansen J. The quality of life impact of peripheral versus central vision loss with a focus on glaucoma versus age-related macular degeneration. Clinical Ophthalmology. 2009; 3 :433–445. [ PMC free article : PMC2724034 ] [ PubMed : 19684867 ]
- Felson DT, Anderson JJ, Hannan MT, Milton RC, Wilson PW, Kiel DP. Impaired vision and hip fracture: The Framingham Study. Journal of the American Geriatric Society. 1989; 37 (6):495–500. [ PubMed : 2715555 ]
- Flanagan N, Jackson A, Hill A. Visual impairment in childhood: Insights from a community-based survey. Child: Care, Health and Development. 2003; 29 (6):493–499. [ PubMed : 14616907 ]
- Fong CSU, Mitchell P, Rochtchina E, Teber ET, Hong T, Wang JJ. Correction of visual impairment by cataract surgery and improved survival in older persons: The Blue Mountains Eye Study cohort. Ophthalmology. 2013; 120 (9):1720–1727. [ PubMed : 23664468 ]
- Fong CSU, Mitchell P, Rochtchina E, De Loryn T, Tan AG, Wang JJ. Visual impairment corrected via cataract surgery and 5-year survival in a prospective cohort. American Journal of Ophthalmology. 2014; 157 (1):163–170. e161. [ PubMed : 24161249 ]
- Freedman BL, Jones SK, Lin A, Stinnett SS, Muir KW. Vision-related quality of life in children with glaucoma. Journal of American Association for Pediatric Ophthalmology and Strabismus. 2014; 18 (1):95–98. [ PMC free article : PMC3973725 ] [ PubMed : 24568998 ]
- Garaigordobil M, Bernarás E. Self-concept, self-esteem, personality traits and psychopathological symptoms in adolescents with and without visual impairment. Spanish Journal of Psychology. 2009; 12 (01):149–160. [ PubMed : 19476228 ]
- Goodman RA, Posner SF, Huang ES, Parekh AK, Koh HK. Defining and measuring chronic conditions: Imperatives for research, policy, program, and practice. Preventing Chronic Disease. 2013; 10 :E66. [ PMC free article : PMC3652713 ] [ PubMed : 23618546 ]
- Gopinath B, Schneider J, McMahon CM, Burlutsky G, Leeder SR, Mitchell P. Dual sensory impairment in older adults increases the risk of mortality: A population-based study. PLoS ONE. 2013; 8 (3):e55054. [ PMC free article : PMC3587637 ] [ PubMed : 23469161 ]
- Harwood RH, Foss A, Osborn F, Gregson R, Zaman A, Masud T. Falls and health status in elderly women following first eye cataract surgery: A randomised controlled trial. British Journal of Ophthalmology. 2005; 89 (1):53–59. [ PMC free article : PMC1772474 ] [ PubMed : 15615747 ]
- Hassell J, Lamoureux E, Keeffe J. Impact of age related macular degeneration on quality of life. British Journal of Ophthalmology. 2006; 90 (5):593–596. [ PMC free article : PMC1857044 ] [ PubMed : 16622089 ]
- Haymes SA, Johnston AW, Heyes AD. Relationship between vision impairment and ability to perform activities of daily living. Ophthalmic and Physiologic Optics. 2002; 22 (2):79–91. [ PubMed : 12014491 ]
- Heine C, Browning CJ. Mental health and dual sensory loss in older adults: A systematic review. Frontiers in Aging Neuroscience. 2014; 6 :83. [ PMC free article : PMC4030176 ] [ PubMed : 24860496 ]
- HHS (U.S. Department of Health and Human Services). Multiple chronic conditions—A strategic framework: Optimum health and quality of life for individuals with multiple chronic conditions. Washington, DC: HHS; 2010.
- Hirneiss C. The impact of a better-seeing eye and a worse-seeing eye on vision-related quality of life. Clinical Ophthalmology. 2014; 8 :1703–1709. [ PMC free article : PMC4159393 ] [ PubMed : 25214763 ]
- Hong T, Mitchell P, Burlutsky G, Samarawickrama C, Wang JJ. Visual impairment and the incidence of falls and fractures among older people: Longitudinal findings from the Blue Mountains Eye Study. Investigative Ophthalmology & Visual Science. 2014; 55 (11):7589–7593. [ PubMed : 25370514 ]
- Hwang W, Weller W, Ireys H, Anderson G. Out-of-pocket medical spending for care of chronic conditions. Health Affairs (Millwood). 2001; 20 (6):267–278. [ PubMed : 11816667 ]
- Ivers RQ, Cumming RG, Mitchell P, Attebo K. Visual impairment and falls in older adults: The Blue Mountains Eye Study. Journal of the American Geriatric Society. 1998; 46 (1):58–64. [ PubMed : 9434666 ]
- Kempen GI, Ballemans J, Ranchor AV, van Rens GH, Zijlstra GA. The impact of low vision on activities of daily living, symptoms of depression, feelings of anxiety and social support in community-living older adults seeking vision rehabilitation services. Quality of Life Research. 2012; 21 (8):1405–1411. [ PMC free article : PMC3438403 ] [ PubMed : 22090173 ]
- Klaver CC, Ott A, Hofman A, Assink JJ, Breteler MM, de Jong PT. Is age-related maculopathy associated with Alzheimer’s disease? The Rotterdam Study. American Journal of Epidemiology. 1999; 150 (9):963–968. [ PubMed : 10547142 ]
- Klein BE, Moss SE, Klein R, Lee KE, Cruickshanks KJ. Associations of visual function with physical outcomes and limitations 5 years later in an older population: The Beaver Dam Eye Study. Ophthalmology. 2003; 110 (4):644–650. [ PubMed : 12689880 ]
- Köberlein J, Beifus K, Schaffert C, Finger RP. The economic burden of visual impairment and blindness: A systematic review. British Medical Journal Open. 2013; 3 (11):e003471. [ PMC free article : PMC3822298 ] [ PubMed : 24202057 ]
- Kulmala J, Era P, Parssinen O, Sakari R, Sipila S, Rantanen T, Heikkinen E. Lowered vision as a risk factor for injurious accidents in older people. Aging Clinical and Experimental Research. 2008; 20 (1):25–30. [ PubMed : 18283225 ]
- Kulp MT, Ciner E, Maguire M, Moore B, Pentimonti J, Pistilli M, Cyert L, Candy TR, Quinn G, Ying GS. Uncorrected hyperopia and preschool early literacy. Ophthalmology. 2016; 123 (4):681–689. [ PMC free article : PMC4808323 ] [ PubMed : 26826748 ]
- Lam BL, Christ SL, Lee DJ, Zheng DD, Arheart KL. Reported visual impairment and risk of suicide: The 1986–1996 National Health Interview Surveys. Archives of Ophthalmology. 2008; 126 (7):975–980. [ PMC free article : PMC2630284 ] [ PubMed : 18625946 ]
- Lamoureux E, Pesudovs K. Vision-specific quality-of-life research: A need to improve the quality. American Journal of Ophthalmology. 2011; 151 (2):195–197. e192. [ PubMed : 21251493 ]
- Langelaan M, de Boer MR, van Nispen RM, Wouters B, Moll AC, van Rens GH. Impact of visual impairment on quality of life: A comparison with quality of life in the general population and with other chronic conditions. Ophthalmic Epidemiology. 2007; 14 (3):119–126. [ PubMed : 17613846 ]
- Lawrence V, Murray J, Ffytche D, Banerjee S. “Out of sight, out of mind”: A qualitative study of visual impairment and dementia from three perspectives. International Psychogeriatrics. 2009; 21 (3):511–518. [ PubMed : 19265571 ]
- Lee DJ, Gomez-Marin O, Lam BL, Zheng DD. Visual acuity impairment and mortality in U.S. adults. Archives of Ophthalmology. 2002; 120 (11):1544–1550. [ PubMed : 12427070 ]
- Lee DJ, Gomez-Marin O, Lam BL, Zheng DD. Visual impairment and unintentional injury mortality: The National Health Interview Survey 1986–1994. American Journal of Ophthalmology. 2003; 136 (6):1152–1154. [ PubMed : 14644228 ]
- Lee DJ, Gomez-Marin O, Lam BL, Zheng DD, Arheart KL, Christ SL, Caban AJ. Severity of concurrent visual and hearing impairment and mortality: The 1986–1994 National Health Interview Survey. Journal of Aging Health. 2007; 19 (3):382–396. [ PubMed : 17496240 ]
- Lew H, Lee HS, Lee JY, Song J, Min K, Kim M. Possible linkage between visual and motor development in children with cerebral palsy. Pediatric Neurology. 2015; 52 (3):338–343. e331. [ PubMed : 25701187 ]
- Lin MY, Gutierrez PR, Stone KL, Yaffe K, Ensrud KE, Fink HA, Sarkisian CA, Coleman AL, Mangione CM. Vision impairment and combined vision and hearing impairment predict cognitive and functional decline in older women. Journal of the American Geriatric Society. 2004; 52 (12):1996–2002. [ PubMed : 15571533 ]
- Lindenberger U, Baltes PB. Sensory functioning and intelligence in old age: A strong connection. Psychology and Aging. 1994; 9 (3):339–355. [ PubMed : 7999320 ]
- Liu T, Cheung S, Schuchard R, Glielmi C, Hu X, He S, Legge GE. Incomplete cortical reorganization in macular degeneration. Investigative Ophthalmology & Visual Science. 2010; 51 (12):6826–6834. [ PMC free article : PMC3055781 ] [ PubMed : 20631240 ]
- Liu Y, Yu C, Liang M, Li J, Tian L, Zhou Y, Qin W, Li K, Jiang T. Whole brain functional connectivity in the early blind. Brain. 2007; 130 (Pt 8):2085–2096. [ PubMed : 17533167 ]
- Lord SR. Visual risk factors for falls in older people. Age and Ageing. 2006; 35 (Suppl 2):ii42–ii45. [ PubMed : 16926203 ]
- Lord SR, Dayhew J. Visual risk factors for falls in older people. Journal of the American Geriatric Society. 2001; 49 (5):508–515. [ PubMed : 11380741 ]
- Lord SR, Clark RD, Webster IW. Visual acuity and contrast sensitivity in relation to falls in an elderly population. Age and Ageing. 1991; 20 (3):175–181. [ PubMed : 1853790 ]
- Lord SR, Ward JA, Williams P, Anstey KJ. Physiological factors associated with falls in older community-dwelling women. Journal of the American Geriatric Society. 1994; 42 (10):1110–1117. [ PubMed : 7930338 ]
- Marron JA, Bailey IL. Visual factors and orientation-mobility performance. American Journal of Optometry and Physiological Optics. 1982; 59 (5):413–426. [ PubMed : 7102800 ]
- Mazzone L, Ducci F, Scoto MC, Passaniti E, ’Arrigo VGD, Vitiello B. The role of anxiety symptoms in school performance in a community sample of children and adolescents. BMC Public Health. 2007; 7 (1):1–6. [ PMC free article : PMC2228292 ] [ PubMed : 18053257 ]
- Moyer VA. Prevention of falls in community-dwelling older adults: U.S. Preventive Services Task Force Recommendation statement. Annals of Internal Medicine. 2012; 157 (3):197–204. [ PubMed : 22868837 ]
- Nevitt MC, Cummings SR, Kidd S, Black D. Risk factors for recurrent nonsyncopal falls. A prospective study. JAMA. 1989; 261 (18):2663–2668. [ PubMed : 2709546 ]
- O’Day B. Employment barriers for people with visual impairments. Journal of Visual Impairment and Blindness. 1999; 93 (10):627–642.
- Ong SY, Ikram MK, Haaland BA, Cheng CY, Saw SM, Wong TY, Cheung CY. Myopia and cognitive dysfunction: The Singapore Malay Eye Study. Investigative Ophthalmology & Visual Science. 2013; 54 (1):799–803. [ PubMed : 23307956 ]
- Orta AÖ, Öztürker ZK, Erkul SÖ, Bayraktar Ş, Yilmaz OF. The correlation between glaucomatous visual field loss and vision-related quality of life. Journal of Glaucoma. 2015; 24 (5):e121–e127. [ PubMed : 25642814 ]
- Park Y, Shin JA, Yang SW, Yim HW, Kim HS, Park YH., Epidemiologic Survey Committee of the Korean Ophthalmologic Society. The relationship between visual impairment and health-related quality of life in Korean adults: The Korea National Health and Nutrition Examination Survey (2008–2012). PLoS ONE. 2015; 10 (7):e0132779. [ PMC free article : PMC4508049 ] [ PubMed : 26192763 ]
- Pascual-Leone A, Amedi A, Fregni F, Merabet LB. The plastic human brain cortex. Annual Reviews of Neuroscience. 2005; 28 :377–401. [ PubMed : 16022601 ]
- Patel I, Turano KA, Broman AT, Bandeen-Roche K, Munoz B, West SK. Measures of visual function and percentage of preferred walking speed in older adults: The Salisbury Eye Evaluation project. Investigative Ophthalmology & Visual Science. 2006; 47 (1):65–71. [ PubMed : 16384945 ]
- Patino CM, McKean-Cowdin R, Azen SP, Allison JC, Choudhury F, Varma R. Central and peripheral visual impairment and the risk of falls and falls with injury. Ophthalmology. 2010; 117 (2):199–206. e191. [ PMC free article : PMC2819614 ] [ PubMed : 20031225 ]
- Pham TQ, Kifley A, Mitchell P, Wang JJ. Relation of age-related macular degeneration and cognitive impairment in an older population. Gerontology. 2006; 52 (6):353–358. [ PubMed : 16902306 ]
- Quigley HA, Cassard SD, Gower EW, Ramulu PY, Jampel HD, Friedman DS. The cost of glaucoma care provided to Medicare beneficiaries from 2002 to 2009. Ophthalmology. 2013; 120 (11):2249–2257. [ PubMed : 23769330 ]
- Rees G, Tee HW, Marella M, Fenwick E, Dirani M, Lamoureux EL. Vision-specific distress and depressive symptoms in people with vision impairment. Investigative Ophthalmology & Visual Science. 2010; 51 (6):2891–2896. [ PubMed : 20164466 ]
- Rein DB, Zhang P, Wirth KE, Lee PP, Hoerger TJ, McCall N, Klein R, Tielsch JM, Vijan S, Saaddine J. The economic burden of major adult visual disorders in the United States. Archives of Ophthalmology. 2006; 124 (12):1754–1760. [ PubMed : 17159036 ]
- Rein DB, Wirth KE, Johnson CA, Lee PP. Estimating quality-adjusted life year losses associated with visual field deficits using methodological approaches. Ophthalmic Epidemiology. 2007; 14 (4):258–264. [ PubMed : 17896306 ]
- Reyes-Ortiz CA, Kuo YF, DiNuzzo AR, Ray LA, Raji MA, Markides KS. Near vision impairment predicts cognitive decline: Data from the Hispanic Established Populations for Epidemiologic Studies of the Elderly. Journal of the American Geriatric Society. 2005; 53 (4):681–686. [ PubMed : 15817017 ]
- Rogers MA, Langa KM. Untreated poor vision: A contributing factor to late-life dementia. American Journal of Epidemiology. 2010; 171 (6):728–735. [ PMC free article : PMC2842219 ] [ PubMed : 20150357 ]
- Roizen N, Kasza K, Karrison T, Mets M, Noble AG, Boyer K, Swisher C, Meier P, Remington J, Jalbrzikowski J. Impact of visual impairment on measures of cognitive function for children with congenital toxoplasmosis: Implications for compensatory intervention strategies. Pediatrics. 2006; 118 (2):e379–e390. [ PubMed : 16864640 ]
- Rovner BW, Casten RJ. Activity loss and depression in age-related macular degeneration. American Journal of Geriatric Psychiatry. 2002; 10 (3):305–310. [ PubMed : 11994218 ]
- Rovner BW, Casten RJ, Tasman WS. Effect of depression on vision function in age-related macular degeneration. Archives of Ophthalmology. 2002; 120 (8):1041–1044. [ PubMed : 12149057 ]
- Rovner BW, Casten RJ, Hegel MT, Tasman WS. Minimal depression and vision function in age-related macular degeneration. Ophthalmology. 2006; 113 (10):1743–1747. [ PubMed : 16893569 ]
- Rovner BW, Casten RJ, Hegel MT, Massof RW, Leiby BE, Ho AC, Tasman WS. Low vision depression prevention trial in age-related macular degeneration. Ophthalmology. 2014; 121 (11):2204–2211. [ PMC free article : PMC4253064 ] [ PubMed : 25016366 ]
- Rubenstein LZ, Josephson KR. The epidemiology of falls and syncope. Clinical Geriatric Medicine. 2002; 18 (2):141–158. [ PubMed : 12180240 ]
- Salt A, Sargent J. Common visual problems in children with disability. Archives of Disease in Childhood. 2014; 99 (12):1163–1168. [ PMC free article : PMC4251159 ] [ PubMed : 25165073 ]
- Saunders GH, Echt KV. An overview of dual sensory impairment in older adults: Perspectives for rehabilitation. Trends in Amplification. 2007; 11 (4):243–258. [ PMC free article : PMC4111537 ] [ PubMed : 18003868 ]
- Sayner R, Carpenter DM, Robin AL, Blalock SJ, Muir KW, Vitko M, Hartnett ME, Lawrence SD, Giangiacomo AL, Tudor G, Goldsmith JA, Sleath B. How glaucoma patient characteristics, self-efficacy and patient-provider communication are associated with eye drop technique. International Journal of Pharmaceutical Practice. 2015; 24 (2):78–85. [ PMC free article : PMC5599214 ] [ PubMed : 26303667 ]
- Schmier JK, Levine JA. Economic impact of progression of age-related macular degeneration. Acta Ophthalmoligica. 2013; 93 (2):105–121.
- Schneck ME, Lott LA, Haegerstrom-Portnoy G, Brabyn JA. Association between hearing and vision impairments in older adults. Ophthalmic and Physiological Optics. 2012; 32 (1):45–52. [ PMC free article : PMC3237734 ] [ PubMed : 21999724 ]
- Schneider JM, Gopinath B, McMahon CM, Leeder SR, Mitchell P, Wang JJ. Dual sensory impairment in older age. Journal of Aging and Health. 2011; 23 (8):1309–1324. [ PubMed : 21596997 ]
- Schneider J, Gopinath B, McMahon C, Teber E, Leeder SR, Wang JJ, Mitchell P. Prevalence and 5-year incidence of dual sensory impairment in an older Australian population. Annals of Epidemiology. 2012; 22 (4):295–301. [ PubMed : 22382082 ]
- Souied EH, Benlian P, Amouyel P, Feingold J, Lagarde JP, Munnich A, Kaplan J, Coscas G, Soubrane G. The epsilon 4 allele of the apolipoprotein E gene as a potential protective factor for exudative age-related macular degeneration. American Journal of Ophthalmology. 1998; 125 (3):353–359. [ PubMed : 9512153 ]
- Spahr J. Presentation at second meeting on Public Health Approaches to Promote Eye Health and Reduce Vision Impairment. Washington, DC: 2015.
- Steinman B, Nguyen A, Pynoos J, Leland N. Falls-prevention interventions for persons who are blind or visually impaired. Insight: Research and Practice in Vision Impairment and Blindness. 2011; 4 :83–91. [ PMC free article : PMC6309321 ] [ PubMed : 30595966 ]
- Swenor BK, Munoz B, West SK. Does visual impairment affect mobility over time? The Salisbury Eye Evaluation study. Investigative Ophthalmology & Visual Science. 2013; 54 (12):7683–7690. [ PMC free article : PMC3835273 ] [ PubMed : 24176902 ]
- Tay T, Wang JJ, Kifley A, Lindley R, Newall P, Mitchell P. Sensory and cognitive association in older persons: Findings from an older Australian population. Gerontology. 2006; 52 (6):386–394. [ PubMed : 16921251 ]
- Tseng VL, Yu F, Lum F, Coleman AL. Risk of fractures following cataract surgery in Medicare beneficiaries. JAMA. 2012; 308 (5):493–501. [ PubMed : 22851116 ]
- Tseng VL, Yu F, Lum F, Coleman AL. Ophthalmology. 2016. (Cataract surgery and mortality in the United States Medicare population). [ PubMed : 26854033 ]
- U.S. Census Bureau. American Community Survey (ACS). 2014. [June 7, 2016]. http://www .census.gov /people/disability/methodology/acs.html .
- Varma R, Lee PP, Goldberg I, Kotak S. An assessment of the health and economic burdens of glaucoma. American Journal of Ophthalmology. 2011; 152 (4):515–522. [ PMC free article : PMC3206636 ] [ PubMed : 21961848 ]
- Vogeli C, Shields AE, Lee TA, Gibson TB, Marder WD, Weiss KB, Blumenthal D. Multiple chronic conditions: Prevalence, health consequences, and implications for quality, care management, and costs. Journal of General and Internal Medicine. 2007; 22 (Suppl 3):391–395. [ PMC free article : PMC2150598 ] [ PubMed : 18026807 ]
- Wang JJ, Mitchell P, Cumming RG, Smith W., Blue Mountains Eye Study. Visual impairment and nursing home placement in older Australians: The Blue Mountains Eye Study. Ophthalmic Epidemiology. 2003; 10 (1):3–13. [ PubMed : 12607154 ]
- Whitson HE, Cousins SW, Burchett BM, Hybels CF, Pieper CF, Cohen HJ. The combined effect of visual impairment and cognitive impairment on disability in older people. Journal of the American Geriatric Society. 2007; 55 (6):885–891. [ PubMed : 17537089 ]
- Whitson HE, Ansah D, Whitaker D, Potter G, Cousins SW, MacDonald H, Pieper CF, Landerman L, Steffens DC, Cohen HJ. Prevalence and patterns of comorbid cognitive impairment in low vision rehabilitation for macular disease. Archives of Gerontology and Geriatrics. 2010; 50 (2):209–212. [ PMC free article : PMC2815114 ] [ PubMed : 19427045 ]
- Whitson HE, Steinhauser K, Ammarell N, Whitaker D, Cousins SW, Ansah D, Sanders LL, Cohen HJ. Categorizing the effect of comorbidity: A qualitative study of individuals’ experiences in a low-vision rehabilitation program. Journal of the American Geriatric Society. 2011; 59 (10):1802–1809. [ PMC free article : PMC3662468 ] [ PubMed : 22091493 ]
- Whitson HE, Malhotra R, Chan A, Matchar DB, Ostbye T. Comorbid visual and cognitive impairment: Relationship with disability status and self-rated health among older Singaporeans. Asia Pacific Journal of Public Health. 2012a; 26 (3):310–319. [ PMC free article : PMC3408775 ] [ PubMed : 22535554 ]
- Whitson HE, Whitaker D, Sanders LL, Potter GG, Cousins SW, Ansah D, McConnell E, Pieper CF, Landerman L, Steffens DC, Cohen HJ. Memory deficit associated with worse functional trajectories in older adults in low-vision rehabilitation for macular disease. Journal of the American Geriatric Society. 2012b; 60 (11):2087–2092. [ PMC free article : PMC3498592 ] [ PubMed : 23126548 ]
- Whitson HE, Malhotra R, Chan A, Matchar DB, Ostbye T. Comorbid visual and cognitive impairment: Relationship with disability status and self-rated health among older Singaporeans. Asia Pacific Journal of Public Health. 2014; 26 (3):310–319. [ PMC free article : PMC3408775 ] [ PubMed : 22535554 ]
- Wittenborn J, Rein D. Cost of vision problems: The economic burden of vision loss and eye disorders in the United States. Chicago, IL: NORC at the University of Chicago; 2013.
- Wittenborn J, Rein D. The potential costs and benefits of treatment for undiagnosed eye disorders. 2016. [September 15, 2016]. (Paper prepared for the Committee on Public Health Approaches to Reduce Vision Impairment and Promote Eye Health). unpublished. http://www .nationalacademies .org/hmd/~/media /Files/Report%20Files /2016/UndiagnosedEyeDisordersCommissionedPaper.pdf .
- Wolff JL, Starfield B, Anderson G. Prevalence, expenditures, and complications of multiple chronic conditions in the elderly. Archives of Internal Medicine. 2002; 162 (20):2269–2276. [ PubMed : 12418941 ]
- Wong TY, Klein R, Nieto FJ, Moraes SA, Mosley TH, Couper DJ, Klein BE, Boland LL, Hubbard LD, Sharrett AR. Is early age-related maculopathy related to cognitive function? The Atherosclerosis Risk in Communities Study. American Journal of Ophthalmology. 2002; 134 (6):828–835. [ PubMed : 12470750 ]
- Woo SJ, Park KH, Ahn J, Choe JY, Jeong H, Han JW, Kim TH, Kim KW. Cognitive impairment in age-related macular degeneration and geographic atrophy. Ophthalmology. 2012; 119 :2094–2101. [ PubMed : 22705343 ]
- Zebardast N, Swenor BK, van Landingham SW, Massof RW, Munoz B, West SK, Ramulu PY. Comparing the impact of refractive and nonrefractive vision loss on functioning and disability. Ophthalmology. 2015; 122 (6):1102–1110. [ PMC free article : PMC4446156 ] [ PubMed : 25813453 ]
- Zhang S, Liang Y, Chen Y, Musch DC, Zhang C, Wang N. Utility analysis of vision-related quality of life in patients with glaucoma and different perceptions from ophthalmologists. Journal of Glaucoma. 2015; 24 (7):508–514. [ PubMed : 25415642 ]
- Zheng DD, Christ SL, Lam BL, Tannenbaum SL, Bokman CL, Arheart KL, McClure LA, Fernandez CA, Lee DJ. Visual acuity and increased mortality: The role of allostatic load and functional status. Investigative Ophthalmology & Visual Science. 2014; 55 (8):5144–5150. [ PMC free article : PMC4580215 ] [ PubMed : 25061115 ]
The EQ-5D is a generic instrument used to measure health-related QOL. The tool rates the impact of disease on a scale of 0 to 1 with a lower score indicating greater effect of the health condition. The EQ-5D has five dimensions—mobility, self-care, usual activities, pain or discomfort, and anxiety or depression.
The authors defined “mild visual impairment” as visual acuity between 20/32 and 20/63; “moderate visual impairment” as visual acuity between 20/80 and 20/60; and “severe visual impairment” as visual acuity worse than or equal to 20/200.
Uncorrected refractive error was defined as a binocular visual acuity of less than or equal to 20/30 that improved to greater than 20/30 with subjective refraction.
Visual acuity was assessed using the Landolt ring chart, which consists of 13 lines in which visual acuity is scored from 0.125 (worst), if the person can only see the first line, to 2.0 (best) if the person can correctly see the last line. Visual acuity between 0.3 and 0.5 in the better eye was defined as lowered vision, and vision better than 0.5 was defined as normal vision.
A friend or relative with whom the participant had at least weekly contact.
The state cost estimates were a function of the states' populations within each age group. State populations were identified for the age groups 0–17, 18–39, 40–64, and 65+ based on the 2011 U.S. Census data. The burden estimate was divided by age for each age group to derive per-person costs for each group, then multiplied by the state population costs for each age group. These estimates do not include state-specific unit cost or utilization estimates ( Wittenborn and Rein, 2013 ).
“These costs are measured separately from other medical costs in MEPS; they are not associated with diagnosis codes and are based on non-confirmed, self-reported expenditures” ( Wittenborn and Rein, 2013, p. 2 ).
- Cite this Page National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Population Health and Public Health Practice; Committee on Public Health Approaches to Reduce Vision Impairment and Promote Eye Health; Welp A, Woodbury RB, McCoy MA, et al., editors. Making Eye Health a Population Health Imperative: Vision for Tomorrow. Washington (DC): National Academies Press (US); 2016 Sep 15. 3, The Impact of Vision Loss.
- PDF version of this title (16M)
In this Page
Other titles in this collection.
- The National Academies Collection: Reports funded by National Institutes of Health
Related information
- PMC PubMed Central citations
- PubMed Links to PubMed
Recent Activity
- The Impact of Vision Loss - Making Eye Health a Population Health Imperative The Impact of Vision Loss - Making Eye Health a Population Health Imperative
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers
The Clinical Problem Solvers
By The Clinical Problem Solvers

What's The Clinical Problem Solvers about?
The Clinical Problem Solvers is a multi-modal venture that works to disseminate and democratize the stories and science of diagnostic reasoning
Twitter: @CPSolvers
Website: clinicalproblemsolving.com

374 374 ratings
Download our free app to listen on your phone

The Clinical Problem Solvers episodes:
Episode 327: wdx #28: “anonymous was a woman”.
In honor of Women’s History month in the United States, Sharmin, Kaylin, and Jane discuss the Matilda Effect, the current state of gender inequality, and reflect on some of the many incredible women scientists that came before us and helped...

Episode 326 – RLR – Whole Body Pain
Episode description Reza and Rabih discuss a case of a patient with whole body pain Student discount https://www.rlrcpsolvers.com/student-discounts/ IMG discount Use coupon code RLRIMG at check out https://rlrcpsolvers.com/annual-plan

Episode 325: Rafael Medina Subspecialty Series – Facial Swelling
In this clinical reasoning Rafael Medina Subspecialty episode, Dr. Mark Heslin presents a case to Dr. Tony Breu of a man presenting with facial swelling. Session facilitator: Youssef Saklawi The goal of this series is to expand access to subspecialty, primary care, and internal medicine-adjacent...

Episode 324: Schema Episode – Joint Pain
Sharmin, Jack, and Youssef discuss their approach to joint pain as they reason through a case presented by Maddy. Monoarticular Arthritis Framework To join us live on Virtual Morning Report (VMR), sign up HERE. Download CPSolvers App here RLRCPSOLVERS

Episode 323: Neurology VMR – Visual Hallucinations
Episode title: Episode 323: Neurology VMR – Visual hallucinations Episode description: We continue our campaign to #EndNeurophobia, with the help of Dr. Aaron Berkowitz. This time, Valeria presents a case of visual hallucinations to Greg and Umbish. Neurology DDx Schema ...

Episode 322: Antiracism in Medicine – Episode 24 – Leveraging Narrative Medicine to Cultivate Antiracist Praxis
CPSolvers: Anti-Racism in Medicine Series Episode 24 – Leveraging Narrative Medicine to Cultivate Antiracist Praxis Show Notes by Sudarshan Krishnamurthy February 13, 2024 Summary: This episode highlights the ways in which we might leverage stories, at the individual and structural...

Episode 321: Spaced Learning Series – AKI, Eosinophilia and Organomegaly
Episode description: SLS team members Priyanka, Kirtan and Anna discuss a case of progressive renal dysfunction in a patient with recent weight loss, peripheral eosinophilia and organomegaly Featuring: Anna FretzKirtan PatoliaPriyanka Athavale SchemasAKIEosinophilia Download CPSolvers App here RLRCPSOLVERS Click here to view the weekly...

Episode 320: WDx #27: “There is No Innocent Eye”
Sharmin and Kaylin sit down with Dr. Denise Davis to discuss communication as a procedure, continuous and incremental improvement, and the interrelationship between social and health justice Denise L. Davis is a general internist and Clinical Professor of Medicine...

Episode 319 – RLR – Profound Fatigue
Episode description Reza and Rabih discuss a case of a patient with profound fatigue Student discount https://www.rlrcpsolvers.com/student-discounts/ IMG discount Use coupon code RLRIMG at check out https://rlrcpsolvers.com/annual-plan

Episode 318: Rafael Medina Subspecialty Series – Headache
In this Rheumatology Rafael Medina Subspecialty episode, Dr. John Landefeld presents a case to Dr. Sarah Goglin of a 74 year old woman presenting with headache. Session facilitator: Şeyma Yıldırım The goal of this session is to expand access to...

Episode 317: Neurology VMR – Generalized Weakness
Episode title: Episode 317: Neurology VMR – Generalized weakness Episode description: We continue our campaign to #EndNeurophobia, with the help of Dr. Aaron Berkowitz. This time, Kirtan presents a case of generalized weakness to Vivek and Hannah. Neurology DDx Schema ...

Episode 316: Dissecting the Learning Process with Rabih & Reza
New Year, old episode! Rabih and Reza reveal all of the math and magic behind becoming better learners. Join live morning reports here! Download CPSolvers App here RLRCPSOLVERS

Episode 315: Schema Episode – Diverticulitis Complications
Ann-Marie, Sharmin, and Maddy discusses their approaches to diverticulitis complications as they talk through a case presented by Jack. Frameworks: Diverticulitis Complications Download CPSolvers App here RLRCPSOLVERS

Episode 314 – RLR – Systemic Stones
Episode description Reza and Rabih discuss a case of a patient with flank pain and systemic features Student discount https://www.rlrcpsolvers.com/student-discounts/ IMG discount Use coupon code RLRIMG at check out https://rlrcpsolvers.com/annual-plan

Episode 313: Rafael Medina Subspecialty Series – Splenomegaly
In this Hepatology Rafael Medina Subspecialty episode, Dr. Christopher Coe presents a case to Dr. Arpan Patel of a woman presenting with fatigue and splenomegaly. Session facilitator: Maddy Conte The goal of this series is to expand access to subspecialty,...

Episode 312: Neurology VMR – Frequent Stumbling
Episode title: Episode 312: Neurology VMR – Frequent stumbling Episode description: We continue our campaign to #EndNeurophobia, with the help of Dr. Aaron Berkowitz. This time, Valeria presents a case of frequent stumbling to Nilayan and Subhangi. Neurology DDx Schema ...

Episode 311: Spaced Learning Series – Hypercalcemia
Episode description: The spaced learning series team discusses a case of severe hypercalcemia, seizure, and vision loss. Featuring: Simone VaisMoses MurdockValeria Roldan SchemasHypercalcemia Download CPSolvers App here RLRCPSOLVERS Click here to view the weekly episode recap email!

Episode 310 – WDx Episode #26: “You are Resilient, even if You Don’t Know It”
Sharmin, Kaylin, and Jane are joined by Dr. Risheen Reejhsinghani, cardiologist extraordinaire, Clinical Associate Professor at Stanford, and Associate Program Director of the cardiology fellowship. They discuss her journey through medical training as an international medical graduate, how her love for cardiology developed, and how her...

Episode 309 – Antiracism in Medicine Series – Episode 23 – Anti-Blackness, Anti-Fatness, and Food Shaming
CPSolvers: Anti-Racism in Medicine Series Episode 23 – Anti-Blackness, Anti-Fatness, and Food Shaming Show Notes by Humza A. Siddiqui October 31, 2023 Summary: This episode highlights the culture of food shaming and anti-fatness as it relates to anti-Blackness. During...

Episode 308 – RLR – 105 Fever
Episode description Reza and Rabih discuss a case of a young woman with a fever of 105 Student discount https://www.rlrcpsolvers.com/student-discounts/ IMG discount Use coupon code RLRIMG at check out https://rlrcpsolvers.com/annual-plan Click here to view the weekly episode recap...

Episode 307: Rafael Medina Subspecialty Series – Fever and Chills
In this Infectious Disease Rafael Medina Subspecialty Episode, Dr. Navila Sharif presents a case to Dr. Natasha Spottiswoode of a patient presenting for fevers and chills. Session facilitator: Youssef Saklawi The goal of this series is to provide greater...

Episode 306: Schema Episode – Severe Acute Liver Injury
Sharmin, Jack, and Maddy discuss their approaches to severe acute liver injury, abdominal pain, and hyperferritinemia as they talk through a case presented by Ann-Marie. Frameworks: Severe Acute Liver Injury Abdominal Pain To join us live on Virtual Morning Report...

Episode 305: Neurology VMR – Shallow Breathing
Episode 305: Neurology VMR – Shallow breathing Episode description: We continue our campaign to #EndNeurophobia, with the help of Dr. Aaron Berkowitz. This time, Vaness presents a case of shallow breathing to Maria and Sridhara. Neurology DDx Schema Vanessa Roque...

Episode 304: Spaced Learning Series – The Journey of 3Hs: Hyperbilirubinemia, Hypoxia and Hemolysis
Episode description: The spaced learning series team discusses a case of a patient with hyperbilirubinemia secondary to acute alcoholic hepatitis, who then developed hypoxia and hemolysis. Featuring: Anna FretzPriyanka AthavaleKirtan Patolia SchemasHyperbilirubinemiaHypoxemiaHemolysis Download CPSolvers App here RLRCPSOLVERS Click here to view the...

Episode 303: WDx #25: “Do Small Things with a Big Heart” with María Jimena Alemán
Sharmin & Kaylin are joined by María Jimena Alemán, CPSolvers co-director of internal operations & future neurologist with a passion for global health. They discuss how her upbringing has informed & shaped her passions & values, how she got involved...

Episode 302 – RLR – Anasarca
Episode description Reza and Rabih discuss a curious case of Anasarca Student discount https://www.rlrcpsolvers.com/student-discounts/ IMG discount Use coupon code RLRIMG at check out https://rlrcpsolvers.com/annual-plan

Episode 301: Rafael Medina Subspecialty Series – Worsening diarrhea
In this Gastroenterology Rafael Medina Subspecialty episode, Dr. Allyson Richardson presents a case to Dr. Ryan Flanagan of a 68 year old woman presenting with chronic diarrhea. Session facilitator: Şeyma Yıldırım The goal of this session is to expand access to...

Episode 300: Schema Episode – Cytopenias
Jack, AMK, and Maddy discuss their approaches to cytopenias and DVTs as they talk through a case presented by Sharmin. Frameworks: Pancytopenia Thrombocytopenia Download CPSolvers App here RLRCPSOLVERS Click here to view the weekly episode recap email!

Episode 299 – Neurology VMR – Clumsiness
Episode 299: Neurology VMR – Clumsiness Episode description: We continue our campaign to #EndNeurophobia, with the help of Dr. Aaron Berkowitz. This time, María presents a case of clumsiness to Andrea and Sridhara. Neurology DDx Schema Andrea Mendez Colmenares @andreamendez92 Andrea...

Episode 298: Spaced Learning Series – Nausea, Vomiting, & Abdominal Pain
Episode description: The spaced learning series team discusses a case of nausea, vomiting and right upper quadrant pain in a pregnant patient. Featuring: Simone Vais Moses Murdock Valeria Roldan

Episode 297: WDx #24 – Clinical Unknown Discussion with Dr. Casey Albin
In this episode of WDx, Dr Casey Albin joins Kiara, Jane, & Sharmin to discuss a clinical unknown. Presented by Kiara, the case starts with the chief concern of difficulty recognizing family members. Casey Albin, MD is an Assistant Professor...

Episode 296 – RLR – A curious case of cough
Episode description RR discuss a grounding case of chest pain Student discount https://www.rlrcpsolvers.com/student-discounts/ IMG discount Use coupon code RLRIMG at check out https://rlrcpsolvers.com/annual-plan

Episode 295: Rafael Medina Subspecialty Series – fatigue in patient living with HIV
In this Infectious Disease Rafael Medina Subspecialty episode, Dr. Jorge Salazar presents a case to Dr. Monica Gandhi of a transgender woman with a recent diagnosis of HIV presenting with fatigue and weight loss. Session facilitator: Maddy Conte The goal...

Episode 294: Schema Episode – Mediastinal Mass
Maddy presents a case to Jack, Sharmin, and Ann Marie of a patient who is found to have a mediastinal mass on imaging. Mediastinal Mass Schema Download CPSolvers App here RLRCPSOLVERS

Episode 293 – Antiracism in Medicine Series – Episode 22 – Live from SGIM 2023: Best of Antiracism Research at the Society of General Internal Medicine’s 2023 Annual Meeting
CPSolvers: Anti-Racism in Medicine Series Episode 22 – Live from SGIM 2023: Best of Antiracism Research at the Society of General Internal Medicine’s 2023 Annual Meeting Show Notes by Alec J. Calac June 22, 2023 Summary: This episode highlights...

Episode 292 – RLR – A Case of Chest Pain to keep you on your toes

Episode 291 – Juneteenth The H&P – History and Perspective – Stories and Conversations with Dr. Kimberly Manning and her Dad, Mr. William Draper, Sr
Dr. Kimberly Manning and her father, Mr. William Draper, commemorate Juneteenth, the holiday that celebrates the day when all remaining enslaved Black Americas were freed in Galveston Texas, on June 19th, 1865, with this hour-long storytelling event.

Episode 290 – Neurology VMR – Vertigo
We continue our campaign to #EndNeurophobia, with the help of Dr. Aaron Berkowitz. This time, Dr. Gabriela Pucci presents a case of right arm weakness to Promise and Ravi. Promise Lee @promiseflee Promise Lee is currently a 3rd year medical...

Episode 289: Spaced Learning Series – Recurrent Presyncope
Episode description: The spaced learning series team discusses a case of intermittent episodes of presyncope in a patient found to have hypoglycemia and polycythemia. Featuring: Anna FretzKirtan PatoliaPriyanka Athavale Schemas:SyncopeHypoglycemiaPolycythemia Download CPSolvers App here RLRCPSOLVERS

Episode 288: WDx #23: Clinical Unknown Discussion with Dr Rebecca Berger
In this episode of WDx, Dr Rebecca Berger joins Kara, Jane, & Sharmin to discuss a clinical unknown. Presented by Kara, the case starts with a young woman presenting with chronic isolated thrombocytopenia. Dr. Rebecca Berger Rebecca is an academic...

Episode 287: RLR – Febrile and Rigid – an episode dedicated to our Rafa Medina
RR dedicate this episode to our beloved Rafa Medina. Rafa’s GoFundMe page.

Episode 286 – Rafael Medina Subspecialty Series – Elevated Creatinine
Maddy Conte and Seyma Yildirim introduce a new series on the podcast: “The Rafael Medina Subspecialty Series,” which will always be in loving memory of our dear friend and CPSolvers family member, Dr. Rafael Medina. Rafa presents a nephrology clinical...

Episode 285: Anti-Racism in Medicine Series – Episode 21 – Psychosocial and Cultural Considerations for Providing Healthcare to Immigrant and Refugee Populations
CPSolvers: Antiracism in Medicine Series Episode 21 – Psychosocial and Cultural Considerations for Providing Healthcare to Immigrant and Refugee Populations Show Notes by Kiersten T. “Gillette” Gillette-Pierce May 2, 2023 Summary: This episode highlights the psychosocial and cultural considerations for...

Episode 284: RLR – Recapping the journey – Moving backwards
RR recap a mystery case presented by Aaron. Student discount https://www.rlrcpsolvers.com/student-discounts/ IMG discount Use coupon code RLRIMG at check out https://rlrcpsolvers.com/annual-plan GlassHealth sponsorship https://twitter.com/GlassHealthHQ https://glass.health/cpsolvers Use promo code CPSOLVERS for one month free!

Episode 283 – Neurology VMR – Right arm weakness
We continue our campaign to #EndNeurophobia, with the help of Dr. Aaron Berkowitz. This time, Dr. Ravi Singh presents a case of right arm weakness to Yazmin and Sridhara. Neurology DDx Schema Yazmin Heredia @minheredia Yazmin is a Mexican Graduate...

Episode 282: Anti-Racism in Medicine Series – Episode 20 – Medical Racism and Indigenous Peoples
CPSolvers: Anti-Racism in Medicine Series Episode 20 – Medical Racism and Indigenous Peoples Show Notes by Sudarshan (“Sud”) Krishnamurthy April 4, 2023 Summary: This episode highlights the checkered past of medicine and the advancements in the field that have occurred...

Episode 281: The Consult Question #8 – Pancytopenia and Rash
Dr. Vipul Kumar presents a fascinating case of pancytopenia and rash to guest discussant, Dr. Anand Patel. Dr. Vipul Kumar MD PhD is a hematology-oncology fellow at UCSF. He is currently in his second year of fellowship and has a clinical...

Episode 280: RLR – Moving backwards
Aaron presents a mystery in reverse to RR Student discount https://www.rlrcpsolvers.com/student-discounts/ IMG discount Use coupon code RLRIMG at check out https://rlrcpsolvers.com/annual-plan GlassHealth sponsorship https://twitter.com/GlassHealthHQ https://glass.health/cpsolvers Use promo code CPSOLVERS for one month free!

Episode 279: Spaced Learning Series – Lower Extremity Weakness and Jaundice
Simone and Moses review their approach to chronic lower extremity weakness in a patient with new-onset jaundice, as Vale presents them a case with a neuro flavor to it. Download CPSolvers App here RLRCPSOLVERS

Episode 278: Schema Episode: Lower Extremity Edema, Thyrotoxicosis, and Pharyngitis
Ann Marie presents a case to Dan, Jack, and Sharmin of a young patient with tachycardia, lower extremity edema, who later develops pharyngitis. Thyrotoxicosis Schema Pharyngitis Schema Peripheral Edema Schema Download CPSolvers App here RLRCPSOLVERS

- Contributors
- Privacy Policy
- Sign Up Here!
Diagnostic frameworks
- Illness scripts
- The IMG Journey
- US Residency Programs VMR
- Inspirational Stories
- Gabriel Talledo’s Scholarship
- Publications
All Frameworks
Abdominal Distension
Abdominal Pain Overview
Abdominal Pain – Image Negative
Abdominal Pain – Image Negative – Action Steps
Abdominal Pain Thought Train
Acute Mesenteric Ischemia
Acute Pancreatitis
AKI – overview
AKI and cancer
Aldosterone – Inappropriate
Altered mental status 1.0
Altered Mental Status 2.0
Altered Mental Status – MIST Negative
Anemia Thought Train
Antibiotic Failure
Aortitis: Paths to the Problem
Bilateral Lower Extremity Weakness
Bone lesions
Bony Back Pain – Overview
Brain Abscess
Bronchiectasis
Bowel Obstruction
Bowel Perforation
Bowel Wall Thickening
Cancer and Hypercalcemia
Cervical Lymphadenopathy
CHF – Hyperacute Heart Failure
Chronic Cough
Chronic Diarrhea Overview
Chronic Diarrhea: 4 quarters approach
Chronic Kidney Disease
Chylothorax
Cirrhosis and Dyspnea
Coagulopathy – Elevated INR and PTT
Community Acquired Pneumonia
Community Acquired Pneumonia – Ddx
Congestive Heart Failure – HFrEF
Constipation
CSF Lymphocytic Pleocytosis
Diabetes – Causes
Diabetes Insipidus
Diarrhea Thought Train
Diffuse Alveolar Hemorrhage – DAH
Diffuse Lymphadenopathy Overview
Diffuse Lymphadenopathy: A Guide
Diverticulitis Complications
Doxycycline Deficiency State
Dyspnea and Cancer
Dyspnea Pyramid
Eosinophilia
Extra-Hepatic Biliary Obstruction
Fever Overview
Fever and Back Pain
Fever and rash
Fever in a returning traveler
Fever – Path to Inflammation
Gastric Mass
Glomerulonephritis
Gram Positive Organisms
Granulomatous Infection – by exposure
HCV – Extrahepatic Manifestations
HCV – Skin Manifestations
Headache (Secondary)
Headache (Thunderclap)
Heart Failure and Weight Loss
Hemolysis: Autoimmune Hemolytic Anemia (AIHA)
Hemolysis: Chronic Hemolysis Complications
Hemolysis: Hemolytic Anemia
Hemolysis: Microangiopathic Hemolytic Anemia (MAHA)
Hemoptysis: The Arc
Hemoptysis: Overview
Hemoptysis: Massive
HIV overview
HIV & Chronic Diarrhea
HIV & Infection
HIV: Non-infectious complications
Hyperammonemia
Hypercalcemia
Hyperkalemia
Hypernatremia
Hypertension Overview
Hypertension – Secondary
Hypoalbuminemia
Hypocalcemia
Hypoglycemia
Hypokalemia – Overview
Hypokalemia – Practical Approach
Hyponatremia
Hypophosphatemia- Overview
Hypophosphatemia – Severe
Hypothermia
Hypothyroidism
Hypoxemia Thought Train
IgM Disorders
Indirect Hyperbilirubinemia
Infection in the inpatient 1.0
Infection in the Inpatient – Secondary Evaluation
Infectious Aortitis: Clues
Inflammation overview
Inflammation Thought Train
Inflammatory Abdominal Pain
Inguinal Lymphadenopathy
Intrarenal AKI
Iron deficiency anemia
Jaundice – Overview
Jaundice – Intrahepatic Cholestasis
Jaundice Thought Train
Kidney Stone Overview
Lactic Acidosis
Lactate Dehydrogenase – LDH
Low Back Pain – Overview
Lung Cancer
Lung cavity
Lung Nodules
Mediastinal Mass
Macrocytic Anemia
Metabolic Acidosis Overview
Metabolic Acidosis: AGMA
Metabolic Acidosis: NAGMA
Metabolic Alkalosis
Monoarticular Arthritis
Nausea and Vomiting Thought Train
Neurologic Complications of Systemic Cancer
Nonstress Fracture
Optic Disc Swelling
Pancreatic Mass
Pancreatic Mass: Simple Approach
Pancytopenia
Parkinson’s Dz – The Time Course
Parkinsonism
Peptic Ulcer Disease
Peripheral Edema
Peripheral Neuropathy
Peritonitis
Petechial Rash
Pharyngitis
Photophobia
Pituitary Adenoma
Pleural effusion
Pleuritic Chest Pain
Pleural Nodularity
Pneumonia Complications
Pneumothorax
Polycythemia
Portal Vein Thrombosis
Postprandial Abdominal Pain
Post-renal AKI
Primary Respiratory Alkalosis
Pulmonary disease with eosinophilia
Pulmonary Function Tests – PFTs
Pulmonary Hypertension
Pulmonary Renal Syndromes
Raynaud’s Syndrome
Severe Acute Liver Injury
Splenomegaly
Specific Back Pain Overview
Subacute Dyspnea on Exertion
Sudden Symptoms
Supraventricular tachycardia – SVT
Thorax and Inflammation
Thrombocytopenia
Thrombocytosis
Thrombosis- Overview
Thrombosis – Bland
Thrombosis on Anticoagulation
Thrombosis – Ultrahypercoagulable
Thyroid Function Test in the Evaluation of Hyperthyroidism
Thyrotoxicosis
Tick borne infection
Transverse Myelitis
Upper GI Bleed
Urinalysis interpretation
Urinary Retention
Urinary Tract Infection
Vaginal Bleeding
Vasculitis Mimics
Vasculopathy – Systemic
Vasculopathy – Skin
Vasculopathy – CNS
Visceral Air
Visual Field Defects
Weakness Thought Train
Weight loss
Wide complex tachycardia

IMAGES
VIDEO
COMMENTS
Outside of the hospital, Kristin enjoys musical theatre, pub-style trivia, making ice-cream, and snuggling on the couch with her husband Andrew and dog Nutmeg. Mansour presents a case of sudden vision loss to Dan, Erica, and Kristin.
Go to: Visual loss is a serious clinical problem, commonly presenting first to non-ophthalmic healthcare professionals. It typically describes a reduction in visual acuity (sharpness/clarity) or field. This may be acute- or gradual-onset, monocular or binocular and transient or permanent, and may affect central and/or peripheral vision.
A year before presentation, painless vision loss had occurred in the left eye, followed by the right eye. ... More Clinical Problem-Solving. Clinical Problem-Solving; Mar 07, 2024; CME; Peeling ...
The evaluation of a patient with acute monocular visual loss begins with a careful history. Details from the patient's description of visual symptoms may offer a preliminary suggestion of whether visual loss results from ocular or optic nerve pathology. Monocular metamorphopsia (wavy, warped images) and positive phenomena such as flashing or ...
Chronic Vision Loss. By Jennifer C Larson, MD. Add to My Bookmarks. Comments. Views 1256. In this interactive case you will learn about evaluating a patient who presents with chronic vision loss, the causes and treatments for chronic vision loss and when to refer a patient to an ophthalmologist. Launch Case.
Amaurosis fugax (from the Greek "amaurosis," meaning dark, and the Latin "fugax," meaning fleeting) refers to a transient loss of vision in one or both eyes [ 1 ]. Varied use of common terminology may cause some confusion when reading the literature. Some suggest that "amaurosis fugax" implies a vascular cause for the visual loss [ 2 ], but the ...
NAION affects approximately 10 out of every 100,000 people over the age of 50. The condition is analogous to an optic-nerve stroke—one that affects the information highway that connects the eye and the brain. It is caused by a loss of blood supply, and therefore oxygen delivery, to the highly metabolically active optic nerve.
PST teaches problem-solving skills in a structured way to enable a participant to identify his problems, generate and select a solution: The ST therapists informed participants that its purpose was to explore the impact of vision loss on their lives: Type I: 3 and 6 mo: TVF, NEI VFQ 25 + supplement, AI, Physical health status, PHQ: Deemer AD ...
The Clinical Problem Solvers. CPSolvers x 100. Exclusive figures, videos, and case challenges uploaded regularly. Start Learning! Latest Content Updates! The goal of this website is to teach clinical reasoning with a focus on diagnosis. Diagnosis is one of the most important clinical skills. You cannot treat a patient or provide a prognosis ...
Introduction. Visual loss is a serious clinical problem, commonly presenting first to non-ophthalmic healthcare professionals. It typically describes a reduction in visual acuity (sharpness/clarity) or field. This may be acute- or gradual-onset, monocular or binocular and transient or permanent, and may affect central and/or peripheral vision.
Clinical Problem-Solving. Share on. ... blurred vision, flushing, tachypnea, tachycardia, and urinary retention but is typically associated with decreased bowel sounds, mydriasis, and dry mucosa ...
clinical problem-solving The new engl and journal of medicine n engl j med 364;9 nejm.org march 3, 2011 871 In this Journal feature, information about a real patient is presented in stages ...
Introduction. Impairments in vision are highly prevalent, affecting roughly 2.2 billion people worldwide. 1 Of these individuals, approximately 36 million are blind, and an estimated 217 million have marked (ie, moderate-to-severe) visual impairment. 2 In the US, age-related vision loss is a leading cause of disability among aging adults, primarily resulting from eye diseases such as macular ...
Join our global community who meet every day to discuss clinical unknowns. All healthcare professionals of all levels of training are more than welcome! ... Virtual Morning Report - December 23, 2023 - sudden vision loss in the left eye. Episode 1163 - Virtual Morning Report - December 22, 2023 - bilateral upper and lower extremity ...
Eye problems constitute 2% to 3% of all primary care and emergency department visits. 1, 2 Conjunctivitis, corneal abrasion, and hordeolum account for more than 50% of eye problems. 1, 2 Disorders ...
Floaters and flashes. Another combination of concerning symptoms are flashes and floaters in combination with monocular vision loss. Flashes and floaters of acute onset are concerning for a retinal detachment. Patients are commonly myopic (short-sighted) and may additionally complain of vision loss as a " curtain drawn " over their vision.
A study by Rein and colleagues (2007) found that the QOL begins to slowly decline with the onset of vision loss, and then decreases more precipitously as measures of visual field defects increase. A systematic literature review of studies that reported QOL in patients with central vision loss or peripheral vision loss, and found that both types of vision loss were associated with similar ...
The Clinical Problem Solvers is a multi-modal venture that works to disseminate and democratize the stories and science of diagnostic reasoning Twitter: @CPSolvers Website: clinicalproblemsolving.com ... Kirtan and Anna discuss a case of progressive renal dysfunction in a patient with recent weight loss, peripheral eosinophilia and organomegaly ...
Vasculopathy - CNS. Visceral Air. Visual Field Defects. Weakness Thought Train. Weight loss. Wide complex tachycardia. Frameworks alphabetically Cardiac Neuro Lung GI Renal Infectious Liver Rheum Blood Electrolytes Endocrine Miscellaneous Icons made by Kara Lau All Frameworks Abdominal DistensionAbdominal Pain OverviewAbdominal Pain - Image ...