Subclinical Hyperthyroidism: Case Report and Review of the Literature
- First Online: 04 January 2022

Cite this chapter

- Karen Tsai 2 , 3 &
- Angela M. Leung 2 , 3
1319 Accesses
Subclinical hyperthyroidism is a biochemical diagnosis defined by a decreased serum thyroid-stimulating hormone (TSH) and normal serum thyroxine (T4) and tri-iodothyronine (T3) concentrations. The clinical presentation can vary widely, ranging from the lack of symptoms to overt symptoms of hyperthyroidism.
We present a case of a 66-year-old female with a history of hypertension, atrial fibrillation, systemic lupus erythematous, pre-diabetes, and osteoporosis who was found to have an incidental finding of subclinical hyperthyroidism. She also notes unintentional weight loss of 10 pounds over 2 months, anxiety, insomnia, heat intolerance, hand tremors, and palpitations which she initially attributed to aging. Her biochemical workup showed a serum TSH of 0.09 mIU/L (reference range, 0.3–4.7 mIU/L), free thyroxine (FT4) of 1.6 ng/dL (reference range, 0.8–1.7 ng/dL), and free tri-iodothyronine (FT3) of 400 (reference range, 222–383 pg/dL). Because of her age, underlying cardiovascular disease, osteoporosis, and TSH level being <0.1 mIU/L, the etiology of her subclinical hyperthyroidism was investigated for potential treatment. This patient was found to have a multinodular goiter, for which she received radioactive iodine therapy and thereafter converted to a euthyroid state. In this case report, we discuss the clinical presentation, workup, and treatment of subclinical hyperthyroidism.
- Subclinical hyperthyroidism
- Thyroid-stimulating hormone
- Thyrotropin
- Thyroid hormones
- Multinodular goiter
This is a preview of subscription content, log in via an institution to check access.
Access this chapter
- Available as PDF
- Read on any device
- Instant download
- Own it forever
- Available as EPUB and PDF
- Compact, lightweight edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
- Durable hardcover edition
Tax calculation will be finalised at checkout
Purchases are for personal use only
Institutional subscriptions
Vadiveloo T, et al. The Thyroid Epidemiology, Audit, and Research Study (TEARS): the natural history of endogenous subclinical hyperthyroidism. J Clin Endocrinol Metab. 2011;96(1):E1–8.
Article CAS Google Scholar
Ross DS, et al. 2016 American thyroid association guidelines for diagnosis and management of hyperthyroidism and other causes of thyrotoxicosis. Thyroid. 2016;26(10):1343–421.
Article Google Scholar
Biondi B, et al. Endogenous subclinical hyperthyroidism affects quality of life and cardiac morphology and function in young and middle-aged patients. J Clin Endocrinol Metab. 2000;85(12):4701–5.
CAS PubMed Google Scholar
Faber J, et al. Normalization of serum thyrotrophin by means of radioiodine treatment in subclinical hyperthyroidism: effect on bone loss in postmenopausal women. Clin Endocrinol. 1998;48(3):285–90.
Aubert CE, et al. The association between subclinical thyroid dysfunction and dementia: the Health, Aging and Body Composition (Health ABC) Study. Clin Endocrinol. 2017;87(5):617–26.
Wijsman LW, et al. Subclinical thyroid dysfunction and cognitive decline in old age. PLoS One. 2013;8(3):e59199.
Download references
Author information
Authors and affiliations.
Division of Endocrinology, Diabetes, and Metabolism, Department of Medicine, UCLA David Geffen School of Medicine, Los Angeles, CA, USA
Karen Tsai & Angela M. Leung
Division of Endocrinology, Diabetes, and Metabolism, Department of Medicine, VA Greater Los Angeles Healthcare System, Los Angeles, CA, USA
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Angela M. Leung .
Editor information
Editors and affiliations.
Thyroid Research Unit, Department of Medicine, Icahn School of Medicine at Mount Sinai and James J. Peters VA Medical Center, New York, NY, USA
Terry F. Davies
Rights and permissions
Reprints and permissions
Copyright information
© 2022 The Editor(s) (if applicable) and The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Tsai, K., Leung, A.M. (2022). Subclinical Hyperthyroidism: Case Report and Review of the Literature. In: Davies, T.F. (eds) A Case-Based Guide to Clinical Endocrinology. Springer, Cham. https://doi.org/10.1007/978-3-030-84367-0_7
Download citation
DOI : https://doi.org/10.1007/978-3-030-84367-0_7
Published : 04 January 2022
Publisher Name : Springer, Cham
Print ISBN : 978-3-030-84366-3
Online ISBN : 978-3-030-84367-0
eBook Packages : Medicine Medicine (R0)
Share this chapter
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research
Featured Clinical Reviews
- Screening for Atrial Fibrillation: US Preventive Services Task Force Recommendation Statement JAMA Recommendation Statement January 25, 2022
- Evaluating the Patient With a Pulmonary Nodule: A Review JAMA Review January 18, 2022
- Download PDF
- CME & MOC
- Share X Facebook Email LinkedIn
- Permissions
Hyperthyroidism : A Review
- 1 Section of Endocrinology, Diabetes, Nutrition, and Weight Management, Boston University Chobanian and Avedisian School of Medicine, Boston, Massachusetts
- Comment & Response Treatment for Hyperthyroidism During Pregnancy—Reply Elizabeth N. Pearce, MD, MSc; Sun Y. Lee, MD, MSc JAMA
- Comment & Response Treatment for Hyperthyroidism During Pregnancy Virginia Y. Watkins, MD; Sarah K. Dotters-Katz, MD, MSHPEd; Jeffrey A. Kuller, MD JAMA
- JAMA Patient Page Patient Information: Hyperthyroidism Rebecca Voelker, MSJ JAMA
Importance Overt hyperthyroidism, defined as suppressed thyrotropin (previously thyroid-stimulating hormone) and high concentration of triiodothyronine (T 3 ) and/or free thyroxine (FT 4 ), affects approximately 0.2% to 1.4% of people worldwide. Subclinical hyperthyroidism, defined as low concentrations of thyrotropin and normal concentrations of T 3 and FT 4 , affects approximately 0.7% to 1.4% of people worldwide. Untreated hyperthyroidism can cause cardiac arrhythmias, heart failure, osteoporosis, and adverse pregnancy outcomes. It may lead to unintentional weight loss and is associated with increased mortality.
Observations The most common cause of hyperthyroidism is Graves disease, with a global prevalence of 2% in women and 0.5% in men. Other causes of hyperthyroidism and thyrotoxicosis include toxic nodules and the thyrotoxic phase of thyroiditis. Common symptoms of thyrotoxicosis include anxiety, insomnia, palpitations, unintentional weight loss, diarrhea, and heat intolerance. Patients with Graves disease may have a diffusely enlarged thyroid gland, stare, or exophthalmos on examination. Patients with toxic nodules (ie, in which thyroid nodules develop autonomous function) may have symptoms from local compression of structures in the neck by the thyroid gland, such as dysphagia, orthopnea, or voice changes. Etiology can typically be established based on clinical presentation, thyroid function tests, and thyrotropin-receptor antibody status. Thyroid scintigraphy is recommended if thyroid nodules are present or the etiology is unclear. Thyrotoxicosis from thyroiditis may be observed if symptomatic or treated with supportive care. Treatment options for overt hyperthyroidism from autonomous thyroid nodules or Graves disease include antithyroid drugs, radioactive iodine ablation, and surgery. Treatment for subclinical hyperthyroidism is recommended for patients at highest risk of osteoporosis and cardiovascular disease, such as those older than 65 years or with persistent serum thyrotropin level less than 0.1 mIU/L.
Conclusions and Relevance Hyperthyroidism affects 2.5% of adults worldwide and is associated with osteoporosis, heart disease, and increased mortality. First-line treatments are antithyroid drugs, thyroid surgery, and radioactive iodine treatment. Treatment choices should be individualized and patient centered.
Read More About
Lee SY , Pearce EN. Hyperthyroidism : A Review . JAMA. 2023;330(15):1472–1483. doi:10.1001/jama.2023.19052
Manage citations:
© 2024
Artificial Intelligence Resource Center
Cardiology in JAMA : Read the Latest
Browse and subscribe to JAMA Network podcasts!
Others Also Liked
Select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Systematic Review
- Open access
- Published: 05 September 2022
Hyperthyroidism and clinical depression: a systematic review and meta-analysis
- Henry Bode ORCID: orcid.org/0000-0002-7515-6996 1 ,
- Beatrice Ivens 1 ,
- Tom Bschor 2 ,
- Guido Schwarzer ORCID: orcid.org/0000-0001-6214-9087 3 ,
- Jonathan Henssler 1 , 4 &
- Christopher Baethge 1
Translational Psychiatry volume 12 , Article number: 362 ( 2022 ) Cite this article
5751 Accesses
8 Citations
7 Altmetric
Metrics details
- Pathogenesis
Hyperthyroidism and clinical depression are common, and there is preliminary evidence of substantial comorbidity. The extent of the association in the general population, however, has not yet been estimated meta-analytically. Therefore we conducted this systematic review and meta-analysis (registered in PROSPERO: CRD42020164791). Until May 2020, Medline (via PubMed), PsycINFO, and Embase databases were systematically searched for studies on the association of hyperthyroidism and clinical depression, without language or date restrictions. Two reviewers independently selected epidemiological studies providing laboratory or ICD-based diagnoses of hyperthyroidism and diagnoses of depression according to operationalized criteria (e.g. DSM) or to cut-offs in established rating scales. All data, including study quality based on the Newcastle-Ottawa Scale, were independently extracted by two authors. Odds ratios for the association of clinical depression and hyperthyroidism were calculated in a DerSimonian-Laird random-effects meta-analysis. Out of 3372 papers screened we selected 15 studies on 239 608 subjects, with 61% women and a mean age of 50. Relative to euthyroid individuals, patients with hyperthyroidism had a higher chance of being diagnosed with clinical depression: OR 1.67 ([95% CI: 1.49; 1.87], I 2 : 6%; prediction interval: 1.40 to 1.99), a result supported in a number of sensitivity and subgroup analyses. The OR was slightly less pronounced for subclinical as opposed to overt hyperthyroidism (1.36 [1.06; 1.74] vs. 1.70 [1.49; 1.93]). This comorbidity calls for clinical awareness and its reasons need investigation and may include neurobiological mechanisms, common genetic vulnerability and a generally heightened risk for clinical depression in patients with chronic somatic disorders.
Similar content being viewed by others

Genome-wide association studies
Emil Uffelmann, Qin Qin Huang, … Danielle Posthuma

The serotonin theory of depression: a systematic umbrella review of the evidence
Joanna Moncrieff, Ruth E. Cooper, … Mark A. Horowitz

Psilocybin microdosers demonstrate greater observed improvements in mood and mental health at one month relative to non-microdosing controls
Joseph M. Rootman, Maggie Kiraga, … Zach Walsh
Introduction
A link between thyroid disorders and depression has been investigated for decades [ 1 ]. Researchers uncovered several possible interactions between thyroid metabolism, the HPT-Axis and mood regulation [ 2 , 3 , 4 , 5 , 6 ], and the association of hypothyroidism with depression has been the focus of various meta-analyses [ 7 , 8 , 9 ]. They yielded positive results, but the extent of the comorbidity may have been overestimated [ 10 ]: In the most recent meta-analysis, we estimated an odds ratio for hypothyroidism and clinical depression of 1.30 [1.08–1.57] in population-based studies [ 11 ].
The association of depression with the other end of the thyroid disorder spectrum, hyperthyroidism, has been much less investigated, although it consists of a common group of conditions [ 12 ]. For example, NHANES III found general population prevalences of 0.7% and 0.5% for subclinical and overt hyperthyroidism, respectively [ 13 ]. A Danish register-based study [ 14 ] found that subjects with hyperthyroidism had a higher likelihood of developing depression than euthyroid controls (Hazard Ratio (HR): 1.54 [1.36–1.74]). Similarly, Williams et al. [ 15 ] found serum T4 to be positively associated with depression. In contrast, an individual patient data meta-analysis of six studies by Wildisen et al. [ 16 ] yielded no relevant association of subclinical hyperthyroid states and depression.
So far, no clear picture of a link between hyperthyroidism and depression emerged. Therefore, we conducted a systematic review and meta-analysis of studies presenting data on established hyperthyroidism—either subclinical or overt—and clinically relevant depression. To reduce bias, we restricted this meta-analysis to epidemiological and population-based studies and did not include studies based on samples from outpatient departments for thyroid or mood disorders.
This is a systematic review and meta-analysis registered in PROSPERO (CRD42020164791). Its reporting is based on the PRISMA 2020 and MOOSE guidelines [ 17 , 18 ].
Data sources and searches
We conducted a systematic search in MEDLINE and PubMed Central via PubMed, in PsycINFO via EBSCOhost, and in Embase to identify epidemiological studies on the association of hyperthyroidism and depression (last update on May 4, 2020). We combined generic terms for depression, hyperthyroidism, and population-based study designs (Supplementary Methods).
Study selection
Inclusion criteria
Study design: Cohort and cross-sectional studies.
Study population: Studies needed to be representative of the general population. If study groups were not randomly sampled from the general population, studies were only eligible if they (1) drew on very broad and diverse populations, such as civil servants or the totality of hospitalized patients in one country, and (2) study reports were not suggestive of biases, i.e. included a complete report of the recruitment and selection process.
Exposure: Hyperthyroid thyroid disorders, either subclinical, overt or, if nothing else was stated, of autoimmune origin (i.e. Graves’ Disease, but not Hashimoto thyroiditis). Disorders needed to be diagnosed by established laboratory methods or had to be drawn from registers employing data with documented reliability.
Outcome: Clinically significant depression, either defined as major depressive disorder (MDD) diagnosis according to established diagnostic systems, e.g. DSM or ICD, or an above-threshold score in established psychopathology rating scales for depression [ 19 ], as specified by study authors. Diagnoses could originate with assessment rating scales, standardized interviews (e.g. WHO-CIDI) or from registers including hospital data with documented reliability. To err on the conservative side we did not associate hyperthyroidism with any change in depression scores, because, in the general population, variations in depression scale scores below a predefined cut-off point for caseness are not indicative of clinical depression and may create pseudo-effects.
Exclusion criteria
Study design: Case-control studies
Data extraction and quality assessment
Titles and abstracts retrieved in the literature search were independently screened by two authors (HB, BI). “Grey” literature was included and no language or date restrictions applied. We searched bibliographies of every article eventually included. All articles potentially eligible were read independently by two authors (HB, BI), and data of included studies were extracted independently by two authors (HB, BI) using an Excel-based standardized data extraction form in accordance with the Cochrane Collaboration Handbook [ 20 ]. Disagreements were solved by discussion with the senior author (CB). If no effect sizes or sufficient data for calculation were reported, we contacted authors by e-mail.
All studies included were rated independently by two authors (HB, BI) for their risk of bias, using the Newcastle-Ottawa Scale (NOS) adaptation for cohort [ 21 ] and cross-sectional studies [ 22 ]. To be rated as “low risk of bias”, studies needed to be categorized in the highest NOS-category, i.e. they needed to receive all or all but one star in the rating system.
Data synthesis and analysis
To account for differences in study settings and methodology we used random effects analyses (DerSimonian & Laird) [ 23 ]. Statistical heterogeneity is reported as I 2 statistic and Tau (τ). We assessed publication bias in funnel plots and Egger’s test [ 24 ] and estimated the role of missing studies in trim-and-fill-analyses [ 25 ]. Prediction intervals were calculated to account for the heterogeneity between studies [ 26 ]. We conducted leave-one-out analyses if forest plots indicated a disproportionate effect of single studies. All calculations have been carried out in Comprehensive Meta-Analysis (CMA) Version 3 [ 27 ] and R [ 28 ], using the packages meta [ 29 ] and metafor [ 30 ].
Primary outcome analysis
The primary outcome is the association of hyperthyroidism and clinical depression. We compared depression prevalence of patients with versus without hyperthyroidism and expressed the results as odds ratio (OR) ± 95% confidence interval (CI). If studies reported effects as risk ratio (RR) or hazard ratio (HR), we transformed these effects into ORs (Supplementary Methods). If studies reported multiple differently adjusted effect sizes, we included those with minimum adjustment to be as coherent as possible with unadjusted or self-calculated effect sizes. The analysis includes all studies reporting results for overt and/or subclinical hyperthyroidism. If a study reported effects for both, only results for overt hyperthyroidism were included to maintain independence. Calculations and formulae are listed in the supplementary information (Supplementary Methods).
Subgroup analyses
We subdivided hyperthyroidism into both its overt and subclinical form, as defined in the studies included, and stratified our primary analyses by gender, risk of bias, intake of thyroid medication, a core group of strictly population-based studies, and assessment of depression. To minimize bias, the subgroup analysis investigating gender-specific effects was conducted only on studies that reported effects for both genders. All of the above stratifications were also repeated in the subgroup analyses of overt and subclinical hyperthyroidism.
Post hoc analyses
We compared studies on older populations (age ≥ 60 years) with studies on subjects of all ages to detect age-specific trends. To compare the effects of hyperthyroidism with those of hypothyroidism, we selected studies reporting an effect for both thyroid disorders and analysed them separately.
Our search yielded 4350 articles. After exclusion of duplicates, 3372 were screened and out of those, 62 were assessed for eligibility in full text. Fifteen studies [ 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 ] are included in this meta-analysis (PRISMA flowchart, Fig. 1 ). Three studies reported effects for overt, 7 for subclinical and 4 for both types of hyperthyroidism. The study of Chen et al. [ 33 ] reported effects for Grave’s disease, which we considered a plausible proxy of overt hyperthyroidism.
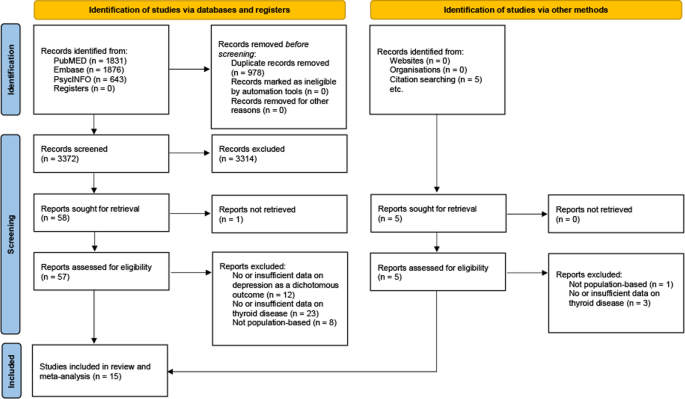
PRISMA flow diagram showing the study selection process.
Table 1 displays characteristics of all studies: Three cohort (72.5% of participants) and 12 cross-sectional studies (27.5%) accounted for a total of 239,608 participants, ranging from 60 [ 41 ] in the smallest to 150,960 [ 44 ] in the largest study. Study size weighted mean age of participants was 50 years. The overall proportion of women was 60.8%.
Six studies reported depression in DSM- or ICD-conforming diagnoses of major depressive disorder. Cut-off points in depression scores were employed in 9 studies.
In 13 studies, authors provided diagnoses of thyroid disorders based upon established laboratory methods, 2 used register data on ICD-based diagnoses. Intake of thyroid or anti-thyroid medication was allowed in 10 studies.
Applying the Newcastle-Ottawa Scale, 3 cohort and 2 cross-sectional studies were rated as carrying a “low risk of bias”.
Full data describing all included studies are presented in the supplementary information (Supplementary Table 1 ).
Pooled analysis of all studies resulted in an OR estimate of 1.67 [1.49–1.87] of clinical depression in all types of hyperthyroidism relative to euthyroidism (Fig. 2 and Table 2 ), no relevant heterogeneity was observed ( I ² = 6.4%, τ = 0.058; prediction interval: 1.40 to 1.99).
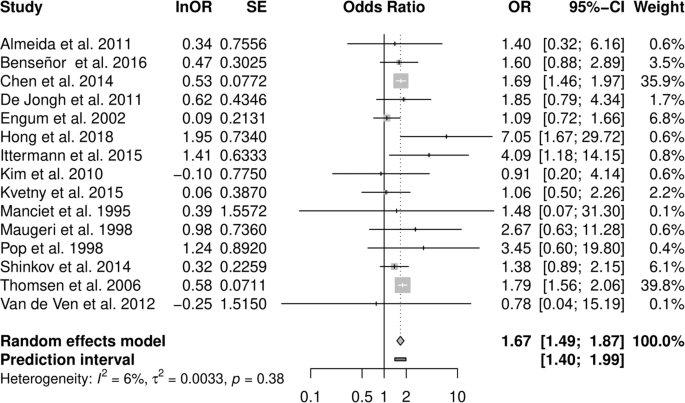
Forest plot of the primary analysis on the association of hyperthyroidism and depression. Odds ratios greater than 1 indicate a stronger association of depression with hyperthyroidism than with euthyroidism, odds ratios smaller than 1 indicate a weaker association.
Subgroup analysis of studies restricted to overt hyperthyroidism resulted in a similar OR of 1.70 [1.49–1.93], and subclinical hyperthyroidism was also associated with depression (1.36 [1.06–1.74], Table 2 ). Stratification by gender, based on results from three studies, revealed an OR of 1.37 [0.91–2.05] among women and 1.84 [1.34–2.54] in men (p-value between effects: 0.257). Strictly population-based studies as well as studies that allowed for thyroid or anti-thyroid medication reported slightly weaker summary associations with depression. A cohort study design and a DSM- or ICD-conforming diagnosis of depression resulted in moderately stronger effects (Supplementary Table 2.1 ). Stratifying subgroup analyses in overt and subclinical hyperthyroidism yielded similar effects (Supplementary Table 2.2 ).
The funnel plot of the primary analysis indicated an asymmetric reporting of effects, with studies missing in the lower right quadrant, indicating the possibility of biased reporting in favour of weaker effects. However, Egger’s test was not positive.
Analysing studies with low risk of bias confirmed the results of the primary subgroup analyses on overt hyperthyroidism, while the association of subclinical hyperthyroidism with depression became negligible (1.08 [0.8–1.46]).
In a leave-one-out analysis, study weight was distributed unevenly with two studies carrying almost 80% of the weight. Exclusion of these large register studies by Chen et al. [ 33 ] and Thomsen et al. [ 44 ] slightly lowered the association (1.61 [1.34–1.93] and 1.58 [1.35–1.85] respectively). In subclinical hyperthyroidism, removal of the study by Engum et al. [ 35 ] increased ORs to 1.52 [1.16–1.99].
Similar to the primary analysis, heterogeneity was low or moderate in subgroup and sensitivity analyses, with I ² generally not exceeding 50% (Supplementary Table 2 ).
Post-hoc analyses on studies on older populations reported effects similar to those of studies on all ages (Supplementary Table 2 ).
In fourteen studies effects for both, hyper- and hypothyroidism were reported [ 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 ]. Thomsen et al. published corresponding data on hypothyroidism in a separate study [ 46 ]. Combining studies on overt and subclinical disease resulted in a nominally weaker association of depression with hypothyroidism (1.36 [1.02–1.82]]) than with hyperthyroidism (1.61 [1.34–1.93]). In overt hyperthyroidism (1.66 [1.22–2.26]) associations were similar to those in overt hypothyroidism (1.69 [0.83–3.45]). Subclinical disease showed equal ORs in hyper- (1.36 [1.06–1.74]) and in hypothyroidism (1.35 [1.05–1.73]). After correction for potentially missing studies (Egger’s test p -value = 0.003) however, the association of hypothyroidism with depression decreased to an OR of 1.14 [0.88–1.47].
To our knowledge, this is the first meta-analysis investigating both subclinical and overt hyperthyroidism and its association with clinical depression in the general population. We found a statistically significant association of hyperthyroidism and depression, with small confidence as well as prediction intervals indicating a robust effect. The results are supported by a variety of subgroup as well as sensitivity analyses and by low heterogeneity. Therefore, even though fewer studies have been carried out on hyper- than on hypothyroidism (OR: 1.30 [1.08–1.57]) [ 11 ], we consider the evidence of an association with clinical depression and the effect itself marginally stronger in hyperthyroidism (OR 1.67 [1.49–1.87]).
These findings challenge earlier research on hyperthyroidism and depression: Wildisen et al. [ 16 ], for example, reported no relevant effect of subclinical hyperthyroidism on BDI-scores. However, they did not analyse patients with overt hyperthyroidism. Of note, more in line with Wildisen and co-authors’ study, our results point to a weaker, if any, association of subclinical hyperthyroidism with clinical depression than in overt hyperthyroidism.
This gradient in effect size may support the notion that it is not primarily autoimmunity that drives the association but possibly the increase in thyroid hormones. Further, in an earlier meta-analysis we could not support an association of TPO-antibody positivity with clinical depression [ 11 ]. At the pathophysiological level, the findings are consistent with several hypotheses of neuroendocrine causes of depression: Dysbalanced, thyroid hormones as key regulators of metabolism can contribute to typical symptoms of depression such as sleep disturbance, weight change, fatigue or psychomotor agitation [ 47 ]. They also stimulate cortical 5-HT secretion [ 48 ] and might act as co-transmitters in the noradrenergic system [ 49 ], influencing monoaminergic transmission in the brain. Several animal models showed that induction of hypo- and hyperthyroid states in rats significantly altered cortical monoamine levels: While in hypothyroidism 5-HT levels decreased in multiple brain regions [ 50 , 51 , 52 ], in hyperthyroidism, norepinephrine concentration as well as the number of 5-HT2-receptors were downregulated simultaneously with an increase in 5-HT levels [ 50 , 52 , 53 , 54 ]. One study reported depression-like behaviour in rats with induced states of both hypo- and hyperthyroidism [ 55 ]. Interestingly, hyperthyroid rats also showed anxiety-like behaviour. Further, both hypo- as well as hyperthyroid disturbances of the HPT-axis can lead to hypercortisolism [ 56 , 57 , 58 , 59 , 60 ], which is often found in patients suffering from depression [ 6 , 61 , 62 , 63 ]. Possibly, inflammation provides a link between thyroid disorders and depression, because with Hashimoto and Graves‘ disease, two of the leading causes of thyroid dysfunction result from autoantibodies against thyroid tissue, are characterized by lymphocytic infiltrates and increased levels of proinflammatory cytokines (such as IL-6 and TNF-alpha), and may have their origins in viral infections [ 64 , 65 ]. Recently, similar factors have been discussed in the etiology of depression [ 66 ], and common pathways may explain the comorbidity described in primary studies and in this meta-analysis.
In principle, the association between thyroid disorders and depression may also be based on a common genetic vulnerability. Some structures involved in brain thyroid hormone metabolism, such as the deiodinase enzymes (DIO) type 1,2 and 3 or the thyroid hormone transporter OATP1C1, may lead to a local state of hormone deficiency when functionally impaired or overactive, facilitating the development of depression potentially regardless of serum thyroid hormone levels [ 4 , 5 , 67 , 68 ]. Studies showed that some DIO2 polymorphisms were indeed associated with worse psychological well-being [ 69 ] and DIO2 expression was reduced in patients suffering from recurrent depressive disorders (rDD) [ 70 ]. DIO1 variants were associated with lifetime MDD in Caucasian female individuals [ 71 ] and DIO1 expression was also found to be decreased in subjects with rDD [ 72 ]. However, other studies could not link DIO expression or certain polymorphisms to depression or impaired well-being [ 73 , 74 ]. Variation of the OATP1C1 gene was connected to fatigue and depression in hypothyroid individuals [ 75 ] and to depression in subjects who suffered from ischemic stroke [ 76 ]. We are not aware, however, of studies investigating the association of depression with genetic variations leading to a brain-specific local state of hyperthyroidism.
In an entirely different approach, the association of hyperthyroidism and clinical depression may be explained by the observation that chronic conditions as such are often related to a greater risk of being depressed [ 77 , 78 , 79 , 80 ]. In this framework, a chronic condition, for example, hyperthyroidism, acts as a stressor and may, particularly in vulnerable people, contribute to the development of clinical depression.
Reverse causation also needs consideration: Subsequent to the elevation of cortisol caused by depression, TRH production might be stimulated and lead to an overproduction of T4 [ 2 , 5 ]. Normalization or, rather, a decrease of T4 levels after successful treatment of depressive disorder has also been observed [ 81 , 82 ]. In general, however, the common paradigm in depression research is one of interdependence, not of one factor causing the other [ 83 , 84 ], for example, with regard to the heightened cardiovascular risk of patients with depression.
A hint towards the predominant direction of the effect responsible for the observed association might be found in our subgroup analyses. Here, studies were stratified by study design, with cohort studies that assured absence of depression at baseline showing stronger effects than cross-sectional studies. While this can be understood as a sign for a stronger effect of hyperthyroidism on depression than vice versa, the analysis was only based on three cohort studies and therefore has to be regarded as preliminary.
A key feature of the present study is its focus on clinical depression. As a result, we may have missed subtle changes in psychopathology. However, not only was there no indication of such an effect in Wildisen and co-authors’ study [ 16 ], but in searching for differences in low and subclinical score ranges lies the risk of inflating small findings of doubtful clinical relevance. The problem of employing subclinical phenomena works also in thyroid parameters: Williams et al. [ 15 ], in their early meta-analysis, took into account the full range of thyroid hormones, physiological and pathological alike. Interestingly, however, even with their very broad approach they arrived at no stronger association than the one in the present study.
At the same time, relying on clinical data derived only from routine examinations in outpatient clinics for mood or metabolism disorders introduces selection bias [ 10 ], hence our restriction to epidemiological studies. In our view, therefore, the OR presented in this study represents a conservative estimate of the association. Since the effect we estimated is moderate it is worth noting that relatively small effect sizes are common in medicine, even in established biomarkers [ 85 ].
With regard to clinical practice, our results suggest that heightened awareness of depression is justified in patients with hyperthyroidism, as is TSH screening among patients with depression. In distinction to our analysis of hypothyroidism and depression—where the observed ORs were 0.71 [0.40–1.25] and 1.48 [1.18–1.85] for men and women respectively [ 11 ]—we did not find a clear-cut gender differential, although men with hyperthyroidism were slightly more affected by clinical depression than women. In fact, for women, our results are inconclusive, as the confidence interval includes a null effect. This applies all the more to the findings regarding women with subclinical hyperthyroidism where no association is apparent. Of note, the data were not sufficient for a subgroup analysis on ethnic differences indicating a need for future research.
In regard to therapy, both conditions, hyperthyroidism and depression, demand guideline-oriented treatment. We are not aware of an established treatment that would target the two diseases in one approach.
Hyperthyroidism is not as prevalent as hypothyroidism in the general population. Assuming a population of 332.5 million people in the US [ 86 ], a hyperthyroidism prevalence of 1.3% [ 13 ], and a 12-months depression prevalence of 6.7% [ 87 ], an OR of 1.67 translates into about 484 thousand people with the comorbidity, 194 thousand of those presumably due to hyperthyroidism. Provided that there are 22.3 million people with depression each year, in a model assuming that hyperthyroidism causes depression, hyperthyroidism contributes about 0.9% to the pandemic of depression.
We focussed on population-based studies, but the term leaves some room for debate: For example, a sample of civil servants, such as the one investigated by Benseñor et al. [ 32 ], is not population-based in the strict sense. However, while it is likely that prevalence differs contingent on the sample, it seems implausible that the association between hyperthyroidism and depression differs meaningfully in such a large and diverse group of people. Reassuringly, sensitivity analysis restricted to strictly population-based studies yielded no substantially different results (Supplementary Table 2 ). In the same vein, exclusion of register studies did not substantially change the summary estimate.
In conclusion, there is an association of hyperthyroidism with clinical depression (1.67 [95% CI: 1.49–1.87]), that is stronger in overt than in subclinical hyperthyroidism, pointing to a possibly biological association of both conditions. This should raise awareness in clinicians and researchers alike: Not only hypothyroid but also, and especially, hyperthyroid patients are at higher risk for depressive disorders and should be monitored for signs of clinical depression. How a hyperthyroid metabolism influences mood is not yet explained and, particularly regarding sex, deserves greater attention in the future research of thyroid–brain interactions.
Whybrow PC, Prange AJ Jr, Treadway CR. Mental changes accompanying thyroid gland dysfunction. a reappraisal using objective psychological measurement. Arch Gen Psychiatry. 1969;20:48–63.
Article CAS PubMed Google Scholar
Bahls SC, de Carvalho GA. The relation between thyroid function and depression: a review. Braz J Psychiatry. 2004;26:41–49.
Article PubMed Google Scholar
Bauer M, Goetz T, Glenn T, Whybrow PC. The thyroid-brain interaction in thyroid disorders and mood disorders. J Neuroendocrinol. 2008;20:1101–14.
Feldman AZ, Shrestha RT, Hennessey JV. Neuropsychiatric manifestations of thyroid disease. Endocrinol Metab Clin North Am. 2013;42:453–76.
Hage MP, Azar ST. The link between thyroid function and depression. J Thyroid Res. 2012;2012:590648.
Article PubMed CAS Google Scholar
Jesulola E, Micalos P, Baguley IJ. Understanding the pathophysiology of depression: from monoamines to the neurogenesis hypothesis model - are we there yet? Behav Brain Res. 2018;341:79–90.
Loh HH, Lim LL, Yee A, Loh HS. Association between subclinical hypothyroidism and depression: an updated systematic review and meta-analysis. BMC Psychiatry. 2019;19:12.
Article PubMed PubMed Central Google Scholar
Siegmann EM, Müller HHO, Luecke C, Philipsen A, Kornhuber J, Grömer TW. Association of depression and anxiety disorders with autoimmune thyroiditis: a systematic review and meta-analysis. JAMA Psychiatry. 2018;75:577–84.
Tang R, Wang J, Yang L, Ding X, Zhong Y, Pan J, et al. Subclinical hypothyroidism and depression: a systematic review and meta-analysis. Front Endocrinol (Lausanne). 2019;10:340.
Article Google Scholar
Baethge C. Autoimmune thyroiditis and depression. JAMA Psychiatry. 2018;75:1204–1204.
Bode H, Ivens B, Bschor T, Schwarzer G, Henssler J, Baethge C. Association of hypothyroidism and clinical depression: a systematic review and meta-analysis. JAMA Psychiatry 2021.
Taylor PN, Albrecht D, Scholz A, Gutierrez-Buey G, Lazarus JH, Dayan CM, et al. Global epidemiology of hyperthyroidism and hypothyroidism. Nat Rev Endocrinol. 2018;14:301–16.
Hollowell JG, Staehling NW, Flanders WD, Hannon WH, Gunter EW, Spencer CA, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87:489–99.
Brandt F, Thvilum M, Almind D, Christensen K, Green A, Hegedüs L, et al. Hyperthyroidism and psychiatric morbidity: evidence from a Danish nationwide register study. Eur J Endocrinol. 2014;170:341–8.
Williams MD, Harris R, Dayan CM, Evans J, Gallacher J, Ben-Shlomo Y. Thyroid function and the natural history of depression: findings from the Caerphilly Prospective Study (CaPS) and a meta-analysis. Clin Endocrinol (Oxf). 2009;70:484–92.
Article CAS Google Scholar
Wildisen L, Del Giovane C, Moutzouri E, Beglinger S, Syrogiannouli L, Collet TH, et al. An individual participant data analysis of prospective cohort studies on the association between subclinical thyroid dysfunction and depressive symptoms. Sci Rep. 2020;10:19111.
Article CAS PubMed PubMed Central Google Scholar
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Bmj. 2021;372:n71.
Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. Jama. 2000;283:2008–12.
Smarr KL, Keefer AL. Measures of depression and depressive symptoms: Beck Depression Inventory-II (BDI-II), Center for Epidemiologic Studies Depression Scale (CES-D), Geriatric Depression Scale (GDS), Hospital Anxiety and Depression Scale (HADS), and Patient Health Questionnaire-9 (PHQ-9). Arthritis Care Res (Hoboken). 2011;63:S454–66.
Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. 2019;10:Ed000142.
PubMed Google Scholar
Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp , Accessed April, 2021.
Modesti PA, Reboldi G, Cappuccio FP, Agyemang C, Remuzzi G, Rapi S, et al. Panethnic differences in blood pressure in Europe: a systematic review and meta-analysis. PLoS ONE. 2016;11:e0147601.
Article PubMed PubMed Central CAS Google Scholar
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88.
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34.
Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–63.
Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta-analyses. Bmj. 2011;342:d549.
Borenstein M, Hedges, L, Higgins, J, Rothstein, H Comprehensive Meta-Analysis Version 3. Biostat: Englewood, NJ, 2013.
Team RCR: A language and environment for statistical computing. R Foundation for Statistical Computing: Vienna, Austria, 2020.
Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health. 2019;22:153–60.
Viechtbauer W. Conducting meta-analyses in R with the metafor Package. 2010; 36:48.
Almeida OP, Alfonso H, Flicker L, Hankey G, Chubb SA, Yeap BB. Thyroid hormones and depression: the Health in Men study. Am J Geriatr Psychiatry. 2011;19:763–70.
Benseñor IM, Nunes MA, Sander Diniz MF, Santos IS, Brunoni AR, Lotufo PA. Subclinical thyroid dysfunction and psychiatric disorders: cross-sectional results from the Brazilian Study of Adult Health (ELSA-Brasil). Clin Endocrinol (Oxf). 2016;84:250–6.
Chen HH, Yeh SY, Lin CL, Chang SN, Kao CH. Increased depression, diabetes and diabetic complications in Graves’ disease patients in Asia. Qjm. 2014;107:727–33.
de Jongh RT, Lips P, van Schoor NM, Rijs KJ, Deeg DJ, Comijs HC, et al. Endogenous subclinical thyroid disorders, physical and cognitive function, depression, and mortality in older individuals. Eur J Endocrinol. 2011;165:545–54.
Engum A, Bjøro T, Mykletun A, Dahl AA. An association between depression, anxiety and thyroid function-a clinical fact or an artefact? Acta Psychiatr Scand. 2002;106:27–34.
Hong JW, Noh JH, Kim DJ. Association between subclinical thyroid dysfunction and depressive symptoms in the Korean adult population: the 2014 Korea National Health and Nutrition Examination Survey. PLoS ONE. 2018;13:e0202258.
Ittermann T, Völzke H, Baumeister SE, Appel K, Grabe HJ. Diagnosed thyroid disorders are associated with depression and anxiety. Soc Psychiatry Psychiatr Epidemiol. 2015;50:1417–25.
Kim JM, Stewart R, Kim SY, Bae KY, Yang SJ, Kim SW, et al. Thyroid stimulating hormone, cognitive impairment and depression in an older korean population. Psychiatry Investig. 2010;7:264–9.
Kvetny J, Ellervik C, Bech P. Is suppressed thyroid-stimulating hormone (TSH) associated with subclinical depression in the Danish General Suburban Population Study? Nord J Psychiatry. 2015;69:282–6.
Manciet G, Dartigues JF, Decamps A, Barberger-Gateau P, Letenneur L, Latapie MJ, et al. The PAQUID survey and correlates of subclinical hypothyroidism in elderly community residents in the southwest of France. Age Ageing. 1995;24:235–41.
Maugeri D, Motta M, Salerno G, Rosso D, Mazzarella R, Salomone S, et al. Cognitive and affective disorders in hyper- and hypothyreotic elderly patients. Arch Gerontol Geriatrics. 1998;26:305–12.
Pop VJ, Maartens LH, Leusink G, van Son MJ, Knottnerus AA, Ward AM, et al. Are autoimmune thyroid dysfunction and depression related? J Clin Endocrinol Metab. 1998;83:3194–7.
CAS PubMed Google Scholar
Shinkov AD, Borisova AM, Kovacheva RD, Vlahov YD, Dakovska LN, Atanassova ID, et al. Influence of serum levels of thyroid-stimulating hormone and anti-thyroid peroxidase antibodies, age and gender on depression as measured by the Zung Self-Rating Depression Scale. Folia Med (Plovdiv). 2014;56:24–31.
Thomsen AF, Kvist TK, Andersen PK, Kessing LV. Increased risk of affective disorder following hospitalisation with hyperthyroidism - a register-based study. Eur J Endocrinol. 2005;152:535–43.
van de Ven AC, Muntjewerff JW, Netea-Maier RT, de Vegt F, Ross HA, Sweep FC, et al. Association between thyroid function, thyroid autoimmunity, and state and trait factors of depression. Acta Psychiatr Scand. 2012;126:377–84.
Thomsen AF, Kvist TK, Andersen PK, Kessing LV. Increased risk of developing affective disorder in patients with hypothyroidism: a register-based study. Thyroid. 2005;15:700–7.
Mullur R, Liu YY, Brent GA. Thyroid hormone regulation of metabolism. Physiol Rev. 2014;94:355–82.
Bauer M, Heinz A, Whybrow PC. Thyroid hormones, serotonin and mood: of synergy and significance in the adult brain. Mol Psychiatry. 2002;7:140–56.
Gordon JT, Kaminski DM, Rozanov CB, Dratman MB. Evidence that 3,3′,5-triiodothyronine is concentrated in and delivered from the locus coeruleus to its noradrenergic targets via anterograde axonal transport. Neuroscience. 1999;93:943–54.
Beley A, Beley P, Bralet J. Influence of hypo- and hyperthyroidism on the turnover rate of noradrenaline, dopamine and serotonin in various rat cerebral structures. Arch Int Physiol Biochim. 1975;83:471–80.
Hassan WA, Aly MS, Rahman TA, Shahat AS. Impact of experimental hypothyroidism on monoamines level in discrete brain regions and other peripheral tissues of young and adult male rats. Int J Dev Neurosci. 2013;31:225–33.
Ito JM, Valcana T, Timiras PS. Effect of hypo- and hyperthyroidism on regional monoamine metabolism in the adult rat brain. Neuroendocrinology. 1977;24:55–64.
Hassan WA, Rahman TA, Aly MS, Shahat AS. Alterations in monoamines level in discrete brain regions and other peripheral tissues in young and adult male rats during experimental hyperthyroidism. Int J Dev Neurosci. 2013;31:311–8.
Sandrini M, Vitale G, Vergoni AV, Ottani A, Bertolini A. Effect of acute and chronic treatment with triiodothyronine on serotonin levels and serotonergic receptor subtypes in the rat brain. Life Sci. 1996;58:1551–9.
Yu D, Zhou H, Yang Y, Jiang Y, Wang T, Lv L, et al. The bidirectional effects of hypothyroidism and hyperthyroidism on anxiety- and depression-like behaviors in rats. Horm Behav. 2015;69:106–15.
Agbaht K, Gullu S. Adrenocortical reserves in hyperthyroidism. Endocrine. 2014;45:136–43.
Gallagher TF, Hellman L, Finkelstein J, Yoshida K, Weitzman ED, Roffwarg HD, et al. Hyperthyroidism and cortisol secretion in man. J Clin Endocrinol Metab. 1972;34:919–27.
Iranmanesh A, Lizarralde G, Johnson ML, Veldhuis JD. Dynamics of 24-hour endogenous cortisol secretion and clearance in primary hypothyroidism assessed before and after partial thyroid hormone replacement. J Clin Endocrinol Metab. 1990;70:155–61.
Seck G, Ndoye O, Mbodj M, Akala A, Cisse F, Niang M, et al. Serum cortisol level variations in thyroid diseases. Dakar Med. 2000;45:30–3.
Google Scholar
Walter KN, Corwin EJ, Ulbrecht J, Demers LM, Bennett JM, Whetzel CA, et al. Elevated thyroid stimulating hormone is associated with elevated cortisol in healthy young men and women. Thyroid Res. 2012;5:13.
Juruena MF, Bocharova M, Agustini B, Young AH. Atypical depression and non-atypical depression: is HPA axis function a biomarker? A systematic review. J Affect Disord. 2018;233:45–67.
Parker KJ, Schatzberg AF, Lyons DM. Neuroendocrine aspects of hypercortisolism in major depression. Horm Behav. 2003;43:60–6.
Belmaker RH, Agam G. Major depressive disorder. N Engl J Med. 2008;358:55–68.
Dong YH, Fu DG. Autoimmune thyroid disease: mechanism, genetics and current knowledge. Eur Rev Med Pharm Sci. 2014;18:3611–8.
CAS Google Scholar
Li Q, Wang B, Mu K, Zhang J-A. The pathogenesis of thyroid autoimmune diseases: new T lymphocytes – Cytokines circuits beyond the Th1−Th2 paradigm. J Cell Physiol. 2019;234:2204–16.
Drevets WC, Wittenberg GM, Bullmore ET, Manji HK. Immune targets for therapeutic development in depression: towards precision medicine. Nat Rev Drug Disco. 2022;21:224–44.
Verloop H, Dekkers OM, Peeters RP, Schoones JW, Smit JW. Genetics in endocrinology: genetic variation in deiodinases: a systematic review of potential clinical effects in humans. Eur J Endocrinol. 2014;171:R123–35.
Dayan CM, Panicker V. Novel insights into thyroid hormones from the study of common genetic variation. Nat Rev Endocrinol. 2009;5:211–8.
Panicker V, Saravanan P, Vaidya B, Evans J, Hattersley AT, Frayling TM, et al. Common variation in the DIO2 gene predicts baseline psychological well-being and response to combination thyroxine plus triiodothyronine therapy in hypothyroid patients. J Clin Endocrinol Metab. 2009;94:1623–9.
Gałecka E, Talarowska M, Maes M, Su KP, Górski P, Kumor-Kisielewska A, et al. Expression levels of interferon-ɣ and type 2 deiodinase in patients diagnosed with recurrent depressive disorders. Pharm Rep. 2018;70:133–8.
Philibert RA, Beach SR, Gunter TD, Todorov AA, Brody GH, Vijayendran M, et al. The relationship of deiodinase 1 genotype and thyroid function to lifetime history of major depression in three independent populations. Am J Med Genet B Neuropsychiatr Genet. 2011;156b:593–9.
Gałecka E, Kumor-Kisielewska A, Orzechowska A, Maes M, Górski P, Szemraj J. Assessment of type 1 and type 3 deiodinase expression levels in depressive disorders. Acta Neurobiol Exp (Wars). 2017;77:225–35.
Gałecka E, Talarowska M, Maes M, Su KP, Górski P, Szemraj J. Polymorphisms of iodothyronine deiodinases (DIO1, DIO3) genes are not associated with recurrent depressive disorder. Pharm Rep. 2016;68:913–7.
Appelhof BC, Peeters RP, Wiersinga WM, Visser TJ, Wekking EM, Huyser J, et al. Polymorphisms in type 2 deiodinase are not associated with well-being, neurocognitive functioning, and preference for combined thyroxine/3,5,3′-triiodothyronine therapy. J Clin Endocrinol Metab. 2005;90:6296–9.
van der Deure WM, Appelhof BC, Peeters RP, Wiersinga WM, Wekking EM, Huyser J, et al. Polymorphisms in the brain-specific thyroid hormone transporter OATP1C1 are associated with fatigue and depression in hypothyroid patients. Clin Endocrinol (Oxf). 2008;69:804–11.
Taroza S, Rastenytė D, Burkauskas J, Podlipskytė A, Kažukauskienė N, Patamsytė V, et al. Deiodinases, organic anion transporter polypeptide polymorphisms and symptoms of anxiety and depression after ischemic stroke. J Stroke Cerebrovasc Dis. 2020;29:105040.
Moldin SO, Scheftner WA, Rice JP, Nelson E, Knesevich MA, Akiskal H. Association between major depressive disorder and physical illness. Psychol Med. 1993;23:755–61.
Patten SB, Williams JVA, Lavorato DH, Wang JL, Jetté N, Sajobi TT, et al. Patterns of association of chronic medical conditions and major depression. Epidemiol Psychiatr Sci. 2018;27:42–50.
Scott KM, Bruffaerts R, Tsang A, Ormel J, Alonso J, Angermeyer MC, et al. Depression-anxiety relationships with chronic physical conditions: results from the World Mental Health Surveys. J Affect Disord. 2007;103:113–20.
Wells KB, Golding JM, Burnam MA. Psychiatric disorder in a sample of the general population with and without chronic medical conditions. Am J Psychiatry. 1988;145:976–81.
Eker SS, Akkaya C, Sarandol A, Cangur S, Sarandol E, Kirli S. Effects of various antidepressants on serum thyroid hormone levels in patients with major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:955–61.
Gitlin M, Altshuler LL, Frye MA, Suri R, Huynh EL, Fairbanks L, et al. Peripheral thyroid hormones and response to selective serotonin reuptake inhibitors. J Psychiatry Neurosci. 2004;29:383–6.
PubMed PubMed Central Google Scholar
Hare DL, Toukhsati SR, Johansson P, Jaarsma T. Depression and cardiovascular disease: a clinical review. Eur Heart J. 2014;35:1365–72.
Khawaja IS, Westermeyer JJ, Gajwani P, Feinstein RE. Depression and coronary artery disease: the association, mechanisms, and therapeutic implications. Psychiatry (Edgmont). 2009;6:38–51.
Ioannidis JP, Panagiotou OA. Comparison of effect sizes associated with biomarkers reported in highly cited individual articles and in subsequent meta-analyses. Jama. 2011;305:2200–10.
United States Census Bureau, U.S. and World Population Clock. https://www.census.gov/popclock/ , Accessed July, 2021.
Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:617–27.
Download references
Acknowledgements
HB and BI were supported by the Koeln Fortune Program/Faculty of Medicine, University of Cologne, Germany, grant numbers 388/2020 and 389/2020.
Author contributors
All authors had full access to all of the data in the study and had final responsibility to submit this work for publication. HB and CB verified the data and take responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: HB, TB, CB. Acquisition, analysis, or interpretation of data: All authors. Drafting of the manuscript: HB, CB. Critical revision of the manuscript for important intellectual content: HB, BI, TB, GS, JH. Statistical analysis: HB, BI, TB, GS, CB. Obtained funding: HB, BI. Administrative, technical, or material support: TB, JH. Supervision: TB, JH, CB.
Supported by the Koeln Fortune Program/Faculty of Medicine, University of Cologne, Germany. Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and affiliations.
Department of Psychiatry and Psychotherapy, Faculty of Medicine, University of Cologne, Cologne, Germany
Henry Bode, Beatrice Ivens, Jonathan Henssler & Christopher Baethge
Department of Psychiatry and Psychotherapy, Faculty of Medicine, Technical University of Dresden, Dresden, Germany
Institute of Medical Biometry and Statistics, Faculty of Medicine and Medical Center, University of Freiburg, Freiburg, Germany
Guido Schwarzer
Department of Psychiatry and Psychotherapy, Charité Universitätsmedizin Berlin, Berlin, Germany
Jonathan Henssler
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Christopher Baethge .
Ethics declarations
Competing interests.
Dr. Schwarzer reported personal fees from Roche Pharma as external statistical consultant outside the submitted work. No other disclosures were reported.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplemental material, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Bode, H., Ivens, B., Bschor, T. et al. Hyperthyroidism and clinical depression: a systematic review and meta-analysis. Transl Psychiatry 12 , 362 (2022). https://doi.org/10.1038/s41398-022-02121-7
Download citation
Received : 14 April 2022
Revised : 09 August 2022
Accepted : 16 August 2022
Published : 05 September 2022
DOI : https://doi.org/10.1038/s41398-022-02121-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
- Case report
- Open access
- Published: 30 March 2024
Plasmapheresis in thyrotoxicosis: a single-center case series
- I. Rami 1 ,
- D. Zerrouki 1 ,
- I. Assarrar 1 ,
- S. Rouf 1 , 2 &
- H. Latrech 1 , 2
Journal of Medical Case Reports volume 18 , Article number: 193 ( 2024 ) Cite this article
49 Accesses
1 Altmetric
Metrics details
Plasmapheresis represent an alternative therapeutic option for hyperthyroidism with thyroid storm or refractory cases. It provides a rapid decrease in plasma thyroid hormones and anti-thyroid antibodies. The aim of this paper was to report our single center’s experience in managing particular situations of hyperthyroidism using apheresis.
Cases presentation
The following case series describes three young African patients (two females, one male) aged 29, 37, and 25 years old, respectively, with Graves’ disease who presented with drug ineffectiveness, drug-induced agranulocytosis, and thyroid storm with multi-organ failure. The three patients underwent plasmapheresis sessions leading to effective decline of thyroid hormone levels and offering a window for processing total thyroidectomy.
Discussion/conclusion
The standard management of thyrotoxicosis and thyroid storm was usually codified by the concomitant use of antithyroid medication, iodine, beta-blockers, and corticosteroids. This medical preparation can be effective in most cases. However, drug toxicity or ineffectiveness can limit the use of such therapeutics. Our paper supports the efficiency and safety of therapeutic plasma exchange in the preoperative management of thyrotoxicosis.
Peer Review reports
Introduction
Thyrotoxicosis is a clinical syndrome characterized by the excess of circulating thyroid hormones. In most instances, the excess comes from increased production by the thyroid gland. In this case, it may be caused by Graves’ disease (GD), toxic multinodular goiter (TMNG), and toxic nodules (TN) [ 1 ].
Management strategies include reducing thyroid hormone synthesis and release, inhibiting the conversion of thyroxin (T4) to triodothyronine (T3), and moderating the peripheral effects of excess thyroid hormone [ 2 , 3 ]. Therefore, there are different options for treatment depending on the etiology. Thionamides are the first line of treatment in the majority of patients with hyperthyroidism period. Other options are radioactive iodine and thyroid surgery [ 4 ].
In patients with severe hyperthyroidism, further therapies are needed in the acute phase to restore the euthyroid status, therapies such as potassium iodide, beta-adrenergic receptor blockers, glucocorticoids, and therapeutic plasma exchange (TPE). Therapeutic plasma exchange is an alternative treatment that was introduced in the 1970s for hyperthyroidism management [ 5 ]. TPE is an extracorporeal blood purification method considered to remove large-molecular-weight substances bound to plasma proteins such as pathogenic auto-antibodies, immunocomplexes, cryoglobulins, cholesterol-containing lipoproteins, and plasma-protein-bound thyroid hormones. Albumin and fresh frozen plasma (FFP) are used as replacement fluids in TPE for thyrotoxic patients [ 6 ].
The indications of TPE for thyrotoxic patients may join category II of apheresis indications, as determined by the American Society for Apheresis (ASFA) [ 7 ]. Nevertheless, there was formerly no clear consensus recommendation for or against its use in patients with hyperthyroidism without thyroid storm [ 7 ].
The effectiveness of the treatment is determined by the volume of blood being processed, the volume of the plasma exchanged in each process, the frequency of exchange, and other technical features. However, as with any invasive process, TPE also has side effects [ 8 , 9 ].
We report in this series, three cases of thyrotoxic patients who required the use of plasma exchanges. The aim of our work was to study the effectiveness and safety of this therapeutic approach in the preoperative management of thyrotoxic patients.
Case presentation
A 25-year-old African man was admitted to the emergency room (ER) for worsening palpitations, and asthenia, without chest pain or dyspnea. He was diagnosed with Graves’ disease 1 year before, with poor compliance with carbimazole and propranolol therapy.
He stopped taking his medication 1 month after diagnosis. A total of 1 year later, he presented with systolic heart failure with dilated cardiomyopathy. He weaned from alcohol and drug abuse 5 years ago. The patient was treated with spironolactone 50 mg/day, propranolol 40 mg twice daily, furosemide 40 mg/day, ramipril 12.5 mg/day, and digoxin 0.25 mg/day.
On initial evaluation, the heart rate was 125 beats per minute (bpm), blood pressure (BP) was at 110/60 mmHg, his temperature was at 36.5°C, respiratory rate 24 breaths/minute, and SaO 2 was 100%. Physical examination found a skinny man who was discreetly agitated but alert and oriented to place and time with a bilateral proptosis. The patient also presented with a non-active Graves’ orbitopathy. We especially noted signs of heart failure, manifested by jugular venous distension, bilateral lower-extremity edema, and hepatomegaly. There were bibasilar crackles over the lungs. Cervical palpation found a symmetric diffusely enlarged and firm thyroid gland.
The electrocardiogram (ECG) showed atrial fibrillation and poor R wave progression with a rate of 130 bpm. Chest X-ray showed mild cardiomegaly. Laboratory investigations showed severe thyrotoxicosis with a free thyroxine (FT4) at 500 pmol/l [normal values (NV): 12–22]. There were also cholestasis and impaired liver function tests. However, liver and cardiac enzymes were not elevated (Table 1 ).
Regarding his liver dysfunction, he benefited from a complementary workup that included negative viral serologies, as well as a liver ultrasound that showed liver damage secondary to heart failure without lesions or abnormalities in the bile ducts.
Transthoracic echocardiogram showed a biventricular dilated cardiomyopathy, with an ejection fraction of less than 34% and global hypokinesis. Pericardial effusion of 3 mm was found too. In addition, he had a pleural effusion objectified on a chest computed tomography (CT) scan. The removed fluid was transudative. Cervical ultrasound noted a voluminous multinodular goiter with nodules classified as EU-TIRADS3, and a thyroid volume at 100 cc.
The patient was transferred to the cardiology intensive care unit (ICU).
He received cardiology resuscitation. He was kept on the same heart medications with propranolol dose optimization to 40 mg three times a day. He was then transferred to the Endocrinology–Diabetology and Nutrition department after stabilization of his heart condition.
In our department, the patient was started on iodide potassium upon admission, and then prednisone was added on the eighth day of his transfer. In view of his deteriorating status and our inability to start on carbimazole owing to worsening liver dysfunction and his heart failure, a decision was made to begin TPE, taking into account the excessive level of FT4 (Table 1 ).
In total the patient received three sessions of TPE over 3 consecutive days, each with 2.5 L of FFP.
After the last plasmapheresis session, there was an exceptional decline in levels of FT4 and transaminases (Table 1 ). Iodide potassium was continued for 14 days, while propranolol was retained until clinical and biological euthyroidism was obtained. Total thyroidectomy was recommended once hyperthyroidism was controlled. However, the patient refused surgery and discontinued all medication. A total of 1 month later, the patient was admitted to the ER for severe arrhythmia and died on the second day of admission.
A 37-year-old African woman was admitted to the ER for fever, chills, mucositis, sore throat, angina, and generalized body aches. She had been diagnosed with Graves’ disease 2 months before admission. She was put on methimazole (15 mg/day) and propranolol (80 mg/day) with good adherence.
On initial evaluation, the temperature was at 39 °C, heart rate was at 105 bpm, BP was at 90/54 mmHg, respiratory rate 25 breaths/minute, and SaO 2 was at 100%. The physical examination was unremarkable except for homogeneous thyroid hypertrophy and proptosis. Blood workup showed an undetectable TSH, significantly elevated levels of free T4, and agranulocytosis (Table 2 ).
Clinical and biological evaluation in this patient resulted in a diagnosis of septic shock secondary to methimazole-induced agranulocytosis (Table 2 ). Therefore, methimazole was stopped, and the patient was admitted to the ICU. She received standard resuscitation measures and antibiotic therapy for urinary tract infection.
The patient improved her absolute neutrophil count (ANC) and inflammatory markers. Table 2 summarizes the results of the first blood workup and follow-up.
The transthoracic echocardiogram was normal, and the cervical ultrasound showed a homogeneous goiter measuring 25 ml in volume.
After stabilization, the patient was transferred to our department to manage the thyrotoxicosis condition. She was put on propranolol 40 mg three times a day (on day 10), prednisolone 60 mg per day (on day 14), potassium iodide 20 drops orally every 8 hour, and cholestyramine 4 g three times a day. A total of 10 days later, we noted a good clinical course. Nevertheless, thyroid hormone levels were still increased (Table 2 ).
Given the agranulocytosis episode and consistently excessive thyroid hormone levels, TPE was highly recommended in this case.
On hospital day 20, the first session of TPE was managed using FFP. The volume of FFP used in the exchange was defined by the formula: Plasma volume = (0.065 × weight (kg)) × (1-hematocrit). A second session was needed (Table 2 ). No incident or complication during or after the plasmapheresis was recorded. Treatment with potassium iodide, propranolol, and prednisolone was discontinued afterward. After maintaining a clinical and biochemical euthyroid state, she underwent a total thyroidectomy.
A 29-year-old African woman was admitted to the Endocrinology Department for thyrotoxicosis with minor side effects (hives) to antithyroid drugs. She has been followed up with for Grave’s disease for 5 years, initially put on carbimazole 40 mg and propranolol 20 mg daily. The course was marked by the occurrence of urticarial lesions, leading to the switch from carbimazole to benzyl-thiouracil. The dose was gradually increased to 150 mg three times daily. However, her thyroid hormones remained dangerously elevated.
Upon physical examination, the heart rate was regular at 88 bpm, the BP was at 120/70 mm Hg, the respiratory rate was at 19 breaths/minute, and the SaO 2 was 100%. The patient also reported episodes of palpitation and diarrhea. Cervical palpation found a thyroid hypertrophy that was responsible for dysphagia. We also noted bilateral proptosis and urticarial lesions.
Blood tests confirmed thyrotoxicosis without abnormalities in the liver or cardiac enzymes. On complete blood counts, we found microcytic anemia that needed venous iron infusion (Table 3 ). ECG and chest X-ray were normal. The transthoracic echocardiogram was normal, while cervical ultrasound showed a regular goiter of 30 ml in volume.
Benzyl-thiouracil was discontinued upon admission for ineffectiveness and urticaria. The patient was then started on propranolol 40 mg three times a day, cholestyramine 4 g three times a day, and prednisone 60 mg daily. Iodide of potassium was administrated later.
On the 11th day of admission, the first session of TPE was accomplished using FFP with an exchange volume of 3750 ml for each session. The patient required six more sessions of plasmapheresis to achieve clinical and biological euthyroidism (Fig. 1 ). Then, she underwent a total thyroidectomy without complications.
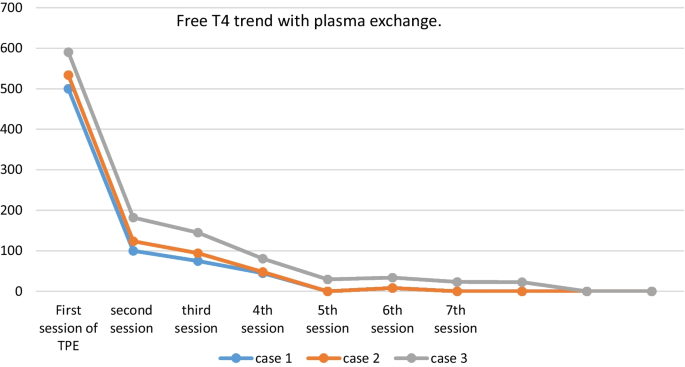
Evolution of FT4 after plasma exchange in the three patients
Thyrotoxicosis is a condition resulting from inappropriate excessive circulating thyroid hormone concentrations. A subtype of thyrotoxicosis, hyperthyroidism, specifically refers to excessive synthesis and secretion of thyroid hormones by the thyroid gland [ 10 ]. The most common cause is Graves’ disease (GD), which accounts for 80% of cases, followed by nodular thyroid goiter and thyroiditis [ 11 ].
Thyroid storm is a severe life-threatening exacerbation of thyrotoxicosis, characterized by the dysfunction of the thermoregulatory, central nervous, digestive, and cardiovascular systems [ 7 ]. Treatment strategies of this rare condition include thionamides, potassium iodide, bile acid sequestrants, glucocorticoids, and beta-blockers [ 1 , 8 ]. Recently, therapeutic plasma exchange is considered more and more as an additional therapeutic option for the management of thyrotoxic patients. In 1970, Ashkar et al. [ 12 ] used this method for the first time in three patients with thyroid storm.
Plasmapheresis is based on extracorporeal separation of plasma from the blood. Plasma is separated from the cellular components of the blood using centrifugation techniques and discarded. The cellular components are then returned to the patient along with replacement fluids such as fresh frozen plasma, albumin, and crystalloids[ 13 ]. TPE needs to be performed by an experienced medical team and used carefully for appropriate indications [ 14 ].
There exists no clear consensus concerning its practice in hyperthyroidism without thyroid storm [ 7 , 15 ]. Indeed, TPE can be performed in thyrotoxic patients with severe symptoms and rapid clinical deterioration, failure or adverse effects of conventional therapy, and obviously, multisystem organ failure[ 13 ]. In the latest guidelines of the American Apheresis Association (ASFA), thyroid storm is designated as a Category IIc and Category III recommendation for TPE [ 16 ]. ASFA recommend that TPE sessions should be executed by multidisciplinary trained medical team, as early as possible and repeated every 24 hours to every 3 days until clinical improvement [ 17 ].
To our best of knowledge, we report the first experience of plasmapheresis for thyrotoxicosis in our country. The three patients had Graves’ disease, and plasmapheresis was indicated for drug side effects (Case 2), thyroid storm complicated by heart failure (Case 1), and drug ineffectiveness (Case 3). Yildirim Simsir et al. [ 18 ] reported the largest series of TPE for thyrotoxicosis. In 46 patients, the most common etiology was Graves’ disease (87%), followed by amiodarone-induced thyrotoxicosis (8,7%), and toxic multinodular goiter (4,3%). In accordance to our cases, plasmapheresis indications were drug side effects (45.7%), drug ineffectiveness (19%), and thyroid storm (6%) [ 5 ].
The number of apheresis sessions in our cases was variable between two to seven sessions with an interval of 24 hours in accordance with the series of Keklik et al. [ 19 ] and Yildirim et al. [ 18 ] where the same interval between TPE sessions was respected. Yildirim et al. [ 18 ] reported a mean of four apheresis sessions [interquartile range (IQR): 3–7] in patients with Graves’ disease, and a mean of three sessions (IQR: 1–7) in patients with non-Graves’ thyrotoxicosis. There was no statistically significant difference between the two groups in terms of the number of sessions ( p = 0.70) according to the cause of thyrotoxicosis. In the series of Keklik et al. [ 22 ], apheresis was applied with a mean of four times (minimum two, maximum nine). An average of 3.4 times (minimum 1, maximum 17) were performed in the study of Ezer et al. [ 20 ]. There is no clear recommendation of number of sessions of TPE. The ASFA recommends continuing sessions until clinical improvement, if there is no adverse effect.[ 17 ].
Plasmapheresis is not completely innocuous. Indeed, the incidence of serious and life-threatening complications of TPE is around 0.025–4.75% [ 21 ]. Transfusion reactions, citrate-related hypocalcaemia, coagulopathy/embolism, and anaphylactic reaction are the most frequent reported side effects. These complications are often catheter-related, and can easily be ruled out by an experienced medical staff. Death is rarely reported and is usually due to the primary disease [ 22 ]. In our series, there were no complications during or after the plasmapheresis sessions, especially those related to catheters (infection, thrombosis). In the study of Yildirim et al. [ 18 ], complications occurred in 6.5%, including catheter infection in two patients, and deep vein thrombosis in a pregnant patient. Another study reported hypotension, citrate-related hypocalcemia, and tachyarrhythmia in the course of myocardial infarction [ 19 ]. Particularly, our cases maintained normal calcium levels before and after the apheresis sessions. This finding is related to the use of heparin instead of citrate in our patients. This technique has recently been used in our facility, and demonstrated its efficacy in managing thyrotoxicosis in our patients. The second and third patients remained well until total thyroidectomy was performed. Unfortunately, the first patient died after refusing surgical treatment and discontinuing all medications against medical advice.
Our paper sheds light on the effectiveness of TPE in the management of refractory, severe thyrotoxicosis. The limiting factor of our case series report is the small number of patients. Our results/conclusions would be even stronger with a larger number of patients. We encourage consideration of this new approach as an effective treatment for patients with complicated or severe, refractory thyrotoxicosis who cannot tolerate, or do not respond to, standard treatment including thfionamides.
In light of the literature and based on our experience, we conclude that TPE is an effective alternate therapeutic option in refractory severe thyrotoxicosis to prepare patients for surgical treatment. It is an appropriate treatment technique to obtain normal thyroid function rapidly and consistently. Thus, TPE should be included in the treatment algorithm for refractory cases or severe/complicated thyrotoxicosis. Therapeutic plasmapheresis should be optimally performed in tertiary centers by an experienced medical team to ensure safety and efficiency.
Availability of data and materials
Not applicable.
Abbreviations
Graves’ disease
Toxic multinodular goiter
Toxic nodules
Triodothyronine
Therapeutic plasma exchange
Fresh frozen plasma
American Society for Apheresis
Emergency room
Blood pressure
Electrocardiogram
Thyroid-stimulating hormone
Intensive care unit
Interquartile range
Ross DS, Burch HB, Cooper DS, Greenlee MC, Laurberg P, Maia AL. American Thyroid Association guidelines for diagnosis and management of hyperthyroidism and other causes of thyrotoxicosis. Thyroid. 2016;2016(26):1343–421.
Article Google Scholar
De Leo S, Lee SYBL. Hyperthyroidism. Lancet. 2016;88(10047):906–18.
Miller A SK. Thyroid storm with multiorgan failure treated with plasmapheresis. Case Rep Endocrinol. 2019;1–5.
Kahaly GJ, Bartalena L, Hegedüs L, Leenhardt L, Poppe KPS. European Thyroid Association guideline for the management of Graves’ hyperthyroidism. Eur Thyroid J. 2018;2018(7):167–86.
Schwartz J, Padmanabhan A, Aqui N, et al . Guidelines on the use of therapeutic apheresis in clinical practice—evidence-based approach from the writing Committee of the American Society for Apheresis: the seventh special issue. J Clin Apher. 2016;31(3):149–62.
PubMed Google Scholar
L W. Thyrotoxic storm. In: Braverman LE, Utiger RD, editors. Werner’s and Ingbar’s the thyroid: a fundamental and clinical text. Lippincott Williams & Wilkins. 2000;679–84.
Carhill A, Gutierrez A, Lakhia R, Nalini R. Surviving the storm: two cases of thyroid storm successfully treated with plasmapheresis. BMJ Case Rep. 2012;2–7.
Angell TE, Lechner MG, Nguyen CT, et al . Clinical features and hospital outcomes in thyroid storm: a retrospective cohort study. J Clin Endocrinol Metab. 2015;100(2):451–9.
Article CAS PubMed Google Scholar
Nayak BBK. Thyrotoxicosis and thyroid storm. Endocrinol Metab Clin North Am. 2006;35(4):663–86.
Sharma A, Marius N, Stan M. Thyrotoxicosis: diagnosis and management. Mayo Clin Proc. 2019;94(6):1048–64.
Article PubMed Google Scholar
Laurberg P, Bülow Pedersen I, Knudsen N. Environmental iodine intake affects the type of nonmalignant thyroid disease. Thyroid. 2001;11(5):457–69.
Ashkar FSKR, Katims RB, Smoak WM III, et al . Thyroid storm treatment with blood exchange and plasmapheresis. JAMA. 1970;214:1275–9.
Muller C, Perrin P, Faller B, et al . Role of plasma exchange in the thyroid storm. Ther Apher Dial. 2011;15(6):522–31.
Basic-Jukic N, Kes P, Glavasa-Boras S, Brunetta B, et al . Complications of therapeutic plasma exchange: experience with treatments. Ther Apher Dial. 2005;9:391–5.
Tietgens STLM. Thyroid storm. Med Clin North Am. 1995;79:169–84.
Szczepiorkowski ZM, Winters JL, Bandarenko N, Kim HC L, ML, Marques MB et al. Apheresis applications committee of the American Society for Apheresis. Guidelines on the use of therapeutic apheresis in clinical practice—evidence-based approach from the Apheresis Applications Committee of the American Society for Apheresis. J Clin Aphe. 2010;25:83–177.
Vinan-Vega M, Mantilla B, Jahan N, Peminda C, Nugent K, Lado-Abeal J, et al . Usefulness of plasmapheresis in patients with severe complicated thyrotoxicosis. Baylor Univ Med Cent Proc. 2020;34(2):279–82. https://doi.org/10.1080/08998280.2020.1852007 .
Simsir IY, Ozdemir M, Duman S, Erdogan M, Donmez A, Ozgen AG. Therapeutic plasmapheresis in thyrotoxic patients. Endocrine. 2018;62(1):144–8. https://doi.org/10.1007/s12020-018-1661-x .
Keklik M, Kaynar L, Yilmaz M, Sivgin S, Solmaz M, Pala C, et al . The results of therapeutic plasma exchange in patients with severe hyperthyroidism: a retrospective multicenter study. Transfus Apher Sci. 2013;48(3):327–30. https://doi.org/10.1016/j.transci.2013.04.010 .
Ezer A, Caliskan K, Parlakgumus A, et al. Preoperative therapeutic plasma exchange in patients with thyrotoxicosis. J Clin Apher. 2009;24(3):111–4. https://doi.org/10.1002/jca.20200 .
Idrose AM. Acute and emergency care for thyrotoxicosis and thyroid storm. Acute Med Surg. 2015;2(3):147–57.
Article PubMed PubMed Central Google Scholar
McLeod BC, Sniecinski I, Ciavarella D, et al . Frequency of immediate adverse effects associated with therapeutic apheresis. Transfus Apher Sci. 1999;39:282–8.
CAS Google Scholar
Download references
Acknowledgements
The authors would like to thank the staff of the Laboratory of Epidemiology, Clinical Research, and Public Health who helped in the sampling procedures.
The authors would like also to acknowledge all medical staff who helped to carry out the study and the school staff who participated.
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and affiliations.
Department of Endocrinology-Diabetology and Nutrition, Faculty of Medicine and Pharmacy, Mohammed VI University Hospital Center, University of Mohammed 1st, 4806, 60049, Oujda, Morocco
I. Rami, D. Zerrouki, I. Assarrar, S. Rouf & H. Latrech
Laboratory of Epidemiology, Clinical Research and Public Health, Faculty of Medicine and Pharmacy, Mohammed VI University Hospital Center, University of Mohammed 1st, 4806, 60049, Oujda, Morocco
S. Rouf & H. Latrech
You can also search for this author in PubMed Google Scholar
Contributions
IR and DZ wrote the manuscript, performed the statistical analysis and helped in the investigation and data curation. They are first co-authors. IA worked on the conceptualization, methodology, and investigation and helped in writing the literature review. SR helped in the conceptualization and methodology, supervised the redaction, and reviewed the manuscript. Professor HL helped in the conceptualization and methodology, supervised the redaction, and reviewed and approved the final draft of the manuscript. All authors approved the final version of the manuscript.
Corresponding author
Correspondence to H. Latrech .
Ethics declarations
Ethics approval and consent to participate.
The study design was approved by the Ethics Board Committee of the Biomedical Research at the Faculty of Medicine, Mohammed First University of Oujda (CERBO), under the number: 22/2020. All of the participants gave their oral consent before enrolling the study.
Consent for publication
Written informed consent was obtained from the patients’ legal guardians for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The Authors declare that there is no conflict of interest.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Rami, I., Zerrouki, D., Assarrar, I. et al. Plasmapheresis in thyrotoxicosis: a single-center case series. J Med Case Reports 18 , 193 (2024). https://doi.org/10.1186/s13256-024-04480-9
Download citation
Received : 26 October 2023
Accepted : 28 February 2024
Published : 30 March 2024
DOI : https://doi.org/10.1186/s13256-024-04480-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Plasmapheresis
- Thyrotoxicosis
- Anti-thyroid agents
- Drug-induced abnormalities
Journal of Medical Case Reports
ISSN: 1752-1947
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
REVIEW article
The pathology of hyperthyroidism.

- Department of Pathology and Laboratory Medicine, University of Pennsylvania Medical Center, Philadelphia, PA, United States
This article reviews those pathologic lesions which are associated with clinical and/or biochemical hyperthyroidism. Beginning with the descriptive pathology of classical Graves' disease and the less common toxic nodular goiter and hyper-functioning thyroid nodules, this paper describes the effects of non-thyroidal hormones, glandular function (including pituitary and hypothalamic lesions), ectopic production of thyroid stimulating proteins by non-thyroidal neoplasms, exogenous drug reactions causing hyper-function and finally conditions associated with a mechanic- destructive cause of hyperthyroidism.
Introduction
Hyperthyroidism is a clinical syndrome characterized by hypermetabolic state due to the increased free serum thyroxine (T4) and/or free triiodothyronine (T3). There are many known factors and pathologies both inherent to the thyroid gland as well of non-thyroidal origin that lead to hyperthyroidism. It can result from hyperplasia and overstimulation of thyroid epithelium, acute destruction of thyroid follicles, and follicular epithelium due to various forms of thyroiditis or metastatic tumors. In addition, various drugs and antineoplastic agents can lead to thyroid dysfunction. In this review we provide a pathologist's perspective on various pathologic features that can be encountered in thyroids of patients with clinical hyperthyroidism.
Toxic Goiter
This condition can be divided into diffuse and nodular types.
Diffuse Toxic Goiter
Most patients with classical hyperthyroidism caused by autoantibodies against the TSH receptors, stimulating thyroid follicular cell receptors show an enlarged hypervascular thyroid without obvious nodularity. This condition known in North America as Graves' disease and in Europe as Basedow disease is a disorder of young usually female patients who present with heat intolerance, tachycardia, tremors, weight loss and orbitopathy ( 1 – 6 ). This disease is characterized by thyroid enlargement with smooth capsule, non-nodular growth, and increased vascularity. Histologically one notes the presence of a diffuse papillary and follicular hyperplasia and varying degrees of lymphocytic infiltration into the thyroid stroma ( 7 – 9 ) (Figures 1 , 2 ). In contrast to classic Hashimoto's disease, the lymphocytes do not infiltrate the follicular cells. The latter often are enlarged and can show cytoplasmic eosinophilia. The nuclei of these cells can also be enlarged and can in extreme cases mimic the nuclei of papillary thyroid carcinoma ( 10 – 12 ). However, the nuclei in Graves' disease tend to maintain a rounded shape and to have internal structure with minimal if any clearing ( 10 – 12 ).
Figure 1 . A case of Graves' disease on low power showing exuberant papillary hyperplasia.
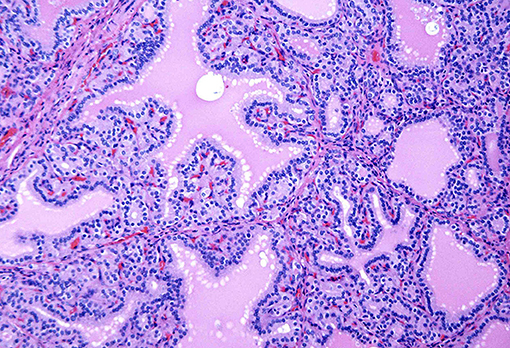
Figure 2 . A case of Graves' disease on medium power showing cells with round nuclei and even chromatin pattern lining the papillae.
There has been controversy regarding whether the presence of Graves' disease can lead to the development of papillary carcinoma and if the two coexist, does the carcinoma behave more aggressively than similar tumors not arising in this background setting. Several studies and our own experience have shown that the data needs to be evaluated systematically ( 13 – 17 ). If a papillary microcarcinoma is discovered incidentally in a surgically removed hyperthyroid gland, the prognosis is excellent. If a clinically evident tumor is identified in a Graves' patient, then the pathological characteristics of that lesion (size, extent, mutlifocality) will determine the prognosis. It does not appear that the background gland influences the prognosis adversely ( 18 ).
Toxic Nodular Goiter
As the name implies is an enlarged gland with multiple nodules of varying sizes. Usually the nodules show the papillary and follicular hyperplasia although the lymphocytic stromal infiltration may be within the nodules and in the non-nodular thyroid ( 9 , 19 ). The correlation between the histology of the nodules and radioactive iodide scan results is fair to poor since nodules with histologic evidence of hyperfunction often are warm or cool on scan. Toxic nodular goiter tends to occur in older individuals and affects males as well as females. In some older patients, the clinical manifestations of the hyperthyroidism may not be related to the thyroid at all; many of these individuals show symptoms related to cardiac disease, frequently atrial fibrillation. This disorder, sometimes referred to as “apathetic hyperthyroidism” needs to be considered by treating clinicians and appropriate laboratory testing will lead to the correct diagnosis ( 20 – 22 ).
Hyperthyroidism Associated With Hyperfunctioning Thyroid Tumors
Most autonomously functioning thyroid tumors are benign that is follicular adenomas or hyperplastic nodules. These lesions are also designated as ‘autonomous nodules” and have been given the acronym “Plummer's disease” ( 23 , 24 ).
Benign hyperfunctional adenomas ( AKA Toxic Adenoma ) are clonal, autonomously functioning follicular proliferations that produce supra-physiological amounts of thyroid hormones causing TSH suppression. These are more common in women and usually present at an older age. Usually, a radioisoptope scan confirms the preoperative diagnosis and most are not subjected to fine-needle aspiration (FNA). However, in rare cases a FNA is performed by a clinician or surgeon without the knowledge of thyroid function tests. In such cases, the FNA specimen is usually cellular and most likely will be diagnosed as a follicular neoplasm (Bethesda Category IV).
On surgical excision the toxic adenoma grossly shows a distinct capsule and may be centrally cystic. These lesions can also show a papillary pattern of growth without nuclear features of papillary carcinoma. The autonomously functioning nodule usually occurs in young females. This lesion also termed “papillary hyperplastic nodule” ( 25 ) (a term coined by the late Dr. Austin Vickery) is an encapsulated or at least circumscribed area in the thyroid composed of exuberant papillary structures often with some follicle formation in the cores of the papillae; the lesions are often centrally cystic and the papillae tend to point toward the center of the nodule. Importantly the nuclei lining these papillary structures are round, have internal structure and are often polarized within the cells (Figures 3 , 4 ). Lymphocytes are rarely found within these lesions ( 11 , 12 ). Most of these nodules are clonal proliferations and are therefore considered adenomas ( 26 – 29 ). (The term “papillary adenoma” would be an appropriate one for these lesions; however, this term is shunned since it has been used to described encapsulated papillary carcinomas in older literature) ( 30 ). Although the great majority of these hyperplastic nodules are not associated with clinical hyperthyroidism, about 15–20% of affected patients do have symptomatic hyperfunction and about another 30% have biochemical hyperthyroid indices ( 25 , 31 , 32 ).
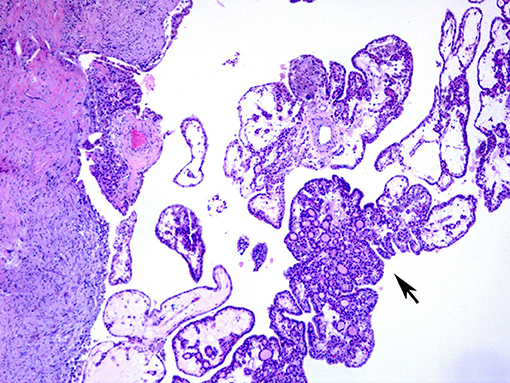
Figure 3 . A case of papillary hyperplastic nodule on low power showing cystic nodule with papillary architecture (arrow).
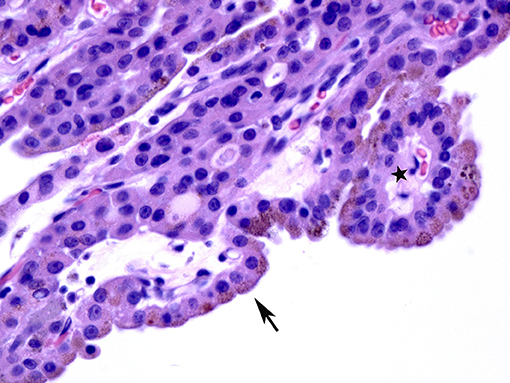
Figure 4 . A case of papillary hyperplastic nodule on high power showing oncocytic cells lining the papillary structures (arrow).
Rarely, malignant tumors of the thyroid may be associated with hyperthyroidism. These are usually but not always follicular carcinomas; some are encapsulated follicular variants of papillary carcinoma ( 33 – 35 ). Although the tumors may lead to hyperfunction while still confined within the gland, many of the affected patients have metastatic disease. Some authors indicate that tumor burden correlates with the degree of hyperthyroidism ( 36 – 41 ).
Another interesting thyroid carcinoma that can present with hyperthyroidism is the rare diffuse follicular variant of papillary carcinoma ( 42 ). A tumor that is most often found in young females who present with goiter, the clinical picture resembles classic Graves' disease or toxic goiter. About 25% of these lesions will show hyperthyroidism and abnormal thyroid function tests. Treatment of the cancer will lead to resolution of the metabolic abnormality ( 40 – 44 ).
Hashitoxicosis
This term originally coined about 40 years ago by the Mayo Clinic group describes patients who present clinically with hyperthyroidism but whose glands show the histology of chronic lymphocytic thyroiditis including oxyphilia (Hürthle cell metaplasia) ( 45 ). This histologic presentation is also often seen in children and very young usually teenage patients who present with thyroid hyperfunction. Often these patients go through a phase of euthyroidism and subsequently hypothyroidism over a period of decades ( 46 – 48 ).
Secondary and Tertiary Hyperthyroidism
When hyperthyroidism is associated with lesions of the pituitary gland or the hypothalamus, it is considered secondary and tertiary hyperthyroidism respectively. In comparison to primary hyper thyroidism, these clinical conditions are extremely rare (< 1% of hyperthyroidism ). The lesion in the pituitary gland is most frequently multifocal thyrotroph hyperplasia rather than a thyroid stimulating hormone (TSH) producing adenoma. Lesions of the hypothalamus producing thyrotropin releasing hormone (TRH) can stimulate the pituitary thyrotrophes to hyper secrete thyroid stimulating hormone (TSH) and subsequently to influence thyroid gland to produce excess thyroid hormone. Lesions of the hypothalamus responsible for this excess TRH include tumors, granulomatous disease (i.e., sarcoid) and other mass producing lesions ( 49 – 53 ).
Hyperthyroidism Due to Struma Ovarii
The presence of thyroid tissue within the ovary is usually seen in benign cystic teratomas also known as dermoid cysts of the ovary ( 54 ). The thyroid in these lesions is often part of a multi-tissue proliferation, that is tissues from all three embryological germ layers are represented. When thyroid tissue is the only or majority of tissue (>50%) in a teratoma (often termed mono dermal teratoma) it is diagnosed as struma ovarii. In most cases the thyroid either appears normal or shows changes consistent with colloid goiter. In rare instances, the thyroid will appear hyperplastic or even show lymphocytic infiltration mimicking thyroiditis. Rarely neoplasms originating in the thyroid gland including papillary carcinoma, follicular carcinoma or even poorly differentiated carcinoma can arise in a background of struma ovarii. Most of these tumors do not involve the ovarian surface and do not spread (these tumors have been designated by some authors as “proliferating struma”) ( 55 ). Unusual situations have been described wherein the struma ovarii or tumors therein may hyper secrete thyroid hormone and lead to clinical hyperthyroidism ( 56 – 58 ).
Hyperthyroidism Associated With Ectopic Production of Thyrotropin (Thyroid Stimulating Hormone- (TSH)) and Thyrotropin Releasing Hormone (TRH)
Rare reported cases of non-endocrine malignant tumors secreting TSH or TRH have been reported. The most common histology is that of hepatocellular carcinoma. The tumor produces these stimulatory hormones and when tumor is entirely removed the levels of hormones drop and hyperthyroidism regresses ( 59 , 60 ).
Hyperthyroidism Associated With Trophoblastic Disease
Gestational trophoblastic disease including hydatidiform mole and choriocarcinoma is associated with marked elevation of beta human chorionic gonadotropin (beta HCG). Because the beta subunit of HCG is identical in chemical structure to one of the subunits of TSH, the HCG elevation can mimic elevated TSH and stimulate the thyroid to produce excess thyroid hormone. Although it is rare to see tissue from the thyroid in these patients, it is expected that the gland would show a hyperplastic appearance with papillae and cellular enlargement. Lymphocytic infiltration would be absent. Treatment of the gestational trophoblastic disease by uterine evacuation followed by chemotherapy usually leads to resolution of the hyperthyroid state ( 61 – 64 ).
Drug Associated Hyperthyroidism
A variety of classes of pharmaceutical agents can cause thyroid dysfunction. It is beyond the purpose of this review to engage in a lengthy discussion of the clinical disorders caused by these drugs. Some of these interfere with metabolism of iodine, others with the production of thyroid hormone and its conversion to active moieties, and still others do not produce abnormalities in thyroid function but cause chemical interference with thyroid function test measurements. Many drugs can affect thyroid function (phenytoin and derivatives, therapies associated with interleukin administration usually in oncology settings) ( 65 – 67 ). The pathologic counterparts for these include lymphocytic infiltration of the gland with or without fibrosis ( 68 ).
Those drugs that cause hyperthyroidism are fewer and they usually exert their effect through interference with the metabolism of iodine. It is rare to see pathological specimens from these patients; the exceptions is the cardiac drug, amiodarone, interleukin containing regimens for chemotherapy and most recently PDL 1 or immune checkpoint inhibitors; these will be discussed below.
Amiodarone Associated Thyroid Dysfunction (AATD)
The literature notes that there are two types of thyroid lesions that are associated with amiodarone, an iodine containing compound used to treat cardiac arrhythmias. Amiodarone induced thyrotoxicosis (AIT) is classified as type I and type II, the former occurs in patients with underlying thyroid disease such as nodular goiter, autonomous nodular goiter or Graves' disease, whereas, Type II is caused by iodine-led destruction of the thyroid follicular epithelium in a normal thyroid gland. Because amiodarone is vital to control the cardiac problems, it is often not possible to wean the patient from the medication or to change to another drug. The first line of therapy in amiodarone induced thyrotoxicosis is treatment with Thionamides in AIT I and glucocorticoids in AIT II. Thyroid excision is undertaken in patients who do not respond to medical therapy in order to treat the hyperthyroidism which often worsens cardiac symptoms ( 69 – 72 ).
If the gland is already pathologically abnormal (nodular thyroid goiter, Graves' disease), the pathology of the resected gland shows follicular disruption with histiocytes infiltrating the follicular epithelium and colloid (Figures 5 , 6 ). Rarely, inflammatory cells are noted within the thyroid parenchyma (Type I). On the other hand if the thyroid is histologically normal (Type II), the pathologic lesions show much milder follicular damage ( 73 – 75 ). These changes are similar to those seen in amiodarone induced pulmonary and liver toxicity ( 76 , 77 ). Ultrastructural studies of both lung and thyroid tissues have shown lysosomal and mitochondrial inclusions in follicular cells consistent with follicle cell destruction ( 77 ). However, this simple explanation is not the only reason for the thyroid dysfunction. For example, co-cultures of amiodarone with human thyrocytes have shown the production of interleukin 6 and the drug also decreases the sodium-iodide symporter mRNA in the follicular cells ( 78 ).
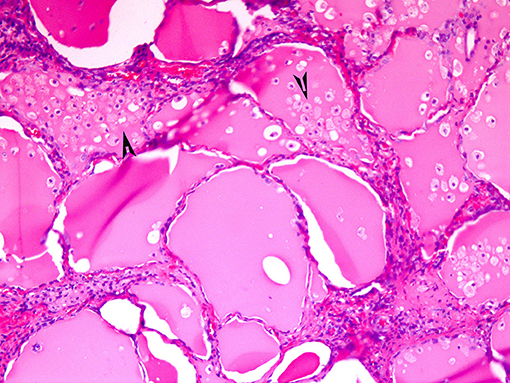
Figure 5 . Amiodarone associated follicular cell damage. Low and high power showing large thyroid follicles filled with colloid and numerous histiocytes (arrow heads, 3A,B).
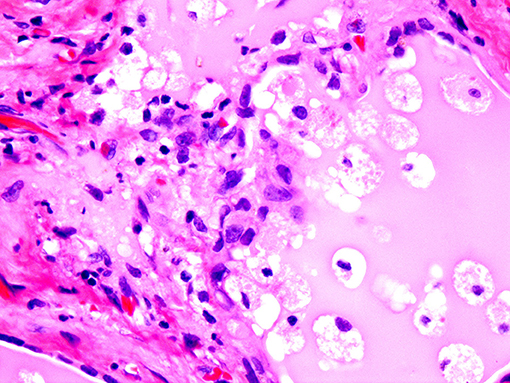
Figure 6 . Same as Figure 5 .
The removal of the thyroid in amiodarone induced hyperthyroidism results in resolution of the hyperfunction and reversion of the cardiac disorder to baseline ( 75 , 79 ).
Hyperthyroidism Associated With Antineoplastic Agents and Targeted Therapies
Thyroid dysfunction can occur in 20–50% patients receiving antineoplastic agents and targeted therapies. High dose IL-2 therapy can lead to hyperthyroidism in 7% of patients. In rare instances, interferon-alpha treatment can lead to classic Graves' disease and even Graves' opthalmopathy; and these condition can persist even after the cessation of therapy ( 67 , 80 ). At present, several tyrosine kinase inhibitors (TK1) are being used to treat different types of malignant neoplasms. TKI can lead to various forms of toxicities including those related to endocrine organs. Transient hyperthyroidism can occur during TKI therapy and is often due to destructive thyroiditis ( 67 , 80 ).
Immune check-point inhibitors with their antitumor activity have shown to improve the survival rates of non-small cell lung carcinoma, melanoma, bladder and renal carcinoma, and ovarian carcinoma. A small number of patients undergoing treatment with immune-checkpoint inhibitors such as anti PD-1/anti-PDL-1 can develop hyperthyroidism ( 81 ).
Mechanico- Destructive Causes of Hyperthyroidism (Non-Hyperthyroid Thyrotoxicosis)
The term mechanico-destructive hyperthyroidism (non-hyperthyroid thyrotoxicosis) has been coined by us to describe those conditions in which relatively rapid destruction of thyroid tissue followed by release of stored thyroid hormone from colloid as follicles or destroyed produces hyperfunction. Both benign and malignant conditions can be associated with this type of hyperthyroidism. An important clinical clue to the possibility of one of these disorders is that in contrast to more common causes of hyperthyroidism, radionucleotide scans show uptakes in the range of 1% or less. This reflects the destruction of the thyroid gland by the inflammatory or neoplastic process; the follicular epithelium is destroyed and cannot take up the radioactive isotope.
Subacute Thyroiditis (“Granulomatous Thyroiditis”; De Quervain Thyroiditis)
This condition is believed to be associated with systemic and or thyroid infection usually viral in nature, is often a painful cause of hyperthyroidism. Patients with this disorder will often present with neck pain which may be referred to the jaw or the chest. In the initial phases of this disease symptoms of hyperthyroidism are often clinically evident. As the gland is replaced by the inflammatory granulomatous process, the follicular epithelium is destroyed, follicles of ruptured and stored thyroid hormone within the colloid is released into the circulation. Unlike usual Graves' disease however the thyroid cannot take up iodide and produce more hormone. Thus, a phase of hypothyroidism is noted until healing occurs ( 82 – 84 ).
Malignant Neoplasms Causing Hyperthyroidism
Malignant neoplasms which are rapidly growing can be associated with this mechanic-destructive type of hyperthyroidism. The tumors most often identified are anaplastic thyroid carcinoma, malignant lymphoma usually primary in the thyroid and of large cell type and poorly differentiated metastatic cancers involving the thyroid (breast carcinoma and lung carcinoma most commonly). Histologically one sees the highly malignant tumor freely infiltrating the thyroid, destroying and replacing the tissue, with rupture of the follicles and release of thyroid hormone containing colloid. The rapidity of the process can lead to market elevation of thyroid hormone and a toxic state simulating thyroid storm ( 85 – 89 ).
In affected patients, there is often near complete destruction of the gland and the eventual development of hypothyroidism. Patients need to be supplemented with thyroid hormone to maintain a euthyroid metabolic state; if treatment of the tumor is successful, regeneration of thyroid follicles may occur from the residual thyroid tissue and as in subacute thyroiditis, normalization of thyroid function may occur ( 86 – 89 ).
This review has described the pathology and clinicopathologic correlations of unusual lesions of the thyroid and extrathyroidal tissues which can show clinical manifestations of hyperthyroidism. Although most of these conditions are rare especially when compared to Graves' disease or toxic nodular goiter, it is important for both the clinician and pathologist to be aware of them as diagnostic considerations.
Author Contributions
VL and ZB have equally contributed to the literature review, drafting the manuscript and obtaining microscopic photographs. Both authors have reviewed the final version of this manuscript before submitting it to topic editors of the journal.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Carnell NE, Valente WA. Thyroid nodules in Graves' disease:classification, characterization, and response to treatment. Thyroid (1998) 8:571–6. doi: 10.1089/thy.1998.8.571
CrossRef Full Text | Google Scholar
2. Gossage A, Munro D. The pathogenesis of Graves' disease. Clin Endocrinol Metab. (1985) 14:299–330. doi: 10.1016/S0300-595X(85)80036-0
PubMed Abstract | CrossRef Full Text | Google Scholar
3. Burman K, Baker J. Immune Mechanisms in Graves' disease. Endocr Rev. (1985) 6:183–223. doi: 10.1210/edrv-6-2-183
4. Karoutsou E, Polymeris A. Pathogenesis of Graves' disease focusing on Graves' ophthalmopathy. Endocr Regul. (2011) 45:209–20. doi: 10.4149/endo_2011_04_209
5. Leovey A, Bako G, Sztojka I, Szabo J, Kalman K, Szabo T. The common incidence of Basedow's-Graves' disease and chronic lymphocytic thyroiditis. Radiobiol Radiother. (1984) 25:769–74.
PubMed Abstract | Google Scholar
6. McKenna TJ. Graves' disease. Lancet (2001) 357:1793–6. doi: 10.1016/S0140-6736(00)04906-0
7. Hirota Y, Tamai H, Hayashi Y, Matsubayashi S, Matsuzuka F, Kuma K, et al. Thyroid function and histology in forty-five patients with hyperthyroid Graves' disease in clinical remission more than ten years after thionamide drug treatment. J Clin Endocrinol Metab. (1986) 62:165–9. doi: 10.1210/jcem-62-1-165
8. Mizukami Y, Matsubara F. [Clinicopathological study on the chronic thyroiditis and Graves' disease –relationship between histological classification and function (author's transl)]. Nihon Naika Gakkai Zasshi (1980) 69:321–9. doi: 10.2169/naika.69.321
9. Spjut H, Warren W, Ackerman L. Clinical-pathologic study of 76 cases of recurrent Graves' disease, toxic (nonexophthalmic) goiter, and nontoxic goiter. Am J Clin Pathol. (1957) 27:367–92. doi: 10.1093/ajcp/27.4.367
10. Albores-Saavedra J, Wu J. The many faces and mimics of papillary thyroid carcinoma. Endocrine Pathol. (2006) 17:1–18. doi: 10.1385/EP:17:1:1
11. Baloch ZW, LiVolsi VA. Cytologic and architectural mimics of papillary thyroid carcinoma. Diagnostic challenges in fine-needle aspiration and surgical pathology specimens. Am J Clin Pathol. (2006) 125(Suppl.):S135–44. doi: 10.1309/YY72M308WPEKL1YY
12. LiVolsi VA. Papillary neoplasms of the thyroid. Pathologic and prognostic features. Am J Clin Pathol. (1992) 97:426–34. doi: 10.1093/ajcp/97.3.426
13. Bitton RN, Sachmechi I, Tabriz MS, Murphy L, Wasserman P. Papillary carcinoma of the thyroid with manifestations resembling Graves' disease. Endocr Pract. (2001) 7:106–9. doi: 10.4158/EP.7.2.106
14. Braga M, Graf H, Ogata A, Batista J, Hakim NC. Aggressive behavior of papillary microcarcinoma in a patient with Graves' disease initially presenting as cystic neck mass. J Endocrinol Invest. (2002) 25:250–3. doi: 10.1007/BF03343999
15. Lucas Martin A, Sanmarti Sala A. [Association of Graves-Basedow disease with thyroid papillary carcinoma:a pathogenic relationship?]. Med Clin. (1987) 89:664–5.
16. Pujadas R, Fernandez F, Camacho L, Foz M. [Association of Graves-Basedow disease with thyroid papillary carcinoma:a pathogenic relationship?]. Med Clin. (1987) 88:786.
17. Valenti TM, Macchia E, Pisa R, Bucalo ML, Russo V, Colletti I, et al. Toxic adenoma and papillary thyroid carcinoma in a patient with Graves' disease. J Endocrinol Invest. (1999) 22:701–4. doi: 10.1007/BF03343633
18. Wei S, Baloch ZW, LiVolsi VA. Thyroid carcinoma in patients with Graves' disease:an institutional experience. Endocrine Pathol. (2015) 26:48–53. doi: 10.1007/s12022-014-9343-6
19. Studer H, Peter H, Gerber H. Toxic nodular goitre. Clin Endocrinol Metab. (1985) 14:351–72. doi: 10.1016/S0300-595X(85)80038-4
20. Thomas FB, Mazzaferri EL, Skillman TG. Apathetic thyrotoxicosis: A distinctive clinical and laboratory entity. Ann Intern Med. (1970) 72:679–85. doi: 10.7326/0003-4819-72-5-679
21. Johnson PC, Kahil ME. Apathetic hyperthyroidism. A type of masked thyrotoxicosis. Tex Med. (1967) 63:59–62.
22. Wu W, Sun Z, Yu J, Meng Q, Wang M, Miao J, et al. A clinical retrospective analysis of factors associated with apathetic hyperthyroidism. Pathobiology (2010) 77:46–51. doi: 10.1159/000272954
23. Miller JM. Plummer's disease. Med Clin North Am. (1975) 59:1203–16. doi: 10.1016/S0025-7125(16)31968-X
24. Messina G, Viceconti N, Trinti B. Diagnostic items and treatment of Plummer's disease:a study on 180 patients. La Clinica Terapeutica (1998) 149:191–5.
25. Vickery AL Jr. Thyroid papillary carcinoma. Pathological and philosophical controversies. Am J Surg Pathol. (1983) 7:797–807. doi: 10.1097/00000478-198307080-00009
26. Apel RL, Ezzat S, Bapat BV, Pan N, LiVolsi VA, Asa SL. Clonality of thyroid nodules in sporadic goiter. Diagn Mol Pathol. (1995) 4:113–21. doi: 10.1097/00019606-199506000-00007
27. Deleu S, Allory Y, Radulescu A, Pirson I, Carrasco N, Corvilain B, et al. Characterization of autonomous thyroid adenoma:metabolism, gene expression, and pathology. Thyroid (2000) 10:131–40. doi: 10.1089/thy.2000.10.131
28. Aeschimann S, Kopp PA, Kimura ET, Zbaeren J, Tobler A, Fey MF, et al. Morphological and functional polymorphism within clonal thyroid nodules. J Clin Endocrinol Metabol. (1993) 77:846–51.
29. Kopp P, Kimura ET, Aeschimann S, Oestreicher M, Tobler A, Fey MF, et al. Polyclonal and monoclonal thyroid nodules coexist within human multinodular goiters. J Clin Endocrinol Metabol. (1994) 79:134–9.
30. Meissner W, Warren S. Tumors of the Thyroid Gland. Washington, DC: Armed Forces Institute of Pathology (1969).
Google Scholar
31. Khurana KK, Baloch ZW, LiVolsi VA. Aspiration cytology of pediatric solitary papillary hyperplastic thyroid nodule. Arch Pathol Lab Med. (2001) 125:1575–8. doi: 10.1043/0003-9985(2001)125<1575:ACOPSP>2.0.CO;2
32. Khayyata S, Barroeta JE, LiVolsi VA, Baloch ZW. Papillary hyperplastic nodule:pitfall in the cytopathologic diagnosis of papillary thyroid carcinoma. Endocr Pract. (2008) 14:863–8. doi: 10.4158/EP.14.7.863
33. Sasaki J, Odaka Y, Kato R, Tada T, Yagawa K, Kowata T, et al. [Hyperfunctioning follicular carcinoma of the thyroid. A case report]. Nippon Geka Gakkai Zasshi (1988) 89:286–91.
34. Michigishi T, Mizukami Y, Shuke N, Satake R, Noguchi M, Aburano T, et al. An autonomously functioning thyroid carcinoma associated with euthyroid Graves' disease. J Nucl Med. (1992) 33:2024–6.
35. Ardito G, Vincenzoni C, Cirielli C, Guidi ML, Corsello MS, Modugno P, et al. Papillary thyroid carcinoma mimicking an autonomous functioning nodule. Eur J Surg Oncol. (1997) 23:569. doi: 10.1016/S0748-7983(97)93397-7
36. Niepomniszcze H, Suarez H, Pitoia F, Pignatta A, Danilowicz K, Manavela M, et al. Follicular carcinoma presenting as autonomous functioning thyroid nodule and containing an activating mutation of the TSH receptor (T620I) and a mutation of the Ki-RAS (G12C) genes. Thyroid (2006) 16:497–503. doi: 10.1089/thy.2006.16.497
37. Siddiqui AR, Karanauskas S. Hurthle cell carcinoma in an autonomous thyroid nodule in an adolescent. Pediatr Radiol. (1995) 25:568–9. doi: 10.1007/BF02015798
38. Smith M, McHenry C, Jarosz H, Lawrence AM, Paloyan E. Carcinoma of the thyroid in patients with autonomous nodules. Am Surg. (1988) 54:448–9.
39. Tfayli HM, Teot LA, Indyk JA, Witchel SF. Papillary thyroid carcinoma in an autonomous hyperfunctioning thyroid nodule:case report and review of the literature. Thyroid (2010) 20:1029–32. doi: 10.1089/thy.2010.0144
40. Siddiqi IN, Friedman J, Barry-Holson KQ, Ma C, Thodima V, Kang I, et al. Characterization of a variant of t(14;18) negative nodal diffuse follicular lymphoma with CD23 expression, 1p36/TNFRSF14 abnormalities, and STAT6 mutations. Mod Pathol. (2016) 29:570–81. doi: 10.1038/modpathol.2016.51
41. Vinciguerra GL, Noccioli N, Bartolazzi A. Diffuse follicular variant of papillary thyroid carcinoma:a case report with a revision of literature. Rare Tumors (2016) 8:6536. doi: 10.4081/rt.2016.6536
42. Sobrinho-Simões M, Soares J, Carneiro F, Limbert E. Diffuse follicular variant of papillary carcinoma of the thyroid: report of eight cases of a distinct aggressive type of thyroid tumor. Surg Pathol. (1990) 3:189–203.
43. Cha YJ, Chang HS, Hong SW. Diffuse follicular variant of papillary thyroid carcinoma in a 69-year-old man with extensive extrathyroidal extension: a case report. J Korean Med Sci. (2013) 28:480–4. doi: 10.3346/jkms.2013.28.3.480
44. Mizukami Y, Nonomura A, Michigishi T, Ohmura K, Noguchi M, Ishizaki T. Diffuse follicular variant of papillary carcinoma of the thyroid. Histopathology (1995) 27:575–7. doi: 10.1111/j.1365-2559.1995.tb00331.x
45. Fatourechi V, McConahey WM, Woolner LB. Hyperthyroidism associated with histologic Hashimoto's thyroiditis. Mayo Clin Proc. (1971) 46:682–9.
46. Mori T. [Hashitoxicosis and Hashimoto's disease with the symptoms of thyrotoxicosis]. Nihon Rinsho (1980) 38:1677–83.
47. Nabhan ZM, Kreher NC, Eugster EA. Hashitoxicosis in children:clinical features and natural history. J Pediatr. (2005) 146:533–6. doi: 10.1016/j.jpeds.2004.10.070
48. Wasniewska M, Corrias A, Salerno M, Lombardo F, Aversa T, Mussa A, et al. Outcomes of children with hashitoxicosis. Horm Res Paediatr. (2012) 77:36–40. doi: 10.1159/000334640
49. Waldhausl W, Bratusch-Marrain P, Nowotny P, Buchler M, Forssmann WG, Lujf A, et al. Secondary hyperthyroidism due to thyrotropin hypersecretion:study of pituitary tumor morphology and thyrotropin chemistry and release. J Clin Endocrinol Metab. (1979) 49:879–87. doi: 10.1210/jcem-49-6-879
PubMed Abstract | CrossRef Full Text
50. Gomez JB, Diaz MA, Jerez ML. [Tertiary hyperthyroidism. Criteria of evaluation]. Rev Med. (1973) 17:231–9.
51. Kourides I, Ridgway E, Weintraub B, Bigos S, Gershengorn M, Maloof F. Thyrotropin-induced hyperthyroidism:use of alpha and beta subunit levels to identify patients with pituitary tumors. J Clin Endocrinol Metab. (1977) 45:534–43. doi: 10.1210/jcem-45-3-534
52. Dorfman SG. Hyperthyroidism. Usual and unusual causes. Arch Internal Med. (1977) 137:995–6. doi: 10.1001/archinte.1977.03630200005005
53. Tolis G, Bird C, Bertrand G, McKenzie J, Ezrin C. Pituitary hyperthyroidism. Am J Med. (1978) 64:177–181. doi: 10.1016/0002-9343(78)90202-4
54. Prat J, Cao D, Carinelli SG, Nogales FF, Vang R, Zaloudek CJ. WHO Classification of Tumours of Female Reproductive Organs. Lyon: IARC (2014).
55. Devaney K, Snyder R, Norris HJ, Tavassoli FA. Proliferative and histologically malignant struma ovarii:a clinicopathologic study of 54 cases. Int J Gynecol Pathol. (1993) 12:333–43. doi: 10.1097/00004347-199310000-00008
56. Yang Q, Yang X, Liu ZZ, Jiang YX, Li JC, Su N, et al. Sonographic and pathologic features of struma ovarii. Zhongguo Yi Xue Ke Xue Yuan Xue Bao (2015) 37:309–14. doi: 10.3881/j.issn.1000-503X.2015.03.012
57. Yassa L, Sadow P, Marqusee E. Malignant struma ovarii. Nat Clin Pract Endocrinol Metabol. (2008) 4:469–72. doi: 10.1038/ncpendmet0887
58. Wei S, Baloch ZW, LiVolsi VA. Pathology of struma ovarii: a report of 96 Cases. Endocrine Pathol. (2015) 26:342–8. doi: 10.1007/s12022-015-9396-1
59. Helzberg JH, McPhee MS, Zarling EJ, Lukert BP. Hepatocellular carcinoma:an unusual course with hyperthyroidism and inappropriate thyroid-stimulating hormone production. Gastroenterology (1985) 88:181–4. doi: 10.1016/S0016-5085(85)80152-9
60. Carri J, Peral F, Surreco M, Lujan A, Leguizamon R, Martinez G, et al. [Fibrolamellar hepatocellular carcinoma:a clinical report with paraneoplastic hyperthyroidism (apropos of a case)]. Acta Gastroenterol Latinoam. (1989) 19:155–64.
61. Berdjis N, Baldauf A, Kittner T, Manseck A, Wirth M. [Paraneoplastic hyperthyroidism in a patient with metastasizing teratocarcinoma and excessively high HCG]. Aktuelle Urol. (2003) 34:407–9. doi: 10.1055/s-2003-43174
62. Kohler S, Tschopp O, Jacky E, Schmid C. Paraneoplastic hyperthyroidism. BMJ Case Rep. (2011) 2011. doi: 10.1136/bcr.04.2011.4163
63. Oosting SF, de Haas EC, Links TP, de Bruin D, Sluiter WJ, de Jong IJ, et al. Prevalence of paraneoplastic hyperthyroidism in patients with metastatic non-seminomatous germ-cell tumors. Ann Oncol. (2010) 21:104–8. doi: 10.1093/annonc/mdp265
64. Solomon CG, Dluhy RG. Paraneoplastic endocrine syndromes. Curr Ther Endocrinol Metab. (1994) 5:537–42.
65. Hein MD, Jackson IM Review:thyroid function in psychiatric illness. Gen Hosp Psychiatry (1990) 12:232–44. doi: 10.1016/0163-8343(90)90060-P
66. Visser WE, de Rijke YB, van Toor H, Visser TJ. Thyroid status in a large cohort of patients with mental retardation:the TOP-R (Thyroid Origin of Psychomotor Retardation) study. Clin Endocrinol. (2011) 75:395–401. doi: 10.1111/j.1365-2265.2011.04089.x
67. Krouse RS, Royal RE, Heywood G, Weintraub BD, White DE, Steinberg SM, et al. Thyroid dysfunction in 281 patients with metastatic melanoma or renal carcinoma treated with interleukin-2 alone. J Immunother Emphasis Tumor Immunol. (1995) 18:272–8. doi: 10.1097/00002371-199511000-00008
68. Rosenbaum A, Maruta T, Richelson E. Drugs that alter mood:lithium. Mayo Clin Proc. (1979) 54:401–7.
69. Amico J, Richardson V, Alpert B, Klein I. Clinical and chemical assessment of thyroid function during therapy with amiodarone. Arch Intern Med. (1984) 144:487–90. doi: 10.1001/archinte.1984.00350150071023
70. Alves L, Rose E, Cahill T. Amiodarone and the thyroid. Ann Intern Med. (1985) 102:412. doi: 10.7326/0003-4819-102-3-412_1
71. Gammage M, Franklyn J. Amiodarone and the thyroid. Q J Med. (1987) 238:83–6.
72. Bogazzi F, Bartalena L, Gasperi M, Braverman LE, Martino E. The various effects of amiodarone on thyroid function. Thyroid (2001) 11:511–9. doi: 10.1089/105072501300176471
73. Smyrk T, Goellner J, Brennan M, Carney J. Pathology of the thyroid in amiodarone assoicated thyrotoxicosis. Am J Surg Pathol. (1987) 11:197–204. doi: 10.1097/00000478-198703000-00004
74. Saad A, Falciglia M, Steward DL, Nikiforov YE. Amiodarone-induced thyrotoxicosis and thyroid cancer:clinical, immunohistochemical, and molecular genetic studies of a case and review of the literature. Arch Pathol Lab Med. (2004) 128:807–10. doi: 10.1043/1543-2165(2004)128<807:ATATCC>2.0.CO;2
75. Elnaggar MN, Jbeili K, Nik-Hussin N, Kozhippally M, Pappachan JM. Amiodarone-induced thyroid dysfunction:a clinical update. Exp Clin Endocrinol Diabetes (2018) 126:333–41. doi: 10.1055/a-0577-7574
76. Marchlinski F, Gansler T, Waxman H, Josephson M, Amiodarone pulmonary toxicity. Ann Intern Med. (1982) 97:839–45. doi: 10.7326/0003-4819-97-6-839
77. Dake M, Madison J, Montgomery C, Shellito JE, Hinchcliffe WA, Winkler ML, et al. Electron microscopic demonstration of lysosomal inclusion bodies in lung, liver, lymph nodes and blood leukocytes of patients with amiodarone pulmonary toxicity. Am J Med. (1985) 78:506–512. doi: 10.1016/0002-9343(85)90346-8
78. Yamazaki K, Mitsuhashi T, Yamada E, Yamada T, Kosaka S, Takano K, et al. Amiodarone reversibly decreases sodium-iodide symporter mRNA expression at therapeutic concentrations and induces antioxidant responses at supraphysiological concentrations in cultured human thyroid follicles. Thyroid (2007) 17:1189–200. doi: 10.1089/thy.2007.0215
79. Bartalena L, Bogazzi F, Chiovato L, Hubalewska-Dydejczyk A, Links TP, Vanderpump M. 2018 European Thyroid Association (ETA) guidelines for the management of amiodarone-associated thyroid dysfunction. Eur Thyroid J. (2018) 7:55–66. doi: 10.1159/000486957
80. Hamnvik OP, Larsen PR, Marqusee E. Thyroid dysfunction from antineoplastic agents. J Natl Cancer Inst. (2011) 103:1572–87. doi: 10.1093/jnci/djr373
81. Guaraldi F, La Selva R, Sama MT, D'Angelo V, Gori D, Fava P, et al. Characterization and implications of thyroid dysfunction induced by immune checkpoint inhibitors in real-life clinical practice:a long-term prospective study from a referral institution. J Endocrinol Invest. (2018) 41:549–56. doi: 10.1007/s40618-017-0772-1
82. Pichler R, Wolfl S, Bogner S, Sulzbacher H, Shamiyeh A, Maschek W. [Subacute thyroiditis with cell destruction and temporary hyperthyroidism in Graves'disease–case report]. Acta Medica Austriaca. (2002) 29:137–40. doi: 10.1046/j.1563-2571.2002.02008.x
83. Woolf PD. Painless thyroiditis as a cause of hyperthyroidism:subacute or chronic lymphocytic? Arch Internal Med. (1978) 138:26–7. doi: 10.1001/archinte.1978.03630250010006
84. Janssen OE. [Atypical presentation of subacute thyroiditis]. Dtsch Med Wochenschr. (2011) 136:519–22. doi: 10.1055/s-0031-1274535
85. De Ridder M, Sermeus AB, Urbain D, Storme GA. Metastases to the thyroid gland-a report of six cases. Eur J Intern Med. (2003) 14:377–9. doi: 10.1016/S0953-6205(03)90005-7
86. Shimaoka K. Thyrotoxicosis due to metastatic involvement of the thyroid. Arch Intern Med. (1980) 140:284–5. doi: 10.1001/archinte.1980.00330140142050
87. Shimaoka K. VanHerle AJ, Dindogru A, Thyrotoxicosis secondary to involvement of the thyroid with malignant lymphoma. J Clin Endocrinol Metab. (1976) 43:64–8. doi: 10.1210/jcem-43-1-64
88. Chung AY, Tran TB, Brumund KT, Weisman RA, Bouvet M. Metastases to the thyroid:a review of the literature from the last decade. Thyroid (2012) 22:258–68. doi: 10.1089/thy.2010.0154
89. Li B, Tang Y, Xiao D, Zhong M, Coexistence of thyroid metastasis carcinoma in the background of HT and primary thyroid mucosa-associated lymphoid tissue B cell lymphoma in a thyroid gland. Ann Hematol. (2010) 89:1053–6. doi: 10.1007/s00277-010-0906-4
Keywords: hyperthyroidism, thyrotoxicosis, non-hyperthyroid, Graves' disease, hyperfunctioning nodules, ectopic hyperthyroidism, drug reactions, mechanico-destructive
Citation: LiVolsi VA and Baloch ZW (2018) The Pathology of Hyperthyroidism. Front. Endocrinol . 9:737. doi: 10.3389/fendo.2018.00737
Received: 07 August 2018; Accepted: 20 November 2018; Published: 03 December 2018.
Reviewed by:
Copyright © 2018 LiVolsi and Baloch. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Virginia A. LiVolsi, [email protected]
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
- Search by keyword
- Search by citation
Page 1 of 8
Novel molecular typing reveals the risk of recurrence in patients with early-stage papillary thyroid cancer
Papillary thyroid cancer (PTC) is an indolent disease with a favorable prognosis but characterized by a high recurrence rate. We aimed to improve precise stratification of recurrence risk in PTC patients with ...
- View Full Text
Evaluating the progression to abnormal thyrotropin in euthyroid preconception women: a population-based study
Abnormal preconception thyrotropin levels were associated with fecundability and adverse fetomaternal outcomes, however, little is known regarding the natural change of serum thyrotropin in euthyroid preconce...
When to settle for SETTLE! A lesson learned from our cases
Spindle epithelial tumor with thymic like elements (SETTLE) is a biphasic tumor composed of epithelial and spindle cell components. It is an uncommon indolent tumor arising in the thyroid gland and most common...

Ultrasound radiomics signature for predicting central lymph node metastasis in clinically node-negative papillary thyroid microcarcinoma
Whether prophylactic central lymph node dissection is necessary for patients with clinically node-negative (cN0) papillary thyroid microcarcinoma (PTMC) remains controversial. Herein, we aimed to establish an ...
Clinical characteristics and outcomes of patients with TSH-secreting pituitary adenoma and Graves’ disease - a case report and systematic review
Coexistence of TSH-secreting pituitary adenoma (TSHoma) and Graves’ disease (GD) is rare and complicates the management decision.
Thyroid storm in pregnancy: a review
Thyroid storm is a state of circulating thyroid hormone excess leading to multiorgan dysfunction and systemic decompensation. It typically occurs in the setting of poorly controlled hyperthyroidism and a preci...
Radiofrequency ablation for thyroid nodules in Ecuador: a cross-sectional study
To describe the demographic characteristics and clinical outcomes following the first cohort of patients with Bening Thyroid Nodule (BTN) and (Papillary Thyroid Microcarcinoma) (PTMC) treated with Radiofrequen...
Twice or thrice weekly levothyroxine provides similar rates of adherence and post-Ramadan euthyroidism compared to daily levothyroxine during Ramadan fasting
Having to take levothyroxine (L-T4) on a daily basis, on an empty stomach is burdensome and may impair adherence, especially during Ramadan fasting. A long half-life and autoregulation of thyroid hormone level...
Assessing the cardiovascular effects of levothyroxine use in an ageing United Kingdom population (ACEL-UK) protocol: a cohort and target trial emulation study
Subclinical hypothyroidism is diagnosed when serum thyroid stimulating hormone levels are higher whilst free thyroxine levels remain within their respective reference ranges. These reference ranges are uniform...
Differential expression of zinc finger CCHC-type superfamily proteins in thyroid carcinoma and their associations with tumor immunity
The zinc-finger CCHC-type (ZCCHC) superfamily proteins are characterized by the shared sequence CX2-CX4-HX4-C and thought to own high affinity to single-stranded nucleic acids, particularly RNAs. In humans, a ...
Is nitric oxide a clue to endemic goitre in highlanders?
Goitre is commonly caused by a lack of iodine in the diet. This condition is particularly prevalent in high-altitude areas where iodine deficiency is common. Here we speculate that inorganic nitrate, the oxida...
Use of thyroid hormones in hypothyroid and euthyroid patients: a 2022 THESIS questionnaire survey of members of the Latin American Thyroid Society (LATS)
Inconsistencies in the medical management of hypothyroidism have been reported between endocrinologists in different countries. This study aimed to identify the attitudes of Latin America thyroid specialists t...
RET/PTC rearrangement in papillary thyroid carcinoma arising in malignant struma ovarii with abdominal wall metastasis and cervical thyroid gland: a case report and review of the literature
Struma ovarii refers to rare mature cystic teratomas containing at least 50% of thyroid tissue, and malignant transformation is known to be even rarer. The synchronous development of malignant struma ovarii an...
Expressions of mitochondria-related genes in pregnant women with subclinical hypothyroidism, and expressions of miRNAs in maternal and cord blood
Subclinical hypothyroidism in pregnancy and definition by upper thyrotropin (TSH) cutoff are controversial. As mitochondria are influenced by thyroid hormones, the purpose in this study was to measure expressi...
Impact of thyroid hormone treatment on maternal pregnancy outcomes in women with subclinical hypothyroidism without TPOAb: a retrospective cross-sectional study
Evidence on the impact of thyroid hormone treatment (LT4) on maternal pregnancy outcomes in women with subclinical hypothyroidism (SCH) without thyroid peroxidase antibodies (TPOAb) positivity is scarce.
Severity of hypothyroidism is inversely associated with impaired quality of life in patients referred to an endocrine clinic
We investigated the association between health-related quality of life (HRQL) and the severity of hypothyroidism at diagnosis in patients referred to a secondary hospital clinic.
Thyroid Research celebrates its 15th year of publication achieving its first Journal impact factor
Correlation of the ultrasound thyroid imaging reporting and data system with cytology findings among patients in uganda.
Ultrasonography is a noninvasive modality for the initial assessment of thyroid nodules. The American College of Radiology Thyroid Imaging Reporting and Data System (ACR TI-RADS) has demonstrated good performa...
Ultrasound characteristics of follicular and parafollicular thyroid neoplasms: diagnostic performance of artificial neural network
Ultrasound is the first-line imaging modality for detection and classification of thyroid nodules. Certain features observable by ultrasound have recently been equated with potential malignancy. This retrospec...
Clinicopathological profile and management of thyroid carcinoma: a Sub-Saharan country experience
In Sudan, there is limited knowledge on the epidemiology, clinical characteristics and pathological patterns of thyroid cancer. To address this shortcoming, we studied the clinical, pathological and treatment ...
Changes in brain structure in subjects with resistance to thyroid hormone due to THRB mutations
Being critical for brain development and neurocognitive function thyroid hormones may have an effect on behaviour and brain structure. Our exploratory study aimed to delineate the influence of mutations in the...
Sonographic changes in the thyroid gland after sclerotherapy with doxycycline can be mistaken for thyroid cancer
The literature considers sclerotherapy to be a safe and effective treatment for benign thyroid cysts. No subsequent diagnostic problems have been reported as a complication. We report the occurrence of focal i...
Histopathological features of asymmetric lacrimal gland enlargement in patients with thyroid eye disease
Lacrimal gland enlargement can be a feature of thyroid eye disease (TED). Unilateral or asymmetric lacrimal gland enlargement is poorly described and may impede diagnosis. We present the histological and clini...
Challenges in the care of patients with RET-altered thyroid cancer: a multicountry mixed-methods study
The discovery of driver oncogenes for thyroid carcinomas and the identification of genomically targeted therapies to inhibit those oncogenes have altered the treatment algorithm in thyroid cancer (TC), while g...
Collision tumor of a papillary and follicular thyroid carcinoma: a case report
Papillary thyroid carcinoma (PTC) and follicular thyroid carcinoma (FTC) are common differentiated thyroid cancers, but the detection of a collision tumor is an extremely rare event.
Macular microvasculature in patients with thyroid-associated orbitopathy: a cross-sectional study
The aim of this study was to evaluate macular blood flow in patients with thyroid-associated orbitopathy (TAO) as compared to healthy subjects. The inflammatory nature of the disease, as well as the vascular c...
Recurrent laryngeal nerve’s course running anteriorly to a thyroid tumor
The thyroid gland's neurovascular relationship is commonly portrayed as the recurrent laryngeal nerve (RLN) coursing posteriorly to the thyroid gland. We report a rare case with the RLN running anteriorly to a...
The clinical significance of low dose biotin supplements (<300μg/day) in the treatment of patients with hypothyroidism: crucial or overestimated?
In the last decade, the combination of the widespread use of streptavidin–biotin technology and biotin–containing supplements (BCS) in the daily clinical practice, have led to numerous reports of erroneous hor...
Decrease of thyroid function after ischemic stroke is related to stroke severity
Thyroid hormones are of fundamental importance for brain function. While low triiodothyronine levels during acute ischemic stroke (AIS) are associated with worse clinical outcomes, dynamics of thyroid function...
Abstracts from the 71 st Annual Meeting of the British Thyroid Association
This article is part of a Supplement: Volume 16 Supplement 1
Determinants and mediating mechanisms of quality of life and disease-specific symptoms among thyroid cancer patients: the design of the WaTCh study
Thyroid cancer (TC) patients are understudied but appear to be at risk for poor physical and psychosocial outcomes. Knowledge of the course and determinants of these deteriorated outcomes is lacking. Furthermo...
A study to evaluate association of nuclear grooving in benign thyroid lesions with RET/ PTC1 and RET/ PTC3 gene translocation
Papillary thyroid carcinoma (PTC) is the most common malignant lesion of the thyroid characterized by unique histological features like nuclear grooving, nuclear clearing, and intra-nuclear inclusions. However...
Hypokalemic periodic paralysis as the first sign of thyrotoxicosis- a rare case report from Somalia
Thyrotoxic hypokalemic periodic paralysis (THPP) is a rare complication of hyperthyroidism characterized by thyrotoxicosis, hypokalemia, and paralysis. It is the most common form of acquired periodic paralysis...
Primary leiomyosarcoma of the thyroid with concurrent papillary thyroid cancer: a rare case report and a review of literature
Leiomyosarcoma (LMS) is a soft tissue malignant tumor that has a predilection to the abdominopelvic and limb smooth muscles. LMS of the thyroid is exceptionally rare. Papillary thyroid cancer (PTC) is the most...
Effect of morning versus night-time administration of proton pump inhibitor (pantoprazole) on thyroid function test in levothyroxine-treated primary hypothyroidism: a prospective cross-over study
One of the common causes of suboptimal control of thyroid stimulating hormone (TSH) in levothyroxine-treated hypothyroidism is coadministration of proton pump inhibitors (PPIs). Morning administration of panto...
Correction to: Age-related variation in thyroid function – a narrative review highlighting important implications for research and clinical practice
The original article was published in Thyroid Research 2023 16 :7
Human exposure to pesticides and thyroid cancer: a worldwide systematic review of the literatures
Thyroid cancer is considered as one of the most prevalent cancers in the world. Some pesticides can play a role as a potentially important risk factor in thyroid cancer by affecting thyroid morphology and thyr...
Correction to: Solitary and multiple thyroid nodules as predictors of malignancy: a systematic review and meta‑analysis
The original article was published in Thyroid Research 2022 15 :22
Analysis of 665 thyroid nodules using both EU-TIRADS and ACR TI-RADS classification systems
Ultrasound-based classification systems allow stratification of thyroid nodules to recommend fine-needle aspiration (FNA) based on their malignancy risk. However, these have discrepancies that may have an impa...
Thyrotoxic periodic paralysis in a Caucasian man without identifiable genetic predisposition: a case report
Thyrotoxic periodic paralysis (TPP) is a rare condition characterized by muscle paralysis, thyrotoxicosis, and hypokalemia. It presents with paralysis of both proximal and distal musculature in upper and lower...
Hyperthyroidism in pregnancy: design and methodology of a Danish multicenter study
Graves’ disease (GD) is the main cause of hyperthyroidism in women of the fertile age. In pregnant women, the disease should be carefully managed and controlled to prevent maternal and fetal complications. Obs...
Colorectal cancer metastases in thyroid: case report and literature review
The thyroid gland is an uncommon site for metastatic deposits from non-thyroid malignancies, occurring in only 1.4 - 3% of surgical specimens where malignancy is suspected. It is even rarer for the source of t...
Age-related variation in thyroid function – a narrative review highlighting important implications for research and clinical practice
Thyroid hormones are key determinants of health and well-being. Normal thyroid function is defined according to the standard 95% confidence interval of the disease-free population. Such standard laboratory ref...
The Correction to this article has been published in Thyroid Research 2023 16 :20
Screening for thyroid disease in pregnancy: a study of Danish clinical practice
Thyroid disease in pregnant women is a matter of clinical awareness, and current clinical guidelines recommend a risk-based screening strategy. This study aimed to evaluate current clinical practice regarding ...
Conservative management of low-risk papillary thyroid carcinoma: a review of the active surveillance experience
The detection of low-risk thyroid carcinoma has increased in recent decades, although disease-specific mortality remained without changes. The high prevalence of occult carcinomas in autopsy studies, and hence...
Targeted therapy with vemurafenib in BRAF(V600E)-mutated anaplastic thyroid cancer
Anaplastic thyroid cancer (ATC) is one of the most aggressive malignancies, representing less than 5% of all thyroid carcinomas. Τhe median survival is limited to months due to the resistance of ATC to surgery...
Lobo-isthmectomy in the management of differentiated thyroid cancer
We have recently witnessed a rapid increase in the incidence of differentiated thyroid carcinoma (DTC), particularly low and very low-risk papillary thyroid carcinoma. Simultaneously, the number of cancer-rela...
Hypothyroidism in hibernating brown bears
Brown bears hibernate throughout half of the year as a survival strategy to reduce energy consumption during prolonged periods with scarcity of food and water. Thyroid hormones are the major endocrine regulato...
Pros and cons of an aggressive initial treatment with surgery and radioiodine treatment in minimally invasive follicular thyroid carcinoma
Currently, surgery alone is the gold standard treatment for minimally invasive follicular thyroid cancer (mi-FTC).
Screening and treatment of brain metastasis from papillary thyroid carcinoma: a case series
The brain metastasis from differentiated thyroid carcinoma (DTC) is a rare condition and its prognosis is poor. The standard protocol for screening and treatment of patients with brain metastases from papillar...
- Editorial Board
- Manuscript editing services
- Instructions for Editors
- Sign up for article alerts and news from this journal
Annual Journal Metrics
2022 Citation Impact 2.2 - 2-year Impact Factor 2.2 - 5-year Impact Factor 0.872 - SNIP (Source Normalized Impact per Paper) 0.532 - SJR (SCImago Journal Rank)
2023 Speed 5 days submission to first editorial decision for all manuscripts (Median) 125 days submission to accept (Median)
2023 Usage 428,111 downloads 98 Altmetric mentions
- More about our metrics
Thyroid Research
ISSN: 1756-6614
- General enquiries: [email protected]

- Heart Failure
- Cardiovascular Clinical Consult
- Adult Immunization
- Hepatic Disease
- Rare Disorders
- Pediatric Immunization
- Implementing The Topcon Ocular Telehealth Platform
- Weight Management
- Men's Health
- Women's Health
- Substance Use
- Kidney Disease
- Complimentary & Alternative Medicine
- Dermatology
- Endocrinology
- Oral Medicine
- Otorhinolaryngologic Diseases
- Gastrointestinal Disorders
- Musculoskeletal Disorders
- Rheumatology
- Pulmonology
Case In Point: Hyperthyroidism: 5 Cases to Hone Your Diagnostic Skills
A 32-year-old woman presents with weight loss of 6.4 kg (14 lb) during the past 8 months and diarrhea of recentonset. Menstruation had ceased 10 weeks earlier. She appears anxious, with pressured speech. Physical examination detectsbaseline sinus tachycardia, sweaty palms, and a diffusely enlarged thyroid gland. Laboratory tests reveal a thyroid-stimulatinghormone (TSH) level of 0.00 µU/mL (normal, 0.45 to 4.5 µU/mL), a free thyroxine (FT4) level of 4.8 ng/dL (normal,0.61 to 1.76 ng/dL), and a positive thyroid-stimulating immunoglobulin (TSI) level with high titer.
Weight Loss and Anxiety in a Young Woman 1. A 32-year-old woman presents with weight loss of 6.4 kg (14 lb) during the past 8 months and diarrhea of recentonset. Menstruation had ceased 10 weeks earlier. She appears anxious, with pressured speech. Physical examination detectsbaseline sinus tachycardia, sweaty palms, and a diffusely enlarged thyroid gland. Laboratory tests reveal a thyroid-stimulatinghormone (TSH) level of 0.00 μU/mL (normal, 0.45 to 4.5 μU/mL), a free thyroxine (FT 4 ) level of 4.8 ng/dL (normal,0.61 to 1.76 ng/dL), and a positive thyroid-stimulating immunoglobulin (TSI) level with high titer.
This is a classic presentation of hyperthyroidism caused by Graves' disease. The diagnosis can be made basedon clinical presentation, physical examination, and selected laboratory tests. Further evaluation with a radioactive iodine(RAI) uptake and thyroid scan would reveal an enlarged gland with increased uptake and confirm the diagnosis.The tests would also yield necessary dosing information if the patient were to be treated with RAI ablation. Alternatively,she could be treated with antithyroid medications or surgery. Subsequent to surgery, thyroid hormone replacementtherapy with levothyroxine would be needed.If this patient were pregnant, treatment options would change. RAI ablation would now be contraindicated, andthe patient could be offered antithyroid treatment with propylthiouracil (PTU). Following pregnancy, RAI ablationwith subsequent thyroid replacement therapy would be recommended.
An Elderly Man With Recent Atrial Fibrillation 2. A 76-year-old man with known heart disease is admitted to the hospital because of new onset of shortness of breath,fatigue, and atrial fibrillation. He denies weight loss, nervousness, and insomnia. There is no evidence of an acute myocardialinfarction or pulmonary embolus. On physical examination, his heart rate is 136 beats per minute; beats are irregularlyirregular; and fine rales are heard at both lung bases. His blood pressure is 152/82 mm Hg, without orthostaticchanges. Results of laboratory tests indicate a hemoglobin level of 14.6 g/dL, a TSH level of 0.02 μU/mL (normal, 0.45to 4.5 μU/mL), and an FT 4 level of 3.3 ng/dL (normal, 0.61 to 1.76 ng/dL).
An elderly patient with hyperthyroidism secondary to Graves' disease, toxic multinodular goiter, or toxic adenomaoften presents without classic symptoms. A diagnosis of Graves' disease is confirmed by measurement of TSIand an RAI uptake and thyroid scan. Imminent treatment includes βblockers (and corticosteroids, if necessary) tocontrol the hyperthyroid state, which is now causing secondary atrial fibrillation and congestive heart failure.βBlockers will control the tachycardia and reduce the risk of heart failure; corticosteroids will block the peripheralconversion of T 4 to triiodothyronine (T 3 ). PTU might be useful for inhibiting intrathyroidal hormone production (oxidationand organification) and the peripheral conversion of T 4 to the metabolically active T 3 . Plans should be madefor RAI ablation, followed by thyroid hormone replacement therapy. In cases such as this, the atrial fibrillation isusually not converted to a normal sinus rhythm until the hyperthyroid state is successfully treated. Anticoagulationtherapy should be considered as well.
Nodule in a Woman With Insomnia 3. A 29-year-old woman presents for her yearly pelvic examination and Pap smear. She complains of insomnia andnervousness. Physical examination reveals an enlargement of the left lobe of the thyroid gland, which suggests the presenceof a nodule. Laboratory test results reveal a TSH level of 0.02 μU/mL (normal, 0.45 to 4.5 μU/mL) and an FT 4 level of2.3 ng/dL (normal, 0.61 to 1.76 ng/dL).
To confirm a diagnosis of hyperthyroidism secondary to toxic adenoma . in a patient with abnormal levels of TSHand FT 4 , fine-needle aspiration and biopsy (FNAB) of the nodule are indicated. An RAI uptake and thyroid scan canbe used to investigate the possibility of "other" adenomas and to help determine the dosage of 131 I for subsequent radioiodinetherapy. Many physicians turn to ultrasonography, but the initial evaluation that consists of TSH and FT 4 measurement and FNAB is sufficient for diagnosis. Ultrasonography cannot distinguish benign from malignanttissue.
Treatment is either surgery (partial thyroidectomy) or RAI ablation. If the nodule persists following treatmentwith RAI ablation, a second tissue biopsy is indicated.
Woman With Tremors Following Weight Loss 4. A 28-year-old hospital nurse presents for evaluation of palpitations and tremors that occur with writing. She hasintentionally lost 10 kg (22 lb) during the past 4 months. She requests a sleeping pill and some lorazepam to relieveher "shakes." She is nervous and fidgets during the examination, and her reflexes demonstrate 2-beat clonus. Laboratorytests reveal a TSH level of 0.01 μU/mL (normal, 0.45 to 4.5 μU/mL)and an FT 4 level of 3.9 ng/dL (normal, 0.61 to1.76 ng/dL). You suspect hyperthyroidism and order an RAI uptake and thyroid scan. Surprisingly, results of the testsreveal "decreased uptake of 123 I and a normal-sized gland, with no hot or cold areas identified."
Factitious hyperthyroidism should be suspected--and confirmed with additional history taking. No doubt thiswoman has been taking exogenous thyroid hormone to facilitate weight loss. This syndrome is often found in hospitaland medical workers who are aware of this hormone's metabolic effect and have access to samples. Factitious hyperthyroidismis distinguished from iatrogenic hyperthyroidism by the fact that the latter occurs in patients for whommore than the recommended dose for the treatment of hypothyroidism is prescribed.Another cause of decreased RAI uptake is subacute thyroiditis. A suppressed serum thyroglobulin level is consistentwith factitious hyperthyroidism; this level is elevated in subacute thyroiditis.
Nervous New Mother 5. A 28-year-old woman presents with complaints of nervousness, trouble sleeping, and weight loss. She is 8 weekspostpartum and wants to return soon to her job as a coronary care unit nurse. Laboratory tests reveal a TSH level of0.03 μU/mL (normal, 0.45 to 4.5 μU/mL) and an FT 4 level of 3.4 ng/dL (normal, 0.61 to 1.76 ng/dL). Results of atest for TSI are negative.
Postpartum thyroiditis is a self-limited disease (lasting 6 to 9 months) that transiently evolves first through astate of hyperthyroidism, then hypothyroidism, before the patient returns to a euthyroid state. 1 Depending on thetime of testing and the clinical presentation, a presumptive diagnosis of hyperthyroidism or hypothyroidism may bemade. Thyroid 123 I uptake measurement is necessary to confirm the diagnosis. Because most patients return to aeuthyroid state, no specific treatment is recommended, although hypothyroidism may persist in 20% to 25% of patientsfor 4 years., 2,3 Serial TSH measurements, explanation of the problem to the patient, and reassurance are indicated. Thetransient symptoms of hyperthyroidism may be managed with β-blockers.
Viking Therapeutics Oral Antiobesity Agent Returns Positive Results in Small Study
The once daily tablet, VK2375, is headed to a phase 2 trial later this year and is an oral version of Viking's investigational injectable GLP-1/GIP mimetic currently in phase 2.

Navigating Cardiovascular Complications of Obesity: Expert Insights for Primary Care
Listen to our latest podcast episode for details on top CVD risk factors to screen patients with obesity for, medications to help prevent CVD, and more.

Medicare Will Cover AOM Semaglutide for Cardiovascular Risk Reduction: A First
CMS says Medicare Part D plans will cover semaglutide for individuals with overweight/obesity who have preexisting CVD, a first after 40 years of legal prohibition.

Obesity & Type 2 Diabetes: An Expert Discussion
In our latest episode, 2 obesity experts discuss the importance of early intervention to reduce risk for complications, suggestions for managing patients in primary care, and more.
Semaglutide 2.4 mg (Wegovy) Wins Expanded Indication to Include Reduced Risk of MACE
The label expansion for semaglutide, based on the SELECT trial, includes data showing reduced risk of CV death of 15% and of death from any cause of 19%.

Novo Nordisk Shares Early Findings for Novel Oral Antiobesity Agent
The novel unimolecular pill amycretin combines a GLP-1 and an amylin agonist and led to weight loss of 13% at 12 weeks in a phase 1 trial that just concluded, the company said.
2 Commerce Drive Cranbury, NJ 08512
609-716-7777


Pregnancy Outcome in Hyperthyroidism: A Case Control Study
- Article contents
- Figures & tables
- Supplementary Data
- Peer Review
- Get Permissions
- Cite Icon Cite
- Search Site
Neelam Aggarawal , Vanita Suri , Rimpi Singla , Seema Chopra , Pooja Sikka , Viral N. Shah , Anil Bhansali; Pregnancy Outcome in Hyperthyroidism: A Case Control Study. Gynecol Obstet Invest 1 February 2014; 77 (2): 94–99. https://doi.org/10.1159/000357615
Download citation file:
- Ris (Zotero)
- Reference Manager
Background: Data comparing pregnancy outcome in hyperthyroid women with euthyroid women are scarce. Hence, this study was carried out to assess the maternal and fetal outcome in pregnant women with hyperthyroidism to ascertain the effect of disease on pregnancy. Methodology: This retrospective study was conducted over a period of 28 years. We compared the maternal and fetal outcomes of 208 hyperthyroid women with 403 healthy controls, between women with well-controlled and uncontrolled disease and amongst women diagnosed with hyperthyroidism before and during pregnancy. Results: Maternal outcome: women with hyperthyroidism were at increased risk for preeclampsia (OR = 3.94), intrauterine growth restriction (OR = 2.16), spontaneous preterm labor (OR = 1.73), preterm birth (OR = 1.7), gestational diabetes mellitus (OR = 1.8), and cesarean delivery (OR = 1.47). Hyperthyroid women required induction of labor more frequently (OR = 3.61). Fetal outcome: newborns of hyperthyroid mothers had lower birth weight than normal ones (p = 0.0001). Women with uncontrolled disease had higher odds for still birth (OR = 8.42; 95% CI: 2.01-35.2) and lower birth weight (p = 0.0001). Conclusions: Obstetrical complications were higher in women with hyperthyroidism than normal women. Outcome was worsened by uncontrolled disease. Women with pregestational hyperthyroidism had better outcomes than those diagnosed with it during pregnancy.
Individual Login
Institutional login.
- Access via Shibboleth and OpenAthens
- Access via username and password
Digital Version
Email alerts, citing articles via, suggested reading.
- Online ISSN 1423-002X
- Print ISSN 0378-7346
INFORMATION
- Contact & Support
- Information & Downloads
- Rights & Permissions
- Terms & Conditions
- Catalogue & Pricing
- Policies & Information
- People & Organization
- Stay Up-to-Date
- Regional Offices
- Community Voice
SERVICES FOR
- Researchers
- Healthcare Professionals
- Patients & Supporters
- Health Sciences Industry
- Medical Societies
- Agents & Booksellers
Karger International
- S. Karger AG
- P.O Box, CH-4009 Basel (Switzerland)
- Allschwilerstrasse 10, CH-4055 Basel
- Tel: +41 61 306 11 11
- Fax: +41 61 306 12 34
- Email: [email protected]
- Experience Blog
- Privacy Policy
- Terms of Use
This Feature Is Available To Subscribers Only
Sign In or Create an Account
Wednesday 3 April 2024
- For readers
- For authors
- For advertisers
- Medicine Today
- Cardiology Today
- Endocrinology Today
- Pain Management Today
- Respiratory Medicine Today
- APSR 2017 Congress, Sydney: video highlights
- Brief Bites For Better Care
- CSANZ 2018 Annual Scientific Meeting, Brisbane: video highlights
- ESC 2017 Congress, Barcelona: video highlights
- ESC 2019 Congress, Paris: video highlights
- Vaccinations for healthy ageing in adults aged over 65 years
- View Current issue
- Past issues
- Supplements
- Feature Articles
- Regular Series
- Clinical News
- Dermatology quiz
- Clinical flowcharts
- Clinical case review
- Something Borrowed
Hashimoto’s thyroiditis. A case study of overt hypothyroidism

- Copied to clipboard
This case describes a 32-year-old man who presents with subtle symptoms and signs but has florid Hashimoto’s thyroiditis. The diagnosis and treatment of Hashimoto’s thyroiditis are straightforward; however, there can be some complexities in how levothyroxine therapy is used.
Case scenario
A 32-year-old man presents to his GP with fatigue, which has been gradual in onset over the past six to 12 months. There has been no weight or appetite change, but he has mild constipation. His sleep patterns are unchanged.
He works in IT, is single and lives alone, following a break up one year prior. His past medical history is unremarkable, except for glandular fever at age 17 years. He is prone to mild anxiety but has not needed formal treatment for this. He has a healthy lifestyle; he exercises regularly and is a nonsmoker who drinks alcohol on weekends only (weekly intake of <60 g). There is a family history of hemithyroidectomy for benign thyroid nodular disease in his mother and his paternal grandfather had pernicious anaemia.
On examination, he is clinically euthyroid with normal blood pressure of 110/70 mmHg and a resting pulse of 60 beats per minute. Neck examination reveals a diffuse nontender goitre, just above normal thyroid size. Eye examination is normal apart from mild periorbital puffiness. There are no peripheral rashes. Cardiovascular, neurological and gastroenterological examinations are unremarkable.
Blood tests reveal a raised thyroid stimulating hormone (TSH) level of 105 mIU/L (reference range [RR] 0.4 to 4.0), free triiodothyronine (FT3) level within the reference range at 3.4 pmol/L (RR 2.6 to 6.0), a low free thyroxine (FT4) level of 8.9 pmol/L (RR 9 to 19) and an antithyroid peroxidase (anti-TPO) antibody titre of greater than 1300 mIU/L (RR <40). Full blood count, serum calcium and biochemistry are normal.
A diagnosis of autoimmune thyroid disease (AITD), specifically Hashimoto’s disease, is made.
What levothyroxine dose is needed initially?
This presentation is consistent with overt hypothyroidism secondary to Hashimoto’s thyroiditis. Thyroid hormone replacement with levothyroxine is indicated. A reasonable starting dose of levothyroxine is 1.6 mcg/kg body weight, with typical starting doses of 50 to 75 mcg daily, although a lower starting dose should be considered in older patients and those with a history of cardiac disease. 1 The dose is related to lean body mass and this generally translates to 75% measured body weight.
When should TSH be remeasured and how should the levothyroxine dose be titrated?
TSH levels can take four to six weeks to fully respond to any adjustments in levothyroxine dosage. It is important to remember that if thyroid function tests are repeated earlier than this the values will not be at steady state.
Which thyroid autoantibodies should be tested in the context of an elevated TSH level? Please advise how to interpret their presence or absence.
Anti-TPO antibodies, also known as thyroid microsomal antibodies (TMAbs), and antithyroglobulin antibodies (TgAbs) are tested at the outset, noting that the former are more likely to be raised. Some authors have suggested that measurement of TgAbs is not needed in AITD (except in the context of monitoring serum thyroglobulin in patients who have had total thyroidectomy for thyroid cancer). 2
Not all patients with AITD will have positive antithyroid antibodies. 3 Serial monitoring of antithyroid antibodies is not recommended as the levels do not guide the effectiveness of therapy; this is done by monitoring the TSH level. In contrast, measurement of TSH-receptor antibodies can be performed every four to six months in the follow up of patients with Graves’ disease as it helps to guide management and predict remission.
What are the indications for arranging imaging in the setting of hypothyroidism and which imaging investigation(s) are appropriate?
Thyroid imaging is not routinely required when diagnosing hypothyroidism unless there is clinical asymmetry or suspected nodularity on clinical examination. The imaging of choice to evaluate this would be a thyroid ultrasound scan. 4 This would not need to be followed by further imaging unless there was a clinical change or new indication, such as nodular disease.
Thyroid isotope functional scanning is not needed in Hashimoto’s disease and can cause confusion as it may show a diffuse increased pattern of isotope uptake (as seen in Graves’ disease). Thyroid isotope scanning is most useful in distinguishing Graves’ from thyroiditis (e.g. subacute or drug-induced), and from toxic nodules.
When would a pituitary or hypothalamic cause of hypothyroidism be suspected?
Secondary hypothyroidism is characterised by a TSH level in the low end of the normal range or below normal, and a FT4 level also low or low-normal (i.e. the TSH level is inappropriately low for the FT4 level). In some healthy individuals, a low-normal TSH level and low-normal FT4 level can be observed. Careful history needs to be evaluated and specialist input should be considered. In secondary hypothyroidism, FT3 levels tend to be low-normal and can be preserved in the normal range until the very late stages of both primary and secondary hypothyroidism (unless the patient is very sick or is taking amiodarone). Trends of thyroid function in different causes of hypothyroidism are outlined in Table 1 .
In hospitalised patients who are unwell, it is important to remember that thyroid function abnormalities can occur transiently as a sick euthyroid picture. This may resemble a pattern of secondary hypothyroidism. In sick euthyroid picture, the FT3 level is often low, with either normal or suppressed TSH and FT4 levels. Unless there is clinical suspicion of secondary hypothyroidism, in which case treatment should be considered, thyroid function should be repeated when the patient is well again.
What initial dose is appropriate for elderly patients or those with cardiac disease?
A starting dose of 25 to 50 mcg levothyroxine per day is recommended, depending on the age, body weight and degree of cardiac dysfunction. In most forms of cardiac disease, including arrhythmia, cardiac failure and ischaemic heart disease, gentle up-titration of levothyroxine should be considered, aiming to avoid over-replacement. 5
Anecdotally, some patients (including young people) experience anxiety symptoms, including palpitations, when levothyroxine is first commenced; even before the TSH has corrected down to normal. It may be necessary to reduce the starting levothyroxine dose further and then titrate upwards more gradually. There are occasional rare cases where TSH normalisation causes intolerable anxiety, and in these cases, a higher TSH level target of above 10 m IU/L might be acceptable, as long as the FT4 level is normal.
When could weekly dosing of levothyroxine be considered?
Ideally levothyroxine should be taken daily but its half-life of one week means that occasional missed doses are not problematic. If compliance is difficult, then the total dose for one week may be taken as a single dose. This approach is sometimes adopted under supervision for patients with psychiatric illness. 6
Should patients use the same type of levothyroxine (i.e. Eutroxsig vs Oroxine vs Eltroxin vs Levoxine) throughout their treatment in case of differences between the brands?
It is generally recommended that patients should continue with the same brand as anecdotally there can be minor differences in the bioavailability of different brands (e.g. a bigger dose of the newer non-refrigerated brand, Eltroxin, is sometimes needed). 7 However, the literature suggests that overall the bioavailability differences between brands are minimal.
What is the appropriate initial treatment target?
The treatment is targeted to achieve a TSH level in the normal range, ideally with resolution of symptoms. Exceptions to this would be in the case of a high-risk thyroid cancer patient, where post-thyroidectomy TSH level targets are individualised according to the evidence-based guidelines. Pregnancy is another circumstance where TSH level targets will differ, especially in relation to trimester and presence of positive anti-TPO antibodies (although this is an area where there is lack of general consensus). 2 Occasionally, patients have poor tolerance of levothyroxine symptomatically, for example those with intercurrent cardiac arrhythmias or significant anxiety, as mentioned earlier; in these cases, it may be acceptable to have a higher TSH level target (e.g. <10 mIU/L) as long as the FT4 level is in the normal range.
If the TSH level is still elevated after four to six weeks of levothyroxine treatment, what are the possible explanations and what is the suggested management?
It may take several months to reach target TSH levels in some patients, requiring ongoing titration of levothyroxine dosing. It is important to check adherence at each dose titration. Adherence should be checked by questioning the patient on when and how they take their tablets. It is advised that levothyroxine is usually taken in the morning on an empty stomach with no food or other medications for at least 30 minutes (or at bedtime, at least two hours after dinner). It is important to avoid calcium and iron tablets at the same time. 8 Impaired absorption may also be a contributing factor in some cases where it is difficult to normalise the TSH level on standard doses of levothyroxine (e.g. levothyroxine absorption in the small bowel may be affected in patients with coeliac disease).
Sometimes the TSH level may normalise but the FT4 level is elevated; this might be explained by the patient having their blood drawn soon after taking their morning levothyroxine dose. Ideally thyroid function testing should be performed pre-dose or six hours after their levothyroxine dose.
A significant change in body weight, especially of lean body mass, may explain a sudden change in levothyroxine dose requirement. It is wise to avoid too high an incremental change in dose at each dose adjustment.
Once the TSH level is in the target range and symptoms have resolved, what is the recommended frequency of monitoring the thyroid function tests?
When stability is achieved, it is acceptable to monitor the TSH level annually (or sooner if symptoms develop in the interim). 4 If frequent recent dose adjustments have been required then the TSH level will need more frequent monitoring. If a woman is considering pregnancy, thyroid function should be checked before conception and then at regular intervals (e.g. four to six weekly, throughout pregnancy). The woman should be advised that once a pregnancy test is positive, she should increase her levothyroxine dose by 30%, to reflect what happens in normal euthyroid women during pregnancy. 9 This is required because thyroid binding globulin levels rise during pregnancy.
What are the indications for referral of patients to a specialist in the setting of hypothyroidism?
Patients who have instability in levothyroxine dosing and persistence of symptoms, such as fatigue, may benefit from specialist referral. Patients may also need reassurance that rising antithyroid antibody titres are not sinister and do not need serial measurement. Levothyroxine requirements can rise by 30% in the first trimester in pregnancy and specialist input should be considered at this crucial stage to maintain the TSH level in the normal range. 4
In cases where patients are using or considering liothyronine (T3) replacement, either alone or in combination with T4 replacement, they should be referred for specialist input as this requires careful evaluation. 7 There is also an emergence of thyroid extract being used by the general population. This is not endorsed by the Therapeutic Goods Administration or the Endocrine Society of Australia. 7
When would you suspect poor adherence to treatment and can you provide some strategies for how to best manage this problem?
If there is significant instability in levothyroxine dosing without clear cause, adherence should be assessed. Persistently elevated TSH levels despite dosage adjustments, or significantly higher levothyroxine doses compared to a weight-based predicted dose requirement should trigger the clinician to question if there is poor adherence. 10
Ways to increase adherence include asking patients to set a reminder for their medication, either as a physical reminder or on their phone. Most refrigerated forms of thyroid hormone replacement come in a metal strip, thus cutting out the weekly required dose and keeping this separate can help keep track of the dose. Nonrefrigerated forms of thyroid hormone replacement can be kept in a weekly pill organiser to help increase compliance. Given the long half-life of thyroid hormone replacement, it is possible to dose every second day or even once a week to help improve compliance. 6
What are some potential drug interactions with levothyroxine and how might these affect the thyroid function test results? What should be the advice to patients taking these medications?
There are multiple medications that may interact with levothyroxine absorption, action or metabolism. Possible drug interactions with levothyroxine and some practical points are outlined in Table 2 .
Case continued
The patient achieves euthyroidism with levothyroxine replacement. His siblings are keen to be evaluated for hypothyroidism and both his brother and sister (aged 23 and 30 years respectively) are found to have normal TSH levels but mildly positive anti-TPO titres. With gradual dose weaning and monitoring of TSH levels, the patient is able to stop taking levothyroxine after three years. He has ongoing annual TSH level surveillance .
A 32-year-old man is diagnosed with florid Hashimoto’s thyroiditis after presenting with fatigue and a small diffuse goitre. His TSH and TMAb levels are very high but his FT3 level remains in the normal range, with a FT4 level just below the normal range. He becomes euthyroid with levothyroxine replacement and achieves remission over three years. TSH level surveillance allowed appropriate levothyroxine adjustment whilst on therapy and also appropriate monitoring to ensure the level remained in the normal range after levothyroxine weaning and cessation. ET
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.

StatPearls [Internet].
Hyperthyroidism.
Philip Mathew ; Jasleen Kaur ; Prashanth Rawla .
Affiliations
Last Update: March 19, 2023 .
- Continuing Education Activity
Hyperthyroidism is a common thyroid disorder with multiple underlying etiologies. This disease is characterized by excess thyroid hormone production. Hyperthyroidism can be overt or subclinical. Overt hyperthyroidism is defined as low or suppressed thyroid stimulating hormone (TSH) levels with elevated triiodothyronine (T3) levels and/or elevated thyroxine (T4) levels. Hyperthyroidism is associated with significant short-term and long-term morbidity. Therefore, early recognition of this condition and timely instruction of appropriate therapy is critical. This activity reviews the etiology, presentation, evaluation, and management of hyperthyroidism and reviews the role of the interprofessional team in evaluating, diagnosing, and managing the condition.
- Review the various etiologies that lead to a presentation of hyperthyroidism.
- Describe the presentation and expected examination findings when evaluating a patient with hyperthyroidism.
- Summarize the various treatment options available for hyperthyroidism, depending on specific etiology.
- Explain the importance of interprofessional team strategies for improving care coordination and communication to aid in prompt diagnosis of hyperthyroidism and improving outcomes in patients diagnosed with the condition.
- Introduction
Hyperthyroidism is a common thyroid disorder. "Hyperthyroidism" defines a syndrome associated with excess thyroid hormone production. [1] It is a common misconception that the terms thyrotoxicosis and hyperthyroidism are synonyms. The term "thyrotoxicosis" refers to a state of excess thyroid hormone exposure to tissues. [1] Although hyperthyroidism can lead to thyrotoxicosis and can be used interchangeably, it is essential to note their differences. For the sake of simplicity, this review will cover a discussion of hyperthyroidism and thyrotoxicosis. Hyperthyroidism has multiple etiologies, clinical manifestations, and treatment modalities.
Hyperthyroidism can be overt or subclinical. Overt hyperthyroidism is defined as low or suppressed thyroid stimulating hormone (TSH) levels with elevated triiodothyronine (T3) levels and/or elevated thyroxine (T4) levels. [1] When T3 levels are elevated with low/suppressed TSH and normal T4 levels, this is called 'T3 toxicosis'. [2] Subclinical hyperthyroidism is low or suppressed TSH with normal T3 and T4 levels. [2] Both overt and subclinical hyperthyroidism are associated with significant long-term complications. [3] [4] [5] [6] [7]
The three most common etiologies of hyperthyroidism include:
- Graves disease (GD)
- Toxic multinodular goiter (TMNG)
- Toxic adenoma (TA) [1]
Other less common etiologies of hyperthyroidism:
- Iodine-induced hyperthyroidism [8]
- TSH (thyroid stimulating hormone)-secreting pituitary adenomas [9]
- Conditions associated with high human chorionic gonadotrophin levels: choriocarcinomas and hydatiform moles in females and germ cell tumors in males [10]
- Ectopic thyroid in struma ovarii (excess thyroid hormone production from ovarian teratomas) [11]
- Extensive metastasis from functionally differentiated thyroid carcinoma (follicular or papillary) [12]
- Drug-induced thyroiditis: amiodarone, lithium, tyrosine kinase inhibitors, interferon-alpha, immune checkpoint inhibitor therapy [13] [14] [15] [16] [17]
- Other thyroiditis: Hashitoxicosis, painless thyroiditis, painful subacute thyroiditis, suppurative thyroiditis, and Riedel thyroiditis [18] [19]
- Factitious thyroiditis (due to excess exogenous thyroid hormone: intentional or unintentional use) [10]
Graves disease is the most common cause of hyperthyroidism in the United States and most Western countries. [20] As Graves disease is autoimmune in etiology, this form of hyperthyroidism tends to manifest itself in younger populations. In older adults and people living in regions of iodine deficiency, toxic multinodular goiter is the most common cause of hyperthyroidism. [21] [22] [23]
Factitious thyroiditis is thyrotoxicosis associated with inappropriate or excessive use of pharmaceutical thyroid hormone. [10] Due to a well-received side effect of weight loss, thyroxine has the potential for abuse. Any history of a hyperthyroid patient should include a medication list and an assessment of possible misuse (whether intentional or unintentional).
- Epidemiology
The prevalence of hyperthyroidism varies worldwide, based on dietary iodine content. [12] Hyperthyroidism is more common in women compared to men. [24] Other risk factors associated with the development of hyperthyroidism include smoking, iodine deficiency, iodine excess, selenium deficiency, genetic factors, and the use of certain drugs. [12] Graves disease is typically seen in younger patients and is the most common cause of hyperthyroidism in this demographic. The incidence of GD is highest between the age group of 30 to 50 years. [25] Toxic multifocal goiter is typically seen in older individuals and is the most common cause of hyperthyroidism in this demographic. [22] Graves disease and toxic multifocal goiter have a female predilection and are typically seen in patients with pertinent family and personal medical histories. Thyroid nodular disease is also more common in women than men by 5- to 15-fold. [26] Autoimmune thyroid disorders like Graves disease are more common in iodine-replete areas, and nodular thyroid diseases are more common in iodine-deficient areas. [12]
The 1977 Whickham Survey evaluated the spectrum of thyroid disorders in County Durham in northeastern England. The Whickham Survey demonstrated a prevalence of hyperthyroidism in women, approximately ten times more than that of men (2.7% versus 0.23%). [27] An incidence of 80 cases per 100,000 women was seen at the 20-year follow-up of the Whickham cohort. [28] The prevalence of hyperthyroidism in the United States was 1.3% in the general population, with 0.5% cases of overt hyperthyroidism and 0.7% cases of subclinical hyperthyroidism. [29] A meta-analysis found the prevalence of hyperthyroidism in Europe to be 0.75%. [24] The prevalence of overt hyperthyroidism is similar in China at 0.78%. [30]
Amiodarone-induced thyrotoxicosis (AIT) is seen in about 6% of the individuals taking the medication in iodine-sufficient areas and about 10% in individuals taking the medication from iodine-deficient areas. [31] [32]
- Pathophysiology
The pathophysiology of hyperthyroidism depends on the particular variant of hyperthyroidism.
Graves Disease
This is an autoimmune process with antibodies against the TSH receptor. An interplay between genetic and environmental factors influences this autoimmune process. The antibodies stimulate the TSH receptor (TSHR), leading to increased production and release of thyroid hormones. The trophic effects on the thyroid also lead to the growth of the thyroid gland. [20]
Toxic Multinodular Goiter
Pathogenesis of TMNG includes the initial phase of development of the nodular disease. This phase is prolonged and present for years before the nodules develop autonomy for thyroid hormone production. The somatic mutations involving the TSHR lead to constitutive activation of the cAMP signaling pathway, resulting the thyroid autonomy. [33] There is a correlation between the size of the nodules and the development of hyperthyroidism. In a previous study, about 93.7% of the patients who developed overt hyperthyroidism had a nodule size greater than 3 cm. [34]
Toxic Adenoma
These are solitary nodules with autonomous thyroid hormone production due to somatic mutations in the TSHR
Iodine-Induced Hyperthyroidism (Jod-Basedow Phenomenon)
This is typically iatrogenic, resulting from excessive iodine intake through diet or administration of iodine-containing medications such as contrast media or amiodarone. [35] [36] Individuals susceptible to this phenomenon include the ones residing in iodine-deficient regions, individuals with underlying thyroid nodular disease, or underlying occult GD or previously treated GD. [8] Hyperthyroidism develops about 2-12 weeks after exposure to excessive iodine. [37] As mentioned previously, the organification of iodide residues into precursor thyroid hormone molecules is relatively self-regulating. Excessive circulating iodide inhibits organification, a process known as the Wolff-Chaikoff effect. This autoregulation is escaped in the Jod-Basedow phenomenon leading to excess thyroid hormone in the presence of excess iodine/iodide.
Amiodarone-Induced Thyrotoxicosis
There are two subtypes of amiodarone-induced thyrotoxicosis (AIT): type 1 and type 2. Type 1 AIT leads to increased thyroid hormone production secondary to excess iodine exposure from amiodarone in the setting of pre-existing thyroid disease (as seen in the Jod-Basedow phenomenon). [38] The pre-existing thyroid disease is usually multinodular goiter or latent Graves disease. Type 2 AIT is destructive thyroiditis due to the direct toxic effects of amiodarone on the thyroid follicular cells. [39]
Thyroiditis results in the transient increase in circulating thyroid hormone resulting from inflammation or destruction of the thyroid follicular cells. Various etiologies of thyroiditis have this common pathophysiology but vary in their clinical presentations. The inflammation or destruction of the thyroid follicular cells can result from autoimmunity (Hashimoto's thyroiditis, painless sporadic thyroiditis, and painless postpartum thyroiditis) or the result of external factors (infections in painful subacute thyroiditis, suppurative thyroiditis, drug-induced thyroiditis). [19]
- History and Physical
Thyroid hormone has physiological effects on multiple organ systems. As a result, the symptoms and signs of hyperthyroidism involve manifestations from multiple organ systems. Clinical manifestations are associated with a hyperadrenergic and hypermetabolic state. Common manifestations include unintentional weight loss (about 10% of patients can gain weight due to increased appetite), palpitations, tremors, heat intolerance, dyspnea on exertion, increased anxiety, irritability, fatigue, muscle weakness, increased frequency of bowel movements (some patients can have significant diarrhea), hair loss, loss of libido, and oligomenorrhea or amenorrhea in women. [20] [40]
Patients with subacute thyroiditis can present with significant anterior neck pain and fever. On physical examination, patients have tachycardia (some can present with atrial fibrillation), hypertension, tremors, warm and moist skin, hyperreflexia, and an anxious appearance. Some patients might have signs of heart failure.
Eye signs of lid lad or lid retraction can be seen in all causes of hyperthyroidism due to a hyperadrenergic state. [41] Eye symptoms and signs of "true orbitopathy' are only seen in patients with Graves disease. These include diplopia, excessive tearing, conjunctival injection, and orbital or retro-orbital pressure proptosis. [42] [43] Other specific physical findings associated with Graves disease are pretibial myxedema (plaques of thick, scaly skin and swelling involving the anterior aspect of lower legs) and acropachy (soft-tissue swelling of the hands and clubbing of the fingers). [44] [45] [46]
Examining the thyroid will reveal a diffuse non-nodular enlargement of the thyroid in Graves disease; a diffuse non-symmetric nodular enlargement can be seen in toxic multinodular goiter, and a single large nodule can be palpated in cases of a toxic adenoma. An exquisitely tender thyroid can be noted in subacute thyroiditis. [47]
When hyperthyroidism is suspected based on clinical features, the patient should undergo an initial evaluation with measurement of TSH, free T4, and total T3 to confirm the diagnosis. Figure 1 illustrates the diagnostic algorithm for hyperthyroidism. Patients with overt hyperthyroidism will have low/suppressed TSH levels with elevated free T4 and total T3 levels. Patients with mild/subclinical hyperthyroidism will have low/suppressed TSH with normal free T4 and total T3 levels. 'T3 toxicosis' is defined as low/suppressed TSH with normal T4 and elevated T3 levels.
Conditions that can interfere with the assessment of TSH include the presence of heterophile antibodies and high biotin intake due to interference with the assays. [48] Heterophile antibodies can lead to a false elevation in TSH levels. High-dose biotin supplementation (5 to 30 mg) can result in falsely low TSH with elevated free T4 levels in vitro. [49]
After the diagnosis of hyperthyroidism has been confirmed, measurement of thyrotropin receptor antibody (TRAb) levels as an initial test for determining the etiology of hyperthyroidism has been shown to reduce the time to diagnosis and is more cost-effective. [50] . Elevated TRAb levels confirm the diagnosis of Graves disease. TRAb levels are measured using TBI or TBII (thyrotropin-binding inhibiting or thyrotropin-binding inhibitory immunoglobulin) assays and TSI (thyroid stimulating immunoglobulin) bioassays. The newer bioassay using the Immulite method for TSI measurement has a high sensitivity and specificity of 98% and 99.9%, respectively, for diagnosing GD. [51] TBII assays used for measuring TRAb levels also have a high sensitivity of 96-97% and specificity of 99% of the diagnosis of GD. [52]
If TRAb levels are normal, the patient should undergo a radioiodine thyroid uptake and scan using an I-123 isotope (enters the thyroid gland through the Na/I symporter). This test is contraindicated in pregnant and lactating women. A capsule containing an I-123 isotope is given a day before the scan is performed. The pattern of uptake of I-123 by the thyroid gland seen on the scan can help determine the diagnosis (see Figure 1). However, this test does not help differentiate between type 1 and type 2 amiodarone-induced thyrotoxicosis, as the uptake will be low in chronic amiodarone use.
- High Uptake/Normal
- Graves disease will have high or normal uptake in a diffuse pattern
- TMNG will have a high or normal uptake in a patch pattern
- TA will have a high or normal uptake with a solitary area of high uptake (corresponding to the known nodule) with low uptake in the remainder of the gland
- Low or Absent Uptake
- Any etiology of thyroiditis is associated with low or absent uptake (Na/I symporters are not functional in inflamed or destroyed thyroid follicular cells)
- Iatrogenic and factitious thyrotoxicosis
Thyroid ultrasound using the color Doppler is another important test that can help determine the underlying etiology. Intrathyroidal arterial flow velocities are measured. [53] Increased (thyroid inferno) and normal flow are seen in Graves disease. Low flow is seen in thyroiditis. [53] This test can help differentiate between type 1 and type 2 amiodarone-induced thyrotoxicosis (AIT). The flow will be high or normal in type 1 AIT (hyperthyroidism due to underlying nodular thyroid disease or occult GD) and low in type 2 AIT (destructive thyroiditis). [54]
- Treatment / Management
Treatment of hyperthyroidism depends on the underlying etiology and can be divided into symptomatic and definitive therapy. The symptoms of hyperthyroidism, such as palpitations, anxiety, and tremor, can be controlled with a beta-adrenergic antagonist such as atenolol. Calcium channel blockers, such as verapamil, can be used as second-line therapy for patients who are beta-blocker intolerant or have contraindications to beta-blocker treatment. [1]
This review will only discuss the treatment for the most common causes of hyperthyroidism: Graves disease, toxic multinodular goiter, and toxic adenoma in non-pregnant patients.
Indications for treatment:
- Overt hyperthyroidism
- Subclinical hyperthyroidism with TSH <0.1 and age >65 years
- Subclinical hyperthyroidism with TSH <0.1 and age <65 years with comorbidities (cardiovascular disease, osteoporosis, or symptomatic)
- Subclinical hyperthyroidism with TSH <0.1 and age <65 years, if TSH still elevated after 3 to 6 months
- Subclinical hyperthyroidism with TSH between 0.1-0.4 and age >65 years, if TSH still elevated after 3 to 6 months
- Subclinical hyperthyroidism with TSH between 0.1-0.4 and age <65 years with comorbidities (cardiovascular disease, osteoporosis, or symptomatic), if TSH still elevated after 3-6 months
There are three definitive treatments for hyperthyroidism: radioactive iodine therapy (RAI), thionamide therapy, and subtotal thyroidectomy. The choice of which definitive treatment modality depends on the etiology, comorbidities, and patient preferences. Historically, radioactive iodine (RAI) has been the preferred treatment for managing Graves disease in the United States. Still, the trend is changing towards increased use of anti-thyroidal drugs (ATD). [55] ATDs have been the preferred treatment for Graves disease in most other countries. [1]
Antithyroid Drugs (ATDs)
Thionamide drugs include methimazole, carbimazole (precursor of methimazole), and propylthiouracil. These drugs are competitive inhibitors of the thyroid peroxidase (TPO) enzyme, resulting in their ability to block thyroid hormone synthesis. Additionally, these drugs may have additional immunosuppressive effects, as shown by their ability to induce remission in patients with Graves disease. [56] [57] Methimazole and propylthiouracil both inhibit thyroid hormone synthesis by thyroid peroxidase. Thyroid peroxidase is the enzyme responsible for converting dietary iodine into iodide. Propylthiouracil (PTU) also lowers peripheral tissue exposure to active thyroid hormone by blocking the extrathyroidal conversion of T4 to T3. Thionamide therapy has no permanent effect on thyroid function, and recurrence of hyperthyroidism is common in patients who discontinue thionamide therapy.
Attaining a euthyroid status typically requires several months after initiation of thionamide therapy. Although methimazole and PTU are equally effective, methimazole is preferred due to once-daily dosing and a relatively better safety profile. An exception to this recommendation is in pregnant patients, in which PTU is preferred. Methimazole is associated with an increased risk of congenital defects, and thus PTU is preferred in managing hyperthyroidism during pregnancy.
- ATA (Americal Thyroid Association) guidelines provide a rough guide for the initial dose of methimazole based on free T4 levels [1]
- Free T4 1-1.5 times upper limit of normal: Start methimazole 5-10 mg daily
- Free T4 1.5-2.0 times the upper limit of normal: Start methimazole 10-20 mg daily
- Free T4 2.0-3.0 times the upper limit of normal: Start methimazole 30-40 mg daily
- PTU is administered in 2-3 doses per day due to its shorter duration of action. The initial dose of 5-150 three times daily is chosen based on the severity of hyperthyroidism. Once the disease is controlled, the dose can be decreased to a maintenance dose of 50 mg 2 to 3 times daily.
- Monitoring: TSH levels remain suppressed for almost six months in patients with Graves disease, so evaluation of free T4 and/or total T3 levels should be done every 4 to 6 weeks.
- Pregnancy: Propylthiouracil is the preferred drug in the first trimester, associated with lower incidence and severity of embryopathy than methimazole. [58] [59] The treatment can be switched to methimazole after 16 weeks of gestation.
- Drug conversions:
- 10 mg of carbimazole is converted to approximately 6 mg of methimazole [1]
- An equivalent dosage ratio of propylthiouracil to methimazole is 20:1. This ratio is recommended for dose conversions when switching between these agents. [60]
These drugs should be continued for at least 12-18 months. TRAb should be assessed at that time to evaluate for remission. If TRAb levels are normal, then thionamide therapy can be discontinued. If TRAb levels are still elevated, the patient remains at high risk for relapse if medication is stopped. Other factors associated with lower remission rates: male gender, smoking, large goiters, higher TRAb titers at the time of diagnosis, presence of orbitopathy, and the need for a high dose of thionamides to maintain euthyroidism. [20]
An older study from the United States showed a 20-30% remission rate for Graves disease using thionamides. [61] European and Japanese populations noted higher remission rates of 50-60%. [62] [63] [64]
Radioactive Iodine (RAI)
RAI (using I-131 isotope) can be the preferred therapy in most patients, especially the ones with high-risk comorbidities who are at high risk for surgery and need definitive management. Patients who have contraindications for the use of thionamides should also undergo RAI. This procedure should be avoided in patients planning a pregnancy in the six months due to the risk of inducing hypothyroidism in the fetus. RAI is also contraindicated in lactating women. Patients will a history of moderate to severe Graves orbitopathy should not undergo treatment with RAI due to the risk of worsening eye disease. Patients with underlying thyroid malignancies should not undergo RAI.
Radioactive iodine-131 leads to the destruction of thyroid follicular cells. In a female patient of reproductive age, it is highly recommended to obtain a beta-hCG to rule out pregnancy before initiation of RAI therapy. Patients on a thionamide (methimazole or propylthiouracil) should be instructed to discontinue this therapy approximately one week before RAI therapy since thionamide administration can interfere with the therapeutic benefit of RAI therapy. Several months are typically needed status post-RAI therapy to achieve euthyroid status.
- Graves disease
- A single fixed dose of 10-15 mCi (370-555 MBq) is sufficient to render a patient with GD hypothyroid. Doses of RAI can be calculated using the size of the thyroid gland and the uptake of RAI. Cure rates are higher with higher doses, up to 85%. [65] [66]
- Toxic multinodular goiter
- A single dose of 15 mCi is usually sufficient. [67] A calculated dose of 150-200 microCi (5.5-7.4 MBq) per gram of thyroid tissue can be used, corrected for 24-hour radioactive iodine uptake. Cure rates are 55% at three months and 80% at six months. [68] Long-term studies have shown that the risk of hypothyroidism after RAI for TMNG is 3-5% by one year, 16% by five years, and 64% by 24 years. [69] [70] [71]
- Toxic adenoma
- A single fixed of 10-20 mCi (370-740 MBq) is usually sufficient. The dose can also be calculated based on nodule size: 150-200 microCi (5.5-7.5 MBq). [72] Long-term studies have shown that the risk of hypothyroidism after RAI for TA is 8% in 1 year and 60% in 20 years. [73]
Typically, patients with GD are evaluated in 4 to 6-week intervals with an assessment of TSH, free T4, and total T3 levels. The monitoring should continue for another six months or till the patient becomes hypothyroid and is on a stable dose of levothyroxine. Failure to achieve euthyroidism after RAI therapy may indicate the need for either repeat RAI therapy (for symptomatic hyperthyroidism) or the initiation of thyroxine therapy (for hypothyroidism).
RAI therapy involves the release of stored thyroid hormone due to the destruction of thyroid follicular cells, leading to transient hyperthyroidism. This is generally well tolerated, although this transient hyperthyroidism is of concern in patients with significant cardiac disease. For patients with cardiac disease, pretreatment with a thionamide to deplete the stored hormone is recommended to avoid the potential exacerbation of the cardiac disease. In addition, the use of beta-blocker therapy is also essential in these patients to minimize beta-adrenergic symptoms.
Preferred in women planning a pregnancy in less than six months, presence of active Graves orbitopathy, patients who experience significant adverse effects with the use of thionamides, when thyroid malignancy is suspected, presence of large compressive goiters, and the presence of co-existing hyperparathyroidism needing surgery. The surgical option should be avoided in patients with significant comorbidities deemed high-risk for undergoing surgery.
Euthyroidism should be achieved before surgery with the use of thionamides. Preoperative SSKI (saturated solutions of potassium iodide), KI (potassium iodide), or Lugol's iodine should be used in patients with Graves disease and TMNG to decrease gland vascularity and decrease intraoperative blood loss. [74] [75]
- Graves disease: Near-total or total thyroidectomy is the surgical procedure of choice in patients with Graves disease, with excellent cure rates. The risk of recurrence or disease persistence with total thyroidectomy is almost 0% versus 8% with sub-total thyroidectomy after five years. [76] [77] [78]
- Toxic multinodular goiter: Surgical option of choice is near-total or total thyroidectomy to avoid recurrences. [79] [80]
- Toxic adenoma: Preferred surgical option is ipsilateral thyroid lobectomy or isthmusectomy, with excellent cure rates and a risk of the treatment failure rate of less than 1%. [81]
After patients undergo near-total or total thyroidectomy, they should be started on weight-based levothyroxine replacement therapy (0.8 mcg/lb or 1.6 mcg/kg). Lower doses should be used in the elderly, especially in patients with a history of cardiovascular disease or arrhythmia.
- Differential Diagnosis
Hyperthyroidism presents with relatively nonspecific signs and symptoms such as palpitations, increased frequency of bowel movements, and weight loss, among others. Therefore, other pathologies should be ruled out as possible explanations for the patient’s symptomatology.
For etiologies of hyperthyroidism, differential diagnoses can be made based on the physical findings of the thyroid gland. Palpation of a normal thyroid gland in the context of hyperthyroidism can be due to Graves disease, painless thyroiditis, or factitious hyperthyroidism (thyrotoxicosis factitia). Graves disease can also present as a non-tender, enlarged thyroid.
Palpation of a tender enlarged thyroid may indicate De Quervain thyroiditis (subacute thyroiditis). Palpation of a single thyroid nodule is likely indicative of thyroid adenoma, and palpation of multiple thyroid nodules strongly indicates toxic multinodular goiter.
Other differential diagnoses include euthyroid hyperthyroxinemia (in which serum total T4 and T3 are elevated, but the TSH level is within normal limits) and struma ovarii.
- Toxicity and Adverse Effect Management
Antithyroid drugs or thionamides are associated with rare but serious adverse effects of agranulocytosis, hepatotoxicity, and vasculitis. Hepatotoxicity is more common with the use of propylthiouracil (2.7%) than methimazole (0.4%). [82] Hepatotoxicity due to methimazole is more likely to be cholestatic, while hepatotoxicity due to PTU is more likely to be hepatocellular. [83] Hematological complications have an incidence of 0.1-0.15% with the use of PTU or methimazole. Of these patients, 89% had agranulocytosis, and 11% had pancytopenia or aplastic anemia. [84] Patients taking PTU and rarely methimazole can develop p-ANCA (anti-neutrophil cytoplasmic antibody) positive small vessel vasculitis. [85] Up to 40% of those taking PTU can develop c-ANCA positivity, but very few develop vasculitis. [86] [87] These medications are also associated with the development of drug-induced lupus. [88] [89] Few cases of hypoglycemia secondary to autoimmune insulin syndrome have been reported using methimazole. [90] [91]
If patients develop an acute febrile illness with symptoms of pharyngitis, they should get blood work done to check complete blood cell counts along with differentials to rule out the development of agranulocytosis. Liver function tests should be assessed in patients who develop a pruritic rash, abdominal pain or bloating, anorexia, nausea, vomiting, fatigue, jaundice, light-colored stool, or dark urine.
The most common complications following total or near-total thyroidectomy include hypocalcemia due to hypoparathyroidism in less than 2% of cases (can be transient or permanent), recurrent or superior laryngeal nerve paralysis in less than 2% of cases (can be temporary or permanent), hemorrhage, and complications related to anesthesia. [92] [93] [94]
Hyperthyroidism secondary to Graves disease or toxic multinodular goiter has overall good outcomes due to high success rates of definitive treatment and efficacy of symptom management. However, as with any disease, the prognosis of particular disease pathology is patient-oriented and reflects management, response to therapy, and compliance with prescribed treatments.
- Complications
Untreated or unmanaged hyperthyroidism can lead to an extreme case of hyperthyroidism, referred to as a thyroid storm. Reflecting the hypermetabolic state of hyperthyroidism, the patient experiencing thyroid storm will present with tachycardia, increased GI motility, diaphoresis, anxiety, fever, and manifestations of multiple organ dysfunction. Thyroid storm is a potentially life-threatening complication of hyperthyroidism, thus requiring immediate attention. The mortality rate is high in individuals more than 60 years of age, of about 16%. [95]
Prolonged untreated or undertreated hyperthyroidism is associated with an increased risk of acute cardiovascular events, atrial fibrillation, ischemic stroke, osteoporosis, infertility, abnormalities of menstrual cycles, and mortality. [96] [97] [98] [99] Subclinical hyperthyroidism has been associated with an increased risk of arrhythmias such as atrial fibrillation, osteoporosis, hip fractures, and mortality. [100] [2] [101]
- Deterrence and Patient Education
Patient education regarding hyperthyroidism is similar to other diseases. Patients should be educated on the importance of compliance with therapy and on the signs and symptoms of extreme hyperthyroidism (thyroid storm).
- Pearls and Other Issues
Acute coronary syndrome (ACS) may be complicated by thyroid dysfunction. A recent study has shown that thyroid dysfunction is seen in up to 23.3% of patients with coronary artery disease and both overt and subclinical hyperthyroidism in 2.5%. [102]
Pregnancy and concurrent thyroid pathology can pose medical management challenges. PTU is recommended in pregnant women presenting with hyperthyroidism due to methimazole’s association with congenital defects. Close monitoring is recommended with PTU administration, as overcorrection can potentially cause fetal hypothyroidism. The thyroid hormone is particularly important due to its role in fetal neurodevelopment. Recent literature indicates that previously recommended TSH cutoffs in pregnant women lead to overcorrection of thyroid disease in pregnant patients. [103] As fetal exposure to thyroid hormone plays a significant role, careful monitoring and close supervision are warranted.
Neonatal thyrotoxicosis results from fetal tissue exposure to excessive thyroid hormone. There are typically two variants of neonatal thyrotoxicosis: autoimmune-mediated and non-autoimmune-mediated. Autoimmune fetal hyperthyroidism involves the transplacental passage of TSH receptor-stimulating antibodies. Hyperthyroidism is usually transient as symptoms cease 5 to 6 months after birth following clearance of maternal antibodies. Non-autoimmune fetal hyperthyroidism is associated with an activating mutation of either the TSH receptor or the GNAS gene (leading to McCune-Albright syndrome). Unlike the autoimmune etiology, the non-autoimmune variant is permanent, long persisting after birth. [104]
- Enhancing Healthcare Team Outcomes
Except for thyroid storm, hyperthyroidism in itself is rarely life-threatening but can pose a significant burden on a patient’s day-to-day routine. Hyperthyroidism can present with many symptoms and, if not managed, can lead to poor quality of life. Because there are many causes of hyperthyroidism, the condition is best managed by an interprofessional team.
Primary care clinicians should educate patients on the importance of medication compliance. In addition, the patient should be informed by the pharmacist that certain products like contrast dyes, expectorants, food supplements, and seaweed tablets may contain high levels of iodine and interfere with therapy.
Inpatient management of a patient with hyperthyroidism does not always necessarily require consultation with an endocrinologist. Still, thyroid storm strongly warrants consultation with an endocrinologist and possible admission to the intensive care unit due to potentially life-threatening complications such as tachycardia and hypertensive crisis. Therefore, nurses and physician assistants involved with patient care should be vigilant about the signs and symptoms of thyroid storm.
As mentioned, any consideration of RAI therapy in a female of reproductive potential should follow a negative beta-hCG, as pregnancy is an absolute contraindication to RAI therapy. Therefore, incorporating a mandatory pregnancy test into an overall care plan would help avoid potentially damaging radiation exposure.
Patients with Graves disease will need an ophthalmology consult. For those who undergo thyroidectomy, lifelong treatment with levothyroxine is required. Pharmacists must review prescriptions, check for drug interactions, and educate patients.
The interprofessional team must communicate with other members to ensure the patient receives the current standard of care treatment.
- Review Questions
- Access free multiple choice questions on this topic.
- Comment on this article.
Algorithm for evaluation of patients presenting with hyperthyroidism Contributed by Jasleen Kaur, MD. Created using BioRender.
Disclosure: Philip Mathew declares no relevant financial relationships with ineligible companies.
Disclosure: Jasleen Kaur declares no relevant financial relationships with ineligible companies.
Disclosure: Prashanth Rawla declares no relevant financial relationships with ineligible companies.
This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) ( http://creativecommons.org/licenses/by-nc-nd/4.0/ ), which permits others to distribute the work, provided that the article is not altered or used commercially. You are not required to obtain permission to distribute this article, provided that you credit the author and journal.
- Cite this Page Mathew P, Kaur J, Rawla P. Hyperthyroidism. [Updated 2023 Mar 19]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.
In this Page
Bulk download.
- Bulk download StatPearls data from FTP
Related information
- PMC PubMed Central citations
- PubMed Links to PubMed
Similar articles in PubMed
- The clinical evaluation of patients with subclinical hyperthyroidism and free triiodothyronine (free T3) toxicosis. [Am J Med. 1994] The clinical evaluation of patients with subclinical hyperthyroidism and free triiodothyronine (free T3) toxicosis. Figge J, Leinung M, Goodman AD, Izquierdo R, Mydosh T, Gates S, Line B, Lee DW. Am J Med. 1994 Mar; 96(3):229-34.
- Hyperthyroidism (Nursing). [StatPearls. 2024] Hyperthyroidism (Nursing). Mathew P, Kaur J, Rawla P, Fortes K. StatPearls. 2024 Jan
- Review A clinical and therapeutic approach to thyrotoxicosis with thyroid-stimulating hormone suppression only. [Am J Med. 2005] Review A clinical and therapeutic approach to thyrotoxicosis with thyroid-stimulating hormone suppression only. Papi G, Pearce EN, Braverman LE, Betterle C, Roti E. Am J Med. 2005 Apr; 118(4):349-61.
- [Autonomy of the thyroid gland: is there an "organ hyperthyroidism" of "euthyroid" patients?]. [Med Klin (Munich). 2000] [Autonomy of the thyroid gland: is there an "organ hyperthyroidism" of "euthyroid" patients?]. Wawrzyn H, Hesch RD. Med Klin (Munich). 2000 Aug 15; 95(8):421-8.
- Review Evaluation of thyroid status in patients with thyrotoxicosis. [Clin Chem. 1996] Review Evaluation of thyroid status in patients with thyrotoxicosis. Braverman LE. Clin Chem. 1996 Jan; 42(1):174-8.
Recent Activity
- Hyperthyroidism - StatPearls Hyperthyroidism - StatPearls
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers
Finished Papers

IMAGES
VIDEO
COMMENTS
Thyrotoxicosis with hyperthyroidism. The most common cause of hyperthyroidism in iodine-sufficient areas is Graves' disease. In Sweden, the annual incidence of Graves' disease is increasing, with 15-30 new cases per 100 000 inhabitants in the 2000s. 6,7 The cause of Graves' disease is thought to be multifactorial, arising from the loss of immunotolerance and the development of ...
Subclinical hyperthyroidism was first described in the 1970s upon the advent of the TSH immunoassay. Over the past couple of decades, increased understanding on this topic has allowed refined recommendations on how diagnosed patients should best be monitored and treated. The condition is a biochemical diagnosis that is defined by a decreased ...
Untreated hyperthyroidism can cause cardiac arrhythmias, heart failure, osteoporosis, and adverse pregnancy outcomes. It may lead to unintentional weight loss and is associated with increased mortality. ObservationsThe most common cause of hyperthyroidism is Graves disease, with a global prevalence of 2% in women and 0.5% in men.
Graves' disease is the consummate ubiquitous cause of hyperthyroidism in developed countries. It is an autoimmune condition in which antibodies against the thyroid-stimulating hormone receptor ...
Subgroup analysis of studies restricted to overt hyperthyroidism resulted in a similar OR of 1.70 [1.49-1.93], and subclinical hyperthyroidism was also associated with depression (1.36 [1.06-1 ...
Case 1. A 26-year-old young woman presented with thyrotoxicosis and headaches from July 2018 and took propranolol irregularly. But the symptoms gradually worsened and developed proptosis, so she came to our department in July 2022, and her thyroid function after admission is shown in Table 1.Unless otherwise specified, the normal ranges of thyroid function are free thyroxine (FT4) 7.64-16.03 ...
Introduction. Hyperthyroidism is defined as an inappropriately high synthesis and/or secretion of thyroid hormones from the thyroid gland. Thyrotoxicosis is the clinical condition where the effect of excess thyroid hormone on the tissues causes systemic clinical manifestations ( 1 ). The prevalence of hyperthyroidism in the United States is 1.2 ...
Plasmapheresis represent an alternative therapeutic option for hyperthyroidism with thyroid storm or refractory cases. It provides a rapid decrease in plasma thyroid hormones and anti-thyroid antibodies. The aim of this paper was to report our single center's experience in managing particular situations of hyperthyroidism using apheresis. The following case series describes three young ...
Introduction. Hyperthyroidism is a clinical syndrome characterized by hypermetabolic state due to the increased free serum thyroxine (T4) and/or free triiodothyronine (T3). There are many known factors and pathologies both inherent to the thyroid gland as well of non-thyroidal origin that lead to hyperthyroidism.
The diagnostic workup for hyperthyroidism includes measuring thyroid-stimulating hormone, free thyroxine (T4), and total triiodothyronine (T3) levels to determine the presence and severity of the ...
A 58-year-old male who complained of frequent chest tightness and typical electrocardiographic changes while in a resting state suffered from hyperthyroidism, for which he was successfully treated with anti-thyroid agents. Hyperthyroidism is associated with an increase in myocardial oxygen consumption that, due to an imbalance of oxygen demand and supply, can cause angina. However, subclinical ...
A rare case of papillary thyroid cancer associated with hyperthyroidism is described here. Case: A 49-year-old male presented to the authors' outpatient clinic with complaints of a painless left-sided anterior neck swelling that had persisted for the past 8 months. He also reported weight loss for the same duration.
Age-related variation in thyroid function - a narrative review highlighting important implications for research and clinical practice. Thyroid hormones are key determinants of health and well-being. Normal thyroid function is defined according to the standard 95% confidence interval of the disease-free population.
Case 537 -- An 11-year-old girl with symptoms of hyperthyroidism. Contributed by Anca V. Florea, MD and Mohamed A. Virji, MD, PhD. CASE HISTORY: An 11-year-old female with no significant past medical history presented with symptoms suggestive of hyperthyroidism (weight loss, heat intolerance).
Unmet Needs in Managing MDDInterpreting Thyroid Function TestsUsing Thyroid Lab TestsTesting & Screening to Optimize Men's Health. Case In Point: Hyperthyroidism: 5 Cases to Hone Your Diagnostic Skills. July 1, 2004. Russell D. White, MD. Article. A 32-year-old woman presents with weight loss of 6.4 kg (14 lb) during the past 8 months and ...
Introduction. Thyroid dysfunction in the elderly is commonly manifested as hypothyroidism. The prevalence of hyperthyroidism in the elderly is approximately 0.5-3%, of which 10-15% of hyperthyroid patients are above 60 years of age [].Graves' disease is the most common cause of hyperthyroidism, accounting for 60-80% of all cases of hyperthyroidism and primarily occurs between 20 and 50 years ...
Abstract. Background: Data comparing pregnancy outcome in hyperthyroid women with euthyroid women are scarce. Hence, this study was carried out to assess the maternal and fetal outcome in pregnant women with hyperthyroidism to ascertain the effect of disease on pregnancy. Methodology: This retrospective study was conducted over a period of 28 years. We compared the maternal and fetal outcomes ...
CASE STUDY . O F GR AVES' DISEASE IN LIBERIA AND SURGICA L INDIC ATION. Jer ry B rown, 3 Atem Geu, 1 Lawuob ah Gbozee, 1. ... A case report of graves' disease in Liberia and surgical indica tion.
3. Case Study - COPD. Health-Illness Concepts Across the Lifespan I. Assignments. 100% (3) 18. 1460 Exam 3 - Actual exam 3. should be 90% accurate as professors throw questions out each. Health-Illness Concepts Across the Lifespan I. Other.
This presentation is consistent with overt hypothyroidism secondary to Hashimoto's thyroiditis. Thyroid hormone replacement with levothyroxine is indicated. A reasonable starting dose of levothyroxine is 1.6 mcg/kg body weight, with typical starting doses of 50 to 75 mcg daily, although a lower starting dose should be considered in older ...
Hyperthyroidism is a common thyroid disorder. "Hyperthyroidism" defines a syndrome associated with excess thyroid hormone production.[1] It is a common misconception that the terms thyrotoxicosis and hyperthyroidism are synonyms. The term "thyrotoxicosis" refers to a state of excess thyroid hormone exposure to tissues.[1] Although hyperthyroidism can lead to thyrotoxicosis and can be used ...
This case study was aimed to highlight the potential of Unani drugs used in metabolic diseases. About 42 million people in India is suffering from thyroid disease8. As compared to hypothyroidism, hyperthyroidism is less common with more complication9. There are very few papers on case studies and research related to
PDF | On Sep 8, 2022, Rakesh Gupta published Case Study on Patient with Hypothyroidism | Find, read and cite all the research you need on ResearchGate
10 question spreadsheets are priced at just .39! Along with your finished paper, our essay writers provide detailed calculations or reasoning behind the answers so that you can attempt the task yourself in the future. Meet Jeremiah! He is passionate about scholarly writing, World History, and Political sciences.