- Share full article
Advertisement
Supported by

What Your Brain Looks Like When It Solves a Math Problem

By Benedict Carey
- July 28, 2016
Solving a hairy math problem might send a shudder of exultation along your spinal cord. But scientists have historically struggled to deconstruct the exact mental alchemy that occurs when the brain successfully leaps the gap from “Say what?” to “Aha!”
Now, using an innovative combination of brain-imaging analyses, researchers have captured four fleeting stages of creative thinking in math. In a paper published in Psychological Science, a team led by John R. Anderson, a professor of psychology and computer science at Carnegie Mellon University, demonstrated a method for reconstructing how the brain moves from understanding a problem to solving it, including the time the brain spends in each stage.
The imaging analysis found four stages in all: encoding (downloading), planning (strategizing), solving (performing the math), and responding (typing out an answer).
“I’m very happy with the way the study worked out, and I think this precision is about the limit of what we can do” with the brain imaging tools available, said Dr. Anderson, who wrote the report with Aryn A. Pyke and Jon M. Fincham, both also at Carnegie Mellon.
To capture these quicksilver mental operations, the team first taught 80 men and women how to interpret a set of math symbols and equations they had not seen before. The underlying math itself wasn’t difficult, mostly addition and subtraction, but manipulating the newly learned symbols required some thinking. The research team could vary the problems to burden specific stages of the thinking process — some were hard to encode, for instance, while others extended the length of the planning stage.
The scientists used two techniques of M.R.I. data analysis to sort through what the participants’ brains were doing. One technique tracked the neural firing patterns during the solving of each problem; the other identified significant shifts from one kind of mental state to another. The subjects solved 88 problems each, and the research team analyzed the imaging data from those solved successfully.
The analysis found four separate stages that, depending on the problem, varied in length by a second or more. For instance, planning took up more time than the other stages when a clever workaround was required. The same stages are likely applicable to solving many creative problems, not just in math. But knowing how they play out in the brain should help in designing curriculums, especially in mathematics, the paper suggests.
“We didn’t know exactly what students were doing when they solved problems,” said Dr. Anderson, whose lab designs math instruction software. “Having a clearer understanding of that will help us develop better instruction; I think that’s the first place this work will have some impact.”
Like the Science Times page on Facebook. | Sign up for the Science Times newsletter.
The Curious World of Mathematics
Earlier this year, mathematicians discovered a unique shape : an “einstein.” Now do-it-yourselfers have found ingenious ways to put it to use .
An online math community faced a problem-solving dilemma when it was asked a simple question: How can you chop a square into four similar rectangles?
What is the sum of an infinite series of natural numbers? The answer may be smaller than you think .
A Texas oil heir was fascinated with one of math’s greatest enigmas: Fermat’s theorem. His private support may have been a critical factor in its solution .
The creative brain: investigation of brain activity during creative problem solving by means of EEG and FMRI
Affiliation.
- 1 Institute of Psychology, University of Graz, Graz, Austria. [email protected]
- PMID: 18266217
- PMCID: PMC6871103
- DOI: 10.1002/hbm.20538
Cortical activity in the EEG alpha band has proven to be particularly sensitive to creativity-related demands, but its functional meaning in the context of creative cognition has not been clarified yet. Specifically, increases in alpha activity (i.e., alpha synchronisation) in response to creative thinking can be interpreted in different ways: As a functional correlate of cortical idling, as a sign of internal top-down activity or, more specifically, as selective inhibition of brain regions. We measured brain activity during creative thinking in two studies employing different neurophysiological measurement methods (EEG and fMRI). In both studies, participants worked on four verbal tasks differentially drawing on creative idea generation. The EEG study revealed that the generation of original ideas was associated with alpha synchronisation in frontal brain regions and with a diffuse and widespread pattern of alpha synchronisation over parietal cortical regions. The fMRI study revealed that task performance was associated with strong activation in frontal regions of the left hemisphere. In addition, we found task-specific effects in parietotemporal brain areas. The findings suggest that EEG alpha band synchronisation during creative thinking can be interpreted as a sign of active cognitive processes rather than cortical idling.
Publication types
- Research Support, Non-U.S. Gov't
- Brain / physiology*
- Brain Mapping / methods*
- Creativity*
- Electroencephalography*
- Magnetic Resonance Imaging*
- Problem Solving / physiology*
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 10 December 2021
Study of EEG characteristics while solving scientific problems with different mental effort
- Yanmei Zhu 1 , 2 ,
- Qian Wang 2 &
- Li Zhang 1
Scientific Reports volume 11 , Article number: 23783 ( 2021 ) Cite this article
4167 Accesses
12 Citations
Metrics details
- Neuroscience
Studying the mental effort in problem-solving is important to the understanding of how the brain allocates cognitive resources to process information. The electroencephalogram is a promising physiological approach to assessing the online mental effort. In this study, we investigate the EEG indicators of mental effort while solving scientific problems. By manipulating the complexity of the scientific problem, the level of mental effort also changes. With the increase of mental effort, theta synchronization in the frontal region and lower alpha desynchronization in the parietal and occipital regions significantly increase. Also, upper alpha desynchronization demonstrates a widespread enhancement across the whole brain. According to the functional topography of brain activity in the theta and alpha frequency, our results suggest that the mental effort while solving scientific problems is related to working memory, visuospatial processing, semantic processing and magnitude manipulation. This study suggests the reliability of EEG to evaluate the mental effort in an educational context and provides valuable insights into improving the problem-solving abilities of students in educational practice.
Similar content being viewed by others
Task prioritization modulates alpha, theta and beta EEG dynamics reflecting proactive cognitive control
Nathalie Liegel, Daniel Schneider, … Stefan Arnau
Salient distractors open the door of perception: alpha desynchronization marks sensory gating in a working memory task
Zsuzsanna Fodor, Csilla Marosi, … Gábor Csukly
From thoughtless awareness to effortful cognition: alpha - theta cross-frequency dynamics in experienced meditators during meditation, rest and arithmetic
Julio Rodriguez-Larios, Pascal Faber, … Kaat Alaerts
Introduction
Mental effort represents the quantity of cognitive resources involved in performing a task 1 , 2 , 3 . It is seen as a combination of the perceived demand characteristics, depth of information processing, and personal expertise 1 , 4 . Perceived demand characteristics mainly depend on the inherent complexity of the task content, which is related to the degree of interaction between various information elements 4 . When element interaction is low, task information can be extracted and learned for one single element at a time since individual elements have minimal references to one another. On the other hand, highly interacting information consists of elements that heavily interrelate, which must be combined and processed simultaneously. Accordingly, the more the number of interacting information a task contains, the higher the cognitive demand and mental effort it elicits 5 . Depth of processing refers to the degree to which a person encodes information. Deeper processing implies a greater degree of semantic or cognitive analysis of input for meaning extraction and comprehension with their existing knowledge, while surface level processing is more concerned with the recognition of physical or sensory features of the stimulus 6 . Thus, deeper processing of presented information requires higher mental effort than a more surface level encoding. Furthermore, developed expertise resulting from repeated practice lowers the mental effort imposed by a familiar task 7 , 8 . Studying the mental effort in problem-solving helps to determine the online processes while individuals are working on these problems and provides a deep understanding of their learning outcomes 9 , 10 . It could also provide implications for the development of effective instructions to improve their learning performance. For example, researchers have found that lead-augmented hypertext system improves the learning performance by visualization of interacting information elements as one undivided unit 5 . This system decreases the mental effort required for the integration of the hypertext nodes with semantic space. However, many of the previous studies usually explored the mental effort imposed by well-controlled working memory tasks or other problems which required little scientific knowledge. Compared with these tasks, scientific problems require the abstract scientific conception and involve complex cognitive processes such as model representation, retrieval of scientific conception and inference calculation. Solving scientific problems will involve a set of brain resources and consequently a high level of mental effort. To our knowledge, mental effort during scientific problem solving is still seldom reported. Research on mental effort in scientific problem solving may shed light on scientific reasoning of students and suggest ways to improve their problem-solving ability in science education.
Various methods, including subjective measures, secondary task measures, and physiological measures, have been applied to the assessment of mental effort 11 , 12 , 13 . Subjective measures evaluate the mental effort by using the subjective rating scales 14 . Secondary task measures estimate the mental effort for the primary task according to accuracy and response time on the secondary task 15 . Physiological measures quantify the mental effort by measuring a variety of physiological factors such as heart rate variability, eye movement and brain activity 16 , 17 , 18 , 19 , 20 . Both subjective and secondary task measures do not allow for a continuous and noninvasive measurement of mental effort. The participant has to interrupt the main task to fill a questionnaire or to perform a secondary task during the main one. In contrast, physiological measures can continuously and objectively monitor online mental effort without interfering with task performance 21 , 22 . Also, physiological measures vary predictably in response to changes of mental state 23 . It can be used to anticipate a mental impairment such as cognitive overload and fatigue 24 , 25 . However, through subjective and secondary task measures, due to lack of the capacity to monitor covert changes in psychophysiological state, it is only possible to detect a mental impairment once it already happened. Moreover, some studies have proved that physiological measures provide higher sensitivity in discriminating different levels of mental workload compared to subjective and secondary task measures 26 , 27 . In these studies, physiological variations reliably represent implicit fluctuations in the mental states, suggesting that physiological measures have sufficient stability and sensitivity to distinguish between different degrees of mental effort.
The electroencephalogram (EEG) is a promising physiological approach to the assessment of the mental effort of students in an educational context 28 , 29 . It can directly monitor an individual’s cognitive state by recording the electrical signals produced by the brain in an authentic environment. Previous studies have examined changes in EEG signals as a function of mental effort 30 , 31 , 32 , 33 . Researchers pointed out that brain activity in the theta and alpha frequency is sensitive to effortful processing 34 , 35 , 36 , 37 . This result was found in the studies about the electrophysiological indicators of mental effort. Most studies that apply the well-controlled working memory tasks, such as N-back tasks or spatial and verbal working memory tasks, have reported both alpha and theta effects on cognitive effort. With the enhancement in memory load due to increased task difficulty, an increase in frontal theta power and decrease in parieto-occipital alpha power are usually observed 38 , 39 , 40 , 41 , 42 , 43 . Research using multitasking also revealed a strong association between mental effort and oscillations in the theta and alpha frequency. They found that a growth in the number of subtasks the participants performed simultaneously increased the amount of theta activity in the frontal region and decreased alpha activity in the parietal region 44 . In addition, a similar pattern was found in studies using arithmetic problems or some real-world simulation tasks like air traffic control 28 , 45 . These studies have shown that changes in the frontal theta and posterior alpha activity are reliable indicators of mental effort elicited by tasks of varying complexity.
Compared with the tasks in the previous work, scientific problems require abstract scientific conception and involve complex cognitive processes. Solving scientific problems activate a set of brain resources and a high level of mental effort. In this case, a refinement of EEG mental effort measure proposed by Smith and Gevins should be considered 46 . Their EEG workload model includes quantification of alpha and theta band activity recorded from the frontal brain area essential for working memory and executive control, centro-parietal area essential for visuospatial processing, occipital area essential for stimulus encoding and semantic memory processing. Changes in EEG-derived regional indices could provide information about the relative activation of different local cortical regions in response to increases in mental effort. Based on these regional indices and specialization of the underlying cortical regions, neurologically meaningful segregation of task effects on mental effort can be speculated. Using this model, Smith and Gevins identified the different levels of mental workload in a flight simulator task. The mental state of an individual, whether in a well-rested state or following a total sleep deprivation, can be determined according to regional indices reflecting both task demands and operator’s state of alertness 46 .
In this study, we attempt to investigate the EEG indicators of mental effort during scientific problem-solving. To this end, we asked students to solve the scientific problems commonly used in the practice of science education while recording their EEG signals. By manipulating the complexity of the scientific problems, students engaged in different levels of mental effort. Their EEG signals during problem-solving were examined. We focused on analyzing the EEG activity in the theta and alpha frequency, because these two bands are particularly important indicators of effortful processing. Besides, brain activity in the lower and upper alpha frequency has been documented to be associated with differential cognitive effects. The lower alpha changes are considered to reflect general task demands, such as attentional processes, while the upper alpha changes are suggested to reflect the task-specific processes such as stimulus encoding, semantic processing and memory access 42 . Although these two alpha bands have different functional specializations, many previous studies employed the entire alpha band to explore EEG indicators of mental effort 44 , 47 , 48 . Some studies used simple working memory tasks to calculate the changes in the lower and upper alpha frequency. They found that the two alpha bands seemed to respond quite similarly in simple tasks 49 . Compared with simple tasks, solving scientific problems involves more complex cognitive processes including attention, working memory, visual processing, semantic memory and multisensory integration. We expected that task-general and task-specific processes during scientific problem solving would be reflected by the lower and upper alpha activity, respectively. For this consideration, both lower and upper alpha bands were examined in this study. We hypothesized that functional specialization of these two alpha oscillations would appear in our task requirements. They would reflect the neural responses to mental effort imposed by the particular cognitive processes while solving scientific problems. According to the functional topography for theta and alpha oscillations, we expected that mental effort imposed by general attention would be associated with posterior lower alpha activity, mental effort by working memory and executive function would be reflected by theta and upper alpha activity in the frontal area, mental effort by task-specific processes of visual processing, semantic processing and multisensory integration would be reflected by upper alpha activity in the occipital and centro-parietal areas. Moreover, with the increase of mental effort, theta oscillation would increase while both alpha bands would decrease.
Behavioral results
Mean reaction time, response accuracy and subjective effort evaluation for the low and high complexity problems are shown in Table 1 and Fig. 1 . In our study, students gave 9.6 ± 0.6 and 7.4 ± 1.6 correct trials for the low and high complexity problems, respectively. Wilcoxon signed-rank test reveals the main effect of problem complexity condition ( Z = − 4.37, p < 0.001, for reaction time; Z = − 4.04, p < 0.001, for response accuracy; Z = − 4.29, p < 0.001, for subjective effort evaluation). The results reflect that high complexity problems are more demanding than the low complexity ones. Students spent more time to solve these problems, but the response accuracy declined significantly. In addition, during the interview session, students responded that these high complexity problems required more effort. All the behavioral results support that our scientific problem design and complexity categorization are reliable. The experiment successfully engaged the students in solving scientific problems with different levels of mental effort.
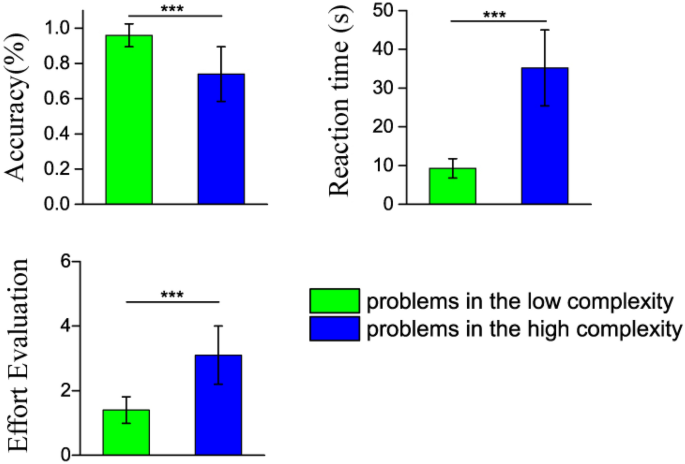
Mean reaction time, response accuracy and subjective effort evaluation for the low and high complexity problems. The behavioral results support that our scientific problem design and complexity categorization are reliable. The experiment successfully engaged the students in solving scientific problems with different levels of mental effort. *** p < 0.001.
Electrophysiological results
Figure 2 gives the neurophysiological results. Figure 2 a illustrates the topographic maps of theta ERS and alpha ERD for the two conditions of problem complexity. Figure 2 b–d gives the statistical results of theta ERS, lower alpha band ERD, and upper alpha band ERD in the different brain regions, respectively. The results indicate that theta activity increases, and alpha activity decrease during scientific problem solving. In addition, the amplitudes of theta ERS and alpha ERD vary across brain regions between the two problem complexity conditions.
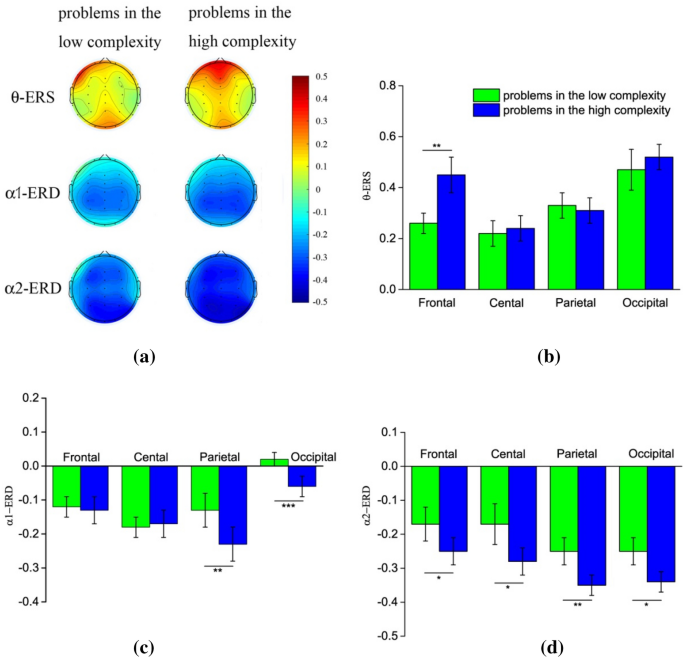
ERS/ERD in the theta and alpha bands while solving the low and high complexity scientific problems. α1 refers to lower alpha band, α2 refers to upper alpha band. With the increase of mental effort, the amplitudes of frontal theta ERS and posterior lower alpha band ERD increase, while the amplitude of upper alpha band ERD demonstrates an extensive enhancement throughout the whole brain. ( a ) Topographical maps of theta ERS and alpha ERD for the two complexity conditions. ( b ) Theta ERS for the two complexity conditions in four brain regions. ( c ) Lower alpha band ERD for the two complexity conditions in four brain regions. ( d ) Upper alpha band ERD for the two complexity conditions in four brain regions.
For the theta ERS, as shown in Fig. 2 b, a two-way repeated measures ANOVA obtains the significant main effect of brain area [ F (3,72) = 6.98, p < 0.01, ƞ 2 = 0.23]. The theta ERS in the frontal and occipital regions are significantly higher than those in the central and parietal regions (both p < 0.05). The results also reveal the significant interaction of the brain area and problem complexity condition [ F (3,72) = 24.13, p < 0.001, ƞ 2 = 0.51]. Simple effect analysis reflects that theta ERS tends to be higher for the high complexity scientific problems than the low complex problems in the frontal region ([ F (1,24) = 14.84, p < 0.01]). There is no significant difference in theta ERS for two complexity conditions in other brain regions (all p > 0.05).
For the lower alpha band ERD, as shown in Fig. 2 c, the statistical results reveal a significant main effect of brain area [ F (3,72) = 9.08, p < 0.001, ƞ 2 = 0.28], with a significantly stronger lower alpha band ERD at the central and parietal regions compared to the frontal and occipital regions (both p < 0.05). The results also show a significant interaction of brain area and problem complexity condition [ F (3,72) = 5.38, p < 0.001, ƞ 2 = 0.18]. Simple effect analysis shows a stronger lower alpha ERD in the parietal ([ F (1,24) = 13.03, p < 0.01]) and occipital regions ([ F (1,24) = 24.58, p < 0.001]) when solving the high complexity problems, in comparison to low complexity problems. Lower alpha band ERD has no significant difference between two complexity conditions in other brain regions (all p > 0.05).
For the upper alpha band ERD, as shown in Fig. 2 d, the results show a significant main effect of problem complexity condition [ F (1,24) = 6.48, p < 0.05, ƞ 2 = 0.21]. Upper alpha band ERD is much stronger for the high complexity problems. The main effect of brain area as well as the interaction of the brain area and problem complexity are both not significant (both p > 0.05). This result suggests a significant global suppression of upper alpha activity when solving high complexity scientific problems. Paired- t test was conducted on the amplitude of upper alpha band ERD between the low and high complexity condition. The results confirm that upper alpha band ERD is significantly stronger across all brain regions ( p < 0.05 in the frontal, central and occipital regions; p < 0.01 in the parietal region.)
In this study, we investigate the EEG characteristics of different mental effort in solving scientific problems. By manipulating the complexity of the problem, students engaged in tasks with different levels of mental effort. Both behavioral data and interview reports show that they spent more mental effort in dealing with the high complexity scientific problems. The different amounts of mental effort are accompanied by theta ERS and alpha ERD, which exhibit the distinct topographical distribution. Particularly, an increase in mental effort significantly enhances the amplitude of frontal theta ERS, parietal and occipital lower alpha band ERD, and brain-wide upper alpha band ERD. These task-induced EEG changes reflect the mental resources required for the specific cognitive processes involved in solving scientific problems.
Brain activity in the frontal theta increases while solving scientific problems
Our findings show that frontal theta activity increases with the enhanced mental effort while solving scientific problems. Frontal theta oscillations are usually observed in EEG studies that use working memory tasks, multiple tasks, and other tasks involving executive functions. The results show that frontal theta activity is positively correlated with task demands on memory load and executive function 35 , 38 , 39 , 40 . Some studies have even found that the degree of theta synchronization is a neural signature of successful information manipulation 50 . Recently, researchers employed the real-world simulation tasks such as flight and air traffic management to investigate the EEG indicators of mental load 25 , 45 . They also found that as cognitive demands on attention, working memory load and task control increase, the enhanced mental effort leads to an increase in the frontal theta activity. These previous studies revealed that frontal theta synchronization is a reliable indicator of mental effort elicited by working memory and executive function.
Consistent with the previous studies, our results further demonstrate that the frontal theta synchronization becomes stronger as mental effort increases when solving the high complexity scientific problems. It suggests that more working memory and control capacity are allocated to accomplish these problems. This result is reasonable since more information and deeper processing are involved in high complexity scientific problems. These problems involve two or more objects. The forces acting on an object will be affected by others as well as the motion state of the system. Students need to analyze each object and the entire system at the same time. Meanwhile, they need to retrieve the physics concepts and apply them to analyze the forces acting on the objects. More interactive elements and greater control requirements expand the amount of information maintained, manipulated and controlled in working memory. Therefore, more mental effort is recruited. In contrast, for the scientific problems with the low complexity, only one object is involved, and there is no complex interaction between objects. Furthermore, in educational practice, it is common to determine the forces acting on a single object in the system. Fewer information elements and the developed expertise make the reasoning process in working memory more automated, thereby reducing online mental effort of the task. Our results suggest that frontal theta activity can reliably reflect the mental effort assigned to working memory and control processes when solving abstract scientific problems.
Brain activity in the alpha frequency decreases while solving scientific problems
In our study, both the lower and upper alpha activity decrease with the increase of mental effort while solving scientific problems. Alpha oscillations are usually found to be negatively correlated with effortful processing 27 , 42 . Alpha desynchronization is suggested to represent an intensified recruitment of relevant mental resources when task demands require more cognitive processing to perform it 51 . Studies on visual or auditory information processing, sensorimotor processing, multitasking and reasoning have proven that the quantification of alpha ERD is a particularly useful and appropriate method for measuring the level and topographical distribution of cortical activation during cognitive task performance. For example, alpha ERD is maximal in the occipital region during the visual task, maximal in the temporal region during the auditory task, and maximal in the centro-parietal region during sensorimotor task 44 , 52 , 53 , 54 , 55 . Moreover, the amplitude of alpha ERD becomes more prominent when task difficulty increases. Besides, brain activity at the lower and upper alpha frequency has been documented to reflect different cognitive demands. Desynchronization of lower alpha has been observed in responses to almost any type of task, and is believed to represent the general task requirements for basic arousal and attentional processing 42 . The ERD map consistently displays that the lower alpha desynchronization is the largest in the parietal area 42 , 56 . Moreover, it is observed that the lower alpha is highly sensitive to practice 37 . Generally, the amplitude of lower alpha activity increases significantly with the increase of practice, suggesting that performance requires lesser mental resources after the individual has completed the task proficiently. In contrast, desynchronization of upper alpha usually emerges at task-relevant sites and is considered to reflect specific task requirements, such as stimulus encoding, semantic processing and memory access 56 , 57 , 58 , 59 . Although these two alpha bands have different functional specializations, many previous studies employed the entire alpha band to explore EEG indicators of mental effort 47 , 48 . Some studies have calculated changes in the lower and upper alpha bands, but used simple tasks such as working memory tasks. They found that the two alpha bands seem to respond very similarly to mental effort in simple tasks 49 . In our study, considering that more complex cognitive processes are involved in solving scientific problems, we calculated the brain activity in the lower and higher alpha band respectively. These two alpha bands demonstrated the different effects on mental effort allocated by the abstract scientific problems.
In line with the previous studies, we found that sensitivity of online mental effort in the lower alpha band located in the parietal and occipital brain areas during solving scientific problems. According to the cognitive effects of lower alpha activity, ERD in the parietal area suggests that more general alertness and attention are imposed on stimulus representation, while ERD in the occipital region reflects that more visual attention is required for stimulus sensory processing 60 , 61 . It is reasonable because more complex visual elements are included in these problems. In addition, compared with the progressive automation of single-object problems in educational practice, complex scientific problems still require sustained and focused attention. Accordingly, the greater amplitude of lower alpha band ERD in the posterior region suggests that a higher level of mental effort is required to gain general attention when solving complex scientific problems.
Different from the previous studies which showed similar activation patterns between lower and upper alpha activity due to the application of simple tasks, we observed a distinct topographical distribution of upper alpha band ERD compared to that of lower alpha band. In our study, the upper alpha band ERD is enhanced throughout the whole brain areas with the increase of mental effort. Our results support the view of functional differences between different alpha bands, and are also in line with the statement that the functional specialization of the lower and upper alpha bands is most prominent in the frontal and central regions as the task demands increase 42 .
It is commonly observed that upper alpha band ERD is the largest at occipital site when processing various visual stimuli 56 . In these visual tasks, researchers found that desynchronization started over occipital site in the upper alpha band, followed by a parietal localized ERD in the lower alpha band. They explained that the early upper alpha band ERD at occipital site reflects visual encoding of the stimulus, including feature extraction, stimulus identification, semantic encoding and memory access, while the following parietal ERD in the lower alpha band reflects attention on encoded visual stimulus 56 . Other studies using semantic processing tasks consistently found that ERD in the occipital region is the most sensitive to the search and retrieval processes of semantic information, and is significantly correlated to the performance of semantic memory 57 . These results reflect that upper alpha in the occipital site is indicative of visual stimulus encoding and semantic memory processing. In our study, students not only need to encode the symbolic diagram and make a mental representation of current information, but also need to retrieve the physics conception from long-term memory and integrate scientific law with information presented in the problem. Mental resources for stimulus encoding and semantic memory processing are particularly higher for the complex scientific problem because it involves interactive objects in the diagram and requires deeper processing for meaning extraction and comprehension. We suggest that this heavier mental effort would be reflected by the stronger upper alpha band ERD in the occipital region.
Upper alpha band ERD in the centro-parietal regions is reported to be essential for visuospatial processing and mathematical ability 54 . It is commonly observed that centro-parietal alpha power decreases in tasks with visually presented stimuli. Several studies have demonstrated that generation of ideas in the figural domain is related to the strong decrease in the upper alpha activity at parietal site, which reflect the high visuospatial processing requirements during mental generation and manipulation of visual representation 62 , 63 . In the field of mathematics, researchers found that the parietal site supports magnitude manipulations in arithmetic tasks 64 . In addition, ERD results revealed that applying procedure strategy to solve more difficult arithmetic problems causes much stronger activation in the parietal regions than using strategy that retrieves answers directly form long-term memory for easier problems 65 . Accordingly, we suggest that the stronger upper alpha band ERD in the centro-parietal region indicates the greater mental effort for visuospatial processing of symbolic diagram in the high complexity problem. Quite similar to the difficult arithmetical problems, complex scientific problems also require a procedure strategy to analyze the forces on each object, rather than directly retrieving the answer from long-term memory. In other words, students need to first analyze the forces based on the motion state of the entire system, and then consider the forces exerted on each object according to its motion state as well as the interaction between adjacent objects. In this case, magnitude manipulations are also involved to calculate and determine the forces due to interaction between objects. The mental effort allocated by these cognitive processes would be reflected by the centro-parietal upper alpha desynchronization.
Frontal upper alpha desynchronization is usually used to examine higher order cognitive functions of executive processes, such as inhibition, and mental manipulation of information 55 , 66 . These cognitive processes are important for learning, reasoning, and comprehension. Stronger frontal upper alpha band ERD suggests that greater mental effort is allocated by executive function and working memory demands in task performance. Researchers further suggest that upper alpha activity at frontal site reflects the degree of executive control of anterior brain region over more posterior regions responsible for cognitive processes including information encoding, memory tracing, visuospatial processing and other mental operations 55 . For example, the activation of frontal brain area of a skilled chess player is lower than that of a lower-skilled player, which suggests that training or practice reduces the need for frontal executive functions since information processing in the posterior regions becomes more automated 55 . These previous investigations reveal that ERD in the frontal upper alpha band reflects mental operation of information and the executive control of cognitive processing. We speculate that the stronger frontal upper alpha ERD is indicative of mental effort exerted by higher executive function and working memory demands to reason the high complexity scientific problems. For these problems, students not only require more mental effort on mental operations of interactive information, but also need more anterior control over relative posterior brain regions responsible for specific cognitive processes.
In summary, we investigated online mental effort in solving scientific problems using the EEG. We suggest that the increase in the frontal theta activity and decrease in the anterior upper alpha activity reflect the mental effort allocated by working memory and cognitive control. The decrease in the centro-parietal upper alpha activity reflects the mental effort imposed by visuospatial processing and magnitude manipulation of information in the scientific problems. The decrease in the upper alpha activity in the occipital area reflects the stimulus encoding and semantic processing of scientific conceptions. The task-induced EEG changes suggest the mental resources required for the specific cognitive processes involved in solving scientific problems. It can provide a deep understanding of students’ learning outcomes. It will also provide implications for the development of effective guidance to improve their ability to solve scientific problems in educational practice.
Participants
Twenty-five healthy students majoring in engineering or science (mean age = 23 ± 1.8 years old, 12 males and 13 females) from Southeast University participated in this study. No physics department students were recruited. All of the participants were right-handed, had normal or correct-to-normal vision, reported no history of psychiatric or neurological disorders, and were not taking any medications. Each participant signed a written informed consent before the experiment and received monetary compensation for participation. Ethical approval for the study was obtained from the ethics committee of Southeast University. All procedures were conducted in accordance with approved guidelines and regulations.
Scientific problems
The scientific problems used in our study are items to analyze the forces acting on objects in various states of motion. Force analysis plays an important role in science learning and is a fundamental step in solving many physics problems. However, it is usually a challenging for students to conduct force analysis, especially for the objects in complex motion state. To solve these problems, students required to analyze the interaction between objects, retrieve scientific conceptions about the relationship between force and motion, as well as make inferences about presented information based on these conceptions. Cognitive resources across various brain regions were recruited and task-related mental effort were allocated. As widely used in science education practice, the problems used in our study were presented as the symbolic diagrams. These symbolic diagrams conveyed precise meanings and combined with rules of force and motion that must be used correctly 67 .
The whole task consisted of 24 scientific problems which were divided into two complex conditions. For the low complexity problems, only one object was considered. The forces acting on the object would be gravity, friction depending on the roughness of the contact surface, the supporting force of the surface, and the pulling or pushing force applied on it if present. For the high complexity problems, two or more objects were involved, so the interaction between the objects had to be carefully analyzed. Typical examples of the scientific problems in the low and high complexity conditions are illustrated in Fig. 3 . The final 24 scientific problems were selected from a bank of 100 force analysis items. The evaluation procedure was conducted by a committee composed of three experienced physics teachers and two university professors from the physics department to ensure the validity of the complexity classification. In the experiment, the students were asked to determine the total amount of force acting on an object. We assumed that the high complexity problems were more demanding and required higher mental effort. We also expected that the different amounts of online mental effort in solving scientific problems would be reflected by EEG signals. In the experiment, we also developed items as controls, in which students were only required to count the number of objects in a particular shape.
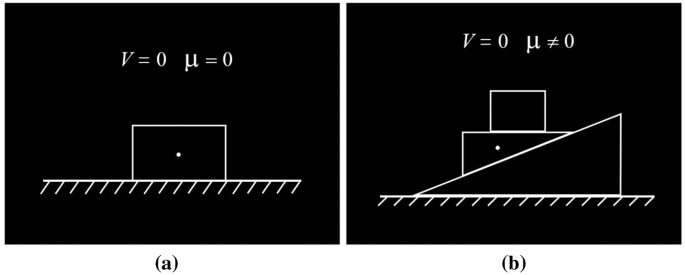
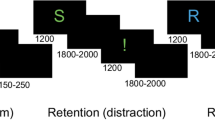
Examples of scientific problems presented in the task. Problems are illustrated as the symbolic diagrams commonly used in science education practice. Students are asked to determine the number of forces acting on the object marked by the small white dot. The velocity of the object ( V ) and the friction of contacting surfaces ( μ ) are shown in the diagram. ( a ) Low complexity scientific problem. ( b ) High complexity scientific problem.
Experimental procedures
A total of 48 stimuli including 24 scientific problems and 24 control items were presented using E-prime 2.0. The stimuli were presented according to the event-related design, as shown in Fig. 4 . Each trial started with the presentation of a central white fixation cross on the black screen for 3000 ms. Then a scientific problem was presented and the participants were required to think silently about the forces acting on the object which was marked with a small white dot. The problem remained on the screen until the participant got the answer and requested the answer options by keystroke (Reaction 1). After that, the scientific problem disappeared from the screen and the answer options were presented. The participant selected the answer from six options by pressing the corresponding reaction button (Reaction 2). Finally, a subjective rating scale was presented to assess the level of effort put into the problem, and the participant responded by pressing the respective button (Reaction 3). After the scientific problem was presented, a control item was showed. An inter-trial interval of 3000 ms was designed as a resting period before presenting the next trial. The purpose of the application of control stimuli was to reset the mental activity of the participant to normal levels and eliminate any stressful effects of the previous scientific problem. Only EEG signals during solving scientific problems were analyzed.
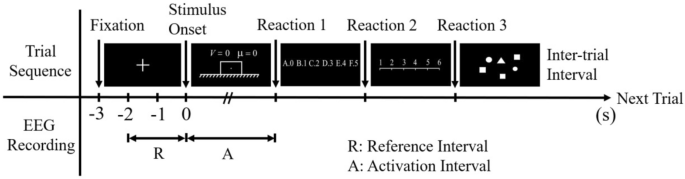
Schematic of a single trial. The time period from the onset of a scientific problem to reaction 1 serves as the activation interval, the time period from 1 to 3 s after the onset of the fixation cross serves as the reference interval.
The participants sat comfortably about 75 cm away from the screen to perform the task and were asked to pay attention to the task while keeping still. Two scientific problems of low and high complexity were used in the practice session for the familiarity of the experiment. The remaining problems were divided into two blocks in the formal experiment procedure. The scientific problems of varying levels of complexity were randomly presented and equally distributed between each block. The participants’ EEG signals in the task were recorded at the same time. After the EEG recording session, participants were interviewed to explain how they solved each scientific problem and how hard they worked on it during the task.
Behavioral data analysis
The behavioral data including reaction time, response accuracy and subjective effort evaluation were recorded by E-prime software. In our study, the reaction time was identified as the time interval between onset of scientific problem and their keystroke for reaction 1. The correctness of response to each question is determined according to their keystroke of answer options for Reaction 2. Subjective effort evaluation was obtained according to their keystroke of Reaction 3. The behavioral data for each student was first averaged for problems in the low and high complexity respectively. Then, the mean and standard deviation of each behavioral data were calculated across all students for each problem condition.
We first performed the Shapiro–Wilk normality test for each behavioral data. It revealed that the data did not conform to a normal distribution (all p < 0.05). Accordingly, we conducted Wilcoxon signed-rank test to analyze the main effect of problem complexity condition on each behavioral data.
We further compared behavioral data between correct and incorrect trials for high complexity problems. For reaction time of correct and incorrect responses, wilcoxon signed-rank test revealed that reaction time of incorrect trials was comparable to that of correct trials ( Z = − 0.44, p = 0.67). The same analysis was conducted to subjective evaluation of mental effort, which also showed no significant difference between two kinds of conditions ( Z = − 0.97, p = 0.33). Additionally, in the interview session, the students also expressed that the amount of effort to solve these difficult problems was high no matter they successfully solved it or not. All these results suggested that the students spent a similar amount of effort to solve the high complexity problems even though they may not obtain the right answer for certain problem. For this reason, we included all trials for further EEG data analysis.
EEG recording and data analysis
EEG was recorded from 32 tin electrodes mounted on an elastic cap according to the international 10–20 system (NeuroScan Inc., Herndon, VA, USA). The electrooculography (EOG) was recorded from two electrodes on the canthi and two electrodes located above and below the right eye. All electrode impedances were maintained below 5 KΩ. EEG and EOG signals were continuously sampled at 500 Hz for off-line analysis. The EEG data were band-pass filtered between 0.5 Hz and 60 Hz. Ocular artifacts were first corrected with an eye-movement correction algorithm which employed a regression analysis in combination with artifact averaging 68 . The continuous EEG data were segmented into epochs covering reference interval and activation interval in each trial. To further remove possible artifacts, the data were submitted to Independent Component Analysis (ICA) using the runica function from the EEGLAB toolbox to clear visible artifacts, such as the components of possible ocular and muscle movements.
Considering that brain activity varies individually, event-related synchronization/ desynchronization (ERS/ERD) of EEG signals was quantified to measure cortical activation and topographical distribution in scientific problem-solving 52 . The amount of ERS/ERD at a given frequency band is defined as the percentage of power increase/decrease during the activation interval relative to the reference interval. The activation interval refers to the time period while working on a task, and the reference interval refers to a pre-stimulus time period without any task demands. In our study, the time period from the onset of a scientific problem to reaction 1 served as the activation interval, and time period from 1 to 3 s after the onset of the fixation cross served as the reference interval, as shown in Fig. 4 .
Figure 5 illustrates the steps of EEG signal processing. For each epoch, EEG time series in the reference and activation intervals were converted into the frequency domain using a fast Fourier transformation (FFT) with a sliding 500 ms window by a step of 25 ms. Therefore, each window overlapped the previous one by 475 sample points. Band power in theta (4–7 Hz), lower alpha (8–10 Hz) and upper alpha (10–12 Hz) frequencies were calculated, respectively. The percentage change in the frequency band power from the activation interval to reference interval in each trial was then quantified. Finally, the calculated change in band power was averaged across all trials for low and high complexity conditions respectively. A positive value represents ERS and a negative value suggests ERD. For statistical analyses, ERS/ERD data were aggregated over different electrode locations as shown in Fig. 6 : frontal (FP1, FP2, F3, Fz and F4), central (C3, Cz and C4), parietal (P3, Pz, and P4) and occipital (O1, Oz, and O2).
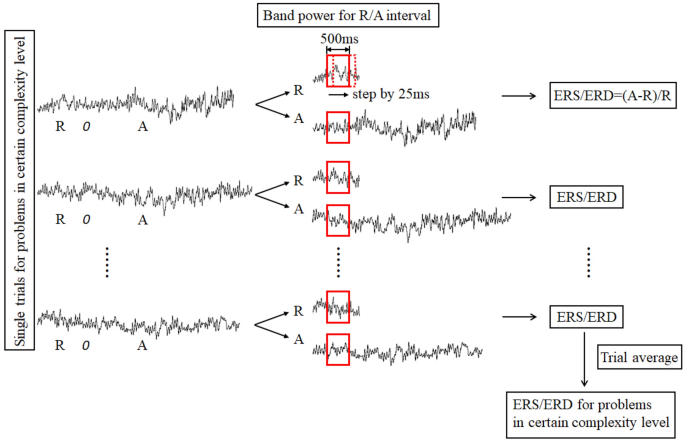
Steps of EEG signal processing to calculate ERS/ERD values.
Division of the brain regions and their included electrodes for ERS/ERD calculation. Frontal (FP1, FP2, F3, Fz and F4), Central (C3, Cz and C4), Parietal (P3, Pz, and P4) and Occipital (O1, Oz, and O2).
For statically analysis, problem complexity condition (low and high complexity) and brain area (frontal, central, parietal and occipital areas) are two within-subjects variables. Therefore, we first conducted the Shapiro–Wilk normality test on each set of EEG data. The statistical results revealed that each set of EEG data was normally distributed (all p > 0.05). Then, we performed the two-way repeated measures ANOVA on ERS/ERD data to analyze the main and interaction effects of problem complexity and brain area. Greenhouse–Geisser correction was applied to correct for violations of the sphericity assumption, all post-hoc tests were bonferroni-corrected.
Kirschner, P. A. & Kirschner, F. Mental effort. In Encyclopedia of the Sciences of Learning 2182–2184 (Springer, 2012).
Chapter Google Scholar
Causse, M., Chua, Z. R., Peysakhovich, V., Del Campo, N. & Matton, N. Mental workload and neural efficiency quantified in the prefrontal cortex using fnirs. Sci. Rep. 7 , 5222 (2017).
Article ADS PubMed PubMed Central Google Scholar
Zammouri, A., Moussa, A. A. & Mebrouk, Y. Brain-computer interface for workload estimation: Assessment of mental efforts in learning processes. Expert Syst. Appl. 112 , 138–147 (2018).
Article Google Scholar
Van Merrienboer, J. J. & Sweller, J. Cognitive load theory and complex learning: Recent developments and future directions. Educ. Psychol. Rev. 17 , 147–177 (2005).
Antonenko, P. D. & Niederhauser, D. S. The influence of leads on cognitive load and learning in a hypertext environment. Comput. Human Behav. 26 , 140–150 (2010).
Craik, F. & Lockhart, R. S. Levels of processing: A framework for memory research. J. Verbal Learn. Verbal Behav. 11 , 671–684 (1972).
Rikers, R. M., Van Gerven, P. W. & Schmidt, H. G. Cognitive load theory as a tool for expertise development. Instr. Sci. 32 , 173–182 (2004).
Fairclough, S. H., Venables, L. & Tattersall, A. The influence task demand and learning on the psychophysiological response. Int. J. Psychophysiol. 56 , 171–184 (2005).
Article PubMed Google Scholar
Tasir, Z. & Pin, O. C. Trainee teachers’ mental effort in learning spreadsheet through self-instructional module based on cognitive load theory. Comput. Educ. 59 , 449–465 (2012).
Olina, Z., Reiser, R., Huang, X., Lim, J. & Park, S. Problem format and presentation sequence: Effects on learning and mental effort among us high school students. Appl. Cogn. Psychol. 20 , 299–309 (2015).
Wetzel, S., Bertel, S., Montag, M. & Zander, S. Spatial task solving on tablets: Analysing mental and physical rotation processes of 12–13 year olds. Educ. Technol. Res. Dev. 68 , 363–381 (2020).
Makransky, G., Terkidsen, T. S. & Mayer, R. E. Role of subjective and objective measures of cognitive processing during learning in explaining the spatial continuity effect. Learn. Instr. 61 , 23–24 (2019).
Matthews, G., Reinerman-Jones, L. E., Barber, D. J. & Abich, J. The psychometrics of mental workload: Multiple measures are sensitive but divergent. Hum. Factors 57 , 125–143 (2015).
Schmeck, A., Opfermann, M., Van Gog, T., Paas, F. & Leutner, D. Measuring cognitive load with subjective rating scales during problem solving: Differences between immediate and delayed ratings. Instr. Sci. 43 , 93–114 (2015).
Brunken, R., Plass, J. L. & Leutner, D. Assessment of cognitive load in multimedia learning with dual-task methodology: Auditory load and modality effects. Instr. Sci. 32 , 115–132 (2004).
Kennedy, D. O. & Scholey, A. B. Glucose administration, heart rate and cognitive performance: effects of increasing mental effort. Psychopharmacol. 149 , 63–71 (2000).
Article CAS Google Scholar
Quirins, M. et al. Conscious processing of auditory regularities induces a pupil dilation. Sci. Rep. 8 , 14819 (2018).
Toth, A. J. & Campbell, M. J. Investigating sex differences, cognitive effort, strategy, and performance on a computerised version of the mental rotations test via eye tracking. Sci. Rep. 9 , 19430 (2019).
Lelis-Torres, N., Ugrinowitsch, H., Apolinario-Souza, T., Benda, R. N. & Lage, G. M. Task engagement and mental workload involved in variation and repetition of a motor skill. Sci. Rep. 7 , 14764 (2017).
Khachouf, O. T., Chen, G., Duzzi, D., Porro, C. A. & Pagnoni, G. Voluntary modulation of mental effort investment: an fmri study. Sci. Rep. 7 , 17191 (2017).
Dirican, A. C. & Gokturk, M. Psychophysiological measures of human cognitive states applied in human computer interaction. Proc. Comput. Sci. 3 , 1361–1367 (2011).
Hogervorst, M. A., Brouwer, A. M. & Van Erp, J. B. E. Combining and comparing EEG, peripheral physiology and eye-related measures for the assessment of mental workload. Front. Neurosci. 8 , 322 (2014).
Article PubMed PubMed Central Google Scholar
Fairclough, S. H. & Venables, L. Prediction of subjective states from psychophysiology: A multivariate approach. Biol. Psychol. 71 , 100–110 (2006).
Byrne, E. A. & Parasuraman, R. Psychophysiology and adaptive automation. Biol. Psychol. 42 , 249–268 (1996).
Article CAS PubMed Google Scholar
Borghini, G., Astolfi, L., Vecchiato, G., Mattia, D. & Babiloni, F. Measuring neurophysiological signals in aircraft pilots and car drivers for the assessment of mental workload, fatigue and drowsiness. Neurosci. Biobehav. Rev. 44 , 58–75 (2014).
Arico, P. et al. Passive BCI in operational environments: Insights, recent advances, and future trends. IEEE Trans. Biomed. Eng. 64 , 1431–1436 (2017).
Stipacek, A., Grabner, R. H., Neuper, C., Fink, A. & Neubauer, A. C. Sensitivity of human eeg alpha band desynchronization to different working memory components and increasing levels of memory load. Neurosci. Lett. 353 , 193–196 (2003).
Walter, C., Rosenstiel, W., Bogdan, M., Gerjets, P. & Spuler, M. Online eeg-based workload adaptation of an arithmetic learning environment. Front. Hum. Neurosci. 11 , 286 (2017).
Gerjets, P., Walter, C., Rosenstiel, W., Bogdan, M. & Zander, T. O. Cognitive state monitoring and the design of adaptive instruction in digital environments: lessons learned from cognitive workload assessment using a passive brain-computer interface approach. Front. Neurosci. 8 , 385 (2014).
Kathner, I., Wriessnegger, S. C., Muller-Putz, G. R., Kubler, A. & Halder, S. Effects of mental workload and fatigue on the P300, alpha and theta band power during operation of an ERP (P300) brain–computer interface. Biol. Psychol. 102 , 118–129 (2014).
Antonenko, P. D., Paas, F., Grabner, R. & Van Gog, T. Using electroencephalography to measure cognitive load. Educ. Psychol. Rev. 22 , 425–438 (2010).
Brouwer, A. M. et al. Estimating workload using EEG spectral power and ERPs in the n-back task. J. Neural. Eng. 9 , 045008 (2012).
Article ADS PubMed Google Scholar
Holm, A., Lukander, K., Korpela, J., Sallinen, M. & Muller, K. M. I. Estimating brain load from the EEG. Sci. World J. 9 , 639–651 (2009).
Spuler, M. et al. EEg-based prediction of cognitive workload induced by arithmetic: A step towards online adaptation in numerical learning. ZDM Math. Educ. 48 , 267–278 (2016).
Kawasaki, M., Kitajo, K. & Yamaguchi, Y. Dynamic links between theta executive functions and alpha storage buffers in auditory and visual working memory. Eur. J. Neurosci. 31 , 1683–1689 (2010).
PubMed PubMed Central Google Scholar
Kwon, G. et al. Individual differences in oscillatory brain activity in response to varying attentional demands during a word recall and oculomotor dual task. Front. Hum. Neurosci. 9 , 381 (2015).
Gevins, A., Smith, M. E., McEvoy, L. & Yu, D. High-resolution EEG mapping of cortical activation related to working memory: Effects of task difficulty, type of processing, and practice. Cereb. Cortex 7 , 374–385 (1997).
Sauseng, P., Griesmayr, B., Freunberger, R. & Klimesch, W. Control mechanisms in working memory: A possible function of EEG theta oscillations. Neurosci. Biobehav. Rev. 34 , 1015–1022 (2010).
Hsieh, L. T. & Ranganath, C. Frontal midline theta oscillations during working memory maintenance and episodic encoding and retrieval. Neuroimage 85 , 721–729 (2014).
Pavlov, Y. G. & Kotchoubey, B. EEG correlates of working memory performance in females. BMC Neurosci. 18 , 26 (2017).
Scharinger, C., Soutschek, A., Schubert, T. & Gerjets, P. When flanker meets the n-back: What EEG and pupil dilation data reveal about the interplay between the two central-executive working memory functions inhibition and updating. Psychophysiology 52 , 1293–1304 (2015).
Fink, A., Grabner, R. H., Neuper, C. & Neubauer, A. C. EEG alpha band dissociation with increasing task demands. Cogn. Brain Res. 24 , 252–259 (2005).
Klimesch, W. EEG alpha and theta oscillations reflect cognitive and memory performance: A review and analysis. Brain Res. Rev. 29 , 169–195 (1999).
Puma, S., Matton, N., Paubel, P. V., Raufaste, E. & El-Yagoubi, R. Using theta and alpha band power to assess cognitive workload in multitasking environments. Int. J. Psychophysiol. 123 , 111–120 (2018).
Dasari, D., Shou, G. & Ding, L. ICA-derived eeg correlates to mental fatigue, effort, and workload in a realistically simulated air traffic control task. Front. Neurosci. 11 , 297 (2017).
Smith, M. E. & Gevins, A. Neurophysiologic monitoring of mental workload and fatigue during operation of a flight simulator in Proceedings of SPIE Defense and Security Symposium, Biomonitoring for Physiological and Cognitive Performance during Military Operations (ed. Caldwell, J. A. & Wesensten, N. J.), 116–126 (Orlando, 2005).
Brouwer, A. M., Hogervorst, M. A., Holewijn, M. & Erp, J. V. Evidence for effects of task difficulty but not learning on neurophysiological variables associated with effort. Int. J. Psychophysiol. 93 , 242–252 (2014).
Dan, A. & Reiner, M. Reduced mental load in learning a motor visual task with virtual 3D method. J. Comput. Assist. Learn. 34 , 84–93 (2017).
Bastiaansen, M. C., Posthuma, D., Groot, P. F. & de Geus, E. J. Event-related alpha and theta responses in a visuo-spatial working memory task. Clin. Neurophysiol. 113 , 1882–1893 (2002).
Itthipuripat, S., Wessel, J. R. & Aron, A. R. Frontal theta is a signature of successful working memory manipulation. Exp. Brain Res. 224 , 255–262 (2013).
Gevins, A. & Smith, M. E. Neurophysiological measures of working memory and individual differences in cognitive ability and cognitive style. Cereb. Cortex 10 , 829–839 (2000).
Pfurtscheller, G. & Aranibar, A. Event-related cortical desynchronization detected by power measurements of scalp eeg. Electroencephalogr. Clin. Neurophysiol. 42 , 817–826 (1977).
Kramer, A. F. Physiological metrics of mental workload: a review of recent progress. In Multiple Task Performance (ed. Damos, D. L.) 279–328 (Taylor and Francis, 1990).
Google Scholar
Winsun, W. V., Sergeant, J. & Geuze, R. The functional significance of event-related desynchronization of alpha rhythm in attentional and activating tasks. Electroencephalogr. Clin. Neurophysiol. 58 , 519–524 (1984).
Grabner, R. H., Neubauer, A. C. & Stern, E. Superior performance and neural efficiency: The impact of intelligence and expertise. Brain Res. Bull. 69 , 422–439 (2006).
Pfurtscheller, G., Neuper, C. & Mohl, W. Event-related desynchronization (ERD) during visual processing. Int. J. Psychophysiol. 16 , 147–153 (1994).
Klimesch, W., Doppelmayr, M., Pachinger, T. & Ripper, B. Brain oscillations and human memory: EEG correlates in the upper alpha and theta band. Neurosci. Lett. 238 , 9–12 (1997).
Krause, C. M. et al. The effects of memory load on event-relaCated EEG desynchronization and synchronization. Clin. Neurophysiol. 111 , 2071–2078 (2000).
Klimesch, W., Vogt, F. & Doppelmayr, M. Interindividual differences in alpha and theta power reflect memory performance. Intelligence 27 , 347–362 (2000).
Malhotra, P., Coulthard, E. J. & Husain, M. Role of right posterior parietal cortex in maintaining attention to spatial locations over time. Brain 132 , 645–660 (2009).
Sauseng, P. et al. A shift of visual spatial attention is selectively associated with human EEG alpha activity. Eur. J. Neurosci. 22 , 2917–2926 (2005).
Rominger, C. et al. The creative brain in the figural domain: Distinct patterns of EEG alpha power during idea generation and idea elaboration. Neuropsychologia 118 , 13–19 (2018).
Jia, W. & Zeng, Y. EEG signals respond differently to idea generation, idea evolution and evaluation in a loosely controlled creativity experiment. Sci. Rep. 11 , 2119 (2021).
Article ADS CAS PubMed PubMed Central Google Scholar
Nelson, B. D. & Shankman, S. A. Visuospatial and mathematical dysfunction in major depressive disorder and/or panic disorder: A study of parietal functioning. Cog. Emot. 30 , 417–429 (2016).
Grabner, R. H. & Smedt, B. D. Neurophysiological evidence for the validity of verbal strategy reports in mental arithmetic. Biol. Psychol. 87 , 128–136 (2011).
Klimesch, W., Doppelmayr, M., Schwaiger, J., Auinger, P. & Winkler, T. Paradoxical’ alpha synchronization in a memory task. Cogn. Brain Res. 7 , 493–501 (1999).
Rosengrant, D., Van Heuvelen, A. & Etkina, E. Do students use and understand free-body diagrams. Phys. Rev. Phys. Educ. Res. 5 , 010108 (2009).
Semlitsch, H. V., Anderer, P., Schuster, P. & Presslich, O. A solution for reliable and valid reduction of ocular artifacts, applied to the P300 ERP. Psychophysiology 23 , 695–703 (1986).
Download references
Acknowledgements
The work is supported by the National Natural Science Foundation of China (Grant Nos. 62077013).
Author information
Authors and affiliations.
School of Early Childhood Education, Nanjing Xiaozhuang University, Nanjing, China
Yanmei Zhu & Li Zhang
School of Biological Science & Medical Engineering, Southeast University, Nanjing, China
Yanmei Zhu & Qian Wang
You can also search for this author in PubMed Google Scholar
Contributions
Y.Z. designed the EEG study and prepared the manuscript. Q.W. carried out the EEG experiment. L.Z. wrote the code to analyze the data. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Yanmei Zhu .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Zhu, Y., Wang, Q. & Zhang, L. Study of EEG characteristics while solving scientific problems with different mental effort. Sci Rep 11 , 23783 (2021). https://doi.org/10.1038/s41598-021-03321-9
Download citation
Received : 20 April 2021
Accepted : 24 November 2021
Published : 10 December 2021
DOI : https://doi.org/10.1038/s41598-021-03321-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Advertisement

- Next Article
INTRODUCTION
Acknowledgments, a brain mechanism for facilitation of insight by positive affect.
- Cite Icon Cite
- Open the PDF for in another window
- Permissions
- Article contents
- Figures & tables
- Supplementary Data
- Peer Review
- Search Site
Karuna Subramaniam , John Kounios , Todd B. Parrish , Mark Jung-Beeman; A Brain Mechanism for Facilitation of Insight by Positive Affect. J Cogn Neurosci 2009; 21 (3): 415–432. doi: https://doi.org/10.1162/jocn.2009.21057
Download citation file:
- Ris (Zotero)
- Reference Manager
Previous research has shown that people solve insight or creative problems better when in a positive mood (assessed or induced), although the precise mechanisms and neural substrates of this facilitation remain unclear. We assessed mood and personality variables in 79 participants before they attempted to solve problems that can be solved by either an insight or an analytic strategy. Participants higher in positive mood solved more problems, and specifically more with insight, compared with participants lower in positive mood. fMRI was performed on 27 of the participants while they solved problems. Positive mood (and to a lesser extent and in the opposite direction, anxiety) was associated with changes in brain activity during a preparatory interval preceding each solved problem; modulation of preparatory activity in several areas biased people to solve either with insight or analytically. Analyses examined whether (a) positive mood modulated activity in brain areas showing responsivity during preparation; (b) positive mood modulated activity in areas showing stronger activity for insight than noninsight trials either during preparation or solution; and (c) insight effects occurred in areas that showed mood-related effects during preparation. Across three analyses, the ACC showed sensitivity to both mood and insight, demonstrating that positive mood alters preparatory activity in ACC, biasing participants to engage in processing conducive to insight solving. This result suggests that positive mood enhances insight, at least in part, by modulating attention and cognitive control mechanisms via ACC, perhaps enhancing sensitivity to detect non-prepotent solution candidates.
This article investigates the neural basis of one way that affect modulates cognition. Specifically, we report changes in brain activity, as measured with fMRI, that occur as affect modulates problem-solving strategies. People can solve problems through methodical, analytic processing, through insight, or through some mix of both (for recent reviews, see Bowden, Jung-Beeman, Fleck, & Kounios, 2005 ; Gilhooly & Murphy, 2005 ). These two strategies or (sets of) processes can co-occur, overlap, and interact, yet they are phenomenologically, behaviorally, and neurologically distinct, as described below. It has previously been demonstrated that positive affect (PA) specifically facilitates people's ability to solve creative or “insight problems,” that is, problems that are more often solved with insight (Rowe, Hirsch, & Anderson, 2007 ; Amabile, Barsade, Mueller, & Staw, 2005 ; Isen, 1999a , 1999b ; Estrada, Young, & Isen, 1994 ; Isen, Daubman, & Nowicki, 1987 ). Therefore, observing brain activity associated with shifts of problem-solving approaches in different affective states provides fertile ground for examining the neural mechanisms of emotion–cognition interactions. Here, we show that distinct affect states change actual cognitive organization to modulate problem-solving processes beyond the well-documented mood–memory congruency effect (Teasdale & Fogarty, 1979 ).
The distinction between insight and analytic solving has been anecdotally recognized for millennia and has been the subject of scientific inquiry for nearly a century (e.g., Duncker, 1945 ; Maier, 1930 ; Kohler, 1917 ). A plethora of behavioral evidence details how these two solving processes differ. Analytic processing involves deliberate application of strategies and operations to gradually approach solution. Insight, which is considered a type of creative cognition, is the process through which people suddenly and unexpectedly achieve solution through processes that are not consciously reportable. Insight solutions tend to involve conceptual reorganization, often occurring after solvers overcome an impasse in their solving effort, and are suddenly able to recognize distant or atypical relations between problem elements that had previously eluded them (Gilhooly & Murphy, 2005 ; Smith & Kounios, 1996 ; Schooler & Melcher, 1995 ; Weisberg, 1994 ; Schooler, Ohlsson, & Brooks, 1993 ; Metcalfe & Weibe, 1987 ; Metcalfe, 1986 ). When solution is achieved, these factors combine to create a unique phenomenological experience, termed the Aha! or Eureka! moment.
PA has been shown to facilitate insight and creative problem solving across a broad range of settings (Rowe et al., 2007 ; Ashby, Isen, & Turken, 1999 ; Isen, 1999b ; Estrada, Isen, & Young, 1997 ; Isen et al., 1987 ). One description of this effect is that PA enhances cognitive flexibility in various settings, such as in classifying material (Isen & Daubman, 1984 ), in negotiation tasks (Carnevale & Isen, 1986 ), in medical diagnoses (Estrada et al., 1994 ), and in creative problem-solving tasks (Isen, Mitzi, Johnson, Mertz, & Robinson, 1985 ; Isen & Daubman, 1984 ). Various explanations have been proposed to explain this facilitation (also see Discussion section ). Briefly, one hypothesis is that PA promotes a more global scope of attention (Bolte, Goschke, & Kuhl, 2003 ; Gasper & Clore, 2002 ), enhancing access to distant or unusual associations (Friedman, Fishbein, Förster, & Werth, 2003 ; Federmeier, Kirson, Moreno, & Kutas, 2001 ; Isen et al., 1985 ), which facilitates creative solutions to classic insight problems such as Duncker's ( 1945 ) candle task (Isen et al., 1987 ) and improves performance (Rowe et al., 2007 ; Isen et al., 1987 ) on the Remote Associates Test (Mednick, 1962 ). Another hypothesis is that PA enhances switching between global and local attentional modes (Baumann & Kuhl, 2005 ) or between strategies (Dreisbach & Goschke, 2004 ), or, similarly, that it enhances selection of different perspectives (Ashby et al., 1999 ).
In contrast, negative affect (NA) states such as anxiety and depression have been associated with deficits in attentional and cognitive control mechanisms (Bishop, Duncan, Brett, & Lawrence, 2004 ; Mayberg et al., 1999 ), often inducing a narrow scope of attention (Easterbrook, 1959 ). Therefore, anxiety in particular should impede cognitive flexibility, problem restructuring, and insight solving.
This study extends the existing literature in two ways. First, we examine not just the facility in solving a particular type of problem, but how mood modulates which strategy , insight or analytic, is preferred (or successful). Second, we measure brain activity as people solve these problems to observe the neural mechanisms of insight problem solving that are modulated by mood.
Insight and analytic problem solving are associated with different patterns of brain activity, measured with both fMRI and EEG, both at the moment people achieve solution (Jung-Beeman et al., 2004 ) and as people prepare for each new problem (Kounios et al., 2006 ). For one thing, the right hemisphere (RH), generally, seems to make stronger contributions as people process insight problems and recognize their solutions (Bowden & Jung-Beeman, 2003a ; Beeman & Bowden, 2000 ; Bowden & Beeman, 1998 ). More specifically, compared with solving problems without insight, solving with insight involves stronger activity in right temporal regions thought to be important for integrating distant semantic associations (Jung-Beeman et al., 2004 ). Additional brain regions showed similar but weaker “insight effects” in the earlier study but manifested strong effects in the current study; these include anterior cingulate, posterior cingulate cortex (PCC), parahippocampal cortex (PHC), right superior frontal gyrus (SFG), and right inferior parietal lobe (IPL).
Additionally, during a brief preparation period prior to the presentation of each problem, various brain regions are more active prior to problems solved with insight than prior to problems solved without insight (Kounios et al., 2006 ). That is, different patterns of brain activity are conducive to solving the subsequent problem with insight versus analytic processing. The distinguishing areas include bilateral temporal areas involved with semantic processing, PCC putatively involved in attention, and ACC thought to be important for cognitive control. Thus, each of these areas represents a reasonable candidate for affect-induced modulation of insight problem solving. The left temporal cortex is more adept at preparing to retrieve many close prepotent associations, whereas activity in the right temporal cortex enhances the readiness to pursue weaker associations (Jung-Beeman, 2005 ). On the other hand, the posterior cingulate is thought to be involved in visuospatial expectancy (Small et al., 2003 ), and the anterior cingulate is more likely to be involved in cognitive control and possibly in switching between solution candidates (or other thought processes), which is likely an important component of insight.
Anterior Cingulate and Insight Processes
We have demonstrated that the rostral portion of the dorsal anterior cingulate cortex (dACC; Brodmann's area [BA] 9, 24, 32) showed a sustained increase in neural activity during the preparatory interval before participants actually see problems, and stronger ACC activity occurs prior to trials solved with insight than those solved more analytically (Kounios et al., 2006 ).
We hypothesized that insights would involve greater cognitive control and restructuring processes, and that the dACC would be involved in the shift and the selection of a new solution path. In tasks involving response competition, cognitive control is thought to be important for the monitoring of competing responses (Weissman, Giesbrecht, Song, Mangun, & Woldorff, 2003 ; Van Veen, Cohen, Botvinick, Stenger, & Carter, 2001 ; MacDonald, Cohen, Stenger, & Carter, 2000 ), in overcoming prepotent responses when strategic processes were less engaged and conflict was high (Carter et al., 2000 ), and in shifting attention (Davis et al., 2005 ; Dreisbach & Goschke, 2004 ; Kondo, Osaka, & Osaka, 2004 ). Such cognitive control mechanisms could be critical for insight because they enable problem solvers to detect competing solution candidates, rely less on dominant associations or strategies, and/or enable shifting attention from a prepotent but irrelevant association to the less potent, but correct, association. This could be an important component of what insight researchers variously term cognitive restructuring and flexibility or “breaking set” and “overcoming functional fixedness.”
Anterior Cingulate, PA, and Insight
One possible mechanism by which PA could facilitate insight is through cognitive restructuring processes. PA is likely to facilitate insight by increasing a person's ability to switch and select alternative cognitive perspectives (Baumann & Kuhl, 2005 ; Dreisbach & Goschke, 2004 ; Isen, 1999b ), reducing perseveration on one particular solution candidate or solving approach, thus increasing the probability of engaging in various cognitive restructuring processes. We propose that PA could modulate activity in ACC (Lane, Reiman, Axelrod, Yun, & Holmes, 1998 ) to make it more open to detecting competing (weak) activations, biasing a shift toward insight solutions. The modulated ACC activity might facilitate one or a combination of mechanisms such as switching between global and local processing modes of attention (Baumann & Kuhl, 2005 ), switching from irrelevant to relevant solving strategies, and/or selecting the correct solution (Dreisbach & Goschke, 2004 ).
ACC appears to be a particularly promising site for interactions between cognitive processes and affect states. Besides its involvement in modulating cognitive processes via attention shifting, conflict detection, response competition, and/or selection mechanisms (Badre & Wagner, 2004 ; Botvinick, Cohen, & Carter, 2004 ; Kerns et al., 2004 ; Dreher & Grafman, 2003 ; Ruff, Woodward, Laurens, & Liddle, 2001 ; Bush, Luu, & Posner, 2000 ), ACC also appears to be involved in emotional processes (Bush et al., 2000 ; Mayberg et al., 1999 ; Drevets & Raichle, 1998 ; Whalen et al., 1998 ). Functional neuroimaging studies show overlapping activation patterns within ACC between cognitive and affective tasks (Fichtenholtz et al., 2004 ; Teasdale et al., 1999 ; Lane et al., 1998 ; Papez, 1937 ). Electrophysiological studies have identified a population of dACC neurons that show increased activity to high- versus low-conflict Stroop tasks, including those with emotional valence (Davis et al., 2005 ). Moreover, cytoarchitectonic studies suggest the involvement of specialized spindle cells of BA 24 that integrate cognitive input with emotional overtones (Nimchinsky et al., 1999 ).
Given ACC's involvement in cognitive control and emotional processes and our prior evidence that activity in ACC prior to solving problems is associated with solution strategy, we predict that affect states will modulate ACC activation and thereby influence insight (versus analytic)-solving processes. Specifically, we hypothesize that PA states will increase activity in ACC before the actual problem onset, biasing the solver toward cognitive processing that is relatively conducive to insight.
Hemispheric Asymmetries, Affect, and Insight
Another possibility can be derived from the following considerations: (1) RH processing seems to make strong contributions to insight solving overall (Jung-Beeman et al., 2004 ; Bowden & Beeman, 1998 ); (2) RH semantic processing activates or maintains activation of a broader set of semantic associations than does LH semantic processing (Faust & Mashal, 2007 ; Beeman et al., 1994 ; Chiarello, 1991 ), and these broad associations seem very relevant for solving with insight; (3) positive mood seems to broaden the overall pattern of semantic associations (Federmeier et al., 2001 ; Isen et al., 1985 ); (4) global or broad attention is associated with RH visual processing, creative problem solving (Ansburg & Hill, 2003 ), and positive mood (Rowe et al., 2007 ; Gasper & Clore, 2002 ); and (5) inducing an approach regulatory focus (with low arousal) increases measures of relative RH activation as well as facilitating creative problem solving (Friedman & Förster, 2005 ). Thus, it remains hypothetically possible that PA will directly increase overall activity in the RH, specifically in the right superior temporal gyrus (STG), which is, cytoarchitectonically more suited than the left STG at integrating distant semantic associates via coarse semantic coding (for a review, see Jung-Beeman, 2005 ). However, such an effect might seem to contradict some established associations between positive mood (or approach focus) and leftward asymmetries in electroencephalographic activity (Herrington, Mohanty, Koven, Fisher, & Stewart, 2005 ; Davidson, 1992 ; Tomarken, Davidson, Wheeler, & Doss, 1992 ). Moreover, to us, it seems intuitively more likely that a global characteristic like positive mood would either modulate all semantic processing (in both hemispheres) to broaden the scope of semantic associations or, more likely, to modulate attention or cognitive control mechanisms that make solvers better able to detect (and utilize) remote associations that are only weakly active (perhaps, mostly due to RH semantic processing).
Insight typically occurs when people initially focus on an incorrect but dominant association (e.g., in Figure 1 , ache can form compounds with tooth and heart but not potato) and need to overcome this impasse and switch to the correct solving strategy to be able to reach a sudden (Aha!) understanding of the solution (Jung-Beeman et al., 2004 ; Bowden & Jung-Beeman, 2003a ). In many studies of insight solving, problems have typically been classified a priori, as either “insight problems” or “noninsight problems” (Weisberg, 1994 ); but because any problem can be solved through insight, through straightforward (incremental, strategic) problem solving, or through a combination of both (Bowden et al., 2005 ), the a priori “insight” classification is not definitive.
Sequence of events within a trial of the CRA task. Each trial began with a central fixation cross, signaling the onset of the preparation interval, which lasted for a variable 0–8 sec, after which the problem was presented. Participants pressed the response buttons bimanually if/when they achieved solution, verbalized the solution at the Solution prompt, and then reported whether they solved the trial with or without insight at the Insight prompt. The intervals between these events were jittered for a variable 0–8 sec.
We exploit this feature by asking participants to report directly which strategy they used predominantly to achieve solutions to directly contrast trials that lead to insight solutions versus those that lead to noninsight solutions. This enables us to examine insight versus noninsight processing while holding task and stimulus type constant. Participants were presented with a large set of compound remote associate (CRA) problems (Bowden & Jung-Beeman, 2003b ). Similar problems (Mednick, 1962 ) are often used as “insight problems” or for creative problem solving (Isen, 1999a ; Isen et al., 1987 ), and the ability to solve them correlates with the ability to solve other classic insight problems (Duncker, 1945 ; Maier, 1930 ). However, they can be solved either analytically or with insight (Bowden & Jung-Beeman, 2003a ; for a review, see Bowden et al., 2005 ). The type of processing involved in successfully solving these problems varies across trials (Kounios et al., 2006 ; Jung-Beeman et al., 2004 ) and across individuals (Kounios et al., 2008 ), making this paradigm a strong candidate for investigating how affect can, in a general (rather than in only a mood-consistent) way, modulate higher level cognition. Specifically, we examine the brain basis of how PA and anxiety modulate solving strategy, tipping the balance of processes toward insight or analytic strategies.
Mood state (including PA and NA and anxiety) and personality measures, gathered prior to the experimental session, were related to performance and neuroimaging measures. For the participants who underwent fMRI scanning, we identified brain regions involved in various aspects of problem solving and correlated the signal change in these regions with the mood and personality indices, as well as identifying areas that showed contrast in the brain activity during problem preparation between high and low positive mood individuals.
Participants and Procedure
All 79 participants were neurologically healthy, right-handed, and native speakers of English. After giving informed consent, all participants completed mood state inventories for the Positive and Negative Affect Schedule (PANAS), the State-Trait Anxiety Inventory (STAI), and a variety of other personality inventories measuring more stable individual traits (the Behavioral Inhibition Scale–Behavioral Activation Scale, the Neuroticism subscale for the Big 5 Personality Mini-Markers, and the Magical Ideation Scale as an indicator of schizotypy). The mood state inventories (PANAS and STAI), given to all participants just before they performed the CRA task, measured the extent that participants were currently experiencing a positive (PANAS) or anxious mood (STAI). We examined correlations between all mood and personality scores and various problem-solving measures (solving rate and proportion of problems solved with insight) as well as fMRI signal change.
After these questionnaires, 52 participants performed the problem-solving task outside the scanner, providing only behavioral data, and 30 participants performed the problem-solving task in the scanner. Data from three participants were excluded—due to poor fMRI signal in two of the participants and due to one participant providing only two analytic responses.
Problem-solving Paradigm
We measured insight and analytical solving of 135 CRA problems (Bowden & Jung-Beeman, 2003b ), adapted from a test of creative cognition (Mednick, 1962 ). For each problem, participants see three problem words (tooth, potato, and heart) and must generate a solution (sweet) that can form a compound word or phrase with each problem word (sweet tooth, sweet potato, sweetheart). The solution word can precede or follow each problem word. Like most problems (even classic “insight problems”), these problems can be solved either with insight or through more methodical or analytical processes. We relied on participants' trial-by-trial judgments to determine the type of processing that led to each solution. This method has reliably shown consistent differences in behavior (Bowden & Jung-Beeman, 2003a ; Beeman & Bowden, 2000 ; Bowden & Beeman, 1998 ) and in brain activity (Kounios et al., 2006 , 2008 ; Jung-Beeman et al., 2004 ). For instance, in our prior EEG study, the neural processes biasing the sudden (Aha!) that led up to an insight solution were associated with increased neural activity (less alpha power) peaking over midfrontal cortex and bilateral temporal cortices for insight versus analytical preparatory processes (Kounios et al., 2006 ). Using a different population sample and methodology, fMRI signal corroborated the EEG findings, specifically isolating ACC as the medial frontal region that revealed increased neural activity for insight versus noninsight preparatory processing, and also showed increased activity within the bilateral temporal cortical areas revealed during EEG (Kounios et al., 2006 ). In another study, about a third of a second prior to the insight solution button press, a burst of EEG gamma activity in the right anterior superior temporal gyrus (aSTG) corresponded to the increase in fMRI solution-related signal within the same region (Jung-Beeman et al., 2004 ). This RH activation likely reflects the processing and integration of a broad range of semantic associations leading to solution (Jung-Beeman et al., 2004 ; Bowden & Jung-Beeman, 2003a ; Bowden & Beeman, 1998 ; Beeman et al., 1994 ).
Prior to the current experiment, participants received instructions to make insight/noninsight judgments, emphasizing that they should respond “insight” if they achieved solution suddenly and surprisingly, possibly by switching their train of thought just prior to solution, and that as soon as they thought of the solution candidate, they were instantly confident it was the solution. In contrast, they should respond “noninsight” if they achieved solution incrementally or by some analytical strategy, for example, by strategically retrieving candidates and testing them out.
Each trial began with a fixation cross that remained on the screen for a variable rest period (from 0, 2, 4, 6, or 8 sec, randomized across all trials), during which participants prepared for the next trial ( Figure 1 ). Such variable delays were used to jitter the events and to optimize deconvolution of the fMRI signal from successive events. After this preparation period, the three problem words (tooth, potato, and heart) were presented on the screen (horizontally centered, just above, at, and just below central fixation) and persisted until participants solved the problem or a 15-sec time limit was reached. Participants attempted to produce a single solution word (sweet) that could form a compound word with each of the problem words. If participants solved the problem, they made a bimanual button press (to avoid biasing laterality of cortical activity) by pressing the two outer buttons with a finger on each hand when they arrived at the solution; after a variable (0–8 sec) delay, a solution prompt appeared, and participants verbalized the solution. After another variable delay (0–8 sec), an insight prompt (“Insight?”) appeared, and participants pressed the two outer buttons with a finger on each hand if they had reached the solution with an insight, or they pressed the two inner buttons if they had reached the solution through analytic noninsight means. After the insight/analytical solution rating, or after 15 sec elapsed on unsolved trials, the next preparation period began.
Image Acquisition
Thirty fMRI participants performed the CRA task during scanning, which for all participants occurred in the same Siemens Trio (3 T) scanner and eight-channel head coil, with the same scanning protocol, at Northwestern's Center for Advanced MRI. Head motion was restricted with plastic calipers built into the coil and a vacuum pillow. The functional imaging sequence was optimized for detection of the BOLD effect (Ogawa et al., 1992 ) including local shimming and 8 sec of scanning prior to data collection to allow the MR signal to reach equilibrium. Functional imaging used a gradient-echo echo-planar sequence (TR = 2 sec for thirty-eight 3-mm slices, TE = 20 msec, matrix size = 64 × 64 in 220-mm field of view). Participants solved problems during four scans of 10 min 20 sec and a final fifth scan that was truncated when participants finished solving problems. Each functional scan was synchronized with the onset of the first problem in that block of trials; timing of subsequent trials was response dependent and not synchronized with image acquisition. Anatomical high-resolution images were acquired in the same plane, with T1-weighted images parallel to the AC–PC plane.
Image Analysis
Functional and anatomical images were coregistered through time, spatially smoothed with a 7.5-mm Gaussian kernel, and fit to a common template. Within each run, voxels were eliminated if the signal magnitude changed more than 20% across successive TRs, or if the mean signal level was below a noise threshold. Functional data were transformed (Collins, Neelin, Peters, & Evans, 1994 ) to a standard stereotaxic atlas (Talairach & Tournoux, 1988 ) with a voxel size of 2.5 mm 3 . The data were analyzed using general linear model analysis, as implemented in AFNI (Ward, http://afni.nimh.nih.gov/afni ), that extracted average estimated responses to each trial type, correcting for linear drift and removing signal changes correlated with head motion as well as signal attributed to other temporally adjacent events to ensure that signal could be isolated to the event of interest. For example, when extracting signal related to preparation events, we included in the analysis the preceding insight ratings, the subsequent problem onsets, and the subsequent solutions to factor out signal more closely tied to those events than to the preparation event. Signal was estimated for all time points (TRs 0–10) within the same model, without regard to any presumed hemodynamic response function.
Areas that turned on, that is, changed their activity, during preparation. We examined overall responsivity corresponding to the preparation interval, manifested as a rise and fall of BOLD signal from onset of the preparation period to peak response and back down to baseline. Specifically, for every voxel, signal corresponding to the peak of the preparation period (TRs 4, 5, and 6 after onset of preparation period; for comparison, there was a peak signal in motor cortex at TR 3, corresponding to the button press from the insight-rating preceding the preparation period) was contrasted with signal corresponding to the points preceding and after the preparation period (TRs 1, 9, and 10). We identified regions of signal change that were consistent across all 27 participants, with a significance threshold combining t values ( p < .005) and cluster size (at least 1500 mm 3 in volume). The dACC, the PCC, and the right angular gyrus (AG) clusters exceeded the above criteria, increasing preparatory activity. Of all these statistically reliable clusters (functionally defined ROIs), the dACC and the right AG were the only two ROIs where preparatory responsivity strongly correlated with positive mood across all 27 participants.
Areas that showed insight-specific activity during preparation or solution. Peak preparatory signal specific to insight trials was calculated by comparing the difference between insight and analytic preparation events for each participant by extracting the mean signal within the three TRs (TRs 4, 5, and 6) corresponding to the expected preparatory hemodynamic peak. For comparison, the preceding insight-rating button press elicited peak signal in motor cortex at 4 sec, just prior to the preparation onset peak signal (6 sec) for each participant. Similarly, peak insight solution-related signal was calculated by examining differences between insight and analytic solution events for each participant by examining the mean signal within the three TRs (TRs 3, 4, and 5—we chose an early time window to minimize contamination from postsolution activity) corresponding to the expected peak signal leading up to the solution point (see Figure 9 for comparison). The subsequent button press elicited peak signal in motor cortex (10 sec) at the solution point. The significance threshold combined cluster size and t values for each voxel within a cluster (set at least 500 mm 3 in volume) in which each voxel was reliably different across participants, t (26) = 3.09, p < .005 uncorrected, for insight versus noninsight preparation and for insight versus noninsight solutions. ACC, PCC, left STG, and right MTG ROI clusters exceeded these criteria, manifesting stronger preparatory peak signal for insight versus analytical trials. Several regions showed stronger peak signal for insight versus analytical solutions including ACC, PCC, right PHC, left MTG, right MTG, right IPL, and right SFG.
Areas that showed mood differences in activity during preparation. To examine how individual differences in affect state influenced successful preparation preceding solved trials, a whole-brain analysis identified regions in which the eight participants highest in PA showed different signal during preparation (as described in A) than did the eight participants lowest in PA. The dACC, ventral ACC (vACC), and PCC all exceeded significance criteria, t (14) = 3.32, p = .005, v > 500 mm 3 , all showing stronger preparatory activation for subjects high in PA than for participants low in PA.
The functional overlap, illustrated in a convergence map, between all the three analyses occurred only within the dACC at (−2, 42, 22). The analysis with the least stringent significance threshold corresponded to a p < .005, combined with a cluster size of at least 500 mm 3 . Thus, the functional overlap between all three analyses, manifesting activation only within the dACC, suggests a much lower probability of a type I error.
In a final set of analyses, we examined whether insight effects (stronger peak signal for insight than for noninsight trials, across all 27 participants) occurred in any of the ROIs defined by the positive mood preparatory effect (item C). We contrasted peak fMRI signal for insight versus noninsight preparation periods (defined above) as well as insight versus noninsight solutions (at the TRs corresponding to the last 2 sec of processing prior to solutions). Within these ROIs, consistently stronger signal for insight than for analytic preparatory events occurred only within the dACC. Similarly, within these mood-sensitive ROIs, stronger signal for insight than for analytic solutions occurred only in the dACC. None of the mood-sensitive ROIs showed stronger signal for analytic than insight trials at preparation or solution. Insight versus analytic signal was not enhanced by positive mood at any other time points (all p > .2).
Behavioral Measures
Participants correctly solved 41.0% ( SD = 11.4) of the problems and identified 50.8% ( SD = 16.3) of their solutions as insight (mean response time = 6.57 sec, SD = 1.31) and 46.8% ( SD = 16.2) of their solutions as analytic/without insight (mean response time = 7.35 sec, SD = 1.23), reliably slower than the insight responses [ t (78) = 3.60, p << .001]. Of trials with responses, 3.96% ( SD = 2.52) were errors.
We examined how affect, assessed by a variety of state, trait, and personality questionnaires, related to problem-solving behavior. The range of scores on the affective scales was somewhat limited. In particular, only 5 of 79 participants had a score higher than 20 on the NA scale, which ranges from 10 to 50. However, some participants had a high score on both the PA and the NA scales, consistent with the assertion that the PA and the NA scales are orthogonal (Watson, Clark, & Tellegen, 1988 ). How should we compare the mood of a person scoring high on PA and NA with the mood of a person scoring high on PA but low on NA? Although results were as strong (sometimes stronger) if we used strict PA scores, we took into account NA scores by using PA–NA as an index of positive mood.
Consistent with prior studies, positive mood modulated solving rates: the top third most positive (PA–NA) participants (mean PA–NA score = 24.0, SD = 3.77; see Table 1 ) solved more problems (mean solved = 60.0; mean solution response time = 6.66 sec) than did the bottom third or least positive mood participants (mean PA–NA score = 5.35; mean solved = 51.3; mean solution response time = 7.19 sec), t (50) = 2.24, p < .05.
Behavior: Positive Mood Enhances Solving Performance and Solving with Insight while Anxiety Inhibits Solving with Insight
For all 79 participants tested, mean number of overall solutions, solutions with insight, and analytical noninsight solutions are given for each participant group ( n = 26); high versus low positive mood was calculated using PA–NA scores from the PANAS inventory; high- versus low-anxiety scores from the STAI inventory (* p < .05, ** p < .01, *** p < .0005). Solved percentages were calculated out of 135 trials; insight and analytical percentages were calculated out of the total solved number.
Positive mood was also related to which type of strategy, by self-report, led to solutions. As predicted, the number of insights differed significantly across the three levels of positive mood [ F (2,76) = 7.364, p = .001]. By contrast, the number of problems solved analytically, that is, without insight, did not differ [ F (2,76) = 1.485, p = .233]. Therefore, positive mood specifically facilitated insights but did not change the rate of analytical solutions ( Figure 3A ). Specifically, the highest positive mood participants solved more problems with insight (mean insights = 34.5; mean insight response time = 6.12 sec) than did the lowest positive mood participants (mean insights = 21.9; mean insight response time = 7.31 sec), t (50) = [3.96], p < .0005. Overall, a regression analysis (partialing out all other mood and personality variables) showed that positive mood (PA–NA) was directly correlated with insight solving [ r (77) = .40, p < .005; Figure 2 ].
Scatterplots for all 79 participants indicating the relation between percent of solutions achieved by insight and (A) positive mood (PA–NA) and (B) anxiety (STAI), both presented in standardized z scores for illustration purposes, with regression lines and values obtained from multiple regression including all mood and personality measures.
Anxiety had the opposite effect (see Figure 3B ) where the third of participants highest in anxiety (mean STAI score = 42.1, SD = 3.77) solved fewer problems with insight (mean insights = 24.1; mean insight response time = 6.12 sec) than did the third of participants, t (50) = [2.75], p < .01, lowest in anxiety (mean STAI score = 24.7; mean insights = 33.1; mean insight response time = 7.31 sec), and anxiety was inversely correlated with solving with insight [ r (77) = −.34, p < .005; Figure 2 ]. However, anxiety did not have a reliable effect on overall solving rates (top versus bottom third), t (50) = [1.277], p = .207. Anxiety enhanced the proportion of solutions achieved analytically without insight, t (50) = [2.189], p = .033, but did not reliably change the raw number of analytical solutions, t (50) = [1.235], p = .222.
Subgroups of participants by high, medium, and low (A) positive mood and (B) anxiety scores, illustrating the number of correct solutions achieved with and without insight.
Imaging Measures
We conducted three analyses to examine the neural basis of the interaction between positive mood and insight solving. In these analyses, we showed that PA modulates participants' preproblem preparatory brain states to specifically facilitate insight solutions by enhancing signal within the rostral region of the dACC (see convergence map in Figure 7 ). These preparatory brain states were assessed by examining fMRI signal corresponding to the variable 0–8 sec rest between the end of one trial and the beginning of the next three-word problem while participants fixate on a centrally located cross and prepare for the next problem (Kounios et al., 2006 ).
(A) Do Brain Regions Showing Signal Change at Preparation Show Mood Effects?
As described in the Methods section , we first identified ROIs that showed changes in neural activity across all preparatory periods preceding trials that participants subsequently solved ( Figure 4 , Table 2 ). Across all participants, we then examined whether this preparatory activity correlated with PA, anxiety, solving rates, or solving strategy (solving with insight or noninsight). This analysis enabled us to investigate if certain regions that “turned on” at preparation were modulated by positive mood and anxiety states.
(A) The ROIs within the dACC (see Table 2 for coordinates) showing strongly increased signal ( p < .0001), across all 27 participants, corresponding to the preparation interval, superimposed on the averaged normalized structural image of all participants. Brain images show (left to right) axial, sagittal, and coronal images (with left hemisphere on left of axial and coronal images). (B) The average signal change across this dACC region for the 20 sec after onset of the preparation interval (which lasted 0–8 sec). (C) Scatterplot illustrating the correlation between positive mood and increased preparatory activity in this dACC region (peak–baseline) across all 27 participants.
Neuroimaging: Positive Mood States Predict Increased Preparatory Activity in ACC to Enhance Solving with Insight
Each value in the correlations section is a correlation value of either positive mood (PA–NA), anxiety (STAI), or overall solving proportion with activity in the corresponding cluster that represents the signal difference between the contrasted conditions as a percent of average signal within the cluster (* p < .05). (A) ROIs identifying significant signal change within the three TRs corresponding to the expected peak preparatory signal (i.e., TRs starting at 6 through 12 sec) compared with the first and last two TRs corresponding to the baseline preparation signal. (B) the positive mood preparatory ROIs with increased fMRI preparatory activity for the top eight participants highest in positive mood than the bottom eight participants lowest in positive mood. (C) ROIs with stronger fMRI peak signal for insight preparation than for analytical noninsight preparation. (D) ROIs with stronger fMRI signal within the three TRs corresponding to the expected peak signal just prior to insight solutions than for analytical solutions. No clusters showed the opposite effect at this strict threshold.
As illustrated by Table 2A , three areas showed increased activation during preparation: dACC, PCC, and the right AG. In two of these regions, as positive mood increased across all 27 participants, so did the amount of preparatory activity: in the ACC [ r (25) = .41, p < .05; see Figure 4C ] and in the right AG [ r (25) = .40, p < .05]. Preparatory activity in the rostral dACC also inversely correlated with anxiety, but this correlation was not statistically reliable [ r (25) = −.34, p = .08; Table 2 ]. Preparatory activity in the PCC showed a mild but nonsignificant positive correlation with overall proportion of problems solved [ r (25) = .36, p = .06; Table 2 ], but no correlation with positive mood.
Hypothetically, deactivations could be equally important to increases in activation. So, for completeness, we performed the same analyses looking at areas that deactivated during preparation. Two areas showed systematic deactivation compared with baseline: the left and the right IFG. This deactivation during preparation was negatively correlated with the overall proportion of problem solved [left IFG: r (25) = −.40, p < .05; right IFG: r (25) = −.50, p < .05; Table 2 ] but did not correlate with any mood variables ( p s > .20). This analysis (item A, Methods section ), therefore, demonstrates that among the ROIs showing changes in neural activity at preparation, only the dACC and right AG increased activation with positive mood.
Does Brain Activity at Preparation Predict Brain Activity at Solution?
We examined whether preparatory brain activity predicted overall solution brain activity, and whether this preparatory activity then correlated with mood in regions showing specific insight effects. As mentioned above, the areas showing overall increased responsivity at preparation included the dACC, the PCC, and the right AG. Each of these areas, therefore, represents a good candidate for preparatory activity predicting overall solution-related activity. To examine where preparatory activity predicted overall solution-related activity, we identified regions that showed solution-related responsivity, similar to the way we defined preparatory ROIs as described by item A ( Methods section ). For instance, we defined solution-related ROIs by subtracting the mean signal across the three TRs corresponding to baseline solution-related signal (TRs 1, 6, and 7) from the mean signal across the three TRs (TRs 3, 4, and 5) corresponding to peak signal leading up to the solution (see Figure 9 for comparison). These solution-related functional ROIs would, therefore, indicate regions of the brain that “turned on” upon arriving at solution. We then looked back at preparatory responsivity within these solution-active ROIs. We found that as preparatory activity increased, so did solution-related responsivity, within one region only: the region of the dACC. This analysis demonstrates that preparatory activity within the dACC partially predicted overall solving activity.
(B) Do Brain Regions Showing Insight Specific Activity at Either Preparation or Solution Correlate with Mood?
We next examined whether preparatory activity correlated with mood in regions identified as showing insight-specific processing (see item B, Methods section ). We identified ROIs that showed an “insight effect,” that is, stronger peak signal for insight versus analytical processes, either during preparation (Kounios et al., 2006 ) or leading up to solution (as in Jung-Beeman et al., 2004 ). Within these “insight effect” regions, we examined whether overall preparatory responsivity (from preparation onset to peak response and back down to baseline) was modulated by positive mood states.
Regions that showed this “insight effect” at preparation—stronger signal during preparation preceding problems that were eventually solved with insight than during preparation preceding analytic solutions—included the ACC, the PCC, and the right and left MTG ( Table 2C ), as previously described (Kounios et al., 2006 ). Within these ROIs, positive mood correlated (across all 27 participants) with preparatory responsivity only in ACC [ r (25) = .40, p < .05; Table 2C ]. This preparatory activity in ACC also inversely correlated with anxiety [ r (25) = −.40, p < .05]. Moreover, the peak of this preparatory activity in ACC correlated with the overall proportion of problems solved [ r (25) = .37, p = .05]. Positive mood did not correlate with preparatory activity observed in other areas showing insight effects during preparation [PCC: r (25) = .23, p = .27; left MTG: r (25) = .22, p = .27; right MTG: r (25) = −.16, p = .42].
We next examined whether positive mood modulated preparatory activity in areas that showed an “insight effect” at solution (see Figure 9 ). We identified several regions showing insight effects at solution, that is, stronger signal for insight solutions than for noninsight solutions. These ROIs included the right aSTG, the ACC, the PCC, the right PHC, the bilateral MTG (stronger in right than left), the right SFG, and the right IPL ( Table 2D ). These data, with more participants and better imaging protocols, match well with earlier results showing smaller effects, but in the same general regions, with right aSTG again showing the largest effect (Jung-Beeman et al., 2004 ). Within all these ROIs showing insight effects at solution, preparatory activity correlated with positive mood only within ACC [ r (25) = .45, p < .05; see Figure 5 , Table 2D ]. Again, ACC preparatory activity negatively correlated with anxiety [ r (25) = −.44, p < .05], whereas preparatory peak signal positively correlated with the overall proportion of problems solved [ r (25) = .37, p = .05].
(A) The ROI within the rostral ACC showing stronger signal for insight ( p < .001) than for noninsight solutions (as in Jung-Beeman et al., 2004 ) across all 27 participants. Brain images show (left to right) axial, sagittal, and coronal images (with left hemisphere on left of axial and coronal images). (B) Scatterplot illustrating the correlation between positive mood and increased preparatory activity (peak–baseline) in this rostral ACC region showing an insight solution effect across all 27 participants.
(C) Are Brain Regions Showing Positive Mood Effects during Preparation Involved in Solving with Insight?
In the above analyses, we identified ROIs by overall preparatory responsivity (item A, Methods section ) and by insight effects (item B, Methods section ) and then found that preparatory activity within ACC ROIs specifically consistently correlated with positive mood across all participants. Analysis C ( Methods section ) does the converse, first identifying ROIs that show mood effects in preparation for all trials, then determining whether an insight effect (stronger signal prior to insight solutions than prior to noninsight solutions) occurred within these ROIs. The positive mood preparatory effect indicated which brain regions manifest increased preparatory responsivity, across all trials, for the eight participants highest in positive mood compared with the eight participants lowest in positive mood, regardless of whether the hemodynamic response demonstrated a rise and fall of signal ( Figure 6 ). 1 For instance, some areas showed decreasing activity during preparation (left and right IFG) but more rapid decreases in low positive mood than in high positive mood participants. ACC and PCC showed more preparatory responsivity for the eight participants highest versus the eight participants lowest in positive mood. In ACC region showing a mood group effect across all trials (specifically, the rostral portion of the dACC; see Figure 6 ), the preparation signal was stronger, across all participants, preceding problems subsequently solved with insight than preceding problems subsequently solved analytically, t (26) = [2.3], p = .03 (see Figure 8 ). In contrast, the PCC region that showed stronger preparation signal for the high positive than for the low positive mood participants did not show any insight effect during preparation ( t < 1.0). We then tested whether these same regions (showing mood effects during preparation) showed insight effects leading up to solution. Indeed, across all participants, there was stronger fMRI signal for insight solutions than for noninsight solutions in the dACC, t (26) = [3.97], p < .0005 (see Figure 9 ), the vACC, t (26) = [3.8], p < .001, and the PCC, t (26) = [3.8], p < .001. These effects were not due to making the insight rating at the end of each trial, as there were no effects within any of these ROIs on the BOLD signal corresponding to the insight rating button press (all t values <1.2).
All ROIs showing stronger signal change (peak–baseline) corresponding to the preparation interval for high positive mood than for low positive mood participants ( p < .005). Reliable clusters include dACC and vACC as well as PCC. (No reliable clusters showed the reverse, that is, stronger signal for low positive mood participants.)
Thus, some brain areas—particularly ACC—in which positive mood modulated activity during the preparation for upcoming trials do seem especially involved in processing that leads to insight solutions. The functional overlap of areas showing both mood and insight effects is illustrated in a convergence map ( Figure 7 ), which shows that only the rostral portion of the dACC manifests the mood–insight correspondence in all three analyses described above.
Convergence map showing all voxels within each of the three types of analyses: Voxels showing reliable signal change (peak–baseline) corresponding to preparation (blue); voxels showing both preparation activity and insight solution effects (green); voxels showing both preparation activity and stronger preparation signal in high than in low positive mood participants (purple); and voxels showing all three effects (black).
Participants higher in positive mood showed different patterns of brain activity during preparation periods preceding each solved problem and solved more problems overall compared with participants lower in positive mood. The mood-related facilitation in solving was limited to solving with insight, as high positive mood participants solved many more problems with insight and somewhat fewer without insight compared with the low positive mood participants. The results reported above used PA–NA scores as an index of positive mood and are maintained or stronger when using PA alone as the index of positive mood. In regression analyses with all mood and personality measures, PA yielded a nominally stronger correlation with insight percentage [ r (77) = .41, p < .0005] than did PA–NA [ r (77) = .40, p < .0005]. Furthermore, the same pattern of HRF peaks and group differences were attained if PA was used rather than PA–NA. However, some subjects scored high on both PA and NA (consistent with prior literature claiming PA and NA scores on the PANAS inventory are orthogonal; e.g., Watson et al., 1988 ), so it is unclear whether they should be considered high in positive mood. Therefore, we decided to consistently use PA minus NA scores throughout all the analyses.
Interestingly, as positive mood seemed to be increasing overall solving productivity, as well as shifting the type of processing employed to specifically facilitate insight solving, anxiety had somewhat the opposite effect, decreasing insight solutions, but not affecting solving performance as reliably or as consistently as positive mood.
The experimental paradigm relies on retrospective self-report measures to categorize solutions as insight versus noninsight. It is, thus, important to note that positive mood affected not just whether participants reported insight, but also their overall ability to solve problems (higher positive mood participants actually solved more of these problems, and all the “extra” solutions were reported to be with insight). Thus, mood affects solving behavior.
This trial-by-trial reporting method does not assume participants solve problems with insight based on a priori categorization of the problems. (In pilot research, participants report that they solved classic insight problems with insight about 65% of the time—with some analytic and “other” solutions—and report that they solve classic analytic problems with insight about 25% of the time). Even if we did rely on relatively more “objective” measures such as GSR measures or warmth ratings, we would still have more confidence in self-report measures. For instance, if a subject reports to have had an insight but shows gradual continuous changes in warmth ratings as he or she progresses toward the solution rather than the sudden discontinuous jump associated with insights upon reaching the solution, we would still have more trust in the subject's self-report assessment rather than warmth ratings.
Moreover, in prior studies, participants manifest different patterns of behavior and neural activity when they report solving (or recognizing solutions) with insight compared with when they report solving without insight. For example, recognizing solutions with insight occurs faster and with more priming of solutions (suggesting semantic activation of the solution prior to solving) than recognizing solutions without insight (e.g., Bowden & Jung-Beeman, 2003a ).
Within the current study, the different solution categories were associated with qualitatively distinct patterns of brain activation preceding solution (see Figure 9 ), including differently shaped hemodynamic response functions; yet there were no consistent differences at the point of insight judgments. This suggests that the decisions were based on some differences in prior processing leading up to solutions rather than post hoc decisions.
Also, both the high and the low positive mood groups showed identical solution latency patterns (in this experiment, slightly faster insight than noninsight solutions) and parallel hemodynamic responses in fMRI signal within each category (insight vs. noninsight), suggesting that high and low positive mood participants used roughly the same processes and decision-making criteria for identifying insight and noninsight solutions.
Besides affecting behavior, positive mood also correlated with brain activity as people prepared for each new problem (in the task-free preparation interval). Specifically, we examined brain regions that changed activity during this preparation period, regions that showed insight effects (more activity during insight than noninsight trials) during this preparation period, and regions that showed insight effects at solution. Across all these analyses, only dACC consistently showed brain activity during this (resting) preparation interval that increased as positive mood increased (see Table 2 , Figure 7 ). The corollary was also true: ACC region that was more responsive (showed greater increase of fMRI signal corresponding to the preparatory period) in highly positive than in less positive participants also showed insight effects across all participants ( Figures 8 and 9 ). All these affect-related effects occurred despite a somewhat limited range of variability in affect (particularly in terms of NA).
Insight preparation effect in mood-sensitive ROI: average percent signal change over time, corresponding to the preparation interval across all voxels in the ROI showing a reliable mood effect (stronger preparation activity in the high than in the low positive mood participants). The blue line shows signal change for preparation prior to problems solved with insight; the pink line shows preparation signal prior to problems solved without insight; and the green line shows the difference, which was near-constant throughout the epoch. The green-shaded region (i.e., TRs starting at 6 sec through TR ending at 12 sec), showing stronger signal for insight versus noninsight preparation (* p < .05), corresponds to the peak signal for the preparation period. For comparison, the preceding button press elicited peak signal in the motor cortex (M1) at 4 sec, which corresponded to the insight rating button press from the prior trial.
Insight solution effect in mood-sensitive ROI: average percent signal change over time, corresponding to the solution interval (i.e., 0 sec corresponds to a solution event 2 sec prior to the solution button press) across all voxels in the ROI showing a reliable mood effect at preparation. The blue line shows signal change prior to problems solved with insight; the pink line shows signal change prior to problems solved without insight; and the green line shows the difference, which was near-constant throughout the epoch. The green-shaded region (TR starting at 4 sec through TR ending at 10 sec) showing greater solution-related signal (* p < .0005) for insight versus noninsight trials corresponds to the peak signal leading up to the solution. For comparison, the subsequent solution button press elicited peak signal in the motor cortex (M1) at 10 sec (i.e., 8 sec after the button press).
Thus, we have strongly demonstrated that positive mood is reliably associated with preparatory states that increase responsivity in the rostral dACC, and that this modulation is associated with processing that leads to insight solutions. We are not arguing that the activation in ACC represents a neural correlate of positive mood or that positive mood states induce insight. We are concluding that positive mood is one factor that enhances activity in the rostral dACC, and that this mediates the shift toward insight solutions.
The precise mechanism by which positive mood facilitates insights through correspondingly modulating cognitive control processes within ACC is not entirely obvious. Cognitive control is itself a multifaceted concept, involving the recruitment of frontal regions—including the dACC, but also DLPFC, particularly in the LH—implicated in the detection of competing responses, overcoming prepotent response tendencies, and switching attention to select the correct response (Hedden & Gabrieli, 2006 ; Kondo et al., 2004 ; Weissman, Warner, & Woldorff, 2004 ; Weissman et al., 2003 ; Carter et al., 2000 ). ACC, specifically, has been implicated in several processes, such as error detection (Carter et al., 1998 ) or conflict monitoring (Botvinick et al., 2004 ; Kerns et al., 2004 ; Weissman et al., 2003 ).
We did not examine conflict monitoring in our study per se, and this study was not designed to tease apart the exact role of ACC in cognitive control. However, we favor a view by which ACC is involved in monitoring not just conflict but a variety of competing responses, such as multiple associations or strategies involved in solving problems. One way of putting it is that ACC sets a parameter of detecting such competing activations that allows either task shielding (ignoring other stimuli or thoughts to remain focused) or task switching (detecting competing stimuli, so that other components of cognitive control networks can switch attention to them; see Dreisbach & Goschke, 2004 ). One mechanism by which PA facilitates insight is by increasing this parameter for detecting multiple competing associations, which provides the solver a better chance of suddenly switching attention to the correct solution (or to solution-related information), thus facilitating insights. In line with our “competing activation” hypothesis, we think that PA enhances insights by possibly enhancing the detection of semantic associations (Rowe et al., 2007 ) facilitating shorter solution RTs, which would also partly explain why insight trials tended to be slightly faster than noninsight trials. In contrast, if insights only involved greater conflict monitoring, we would predict longer RTs for insight versus noninsight trials in our task.
PA previously has been linked to modulation of cognitive control processes to enhance cognitive flexibility, at the expense of perseveration or maintained focus (Dreisbach & Goschke, 2004 ). Further, prior theoretical explanations have attributed increases in cognitive flexibility to the effect of PA at enhancing phasic dopaminergic activity in the ACC and the pFC (Ashby, Valentin, & Turken, 2002 ; Ashby et al., 1999 ), consistent with other models of dopamine's effect on cognitive control (e.g., Daw, O'Doherty, Dayan, Seymour, & Dolan, 2006 ; Braver, Barch, & Cohen, 1999 ).
When people encounter a problem to solve (or any input to understand), they frequently engage multiple possible solving mechanisms. However, under various circumstances, different mechanisms are favored—due to individual states or traits or due to the problem itself (which is why some problems are more likely to be solved with insight and others more analytically; Bowden et al., 2005 ; Ansburg & Hill, 2003 ; Oelling & Knoblich, 2003 ). PA likely shifts the balance of which mechanisms will be most effective. As noted in the introduction, solving problems with insight requires cognitive flexibility (hence cognitive control) because it benefits from “cognitive restructuring” of the problem, enabling the solver to pursue a new strategy or a new set of associations. Several putative mechanisms could explain (in whole or in part) how PA enhances such flexibility. It may alter the selection process through which information enters working memory (Ashby et al., 1999 , 2002 ); it may tip the balance toward a more global focus of attention (Gasper & Clore, 2002 ) or a broader attention to both external visual space and internal conceptual space (Rowe et al., 2007 ) allowing more problem elements to simultaneously influence solution efforts; and it may facilitate switching between different modes of attention (Baumann & Kuhl, 2005 ; Kondo et al., 2004 ) or switching from irrelevant to relevant solving strategies (Dreisbach & Goschke, 2004 ). These putative mechanisms may overlap or may work in combination. The bottom line is that solvers appear to be better able to switch from pursuing a dominant but errant set of associations to a solution-relevant set.
Note that such a proposal does not mean that PA facilitates solutions by directly enhancing access to a broader range of semantic associations, for example, by increasing RH semantic processing. Recall that another hypothetical mechanism by which positive mood could facilitate insight would be through enhanced RH processing, given the demonstrated importance of RH semantic processing for processing a broad set of semantic associations (Chiarello, 1998 ; Beeman et al., 1994 ) generally and for insight solutions specifically (Jung-Beeman et al., 2004 ; Bowden & Beeman, 1998 ). Several pieces of evidence suggest that PA could enhance relative RH activation. First, PA increases sensitivity to a larger range of semantic associations (Fredrickson & Branigan, 2005 ; Federmeier et al., 2001 ), which, as noted, is characteristic of RH semantic processing. Second, induced positive mood increases a global focus of attention (Gasper & Clore, 2002 ), which is usually associated with RH visual attention, whereas a local focus of attention is associated with LH processing. Third, inducing an approach regulatory focus (which is often associated with PA) enhances both overall RH activation, as measured by a line-bisection task, and creativity (e.g., Friedman & Förster, 2005 ). Finally, compared with people who solve anagrams analytically, people who solve with insight show increased brain activity at rest in mostly right-lateralized regions according to resting-state EEG (Kounios et al., 2008 ). However, a great deal of research using frontal asymmetries during resting-state EEG associates LH activity with PA or approach regulatory focus (e.g., Sutton & Davidson, 1997 ). Further, effects that shift processing toward biases that are associated with one or the other hemisphere could occur due to modulation of medial attention or cognitive control-related processes.
Regardless, in the current experiment, PA did correlate with signal change during the preparation period in one lateral (rather than midline) cortical region, the AG of the RH; however, this area did not show other mood-related effects nor did it show an “insight effect” (stronger activity for insight than noninsight trials) at either solution or preparation period. Rather than simply increasing RH semantic processing, it appears that PA heightens solvers' sensitivity to solution-relevant processing, which may often occur within the RH semantic processing network (Jung-Beeman, 2005 ), working in cooperation with cognitive control processes in the frontal cortex to make the switch to converge to the correct solution. Still, it remains possible that a wider range of assessed (or induced) PA would reveal enhanced RH relative activation associated with a high positive mood.
There are several potential alternative explanations that can be considered and rejected. First, one might wonder whether positive mood did not alter the processing that led to solution but instead simply affected participants' willingness to label a solution as “insight.” This is unlikely, as we mentioned earlier, because participants higher in positive mood actually solved more problems than participants lower in positive mood—they solved more with insight and almost equally as many without insight as the lower positive mood group. Moreover, the high and low positive mood subgroups showed similar solution reaction times for insight versus noninsight solutions (for both groups, slightly faster insight than noninsight solutions). Furthermore, both subgroups showed nearly identical hemodynamic responses for insight solutions and likewise for noninsight solutions; that is, the solution types differed but the groups did not, suggesting that both groups used the same processes for solutions they labeled as insight.
Given that insight solutions were (in this study) faster than noninsight solutions, the possibility arises that participants higher in positive mood were more likely to adopt simpler decision heuristics before responding that they achieved solution. For instance, positive mood has been suggested to the use of “satisficing” rather than optimizing solving strategy (Kaufmann & Vosburg, 1997 ) or even suggested to be related to reduced overall cognitive capacity (Mackie & Worth, 1989 ). However, such a strategy should lead to more premature and incorrect responses, that is, trials on which participants press the button indicating solution, but then give an incorrect response. Yet high and low positive mood participants gave equally few incorrect responses ( p > .20); indeed, in other studies, participants who demonstrate a preference to solve without insight are more likely to make incorrect responses (Kounios et al., 2008 ).
Another possibility to consider is that PA enhances all neural activity (or perhaps enhances hemodynamic response, such as caffeine does), and that the PA-associated enhancements during preparation only occur in ACC because that is the primary area showing increased signal during that epoch. However, we observed no PA-related enhancement of signal change in brain areas showing large responses corresponding to either problem onset or solution (e.g., the insight effect in right aSTG was no bigger in high positive mood than in low positive mood participants).
Given that the “Aha!” experience has an affective component, we also considered the possibility that differences during the preparation period were remnants of activity from the preceding trial. Immediately before the preparation period, participants made their insight versus noninsight rating of the prior trial (if it was solved). However, hemodynamic responses directly related to these ratings did not differ depending on the type of rating made (no reliable clusters of activation were observed). The enhanced activation of dACC also did not relate to whether the prior trial was solved at all, so it was not a form of increased attention in response to failure or error evaluation on the prior trial (Bush et al., 2000 ).
The difference between insight preparation and noninsight preparation cannot be attributed to simple lack of attention because we analyzed only preparation periods preceding problems that were solved, not solved versus unsolved problems. Moreover, the mood-related difference in preparation activity within the dACC was not attributable to increased arousal (Critchley, Tang, Glaser, Butterworth, & Dolan, 2005 ) because if anything, it was inversely related to anxiety. If increased arousal drove the effect, then it should be stronger in high- rather than low-anxiety participants. Indeed, given the inverse relation between positive mood and anxiety, it is possible that some effects discussed here could be attributable to lack of anxiety (Beversdorf, White, Chever, Hughes, & Bornstein, 2002 ; Beversdorf, Hughes, Steinberg, Lewis, & Heilman, 1999 ) rather than presence of positive mood. However, all behavioral and neuroimaging measures correlated more consistently with increasing positive mood than with decreasing anxiety, whereas few of the effects correlated with the anxiety measure. Further, the effects of PA have been shown to be distinct from “affectless arousal” (Isen et al., 1987 ). If anything, arousal is thought to impede creativity, facilitating a narrow range of attention and perseveration on the prepotent response, thereby inhibiting overall cognitive flexibility (Kischka et al., 1996 ; Martindale, 1995 ; Easterbrook, 1959 ).
Finally, others have noted increased activation during what they term the default state of attention in MPFC (including dACC) and PCC (Raichle et al., 2001 ). It is at least possible that mood-associated changes in ACC in the current study reflect modulation of a default state network. However, we have no assessment of such default activation in the current study, so it would be a leap to make solid claims one way or the other.
Whether default state or task-related preparation, positive mood enhances activity within dACC in a manner conducive to solving with insight. This modulation may promote a more global (Gasper & Clore, 2002 ) or diffuse focus of attention, which has previously been linked to improved insight or creative problem solving (Rowe et al., 2007 ; Ansburg & Hill, 2003 ). Thus, we believe that one mechanism by which positive mood facilitates the shift toward an insight is by modulating ACC activity, at both the preparation and the solution time periods, in a manner that enhances the detection of multiple competing associations. Therefore, a solver focused on an incorrect association (or solution path) is better “prepared” to detect and to switch attention to the correct association; if this attention suddenly brings the correct solution into awareness, the solver experiences an “Aha!”
Conclusions
We examined the relation between various mood states (including positive mood and anxiety) and personality measures, assessed prior to the experiment, and brain activity immediately preceding and during problem solving. We found that positive mood enhanced overall solving for these insight-like verbal problems and particularly increased the likelihood of solving them with insight. We demonstrated that these effects were related to brain activity in ACC during the preparation interval prior to each trial. Specifically, activity increased in ACC more for high positive than for low positive mood participants. ACC was the only region showing sensitivity to multiple measures of this mood–insight association, providing strong evidence that positive mood states alter preparatory activity in ACC biasing participants to engage in problem processing that is conducive to solving with insight. These results have important implications for neural accounts of both general analytic problem solving and creative insight solving. Previous research has demonstrated that positive mood broadens the scope of attention to both external visual space and internal conceptual space (Rowe et al., 2007 ). The current work illustrates a neural basis for this modulation of problem solving by positive mood. Further, it suggests that positive mood enhances insight and creative problem solving, at least in part, by modulating attentional and cognitive control mechanisms within ACC to allow more sensitivity to detect competing solution candidates.
This research was supported in part by NIH/NIDCD through grants R01 DC-04052 to Mark Jung-Beeman and R01 DC-04818 to John Kounios. We thank Edward Bowden, Paul Reber, Nondas Loudas, Jason Haberman, Zoe Clancy, Joel Voss, and John Rudoy for their assistance and input on this project.
Reprint requests should be sent to Karuna Subramaniam or Mark Jung Beeman, Department of Psychology and Cognitive Brain Mapping Group, Northwestern University, 2029 Sheridan Road, Evanston, IL 60208-2710, or via e-mail: [email protected]; [email protected].
Although the top third of participants would technically be nine participants, matching PA scores made it impossible to use more than eight participants on either end of the distribution.
Email alerts
Related articles, related book chapters, affiliations.
- Online ISSN 1530-8898
- Print ISSN 0898-929X
A product of The MIT Press
Mit press direct.
- About MIT Press Direct
Information
- Accessibility
- For Authors
- For Customers
- For Librarians
- Direct to Open
- Open Access
- Media Inquiries
- Rights and Permissions
- For Advertisers
- About the MIT Press
- The MIT Press Reader
- MIT Press Blog
- Seasonal Catalogs
- MIT Press Home
- Give to the MIT Press
- Direct Service Desk
- Terms of Use
- Privacy Statement
- Crossref Member
- COUNTER Member
- The MIT Press colophon is registered in the U.S. Patent and Trademark Office

This Feature Is Available To Subscribers Only
Sign In or Create an Account
EEG correlation during the solving of simple and complex logical–mathematical problems
- Published: 21 February 2019
- Volume 19 , pages 1036–1046, ( 2019 )
Cite this article
- Jahaziel Molina del Río 1 , 2 ,
- Miguel Angel Guevara 2 ,
- Marisela Hernández González 2 ,
- Rosa María Hidalgo Aguirre 1 , 2 &
- Manuel Alejandro Cruz Aguilar 3
4379 Accesses
11 Citations
Explore all metrics
Solving logical–mathematical word problems is a complex task that requires numerous cognitive operations, including comprehension, reasoning, and calculation. These abilities have been associated with activation of the parietal, temporal, and prefrontal cortices. It has been suggested that the reasoning involved in solving logical–mathematical problems requires the coordinated functionality of all these cortical areas. In this study was evaluated the activation and electroencephalographic (EEG) correlation of the prefrontal, temporal, and parietal regions in young men while solving logical–mathematical word problems with two degrees of difficulty: simple and complex. During the solving of complex problems, higher absolute power and EEG correlation of the alpha and fast bands between the left frontal and parietal cortices were observed. A temporal deactivation and functional decoupling of the right parietal-temporal cortices also were obtained. Solving complex problems probably require activation of a left prefrontal-parietal circuit to maintain and manipulate multiple pieces of information. The temporal deactivation and decreased parietal-temporal correlation could be associated to text processing and suppression of the content-dependent reasoning to focus cognitive resources on the mathematical reasoning. Together, these findings support a pivotal role for the left prefrontal and parietal cortices in mathematical reasoning and of the temporal regions in text processing required to understand and solve written mathematical problems.
Similar content being viewed by others

Using Brain Computer Interaction to Evaluate Problem Solving Abilities
Oscillatory electroencephalographic patterns of arithmetic problem solving in fourth graders
Clemens Brunner, Nikolaus A. Koren, … Stephan E. Vogel

Studying Functional Brain Networks to Understand Mathematical Thinking: A Graph-Theoretical Approach
Avoid common mistakes on your manuscript.
Solving logical–mathematical word problems is a complex task that requires numerous cognitive operations, including comprehension, reasoning, calculation, knowledge of previously learned mathematical rules, as well as manipulating the new information given by the components of the problem itself. Leron ( 2004 ) has proposed that, regardless of the degree of difficulty, solving logical–mathematical problems requires three levels of mathematical thinking. The first level corresponds to rudimentary arithmetic or basic mathematical skills, such as subitizing or addition and subtraction. The second, known as informal mathematics, refers to the use of diagrams, figures, and everyday analogies; while the third, called formal mathematics, is characterized by the use of logical relations or syllogisms—like “if P, then Q”—which have been associated with the act of reasoning (Goel & Dolan, 2003 ), one of the pivotal processes required to solve problems of this kind (Leron, 2003 ; Mahmood, Othman, & Yusof, 2012 ). All three levels of mathematical thinking require the participation of cognitive processes that have been studied and consolidated in the triple code model proposed by Dehaene and Cohen ( 1995 ). This code consists of three categories of mental representations of numbers—namely, the verbal word frame, the visual Arabic number form, and the analog magnitude representation.
Neuroimaging techniques have revealed the neural bases of the aforementioned categories. The first activation pathway depends on the modality of the stimuli, so that if the problem is presented verbally, activation corresponds to the auditory verbal network (Eger, Sterzer, Russ, Giraud, & Kleinschmidt, 2003 ), while if the elements of the problem are received through the visual pathway, recognition of the numbers is associated with the visual network, including the inferior and mid occipital cortex. For subitizing and counting (Piazza, Mechelli, Butterworth, & Price, 2002 ) with semantic or asemantic associations (Fias, 2001 ), an area often activated in both cases is the parietal region, which is associated with the magnitude of the quantities involved (Dehaene, Molko, Cohen, & Wilson, 2004 ; Eger et al., 2003 ; Le Clec’H et al., 2000 ).
One of the main cognitive processes required to solve mathematical problems is working memory (Ashcraft & Krause, 2007 ; DeStefano & LeFevre, 2004 ), which involves the prefrontal cortex, especially the dorsolateral region (Zago et al., 2001 ). In fact, it has been reported that subjects with damage to the frontal region show deficits in the manipulation of multiple pieces of information, though their basic arithmetical processes remain intact (Besnard et al., 2014 ). Similarly, greater activation of the dorsolateral prefrontal cortex has been observed in subjects as they attempt to solve problems that required two operations to obtain the answer, compared with those that required only one (Prabhakaran, Rypma, & Gabrieli, 2001 ).
The parietal region, especially the intraparietal sulcus, participates in solving mathematical problems (Knops & Willmes, 2014 ). Activation of these areas seems to depend on the characteristics of the problem, since greater activation of the intraparietal sulcus has been reported during solving of two-digit problems (e.g., 15x32) compared with one-digit problems (e.g., 7 × 3; Zago et al., 2001 ). Some studies have associated simple calculation processes with parietal or parieto-occipital regions (Burbaud et al., 1995 ; Dehaene, Spelke, Pinel, Stanescu, & Tsivkin, 1999 ; Levin et al., 1996 ), as well as algebra processing (Waisman, Leikin, Shaul, & Leikin, 2014 ), whereas more complex mathematical tasks that require multiple calculations with intermediate steps seem to require frontal regions as well (Burbaud et al., 1995 ; Grafman, Passafiume, Faglioni, & Boiler, 1982 ; Jackson & Warrington, 1986 ). This may be due to an increase in the demands on working memory that are necessary to maintain and manipulate the intermediate products during the problem-solving process.
Another cortical area that participates in information retrieval when people attempt to solve word problems is the temporal cortex (Prabhakaran et al., 2001 ; Zago et al., 2001 ). In addition, a pivotal role of this cortical area has been demonstrated in text processing (Fasotti, Eling, & Bremer, 1992 ; Fasotti, Eling, & Houtem, 1994 ), since previous studies that analyzed the comprehension and reasoning of textual material have shown prominent activations in temporal and frontal regions (Haier & Camilla, 1995 ; Just, Carpenter, Keller, Eddy, & Thulborn, 1996 ; Nichelli et al., 1995 ; Partiot, Grafman, Sadato, Flitman, & Wild, 1996 ).
Recording electroencephalographic activity (EEG) is a noninvasive procedure with high temporal resolution that allows researchers to obtain information on brain functioning during different behavioral and cognitive states. The data obtained from EEG recordings are grouped in bands by frequency. The most commonly used bands are delta (δ, 1.5–3.5 Hz), theta (θ, 3.5–7.5 Hz), alpha1 (α1, 7.5–10.5 Hz), alpha2 (α2, 10.5–13.5 Hz), beta1 (β1, 13.5–19.5 Hz), beta2 (β2, 19.5–30 Hz), and gamma (γ, 31–50 Hz). The low frequencies in frontal regions (e.g., theta and delta) have been related to facilitating working memory tasks (Carrillo-de la Peña & García-Larrea, 2007 ) and identifying the elements of cognitive tasks (Cunillera et al., 2012 ). In posterior regions, in contrast, low frequencies have been associated with the performance of visual spatial tasks during continuous periods (Jap, Lal, & Fischer, 2010 ). Changes in the alpha range have been related to attentional processes and the inhibition of irrelevant information (Cooper, Croft, Dominey, Burgess, & Gruzelier, 2003 ; Foxe & Snyder, 2011 ; Payne, Guillory, & Sekuler, 2013 ; Sauseng, Klimesch, Schabus, & Doppelmayr, 2005 ), while the fast bands (beta1, beta2, gamma) appear to be associated with interneuronal communication of inhibitory networks (Whittington, Traub, Kopell, Ermentrout, & Buhl, 2000 ) and information transfer between regions (Engel & Fries, 2010 ).
The interaction that occurs among different brain areas to perform cognitive processes is determined by their anatomical and functional connections (Mesulam, 1994 ). The former consists of bundles of axons that form fasciculi and connect different brain regions, such as the frontal and parietal areas, through the superior longitudinal fasciculus, and the temporal pole with frontal regions through the uncinated fasciculus (Gerig, Gouttard, & Corouge, 2004 ; Mori et al., 2002 ). Functional connections—or synchronization—among brain areas has been studied by electroencephalographic correlation (rEEG), which can provide an index of short-range and long-range functional relations between brain areas (Guevara and Corsi-Cabrera 1996 ; Harris & Gordon, 2015 ; Shaw, 1984 ; Shaw, O’Connor, & Ongley, 1977 ; Thatcher, Biver, & North, 2007 ; Varela, Lachaux, Rodriguez, & Martinerie, 2001 ).
Electroencephalographic studies conducted while subjects are solving mathematical problems have revealed participation by the left central, temporal, parietal, and right frontal regions (Wang, Chen, Zhao, & Zou, 2010 ) and the posterior parietal area (Rousell, Catherwood, Edgar, & Design, 2012 ). Also, there are reports that activation of, and synchronization between, frontal and parieto-occipital areas in the theta and alpha bands increase during working memory tasks and in relation to the degree of difficulty of the problem (Dimitriadis, Sun, Thakor, & Bezerianos, 2016 ; Sauseng et al., 2004 ; Zarjam, Epps, Lovell, & Fang Chen, 2012 ). Subhani, Malik, Kamil, and Saad ( 2016 ) observed a characteristic EEG coherence profile during the solving of mathematical problems that included a stress condition. Meanwhile, using depth electrodes in human intracranial recordings, Halgren, Boujon, Clarke, Wang, and Chauvel ( 2002 ) observed phase synchrony in the alpha band in occipital, parietal, frontal, and Rolandic regions during periods of mental calculation and working memory maintenance. Additionally, the magnetoencephalographic recordings of human cortical ongoing activity made by Palva, Palva, and Kaila ( 2005 ) showed that mental calculation is associated with enhanced frontoparietal alpha band phase synchrony.
Although activation of several cortical areas has been associated with the demands of the mathematical tasks presented, we do not know how the degree of EEG synchronization between the prefrontal and posterior cortices changes during the reasoning or syllogism applied to solve logical–mathematical word problems. Thus, the aim of this study was to characterize the activation (absolute power) and degree of cortical EEG correlation in young men while they solved simple and complex logical–mathematical word problems. Considering the pivotal role that the prefrontal and parietal regions play in manipulating multiple pieces of information, mental calculation, and working memory, we hypothesized that higher activation and enhanced synchronization of the alpha and fast bands between these cortical areas would be found while subjects attempted to solve complex mathematical problems. Similarly, in light of the participation of temporal regions in information retrieval and text processing, we expected to detect changes in the alpha and fast bands as participants sought to solve such complex problems. Finally, better performance was predicted on simple problems, because it is assumed that complex problems require higher cognitive demands.
Material and method
Participants.
Eighteen young men with a mean age of 21.6 (±2.2) years participated in the study. All were healthy and right-handed with no prior history of neurological or psychiatric disorders, learning disabilities, drug abuse, or chronic illness. Participants were asked to refrain from drinking caffeine or alcohol during the 12 hours prior to the recording sessions, and to arrive with clean, dry hair. All participants had an intellectual coefficient (IQ) equal to or greater than 80, as measured by the Shipley-2 intelligence scale (Shipley, Gruber, Martin, & Klein, 2009 ), and showed normal-to-above-normal parameters of attention and memory, as measured by the Digit Detection and Visual Detection subtests, and successive series of the NEUROPSI test (Ostrosky et al., 2012 ). Informed consent was obtained from all participants, according to the guidelines of the Institutional Ethics Committee. Finally, all procedures involved in the experiment were approved by this Committee and performed in accordance with the ethical standards laid down in the 1964 Helsinki Declaration and its later amendments, or comparable ethical standards.
Mathematical problems
A total of 40 logical–mathematical word problems were displayed consecutively on a 32-in. computer screen placed at a distance of 1 meter from participants. The problems in sentence form, as well as the answer (in numeric values), were simultaneously presented on the screen. Taking into account that the answer was always present below the written problem, once the participants solved the problem, they could decide if that answer was correct or incorrect by pressing one of two answer keys on a computer keyboard: “B” if they were correct, “M” if not. The time for each problem (simple or complex) finished when the participant press the answer key. Two types of logical–mathematical problems were used: 20 classified as “simple” and 20 as “complex.” Text processing was required in both cases. The simple problems consisted of eight words, while the complex problems averaged 25 words. The former consisted of one-digit quantities and required only one operation to obtain the answer. The problem formula was x = a + b . The complex problems, in contrast, consisted of two-digit quantities and required three or more operations. The problem formula was x = a + ( a − b ) + [{ a + ( a − b )} + c ] (see Table 1 ). Clearly, the text-processing demands of the problems that required three or more operations were greater than those of the one-operation problems. To eliminate the possibility that participants might find it easier to solve simple problems after analyzing more challenging ones, all test sessions began with the simple problems, followed by the complex ones. The following parameters were measured while participants tried to solve both types of mathematical word problems: number of correct answers and response times.
The response time was considered because the written problem appears on the screen until the participant presses the corresponding answer key. This included the time each participant took to read, reason, and verify the response, to finally press the answer key corresponding to “correct” or “incorrect.”
EEG recording and procedure
Electrode placement followed the international 10–20 system (Jasper, 1958 ). Due to the specific interests of this study, the recording sites were F3 and F4, considered prefrontal areas; T3 and T4, considered temporal areas; and P3 and P4, considered parietal areas (Herwig, Satrapi, & Schönfeldt-Lecuona, 2003 ; Homan, 1978 ; Okamoto et al., 2004 ). EEGs were recorded continuously with eyes open in two conditions: while solving (1) simple and (2) complex problems. In each condition, participants were awake in a sitting position with their heads supported by the head rest of a comfortable chair. All derivations were referred to linked ears with the ground electrode placed on the forehead. EEGs were recorded using a NEXUS 32 device at 24 bits resolution and filters set at 1–50 Hz. Impedance for the EEG electrodes was maintained below 10 kOhms. BioTrace+ ® software was used to sample (512 Hz) and store the EEG data for off-line processing.
Epoch rejection was performed by both visual and computer-assisted assessment. Signals were examined off-line to identify saturated epochs or those that showed noise due to muscle activity, eye movement, or heartbeat. Those epochs were removed by means of a computer program (CHECASEN; Guevara et al. 2010 ). An off-line digital filter was applied for the frequencies below 1 Hz and above 50 Hz using another computer program (FILDIG; Guevara et al. 2005 ). All artifact-free EEG segments were analyzed using the EEG bands program (Guevara et al. 2014 ), which used fast Fourier transform to calculate absolute power (AP) and electroencephalographic correlations (rEEG) by applying the Pearson’s correlation coefficient between intrahemispheric (F3–T3, F3–P3, T3–P3, F4–T4, F4–P4, T4–P4) and interhemispheric electrodes (F3–F4, T3–T4, P3–P4) for six frequency bands: delta (δ, 1.5–3.5 Hz), theta (θ, 3.5–7.5 Hz), alpha1 (α1, 7.5–10.5 Hz), alpha2 (α2, 10.5–13.5 Hz), beta1 (β1, 13.5–19.5 Hz), beta2 (β2, 19.5–30 Hz), and gamma (γ, 31–50 Hz).
Statistical analyses
Behavioral analysis.
The number of correct answers and the sum of all response times from each participant were obtained. These parameters were then compared between the two types of problem using a Student’s t test for correlated groups, considering a significance level equal to, or below, a p value of .05 for all comparisons.
EEG analysis
For each type of mathematical problem, sixty 2-s EEG segments were selected and analyzed for each participant—that is, sixty 2-s EEG segments representatives of the simple problems and sixty 2-s EEG segments representatives of the complex problems. Specifically, the start of the EEG recording coincided with the start of the sequence of the 20 simple problems, and after that, with the start of the sequence of the 20 complex problems. Only for the EEG analysis, these segments were aligned at the beginning of the reading of each mathematical problem. Thus, the EEGs were recorded continuously while participants read the problem and perform the mental calculations required to solve it. This alignment at the begin of reading was used for all 20 simple and 20 complex problems to ensure that we would obtain EEG segments representative of the reading, comprehension, reasoning, and solving phases of the logical–mathematical problems. Selection of EEG segments was performed manually during the posterior analysis of the signals, striving to include EEG segments that were representative of all problems and of all phases.
Before conducting the statistical analyses, and to approximate a normal distribution, the AP values were transformed into logarithms and the correlation data into Fisher Z values. EEG AP values (in log) and Z correlation scores for each EEG band were compared between conditions using the Student’s t test for correlated groups. The effect sizes ( g ) of the statistical tests were calculated following Cohen ( 1988 ), and the level of significance was set at p < .05 for all comparisons.
Behavioral results
During performance of the simple logical–mathematical word problems, participants achieved a higher number of correct answers and had a lower mean response time than while solving the complex logical–mathematical word problems (see Table 2 ).
Absolute power
While participants were solving the complex problems, a higher AP of the theta ( t = −4.685, p = .00021, effect size [ d ] = 1.085) and alpha1 ( t = −2.346, p = .03135, d = 0.549) bands at F3, as well as of the alpha1 ( t = −2.721, p = .01454, d = 0.6411) and alpha2 ( t = −2.137, p = .04739, d = 0.506) bands at the P3 derivation were observed, compared with performance on the simple problems. Lower APs were obtained for the fast frequencies, beta1 ( t = 2.694, p = .01537, d = 0.637) and gamma band ( t = 2.273, p = .03627, d = 0.535) only in the right temporal area (T4) while participants solved the complex problems compared with the simple ones (see Fig. 1 ).
Mean and mean differences (MD) ± 1 SE of the absolute power (in natural logarithms; Ln) for each frequency band recorded in the left (F3, T3, and P3) and right (F4, T4, and P4) cortices of the young men while solving simple and complex logical-mathematical word problems. * p < .05, as compared with simple problems. Note. SE = standard error
Interhemispheric EEG correlation
During performance of the complex problems, a higher interfrontal (F3–F4) correlation in the alpha1 ( t = −4.001, p = .00093, d = 0.960), alpha2 ( t = −2.53, p = .02159, d = 0.589), and gamma bands ( t = −2.195, p = .04235, d = 0.510) was obtained compared with the simple problem condition. A similar increase of the correlation was obtained between the T3–T4 derivations in the alpha1 band ( t = −2.928, p = .00939, d = 0.691), whereas between the P3–P4 derivations, a higher correlation of the alpha1 ( t = −3.071, p = .00692, d = 0.718) and gamma ( t = −2.137, p = .04743, d = 0.492) bands was observed while participants solved the complex problems compared with the simple ones (see Fig. 2 ).
Mean and mean differences (MD) ± 1 SE of the interhemispheric correlation (in z values) among the prefrontal (F3–F4), temporal (T3–T4), and parietal (P3–P4) cortices for the different frequency bands recorded in young men while solving simple and complex logical-mathematical word problems. * p < .05, as compared with simple problems. Note. SE = standard error
Intrahemispheric EEG correlation
With respect to the left intrahemispheric correlation, a higher value was observed between the F3–P3 derivations in the fast frequencies, beta1 ( t = −2.373, p = .02973, d = 0.557), beta2 ( t = −2.342, p = .03159, d = 0.557), and gamma ( t = −2.435, p = .02620, d = 0.569)], compared with simple problems (see Fig. 3 ). In contrast to our observations of the left hemisphere, in the right hemisphere a lower correlation in the delta ( t = 2.395, p = .02839, d = 0.561), theta ( t = 2.377, p = .02945, d = 0.571), alpha1 ( t = 2.213, p = .04087, d = 0.525), and beta1 ( t = 2.442, p = .02581, d = 0.576) bands was obtained between the T4–P4 derivations during performance of the complex problems compared with the simple ones (see Fig. 3 ).
Mean and mean differences (MD) ± 1 SE of the intrahemispheric correlation (in z values) between the left (F3–T3, F3–P3, and P3–T3) and right (F4–T4, F4–P4, and P4–T4) derivations for the different frequency bands recorded in young men while solving simple and complex logical-mathematical word problems. * p < .05, as compared with simple problems. Note. SE = standard error
The present study compared the cortical activation and degree of electroencephalographic synchronization between different cortical areas while participants were solving simple versus complex logical–mathematical word problems. The EEG pattern associated with the solving of the simple problems was different from the one observed during the solving of complex problems, which featured a higher AP of the theta and alpha bands in the left frontal and parietal cortices. These results agree with other reports (Dimitriadis et al., 2016 ; Prabhakaran et al., 2001 ; Zago et al., 2001 ) that have shown a higher activation of frontal and parietal areas associated with the increased difficulty of problems. Indeed, our behavioral data showed that participants had fewer correct answers and longer response times when solving the complex problems. These findings reflect the greater complexity of those problems.
Prevalence of the alpha and theta bands has been linked to the maintenance and manipulation of information (Kawasaki, Kitajo, & Yamaguchi, 2010 ). In effect, studies have shown that theta is related to the coding phase of short-term memory tasks (Klimesch, Doppelmayr, Russegger, & Pachinger, 1996 ), with both information maintenance (Sarnthein, Petsche, Rappelsberger, Shaw, & von Stein, 1998 ) and retrieval (Klimesch et al., 2001 ), and with differences in memory load (Sauseng et al., 2005 ). Changes in the alpha range, in contrast, have been associated with attentional processes, inhibition of irrelevant information (Cooper et al., 2003 ; Foxe & Snyder, 2011 ; Payne et al., 2013 ; Sauseng et al., 2005 ) and mental calculation (Halgren et al., 2002 ; Palva et al., 2005 ). Thus, considering that the prefrontal and parietal cortices are involved in mathematical reasoning and calculation processes (Prabhakaran et al., 2001 ), it is not surprising that these two cortical areas show greater activation of the theta and alpha bands during the solving of complex problems.
Contrary to the activation of the left frontal and parietal regions during the solving of complex problems, a lower activation (indicated by the lower AP of the fast frequencies) was obtained in the right temporal areas. Decreases in temporal regions have been evidenced in other studies; for example, using positron emission tomography, Dehaene et al. ( 1999 ) reported a decreased blood flow in the temporal regions during multiplication retrieval compared with a rest condition. Similarly, Zago et al. ( 2001 ) reported that during processes of mental calculation that required several intermediate steps to reach a solution (compute condition), temporal deactivation was larger than during the solving of mathematical problems that required only a memory retrieval strategy. Thus, it is probable that—as Zago et al. ( 2001 ) suggest—the mental calculations required to solve the complex mathematical problems presented in our study induced a temporal inhibition that was related to cognitive demand and the degree of difficulty of the calculation task. Previous studies that analyzed the comprehension and reasoning of textual material have shown prominent activations in temporal and frontal regions (Fasotti et al., 1992 ; Fasotti et al., 1994 ; Grafman et al., 1982 ; Haier & Camilla, 1995 ; Jackson & Warrington, 1986 ; Just et al., 1996 ; Nichelli et al., 1995 ; Partiot et al., 1996 ). The fact that the text-processing demands of the problems presented in our study requiring three or more operations were greater than those of the one-operation problems suggests that decreased activation of the temporal areas could be associated with both the higher cognitive demands required to understand the sentences and the greater complexity of the mental calculations required to solve the complex problems even more convincing.
Taken together, these data show that the solving of complex logical–mathematical word problems requires simultaneous activation of the prefrontal and parietal cortices, accompanied by lower activation of temporal areas. This EEG pattern could represent a characteristic cortical functioning that involves, on the one hand, activation of a left prefrontoparietal network associated with mathematical reasoning and calculation and, on the other, deactivation of the right temporal region associated with the greater complexity of the text processing and mental calculation.
An additional question of interest is the meaning of the left and right activations observed in this study. Insights concerning the asymmetric roles of the frontal, parietal, and temporal lobes in mathematical reasoning can be drawn from other research, which has reported a pronounced lateralization during mathematical tasks. In fact, several studies on number processing and calculation have concluded that numerical deficits are observed more frequently after left lesions than after right ones (Cipolotti, Butterworth, & Denes, 1991 ; Lemer, Dehaene, Spelke, & Cohen, 2003 ). Similarly, there are reports that arithmetical tasks usually lead to a strong left dominance (Delazer et al., 2003 ; Zago et al., 2001 ), though increased activity in the right areas has also been reported (Stanescu-Cosson et al., 2000 ). Thus, the higher AP found in our study in the left prefrontal and parietal areas concurs with the research mentioned above and supports the notion that reasoning and mental calculation involve greater participation by cortical areas located in the left hemisphere.
In our study, the solving of the complex problems was also associated with higher interprefrontal (F3–F4) and interparietal (P3–P4) correlations, specifically in the alpha and gamma bands, and with a higher intertemporal correlation (T3–T4) in alpha1. The alpha band has been associated with attentional processes, while gamma has been related to high executive demands (Başar, Başar-Eroglu, Karakaş, & Schürmann, 2001 ), retention processes (Sarnthein et al., 1998 ), and information transfer between regions (Engel & Fries, 2010 ). Also, a higher phase synchrony in the alpha band has been reported in occipital, parietal, and frontal regions during periods of mental calculation and working memory (Halgren et al., 2002 ). Our data agree with those results because, in effect, a higher functional synchronization between hemispheres was observed, specifically in the alpha and gamma range, as our participants solved the complex problems. Hence, we can suggest that a higher degree of alpha and gamma synchronization between hemispheres could be a requisite for maintaining longer sustained attention, manipulating the larger amount of information (Anokhin, Lutzenberger, & Birbaumer, 1999 ; Jap et al., 2010 ) and performing the reasoning and mental calculations necessary to solve complex problems.
In addition to the higher correlation between cortical regions of both hemispheres, participants also showed a higher correlation of the fast frequencies (beta1, beta2, gamma) between prefrontoparietal regions of the left hemisphere while solving the complex problems. The fast bands (beta1, beta2, gamma) have been related to interneuronal communication of inhibitory networks (Whittington et al., 2000 ) and high executive demands (Ahmed & Cash, 2013 ; Başar et al., 2001 ; Fries, Reynolds, Rorie, Desimone, & Reynolds, 2001 ; Haig, Gordon, Wright, Meares, & Bahramali, 2000 ; Paul et al., 2005 ). Indeed, gamma phase synchrony has been proposed as an index of integrative network processing (Phillips & Singer, 1997 ). Thus, the higher synchronization of the fast bands between left prefrontoparietal regions could be related to a greater active maintenance and transfer of information between the two cortices, which could be required to perform the higher number of mental calculations involved in solving complex problems.
Unlike the higher correlation observed in the left hemisphere, in the right hemisphere a lower intrahemispheric correlation, mainly of the slow bands (delta, theta, alpha) and beta1 was seen between right parietal and temporal regions while participants solved the complex problems compared with the simple ones.
The temporal region, as mentioned above, is strongly involved in language processing, and a clear left hemispheric asymmetry has been reported in relation to both sentence listening and reading (Geschwind & Levitsky, 1986 ; Toga & Thompson, 2003 ). The left parietal cortex also plays a key role in mental arithmetic and reasoning (Jackson & Warrington, 1986 ); thus, the lower synchronization of the different EEG bands between right temporal and parietal areas confirms the dominance of the left hemisphere in the reasoning and mental calculation processes that predominated as participants solved the complex logical–mathematical word problems. Support for such hemispheric asymmetry comes from other studies (Deglin & Kinsbourne, 1996 ; Nathan, Kintsch, & Young, 1992 ; Wharton & Grafman, 1998 ), which have proposed that the right hemisphere participates in content-dependent reasoning (using real-world knowledge that is often spatial in nature), whereas the left hemisphere participates in the mathematical formalization of the problem (which is often abstract, symbolic, and nonspatial; Greeno, 1989 ; Kintsch & Greeno, 1985 ). Word problems require making inferences concerning content-dependent reasoning in relation to the mathematical formalization of the problem in order to perform successful mathematical reasoning. The results of our study show that a lower correlation between right parietal and temporal areas occurs during the solving of complex mathematical problems, which could reflect the lesser content-dependent reasoning that participants use to make greater cognitive resources available to focus directly on the mathematical reasoning of the problem.
An important point that must be considered here is that the EEG segments analyzed included segments that were representative of the word-reading, text comprehension, reasoning, and mental calculation phases of both types of problems. Although it is impossible to identify specific EEG changes for each phase, these data support the idea that the functioning of the prefrontal, parietal and temporal cortices in each hemisphere varies according to the difficulty of the task and the degree of cognitive demands (specific mental processes) required to solve each type of mathematical word problem.
In summary, the data from the present study show that solving complex problems require a characteristic pattern of activation and functional synchronization between cortices: (1) an increased left activation (frontal and parietal) associated to a deactivation of the right temporal cortex, (2) an increased rINTER between the three cortices, (3) an, increased rINTRA between left prefrontalparietal cortices, and (4) a functional decoupling of the right parietal-temporal cortices.
The increased intertemporal correlation in the alpha1 band and the decreased right parietal-temporal correlation in almost all the EEG bands could be explained considering the pivotal role that the temporal cortex plays in the text processing. In fact, as was mentioned, it is probably that an increased correlation between cortices of both hemispheres allows for more sustained attention, manipulating the larger amount of information (Anokhin et al., 1999 ; Jap et al., 2010 ) and performing the text reasoning and mental calculations necessary to solve complex problems. On the other hand, taking into account that word problems require making inferences concerning content-dependent reasoning, it is likely that the lower right parietal-temporal correlation was associated with the text processing so that lower content-dependent reasoning was used for the participants to focus directly on the mathematical reasoning of the problem.
In conclusion, the data from the present study show that complex problems require activation of a left prefrontal-parietal circuit, probably to maintain and manipulate multiple pieces of information, together with a functional decoupling of the right parietal-temporal cortices to suppress content-dependent reasoning and focus more cognitive resources on the mathematical reasoning required to solve the problem.
Finally, our study has some limitations, including the degree of difficulty and considerable variation in the length of the texts that described the simple and complex logical–mathematical problems. This was revealed by the significant differences in both the number of correct answers and response times between the simple and complex problems (see Table 2 ). One approach to correct this could be to analyze only an equivalent unit of time from the different trials (e.g., the first few seconds of each problem). However, the risk of analyzing the initial seconds in these different problem types is that it might isolate only the neural substrates involved in text-processing instead of the substrates involved in mathematical reasoning. A second issue is that, due to technical limitations, we were unable to record and analyze the specific EEG activity of the different phases involved in solving the mathematical word problems presented (i.e., word reading, text comprehension, reasoning, and mental calculation). Third, our participants were undergraduate students, so it remains to be determined to what degree our findings can be extrapolated to other populations. These issues may be resolved in future EEG studies.
Ahmed, O. J., & Cash, S. S. (2013). Finding synchrony in the desynchronized EEG: The history and interpretation of gamma rhythms. Frontiers in Integrative Neuroscience, 7 , 58. https://doi.org/10.3389/fnint.2013.00058
Article PubMed PubMed Central Google Scholar
Anokhin, A. P., Lutzenberger, W., & Birbaumer, N. (1999). Spatiotemporal organization of brain dynamics and intelligence: An EEG study in adolescents. International Journal of Psychophysiology, 33 (3), 259–273.
Article PubMed Google Scholar
Ashcraft, M. H., & Krause, J. A. (2007). Working memory, math performance, and math anxiety. Psychonomic Bulletin & Review, 14 (2), 243–248. https://doi.org/10.3758/BF03194059
Article Google Scholar
Başar, E., Başar-Eroglu, C., Karakaş, S., & Schürmann, M. (2001). Gamma, alpha, delta, and theta oscillations govern cognitive processes. International Journal of Psychophysiology, 39 (2), 241–248. https://doi.org/10.1016/S0167-8760(00)00145-8
Besnard, J., Allain, P., Aubin, G., Chauviré, V., Etcharry-Bouyx, F., & Le Gall, D. (2014). An integrative view of Luria’s perspective on arithmetic problem solving: the two sides of environmental dependency. Journal of Clinical and Experimental Neuropsychology, 36 (1), 88–109. https://doi.org/10.1080/13803395.2013.870135
Burbaud, P., Degreze, P., Lafon, P., Franconi, J., Bouligand, B., Bioulac, B., . . . Allard, M. (1995). Lateralization of prefrontal activation during internal mental calculation: A functional magnetic resonance imaging study. Journal of Neurophysiology, 74 , 2194–2200.
Carrillo-de la Peña, M. T., & García-Larrea, L. (2007). Right frontal event related EEG coherence (ERCoh) differentiates good from bad performers of the Wisconsin Card Sorting Test (WCST). Clinical Neurophysiology, 37 , 63–75.
Cipolotti, L., Butterworth, B., & Denes, G. A. (1991). Specific deficit for numbers in a case of dense acalculia. Brain, 114 , 2619–2637.
Cohen, J. (1988). Statistical power analysis for the behavioral sciences (2nd ed.). Mahwah, NJ: Erlbaum.
Google Scholar
Cooper, N. R., Croft, R. J., Dominey, S. J., Burgess, A. P., & Gruzelier, J. H. (2003). Paradox lost? Exploring the role of alpha oscillations during externally vs. internally directed attention and the implications for idling and inhibition hypotheses. International Journal of Psychophysiology, 47 (1), 65–74. https://doi.org/10.1016/S0167-8760(02)00107-1
Cunillera, T., Fuentemilla, L., Periañez, J., Marco-Pallarés, J., Krämer, U. M., Càmara, E., . . . Rodríguez-Fornells, A. (2012). Brain oscillatory activity associated with task switching and feedback processing. Cognitive Affective and Behavioral Neuroscience, 12, 16–33.
Deglin, V. L., & Kinsbourne, M. (1996). Divergent thinking styles of the hemispheres: How syllogisms are solved during transitory hemisphere suppression. Brain and Cognition, 31 , 285–307.
Dehaene, S., & Cohen, L. (1995). Towards an anatomical and functional model of number processing . Mahwah, NJ: Erlbaum.
Dehaene, S., Molko, N., Cohen, L., & Wilson, A. J. (2004). Arithmetic and the brain. Current Opinion in Neurobiology, 14 (2), 218–224. https://doi.org/10.1016/j.conb.2004.03.008
Dehaene, S., Spelke, E., Pinel, P., Stanescu, R., & Tsivkin, S. (1999). Sources of mathematical thinking: Behavioral and brain-imaging evidence. Science, 284 , 970–974.
Delazer, M., Domahs, F., Bartha, L., Brenneis, C., Lochy, A., Trieb, T., & Benke, T. (2003). Learning complex arithmetic—An fMRI study. Cognitive Brain Research , 18, 76–88.
DeStefano, D., & LeFevre, J. (2004). The role of working memory in mental arithmetic. European Journal of Cognitive Psychology, 16 (3), 353–386. https://doi.org/10.1080/09541440244000328
Dimitriadis, S. I., Sun, Y., Thakor, N. V., & Bezerianos, A. (2016). Causal interactions between Frontal θ − Parieto-Occipital α2 predict performance on a mental arithmetic task. Frontiers in Human Neuroscience,10 , 1–17. https://doi.org/10.3389/fnhum.2016.00454
Eger, E., Sterzer, P., Russ, M. O., Giraud, A. L., & Kleinschmidt, A. (2003). A supramodal number representation in human intraparietal cortex. Neuron, 37 (4), 719–725. https://doi.org/10.1016/S0896-6273(03)00036-9
Engel, A. K., & Fries, P. (2010). Beta-band oscillations—Signalling the status quo?. Current Opinion in Neurobiology, 20 , 156–165.
Fasotti, L., Eling, P. A. T. M., & Bremer, J. J. C. B. (1992). The internal representation of arithmetic word problem sentences. Brain and Cognition, 20 , 245–263.
Fasotti, L., Eling, P. A. T. M., & Houtem, J. V. (1994). Categorization of arithmetic word problems by normal, frontal and posterior-injured patients. Journal of Clinical and Experimental Neuropsychology, 16 , 723–733.
Fias, W. (2001). Two routes for the processing of verbal numbers: Evidence from the SNARC effect. Psychological Research, 65 (4), 250–259. https://doi.org/10.1007/s004260100065
Foxe, J. J., & Snyder, A. C. (2011). The role of alpha-band brain oscillations as a sensory suppression mechanism during selective attention. Frontiers in Psychology, 2 , 1–13. https://doi.org/10.3389/fpsyg.2011.00154
Fries, P., Reynolds, J., Rorie, A., Desimone, R., & Reynolds, J. (2001). Modulation of oscillatory neuronal synchronization by selective visual attention. Science, 291 (5508), 1560–1563. https://doi.org/10.1126/science.1055465
Gerig, G., Gouttard, S., & Corouge, I. (2004). Analysis of brain white matter via fiber tract modeling. Conference Proceedings: Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE Engineering in Medicine and Biology Society, 6, 4421–4424. https://doi.org/10.1109/IEMBS.2004.1404229
Geschwind, N., & Levitsky, W. (1986). Left–right asymmetry in temporal speech region. Science, 161 , 186–187.
Goel, V., & Dolan, R. J. (2003). Reciprocal neural response within lateral and ventral medial prefrontal cortex during hot and cold reasoning. NeuroImage, 20 (4), 2314–2321. https://doi.org/10.1016/j.neuroimage.2003.07.027
Grafman, J., Passafiume, D., Faglioni, P., & Boiler, F. (1982). Calculation disturbances in adults with focal hemispheric damage. Cortex, 18 , 37–50.
Greeno, J. G. (1989). Situation models, mental models, and generative knowledge. In D. K. K. Kotovsky (Ed.), Complex information processing: The impact of Herbert A. Simon (pp. 35–55). Hillsdale, NJ: Erlbaum.
Guevara, M. A., & Corsi-Cabrera, M. (1996). EEG coherence or EEG correlation? International Journal of Psychophysiology, 23 , 145–153. https://doi.org/10.1016/S0167-8760(96)00038-4 .
Guevara, M.A., Ramos, J., Hernández-González, M., & Corsi-Cabrera, M. (2005). FILDIG: a program to filter brain electrical signals in the frequency domain. Computer Methods & Programs in Biomedicine, 80 , 93–186.
Guevara, M. A., Sanz-Martin, A., Corsi-Cabrera, M., Amezcua, C., & Hernández-González, M. (2010). CHECASEN: programa para revisar señales EEG fuera de línea. Revista Mexicana de Ingeniería Biomédica, 31 (2), 135–141.
Guevara, M. A., Sanz-Martin, A., & Hernández-González, M. (2014). EEGbands: A computer program to statistically analyze parameters of electroencephalographic signals. Journal of Behavioral and Brain Science, 4 , 308–324.
Haier, R. J., & Camilla, P. B. (1995). Sex differences and lateralization in temporal lobe glucose metabolism during mathematical reasoning. Developmental Neuropsychology, 11 , 405–414.
Haig, A. R., Gordon, E., Wright, J. J., Meares, R. A., & Bahramali, H. (2000). Synchronous cortical gamma-band activity in task-relevant cognition. NeuroReport, 11 (4), 669–675. https://doi.org/10.1097/00001756-200003200-00004
Halgren, E., Boujon, C., Clarke, J., Wang, C., & Chauvel, P. (2002). Rapid distributed fronto-parieto-occipital processing stages during working memory in humans. Cerebral Cortex, 12 (7), 710–728. https://doi.org/10.1093/cercor/12.7.710
Harris, A. Z., & Gordon, J. A. (2015). Long-range neural synchrony in behavior. Annual Review of Neuroscience, 38 (1), 171–194. https://doi.org/10.1146/annurev-neuro-071714-034111
Herwig, U., Satrapi, P., & Schönfeldt-Lecuona, C. (2003). Using the international 10-20 EEG system for positioning of transcranial magnetic stimulation. Brain Topography, 16 (2), 95–99.
Homan, R. W. (1978). Cerebral location of international 10–20 system electrode placement. Electroencephalography and Clinical Neurophysiology, 66 , 376–382.
Jackson, M., & Warrington, E. K. (1986). Arithmetic skills in patients with unilateral cerebral lesions. Cortex, 22 , 611–620.
Jap, B. T., Lal, S., & Fischer, P. (2010). Inter-hemispheric electroencephalography coherence analysis: Assessing brain activity during monotonous driving. International Journal of Psychophysiology, 76 (3), 169–173. https://doi.org/10.1016/j.ijpsycho.2010.03.007 .
Jasper, H. H. (1958). Report of the committee on methods of clinical examination in electroencephalography. Electroencephalography and Clinical Neurophysiology, 10 (2), 370–375. https://doi.org/10.1016/0013-4694(58)90053-1
Just, M. A., Carpenter, P. A., Keller, T. A., Eddy, W. P., & Thulborn, K. R. (1996). Brain activation modulated by sentence comprehension. Science, 274 , 114–116.
Kawasaki, M., Kitajo, K., & Yamaguchi, Y. (2010). Dynamic links between theta executive functions and alpha storage buffers in auditory and visual working memory. European Journal of Neuroscience, 31 (9), 1683–1689. https://doi.org/10.1111/j.1460-9568.2010.07217
Kintsch, W., & Greeno, J. G. (1985). Understanding and solving word arithmetic problems. Psychological Review, 92 , 109-129.
Klimesch, W., Doppelmayr, M., Russegger, H., & Pachinger, T. (1996). Theta band power in the human EEG and the encoding of new information. NeuroReport, 7 , 1235–1240. https://doi.org/10.1097/00001756-199605170-00002
Klimesch, W., Doppelmayr, M., Stadler, W., Pöllhuber, D., Sauseng, P., & Roehm, D. (2001). Episodic retrieval is reflected by a process specific increase in human electroencephalographic theta activity. Neuroscience Letters, 302 (1), 49–52. https://doi.org/10.1016/S0304-3940(01)01656-1
Knops, A., & Willmes, K. (2014). Numerical ordering and symbolic arithmetic share frontal and parietal circuits in the right hemisphere. NeuroImage, 84 , 786–795. https://doi.org/10.1016/j.neuroimage.2013.09.037
Le Clec’H, G., Dehaene, S., Cohen, L., Mehler, J., Dupoux, E., Poline, J. B., & Le Bihan, D. (2000). Distinct cortical areas for names of numbers and body parts independent of language and input modality. NeuroImage,12 (4), 381–391. https://doi.org/10.1006/nimg.2000.0627
Lemer, C., Dehaene, S., Spelke, E., & Cohen, L. (2003). Approximated quantities and exact number words: Dissociable systems . Neuropsychologia, 41 , 1942–1958.
Leron, U. (2003). Origins of mathematical thinking: A synthesis. European Research in Mathematics Education III . Retrieved from http://www.dm.unipi.it/~didattica/CERME3/proceedings/Groups/TG1/TG1_leron_cerme3.pdf
Leron, U. (2004). Mathematical thinking and human nature: Consonance and conflict. PME, 28 (3), 217–224.
Levin, H. S., Scheller, J., Rickard, T., Grafman, J., Martinkowski, K., Winslow, M., & Mirvis, S. (1996). Dyscalculia and dyslexia after right hemisphere injury in infancy. Archives of Neurology, 53 , 88–96.
Mahmood, A., Othman, M. F., & Yusof, Y. M. (2012). A conceptual framework for mathematical ability analysis through the lens of cultural neuroscience. Procedia - Social and Behavioral Sciences, 56 , 175–182. https://doi.org/10.1016/j.sbspro.2012.09.644
Mesulam, M. M. (1994). Neurocognitive networks and selectively distributed processing. Revue Neurologique (Paris), 150 , 564–569.
Mori, S., Kaufmann, W. E., Davatzikos, C., Stieltjes, B., Amodei, L., Fredericksen, K., & Van Zijl, P. C. M. (2002). Imaging cortical association tracts in the human brain using diffusion-tensor-based axonal tracking. Magnetic Resonance in Medicine , 47 (2), 215–223. https://doi.org/10.1002/mrm.10074
Nathan, M. J., Kintsch, W., & Young, E. (1992). A theory of algebra-word-problem comprehension and its implications for the design of learning environments. Cognition and Instruction, 9 , 329–389.
Nichelli, P., Grafman, J., Pietrini, P., Clark, K., Lee, K. Y., & Miletich, R. (1995). Where the brain appreciates the moral of a story. Cognitive Neuroscience and Neuropsychology, 6 , 2309–2313.
Okamoto, M., Dan, H., Sakamoto, K., Takeo, K., Shimizu, K., Kohno, S., & Dan, I. (2004). Three-dimensional probabilistic anatomical cranio-cerebral correlation via the international 10–20 system oriented for transcranial functional brain mapping. NeuroImage, 21 , 99–111. https://doi.org/10.1016/j.neuroimage.2003.08.026
Ostrosky, F., Gómez, E., Matute, E., Rosselli, M., Ardila, A., & Pineda, D. (2012). NEUROPSI: Atención y memoria [NEUROPSI: Memory and attention] . Mexico City, Mexico: Manual Moderno.
Palva, J. M., Palva, S., & Kaila, K. (2005). Phase synchrony among neuronal oscillations in the human cortex. The Journal of Neuroscience, 25 , 3962–3972. https://doi.org/10.1523/JNEUROSCI.4250-04.2005
Partiot, A., Grafman, J., Sadato, N., Flitman, S., & Wild, K. (1996). Brain activation during script event processing. NeuroReport, 7 , 761–766.
Paul, R. H., Richard, C., Lawrence, J., Goldberg, E., Williams, L. M., Cooper, N., . . . Gordon, E. (2005). Age-dependent change in executive function and gamma 40 Hz phase synchrony. Journal of Integrative Neuroscience, 4 (01), 63–76. https://doi.org/10.1142/S0219635205000690
Payne, L., Guillory, S., & Sekuler, R. (2013). Attention-modulated alpha-band oscillations protect against intrusion of irrelevant information. Journal of Cognitive Neuroscience, 25 (9), 1463–1476. https://doi.org/10.1162/jocn_a_00395
Phillips, W. A., & Singer, W. (1997). In search of common foundations for cortical computation. Behavioral and Brain Sciences, 20 (4), 657–683. https://doi.org/10.1017/S0140525X9700160X
Piazza, M., Mechelli, A., Butterworth, B., & Price, C. J. (2002). Are subitizing and counting implemented as separate or functionally overlapping processes. NeuroImage, 15 , 435–446. https://doi.org/10.1006/nimg.2001.0980
Prabhakaran, V., Rypma, B., & Gabrieli, J. D. (2001). Neural substrates of mathematical reasoning: A functional magnetic resonance imaging study of neocortical activation during performance of the necessary arithmetic operations test. Neuropsychology, 15 (1), 115–127. https://doi.org/10.1037/0894-4105.15.1.115
Rousell, M., Catherwood, D., Edgar, G., & Design, A. (2012). An EEG case study of arithmetical reasoning by four individuals varying in imagery and mathematical ability: Implications for mathematics education. Word Academy of Science, Engineering and Technology, 71 , 1946–1948.
Sarnthein, J., Petsche, H., Rappelsberger, P., Shaw, G. L., & Von Stein, A. (1998). Synchronization between prefrontal and posterior association cortex during human working memory. Proceedings of the National Academy of Sciences, 95 (12), 7092–7096. https://doi.org/10.1073/pnas.95.12.7092
Sauseng, P., Klimesch, W., Doppelmayr, M., Hanslmayr, S., Schabus, M., & Gruber, W. R. (2004). Theta coupling in the human electroencephalogram during a working memory task. Neuroscience Letters, 354 (2), 123–126. https://doi.org/10.1016/j.neulet.2003.10.002
Sauseng, P., Klimesch, W., Schabus, M., & Doppelmayr, M. (2005). Fronto-parietal EEG coherence in theta and upper alpha reflect central executive functions of working memory. International Journal of Psychophysiology, 57 (2), 97–103. https://doi.org/10.1016/j.ijpsycho.2005.03.018
Shaw, C. (1984). Correlation and coherence analysis a selective tutorial review of the EEG. International Journal of Psychophysiology, 1 (3), 255–266. https://doi.org/10.1016/0167-8760(84)90045-X
Shaw, J. C., O’Connor, K. P., & Ongley, C. (1977). The EEG as a measure of cerebral functional organization. British Journal of Psychiatry, 130 (3), 260–264.
Shipley, W. C., Gruber, C. P., Martin, T. A., & Klein, A. M. (2009). Shipley-2: Escala breve de inteligencia [Shipley-2: Brief intelligence scale]. Mexico City, Mexico: Manual Moderno.
Stanescu-Cosson, R., Pinel, P., van de Moortele, P. F., Le Bihan, D., Cohen, L., & Dehaene, S. (2000). Understanding dissociations in dyscalculia: A brain imaging study of the impact of number size on the cerebral networks for exact and approximate calculation. Brain, 123 , 2240–2255.
Subhani, A. R., Malik, A. S., Kamil, N., & Saad, M. N. M. (2016). Difference in brain dynamics during arithmetic task performed in stress and control conditions. IECBES 2016: IEEE-EMBS Conference on Biomedical Engineering and Sciences, 1 , 695–698. https://doi.org/10.1109/IECBES.2016.7843539
Thatcher, R. W., Biver, C. J., & North, D. (2007). Spatial-temporal current source correlations and cortical connectivity. Clinical EEG and Neuroscience: Official Journal of the EEG and Clinical Neuroscience Society (ENCS),38 (1), 35–48. https://doi.org/10.1177/155005940703800109
Toga, A. W., & Thompson, P. M. (2003). Mapping brain asymmetry. Nature Reviews Neuroscience, 4 , 37–48.
Varela, F., Lachaux, J., Rodriguez, E., & Martinerie, J. (2001). The brain web: Phase synchronization and large-scale integration. Nature Reviews, 2 , 229–239.
Waisman, I., Leikin, M., Shaul, S., & Leikin, R. (2014). Brain activity associated with translation between graphical and symbolic representations of functions in generally gifted and excelling in mathematics adolescents. International Journal of Science and Mathematics Education, 12 , 669–696. https://doi.org/10.1007/s10763-014-9513-5
Wang, X., Chen, Z., Zhao, L., & Zou, S. (2010). The study of mental arithmetic load by EEG data. International Conference on Multimedia Technology , 2010, 1–4. https://doi.org/10.1109/ICMULT.2010.5629620
Wharton, C., & Grafman, J. (1998). Deductive reasoning and the brain. Trends in Cognitive Sciences, 2 , 54–59.
Whittington, M. A., Traub, R. D., Kopell, N., Ermentrout, B., & Buhl, E. H. (2000). Inhibition-based rhythms: Experimental and mathematical observations on network dynamics. International Journal of Psychophysiology, 38 (3), 315–336. https://doi.org/10.1016/S0167-8760(00)00173-2
Zago, L., Pesenti, M., Mellet, E., Crivello, F., Mazoyer, B., & Tzourio-Mazoyer, N. (2001). Neural correlates of simple and complex mental calculation. NeuroImage, 13 (2), 314–27. https://doi.org/10.1006/nimg.2000.0697
Zarjam, P., Epps, J., Lovell, N. H., & Fang Chen, F. (2012). Characterization of memory load in an arithmetic task using non-linear analysis of EEG signals. Annual International Conference of the IEEE Engineering in Medicine and Biology Society, 2012 , 3519–3522. https://doi.org/10.1109/EMBC.2012.6346725
Download references
Author information
Authors and affiliations.
Laboratorio de Neuropsicología, Centro Universitario de los Valles, Universidad de Guadalajara, Carretera Guadalajara-Ameca Km. 45.5, C.P. 46600, Ameca, Jalisco, México
Jahaziel Molina del Río & Rosa María Hidalgo Aguirre
Laboratorio de Correlación Electroencefalográfica y Conducta, Instituto de Neurociencias, Universidad de Guadalajara, Francisco de Quevedo, 180. Col. Arcos-Vallarta, C.P. 44130, Guadalajara, Jalisco, México
Jahaziel Molina del Río, Miguel Angel Guevara, Marisela Hernández González & Rosa María Hidalgo Aguirre
Dirección de Investigaciones en Neurociencias, Laboratorio de Cronobiología y Sueño, Instituto Nacional de Psiquiatría “Ramón de la Fuente Muñiz”, Mexico City, México
Manuel Alejandro Cruz Aguilar
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Miguel Angel Guevara .
Ethics declarations
Conflict of interest, additional information, publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Reprints and permissions
About this article
Molina del Río, J., Guevara, M.A., Hernández González, M. et al. EEG correlation during the solving of simple and complex logical–mathematical problems. Cogn Affect Behav Neurosci 19 , 1036–1046 (2019). https://doi.org/10.3758/s13415-019-00703-5
Download citation
Published : 21 February 2019
Issue Date : 15 August 2019
DOI : https://doi.org/10.3758/s13415-019-00703-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- EEG correlation
- Mathematical word problems
- Mental calculation
- Prefrontal cortex
- Find a journal
- Publish with us
- Track your research

We apologize for the inconvenience...
To ensure we keep this website safe, please can you confirm you are a human by ticking the box below.
If you are unable to complete the above request please contact us using the below link, providing a screenshot of your experience.
https://ioppublishing.org/contacts/
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- HHS Author Manuscripts

Memory and cognitive control circuits in mathematical cognition and learning
Stanford Cognitive and Systems Neuroscience Laboratory, Palo Alto, CA
Numerical cognition relies on interactions within and between multiple functional brain systems, including those subserving quantity processing, working memory, declarative memory, and cognitive control. This chapter describes recent advances in our understanding of memory and control circuits in mathematical cognition and learning. The working memory system involves multiple parietal–frontal circuits which create short-term representations that allow manipulation of discrete quantities over several seconds. In contrast, hippocampal–frontal circuits underlying the declarative memory system play an important role in formation of associative memories and binding of new and old information, leading to the formation of long-term memories that allow generalization beyond individual problem attributes. The flow of information across these systems is regulated by flexible cognitive control systems which facilitate the integration and manipulation of quantity and mnemonic information. The implications of recent research for formulating a more comprehensive systems neuroscience view of the neural basis of mathematical learning and knowledge acquisition in both children and adults are discussed.
1 INTRODUCTION
Knowledge of numerical magnitude and manipulations of symbolic and nonsymbolic quantity (number sense) are critical building blocks from which all mathematical knowledge is constructed. These basic building blocks rely on visual and auditory association cortices which help decode the visual form and phonological features of numerical stimuli, and the parietal attention system ( Dehaene et al., 2003 ) which helps to build semantic representations of quantity ( Ansari, 2008 ) from visuo-spatial primitives including object identification, spatial attention, eye gaze, and pointing ( Simon et al., 2002 ). With increased proficiency, these functions are sub-served by a “core” visuospatial number system anchored in the fusiform gyrus (FG) and intraparietal sulcus (IPS).
The IPS subdivision of the posterior parietal cortex has been the focus of most neurobiological research on mathematical cognition and learning ( Ansari, 2008 ; Butterworth, 1999 ; Dehaene et al., 2003 ). The IPS and FG form core building blocks from which number form and quantity representations are constructed in the brain ( Ansari, 2008 ). But these regions do not function in isolation. They receive input from multiple brain regions and send outputs to several others. Furthermore, the development of core systems is supported by its engagement with multiple brain systems. Recent research is beginning to emphasize a multisystem approach ( Arsalidou and Taylor, 2011 ; Fias et al., 2013 ; Qin et al., 2014 ). Multiple distributed neural processes involved in number form, magnitude and quantity representations, working memory, and declarative memory have been identified as being important for numerical problem solving and mathematical learning ( Fig. 1 ). This chapter synthesizes emerging findings on multiple memory and cognitive control systems which play a critical, but heretofore underappreciated, role in mathematical cognition in adults, as well as in scaffolding children’s mathematics learning and skill development.

Schematic diagram of memory and control circuits. The fusiform gyrus (FG) in inferior temporal cortex decodes number form and together with the intraparietal sulcus (IPS) in the parietal cortex which helps builds visuospatial representations of numerical quantity (shown in green , light gray in the print version, boxes and links ). Distinct parietal–frontal circuits differentially link the IPS and supramarginal gyrus (SMG) with the frontal eye field (FEF) and dorsolateral prefrontal cortex (DLPFC), respectively. These circuits facilitate visuospatial working memory for objects in space and create a hierarchy of short-term representations that allow manipulation of multiple discrete quantities over several seconds. The declarative memory system anchored in the medial temporal cortex (MTL)—the hippocampus, specifically, plays an important role in long-term memory formation and generalization beyond individual problem attributes. Finally, prefrontal control circuits (shown in red , dark gray in the print version) anchored in the anterior insula (AI), ventrolateral prefrontal cortex (VLPFC), and DLPFC serve as flexible hubs for integrating information across attentional and memory systems, thereby facilitating goal-directed problem solving and decision making.
We focus on two memory systems—working memory and declarative memory— that play distinct roles in mathematical cognition and learning. The working memory system anchored in parietal–frontal circuits creates short-term representations that allow manipulation of multiple discrete quantities over several seconds. In contrast, declarative memory systems anchored in hippocampal–frontal circuits play an important role in formation of associative memories and binding of new and old information, contributing to long-term memory and generalization beyond individual problem attributes. Both these processes require flexible integration of functional circuits anchored in prefrontal cognitive control systems. We describe cognitive control systems which guide allocation of attention resources and retrieval of facts from memory in the service of goal-directed numerical problem solving.
The roles of the two memory systems are greatly amplified during key developmental stages of learning, and their involvement in mathematical cognition and learning is best studied in the context of cognitive development. There is now growing evidence to suggest that functional circuits engaged by children are not the same as those engaged by adults who have evolved multiple strategies for learning and cognitive skill acquisition. Studies comparing children with adults are therefore likely to be insensitive to major changes that occur during specific stages of development. A number of scaffolding systems are likely to be engaged during development, and new studies are beginning to investigate the role of parietal–frontal and hippocampal–frontal circuits that might otherwise be missed in studies involving adults. The precise nature of this engagement is a function of developmental stage, domain knowledge, problem complexity, and individual proficiency in use of efficient problem-solving strategies. Accordingly, this review has a strong focus on both typical and atypical neurodevelopmental processes associated with the two memory systems and their associated prefrontal cognitive control systems.
The next sections are organized as follows. In Section 2, we first consider the relation between core systems and working memory and describe multiple parietal–frontal working memory circuits anchored in different subdivisions of the posterior parietal cortex, with a specific focus on functional circuits associated with the IPS and supramarginal gyrus. The role of parietal–frontal working memory circuits in the typical and atypical development of mathematical cognition is then discussed. In Section 3, we turn to the declarative memory system highlighting key hippocampal–prefrontal circuits. Emerging findings on the role of the hippocampal memory system in mathematical learning and development are then described. In Section 4, we consider how distinct prefrontal cortex (PFC) control systems facilitate mathematical cognition and learning. Section 5 summarizes the main points of this review. This chapter builds on related topical reviews ( Menon, 2015 , 2016 ) and attempts an integrative view of distinct, but overlapping, memory and cognitive control circuits involved in mathematical cognition and learning.
2 PARIETAL–FRONTAL WORKING MEMORY SYSTEMS
2.1 core and noncore parietal systems overlap in the ips.
Parietal–frontal circuits play a prominent role in mathematical cognition. Functional neuroimaging research has revealed significant overlap in multiple parietal and prefrontal cortical regions involved in working memory and numerical problem solving ( Arsalidou and Taylor, 2011 ; Metcalfe et al., 2013 ; Rottschy et al., 2012 ). Common patterns of coactivation have most prominently been detected in the IPS, supramarginal gyrus, premotor cortex, and ventral and dorsal aspects of the lateral PFC.
A critical locus of intersection between “core” number system and “noncore” working memory systems is the IPS, a region important for representing and manipulating numerical quantity. It is now well known that “core” IPS regions implicated in quantity and numerosity judgement are also involved in a broader class of cognitive functions, including sequential ordering and manipulation of working memory contents for nonnumerical stimuli, as shown in several elegant studies by Fias and colleagues ( Van Opstal et al., 2009 ). Furthermore, these IPS regions are also integral to short-term visual WM for object locations in space over a period lasting 1–2 s ( Luck and Vogel, 2013 ). Thus, multiple lines of evidence suggest that parietal systems for numerical quantity processing and working memory show a prominent overlap in the parietal cortex. The crucial point these examples illustrate is that the distinction between “core” quantity and “noncore” working memory systems is not functionally segregated and that they draw on similar mechanisms for dynamic manipulation of representations over a timescale of several seconds.
2.2 MULTIPLE PARIETAL–FRONTAL WORKING MEMORY CIRCUITS
Analysis of intrinsic functional circuits associated with the posterior parietal cortex hints at multiple parietal–frontal working memory-related circuits involved in mathematical cognition. The three distinct subdivisions of the inferior parietal cortex, the IPS, supramarginal gyrus, and angular gyrus are associated with distinct but overlapping parietal–frontal circuits ( Fig. 2 ). These circuits contribute to different aspects of mathematical cognition and learning by virtue of their differential large-scale functional organization. The IPS is part of an intrinsically connected parietal–frontal system that includes the frontal eye fields, supplementary motor area, anterior insula, and ventrolateral PFC ( Corbetta and Shulman, 2002 ; Corbetta et al., 2008 ; Menon and Uddin, 2010 ; Supekar and Menon, 2012 ; Uddin et al., 2010a ). A key distinguishing feature of the IPS is its connectivity with the frontal eye field: the IPS is more strongly connected with this region than the supramarginal gyrus ( Uddin et al., 2010a ). In contrast, the supramarginal gyrus is more tightly linked to the dorsolateral PFC, together with which it forms the canonical parietal–frontal central executive network ( Bressler and Menon, 2010 ). The IPS also shows a distinct pattern of connectivity from the adjoining angular gyrus. The angular gyrus is strongly connected with ventromedial PFC and posterior cingulate regions, comprising the default mode network ( Greicius et al., 2003 , 2004 ; Raichle et al., 2001 ), a system with no direct involvement in working memory. Thus, the IPS and supramarginal gyrus form distinct parietal–frontal working memory-related circuits.

Parietal–frontal circuits associated with the IPS. Parietal–frontal circuits identified using intrinsic functional connectivity analysis of the intraparietal sulcus (IPS), a “core” region involved in basic magnitude judgment and arithmetic. (A) IPS region of interests (ROIs) derived from cytoarchitectonic maps for the three subdivisions of the IPS: hIP2 is the lateral and anterior subdivision of the IPS ( blue , dark gray in the print version); hIP1 is the subdivision located posterior to hIP2 ( green , gray in the print version); and hIP3 is the posterior subdivision of the IPS ( red , light gray in the print version). (B) Functional connectivity maps associated with hIP1, hIP2, and hIP3. The color ( different gray shades in the print version) code represents voxels correlated with each source ROI. The IPS has significant connectivity with distributed frontal (MFG and PMC) and parietal (SMG and SPL) cortical regions in both hemispheres. Additional functional circuits associated with the ventral-occipital temporal cortex are not shown. hIP , human intraparietal; MFG , middle frontal gyrus; PMC , premotor cortex; SMG , supramarginal gyrus; SPL , superior parietal lobule.
Adapted from Uddin, L.Q., Supekar, K., Amin, H., Rykhlevskaia, E., Nguyen, D.A., Greicius, M.D., Menon, V., 2010a. Dissociable connectivity within human angular gyrus and intraparietal sulcus: evidence from functional and structural connectivity. Cereb. Cortex 20, 2636–2646.
It should be noted that the IPS itself is not a homogeneous structure. Cytoarchitectonic maps obtained from postmortem brains suggest that the human IPS has a more finely grained parcellation than previously suggested by the classical Brodmann map ( Caspers et al., 2008 ). While all three subdivisions identified to date have strong links to the frontal eye field, their large-scale connectivity patterns can also be dissociated. The anterior-most IPS subdivisions (hIP2 and hIP1) are linked with ventral premotor cortex, anterior insula, and dorsolateral PFC, while the posterior-most IPS subdivision (hIP3) has higher connectivity with extrastriate visual areas ( Fig. 2 ). This functional connectivity profile suggests a strong coupling between the anterior IPS and PFC control regions. In sharp contrast, the posterior IPS region shows strong coupling with posterior occipital regions. Thus, connectivity analyses of networks associated with the IPS suggest a general principle of organization whereby posterior IPS regions that are closely linked to the visual system translate stimuli into motor action through anterior IPS connections with the PFC.
A key point suggested by extant findings is that IPS and supramarginal gyrus involved in mathematical cognition form overlapping but segregated working memory circuits. Precisely how these circuits work to serve the dual purpose of segregation and integration of core and noncore parietal systems involved in mathematical cognition and learning is less clear. A crucial hint comes from their distinct functional roles. Unlike the IPS, the supramarginal gyrus is not critically involved in quantity judgement per se, but it is strongly engaged during numerical problem-solving tasks that require active storage and manipulation of the contents of working memory. It is likely that the supramarginal gyrus provides a more general mechanism for manipulating the contents of working memory in a more flexible and context-dependent manner over an extended period of time, thus freeing up “core” IPS resources necessary for quantity processing.
2.3 PARIETAL–FRONTAL WORKING MEMORY SYSTEMS IN MATHEMATICAL COGNITION AND ITS DEVELOPMENT
The particular emphasis on parietal–frontal working memory systems in mathematical cognition is most prominent in developmental studies. This has origins in children’s immature problem-solving abilities which require them to break down numerical problems into more basic components. The use of such strategies requires greater reliance on working memory systems for problem solving in children. For example, children rely more on counting strategies during simple arithmetic problem solving and need to access multiple working memory components including short-term storage, rule-based manipulation, and updating of the stored contents. With increased proficiency and a shift to fact retrieval strategies, there is less demand and need for working memory resources ( Bailey et al., 2012 ; Geary et al., 2007a ). Consistent with this view, neuroimaging studies in typical and atypical development have provided strong evidence for coactivation of parietal and prefrontal regions that overlap with parietal–frontal circuits highlighted in the previous section.
The involvement of working memory in mathematical cognition had initially been surmised based on overlapping responses in parietal and PFC in the two domains ( Cantlon et al., 2009 ; Grabner et al., 2009 ; Ischebeck et al., 2007 ; Rivera et al., 2005 ). Studies of typical development provided initial evidence for the changing role of working memory with age. For example, Rivera and colleagues found that relative to adults, children tend to engage the posterior parietal cortex less, and the PFC more, when solving arithmetic problems, likely reflecting the increased role of visuospatial processing and the concurrent decrease in demands on cognitive control. Other studies have more directly addressed the link between working memory abilities and numerical problem-solving skills. Dumontheil and Klingberg (2012) found that IPS activity during a visuospatial working memory task predicted arithmetic performance 2 years later in a sample of 6–16-year-old children and adolescents. This finding further reinforced the role of parietal–frontal working memory systems in mathematical cognition and pointed to the overlap between core and noncore functional systems in the IPS.
More detailed analyses of the neural correlates of individual components of working memory have provided evidence for the fractionation of neurofunctional systems associated with distinct working memory components during numerical problem solving ( Arsalidou and Taylor, 2011 ; Metcalfe et al., 2013 ; Rottschy et al., 2012 ). Analysis of the relation between the central executive, phonological and visuospatial components of working memory, and brain activation during an arithmetic verification task in a large group of 7–9-year-old children revealed that visuospatial working memory is a strong predictor of mathematical ability in children in this age group and is associated with increased problem complexity-related responses in left dorsolateral and right ventrolateral PFC as well as in the bilateral IPS and supramarginal gyrus. Metcalfe and colleagues also found that visuospatial working memory and the central executive component were associated with largely distinct patterns of brain responses during arithmetic problem solving, and overlap was only observed in the ventral aspects of the left supramarginal gyrus, suggesting that this region is an important locus for the integration of information in working memory during numerical problem solving in children ( Ansari, 2008 ; Dehaene et al., 2003 ; Kawashima et al., 2004 ; Kucian et al., 2008 ; Menon et al., 2000 ; Rivera et al., 2005 ; Rosenberg-Lee et al., 2014 ).
Finally, analysis of intrinsic functional connectivity suggests that a network of prefrontal cortical areas supports the longitudinal development of numerical abilities. Intrinsic functional connectivity between the IPS and dorsolateral and ventrolateral PFC predicted longitudinal gains in numerical problem-solving abilities over a 6-year period in children ( Evans et al., 2015 ). These findings further confirm the pivotal role of overlapping parietal–frontal circuits in children’s mathematical skill development.
2.4 PARIETAL–FRONTAL IMPAIRMENTS IN CHILDREN WITH MATHEMATICAL DISABILITIES
Although mathematical disability (MD) was initially conceptualized as a disorder of a single brain region characterized by a localized deficit in the IPS ( Cohen Kadosh et al., 2007 ; Isaacs et al., 2001 ; Price et al., 2007 ), more recently, prominent neurocognitive models of MD have posited that the disorder stems from more extensive functional aberrations in a distributed network of brain areas encompassing not only posterior parietal, but also prefrontal, as well as ventral temporal–occipital cortices that are known to serve multiple cognitive functions necessary for successful numerical problem solving. Studies have variably reported aberrant responses in the IPS, supramarginal gyrus, and multiple prefrontal cortical areas implicated in working memory ( Butterworth et al., 2011 ; Davis et al., 2009 ; Iuculano et al., 2015 ; Kaufmann et al., 2009a ; Kucian and von Aster, 2015 ; Kucian et al., 2006 , 2011 ; Menon, 2014 ; Price et al., 2007 ; Rosenberg-Lee et al., 2014 ).
Behavioral studies have shown that disruptions to working memory are a prominent factor contributing to persistent deficits in arithmetic problem solving in children with MD ( Geary et al., 2007b ). Evidence to date suggests that visuospatial working memory is a specific source of vulnerability in symbolic numerical calculation deficits and thus needs to be seriously considered as a key component in neurobiological and developmental models of typical and atypical mathematical skill acquisition ( Ashkenazi et al., 2013 ). Rotzer et al. (2009) found that compared to typically developing children, children with low math abilities had lower visuospatial abilities and lower activity levels in the right anterior IPS, ventrolateral PFC, and insular cortex during a visuospatial working memory task. In a different vein, Ashkenazi et al. (2013) identified impaired working memory components in children with MD and then examined their role in modulating brain responses to numerical problem solving. Children with MD had specific deficits in visuospatial working memory in addition to deficits in arithmetic task performance, even though they were matched on IQ and verbal abilities to their typically developing peers. Crucially, activations in IPS, and dorsolateral and ventrolateral PFC are positively correlated with visuospatial working memory ability in typically developing children, but no such relation was seen in children with MD. This result suggests that children with MD fail to appropriately exploit parietal–frontal working memory resources during problem solving.
2.5 HYPERACTIVE PARIETAL–FRONTAL WORKING MEMORY CIRCUITS IN CHILDREN WITH MD
While previous studies have focused on regional profiles of deficits, understanding cognitive deficits requires knowledge not only about aberrations in localized patterns of brain responses but also distributed functional circuits that might be impaired or organized in unusual ways ( Uddin et al., 2010b ). This is particularly true for numerical problem solving which typically requires the coordinated interaction of multiple brain regions ( Rosenberg-Lee et al., 2011 ; Varma and Schwartz, 2008 ). A few recent studies have begun to probe parietal–frontal circuits in children with MD.
Analysis of both task and task-free data suggests that parietal–frontal working memory circuits are impaired in children with MD. Surprisingly, contrary to what might be predicted, relative to typically developing children, children with MD show hyperconnectivity of the IPS, rather than reduced connectivity, with several cortical areas ( Rosenberg-Lee et al., 2014 ). Hyperconnectivity of the IPS with multiple regions in lateral PFC and parietal regions is most prominent in affected children. These regions include the bilateral ventrolateral and dorsolateral PFC and the supramarginal gyrus. This pattern of hyperconnectivity was observed during both a simpler addition task and a more cognitively challenging subtraction task ( Fig. 3 ). Thus, children with MD engage multiple parietal–frontal working memory circuits differently from their typically developing peers—they require greater engagement of these circuits despite lower performance levels. The greater engagement of these circuits may arise from the activation of problem-irrelevant information that in turn disrupts problem solving. This view is consistent with behavioral studies that show the intrusion of problem-irrelevant information into working memory when children with MD attempt to retrieve arithmetic answers from long-term memory ( Barrouillet et al., 1997 ; Geary et al., 2000 , 2012 ). Further studies are needed to disentangle intrusive vs compensatory effects in this complex pattern of hyperconnectivity in parietal–frontal working memory circuits.

Aberrant parietal–frontal response and hyperconnectivity in children with mathematical disability. (A) Brain areas that showed significant main effect of group during problem solving involving addition and subtraction operations in children with mathematical disability (MD), compared to TD children. Signal levels demonstrate that, when activations are compared to a low-level passive fixation condition, arithmetic processing is associated with hyperactivation in the MD group in multiple brain areas including: left lingual gyrus (LG), left fusiform gyrus (FG), right intraparietal sulcus (IPS), right anterior insula, superior frontal gyrus (SFG) bilaterally, and right supplementary motor area (SMA), and right inferior frontal gyrus (IFG). (B) Brain areas that showed a significant group (MD, TD) × operation (addition, subtraction) interaction. Compared to TD children, children with MD showed hyperactivation in several posterior brain regions for subtraction (Sub), compared to addition (Add). These regions included bilateral posterior IPS, right anterior IPS, right superior parietal lobe (SPL), left angular gyrus (AG), and left FG. (C) Effective connectivity of the IPS during arithmetic problem solving in the MD (shown in yellow, white in the print version) and TD (shown in red , dark gray in the print version) groups. Note the more extensive connectivity in the MD group. Main effect of group is shown. (D) Brain regions that showed greater IPS connectivity in the MD group included multiple frontal, parietal and occipital regions: bilateral angular gyrus (AG), left supramarginal gyrus (SMG), right middle frontal gyrus (MFG), right inferior frontal gyrus (IFG), posteromedial cortex (PMC), and ventral medial prefrontal cortex (vmPFC).
Adapted from Rosenberg-lee, M., Ashkenazi, S., Chen, T., Young, C.B., Geary, D.C., Menon, V., 2014. Brain hyper-connectivity and operation-specific deficits during arithmetic problem solving in children with developmental dyscalculia. Dev. Sci. 18, 351–372.
Notably, this pattern of hyperconnectivity is also manifest in intrinsic functional circuits ( Fig. 4 ). Compared to their typically developing peers, children with MD show aberrant IPS connectivity with multiple prefrontal and parietal regions ( Jolles et al., 2016 ). Specifically, children with MD show greater functional connectivity between left and right IPS, as well as between IPS and dorsolateral and ventrolateral PFC. It is plausible that intrinsic hyperconnectivity in these parietal–frontal circuits in children with MD may underlie the increased activation and connectivity of these regions reported in several studies of numerical problem solving ( Iuculano et al., 2015 ; Kaufmann et al., 2009a , b , 2011 ; Rosenberg-Lee et al., 2014 ). Interestingly, however, not all studies have found greater parietal–frontal activation in children with MD, but discrepancies between studies may be explained by the type of baseline conditions used. Specifically, it has been suggested that MD is characterized by reduced modulation of brain responses with increasing task complexity, rather than reduced activation per se ( Ashkenazi et al., 2012 ). For example, children with MD show reduced activation in parietal–frontal regions for small vs large distances in number comparison tasks ( Mussolin et al., 2010 ; Price et al., 2007 ; but see Kucian, 2011 ), and for complex vs simple problems in arithmetic tasks ( Ashkenazi et al., 2012 ). Finally, aberrant connectivity within these parietal–frontal areas in children with MD is consistent with deficits in spatial attention that have been reported in domains outside mathematical problem solving ( Ashkenazi and Henik, 2010 ; Szucs et al., 2013 ).

Parietal hyperconnectivity in children with MD. Brain areas that showed greater IPS connectivity in children with MD compared to typically developing (TD) children. (A) Children with MD showed hyperconnectivity between bilateral IPS and multiple dorsal frontal and parietal cortical regions, between bilateral IPS and right hemisphere SMG and STG, and between left IPS and right putamen. (B) Results were almost identical when examining a subset of 14 MD children who scored at or below 85 on the numerical operations subtest of the WIAT-II (MD*). Greater connectivity for MD>TD in red ( dark gray in the print version) (left IPS), blue ( gray in the print version) (right IPS), and green ( light gray in the print version) (both left and right IPS). Coordinates are in MNI space. FP , frontal pole; IPS , intraparietal sulcus; SFG , superior frontal gyrus; SMG , supramarginal gyrus; SPL , superior parietal lobe; STG , superior temporal gyrus.
Adapted from Jolles, D., Ashkenazi, S., Kochalka, J., Evans, T., Richardson, J., Rosenberg-lee, M., Zhao, H., Supekar, K., Chen, T., Menon, V., 2016. Parietal hyper-connectivity, aberrant brain organization, and circuitbased biomarkers in children with mathematical disabilities. Dev. Sci.
An important question for future research is how aberrant parietal circuits impact the ability to modulate parietal–frontal responses in a context-specific manner and how this in turn influences skill development and learning.
3 HIPPOCAMPAL–FRONTAL DECLARATIVE MEMORY SYSTEM
3.1 the medial temporal lobe: a system for associative learning.
Over the past few years, evidence has been accumulating for the differential involvement of the declarative memory system in mathematical learning, especially during key stages of skill acquisition in children ( Qin et al., 2014 ). The importance of the medial temporal lobe, particularly its hippocampal subdivision, in learning and memory for events in space and time is well known ( Davachi, 2006 ; Davachi et al., 2003 ; Diana et al., 2007 ; Eichenbaum et al., 2007 ; Tulving, 1983 ). Theories of memory consolidation posit that the hippocampus plays an important role in the early stages of learning and retrieval, but its involvement decreases over time with concomitant increase in reliance on neocortical memory systems ( Eichenbaum et al., 2007 ). This might explain why despite its critical role in learning and memory formation, hippocampal contributions to mathematics learning and cognitive development more broadly have received little attention until recently.
3.2 HIPPOCAMPAL–FRONTAL CORTEX CIRCUITS
The hippocampus forms the structural core of the declarative memory system. Research over the past two decades has clarified the specific roles of its functional subdivisions in different aspects of encoding and retrieval of novel information ( Kumaran et al., 2009 ; Schacter et al., 1998 , 2007 ; Tulving, 1983 , 2002 ). The hippocampus and its associated functional circuits play an important role in memory encoding and retrieval in both children and adults ( Ghetti et al., 2010 ; Menon et al., 2005 ; Ofen et al., 2007 ). The hippocampus is thought to contribute to declarative memory through binding inputs from multiple cortical areas ( Davachi, 2006 ; Eichenbaum, 2004 ; Eichenbaum et al., 2007 ), while its functional interactions with the PFC are thought to facilitate memory formation and retrieval through cognitive control processes acting on the contents of memory ( Qin et al., 2007 , 2009 , 2011a , b ). Declarative memory relies on the coordinated interactions of distributed brain areas, most prominently, the hippocampus and the PFC ( Diekelmann et al., 2009 ; Frankland and Bontempi, 2005 ; McGaugh, 2000 ; Norman and O’Reilly, 2003 ; Qin et al., 2011a ; Simons and Spiers, 2003 ). These studies suggest that newly acquired memories are strongly dependent on the hippocampus and its interactions with the PFC, and become increasingly independent of the hippocampus and MTL over time.
3.3 HIPPOCAMPAL–PREFRONTAL COACTIVATION IN CHILDREN’S MATHEMATICAL SKILL DEVELOPMENT
The first evidence for the differential engagement of the hippocampal memory system in arithmetic skill acquisition came from a cross-sectional study in children, adolescents, and adults who ranged in age from 8 to 19 ( Rivera et al., 2005 ). Importantly, children exhibited significantly greater engagement of multiple medial temporal lobe regions including the hippocampus. Similarly, De Smedt et al. (2011) found greater hippocampal response in children compared to adults when solving addition problems; hippocampal activation was not detected for subtraction problems which are less well rehearsed and more difficult to memorize because subtraction problems are not commutative. These findings highlight the dynamic role of the hippocampus in the maturation of memory-based problem-solving strategies and its greater engagement in childhood followed by decreased involvement in adolescence and adulthood ( Fig. 5 ).

Longitudinal developmental changes in medial temporal lobe engagement and connectivity. (A) Longitudinal changes in hippocampal engagement during childhood, and further development through adolescence into adulthood. (a) Right hippocampus response showing main effect of group across children (at Time 1 and Time 2), adolescents, and adults. (b) Bar graphs depict developmental changes in the functionally defined hippocampus cluster. (c) Bar graphs show developmental changes in engagement of anatomically defined left and right hippocampal regions of interest. (B) Longitudinal changes in hippocampal–neocortical functional circuits in relation to individual improvements in children’s use of memory-based problem-solving strategies. (a) Right hippocampus seed region used in effective connectivity (ie, psychophysiological interaction) analysis. (b, c) Left and right dorsolateral prefrontal cortex (DLPFC) and the left intraparietal sulcus (IPS) regions that showed increased effective connectivity with the hippocampus, as a function of longitudinal improvements in retrieval fluency from Time 1 to Time 2. (d–f) Scatter plots depict the relation between longitudinal changes in retrieval fluency ( x -axes) and changes in effective connectivity strength from Time 1 to Time 2 ( y -axes).
Adapted from Qin, S., Cho, S., Chen, T., Rosenberg-Lee, M., Geary, D.C., Menon, V., 2014. Hippocampalneocortical functional reorganization underlies children’s cognitive development. Nat. Neurosci. 17, 1263–1269.
As noted earlier, children’s gains in problem-solving skills during the elementary school years are characterized by the gradual replacement of inefficient procedural strategies with direct retrieval of domain-relevant facts ( Cho et al., 2011 ; Geary, 2011 ; Geary and Brown, 1991 ; Geary and Hoard, 2003 ). Cho et al. (2012) examined neurodevelopmental changes related to increased use of retrieval strategies and found that higher retrieval fluency was associated with greater response in multiple brain regions, including the hippocampus and parahippocampal gyrus subdivisions of the medial temporal lobe. Thus, children’s use of retrieval strategies, far from being idiosyncratic, is in fact associated with a predictable profile of hippocampal responses. A related study found that retrieval and counting strategies were associated with different activation patterns in hippocampal regions important for memory encoding and retrieval, including bilateral hippocampus and parahippocampal gyrus ( Cho et al., 2011 , 2012 ). The existence of decodable fine-scale pattern differences in fMRI signals suggests not only that the hippocampus is differentially engaged in relation to retrieval but also that the underlying neural resources are accessed and used differently in each strategy.
Qin and colleagues further investigated the transition from procedure-based to memory-based problem-solving strategies using longitudinal fMRI data from 7- to 9-year-old children ( Qin et al., 2014 ). Children’s transition from counting to memory-based retrieval strategies over a 1.2-year interval was mediated by increased hippocampal activation and decreased parietal–frontal engagement. Following an initial increase in hippocampal engagement during middle childhood, this hippocampal dependency decreased during adolescence and adulthood despite further improvements in memory-based problem solving. This pattern of initial increase and subsequent decrease in activation provides novel support for models of long-term memory consolidation which posit that the hippocampus plays a time-limited role in the early phase of knowledge acquisition ( McClelland et al., 1995 ; Tse et al., 2007 ). Consistent with this pattern of developmental change, previous studies in adults have reported no reliable hippocampal engagement during arithmetic tasks. Thus, the hippocampal system is critical to children’s early learning of arithmetic facts ( Cho et al., 2011 , 2012 ; De Smedt et al., 2011 ), while retrieval is largely dependent on the neocortex in adults ( Dehaene et al., 2003 ; Menon, 2014 ).
3.4 HIPPOCAMPAL–FRONTAL CIRCUITS IN CHILDREN’S MATHEMATICAL SKILL DEVELOPMENT AND LEARNING
Dynamic coordination between the hippocampus and PFC plays an important role in memory formation ( Norman and O’Reilly, 2003 ; Qin et al., 2011a ; Simons and Spiers, 2003 ), and connectivity analyses are beginning to shed light on how the hippocampus and PFC interact to support memory formation for arithmetic facts. Analysis of task-related hippocampus connectivity has identified distributed functional circuits associated with retrieval fluency. In particular, right hippocampal connectivity with bilateral ventrolateral and dorsolateral PFC is strongly correlated with retrieval fluency ( Cho et al., 2012 ). Analysis of longitudinal data has further clarified the relation between hippocampal–prefrontal circuits and individual differences in children’s mathematical skill development. In particular, the shift from counting to memory-based retrieval strategy and increased hippocampal activation is accompanied by decreased parietal–frontal engagement. Longitudinal improvements in retrieval fluency are best predicted by increased functional connectivity in hippocampal–neocortical circuits ( Qin et al., 2014 ). Increased hippocampal functional coupling with prefrontal and parietal cortices is positively correlated with individual gains in memory-based strategy use. Finally, in a tutoring study designed to facilitate rapid retrieval, hippocampal–PFC functional circuits predicted performance gains over an 8-week interval ( Fig. 6 ). Children who exhibited higher intrinsic functional connectivity in these circuits prior to tutoring showed the greatest performance improvement in math problem solving ( Supekar et al., 2013 ). Hippocampal– neocortical circuit reorganization therefore plays an important role in children’s shift from effortful counting to more efficient memory-based problem solving.

Medial temporal lobe structure and connectivity predict children’s math learning. (A) Gray matter volume in hippocampus correlates with improvement in arithmetic performance in response to 8 weeks of one-to-one math tutoring. (B) Intrinsic functional connectivity of the hippocampus correlates with improvement in arithmetic performance in response to 8 weeks of one-to-one math tutoring. Performance gains were predicted by hippocampal connectivity with the left dorsolateral prefrontal cortex (L DLPFC), left ventrolateral prefrontal cortex (L VLPFC), right supplementary motor area (R SMA), left basal ganglia (L BG), and right middle temporal gyrus (R MTG). Composite 3D view of connectivity network is shown in the central panel with the right hippocampus seed ROI highlighted in red ( gray in the print version) and voxels showing peak connectivity with the hippocampus highlighted in green ( light gray in the print version). Surrounding panels show brain areas correlated with performance gains with tutoring. Scatter plots in each panel are based on voxels showing peak connectivity.
Adapted from Supekar, K., Swigart, A.G., Tenison, C., Jolles, D.D., Rosenberg-Lee, M., Fuchs, L., Menon, V., 2013. Neural predictors of individual differences in response to math tutoring in primary-grade school children. Proc. Natl. Acad. Sci. U.S.A. 110, 8230–8235.
4 COGNITIVE CONTROL SYSTEMS IN MATHEMATICAL COGNITION
4.1 flexible hubs for cognitive control.
Prefrontal control processes are important for virtually every complex cognitive task, including mathematical cognition. The role of both working memory and declarative memory systems in mathematical cognition must therefore be considered in the context of cognitive control processes that support flexible problem solving and learning. Prefrontal control processes serve several functions in numerical cognition including maintenance of attention on goal-relevant numerical representations, manipulation of information in working memory, inhibition of irrelevant information, and implementation of task-relevant activations. Implementation of such control relies on dynamic functional interactions between multiple frontal regions ( Cai et al., 2014 , 2016 ; Cole et al., 2013 ; Ham et al., 2013 ; Seeley et al., 2007 ; Sridharan et al., 2008 ), and recent research has begun to elucidate the role of parietal–frontal and hippocampal–frontal circuits in different aspects of cognitive control during mathematical cognition.
4.2 DYNAMIC PARIETAL–FRONTAL CONTROL SIGNALS
As noted in Section 2, a common recurring theme in numerical problem solving is the coengagement of parietal and prefrontal regions associated with working memory. Children as young as 7 show reliable, and consistent, patterns of brain activity during arithmetic problem solving in multiple PFC regions ( Houde et al., 2010 ). Commonly activated PFC regions include the anterior insula, and ventrolateral and dorsolateral PFC ( Fig. 1 ). It should be noted that these regions are also implicated in a wide range of cognitive control tasks in adults as well as children ( Cole et al., 2013 ; Cai et al., 2014 , 2016 ; Ham et al., 2013 ; Seeley et al., 2007 ; Sridharan et al., 2008 ; Ordaz et al., 2013 ).This profile of anatomical overlap suggests a common mechanism by which maturation of basic cognitive control can influence skill development across multiple cognitive domains.
Efficient control requires the concerted coordination between multiple brain regions, and there is growing evidence to suggest that this is implemented via dedicated neurocognitive networks. Two-key networks play a fundamental role in cognitive control processes in the human brain: the insula–cingulate salience network, which includes the anterior insula and anterior cingulate cortex, and the dorsal parietal–frontal working memory network, which includes the ventrolateral and dorsolateral PFC and supramarginal gyrus. Supekar and Menon (2012) examined functional connectivity and dynamic causal interactions between the major nodes of these networks to investigate the maturation of control processes underlying numerical problem-solving skills in 7–9-year-old children, relative to adults. They found that, by age 9, the anterior insula node of the salience network is a major causal hub initiating control signals during problem solving ( Fig. 7 ). The anterior insula, part of a larger network of regions previously shown to be important for salience processing and generating influential control signals, showed weaker influence over the ventrolateral and dorsolateral PFC and anterior cingulate cortex in children compared to adults. Despite higher levels of PFC activation in children, the strength of their causal modulatory influences to the parietal cortex was significantly weaker relative to adults.

Developmental changes in causal network interactions during arithmetic problem solving. (A) Activation of the salience and parietal–frontal central executive networks in (a) children and (b) adults. (c) Task-related signal change in ROIs within the nodes of the two networks. Compared to adults, children showed stronger activation in the right anterior insula (AI) and weaker activation in the right posterior parietal cortex (PPC) (** p <0.01, FDR corrected). (B) Dynamic causal analysis of the five-key nodes of the salience network ( blue , gray in the print version, rectangles ), and parietal–frontal network ( green , light gray in the print version, rectangles ) in (a) children and (b) adults. (c) Weaker causal interactions in children, compared to adults. VLPFC , ventrolateral prefrontal cortex; DLPFC , dorsolateral prefrontal cortex; ACC , anterior cingulate cortex.
Adapted from Supekar, K., Menon, V., 2012. Developmental maturation of dynamic causal control signals in higher-order cognition: a neurocognitive network model. PLoS Comput. Biol. 8, e1002374.
Notably, weaker PFC control signals were associated with lower levels of arithmetic performance, and network interactions better predicted reaction time in both children and adults. In children, the strength of casual signals from the anterior insula to supramarginal gyrus and ventrolateral PFC predicted reaction times, while the strength of anterior insula to supramarginal gyrus, ventrolateral PFC, and anterior cingulate cortex predicted reaction times in adults. Reaction times were better predicted in adults, compared to children. It is noteworthy that even though a different set of links predicted reaction times in both groups, the anterior insula to supramarginal gyrus link was common in both. Similar results were observed when accuracy instead of reaction time was used as the performance measure. Thus, multiple PFC control signals contribute to efficient problem-solving skills in adults and weak signaling mechanisms contribute to lower levels of performance in children.
4.3 DYNAMIC HIPPOCAMPAL–FRONTAL CONTROL SIGNALS
As described in Section 3, both the ventrolateral and dorsolateral PFC are associated with increased use of memory-based strategies in children ( Cho et al., 2012 ). The ventrolateral PFC is known to play a prominent role in cognitive control over memory retrieval processes both in adults ( Badre and Wagner, 2007 ; Koechlin et al., 2003 ; Miller, 2000 ) and in children ( Adleman et al., 2002 ; Bunge and Wright, 2007 ; Houde et al., 2010 ; Kwon et al., 2002 ). These control processes are thought to be important for accurate retrieval of relevant facts and inhibition of irrelevant information ( Destefano and Lefevre, 2004 ; Kaufmann, 2002 ; Kaufmann et al., 2004 ; Logie et al., 1994 ).
Dynamic causal modeling of fMRI data has provided further insights into the temporal profile of interactions between the hippocampus and PFC regions involved in mediating retrieval fluency. Causal analysis has revealed strong bidirectional interactions between the hippocampus and both the left ventrolateral and dorsolateral PFC ( Fig. 8 ). Crucially, causal influences from the left ventrolateral PFC to the hippocampus act as the main “top-down” component, while causal influences from the hippocampus to the left dorsolateral PFC serve as the main “bottom-up” component of this retrieval network. While still preliminary, these analyses highlight the differential contribution of hippocampal–prefrontal circuits to the early development of retrieval fluency in arithmetic problem solving and provide a novel framework for studying dynamic developmental processes involving the hippocampus and PFC that accompany the maturation of cognitive skills. Further research is needed to investigate how these processes contribute to concomitant improvements in cognitive control over retrieval, including successful inhibition of irrelevant information, such as incorrect answers, intermediate steps, and operand intrusions ( Barrouillet and Lepine, 2005 ; Passolunghi and Siegel, 2004 ).

Hippocampal–prefrontal cortex circuits in children’s fact retrieval. (A) Functional connectivity of right hippocampus ( top-left inset ), showing greater effective connectivity during addition problem solving when compared with a control task. Greater connectivity was observed in the bilateral hippocampus, bilateral VLPFC, bilateral DLPFC, left SFG, bilateral insula, bilateral LG, bilateral PHG, and right FG. (B) Dynamic causal interactions in the hippocampal–prefrontal retrieval network. Analysis of causal interactions between left VLPFC, left and right DLPFC, and right hippocampus. Both the left VLPFC and the left DLPFC showed highly significant direct causal influences with the right hippocampus. Causal links depicted were all significant using p <0.01, after Bonferroni correction. VLPFC , ventrolateral prefrontal cortex; DLPFC , dorsolateral prefrontal cortex; SFG , superior frontal gyrus; PHG , parahippocampal gyrus; LG , lingual gyrus; FG , fusiform gyrus.
Adapted from Cho, S., Ryali, S., Geary, D.C., Menon, V., 2011. How does a child solve 7+8? Decoding brain activity patterns associated with counting and retrieval strategies. Dev. Sci. 14, 989–1001.
5 SUMMARY AND CONCLUSIONS
Multiple lines of evidence affirm that numerical cognition relies on interactions within and between multiple functional brain circuits, including those underlying numerical quantity representations (FG–IPS), working memory (IPS–SMG–ventrolateral and dorsolateral PFC), declarative memory (hippocampus–ventrolateral and dorsolateral PFC), and cognitive control (anterior insula–ventrolateral and dorsolateral PFC) ( Fig. 1 ). We have highlighted the role of distinct memory and cognitive control systems that play distinct roles in mathematical cognition and learning.
We have reviewed evidence that the parietal–frontal working memory system is engaged during a wide range of numerical problem-solving tasks. Multiple working memory circuits anchored in different subdivisions of the inferior parietal cortex help create short-term representations that support the manipulation of multiple discrete quantities over several seconds. The IPS and supramarginal gyrus are associated with differential patterns of connectivity with the frontal eye field and ventrolateral and dorsolateral PFC. Findings to date suggest that the IPS plays an essential role not only in quantity representations but also in maintaining quantity-related information in short-term working memory. Rule-based and context-specific manipulation of these representations in working memory is in turn supported by multiple prefrontal cortical areas—here the supramarginal gyrus emerges as a key locus for integrating frontal control systems with quantity representations supported by the IPS.
The hippocampal–frontal declarative memory system has an entirely different role in mathematical cognition and learning. This system plays a critical, but time limited, role in the early phase of knowledge acquisition, and this hippocampal dependence is reduced following reconfiguration of neocortical connections and stabilization of newly acquired knowledge. Hippocampal–neocortical reorganization facilitates fluent retrieval and long-term neocortical memory consolidation in children, eventually resulting in retrieval processes that are independent of the hippocampus ( Qin et al., 2014 ).
The working memory and declarative memory systems intersect most prominently in the ventrolateral and dorsolateral PFC, which together with the anterior insula likely serve as flexible hubs for integrating information across attentional and memory systems. As reviewed in Section 4, weak control signals from these PFC regions negatively impact the ability to maintain task-relevant representations needed for achieving mature levels of performance. Better understanding of control processes mediated by these PFC regions is essential for a more mechanistic characterization of mathematical cognition, skill development, and learning.
A challenging question for future research is to understand how the distinct functional circuits highlighted in this review interact dynamically to support different aspects of mathematical cognition and learning and how they change with different stages of development. Addressing this question will require developing appropriate computational models of dynamic causal interactions between brain regions, analyzing different stages of information processing, and utilizing more appropriate experimental designs that involve the controlled manipulation of symbolic and nonsymbolic quantity representations in posterior brain regions including the FG and IPS.
- Adleman NE, Menon V, Blasey CM, White CD, Warsofsky IS, Glover GH, Reiss AL. A developmental fMRI study of the Stroop color-word task. Neuroimage. 2002; 16 :61–75. [ PubMed ] [ Google Scholar ]
- Ansari D. Effects of development and enculturation on number representation in the brain. Nat Rev Neurosci. 2008; 9 :278–291. [ PubMed ] [ Google Scholar ]
- Arsalidou M, Taylor MJ. Is 2 + 2=4? Meta-analyses of brain areas needed for numbers and calculations. Neuroimage. 2011; 54 :2382–2393. [ PubMed ] [ Google Scholar ]
- Ashkenazi S, Henik A. Attentional networks in developmental dyscalculia. Behav Brain Funct. 2010; 6 :2. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Ashkenazi S, Rosenberg-Lee M, Tenison C, Menon V. Weak task-related modulation and stimulus representations during arithmetic problem solving in children with developmental dyscalculia. Dev Cogn Neurosci. 2012; 2 :S152–S166. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Ashkenazi S, Rosenberg-Lee M, Metcalfe AW, Swigart AG, Menon V. Visuo-spatial working memory is an important source of domain-general vulnerability in the development of arithmetic cognition. Neuropsychologia. 2013; 51 :2305–2317. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Badre D, Wagner AD. Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia. 2007; 45 :2883–2901. [ PubMed ] [ Google Scholar ]
- Bailey DH, Littlefield A, Geary DC. The codevelopment of skill at and preference for use of retrieval-based processes for solving addition problems: individual and sex differences from first to sixth grades. J Exp Child Psychol. 2012; 113 :78–92. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Barrouillet P, Lepine R. Working memory and children’s use of retrieval to solve addition problems. J Exp Child Psychol. 2005; 91 :183–204. [ PubMed ] [ Google Scholar ]
- Barrouillet P, Fayol M, Lathuliere E. Selecting between competitors in multiplication tasks: an explanation of the errors produced by adolescents with learning difficulties. Int J Behav Dev. 1997; 21 :253–275. [ Google Scholar ]
- Bressler SL, Menon V. Large-scale brain networks in cognition: emerging methods and principles. Trends Cogn Sci. 2010; 14 :277–290. [ PubMed ] [ Google Scholar ]
- Bunge SA, Wright SB. Neurodevelopmental changes in working memory and cognitive control. Curr Opin Neurobiol. 2007; 17 :243–250. [ PubMed ] [ Google Scholar ]
- Butterworth B. The Mathematical Brain. Macmillan; London: 1999. [ Google Scholar ]
- Butterworth B, Varma S, Laurillard D. Dyscalculia: from brain to education. Science. 2011; 332 :1049–1053. [ PubMed ] [ Google Scholar ]
- Cai W, Ryali S, Chen T, Li CS, Menon V. Dissociable roles of right inferior frontal cortex and anterior insula in inhibitory control: evidence from intrinsic and task-related functional parcellation, connectivity, and response profile analyses across multiple datasets. J Neurosci. 2014; 34 :14652–14667. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Cai W, Chen T, Ryali S, Kochalka J, Li CS, Menon V. Causal interactions within a frontal-cingulate-parietal network during cognitive control: convergent evidence from a multisite-multitask investigation. Cereb Cortex. 2016; 26 :2140–2153. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Cantlon JF, Libertus ME, Pinel P, Dehaene S, Brannon EM, Pelphrey KA. The neural development of an abstract concept of number. J Cogn Neurosci. 2009; 21 :2217–2229. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Caspers S, Eickhoff SB, Geyer S, Scheperjans F, Mohlberg H, Zilles K, Amunts K. The human inferior parietal lobule in stereotaxic space. Brain Struct Funct. 2008; 212 :481–495. [ PubMed ] [ Google Scholar ]
- Cho S, Ryali S, Geary DC, Menon V. How does a child solve 7 + 8? Decoding brain activity patterns associated with counting and retrieval strategies. Dev Sci. 2011; 14 :989–1001. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Cho S, Metcalfe AW, Young CB, Ryali S, Geary DC, Menon V. Hippocampal-prefrontal engagement and dynamic causal interactions in the maturation of children’s fact retrieval. J Cogn Neurosci. 2012; 24 :1849–1866. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Cohen Kadosh R, Cohen Kadosh K, Schuhmann T, Kaas A, Goebel R, Henik A, Sack AT. Virtual dyscalculia induced by parietal-lobe TMS impairs automatic magnitude processing. Curr Biol. 2007; 17 :689–693. [ PubMed ] [ Google Scholar ]
- Cole MW, Reynolds JR, Power JD, Repovs G, Anticevic A, Braver TS. Multi-task connectivity reveals flexible hubs for adaptive task control. Nat Neurosci. 2013; 16 :1348–1355. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Corbetta M, Shulman G. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002; 3 :201–215. [ PubMed ] [ Google Scholar ]
- Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008; 58 :306–324. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Davachi L. Item, context and relational episodic encoding in humans. Curr Opin Neurobiol. 2006; 16 :693–700. [ PubMed ] [ Google Scholar ]
- Davachi L, Mitchell JP, Wagner AD. Multiple routes to memory: distinct medial temporal lobe processes build item and source memories. Proc Natl Acad Sci USA. 2003; 100 :2157–2162. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Davis N, Cannistraci CJ, Rogers BP, Gatenby JC, Fuchs LS, Anderson AW, Gore JC. Aberrant functional activation in school age children at-risk for mathematical disability: a functional imaging study of simple arithmetic skill. Neuropsychologia. 2009; 47 :2470–2479. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- De Smedt B, Holloway ID, Ansari D. Effects of problem size and arithmetic operation on brain activation during calculation in children with varying levels of arithmetical fluency. Neuroimage. 2011; 57 :771–781. [ PubMed ] [ Google Scholar ]
- Dehaene S, Piazza M, Pinel P, Cohen L. Three parietal circuits for number processing. Cogn Neuropsychol. 2003; 20 :487–506. [ PubMed ] [ Google Scholar ]
- Destefano D, Lefevre JA. The role of working memory in mental arithmetic. Eur J Cogn Psychol. 2004; 16 :353–386. [ Google Scholar ]
- Diana RA, Yonelinas AP, Ranganath C. Imaging recollection and familiarity in the medial temporal lobe: a three-component model. Trends Cogn Sci. 2007; 11 :379–386. [ PubMed ] [ Google Scholar ]
- Diekelmann S, Wilhelm I, Born J. The whats and whens of sleep-dependent memory consolidation. Sleep Med Rev. 2009; 13 :309–321. [ PubMed ] [ Google Scholar ]
- Dumontheil I, Klingberg T. Brain activity during a visuospatial working memory task predicts arithmetical performance 2 years later. Cereb Cortex. 2012; 22 :1078–1085. [ PubMed ] [ Google Scholar ]
- Eichenbaum H. Hippocampus: cognitive processes and neural representations that underlie declarative memory. Neuron. 2004; 44 :109–120. [ PubMed ] [ Google Scholar ]
- Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annu Rev Neurosci. 2007; 30 :123–152. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Evans TM, Kochalka J, Ngoon TJ, Wu SS, Qin S, Battista C, Menon V. Brain structural integrity and intrinsic functional connectivity forecast 6 year longitudinal growth in children’s numerical abilities. J Neurosci. 2015; 35 :11743–11750. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Fias W, Menon V, Szucs D. Multiple components of developmental dyscalculia. Trends Neurosci Educ. 2013; 2 :43–47. [ Google Scholar ]
- Frankland PW, Bontempi B. The organization of recent and remote memories. Nat Rev Neurosci. 2005; 6 :119–130. [ PubMed ] [ Google Scholar ]
- Geary DC. Cognitive predictors of achievement growth in mathematics: a 5-year longitudinal study. Dev Psychol. 2011; 47 :1539–1552. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Geary DC, Brown SC. Cognitive addition: strategy choice and speed-of-processing differences in gifted, normal, and mathematically disabled children. Dev Psychol. 1991; 27 :398–406. [ Google Scholar ]
- Geary DC, Hoard MK. Learning disabilities in basic mathematics: deficits in memory and cognition. In: Royer JM, editor. Mathematical Cognition. Information Age Publishing; Greenwich, CT: 2003. pp. 93–115. [ Google Scholar ]
- Geary DC, Hamson CO, Hoard MK. Numerical and arithmetical cognition: a longitudinal study of process and concept deficits in children with learning disability. J Exp Child Psychol. 2000; 77 :236–263. [ PubMed ] [ Google Scholar ]
- Geary D, Hoard M, Nugent L, Byrd-Craven J, Berch D, Mazzocco M. Strategy use, long-term memory, and working memory capacity. In: Berch DB, Mazzocco MMM, editors. Why Is Math So Hard For Some Children? Paul H Brookes Publishing Co; Baltimore, MD: 2007a. pp. 83–105. [ Google Scholar ]
- Geary DC, Hoard MK, Byrd-Craven J, Nugent L, Numtee C. Cognitive mechanisms underlying achievement deficits in children with mathematical learning disability. Child Dev. 2007b; 78 :1343–1359. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Geary DC, Hoard MK, Bailey DH. Fact retrieval deficits in low achieving children and children with mathematical learning disability. J Learn Disabil. 2012; 45 :291–307. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Ghetti S, Demaster DM, Yonelinas AP, Bunge SA. Developmental differences in medial temporal lobe function during memory encoding. J Neurosci. 2010; 30 :9548–9556. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Grabner RH, Ansari D, Koschutnig K, Reishofer G, Ebner F, Neuper C. To retrieve or to calculate? Left angular gyrus mediates the retrieval of arithmetic facts during problem solving. Neuropsychologia. 2009; 47 :604–608. [ PubMed ] [ Google Scholar ]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci USA. 2003; 100 :253–258. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci USA. 2004; 101 :4637–4642. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Ham T, Leff A, de Boissezon X, Joffe A, Sharp DJ. Cognitive control and the salience network: an investigation of error processing and effective connectivity. J Neurosci. 2013; 33 :7091–7098. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Houde O, Rossi S, Lubin A, Joliot M. Mapping numerical processing, reading, and executive functions in the developing brain: an fMRI meta-analysis of 52 studies including 842 children. Dev Sci. 2010; 13 :876–885. [ PubMed ] [ Google Scholar ]
- Isaacs EB, Edmonds CJ, Lucas A, Gadian DG. Calculation difficulties in children of very low birthweight: a neural correlate. Brain. 2001; 124 :1701–1707. [ PubMed ] [ Google Scholar ]
- Ischebeck A, Zamarian L, Egger K, Schocke M, Delazer M. Imaging early practice effects in arithmetic. Neuroimage. 2007; 36 :993–1003. [ PubMed ] [ Google Scholar ]
- Iuculano T, Rosenberg-Lee M, Richardson J, Tenison C, Fuchs L, Supekar K, Menon V. Cognitive tutoring induces widespread neuroplasticity and remediates brain function in children with mathematical learning disabilities. Nat Commun. 2015; 6 :8453. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Jolles D, Ashkenazi S, Kochalka J, Evans T, Richardson J, Rosenberg-lee M, Zhao H, Supekar K, Chen T, Menon V. Parietal hyper-connectivity, aberrant brain organization, and circuit-based biomarkers in children with mathematical disabilities. Dev Sci. 2016; 19 :1–19. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kaufmann L. More evidence for the role of the central executive in retrieving arithmetic facts—a case study of severe developmental dyscalculia. J Clin Exp Neuropsychol. 2002; 24 :302–310. [ PubMed ] [ Google Scholar ]
- Kaufmann L, Lochy A, Drexler A, Semenza C. Deficient arithmetic fact retrieval— storage or access problem? A case study. Neuropsychologia. 2004; 42 :482–496. [ PubMed ] [ Google Scholar ]
- Kaufmann L, Vogel SE, Starke M, Kremser C, Schocke M. Numerical and non-numerical ordinality processing in children with and without developmental dyscalculia: evidence from fMRI. Cogn Dev. 2009a; 24 :486–494. [ Google Scholar ]
- Kaufmann L, Vogel SE, Starke M, Kremser C, Schocke M, Wood G. Developmental dyscalculia: compensatory mechanisms in left intraparietal regions in response to nonsymbolic magnitudes. Behav Brain Funct. 2009b; 5 :35. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kaufmann L, Wood G, Rubinsten O, Henik A. Meta-analyses of developmental fMRI studies investigating typical and atypical trajectories of number processing and calculation. Dev Neuropsychol. 2011; 36 :763–787. [ PubMed ] [ Google Scholar ]
- Kawashima R, Taira M, Okita K, Inoue K, Tajima N, Yoshida H, Sasaki T, Sugiura M, Watanabe J, Fukuda H. A functional MRI study of simple arithmetic—a comparison between children and adults. Cogn Brain Res. 2004; 18 :227–233. [ PubMed ] [ Google Scholar ]
- Koechlin E, Ody C, Kouneiher F. The architecture of cognitive control in the human prefrontal cortex. Science. 2003; 302 :1181–1185. [ PubMed ] [ Google Scholar ]
- Kucian K. Non-symbolic numerical distance effect in children with and without developmental dyscalculia: a parametric fMRI study. Dev Neuropsychol. 2011; 36 :741. [ PubMed ] [ Google Scholar ]
- Kucian K, von Aster M. Developmental dyscalculia. Eur J Pediatr. 2015; 174 :1–13. [ PubMed ] [ Google Scholar ]
- Kucian K, Loenneker T, Dietrich T, Dosch M, Martin E, von Aster M. Impaired neural networks for approximate calculation in dyscalculic children: a functional MRI study. Behav Brain Funct. 2006; 2 :31. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kucian K, von Aster M, Loenneker T, Dietrich T, Martin E. Development of neural networks for exact and approximate calculation: a fMRI study. Dev Neuropsychol. 2008; 33 :447–473. [ PubMed ] [ Google Scholar ]
- Kucian K, Grond U, Rotzer S, Henzi B, Schonmann C, Plangger F, Galli M, Martin E, von Aster M. Mental number line training in children with developmental dyscalculia. Neuroimage. 2011; 57 :782–795. [ PubMed ] [ Google Scholar ]
- Kumaran D, Summerfield JJ, Hassabis D, Maguire EA. Tracking the emergence of conceptual knowledge during human decision making. Neuron. 2009; 63 :889–901. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kwon H, Reiss AL, Menon V. Neural basis of protracted developmental changes in visuo-spatial working memory. Proc Natl Acad Sci USA. 2002; 99 :13336–13341. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Logie RH, Gilhooly KJ, Wynn V. Counting on working memory in arithmetic problem solving. Mem Cognit. 1994; 22 :395–410. [ PubMed ] [ Google Scholar ]
- Luck SJ, Vogel EK. Visual working memory capacity: from psychophysics and neurobiology to individual differences. Trends Cogn Sci. 2013; 17 :391–400. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- McClelland JL, Mcnaughton BL, O’Reilly RC. Why there are complementary learning systems in the hippocampus and neocortex: insights from the successes and failures of connectionist models of learning and memory. Psychol Rev. 1995; 102 :419–457. [ PubMed ] [ Google Scholar ]
- McGaugh JL. Memory—a century of consolidation. Science. 2000; 287 :248–251. [ PubMed ] [ Google Scholar ]
- Menon V. Arithmetic in child and adult brain. In: Cohen Kadosh R, Dowker A, editors. Handbook of Mathematical Cognition. Oxford University Press; Oxford: 2014. pp. 502–530. [ Google Scholar ]
- Menon V. A neurodevelopmental perspective on the role of memory systems in children’s math learning. In: Berch DB, Geary DC, Mann Koepke K, editors. Development of Mathematical Cognition-Neural Substrates and Genetic Influences. Elsevier Science Publishing Co Inc.; United States: 2015. pp. 79–107. In revision. [ Google Scholar ]
- Menon V. Working memory in children’s math learning and its disruption in dyscalculia. Curr Opin Behav Sci. 2016 in press. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010; 214 :655–667. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Menon V, Rivera SM, White CD, Eliez S, Glover GH, Reiss AL. Functional optimization of arithmetic processing in perfect performers. Cogn Brain Res. 2000; 9 :343–345. [ PubMed ] [ Google Scholar ]
- Menon V, Boyett-Anderson JM, Reiss AL. Maturation of medial temporal lobe response and connectivity during memory encoding. Brain Res Cogn Brain Res. 2005; 25 :379–385. [ PubMed ] [ Google Scholar ]
- Metcalfe AWS, Ashkenazi S, Rosenberg-Lee M, Menon V. Fractionating the neural correlates of individual working memory components underlying arithmetic problem solving skills in children. Dev Cogn Neurosci. 2013; 6 :162–175. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Miller EK. The prefrontal cortex and cognitive control. Nat Rev Neurosci. 2000; 1 :59–65. [ PubMed ] [ Google Scholar ]
- Mussolin C, De Volder A, Grandin C, Schlogel X, Nassogne MC, Noël MP. Neural correlates of symbolic number comparison in developmental dyscalculia. J Cogn Neurosci. 2010; 22 :860–874. [ PubMed ] [ Google Scholar ]
- Norman KA, O’Reilly RC. Modeling hippocampal and neocortical contributions to recognition memory: a complementary-learning-systems approach. Psychol Rev. 2003; 110 :611–646. [ PubMed ] [ Google Scholar ]
- Ofen N, Kao YC, Sokol-Hessner P, Kim H, Whitfield-Gabrieli S, Gabrieli JD. Development of the declarative memory system in the human brain. Nat Neurosci. 2007; 10 :1198–1205. [ PubMed ] [ Google Scholar ]
- Ordaz S, Foran W, Velanova K, Luna B. Longitudinal growth curves of brain function underlying inhibitory control through adolescence. J Neurosci. 2013; 33 :18109–18124. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Passolunghi MC, Siegel LS. Working memory and access to numerical information in children with disability in mathematics. J Exp Child Psychol. 2004; 88 :348–367. [ PubMed ] [ Google Scholar ]
- Price GR, Holloway I, Rasanen P, Vesterinen M, Ansari D. Impaired parietal magnitude processing in developmental dyscalculia. Curr Biol. 2007; 17 :R1042–R1043. [ PubMed ] [ Google Scholar ]
- Qin S, Piekema C, Petersson KM, Han B, Luo J, Fernandez G. Probing the transformation of discontinuous associations into episodic memory: an event-related fMRI study. Neuroimage. 2007; 38 :212–222. [ PubMed ] [ Google Scholar ]
- Qin S, Rijpkema M, Tendolkar I, Piekema C, Hermans EJ, Binder M, Petersson KM, Luo J, Fernandez G. Dissecting medial temporal lobe contributions to item and associative memory formation. Neuroimage. 2009; 46 :874–881. [ PubMed ] [ Google Scholar ]
- Qin S, Hermans EJ, Rijpkema M, Fernández G. Adaptive Memory: Imaging Medial Temporal and Prefrontal Memory Systems. Radboud Universiteit Nijmegen, PhD; Nijmegen, the Netherlands: 2011a. [ Google Scholar ]
- Qin S, van Marle HJ, Hermans EJ, Fernandez G. Subjective sense of memory strength and the objective amount of information accurately remembered are related to distinct neural correlates at encoding. J Neurosci. 2011b; 31 :8920–8927. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Qin S, Cho S, Chen T, Rosenberg-Lee M, Geary DC, Menon V. Hippocampal-neocortical functional reorganization underlies children’s cognitive development. Nat Neurosci. 2014; 17 :1263–1269. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Raichle ME, Macleod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci USA. 2001; 98 :676–682. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Rivera SM, Reiss AL, Eckert MA, Menon V. Developmental changes in mental arithmetic: evidence for increased functional specialization in the left inferior parietal cortex. Cereb Cortex. 2005; 15 :1779–1790. [ PubMed ] [ Google Scholar ]
- Rosenberg-Lee M, Barth M, Menon V. What difference does a year of schooling make? Maturation of brain response and connectivity between 2nd and 3rd grades during arithmetic problem solving. Neuroimage. 2011; 57 :796–808. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Rosenberg-Lee M, Ashkenazi S, Chen T, Young CB, Geary DC, Menon V. Brain hyper-connectivity and operation-specific deficits during arithmetic problem solving in children with developmental dyscalculia. Dev Sci. 2014; 18 :351–372. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Rottschy C, Langner R, Dogan I, Reetz K, Laird AR, Schulz JB, Fox PT, Eickhoff SB. Modelling neural correlates of working memory: a coordinate-based meta-analysis. Neuroimage. 2012; 60 :830–846. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Rotzer S, Loenneker T, Kucian K, Martin E, Klaver P, von Aster M. Dysfunctional neural network of spatial working memory contributes to developmental dyscalculia. Neuropsychologia. 2009; 47 :2859–2865. [ PubMed ] [ Google Scholar ]
- Schacter DL, Norman KA, Koutstaal W. The cognitive neuroscience of constructive memory. Annu Rev Psychol. 1998; 49 :289–318. [ PubMed ] [ Google Scholar ]
- Schacter DL, Addis DR, Buckner RL. Remembering the past to imagine the future: the prospective brain. Nat Rev Neurosci. 2007; 8 :657–661. [ PubMed ] [ Google Scholar ]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007; 27 :2349–2356. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Simon O, Mangin JF, Cohen L, Le Bihan D, Dehaene S. Topographical layout of hand, eye, calculation, and language-related areas in the human parietal lobe. Neuron. 2002; 33 :475–487. [ PubMed ] [ Google Scholar ]
- Simons JS, Spiers HJ. Prefrontal and medial temporal lobe interactions in long-term memory. Nat Rev Neurosci. 2003; 4 :637–648. [ PubMed ] [ Google Scholar ]
- Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci USA. 2008; 105 :12569–12574. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Supekar K, Menon V. Developmental maturation of dynamic causal control signals in higher-order cognition: a neurocognitive network model. PLoS Comput Biol. 2012; 8 :e1002374. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Supekar K, Swigart AG, Tenison C, Jolles DD, Rosenberg-Lee M, Fuchs L, Menon V. Neural predictors of individual differences in response to math tutoring in primary-grade school children. Proc Natl Acad Sci USA. 2013; 110 :8230–8235. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Szucs D, Devine A, Soltesz F, Nobes A, Gabriel F. Developmental dyscalculia is related to visuo-spatial memory and inhibition impairment. Cortex. 2013; 49 :2674–2688. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Tse D, Langston RF, Kakeyama M, Bethus I, Spooner PA, Wood ER, Witter MP, Morris RG. Schemas and memory consolidation. Science. 2007; 316 :76–82. [ PubMed ] [ Google Scholar ]
- Tulving E. Elements of Episodic Memory. Oxford University Press; New York: 1983. [ Google Scholar ]
- Tulving E. Episodic memory: from mind to brain. Annu Rev Psychol. 2002; 53 :1–25. [ PubMed ] [ Google Scholar ]
- Uddin LQ, Supekar K, Amin H, Rykhlevskaia E, Nguyen DA, Greicius MD, Menon V. Dissociable connectivity within human angular gyrus and intra-parietal sulcus: evidence from functional and structural connectivity. Cereb Cortex. 2010a; 20 :2636–2646. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Uddin LQ, Supekar K, Menon V. Typical and atypical development of functional human brain networks: insights from resting-state fMRI. Front Syst Neurosci. 2010b; 4 :21. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Van Opstal F, Fias W, Peigneux P, Verguts T. The neural representation of extensively trained ordered sequences. Neuroimage. 2009; 47 :367–375. [ PubMed ] [ Google Scholar ]
- Varma S, Schwartz DL. How should educational neuroscience conceptualise the relation between cognition and brain function? Mathematical reasoning as a network process. Educ Res. 2008; 50 :149–161. [ Google Scholar ]
January 25, 2008
What Are We Thinking When We (Try to) Solve Problems?
New research indicates what happens in the brain when we're faced with a dilemma
By Nikhil Swaminathan
Aha! Eureka! Bingo! "By George, I think she's got it!" Everyone knows what it's like to finally figure out a seemingly impossible problem. But what on Earth is happening in the brain while we're driving toward mental pay dirt ? Researchers eager to find out have long been on the hunt, knowing that such information could one day provide priceless clues in uncovering and fixing faulty neural systems believed to be behind some mental illnesses and learning disabilities.
Researchers at Goldsmiths, University of London report in the journal PLoS ONE that they monitored action in the brains of 21 volunteers with electroencephalography (EEG) as they tackled verbal problems in an attempt to uncover what goes through the mind—literally—in order to observe what happens in the brain during an "aha!" moment of problem solving.
"This insight is at the core of human intelligence … this is a key cognitive function that the human can boast to have," says Joydeep Bhattacharya, an assistant professor in Goldsmiths's psychology department. "We're interested [in finding out] whether—there is a sudden change that takes place or something that changes gradually [that] we're not consciously aware of," he says. The researchers believed they could pin down brain signals that would enable them to predict whether a person could solve a particular problem or not.
On supporting science journalism
If you're enjoying this article, consider supporting our award-winning journalism by subscribing . By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
In many cases, the subjects hit a wall, or what researchers refer to as a "mental impasse." If the participants arrived at this point, they could press a button for a clue to help them untangle a problem. Bhattacharya says blocks correlated with strong gamma rhythms (a pattern of brain wave activity associated with selective attention) in the parietal cortex, a region in the upper rear of the brain that has been implicated in integrating information coming from the senses. The research team noticed an interesting phenomenon taking place in the brains of participants given hints: The clues were less likely to help if subjects had an especially high gamma rhythm pattern. The reason, Bhattacharya speculates, is that these participants were, in essence, locked into an inflexible way of thinking and less able to free their minds, and thereby unable to restructure the problem before them.
"If there's excessive attention, it somehow creates mental fixation," he notes. "Your brain is not in a receptive condition."
At the end of each trial, subjects reported whether or not they had a strong "Aha!" moment. Interestingly, researchers found that subjects who were aware that they had found a new way to tackle the problem (and so, had consciously restructured their thinking) were less likely to feel as if they'd had eureka moment compared to more clueless candidates.
"People experience the "Aha!" feeling when they are not consciously monitoring what they are thinking," Bhattacharya says, adding that the sentiment is more of an emotional experience he likens to relief. "If you're applying your conscious brain information processing ability, then you're alpha." (Alpha brain rhythms are associated with a relaxed and open mind; volunteers who unwittingly solved problems showed more robust alpha rhythms than those who knowingly adjusted their thinking to come up with the answer.)
He says the findings indicate that it's better to tackle problems with an open mind than by concentrating too hard on them. In the future, Bhattacharya says, his team will attempt to predict in real-time whether a stumped subject will be able to solve a vexing problem and, also, whether they can manipulate brain rhythms to aid in finding a solution.
The second probe into problem-solving focused on the anterior cingulate cortex (ACC), a region in the front of the brain tied to functions such as decision making, conflict monitoring and reward feedback. A team at the University of Lyon's Stem Cell and Brain Research Institute in Bron, France reports in Neuron that it verified that the ACC helps detect errors during problem solving (as previously discovered), but also that it does so by acting more as a general guide, monitoring and scoring the steps involved in problem solving, pointing out miscalculations as well as success.
The team discovered this by recording electrical activity in the brains of two male rhesus monkeys as they tried to determine which targets on a screen would result in a tasty drink of juice. "When you're trying to solve a problem, you need to search; when you discover the solution, you need to stop searching," says study co-author Emmanuel Procyk, coordinator of the Institute's Department of Integrative Neurobiology. "We need brain areas to do that."
He says that researchers observed increased neuronal activity in the animals' ACCs when they began searching. When the monkeys hit the jackpot, there was still heightened activity in the ACC (though only a selective population of nerve cells remained hopped up), indicating that the region is responsible for more than simply alerting the rest of the brain when errors are made. Once the monkeys got the hang of it—and routinely pressed the correct target—ACC activity slowed.
"What we think based on this experiment and other experiments," Procyk says, "is that this structure is very important in valuing things." It essentially scores each of the monkey's behaviors as successful or not successful. "It is an area," he adds, "that will help to decide when to shift from the functioning that goes on when [the brain is] learning to when the learning [is] done."
Procyk says that if this system is compromised, it could have implications for issues such as drug dependency. If the ACC is functioning abnormally, he says, it could overvalue drugs, leading to addiction. (Other studies have shown that an impaired cingulate cortex can result in maladaptive social behavior and disrupted cognitive abilities.)
Alas, the ultimate "Aha!" moment for researchers probing problem solving is likely is far off, but at least the latest research may help them avoid an impasse.
Academia.edu no longer supports Internet Explorer.
To browse Academia.edu and the wider internet faster and more securely, please take a few seconds to upgrade your browser .
Enter the email address you signed up with and we'll email you a reset link.
- We're Hiring!
- Help Center

The creative brain: Investigation of brain activity during creative problem solving by means of EEG and FMRI

2009, Human Brain Mapping
Related Papers
Intelligence
Mathias Benedek , Saskia Jaarsveld
International Journal of Psychophysiology
Aljoscha Neubauer
Frontiers in Human Neuroscience
Ilona Papousek , Mathias Benedek
Ilona Papousek
Dalvir Singh
In recent times, neurophysiological measurement methods such as EEG and fMRI are widely used in an Engineering field to study designer’s brain activity during creative thinking. In literature, many researchers reported the synchronization and desynchronization of EEG activity in specific brain cortex during creative thinking. However, we do not find many studies associated to comparison of designer’s brain activity during creativity/non-creativity related task demands. The chief objective of present thesis is to investigate the power of brain activity using EEG comparison between creative and non-creative design task. For psychometric measures of creative thinking, Torrance Test of Creative Thinking (TTCT) (Torrance, 1966) is widely used. In present thesis, we use modified TTCT according to our experiment requirement. The test was decomposed between creative and non-creative design task. In creative design task, designers were instructed to think creatively whereas in non-creative d...
Psychophysiology
Lisa Marshall
Neuropsychologia
Jarl Risberg
Mathias Benedek , Aljoscha Neubauer
Neuroscience Letters
RELATED PAPERS
SIAM Journal on Matrix Analysis and Applications
Jesse Barlow
FLEKS - Scandinavian Journal of Intercultural Theory and Practice
Solveig Moldrheim
Jiří Bezecný
David Knights
Indian Journal of Case Reports
Lalit Bhardwaj
Cancer discovery
Marina P Gehring
Alba Denise Q. Ferreira
Matilde Said
Hussain othman
De-Quan Yang
Veterinária e Zootecnia
Goreti Reis
Edileine Dellalibera
© 2022. Published by AHFE Open Access.
Patricia Castillo
New biotechnology
Journal of neuroinflammation
Emmanuel Victor
Ethology Ecology & Evolution
Dariusz Wysocki
Antonio Jose Sabido Guzman
Adebisi Awodun
Nature Reviews Neuroscience
K. Slinning
Tropical and Subtropical Agroecosystems
Roberto Carlos Barrientos Medina
Journal of the European Academy of Dermatology and Venereology
Jerry Bouquot
International Journal for Parasitology
Nik Ahmad Irwan Izzauddin Nik Him
Complex Systems
fernando pazos
DergiPark (Istanbul University)
Aliq Bağırov
Ivancica Ternjej
See More Documents Like This
RELATED TOPICS
- We're Hiring!
- Help Center
- Find new research papers in:
- Health Sciences
- Earth Sciences
- Cognitive Science
- Mathematics
- Computer Science
- Academia ©2024
Brain's Problem-solving Function At Work When We Daydream
A new University of British Columbia study finds that our brains are much more active when we daydream than previously thought.
The study, published in the Proceedings of the National Academy of Sciences , finds that activity in numerous brain regions increases when our minds wander. It also finds that brain areas associated with complex problem-solving – previously thought to go dormant when we daydream – are in fact highly active during these episodes.
"Mind wandering is typically associated with negative things like laziness or inattentiveness," says lead author, Prof. Kalina Christoff, UBC Dept. of Psychology. "But this study shows our brains are very active when we daydream – much more active than when we focus on routine tasks."
For the study, subjects were placed inside an fMRI scanner, where they performed the simple routine task of pushing a button when numbers appear on a screen. The researchers tracked subjects' attentiveness moment-to-moment through brain scans, subjective reports from subjects and by tracking their performance on the task.
The findings suggest that daydreaming – which can occupy as much as one third of our waking lives – is an important cognitive state where we may unconsciously turn our attention from immediate tasks to sort through important problems in our lives.
Until now, the brain's "default network" – which is linked to easy, routine mental activity and includes the medial prefrontal cortex (PFC), the posterior cingulate cortex and the temporoparietal junction – was the only part of the brain thought to be active when our minds wander.
However, the study finds that the brain's "executive network" – associated with high-level, complex problem-solving and including the lateral PFC and the dorsal anterior cingulate cortex – also becomes activated when we daydream.
"This is a surprising finding, that these two brain networks are activated in parallel," says Christoff. "Until now, scientists have thought they operated on an either-or basis – when one was activated, the other was thought to be dormant." The less subjects were aware that their mind was wandering, the more both networks were activated.
The quantity and quality of brain activity suggests that people struggling to solve complicated problems might be better off switching to a simpler task and letting their mind wander.
"When you daydream, you may not be achieving your immediate goal – say reading a book or paying attention in class – but your mind may be taking that time to address more important questions in your life, such as advancing your career or personal relationships," says Christoff.
The research team included members who are now at Stanford University and University of California, Santa Barbara.
- Brain-Computer Interfaces
- Neuroscience
- Intelligence
- Brain Injury
- Learning Disorders
- Language Acquisition
- Disorders and Syndromes
- Human brain
- Functional neuroimaging
- Psychedelic drug
Story Source:
Materials provided by University of British Columbia . Note: Content may be edited for style and length.
Journal Reference :
- Kalina Christoff, Alan M. Gordon, Jonathan Smallwood, Rachelle Smith, and Jonathan W. Schooler. Experience sampling during fMRI reveals default network and executive system contributions to mind wandering . Proceedings of the National Academy of Sciences , 2009; DOI: 10.1073/pnas.0900234106
Cite This Page :
Explore More
- Illuminating Oxygen's Journey in the Brain
- DNA Study IDs Descendants of George Washington
- Heart Disease Risk: More Than One Drink a Day
- Unlocking Supernova Stardust Secrets
- Why Do Some Memories Become Longterm?
- Cell Division Quality Control 'Stopwatch'
- What Controls Sun's Differential Rotation?
- Robot, Can You Say 'Cheese'?
- Researchers Turn Back the Clock On Cancer Cells
- Making Long-Term Memories: Nerve-Cell Damage
Trending Topics
Strange & offbeat.

IMAGES
VIDEO
COMMENTS
For instance, brain activity has been investigated in response to divergent (as opposed to convergent) thinking [Mölle et al., 1999; Razumnikova, 2000], during insightful problem solving or the subjective experience of "AHA!" [Jung‐Beeman et al., 2004], likewise during the performance of classic creativity tasks such as the alternate or ...
Scans show brain activity during four distinct stages of problem solving. ... reconstructing how the brain moves from understanding a problem to solving it, including the time the brain spends in ...
Specifically, increases in alpha activity (i.e., alpha synchronisation) in response to creative thinking can be interpreted in different ways: As a functional correlate of cortical idling, as a sign of internal top-down activity or, more specifically, as selective inhibition of brain regions. We measured brain activity during creative thinking ...
Difficulty, but not accuracy and strategy, modulate brain activity during problem solving. To relate brain function to behavioral measures impacting student success, we tested our hypotheses that ...
According to the functional topography of brain activity in the theta and alpha frequency, our results suggest that the mental effort while solving scientific problems is related to working memory ...
Additionally, during a brief preparation period prior to the presentation of each problem, various brain regions are more active prior to problems solved with insight than prior to problems solved without insight (Kounios et al., 2006).That is, different patterns of brain activity are conducive to solving the subsequent problem with insight versus analytic processing.
Cortical activity in the EEG alpha band has proven to be particularly sensitive to creativity-related demands, but its functional meaning in the context of creative cognition has not been clarified yet. Specifically, increases in alpha activity (i.e., alpha synchronization) in response to creative thinking can be interpreted in different ways: As a functional correlate of cortical idling, as a ...
Human Brain Mapping is a functional neuroanatomy and neuroimaging journal where all ... Investigation of brain activity during creative problem solving by means of EEG and FMRI. Andreas Fink ... as selective inhibition of brain regions. We measured brain activity during creative thinking in two studies employing different neurophysiological ...
DOI: 10.1002/hbm.20538 Corpus ID: 6027287; The creative brain: Investigation of brain activity during creative problem solving by means of EEG and FMRI @article{Fink2009TheCB, title={The creative brain: Investigation of brain activity during creative problem solving by means of EEG and FMRI}, author={Andreas Fink and Roland H. Grabner and Mathias Benedek and Gernot Reishofer and Verena ...
Yet, as mentioned above, investigating the neurocognitive mechanisms of remote elements combination is central to the understanding of crea-tive thinking. In the present study, we jointly investigated the EEG correlates of semantic remoteness and insight solving using the CAT (Bendetowicz et al., 2017, 2018).
This pattern of brain network connectivity has been reported across domain-general creative problem solving (e.g., divergent thinking) and domain-specific artistic performance (e.g., poetry composition, musical improvisation, and visual art production). ... Default network activity during creative cognition appears to reflect the spontaneous ...
During the solving of complex problems, higher absolute power and EEG correlation of the alpha and fast bands between the left frontal and parietal cortices were observed. ... Brain activity associated with translation between graphical and symbolic representations of functions in generally gifted and excelling in mathematics adolescents ...
5. We are looking for general patterns of problem-solving activities and the part of the brain used for problem-solving activities. 3. Results and Discussion 3.1 Brain Activities during the Problem Solving Process The process of problem-solving is a mental activity that involves many parts of the brain. In education,
As noted in Section 2, a common recurring theme in numerical problem solving is the coengagement of parietal and prefrontal regions associated with working memory. Children as young as 7 show reliable, and consistent, patterns of brain activity during arithmetic problem solving in multiple PFC regions (Houde et al., 2010).
For instance, brain activity has been investigated in response to divergent (as opposed to convergent) thinking [Mo¨lle et al., 1999; Razumnikova, 2000], during insightful problem solving or the subjective experience of ''AHA!'' [Jung-Beeman et al., 2004], likewise during the performance of classic creativity tasks such as
2000], during insightful problem solving or the subjective experience of ''AHA!' ' [Jung-Beeman et al., 2004], likewise during the performance of classic creativity tasks such as
A team at the University of Lyon's Stem Cell and Brain Research Institute in Bron, France reports in Neuron that it verified that the ACC helps detect errors during problem solving (as previously ...
Brain activity during constraint relaxation 2 From the period of impasse, the problem is unconsciously solved through sudden inspiration. At least, consciously, there is no perceived forerunner of µaha¶ moment. The insight problem-solving process has long been studied in psychology and neuroscience (Weisberg, 2015; Shen et al., 2017, 2018 ...
r Human Brain Mapping 30:734-748 (2009) r The Creative Brain: Investigation of Brain Activity During Creative Problem Solving by Means of EEG and fMRI Andreas Fink,1* Roland H. Grabner,2 Mathias Benedek,3 Gernot Reishofer,4 Verena Hauswirth,1 Maria Fally,1 Christa Neuper,1 Franz Ebner,5 and Aljoscha C. Neubauer1 1 Institute of Psychology, University of Graz, Graz, Austria Institute for ...
Activity in numerous brain regions increases when our minds wander, according to new research. Psychologists found that brain areas associated with complex problem-solving -- previously thought to ...