An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Case Rep Womens Health
- v.33; 2022 Jan

A case report of successful vaginal delivery in a patient with severe uterine prolapse and a review of the healing process of a cervical incision
Associated data.
The incidence of severe uterine prolapse during childbirth is approximately 0.01%. Moreover, to the best of our knowledge, no reports detail the healing process of the cervix during uterine involution. This report describes successful vaginal delivery and the healing process of postpartum uterine prolapse and cervical tears in a patient with severe uterine prolapse.
Case presentation
A patient in her 40s (gravida 3, para 1, abortus 1) with severe uterine prolapse successfully delivered a live female baby weighing 3190 g at 38 + 5 weeks of gestation by assisted vaginal delivery. Uterine prolapse had improved to approximately 2° by 2 months postoperatively. On postpartum day 4, during the healing process of cervical laceration, the thread loosened in a single layer of continuous sutures due to uterine involution, and poor wound healing was observed. The wound was subsequently re-sutured with a two-layer single ligation suture (Gambee suture + vertical mattress suture). However, on postpartum day 11, a large thread ball was hindering the healing of the muscle layer, which improved with re-suturing.
Although vaginal delivery in a patient with severe uterine prolapse is possible in some cases, the cervix should be sutured, while considering cervical involution after delivery.
- • Pregnancies with complete uterine prolapse are exceedingly rare.
- • When suturing the uterus, involution after delivery should be considered.
- • This is the first report on the healing process of cervical canal lacerations.
1. Introduction
Pregnancies complicated by uterine prolapse are rare, and cases of severe uterine prolapse are rarer [ 1 ]. Recommendations regarding the management of this infrequent but potentially fatal condition are scarce [ 1 ]. Pregnancies with severe uterine and cervical prolapse have been reported; however, the delivery method is complicated in most cases, and few reports of successful vaginal delivery despite severe cervical edema have been reported in the literature [ 2 ]. This report describes a case of vaginal delivery in a patient with severe uterine prolapse and presents the first description of the healing process after a Duhrssen incision performed on a prolapsed cervix.
2. Case Presentation
A 43-year-old woman, gravida 3, para 1, abortus 1, implanted with frozen-thawed embryos was diagnosed with high-risk pregnancy at her previous hospital. Her obstetric history included a term, vaginal singleton delivery (3298 g, male neonate) 4 years earlier, which was complicated by a retained placenta. The placenta was removed manually. Thereafter, no uterine prolapse was observed.
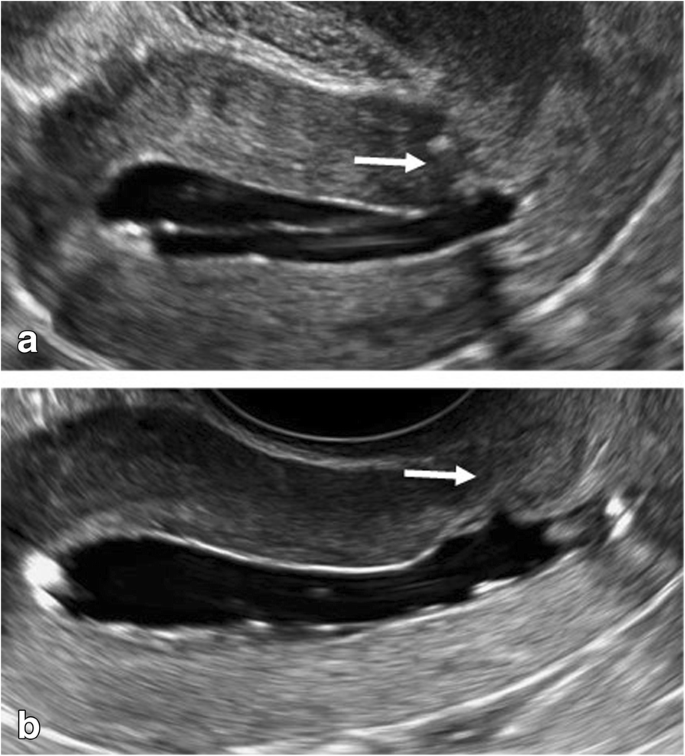
She had been undergoing treatment for hypertension and dermatomyositis since 2018. She had been prescribed nifedipine CR 20 mg/day for the hypertension and steroids (prednisolone) 4.0 mg/day and tacrolimus 3.4 mg/day for the dermatomyositis. As the symptoms of dermatomyositis improved in 2019, the pregnancy was approved. She had also undergone sub-plasmalemmal myomectomy 10 years previously. She was otherwise fit and had a normal body mass index; she did not smoke and had no allergies. During the index pregnancy, at the 19-week check-up, her cervix was found to have descended. Subsequent regular ultrasound scans at 24, 26, 30, 32, and 34 weeks of gestation demonstrated normal fetal morphology, with normal and concordant fetal growth. An ultrasound scan at 36 weeks showed that the cervical canal was very long (8.8 cm) and closed ( Fig. 1 ). A 95-mm self-removable donut-shaped pessary (donut support for 3° prolapse/procidentia; CooperSurgical Milex™, 75 Corporate Drive Trumbull, CT 06611 USA) appeared to drop out spontaneously. Color Doppler imaging with 3D transvaginal ultrasonography revealed cervical edema ( Fig. 2 ). Color Doppler examination did not reveal any obvious blood flow. She was admitted to hospital at 37 + 2 weeks due to a worsening skin rash over the perineum and difficulty in washing. Her blood pressure was stable, her general condition was good, and there were no abnormal clinical or biochemical findings suggestive of an infection.

Photographs at the onset of uterine prolapse at 36 weeks of gestation.

Cervical edema and cervical length of 8.8 cm, color Doppler with 3D transvaginal ultrasonography.
A, Sagittal section; B, Coronal section; C, Transverse section; D, Color Doppler.
In the pre-induction state of the cervix, the Bishop scoring system showed that the cervical os was dilated by 4 cm, effaced 0%, station −3, soft, and anterior 7 points. Induction with oxytocin was commenced at 38 weeks and 4 days of gestation for planned delivery, but there were no effective contractions. The next day, the cervix was dilated by 6 cm and effaced 30%.
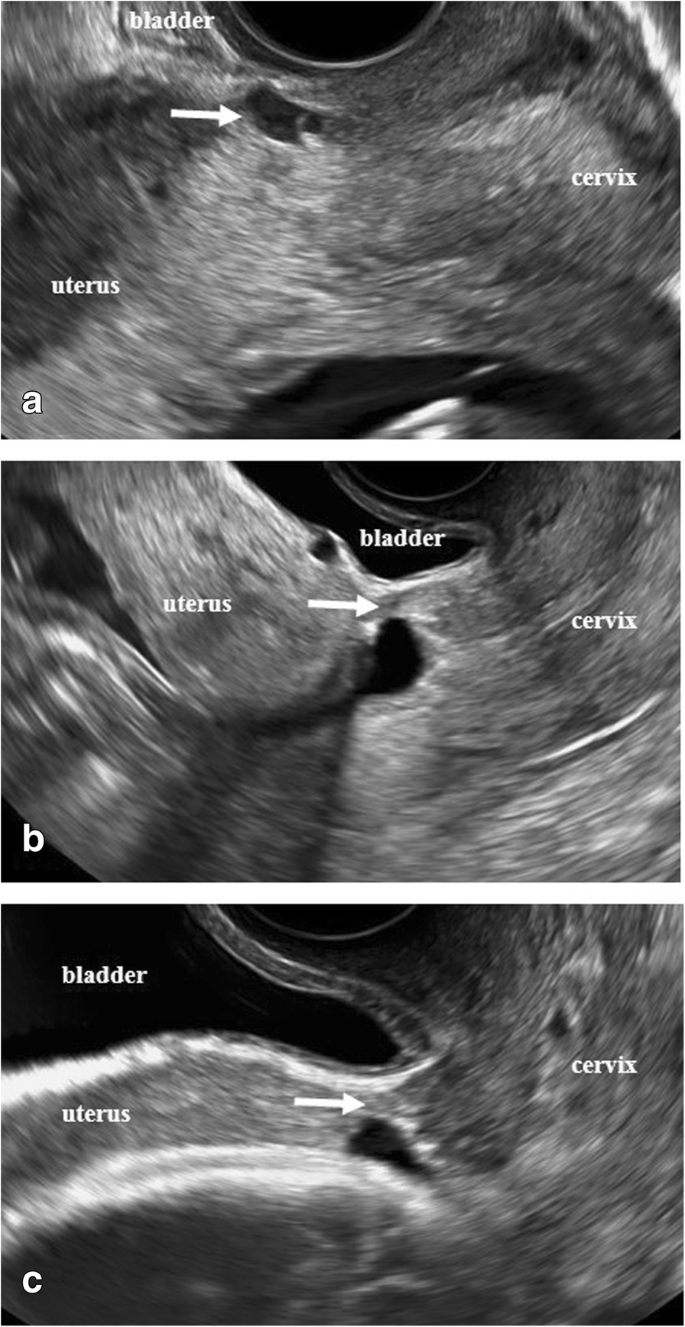
With the patient's consent, we performed an artificial rupture of the membranes, which led to labor progression. After an intramuscular injection of butyl scopolamine, her cervix was softened for a short time. The cervix was completely prolapsed outside the vagina, but the station had reached +1 in relation to the pubic symphysis. The 95-mm donut-type pessary was easily removed from the soft birth canal, and vacuum extraction delivery (Kiwi®) was deemed feasible. Cervix-holding forceps were used, and a Duhrssen incision was made under visualization as labor progressed. A cervical Duhrssen incision of approximately 5 cm was made, and the length of the incision was extended to 10 cm. We did not perform a perineal incision. After three traction cycles, a live female neonate weighing 3190 g was delivered, with Apgar scores of 8/9 (1st and 5th minutes) and a pH of 7.319. The delivery was complicated with a retained placenta and massive postpartum hemorrhage (PPH), with blood loss of 2500 mL. The cervix was sutured, as usual, using 0 Vicryl® sutures in one continuous layer from 5 mm above the upper edge of incision, and the surface of the suture appeared to be fine. However, on discharge examination on the 4th day post-delivery, a complete breakdown of the repair of approximately 5 cm of the cervical canal was identified at the introitus. After she was returned to the operating theatre, debridement was performed; the first layer was sutured with a single ligation using the Gambee suture (2–0 Vicryl®), and the second layer was sutured with an end-to-end single ligation using the mattress suture technique (2–0 Vicryl®). On the 7th day after re-suturing (11th day after delivery), the uterus was markedly involuted, and uterine prolapse somewhat improved, but the ligature threads were collected in one place and became a single ball of threads. All sutures were removed, and the wound was re-sutured with two Z-sutures using a 3–0 Vicryl® thread. Sutures were removed 1 month postpartum, and complete wound healing was confirmed. Two months post-surgery, uterine prolapse had improved to a 2° prolapse. The healing process of cervical laceration and cervical canal prolapse is shown in Fig. 3 .

Healing process of cervical laceration and cervical canal prolapse.
A: Cervical laceration suture surface on the day of delivery.
B: On postpartum day 4, suture failure was noted due to a continuous 1-layer suture (0 mesh thread (Vicryl®), absorbable thread).
C: On postpartum day 4, stitches were removed and debridement was performed.
D: On postpartum day 4, re-suture and first-layer Gambee suture (2–0 mesh thread, absorbable (Vicryl®)) were performed.
E: On postpartum day 4, re-suture and second-layer horizontal mattress suture (2–0 mesh thread, absorbable (Vicryl®)) were performed.
F: On postpartum day 11, the thread had formed into a ball, which interfered with viability. The wound was re-sutured using a single ligation due to suture failure (3–0 mesh thread, absorbable (Vicryl®)).
G: On postpartum day 28, uterine prolapse improved to 2°–3°, and the wound became viable.
3. Discussion
Uterine and cervical prolapse are rare in pregnancy. Uterine prolapse during pregnancy is a precarious event, with an incidence of 1 in 10,000–15,000 pregnancies [ 3 ]. It can be associated with minor cervical desiccation and ulceration or devastating maternal fatalities. Complications include urinary retention, preterm labor, premature delivery, fetal demise, maternal sepsis, and urinary retention [ 1 ].
Prolapse development in pregnancy is likely due to an escalation of the physiological changes in pregnancy, leading to a weakening of pelvic organ support [ 4 ]. Increased cortisol and progesterone levels during pregnancy may contribute to uterine relaxation. In our case, the patient did not have any congenital risk factors. However, she had developed dermatomyositis after her last delivery and had been prescribed prednisolone 4 mg/day which she had taken consistently since before pregnancy. In a previously reported case study, pessary insertion resulted in the temporary improvement of uterine prolapse [ 5 , 6 ], but, in our case, a 95-mm donut pessary (CooperSurgical Milex™) easily slipped out of the soft birth canal. According to a case review of pregnancies with uterine prolapse reported by Kana et al. [ 7 ], since 1997, vaginal delivery could be performed in only 2 of 16 patients with cervical edema, and none of them was diagnosed with complete uterine prolapse. Jeong et al. [ 8 ] reported that, since 2002, only 1 of 14 patients with uterine prolapse was able to be delivered vaginally. These results suggest that this study reports a valuable case of vaginal delivery. In fact, the two cases reported recently were both delivered by cesarean section [ 9 , 10 ].
We performed vaginal delivery, and a Duhrssen incision was stitched with minimal blood loss. In active labor, a prolapsed cervix that is enlarged and edematous can be managed with a topical concentrated magnesium solution to treat cervical dystocia and lacerations [ 11 ]. Verma et al. reported that two incisions approximately 3 cm long each were made at 2 o'clock and 10 o'clock positions on the cervix using tissue-cutting scissors [ 12 ]. In contrast, our approach involves using one incision that is approximately 5 cm long at the 6 o'clock position on the cervix using tissue-cutting scissors. Cervical laceration suturing was performed, and we observed that a single layer of continuous sutures had lost tension and was loose due to the significant involution of the cervix. Moreover, the external os was found outside the vagina; thus, the wound could not be covered, and thread traction could not prevent incision separation at 6 o'clock. After debridement, Gambee sutures and horizontal mattress sutures were used to re-suture the wound, but on postpartum examination on day 11, the ligature thread from the single ligation suture had formed a ball of threads. We re-sutured the wound using 3–0 Vicryl® sutures, and the wound was stabilized. The cervix was edematous and markedly dilated; therefore, any sutures are likely to be extruded as the cervix involutes and the edema improves. A technique that results in the end-to-end apposition of the incision is more likely to succeed as it allows for clot formation and healing by primary intention with less reliance on suture tension. Nevertheless, the majority of such cases have been managed by cesarean section. This vaginal delivery resulted in a prolonged process of labor induction, cervical incision (which likely induced long-term cervical incompetence), three trips to the operating room, a retained placenta, and a massive PPH; however, direct causality of the last two factors is unknown. Avoiding cesarean section in this case resulted in an unavoidable burden on the patient after delivery. Although we do not necessarily recommend vaginal delivery, we report it as an option. An extensive literature search listing the keywords “healing process, cervical canal lacerations, single ligation suture, and continuous sutures” revealed that this is the first report on the healing process of cervical canal lacerations.
4. Conclusion
Vaginal delivery of a pregnancy complicated by severe uterine prolapse is possible in some cases. Given the paucity of data on the management and pregnancy outcomes of patients with uterine anomalies, such cases should be considered high risk, and individualized care should be given to patients to optimize outcomes. Moreover, when suturing the uterus cervix, uterus cervical involution after delivery should be considered. This case report adds to the literature as it highlights the management of a rare obstetric condition.
The following is the supplementary data related to this article.
Video of assisted vaginal delivery and salvage from cervical dystocia in uterocervical prolapse: Duhrssen incision
Supplementary material
Contributors
Jota Maki was responsible for the conceptualization of the case report, was the main author, conducted the literature review, and drafted the manuscript, and was involved in the patient’s treatment, from outpatient care to hospitalization and discharge.
Tomohiro Mitoma was responsible for the conceptualization of the case report, was involved in the case, and reviewed and revised the manuscript.
Sakurako Mishima was responsible for the conceptualization of the case report, and reviewed the draft.
Akiko Ohira was responsible for the conceptualization of the case report, and reviewed the draft.
Kazumasa Tani was responsible for the conceptualization of the case report, and reviewed the draft.
Eriko Eto was responsible for the conceptualization of the case report, was involved in the case, and reviewed and revised the manuscript.
Kei Hayata was responsible for the conceptualization of the case report, and reviewed the draft.
Hisashi Masuyama was responsible for the conceptualization of the case report, was involved in the case, and reviewed and revised the manuscript.
All authors approved the final version of the paper and take full responsibility for the work.
This work did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Patient consent
The patient provided informed consent for the publication of this report and all accompanying images.
Provenance and peer review
This case report was not commissioned and was peer reviewed.
Acknowledgements
We thank the obstetrics surgeons and other healthcare personnel involved in the treatment of patients at the Department of Obstetrics and Gynecology, Okayama University Hospital.
Conflict of interest statement
The authors declare that they have no conflict of interest regarding the publication of this case report.
- Case report
- Open access
- Published: 21 June 2018
Normal vaginal delivery at term after expectant management of heterotopic caesarean scar pregnancy: a case report
- Olga Vikhareva 1 , 2 ,
- Ekaterina Nedopekina 1 , 2 &
- Andreas Herbst 1 , 2
Journal of Medical Case Reports volume 12 , Article number: 179 ( 2018 ) Cite this article
14k Accesses
8 Citations
1 Altmetric
Metrics details
Heterotopic pregnancy with a combination of a caesarean scar pregnancy and an intrauterine pregnancy is rare and has potentially life-threatening complications.
Case presentation
We describe the case of a 27-year-old white woman who had experienced an emergency caesarean delivery at 39 weeks for fetal distress with no postpartum complications. This is a report of the successful expectant management of a heterotopic scar pregnancy. The gestational sac implanted into the scar area was non-viable. The woman was treated expectantly and had a normal vaginal delivery at 37 weeks of gestation.
Expectant management under close monitoring can be appropriate in small non-viable heterotopic caesarean scar pregnancies.
Peer Review reports
Heterotopic caesarean scar pregnancy (CSP), in which one gestational sac is located in the caesarean scar area and the other one is a normal intrauterine pregnancy, is rare and may have potentially life-threatening complications. The correct management of this condition is unclear. It is a challenge to manage a heterotopic CSP with preservation of the intrauterine pregnancy minimizing the risks for mother and child.
Transvaginal sonography is a valuable diagnostic tool in the management of such pregnancies [ 1 ]. Currently, we offer women with one previous caesarean section participation in an ongoing study of transvaginal ultrasound examinations in each trimester. The study provides support to identify patients with high risk of uterine rupture/potential placental complications to make an individual plan for pregnancy surveillance and delivery.
We have not found previous reports on successful expectant management of spontaneous heterotopic CSP with the preservation of intrauterine pregnancy resulting in a normal vaginal delivery.
We describe the case of a 27-year-old white woman who had experienced an emergency caesarean delivery at 39 weeks for fetal distress with no postpartum complications. As part of our ongoing study “Vaginal delivery after caesarean section”, she underwent saline contrast sonohysterography 6 months after the caesarean section. The caesarean scar had a small indentation and the remaining myometrium over the defect was 7.5 mm (Fig. 1a ).
Saline contrast sonohysterography images. The arrows indicate the caesarean section scar 6 months after the index caesarean ( a ) and 6 months after the end of the heterotopic caesarean scar pregnancy by vaginal delivery ( b ). The thickness of the remaining myometrium appeared almost unchanged
In the current pregnancy, she had a dating scan at around 11 weeks with no remarks. She came for a transvaginal ultrasound examination at around 13 weeks as part of our study. This scan revealed a duplex pregnancy with one viable intrauterine fetus with normal anatomy and placenta located high on the anterior wall and a small gestational sac (8 mm) with a yolk sac without embryo was located in the caesarean scar (Fig. 2a ). There was no extensive vascularity surrounding the sac. One corpus luteum was found in each of the two ovaries. She was asymptomatic.
Transvaginal sonographic images. The arrows indicate the appearance of the cesarean scar area at the presence of the scar pregnancy at 13 + 2 ( a ) and after reabsorption of the scar pregnancy at 22 + 0 ( b ) and at 30 + 2 ( c ) weeks of gestation
She was informed that not enough evidence existed to advise a specific management of this condition. After discussion with her and her husband, expectant management was chosen with a new ultrasound examination after 5 weeks.
She came to our ultrasound department at 18 weeks, 22 weeks, and 30 weeks of gestation. She remained asymptomatic. The ectopic gestational sac was not visualized with transvaginal or transabdominal scans at the 18 weeks examination (Fig. 2b ). The niche in the scar and the thickness of the thinnest part of the remaining myometrium appeared unchanged at all visits. The intrauterine pregnancy developed normally with no signs of abnormal placentation. At 30 weeks of gestation the ultrasound appearance of the scar area did not indicate any contraindications for vaginal delivery. The thickness of the lower uterine segment (LUS) was 4.9 mm (Fig. 2c ). In agreement with our patient, vaginal delivery was planned. The staff of the labor ward was fully informed.
She was admitted to the labor ward with irregular contractions in week 37 + 0. Her cervix dilated to 3 cm with no further progress. Due to that oxytocin augmentation was administered for 3 hours. The duration of active labor was 6.5 hours. A healthy male neonate weighing 2985 g was delivered, with Apgar scores 9–10 at 1 and 5 minutes and umbilical cord pH 7.27. The placenta delivered spontaneously and total blood loss was 250 ml. The postpartum period was without any complications, and she was discharged home the next day.
At a follow-up visit 6 months postpartum, saline contrast sonohysterography showed no signs of the previous CSP, and the remaining myometrium over the hysterotomy scar defect was 5.7 mm (Fig. 1b ).
Ethical approval for the ongoing study was obtained by the Ethics Committee of the Medical Faculty of Lund University, Sweden, reference number 2013/176. Our patient has given permission for publication of this case report in a scientific journal.
Discussion and conclusions
Management of heterotopic CSP with an intrauterine gestation is a challenge. Treatment options for CSP include expectant management, and medical or surgical termination [ 1 , 2 , 3 , 4 ].
The use of methotrexate has been reported in management of ectopic gestations, but in heterotopic pregnancies with preservation of intrauterine pregnancy this may cause a teratogenic effect with fetal anomalies [ 5 , 6 ].
A few case reports have described treatment of heterotopic CSP viable pregnancies with local injection of potassium chloride. This method is commonly used for fetal reduction in multiple pregnancy [ 7 , 8 , 9 ]. Treatment with potassium chloride is associated with an increased risk of abdominal pain, pregnancy loss, excessive vaginal bleeding, prematurity, need for subsequent surgery, and spontaneous rupture of membranes and subsequent chorioamnionitis [ 1 , 8 , 9 , 10 , 11 , 12 ].
Laparoscopic treatment can be an option for removal of an ectopic scar pregnancy, but there is increased risk of hemorrhage and miscarriage [ 7 , 13 , 14 , 15 ].
Michaels et al. suggested that expectant management can be appropriate in early gestations with no heartbeat, often resulting in complete absorption of the trophoblast [ 10 ]. Our patient had no bleeding or abdominal pain. The gestational sac located in the scar was non-viable with no extensive vascularity.
It is difficult to study possible changes in the tissues of the caesarean scar area after reabsorption of CSP. With ultrasound one can appreciate the thickness of LUS during the pregnancy, but not the quality of the myometrium.
Interestingly, it was found that our patient had a small defect in the uterine scar detected at ultrasound 6 months after caesarean section. Jurkovic et al. reported 18 cases of CSP over a 4-year period [ 1 ]. They observed that the majority of scars were well-healed. These data suggest that the size of a defect in the scar does not increase the risk of CSP; however, more studies are needed.
Our patient had a “normal” dating scan at 11 weeks. The early diagnosis of a heterotopic CSP is easy to miss, in particular with presence of an intrauterine viable embryo. Serum human chorionic gonadotropin (hCG) is of little value as long as an intrauterine pregnancy is ongoing. Transvaginal ultrasound is the best tool for diagnosis and management of such pregnancies. In our ongoing study “Vaginal delivery after caesarean section” we assess multiple parameters: scar area/scar pregnancy/potential placental complications. These ultrasound characteristics and clinical evaluation together with close monitoring provide support for the obstetrician in management of these women.
The literature is sparse and we still lack evidence and strong clinical guidelines to manage heterotopic pregnancies. Each woman diagnosed with scar implantation should receive an individual approach.
Abbreviations
Caesarean scar pregnancy
Human chorionic gonadotropin
Lower uterine segment
Jurkovic D, Hillaby K, Woelfer B, Lawrence A, Salim R, Elson CJ. First trimester diagnosis and management of pregnancies implanted into the lower uterine segment Cesarean section scar. Ultrasound Obstet Gynecol. 2003;21(3):220–7.
Article PubMed CAS Google Scholar
Osborn DA, Williams TR, Craig BM. Cesarean scar pregnancy: sonographic and magnetic resonance imaging findings, complications, and treatment. J Ultrasound Med. 2012;31:1449–56.
Article PubMed Google Scholar
Uysal F, Usal A, Adam G. Cesarean scar pregnancy: diagnosis, management, and follow-up. J Ultrasound Med. 2013;32:1295–300.
Litwicka K, Greco E. Caesarean scar pregnancy: a review of management options. Curr Opin Obstet Gynecol. 2011;23:415–21.
Jordan RL, Wilson JG, Schumacher HJ. Embryotoxicity of the folate antagonist methotrexate in rats and rabbits. Teratology. 1977;15(1):73–9.
Timor-Tritsch IE. Is it safe to use methotrexate for selective injection in heterotopic pregnancy? Am J Obstet Gynecol. 1998;178:193–4.
PubMed CAS Google Scholar
Timor-Tritsch IE, Monteagudo A. Unforeseen consequences of the increasing rate of cesarean deliveries: early placenta accreta and cesarean scar pregnancy. A review. Am J Obstet Gynecol. 2012;207(1):14–29.
Mohammed ABF, Farid I, Ahmed B, Ghany EA. Obstetric and neonatal outcome of multifetal pregnancy reduction. Middle East Fertil Soc J. 2015;20(3):176–81.
Article Google Scholar
AlShelaly UE, Al-Mousa NH, Kurdi WI. Obstetric outcomes in reduced and non-reduced twin pregnancies. Saudi Med J. 2015;36(9):1122–5.
Article PubMed PubMed Central Google Scholar
Michaels AY, Washburn EE, Pocius KD, Benson CB, Doubilet PM, Carusi DA. Outcome of cesarean scar pregnancies diagnosed sonographically in the first trimester. J Ultrasound Med. 2015;34(4):595–9.
Salomon LJ, Fernandez H, Chauveaud A, Doumerc S, Frydman R. Successful management of a heterotopic Caesarean scar pregnancy: potassium chloride injection with preservation of the intrauterine gestation: case report. Hum Reprod. 2003;18(1):189–91.
Monteagudo A, Minior VK, Stephenson C, Monda S, Timor-Tritsch IE. Non-surgical management of live ectopic pregnancy with ultrasound-guided local injection: a case series. Ultrasound Obstet Gynecol. 2005;25(3):282–8.
Wang CJ, Chao AS, Yuen LT, Wang CW, Soong YK, Lee CL. Endoscopic management of cesarean scar pregnancy. Fertil Steril. 2006;85(2):494.e1-4.
Ash A, Smith A, Maxwell D. Caesarean scar pregnancy. BJOG. 2007;114(3):253–63.
Demirel LC, Bodur H, Selam B, Lembet A, Ergin T. Laparoscopic management of heterotopic cesarean scar pregnancy with preservation of intrauterine gestation and delivery at term: case report. Fertil Steril. 2009;91(4):1293.e5-7.
Download references
Availability of data and materials
Data are available in internal digitalized record systems of Skåne University Hospital.
Author information
Authors and affiliations.
Department of Obstetrics and Gynaecology, Skåne University Hospital Malmö, Lund University, Box 117, 221 00, Lund, Sweden
Olga Vikhareva, Ekaterina Nedopekina & Andreas Herbst
Department of Obstetrics and Gynaecology, Skåne University Hospital, Lund University, Jan Waldenströms gata 47, SE-20502, Malmö, Sweden
You can also search for this author in PubMed Google Scholar
Contributions
All authors EN, AH, and OV contributed to the analysis and interpretation of the data. The first draft was written by EN and OV and all the authors revised it critically and approved the final version to be published.
Corresponding author
Correspondence to Olga Vikhareva .
Ethics declarations
Ethics approval and consent to participate.
Ethical approval was obtained by the Ethics Committee of the Medical Faculty of Lund University, Sweden, reference number 2013/176.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Reprints and permissions
About this article
Cite this article.
Vikhareva, O., Nedopekina, E. & Herbst, A. Normal vaginal delivery at term after expectant management of heterotopic caesarean scar pregnancy: a case report. J Med Case Reports 12 , 179 (2018). https://doi.org/10.1186/s13256-018-1713-0
Download citation
Received : 13 February 2018
Accepted : 08 May 2018
Published : 21 June 2018
DOI : https://doi.org/10.1186/s13256-018-1713-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Heterotopic caesarean scar pregnancy
- Expectant management
- Vaginal delivery
Journal of Medical Case Reports
ISSN: 1752-1947
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]

LEE T. DRESANG, MD, AND NICOLE YONKE, MD, MPH
Am Fam Physician. 2015;92(3):202-208
Author disclosure: No relevant financial affiliations.
Most of the nearly 4 million births in the United States annually are normal spontaneous vaginal deliveries. In the first stage of labor, normal birth outcomes can be improved by encouraging the patient to walk and stay in upright positions, waiting until at least 6 cm dilation to diagnose active stage arrest, providing continuous labor support, using intermittent auscultation in low-risk deliveries, and following the Centers for Disease Control and Prevention guidelines for group B streptococcus prophylaxis. Most women with a low transverse uterine incision are candidates for a trial of labor after cesarean delivery and should be counseled accordingly. Pain management during labor includes complementary modalities and systemic opioids, epidural anesthesia, and pudendal block. Outcomes in the second stage of labor can be improved by using warm perineal compresses, allowing women more time to push before intervening, and offering labor support. Delayed pushing increases the length of the second stage of labor and does not affect the rate of spontaneous vaginal delivery. A tight nuchal cord can be clamped twice and cut before delivery of the shoulders, or the baby may be delivered using a somersault maneuver in which the cord is left nuchal and the distance from the cord to placenta minimized by pushing the head toward the maternal thigh. After delivery, skin-to-skin contact with the mother is recommended. Beyond 35 weeks' gestation, there is no benefit to bulb suctioning the nose and mouth. Postpartum maternal and neonatal outcomes can be improved through delayed cord clamping, active management to prevent postpartum hemorrhage, careful examination for external anal sphincter injuries, and use of absorbable synthetic suture for second-degree perineal laceration repair. Practices that will not improve outcomes and may result in negative outcomes include discontinuation of epidurals late in labor and routine episiotomy.
Out of the nearly 4 million births in the United States in 2013, approximately 3 million were vaginal deliveries. 1 Accurate pregnancy dating is essential for anticipating complications and preparing for delivery. A woman's estimated due date is 40 weeks from the first day of her last menstrual period. If ultrasonography is performed, the due date calculated by the first ultrasound will either confirm or change the due date based on the last menstrual period ( Table 1 ) . 2 If reproductive technology was used to achieve pregnancy, dating should be based on the timing of embryo transfer. 2
When describing how a pregnancy is dated, “by last menstrual period” means ultrasonography has not been performed, “by X-week ultrasonography” means that the due date is based on ultrasound findings only, and “by last menstrual period consistent with X-week ultrasound findings” means ultrasonography confirmed the estimated due date calculated using the last menstrual period.
Table 2 defines the classifications of terms of pregnancies. 3 Maternity care clinicians can learn more from the American Academy of Family Physicians (AAFP) Advanced Life Support in Obstetrics (ALSO) course ( https://www.aafp.org/also ). Emergency medical technicians, medical students, and others with limited maternity care experience may benefit from the AAFP Basic Life Support in Obstetrics course ( https://www.aafp.org/blso ), which offers a module on normal labor and delivery. The Global ALSO manual ( https://www.aafp.org/globalalso ) provides additional training for normal delivery in low-resource settings.
Onset of Labor
Labor begins when regular uterine contractions cause progressive cervical effacement and dilation. The risk of infection increases after rupture of membranes, which may occur before or during labor. Induction is recommended for a term pregnancy if the membranes rupture before labor begins. 4 Intrapartum antibiotic prophylaxis is indicated if the patient is positive for group B streptococcus at the 35- to 37-week screening or within five weeks of screening if performed earlier in pregnancy, or if the patient has group B streptococcus bacteriuria in the current pregnancy or had a previous infant with group B streptococcus sepsis. 5 If the group B streptococcus status is unknown at the time of labor, the patient should receive prophylaxis if she is less than 37 weeks' gestation, the membranes have been ruptured for 18 hours or more, she has a low-grade fever of at least 100.4°F (38°C), or an intrapartum nucleic acid amplification test result is positive. 5
The First Stage of Labor
The first stage of labor begins with regular uterine contractions and ends with complete cervical dilation (10 cm). Reanalysis of data from the National Collaborative Perinatal Project (including 39,491 deliveries between 1959 and 1966) and new data from the Consortium on Safe Labor (including 98,359 deliveries between 2002 and 2008) have led to reevaluation of the normal labor curve. Latent labor lasting many hours is normal and is not an indication for cesarean delivery. 6 – 8 Active labor with more rapid dilation may not occur until 6 cm is achieved. Cesarean delivery for failure to progress in active labor is indicated only if the woman is 6 cm or more dilated with ruptured membranes, and she has no cervical change for at least four hours of adequate contractions (more than 200 Montevideo units per intrauterine pressure catheter) or inadequate contractions for at least six hours. 8 If possible, the membranes should be ruptured before diagnosing failure to progress. Labor can be significantly longer in obese women. 9 Walking, an upright position, and continuous labor support in the first stage of labor increase the likelihood of spontaneous vaginal delivery and decrease the use of regional anesthesia. 10 , 11
Most women who have had a prior cesarean delivery with a low transverse uterine incision are candidates for labor after cesarean delivery (LAC) and should be counseled accordingly. 12 A recent AAFP guideline concludes that planned labor and vaginal delivery are an appropriate option for most women with a previous cesarean delivery. 13 Women who may want more children should be encouraged to try LAC because the risk of pregnancy complications increases with increasing number of cesarean deliveries. 12 The risk of uterine rupture with cesarean delivery is less than 1%, and the risk of the infant dying or having permanent brain injury is approximately one in 2,000 (the same as for vaginal delivery in primiparous women). 14 Based on the clinical scenario, women with two prior cesarean deliveries may also try LAC. 12 Contraindications to vaginal delivery are outlined in Table 3 .
In low-risk deliveries, intermittent auscultation by handheld Doppler ultrasonography has advantages over continuous electronic fetal monitoring. Although continuous electronic fetal monitoring is associated with a decrease in the rare outcome of neonatal seizures, it is associated with an increase in cesarean and assisted vaginal deliveries with no other improvement in neonatal outcomes. 15 When electronic fetal monitoring is employed, the National Institute of Child Health and Human Development definitions and categories should be used ( Table 4 ) . 16
Pain management includes nonpharmacologic and pharmacologic methods. 17 Nonpharmacologic approaches include acupuncture and acupressure 18 ; other complementary and alternative therapies, including audioanalgesia, aromatherapy, hypnosis, massage, and relaxation techniques 19 ; sterile water injections 17 ; continuous labor support 11 ; and immersion in water. 20 Pharmacologic analgesia includes systemic opioids, nitrous oxide, epidural anesthesia, and pudendal block. 17 , 21 Although epidurals provide better pain relief than systemic opioids, they are associated with a significantly longer second stage of labor; an increased rate of oxytocin (Pitocin) augmentation; assisted vaginal delivery; and an increased risk of maternal hypotension, urinary retention, and fever. 22 Cesarean delivery for abnormal fetal heart tracings is more common in women with epidurals, but there is no significant difference in overall cesarean delivery rates compared with women who do not have epidurals. 22 Discontinuing an epidural late in labor does not increase the likelihood of vaginal delivery and increases inadequate pain relief. 23
The Second Stage of Labor
The second stage begins with complete cervical dilation and ends with delivery. The fetal head comes below the pubic symphysis and then extends. Pushing can begin once the cervix is fully dilated. Although delayed pushing or “laboring down” shortens the duration of pushing, it increases the length of the second stage and does not affect the rate of spontaneous vaginal delivery. 24 Arrest of the second stage of labor is defined as no descent or rotation after two hours of pushing for a multiparous woman without an epidural, three hours of pushing for a multiparous woman with an epidural or a nulliparous woman without an epidural, and four hours of pushing for a nulliparous woman with an epidural. 8 A prolonged second stage in nulliparous women is associated with chorioamnionitis and neonatal sepsis in the newborn. 25
If the fetus is in the occipitotransverse or occipitoposterior position in the second stage, manual rotation to the occipitoanterior position decreases the likelihood of operative vaginal and cesarean delivery. 26 Fetal position can be determined by identifying the sagittal suture with four suture lines by the anterior (larger) fontanelle and three by the posterior fontanelle. The position of the ears can also be helpful in determining fetal position when a large amount of caput is present and the sutures are difficult to palpate. Bedside ultrasonography is helpful when position is unclear by examination findings.
Women may push in any position that they prefer. Potential positions include on the back, side, or hands and knees; standing; or squatting. Women without epidurals who deliver in upright positions (kneeling, squatting, or standing) have a significantly reduced risk of assisted vaginal delivery and abnormal fetal heart rate pattern, but an increased risk of second-degree perineal laceration and an estimated blood loss of more than 500 mL. 27 Flexing the hips and legs increases the pelvic inlet diameter, allowing more room for delivery.
Second stage warm perineal compresses have been associated with a reduction in third- and fourth-degree perineal lacerations. 28 Studies have not shown benefit to keeping hands on vs. hands off the fetal head and maternal perineum during delivery. 29 Although not well studied, shorter pushes as the head is crowning are encouraged by many clinicians in an attempt to decrease perineal lacerations. Also, delivering between contractions may decrease perineal lacerations. 30 Routine episiotomy should not be performed. Episiotomy is associated with more severe perineal trauma, increased need for suturing, and more healing complications. 31
Once the infant's head is delivered, the clinician can check for a nuchal cord. The cord may be wrapped around the neck one or more times. If the nuchal cord is loose, it can be gently pulled over the head if possible or left in place if it does not interfere with delivery. A tight nuchal cord can be clamped twice and cut before delivery of the shoulders, although this may be associated with increased neonatal complications, including hypovolemia, anemia, shock, hypoxic-ischemic encephalopathy, cerebral palsy, and death according to case reports. The tight nuchal cord itself may contribute to some of these outcomes, however. 32 Another option for a tight nuchal cord is the somersault maneuver (carefully delivering the anterior and posterior shoulder, and then delivering the body by somersault while the head is kept next to the maternal thigh). After delivery, the cord can be removed from the neck. 32 A video of the somersault maneuver is available at https://www.youtube.com/watch?v=WaJ6sZ4nfnQ . More research on the safety and effectiveness of this maneuver is needed.
After delivery of the head, gentle downward traction should be applied with one gloved hand on each side of the fetal head to facilitate delivery of the shoulders. After the anterior shoulder delivers, the clinician pulls up gently, and the rest of the body should deliver easily. The vigorous newborn should be placed directly in contact with the mother's skin and covered with a blanket. Skin-to-skin contact is associated with decreased time to the first feeding, improved breastfeeding initiation and continuation, higher blood glucose level, decreased crying, and decreased hypothermia. 33 After delivery, quick drying of the newborn helps prevent hypothermia and stimulates crying and breathing. Beyond 35 weeks' gestation, there is no benefit to bulb suctioning the nose and mouth; earlier gestational ages have not been studied. 34
Delayed cord clamping, defined as waiting to clamp the umbilical cord for one to three minutes after birth or until cord pulsation has ceased, is associated with benefits in term infants, including higher birth weight, higher hemoglobin concentration, improved iron stores at six months, and improved respiratory transition. 35 Benefits are even greater with preterm infants. 36 However, delayed cord clamping is associated with an increase in jaundice requiring phototherapy. 35 Delayed cord clamping is indicated with all deliveries unless urgent resuscitation is needed. It is not necessary to keep the newborn below the level of the placenta before cutting the cord. 37 The cord should be clamped twice, leaving 2 to 4 cm of cord between the newborn and the closest clamp, and then the cord is cut between the clamps.
The Third Stage of Labor
The third stage begins after delivery of the newborn and ends with the delivery of the placenta. The average length of the third stage of labor is eight to nine minutes. 38
The greatest risk in the third stage is postpartum hemorrhage, which was recently redefined as 1,000 mL or more of blood loss or signs and symptoms of hypovolemia. 39 The median blood loss with vaginal delivery is 574 mL. 40 Blood loss is often underestimated by as much as 30%, and underestimation increases with increasing blood loss. 41 The risk of hemorrhage increases after 18 minutes and is six times greater after 30 minutes. 38 Postpartum hemorrhage is most commonly caused by atony (70% of cases). 42 Other causes include vaginal or cervical lacerations, uterine inversion, retained products of conception, and coagulopathy. 42 Table 5 lists risk factors for postpartum hemorrhage. 42
Active management of the third stage of labor (AMTSL), which is recommended by the World Health Organization, 43 is associated with a reduction in the risk of hemorrhage, both greater than 500 mL and greater than 1,000 mL, maternal hemoglobin level of less than 9 g per dL (90 g per L) after delivery, need for maternal blood transfusion, and need for more uterotonics in labor or in the first 24 hours after delivery. 44 However, AMTSL is also associated with an increase in postpartum maternal diastolic blood pressure, emesis, and use of analgesia and a decrease in neonatal birth weight. 44 Although AMTSL has traditionally consisted of oxytocin (10 IU intramuscularly or 20 IU per L intravenously at 250 mL per hour) and early cord clamping, the most important component now appears to be the administration of oxytocin. 43 , 44 Early cord clamping is no longer a component because it does not decrease postpartum hemorrhage and may be associated with neonatal harm. 35 , 44 Delayed cord clamping may avoid interfering with early transplacental transfusion and avoid the increase in maternal blood pressure and decrease in fetal weight associated with traditional AMTSL. 44 More research is needed regarding the effects of individual components of AMTSL. 44
Cervical, vaginal, and perineal lacerations should be repaired if there is bleeding. Second-degree laceration repairs are best performed in a continuous manner with absorbable synthetic suture. Compared with interrupted sutures, continuous repair of second-degree perineal lacerations is associated with less analgesia use, less short-term pain, and less need for suture removal. 45 Compared with catgut (chromic) sutures, synthetic sutures (polyglactin 910 [Vicryl], polyglycolic acid [Dexon]) are associated with less pain, less analgesia use, and less need for resuturing. However, synthetic sutures are associated with increased need for unabsorbed suture removal. 46
There are no quality randomized controlled trials assessing repair vs. nonrepair of second-degree perineal lacerations. 47 External anal sphincter injuries are often unrecognized, which can lead to fecal incontinence. 48 Knowledge of perineal anatomy and careful visual and digital examination can increase external anal sphincter injury detection. 48
Data Sources : A PubMed search was completed in Clinical Queries using key terms including labor and obstetric, delivery and obstetric, labor stage and first, labor stage and second, labor stage and third, doulas, anesthesia and epidural, and postpartum hemorrhage. The search included meta-analyses, randomized controlled trials, clinical trials, and reviews. We also searched the Cochrane database, Essential Evidence Plus, the National Guideline Clearinghouse database, and the U.S. Preventive Services Task Force. Search dates: September 4, 2014, and April 23, 2015.
This article is one in a series on “Advanced Life Support in Obstetrics (ALSO),” initially established by Mark Deutchman, MD, Denver, Colo. The coordinator of this series is Larry Leeman, MD, MPH, ALSO Managing Editor, Albuquerque, N.M.
Martin JA, Hamilton BE, Osterman MJ, Curtin SC, Matthews TJ. Births: final data for 2013. Natl Vital Stat Rep. 2015;64(1):1-65.
Committee opinion no 611: method for estimating due date. Obstet Gynecol. 2014;124(4):863-866.
Spong CY. Defining “term” pregnancy: recommendations from the Defining “Term” Pregnancy Workgroup. JAMA. 2013;309(23):2445-2446.
Practice bulletins no. 139: premature rupture of membranes. Obstet Gynecol. 2013;122(4):918-930.
Verani JR, McGee L, Schrag SJ Division of Bacterial Diseases, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention. Prevention of perinatal group B streptococcal disease—revised guidelines from CDC, 2010. MMWR Recomm Rep. 2010;59(RR-10):1-36.
Laughon SK, Branch DW, Beaver J, Zhang J. Changes in labor patterns over 50 years. Am J Obstet Gynecol. 2012;206(5):419.e1-9.
Zhang J, Landy HJ, Branch DW, et al.; Consortium on Safe Labor. Contemporary patterns of spontaneous labor with normal neonatal outcomes. Obstet Gynecol. 2010;116(6):1281-1287.
Spong CY, Berghella V, Wenstrom KD, Mercer BM, Saade GR. Preventing the first cesarean delivery: summary of a joint Eunice Kennedy Shriver National Institute of Child Health and Human Development, Society for Maternal-Fetal Medicine, and American College of Obstetricians and Gynecologists Workshop. Obstet Gynecol. 2012;120(5):1181-1193.
Kominiarek MA, Zhang J, Vanveldhuisen P, Troendle J, Beaver J, Hibbard JU. Contemporary labor patterns: the impact of maternal body mass index. Am J Obstet Gynecol. 2011;205(3):244.e1-8.
Lawrence A, Lewis L, Hofmeyr GJ, Styles C. Maternal positions and mobility during first stage labour. Cochrane Database Syst Rev. 2013;10:CD003934.
Hodnett ED, Gates S, Hofmeyr GJ, Sakala C. Continuous support for women during childbirth. Cochrane Database Syst Rev. 2013;7:CD003766.
American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 115: vaginal birth after previous cesarean delivery. Obstet Gynecol. 2010;116(2 pt 1):450-463.
American Academy of Family Physicians. Clinical practice guideline: planning for labor and vaginal birth after cesarean. January 2015. https://www.aafp.org/pvbac . Accessed April 23, 2015.
Landon MB, Hauth JC, Leveno KJ, et al.; National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Maternal and perinatal outcomes associated with a trial of labor after prior cesarean delivery. N Engl J Med. 2004;351(25):2581-2589.
Alfirevic Z, Devane D, Gyte GM. Continuous cardiotocography (CTG) as a form of electronic fetal monitoring (EFM) for fetal assessment during labour. Cochrane Database Syst Rev. 2013;5:CD006066.
American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 106: intrapartum fetal heart rate monitoring: nomenclature, interpretation, and general management principles. Obstet Gynecol. 2009;114(1):192-202.
Schrock SD, Harraway-Smith C. Labor analgesia. Am Fam Physician. 2012;85(5):447-454.
Smith CA, Collins CT, Crowther CA, Levett KM. Acupuncture or acupressure for pain management in labour. Cochrane Database Syst Rev. 2011;7:CD009232.
Smith CA, Collins CT, Cyna AM, Crowther CA. Complementary and alternative therapies for pain management in labour. Cochrane Database Syst Rev. 2006;4:CD003521.
Cluett ER, Burns E. Immersion in water in labour and birth. Cochrane Database Syst Rev. 2009;2:CD000111.
Ullman R, Smith LA, Burns E, Mori R, Dowswell T. Parenteral opioids for maternal pain relief in labour. Cochrane Database Syst Rev. 2010;9:CD007396.
Anim-Somuah M, Smyth RM, Jones L. Epidural versus non-epidural or no analgesia in labour. Cochrane Database Syst Rev. 2011;12:CD000331.
Torvaldsen S, Roberts CL, Bell JC, Raynes-Greenow CH. Discontinuation of epidural analgesia late in labour for reducing the adverse delivery outcomes associated with epidural analgesia. Cochrane Database Syst Rev. 2004;4:CD004457.
Tuuli MG, Frey HA, Odibo AO, et al. Immediate compared with delayed pushing in the second stage of labor. Obstet Gynecol. 2012;120(3):660-668.
Laughon SK, Berghella V, Reddy UM, Sundaram R, Lu Z, Hoffman MK. Neonatal and maternal outcomes with prolonged second stage of labor [published correction appears in Obstet Gynecol . 2014;124(4):842]. Obstet Gynecol. 2014;124(1):57-67.
Le Ray C, Deneux-Tharaux C, Khireddine I, Dreyfus M, Vardon D, Goffinet F. Manual rotation to decrease operative delivery in posterior or transverse positions. Obstet Gynecol. 2013;122(3):634-640.
Gupta JK, Hofmeyr GJ, Shehmar M. Position in the second stage of labour for women without epidural anaesthesia. Cochrane Database Syst Rev. 2012;5:CD002006.
Aasheim V, Nilsen AB, Lukasse M, Reinar LM. Perineal techniques during the second stage of labour for reducing perineal trauma. Cochrane Database Syst Rev. 2011;12:CD006672.
Albers LL, Sedler KD, Bedrick EJ, Teaf D, Peralta P. Midwifery care measures in the second stage of labor and reduction of genital tract trauma at birth: a randomized trial. J Midwifery Womens Health. 2005;50(5):365-372.
Bowe NL. Intact perineum: a slow delivery of the head does not adversely affect the outcome of the newborn. J Nurse Midwifery. 1981;26(2):5-11.
Carroli G, Mignini L. Episiotomy for vaginal birth. Cochrane Database Syst Rev. 2009;1:CD000081.
Mercer JS, Skovgaard RL, Peareara-Eaves J, et al. Nuchal cord management and nurse-midwifery practice. J Midwifery Womens Health. 2005;50(5):373-379.
Moore ER, Anderson GC, Bergman N, Dowswell T. Early skin-to-skin contact for mothers and their healthy newborn infants. Cochrane Database Syst Rev. 2012;5:CD003519.
Kelleher J, Bhat R, Salas AA, et al. Oronasopharyngeal suction versus wiping of the mouth and nose at birth. Lancet. 2013;382(9889):326-330.
McDonald SJ, Middleton P, Dowswell T, Morris PS. Effect of timing of umbilical cord clamping of term infants on maternal and neonatal outcomes. Cochrane Database Syst Rev. 2013;7:CD004074.
Committee on Obstetric Practice, American College of Obstetricians and Gynecologists. Committee opinion no. 543: timing of umbilical cord clamping after birth. Obstet Gynecol. 2012;120(6):1522-1526.
Vain NE, Satragno DS, Gorenstein AN, et al. Effect of gravity on volume of placental transfusion. Lancet. 2014;384(9939):235-240.
Magann EF, Evans S, Chauhan SP, Lanneau G, Fisk AD, Morrison JC. The length of the third stage of labor and the risk of postpartum hemorrhage. Obstet Gynecol. 2005;105(2):290-293.
American College of Obstetricians and Gynecologists. Obstetric data definitions. 2014. http://www.acog.org/-/media/Departments/Patient-Safety-and-Quality-Improvement/2014reVITALizeObstetricDataDefinitionsV10.pdf . Accessed April 23, 2015.
Stafford I, Dildy GA, Clark SL, Belfort MA. Visually estimated and calculated blood loss in vaginal and cesarean delivery. Am J Obstet Gynecol. 2008;199(5):519.e1-7.
Al Kadri HM, Al Anazi BK, Tamim HM. Visual estimation versus gravimetric measurement of postpartum blood loss: a prospective cohort study. Arch Gynecol Obstet. 2011;283(6):1207-1213.
Anderson JM, Etches D. Prevention and management of postpartum hemorrhage. Am Fam Physician. 2007;75(6):875-882.
Tunçalp O, Souza JP, Gülmezoglu M World Health Organization. New WHO recommendations on prevention and treatment of postpartum hemorrhage. Int J Gynaecol Obstet. 2013;123(3):254-256.
Begley CM, Gyte GM, Devane D, McGuire W, Weeks A. Active versus expectant management for women in the third stage of labour. Cochrane Database Syst Rev. 2015;3:CD007412.
Kettle C, Dowswell T, Ismail KM. Continuous and interrupted suturing techniques for repair of episiotomy or second-degree tears. Cochrane Database Syst Rev. 2012;11:CD000947.
Kettle C, Dowswell T, Ismail KM. Absorbable suture materials for primary repair of episiotomy and second degree tears. Cochrane Database Syst Rev. 2010;6:CD000006.
Elharmeel SM, Chaudhary Y, Tan S, et al. Surgical repair of spontaneous perineal tears that occur during childbirth versus no intervention. Cochrane Database Syst Rev. 2011;8:CD008534.
Andrews V, Sultan AH, Thakar R, Jones PW. Occult anal sphincter injuries—myth or reality?. BJOG. 2006;113(2):195-200.
Continue Reading
More in AFP
More in pubmed.
Copyright © 2015 by the American Academy of Family Physicians.
This content is owned by the AAFP. A person viewing it online may make one printout of the material and may use that printout only for his or her personal, non-commercial reference. This material may not otherwise be downloaded, copied, printed, stored, transmitted or reproduced in any medium, whether now known or later invented, except as authorized in writing by the AAFP. See permissions for copyright questions and/or permission requests.
Copyright © 2024 American Academy of Family Physicians. All Rights Reserved.
We have a new app!
Take the Access library with you wherever you go—easy access to books, videos, images, podcasts, personalized features, and more.
Download the Access App here: iOS and Android . Learn more here!
- Remote Access
- Save figures into PowerPoint
- Download tables as PDFs

Chapter 131. Normal Spontaneous Vaginal Delivery
- Download Chapter PDF
Disclaimer: These citations have been automatically generated based on the information we have and it may not be 100% accurate. Please consult the latest official manual style if you have any questions regarding the format accuracy.
Download citation file:
- Search Book
Jump to a Section
- Introduction
- Anatomy and Pathophysiology
- Indications
- Contraindications
- Initial Assessment
- Patient Preparation
- Alternative Techniques
- Complications
- Full Chapter
- Supplementary Content
The Emergency Physician will, on occasion, be required to handle the delivery of an infant when an Obstetrician or Family Physician is not available. The management of normal labor and delivery requires a basic understanding of the mechanisms of labor, the assessment and treatment of the mother, the safe delivery of the infant, and careful observation of both in the immediate postpartum period.
Labor is defined as repetitive uterine contractions leading to cervical change (dilation and effacement). The mechanisms of labor, also known as the cardinal movements of labor, describe the changes in the position of the fetal head as it travels through the birth canal. The safe delivery of the infant is the ultimate goal of labor.
Pelvic Anatomy
A successful vaginal delivery is dependent on the adequacy of the female pelvis. Through the use of clinical pelvimetry, physicians are able to make an assessment if adequate space exists for the passage of the fetus during prenatal visits. In theory, the most useful planes to measure are the pelvic inlet and the midplane. Evaluating the pelvic inlet is done by measuring the diagonal conjugate ( Figures 131-1 A & B ). When assessing the midplane, a measurement is taken of the ischial interspinous or bi-ischial diameter ( Figure 131-1 C ). Inadequate measurements indicate potential problems that may result in fetal entrapment, shoulder dystocia, or prolonged labor. 1
Figure 131-1.

Measure pelvic distances to determine if there may be difficulties during the delivery. A. The pelvic conjugate diameters. B. Measuring the diagonal conjugate. C. The ischial interspinous distance.
Sign in or create a free Access profile below to access even more exclusive content.
With an Access profile, you can save and manage favorites from your personal dashboard, complete case quizzes, review Q&A, and take these feature on the go with our Access app.
Best of the Blogs
Pop-up div successfully displayed.
This div only appears when the trigger link is hovered over. Otherwise it is hidden from view.
Please Wait
Labour and Delivery Care Module: 5. Conducting a Normal Delivery
Study session 5 conducting a normal delivery, introduction.
In the previous study sessions of this Module, you were introduced to the definition, signs, symptoms and stages of labour and the use of the partograph. You also learned about care of the woman in labour. In this session, you will learn how to assist the woman in the second stage of a normal labour and how to deliver the baby. The second stage is the part of labour when the mother pushes the baby out of the uterus and down the vagina, and the baby is born. Second stage begins when the cervix is completely dilated and ends when the baby is delivered.
During the second stage, the mother’s passive control during the long hours of the first stage of labour is replaced by intense physical effort and exertion for a comparatively short period. The mother and her support person require stamina, courage and confidence from the birth attendant. A healthy outcome for the mother and her baby depends upon your competence in providing quality care and the successful partnership between you and the mother.
Learning Outcomes for Study Session 5
When you have studied this session you should be able to:
5.1 Define and use correctly all of the key words printed in bold . (SAQs 5.1, 5.2 and 5.3)
5.2 Describe the signs of second stage labour and explain what is happening to the mother and the baby as it moves down the birth canal. (SAQs 5.1 and 5.2)
5.3 Describe how you would assess if the second stage is progressing normally and identify the warning signs that sufficient progress is not being made. (SAQs 5.1 and 5.2)
5.4 Describe how you would conduct the normal delivery of a healthy baby and give it immediate newborn care. (SAQs 5.3 and 5.4)
5.5 Explain how you would support bonding between mother and newborn after the delivery. (SAQ 5.5)
5.1 Recognising the signs of second stage labour
The only positive sign in diagnosing second stage of labour is full dilatation of the cervix. The only way you can be certain the cervix is dilated all the way is to do a vaginal examination. But remember: repeated vaginal exams can cause infection. It is better not to do a vaginal exam frequently (less than 4 hours interval) unless:
- When you count the fetal heart beat it is outside the normal range (outside 120–160 beats per minute).
- There is a sudden gush of amniotic fluid, which may indicate that there is a risk for cord prolapse or placental abruption.
- You detect signs of second stage of labour beginning before the next scheduled vaginal examination. (See Box 5.1 for signs of second stage.)
With experience, you can usually tell when the mother is ready to push without doing a vaginal exam.
Box 5.1 Signs of second stage
If the mother has two or more of these signs, she is probably in second stage of labour:
- She feels an uncontrollable urge to push (she may say she needs to pass stool)
- She may hold her breath or grunt during contractions
- She starts to sweat
- Her mood changes — she may become sleepy or more focused
- Her external genitals or anus begin to bulge out during contractions
- She feels the baby’s head begin to move into the vagina
- A purple line appears between the mother’s buttocks as they spread apart from the pressure of the baby’s head.
5.1.1 What happens during second stage of labour?
During second stage, when the baby is high in the vagina, you can see the mother’s genitals bulge during contractions. Her anus may open a little. Between contractions, her genitals relax (Figure 5.1).
Each contraction (and each push from the mother) moves the baby further down. Between contractions, the mother’s uterus relaxes and pulls the baby back up a little (but not as far as it was before the contraction).
After a while, you can see a little of the baby’s head coming down the vagina during contractions. The baby moves like an ocean tide: in and out, in and out, but each time closer to birth (Figures 5.2a–d).
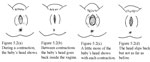
When the baby’s head stretches the vaginal opening to about the size of the palm of your hand (Figure 5.3), the head will stay at the opening - even between contractions. This is called crowning . Once the head is born, the rest of the body usually slips out easily with one or two pushes.
5.1.2 How does the baby move through the birth canal?
Figure 5.4 shows the movement of the baby through the birth canal. Babies move this way if they are positioned head-first, with their backs toward their mother’s bellies. But many babies do not face this way. A baby who faces the mother’s front, or who is breech, moves in a different way. Watch each birth closely to see how babies in different positions move.
5.2 Help the mother and baby have a safe birth
Continue to check the mother’s vital signs as you have been doing during the first stage of labour.
5.2.1 Check the baby’s heart beat
The baby’s heartbeat is harder to hear in second stage because the heart is usually lower in the mother’s belly. If you are experienced, you may be able to hear the baby’s heart between contractions. You can hear it best very low in the mother’s belly, near the pubic bone (Figure 5.5). It is OK for the heartbeat to be as slow as 100 beats a minute during a pushing contraction. But it should come right back up to the normal rate as soon as the contraction is over.
What is the normal fetal heatbeat?
Between 120 and 160 beats per minute.
If the baby’s heartbeat does not come back up within 1 minute, or stays slower than 100 beats a minute for more than a few minutes, the baby may be in trouble. Ask the mother to change position (to lie on her side), and check the baby’s heartbeat again. If it is still slow, ask the mother to stop pushing for a few contractions. Make sure she takes deep, long breaths so that the baby will get adequate oxygen.
5.2.2 Support the mother’s pushing

When the cervix is fully dilated, the mother’s body will push the baby out. Some healthcare providers get very excited during the pushing stage. They yell at mothers, ‘Push! Push!’ but mothers do not usually need much help to push. Their bodies push naturally, and when they are encouraged and supported, women will usually find the way to push that feels right and gets the baby out.
If a mother has difficulty pushing, do not scold or threaten her. And never insult or hit a woman to make her push. Upsetting or frightening her can slow the birth. Instead, explain how to push well (Figure 5.6). Each contraction is a new chance. Praise her for trying.
Tell the mother when you see her outer genitals bulge. Explain that this means the baby is coming down. When you see the head, let the mother touch it. This may also help her to push better.
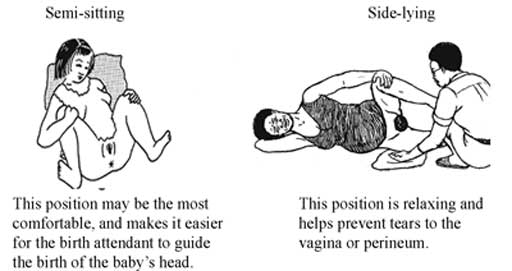
Let the mother choose the position that feels good to her. You already learned about different positions in first stage in Study Session 3. But note that it is not good for the mother to lie flat on her back during a normal birth. Lying flat can squeeze the blood vessels that bring blood to the baby and the mother, and can make the birth slower.
5.2.3 Watch for warning signs
Watch the speed of each birth. If the birth is taking too long, take the woman to a hospital. This is one of the most important things you can do to prevent serious problems or even death of women in labour.
First babies may take a full 2 hours of strong contractions and good pushing to be born. Second and later babies usually take less than 1 hour of pushing. Watch how fast the baby’s head is moving down through the birth canal. As long as the baby continues to move down (even very slowly), and the baby’s heartbeat is normal, and the mother has strength, then the birth is normal and healthy. The mother should continue to push until the head crowns.
But pushing for a long time with no progress can cause serious problems, including fistula, uterine rupture (you will learn about this in Study Session 10 of this Module), or even death of the baby or mother. If you do not see the mother’s genitals bulging after 30 minutes of strong pushing, or if the mild bulging does not increase, the head may not be coming down. If the baby is not moving down at all after 1 hour of pushing, the mother needs help.
Therefore, refer immediately if the woman stayed (couldn’t deliver) in the second stage for more than:
- 1 hour with no good progress (multigravida woman)
- 2 hours with no good progress (primigravida).
5.3 Conducting delivery of the baby
Your skill and judgment are crucial factors in minimising trauma for the mother and ensuring a safe delivery for the baby. These qualities are acquired through experience but certain basic principles must be applied whatever the expertise you have. These are:
- Observation of progress of the labour
- Prevention of infection
- Emotional and physical comfort of the mother
- Anticipation of normal events
- Recognition of abnormal labour or fetal distress.
5.3.1 Prevent tears in the vaginal opening
The birth of the baby’s head may tear the mother’s vaginal opening. But you can prevent tears by supporting the vagina during the birth. In some communities, circumcision of girls (also called female genital cutting) is common. This harmful traditional practice causes scars that may not stretch enough to let the baby out or the scar may tear as the baby is born.
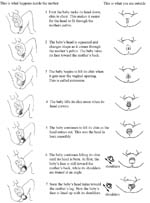
5.3.2 Delivery of the head

Wash your hands well and put on sterile gloves and other protective materials.
Clean the perineal area using antispetic and (if you have them) put clean drapes (cloths) over the mother’s thighs.
Press one hand firmly on the perineum (the skin between the opening of the vagina and the anus). This hand will keep the baby’s chin close to its chest — making it easier for the head to come out (Figure 5.8). Use a piece of cloth or gauze to cover the mother’s anus; some faeces (stool) may be pushed out with the baby’s head.
Use your other hand to apply gentle downward pressure on the top of the baby’s head to keep the baby’s head flexed (bent downwards).
Once the head has crowned, the head is born by the extension of the face, which appears at the perineum.
Clear the baby’s nose and mouth. When the head is born, and before the rest of the body comes out, you may need to help the baby breathe by clearing its mouth and nose. If the baby has some mucus or water in its nose or mouth, wipe it gently with a clean cloth wrapped around your finger.
5.3.3 Check if the cord is around the baby’s neck
If there is a rest between the birth of the head and the birth of the shoulders, feel for the cord around the baby’s neck.
- If the cord is wrapped loosely around the neck, loosen it so it can slip over the baby’s head or shoulders.
- If the cord is very tight, or if it is wrapped around the neck more than once, try to loosen it and slip it over the head.
- If you cannot loosen the cord, and if the cord is preventing the baby from coming out, you may have to clamp and cut it.
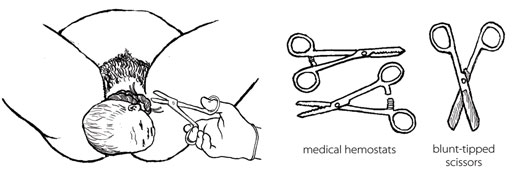
If you can, use medical hemostats (clamps) and blunt-tipped scissors for clamping and cutting the cord in this situation. If you do not have them, use clean string and a new razor. Clamp or tie in two places and cut in between (Figure 5.9). Be very careful not to cut the mother or the baby’s neck.
5.3.4 Delivery of the shoulders
After the baby’s head is born and he or she turns to face the mother’s leg, wait for the next contraction. Ask the mother to give a gentle push as soon as she feels the contraction. Usually, the baby’s shoulders will slip right out. To prevent tearing, try to bring out one shoulder at a time (Figure 5.10).
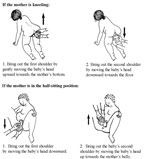
5.3.5 Delivery of the baby’s body
After the shoulders are born, the rest of the body usually slides out without any trouble. Remember that new babies are wet and slippery. Be careful not to drop the baby!
Put the baby on the mother’s abdomen, dry the baby with a clean cloth and then put a new, clean blanket over him or her to keep the baby warm. Be sure the top of the baby’s head is covered with a hat or blanket. If everything seems OK, give the baby the chance to breastfeed right away. You do not have to wait until the placenta comes out or the cord is cut.
5.3.6 Cutting the cord
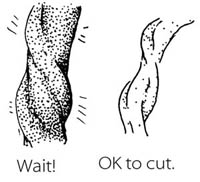
Most of the time, there is no need to hurry to cut the cord right away. Leaving the cord attached will help the baby to have enough iron in his or her blood, because some of the blood in the placenta drains along the cord and into the baby. It will also keep the baby on the mother’s belly which is the best place to be right now. Wait until the cord stops pulsing and looks like it is mostly empty of blood.
BUT if the mother is known to be HIV-infected or her HIV status is not known, it is better to cut the cord soon after you have dried the baby and made sure that he or she is warm.
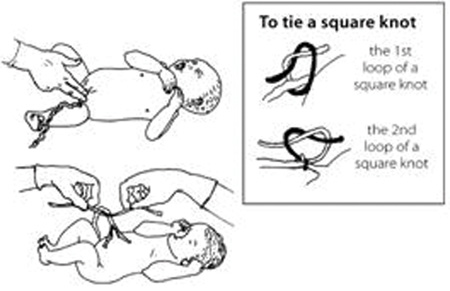
Use a sterile string or sterile clamp to tightly tie or clamp the cord about two finger widths from the baby’s belly. (The baby’s risk of getting tetanus is greater when the cord is cut far from the body.) Tie a square knot (Figure 5.11).
Put another sterile string or clamp one finger from the first knot. And, if you do not have a clamp on the cord on the mother’s side, add a third knot two fingers from the second knot. Putting a double knot on the cord reduces the risk of bleeding.
Cut after the second tie (e.g. the first tie is approximately 3 cm from the baby’s abdomen and the second is approximately 5 cm). Cut after the 5 cm tie with a sterile razor blade or sterile scissors.

5.4 Immediate care of the newborn baby
Essential newborn care includes the following actions. But note that you will learn about resuscitation of the newborn who is not breathing adequately in Study Session 6.
5.4.1 Clean childbirth and cord care
Principles of cleanliness are essential in both home and health post childbirth to prevent infection to the mother and baby. These are:
- Clean your hands
- Clean the mother’s perineum
- Nothing unclean introduced vaginally
- Clean delivery surface
- Cleanliness in cord clamping and cutting.
The stump of the umbilical cord must be kept clean and dry to prevent infection. Wash it with soap and clean water only if it is soiled. Remember:
- Do not apply dressings or substances of any kind
- If the cord bleeds, re-tie it.
It usually falls off 4–7 days after birth, but until this happens, place the cord outside the nappy to prevent contamination with urine/faeces.
5.4.2 Check the newborn
Most babies are alert and strong when they are born. Other babies start slow, but as the first few minutes pass, they breathe and move better, get stronger, and become less blue. Immediately after delivery, clear airways and stimulate the baby while drying. To see how healthy the baby is, watch for:
- Breathing: babies should start to breathe normally within seconds after birth. Babies who cry after birth are usually breathing well. But many babies breathe well and do not cry at all.
- Colour: the baby’s skin should be a normal colour – not pale or bluish.
- Muscle tone: the baby should move his or her arms and legs vigorously.
All of these things should be checked simultaneously within the first minute after birth. You will learn about this in detail in Study Session 6 of this Module.
5.4.3 Warmth and bonding
Newborn babies are at increased risk of getting extremely cold. The mother and the baby should be kept skin-to-skin contact, covered with a clean, dry blanket. This should be done immediately after the birth, even before you cut the cord.
The mother’s body will keep the baby warm, and the smell of the mother’s milk will encourage him or her to suck. Be gentle with a new baby. The first hour is the best time for the mother and baby to be together, and they should not be separated. This time together will also help to start breastfeeding as early as possible.
5.4.4 Early breastfeeding
If everything is normal after the birth, the mother should breastfeed her baby right away (Figure 5.12). She may need some help getting started. The first milk to come from the breast is yellowish and is called colostrum . Some women think that colostrum is bad for the baby and do not breastfeed in the first day after the birth. But colostrum is very important! It is full of protein and helps to protect the baby from infections.
- Breastfeeding makes the uterus contract. This helps the placenta come out, and it may help prevent heavy bleeding.
- Breastfeeding helps the baby to clear fluid from his nose and mouth and breathe more easily.
- Breastfeeding is a good way for the mother and baby to begin to know each other.
- Breastfeeding comforts the baby.
- Breastfeeding can help the mother relax and feel good about her new baby.
If the baby does not seem able to breastfeed, see if it has a lot of mucus in his or her nose. To help the mucus drain, lay the baby across the mother’s chest with its head lower than its body. Stroke the baby’s back from the waist up to the shoulders. After draining the mucus, help the mother to put the baby to the breast again. You will learn a lot more about breastfeeding in the next Module in this curriculum on Postnatal Care .
Summary of Study Session 5
In Study Session 5 you learned that:
- The second stage of labour begins when the cervix is completely dilated and ends when the baby is delivered. Close attention, skilled care and prompt action are needed from you for a safe clean birth.
- The signs of second stage are when the mother feels an uncontrollable urge to push, she holds her breath or grunts during contractions, she starts to sweat, her mood changes, her external genitals or anus begin to bulge out during contractions, she feels the baby’s head begin to move into the vagina, a purple line appears between her buttocks.
- Check the mother’s vital signs, the fetal heart beat and the descent of the baby’s head at intervals to ensure that labour is progressing normally.
- Watch for warning signs that labour is not progressing sufficiently during the second stage and take appropriate action to refer the mother.
- Support the mother’s pushing during the time of actual delivery.
- If the cord is trapped around the baby’s neck, cut it before the body is delivered — but make sure the mother pushes hard to get the baby out fast.
- Maintain cleanliness throughout the entire process of labour and delivery to prevent infection to the mother and baby.
- Keep the newborn baby warm and make sure it is breathing well.
- Initiate early breast feeding.
Self-Assessment Questions (SAQs) for Study Session 5
Now that you have completed this study session, you can assess how well you have achieved its Learning Outcomes by answering the questions below Case Study 3.1. Write your answers in your Study Diary and discuss them with your Tutor at the next Study Support Meeting. You can check your answers with the Notes on the Self-Assessment Questions at the end of this Module.
SAQ 5.1 (tests Learning Outcomes 5.1, 5.2 and 5.3)
Which of the following statements is false ? In each case, explain what is incorrect.
A Full dilatation of the cervix to 10 cm is the most important sign that the second stage of labour is beginning.
B In second stage, the mother’s genitals tend to bulge during contractions and relax between contractions.
C Crowning is the name given to the moment when the baby’s head is completely born.
D In a normal delivery, the baby moves down the birth canal facing the front of the mother’s body, with its back towards her backbone.
E While it is still in the birth canal, the baby’s heartbeat tends to get faster during a contraction.
F Let the mother choose the position that she feels most comfortable in when she gets the urge to push in the second stage of labour.
A is true. Full dilatation of the cervix to 10 cm is the most important sign that second stage of labour is beginning.
B is true. In second stage, the mother’s genitals tend to bulge during contractions and relax between contractions.
C is false. Crowning is when the top of the baby’s head stretches the vaginal opening to the size of your hand and it stays in the opening even between contractions.
D is false. In a normal delivery, the baby moves down the birth canal facing the back of the mother’s body, with its own back towards her belly.
E is false. While it is still in the birth canal, the baby’s heartbeat tends to get slower (not faster) during a contraction.
F is true. You should let the mother choose the position that she feels most comfortable in when she gets the urge to push in the second stage of labour.
SAQ 5.2 (tests Learning Outcome 5.3)
List four warning signs that second stage labour may not be progressing normally.
Warning signs that second stage may not be progressing normally include:
- Fetal heartbeat stays above or below the normal range (120-160 beats per minute) even between contractions of the mother’s uterus.
- A sudden gush of amniotic fluid leaves the vagina, which may indicate a cord prolapse or placental abruption.
- A multigravida mother has been pushing for 1 hour without the baby moving down the birth canal, or a primigravida mother has been pushing for 2 hours with no good progress.
- Baby is not descending and there are signs that it is developing caput or excessive moulding of the fetal skull.
SAQ 5.3 (tests Learning Outcome 5.4)
Imagine that the baby’s head has been born and you are waiting for the next contraction to deliver the baby’s shoulders. What should you do if you find that the umbilical cord is wrapped around the baby’s neck?
First, try to loosen the cord and slip it over the baby’s head. If you cannot loosen it and it is preventing the baby from being delivered, clamp the cord in two places (or tie it with very clean string) and cut it in between the clamps. Be careful not to cut the mother or the baby’s neck.
SAQ 5.4 (tests Learning Outcomes 5.4 and 5.5)
Rearrange the following actions into the correct order during delivery of the baby and immediately afterwards.
A Once the baby’s head is born, help it to breathe by clearing its nose and mouth.
B Wash your hands well and put on sterile gloves and other protective clothing.
C To prevent tearing of the mother’s birth vagina or perineum, deliver the baby’s shoulders one at a time.
D Press one hand firmly over the mother’s perineum.
E When the baby has been completely delivered, put it on the mother’s abdomen and dry it with a clean cloth.
F Clean the mother’s perineal area with antiseptic.
G Clamp or tie the cord in two places and cut it in between the clamps.
H Use your other hand to apply gentle downward pressure on the top of the baby’s head to keep it flexed (bent downwards).
I Cover the baby to keep it warm and give it a chance to breastfeed straight away.
J Use a piece of cloth or gauze to cover the mother’s anus in case any faeces come out with the baby.
K Check that the cord is not around the baby’s neck.
The correct sequence is as follows:
SAQ 5.5 (tests Learning Outcome 5.5)
What do you do to help bonding between the mother and her newborn baby?
To help bonding between the mother and her newborn baby you place the baby on the mother’s abdomen as soon as it is born, and give it an early opportunity to breastfeed. Do not separate the mother and her baby during at least the first hour after the birth.
Except for third party materials and/or otherwise stated (see terms and conditions ) the content in OpenLearn is released for use under the terms of the Creative Commons Attribution-NonCommercial-Sharealike 2.0 licence . In short this allows you to use the content throughout the world without payment for non-commercial purposes in accordance with the Creative Commons non commercial sharealike licence. Please read this licence in full along with OpenLearn terms and conditions before making use of the content.
When using the content you must attribute us (The Open University) (the OU) and any identified author in accordance with the terms of the Creative Commons Licence.
The Acknowledgements section is used to list, amongst other things, third party (Proprietary), licensed content which is not subject to Creative Commons licensing. Proprietary content must be used (retained) intact and in context to the content at all times. The Acknowledgements section is also used to bring to your attention any other Special Restrictions which may apply to the content. For example there may be times when the Creative Commons Non-Commercial Sharealike licence does not apply to any of the content even if owned by us (the OU). In these stances, unless stated otherwise, the content may be used for personal and non-commercial use. We have also identified as Proprietary other material included in the content which is not subject to Creative Commons Licence. These are: OU logos, trading names and may extend to certain photographic and video images and sound recordings and any other material as may be brought to your attention.
Unauthorised use of any of the content may constitute a breach of the terms and conditions and/or intellectual property laws.
We reserve the right to alter, amend or bring to an end any terms and conditions provided here without notice.
All rights falling outside the terms of the Creative Commons licence are retained or controlled by The Open University.
Head of Intellectual Property, The Open University
5.1 Normal delivery
5.1.1 general recommendations.
Personnel should wear personal protective equipment (gloves, goggles, clothing and eye protection) to prevent infection from blood and other body fluids.
Ensure a calm reassuring environment and provide the woman as much privacy as possible during examinations and delivery. Encourage her to move about freely if desired and to have a person of her choice to accompany her.
Anticipate the need for resuscitation at every birth. The necessary equipment should be ready at hand and ready for use.
5.1.2 Diagnosing the start of labour
- Onset of uterine contractions: intermittent, rhythmic pains accompanied by a hardening of the uterus, progressively increasing in strength and frequency;
- in a primipara, the cervix will first efface then, dilate;
- in a multipara, effacement and dilation occur simultaneously.
Repeated contractions without cervical changes should not be considered as the start of labour. Repeated contractions that are ineffective (unaccompanied by cervical changes) and irregular, which spontaneously stop and then possibly start up again, represent false labour. In this case, do not rupture the membranes, do not administer oxytocin.
Likewise, cervical dilation with few or no contractions should not be considered the start of labour. Multiparous women in particular may have a dilated cervix (up to 5 cm) at term before the onset of labour. If in doubt, in both cases, re-examine 4 hours later. If the cervix has not changed labour has not begun and the woman does not need to be admitted to the delivery room.
5.1.3 Stages of labour
First stage: dilation and foetal descent, divided into 2 phases.
1) Latent phase: from the start of labour to approximately 5 cm of dilation. Its duration varies depending on the number of prior deliveries. 2) Active phase: from approximately 5 cm to complete dilation [1] Citation 1. World Health Organization. WHO recommendations Intrapartum care for a positive childbirth experience, Geneva, 2018. http://apps.who.int/iris/bitstream/handle/10665/272447/WHO-RHR-18.12-eng.pdf?ua=1 [Accessed 18 june 2018] . During this phase the cervix dilates faster than during the latent phase. The time to dilate varies with the number of previous deliveries. As a rule, it does not last longer than 10 hours in a multipara and 12 hours in a primipara.
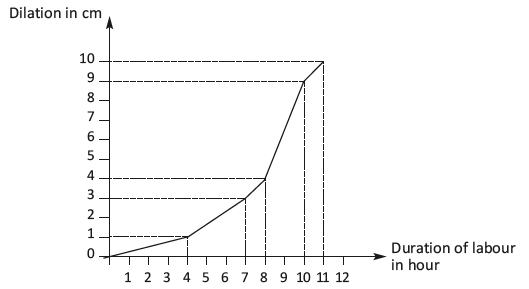
Second stage: delivery of the infant
Begins at full dilation.
Third stage: delivery of the placenta
See Chapter 8 .
5.1.4 First stage: dilation and descent of the foetus
The indicators being monitored are noted on the partograph ( Section 5.2 ).
Uterine contractions
- Contractions progressively increase in strength and frequency: sometimes 30 minutes apart early in labour; closer together (every 2 to 3 minutes) at the end of labour.
- A contraction can last up to a minute.
- The uterus should relax between contractions.
- Watch the shape of the uterus in order to spot a Bandl’s ring (Chapter 3, Section 3.3.2 ).
General condition of the patient
- Monitor the heart rate, blood pressure and temperature every 4 hours or more often in case of abnormality.
- Ask the woman to empty her bladder regularly (e.g. every 2 hours).
- Keep the woman hydrated (offer her water or tea).
- Encourage the woman to move about freely during labour. Position changes and walking around help relieve the pain, enhances the progress of labour and helps foetal descent. Pain can also be relieved by massage or hot or cold compresses. Midwife support helps manage pain.
- Routinely insert an IV line in the following situations: excessively large uterus (foetal macrosomia, multiple pregnancy or polyhydramnios), known anaemia and hypertension.
Foetal heart rate
Foetal heart rate monitoring.
Use a Pinard stethoscope or foetal Doppler, every 30 minutes during the active phase and every 5 minutes during active second stage, or as often as possible. Listen to and count for at least one whole minute immediately after the contraction. Normal foetal heart rate is 110 to 160 beats per minute. The foetal heart rate may slow down during a contraction. If it becomes completely normal again as soon as the uterus relaxes, there is probably no foetal distress. If the foetal heart rate heard immediately after the end of a contraction is abnormal (less than 100 beats per minute or more than 180 beats per minute), continue foetal heart rate monitoring for the next 3 contractions to confirm the abnormality.
Management of abnormal foetal heart rate
- Insert an IV line.
- Check maternal vital signs: heart rate, blood pressure and temperature.
- Check the uterine tonus. If hypertonic, look for excessive administration of oxytocin (which should therefore be stopped) or placental abruption (Chapter 3, Section 3.2 ).
- Check the colour of the amniotic fluid: meconium-stained (greenish) amniotic fluid combined with foetal heart rate abnormalities is suggestive of true foetal distress.
- Stop administering oxytocin if an infusion is in progress.
- Check for vaginal bleeding: bleeding may suggest placental abruption or uterine rupture.
- Raise the patient or place her on her left side. Laying on her back the uterus creates pressure on the vena cava, which may be the cause of low foetal heart rate.
- Correct possible hypotension by fluid replacement (Ringer lactate) to bring the systolic blood pressure ≥ 90 mmHg.
- Perform a vaginal examination to look for cord prolapse.
- If the foetal heart rate is more than 180 beats/minute:
The most common cause is maternal febrile infection.
- Look for the cause of the infection (uterine infection, pyelonephritis, malaria, etc.) and treat.
- Treat the fever (paracetamol).
- In case of fever of unknown origin, administer antibiotics as for a prolonged rupture of membranes (Chapter 4, Section 4.9 ).
If the abnormal foetal heart rate persists or the amniotic fluid becomes stained with meconium, deliver quickly. If the cervix is fully dilated and the head engaged, perform instrumental delivery (vacuum extractor or forceps, depending on the operator’s skill and experience); otherwise consider caesarean section.
Dilation during active phase
- The cervix should remain soft, and dilate progressively. Dilation should be checked by vaginal examination every 4 hours if there are no particular problems (Figures 5.2).
- No progress in cervical dilation between two vaginal examinations is a warning sign.
- Action must be taken if there is no progress for 4 hours: artificial rupture of membranes, administration of oxytocin, caesarean section, depending on the circumstances.
Figures 5.2 - Estimating cervical dilation
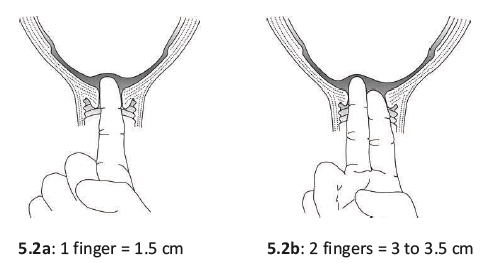
Amniotic sac
- The amniotic sac bulges during contractions and usually breaks spontaneously after 5 cm of dilation or at full dilation during delivery. Immediately after rupture, check the foetal heart rate and if necessary perform a vaginal examination in order to identify a potential prolapse of the umbilical cord ( Section 5.4 ). Once the membranes are ruptured, always use sterile gloves for vaginal examination.
- Note the colour of the amniotic fluid: clear, blood-stained, or meconium-stained.
- Meconium staining by itself, without abnormal foetal heart rate, is not diagnostic of foetal distress, but does require closer monitoring—in particular, a vaginal examination every 2 hours. Action must be taken if dilation fails to progress after 2 hours.
Foetal progress
- Assess foetal descent by palpating the abdomen (portion of the foetal head felt above the symphysis pubis) before performing the vaginal examination.
- At each vaginal examination, in addition to dilation, check the presentation, the position and the degree of foetal descent.
- Look for signs that the foetal head is engaged:
On vaginal examination, the presenting part prevents the examiner's fingers from reaching the sacral concavity (Figures 5.3a and 5.3b). The presence of caput (benign diffuse swelling of the foetal head) can lead to the mistaken conclusion that the foetal head is engaged. The distance between the foetal shoulder and the upper edge of the symphysis pubis is less than 2 finger widths (Figures 5.3c and 5.3d).
- Use reference points on the foetal skull to determine the position of the head in the mother's pelvis. It is easier to determine the position of the head after the membranes have ruptured, and the cervix is more than 5 cm dilated. When the head is well flexed, the anterior (diamond-shaped) fontanelle is not palpable; only the sagittal suture and the posterior (triangular) fontanelle are. The posterior fontanelle is the landmark for the foetal occiput, and thus helps give the foetal position. In most cases, once the head is engaged, rotation of the head within the pelvis brings the foetal occiput under the mother's symphysis, with the posterior fontanelle along the anterior midline.
5.1.5 Second stage: delivery of the infant
This stage is often rapid in a multipara, and slower in a primipara. It should not, however, take longer than 2 hours in a multipara and 3 hours in a primipara
If there is a traditional delivery position and no specific risk for the mother or child has been established, it is possible to assist a delivery in a woman on her back, on her left side, squatting or on all fours (Figures 5.4).
Figures 5.4 - Delivery position
- Rinse the vulva and perineum with clean water.
- The bladder should be emptied, naturally if possible. In cases of urinary retention only, insert a urinary catheter using sterile technique (sterile gloves; sterile, single use catheter).
- If labour is progressing well and there is no foetal heart rate abnormality, let the woman follow her own urge to push. In other cases, expulsive effort should be directed. The woman should push during the uterine contraction. Pushing may be done either with held breath (after a deep inhalation, glottis closed, abdominal muscles and diaphragm contracted, directed toward the perineum) or with exhalation. Expulsive effort is maintained for long as possible: in general, 2 to 3 pushes per contraction.
- Between contractions, the woman should rest and breathe deeply. The birth attendant should monitor the foetal heart rate after each contraction.
- The head begins to stretch the perineum, which becomes progressively thinner; the vaginal opening distends, the labia spread apart, and the occiput appears. In a cephalic presentation, the head usually emerges occiput anterior: the infant is born looking down, the occiput pivoting against the symphysis (Figures 5.5). The head goes into slight extension. The birth attendant must guide this motion and prevent any abrupt expulsive movement, with one hand supporting the occiput. The other hand can support the chin through the perineum. Cover the anal area with a compress (Figures 5.6).
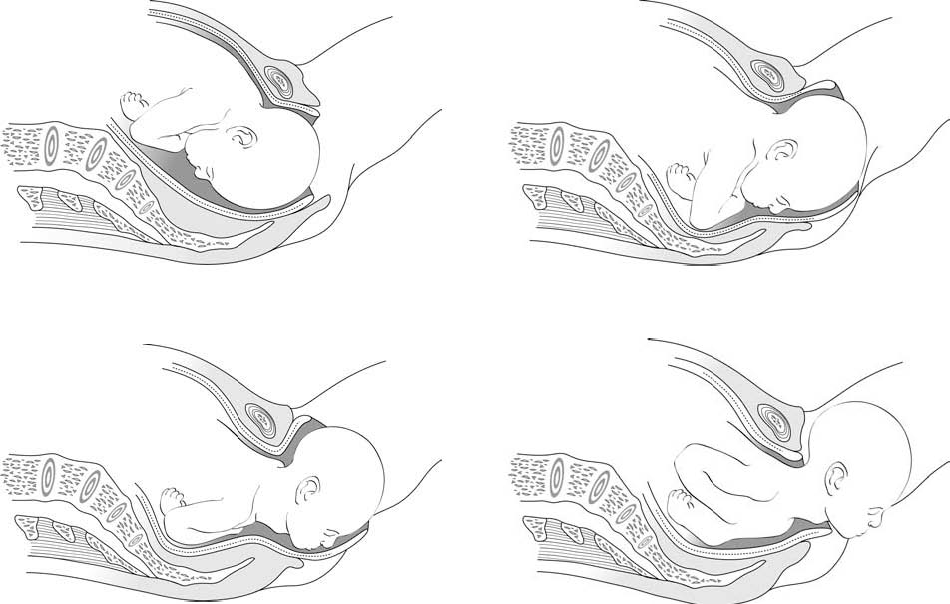
During this final phase—an active one for the birth attendant—the woman should stop all expulsive efforts and breathe deeply. With one hand, the birth attendant controls the extension of the head and moves it slightly side-to-side, in order to gradually free the parietal protuberances; if necessary (not routinely), the chin can be lifted with the other hand (Figure 5.7).
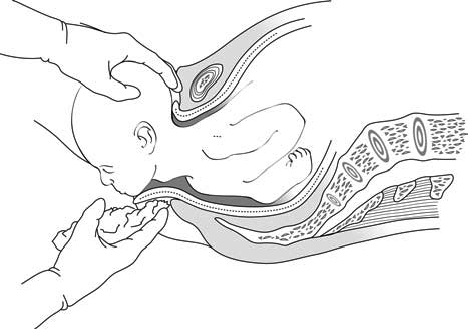
- At the moment of delivery, the perineum is extremely distended. Controlling the expulsion can help reduce the risk of a tear. Episiotomy ( Section 5.8 ) is not routinely indicated. In an occiput-posterior delivery (Figure 5.8), where perineal distension is at a maximum, episiotomy may be helpful.
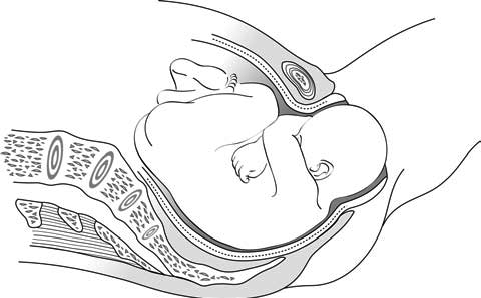
- The head, once delivered, rotates spontaneously by at least 90°. The birth attendant helps this movement by grasping the head in both hands and exerting gentle downward traction to bring the anterior shoulder under the symphysis and then deliver it then, smooth upward traction to deliver the posterior shoulder (Figures 5.9).
To reduce the risk of perineal tears, control the delivery of the posterior shoulder.
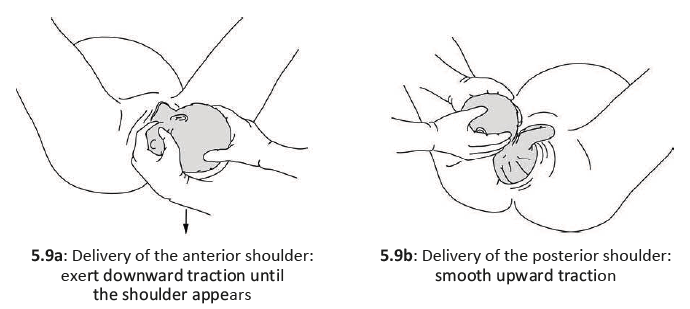
- Place the neonate on mother's chest. For neonatal care, see Chapter 10, Section 10.1 .
5.1.6 Oxytocin administration
Administer oxytocin to the mother immediately and then deliver the placenta (Chapter 8, Section 8.1.2 ).
5.1.7 Umbilical cord clamping
See Chapter 10, Section 10.1.1 .
- 1. World Health Organization. WHO recommendations Intrapartum care for a positive childbirth experience, Geneva, 2018. http://apps.who.int/iris/bitstream/handle/10665/272447/WHO-RHR-18.12-eng.pdf?ua=1 [Accessed 18 june 2018]
- Research article
- Open access
- Published: 18 June 2018
Factors promoting or inhibiting normal birth
- Samantha J. Prosser 1 , 2 ,
- Adrian G. Barnett 2 &
- Yvette D. Miller ORCID: orcid.org/0000-0002-5393-7142 2
BMC Pregnancy and Childbirth volume 18 , Article number: 241 ( 2018 ) Cite this article
62k Accesses
33 Citations
111 Altmetric
Metrics details
In response to rising rates of medical intervention in birth, there has been increased international interest in promoting normal birth (without induction of labour, epidural/spinal/general anaesthesia, episiotomy, forceps/vacuum, or caesarean section). However, there is limited evidence for how best to achieve increased rates of normal birth. In this study we examined the role of modifiable and non-modifiable factors in experiencing a normal birth using retrospective, self-reported data.
Women who gave birth over a four-month period in Queensland, Australia, were invited to complete a questionnaire about their preferences for and experiences of pregnancy, labour, birth, and postnatal care. Responses ( N = 5840) were analysed using multiple logistic regression models to identify associations with four aspects of normal birth: onset of labour, use of anaesthesia, mode of birth, and use of episiotomy. The probability of normal birth was then estimated by combining these models.
Overall, 28.7% of women experienced a normal birth. Probability of a normal birth was reduced for women who were primiparous, had a history of caesarean, had a multiple pregnancy, were older, had a more advanced gestational age, experienced pregnancy-related health conditions (gestational diabetes, low-lying placenta, high blood pressure), had continuous electronic fetal monitoring during labour, and knew only some of their care providers for labour and birth. Women had a higher probability of normal birth if they lived outside major metropolitan areas, did not receive private obstetric care, had freedom of movement throughout labour, received continuity of care in labour and birth, did not have an augmented labour, or gave birth in a non-supine position.
Conclusions
Our findings highlight several relevant modifiable factors including mobility, monitoring, and care provision during labour and birth, for increasing normal birth opportunity. An important step forward in promoting normal birth is increasing awareness of such relationships through patient involvement in informed decision-making and implementation of this evidence in care guidelines.
Peer Review reports
The term ‘normal birth’ in academic literature and health policy has more generally come to refer to a birth without, or with limited, clinical intervention. A 2007 consensus statement by the Maternity Care Working Party, Making Normal Birth a Reality , called for a standard definition of normal birth to increase confidence in the auditing and monitoring of practice trends [ 1 ]. The resulting definition described normal birth as an unassisted vaginal birth without induction of labour; epidural, spinal or general anaesthesia; or episiotomy [ 2 ]. Unlike some definitions, Werkmeister’s definition [ 2 ] is limited to the process of birth and does not extend to outcomes of birth such as vertex presentation and intact perineum.
The 1990s and 2000s saw a steady increase in rates of medical intervention during labour and birth across a number of developed countries [ 3 ]. While such procedures can be life-saving, they also bear risk to women [ 4 , 5 , 6 , 7 ] and their babies [ 8 , 9 ] and thus should be limited to instances of medical necessity. There has been increasing international interest in promoting normal birth and progression towards less medicalised models of birth in response to the rising rate of intervention. In 2005, The Royal College of Midwives launched the Campaign for Normal Birth within the UK, now continued as part of the Better Births Initiative [ 10 ]. Care providers in Canada have published a Joint Policy Statement on Normal Birth to support, promote and protect normal birth for women [ 11 ]. In Australia, current maternity care reform ( National Maternity Services Plan: 2010 ) [ 12 ] is based on an underlying philosophy of birth as a normal physiological event. Normal birth guidelines were published in the Australian state of Queensland in 2012 to protect, promote and support normal birth [ 13 ]. Additionally, the Towards Normal Birth in New South Wales policy directive required all birthing facilities in that Australian state to have a written policy for normal birth by 2015 [ 14 ].
Despite increasing international interest there is relatively limited evidence for how to best facilitate normal birth as a multi-dimensional construct. To date, very few studies have examined determinants of normal birth in this way (i.e., based on Werkmeister’s definition) [ 2 ]. The Birthplace in England national prospective cohort study [ 15 ] found that the rate of normal birth for low risk women differed depending on the place in which they were giving birth. Normal birth was more likely in freestanding midwifery units or at home than within obstetric units. More recently, Miller and colleagues [ 16 ] examined a wider range of predictors and reported that women were more likely to experience normal birth if they were in a public rather than private facility, did not have continuous fetal monitoring, were able to move freely during their labour, gave birth in a non-supine position, or delivered their baby outside standard business hours. Primiparous women were less likely to experience normal birth [ 16 ]. These studies identify a number of modifiable factors that can be used to inform practice aimed at promoting normal birth. However, the samples were limited to low risk women or women who had a vaginal birth, respectively, and findings may not generalise to all women (for example, as a means of preventing intrapartum caesarean or for women with obstetric risk factors).
Other research on factors that may reduce intervention during birth has focused on individual procedures, such as induction of labour or caesarean section. In regards to modifiable factors, characteristics of care are one source of variation. There is greater use of intervention in private birthing facilities than in public facilities, including induction of labour, epidural anaesthesia, episiotomy, instrumental vaginal birth, and caesarean section [ 17 , 18 , 19 , 20 , 21 ]. These differences are not attributable to case mix [ 22 , 23 ]. Rates of intervention have also been shown to increase for women outside midwifery-led continuity models of care [ 24 ], who receive care from a larger number of nurses in labour [ 25 ], give birth during business hours [ 26 , 27 ], or have continuous electronic fetal monitoring during labour [ 28 ]. More specifically, some practices have been associated with reduced need for regional anaesthesia during labour, such as water immersion during first stage labour [ 29 ], upright positioning and freedom of movement [ 30 ] and continuous one-to-one support [ 31 ]. There is some consistency between these findings and those directly evaluating associations with normal birth as an outcome.
Less modifiable characteristics have also been associated with increased intervention during birth. Rates of intervention are typically increased for women with obstetric conditions such as gestational diabetes, pre-eclampsia or placenta praevia [ 18 , 32 , 33 ]. While not a risk factor in and of itself, primiparity is linked to higher rates of induction [ 32 , 34 ], epidural usage [ 35 ], and caesarean section [ 36 ]. Furthermore, women with a history of caesarean section are at a high likelihood of having a repeat caesarean for this reason only [ 37 ]. Women with a body mass index (BMI) in the ‘obese’ range are at greater odds having an induced labour [ 32 ] and a caesarean section [ 18 , 38 , 39 ] and older maternal age is often associated greater use of intervention [ 40 ]. However, the relationship between age and intervention is not always consistent, may be indirect, and may depend on whether women have given birth previously [ 41 ].
The aim of this study was to expand current evidence for factors which may promote or inhibit women achieving a normal birth. We examined a range of both modifiable (e.g. continuity of care) and non-modifiable (e.g. parity) factors, using secondary analysis of retrospective, self-reported, population-level survey data of women’s maternity care experience. Additionally, we aimed to expand on the methods used in previous studies by providing the probabilities of achieving normal birth as a singular construct among all women, irrespective of their risk status.
Background/sampling
All women who gave birth in Queensland, Australia, across a 4-month period (October 2011 to January 2012, inclusive) were invited by the Queensland Registry of Births, Deaths and Marriages to complete the 2012 Having a Baby in Queensland Survey; a cross-sectional evaluation of women’s experiences of pregnancy, labour, birth, and postnatal care [ 42 ]. Women who experienced a stillbirth or neonatal death were sent a tailored survey and not included in the current study. Women received the survey 3 to 4 months after birth and could complete it either online, on paper (returned using a provided reply-paid envelope), or over the telephone with a trained female interviewer and a translator if required. A reminder/thank you postcard was sent to all women approximately two weeks after the initial mail-out. This survey and all subsequent analyses received clearance from The University of Queensland’s Behavioural & Social Sciences Ethical Review Committee . Completion of the anonymous survey was taken as evidence of consent to participate.
For women who had a live birth, the usable response rate was 30.4% (5840 out of 19,194). The respondent sample was approximately representative of the 2011 birthing population of Queensland for mode of birth, previous caesarean section, plurality of pregnancy, area of residence, infant birthweight and gestational age at birth [ 43 , 44 ]. The sample under-represented younger women (aged less than 20 years), Aboriginal and/or Torres Strait Islander women, and women birthing in public facilities. Respondent characteristics are compared with those of the 2011 Queensland birthing population in Additional file 1 .
Outcome variables
Normal birth was defined according to the Werkmeister and colleagues definition [ 2 ]. Women were categorised as having experienced a normal birth if they did not have an induction of labour, epidural, spinal and/or general anaesthesia, an assisted vaginal birth (with forceps and/or ventouse) or caesarean section, or an episiotomy. Normal birth was broken into four categorical time-dependent outcomes: onset of labour, anaesthesia, mode of birth, and episiotomy. This was to allow the inclusion of variables that may not have been relevant for all women (e.g. water immersion in labour which was only relevant for women who experienced labour). For each subsequent outcome variable, only women who were still eligible for meeting normal birth criteria were included. For example, only those who had a spontaneous onset of labour were included in analyses examining use of anaesthesia during labour.
Onset of labour was assessed through a series of questions about whether women had experienced medical or surgical procedures to induce labour, and whether women had experienced any labour at all. Women were coded as having a spontaneous labour if they did not have prostaglandins, artificial rupture of membranes, synthetic oxytocin, or a balloon catheter for the purposes of inducing labour. Women who had a spontaneous labour were coded according to whether they used anaesthesia during labour using the item ‘ Did you have an epidural or spinal (anaesthetic injection in your back) for pain relief during labour? ’. For women who did not use anaesthesia during labour we assessed mode of birth by asking ‘ How was your baby born? ’ with five response options: an unassisted vaginal birth , a vaginal birth assisted with a vacuum , a vaginal birth assisted with forceps , a vaginal birth assisted by forceps and a vacuum , a caesarean birth . Women who had an unassisted vaginal birth were then further categorised using the item, ‘ During your birth, did you have an episiotomy (cut with scissors or a scalpel) to enlarge your vaginal opening? ’, according to whether they had an episiotomy. The remaining women who did not have an episiotomy were those who had a normal birth.
Independent variables
Based on previous literature we selected a range of independent variables, including demographic and obstetric characteristics, antenatal and intrapartum care, experiences during labour and birth, and organisational factors. These variables are detailed in Additional file 2 .
Statistical models
Multiple logistic regression models were used to estimate the associations between the independent variables and the four binary outcomes: onset of labour, anaesthesia, mode of birth, and episiotomy. Results are presented as probability ratios. As a simple example, if the probability of a normal birth was 0.2 for women in the reference group and 0.1 in another group then the probability ratio would be 0.5 (0.1 divided by 0.2), meaning that the probability was halved compared with the reference group. These probability ratios are used instead of odds ratios (the ratios of odds) which are often less intuitive and prone to misinterpretation – particularly with highly prevalent outcomes.
To estimate the probability of a normal birth we multiplied the estimated probabilities from the four logistic models (the probabilities of a spontaneous onset of labour, no anaesthesia, an unassisted vaginal birth, and no episiotomy). For example, if a woman had a 0.80 probability at each of the four stages than her probability of a normal birth would be 0.80 4 = 0.41.
We used a Bayesian paradigm to fit the logistic models so that we could multiply together the four probabilities and create a mean and 95% credible interval for the probability of normal birth. Ninety-five per cent credible intervals are like 95% confidence intervals but have the simpler interpretation of having a 95% probability of containing the true value.
Relationships between the independent variables were checked for co-linearity using variance inflation factors. Type of facility (public or private) and place of birth were found to have too much overlap with model of care, and were excluded for this reason. For water immersion during birth there was perfect prediction, with all women birthing in water experiencing a normal birth. This variable was excluded from the analysis as it is likely a proxy for an intervention-free birth rather than a predictor. Continuous variables were centred and scaled to help with the interpretation of the regression models.
Analyses were conducted using R version 3.0.2 (2013–09-25) software combined with JAGS to run the models [ 45 , 46 ]. We used a burn-in of 10,000 MCMC iterations followed by 10,000 samples thinned by 3.
Of the total sample, 53.1% had a spontaneous labour, 47.5% birthed without an epidural, spinal or general anaesthesia, 53.7% had an unassisted vaginal birth and 53.2% gave birth vaginally without an episiotomy. Combining these four outcomes, the overall rate of normal birth was 28.7% and was 44.3% among women who had a vaginal birth. Descriptive information relative to the independent variables is provided in Additional file 3 .
Probability of having spontaneous labour
Women had higher probability of experiencing spontaneous labour if they had completed secondary education. Relative to private obstetric care, women had a higher probability of having a spontaneous labour if they were receiving GP shared care, standard public care, public midwifery continuity care, or private midwifery care.
The probability of experiencing spontaneous labour was lower for women with gestational diabetes, high blood pressure, other risk factors, or a multiple pregnancy. Women who were primiparous, multiparous with a history of caesarean, older, of higher BMI, or of later gestational age were also at a lower probability of having a spontaneous labour. The mean probability ratios and 95% credible intervals are presented in Table 1 .
Probability of not using Anaesthesia
The probability of not using anaesthesia during labour was higher for women living in inner and outer regional areas, receiving GP shared care, standard public care, or public midwifery continuity care, not receiving augmentation of labour, experiencing freedom of movement throughout labour, or who had continuity of carer for labour/birth (see Table 1 ).
Women had a lower probability of not using anaesthesia if they were: primiparous, multiparous with a history of caesarean, older, of a later gestational age at birth, having a multiple birth, experiencing high blood pressure, having continuous electronic fetal monitoring during labour, or receiving care from only some known care providers during labour and birth.
Probability of unassisted vaginal birth
The probability of having an unassisted vaginal birth was reduced for women who were: primiparous, multiparous with a history of caesarean, having a multiple birth, having continuous electronic fetal monitoring, felt rushed during their labour, or received their labour and birth care from only some known care providers (see Table 1 ).
Probability of not having an episiotomy
Women had a higher probability of avoiding episiotomy if they received standard public care or private midwifery care, or if they gave birth in a non-supine position. The probability of avoiding episiotomy was lower for primiparous women and multiparous women with a previous caesarean section. Mean probability ratios and 95% credible intervals are presented in Table 1 .
Probability of normal birth
The probabilities of the four outcomes were multiplied to give the overall probability of normal birth for each independent variable (see Table 2 ). Overall, the probability of having a normal birth was higher for women: living in inner and outer regional areas; receiving GP shared care, standard public care, public midwifery continuity care, or private midwifery care; who had freedom of movement throughout labour; received continuity of care in labour and birth; who had no augmentation of labour; and who gave birth in a non-supine position.
The probability of having a normal birth was reduced for women who were primiparous, multiparous with a previous caesarean, older, had a multiple pregnancy, had a later gestational age, had gestational diabetes, had a low-lying placenta, had high blood pressure, experienced other risk factors, had continuous electronic fetal monitoring in labour, and had only some known care providers for labour and birth.
This study aimed to identify factors which may facilitate or impede normal birth, using the definition of Werkmeister and colleagues [ 2 ] where normal birth is an unassisted vaginal birth without induction of labour, epidural or general anaesthesia, or episiotomy. We calculated the probability of normal birth based on a range of modifiable and non-modifiable characteristics with retrospective, self-reported data. We found that less than half of women birthing vaginally (44.3%) and less than one-third of all birthing women (28.7%) experienced a normal birth. While consistent with previous Australian research [ 16 ], this rate is lower than estimated rates of normal birth (as defined by Werkmeister and colleagues) in Scotland (35.5%) [ 47 ] and England (42%) [ 48 ].
We identified several modifiable and non-modifiable factors that can inhibit or promote normal birth. In regards to factors that were non-modifiable at the time of pregnancy, women were less likely to experience normal birth if they were older, were primiparous, had previously given birth by caesarean, were having a multiple birth, had obstetric health concerns (including high blood pressure, low-lying placenta, gestational diabetes, or other risk factors), were at an earlier gestational age, or lived in a major city relative to an inner or outer regional area. These characteristics align with findings from previous studies of normal birth [ 16 ] and the broader literature around risk factors for medical intervention in birth [ 18 , 32 , 41 ].
Perhaps of greater clinical utility in the promotion of normal birth are the modifiable factors (after accounting for clinical risk) we found to change women’s likelihood of normal birth. The probability of normal birth was higher for women who received care in a model other than private obstetric care, had continuity of care during labour and birth, knew none rather than some of their care providers, had freedom of movement during labour, did not have continuous fetal monitoring or augmentation, or birthed in a non-supine position. Despite the sample and methodological differences in this study, these findings are largely consistent with those of Miller and colleagues [ 16 ].
The higher rate of obstetric intervention in private facilities is well-established [ 17 , 18 , 19 , 20 , 21 ]. By using model of care rather than place of birth, we allowed exploration of potential variation among different types of public care models. Relative to private obstetric care, all other models resulted in increased likelihood of normal birth for women. Women receiving GP shared care or public midwifery continuity care were at least twice as likely to have a normal birth, while it was three times higher for women in private midwifery care. Private obstetric care is the primary model of care in private facilities and is based around a medicalised view of birth. This contrasts with a more naturalist philosophy typically adopted in both public and private midwifery-led models [ 49 ]. These philosophical differences, along with the differing clinical skills of lead care providers, are likely to contribute to the disparate probabilities of normal birth for women receiving care in these models. Risk alone cannot account for the higher rate of intervention in private hospitals as discrepancies in outcomes remain after accounting for obstetric and social risk factors [ 18 ]. The relationship between model of care and normal birth is relevant for women’s decision-making about their care, however the associated risks and benefits of different models are infrequently discussed at the time this choice is made [ 50 ].
We also found that normal birth was less likely when women knew some rather than none of their care providers. It is possible that this may relate to differences in the organisation of labour and birth care between private and public models of care (and thus, private and public birth facilities). As we have demonstrated, models of care provided in public facilities (GP shared care, public midwifery continuity care and standard public care) increase the probability of achieving a normal birth, and may be less likely to provide a known care provider at the time of labour and birth care. However, having previously known care providers for labour and birth may be less relevant than having continuity of carer throughout this time. We found that women with continuity of carer for labour and birth (after accounting for known care providers and model of care) were 24% more likely to have a normal birth. Having continuous support during labour is argued to provide emotional support and comfort, information and advocacy, enhancing women’s sense of control and reducing the need for medical intervention [ 31 ]. While continuity of care across pregnancy, birth and beyond may be the ideal, having continuity of carer for labour and birth may be a cost-effective solution to reducing intervention where resources are not available for full continuity.
Our findings for a greater probability of normal birth among women who didn’t have continuous electronic fetal monitoring, had freedom of movement, or who birthed in a non-supine position are consistent with previous studies about normal birth [ 16 ]. Other research has identified that upright positioning during the first stage of labour can reduce the use of epidural anaesthesia [ 30 ] and the incidence of instrumental delivery [ 51 ], both factors that prevent a normal birth. Despite calls that the risks and benefits of fetal monitoring during labour should be discussed [ 52 , 53 ], many women report that they are either not informed or not involved in decisions about its use [ 54 ]. The extent to which upright positioning and mobility during labour and birth are encouraged by care providers remains unclear.
In contrast to a previous study of normal birth [ 16 ], we did not identify timing of birth to be associated with the probability of experiencing normal birth. Rates of episiotomy [ 26 ] and instrumental vaginal birth [ 55 ] are shown to be higher during regular working hours, while the declining rate of weekend births appears to be related to the increasing rate of caesarean section [ 27 ]. Our lack of an association is potentially due to the stage at which timing of birth was introduced in the analysis. We did not examine timing of birth as a predictor among women who had an induction of labour or a planned caesarean – interventions where the effect of staff availability and scheduling logistics are likely to play a greater role in timing of birth. We also did not identify a relationship between water immersion during labour and normal birth. Aligned with our findings, a review of water immersion during the first stage of labour also found no effect on rates of assisted vaginal birth or caesarean section [ 29 ]. However, we also did not replicate the association between water immersion and the reduced need for epidural or spinal anaesthesia identified in this review [ 29 ]. Our measurement of water immersion during labour did not account for duration, which may result in a less consistent relationship. There is currently a lack of evidence and need for further research around the optimal conditions for water immersion during labour and how factors such as duration, depth of immersion, temperature and mobility may affect this.
A strength of this study is that it appears to be the first to have examined probabilities of normal birth as a multi-dimensional construct for a wide range of predictors and with a non-restricted sample of women. The study also used population-level sampling to invite participation and was uniquely able to use a combination of clinical and experiential factors due to the self-reported nature of the data, which has not been possible using clinical reporting alone. A high level of concordance between maternal self-report and medical records has been repeatedly demonstrated for clinical information pertaining to birth [ 56 , 57 , 58 ].
While the respondent sample was representative of the Queensland birthing population on many key clinical characteristics, some characteristics were under-represented. We could have used sampling weights to provide a closer match to the Queensland birthing population; however such weights are generally more important for estimating prevalence, whereas our study was concerned with the association between variables which we assumed were generalisable from our sample to the wider population. We also did not examine the inter-relationships between different predictors of normal birth. While we identified certain groups of women at a reduced likelihood of achieving a normal birth, such as primiparous women or those with a history of caesarean section, we did not examine how the chances of normal birth may be increased for such groups. Future research may wish to consider potential interactions between modifiable and non-modifiable characteristics to determine how intervention could be reduced among particular groups of women.
Despite increasing interest in normal birth, actual rates remain low and research for how to facilitate this is minimal. Models of care with a more natural philosophy of birth, not limiting women’s freedom of movement and position during labour and birth, and continuity of care provider throughout labour and birth are shown to increase the likelihood of achieving a normal birth. To support the desired promotion of normal birth, care providers and women must be made aware of existing evidence for how care and treatment related factors influence normal birth outcomes. Pragmatic evaluation research is needed for how policies that relate to facilitating factors affect women’s experience of normal birth.
Abbreviations
Body mass index
Credible intervals
Caesarean section
General practitioner
Maternity Care Working Party. Making normal birth a reality. Consensus statement from the maternity care working party: our shared views about the need to recognise, facilitate and audit normal birth. UK: National Childbirth Trust, Royal College of Midwives: Royal College of Obstetricians and Gynaecologists; 2007. http://bhpelopartonormal.pbh.gov.br/estudos_cientificos/arquivos/normal_birth_consensus.pdf .
Werkmeister G, Jokinen M, Mahmood T, Newborne M. Making normal labour and birth a reality – developing a multi disciplinary consensus. Midwifery. 2008;24:256–9.
Article Google Scholar
Declercq E, Young R, Cabral H, Ecker J. Is a rising cesarean delivery rate inevitable? Trends in industrialized countries, 1987 to 2007. Birth. 2011;38:99–104.
Anim-Somuah M, Smyth RMD, Jones L. Epidural versus non-epidural or no analgesia in labour. Cochrane Database Syst Rev. 2011;(12) Art. No.: CD000331
Bernitz S, Øian P, Rolland R, Sandvik L, Blix E. Oxytocin and dystocia as risk factors for adverse birth outcomes: a cohort of low-risk nulliparous women. Midwifery. 2014;30:364–70.
Grivell RM, Reilly AJ, Oakey H, Chan A, Dodd JM. Maternal and neonatal outcomes following induction of labor: a cohort study. Acta Obstet Gyn Scan. 2011;91:198–203.
Knight M, Kurinczuk JJ, Spark P, Brocklehurst P. Cesarean delivery and peripartum hysterectomy. Obstet Gynecol. 2008;111:97–105.
Allen VM, Stewart A, O’Connell CM, Baskett TF, Vincer M, Allen AC. The influence of changing post-term induction of labour patterns on severe neonatal morbidity. J Obstet Gynaecol Can. 2012;34:330–40.
MacDorman MF, Declercq E, Menacker F, Malloy MH. Neonatal mortality for primary cesarean and vaginal births to low-risk women: application of an “intention-to-treat” model. Birth. 2008;35:3–8.
The Royal College of Midwives Better Birth Initiative. https://www.rcm.org.uk/better-births-initiative . Accessed 15 June 2018.
Society of Obstetricians and Gynaecologists of Canada. Association of Women's health obstetric and neonatal nurses of Canada, Canadian Association of Midwives, College of Family Physicians of Canada, Society of Rural Physicians of Canada. Joint policy statement on normal birth. J Obstet Gynaecol Can. 2008;30:1163–5.
Australian Health Ministers’ Conference. National Maternity Services Plan. Canberra: ACT: Commonwealth of Australia; 2010. p. 2011.
Google Scholar
Queensland Maternity and Neonatal Clinical Guidelines Program. Queensland Maternity and Neonatal Clinical Guideline: Normal birth. Brisbane: Queensland Government; 2012.
New South Wales Health. Maternity – Towards Normal Birth in NSW. Doc No. PD2010_45. Sydney: Department of Health; 2010.
The Birthplace in England Collaborative Group. Perinatal and maternal outcomes by planned place of birth for healthy women with low risk pregnancies: the birthplace in England national prospective cohort study. Brit Med J. 2011;343:d7400.
Miller YD, Prosser SJ, Thompson R. Back to normal: a retrospective, cross-sectional study of the multi-factorial determinants of normal birth in Queensland, Australia. Midwifery. 2015;31:818–27.
Robson SJ, Laws P, Sullivan EA. Adverse outcomes of labour in public and private hospitals in Australia: a population-based descriptive study. Med J Aust. 2009;190:474–7.
PubMed Google Scholar
Miller YD, Prosser SJ, Thompson R. Going public: do risk and choice explain differences in caesarean birth rates between public and private places of birth in Australia? Midwifery. 2012;28:627–35.
Dahlen HG, Tracy S, Tracy M, Bisits A, Brown C, Thornton C. Rates of obstetric intervention among low risk women giving birth in private and public hospitals in NSW: a population-based descriptive study. BMJ Open. 2012;2:e001723.
Einarsdóttir K, Stock S, Haggar F. Neonatal complications in public and private patients: a retrospective cohort study. BMJ Open. 2013;3:e002786.
Dahlen HG, Tracy S, Tracy M, Bisits A, Brown C, Thornton C. Rates of obstetric intervention and associated perinatal mortality and morbidity among low-risk women giving birth in private and public hospitals in NSW (2000–2008): a linked data population-based cohort study. BMJ Open. 2014;4:e004551.
Howell S, Johnston T, Macleod SL. Trends and determinants of caesarean sections births in Queensland, 1997-2006. Aust N Z J Obstet Gynaecol. 2009;49:606–11.
Roberts CL, Tracy S, Peat B. Rates for obstetric intervention among private and public patients in Australia: population based descriptive study. BMJ. 2000;321:137–41.
Article CAS Google Scholar
Sandall J, Saltani H, Gates S, Shennan A, Devane D. Midwife-led continuity models versus other models of care for childbearing women. Cochrane Database Syst Rev. 2015;(9) Art. No.: CD004667
Gagnon AJ, Meier KM, Waghorn K. Continuity of nursing care and its link to cesarean birth rate. Birth. 2007;34:26–31.
Allen RE, Hanson RW. Episiotomy in low risk vaginal deliveries. J Am Board Fam Pract. 2005;18:8–12.
Lerchl A. Where are the Sunday babies? III. Caesarean sections, decreased weekend births, and midwife involvement in Germany. Naturwissenschaften. 2008;95:165–70.
Alfirevic Z, Devane D, Gyte GML, Cuthbert A. Continuous cardiotocography (CTG) as a form of electronic fetal monitoring (EFM) for fetal assessment during labour. Cochrane Database Syst Rev. 2017;(2. Art. No.: CD006066) https://doi.org/10.1002/14651858.CD006066.pub3 .
Cluett ER, Burns E. Immersion in water in labour and birth. Cochrane Database Syst Rev. 2009;(2) Art. No.: CD000111
Lawrence A, Lewis L, Hofmeyr GJ, Dowswell T, Styles C. Maternal positions and mobility during first stage labour. Cochrane Database Syst Rev. 2013;(8) Art. No.:CD003934
Hodnett ED, Gates S, Hofmeyr G, Sakala C. Continuous support for women during childbirth. Cochrane Database Syst Rev. 2013; Issue 7: Art. No.: CD003766.
Humphrey T, Tucker JS. Rising rates of obstetric interventions: exploring the determinants of induction of labour. J Public Health. 2009;31:88–94.
Guidelines Committee of the Royal College of Obstetricians and Gynaecologists. Green–top Guideline No. 27. Placenta Praevia, Placenta Praevia Accreta and Vasa Praevia: Diagnosis and Management. London: Royal College of Obstetricians and Gynaecologists; 2011.
Laughon SK, Zhang J, Grewal J, Sundaram R, Beaver J, Reddy UM. Induction of labor in a contemporary obstetric cohort. Am J Obstet Gynecol. 2012;206:486e1–9.
Jeschke E, Ostermann T, Dippong N, Brauer D, Pumpe J, Meißner S, Matthes H. Identification of maternal characteristics associated with the use of epidural analgesia. J Obstet Gynaecol. 2012;32:342–6.
Janssens S, Wallace KL, Chang AMZ. Prepartum and intrapartum caesarean section rates at mater mothers’ hospital Brisbane 1997–2005. Aust N Z J Obstet Gynaecol. 2008;48:564–9.
Prosser SJ, Miller YD, Thompson R, Redshaw M. Why 'down under' is a cut above: a comparison of rates of and reasons for caesarean section in England and Australia. BMC Pregnancy Childbirth. 2014;14:149.
Dzakpasu S, Fahey J, Kirby RS, Tough SC, Chalmers B, Heaman MI, et al. Contribution of prepregnancy body mass index and gestational weight gain to caesarean birth in Canada. BMC Pregnancy Childbirth. 2014;14:106.
Poobalan AS, Aucott LS, Gurung T, Smith WCS, Bhattacharya S. Obesity as an independent risk factor for elective and emergency caesarean delivery in nulliparous women – systematic review and meta-analysis of cohort studies. Obes Rev. 2009;10:28–35.
Wills R, Johnston T. Statbite 56: Morbidity and mortality associated with older maternal age at Birth. Brisbane: Queensland Health; 2013. https://www.health.qld.gov.au/__data/assets/pdf_file/0028/361486/statbite56.pdf .
Essex H, Green J, Baston H, Pickett K. Which women are at an increased risk of a caesarean section or an instrumental vaginal birth in the UK: an exploration within the millennium cohort study. BJOG. 2013;120:732–43.
Prosser SJ, Miller YD, Armanasco A, Hennegan J, Porter J, Thompson R. Findings from the having a baby in Queensland survey, 2012. Brisbane: Queensland Centre for Mothers & Babies, The University of Queensland; 2013.
Li Z, Zeki R, Hilder L, Sullivan EA. Australia’s mothers and babies 2011. Perinatal statistics series no. 28. Cat. no. PER 59. Canberra: AIHW National Perinatal Epidemiology and Statistics Unit; 2013.
Queensland Health. Perinatal statistics: Queensland 2011. Brisbane: Queensland Department of Health; 2014.
Plummer M. Rjags: Bayesian graphical models using MCMC. R package version. 2013:3–11.
R Core Team. R: A Language and Environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2013.
Information and Statistics Division, Scotland Births in Scottish Hospitals, year ending 31 March 2012. 2013. Data reported at https://www.nct.org.uk/professional/research/maternity-statistics/maternity-statistics-scotland . Accessed 15 June 2018.
Dodwell M. Normal birth rates in England. NCT Perspective. 2012;16:16–7.
Klein MC, Kaczorowski J, Hall WA, Fraser W, Liston RM, Eftekhary S, et al. The attitudes of Canadian maternity care practitioners towards labour and birth: many differences but important similarities. J Obstet Gynaecol Can. 2009;31:827–40.
Stevens G, Thompson R, Kruske S, Watson B, Miller YD. What are pregnant women told about models of maternity care in Australia? A retrospective study of women's reports. Patient Educ Couns. 2014;97:114–21.
Gupta JK, Hofmeyr J, Shehmar M. Position in the second stage of labour for women without epidural anaesthesia. Cochrane Database Syst Rev. 2012;(5) Art. No.: CD002006
Queensland Clinical Guidelines. Intrapartum fetal surveillance. Brisbane: Queensland Government; 2015.
Wood SH. Should women be given a choice about fetal assessment in labor? MCN Am J Matern Child Nurs. 2003;28:292–8.
Thompson R, Miller YD. Birth control: to what extent do women report being informed and involved in decisions about pregnancy and birth procedures? BMC Pregnancy Childbirth. 2014;14:62.
Webb DA, Culhane J. Time of day variation in rates of obstetric intervention to assist in vaginal delivery. J Epidemiol Commun H. 2002;56:577–8.
Bat-Erdene U, Metcalfe A, McDonald SW, Tough SC. Validation of Canadian mothers’ recall of events in labour and delivery with electronic health records. BMC Pregnancy Childbirth. 2013;13(Suppl 1):S3.
Gartland D, Lansakara N, Flood M, Brown SJ. Assessing obstetric risk factors for maternal morbidity: congruity between medical records and mothers’ reports of obstetric exposures. Am J Obstet Gynecol. 2012;206(152):e1–10.
Rice F, Lewis A, Harold G, van den Bree M, Boivin J, Hay DF. Agreement between maternal report and antenatal records for a range of pre and peri-natal factors: the influence of maternal and child characteristics. Early Hum Dev. 2006;83:497–504.
Download references
Acknowledgements
We are grateful to the women who provided survey data. The Queensland Registry of Births, Deaths and Marriages contacted women to invite them to participate on behalf of the Queensland Centre for Mothers & Babies to ensure women’s privacy was protected. Computational resources and services used in this work were provided by the High Performance Computer and Research Support Unit, Queensland University of Technology, Brisbane, Australia. We acknowledge Rachel Thompson for her insights and contribution to a previous paper on normal birth that informed the current study.
The research on which this article is based was conducted as part of a Statewide survey program funded by the Queensland Government, at The University of Queensland. The views expressed are not necessarily those of the aforementioned bodies.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and affiliations.
School of Psychology, The University of Queensland, Brisbane, Australia
Samantha J. Prosser
School of Public Health & Social Work, Institute of Health and Biomedical Innovation, Queensland University of Technology, Kelvin Grove, Brisbane, QLD, 4059, Australia
Samantha J. Prosser, Adrian G. Barnett & Yvette D. Miller
You can also search for this author in PubMed Google Scholar
Contributions
The authors worked jointly to design the study. YM and SP developed the measures and collected the data. AB analysed the data and SP drafted the manuscript. All authors contributed provided critical feedback throughout the drafting of the manuscript and approved a final version for publication.
Corresponding author
Correspondence to Yvette D. Miller .
Ethics declarations
Ethics approval and consent to participate.
Ethics approval for this study was granted by The University of Queensland Human Research Ethics Committee on 14th December, 2011 (#2011001083). Completion of the anonymous survey was taken as evidence of consent to participate.
Competing interests
The author(s) declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional files
Additional file 1:.
Comparison of Respondent Sample and Population Characteristics. This file contains two tables comparing maternal and infant characteristics of the sample population with those of the Queensland, Australia, birthing population in 2011. (PDF 642 kb)
Additional file 2:
Measurement of independent variables examined for associations with normal birth. This file contains a table detailing the independent variables used in the analysis, describing how they were measured in the Having a Baby in Queensland 2012 Survey. (PDF 484 kb)
Additional file 3:
Sample characteristics of women as frequencies and percentages. This file presents a table outlining the characteristics of women in the study sample. Means and standard deviations, or frequencies and percentages where relevant, are provided. (PDF 486 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Reprints and permissions
About this article
Cite this article.
Prosser, S.J., Barnett, A.G. & Miller, Y.D. Factors promoting or inhibiting normal birth. BMC Pregnancy Childbirth 18 , 241 (2018). https://doi.org/10.1186/s12884-018-1871-5
Download citation
Received : 13 February 2017
Accepted : 31 May 2018
Published : 18 June 2018
DOI : https://doi.org/10.1186/s12884-018-1871-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Normal birth
- Spontaneous labour
- Midwifery-led care
- Vaginal birth
- Patient-reported data
BMC Pregnancy and Childbirth
ISSN: 1471-2393
- Submission enquiries: [email protected]
- General enquiries: [email protected]
Advertisement
Was Today’s Earthquake Connected to the Solar Eclipse?
The tidal forces on Earth grow as the sun, moon and Earth begin to align, a configuration that can lead to a solar eclipse. But the results of several studies of the relationship between earthquakes and tides are inconclusive, a geophysicist said.
- Share full article

By Katrina Miller
- April 5, 2024
With a total solar eclipse set to pass through the United States on Monday, it is easy to imagine a linkage between unusual events in the heavens and on Earth. But geoscientists were cautious about making such a connection.
Earthquakes happen along fault lines, or cracks between two blocks of rock on Earth’s crust. Tides stretch and squish the land on Earth just as they contribute to waves in the ocean, and those tidal forces grow as the sun, moon and Earth begin to align — a configuration that sometimes creates a solar eclipse.
One theory is that this may introduce additional stress along Earth’s fault lines.
“We do know that the relative position of the Earth and the moon and the sun does exert tidal forces,” said William Frank, a geophysicist at the Massachusetts Institute of Technology. “And we know that changes the stress that can be on a fault that can host an earthquake.”
But the results of several studies of the relationship between earthquakes and tides are inconclusive, according to Seth Stein, a geophysicist at Northwestern University. “If there’s any effect, it would be incredibly weak,” he said.
Earthquakes are driven most often by the motion between two tectonic plates making up Earth’s crust — either when two plates slide along each other in opposite directions, or when one slides under the other.
Both types of movements introduce strain at the junction, which often gets relieved by an earthquake.
But at the moment, it’s difficult to say that plate motion was responsible for the quake that shook the Northeast Friday morning.
“It’s not quite as obvious, because there is no tectonic plate boundary that is active,” Dr. Frank said.
Still, he added, fault lines from past activity are everywhere on Earth’s crust.
“Some of these faults can still be storing stress and be closure to failure,” he said. “And it can just require a little bit more to push it over the edge.”
Katrina Miller is a science reporting fellow for The Times. She recently earned her Ph.D. in particle physics from the University of Chicago. More about Katrina Miller

IMAGES
VIDEO
COMMENTS
She was otherwise fit and had a normal body mass index; she did not smoke and had no allergies. During the index pregnancy, at the 19-week check-up, her cervix was found to have descended. Subsequent regular ultrasound scans at 24, 26, 30, 32, and 34 weeks of gestation demonstrated normal fetal morphology, with normal and concordant fetal growth.
Case B Normal Delivery in 15-year-old primipara Original Scenario Developer(s): S. Vaughn, RN, MPH ... Case studies in maternity and women's health. Clifton Park, NY: Thomson-Delmar Learning. Kelly, P., Vottero, B., Christie-McAuliffe, C. (2014). Introduction to Quality and Safety Education for Nurses.
VBAC will have 2-3 times increased chances of uterine rupture. Majority of published studies have reported successful delivery of 72-75% in VBAC cases. Client will be fasting and on intravenous infusion in the early stage of labour. Continuous fetal heart monitoring will be conducted during the entire labour process.
We describe the case of a 27-year-old white woman who had experienced an emergency caesarean delivery at 39 weeks for fetal distress with no postpartum complications. As part of our ongoing study "Vaginal delivery after caesarean section", she underwent saline contrast sonohysterography 6 months after the caesarean section.
Reference. Promote walking and upright positions (kneeling, squatting, or standing) for the mother in the first stage of labor. A. 10. Provide continuous support during labor and delivery. A. 11 ...
Data from number a studies have suggested that normal labor can progress at a rate much slower than that ... but it is considered normal up to 30 minutes after delivery of the fetus. ... Hussain J, Maclennan FA, et al. Factors associated with a prolonged second state of labour--a case-controlled study of 364 nulliparous labours. J Obstet ...
A normal diagonal conjugate measures approximately 12.5 cm, with the critical distance being 10 cm. To measure the diagonal conjugate place the tip of the middle finger at the sacral promontory and note the point on the hand that contacts the pubic symphysis ( Figure 131-1 B ).
Conducting a Normal Delivery Study Session 5 Conducting a Normal Delivery Introduction. ... you can assess how well you have achieved its Learning Outcomes by answering the questions below Case Study 3.1. Write your answers in your Study Diary and discuss them with your Tutor at the next Study Support Meeting. You can check your answers with ...
5.1.1 General recommendations. Personnel should wear personal protective equipment (gloves, goggles, clothing and eye protection) to prevent infection from blood and other body fluids. Ensure a calm reassuring environment and provide the woman as much privacy as possible during examinations and delivery. Encourage her to move about freely if ...
A Case Study of G2 P2 (2002) PU of 37 1/7 weeks AOG by UTZ delivered spontaneously to a Live Baby Boy via Midline (Median) Episiotomy Repaired ... Olongapo City. Her first delivery was delivered via Normal Spontaneous Delivery with right mediolateral episiotomy repaired at James L. Gordon Memorial Hospital attended by doctors, nurses, and ...
Case Study on Normal Vaginal Delivery (4) (2) - Free download as Word Doc (.doc / .docx), PDF File (.pdf), Text File (.txt) or view presentation slides online. case study
Background In response to rising rates of medical intervention in birth, there has been increased international interest in promoting normal birth (without induction of labour, epidural/spinal/general anaesthesia, episiotomy, forceps/vacuum, or caesarean section). However, there is limited evidence for how best to achieve increased rates of normal birth. In this study we examined the role of ...
A recent study reported that pregnancy during intensive renal replacement therapy and multidisciplinary team management may result in significantly better outcomes for both mother and baby. Herein, we report a case of successful pregnancy and delivery after peritoneal dialysis in a patient who was misdiagnosed as primary infertility. 2 Case report
RAMOS-Final-NSD-Case-Study - Free download as Word Doc (.doc / .docx), PDF File (.pdf), Text File (.txt) or read online for free. A 21-year-old woman presented at 38 weeks and 5/7 days in spontaneous labor. She delivered a healthy baby girl via normal spontaneous vaginal delivery with mediolateral episiotomy. The stages of labor and mechanisms of delivery are described, including engagement ...
The remainder of the examination was normal. The hemoglobin level was 7.7 g per deciliter (reference range, 12.0 to 16.0), which was similar to the patient's baseline level. ... are derived from ...
Case Study #1: Labor and Delivery. SITUATION: Mrs. M. is a 27-y/o gravida 3, para 2, who was admitted at term at 0630. She stated that she had been having contractions at 7 to 10-minute intervals since 1600 the previous day.
The Researchers ABSTRACT This case study aims to provide the students and readers with nursing research related and nursing practice related further understanding about Normal Spontaneous Delivery. A spontaneous vaginal delivery (SVD) occurs when a pregnant female goes into labor without the use of drugs or techniques to induce labor, and delivers her baby in the normal manner, without forceps ...
Normal Spontaneous Delivery Case Study - Free download as Word Doc (.doc / .docx), PDF File (.pdf), Text File (.txt) or read online for free. Scribd is the world's largest social reading and publishing site.
A CASE STUDY ON NORMAL SPONTANEOUS DELIVERY. Manuel V. Gallego Cabanatuan City General Hospital (Delivery Room) Presented By: Payra, Divina May L. (Head Nursing) Gallardo, Khristine Bernadette Reyes, Deane Carmina C. San Pedro, Kimberly Tajorda, Jandaria Tarah Mae Villamia, Lyka C. NEUST SN' NORMAL SPONTANEOUS DELIVERY (Case Study)
Republic of the Philippines ISABELA STATE UNIVERSITY Echague, Isabela A Case Study of Postpartum Normal Spontaneous Delivery Submitted to the Faculty and Staff of the College of Nursing Submitted by: GROUP 2, BSN 2-2 S.Y. 2019 Agustin, Honnie Gie B. Deloso, Julie Ann F. Florentin, Mariel Joy M. Magtanong, Karen Joyce C. Magtanong, Kathleen Joy ...
Introduction. Preventing preterm birth (PTB, defined as delivery <37 weeks' gestation) is a global priority. Of the estimated 15 million babies born preterm each year [Citation 1, Citation 2], approximately one million will die from complications, and many more will be left with lifelong disabilities [Citation 3].PTB prediction in otherwise low-risk women could improve neonatal outcomes by ...
The tidal forces on Earth grow as the sun, moon and Earth begin to align, a configuration that can lead to a solar eclipse. But the results of several studies of the relationship between ...