Have a language expert improve your writing
Run a free plagiarism check in 10 minutes, generate accurate citations for free.
- Knowledge Base
Methodology
- What Is a Case-Control Study? | Definition & Examples

What Is a Case-Control Study? | Definition & Examples
Published on February 4, 2023 by Tegan George . Revised on June 22, 2023.
A case-control study is an experimental design that compares a group of participants possessing a condition of interest to a very similar group lacking that condition. Here, the participants possessing the attribute of study, such as a disease, are called the “case,” and those without it are the “control.”
It’s important to remember that the case group is chosen because they already possess the attribute of interest. The point of the control group is to facilitate investigation, e.g., studying whether the case group systematically exhibits that attribute more than the control group does.
Table of contents
When to use a case-control study, examples of case-control studies, advantages and disadvantages of case-control studies, other interesting articles, frequently asked questions.
Case-control studies are a type of observational study often used in fields like medical research, environmental health, or epidemiology. While most observational studies are qualitative in nature, case-control studies can also be quantitative , and they often are in healthcare settings. Case-control studies can be used for both exploratory and explanatory research , and they are a good choice for studying research topics like disease exposure and health outcomes.
A case-control study may be a good fit for your research if it meets the following criteria.
- Data on exposure (e.g., to a chemical or a pesticide) are difficult to obtain or expensive.
- The disease associated with the exposure you’re studying has a long incubation period or is rare or under-studied (e.g., AIDS in the early 1980s).
- The population you are studying is difficult to contact for follow-up questions (e.g., asylum seekers).
Retrospective cohort studies use existing secondary research data, such as medical records or databases, to identify a group of people with a common exposure or risk factor and to observe their outcomes over time. Case-control studies conduct primary research , comparing a group of participants possessing a condition of interest to a very similar group lacking that condition in real time.
Receive feedback on language, structure, and formatting
Professional editors proofread and edit your paper by focusing on:
- Academic style
- Vague sentences
- Style consistency
See an example

Case-control studies are common in fields like epidemiology, healthcare, and psychology.
You would then collect data on your participants’ exposure to contaminated drinking water, focusing on variables such as the source of said water and the duration of exposure, for both groups. You could then compare the two to determine if there is a relationship between drinking water contamination and the risk of developing a gastrointestinal illness. Example: Healthcare case-control study You are interested in the relationship between the dietary intake of a particular vitamin (e.g., vitamin D) and the risk of developing osteoporosis later in life. Here, the case group would be individuals who have been diagnosed with osteoporosis, while the control group would be individuals without osteoporosis.
You would then collect information on dietary intake of vitamin D for both the cases and controls and compare the two groups to determine if there is a relationship between vitamin D intake and the risk of developing osteoporosis. Example: Psychology case-control study You are studying the relationship between early-childhood stress and the likelihood of later developing post-traumatic stress disorder (PTSD). Here, the case group would be individuals who have been diagnosed with PTSD, while the control group would be individuals without PTSD.
Case-control studies are a solid research method choice, but they come with distinct advantages and disadvantages.
Advantages of case-control studies
- Case-control studies are a great choice if you have any ethical considerations about your participants that could preclude you from using a traditional experimental design .
- Case-control studies are time efficient and fairly inexpensive to conduct because they require fewer subjects than other research methods .
- If there were multiple exposures leading to a single outcome, case-control studies can incorporate that. As such, they truly shine when used to study rare outcomes or outbreaks of a particular disease .
Disadvantages of case-control studies
- Case-control studies, similarly to observational studies, run a high risk of research biases . They are particularly susceptible to observer bias , recall bias , and interviewer bias.
- In the case of very rare exposures of the outcome studied, attempting to conduct a case-control study can be very time consuming and inefficient .
- Case-control studies in general have low internal validity and are not always credible.
Case-control studies by design focus on one singular outcome. This makes them very rigid and not generalizable , as no extrapolation can be made about other outcomes like risk recurrence or future exposure threat. This leads to less satisfying results than other methodological choices.
If you want to know more about statistics , methodology , or research bias , make sure to check out some of our other articles with explanations and examples.
- Student’s t -distribution
- Normal distribution
- Null and Alternative Hypotheses
- Chi square tests
- Confidence interval
- Quartiles & Quantiles
- Cluster sampling
- Stratified sampling
- Data cleansing
- Reproducibility vs Replicability
- Peer review
- Prospective cohort study
Research bias
- Implicit bias
- Cognitive bias
- Placebo effect
- Hawthorne effect
- Hindsight bias
- Affect heuristic
- Social desirability bias
Prevent plagiarism. Run a free check.
A case-control study differs from a cohort study because cohort studies are more longitudinal in nature and do not necessarily require a control group .
While one may be added if the investigator so chooses, members of the cohort are primarily selected because of a shared characteristic among them. In particular, retrospective cohort studies are designed to follow a group of people with a common exposure or risk factor over time and observe their outcomes.
Case-control studies, in contrast, require both a case group and a control group, as suggested by their name, and usually are used to identify risk factors for a disease by comparing cases and controls.
A case-control study differs from a cross-sectional study because case-control studies are naturally retrospective in nature, looking backward in time to identify exposures that may have occurred before the development of the disease.
On the other hand, cross-sectional studies collect data on a population at a single point in time. The goal here is to describe the characteristics of the population, such as their age, gender identity, or health status, and understand the distribution and relationships of these characteristics.
Cases and controls are selected for a case-control study based on their inherent characteristics. Participants already possessing the condition of interest form the “case,” while those without form the “control.”
Keep in mind that by definition the case group is chosen because they already possess the attribute of interest. The point of the control group is to facilitate investigation, e.g., studying whether the case group systematically exhibits that attribute more than the control group does.
The strength of the association between an exposure and a disease in a case-control study can be measured using a few different statistical measures , such as odds ratios (ORs) and relative risk (RR).
No, case-control studies cannot establish causality as a standalone measure.
As observational studies , they can suggest associations between an exposure and a disease, but they cannot prove without a doubt that the exposure causes the disease. In particular, issues arising from timing, research biases like recall bias , and the selection of variables lead to low internal validity and the inability to determine causality.
Sources in this article
We strongly encourage students to use sources in their work. You can cite our article (APA Style) or take a deep dive into the articles below.
George, T. (2023, June 22). What Is a Case-Control Study? | Definition & Examples. Scribbr. Retrieved April 9, 2024, from https://www.scribbr.com/methodology/case-control-study/
Schlesselman, J. J. (1982). Case-Control Studies: Design, Conduct, Analysis (Monographs in Epidemiology and Biostatistics, 2) (Illustrated). Oxford University Press.
Is this article helpful?
Tegan George
Other students also liked, what is an observational study | guide & examples, control groups and treatment groups | uses & examples, cross-sectional study | definition, uses & examples, what is your plagiarism score.
- Alzheimer's & Dementia
- Asthma & Allergies
- Atopic Dermatitis
- Breast Cancer
- Cardiovascular Health
- Environment & Sustainability
- Exercise & Fitness
- Headache & Migraine
- Health Equity
- HIV & AIDS
- Human Biology
- Men's Health
- Mental Health
- Multiple Sclerosis (MS)
- Parkinson's Disease
- Psoriatic Arthritis
- Sexual Health
- Ulcerative Colitis
- Women's Health
- Nutrition & Fitness
- Vitamins & Supplements
- At-Home Testing
- Men’s Health
- Women’s Health
- Latest News
- Medical Myths
- Honest Nutrition
- Through My Eyes
- New Normal Health
- 2023 in medicine
- Why exercise is key to living a long and healthy life
- What do we know about the gut microbiome in IBD?
- My podcast changed me
- Can 'biological race' explain disparities in health?
- Why Parkinson's research is zooming in on the gut
- Health Hubs
- Find a Doctor
- BMI Calculators and Charts
- Blood Pressure Chart: Ranges and Guide
- Breast Cancer: Self-Examination Guide
- Sleep Calculator
- RA Myths vs Facts
- Type 2 Diabetes: Managing Blood Sugar
- Ankylosing Spondylitis Pain: Fact or Fiction
- Our Editorial Process
- Content Integrity
- Conscious Language
- Health Conditions
- Health Products
What is a case-control study in medical research?

A case-control study is a type of medical research investigation often used to help determine the cause of a disease, particularly when investigating a disease outbreak or rare condition.
If public health scientists want a quick and easy way to highlight clues about the cause of a new disease outbreak, they can compare two groups of people: Cases, the term for people who already have the disease, and controls, or people not affected by the disease.
Other terms used to describe case-control studies include epidemiological, retrospective, and observational.
What is a case-control study?

A case-control study is a way of carrying out a medical investigation to confirm or indicate what is likely to have caused a condition.
They are usually retrospective, meaning that the researchers look at past data to test whether a particular outcome can be linked back to a suspected risk factor and prevent further outbreaks.
Prospective case-control studies are less common. These involve enrolling a specific selection of people and following that group while monitoring their health. Cases emerge as people who develop the disease or condition under investigation as the study progresses. Those unaffected by the disease form the control group.
To test for specific causes, the scientists need to create a hypothesis about possible causes of the outbreak or disease. These are known as risk factors.
They compare how often the people in the group of cases had been exposed to the suspected cause against how often members of the control group had been exposed. If more participants in the case group experience the risk factor, this suggests that it is a likely cause of the disease.
Researchers might also uncover likely risk factors not mentioned in their hypothesis by studying the medical and personal histories of the people in each group. A pattern may emerge that links the condition to certain factors.
If a specific risk factor has already been identified for a disease or condition, such as age, sex, smoking, or eating red meat, the researchers can use statistical methods to adjust the study to account for that risk factor, helping them to identify other possible risk factors more easily.
Case-control research is a vital tool used by epidemiologists, or researchers who look into the factors affecting health and illness of populations.
Just one risk factor could be investigated for a particular outcome. A good example of this is to compare the number people with lung cancer who have a history of smoking with the number who do not. This will indicate the link between lung cancer and smoking.
Why is it useful?
There are multiple reasons for the use of case-control studies.
Relatively quick and easy
Case-control studies are usually based on past data, so all of the necessary information is readily available, making them quick to carry out. Scientists can analyze existing data to look at health events that have already happened and risk factors that have already been observed.
A retrospective case-control study does not require scientists to wait and see what happens in a trial over a period of days, weeks, or years.
The fact that the data is already available for collation and analysis means that a case-control study is useful when quick results are desired, perhaps when clues are sought for what is causing a sudden disease outbreak.
A prospective case-control study may also be helpful in this scenario as researchers can collect data on suspected risk factors while they monitor for new cases.
The time-saving advantage offered by case-control studies also means they are more practical than other scientific trial designs if the exposure to a suspected cause occurs a long time before the outcome of a disease.
For example, if you wanted to test the hypothesis that a disease seen in adulthood is linked to factors occurring in young children, a prospective study would take decades to carry out. A case-control study is a far more feasible option.
Does not need large numbers of people
Numerous risk factors can be evaluated in case-control studies since they do not require large numbers of participants to be statistically meaningful. More resources can be dedicated to the analysis of fewer people.
Overcomes ethical challenges
As case-control studies are observational and usually about people who have already experienced a condition, they do not pose the ethical problems seen with some interventional studies.
For example, it would be unethical to deprive a group of children of a potentially lifesaving vaccine to see who developed the associated disease. However, analyzing a group of children with limited access to that vaccine can help determine who is at most risk of developing the disease, as well as helping to guide future vaccination efforts.
Limitations
While a case-control study can help to test a hypothesis about the link between a risk factor and an outcome, it is not as powerful as other types of study in confirming a causal relationship.
Case-control studies are often used to provide early clues and inform further research using more rigorous scientific methods.
The main problem with case-control studies is that they are not as reliable as planned studies that record data in real time, because they look into data from the past.
The main limitations of case-control studies are:
‘Recall bias’
When people answer questions about their previous exposure to certain risk factors their ability to recall may be unreliable. Compared to people not affected by a condition, individuals with a certain disease outcome may be more likely to recall a certain risk factor, even if it did not exist, because of a temptation to make their own subjective links to explain their condition.
This bias may be reduced if data about the risk factors – exposure to certain drugs, for example – had been entered into reliable records at the time. But this may not be possible for lifestyle factors, for example, because they are usually investigated by questionnaire.
An example of recall bias is the difference between asking study participants to recall the weather at the time of the onset of a certain symptom, versus an analysis of scientifically measured weather patterns around the time of a formal diagnosis.
Finding a measurement of exposure to a risk factor in the body is another way of making case-control studies more reliable and less subjective. These are known as biomarkers. For example, researchers may look at results of blood or urine tests for evidence of a specific drug, rather than asking a participant about drug use.
Cause and effect
An association found between a disease and a possible risk does not necessarily mean one factor directly caused the other.
In fact, a retrospective study can never definitively prove that a link represents a definite cause, as it is not an experiment. There are, though, questions that can be used to test the likelihood of a causal relationship, such as the extent of the association or whether there is a ‘dose response’ to increasing exposure to the risk factor.
One way of illustrating the limitations of cause-and-effect is to look at associations found between a cultural factor and a particular health effect. The cultural factor itself, such as a certain type of exercise, may not be causing the outcome if the same cultural group of cases shares another plausible common factor, such as a certain food preference.
Some risk factors are linked to others. Researchers have to take into account overlaps between risk factors, such as leading a sedentary lifestyle, being depressed, and living in poverty.
If researchers conducting a retrospective case-control study find an association between depression and weight gain over time, for example, they cannot say with any certainty that depression is a risk factor for weight gain without bringing in a control group containing people who follow a sedentary lifestyle.
‘Sampling bias’
The cases and controls selected for study may not truly represent the disease under investigation.
An example of this occurs when cases are seen in a teaching hospital, a highly specialized setting compared with most settings in which the disease may occur. The controls, too, may not be typical of the population. People volunteering their data for the study may have a particularly high level of health motivation.
Other limitations
There are other limitations to case-control studies. While they are good for studying rare conditions, as they do not require large groups of participants, they are less useful for examining rare risk factors, which are more clearly indicated by cohort studies.
Finally, case-control studies cannot confirm different levels or types of the disease being investigated. They can look at only one outcome because a case is defined by whether they did or did not have the condition.
Last medically reviewed on May 16, 2018
- Public Health
- Clinical Trials / Drug Trials
- Pharma Industry / Biotech Industry
How we reviewed this article:
- Introduction to study designs – case-control studies. (n.d.) https://www.healthknowledge.org.uk/e-learning/epidemiology/practitioners/introduction-study-design-ccs
- Mann, C. J. (2003). Observational research methods. Research design II: cohort, cross sectional , and case-control studies. Emergency Medicine Journal, 20 , 54-60 http://emj.bmj.com/content/emermed/20/1/54.full.pdf
- Prospective vs. retrospective studies. (n.d.) https://www.statsdirect.com/help/default.htm#basics/prospective.htm
Share this article
Latest news
- High blood pressure during middle age could increase dementia risk
- Is prescribing beta-blockers following a heart attack necessary?
- Early-onset cancer: Faster biological aging may be driving rates in young adults
- Weight-loss drugs like Wegovy may help treat heart failure in diabetes, obesity
- A 'balanced' diet is better than a vegetarian one in supporting brain health
Related Coverage
Systematic reviews and meta-analyses are a reliable type of research. Medical experts base guidelines for the best medical treatments on them.
A randomized controlled trial is one of the best ways of keeping the bias of the researchers out of the data and making sure that a study gives the…
Another year has come and gone, and we are about to step into a new decade. But what have the past 12 months meant for medical research?
Clinical trials are carried out to ensure that medical practices and treatments are safe and effective. People with a health condition may choose to…
The past 12 months have seen discoveries, breakthroughs, and innovations in medical research. MNT take you on a journey through 2017's highlights.
- En español – ExME
- Em português – EME
Case-control and Cohort studies: A brief overview
Posted on 6th December 2017 by Saul Crandon

Introduction
Case-control and cohort studies are observational studies that lie near the middle of the hierarchy of evidence . These types of studies, along with randomised controlled trials, constitute analytical studies, whereas case reports and case series define descriptive studies (1). Although these studies are not ranked as highly as randomised controlled trials, they can provide strong evidence if designed appropriately.
Case-control studies
Case-control studies are retrospective. They clearly define two groups at the start: one with the outcome/disease and one without the outcome/disease. They look back to assess whether there is a statistically significant difference in the rates of exposure to a defined risk factor between the groups. See Figure 1 for a pictorial representation of a case-control study design. This can suggest associations between the risk factor and development of the disease in question, although no definitive causality can be drawn. The main outcome measure in case-control studies is odds ratio (OR) .
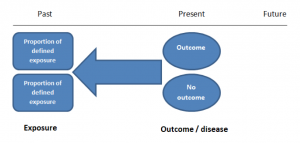
Figure 1. Case-control study design.
Cases should be selected based on objective inclusion and exclusion criteria from a reliable source such as a disease registry. An inherent issue with selecting cases is that a certain proportion of those with the disease would not have a formal diagnosis, may not present for medical care, may be misdiagnosed or may have died before getting a diagnosis. Regardless of how the cases are selected, they should be representative of the broader disease population that you are investigating to ensure generalisability.
Case-control studies should include two groups that are identical EXCEPT for their outcome / disease status.
As such, controls should also be selected carefully. It is possible to match controls to the cases selected on the basis of various factors (e.g. age, sex) to ensure these do not confound the study results. It may even increase statistical power and study precision by choosing up to three or four controls per case (2).
Case-controls can provide fast results and they are cheaper to perform than most other studies. The fact that the analysis is retrospective, allows rare diseases or diseases with long latency periods to be investigated. Furthermore, you can assess multiple exposures to get a better understanding of possible risk factors for the defined outcome / disease.
Nevertheless, as case-controls are retrospective, they are more prone to bias. One of the main examples is recall bias. Often case-control studies require the participants to self-report their exposure to a certain factor. Recall bias is the systematic difference in how the two groups may recall past events e.g. in a study investigating stillbirth, a mother who experienced this may recall the possible contributing factors a lot more vividly than a mother who had a healthy birth.
A summary of the pros and cons of case-control studies are provided in Table 1.
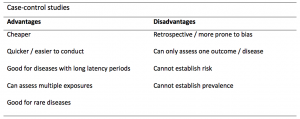
Table 1. Advantages and disadvantages of case-control studies.
Cohort studies
Cohort studies can be retrospective or prospective. Retrospective cohort studies are NOT the same as case-control studies.
In retrospective cohort studies, the exposure and outcomes have already happened. They are usually conducted on data that already exists (from prospective studies) and the exposures are defined before looking at the existing outcome data to see whether exposure to a risk factor is associated with a statistically significant difference in the outcome development rate.
Prospective cohort studies are more common. People are recruited into cohort studies regardless of their exposure or outcome status. This is one of their important strengths. People are often recruited because of their geographical area or occupation, for example, and researchers can then measure and analyse a range of exposures and outcomes.
The study then follows these participants for a defined period to assess the proportion that develop the outcome/disease of interest. See Figure 2 for a pictorial representation of a cohort study design. Therefore, cohort studies are good for assessing prognosis, risk factors and harm. The outcome measure in cohort studies is usually a risk ratio / relative risk (RR).
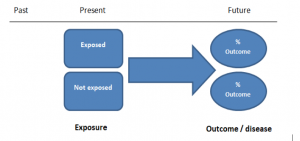
Figure 2. Cohort study design.
Cohort studies should include two groups that are identical EXCEPT for their exposure status.
As a result, both exposed and unexposed groups should be recruited from the same source population. Another important consideration is attrition. If a significant number of participants are not followed up (lost, death, dropped out) then this may impact the validity of the study. Not only does it decrease the study’s power, but there may be attrition bias – a significant difference between the groups of those that did not complete the study.
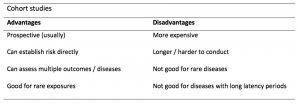
Cohort studies can assess a range of outcomes allowing an exposure to be rigorously assessed for its impact in developing disease. Additionally, they are good for rare exposures, e.g. contact with a chemical radiation blast.
Whilst cohort studies are useful, they can be expensive and time-consuming, especially if a long follow-up period is chosen or the disease itself is rare or has a long latency.
A summary of the pros and cons of cohort studies are provided in Table 2.
The Strengthening of Reporting of Observational Studies in Epidemiology Statement (STROBE)
STROBE provides a checklist of important steps for conducting these types of studies, as well as acting as best-practice reporting guidelines (3). Both case-control and cohort studies are observational, with varying advantages and disadvantages. However, the most important factor to the quality of evidence these studies provide, is their methodological quality.
- Song, J. and Chung, K. Observational Studies: Cohort and Case-Control Studies . Plastic and Reconstructive Surgery.  2010 Dec;126(6):2234-2242.
- Ury HK. Efficiency of case-control studies with multiple controls per case: Continuous or dichotomous data . Biometrics . 1975 Sep;31(3):643–649.
- von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies.  Lancet 2007 Oct;370(9596):1453-14577. PMID: 18064739.
Saul Crandon
Leave a reply cancel reply.
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
No Comments on Case-control and Cohort studies: A brief overview
Very well presented, excellent clarifications. Has put me right back into class, literally!
Very clear and informative! Thank you.
very informative article.
Thank you for the easy to understand blog in cohort studies. I want to follow a group of people with and without a disease to see what health outcomes occurs to them in future such as hospitalisations, diagnoses, procedures etc, as I have many health outcomes to consider, my questions is how to make sure these outcomes has not occurred before the “exposure disease”. As, in cohort studies we are looking at incidence (new) cases, so if an outcome have occurred before the exposure, I can leave them out of the analysis. But because I am not looking at a single outcome which can be checked easily and if happened before exposure can be left out. I have EHR data, so all the exposure and outcome have occurred. my aim is to check the rates of different health outcomes between the exposed)dementia) and unexposed(non-dementia) individuals.
Very helpful information
Thanks for making this subject student friendly and easier to understand. A great help.
Thanks a lot. It really helped me to understand the topic. I am taking epidemiology class this winter, and your paper really saved me.
Happy new year.
Wow its amazing n simple way of briefing ,which i was enjoyed to learn this.its very easy n quick to pick ideas .. Thanks n stay connected
Saul you absolute melt! Really good work man
am a student of public health. This information is simple and well presented to the point. Thank you so much.
very helpful information provided here
really thanks for wonderful information because i doing my bachelor degree research by survival model
Quite informative thank you so much for the info please continue posting. An mph student with Africa university Zimbabwe.
Thank you this was so helpful amazing
Apreciated the information provided above.
So clear and perfect. The language is simple and superb.I am recommending this to all budding epidemiology students. Thanks a lot.
Great to hear, thank you AJ!
I have recently completed an investigational study where evidence of phlebitis was determined in a control cohort by data mining from electronic medical records. We then introduced an intervention in an attempt to reduce incidence of phlebitis in a second cohort. Again, results were determined by data mining. This was an expedited study, so there subjects were enrolled in a specific cohort based on date(s) of the drug infused. How do I define this study? Thanks so much.
thanks for the information and knowledge about observational studies. am a masters student in public health/epidemilogy of the faculty of medicines and pharmaceutical sciences , University of Dschang. this information is very explicit and straight to the point
Very much helpful
Subscribe to our newsletter
You will receive our monthly newsletter and free access to Trip Premium.
Related Articles

Cluster Randomized Trials: Concepts
This blog summarizes the concepts of cluster randomization, and the logistical and statistical considerations while designing a cluster randomized controlled trial.

Expertise-based Randomized Controlled Trials
This blog summarizes the concepts of Expertise-based randomized controlled trials with a focus on the advantages and challenges associated with this type of study.

An introduction to different types of study design
Conducting successful research requires choosing the appropriate study design. This article describes the most common types of designs conducted by researchers.
Have a language expert improve your writing
Run a free plagiarism check in 10 minutes, automatically generate references for free.
- Knowledge Base
- Methodology
- What Is a Case-Control Study? | Definition & Examples
What Is a Case-Control Study? | Definition & Examples
Published on 4 February 2023 by Tegan George .
A case-control study is an experimental design that compares a group of participants possessing a condition of interest to a very similar group lacking that condition. Here, the participants possessing the attribute of study, such as a disease, are called the ‘case’, and those without it are the ‘control’.
It’s important to remember that the case group is chosen because they already possess the attribute of interest. The point of the control group is to facilitate investigation, e.g., studying whether the case group systematically exhibits that attribute more than the control group does.
Table of contents
When to use a case-control study, examples of case-control studies, advantages and disadvantages of case-control studies, frequently asked questions.
Case-control studies are a type of observational study often used in fields like medical research, environmental health, or epidemiology. While most observational studies are qualitative in nature, case-control studies can also be quantitative , and they often are in healthcare settings. Case-control studies can be used for both exploratory and explanatory research , and they are a good choice for studying research topics like disease exposure and health outcomes.
A case-control study may be a good fit for your research if it meets the following criteria.
- Data on exposure (e.g., to a chemical or a pesticide) are difficult to obtain or expensive.
- The disease associated with the exposure you’re studying has a long incubation period or is rare or under-studied (e.g., AIDS in the early 1980s).
- The population you are studying is difficult to contact for follow-up questions (e.g., asylum seekers).
Retrospective cohort studies use existing secondary research data, such as medical records or databases, to identify a group of people with a common exposure or risk factor and to observe their outcomes over time. Case-control studies conduct primary research , comparing a group of participants possessing a condition of interest to a very similar group lacking that condition in real time.
Prevent plagiarism, run a free check.
Case-control studies are common in fields like epidemiology, healthcare, and psychology.
You would then collect data on your participants’ exposure to contaminated drinking water, focusing on variables such as the source of said water and the duration of exposure, for both groups. You could then compare the two to determine if there is a relationship between drinking water contamination and the risk of developing a gastrointestinal illness. Example: Healthcare case-control study You are interested in the relationship between the dietary intake of a particular vitamin (e.g., vitamin D) and the risk of developing osteoporosis later in life. Here, the case group would be individuals who have been diagnosed with osteoporosis, while the control group would be individuals without osteoporosis.
You would then collect information on dietary intake of vitamin D for both the cases and controls and compare the two groups to determine if there is a relationship between vitamin D intake and the risk of developing osteoporosis. Example: Psychology case-control study You are studying the relationship between early-childhood stress and the likelihood of later developing post-traumatic stress disorder (PTSD). Here, the case group would be individuals who have been diagnosed with PTSD, while the control group would be individuals without PTSD.
Case-control studies are a solid research method choice, but they come with distinct advantages and disadvantages.
Advantages of case-control studies
- Case-control studies are a great choice if you have any ethical considerations about your participants that could preclude you from using a traditional experimental design .
- Case-control studies are time efficient and fairly inexpensive to conduct because they require fewer subjects than other research methods .
- If there were multiple exposures leading to a single outcome, case-control studies can incorporate that. As such, they truly shine when used to study rare outcomes or outbreaks of a particular disease .
Disadvantages of case-control studies
- Case-control studies, similarly to observational studies, run a high risk of research biases . They are particularly susceptible to observer bias , recall bias , and interviewer bias.
- In the case of very rare exposures of the outcome studied, attempting to conduct a case-control study can be very time consuming and inefficient .
- Case-control studies in general have low internal validity and are not always credible.
Case-control studies by design focus on one singular outcome. This makes them very rigid and not generalisable , as no extrapolation can be made about other outcomes like risk recurrence or future exposure threat. This leads to less satisfying results than other methodological choices.
A case-control study differs from a cohort study because cohort studies are more longitudinal in nature and do not necessarily require a control group .
While one may be added if the investigator so chooses, members of the cohort are primarily selected because of a shared characteristic among them. In particular, retrospective cohort studies are designed to follow a group of people with a common exposure or risk factor over time and observe their outcomes.
Case-control studies, in contrast, require both a case group and a control group, as suggested by their name, and usually are used to identify risk factors for a disease by comparing cases and controls.
A case-control study differs from a cross-sectional study because case-control studies are naturally retrospective in nature, looking backward in time to identify exposures that may have occurred before the development of the disease.
On the other hand, cross-sectional studies collect data on a population at a single point in time. The goal here is to describe the characteristics of the population, such as their age, gender identity, or health status, and understand the distribution and relationships of these characteristics.
Cases and controls are selected for a case-control study based on their inherent characteristics. Participants already possessing the condition of interest form the “case,” while those without form the “control.”
Keep in mind that by definition the case group is chosen because they already possess the attribute of interest. The point of the control group is to facilitate investigation, e.g., studying whether the case group systematically exhibits that attribute more than the control group does.
The strength of the association between an exposure and a disease in a case-control study can be measured using a few different statistical measures , such as odds ratios (ORs) and relative risk (RR).
No, case-control studies cannot establish causality as a standalone measure.
As observational studies , they can suggest associations between an exposure and a disease, but they cannot prove without a doubt that the exposure causes the disease. In particular, issues arising from timing, research biases like recall bias , and the selection of variables lead to low internal validity and the inability to determine causality.
Sources for this article
We strongly encourage students to use sources in their work. You can cite our article (APA Style) or take a deep dive into the articles below.
George, T. (2023, February 04). What Is a Case-Control Study? | Definition & Examples. Scribbr. Retrieved 9 April 2024, from https://www.scribbr.co.uk/research-methods/case-control-studies/
Schlesselman, J. J. (1982). Case-Control Studies: Design, Conduct, Analysis (Monographs in Epidemiology and Biostatistics, 2) (Illustrated). Oxford University Press.
Is this article helpful?
Tegan George
Other students also liked, what is an observational study | guide & examples, control groups and treatment groups | uses & examples, cross-sectional study | definitions, uses & examples.
- Introduction
- Conclusions
- Article Information
The shaded area indicates the upper and lower limits of the 95% CIs.
DOAC indicates direct oral anticoagulant; IRR, incidence rate ratio; and VKA, vitamin K antagonist.
eMethods 1. Interaction
eMethods 2. Time-Conditional Propensity Score-Matched Analysis
eFigure. Flowchart of Patient Selection in Study Cohort and Case-Control Selection
eTable 1. ICD-10 Codes Used to Define Major Bleeding
eTable 2. Crude and Adjusted IRRs of Major Bleeding Associated With the Continuous Duration of Concomitant Use of SSRIs With OACs, Compared With OAC Use Alone
eTable 3. Crude and Adjusted IRRs of Major Bleeding Associated With Concomitant Use of SSRIs With OACs, Stratified by Age, Sex, History of Bleeding, and History of Chronic Kidney Disease
eTable 4. Crude and Adjusted IRRs of Major Bleeding Associated With Concomitant Use of Strong and Moderate SSRIs With OACs
eTable 5. Crude and Adjusted IRRs of Any Bleeding Associated With Concomitant Use of SSRIs With OACs, Compared With OAC Use Alone
eTable 6. Assessment of Additive and Multiplicative Interaction Between SSRIs and OACs, With Respect to Major Bleeding
eTable 7. Crude and Adjusted IRRs of Major Bleeding Associated With Concomitant Use of SSRIs With OACs, Varying the Exposure Assessment Window
eTable 8. Crude and Adjusted IRRs of Major Bleeding Associated With Concomitant Use of SSRIs With OACs, With Covariates Measured Prior to Cohort Entry
eTable 9. Crude and Adjusted IRRs of Major Bleeding Associated With Concomitant Use of SSRIs With OACs, After Multiple Imputation of Missing BMI and Smoking Values
eTable 10. Crude and Adjusted IRRs of Major Bleeding Associated With Concomitant Use of SSRIs With OACs, Compared With OAC Use Alone, by Type of OAC and Excluding Patients With Valvular AF
eTable 11. Adjusted HRs of Major Bleeding Associated With Concomitant Use of SSRIs With OACs Compared With OAC Use Alone, in a Time-Conditional Propensity Score-Matched Analysis
eTable 12. Crude and Adjusted IRRs of Major Bleeding Associated With Concomitant Use of SSRIs With OACs, With Adjustment for Additional Comedications Interacting With OACs
eReferences.
Data Sharing Statement
See More About
Sign up for emails based on your interests, select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
Get the latest research based on your areas of interest.
Others also liked.
- Download PDF
- X Facebook More LinkedIn
Rahman AA , Platt RW , Beradid S , Boivin J , Rej S , Renoux C. Concomitant Use of Selective Serotonin Reuptake Inhibitors With Oral Anticoagulants and Risk of Major Bleeding. JAMA Netw Open. 2024;7(3):e243208. doi:10.1001/jamanetworkopen.2024.3208
Manage citations:
© 2024
- Permissions
Concomitant Use of Selective Serotonin Reuptake Inhibitors With Oral Anticoagulants and Risk of Major Bleeding
- 1 Department of Epidemiology, Biostatistics and Occupational Health, McGill University, Montreal, Quebec, Canada
- 2 Centre for Clinical Epidemiology, Lady Davis Institute for Medical Research, Jewish General Hospital, Montreal, Quebec, Canada
- 3 Department of Pediatrics, McGill University, Montreal, Quebec, Canada
- 4 Department of Psychiatry, McGill University, Montreal, Quebec, Canada
- 5 Department of Neurology and Neurosurgery, McGill University, Montreal, Quebec, Canada
- 6 Department of Medicine, McGill University, Montreal, Quebec, Canada
Question Is there an association between concomitant use of selective serotonin reuptake inhibitors (SSRIs) and oral anticoagulants (OACs) and the risk of major bleeding among patients with atrial fibrillation compared with OAC use alone?
Findings In this nested case-control study comprising 42 190 cases with major bleeding matched to 1 156 641 controls, concomitant SSRI and OAC use was associated with a 33% increased risk of major bleeding compared with OAC use alone; this risk was highest in the first few months of concomitant use and was substantially lower after 6 months.
Meaning This study suggests that concomitant use of SSRIs and OACs may be a risk factor for bleeding and should be closely monitored, particularly within the initial months of treatment.
Importance Selective serotonin reuptake inhibitors (SSRIs) are commonly prescribed antidepressants associated with a small increased risk of major bleeding. However, the risk of bleeding associated with the concomitant use of SSRIs and oral anticoagulants (OACs) has not been well characterized.
Objectives To assess whether concomitant use of SSRIs with OACs is associated with an increased risk of major bleeding compared with OAC use alone, describe how the risk varies with duration of use, and identify key clinical characteristics modifying this risk.
Design, Setting, and Participants A population-based, nested case-control study was conducted among patients with atrial fibrillation initiating OACs between January 2, 1998, and March 29, 2021. Patients were from approximately 2000 general practices in the UK contributing to the Clinical Practice Research Datalink. With the use of risk-set sampling, for each case of major bleeding during follow-up, up to 30 controls were selected from risk sets defined by the case and matched on age, sex, cohort entry date, and follow-up duration.
Exposures Concomitant use of SSRIs and OACs (direct OACs and vitamin K antagonists [VKAs]) compared with OAC use alone.
Main Outcomes and Measures The main outcome was incidence rate ratios (IRRs) of hospitalization for bleeding or death due to bleeding.
Results There were 42 190 patients with major bleeding (mean [SD] age, 74.2 [9.3] years; 59.8% men) matched to 1 156 641 controls (mean [SD] age, 74.2 [9.3] years; 59.8% men). Concomitant use of SSRIs and OACs was associated with an increased risk of major bleeding compared with OACs alone (IRR, 1.33; 95% CI, 1.24-1.42). The risk peaked during the initial months of treatment (first 30 days of use: IRR, 1.74; 95% CI, 1.37-2.22) and persisted for up to 6 months. The risk did not vary with age, sex, history of bleeding, chronic kidney disease, and potency of SSRIs. An association was present both with concomitant use of SSRIs and direct OACs compared with direct OAC use alone (IRR, 1.25; 95% CI, 1.12-1.40) and concomitant use of SSRIs and VKAs compared with VKA use alone (IRR, 1.36; 95% CI, 1.25-1.47).
Conclusions and Relevance This study suggests that among patients with atrial fibrillation, concomitant use of SSRIs and OACs was associated with an increased risk of major bleeding compared with OAC use alone, requiring close monitoring and management of risk factors for bleeding, particularly in the first few months of use.
Antidepressant medications are among the most frequently prescribed class of drugs worldwide, with up to 19% of individuals aged 60 years or older in the US reporting use of an antidepressant over the past 30 days. 1 Selective serotonin reuptake inhibitors (SSRIs) are the most widely used antidepressant medications and are often recommended over other classes of antidepressants for the treatment of major depressive disorder due to their comparable efficacy and favorable safety profile. 2 , 3 However, SSRIs have been shown to increase the risk of major bleeding, 4 - 14 possibly owing to their inhibition of platelet activation during hemostasis. 2 Although the absolute risk remains low for most individuals who use SSRIs, 11 , 12 , 15 coprescription with drugs such as oral anticoagulants (OACs) may be consequential. Concomitant use of SSRIs and OACs is common given the prevalence of mental health disorders. 16
Some observational studies have assessed the association between concomitant use of SSRIs and OACs and the risk of major bleeding. However, some had notable limitations, including exposure misclassification, 17 possible informative censoring, 18 , 19 residual confounding, 19 - 21 and limited statistical power. 20 , 22 - 24 Gaps in evidence that may inform the coprescription of SSRIs and OACs include whether the risk varies with demographic or clinical characteristics or between direct OACs (DOACs) and vitamin K antagonists (VKAs). In addition, data on the risk of specific types of major bleeding are limited. 25
To address these knowledge gaps, we conducted a population-based, nested case-control study to assess whether the concomitant use of SSRIs and OACs was associated with the risk of major bleeding compared with OAC use alone among patients with atrial fibrillation (AF). We also assessed whether the risk varied by duration of use, relevant demographic and other risk factors, potency of SSRIs, and OAC type.
In this population-based, nested case-control study, we used the UK Clinical Practice Research Datalink (CPRD GOLD and Aurum databases), a large primary care database of electronic medical records that contains demographic and lifestyle information, medical diagnoses, prescriptions, and referrals for more than 60 million patients from more than 2000 general practices. 26 , 27 These data are representative of the UK population in terms of age, sex, and race and ethnicity. 26 , 27 Drug prescriptions issued by the general practitioner are automatically recorded at the time of prescription. 26 , 27 Quality control audits of the CPRD are regularly conducted to ensure the accuracy and completeness of data. 26 , 27 The CPRD was linked with the Hospital Episodes Statistics repository, which contains details of inpatient and day case admissions, 28 and the Office for National Statistics database, which contains electronic death certificates. 29 The study protocol was approved by the CPRD Research Data Governance (No. 22_001906) and the Research Ethics Board of the Jewish General Hospital in Montreal, Canada, which also waived the need for patient informed consent as the data were deidentified. The study followed the Strengthening the Reporting of Observational Studies in Epidemiology ( STROBE ) reporting guideline. 30
We conducted a population-based study with a nested case-control approach to analysis because of its computational efficiency compared with a full-cohort analysis given the time-varying nature of both medications of interest, the size of the cohort, and the long duration of follow-up. 31 We first identified all patients aged 18 years or older with an incident diagnosis of AF between January 2, 1998, and March 29, 2021, and at least 1 year of registration with the practice before AF diagnosis. From this base cohort, we selected those with a prescription for an OAC (apixaban, dabigatran, edoxaban, rivaroxaban, or warfarin) after AF diagnosis, with the date of first prescription defined as study cohort entry. We excluded patients who received OACs any time before cohort entry or SSRIs 6 months prior to cohort entry. We also excluded patients with hyperthyroidism in the year prior to cohort entry because AF in association with hyperthyroidism rarely requires long-term oral anticoagulation. Patients meeting these criteria were followed up until a first major bleeding event, death, end of registration with the practice, or end of the study period (March 29, 2021), whichever occurred first.
We identified cases as patients with a first recorded diagnosis of major bleeding during follow-up, defined as hospitalization with a primary diagnosis of major bleeding or death with bleeding as the primary cause, using relevant International Statistical Classification of Diseases and Related Health Problems, Tenth Revision ( ICD-10 ) codes (eTable 1 in Supplement 1 ); elective hospitalizations were not considered. ICD-10 codes for bleeding have shown good positive predictive values between 81% and 95%. 32 - 34 The index date was the date of hospital admission. For each case, we randomly selected up to 30 controls among the cohort members from the risk sets defined by the case. Each risk set, at each case’s index date, included all individuals who did not experience major bleeding and thus were still at risk up to that point in follow-up time, matched on age, sex, calendar date of cohort entry (±6 months), and duration of follow-up. Thus, as per the risk-set sampling approach, cases were eligible for selection as controls prior to becoming a case, and patients may have been selected as controls for multiple cases. 35 , 36 The index date for controls was the date resulting in the same duration of follow-up for cases and controls.
We identified prescriptions of SSRIs (citalopram, escitalopram, fluoxetine, fluvoxamine, paroxetine, or sertraline) and OACs for all cases and their matched controls between cohort entry and the index date. Exposure was defined in 4 mutually exclusive categories: concomitant use of SSRIs and OACs, OAC use alone, nonuse, and other use. We considered patients as concomitant users of SSRIs and OACs if the duration of their last prescription for both medications covered or ended 30 days before the index date. Similarly, we considered patients as users of OACs alone if their last prescription for an OAC covered or ended 30 days before the index date, without a prescription for an SSRI in this period—this was the reference category. Users of SSRIs alone, non-SSRI antidepressants alone, or non-SSRI antidepressants concomitantly with SSRIs and/or OACs were classified separately (other use). Finally, nonusers were those not exposed to any medications of interest on or 30 days before the index date.
We adjusted all models for the following comorbidities based on substantive knowledge, measured at or earlier than 365 days (1 year) before the index date: smoking, alcohol abuse, body mass index (BMI; calculated as weight in kilograms divided by height in meters squared) (<25, 25-29.9, or ≥30.0), depression, hypertension, diabetes, stroke or transient ischemic attack, coronary artery disease, congestive heart failure, peripheral arterial disease, disorders of hemostasis, cancer (other than nonmelanoma skin cancer), liver disease, chronic kidney disease, chronic obstructive pulmonary disease, anemia, and venous thromboembolism. We also included history of bleeding at any time before cohort entry and the time between incident AF diagnosis and first OAC prescription. Diabetes and hypertension were defined using diagnostic codes or relevant medications. All models were also adjusted for use of the following drugs measured between 365 days (1 year) and 730 days (2 years) prior to the index date: angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, β-blockers, calcium channel blockers, thiazide diuretics, other diuretics, antiplatelets, lipid-lowering drugs (including statins), antipsychotics, non-SSRI antidepressants, nonsteroidal anti-inflammatory drugs, proton pump inhibitors, and H 2 receptor blockers. We considered the number of hospitalizations in the year before cohort entry as a surrogate marker for overall health. Finally, we adjusted for socioeconomic status using the Index of Multiple Deprivation, categorized in deciles. 37
We used conditional logistic regression to compute odds ratios of major bleeding associated with concomitant use of SSRIs and OACs compared with OAC use alone, adjusting for the covariates listed. In a nested case-control approach, odds ratios are unbiased estimators of incidence rate ratios (IRRs) with very limited loss in precision. 36 In secondary analyses, we assessed whether the risk of major bleeding varied according to age, sex, chronic kidney disease, history of bleeding, type of OAC (DOACs or VKAs), and potency of SSRIs (strong or moderate serotonin reuptake inhibitors based on the dissociation constant). 38 Next, among patients continuously exposed to OACs and concomitantly exposed to SSRIs and OACs at the index date, we investigated whether the risk of major bleeding varied with the duration of continuous concomitant use of SSRIs in 3 prespecified categories (≤30 days, 31-180 days, or >180 days) compared with OAC use alone. These categories were selected because SSRIs were reported to exert antiplatelet action as early as 2 to 3 weeks after initiation. 39 , 40 We defined continuous exposure to SSRIs and OACs separately, allowing a 30-day grace period between consecutive prescriptions where patients were still considered exposed. Patients were then considered concomitant users on any given day if exposed to both drugs on that day. In addition, we used a restricted cubic spline with 5 interior knots to produce a smooth curve of the IRR as a function of continuous duration of use. We also estimated the risk in specific anatomical locations, including gastrointestinal bleeding, intracranial hemorrhage, and other major bleeding. We assessed the risk of any bleeding associated with concomitant use of SSRIs and OACs. For this analysis, we repeated the selection of cases and controls already described, with cases defined using relevant diagnostic codes in primary electronic medical records. Finally, we assessed whether an interaction was present between SSRIs and OACs with respect to the risk of major bleeding on both the additive and multiplicative scales (eMethods 1 in Supplement 1 ). In other words, we assessed whether the joint association of the 2 exposures departed from the sum or product of their individual associations with the risk of bleeding, although an additive interaction has been described as most indicative of biological or mechanistic interaction. 41 , 42
We performed 4 sensitivity analyses to assess the robustness of the results. First, to explore potential exposure misclassification, we considered only prescriptions that covered the index date and, next, those that covered or ended within 15 days before the index date. Second, to account for the potential adjustment for covariates affected by exposure, all covariates were measured at or prior to cohort entry. Third, we implemented multiple imputation by chained equations for missing values of BMI and smoking, combining results from 5 imputed datasets. 43 Fourth, we repeated the analysis by type of OAC, excluding patients with a history of valvular surgery or rheumatic valvular disease before cohort entry because DOACs are not indicated for patients with valvular AF. 44 - 46
We conducted a supplementary time-conditional propensity score–matched analysis to further explore the potential for residual confounding. 47 , 48 In brief, among the base cohort of patients with incident AF initiating OACs, we matched each patient initiating SSRIs to a patient using OACs alone up to that point in time with the same age (±1 year), sex, calendar date of OAC initiation (±1 year), and time-conditional propensity score (eMethods 2 in Supplement 1 ). Finally, we conducted a post hoc analysis, repeating the primary analysis with additional adjustment for the following comedications reported to interact with OACs, measured between 1 and 2 years before the index date: clarithromycin, erythromycin, penicillin, azole antifungals, quinidine, amiodarone, dronedarone, propafenone, allopurinol, oral corticosteroids, tamoxifen, valproic acid, cyclosporin, tacrolimus, disulfiram, methylphenidate, and sulfamethoxazole. All analyses were performed with a 2-sided hypothesis test, and P < .05 was considered statistically significant, without adjustment for multiple comparisons, using SAS, version 9.4 (SAS Institute Inc).
After applying all eligibility criteria, the cohort included 331 305 patients (mean [SD] age, 73.7 [10.8] years; 57.1% men) with incident AF initiating OACs (eFigure in Supplement 1 ). During a mean (SD) follow-up of 4.6 (4.0) years, 42 391 patients were hospitalized with major bleeding, yielding an incidence rate of 27.9 per 1000 person-years (95% CI, 27.7-28.2 per 1000 person-years). Among those, 42 190 cases (mean [SD] age, 74.2 [9.3] years; 59.8% men) were matched to 1 156 641 controls (mean [SD] age, 74.2 [9.3] years; 59.8% men). As anticipated, risk factors for major bleeding were more prevalent among cases than controls ( Table 1 ).
Concomitant use of SSRIs and OACs was associated with an increased risk of major bleeding compared with OAC use alone (IRR, 1.33; 95% CI, 1.24-1.42) ( Table 2 ). The risk was the highest during the first 30 days of continuous use (IRR, 1.74; 95% CI, 1.37-2.22), and decreased thereafter (eTable 2 in Supplement 1 ). This trend was also observed when modeling the IRR flexibly as a spline of the duration of continuous use ( Figure 1 ). The risk did not vary according to age, sex, history of major bleeding, chronic kidney disease ( Figure 2 ; eTable 3 in Supplement 1 ), or potency of SSRIs (eTable 4 in Supplement 1 ). The risk of major bleeding was associated with concomitant use of SSRIs and DOACs compared with DOACs alone (IRR, 1.25; 95% CI, 1.12-1.40) and with concomitant use of SSRIs and VKAs compared with VKAs alone (IRR, 1.36; 95% CI, 1.25-1.47) ( Table 3 ). With respect to types of major bleeding, the association was present for intracranial hemorrhage, gastrointestinal bleeding, and other major bleeding ( Table 2 ). Last, concomitant use of SSRIs and OACs was also associated with the risk of any bleeding (IRR, 1.22; 95% CI, 1.16-1.28) compared with OAC use alone (eTable 5 in Supplement 1 ).
In the assessment of interaction, a small superadditive interaction may have been present, although the estimate was not statistically significant (relative excess risk due to interaction [RERI], 0.10; 95% CI, −0.07 to 0.27) (eTable 6 in Supplement 1 ). Based on the estimated RERI, the interaction may be associated with approximately 5% of all major bleeding. In addition, there was limited evidence of a multiplicative interaction. Results from sensitivity analyses were consistent with those of the primary analysis (eTables 7-10 in Supplement 1 ). Finally, the association remained in the time-conditional propensity score–matched analysis, although slightly attenuated (adjusted hazard ratio, 1.23; 95% CI, 1.08-1.40) (eTable 11 in Supplement 1 ), and was consistent in the post hoc analysis (eTable 12 in Supplement 1 ).
In this population-based, nested case-control study, the concomitant use of SSRIs and OACs was associated with a 33% increased risk of major bleeding. The association was the strongest for the first few months of concomitant use. The overall risk remained consistent regardless of age, sex, potency of SSRIs, history of major bleeding, or chronic kidney disease as well as type of OAC. Concomitant use was individually associated with gastrointestinal bleeding, intracranial hemorrhage, and other major bleeding. Interaction between SSRIs and OACs, if any, was limited.
In light of these findings, the risk of major bleeding may be a pertinent safety consideration for patients using SSRIs and OACs concomitantly. This finding has been echoed in the summary of product characteristics for different OACs, which describes SSRIs as interacting drugs given that they independently increase the risk of bleeding. Although clinical guidelines for the management of major depressive disorder have acknowledged the risk of bleeding associated with SSRIs, the potential for interaction with OACs was either not discussed or based on very limited evidence. 49 , 50 Likewise, guidelines from Canadian, US, and European cardiology associations for the management of AF suggest consideration of drug-drug interactions when prescribing OACs, 44 - 46 with nonsteroidal anti-inflammatory drugs being the only class of drugs cited. 45 Although the European Heart Rhythm Association lists SSRIs as drugs with pharmacodynamic interactions with DOACs, no evidence was cited. 51
The risk of major bleeding associated with the concomitant use of SSRIs and OACs has been assessed in previous observational studies, although results were inconsistent. 7 , 17 - 20 , 22 - 24 , 52 Limitations of previous studies included residual confounding, 19 , 20 , 22 , 24 varying exposure definitions, limited statistical power, 20 , 22 - 24 and the assessment of the concomitant use of SSRIs and OACs being a secondary objective. 7 , 18 In line with our results, a systematic review and meta-analysis of 8 observational studies suggested an increased risk of major bleeding associated with the concomitant use of SSRIs and OACs (hazard ratio, 1.35; 95% CI, 1.14-1.58) compared with OAC use alone. 25 However, several knowledge gaps were identified, including the risk of major bleeding in important patient subgroups. The present study confirmed that compared with OACs alone, concomitant use of SSRIs and OACs increased the risk of major bleeding among patients 60 years of age or older and among both sexes. One study previously assessed this association in these patient subgroups; however, statistical power was limited. 22 Furthermore, we showed that the association remained similar irrespective of patients’ history of major bleeding or chronic kidney disease, both important factors in the HAS-BLED (Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile International Normalized Ratio, Elderly [>65 Years], Drugs/Alcohol Concomitantly) score for major bleeding risk. 53 Our findings also suggested that the concomitant use of SSRIs with both DOACs and VKAs was associated with an increased risk of major bleeding, with a possible lower risk with DOACs, although 95% CIs overlapped. A cohort study of patients from the ROCKET-AF (Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared With Vitamin K Antagonism for Prevention of Embolism and Stroke Trial in Atrial Fibrillation) trial suggested that the concomitant use of SSRIs and warfarin may increase the risk of major bleeding compared with rivaroxaban; however, the results were limited by high uncertainty and potential for selection bias and confounding. 20 In another nested case-control study of nursing home residents, similar increases in risk were associated with the concomitant use of SSRIs and DOACs and of SSRIs and VKAs; however, statistical power was low. 23
The increased risk of major bleeding with the concomitant use of SSRIs and OACs may occur through multiple mechanisms of action. During hemostasis, serotonin is released by platelets to enhance platelet activation and aggregation and prime them to interact with coagulation factors. 54 Selective serotonin reuptake inhibitors block the serotonin reuptake transporter on platelet membranes and reduce serotonin content within platelets by up to 80% to 90%, 39 , 40 decreasing the potency of hemostasis over time. In addition, some SSRIs, such as fluoxetine and fluvoxamine, inhibit the 1A2 and 2C9 isozymes of the hepatic cytochrome P450 enzyme, which play a key role in the metabolism of warfarin. 55 Nonetheless, the interaction analysis suggests that the joint association of SSRIs and OACs is mainly owing to their individual risks of major bleeding; hence, any additional risk posed by pharmacokinetic interaction is likely minimal.
Although the increased risk of major bleeding does not suggest withholding treatment with either SSRIs or OACs, measures can be taken to mitigate this risk. Studies suggest that DOACs have lower potential for pharmacokinetic interactions with SSRIs than VKAs, and guidelines also recommend them over VKAs for the management of nonvalvular AF. 44 - 46 , 55 , 56 Taken together with the findings in this study, DOACs may also be preferred for patients concomitantly using SSRIs. On the other hand, the risk of major bleeding was similar between SSRIs with more potent inhibition and SSRIs with less potent serotonin inhibition; thus; changing the SSRI may not be associated with bleeding risk. Finally, coprescription of proton pump inhibitors has also been suggested to prevent gastrointestinal bleeding. 51 , 57 Overall, risk factors for bleeding should be monitored and managed to improve the safety of the concomitant use of SSRIs and OACs. 51 Close monitoring is particularly essential within the first few months of concomitant use.
This study has notable strengths. First, the selection of a large study population from routine care settings enhanced generalizability and provided sufficient statistical power to generate precise estimates in primary and secondary analyses. Second, selection bias was unlikely because we analyzed a well-defined cohort and used a nested case-control approach. Third, the assessment of additive and multiplicative interactions provided evidence suggesting that any biological interaction between use of SSRIs and OACs and the risk of major bleeding may only be marginally synergistic. 42
This study also has some limitations. Residual confounding may affect the results given the observational nature of the study. The baseline risk for major bleeding may differ between patients concomitantly using SSRIs and those who were not. To mitigate potential bias, we adjusted for several potential confounders, including some lifestyle risk factors (such as BMI, smoking, and alcohol abuse). Furthermore, the results remained consistent in the time-conditional propensity score–matched analysis and in a post hoc analysis adjusted for additional comedications. Another consideration is that prescriptions recorded in the CPRD are those issued by general practitioners; hence, misclassification of exposure is possible if patients do not follow the treatment regimen. Prescriptions also do not include those issued by specialists, although AF as well as mild and moderate depression are managed mainly by general practitioners in the UK. 58 , 59 To explore the potential for misclassification, we varied the exposure assessment window in sensitivity analyses, which produced results consistent with the main results. Finally, outcome misclassification through inaccurate recording of bleeding in the Hospital Episodes Statistics repository may occur. In addition, the physician’s judgment may be influenced by knowledge of patient treatment. To mitigate bias, we considered only primary diagnoses and did not include elective hospitalizations.
In this large population-based, nested case-control study of patients with AF, the concomitant use of SSRIs and OACs was associated with an increased risk of major bleeding compared with OACs alone. To minimize the risk of bleeding, individual modifiable risk factors should be controlled, and patients should be closely monitored, particularly during the first few months of concomitant use.
Accepted for Publication: January 26, 2024.
Published: March 22, 2024. doi:10.1001/jamanetworkopen.2024.3208
Open Access: This is an open access article distributed under the terms of the CC-BY License . © 2024 Rahman AA et al. JAMA Network Open .
Corresponding Author: Christel Renoux, MD, PhD, Centre for Clinical Epidemiology, Lady Davis Institute for Medical Research, Jewish General Hospital, 3755 Côte Sainte Catherine H 416.1, Montreal, QC H3T 1E2, Canada ( [email protected] ).
Author Contributions: Mr Rahman and Dr Renoux had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Rahman, Platt, Boivin, Rej, Renoux.
Acquisition, analysis, or interpretation of data: Rahman, Platt, Beradid, Rej, Renoux.
Drafting of the manuscript: Rahman, Boivin, Renoux.
Critical review of the manuscript for important intellectual content: Rahman, Platt, Beradid, Rej, Renoux.
Statistical analysis: Rahman, Beradid, Renoux.
Administrative, technical, or material support: Renoux.
Supervision: Platt, Boivin, Rej, Renoux.
Conflict of Interest Disclosures: Dr Platt reported receiving personal fees from Biogen, Boehringer Ingelheim, Merck, Nant Pharma, and Pfizer outside the submitted work. Dr Rej reported receiving a Clinician-Scientist Salary Award from Fonds de Recherche Quebec Sante; receiving grants from Mitacs; serving on a steering committee for AbbVie; and holding shares in Aifred outside the submitted work. No other disclosures were reported.
Funding/Support: Mr Rahman was supported by a Tomlinson Doctoral Fellowship from McGill University, a Canada Graduate Scholarship–Doctoral from the Canadian Institutes of Health Research, and a stipend from the Drug Safety and Effectiveness Cross-Training Program.
Role of the Funder/Sponsor: The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Data Sharing Statement: See Supplement 2 .
Additional Contributions: The authors would like to thank Sophie Dell’Aniello, MSc, Centre for Clinical Epidemiology, Lady Davis Institute for Medical Research, Jewish General Hospital, for providing technical support in the analysis of data. She was not compensated for her contribution.
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
- Search Menu
- Advance articles
- Editor's Choice
- 100 years of the AJE
- Collections
- Author Guidelines
- Submission Site
- Open Access Options
- About American Journal of Epidemiology
- About the Johns Hopkins Bloomberg School of Public Health
- Journals Career Network
- Editorial Board
- Advertising and Corporate Services
- Self-Archiving Policy
- Dispatch Dates
- Journals on Oxford Academic
- Books on Oxford Academic

- < Previous
Cohort Studies Versus Case-Control Studies on Night-Shift Work and Cancer Risk: The Importance of Exposure Assessment
- Article contents
- Figures & tables
- Supplementary Data
Kyriaki Papantoniou, Johnni Hansen, Cohort Studies Versus Case-Control Studies on Night-Shift Work and Cancer Risk: The Importance of Exposure Assessment, American Journal of Epidemiology , Volume 193, Issue 4, April 2024, Pages 577–579, https://doi.org/10.1093/aje/kwad227
- Permissions Icon Permissions
It is a general assumption that the prospective cohort study design is the gold standard approach and is superior to the case-control study design in epidemiology. However, there may be exceptions if the exposure is complex and requires collection of detailed information on many different aspects. Night-shift work, which impairs circadian rhythms, is an example of such a complex occupational exposure and may increase the risks of breast, prostate, and colorectal cancer. So far, for logistical reasons, investigators in cohort studies have assessed shift work rather crudely, lacking information on full occupational history and relevant shift-work metrics, and have presented mostly null findings. On the other hand, most cancer case-control studies have assessed the lifetime occupational histories of participants, including collection of detailed night-shift work metrics (e.g., type, duration, intensity), and tend to show positive associations. In this commentary, we debate why cohort studies with weak exposure assessment and other limitations might not necessarily be the preferred or less biased approach in assessing the carcinogenicity of night-shift work. Furthermore, we propose that risk-of-bias assessment and comparison of associations between studies with low versus high risks of bias be considered in future synthesis of the evidence.
Email alerts
Citing articles via, looking for your next opportunity.
- Recommend to your Library
Affiliations
- Online ISSN 1476-6256
- Print ISSN 0002-9262
- Copyright © 2024 Johns Hopkins Bloomberg School of Public Health
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
ORIGINAL RESEARCH article
Measles outbreak investigation in tocha district, southwestern ethiopia: an unmatched case–control study.

- 1 Ethiopian Field Epidemiology Laboratory Training Program (FETP), Jimma University, Jimma, Ethiopia
- 2 Lecturer of Biostatistics, Department of Epidemiology, Jimma University, Jimma, Ethiopia
- 3 Ethiopian Field Epidemiology Laboratory Training Program (EFETP), Jimma University, Jimma, Ethiopia
- 4 School of Public Health, College of Medicine and Health Science, Mizan–Tepi University, Mizan, Ethiopia
Background: Measles continues to be a public health challenge in Ethiopia. Rumors of suspected measles were notified on April 8, 2023 from Tocha district. We conducted an assessment to describe measles outbreak and determine risk factors for measles infection in the Tocha district of the Dawuro zone, Southwest Ethiopia.
Methods: We conducted a 1:2 unmatched case–control studies from April to May 2023. We took all 147 cases registered on line list for descriptive analyses. We used a total of 74 randomly selected cases and 147 controls for case–control part. Any person in Tocha district with laboratory-confirmed measles IgM antibody; or any suspected person epidemiologically linked to confirmed measles cases from March 23 to April 26 2023, were included in the case. Neighborhood who did not fulfill this standard case definition were included in controls. Data were collected using standardized questionnaires deployed on Kobo Collect. Descriptive analyses were conducted using Epi info version 7.2.5.0. The analyses were performed using Statistical Package for Social Science (SPSS) version 26. Binary logistic regression analyses were utilized to select candidate variables. We conducted multiple logistic regression analysis to identify determinants of measles infection at a p value ≤0.05 with 95% confidence interval.
Results: The overall attack rate of 22.64/10,000 for general population and 104.59/10,000 among under-five children were attributed to the outbreak with a case fatality rate of 2.72%. Vaccine coverage in the last year and this year were 73.52 and 53.88%, respectively, while vaccine effectiveness in the district was 79%. Poor house ventilation (AOR = 3.540, 95% CI: 1.663–7.535) and having contact history with the case (AOR = 2.528, 95% CI: 1.180–4.557) were positively related to measles infection while being previously vaccinated for measles (AOR = 0.209, 95% CI: 0.180–4.577) reduce risk of measles infections.
Conclusion: The highest attack rate was observed among children under 5 years of age, with a case fatality rate of 2.72%. Vaccination coverage was less than what expected to develop herd immunity. Strategies to increase vaccination coverage and strengthening surveillance systems for rumor identification and early responses to prevent person to person transmission are recommended.
Introduction
Measles is a highly infectious disease caused by a virus belonging to the paramyxovirus family and is characterized by typical symptoms of fever, a flat red spot rash with raised bumps, cough, runny nose, and pink eye ( 1 , 2 ). The primary mode of transmission is respiratory secretions expelled by an infected individual ( 2 – 4 ). According to a recent WHO report, an estimated 9.5 million cases and 128,000 people died from measles in 2021, most of whom were under 5 years of age ( 4 ). The globe experienced 21 significant and disruptive measles outbreaks in a year, with a large majority of cases from developing countries, including Africa. Ethiopia is among the top five countries affected by measles cases ( 5 , 6 ).
In developing countries such as Ethiopia, repeated measles outbreaks not only affect infected individuals but also disrupt the country’s health system and socioeconomic status ( 7 ). Outbreaks often result in a surge of cases, devastating healthcare facilities and healthcare workers. In addition, measles outbreaks can disrupt and divert attention and resources away from other essential and routine health care, such as maternal and child health (MCH) services ( 8 , 9 ). Some measures taken to manage outbreaks can also result in increased medical costs, lost productivity due to illness and caretaking responsibilities, and potential school closures to prevent further spread of the virus ( 10 , 11 ).
As part of the sustainable development goal (SDG), the international community has developed an ambitious goal of eliminating measles by 2030 ( 12 , 13 ). To achieve this, children 9–59 months old have to develop herd immunity with 95% coverage for two doses of measles-containing virus (MCV). Despite this, many countries have not currently had the impact required to end measles by 2030 ( 4 – 6 ). Only three countries in Africa were able to meet this target by 2020 ( 6 ).
With 1,953 cases in 2021, there will be an almost fivefold increase in confirmed measles cases in Ethiopia in 2022 ( 14 ). The 2019 Ethiopian Demographic and Mini-Health Survey (EDHS) showed that coverage of first and second doses of the measles vaccine were 59 and 9%, respectively, falling far below the target for measles elimination ( 15 ). Along with this, the continued vulnerability of vaccinated children to measles infection has overwhelmed the conditions ( 16 , 17 ).
Multiple factors may contribute to these problems in Ethiopia, including low population immunity, concurrent epidemics, conflict, forced displacement, and other humanitarian crises that disrupt childhood vaccinations ( 14 ). In addition, cultural beliefs, limited awareness, and behavioral factors can act as barriers to seeking early treatment and utilizing other disease management strategies ( 14 , 16 , 17 ). Comprehensive rumor identification and prompt responses are needed to prevent and manage such problems ( 13 , 18 ).
Rumors of suspected measles cases were reported from the Tocha district of the Dawuro Zone on April 8, 2023. This outbreak investigation was conducted to confirm the rumor and identify potential risk factors for measles infections and implement necessary public preventive and control measures in the affected areas.

Methods and materials
Study area and period.
The study was conducted in Tocha district from April 10 to May 30, 2023. The district is located in the Dawuro Zone of southwest Ethiopia. The district consists of 15 kebeles, with 14 rural kebeles and 1 urban kebele. From these, the Geda Mela, Bobi, Shechi, and Shada kebeles were located in hard-to-reach areas. The total population of the district was 64,917, with 31,808 males and 33,109 females. Among the total population, 10,917 (15.6%) were children under the age of five, and 2,071 (3.19%) were infants under 1 year old. The district has a total of 13,248 households with an average family size of 4.9. Currently, the district has 15 health posts, 1 primary hospital operated by the government, 3 private clinics, and 1 private pharmacy. The primary hospital in the district offers static immunization services, while health posts provide outreach services ( 19 , 20 ).
The outbreak primarily occurred in Geda Mela and Gani Denefaa kebeles, situated approximately 12 kilometers and 9 kilometers from Tocha town, respectively ( Figure 1 ).
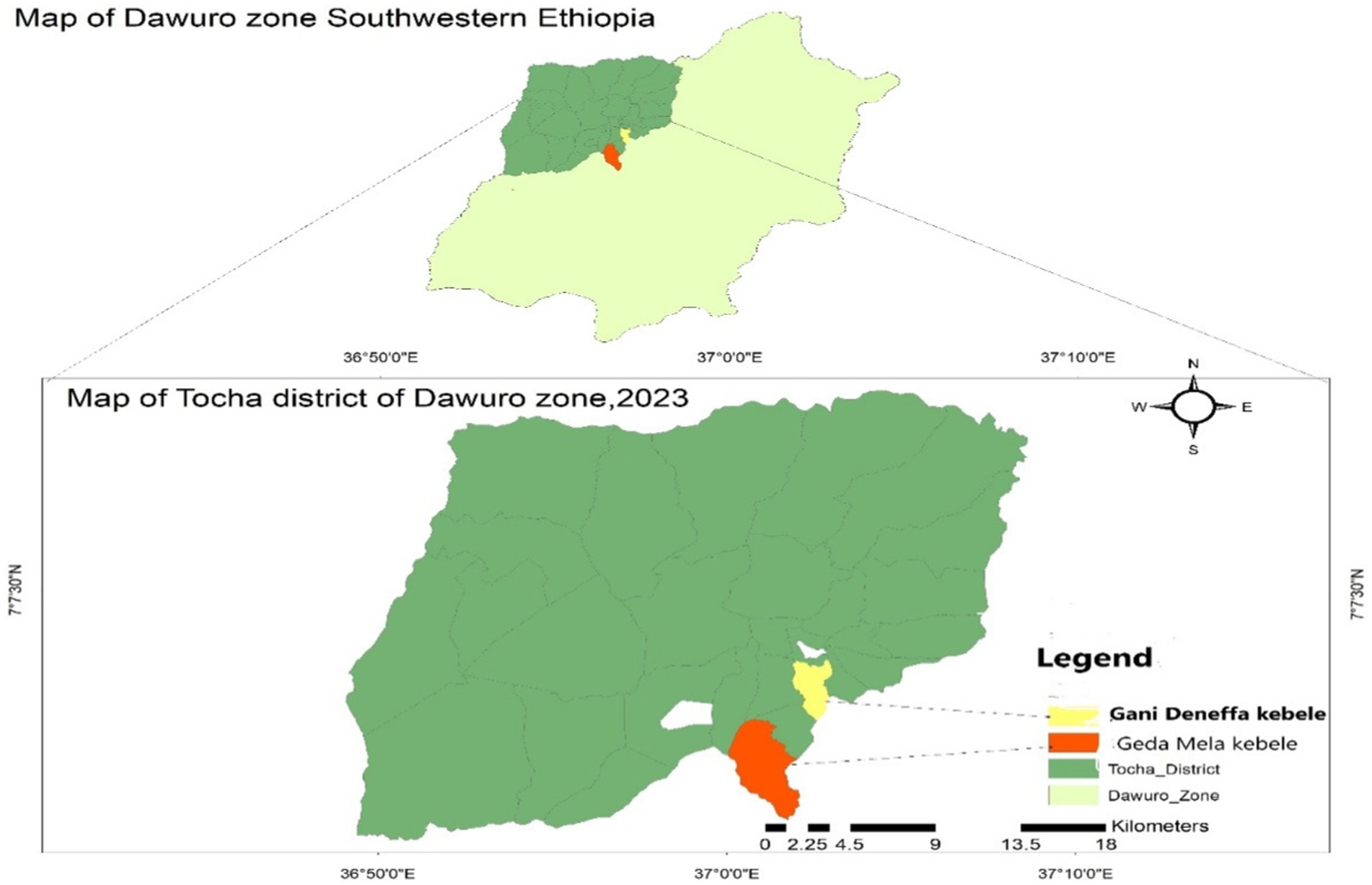
Figure 1 . Map of Tocha district Dawuro Zone, Southwest Ethiopia, 2023.
Study design
An unmatched case–control study was conducted to describe the measles outbreak and to determine risk factors for measles infection.
Source and study population
All populations of Tocha district were the source population. People in Tocha district who fulfilled the standard measles case definition and lived in selected kebeles were considered cases. Other people living in similar kebeles but who did not complete the standard case definition of measles were considered controls. Any person in Tocha district with laboratory-confirmed measles IgM antibody; or any suspected person epidemiologically linked to confirmed measles cases from March 23 to April 262,023, were included in the case. Those who had a vaccination history in the past 2 weeks were excluded from cases, provided that they may have developed measles symptoms as a result of an adverse event following immunization. Those who had a previous history of measles infection were excluded from control because they develop immunity against measles.
Sample size determination
Prior to sample size determination, a sampling frame was established by the following procedure. Immediately after the rumors were reported, five blood samples were obtained from suspected cases and dispatched to the National Public Health Laboratory for measles outbreak confirmation. Accordingly, all five samples tested positive for IgM antibodies.
For the descriptive analysis, we included all 147 registered measles cases on the line lists. However, we determined the sample size for the case–control study using the double population proportion formula assisted by Epi Info software version 7.2.5.0 considering the following assumptions for vaccination status: proportion of control exposed ( p = 67%, AOR = 2.84), 95% confidence interval, power 80%, and ratio of case to control 1:2 ( 16 , 17 ). Finally, a large sample size was selected from the three-sample size and found to be 221 for vaccination status. This sample size was finally split into 74 cases and 147 controls. We identified cases that fulfilled the measles case definition by performing active case searches in the affected kebeles and from line lists in health facilities.
Sampling technique
We selected the two-measles affected kebeles of Tocha district, namely, Geda Mela and Gani Denefaa, in the study. We employed a simple random sampling technique to select cases from line lists of measles cases in the district. Then, we conducted a house-to-house survey to collect data from selected cases in the affected kebeles.
Controls were participants from adjacent neighborhoods of cases who did not fulfill measles case definition, identified from randomly selection. They were all selected from kebeles (the smallest administrative structure) from which cases were selected 42 days after the incubation period of the last case.
Data collection and procedures
The data were collected using a structured researcher-administered questionnaire adapted from related literature ( 2 , 16 , 21 ), reviewing line lists, patients’ medical records, and observation checklists. The data were collected by two Field Epidemiology residents using the Kobo Collect platform Android version 2022.4.4 under daily supervision.
Observation checklists and recorded secondary data (line lists, patients’ medical records) were used in a descriptive analysis. The measles line lists include the patients’ age and sex, signs and symptoms, date of onset, admission status, vaccination status, laboratory results, treatment outcomes and others. Observation data were used to assess the availability and functionality of refrigerators, vaccine carriers, ice packs, and the overall management of the cold chain system of the district health office.
Questionnaires for case–control studies also collected sociodemographic information, ventilation status, exposure history (contact history with measles cases, travel history and vaccination status), knowledge about measles, and measles clinical features (signs and symptoms).
Variables and measurements
The outcome variable was measles infection, which was measured based on standard measles case definitions. Accordingly, any suspected person epidemiologically linked to confirmed measles cases who developed fever, maculopapular rash with cough or coryza, conjunctivitis and/or laboratory-confirmed IgM antibody for measles was said to be infected by measles ( 1 ).
Independent variables include sociodemographic variables, ventilation status, exposure history (contact history with measles cases, travel history and vaccination status), and knowledge about measles.
House ventilation status
Refers to the mechanism to allow exchange of indoor and outdoor air to reduce the risk of measles transmission. We measured the ventilation status using two questions, and the house was said to have good ventilation if it had at least one window that was opened on a daily basis. Otherwise, the house is labeled poorly ventilated.
Measles exposure history
The condition that puts a person at risk of measles infection. We measured this condition using three interrelated concepts (contact history with active measles cases, travel history to measles outbreak sites, and vaccination status). Persons who had a history of exposure during these days were said to have a history of contact with active measles cases ( 1 , 2 ). In addition, a person who traveled to known measles outbreak places was considered to have a travel history ( 2 , 16 ).
Vaccination status
Data about vaccination status were collected both from immunization cards and historical recall of participants. A person was said to be vaccinated if he/she took a minimum of a single dose of measles-containing vaccine (MCV) ( 2 , 16 ).
Knowledge about measles
Knowledge about measles was measured considering most susceptibility status, measles mode of transmission, signs and symptoms, treatment options and prevention methods. Participants were given a score of one ( 1 ) for each correct answer and zero otherwise. All correct answers were added together, and the mean score was calculated. Finally, participants who scored greater than the mean score were declared as having good knowledge about measles ( 22 ).
Suspected measles case definition
Any person with fever, nonvesicular generalized maculopapular rash and cough, coryza or conjunctivitis, or any person in whom the clinician suspects measles ( 1 ).
Confirmed measles case definition
A suspected case with laboratory confirmed measles IgM antibody or epidemiologically linked to confirmed measles cases in an measles outbreak ( 1 ).
Epidemiologically linked case
A suspected measles case with no specimen collected for serologic confirmation but linked (in place, person, and time) to a laboratory-confirmed case, that is, living in the same or a neighboring district with a confirmed measles case where there is a likelihood of transmission; onset of rash of the two cases being within 30 days of each other ( 1 ).
Measles outbreak
Measles outbreak is declared when 3 or more measles infections are laboratory confirmed for measles IgM antibody in a specific district in a month ( 1 ).
Compatible case
Not epidemiologically linked to laboratory confirmed cases. A suspected case which has not been adequately investigated ( 1 ).
Measles death
Defined as any death from an illness that occurs in a confirmed measles case or epidemiologically linked case of measles within 1 month of the onset of rash ( 1 ).
Vaccine effectiveness
Measure the proportion of reduction in measles cases among vaccinated persons. It was determined by calculating the percentage reduction in the incidence rate of measles among unvaccinated individuals and determining the percentage of reduction in risks of cases among vaccinated persons relative to unvaccinated persons. The formula for calculating vaccine effectiveness (VE) is (1-OR) *100. In Ethiopia, this calculation was performed for children aged 9 months to 59 months, provided that the routine measles immunization program starts at 9 months of age and the outbreak in Tocha district primarily affected children under 5 years old ( 2 , 23 ).
Laboratory investigation
We obtained 5 mL of blood samples from five ( 5 ) suspected measles cases, using a sterile syringe and needle. Samples were then placed in a sterile tube labeled with the individuals’ details and the date. After separating the serum from the blood, we stored the labeled tubes in a cooler with four ice packs in a vaccine carrier. Subsequently, we sent both the samples and a form detailing the cases to the national health lab for IgM antibody testing. All the five samples tested positive for IgM antibodies.
Data analysis procedures
The collected data were cleaned and exported to Epinfo version 7.2.5 software for descriptive analyses and Statistical Package for Social Science (SPSS) version 26 for analytical study. Descriptions of cases by person, place and time along the attack rate (AR), case fatality rate (CFR) and vaccine effectiveness were calculated. Bivariate logistic regressions were utilized to select candidate variables with a p value of ≤0.25. Multiple logistic regression was performed to identify predictors for measles infection. The level of statistical significance was declared at a p value ≤0.05 with a 95% confidence interval (CI).
Data quality management
The measles case definition was clearly established to ensure that all cases included in the study met the specific criteria outlined, minimizing the risk of misclassification bias. To prevent selection bias and enhance the representativeness of the study findings, both cases and controls were recruited from the same population. Data were collected by Field Epidemiology residents. The supervisor conducted daily checks to ensure the completeness and consistency of the collected data.
Review of the outbreak
According to interview result with family members, the index case for the outbreak was a 3-year-old female child who had a travel history to the adjacent Dali town of dawuro zone along with her mother on March 9, 2023. After returning back to Geda Mella kebele, the mother used to bring the child in most of community gathering she participated, like spiritual worshiping conference, market places, and mourning. The case was unvaccinated against measles and started to show rushes and fever on March 23, 2023. The mother gave anti pain bought from rural private drug vender. Later on, the child developed coughing and unable to feed and passed away on April 2, 2023.
During the time, there were many other similar cases in the community that were not brought to health facilities. However, rumors for suspected measles cases were received on April 8, 2023. On the date, the district health office has mobilized to confirm the cases. The zonal health department and regional health bureau took measurements on the next day. A multidisciplinary team engaged on outbreak investigation in the affected kebeles. Blood samples were collected from five suspected cases and sent to the Ethiopian public health institute (EPHI) for laboratory confirmation on April 9, 2023. All of the five suspected cases were found to be positive for measles IgM antibody, which was enough to confirm measles outbreak in Tocha district by April 11, 2023. 3(60%) cases reported to show first rush on April 5 while the rest 2 (40%) on April 6 ( Figure 2 ). The remaining 142 cases were not laboratory tested but, epidemiologically linked with confirmed measles cases. Therefore, the total number of measles cases during the outbreak was 147, with an overall attack rate (AR) of 22.64 per 10,000 population. The outbreak ended with four related deaths with a case fatality rate (CFR) of 2.72%.
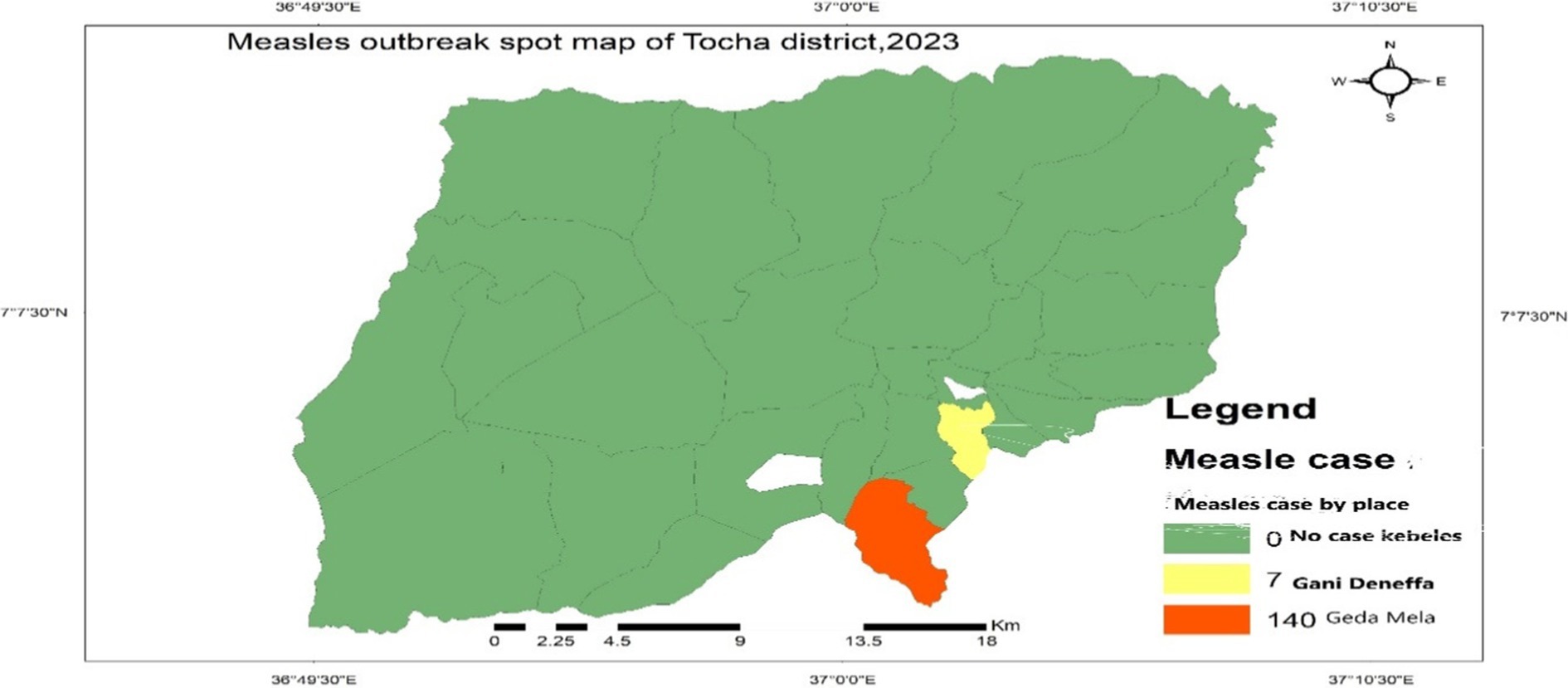
Figure 2 . Measles cases by place in Tocha district, 2023.
Descriptive epidemiology
Cases by time.
The Epi curve showed that the first case was registered on March 23, 2023. The peak of the outbreak was observed on April 8, 2023, and the last case occurred on April 26, 2023. It is known that the measles incubation period ranges from 7 to 18 days with an average of 14 days ( 1 ). With these data, the possible earliest and last dates of exposure were estimated to be March 16, 2023, and April 7, 2023, respectively. Therefore, the estimated date of exposure for the first case of measles in Torcha district was estimated to be from March 16, 2023, to April 7, 2023. This indicated that the outbreak lasted for two incubation periods ( Figure 2 ).
Cases by place
A total of 147 cases of measles, accompanied by four deaths, were reported from Geda Mela and Gani Denefaa kebeles of Tocha district, with an overall attack rate (AR) of 22.64/10,000 of the population. The majority of cases were reported from Geda Mela kebeles, with an attack rate of 232.37/10,000 population. Geda Mela was the remotest of all other kebeles in the district, situated approximately 12 kilometers from Tocha town in a hard-to-reach area, which makes it difficult to deliver routine immunization services ( Figure 3 ).
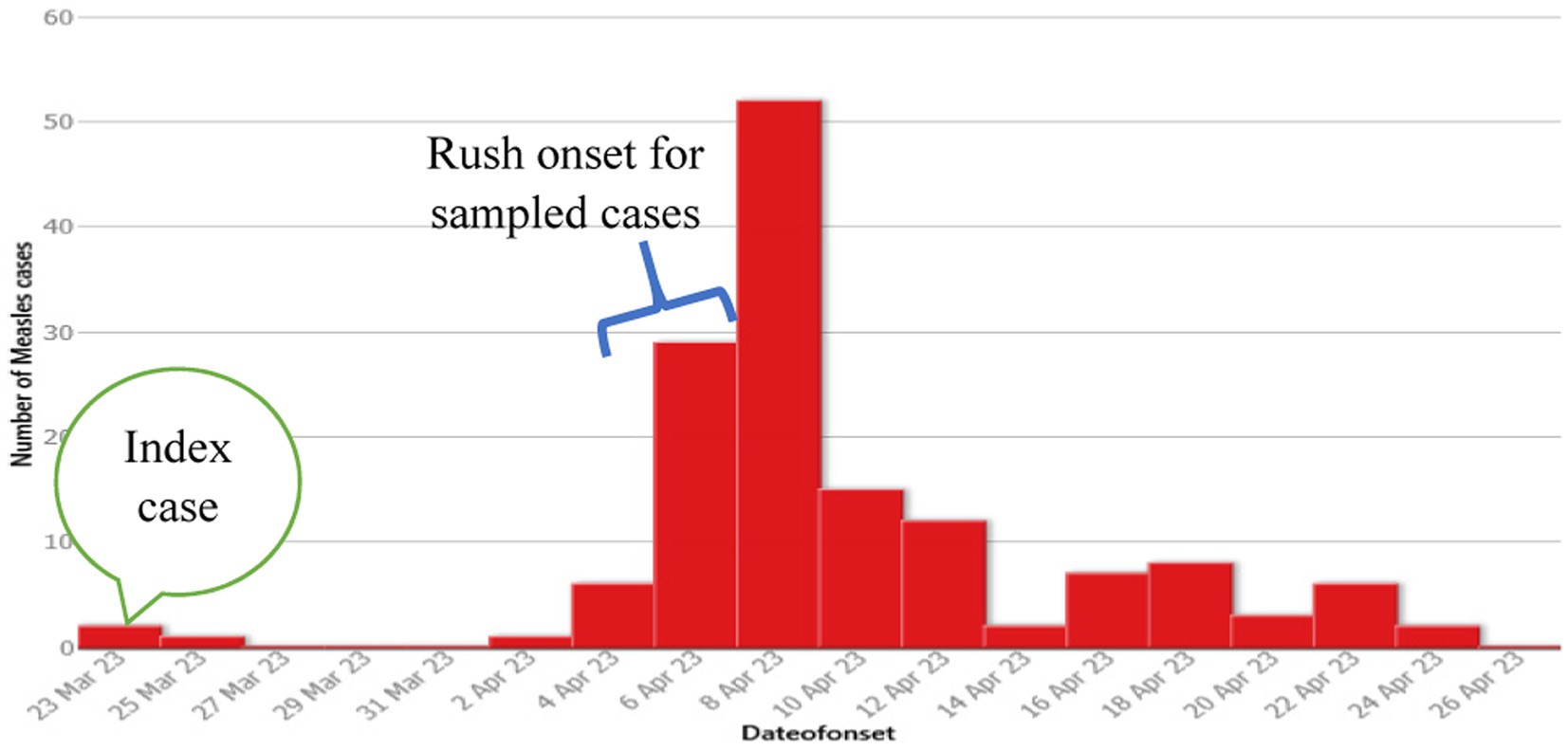
Figure 3 . Number of measles cases by date of onset of rash, Tocha district, of the Dawuro Zone from March 23 to April 26, 2023.
Cases by person
Among the total measles cases, 74 (50.34%) were males. The attack rates of measles in females and males were almost similar, with 22.05 and 23.26 per 10,000 people in the district, respectively. In addition, the age-specific attack rate for children under 5 years of age was 104.59/10,000 population ( Table 1 ).
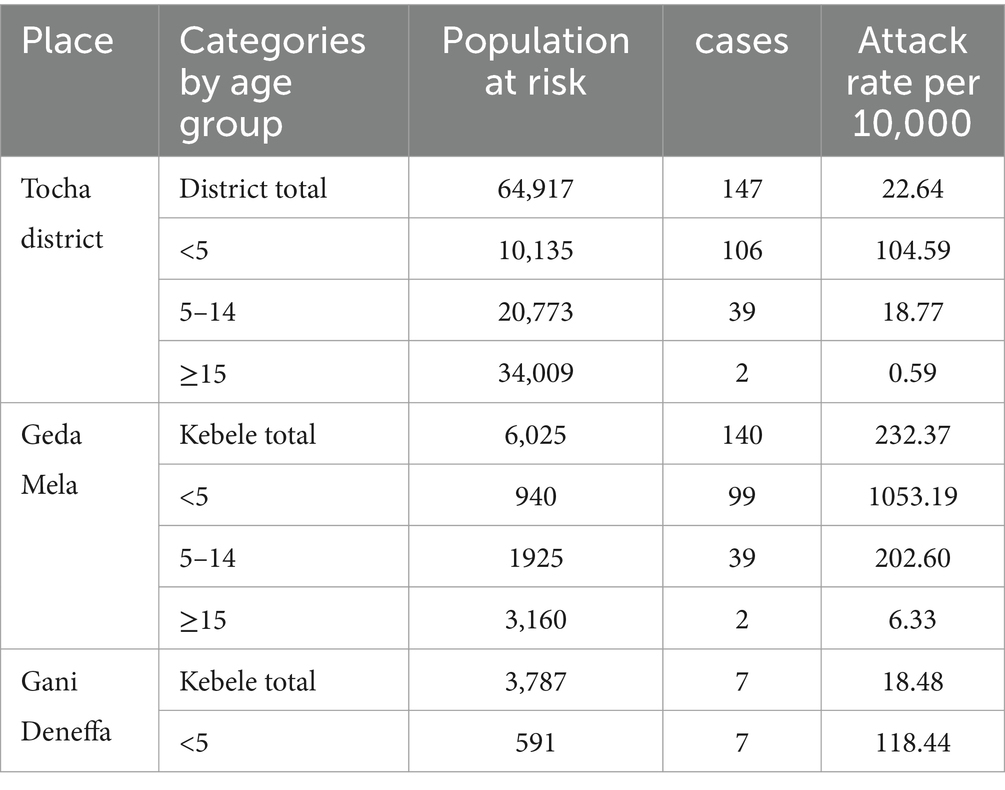
Table 1 . Measles outbreak attack rate in Tocha district, SWE 2023.
Clinical features of cases
Based on the findings of this study, all 147 cases exhibited signs and symptoms consistent with the standard measles case definition. All cases developed fever and generalized maculopapular rashes. Almost all 145 (98.63%) patients experienced cough. Additionally, patients who developed conjunctivitis and coryza accounted for 12 (8.16%) and 4 (2.72%) patients, respectively. A total of 19 (12.92%) patients were admitted with at least one measles complication. Of these, 6 (4.08%) had developed severe pneumonia, 11 (7.48%) had diarrhea with severe pneumonia, one case experienced severe acute malnutrition, and the remaining case developed tonsillitis abscess with diarrhea.
Vaccination status of cases
Among the 147 registered measles cases, almost two-thirds (94, 63.95%) had received at least one dose of measles-containing vaccine (MCV). Approximately one in four cases, 37 (25.17%), were unvaccinated for measles. Fourteen (9.52%) were ineligible for the first dose of MCV, while the vaccination status of two cases, 2 (1.36%), was unknown ( Figure 4 ).
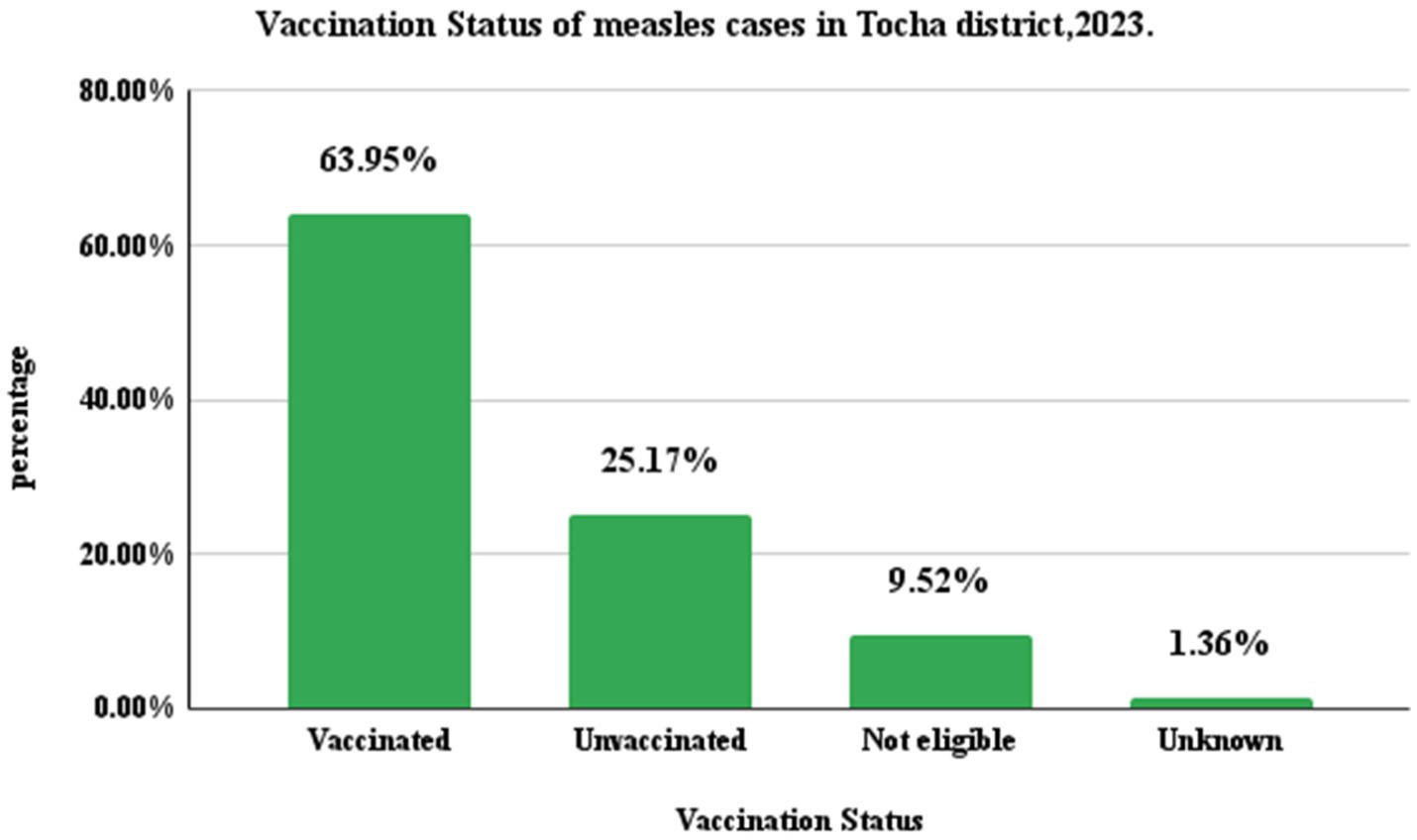
Figure 4 . Measles cases by vaccination status in Tocha district of Dawuro Zone, Southwest Ethiopia, 2023.
Measles cases final classifications
Among the registered measles cases, 74 (50.34%) were confirmed by epidemiological link. From these, 38 (51.35%) were female and 54 (72.97%) were children under the age of five. Patients who were not vaccinated for measles accounted for 51(68.92%). From the 68 (46.26%) compatible cases, 35(74.23%) were male. Under five children and those who were not vaccinated for measles accounted for 47 (63.51%) and 43 (58.11%), respectively. In addition, among the five laboratory confirmed cases, 3(60%) were male and all of them were under five children with no history of measles vaccination ( Figure 5 ).
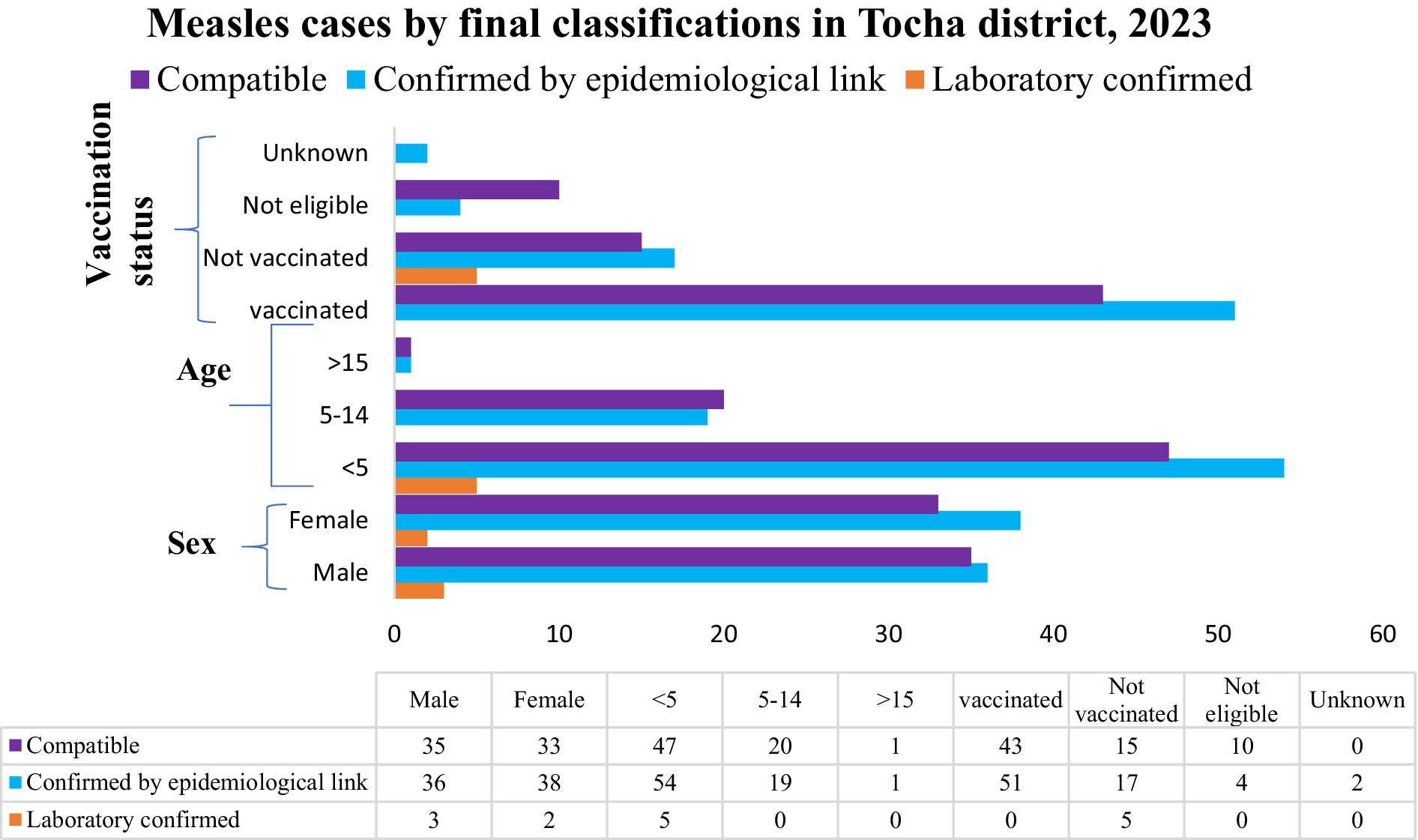
Figure 5 . Measles cases status final classification in Tocha district of Dawuro Zone, Southwest Ethiopia, 2023.
Vaccination coverage and cold chain management
In the district, the measles vaccination coverage in 2022 for MCV1 and MCV2 was 1,497 (73.52%) and 1,097 (53.88%), respectively. In 2023, the measles vaccination coverage was 1,112 (71.60%) for MCV1 and 970 (62.46%) for MCV2. From these data, the calculated dropout rates of measles vaccination in 2022 and 2023 were 26.72 and 12.76%, respectively. Only Tocha Primary Hospital and six health posts in the district had a refrigerator. From these, two refrigerators were not functional, including that of Geda Mella Kebele. All the assessed health facilities had an adequate supply of vaccine carriers and ice packs. However, power interruptions were common in the district, so the refrigerators were supplied with kerosene and solar energy ( Figure 6 ).
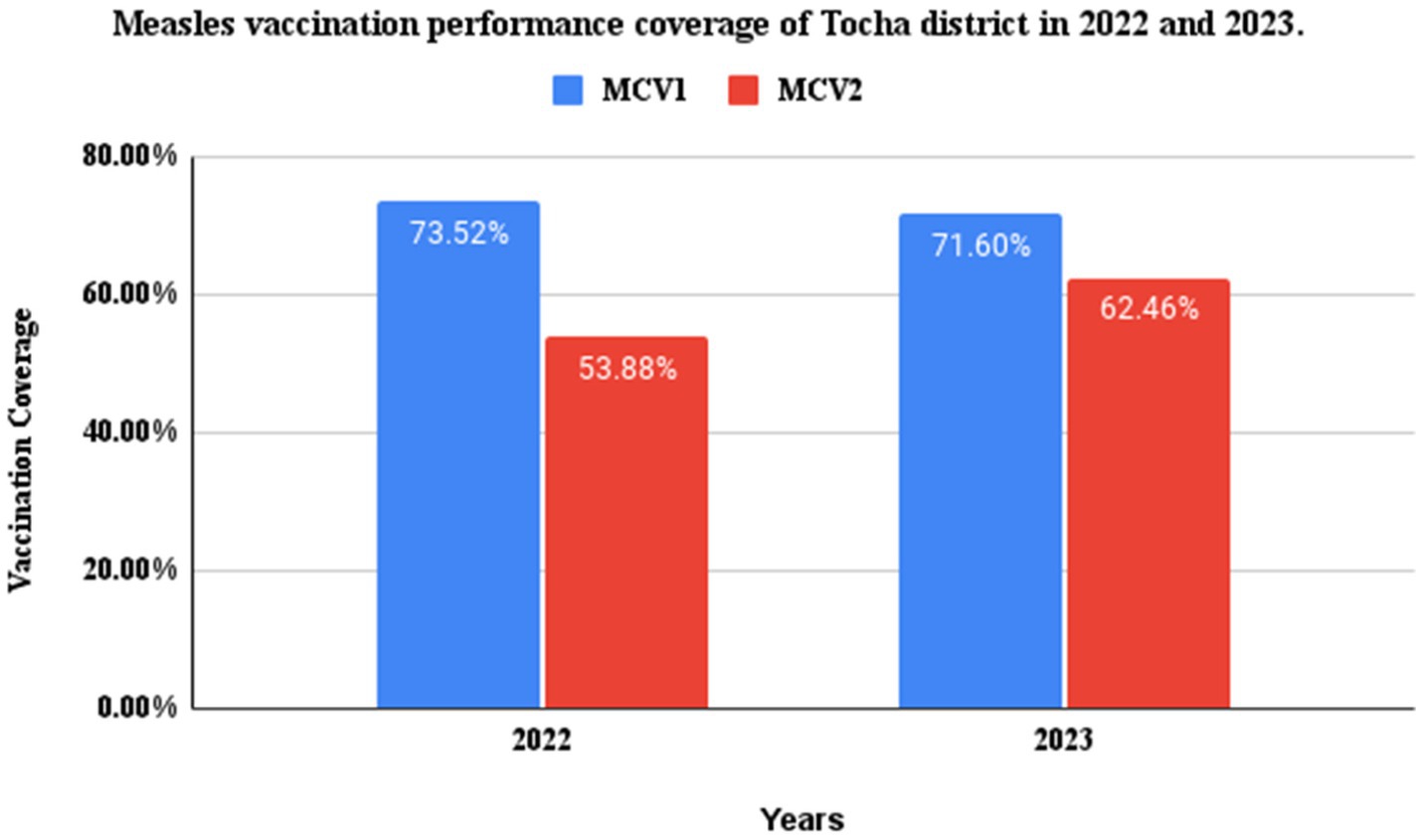
Figure 6 . MCV1 and 2 performance coverage of Tocha district, Southwest Ethiopia, in 2022 and 2023.
Analytical epidemiology
Socioeconomic characteristics of participants.
Two hundred twenty-one (74 cases and 147 controls) participants were involved in this study, and all of them were from the Dawuro ethnic background. The mean age of the participants was 3.60 (SD =1.62) for the case group and 3.12 (SD = 1.32) for the control group at the time of survey. Regarding gender, 46 (62.16%) cases and 81 (55.10%) controls were male. The majority of mothers/caregivers in both groups followed the Protestant religion, with 62 (83.78%) of the cases and 123 (83.67%) controls. Approximately two-thirds of caregivers, 53 (71.62%) of cases and 95 (64.35%) of controls, had engaged in farming. Caregivers who did not attend school accounted for 42 (56.77%) cases and 73 (49.67%) controls. Regarding housing conditions, 45 (60.8%) cases and 127 (83.4%) controls lived in houses with good ventilation status.
Caregivers who had good knowledge about measles accounted for 35 (47.3%) cases and 100 (68%) controls. A higher percentage of cases (47, 63.5%) than controls (62, 41.1%) had a contact history. Regarding vaccination status, approximately 30 (40.54%) of the cases and 117 (79.59%) of the controls were vaccinated ( Table 2 ).
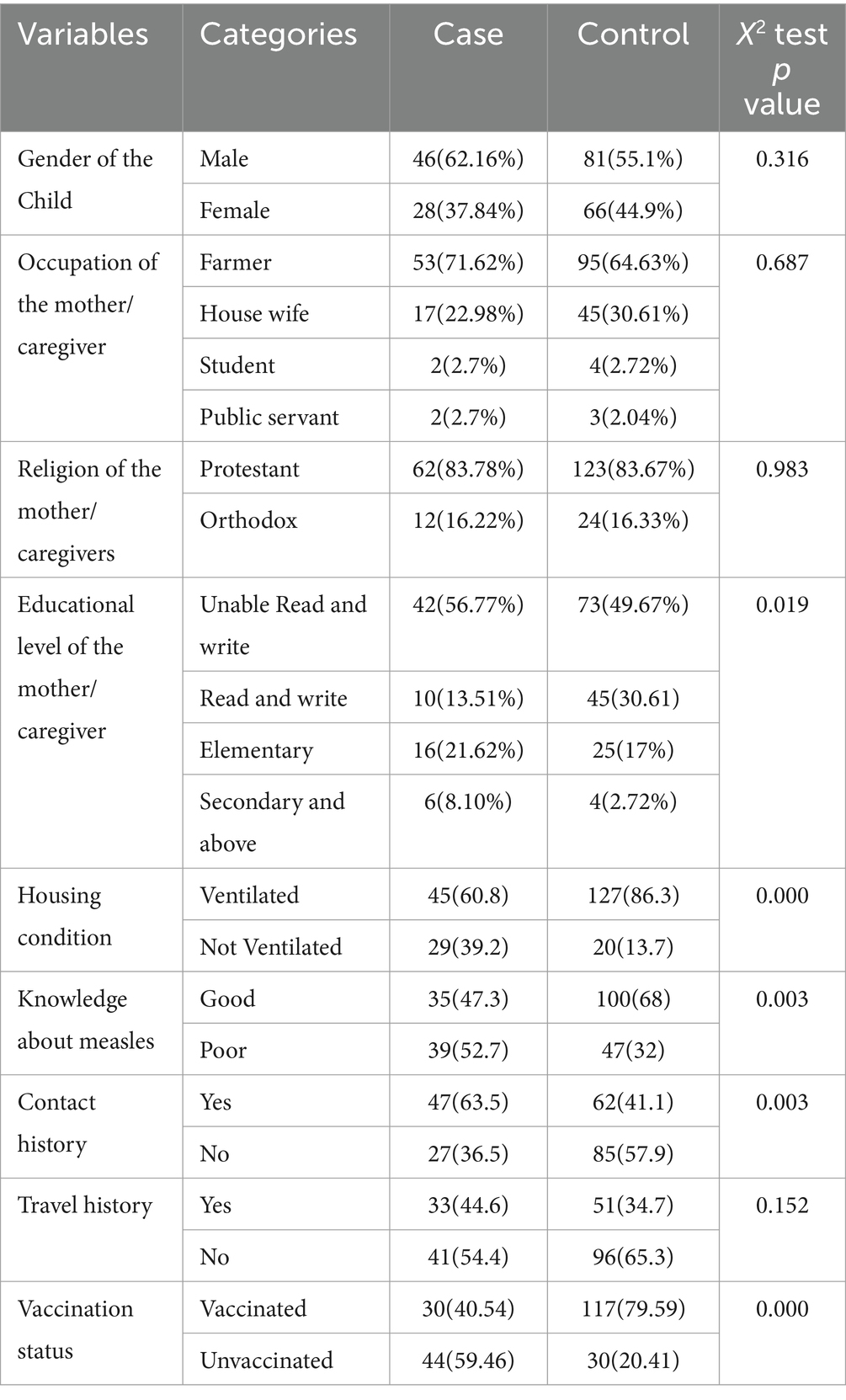
Table 2 . Sociodemographic characteristics in cases and controls of measles and the respondents in Tocha district, 2023.
In this measles outbreak investigation, vaccine effectiveness was found to be 79%.
Factors associated with measles infections
In the bivariate binary logistic regression, family size, vaccination status, contact history with suspected or confirmed measles cases, age of the child, knowledge about measles transmissions, travel history and house ventilation status were identified as significant predictors of measles infection at p values less than 0.25. After controlling for possible confounders in multivariate logistic regression, however, only vaccination status, contact history and house ventilation status continued to have a statistical association with measles infection. Accordingly, being previously vaccinated for measles reduce risk of measles infections by 79% compared with unvaccinated people (AOR = 0.209, 95% CI: 0.180–4.577).
On the other hand, the odds of contracting measles are increased by 3.540 factors for people living in poorly ventilated houses compared to those who live in ventilated houses (AOR = 3.540, 95% CI: 1.663–7.535). Similarly, people who had contact history with known or suspected measles cases were 2.528 times more likely to be infected with measles than their counterparts (AOR = 2.528, 95% CI: 1.180–4.557) ( Table 3 ).
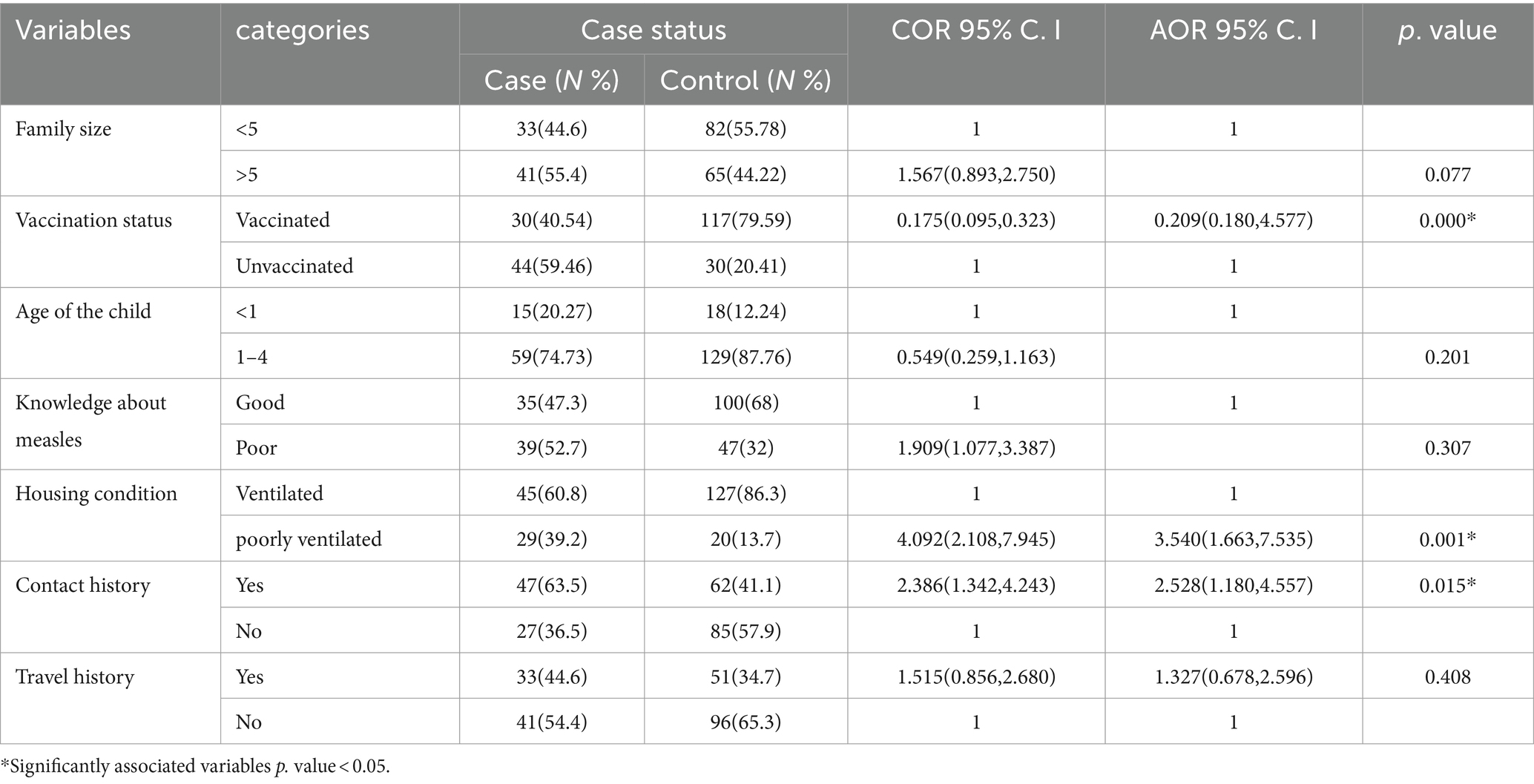
Table 3 . Bivariate and multivariable logistic regression analysis of measles infection in Tocha district, Southwest Ethiopia, 2023.
The World Health Organization and other partners strive to eliminate measles by 2030, with 95% coverage of measles vaccination among children 9–24 months old ( 13 ). Outbreak investigation and timely response are other pillars among the core components of measles elimination strategies worldwide ( 24 , 25 ). The aim of this study was to describe the magnitudes of measles cases and determinants of measles infection that contributed to measles outbreaks in the Tocha district of the Dawuro zone.
In the study area, the overall measles AR was 22.64 per 10,000 population. The AR was higher among under-five children, with 104.59 cases per 10,000 population. Similarly, the AR was higher among residents of Geda Mela kebele, with 232.37 per 10,000 population. This finding was consistent with a similar study from the Garda Marta District of the Gofa zone ( 16 ). However, it is higher than the measles outbreak investigation findings from Yemen ( 26 ), Guradamole District of Bale Zone ( 17 ), Basso Liben District of Amhara region ( 27 ) and Nunukumba District, East Wollega Zone ( 28 ). This might be due to the large number of unvaccinated children in Tocha district, which made the under-five children more susceptible to measles infection ( 16 , 17 ).
In addition, the poor cold chain management and low vaccination coverage of the district in general and Geda Mella kebele in particular could have contributed to the outbreak. Although all health posts had icepacks during the survey, only one-third of them had refrigerators. These findings may imply the need to find a lasting strategy for appropriate cold chain management in the districtSimilarly, the case fatality rate (CFR) of measles infection in the district was 2.72%. The findings of our study were lower than those of studies from the Gurada Mole district of the Bale Zone ( 17 ) and the expected CFR during measles outbreaks ( 1 ). The relatively lower CFR observed in this study may be attributed to unregistered deaths at the community level. However, this finding is higher compared to the study conducted in South Sudan ( 29 ), Garda Marta district of Gofa zone ( 16 ), and Ginnir district of Bale zone ( 21 ). This difference could be attributed to a delayed outbreak response, as the first response was initiated after the index cases had passed away. This may call for strengthening the surveillance system of Southwest Ethiopia in general and the Dawuro zone and Tocha district in particular.
In line with these findings, the district measles vaccination coverage of MCV1 and MCV2 over the past two consecutive years (2022–2023) was 73.52 and 53.88%, respectively. In addition, the district measles vaccination dropout rates over these 2 years were 26.72 and 12.76%, respectively. These were far below the target of WHO measles immunization coverage as the strategy to eliminate measles by 2030 ( 13 , 24 ).
The difficult topography of the local landscape and the fact that the health post was situated very far from densely populated residences in hard-to-reach areas might also enable health extension workers to trace and vaccinate unvaccinated children in the community. One HEW serves more than 6000 people in highly affected kebeles, such as Geda Mella, which may also affect the quality and equity of vaccination services in the district. The Ethiopian health indicator 2021 suggested that one health post should have to serve 5000 people ( 30 ).
Our multivariable analysis demonstrated that being previously vaccinated for measles had reduced risk of acquiring measles infection by 79%. This finding aligns with another measles outbreak investigation from Garda Marta, Sinana district of Oromia and Sekota Zuria district of Amhara regions ( 16 , 31 , 32 ). This may call for strengthening the vaccination strategy to decrease the risk of being at risk of measles infection among 9–59-month age groups. However, this was lower compared with similar study findings from the Ginnir districts of the Bale zone ( 21 ). It was also below the WHO-confirmed measles vaccine protective ability that could be achieved among children who received MCV1 ( 2 , 24 ). This might be attributed by the poor cold chain management of the district in general and Geda Mella kebele in particular that made even the vaccinated children susceptible to measles infection. Although all health posts had icepacks during the survey, only one-third of them had refrigerators. These findings may imply the need to find a lasting strategy for appropriate cold chain management in the district.
Additionally, we found that individuals who had contact history with measles cases had 2.528 times the odds of acquiring measles infection compared to their counterparts. This finding is consistent with a study conducted in the rural district of Ethiopia, the Garda Marta district of Gofa zone and Yemen ( 26 , 16 , 31 ). In fact, measles-infected persons are highly likely to transmit the virus from 4 days prior to the onset of the rash to 4 days after the rash erupts ( 1 ). Public health surveillance systems should be strengthened in early rumor identification and management and early response and mitigation to control diseases in elimination phases, such as measles.
Finally, the likelihood of contracting measles is increased by 3.540 factors for people living in poorly ventilated houses compared to those who live in ventilated houses. A systematic review and study findings from America also confirmed the existence of an association between poor house ventilation and the spread of airborne infections, such as measles ( 33 , 34 ). This demonstrates the need to take measures to reduce overload and increase ventilation in suspected measles cases.
Strengths and limitations of the study
Due to the nature of case control study design, it was difficult to control for unobserved/observed characteristics among control/case. We used a 1:2 case control to increase probability of getting sufficient number of controls with the same characteristics as cases. In addition, the fact that the care givers may not recognize exact information about some data like vaccination history, these may led to recall bias. However, we tried to manage this by observing vaccination card for those who could show it. Furthermore, we could not get data over the past 5 years to calculate vaccination coverage over 5 years and measles antibody titers by age group.
The highest AR was noticed among children under-5 years of age, with a CFR of 2.72%. Vaccination coverage and VE among children 9–59 months old were less than expected to develop herd immunity. Strategies to increase vaccination coverage and strengthen surveillance systems for rumor identification and early responses to control the spread of infections via contact and poor ventilation of houses are recommended.
Public health actions/interventions
In response to the measles outbreak, we took various measures to ensure effective treatment and control of the disease in collaboration with the Southwest Ethiopia People Regional Health Bureau, the Public Health Institute, and the Tocha District Health Office of the Dawuro Zone. Measles cases with complications were treated with antibiotics, oral rehydration salts and supplementary feedings as necessary.
Following confirmation of the outbreak, a catch-up vaccination campaign was conducted, along with a joint nutritional screening. A total of 877 children were vaccinated during the campaign. Additionally, all 6- to 59-month-old children were supplemented with vitamin A to boost the immune response and prevent further complications. We also conducted capacity building on job orientation for health workers and HEWs on measles case definitions. Health education was carried out in schools and churches, with key messages prepared on measles prevention and control measures.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Southwest People Regional Health Bureau Public Health Institute Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from primarily isolated as part of your previous study for which ethical approval was obtained. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements. Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
ST: Conceptualization, Formal analysis, Investigation, Methodology, Supervision, Validation, Writing – original draft, Writing – review & editing. NA: Conceptualization, Investigation, Writing – review & editing. HA: Conceptualization, Data curation, Methodology, Writing – review & editing. GF: Conceptualization, Data curation, Supervision, Validation, Writing – review & editing. GM: Data curation, Methodology, Software, Validation, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The research was conducted with financial support from the Ethiopian Field Epidemiology and Laboratory Training Program (EFELTP). The supporting bodies played no role in the design of the study and data collection, analysis, interpretation, and manuscript preparation.
Acknowledgments
We would like to thank the data collectors and supervisors for their cooperation. We also extend our appreciation to the Tocha district community for their kind support during the data collection period.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AR, Attack Rate; CCO, Chief Clinical Officer; CEO, Chief Executive Officer; CFR, Case Fatality Rate; COVID-19, Coronavirus Disease 2019; EDHS, Ethiopian Demographic and Health Survey; EPHI, Ethiopian Public Health Institute; GAVI, Gavi, The Vaccine Alliance; IA2030, Immunization Agenda 2030; MCV1, First dose of Measles-Containing Vaccine; MCV2, Second Dose of Measles-Containing Vaccine; MCH, Maternal and Child Health; MRSF, Measles and Rubella Strategic Framework; SDG, Sustainable Development Goal; SWEPRHB, South West Ethiopia People’s Regional Health Bureau; PHC, Primary Health Care; UNICEF, United Nations Children’s Fund; VE, Vaccine Effectiveness; WHO, World Health Organization.
1. EPHI. Guideline on measles surveillance and outbreak management 3rd, (2012) Addis Ababa Ethiopia. 3. 1–82 Ethiopian Public Health Institute
Google Scholar
2. World Health Organization. African regional guidelines for measles and rubella surveillance Geneva: World Health Organization (2020):1–82.
3. WHO. Guide for clinical case management and infection prevention and control during a measles outbreak . Geneva: World Health Organization (2020).
4. WHO. Global Measles fact sheet . Geneva: World Health Organization (2023). Available at: https://www.who.int/news-room/fact-sheets/detail/measles .
5. UNICEF and WHO. Warn of perfect storm of conditions for measles outbreaks, affecting children , (2022). Available at: https://www.who.int/news/item/27-04-2022 .
6. World Health Organization. Vaccine-preventable disease outbreaks on the rise in Africa , (2022). Available at: https://www.afro.who.int/news/vaccine-preventable-disease-outbreaks-rise-africa .
7. CDC. Health Cost: Measles and rubella can cause serious illness, birth defects, and death , (2023). Available at: https://www.cdc.gov/globalhealth/measles/why/index.html .
8. Chen, SY, Anderson, S, Kutty, PK, Lugo, F, McDonald, M, Rota, PA, et al. Health care–associated measles outbreak in the United States after an importation: challenges and economic impact. J Infect Dis . (2011) 203:1517–25. doi: 10.1093/infdis/jir115
PubMed Abstract | Crossref Full Text | Google Scholar
9. Dayan, GH, Ortega-Sánchez, IR, CW, LB, and Quinlisk, MP Team IMR. The cost of containing one case of measles: the economic impact on the public health infrastructure—Iowa, 2004. Pediatrics . (2005) 116:e1–4. doi: 10.1542/peds.2004-2512
Crossref Full Text | Google Scholar
10. Meyers, EC, Solorzano, BR, James, J, Ganzer, PD, Elaine, S, Rennaker, RL, et al. HHS public access. Physiol Behav . (2018) 176:100–6.
11. Mac McCullough, J, Fowle, N, Sylvester, T, Kretschmer, M, Ayala, A, Popescu, S, et al. Cost analysis of 3 concurrent public health response events: financial impact of measles outbreak, super bowl surveillance, and Ebola surveillance in Maricopa County. J Public Heal Manag Pract . (2019) 25:357–65. doi: 10.1097/PHH.0000000000000818
12. World Health Organization (WHO). Monitoring health for the SDGs, sustainable development goal . Geneva: World Health Statistics (2019).
13. WHO. Measles and rubella strategic framework 2021–2030 . Geneva: World Health Organization; (2020). 1–34 p.
14. World Health Organization. Disease outbreak news; measles . Ethiopia: World Health Organization (2023). Available at: https://www.who.int/emergencies/disease-outbreak-news/item/2023-DON460 .
15. EPHI. Ethiopia Mini Demographic and Health Survey . Rockville, Maryland, USA: EPHI and ICF (2019).
16. Bukuno, S. Measles outbreak investigation in Garda Marta District, Southwestern Ethiopia, 2022: Community-Based Case-Control Study. Infect Drug Resist . (2023) 16:2681–94. doi: 10.2147/IDR.S405802
17. Tsegaye, G, Gezahegn, Y, Tesfaye, A, Mulatu, G, Bulcha, GG, and Berhanu, N. Measles outbreak investigation in Guradamole District of bale zone, South Eastern Ethiopia, 2021. Infect Drug Resist . (2022) 15:669–83. doi: 10.2147/IDR.S343270
18. PHEM. Federal Democratic Republic of Ethiopia:Public Health Emergency Management (PHEM) . Addis Ababa: Ethiopian Public Health Institute (2022).
19. Dawuro Health Office. Dawuro zone health department performance report . Dawuro Zone: Dawuro Health Office (2022).
20. Dawuro Health Office. Dawuro Zone Health department performance Report of 2023 . Tarcha: Dawuro Health Office.
21. Kalil, FS, Gemeda, DH, Bedaso, MH, and Wario, SK. Measles outbreak investigation in Ginnir District of bale zone, Oromia region, Southeast Ethiopia, May 2019. Pan Afr Med J . (2020) 36:1–12. doi: 10.11604/pamj.2020.36.20.21169
22. Ibrahim, U. Assessment of knowledge, attude, and practices of measles prevention among mothers of under five years attending under 5 clinic in Bauchi Town. Int J Sci Eng Res . (2014) 5:1050–3.
23. Orenstein, WA, Bernier, RH, Dondero, TJ, Hinman, AR, Marks, JS, Bart, KJ, et al. Field evaluation of vaccine efficacy. Bull World Health Organ . (1985) 63:1055–68.
PubMed Abstract | Google Scholar
24. States, M, Preuve, T, Advi-, WHOS, Grade, T, and Sage, T. Measles vaccines: WHO position paper – April 2017. Wkly Epidemiol Rec . (2017) 92:205–27.
25. The World Health Organisation. Table 1: WHO recommendations for routine immunization . Nezerland, Geneva: WHO (2020).
26. May, F, Ali, A, Nassar, H, Abdullah, M, Amad, A, and Qasim, M. Risk factors for measles outbreak in Ataq and Habban districts, Shabwah governorate, Yemen, February to May 2018. BMC Infect Dis . (2021) 21:1–7. doi: 10.1186/s12879-021-06207-3
27. Tesfaye, A, Sisay, A, Sofoniyas, G, Yimer, S, Addisu, M, and Chalachew, G. Measles outbreak investigation in basso Liben District, Amhara Region, Ethiopia 2017. J Infect Dis Immun . (2021) 13:1–6. doi: 10.5897/JIDI2017.0171
28. Babure, ZK, and Tufa, AF. Evaluation of Measles Outbreak Response Activities and Surveillance System Performance in Nunukumba District, East Wollega Zone of Oromia Region, Ethiopia, June 2020. J Women’s Health Care . (2021) 10:516. doi: 10.35248/2167-0420.21.10.516
29. Mokaya, EN, Isaac, Z, and Anyuon, NA. Measles outbreak investigation in aweil east county, South Sudan. Pan Afr Med J . (2021) 40:40. doi: 10.11604/pamj.2021.40.87.28370
30. Policy, planning, monitoring and evaluation directorate M. HMIS indicators reference guide , Ethiopian Health Policy Planning, Monitoring and Directorate. vol. 126 (2022).
31. Girmay, A, and Dadi, AF. Being unvaccinated and having a contact history increased the risk of measles infection during an outbreak: a finding from measles outbreak investigation in rural district of Ethiopia. BMC Infect Dis . (2019) 19:1–7. doi: 10.1186/s12879-019-3973-8
32. Mebrate, M, Hailu, C, and Alemu, S. Measles outbreak investigation in Kasoshekumer kebele, Sinana district, south-eastern Oromia, Ethiopia: a case–control study. SAGE Open Med . (2023) 11:205031212311691. doi: 10.1177/20503121231169182
33. Li, Y, Leung, GM, Tang, JW, Yang, X, Chao, CYH, Lin, JZ, et al. Role of ventilation in airborne transmission of infectious agents in the built environment? A multidisciplinary systematic review. Indoor Air . (2007) 17:2–18. doi: 10.1111/j.1600-0668.2006.00445.x
34. Aizmi, P, Keshavarz, Z, Laurent, JGC, Luna, ML, and Allen, J. Estimating the nationwide transmission risk of measles in US schools and impacts of vaccination and supplemental infection control strategies. BMC Infect Dis . (2020) 20:1–22. doi: 10.1186/s12879-020-05200-6
Keywords: measles, outbreak, Tocha, SWE, Dawuro
Citation: Fikadu S, Admasu N, Abebe H, Feyisa GC and Midaksa G (2024) Measles outbreak investigation in Tocha district, southwestern Ethiopia: an unmatched case–control study. Front. Public Health . 12:1331798. doi: 10.3389/fpubh.2024.1331798
Received: 01 November 2023; Accepted: 27 March 2024; Published: 10 April 2024.
Reviewed by:
Copyright © 2024 Tefera, Admasu, Abebe, Feyisa and Midaksa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Simon Fikadu Tefera, [email protected]
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
What to know about the crisis of violence, politics and hunger engulfing Haiti

A long-simmering crisis over Haiti’s ability to govern itself, particularly after a series of natural disasters and an increasingly dire humanitarian emergency, has come to a head in the Caribbean nation, as its de facto president remains stranded in Puerto Rico and its people starve and live in fear of rampant violence.
The chaos engulfing the country has been bubbling for more than a year, only for it to spill over on the global stage on Monday night, as Haiti’s unpopular prime minister, Ariel Henry, agreed to resign once a transitional government is brokered by other Caribbean nations and parties, including the U.S.
But the very idea of a transitional government brokered not by Haitians but by outsiders is one of the main reasons Haiti, a nation of 11 million, is on the brink, according to humanitarian workers and residents who have called for Haitian-led solutions.
“What we’re seeing in Haiti has been building since the 2010 earthquake,” said Greg Beckett, an associate professor of anthropology at Western University in Canada.

What is happening in Haiti and why?
In the power vacuum that followed the assassination of democratically elected President Jovenel Moïse in 2021, Henry, who was prime minister under Moïse, assumed power, with the support of several nations, including the U.S.
When Haiti failed to hold elections multiple times — Henry said it was due to logistical problems or violence — protests rang out against him. By the time Henry announced last year that elections would be postponed again, to 2025, armed groups that were already active in Port-au-Prince, the capital, dialed up the violence.
Even before Moïse’s assassination, these militias and armed groups existed alongside politicians who used them to do their bidding, including everything from intimidating the opposition to collecting votes . With the dwindling of the country’s elected officials, though, many of these rebel forces have engaged in excessively violent acts, and have taken control of at least 80% of the capital, according to a United Nations estimate.
Those groups, which include paramilitary and former police officers who pose as community leaders, have been responsible for the increase in killings, kidnappings and rapes since Moïse’s death, according to the Uppsala Conflict Data Program at Uppsala University in Sweden. According to a report from the U.N . released in January, more than 8,400 people were killed, injured or kidnapped in 2023, an increase of 122% increase from 2022.
“January and February have been the most violent months in the recent crisis, with thousands of people killed, or injured, or raped,” Beckett said.

Armed groups who had been calling for Henry’s resignation have already attacked airports, police stations, sea ports, the Central Bank and the country’s national soccer stadium. The situation reached critical mass earlier this month when the country’s two main prisons were raided , leading to the escape of about 4,000 prisoners. The beleaguered government called a 72-hour state of emergency, including a night-time curfew — but its authority had evaporated by then.
Aside from human-made catastrophes, Haiti still has not fully recovered from the devastating earthquake in 2010 that killed about 220,000 people and left 1.5 million homeless, many of them living in poorly built and exposed housing. More earthquakes, hurricanes and floods have followed, exacerbating efforts to rebuild infrastructure and a sense of national unity.
Since the earthquake, “there have been groups in Haiti trying to control that reconstruction process and the funding, the billions of dollars coming into the country to rebuild it,” said Beckett, who specializes in the Caribbean, particularly Haiti.
Beckett said that control initially came from politicians and subsequently from armed groups supported by those politicians. Political “parties that controlled the government used the government for corruption to steal that money. We’re seeing the fallout from that.”

Many armed groups have formed in recent years claiming to be community groups carrying out essential work in underprivileged neighborhoods, but they have instead been accused of violence, even murder . One of the two main groups, G-9, is led by a former elite police officer, Jimmy Chérizier — also known as “Barbecue” — who has become the public face of the unrest and claimed credit for various attacks on public institutions. He has openly called for Henry to step down and called his campaign an “armed revolution.”
But caught in the crossfire are the residents of Haiti. In just one week, 15,000 people have been displaced from Port-au-Prince, according to a U.N. estimate. But people have been trying to flee the capital for well over a year, with one woman telling NBC News that she is currently hiding in a church with her three children and another family with eight children. The U.N. said about 160,000 people have left Port-au-Prince because of the swell of violence in the last several months.
Deep poverty and famine are also a serious danger. Gangs have cut off access to the country’s largest port, Autorité Portuaire Nationale, and food could soon become scarce.
Haiti's uncertain future
A new transitional government may dismay the Haitians and their supporters who call for Haitian-led solutions to the crisis.
But the creation of such a government would come after years of democratic disruption and the crumbling of Haiti’s political leadership. The country hasn’t held an election in eight years.
Haitian advocates and scholars like Jemima Pierre, a professor at the University of British Columbia, Vancouver, say foreign intervention, including from the U.S., is partially to blame for Haiti’s turmoil. The U.S. has routinely sent thousands of troops to Haiti , intervened in its government and supported unpopular leaders like Henry.
“What you have over the last 20 years is the consistent dismantling of the Haitian state,” Pierre said. “What intervention means for Haiti, what it has always meant, is death and destruction.”

In fact, the country’s situation was so dire that Henry was forced to travel abroad in the hope of securing a U.N. peacekeeping deal. He went to Kenya, which agreed to send 1,000 troops to coordinate an East African and U.N.-backed alliance to help restore order in Haiti, but the plan is now on hold . Kenya agreed last October to send a U.N.-sanctioned security force to Haiti, but Kenya’s courts decided it was unconstitutional. The result has been Haiti fending for itself.
“A force like Kenya, they don’t speak Kreyòl, they don’t speak French,” Pierre said. “The Kenyan police are known for human rights abuses . So what does it tell us as Haitians that the only thing that you see that we deserve are not schools, not reparations for the cholera the U.N. brought , but more military with the mandate to use all kinds of force on our population? That is unacceptable.”
Henry was forced to announce his planned resignation from Puerto Rico, as threats of violence — and armed groups taking over the airports — have prevented him from returning to his country.

Now that Henry is to stand down, it is far from clear what the armed groups will do or demand next, aside from the right to govern.
“It’s the Haitian people who know what they’re going through. It’s the Haitian people who are going to take destiny into their own hands. Haitian people will choose who will govern them,” Chérizier said recently, according to The Associated Press .
Haitians and their supporters have put forth their own solutions over the years, holding that foreign intervention routinely ignores the voices and desires of Haitians.
In 2021, both Haitian and non-Haitian church leaders, women’s rights groups, lawyers, humanitarian workers, the Voodoo Sector and more created the Commission to Search for a Haitian Solution to the Crisis . The commission has proposed the “ Montana Accord ,” outlining a two-year interim government with oversight committees tasked with restoring order, eradicating corruption and establishing fair elections.
For more from NBC BLK, sign up for our weekly newsletter .
CORRECTION (March 15, 2024, 9:58 a.m. ET): An earlier version of this article misstated which university Jemima Pierre is affiliated with. She is a professor at the University of British Columbia, Vancouver, not the University of California, Los Angeles, (or Columbia University, as an earlier correction misstated).
Patrick Smith is a London-based editor and reporter for NBC News Digital.
Char Adams is a reporter for NBC BLK who writes about race.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Ann Indian Acad Neurol
- v.16(4); Oct-Dec 2013
Design and data analysis case-controlled study in clinical research
Sanjeev v. thomas.
Department of Neurology, Sree Chitra Tirunal Institute for Medical Sciences and Technology, Trivandrum, Kerala, India
Karthik Suresh
1 Department of Pulmonary and Critical Care Medicine, Johns Hopkins University School of Medicine, Louiseville, USA
Geetha Suresh
2 Department of Justice Administration, University of Louisville, Louiseville, USA
Clinicians during their training period and practice are often called upon to conduct studies to explore the association between certain exposures and disease states or interventions and outcomes. More often they need to interpret the results of research data published in the medical literature. Case-control studies are one of the most frequently used study designs for these purposes. This paper explains basic features of case control studies, rationality behind applying case control design with appropriate examples and limitations of this design. Analysis of sensitivity and specificity along with template to calculate various ratios are explained with user friendly tables and calculations in this article. The interpretation of some of the laboratory results requires sound knowledge of the various risk ratios and positive or negative predictive values for correct identification for unbiased analysis. A major advantage of case-control study is that they are small and retrospective and so they are economical than cohort studies and randomized controlled trials.
Introduction
Clinicians think of case-control study when they want to ascertain association between one clinical condition and an exposure or when a researcher wants to compare patients with disease exposed to the risk factors to non-exposed control group. In other words, case-control study compares subjects who have disease or outcome (cases) with subjects who do not have the disease or outcome (controls). Historically, case control studies came into fashion in the early 20 th century, when great interest arose in the role of environmental factors (such as pipe smoke) in the pathogenesis of disease. In the 1950s, case control studies were used to link cigarette smoke and lung cancer. Case-control studies look back in time to compare “what happened” in each group to determine the relationship between the risk factor and disease. The case-control study has important advantages, including cost and ease of deployment. However, it is important to note that a positive relationship between exposure and disease does not imply causality.
At the center of the case-control study is a collection of cases. [ Figure 1 ] This explains why this type of study is often used to study rare diseases, where the prevalence of the disease may not be high enough to permit for a cohort study. A cohort study identifies patients with and without an exposure and then “looks forward” to see whether or not greater numbers of patients with an exposure develop disease.

Comparison of cohort and case control studies
For instance, Yang et al . studied antiepileptic drug (AED) associated rashes in Asians in a case-control study.[ 1 ] They collected cases of confirmed anti-epileptic induced severe cutaneous reactions (such as Stevens Johnson syndrome) and then, using appropriate controls, analyzed various exposures (including type of [AED] used) to look for risk factors to developing AED induced skin disease.
Choosing controls is very important aspect of case-control study design. The investigator must weigh the need for the controls to be relevant against the tendency to over match controls such that potential differences may become muted. In general, one may consider three populations: Cases, the relevant control population and the population at large. For the study above, the cases include patients with AED skin disease. In this case, the relevant control population is a group of Asian patients without skin disease. It is important for controls to be relevant: In the anti-epileptic study, it would not be appropriate to choose a population across ethnicities since one of the premises of the paper revolves around particularly susceptibility to AED drug rashes in Asian populations.
One popular method of choosing controls is to choose patients from a geographic population at large. In studying the relationship between non-steroidal anti-inflammatory drugs and Parkinson's disease (PD), Wahner et al . chose a control population from several rural California counties.[ 2 ] There are other methods of choosing controls (using patients without disease admitted to the hospital during the time of study, neighbors of disease positive cases, using mail routes to identify disease negative cases). However, one must be careful not to introduce bias into control selection. For instance, a study that enrolls cases from a clinic population should not use a hospital population as control. Studies looking at geography specific population (e.g., Neurocysticercosis in India) cannot use controls from large studies done in other populations (registries of patients from countries where disease prevalence may be drastically different than in India). In general, geographic clustering is probably the easiest way to choose controls for case-control studies.
Two popular ways of choosing controls include hospitalized patients and patients from the general population. Choosing hospitalized, disease negative patients offers several advantages, including good rates of response (patients admitted to the hospital are generally already being examined and evaluated and often tend to be available to further questioning for a study, compared with the general population, where rates of response may be much lower) and possibly less amnestic bias (patients who are already in the hospital are, by default, being asked to remember details of their presenting illnesses and as such, may more reliably remember details of exposures). However, using hospitalized patients has one large disadvantage; these patients have higher severity of disease since they required hospitalization in the first place. In addition, patients may be hospitalized for disease processes that may share features with diseases under study, thus confounding results.
Using a general population offers the advantage of being a true control group, random in its choosing and without any common features that may confound associations. However, disadvantages include poor response rates and biasing based on geography. Administering long histories and questions regarding exposures are often hard to accomplish in the general population due to the number of people willing (or rather, not willing) to undergo testing. In addition, choosing cases from the general population from particular geographic areas may bias the population toward certain characteristics (such as a socio-economic status) of that geographic population. Consider a study that uses cases from a referral clinic population that draws patients from across socio-economic strata. Using a control group selected from a population from a very affluent or very impoverished area may be problematic unless the socio-economic status is included in the final analysis.
In case-controls studies, cases are usually available before controls. When studying specific diseases, cases are often collected from specialty clinics that see large numbers of patients with a specific disease. Consider for example, the study by Garwood et al .[ 3 ] which looked at patients with established PD and looked for associations between prior amphetamine use and subsequent development various neurologic disorders. Patients in this study were chosen from specialty clinics that see large numbers of patients with certain neurologic disorders. Case definitions are very important when planning to choose cases. For instance, in a hypothetical study aiming to study cases of peripheral neuropathy, will all patients who carry a diagnosis of peripheral neuropathy be included? Or, will only patients with definite electromyography evidence of neuropathy be included? If a disease process with known histopathology is being studied, will tissue diagnosis be required for all cases? More stringent case definitions that require multiple pieces of data to be present may limit the number of cases that can be used in the study. Less stringent criteria (for instance, counting all patients with the diagnosis of “peripheral neuropathy” listed in the chart) may inadvertently choose a group of cases that are too heterogeneous.
The disease history status of the chosen cases must also be decided. Will the cases being chosen have newly diagnosed disease, or will cases of ongoing/longstanding disease also be included? Will decedent cases be included? This is important when looking at exposures in the following fashion: Consider exposure X that is associated with disease Y. Suppose that exposure X negatively affects disease Y such that patients that are X + have more severe disease. Now, a case-control study that used only patients with long-standing or ongoing disease might miss a potential association between X and Y because X + patients, due to their more aggressive course of disease, are no longer alive and therefore were not included in the analysis. If this particular confounding effect is of concern, it can be circumvented by using incident cases only.
Selection bias occurs when the exposure of interest results in more careful screening of a population, thus mimicking an association. The classic example of this phenomenon was noted in the 70s, when certain studies noted a relationship between estrogen use and endometrial cancer. However, on close analysis, it was noted that patients who used estrogen were more likely to experience vaginal bleeding, which in turn is often a cause for close examination by physicians to rule out endometrial cancer. This is often seen with certain drug exposures as well. A drug may produce various symptoms, which lead to closer physician evaluation, thus leading to more disease positive cases. Thus, when analyzed in a retrospective fashion, more of the cases may have a particular exposure only insofar as that particular exposure led to evaluations that resulted in a diagnosis, but without any direct association or causality between the exposure and disease.
One advantage of case-control studies is the ability to study multiple exposures and other risk factors within one study. In addition, the “exposure” being studied can be biochemical in nature. Consider the study, which looked at a genetic variant of a kinase enzyme as a risk factor for development of Alzheimer's disease.[ 4 ] Compare this with the study mentioned earlier by Garwood et al .,[ 3 ] where exposure data was collected by surveys and questionnaires. In this study, the authors drew blood work on cases and controls in order to assess their polymorphism status. Indeed, more than one exposure can be assessed in the same study and with planning, a researcher may look at several variables, including biochemical ones, in single case-control study.
Matching is one of three ways (along with exclusion and statistical adjustment) to adjust for differences. Matching attempts to make sure that the control group is sufficiently similar to the cases group, with respects to variables such as age, sex, etc., Cases and controls should not be matched on variables that will be analyzed for possible associations to disease. Not only should exposure variables not be included, but neither should variables that are closely related to these variables. Lastly, overmatching should be avoided. If the control group is too similar to the cases group, the study may fail to detect the difference even if one exists. In addition, adding matching categories increases expense of the study.
One measure of association derived from case control studies are sensitivity and specificity ratios. These measures are important to a researcher, to understand the correct classification. A good understanding of sensitivity and specificity is essential to understand receiver operating characteristic curve and in distinguishing correct classification of positive exposure and disease with negative exposure and no disease. Table 1 explains a hypothetical example and method of calculation of specificity and sensitivity analysis.
Hypothetical example of sensitivity, specificity and predictive values

Interpretation of sensitivity, specificity and predictive values
Sensitivity and specificity are statistical measures of the performance of a two by two classification of cases and controls (sick or healthy) against positives and negatives (exposed or non-exposed).[ 5 ] Sensitivity measures or identifies the proportion of actual positives identified as the percentage of sick people who are correctly identified as sick. Specificity measures or identifies the proportion of negatives identified as the percentage of healthy people who are correctly identified as healthy. Theoretically, optimum prediction aims at 100% sensitivity and specificity with a minimum of margin of error. Table 1 also shows false positive rate, which is referred to as Type I error commonly stated as α “Alpha” is calculated using the following formula: 100 − specificity, which is equal to 100 − 90.80 = 9.20% for Table 1 example. Type 1 error is also known as false positive error is referred to as a false alarm, indicates that a condition is present when it is actually not present. In the above mentioned example, a false positive error indicates the percent falsely identified healthy as sick. The reason why we want Type 1 error to be as minimum as possible is because healthy should not get treatment.
The false negative rate, which is referred to as Type II error commonly stated as β “Beta” is calculated using the following formula: 100 − sensitivity which is equal to 100 − 73.30 = 26.70% for Table 1 example. Type II error is also known as false negative error indicates that a condition is not present when it should have been present. In the above mentioned example, a false negative error indicates percent falsely identified sick as healthy. A Type 1 error unnecessarily treats a healthy, which in turn increases the budget and Type II error would risk the sick, which would act against study objectives. A researcher wants to minimize both errors, which not a simple issue because an effort to decrease one type of error increases the other type of error. The only way to minimize both type of error statistically is by increasing sample size, which may be difficult sometimes not feasible or expensive. If the sample size is too low it lacks precision and it is too large, time and resources will be wasted. Hence, the question is what should be the sample size so that the study has the power to generalize the result obtained from the study. The researcher has to decide whether, the study has enough power to make a judgment of the population from their sample. The researcher has to decide this issue in the process of designing an experiment, how large a sample is needed to enable reliable judgment.
Statistical power is same as sensitivity (73.30%). In this example, large number of false positives and few false negatives indicate the test conducted alone is not the best test to confirm the disease. Higher statistical power increase statistical significance by reducing Type 1 error which increases confidence interval. In other words, larger the power more accurately the study can mirror the behavior of the study population.
The positive predictive values (PPV) or the precision rate is referred to as the proportion of positive test results, which means correct diagnoses. If the test correctly identifies all positive conditions then the PPV would be 100% and negative predictive value (NPV) would be 0. The calculative PPV in Table 1 is 11.8%, which is not large enough to predict cases with test conducted alone. However, the NPV 99.9% indicates the test correctly identifies negative conditions.
Clinical interpretation of a test
In a sample, there are two groups those who have the disease and those who do not have the disease. A test designed to detect that disease can have two results a positive result that states that the disease is present and a negative result that states that the disease is absent. In an ideal situation, we would want the test to be positive for all persons who have the disease and test to be negative for all persons who do not have the disease. Unfortunately, reality is often far from ideal. The clinician who had ordered the test has the result as positive or negative. What conclusion can he or she make about the disease status for his patient? The first step would be to examine the reliability of the test in statistical terms. (1) What is the sensitivity of the test? (2) What is the specificity of the test? The second step is to examine it applicability to his patient. (3) What is the PPV of the test? (4) What is the NPV of the test?
Suppose the test result had come as positive. In this example the test has a sensitivity of 73.3% and specificity of 90.8%. This test is capable of detecting the disease status in 73% of cases only. It has a false positivity of 9.2%. The PPV of the test is 11.8%. In other words, there is a good possibility that the test result is false positive and the person does not have the disease. We need to look at other test results and the clinical situation. Suppose the PPV of this test was close to 80 or 90%, one could conclude that most likely the person has the disease state if the test result is positive.
Suppose the test result had come as negative. The NPV of this test is 99.9%, which means this test gave a negative result in a patient with the disease only very rarely. Hence, there is only 0.1% possibility that the person who tested negative has in fact the disease. Probably no further tests are required unless the clinical suspicion is very high.
It is very important how the clinician interprets the result of a test. The usefulness of a positive result or negative result depends upon the PPV or NPV of the test respectively. A screening test should have high sensitivity and high PPV. A confirmatory test should have high specificity and high NPV.
Case control method is most efficient, for the study of rare diseases and most common diseases. Other measures of association from case control studies are calculation of odds ratio (OR) and risk ratio which is presented in Table 2 .
Different ratio calculation templates with sample calculation

Absolute risk means the probability of an event occurring and are not compared with any other type of risk. Absolute risk is expressed as a ratio or percent. In the example, absolute risk reduction indicates 27.37% decline in risk. Relative risk (RR) on the other hand compares the risk among exposed and non-exposed. In the example provided in Table 2 , the non-exposed control group is 69.93% less likely compared to exposed cases. Reader should keep in mind that RR does not mean increase in risk. This means that while a 100% likely risk among those exposed cases, unexposed control is less likely by 69.93%. RR does not explain actual risk but is expressed as relative increase or decrease in risk of exposed compared to non-exposed.
OR help the researcher to conclude whether the odds of a certain event or outcome are same for two groups. It calculates the odds of a health outcome when exposed compared to non-exposed. In our example an OR of. 207 can be interpreted as the non-exposed group is less likely to experience the event compared to the exposed group. If the OR is greater than 1 (example 1.11) means that the exposed are 1.11 times more likely to be riskier than the non-exposed.
Event rate for cases (E) and controls (C) in biostatistics explains how event ratio is a measure of how often a particular statistical exposure results in occurrence of disease within the experimental group (cases) of an experiment. This value in our example is 11.76%. This value or percent explains the extent of risk to patients exposed, compared with the non-exposed.
The statistical tests that can be used for ascertain an association depends upon the variable characteristics also. If the researcher wants to find the association between two categorical variables (e.g., a positive versus negative test result and disease state expressed as present or absent), Cochran-Armitage test, which is same as Pearson Chi-squared test can be used. When the objective is to find the association between two interval or ratio level (continuous) variables, correlation and regression analysis can be performed. In order to evaluate statistical significant difference between the means of cases and control, a test of group difference can be performed. If the researcher wants to find statically significant difference among means of more than two groups, analysis of variance can be performed. A detailed explanation and how to calculate various statistical tests will be published in later issues. The success of the research directly and indirectly depends on how the following biases or systematic errors, are controlled.
When selecting cases and controls, based on exposed or not-exposed factors, the ability of subjects to recall information on exposure is collected retrospectively and often forms the basis for recall bias. Recall bias is a methodological issue. Problems of recall method are: Limitations in human ability to recall and cases may remember their exposure with more accuracy than the controls. Other possible bias is the selection bias. In case-control studies, the cases and controls are selected from the same inherited characteristics. For instance, cases collected from referral clinics often exposed to selection bias cases. If selection bias is not controlled, the findings of association, most likely may be due to of chance resulting from the study design. Another possible bias is information bias, which arises because of misclassification of the level of exposure or misclassification of disease or other symptoms of outcome itself.
Case control studies are good for studying rare diseases, but they are not generally used to study rare exposures. As Kaelin and Bayona explains[ 6 ] if a researcher want to study the risk of asthma from working in a nuclear submarine shipyard, a case control study may not be a best option because a very small proportion of people with asthma might be exposed. Similarly, case-control studies cannot be the best option to study multiple diseases or conditions because the selection of the control group may not be comparable for multiple disease or conditions selected. The major advantage of case-control study is that they are small and retrospective and so they are economical than cohort studies and randomized controlled trials.
Source of Support: Nil
Conflict of Interest: Nil
- Skip to main content
- Keyboard shortcuts for audio player
Ex-assistant principal charged with child neglect in case of boy who shot teacher
The Associated Press

Signs stand outside Richneck Elementary School in Newport News, Va., Jan. 25, 2023. Denise Lavoie/AP hide caption
Signs stand outside Richneck Elementary School in Newport News, Va., Jan. 25, 2023.
NEWPORT NEWS, Va. — A former assistant principal at a Virginia elementary school has been charged with felony child neglect more than a year after a 6-year-old boy brought a gun to class and shot his first-grade teacher .
A special grand jury in Newport News found that Ebony Parker showed a reckless disregard for the lives of Richneck Elementary School students on Jan. 6, 2023, according to indictments unsealed Tuesday.
Parker and other school officials already face a $40 million negligence lawsuit from the teacher who was shot, Abby Zwerner. She accuses Parker and others of ignoring multiple warnings the boy had a gun and was in a "violent mood" the day of the shooting.
Criminal charges against school officials following a school shootings are quite rare, experts say. Parker, 39, faces eight felony counts, each of which is punishable by up to five years in prison.
The Associated Press left a message seeking comment Tuesday with Parker's attorney, Curtis Rogers.

Shots - Health News
'say something' tip line in schools flags gun violence threats, study finds.
Court documents filed Tuesday reveal little about the criminal case against Parker, listing only the counts and a description of the felony charge. It alleges that Parker "did commit a willful act or omission in the care of such students, in a manner so gross, wanton and culpable as to show a reckless disregard for human life."
Newport News police have said the student who shot Zwerner retrieved his mother's handgun from atop a dresser at home and brought the weapon to school concealed in a backpack.
Zwerner's lawsuit describes a series of warnings that school employees gave administrators before the shooting. The lawsuit said those warnings began with Zwerner telling Parker that the boy "was in a violent mood," had threatened to beat up a kindergartener and stared down a security officer in the lunchroom.
The lawsuit alleges that Parker "had no response, refusing even to look up" when Zwerner expressed her concerns.
When concerns were raised that the child may have transferred the gun from his backpack to his pocket, Parker said his "pockets were too small to hold a handgun and did nothing," the lawsuit states.

Architecture
With gun control far from sight, schools redesign for student safety.
A guidance counselor also asked Parker for permission to search the boy, but Parker forbade him, "and stated that John Doe's mother would be arriving soon to pick him up," the lawsuit stated.
Zwerner was sitting at a reading table in front of the class when the boy fired the gun, police said. The bullet struck Zwerner's hand and then her chest, collapsing one of her lungs. She spent nearly two weeks in the hospital and has endured multiple surgeries as well as ongoing emotional trauma, according to her lawsuit.
Parker and the lawsuit's other defendants, which include a former superintendent and the Newport News school board, have tried to block Zwerner's lawsuit.
They've argued that Zwerner's injuries fall under Virginia's workers' compensation law. Their arguments have been unsuccessful so far in blocking the litigation. A trial date for Zwerner's lawsuit is slated for January.
Prosecutors had said a year ago that they were investigating whether the "actions or omissions" of any school employees could lead to criminal charges.
What schools can (and can't) do to prevent school shootings
Howard Gwynn, the commonwealth's attorney in Newport News, said in April 2023 that he had petitioned a special grand jury to probe if any "security failures" contributed to the shooting. Gwynn wrote that an investigation could also lead to recommendations "in the hopes that such a situation never occurs again."
It is not the first school shooting to spark a criminal investigation into school officials. For instance, a former school resource officer was acquitted of all charges last year after he was accused of hiding during the Parkland school massacre in 2018.
Chuck Vergon, a professor of educational law and policy at the University of Michigan-Flint, told The AP last year that it is rare for a teacher or school official to be charged in a school shooting because allegations of criminal negligence can be difficult to prove.
More often, he said, those impacted by school shootings seek to hold school officials liable in civil court.
- elementary school
The effects of case/control ratio and sample size on genome-wide association studies: A simulation study
Affiliations.
- 1 Faculty of Veterinary Medicine, Department of Animal Science, Siirt University, Siirt, Turkey.
- 2 Faculty of Veterinary Medicine, Department of Obstetrics and Gynecology, Van Yüzüncü Yıl University, Van, Turkey.
- PMID: 38581306
- PMCID: PMC10998454
- DOI: 10.1002/vms3.1444
Background: Genome-wide association studies (GWAS) is a useful tool for the detection of disease or quantitative trait-related genetic variations in the veterinary field. For a binary trait, a case/control experiment is designed in GWAS. However, there is limited information on the optimal case/control and sample size in GWAS.
Objectives: In this study, it was aimed to detect the effects of case/control ratio and sample size for GWAS using computer simulation under certain assumptions.
Method: Using the PLINK software, we simulated three different disease scenarios. In scenario 1, we simulated 10 different case/control ratios with increasing ratio of cases to controls. In scenario 2, we did versa of scenario 1 with the increasing ratio of controls to cases. In scenarios 1 and 2, sample size gradually was increased with the change case/control ratios. In scenario 3, the total sample size was fixed to 2000 to see real effects of case/control ratio on the number of disease-related single nucleotide polymorphisms (SNPs).
Results: The results showed that the number of disease-related SNPs were the highest when the case/control ratio is close to 1:1 in scenarios 1 and 2 and did not change with an increase in sample size. Similarly, the number of disease-related SNPs was the highest in case/control ratios 1:1 in scenario 3. However, unbalanced case/control ratio caused the detection of lower number of disease-related SNPs in scenario 3. The estimated average power of SNPs was highest when case/control ratio is 1:1 in all scenarios.
Conclusions: All findings led to the conclusion that an increase in sample size may enhance the statistical power of GWAS when the number of cases is small. In addition, case/control ratio 1:1 may be the optimal ratio for GWAS. These findings may be valuable not only for veterinary field but also for human clinical experiments.
Keywords: GWAS; case/control ratio; diseases; sample size; simulation.
© 2024 The Authors. Veterinary Medicine and Science published by John Wiley & Sons Ltd.
- Computer Simulation
- Genome-Wide Association Study* / methods
- Genome-Wide Association Study* / veterinary
- Polymorphism, Single Nucleotide*
- Sample Size

IMAGES
VIDEO
COMMENTS
Case-control studies are a type of observational study often used in fields like medical research, environmental health, or epidemiology. While most observational studies are qualitative in nature, case-control studies can also be quantitative, and they often are in healthcare settings. Case-control studies can be used for both exploratory and ...
A case-control study is a type of observational study commonly used to look at factors associated with diseases or outcomes.[1] The case-control study starts with a group of cases, which are the individuals who have the outcome of interest. The researcher then tries to construct a second group of individuals called the controls, who are similar to the case individuals but do not have the ...
Case-control studies are one of the major observational study designs for performing clinical research. The advantages of these study designs over other study designs are that they are relatively quick to perform, economical, and easy to design and implement. Case-control studies are particularly appropriate for studying disease outbreaks, rare diseases, or outcomes of interest. This article ...
A case control study is a retrospective, observational study that compares two existing groups. Researchers form these groups based on the existence of a condition in the case group and the lack of that condition in the control group. They evaluate the differences in the histories between these two groups looking for factors that might cause a ...
A case-control study is a type of observational study commonly used to look at factors associated with diseases or outcomes. The case-control study starts with a group of cases, which are the individuals who have the outcome of interest. The researcher then tries to construct a second group of individuals called the controls, who are similar to ...
General Overview of Case-Control Studies. In observational studies, also called epidemiologic studies, the primary objective is to discover and quantify an association between exposures and the outcome of interest, in hopes of drawing causal inference. Observational studies can have a retrospective study design, a prospective design, a cross ...
A case-control study (also known as case-referent study) is a type of observational study in which two existing groups differing in outcome are identified and compared on the basis of some supposed causal attribute. Case-control studies are often used to identify factors that may contribute to a medical condition by comparing subjects who ...
Abstract. Case-control studies are observational studies in which cases are subjects who have a characteristic of interest, such as a clinical diagnosis, and controls are (usually) matched subjects who do not have that characteristic. After cases and controls are identified, researchers "look back" to determine what past events (exposures ...
A case-control study can help provide extra insight on data that has already been collected. A case-control study is a way of carrying out a medical investigation to confirm or indicate what is ...
Case-control studies are time-efficient and less costly than RCTs, particularly when the outcome of interest is rare or takes a long time to occur, because the cases are identified at study onset and the outcomes have already occurred with no need for a long-term follow-up. The case-control design is useful in exploratory studies to assess a ...
Introduction. A case-control study is designed to help determine if an exposure is associated with an outcome (i.e., disease or condition of interest). In theory, the case-control study can be described simply. First, identify the cases (a group known to have the outcome) and the controls (a group known to be free of the outcome).
Examples. A case-control study is an observational study where researchers analyzed two groups of people (cases and controls) to look at factors associated with particular diseases or outcomes. Below are some examples of case-control studies: Investigating the impact of exposure to daylight on the health of office workers (Boubekri et al., 2014).
Case-control studies. Case-control studies are retrospective. They clearly define two groups at the start: one with the outcome/disease and one without the outcome/disease. They look back to assess whether there is a statistically significant difference in the rates of exposure to a defined risk factor between the groups.
The main advantages of a nested case-control study are as follows: (1) cost reduction and effort minimization, as only a fraction of the parent cohort requires the necessary outcome assessment; (2) reduced selection bias, as both case and control subjects are sampled from the same population; and (3) flexibility in analysis by allowing testing of a hypotheses in the future that is not ...
Case-control studies are one of the major observational study designs for performing clinical research. The advantages of these study designs over other study designs are that they are relatively quick to perform, economical, and easy to design and implement. Case-control studies are particularly appropriate for studying disease outbreaks, rare ...
Case-control studies are a type of observational study often used in fields like medical research, environmental health, or epidemiology. While most observational studies are qualitative in nature, case-control studies can also be quantitative, and they often are in healthcare settings. Case-control studies can be used for both exploratory and ...
The purpose of this article is to present in elementary mathematical and statistical terms a simple way to quickly and effectively teach and understand case-control studies, as they are commonly done in dynamic populations-without using the rare disease assumption. Our focus is on case-control studies of disease incidence ('incident case ...
Case-control studies are the most efficient design for rare diseases and require a much smaller study sample than cohort studies. Additionally, investigators can avoid the logistical challenges of following a large sample over time. Thus, case-control studies also allow more intensive
Case-Control Studies. Keeler, Courtney PhD; Curtis, Alexa Colgrove PhD, MPH, FNP, PMHNP. Author Information . Courtney Keeler is an associate professor and Alexa Colgrove Curtis is assistant dean of graduate nursing and director of the MPH-DNP dual degree program, both at the University of San Francisco School of Nursing and Health Professions.
In a case-control study, a number of cases and noncases (controls) are identified, and the occurrence of one or more prior exposures is compared between groups to evaluate drug-outcome associations ( Figure 1 ). A case-control study runs in reverse relative to a cohort study. 21 As such, study inception occurs when a patient experiences ...
Findings In this nested case-control study comprising 42 190 cases with major bleeding matched to 1 156 641 controls, concomitant SSRI and OAC use was associated with a 33% increased risk of major bleeding compared with OAC use alone; this risk was highest in the first few months of concomitant use and was substantially lower after 6 months.
On the other hand, most cancer case-control studies have assessed the lifetime occupational histories of participants, including collection of detailed night-shift work metrics (e.g., type, duration, intensity), and tend to show positive associations. In this commentary, we debate why cohort studies with weak exposure assessment and other ...
Genome-wide association studies (GWAS) is a useful tool for the detection of disease or quantitative trait-related genetic variations in the veterinary field. For a binary trait, a case/control experiment is designed in GWAS. However, there is limited information on the optimal case/control and sample size in GWAS. Objectives
An unmatched case-control study was conducted to describe the measles outbreak and to determine risk factors for measles infection. Source and study population. All populations of Tocha district were the source population. People in Tocha district who fulfilled the standard measles case definition and lived in selected kebeles were considered ...
ABSTRACT. Background: In the present study, we explored the association between major dietary patterns, odds, and severity of anxiety disorders, which has not been clarified to date. Methods: This case-control study was conducted on 85 patients who were group-matched by gender with 170 healthy subjects. Dietary intakes were evaluated applying a 147-item validated food frequency questionnaire ...
Cohort studies and case-control studies are two primary types of observational studies that aid in evaluating associations between diseases and exposures. In this review article, we describe these study designs, methodological issues, and provide examples from the plastic surgery literature. Keywords: observational studies, case-control study ...
Chaos has gutted Port-au-Prince and Haiti's government, a crisis brought on by decades of political disruption, a series of natural disasters and a power vacuum left by the president's assassination.
Introduction. Clinicians think of case-control study when they want to ascertain association between one clinical condition and an exposure or when a researcher wants to compare patients with disease exposed to the risk factors to non-exposed control group. In other words, case-control study compares subjects who have disease or outcome (cases ...
A former assistant principal at a Virginia elementary school has been charged with felony child neglect more than a year after a 6-year-old boy brought a gun to class and shot a teacher.
Background: Genome-wide association studies (GWAS) is a useful tool for the detection of disease or quantitative trait-related genetic variations in the veterinary field. For a binary trait, a case/control experiment is designed in GWAS. However, there is limited information on the optimal case/control and sample size in GWAS.