

Calculating Percent Yield

Core Concepts
In this tutorial, you will learn calculating percent yield and theoretical yield are, and how to calculate it. In addition, you will walk through an example calculation.
Topics Covered in Other Articles
- Calculating Molar Mass
- How to Read Periodic Table
- Molecular vs. Empirical Formula
- Chemical Reactions Made Easy
What is Percent Yield?
When performing an experiment, there is a maximum yield you can obtain if there are perfect reaction conditions; this is the theoretical yield . However, even if you follow an experiment correctly, it is likely that you will not have a perfect yield of the product; the amount of product you end up with is your actual yield .
The percentage of the theoretical yield you obtained in your experiment is the percent yield . Let’s learn how to calculate it below!

How to Calculate Percent Yield
You can use the equation below to calculate your yield from an experiment:
What is Theoretical Yield
The theoretical yield is the maximum amount of product that can be obtained from a chemical reaction. It is calculated based on the stoichiometry of the reaction, which is the study of the proportions of the reactants and products in a chemical reaction. The theoretical yield is determined by using the balanced chemical equation for the reaction and the known amounts of the reactants.
Calculating Theoretical Yield
First, you should calculate the theoretical yield of your experiment; usually, this will involve stoichiometric calculations . By looking at the chemical equation and information given, you can get an idea of what is reacting and how the product is forming.
The next step is to identify the limiting reagent, since no more product can be formed once the limiting reagent runs out.
You can then use dimensional analysis to see how much product can be formed based on the amount of limiting reagent that is given. This is the theoretical yield of your experiment.
Calculating Actual Yield
If you are physically doing an experiment, your actual yield will be the amount of product you weigh out on your balance. If you are doing a word problem, the actual yield may be given within the problem.
Percent Yield Equation
The last step, once you have both theoretical and actual yield, is plugging the numbers into the equation. Dividing the actual by the theoretical gives you the fraction of product you made. Multiplying that by 100 gives you the percent yield.
Calculating Percent Yield Example
Now that we know the steps to calculate yield, let’s walk through an example:
First step is to find limiting reagent & theoretical yield of water:
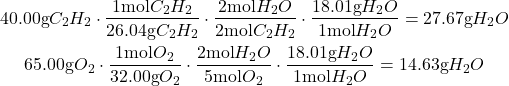
Using dimensional analysis on both reagents, water is found to produce a lower amount of product than oxygen; because of this water is our limiting reagent.
For more example questions to try, click here !
Calculating Percent Yield Practice Problems
MgCl 2 and AgNO 3 react according to the following equation:
Calculating Percent Yield Practice Problem Solutions
Further Reading
- Common Polyatomic Ions
- Kinetic Molecular Theory
- E1 Reaction
- E2 Reaction
Percent Error & Percent Yield Quiz
This online quiz is intended to give you extra practice in percent error and percent yield calculations with descriptions of a variety of chemical reactions.
Select your preferences below and click 'Start' to give it a try!

Understanding Percent Yield and Theoretical Yield
- The Albert Team
- Last Updated On: March 25, 2024

A fundamental concept that every budding chemist must grasp is calculating percent yield, a measure that bridges the theoretical world of chemistry with its practical applications. But what exactly is this value, and why is it so crucial in the realm of chemical reactions?
Percent yield is a key indicator used by chemists to determine the efficiency of a reaction. It compares the amount of product actually obtained from a reaction to the amount that theoretically could be produced. Understanding this concept is not just about crunching numbers; it’s about connecting the dots between the lab predictions and what transpires during a chemical reaction.
This post will guide you through the essentials of finding theoretical yield and calculating percent yield. Whether you’re conducting experiments in the lab or tackling problem sets in class, mastering these calculations will enhance your chemistry toolkit, enabling you to predict and analyze the outcomes of reactions with greater precision. So, let’s dive into the world of theoretical and percent yield, where the beauty of chemistry meets the rigor of mathematics!
What We Review
How to Find Theoretical Yield
Definition and importance.
Before we dive into the how-tos, let’s define theoretical yield. The definition is the amount of product that will theoretically be produced in a chemical reaction based on the limiting reactant and the stoichiometry of the reaction. It’s the maximum possible amount of product you can get under perfect conditions where everything goes exactly as planned.
But why is this theoretical amount important? In chemistry, predicting how much product a reaction can produce is essential. This prediction helps in planning and optimizing reactions, whether you’re synthesizing a new compound in a research lab or just performing a class experiment. Therefore, it’s all about efficiency and anticipation – knowing how much you can expect helps evaluate a reaction’s success and efficiency.
Breaking Down the Steps
With this in mind, let’s break down the steps:
- Balanced Chemical Equation : Everything starts with a balanced chemical equation. This equation tells you the ratio in which reactants combine and products form, which is crucial for the next steps.
- Identify the Limiting Reactant : The limiting reactant is the substance that will be used up first in the reaction, determining the maximum amount of product that can be formed. Compare the mole ratios of the reactants to figure out which one is the limiting reactant.
- Calculate Moles of Product : Using stoichiometry, convert the moles of the limiting reactant to moles of the desired product based on the coefficients in the balanced equation.
- Find Theoretical Yield : Finally, convert the moles of the product to grams (or any other unit, depending on the context) using its molar mass. This conversion gives you the theoretical yield of the product.
Let’s further illustrate this with a simple example. Imagine you’re reacting 2 moles of hydrogen ( \text{H}_2 ) with 1 mole of oxygen ( \text{O}_2 ) to produce water ( \text{H}_2\text{O} ). According to the balanced equation 2\text{H}_2 + \text{O}_2 \rightarrow 2\text{H}_2\text{O} , you can theoretically get 2 moles of water from 2 moles of hydrogen and 1 mole of oxygen. If hydrogen is your limiting reactant, then you can expect to produce 2 moles of water, which is your theoretical yield.
Finding Theoretical Yield: Practical Tips
Common challenges and solutions.
Finding the theoretical yield in a chemical reaction is a fundamental skill in chemistry, but it can come with its own set of challenges. For example, here are some common issues students might face and how to overcome them:
- Misinterpreting the Limiting Reactant : One common mistake is incorrectly identifying the limiting reactant. To avoid this, carefully compare the mole ratios of the reactants used to the ratios in the balanced equation. Remember, the limiting reactant is the one that would run out first, limiting the amount of product formed.

- Calculation Errors : When converting between moles and grams, double-check your calculations. A simple arithmetic mistake can throw off your entire result. Using a calculator and rechecking your steps can minimize these errors.
- Forgetting Units : Always include units in your calculations. Whether you’re working with moles, grams, or liters, keeping track of your units can prevent mix-ups and help ensure your calculations are correct.
Tools and Resources
In addition to understanding the common pitfalls, here are some tools and resources that can help you master finding theoretical yields:
- Stoichiometry Calculators : There are many online calculators available that can help you with stoichiometry problems. While it’s important to understand the process yourself, these tools can be useful for checking your work.

- Chemistry Textbooks and Guides : Don’t overlook the value of a good chemistry textbook or guide. These resources often provide step-by-step examples and can be great references when you’re stuck.
- Educational Videos and Tutorials : Visual learners might benefit from video tutorials available on educational platforms. The step-by-step process can provide a clearer understanding of how to find theoretical yield.
Overall, by being mindful of these challenges and utilizing available resources, you can enhance your ability to accurately find the theoretical amount of product in chemical reactions, a crucial skill in chemistry.
How to Calculate Percent Yield
Understanding the percent yield formula.
Percent yield is a crucial concept in chemistry, representing the efficiency of a reaction. The formula for this is:
- Actual Yield : The quantity of product actually produced in the reaction.
- Theoretical Yield : The amount of product predicted by stoichiometry.
This formula calculates the percentage of the predicted amount that was actually obtained in the experiment.
Step-by-Step Guide to Calculate Percent Yield
Follow these steps:
- Determine the Theoretical Yield : Use stoichiometry to find the theoretical yield, ensuring it is in the same units as the actual yield.
- Measure the Actual Yield : Obtain this from your experimental data.
- Apply the Percent Yield Formula : Insert your actual and theoretical yields into the percent yield formula to find the efficiency of your reaction.
Example Problem
If a reaction has a theoretical yield of 10.0 \text{ grams} and an actual yield of 8.0 \text{ grams} , the percent yield is calculated as:
This indicates that the reaction had an 80\% yield, meaning 80\% of the predicted product was successfully produced.
Practice Problem s
Test your knowledge with these practice problems on theoretical and percent yield. Solve the problems below before moving on to the answer section.
Practice Problem 1
Given the balanced equation 2 H_2 + O_2 \rightarrow 2 H_2O , calculate the theoretical yield of H_2O if you start with 5.0 moles of H_2 and an excess of O_2 .
Practice Problem 2
For the reaction P_4 + 6 Cl_2 \rightarrow 4 PCl_3 , if the theoretical yield of PCl_3 is 150.0 grams and the actual yield obtained from the experiment is 115.0 grams, calculate the percent yield.
Practice Problem 3
In the reaction N_2 + 3 H_2 \rightarrow 2 NH_3 , if you start with 10.0 moles of N_2 and 10.0 moles of H_2 , determine the limiting reactant and calculate the theoretical yield of NH_3 .
Practice Problem 4
Given the reaction C + 2 H_2 \rightarrow CH_4 , if you start with 12.0 grams of carbon and have an excess of hydrogen, first convert the mass of carbon to moles, determine the theoretical yield of methane in moles, and then calculate the percent yield if the actual yield is 22.0 grams of CH_4 .
Practice Problem Answers
Here are the solutions to the practice problems. Check your work and understand each step to improve your grasp of theoretical and percent yield calculations.

Practice Problem 1 Answer
For the equation 2 H_2 + O_2 \rightarrow 2 H_2O , with 5.0 moles of H_2 and an excess of O_2 , the theoretical yield of H_2O is calculated as follows:
Practice Problem 2 Answer
For the reaction P_4 + 6 Cl_2 \rightarrow 4 PCl_3 , with a theoretical yield of 150.0 grams and an actual yield of 115.0 grams, the percent yield is:
Practice Problem 3 Answer
In the reaction N_2 + 3 H_2 \rightarrow 2 NH_3 , starting with 10.0 moles of N_2 and 10.0 moles of H_2 , you must complete two stoichiometry problems, one for each reactant, to determine which would be the limiting reactant:
Since H_2 produces the least amount of NH_3 , it is the limiting reactant. This also tells you the theoretical yield, which will be 6.67 moles of NH_3 .
Practice Problem 4 Answer
For the reaction C + 2 H_2 \rightarrow CH_4 , converting 12.0 grams of carbon to moles:
The theoretical yield of CH_4 is 1.00 mole (since 1 mole of C produces 1 mole of CH_4 ). If the actual yield is 22.0 grams of CH_4 :
First, convert the amount obtained to moles:
Then, calculate the percent yield:
(Note: The percent can be over 100% due to measurement errors or impurities in the reactants.)
Congratulations on working through the fundamentals of theoretical and percent yield! These concepts are not just abstract numbers; they are essential tools that chemists use to measure the efficiency and success of their reactions. Understanding how to calculate theoretical helps you predict the maximum amount of product that can be produced in a reaction while determining percent yield gives you insight into the reaction’s efficiency in a real-world lab setting.
By mastering these calculations, you’re not just learning to crunch numbers—you’re gaining valuable skills that will help you in practical lab work and deepen your understanding of chemical processes. Remember, practice is key to becoming proficient in these concepts. The more you work through different problems and scenarios, the more intuitive these calculations will become.
So, keep challenging yourself with more complex reactions and different types of calculations. Your journey into the fascinating world of chemistry is just beginning, and these foundational skills will serve as stepping stones to more advanced topics and experiments. Keep exploring, stay curious, and enjoy the process of discovery in the wonderful world of chemistry!
Interested in a school license?
Popular posts.

AP® Score Calculators
Simulate how different MCQ and FRQ scores translate into AP® scores

AP® Review Guides
The ultimate review guides for AP® subjects to help you plan and structure your prep.

Core Subject Review Guides
Review the most important topics in Physics and Algebra 1 .

SAT® Score Calculator
See how scores on each section impacts your overall SAT® score

ACT® Score Calculator
See how scores on each section impacts your overall ACT® score

Grammar Review Hub
Comprehensive review of grammar skills

AP® Posters
Download updated posters summarizing the main topics and structure for each AP® exam.
Interested in a school license?

Bring Albert to your school and empower all teachers with the world's best question bank for: ➜ SAT® & ACT® ➜ AP® ➜ ELA, Math, Science, & Social Studies aligned to state standards ➜ State assessments Options for teachers, schools, and districts.

Chemistry Steps

General Chemistry
Stoichiometry.
This is a comprehensive, end-of-chapter set of practice problems on stoichiometry that covers balancing chemical equations, mole-ratio calculations, limiting reactants, and percent yield concepts.
The links to the corresponding topics are given below.
- The Mole and Molar Mass
- Molar Calculations
- Percent Composition and Empirical Formula
- Stoichiometry of Chemical Reactions
Limiting Reactant
- Reaction/Percent Yield
- Stoichiometry Practice Problems
Balance the following chemical equations:
a) HCl + O 2 → H 2 O + Cl 2
b) Al(NO 3 ) 3 + NaOH → Al(OH) 3 + NaNO 3
c) H 2 + N 2 → NH 3
d) PCl 5 + H 2 O → H 3 PO 4 + HCl
e) Fe + H 2 SO 4 → Fe 2 (SO 4 ) 3 + H 2
f) CaCl 2 + HNO 3 → Ca(NO 3 ) 2 + HCl
g) KO 2 + H 2 O → KOH + O 2 + H 2 O 2
h) Al + H 2 O → Al 2 O 3 + H 2
i) Fe + Br 2 → FeBr 3
j) Cu + HNO 3 → Cu(NO 3 ) 2 + NO 2 + H 2 O
k) Al(OH) 3 → Al 2 O 3 + H 2 O
l) NH 3 + O 2 → NO + H 2 O
m) Ca(AlO 2 ) 2 + HCl → AlCl 3 + CaCl 2 + H 2 O
n) C 5 H 12 + O 2 → CO 2 + H 2 O
o) P 4 O 10 + H 2 O → H 3 PO 4
p) Na 2 CrO 4 + Pb(NO 3 ) 2 → PbCrO 4 + NaNO 3
q) MgCl 2 + AgNO 3 → AgCl + Mg(NO 3 ) 2
r) KClO 3 → KClO 4 + KCl
s) Ca(OH) 2 + H 3 PO 4 → Ca 3 (PO 4 ) 2 + H 2 O
Consider the balanced equation:
C 5 H 12 + 8 O 2 → 5CO 2 + 6H 2 O
Complete the table showing the appropriate number of moles of reactants and products.
How many grams of CO 2 and H 2 O are produced from the combustion of 220. g of propane (C 3 H 8 )?
C 3 H 8 (g) + 5O 2 (g) → 3CO 2 (g) + 4H 2 O(g)
How many grams of CaCl 2 can be produced from 65.0 g of Ca(OH) 2 according to the following reaction,
Ca(OH) 2 + 2HCl → CaCl 2 + 2H 2 O
How many moles of oxygen are formed when 75.0 g of Cu(NO 3 ) 2 decomposes according to the following reaction?
2Cu(NO 3 ) 2 → 2CuO + 4NO 2 + O 2
How many grams of MnCl 2 can be prepared from 52.1 grams of MnO 2 ?
MnO 2 + 4HCl → MnCl 2 + Cl 2 + 2H 2 O
Determine the mass of oxygen that is formed when an 18.3-g sample of potassium chlorate is decomposed according to the following equation:
2KClO 3 (s) → 2KCl(s) + 3O 2 (g).
How many grams of H 2 O will be formed when 48.0 grams H 2 are mixed with excess hydrogen gas?
2H 2 + O 2 → 2H 2 O
Consider the chlorination reaction of methane (CH4):
CH 4 (g) + 4Cl 2 (g) → CCl 4 (g) + 4HCl(g)
How many moles of CH 4 were used in the reaction if 51.9 g of CCl4 were obtained?
How many grams of Ba(NO 3 ) 2 can be produced by reacting 16.5 g of HNO 3 with an excess of Ba(OH) 2 ?
Ethanol can be obtained by fermentation – a complex chemical process breaking down glucose to ethanol and carbon dioxide.
C 6 H 12 O 6 → 2C 2 H 5 OH + 2CO 2 glucose ethanol
How many mL of ethanol (d =0.789 g/mL) can be obtained by this process starting with 286 g of glucose?
36.0 g of butane (C 4 H 10 ) was burned in an excess of oxygen and the resulting carbon dioxide (CO 2 ) was collected in a sealed vessel.
2C 4 H 10 + 13O 2 → 8CO 2 + 10H 2 O
How many grams of LiOH will be necessary to consume all the CO 2 from the first reaction?
2LiOH + CO 2 → Li 2 CO 3 + H 2 O
13. Which statement about limiting reactant is correct?
a) The limiting reactant is the one in a smaller quantity.
b) The limiting reactant is the one in greater quantity.
c) The limiting reactant is the one producing less product.
d) The limiting reactant is the one producing more product.
Find the limiting reactant for each initial amount of reactants.
4NH 3 + 5O 2 → 4NO + 6H 2 O
a) 2 mol of NH 3 and 2 mol of O 2
b) 2 mol of NH 3 and 3 mol of O 2
c) 3 mol of NH 3 and 3 mol of O 2
d) 3 mol of NH 3 and 2 mol of O 2
Note: This is not a multiple-choice question. Each row represents a separate question where you need to determine the limiting reactant.
How many g of hydrogen are left over in producing ammonia when 14.0 g of nitrogen is reacted with 8.0 g of hydrogen?
N 2 (g) + 3 H 2 (g) → 2 NH 3 (g)
How many grams of PCl 3 will be produced if 130.5 g Cl 2 is reacted with 56.4 g P 4 according to the following equation?
6Cl 2 (g) + P 4 (s) → 4PCl 3 (l)
How many grams of sulfur can be obtained if 12.6 g H 2 S is reacted with 14.6 g SO 2 according to the following equation?
2H 2 S(g) + SO 2 (g) → 3S(s) + 2H 2 O(g)
The following equation represents the combustion of octane, C 8 H 18 , a component of gasoline:
2C 8 H 18 (g) + 25O 2 (g) → 16CO 2 (g) + 18H 2 O(g)
Will 356 g of oxygen be enough for the complete combustion of 954 g of octane?
When 140.0 g of AgNO 3 was added to an aqueous solution of NaCl, 86.0 g of AgCl was collected as a white precipitate. Which salt was the limiting reactant in this reaction? How many grams of NaCl were present in the solution when AgNO 3 was added?
AgNO 3 (aq) + NaCl(aq) → AgCl(s) + NaNO 3 (aq)
Consider the reaction between MnO 2 and HCl:
MnO 2 + 4HCl → MnCl 2 + Cl 2 + 2H 2 O
What is the theoretical yield of MnCl 2 in grams when 165 g of MnO 2 is added to a solution containing 94.2 g of HCl?
Percent Yield
21. In a chemistry experiment, a student obtained 5.68 g of a product. What is the percent yield of the product if the theoretical yield was 7.12 g?
When 38.45 g CCl 4 is reacted with an excess of HF, 21.3 g CCl 2 F 2 is obtained. Calculate the theoretical and percent yields of this reaction.
CCl 4 + 2HF → CCl 2 F 2 + 2HCl
Iron(III) oxide reacts with carbon monoxide according to the equation:
Fe 2 O 3 ( s ) + 3CO( g ) → 2Fe( s ) + 3CO 2 ( g )
What is the percent yield of this reaction if 623 g of iron oxide produces 341 g of iron?
Determine the percent yield of the reaction if 77.0 g of CO 2 are formed from burning 2.00 moles of C 5 H 12 in 4.00 moles of O 2 .
C 5 H 12 + 8 O 2 → 5CO 2 + 6H 2 O
The percent yield for the following reaction was determined to be 84%:
N 2 ( g ) + 2H 2 ( g ) → N 2 H 4 ( l )
How many grams of hydrazine (N 2 H 4 ) can be produced when 38.36 g of nitrogen reacts with 6.68 g of hydrogen?
Silver metal can be prepared by reducing its nitrate, AgNO 3 with copper according to the following equation:
Cu( s ) + 2AgNO 3 ( aq ) → Cu(NO 3 ) 2 ( aq ) + 2Ag( s )
What is the percent yield of the reaction if 71.5 grams of Ag was obtained from 132.5 grams of AgNO 3 ?
Industrially, nitric acid is produced from ammonia by the Ostwald process in a series of reactions:
4NH 3 ( g ) + 5O 2 ( g ) → 4NO( g ) + 6H 2 O( l )
2NO( g ) + O 2 ( g ) → 2NO 2 ( g )
2NO 2 ( g ) + H 2 O( l ) → HNO 3 ( aq ) + HNO 2 ( aq )
Considering that each reaction has an 85% percent yield, how many grams of NH 3 must be used to produce 25.0 kg of HNO 3 by the above procedure?
Aspirin (acetylsalicylic acid) is widely used to treat pain, fever, and inflammation. It is produced from the reaction of salicylic acid with acetic anhydride. The chemical equation for aspirin synthesis is shown below:
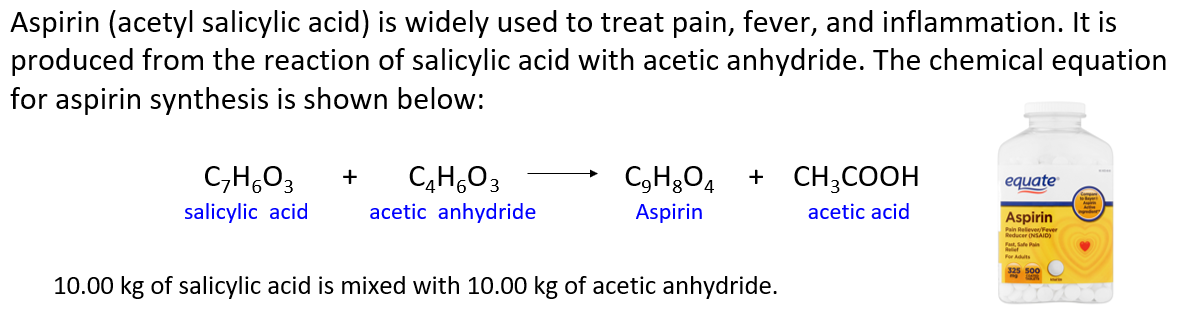
In one container, 10.00 kg of salicylic acid is mixed with 10.00 kg of acetic anhydride.
a) Which reactant is limiting? Which is in excess? b) What mass of excess reactant is left over? c) What mass of aspirin is formed assuming 100% yield (Theoretical yield)? d) What mass of aspirin is formed if the reaction yield is 70.0% ? e) If the actual yield of aspirin is 11.2 kg, what is the percent yield? f) How many kg of salicylic acid is needed to produce 20.0 kg of aspirin if the reaction yield is 85.0% ?
3 thoughts on “Stoichiometry Practice Problems”
You forgot the subscript 3 for O in the molecular formula for acetic anhydride and the reaction is not balanced as written. For part F) it’s 18.1 kg and not1.81 kg as written in the final line of the solution.
Thanks for letting me know! Fixed.
You’re welcome!
Leave a Comment Cancel reply
Notify me of followup comments via e-mail. You can also subscribe without commenting.

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

12.9: Theoretical Yield and Percent Yield
- Last updated
- Save as PDF
- Page ID 53797

Can we save some money?
The world of pharmaceutical production is an expensive one. Many drugs have several steps in their synthesis and use costly chemicals. A great deal of research takes place to develop better ways to make drugs faster and more efficiently. Studying how much of a compound is produced in any given reaction is an important part of cost control.
Percent Yield
Chemical reactions in the real world do not always go exactly as planned on paper. In the course of an experiment, many things will contribute to the formation of less product than would be predicted. Besides spills and other experimental errors, there are often losses due to an incomplete reaction, undesirable side reactions, etc. Chemists need a measurement that indicates how successful a reaction has been. This measurement is called the percent yield.
To compute the percent yield, it is first necessary to determine how much of the product should be formed based on stoichiometry. This is called the theoretical yield , the maximum amount of product that could be formed from the given amounts of reactants. The actual yield is the amount of product that is actually formed when the reaction is carried out in the laboratory. The percent yield is the ratio of the actual yield to the theoretical yield, expressed as a percentage:
\[\text{Percent Yield} = \frac{\text{Actual Yield}}{\text{Theoretical Yield}} \times 100\%\nonumber \]
Percent yield is very important in the manufacture of products. Much time and money is spent improving the percent yield for chemical production. When complex chemicals are synthesized by many different reactions, one step with a low percent yield can quickly cause a large waste of reactants and unnecessary expense.
Typically, percent yields are understandably less than \(100\%\) because of the reasons previously indicated. However, percent yields greater than \(100\%\) are possible if the measured product of the reaction contains impurities that cause its mass to be greater than it actually would be if the product was pure. When a chemist synthesizes a desired chemical, he or she is always careful to purify the products of the reaction.
Example \(\PageIndex{1}\): Calculating the Theoretical Yield and the Percent Yield
Potassium chlorate decomposes upon slight heating in the presence of a catalyst, according to the reaction below.
\[2 \ce{KClO_3} \left( s \right) \rightarrow 2 \ce{KCl} \left( s \right) + 3 \ce{O_2} \left( g \right)\nonumber \]
In a certain experiment, \(40.0 \: \text{g} \: \ce{KClO_3}\) is heated until it completely decomposes. What is the theoretical yield of oxygen gas? The experiment is performed, the oxygen gas is collected, and its mass is found to be \(14.9 \: \text{g}\). What is the percent yield for the reaction?
First, we will calculate the theoretical yield based on the stoichiometry.
Step 1: List the known quantities and plan the problem.
- Given: Mass of \(\ce{KClO_3} = 40.0 \: \text{g}\)
- Molar mass \(\ce{KClO_3} = 122.55 \: \text{g/mol}\)
- Molar mass \(\ce{O_2} = 32.00 \: \text{g/mol}\)
- theoretical yield O 2 = ? g
Apply stoichiometry to convert from the mass of a reactant to the mass of a product:
\[\text{g} \: \ce{KClO_3} \rightarrow \text{mol} \: \ce{KClO_3} \rightarrow \text{mol} \: \ce{O_2} \rightarrow \text{g} \: \ce{O_2} \nonumber\nonumber \]
Step 2: Solve.
\[40.0 \: \text{g} \: \ce{KClO_3} \times \frac{1 \: \text{mol} \: \ce{KClO_3}}{122.55 \: \text{g} \: \ce{KClO_3}} \times \frac{3 \: \text{mol} \: \ce{O_2}}{2 \: \text{mol} \: \ce{KClO_3}} \times \frac{32.00 \: \text{g} \: \ce{O_2}}{1 \: \text{mol} \: \ce{O_2}} = 15.7 \: \text{g} \: \ce{O_2} \nonumber\nonumber \]
The theoretical yield of \(\ce{O_2}\) is \(15.7 \: \text{g}\).
Step 3: Think about your result.
The mass of oxygen gas must be less than the \(40.0 \: \text{g}\) of potassium chlorate that was decomposed.
Now we will use the actual yield and the theoretical yield to calculate the percent yield.
- Actual yield \(= 14.9 \: \text{g}\)
- Theoretical yield \(= 15.7 \: \text{g}\)
- Percent yield = ? %
\[\text{Percent Yield} = \frac{\text{Actual Yield}}{\text{Theoretical Yield}} \times 100\% \nonumber\nonumber \]
Use the percent yield equation above.
\[\text{Percent Yield} = \frac{14.9 \: \text{g}}{15.7 \: \text{g}} \times 100\% = 94.9\% \nonumber\nonumber \]
Since the actual yield is slightly less than the theoretical yield, the percent yield is just under \(100\%\).
- Theoretical yield is calculated based on the stoichiometry of the chemical equation.
- The actual yield is experimentally determined.
- The percent yield is determined by calculating the ratio of actual yield/theoretical yield.
- What do we need in order to calculate theoretical yield?
- If I spill some of the product before I weigh it, how will that affect the actual yield?
- How will spilling some of the product affect the percent yield?
- I make a product and weigh it before it is dry. How will that affect the actual yield?
If you're seeing this message, it means we're having trouble loading external resources on our website.
If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked.
To log in and use all the features of Khan Academy, please enable JavaScript in your browser.
AP®︎/College Chemistry
Course: ap®︎/college chemistry > unit 4.
- Stoichiometry
- Worked example: Calculating amounts of reactants and products
Limiting reactant and reaction yields
- Worked example: Calculating the amount of product formed from a limiting reactant
- Worked example: Relating reaction stoichiometry and the ideal gas law
Limiting reactant and theoretical yield
Example 1: using the limiting reactant to calculate theoretical yield, step 1: convert reactant masses to moles, step 2: find the limiting reactant, step 3: calculate the theoretical yield, percent yield, example 2: calculating percent yield, step 1: find moles of the limiting reactant, step 2: determine the theoretical yield (in grams), step 3: calculate the percent yield.
- " Chemical Reactions " from UC Davis ChemWiki, CC BY-NC-SA 3.0
- " Stoichiometry and Balancing Reactions " from UC Davis ChemWiki, CC BY-NC-SA 3.0
- " Reaction Stoichiometry " from Boundless Chemistry , CC BY-SA 4.0
Want to join the conversation?
- Upvote Button navigates to signup page
- Downvote Button navigates to signup page
- Flag Button navigates to signup page


IMAGES
VIDEO
COMMENTS
Study with Quizlet and memorize flashcards containing terms like For the balanced equation shown below, if the reaction of 90.7 grams of F2 produces a 73.7% yield, how many grams of O2 would be produced ? 2F2+2H2O=>4HF+O2, For the balanced equation shown below, if the reaction of 99.8 grams of O2 produces 51.0 grams of CO2, what is the percent yield? 4C2H5Cl+13O2=>8CO2+10H2O+2Cl2, For the ...
PROBLEM \(\PageIndex{6}\) Freon-12, CCl 2 F 2, is prepared from CCl 4 by reaction with HF. The other product of this reaction is HCl. Outline the steps needed to determine the percent yield of a reaction that produces 12.5 g of CCl 2 F 2 from 32.9 g of CCl 4.Freon-12 has been banned and is no longer used as a refrigerant because it catalyzes the decomposition of ozone and has a very long ...
Learn about the percent yield of chemical reactions. The practice problems will address finding the percent yield from a single reactant, from two reactants considering the limiting reactant and determining the amounts of reactants needed at a given percent yield. Check the answers and the solutions below.
13 PRACTICE PROBLEM. Calcium hydroxide is produced by the reaction of calcium oxide reacts with water as shown in the reaction below: CaO (s) + H 2 O (I) → Ca (OH) 2 (s) Calculate the percent yield if a 4.89-g sample of CaO is reacted with excess water and 5.63 g of Ca (OH) 2 is produced. 3.
Percent Yield Practice Problems. 7 problems. Previous Topic. 1 PRACTICE PROBLEM. The reactants below combine according to the following equation: M 2 + 3 X 2 → 2 MX 3. The initial amounts of M 2 and X 2 are shown in Figure A (M = gray; X = orange) while the actual yield is shown in Figure B. What is the percent yield of the reaction?
The percent yield of a reaction is the ratio of the actual yield to the theoretical yield, expressed as a percentage. 7.3 Limiting Reactant and Percent Yield Problems is shared under a not declared license and was authored, remixed, and/or curated by LibreTexts.
Magnesium oxide can be made by heating magnesium metal in the presence of oxygen. The balanced equation for the reaction is: 2 Mg (s) + O2 ( g)¡2 MgO (s) When 10.1 g of Mg reacts with 10.5 g O2, 11.9 g MgO is collected. Determine the limiting reactant. Determine the theoretical yield. Determine the percent yield for the reaction.
Percent Yield Calculations Practice Problems 1) A reaction with a·calculated yield of 9.23 g produced 7.89 g of product. What is the percent yield for this reaction? 2) 5.96 g of ammonia (17.031 g/mol) react completely according to the following reaction: 2 NH3(g) + C02(g)
Calculating Percent Yield Practice Problems. Problem 1. You mix of MgCl 2 and of AgNO 3 and recover of AgCl. Calculate the yield. MgCl 2 and AgNO 3 react according to the following equation: Problem 2. You drop of elemental sodium (Na) into of water and collect of H 2. Calculate the percent yield.
If the reaction of 6.5 grams of C6H12O6 produces 2.5 grams of CO2, what is the percent yield? 1. If the reaction of 125 grams of C6H6O3 reacts in excess of oxygen (O2) and produces 51 grams of H2O ...
explaining percent yield, how to do percent yield problems from simple percent to using stoichiometry to using limiting reactantsCC Academy videos are easy 1...
Practice some actual yield and percentage problems below. 1. For the balanced equation shown below, if the reaction of 40.8 grams of C6H6O3 produces a 39.0% yield, how many grams of H2O would be produced ? C6H6O3+6O2=>6CO2+3H2O. 2. For the balanced equation shown below, if the reaction of 20.7 grams of CaCO3 produces 6.81 grams of CaO, what is ...
This quiz helps you practice calculating percent error and percent yield in hundreds of chemical reactions.
Practice Problems. Test your knowledge with these practice problems on theoretical and percent yield. Solve the problems below before moving on to the answer section. Practice Problem 1. Given the balanced equation 2 H_2 + O_2 \rightarrow 2 H_2O, calculate the theoretical yield of H_2O if you start with 5.0 moles of H_2 and an excess of O_2.
If you were good, that means you'd be equal to or greater than 70% and then you have a poor yield. If you're percent. Yield was less than 40%. Now, with percent yield comes the percent yield formula and the percent yield formula equals actual yield over theoretical yield. Times 100 remember, we have our purple box here.
7.4NH Percent Yield (For additional information see page 317 in the book) Practice Problems - Work these on a Separate sheet, You will need the room. 1. Start with an Easy One: Be + 2 HCl BeCl 2 + H 2 The theoretical yield of beryllium chloride was 10.7 grams. If the reaction actually yields 4.5 grams, what was the percent yield?
Stoichiometry Practice Problems. This is a comprehensive, end-of-chapter set of practice problems on stoichiometry that covers balancing chemical equations, mole-ratio calculations, limiting reactants, and percent yield concepts. The links to the corresponding topics are given below. The Mole and Molar Mass.
percentyield = actualyield theoreticalyield × 100%. Actual and theoretical yields may be expressed as masses or molar amounts (or any other appropriate property; e.g., volume, if the product is a gas). As long as both yields are expressed using the same units, these units will cancel when percent yield is calculated.
Tollens' and Benedict's Test 8m. Reduction of Aldehydes and Ketones 7m. Hemiacetal and Acetal Formation 6m. 16. Carboxylic Acids and Their Derivatives 42m. Naming Carboxylic Acids 11m. Naming Esters 8m. Naming Amides 6m. Learn Percent Yield with free step-by-step video explanations and practice problems by experienced tutors.
Use the percent yield equation above. Step 2: Solve. Percent Yield = 14.9 g 15.7 g × 100% = 94.9% Percent Yield = 14.9 g 15.7 g × 100 % = 94.9 %. Step 3: Think about your result. Since the actual yield is slightly less than the theoretical yield, the percent yield is just under 100% 100 %. How To Calculate Theoretical Yield and Percent Yield.
Step 3: Calculate the theoretical yield. Our final step is to determine the theoretical yield of AlCl 3 in the reaction. Remember that the theoretical yield is the amount of product that is produced when the limiting reactant is fully consumed. In this case, the limiting reactant is Cl A 2 , so the maximum amount of AlCl 3 that can be formed is ...
Theoretical, Actual and Percent Yield Problems - Chemistry Tutorial. Skip to main content. General Chemistry Start typing, then use the up and down arrows to select an option from the list. ... Related Practice. Guided course. 03:09. Percent Yield. Jules Bruno. 1993. views. 23. rank. Guided course. 04:43. Percent Yield Example 1. Jules Bruno ...