

Choose Your Test
Sat / act prep online guides and tips, what is dynamic equilibrium definition and examples.
General Education

Dynamic equilibrium is an important concept in chemistry. But what is dynamic equilibrium exactly? How can something be dynamic but also at equilibrium? Keep reading to learn the best dynamic equilibrium definition, common dynamic equilibrium examples, and how dynamic and static equilibrium may look the same but are in fact very different.
What Is Dynamic Equilibrium?
Chemical reactions can either go in both directions (forward and reverse) or only in one direction. The ones that go in two directions are known as reversible reactions, and you can identify them by the arrows going in two directions, like the example below.
H2O(l) ⇌ H + (aq) + OH - (aq)
Dynamic equilibrium only occurs in reversible reactions, and it’s when the rate of the forward reaction is equal to the rate of the reverse reaction. These equations are dynamic because the forward and reverse reactions are still occurring, but the two rates are equal and unchanging, so they’re also at equilibrium.
Dynamic equilibrium is an example of a system in a steady state. This means the variables in the equation are unchanging over time (since the rates of reaction are equal). If you look at a reaction in dynamic equilibrium, it’ll look like nothing is happening since the concentrations of each substance stay constant. However, reactions are actually continuously occurring.
Dynamic equilibrium doesn't just occur in chemistry labs though; you've witnessed an dynamic equilibrium example every time you've had a soda. In a sealed bottle of soda, carbon dioxide is present in both the liquid/aqueous phase and the gaseous phase (bubbles). The two phases of carbon dioxide are in dynamic equilibrium inside the sealed soda bottle since the gaseous carbon dioxide is dissolving into the liquid form at the same rate that the liquid form of carbon dioxide is being converted back to its gaseous form.
The equation looks like this: C O 2 (g) ⇌ C O 2 (aq).
Changing the temperature, pressure, or concentration of a reaction can shift the equilibrium of an equation and knock it out of dynamic equilibrium. This is why, if you open a soda can and leave it out for a long time, eventually it'll become "flat" and there will be no more bubbles. This is because the soda can is no longer a closed system and the carbon dioxide can interact with the atmosphere. This moves it out of dynamic equilibrium and releases the gaseous form of carbon dioxide until there are no more bubbles.
Dynamic Equilibrium Examples
Any reaction will be in dynamic equilibrium if it’s reversible and the rates of the forward and reverse reactions are equal. For example, say that you prepare a solution that is saturated with an aqueous solution of NaCl. If you then add solid crystals of NaCl, the NaCl will be simultaneously dissolving and recrystallizing within the solution. The reaction, NaCl(s) ⇌ Na + (aq) + Cl - (aq), will be in dynamic equilibrium when the rate of the dissolution of the NaCl equals the rate of recrystallization.
Another example of dynamic equilibrium is NO 2 (g) + CO(g) ⇌ NO(g) + C O 2 (g) (again, as long as the two rates are equal). Nitrogen dioxide ( NO 2 ) reacts with carbon monoxide (CO) to form nitrogen oxide (NO) and carbon dioxide (C O 2 ), and, in the reverse reaction, nitrogen oxide and carbon dioxide react to form nitrogen dioxide and carbon monoxide.
If you’re observing a reaction, you can tell it’s not at dynamic equilibrium if you can see changes occurring in the amounts of reactants or products. (If you can’t see any changes, that doesn’t guarantee it’s at dynamic equilibrium, since it may be at static equilibrium or the changes may be too small to see with the naked eye.)
An example of an equation that could never be at dynamic equilibrium is: 4 Fe(s) + 6 H 2 O(l) + 3O 2 (g) → 4 Fe( OH ) 3 (s). This is an equation for the formation of rust. We can see that it’ll never be in dynamic equilibrium because the arrow for the reaction only goes one way (which is why a rusty car won’t become shiny again on its own).

There's no dynamic equilibrium for this car!
Dynamic Equilibrium vs Static Equilibrium
If you observe reactions at dynamic equilibrium and reactions at static equilibrium, neither will have visible changes occurring, and it'll look like nothing is happening. However, reactions at static equilibrium are actually very different from those at dynamic equilibrium.
Static equilibrium (also known as mechanical equilibrium) is when the reaction has stopped and there is no movement at all between the reactants and products. The reaction is complete and the forward and reverse reaction rates are both 0.
While reactions at dynamic equilibrium are reversible (can proceed in either direction), those at static equilibrium are irreversible and can only proceed in one direction. However, both dynamic equilibrium and static equilibrium are examples of systems at steady state, in which the net force action on the systems is zero.
Below is a chart showing the key differences between dynamic and static equilibrium.
How Does Dynamic Equilibrium Relate to Rate Constants?
When a reaction is at dynamic equilibrium, the reaction will have a specific rate constant, known as the equilibrium constant, or K eq .
The equilibrium constant, or rate constant, is a coefficient that shows the reaction quotient (or the relative amounts of products and reactants in the reaction at a given point in time) when the reaction is at equilibrium. The value of the equilibrium constant will tell you the relative amounts of product and reactant at equilibrium.
If K eq is >1000, at equilibrium there will be mostly product.
If K eq is between .001 and 1000, at equilibrium there will be a significant amount of both product and reactant.
If K eq is <.001, at equilibrium there will be mostly reactant.
For the reaction a A + b B⇌ c C+ d D, A and B represent the reactants and C and D represent the products.
The equation for the equilibrium constant is K eq =[C] c [D] d /[A] a [B] b .
Take the reaction N 2 (g)+O 2 (g)⇋2NO(g).
Using the equation for the equilibrium constant, K eq is equal to [NO] 2 /[N 2 ][O 2 ]. You would either leave the equation like this, or, if you're given equilibrium concentrations/the equilibrium constant, you can plug those in to find any missing values.
Say we know the concentrations of both [ N 2 ] and [ O 2 ]=.15 M and the concentration of [NO] is 1.1 M.
Plugging in those values would give you: K eq = ( 1.1) 2 /(.15)(.15) or 1.21/.0225.
You can solve and find that K eq = 53.8.
Since K eq is between .001 and 1000, there will be a significant amount each of NO, O 2 , and N 2 at equilibrium.

Summary: What Is Dynamic Equilibrium?
What is the best dynamic equilibrium definition? Dynamic equilibrium occurs when, for a reversible reaction, the rate of the forward reaction equals the rate of the reverse reaction. Since the two rates are equal, it looks like nothing is happening, but in reality the reaction is continuously occurring at its stable rate.
In contrast, reactions at stable equilibrium are complete and no further reaction is occurring.
The equation for the equilibrium constant is K eq =[C] c [D] d /[A] a [B] b .
What's Next?
Writing a research paper for school but not sure what to write about? Our guide to research paper topics has over 100 topics in ten categories so you can be sure to find the perfect topic for you.
Want to know the fastest and easiest ways to convert between Fahrenheit and Celsius? We've got you covered! Check out our guide to the best ways to convert Celsius to Fahrenheit (or vice versa).
Are you studying clouds in your science class? Get help identifying the different types of clouds with our expert guide.

Christine graduated from Michigan State University with degrees in Environmental Biology and Geography and received her Master's from Duke University. In high school she scored in the 99th percentile on the SAT and was named a National Merit Finalist. She has taught English and biology in several countries.
Student and Parent Forum
Our new student and parent forum, at ExpertHub.PrepScholar.com , allow you to interact with your peers and the PrepScholar staff. See how other students and parents are navigating high school, college, and the college admissions process. Ask questions; get answers.

Ask a Question Below
Have any questions about this article or other topics? Ask below and we'll reply!
Improve With Our Famous Guides
- For All Students
The 5 Strategies You Must Be Using to Improve 160+ SAT Points
How to Get a Perfect 1600, by a Perfect Scorer
Series: How to Get 800 on Each SAT Section:
Score 800 on SAT Math
Score 800 on SAT Reading
Score 800 on SAT Writing
Series: How to Get to 600 on Each SAT Section:
Score 600 on SAT Math
Score 600 on SAT Reading
Score 600 on SAT Writing
Free Complete Official SAT Practice Tests
What SAT Target Score Should You Be Aiming For?
15 Strategies to Improve Your SAT Essay
The 5 Strategies You Must Be Using to Improve 4+ ACT Points
How to Get a Perfect 36 ACT, by a Perfect Scorer
Series: How to Get 36 on Each ACT Section:
36 on ACT English
36 on ACT Math
36 on ACT Reading
36 on ACT Science
Series: How to Get to 24 on Each ACT Section:
24 on ACT English
24 on ACT Math
24 on ACT Reading
24 on ACT Science
What ACT target score should you be aiming for?
ACT Vocabulary You Must Know
ACT Writing: 15 Tips to Raise Your Essay Score
How to Get Into Harvard and the Ivy League
How to Get a Perfect 4.0 GPA
How to Write an Amazing College Essay
What Exactly Are Colleges Looking For?
Is the ACT easier than the SAT? A Comprehensive Guide
Should you retake your SAT or ACT?
When should you take the SAT or ACT?
Stay Informed
Get the latest articles and test prep tips!
Looking for Graduate School Test Prep?
Check out our top-rated graduate blogs here:
GRE Online Prep Blog
GMAT Online Prep Blog
TOEFL Online Prep Blog
Holly R. "I am absolutely overjoyed and cannot thank you enough for helping me!”
- Study Guides
- Equilibrium Analysis
- Economic Policy
- Economic Analysis
- Economics Defined
- Macroeconomics
- Microeconomics
- Nominal GDP, Real GDP, and Price Level
- Unemployment Rate
- Aggregate Demand (AD) Curve
- Aggregate Supply (AS) Curve
- Combining AD and AS Supply Curves
- The Classical Theory
- The Keynesian Theory
- Supply of Money
- Definition of Money
- Functions of Money
- The Demand for Money
- Fiscal Policy
- Monetary Policy
- Consumer Equilibrium
- Consumer Equilibrium Changes in Prices
- Individual Demand Market Demand
- Consumer Surplus
- Utility and Preferences
- Production Costs and Firm Profits
- Long‐Run Costs
- Production of Goods
- Long-Run Supply
- Conditions for Perfect Competition
- Demand in a Perfectly Competitive Market
- Short-Run Supply
- Monopoly in the Long-Run
- Costs of Monopoly
- Conditions for Monopoly
- Demand in a Monopolistic Market
- Profit Maximization
- Monopolistic Competition in the Long-run
- Conditions for an Oligopolistic Market
- Kinked-Demand Theory of Oligopoly
- Cartel Theory of Oligopoly
- Monopolists: Profit Maximization
- Equilibrium in a Perfectly Competitive Market
- Labor Demand and Supply in a Monopsony
- Equilibrium in a Monopsony Market
- Labor Demand and Supply in a Perfectly Competitive Market
- Capital, Loanable Funds, Interest Rate
- Present Value and Investment Decisions
- Measures of Capital
The algebraic approach to equilibrium . The algebraic approach to equilibrium analysis is to solve, simultaneously, the algebraic equations for demand and supply. In the example given above, the demand equation for good X was
To solve simultaneously, one first rewrites either the demand or the supply equation as a function of price. In the example above, the supply curve may be rewritten as follows:
Substituting this expression into the demand equation, one can solve for the equilibrium price:
The equilibrium price of good X is found to be $2. Substituting the equilibrium price of 2 into the rewritten supply equation for good X , one has:
The equilibrium quantity is found to be 4 units of good X .
A graphical depiction of equilibrium . The graphical approach to equilibrium analysis is illustrated in Figure . The equilibrium price and quantity are determined by the intersection of the two curves. The equilibrium quantity is 4 units of good X , and the equilibrium price is $2 per unit of good X . This result is the same as the one obtained by simultaneously solving the algebraic equations for demand and supply.
A price of $2 and a quantity of 4 units of X are the equilibrium price and quantity only when the demand and supply for good X are exactly as depicted in Figure . If either the demand curve or the supply curve shifts, the equilibrium price and quantity change. Examples of shifts in the demand and supply curves and the resultant changes in equilibrium are illustrated in Figures (a) and (b). In Figure (a), a shift to left of the demand curve, from D A to D B , leads to a decrease in both the equilibrium price and quantity of good X , while a shift to the right of the demand curve, from D A to D C , leads to an increase in both the equilibrium price and quantity of good X , assuming supply is held constant‐the ceteris paribus assumption. In Figure (b), a shift to the left of the supply curve, from S A to S B , leads to an increase in the equilibrium price of good X but a decrease in the equilibrium quantity of good X , assuming demand is held constant. A shift to the right of the supply curve, from S A to S C , leads to a decrease in the equilibrium price of good X but an increase in the equilibrium quantity of good X , again assuming that demand is held constant.
Previous Microeconomics
Next Elasticity

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

3.6: Equilibrium Analysis for a Rigid Body
- Last updated
- Save as PDF
- Page ID 51709

- Jacob Moore & Contributors
- Pennsylvania State University Mont Alto via Mechanics Map
For an rigid body in static equilibrium—that is, a non-deformable body where forces are not concurrent—the sum of both the forces and the moments acting on the body must be equal to zero. The addition of moments (as opposed to particles, where we only looked at the forces) adds another set of possible equilibrium equations, allowing us to solve for more unknowns as compared to particle problems.
Moments, like forces, are vectors. This means that our vector equation needs to be broken down into scalar components before we can solve the equilibrium equations. In a two-dimensional problem, the body can only have clockwise or counterclockwise rotation (corresponding to rotations about the \(z\) axis). This means that a rigid body in a two-dimensional problem has three possible equilibrium equations; that is, the sum of force components in the \(x\) and \(y\) directions, and the moments about the \(z\) axis. The sum of each of these will be equal to zero.
For a two-dimensional problem, we break our one vector force equation into two scalar component equations. \[ \sum \vec{F} \, = \, 0 \]
\[ \sum F_x \, = \, 0\, ; \,\, \sum F_y \, = \, 0 \] The one moment vector equation becomes a single moment scalar equation. \[ \sum \vec{M} \, = \, 0 \]
\[ \sum M_z \, = \, 0 \]
If we look at a three-dimensional problem we will increase the number of possible equilibrium equations to six. There are three equilibrium equations for force, where the sum of the components in the \(x\), \(y\), and \(z\) directions must be equal to zero. The body may also have moments about each of the three axes. The second set of three equilibrium equations states that the sum of the moment components about the \(x\), \(y\), and \(z\) axes must also be equal to zero.
We break the forces into three component equations. \[ \sum \vec{F} \, = \, 0 \]
\[ \sum F_x \, = \, 0 \, ; \,\, \sum F_y \, = \, 0 \, ; \,\, \sum F_z \, = \, 0 \]
Then we also break the moments into three component equations. \[ \sum \vec{M} \, = \, 0 \]
\[ \sum M_x \, = \, 0 \, ; \,\, \sum M_y \, = \, 0 \, ; \,\, \sum M_z \, = \, 0 \]
Finding the Equilibrium Equations
As with particles, the first step in finding the equilibrium equations is to draw a free body diagram of the body being analyzed. This diagram should show all the force vectors acting on the body. In the free body diagram, provide values for any of the known magnitudes, directions, and points of application for the force vectors and provide variable names for any unknowns (either magnitudes, directions, or distances).
Next you will need to choose the \(x\), \(y\), and \(z\) axes. These axes do need to be perpendicular to one another, but they do not necessarily have to be horizontal or vertical. If you choose coordinate axes that line up with some of your force vectors you will simplify later analysis.
Once you have chosen axes, you need to break down all of the force vectors into components along the \(x\), \(y\) and \(z\) directions (see the vectors page in Appendix 1 page for more details on this process). Your first equation will be the sum of the magnitudes of the components in the \(x\) direction being equal to zero, the second equation will be the sum of the magnitudes of the components in the \(y\) direction being equal to zero, and the third (if you have a 3D problem) will be the sum of the magnitudes in the \(z\) direction being equal to zero.
Next you will need to come up with the the moment equations. To do this you will need to choose a point to take the moments about. Any point should work, but it is usually advantageous to choose a point that will decrease the number of unknowns in the equation. Remember that any force vector that travels through a given point will exert no moment about that point. To write out the moment equations, simply sum the moments exerted by each force (adding in pure moments shown in the diagram) about the given point and the given axis, and set that sum equal to zero. All moments will be about the \(z\) axis for two-dimensional problems, though moments can be about the \(x\), \(y\) and \(z\) axes for three-dimensional problems.
Once you have your equilibrium equations, you can solve these formulas for unknowns. The number of unknowns that you will be able to solve for will again be the number of equations that you have.
Example \(\PageIndex{1}\)
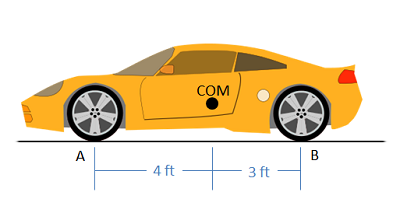
The car below has a weight of 1500 lbs with the center of mass 4 ft behind the front wheels of the car. What are the normal forces on the front and the back wheels of the car?
Example \(\PageIndex{2}\)
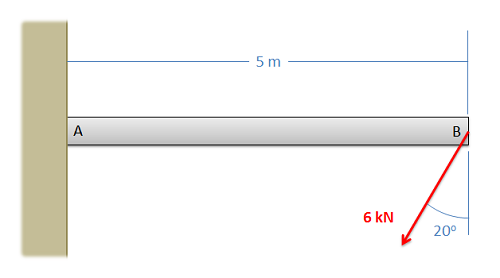
A 5-meter-long beam has a fixed connection to a wall at point A and a force acting as shown at point B. What are the reaction forces acting on the beam at point A?
Example \(\PageIndex{3}\)
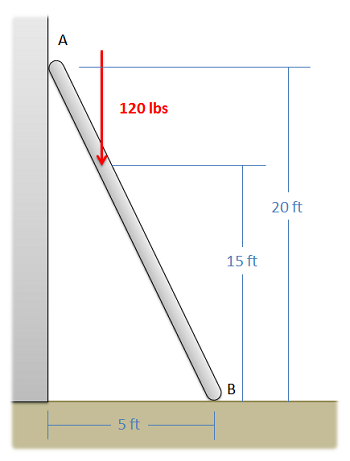
A ladder with negligible mass is supporting a 120-lb person as shown below. If the contact point at A is frictionless, and the contact point at B is a rough connection, determine the forces acting at contact points A and B.
Example \(\PageIndex{4}\)
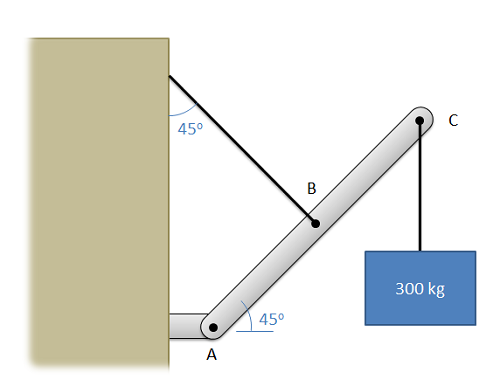
Member ABC is 6 meters long, with point B being at its midpoint. Determine all forces acting on member ABC.
Example \(\PageIndex{5}\)
While sitting in a chair, a person exerts the forces in the diagram below. Determine all forces acting on the chair at points A and B. (Assume A is frictionless and B is a rough surface).
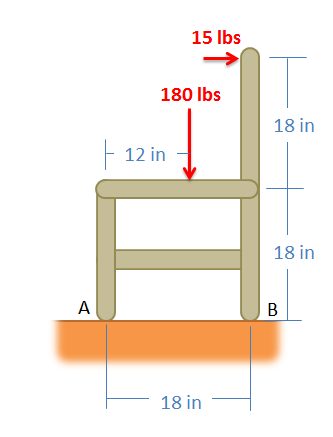
Example \(\PageIndex{6}\)
The trailer shown below consists of a deck with a weight of 250 lbs on an axle with wheels with a weight of 350 lbs. Assume the weight forces act in the center of each component. If we wish the tongue weight (\(F_T\)) of the unloaded trailer to be 50 lbs, what is the distance \(d\) from the front where we must place the axle?
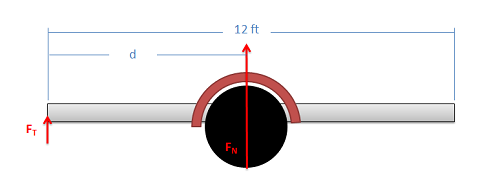
Example \(\PageIndex{7}\)
A 12-inch-by-24-inch flat steel sign is supported by two cables, each 6 inches from the edge of the sign. The sign has a weight of 10 lbs, and the wind is causing the sign to sit at an angle of 10 degrees from vertical (the \(y\) axis). If we treat the wind as a point force acting in the negative \(z\) direction on the center of the sign, how strong must the wind force be to cause this ten-degree angle?
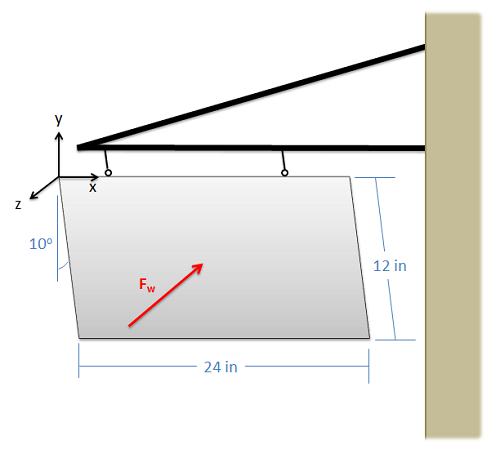
Example \(\PageIndex{8}\)
A sixty-kilogram acoustic panel is suspended by three cables as shown below. Assuming the panel has a uniformly distributed weight, what is the tension in each of the cables?
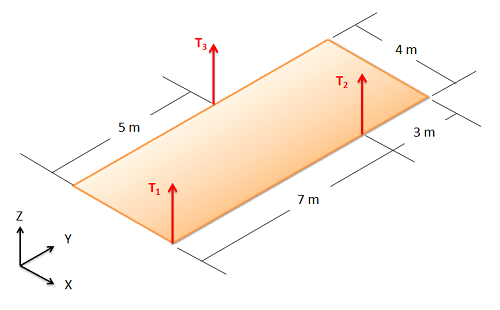
If you're seeing this message, it means we're having trouble loading external resources on our website.
If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked.
To log in and use all the features of Khan Academy, please enable JavaScript in your browser.
AP®︎/College Macroeconomics
Course: ap®︎/college macroeconomics > unit 1.
- Market equilibrium
- Changes in market equilibrium
- Changes in equilibrium price and quantity when supply and demand change
Lesson summary: Market equilibrium, disequilibrium, and changes in equilibrium
- Market equilibrium and disequilibrium
Changes in equilibrium
Equilibrium, disequilibrium, key graphical models - the market model, changes in supply, changes in demand, changes in both demand and supply.
- Price is higher and quantity is higher
- Price is higher and quantity is lower
- Price is lower and quantity is higher
- Price is lower and quantity is lower
Common Misperceptions
- When showing an equilibrium price and quantity, it is important to clearly label these on the appropriate axis, not just the interior of the graph. Remember that the point on either axis represents the market price and the market quantity, not a point in the middle of the graph.
- When both supply and demand change at the same time, we will not be able to make a statement about what happens to both price and quantity, one of these will be uncertain.
Discussion Questions
- When both supply and demand increase at the same time, why can't we tell what will happen to the equilibrium price?
- Can you think of an example of a good in your own life for which there was a shortage?
- What happened to the price of that good?
- Using a correctly labeled graph, show the impact on equilibrium price and quantity in the market for pumpkin spiced lattes if the cost of producing them increases. Explain An increase in the cost of production causes a decrease in supply, and increase in equilibrium price, and a decrease in equilibrium quantity, as in Figure 5 . Figure 5: Pumpkin spiced lattes following an increase in production costs
Want to join the conversation?
- Upvote Button navigates to signup page
- Downvote Button navigates to signup page
- Flag Button navigates to signup page
- Search Search Please fill out this field.
What Is Equilibrium?
Understanding equilibrium, special considerations, equilibrium vs. disequilibrium.
- Equilibrium FAQs
- Guide to Microeconomics
Equilibrium Price: Definition, Types, Example, and How to Calculate
James Chen, CMT is an expert trader, investment adviser, and global market strategist.
:max_bytes(150000):strip_icc():format(webp)/photo__james_chen-5bfc26144cedfd0026c00af8.jpeg)
Equilibrium is the state in which market supply and demand balance each other, and as a result prices become stable. Generally, an over-supply of goods or services causes prices to go down, which results in higher demand—while an under-supply or shortage causes prices to go up resulting in less demand.
The balancing effect of supply and demand results in a state of equilibrium.
Key Takeaways
- A market is said to have reached equilibrium price when the supply of goods matches demand.
- A market in equilibrium demonstrates three characteristics: the behavior of agents is consistent, there are no incentives for agents to change behavior, and a dynamic process governs equilibrium outcomes.
- There are several types of equilibrium used in economics.
- Disequilibrium is the opposite of equilibrium and it is characterized by changes in conditions that affect market equilibrium.
- In reality, markets are never in perfect equilibrium, although prices do tend toward it.
Investopedia / Paige McLaughlin
The equilibrium price is where the supply of goods matches demand. When a major index experiences a period of consolidation or sideways momentum, it can be said that the forces of supply and demand are relatively equal and the market is in a state of equilibrium.
Economists find that prices tend to fluctuate around the equilibrium levels . If the price rises too high, market forces will incentivize sellers to come in and produce more. If the price is too low, additional buyers will bid up the price. These activities keep the equilibrium level in relative balance over time.
Economists like Adam Smith believed that a free market would tend toward equilibrium. For example, a dearth of any one good would create a higher price generally, which would reduce demand, leading to an increase in supply provided the right incentive. The same would occur in reverse order provided there was excess in any one market.
Modern economists point out that cartels or monopolistic companies can artificially hold prices higher and keep them there in order to reap higher profits. The diamond industry is a classic example of a market where demand is high, but supply is made artificially scarce by companies selling fewer diamonds in order to keep prices high.
As noted by Paul Samuelson in his 1983 work Foundations of Economic Analysis, the term equilibrium with respect to a market is not necessarily a good thing from a normative perspective, and making that value judgment could be a misstep.
Markets can be in equilibrium, but it may not mean that all is well. For example, the food markets in Ireland were at equilibrium during the great potato famine in the mid-1800s. Higher profits from selling to the British made it so the Irish and British market was at an equilibrium price that was higher than what consumers could pay, and consequently, many people starved.
When markets aren't in a state of equilibrium, they are said to be in disequilibrium . Disequilibrium can happen in a flash in a more stable market or can be a systematic characteristic of certain markets.
At times disequilibrium can spill over from one market to another—for instance, if there aren’t enough transport companies or resources available to ship coffee internationally then the coffee supply for certain regions could be reduced, affecting the equilibrium of coffee markets. Economists view many labor markets as being in disequilibrium due to how legislation and public policy protect people and their jobs, or the amount they are compensated for their labor.
Types of Equilibrium
Economic equilibrium.
Economic equilibrium refers broadly to any state in the economy where forces are balanced. This can be related to prices in a market where supply is equal to demand, but can also represent the level of employment, interest rates, and so on.
Competitive Equilbrium
The process by which equilibrium prices are reached is through a process of competition . Among sellers to be the low-cost producer to grab the largest market share, and also among buyers to snatch up the best deals.
General Equilibrium
General equilibrium considers the aggregation of forces occurring at the macro-economic level, and not the micro forces of individual markets. It is a cornerstone of Walrasian economics.
Underemployment Equilibrium
Economists have found that there is a level of persistent unemployment that is observed when there is general equilibrium in an economy. This is known as underemployment equilibrium , and is predicted by Keynesian economic theory .
Lindahl Equilibrium
Lindahl equilibrium is a special case where, in theory, the optimal amount of public goods is produced and the cost of public goods is fairly shared among everyone. It describes an ideal state rarely, if ever, achieved in reality, but is used to help craft tax policy and is an important concept in welfare economics .
Intertemporal Equilibrium
Because prices may swing above or below the equilibrium level due to proximate changes in supply or demand at a given moment, it is best to look at this effect over time, known as intertemporal equilibrium . The concept is also used in understanding how firms and households budget and smooth spending over longer time horizons.
Nash Equilibrium
In game theory , Nash equilibrium is a state of play whereby the optimal strategy involves considering the optimal strategy of the other player or opponent.
The prisoner's dilemma is a common situation in game theory that exemplifies the Nash equilibrium.
Example of Equilibrium
A store manufactures 1,000 spinning tops and retails them at $10 per piece. But no one is willing to buy them at that price. To pump up demand, the store reduces its price to $8. There are 250 buyers at that price point. In response, the store further slashes the retail cost to $5 and garners five hundred buyers in total. Upon further reduction of the price to $2, one thousand buyers of the spinning top materialize. At this price point, supply equals demand. Hence $2 is the equilibrium price for the spinning tops.
What Happens During Market Equilibrium?
When a market is in equilibrium, prices reflect an exact balance between buyers (demand) and sellers (supply). While elegant in theory, markets are rarely in equilibrium at a given moment. Rather, equilibrium should be thought of as a long-term average level.
How Do You Calculate Equilibrium Price?
In economics, the equilibrium price is calculated by setting the supply function and demand function equal to one another and solving for the price.
What Is Equilibrium Quantity?
The amount supplied that exactly equals demand is the equilibrium quantity . In such a case, there will neither be an oversupply nor a shortage.
Paul A. Samuelson. "Foundations of Economic Analysis." Harvard University Press, 1983.
:max_bytes(150000):strip_icc():format(webp)/sphere-balancing-on-the-edge-of-a-cube--3d-rendering-1023149598-1ce2e2961a144d51a388df2a4de9fe38.jpg)
- Terms of Service
- Editorial Policy
- Privacy Policy
- Your Privacy Choices
Equilibrium
What is the meaning of equilibrium.
An equilibrium represents a state in a process when the observable properties such as colour, temperature, pressure, concentration etc do not show any change.
The word equilibrium means ‘balance’ which indicates that a chemical reaction represents a balance between the reactants and products taking part in the reaction. The equilibrium state is also noticed in certain physical processes such as the melting point of ice at 0℃, both ice and water are present at equilibrium.
In the case of physical processes such as the melting of solid, dissolution of salt in water etc., the equilibrium is called physical equilibrium while the equilibrium associated with chemical reaction is known as a chemical equilibrium .
Table of Contents
Equilibrium in physical changes, equilibrium in chemical changes, characteristics of equilibrium states, related topics on chemical equilibrium, ionic equilibrium, related topic on ionic equilibrium.
This equilibrium is associated with the physical process. These are:
(i) Solid ⇋ Liquid equilibrium
eg. H 2 O(s) ⇋ H 2 O(l) rate of melting of ice = rate of freezing of ice
(ii) Liquid ⇋ Gas equilibrium
eg. H 2 O(l) ⇋ H 2 O(g)
(iii) Solid ⇋ Gas equilibrium
eg. I 2 (s) ⇋ I 2 (vapour)
Also read : Physical equilibrium
The chemical equilibrium in a reversible reaction is the state at which both forward and backward reactions occur at the same speed.
The stage of the reversible reaction at which the concentration of the reactants and products do not change with time is called the equilibrium state.
The state in which the measurable properties of the system such as pressure, density, colour or concentration do not undergo any further noticeable changes with time under a given set of conditions is said to be a state of equilibrium.

(i) Equilibrium state can only be achieved if a reversible reaction is carried out in closed space.
(ii) Chemical equilibrium at a given temperature is characterised by the constancy of certain properties such as pressure, concentration, density or colour.
(iii) At equilibrium each reactant and each product have a fixed concentration and it is independent of the fact whether we start the reaction with the reactants or with the products.
2HI ⇋ H 2 + I 2
H 2 + I 2 ⇋ 2HI

(iv) Equilibrium state attained in a lesser time by the use of a positive catalyst.
(v) It is dynamic in nature. However, the reaction seems to have come to standstill because the concentration of reactants and products do not change.
- The law of mass action
- Equilibrium constant
- Standard free energy change and equilibrium constant
- Le Chatelier Principle
Chemical reactions also take place in a solution in which generally ions participate. The substance which forms an ion in the solution is called the electrolyte. The equilibrium is present between the unionised molecules of a particular substance and the ion formed in the solution is known as ionic Equilibrium .
The ionic compounds are generally acids, bases and salts. Therefore the ionic equilibrium is present in them when dissolved in water and any other solvent. Since all of them are electrolytes, the equilibrium constant is related to the strength of these electrolytes i.e. the number of ions which they furnish in solution.
Strong and Weak electrolytes:
The strength of an electrolyte is expressed in terms of the degree of ionisation(α).
\(\begin{array}{l}Degree of ionization (\alpha) = \frac{No. of moles dissociated}{Total no. of moles} \end{array} \)
The electrolytes which are almost completely ionized are called strong electrolytes while those ionized to smaller extent are known as weak electrolytes .
- For strong electrolytes, α is equal to 1.
- For weak electrolytes, α is equal to less than 1.
There is no equilibrium in strong electrolytes because when it is dissolved in a solvent it ionises completely. But weak electrolytes are ionized to a small extent. Therefore there is an equilibrium between the unionized electrolytes and ions formed in the solution.
- CH 3 COOH + H 2 O ⇌ CH 3 COO – + H 3 O +
- NH 4 OH + H 2 O ⇌ NH 4 + + OH –
- Ostwald Dilution Law
- Theories of Acids and Bases
- pH of acid and base
- Salt Hydrolysis
- Buffer solutions
- Solubility Product
- Common ion effect

Frequently Asked Questions on Equilibrium
What is equilibrium and what is an example.
The state in which the measurable properties of the system such as pressure, density, colour or concentration do not undergo any further noticeable changes with time under a given set of conditions is said to be a state of equilibrium. Example: 2HI ⇋ H 2 + I 2
What are K P and K C in equilibrium?
Kp is the equilibrium constant used when partial pressures of the species are given and Kc is the equilibrium constant used when equilibrium concentrations are expressed in molarity.
Why is equilibrium important in chemistry?
When the quantities of reactants and products are stable – their ratio does not change a chemical reaction is in equilibrium. This suggests that the reaction has reached a point where the reactant and product amounts remain constant over time, since the forward and backward reactions are at the same rate.
What is the pH formula?
pH is described as the negative of the logarithm of the molar hydronium-ion concentration. pH formula is -log [H 3 O + ].
Leave a Comment Cancel reply
Your Mobile number and Email id will not be published. Required fields are marked *
Request OTP on Voice Call
Post My Comment
- Share Share
Register with BYJU'S & Download Free PDFs
Register with byju's & watch live videos.


- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

6.4: Equilibrium Constants for Chemical Reactions
- Last updated
- Save as PDF
- Page ID 220701

- David Harvey
- DePauw University
Several types of chemical reactions are important in analytical chemistry, either in preparing a sample for analysis or during the analysis. The most significant of these are precipitation reactions, acid–base reactions, complexation reactions, and oxidation–reduction reactions. In this section we review these reactions and their equilibrium constant expressions.
Another common name for an oxidation–reduction reaction is a redox reaction, where “red” is short for reduction and “ox” is short for oxidation.
Precipitation Reactions
In a precipitation reaction, two or more soluble species combine to form an insoluble precipitate . The most common precipitation reaction is a metathesis reaction in which two soluble ionic compounds exchange parts. For example, if we add a solution of lead nitrate, Pb(NO 3 ) 2 , to a solution of potassium chloride, KCl, a precipitate of lead chloride, PbCl 2 , forms. We usually write a precipitation reaction as a net ionic equation, which shows only the precipitate and those ions that form the precipitate; thus, the precipitation reaction for PbCl 2 is
\[\mathrm{Pb}^{2+}(a q)+2 \mathrm{Cl}^{-}(a q) \rightleftharpoons \mathrm{PbCl}_{2}(s) \nonumber\]
When we write the equilibrium constant for a precipitation reaction, we focus on the precipitate’s solubility; thus, for PbCl 2 , the solubility reaction is
\[\mathrm{PbCl}_{2}(s)\rightleftharpoons \mathrm{Pb}^{2+}(a q)+2 \mathrm{Cl}^{-}(a q) \nonumber\]
and its equilibrium constant, or solubility product , K sp , is
\[K_{\mathrm{sp}}=\left[\mathrm{Pb}^{2+}\right]\left[\mathrm{Cl}^{-}\right]^{2} \label{6.1}\]
Even though it does not appear in the K sp expression, it is important to remember that equation \ref{6.1} is valid only if PbCl 2 (s) is present and in equilibrium with Pb 2 + and Cl – . You will find values for selected solubility products in Appendix 10 .
Acid–Base Reactions
A useful definition of acids and bases is that independently introduced in 1923 by Johannes Brønsted and Thomas Lowry. In the Brønsted‐Lowry definition, an acid is a proton donor and a base is a proton acceptor. Note the connection between these definitions—defining a base as a proton acceptor implies there is an acid available to donate the proton. For example, in reaction \ref{6.2} acetic acid, CH 3 COOH, donates a proton to ammonia, NH 3 , which serves as the base.
\[\mathrm{CH}_{3} \mathrm{COOH}(aq)+\mathrm{NH}_{3}(aq) \rightleftharpoons \mathrm{NH}_{4}^{+}(aq)+\mathrm{CH}_{3} \mathrm{COO}^{-}(aq) \label{6.2}\]
When an acid and a base react, the products are a new acid and a new base. For example, the acetate ion, CH 3 COO – , in reaction \ref{6.2} is a base that can accept a proton from the acidic ammonium ion, \(\text{NH}_4^+\), forming acetic acid and ammonia. We call the acetate ion the conjugate base of acetic acid, and we call the ammonium ion the conjugate acid of ammonia.
Strong and Weak Acids
The reaction of an acid with its solvent (typically water) is an acid dissociation reaction. We divide acids into two categories—strong and weak—based on their ability to donate a proton to the solvent. A strong acid, such as HCl, almost completely transfers its proton to the solvent, which acts as the base.
\[\mathrm{HCl}(a q)+\mathrm{H}_{2} \mathrm{O}(l) \rightarrow \mathrm{H}_{3} \mathrm{O}^{+}(a q)+\mathrm{Cl}^{-}(a q) \nonumber\]
We use a single arrow (\(\rightarrow\)) in place of the equilibrium arrow (\(\rightleftharpoons\)) because we treat HCl as if it dissociates completely in an aqueous solution. In water, the common strong acids are hydrochloric acid (HCl), hydroiodic acid (HI), hydrobromic acid (HBr), nitric acid (HNO 3 ), perchloric acid (HClO 4 ), and the first proton of sulfuric acid (H 2 SO 4 ).
The strength of an acid is a function of the acid and the solvent. For example, HCl does not act as a strong acid in methanol. In this case we use the equilibrium arrow when writing the acid–base reaction.
\[\mathrm{HCl}+\mathrm{CH}_{3} \mathrm{OH}\rightleftharpoons \mathrm{CH}_{3} \mathrm{OH}_{2}^{+}+\mathrm{Cl}^{-} \nonumber\]
A weak acid, of which aqueous acetic acid is one example, does not completely donate its acidic proton to the solvent. Instead, most of the acid remains undissociated with only a small fraction present as the conjugate base.
\[\mathrm{CH}_{3} \mathrm{COOH}(a q)+\mathrm{H}_{2} \mathrm{O}(l)\rightleftharpoons \mathrm{H}_{3} \mathrm{O}^{+}(a q)+\mathrm{CH}_{3} \mathrm{COO}^{-}(a q) \nonumber\]
The equilibrium constant for this reaction is an acid dissociation constant , K a , which we write as
\[K_{a}=\frac{\left[\mathrm{CH}_{3} \mathrm{COO}^{-}\right]\left[\mathrm{H}_{3} \mathrm{O}^{+}\right]}{\left[\mathrm{CH}_{3} \mathrm{COOH}\right]}=1.75 \times 10^{-5} \nonumber\]
The magnitude of K provides information about a weak acid's relative strength, with a smaller K a corresponding to a weaker acid. The ammonium ion, \(\text{NH}_4^+\), for example, has a K a of \(5.702 \times 10^{-10}\) and is a weaker acid than acetic acid.
Earlier we noted that we omit pure solids and pure liquids from equilibrium constant expressions. Because the solvent, H 2 O, is not pure, you might wonder why we have not included it in acetic acid’s K a expression. Recall that we divide each term in an equilibrium constant expression by its standard state value. Because the concentration of H 2 O is so large—it is approximately 55.5 mol/L—its concentration as a pure liquid and as a solvent are virtually identical. The ratio
\[\frac{\left[\mathrm{H}_{2} \mathrm{O}\right]}{\left[\mathrm{H}_{2} \mathrm{O}\right]^{\circ}} \nonumber\]
is essentially 1.00.
A monoprotic weak acid, such as acetic acid, has only a single acidic proton and a single acid dissociation constant. Other acids, such as phosphoric acid, have multiple acidic protons, each characterized by an acid dissociation constant. We call such acids polyprotic . Phosphoric acid, for example, has three acid dissociation reactions and three acid dissociation constants.
\[\mathrm{H}_{3} \mathrm{PO}_{4}(a q)+\mathrm{H}_{2} \mathrm{O}(l) \rightleftharpoons \mathrm{H}_{3} \mathrm{O}^{+}(a q)+\mathrm{H}_{2} \mathrm{PO}_{4}^{-}(a q) \nonumber\]
\[K_{\mathrm{al}}=\frac{\left[\mathrm{H}_{2} \mathrm{PO}_{4}^{-}\right]\left[\mathrm{H}_{3} \mathrm{O}^{+}\right]}{\left[\mathrm{H}_{3} \mathrm{PO}_{4}\right]}=7.11 \times 10^{-3} \nonumber\]
\[\mathrm{H}_{2} \mathrm{PO}_{4}^-(a q)+\mathrm{H}_{2} \mathrm{O}(l)\rightleftharpoons \mathrm{H}_{3} \mathrm{O}^{+}(a q)+\mathrm{HPO}_{4}^{2-}(a q) \nonumber\]
\[K_{a 2}=\frac{\left[\mathrm{HPO}_{4}^{2-}\right]\left[\mathrm{H}_{3} \mathrm{O}^{+}\right]}{\left[\mathrm{H}_{2} \mathrm{PO}_{4}^-\right]}=6.32 \times 10^{-8} \nonumber\]
\[\mathrm{HPO}_{4}^{2-}(a q)+\mathrm{H}_{2} \mathrm{O}({l})\rightleftharpoons \mathrm{H}_{3} \mathrm{O}^{+}(a q)+\mathrm{PO}_{4}^{3-}(a q) \nonumber\]
\[K_{\mathrm{a} 3}=\frac{\left[\mathrm{PO}_{4}^{3-}\right]\left[\mathrm{H}_{3} \mathrm{O}^{+}\right]}{\left[\mathrm{HPO}_{4}^{2-}\right]}=4.5 \times 10^{-13} \nonumber\]
The decrease in the acid dissociation constants from K a1 to K a3 tells us that each successive proton is harder to remove. Consequently, H 3 PO 4 is a stronger acid than \(\text{H}_2\text{PO}_4^-\), and \(\text{H}_2\text{PO}_4^-\) is a stronger acid than \(\text{HPO}_4^{2-}\).
Strong and Weak Bases
The most common example of a strong base is an alkali metal hydroxide, such as sodium hydroxide, NaOH, which completely dissociates to produce hydroxide ion.
\[\mathrm{NaOH}(s) \rightarrow \mathrm{Na}^{+}(a q)+\mathrm{OH}^{-}(a q) \nonumber\]
A weak base, such as the acetate ion, CH 3 COO – , only partially accepts a proton from the solvent, and is characterized by a base dissociation constant , K b . For example, the base dissociation reaction and the base dissociation constant for the acetate ion are
\[\mathrm{CH}_{3} \mathrm{COO}^{-}(a q)+\mathrm{H}_{2} \mathrm{O}(l)\rightleftharpoons \mathrm{OH}^{-}(a q)+\mathrm{CH}_{3} \mathrm{COOH}(a q) \nonumber\]
\[K_{\mathrm{b}}=\frac{\left[\mathrm{CH}_{3} \mathrm{COOH}\right]\left[\mathrm{OH}^{-}\right]}{\left[\mathrm{CH}_{3} \mathrm{COO}^{-}\right]}=5.71 \times 10^{-10} \nonumber\]
A polyprotic weak base, like a polyprotic acid, has more than one base dissociation reaction and more than one base dissociation constant.
Amphiprotic Species
Some species can behave as either a weak acid or as a weak base. For example, the following two reactions show the chemical reactivity of the bicarbonate ion, \(\text{HCO}_3^-\), in water.
\[\mathrm{HCO}_{3}^{-}(a q)+\mathrm{H}_{2} \mathrm{O}(l)\rightleftharpoons \mathrm{H}_{3} \mathrm{O}^{+}(a q)+\mathrm{CO}_{3}^{2-}(a q) \label{6.3}\]
\[\mathrm{HCO}_{3}^{-}(a q)+\mathrm{H}_{2} \mathrm{O}(l)\rightleftharpoons \mathrm{OH}^{-}(a q)+\mathrm{H}_{2} \mathrm{CO}_{3}(a q) \label{6.4}\]
A species that is both a proton donor and a proton acceptor is called amphiprotic . Whether an amphiprotic species behaves as an acid or as a base depends on the equilibrium constants for the competing reactions. For bicarbonate, the acid dissociation constant for reaction \ref{6.3}
\[K_{a 2}=\frac{\left[\mathrm{CO}_{3}^{2-}\right]\left[\mathrm{H}_{3} \mathrm{O}^{+}\right]}{\left[\mathrm{HCO}_{3}^{-}\right]}=4.69 \times 10^{-11} \nonumber\]
is smaller than the base dissociation constant for reaction \ref{6.4}.
\[K_{\mathrm{b} 2}=\frac{\left[\mathrm{H}_{2} \mathrm{CO}_{3}\right]\left[\mathrm{OH}^{-}\right]}{\left[\mathrm{HCO}_{3}^{-}\right]}=2.25 \times 10^{-8} \nonumber\]
Because bicarbonate is a stronger base than it is an acid, we expect that an aqueous solution of \(\text{HCO}_3^-\) is basic.
Dissociation of Water
Water is an amphiprotic solvent because it can serve as an acid or as a base. An interesting feature of an amphiprotic solvent is that it is capable of reacting with itself in an acid–base reaction.
\[2 \mathrm{H}_{2} \mathrm{O}(l)\rightleftharpoons \mathrm{H}_{3} \mathrm{O}^{+}(a q)+\mathrm{OH}^{-}(a q) \label{6.5}\]
We identify the equilibrium constant for this reaction as water’s dissociation constant, K w ,
\[K_{w}=\left[\mathrm{H}_{3} \mathrm{O}^{+}\right]\left[\mathrm{OH}^{-}\right]=1.00 \times 10^{-14} \label{6.6}\]
at a temperature of 24 o C. The value of K w varies substantially with temperature. For example, at 20 o C K w is \(6.809 \times 10^{-15}\), while at 30 o C K w is \(1.469 \times 10^{-14}\). At 25 o C, K w is \(1.008 \times 10^{-14}\), which is sufficiently close to \(1.00 \times 10^{-14}\) that we can use the latter value with negligible error.
An important consequence of equation \ref{6.6} is that the concentration of H 3 O + and the concentration of OH – are related. If we know [H 3 O + ] for a solution, then we can calculate [OH – ] using equation \ref{6.6}.
Example \(\PageIndex{1}\)
What is the [OH – ] if the [H 3 O + ] is \(6.12 \times 10^{-5}\) M?
\[\left[\mathrm{OH}^{-}\right]=\frac{K_{w}}{\left[\mathrm{H}_{3} \mathrm{O}^{+}\right]}=\frac{1.00 \times 10^{-14}}{6.12 \times 10^{-5}}=1.63 \times 10^{-10} \nonumber\]
The pH Scale
Equation \ref{6.6} allows us to develop a pH scale (\(\text{pH} = - \log [\text{H}_3\text{O}^+]\)) that indicates a solution’s acidity. When the concentrations of H 3 O + and OH – are equal a solution is neither acidic nor basic; that is, the solution is neutral. Letting
\[\left[\mathrm{H}_{3} \mathrm{O}^{+}\right]=\left[\mathrm{OH}^{-}\right] \nonumber\]
substituting into equation \ref{6.6}
\[K_{w}=\left[\mathrm{H}_{3} \mathrm{O}^{+}\right]^{2}=1.00 \times 10^{-14} \nonumber\]
and solving for [H 3 O + ] gives
\[\left[\mathrm{H}_{3} \mathrm{O}^{+}\right]=\sqrt{1.00 \times 10^{-14}}=1.00 \times 10^{-7} \nonumber\]
A neutral solution of water at 25 o C has a hydronium ion concentration of \(1.00 \times 10^{-7}\) M and a pH of 7.00. In an acidic solution the concentration of H 3 O + is greater than that for OH – , which means that
\[\left[\mathrm{H}_{3} \mathrm{O}^{+}\right]>1.00 \times 10^{-7} \mathrm{M} \nonumber\]
The pH of an acidic solution, therefore, is less than 7.00. A basic solution, on the other hand, has a pH greater than 7.00. Figure \(\PageIndex{1}\) shows the pH scale and pH values for some representative solutions.
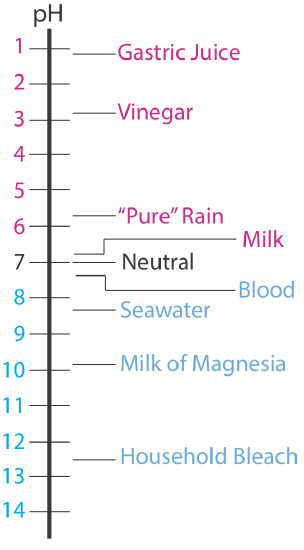
Tabulating Values for K a and K b
A useful observation about weak acids and weak bases is that the strength of a weak base is inversely proportional to the strength of its conjugate weak acid. Consider, for example, the dissociation reactions of acetic acid and acetate.
\[\mathrm{CH}_{3} \mathrm{COOH}(a q)+\mathrm{H}_{2} \mathrm{O}(l)\rightleftharpoons \ \mathrm{H}_{3} \mathrm{O}^{+}(a q)+\mathrm{CH}_{3} \mathrm{COO}^{-}(a q) \label{6.7}\]
\[\mathrm{CH}_{3} \mathrm{COO}^{-}(a q)+\mathrm{H}_{2} \mathrm{O}(l)\rightleftharpoons \mathrm{OH}^{-}(a q)+\mathrm{CH}_{3} \mathrm{COOH}(a q) \label{6.8}\]
Adding together these two reactions gives the reaction
\[2 \mathrm{H}_{2} \mathrm{O}(l)\rightleftharpoons \mathrm{H}_{3} \mathrm{O}^{+}(a q)+\mathrm{OH}^{-}(a q) \nonumber\]
for which the equilibrium constant is K w . Because adding together two reactions is equivalent to multiplying their respective equilibrium constants, we may express K w as the product of K a for CH3COOH and K b for CH3COO –.
\[K_{\mathrm{w}}=K_{\mathrm{a}, \mathrm{CH}_{3} \mathrm{COOH}} \times K_{\mathrm{b}, \mathrm{CH}_{3} \mathrm{COO}^{-}} \nonumber\]
For any weak acid, HA, and its conjugate weak base, A – , we can generalize this to the following equation
\[K_{\mathrm{w}}=K_{\mathrm{a}, \mathrm{HA}} \times K_{\mathrm{b}, \mathrm{A}^{-}} \label{6.9}\]
where HA and A – are a conjugate acid–base pair. The relationship between K a and K b for a conjugate acid–base pair simplifies our tabulation of acid and base dissociation constants. Appendix 11 includes acid dissociation constants for a variety of weak acids. To find the value of K b for a weak base, use equation \ref{6.9} and the K a value for its corresponding weak acid.
A common mistake when using equation \ref{6.9} is to forget that it applies to a conjugate acid–base pair only.
Example \(\PageIndex{2}\)
Using Appendix 11 , calculate values for the following equilibrium constants.
- K b for pyridine, C 5 H 5 N
- K b for dihydrogen phosphate, \(\text{H}_2\text{PO}_4^-\)
\[\text { (a) } K_{\mathrm{b}, \mathrm{C}_5 \mathrm{H}_{5} \mathrm{N}}=\frac{K_{\mathrm{w}}}{K_{\mathrm{a}, \mathrm{C}_{\mathrm{5}} \mathrm{H}_{5} \mathrm{NH}^{+}}}=\frac{1.00 \times 10^{-14}}{5.90 \times 10^{-6}}=1.69 \times 10^{-9} \nonumber\]
\[\text { (b) } K_{\mathrm{b}, \mathrm{H}_2 \mathrm{PO}_{4}^- }=\frac{K_{\mathrm{w}}}{K_{\mathrm{a}, \mathrm{H}_{\mathrm{3}} \mathrm{PO}_{4} }}=\frac{1.00 \times 10^{-14}}{7.11 \times 10^{-3}}=1.41 \times 10^{-12} \nonumber\]
When finding the K b value for a polyprotic weak base, be careful to choose the correct K a value. Remember that equation \ref{6.9} applies to a conjugate acid–base pair only. The conjugate acid of \(\text{H}_2\text{PO}_4^-\) is H 3 PO 4 , not \(\text{HPO}_4^{2-}\).
Exercise \(\PageIndex{1}\)
Using Appendix 11 , calculate K b values for hydrogen oxalate, \(\text{HC}_2\text{O}_4^-\), and for oxalate, \(\text{C}_2\text{O}_4^{2-}\).
The K b for hydrogen oxalate is
\[K_{\mathrm{b}, \mathrm{HC}_{2} \mathrm{O}_{4}^-}=\frac{K_{\mathrm{w}}}{K_{\mathrm{a}, \mathrm{H}_{2} \mathrm{C}_{2} \mathrm{O}_{4}}}=\frac{1.00 \times 10^{-14}}{5.60 \times 10^{-2}}=1.79 \times 10^{-13} \nonumber\]
and the K b for oxalate is
\[K_{\mathrm{b}, \mathrm{C}_{2} \mathrm{O}_{4}^{2-}}=\frac{K_{\mathrm{w}}}{K_{\mathrm{a}, \mathrm{HC}_{2} \mathrm{O}_{\mathrm{4}}^-}}=\frac{1.00 \times 10^{-14}}{5.42 \times 10^{-5}}=1.85 \times 10^{-10} \nonumber\]
As we expect, the K b value for \(\text{C}_2\text{O}_4^{2-}\) is larger than that for \(\text{HC}_2\text{O}_4^-\).
Complexation Reactions
A more general definition of acids and bases was proposed in 1923 by G. N. Lewis. The Brønsted‐Lowry definition of acids and bases focuses on an acid’s proton‐donating ability and a base’s proton‐accepting ability. Lewis theory, on the other hand, uses the breaking and the forming of covalent bonds to describe acids and bases. In this treatment, an acid is an electron pair acceptor and a base in an electron pair donor. Although we can apply Lewis theory to the treatment of acid–base reactions, it is more useful for treating complexation reactions between metal ions and ligands.
The following reaction between the metal ion Cd 2 + and the ligand NH 3 is typical of a complexation reaction.
\[\mathrm{Cd}^{2+}(a q)+4: \mathrm{NH}_{3}(a q)\rightleftharpoons \mathrm{Cd}\left( : \mathrm{NH}_{3}\right)_{4}^{2+}(a q) \label{6.10}\]
The product of this reaction is a metal–ligand complex . In writing this reaction we show ammonia as :NH 3 , using a pair of dots to emphasize the pair of electrons that it donates to Cd 2 + . In subsequent reactions we will omit this notation.
Metal-Ligand Formation Constants
We characterize the formation of a metal–ligand complex by a formation constant , K f . For example, the complexation reaction between Cd 2 + and NH 3 , reaction \ref{6.10}, has the following equilibrium constant.
\[K_{f}=\frac{\left[\mathrm{Cd}\left(\mathrm{NH}_{3}\right)_{4}^{2+}\right]}{\left[\mathrm{Cd}^{2+}\right]\left[\mathrm{NH}_{3}\right]^{4}}=5.5 \times 10^{7} \label{6.11}\]
The reverse of reaction \ref{6.10} is a dissociation reaction, which we characterize by a dissociation constant , K d , that is the reciprocal of K f .
Many complexation reactions occur in a stepwise fashion. For example, the reaction between Cd 2 + and NH 3 involves four successive reactions.
\[\mathrm{Cd}^{2+}(a q)+\mathrm{NH}_{3}(a q) \rightleftharpoons \mathrm{Cd}\left(\mathrm{NH}_{3}\right)^{2+}(a q) \label{6.12}\]
\[\mathrm{Cd}\left(\mathrm{NH}_{3}\right)^{2+}(a q)+\mathrm{NH}_{3}(a q)\rightleftharpoons \mathrm{Cd}\left(\mathrm{NH}_{3}\right)_{2}^{2+}(a q) \label{6.13}\]
\[\mathrm{Cd}\left(\mathrm{NH}_{3}\right)_{2}^{2+}(a q)+\mathrm{NH}_{3}(a q)\rightleftharpoons \mathrm{Cd}\left(\mathrm{NH}_{3}\right)_{3}^{2+}(a q) \label{6.14}\]
\[\mathrm{Cd}\left(\mathrm{NH}_{3}\right)_{3}^{2+}(a q)+\mathrm{NH}_{3}(a q)\rightleftharpoons \mathrm{Cd}\left(\mathrm{NH}_{3}\right)_{4}^{2+}(a q) \label{6.15}\]
To avoid ambiguity, we divide formation constants into two categories. A stepwise formation constant , which we designate as K i for the i th step, describes the successive addition of one ligand to the metal–ligand complex from the previous step. Thus, the equilibrium constants for reactions \ref{6.12}–\ref{6.15} are, respectively, K 1 , K 2 , K 3 , and K 4 . An overall, or cumulative formation constant , which we designate as \(\beta_i\), describes the addition of i ligands to the free metal ion. The equilibrium constant in equation \ref{6.11} is correctly identified as \(\beta_4\), where
\[\beta_{4}=K_{1} \times K_{2} \times K_{3} \times K_{4} \nonumber\]
\[\beta_{n}=K_{1} \times K_{2} \times \cdots \times K_{n}=\prod_{i=1}^{n} K_{i} \nonumber\]
Stepwise and overall formation constants for selected metal–ligand complexes are in Appendix 12 .
Metal-Ligand Complexation and Solubility
A formation constant describes the addition of one or more ligands to a free metal ion. To find the equilibrium constant for a complexation reaction that includes a solid, we combine appropriate K sp and K f expressions. For example, the solubility of AgCl increases in the presence of excess chloride ions as the result of the following complexation reaction.
\[\operatorname{AgCl}(s)+\mathrm{Cl}^{-}(a q)\rightleftharpoons\operatorname{Ag}(\mathrm{Cl})_{2}^{-}(a q) \label{6.16}\]
We can write this reaction as the sum of three other equilibrium reactions with known equilibrium constants—the solubility of AgCl, which is described by its K sp reaction
\[\mathrm{AgCl}(s) \rightleftharpoons \mathrm{Ag}^{+}(a q)+\mathrm{Cl}^{-}(a q) \nonumber\]
and the stepwise formation of \(\text{AgCl}_2^-\), which is described by K 1 and K 2 reactions.
\[\mathrm{Ag}^{+}(a q)+\mathrm{Cl}^{-}(a q) \rightleftharpoons \operatorname{Ag} \mathrm{Cl}(a q) \nonumber\]
\[\operatorname{AgCl}(a q)+\mathrm{Cl}^{-}(a q) \rightleftharpoons \operatorname{AgCl}_{2}^{-}(a q) \nonumber\]
The equilibrium constant for reaction \ref{6.16}, therefore, is \(K_\text{sp} \times K_1 \times K_2\).
Example \(\PageIndex{3}\)
Determine the value of the equilibrium constant for the reaction
\[\mathrm{PbCl}_{2}(s)\rightleftharpoons \mathrm{PbCl}_{2}(a q) \nonumber\]
We can write this reaction as the sum of three other reactions. The first of these reactions is the solubility of PbCl 2 (s) , which is described by its K sp reaction.
The remaining two reactions are the stepwise formation of PbCl 2 (aq) , which are described by K 1 and K 2 .
\[\mathrm{Pb}^{2+}(a q)+\mathrm{Cl}^{-}(a q)\rightleftharpoons \mathrm{PbCl}^{+}(a q) \nonumber\]
\[\mathrm{PbCl}^{+}(a q)+\mathrm{Cl}^{-}(a q)\rightleftharpoons \mathrm{PbCl}_{2}(a q) \nonumber\]
Using values for K sp , K 1 , and K 2 from Appendix 10 and Appendix 12 , we find that the equilibrium constant is
\[K=K_{\mathrm{sp}} \times K_{1} \times K_{2}=\left(1.7 \times 10^{-5}\right) \times 38.9 \times 1.62=1.1 \times 10^{-3} \nonumber\]
Exercise \(\PageIndex{2}\)
What is the equilibrium constant for the following reaction? You will find appropriate equilibrium constants in Appendix 10 and Appendix 12 .
\[\operatorname{Ag} \mathrm{Br}(s)+2 \mathrm{S}_{2} \mathrm{O}_{3}^{2-}(a q)\rightleftharpoons\operatorname{Ag}\left(\mathrm{S}_{2} \mathrm{O}_{3}\right)_2^{3-}(a q)+\mathrm{Br}^{-}(a q) \nonumber\]
We can write the reaction as a sum of three other reactions. The first reaction is the solubility of AgBr(s), which we characterize by its K sp .
\[\operatorname{AgBr}(s)\rightleftharpoons\operatorname{Ag}^{+}(a q)+\mathrm{Br}^{-}(a q) \nonumber\]
The remaining two reactions are the stepwise formation of \(\text{Ag(S}_2\text{O}_3)_2^{3-}\), which we characterize by K 1 and K 2 .
\[\mathrm{Ag}^{+}(a q)+\mathrm{S}_{2} \mathrm{O}_{3}^{2-}(a q)\rightleftharpoons\operatorname{Ag}\left(\mathrm{S}_{2} \mathrm{O}_{3}\right)^{-}(a q) \nonumber\]
\[\operatorname{Ag}\left(\mathrm{S}_{2} \mathrm{O}_{3}\right)^{-}(a q)+\mathrm{S}_{2} \mathrm{O}_{3}^{2-}(a q)\rightleftharpoons\operatorname{Ag}\left(\mathrm{S}_{2} \mathrm{O}_{3}\right)_{2}^{3-}(a q) \nonumber\]
Using values for K sp , K 1 , and K 2 from Appendix 10 and Appendix 12 , we find that the equilibrium constant for our reaction is
\[K=K_{sp} \times K_{1} \times K_{2}=\left(5.0 \times 10^{-13}\right)\left(6.6 \times 10^{8}\right)\left(7.1 \times 10^{4}\right)=23 \nonumber\]
Oxidation–Reduction (Redox) Reactions
An oxidation–reduction reaction occurs when electrons move from one reactant to another reactant. As a result of this transfer of electrons, the reactants undergo a change in oxidation state. Those reactant that increases its oxidation state undergoes oxidation , and the reactant that decreases its oxidation state undergoes reduction . For example, in the following redox reaction between Fe 3 + and oxalic acid, H 2 C 2 O 4 , iron is reduced because its oxidation state changes from +3 to +2.
\[2 \mathrm{Fe}^{3+}(a q)+\mathrm{H}_{2} \mathrm{C}_{2} \mathrm{O}_{4}(a q)+2 \mathrm{H}_{2} \mathrm{O}(l)\rightleftharpoons \\ {2 \mathrm{Fe}^{2+}(a q)+2 \mathrm{CO}_{2}(g)+2 \mathrm{H}_{3} \mathrm{O}^{+}(a q)} \label{6.17}\]
Oxalic acid, on the other hand, is oxidized because the oxidation state for carbon increases from +3 in H 2 C 2 O 4 to +4 in CO 2 .
We can divide a redox reaction, such as reaction \ref{6.17}, into separate half‐reactions that show the oxidation and the reduction processes.
\[\mathrm{H}_{2} \mathrm{C}_{2} \mathrm{O}_{4}(a q)+2 \mathrm{H}_{2} \mathrm{O}(l)\rightleftharpoons 2 \mathrm{CO}_{2}(g)+2 \mathrm{H}_{3} \mathrm{O}^{+}(a q)+2 e^{-} \nonumber\]
\[\mathrm{Fe}^{3+}(a q)+e^{-} \rightleftharpoons \mathrm{Fe}^{2+}(a q) \nonumber\]
It is important to remember, however, that an oxidation reaction and a reduction reaction always occur as a pair. We formalize this relationship by identifying as a reducing agent the reactant that is oxidized, because it provides the electrons for the reduction half‐reaction. Conversely, the reactant that is reduced is an oxidizing agent . In reaction \ref{6.17}, Fe 3 + is the oxidizing agent and H 2 C 2 O 4 is the reducing agent.
The products of a redox reaction also have redox properties. For example, the Fe 2 + in reaction \ref{6.17} is oxidized to Fe 3 + when CO 2 is reduced to H 2 C 2 O 4 . Borrowing some terminology from acid–base chemistry, Fe 2 + is the conjugate reducing agent of the oxidizing agent Fe 3 + , and CO 2 is the conjugate oxidizing agent of the reducing agent H 2 C 2 O 4 .
Thermodynamics of Redox Reactions
Unlike precipitation reactions, acid–base reactions, and complexation reactions, we rarely express the equilibrium position of a redox reaction with an equilibrium constant. Because a redox reaction involves a transfer of electrons from a reducing agent to an oxidizing agent, it is convenient to consider the reaction’s thermodynamics in terms of the electron.
For a reaction in which one mole of a reactant undergoes oxidation or reduction, the net transfer of charge, Q , in coulombs is
\[Q=n F \nonumber\]
where n is the moles of electrons per mole of reactant, and F is Faraday’s constant (96485 C/mol). The free energy, ∆ G , to move this charge, Q , over a change in potential , E , is
\[\triangle G=E Q \nonumber \]
The change in free energy (in kJ/mole) for a redox reaction, therefore, is
\[\Delta G=-n F E \label{6.18}\]
where ∆ G has units of kJ/mol. The minus sign in equation \ref{6.18} is the result of a different convention for assigning a reaction’s favorable direction. In thermodynamics, a reaction is favored when ∆ G is negative, but an oxidation‐reduction reaction is favored when E is positive. Substituting equation \ref{6.18} into equation 6.2.3
\[-n F E=-n F E^{\circ}+R T \ln Q_r \nonumber\]
and dividing by – nF , leads to the well‐known Nernst equation
\[E=E^{\circ}-\frac{R T}{n F} \ln Q_r \nonumber\]
where E o is the potential under standard‐state conditions. Substituting appropriate values for R and F , assuming a temperature of 25 o C (298 K), and switching from ln to log gives the potential in volts as
\[E=E^{\mathrm{o}}-\frac{0.05916}{n} \log Q_r \label{6.19}\]
Standard Potentials
A redox reaction’s standard potential , E o , provides an alternative way of expressing its equilibrium constant and, therefore, its equilibrium position. Because a reaction at equilibrium has a ∆ G of zero, the potential, E , also is zero at equilibrium. Substituting these values into equation \ref{6.19} and rearranging provides a relationship between E o and K
\[E^{\circ}=\frac{0.05916}{n} \log K \label{6.20}\]
A standard potential is the potential when all species are in their standard states. You may recall that we define standard state conditions as follows: all gases have unit partial pressures, all solutes have unit concentrations, and all solids and liquids are pure.
We generally do not tabulate standard potentials for redox reactions. Instead, we calculate E o using the standard potentials for the corresponding oxidation half‐reaction and reduction half‐reaction. By convention, standard potentials are provided for reduction half‐reactions. The standard potential for a redox reaction, E o , is
\[E^{\circ}=E_{red}^{\circ}-E_{ox}^{\circ} \nonumber\]
where \(E_{red}^{\circ}\) and \(E_{ox}^{\circ}\) are the standard reduction potentials for the reduction half‐reaction and the oxidation half‐reaction.
Because we cannot measure the potential for a single half‐reaction, we arbitrarily assign a standard reduction potential of zero to a reference half‐reaction
\[2 \mathrm{H}_{3} \mathrm{O}^{+}(a q)+2 e^{-}\rightleftharpoons 2 \mathrm{H}_{2} \mathrm{O}(l)+\mathrm{H}_{2}(g) \nonumber\]
and report all other reduction potentials relative to this reference. Appendix 13 contains a list of selected standard reduction potentials. The more positive the standard reduction potential, the more favorable the reduction reaction is under standard state conditions. For example, under standard state conditions the reduction of Cu 2 + to Cu ( E o = +0.3419 V) is more favorable than the reduction of Zn 2 + to Zn ( E o = –0.7618 V).
Example \(\PageIndex{4}\)
Calculate (a) the standard potential, (b) the equilibrium constant, and (c) the potential when [Ag + ] = 0.020 M and [Cd 2 + ] = 0.050 M, for the following reaction at 25 o C.
\[\mathrm{Cd}(s)+2 \mathrm{Ag}^{+}(a q)\rightleftharpoons2 \mathrm{Ag}(s)+\mathrm{Cd}^{2+}(a q) \nonumber\]
(a) In this reaction Cd is oxidized and Ag + is reduced. The standard cell potential, therefore, is
\[E^{\circ} = E^{\circ}_{\text{Ag}^+/ \text{Ag}} - E^{\circ}_{\text{Cd}^{2+}/ \text{Cd}} = 0.7996 - (-0.4030) = 1.2026 \ \text{V} \nonumber\]
(b) To calculate the equilibrium constant we substitute appropriate values into equation \ref{6.20}.
\[E^{\circ}=1.2026 \ \mathrm{V}=\frac{0.05916 \ \mathrm{V}}{2} \log K \nonumber\]
Solving for K gives the equilibrium constant as
\[\begin{array}{l}{\log K=40.6558} \\ {K=4.527 \times 10^{40}}\end{array} \nonumber\]
(c) To calculate the potential when [Ag + ] is 0.020 M and [Cd 2 + ] is 0.050M, we use the appropriate relationship for the reaction quotient, Q r , in equation \ref{6.19}.
\[\begin{array}{c}{E=E^{\circ}-\frac{0.05916 \ \mathrm{V}}{n} \log \frac{\left[\mathrm{Cd}^{2+}\right]}{\left[\mathrm{Ag}^{+}\right]^{2}}} \\ {E=1.2026 \ \mathrm{V}-\frac{0.05916 \ \mathrm{V}}{2} \log \frac{0.050}{(0.020)^{2}}=1.14 \ \mathrm{V}}\end{array} \nonumber\]
Exercise \(\PageIndex{3}\)
For the following reaction at 25 o C
\[5 \mathrm{Fe}^{2+}(a q)+\mathrm{MnO}_{4}^{-}(a q)+8 \mathrm{H}^{+}(a q) \rightleftharpoons 5 \mathrm{Fe}^{3+}(a q)+\mathrm{Mn}^{2+}(a q)+4 \mathrm{H}_{2} \mathrm{O}(l) \nonumber\]
calculate (a) the standard potential, (b) the equilibrium constant, and (c) the potential under these conditions: [Fe 2 + ] = 0.50 M, [Fe 3 + ] = 0.10 M, [\(\text{MnO}_4^{-}\)] = 0.025 M, [Mn 2 + ] = 0.015 M, and a pH of 7.00. See Appendix 13 for standard state reduction potentials.
The two half‐reactions are the oxidation of Fe 2 + and the reduction of \(\text{MnO}_4^-\).
\[\mathrm{Fe}^{2+}(a q) \rightleftharpoons \mathrm{Fe}^{3+}(a q)+e^{-} \nonumber\]
\[\mathrm{MnO}_{4}^{-}(a q)+8 \mathrm{H}^{+}(a q)+5 e^{-} \rightleftharpoons \mathrm{Mn}^{2+}(a q)+4 \mathrm{H}_{2} \mathrm{O}(l) \nonumber\]
From Appendix 13 , the standard state reduction potentials for these half‐reactions are
\[E_{\text{Fe}^{3+}/\text{Fe}^{2+}}^{\circ} = 0.771 \ \text{V and } E_{\text{MnO}_4^-/\text{Mn}^{2+}}^{\circ} = 1.51 \ \text{V} \nonumber\]
(a) The standard state potential for the reaction is
\[E^{\circ} = E_{\text{MnO}_4^-/\text{Mn}^{2+}}^{\circ} - E_{\text{Fe}^{3+}/\text{Fe}^{2+}}^{\circ} = 1.51 \ \text{V} - 0.771 \ \text{V } = 0.74 \ \text{V} \nonumber\]
(b) To calculate the equilibrium constant we substitute appropriate values into equation \ref{6.20}.
\[E^{\circ}=0.74 \ \mathrm{V}=\frac{0.05916}{5} \log K \nonumber\]
Solving for K gives its value as \(3.5 \times 10^{62}\).
(c) To calculate the potential under these non‐standard state conditions, we make appropriate substitutions into the Nernst equation.
\[E=E^{\circ}-\frac{R T}{n F} \ln \frac{\left[\mathrm{Mn}^{2+}\right]\left[\mathrm{Fe}^{3+}\right]^{5}}{\left[\mathrm{MnO}_{4}^{-}\right]\left[\mathrm{Fe}^{2+}\right]^{5}\left[\mathrm{H}^{+}\right]^{8}} \nonumber\]
\[E=0.74-\frac{0.05916}{5} \log \frac{(0.015)(0.10)^{5}}{(0.025)(0.50)^{5}\left(1 \times 10^{-7}\right)^{8}}=0.12 \ \mathrm{V} \nonumber\]
When writing precipitation, acid–base, and metal–ligand complexation reactions, we represent acidity as H 3 O + . Redox reactions more commonly are written using H + instead of H 3 O + . For the reaction in Exercise \(\PageIndex{3}\), we could replace H + with H 3 O + and increase the stoichiometric coefficient for H 2 O from 4 to 12.
An Overview of General Equilibrium Theory
- First Online: 15 November 2011
Cite this chapter

- Manuel Alejandro Cardenete Ph.D. 4 ,
- Ana-Isabel Guerra Ph.D. 4 &
- Ferran Sancho Ph.D. 5
Part of the book series: Springer Texts in Business and Economics ((STBE))
1820 Accesses
As economists we are usually interested in how production is organized and in how whatever is produced is eventually distributed among consumers. All these activities take place within specific institutions we know as markets. What condition markets’ outcomes, i.e. prices of goods and services and quantities traded, are agents’ behavioral characteristics and the market mechanisms that emanate from them, namely, the so-called law and supply and demand. It is common to distinguish two large and distinct groups of agents—households and firms. Each of these groups plays a different role in the marketplace and in the whole economic system as well, depending on the particular type of commodity being traded.
- Equilibrium Price
- Excess Demand
- Initial Endowment
- Walrasian Equilibrium
- Feasible Allocation
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
This is a preview of subscription content, log in via an institution to check access.
Access this chapter
- Available as PDF
- Read on any device
- Instant download
- Own it forever
- Available as EPUB and PDF
- Durable hardcover edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
Tax calculation will be finalised at checkout
Purchases are for personal use only
Institutional subscriptions
Walras ( 1874a , b ) was wrong when posing and facing the question about the uniqueness of equilibrium. In fact, aggregate excess demand function and not only individual excess demand functions must present additional properties for the equilibrium to become unique and stable, since not all the properties of the later are inherited into the former. This is the so-called Sonnenschein-Mantel-Debreu Theorem (1973, 1974, 1974). The concept of uniqueness is also related to the concept of stability (Arrow and Hurwicz 1958 , 1959 ). An excellent exposition of uniqueness and stability of equilibrium for pure exchange economies and economies with production can be found in Elements of General Equilibrium Analysis (1998), Chap. III, written by Timothy J. Kehoe.
Arrow, K. J. (1951). An extension of the basic theorems of classical welfare economics. In J. Neyman (Ed.), Proceedings of the Second Berkeley Symposium on Mathematical Statistics and Probability .
Google Scholar
Arrow, K. J., & Debreu, G. (1954). Existence of equilibrium for a competitive economy. Econometrica, 22 , 265–290.
Article Google Scholar
Arrow, K. L., & Hahn, F. H. (1971). General competitive analysis . San Francisco: Ed. Holden-Day.
Arrow, K. J., & Hurwicz, L. (1958). On the stability of the competitive equilibrium I. Econometrica, 26 , 522–552.
Arrow, K. J., & Hurwicz, L. (1959). On the stability of the competitive equilibrium II. Econometrica, 27 , 82–109.
Brouwer, L. E. J. (1911–1912). Uber Abbildung von Mannigfaltigkeiten. Mathematische Annalen, 71 , 97–115.
Debreu, G. (1952). A social equilibrium existence theorem. Proceedings of the National Academy of Sciences, 38 (10), 886–893.
Debreu, G. (1956). Market equilibrium . Cowles Foundation Discussion Papers 10, Cowles Foundation for Research in Economics, Yale University, New Haven.
Debreu, G. (1970). Economies with a finite set of equilibria. Econometrica, 38 , 387–392.
Gale, D. (1955). The law of supply and demand. Mathematica Scandinavia, 3 , 155–169.
Kakutani, S. (1941). A generalization of Brouwer’s fixed point theorem. Duke Mathematical Journal, 8 , 457–459.
Kehoe, T. J. (1980). An index theorem for general equilibrium models with production. Econometrica, 48 , 1211–1232.
Kehoe, T. J. (1985). The comparative statistics of tax models. Canadian Journal of Economics, 18 , 314–334.
Kehoe, T. J. (1991). Multiplicity of equilibria and comparative statistics. In W. Hildenbrand & H. F. Sonnenschein (Eds.), Handbook of mathematical economics (Vol. 4, pp. 2049–2143). Amsterdam: North-Holland.
Kehoe, T. J. (1992). Gross substitutability and the weak axiom of revealed preference. Journal of Mathematical Economics, 21 , 37–50.
Kehoe, T. J. (1998). Uniqueness and stability. In A. Kirman (Ed.), Elements of general equilibrium analysis . Malden: Blackwell.
Kehoe, T. J., & Mas-Colell, A. (1984). An observation on gross substitutability and the weak axiom of revealed preference. Economics Letters, 15 , 241–243.
Kehoe, T. J., & Whalley, J. (1985). Uniqueness of equilibrium in large-scale numerical general equilibrium models. Journal of Public Economics, 28 , 247–254.
Mas-Colell, A. (1991). On the uniqueness of equilibrium once again. In W. A. Barnett (Ed.), Equilibrium theory and applications . Cambridge: Cambridge University Press.
Nikaido, H. (1956). On the classical multilateral exchange problem. Metroeconomica, 8 , 135–145.
Villar, A. (1996). General equilibrium with increasing returns . Berlin: Springer.
Book Google Scholar
Wald, A. (1936a). Uber die Produktionsgleichungen der okonomischen Wertlehre. Ergebnisse eines mathematischen Kolloquiums, 7 (1934–35), 1–6.
Wald, A. (1936b). Uber einige Gleichungssysteme der mathematischen Okonomie. Zeitschrift fur Nationalokonomie, 7 , 637–670. Translated as On some systems of equations of mathematical economics. Econometrica, 19 , 368–403, 1951.
Walras, L. (1874a). Principe d’une théorie mathématique de l’échange. Journal des economists, 34 , 5–21.
Walras, L. (1874b). Eléments d’economie politique pure, ou Théorie de la richesse social . Laussane: L. Corbaz. Final edition. Ed: Paris: Pichon et Durand-Auzias, 1926, reedite in 1952.
Download references
Author information
Authors and affiliations.
University Pablo de Olavide Economics, Ctra. Utrera, km. 1, s/n, 41013, Seville, Spain
Manuel Alejandro Cardenete Ph.D. & Ana-Isabel Guerra Ph.D.
Universitat Autònoma de Barcelona Economics, Campus de Bellaterra-Edifici B, 08193, Cerdanyola del Vallès, Spain
Ferran Sancho Ph.D.
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Manuel Alejandro Cardenete Ph.D. .
Rights and permissions
Reprints and permissions
Copyright information
© 2012 Springer-Verlag Berlin Heidelberg
About this chapter
Cardenete, M.A., Guerra, AI., Sancho, F. (2012). An Overview of General Equilibrium Theory. In: Applied General Equilibrium. Springer Texts in Business and Economics. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-24746-0_2
Download citation
DOI : https://doi.org/10.1007/978-3-642-24746-0_2
Published : 15 November 2011
Publisher Name : Springer, Berlin, Heidelberg
Print ISBN : 978-3-642-24745-3
Online ISBN : 978-3-642-24746-0
eBook Packages : Business and Economics Economics and Finance (R0)
Share this chapter
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research
Particular Equilibrium Analysis: Definition, Importance and Limitations
Let us make an in-depth study of Particular Equilibrium Analysis:- 1. Definition of Particular Equilibrium Analysis 2. Importance of Particular Equilibrium Analysis 3. Limitations.
Definition of Particular Equilibrium Analysis:
Partial equilibrium analysis is the counterpart of microeconomic analysis. It is also preferably called ‘particular equilibrium’ analysis because the word ‘partial’ smacks of incompleteness of the analysis when there is nothing incomplete about it.
A large part of the economic analysis is built around the concept of equilibrium of a consumer, a resource owner, a firm or an industry.
It concerns itself with movements of particular economic units or particular industries towards equilibrium positions in response to the given economic conditions facing them. Thus, a household, with its given tastes and preferences, is confronted with given income and with given prices of services and goods.
ADVERTISEMENTS:
The household must adjust its purchases so as to move towards equilibrium. A business firm, when faced with given demand conditions for its product, technological conditions and resource suppliers, adjusts its production so as to move towards equilibrium.
The resource owner has fixed quantities of resources with him which lie can place in employment. He is confronted with given alternatives of employment, with given resource price offers. He makes his equilibrium adjustment on the basis of given data.
An industry also tends towards equilibrium when firms move out of it so as to avoid losses which they may incur if they remain in it or when new firms enter the industry (if entry is free and possible) to reap the unusually high profits that are expected to be reaped. Changes in the given data facing economic units and industries change the position of equilibrium towards which each is tending, and thus cause movements towards the new positions.
According to George J. Stigler, a partial equilibrium is one which is based only on a restricted range of data. A standard example is the determination of price of a single product, the prices of all other products being held fixed during the analysis.
Marshall explained the partial-equilibrium method thus:
“The forces to be dealt with are, however, so numerous that it is best to take a few at a lime and to work out a number of partial solutions as auxiliaries to our main study. Thus, we begin by isolating the primary relations of supply, demand and price in regard to a particular commodity. We reduce to inaction all other forces by the phase ‘other things being equal’. We do not suppose that they are inert, but for the time being we ignore their activity. This scientific device is a great deal older than science; it is the method by which consciously or unconsciously, sensible men dealt from time immemorial with every difficult problem of ordinary life.”
Importance of Particular Equilibrium Analysis :
Particular equilibrium analysis is especially suited to two types of problems.
First, those problems which are confined to an industry or a sector of the economy and which do not produce any disturbances in the rest of the economy. For example, if the workers of a small toy-manufacturing firm go on strike and if they are fairly distributed in different residential areas of the city, their strike will not produce any disturbance anywhere else but in the firm and its workers.
Particular equilibrium analysis can easily be used to give answers to most of the economic problems emanating from the strike.
Second, it can be used to analyse the immediate or primary effects of economic disturbance of any type. For example, if the Government of India chalks out a programme of railway wagon and machinery exports to the U.S.S.R., particular equilibrium analysis can be helpful in analysing the first-order effects on the iron and steel industry of India—the effect on steel prices, its output, its profits, wages and employment in it. Of course, the effect of such a programme will by no means remain limited to iron and steel industry alone.
Limitations of Particular Equilibrium Analysis :
The limitations of particular equilibrium analysis are obvious.
Firstly, it is based on the assumption that an economic disturbance in a particular industry has only located effects. In reality, such cases are few and far between.
Secondly, particular equilibrium analysis is easy to follow and use. In Marshall’s view, particular equilibrium gives us simpler propositions and simpler analysis. He was sensitively aware of the limitations of the human mind.
In his view, the best we can do is to examine a commodity from the angle of such variables as price, cost and quantities. We cannot easily examine every aspect, every repercussion. He, therefore, applied this mind mainly to the analysis of the equilibrium of consumer, a firm and an industry.
Related Articles:
- Difference between Particular Equilibrium and General Equilibrium
- Difference between Firm and Industry’s Equilibrium
- Break-Even Analysis: Introduction, Assumptions and Limitations
- General Equilibrium Analysis: Meaning, Nature, Objectives and Uses | Macro Economics
Equilibrium in National Income Analysis
Even though the discussion of static analysis has hitherto been restricted to market models in various guises—linear and nonlinear, one-commodity and multicommodity, specific and general it, of course, has applications in other areas of economics also. As an example, we may cite the simplest Keyncsian national-income model,
C = a +br where Y and C stand for the endogenous variables national income and (planned) consumption expenditure, respectively, and lo and Co represent the exogenously determined investment and government expenditures. The first equation is an equilibrium condition (national income = total planned expenditure). The second, the consumption function, is behavioral. The two parameters in the consumption function, a and b, stand for the autonomous consumption expenditure and the marginal propensity to consume, respectively It is quite clear that these two equations in two endogenous variables are neither functionally dependent upon, nor inconsistent with, each other. Thus wc would be able to find the equilibrium values of income and consumption expenditure, Y* and C*, in terms of the parameters a and b and the exogenous variables A> and Go.
Substitution of the second equation into the first will reduce (3.23) to a single equation in one variable, Y:
Y=a + bY+Io + Go or (1 - h)Y = a + Id 4- Go (collecting terms involving Y)
To find the solution value of Y (equilibrium national income), we only have to divide through by (1 - b) :
Note, again, that the solution value is expressed entirely in terms of the parameters and exogenous variables, the given data of the model. Putting (3.24) into the second equation of (3.23) will then yield the equilibrium level of consumption expenditure:
1 -b g(l -b) + b(a + /q + Gq) _ a + b{I0 + <jq) 1-b ~ 1-b
This is again expressed entirely in terms of the given data.
Both Y* and C* have the expression (I - b) in the denominator; thus a restriction b ^ 1 is necessary to avoid division by zero. Since b, the marginal propensity to consume, has been assumed to be a positive fraction, this restriction is automatically satisfied. For Y* and C* to be positive, moreover, the numerators in (3.24) and (3.25) must be positive. Since the exogenous expenditures /q and Go are normally positive, as is the parameter a (the vertical intercept of the consumption function), the sign of the numerator expressions will work out, too.
As a check on our calculation, we can add the C* expression in (3.25) to (/<> 4- G{>) and verify that the sum is equal to the Y* expression in (3.24).
This model is obviously one of extreme simplicity and crudity, but other models ol national-income determination, in varying degrees of complexity and sophistication, can be constructed as well. In each case, however, the principles involved in the construction and analysis of the model are identical with those already discussed. Kor this reason, we shall not go into further illustrations here. A more comprehensive national-income model, involving the simultaneous equilibrium of the money market and the goods market, will be discussed in Sec. 8.6.
EXERCISE 3.5
1. Given the following model: y = o
C = a + b{Y -T) {a>0f 0 < b < 1) [T: taxes] T = d+tY {d > Q, 0 < t < 1) [f: income tax rate]
(a) How many endogenous variables are there?
2. Let the national-income model be: Y = C 4 k 4 C
C = o - b{Y - T0) (o >0, 0 < b < 1)
(□) Identify the endogenous variables.
(6) Give the economic meaning of the parameter g.
(c) Find the equilibrium national income,
(d) What restriction on the parameters is needed for a solution to exist?
3. Find Y~ and C* from the following:
Linear Models and Matrix Algebra
For the one-commodity model (3.1)f the solutions P* and Q* as expressed in (3.4) and (3.5), respectively, arc relatively simple, even though a number of parameters arc involved. As more and more commodities arc incorporated into the model, such solution formulas quickly become cumbersome and unwieldy. That was why we had to resort to a little shorthand, even for the two-commodity case—in order that the solutions (3.14) and (3.15) can still be written in a relatively concise fashion. We did not attempt to tackle any three- or four-commodity models, even in the linear version, primarily because we did not yet have at our disposal a method suitable for handling a large system of simultaneous equations. Such a method is found in maim algebra, the subject of this chapter and the next.
Matrix algebra can enable us to do many things. In the first place, it provides a compact way of writing an equation system, even an extremely large one. Second, it leads to a way of testing the existence of a solution by evaluation of a determinant—a concept closely related to that of a matrix. Third, it gives a method of finding that solution (if it exists). Since equation systems are encountered not only in static analysis but also in comparative-static and dynamic analyses and in optimization problems, you will lind ample application of matrix algebra in almost every chapter that is to follow. This is why it is desirable to introduce matrix algebra early.
However, one slight catch is that matrix algebra is applicable only to //«ear-equation systems. How realistically linear equations can describe actual economic relationships depends, of course, on the nature of the relationships in question. In many cases, even if some sacrifice of realism is entailed by the assumption of linearity, an assumed linear relationship can produce a sufficiently close approximation to an actual nonlinear relationship to warrant its use.
In other cases, while preserving the nonlinearity in the model, we can effect a transformation of variables so as to obtain a linear relation to work with. For example, the nonlinear function v = ax'
can be readily transformed, by taking the logarithm on both sides, into the function logy = log a -|-Mogx which is linear in the two variables (logy) and (log.v). (Logarithms will be discussed in more detail in Chap. 10.). More importantly, in many applications such as comparative-static analysis and optimization problems, discusscd subsequently, although the original formulation of the economic model is nonlinear in nature, linear equation systems will emerge in the course of analysis. Thus the linearity restriction is not nearly as restrictive as it may first appear.
Continue reading here: Matrices and Vectors
Was this article helpful?
Related Posts
- General Market Equilibrium
- The Nature of Mathematical Economics
- The Nature of Comparative Statics
- Variables Constants and Parameters
- Equations and Identities - Mathematical Economics
- Conditions for Profit Maximization
Readers' Questions
What are the algebric method of determine national income in the economy?
The algebraic methods used to determine national income in an economy include: Income approach: This method calculates national income by summing up the total factor incomes earned in an economy. It considers wages, salaries, rent, interest, and profits as the main components of national income. Production or value-added approach: This method calculates national income by adding up the value-added at each stage of production within an economy. It measures the difference between the value of inputs and outputs at each stage, ultimately arriving at the total value-added in the economy. Expenditure approach: This method calculates national income by summing up the total expenditures made in an economy. It considers consumption expenditure, investment expenditure, government spending, and net exports (exports minus imports). These algebraic methods are often used in conjunction with national income accounting systems and data to accurately measure and determine the level of national income in an economy.
What is the meaning of the parameter g in economics?
In economics, the parameter "g" commonly refers to the growth rate of an economy's Gross Domestic Product (GDP). It represents the rate at which the total value of goods and services produced within a country increases over a given period of time. The growth rate is typically measured on an annual basis and is influenced by various factors such as government policies, investment, technological advancements, consumer spending, and international trade. The value of "g" is crucial for assessing the overall health and performance of an economy.
What is parameter in national income?
Parameter in national income is defined as any factor that affects the overall level of national income. These can include population, consumer spending, investment, wages, productivity, monetary, fiscal and trade policies.
What variable is needed to for a unique solution to exist national income?
National income is the total amount of money that a country earns from the production of goods and services in a given period of time. In order for a unique solution to exist, it is necessary to have a measure of national income, such as gross domestic product (GDP), as a reference point. This measure is used to consider how much a country earns and how it is distributed among its citizens, businesses, and government. National income is a key indicator of a country's economic health and stability.
What parameters are needed for a national income model?
The following parameters are typically needed for a national income model: Consumption (C): The total amount of goods and services consumed by households, business, and government. Investment (I): The amount of capital goods purchased by households and businesses, such as buildings, machines, and vehicles. Government expenditure (G): Total spending by government entities on goods and services, such as infrastructure, public services, and other purchases. Net exports (NX): The value of exports minus the value of imports. Labor force (L): The amount of workers available in the economy. Capital stock (K): The amount of capital goods available in the economy. Tax rate (T): The rate of taxation imposed by governments on their citizens and businesses. Labor productivity (LP): The level of output produced per unit of labor or capital.
What is the economic meaning of the parameters a, b, d, and t?
A, B, D, and T are all examples of economic parameters, which are variables that measure one or multiple aspects of the macroeconomic environment. A is typically an elasticity parameter, which measures the responsiveness of demand to changes in price. B is usually a budget parameter, which measures an individual’s or an organization’s budget constraints. D is usually an income parameter, which measures an individual’s or an organization’s income level. T is usually a tax parameter, which measures levels of taxation.
What is the economic meaning of the parameter g?
G is a parameter that represents the rate of growth in an economy. It is used to calculate the change in the level of economic output, measured as the sum of consumption, investment, government spending, and foreign trade. G is a useful tool in measuring economic progress over time and is often used to compare economic performance between countries.
What restrictions on the parameters is needed for a solution to exist?
The parameters must be suitable for the type of problem being solved. For instance, if the problem requires two values to be equal, then the parameters must have a value that will satisfy the equation and allow a solution to exist. If the parameters must be within a certain range, then the values must fall within that range in order for a solution to exist.
What restrictions on the parameter is needed for consumption to exist?
The parameters needed for consumption to exist are as follows: 1) The availability of resources: People must have access to the resources that are necessary for consumption in order for it to take place. 2) Income: A person must have sufficient income to purchase the goods and services that are desired for consumption. 3) Prices: Prices of goods and services must be set at a level that is affordable for the consumer. 4) Credit: Access to credit or other forms of financing must be available in order to enable people to consume larger items that have a higher associated cost.
What restrictions on the parameters is neede for equilibrium national income to exist?
Wages must be equal to the marginal product of labour. The marginal propensity to consume must be greater than zero. Prices must be flexible and not fixed. Supply and demand for goods and services must be in equilibrium. Production must exceed consumption. Interest rates must be stable. Saving must be greater than or equal to investment. Fiscal and monetary policies must be consistent with full employment.
What restriction on the parameters is needed for a solution to exist?
In order to have a solution, all the parameters of the problem must satisfy the necessary conditions for the problem. This can include restrictions on the range of values, constraints on the relationship between the parameters, or any other conditions that are necessary for a valid solution to exist.
- International edition
- Australia edition
- Europe edition

Trump’s bizarre, vindictive incoherence has to be heard in full to be believed
Excerpts from his speeches do not do justice to Trump’s smorgasbord of vendettas, non sequiturs and comparisons to famous people
Donald Trump’s speeches on the 2024 campaign trail so far have been focused on a laundry list of complaints, largely personal, and an increasingly menacing tone.
He’s on the campaign trail less these days than he was in previous cycles – and less than you’d expect from a guy with dedicated superfans who brags about the size of his crowds every chance he gets. But when he has held rallies, he speaks in dark, dehumanizing terms about migrants, promising to vanquish people crossing the border. He rails about the legal battles he faces and how they’re a sign he’s winning, actually. He tells lies and invents fictions. He calls his opponent a threat to democracy and claims this election could be the last one.
Trump’s tone, as many have noted, is decidedly more vengeful this time around, as he seeks to reclaim the White House after a bruising loss that he insists was a steal. This alone is a cause for concern, foreshadowing what the Trump presidency redux could look like. But he’s also, quite frequently, rambling and incoherent, running off on tangents that would grab headlines for their oddness should any other candidate say them.
Journalists rightly chose not to broadcast Trump’s entire speeches after 2016, believing that the free coverage helped boost the former president and spread lies unchecked. But now there’s the possibility that stories about his speeches often make his ideas appear more cogent than they are – making the case that, this time around, people should hear the full speeches to understand how Trump would govern again.
Watching a Trump speech in full better shows what it’s like inside his head: a smorgasbord of falsehoods, personal and professional vendettas, frequent comparisons to other famous people, a couple of handfuls of simple policy ideas, and a lot of non sequiturs that veer into barely intelligible stories.
Curiously, Trump tucks the most tangible policy implications in at the end. His speeches often finish with a rundown of what his second term in office could bring, in a meditation-like recitation the New York Times recently compared to a sermon. Since these policies could become reality, here’s a few of those ideas:
Instituting the death penalty for drug dealers.
Creating the “Trump Reciprocal Trade Act”: “If China or any other country makes us pay 100% or 200% tariff, which they do, we will make them pay a reciprocal tariff of 100% or 200%. In other words, you screw us and we’ll screw you.”
Indemnifying all police officers and law enforcement officials.
Rebuilding cities and taking over Washington DC, where, he said in a recent speech, there are “beautiful columns” put together “through force of will” because there were no “Caterpillar tractors” and now those columns have graffiti on them.
Issuing an executive order to cut federal funding for any school pushing critical race theory, transgender and other inappropriate racial, sexual or political content.
Moving to one-day voting with paper ballots and voter ID.
This conclusion is the most straightforward part of a Trump speech and is typically the extent of what a candidate for office would say on the campaign trail, perhaps with some personal storytelling or mild joking added in.
But it’s also often the shortest part.
Trump’s tangents aren’t new, nor is Trump’s penchant for elevating baseless ideas that most other presidential candidates wouldn’t, like his promotion of injecting bleach during the pandemic.
But in a presidential race among two old men that’s often focused on the age of the one who’s slightly older, these campaign trail antics shed light on Trump’s mental acuity, even if people tend to characterize them differently than Joe Biden’s. While Biden’s gaffes elicit serious scrutiny, as writers in the New Yorker and the New York Times recently noted, we’ve seemingly become inured to Trump’s brand of speaking, either skimming over it or giving him leeway because this has always been his shtick.
Trump, like Biden, has confused names of world leaders (but then claims it’s on purpose ). He has also stumbled and slurred his words. But beyond that, Trump’s can take a different turn. Trump has described using an “iron dome” missile defense system as “ding, ding, ding, ding, ding, ding. They’ve only got 17 seconds to figure this whole thing out. Boom. OK. Missile launch. Whoosh. Boom.”
These tangents can be part of a tirade, or they can be what one can only describe as complete nonsense.
During this week’s Wisconsin speech, which was more coherent than usual, Trump pulled out a few frequent refrains: comparing himself, incorrectly , to Al Capone, saying he was indicted more than the notorious gangster; making fun of the Georgia prosecutor Fani Willis’s first name (“It’s spelled fanny like your ass, right? Fanny. But when she became DA, she decided to add a little French, a little fancy”).

He made fun of Biden’s golfing game, miming how Biden golfs, perhaps a ding back at Biden for poking Trump about his golf game. Later, he called Biden a “lost soul” and lamented that he gets to sit at the president’s desk. “Can you imagine him sitting at the Resolute Desk? What a great desk,” Trump said.
One muddled addition in Wisconsin involved squatters’ rights, a hot topic related to immigration now: “If you have illegal aliens invading your home, we will deport you,” presumably meaning the migrant would be deported instead of the homeowner. He wanted to create a federal taskforce to end squatting, he said.
“Sounds like a little bit of a weird topic but it’s not, it’s a very bad thing,” he said.
These half-cocked remarks aren’t new; they are a feature of who Trump is and how he communicates that to the public, and that’s key to understanding how he is as a leader.
The New York Times opinion writer Jamelle Bouie described it as “something akin to the soft bigotry of low expectations”, whereby no one expected him to behave in an orderly fashion or communicate well.
Some of these bizarre asides are best seen in full, like this one about Biden at the beach in Trump’s Georgia response to the State of the Union:
“Somebody said he looks great in a bathing suit, right? And you know, when he was in the sand and he was having a hard time lifting his feet through the sand, because you know sand is heavy, they figured three solid ounces per foot, but sand is a little heavy, and he’s sitting in a bathing suit. Look, at 81, do you remember Cary Grant? How good was Cary Grant, right? I don’t think Cary Grant, he was good. I don’t know what happened to movie stars today. We used to have Cary Grant and Clark Gable and all these people. Today we have, I won’t say names, because I don’t need enemies. I don’t need enemies. I got enough enemies. But Cary Grant was, like – Michael Jackson once told me, ‘The most handsome man, Trump, in the world.’ ‘Who?’ ‘Cary Grant.’ Well, we don’t have that any more, but Cary Grant at 81 or 82, going on 100. This guy, he’s 81, going on 100. Cary Grant wouldn’t look too good in a bathing suit, either. And he was pretty good-looking, right?”
Or another Hollywood-related bop, inspired by a rant about Willis and special prosecutor Nathan Wade’s romantic relationship:
“It’s a magnificent love story, like Gone With the Wind. You know Gone With the Wind, you’re not allowed to watch it any more. You know that, right? It’s politically incorrect to watch Gone With the Wind. They have a list. What were the greatest movies ever made? Well, Gone With the Wind is usually number one or two or three. And then they have another list you’re not allowed to watch any more, Gone With the Wind. You tell me, is our country screwed up?”
He still claims to have “done more for Black people than any president other than Abraham Lincoln” and also now says he’s being persecuted more than Lincoln and Andrew Jackson:
“ All my life you’ve heard of Andrew Jackson, he was actually a great general and a very good president. They say that he was persecuted as president more than anybody else, second was Abraham Lincoln. This is just what they said. This is in the history books. They were brutal, Andrew Jackson’s wife actually died over it.”
You not only see the truly bizarre nature of Trump’s speeches when viewing them in full, but you see the sheer breadth of his menace and animus toward those who disagree with him.
His comments especially toward migrants have grown more dehumanizing. He has said they are “poisoning the blood” of the US – a nod at Great Replacement Theory, the far-right conspiracy that the left is orchestrating migration to replace white people. Trump claimed the people coming in were “prisoners, murderers, drug dealers, mental patients and terrorists, the worst they have”. He has repeatedly called migrants “animals”.

“Democrats said please don’t call them ‘animals’. I said, no, they’re not humans, they’re animals,” he said during a speech in Michigan this week.
“In some cases they’re not people, in my opinion,” he said during his March appearance in Ohio. “But I’m not allowed to say that because the radical left says that’s a terrible thing to say. “These are animals, OK, and we have to stop it,” he said.
And he has turned more authoritarian in his language, saying he would be a “dictator on day one” but then later said it would only be for a day. He’s called his political enemies “vermin”: “We pledge to you that we will root out the communists, Marxists, fascists and the radical left thugs that live like vermin within the confines of our country,” he said in New Hampshire in late 2023.
At a speech in March in Ohio about the US auto industry he claimed there would be a “bloodbath” if he lost, which some interpreted as him claiming there would be violence if he loses the election.
Trump’s campaign said later that he meant the comment to be specific to the auto industry, but now the former president has started saying Biden created a “border bloodbath” and the Republican National Committee created a website to that effect as well.
It’s tempting to find a coherent line of attack in Trump speeches to try to distill the meaning of a rambling story. And it’s sometimes hard to even figure out the full context of what he’s saying, either in text or subtext and perhaps by design, like the “bloodbath” comment or him saying there wouldn’t be another election if he doesn’t win this one.
But it’s only in seeing the full breadth of the 2024 Trump speech that one can truly understand what kind of president he could become if he won the election.
“It’s easiest to understand the threat that Trump poses to American democracy most clearly when you see it for yourself,” Susan B Glasser wrote in the New Yorker. “Small clips of his craziness can be too easily dismissed as the background noise of our times.”
If you ask Trump himself, of course, these are just examples that Trump is smart.
“The fake news will say, ‘Oh, he goes from subject to subject.’ No, you have to be very smart to do that. You got to be very smart. You know what it is? It’s called spot-checking. You’re thinking about something when you’re talking about something else, and then you get back to the original. And they go, ‘Holy shit. Did you see what he did?’ It’s called intelligence.”
- Donald Trump
- US elections 2024
- Republicans
- US politics
Most viewed
Help | Advanced Search
Mathematics > Optimization and Control
Title: a mean-field model of optimal investment.
Abstract: We establish the existence and uniqueness of the equilibrium for a stochastic mean-field game of optimal investment. The analysis covers both finite and infinite time horizons, and the mean-field interaction of the representative company with a mass of identical and indistinguishable firms is modeled through the time-dependent price at which the produced good is sold. At equilibrium, this price is given in terms of a nonlinear function of the expected (optimally controlled) production capacity of the representative company at each time. The proof of the existence and uniqueness of the mean-field equilibrium relies on a priori estimates and the study of nonlinear integral equations, but employs different techniques for the finite and infinite horizon cases. Additionally, we investigate the deterministic counterpart of the mean-field game under study.
Submission history
Access paper:.
- HTML (experimental)
- Other Formats
References & Citations
- Google Scholar
- Semantic Scholar
BibTeX formatted citation
Bibliographic and Citation Tools
Code, data and media associated with this article, recommenders and search tools.
- Institution
arXivLabs: experimental projects with community collaborators
arXivLabs is a framework that allows collaborators to develop and share new arXiv features directly on our website.
Both individuals and organizations that work with arXivLabs have embraced and accepted our values of openness, community, excellence, and user data privacy. arXiv is committed to these values and only works with partners that adhere to them.
Have an idea for a project that will add value for arXiv's community? Learn more about arXivLabs .
Mitch McConnell's support of the TikTok bill is a big deal, but that doesn't mean the app is doomed
- The head of the GOP Senate caucus cast his support on Monday for the TikTok bill.
- Despite passing in a bipartisan fashion in the House, there's no guarantee it'll pass the Senate.
- Several senators have said they think it would get struck down in court.

Senate Minority Leader Mitch McConnell declared his support on Monday for a bill circulating through Congress that would effectively ban TikTok , but the app's immediate demise is no guarantee.
Speaking on the Senate floor, McConnell said China uses the app and its user data to spy on the "170 million Americans" who are active TikTok users and pointed to a recent report from Microsoft about how the rivaling country used social media to influence elections.
"All sorts of social media platforms can be fountains of disinformation and propaganda," McConnell said. "Just look at last week's news about the PRC's efforts to manipulate Taiwan's elections with Twitter accounts driven by AI."
But even with the support of McConnell, there might not be 50 senators on either side of the aisle who'd vote for the bill, officially called the Protecting Americans from Foreign Adversary Controlled Applications Act.
After passing in the House of Representatives in a bipartisan landslide in mid-March, the bill now sits in the Senate Committee On Commerce, Science, and Transportation.
Related stories
Sen. Maria Cantwell, the committee's chair, has supported the idea of regulating TikTok in the past but hasn't completely supported this particular bill, instead preferring the one she drew up in 2023 that she never introduced.
According to Punchbowl News , Cantwell told reporters Monday night that she doesn't think the House's bill would stand in a court of law.
"But you also can't, as a Congress, just decide one day, 'Ah!' And pass a law," she said. "Well, you can, I just don't know if it'll hold up in court… Let's get something that can be upheld."
A handful of Republican and Democratic senators previously said they were concerned about TikTok and its parent company, ByteDance, being specifically named in the proposed legislation.
"You don't want to establish a precedent on naming an individual company," Sen. Todd Young said.
Cantwell's reportedly scheduled to meet with Senate Majority Leader Chuck Schumer and an advocate for the bill, Senate Intelligence Committee Chair Mark Warner, this week, which very well may determine TikTok's future in the US.
In a " Dear Colleague " letter detailing his goals post-Easter recess, Schumer didn't guarantee he'll bring the TikTok bill to a vote but did say he hoped "our Senate Republican colleagues don't allow the ultra-right wing of their party to derail progress on these bipartisan bills."
President Joe Biden has already said he'll sign the bill into law if it passes through the Senate. According to a March report from The New York Times, Biden's White House staffers have lobbied senators to try to get them on board.
Watch: TikTok could be banned in US after House vote
- Main content

IMAGES
VIDEO
COMMENTS
A given chemical reaction system is defined by a balanced net chemical equation which is conventionally written as. reactants→ products (11.1.1) (11.1.1) reactants → products. The first thing we need to know about a chemical reaction represented by a balanced equation is whether it can actually take place.
6.2: Thermodynamics and Equilibrium Chemistry. Thermodynamics is the study of thermal, electrical, chemical, and mechanical forms of energy. The study of thermodynamics crosses many disciplines, including physics, engineering, and chemistry. Of the various branches of thermodynamics, the most important to chemistry is the study of how energy ...
These equations are dynamic because the forward and reverse reactions are still occurring, but the two rates are equal and unchanging, so they're also at equilibrium. Dynamic equilibrium is an example of a system in a steady state. This means the variables in the equation are unchanging over time (since the rates of reaction are equal).
A graphical depiction of equilibrium. The graphical approach to equilibrium analysis is illustrated in Figure . The equilibrium price and quantity are determined by the intersection of the two curves. The equilibrium quantity is 4 units of good X, and the equilibrium price is $2 per unit of good X. This result is the same as the one obtained by ...
The analysis of a compound structure must always begin with the analysis of the complimentary structure, as the complimentary structure is supported by the primary structure. Using the equations of equilibrium, the support reactions of the beam are determined as follows: Analysis of the complimentary structure CB. Figure 3.13e.
The one moment vector equation becomes a single moment scalar equation. ∑M = 0 (3.6.3) (3.6.3) ∑ M → = 0. ∑Mz = 0 (3.6.4) (3.6.4) ∑ M z = 0. If we look at a three-dimensional problem we will increase the number of possible equilibrium equations to six. There are three equilibrium equations for force, where the sum of the components in ...
By comparing Q to K c , we can tell if the reaction is at equilibrium because Q = K c at equilibrium. If we calculate Q using the concentrations above, we get: Q = [ SO 3] 2 [ SO 2] 2 [ O 2] = [ 2.2] 2 [ 3.6] 2 [ 0.087] = 4.3. Because our value for Q is equal to K c , we know the new reaction is also at equilibrium.
The equilibrium is the only price where quantity demanded is equal to quantity supplied. At a price above equilibrium, like 1.8 dollars, quantity supplied exceeds the quantity demanded, so there is excess supply. At a price below equilibrium, such as 1.2 dollars, quantity demanded exceeds quantity supplied, so there is excess demand.
Instead is would reach chemical equilibrium ( reactants ⇌ products reactants ⇌ products ). Chemical equilibrium occurs when the number of particles becoming products is equal to the number of particles becoming reactants. A dynamic equilibrium is a state where the rate of the forward reaction is equal to the rate of the reverse reaction.
in a market setting, disequilibrium occurs when quantity supplied is not equal to the quantity demanded; when a market is experiencing a disequilibrium, there will be either a shortage or a surplus. equilibrium price. the price in a market at which the quantity demanded and the quantity supplied of a good are equal to one another; this is also ...
The Economic Analysis of Systems: Some systems are called Òequilibrium systems.Ó These systems have a tendency to ... equilibrium has alternative meanings and no single precise definition. Equilibrium is not easily defined because a different perspective will result in a different definition of equilibrium. Below, we offer two definitions of ...
Abstract. The need for scientific analysis springs from a fundamental bias of our natural and social environment against sameness and constancy. Physical objects that are similar in specified attributes will vary among themselves in the exact degree to which they possess such attributes. Static variation of this type is compounded with dynamic ...
Meaning of General Equilibrium Analysis: As against partial equilibrium analysis, general equilibrium analysis is concerned with economic system as a whole. It recognises the fact that economic system is a network in which all the parts are mutually dependent on one another and in mutual interaction with one another.
Equilibrium is the state in which market supply and demand balance each other and, as a result, prices become stable. Generally, when there is too much supply for goods or services, the price goes ...
Equilibrium analysis can be viewed as sort of thought experiment where to organize your thinking passage of time is held constant. ... The most famous definition of equilibrium is perhaps equilibrium in markets as equality between demand and supply of the goods, in particular the definition of equilibrium in walrasian model, as in modern ...
Equilibrium - The state in which the measurable properties of the system such as pressure, density, colour or concentration do not undergo any further noticeable changes with time under a given set of conditions is said to be a state of equilibrium. To learn more about Classification, Example, Description, Characteristics and FAQs of equilibrium, Visit BYJU'S.
Equilibrium would be lost if either any of the competition would suddenly release the rope. A structure is in equilibrium when all forces or moments acting upon it are balanced. This means that each and every force acting upon a body, or part of the body, is resisted by either another equal and opposite force or set of forces whose net result ...
Scitovsky Contours and cost-bene t analysis 20 6. Excess demand functions 22 6.1. Notation 22 6.2. Aggregate excess demand in an exchange economy 22 6.3. Aggregate excess demand 25 ... GENERAL EQUILIBRIUM THEORY 9 be endowed with non-negative quantities of each good. These are the goods that are sold in market, at the prevailing market prices ...
Acid-Base Reactions. A useful definition of acids and bases is that independently introduced in 1923 by Johannes Brønsted and Thomas Lowry. In the Brønsted‐Lowry definition, an acid is a proton donor and a base is a proton acceptor. Note the connection between these definitions—defining a base as a proton acceptor implies there is an acid available to donate the proton.
In partial equilibrium analysis, the determination of the price of a good is simplified by just looking at the price of one good, and assuming that the prices of all other goods remain constant. The Marshallian theory of supply and demand is an example of partial equilibrium analysis. Partial equilibrium analysis is adequate when the first ...
Partial equilibrium implies analyzing one market in isolation from all other markets. This is in fact the very definition of partial equilibrium analysis where only direct effects are taken into account while omitting possible indirect and induced or feedback impacts that occur simultaneously in other interrelated markets.
Partial equilibrium analysis is the counterpart of microeconomic analysis. It is also preferably called 'particular equilibrium' analysis because the word 'partial' smacks of incompleteness of the analysis when there is nothing incomplete about it. A large part of the economic analysis is built around the concept of equilibrium of a ...
Y=a + bY+Io + Go or (1 - h)Y = a + Id 4- Go (collecting terms involving Y) To find the solution value of Y (equilibrium national income), we only have to divide through by (1 - b) : Note, again, that the solution value is expressed entirely in terms of the parameters and exogenous variables, the given data of the model.
Trump's tone, as many have noted, is decidedly more vengeful this time around, as he seeks to reclaim the White House after a bruising loss that he insists was a steal.
A mean-field model of optimal investment. We establish the existence and uniqueness of the equilibrium for a stochastic mean-field game of optimal investment. The analysis covers both finite and infinite time horizons, and the mean-field interaction of the representative company with a mass of identical and indistinguishable firms is modeled ...
What is the meaning of cohesion reduction factor, defined in Geotechnical option dialog, while spread footing design, according to EN-1997 in Robot Structural Analysis. Cohesion reduction factor is used to consider soil cohesion while sliding verification. By default it is set to 0 (cohesion is not considered)
SWOT Analysis is a strategic planning tool utilized to evaluate the strengths, weaknesses, opportunities, and threats involved in a project or in a business venture. Advertisements. It aids individuals and organizations in identifying both internal and external factors that could impact their objectives. By analyzing strengths and weaknesses ...
Mitch McConnell's support of the TikTok bill is a big deal, but that doesn't mean the app is doomed. Analysis by Madison Hall. Apr 9, 2024, 9:29 AM PDT. Senate Minority Leader Mitch McConnell ...