- Support CCR
- Edit User Profile
- Username Reminder
- Password Reset
- Journal Watch
- Hot Guidelines

Paper of the Day
- My Subscription
- COVID-STEROID2
- COVID-19 Anticoagulation
- RECOVERY-RS
- REMAP-CAP IL-6
- CCR Down Under
- B-FREE Trial
- COVID-19 Vitamin C
- REMAP-CAP Statin Domain
- COMBAT-COVID
- COVID-19 IL-6
- SYNAPSE-ICU
- COAST Trial
- INTUBE Study
- REMAP-CAP Series
- Circulatory
- Resuscitation
- Respiratory
- Gastrointestinal
- Haematological
- Miscellaneous
- Critical Care
- Anaesthesia
- Emergency Medicine
- Methodology
- Visual Abstracts
Critical Care Reviews Meeting 2024
June 12th to 14th, titanic belfast, latest research.
Sharshar. A randomized clinical trial to evaluate the effect of post-intensive care multidisciplinary consultations on mortality and the quality of life at 1 year. Intensive Care Med 2024;epublished April 8th
Lamy. Topical Versus Intravenous Tranexamic Acid in Patients Undergoing Cardiac Surgery: The DEPOSITION Randomized Controlled Trial. Circulation 2024;epublished April 8th
Møller. Microaxial Flow Pump or Standard Care in Infarct-Related Cardiogenic Shock. N Engl J Med. 2024;epublished April 7th.
Yndigegn. Beta-Blockers after Myocardial Infarction and Preserved Ejection Fraction. N Engl J Med. 2024;epublished April 7th
Butler. Empagliflozin after Acute Myocardial Infarction. N Engl J Med. 2024;epublished April 6th
Liu. Effectiveness and safety of Shenfu injection in septic patients with hypoperfusion: A multi-center, open-label, randomized, controlled trial. J Intensive Med. 2024;epublished April 4th
Updated April 8th
Latest Guidelines
Canadian Cardiovascular Society/Canadian Cardiovascular Critical Care Society/Canadian Association of Interventional Cardiology Clinical Practice Update on Optimal Post Cardiac Arrest and Refractory Cardiac Arrest Patient Care. Can J Cardiol 2024;40(4):524-539
Aiza-Haddad. Guidelines for the management of coagulation disorders in patients with cirrhosis. Rev Gastroenterol Mex 2024;epublished April 9th
Management of patients with an electrical storm or clustered ventricular arrhythmias: a clinical consensus statement of the European Heart Rhythm Association of the ESC—endorsed by the Asia-Pacific Heart Rhythm Society, Heart Rhythm Society, and Latin-American Heart Rhythm Society. EP Europace 2024;26(4):euae049
Williams. The management of heart failure cardiogenic shock: an international RAND appropriateness panel. Crit Care 2024;28:105
Updated April 11th
Latest Narrative Reviews
Douflé. Head-to-toe bedside ultrasound for adult patients on extracorporeal membrane oxygenation. Intensive Care Med 2024;epublished April 10th
Wang. Gut-brain axis in the pathogenesis of sepsis-associated encephalopathy. Neurobiol Dis 2024;195:106499
Robba. Contemporary management of aneurysmal subarachnoid haemorrhage. An update for the intensivist. Intensive Care Med 2024;epublished April 10th
El-Menyar. Cardiac arrest, stony heart, and cardiopulmonary resuscitation: An updated revisit. World J Cardiol 2024;16(3):126-136
Strauß. Biomarkers of acute kidney injury: From discovery to the future of clinical practice. J Clin Anesth. 2024;95:111458
Zhao. Glucocorticoid therapy for acute respiratory distress syndrome: Current concepts. J Intensive Med 2024;epublished April 1st
Latest CCR Content
Alzerwi. Surgical management of acute pancreatitis: Historical perspectives, challenges, and current management approaches. World J Gastrointest Surg 2023;15(3):307-322
Saturday, April 13h
Join us to read one paper per day and cover the spectrum of critical care across 2024. Previous papers of the day are available here .

Newsletter 643
Critical Care Reviews Newsletter 643, bringing you the best critical care research and open access articles for the week April 1st to 7th, 2024
Subscriptions are available to individuals and departments
added April 8th

Jessical Spence (Hamilton, Canada) talks about the B-FREE trial, investigating bendrodiazepine-free anaesthesia for the prevetion in delirium in cardiac surgical patients.
Added April 7th
Latest CCR24 Trial Announcements

CCR24 Announcement
Continuous vs intermittent carbapenem infusion
Added March 7th

Propofol vs Dexmedetomidine vs Clonidine
Added March 5th

Steroids for non-COVID-19 Community-Acquired Pneumonia
Added March 1st

- Critical Care Medicine
Explore the latest in critical care medicine, including management of respiratory failure, sepsis, HAI prevention, end-of-life care, and more.
Publication
Article type.
In this narrative medicine essay, a pediatric critical care physician mulls a discussion about a patient’s status with a male colleague in front of her all-female medical team, a discussion that led her to question herself until she concludes that she can’t be all things to all people at all times.
This cohort study characterizes heterogeneity in cardiac function prior to sepsis and describes associations with hospitalization outcomes and mortality.
This cross-sectional study investigates perioperative oxygen saturation differences in Black and White infants with single ventricles undergoing stage 1 palliation.
This cohort study describes and compares clinical and radiographic features, treatment responses, and outcomes of neuroinvasive West Nile virus infection in individuals with and without immunosuppression.
This cohort study examines whether the use of an artificial intelligence–enabled deterioration model is associated with a decrease in the risk of escalations in care in hospitalized patients.
This randomized clinical trial assesses the effect of targeting a lower vs higher oxygenation level on 90-day survival without need for life support among intensive care unit patients with COVID-19 and severe hypoxemia.
This cohort study examines whether peripheral oxygenation-saturation targets on mortality would differ by individual patient characteristics among 2 temporally and geographically distinct randomized trials of lower vs higher Sp o 2 targets in critically ill patients receiving mechanical ventilation
This JAMA Guide to Statistics and Methods article explains effect score analyses, an approach for evaluating the heterogeneity of treatment effects, and examines its use in a study of oxygen-saturation targets in critically ill patients.
- Oxygen Supplementation in COVID-19—How Much Is Enough? JAMA Opinion March 19, 2024 Respiratory Failure and Ventilation Pulmonary Medicine Coronavirus (COVID-19) Full Text | pdf link PDF
- Your Mileage May Vary: Toward Personalized Oxygen Supplementation JAMA Opinion March 19, 2024 Respiratory Failure and Ventilation Pulmonary Medicine Full Text | pdf link PDF
This cohort study assesses whether severe sepsis during treatment for childhood acute leukemia is associated with increased incidence of long-term organ dysfunction among adult survivors using observational data from the St Jude Lifetime Cohort Study.
This cohort study examines the natural history and response to treatment of sodium glucose cotransporter 2 (SGLT2) inhibitor–associated ketoacidosis compared with that of type 1 diabetes–associated ketoacidosis.
This cluster randomized clinical trial assesses the extent to which video laryngoscopy compared with direct laryngoscopy might facilitate intubation in patients undergoing surgical procedures during routine clinical practice.
This cohort study assesses changes in intensive care unit (ICU) admission rate, hospital length of stay, and use of noninvasive and invasive ventilation after the adoption of weight-based high-flow nasal cannula protocol in hospitalized patients aged 0 to 24 months.
This cohort study assesses the association between number of recent pediatric intensive care unit (PICU) critical illness episodes and survival among children with severe neurologic impairment.
- Bringing Pediatric Chronic Critical Illness Into Acute Focus JAMA Network Open Opinion March 15, 2024 Pediatrics Neurology Child Development Full Text | pdf link PDF open access
This cross-sectional study examines the prevalence of anxiety, depression, posttraumatic stress disorder, and physical somatic symptoms in adult and pediatric patients with induced laryngeal obstruction.
This cohort study evaluates prevalence and factors associated with bacteremia from a presumed urinary source in inpatients with asymptomatic bacteriuria with or without altered mental status.
This cohort study assesses in-hospital mortality among adults at US rural and urban hospitals during vs before the COVID-19 pandemic for non–COVID-19 time-sensitive conditions.
- Transfer as Treatment in Rural Hospitals JAMA Network Open Opinion March 12, 2024 Health Policy Acute Coronary Syndromes Cardiology Infectious Diseases Percutaneous Coronary Intervention Full Text | pdf link PDF open access
Select Your Interests
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
- Open access
- Published: 04 February 2020
Nutrition therapy in critical illness: a review of the literature for clinicians
- Kate J. Lambell ORCID: orcid.org/0000-0002-4816-3023 1 , 2 , 3 ,
- Oana A. Tatucu-Babet 1 ,
- Lee-anne Chapple 4 , 5 ,
- Dashiell Gantner 1 , 6 &
- Emma J. Ridley 1 , 2
Critical Care volume 24 , Article number: 35 ( 2020 ) Cite this article
48k Accesses
67 Citations
116 Altmetric
Metrics details
A Letter to this article was published on 19 June 2020
Nutrition therapy during critical illness has been a focus of recent research, with a rapid increase in publications accompanied by two updated international clinical guidelines. However, the translation of evidence into practice is challenging due to the continually evolving, often conflicting trial findings and guideline recommendations. This narrative review aims to provide a comprehensive synthesis and interpretation of the adult critical care nutrition literature, with a particular focus on continuing practice gaps and areas with new data, to assist clinicians in making practical, yet evidence-based decisions regarding nutrition management during the different stages of critical illness.
In recent years, there has been much interest in the role of nutrition therapy in critical illness with an increase in publications and two updated international clinical guidelines [ 1 , 2 ]. However, trial findings and guideline recommendations continue to be conflicting, making the translation of evidence into practice challenging. Further, it is becoming evident that the stage of critical illness and individual factors such as body composition may be important when considering how individuals might respond to nutrition interventions [ 3 , 4 ]. This narrative review aims to provide a summary and interpretation of the adult critical care nutrition literature, with a particular focus on continuing practice gaps and areas with new data, to help clinicians make practical, yet evidence-based decisions regarding nutrition management during critical illness.
The metabolic response to critical illness and the role of nutrition therapy
It is recognised that ‘one-size fits all’ and ‘set and forget’ approaches to nutrition do not adequately address the complex metabolic, hormonal, and immunological changes that occur with critical illness [ 3 , 5 ]. It is essential that clinicians understand these processes and the impact on nutrient metabolism [ 4 ]. In 1942, Cuthbertson described two distinct metabolic phases during acute illness—the ‘ebb’ or early shock phase, followed by the ‘flow’ or catabolic phase [ 6 ]. In brief, the ‘ebb’ phase is characterised by haemodynamic instability and hormonal changes (including insulin resistance) in order to prioritise the delivery of energy substrates to vital tissues [ 6 , 7 ]. This survival mechanism results in endogenous glucose production as well as lower energy expenditure compared to pre-injury [ 4 ]. The ‘flow’ phase involves the breakdown of tissue (including lean muscle tissue) in order to provide substrates to cover the immediate needs for the ‘fight or flight’ response and to reduce the risk of bleeding and infection [ 4 ]. More recently, a third, anabolic recovery phase has been described [ 3 ]. It is during this recovery phase when resynthesis of lost tissue can take place and the body may be more metabolically able to process delivered nutrients [ 3 , 4 ]. Currently, there is no known clinical marker to identify when an individual shifts from one phase of critical illness to another. For the purposes of this review which aims to provide practical recommendations, we have adapted the terminology from the 2019 European Society of Parenteral and Enteral Nutrition (ESPEN) critical care guideline to describe the different stages of critical illness: ICU day 1–2 (acute early phase), ICU day 3–7 (acute late phase), and after ICU day 7 (recovery phase) [ 2 ].
While it is considered that nutrition may be more physiologically available and hence more important in the later phase of illness, due to the average intensive care unit (ICU) length of stay (LOS), the majority of nutrition trials have provided nutrition interventions in the acute phases of illness (regardless of the intended trial intervention period). Traditionally, it was thought that aggressive nutrition in the early stages of critical illness may improve clinical outcomes. However, evidence from recent randomised controlled trials (RCTs) does not support this, finding no benefit or harm with early nutrition delivery [ 8 , 9 , 10 , 11 ]. An explanation for this may be because a substantial amount of energy was provided in a period of critical illness where energy expenditure is decreased and endogenous production is enhanced [ 4 ]. Specifically, harm was observed in The Early Parenteral Nutrition Completing Enteral Nutrition in Adult Critically ill Patients (EPaNIC) trial, the largest nutrition trial in critical illness [ 10 ]. In a study of 4640 mixed ICU patients ( n = 2818 (61%) cardiac surgery patients) who were eligible to receive EN, late initiation of PN (started on day 8 of the ICU stay) led to an increase in the proportion of patients discharged alive and earlier from ICU and hospital (hazard ratio (HR) 1.06; 95% CI 1.00–1.13; p = 0.04 for both) when compared to PN commenced within 48 h of ICU admission [ 10 ]. Late initiation PN also led to a reduction in infectious complications (22.8% vs 26.2%, p = 0.008), cholestasis, duration of mechanical ventilation (MV), duration of renal replacement therapy, and health care costs [ 10 ]. Most recently, results from the largest enteral nutrition (EN) trial, The Augmented versus Routine approach to Giving Energy Trial (TARGET), support the theory that augmented energy delivery in the early phase of illness does not improve clinical outcomes compared to standard care [ 8 ]. This pragmatic prospective RCT of 3957 patients assessed 90-day mortality with augmented energy delivery (based on a predictive estimate of 1 ml/kg ideal body weight for height per day), compared to routine care [ 8 ]. Energy delivery was 50% higher in the intervention group (~ 30 kcal/kg ideal body weight/day) over the median 6-day nutrition delivery period (and approximated clinician estimated energy aims) but did not impact mortality or any secondary clinical outcomes [ 8 ]. However, it must be noted this study included a very ‘general’ (or unselected) population and that overfeeding may have occurred. Further post hoc work may increase the understanding and clinical implications of these results. Lack of benefit has also been observed with hypocaloric (low energy and adequate protein) and trophic (low energy and protein) feeding strategies compared to standard care, also provided early in critical illness and for short periods [ 9 , 12 ]. The results of these trials support the hypothesis that for mixed ICU patients, nutrition interventions in the acute early and acute late phase of critical illness may not impact clinical outcomes and may cause harm in some groups. Therefore, less than 100% of energy expenditure should be targeted in this period due to endogenous glucose production. It remains unknown whether nutrition interventions continued for longer, impact functional recovery and quality of life [ 3 ].
Guidelines for nutrition therapy in critical illness
There are currently four international clinical practice guidelines available to inform the nutrition management of critically ill patients [ 1 , 2 , 13 , 14 ]. Table 1 summarises each guideline and outlines key recommendations and their level of supporting evidence.
Energy in critical illness
Determination of energy requirements is one of the most significant challenges in critical illness and is of vital importance as prescribed targets are used to guide nutrition delivery. Predictive equations that estimate energy expenditure are the most commonly used method due to their ease of application but are often inaccurate compared to measured energy expenditure using indirect calorimetry [ 15 ]. Table 2 summarises why predictive equation estimates vary from measured energy expenditure [ 16 , 17 ]. Importantly, inaccuracies increase at the extremes of weight, in the most severely unwell, and in older and more malnourished populations [ 16 , 18 ]. Despite these failings, predictive equations continue to be widely used and are recommended in international clinical guidelines in the absence of indirect calorimetry [ 1 , 2 ].
Estimating energy expenditure via VO 2 and VCO 2
Due to the persistent inaccuracies associated with the use of predictive equations, other methods (many of which have existed for some time) have been recently recommended in the 2019 ESPEN critical care guideline in the absence of indirect calorimetry [ 2 ]. Resting energy expenditure (REE) can be estimated via VCO 2 (carbon dioxide production) from the ventilator and the rewritten Weir formula (REE = VCO 2 × 8.19) or using VO 2 (oxygen consumption) from a pulmonary artery catheter via the Fick method [ 19 , 20 , 21 , 22 ]. A recent study in 84 critically ill patients reported a higher level of agreement between energy requirements estimated by the VCO 2 method and measured REE compared to other predictive equations [ 20 ]. There are methodological limitations to note with this method: an assumed normal respiratory quotient (RQ) of 0.85 is used, which is the RQ of most nutritional products (with RQ = VCO 2 /VO 2 , normally ranging between 0.67 and 1.2 depending on the proportion of carbohydrate, fat, and protein being combusted) [ 23 ]. However, in critical illness, RQ may also be influenced by endogenous glucose production and by periods of hypo- and hyper-ventilation, and is likely to fluctuate between populations [ 19 , 20 ].
Measuring energy expenditure in the critically ill—indirect calorimetry
Indirect calorimetry allows for the measurement of VO 2 and VCO 2 through the ventilator and is the gold standard method for measuring REE in critical illness when ideal test conditions are implemented [ 24 ]. Both the European (ESPEN) and American (ASPEN/SCCM) clinical practice guidelines recommend the use of indirect calorimetry to measure energy expenditure (Table 1 ) [ 1 , 2 ].
Despite the guideline recommendations, only three single-centre RCTs have investigated the impact of delivering energy according to a measured energy expenditure (via indirect calorimetry) compared to energy delivery using a 25-kcal/kg/day estimate (standard care) on clinical outcomes. The first, published in 2011, included 130 patients and observed a trend towards reduced hospital mortality (primary outcome) in the intervention group using intention to treat (ITT) analysis ( n = 21/65, 32.3%, vs 31/65, 47.7%, p = 0.058) [ 25 ]. However, infectious complications ( n = 37 vs 20, p = 0.05) and mean (± standard deviation) duration of MV (16.1 ± 14.7 vs 10.5 ± 8.3 days, p = 0.03) and ICU LOS (17.2 ± 14.6 vs 11.7 ± 8.4 days, p = 0.04) were increased in the intervention group compared to standard care [ 25 ]. In a more recent and slightly larger trial of 203 patients, no differences were observed in the primary outcome (self-reported physical component summary score of SF-36 at 6 months) between intervention and control in the ITT analysis ( n = 199, 22.9 vs 23.0, p = 0.99, respectively) or in any clinically important secondary outcomes [ 11 ]. However, in a post hoc analysis, a longer median (interquartile range) ICU LOS was observed in the intervention group (8 (5–25) vs 7 (4–12) days, p = 0.03) [ 11 ]. Lastly, in a pilot study ( n = 40), no statistically significant differences were observed between groups in the primary outcome of change in bioelectrical impedance phase angle (related to nutritional status and prognosis) from baseline to ICU discharge [ 26 ]. However, a declining trend in mean phase angle was observed in the standard care group (3.31 ± 1.34° to 2.95 ± 1.15°, p = 0.077), and a significantly shorter ICU LOS was reported in the intervention versus the standard care group (13 ± 8 vs 24 ± 20 days, p < 0.05) [ 26 ].
Consistently across all three RCTs, indirect calorimetry was feasible and energy targets were more closely met when using indirect calorimetry in place of fixed-energy prescription. Methodological characteristics must be noted in interpreting these results; all studies were unblinded and single-centre in design and were likely underpowered to demonstrate true differences in clinical and functional recovery outcomes. Further, these studies aimed to meet 100% of indirect calorimetry targets early in the ICU admission, which recent evidence suggests is not beneficial, and there was limited investigation into high-risk subgroups in which indirect calorimetry may have avoided harm by under- or overfeeding (i.e. obesity). Despite this, these studies do not suggest that indirect calorimetry to guide energy delivery is superior to using predictive equations with regard to improving clinical outcomes.
Measurement or estimation of energy expenditure?
Regardless if energy expenditure is measured or estimated, there is no consensus on how much energy should be provided. Based on current evidence, the most significant benefit of using indirect calorimetry is to personalise energy prescription and avoid under- or overdelivery of energy across the different phases of critical illness. For this reason, it is the opinion of the authors that if indirect calorimetry is available, it should be used primarily in patients where the clinicians are concerned about under- or overestimating energy needs (i.e. obese and underweight individuals) [ 27 ]. When used, clinicians should aim for high-quality tests by reaching a steady test state (defined as a variation in VO 2 and VCO 2 less than 10% over five consecutive minutes), conduct tests for ≥ 30 min, and repeat tests at least weekly (or more frequently if clinically indicated) [ 24 ].
For the majority of clinicians, current practice will continue to include the use of a predictive equation for estimation of energy needs. Clinicians must be aware that accurate estimation of energy expenditure with a predictive equation requires considerable knowledge of the underlying patient condition, the factors that alter the metabolic response to illness, and the limitations of the equation being used. It is also important to consider that delivery of calories to meet measured or estimated energy expenditure may not equate to what should be provided to improve outcomes. This may be particularly relevant in the acute early phase of critical illness where endogenous substrate mobilisation provides a substantial portion of the energy requirement and insulin resistance occurs, and therefore, a conservative energy target should be the aim [ 28 ]. Energy prescription and energy delivery (including non-nutritional sources such as dextrose and propofol) should be regularly reviewed in the context of the patient’s clinical condition and metabolic phase to prevent considerable under- or overfeeding [ 29 ].
Protein in critical illness
In states of stress, such as in critical illness, the synthesis of acute phase proteins and those involved in immune function increase to support recovery [ 30 ]. Rapid and significant loss of skeletal muscle mass occurs to provide precursor amino acids to aid this process [ 31 ]. Despite a lack of definitive evidence, clinical guidelines recommend protein delivery of between 1.2 and 2 g/kg/day (Table 1 ) based on the assumption that like energy, delivery of adequate protein will attenuate skeletal muscle wasting and improve clinical outcomes. The ASPEN/SCCM guidelines also make recommendations for higher protein provision in specific clinical conditions (i.e. burns, obesity, and multi-trauma), which again are based on limited, primarily observational data and expert opinion [ 1 ]. The variation in the clinical guideline recommendations for protein delivery reflects the lack of good quality trials investigating the role of protein provision on clinical outcomes.
Protein delivery and clinical outcomes
Higher protein provision has been associated with improved survival in a number of observational studies [ 32 , 33 , 34 , 35 , 36 ]. Conversely, higher protein delivery during ICU admission has led to increased urea production and has been associated with increased muscle wasting in a small observational study [ 10 , 11 , 31 , 37 ].
In RCTs aiming to compare high versus lower protein delivery in critical illness, no benefit has been shown with an increased protein dose, although most have been underpowered to demonstrate an effect on clinical outcomes [ 11 , 37 , 38 , 39 ]. The largest RCT ( n = 474) investigating intravenous protein provided at a dose of up to 100 g/day compared to standard care found no impact on the primary outcome of renal dysfunction [ 37 ]. A smaller RCT compared intravenous protein at a dose of either 0.8 g/kg ( n = 60) or 1.2 g/kg ( n = 59) delivered over ten days while controlling for energy intake [ 38 ]. While there was no difference in the primary outcome of handgrip strength, the group who received the higher protein dose had less fatigue and higher forearm thickness (using ultrasound) at day 7 [ 38 ]. However, these findings may be impacted by unadjusted confounders and must be interpreted with caution [ 40 ].
Timing of protein delivery may also influence clinical outcomes. Two observational studies have reported increased survival with early increased protein delivery (day 3–4) [ 32 , 33 ]. In the largest study ( n = 2253), early protein delivery (> 0.7 g/kg/day versus ≤ 0.7 g/kg/day) was associated with increased survival (adjusted HR 0.83, 95% CI 0.71–0.97, p = 0.017) [ 33 ]. Contrary to these findings, in a post hoc secondary analysis of the EPaNIC trial, a cumulative protein dose, rather than the cumulative glucose dose, early during ICU stay was associated with delayed ICU discharge [ 41 ]. Further, a single-centre retrospective cohort study ( n = 455) reported a lower protein intake (< 0.8 g/kg/day) before day 3 and high protein intake (> 0.8 g/kg/day) after day 3 was associated with lower 6-month mortality (adjusted HR 0.609; 95% CI 0.480–0.772, p < 0.001) compared to patients with overall high protein intake [ 42 ]. Prospective, randomised data is required to inform the most appropriate amount and timing of protein to deliver to critically ill patients. Adequately powered RCTs are urgently needed to better understand the impact of both protein dose and timing on clinical outcomes in critical illness. Such trials should ideally control for energy delivery, by ensuring it is consistent across both the intervention and control groups.
How much energy and protein do patients get in clinical practice?
One of the most important pieces of information that clinicians should consider is that patients do not receive the energy and protein dose that is prescribed. In a recent retrospective observational study of 17,524 patients, the mean ± standard deviation energy and protein received was 56 ± 30% and 52 ± 30% of the intended aim, respectively [ 43 ]. This has consistently been shown across different time periods and geographical regions [ 44 ]. The reasons for this are multifactorial, including interruptions to EN for procedures, delayed initiation of nutrition, and gastrointestinal intolerance [ 45 ].
What energy and protein targets should clinicians aim for?
In light of the current evidence, the authors support the gradual introduction of nutrition therapy during the acute phases of critical illness, with energy and protein targets outlined in Fig. 1 . In patients who are ‘at risk’ of refeeding syndrome, it is crucial that nutrition therapy is introduced slowly, and electrolytes are monitored closely and replaced as necessary [ 46 ]. If hypophosphatemia is present (e.g. < 0.65 mmol/l) in the first few days after starting nutrition therapy, then energy delivery should be restricted to ~ 50% requirements for 2–3 days [ 47 ].
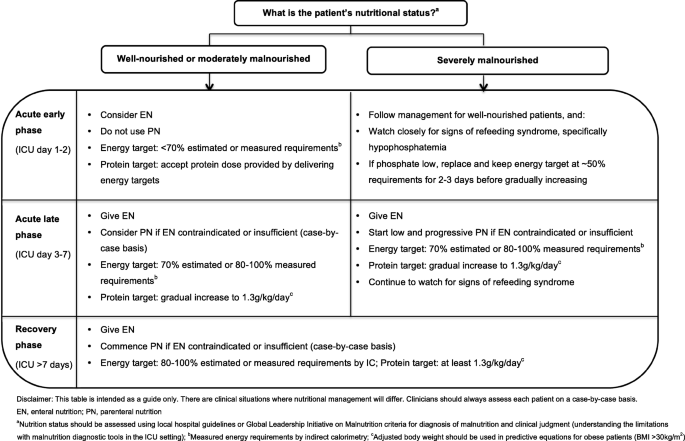
Recommendations for nutritional management by nutritional status and phase of critical illness
- Enteral nutrition
When to start?
Early provision of EN (within 48 h of ICU admission) in patients who are mechanically ventilated is an established standard of care and supported by all clinical guidelines [ 1 , 2 , 13 , 14 ].
How should EN be delivered?
The most common method of delivering EN in ICU is via a gastric tube, with a continuous hourly infusion. However, this continuous supply of nutrients does not mimic normal volitional intake which is most commonly in the form of boluses followed by periods of fasting. Recently, it has been proposed that bolus (intermittent) feeding may be more physiologic and therefore superior to continuous feeding [ 48 ]. A systematic review was conducted as part of the recent ESPEN guidelines to investigate whether bolus EN has an advantage over continuously administered EN [ 2 ]. Including 5 small prospective studies and 236 patients, a significant reduction in diarrhoea was observed with continuous versus bolus administration of EN (RR 0.42, 95% CI 0.19–0.91, p = 0.03) [ 2 ]. No differences in gastric residual volume, rates of aspiration, or pneumonia were observed. It has also been suggested that muscle protein synthesis may be improved when EN is delivered via a bolus when compared to continuous delivery, and a phase II multicentre RCT has recently completed recruitment to investigate this question ( ClinicalTrials.gov NCT02358512) [ 5 , 48 ]. Moving from continuous to bolus delivery of EN in the ICU is a significant change to practice in most countries, which would require a variation in feeding protocols and extensive education of clinical staff. Due to the considerable practice change associated, until definitive evidence is available to support one method of delivery over the other, it is reasonable that clinicians continue to deliver EN via a continuous infusion.
EN delivery—an ongoing challenge
International guidelines are unanimous in favouring EN delivery into the stomach or small bowel over parenteral nutrition (PN) [ 1 , 2 ]. Due to continued and consistent recommendations to meet energy requirements over many years, many strategies to ‘optimise’ EN delivery closer to predicted targets have been tested, including the use of evidence-based feeding protocols, small bowel feeding tubes, prokinetic drugs, and increase of the acceptable gastric residual volume [ 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 ]. Guideline recommendations to maximise EN delivery are summarised in Table 3 . Despite the implementation of such interventions, these trials have observed modest to no increase in nutrition delivery and none have demonstrated a beneficial effect on clinical outcomes, potentially related to the disconnect between ‘delivery’ and ‘utilisation’ of nutrients.
- Parenteral nutrition
PN is indicated when the delivery of nutrients via the gastrointestinal tract is contraindicated or insufficient. PN can be provided either as a full source of nutrition (exclusive PN) or as an additional nutrition source when full requirements are not able to be met by oral intake or EN (supplemental PN). Recent RCT evidence has indicated there are no differences in clinical outcomes, including mortality and infective complications, when PN is provided versus EN in a modern day ICU setting and when energy provided is comparable in both groups [ 59 , 60 ]. Guideline recommendations for when to commence PN differ and are outlined in Table 1 . Due to the potential harm with early PN, it is the opinion of the authors that if oral intake or EN is contraindicated, then PN should only be considered between ICU days 3 and 7 and that supplemental PN be considered on an individual case-by-case basis (Fig. 1 ).
Body composition analysis
The measurement of weight and muscularity is important in the assessment of nutrition status and monitoring the effectiveness of nutrition interventions [ 61 ]. However, due to the extreme fluid shifts that critically ill patients experience, measured weight and/or muscularity assessed by traditional bedside methods (e.g., subjective physical assessment, mid-arm muscle circumference) may be inaccurate in this patient population [ 62 , 63 , 64 ]. Table 4 summarises the emerging tools for assessment of muscularity in the ICU setting: computed tomography image analysis, bioimpedance analysis, and ultrasound. Currently, these methods for assessing muscle mass and quality are mostly limited to research [ 64 , 65 , 66 ]. There is an essential need to evaluate which bedside tools can accurately measure muscle mass, and identify those individuals with lower than normal muscularity, as well as to better understand the clinical importance of changes in muscle health and the interface with nutrition interventions in critical illness.
Nutritional management in critically ill subgroups
RCTs conducted to date have focused on key practice questions, but included heterogenic populations. These studies have not shown clinical benefit with nutrition interventions for reasons previously discussed though there are several patient subgroups that may still benefit from nutrition interventions. In an attempt to investigate such groups, a number of large RCTs have included pre-planned subgroup analysis (e.g. response to the intervention according to differing BMI category). However, results from these types of analyses must be interpreted with caution as the sample size may be small. Moreover, if a benefit or harm is observed in a subgroup, but the overall trial result suggests no difference, it must be considered that another subgroup hidden in the heterogeneous population may have experienced the opposite effect.
Malnourished
The diagnosis of malnutrition in critically ill patients is challenging. Diagnostic tools, such as the widely used Subjective Global Assessment (SGA) and criteria outlined in the recent Global Leadership Initiative in Malnutrition (GLIM) recommendations, rely heavily on obtaining accurate anthropometrical data, weight and diet history, and the assessment of muscle mass, all of which are difficult to acquire in the acute early phase of ICU admission [ 61 ]. For this reason, RCT evidence attempting to investigate if patients who may be malnourished respond differently to nutrition is limited to subgroup analysis in patients with differing BMI categories or nutrition risk scores [ 10 , 12 , 67 ]. To date, no benefit has been observed when more or less nutrition is provided in these subgroups although the numbers included are often small. Further, BMI is a poor surrogate measure for malnutrition, and commonly used nutrition risk scores have not been well validated, which limits any conclusions on how nutrition therapy may affect outcomes in this vulnerable subgroup [ 2 ]. Despite the lack of evidence in this area, the authors support minimising progression of malnutrition. Where possible, clinicians should use local hospital guidelines or the recent GLIM criteria, combined with clinical judgement to diagnose malnutrition. As outlined in Fig. 1 , in severely malnourished patients, we encourage early low dose nutrition therapy in the acute early phase, with a slow progression to target during the acute late phase, while carefully monitoring for refeeding syndrome.
The unique and complex care needs of obese patients (BMI ≥ 30 kg/m 2 ) are amplified when they become critically ill and include a greater risk of insulin resistance and loss of lean muscle mass, and wide variations in macronutrient metabolism, which makes nutrition management complex [ 4 , 68 ]. There is currently very limited, low-quality evidence to inform nutrition provision in the critically ill obese patient, and as a result, the latest clinical guidelines provide inconsistent recommendations regarding energy and protein targets (Table 1 ).
In the TARGET trial, 1423 obese critically ill patients were included, representing the largest population of obese patients in an ICU nutrition study [ 8 ]. While not statistically significant, the obese subgroup was the only pre-specified subgroup where the point estimate sat on the side of benefit with greater energy delivery [ 8 ]. These results require formal evaluation in a robust, adequately powered and blinded clinical trial; however, they highlight that obese patients may respond differently to nutrition delivery than non-obese individuals and that there is a critical need for further research in this patient group.
In the absence of definitive evidence of the impact on functional recovery in particular, it is the opinion of the authors that obese patients should be managed like any other patient admitted to the ICU. If predictive equation estimates are used, a method to adjust body weight should be used in the nutrition prescriptions (not actual weight), and delivery monitored carefully with the knowledge that most predictive equations significantly underestimate requirements in this group [ 69 ]. It may be appropriate to consider a weight loss regime in the recovery phase once the acute illness has resolved.
The non-ventilated patient
Critically ill patients who are not intubated may have prolonged periods of inadequate oral intake. In a prospective observational study, 50 patients who were not receiving any EN or PN were studied for 7 days following endotracheal extubation [ 70 ]. The average daily energy and protein intake failed to exceed 50% of daily requirements on all 7 days for the entire population [ 70 ]. To prevent malnutrition, it is important that clinicians monitor the oral intake of awake patients and the authors support the ESPEN guideline recommendation that medical nutrition therapy should be considered for all patients staying in the ICU for > 2 days regardless of their ventilation status [ 2 ].
The limited data available indicates that the predominant mode of nutrition following an ICU admission is via the oral route and nutrition intake in this period remains below clinician recommendations. In 32 patients from 2 centres, nutrition intake was assessed 3 times per week in the post-ICU phase [ 71 ]. Oral nutrition was the most common type of nutrition therapy (55% of study days) [ 71 ]. The median [interquartile range] energy and protein intake was 79% [41–108%] and 73% [44–98%], respectively; however, considerable variation was observed depending on the type of nutrition therapy provided, with energy and protein provision the lowest in patients who received no additional oral nutrition supplements (37% [21–66%] of target energy and 48% [13–63%] protein) [ 71 ]. A second single-centre study of patients with traumatic brain injury indicated poorer intake post-ICU compared to in ICU, and nutritional deficit was significantly greater in patients who consumed oral nutrition alone compared to those receiving artificial nutrition support [ 72 ]. Despite this, dietitians spent just 20% of their time managing patients receiving oral nutrition therapy and saw the patients a mean of 2.2 (1.0) times per week for 34 (20) min per occasion on the post-ICU ward [ 72 ]. The predominant issues impacting nutrition intake are reported as appetite, disinterest in food, and taste changes [ 73 ].
Unfortunately, non-individualised, ‘one-size fits all’ processes to the management of nutrition are likely impacting on nutrition adequacy in the post-ICU period. In one of the only studies investigating processes that impact nutrition in the post-ICU period, it was found that of nine patients transferred to the post-ICU ward, six had their gastric tube removed on the advice of the medical team without assessment of nutrition intake [ 73 ]. Early removal of gastric tubes may improve patient comfort and is encouraged by many post-surgical protocols, but has the potential to negatively impact nutrition intake [ 73 ]. The decision to remove a tube should be made on a case-by-case basis and after consultation with the patient, the treating team, and the dietitian [ 74 ]. Among other possible causes, it is plausible that inadequate nutrition following critical illness may result in significant energy and protein deficit and may explain the lack of benefit in long-term outcomes observed in nutrition studies that have delivered an intervention in the acute early and late phases. This is an important knowledge gap for investigation and to provide initial insights; a multicentre RCT is underway ( ClinicalTrials.gov NCT03292237).
Results from recent large-scale trials highlight that in heterogeneous groups of patients, full feeding in the acute phases of critical illness does not provide an advantage over trophic feeding and may be harmful. It remains uncertain what impact specific nutrition interventions have in the recovery phase of illness and in specific subgroups who may respond differently to nutrition interventions. The effect of nutrition delivery on other clinically meaningful outcomes, such as muscle health and physical function, is also insufficiently studied. We recommend nutrition prescriptions that tailor for pre-admission nutrition status, and severity and stage of illness. Particular attention should be paid to patients that are in (or likely to stay in) ICU for greater than a week, with ongoing monitoring of nutrition delivery and regular review of measured or estimated nutrition requirements.
Availability of data and materials
Not applicable.
Abbreviations
American Society of Parenteral and Enteral Nutrition/Society of Critical Care Medicine
European Society of Parenteral and Enteral Nutrition
Intensive care unit
Randomised control trial
Taylor BE, McClave SA, Martindale RG, Warren MM, Johnson DR, Braunschweig C, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). Crit Care Med. 2016;44(2):390–438.
Article PubMed Google Scholar
Singer P, Blaser AR, Berger MM, Alhazzani W, Calder PC, Casaer MP, et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin Nutr. 2019;38(1):48–79.
Wischmeyer PE. Tailoring nutrition therapy to illness and recovery. Crit Care. 2017;21(Suppl 3):316.
Article PubMed PubMed Central Google Scholar
Preiser J-C. The stress response of critical illness: metabolic and hormonal aspects. Switzerland: Springer Cham; 2016. [cited 2019 July 11]. Available from: http://ezproxy.lib.monash.edu.au/login?url=http://link.springer.com/10.1007/978-3-319-27687-8
Book Google Scholar
Bear DE, Wandrag L, Merriweather JL, Connolly B, Hart N, Grocott MPW, et al. The role of nutritional support in the physical and functional recovery of critically ill patients: a narrative review. Crit Care. 2017;21(1):226.
Cuthbertson DP. Post-shock metabolic response. Lancet. 1942;239(6189):433–7.
Article Google Scholar
Marik PE, Bellomo R. Stress hyperglycemia: an essential survival response! Crit Care. 2013;17(2):305.
Target Investigators ftACTG, Chapman M, Peake SL, Bellomo R, Davies A, Deane A, et al. Energy-dense versus routine enteral nutrition in the critically ill. N Engl J Med. 2018;379(19):1823–34.
Rice TW, Wheeler AP, Thompson BT, Steingrub J, Hite RD, Moss M, et al. Initial trophic vs full enteral feeding in patients with acute lung injury: the EDEN randomized trial. JAMA. 2012;307(8):795–803.
Article PubMed CAS Google Scholar
Casaer MP, Mesotten D, Hermans G, Wouters PJ, Schetz M, Meyfroidt G, et al. Early versus late parenteral nutrition in critically ill adults. N Engl J Med. 2011;365(6):506–17.
Article CAS PubMed Google Scholar
Allingstrup MJ, Kondrup J, Wiis J, Claudius C, Pedersen UG, Hein-Rasmussen R, et al. Early goal-directed nutrition versus standard of care in adult intensive care patients: the single-centre, randomised, outcome assessor-blinded EAT-ICU trial. Intensive Care Med. 2017;43(11):1637–47.
Arabi YM, Aldawood AS, Haddad SH, Al-Dorzi HM, Tamim HM, Jones G, et al. Permissive underfeeding or standard enteral feeding in critically ill adults. N Engl J Med. 2015;372(25):2398–408.
Reintam Blaser A, Starkopf J, Alhazzani W, Berger MM, Casaer MP, Deane AM, et al. Early enteral nutrition in critically ill patients: ESICM clinical practice guidelines. Intensive Care Med. 2017;43(3):380–98.
Critical Care Nutrition. The Canadian clinical practice guidelines (2015). Available from: http://www.criticalcarenutrition.com [Accessed 24 Jul 2019].
Google Scholar
Tatucu-Babet OA, Ridley EJ, Tierney AC. Prevalence of underprescription or overprescription of energy needs in critically ill mechanically ventilated adults as determined by indirect calorimetry: a systematic literature review. JPEN J Parenter Enteral Nutr. 2016;40(2):212–25.
Frankenfield DC, Coleman A, Alam S, Cooney RN. Analysis of estimation methods for resting metabolic rate in critically ill adults. JPEN J Parenter Enteral Nutr. 2009;33(1):27–36.
Walker RN, Heuberger RA. Predictive equations for energy needs for the critically ill. Respir Care. 2009;54(4):509–21.
PubMed Google Scholar
Reeves MM, Capra S. Predicting energy requirements in the clinical setting: are current methods evidence based? Nutr Rev. 2003;61(4):143–51.
Kagan I, Zusman O, Bendavid I, Theilla M, Cohen J, Singer P. Validation of carbon dioxide production (VCO2) as a tool to calculate resting energy expenditure (REE) in mechanically ventilated critically ill patients: a retrospective observational study. Crit Care. 2018;22(1):186.
Article CAS PubMed PubMed Central Google Scholar
Stapel SN, de Grooth HJ, Alimohamad H, Elbers PW, Girbes AR, Weijs PJ, et al. Ventilator-derived carbon dioxide production to assess energy expenditure in critically ill patients: proof of concept. Crit Care. 2015;19:370.
Flancbaum L, Choban PS, Sambucco S, Verducci J, Burge JC. Comparison of indirect calorimetry, the Fick method, and prediction equations in estimating the energy requirements of critically ill patients. Am J Clin Nutr. 1999;69(3):461–6.
Basile-Filho A, Martins MA, Marson F, Evora PRB. An easy way to estimate energy expenditure from hemodynamic data in septic patients. Acta Cirurgica Brasileira. 2008;23:112–7.
Gupta RD, Ramachandran R, Venkatesan P, Anoop S, Joseph M, Thomas N. Indirect calorimetry: from bench to bedside. Indian J Endocrinol Metab. 2017;21(4):594–9.
McClave SA, Spain DA, Skolnick JL, Lowen CC, Kieber MJ, Wickerham PS, et al. Achievement of steady state optimizes results when performing indirect calorimetry. JPEN J Parenter Enteral Nutr. 2003;27(1):16–20.
Singer P, Anbar R, Cohen J, Shapiro H, Shalita-Chesner M, Lev S, et al. The tight calorie control study (TICACOS): a prospective, randomized, controlled pilot study of nutritional support in critically ill patients. Intensive Care Med. 2011;37(4):601–9.
Gonzalez-Granda A, Schollenberger A, Haap M, Riessen R, Bischoff SC. Optimization of nutrition therapy with the use of calorimetry to determine and control energy needs in mechanically ventilated critically ill patients: the ONCA study, a randomized, prospective pilot study. JPEN J Parenter Enteral Nutr. 2019;43(4):481–9.
Oshima T, Berger MM, De Waele E, Guttormsen AB, Heidegger CP, Hiesmayr M, et al. Indirect calorimetry in nutritional therapy. A position paper by the ICALIC study group. Clin Nutr. 2017;36(3):651–62.
Wernerman J, Christopher KB, Annane D, Casaer MP, Coopersmith CM, Deane AM, et al. Metabolic support in the critically ill: a consensus of 19. Crit Care. 2019;23(1):318.
Charriere M, Ridley E, Hastings J, Bianchet O, Scheinkestel C, Berger MM. Propofol sedation substantially increases the caloric and lipid intake in critically ill patients. Nutrition. 2017;42:64–8.
Wolfe RR. The underappreciated role of muscle in health and disease. Am J Clin Nutr. 2006;84(3):475–82.
Puthucheary ZA, Rawal J, McPhail M, Connolly B, Ratnayake G, Chan P, et al. Acute skeletal muscle wasting in critical illness. JAMA. 2013;310(15):1591–600.
Weijs PJ, Looijaard WG, Beishuizen A, Girbes AR, Oudemans-van Straaten HM. Early high protein intake is associated with low mortality and energy overfeeding with high mortality in non-septic mechanically ventilated critically ill patients. Crit Care. 2014;18(6):701.
Bendavid I, Zusman O, Kagan I, Theilla M, Cohen J, Singer P. Early administration of protein in critically ill patients: a retrospective cohort study. Nutrients. 2019;11(1):106.
Allingstrup MJ, Esmailzadeh N, Wilkens Knudsen A, Espersen K, Hartvig Jensen T, Wiis J, et al. Provision of protein and energy in relation to measured requirements in intensive care patients. Clin Nutr. 2012;31(4):462–8.
Compher C, Chittams J, Sammarco T, Nicolo M, Heyland DK. Greater protein and energy intake may be associated with improved mortality in higher risk critically ill patients: a multicenter, multinational observational study. Crit Care Med. 2017;45(2):156–63.
Nicolo M, Heyland DK, Chittams J, Sammarco T, Compher C. Clinical outcomes related to protein delivery in a critically ill population: a multicenter, multinational observation study. JPEN J Parenter Enteral Nutr. 2016;40(1):45–51.
Doig GS, Simpson F, Bellomo R, Heighes PT, Sweetman EA, Chesher D, et al. Intravenous amino acid therapy for kidney function in critically ill patients: a randomized controlled trial. Intensive Care Med. 2015;41(7):1197–208.
Ferrie S, Allman-Farinelli M, Daley M, Smith K. Protein requirements in the critically ill: a randomized controlled trial using parenteral nutrition. JPEN J Parenter Enteral Nutr. 2016;40(6):795–805.
Fetterplace K, Deane AM, Tierney A, Beach LJ, Knight LD, Presneill J, et al. Targeted full energy and protein delivery in critically ill patients: a pilot randomized controlled trial (FEED trial). JPEN J Parenter Enteral Nutr. 2018;42(8):1252–62.
Casaer MP, Van den Berghe G. Comment on “protein requirements in the critically ill: a randomized controlled trial using parenteral nutrition”. JPEN J Parenter Enteral Nutr. 2016;40(6):763.
Casaer MP, Wilmer A, Hermans G, Wouters PJ, Mesotten D, Van den Berghe G. Role of disease and macronutrient dose in the randomized controlled EPaNIC trial: a post hoc analysis. Am J Respir Crit Care Med. 2013;187(3):247–55.
Koekkoek W, van Setten CHC, Olthof LE, Kars J, van Zanten ARH. Timing of PROTein INtake and clinical outcomes of adult critically ill patients on prolonged mechanical VENTilation: the PROTINVENT retrospective study. Clin Nutr. 2019;38(2):883–90.
Ridley EJ, Peake SL, Jarvis M, Deane AM, Lange K, Davies AR, et al. Nutrition therapy in Australia and New Zealand intensive care units: an international comparison study. JPEN J Parenter Enteral Nutr. 2018;42(8):1349–57.
Cahill NE, Dhaliwal R, Day AG, Jiang X, Heyland DK. Nutrition therapy in the critical care setting: what is “best achievable” practice? An international multicenter observational study. Crit Care Med. 2010;38(2):395–401.
Passier RH, Davies AR, Ridley E, McClure J, Murphy D, Scheinkestel CD. Periprocedural cessation of nutrition in the intensive care unit: opportunities for improvement. Intensive Care Med. 2013;39(7):1221–6.
Mehanna HM, Moledina J, Travis J. Refeeding syndrome: what it is, and how to prevent and treat it. BMJ. 2008;336(7659):1495–8.
Doig GS, Simpson F, Heighes PT, Bellomo R, Chesher D, Caterson ID, et al. Restricted versus continued standard caloric intake during the management of refeeding syndrome in critically ill adults: a randomised, parallel-group, multicentre, single-blind controlled trial. Lancet Respir Med. 2015;3(12):943–52.
Patel JJ, Rosenthal MD, Heyland DK. Intermittent versus continuous feeding in critically ill adults. Curr Opin Clin Nutr Metab Care. 2018;21(2):116–20.
Doig GS, Simpson F, Finfer S, Delaney A, Davies AR, Mitchell I, et al. Effect of evidence-based feeding guidelines on mortality of critically ill adults: a cluster randomized controlled trial. JAMA. 2008;300(23):2731–41.
Heyland DK, Lemieux M, Shu L, Quisenberry K, Day AG. What is “best achievable” practice in implementing the enhanced protein-energy provision via the enteral route feeding protocol in intensive care units in the United States? Results of a multicenter, quality improvement collaborative. JPEN J Parenter Enteral Nutr. 2016;42(2):308–17.
Barr J, Hecht M, Flavin KE, Khorana A, Gould MK. Outcomes in critically ill patients before and after the implementation of an evidence-based nutritional management protocol. Chest. 2004;125(4):1446–57.
Heyland DK, Murch L, Cahill N, McCall M, Muscedere J, Stelfox HT, et al. Enhanced protein-energy provision via the enteral route feeding protocol in critically ill patients: results of a cluster randomized trial. Crit Care Med. 2013;41(12):2743–53.
Martin CM, Doig GS, Heyland DK, Morrison T, Sibbald WJ, Southwestern Ontario Critical Care Research N. Multicentre, cluster-randomized clinical trial of algorithms for critical-care enteral and parenteral therapy (ACCEPT). CMAJ. 2004;170(2):197–204.
PubMed PubMed Central Google Scholar
Montejo JC, Minambres E, Bordeje L, Mesejo A, Acosta J, Heras A, et al. Gastric residual volume during enteral nutrition in ICU patients: the REGANE study. Intensive Care Med. 2010;36(8):1386–93.
Reignier J, Mercier E, Le Gouge A, Boulain T, Desachy A, Bellec F, et al. Effect of not monitoring residual gastric volume on risk of ventilator-associated pneumonia in adults receiving mechanical ventilation and early enteral feeding: a randomized controlled trial. JAMA. 2013;309(3):249–56.
Poulard F, Dimet J, Martin-Lefevre L, Bontemps F, Fiancette M, Clementi E, et al. Impact of not measuring residual gastric volume in mechanically ventilated patients receiving early enteral feeding: a prospective before-after study. JPEN J Parenter Enteral Nutr. 2010;34(2):125–30.
Davies AR. Enteral nutrition in ICU: small bowel or stomach? And how much? Crit Care Resusc. 2012;14(2):99–100.
Nguyen NQ, Chapman MJ, Fraser RJ, Bryant LK, Holloway RH. Erythromycin is more effective than metoclopramide in the treatment of feed intolerance in critical illness. Crit Care Med. 2007;35(2):483–9.
Harvey SE, Parrott F, Harrison DA, Bear DE, Segaran E, Beale R, et al. Trial of the route of early nutritional support in critically ill adults. N Engl J Med. 2014;371(18):1673–84.
Reignier J, Boisrame-Helms J, Brisard L, Lascarrou JB, Ait Hssain A, Anguel N, et al. Enteral versus parenteral early nutrition in ventilated adults with shock: a randomised, controlled, multicentre, open-label, parallel-group study (NUTRIREA-2). Lancet. 2018;391(10116):133–43.
Cederholm T, Jensen GL, Correia M, Gonzalez MC, Fukushima R, Higashiguchi T, et al. GLIM criteria for the diagnosis of malnutrition - a consensus report from the global clinical nutrition community. J Cachexia Sarcopenia Muscle. 2019;10(1):207–17.
Sheean PM, Peterson SJ, Gomez Perez S, Troy KL, Patel A, Sclamberg JS, et al. The prevalence of sarcopenia in patients with respiratory failure classified as normally nourished using computed tomography and subjective global assessment. JPEN J Parenter Enteral Nutr. 2014;38(7):873–9.
Campbell IT, Watt T, Withers D, England R, Sukumar S, Keegan MA, et al. Muscle thickness, measured with ultrasound, may be an indicator of lean tissue wasting in multiple organ failure in the presence of edema. Am J Clin Nutr. 1995;62(3):533–9.
Earthman CP. Body composition tools for assessment of adult malnutrition at the bedside: a tutorial on research considerations and clinical applications. JPEN J Parenter Enteral Nutr. 2015;39(7):787–822.
Mundi MS, Patel JJ, Martindale R. Body composition technology: implications for the ICU. Nutr Clin Pract. 2019;34(1):48–58.
Paris M, Mourtzakis M. Assessment of skeletal muscle mass in critically ill patients: considerations for the utility of computed tomography imaging and ultrasonography. Curr Opin Clin Nutr Metab Care. 2016;19(2):125–30.
Wischmeyer PE, Hasselmann M, Kummerlen C, Kozar R, Kutsogiannis DJ, Karvellas CJ, et al. A randomized trial of supplemental parenteral nutrition in underweight and overweight critically ill patients: the TOP-UP pilot trial. Crit Care. 2017;21(1):142.
Dickerson RN. Metabolic support challenges with obesity during critical illness. Nutrition. 2019;57:24–31.
Frankenfield DC, Ashcraft CM, Galvan DA. Prediction of resting metabolic rate in critically ill patients at the extremes of body mass index. JPEN J Parenter Enteral Nutr. 2013;37(3):361–7.
Peterson SJ, Tsai AA, Scala CM, Sowa DC, Sheean PM, Braunschweig CL. Adequacy of oral intake in critically ill patients 1 week after extubation. J Am Diet Assoc. 2010;110(3):427–33.
Ridley EJ, Parke RL, Davies AR, Bailey M, Hodgson C, Deane AM, et al. What happens to nutrition intake in the post-intensive care unit hospitalization period? An observational cohort study in critically ill adults. JPEN J Parenter Enteral Nutr. 2019;43(1):88–95.
Chapple LS, Deane AM, Heyland DK, Lange K, Kranz AJ, Williams LT, et al. Energy and protein deficits throughout hospitalization in patients admitted with a traumatic brain injury. Clin Nutr. 2016;35(6):1315–22.
Merriweather J, Smith P, Walsh T. Nutritional rehabilitation after ICU - does it happen: a qualitative interview and observational study. J Clin Nurs. 2014;23(5–6):654–62.
Stratton RJ, Stubbs RJ, Elia M. Short-term continuous enteral tube feeding schedules did not suppress appetite and food intake in healthy men in a placebo-controlled trial. J Nutr. 2003;133(8):2570–6.
Download references
Author information
Authors and affiliations.
Australian and New Zealand Intensive Care Research Centre, School of Public Health and Preventive Medicine, Monash University, Level 3, 555 St Kilda Rd, Melbourne, VIC, 3004, Australia
Kate J. Lambell, Oana A. Tatucu-Babet, Dashiell Gantner & Emma J. Ridley
Nutrition Department, Alfred Health, Melbourne, Australia
Kate J. Lambell & Emma J. Ridley
Department of Dietetics, Nutrition and Sport, La Trobe University, Melbourne, Australia
Kate J. Lambell
Discipline of Acute Care Medicine, University of Adelaide, Adelaide, Australia
Lee-anne Chapple
Intensive Care Research, Royal Adelaide Hospital, Adelaide, Australia
Intensive Care Unit, Alfred Health, Melbourne, Australia
Dashiell Gantner
You can also search for this author in PubMed Google Scholar
Contributions
EJR conceived of the idea. KJL and EJR drafted the manuscript. All authors reviewed and commented on the manuscript and approved the final draft.
Corresponding author
Correspondence to Kate J. Lambell .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
EJR has received an honorarium and has unrestricted, investigator-initiated grant funding from Baxter Healthcare Corporation for trial Clinicaltrials.gov NCT03292237. The other authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Reprints and permissions
About this article
Cite this article.
Lambell, K.J., Tatucu-Babet, O.A., Chapple, La. et al. Nutrition therapy in critical illness: a review of the literature for clinicians. Crit Care 24 , 35 (2020). https://doi.org/10.1186/s13054-020-2739-4
Download citation
Received : 14 July 2019
Accepted : 14 January 2020
Published : 04 February 2020
DOI : https://doi.org/10.1186/s13054-020-2739-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Critical illness
- Intensive care
- Nutrition therapy
Critical Care
ISSN: 1364-8535
- Submission enquiries: [email protected]
- Open access
- Published: 13 October 2022
Critical care nurses’ knowledge and attitudes and their perspectives toward promoting advance directives and end-of-life care
- Mu-Hsing Ho 1 na1 ,
- Hsiao-Chi Liu 2 na1 ,
- Jee Young Joo 3 ,
- Jung Jae Lee 1 &
- Megan F. Liu 4
BMC Nursing volume 21 , Article number: 278 ( 2022 ) Cite this article
4517 Accesses
4 Citations
3 Altmetric
Metrics details
End-of-life care can be a difficult and challenging process for critical care nurses in intensive care units (ICUs) due to the care plan shifts from providing life-sustaining measures to end-of-life care. The aims of this study were to assess critical care nurses' perceived knowledge and attitudes toward end-of-life care, as well as their perspectives on promoting advance directives and the associated factors.
A cross-sectional study was undertaken in an acute major metropolitan medical center in northern Taiwan between February and March 2020, and 250 critical care nurses were invited to participate in the study. Data on demographics, self-perceived knowledge of end-of-life care, attitudes toward end-of-life care, and perspectives of promoting advance directives were collected. A multiple linear regression model with stepwise selection was used to identify factors associated with their perspectives of promoting advance directives.
The law related to end-of-life care was rated as the least familiar part of the self-perceived end-of-life care knowledge, while ‘I have sufficient knowledge to care for patients who have accepted end-of-life care’ was the lowest level of agreement in attitude scores among critical care nurses. Increased levels of perceived knowledge ( β = 0.134; p = 0.045) and attitudes ( β = 0.423; p < 0.001) toward end-of-life care were associated with the perspectives of promoting advance directives. Nurses who worked in cardiac ( β = -0.234; p < 0.001) and respiratory ICUs ( β = -0.135; p = 0.024) had less motivation to promote advance directives ( F = 16.943; p < 0.001).
Given their important contributions to ICU care services, appropriate and meaningful support is required to optimize critical care nurses' involvement in end-of-life care. This study demonstrated a significant impact on perspectives of promoting advance directives of critical care nurse participants. Findings from this study can inform the design of effective nurse support programs to enhance the promotion of advance directives in intensive care settings.
Peer Review reports
Introduction
In Taiwan, the Hospice Palliative Care Act was enacted into law in 2000, providing patients suffering from a terminal illness who were certified by two physicians the authority to sign advance directives and do not resuscitate (DNR) orders [ 1 ]. A directive can be made in one of two ways “ (1) it can be signed by a competent terminally ill patient or (2) it can be signed by a surrogate decision-maker, most commonly a close family member when a patient is unable to make such a decision for themselves due to dementia, delirium, or other types of cognitive impairment ” [ 1 ]. Advance directives are regarded as “a declaration, typically in writing, in which a person stated when mentally competent the type of health care he would desire to have at a future time when he is no longer capable [ 2 ].” Advance directives can assist healthcare personnel in respecting and following the preferred treatment of patients [ 3 ]. Before deciding on a DNR directive, the physician must consult with the patients and their family members. Initially, this used to exclusively apply to cancer patients in terminal condition, allowing them to receive hospice palliative treatment and make a DNR decision. Later modifications in 2009 increased insurance coverage of hospice and palliative care treatments to include non-malignant terminal conditions such as heart failure, chronic renal failure, liver cirrhosis and severe dementia. One of the most often used advance directives in end-of-life care is that when a person refuses to accept cardiopulmonary resuscitation (CPR) in the case of cardiac or respiratory arrest [ 1 ].
End-of-life care can be a difficult and challenging process in an intensive care unit (ICU) because many terminal illnesses involve withholding or withdrawing life-sustaining therapies, and in such situations, the role of critical care nurses shifts from providing life-sustaining measures to end-of-life care [ 4 , 5 ]. Critical care nurses face these terminal illnesses and provide direct care to critically ill patients in the ICU, and they are in the best position to promote advance directives and provide end-of-life care. However, a study in Taiwan discovered that critical care nurses have significantly lower knowledge of and attitudes toward advance directives than ICU physicians. Also, a disagreement about end-of-life decision-making between ICU physician and nurses was found regarding who initiated conversation about end-of-life care discussion [ 6 ]. Insufficient knowledge and low awareness of advance directives are likely to be the reasons that result in challenges for critical care nurses to provide end-of-life care in ICUs [ 7 ]. Given the importance of the involvement of critical care nurses in end-of-life care such as decision-making and promoting advance directives, more strategies for practice development to prepare and support nurses in providing end-of-life care in intensive care settings are needed [ 6 , 8 ]. Understanding the perceived knowledge of and attitudes toward end-of-life care can inform further development of strategies to support nurses in providing end-of-life care and promoting advance directives. Thus, this study aimed to assess (1) critical care nurses' self-perceived end-of-life care knowledge and attitudes toward terminal patients, as well as their perspectives of promoting advance directives; and (2) the factors associated with critical care nurses’ perspectives of promoting advance directives.
Research design and setting
A cross-sectional survey was conducted among critical care nurses between February and March 2020. The research site was a major acute-care metropolitan medical center with approximately 120 ICU beds located in northern Taiwan.
Participants
Critical care nurses who met the selection criteria were invited to participate in this study. The inclusion criteria were a status as a registered nurse working in an ICU and agreed to participate in this study. Nurses who were receiving a temporary ICU training in the ICU were excluded. Critical care nurses who worked in a pediatric or neonatal ICU or the emergency room were also excluded, given the nature of nursing practice and workload, admitted patients, and the equipment/medications used in between adult ICU, pediatric or neonatal ICU, and the emergency room were different in Taiwan. G* power vers. 3.0.10 software was used to estimate the sample size [ 9 ]. The statistical test and model settings for the sample size estimation were as follow: F test as the test family; linear multiple regression: fixed model; R 2 deviation from zero as the statistical test with a prior power analysis (given α , power, and effect size); and parameter settings ( α = 0.05, 1– β = 0.95. f 2 effect size = 0.08, and number of predictors = 3). Parameter settings were established according to a previous study on perspectives of promoting advance directives as an outcome [ 10 ]. An estimated sample size of 219 participants was considered sufficient.
Ethical approval
The study protocol was reviewed and approved by the Hospital Institutional Review Board of the research site. Ethical approval was granted with approval no.: 108152-F. Written consent was obtained prior to the commencement of data collection. Subjects who were invited to participate could choose to discontinue their participation at any time.
Data collection
A survey was developed based on a literature review and expert input. The tool was comprised of four parts that had to be completed, namely demographics, self-perceived knowledge of end-of-life care, attitudes toward end-of-life care, and perspectives of promoting advance directives. The survey was distributed by a research assistant to potential participants in person who agreed to join the study.
Demographic characteristics
Information of critical care nurses we collected included gender, age, educational level, years working as an ICU nurse, type of their workplace ICU, and continuing education in end-of-life care per year (hours).
Self-perceived end-of-life care knowledge of terminal patients
In total, 23 questions and a five-point Likert scale were used. Positively keyed questions were assigned 5 as “very familiar”, 4 as “familiar”, 3 as “neither familiar nor unfamiliar”, 2 as “unfamiliar”, or 1 as “very unfamiliar”. The higher the score, the higher the degree of self-perceived end-of-life care knowledge of critical care nurses, and the higher the tendency that critical care nurses would provide end-of-life care. This scale was developed to examine self-perceived end-of-life care knowledge towards terminal patients among critical care nurses. The content validity and the internal consistency (Cronbach’s alpha = 0.94) were established [ 11 ].
End-of-life care attitudes towards terminal patients
In total, 17 questions and a five-point Likert scale were adopted, with 5 as “extremely agree”, 4 as “agree”, 3 as “neither agree nor disagree”, 2 as “disagree”, or 1 as “extremely disagree”. The higher the score, the more positive care attitudes towards terminal patients from critical care nurses, and the higher the tendency that the critical care nurses would provide end-of-life care. This scale aimed to assess end-of-life care attitudes among critical care nurses towards terminal patients. The content validity and the internal consistency (Cronbach’s alpha = 0.85) were confirmed [ 11 ].
Perspectives of promoting advance directives
Perspectives of promoting advance directives scale were adopted from Hsieh et al. (2010) who developed a test for Taiwanese nursing staff. In total, 24 questions with a five-point Likert scale were used, with 1 as “extremely disagree”, 2 as “disagree”, 3 as “neither agree nor disagree”, 4 as “agree”, and 5 as “extremely agree”. Negatively keyed questions were inversely scored when summary scores were computed. The total score of the questionnaire ranged 24 ~ 120 points. The higher the score, the more-positive attitudes nursing staff had towards promoting advance directives. A lower score indicated that there would be challenges or negative attitudes towards discussing advance directives between nursing staff and patients. The content validity and internal consistency (Cronbach’s alpha = 0.80) were confirmed [ 10 ].
Data analysis
All data were entered into SPSS© vers. 25.0 for analysis (IBM SPSS Statistics for Windows, vers. 25.0. IBM, Armonk, NY, USA). Descriptive analyses, including the mean, standard deviation (SD), and frequency distributions were used to summarize data relating to demographics, self-perceived knowledge of end-of-life care, attitudes toward end-of-life care, and perspectives of promoting advance directives. Multiple imputation was employed to handle missing values. A multiple linear regression model with stepwise selection was used to identify factors associated with perspectives of promoting advance directives. All variables including demographics were entered in the regression model, and significant variables were selected and included in the final model. The variance inflation factor (VIF) was also examined to discover potential multicollinearity issues between variables. Statistical significance for all tests was set to p < 0.05.
In total, 250 responses were received. Of these, 88.4% ( n = 220) of subjects were female, and 76.0% ( n = 341) were aged 20 ~ 30 years (Table 1 ). Ninety-two percent ( n = 231) of participants had an undergraduate education level, and more than half ( n = 148; 59.2%) had been an ICU nurse for 1 ~ 5 years. Most ( n = 76; 30.4%) participants worked in a medical ICU, followed by surgical ICUs ( n = 57; 22.8%) and cardiac ICUs ( n = 54; 21.6%). Almost all participants ( n = 244; 98.0%) received ≤ 5 h of continuing education in end-of-life care per year.
Self-perceived end-of-life care knowledge of and attitudes towards terminal patients
Critical care nurses reported that ‘I am familiar with the importance of collaboration among end-of-life care team members’ (mean = 4.17; SD = 0.54), ‘I am familiar with the purpose of end-of-life care’ (mean = 4.12; SD = 0.51), and ‘I am familiar with the role and function of an end-of-life care team member’ (mean = 4.12; SD = 0.53) were the most familiar self-perceived aspects of end-of-life care knowledge towards terminal patients, demonstrating higher degree of self-perceived end-of-life care knowledge than other items. The least familiar part of the self-perceived end-of-life care knowledge with the lowest score was ‘I am familiar with the laws relating to end-of-life care’ (mean = 3.42; SD = 0.92), showing that the nurses had lower degree of self-perceived end-of-life care knowledge regarding the laws relating to end-of life care (Table 2 ). As for the end-of-life care attitudes of critical care nurses towards terminal patients, ‘I believe that providing end-of-life care information to patients and their family is beneficial’ (mean = 4.40; SD = 0.57), ‘I will agree to disconnect the ventilator under legal conditions for a terminal patient’ (mean = 4.38; SD = 0.58), and ‘I believe that high-quality end-of-life care can reduce medical disputes’ (mean = 4.34; SD = 0.62) were the most familiar items, while ‘I have sufficient knowledge to care for patients who have accepted end-of-life care’ was the item with the lowest level of agreement in attitude scores (Table 3 ).
Critical care nurses’ perspectives of promoting advance directives
Figure 1 shows that statements with higher agreement of perspectives of promoting advance directives were ‘Discussing advance directives with patients improves the quality of their end-of-life’ (mean = 4.32; SD = 0.53), ‘Discussing advance directives with patients respects patients’ values’ (mean = 4.31; SD = 0.52), and ‘Discussing advance directives with patients allows for a better understanding of patients’ expectations of their treatment plans (mean = 4.26; SD = 0.54)’, and the statement with the lowest agreement was ‘I am worried that discussing advance directives with patients may cause annoyance or discomfort’ (mean = 2.56; SD = 1.01).
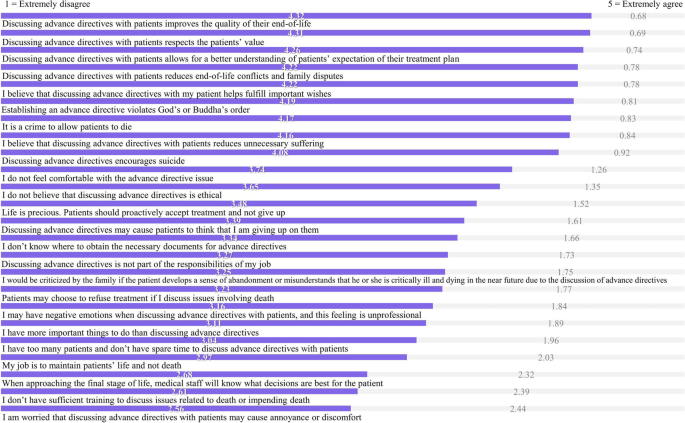
Statements of perspectives of promoting advance directives among critical care nurses. Note . Ordered by mean values, the higher the score, the more-positive attitude critical care nurses had towards promoting advance directives. A lower score (1 or 2) indicates that there would be challenges or negative attitudes towards discussing advance directives between critical care nurses and patients
Factors associated with perspectives of promoting advance directives
The stepwise multiple regression analysis revealed that critical care nurses self-perceived end-of-life care knowledge ( β = 0.134; p = 0.045) and attitudes ( β = 0.423; p < 0.001) towards terminal patients were factors associated with perspectives of promoting advance directives. Nurses with higher self-perceived end-of-life care knowledge and more-positive end-of-life care attitudes had higher motivation to promote advance directives. Also, compared to nurses who worked in medical ICUs, those who worked in cardiac ( β = -0.234; p < 0.001) and respiratory ( β = -0.135; p = 0.024) ICUs had lower motivation to promote advance directives ( F = 16.943; p < 0.001). All VIFs were < 10 which showed that no multicollinearity issue existed in the regression model (Table 4 ).
This study assessed critical care nurses' self-perceived knowledge of and attitudes toward end-of-life care, as well as their perspectives of promoting advance directives. Critical care nurses demonstrated high perceived knowledge of end-of-life care on items about the purpose of end-of-life care, the role and function of end-of-life care team members, and the importance of collaboration among end-of-life care team members. This shows that critical care nurses are equipped with fundamental knowledge of end-of-life care and understand that teamwork with other healthcare professionals in the end-of-life care team is important. Other research suggested that interprofessional interventions and interdisciplinary teamwork have the potential to support ICU staff to provide better end-of-life care [ 12 , 13 , 14 ]. The least familiar item among critical care nurses was about laws relating to end-of-life care, which referred to the Hospice Palliative Care Act in Taiwan [ 1 ]. Future studies, interventions, and continuing education can consider putting more efforts in enhancing critical care nurses’ understanding of end-of-life care law.
In line with previous studies that attitudes toward end-of-life in critical care nurses are positive [ 6 , 7 , 11 ], both self-perceived knowledge of and attitudes toward end-of-life care were found to be significant factors of perspectives of promoting advance directives, and future studies can be informed by our findings to develop tailor-made interventions for getting critical care nurses involved and engaged in end-of-life care and decision-making processes [ 15 ]. The role of critical care nurses being actively involved in end-of-life care and discussions has been highlighted, and they are essential partners in the end-of-life decision-making process within the end-of-life care team [ 16 ]. Increasing nurses’ knowledge and confidence to provide input in end-of-life discussions needs more research attention [ 17 , 18 ].
Our findings also revealed that critical care nurses realized that discussing advance directives with patients improved their quality of end-of-life, respected their values, and allowed for a better understanding of patients’ expectations of their treatment plans. In addition, critical care nurses were not worried that discussing advance directives with patients might cause annoyance or discomfort. This is dissimilar to a previous study that assessed perspectives of promoting advance directives among nurses working in a hemodialysis room, in which more than 65% of those nurses were worried that discussing advance directives with patients might cause annoyance or discomfort [ 10 ]. A possible reason might be that as critical care nurses are frontline care providers who care for critically ill patients, they understand that advance directives would be one of the options to provide better end-of-life care and they have to face many patients with terminal illnesses and dying patients [ 19 , 20 ]. Also, we identified that compared to nurses who work in medical ICUs, those who work in cardiac and respiratory ICUs have lower motivation to promote advance directives. This is likely because mortality rates in and the severity of diseases are higher in medical ICUs in Taiwan. We suggest adding advance directives-relevant information on laws into the content of continuing education for end-of-life care, particularly for cardiac and respiratory ICU nurses. Educational strategies such as simulated education with role-playing scenarios to introduce advance directives to patients would likely be effective in improving nurses’ end-of-life care [ 21 , 22 ]. Furthermore, the dignity of patients with palliative needs were also needed to be considered when developing the aforementioned educational strategies for nurses to promote palliative care. As a comprehensive integrated review has summarized a model of dignity that identifying several themes such as family care and support, social justice, reliable health care, which are highly relevant to be embedded in providing nursing care [ 23 ].
Study limitations
The main limitation of this study to be acknowledged is that the survey was only distributed to participants in a major acute-care metropolitan medical center located in northern Taiwan. The nonprobability sampling method used in this study to choose the hospital may influence the external validity of the study, and therefore, the results may not be generalizable to other areas in Taiwan due to representative bias. Future research should enroll more participants that particularly reflect different areas to conduct a multicenter study to compare the results. Nevertheless, this study discovered critical care nurses' perceived knowledge and attitudes toward end-of-life care, as well as their perspectives on promoting advance directives and the associated factors using validated instruments. Findings from this study can inform the design of effective nurse support programs to enhance the promotion of advance directives in intensive care settings.
Conclusions
Self-perceived knowledge and attitudes toward end-of-life care, and perspectives of promoting advance directives of critical care nurses were assessed. We found that increased levels of perceived knowledge and attitudes toward end-of-life care were associated with the perspectives of promoting advance directives. Nurses who worked in cardiac and respiratory ICUs had less motivation to promote advance directives. This study demonstrated a significant impact of perspectives of promoting advance directives of critical care nurse participants. Given their important contribution to ICU care services, appropriate and meaningful support is required to optimize critical care nurses' involvement in end-of-life care.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Hospice palliative care act. Available from: https://law.moj.gov.tw/ENG/LawClass/LawAll.aspx?pcode=L0020066 . Accessed 30 Sep 2022.
Introduction of the concept of advance directives in Hong Kong. Available from: https://www.gov.hk/en/residents/government/publication/consultation/docs/2010/AdvanceDirectives.pdf . Accessed 30 Sep 2022.
Ford DW, Koch KA, Ray DE, Selecky PA. Palliative and end-of-life care in lung cancer: diagnosis and management of lung cancer, 3rd ed: American college of chest physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 Suppl):e498S-e512S.
Article Google Scholar
Ozga D, Woźniak K, Gurowiec PJ. Difficulties perceived by ICU nurses providing end-of-life care: a qualitative study. Glob Adv Health Med. 2020;9:2164956120916176.
Kisorio LC, Langley GC. Intensive care nurses’ experiences of end-of-life care. Intensive Crit Care Nurs. 2016;33:30–8.
Ke YX, Hu SH, Takemura N, Lin CC. Perceived quality of palliative care in intensive care units among doctors and nurses in Taiwan. Int J Qual Health Care. 2019;31(10):741–7.
PubMed Google Scholar
Velasco-Sanz TR, Rayón-Valpuesta E. Advance directives in intensive care: health professional competences. Med Intensiva. 2016;40(3):154–62.
Article CAS Google Scholar
Riegel M, Randall S, Ranse K, Buckley T. Healthcare professionals’ values about and experience with facilitating end-of-life care in the adult intensive care unit. Intensive Crit Care Nurs. 2021;65:103057.
Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175–91.
Hsieh LY, Lin SY. A pilot study on the perspectives of hemodialysis room nurses on promoting advance directives. J Nurs. 2010;57(4):59–67.
Google Scholar
Su LH, Huang ML, Lin YL, Wu CK, Lin FR, Lin HM, Huang SJ. Knowledge and attitudes of clinical nurses regarding end-of-life care: survey in a regional hospital in central Taiwan. Taiwan J Hosp Palliat Care. 2008;13(4):431–46.
Blythe JA, Kentish-Barnes N, Debue AS, Dohan D, Azoulay E, Covinsky K, Matthews T, Curtis JR, Dzeng E. An interprofessional process for the limitation of life-sustaining treatments at the end of life in France. J Pain Symptom Manage. 2022;63(1):160–70.
Schwarzkopf D, Pausch C, Kortgen A, Guenther A, Reinhart K, Hartog CS. Quality improvement of end-of-life decision-making and communication in the ICU : effect on clinicians’ burnout and relatives’ distress. Med Klin Intensivmed Notfmed. 2020;115(7):600–8.
Graham R, Lepage C, Boitor M, Petizian S, Fillion L, Gélinas C. Acceptability and feasibility of an interprofessional end-of-life/palliative care educational intervention in the intensive care unit: a mixed-methods study. Intensive Crit Care Nurs. 2018;48:75–84.
Badır A, Topçu İ, Türkmen E, Göktepe N, Miral M, Ersoy N, Akın E. Turkish critical care nurses’ views on end-of-life decision making and practices. Nurs Crit Care. 2016;21(6):334–42.
Benbenishty J, Ganz FD, Anstey MH, Barbosa-Camacho FJ, Bocci MG, Çizmeci EA, Dybwik K, Ingels C, Lautrette A, Miranda-Ackerman RC, et al. Changes in intensive care unit nurse involvement in end of life decision making between 1999 and 2016: Descriptive comparative study. Intensive Crit Care Nurs. 2022;68:103138.
Khater WA, Akhu-Zaheya LM, Al-Nabulsi HW, Shattnawi KK, Shamieh O, Joseph R. Barriers to implementing palliative care in intensive care units: perceptions of physicians and nurses in Jordan. Int J Palliat Nurs. 2021;27(2):98–106.
Henao-Castaño ÁM, Rivera-Romero N, Garzón HPO. Health care at the end of life: experience of nurses at the adult intensive care unit. Crit Care Nurs Q. 2021;44(4):387–92.
Bayuo J, Anago EK, Agyei FB, Salifu Y, Kyei Baffour P, Atta Poku C. “Resuscitate and push”: end-of-life care experiences of healthcare staff in the emergency department - a hermeneutic phenomenological study. J Palliat Care. 2022;37(4):494–502.
Jang SK, Park WH, Kim HI, Chang SO. Exploring nurses’ end-of-life care for dying patients in the ICU using focus group interviews. Intensive Crit Care Nurs. 2019;52:3–8.
Novaes LMS, Paiva E, O’Mahony A, Garcia ACM. Roleplay as an educational strategy in palliative care: a systematic integrative review. Am J Hosp Palliat Care. 2022;39(5):570–80.
Tamaki T, Inumaru A, Yokoi Y, Fujii M, Tomita M, Inoue Y, Kido M, Ohno Y, Tsujikawa M. The effectiveness of end-of-life care simulation in undergraduate nursing education: a randomized controlled trial. Nurse Educ Today. 2019;76:1–7.
Dakessian Sailian S, Salifu Y, Saad R, Preston N. Dignity of patients with palliative needs in the Middle East: an integrative review. BMC Palliat Care. 2021;20(1):112.
Download references
Acknowledgements
We would like to thank the critical care nurses who so generously participated in the survey.
Author information
Mu-Hsing Ho and Hsiao-Chi Liu contributed equally to this work.
Authors and Affiliations
School of Nursing, LKS Faculty of Medicine, The University of Hong Kong, 5/F, Academic Building 3 Sassoon Road, Pokfulam, Hong Kong, Hong Kong
Mu-Hsing Ho & Jung Jae Lee
Department of Nursing, Far Eastern Memorial Hospital, 21 Nanya S. Rd., Sec. 2, Banciao Dist., New Taipei City, 220, Taiwan
Hsiao-Chi Liu
College of Nursing, Gachon University, 191 Hambakmoero, Yeonsu-gu, Incheon, Korea
Jee Young Joo
School of Gerontology and Long-Term Care, College of Nursing, Taipei Medical University, 250 Wu-Hsing Street, Taipei, 11031, Taiwan
Megan F. Liu
You can also search for this author in PubMed Google Scholar
Contributions
MHH: design of the work, the acquisition, analysis, interpretation of data, have drafted the work; HCL: analysis, interpretation of data; JYJ: design of the work, interpretation of data; JJL: analysis, interpretation of data, have drafted the work; MFL: design of the work, analysis, interpretation of data, have drafted the work. The author(s) read and approved the final manuscript.
Corresponding author
Correspondence to Megan F. Liu .
Ethics declarations
Ethics approval and consent to participate.
The study protocol was reviewed and approved by the Far Eastern Memorial Hospital Research Ethics Review Committee in Taiwan for ethical considerations (approval number: 108152-F). All methods were carried out in accordance with the Declaration of Helsinki. Informed consent was obtained from all participants. Legally Authorized Representatives of illiterate participants provided informed consent for the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Ho, MH., Liu, HC., Joo, J.Y. et al. Critical care nurses’ knowledge and attitudes and their perspectives toward promoting advance directives and end-of-life care. BMC Nurs 21 , 278 (2022). https://doi.org/10.1186/s12912-022-01066-y
Download citation
Received : 07 July 2022
Accepted : 10 October 2022
Published : 13 October 2022
DOI : https://doi.org/10.1186/s12912-022-01066-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Advance directive
- Critical care nurse
- End-of-life
BMC Nursing
ISSN: 1472-6955
- General enquiries: [email protected]
Burnout in critical care nurses: a literature review
Affiliation.
- 1 Grey Nuns Community Hopsital, Edmonton, AB. [email protected]
- PMID: 23342935
Burnout and its development in critical care nurses is a concept that has received extensive study, yet remains a problem in Canada and around the world. Critical care nurses are particularly vulnerable to developing burnout due to the chronic occupational stressors they are exposed to, including high patient acuity, high levels of responsibility, working with advanced technology, caring for families in crisis, and involved in morally distressing situations, particularly prolonging life unnecessarily. The purpose of this article is to explore how the chronic stressors that critical care nurses are exposed to contribute to the development of burnout, and strategies for burnout prevention. A review of the literature between the years 2007 and 2012 was conducted and included the search terms burnout, moral distress, compassion fatigue, intensive care, critical care, and nursing. The search was limited to the adult population, English language, and Western cultures. The results revealed that nurse managers play a crucial role in preventing burnout by creating a supportive work environment for critical care nurses. Strategies for nurse managers to accomplish this include being accessible to critical care nurses, fostering collegial relationships among the different disciplines, and making a counsellor or grief team available to facilitate debriefing after stressful situations, such as a death. In addition, critical care nurses can help prevent burnout by being a support system for each other and implementing self-care strategies.
Publication types
- Burnout, Professional / prevention & control*
- Burnout, Professional / psychology*
- Critical Care*
- Nurses / psychology*
- Risk Factors
- Social Support
- Stress, Psychological / prevention & control
- Stress, Psychological / psychology

- Center on Health Equity and Access
- Health Care Cost
- Health Care Delivery
- Value-Based Care
Expert Analysis Reveals Critical Insights for Optimizing Care in LA-NSCLC
Authors conducted a systematic literature review to gather evidence on the appropriateness of recommended treatments for locally advanced non–small cell lung cancer (LA-NSCLC) from the American Radium Society Appropriate Use Criteria Thoracic Committee.
An expert analysis has concluded that evidence-based guidance from the American Radium Society Appropriate Use Criteria (ARS AUC) Thoracic Committee on several treatment modalities for locally advanced non–small cell lung cancer (LA-NSCLC)—which accounts for 20% to 30% of lung cancer cases at diagnosis—does have utility for optimizing multidisciplinary care in this challenging patient population.
Findings were published online today in JAMA Oncology , with the study investigators noting the need for their review stems from the most recent appropriate use criteria for these patients came out in 2014 and the publication since then of studies examining radiation therapy technique and dosing, and systemic therapy selection. Their systematic literature review involved relevant articles from PubMed—69 references comprising 30 well-designed studies, 9 moderately well-designed studies, 2 studies with design limitations, and 28 nonprimary data references—that were published between January 1966 and December 2022 and focused on combined chemoradiation therapy, dose escalation, consolidation systemic therapy, and palliative radiotherapy.
“Information regarding the treatment of recurrent disease either locally or with oligometastatic disease after primary treatment for LA-NSCLC is increasingly available and should be integrated into modern clinical decision-making,” they wrote. “Additionally, the use of molecular markers such as EGFR, ALK, and PD-L1 has significantly informed systemic therapy options for many patients with NSCLC, and their integration into LA-NSCLC treatment pathways needs to be clarified.”
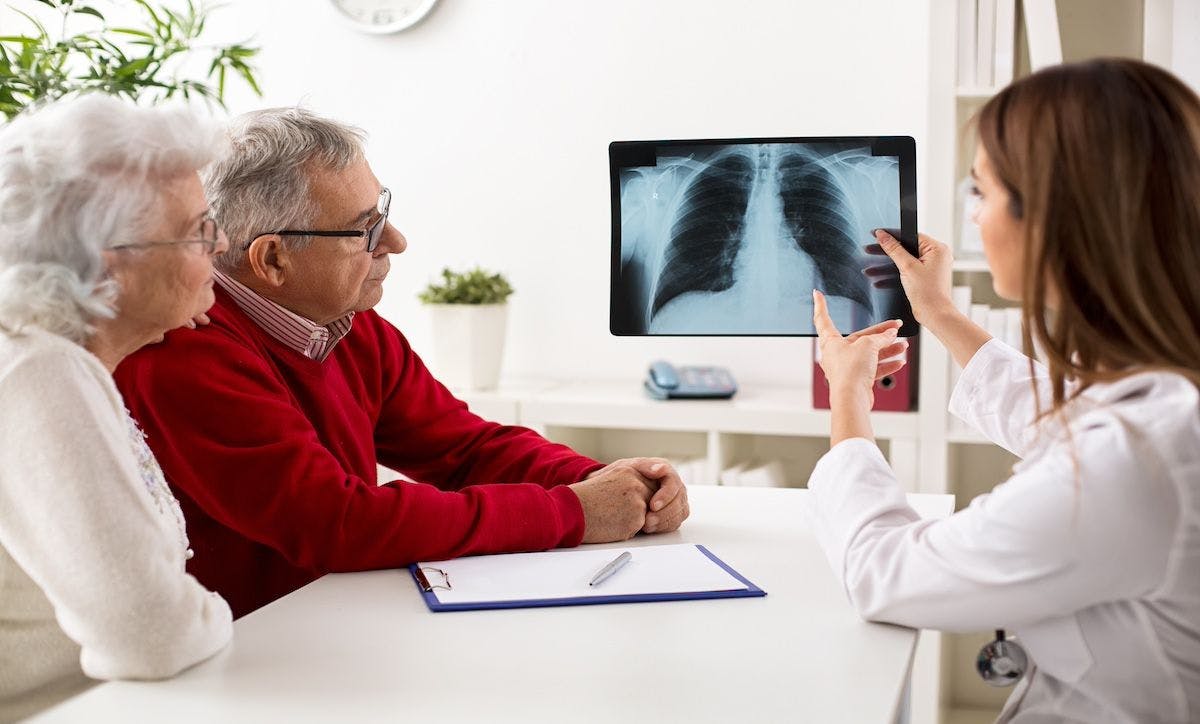
Study authors also highlight the importance of multidisciplinary input and shared decision-making with patients who have LA-NSCLC during treatment discussions Image credit: | Image Credit: didesign - stock.adobe.com
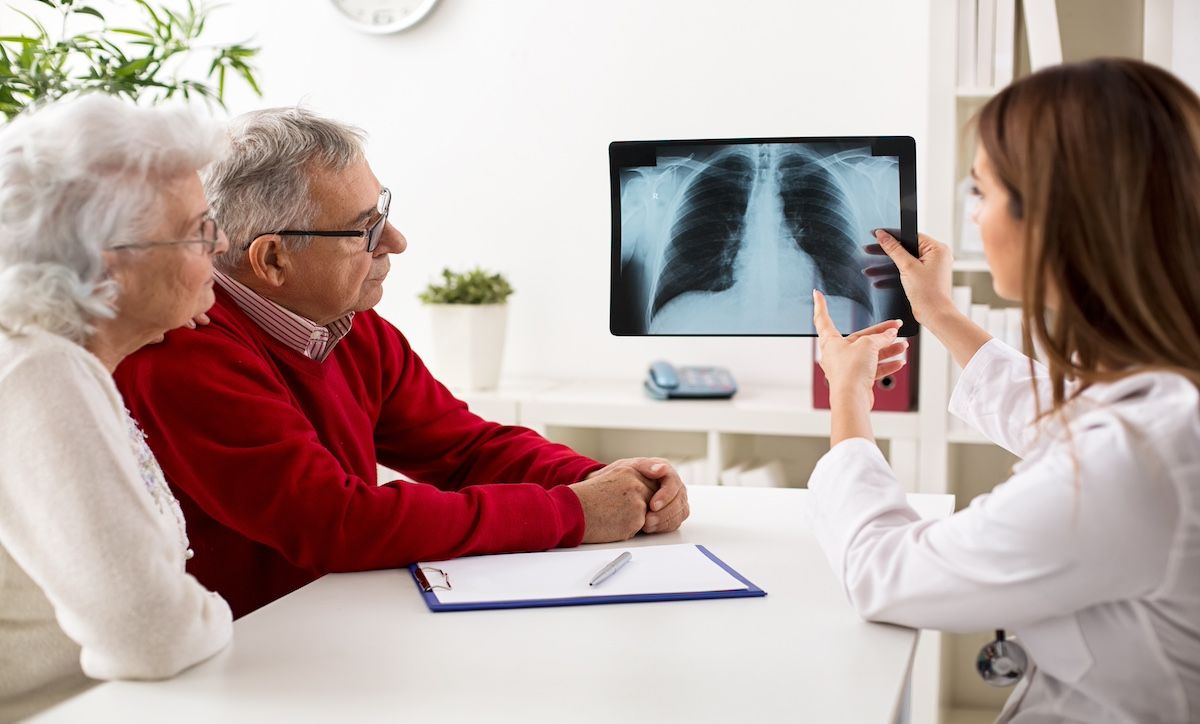
This consensus-based document encompasses key practice paradigms and 6 typical clinical scenarios in patients with unresectable disease, which covers most patients who have LA-NSCLC, the authors noted:
- Treatment of a patient with unresectable LA-NSCLC and good performance status
- Treatment of a patient with unresectable LA-NSCLC and good performance status and bulky disease
- Treatment of a patient with EGFR-positive unresectable LA-NSCLC and good performance status
- Treatment of a symptomatic patient with unresectable LA-NSCLC and poor performance status
- Treatment of an asymptomatic patient with unresectable LA-NSCLC and poor performance status
- Treatment of a patient with unresectable LA-NSCLC and local recurrence
The ARS AUC Thoracic Committee, citing a lack of uniform guidance, delivers 3 summary recommendations from their review:
- For unresectable patients who have LA-NSCLC, appropriate standard-of-care disease management should encompass combined concurrent radical (60-70 Gy) platinum-based chemoradiation and consolidation therapy with durvalumab per PD-L1/ EGFR status.
- Intensity-modulated radiotherapy, because it is conformal in delivery and widely available, should be used when clinicians are planning how to best deliver radiotherapy regimens; tumor bulk and treatment availability, however, may necessitate a switch to 3-dimensional conformal radiotherapy or intensity-modulated proton therapy.
- In patient cases with either poor performance status and/or pulmonary status, potential for treatment-related toxicity should be a primary consideration, with the authors citing the variety of available palliative and radical fractionation schedules and the benefits of either sequential therapy or radiation therapy alone vs concurrent regimens.
“Treatment appropriateness of a variety of LA-NSCLC scenarios was assessed by a consensus-based modified Delphi approach using a range of 3 points to 9 points to denote consensus agreement,” the authors explained regarding how they arrived at their recommendations.
The authors also highlight the importance of multidisciplinary input and shared decision-making with patients who have LA-NSCLC during treatment discussions, concluding that “various surgical, radiotherapeutic, and systemic options are available to manage this challenging patient population.”
Rodrigues G, Higgins KA, Rimner A, et al. American Radium Society appropriate use criteria for unresectable locally advanced non–small cell lung cancer. JAMA Oncol . Published online April 11, 2024. doi:10.1001/jamaoncol.2024.0294

Responsibility for Addressing Health-Related Social Needs Stretches Across Public Health, Health Systems, and Plans
By prioritizing well-being, both the public and private sectors can come together in partnerships to address social needs and social determinants of health.

Navigating Health Literacy, Social Determinants, and Discrimination in National Health Plans
On this episode of Managed Care Cast, we're talking with the authors of a study published in the February 2024 issue of The American Journal of Managed Care® about their findings on how health plans can screen for health literacy, social determinants of health, and perceived health care discrimination.
NSAIDs vs bDMARDs: Sex Disparities in Treatment Initiation for Axial Spondyloarthritis
New research examining treatment initiation patterns among patients with axial spondyloarthritis revealed significant sex-based disparities, shedding light on the process from diagnosis to therapeutic intervention.

Drs Raymond Thertulien, Joseph Mikhael on Racial Disparities in Multiple Myeloma Care Access
In the wake of the 2023 American Society of Hematology Annual Meeting and Exposition, Raymond Thertulien, MD, PhD, of Novant Health, and Joseph Mikhael, MD, MEd, FRCPC, FACP, chief medical officer of the International Myeloma Foundation, discussed health equity research highlights from the meeting and drivers of racial disparities in multiple myeloma outcomes.

What We’re Reading: Medicaid Coverage Unwinding; Subscription-Based Health Care; Measles Surging
Nearly a quarter of adults disenrolled from Medicaid are now uninsured; a new bill in Alaska will allow primary care providers to offer care based on monthly fees; the CDC warns of a 17-fold increase in measles cases in the first quarter of 2024.

Radiographic Progression Low for High-Risk Patients With axSpA on Both Secukinumab and an Adalimumab Biosimilar
Patients with radiographic axial spondyloarthritis (ax-SpA) and a high risk of radiographic progression had similar low progression on both secukinumab and an adalimumab biosimilar.
2 Commerce Drive Cranbury, NJ 08512
609-716-7777

Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Perspective
- Open access
- Published: 09 April 2024

The potential for artificial intelligence to transform healthcare: perspectives from international health leaders
- Christina Silcox 1 ,
- Eyal Zimlichmann 2 , 3 ,
- Katie Huber ORCID: orcid.org/0000-0003-2519-8714 1 ,
- Neil Rowen 1 ,
- Robert Saunders 1 ,
- Mark McClellan 1 ,
- Charles N. Kahn III 3 , 4 ,
- Claudia A. Salzberg 3 &
- David W. Bates ORCID: orcid.org/0000-0001-6268-1540 5 , 6 , 7
npj Digital Medicine volume 7 , Article number: 88 ( 2024 ) Cite this article
845 Accesses
35 Altmetric
Metrics details
- Health policy
- Health services
Artificial intelligence (AI) has the potential to transform care delivery by improving health outcomes, patient safety, and the affordability and accessibility of high-quality care. AI will be critical to building an infrastructure capable of caring for an increasingly aging population, utilizing an ever-increasing knowledge of disease and options for precision treatments, and combatting workforce shortages and burnout of medical professionals. However, we are not currently on track to create this future. This is in part because the health data needed to train, test, use, and surveil these tools are generally neither standardized nor accessible. There is also universal concern about the ability to monitor health AI tools for changes in performance as they are implemented in new places, used with diverse populations, and over time as health data may change. The Future of Health (FOH), an international community of senior health care leaders, collaborated with the Duke-Margolis Institute for Health Policy to conduct a literature review, expert convening, and consensus-building exercise around this topic. This commentary summarizes the four priority action areas and recommendations for health care organizations and policymakers across the globe that FOH members identified as important for fully realizing AI’s potential in health care: improving data quality to power AI, building infrastructure to encourage efficient and trustworthy development and evaluations, sharing data for better AI, and providing incentives to accelerate the progress and impact of AI.
Similar content being viewed by others

Guiding principles for the responsible development of artificial intelligence tools for healthcare
Kimberly Badal, Carmen M. Lee & Laura J. Esserman
A short guide for medical professionals in the era of artificial intelligence
Bertalan Meskó & Marton Görög
Reporting guidelines in medical artificial intelligence: a systematic review and meta-analysis
Fiona R. Kolbinger, Gregory P. Veldhuizen, … Jakob Nikolas Kather
Introduction
Artificial intelligence (AI), supported by timely and accurate data and evidence, has the potential to transform health care delivery by improving health outcomes, patient safety, and the affordability and accessibility of high-quality care 1 , 2 . AI integration is critical to building an infrastructure capable of caring for an increasingly aging population, utilizing an ever-increasing knowledge of disease and options for precision treatments, and combatting workforce shortages and burnout of medical professionals. However, we are not currently on track to create this future. This is in part because the health data needed to train, test, use, and surveil these tools are generally neither standardized nor accessible. This is true across the international community, although there is variable progress within individual countries. There is also universal concern about monitoring health AI tools for changes in performance as they are implemented in new places, used with diverse populations, and over time as health data may change.
The Future of Health (FOH) is an international community of senior health care leaders representing health systems, health policy, health care technology, venture funding, insurance, and risk management. FOH collaborated with the Duke-Margolis Institute for Health Policy to conduct a literature review, expert convening, and consensus-building exercise. In total, 46 senior health care leaders were engaged in this work, from eleven countries in Europe, North America, Africa, Asia, and Australia. This commentary summarizes the four priority action areas and recommendations for health care organizations and policymakers that FOH members identified as important for fully realizing AI’s potential in health care: improving data quality to power AI, building infrastructure to encourage efficient and trustworthy development and evaluations, sharing data for better AI, and providing incentives to accelerate the progress and impact of AI.
Powering AI through high-quality data
“Going forward, data are going to be the most valuable commodity in health care. Organizations need robust plans about how to mobilize and use their data.”
AI algorithms will only perform as well as the accuracy and completeness of key underlying data, and data quality is dependent on actions and workflows that encourage trust.
To begin to improve data quality, FOH members agreed that an initial priority is identifying and assuring reliable availability of high-priority data elements for promising AI applications: those with the most predictive value, those of the highest value to patients, and those most important for analyses of performance, including subgroup analyses to detect bias.
Leaders should also advocate for aligned policy incentives to improve the availability and reliability of these priority data elements. There are several examples of efforts across the world to identify and standardize high-priority data elements for AI applications and beyond, such as the multinational project STANDING Together, which is developing standards to improve the quality and representativeness of data used to build and test AI tools 3 .
Policy incentives that would further encourage high-quality data collection include (1) aligned payment incentives for measures of health care quality and safety, and ensuring the reliability of the underlying data, and (2) quality measures and performance standards focused on the reliability, completeness, and timeliness of collection and sharing of high-priority data itself.
Trust and verify
“Your AI algorithms are only going to be as good as the data and the real-world evidence used to validate them, and the data are only going to be as good as the trust and privacy and supporting policies.”
FOH members stressed the importance of showing that AI tools are both effective and safe within their specific patient populations.
This is a particular challenge with AI tools, whose performance can differ dramatically across sites and over time, as health data patterns and population characteristics vary. For example, several studies of the Epic Sepsis Model found both location-based differences in performance and degradation in performance over time due to data drift 4 , 5 . However, real-world evaluations are often much more difficult for algorithms that are used for longer-term predictions, or to avert long-term complications from occurring, particularly in the absence of connected, longitudinal data infrastructure. As such, health systems must prioritize implementing data standards and data infrastructure that can facilitate the retraining or tuning of algorithms, test for local performance and bias, and ensure scalability across the organization and longer-term applications 6 .
There are efforts to help leaders and health systems develop consensus-based evaluation techniques and infrastructure for AI tools, including HealthAI: The Global Agency for Responsible AI in Health, which aims to build and certify validation mechanisms for nations and regions to adopt; and the Coalition for Health AI (CHAI), which recently announced plans to build a US-wide health AI assurance labs network 7 , 8 . These efforts, if successful, will assist manufacturers and health systems in complying with new laws, rules, and regulations being proposed and released that seek to ensure AI tools are trustworthy, such as the EU AI Act and the 2023 US Executive Order on AI.
Sharing data for better AI
“Underlying these challenges is the investment required to standardize business processes so that you actually get data that’s usable between institutions and even within an institution.”
While high-quality internal data may enable some types of AI-tool development and testing, this is insufficient to power and evaluate all AI applications. To build truly effective AI-enabled predictive software for clinical care and predictive supports, data often need to be interoperable across health systems to build a diverse picture of patients’ health across geographies, and reliably shared.
FOH members recommended that health care leaders work with researchers and policymakers to connect detailed encounter data with longitudinal outcomes, and pilot opportunities across diverse populations and systems to help assure valid outcome evaluations as well as address potential confounding and population subgroup differences—the ability to aggregate data is a clear rate-limiting step. The South African National Digital Health Strategy outlined interventions to improve the adoption of digital technologies while complying with the 2013 Protection of Personal Information Act 9 . Although challenges remain, the country has made progress on multiple fronts, including building out a Health Patient Registration System as a first step towards a portable, longitudinal patient record system and releasing a Health Normative Standards Framework to improve data flow across institutional and geographic boundaries 10 .
Leaders should adopt policies in their organizations, and encourage adoption in their province and country, that simplify data governance and sharing while providing appropriate privacy protections – including building foundations of trust with patients and the public as previously discussed. Privacy-preserving innovations include ways to “share” data without movement from protected systems using approaches like federated analyses, data sandboxes, or synthetic data. In addition to exploring privacy-preserving approaches to data sharing, countries and health systems may need to consider broad and dynamic approaches to consent 11 , 12 . As we look to a future where a patient may have thousands of algorithms churning away at their data, efforts to improve data quality and sharing should include enabling patients’ access to and engagement with their own data to encourage them to actively partner in their health and provide transparency on how their data are being used to improve health care. For example, the Understanding Patient Data program in the United Kingdom produces research and resources to explain how the National Health Service uses patients’ data 13 . Community engagement efforts can further assist with these efforts by building trust and expanding understanding.
FOH members also stressed the importance of timely data access. Health systems should work together to establish re-usable governance and privacy frameworks that allow stakeholders to clearly understand what data will be shared and how it will be protected to reduce the time needed for data use agreements. Trusted third-party data coordinating centers could also be used to set up “precertification” systems around data quality, testing, and cybersecurity to support health organizations with appropriate data stewardship to form partnerships and access data rapidly.
Incentivizing progress for AI impact
“Unless it’s tied to some kind of compensation to the organization, the drive to help implement those tools and overcome that risk aversion is going to be very high… I do think that business driver needs to be there.”
AI tools and data quality initiatives have not moved as quickly in health care due to the lack of direct payment, and often, misalignment of financial incentives and supports for high-quality data collection and predictive analytics. This affects both the ability to purchase and safely implement commercial AI products as well as the development of “homegrown” AI tools.
FOH members recommended that leaders should advocate for paying for value in health – quality, safety, better health, and lower costs for patients. This better aligns the financial incentives for accelerating the development, evaluation, and adoption of AI as well as other tools designed to either keep patients healthy or quickly diagnose and treat them with the most effective therapies when they do become ill. Effective personalized health care requires high-quality, standardized, interoperable datasets from diverse sources 14 . Within value-based payments themselves, data are critical to measuring quality of care and patient outcomes, adjusted or contextualized for factors outside of clinical control. Value-based payments therefore align incentives for (1) high-quality data collection and trusted use, (2) building effective AI tools, and (3) ensuring that those tools are improving patient outcomes and/or health system operations.
Data have become the most valuable commodity in health care, but questions remain about whether there will be an AI “revolution” or “evolution” in health care delivery. Early AI applications in certain clinical areas have been promising, but more advanced AI tools will require higher quality, real-world data that is interoperable and secure. The steps health care organization leaders and policymakers take in the coming years, starting with short-term opportunities to develop meaningful AI applications that achieve measurable improvements in outcomes and costs, will be critical in enabling this future that can improve health outcomes, safety, affordability, and equity.
Data availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Abernethy, A. et al. The promise of digital health: then, now, and the future. NAM Perspect. 6 (2022).
Akpakwu, E. Four ways AI can make healthcare more efficient and affordable. World Economic Forum https://www.weforum.org/agenda/2018/05/four-ways-ai-is-bringing-down-the-cost-of-healthcare/ (2018).
STANDING Together. https://www.datadiversity.org/home .
Wong, A. et al. External validation of a widely implemented proprietary sepsis prediction model in hospitalized patients. JAMA Intern Med 181 , 1065–1070 (2021).
Article PubMed Google Scholar
Ross, C. STAT and MIT rooted out the weaknesses in health care algorithms. Here’s how we did it. STAT https://www.statnews.com/2022/02/28/data-drift-machine-learning/ (2022).
Locke, T., Parker, V., Thoumi, A., Goldstein, B. & Silcox, C. Preventing bias and inequities in AI-enabled health tools . https://healthpolicy.duke.edu/publications/preventing-bias-and-inequities-ai-enabled-health-tools (2022).
Introducing HealthAI. The International Digital Health and AI Research Collaborative (I-DAIR) https://www.i-dair.org/news/introducing-healthai (2023).
Shah, N. H. et al. A nationwide network of health AI assurance laboratories. JAMA 331 , 245 (2024).
Singh, V. AI & Data in South Africa’s Health Sector . https://policyaction.org.za/sites/default/files/PAN_TopicalGuide_AIData6_Health_Elec.pdf (2020).
Zharima, C., Griffiths, F. & Goudge, J. Exploring the barriers and facilitators to implementing electronic health records in a middle-income country: a qualitative study from South Africa. Front. Digit. Health 5 , 1207602 (2023).
Article PubMed PubMed Central Google Scholar
Lee, A. R. et al. Identifying facilitators of and barriers to the adoption of dynamic consent in digital health ecosystems: a scoping review. BMC Med. Ethics 24 , 107 (2023).
Article CAS PubMed PubMed Central Google Scholar
Stoeklé, H. C., Hulier-Ammar, E. & Hervé, C. Data medicine: ‘broad’ or ‘dynamic’ consent? Public Health Ethics 15 , 181–185 (2022).
Article Google Scholar
Understanding Patient Data. Understanding Patient Data http://understandingpatientdata.org.uk/ .
Chén, O. Y. & Roberts, B. Personalized health care and public health in the digital age. Front. Digit. Health 3 , 595704 (2021).
Download references
Acknowledgements
The authors acknowledge Oranit Ido and Jonathan Gonzalez-Smith for their contributions to this work. This study was funded by The Future of Health, LLC. The Future of Health, LLC, was involved in all stages of this research, including study design, data collection, analysis and interpretation of data, and the preparation of this manuscript.
Author information
Authors and affiliations.
Duke-Margolis Institute for Health Policy, Duke University, Washington, DC, USA &, Durham, NC, USA
Christina Silcox, Katie Huber, Neil Rowen, Robert Saunders & Mark McClellan
Sheba Medical Center, Ramat Gan, Israel
Eyal Zimlichmann
Future of Health, Washington, DC, USA
Eyal Zimlichmann, Charles N. Kahn III & Claudia A. Salzberg
Federation of American Hospitals, Washington, DC, USA
Charles N. Kahn III
Division of General Internal Medicine, Brigham and Women’s Hospital, Boston, MA, USA
David W. Bates
Harvard Medical School, Boston, MA, USA
Department of Health Policy and Management, Harvard T. H. Chan School of Public Health, Boston, MA, USA
You can also search for this author in PubMed Google Scholar
Contributions
C.S., K.H., N.R., and R.S. conducted initial background research and analyzed qualitative data from stakeholders. All authors (C.S., E.Z., K.H., N.R., R.S., M.M., C.K., C.A.S., and D.B.) assisted with conceptualization of the project and strategic guidance. C.S., K.H., and N.R. wrote initial drafts of the manuscript. All authors contributed to critical revisions of the manuscript and read and approved the final manuscript.
Corresponding author
Correspondence to David W. Bates .
Ethics declarations
Competing interests.
C.S., K.H., N.R., and C.A.S. declare no competing interests. E.Z. reports personal fees from Arkin Holdings, personal fees from Statista and equity from Valera Health, Profility and Hello Heart. R.S. has been an external reviewer for The John A. Hartford Foundation, and is a co-chair for the Health Evolution Summit Roundtable on Value-Based Care for Specialized Populations. M.M. is an independent director on the boards of Johnson & Johnson, Cigna, Alignment Healthcare, and PrognomIQ; co-chairs the Guiding Committee for the Health Care Payment Learning and Action Network; and reports fees for serving as an adviser for Arsenal Capital Partners, Blackstone Life Sciences, and MITRE. C.K. is a Profility Board member and additionally reports equity from Valera Health and MDClone. D.W.B. reports grants and personal fees from EarlySense, personal fees from CDI Negev, equity from Valera Health, equity from Clew, equity from MDClone, personal fees and equity from AESOP, personal fees and equity from Feelbetter, equity from Guided Clinical Solutions, and grants from IBM Watson Health, outside the submitted work. D.W.B. has a patent pending (PHC-028564 US PCT), on intraoperative clinical decision support.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Silcox, C., Zimlichmann, E., Huber, K. et al. The potential for artificial intelligence to transform healthcare: perspectives from international health leaders. npj Digit. Med. 7 , 88 (2024). https://doi.org/10.1038/s41746-024-01097-6
Download citation
Received : 30 October 2023
Accepted : 29 March 2024
Published : 09 April 2024
DOI : https://doi.org/10.1038/s41746-024-01097-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
- Open access
- Published: 27 October 2022
Sanfilippo syndrome: consensus guidelines for clinical care
- Nicole Muschol 1 ,
- Roberto Giugliani 2 ,
- Simon A. Jones 3 ,
- Joseph Muenzer 4 ,
- Nicholas J. C. Smith 5 ,
- Chester B. Whitley 6 ,
- Megan Donnell 7 ,
- Elise Drake 8 ,
- Kristina Elvidge 7 ,
- Lisa Melton 7 ,
- Cara O’Neill ORCID: orcid.org/0000-0002-9377-259X 8 on behalf of
MPS III Guideline Development Group
Orphanet Journal of Rare Diseases volume 17 , Article number: 391 ( 2022 ) Cite this article
12k Accesses
11 Citations
8 Altmetric
Metrics details
Sanfilippo syndrome is a group of rare, complex, and progressive neurodegenerative lysosomal storage disorders that is characterized by childhood dementia. The clinical management of patients with progressive neurological decline and multisystem involvement requires a multidisciplinary team with experience in the management of neurodegenerative disorders. Best practice guidelines for the clinical management of patients with these types of rare disorders are critical to ensure prompt diagnosis and initiation of appropriate care. However, there are no published standard global clinical care guidelines for patients with Sanfilippo syndrome. To address this, a literature review was conducted to evaluate the current evidence base and to identify evidence gaps. The findings were reviewed by an international steering committee composed of clinical experts with extensive experience in managing patients with Sanfilippo syndrome. The goal was to create a consensus set of basic clinical guidelines that will be accessible to and informed by clinicians globally, as well as providing a practical resource for families to share with their local care team who may not have experience with this rare disease. This review distills 178 guideline statements into an easily digestible document that provides evidence-based, expert-led recommendations for how to approach common management challenges and appropriate monitoring schedules in the care of patients with Sanfilippo syndrome.
Sanfilippo syndrome (mucopolysaccharidosis type III [MPS III]) is a group of inherited lysosomal storage disorders, manifesting progressive central nervous system (CNS) and systemic disease in childhood, with progressive neurocognitive deterioration and loss of functional abilities, and premature death [ 1 ]. There are four autosomal recessive subtypes (types A, B, C, and D) of Sanfilippo syndrome. Each subtype is caused by a deficiency of a different enzyme that degrades the ubiquitous glycosaminoglycan (GAG) heparan sulfate (Table 1 ), which leads to substrate accumulation and cellular dysfunction [ 2 ].
The combined estimated prevalence of Sanfilippo syndrome (types A, B, C, and D) is between 1:50,000 and 1:250,000 depending on the population studied [ 3 ]. Sanfilippo syndrome type A is the most common subtype globally; however, the prevalence of subtypes can vary depending on region, with Sanfilippo syndrome type A being more prevalent in Northern Europe and Eastern Europe than in Mediterranean countries [ 4 , 5 , 6 ]. In contrast, Sanfilippo syndrome type B is the most prevalent subtype in Southern Europe [ 4 , 7 ]. Sanfilippo syndrome types C and D are much less common overall, with estimated global incidences of 1:1,500,000 and 1:1,000,000, respectively [ 1 ]. However, the total number of patients with Sanfilippo syndrome is most likely underestimated owing to delayed or missed diagnoses, particularly for the most slowly progressing phenotypes.
The age at onset and extent and rate of progression in patients with Sanfilippo syndrome vary greatly between those with different subtypes (ie types A, B, C, and D) and within those with the same subtype (eg type A only). The behavioral, cognitive, and physical findings in patients with Sanfilippo syndrome present as a clinical spectrum from early-onset, rapidly progressive disease with death in late childhood and adolescence, to slower progressing forms that present in later childhood with survival into adulthood. In rare cases, more indolent disease with onset in adulthood is also observed in patients with Sanfilippo syndrome [ 2 ]. The natural history of Sanfilippo syndrome, while traditionally considered across three broad symptomatic phases, remains variable between individuals and is best considered as a phenotypic continuum. Typical disease manifests in patients between 1 and 4 years of age with presentation of mild global developmental or speech delay, usually after a period of normal development with somatic manifestations such as recurrent ear, nose, and throat (ENT) disease and/or bowel disturbance [ 2 ]. Behavioral difficulties include: hyperactivity, (hyper) orality and/or preservative chewing, temper tantrums, lack of fear (for danger), disobedience or unresponsiveness to discipline, and destructive behavior [ 8 , 9 , 10 , 11 ]. Physical manifestations in patients with Sanfilippo syndrome can include musculoskeletal, respiratory, gastrointestinal, cardiovascular complications, vision and hearing loss, and dental issues; these manifestations can further exacerbate the neurocognitive and behavioral challenges in these patients [ 2 , 9 , 12 ]. In the later phase of Sanfilippo syndrome, patients show a decline in engagement with their environment, dementia, and progressive loss of motor function. Patients may develop seizures, dysphagia, and become fully bedridden [ 2 , 13 , 14 , 15 ]. For patients with severe forms of Sanfilippo syndrome, death usually occurs within their second decade of life [ 2 , 16 , 17 , 18 ]. In contrast, patients with attenuated phenotypes of the disorder have a more variable life span, in rare cases surviving into their seventh decade of life [ 14 , 19 ].
Patient care requires a collaborative specialist health and community-based multidisciplinary team with experience in the management of Sanfilippo syndrome. There is currently no disease-modifying therapy available for patients with Sanfilippo syndrome. However, disease-specific therapies for Sanfilippo syndrome are being studied (including forms of enzyme replacement therapy, substrate reduction therapy, hematopoietic stem cell transplantation, and gene therapy), with some reaching the mid-to-late stages of clinical development. In lieu of these emergent therapies, management focuses on supportive interventions to maintain function, optimize ability, and maximize quality of life for patients with Sanfilippo syndrome and their families.
Best practice guidelines for the clinical management of rare diseases are critical to ensure prompt diagnosis and initiation of appropriate care. Such guidelines allow physicians and other healthcare professionals to make recommendations based on the best available evidence, to improve the consistency of diagnosis and clinical management across treatment centers, and to enable affected families to make informed decisions regarding therapy. For patients with Sanfilippo syndrome, there are currently no published, standard global clinical care guidelines.
Here, a collaboration between Cure Sanfilippo Foundation (USA) and Sanfilippo Children’s Foundation (Australia) was initiated in mid-2017 to investigate current best practice in the clinical management of patients with Sanfilippo syndrome. A literature review and gap analysis were conducted to evaluate the current evidence base, and the findings were reviewed by an international steering committee consisting of clinical experts with extensive experience in managing patients with Sanfilippo syndrome. The goal was to create a consensus set of basic clinical guidelines for patients with Sanfilippo syndrome that will be accessible to and informed by clinicians globally, as well as providing a practical resource for families to share with their local care team who may not have experience with this rare disease. Here, 178 guideline statements are distilled into an easily digestible document that provides recommendations based on evidence and consensus clinical expertise for how to approach common management challenges in the care of patients with Sanfilippo syndrome. This review is a first step in establishing basic care guidance and will require updates as Sanfilippo syndrome becomes further characterized and should new therapies become available.
A consultative survey-based technique was used to reach consensus on best practice for the management of patients with Sanfilippo syndrome. An overview of the consensus process is shown in Fig. 1 .
Flow chart for the development of consensus guideline statements. *One statement was subsequently refined by the steering committee during development of the guidelines
A steering committee was formed, consisting of clinical experts from Australia (including members of Sanfilippo Children’s Foundation), Brazil, Germany, the UK, and the USA (including members of Cure Sanfilippo Foundation), each with extensive experience in managing patients with Sanfilippo syndrome. A comprehensive literature review and gap analysis was conducted by members of the steering committee to consolidate the best available published information on the management of patients with Sanfilippo syndrome and to identify evidence gaps. The search terms used are detailed in the supplemental material. Publications reviewed in detail included any published article containing information on patients with Sanfilippo syndrome (types A, B, C, and D), including articles referencing mucopolysaccharidoses in general to delineate information specific to Sanfilippo syndrome.
A network of expert clinicians was invited to join a Guideline Development Group to consider the findings of the literature review and draft initial guideline statements for their area of expertise. In addition to the steering committee members, the Guideline Development Group included 29 clinicians (35 in total) with experience of Sanfilippo syndrome from nine countries (Additional file 1 : Table S1). Collectively, the expert clinicians represented the following focus areas: neurology, metabolic and/or genetic diseases, orthopedics, gastroenterology, ophthalmology, cardiology, dentistry, ENT (including audiology), rehabilitative therapies (speech therapy, occupational therapy, behavioral therapy, and physical therapy), developmental pediatrics, anesthesiology, endocrinology, and integrative medicine (including nutrition and supplements). A total of 185 draft guideline statements were developed by the Guideline Development Group and refined or expanded upon by the steering committee. The draft guideline statements were sent to 166 clinicians from five continents with a survey asking them to rate their level of agreement with each statement on a 5-point Likert scale, as follows: Strongly agree, Agree, Neutral, Disagree, or Strongly disagree. They were also given the option ‘Not my area of expertise’ and asked to provide comments, particularly if they disagreed with a statement.
Consensus was defined as ≥ 75% of responses being ‘Strongly Agree’ or ‘Agree’, excluding responses of ‘Not my area of expertise’. This consensus threshold was determined by a literature review and applied to the rare disease field by the steering committee. The most common definition of consensus for Delphi studies is percent agreement, with ≥ 75% being the median threshold to define consensus [ 20 ]. No participants were compensated for their involvement.
Responses were received from 64 clinicians representing 21 specialty areas. Clinicians were based in 14 countries across five continents, as follows: 29.7% (n = 19) in North America, 26.6% (n = 17) in Europe, 23.4% (n = 15) in Australasia, 12.5% (n = 8) in South America, and 7.8% (n = 5) in Asia. Of the clinicians surveyed, 59% (n = 38) had cared for ≥ 10 patients with Sanfilippo syndrome in their career, and 28% (n = 18) had cared for > 30 patients with Sanfilippo syndrome. Consensus (defined as ≥ 75% responses of ‘Strongly Agree’ or ‘Agree’, excluding ‘Not my area of expertise’) was reached for 173 (94%) of 185 statements. After a steering committee review of the 12 non-consensus items, consensus on four of the statements was not reached and they were omitted. The remaining eight statements were revised based on comments from participants and recommendations from the steering committee. The eight revised were then distributed to the same global clinical email list. Of these eight statements, consensus was reached for five, while three were removed. A full list of all guideline statements and their level of consensus can be found in Additional file 1 : Table S2.
Following the consensus-forming process, the steering committee convened to review the 178 guideline statements and discuss how to distill them into a practical and user-friendly format. As part of this process, consensus recommendations were divided into 156 core statements that tackle the most pressing needs faced by patients with Sanfilippo syndrome, and 22 supplemental statements that address some of the less common aspects of diagnosing and managing the disease, or areas that require further evidence. Additional refinement of the resulting guidance by the steering committee’s clinical experts was made in select areas, based on their collective clinical experience and consideration of any risks associated with recommended procedures. These few instances are noted where they occur.
Optimal management relies on early diagnosis
Early diagnosis of Sanfilippo syndrome is critical to ensure the optimal care for patients and their families by enabling access to specific supportive interventions to maximize peak abilities, slow rate of decline, and improve quality of life. In addition to accessing appropriate education and developmental therapies, early diagnosis enables patients to participate in clinical trials and/or receive treatments as they emerge, and affords timely genetic counseling of affected families. However, diagnoses delayed by > 2 years are not uncommon in patients with Sanfilippo syndrome [ 21 , 22 , 23 , 24 ]. Potential reasons for delays include a lack of disease awareness, the absence or subtle presentation of somatic symptoms, and neurological symptoms that can be mistakenly considered as idiopathic developmental delays and behavioral challenges [ 23 ]. Furthermore, Sanfilippo syndrome is not included in newborn screening programs and patients often receive diagnoses such as idiopathic developmental delay, attention deficit hyperactivity disorder (ADHD), and/or autism without sufficient medical workup to identify Sanfilippo syndrome as the underlying genetic disorder [ 18 , 25 , 26 ].
A recent consensus-forming process identified eight signs and symptoms that presented early in neonates and infants that may, alone or in combination, raise suspicion of Sanfilippo syndrome [ 27 ]. Such signs and symptoms included coarse facial features, persistent hirsutism and/or prominent eyebrows, which have been reported as signs that are suggestive of Sanfilippo syndrome and should prompt referral to a metabolic physician and/or developmental specialist [ 27 ]. Somatic signs should also be viewed in the context of neurocognitive features. For example, early somatic signs that are not specific to Sanfilippo syndrome (eg frontal bossing and macrocephaly) become noteworthy when present alongside neurocognitive features (eg speech delay). Similarly, while episodic irritability and gastrointestinal discomfort, umbilical or inguinal hernia, and upper respiratory congestion are considered prevalent among neonates and infants [ 28 ], any of these conditions, including those listed previously, should raise suspicion of MPS diseases but particularly for Sanfilippo syndrome when present alongside the characteristic neurobehavioral features. Table 2 shows a list of neurological and somatic features that (alone or in combination) should raise suspicion of Sanfilippo syndrome. Sanfilippo syndrome should be considered in patients of all ages, not only young children, since slower progressing forms of the disorder are noted. For example, investigation for Sanfilippo syndrome is warranted in adults who show signs of early-onset dementia, vision loss with retinitis pigmentosa, and/or adult-onset cardiomyopathy [ 29 ]. Where such suspicion exists, screening and/or diagnostic tests should be initiated by the primary care provider to avoid delay of diagnosis, in conjunction with referral to an appropriate specialist.
Confirming a diagnosis of Sanfilippo syndrome
In individuals with clinical features suggestive of Sanfilippo syndrome, confirmation of a diagnosis requires at least two biochemical or genetic markers of the disorder to be present: evidence of GAG accumulation (eg increased total GAGs or the more specific component substrate, heparan sulfate, in the urine or blood), decreased lysosomal enzyme activity, and/or evidence of pathogenic or likely pathogenic variants via molecular testing [ 30 , 31 ].
GAG analysis
Analysis of urine GAGs using quantitative and qualitative approaches in a first morning void sample are accepted biochemical diagnostic tests, with a sample from a random time also being acceptable. A sterile sample is not required. Quantitative analysis of urine for the presence of GAG biomarkers using the spectrophotometric compound dimethylmethylene blue is often used as a first-line screen for MPS disorders [ 32 , 33 , 34 , 35 ]. The use of age-related reference ranges is strongly recommended owing to the natural decrease in GAG levels with age, both in affected patients and healthy individuals. The recommended qualitative GAG assay is GAG electrophoresis [ 31 , 36 , 37 ]. Notably, however, both quantitative and qualitative urine GAG tests can be insensitive, particularly if the urine is dilute, and cannot rule out Sanfilippo syndrome owing to the significant rate of false-negatives [ 31 , 32 , 33 , 34 , 35 , 36 , 37 ]. Therefore, in cases of increased clinical suspicion with a negative urine GAG screen, follow-up with enzyme analysis or genetic testing is recommended. Semi-quantitative urine screening assays using cationic dyes on filter paper (eg the Berry spot test) have relatively high rates of false-positives and false-negatives and should no longer be used [ 30 ].
The analysis of GAGs is being replaced by the analysis of specific GAG species (eg heparan sulfate) using tandem mass spectrometry because of increased sensitivity and specificity to these species [ 38 ]. Tandem mass spectrometry is now routinely used in some laboratories and should become the predominant strategy for GAG analysis in the future.
Enzyme analysis
As above, Sanfilippo syndrome is caused by deficiencies in one of four enzymes that are associated with a defect in heparan sulfate metabolism [ 39 , 40 , 41 , 42 ]. Enzymatic analysis using blood leukocytes or cultured fibroblasts is the recommended gold standard for confirmation of diagnosis of Sanfilippo syndrome and can be considered as a first-line test, particularly when there are difficulties obtaining a suitable urine sample and/or shipping in adequate conditions (ie < 4 °C, delivered within 24 h) [ 43 ]. At the very least, enzyme analysis should be performed in patients with increased GAGs (heparan sulfate) or if clinical suspicion is increased [ 43 ]. Enzyme activity and the presence of heparan sulfate fragments can also be measured by mass spectroscopy in dried blood spots (DBS), which offers considerable practical advantages (eg sample collection, storage, and transport), and multiple enzyme activity tests can be performed on a single sample [ 44 , 45 ]. In addition, parameters such as sample viscosity, hematocrit level, and contamination during the drying process can affect the sensitivity, reproducibility, and overall accuracy of DBS measurement [ 46 ]. Therefore, a deficient enzyme result in DBS samples should be confirmed by an enzyme assay in leukocytes or fibroblasts, and/or by molecular genetic analyses [ 31 ]. False-negatives have not been reported in newborn screening pilot studies with this methodology, but clinical suspicion beyond the newborn period should always trigger laboratory testing.
Multiplex tandem mass spectrometry provides the potential to assay all enzymes simultaneously via high-throughput screening [ 47 ]. Alternatively, each enzyme can be assessed individually and ordered in a sequence according to the relative frequency of disease subtypes in the region; however, this assay is labor intensive and good quality control is essential [ 43 ]. The clinical features of Sanfilippo syndrome are similar to other conditions, such as multiple sulfatase deficiency and mucolipidosis. Therefore, if the sulfatase enzymes for Sanfilippo syndrome type A (heparan- N -sulfatase) or type D (N-acetylglucosamine 6-sulfatase) are deficient, then at least one other sulfatase should be assayed to rule out multiple sulfatase deficiency [ 31 ]. Conversely, if multiple lysosomal enzymes are elevated, the diagnosis of mucolipidosis should be suspected and confirmed by DNA testing.
Molecular genetic testing
A suspected diagnosis of Sanfilippo syndrome can be confirmed by molecular genetic testing or mutation analysis [ 31 ]. Molecular genetic testing should be offered to all patients as it enables cascade molecular screening of undiagnosed siblings or extended family members and family members who are carriers [ 31 ], thereby enabling appropriate genetic counseling and informed family planning [ 30 ]. In addition, the findings of molecular testing may inform clinical expectations of disease progression according to the pathogenicity of the mutation and knowledge regarding the correlation of genotype with phenotype [ 30 ]. Molecular testing results may also impact the patient’s eligibility for clinical trials and future therapies.
In instances where a patient’s primary diagnosis is made based on a molecular genetic diagnosis, a confirmatory biochemical assay should be conducted to confirm the pathogenicity of the mutations [ 48 , 49 ]. When a patient is identified as homozygous for a mutation that is pathogenic for Sanfilippo syndrome or heterozygous for two known pathogenic mutations, a diagnosis can be made with reasonable confidence if the patient has a clinical phenotype consistent with Sanfilippo syndrome.
Prenatal diagnosis
Prenatal diagnosis is feasible for Sanfilippo syndrome in the context of a known familial diagnosis. The main methods used to collect material for prenatal testing are amniocentesis and chorionic villus sampling, which enable biochemical and molecular testing of fetal-derived tissues. If there is an older sibling with a confirmed diagnosis of Sanfilippo syndrome who has two known mutations, prenatal diagnosis may be made with molecular testing alone [ 1 , 27 , 44 ].
Newborn screening
Newborn screening provides the opportunity to diagnose patients as early as possible and enable prompt intervention with optimal outcomes when disease-specific therapies are approved [ 31 ]. Given the progressive and seemingly irreversible nature of the neurological manifestations of Sanfilippo syndrome, the adoption of all available measures to detect patients as early as possible should become standard practice. Most newborns with Sanfilippo syndrome are asymptomatic at birth; therefore, the identification of biochemical or genetic markers of Sanfilippo syndrome in newborns is crucial [ 50 ].
A suitable method for the screening of newborns is one that is rapid, cost-effective, sensitive, and widely available [ 50 ]. Newborn screening for MPS disorders has been studied with several methods, including GAG assay in urine, GAG assay in DBS with ultra-performance liquid chromatography combined with tandem mass spectrometry, fluorometric enzyme assay, digital microfluidics enzyme assay, and enzyme and/or substrate assay with tandem mass spectrometry (MS/MS) [ 50 ]. Overall, the recommended approach for the screening of MPS disorders is the analysis of enzyme activity in DBS with MS/MS or fluorometry to identify MPS subtypes, which is not possible with GAG assays. Newborn screening with molecular genetics tools is being considered; however, these tools are less readily available compared with biochemical testing [ 50 ].
In countries with newborn screening facilities, Sanfilippo syndrome is generally not included in routine screening programs; however, pilot studies for Sanfilippo syndrome types A and B are in progress. As disease-specific therapies become available and improve the lives of patients with Sanfilippo syndrome, the ethical and clinical imperative for early (pre-symptomatic) diagnosis will strengthen. Moreover, although newborn screening cannot determine disease severity, such programs provide timely information that may inform planning for families, even prior to the availability of commercially approved treatments.
General principles and goals of management
In the absence of a disease-modifying treatment for Sanfilippo syndrome, the primary goal of management should be to optimize the quality of life for patients and their families. This requires a holistic approach that considers the wide-ranging and complex medical needs of patients with this condition. A key step in this process is the establishment of a multidisciplinary team of healthcare professionals to work collaboratively and in partnership with patients with Sanfilippo syndrome and their families. This multidisciplinary team would include (but is not limited) to physicians, nurses, therapists (eg physical, occupational, and speech), dieticians, psychologists, social workers, special educators, and counselors. A supervising clinician should oversee the coordination of care. Comprehensive care should be initiated as early as possible, ideally immediately after diagnosis, and the frequency of clinic visits and assessments should be tailored to meet the individual needs of each patient with Sanfilippo syndrome and their family. Frequent communication with families is important to align on care goals and plans, and to ensure that the best interests and values of patients and their families remain at the heart of the decision-making process.
Different assessments and interventions are required for patients with Sanfilippo syndrome depending on their level of disease progression (Table 3 ), and treatment plans should be modified according to each patient’s needs. For example, during the pre- and early symptomatic time frames, it is important to establish a multidisciplinary care team, initiate supportive care measures, conduct forward planning with families around future care needs, and provide genetic counseling as a part of family planning. As a patient’s disease progresses, supportive care measures need to be increased to alleviate the burden of symptoms and to support engagement in everyday activities as much as possible. For patients showing signs of pain, distress or behavioral changes of undetermined etiology, systemic assessments of likely causes of pain are recommended (Table 4 ). In the later stages of the disease, the maintenance of quality of life and prevention of complications become the priorities of care.
In addition to their impact on the patient, neurodegenerative conditions such as Sanfilippo syndrome can have a strong negative impact on the psychosocial functioning and quality of life of family members [ 51 , 52 , 53 , 54 , 55 ]. Parents and caregivers face potentially traumatic medical events followed by short- and long-term stress [ 56 ], putting them at risk of developing parental post-traumatic stress disorder (PTSD) [ 56 , 57 , 58 ]. For example, 22% of parents of children with Sanfilippo syndrome in the Netherlands were found to be suffering from PTSD, compared with 3.8% of parents in the general population of the same country [ 59 ]. The presence of parental PTSD can, in turn, have a significant influence on the psychological wellbeing of the affected child [ 60 ]. Thus, the adoption of a trauma-informed approach to caring and supporting families affected by Sanfilippo syndrome is imperative [ 61 ].
Managing the neurological challenges of Sanfilippo syndrome
Monitoring of neurodevelopment.
Progressive CNS degeneration is a characteristic feature in patients with Sanfilippo syndrome, with neurological plateau and eventual regression following initial normative gains in neurodevelopment [ 1 , 62 ]. Clinical heterogeneity exists between and within the four disease subtypes and the rate of neurocognitive decline varies. While broad genotype–phenotype correlations have been recognized in some cases [ 15 ], these are not universal [ 1 , 62 ]. Patients should therefore undergo detailed neurological evaluation at diagnosis and regular monitoring (eg every 6–12 months) thereafter to detect changes in cognition, motor function, and behavior.
The most well-characterized neurocognitive phenotypes are Sanfilippo subtypes A and B. In these forms of the disease, patients generally continue to acquire cognitive skills until the age of 2.5–4 years depending on the subtype and severity phenotype [ 62 ]. Data for Sanfilippo syndrome types C and D are limited [ 63 ]. However, depending on phenotype, the timing of developmental plateau and pace of decline can vary. Speech and language delay are the most frequent initial symptoms, and language delay may be apparent by the age of 2 years, before cognitive decline starts [ 21 , 64 ]. Conductive hearing loss secondary to middle ear disease is commonly comorbid, along with the development of high frequency sensorineural hearing loss [ 21 ], and further impacts the acquisition of critical early language skills. Indeed, in this context, the effective management of middle ear disease and sensorineural hearing loss typically results in significant improvements. Monitoring of neurocognitive function is recommended on an ongoing basis (or at a frequency appropriate to each individual’s needs) to help families identify areas of strength and interval loss of skills. In addition, this monitoring will help to support discussions focused on helping families adjust to progression of the disorder, educational needs, and supportive interventions in the later phases of the disease. There are many psychometric measures that can be used to evaluate cognitive function in patients with Sanfilippo syndrome [ 65 ]. The Bayley Scales of Infant and Toddler Development, Third Edition (Bayley-III) is one of the most frequently used in clinical studies [ 62 ]. However, use of a specific instrument for clinical care purposes did not reach consensus in our survey. Clinicians may use an available tool that is best suited for monitoring their individual patient over time; recognizing that use of measures that align with published studies may allow for a more informed comparison of the patient’s results with existing disease natural history data.
In addition to assessing neurocognitive function, magnetic resonance imaging (MRI) of the brain should be conducted at baseline and as clinically indicated. Neurodegeneration in patients with Sanfilippo syndrome can be represented by decreases in the volume of cortical and subcortical parenchyma, with secondary increases in ventricular volume on MRI over time [ 63 ]. These changes occur in parallel with cognitive decline and are much more severe in patients with rapidly progressing phenotypes than in those with slowly progressing phenotypes [ 64 ]. Triggers for ordering an MRI of the brain, beyond the baseline assessment, may include extreme behavioral changes, unexplained pain or distress, suspicion of headaches, suspicion of elevated intracranial pressure, and sudden neurological or functional declines. Opportunistic neuroimaging may also be considered during anesthesia for another reason, provided that the risks and benefits are weighed and discussed with the family.
Motor function
Assessment of gross motor and fine motor function is recommended at diagnosis of Sanfilippo syndrome and then every 6–12 months, or more frequently if clinically indicated. Fine motor skills reach a plateau at approximately 2–3 years of age in patients with Sanfilippo syndrome types A and B with typical progression of the disorder (mirroring cognitive decline), whereas development of gross motor skills tends to be preserved until later. In this regard, one study of patients with Sanfilippo syndrome types A, B, and C reported the onset of clumsiness in walking at a median age of 7 years, 7.5 years, and 9 years, respectively, and loss of unassisted sitting at 10.5 years, 14 years, and 13.5 years, respectively [ 22 ]. Given cognitive impairment and hearing loss, difficulties in following instructions together with poor imitative skills may impair the ability of patients with Sanfilippo syndrome to perform motor tasks after this age.
Walking and gait should be assessed at baseline then every 6–12 months, or as needed. Clinicians should be particularly mindful of functional impairments and the development of movement disorders such as dystonia, ataxia, and dyskinesias (including tics, myoclonus, and choreoathetosis). As the disease progresses, patients may require more time to initiate or complete a task owing to the development of motor apraxia and challenges with motor planning. This additional time should be accommodated in clinical exams, formal testing, and during educational and therapeutic activities. Consideration should be given to the needs for medical equipment such as orthotics and bracing, and when to refer to orthopedics, physiotherapy, or other supportive care functions.
Considerations for neurobehavioral, psychological, and psychiatric care
Changes in neurocognition at 2–4 years of age typically coincide with the appearance of behavioral difficulties, including hyperactivity, hyperorality and/or preservative chewing, temper tantrums, disobedience or unresponsiveness to discipline, decreased attention, and severe sleep disturbance [ 4 , 8 , 10 , 19 , 66 , 67 , 68 ]. Most patients with Sanfilippo syndrome develop autistic-like behaviors (primarily social and emotional abnormalities from approximately 4 years of age [ 25 , 69 ]), and as cognitive function declines, many patients display disinhibited behaviors [ 70 ]. For patients with slowly progressing disease who survive into adulthood, reported behavioral problems include motor restlessness, screaming, sensitivity to touch or temperature changes, anxiety, crying fits, aggressive behavior, stereotypic speech, and irritability [ 19 ]. Another report found that adults with Sanfilippo syndrome tend to engage less in interactions and become withdrawn [ 9 ].
The management of behavioral symptoms requires a holistic approach of understanding the behavior in the context of the cognitive skill level, creating a safe environment at home and school for the patient, and providing a routine and structure in addition to any pharmacologic approaches [ 9 , 71 ]. Developmental testing should be conducted in an environment familiar to the patient by a tester who has an established rapport with the patient and has familiarized themselves with the behavioral characteristics of Sanfilippo syndrome prior to testing. When evaluating and monitoring adaptive behavior skills, the Vineland Adaptive Behavior Scale should be used as at least one of the measures [ 72 , 73 , 74 ]. When considering behavior-modifying medications, careful consideration of the following is needed to formulate a proper treatment strategy: any physical problem (eg pain), musculoskeletal problems, gastrointestinal disturbances, seizures, dental problems, and communication challenges.
The identification of negative stimuli (eg pain, an unfamiliar situation or environment, or the association of an unpleasant feeling with a specific location) is needed to prevent or mitigate abnormal behavior [ 9 , 71 ]. The input of parents and caregivers should be encouraged to help calm and comfort the patient when completing necessary medical tests and exams. If necessary, tests may be conducted under anesthesia when coordinated with other procedures.
Early identification of behavioral changes and sleep problems will help to enable effective management and referral to appropriate specialized services [ 4 , 15 ]. Regular neurologic assessments are recommended at baseline and then every 6–12 months, and more frequently if clinically indicated [ 18 ]. Evaluations should monitor the appearance or changes in sleep disturbances, seizure activity, neuromuscular tone, movement disorders, and behavior.
The identification of psychiatric symptom clusters in the context of the developmental age-equivalent profile of each patient is helpful when considering interventions to manage the neurobehavioral aspects of Sanfilippo syndrome. These clusters include sleep, ADHD and autistic behaviors, social communication difficulties, speech and language difficulties, sensory difficulties, and anxiety. Applied behavioral analysis therapy, where available and tailored to the individual patient, should be supported to enhance communication skills, maintain motor abilities, reduce unsafe behaviors, and reduce behaviors that interfere with learning and engagement as it has been found to be beneficial for some patients with Sanfilippo syndrome [ 75 ].
Several groups of behavior-modifying medications have been administered to patients with MPS disorders; however, published evidence for the use and long-term effectiveness of these agents in patients with Sanfilippo syndrome is limited [ 9 ]. Therefore, the prescription of psychiatric medication targeted to mitigate behavioral symptoms should be accompanied by an evaluation of contraindicated risks. Such evaluation is particularly important given that Sanfilippo syndrome is a multisystem disease and the impact of psychotropic medication on cardiac, hepatic, and renal systems needs to be taken into account. Use of stimulant medications, mood stabilizers, antipsychotics, and antianxiety drugs may be considered on a case-by-case basis and with short-term trial periods following a review of the potential risks and benefits with the patient’s family.
Seizure management
Seizures have been reported in patients with MPS disorders in which GAG accumulation in the brain is speculated to trigger alterations in neuronal connectivity and signaling, and release of inflammatory mediators [ 4 , 76 ]. Approximately 26–52% of patients with Sanfilippo syndrome will develop seizures and epilepsy in the later stages of the disease [ 4 , 14 , 15 , 68 , 76 ]. While the prevalence of seizures does not differ greatly between patients with the four subtypes of Sanfilippo syndrome, the age of onset of seizures appears to be somewhat earlier in patients with type A than in those with other subtypes [ 4 , 14 , 15 , 17 , 19 , 22 , 77 ], and the incidence of seizures has been found to increase with advancing neurocognitive deterioration [ 4 ].
Patients with Sanfilippo syndrome typically present with generalized tonic–clonic seizures [ 19 , 22 , 78 , 79 ]. A study of electroencephalography (EEG) records of patients at different stages of Sanfilippo syndrome found that progressive EEG changes correlated with age and disease progression [ 80 ]. While patients younger than 3 years had normal background activity while awake, slowing of occipital-dominant rhythm and background activity at wakefulness could be observed after 6 years of age and became more severe after 11 years of age. Non-convulsive status also was noted in a couple of patients [ 80 ]. EEG abnormalities during sleep have also been reported [ 79 ]. Nocturnal seizures can disrupt sleep hygiene, which in turn can exacerbate and contribute to diurnal somnolence, disturbed concentration, and neurobehavioral lability [ 76 ].
Optimal management of patients with epileptic seizures requires a correct diagnosis. Clinicians should have a high index of suspicion in monitoring for epileptic activity (convulsive and non-convulsive) in patients with Sanfilippo syndrome. However, seizures can be difficult to detect in patients with Sanfilippo syndrome as they often become evident by alterations and/or abnormalities in mental status, behavior, and/or cognition, which are inherent features of the disease [ 81 , 82 ]. The occurrence of absence seizures and non-convulsive status epilepticus can be subtle and difficult to monitor. A diagnostic workup for seizures in patients with Sanfilippo syndrome should include electrophysiological examination by EEG, and prolonged video EEG or in-home mobile EEG may be warranted to detect more subtle seizure activity and nocturnal seizures.
Both convulsive and non-convulsive epilepsy should be adequately treated according to the patient's individual needs and medication history. Literature discussing the treatment of epileptic seizures in patients with Sanfilippo syndrome is limited. Anecdotal evidence based on the experience of experts in the treatment of epilepsy in patients with MPS disorders indicates there are not clinically significant differences in seizure control and management between patients with Sanfilippo syndrome and other patients with epilepsy [ 76 ]. Therefore, standard protocols for the treatment of seizures should be followed [ 53 ]. Preference should be given to anti-epileptic drugs with fewer drug–drug interactions that do not require the monitoring of therapeutic drug levels.
Sleep alterations are an almost constant feature of Sanfilippo syndrome, affecting 87–92% of patients [ 67 , 83 ]. Features include difficulties with settling, frequent nocturnal waking and wandering, and greater daytime sleep compared with healthy individuals [ 2 , 10 , 84 ]. The unrelenting nature of sleep disturbances places a heavy burden on both the patient and their family, and can cause great distress [ 10 , 84 ].
In patients with sleep disturbance, medical workup should include consideration of the presence of disordered movement or seizure activity [ 85 ], iron deficiency in the setting of restless legs, pain or intercurrent illness, esophageal reflux, dental disease, and disordered breathing or sleep apnea during sleep. Sleep disturbance should be addressed with a multimodal approach that includes sleep hygiene counseling, implementing behavioral strategies, addressing the safety of the environment (eg securing the door to prevent harm from wandering, removing items that may cause choking, removing or covering hard surfaces, enclosed specialty beds, and avoiding furniture that could be toppled over), treating circadian rhythm disturbance, and other comorbid medical factors. The use of sleep diaries is encouraged for monitoring changes, evolution of sleep disturbance, and response to interventions.
Sleep apnea is well described as a cause of sleep disturbance in patients with MPS disorders [ 86 , 87 ]. A history of sleep apnea and snoring should be sought in all patients with Sanfilippo syndrome who also have sleep disturbance [ 87 ], and diagnosis and management of sleep apnea should be made under the guidance of a pulmonologist and/or otolaryngologist depending on etiology. If the patient has signs and symptoms of obstructive sleep apnea along with adenoid and/or tonsillar hypertrophy, removal of adenoids and/or tonsils should be performed without delay. This may need to be repeated if tissue regrowth and obstructive sleep apnea recurs later. Continuous positive airway pressure (CPAP) therapy should be considered for patients who display the presence of obstructive sleep apnea that persists after adenoidectomy and/or tonsillectomy. The implementation of CPAP will likely require additional longer-term behavioral or other supports to help increase the patient’s acceptance of the device. The ongoing follow-up of patients who are receiving medication for respiratory and sleep disorders is recommended, the frequency of which will depend on the severity of the respiratory disease and sleep-disordered breathing.
Patients with Sanfilippo syndrome may experience disruption of their circadian rhythm [ 86 , 88 ], which may be partly addressed by melatonin supplementation [ 9 , 10 , 71 ]. If melatonin is started, it is recommended to begin at a low dose (0.5–2 mg) and then be titrated up to higher doses per the patient’s response [ 10 , 89 ]. The typically recommended dose is 2–10 mg at bedtime, but a higher dose is occasionally needed.
Managing the airway
Respiratory management.
Respiratory tract and sinopulmonary infections are common in patients with Sanfilippo syndrome [ 12 ], and respiratory complications such as pneumonia have been reported as the primary cause of death in these patients [ 90 ]. However, behavioral disturbances in patients with Sanfilippo syndrome may mask the typical signs of respiratory infection, leading to diagnosis after the respiratory infection has advanced. Therefore, clinicians should consider a diagnosis of pneumonia in patients with Sanfilippo syndrome and should order early diagnostic radiology when a respiratory infection is suspected, with prompt treatment with antibiotics when pneumonia is confirmed.
As part of routine clinical care, patients with Sanfilippo syndrome should undergo regular clinical assessments and physical examination to facilitate the early detection of respiratory or other complications. These steps should include assessment of vital signs (eg respiratory rate, heart rate, height, and weight) and routine respiratory examination (including the nose and oropharynx). Investigation of clinical history should include sleep hygiene, quality and duration, symptoms of sleep-disordered breathing, history of respiratory symptoms (eg chronic cough), history of pneumonia, history of oral secretions, history of difficulty feeding, history of gastroesophageal disease, and history of nasal secretions and/or nasal congestion. Abnormal findings may prompt measurement of oxygen saturation and non-invasive monitoring of carbon dioxide, if available. Excessive oral secretions may be managed by manual suction and/or medications such as atropine or glycopyrrolate [ 84 , 91 , 92 ].
Routine childhood vaccinations should be given per the standard of care schedule, including annual influenza vaccines. Pneumovax 23 is recommended for patients with Sanfilippo syndrome, in accordance with the guidelines for those who are at increased risk of pneumococcal disease [ 12 ]. While the potential increased risk of serious illness owing to COVID-19 infection in patients with Sanfilippo syndrome is considered likely, there is limited experience in these patients and vaccination is recommended in line with accepted global protocols.
Anesthesia and peri-operative care
Patients with Sanfilippo syndrome may require anesthesia for surgical interventions (eg dental extractions and tonsillectomy) to help manage their disease or to carry out evaluations such as MRI, lumbar puncture, or echocardiography [ 93 , 94 ]. Complications during anesthesia and surgery can occur in patients with Sanfilippo syndrome [ 94 , 95 ], albeit typically at a lower rate than in patients with other MPS disorders [ 96 ]. A retrospective analysis of 126 cases of anesthesia in 37 patients with Sanfilippo syndrome found that the most common anesthesia-related complications were bradycardia or tachycardia (2.4% of anesthesia events), respiratory insufficiency (1.6%), hypoxemia (1.6%), and atelectasis (1.6%) [ 96 ].
To mitigate the respiratory risks in patients with Sanfilippo syndrome, sedation and anesthesia events should always be conducted in the hospital setting with experienced anesthesia personnel available and ready to manage complex airway emergencies. In situations where a procedure or evaluation would be most efficiently and humanely conducted under anesthesia, the number of such anesthesia events should be minimized within reason by combining procedures and coordinating efforts with the multidisciplinary team, as much as possible.
For patients with behavioral and cognitive challenges, patient-centered accommodations should be considered to ensure their safety and wellbeing before and after anesthesia [ 93 , 94 , 95 , 97 ]. Such accommodations may include: allowing parent/caregiver access to the patient during the induction of anesthesia and upon emergence/recovery; providing a low-stimulus environment with the ability to secure/close doors to reduce the risk of the patient escaping; the use of distraction techniques and items; consideration of safety concerns with regards to impulsivity, hyperactivity, and flight risk; and provision for additional staff to supervise as appropriate to meet the needs of each patient.
Prior to anesthesia, nursing and medical providers should review advanced directives, baseline pain, and comfort care needs with the patient and their family. Pre-operative anesthetic review and airway assessment should be conducted prior to the day of the scheduled procedure to allow time to have any necessary equipment and staff available for the sedation event. Unless contraindicated, chronic medications should be given on the day of anesthesia within the confines of fasting guidelines, particularly anticonvulsants and neurobehavioral medications.
While anesthesia-related airway issues are less common in Sanfilippo syndrome than in other mucopolysaccharidoses, when they do occur, they can be serious.
When preparing for anesthesia in a patient with Sanfilippo syndrome, providers should be prepared for a potentially difficult laryngoscopy and intubation [ 93 , 94 , 95 , 97 ]. If an upper airway obstruction which may complicate intubation is suspected, a pre-operative flexible endoscopy (nasopharyngolaryngoscopy) is recommended in order to inspect the upper airway. Laryngeal mask airway is a good alternative to tracheal intubation for many patients in whom a native airway is not feasible or if the procedure is short and non-invasive (eg MRI scan). General anesthesia with a native airway (without pharyngeal or laryngeal intubation) may be considered for patients with Sanfilippo syndrome; however, the use of standard airway maneuvers (eg chin lift, shoulder roll, CPAP, and oral or nasal airways) and adjuncts (eg CPAP) may be needed [ 93 , 94 , 95 , 97 ].
Somatic manifestations of MPS III
Ent and audiology considerations.
Hearing loss is common in patients with Sanfilippo syndrome and can contribute to speech delay and behavioral and learning problems [ 9 ]. Hearing loss can be conductive, sensorineural, or mixed due to a combination of dysostosis of the ossicles of the middle ear, inner ear abnormalities, and frequent otitis media and impaired neurological function [ 12 ]. To ensure early detection, ENT examination and audiologic testing should be conducted immediately after diagnosis, with a follow-up at least every 12 months and more frequently if there are recurrent episodes of otitis or suspected changes in hearing. When there is identified hearing loss or otitis media with effusion, follow-up may need to be more frequent based on the individual patient.
When detected, the early and aggressive management of hearing impairment and ear effusion should be performed to optimize language development during critical developmental windows. ENT surgery remains a fundamental therapeutic procedure for reducing the frequency and severity of ear infections, even if the interventions are not curative [ 86 , 98 ]. If a conductive type of hearing loss is detected owing to effusion in the ear (lasting more than 2 months bilaterally or 4 months unilaterally), grommets (ventilation tubes) should be inserted without delay to maximize hearing and reduce symptoms.
Audiology evaluation should include assessments of both air and bone conduction. Where hearing assessment is needed, and behavioral testing is not possible, auditory brainstem response testing under sedation or general anesthesia should be considered. Decisions on the use of hearing devices should be made in close collaboration with the family, and the hearing needs of the patient should always be clearly documented in their records and care plans with accompanying advice on communication and hearing supports, particularly in the educational setting. Standard ‘behind the ear’ hearing aids should be considered for patients with hearing loss. Challenging behavior should not be used as an excuse to dismiss a trial of hearing aids, particularly in the educational setting.
Ear disease may also present with an impairment in balance. Balance problems can significantly impact quality of life, particularly mobility, and may be overlooked in patients who are unable to communicate their symptoms effectively. Considering ear disease as a factor in emerging or worsening balance problems may uncover a potentially treatable etiology. ENT physicians can aid in specialized evaluation of these concerns.
Ophthalmologic considerations
A proportion of patients with Sanfilippo syndrome have affected eyesight, though the timing and progression of visual impairment has not been well studied to date. Pigmentary retinopathy is considered a prominent ocular manifestation in patients with Sanfilippo syndrome [ 29 ], with severity ranging from subclinical electroretinography features to moderate-to-severe clinical disease that leads to problems such as nyctalopia (night blindness) and overall decreased vision [ 99 , 100 , 101 , 102 ]. The corneas of patients with Sanfilippo syndrome type A and type B appear clear but have increased mean fibril diameter and fibril spacing [ 101 , 103 ]. Optic atrophy and disk swelling have also been reported [ 104 ].
Routine ophthalmologic examination is recommended every 12 months and more frequently if clinically indicated. Ophthalmologic assessment should include assessment of vision in both eyes, orthoptic assessment, refraction, examination of anterior and posterior segment of the eye (including examination of the cornea, retina, and optic nerve), and measurement of intraocular pressure. Patients with behavioral challenges may require examination under anesthesia, in which case the risks and benefits must be weighed.
Given that clinical signs of vision loss may be difficult to detect or absent in patients with impaired communication, input from caregivers is essential. An electroretinogram can confirm the diagnosis when retinopathy is suspected owing to symptoms of night blindness or impaired vision in low light, visual field loss or reduction in vision, or signs of pigmentary retinal change, but the benefits of knowing versus the risk of anesthesia must be weighed.
Patients with Sanfilippo syndrome and visual impairment should be provided with access to low-vision supports and services in the home, community, and educational settings. Support for vision impairment should be included in educational settings as part of their individualized educational plan.
Dental care
The dental features of patients with Sanfilippo syndrome are not well described compared with those of other MPS disorders [ 105 ], with disease-specific observations limited to generalized obliteration of pulp chambers and root canals [ 106 , 107 ]. However, patients with MPS disorders are typically considered at high risk of dental disease [ 105 ]. Therefore, basic good oral hygiene is recommended with twice-daily brushing and avoidance of drinking sugary beverages on a regular basis.
Regular dental visits, preventive fluoride applications, and dental treatment must be included in the multidisciplinary team approach. Oral health problems should be ruled out in the setting of behavioral changes, agitation, distress, changes in sleep patterns, changes in eating habits, or a change in oral sensory behaviors.
In patients who have challenges clearing food from the oral cavity or who take daily sweetened liquid medications, water should be offered or teeth wiped after meals and snacks. As brushing the teeth can be challenging in patients who have a sensory aversion to or do not understand this task, supports such as three-sided toothbrushes, bite blocks, and distraction techniques may be helpful. Dental sealants are recommended to prevent and/or arrest dental caries in primary and/or permanent molars, and they should be monitored for integrity at each dental visit and restored as indicated. If sedation is required for dental procedures, dental care should take place in a tertiary care facility with experienced anesthesia staff.
Nutritional and gastrointestinal management
Gastrointestinal disturbances are common in patients with Sanfilippo syndrome and typically include chronic or recurrent non-infectious loose stools and/or constipation [ 12 ]. Stooling issues can be a source of discomfort and distress for patients, which may manifest through an increase in behavioral disturbances, heightened sleep disturbance, or other alternative expressions of pain. To mitigate discomfort and distress, therapeutic maintenance regimens should aim for consistent and adequate stool elimination to maintain the comfort and health of the patient.
Diarrhea is typically episodic for patients with Sanfilippo syndrome but can be persistent in some individuals and can be exacerbated by frequent antibiotic treatment or recurrent infections. The management of diarrhea includes medications when needed (eg synthetic opioids to reduce gut motility). The care plan should note, particularly for all care providers in the educational and therapeutic settings, that non-infectious Sanfilippo syndrome-related diarrhea should not be a cause for exclusion from educational and therapeutic activities.
No specific dietary plan has been studied in Sanfilippo syndrome to guide dietary recommendations, outside of general advice for a healthy diet. Monitoring and restoration of micronutrient deficiencies is recommended to support metabolic functions. Patients should also be monitored for gastroesophageal reflux, which may contribute to increased behavioral distress or increased sleep disturbance. Where present, a trial of anti-reflux medication, diet modification, or a combination thereof should be considered.
Assessment of eating, drinking, and swallowing abilities should be performed by a speech–language–feeding therapist at diagnosis and then monitored at least yearly if clinically indicated. Steering committee clinicians further recommend that primary care providers elicit history regarding any safety concerns with eating, drinking, and swallowing routinely at scheduled visits, prompting further referral as needed. Clinical assessments provide the best information when conducted at mealtimes and in a variety of settings (eg home and school) to observe any accompanying behavioral and cognitive challenges around mealtimes.
Referral to a dietician is recommended for patients with Sanfilippo syndrome who have a substantially self-limited diet, experience weight loss or poor growth, have sensory needs limiting proper nutrition, or experience a decline of oromotor skills that impairs normal caloric intake within a reasonable time frame. Such referral should be made in conjunction with referral to or consultation with a speech–language–feeding specialist. Diet and fluid modifications should be made using the International Dysphagia Diet Standardization Initiative framework ( https://iddsi.org/framework/ ) in consultation with a trained speech–language–feeding therapist. In instances of inadequate nutrition via oral feeding or presence of significant risk of aspiration or history of aspiration pneumonia, placement of an enteral feeding tube should be considered jointly with the patient’s family. When red flags are present for pharyngeal dysfunction (eg cough, wet voice, or recurrent lower respiratory tract infections), the patient should be referred for a Videofluoroscopic Swallowing Study in consultation with a speech–language–feeding therapist.
Other gastrointestinal manifestations of Sanfilippo syndrome include elevations in liver enzymes (alanine aminotransferase ≤ 3.5 times upper limit of normal [ULN]; aspartate aminotransferase ≤ 1.5 × ULN) and hepatomegaly, which do not typically require intervention. Hernias of the umbilicus and inguinal area should be monitored on routine examination and may require intervention if they become problematic.
Cardiac manifestations
In rare cases, cardiac manifestations may require intervention in patients with Sanfilippo syndrome. GAG accumulation can lead to cardiomyopathy, low-grade valve disease and/or dysplastic valves, arrhythmia owing to heparan sulfate accumulation in the conduction system, and other complications that may be problematic in patients surviving to adulthood [ 108 , 109 ]. For example, at least two case studies describe adults with Sanfilippo syndrome types A and C presenting with symptomatic atrioventricular block that required implantation of a pacemaker [ 110 , 111 ].
In a study of 30 patients with Sanfilippo syndrome (n = 16 aged < 18 years), none of the individuals had significant signs or symptoms of cardiac disease, but subclinical systolic and diastolic dysfunction and valvular abnormalities were prevalent, and about 16% had a first-degree atrioventricular block on electrocardiography (ECG) [ 108 ].
All individuals with Sanfilippo syndrome should have baseline cardiac evaluation at diagnosis to include a physical exam, vital signs (eg blood pressure), echocardiogram, and ECG. Thereafter, an echocardiogram is recommended every 24 months if no abnormalities are noted at the initial echocardiogram. If abnormalities are noted on initial or subsequent echocardiograms, the frequency should increase to every 12 months.
A 12-lead ECG and rhythm strip is recommended every 12 months in patients with Sanfilippo syndrome, and as needed owing to the difficulty of assessing symptoms in these patients. If an ECG is abnormal, a Holter monitor should be placed for at least 24–48 h for a more thorough evaluation.
Management of orthopedic complications
Orthopedic complications are a source of discomfort and distress in patients with Sanfilippo syndrome, often involving changes to the hips and spine [ 112 ]. Osteonecrosis of the femoral head and hip dysplasia can be a source of particularly severe discomfort, and intervention should be considered on a case-by-case basis. Complications requiring surgical intervention include progressive scoliosis [ 112 ]. Low bone mass and vitamin D insufficiency or deficiency are prevalent, and patients with decreased mobility or a history of receiving anti-epileptic medication are at risk for osteoporosis and fractures [ 113 ].
Patients with Sanfilippo syndrome should undergo a thorough orthopedic examination at the time of diagnosis. Consensus statements additionally endorse X-ray evaluation of the hips and spine at diagnosis and every 1–2 years from 7 years of age onwards, or sooner if clinically indicated. However, after thoughtful review, the expert clinician steering committee recommends further refinement of this guidance based on accessibility of specialists and procedural risks. It is felt that for patients without overt musculoskeletal symptoms, the initial orthopedic evaluation may be performed by the primary care clinician through a close musculoskeletal exam and a baseline X-ray of the hips and spine. Weighing the risks of cumulative radiation exposure with repeated monitoring X-rays, the steering committee suggests that radiography be performed at the time of diagnosis and then repeated only as clinically indicated. Unless there is clinical suspicion, monitoring for cervical spine instability is not recommended. In addition to the routine musculoskeletal exam, annual visits should also monitor for trigger finger, genu valgus deformity, abnormal spinal curvature, femoral anteversion, and tibial torsion that do not appear to improve or are worsening with age, with referral to an orthopedic specialist as clinically indicated. Given that pain may be difficult to assess and localize in patients with cognitive impairment and behavioral disturbances, radiographic studies of the hips should be considered in the evaluation of otherwise unexplained signs of discomfort or pain.
Facilitating day-to-day activities and maintaining quality of life
Management of pain and distress.
Although the neurological features of Sanfilippo syndrome may be the primary focus of patient care, physical manifestations such as pain and discomfort due to musculoskeletal or gastrointestinal problems can further exacerbate the neurocognitive and behavioral challenges experienced by individuals with this condition [ 9 ]. Thus, after appropriate evaluation for treatable medical complications, management of pain should be a fundamental part of the care of patients with Sanfilippo syndrome, with the aim of improving their quality of life and maintaining mobility. Notably, patients with cognitive impairment may express pain or discomfort via a range of behaviors that are non-classical and individual to them [ 114 ].
There should be a low threshold for investigation of sources of pain in patients with escalating abnormal behaviors or acutely worsening sleep disturbances, or signs of significantly increased agitation. Investigation for sources of pain or discomfort may include consideration of headaches (with consideration of alterations in intracranial pressure or symptoms of normal-pressure hydrocephalus), abdominal discomfort (eg acid reflux, ulcers, intestinal gas pain, and constipation), joint disease (eg arthralgia, arthritis, and osteonecrosis of femoral head), ENT issues (eg otitis and sinusitis), and dental-related pain (Table 4 ). In the case of persistent pain, distress, or agitation for which outpatient evaluation has been unrevealing, admission to hospital for thorough efficient medical workup is recommended. This workup should include hip and spine X-ray; dental examination for signs of decay; abdominal imaging to investigate potential constipation or other obstruction; eye exam with consideration for signs of elevated intracranial or intraocular pressure; complete blood count with differential to check for infection or anemia; measurement of electrolytes; and if other investigations are not conclusive, brain imaging (MRI or computerized tomography) for ventriculomegaly, atrophy, or intracranial bleed is suggested [ 115 ]. It is acknowledged that communicating hydrocephalus (due to defective cerebrospinal fluid reabsorption) is less common in patients with Sanfilippo syndrome than in other MPS disorders and can be difficult to distinguish from brain atrophy in MRI scans [ 116 ]. However, given that patients with GAG accumulation in MPS I and MPS II also have abnormal cerebrospinal fluid reabsorption [ 117 , 118 ], it is reasonable to consider as a potential cause of pain and distress in patients with Sanfilippo syndrome and should be investigated. In the evaluation of increased intracranial pressure, evidence of papilledema may indicate elevated pressure but is not a reliable indicator of chronically elevated intracranial pressure; therefore, a normal fundus does not exclude the presence of intracranial pressure-related symptoms.
Standardized pain assessments appropriate for the patient’s cognitive level and/or caregiver proxy assessments should be included in regular follow-up visits for patients with Sanfilippo syndrome. For patients who have a limited ability to communicate, the revised Non-Communicating Children's Pain Checklist is recommended [ 119 ].
Special education, physical, occupational, speech, and complementary therapy interventions
Patients with Sanfilippo syndrome have unique needs and developmental trajectories that require careful and informed consideration throughout their period of care. Changes in neurocognition, speech, language, and motor skills may be subtle and not obvious from day to day or even month to month. Without careful and consistent monitoring of these outcomes by appropriately trained individuals, which interventions are benefiting a patient and whether alternative approaches need to be adapted are impossible to determine. In this regard, the consistent use of established measurement tools is recommended to track motor skills over time (eg the Peabody Developmental Motor Scales II, the Bayley-III motor domain and the Bruininks–Oseretsky test of motor proficiency, second edition) [ 74 ], with the selection of measures that are most appropriate for the patient [ 120 ].
Whereas for many children who do not have neurodegenerative disorders, goals for receiving supportive interventions are set based on an anticipated improvement in relevant skills, the natural course of Sanfilippo syndrome means that children beyond a certain point in their disease process will instead either lose or never acquire such skills. Therefore, supportive interventions should strive to maintain existing skill levels rather than require improvement in a patient’s abilities for them to qualify for continued access to such services. Similarly, therapeutic goals for rehabilitative therapies such as physical or speech therapy should focus on prolonging skills for as long as possible and improving quality of life and functional access to educational and social environments. Cognitive impairment and the progressive nature of Sanfilippo syndrome should not preclude an affected patient’s access to vision, hearing, behavioral, or any other support services; the patient should be recommended access to these services even after a decline in the relevant skills and abilities.
The provision of an appropriate high-quality, enriching educational environment with regular opportunities for peer engagement helps to ensure maximal developmental gains and skill maintenance [ 9 , 12 , 67 , 71 ]. Consistent routines and structured schedules can have a positive influence on the behavior and quality of life of patients with Sanfilippo syndrome. As much as possible, these patients deserve stimulation and inclusion, even when processes of deterioration have begun. Providing an individual aide in the school setting is helpful and often necessary to maintain the safety of the patient and others in the classroom, as well as to maximize the child's attention, adequately reinforce their attempts to communicate, and support them during educational activities.
Speech regression can contribute to the distress and frustration experienced by the patient and their family, with dysfluencies and speech apraxia as early warning signs of regression in patients with Sanfilippo syndrome [ 11 ]. An array of augmentative and alternative communication (AAC) methods can be used to augment, complement, or replace speech for patients with complex communication needs [ 121 ]. Such methods range from basic tools (eg picture boards and single voice output buttons) to smart devices and dedicated AAC devices that integrate hardware and software to support a patient’s communication needs. The potential benefits of these approaches should be considered on a patient-by-patient basis, noting that patients with Sanfilippo syndrome may require longer and more intensive therapy to achieve success with these methods. The above approaches should be applied to patients with Sanfilippo syndrome as early as possible (ie during maximal cognitive capacity and even prior to loss of verbal speech). AAC methods should be initiated by a trained professional on a trial basis to determine suitability and feasibility, and then generalized for use in home and educational settings as quickly as possible. The type of AAC may need to be adjusted over time per the patient’s need, ability, and level of engagement. Behavioral therapies are helpful in conjunction to increase acceptance and positively reinforce the use of these communication tools.
Regular physical therapy may reduce physical discomfort and support some aspects of mobility in patients with Sanfilippo syndrome, which can have beneficial effects on inattention or other behaviors that may be driven by pain or frustration, as well as bone health, gastrointestinal motility, avoidance of pressure sores, and enabling them to maintain access to their environment. Thus, it is our opinion that physical therapy should be considered as early as possible and be performed regularly prior to, during, and beyond the decline of gross motor skills to maintain mobility and function and reduce development of contractures. The range of motion in the upper and lower extremities should be assessed at diagnosis, on the first visit to any new physical therapy provider, and at least every 6 months. Orthotic bracing may help balance, foot and ankle positioning, and to improve and maintain gait function and mobility for longer.
To help facilitate daily activities for patients with Sanfilippo syndrome, supportive equipment needs should be discussed, and appropriate prescriptions and referrals as needed made every 6 months. A forward-looking, proactive approach is warranted to ensure that required adaptive equipment can be obtained when needed, including a wheelchair or medical stroller, stander, bath seat, activity chair, safety beds, lifts, or specialized car seats. Similarly, adaptations to the home or school environment may be needed owing to the patients’ lack of safety sense and cognitive decline with preserved motor skills.
Although patients with Sanfilippo syndrome are generally of normal weight and height for their gestational age at birth, adults with Sanfilippo syndrome are generally of short stature [ 122 , 123 ]. In a study of 182 patients with Sanfilippo syndrome in Germany, accelerated growth was observed in the first year of life, followed by decelerated growth from 4.5 to 5.0 years of age onwards. On reaching adulthood, these patients were shorter than expected based on the height of their respective parents [ 123 ]. Similarly, growth charts of patients with Sanfilippo syndrome in the Netherlands showed significantly slowed growth from 6 years of age onwards [ 122 ]. Disease-specific growth charts are important tools for tracking growth and recognizing deviation from normal and help physicians to counsel parents regarding growth expectations. Therefore, growth should be monitored and plotted on Sanfilippo syndrome-specific growth curves [ 123 ].
The onset of puberty may be advanced in patients with Sanfilippo syndrome [ 124 , 125 ]. If signs of early puberty are present, referral to a pediatric endocrinologist is warranted. The use of gonadotrophin-releasing hormone agonists is not contraindicated for patients with Sanfilippo syndrome and should be considered in consultation with the patient and their family.
Family support
Coming to terms with a diagnosis of Sanfilippo syndrome—a condition that many people may never have heard of—can be incredibly challenging for family members. Caregivers should be provided with counseling on the natural history of disease progression in the absence of a disease-modifying treatment, both at the time of diagnosis and throughout the disease course. Understanding what the expected symptoms of the disease are can help to ‘normalize’ the patient’s challenging behaviors, sleep, and other concerns during other times of high stress or changing symptom patterns [ 53 , 126 , 127 ]. However, this should not supplant the need to assess for treatable modifiers of symptoms that may improve the quality of life for the patient and their family.
Given that family members living with or caring for an individual with Sanfilippo syndrome will most likely experience significant psychological stress and social challenges, proactive intermittent assessment of caregivers’ anxiety, depression, and chronic traumatic stress with appropriate referral is warranted [ 126 ]. Patient advocacy groups provide a forum for peer-to-peer support and can facilitate the provision of services and financial aid grants available from the government and other community resources. Palliative care teams should be engaged, with regular monitoring of the service needs of the family and patient as these needs vary in intensity and type depending on the age and extent of disease progression.
While there are no approved therapies for patients with Sanfilippo syndrome, disease-specific therapies are being developed and a number of clinical trials have been attempted or are ongoing (eg intravenous and intrathecal enzyme replacement therapy, substrate reduction therapy, autologous stem cell-based lentiviral gene therapy, and adeno-associated viral vector gene therapy). In lieu of the promise provided by these novel therapies, this review aims to distill the best available guidance on how to recognize, diagnose, and care for patients with this devastating, progressive, and life-limiting disease based on the broad consensus of a multidisciplinary panel of expert clinicians from nine countries.
To our knowledge, this is the first example of a consensus guideline for the management of patients with Sanfilippo syndrome. Its development was led by a steering committee consisting of internationally renowned experts in Sanfilippo syndrome. The recommendations described here reflect the current understanding and experience of caring for patients with this condition. While guidelines support consistency in care, clinical judgment should be used to determine if deviation from the described schedule is appropriate based on the patient’s clinical history, extent of organ manifestations, variability of disease phenotype, and in collaboration with the family as to the potential burden of assessments.
It is acknowledged that a potential limitation of these guidelines is the fact that they do not fully encapsulate local variations in terminologies and different cultural systems of care, as some modifications to guideline statements were needed to ensure their applicability across the global healthcare landscape. Furthermore, given the large number of statements required to capture the available published evidence and experience of current clinical practice, not all issues and theories associated with the management of Sanfilippo syndrome could be covered within this review, although a full list of the recommendations that reached consensus are provided in Additional file 1 : Table S2. Some of the statements put forward for consideration did not reach consensus owing to variations in local practice or lack of supporting evidence. For example, consensus was not reached on the appropriateness of routine monitoring of bone density using a dual-energy X-ray absorptiometry scan to assess for fracture risk in patients with prolonged functional immobility. Similarly, routine monitoring for retinal disease via electroretinogram and/or optical coherence in patients who do not have overt visual impairment remains a subject of debate. However, we do not consider that the guidance provided in this document is affected substantially by the omission of these statements. As is common in most rare diseases, some features of the disease are not as well represented in the literature as others. In such areas, there is an increased risk of bias from expert opinion based on clinicians’ individual clinical experience. We attempted to mitigate the impact of this potential bias by including a large number of clinicians with significant experience in Sanfilippo syndrome across a wide range of specialties and geographic locations so that their collective experience would offer a more comprehensive perspective.
Family and patient burden is an important consideration in medical-decision making. The extent of burden that is experienced or anticipated by each patient and family unit is unique. Further, benefits of monitoring procedures may not be immediately evident but rather may be appreciated at a later time point when disease symptoms evolve and the team then has a baseline from which to compare, allowing for better-informed clinical decisions. In this set of guidelines, we aim to respect the autonomy of patients and families by bringing consensus recommendations for proactive care together in one document, thus allowing them to make their own informed, individual decisions regarding benefit–risk–burden calculations in consultation with their care team.
As clinical experience of managing patients with Sanfilippo syndrome continues to grow, along with our understanding of the underlying disease pathways, the strategies described in this review will most likely require updates to reflect the closing of remaining knowledge gaps. The continued study of patients with Sanfilippo syndrome via observational studies, clinical registries, and preclinical studies is essential to ensure that progress continues to be made. Ultimately, the availability of the first disease-specific therapies for Sanfilippo syndrome will result in a major transformation of the clinical landscape and prospects for the patients and families affected by this disorder. The guidance contained here should therefore be reviewed and updated regularly by a panel of appropriately qualified experts.
Conclusions
Sanfilippo syndrome is a complex neurodegenerative disease that, until now, had no published standard global clinical care guideline. This document, created through collaboration between Cure Sanfilippo Foundation (USA) and Sanfilippo Children’s Foundation (Australia), distills 178 guideline statements into an easily digestible document that provides evidence-based, expert-led recommendations. This review is intended to help the provision of consistent care to the patients and families affected by Sanfilippo syndrome, as well as facilitating interventions to improve their quality of life.
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Andrade F, Aldamiz-Echevarria L, Llarena M, Couce ML. Sanfilippo syndrome: overall review. Pediatr Int. 2015;57(3):331–8.
Article PubMed Google Scholar
Valstar MJ, Ruijter GJ, van Diggelen OP, Poorthuis BJ, Wijburg FA. Sanfilippo syndrome: a mini-review. J Inherit Metab Dis. 2008;31(2):240–52.
Article CAS PubMed Google Scholar
Khan SA, Peracha H, Ballhausen D, Wiesbauer A, Rohrbach M, Gautschi M, et al. Epidemiology of mucopolysaccharidoses. Mol Genet Metab. 2017;121(3):227–40.
Article CAS PubMed PubMed Central Google Scholar
Héron B, Mikaeloff Y, Froissart R, Caridade G, Maire I, Caillaud C, et al. Incidence and natural history of mucopolysaccharidosis type III in France and comparison with United Kingdom and Greece. Am J Med Genet A. 2011;155A(1):58–68.
Poorthuis BJ, Wevers RA, Kleijer WJ, Groener JE, de Jong JG, van Weely S, et al. The frequency of lysosomal storage diseases in The Netherlands. Human Genet. 1999;105(1–2):151–6.
Article CAS Google Scholar
Zelei T, Csetneki K, Voko Z, Siffel C. Epidemiology of Sanfilippo syndrome: results of a systematic literature review. Orphanet J Rare Dis. 2018;13(1):53.
Article PubMed PubMed Central Google Scholar
Malm G, Lund AM, Mansson JE, Heiberg A. Mucopolysaccharidoses in the Scandinavian countries: incidence and prevalence. Acta Paediatr. 2008;97(11):1577–81.
Bax MC, Colville GA. Behaviour in mucopolysaccharide disorders. Arch Dis Child. 1995;73(1):77–81.
Escolar ML, Jones SA, Shapiro EG, Horovitz DDG, Lampe C, Amartino H. Practical management of behavioral problems in mucopolysaccharidoses disorders. Mol Genet Metab. 2017;122S:35–40.
Fraser J, Wraith JE, Delatycki MB. Sleep disturbance in mucopolysaccharidosis type III (Sanfilippo syndrome): a survey of managing clinicians. Clin Genet. 2002;62(5):418–21.
Valstar MJ, Marchal JP, Grootenhuis M, Colland V, Wijburg FA. Cognitive development in patients with Mucopolysaccharidosis type III (Sanfilippo syndrome). Orphanet J Rare Dis. 2011;6:43.
Wagner VF, Northrup H. Mucopolysaccharidosis type III. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Mirzaa G, et al., editors. Gene Reviews ® . Seattle (WA) 1993.
Valstar MJ, Bertoli-Avella AM, Wessels MW, Ruijter GJ, de Graaf B, Olmer R, et al. Mucopolysaccharidosis type IIID: 12 new patients and 15 novel mutations. Hum Mutat. 2010;31(5):E1348–60.
CAS PubMed Google Scholar
Valstar MJ, Bruggenwirth HT, Olmer R, Wevers RA, Verheijen FW, Poorthuis BJ, et al. Mucopolysaccharidosis type IIIB may predominantly present with an attenuated clinical phenotype. J Inherit Metab Dis. 2010;33(6):759–67.
Valstar MJ, Neijs S, Bruggenwirth HT, Olmer R, Ruijter GJ, Wevers RA, et al. Mucopolysaccharidosis type IIIA: clinical spectrum and genotype–phenotype correlations. Ann Neurol. 2010;68(6):876–87.
Meyer A, Kossow K, Gal A, Muhlhausen C, Ullrich K, Braulke T, et al. Scoring evaluation of the natural course of mucopolysaccharidosis type IIIA (Sanfilippo syndrome type A). Pediatrics. 2007;120(5):e1255–61.
Ruijter GJ, Valstar MJ, van de Kamp JM, van der Helm RM, Durand S, van Diggelen OP, et al. Clinical and genetic spectrum of Sanfilippo type C (MPS IIIC) disease in The Netherlands. Mol Genet Metab. 2008;93(2):104–11.
Wijburg FA, Wegrzyn G, Burton BK, Tylki-Szymanska A. Mucopolysaccharidosis type III (Sanfilippo syndrome) and misdiagnosis of idiopathic developmental delay, attention deficit/hyperactivity disorder or autism spectrum disorder. Acta Paediatr. 2013;102(5):462–70.
Moog U, van Mierlo I, van Schrojenstein HMJ, Valk L, Spaapen L, Maaskant MA, Curfs LMG. Is Sanfilippo type B in your mind when you see adults with mental retardation and behavioral problems? Am J Med Genet Part C Semin Med Genet. 2007;145C(3):293–301.
Diamond IR, Grant RC, Feldman BM, Pencharz PB, Ling SC, Moore AM, et al. Defining consensus: a systematic review recommends methodologic criteria for reporting of Delphi studies. J Clin Epidemiol. 2014;67(4):401–9.
Buhrman D, Thakkar K, Poe M, Escolar ML. Natural history of Sanfilippo syndrome type A. J Inherit Metab Dis. 2014;37(3):431–7.
Delgadillo V, del Mar OM, Gort L, Coll MJ, Pineda M. Natural history of Sanfilippo syndrome in Spain. Orphanet J Rare Dis. 2013;8:189.
Kuiper GA, Meijer OLM, Langereis EJ, Wijburg FA. Failure to shorten the diagnostic delay in two ultra-orphan diseases (mucopolysaccharidosis types I and III): potential causes and implications. Orphanet J Rare Dis. 2018;13(1):2.
Vieira T, Schwartz I, Munoz V, Pinto L, Steiner C, Ribeiro M, et al. Mucopolysaccharidoses in Brazil: what happens from birth to biochemical diagnosis? Am J Med Genet A. 2008;146A(13):1741–7.
Shapiro E, King K, Ahmed A, Rudser K, Rumsey R, Yund B, et al. The neurobehavioral phenotype in mucopolysaccharidosis type IIIB: an exploratory study. Mol Genet Metab Rep. 2016;6:41–7.
Wolfenden C, Wittkowski A, Jones SA, Rust S, Hare DJ. Autism spectrum disorder symptomatology in children with mucopolysaccharide disease type III. Br J Learn Disabil. 2019;47:5–11.
Article Google Scholar
Escolar M, Bradshaw J, Tharp Byers V, Giugliani R, Golightly L, Marques Lourenço C, et al. Development of a clinical algorithm for the early diagnosis and mucopolysaccharidosis III. J Inborn Errors Metab Screen. 2020;8: e20200002.
Kelly KB, Ponsky TA. Pediatric abdominal wall defects. Surg Clin North Am. 2013;93(5):1255–67.
Nijmeijer SCM, van den Born LI, Kievit AJA, Stepien KM, Langendonk J, Marchal JP, et al. The attenuated end of the phenotypic spectrum in MPS III: from late-onset stable cognitive impairment to a non-neuronopathic phenotype. Orphanet J Rare Dis. 2019;14(1):249.
Bodamer OA, Giugliani R, Wood T. The laboratory diagnosis of mucopolysaccharidosis III (Sanfilippo syndrome): a changing landscape. Mol Genet Metab. 2014;113(1–2):34–41.
Lehman TJ, Miller N, Norquist B, Underhill L, Keutzer J. Diagnosis of the mucopolysaccharidoses. Rheumatology. 2011;50(Suppl 5):v41–8.
de Jong JG, Wevers RA, Liebrand-van Sambeek R. Measuring urinary glycosaminoglycans in the presence of protein: an improved screening procedure for mucopolysaccharidoses based on dimethylmethylene blue. Clin Chem. 1992;38(6):803–7.
Komosinska-Vassev K, Blat D, Olczyk P, Szeremeta A, Jura-Poltorak A, Winsz-Szczotka K, et al. Urinary glycosaminoglycan (uGAG) excretion in healthy pediatric and adolescent population. Clin Biochem. 2014;47(13–14):1341–3.
Whitley CB, Draper KA, Dutton CM, Brown PA, Severson SL, France LA. Diagnostic test for mucopolysaccharidosis. II. Rapid quantification of glycosaminoglycan in urine samples collected on a paper matrix. Clin Chem. 1989;35(10):2074–81.
Whitley CB, Ridnour MD, Draper KA, Dutton CM, Neglia JP. Diagnostic test for mucopolysaccharidosis. I. Direct method for quantifying excessive urinary glycosaminoglycan excretion. Clin Chem. 1989;35(3):374–9.
Byers S, Rozaklis T, Brumfield LK, Ranieri E, Hopwood JJ. Glycosaminoglycan accumulation and excretion in the mucopolysaccharidoses: characterization and basis of a diagnostic test for MPS. Mol Genet Metab. 1998;65(4):282–90.
Giudici TA, Sunico H, Blaskovics M. Diagnostic screening for mucopolysaccharidoses types I-VII by fluorophore-labelled carbohydrate PAGE. J Inherit Metab Dis. 1996;19(2):263–6.
Kubaski F, Osago H, Mason RW, Yamaguchi S, Kobayashi H, Tsuchiya M, et al. Glycosaminoglycans detection methods: applications of mass spectrometry. Mol Genet Metab. 2017;120(1–2):67–77.
Fedele AO. Sanfilippo syndrome: causes, consequences, and treatments. Appl Clin Genet. 2015;8:269–81.
Klein U, Kresse H, von Figura K. Sanfilippo syndrome type C: deficiency of acetyl-CoA:alpha-glucosaminide N -acetyltransferase in skin fibroblasts. Proc Natl Acad Sci USA. 1978;75(10):5185–9.
Kresse H. Mucopolysaccharidosis 3 A (Sanfilippo A disease): deficiency of a heparin sulfamidase in skin fibroblasts and leucocytes. Biochem Biophys Res Commun. 1973;54(3):1111–8.
von Figura K. Human alpha- N -acetylglucosaminidase. 1. Purification and properties. Eur J Biochem. 1977;80(2):525–33.
Karpova EA, Voznyi Ya V, Keulemans JL, Hoogeveen AT, Winchester B, Tsvetkova IV, et al. A fluorimetric enzyme assay for the diagnosis of Sanfilippo disease type A (MPS IIIA). J Inherit Metab Dis. 1996;19(3):278–85.
Yi F, Hong X, Kumar AB, Zong C, Boons GJ, Scott CR, et al. Detection of mucopolysaccharidosis III-A (Sanfilippo Syndrome-A) in dried blood spots (DBS) by tandem mass spectrometry. Mol Genet Metab. 2018;125(1–2):59–63.
Khaledi H, Gelb MH. Tandem mass spectrometry enzyme assays for multiplex detection of 10-mucopolysaccharidoses in dried blood spots and fibroblasts. Anal Chem. 2020;92(17):11721–7.
Zakaria R, Allen KJ, Koplin JJ, Roche P, Greaves RF. Advantages and challenges of dried blood spot analysis by mass spectrometry across the total testing process. EJIFCC. 2016;27(4):288–317.
CAS PubMed PubMed Central Google Scholar
Wolfe BJ, Ghomashchi F, Kim T, Abam CA, Sadilek M, Jack R, et al. New substrates and enzyme assays for the detection of mucopolysaccharidosis III (Sanfilippo Syndrome) types A, B, C, and D by tandem mass spectrometry. Bioconjug Chem. 2012;23(3):557–64.
Selmer KK, Gilfillan GD, Stromme P, Lyle R, Hughes T, Hjorthaug HS, et al. A mild form of mucopolysaccharidosis IIIB diagnosed with targeted next-generation sequencing of linked genomic regions. Eur J Human Genet. 2012;20(1):58–63.
Zeng Q, Fan Y, Wang L, Huang Z, Gu X, Yu Y. Molecular defects identified by whole exome sequencing in a child with atypical mucopolysaccharidosis IIIB. J Pediatr Endocrinol Metab. 2017;30(4):463–9.
Arunkumar N, Langan TJ, Stapleton M, Kubaski F, Mason RW, Singh R, et al. Newborn screening of mucopolysaccharidoses: past, present, and future. J Human Genet. 2020;65(7):557–67.
Hatzmann J, Heymans HS, Ferrer-i-Carbonell A, van Praag BM, Grootenhuis MA. Hidden consequences of success in pediatrics: parental health-related quality of life: results from the Care Project. Pediatrics. 2008;122(5):e1030–8.
Kuratsubo I, Suzuki Y, Orii KO, Kato T, Orii T, Kondo N. Psychological status of patients with mucopolysaccharidosis type II and their parents. Pediatr Int. 2009;51(1):41–7.
Malcolm C, Hain R, Gibson F, Adams S, Anderson G, Forbat L. Challenging symptoms in children with rare life-limiting conditions: findings from a prospective diary and interview study with families. Acta Paediatr. 2012;101(9):985–92.
Somanadhan S, Larkin PJ. Parents’ experiences of living with, and caring for children, adolescents and young adults with mucopolysaccharidosis (MPS). Orphanet J Rare Dis. 2016;11(1):138.
Weber SL, Segal S, Packman W. Inborn errors of metabolism: psychosocial challenges and proposed family systems model of intervention. Mol Genet Metab. 2012;105(4):537–41.
Kazak AE, Kassam-Adams N, Schneider S, Zelikovsky N, Alderfer MA, Rourke M. An integrative model of pediatric medical traumatic stress. J Pediatr Psychol. 2006;31(4):343–55.
Pinquart M. Posttraumatic stress symptoms and disorders in parents of children and adolescents with chronic physical illnesses: a meta-analysis. J Trauma Stress. 2019;32(1):88–96.
Stuber ML, Shemesh E. Post-traumatic stress response to life-threatening illnesses in children and their parents. Child Adolesc Psychiatr Clin N Am. 2006;15(3):597–609.
Conijn T, Nijmeijer SCM, van Oers HA, Wijburg FA, Haverman L. Psychosocial functioning in parents of MPS III patients. JIMD Rep. 2019;44:33–41.
Selimbasic Z, Sinanovic O, Avdibegovic E. Psychosocial problems among children of parents with posttraumatic stress disorder. Med Arch. 2012;66(5):304–8.
Conijn T, Haverman L, Wijburg FA, De Roos C. Reducing posttraumatic stress in parents of patients with a rare inherited metabolic disorder using eye movement desensitization and reprocessing therapy: a case study. Orphanet J Rare Dis. 2021;16(1):126.
Shapiro EG, Eisengart JB. The natural history of neurocognition in MPS disorders: a review. Mol Genet Metab. 2021;133(1):8–34.
Shapiro EG, Jones SA, Escolar ML. Developmental and behavioral aspects of mucopolysaccharidoses with brain manifestations – neurological signs and symptoms. Mol Genet Metab. 2017;122S:1–7.
Shapiro EG, Nestrasil I, Delaney KA, Rudser K, Kovac V, Nair N, et al. A prospective natural history study of mucopolysaccharidosis type IIIA. J Pediatr. 2016;170(278–87):e1–4.
Google Scholar
Janzen D, Delaney KA, Shapiro EG. Cognitive and adaptive measurement endpoints for clinical trials in mucopolysaccharidoses types I, II, and III: a review of the literature. Mol Genet Metab. 2017;121(2):57–69.
Colville GA, Watters JP, Yule W, Bax M. Sleep problems in children with Sanfilippo syndrome. Dev Med Child Neurol. 1996;38(6):538–44.
Cross EM, Hare DJ. Behavioural phenotypes of the mucopolysaccharide disorders: a systematic literature review of cognitive, motor, social, linguistic and behavioural presentation in the MPS disorders. J Inherit Metab Dis. 2013;36(2):189–200.
Gilkes JA, Heldermon CD. Mucopolysaccharidosis III (Sanfilippo syndrome) – disease presentation and experimental therapies. Pediatr Endocrinol Rev. 2014;12(Suppl 1):133–40.
PubMed Google Scholar
Rumsey RK, Rudser K, Delaney K, Potegal M, Whitley CB, Shapiro E. Acquired autistic behaviors in children with mucopolysaccharidosis type IIIA. J Pediatr. 2014;164(5):1147–51.e1.
Potegal M, Yund B, Rudser K, Ahmed A, Delaney K, Nestrasil I, et al. Mucopolysaccharidosis type IIIA presents as a variant of Kluver-Bucy syndrome. J Clin Exp Neuropsychol. 2013;35(6):608–16.
Cleary MA, Wraith JE. Management of mucopolysaccharidosis type III. Arch Dis Child. 1993;69(3):403–6.
Shapiro EG, Escolar ML, Delaney KA, Mitchell JJ. Assessments of neurocognitive and behavioral function in the mucopolysaccharidoses. Mol Genet Metab. 2017;122S:8–16.
van der Lee JH, Morton J, Adams HR, Clarke L, Ebbink BJ, Escolar ML, et al. Cognitive endpoints for therapy development for neuronopathic mucopolysaccharidoses: results of a consensus procedure. Mol Genet Metab. 2017;121(2):70–9.
van der Lee JH, Morton J, Adams HR, Clarke L, Eisengart JB, Escolar ML, et al. Therapy development for the mucopolysaccharidoses: updated consensus recommendations for neuropsychological endpoints. Mol Genet Metab. 2020;131(1–2):181–96.
Schreck KA, Helsel C, Paxon A, Weston K, Daniels M. Regression trends and treatment effectiveness to improve quality of life for a pre-adolescent girl with MPS IIIA. J Develop Physical Dis. 2018;30:545–58.
Scarpa M, Lourenco CM, Amartino H. Epilepsy in mucopolysaccharidosis disorders. Mol Genet Metab. 2017;122S:55–61.
Bonanni P, Volzone A, Randazzo G, Antoniazzi L, Rampazzo A, Scarpa M, et al. Nocturnal frontal lobe epilepsy in mucopolysaccharidosis. Brain Dev. 2014;36(9):826–9.
Barone R, Nigro F, Triulzi F, Musumeci S, Fiumara A, Pavone L. Clinical and neuroradiological follow-up in mucopolysaccharidosis type III (Sanfilippo syndrome). Neuropediatrics. 1999;30(5):270–4.
Kriel RL, Hauser WA, Sung JH, Posalaky Z. Neuroanatomical and electroencephalographic correlations in Sanfilippo syndrome, type A. Arch Neurol. 1978;35(12):838–43.
Barone R, Cocuzza MD, Guida C, Miano G, Sofia V, Fiumara A. EEG features in patients with mucopolysaccharidoses III at different disease stages. J Inherit Metab Dis. 2016;39(Suppl 1):S186.
Grioni D, Contri M, Furlan F, Rigoldi M, Rovelli A, Parini R. Epilepsy in mucopolysaccharidosis: clinical features and outcome. In: Parini R, Andria G, editors. Lysosomal storage diseases: early diagnosis and new treatments. Montrouge: John Libbey Eurotext; 2010. p. 73–80.
Kaplan PW. Prognosis in nonconvulsive status epilepticus. Epileptic Disord. 2000;2(4):185–93.
Barone R, Pellico A, Pittala A, Gasperini S. Neurobehavioral phenotypes of neuronopathic mucopolysaccharidoses. Ital J Pediatr. 2018;44(Suppl 2):121.
Mahon LV, Lomax M, Grant S, Cross E, Hare DJ, Wraith JE, et al. Assessment of sleep in children with mucopolysaccharidosis type III. PLoS One. 2014;9(2): e84128.
Abramova AA, Attarian HP, Dolgova SM, Belyakova-Bodina AI, Iakovenko EV, Broutian AG. Sleep-related hypermotor epilepsy in a patient with mucopolysaccharidosis type III. Sleep Sci. 2021;14:97–100.
Bianchi PM, Gaini R, Vitale S. ENT and mucopolysaccharidoses. Ital. J Pediatr. 2018;44(Suppl 2):127.
Leighton SE, Papsin B, Vellodi A, Dinwiddie R, Lane R. Disordered breathing during sleep in patients with mucopolysaccharidoses. Int J Pediatr Otorhinolaryngol. 2001;58(2):127–38.
Mumford RA, Mahon LV, Jones S, Bigger B, Canal M, Hare DJ. Actigraphic investigation of circadian rhythm functioning and activity levels in children with mucopolysaccharidosis type III (Sanfilippo syndrome). J Neurodev Disord. 2015;7(1):31.
Fraser J, Gason AA, Wraith JE, Delatycki MB. Sleep disturbance in Sanfilippo syndrome: a parental questionnaire study. Arch Dis Child. 2005;90(12):1239–42.
Lavery C, Hendriksz CJ, Jones SA. Mortality in patients with Sanfilippo syndrome. Orphanet J Rare Dis. 2017;12(1):168.
Bachrach SJ, Walter RS, Trzcinski K. Use of glycopyrrolate and other anticholinergic medications for sialorrhea in children with cerebral palsy. Clin Pediatr (Phila). 1998;37(8):485–90.
Moores C, Rogers JG, McKenzie IM, Brown TC. Anaesthesia for children with mucopolysaccharidoses. Anaesth Intensive Care. 1996;24(4):459–63.
Cingi EC, Beebe DS, Whitley CB, Belani KG. Anesthetic care and perioperative complications in children with Sanfilipo syndrome type A. Paediatr Anaesth. 2016;26(5):531–8.
Cohen MA, Stuart GM. Delivery of anesthesia for children with mucopolysaccharidosis type III (Sanfilippo syndrome): a review of 86 anesthetics. Paediatr Anaesth. 2017;27(4):363–9.
Kamata M, McKee C, Truxal KV, Flanigan KM, McBride KL, Aylward SC, et al. General anesthesia with a native airway for patients with mucopolysaccharidosis type III. Paediatr Anaesth. 2017;27(4):370–6.
Ammer LS, Dohrmann T, Muschol NM, Lang A, Breyer SR, Ozga AK, et al. Disease manifestations in mucopolysaccharidoses and their impact on anaesthesia-related complications a retrospective analysis of 99 patients. J Clin Med. 2021;10(16):3518.
Scaravilli V, Zanella A, Ciceri V, Bosatra M, Flandoli C, La Bruna A, et al. Safety of anesthesia for children with mucopolysaccharidoses: a retrospective analysis of 54 patients. Paediatr Anaesth. 2018;28(5):436–42.
Mesolella M, Cimmino M, Cantone E, Marino A, Cozzolino M, Della Casa R, et al. Management of otolaryngological manifestations in mucopolysaccharidoses: our experience. Acta Otorhinolaryngol Ital. 2013;33(4):267–72.
Caruso RC, Kaiser-Kupfer MI, Muenzer J, Ludwig IH, Zasloff MA, Mercer PA. Electroretinographic findings in the mucopolysaccharidoses. Ophthalmology. 1986;93(12):1612–6.
Del Monte MA, Maumenee IH, Green WR, Kenyon KR. Histopathology of Sanfilippo’s syndrome. Arch Ophthalmol. 1983;101(8):1255–62.
François J. Ocular manifestations of the mucopolysaccharidoses. Ophthalmologica. 1974;169(5):345–61.
Wilkin J, Kerr NC, Byrd KW, Ward JC, Iannaccone A. Characterization of a case of pigmentary retinopathy in Sanfilippo syndrome type IIIA associated with compound heterozygous mutations in the SGSH gene. Ophthalmic Genet. 2016;37(2):217–27.
Alroy J, Haskins M, Birk DE. Altered corneal stromal matrix organization is associated with mucopolysaccharidosis I III and VI Exp. Eye Res. 1999;68(5):523–30.
Collins ML, Traboulsi EI, Maumenee IH. Optic nerve head swelling and optic atrophy in the systemic mucopolysaccharidoses. Ophthalmology. 1990;97(11):1445–9.
Yoon JH, Lee HI, Jang JH, Choi SH, Chang HS, Hwang YC, et al. Oral manifestation and root canal therapy of the patient with mucopolysaccharidosis. Restor Dent Endod. 2019;44(2): e14.
Mellara Tde S, Azevedo DT, Faria G, Nelson Filho P, Queiroz AM, Brentegani LG. Dental findings and management in a mucopolysaccharidosis type IIIB patient. J Dent Child (Chic). 2012;79(3):176–80.
Webman MS, Hirsch SA, Webman H, Stanley HR. Obliterated pulp cavities in the Sanfilippo syndrome (mucopolysaccharidosis III). Oral Surg Oral Med Oral Pathol. 1977;43(5):734–8.
Nijmeijer SCM, de Bruin-Bon R, Wijburg FA, Kuipers IM. Cardiac disease in mucopolysaccharidosis type III. J Inherit Metab Dis. 2019;42(2):276–85.
Wilhelm CM, Truxal KV, McBride KL, Kovalchin JP, Flanigan KM. Natural history of echocardiographic abnormalities in mucopolysaccharidosis III. Mol Genet Metab. 2018;124(2):131–4.
Kato R, Miyahara H, Kawano T, Matsuzuka A, Noda K, Izumi T. Heparan sulfate storage in the cardiac conduction system triggers atrioventricular block. Brain Dev. 2017;39(5):418–21.
Misumi I, Chikazawa S, Ishitsu T, Higuchi S, Shimazu T, Ikeda C, et al. Atrioventricular block and diastolic dysfunction in a patient with Sanfilippo C. Intern Med. 2010;49(21):2313–6.
White KK, Karol LA, White DR, Hale S. Musculoskeletal manifestations of Sanfilippo syndrome (mucopolysaccharidosis type III). J Pediatr Orthop. 2011;31(5):594–8.
Nur BG, Nur H, Mihci E. Bone mineral density in patients with mucopolysaccharidosis type III. J Bone Miner Metab. 2017;35(3):338–43.
Hauer J, Houtrow AJ. Pain assessment and treatment in children with significant impairment of the central nervous system. Pediatrics. 2017;139(6):e20171002.
Truxal KV, Fu H, McCarty DM, McNally KA, Kunkler KL, Zumberge NA, et al. A prospective one-year natural history study of mucopolysaccharidosis types IIIA and IIIB: implications for clinical trial design. Mol Genet Metab. 2016;119(3):239–48.
Zafeiriou DI, Batzios SP. Brain and spinal MR imaging findings in mucopolysaccharidoses: a review. Am J Neuroradiol. 2013;34(1):5–13.
Kulkarni MV, Williams JC, Yeakley JW, Andrews JL, McArdle CB, Narayana PA, et al. Magnetic resonance imaging in the diagnosis of the cranio-cervical manifestations of the mucopolysaccharidoses. Magn Reson Imaging. 1987;5(5):317–23.
Kwee RM, Kwee TC. Virchow-Robin spaces at MR imaging. Radiographics. 2007;27(4):1071–86.
Breau LM, McGrath PJ, Camfield CS, Finley GA. Psychometric properties of the non-communicating children’s pain checklist-revised. Pain. 2002;99(1–2):349–57.
Guarany NR, Schwartz IV, Guarany FC, Giugliani R. Functional capacity evaluation of patients with mucopolysaccharidosis. J Pediatr Rehabil Med. 2012;5(1):37–46.
Elsahar Y, Hu S, Bouazza-Marouf K, Kerr D, Mansor A. Augmentative and alternative communication (AAC) advances: a review of configurations for individuals with a speech disability. Sensors. 2019;19(8):1911.
Article PubMed Central Google Scholar
de Ruijter J, Broere L, Mulder MF, van der Ploeg AT, Rubio-Gozalbo ME, Wortmann SB, et al. Growth in patients with mucopolysaccharidosis type III (Sanfilippo disease). J Inherit Metab Dis. 2014;37(3):447–54.
Muschol NM, Pape D, Kossow K, Ullrich K, Arash-Kaps L, Hennermann JB, et al. Growth charts for patients with Sanfilippo syndrome (mucopolysaccharidosis type III). Orphanet J Rare Dis. 2019;14(1):93.
Concolino D, Muzzi G, Pisaturo L, Piccirillo A, Di Natale P, Strisciuglio P. Precocious puberty in Sanfilippo IIIA disease: diagnosis and follow-up of two new cases. Eur J Med Genet. 2008;51(5):466–71.
Tylki-Szymanska A, Metera M. Precocious puberty in three boys with Sanfilippo A (mucopolysaccharidosis III A). J Pediatr Endocrinol Metab. 1995;8(4):291–3.
Grant S, Cross E, Wraith JE, Jones S, Mahon L, Lomax M, et al. Parental social support, coping strategies, resilience factors, stress, anxiety and depression levels in parents of children with MPS III (Sanfilippo syndrome) or children with intellectual disabilities (ID). J Inherit Metab Dis. 2013;36(2):281–91.
Nidiffer FD, Kelly TE. Developmental and degenerative patterns associated with cognitive, behavioural and motor difficulties in the Sanfilippo syndrome: an epidemiological study. J Ment Defic Res. 1983;27(Pt 3):185–203.
Download references
Acknowledgements
The authors are grateful to Jonathan Morton PhD of Comradis Limited (Oxford, UK) for his assistance in writing this manuscript, funded by Cure Sanfilippo Foundation, and to the many clinicians globally who participated in the online survey to establish consensus.
Funding to support development of these consensus guidelines was provided in part by Global Genes, BioMarin Pharmaceutical Inc, Cure Sanfilippo Foundation, and Sanfilippo Children’s Foundation. Global Genes and BioMarin were not involved in any stages of the process and did not influence the design or content of the resulting guidance statements or manuscript .
Author information
Authors and affiliations.
Department of Pediatrics, International Center for Lysosomal Disorders (ICLD), University Medical Center Hamburg-Eppendorf, Hamburg, Germany
Nicole Muschol
DASA, Federal University of Rio Grande do Sul (UFRGS), Hospital de Clinicas de Porto Alegre (HCPA), Casa dos Raros, Porto Alegre, Brazil
Roberto Giugliani
University of Manchester, Manchester, UK
Simon A. Jones
University of North Carolina at Chapel Hill, Chapel Hill, NC, USA
Joseph Muenzer
Department of Neurology and Clinical Neurophysiology, Women’s and Children’s Health Network and the Discipline of Paediatrics, University of Adelaide, Adelaide, Australia
Nicholas J. C. Smith
University of Minnesota, Minneapolis, MN, USA
Chester B. Whitley
Sanfilippo Children’s Foundation, Freshwater, NSW, Australia
Megan Donnell, Kristina Elvidge & Lisa Melton
Cure Sanfilippo Foundation, Columbia, SC, USA
Elise Drake & Cara O’Neill
You can also search for this author in PubMed Google Scholar
Contributions
MD and CO’N devised the project and the main conceptual ideas and were in charge of overall direction and planning. KE performed the literature review and devised, distributed, and analyzed the survey. CO’N revised guideline statements and ED, LM, and KE provided administrative and technical support. CO’N, MD, and ED additionally provided the caregiver perspective. RG, SAJ, JM, NM, NJCS, and CBW were members of the steering committee who guided the direction of the project. They recommended members for the Guideline Development Group, reviewed guideline statements, and identified and filled gaps in recommendations. CO’N and NM took the lead in writing the manuscript with help from JM. All authors provided critical feedback and helped shape the research, analysis, and manuscript.
Corresponding author
Correspondence to Cara O’Neill .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Competing interests.
Nicole Muschol, Roberto Giugliani, Joseph Muenzer, Chester B. Whitley, Nicholas J. C. Smith, and Simon A. Jones have no competing interests in relation to this manuscript. Megan Donnell is on the Board of Sanfilippo Children's Foundation and has no competing interests in relation to this manuscript. Kristina Elvidge and Lisa Melton are employees of Sanfilippo Children’s Foundation and have no competing interests in relation to this manuscript. Cara O’Neill is an employee of Cure Sanfilippo Foundation and has no competing interests in relation to this manuscript. Elise Drake is a volunteer of Cure Sanfilippo Foundation and has no competing interests in relation to this manuscript.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
. Supplemental methods and results.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Muschol, N., Giugliani, R., Jones, S.A. et al. Sanfilippo syndrome: consensus guidelines for clinical care. Orphanet J Rare Dis 17 , 391 (2022). https://doi.org/10.1186/s13023-022-02484-6
Download citation
Received : 07 April 2022
Accepted : 15 August 2022
Published : 27 October 2022
DOI : https://doi.org/10.1186/s13023-022-02484-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Mucopolysaccharidosis type III
- Sanfilippo syndrome
- Recommendations
Orphanet Journal of Rare Diseases
ISSN: 1750-1172
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Int J Emerg Med

Clinical care review systems in healthcare: a systematic review
Laura e. walker.
1 Department of Emergency Medicine and Health Sciences Research, Mayo Clinic, 200 First St. SW, Rochester, MN 55905 USA
David M. Nestler
Torrey a. laack, casey m. clements, patricia j. erwin.
2 Mayo Clinic Libraries and Health Sciences Research, Mayo Clinic, Rochester, MN USA
Lori Scanlan-Hanson
M. fernanda bellolio, associated data.
Clinical care review is the process of retrospectively examining potential errors or gaps in medical care, aiming for future practice improvement. The objective of our systematic review is to identify the current state of care review reported in peer-reviewed publications and to identify domains that contribute to successful systems of care review.
A librarian designed and conducted a comprehensive literature search of eight electronic databases. We evaluated publications from January 1, 2000, through May 31, 2016, and identified common domains for care review. Sixteen domains were identified for further abstraction.
We found that there were few publications that described a comprehensive care review system and more focus on individual pathways within the overall systems. There is inconsistent inclusion of the identified domains of care review.
While guidelines for some aspects of care review exist and have gained traction, there is no comprehensive standardized process for care review with widespread implementation.
Electronic supplementary material
The online version of this article (10.1186/s12245-018-0166-y) contains supplementary material, which is available to authorized users.
Clinical care review is the process of retrospectively examining potential errors or gaps in medical care, with a goal of future practice improvement. This goes by many different names, sometimes with different audiences or case types, including peer review, adverse event review, sentinel event review, and root cause analysis. The concept of care review is widely accepted and encouraged among safety and quality healthcare leaders. However, a paucity of literature exists discussing and describing the current state of clinical care review.
The challenges and risks of contemporary medical care are well described. Medical error and its resulting outcomes have been defined and measured in many different ways, leading to varying quantifications of the effects [ 1 ]. The Institute of Medicine’s (IOM) 1999 report entitled “To Err is Human” [ 2 ] estimated that as many as 44,000 to 98,000 deaths annually in the USA occur as a result of medical error. Publication of “To Err is Human” was a landmark event in the recognition of the role of adverse events in medical care in the USA. This represents a shift in the focus on adverse events to look toward systems issues as a cause or error and a call to identify and act to prevent medical error. The National Quality Foundation estimates that, in 2010, medical errors affected 15.5% of Medicare beneficiaries, with nearly half of these errors considered preventable [ 3 ]. More recently, Makary and Daniel estimated that as many as 250,000 deaths per year in the USA are due to medical error, making it the third leading cause of death by their estimation [ 1 ]. Review of adverse events allows for investigation into, and classification of the causes of, the event and presents an opportunity to modify systems and behaviors to prevent future similar errors. As a part of the strategic approach for increasing safety, the IOM’s “To Err is Human” recommended “Identifying and learning from errors by ... encouraging health care organizations and practitioners to develop and participate in voluntary reporting systems.” They went on to say “Such systems can focus on a much broader set of errors, mainly those that do no or minimal harm, and help detect system weaknesses that can be fixed before the occurrence of serious harm, thereby providing rich information to health care organizations in support of their quality improvement efforts” [ 1 ].
Given the long standing call for clinical care review, with limited literature to inform care review systems, we conducted a qualitative systematic review to identify characteristics discussed in existing models for care review. The objectives are to (1) describe the current state of care review and (2) identify elements from published care review systems that contribute to their success. This systematic review will allow for a more complete evaluation of the current state of clinical care review and will identify areas for future scholarly activity.
This is a qualitative systematic review of studies describing and evaluating care review systems. This study was exempt from our IRB review. This report adheres to the recommendations made in the preferred reporting items for systematic reviews (PRISMA) statement [ 4 ]. A protocol was written before the beginning of the investigation.
We included original research studies with any methodological design including cohort studies, case controls, and randomized trials, as well as commentaries, narrative reviews, letters to the editor, and abstracts in peer-reviewed journals that reported models for care review. Search results were limited to publications after January 1, 2000, to focus on publications since the release of “To Err is Human” [ 1 ]—a turning point in the way adverse events are analyzed and regarded. In choosing relevant publications, some articles described their process as the main purpose of the article, while others incidentally described a care review process, while instead focusing on a specific intervention or aspect of their mechanism for review. Either was acceptable, as they both shed light on a review system for analysis.
All types of patients and hospital settings were included, as well as recommendations from professional organizations and companies. This study’s investigators are physicians with involvement in quality improvement, adverse event identification and management, patient safety, and leadership of committees for clinical care review.
A senior expert librarian (P.E.) designed and conducted a comprehensive search of eight electronic databases, including Ovid MEDLINE, Ovid EMBASE, EBSCO CINAHL, Ovid CENTRAL, Ovid Cochrane Database of Systematic Reviews, Web of Science, and Scopus. Our search was done on June 10, 2016, and includes publications from January 1, 2000, through May 31, 2016. We included published conference abstracts in our search. There was no language restriction to the search strategy. Bibliography and reference lists of the articles obtained through database search were reviewed to identify additional publications for inclusion. The search strategy can be found in the Additional file 1 .
Qualitative assessment and data abstraction process
Two investigators (L.W. and D.N.) identified common domains in the initial literature review to determine which data to abstract, and included additional variables determined to be clinically important based on their experience in the clinical care review process and practice improvement. Domains included were description of systems improvement, educational output and feedback, description of a standardized process and referral mechanism, consideration of the case outcome, deliverables of the review system including non-punitive process and recognition of excellence, multidisciplinary involvement, dedicated process leadership, reviewer training, case blinding/anonymity, and implementation of improvement recommendations by the investigating group. These are further described in Table 1 .
Descriptions of the 16 domains of care review
In phase I of the review, one investigator (L.W.) independently screened all titles yielded by the initial search strategy for possible inclusion. After identifying appropriateness for possible inclusion, phase II consisted of two reviewers (L.W. and D.N.) independently evaluating the abstracts of publications identified in phase I. The publications from phase II were then retrieved in full text and assessed for inclusion of domain abstraction in phase III by two independent reviewers. The agreed-upon articles were assessed by independent reviewers in duplicate, to abstract the identified domains of care review in phase III.
In phase II, disagreement between reviewers was reconciled by discussion and consensus. The investigators were not blinded to the authors, journals, or results of studies. In phase III, disagreements on the data abstraction were resolved by a third independent reviewer who assessed the article and determined if the theme was included in the care review process. Descriptions of the 16 domains were supplied to all reviewers prior to data abstraction for reference.
Critical appraisal is the process of systematically examining research evidence to assess its validity, results, and relevance before using it to inform a decision. Instruments developed to support quality appraisal usually share some basic criteria for the assessment of qualitative research. These include the need for research to have been conducted ethically, the consideration of relevance to inform practice or policy, the use of appropriate and rigorous methods, and the clarity, coherence of reporting, address of reliability, validity, and objectivity [ 5 ].
In considering the most appropriate instruments to use for critical appraisal, we considered using the Cochrane Collaboration Bias Appraisal Tool [ 6 ] and a modified Newcastle-Ottawa Scale tool [ 7 ]. The nature of our qualitative data abstraction precluded the use of these tools. While the studies we evaluated may have included randomized controlled trials and been at risk for bias, the results of the publications evaluated were not typically relevant to our goal of qualitative domain abstraction. Many publications we evaluated were narrative in nature—describing a process without presentation of data, either qualitative or quantitative. For those publications that did present data relevant to our domains, the effect of bias within the study was felt to be unlikely to impact our qualitative data collection because the abstracted domains—descriptions of processes—were not affected by the results of the studies. We reviewed the items described in the Standards for Reporting Qualitative Research (SRQR) [ 8 ] and the Enhancing Transparency in Reporting the Synthesis of Qualitative Research (ENTREQ) [ 9 ] statement. The SRQR and ENTREQ aim to improve the transparency of all aspects of qualitative research by providing clear standards for reporting qualitative research. When assessing the risk of bias, we decided not to exclude articles based on their quality assessment. All potentially valuable insights were included. From each study, we extracted the domains relevant to care review processes. We tabulated the results and created graphics based on frequencies. No quantitative data was appropriate for abstraction, so we did not perform a meta-analysis.
The initial library search strategy identified 1318 titles for review. In phase I, 440 abstracts were reviewed, 76 of which were selected for full-text review in phase II. Fifteen articles from outside sources and bibliography review were also identified and reviewed. In total, 91 full-text articles were assessed, and after reconciliation between two independent reviewers, 47 articles were initially found to be appropriate for inclusion in our analysis of the domains of care review. One article was removed in the abstraction process, as both reviewers independently determined that it did not meet inclusion criteria [ 10 ] leading to 46 unique articles reviewed.
Domains were abstracted by two independent reviewers for each of the 46 articles in phase III. Articles that described a care review process from the same institution were consolidated to reflect the most complete view of that process possible, as aspects may have been reported differently in multiple articles/abstracts. Figure 1 shows the study selection process. Ultimately, we evaluated the care review systems from 35 unique institutions.

Study selection process
Study characteristics
Among the 46 studies, 35 represented unique institutions and 11 were same authors/institutions describing different aspects of the process or domains. The types of articles identified included 14 descriptive [ 11 – 24 ], three editorials [ 25 – 27 ] 15 prospective [ 28 – 42 ], seven quality improvement projects [ 12 , 43 – 48 ], and ten retrospective [ 11 , 30 , 49 – 56 ]. The 16 domains of successful care review that were identified for abstraction are presented and defined in Table 1 .
The percentage of frequency of each component is shown in Fig. 2 . The most commonly identified component of a care review process was utilizing an analysis of systems issues contributing to the case (32 institutions, 91.4%), followed by utilizing a standardized process for case review (30 institutions, 85.7%) and use of a structured case classification system (28 institutions, 80.0%). The least common components identified were recognition of excellence and use of case blinding/anonymity in reviews (5 institutions, 14.3%). Some articles were consistent with more than one article type and were classified as both.

Frequency of domain identification
Table 2 shows the distribution of all components in the full-text articles reviewed, with consolidation of same-institutions. No article/institution identified all 16 items evaluated by reviewers. Two institutions identified 14 of 16 items: Lehigh Valley and Johns Hopkins.
Distribution of care review domains
Our systematic review shows that, in the first two decades since the IOM report calling for improved safety systems, there have been few articles outlining a comprehensive clinical care review process. Additionally, most articles discuss their care review systems in the context of describing an aspect of their process, or corresponding improvement initiative.
Systems analysis—defined as the assessment of the effects of external forces such as policies, workflows, and software such as the electronic medical record on the critical event—was the most commonly identified care review process characteristic. Many identified articles describe the importance of evaluating how a person works within a system, rather than in isolation, to identify improvement opportunities. Assuming individuals are properly motivated with benign intent, looking at the system surrounding, the care avoids an antagonistic approach and supports the IOM’s underlying reasons for calling for care review processes—to prevent future errors.
Similarly, standardized processes and structured case classification were frequently discussed in the literature. To meet the IOM’s recommendations for creating care review “systems,” having a standardized process that uses structured classification is likely necessary. Without standardization, reviews would likely be sporadic, inefficient, and challenging to implement and subsequently inform future practice. Without structured classification, one could assume that conclusions would also be difficult to interpret.
Although some of these characteristics were common among reported care review systems, others are only rarely reported. Recognition of excellence and blinding of cases were reported in just five (14%) of the reports. Institutions that recognize excellence while performing care reviews were supportive of the practice, and one can understand why this would support the culture needed to have an effective care review system, and perhaps designers of future care review systems may wish to consider implementing this component. Similarly, anonymous review, or blinding, is intended to reduce bias and may allow a more objective review of each case. However, its infrequent mention may be indicative of unpublished prior experiences that may have supported avoiding this practice. From our experience, these are controversial topics, and future work is needed to understand the effects of specific characteristics on the overall care review process.
One additional characteristic that review processes must be supported by a functioning organizational system should receive particular attention. Although this was specifically identified by only 19 organizations, the downstream benefit to reviewing an episode of care and making recommendations for change in a non-functional system is likely lost. Key stakeholders in the process (physicians, nurse practitioners, physician assistants, nursing staff, support staff, etc.) are seemingly necessary for the care review process, and the administrative and leadership structure must be supportive of recommendations for change after care review is completed. This combination is strongly conducive to a process that engenders trust from the care team, which in turn bolsters the system as cases are referred for review, and staff engage in further problem solving.
Limitations
The articles evaluated come from a variety of settings—from consulting firms to in situ care review systems. Some authors strove to describe a comprehensive local practice, while others focused primarily on a particular component of a larger system. This heterogeneity limits the generalizability as the variability from one system to another may indicate institution- or system-specific adaptations to facilitate the process. A solution for one setting may not represent a good solution for another. We included articles from institutions and consulting firms describing or self-reporting care review systems, and it is not possible to know the true effectiveness of the processes described when removed from clinical context. It may be that there is an over-emphasis on some areas of care review believed to be ideal that are not practiced as described, and also possible that not all aspects of a process are represented. Particularly in the articles that discuss the care review process as the context for a specific project, it is possible that not all the details on the over-arching system of care review in place are described resulting in abstraction of domains in what is an incomplete description.
The domains we used during abstraction were determined by screening the included articles and supplemented by expert opinion. It is possible that there are additional variables that are more important, but less common, and were not included in our analysis. It is possible that a care review process we reviewed may include some of the 16 characteristics but did not specifically mention them in the articles reviewed. Additionally, the qualitative nature of the abstraction and interpretation of each item definitions are complex and may lead to less reliable results.
In an effort to reduce the effects of bias and definition complexity, all articles were reviewed in duplicate—both for inclusion in the study as well as abstraction of data. Disagreements were resolved by discussion and consensus for article inclusion and adjudicated by a third reviewer for the domain abstraction.
Despite increased discussion among institutions such as IOM and the National Patient Safety Foundation, in the last 16 years, there have been relatively few publications describing clinical care review processes and no clear evidence of a cultural shift to embrace clinical care review in an organized fashion. We have identified 16 domains of focus in a care review process and found that the approach to care review is highly variable as represented in the literature.
Future research
The effects of different aspects of care review processes have not been well studied. This presents an opportunity to evaluate processes that are present in many hospitals and health systems and identify truly effective, rather than simply common, practices, as identified within.
Additional file
Search strategy. (DOCX 14 kb)
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Authors’ contributions
All the authors have contributed substantially to this manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Laura E. Walker, Email: [email protected] .
David M. Nestler, Email: [email protected] .
Torrey A. Laack, Email: [email protected] .
Casey M. Clements, Email: [email protected] .
Patricia J. Erwin, Email: [email protected] .
Lori Scanlan-Hanson, Email: [email protected] .
M. Fernanda Bellolio, Phone: 507-775-5607, Email: [email protected] .

IMAGES
VIDEO
COMMENTS
Since the 1990s, time-limited trials have been described as an approach to navigate uncertain benefits and limits of life-sustaining therapies in patients with critical illness. In this review, we aim to synthesize the evidence on time-limited trials in critical care, establish what is known, and highlight important knowledge gaps.
Abstract. Critically reviewing the literature is an indispensible skill which is used throughout a research career. This demystifies the processes involved in systematically and critically reviewing the literature to demonstrate knowledge, identify research ideas and questions, position research and develop theory.
Critical Care Reviews Newsletter 643, bringing you the best critical care research and open access articles for the week April 1st to 7th, 2024. Subscriptions are available to individuals and departments. added April 8th. Podcast .
Critical Care Medicine. Explore the latest in critical care medicine, including management of respiratory failure, sepsis, HAI prevention, end-of-life care, and more. In this narrative medicine essay, a pediatric critical care physician mulls a discussion about a patient's status with a male colleague in front of her all-female medical team ...
A formal literature review is an evidence-based, in-depth analysis of a subject. There are many reasons for writing one and these will influence the length and style of your review, but in essence a literature review is a critical appraisal of the current collective knowledge on a subject. Rather than just being an exhaustive list of all that ...
These articles have been selected based on the authors annual review of key critical care, emergency medicine and medicine journals and their opinion of the importance of study findings as it pertains to the care of critically ill ED patients. ... The 65 trial is an important contribution to the 2020 critical care literature and addresses an ...
This narrative review aims to provide a summary and interpretation of the adult critical care nutrition literature, with a particular focus on continuing practice gaps and areas with new data, to help clinicians make practical, yet evidence-based decisions regarding nutrition management during critical illness.
Introduction. Sepsis is a global health problem that increases morbidity and mortality rates worldwide and which is one of the most common complications documented in intensive care units (ICUs) [].About 48.9 million cases of sepsis and 11 million sepsis-related deaths were documented in 2017 worldwide [].Sepsis is an emergency condition leading to several life-threatening complications, such ...
Nurse Practitioners and Physician Assistants in Acute and Critical Care: A Concise Review of the Literature and Data 2008-2018; 366: CREATING A SURGICAL AND CRITICAL CARE NURSE PRACTITIONER FELLOWSHIP AT UCSF; Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016
Aims: The comprehensive review sought to examine the impact of Critical Care Nurse Practitioner models, roles, activities and outcomes. Method: The Medical Literature Analyses and Retrieval (MEDLINE), The Cumulative Index of Nursing and Allied Health Literature (CINAHL); PubMED; PROQUEST; ScienceDirect; and the Cochrane database were accessed for the review.
This inclusive review of literature has highlighted the lacunae and areas of dissonance both in the literature and in clinical practice in relation to the critical care trajectory as experienced by survivors of critical illness and their families. ... Critical care nurses can and should play a role in preparing and supporting patients and ...
This review examines the experiences of Newly Qualified Nurses (NQNs) working in Critical Care, an environment traditionally reserved for experienced nurses, due to the complexity of patients and specialist skills required. 1 Recent decline in the nursing workforce has been acutely felt in specialist areas, including Critical Care, with higher staffing requirements. 2 This has led to increased ...
The search terms sleep, evaluating sleep and critical care were used. An integrative review method was used to analyse the data. Results. According to the 52 articles of this review, there is a wide variety of methods to evaluate patients' sleep in critical care by observation, by asking for patient's own perception and by objective measures.
For this research, a scoping review was carried out to address the research questions highlighted below. This intent of this scoping review was to gather and synthesize research evidence on current literature concerning critical care nurse burnout and resiliency (Arksey and O'Malley, 2005, Mealer et al., 2014, Rushton et al., 2015).
Nutrition therapy during critical illness has been a focus of recent research, with a rapid increase in publications accompanied by two updated international clinical guidelines. However, the translation of evidence into practice is challenging due to the continually evolving, often conflicting trial findings and guideline recommendations. This narrative review aims to provide a comprehensive ...
End-of-life care can be a difficult and challenging process for critical care nurses in intensive care units (ICUs) due to the care plan shifts from providing life-sustaining measures to end-of-life care. The aims of this study were to assess critical care nurses' perceived knowledge and attitudes toward end-of-life care, as well as their perspectives on promoting advance directives and the ...
The Clinical Pharmacy and Pharmacology Literature Update working group in the Society of Critical Care Medicine, Section on Clinical Pharmacy and Pharmacology, reviews major critical care journals and provides synopses to its members on a monthly basis in addition to an annual review of most impactful articles relevant to critical care ...
A review of the literature between the years 2007 and 2012 was conducted and included the search terms burnout, moral distress, compassion fatigue, intensive care, critical care, and nursing. The search was limited to the adult population, English language, and Western cultures. The results revealed that nurse managers play a crucial role in ...
A consensus committee of adult critical care nursing experts from the United Kingdom met in 2018 to evaluate and review the literature relating to oral care, its application in reducing pneumonia in critically ill adults and to make recommendations for practice. An elected national board member for the British Association of Critical Care ...
Their systematic literature review involved relevant articles from PubMed—69 references comprising 30 well-designed studies, 9 moderately well-designed studies, 2 studies with design limitations ...
The Future of Health (FOH), an international community of senior health care leaders, collaborated with the Duke-Margolis Institute for Health Policy to conduct a literature review, expert ...
Literature reviews play a critical role in scholarship because science remains, first and foremost, a cumulative endeavour (vom Brocke et al., 2009). As in any academic discipline, rigorous knowledge syntheses are becoming indispensable in keeping up with an exponentially growing eHealth literature, assisting practitioners, academics, and graduate students in finding, evaluating, and ...
Introduction. Critical care nursing deals with specific human responses to actual or potentially life-threatening problems. 1 According to the World Federation of Societies of Intensive and Critical Care Medicine, critical care is 'a multidisciplinary and interprofessional specialty dedicated to the comprehensive management of patients having, or at risk of developing, acute, life ...
Background: Given the growing life expectancy, the likelihood increases that health-care providers are confronted with older people having an adult child with a life-limiting disease. Aim: This literature review aimed to (1) explore the experiences of aged parents with regard to their position and role as a parent of an adult child with a life-limiting illness, (2) detect gaps in the existing ...
Sanfilippo syndrome is a group of rare, complex, and progressive neurodegenerative lysosomal storage disorders that is characterized by childhood dementia. The clinical management of patients with progressive neurological decline and multisystem involvement requires a multidisciplinary team with experience in the management of neurodegenerative disorders. Best practice guidelines for the ...
Given the long standing call for clinical care review, with limited literature to inform care review systems, we conducted a qualitative systematic review to identify characteristics discussed in existing models for care review. ... Critical appraisal is the process of systematically examining research evidence to assess its validity, results ...