An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Scientific Reports
- PMC10279665


Malaria surveillance, outbreak investigation, response and its determinant factors in Waghemra Zone, Northeast Ethiopia: unmatched case–control study
Habtu debash.
1 Department of Medical Laboratory Sciences, College of Medicine and Health Sciences, Wollo University, Dessie, Ethiopia
Marye Nigatie
3 Department of Medical Laboratory Sciences, College of Health Sciences, Woldia University, Woldia, Ethiopia
Habtye Bisetegn
Daniel getacher feleke.
4 Department of Microbiology, Immunology and Parasitology, College of Health Sciences, Addis Ababa University, Addis Ababa, Ethiopia
Gebru Tesfaw
2 Department of Internal Medicine, School of Medicine, Wollo University, Dessie, Ethiopia
Askale Amha
5 Waghemra Zone Health Department, Sekota, Ethiopia
Megbaru Alemu Abate
6 Department of Medical Laboratory Sciences, College of Medicine and Health Sciences, Bahirdar University, Bahirdar, Ethiopia
7 The University of Queensland, School of Public Health, Brisbane, Australia
Alemu Gedefie
Associated data.
All relevant data are included in the published article.
Malaria is a major global public health concern, with around half of the world's population at risk of infection. It is one of the most common epidemic-prone diseases, resulting in on-going epidemics and significant public health problems. On September 12, 2022, Waghemra Zone malaria monitoring data revealed that the district was suffering an unusually high number of malaria cases. Therefore, the aim of this study was to assess the occurrence of malaria outbreaks and investigate contracting factors in Waghemra Zone, Northeast Ethiopia. A community-based case–control study with a 1:1 ratio was employed at Waghemra Zone from September 14 to November 27, 2022. A total of 260 individuals (130 cases and 130 controls) were included in the study. A structured questionnaire was used to collect the data. Malaria cases were confirmed by either microscopy or malaria rapid diagnostic tests. The magnitude of the outbreak was described by place, person, and time. A multivariable logistic regression analysis was conducted to identify malaria risk factors. A total of 13,136 confirmed cases of malaria were detected in the Waghemra zone, with an overall attack rate of 26.5 per 1000 and slide positivity rate was 43.0%. The predominant species was Plasmodium falciparum accounting for 66.1%. Children under five years old (AOR = 5.1; 95% CI 2.6–23.0), the presence of artificial water-holding bodies (AOR: 2.7; 95% CI 1.340–5.420), intermittent rivers closer to the living house (AOR = 4.9; 95% CI 2.51–9.62), sleeping outside a home (AOR = 4.9; 95% CI 2.51–9.62), and a lack of knowledge about malaria transmission and prevention (AOR: 9.7; 95% CI 4.459–20.930) were factors associated with malaria contraction. The overall attack rate for malaria during this outbreak was high. Children less than five years, the presence of mosquito breeding sites, staying outdoors overnight, and a lack of knowledge on malaria transmission and prevention were predictors of malaria. Early management of local vector breeding places, as well as adequate health education on malaria transmission and prevention methods, should be provided to the community to prevent such outbreaks in the future.
Introduction
Malaria is a widespread and debilitating tropical disease caused by Plasmodium species and transmitted through the bites of infected female Anopheles mosquitoes 1 . According to the World Health Organization's (WHO) 2021 malaria report, the WHO African regions continue to suffer the greatest burden of malaria. The African Region accounted for 95% of all malaria cases (228 million) and 96% of all malaria deaths (602 000) in 2020, with children under the age of five accounting for 80% of all malaria deaths in the region. Malaria services were hampered beginning in 2020 because of the Covid-19 epidemic, adding to the region's malaria load 2 .
Malaria is a major public health issue in Ethiopia, where it is estimated that 68% of the population resides 3 . Despite widespread deployment of malaria prevention strategies such as early diagnosis and treatment, indoor residual spraying, and mass distribution campaigns of long-lasting insecticide-treated bed nets 4 , Ethiopia has the highest incidence of malaria cases. Malaria is mostly an endemic disease in the country, and outbreaks sometimes happen. Its transmissions peak between September and December, following the main rainy season, and between June and August 3 .
Recurrent outbreaks and epidemics are linked to cyclical weather fluctuations in the country, which lead to enhanced vector survival. Other triggering factors include exceptional local weather events and activities that result in environmental alteration, increasing vector populations, and increasing population vulnerability to famine, starvation, and conflict 3 , 5 . More than 542,000 people have been displaced as a result of internal conflict in Amhara region Ethiopia. The Waghemra zone has been severely affected by this internal conflict 6 . The conflict has led to the deterioration of health services, the interruption of anti-malarial treatments, and the movement of people, which has resulted in the failure of efforts to keep malaria under control and the likelihood of an outbreak 7 .
The Waghemra zone is one of the most malaria-prevalent areas in the Amhara region of northeast Ethiopia. On September 12, 2022, malaria monitoring data obtained from the Zone Health Office revealed that the districts were experiencing an exceptionally high number of malaria cases. In WHO epidemiologic week 36 of 2022, a total of 190 malaria cases were registered, compared to only 122 cases in the same epidemiologic week during the threshold period (2016–2020). On September 14, 2022, a rapid response team was dispatched to the affected districts to confirm the existence of the outbreak, identify risk factors, and aid in intervention actions.
Understanding the causes of outbreaks in these areas allows for early case management, identification of variables that maintain the disease, and the design of more effective preventative and control methods to facilitate malaria elimination by 2030. As a result, the goal of this study was to confirm the occurrence of the outbreak, identify gaps and risk factors that contributed to the outbreak's existence, and provide appropriate public health intervention for the outbreak in the Waghemra zone.
Materials and methods
Waghemra Zone is one of eleven zones in Amhara region of Ethiopia. The Waghemra zone is defined by the following latitude and longitude coordinates: 12° 45′ 54" N, 38° 50′ 34.8"E and has an elevation of 1498 m. In terms of health care, it has 136 health posts, 34 health centers, one general hospital, and two primary hospitals. This zone is divided into eight districts with a total population of 536,129 people. Data was collected from Ziquala, Sahala, Abergelie, Dehana, Sekota Zuria, Sekota Town and Gazgibla districts. However, due to the presence of war during data collection in the Tsagbji district and some kebeles in the Abergele district were excluded. The outbreak occurs in all districts, but the severity varies. The area's average yearly temperature and rainfall are 26 °C and 786 mm, respectively. The climate and topography of the study areas are conducive to Anopheles mosquito breeding, and malaria transmission is prevalent.
Study design and period
Community based unmatched case–control study was conducted from September 14 to November 27, 2022.
Source population, study subject and variables
People living in the Amhara region's Waghemra zone who are at risk of malaria are the source population. And the specific study subjects for these cases were febrile patients who tested positive for malaria parasites by either Rapid Diagnostic test (RDT) or a microscope. Controls, on the other hand, were classified as having no signs and symptoms of acute febrile illness one month before data collection. A non-febrile, apparently healthy person living in the same village as the active case patient from September 14 to November 27, 2022, was studied as a control subject. Controls were selected regardless of their age, gender, educational status, physiological status, and socio-economic status. The independent variables were socio-demographic and economic characteristics, behavioral factors like Insecticide-Treated Nets (ITN) use, Indoor Residual Spray (IRS), sleeping area at night and environmental factors.
Descriptive and analytical epidemiology
Confirm the diagnosis and verify the existence of the outbreak.
Malaria data from the last six years (2016–2021) were analyzed at the Waghemra zone health office to determine the epidemic threshold level. However, because of the inadequacy of the most recent year's (2021) data, the previous five years' (2016–2020) weekly malaria case reports were utilized. Then epidemic threshold level was defined by comparing weekly data with similar weeks in 2022, and an epidemic curve was produced. A rise beyond the weekly threshold was recorded, indicating an outbreak. On September 12, 2022 (week 36), an early warning alarm was received from the Waghemra zone. The Zonal public health emergency management case team decided to investigate or confirm the outbreak and intervene after receiving a request from the zone health office and analyzing regular surveillance data. A number of malaria cases have been recorded; the slide positivity rate and attack rate were calculated as the number of confirmed malaria cases per 100 and 1000 population, respectively.
Sample size determination and sampling technique
The sample size was calculated using Epi-Info version 7.2.1 by taking an 80% power,, an odds ratio of 3.32 for the presence of artificial water holding bodies near the home, the percentage of exposed controls of 21.3% 8 , and the case-to-control ratio of 1:1. The total sample size was 118. Considering a design effect of 2 and 10% non-response rate, the final sample size became 260, with 130 cases and 130 controls .
A multi-stage random sampling method was used to enrol the study participants. Waghemra zone has eight districts, and of them, three (Ziquala, Sahala, and Abergelie) were purposefully selected. In each district, two kebeles were selected randomly using a lottery method. Accordingly, Tsitsika and Netsawork, Silazge and Meharit, and Saka and Debre-brihan kebeles were selected from Ziquala, Sahala, and Abergele districts, respectively. The total households for each village were available at their nearest health center or health post, which is stored as a family card folder. Based on this, the total sample size was proportionally allocated as 60, 43, 52, 33, 47, and 25 to Tsitsika, Netsawork, Silazge, Meharit, Saka, and Debre-brihan kebeles, respectively. All cases and controls were selected from the same community or neighbour for the controls at the same time. The lottery method was applied to select individual participants in the selected household.
Data collection
Six health extension workers and six laboratory technologists collected data using a structured questionnaire under the supervision of the principal investigator and the zonal public health emergency management case team. The questionnaire utilized in the study was prepared by reviewing the literatures 7 – 9 . Data collectors and supervisors received one day of training to ensure data quality. A review of weekly Integrated Disease Surveillance and Response (IDSR) reports at various levels (district health office and health facilities) was done. For adults, selected cases and controls were interviewed directly; for children, parents were involved in the interview process. But each participant gave blood for malaria diagnosis.
Laboratory methods
At Waghemra Zone health facilities, laboratory technologists utilized a light microscope to detect malaria parasites. During power outages, RDTs were used in healthcare facilities. Furthermore, at time of outbreak investigation, health extension workers and surveillance teams employed RDTs to identify confirmed malaria cases at health posts and the community level.
Environmental and vector control assessment
The environmental impact, as well as the ownership and use of ITNs were assessed. Selected case patients and controls were asked questions regarding the existence of mosquito breeding places in and around their compound. The potential breeding sites of Anopheles mosquitoes, such as uncovered plastic water containers, old tires, stagnant water, and broken glasses in the home or outside the home were evaluated. Furthermore, we assessed for the presence of anopheles’ larvae in stagnant water.
Data processing and analysis
Data were entered into Epi-Info 7.2.0.1 and analyzed using Statistical Package for Social Science version 26 (SPSS-26). The outbreak's scope was described in terms of person, place and time. The significance of risk factors for the outbreak was determined using logistic regression. Variables with p-value < 0.25 in bivariate analysis were entered in multiple logistic regression analysis to examine the effect of an independent variables on the outcome variable. The association between dependent and independent variables was determined using Odds Ratio (OR) of 95% Confidence Interval (CI) at p-value less than 0.05 was regarded as statistically significant.
Ethical consideration
Ethical clearance was obtained from the ethical review committee of College of Medicine and Health Sciences, Wollo University on the date 16/8/2022 with a protocol number of CMHS/201/2022. Supportive letters were also obtained from the Waghemra Zone Health Office. Written informed consent and assent were obtained from participants or caregivers. Positive cases were treated according to national malaria guidelines. The information obtained was made anonymous and de-identified prior to analysis to ensure confidentiality. The study was also conducted in accordance with the Helsinki Declaration.
Socio demographic characteristics
During the study period, 260 eligible study participants were selected and interviewed, making the response rate 100. The study included 155(59.6%) males and 105 (40.4%) females. The majority of the participants were between the ages of 15 and 45. In terms of occupation and education, 124 (47.7%) were farmers, while 227 (68.8%) were illiterate (Table (Table1 1 ).
Socio-demographic characteristics of study participants of malaria outbreak, Waghemra Zone, Northeast Ethiopia, 2022.
Descriptive result
Description of cases by person and place.
During the outbreak investigation period from WHO weeks 29 to 47, a total of 13,136 confirmed cases of malaria from the Waghemra zone were detected. Total slide positivity rate (TPR) and attack rate (AR) were 43.0% and 26.5%, respectively. From all malaria confirmed cases, the most affected age group was > 15 years (65.6%), followed by 5–14 years (24.0%), and below 5 years (10.4%). The districts with the largest proportions of malaria-confirmed patients were Ziquala, Sahala, and Abergele, with 37.9%, 37.2%, and 10.2%, respectively. On the other hand, the highest attack rate was observed in the Sahala, Ziquala, and Abergele districts, with rates of 172.2, 113.2, and 28.9, respectively. Plasmodium falciparum responsible for 8681 (66.1%) of the infections, while P. vivax responsible for 3875 (29.5%) (Table (Table2 2 ).
Distribution of malaria cases by cluster, age group and attack rate in Waghemra Zone, Northeast Ethiopia, 2022.
PF: Plasmodium falciparum; PV: Plasmodium vivax.
Description of cases by time
The Waghemra Zone Health Department was informed that the number of malaria cases had exceeded the threshold in the WHO epidemiologic week 36/2022. The number of malaria patients steadily increased and peaked in week 42. Then it steadily decreased from week 43 to week 47 but was not controlled till this investigation was completed (Fig. 1 ). The intervention began with mass diagnosis using RDT and microscopy, and the positive cases were treated with artemisinin-based combination therapy and chloroquine for infection with P. falciparum and P. vivax , respectively. Health education, environmental management, distribution of ITN and the use of Abet chemicals to larvicide stagnant water were also applied.

Malaria outbreak line graph by WHO epidemiologic week in Waghemra zone, Northeast Ethiopia, 2022.
Analytic results
Factors associated with malaria outbreaks.
In a multivariable analysis, children under the age of five were five times more likely than those over the age of 45 to contract malaria (Adjusted Odds ratio (AOR) = 5.1; 95% Confidence Interval (CI) 2.6–23.0). People who were living in households where artificial water-holding bodies were thus 2.7 times more at risk of getting malaria infection than their counterparts (AOR: 2.7; 95% CI 1.340–5.420). Similarly, the presence of intermittent rivers closes to the community within 1 km distance increased the likelihood of getting malaria than those far away from it (AOR: 9.4; 95% CI 4.8–18.0). Likewise, children who stayed outside at night had an almost five-fold greater risk of acquiring malaria compared to those who did not (AOR = 4.9; 95% CI 2.51–9.62). Furthermore, higher odds of malaria were noted among those who had no knowledge on malaria transmission, prevention and control mechanisms (AOR: 9.7; 95% CI 4.459–20.930) (Table (Table3 3 ).
Bivariable and multivariable analysis of risk factors for malaria outbreak in Waghemra Zone, Northeast Ethiopia, 2022.
*Significant variable in bivariate analysis ** significant variables in multivariable analysis.
Public health interventions
Early diagnosis and treatment.
During the investigation period, an active case detection was conducted using RDT or microscopy, as well as early case management in accordance with national malaria treatment standards 9 . Temporary diagnosis and treatment sites were established to control and prevent further transmission through early treatment.
Environmental assessment
There were many mosquito breeding sites detected in the districts, which could be the source of the outbreak. In most of houses, unnecessary weeds, fake water-holding containers, especially damaged gutters, unused cans, unused old ties and stagnant waters were observed. Environmental management such as filling, draining, and clearing were carried out in an area larger than 432,157 square meters in a selected Anopheles mosquito breeding site. The community was involved in both the opening of temporarily stagnant water and the administration of larvicide (abet insecticide) at the breeding location. In this environmental management a total of 8,654 people were participated.
Vector control activities
The zone fast response team assessed and provided vector control activities in the study area. In all households in the Waghemira zone, indoor residual spray chemicals were not sprayed due to conflict in the last year. The fast response team, sprayed anti-larval chemical (abate) on stagnant water with an approximate area of 432,157 square meters. Fifty homes from each affected kebeles were randomly selected and visited to look for new malaria cases and assess the use of insecticide-treated bed nets at night. Even though every household had at least one insecticide-treated bed net, only 42.6% of them hung it directly on the bedding, with the rest hanging it underneath the beds and elsewhere in the house Moreover, about 22.6% of the household nets were damaged. The response teams then distributed over 3100 ITNs to the community.
Health education and communication
Health professionals were mobilized and assigned to the affected village for an active case search and early case management in the community. In addition, health education was given to 15,890 people about the cause, transmission, prevention, and control of malaria. Communicating and discussing the trend of the malaria situation with health facilities, Woreda, and zone health departments, and there was also multi-sectorial integration for social mobilization and prevention of malaria.
Based on five years of epidemiological records of malaria cases, the study findings showed the presence of a malaria outbreak in the study area. The malaria outbreak investigation included WHO weeks 36 to 47. Overall, the outbreak decreased but was not controlled due to inadequate environmental and vector control interventions in affected areas. For the past year, there has been an internal conflict in the study area, which has resulted in the deterioration of the health system and the interruption of malarial prevention measures, which have kept malaria under control.
The national malaria prevention and control strategies recommend the application of the IRS at least once a year with 100% coverage and at least one ITN per two people in high malaria-risk areas 10 . Despite this fact, prior to the outbreak, IRS was not applied, early replacement of ITN was not done, and there were multiple mosquito breeding sites. Households that had been using the ITN for purposes other than their intended purpose were also observed. This could be due to poor monitoring of the communities after distributing the ITN. The districts were also inadequately prepared for the outbreak, leading to a shortage of resources. This negatively affected outbreak control and resulted in the outbreak taking longer to contain. A similar finding was reported in Binga district, Zimbabwe 11 .
The overall attack rate (AR) was 26.5 cases per 1000 population; this finding was higher than a study done in Argoba district, South Wello Zone (AR: 1.8) 12 , Laelay Adyabo district, Northern Ethiopia (AR: 12.1) 13 , and India (AR: 15.1) 14 . However, this finding was lower than a study done in the Abergelle district, North Ethiopia (AR: 33.1) 15 , Simada district, Northwest Ethiopia (AR: 200) 8 , Afar region, Ethiopia (AR: 36.7) 16 , Bolosso Sore district, Southern Ethiopia (AR: 36.4) 17 , BenaTsemay district, Southern Ethiopia (AR: 114) 18 , and Kole district, Uganda (AR = 68) 19 . This difference might be attributed to prevention and control efforts, community level of awareness, internal conflict, and area differences in the burden of malaria and duration of the disease.
The AR was highest in Sahala, Ziquala, and Abergele districts, with rates of 172.2, 113.2, and 28.9 per 1,000 populations, respectively. This might be due to the presence of multiple mosquito breeding sites near residents of these districts compared to the other districts. Moreover, these districts are extremely hot and low-land areas with a high malaria burden. This was in line with a study done in the Metema district and in the Amhara Regional State, Ethiopia 20 , 21 . This could be due to high temperatures in the area, which are conducive to mosquito development rates, biting rates, and parasite survival within the mosquito 22 .
The greatest number of malaria cases was found in patients above the age of 15 (8621 out of 13,136). This finding was in line with studies from Abergele district Northeast Ethiopia 23 , Ankasha district, North Ethiopia 9 , and BenaTsemay district, Southern Ethiopia 18 . This might be due to the fact that the majority of the adolescents were spending more time outdoors in this area for farming, livestock-keeping, and fishing activities that exposed them to mosquito bites. This implies that the regional health bureau needs to give more focus and extend medical services to people who are engaged in farming, livestock keeping, and fishing.
The predominant Plasmodium species detected in this study was P. falciparum (66.1%), followed by P. vivax (29.5%). This was in agreement with other previous studies done in Argoba district, Northeast Ethiopia 12 , and Abergele district, Northern Ethiopia 15 . However, it disagreed with the national malaria parasite distribution pattern of Ethiopia, which showed that P. falciparum and P. vivax accounted for 60 and 40% of the malaria cases in the country, respectively 24 . This variation could be due to the fact that this study was limited to a small malaria-endemic setting in the country, which could have caused the species prevalence to vary. In addition, P. falciparum is a common species in the lowlands.
Malaria outbreaks are frequently complicated and multi-factorial, including both natural and man-made causes 25 . This case–control study verified the occurrence of a malaria outbreak in the Waghemra zone. Age, the availability of artificial water-holding bodies, nearby stagnant water, sleeping outside overnight, and a lack of knowledge about malaria transmission and prevention all contributed to the epidemic's existence. As a result, children under the age of five were nearly five times more likely than individuals over the age of 45 to contract malaria. This finding was congruent with research undertaken in the Bena Tsemay district of southern Ethiopia 18 . Malaria immunity develops slowly after multiple infections, and it takes at least five years for children to establish immunity 26 .
Furthermore, people who live near artificial water-holding bodies and stagnant water were more likely to be exposed to the malaria parasite than their counterparts. A similar conclusion was reached in research conducted in Simada district, Northwest Ethiopia, which found a link between staying near such water sources and contracting malaria 8 . Stagnant water created by heavy rains provides an ideal breeding environment for mosquitoes and contributes to malaria epidemics 8 , 16 . Similarly, people who stayed outside at night were approximately five times more likely to be infected with malaria than those who did not. This finding was supported by a report from the Ziquala, Armachiho, and Dembia districts of the Amhara region in Ethiopia 27 – 29 . This could be explained by the exophagic-exophilic biting behaviours of mosquitoes 30 . Moreover, a lack of knowledge regarding malaria transmission and control was a risk factor for disease development. Malaria education is crucial for minimizing exposure to the disease and its negative health consequences 8 , 31 , 32 .
During the investigation period, active case searching, treatment and management were carried out in accordance with national malaria treatment guidelines. Aside from that, environmental management activities such as filing, draining and clearing temporarily stagnant water were done with community involvement. At the time of data collection period, larvicide (abet chemical) was sprayed on Anopheles mosquito breeding sites. Moreover, the malaria surveillance team provided health education on disease transmission and prevention, and distributed over 3100 ITN to the community. However, due to a scarcity of chemicals, indoor residual spraying of houses in impacted kebeles is now being delayed. This outbreak scenario exemplified the critical role of long-term environmental and vector control intervention through well-organized malaria strategies and programs in preventing and controlling malaria infections. Malaria control and elimination require cross-sectoral collaboration as well as close monitoring and assessment of prevention and control initiatives.
Conclusion and recommendations
Following a year of internal conflict, a malaria outbreak was confirmed in Waghemra Zone. The predominant Plasmodium species identified was P. falciparum , and the outbreak was linked to being under five age, the existence of vector-breeding areas, people staying outdoors overnight, and a lack of knowledge about malaria transmission and control. The response to the outbreak included early diagnosis and treatment, environmental change, vector control, and awareness raising, which resulted in a reduction but not complete control of the outbreak. To prevent future malaria outbreaks in the study area, we recommended that the Waghemira Zone health office, Amhara regional health bureau, and other concerned sectors implement the following malaria prevention and control techniques: Those include raising community knowledge about malaria, mobilizing to disrupt mosquito breeding areas, scheduling indoor residual spraying activities, and monitoring malaria case trends on a weekly basis.
Ethical approval and consent to participate
Ethical clearance was obtained from the ethical review committee of College of Medicine and Health Sciences, Wollo University on the date 16/8/2022 with a protocol number of CMHS/201/2022. Permission was obtained from Waghemra Zone Health Office and each district health office where the study was conducted. This study was conducted in accordance with the Declaration of Helsinki. After briefly describing the significance of the study, the participants or children’s parents or guardians signed informed written consent. Confidentiality of the data was maintained. Finally, participants who were infected with the Plasmodium parasite received antimalarial treatment according to the national malaria treatment guidelines.
Acknowledgements
The authors thank the study participants, data collectors, Waghemra Zone Health Office. The authors would like to also thank district health offices, kebele leaders, health extension workers, health facility administrative and medical laboratory staffs for their support and unreserved cooperation in making this study to be a fruitful work.
Abbreviations
Author contributions.
Habtu Debash, Marye Nigatie, Habtye Bisetegn and Daniel Getacher Feleke conceived and designed the study, prepared the proposal, supervised data collection, analyzed, and interpreted the data. Habtu Debash, Gebru Tesfaw, Askale Amha, Megbaru Alemu, and Alemu Gedefie had participated in data collection, data analysis, and interpretation of the result, collecting scientific literature, critical appraisal of articles for inclusion, analysis, and interpretation of the findings. Habtu Debash drafted and prepared the manuscript for publication. Habtye Bisetegn, Marye Nigatie, Daniel Getacher Feleke and Alemu Gedefie critically reviewed the manuscript. All the authors have read and approved the final version of the manuscript and agreed to be accountable for all aspects of the work.
The research project was not funded by any organization.
Data availability
Competing interests.
The authors declare no competing interests.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
- Open access
- Published: 12 October 2022
Malaria outbreak facilitated by increased mosquito breeding sites near houses and cessation of indoor residual spraying, Kole district, Uganda, January-June 2019
- Maureen Nabatanzi 1 ,
- Vivian Ntono 1 ,
- John Kamulegeya 1 ,
- Benon Kwesiga 1 ,
- Lilian Bulage 1 ,
- Bernard Lubwama 2 ,
- Alex. R. Ario 1 &
- Julie Harris 3
BMC Public Health volume 22 , Article number: 1898 ( 2022 ) Cite this article
2150 Accesses
3 Citations
12 Altmetric
Metrics details
In June 2019, surveillance data from the Uganda’s District Health Information System revealed an outbreak of malaria in Kole District. Analysis revealed that cases had exceeded the outbreak threshold from January 2019. The Ministry of Health deployed our team to investigate the areas and people affected, identify risk factors for disease transmission, and recommend control and prevention measures.
We conducted an outbreak investigation involving a matched case-control study. We defined a confirmed case as a positive malaria test in a resident of Aboke, Akalo, Alito, and Bala sub-counties of Kole District January–June 2019. We identified cases by reviewing outpatient health records. Exposures were assessed in a 1:1 matched case-control study (n = 282) in Aboke sub-county. We selected cases systematically from 10 villages using probability proportionate to size and identified age- and village-matched controls. We conducted entomological and environmental assessments to identify mosquito breeding sites. We plotted epidemic curves and overlaid rainfall, and indoor residual spraying (IRS). Case-control exposures were combined into: breeding site near house, proximity to swamp and breeding site, and proximity to swamp; these were compared to no exposure in a logistic regression analysis.
Of 18,737 confirmed case-patients (AR = 68/1,000), Aboke sub-county residents (AR = 180/1,000), children < 5 years (AR = 94/1,000), and females (AR = 90/1,000) were most affected. Longitudinal analysis of surveillance data showed decline in cases after an IRS campaign in 2017 but an increase after IRS cessation in 2018–2019. Overlay of rainfall and case data showed two malaria upsurges during 2019, occurring 35–42 days after rainfall increases. Among 141 case-patients and 141 controls, the combination of having mosquito breeding sites near the house and proximity to swamps increased the odds of malaria 6-fold (OR = 6.6, 95% CI = 2.24–19.7) compared to no exposures. Among 84 abandoned containers found near case-patients’ and controls’ houses, 14 (17%) had mosquito larvae. Adult Anopheles mosquitoes, larvae, pupae, and pupal exuviae were identified near affected houses.
Stagnant water formed by increased rainfall likely provided increased breeding sites that drove this outbreak. Cessation of IRS preceded the malaria upsurges. We recommend re-introduction of IRS and removal of mosquito breeding sites in Kole District.
Peer Review reports
Malaria is transmitted to humans when they are bitten by infective female Anopheles mosquitoes with Plasmodium parasite sporozoites in the salivary glands. Malaria is endemic in Uganda; 90–95% of the country has stable malaria transmission [ 1 ]. Anopheles gambiae and Anopheles funestus species, which are endophagic and endophilic (bite and rest indoors), are the most common malaria vectors in Uganda [ 1 ]. Malaria transmission intensity partly depends on the vector density, which is in turn dependent on favorable temperatures and the presence of mosquito breeding sites. In Uganda, transmission is ongoing throughout the year, with two annual peaks that typically follow the two rainy seasons in March–May and August–October [ 2 ].
Uganda has reported multiple, geographically diverse malaria outbreaks over the last 20 years [ 3 , 4 , 5 , 6 ]. In 2017, nearly 20% of Ugandans suffered at least one episode of malaria, and malaria was responsible for 5% of all deaths in the country [ 7 ]. Despite a 52% decline in national malaria-related deaths between 2016/2017 and 2017/2018, malaria prevalence was 9% among children under 5 in 2018/2019 financial year [ 8 , 9 ]. Through the Uganda Malaria Reduction Strategic Plan (UMRSP) 2014–2020, the Ministry of Health (MoH) implemented activities to reduce annual malaria morbidity, mortality, and parasite prevalence. This involved case and fever management, referral, provision of essential diagnostics and antimalarials, behavioral change communication and technical support to affected districts. Long-lasting insecticide-treated nets (LLINs) were distributed continuously through antenatal and immunization clinics and nationally every three years, and indoor residual spraying (IRS) was conducted annually in selected districts to control vectors [ 10 ].
Kole District (altitude: 1,150 m above sea level) is located in Lango sub-region of northern Uganda and has a population of approximately 280,000 persons [ 11 ]. It has two seasonal rainfall peaks in March to May and September to November, with annual rainfall ranging from 875 mm to 1,500 mm. As of 2019, Kole District had 16 health facilities, including one Health Centre (HC) IV, five HC IIIs, six HC IIs and four clinics. All have capacity to test for and treat malaria.
Ten districts in Eastern and mid-Northern Uganda, including Kole, received IRS annually during 2009–2014, which contributed to reducing the malaria burden. However, during 2014–2016, IRS support shifted to other districts, leading to increases in malaria occurrence in the former 10 districts in Eastern and mid-Northern Uganda. As an intervention to address this resurgence, single round of IRS was conducted in 2017 [ 8 ]. Since that time, no additional IRS campaigns have been carried out in the area, and Kole, like other districts in Northern Uganda, continues to experience seasonal malaria outbreaks [ 3 ].
In June 2019, routine analysis of malaria surveillance data from Uganda’s District Health Information System (DHIS2) showed a malaria outbreak in Kole District. We plotted a malaria normal channel graph, a plot of weekly confirmed malaria cases in Kole District over the previous five years (2013–2018) analyzed into upper (75th percentile) and lower (25th percentile) epidemic thresholds of expected cases, and compared to 2019 cases [ 12 ]. Starting in January to June 2019, malaria cases exceeded the upper epidemic threshold. Further disaggregation of the data showed four sub-counties were the most highly affected. The MoH deployed a study team composed of national rapid response members, district and community health workers to respond to this outbreak. The team investigated to determine the extent, identify risk factors for increased transmission in Kole District, and to recommend control and prevention measures.
Outbreak area
We extracted malaria surveillance data for Kole District from the District Health Information System (DHIS2). We computed malaria cases by sub-county and drew malaria channel graphs to identify sub-counties with the highest burden of cases during the outbreak period. The four most-affected sub-counties: Aboke, Akalo, Alito, and Bala were selected for the investigation of the outbreak. In the sub-counties, we purposively selected and visited Aboke HC IV, Akole HC III, Apalabarowoo HC III, Bala HC III and Opeta HC III. During our investigation, the district health team informed us of antimalaria stockouts at lower-level public health facilities, which led to referral of cases to these five health facilities.
Case definition and finding
We defined a confirmed case as a positive malaria result by the histidine-rich protein II rapid diagnostic test (mRDT) or microscopy in a resident of the four most-affected sub-counties (Aboke, Akalo, Alito, and Bala sub-counties) from 1 January to 30 June 2019. We purposively reviewed outpatient health records in five higher-level health facilities (1 HC IV and 4 HC IIIs) to search for confirmed malaria cases in these sub-counties. This purposive selection was based on information by the district health team that antimalaria stockouts at lower-level public health facilities had led to referral of cases to these five. Using the out-patient records, we line-listed all case-patients who fit the case definition. For each case-patient, we abstracted information on confirmatory diagnostic test done, age, sex, village, parish, and sub-county of residence.
Descriptive epidemiology
Using the line list, we described case-patients by person, place, and time. We defined attack rate as the number of malaria cases during January to June 2019 divided by the population at risk. Populations at risk used were extracted from the 2019 Uganda National Population Census projections for Kole District [ 11 ]. Consequently, we computed attack rates by age-group, sex, sub-county, parish and village; groups with the highest attack rates were classified as the most affected. We drew a map of the district indicating affected sub-counties. Rainfall data for Kole District for January to June 2019 were abstracted from the online weather resource AccuWeather Inc. [ 13 ]. An epidemic curve was drawn to describe the distribution of malaria cases in the district during January to June 2019 and. rainfall data superimposed over the curve. Another epidemic curve of malaria cases in Kole District from 2016 to 2019 was drawn with data on IRS obtained from MoH records [ 8 ] superimposed over the cases. Using surveillance data from the DHIS2, we plotted a graph showing trends in confirmed malaria cases in Kole and included IRS interventions in the district from 2016 to 2019.
Environmental assessment
In Aboke sub-county, we selected Ogwangacuma Parish which had the highest attack rate (345 per 1,000) and in turn selected Aweingwec Village that reported the highest number of malaria cases (n = 2,392) during January – June 2019. In Aweingwec Village, we conducted transect walks by systematically walking with community health workers to explore the environment for stagnant water, swamps and potential risk factors for mosquito breeding and malaria transmission. We identified active and potential breeding sites for mosquitoes near houses and the environment.
Entomological assessment
In Aboke sub-county, we selected Akwirididi parish, one of the two most affected parishes, to conduct entomological assessments. In 2019, Akwirididi had 28 villages and 2,748 households, from which we selected a random sample of 20 houses to assess the mosquito density. In each house, we used the pyrethrum spray catch method to collect indoor resting mosquitoes by spraying pyrethrum insecticide inside the house and collecting mosquitoes that were knocked down on a white sheet laid on the ground. We conducted daily pyrethrum spray catches from 6 to 10 am during the 13–15 July 2019. The dead mosquitoes were collected using forceps, packed in petri dishes, and transported to the laboratory for counting and identification [ 14 ]. The mosquito indoor resting density (IRD) was computed using the formula:
\({\text{IRD}}=\frac{{\left( {{\text{no}}{\text{.}}\;of\;{\text{mosquitoes collected indoors}} \div {\text{no}}{\text{.of houses}}} \right)}}{{{\text{number of mornings}}}}\)
At breeding sites around the sampled houses, scoops were used to collect larvae, pupae, and pupal exuviae; strainers and filter cloths were used to remove excess water. Residual material was then transported to the laboratory for counting and identification.
Hypothesis generation interviews
In Aboke, the most affected sub-county, we purposively selected Ogwangacuma parish because it had the highest attack rate. In this parish, we conveniently sampled 20 case-patients. The community health workers on our team introduced the purpose of the investigation and supported translation from the local language when necessary. Case-patients were interviewed about possible behavioral and environmental exposures associated with malaria transmission; we also observed their environment for potential risk factors. The exposure variables explored included living close to swampy areas, human activities in and around swamps, presence of stagnant water near houses following rainfall (present during our visits), and LLIN use during the two weeks before symptom onset.
Case-control study
We conducted a case-control study to test the generated hypotheses in two parishes of Aboke sub-county. The parishes of Ogwangacuma and Akwirididi were selected because of their high attack rates. From these two parishes, we further selected the ten most affected villages. The number of cases and controls selected from each affected village was estimated using the probability proportionate to size sampling method where each village contributed persons proportional to the village’s attack rate [ 3 ].
We defined a case-patient as a resident of Ogwangacuma or Akwirididi Parish in Aboke sub-county with evidence of a positive malaria RDT in the previous four weeks (8 June to 8 July 2019). For each case-patient, evidence of malaria RDT was abstracted from health facility out-patient records. We defined a control as a resident of Ogwangacuma or Akwirididi Parish with no signs or symptoms of malaria and no positive test for malaria in the same previous four weeks. Cases and controls were matched by village of residence and age (within 5 years). We used a case to control ratio of 1:1, selecting 141 cases and 141 controls (n = 282).
We used systematic sampling to select cases and controls. A list of all houses per village was obtained from the respective local council leaders and used as the sampling frame from which we calculated the sampling interval. All houses in the sampling frame were assigned a number and OpenEpi™ was used to generate one random number which served as the starting point for selecting the first house from which to select a case-patient. After this, we used the sampling interval to select the remaining cases. The remaining houses were assigned numbers and random numbers generated in OpenEpi™ and used to select matching controls. If the house had a case-patient or didn’t have an age-matched person, it was replaced by a neighboring house. We administered a questionnaire to each case-patient with questions on demographics and exposure to malaria risk factors during the two weeks before symptom onset. The same questionnaire was administered to controls to assess exposure to malaria risk factors during the two weeks before their matched case-patient’s symptom onset. For case-patients or controls who were minors, the questionnaire was administered to guardians. At selected houses, we looked out for abandoned containers with stagnant water and visible mosquito larvae. Any vessel found in the open around the house but no longer in use that could store an amount of water to allow mosquitoes to lay their eggs was considered as an abandoned container.
Data management and analysis
Data were first entered, cleaned in Microsoft Excel before being imported into Epi Info 7.2 to generate descriptive statistics. In Epi Info, we analyzed the case-control data by creating the following combined exposure categories: [ 1 ] Breeding site near house (a combination of either abandoned containers or stagnant water near house), [ 2 ] Proximity to swamp (a combination of either house < 500 m of swamp exposures or farm < 500 m of swamp exposures), [ 3 ] Combination of breeding site near house and proximity to swamp, and [ 4 ] Common reference category (no breeding site near house and no proximity to swamp). This enabled us to compare the individual effect of each of the combined exposures (categories 1 and 2), and the joint effect of all the exposures (category 3) to a common reference of no exposures (category 4). Using logistic regression analysis, we computed odds ratios (OR) and their 95% confidence intervals.
We line-listed 18,737 confirmed case-patients in the four most affected sub-counties of Kole District (Aboke, Akalo, Alito, and Bala). The overall attack rate (AR) was 68/1,000 with no deaths. The median age was 12 years (range: <1 to 98 years). Children under 5 years were the most affected (AR = 94/1,000) followed by children aged 5–18 years (71/1,000). Females (AR = 90/1,000) were more affected than the males (AR = 45/1,000) (Table 1 ).
Of the four sub-counties visited, Aboke had a higher attack rate (AR = 180/1,000) in comparison to Alito, Akalo and Bala (Fig. 1 ).
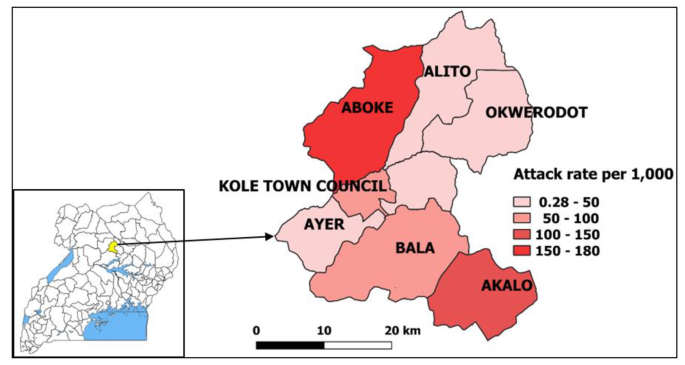
Map of affected sub-counties during a malaria outbreak in Kole District, Northern Uganda, January-June 2019. Inset: location of Kole District in Uganda. ( Note. Results are presented for 7 sub-counties instead of the 4 visited due to the referrals from health facilities located in other sub-counties during the period considered for the investigation. The outpatient department register collects data on village, parish, and sub-county of residence of the case-patients.)
The epidemic curve showed peaks in malaria cases on 9 April and 21 May 2019. The peaks in malaria cases followed increases in rainfall by 35-42-day intervals. We also observed peak-to-peak increases; May’s peak was the highest following the second increase in rainfall (Fig. 2 ).
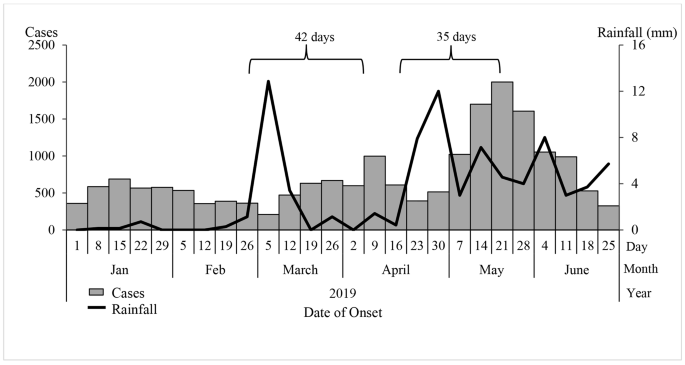
Weekly confirmed cases (red bars) and weekly rainfall (blue line) during a malaria outbreak in Kole District, Uganda, January-June 2019
A graph of confirmed malaria cases in Kole District from 2016 to 2019 showed annual seasonal peaks in malaria cases during May-July and October-November (Fig. 3 ). During May and June 2017, Kole District conducted a mass indoor residual spraying (IRS) campaign, which appeared to reduce cases over the following year. Monthly cases in 2019 were high in comparison to 2016, 2017 and 2018.
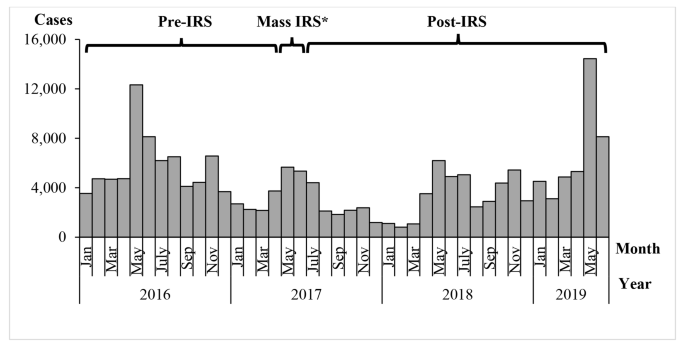
Monthly confirmed malaria cases and timing of mass indoor residual spraying in Kole District, Uganda, 2016–2019. Note*: In addition to the mass IRS, an LLIN distribution campaign was conducted
Entomological assessment findings
Around the 20 houses we visited, we identified any stagnant water containing areas or containers with mosquito larvae as sites for breeding. From these 20 houses, 262 adult Anopheles mosquitoes were identified during knockdown. Of the 262 adult mosquitoes, 204 (78%) were female, of whom 140 (69%) were Anopheles gambiae and 64 (31%) were Anopheles funestus. Among these, 171 (84%) were freshly fed. The average indoor resting density of malaria vectors was 4 mosquitoes per house per night. In stagnant water near the 20 houses, we identified an average of 10 Anopheles larvae, four Anopheles pupae, and multiple Anopheles exuviae per 500ml scoop; these were of gambiae and fenustus species.
Environmental assessment findings
In Aboke sub-county, the main economic activity was subsistence farming. On rice farms in swampy areas, we identified stagnant water with visible Anopheles mosquito larvae. We also identified man-made ponds being used for fish farming. These were surrounded by ditches which had filled with rainwater that had stagnated; we found Anopheles mosquito larvae in the ditches.
Hypothesis generation findings
Among the 20 case-patients interviewed, 17 (85%) lived within 500 m of a swamp, 15 (75%) farmed within 500 m of a swamp and 11 (55%) had stagnant water near their house. Based on the descriptive epidemiology, environmental and entomological assessments, and interview findings, the study team hypothesized that stagnation of rain water in swampy areas, ditches, and around houses favored mosquito breeding.
Case-control study findings
Among 141 case-patients and 141 controls, having breeding sites near the house either as abandoned containers or as stagnant water (OR = 1.09, 95% CI = 0.24–5.02) was not associated with malaria infection. Proximity to swamps either as farm or house less than 500 m to the swamp (OR = 1.05, 95% CI = 0.45–2.4) was also not associated with malaria infection. Further analysis of the risky exposures in combination revealed a possible combined effect. The combination of having breeding sites near house and proximity to swamps increased the odds of malaria 6-fold (OR = 6.6, 95% CI = 2.24–19.7) (Table 2 ). We identified a total of 84 abandoned containers near participants’ houses, 14 (17%) of which had visible mosquito larvae. Examples of abandoned containers identified included old jerry cans, saucepans and basins.
Of the 282 study participants, 227 (80%) reported using an LLIN the previous night that is, 80% (113/141) of case-patients compared to 81% (114/141) controls.
There was in increase in malaria cases in Kole District in 2019. While IRS in 2017 appeared to reduce the malaria levels in 2017 and early 2018, its effect appeared to have worn off by 2019. Peaks in malaria cases followed rains in 2019. Persons living in Aboke sub-county, children under five years, and women were the more affected by this outbreak in comparison to other groups. There were many freshly-fed adult female mosquitoes in houses in the affected area, implying that residents were being actively bitten even during our investigation period, which occurred after the peak of cases. Risky exposures associated with malaria included having abandoned containers and stagnant water near work or house.
In Uganda, the main malaria control measures are IRS, distribution of LLINs, accurate diagnosis and prompt treatment, and intermittent preventive treatment of pregnant women [ 10 ]. In 2017, the MoH conducted a routine LLIN distribution that achieved 88% national coverage [ 8 ], which, complemented by the mass IRS, should have been sufficient to have a protective effect. However, high LLIN coverage rates don’t always reflect use; the 2018/2019 Uganda Malaria Indicator Survey reported national net use of 59% [ 9 ]. Although reported net use in our study was 80%,in areas with favorable vector and rainfall conditions, regular LLIN use should be combined with other interventions such as IRS to reduce the mosquito population sufficiently to impact malaria infection rates [ 4 ]. However, the expense of IRS often precludes its regular application or universal coverage.
We noted increases in malaria peaks approximately 5–6 weeks after rainfall peaks. This is a well-described phenomenon in the malaria literature and has been reported previously [ 3 , 4 ]. This first increase in rainfall, during early March of 2019 could have facilitated an increase in mosquito breeding sites. Successive peaks in rainfall could have favored three mosquito breeding cycles of two weeks each, resulting in a generational increase in mosquito density[ 15 ]. However, rainfall itself is not enough to guarantee mosquito breeding. Opportunities for breeding sites exist when there is stagnant water and flooding near places where people live, work or rest [ 3 , 4 , 16 ]. Farming in swamps can also modify the water temperature, resulting in favorable conditions for mosquito breeding [ 17 ]. We found pools of water in open abandoned containers surrounding houses, as well as stagnant water near houses resulting from flooding which served as sites for mosquitoes to breed. In our study, breeding sites near houses, and having farms or houses close to swamps increased odds of malaria infection. This combined effect of exposures emphasizes the need for multiple environmental and behavioral interventions to reduce risk of malaria exposure. Malaria prevention messages to the public in this area should emphasize responsible land use practices to reduce the creation of mosquito breeding habitats in the environment [ 18 , 19 ].
We identified children under five years of age as the most affected by malaria. This disproportionate burden has been widely reported both in Uganda and globally [ 4 , 8 , 16 , 20 ]. In addition, females were twice as affected as males., a finding reported previously in multiple districts of Uganda [ 21 ]. Females in Kole District might engage in activities that increase their exposure to mosquitoes. During our study, we observed that cooking areas were outside the house, meaning that women would likely be exposed in the evenings while preparing meals. It should be noted that in comparison to males, females are also more likely to report fevers to health facilities and have more opportunities to be tested for malaria during child health care or antenatal visits [ 21 ]. However, pregnancy may also increase susceptibility [ 22 ]. In Uganda, prevalence of malaria during pregnancy was 30% in 2017, increasing the risk of maternal anemia and low birth weight babies [ 10 ]. Malaria control initiatives in this area – and likely other high-transmission areas in Uganda – should increase their targeting of pregnant women and children under five years.
Beyond the morbidity and mortality, malaria infections have negative socioeconomic implications, including treatment expenditures, lost work and school days, decreased productivity, and sometimes the loss of a household breadwinner [ 23 ]. The 2014–2020 Uganda Malaria Reduction Strategic Plan aimed to accelerate nationwide scale up of cost-effective malaria prevention and treatment interventions [ 24 ]. When scaled up, the combination of IRS, distribution of LLINs, and test-and-treat interventions contributed to a 27% reduction in the national incidence of malaria between 2017 and 2018 [ 8 ]. Ugandan researchers estimated that using a district-led approach for IRS, the overall cost per structure sprayed is UGX 28,400 (8 US$) and the average cost per person protected is UGX 7,200 (2 US$) [ 25 ]. However, costs are increased by additional measures, such as environmental compliance; a previous recent IRS in Uganda cost approximately USD 12 million to cover just 10 districts (unpublished data). In contrast, the cost of treating malaria is estimated to be between UGX 1,500 (0.41 US$) and UGX 13,800 (3.88 US$) per person per month [ 24 ]. Thus, while consistent IRS, removal of vector breeding sites, and consistent distribution and use of LLINs in the affected areas are effective, they may not be economically feasible. Community leaders can be encouraged to conduct education campaigns that raise awareness and encourage the use of LLINs and removal of stagnant water to address risk where IRS is not economically feasible.
Limitations
During the search for cases, we did not review health records from the integrated community case management of malaria (iCCM) for children under five years in Kole District. This might have led to an underestimation of the magnitude of the outbreak among children under five years. In addition, most persons visit Health Center II (lower-level health facilities) first for malaria treatment. However, we did not visit these facilities to search for cases due to reported antimalarial stockouts during the outbreak period; instead, we visited the five health facilities where suspected cases from the lower health facilities were referred. Finally, given the high attack rates we reported in our investigation, it is possible that controls were infected but asymptomatic at the time of interview. This could have introduced a misclassification bias and underestimated our associations.
Stagnant water near houses likely facilitated this outbreak through increases in mosquito breeding sites following rains. Inadequate preventive measures such as absence of IRS likely facilitated vector-human contact to enhance the outbreak. Re-introduction of IRS, re-distribution of LLIN in Kole District, and sensitizing communities about removing mosquito breeding sites might reduce the risk of future outbreaks.
Public health actions
We sensitized the community members and leaders on malaria and easy preventive actions like removing abandoned containers around their houses that act as mosquito breeding sites and consistent use of LLIN.
Data Availability
The datasets upon which our findings are based belong to the Uganda Public Health Fellowship Program. For confidentiality reasons the datasets are not publicly available. However, the data sets can be availed upon reasonable request from the corresponding author and with permission from the Uganda Public Health Fellowship Program.
Abbreviations
Attack rate.
Confidence interval.
District Health Information Software 2.
Health Centre.
integrated community case management of malaria.
Indoor residual spraying.
Long-lasting insecticide-treated bed nets.
Uganda Ministry of Health.
National Malaria Control Division.
Odds ratio.
Rapid diagnostic test.
Uganda Bureau of Statistics.
World Health Organization.
Statistics UBo, Hospital M, Macro I, Programme NMC. Uganda Malaria Indicator Survey, 2009: Uganda Bureau of Statistics; 2010.
Kamya MR, Arinaitwe E, Wanzira H, Katureebe A, Barusya C, Kigozi SP, et al. Malaria transmission, infection, and disease at three sites with varied transmission intensity in Uganda: implications for malaria control. Am J Trop Med Hyg. 2015;92(5):903–12.
Article Google Scholar
Nsereko G, Kadobera D, Okethwangu D, Nguna J, Rutazaana D, Kyabayinze DJ, et al. Malaria Outbreak Facilitated by Appearance of Vector-Breeding Sites after Heavy Rainfall and Inadequate Preventive Measures: Nwoya District, Northern Uganda, February-May 2018. J Environ public health. 2020;2020:5802401.
Simple O, Mindra A, Obai G, Ovuga E, Odongo-Aginya EI. Influence of Climatic Factors on Malaria Epidemic in Gulu District, Northern Uganda: A 10-Year Retrospective Study. Malar Res Treat. 2018;2018:8.
Google Scholar
Yeka A, Gasasira A, Mpimbaza A, Achan J, Nankabirwa J, Nsobya S, et al. Malaria in Uganda: challenges to control on the long road to elimination: I. Epidemiology and current control efforts. Acta Trop. 2012;121(3):184–95.
Ogwang R, Akena G, Yeka A, Osier F, Idro R. The 2015–2016 malaria epidemic in Northern Uganda; What are the implications for malaria control interventions? Acta Trop. 2018;188:27–33.
Today ON. Uganda reports malaria upsurge: 40% increase: Outbreak News Today; 2019 [updated 18/08/2019. Available from: http://outbreaknewstoday.com/uganda-reports-malaria-upsurge-40-percent-increase-31674/ .
MoH. National Malaria Annual Report 2017–2018. Kampala, Uganda: National Malaria Control Division, Surveillance Monitoring & Evaluation Unit, Division NMC; 2019.
NMCD U, and ICF. 2018-19 Uganda Malaria Indicator Survey (UMIS): Atlas of Key Indicators. Kampala, Uganda and Rockville, Maryland, USA, (NMCD) UNMCD; 2019.
USAID. Presidential Malaria Initiative Uganda. Operational Plan M FY 2019. In: Services USDoHaH, editor. U.S.A2019.
Statistics UBo. Population and Censuses Kampala, Uganda: UBOS 2019 [Available from: https://www.ubos.org/publications/statistical/20/ .
WHO. Malaria surveillance, monitoring & evaluation: a reference manual. Switzerland: Geneva; 2018.
AccuWeather. Uganda Weather 2019 [Available from: https://www.accuweather.com/en/ug/alito/1042300/may-weather/1042300 .
WHO. Manual on Practical Entomology in Malaria: Part II Methods and Techniques. Geneva: Diseases WDoMaoP; 1975.
Organization WH. Malaria control in complex emergencies: an inter-agency field handbook. World Health Organization; 2005.
Hajison PL, Feresu SA, Mwakikunga BW. Malaria in children under-five: A comparison of risk factors in lakeshore and highland areas, Zomba district, Malawi. PLoS ONE. 2018;13(11):e0207207.
Lindblade KA, Walker ED, Onapa AW, Katungu J, Wilson ML. Land use change alters malaria transmission parameters by modifying temperature in a highland area of Uganda. Tropical Med Int Health. 2000;5(4):263–74.
Article CAS Google Scholar
Paul P, Kangalawe RYM, Mboera LEG. Land-use patterns and their implication on malaria transmission in Kilosa District, Tanzania. Tropical Diseases, Travel Medicine and Vaccines. 2018;4(1):6.
Vanwambeke SO, Lambin EF, Eichhorn MP, Flasse SP, Harbach RE, Oskam L, et al. Impact of Land-use Change on Dengue and Malaria in Northern Thailand. EcoHealth. 2007;4(1):37–51.
WHO. World malaria report 2018. Switzerland: Geneva; 2018.
Okiring J, Epstein A, Namuganga JF, Kamya EV, Nabende I, Nassali M, et al. Gender difference in the incidence of malaria diagnosed at public health facilities in Uganda. Malar J. 2022;21(1):22.
Organization WH. Gender, health and malaria. 20, Avenue Appia, Geneva 27; 2007.
MoH. National Malaria Control Program Kampala. Uganda2019 [Available from: https://www.health.go.ug/programs/national-malaria-control-program/ .
Ministry of Health U. THE UGANDA MALARIA REDUCTION STRATEGIC. PLAN 2014–2020. In: Program NMC, editor. Kampala, Uganda2014.
Tonny Odokonyero GA, Kirigwajjo M. Freddie Ssengooba. Financing Indoor Residual Spraying in Uganda: Cost-cutting options. Economic Ploicy Research Centre; 2020.
Download references
Acknowledgements
We would like to appreciate the Uganda Public Health Fellowship Program for the technical support provided during the preparation and conducting the investigation. We appreciate the Uganda Ministry of Health- National Malaria Control Division and Kole District health team for supporting the outbreak response efforts.
This project was supported by the President’s Emergency Plan for AIDS Relief (PEPFAR) through the US Centers for Disease Control and Prevention Cooperative Agreement number GH001353–01 through Makerere University School of Public Health to the Uganda Public Health Fellowship Program, MoH. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the US Centers for Disease Control and Prevention, the Department of Health and Human Services, Makerere University School of Public Health, or the MoH. The staff of the funding body provided technical guidance in the design of the study, ethical clearance and collection, analysis, and interpretation of data and in writing the manuscript.
Author information
Authors and affiliations.
Uganda Public Health Fellowship Program, Ministry of Health, Kampala, Uganda
Maureen Nabatanzi, Vivian Ntono, John Kamulegeya, Benon Kwesiga, Lilian Bulage & Alex. R. Ario
Integrated Epidemiology, Surveillance and Public Health Emergencies Department, Ministry of Health, Kampala, Uganda
Bernard Lubwama
US Centers for Disease Control and Prevention, Kampala, Uganda
Julie Harris
You can also search for this author in PubMed Google Scholar
Contributions
Conceived and designed the study, acquired, analyzed, interpreted the data and wrote the first draft of the manuscript: MN, VN and JK. Contributed substantially to analysis and interpretation of data: BL. Critically reviewed the paper for important intellectual content: LB, BK, ARA and JH. All authors read and approved the manuscript.
Corresponding author
Correspondence to Maureen Nabatanzi .
Ethics declarations
Ethics approval and consent to participate.
This investigation was in response to a public health emergency and was therefore determined to be non-research. The MoH gave the directive and approval to investigate this outbreak. The Office of the Associate Director for Science, Centers for Global Health, CDC, determined that this activity was not human subject research, and its primary intent was public health practice or a disease control activity (specifically, epidemic or endemic disease control activity). All methods were carried out in accordance with relevant guidelines for outbreak investigations approved by the MoH. Verbal informed consent in the local language was sought from respondents or care-takers of diseased children (participants under 18). They were informed that their participation was voluntary and their refusal would not result in any negative consequences. To protect the confidentiality of the respondents, each was assigned a unique identifier which was used instead of their names.
Competing interests
The authors declare that they had no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Nabatanzi, M., Ntono, V., Kamulegeya, J. et al. Malaria outbreak facilitated by increased mosquito breeding sites near houses and cessation of indoor residual spraying, Kole district, Uganda, January-June 2019. BMC Public Health 22 , 1898 (2022). https://doi.org/10.1186/s12889-022-14245-y
Download citation
Received : 29 April 2022
Accepted : 21 September 2022
Published : 12 October 2022
DOI : https://doi.org/10.1186/s12889-022-14245-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Stagnant water
BMC Public Health
ISSN: 1471-2458
- Submission enquiries: [email protected]
- General enquiries: [email protected]
Clinical Case Study 1: Fever 6 months after a visit to Pakistan
A 44-year-old man is seen at a physician’s office in the United States, during a week-end, for suspected malaria.
The patient was born in Pakistan but has lived in the United States for the past 12 years. He travels frequently back to Pakistan to visit friends and relatives. His last visit there was for two months, returning 11 months before the current episode. He did not take malaria prophylaxis then.
Five weeks ago, he was diagnosed with malaria and treated at a local hospital. The blood smear at that time was reported by the hospital as positive for malaria, species undetermined. He was then treated with 2 days of IV fluids (nature unknown) and tablets (nature unknown), and recovered.
The patient now presents with a history of low grade fever for the past few days, with no other symptoms. A blood smear is taken and examined at a hospital laboratory by the technician (no pathologist is available on this week-end). Through a telephone discussion, the technician states that she sees 4 parasites per 1000 red blood cells, with rings, “other forms with up to four nuclei,” and that some of the infected red blood cells are enlarged and deformed.
Question 1: What is your most probable diagnosis?
Not Malaria
That is incorrect. Please, try another answer.
Plasmodium falciparum
Plasmodium vivax
That is correct.
This is the most probable diagnosis. The reported microscopic findings are compatible with P. vivax: some infected red cells are enlarged and deformed, and the “other forms with four nuclei” are compatible with the presence of schizonts. Plasmodium vivax does occur in Pakistan, where it is found in slightly more than 50% of malaria cases.
The history suggests a relapse of P. vivax malaria, following an earlier episode five weeks ago. The earlier treatment apparently did not include primaquine, thus allowing the persistence of hypnozoites which caused this relapse.
An alternate explanation would be that the earlier infection was caused by chloroquine-resistant P. vivax (which has been reported in Pakistan), with recrudescence of blood-stage parasites occurring after an unsuccessful earlier treatment (if indeed the earlier treatment included chloroquine). However, recrudescences usually occur within 28 days of the intial episode, rather than at five weeks as described here.
The other species are less likely:
- While P. falciparum does occur in Pakistan (slightly less than 50% of malaria cases), this patient reportedly did not develop symptoms until 10 months after departure from the exposure area: most cases of P. falciparum would have become symptomatic earlier.
- P. ovale occurs mainly in Africa and has been found only occasionally in Asia (in the western Pacific).
- P. malariae occurs worldwide, but its distribution is spotty, and its frequency in Pakistan is low to negligible.
- Babesia would not fit with the microscopic description; in addition, babesiosis has not been reported in Pakistan, although admittedly the disease might have escaped detection.
Plasmodium ovale
Plasmodium malariae
Question 2: What treatment approach would you recommend, based on this clinical history and on the fact that the microscopy findings will not be confirmed by a pathologist for at least 24 hours?
Do not start treatment until a formal microscopic diagnosis is made (in 12-24 hours)
Treat as if chloroquine-sensitive Plasmodium falciparum malaria
A reasonable option, signifying that in the absence of definitive microscopic diagnosis, you prefer to play it safe and treat the patient for the most dangerous and rapidly progressing infection possible.
The safest course of action is to initially admit all cases of proven or suspected P. falciparum to the hospital until one can begin treatment and ensure that they are improving clinically and parasitologically.
However in this case, if the patient is only minimally symptomatic, one might elect against hospitalization and instead treat as an outpatient provided that close follow-up can be arranged. Once the definitive microscopic diagnosis is made the following day, you can always switch treatment.
Treat as if chloroquine-resistant Plasmodium falciparum malaria
Treat as if Plasmodium vivax malaria
P. Vivax schizont
The diagnosis of P. vivax malaria is later confirmed by review of a blood smear available from the first episode (Figure), and by a PCR positive for P. vivax on blood collected during the current episode.
The microscopic diagnosis of P. vivax is based on the following:
- The infected red cells are enlarged and deformed;
- The schizont shown contains 20 merozoites (schizonts of P. malariae and P. ovale have fewer merozoites; and in P. falciparum , schizonts are not usually seen in the peripheral blood);
- The round gametocyte shown, contained in an enlarged red cell. (In this case, the typical Schüffner’s dots were not visible, probably due to staining problems.)
Question 3. To prevent further relapses from dormant liver stages, what would you recommend?
No further measures needed
A lab test to determine if the patient has dormant liver stages
Treatment immediately with a drug that kills dormant liver stages
A lab test, followed by treatment with a drug that kills dormant liver stages
You should exclude G6PD deficiency first, then give the patient primaquine, 30 mg per day for 14 days.
In case of G6PD deficiency, consultation with an expert in infectious diseases or tropical medicine is advised to discuss options for relapse prevention. For some patients with partial G6PD deficiency, an alternative regimen of primaquine 45 mg weekly for 8 weeks can sometime be used. Alternatively, weekly chloroquine prophylaxis may also be considered. Treatment with primaquine is justified because this patient probably has already had a relapse, and is at risk for further relapses. No test exists to detect the presence of liver stage parasites.
Question 4. Should this patient have taken preventive measures against malaria for his visit to Pakistan, considering that he was born there?
Even to visit friends and relatives, preventive measures must be taken. Chloroquine-resistant Plasmodium falciparum occurs in Pakistan, and thus the drugs recommended would be atovaquone-proguanil (Malarone®), doxycycline or mefloquine. Other preventive measures against mosquito bites also apply. Even though the patient was born in Pakistan, whatever acquired immunity he has developed would most likely have waned; negligence of preventive measures often occurs in individuals visiting friends and relatives , a situation that needs to be remedied.
Main Points
Travelers to Pakistan (including those visiting friends and relatives) need to take prophylaxis (atovaquone-proguanil [Malarone®], doxycycline or mefloquine).
Clinical history and travel history, and careful microscopic examination, probably would have directed the diagnosis toward P. vivax during the earlier episode, so that the relapse could have been prevented.
P. vivax malaria should be treated with chloroquine, except when acquired in Papua New Guinea and Indonesia, areas with high prevalence of chloroquine-resistant P. vivax . After a normal G6PD test, patients should get a radical cure with primaquine (30 mg per day for 14 days).
To receive email updates about this page, enter your email address:
New! Locally Acquired Cases of Malaria in Florida, Texas, Maryland, and Arkansas
New! Update to Guidance for use of Artemether-Lumefantrine (Coartem®) in Pregnancy for Uncomplicated Malaria New! Discontinuation of CDC’s Distribution of Intravenous Artesunate as Commercial Drug Guidance for Malaria Diagnosis in Patients Suspected of Ebola Infection in the United States -->
See all Malaria Notices
- New! Malaria is a Serious Disease
- New! La malaria (paludismo) es una enfermedad grave
- How to Report a Case of Malaria
- CDC Yellow Book
- Red Pages: Malaria-endemic areas by country
- Drugs for Prevention
- Choosing a Drug to Prevent Malaria
- Drugs for Treatment in the U.S.
- Frequently Asked Questions (FAQs)
- Blood Banks
Click here for contact information
Exit Notification / Disclaimer Policy
- The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website.
- Linking to a non-federal website does not constitute an endorsement by CDC or any of its employees of the sponsors or the information and products presented on the website.
- You will be subject to the destination website's privacy policy when you follow the link.
- CDC is not responsible for Section 508 compliance (accessibility) on other federal or private website.
We've detected unusual activity from your computer network
To continue, please click the box below to let us know you're not a robot.
Why did this happen?
Please make sure your browser supports JavaScript and cookies and that you are not blocking them from loading. For more information you can review our Terms of Service and Cookie Policy .
For inquiries related to this message please contact our support team and provide the reference ID below.
Impact of aerosol concentration changes on carbon sequestration potential of rice in a temperate monsoon climate zone during the COVID-19: a case study on the Sanjiang Plain, China
- Research Article
- Published: 06 April 2024
Cite this article
- Xiaokang Zuo 1 &
- Hanxi Wang ORCID: orcid.org/0000-0003-4130-6981 1 , 2
The emission reduction of atmospheric pollutants during the COVID-19 caused the change in aerosol concentration. However, there is a lack of research on the impact of changes in aerosol concentration on carbon sequestration potential. To reveal the impact mechanism of aerosols on rice carbon sequestration, the spatial differentiation characteristics of aerosol optical depth (AOD), gross primary productivity (GPP), net primary productivity (NPP), leaf area index (LAI), fraction of absorbed photosynthetically active radiation (FPAR), and meteorological factors were compared in the Sanjiang Plain. Pearson correlation analysis and geographic detector were used to analyze the main driving factors affecting the spatial heterogeneity of GPP and NPP. The study showed that the spatial distribution pattern of AOD in the rice-growing area during the epidemic was gradually decreasing from northeast to southwest with an overall decrease of 29.76%. Under the synergistic effect of multiple driving factors, both GPP and NPP increased by more than 5.0%, and the carbon sequestration capacity was improved. LAI and FPAR were the main driving factors for the spatial differentiation of rice GPP and NPP during the epidemic, followed by potential evapotranspiration and AOD. All interaction detection results showed a double-factor enhancement, which indicated that the effects of atmospheric environmental changes on rice primary productivity were the synergistic effect result of multiple factors, and AOD was the key factor that indirectly affected rice primary productivity. The synergistic effects between aerosol-radiation-meteorological factor-rice primary productivity in a typical temperate monsoon climate zone suitable for rice growth were studied, and the effects of changes in aerosol concentration on carbon sequestration potential were analyzed. The study can provide important references for the assessment of carbon sequestration potential in this climate zone.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Data availability
Data used in this research are available upon request from the corresponding author.
Breiman L (2001) Random forests. Mach Learn 45(1):5–32. https://doi.org/10.1023/a:1010933404324
Article Google Scholar
Cao S, Sanchez-Azofeifa GA, Duran SM, Calvo-Rodriguez S (2016) Estimation of aboveground net primary productivity in secondary tropical dry forests using the Carnegie–Ames–Stanford approach (CASA) model. Environ Res Lett 11(7):075004. https://doi.org/10.1029/2007JG000603
Article CAS Google Scholar
Chandra MA, Bedi SS (2021) Survey on SVM and their application in image classification. Int J Inform Technol 13:1–11. https://doi.org/10.1007/s41870-017-0080-1
Chang Q, Xiao X, Jiao W, Wu X, Doughty R, Wang J, Qin Y (2019) Assessing consistency of spring phenology of snow-covered forests as estimated by vegetation indices, gross primary production, and solar-induced chlorophyll fluorescence. Agr Forest Meteorol 275:305–316. https://doi.org/10.1016/j.agrformet.2019.06.002
Chen J, He L, Wen Z, Liao H, Wang B, Cui L, Li G (2017) Carbon sequestration potential of reed swamp wetland vegetation in the estuary of the Liaohe Delta. J Ecol 37(16):5402–5410. https://doi.org/10.5846/stxb201605241004
Cheng Y, Yu Q, Liu J, Cao X, Zhong Y, Du Z, Liang L, Geng G, Ma W, Qi H, Zhang Q, He K (2021) Dramatic changes in Harbin aerosol during 2018–2020: the roles of open burning policy and secondary aerosol formation. Atmo Chem Phys 21(19):15199–15211. https://doi.org/10.5194/acp-21-15199-2021
Chipman HA, George EI, McCulloch RE (1998) Bayesian CART model search. J Am Stat Assoc 93(443):935–948. https://doi.org/10.1080/01621459.1998.10473750
Duan J, Ju T, Wang Q, Li F, Fan J, Huang R, Liang Z, Zhang G, Geng T (2021) Absorbable aerosols based on OMI data: a case study in three provinces of Northeast China. Environ Monit Assess 193:479. https://doi.org/10.1007/s10661-021-09249-x
Ezhova E, Ylivinkka I, Kuusk J, Komsaare K, Vana M, Krasnova A, Noe S, Arshinov M, Belan B, Park S, Lavrič JV, Heimann M, Petäjä T, Vesala T, Mammarella I, Kolari P, Bäck J, Rannik Ü, Kerminen V, Kulmala M (2018) Direct effect of aerosols on solar radiation and gross primary production in boreal and hemiboreal forests. Atmos Chem Phys 18(24):17863–17881. https://doi.org/10.5194/acp-18-17863-2018
Fan J, Wang Y, Rosenfeld D, Liu X (2016) Review of aerosol–cloud interactions: mechanisms, significance, and challenges. J Atmos Sci 73(11):4221–4252. https://doi.org/10.1175/JAS-D-16-0037.1
Flamant C, Gaetani M, Chaboureau JP, Chazette P, Cuesta J, Piketh SJ, Formenti P (2022) Smoke in the river: an aerosols, radiation, and clouds in southern Africa (AEROCLO-SA) case study. Atmos Chem Physi 22(8):5701–5724. https://doi.org/10.5194/acp-22-5701-2022
Gao M (2020) Environmental effect condition (air temperature) of aerosols on gross primary productivity of vegetation. Int J Ecol 9(2):210–222. https://doi.org/10.12677/IJE.2020.92027
Gao X, Gu F, Mei X, Hao W, Li H, Gong D, Li X (2018) Light and water use efficiency as influenced by clouds and/or aerosols in a rainfed spring maize cropland on the loess plateau. Crop Sci 58(2):853–862. https://doi.org/10.2135/cropsci2017.06.0341
Ge W, Deng L, Wang F, Han J (2021) Quantifying the contributions of human activities and climate change to vegetation net primary productivity dynamics in China from 2001 to 2016. Sci Total Environ 773:145648. https://doi.org/10.1016/j.scitotenv.2021.145648
Greenwald R, Bergin MH, Xu J, Cohan D, Hoogenboom G, Chameides WL (2006) The influence of aerosols on crop production: a study using the CERES crop model. Agr Syst 89(2-3):390–413. https://doi.org/10.1016/j.agsy.2005.10.004
Gu L, Baldocchi DD, Wofsy SC, Munger JW, Michalsky JJ, Urbanski SP, Boden TA (2003) Response of a deciduous forest to the Mount Pinatubo eruption: enhanced photosynthesis. Science 299(5615):2035–2038. https://doi.org/10.1126/science.1078366
Haywood JM, Abel SJ, Barrett PA, Bellouin N, Blyth A, Bower KN, Brooks M, Carslaw K, Che HC, Coe H, Cotterell MI, Crawford I, Cui Z, Davies N, Dingley B, Field P, Formenti P, Gordon H, Graaf MD et al (2021) The cloud–aerosol–radiation interaction and forcing: the year 2017 (CLARIFY-2017) measurement campaign. Atmos Chem Phys 21(2):1049–1084. https://doi.org/10.5194/acp-21-1049-2021
Ho T (1998) The random subspace method for constructing decision forests. IEEE Tran Pattern Anal Mach Intell 20(8):832–844. https://doi.org/10.1109/34.709601
Huang S, Cai N, Pacheco PP, Narrandes S, Wang Y, Xu W (2018) Applications of support vector machine (SVM) learning in cancer genomics. Cancer Genom Proteom 15(1):41–51. https://doi.org/10.21873/cgp.20063
Jiang L, Chen X, Zhu H (2021) Spatial distribution characteristics of urban nursing homes in China and their divergent causes. J Geogr 76(8):1951–1964. https://doi.org/10.11821/dlxb202108010
Jiang S, Huang Y, Zhao L, Cui N, Wang Y, Hu X, Zheng S, Zou Q, Feng Y, Guo L (2022) Effects of clouds and aerosols on ecosystem exchange, water and light use efficiency in a humid region orchard. Sci Total Environ 811:152377. https://doi.org/10.1016/j.scitotenv.2021.152377
Kang S, Hao X, Du T, Tong L, Su X, Lu H, Li X, Huo Z, Li S, Ding R (2017) Improving agricultural water productivity to ensure food security in China under changing environment: from research to practice. Agr Water Manag 179:5–17. https://doi.org/10.1016/j.agwat.2016.05.007
Kong X, Zhao J, Xu H, Xu J (2019) Assessment of atmospheric aerosol direct radiation effect on maize yield in China based on APSIM model. Chin J Ecol Agr 27(7):994–1003. https://doi.org/10.13930/j.cnki.cjea.181071
Kumar N, Middey A (2022) Interaction of aerosol with meteorological parameters and its effect on the cash crop in the Vidarbha region of Maharashtra, India. Int J Biometeorol 66(7):1473–1485. https://doi.org/10.1007/s00484-022-02296-0
Lau WKM, Kim KM, Leung LR (2017) Changing circulation structure and precipitation characteristics in Asian monsoon regions: greenhouse warming vs. aerosol effects. Geosci Lett 4:1–11. https://doi.org/10.1186/s40562-017-0094-3
Le TH, Wang Y, Liu L, Yang J, Yung YL, Li G, Seinfeld JH (2020) Unexpected air pollution with marked emission reductions during the COVID-19 outbreak in China. Science 369(6504):702–706. https://doi.org/10.1126/science.abb7431
Li B, Liu Z, Huang F, Yang XG, Liu ZJ, Wan W, Wang J, Xu Y, Li Z, Ren T (2021b) Ensuring national food security by strengthening high-productivity black soil granary in Northeast China. Bull Chin Acad Sci 36(10):1184–1193. https://doi.org/10.16418/j.issn.1000-3045.20210706003
Li M, Zhang R, Luo H, Gu S, Qin Z (2022) Crop mapping in the Sanjiang Plain using an improved object-oriented method based on google earth engine and combined growth period attributes. Remote Sens 14:273. https://doi.org/10.3390/rs14020273
Li X, Liang H, Cheng W (2021a) Evaluation and comparison of light use efficiency models for their sensitivity to the diffuse PAR fraction and aerosol loading in China. Int J Appl Earth Obs Geoinf 95:102269. https://doi.org/10.1016/j.jag.2020.102269
Li Y, Shiraiwa M (2019) Timescales of secondary organic aerosols to reach equilibrium at various temperatures and relative humidities. Atmos Chem Physi 19(9):5959–5971. https://doi.org/10.5194/acp-19-5959-2019
Liu X, Ning J, Dong F, Yu J, Du G, Kuang W (2017) Spatial-temporal variation characteristics of vegetation NPP of northern Sanjiang plain from 2000 to 2013. J Northeast Agr Univ 48(7):63–71. https://doi.org/10.19720/j.cnki.issn.1005-9369.2017.07.007
Liu X, Xu J, Yang S, Zhang J, Wang Y (2018) Vapor condensation in rice fields and its contribution to crop evapotranspiration in the subtropical monsoon climate of China. J Hydrometeorol 19(6):1043–1057. https://doi.org/10.1175/JHM-D-17-0201.1
Lv F, Deng L, Zhang Z, Wang Z, Wu Q, Qiao J (2022) Multiscale analysis of factors affecting food security in China, 1980–2017. Environ Sci Pollut Res 29(5):6511–6525. https://doi.org/10.1007/s11356-021-16125-1
Ma W, Ding J, Wang J, Zhang J (2022) Effects of aerosol on terrestrial gross primary productivity in Central Asia. Atmos Environ 288:119294. https://doi.org/10.1016/j.atmosenv.2022.119294
Miao C, He X, Gao Z, Chen W, He B (2023) Assessing the vertical synergies between outdoor thermal comfort and air quality in an urban street canyon based on field measurements. Build Environ 227(109810):109810. https://doi.org/10.1016/j.buildenv.2022.109810
Mo X, Chen X, Hu S, Liu S, Xia J (2017) Attributing regional trends of evapotranspiration and gross primary productivity with remote sensing: a case study in the North China Plain. Hydrol Earth Syst Sci 21(1):295–310. https://doi.org/10.5194/hess-21-295-2017
Moazenzadeh R, Mohammadi B, Shamshirband S, Chau KW (2018) Coupling a firefly algorithm with support vector regression to predict evaporation in northern Iran. Eng Appl Comput Fluid Mech 12(1):584–597. https://doi.org/10.1080/19942060.2018.1482476
Paul A, Mukherjee DP, Das P, Gangopadhyay A, Chintha AR, Kundu S (2018) Improved random forest for classification. IEEE Tran Image Process 27(8):4012–4024. https://doi.org/10.1109/TIP.2018.2834830
Pei Y, Dong J, Zhang Y, Yang J, Zhang Y, Jiang C, Xiao X (2020) Performance of four state-of-the-art GPP products (VPM, MOD17, BESS, and PML) for grasslands in drought years. Ecol Inform 56:101052. https://doi.org/10.1016/j.ecoinf.2020.101052
Ren Y, Wang C, Zhao Y (2010) Review on impact of atmospheric aerosol radiation effect on crops and ecological system. China Agr Weather 31(4):533–540. https://doi.org/10.3969/j.issn.1000-6362.2010.04.009
Rosenfeld D, Sherwood S, Wood R, Donner L (2014) Climate effects of aerosol-cloud interactions. Science 343(6169):379–380. https://doi.org/10.1126/science.1247490
Shu Y, Liu S, Wang Z, Xiao J, Shi Y, Peng X, Gao H, Wang Y, Yuan W, Yan W, Ning Y, Li Q (2022) Effects of aerosols on gross primary production from ecosystems to the globe. Remote Sens 14(12):2759. https://doi.org/10.3390/rs14122759
Singh P, Vaishya A, Rastogi S, Babu S (2020) Seasonal heterogeneity in aerosol optical properties over the subtropical humid region of northern India. J Atmos Solar-Terr Phy 201:105246. https://doi.org/10.1016/j.jastp.2020.105246
Sun Q, Lu C, Guo H, Yan L, He X, Wu C (2021) Impact of land use change on water balance in the Sanjiang Plain. Adv Water Sci 32(5):694–706. https://doi.org/10.14042/j.cnki.32.1309.2021.05.005
Sun Y, Wang Z, Fu P, Jiang Q, Yang T, Li J, Ge X (2013) The impact of relative humidity on aerosol composition and evolution processes during wintertime in Beijing, China. Atmos Environ 77:927–934. https://doi.org/10.1016/j.atmo-senv.2013.06.019
Sun Z, Wang X, Zhang X, Tani H, Guo E, Yin S, Zhang T (2019) Evaluating and comparing remote sensing terrestrial GPP models for their response to climate variability and CO 2 trends. Sci Total Environ 668:696–713. https://doi.org/10.1016/j.scitotenv.2019.03.025
Thorsen TJ, Ferrare RA, Kato S, Winker DM (2020) Aerosol direct radiative effect sensitivity analysis. J Clim 33(14):6119–6139. https://doi.org/10.1175/JCLI-D-19-0669.1
Tian J, Wang Q, Zhang Y, Yan M, Liu H, Zhang N, Ran W, Cao J (2021) Impacts of primary emissions and secondary aerosol formation on air pollution in an urban area of China during the COVID-19 lockdown. Environ Int 150:106426. https://doi.org/10.1016/j.envint.2021.106426
Tie X, Huang R, Dai W, Cao J, Long X, Su X, Zhao S, Wang Q, Li G (2016) Effect of heavy haze and aerosol pollution on rice and wheat productions in China. Sci Rep 6:29612. https://doi.org/10.1038/srep29612
Velavan TP, Meyer CG (2020) The COVID-19 epidemic. Trop Med Int Health 25(3):278. https://doi.org/10.1111/tmi.13383
Wang H, Liang H, Gao D (2017) Occurrence and risk assessment of phthalate esters (PAEs) in agricultural soils of the Sanjiang Plain, northeast China. Environ Sci Pollut Res 24:19723–19732. https://doi.org/10.1007/s11356-017-9646-5
Wang J, Xu C (2017) Geodetectors: principles and prospects. J Geogr 72(1):116–134. https://doi.org/10.11821/dlxb201701010
Wang W, He B (2023) Co-occurrence of urban heat and the COVID-19: impacts, drivers, methods, and implications for the post-pandemic era. Sustain Cities Soc 90:104387. https://doi.org/10.1016/j.scs.2022.104387
Wei S, Yi C, Fang W, Hendrey G (2017) A global study of GPP focusing on light-use efficiency in a random forest regression model. Ecosphere 8(5):e01724. https://doi.org/10.1002/ecs2.1724
Xiao Z, Miao Y, Zhu S, Yu Y, Du X, Che H (2022) Relationship between aerosol pollution and different types of precipitation in autumn and winter in North China. J Meteorol 80(6):986–998. https://doi.org/10.11676/qxxb2022.066
Xu Y, Lin L (2017) Pattern scaling based projections for precipitation and potential evapotranspiration: sensitivity to the composition of GHGs and aerosols forcing. Climatic Change 140(3-4):635–647. https://doi.org/10.1007/s10584-016-1879-7
Yang H, Zhong X, Deng S, Xu H (2021) Assessment of the impact of LUCC on NPP and its influencing factors in the Yangtze River basin, China. Catena 206:105542. https://doi.org/10.1016/j.catena.2021.105542
Zhang H, Zhang YD, Huang Y, Huan G, Bai W (2023) Study on water consumption and growth characteristics of rice under different irrigation modes. Water-saving Irrig 48(4):25–31. https://doi.org/10.12396/jsgg.2022339
Zhang L, Wang Z, Du G, Chen Z (2022) Analysis of the climatic basis for the change of cultivated land area in Sanjiang Plain of China. Front Earth Sci 10:590. https://doi.org/10.3389/feart.2022.862141
Zhang Y, Boucher O, Ciais P, Li L, Bellouin N (2020) How to reconstruct diffuse radiation scenario for simulating GPP in land surface models? Geosci Model Dev 2020:1–15. https://doi.org/10.5194/gmd-2020-267
Zhang Y, Li W, Zhu Q, Chen H, Fang X, Zhang T, Zhao P, Peng C (2015) Monitoring the impact of aerosol contamination on the drought-induced decline of gross primary productivity. Int J Appl Earth Obs Geoinf 36:30–40. https://doi.org/10.1016/j.jag.2014.11.006
Zhang Y, Xiao X, Wu X, Zhou S, Zhang G, Qin Y, Dong J (2017) A global moderate resolution dataset of gross primary production of vegetation for 2000–2016. Sci Data 4(1):1–13. https://doi.org/10.1038/sdata.2017.165
Zhang Z, Liu Q, Ruan Y, Tan Y (2021) Estimation of aerosol radiative effects on terrestrial gross primary productivity and water use efficiency using the process-based model and satellite data. Atmos Res 247:105245. https://doi.org/10.1016/j.atmosres.2020.105245
Zheng C, Zhao C, Zhu Y, Shi XQ, Wu XL, Chen TM, Wu F, Qiu YM (2017) Analysis of influential factors for the relationship between PM 2.5 and AOD in Beijing. Atmos Chem Phys 17(21):13473–13489. https://doi.org/10.5194/acp-17-13473-2017
Zhou H, Yue X, Lei Y, Tian C, Zhu J, Ma Y, Cao Y, Yin X, Zhang Z (2022) Distinguishing the impacts of natural and anthropogenic aerosols on global gross primary productivity through diffuse fertilization effect. Atmos Chem Phys 22(1):693–709. https://doi.org/10.5194/acp-22-693-2022
Zimmerman RK, Balasubramani GK, Norwalk MP, Eng H, Urbanski L, Jackson LA, Mclean HQ, Belongia EA, Monto AS, Malosh RE, Gaglani M, Clipper L, Flannery B, Wisniewski SR (2016) Classification and regression tree (CART) analysis to predict influenza in primary care patients. BMC Infect Dis 16(1):1–11. https://doi.org/10.1186/s12879-016-1839-x
Download references
Acknowledgements
The authors thank the reviewers for their valuable comments, and the authors thank the editor for his efforts in this paper.
This research was funded by the High-level Talent Foundation Project of Harbin Normal University (No. 1305123005).
Author information
Authors and affiliations.
Heilongjiang Province Key Laboratory of Geographical Environment Monitoring and Spatial Information Service in Cold Regions/School of Geographical Sciences, Harbin Normal University, Harbin, 150025, China
Xiaokang Zuo & Hanxi Wang
Heilongjiang Province Collaborative Innovation Center of Cold Region Ecological Safety, Harbin, 150025, China
You can also search for this author in PubMed Google Scholar
Contributions
Xiaokang Zuo: Ms. Huang drafted the initial manuscript, analyzed data, reviewed the manuscript, and approved the final manuscript as submitted. Hanxi Wang: Mr. Wang conceptualized and designed the study, reviewed and revised the manuscript, and approved the final manuscript as submitted. All authors approved the final manuscript as submitted and agreed to be accountable for all aspects of the work.
Corresponding author
Correspondence to Hanxi Wang .
Ethics declarations
Ethics approval.
Not applicable
Consent to participate
Consent for publication, competing interests.
The authors declare no competing interests.
Additional information
Responsible Editor: Zhihong Xu
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
(DOCX 20 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions

About this article
Zuo, X., Wang, H. Impact of aerosol concentration changes on carbon sequestration potential of rice in a temperate monsoon climate zone during the COVID-19: a case study on the Sanjiang Plain, China. Environ Sci Pollut Res (2024). https://doi.org/10.1007/s11356-024-33149-5
Download citation
Received : 24 December 2023
Accepted : 26 March 2024
Published : 06 April 2024
DOI : https://doi.org/10.1007/s11356-024-33149-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Aerosol optical depth (AOD)
- Gross primary productivity (GPP)
- Net primary productivity (NPP)
- Driving factors
- Carbon sequestration
- Find a journal
- Publish with us
- Track your research
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 26 October 2023
Evidence for a role of Anopheles stephensi in the spread of drug- and diagnosis-resistant malaria in Africa
- Tadele Emiru 1 na1 ,
- Dejene Getachew 2 na1 ,
- Maxwell Murphy ORCID: orcid.org/0000-0003-0332-4388 3 na1 ,
- Luigi Sedda ORCID: orcid.org/0000-0002-9271-6596 4 na1 ,
- Legesse Alamerie Ejigu 1 ,
- Mikiyas Gebremichael Bulto 1 ,
- Isabel Byrne 5 ,
- Mulugeta Demisse 1 ,
- Melat Abdo 1 ,
- Wakweya Chali 1 , 6 ,
- Aaron Elliott 3 ,
- Eric Neubauer Vickers 3 ,
- Andrés Aranda-Díaz 3 ,
- Lina Alemayehu 1 ,
- Sinknesh W. Behaksera 1 ,
- Gutema Jebessa 1 ,
- Hunduma Dinka 2 ,
- Tizita Tsegaye 1 ,
- Hiwot Teka 7 ,
- Sheleme Chibsa 7 ,
- Peter Mumba 7 ,
- Samuel Girma 7 ,
- Jimee Hwang ORCID: orcid.org/0000-0002-9890-2664 8 ,
- Melissa Yoshimizu 9 ,
- Alice Sutcliffe 10 ,
- Hiwot Solomon Taffese 11 ,
- Gudissa Aseffa Bayissa 11 ,
- Sarah Zohdy 10 ,
- Jon Eric Tongren ORCID: orcid.org/0000-0002-4879-3228 8 ,
- Chris Drakeley ORCID: orcid.org/0000-0003-4863-075X 5 ,
- Bryan Greenhouse 3 ,
- Teun Bousema ORCID: orcid.org/0000-0003-2666-094X 6 &
- Fitsum G. Tadesse ORCID: orcid.org/0000-0003-1931-1442 1 , 5 , 6
Nature Medicine volume 29 , pages 3203–3211 ( 2023 ) Cite this article
5617 Accesses
10 Citations
133 Altmetric
Metrics details
- Risk factors
Anopheles stephensi , an Asian malaria vector, continues to expand across Africa. The vector is now firmly established in urban settings in the Horn of Africa. Its presence in areas where malaria resurged suggested a possible role in causing malaria outbreaks. Here, using a prospective case–control design, we investigated the role of An. stephensi in transmission following a malaria outbreak in Dire Dawa, Ethiopia in April–July 2022. Screening contacts of patients with malaria and febrile controls revealed spatial clustering of Plasmodium falciparum infections around patients with malaria in strong association with the presence of An. stephensi in the household vicinity . Plasmodium sporozoites were detected in these mosquitoes. This outbreak involved clonal propagation of parasites with molecular signatures of artemisinin and diagnostic resistance. To our knowledge, this study provides the strongest evidence so far for a role of An. stephensi in driving an urban malaria outbreak in Africa, highlighting the major public health threat posed by this fast-spreading mosquito.
Similar content being viewed by others

A guide to vaccinology: from basic principles to new developments
Andrew J. Pollard & Else M. Bijker

Infectious disease in an era of global change
Rachel E. Baker, Ayesha S. Mahmud, … C. Jessica E. Metcalf

Metagenomic analysis of individual mosquito viromes reveals the geographical patterns and drivers of viral diversity
Yuan-Fei Pan, Hailong Zhao, … Mang Shi
The promising decline in malaria burden has slowed since 2015. This is particularly evident in Africa, the continent that carries the largest malaria prevalence 1 . Malaria control programs in Africa traditionally focus on rural settings, where most infections occur, but malaria is of increasing concern in urban settings 2 . Given the rapid urbanization in Africa 3 , urban malaria transmission can result in a considerable health burden 4 . Urban malaria is classically associated with importation from areas of intense transmission 5 but can be exacerbated by the adaptation of existing malaria vectors to urban environments 6 and the emergence of urban malaria vectors such as Anopheles stephensi 7 .
An. stephensi is distinct from other Anopheles species that are traditional vectors in (rural) Africa, with its preference for artificial water storage containers that are common in urban settings 8 , 9 . Native to the Indian subcontinent and the Persian Gulf 10 , An. stephensi is now rapidly expanding its geographic range westward (Fig. 1a ) 7 . First detected in Africa in Djibouti in 2012 (ref. 11 ), An. stephensi has spread across the Horn of Africa; its range now includes Ethiopia (2016) 12 , Sudan (2016) 13 , Somalia (2019) 14 , Eritrea (2022) 15 and beyond: Yemen (2021) 16 , Kenya (2022) 17 , Ghana (2022) 15 and Nigeria (2020) 15 . In the Horn of Africa, the vector was found firmly established 18 and abundantly present in manmade aquatic habitats in the driest months of the year, when endemic vectors such as An. arabiensis are largely absent, demonstrating how well adapted the mosquito is to perennial persistence and urban ecology. This poses a risk of year-round malaria transmission. In recognition of the potentially devastating consequences of An. stephensi advancing across Africa, the World Health Organization (WHO) urgently requested more data on its distribution and released a strategy to mitigate its spread 19 .
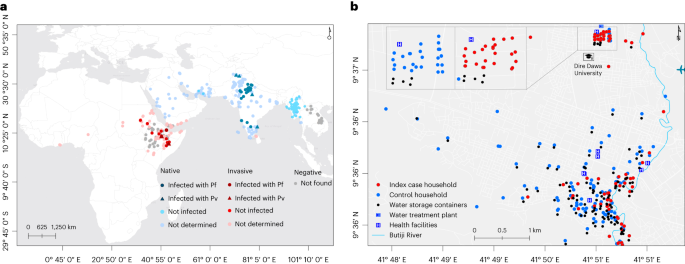
a , The global distribution of An. stephensi where it is native (blue) and invasive (red) is shown, together with sporozoite infection detection outcomes where it was found infected and not infected with P. falciparum (Pf) and P. vivax (Pv). Sites where An. stephensi was observed but mosquitoes were not tested for the presence of sporozoites are also shown (not determined). Settings where dedicated entomological surveillance did not detect An. stephensi mosquitoes are indicated by gray circles (negative). b , The locations of case (red) and control (green) households/dormitories surveyed in this study are shown, together with water storage containers (black), water treatment plant (in the university campus), health facilities (H) and Butiji River in Dire Dawa City. Source: the global map ( a ) was modified on the basis of the malaria threats map 7 of the WHO.
Source data
In addition to the invasive An. stephensi and widespread high prevalence of insecticide resistance, the Horn of Africa region is disproportionately affected by other emerging biological threats for malaria control, including the emergence of Plasmodium falciparum parasites with drug resistance (Uganda 20 , Rwanda 21 and Eritrea 22 ) and histidine-rich protein 2 ( pfhrp2 ) and pfhrp3 gene deletions (Ethiopia 23 , Eritrea 24 and Djibouti 25 ) that could compromise the utility of widely used rapid diagnostic tests (RDTs). Because of its abundant and species-specific expression by P. falciparum parasites, histidine-rich protein 2 (HRP2)-based RDTs are commonly used for the diagnosis of P. falciparum . Recent reports of expansion of parasites with pfhrp2 / pfhrp 3 gene deletions and drug resistance, together with a highly efficient invasive mosquito in the region, threaten the major gains documented in recent decades.
In addition to being an efficient vector for both P. falciparum and P. vivax in its native geographical range 10 , An. stephensi was recently confirmed as being susceptible to local parasites in Ethiopia (Fig. 1a ) 9 , 18 and a resurgence of malaria was reported in Djibouti following its detection in 2012 (ref. 26 ), although direct evidence for a role of An. stephensi in this resurgence was unavailable. In this Article, following a report of a dry-season upsurge in malaria cases in Dire Dawa City, Ethiopia, where An. stephensi was recently documented 8 , we prospectively investigated its role in malaria transmission through responsive epidemiological and entomological surveillance (Fig. 1b ).
Malaria outbreaks in Dire Dawa City and its university
Clinical malaria incidence data (diagnosed by microscopy) collected from public and private health facilities ( n = 34) showed an overall statistically significant trend of increasing number of malaria-positive cases between 2019 and 2022 (Mann–Kendall statistical test τ = 0.42, P < 0.001). A 12-fold increase was observed (Extended Data Table 1 and Supplementary Fig. 1 ) for malaria incidence in Dire Dawa during the dry months (January–May) of 2022 (2,425 cases) compared with 2019 (205 cases). A similar increasing trend was observed using District Health Information System 2 (DHIS2) data (Fig. 2a and Supplementary Fig. 1 ). Patients reported at both public and private health facilities, with the latter contributing to 15.8% of patients diagnosed for malaria in the past 4 years with an increasing trend from 17.7% in 2019 to 25.9% in 2021, which later declined to 5.7% during the outbreak (2022). In 2022, 76% of all reported malaria cases originated from only three public health facilities: Dire Dawa University (DDU) students’ clinic (42%), Sabiyan Hospital (19%) and Goro Health Center (15%). At DDU campus, 94% (1,075 out of 1,141) of clinical malaria episodes occurred in the male student population living in the university’s single-sex dormitories.
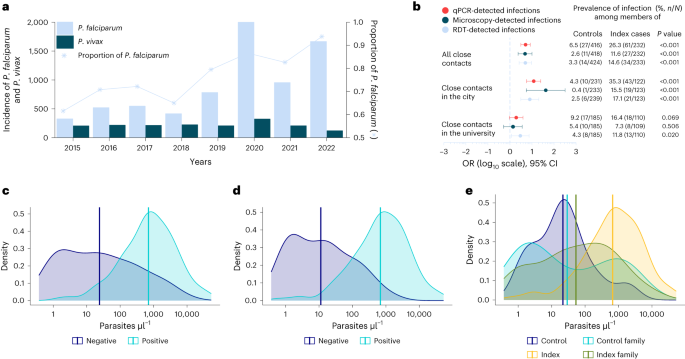
a , b , Malaria trends using DHIS2 data ( a ) are shown, with the prevalence and odds of detecting additional infections in close contacts of cases compared with controls in Dire Dawa, separately for all close contacts, contacts in the city and the university ( b ). ORs were obtained from univariate logistic regression, with diagnostic test results as outcome and site as predictor. Univariate logistic models were fitted for each diagnostic test. ORs are shown on a log 10 scale ( x axis), together with their 95% CI bars and respective P values (estimated from Wald test). Numbers to the right of the forest plot indicate the proportion of positive cases by respective diagnostic test (color coded and embedded in the figure) among control and index household/dormitory members. c – e , Parasite density per microliter distributions and their respective averages determined by 18S-based qPCR among HRP2-based RDT-positive ( n = 113) and RDT-negative ( n = 88) infections ( c ) and microscopy-positive ( n = 129) and microscopy-negative ( n = 71) infections ( d ) are shown, together with distribution among index cases ( n = 99), contacts of index cases ( n = 61), controls ( n = 14) and contacts of controls ( n = 27) ( e ).
We conducted a prospective case–control study to identify risk factors associated with this sudden rise in malaria in the city (Goro Health Center) and DDU (Fig. 1b ). In the city we recruited 48 microscopy-confirmed febrile malaria cases plus 125 case household members and 109 febrile controls without microscopy-confirmed malaria who had attended the same clinic within 72 h, plus 241 control household members. At DDU we recruited 53 students with clinical malaria and 110 dorm-mates and 80 uninfected febrile students with 186 dorm-mates. Details of individual and household characteristics are presented in Table 1 . Both index cases and controls were febrile either at the time of recruitment or within 48 h (self-reported) before attending the clinics. Family members/dorm-mates were recruited irrespective of symptoms. Fever was detected in a minority of recruited family/dormitory members of the controls (1.4%, 6 out of 424) and index cases (6.0%, 14 out of 233; Extended Data Table 2 ). The responsive case–control study unit was household/dormitory; no plausible risk factors were defined a priori, and neither a sex/gender nor Plasmodium species stratification was considered in the study design. The outbreak at the university campus occurred at a fine spatial scale (20 dormitory buildings in a 45,450-m 2 area); the dormitories affected by malaria were occupied by male students only (Extended Data Table 1 ). Despite Dire Dawa being historically coendemic for P. falciparum and P. vivax , the proportion of malaria cases due to P. falciparum increased from 61% in 2015 to 93% in 2022 (Fig. 2a ). All index cases that we recruited ( n = 101) and the additional infections detected ( n = 102) in this study were found to be P. falciparum , with the exception of two P. vivax infections detected by 18S-based quantitative polymerase chain reaction (qPCR). Plasmodium infection was detected in 14 controls by 18S-based qPCR. The parasite density in these infections—which were all P. falciparum —was very low (median parasitemia was 21 parasites µl –1 ) and thus lies below the detection limits of conventional diagnostics. Only two of these infections had parasitemia >100 parasites µl –1 (278 and 1,822 parasites µl –1 ).
Mosquito exposure and infection prevalence in malaria contacts
The results obtained from case–control analysis showed that members of index cases and controls had different levels of mosquito exposure (Extended Data Table 3 ). In entomological surveillance, all households and dormitories were surveyed for adult mosquitoes (indoors, outdoors and in animal shelters if present) and immature stages of Anopheles in waterbodies present within a 100-m radius. Members of a case household/dormitory were more likely to be living close to An. stephensi- positive sites, defined as the presence of larvae within a 100-m radius from the household/dormitory (odds ratio (OR) 5.0, 95% confidence interval (CI) 2.8–9.4, P < 0.001), to adult mosquito resting sites (OR 1.9, 95% CI 0.9–4.0, P = 0.068) or to natural/manmade waterbodies in general (OR 1.6, 95% CI 1.2–2.2, P = 0.002). The odds of using an aerosol insecticide spray were 58% lower among members of index cases compared with controls (OR 0.42, 95% CI 0.23–0.72, P < 0.001).
In the city, P. falciparum qPCR-detected infections were significantly more common (OR 12.0, 95% CI 5.8–25.1, P < 0.001; Fig. 2b ) among case household members (35.3%, 43 out of 122) than control household members (4.3%, 10 out of 233), with a similar trend for microscopy- (OR 42.4, 95% CI 5.6–320.8, P < 0.001) and RDT-detected infections (OR 8.0, 95% CI 3.1–20.4, P < 0.001). At DDU, despite all students living in close proximity (20 buildings in a 45,450-m 2 area), dorm-mates of malaria cases were three times more likely (OR 3.0, 95% CI 1.2–7.4, P = 0.020; Fig. 2b ) to be P . falciparum positive by RDT (11.8%, 13 out of 110) compared with dorm-mates of controls (4.3%, 8 out of 185). One-quarter of microscopy-positive infections (34 out of 136) were negative by HRP2-based RDT (sensitivity 75.0, 95% CI 72.2–77.8, specificity 97.0, 95% CI 95.9–98.1; Extended Data Table 4 ), with different proportions of HRP2-based RDT-negative infections in the city (10.3%, 7 out of 68) and the university (39.7%, 27 out of 68). HRP2-based RDTs are those most commonly used in the diagnosis of P. falciparum in the area. Recent reports of expansion of parasites with pfhrp2 / pfhrp 3 gene deletion threaten the important role of these RDTs in the diagnosis of malaria. qPCR detected considerably more infections, with the likelihood of infections being missed by RDT (Fig. 2c ) or microscopy (Fig. 2d ) being dependent on parasite density and, for RDT, pfhrp2 gene deletion status (Extended Data Table 5 ). Parasite densities were higher in RDT-positive infections (geometric mean 702 parasites µl –1 , 95% CI 495–997) than RDT-negative infections (geometric mean 24, 95% CI 14–42, P < 0.001). Similarly, parasite densities were higher in microscopy-positive infections (683 parasites µl –1 , 95% CI 488–956) than in microscopy-negative infections (11 parasites µl –1 , 95% CI 7–19, P < 0.001; Extended Data Table 5 ). Median parasite density (per microliter) as determined by qPCR for infections that were RDT negative but microscopy positive was 357,236 (interquartile range (IQR) 51,440–1,790,966, n = 31), strongly suggestive of pfhrp2 / pfhrp 3 gene deletion in these infections. Parasite density distributions were not different between university students (geometric mean 158 parasites µl –1 , 95% CI 94–265) and city residents (163 parasites µl –1 , 95% CI 91–291, P = 0.132; Supplementary Fig. 2 ). As expected, parasitemia was higher in index cases (geometric mean 669 P. falciparum parasites µl –1 , 95% CI 442–1012; Fig. 2e ) compared with malaria-infected controls (21 parasites µl –1 , 95% CI 7–67, P < 0.001), malaria-infected control family members (29 parasites µl –1 , 95% CI 9–97, P = 0.005) and malaria-infected index family members (53 parasites µl –1 , 95% CI 27–107, P < 0.001).
An. stephensi dominates and carries P. falciparum sporozoites
Anopheles larvae were detected in 3% (26 out of 886) of aquatic habitats, which were either artificial ( n = 17) or natural ( n = 9). An. stephensi was the only species detected in artificial containers ( n = 414 larvae), of which the majority were metal and plastic barrels and jerrycans, and was the predominant species detected at stream edges (57% larvae, 160 out of 280; Extended Data Table 6 ). Adult Anopheles spp. mosquitoes were detected in the majority of examined animal shelters (18 out of 24), water storage tankers (4 out of 4), manholes (7 out of 7), inside (22 out of 508) and outside (7 out of 305) the index and control households/dormitories using Prokopack aspirators, with nearly all identified as An. stephensi (97%, 599 out of 618; Extended Data Table 7 ). All mosquitoes morphologically identified as An. stephensi and tested molecularly ( n = 90) were confirmed to be this species, except four for which the ITS2 -based PCR experiment failed (Supplementary Fig. 3 )—which may have been the result of loss of genetic material during extraction. Fully engorged adult-caught An. stephensi (195 out of 599) and An. gambiae (5 out of 16) mosquitoes (Extended Data Table 8 ) were tested for bloodmeal sources in a multiplex PCR assay that amplifies the cytochrome-b gene: for cow, dog, goat and human. Goats or cows were the main recent bloodmeal sources of An. stephensi (98%, 96 out of 98) and An. gambiae s.l. (80%, 4 out of 5), but only An. stephensi (2 out of 98) had recently fed on humans. Bloodmeal source was undetermined for one-half ( n = 96) of fully engorged ( n = 199) An. stephensi mosquitoes tested in this study. P. falciparum sporozoites, indicative of transmission following natural blood feeding, based on sporozoite and PCR-based detection, were confirmed only in An. stephensi (0.5%, 3 out of 599).
Overlapping clusters of P . falciparum and An. stephensi abundance
Spatial analysis of P. falciparum infection localities within the city demonstrated significant evidence for clustering (global Moran’s I test 0.020; P < 0.001; Fig. 3a ) in the study area, and 11 significant clusters of P. falciparum infection (detectable by microscopy and/or RDT) were detected. An. stephensi larvae and/or adult mosquitoes were more often detected near index cases (14.9%) than controls (4.3%, P = 0.020; Fig. 3b ), and this overlapped with clusters of P. falciparum infections (Fig. 3c ). Sporozoite-infected mosquitoes were also found in close proximity (Fig. 3b ). In the city, clusters of households with higher infection prevalence were all situated within 200 m of Butiji River.
a – c , Statistically significant evidence for global spatial clustering of household P. falciparum infections prevalence ( a ) and An. stephensi mosquitoes ( b ) and an overlap between the two ( c ) were observed. Eleven clusters of households were found (A) in the city ( P < 0.05) by one-sided local Anselin Moran’s I test (pseudo P values calculated from 9,999 random permutations): high–high ( n = 6) whereby households had high P. falciparum prevalence, low–low clusters ( n = 5) whereby households had low P. falciparum prevalence, and high–low outlier clusters ( n = 2) whereby high P. falciparum prevalence households were surrounded by low P. falciparum prevalence households, or vice versa. Locations of An. stephensi mosquitoes found infected ( n = 3) are shown in dark-red circles and triangles ( b ). d , e , A map displaying case incidence colored by genetic cluster (lineage 1 in blue and lineage 2 in red) are shown along with timelines that cases were identified ( d ) and their spatial distribution ( e ).
An. stephensi presence is strongly linked with P. falciparum positivity
We next evaluated risk factors for being infected with P. falciparum (Table 2 ). Male sex (OR 3.0, 95% CI 1.7–5.4, P = 0.001) and being above 15 years of age (OR 4.3, 95% CI 1.2–15.7, P = 0.029) were risk factors associated with P. falciparum infection positivity, while using aerosol insecticide sprays was found protective from malaria (OR 0.3, 95% CI 0.1–0.8, P = 0.016). The results further show that those individuals residing in households/dormitories with An. stephensi positivity (larvae/adult/indoor/outdoor) had a higher risk of malaria infection (OR 3.7, 95% CI 1.7–6.5, P < 0.001) compared with individuals in households/dormitories where An. stephensi was not detected.
Parasites with signatures of artemisinin and diagnostic resistance
We attempted to sequence 18S qPCR-positive samples and of these the sequencing was successful for 71% ( n = 131) of the samples. All blood samples were collected from patients before treatment was provided, and thus represent the composition of parasites in the blood. Genotyping of 131 infections at 162 microhaplotype loci by amplicon sequencing uncovered that 90% of infections were monoclonal and nearly all were closely related to other detected infections, with 98% falling into one of two distinct, nearly clonal lineages. Lineage 1 was the most common, almost completely homogeneous, observed throughout the study period, and distributed widely throughout both study sites (Fig. 3d,e and Table 3 ). Lineage 2 accounted for 15% of infections and contained some genetic diversity, with only 13 of 20 infections highly related to each other. Highly related infections within lineage 2 were not detected until May 2022, with most (11/13) detected at DDU (Fig. 3c ). Infections within dormitories were not restricted to a single lineage; half (7/14) of all dormitories with more than one infection had infections from both lineages detected. Of concern was that 14 out of 20 lineage 2 infections carried the R622I mutation in the kelch13 gene—which has been associated with reduced susceptibility to artemisinins in Eritrea 22 —along with evidence of P. falciparum pfhrp2 and pfhrp3 gene deletions—which are associated with false negativity of HRP2-based RDTs. Consistent with evidence of deletions of these genes, the majority of lineage 2 parasites (70.0%, 14/20) tested negative on HRP2-based RDT but were positive by microscopy. Lineage 1 infections did not contain pfhrp2 deletions, most were detectable by RDT (71.6%, 78/109), and only 2.8% ( n = 3) contained the kelch 13 R622I mutation, but all had evidence of pfhrp3 deletions and the quintuple mutation in pfdhfr and pfdhps associated with antifolate resistance. Of the successfully sequenced microscopically detectable but RDT-negative infections ( n = 24), some were found to be pfhrp2 and pfhrp3 double gene deleted (37.5%, 9/24) while the rest were only pfhrp3 gene deleted (62.5%, 15/24). Interestingly, most infections from lineage 2 containing the R622I mutation (11/14) exhibited incomplete antifolate resistance, lacking the pfdhfr 59 mutation. A single monoclonal infection with low relatedness within lineage 2 showed unique features: elevated pfmdr copy number, heterozygous for the pfmdr1 184 mutation, while being the only infection with a wild-type pfcrt genotype. There was no significant association between lineage 1 and lineage 2 with self-reported uptake of vector control measures (bed net utilization, insecticide residual spray and repellent use), travel history, age, sex, educational level, occupation or infection detection by microscopy (Extended Data Table 9 ). In contrast, a larger proportion of lineage 2 infections were undetected by RDT, as described above. These data, showing primarily clonal transmission of two distinct parasite lineages that did not intermix, are consistent with increased transmission occurring on the background of an exceedingly small parasite population, with more recent spread of a parasite lineage containing mutations that are concerning for drug and diagnostic resistance.
Our findings raise concern about urban malaria associated with the presence of An. stephensi . First detected in 2018 in Dire Dawa 8 , An. stephensi is now perennially present in the city and was found infected with P. falciparum 18 . In 2014, no Anopheles developmental stages were detected in containers in Dire Dawa 27 , supporting the notion of its recent introduction in the area. In the years following its first detection (between 2019 and 2022), a 12-fold increase in malaria incidence that was predominantly P. falciparum was observed in the city. The spatial overlap and association between malaria infection and the presence of An. stephensi , the detection of sporozoites in adult mosquitoes and the clonal propagation of parasites that we report here provide the strongest evidence so far for a role of An. stephensi in driving an urban malaria outbreak in Africa. This, to our knowledge, is the first direct evidence of the role of An. stephensi in transmitting malaria in Africa and corroborates recent reports from Djibouti of exponential increases in malaria cases in the years following detection of the species 26 .
The outbreak in the university campus was localized and the dormitories affected by malaria were occupied by male students only. However, in the population of Dire Dawa City, male sex and older age were predictors of malaria positivity. Higher parasite prevalence in males compared with females has been reported in Ethiopia 28 , other African countries 29 and Brazil 30 , and is commonly described in South East Asia 31 . Common explanations are increased risk due to employment and sociobehavioral factors (for example, use of preventive measures, sleeping times and forest work). There may be other behavioral differences between males and females involving crepuscular activities consistent with biting times for An. stephensi , which is exophilic and exophagic 32 . In our setting, chewing khat outdoors is done predominately by men 33 again increasing exposure to vectors. There is limited evidence for sex-associated biological differences in infection acquisition or infection consequences, with the exception of the well-established role of pregnancy in malaria risk 34 . The recently described longer infection duration in males compared with females 35 suggests that there may be differences in infection kinetics/responses to infections between sexes that may in turn impact the epidemiology of malaria infection.
Interestingly, this outbreak only involved P. falciparum infections despite the co-occurrence of P. vivax in the region. We previously demonstrated that An. stephensi is highly susceptible to Ethiopian P. vivax isolates 9 and an increase in P. vivax cases coincided with a rise in An. stephensi mosquitoes in Djibouti 26 . Epidemiological circumstances at the start of the outbreak, notably the extent of the human infectious reservoir for Plasmodium infections, may have been more favorable for P. falciparum in our setting. In sympatric settings, it is well known that P. falciparum is more prone to epidemic expansion than P. vivax 36 , 37 . There is a large and increasing body of evidence (including our own work from Ethiopia) 38 , 39 showing that asymptomatic P. falciparum infections can be highly infectious to mosquitoes and that the level of infectivity depends on the circulating parasite biomass (that is, parasite density in asymptomatic carriers). Related studies on the human infectious reservoir for P. falciparum have also demonstrated that a limited number of individuals, sometimes with asymptomatic infections, may be highly infectious to mosquitoes 39 . This hypothesis is supported by the limited genetic diversity of parasites detected in this study. We speculate that, at the start of the outbreak, the asymptomatic infectious reservoir for P. falciparum was larger than for P. vivax and that a small number of infected individuals may have been responsible for initiating the current outbreak. The continued increase in the proportion of P. falciparum infections between 2015 and 2022 in Dire Dawa and the timing of the outbreak supports this notion. Although sporozoite rates are difficult to compare between sites, times and species, since they depend on many factors including mosquito age and survival, the 0.5% P. falciparum sporozoite positivity that we observed is similar to that observed previously in An. stephensi in Dire Dawa and other areas in Ethiopia 18 as well as sporozoite rates in An. arabiensis , a native malaria vector in Ethiopia 40 . We consider a comparison with other areas with markedly different parasite populations and transmission intensity less relevant although sporozoite rates of An. stephensi in Afghanistan (0.8%) and India (0.6%) are in the same range as we observed 41 . Higher sporozoite rates are more likely to be associated with sustained endemicity (with entomological inoculation rate >1) and are typically associated with microscopy parasite prevalence between 10% and 40% (ref. 42 ). Continuous entomological and clinical surveillance would provide further evidence if this was the case in Dire Dawa. In contrast, asymptomatic P. vivax infections have typically too low parasite densities to infect mosquitoes 38 , 43 . Since P. vivax sporozoites have been detected in An. stephensi mosquitoes previously from the same setting 18 , it is possible that future malaria outbreaks caused by An. stephensi would also involve P. vivax .
The trends in increased parasite carriage among individuals living in proximity of malaria cases were most apparent for conventional diagnostics (RDT and microscopy) but not for qPCR. This is probably to reflect the age of infections with recent infections (that is, acquired during the outbreak under examination) being more likely to be of higher parasite density while low-density infections that are detectable by qPCR to mainly reflect old infections that may have been acquired many months before the study 44 . Historical transmission levels influence the size of the submicroscopic reservoir through acquired immunity 45 . As Dire Dawa was previously endemic 46 , some low-density infections may persist and affect the interpretation of the extent of the outbreak. The relatively high-density (microscopy-detected) asymptomatic infections provided a better description of the current outbreak 38 .
In addition to the role for the invasive An. stephensi , two other biological threats for the control of P. falciparum were identified in our study: drug resistance and diagnostic resistance. The high prevalence of parasites with the R622I mutation in the kelch13 gene is of particular concern. Although it should be noted that parasite strains were not directly tested for resistance ex vivo in the current study, a recent study identified this as a variant linked with partial drug resistance in Eritrea 22 . Following the first report in 2016 from northwest Ethiopia 47 , parasites carrying the R622I variant are reported to be expanding in the same setting 48 , more widely in the country 49 and elsewhere in the Horn of Africa 50 . In addition to evidence for artemisinin-resistant parasites, mutations conferring chloroquine and antifolate resistance were common in the parasite population responsible for this outbreak. Similarly, pfhrp2 and pfhrp3 gene deletions with phenotypic evidence of RDT negativity were detected in our study. Despite its first report from Peru 51 , the Horn of Africa (Ethiopia 23 , Eritrea 24 , Sudan 52 , South Sudan 53 and Djibouti 25 ) is disproportionately affected by diagnostic challenges of infections with pfhrp2 / pfhrp 3 deletions. Co-occurrence of parasites with pfhrp2 / pfhrp 3 gene deletions and the R622I mutation was recently reported from other sites in Ethiopia 49 . So far, no evidence exists if the drug resistance conferring kelch13 mutation (R622I) and pfhrp2 / pfhrp 3 gene deletions have co-evolved in the region or if this is a matter of coincidence. Even without the evidence of co-evolution, the convergence of the three biological threats ( kelch13 mutation, pfhrp2 / pfhrp 3 gene deletion, and An. stephensi playing a role in sustaining transmission of these parasites) is concerning for the region and the entire continent at large.
In this study we concurrently examined parasite carriage and spatial clustering in humans and mosquitoes as well as genetic linkage analysis to demonstrate a highly plausible role for An. stephensi in an outbreak of P. falciparum infections that carry diagnostic and drug resistance markers in Ethiopia. Our data, demonstrating An. stephensi being abundant both in artificial and natural aquatic habitats in the driest months of the year, highlight how well-adapted the mosquito is to perennial persistence and urban ecology. While our outbreak investigation was performed shortly after the mosquito species was first detected in the area 8 , routine vector surveillance was sparse and we cannot draw firm conclusions on the timing of An. stephensi introduction in the area. Additionally, limited sensitivity of methodologies for sampling exophagic adult mosquitoes may have resulted in an underestimation of mosquito exposure and reduced precision of sporozoite prevalence estimates. Common adult mosquito collection methods have limited sensitivity for this invasive exophilic/exophagic species. Enhanced surveillance in this study revealed outdoor resting sites (manholes, water storage tankers and animal shelters) that offer opportunities for targeted vector control and highlight the behavioral plasticity of this invasive mosquito which makes it less amenable to conventional control approaches. Our data on the use of protective measures (for example, repellents) were insufficiently detailed to explore how effective these measures are against An. stephensi . Future studies should address this. Considering the very high level of resistance of An. stephensi to the major insecticides in Ethiopia 18 , 54 , the repellent effect of the aerosol sprays is one explanation for the protective association observed in this study 55 . Most sprays contain repellents such as N , N -diethyl-meta-toluamide (DEET) or permethrin. Permethrin and DEET have strong repellent effects on both Plasmodium -infected and uninfected An. stephensi mosquitoes 55 .
In terms of public health consequences, the spread of An. stephensi in rapidly expanding urban settings could pose a challenge to malaria control programs in Africa for four main reasons: (1) its year round persistence due to its ability to exploit manmade containers that are abundantly present in rapidly expanding urban settings; (2) its ability to evade standard vector control tools given its unique ecology and resistance to many of the currently available insecticides; (3) its ability to efficiently transmit both P. falciparum and P. vivax in the region; and (4) its confirmed role in sustaining the transmission of drug and diagnostic resistant parasites demonstrated in this study that highlights a concerning convergence of biological threats for malaria control in the Horn of Africa and beyond. There is an urgent need for intensified surveillance to identify the extent of the distribution of this vector and to develop and implement tailored control measures. While there is an increasing body of high-quality evidence of the spread of An. stephensi across the African continent, pragmatic studies on how to address this novel malaria threat are largely absent. Given increasing reports of An. stephensi in West and East Africa, the time window during which elimination of this mosquito from (parts of) Africa is possible is rapidly closing.
Description of the study area
Dire Dawa, located 515 km southeast of Addis Ababa (capital of Ethiopia) and 311 km west of Djibouti, is a logistics hub for transportation of goods and cargo (Fig. 1b ). Of its total population (445,050), 74% live in an urban area which is only 2.3% of the 1,288 km 2 Dire Dawa City administrative land (UN-HABITAT, 2008). The area has a warm and dry climate with low level of precipitation (annual average rainfall of 624 mm), and an annual temperature ranging from 19 °C to 32 °C. Malaria incidence has historically been low (an annual parasite clinical incidence of <5 per 1,000 people between 2014 and 2019), with strong seasonality (August to November being the peak season), and sympatric P. falciparum and P. vivax infections.
We obtained public health data, collected through the District Health Information System 2 (DHIS2), to analyze the trend in malaria cases between 2015 and 2022. In the Ethiopian malaria case management guideline, microscopy is recommended for diagnosis at the health center level and above. RDTs are recommended to be used only at the health post level by community health extension workers, in rural settings. In all of the facilities located in Dire Dawa, microscopy was used for diagnosis. The DHIS2 data do not capture cases detected at private health facilities. The recent ‘Global framework for the response to malaria in urban areas’ by the WHO 4 states that “In some urban settings, the private sector is a major source of malaria diagnosis and treatment. However, it is poorly integrated into the surveillance system”. To give context on how much is being managed by the private sector in Dire Dawa, we have collected 4 years of data (January 2019 to May 2022) from 34 out 39 health facilities (both private and public) that are located within the city administration. This included 2 public and 5 private hospitals, 15 health centers (funded publicly) and 17 clinics (private). Some private clinics ( n = 5) refused to provide data or provided incomplete data. Goro Health Center and DDU students’ clinic were selected for the current study based on the highest number of cases they reported before the start of the study (January–February 2022). In fact, together, the two health facilities reported 56% of the total cases in the city in 2022 (January–May). As in all public universities in Ethiopia, students live within campus with full and shared accommodation provided by the government. At DDU, an average of six students of the same sex and year of study share a dormitory on a three-story building that has an average of 67 dormitories. Routine healthcare service is provided in a university dedicated students’ clinic.
Study design and procedure
To ascertain the effect of exposure to An. stephensi on malaria, we employed a case–control study where identification of patients was done prospectively to capture co-occurrent characteristics in terms of exposure and risk factors. We recruited consecutive patients with criteria described below in a 1:2 ratio (one case:two controls) unmatched study design. Study protocol was approved by the Institutional Ethical Review Board of Armauer Hansen Research Institute (AHRI)/All Africa Leprosy Education, Research, and Training Center ethics review committee (AF-10-015.1, PO/07/19). We obtained informed written consent from all participants and guardians or parents for minors.
Recruitment of participants
Patients with (history within 48 h) fever that presented at the two health facilities and tested positive for malaria by microscopy were recruited as index cases (index) from April to July 2022. We recruited febrile patients who attended the same clinic and tested negative for malaria as controls within 72 h of when the index was identified. The index and controls were followed to their homes, and their household/dormitory members were tested for malaria and their households/dormitories were screened for Anopheles mosquitoes (larvae and adult). Household/dormitory members of cases and controls who were willing to participate in the reactive case detection were included irrespective of their symptoms. Households were surveyed for mosquitoes when the head of the household and members of the dormitory gave consent to allow the study team to use mosquito collection methods in their houses/dormitories. Families of cases or controls who were not available within 72 h of recruitment of the cases or controls irrespective of their symptoms were excluded as well as individuals or family members who were unwilling/refused to give informed written consent. It is noticeable that, although the study was unmatched due to the difficulty in recruiting matched controls in geographical proximity of the cases, their general characteristics were very similar. Detailed characteristics of study participants are presented in Table 1 .
Sample size
We planned an unmatched case:control ratio of approximately 1:2 (ref. 56 ) with prospective case identification until the stopping rule was achieved. The choice of the case:control ratio was based on a logistic regression model aimed to detect an OR of at least 2, assuming an exposure of 20% in controls at household level, where the exposure was defined as presence of An. stephensi . The power analysis was conducted in epiR package (R-cran software), and the stopping rule was set to a power of 70% for the study to be sufficiently powered to detect differences between the presence of malaria on An. stephensi exposure at household level. The controls were selected from the same population as the cases and post-stratification applied. Data from cases and controls were reviewed regularly, and final sample size was set to 290 with 101 cases and 189 controls. The recruitment of case household and control household members was done to include reactive case detection and improve the power of the study (as well as the OR minimum detection).
Data collection
Data on the sociodemographic, epidemiological, intervention and travel history were collected verbally using pre-tested questionnaires which were uploaded to mobile tablets using REDCap tools. The entomological survey data and intervention availability were scored by the study data collectors. Malaria case incidence data (from January 2019 to May 2022) were collected from the records of both private and public health facilities ( n = 34).
Blood samples collection
Finger prick blood samples (~0.5 ml), collected in BD K 2 EDTA Microtainer tubes, were used to diagnose malaria using RDT (Abbott Bioline Malaria Ag Pf/Pv HRP2/LDH) and microscopy, and to prepare dried blood spots (DBS) on 3MM Whatman filter paper (Whatman). The remaining blood was separated into cell pellet and plasma. Slide films were confirmed by expert microscopists. Sociodemographic, epidemiological, intervention utilization, and history of travel and malaria were collected from all study participants.
Entomological surveys
We surveyed immature stages of Anopheles mosquitoes within a 100-m radius of the index and control houses/dormitories targeting both manmade water storage containers and natural habitats including riverbeds and stream edges. We checked each aquatic habitat for 10 min from 9:00 to 11:00 and 15:00 to 17:00 for the presence of Anopheles mosquitoes’ larvae or pupae aiming for ten dips per habitat (using a standard dipper with 350 ml capacity). Characteristics of water holding containers (permanency of habitat, lid status, purpose, volume, presence of shade, type, turbidity, temperature and water source) were recorded for each habitat (Extended Data Table 6 ). We searched adult mosquitoes using Prokopack aspirators for 10 min between 6:00 and 8:00 indoor, outdoor and in animal shelters located within the compound of the household or inside and outside the dormitories at the university (Extended Data Table 7 ). Mosquito surveys (immature and adult) were done within 48–72 h of when the index/control was recruited.
Conventional adult mosquito collection methods such as Centers for Disease Control and Prevention light traps and pyrethrum spray sheet have limited sensitivity for this invasive species mainly related with its unique resting behavior 21 . To supplement the evidence generated from the case–control study and examine the resting sites of the adult Anopheles mosquitoes in detail in the study area, additional adult mosquito surveys were done targeting potential resting sites including animal shelters and manholes within the study time and area. Informed by these preliminary findings, surveys were systematized in three fortnightly rounds during the study period. In the city, households with ( n = 15) and without ( n = 15) animal shelters were included (Extended Data Table 7 ). At DDU, two dormitory buildings which reported the highest number of malaria cases and their surroundings were selected. Adult mosquitoes were surveyed indoor, outdoor, in animal shelters, in overhead tanks and in manholes using Prokopack aspirators for 10 min between 6:00 and 8:00. Animal shelters were not available at DDU. Adult-caught mosquitoes (sorted on the basis of their abdominal status), and those raised from aquatic stages, were morphologically identified to the species level 22 (Extended Data Table 8 ). Anopheles mosquitoes were individually preserved in tubes that contained silica gel desiccant in zipped bags and transported to the lab at the AHRI for further analysis. The global positioning system (GPS) coordinates of the households and immature and adult mosquito collection sites were recorded using GARMIN handheld GPS navigator (GARMIN GPSMAP 64S).
Laboratory procedures
Nucleic acid extraction from whole blood and parasite quantification, and genotyping.
Blood samples in ethylenediaminetetraacetic acid (EDTA) tubes were used to extract genomic DNA using MagMAX magnetic bead-based technology DNA multi-sample kit on KingFisher Flex robotic extractor machine (Thermo Fisher Scientific). Fifty microliters of whole blood input was eluted in a 150 μl low-salt elution buffer. Multiplex qPCR targeting the 18S rRNA small subunit gene for P. falciparum and P. vivax was run using primer and probe sequences described by Hermsen 57 and Wampfler 58 using TaqMan Fast Advanced Master Mix (Applied Biosystems). P. falciparum parasites were quantified using standard curves generated from a serial dilution of NF54 ring stage parasites (10 6 to 10 3 parasites ml −1 ). For P. vivax , parasite quantification was done using plasmid constructs to infer copy numbers by running serial dilutions (10 7 to 10 3 copies µl −1 ) of plasmids having the amplicon. Serial dilutions of the standard curves were generated in duplicate on each plate. Multiplexed amplicon sequencing was performed on qPCR-positive samples with reagents and protocol as in Tessema et al. 59 . DNA was amplified for 15 or 20 cycles in multiplexed PCR, depending on parasitemia and ability to amplify, and for 15 cycles for indexing PCR. The primer pools used in this study comprised high-diversity microhaplotype targets ( n = 162), polymorphisms associated with drug resistance, and targets in and adjacent to pfhrp2 and pfhrp3 to assess for gene deletion (Primer pools 1A and 5 as described in protocols.io repository) 60 . Amplified libraries were sequenced in a NextSeq 2000 or a MiniSeq instrument using 150PE reads with 10% PhiX.
Nucleic acid extraction from mosquitoes, assessment of infectivity and bloodmeal source and confirmation of morphological species identification
Infection detection in wild caught mosquitoes is commonly based on an enzyme-linked immunosorbent assay (ELISA)-based protocol that targets circumsporzoite protein (CSP) that is expressed on the surface of Plasmodium sporozoites. Low-level expression of CSP at stages of sporogony before the parasites migrate to the salivary gland might interfere with signal detected 61 . Several studies have reported false positive results when targeting CSP especially in zoophilic mosquitoes 62 , 63 . The false positive results could lead to an overestimation of mosquito infection rates. To achieve a conservative estimate of mosquito infection rates, we implemented stringent steps as indicated below:
Bisected mosquitoes : We observed previously 61 that a signal detected from an earlier stage of sporogony might interfere with interpretation of sporozoite detection, probably causing false positive results. We bisected the mosquitoes anterior to the thorax–abdomen junction under a stereo microscope before processing them for infection detection 64 . The head and thoraces were processed and stored separately from the abdomen of the mosquitoes. We only used the head and thorax part for infection detection following homogenization in a robust semi-high-throughput mini-bead beater protocol we developed previously 65 . The heads and thoraces of the mosquitoes were homogenized in 150 µl molecular-grade water that contains 0.2 g zirconium bead (1 mm diameter) using a Mini-Bead Beater 96. Part of the homogenate (50 µl) was used for nucleic acid extraction using cetyl trimethyl ammonium bromide 62 ; 100 µl grinding buffer (0.5% w/v case in, 0.1 N NaOH in 10 mM PBS, pH 7.4, and 0.5% IGPAL CA-630) was added to the remaining that was used to screen samples for circumsporozoite in bead-based assay.
Circumsporozoite bead-based assay : We adopted the most advanced (highly sensitive) bead-based assay for infection detection in mosquitoes 66 by targeting CSP. Antibody-coupled magnetic beads and biotinylated secondary antibodies were obtained from the Centers for Disease Control and Prevention, Division of Parasitic Diseases and Malaria, Entomology Branch, Atlanta, GA, USA, and implemented as described before 7 and were run using MagPix immunoanalyzer (Luminex Corp, CN-0269-01).
Quality control to reduce cross-reactivity : The bead-based assay we adopted may eliminate false negatives due to lower limit of detection than previous ELISA-based assays 66 but also brings a challenge of enhanced detection of cross-reacting proteins. To reduce this chance, mosquito homogenate was boiled at 100 °C before processing to eliminate false positives that may be caused by heat-unstable cross-reactive proteins to strengthen the validity of the results. To ascertain this specificity issue, we have included colony-maintained An. arabiensis and An. stephensi mosquitoes fed on sugar solution and patients’ blood in direct membrane feeding assays (had infection status determined morphologically in the same mosquito batches) that were used as negative and positive controls, respectively. Plasmodium -infected mosquitoes were used as positive controls along with sugar-fed mosquitoes as negative controls in every extraction round (Supplementary Fig. 3 and Supplementary Tables 1 and 2 ).
Retesting and confirmatory 18S-based species-specific PCR : Samples with higher mean fluorescence intensity signal than the negative controls plus three standard deviations and a representative set of mosquitoes that gave low signal were rerun to confirm the observations. Genomic DNA extracted from the head and thoraces of all mosquitoes was tested on a PCR that targeted 18S small ribosomal subunit gene as a confirmatory test. Only mosquito samples positive by the CSP-based assays and 18S-based PCR were considered infected.
Nucleic acid was extracted from the abdomen of fully engorged mosquitoes for bloodmeal source identification following the same procedure used for the head and thoraces using a cetyltrimethylammonium bromide (CTAB)-based method as described before 67 . A multiplex PCR assay that amplifies the cytochrome b gene based on Kent and Norris 68 was used for bloodmeal source analysis. We have introduced slight modifications to improve product size separation on gel electrophoresis. The multiplex of cow and dog was separately done from the multiplex of goat and human. The optimized PCR thermal cycler conditions were: 5 min at 95 °C as an initial denaturation followed by 40 cycles of denaturation at 95 °C for 60 s, annealing at 56 °C for 60 s for cow and dog multiplex, and 62 °C for goat and human multiplex, followed by an extension at 72 °C for 60 s, and 1 cycle of the final extension at 72 °C for 7 min.
Confirmation of the Anopheles morphological identification was done following a recently published protocol that targets the ITS2 gene 69 . An. stephensi diagnostic amplicon of 438 bp size was expected while a universal amplicon of varying sizes (>600 bp), depending on the length of ITS2 in a particular species, was expected in this multiplex protocol (Supplementary Fig. 4 ). The universal amplicon was used to serve as an internal control to rule out PCR failure.
Data management and analysis
Data management.
Study data collection tools (mobile application version 5.20.11) were prepared and managed using REDCap electronic data capture tools hosted at AHRI. CSV files exported from REDCap were analyzed using STATA 17 (StataCorp), RStudio v.2022.12.0.353 (Posit, 2023), QGIS v.3.22.16 (QGIS Development Team, 2023, QGIS Geographic Information System, Open Source Geospatial Foundation Project), GraphPad Prism 5.03 (GraphPad Software) and RStudio using packages lme4 (generalized linear mixed models) and dcifer 70 (pairwise relatedness analysis on P. falciparum genotypes in diverse loci).
Description of study variables
We collected the following variables in this study:
Sociodemographic: sex, age, educational level and occupation
Household characteristics: main materials used for building the household, fuel source, water source and presence of water bodies near the household/dormitory, and presence of livestock
Intervention: presence, number, and condition of bed nets, use of bed nets, use of smoke repellents or aerosol mosquito spray, and history of insecticide residual spray
Diagnosis and treatment: malaria test result by RDTs and microscopy, temperature, presence of symptoms and treatment history, and pregnancy status
Human behavior: travel history, health seeking behavior, sleeping and waking time, and sleeping place
Entomological survey: mosquito collection method and time of collection, mosquito species detected and density, Anopheles species detected and density, abdominal status of mosquitoes detected, type of aquatic habitat near the household/dormitory, and type and characteristics of water sources detected within 100-m radius around the household/dormitory
Bioinformatic analysis
FASTQ files from multiplexed amplicon sequencing of P. falciparum were subjected to filtering, demultiplexing and allele inference using a Nextflow-based pipeline 71 . We used cut adapt to demultiplex reads for each locus based on the locus primer sequences (no mismatches or indels allowed), filter reads by length (100 base pairs) and quality (default NextSeq quality trimming). We used dada2 to infer variants and remove chimeras. Reads with a Phred quality score of less than 5 were truncated. The leftmost base was trimmed and trimmed reads of less than 75 base pairs were filtered out. Default values were used for all other parameters. We then aligned alleles to their reference sequence and filtered out reads with low alignment. We masked homopolymers and tandem repeats to avoid false positives.
Genetic analysis
Pairwise relatedness analysis was performed on P. falciparum genotypes in diverse loci using Dcifer with default settings 70 . Pairwise relatedness was only considered between samples where the lower 95% CI of estimated relatedness was greater than 0.1. Point estimates of pairwise relatedness that satisfied this threshold were then binned into low, medium and high relatedness at greater than 0.2, 0.5 and 0.9 respectively. Samples were then clustered based on pairwise relatedness. Drug resistance marker genotypes were extracted from loci of interest. Evidence of pfhrp2 and pfhrp3 deletions were identified from a drop in normalized coverage in amplicons within and surrounding pfhrp2 and pfhrp3 . Complexity of infection was estimated by taking the 0.97 quantile (fifth highest number) of observed alleles across loci to minimize the impact of false positives on estimates.
Epidemiological analysis
We used standard case–control analyses to examine the association between risk factors and malaria infection. It calculates point estimates and CIs for the OR along with the significance level based on the chi-squared test. Continuous variables were presented as median and IQR. Tests of association between two categorical variables were performed using chi-squared test on contingency tables. Mann–Kendall statistical test was used to test for monotonic (increasing or decreasing) trends of malaria cases using the secondary data obtained from the private and public health facilities at the city and DDU.
Spatial data analysis
As the dormitories within the university study site were located within a small area (20 buildings in 45,450 m 2 area), clustering of prevalence data was assessed in the city only. The prevalence of malaria by RDT and/or microscopy was calculated for each household. Global and local Moran’s I calculations were used to estimate the level of spatial autocorrelation within household prevalence data. The statistical strength of association for global Moran’s I was calculated using Monte-Carlo methods based on 9,999 times permutations of the prevalence data. The Euclidean distance from the river to every site where adult or larval An. stephensi were located were calculated in meters.
Statistical analysis
To identify the association of An. stephensi and other risk factors for malaria positivity and quantify the variation in a parasite positive outcome in Dire Dawa, we employed a multilevel logistic regression model with nested random effects (heterogeneous household and case–control group variances) to account for intra-class correlation 72 . The covariates included for the multi-level logistic regression analysis with random effect are listed in detail in Supplementary Table 3 . Having more than 30 potential covariates associated to malaria, more than one billion models for exhaustive best model searching (excluding interactions between covariates), we reduced the number of covariates to a manageable size by considering univariate generalized mixed models (with case index as random effect instead of setting which were not contributing to the differences in malaria positivity for cases and controls) and considering only the covariates with P value lower than 0.3 within these models (Supplementary Table 3 ). The decision to use case/control as random effect instead of fixed effect came from preliminary analysis that considered the best candidate(s) for random effects. Variable selection was performed by testing 2,000+ binomial logistic mixed models (number of tested models depending on initial screening). During the initial screening, a candidate variable was selected if its P value, obtained from a Wald test applied to the variable’s estimated coefficient in logistic regression, was lower than 0.3. The models were ranked on the basis of their Akaike Information Criteria (AIC) and the Bayes information criteria (BIC) values, with the top model being the one with the lowest AIC value 73 . Variable selection was repeated for three different response variables: model 1 with response RDT/microscopy, model 2 with response RDT/microscopy/qPCR, and, finally, model 3 with response qPCR. As a result, only five of the 12 factors assessed for individual and household characteristics (sex, age, An. stephensi larvae and/or adult presence, natural waterbody existence, and use of aerosol insecticide spray) were included for the final model (Supplementary Table 4 ). We also explored interactions between gender, age and site.
After model selection with several model outcomes and distribution (Supplementary Table 4 ), the binomial model with outcome represented by malaria positivity (positive/negative) using RDT and/or microscopy best represented the relationship between malaria and risk factors (Supplementary Table 4 ) 74 . In this model, the employment of geographic unit’s effects such as household and area setting (city versus university) enabled us to control for unknown variations by including them as random effects in the model. In fact, individuals living in the same household may share exposures that can determine similarities in malaria transmission as well as in the larger setting (city versus university).
Let y ij denote the malaria outcome of the i th individual in the j th household or cluster, identified by the RDT and/or microscopy with probability π ij where y ij = 1 denotes the individual tested positive, while y ij = 0 denotes the individual tested negative for malaria. A multilevel logistic regression model with random effects for the outcome y ij is given by
where X ij = (1, x 1 ij ,…, x pij ) is vector of p explanatory variables or covariates measured on the i individual and on the j household (cluster), β is vector of fixed regression coefficients or parameters and u j is a random effect varying over household and case control.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All the data used in the paper are available on dryad (linked with the ORCID: https://orcid.org/0000-0003-1931-1442 ). Sequence data are deposited on NCBI with the BioProject accession number PRJNA962166 . Raw data of the study will be available in the future upon request following signing of data sharing agreement, abiding to institutional and international data sharing guidelines. Source data are provided with this paper.
Code availability
The R codes used to run the analyses reported in this study can be found at https://github.com/legessealamerie/DD-Stephensi and https://github.com/EPPIcenter/mad4hatter .
World malaria report 2022. World Health Organization https://www.who.int/publications/i/item/9789240064898 (2022).
Wilson, M. L. et al. Urban malaria: understanding its epidemiology, ecology, and transmission across seven diverse ICEMR network sites. Am. J. Trop. Med Hyg. 93 , 110–123 (2015).
PubMed PubMed Central Google Scholar
Productive, livable cities will open Africa’s doors to the world. The World Bank https://www.worldbank.org/en/region/afr/publication/africa-cities-opening-doors-world (2017).
Global framework for the response to malaria in urban areas. World Health Organization https://books.google.com.et/books?hl=en&lr=&id=hF-qEAAAQBAJ&oi=fnd&pg=PR5&dq=urban+malaria+framework+who&ots=wv_CFQdXdP&sig=nKgU0hKlXS3Hk3ErQtWNliEbrIU&redir_esc=y#v=onepage&q=urban%20malaria%20framework%20who&f=false (2022).
Molina Gomez, K. et al. Characterizing the malaria rural-to-urban transmission interface: the importance of reactive case detection. PLoS Negl. Trop. Dis. 11 , e0005780 (2017).
Azrag, R. S. & Mohammed, B. H. Anopheles arabiensis in Sudan: a noticeable tolerance to urban polluted larval habitats associated with resistance to Temephos. Malar. J. 17 , 204 (2018).
Malaria threats map. World Health Organization https://apps.who.int/malaria/maps/threats/#/maps?theme=invasive&map (2023).
Balkew, M. et al. Geographical distribution of Anopheles stephensi in eastern Ethiopia. Parasit. Vectors 13 , 35 (2020).
Tadesse, F. G. et al. Anopheles stephensi mosquitoes as vectors of Plasmodium vivax and falciparum , Horn of Africa, 2019. Emerg. Infect. Dis. 27 , 603–607 (2021).
Sinka, M. E. et al. The dominant Anopheles vectors of human malaria in the Asia-Pacific region: occurrence data, distribution maps and bionomic précis. Parasit. Vectors 4 , 89 (2011).
Faulde, M. K., Rueda, L. M. & Khaireh, B. A. First record of the Asian malaria vector Anopheles stephensi and its possible role in the resurgence of malaria in Djibouti, Horn of Africa. Acta Trop. 139 , 39–43 (2014).
PubMed Google Scholar
Carter, T. E. et al. First detection of Anopheles stephensi Liston, 1901 (Diptera: Culicidae) in Ethiopia using molecular and morphological approaches. Acta Trop. 188 , 180–186 (2018).
Ahmed, A. et al. Invasive malaria vector Anopheles stephensi mosquitoes in Sudan, 2016–2018. Emerg. Infect. Dis. 27 , 2952–2954 (2021).
Ali, S., Samake, J. N., Spear, J. & Carter, T. E. Morphological identification and genetic characterization of Anopheles stephensi in Somaliland. Parasit. Vectors 15 , 247 (2022).
CAS PubMed PubMed Central Google Scholar
Partners convening: a regional response to the invasion of Anopheles stephensi in Africa: meeting report, 8–10 March 2023. World Health Organization https://apps.who.int/iris/bitstream/handle/10665/369368/9789240075535-eng.pdf?sequence=1 (2023).
Allan, R. et al. Confirmation of the presence of Anopheles stephensi among internally displaced people’s camps and host communities in Aden city, Yemen. Malar J. 22 , 1 (2022).
Google Scholar
Ochomo, E. O. et al. Molecular surveillance leads to the first detection of Anopheles stephensi in Kenya. Preprint at Research Square https://doi.org/10.21203/rs.3.rs-2498485/v2 (2023).
Balkew, M. et al. An update on the distribution, bionomics, and insecticide susceptibility of Anopheles stephensi in Ethiopia, 2018–2020. Malar. J. 20 , 263 (2021).
WHO Initiative to Stop the Spread of Anopheles stephensi in Africa https://iris.who.int/bitstream/handle/10665/372259/WHO-UCN-GMP-2023.06-eng.pdf?sequence=1 (World Health Organization, 2022).
Balikagala, B. et al. Evidence of artemisinin-resistant malaria in Africa. N. Engl. J. Med. 385 , 1163–1171 (2021).
CAS PubMed Google Scholar
Uwimana, A. et al. Emergence and clonal expansion of in vitro artemisinin-resistant Plasmodium falciparum kelch13 R561H mutant parasites in Rwanda. Nat. Med. 26 , 1602–1608 (2020).
Mihreteab, S. et al. Increasing prevalence of artemisinin-resistant HRP2-negative malaria in Eritrea. N. Engl. J. Med. 389 , 1191–1202 (2023).
Feleke, S. M. et al. Plasmodium falciparum is evolving to escape malaria rapid diagnostic tests in Ethiopia. Nat. Microbiol. 6 , 1289–1299 (2021).
Berhane, A. et al. Major threat to malaria control programs by Plasmodium falciparum lacking histidine-rich protein 2, Eritrea. Emerg. Infect. Dis. 24 , 462–470 (2018).
Iriart, X. et al. Misdiagnosis of imported falciparum malaria from African areas due to an increased prevalence of pfhrp2 / pfhrp3 gene deletion: the Djibouti case. Emerg. Microbes Infect. 9 , 1984–1987 (2020).
Seyfarth, M., Khaireh, B. A., Abdi, A. A., Bouh, S. M. & Faulde, M. K. Five years following first detection of Anopheles stephensi (Diptera: Culicidae) in Djibouti, Horn of Africa: populations established—malaria emerging. Parasitol. Res 118 , 725–732 (2019).
Getachew, D., Tekie, H., Gebre-Michael, T., Balkew, M. & Mesfin, A. Breeding sites of Aedes aegypti : potential dengue vectors in Dire Dawa, East Ethiopia. Interdiscip. Perspect. Infect. Dis. 2015 , 706276 (2015).
Loha, E. & Lindtjørn, B. Predictors of Plasmodium falciparum malaria incidence in Chano Mille, South Ethiopia: a longitudinal study. Am. J. Trop. Med. Hyg. 87 , 450–459 (2012).
Landgraf, B., Kollaritsch, H., Wiedermann, G. & Wernsdorfer, W. H. Parasite density of Plasmodium falciparum malaria in Ghanaian schoolchildren: evidence for influence of sex hormones? Trans. R. Soc. Trop. Med. Hyg. 88 , 73–74 (1994).
Camargo, L. et al. Hypoendemic malaria in Rondonia (Brazil, western Amazon region): seasonal variation and risk groups in an urban locality. Am. J. Trop. Med. Hyg. 55 , 32–38 (1996).
Bannister-Tyrrell, M. et al. Forest goers and multidrug-resistant malaria in Cambodia: an ethnographic study. Am. J. Trop. Med. Hyg. 100 , 1170–1178 (2019).
Bruce-Chwatt, L. J., Garrett-Jones, C. & Weitz, B. Ten years’ study (1955–64) of host selection by anopheline mosquitos. Bull. World Health Organ. 35 , 405–439 (1966).
Haile, D. & Lakew, Y. Khat chewing practice and associated factors among adults in Ethiopia: further analysis using the 2011 demographic and health survey. PLoS ONE 10 , e0130460 (2015).
Desai, M. et al. Epidemiology and burden of malaria in pregnancy. Lancet Infect. Dis. 7 , 93–104 (2007).
Briggs, J. et al. Sex-based differences in clearance of chronic Plasmodium falciparum infection. eLife 9 , e59872 (2020).
Montenegro, C. C. et al. Plasmodium falciparum outbreak in native communities of Condorcanqui, Amazonas, Perú. Malar. J. 20 , 88 (2021).
Obaldia, N. 3rd et al. Clonal outbreak of Plasmodium falciparum infection in eastern Panama. J. Infect. Dis. 211 , 1087–1096 (2015).
Tadesse, F. G. et al. The relative contribution of symptomatic and asymptomatic Plasmodium vivax and Plasmodium falciparum infections to the infectious reservoir in a low-endemic setting in Ethiopia. Clin. Infect. Dis. 66 , 1883–1891 (2018).
Andolina, C. et al. Sources of persistent malaria transmission in a setting with effective malaria control in eastern Uganda: a longitudinal, observational cohort study. Lancet Infect. Dis. 21 , 1568–1578 (2021).
Kibret, S., Wilson, G. G., Tekie, H. & Petros, B. Increased malaria transmission around irrigation schemes in Ethiopia and the potential of canal water management for malaria vector control. Malar. J. 13 , 360 (2014).
Moin-Vaziri, V. et al. Molecular detection of Plasmodium infection among Anophelinae mosquitoes and differentiation of biological forms of Anopheles stephensi collected from malarious areas of Afghanistan and Iran. Ethiop. J. Health Sci. 32 , 269–278 (2022).
Smith, D. L., Dushoff, J., Snow, R. W. & Hay, S. I. The entomological inoculation rate and Plasmodium falciparum infection in African children. Nature 438 , 492–495 (2005).
Kiattibutr, K. et al. Infectivity of symptomatic and asymptomatic Plasmodium vivax infections to a Southeast Asian vector, Anopheles dirus . Int. J. Parasitol. 47 , 163–170 (2017).
Okell, L. C. et al. Factors determining the occurrence of submicroscopic malaria infections and their relevance for control. Nat. Commun. 3 , 1237 (2012).
Whittaker, C. et al. Global patterns of submicroscopic Plasmodium falciparum malaria infection: insights from a systematic review and meta-analysis of population surveys. Lancet Microbe 2 , e366–e374 (2021).
Nega, D. et al. Baseline malaria prevalence at the targeted pre-elimination districts in Ethiopia. BMC Public Health 21 , 1996 (2021).
Bayih, A. G. et al. A unique Plasmodium falciparum K13 gene mutation in Northwest Ethiopia. Am. J. Trop. Med. Hyg. 94 , 132–135 (2016).
Alemayehu, A. et al. Expansion of the Plasmodium falciparum Kelch 13 R622I mutation in Northwest Ethiopia. Research Square https://www.researchsquare.com/article/rs-171038/latest.pdf (2021).
Fola, A. A. et al. Plasmodium falciparum resistant to artemisinin and diagnostics have emerged in Ethiopia. Nat. Microbiol. 8 , 1911–1919 (2023).
Wang, X. et al. Molecular surveillance of Pfcrt and k13 propeller polymorphisms of imported Plasmodium falciparum cases to Zhejiang Province, China between 2016 and 2018. Malar. J. 19 , 59 (2020).
Gamboa, D. et al. A large proportion of P. falciparum isolates in the Amazon region of Peru lack pfhrp2 and pfhrp3 : implications for malaria rapid diagnostic tests. PLoS ONE 5 , e8091 (2010).
Prosser, C. et al. Plasmodium falciparum histidine-rich protein 2 and 3 gene deletions in strains from Nigeria, Sudan, and South Sudan. Emerg. Infect. Dis. 27 , 471–479 (2021).
Lynch, E. et al. Evaluation of HRP2 and pLDH-based rapid diagnostic tests for malaria and prevalence of pfhrp2/3 deletions in Aweil, South Sudan. Malar. J. 21 , 261 (2022).
Yared, S. et al. Insecticide resistance in Anopheles stephensi in Somali Region, eastern Ethiopia. Malar. J. 19 , 180 (2020).
Robert, L. L., Schneider, I. & Wirtz, R. A. Deet and permethrin as protectants against malaria-infected and uninfected Anopheles stephensi mosquitoes. J. Am. Mosq. Control Assoc. 7 , 304–306 (1991).
Smith, P. G. & Day, N. E. The design of case–control studies: the influence of confounding and interaction effects. Int. J. Epidemiol. 13 , 356–365 (1984).
Hermsen, C. C. et al. Detection of Plasmodium falciparum malaria parasites in vivo by real-time quantitative PCR. Mol. Biochem. Parasitol. 118 , 247–251 (2001).
Wampfler, R. et al. Strategies for detection of Plasmodium species gametocytes. PLoS ONE 8 , e76316 (2013).
Tessema, S. K. et al. Sensitive, highly multiplexed sequencing of microhaplotypes from the Plasmodium falciparum heterozygome. J. Infect. Dis. 225 , 1227–1237 (2022).
Aranda-Diaz, A. & Neubauer Vickers, E. MAD4HatTeR V.3. protocols.io https://doi.org/10.17504/protocols.io.14egn779mv5d/v3 (2022).
Hendershot, A. L. et al. A comparison of PCR and ELISA methods to detect different stages of Plasmodium vivax in Anopheles arabiensis . Parasit. Vectors 14 , 473 (2021).
Durnez, L. et al. False positive circumsporozoite protein ELISA: a challenge for the estimation of the entomological inoculation rate of malaria and for vector incrimination. Malar. J. 10 , 195 (2011).
Bashar, K., Tuno, N., Ahmed, T. U. & Howlader, A. J. False positivity of circumsporozoite protein (CSP)-ELISA in zoophilic anophelines in Bangladesh. Acta Trop. 125 , 220–225 (2013).
Foley, D. H. et al. Mosquito bisection as a variable in estimates of PCR-derived malaria sporozoite rates. Malar. J. 11 , 145 (2012).
Graumans, W. et al. Semi-high-throughput detection of Plasmodium falciparum and Plasmodium vivax oocysts in mosquitoes using bead-beating followed by circumsporozoite ELISA and quantitative PCR. Malar. J. 16 , 356 (2017).
Sutcliffe, A. C. et al. Adaptation of ELISA detection of Plasmodium falciparum and Plasmodium vivax circumsporozoite proteins in mosquitoes to a multiplex bead-based immunoassay. Malar. J. 20 , 377 (2021).
Echeverry, D. F. et al. Fast and robust single PCR for Plasmodium sporozoite detection in mosquitoes using the cytochrome oxidase I gene. Malar. J. 16 , 230 (2017).
Kent, R. J. & Norris, D. E. Identification of mammalian blood meals in mosquitoes by a multiplexed polymerase chain reaction targeting cytochrome B. Am. J. Trop. Med. Hyg. 73 , 336–342 (2005).
Singh, O. P. et al. Molecular tools for early detection of invasive malaria vector Anopheles stephensi mosquitoes. Emerg. Infect. Dis. 29 , 36–44 (2023).
Gerlovina, I., Gerlovin, B., Rodríguez-Barraquer, I. & Greenhouse, B. Dcifer: an IBD-based method to calculate genetic distance between polyclonal infections. Genetics 222 , iyac126 (2022).
mad4hatter. GitHub https://github.com/EPPIcenter/mad4hatter
Goldstein, H. & Browne, W. in Contemporary Psychometrics (eds Maydeu-Olivares, A. & McArdle, J. J.) Ch. 14 (Taylor & Francis Group, 2005).
Burnham, K. P. & Anderson, D. R. (eds) Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach 2nd edn (Springer, 2002).
Austin, P. C., Goel, V. & van Walraven, C. An introduction to multilevel regression models. Can. J. Public Health 92 , 150–154 (2001).
Download references
Acknowledgements
F.G.T. was supported by the Bill and Melinda Gates Foundation (ACHIDES; INV-005898 and EMAGEN; INV-035257) and Wellcome Trust Early Career Award (102348). L.S. was supported by the Wellcome Trust NIHR–Wellcome Partnership for Global Health Research Collaborative Award (CEASE; 220870/Z/20/Z). T.B. was supported by a European Research Council Consolidator Grant (ERC-CoG 864180; QUANTUM) and a VICI fellowship from the Dutch Research Council (NWO; grant number 09150182210039). B.G. was supported by NIH/NIAID K24 AI144048. We acknowledge the WHO and the countries that provided the underlying data for the Global Malaria Threats map that we used to make Fig. 1a . The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript. The findings and conclusions in this paper are those of the authors and do not necessarily represent the official position of the US Centers for Disease Control and Prevention.
Author information
These authors contributed equally: Tadele Emiru, Dejene Getachew, Maxwell Murphy, Luigi Sedda.
Authors and Affiliations
Armauer Hansen Research Institute, Addis Ababa, Ethiopia
Tadele Emiru, Legesse Alamerie Ejigu, Mikiyas Gebremichael Bulto, Mulugeta Demisse, Melat Abdo, Wakweya Chali, Lina Alemayehu, Sinknesh W. Behaksera, Gutema Jebessa, Tizita Tsegaye & Fitsum G. Tadesse
Adama Science and Technology University, Adama, Ethiopia
Dejene Getachew & Hunduma Dinka
EPPIcenter program, Division of HIV, ID and Global Medicine, University of California, San Francisco, San Francisco, CA, USA
Maxwell Murphy, Aaron Elliott, Eric Neubauer Vickers, Andrés Aranda-Díaz & Bryan Greenhouse
Lancaster Ecology and Epidemiology Group, Lancaster Medical School, Lancaster University, Lancaster, UK
Luigi Sedda
London School of Hygiene and Tropical Medicine, London, UK
Isabel Byrne, Chris Drakeley & Fitsum G. Tadesse
Radboudumc, Nijmegen, the Netherlands
Wakweya Chali, Teun Bousema & Fitsum G. Tadesse
U.S. President’s Malaria Initiative, USAID, Addis Ababa, Ethiopia
Hiwot Teka, Sheleme Chibsa, Peter Mumba & Samuel Girma
U.S. President’s Malaria Initiative, Malaria Branch, US Centers for Disease Control and Prevention, Atlanta, GA, USA
Jimee Hwang & Jon Eric Tongren
U.S. President’s Malaria Initiative, USAID, Washington DC, DC, USA
Melissa Yoshimizu
U.S. President’s Malaria Initiative, Entomology Branch, US Centers for Disease Control and Prevention, Atlanta, GA, USA
Alice Sutcliffe & Sarah Zohdy
Federal Ministry of Health, Addis Ababa, Ethiopia
Hiwot Solomon Taffese & Gudissa Aseffa Bayissa
You can also search for this author in PubMed Google Scholar
Contributions
F.G.T., T.B., C.D., J.E.T., S.Z., G.A.B., H.S.T., J.H., M.Y., S.G., P.M., S.C., H.T., H.D. and T.E. conceived the study; T.E., D.G., M.G.B., G.J., T.T. and F.G.T. executed the study data and sample collection; M.M., M.A., W.C., A.E., E.N.V., A.A.-D., L.A., S.W.B., A.S. and F.G.T. run the laboratory experiments; T.E., M.M., L.S., L.A.E., M.G.B., I.B., M.D., C.D., B.G., T.B. and F.G.T. analyzed the data; T.E., D.G., M.M., L.S., L.A.E., M.G.B., I.B., M.D., G.A.B., H.S.T., J.H., M.Y., A.S., S.Z., J.E.T., C.D., B.G., T.B. and F.G.T. drafted the paper. All authors read and approved the final version.
Corresponding author
Correspondence to Fitsum G. Tadesse .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Peer review
Peer review information.
Nature Medicine thanks David Fidock, Didier Menard, and Tamar Carter for their contribution to the peer review of this work. Primary Handling Editor: Alison Farrell, in collaboration with the Nature Medicine team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Supplementary information, supplementary information.
Supplementary information.
Reporting Summary
Source data tables 1–3.
This file contains the source dataset for the three tables that are part of the main text of the paper. It contains three tabs that are labeled: Source_Data_Table_1, Source_Data_Table_2 and Source_Data_Table_3.
Source Data Fig. 1
Global An. stephensi distribution (native and invasive range) and detection of Plasmodium infection for Fig. 1a and geographic location of the study area (households/dormitories, aquatic habitats and health facilities) for Fig. 1b source data.
Source Data Fig. 2
Source data obtained from the national DHIS2 repository for Dire Dawa between January 2013 and May 2022 and data collected during the study period from the study participants infection status (as measured by RDTs, microscopy and quantitative species-specific 18S-based PCR) in separate Excel sheet for Fig. 2a–e.
Source Data Fig. 3
Source data on the geographic location of infections detected in the study households (Fig. 3a), Anopheles mosquito presence (Fig. 3b) and overlap of Plasmodium infection detected in study participants and An. stephensi in the respective households (Fig. 3c) and pairwise genetic relatedness data (Fig. 3d) and their clustering (Fig. 3e).
Source Data Extended Data Table 1
Source data collected from 34 private and public health facilities located in Dire Dawa for the period between January 2019 and May 2022.
Source Data Extended Data Table 2
Source data on symptom status (axillary temperature ≥37.5 °C) by study site and participant category.
Source Data Extended Data Table 3
Sociodemographic, intervention utilization and malaria predisposing factors related to malaria infection source data.
Source Data Extended Data Table 4
Infection status (as measured by RDTs, microscopy and qPCR) source data.
Source Data Extended Data Table 5
Source data on infection status (as measured by RDTs, microscopy and qPCR as well as parasite density determined by the latter).
Source Data Extended Data Table 6
Source data related to adult and larvae mosquito surveys. Individual adult mosquito data including date, method and place of collection, and abdominal status included.
Source Data Extended Data Table 7
Source data extended data table 8, source data extended data table 9.
Source data collected during the study period from the study participants on infection status (as measured by RDTs and microscopy), sociodemographic status, intervention utilization and malaria predisposing factors related to parasite lineages.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Emiru, T., Getachew, D., Murphy, M. et al. Evidence for a role of Anopheles stephensi in the spread of drug- and diagnosis-resistant malaria in Africa. Nat Med 29 , 3203–3211 (2023). https://doi.org/10.1038/s41591-023-02641-9
Download citation
Received : 22 April 2023
Accepted : 12 October 2023
Published : 26 October 2023
Issue Date : December 2023
DOI : https://doi.org/10.1038/s41591-023-02641-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
- Open access
- Published: 03 February 2023
Leveraging innovation technologies to respond to malaria: a systematized literature review of emerging technologies
- Moredreck Chibi 1 ,
- William Wasswa 1 ,
- Chipo Ngongoni 1 ,
- Ebenezer Baba 2 &
- Akpaka Kalu 2
Malaria Journal volume 22 , Article number: 40 ( 2023 ) Cite this article
5210 Accesses
2 Altmetric
Metrics details
In 2019, an estimated 409,000 people died of malaria and most of them were young children in sub-Saharan Africa. In a bid to combat malaria epidemics, several technological innovations that have contributed significantly to malaria response have been developed across the world. This paper presents a systematized review and identifies key technological innovations that have been developed worldwide targeting different areas of the malaria response, which include surveillance, microplanning, prevention, diagnosis and management.
A systematized literature review which involved a structured search of the malaria technological innovations followed by a quantitative and narrative description and synthesis of the innovations was carried out. The malaria technological innovations were electronically retrieved from scientific databases that include PubMed, Google Scholar, Scopus, IEEE and Science Direct. Additional innovations were found across grey sources such as the Google Play Store, Apple App Store and cooperate websites. This was done using keywords pertaining to different malaria response areas combined with the words “innovation or technology” in a search query. The search was conducted between July 2021 and December 2021. Drugs, vaccines, social programmes, and apps in non-English were excluded. The quality of technological innovations included was based on reported impact and an exclusion criterion set by the authors.
Out of over 1000 malaria innovations and programmes, only 650 key malaria technological innovations were considered for further review. There were web-based innovations (34%), mobile-based applications (28%), diagnostic tools and devices (25%), and drone-based technologies (13%.
Discussion and conclusion
This study was undertaken to unveil impactful and contextually relevant malaria innovations that can be adapted in Africa. This was in response to the existing knowledge gap about the comprehensive technological landscape for malaria response. The paper provides information that countries and key malaria control stakeholders can leverage with regards to adopting some of these technologies as part of the malaria response in their respective countries.
The paper has also highlighted key drivers including infrastructural requirements to foster development and scaling up of innovations. In order to stimulate development of innovations in Africa, countries should prioritize investment in infrastructure for information and communication technologies and also drone technologies. These should be accompanied by the right policies and incentive frameworks.
In sub-Saharan Africa, malaria is the leading cause of death for children under 5. It has been reported that malaria infection during pregnancy increases the risk of maternal mortality and neonatal mortality [ 1 ]. According to the World Health Organization (WHO), there were 229 million cases of malaria in 2019 compared to 228 million cases in 2018. The estimated number of malaria deaths stood at 409,000 in 2019, compared with 411,000 deaths in 2018. Children under 5 years of age are the most vulnerable group affected by malaria and in 2019 they accounted for 67% (274,000) of all malaria deaths worldwide. The WHO African Region continues to carry a disproportionately high share of the global malaria burden. In 2019, the region was home to 94% of all malaria cases and deaths with six countries accounting for approximately half of all malaria deaths worldwide: Nigeria (23%), the Democratic Republic of the Congo (11%), United Republic of Tanzania (5%), Burkina Faso (4%), Mozambique (4%) and Niger (4%) [ 2 ].
Knowledge, learning and innovation are key to addressing, minimizing and tackling these disparities. One example of this is the knowledge hub developed by WHO called MAGICapp which aims to give living evidence and resources for tackling malaria interventions. It contains all official WHO recommendations for malaria prevention (vector control and preventive chemotherapies) and case management (diagnosis and treatment). The resources serve as a guide on the strategic use of information to drive impact, surveillance, monitoring and evaluation; operational manuals, handbooks, and frameworks; and a glossary of key terms and definitions. So, this paper aligns with identifying and adding discourse into the importance of reviews especially from a technological perspective.
To understand the advances in malaria services, various scholars have undertaken reviews across vast thematic areas of malaria interventions. In a quest to inform policy, Garner et al. [ 3 ] conducted an analysis of why Cochrane Reviews are important in malaria interventions. They noted that it is important for researchers to collaborate across regions and in understanding new preventive interventions. Their aim was to inform policymakers to understand the importance of reviews in identification of trends that are occurring in malaria interventions. Other aspects that have been looked at through reviews are the costs and cost-effectiveness aligned with malaria control interventions. White et al. [ 4 ] looked at interventions from studies published between 2000 and 2010 looking at the role of infection detection technologies for malaria elimination and eradication and the costs related to them in order to assess how accessible interventions are across regions. More recently, Conteh et al. [ 5 ] also carried on with assessing the unit cost and cost-effectiveness of malaria control during the period of January 1, 2005, and August 31, 2018. The aim was to see how resource allocation can be planned proactively according to costs, though they did highlight that care in methodological and reporting standards is required to enhance data transferability.
In a bid to combat malaria epidemic, several technological innovations have been developed all over the world that have contributed significantly to malaria response. Adeola et al. [ 6 ] reviewed the use of spatial technology for malaria epidemiology in South Africa between 1930 and 2013. The focus was on the use of statistical and mathematical models as well as geographic information science (GIS) and remote sensing (RS) technology for malaria research to create a robust malaria warning system. The mathematical modelling is also aligned with agent-based modelling which Smith et al. [ 7 ] highlighted through their analysis of 90 articles published between 1998 and May 2018 characterizing agent-based models (ABMs) relevant to malaria transmission. The aim was to provide an overview of key approaches utilized in malaria prevention. Such technologies feed into modelling sites and interventions to project various outcomes. From a platform centric perspective, Vasiman et al. [ 8 ] analysed how different mobile phone devices and handheld microscopes work as diagnostic platforms for malaria in low-resource settings. Malaria diagnostics tests and methods have also been reviewed as being key in the successful control and elimination programmes [ 9 ]. Mobile health has been found to play a key role in supporting health workers in the diagnosis and treatment of malaria in sub-Saharan Africa [ 10 ].
To add to this discourse, this paper presents a holistic systematized review of key technological innovations that have been developed worldwide targeting different areas of the malaria response, which include surveillance, microplanning, prevention, diagnosis, and management. A systematized review was utilized in this study as data sources that included unconventional grey sources was utilized and the review gravitated more towards being narrative with tabular accompaniments as compared to the systematic literature reviews that are less narrative [ 11 ]. The study was undertaken with the view to provide African countries and key stakeholders with information relating to technologies that can be adapted in their different contexts as they strengthen malaria response strategies.
Scientific databases literature search
This study adopted a systematic search strategy to identify the publications with innovations related to malaria surveillance, microplanning, prevention, diagnosis, and management from 5 scientific databases (PubMed, Google Scholar, Scopus, IEEE and Science Direct). The keywords used were malaria surveillance, microplanning, prevention, diagnosis and management combined with the words “innovations” or “technologies” in a search query. Innovations deemed not relevant to the scope of this research by the authors include drugs, vaccines, social programmes. Only papers reporting design, implementation or evaluation of malaria technological innovations were considered in this paper. The process was shown in Fig. 1 . The quality of technological innovations included was based on reported impact and judgement by the authors.
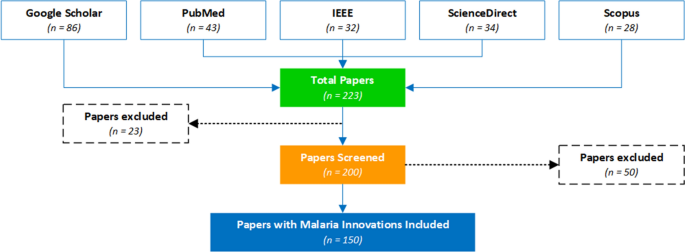
PRISMA flow chart for the malaria innovations literature search
Search through technology platforms e.g., google play store and apple app store
This study also adopted a systematic search strategy to identify the mobile apps related to malaria surveillance, microplanning, prevention, diagnosis and management available in the Google Play and Apple App stores. Keywords such as malaria surveillance, microplanning, prevention, diagnosis, and management were used in the search. The search was conducted between July 2021 and December 2021. The applications had to have a description, be in English, have 1000 + installs and reviews to be included in the analysis. The applications that did not meet these criteria were excluded. The core research question was: What mobile-based innovations are available for malaria interventions that can be adopted by the countries in the WHO Africa region for use across the continuum of the malaria response ? The resultant apps considered for this study were 260 as shown in Fig. 2 .
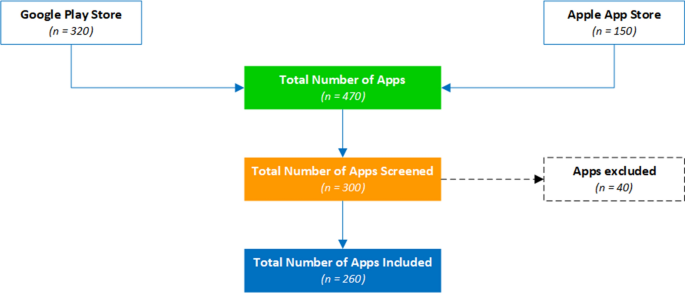
PRISMA flow chart for the mobile apps
Web search using a custom web-content mining algorithm
A custom web-content mining algorithm was also developed to search for malaria innovations and technologies published on different cooperate organizational websites, social media channels like twitter, and media channels like legit news websites like CNN. These technological innovations were collated between July 2021 and December 2021. The innovation name, description, Intellectual Property owner, web link to the innovation and geographical location were collated. Innovations that did not have functional and tested prototypes and were not related to addressing malaria interventions were excluded. The number of innovations surpassed 1000 however after screening, only 240 key technological innovations were selected that best fit the selection criteria.
A total of 650 malaria innovations (260 from Google play and Apple App store, 150 from scientific databases and 240 from web content mining) were considered for detailed review.
The review has identified innovations for malaria in the following technological thematic areas; web-based innovations (34%), mobile-based applications (28%), diagnostic tools and other devices (25%), and drone-based technologies (13%).
Web-based innovations
The web-based technologies include GIS systems [ 12 ]. An example is the Malaria Atlas Project (MAP), developed at the Telethon Kids Institute, Perth, Western Australia. MAP is a web platform that displays time aware raster and survey point data for malaria incidence, endemicity, and mosquito distribution. MAP has been designated as a WHO Collaborating Centre in Geospatial Disease Modelling. The impact of the Atlas Project has been validated in Sokoto Nigeria by Nakakana et al. [ 13 ]. The study concluded that the prevalence of malaria and its transmission intensity in Sokoto are similar to the Malaria Atlas Project predictions for the area and that is essential in modellings various aspects of malaria control planning purposes.
Other innovations like malariaAtlas which is an open-access R-interface on the Malaria Atlas Project, collates malariometric data, providing reproducible means of accessing such data within a freely available and commonly used statistical software environment [ 14 ]. A team from the University of Queensland developed a GIS-based spatial decision support system (SDSS) used to automatically locate and map the distribution of confirmed malaria cases, rapidly classify active transmission foci, and guide targeted responses in elimination zones. This has been implemented and evaluated in the Solomon Islands and Vanuatu in a study by Kelly et al. [ 15 ] and 82.5% of confirmed malaria cases were automatically geo-referenced and mapped at the household level, with 100% of remaining cases geo-referenced at a village level using the system. The GIS-based spatial decision support system has also been implemented in other countries like Vietnam. In Korea, the Malaria Vulnerability Map Mobile System which consists of a system database construction, malaria risk calculation function, visual expression function, and website and mobile application has been developed for use in Incheon [ 16 ]. The Malaria Decision Analysis Support Tool (MDAST) project promotes evidence-based, multi-sectoral malaria control policy-making in Kenya, Tanzania, and Uganda, serving as a pilot for such a programme in other malaria-prone countries [ 17 ].
In Zanzibar, the Malaria Case Notification (MCN) System was developed and the performance evaluation of the tool by Khandekar [ 18 ] showed that while a surveillance system can automate data collection and reporting, its performance will still rely heavily on health worker performance, community acceptance, and infrastructure within a country. A study by Mody et al. [ 19 ] showed that the use of telemedicine and e-health technologies shows promise for the remote diagnosis of malaria and hence several systems been developed. ProMED Mail (PMM) is an open and free to use, global, e-health based surveillance system from the International Society for Infectious Diseases with several use cases for malaria [ 20 , 21 ]. The Epidemic Prognosis Incorporating Disease and Environmental Monitoring for Integrated Assessment (EPIDEMIA) computer system was designed and implemented to integrate disease surveillance with environmental monitoring in support of operational malaria forecasting in the Amhara region of Ethiopia [ 22 ]. Table 1 summarizes some of the technologies.
Mobile applications-based technologies
This study has also revealed that several mobile-based malaria innovations have been developed which include smart mobile apps, Short Message Service (SMS) based apps and Unstructured Supplementary Service Data (USSD) based applications for use across the continuum of the malaria response. In India the Mobile-based Surveillance Quest using IT (MoSQuIT) is being used to automate and streamline malaria surveillance for all stakeholders involved, from health workers in rural India to medical officers and public health decision-makers. Malaria Epidemic Early Detection System (MEEDS) is a groundbreaking mHealth system used in Zanzibar by health facilities to report new malaria cases through mobile phones. Coconut Surveillance is an open-source mobile software application designed by malaria experts specifically for malaria control and elimination and it has become an essential tool for the Zanzibar Malaria Elimination Programme [ 23 ]. The SMS for Life initiative is a ‘public-private’ project that harnesses everyday technology to eliminate stock-outs and improve access to essential medicines in sub-Saharan Africa with a health focus on malaria and other vector borne diseases. This has been implemented and evaluated in Tanzania [ 24 ]. In Mozambique Community Health Workers (CHWs) use inSCALE CommCare tool for decision support, immediate feedback and multimedia audio and images to improve adherence to protocols.
Additional surveillance apps include the likes of the DHS mobile app for Malaria Indicator Surveys and Solution for Community Health-workers (SOCH) mobile app is a comprehensive mobile application tool for disease surveillance, workforce management and supply chain management for malaria elimination [ 25 ]. The National Malaria Case-Based Reporting App (MCBR) is a mobile phone application for malaria case-based reporting to advance malaria surveillance in Myanmar [ 26 ]. Mobile apps have also been used to support distribution of medicines like the Net4Schs App, an android application that is used for data capturing, processing and reporting on School Long-lasting insecticidal nets (LLINs) distribution activities. Apps have also been developed to support malaria screening and diagnosis for example the NLM Malaria Screener is a diagnostic app that assists users in the diagnosis of malaria and in the monitoring of malaria patients. This has been validated in several studies and it is reported that it makes the screening process faster, more consistent, and less dependent on human expertise [ 27 ]. Additional diagnostic apps include the Malaria System MicroApp which is a mobile device-based tool for malaria diagnosis [ 28 ], the Malaria Hero app is a web based mobile app for diagnosis of malaria, and LifeLens is a smartphone app that can detect malaria. Some key technologies are summarized in Table 2 .
Other notable mobile apps that have also been used in malaria management include CommCare’s usage in in Mozambique for integrated community case management in the remote communities. This has been reported to strengthen Community-Based Health [ 29 ]. Another app, FeverTracker, has been used for malaria surveillance and patient information management in India. There has also been a number of educational and knowledge base apps. These are the likes of Malaria Consultant, a mobile application designed to educate individuals on malaria and its prevention; the WHO Malaria toolkit App that brings together the content of the latest world malaria report and of the consolidated WHO Guidelines for malaria. This includes operational manuals for carrying out malaria interventions and other technical documents in one easy to navigate resource. Another interesting area where mobile apps have been used is in malaria prevention and such apps include those that scare away mosquitoes using high frequency sounds, and these include Anti Mosquito Repellent Sound App.
Drone-based technologies
This review has revealed that drone technologies can greatly help in malaria control programmes. The drones can be used in developing genetically-based vector control tools [ 30 ], delivering massive aerial spraying to kill mosquito larvae [ 31 ], identifying mosquito larvae sites using aerial imaging [ 32 ] and in delivering drugs and vaccines [ 33 ]. Anti-malaria drones have been widely used to spray biological insecticides in rice fields and swamps to reduce the emerging mosquito populations. This has been successful in Kenya, Tanzania, India, Rwanda and Zanzibar. In Zanzibar, the Agras MG-1S drones were used to spray 10 L of a biodegradable agent called Aquatain; a chemical that has been used to cover drinking water basins. Drones have also been used to collect data to identify mosquito breeding sites so that the larvae can be controlled, reducing the number of adult mosquitoes able to spread malaria. For example in Malawi and near Lake Victoria the DJI Phantom low-cost drones are being used to survey and find mosquito breeding grounds. A new trial using ‘gene drive’ technology is currently taking place in Burkina Faso where the trial will see the release of genetically modified mosquitoes in an attempt to wipe out the female carriers of the disease [ 34 ].
Diagnostic tools including other devices developed for malaria interventions
Devices that have been developed to respond to malaria include the SolarMal device, a solar-powered mosquito trapper being piloted in Kenya [ 35 ]. The Solar Powered Mosquito Trap (SMOT) is baited with a synthetic odor blend that mimics human odor to lure host-seeking malaria mosquitoes. Other devices such as the ThermaCell Patio Shield Mosquito Repellants developed by ThermaCell are shield lanterns that repel mosquitoes by creating a 15-foot zone of protection. Several devices have also been developed to improve malaria diagnosis and these include the Nanomal DNA analyzer a simple, rapid and affordable point-of-care (POC) handheld diagnostic nanotechnology device to confirm malaria diagnosis and detect drug resistance in malaria parasites in minutes and at the patient’s side, by analysis of mutations in malaria DNA using a range of proven nanotechnologies. Medication Events Monitoring Device (MEMS) have also been greatly used to monitor medication adherence to malaria drugs [ 36 ]. Malaria Rapid Diagnostic Tests (RDTs), sometimes called dipsticks or Malaria Rapid Diagnostic Devices (MRDDS), are simple immunochromatographic tests that identify specific antigens of malaria parasites in whole or peripheral blood. They are categorized into dipstick, cassette or hybrids. Dipstick RDTs are cheap and readily available on market [ 37 ]. An example is the OptiMAL dipstick [ 38 ]. Cassette RDTS are complex and require much time for results to be read but are much safer to use.
This research has culminated into insightful conclusions from the systematized review of the malaria technological Innovations and has been the foundation of the collated database that can be accessed via the WHO AFRO marketplace platform. This is a platform that has been developed to showcase various technologies and innovations that can be applied for different disease areas. This focused on technologies relevant for malaria response. The identified intervention technologies and focus areas provide ways of identifying key leverage points in strengthening the health systems and making tangible impact towards various mandates to fight the scourge of malaria. More importantly highlighting these trends empowers innovators and policy makers on the continent to make informed decisions on applying frugal design to develop affordable, locally manufactured, functional and sustainable innovations fit for the African continent. Furthermore, the marketplace platform provides implementation insights to African nations on the adoption of some of the technological innovations from this study.
The review has highlighted that mobile applications are a vital component of malaria response programmes and are increasingly being used along the different response areas, such as surveillance (malaria data capturing apps like Coconut Surveillance and DHS mobile app), microplanning (drug delivery and distribution management apps like Net4Schs App), prevention (mosquito repelling like Anti Mosquito Repellent Sound App), diagnosis (AI driven slide analysis apps like LifeLens and Malaria Screener), management (telehealth like the Malaria Consultant) and the provision of support for health services [decision support like the solution for Community Health-workers (SOCH) app] as outlined in Fig. 3 . Their impact has been validated in several studies [ 27 , 39 ].
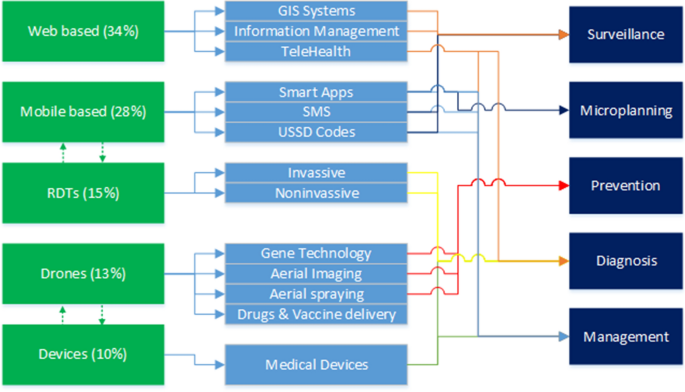
Analysis of the innovations by category, application and target outcome
In 2019, 93% of the global population was covered by a mobile broadband signal. In Sub-Saharan Africa, 3G coverage expanded to 75% compared to 63% in 2017, while 4G doubled to nearly 50% compared to 2017 [ 40 ]. This implies that mobile solutions can substantially mitigate many of the health system limitations prevalent mostly in African countries where malaria is endemic. A substantial number of mobile applications have been developed for surveillance of malaria control programs in Africa such as inSCALE (Mozambique), Coconut Surveillance (Zanzibar), CommCare (Senegal) and DHIS2 (Zimbabwe, and South Africa). This shows that mobile-based apps give a larger footprint and a high level of agility to malaria response. Nevertheless, limited connectivity and erratic energy supplies have been key factors affecting the levels of adoption and some apps have been reported to have a high level of complexity. This has also been reported in other studies [ 41 , 42 ].
Moreover, it has been noted that most of these apps are independent with limited capability for interoperability. Hence there is a need to develop open standards for mobile technologies for malaria control. For example, surveillance applications should be able to have geolocation capabilities and use exiting open-source platforms like OpenStreetMap, OpenDataKit & OpenMapKit; work online and offline mode to enable usage in resource constraints areas, ease of use to enable usage with little or no training and should support different languages including local languages. This calls for more research and implementation of natural language processing frameworks for use in mobile apps in Africa, which can assist with data analytics as well. Furthermore, aligning app development with standards such as the Fast Healthcare Interoperability Resources (FHIR) which facilitate interoperability between legacy health care systems and technology is important.
Superseding technological interoperability, there needs to be platform integration and overall visibility particularly on innovations that target malaria diagnosis, surveillance and management. However, it should be noted that systemically there has been launching of different applications for different malaria interventions which may confuse the public in terms of usage. Therefore, a single application or platform integrating several services such as Coconut Surveillance and owned and managed by a reputable malaria organization or the ministries of health may benefit citizens by allowing them to access services from a single and trusted application. Misinformation and misdiagnosis from publicly available medical apps is a health threat to the public as reported by [ 43 ].
Most of the reviewed web systems depend on data or are used to collect large amounts of malaria data to support decision-making. Hence a need for national malaria control and elimination information systems that can utilize regional and global structures, prioritizing cross-border intelligence sharing information regarding disease transmission hotspots, outbreaks, and human movement. Such systems can also be very useful in responding to pandemics like COVID-19 and other infectious outbreaks. There is also a need to have malaria related data centrally stored and managed by the Ministry of Health or malaria control programmes to guide decision-making at all levels of malaria response among the different stakeholders. Hospitals and clinics have also developed standalone patient information management systems in addition to the national health information management systems like OpenMRS and DHIS2. However, there is no communication between the different patient’s information management systems hence a need for development of open data standard driven systems and APIs to enforce interoperability among health systems in Africa. An effective information system must receive data from other sources, process it and send it back to other systems being used in malaria programme, particularly at the community level.
In malaria control, larval source management is very difficult to archive in rural areas due to perceived difficulties in identifying target areas [ 44 ]. Drones can capture extremely detailed images of the landscape, opening the possibility of replacing the time-consuming hunt for mosquito larvae on the ground with identifying habitat through aerial imagery. The review has shown that this has been used in several countries for example in Malawi and near Lake Victoria using DJI Phantom; low-cost drones that survey wilderness to find mosquito breeding grounds using Geospatial technology. Geospatial technology is rapidly evolving and now can be archived using remotely sensed data [ 45 ]. In Zanzibar, drones have been used to spray rice fields with a thin, non-toxic film as a strategy to eliminate mosquitoes. The review has shown that drones are a possible solution in malaria control programmes as also indicated in other studies [ 45 , 46 ]. The review also showed that rapid diagnostics tools offer fast turnaround services while circumventing obstacles faced when using microscopy in peripheral health care settings, including cost of equipment, reagents, and the need for electricity and skilled personnel [ 47 ].
This study has reviewed key emerging technologies used in malaria control programmes. The review revealed various technological applications that have been developed in response to malaria including surveillance, microplanning, prevention, diagnosis and management. Although breakthrough innovative platforms have been made available, one key challenge remained, which is lack of integration of key end-to-end components and functionalities to facilitate effective and efficient malaria response and to reduce fragmentation.
The review has also revealed several stakeholders in malaria control hence a need for mechanisms that promote the exchange of evidence between scientific, policy, and programme management communities for analysing the potential outcomes of the different malaria control strategies and interventions. In many malaria-endemic areas in Africa, the communication gap between policy makers, health workers, and patients is a significant barrier to efficient malaria control.
Furthermore, artificial intelligence (AI) has been widely used in the reviewed technological innovations, however there is an urgent need to provide reliable datasets, develop local AI expertise among WHO African member states, implement data protection and privacy acts; and put in place health innovation clusters to bring the different stakeholders together to develop and adopt appropriate technologies to solve the intended challenges.
Limitations of this work and future prospects
The main limitation of this work was that some applications were overlapping among the response areas and hence the decision to place an innovation under a given category was based on the judgement of the authors. Another limitation is the fact that this work is not aimed at analysing the total landscape of all malaria innovations. Only those that met the inclusion criteria and deemed relevant by the authors were included hence some innovations might not have been captured but we will be subjected to continuous update on the global database for malaria innovations at https://innov.afro.who.int/emerging-technological-innovations/7-malaria-innovations . Future research can focus on reviewing the technologies that are open source dedicated to malaria, and publishing findings that can be used by medical practitioners, application developers, and governments to collaborate in the process of containing the spread of malaria.
Availability of data and materials
The data used in this report is available to readers.
Ryan SJ, Lippi CA, Zermoglio F. Shifting transmission risk for malaria in Africa with climate change: a framework for planning and intervention. Malar J. 2020;19:170.
Article Google Scholar
WHO. World malaria report 2019. Geneva: World Health Organization; 2019.
Google Scholar
Garner P, Gelband H, Graves P, Jones K, Maclehose H, Olliaro P, et al. Systematic reviews in malaria: global policies need global reviews. Infect Dis Clin North Am. 2009;23:387–404.
White MT, Conteh L, Cibulskis R, Ghani AC. Costs and cost-effectiveness of malaria control interventions—a systematic review. Malar J. 2011;10:337.
Conteh L, Shuford K, Agboraw E, Kont M, Kolaczinski J, Patouillard E. Costs and cost-effectiveness of malaria control interventions: a systematic literature review. Value Health. 2021;24:1213–22.
Adeola AM, Botai JO, Olwoch JM, Rautenbach AC, Kalumba AM, Tsela PL, et al. Application of geographical information system and remote sensing in malaria research and control in South Africa: a review. South Afr J Infect Dis. 2015;30:114–21.
Smith NR, Trauer JM, Gambhir M, Richards JS, Maude RJ, Keith JM, et al. Agent-based models of malaria transmission: a systematic review. Malar J. 2018;17:299.
Vasiman A, Stothard JR, Bogoch II. Mobile phone devices and handheld microscopes as diagnostic platforms for malaria and neglected tropical diseases (NTDs) in low-resource settings: a systematic review, historical perspective and future outlook. Adv Parasitol. 2019;103:151–73.
Mbanefo A, Kumar N. Evaluation of malaria diagnostic methods as a key for successful control and elimination programs. Trop Med Infect Dis. 2020;5:E102.
Osei E, Kuupiel D, Vezi PN, Mashamba-Thompson TP. Mapping evidence of mobile health technologies for disease diagnosis and treatment support by health workers in sub-Saharan Africa: a scoping review. BMC Med Inform Decis Mak. 2021;21:11.
Grant MJ, Booth A. A typology of reviews: an analysis of 14 review types and associated methodologies. Health Inf Libr J. 2009;26:91–108.
Kurland KS, Gorr WL. GIS tutorial for health. ESRI, Inc.; 2007.
Nakakana UN, Mohammed IA, Onankpa BO, Jega RM, Jiya NM. A validation of the malaria Atlas project maps and development of a new map of malaria transmission in Sokoto, Nigeria: a cross-sectional study using geographic information systems. Malar J. 2020;19:149.
Pfeffer DA, Lucas TCD, May D, Harris J, Rozier J, Twohig KA, et al. Malaria Atlas: an R interface to global malariometric data hosted by the malaria Atlas project. Malar J. 2018;17:352.
Kelly GC, Hale E, Donald W, Batarii W, Bugoro H, Nausien J, et al. A high-resolution geospatial surveillance-response system for malaria elimination in Solomon Islands and Vanuatu. Malar J. 2013;12:108.
Kim JY, Eun SJ, Park DK. Malaria vulnerability map mobile system development using GIS-based decision-making technique. Mob Inf Syst. 2018;12:1–9.
Brown Z, Kramer R, Mutero C, Kim D, Miranda ML, Amenshewa B, et al. Stakeholder development of the malaria decision analysis support tool (MDAST). Malar J. 2012;11(Suppl 1):P15.
Khandekar E. Performance evaluation of zanzibar’s malaria case notification (MCN) surveillance system: the assessment of timeliness and stakeholder interaction. Thesis, MSc Duke Global Health Institute, 2015.
Murray CK, Mody RM, Dooley DP, Hospenthal DR, Horvath LL, Moran KA, et al. The remote diagnosis of malaria using telemedicine or e-mailed images. Mil Med. 2006;171:1167–71.
Madoff LC, Freedman DO. Detection of infectious diseases using unofficial sources infectious diseases. In: Petersen E, Chen LH, Schlagenhauf P, editors. A geographic guide. Hoboken: Wiley Online; 2011.
Woodall JP. Global surveillance of emerging diseases: the ProMED-mail perspective. Cad Saúde Pública. 2001;17:147–54.
Merkord CL, Liu Y, Mihretie A, Gebrehiwot T, Awoke W, Bayabil E, et al. Integrating malaria surveillance with climate data for outbreak detection and forecasting: the EPIDEMIA system. Malar J. 2017;16:89.
Article CAS Google Scholar
Sanches P, Brown B. Data bites man: the production of malaria by technology. Proc ACM Hum-Comput Interact. 2018;2:153.
Barrington J, Wereko-Brobby O, Ward P, Mwafongo W, Kungulwe S. SMS for life: a pilot project to improve anti-malarial drug supply management in rural Tanzania using standard technology. Malar J. 2010;9:298.
Rajvanshi H, Jain Y, Kaintura N, Soni C, Chandramohan R, Srinivasan R, et al. A comprehensive mobile application tool for disease surveillance, workforce management and supply chain management for malaria elimination demonstration project. Malar J. 2021;20:91.
Oo Win Han, Htike Win, Cutts JC, Win KM, Thu KM, Oo MC, et al. A mobile phone application for malaria case-based reporting to advance malaria surveillance in Myanmar: a mixed methods evaluation. Malar J. 2021;20:167.
Yu H, Yang F, Rajaraman S, et al. Malaria screener: a smartphone application for automated malaria screening. BMC Infect Dis. 2020;20:825.
Oliveira AD, Prats C, Espasa M, Serrat FZ, Sales CM, Silgado A, et al. The malaria system microapp: a new, mobile device-based tool for malaria diagnosis. JMIR Res Protoc. 2017;6: e70.
Svoronos T. CommCare: automated quality improvement to strengthen community-based health the need for quality improvement for CHWs. Weston: D-Tree Int Publisher; 2010.
James S, Collins FH, Welkhoff PA, Emerson C, Godfray HC, Gottlieb M, et al. Pathway to deployment of gene drive mosquitoes as a potential biocontrol tool for elimination of malaria in sub-Saharan Africa: recommendations of a scientific working group. Am J Trop Med Hyg. 2018;98(Suppl 6):1–49.
Choi L, Majambere S, Wilson AL. Larviciding to prevent malaria transmission. Cochrane Database Syst Rev. 2019. https://doi.org/10.1002/14651858.CD012736.pub2 .
Thompson DR, de la Torre JM, Barker CM, Holeman J, Lundeen S, Mulligan S, et al. Airborne imaging spectroscopy to monitor urban mosquito microhabitats. Remote Sens Environ. 2013;137:226–33.
Stanford Graduate School of Business. Zipline : Lifesaving deliveries by drone. Stanford Business. 2019. https://www.gsb.stanford.edu/faculty-research/case-studies/zipline-lifesaving-deliveries-drone .
Pare Toe L, Barry N, Ky AD, Kekele S, Meda W, Bayala K, et al. Small-scale release of non-gene drive mosquitoes in Burkina Faso: from engagement implementation to assessment, a learning journey. Malar J. 2021;20:395.
Hiscox A, Maire N, Kiche I, Silkey M, Homan T, Oria P, et al. The SolarMal project: innovative mosquito trapping technology for malaria control. Malar J. 2012;11(Suppl 1):O45.
Teshome EM, Oriaro VS, Andango PEA, Prentice AM, Verhoef H. Adherence to home fortification with micronutrient powders in Kenyan pre-school children: self-reporting and sachet counts compared to an electronic monitoring device. BMC Public Health. 2018;18:205.
Jelinek T, Grobusch MP, Nothdurft HD. Use of dipstick tests for the rapid diagnosis of malaria in nonimmune travelers. J Travel Med. 2000;7:175–9.
Tagbor H, Bruce J, Browne E, Greenwood B, Chandramohan D. Performance of the OptiMAL ® dipstick in the diagnosis of malaria infection in pregnancy. Ther Clin Risk Manag. 2008;4:631–6.
Visser T, Ramachandra S, Pothin E, Jacobs J, Cunningham J, Le Menach A, et al. A comparative evaluation of mobile medical APPS (MMAS) for reading and interpreting malaria rapid diagnostic tests. Malar J. 2021;20:39.
GSMA. Mobile Internet Connectivity 2019 Sub-Saharan Africa Factsheet. 2019. https://www.gsma.com/mobilefordevelopment/wp-content/uploads/2019/07/Mobile-Internet-Connectivity-SSA-Factsheet.pdf .
Murugesan S. Mobile apps in Africa. IT Prof. 2013. https://doi.org/10.1109/MITP.2013.83 .
Shead DC, Chetty S. Smartphone and app usage amongst South African anaesthetic service providers. South Afr J Anaesth Analg. 2021;27:76–82.
Swire-Thompson B, Lazer D. Public health and online misinformation: challenges and recommendations. Annu Rev Public Health. 2019;41:433–51.
Stanton MC, Kalonde P, Zembere K, Spaans RH, Jones CM. The application of drones for mosquito larval habitat identification in rural environments: a practical approach for malaria control? Malar J. 2021;20:244.
Kullmann K. The drone’s eye: applications and implications for landscape architecture. Landscape Res. 2018;43:7.
Liardon JL, Hostettler L, Zulliger L, Kangur K, Shaik NS, Barry DA. Lake imaging and monitoring aerial drone. HardwareX. 2018;3:146–59.
Boyce MR, O’Meara WP. Use of malaria RDTs in various health contexts across sub-Saharan Africa: a systematic review. BMC Public Health. 2017;17:470.
Download references
Acknowledgements
Author information, authors and affiliations.
World Health Organization Africa Region, Brazzaville, Republic of Congo
Moredreck Chibi, William Wasswa & Chipo Ngongoni
Tropical and Vector Borne Diseases, Universal Health Coverage/Communicable and Non Communicable Disease Cluster, World Health Organization Africa Region, Brazzaville, Republic of Congo
Ebenezer Baba & Akpaka Kalu
You can also search for this author in PubMed Google Scholar
Contributions
MC lead the conceptualization and designing of the study, and writing of the manuscript. WW contributed with data mining, analytics and writing the manuscript. CN contributed with systematized literature review and reviewing the manuscript. EB contributed to conceptualizing the study and reviewing of the draft manuscript. AK contributed to reviewing the draft manuscript and providing expert oversight. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Moredreck Chibi .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
The author reports no conflicts of interest for this work and given their consent for the publication.
Competing interests
The authors declared no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Chibi, M., Wasswa, W., Ngongoni, C. et al. Leveraging innovation technologies to respond to malaria: a systematized literature review of emerging technologies. Malar J 22 , 40 (2023). https://doi.org/10.1186/s12936-023-04454-0
Download citation
Received : 23 January 2022
Accepted : 14 January 2023
Published : 03 February 2023
DOI : https://doi.org/10.1186/s12936-023-04454-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Emerging technologies
Malaria Journal
ISSN: 1475-2875
- Submission enquiries: [email protected]

IMAGES
COMMENTS
This case-control study verified the occurrence of a malaria outbreak in the Waghemra zone. Age, the availability of artificial water-holding bodies, nearby stagnant water, sleeping outside overnight, and a lack of knowledge about malaria transmission and prevention all contributed to the epidemic's existence.
A community-based case-control study with a 1:1 ratio was employed at Waghemra Zone from September 14 to November 27, 2022. ... the study findings showed the presence of a malaria outbreak in ...
An unmatched 1:1 case-control study was conducted in October 2017. This study employed the stages used in the investigation of an outbreak guidelines recommended by the Centre for Disease Control and Prevention (CDC) [].This is a guide to real time stepwise investigation of acute public health events at a local, national, or multinational level.
This documentary video discusses the epidemiology of malaria; strategies for prevention, including vector control and vaccines; and the pipeline of promising new drugs for the fight to eliminate ma...
Eswatini was the first country in sub-Saharan Africa to pass a National Malaria Elimination Policy in 2011, and later set a target for elimination by the year 2020. This case study aimed to review the malaria surveillance data of Eswatini collected over 8 years between 2012 and 2019 to evaluate the country's efforts that targeted malaria elimination by 2020.
Purulia is a malaria-prone district in West Bengal, India, with approximately half of the blocks defined as malaria endemic. We analyzed the malaria case in each block of the Purulia district from ...
In 2020, malaria was estimated to have resulted in 627,000 deaths and 241 million cases, with 77% of deaths in children <5 years of age 1. Overall, 90% of malaria cases and deaths are reported in ...
It is also evident that a malaria outbreak ensued, with the rate of transmission surging five-fold, with 2.1 million additional cases in 2022 over 2021. The floods damaged 1000 health facilities in the country, leaving millions of people without access to health care in affected districts, and driving up the number of deaths from malaria ...
The estimated number of global malaria cases in 2022 exceeded pre-COVID-19 pandemic levels in 2019, according to WHO's 2023 World malaria report. Several threats to the malaria global response are highlighted in the report, including climate change. The report, an annual assessment of global trends in malaria control and elimination, noted ...
We conducted an outbreak investigation involving a matched case-control study. We defined a confirmed case as a positive malaria test in a resident of Aboke, Akalo, Alito, and Bala sub-counties of Kole District January-June 2019. We identified cases by reviewing outpatient health records. Exposures were assessed in a 1:1 matched case-control ...
Overview. Malaria is a life-threatening disease spread to humans by some types of mosquitoes. It is mostly found in tropical countries. It is preventable and curable. The infection is caused by a parasite and does not spread from person to person. Symptoms can be mild or life-threatening. Mild symptoms are fever, chills and headache.
A 1:1 unmatched case-control study involving 80 cases and 80 controls was conducted using interviewer-administered questionnaires at household level. ... The malaria outbreak at Tongogara refugee camp reemphasizes the role of behavioural factors in malaria transmission. Intensified health education to address human behaviours that expose ...
P. vivax malaria should be treated with chloroquine, except when acquired in Papua New Guinea and Indonesia, areas with high prevalence of chloroquine-resistant P. vivax. After a normal G6PD test, patients should get a radical cure with primaquine (30 mg per day for 14 days). Interactive clinical case-study dealing with a 44 year old man with ...
Our aim was to assess whether a combination of seasonal climate forecasts, monitoring of meteorological conditions, and early detection of cases could have helped to prevent the 2002 malaria emergency in the highlands of western Kenya. Seasonal climate forecasts did not anticipate the heavy rainfall. Rainfall data gave timely and reliable early warnings; but monthly surveillance of malaria out ...
A summary of the main findings, limitations and policy implications of our study is shown in Table 1. The pervasive and potentially large consequences of COVID-19 on African communities, such as ...
The malaria elimination case‑study series ... epidemic Occurrence of cases in excess of the number expected in a given place and time. eradication Permanent reduction to zero of the worldwide incidence of infection caused by human malaria parasites as a result of deliberate efforts. Intervention measures are no longer needed once eradication ...
African highlands often suffer of devastating malaria epidemics, sometimes in conjunction with complex emergencies, making their control even more difficult. In 2000, Burundian highlands experienced a large malaria outbreak at a time of civil unrest, constant insecurity and nutritional emergency. ... The objective of this case study is to ...
Malaria season should be over in Burkina Faso and Mali. This year, that's not the case, and health workers from drugmaker Novartis AG are still enrolling young patients into a study for a new ...
The study showed that the spatial distribution pattern of AOD in the rice-growing area during the epidemic was gradually decreasing from northeast to southwest with an overall decrease of 29.76%. ... Fan J, Huang R, Liang Z, Zhang G, Geng T (2021) Absorbable aerosols based on OMI data: a case study in three provinces of Northeast China. Environ ...
Here, using a prospective case-control design, we investigated the role of An. stephensi in transmission following a malaria outbreak in Dire Dawa, Ethiopia in April-July 2022.
In 2019, an estimated 409,000 people died of malaria and most of them were young children in sub-Saharan Africa. In a bid to combat malaria epidemics, several technological innovations that have contributed significantly to malaria response have been developed across the world. This paper presents a systematized review and identifies key technological innovations that have been developed ...