Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Published: 23 November 2021

The health effects of vitamin D supplementation: evidence from human studies
- Roger Bouillon ORCID: orcid.org/0000-0002-6446-3763 1 ,
- Despoina Manousaki 2 ,
- Cliff Rosen ORCID: orcid.org/0000-0003-3436-8199 3 ,
- Katerina Trajanoska 4 ,
- Fernando Rivadeneira ORCID: orcid.org/0000-0001-9435-9441 5 &
- J. Brent Richards 6 , 7
Nature Reviews Endocrinology volume 18 , pages 96–110 ( 2022 ) Cite this article
112k Accesses
172 Citations
206 Altmetric
Metrics details
- Endocrine system
- Endocrine system and metabolic diseases
Vitamin D supplementation can prevent and cure nutritional rickets in infants and children. Preclinical and observational data suggest that the vitamin D endocrine system has a wide spectrum of skeletal and extra-skeletal activities. There is consensus that severe vitamin D deficiency (serum 25-hydroxyvitamin D (25OHD) concentration <30 nmol/l) should be corrected, whereas most guidelines recommend serum 25OHD concentrations of >50 nmol/l for optimal bone health in older adults. However, the causal link between vitamin D and many extra-skeletal outcomes remains unclear. The VITAL, ViDA and D2d randomized clinical trials (combined number of participants >30,000) indicated that vitamin D supplementation of vitamin D-replete adults (baseline serum 25OHD >50 nmol/l) does not prevent cancer, cardiovascular events, falls or progression to type 2 diabetes mellitus. Post hoc analysis has suggested some extra-skeletal benefits for individuals with vitamin D deficiency. Over 60 Mendelian randomization studies, designed to minimize bias from confounding, have evaluated the consequences of lifelong genetically lowered serum 25OHD concentrations on various outcomes and most studies have found null effects. Four Mendelian randomization studies found an increased risk of multiple sclerosis in individuals with genetically lowered serum 25OHD concentrations. In conclusion, supplementation of vitamin D-replete individuals does not provide demonstrable health benefits. This conclusion does not contradict older guidelines that severe vitamin D deficiency should be prevented or corrected.
Vitamin D and calcium supplementation can cure nutritional rickets and can modestly decrease the risk of major fractures in older adults with poor vitamin D status or calcium intake.
Large supplementation trials recruiting vitamin D-replete adults (serum 25OHD concentration >50 nmol/l) have demonstrated no effects on the incidence of cancer, cardiovascular events or type 2 diabetes mellitus (T2DM) and no benefits in terms of bone density and the risk of falls.
Post-hoc analysis of large supplementation trials has suggested that supplementation of individuals with vitamin D deficiency modestly delays age-related bone loss and progression to T2DM, and improves lung function.
A meta-analysis suggested that vitamin D supplementation results in a modest decrease in cancer mortality.
Over 60 Mendelian randomization studies have examined causal links between genetically lower vitamin D levels and health outcomes; most studies generated null effects except four studies that demonstrated an increased risk of multiple sclerosis.
In conclusion, supplementation of vitamin D-replete individuals does not generate overall health benefits; however, correction of severe vitamin D deficiency remains essential.
Similar content being viewed by others

Vitamin D deficiency 2.0: an update on the current status worldwide
Karin Amrein, Mario Scherkl, … Oliver Malle

Absence of causal association between Vitamin D and bone mineral density across the lifespan: a Mendelian randomization study
Yanchao Tang, Feng Wei, … Xiaoguang Liu
Targeted 25-hydroxyvitamin D concentration measurements and vitamin D3 supplementation can have important patient and public health benefits
William B. Grant, Fatme Al Anouti & Meis Moukayed
Introduction
There is consensus that daily intake of 400 IU of vitamin D can prevent nutritional rickets in infants and children 1 . However, the skeletal effects of vitamin D deficiency in adults and older adults (aged >65 years), and the potential extra-skeletal effects of vitamin D are more controversial. Some people consider that vitamin D supplementation is futile 2 . By contrast, others have suggested that the vitamin D intake requirement is much higher than currently achieved by the general population and that people should aim to achieve 25-hydroxyvitamin D (25OHD, the major marker of vitamin D status) concentrations similar to those found in certain tribes in equatorial Africa with a sun exposure lifestyle that might be similar to that of early humans 3 , 4 , 5 , 6 , 7 .
The potential extra-skeletal effects of the vitamin D endocrine system (which refers to vitamin D in its active form, its precursors and metabolites, and vitamin D receptor) are based on several arguments. For example, the vitamin D receptor (VDR) and CYP27B1 (the enzyme primarily responsible for producing the active form of vitamin D, 1,25-dihydroxyvitamin D or 1,25(OH) 2 D 3 ) are widely expressed, including in tissues that are not involved in calcium or phosphate transport (Fig. 1 ). In addition, ~3% of the human and mouse genomes are under the direct or indirect control of 1,25(OH) 2 D 3 (refs 8 , 9 ). Finally, many diseases and illnesses in humans are associated with a poor vitamin D status, as measured by low serum levels of 25OHD. Therefore, one of the major clinical questions in the field is whether poor vitamin D status plays a causal role in the diseases and conditions associated with low 25OHD levels, such as cancer, impaired muscle strength and falls, and immune, metabolic or cardiovascular diseases. Furthermore, if the link is causal, the threshold serum level of 25OHD below which the risk of these diseases is increased must be identified 8 .

The potential skeletal and extra-skeletal target tissues and effects of the vitamin D endocrine system (vitamin D and vitamin D receptor) as based on preclinical and observational studies, Mendelian randomization studies and randomized controlled trials (RCTs). In vitro studies have identified many molecular and genetic targets of vitamin D action. Animal models have confirmed a variety of skeletal and extra-skeletal actions. Human observational data are largely in line with preclinical data. However, Mendelian randomization studies and RCTs have not confirmed such a widespread action profile in vitamin D-replete adults. Therefore, the health consequences of poor vitamin D status remain controversial. The strength of the relationship between the vitamin D endocrine system and health effects are indicated by the arrow thickness. FGF23, fibroblast growth factor 23; PTH, parathyroid hormone.
Up to about a decade ago, there was tremendous uncertainty about vitamin D supplementation for the maintenance of adequate health levels. Large, randomized placebo-controlled trials with clinically important outcomes and/or surrogates had yet to be performed and only a few meta-analyses of randomized controlled trials (RCTs) were available. On the other hand, many observational studies had been conducted that were confounded by multiple variables. The Institute of Medicine (IOM) guidelines were developed to provide an answer based on the best available evidence at that time 10 . The IOM Committee established minimal dosing to maintain adequate serum levels of 25OHD in young and older individuals and established the tolerable upper limits of supplementation. The Committee also examined the totality of evidence relating vitamin D supplementation to numerous outcomes, and concluded that large randomized trials were needed to establish efficacy and safety. Other societies, such as the Endocrine Society 11 , and governmental organizations also generated a variety of guidelines 12 . The minimal serum level of 25OHD that was considered to provide vitamin D sufficiency varied from 30 nmol/l (UK Scientific Advisory Committee on Nutrition 13 ), to 50 nmol/l (IOM and many other governmental guidelines) 12 , 14 , 75 nmol/l (Endocrine Society and some other societies) 11 and even >100 nmol/l (refs 12 , 14 ). Of note, serum levels of >100 nmol/l are found in people living in Africa under conditions of sun exposure supposed to be similar to that of early humans.
In this Review, we summarize the results of recent (2017–2020) RCTs as well as Mendelian randomization studies, while not reviewing observational studies, which have been well-documented previously 9 . We have chosen these two study designs because they are both types of causal inference studies and can help provide insights into the role of vitamin D in the aetiology of common diseases. The reviewed studies do not provide evidence that vitamin D supplementation prevents negative health outcomes in vitamin D-replete adults. However, all these studies reveal new suggestions for potential effects of vitamin D supplementation. Note that throughout the text vitamin D refers to vitamin D 3 unless otherwise specified.
RCTs: 2017–2020
Many small-scale RCTs have been published over the past few years. In addition, several large studies have generated a wealth of new data (Table 1 ; Supplementary Box 1 ). The new major RCTs deal with more than 35,000 study participants who have a generally better health profile than participants in older studies. These studies used higher dosages than previous studies and the volunteers were mostly vitamin D-replete at baseline. These differences might explain why these large RCTs generated mostly null results in the intention-to-treat (ITT) analysis.
The largest trial to date is the VITAL study 15 that recruited more than 25,000 adults from 44 centres in the USA and evaluated daily doses (2,000 IU) of vitamin D for a mean duration of 5.3 years. The Vitamin D Assessment Study (ViDA study) 16 evaluated the effects of monthly high-dose vitamin D supplementation in more than 5,000 adults in New Zealand followed for a mean duration of 3.3 years. The primary aim of the D2d study of 2,423 US participants was to evaluate the effects of a daily dose of vitamin D (4,000 IU per day) for a mean duration of 2.5 years on the conversion of prediabetes to type 2 diabetes mellitus (T2DM) 17 . The DO-HEALTH study evaluated the effects of vitamin D (2,000 IU per day) in 2,157 older adults in Europe for a duration of 3 years 18 . The Calgary study was not really a megatrial, as it included only 311 Canadian adults and explored the effects on bone structure and quality and the safety of daily high-dose vitamin D (4,000 and 10,000 IU versus 400 IU) for 3 years 18 , 19 , 20 .
Mendelian randomization studies
Mendelian randomization is an established genetic epidemiological method, which can be used to test whether genetically decreased 25OHD levels are associated with increased risk of disease. To do this, Mendelian randomization uses single nucleotide polymorphisms (SNPs) that are associated with 25OHD levels in genome-wide association studies (GWAS) as instruments to infer 25OHD levels. Depending on their number, these SNPs can explain from 2% to 10% of the variance in 25OHD levels. This approach offers an alternative analytical technique able to reduce bias from confounding and reverse causation present in observational studies and re-estimates observations in a framework enabling causal inference (Supplementary Box 2 ). The very large number of Mendelian randomization studies of vitamin D have also generated mostly null results; however, they have been handicapped by the low power to predict decreased serum 25OHD concentrations.
Effects of vitamin D on health outcomes
Many observational studies suggest a link between low vitamin D status and T2DM 9 .
Evidence from RCTs
In the large D2d RCT of patients with prediabetes (Table 1 ), vitamin D supplementation only showed a non-significant trend to slow down the progression of prediabetes into T2DM. The study design intentionally included people with a high risk of progression to T2DM, who received vitamin D (4,000 IU per day). In the ITT analysis, the hazard ratio for the development of T2DM in the group receiving vitamin D was 0.88 (95% CI 0.75–1.04; P = 0.12) compared with the placebo group. In a post hoc analysis, however, a significant effect was observed in individuals with a baseline BMI below 30 mg/m 2 , severe vitamin D deficiency at baseline, perfect adherence to treatment during the study or serum 25OHD above 100 nmol/l throughout the study 21 (Tables 2 , 3 ). Analysis of the combined data from the D2d trial and two other trials specifically designed and conducted to investigate the effectiveness of vitamin D supplementation in preventing T2DM showed that vitamin D supplementation (when compared with placebo) reduced the risk of developing T2DM from 23% to 13% (a 10% reduction) in persons with prediabetes not selected for vitamin D deficiency 22 . This finding is in line with two meta analyses published in 2020 dealing with eight 23 and seven 24 RCTs in people with prediabetes. These meta-analyses concluded that vitamin D supplementation decreased the risk to progress to T2DM by about 10%, especially when using doses above 1,000 IU per day and in participants without obesity. Participant-level meta-analysis of these trials might provide a better estimate of risk reduction and identify populations of patients with prediabetes who are likely to benefit the most from vitamin D supplementation.
Evidence from Mendelian randomization
Since 2015, seven large Mendelian randomization studies have investigated the causal effect of genetically altered 25OHD levels on risk of T2DM and related traits (Supplementary Box 3 ). These Mendelian randomization studies included very large numbers of participants and mostly recruited white individuals and Chinese individuals. One study 25 generated conflicting results, as part of the study using only two SNPs concluded that high predicted serum levels of 25OHD protected against T2DM (OR 0.86 of T2DM for a 25 nmol/l higher 25OHD concentration than that seen in the general population). However, in a slightly larger group of the same study that included two additional SNPs, the odds ratio became insignificant (Supplementary Box 3 ). All the other Mendelian randomization studies, including more than 500,000 volunteers, did not find a significant odds ratio for the relationship between predicted 25OHD and risk of T2DM.
Vitamin D and T2DM — summary
Although observational data have consistently confirmed lower serum 25OHD concentrations in patients with T2DM or the metabolic syndrome 9 , most Mendelian randomization studies have not supported these conclusions. Importantly, the large D2d RCT only showed a non-significant trend to slow down the progression of prediabetes into T2DM. In a small subgroup of individuals with overweight (rather than obesity) and prediabetes, supplementation provided some modest benefit, albeit lower than lifestyle modifications or metformin 26 . Furthermore, analysis of the combined results of the D2d trial and two other trials showed that vitamin D supplementation reduced the risk of developing T2DM in people with prediabetes not selected for vitamin D deficiency 22 . Additional studies or more in-depth analysis of the existing studies are needed to validate these findings. In summary, the evidence from large-scale Mendelian randomization studies and RCTs are convergent and do not support the use of vitamin D supplementation for the prevention of T2DM.
Strong preclinical data exist that link vitamin D with cell cycle control and cancer. Furthermore, many observational studies have associated poor vitamin D status with increased risk of cancer or poor prognosis 27 .
The largest RCT (VITAL) did not find an effect of daily vitamin D supplementation on invasive cancer incidence (HR 0.96, 95% CI 0.88–1.06) in US adults during a 5.3-year follow-up 15 . Further subanalysis (not statistically corrected for multiple comparisons) revealed a significant reduction in cancer risk in individuals with a normal BMI (<25 kg/m 2 ) and a trend for decreased cancer risk in African Americans. Baseline serum 25OHD concentrations did not influence cancer incidence or mortality but the number of participants with vitamin D deficiency at baseline (<50 nmol/l) was low (~10% of the total cohort) 28 . In the ViDA trial in New Zealand adults, monthly vitamin D supplementation did not modify cancer incidence (overall or specific types of cancer, excluding non-melanoma skin cancers) with an overall hazard ratio of 1.01 (95% CI 0.81–1.25) 16 .
Cancer mortality, as evaluated in a Cochrane systematic review 29 , was modestly decreased by vitamin D supplementation in four RCTs (44,492 participants), with a relative risk (RR) for cancer mortality of 0.88 (95% CI 0.78–0.98) in individuals receiving a mean daily dose of 1,146 IU (compared with no supplementation) during a mean follow-up of 6.3 years. Cancer mortality was also evaluated in several large RCTs (Supplementary Box 4 ). In the ITT analysis of the VITAL trial, a non-significant trend of reduction in total cancer mortality (HR 0.83, 95% CI 0.67–1.02) was observed in the vitamin D supplementation group. When excluding cancer deaths during the first year, or the first and second year after randomization, a significant reduction in cancer mortality was observed in the vitamin D supplementation group compared with no supplementation (HR 0.75, 95% CI 0.59–0.96). In a Kaplan–Meier plot, the cumulative increased risk of cancer mortality was visible from year 4 of follow-up onwards 28 . In the ViDA trial, however, the number of cancer deaths was not influenced by vitamin D supplementation (HR 0.97), even after exclusion of cancer deaths registered in the first year after randomization (HR 0.95) 16 . This discrepancy might be related to the short duration of follow-up. The ViDA trial lasted <4 years, whereas the effect of vitamin D supplementation in the VITAL study was only significant 4 years after randomization. An updated summary from the VITAL study 28 confirmed a small but significant effect on cancer death in vitamin D-supplemented individuals (HR 0.87, 95% CI 0.79–0.96; P = 0.005). As the final serum concentration of 25OHD in the VITAL trial (~110 nmol/l) and the ViDA trial (~125 nmol/l or 50 ng/ml) were in the high normal range it is unlikely that higher doses would be more effective.
The Ovarian Cancer Association Consortium (10,065 patients with ovarian cancer, 21,654 control individuals) 30 found a 27% increase in the risk of epithelial ovarian cancer per 20 nmol/l decrease in genetically determined 25OHD serum concentration (OR 1.27, 95% CI 1.06–1.51). However, the results were not corroborated by another Mendelian randomization study 31 which also showed no evidence of an association between 25OHD and risk of colorectal, breast, prostate, lung and pancreatic cancer or neuroblastoma. Similar findings were reported in a separate study 32 in relation to total incident cancer and cancer subtypes such as breast, colorectal and lung cancer in 23,294 women. A null effect of genetically determined 25OHD on colorectal carcinoma was confirmed in men and women after including two additional SNPs 33 . Similarly, a large-scale two-sample Mendelian randomization study (122,977 patients with breast cancer and 79,148 patients with prostate cancer) did not show any effects of genetically predicted 25OHD concentrations on these cancers (Supplementary Box 5 ). Evidence from Mendelian randomization also refutes a link between 25OHD concentrations with risk of oesophageal adenocarcinoma 34 , melanoma and non-melanoma skin cancer 35 (Supplementary Box 5 ).
Vitamin D and cancer — summary
No effects of vitamin D supplementation on cancer risk were observed in the large VITAL and ViDA trials. In line with prior studies and Mendelian randomization results, it thus seems clear that vitamin D supplementation in vitamin D-replete adults does not change cancer risk. However, a subanalysis of the VITAL trial showed that vitamin D supplementation might have some minor benefits in individuals with a normal BMI, but this finding was not corrected for multiple end point analysis 15 . In addition, several independent trials have suggested, in post hoc analysis, potential benefits of vitamin D supplementation on cancer mortality, especially when the follow-up is longer than 4 years 28 (Supplementary Box 4 ). Therefore, a link between vitamin D status and cancer incidence or mortality cannot be excluded, but will be very difficult to verify. Small changes in vitamin D status are unlikely to affect cancer incidence based on several Mendelian randomization studies.
Cardiovascular events
Major cardiovascular events.
The results of any observational studies in humans are in line with preclinical data and have demonstrated a consistent association between low vitamin D status and increased risk of cardiovascular diseases, hypertension and cardiovascular events, including ischaemic cardiac events, cardiomyopathy, congestive heart failure, stroke and even cardiovascular mortality. In a meta-analysis of nearly 850,000 individuals, low serum 25OHD concentrations were associated with an increased risk of cardiovascular events (RR 1.43, comparing individuals with the lowest vitamin D status with individuals with a better vitamin D status) 36 .
Two large RCTs (VITAL and ViDA) were designed to include cardiovascular events as one of their primary end points 15 , 37 . During the 5.3 years of follow-up in the VITAL trial, the hazard ratio for the expanded composite end point of major cardiovascular events including coronary revascularization was 0.97 (95% CI 0.85–1.12) in the vitamin D supplementation group, compared with placebo. A similar hazard ratio was found for cardiovascular death (HR 1.11, 95% CI 0.88–1.40), or death from any cause. Exclusion of cardiovascular events or deaths during the first 2 years of follow-up did not change the overall results. Similarly, in the ViDA study, the primary outcome of major cardiovascular events was not influenced by monthly vitamin D supplementation over 3.3 years 37 . The adjusted hazard ratio for a combination of major cardiovascular events in the vitamin D supplementation group was 1.02 (95% CI 0.87–1.20) compared with placebo, and such null findings also applied for a large list of secondary end points (myocardial infarction, heart failure, stroke and hypertension, among others), or cardiovascular deaths. Findings were not dependent on the baseline serum 25OHD concentration or previous cardiovascular status. When the results of these two major trials (including together more than 30,000 participants) were combined with those of previous studies evaluating the potential effects of vitamin D supplementation, a similar general conclusion of no effect of vitamin D supplementation was reached. An analysis of 21 RCTs including more than 80,000 participants showed that major cardiovascular events were not influenced by vitamin D supplementation 38 . The hazard ratios for myocardial infarction, stroke or cardiovascular death were all close to 1 and the 95% confidence intervals included the null. The results are uniformly concordant despite variation in target groups, baseline vitamin D status and vitamin dosage or regimens. Furthermore, vitamin D supplementation of largely vitamin D-replete participants did not significantly reduce first or recurrent hospitalization rates for heart failure compared with no supplementation in the VITAL Heart Failure study (HR 0.93, 95% CI 0.78–1.11; non-significant).
To date, six Mendelian randomization studies have investigated the effect of genetically altered 25OHD levels on cardiovascular events and related outcomes (Supplementary Box 6 ). These studies evaluated the effects of genetically altered 25OHD concentrations (based on two to six SNPs) in more than a million European and Chinese adults and found no significant effects on any cardiovascular event or mortality 39 , 40 , 41 , 42 . A 2020 study 43 , using a substantially larger number of SNPs (242 SNPs associated with 25OHD levels adjusted for BMI, and 232 SNPs associated to 25OHD levels without adjustment for BMI), showed a non-significant odds ratio for coronary artery disease in people with genetically lowered 25OHD levels of 0.98 (95% CI −0.06–0.02) compared with those with normal or high 25OHD level in a sample of 417,580 white British individuals from the UK Biobank.
Hypertension
Observational data also link hypertension with low vitamin D status but this apparent association could have been due to many other confounding factors (for example, related to lifestyle). Causal inference studies, such as RCTs and Mendelian randomization studies, should provide insights that reduce the risk of confounding. The data on blood pressure effects of vitamin D supplementation in the VITAL trial (VITAL Hypertension) are not yet available (NCT01653678; as of October 2021). The ViDA trial, however, studied extensively the effects of vitamin D supplementation in a subgroup of participants using a state of the art invasive technology (suprasystolic oscillometry) 44 . After a mean follow-up of 1.1 years, vitamin D supplementation generated null effects. In participants with vitamin D deficiency at baseline (<50 nmol/l), brachial systolic and diastolic blood pressure decreased by 3 mmHg to 5 mmHg (not statistically significant); however, aortic systolic blood pressure (−7.5 mmHg, P = 0.03) and other parameters (augmentation index, pulse wave velocity, peak reservoir pressure and backward pressure amplitude) improved on correction of baseline vitamin D deficiency 44 . The DO-HEALTH trial in European older adults did not find any effect of vitamin D supplementation on systolic or diastolic blood pressure 18 .
The evidence from Mendelian randomization studies on the effects of predicted serum 25OHD levels on hypertension, systolic and diastolic blood pressure is consistent across five large studies, and overall does not support any of these outcomes (Supplementary Box 6 ). Specifically, a study in 146,581 European individuals 45 , using two SNPs in the two vitamin D synthesis genes showed a marginal decrease in diastolic blood pressure of 0.29 mmHg per 10% increase in 25OHD level. There was no significant effect on systolic blood pressure, and the Mendelian randomization odds ratio for hypertension was 0.92 per 10% increase in 25OHD level (95% CI 0.87–0.97). A 2019 study, using six 25OHD-related SNPs 46 , failed to show any evidence of a causal association between 25OHD levels and systolic blood pressure, diastolic blood pressure or hypertension. Finally, using up to 252 SNPs as instruments for estimating levels of 25OHD, the most recent Mendelian randomization study in this field published in 2020 (ref. 43 ) showed a marginal effect of 25OHD levels on risk of hypertension (Mendelian randomization OR 0.97 per unit increase in rank-based inverse normal-transformed 25OHD level, 95% CI 0.94–1.0) in 417,580 White British individuals from UK Biobank. After adjusting for BMI, this association became non-significant. In non-European populations, Mendelian randomization results thus far are consistent with those in Europeans. Specifically, a Mendelian randomization study 47 on 2,591 Korean adults failed to show any causal effect of 25OHD levels on systolic blood pressure, diastolic blood pressure or risk of hypertension. A Mendelian randomization study 48 in 10,655 Chinese individuals showed equally a null effect of 25OHD on systolic and diastolic blood pressure.
Vitamin D and cardiovascular disease — summary
In summary, convergent evidence from Mendelian randomization studies and RCTs demonstrates that vitamin D supplementation does not decrease the risk of cardiovascular disease. The link between vitamin D status and a variety of cardiovascular events or risk factors was tested previously in mostly small-scale studies. The 2017–2020 megatrials (Table 1 ) and Mendelian randomization studies clearly confirm the lack of benefit of vitamin D supplementation in vitamin D-replete adults. This conclusion most likely also applies to people with vitamin D deficiency as based on subgroup analyses of the VITAL and ViDA trials. Unfortunately, both studies recruited very few participants with severe vitamin D deficiency. A dedicated detailed analysis of the ViDA trial suggested some modest benefits on central (but not peripheral) blood pressure, but the implications of this observations are limited in view of the small scale of this ViDA substudy 44 .
Musculoskeletal effects and falls
Vitamin d and bone health.
Severe vitamin D deficiency is the leading cause of nutritional rickets 1 . The importance of more modest vitamin D deficiency than seen in nutritional rickets for the skeleton of adults and older adults is disputed. Supplementation with vitamin D only is unlikely to be able to reduce fracture risk in older adults; 2 , 49 however, a combination of calcium and vitamin D supplementation can modestly reduce hip and non-vertebral fracture incidence in this population 2 , 50 , 51 . This conclusion is in line with a 2019 overview and meta-analysis on vitamin D and calcium supplementation and fractures 52 , which concluded from observational data (39,0141 participants) that a 25 nmol/l increase in the serum 25OHD concentration reduces the risk of any fracture or hip fracture by 7% and 20%, respectively (both statistically significant). A similar conclusion was reached in another meta-analysis 53 .
Several large RCTs have generated new results regarding the effects of vitamin D supplementation on the adult skeleton. The VITAL Bone Health study is an ancillary study of the VITAL trial, including a subcohort of 771 participants (men aged ≥50 years and women aged ≥55 years; not taking bone active medications) evaluated at baseline and after 2 years (89% retention), and aims to evaluate the effects of vitamin D on bone structure and architecture. Supplemental vitamin D (compared with placebo) had no effect on 2-year changes in areal bone mineral density (BMD) at the spine, femoral neck, total hip or whole body, or on measures of bone structure. This conclusion remained valid in a subgroup analysis, including individuals with the lowest vitamin D status (as measured by total 25OHD) at baseline. New technology allows the direct measurement of free (non-protein-bound) 25OHD as an alternative strategy to define vitamin D status 54 . In participants of the VITAL trial with the lowest directly measured free 25OHD concentrations, vitamin D supplementation generated a slight increase in spine areal BMD (0.75% in the vitamin D group versus 0% in the placebo group; P = 0.043) and attenuation in loss of total hip areal BMD (−0.42% in the vitamin D group versus −0.98% in the placebo group; P = 0.044), yet such results might not survive multiple testing correction 55 . The ViDA trial did not find an effect of monthly vitamin D supplementation on the incidence of non-vertebral fractures (RR 1.19, 95% CI 0.94–1.50; non-significant) compared with no supplementation 56 . In participants with baseline vitamin D deficiency (<50 nmol/l), the HR for non-vertebral fractures was 0.94 compared with that in vitamin D-replete participants (95% CI 0.58–1.52). This conclusion was confirmed in the DO-HEALTH trial 18 .
A well-validated risk factor for fracture, such as BMD, might provide more information on the possible effects of vitamin D supplementation. In a subgroup of participants in the ViDA trial ( n = 452) 57 , the loss of BMD during follow-up was about 0.5% lower in the vitamin D group compared with the control group. This difference was statistically significant for the femoral neck and total hip but not for the lumbar spine or total body BMD. However, in the small ( n = 30) group of participants with a baseline serum 25OHD concentration of <30 nmol/l, BMD of the lumbar spine increased significantly by 3.1% compared with that in controls. These data indicate that correction of severe vitamin D deficiency might improve bone density, but not when given to vitamin D-replete people. A smaller RCT in Scottish adults confirmed that vitamin D supplementation (daily dose of 1,000 IU) increased BMD in individuals with a baseline serum 25OHD concentration of <30 nmol/l but not in people with a better vitamin D status at baseline 58 . These results are also in line with a RCT in US adults randomized to receive placebo, 800 IU of vitamin D or high-dose vitamin D (50,000 IU per day for 2 weeks followed by 50,000 IU per 2 weeks for 1 year), which concluded that neither low-dose nor high-dose vitamin D improved bone density in participants with a mean baseline serum 25OHD of 50 nmol/l (ref. 59 ). The same conclusion was drawn from a RCT of vitamin D supplementation in Black American women, as increasing baseline serum 25OHD concentrations of 55 nmol/l to concentrations above 75 nmol/l by vitamin D supplementation did not change the rate of bone loss during 3 years of follow-up 60 . Similarly, Finnish children below the age of 2 years who received 1,200 IU of vitamin D per day for ~2 years did not have better bone density (measured by peripheral quantitative CT (pQCT)) compared with children receiving the standard dose of 400 IU per day 61 . This finding is not totally unexpected, as the baseline serum 25OHD concentration was higher (80 nmol/l) than expected in this study due to the introduction of vitamin D supplementation of food in Finland.
The Calgary study was designed to evaluate the effect of long-term high-dose vitamin D on bone mass and quality. A daily dose of 400 IU, 4,000 IU or 10,000 IU of vitamin D for 3 years in Canadian adults did not increase BMD, but rather slightly decreased BMD, as measured by the best available methodology (high-resolution pQCT) 19 . Indeed, BMD at the radius and tibia significantly decreased by 3.5% and 1.7 %, respectively in the 10,000 IU per day group compared with the 400 IU per day group, whereas the decrease at both sites was not statistically significant in the 4,000 IU per day group compared with the 400 IU per day group. This study does demonstrate that vitamin D supplementation in vitamin D-replete adults (baseline serum 25OHD concentration of about 75 nmol/l) does not improve bone mass or quality. Moreover, very high doses might even have negative effects, as a small percentage of participants developed hypercalciuria or hypercalcaemia, which quickly resolved after adjustment of dosing. Of course, this finding might imply that regular follow-up is desirable when using such dosages 19 , 62 .
Many Mendelian randomization studies showed no causal effect of vitamin D status on a variety of bone traits in populations of European and non-European ancestry. An early Mendelian randomization study 63 found that genetically predicted one standard deviation increase in 25OHD was not associated with increased femoral neck BMD, lumbar spine BMD or estimated BMD change. Similar results were observed in relation to total body BMD 64 . A more powered Mendelian randomization analysis 65 (37,857 patients with fracture and 227,116 control individuals) also did not support a causal effect of 25OHD on fracture risk. However, a Mendelian randomization study in children 66 showed that haplotypes associating with low 25OHD were associated with low pQCT parameters (BMD, cross-sectional area and cortical density) in 2-year-old children. Finally, evidence from Mendelian randomization studies 67 refutes causal associations between predicted serum 25OHD concentrations and either BMD or bone metabolism markers found in 1,824 postmenopausal Chinese women (Supplementary Box 6 ).
Vitamin D and muscle function or falls
In mice, total deletion of VDR generates structural and functional consequences for skeletal and cardiac muscle 9 . Furthermore, humans with congenital CYP27B1 mutations or patients with severe combined deficiency of 25OHD and 1,25(OH) 2 D due to chronic renal failure develop severe muscle weakness that rapidly improves after treatment with 1,25(OH) 2 D 9 . Several meta-analyses have come to different conclusions regarding the consequences of vitamin D supplementation on muscle strength, with both positive 68 and null effects 69 . In addition, ample literature is available supporting a link between poor vitamin D status and increased risk of falls, but hesitance remains regarding causality 70 . High boluses of vitamin D, however, might transiently increase the risk of falls in older women 71 . High-dose continuous vitamin D supplementation to increase serum 25OHD concentrations to above 112 nmol/l might also induce an increased risk of falls in older men and women 72 , 73 , 74 . However, the large ViDA trial showed that monthly 100,000 IU doses of vitamin D did not reduce or increase the risk of falls. The hazard ratio for falls was 0.99 (95% CI 0.92–1.07) in the overall cohort who were treated with vitamin D compared with those receiving placebo and 1.07 (95% CI 0.91–1.25) in vitamin D-supplemented participants with baseline serum 25OHD concentrations below 50 nmol/l (ref. 56 ). The VITAL trial also looked at the effects of daily vitamin D supplementation on physical disability and falls in the SRURDY study 75 and found a non-significant (OR 0.97, 95% CI 0.91–1.25) effect of vitamin D supplementation on the risk of two falls or injurious falls requiring support from a doctor or hospital 76 . In further exploratory analysis, the same conclusion was reached when the baseline serum concentration of 25OHD was taken into account.
To our knowledge, no Mendelian randomization studies so far have examined the causal association between genetically estimated 25OHD levels and muscle traits or falls.
Vitamin D and musculoskeletal effects — summary
Of note, the 2017–2020 megatrials did not address the question of vitamin D supplementation and rickets, as there is consensus in all vitamin D guidelines from the past decade that serum 25OHD concentrations below 30 nmol/l are a risk factor for rickets or osteomalacia 12 . A daily vitamin D dose of 400 IU can prevent rickets and osteomalacia and increase serum concentrations of 25OHD well above 30 nmol/l (12 ng/ml) 77 . However, ~7% of the world population lives with severe vitamin D deficiency, with this percentage being much higher in the Middle East, North Africa and many countries in Asia 78 .
The role of vitamin D in the skeleton of adults and older adults is more disputed. The 2017–2020 megatrials were not designed to primarily evaluate the effect of vitamin D supplementation on fracture risk in older adults. These trials 15 , 16 recruited mostly vitamin D-replete adults with a fairly low risk of fracture. Even the DO-HEALTH trial in older, less vitamin D-replete, adults (compared with the other megatrials) did not find an effect on non-vertebral fractures 18 . However, the ViDA trial demonstrated that correction of severe vitamin D deficiency (<30 nmol/l) prevents age-related bone loss in adults. By contrast, the 2017–2020 megatrials demonstrate that vitamin D supplementation in vitamin D-replete adults does not improve bone mass, density or quality 16 .
Taken together, the findings indicate that supplementation with vitamin D only does not have a beneficial effect on fracture risk in vitamin D-replete, mostly white adults. However, combined calcium and vitamin D supplementation in older adults, especially those with poor vitamin D status and poor calcium intake, might decrease the risk of hip fractures and other major fractures by about 20% 51 . Therefore, most recent guidelines recommend a daily vitamin D supplement of about 800 IU of vitamin D combined with a good calcium intake (above 1,000 mg per day) in all older adults with a high risk or documented vitamin D deficiency. Of note, the Calgary study demonstrated that high daily doses of vitamin D (4,000 and especially 10,000 IU per day) might decrease BMD and bone quality 19 , 20 . Therefore, the optimal dose in vitamin D-deficient older adults should be at least 800 IU per day but not more than 4,000 IU per day.
Meta-analyses of older studies suggested a modest decrease in the risk of falls in older, mostly vitamin D-deficient, adults 79 . However, the ViDA trial did not confirm this finding as vitamin D supplementation did not change the risk of falls. The New Zealand population was younger and had a better vitamin D status than the participants in the older studies. There might also be a U-shaped relationship as very high vitamin status, especially due to high bolus doses, might increase the risk of falls 72 , 73 , 74 .
Lung function and respiratory effects
Vitamin d and respiratory infections or lung function.
The lung is increasingly recognized as an important target tissue for vitamin D. Observational data link poor vitamin D status with several inflammatory lung diseases or impaired lung function 80 , 81 , 82 . The most recent analysis published in 2019 (ref. 83 ) evaluated 10,933 participants in 25 RCTs and found a significant overall reduction in acute respiratory infections following vitamin D supplementation (OR 0.88, 95% CI 0.81–0.96) compared with no supplementation. The number needed to treat for benefit was 33. Subgroup analysis revealed that the greatest benefits were found in people with severe vitamin D deficiency (<25 nmol/l) at baseline (OR 0.58, 95% CI 0.40–0.82). Subgroup analysis revealed that intermittent (monthly or less frequent) doses of vitamin D did not generate protection, whereas daily or weekly vitamin D supplementation was more effective for preventing acute respiratory infections (OR 0.81, 95% CI 0.072–0.91). In the ViDA trial, however, no effects of vitamin D supplementation were found on acute respiratory infections in older adults 84 . This finding is not a total surprise as the lack of effects might be due to the intermittent dosing and/or adequate vitamin D status at baseline, and therefore might not contradict the findings of the 2019 meta-analysis 83 . In addition, the European DO-HEALTH trial did not show an effect on infections in general nor on upper respiratory infections 18 .
Several small-scale studies (eight RCTs) did not find an improvement in lung function (as measured in terms of forced expiratory volume in 1 s (FEV1)) in patients with chronic obstructive pulmonary disease (COPD) who were randomized to receive vitamin D supplementation 85 . A substudy of the ViDA trial, however, evaluated the effects of monthly vitamin D supplementation 86 in 442 adults treated for 1.1 years. Overall, in the ITT analysis, no significant effects were observed on FEV1. However, subgroup analysis revealed some beneficial effects, especially in subjects with existing lung problems such as asthma, COPD or a history of smoking (Table 2 ). To date, no Mendelian randomization studies have been performed that examined 25OHD levels, COPD and lung function.
Vitamin D and COVID-19
In view of the enormous health implications of the coronavirus disease 19 (COVID-19) pandemic caused by the worldwide spread of severe acute respiratory syndrome coronavirus 2, a possible link with poor vitamin D status and the risk or severity of COVID-19 has received great attention. Seven studies so far compared serum 25OHD concentrations in patients with COVID-19 compared with individuals without COVID-19 (ref. 87 ) and found a lower level (mean difference of about 12 nmol/l) in patients with COVID-19; however, in many studies the sampling did not take place at the same time in both groups. In addition, these studies were unable to control for confounding factors, a major problem due to the large number of similarities in the risk factors for vitamin D deficiency and COVID-19. About 31 studies looked at a possible link between vitamin D status and severity of the outcome of COVID-19. Lower serum concentrations of 25OHD were associated with greater mortality, greater need for intensive care treatment or increased severity of illness in general compared with better vitamin D status. However, this finding was based on observational studies. One placebo-controlled intervention study using a bolus dose of vitamin D (200,000 IU) did not reveal a beneficial effect in patients hospitalized with COVID-19 with a mean baseline 25OHD concentration of 50 nmol/l (ref. 88 ). However, one pilot study (which was not placebo-controlled) showed a marked reduction in the need for intensive care treatment in patients hospitalized for COVID-19 and treated with a high dose of 25OHD (calcifediol) at the time of admission 89 . Therefore, the link between vitamin D status and COVID-19 is unsettled so far, but many trials are ongoing that might clarify this question.
In 2021, a Mendelian randomization study assessed the causal role of serum 25OHD levels on COVID-19 susceptibility and disease severity 90 . Using data from 11,181 patients with COVID-19 and 116,456 control individuals from the Host Genetics Initiative, and six vitamin D SNPs that explain 2.5% of the variance in serum 25OHD levels, this study did not show any association between genetically decreased 25OHD and COVID-19 susceptibility or severity. These results were confirmed in a separate Mendelian randomization study using 81 25OHD SNPs that explain 4.3% of the variance in serum 25OHD levels, which also showed no effect of genetically determined 25OHD levels on risk of COVID-19-related hospitalization 91 .
Vitamin D and asthma
Research investigating the potential effects of vitamin D status on asthma has largely focused on a possible link between prenatal or maternal vitamin D status and wheezing or asthma in the offspring. A meta-analysis of four prospective studies and three RCTs concluded that vitamin D intake (~800 IU per day) by women during pregnancy is inversely related to wheezing or asthma in their offspring during up to 3 years of follow-up 92 . However, a longer follow-up did not confirm this conclusion: vitamin D supplementation during the prenatal period alone did not influence the 6-year incidence of asthma and recurrent wheeze among children who were at risk of asthma 93 . Two Mendelian randomization studies have investigated the causal association between vitamin D and asthma. A large study (n > 160,000 children and adults) 94 found odds ratios of 1.03 (95% CI 0.90–1.19) for asthma and 0.95 (95% CI 0.69–1.31) for childhood-onset asthma per standard deviation of log-transformed decrease in serum 25OHD (Supplementary Box 6 ). These findings suggest that vitamin D levels probably do not have clinically relevant effects on the risk of asthma.
Vitamin D and respiratory effects — summary
The vitamin D endocrine system influences all cells and most cytokines of the immune system 9 . The innate immune system is stimulated by 1,25(OH) 2 D and this is in line with a decreased risk of upper respiratory infections with vitamin D supplementation in individuals with vitamin D deficiency 83 . Meta-analysis of intervention studies suggested a benefit of vitamin D supplementation of participants with severe vitamin D deficiency and COPD, asthma, or similar lung diseases, and on reducing the risk of acute upper respiratory infections in severely deficient individuals 83 . However, where tested, these findings have not been supported by Mendelian randomization studies 90 . According to the results of the LUNG-ViDA trial, vitamin D supplementation might modestly improve expiratory lung function 85 . If confirmed, such data would imply that the lung is a clinically relevant target issue for vitamin D. Of note, currently there are insufficient RCTs to evaluate the potential benefit of vitamin D or calcifediol supplementation on the risk or severity of COVID-19.
Autoimmune diseases
Observational studies have, in line with preclinical data, made a link between poor vitamin D status and increased risk of infection or risk of autoimmune diseases (such as multiple sclerosis (MS), inflammatory bowel diseases or type 1 diabetes mellitus) 95 . RCTs in humans dealing with infections have mainly focused on upper respiratory infections and an overview is presented in the previous section. Unfortunately, no major RCTs have addressed the possible primary or secondary prevention of the major human autoimmune diseases. So far, the 2017–2020 megatrials (Table 1 ) have not shown results related to autoimmune diseases.
Currently, strong evidence exists that supports a causal association between genetically low serum 25OHD levels and increased risk of MS 96 , 97 , 98 , 99 . The most recent Mendelian randomization study from 2020 evaluated data from The International Multiple Sclerosis Genetics Consortium discovery phase GWAS (14,802 MS and 26,703 controls from the USA, Europe, Australia and some Asian countries) 97 using six SNPs associated with serum levels of 25OHD and found that each genetically determined unit increase in log-transformed 25(OH)D 3 was associated with an odds ratio for MS of 0.57 (95% CI 0.41–0.81; P = 0.001) (Table 4 ). This effect applies to adult-onset and childhood-onset MS.
Earlier Mendelian randomization evidence 100 did not support causality of predicted serum 25OHD levels in systemic lupus erythematosus or rheumatoid arthritis. Consistent null effects on rheumatoid arthritis were found in a 2020 Mendelian randomization study in participants from the UK Biobank, using ~220 vitamin D-associated SNPs as instruments 43 . Null effects of predicted serum 25OHD levels were also shown in Mendelian randomization studies on Crohn’s disease (odds ratio for 10 nmol/l higher 25OHD of 1.04, 95% CI 0.93–1.16) and ulcerative colitis (OR 1.13, 95% CI 1.06–1.21) 101 . Similarly, no effect on ulcerative colitis was found in participants from the UK Biobank 43 . The UK Biobank study also did not support a causal role of vitamin D on allergic rhinitis. Finally, Mendelian randomization 94 does not support causal effects of 25OHD on atopic dermatitis. A 2021 Mendelian randomization study on type 1 diabetes mellitus did not support causal effects of genetically lowered 25OHD levels on the risk of this disease 102 .
In summary, the adaptive immune system is downregulated by 1,25(OH) 2 D and therefore vitamin D deficiency might predispose to autoimmune diseases 9 . Observational studies have suggested this effect might apply to humans, but too few intervention studies have been conducted to evaluate this statement. Four independent Mendelian randomization studies agree, however, that individuals with genetically driven lower serum 25OHD concentrations have an increased risk of developing MS, either during adolescence or adulthood (Table 4 ).
Intervention studies as summarized in a Cochrane review from 2016 (ref. 103 ) dealing with 22 RCTs including 3,725 pregnant women, concluded that vitamin D supplementation significantly reduced the risk of pre-eclampsia (RR 0.48), gestational diabetes mellitus (RR 0.51) and low birthweight (<2,500 g; RR 0.55) compared with no supplementation. An update of these data 104 largely confirmed these observations. However, a large RCT in pregnant Bangladeshi women with severe vitamin D deficiency (baseline mean serum 25OHD about 25 nmol/l) supplemented from week 17–24 onwards with placebo or vitamin D (three groups receiving 4,200, 16,800 or 28,000 IU per week) until birth did not find a beneficial effect on fetal or neonatal parameters of length, weight or head circumference, either at birth or at one year of age ( n = 1,164 infants) 105 .
To date only one Mendelian randomization study 106 has examined the causal effect of predicted serum 25OHD levels on gestational hypertension and pre-eclampsia. Overall, the evidence was weak supporting a causal effect of vitamin D status on gestational hypertension (OR 0.90, 95% CI 0.78–1.03) or pre-eclampsia (OR 0.98, 95% CI: 0.89–1.07) per 10% decrease in serum 25OHD (Supplementary Box 5 ).
In summary, pregnant women more frequently have a poor vitamin D status than non-pregnant women of the same age but the absolute and relative values vary from country to country. Several meta-analyses have suggested that vitamin D supplementation might modestly decrease maternal morbidity and improve the health of their offspring 103 , 104 . However, a 2018 large RCT in Bangladeshi women with severe vitamin D deficiency did not confirm this observation 105 . Therefore, the effects of poor vitamin D status during pregnancy on pregnancy outcomes for mother and infant remains unsettled.
Patients in intensive care
Patients with severe acute illness requiring intensive care frequently have low serum concentrations of 25OHD and this poor vitamin D status is linked with increased morbidity and mortality 107 , 108 . Two major RCTs so far in patients in intensive care units (ICU) have generated conflicting results. In the VITdAL-ICU trial, patients in the ICU were randomized to either placebo ( n = 243) or high-dose oral vitamin D ( n = 249) (starting dose 540,000 IU followed by monthly maintenance doses of 90,000 IU for 5 months). Mean baseline serum 25OHD concentrations were low (33 nmol/l) and increased to ~82 nmol/l at day 3. Length of stay in the ICU or hospital, mortality in the ICU, in-hospital mortality and mortality at 6 months did not improve with the intervention. In a predefined subgroup with severe vitamin D deficiency who received the intervention (<30 nmol/l), hospital mortality (HR 0.56, 95% CI, 0.35–0.90) and 6-month mortality (HR 0.60, 95% CI, 0.39–0.93) were significantly decreased compared with patients with severe vitamin D deficiency who received placebo 107 . In the much larger Amrein ICU trial 108 , 1,059 patients in the ICU with vitamin D deficiency (<50 nmol/l) received either placebo or a single oral high dose of vitamin D (540,000 IU). This dose increased mean serum 25OHD concentration at day 3 to a mean concentration of 117 ± 58 nmol/l in comparison with the control group (mean concentration 28 ± 14 nmol/ml). The primary end point (90-day mortality) and other non-fatal outcomes were similar in the two groups. Although all patients in both studies were admitted to ICUs, the US patients in the VITdAL-ICU trial were probably less sick than those in the Amrein trial 107 as indicated by the percentage of patients requiring mechanical ventilation (32% in the US trial).
Effects of vitamin D supplementation on safety outcomes
In all vitamin D supplementation RCTs, some safety end points have been reported in addition to mortality (see next section). No effects were found on serum calcium or calciuria unless very high doses were used, such as 4,000–10,000 IU per day in the Calgary study. Even in these circumstances, hypercalcaemia was infrequent and occurred transiently after changes in treatment modality 19 , 62 . A modestly increased risk of kidney stones was observed in the WHI trial 109 , but this effect was not seen in the more recent 2017–2020 megatrials (that is, ViDA, VITAL and D2d; Table 1 ). Furthermore, no changes in kidney function were found in these large trials. Skeletal consequences were either null effects, slight (beneficial) increases in BMD in subgroups with poor vitamin D status at baseline, or a modest but significant decrease in BMD during high-dose (10,000 IU per day) therapy in the Calgary study 19 . An increased risk of fractures in patients receiving high intermittent bolus doses has been reported 71 , 110 . Similarly, an increased risk of falls has been reported when either high intermittent doses 71 or high continuous doses were used 72 , 73 . Importantly, the 2017–2020 megatrials (that is, ViDA, VITAL and D2d), with detailed evaluation of about 30,000 participants for 2–5 years, did not discover notable adverse effects. These findings indicate that a daily dose (or dose equivalent) of 2,000–4,000 IU can be considered as safe in an adult (even vitamin D-replete) population. High-dose vitamin D also did not modify arterial calcifications during a 3-year follow up in the Calgary study 111 .
Effects of vitamin D supplementation on mortality
Observational data have repeatedly linked poor vitamin D status with increased mortality. This effect was extensively documented in several NHANES studies based on representative samples of the US population and confirmed after validation of serum 25OHD concentrations according to standards generated by the US National Institute of Standards and Technology 112 . To decrease the possible effect of reverse causation, people who died within the first 3 years after 25OHD measurements were excluded from the analysis; however, the same association between poor vitamin D status and increased mortality remained 112 . Using a combination of several European prospective studies, mortality was also higher in the population with the poorest vitamin D status compared with the vitamin D-replete population 113 . A 2019 large long-term (>10 years) Finnish study concluded that people with the highest tertile of 25OHD concentrations (>50 nmol/l) had a mortality odds ratio of 0.77 (95% CI 0.71–0.84) compared with people with the lowest tertile of 25OHD concentrations, even in a multivariate model with correction of multiple co-variables 114 .
As nearly all long-term vitamin D supplementation trials include data on mortality, several meta-analyses have shown the effects of vitamin D supplementation on mortality. Extensive meta-analyses published in 2014 showed a modest decrease in overall mortality in participants randomized to vitamin D supplementation; based on 22 RCTs, the risk of death decreased by 11% 36 . A 2014 Cochrane analysis 29 evaluated 56 RCTs including 95,286 participants (mostly healthy women older than 70 years) with a mean follow-up of 4.4 years. Vitamin D supplementation significantly reduced all-cause mortality (RR 0.94, 95% CI 0.91–0.98; P = 0.002) compared with no supplementation. This finding implies that vitamin D supplementation of 150 women for 5 years prevented one additional death. Vitamin D supplementation also decreased cancer mortality (RR 0.88, 95% CI 0.78–0.98; P = 0.02) compared with no supplementation 29 .
In the 2017–2020 megatrials (that is, VITAL, ViDA and D2d), overall mortality was much lower than shown in the previous meta-analyses 29 , 36 and did not show an effect of vitamin D supplementation on overall mortality 15 . A new meta-analysis of 52 RCTs including a total of 75,454 participants concluded that vitamin D (either vitamin D 3 or D 2 ) supplementation did not change mortality (RR 0.98, 95% CI 0.95–1.02) compared with no supplementation 115 . A subanalysis, however, found that vitamin D 3 (instead of D 2 ) supplementation trials tended to reduce mortality (RR 0.95, 95% CI 1.90–1.00; P = 0.06), whereas this was not the case for vitamin D 2 supplementation trials. These new findings conflict with the 2014 reports 112 . The difference could be partly because the 2019 meta-analysis did not include ten RCTs including ~50,000 participants using a combination of vitamin D and calcium supplementation. However, the 2019 meta-analysis did include two megatrials (VITAL and ViDA) that evaluated the effects of vitamin D supplementation in a younger population of mostly vitamin D-replete participants 115 .
In a large-scale population Mendelian randomization study (10,349 deaths in 95,766 total participants) 116 , the odds ratios for a genetically determined lower 25OHD concentration was 1.30 (95% CI 1.05–1.61) for all-cause mortality, 0.77 (95% CI 0.55–1.08) for cardiovascular mortality, 1.43 (95% CI 1.02–1.99) for cancer mortality and 1.44 (95% CI 1.01–2.04) for other types of mortality. Similar point estimates and effect sizes, whose 95% confidence intervals included the null, were found for all-cause mortality in two follow-up Mendelian randomization studies 46 , 117 . Nevertheless, both studies may have been underpowered to detect existing causal associations. Finally, evidence from Mendelian randomization 118 did not support an association between 25OHD concentrations and cancer mortality in a sample of 6,998 deaths from cancer. These data provide some evidence that genetically lowered vitamin D levels might increase overall mortality risks, but the results have not been consistent across studies, or across causes of mortality.
If vitamin D supplementation exerts beneficial effects on extra-skeletal health outcomes and major diseases, then it is likely to have some effects on mortality, especially in older adults with poor vitamin D status. Large meta-analyses dealing mostly with women older than 70 years 29 , 36 showed a 6–11% reduction in mortality; however, adding the newest 2017–2020 megatrials eliminated this effect, possible because these new trials recruited a younger population.
Discordance between studies
Preclinical data are mostly in line with the very large number of observational studies linking very poor vitamin D status with skeletal and extra-skeletal health effects (Fig. 1 ). However, Mendelian randomization studies and the majority of RCTs do not confirm the causality of these associations. Several possible reasons exist for this discrepancy. Most importantly, serum 25OHD levels are a highly confounded variable. Specifically, serum 25OHD levels are affected by a host of health behaviours, the presence of obesity, socioeconomic status and education levels. Although most observational studies have attempted to control for such confounding through multivariable adjustment, such approaches depend upon the degree of accuracy of measurement of the confounders, knowledge that such confounding takes place, and most often that the nature of the confounding relationship (linear versus nonlinear) is known. Furthermore, statistical adjustment for confounding variables can only be accomplished if the confounding variables are known.
The concordance between 25OHD Mendelian randomization studies and RCTs is striking and suggests that Mendelian randomization might be a more relevant way to begin to understand the effect of 25OHD levels on risk of disease than observational studies. Perhaps the vitamin D endocrine system only has a role in these extra-skeletal effects in people with prolonged and very severe vitamin D deficiency. Studies in countries or population groups with severe vitamin D deficiency who need improved vitamin D status anyway might be the ideal approach to better understand the effect of vitamin D supplementation in individuals with severe vitamin D deficiency. Most RCTs and Mendelian randomization studies have been undertaken in individuals from the general population in which the rates of severe vitamin D deficiency are low.
Of note, the available Mendelian randomization studies were not able to predict large variations in serum 25OHD concentrations (usually only about 5% difference or less). However, this low degree of variance would affect the statistical power of a study but not introduce bias. New techniques will soon enable us to use a much larger number of SNPs than used in current studies (usually based on less than six SNPs), thereby allowing much larger variations in serum 25OHD concentrations to be predicted. Most RCTs did not last longer than 3–5 years. In such short-term scenarios, answering the question of causality is extremely difficult. This fact implies that only very long-term improvements in vitamin D status might generate beneficial effects. However, Mendelian randomization studies provide estimates of the effect of a lifetime of genetically lowered vitamin D levels and such Mendelian randomization studies have generally produced null findings.
Reverse causality remains a valid rationale to explain the discordance between observational and intervention studies. The most plausible hypothesis states that individuals with any health problems are less likely to regularly engage in outdoor activity and less exposure to sunlight results in lower vitamin D status. Another mechanism of reverse causality might be that the activity of hepatic 25-hydroxylase is decreased in many major diseases and this decrease could cause low serum 25OHD concentrations. Indeed, data in mice demonstrate that diet-induced obesity, type 1 diabetes mellitus or T2DM, fasting and exposure to glucocorticoids substantially decrease the gene and protein expression of CYP2R1, thereby decreasing the overall 25-hydroxylase activity 119 , 120 , 121 . This finding implies that decreased 25OHD concentrations are the consequence of disease, rather than involved in the origin of these metabolic diseases. Of course, these data from mice need confirmation in humans. Finally, many diseases other than those described in this Review (including brain-related diseases) are linked with poor vitamin D status; however, causality is doubtful without adequate Mendelian randomization studies or RCTs.
Future Mendelian randomization studies
Improved understanding of the genetic determinants of 25OHD has helped re-assess the role of vitamin D in the aetiology of complex diseases through Mendelian randomization. Taken together, the evidence from over 60 Mendelian randomization studies published to date assessing the role vitamin D does not support a causal role for the large majority of studied outcomes. Despite this null data, in the few cases where the evidence from Mendelian randomization supported a causal role of vitamin D status, such as in the example of MS, these results had important clinical implications. For instance, clinical care guidelines for the use of vitamin D in preventing MS in those at risk were published by the MS Society of Canada 122 .
The earlier Mendelian randomization studies used, as instruments for 25OHD levels, SNPs within the four genes related to 25OHD synthesis and metabolism ( DHCR7 , CYP2R1 , GC and CYP24A1 ), which together explained 2.4% of the variance in 25OHD levels 123 . Later Mendelian randomization studies combined the aforementioned four SNPs with two SNPs in SEC23A and AMDHD1 (both genes without clear role in the vitamin D metabolic pathway), and thereby explained ~5.3% of the variance in 25OHD levels. The identification of over 150 25OHD-associated genetic variants in 2020, which explain a considerable portion of the variance in 25OHD levels (~10.5%) 43 , has enabled a deeper understanding of the genetic determinants contributing to variation in circulating 25OHD levels. These newly identified SNPs will probably enable improved instrumentation of vitamin D in Mendelian randomization studies. Moreover, with the emergence of large-scale GWAS in densely phenotyped biobanks, we anticipate that more powerful vitamin D Mendelian randomization studies will be published that utilize the optimized set of genetic instruments. Such new studies should revisit previously studied diseases and investigate new disease outcomes, to further aid causal effect estimation.
Conclusions
In conclusion, the data generated by the 2017–2020 megatrials of vitamin D supplementation in largely vitamin D-replete adults (Table 1 ) demonstrate that increasing the serum 25OHD concentration into the high normal range (based on the IOM and most recent guidelines published over the past decade 12 , in the range of 50–125 nmol/l or 20–50 ng/ml) does not generate benefits for global health or major diseases or medical events such as cancer, cardiovascular events, T2DM, falls or fractures. Therefore, no reason exists at present to recommend vitamin D supplementation of already vitamin D-replete individuals. These data do not contradict the causal link between severe vitamin D deficiency and rickets, or the need to correct severe deficiency at any age. Similarly, the 2017–2020 trials do not contradict the probable beneficial effects of combined supplementation of calcium and vitamin D in older adults with poor vitamin D and calcium status on their risks of fracture or falls.
A few hints have emerged that vitamin D supplementation might have some extra-skeletal benefits, especially in people with severe vitamin D deficiency (such as reduced progression to T2DM, decreased numbers of infections, increased lung function and decreased cancer or overall mortality) (Tables 2 , 3 ). These suggestions are largely based on subgroup or post hoc analyses and thus should not result in the systematic recommendation of vitamin D supplements in such populations but might guide the correct design of future studies.
Arguments have been put forward that daily doses of ≥4,000 IU of vitamin D convey some risks other than simple hypercalcaemia or hypercalciuria. Such doses, or the equivalent of serum 25OHD concentrations well above 112 nmol/l or 45 ng/ml bring no benefits, but might be harmful in some people (for example, in causing loss of BMD or increasing the risk of falls). The same is true for intermittent high-dose boluses of vitamin D. Unfortunately, about 3% of the US population as screened by NHANES use such high dose vitamin D supplements.
Over the past few decades, vitamin D has been a hot topic for scientists and lay people alike, who frequently suggest that vitamin D supplementation might generate a wide variety of health benefits. The data discussed in the present Review might well dampen such enthusiasm. However, a large number of intervention studies (and most probably Mendelian randomization studies) are still ongoing, and these might help provide a better understanding of who would benefit from vitamin D supplementation.
In conclusion, it seems that far too many people with severe vitamin D deficiency (~7% of the world population) do not take or even have access to normal doses of vitamin D. About a third of the world population lives with suboptimal (below 20 ng/ml) serum 25OHD concentrations 78 . However, many vitamin D-replete people take vitamin D supplements without clear benefits. In addition, a small percentage of the population takes higher doses than the upper limit of safe intake. Therefore, we recommend that vitamin D be used wisely and “giveth to those who needeth” 7 .
Munns, C. F. et al. Global consensus recommendations on prevention and management of nutritional rickets. J. Clin. Endocrinol. Metab. 101 , 394–415 (2016).
Article CAS PubMed PubMed Central Google Scholar
Bolland, M. J., Grey, A. & Avenell, A. Effects of vitamin D supplementation on musculoskeletal health: a systematic review, meta-analysis, and trial sequential analysis. Lancet Diabetes Endocrinol. 6 , 847–858 (2018).
Article CAS PubMed Google Scholar
Luxwolda, M. F., Kuipers, R. S., Kema, I. P., Dijck-Brouwer, D. A. & Muskiet, F. A. Traditionally living populations in East Africa have a mean serum 25-hydroxyvitamin D concentration of 115 nmol/l. Br. J. Nutr. 108 , 1557–1561 (2012).
Luxwolda, M. F. et al. Vitamin D status indicators in indigenous populations in East Africa. Eur. J. Nutr. 52 , 1115–1125 (2013).
Holick, M. F. & Grant, W. B. Vitamin D status and ill health. Lancet Diabetes Endocrinol. 2 , 273–274 (2014).
Article PubMed Google Scholar
Bouillon, R., Lips, P. & Bilezikian, J. P. Vitamin D supplementation and musculoskeletal health. Lancet Diabetes Endocrinol. 7 , 85–86 (2019).
Lips, P., Bilezikian, J. P. & Bouillon, R. Vitamin D: giveth to those who needeth. JBMR 4 , e10232 (2020).
CAS Google Scholar
Ebeling, P. R. et al. Management of endocrine disease: therapeutics of vitamin D. Eur. J. Endocrinol. 179 , R239–R259 (2018).
Bouillon, R. et al. Skeletal and extraskeletal actions of vitamin D: current evidence and outstanding questions. Endocr. Rev. 40 , 1109–1151 (2019).
Institute of Medicine. Dietary Reference Intakes for Calcium and Vitamin D (National Academies Press, 2011)
Holick, M. F. et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 96 , 1911–1930 (2011).
Bouillon, R. Comparative analysis of nutritional guidelines for vitamin D. Nat. Rev. Endocrinol. 13 , 466–479 (2017).
Scientific Advisory Committee on Nutrition (SACN). Vitamin D and health. GOV.UK https://www.gov.uk/government/publications/sacn-vitamin-d-and-health-report (2016).
Lips, P. et al. Current vitamin D status in European and Middle East countries and strategies to prevent vitamin D deficiency; a position statement of the European Calcified Tissue Society. Eur. J. Endocrinol. 180 , 23–54 (2019).
Article Google Scholar
Manson, J. E. et al. Vitamin D supplements and prevention of cancer and cardiovascular disease. N. Engl. J. Med. 380 , 33–44 (2019).
Scragg, R. et al. Monthly high-dose vitamin D supplementation and cancer risk: a post hoc analysis of the vitamin D assessment randomized clinical trial. JAMA Oncol. 4 , e182178 (2018).
Article PubMed PubMed Central Google Scholar
Pittas, A. G. et al. Vitamin D supplementation and prevention of type 2 diabetes. N. Engl. J. Med. 381 , 520–530 (2019).
Bischoff-Ferrari, H. A. et al. Effect of vitamin D supplementation, omega-3 fatty acid supplementation, or a strength-training exercise program on clinical outcomes in older adults: the DO-HEALTH Randomized Clinical Trial. JAMA 324 , 1855–1868 (2020).
Burt, L. A. et al. Effect of high-dose vitamin D supplementation on volumetric bone density and bone strength: a randomized clinical trial. JAMA 322 , 736–745 (2019).
Billington, E. O. et al. Safety of high-dose vitamin D supplementation: secondary analysis of a randomized controlled trial. J. Clin. Endocrinol. Metab. 105 , 1261–1273 (2020).
Dawson-Hughes, B. et al. Intratrial exposure to vitamin D and new-onset diabetes among adults with prediabetes: a secondary analysis from the Vitamin D and Type 2 Diabetes (D2d) Study. Diabetes Care 43 , 2916–2922 (2020).
Pittas, A. G., Jorde, R., Kawahara, T. & Dawson-Hughes, B. Vitamin D supplementation for prevention of type 2 diabetes mellitus: to D or not to D? J. Clin. Endocrinol. Metab. 105 , 3721–3733 (2020).
Article PubMed Central Google Scholar
Zhang, Y. et al. Effects of Vitamin D supplementation on prevention of type 2 diabetes in patients with prediabetes: a systematic review and meta-analysis. Diabetes Care 43 , 1650–1658 (2020).
Barbarawi, M. et al. Effect of vitamin D supplementation on the incidence of diabetes mellitus. J. Clin. Endocrinol. Metab. 105 , 2857–2868 (2020).
Lu, L. et al. Association of vitamin D with risk of type 2 diabetes: a Mendelian randomisation study in European and Chinese adults. PLoS Med. 15 , e1002566 (2018).
Diabetes Prevention Program Research Group. Long-term effects of lifestyle intervention or metformin on diabetes development and microvascular complications over 15-year follow-up: the Diabetes Prevention Program Outcomes Study. Lancet Diabetes Endocrinol. 3 , 866–875 (2015).
Feldman, D., Krishnan, A. V., Swami, S., Giovannucci, E. & Feldman, B. J. The role of vitamin D in reducing cancer risk and progression. Nat. Rev. Cancer 14 , 342–357 (2014).
Manson, J. E., Bassuk, S. S., Buring, J. E. & Group, V. R. Principal results of the VITamin D and OmegA-3 TriaL (VITAL) and updated meta-analyses of relevant vitamin D trials. J. Steroid Biochem. Mol. Biol. 198 , 105522 (2020).
Bjelakovic, G. et al. Vitamin D supplementation for prevention of cancer in adults. Cochrane Database Syst. Rev. 6 , CD007469 (2014).
Google Scholar
Ong, J. S. et al. Association of vitamin D levels and risk of ovarian cancer: a Mendelian randomization study. Int. J. Epidemiol. 45 , 1619–1630 (2016).
Dimitrakopoulou, V. I. et al. Circulating vitamin D concentration and risk of seven cancers: Mendelian randomisation study. BMJ 359 , j4761 (2017).
Chandler, P. D. et al. Association between vitamin D genetic risk score and cancer risk in a large cohort of U.S. women. Nutrients 10 , 55 (2018).
He, Y. et al. Exploring causality in the association between circulating 25-hydroxyvitamin D and colorectal cancer risk: a large Mendelian randomisation study. BMC Med. 16 , 142 (2018).
Dong, J. et al. No association between vitamin D status and risk of Barrett’s esophagus or esophageal adenocarcinoma: a Mendelian randomization study. Clin. Gastroenterol. Hepatol. 17 , 2227–2235.e1 (2019).
Winslow, U. C., Nordestgaard, B. G. & Afzal, S. High plasma 25-hydroxyvitamin D and high risk of nonmelanoma skin cancer: a Mendelian randomization study of 97 849 individuals. Br. J. Dermatol. 178 , 1388–1395 (2018).
Chowdhury, R. et al. Vitamin D and risk of cause specific death: systematic review and meta-analysis of observational cohort and randomised intervention studies. BMJ 348 , g1903 (2014).
Scragg, R. et al. Effect of monthly high-dose vitamin D supplementation on cardiovascular disease in the Vitamin D Assessment Study: a randomized clinical trial. JAMA Cardiol. 2 , 608–616 (2017).
Barbarawi, M. et al. Vitamin D supplementation and cardiovascular disease risks in more than 83000 individuals in 21 randomized clinical trials: a meta-analysis. JAMA Cardiol. 4 , 765–776 (2019).
Afzal, S., Brondum-Jacobsen, P., Bojesen, S. E. & Nordestgaard, B. G. Genetically low vitamin D concentrations and increased mortality: Mendelian randomisation analysis in three large cohorts. BMJ 349 , g6330 (2014).
Manousaki, D., Mokry, L. E., Ross, S., Goltzman, D. & Richards, J. B. Mendelian randomization studies do not support a role for vitamin D in coronary artery disease. Circ. Cardiovasc. Genet. 9 , 349–356 (2016).
Larsson, S. C. et al. Serum 25-hydroxyvitamin D concentrations and ischemic stroke and its subtypes. Stroke 49 , 2508–2511 (2018).
Huang, T. et al. Vitamin D and cause-specific vascular disease and mortality: a Mendelian randomisation study involving 99,012 Chinese and 106,911 European adults. BMC Med. 17 , 160 (2019).
Revez, J. A. et al. Genome-wide association study identifies 143 loci associated with 25 hydroxyvitamin D concentration. Nat. Commun. 11 , 1647 (2020).
Sluyter, J. D. et al. Effect of monthly, high-dose, long-term vitamin D supplementation on central blood pressure parameters: a randomized controlled trial substudy. J. Am. Heart Assoc. 6 , e006802 (2017).
Vimaleswaran, K. S. et al. Association of vitamin D status with arterial blood pressure and hypertension risk: a Mendelian randomisation study. Lancet Diabetes Endocrinol. 2 , 719–729 (2014).
Meng, X. et al. Phenome-wide Mendelian-randomization study of genetically determined vitamin D on multiple health outcomes using the UK Biobank study. Int. J. Epidemiol. 48 , 1425–1434 (2019).
Kwak, S. Y., Cho, Y., Oh, H. & Shin, M. J. Association of circulating 25-hydroxyvitamin D levels with hypertension and blood pressure values in Korean adults: a Mendelian randomization study on a subset of the Korea National Health and Nutrition Survey 2011-2012 population. Nutr. Res. Pract. 13 , 498–508 (2019).
Chen, C. et al. Association of 25-hydroxyvitamin D with cardiometabolic risk factors and metabolic syndrome: a Mendelian randomization study. Nutr. J. 18 , 61 (2019).
Boonen, S. et al. Need for additional calcium to reduce the risk of hip fracture with vitamin D supplementation: evidence from a comparative metaanalysis of randomized controlled trials. J. Clin. Endocrinol. Metab. 92 , 1415–1423 (2007).
Bolland, M. J., Grey, A., Gamble, G. D. & Reid, I. R. The effect of vitamin D supplementation on skeletal, vascular, or cancer outcomes: a trial sequential meta-analysis. Lancet Diabetes Endocrinol. 2 , 307–320 (2014).
Avenell, A., Mak, J. C. & O’Connell, D. Vitamin D and vitamin D analogues for preventing fractures in post-menopausal women and older men. Cochrane Database Syst. Rev. 4 , CD000227 (2014).
Yao, P. et al. Vitamin D and calcium for the prevention of fracture: a systematic review and meta-analysis. JAMA Netw. Open 2 , e1917789 (2019).
Chakhtoura, M., Chamoun, N., Rahme, M. & Fuleihan, G. E. Impact of vitamin D supplementation on falls and fractures–a critical appraisal of the quality of the evidence and an overview of the available guidelines. Bone 131 , 115112 (2020).
Bikle, D., Bouillon, R., Thadhani, R. & Schoenmakers, I. Vitamin D metabolites in captivity? Should we measure free or total 25(OH)D to assess vitamin D status? J. Steroid Biochem. Mol. Biol. 173 , 105–116 (2017).
LeBoff, M. S. et al. Effects of supplemental vitamin D on bone health outcomes in women and men in the VITamin D and OmegA-3 TriaL (VITAL). J. Bone Miner. Res. 35 , 883–893 (2020).
Khaw, K. T. et al. Effect of monthly high-dose vitamin D supplementation on falls and non-vertebral fractures: secondary and post-hoc outcomes from the randomised, double-blind, placebo-controlled ViDA trial. Lancet Diabetes Endocrinol. 5 , 438–447 (2017).
Reid, I. R. et al. Effect of monthly high-dose vitamin D on bone density in community-dwelling older adults substudy of a randomized controlled trial. J. Intern. Med. 282 , 452–460 (2017).
Macdonald, H. M. et al. 25-hydroxyvitamin D threshold for the effects of vitamin D supplements on bone density: secondary analysis of a randomized controlled trial. J. Bone Miner. Res. 33 , 1464–1469 (2018).
Hansen, K. E. et al. Treatment of vitamin D insufficiency in postmenopausal women: a randomized clinical trial. JAMA Intern. Med. 175 , 1612–1621 (2015).
Aloia, J. et al. Vitamin D supplementation in elderly black women does not prevent bone loss: a randomized controlled trial. J. Bone Miner. Res. 33 , 1916–1922 (2018).
Rosendahl, J. et al. Effect of higher vs standard dosage of vitamin D3 supplementation on bone strength and infection in healthy infants: a randomized clinical trial. JAMA Pediatrics 172 , 646–654 (2018).
Bouillon, R. Safety of high-dose vitamin D supplementation. J. Clin. Endocrinol. Metab. 105 , 1290–1291 (2020).
Larsson, S. C., Melhus, H. & Michaelsson, K. Circulating serum 25-hydroxyvitamin D levels and bone mineral density: Mendelian randomization study. J. Bone Miner. Res. 33 , 840–844 (2018).
Sun, J. Y. et al. Circulating serum vitamin D levels and total body bone mineral density: a Mendelian randomization study. J. Cell Mol. Med. 23 , 2268–2271 (2019).
Trajanoska, K. et al. Assessment of the genetic and clinical determinants of fracture risk: genome wide association and Mendelian randomisation study. BMJ 362 , k3225 (2018).
Kampe, A. et al. Genetic variation in GC and CYP2R1 affects 25-hydroxyvitamin D concentration and skeletal parameters: a genome-wide association study in 24-month-old Finnish children. PLoS Genet. 15 , e1008530 (2019).
Li, S. S. et al. Genetically low vitamin D Levels, bone mineral density, and bone metabolism markers: a Mendelian randomisation study. Sci. Rep. 6 , 33202 (2016).
Beaudart, C. et al. The effects of vitamin D on skeletal muscle strength, muscle mass, and muscle power: a systematic review and meta-analysis of randomized controlled trials. J. Clin. Endocrinol. Metab. 99 , 4336–4345 (2014).
Tabrizi, R. et al. The effects of vitamin D supplementation on muscle function among postmenopausal women: a systematic review and meta-analysis of randomized controlled trials. EXCLI J. 18 , 591–603 (2019).
PubMed PubMed Central Google Scholar
Girgis, C. M., Clifton-Bligh, R. J., Hamrick, M. W., Holick, M. F. & Gunton, J. E. The roles of vitamin D in skeletal muscle: form, function, and metabolism. Endocr. Rev. 34 , 33–83 (2013).
Sanders, K. M. et al. Annual high-dose oral vitamin D and falls and fractures in older women: a randomized controlled trial. JAMA 303 , 1815–1822 (2010).
Bischoff-Ferrari, H. A. et al. Monthly high-dose vitamin D treatment for the prevention of functional decline: a randomized clinical trial. JAMA Intern. Med. 176 , 175–183 (2016).
Gallagher, J. C. Vitamin D and falls–the dosage conundrum. Nat. Rev. Endocrinol. 12 , 680–684 (2016).
Smith, L. M., Gallagher, J. C. & Suiter, C. Medium doses of daily vitamin D decrease falls and higher doses of daily vitamin D3 increase falls: a randomized clinical trial. J. Steroid Biochem. Mol. Biol. 173 , 317–322 (2017).
Michos, E. D. et al. Rationale and design of the Study To Understand Fall Reduction and Vitamin D in You (STURDY): a randomized clinical trial of vitamin D supplement doses for the prevention of falls in older adults. Contemp. Clin. trials 73 , 111–122 (2018).
LeBoff, M. S. et al. VITamin D and OmegA-3 TriaL (VITAL): effects of vitamin D supplements on risk of falls in the US population. J. Clin. Endocrinol. Metab. 105 , 2929–2938 (2020).
Cashman, K. D., Ritz, C., Kiely, M. & Odin, C. Improved dietary guidelines for vitamin D: application of individual participant data (IPD)-level meta-regression analyses. Nutrients 9 , 469 (2017).
Bouillon, R. Vitamin D status in Africa is worse than in other continents. Lancet Glob. Health 8 , e20–e21 (2020).
Bischoff-Ferrari, H. A. et al. Effect of vitamin D on falls: a meta-analysis. JAMA 291 , 1999–2006 (2004).
Lange, N. E., Sparrow, D., Vokonas, P. & Litonjua, A. A. Vitamin D deficiency, smoking, and lung function in the Normative Aging Study. Am. J. Respir. Crit. Care Med. 186 , 616–621 (2012).
Janssens, W. et al. Vitamin D deficiency is highly prevalent in COPD and correlates with variants in the vitamin D-binding gene. Thorax 65 , 215–220 (2010).
Maes, K., Serre, J., Mathyssen, C., Janssens, W. & Gayan-Ramirez, G. Targeting vitamin D deficiency to limit exacerbations in respiratory diseases: utopia or strategy with potential? Calcif. Tissue Int. 106 , 76–87 (2020).
Martineau, A. R. et al. Vitamin D supplementation to prevent acute respiratory infections: individual participant data meta-analysis. Health Technol. Assess. 23 , 1–44 (2019).
Camargo, C. A. et al. Effect of monthly high-dose vitamin D supplementation on acute respiratory infections in older adults: a randomized controlled trial. Clin. Infect. Dis. 71 , 311–317 (2019).
Chen, F. Y., Xiao, M., Ling, B., Liu, L. & Chen, L. Vitamin D does not improve lung function decline in COPD: a meta-analysis. Eur. Rev. Med. Pharmacol. Sci. 23 , 8637–8644 (2019).
PubMed Google Scholar
Sluyter, J. D. et al. Effect of monthly, high-dose, long-term vitamin D on lung function: a randomized controlled trial. Nutrients 9 , 1353 (2017).
Bassatne, A. et al. The link between COVID-19 and vitamin D (VIVID): a systematic review and meta-analysis. Metabolism 119 , 15475 (2021).
Murai, I. H. et al. Effect of a single high dose of vitamin D3 on hospital length of stay in patients with moderate to severe COVID-19: a randomized clinical trial. JAMA 325 , 1053–1060 (2021).
Entrenas Castillo, M. et al. Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: a pilot randomized clinical study. J. Steroid Biochem. Mol. Biol. 203 , 105751 (2020).
Amin, H. & Drenos, F. No evidence that vitamin D is able to prevent or affect the severity of COVID-19 in individuals with European ancestry: a Mendelian randomisation study of open data. BMJ Nutr. Prev. Health 4 , 42–48 (2021).
Butler-Laporte, G. et al. Vitamin D and COVID-19 susceptibility and severity in the COVID-19 Host Genetics Initiative: a Mendelian randomization study. PLoS Med. 18 , e1003605 (2021).
Li, W. et al. Vitamin D supplementation during pregnancy and the risk of wheezing in offspring: a systematic review and dose-response meta-analysis. J. Asthma 56 , 1266–1273 (2019).
Litonjua, A. A. et al. Six-year follow-up of a trial of antenatal vitamin D for asthma reduction. N. Engl. J. Med. 382 , 525–533 (2020).
Manousaki, D. et al. Vitamin D levels and susceptibility to asthma, elevated immunoglobulin E levels, and atopic dermatitis: a Mendelian randomization study. PLoS Med. 14 , e1002294 (2017).
Murdaca, G. et al. Emerging role of vitamin D in autoimmune diseases: an update on evidence and therapeutic implications. Autoimmun. Rev. 18 , 102350 (2019).
Mokry, L. E. et al. Vitamin D and risk of multiple sclerosis: a mendelian randomization study. PLoS Med. 12 , e1001866 (2015).
Jacobs, B. M., Noyce, A. J., Giovannoni, G. & Dobson, R. BMI and low vitamin D are causal factors for multiple sclerosis: a Mendelian randomization study. Neurol. Neuroimmunol. Neuroinflamm 7 , e662 (2020).
Gianfrancesco, M. A. et al. Evidence for a causal relationship between low vitamin D, high BMI, and pediatric-onset MS. Neurology 88 , 1623–1629 (2017).
Rhead, B. et al. Mendelian randomization shows a causal effect of low vitamin D on multiple sclerosis risk. Neurol. Genet. 2 , e97 (2016).
Bae, S. C. & Lee, Y. H. Vitamin D level and risk of systemic lupus erythematosus and rheumatoid arthritis: a Mendelian randomization. Clin. Rheumatol. 37 , 2415–2421 (2018).
Lund-Nielsen, J. et al. Vitamin D and inflammatory bowel disease: Mendelian randomization analyses in the Copenhagen studies and UK biobank. J. Clin. Endocrinol. Metab. 103 , 3267–3277 (2018).
Manousaki, D. et al. Vitamin D levels and risk of type 1 diabetes: a Mendelian randomization study. PLoS Med. 18 , e1003536 (2021).
Palacios, C., De-Regil, L. M., Lombardo, L. K. & Pena-Rosas, J. P. Vitamin D supplementation during pregnancy: updated meta-analysis on maternal outcomes. J. Steroid Biochem. Mol. Biol. 164 , 148–155 (2016).
Palacios, C., Kostiuk, L. K. & Pena-Rosas, J. P. Vitamin D supplementation for women during pregnancy. Cochrane Database Syst. Rev. 7 , CD008873 (2019).
Roth, D. E. et al. Vitamin D supplementation in pregnancy and lactation and infant growth. N. Engl. J. Med. 379 , 535–546 (2018).
Magnus, M. C. et al. Vitamin D and risk of pregnancy related hypertensive disorders: Mendelian randomisation study. BMJ 361 , k2167 (2018).
Amrein, K. et al. Effect of high-dose vitamin D3 on hospital length of stay in critically ill patients with vitamin D deficiency: the VITdAL-ICU randomized clinical trial. JAMA 312 , 1520–1530 (2014).
National Heart, L. et al. Early high-dose vitamin D3 for critically ill, vitamin D-deficient patients. N. Engl. J. Med. 381 , 2529–2540 (2019).
Wactawski-Wende, J. et al. Calcium plus vitamin D supplementation and the risk of colorectal cancer. N. Engl. J. Med. 354 , 684–696 (2006).
Smith, H. et al. Effect of annual intramuscular vitamin D on fracture risk in elderly men and women–a population-based, randomized, double-blind, placebo-controlled trial. Rheumatology 46 , 1852–1857 (2007).
Billington, E. O. et al. Effect of high-dose vitamin D supplementation on peripheral arterial calcification: secondary analysis of a randomized controlled trial. Osteoporos. Int. 31 , 2141–2150 (2020).
Durazo-Arvizu, R. A. et al. The reverse J-shaped association between serum total 25-Hydroxyvitamin D concentration and all-cause mortality: the impact of assay standardization. Am. J. Epidemiol. 185 , 720–726 (2017).
Gaksch, M. et al. Vitamin D and mortality: individual participant data meta-analysis of standardized 25-hydroxyvitamin D in 26916 individuals from a European consortium. PLoS ONE 12 , e0170791 (2017).
Mattila, T. et al. Airway obstruction, serum vitamin D and mortality in a 33-year follow-up study. Eur. J. Clin. Nutr. 73 , 1024–1032 (2019).
Zhang, Y. et al. Association between vitamin D supplementation and mortality: systematic review and meta-analysis. BMJ 366 , l4673 (2019).
Ordonez-Mena, J. M. et al. Genetic variants in the vitamin D pathway, 25(OH)D levels, and mortality in a large population-based cohort study. J. Clin. Endocrinol. Metab. 102 , 470–477 (2016).
Aspelund, T. et al. Effect of genetically low 25-hydroxyvitamin D on mortality risk: Mendelian randomization analysis in 3 large European cohorts. Nutrients 11 , 74 (2019).
Article CAS PubMed Central Google Scholar
Ong, J. S. et al. Vitamin D and overall cancer risk and cancer mortality: a mendelian randomization study. Hum. Mol. Genet. 27 , 4315–4322 (2018).
CAS PubMed Google Scholar
Bouillon, R. & Bikle, D. Vitamin D metabolism revised: fall of dogmas. J. Bone Miner. Res. 34 , 1985–1992 (2019).
Aatsinki, S. M. et al. Fasting-induced transcription factors repress vitamin D bioactivation, a mechanism for vitamin D deficiency in diabetes. Diabetes 68 , 918–931 (2019).
Roizen, J. D. et al. Obesity decreases hepatic 25-hydroxylase activity causing low serum 25-hydroxyvitamin D. J. Bone Miner. Res. 34 , 1068–1073 (2019).
Atkinson, S. A. Recommendations on vitamin D needs in multiple sclerosis from the MS Society of Canada. Public Health Nutr. 23 , 1278–1279 (2020).
Wang, T. J. et al. Common genetic determinants of vitamin D insufficiency: a genome-wide association study. Lancet 376 , 180–188 (2010).
Wu, H. X. et al. Effects of vitamin D supplementation on the outcomes of patients with pulmonary tuberculosis: a systematic review and meta-analysis. BMC Pulm. Med. 18 , 108 (2018).
Download references
Author information
Authors and affiliations.
Clinical and Experimental Endocrinology, Department of Chronic Diseases and Metabolism, KU Leuven, Leuven, Belgium
Roger Bouillon
Research Center of the Sainte-Justine University Hospital, University of Montreal, Montreal, Quebec, Canada
Despoina Manousaki
Maine Medical Center Research Institute, Scarborough, ME, USA
Cliff Rosen
Department of Internal Medicine, Erasmus MC University Medical Center, Rotterdam, Netherlands
Katerina Trajanoska
Translational Skeletal Genomics, Department of Internal Medicine, Erasmus MC University Medical Center, Rotterdam, Netherlands
Fernando Rivadeneira
Centre for Clinical Epidemiology, Lady Davis Institute for Medical Research, Jewish General Hospital, Montreal, Quebec, Canada
J. Brent Richards
Departments of Medicine, Human Genetics, Epidemiology and Biostatistics, McGill University, Montreal, Quebec, Canada
You can also search for this author in PubMed Google Scholar
Contributions
R.B., D.M. and K.T. researched data for the article. All authors contributed substantially to discussion of the content. R.B. and D.M. wrote the article. All authors reviewed and/or edited the manuscript before submission.
Corresponding author
Correspondence to Roger Bouillon .
Ethics declarations
Competing interests.
R.B. received modest speaking or consultancy fees from Fresenius, Abiogen, FAES Farma, Ceres and Proctor and Gamble. J.B.R. has worked as a consultant to GlaxoSmithKline and Deerfield Capital. The other authors declare no competing interests
Additional information
Peer review information.
Nature Reviews Endocrinology thanks D. Bikle, C. Carlberg, G. Jones and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions.
Reprints and permissions
About this article
Cite this article.
Bouillon, R., Manousaki, D., Rosen, C. et al. The health effects of vitamin D supplementation: evidence from human studies. Nat Rev Endocrinol 18 , 96–110 (2022). https://doi.org/10.1038/s41574-021-00593-z
Download citation
Accepted : 25 October 2021
Published : 23 November 2021
Issue Date : February 2022
DOI : https://doi.org/10.1038/s41574-021-00593-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Clinical data mining: challenges, opportunities, and recommendations for translational applications.
- Huimin Qiao
- Yijing Chen
Journal of Translational Medicine (2024)
Tremendous Fidelity of Vitamin D3 in Age-related Neurological Disorders
- Manjari SKV
- Sharon Mariam Abraham
- Pragya Komal
Molecular Neurobiology (2024)
Vitamin D and human health: evidence from Mendelian randomization studies
- Aiping Fang
- Edward L. Giovannucci
European Journal of Epidemiology (2024)
Geographic variation in bone mineral density and prevalent fractures in the Canadian longitudinal study on aging
- N. Hassanabadi
- S. N. Morin
Osteoporosis International (2024)
Metabolic bone disorders and the promise of marine osteoactive compounds
- Alessio Carletti
- Paulo Jorge Gavaia
- Vincent Laizé
Cellular and Molecular Life Sciences (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
- Introduction
- Conclusions
- Article Information
KQ indicates key question.
Size of each data marker indicates the weight of the study in the analysis. Weights are from random-effects analysis. To calculate the absolute risk difference in percentage points, multiply value by 100 (eg, 0.009 multiplied by 100 = 0.9 percentage points).
Size of each data marker indicates the weight of the study in the analysis. Weights are from random-effects analysis. To calculate the RD in percentage points, multiply value by 100 (eg, 0.009 multiplied by 100 = 0.9 percentage points).
eTables 1-19
eFigures 1-8
List of Excluded Studies
eReferences
- USPSTF Recommendation: Screening for Vitamin D Deficiency in Adults JAMA US Preventive Services Task Force April 13, 2021 This 2021 US Preventive Services Task Force Recommendation Statement concludes that current evidence is insufficient to assess the balance of benefits and harms of screening for vitamin D deficiency in asymptomatic adults (I statement). US Preventive Services Task Force; Alex H. Krist, MD, MPH; Karina W. Davidson, PhD, MASc; Carol M. Mangione, MD, MSPH; Michael Cabana, MD, MA, MPH; Aaron B. Caughey, MD, PhD; Esa M. Davis, MD, MPH; Katrina E. Donahue, MD, MPH; Chyke A. Doubeni, MD, MPH; John W. Epling Jr, MD, MSEd; Martha Kubik, PhD, RN; Li Li, MD, PhD, MPH; Gbenga Ogedegbe, MD, MPH; Douglas K. Owens, MD, MS; Lori Pbert, PhD; Michael Silverstein, MD, MPH; James Stevermer, MD, MSPH; Chien-Wen Tseng, MD, MPH, MSEE; John B. Wong, MD
- USPSTF 2021 Recommendations on Screening for Asymptomatic Vitamin D Deficiency in Adults JAMA Editorial April 13, 2021 Sherri-Ann M. Burnett-Bowie, MD, MPH; Anne R. Cappola, MD, ScM
- Patient Information: Screening for Vitamin D Deficiency in Adults JAMA JAMA Patient Page April 13, 2021 This JAMA Patient Page summarizes the US Preventive Services Task Force’s 2021 recommendation that current evidence is insufficient to assess the balance of benefits and harms of screening for vitamin D deficiency in asymptomatic adults (I statement). Jill Jin, MD, MPH
- USPSTF Still Finds Insufficient Evidence to Support Screening for Vitamin D Deficiency JAMA Network Open Editorial April 13, 2021 Erin D. Michos, MD, MHS; Rita R. Kalyani, MD, MHS; Jodi B. Segal, MD, MPH
See More About
Select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
Others Also Liked
- Download PDF
- X Facebook More LinkedIn
Kahwati LC , LeBlanc E , Weber RP, et al. Screening for Vitamin D Deficiency in Adults : Updated Evidence Report and Systematic Review for the US Preventive Services Task Force . JAMA. 2021;325(14):1443–1463. doi:10.1001/jama.2020.26498
Manage citations:
© 2024
- Permissions
Screening for Vitamin D Deficiency in Adults : Updated Evidence Report and Systematic Review for the US Preventive Services Task Force
- 1 RTI International–University of North Carolina at Chapel Hill Evidence-based Practice Center, Chapel Hill, North Carolina
- 2 RTI International, Research Triangle Park, North Carolina
- 3 Kaiser Permanente Center for Health Research, Portland, Oregon
- 4 Cecil G. Sheps Center for Health Services Research, University of North Carolina at Chapel Hill
- 5 Gillings School of Global Public Health and Eshelman School of Pharmacy, University of North Carolina at Chapel Hill
- Editorial USPSTF 2021 Recommendations on Screening for Asymptomatic Vitamin D Deficiency in Adults Sherri-Ann M. Burnett-Bowie, MD, MPH; Anne R. Cappola, MD, ScM JAMA
- Editorial USPSTF Still Finds Insufficient Evidence to Support Screening for Vitamin D Deficiency Erin D. Michos, MD, MHS; Rita R. Kalyani, MD, MHS; Jodi B. Segal, MD, MPH JAMA Network Open
- US Preventive Services Task Force USPSTF Recommendation: Screening for Vitamin D Deficiency in Adults US Preventive Services Task Force; Alex H. Krist, MD, MPH; Karina W. Davidson, PhD, MASc; Carol M. Mangione, MD, MSPH; Michael Cabana, MD, MA, MPH; Aaron B. Caughey, MD, PhD; Esa M. Davis, MD, MPH; Katrina E. Donahue, MD, MPH; Chyke A. Doubeni, MD, MPH; John W. Epling Jr, MD, MSEd; Martha Kubik, PhD, RN; Li Li, MD, PhD, MPH; Gbenga Ogedegbe, MD, MPH; Douglas K. Owens, MD, MS; Lori Pbert, PhD; Michael Silverstein, MD, MPH; James Stevermer, MD, MSPH; Chien-Wen Tseng, MD, MPH, MSEE; John B. Wong, MD JAMA
- JAMA Patient Page Patient Information: Screening for Vitamin D Deficiency in Adults Jill Jin, MD, MPH JAMA
Importance Low serum vitamin D levels have been associated with adverse clinical outcomes; identifying and treating deficiency may improve outcomes.
Objective To review the evidence about screening for vitamin D deficiency in adults.
Data Sources PubMed, EMBASE, the Cochrane Library, and trial registries through March 12, 2020; bibliographies from retrieved articles, outside experts, and surveillance of the literature through November 30, 2020.
Study Selection Fair- or good-quality, English-language randomized clinical trials (RCTs) of screening with serum 25-hydroxyvitamin D (25[OH]D) compared with no screening, or treatment with vitamin D (with or without calcium) compared with placebo or no treatment conducted in nonpregnant adults; nonrandomized controlled intervention studies for harms only. Treatment was limited to studies enrolling or analyzing participants with low serum vitamin D levels.
Data Extraction and Synthesis Two reviewers assessed titles/abstracts and full-text articles, extracted data, and assessed study quality; when at least 3 similar studies were available, meta-analyses were conducted.
Main Outcomes and Measures Mortality, incident fractures, falls, diabetes, cardiovascular events, cancer, depression, physical functioning, and infection.
Results Forty-six studies (N = 16 205) (77 publications) were included. No studies directly evaluated the health benefits or harms of screening. Among community-dwelling populations, treatment was not significantly associated with mortality (pooled absolute risk difference [ARD], 0.3% [95% CI, −0.6% to 1.1%]; 8 RCTs, n = 2006), any fractures (pooled ARD, −0.3% [95% CI, −2.1% to 1.6%]; 6 RCTs, n = 2186), incidence of diabetes (pooled ARD, 0.1% [95% CI, −1.3% to 1.6%]; 5 RCTs, n = 3356), incidence of cardiovascular disease (2 RCTs; hazard ratio, 1.00 [95% CI, 0.74 to 1.35] and 1.09 [95% CI, 0.68 to 1.76]), incidence of cancer (2 RCTs; hazard ratio, 0.97 [95% CI, 0.68 to 1.39] and 1.01 [95% CI, 0.65 to 1.58], or depression (3 RCTs, various measures reported). The pooled ARD for incidence of participants with 1 or more falls was −4.3% (95% CI, −11.6% to 2.9%; 6 RCTs). The evidence was mixed for the effect of treatment on physical functioning (2 RCTs) and limited for the effect on infection (1 RCT). The incidence of adverse events and kidney stones was similar between treatment and control groups.
Conclusions and Relevance No studies evaluated the direct benefits or harms of screening for vitamin D deficiency. Among asymptomatic, community-dwelling populations with low vitamin D levels, the evidence suggests that treatment with vitamin D has no effect on mortality or the incidence of fractures, falls, depression, diabetes, cardiovascular disease, cancer, or adverse events. The evidence is inconclusive about the effect of treatment on physical functioning and infection.
Vitamin D has a variety of actions on calcium homeostasis, bone metabolism, and other cellular regulatory functions. 1 - 3 Vitamin D deficiency refers to serum levels of vitamin D (serum total hydroxyvitamin D, or 25[OH]D) that are inadequate to support bodily needs. Serum total 25(OH)D is currently considered the best marker of vitamin D status. 4 , 5 However, there is no consensus regarding the serum level of 25(OH)D that represents optimal health or deficiency. 1 , 5 , 6
The rationale for screening for vitamin D deficiency among asymptomatic adults is to identify low serum vitamin D levels that place persons at risk for deficiency and offer treatment before potential adverse clinical outcomes (falls, fractures, and other outcomes) occur. In 2014, the US Preventive Services Task Force (USPSTF) concluded that the evidence was insufficient to assess the balance of benefits and harms of screening for vitamin D deficiency in adults (I statement). This review was conducted for the USPSTF to inform an update of its 2014 recommendation. 7 - 9
The analytic framework and key questions (KQs) that guided the review are shown in Figure 1 . Detailed methods, evidence tables, supplemental analyses, and contextual information are available in the full evidence report. 10
PubMed, the Cochrane Library, and EMBASE were searched for English-language articles published from January 1, 2013, through March 12, 2020. ClinicalTrials.gov, Cochrane Register of Controlled Trials, and the World Health Organization International Clinical Trials Registry Platform were also searched. To supplement systematic electronic searches (eMethods in the Supplement ), reference lists of pertinent articles and studies suggested by reviewers were searched. Ongoing surveillance was conducted through article alerts and targeted searches of journals to identify major studies published in the interim that may affect the conclusions or understanding of the evidence and the related USPSTF recommendation. The last surveillance was conducted on November 30, 2020.
Two investigators independently reviewed titles, abstracts, and full-text articles using prespecified inclusion criteria for each KQ (eMethods in the Supplement ); disagreements about inclusion were resolved by discussion or by a third reviewer. For all KQs, randomized clinical trials (RCTs) conducted in nonpregnant adults were eligible for selection. For KQ1 and KQ2, studies that were conducted among participants not known to have vitamin D deficiency were eligible for selection. For KQ3 and KQ4, studies that either enrolled participants with known deficiency (defined as serum vitamin D level less than 30 ng/mL [to convert to nmol/L, multiply by 2.496]) or reported findings for a subgroup of participants with known deficiency were eligible, as were nested case-control studies within RCTs. For KQ1 and KQ2, studies that evaluated screening using total serum 25(OH)D were eligible, and for KQ3 and KQ4, studies that evaluated treatment with oral or injectable vitamin D 2 or vitamin D 3 of any dosage with or without concomitant calcium were eligible. For KQ1 and KQ3, studies reporting health outcomes, such as mortality, falls, fractures, incident disease (eg, diabetes, cancer, cardiovascular event, and others), and validated quality of life, and self-reported physical functioning measures were eligible; studies reporting only changes in serum vitamin D levels, intermediate physiologic outcomes (eg, bone mineral density, blood pressure), or physical fitness/muscle strength measures were not eligible. For KQ2 and KQ4, studies reporting harms from screening (eg, anxiety, labeling) or harms from treatment (eg, toxicity, nephrolithiasis, adverse events) were eligible; nonrandomized controlled intervention studies, cohort studies, and case-control studies were also eligible for selection.
English-language studies that met all study selection criteria, were fair or good methodological quality, and were conducted in countries categorized as very highly developed by the 2016 United Nations Human Development Index were included. 11 Studies included in the prior 2014 review for the USPSTF were reassessed against the study selection and methodological quality criteria for this update.
For each included study, 1 reviewer abstracted relevant study characteristics (ie, population, intervention, comparator) and data for eligible outcomes into a structured form. A second reviewer checked all data for completeness and accuracy. Two senior reviewers independently assessed each study’s methodological quality using predefined criteria established by the USPSTF (eMethods in the Supplement ) and others. 12 Disagreements in study quality ratings were resolved through discussion or with a third senior reviewer.
Data were synthesized in tabular and narrative formats. When at least 3 similar studies were available, a quantitative synthesis was performed using random-effects models with the inverse-variance weighted method of DerSimonian and Laird in Stata version 16 (StataCorp) to generate pooled estimates of the absolute risk difference (ARD), the relative risk ratio (RR), the incidence rate difference, or the incidence rate ratio. 13 Analyses were stratified based on study population (community dwelling vs institutionalized) when possible. For rare event outcomes, such as mortality, sensitivity analyses were also conducted using other estimators and models with and without continuity corrections to assess robustness of the main findings. Significance testing was based on the exclusion of the null value by the 95% confidence interval around the pooled estimate.
The strength of evidence was assessed based on the Agency for Healthcare Quality and Research Methods Guide for Effectiveness and Comparative Effectiveness Reviews , which specifies the assessment of study limitations, directness, consistency, precision, and reporting bias for each intervention comparison and major outcome of interest. 14 Two senior reviewers independently developed initial strength-of-evidence assessments for each relevant outcome and comparison across the KQs; disagreements were resolved through discussion or input of a third senior reviewer.
Forty-six studies (N = 16 205) from 77 publications were included ( Figure 2 ). Twenty-seven studies of treatment benefits (KQ3) 15 - 59 and 36 studies evaluating the harms of treatment (KQ4) 15 - 19 , 21 - 29 , 35 , 36 , 39 - 43 , 58 - 88 were identified. Study characteristics of included RCTs are described in Table 1 . A list of full-text articles screened but excluded is provided in the Supplement .
Key Question 1a. Does screening for vitamin D deficiency improve health outcomes?
Key Question 1b. Does screening efficacy vary among patient subpopulations at higher risk for vitamin D deficiency (eg, persons residing in institutions, persons with obesity, persons with low levels of sun exposure, or older adults) or vary by race/ethnicity?
No studies were identified.
Key Question 2. What are the harms of screening for vitamin D deficiency?
Key Question 3a. Does treatment of vitamin D deficiency with vitamin D improve health outcomes?
Key Question 3b. Does treatment efficacy vary among patient subpopulations at higher risk for vitamin D deficiency (eg, persons residing in institutions, persons with obesity, persons with low levels of sun exposure, or older adults) or vary by race/ethnicity?
Twenty-six RCTs 15 - 29 , 35 - 59 and 1 nested case-control study from the Women’s Health Initiative (WHI) Calcium and Vitamin D RCT 30 - 34 reported eligible outcomes. Nine RCTs were assessed as good quality, 17 , 20 , 22 , 26 , 27 , 41 , 46 , 54 , 57 and the rest were assessed as fair quality. Detailed study characteristics, outcomes, and individual study methodological quality are described in eTables 1-7 and 13-17 in the Supplement .
Five studies were conducted exclusively or predominantly among populations in nursing homes or homes for the elderly (ie, “institutionalized” settings) 16 , 19 , 35 , 42 ; the rest were conducted exclusively or predominantly among community-dwelling populations. The mean age of included populations ranged from 36 to 85, but 54% were conducted among study populations with a mean age of 60 years or older. Twelve studies were conducted exclusively among female populations. 16 - 19 , 21 , 22 , 26 , 30 , 39 , 42 , 52 , 58 The race/ethnicity of the studied populations included multiple races and ethnicities in 9 studies, 15 , 21 , 22 , 26 , 30 , 46 , 53 , 54 , 57 was exclusively White in 1 study, 58 was mostly Latino in 1 study, 20 and was not reported in the remaining studies.
Nine studies 17 , 18 , 21 , 22 , 35 , 36 , 43 , 52 , 57 enrolled participants with serum vitamin D levels less than 20 ng/mL, and 5 studies enrolled participants using thresholds between 20 and 30 ng/mL. 15 , 20 , 26 , 41 , 51 Eight studies did not require participants to meet specific serum vitamin D–level criteria for enrollment, but the mean baseline serum vitamin D levels reported among the enrolled populations suggested that 90% or more of the enrolled participants had baseline serum levels less than 30 ng/mL. 16 , 19 , 25 , 27 , 39 , 42 , 44 , 58 Five studies did not require participants to be vitamin D deficient for enrollment but reported results separately for the subgroup of participants with serum levels less than 20 ng/mL. 30 , 37 , 46 , 53 , 54 Vitamin D assays used by studies varied.
All studies used vitamin D 3 as part of the active treatment intervention. Most studies used daily doses, which varied from as low as 400 IU to as high as 4000 IU. Two studies used a high initial loading dose, followed by lower monthly doses 26 , 54 ; 1 of these studies also titrated the dose to reach a target serum level of 30 ng/mL. 26 One study titrated the weekly dose to achieve a target serum level between 65 ng/mL and 90 ng/mL, resulting in an average weekly dose of 88 865 IU. 20 The rest of the studies used weekly, twice weekly, twice monthly, or monthly doses. Two studies used a no-intervention control group 39 , 42 ; the rest used placebo controls. Four studies included various doses of oral calcium as part of the active treatment intervention. 18 , 19 , 39 , 42 Six studies provided calcium to both the active vitamin D treatment group and control group. 16 , 21 , 22 , 43 , 51 , 52 Treatment duration ranged from 8 weeks to 7 years.
Twelve RCTs 18 , 19 , 21 , 22 , 25 - 27 , 35 , 39 , 42 - 44 reported all-cause mortality outcomes over 4 months to 3 years (eTable 4 in the Supplement ); however, none evaluated mortality as a primary study aim. The pooled ARD comparing vitamin D treatment with control among studies conducted in community-dwelling populations was 0.3 percentage points (95% CI, −0.6% to 1.1%; 2006 participants; 8 RCTs; I 2 = 0%), and the pooled RR was 1.13 (95% CI, 0.39 to 3.28) ( Figure 3 ). Because events were rare, sensitivity analyses were conducted using alternative pooling methods, and ARD estimates were stable (eResults and eTables 18 and 19 in the Supplement ). The findings from the WHI nested case-control study were consistent with the findings from the RCTs. 30 , 34
Nine RCTs 17 , 19 , 26 , 27 , 35 , 44 , 51 , 52 , 54 reported fracture outcomes over 12 weeks to 3.3 years (eTable 5 in the Supplement ); studies varied by type of fracture reported and ascertainment methods. The pooled ARD comparing vitamin D treatment with control among studies conducted in community-dwelling participants for incidence of fractures was −0.3 percentage points (95% CI, −2.1% to 1.6%; 2186 participants; 6 RCTs; I 2 = 13.0%), and the pooled RR was 0.84 (95% CI, 0.58 to 1.21) ( Figure 4 ). Findings from the WHI nested case-control study were consistent with findings from the RCTs. 30 Four RCTs 19 , 35 , 44 , 52 reported the incidence of hip fracture, but only 1 was conducted among community-dwelling populations 52 ; only 1 hip fracture occurred, leading to an imprecise effect estimate (eFigure 1 in the Supplement ).
Eleven RCTs reported fall outcomes over 1 to 3 years among either community-dwelling or institutionalized populations (eTable 6 in the Supplement ). 16 , 19 , 26 , 27 , 39 , 46 , 51 , 52 , 54 , 57 , 58 , 89 Four RCTs reported the number of participants who experienced 1 or more falls, 19 , 27 , 54 , 57 1 RCT reported the number of participants who experienced 2 or more falls, 89 2 RCTs reported the total number of falls experienced in each treatment group, 26 , 58 and 4 RCTs reported both outcomes. 16 , 39 , 51 , 52 The pooled ARD comparing vitamin D treatment with control for the incidence of participants with 1 or more falls among community-dwelling populations was −4.3 percentage points (95% CI, −11.6% to 2.9%; 2633 participants; 6 RCTs; I 2 = 70.1%), and the RR was 0.90 (95% CI, 0.75 to 1.08) ( Figure 5 ). Heterogeneity was high, as indicated by the I 2 statistic.
The 2 studies observing a more than 10–percentage-point absolute decrease in incidence were conducted by the same research team using similar methods and calcium controls 51 , 52 ; findings were statistically significant in only 1 of the studies. 51 The other 4 studies observed smaller effects ranging from a decrease of 4.6 percentage points to an increase of 3.1 percentage points; these findings were not statistically significant. 27 , 39 , 54 , 57 In the RCT reporting on the incidence of 2 or more falls, no significant difference was observed between vitamin D and placebo groups among participants with baseline vitamin D levels less than 12 ng/mL (adjusted odds ratio, 1.03 [95% CI, 0.59 to 1.79]) or for participants with baseline levels between 12 and 20 ng/mL (adjusted odds ratio, 1.13 [95% CI, 0.87 to 1.48]). 46 , 89
Vitamin D treatment was associated with fewer total falls compared with control in studies conducted among community-dwelling populations (incidence rate difference, 0.10 fewer falls per person-year [95% CI, −0.19 to −0.002]; 2838 person-years; 6 RCTs; I 2 = 76.9%; incidence rate ratio, 0.76 [95% CI, 0.57 to 0.94]) ( Figure 6 ).
Studies also reported on the incidence of other morbidities, including diabetes, cardiovascular disease, cancer, depression, and infection, and on physical functioning (eTable 7 in the Supplement ). Five RCTs, all conducted among community-dwelling populations, reported on incident diabetes over 1 to 7 years, although ascertainment methods varied. 20 , 31 , 37 , 53 , 58 The pooled ARD for incident diabetes was 0.1 percentage points (95% CI, −1.3% to 1.6%; 3356 participants; 5 RCTs; I 2 = 0%), and the pooled RR was 0.96 (0.80 to 1.15) (eFigure 2 in the Supplement ).
Two RCTs conducted among community-dwelling populations reported the effect of vitamin D treatment on the incidence of cardiovascular disease and cancer among subgroups of participants with serum levels less than 20 ng/mL at baseline. 46 , 53 No statistically significant differences in cardiovascular events (subgroup n = 2000; hazard ratio [HR], 1.09 [95% CI, 0.68 to 1.76] over 5.3 years 46 and subgroup n = 1270; HR, 1.00 [95% CI, 0.74 to 1.53] over 3.3 years 54 , 55 ) or incident invasive cancer (HR, 1.01 [95% CI, 0.65 to 1.58] 90 and HR, 0.97 [95% CI, 0.68 to 1.39] 46 ) were observed in either trial. No statistically significant associations were observed between vitamin D treatment and incident breast or colorectal cancer over 7 years in the WHI nested case-control study among participants with low serum vitamin D levels at baseline. 32 , 33
Three RCTs 36 , 41 (subgroup n = 1328, 46 , 91 n = 243, 39 and n = 408 34 ) reported on depression outcomes over 5.3 years, 16 weeks, and 26 weeks, respectively, and found no statistically significant differences between treatment and control as measured by various validated depression symptom rating scales. Two RCTs (n = 230 24 and n = 100 13 ) reported measures of physical functioning (eg, fibromyalgia impact questionnaire at 8 weeks, 13 modified Stanford Health Assessment Questionnaire 24 at 1 year); findings were mixed. One RCT 37 (subgroup n = 173) reported on incident urinary tract infection over 5 years of follow-up (HR, 0.53 [95% CI, 0.17 to 1.64]).
One of the RCTs conducted in institutional settings reported mortality (1 participant), but this was not reported by group, so it could not be included in the quantitative synthesis. 35 Among the 3 RCTs conducted among institutionalized populations, an absolute risk decrease ranging from 2.2 to 5.6 percentage points was observed; however, no individual study estimates were precise enough to exclude the null effect ( Figure 3 ). When pooled, the ARD was −2.8 percentage points (95% CI, −5.5% to −0.2%; 3409 participants; I 2 = 0%). The RR was 0.86 (95% CI, 0.74 to 0.99). Data were limited for evaluating effects among other subgroups, but for mortality, fractures, and falls, no differences between men and women or among studies using lower thresholds to define deficiency (eg, <20 ng/mL) for enrollment or calcium cointerventions were observed (eFigures 3-8 in the Supplement ).
Only 1 study reported benefits of vitamin D treatment stratified by race or ethnicity. 22 , 23 In this study, no mortality events occurred among either the White or African American populations enrolled. With the exception of 1 study conducted primarily among a Latino population, 20 the studies reporting the race or ethnicity of the enrolled population were conducted among exclusively or majority White populations. Thus, the ability to determine the influence of race/ethnicity on benefit outcomes was limited.
Key Question 4a. What are the harms of treatment of vitamin D deficiency with vitamin D?
Key Question 4b. Do harms vary among patient subpopulations at higher risk for vitamin D deficiency (eg, persons residing in institutions, persons with obesity, persons with low levels of sun exposure, or older adults) or vary by race/ethnicity?
Thirty-six RCTs 15 - 19 , 21 - 29 , 35 , 36 , 39 - 43 , 58 - 88 reported on harms of treatment; 16 of these were also included for KQ3. Nine of the studies were assessed as good quality 17 , 22 , 26 , 27 , 41 , 63 , 74 , 77 , 84 ; the rest were assessed as fair quality. See the Supplement for additional study characteristics (eTables 1-3) and individual study quality ratings (eTables 15 and 16).
Four studies were conducted among institutionalized populations, 16 , 19 , 35 , 42 2 were conducted among mixed community-dwelling and institutionalized populations, 43 , 66 and the rest were conducted exclusively in community-dwelling populations. Four studies exclusively enrolled Black participants. 60 , 61 , 74 , 82 Three studies evaluated vitamin D 2 as a 2000 IU daily dose, 69 a 50 000 IU weekly dose, 63 or a single 100 000 IU dose. 71 The rest of the studies evaluated various daily, weekly, monthly, or single doses of vitamin D 3 . In the studies using daily doses, the doses ranged from as low as 400 IU to as high as 4000 IU, and the studies using weekly doses ranged from 20 000 IU to 50 000 IU. Nine studies provided calcium to both the active vitamin D treatment group and the control group. 16 , 21 , 22 , 43 , 60 , 61 , 65 , 74 , 84 The rest of the included studies did not include any calcium as part of the active or control intervention. The duration of the intervention ranged from a single, 1-time dose to 3 years; however, the duration of intervention was less than 6 months in 22 of the 36 studies.
No studies specified adverse events as primary outcomes. With 1 exception, 39 primary outcomes included laboratory (eg, serum vitamin D level), imaging (eg, bone mineral density), or physical strength (eg, grip strength) measures. Seven studies collected data on adverse events at study visits, 16 , 43 , 65 , 67 , 72 , 77 , 86 2 used follow-up telephone calls, 25 , 63 1 used a toll-free call-in line available to participants to report adverse events, 84 and 1 used multiple methods. 41 Fourteen studies did not report how adverse events were ascertained. 15 , 17 , 18 , 35 , 36 , 58 , 60 , 68 - 71 , 73 , 82 , 88 Consistent definitions for total and serious adverse events were not used across studies.
Twenty-four studies (n = 3938) reported overall adverse events (eTable 8 in the Supplement ). 15 - 18 , 25 , 35 , 41 , 43 , 58 , 60 , 63 , 65 , 67 - 73 , 77 , 82 , 84 , 86 , 88 The incidence of adverse events varied by study, ranging from 0% to 92% across the treatment and control groups. However, within any given study, the incidence of adverse events was generally similar between treatment and control groups. Seven studies reported no adverse events. 15 , 35 , 60 , 70 , 71 , 73 , 82 However, 1 of the studies that reported no adverse events did in fact note adverse effects (eg, nausea) and discontinuations from the study. 35 Of the 14 studies reporting total adverse events by group, only 3 conducted statistical significance testing, and all reported no significant differences between groups. 18 , 77 , 86 Although many studies did not list the specific adverse events experienced by participants, those that did reported the following types of adverse events: abdominal discomfort, gastrointestinal issues, fatigue, musculoskeletal symptoms, nontoxic goiter, light-headedness, severe headaches, nausea, rash/hives, weakness, numbness, constipation, and itching. 16 , 35 , 60 , 63 , 65 , 72 , 86
Sixteen RCTs (n = 3912) reported serious adverse events (eTable 9 in the Supplement ). 17 , 18 , 21 , 22 , 27 , 36 , 43 , 58 , 60 , 61 , 63 , 68 , 72 , 78 , 84 , 88 The incidence of serious adverse events ranged from 0% to 29.4% across the groups within the studies; the incidence appeared similar between treatment and control groups, although formal statistical significance testing was not conducted in any study. Seven studies (n = 1702) reported 0 serious adverse events overall. 17 , 36 , 60 , 63 , 72 , 84 , 88 Five studies (n = 1341) reported serious adverse events, but authors indicated that these were most likely unrelated to the study medication. 21 , 22 , 27 , 58 , 61
Ten RCTs (n = 2120) reported on kidney stones (eTable 11 in the Supplement ). 19 , 21 , 22 , 25 , 26 , 43 , 61 , 65 , 66 , 88 In all but 1 of those studies, the incidence of kidney stones was reported in 0% of both the active treatment and control groups. In the study reporting more than 0 events, 1 participant in the lower-dose vitamin D group (800 IU daily) reported a kidney stone; no kidney stones were reported in the placebo group or in the higher-dose vitamin D group (50 000 IU twice monthly). 26 This study did not use calcium as part of the active treatment or control intervention.
Discontinuations due to adverse events and various other specific harms are detailed in the eResults and eTables 10 and 12 in the Supplement .
Data were too limited to evaluate differences in harms by subgroups of participants.
This review is an updated report regarding screening for vitamin D deficiency in adults. However, no studies were identified that evaluated screening for vitamin D deficiency; thus, this evidence report was limited to an evaluation of the benefits and harms of vitamin D treatment among participants at risk for deficiency based on low serum vitamin D levels. Compared with the 2014 review for the USPSTF on this topic, 8 , 9 23 new RCTs were added, and 4 RCTs were excluded. Table 2 summarizes the evidence by KQ and provides an assessment of the strength of evidence.
For benefits of treatment (KQ3) among community-dwelling populations, the strength of evidence was assessed as moderate for no benefit for mortality, any fractures, incident diabetes, cardiovascular disease, and incident cancer. For these outcomes, the strength of evidence was downgraded for study limitations or imprecision. The strength of evidence was assessed as low for no benefit for hip fractures and depression because of study limitations and imprecision. The strength of evidence for incidence of falls was assessed as low for no benefit; it was downgraded because of inconsistency between the various fall measures (incidence vs total falls) and for imprecision in effect estimates. The strength of evidence for physical functioning and infection was assessed as insufficient because of inconsistency, imprecision, and study limitations. For harms of treatment (KQ4), the strength of evidence was assessed as low for no harm for total adverse events, serious adverse events, discontinuations due to adverse events, kidney stones, and other harms. The strength of evidence was downgraded for these outcomes because of imprecision and study limitations. Although studies were consistent in demonstrating no difference in harms between active treatment and control groups, the absolute incidence of reported adverse events varied vastly across studies, likely because of different approaches to defining and ascertaining these outcomes across the studies.
Despite a reasonable number of studies reporting falls outcomes, the body of evidence demonstrated mixed findings. Among the studies reporting the incidence of 1 or more falls, a numerical but not statistically significant decrease (pooled ARD, −4.3%) was observed among community-dwelling populations. The most recent good-quality trial reported the incidence of 2 or more falls among subgroups of participants with low vitamin D levels and also found no significant association, although effect estimates were imprecise. Among the studies reporting total number of falls, a small but statistically significant decrease (−0.1 falls per person-year) in the total number of falls was observed. Estimates for both types of outcomes were inconsistent and imprecise. Some studies reported both outcomes, but others reported only 1 of these outcomes, raising the possibility of selective outcome reporting. One hypothesis to explain the difference between these 2 outcomes is that although vitamin D may not prevent a first fall, it may have some benefit in preventing repeat falls.
A related systematic review on behalf of the USPSTF recommendation for fall prevention in community-dwelling populations at increased risk of falls found mixed findings for vitamin D interventions. 92 There was also evidence of possible harms from high-dose vitamin D in such populations, resulting in a recommendation against vitamin D supplementation in community-dwelling adults 65 years or older. 92 , 93 The falls prevention review excluded studies conducted among vitamin D–deficient populations; thus, additional evidence specifically in vitamin D–deficient populations is needed to be able to draw definitive conclusions about the effect of screening for vitamin D deficiency on falls among community-dwelling adults.
Findings regarding benefits of treatment in this review are not directly comparable with those from other reviews of vitamin D supplementation because this review was focused specifically on persons with low vitamin D levels (ie, less than 20 or 30 ng/mL) and other differences in study selection criteria. Despite these differences, the findings from this review are largely consistent with those from other reviews conducted in broader populations with respect to most outcomes.
This evidence review had several limitations. First, no available evidence that directly evaluated the health benefits and harms of screening (KQ1 and KQ2) was identified. Second, studies selected for this review included some conducted in institutionalized settings. However, the synthesis and strength of evidence assessment focused mainly on community-dwelling populations because USPSTF recommendations are for clinical preventive services in or referred from primary care settings. Studies focused on populations with a specific clinical condition to evaluate the treatment of vitamin D deficiency for the alleviation of specific symptoms or issues associated with that condition were not included. Third, the comparative benefits or harms of various vitamin D doses, formulations, or durations of treatment were not assessed. Fourth, this review included studies that enrolled participants based on 25(OH)D levels that used various assays and that may not have been standardized according to current criteria from the Vitamin D Standardization Program. 94 Fifth, for the trials enrolling participants unselected with respect to vitamin D status, only findings from the vitamin D–deficient subgroups were reported. Findings from the overall population were not included, but these may be eligible to be included in the next update of a related review of vitamin D supplementation conducted on behalf of the USPSTF. 95
No studies evaluated the direct benefit or harms of screening for vitamin D deficiency. Among asymptomatic, community-dwelling populations with low vitamin D levels, the evidence suggests that treatment with vitamin D (with or without calcium) has no effect on mortality or incidence of fractures, falls, depression, diabetes, cardiovascular disease, cancer, or adverse events. The evidence is inconclusive about the effect of treatment on physical functioning and infection.
Corresponding Author: Leila C. Kahwati, MD, MPH, RTI International, 3040 E Cornwallis Rd, Research Triangle Park, NC 27709 ( [email protected] ).
Accepted for Publication: December 21, 2020.
Author Contributions: Dr Kahwati had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Kahwati, LeBlanc, Palmieri Weber, Clark, Viswanathan.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Kahwati, LeBlanc, Giger, Clark, Suvada, Guisinger.
Critical revision of the manuscript for important intellectual content: Kahwati, LeBlanc, Palmieri Weber, Suvada, Viswanathan.
Statistical analysis: Kahwati, Weber, Clark, Suvada.
Obtained funding: Kahwati, Viswanathan.
Administrative, technical, or material support: Kahwati, Palmieri Weber, Giger, Clark, Suvada, Guisinger, Viswanathan.
Supervision: Kahwati.
Conflict of Interest Disclosures: None reported.
Funding/Support: This research was funded under contract HHSA-290-2015-00011-I, Task Order 11, from the Agency for Healthcare Research and Quality (AHRQ) under a contract to support the US Preventive Services Task Force (USPSTF).
Role of the Funder/Sponsor: Investigators worked with USPSTF members and AHRQ staff to develop the scope, analytic framework, and key questions for this review. AHRQ had no role in study selection, quality assessment, or synthesis. AHRQ staff provided project oversight, reviewed the report to ensure that the analysis met methodological standards, and distributed the draft for peer review. Otherwise, AHRQ had no role in the conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript findings.
Disclaimer: The opinions expressed in this document are those of the authors and do not reflect the official position of AHRQ or the US Department of Health and Human Services.
Additional Contributions: We acknowledge the following individuals for their contributions to this project: AHRQ staff Howard Tracer, MD, andTracy Wolff, MD, MPH; former AHRQ staff Quyen Ngo-Metzger, MD, MPH; current and former members of the USPSTF who contributed to topic deliberations; and RTI International–University of North Carolina Evidence-based Practice Center staff B. Lynn Whitener, DrPH, Carol Woodell, BSPH, Sharon Barrell, MA, and Loraine Monroe. USPSTF members, peer reviewers, and federal partner reviewers did not receive financial compensation for their contributions.
Additional Information: A draft version of the full evidence report underwent external peer review from 4 content experts (John Aloia, MD, New York University Winthrop Bone Mineral Research Center; JoAnn E. Manson, MD, MPH, DrPH, Harvard Medical School; Clifford Rosen, MD, Maine Medical Center Research Institute; and Christopher Sempos, PhD, Vitamin D Standardization Program LLC) and 4 individuals from 3 federal partner reviewers (2 from the National Institutes of Health, 1 from the Centers for Disease Control and Prevention). Comments from reviewers were presented to the USPSTF during its deliberation of the evidence and were considered in preparing the final evidence review.
Editorial Disclaimer: This evidence report is presented as a document in support of the accompanying USPSTF Recommendation Statement. It did not undergo additional peer review after submission to JAMA .
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
The Role of Vitamin D in Health and Disease: A Narrative Review on the Mechanisms Linking Vitamin D with Disease and the Effects of Supplementation
- Review Article
- Published: 06 May 2023
- Volume 83 , pages 665–685, ( 2023 )
Cite this article
- Eleni Rebelos 1 , 2 ,
- Nikolaos Tentolouris 3 &
- Edward Jude ORCID: orcid.org/0000-0002-3186-4122 4 , 5 , 6
4623 Accesses
13 Citations
25 Altmetric
Explore all metrics
Vitamin D insufficiency or deficiency (VDD) is a very prevalent condition in the general population. Vitamin D is necessary for optimal bone mineralization, but apart from the bone effects, preclinical and observational studies have suggested that vitamin D may have pleiotropic actions, whereas VDD has been linked to several diseases and higher all-cause mortality. Thus, supplementing vitamin D has been considered a safe and inexpensive approach to generate better health outcomes—and especially so in frail populations. Whereas it is generally accepted that prescribing of vitamin D in VDD subjects has demonstrable health benefits, most randomized clinical trials, although with design constraints, assessing the effects of vitamin D supplementation on a variety of diseases have failed to demonstrate any positive effects of vitamin D supplementation. In this narrative review, we first describe mechanisms through which vitamin D may exert an important role in the pathophysiology of the discussed disorder, and then provide studies that have addressed the impact of VDD and of vitamin D supplementation on each disorder, focusing especially on randomized clinical trials and meta-analyses. Despite there already being vast literature on the pleiotropic actions of vitamin D, future research approaches that consider and circumvent the inherent difficulties in studying the effects of vitamin D supplementation on health outcomes are needed to assess the potential beneficial effects of vitamin D. The evaluation of the whole vitamin D endocrine system, rather than only of 25-hydroxyvitamin D levels before and after treatment, use of adequate and physiologic vitamin D dosing, grouping based on the achieved vitamin D levels rather than the amount of vitamin D supplementation subjects may receive, and sufficiently long follow-up are some of the aspects that need to be carefully considered in future studies.
Similar content being viewed by others
Association between Mediterranean diet and dementia and Alzheimer disease: a systematic review with meta-analysis
Daniele Nucci, Andrea Sommariva, … Vincenza Gianfredi
The vitamin D deficiency pandemic: Approaches for diagnosis, treatment and prevention
Michael F. Holick
Vitamin D Deficiency: Defining, Prevalence, Causes, and Strategies of Addressing
Kevin D. Cashman
Avoid common mistakes on your manuscript.
1 Introduction
Vitamin D insufficiency or deficiency (VDD) have been identified as very prevalent conditions in the general population, with some authors coining the use of the terms of “vitamin D deficiency epidemic, or pandemic” [ 1 , 2 ]. Other than the well-known effects of vitamin D on bone metabolism, vitamin D exerts pleiotropic actions. On one hand, VDD is associated with a series of adverse health conditions; on the other, supplementation with vitamin D is a low‐cost and safe intervention, making it an attractive therapeutic option in the clinician’s and researcher’s armature. These facts have contributed to the “explosion” in the interest of the scientific community on the understanding of the pleiotropic actions of vitamin D, among which is its immunomodulating effects.
Currently, there is vast research on the effects of vitamin D on human homeostasis, mechanisms of action, and supplementation outcomes. Up to June 2022, a PUBMED (MeSH) search on vitamin D yielded 65,758 results, with abrupt increases in the scientific publications in the last two decades. However, many of the published studies that have linked decreased vitamin D levels with poorer health outcomes are of associative nature, making the evidence whether vitamin D per se contributes or not to poor health relatively weak. In this narrative review, we present the links between VDD and a variety of diseases such as infections, COVID-19, type 2 diabetes (T2D), hypertension, cardiovascular, gastrointestinal, neurodegenerative and autoimmune diseases, and also the impact of vitamin D supplementation. First, we describe briefly, the mechanisms through which vitamin D could have an impact on the discussed pathology (Fig. 1 ). Then, we provide the available evidence from purely an association point of view. Since association does not prove causation, and there are often undetected confounders in reported associations, we then focused on meta-analyses and systematic reviews where vitamin D administration has been tested in the treatment or prognosis of the disease in question. Original articles and/or meta-analyses on the supplementation of vitamin D outcomes are also provided if available.
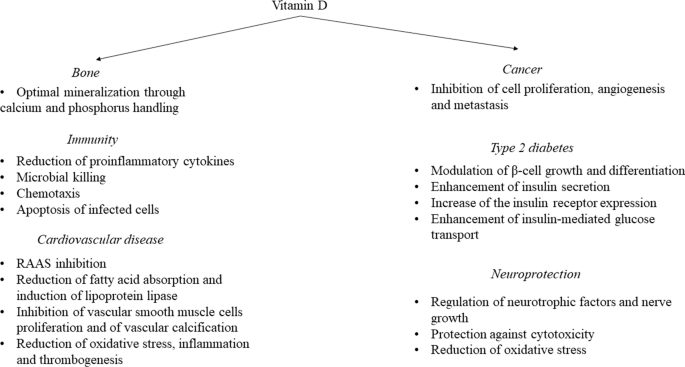
Mechanisms through which vitamin D may impact on bone health, immunity, cancer, cardiovascular disease, and neuroprotection
1.1 Regulation of Vitamin D
Vitamin D exists in two major forms: vitamin D2 (ergocalciferol) and vitamin D3 (cholecalciferol); the former is obtained with diet mainly from fungi and also plants, whereas the latter can be either obtained with diet (animal products) or synthesized in the skin from the conversion of the cholesterol precursor 7-dehydrocholesterol after exposure to adequate ultraviolet B radiation. Sun exposure for vitamin D synthesis may be efficient only when the angle of sun rays is more than 45°. As a result of this, inhabitants of the northern hemisphere do not receive sufficient amounts of vitamin D through skin synthesis during winter months, and in some northern areas, defective sun exposure may last up to 6 months of the year [ 3 ]. Moreover, a typical Western diet is poor in vitamin D [ 4 ]. To increase vitamin D ingestion, some countries have applied a policy of enriching milk products [ 5 , 6 ] and margarine [ 7 ] with vitamin D, while also the use of light bulbs for artificial UVB exposure is another tool to increase vitamin D synthesis.
Vitamin D needs to undergo activation, which consists of two consecutive hydroxylations; the first in the liver and the second predominantly in the kidneys, but also in extrarenal tissues. In the liver, cholecalciferol is quickly hydroxylated by the enzyme 25-hydroxylase (a CYP450-dependent enzyme also known as CYP2R1) yielding 25-hydroxyvitamin D (25(OH)D) in an uncontrolled process [ 8 ]. Low plasma calcium or phosphate levels regulate parathyroid hormone (PTH) and fibroblast growth factor 23 (FGF-23) levels, leading to the 1α-hydroxylation of 25(OH)D in the kidney and particularly in the mitochondria of the proximal convoluted tubule cells by the 1-hydroxylase enzyme (CYP27B1), resulting in the active vitamin D (1,25(OH) 2 D) [ 9 ] (Fig. 2 ). The 1α-hydroxylation may also occur in extrarenal tissues (epithelial tissues, placenta, bone, endocrine glands, brain, liver, and endothelium [ 10 , 11 ]), and especially in immune cells [ 12 ]. The 1,25(OH) 2 D can then de-activate 1α-hydroxylase and stimulate the 24-hydroxylase enzyme, which destroys 25(OH)D, providing a negative feedback loop that controls active vitamin D levels. The 24-hydroxylation of 25(OH)D yields 24,25(OH) 2 D, the inactive metabolite, the formation of which, along with saturation of the synthesis of vitamin D in the skin, guards against vitamin D intoxication. Even though the active form of vitamin D is 1,25(OH) 2 D, conventional blood tests measure 25(OH)D because of its long half-life (~15 days) [ 13 ], making it a suitable marker of vitamin D storage. In contrast, circulating 1,25(OH) 2 D does not reflect vitamin D status because of its short half-life of a few hours and its tight regulation by PTH, calcium, and phosphate levels [ 14 ]. The direct measurement of free (non-protein bound) 25(OH)D is also possible, with some authors proposing that the contemporaneous assessment of total and free 25(OH)D levels, as well as vitamin D binding protein (VDBP) and PTH should be measured in assessing vitamin D status and the effect of vitamin D supplementation on clinical outcomes [ 15 , 16 , 17 , 18 ].
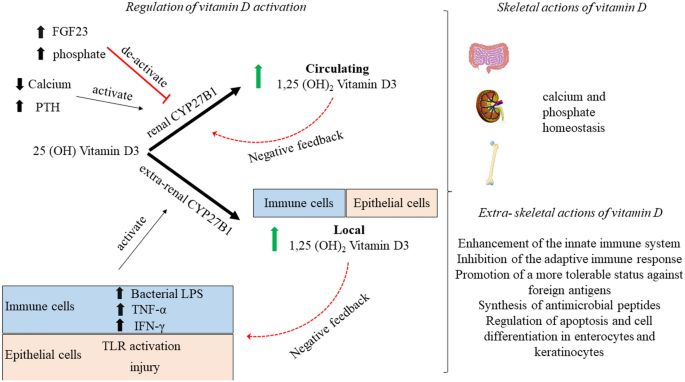
Schematic representation of 1α-hydroxylation of 25 (OH)D in the active form in renal and extrarenal tissues. Several tissues have been described to have the CYP27B1 enzyme responsible for the 1α-hydroxylation of 25 (OH) D, but here emphasis is given in the immune and epithelial cells. Of note is that the control of the CYP27B1 activity differs between renal and extrarenal tissues. FGF23 fibroblast growth factor, IFN-γ interferon gamma, PTH parathyroid hormone, TLR toll-like receptor, TNF-α tumor necrosis factor alpha
The 1,25(OH) 2 D binds to the vitamin D receptor (VDR), a member of the nuclear receptor family of ligand-regulated transcription factors, which then forms a heterodimer with the retinoid X receptor. The heterodimer enters the cell nucleus and binds to vitamin D responsive elements (VDRE) in DNA, resulting in regulation of the expression of key genes in target organs to yield its actions. This is the basis for the genomic actions of vitamin D. Genomic actions of vitamin D require hours before any effects can be noticed. However, vitamin D also exerts actions that are rapid (within seconds to minutes); these are the nongenomic actions of vitamin D that are yielded without gene activation. The nongenomic actions of vitamin D may occur when vitamin D activates the VDR found outside the nucleus [ 19 ]. Furthermore, it has been suggested that vitamin D may also have a membrane receptor, which could explain the rapid nongenomic actions of vitamin D. However, the membrane target of vitamin D is currently not fully elucidated [ 20 ].
1.2 The Difficulty in Assessing the Effects of Vitamin D Supplementation in Health Outcomes
Vitamin D is a nutrient, but the major determinant of vitamin D levels is dependent on skin synthesis following sunlight exposure. Thus, placebo-controlled randomized controlled trials (RCTs) assessing the effects of vitamin D on health outcomes differ greatly from standard RCTs using drugs, since it is impossible to exclude vitamin D intake or sunlight exposure in the placebo arms of the vitamin D trials. [ 21 ]. Moreover, since VDD is a very prevalent condition, some RCTs (for instance, the large VITAL study [ 22 , 23 ]) also allow supplementation with low doses of vitamin D in the placebo group. While most RCTs are done in the general population to increase generalizability of the study results, it is well known that anthropometric characteristics of the study participants such as age, body mass index (BMI), and even skin pigmentation may affect the intake or metabolism of vitamin D, and therefore constitute confounders [ 24 , 25 ]. However, RCTs on vitamin D typically use standard doses of supplementation rather than personalized doses based on the characteristics of the participants. Moreover, in several RCTs, baseline and on-treatment 25(OH)D are not monitored; this is again a great confounder of the study results since subjects on the placebo arm may actually achieve higher 25(OH)D levels compared with subjects on treatment. Even when plasma 25(OH)D levels are monitored, there is large variance in the results, especially if the widely used immunoassay methodology is used [ 26 ]. Thus, data from different studies are not always comparable and could not be used in meta-analyses. Finally, the dose-response between vitamin D and its health effects is “S shaped” [ 21 , 27 ]. This implies that, on one hand, in subjects with VDD, large doses of vitamin D supplementation would be needed to elicit any meaningful effect, while on the other, supplementation in vitamin D replete subjects would not yield any effect. These are important confounders that make the interpretation and the execution of an RCT on vitamin D much more demanding compared with a drug RCT, and are expected to have affected the results of the RCTs that are presented in the following chapters.
2 Classical Vitamin D Actions
2.1 vitamin d and bone.
Mechanisms Vitamin D exerts both direct and indirect actions on bone [ 28 ]. Vitamin D is a major determinant of mineral homeostasis, promoting intestinal calcium and phosphorus absorption, which are required for optimal mineralization of bone. Vitamin D also exerts direct actions on bone. The direct actions of vitamin D on bone are more complex to demonstrate, and studies on VDR or CYP27B1 knockout animal models treated with a rescue high-calcium, high-phosphorus, and high-lactose diet have shown that even though severe bone abnormalities such as rickets (i.e., defective mineralization of the growth plate and adjacent metaphysis in the growing skeleton) and osteomalacia (i.e., the accumulation of unmineralized osteoid at sites other than the growing metaphysis) are prevented [ 29 , 30 ], changes in osteoblast number, mineral apposition rate, and bone volume remain [ 31 ]. Indeed, as reviewed in [ 28 ], direct effects of vitamin D on osteoblasts proliferation and survival and in the mineralization process have been shown.
Even though it is well established that acquired or genetic alterations in the vitamin D endocrine system can lead to rickets and osteomalacia and that, vice versa, treatment with an adequate quantity of vitamin D prevents rickets, osteomalacia [ 32 ], and renal osteodystrophy, the role of vitamin D in the skeleton of adults and older adults is often disputed.
In the large Vitamin D Assessment (VIDA) study—a trial in which participants were randomized to receive either 100,000 IU vitamin D3 or placebo monthly—correction of severe vitamin D deficiency led to improvement in bone mass density (BMD) [ 33 ], whereas vitamin D supplementation in already vitamin D replete adults was not associated with improved bone mass density (BMD) or bone quality [ 33 ]. Moreover, no effect was found in the VIDA trial in risk of fractures or falls after vitamin D supplementation in either the whole dataset or the vitamin D deplete group compared with placebo [ 33 ]. In the other large RCT Vitamin D and OmegA-3 TriaL (VITAL), supplemental vitamin D3 (2000 IU/d) was compared with placebo. Also in this study, vitamin D supplementation did not affect BMD of the spine, hip, or whole body, and this lack of effect was independent of baseline 25(OH)D levels [ 23 ]. However, among subjects with baseline free vitamin D levels below the median (< 14.2 pmol/L), those receiving vitamin D supplementation showed a slight increase in spine aBMD (0.75% versus 0%; p = 0.043) and attenuation in loss of total hip aBMD (−0.42% versus −0.98%; p = 0.044) compared with placebo [ 23 ]. In the Calgary study, the long-term outcomes of vitamin D supplementation at 400, 4000, and 10,000 IU per day were compared. It was found that subjects receiving the very high dose of vitamin D supplementation had decreased BMD at the radius and tibia compared with subjects receiving 400 IU daily [ 34 ], while no differences in BMD were noted between the 4000 and 400 IU groups. Moreover, very high-dose vitamin D supplementation (4000 and 10,000 IU/day) may result in hypercalciuria and/or hypercalcaemia [ 34 ]. The decrease in BMD with very high doses of vitamin D may be due to excessive bone resorption by increasing the number and activity of osteoclasts directly [ 35 ], or indirectly through activation of osteoblasts, which in turn activate osteoclastogenesis [ 36 ]. Another important aspect related to bone health often evaluated in clinical studies is the risk of fractures. In a large meta-analyses conducted by Bolland et al., administration of vitamin D had no effect on total fracture [36 trials; n = 44.790, relative risk (RR) 1.00, 95% confidence intervals (CI) 0.93–1.07], hip fracture (20 trials; n = 36.655, RR 1.11; 95% CI 0.97–1.26), or falls (37 trials; n = 34.144, RR 0.97; 95% CI 0.93–1.02), and similar results were found when comparing randomized controlled trials (RCTs) of high-dose versus low-dose vitamin D [ 37 ]. Moreover, regarding hip fractures, this meta-analysis showed that, whereas there is reliable evidence that vitamin D supplementation does not reduce hip fractures, it is uncertain whether it might increase the risk of hip fractures [ 37 ]. On the contrary, a meta-analysis of eight studies including 30,970 participants showed that the combined administration of vitamin D and calcium can reduce the risk of total fractures by 15% [odds ratio (OR) 0.85; 95% CI 0.73–0.98] and the risk of hip fractures by 30% (OR 0.70; 95% CI 0.56–0.87) [ 38 ].
2.2 Vitamin D, Muscle Strength, Muscle Mass, Muscle Power, and Risk of Falls
Mechanisms VDD has been associated with musculoskeletal dysfunction, a reduction in muscle strength and size, and increased intramuscular noncontractile tissue [ 39 , 40 ].
One of the largest meta-analyses evaluated the effect of vitamin D supplementation on muscle strength, including data of 29 RCTs involving 5533 subjects. It demonstrated that vitamin D supplementation had a small but significant effect on improving global muscle strength (SMD 0.17, 95% CI 0.03–0.31, p = 0.02), and in particular there was a significant positive effect of vitamin D supplementation on lower limb muscle strength (SMD 0.19; 95% CI 0.05–0.34; p = 0.01), but not on grip strength (SMD 0.01; 95% CI 0.06–0.07; p = 0.87) [ 41 ]. In a subgroup analyses, it was further demonstrated that the improvement in muscle strength was greater in patients who at baseline had 25(OH)D values < 30 nmol/L, compared with those who had 25(OH) D ≥ 30 nmol/L. Moreover, a meta-regression showed a significant association between changes in 25(OH)D concentration and changes in muscle strength [slope 95% CI 0.01 (0.00, 0.01); p = 0.01]. With regards to age, vitamin D supplementation in subjects older than 65 years resulted in a significant improvement of muscle strength (SMD 0.25; 95% CI 0.01–0.48), whereas supplementation in younger people did not (SMD 0.03; 95% CI 0.08–0.14) [ 41 ]. This meta-analysis also assessed the effects of vitamin D supplementation on muscle mass and muscle power, even though a limited number of studies had assessed these outcomes (six and five studies, with a total of only 538 and 245 subjects, respectively). It was shown that vitamin D supplementation does not improve muscle mass or muscle power [ 41 ].
An improvement in lower limb muscle strength could be a promising mechanism through which vitamin D supplementation could reduce the risk of falls, since, on one hand, quadriceps strength is a significant predictor of falls [ 42 ] and, on the other hand, VDD has also been linked to an increased risk of falls [ 43 , 44 ]. Thus, whether vitamin D supplementation confers protection from falls has received a lot of interest, but meta-analyses on this topic have yielded conflicting results. Early meta-analyses reported beneficial effects of vitamin D supplementation on reducing falls, and two analyses reported that vitamin D supplementation combined with calcium, but not vitamin D supplementation alone, reduces the risk of falls [ 43 , 45 ]. However, subsequent meta-analyses reported neutral effects of vitamin D supplementation on falls [ 46 ], and when very high doses of vitamin D supplementation were used, there was an increased risk of falls [ 47 , 48 ].
In a 2014 trial, a sequential meta-analysis approach to reduce the risk of false positive effects, Bolland et al. analyzed data from 20 RCTs ( n = 29,535). They reported that vitamin D supplementation did not reduce the relative risk for falls by 15% or more, and similar null effects were reported when they performed a sensitivity analysis, reducing the risk reduction threshold at 10% [ 49 ]. There were no differences in the effects of vitamin D supplementation alone or vitamin D and calcium supplementation on the risk of falls. Based on their approach, the authors concluded that it is unlikely that similar future trials may alter these negative conclusions of vitamin D supplementation on the rick of falls [ 50 ]. The null effects of vitamin D supplementation on reducing the risk of falls were replicated in a subsequent meta-analysis of the same group in 2018, including data of 37 trials and a total of 34,144 subjects (RR 0.97; 95% CI 0.93–1.02). Of note is that in this meta-analysis, vitamin D supplementation did not decrease the RR of falls by 7.5%—i.e., the efficacy of vitamin D supplementation at a lower RR threshold was tested but still no clinically meaningful effect of vitamin D supplementation on reducing the risk of falls was found [ 37 ].
3 Non-Classical Vitamin D Actions
3.1 vitamin d and hypertension.
Mechanisms Preclinical studies have shown that VDD may predispose to hypertension through upregulation of the renin–angiotensin–aldosterone system (RAAS) and increased vascular resistance and vasoconstriction [ 51 , 52 , 53 ]. On the other hand, VDR activation has been shown to inhibit intrarenal mRNA levels and protein expression of key components of the RAAS [ 51 ].
Evidence shows that vitamin D supplementation is effective in reducing blood pressure in patients with hypertension and VDD [ 54 ]. Once again, the modality of vitamin D supplementation impacts the outcome, with daily [ 55 , 56 , 57 ] or weekly [ 58 ] administrations of vitamin D improving hypertension outcomes, whereas large bolus vitamin D dosing (e.g., 100,000 IU VD every 2 months) failed to reduce blood pressure in vitamin D deficient subjects [ 59 ]. Large doses of vitamin D might also have detrimental vascular effects, since they can result in vascular calcification [ 60 ]. On the contrary, vitamin D supplementation in vitamin D replete subjects has null effects on lowering blood pressure [ 61 ]. Antihypertensive medications may also affect whether vitamin D supplementation will affect blood pressure. For instance, Bernini et al. did not find any effect of acute or chronic vitamin D supplementation on RAAS in patients with essential hypertension on RAAS inhibitor treatment [ 55 ]; however, they also showed that chronic vitamin D receptor activation in drug-free essential hypertensives suppresses RAAS components [ 62 ]. This evidence further underlines that the blood pressure effects of vitamin D in humans are dependent on the activity of RAAS.
Low serum 25(OH)D levels have also been associated with an increased risk of developing hypertension [ 53 ], which raises the question of whether vitamin D supplementation can impact the incidence of hypertension, and this is of great clinical interest. It is important to note that to evaluate the effects of vitamin D supplementation on the incidence of chronic diseases, such as hypertension, the intervention period should be long enough (> 5 years) to record a sufficient number of events [ 54 ].
However, in the VITAL study (intervention for 5 years), vitamin D supplementation did not reduce the incidence of cardiovascular events [ 63 ], and there was no specific mention of whether the incidence of hypertension was affected. DO-HEALTH was a RCT on adults aged 70 years or older without major comorbidities. Treatment with 2000 IU/day of vitamin D did not improve systolic (SBP) or diastolic blood pressure (DBP) compared with placebo [ 64 ]. However, as the authors pointed out, in this trial only 40.7% of individuals had 25(OH)D levels less than 20 ng/ml at baseline, and all participants were allowed to take up to 800 IU/day of vitamin D outside the study medication [ 64 ].
3.2 Cardiovascular Events
Mechanisms VDR is expressed in endothelial cells, vascular smooth muscle cells, and cardiac myocytes [ 65 ]. Vitamin D preserves endothelial function through inhibition of the proliferation of vascular smooth muscle cells [ 66 ], and also reduces oxidative stress, inflammation, and thrombogenesis [ 67 ]. It has also been suggested that it can modify lipid metabolism by increasing the activity of lipoprotein lipase in adipose tissue [ 68 ] and by reducing fatty acid absorption [ 69 ]. As discussed earlier, it can also reduce RAAS activity, thereby decreasing blood pressure.
In a meta-analysis of nearly 850,000 individuals, patients were divided into tertiles for 25(OH)D supplementation. Patients on the lower tertile of 25(OH)D concentrations had an increased risk of death from cardiovascular disease compared with patients on the top thirds of 25(OH) D concentrations (RR 1.35; 95% CI 1.13–1.61) [ 70 ]. Moreover, another meta-analysis showed that subjects in the lowest quintile of 25(OH)D concentration had an increased risk of cardiovascular mortality compared with subjects in the highest quintile (RR 1.41, 95% CI 1.18–1.68 in subjects without a history of cardiovascular disease and RR 1.65, 95% CI 1.22–2.22 in subjects with a history of cardiovascular disease) [ 71 ]. In a recent large cohort study in 24,311 patients with T2D and 67.789 subjects with prediabetes (i.e., a study population with high CVD risk) it was shown that 25(OH)D levels were inversely and independently associated with the risk of incident cardiovascular outcomes and all-cause mortality. Moreover, in a recent large cohort study in 24,311 patients with T2D and 67,789 subjects with prediabetes (i.e.. a study population at increased risk for CVD [ 72 ]), 25(OH)D was associated with lower risk of incident CVD events and mortality [ 73 ]. In a dose-response analysis, it was shown that increasing 25(OH)D up to 50–60 nmol/L decreased mortality and cardiovascular events [ 73 ].
However, in the two large RCTs (VITAL and VIDA) with long follow-up, supplementation with vitamin D did not impact on major cardiovascular events or cardiovascular death compared with placebo [ 63 , 74 ]. The same conclusion was reached by Barbarawi and colleagues analyzing data of 21 RCTs with a total of 83,000 individuals [ 75 ].
Whether vitamin D supplementation affects the risk factors for CVD has also been investigated. Earlier systematic reviews and meta-analyses have reported a null effect of vitamin D supplementation on the modification of CVD risk factors [ 49 , 76 , 77 , 78 ]. Mirhosseini et al. recently performed a systematic review and meta-analysis with stringent inclusion criteria, including only studies in which the duration of vitamin D supplementation was at least 3 months; studies using a daily, weekly, or monthly frequency of vitamin D dosage; and studies where baseline and post-intervention serum 25 (OH)D levels were included. Eighty-one studies met the selection criteria. The authors showed that supplementation of vitamin D led to a reduction of SBP and DBP, a reduction of total cholesterol and triglycerides, an increase in HDL, and a reduction in high-sensitivity C-reactive protein (hs-CRP) [ 79 ]. In subgroup analyses, they also reported dose-effect responses comparing studies in which subjects received ≥ 4000 IU/day with studies in which patients received < 4000 IU/day. They showed that trials with vitamin D supplementation ≥ 4000 IU/day had greater reductions in SBP, DBP, and hs-CRP. Similar effects were reported when serum 25(OH)D levels higher or lower than 86 nmoL/L were considered, with subjects with higher 25(OH)D levels showing greater reductions in SBP, DBP, and hs-CRP. On the contrary, lipid changes were not associated with the dose or the achieved serum 25(OH)D concentrations [ 79 ]. As the authors state, the discrepancy with earlier systematic reviews and meta-analyses could be attributed to the quality of the studies included (small sample sizes, too low doses of vitamin D supplementation, and too narrow intervention length). The effect of vitamin D supplementation on markers of arterial stiffness [i.e., pulse wave velocity (PWV) and augmentation index (AI)] was also assessed, but the numbers of studies that evaluated these markers was small (11 and 10 studies, respectively). While there were no overall effects of vitamin D supplementation on these markers, subgroup analyses found that AI was lower in patients with serum 25(OH)D concentrations ≥ 86 nmol/L and in patients receiving vitamin D doses ≥ 4000 IU/day, with the authors concluding that vitamin D supplementation may improve the markers of arterial stiffness [ 79 ]. These results seem in line with the results of a prespecified analysis of a subsample of participants in the Vitamin D Assessment (VIDA) study who underwent suprasystolic oscillometry [ 74 ]. The VIDA study showed that monthly high-dose (i.e., 100,000 IU/month) supplementation with vitamin D led to improvements in AI, PWV, peak reservoir pressure, and backward pressure amplitude [ 74 ]. Aortic systolic blood pressure also improved, whereas SBP and DBP showed only small, nonsignificant reductions [ 74 ].
3.3 Acute Respiratory Tract Infection and Influenza
Mechanisms Vitamin D is involved in the control of both the innate and adaptive immune response. Virtually all immune cells express VDR and CYP27B1, and it has been shown that macrophages, activated T and B cells, dendritic cells, and endothelial cells lining the upper and lower respiratory tracts can hydroxylate 25(OH)D into the active form[ 80 , 81 , 82 ]. Neutrophils express VDR, but it seems that they do not possess CYP27B1 [ 83 ]. Evidence suggests that 1,25(OH) 2 D controls the innate immune response through a negative feedback loop on macrophages and other immune cells. More specifically, IFNγ-activated macrophages induce 1,25(OH) 2 D release, which in turn activates VDR on macrophages, suppressing the expression of key genes producing proinflammatory proteins [ 84 ]. Regarding regulation of adaptive immune responses, 1,25(OH) 2 D has been shown both to inhibit proliferation and differentiation of activated human B cells [ 85 ], to inhibit T helper cells, and also to promote Treg cells [ 86 ]; the net outcome of these effects would be to limit inflammatory processes. In the specific case of influenza virus, it has been shown that incubation of human lung A549 epithelial cells with 1,25(OH) 2 D before or after exposure to influenza A virus led to decreased production of TNF-α, IFN-β, and IFN-stimulated gene-15, and downregulated interleukin (IL-8 and IL-6 RNA levels [ 87 ]. An extensive review of the mechanisms through which vitamin D modulates and controls the immune responses has been performed recently [ 81 ].
A negative linear association among vitamin D levels and lung infections and function has been established in a large cross-sectional study of 6789 subjects, where for each 10 nM/L increase in vitamin D levels, the risk of infection was reduced by 7% [ 88 ]. Negative associations between vitamin D levels and the risk [ 89 ] or severity of pneumonia have also been described [ 90 ].
Urashima et al. performed an RCT in children ( N = 167 on vitamin D and N = 167 on placebo) receiving either a daily supplement of vitamin D (1200 IU/day) or placebo. They found that patients treated with vitamin D had a lower incidence of influenza A compared with placebo (incidence of influenza A, 10.8% in the vitamin D group versus 18.6% in the placebo group; RR 0.58; 95% CI 0.34–0.99; p = 0.04) [ 91 ]. Apart from these positive outcomes of higher vitamin D levels and of vitamin D supplementation on influenza and other lung infections, other studies have reported neutral [ 92 ] or even negative results [ 93 ] of vitamin D supplementation on the outcomes of lung infections. It is not clear if this discrepancy is due to methodological issues [low vitamin D supplementation [ 92 ] or weak endpoints used (questionnaires on self-reported symptoms) [ 93 ]], characteristics of the study population, or are dependent on the baseline vitamin D status. For instance, in another RCT conducted by Urashima et al. investigating the effects of vitamin D supplementation during the 2009 H1N1 pandemic, they showed that subjects in the vitamin D group (2000 IU/day) had a lower incidence of influenza A or B compared with the placebo group during the first month of intervention, whereas there was a higher incidence of infection during the second month [ 94 ]. It would be tempting to hypothesize that at the beginning of the intervention, vitamin D levels were low, allowing the treatment to show a positive protective effect, whereas once vitamin D levels were restored, the vitamin D had no impact in the prevention of infection. Unfortunately, in this study, serum levels of 25(OH)D were not measured, which could have explained the reasons for this difference at the two time periods, and thus this suggestion is speculative. A meta-analysis of 25 RCTs (including a total of 10,933 participants) supports the protective effects of vitamin D on acute lung infections. More specifically, vitamin D supplementation reduced the risk of acute respiratory infections among all participants [adjusted OR (aOR) 0.88; 95% CI 0.81–0.96; heterogeneity p < 0.001]. Importantly, the protective effects were seen in individuals receiving daily or weekly vitamin D (aOR 0.81;95% CI 0.72–0.91), but not in those receiving bolus doses (aOR 0.97; 95% CI 0.86–1.10; p = 0.05). Moreover, among subjects receiving daily or weekly vitamin D, protective effects of vitamin D were stronger in those who baseline 25(OH)D concentrations < 25 nmol/l (aOR 0.30; 95% CI 0.17–0.53) compared with those with baseline 25(OH)D ≥ 25 nmol/L (aOR 0.75; 95% CI 0.60–0.95; p for interaction = 0.006) [ 95 ]. In a more recent meta-analysis by the same group, including data from 43 RCTs and a total of 48,488 participants, the protective effect of vitamin D supplementation when given using a daily dosing regimen, at daily dose equivalents of 400–1000 IU on acute respiratory infections was confirmed [ 96 ].
3.4 Tuberculosis
Vitamin D was used in the pre-antibiotic era for the treatment of patients with tuberculosis (TB), when the ancient Greeks had first introduced “heliotherapy” (i.e., sunlight exposure) to treat TB [ 97 ]. Moreover, in preclinical studies, it has been shown that 1,25(OH) 2 D induces antimycobacterial activity in vitro in monocytes and macrophages [ 98 , 99 ]. However, recent controlled trials and meta-analyses have produced either minimal or null effects in a variety of TB-associated outcomes. A systematic review showed that serum vitamin D levels are not associated with the incidence of latent tuberculosis infection [ 100 ]. As the authors pointed out, different 25(OH)D assays were used in the studies included, which have differences in their sensitivity and precision, and that may have affected the results of the meta-analysis. In a RCT on TB contacts, it was shown that a single dose of 2.5 mg vitamin D (i.e., 100,000 IU) suppressed recombinant Mycobacterium growth through Bacillus Calmette–Guérin (BCG)-lux analysis at 24 h but not at 96 h, suggesting improved innate but unmodified acquired immunity against mycobacteria compared with placebo [ 101 ]. In a large RCT on children with a negative Quantiferon test at randomization, supplementation with a weekly dose of 14,000 IU vitamin D for 3 years did not result in a lower risk of tuberculosis infection, tuberculosis disease, or acute respiratory infection compared with placebo [ 102 ]. Finally, in the, thus far, largest meta-analysis investigating the effects of vitamin D supplementation on patients with pulmonary TB, vitamin D supplementation resulted in an increase in lymphocyte count, an improvement in chest radiography (mean number of zones involved), and an increased proportion of sputum smear and culture conversion. On the contrary, compared with placebo, vitamin D yielded null effects on time to sputum smear and culture conversion, and on mortality [ 103 ].
3.5 COVID-19
Considering the previous implications of vitamin D in acute respiratory tract infections, soon after the outbreak of the COVID-19 pandemic the research community started investigating whether vitamin D supplementation may have an impact in preventing infection with Severe acute respiratory syndrome coronavirus (SARS-COV2), or on the severity of COVID-19. This was especially important at the beginning of the pandemic when the medical community had almost no treatments in the fight against COVID-19.
Mechanisms Several mechanisms have been proposed through which vitamin D could offer protection against COVID-19. First, by regulating the innate immune response, vitamin D induces the production of the antimicrobial peptides cathelicidin (or LL-37) and β defensin, blocking the viral entry into cells [ 104 ]. Because of the actions of vitamin D on the adaptive immune system, and specifically the shift away from a proinflammatory state, it reduces the risk of cytokine storm, which is particularly detrimental in severe cases of COVID-19 [ 105 ]. Finally, through regulation of the renin–angiotensin–aldosterone system (RAAS), it suppresses the angiotensin converting enzyme (ACE) while it induces ACE2, leading to a reduction of angiotensin 2 and an increase in angiotensin 1–7. These enzymatic changes restore the ACE: ACE2 imbalance induced by SARS-CoV-2 infection and reduce the risk of vasoconstriction and acute respiratory distress syndrome (ARDS) [ 105 ].
Observational studies have shown that patients with VDD have an increased risk for COVID-19 [ 106 ], and in the, thus far, largest observational study, we have shown that vitamin D insufficiency or deficiency is associated with a 2.3–3.6 times higher risk of severe COVID-19, necessitating hospital admission [ 107 ].
A small, nonrandomized study showed that administration of high doses of vitamin D before SARS-CoV-2 infection was associated with less severe COVID-19 and better survival in older frail patients [ 108 ]. Castillo and colleagues performed a pilot study on 76 consecutive patients hospitalized for COVID-19 [ 109 ]. Patients at admission and on top of optimal medical treatment were randomized in a 2:1 ratio to receive or not high doses of calcifediol. It was shown that calcifediol supplementation significantly reduced the need for intensive care unit (ICU) treatment [ 109 ]. On the contrary, Murai and colleagues randomized 240 subjects to receive either a 200,000 IU vitamin D bolus or placebo. Mean time lag from symptom onset to randomization was relatively long (i.e., mean of 10.3 days). They found that there was no difference in in-hospital stay length, mortality, admission to ICU, or need for mechanical ventilation between the vitamin D and placebo groups [ 110 ]. These (negative) results were also confirmed in a post hoc analysis involving only patients with VDD at baseline ( N = 115) [ 110 ]. In a systematic meta-analysis of our group, including data from nine studies and a total of 2078 patients, we found that vitamin D supplementation was associated with a significant reduction in the need for ICU admission, whereas vitamin D supplementation did not confer protection from COVID-19 mortality [ 111 ]. These results are essentially in line with a previous meta-analysis conducted by Shah et al., which was performed earlier and thus had a smaller sample size ( N = 532) of COVID-19 patients [ 112 ]. Moreover, in our study we performed a meta-regression analysis to identify the effect of dose supplementation; although no significant relationship was found between the dose of supplementation and either severity of disease or mortality, it was shown that low versus high vitamin D supplementation protected from severe disease requiring admission to ICU [ 111 ].
In the systematic review and meta-analysis by Pal et al. [ 113 ] including data from 13 studies, it was shown that supplementation with vitamin D was associated with improved clinical outcomes in COVID-19 (including mortality) patients, especially when vitamin D is administered in patients after the diagnosis of COVID-19. Based on this finding, the authors suggested that vitamin D can be used as a potential treatment addition in patients with COVID-19. However, it should be noted that in their analysis, only three studies were included where vitamin D supplementation was given before COVID-19 diagnosis [ 113 ]. Overall, the discrepancies in the results of vitamin D supplementation on COVID-19 outcomes may have been affected by relatively small sample sizes, and patient’ heterogeneity.
In a recently published phase 3 RCT (CORONAVIT) the investigators assessed the effect of vitamin D supplementation for 6 months on the incidence of all-cause acute respiratory tract infection and COVID-19 [ 114 ]. In this study, a test-and-treat approach was selected in which only subjects with 25(OH)D levels < 75 mmol/L were enrolled to receive low (800 IU/day) or high (3200 IU/day) vitamin D supplementation, and were compared with subjects who were not offered vitamin D supplementation (in the intention to treat, N = 1515, 1515, and 2949 for the low dose, high dose, and no supplementation, respectively). It was found that correction of suboptimal vitamin D levels with either supplementation dose was not associated with a reduction in risk of all-cause acute respiratory tract infection or infection from COVID-19 [ 114 ].
3.6 Type 2 Diabetes (T2D)
Mechanisms Preclinical studies have shown that vitamin D may modulate β-cell growth and differentiation, enhance insulin secretion [ 115 , 116 ], increase the expression of the insulin receptor [ 117 ], and enhance insulin-mediated glucose transport [ 118 ].
However, studies in humans assessing the effect of vitamin D supplementation on insulin secretion and insulin action with gold standard methods have not confirmed these findings. More specifically, in the Tromsö study, a case-control and RCT study, 104 nondiabetic subjects with low serum 25(OH)D levels at baseline were randomized to receive either 20,000 IU twice weekly or placebo. A hyperglycemic clamp was performed at baseline and 6 months after treatment, showing that vitamin D supplementation did not increase first- or second-phase insulin secretion, or insulin sensitivity (assessed as the insulin sensitivity index, ISI) compared with placebo [ 119 ]. Similar null effects of vitamin D on insulin secretion (assessed with the intravenous glucose tolerance test, IVGTT) were reported after 3 months of vitamin D supplementation on nondiabetic subjects with low baseline 25(OH)D receiving 50,000 IU/week compared with placebo [ 120 ]. These results were confirmed in a meta-analysis that included 12 RCTs and a total of 1181 participants with BMI > 23 kg/m 2 . It was shown that vitamin D supplementation did not modify whole-body insulin sensitivity (assessed by the HOMA-IR)[ 121 ]. Of note, tissue-specific insulin sensitivity may also be assessed using fluorodeoxyglucose positron emission tomography studies in conjunction with a euglycemic hyperinsulinemic clamp [ 122 , 123 , 124 , 125 ], but to the best of our knowledge, thus far, it has not been assessed whether there is any correlation between the vitamin D status and tissue-specific insulin sensitivity, or whether vitamin D supplementation may affect tissue-specific insulin sensitivity.
Several association studies have shown an inverse association among serum 25(OH)D levels and fasting glucose [ 126 , 127 ], glycated hemoglobin (HbA 1c ) 1c [ 128 ], insulin resistance, and prevalence of T2D [ 129 ].
In a large RCT in patients with prediabetes at a high risk of progression to T2D, supplementation with 4000 IU/day of vitamin D led to a nonsignificant tendency to slower progression to T2D compared with placebo. However, in a post hoc analysis on patients without obesity, severe vitamin D deficiency at baseline and excellent adherence to treatment during the intervention period, a significant effect in decreasing the progression to T2D was seen [ 130 ]. This finding was confirmed in two recent systematic reviews and meta-analyses. In a meta-analysis by Barbarawi et al., data from nine RCTs and a total of 43,559 patients were assessed. While in the whole population vitamin D supplementation did not affect the incidence of T2D, post hoc analyses according to the vitamin D dosage showed that subjects receiving ≥ 1000 IU/day had significantly lower incidence of T2D (RR 0.88; 95% CI, 0.79–0.99; p = 0.03). Moreover, patients without obesity who received high-dose treatment had a lower relative risk of T2D (RR 0.68; 95% CI 0.53–0.89; p = 0.005), while no benefit was seen in patients with obesity [ 131 ]. In the study by Zhang et al. analyzing data of eight RCTS and 4896 participants, vitamin D supplementation reduced the incidence of T2D (RR 0.89; 95% CI 0.80–1.00; p = 0.04). Similarly to the results of Barbarawi et al., subgroup analyses showed that vitamin D supplementation lowered the risk of new-onset T2D only among non-obese patients, whereas a difference with respect to dose received was not reported [ 132 ]. The authors also reported that from five trials in 1080 participants, reversion from prediabetes to normoglycemia was significantly increased by vitamin D supplementation (RR 1.48; 95% CI 1.14–1.92) [ 132 ].
The effect of vitamin D supplementation on glycemic control in patients with T2D has also been assessed. Wu et al. assessed 24 studies; supplementation of vitamin D improved HbA 1c levels [standardized mean difference (SMD) −0.25 (−0.45 to −0.05)] and this effect was larger among patients with vitamin D deficiency at baseline [SMD −0.39 (−0.67 to −0.10)] and in patients with BMI < 30 kg/m 2 [SMD −0.30 (−0.54 to −0.07)] [ 133 ]. On the contrary, a subsequent systematic review and meta-analysis by Li and colleagues showed that vitamin D supplementation did not influence fasting blood glucose, HbA 1c , or fasting insulin levels, whereas HOMA-IR (i.e., an index of insulin resistance) was improved [ 134 ].
There is also evidence that VDD is associated with gestational diabetes mellitus (GDM). In a meta-analysis including seven observational studies and a total of 2146 subjects, of whom 433 developed GDM, it was shown that 25(OH)D levels < 50 nmol/L were associated with development of GDM (OR 1.61; 95% CI 1.19–2.17; p = 0.002) [ 135 ]. In a recent systematic review and meta-analysis in a small number of women, supplementation with 2000 IU of vitamin D per day did not affect the incidence of GDM compared with placebo ( N = 95 on vitamin D and N = 88 placebo). However, in seven studies including a total of 1722 women comparing the effect of vitamin D supplementation > 2000 IU/day and ≤ 2000 IU/day, it was shown that the incidence of GDM was reduced in the group receiving > 2000 IU of vitamin D per day (RR = 0.70; 95% CI 0.51–0.95; p = 0.02) [ 136 ].
3.7 Diabetic Neuropathy and Diabetic Foot Ulcers (DFU)
Mechanisms The role of vitamin D in the function of peripheral nervous system has not been extensively studied [ 137 ]. Studies have suggested that vitamin D may be involved in pain perception [ 138 ] and that it can induce nerve-growth factor synthesis in human cell lines [ 139 ]. Low vitamin D levels have been also reported to impair the differentiation and proliferation of keratinocytes and skin fibroblasts, and to delay DFU healing [ 140 , 141 , 142 ]. Vitamin D has been shown to induce production of antimicrobial peptides in keratinocyte cells from DFU [ 143 ]. Preclinical studies have shown that topical application of vitamin D promotes wound healing in a dose-dependent manner [ 144 ], and activates the expression of angiogenic molecules in keratinocytes and the migration of endothelial and keratinocyte cells in a diabetic foot ulceration model [ 145 ].
Studies have shown that VDD is associated with painful diabetic neuropathy, diabetic foot ulceration, and diabetic foot infections [ 146 , 147 , 148 ]. Two recent meta-analyses including a total of 1115 and 1644 patients with T2D showed that severe VDD [i.e., 25(OH) D < 10 ng/ml] is associated with increased risk of diabetic foot ulceration (OR 3.2; 95% CI 2.4–4.3 [ 149 ] and OR 3.6; 95% CI 2.9–4.4; p < 0.0001) [ 150 ], respectively. In a small RCT on 60 patients with grade 3 DFU according to the “Wagner–Meggit” criteria, patients were randomized to receive either 50,000 IU of vitamin D every 2 weeks or placebo for 12 weeks. Vitamin D supplementation was shown to reduce the ulcer length, width, depth, and erythema rate [ 151 ]. A later RCT compared high-dose vitamin D supplementation with 170 μg/day (i.e., 6800 IU) compared with low dose (20 μg/day, i.e., 800 IU) for 48 weeks of treatment. The intention-to-treat analysis showed that patients receiving high-dose supplementation had a higher rate of ulcer healing (70% versus 35%, p = 0.01, in the high versus low supplementation group) [ 152 ].
3.8 Neuroprotection
Mechanisms VDR and 1α-hydroxylase are expressed throughout the brain, and they are particularly highly expressed in the substantia nigra and in the hippocampus [ 153 , 154 ], two important regions for Parkinson’s disease and cognition, respectively. It has been suggested that vitamin D may confer neuroprotection through several mechanisms, including regulation of neurotrophic factors and of nerve growth, protection against cytotoxicity, and reduced oxidative stress [ 155 , 156 , 157 ]. Vitamin D has also been implicated in the regulation of acetylcholine and clearing of amyloid beta [ 158 ].
Considering the high expression of VDR and 1α-hydroxylase in substantia nigra, the impact of VDD on Parkinson’s disease has been studied, yielding conflicting results. In a large prospective study from Finland ( N = 3173), patients in the highest quartile for baseline serum vitamin D levels had a 65% lower risk of developing Parkinson’s disease than those in the lowest quartile, suggesting that lower levels of vitamin D in mid-life may increase the risk of Parkinson’s disease [ 159 ]. However, later studies in an even larger study sample in the USA failed to confirm this association [ 160 ].
The literature regarding vitamin D levels and Parkinson’s disease severity appears more consistent. Cross-sectional studies have consistently reported an association between vitamin D levels and the motor disability in Parkinson’s disease: the lower the serum vitamin D levels, the worse the motor function [ 161 , 162 ]. However, it is not clear whether vitamin D may modify the severity of the disease, or whether these associations are due to “inverse causality,” since patients suffering worse motor symptoms are also expected to move less and get lower sun exposure.
A small RCT assessed whether high-dose vitamin D supplementation (10,000 IU/day) for 4 months improved balance in patients with Parkinson’s disease compared with placebo. Even though, in the whole dataset, vitamin D supplementation seemed not to have any impact on balance, as measured by the sensory organization test, a post hoc analysis showed that supplementation with vitamin D in younger patients (52–66 years of age) improved balance compared with older participants [ 163 ].
With regard to cognitive function in the general population, whereas numerous studies have shown an association between low vitamin D levels and worse cognition [ 164 , 165 ], intervention studies have failed to show benefits from vitamin D supplementation [ 165 ].
The effects of VDD and vitamin D deficiency on multiple sclerosis (MS) have also been studied and they are presented in the chapter regarding autoimmunity.
Mechanisms Early studies have shown that 1,25(OH) 2 D analogs have potent antiproliferative and pro-differentiating effects on cancer cells in vitro [ 166 ]. Also, vitamin D decreases tumor invasiveness, angiogenesis, and metastatic propensity [ 167 , 168 ].
Systematic reviews and meta-analyses on the levels of vitamin D and mortality outcomes in cancer patients have shown that higher vitamin D levels are protective in a series of cancers such as breast cancer [ 169 ], colorectal cancer [ 170 ], prostate cancer [ 171 ], and hematological malignancies [ 172 ]. However, these promising data, based on observational studies, may be biased by a generally better health status and/or a healthier lifestyle (e.g., exercising with greater sunlight exposure) in the subjects who had higher levels of 25(OH)D.
In the large VITAL RCT ( N = 25,871), participants were randomized to receive 2000 IU of vitamin D or placebo daily, and omega-3 fatty acids or placebo in a two-by-two factorial design (for a median follow-up time of 5.3 years). Participants had no history of cancer (except nonmelanoma skin cancer) [ 63 ]. Supplementation with vitamin D did not significantly reduce the primary endpoint of total invasive cancer incidence (HR 0.96; 95% CI 0.88–1.06), but there was a trend for reducing total cancer mortality (HR 0.83; 95% CI 0.67–1.02) [ 63 ]. The authors then accounted for latency, and after excluding events within the first or second year of supplementation, the vitamin D intervention significantly decreased the risk of mortality (HR 0.79; 95% CI 0.63–0.99 after excluding the first and second year cases, respectively). The effect of vitamin D supplementation on cancer mortality was evident in the cumulative incidence curves at 4 years of supplementation. Interestingly, the authors also assessed whether baseline participants’ characteristics could affect the results of the supplementation, and found a significant interaction with BMI, with lean participants having a significant reduction in cancer risk (HR 0.76; 95% CI 0.63–0.90), whereas overweight and obese individuals did not [ 63 ].
Earlier RCTs have generally produced null effects of vitamin D supplementation on cancer-related risk reduction, but these studies were either smaller or had methodological problems (low vitamin D supplementation [ 173 , 174 ] or intermittent bolus dosing [ 175 , 176 ]). In a meta-analysis, also including the VITAL trial, the protective effect of vitamin D supplementation on cancer mortality was confirmed (HR 0.87; 95% CI 0.79–0.96), whereas there was no effect on cancer incidence (HR 0.98; 95% CI 0.93–1.03) [ 177 ].
3.10 Inflammatory Bowel Disease (IBD)
Mechanisms IL-10 knockout mice is an animal model used for the study of IBD; these animals spontaneously develop enterocolitis within 5–8 weeks of birth due to an uncontrolled immune response to resident intestinal flora [ 178 , 179 ]. People who have an IL-10 gene polymorphism also have an increased risk of developing colitis [ 180 ]. In the animal model, it has been shown that VDD exacerbates the symptoms of IBD and increases morbidity and mortality in the affected mice, whereas supplementation with vitamin D improves symptoms and reduces inflammation and mortality [ 181 ]. Patients suffering from IBD are at risk for VDD, since they often undergo small-bowel resection, and are treated with cholestyramine to control postresectional diarrhea caused by malabsorprion of bile acids. Both these factors contribute to bile acids loss, which are essential for vitamin D absorption [ 182 ]. It has been hypothesized that vitamin D supplementation may reduce inflammation in patients with IBD through decreasing intestinal permeability and increasing the levels of cathelicidin, a peptide that reduces inflammation and promotes healing [ 183 , 184 ].
A systematic review and meta-analysis on data from 900 IBD patients showed that VDD is a very prevalent condition in these patients, affecting 38.1% of patients with Crohn’s disease (CD) and 31.6% of patients with ulcerative colitis (UC) [ 185 ]. Moreover, in a recent systematic review and meta-analysis by Gubatan and colleagues, it was shown that low vitamin D levels were associated with increased odds of clinically active disease and increased odds of clinical relapse among all IBD patients and separately for both CD and UC [ 186 ]. Mucosal inflammation and quality of life were also assessed, and it was shown that among all patients, low 25(OH)D levels were associated with increased odds of mucosal inflammation and lower quality of life among all patients and in patients with CD, but not in patients with UC. As the authors argued for the quality of life in UC patients, results may have been underpowered due to the smaller sample size of patients with UC compared with CD in the included studies. On the contrary, the sample sizes were similar for UC and CD regarding the mucosal inflammation outcome, with the authors suggesting that vitamin D may play a specific role in the pathogenesis of CD, and also that VDD may be more suggestive of mucosal inflammation in CD since small bowel inflammation (thus affecting vitamin D absorption) is characteristic of CD but not of UC [ 186 ].
Some small studies have assessed the effect of vitamin D supplementation on clinical relapse based on validated scores, serum CRP levels, and quality of life, yielding conflicting results [ 183 , 187 ]. In a recent, relatively large RCT assessing the effect of vitamin D supplementation on the outcomes of CD using a more robust endpoint (i.e., endoscopic recurrence), 143 patients with CD who had recently undergone ileocecal or ileocolonic resection with ileocolonic anastomosis were randomized to receive 25,000 IU of vitamin D weekly compared with placebo. Even though serum vitamin D levels were doubled in the vitamin D group, the intervention did not affect endoscopic or clinical recurrence compared with placebo [ 188 ].
3.11 Autoimmune Disorders
Mechanisms Activation of the VDR by 1,25(OH) 2 D has been shown to inhibit the differentiation and proliferation of B and T helper lymphocytes, promoting a shift from an inflammatory to a more tolerant immune status [ 189 ]. Also, 1,25(OH) 2 D inhibits the production of proinflammatory Th1 cytokines while stimulating Th2 and regulatory T-cell activity [ 190 ]. Independent of VDR activation, 1,25(OH) 2 D and other vitamin D hydroxyl-metabolites can bind to RORa and RORg, and result in IL17 inhibition [ 191 , 192 ]. Both these pathways have been implicated in the protective role of vitamin D from autoimmune disorders. An acquired form of vitamin D resistance has also been hypothesized to play a role in the development of autoimmune disorders [ 193 ].
VDD has been described in a series of autoimmune disorders, comprising IBD (discussed in the paragraph above), rheumatoid arthritis, Sjogren’s disease, autoimmune thyroiditis, multiple sclerosis (MS), type 1 diabetes, and psoriasis [ 194 , 195 , 196 , 197 ]. In this paragraph, we will focus mainly on MS, since the effects of vitamin D on MS have been thoroughly investigated, and to the recent positive findings of the VITAL trial. Of particular interest is also the fact that the CYP27B1 gene, which codes for 25(OH)D 1α-hydroxylase, lies within a genomic region associated with MS, as shown in genome-wide association studies [ 198 ]. Indeed, evidence suggests a casual association between genetically induced VDD and increased risk of MS [ 199 , 200 ].
Several studies have thus shown that patients with MS have lower levels of 25(OH)D compared with healthy subjects [ 201 ], and this finding was confirmed in a 2014 systematic review and meta-analysis, including 11 studies and a total of 1007 patients and 829 healthy subjects [ 202 ].
Vitamin D has also been used in the treatment of MS, with investigators applying varying doses of vitamin D supplementation from low to extremely high doses. In particular, the “Coimbra protocol” is a protocol of very high doses of vitamin D supplementation, which was originally applied in patients with autoimmune skin disorders (psoriasis and vitiligo) [ 203 ]. This protocol has also been applied in MS, with supplementation of vitamin D as high as 1000 IU/kg of body weight per day [ 193 ]. A relatively recent systematic review and meta-analysis on the effects of vitamin D supplementation for the treatment of MS has yielded substantially negative results [ 204 ]. More specifically, McLaughlin and colleagues evaluated three outcome measures [annualized relapse rate, expanded disability status scale (EDSS) and new gadolinium-enhancing lesions]. Vitamin D supplementation did not improve any of the tested outcomes [ 204 ]. However, as the authors discussed in their article, there could be a potential clinically meaningful treatment effect in favor of vitamin D supplementation in the placebo-controlled studies, suggesting that more well-planned and placebo-controlled studies are needed. Of note, in this meta-analysis, high-dose vitamin D supplementation had a significantly worse outcome in terms of relapse rate compared with low dose [ 204 ].
In the VITAL trial (i.e., a randomized, double blind, placebo-controlled study with a two-by-two factorial design), the potential benefits of vitamin D supplementation with 2000 IU of cholecalciferol per day with or without of omega 3 fatty acids (1 g/day) on autoimmunity were assessed in 25,871 participants [ 22 ]. The mean age of the participants was 67 years. The impact on autoimmunity was assessed by the total confirmed incidence of autoimmune diseases during the 5 years of observation. In particular, annual questionnaires were filled in, inquiring for new onset of rheumatoid arthritis, polymyalgia reumatica, psoriasis, autoimmune thyroiditis, and IBD. They found that subjects on the vitamin D arm had decreased risk for new onset of autoimmune diseases by 22% compared with the placebo group (adjusted HR 0.78; 95% CI 0.61–0.99; p = 0.05). Moreover, after excluding the first 2 years of follow-up to evaluate the latency of the intervention effect, it was confirmed that vitamin D supplementation reduces the incidence of autoimmune diseases and the effect was even stronger (adjusted HR 0.61; 95% CI 0.43–0.86; p = 0.005). Of interest, in a prespecified subgroup analyses, a significant interaction between BMI and the effect of vitamin D supplementation was found with participants with lower BMI being more protected compared with subjects with obesity in whom vitamin D supplementation did not seem to reduce the incidence of autoimmune diseases (adjusted HR 0.47, 95% CI 0.29–0.77 for BMI 18 kg/m 2 ; adjusted HR 0.69, 95% CI 0.52–0.90 for BMI 25 kg/m 2 ; adjusted HR 0.90, 95% CI 0.69–1.19 for BMI 30 kg/m 2 ). Considering the important positive results of this trial, the follow-up period of this study has been extended and more results on the effects of vitamin D supplementation on the incidence of autoimmune diseases are expected.
4 Mendelian Randomization Studies
Bouillon and colleagues reviewed Mendelian randomization studies on the effects of genetically determined low 25(OH)D levels on a variety of conditions such as T2D, cancer, cardiovascular disease, COVID-19, and asthma, showing null effects [ 205 ]. Genetic VDD was shown to associate with increased risk for multiple sclerosis [ 205 ]. In a very recent study, the approach of Mendelian randomization was also used to assess the association of genetically determined 25(OH)D with mortality [ 206 ]. In this study, genetic data of 307,601 participants from the UK Biobank were analyzed, showing evidence of a causal relationship between genetically predicted 25(OH)D and all-mortality outcomes (all-cause, cancer, CVD, and respiratory). More specifically, an L-shaped relationship was described among all-cause mortality, cancer mortality, and CVD mortality with 25(OH)D levels, with the strongest association at concentrations below 25 nmol/L, while the association plateaued at 50 nmol/L. The association of respiratory mortality and 25(OH)D levels was linear [ 206 ].
5 Targets for Vitamin D Supplementation
Even though daily vitamin D requirements may be met through synthesis of vitamin D from 7-dehydrocholesterol in the skin after sunlight exposure, deficiency in vitamin D levels is a very common condition. Serum concentrations of 25(OH)D < 10 ng/ml (i.e., 25 nmol/L) are generally indicative of VDD, but the proposed target cut-offs of ideal vitamin D levels vary across organizations. According to the Endocrine Society Practice Guidelines on vitamin D, VDD is defined as a serum 25(OH)D < 20 ng/ml (i.e., 50 nmol/L), insufficiency as 21–29 ng/ml (i.e., 52.5–72.5 nmol/L), and sufficiency as at least 30 ng/ml (i.e., 75 nmol/L) for maximum musculoskeletal health [ 207 ]. These cut-offs have also been endorsed by other organizations such as the American Association for Clinical Endocrinologists, the American Geriatric Society, the National Osteoporosis Foundation, and the International Osteoporosis Foundation [ 208 ]. Whereas, according to the World Health Organization (WHO) and the current National Institute for Health and Clinical Care Excellence (NICE), UK guidelines, VDD is defined as a serum 25(OH)D < 10 ng/ml (i.e., 25 nmol/L) and insufficiency as 10–20 ng/ml (i.e., 25–50 nmol/L) [ 209 ].
More aggressive supplementation should be followed in the elderly and subjects with low exposure to sunshine (dark skinned people, people with poor exposure to sunlight due to cultural reasons, institutionalized patients) and poor nutrition. Despite general recommendations, clinicians should tailor vitamin D prescriptions accounting for several parameters, (obesity, nutritional status, diet, sunlight exposure) since one-size-fits-all recommendations of vitamin D supplementation are doomed to fail. For instance, it has been shown that patients with obesity require two to three times higher vitamin D supplementation to treat VDD [ 210 , 211 ]. Even though toxicity from vitamin D is extremely rare, as with all treatments, moderation is safer than exaggeration. Interestingly the clinical utility of these cut-offs has been confirmed in large studies on mortality. Apart from the recent Mendelian randomization study showing higher mortality at 25(OH)D levels below 25 nmol/L, with the association plateauing at the deficiency cut-off level (50 nmol/L) [ 206 ], similar results were also yielded from the institute of medicine. In this report, a J-curve was shown in the relationship between mortality and blood levels of 25(OH)D, with a significant decline in mortality when 25(OH)D approached 30 ng/mL and then a slight increase that was apparent at 50 ng/mL [ 212 ]. However, some authors have argued that the increased mortality seen for 25(OH)D > 50 ng/ml, may be attributed to previous long-standing VDD for which subjects were treated [ 213 ].
Still, despite apparent optimal per os supplementation, many subjects do not achieve normal vitamin D levels. Predictive equations to guide vitamin D replacement doses have been formulated, such as the one by Singh et al., proposing a formula that accounts for initial vitamin D levels, age, BMI, serum albumin concentration, and desired change in vitamin D levels to estimate the optimal and personalized dose of vitamin D replacement needed [ 214 ]. Whether the application of this formula corrects vitamin D levels has not been confirmed in large-scale clinical studies.
6 Discussion
Despite the pleiotropic actions of vitamin D, most RCTs on the effect of vitamin D supplementation on improving a disease outcome have been negative. There are several inherent difficulties in studying the effects of vitamin D supplementation using standard RCT designs, as discussed in chapter 1.2. Still, several other considerations can be made. First, to be able to modify the course of a chronic disease, long follow-up is needed. This was the purpose of the recent large VIDA and VITAL RCTs [ 23 , 176 ]. The mode of supplementation often varies, with some authors preferring intermittent bolus dosing and others daily dosing. Evidence suggests that intermittent bolus dosing (and generally extremely high dosing) should be avoided since it can even generate harmful events. Intermittent bolus dosing would also go against the ideal scenario in which there would be no fluctuations in the circulating levels of vitamin D, considering its multisystem homeostatic role. On the other hand larger doses (i.e., ~1000–2000 IU/day) should be preferred over too-small doses (i.e., ~400–800 IU/day) to expect any meaningful effect. Finally, it may be more appropriate in future investigations to compare subjects with high 25(OH)D vitamin levels with those not achieving normalization of vitamin D levels, rather than continue comparing groups based on the amount of vitamin D supplementation received.
Even though experts still debate the optimal cut-offs of 25(OH)D levels, it could be that these vary according to the disease of interest [ 215 ]. For instance, even though levels higher than 30 ng/ml are considered the target for maximum musculoskeletal health, it could be that this cut-off should be placed higher when vitamin D is given for its immunomodulation effects. Indeed, in the practice guidelines published in 2018 by Pludowski et al., 25(OH)D values in the range 30–50 ng/ml were recommended to achieve the pleiotropic actions of vitamin D and for optimal overall health [ 216 ]. Future studies should probably contemporaneously assess total and free 25(OH)D levels, as well as DBP and PTH values. This would be an important advancement in the planning of RCTs if we consider the studies by Carlberg and colleagues [ 217 ]. These investigators gave 0, 1600, or 3200 IU of vitamin D daily for 5 months to elderly prediabetic subjects. After assessing PTH response and other vitamin D biomarkers, they showed that 24% of their studied subjects were low responders, 51% mid responders, and 25% high responders [ 217 ], and similar rates were found also in healthy young individuals [ 218 ]. These studies set the groundwork, demonstrating that in humans in vivo, there is a spectrum of responsiveness to vitamin D supplementation, or a varying degree of vitamin D resistance.
7 Conclusions
The present narrative review provides an overview of the current evidence regarding the applications of vitamin D in a series of diseases. Despite the inherent difficulties in assessing the effects of vitamin D supplementation in RCTs, vitamin D supplementation has been shown to decrease acute respiratory infections, cancer mortality, and the incidence of T2D and autoimmune diseases. Moreover, subjects without obesity seem to benefit more from vitamin D supplementation, a finding that warrants further investigation. It also clearly emerges that VDD should be treated as it is associated with poor health outcomes and increased morbidity and mortality. However, vitamin D supplementation in vitamin D replete subjects does not seem to induce any clinically meaningful benefits. Considering that universal testing for vitamin D is not possible and is expensive, in everyday clinical practice it should be advisable to give vitamin D supplementation, which is cheap, well-tolerated, and easily available. In research settings, a holistic approach when studying the effects of vitamin D supplementation, such as evaluation of the whole vitamin D endocrine system, rather than only of 25(OH)D levels before and after treatment, the use of adequate and physiologic vitamin D dosing, controlling for the amount of vitamin D supplementation subjects on the placebo arms may receive, and sufficiently long follow-up are some aspects that need to be carefully considered in future studies.
Data Availability
Not applicable.
Naeem Z. Vitamin D deficiency—an ignored epidemic. Int J Health Sci (Qassim). 2010;4:V–VI.
Holick MF. The vitamin D deficiency pandemic: approaches for diagnosis, treatment and prevention. Rev Endocr Metab Disord Germ. 2017;18:153–65.
Article CAS Google Scholar
Hoseinzadeh E, Taha P, Wei C, Godini H, Ashraf GM, Taghavi M, et al. The impact of air pollutants, UV exposure and geographic location on vitamin D deficiency. Food Chem Toxicol Int J Publ Br Ind Biol Res Assoc England. 2018;113:241–54.
Saternus R, Vogt T, Reichrath J. A critical appraisal of strategies to optimize vitamin D status in Germany, a population with a western diet. Nutrients. Switzerland. 2019;11:2682.
Grønborg IM, Tetens I, Christensen T, Andersen EW, Jakobsen J, Kiely M, et al. Vitamin D-fortified foods improve wintertime vitamin D status in women of Danish and Pakistani origin living in Denmark: a randomized controlled trial. Eur J Nutr Germ. 2020;59:741–53.
Article Google Scholar
Jääskeläinen T, Itkonen ST, Lundqvist A, Erkkola M, Koskela T, Lakkala K, et al. The positive impact of general vitamin D food fortification policy on vitamin D status in a representative adult Finnish population: evidence from an 11-y follow-up based on standardized 25-hydroxyvitamin D data. Am J Clin Nutr United States. 2017;105:1512–20.
Pilz S, März W, Cashman KD, Kiely ME, Whiting SJ, Holick MF, et al. Rationale and plan for vitamin D food fortification: a review and guidance paper. Front Endocrinol (Lausanne). Switzerland; 2018;9:373.
Jones G. Pharmacokinetics of vitamin D toxicity. Am J Clin Nutr United States. 2008;88:582S-586S.
Tanaka Y, DeLuca HF. Stimulation of 1,25-dihydroxyvitamin D3 production by 1,25-dihydroxyvitamin D3 in the hypocalcaemic rat. Biochem J. 1983;214:893–7.
Article CAS PubMed PubMed Central Google Scholar
Bikle DD, Patzek S, Wang Y. Physiologic and pathophysiologic roles of extra renal CYP27b1: case report and review. Bone Rep United States. 2018;8:255–67.
Google Scholar
Saponaro F, Saba A, Zucchi R. An update on vitamin D metabolism. Int J Mol Sci. Switzerland. 2020;21:6573.
Beard JA, Bearden A, Striker R. Vitamin D and the anti-viral state. J Clin Virol Off Publ Pan Am Soc Clin Virol Netherlands. 2011;50:194–200.
Jones KS, Assar S, Harnpanich D, Bouillon R, Lambrechts D, Prentice A, et al. 25(OH)D2 half-life is shorter than 25(OH)D3 half-life and is influenced by DBP concentration and genotype. J Clin Endocrinol Metab. 2014;99:3373–81.
Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium. Dietary reference intakes for calcium and vitamin D. In: Ross AC, Taylor CL, Yaktine AL, et al., editors. Washington (DC): National Academies Press; 2011.
Tsuprykov O, Chen X, Hocher C-F, Skoblo R, Yin L, Hocher B. Why should we measure free 25(OH) vitamin D? J Steroid Biochem Mol Biol England. 2018;180:87–104.
Yuan C, Shui IM, Wilson KM, Stampfer MJ, Mucci LA, Giovannucci EL. Circulating 25-hydroxyvitamin D, vitamin D binding protein and risk of advanced and lethal prostate cancer. Int J Cancer. 2019;144:2401–7.
Article CAS PubMed Google Scholar
Qi L, Ma W, Heianza Y, Zheng Y, Wang T, Sun D, et al. Independent and synergistic associations of biomarkers of vitamin D status with risk of coronary heart disease. Arterioscler Thromb Vasc Biol. 2017;37:2204–12.
Yu C, Xue H, Wang L, Chen Q, Chen X, Zhang Y, et al. Serum bioavailable and free 25-hydroxyvitamin D levels, but not its total level, are associated with the risk of mortality in patients with coronary artery disease. Circ Res United States. 2018;123:996–1007.
Norman AW, Nemere I, Zhou LX, Bishop JE, Lowe KE, Maiyar AC, et al. 1,25(OH)2-vitamin D3, a steroid hormone that produces biologic effects via both genomic and nongenomic pathways. J Steroid Biochem Mol Biol England. 1992;41:231–40.
Zmijewski MA, Carlberg C. Vitamin D receptor(s): in the nucleus but also at membranes? Exp Dermatol Den. 2020;29:876–84.
Boucher BJ. Why do so many trials of vitamin D supplementation fail? Endocr Connect England. 2020;9:R195-206.
Hahn J, Cook NR, Alexander EK, Friedman S, Walter J, Bubes V, et al. Vitamin D and marine omega 3 fatty acid supplementation and incident autoimmune disease: VITAL randomized controlled trial. BMJ England. 2022;376:e066452.
LeBoff MS, Chou SH, Murata EM, Donlon CM, Cook NR, Mora S, et al. Effects of supplemental vitamin D on bone health outcomes in women and men in the VITamin D and OmegA-3 TriaL (VITAL). J Bone Miner Res Off J Am Soc Bone Miner Res. 2020;35:883–93.
Chalcraft JR, Cardinal LM, Wechsler PJ, Hollis BW, Gerow KG, Alexander BM, et al. Vitamin D synthesis following a single bout of sun exposure in older and younger men and women. Nutrients. Switzerland. 2020;12:2237.
Vranić L, Mikolašević I, Milić S. Vitamin D deficiency: consequence or cause of obesity? Medicina (Kaunas). Switzerland. 2019;55:541.
Bedner M, Lippa KA, Tai SS-C. An assessment of 25-hydroxyvitamin D measurements in comparability studies conducted by the Vitamin D Metabolites Quality Assurance Program. Clin Chim Acta. Netherlands. 2013;426:6–11.
Lappe JM, Heaney RP. Why randomized controlled trials of calcium and vitamin D sometimes fail. Dermatoendocrinol United States. 2012;4:95–100.
van Driel M, van Leeuwen JPTM. Vitamin D and bone: a story of endocrine and auto/paracrine action in osteoblasts. Nutrients. Switzerland. 2023;15:480.
Amling M, Priemel M, Holzmann T, Chapin K, Rueger JM, Baron R, et al. Rescue of the skeletal phenotype of vitamin D receptor-ablated mice in the setting of normal mineral ion homeostasis: formal histomorphometric and biomechanical analyses. Endocrinology United States. 1999;140:4982–7.
CAS Google Scholar
Dardenne O, Prud’homme J, Hacking SA, Glorieux FH, St-Arnaud R. Correction of the abnormal mineral ion homeostasis with a high-calcium, high-phosphorus, high-lactose diet rescues the PDDR phenotype of mice deficient for the 25-hydroxyvitamin D-1alpha-hydroxylase (CYP27B1). Bone. United States; 2003;32:332–40.
Panda DK, Miao D, Bolivar I, Li J, Huo R, Hendy GN, et al. Inactivation of the 25-hydroxyvitamin D 1alpha-hydroxylase and vitamin D receptor demonstrates independent and interdependent effects of calcium and vitamin D on skeletal and mineral homeostasis. J Biol Chem United States. 2004;279:16754–66.
Bouillon R, Antonio L. Nutritional rickets: Historic overview and plan for worldwide eradication. J Steroid Biochem Mol Biol England. 2020;198:105563.
Scragg R. The vitamin D Assessment (ViDA) study—design and main findings. J Steroid Biochem Mol Biol England. 2020;198:105562.
Burt LA, Billington EO, Rose MS, Raymond DA, Hanley DA, Boyd SK. Effect of high-dose vitamin D supplementation on volumetric bone density and bone strength: a randomized clinical trial. JAMA. 2019;322:736–45.
Suda T, Takahashi N, Abe E. Role of vitamin D in bone resorption. J Cell Biochem United States. 1992;49:53–8.
Rodan GA, Martin TJ. Role of osteoblasts in hormonal control of bone resorption—a hypothesis. Calcif Tissue Int United States. 1981;33:349–51.
Bolland MJ, Grey A, Avenell A. Effects of vitamin D supplementation on musculoskeletal health: a systematic review, meta-analysis, and trial sequential analysis. Lancet Diabetes Endocrinol England. 2018;6:847–58.
Weaver CM, Alexander DD, Boushey CJ, Dawson-Hughes B, Lappe JM, LeBoff MS, et al. Calcium plus vitamin D supplementation and risk of fractures: an updated meta-analysis from the National Osteoporosis Foundation. Osteoporos Int J Establ Result Coop Betw Eur Found Osteoporos Natl Osteoporos Found USA. 2016;27:367–76.
Kalyani RR, Corriere M, Ferrucci L. Age-related and disease-related muscle loss: the effect of diabetes, obesity, and other diseases. Lancet Diabetes Endocrinol. 2014;2:819–29.
Article PubMed PubMed Central Google Scholar
Bignotti B, Cadoni A, Martinoli C, Tagliafico A. Imaging of skeletal muscle in vitamin D deficiency. World J Radiol. 2014;6:119–24.
Beaudart C, Buckinx F, Rabenda V, Gillain S, Cavalier E, Slomian J, et al. The effects of vitamin D on skeletal muscle strength, muscle mass, and muscle power: a systematic review and meta-analysis of randomized controlled trials. J Clin Endocrinol Metab United States. 2014;99:4336–45.
Scott D, Stuart AL, Kay D, Ebeling PR, Nicholson G, Sanders KM. Investigating the predictive ability of gait speed and quadriceps strength for incident falls in community-dwelling older women at high risk of fracture. Arch Gerontol Geriatr Netherlands. 2014;58:308–13.
Murad MH, Elamin KB, Abu Elnour NO, Elamin MB, Alkatib AA, Fatourechi MM, et al. Clinical review: the effect of vitamin D on falls: a systematic review and meta-analysis. J Clin Endocrinol Metab United States. 2011;96:2997–3006.
Bischoff-Ferrari HA, Dawson-Hughes B, Willett WC, Staehelin HB, Bazemore MG, Zee RY, et al. Effect of vitamin D on falls: a meta-analysis. JAMA United States. 2004;291:1999–2006.
Guo J-L, Tsai Y-Y, Liao J-Y, Tu H-M, Huang C-M. Interventions to reduce the number of falls among older adults with/without cognitive impairment: an exploratory meta-analysis. Int J Geriatr Psychiatry England. 2014;29:661–9.
Kärkkäinen MK, Tuppurainen M, Salovaara K, Sandini L, Rikkonen T, Sirola J, et al. Does daily vitamin D 800 IU and calcium 1000 mg supplementation decrease the risk of falling in ambulatory women aged 65–71 years? A 3-year randomized population-based trial (OSTPRE-FPS). Maturitas Irel. 2010;65:359–65.
Glendenning P, Zhu K, Inderjeeth C, Howat P, Lewis JR, Prince RL. Effects of three-monthly oral 150,000 IU cholecalciferol supplementation on falls, mobility, and muscle strength in older postmenopausal women: a randomized controlled trial. J Bone Miner Res Off J Am Soc Bone Miner Res United States. 2012;27:170–6.
Sanders KM, Stuart AL, Williamson EJ, Simpson JA, Kotowicz MA, Young D, et al. Annual high-dose oral vitamin D and falls and fractures in older women: a randomized controlled trial. JAMA United States. 2010;303:1815–22.
Bolland MJ, Grey A, Gamble GD, Reid IR. The effect of vitamin D supplementation on skeletal, vascular, or cancer outcomes: a trial sequential meta-analysis. Lancet Diabetes Endocrinol England. 2014;2:307–20.
Bolland MJ, Grey A, Gamble GD, Reid IR. Vitamin D supplementation and falls: a trial sequential meta-analysis. Lancet Diabetes Endocrinol England. 2014;2:573–80.
Yuan W, Pan W, Kong J, Zheng W, Szeto FL, Wong KE, et al. 1,25-Dihydroxyvitamin D3 suppresses renin gene transcription by blocking the activity of the cyclic AMP response element in the renin gene promoter. J Biol Chem United States. 2007;282:29821–30.
Li YC. Molecular mechanism of vitamin D in the cardiovascular system. J Investig Med Off Publ Am Fed Clin Res. 2011;59:868–71.
Chen S, Sun Y, Agrawal DK. Vitamin D deficiency and essential hypertension. J Am Soc Hypertens. 2015;9:885–901.
Chen S, Gemelga G, Yeghiazarians Y. Is vitamin D supplementation an effective treatment for hypertension? Curr Hypertens Rep. United States. 2022.
Bernini G, Carrara D, Bacca A, Carli V, Virdis A, Rugani I, et al. Effect of acute and chronic vitamin D administration on systemic renin angiotensin system in essential hypertensives and controls. J Endocrinol Investig Italy. 2013;36:216–20.
Forman JP, Scott JB, Ng K, Drake BF, Suarez EG, Hayden DL, et al. Effect of vitamin D supplementation on blood pressure in blacks. Hypertens (Dallas, Tex 1979). 2013;61:779–85.
Bricio-Barrios JAR, Palacios-Fonseca AJMS, Del Toro-Equihua M, Sanchez-Ramirez CA. Effect of calcitriol supplementation on blood pressure in older adults. J Nutr Gerontol Geriatr. United States. 2016;35:243–52.
Sheikh V, Mozaianimonfared A, Gharakhani M, Poorolajal J, Ph D. Effect of vitamin D supplementation versus placebo on essential hypertension in patients with vitamin D deficiency: a double-blind randomized clinical trial. J Clin Hypertens (Greenwich). 2020;22:1867–73.
Witham MD, Ireland S, Houston JG, Gandy SJ, Waugh S, Macdonald TM, et al. Vitamin D therapy to reduce blood pressure and left ventricular hypertrophy in resistant hypertension: randomized, controlled trial. Hypertens (Dallas, Tex 1979) United States. 2014;63:706–12.
Wang J, Zhou JJ, Robertson GR, Lee VW. Vitamin D in vascular calcification: a double-edged sword? Nutrients. 2018;10:652.
Jorde R, Sneve M, Torjesen P, Figenschau Y. No improvement in cardiovascular risk factors in overweight and obese subjects after supplementation with vitamin D3 for 1 year. J Intern Med England. 2010;267:462–72.
Carrara D, Bernini M, Bacca A, Rugani I, Duranti E, Virdis A, et al. Cholecalciferol administration blunts the systemic renin-angiotensin system in essential hypertensives with hypovitaminosis D. J Renin Angiotensin Aldosterone Syst England. 2014;15:82–7.
Manson JE, Cook NR, Lee I-M, Christen W, Bassuk SS, Mora S, et al. Vitamin D supplements and prevention of cancer and cardiovascular disease. N Engl J Med. 2019;380:33–44.
Bischoff-Ferrari HA, Vellas B, Rizzoli R, Kressig RW, da Silva JAP, Blauth M, et al. Effect of vitamin D supplementation, omega-3 fatty acid supplementation, or a strength-training exercise program on clinical outcomes in older adults: the DO-HEALTH randomized clinical trial. JAMA. 2020;324:1855–68.
Challoumas D, Stavrou A, Pericleous A, Dimitrakakis G. Effects of combined vitamin D–calcium supplements on the cardiovascular system: should we be cautious? Atherosclerosis Ireland. 2015;238:388–98.
Davies MR, Hruska KA. Pathophysiological mechanisms of vascular calcification in end-stage renal disease. Kidney Int United States. 2001;60:472–9.
Carvalho LSF, Sposito AC. Vitamin D for the prevention of cardiovascular disease: are we ready for that? Atherosclerosis Ireland. 2015;241:729–40.
Wang J-H, Keisala T, Solakivi T, Minasyan A, Kalueff AV, Tuohimaa P. Serum cholesterol and expression of ApoAI, LXRbeta and SREBP2 in vitamin D receptor knock-out mice. J Steroid Biochem Mol Biol England. 2009;113:222–6.
Christensen R, Lorenzen JK, Svith CR, Bartels EM, Melanson EL, Saris WH, et al. Effect of calcium from dairy and dietary supplements on faecal fat excretion: a meta-analysis of randomized controlled trials. Obes Rev Off J Int Assoc Study Obes England. 2009;10:475–86.
Chowdhury R, Kunutsor S, Vitezova A, Oliver-Williams C, Chowdhury S, Kiefte-de-Jong JC, et al. Vitamin D and risk of cause specific death: systematic review and meta-analysis of observational cohort and randomised intervention studies. BMJ. 2014;348: g1903.
Schöttker B, Jorde R, Peasey A, Thorand B, Jansen EHJM, de Groot L, et al. Vitamin D and mortality: meta-analysis of individual participant data from a large consortium of cohort studies from Europe and the United States. BMJ. 2014;348: g3656.
Huang Y, Cai X, Mai W, Li M, Hu Y. Association between prediabetes and risk of cardiovascular disease and all cause mortality: systematic review and meta-analysis. BMJ England. 2016;355:i5953.
Zhang P, Guo D, Xu B, Huang C, Yang S, Wang W, et al. Association of serum 25-hydroxyvitamin D with cardiovascular outcomes and all-cause mortality in individuals with prediabetes and diabetes: results from the UK biobank prospective cohort study. Diabetes Care United States. 2022;45:1219–29.
Sluyter JD, Camargo CAJ, Stewart AW, Waayer D, Lawes CMM, Toop L, et al. Effect of monthly, high-dose, long-term vitamin D Supplementation on central blood pressure parameters: a randomized controlled trial substudy. J Am Heart Assoc. 2017;6:e006802.
Barbarawi M, Kheiri B, Zayed Y, Barbarawi O, Dhillon H, Swaid B, et al. Vitamin D supplementation and cardiovascular disease risks in more than 83 000 individuals in 21 randomized clinical trials: a meta-analysis. JAMA Cardiol. 2019;4:765–76.
Wang L, Manson JE, Song Y, Sesso HD. Systematic review: Vitamin D and calcium supplementation in prevention of cardiovascular events. Ann Intern Med United States. 2010;152:315–23.
Mao P-J, Zhang C, Tang L, Xian Y-Q, Li Y-S, Wang W-D, et al. Effect of calcium or vitamin D supplementation on vascular outcomes: a meta-analysis of randomized controlled trials. Int J Cardiol Netherlands. 2013;169:106–11.
Beveridge LA, Struthers AD, Khan F, Jorde R, Scragg R, Macdonald HM, et al. Effect of vitamin D supplementation on blood pressure: a systematic review and meta-analysis incorporating individual patient data. JAMA Intern Med. 2015;175:745–54.
Mirhosseini N, Rainsbury J, Kimball SM. Vitamin D supplementation, serum 25(OH)D concentrations and cardiovascular disease risk factors: a systematic review and meta-analysis. Front Cardiovasc Med. 2018;5:87.
Stoffels K, Overbergh L, Giulietti A, Verlinden L, Bouillon R, Mathieu C. Immune regulation of 25-hydroxyvitamin-D3-1alpha-hydroxylase in human monocytes. J Bone Miner Res Off J Am Soc Bone Miner Res United States. 2006;21:37–47.
L Bishop E, Ismailova A, Dimeloe S, Hewison M, White JH. Vitamin D and immune regulation: antibacterial, antiviral, anti-inflammatory. JBMR Plus. England. 2021;5:e10405.
Zehnder D, Bland R, Williams MC, McNinch RW, Howie AJ, Stewart PM, et al. Extrarenal expression of 25-hydroxyvitamin d(3)-1 alpha-hydroxylase. J Clin Endocrinol Metab United States. 2001;86:888–94.
Szymczak I, Pawliczak R. The active metabolite of vitamin D3 as a potential immunomodulator. Scand J Immunol England. 2016;83:83–91.
Helming L, Böse J, Ehrchen J, Schiebe S, Frahm T, Geffers R, et al. 1alpha,25-dihydroxyvitamin D3 is a potent suppressor of interferon gamma-mediated macrophage activation. Blood United States. 2005;106:4351–8.
Sundaram ME, Coleman LA. Vitamin D and influenza. Adv Nutr. 2012;3:517–25.
Bruce D, Ooi JH, Yu S, Cantorna MT. Vitamin D and host resistance to infection? Putting the cart in front of the horse. Exp Biol Med (Maywood). 2010;235:921–7.
Khare D, Godbole NM, Pawar SD, Mohan V, Pandey G, Gupta S, et al. Calcitriol [1, 25[OH]2 D3] pre- and post-treatment suppresses inflammatory response to influenza A (H1N1) infection in human lung A549 epithelial cells. Eur J Nutr Germ. 2013;52:1405–15.
Berry DJ, Hesketh K, Power C, Hyppönen E. Vitamin D status has a linear association with seasonal infections and lung function in British adults. Br J Nutr England. 2011;106:1433–40.
Aregbesola A, Voutilainen S, Nurmi T, Virtanen JK, Ronkainen K, Tuomainen T-P. Serum 25-hydroxyvitamin D3 and the risk of pneumonia in an ageing general population. J Epidemiol Community Health England. 2013;67:533–6.
Mamani M, Muceli N, Ghasemi Basir HR, Vasheghani M, Poorolajal J. Association between serum concentration of 25-hydroxyvitamin D and community-acquired pneumonia: a case-control study. Int J Gen Med. 2017;10:423–9.
Urashima M, Segawa T, Okazaki M, Kurihara M, Wada Y, Ida H. Randomized trial of vitamin D supplementation to prevent seasonal influenza A in schoolchildren. Am J Clin Nutr United States. 2010;91:1255–60.
Li-Ng M, Aloia JF, Pollack S, Cunha BA, Mikhail M, Yeh J, et al. A randomized controlled trial of vitamin D3 supplementation for the prevention of symptomatic upper respiratory tract infections. Epidemiol Infect England. 2009;137:1396–404.
Jorde R, Witham M, Janssens W, Rolighed L, Borchhardt K, de Boer IH, et al. Vitamin D supplementation did not prevent influenza-like illness as diagnosed retrospectively by questionnaires in subjects participating in randomized clinical trials. Scand J Infect Dis. 2012;44:126–32.
Urashima M, Mezawa H, Noya M, Camargo CAJ. Effects of vitamin D supplements on influenza A illness during the 2009 H1N1 pandemic: a randomized controlled trial. Food Funct England. 2014;5:2365–70.
Martineau AR, Jolliffe DA, Hooper RL, Greenberg L, Aloia JF, Bergman P, et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. 2017;356: i6583.
Jolliffe DA, Camargo CAJ, Sluyter JD, Aglipay M, Aloia JF, Ganmaa D, et al. Vitamin D supplementation to prevent acute respiratory infections: a systematic review and meta-analysis of aggregate data from randomised controlled trials. Lancet Diabetes Endocrinol England. 2021;9:276–92.
Martineau AR, Honecker FU, Wilkinson RJ, Griffiths CJ. Vitamin D in the treatment of pulmonary tuberculosis. J Steroid Biochem Mol Biol England. 2007;103:793–8.
Rook GA, Steele J, Fraher L, Barker S, Karmali R, O’Riordan J, et al. Vitamin D3, gamma interferon, and control of proliferation of Mycobacterium tuberculosis by human monocytes. Immunology. 1986;57:159–63.
CAS PubMed PubMed Central Google Scholar
Crowle AJ, Ross EJ, May MH. Inhibition by 1,25(OH)2-vitamin D3 of the multiplication of virulent tubercle bacilli in cultured human macrophages. Infect Immun. 1987;55:2945–50.
Cao Y, Wang X, Liu P, Su Y, Yu H, Du J. Vitamin D and the risk of latent tuberculosis infection: a systematic review and meta-analysis. BMC Pulm Med. 2022;22:39.
Martineau AR, Wilkinson RJ, Wilkinson KA, Newton SM, Kampmann B, Hall BM, et al. A single dose of vitamin D enhances immunity to mycobacteria. Am J Respir Crit Care Med United States. 2007;176:208–13.
Ganmaa D, Uyanga B, Zhou X, Gantsetseg G, Delgerekh B, Enkhmaa D, et al. Vitamin D supplements for prevention of tuberculosis infection and disease. N Engl J Med. 2020;383:359–68.
Wu H-X, Xiong X-F, Zhu M, Wei J, Zhuo K-Q, Cheng D-Y. Effects of vitamin D supplementation on the outcomes of patients with pulmonary tuberculosis: a systematic review and meta-analysis. BMC Pulm Med. 2018;18:108.
Bilezikian JP, Bikle D, Hewison M, Lazaretti-Castro M, Formenti AM, Gupta A, et al. Mechanisms in endocrinology: vitamin D and COVID-19. Eur J Endocrinol England. 2020;183:R133–47.
Charoenngam N, Shirvani A, Holick MF. Vitamin D and its potential benefit for the COVID-19 pandemic. Endocr Pract Off J Am Coll Endocrinol Am Assoc Clin Endocrinol. 2021;27:484–93.
Katz J, Yue S, Xue W. Increased risk for COVID-19 in patients with vitamin D deficiency. Nutrition. United States; 2021;84:111106.
Jude EB, Ling SF, Allcock R, Yeap BXY, Pappachan JM. Vitamin D deficiency is associated with higher hospitalization risk from COVID-19: a retrospective case-control study. J Clin Endocrinol Metab United States. 2021;106:e4708–15.
Annweiler G, Corvaisier M, Gautier J, Dubée V, Legrand E, Sacco G, et al. Vitamin D supplementation associated to better survival in hospitalized frail elderly COVID-19 patients: the GERIA-COVID quasi-experimental study. Nutrients. 2020;12:3377.
Entrenas Castillo M, Entrenas Costa LM, Vaquero Barrios JM, Alcalá Díaz JF, López Miranda J, Bouillon R, et al. Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: a pilot randomized clinical study. J Steroid Biochem Mol Biol. 2020;203: 105751.
Murai IH, Fernandes AL, Sales LP, Pinto AJ, Goessler KF, Duran CSC, et al. Effect of a single high dose of vitamin D3 on hospital length of stay in patients with moderate to severe COVID-19: a randomized clinical trial. JAMA. 2021;325:1053–60.
Tentolouris N, Samakidou G, Eleftheriadou I, Tentolouris A, Jude EB. The effect of vitamin D supplementation on mortality and intensive care unit admission of COVID-19 patients. A systematic review, meta-analysis and meta-regression. Diabetes Metab Res Rev. 2022;38:e3517.
Shah K, Saxena D, Mavalankar D. Vitamin D supplementation, COVID-19 and disease severity: a meta-analysis. QJM. 2021;114:175–81.
Pal R, Banerjee M, Bhadada SK, Shetty AJ, Singh B, Vyas A. Vitamin D supplementation and clinical outcomes in COVID-19: a systematic review and meta-analysis. J Endocrinol Investig. 2022;45:53–68.
Jolliffe DA, Holt H, Greenig M, Talaei M, Perdek N, Pfeffer P, et al. Effect of a test-and-treat approach to vitamin D supplementation on risk of all cause acute respiratory tract infection and covid-19: phase 3 randomised controlled trial (CORONAVIT). BMJ. England. 2022;378:e071230.
Takiishi T, Gysemans C, Bouillon R, Mathieu C. Vitamin D and diabetes. Endocrinol Metab Clin N Am United States. 2010;39:419–46 ( table of contents ).
Cade C, Norman AW. Vitamin D3 improves impaired glucose tolerance and insulin secretion in the vitamin D-deficient rat in vivo. Endocrinology United States. 1986;119:84–90.
Szymczak-Pajor I, Drzewoski J, Śliwińska A. The molecular mechanisms by which vitamin D prevents insulin resistance and associated disorders. Int J Mol Sci. Switzerland. 2020;21:6644.
Maestro B, Campión J, Dávila N, Calle C. Stimulation by 1,25-dihydroxyvitamin D3 of insulin receptor expression and insulin responsiveness for glucose transport in U-937 human promonocytic cells. Endocr J Jpn. 2000;47:383–91.
Grimnes G, Figenschau Y, Almås B, Jorde R, Vitamin D. insulin secretion, sensitivity, and lipids: results from a case-control study and a randomized controlled trial using hyperglycemic clamp technique. Diabetes. 2011;60:2748–57.
Mitchell DM, Leder BZ, Cagliero E, Mendoza N, Henao MP, Hayden DL, et al. Insulin secretion and sensitivity in healthy adults with low vitamin D are not affected by high-dose ergocalciferol administration: a randomized controlled trial. Am J Clin Nutr. 2015;102:385–92.
Jamka M, Woźniewicz M, Jeszka J, Mardas M, Bogdański P, Stelmach-Mardas M. The effect of vitamin D supplementation on insulin and glucose metabolism in overweight and obese individuals: systematic review with meta-analysis. Sci Rep England. 2015;5:16142.
Rebelos E, Mari A, Oikonen V, Iida H, Nuutila P, Ferrannini E. Evaluation of renal glucose uptake with [(18)F]FDG-PET: methodological advancements and metabolic outcomes. Metabolism. United States; 2023;141:155382.
Rebelos E, Bucci M, Karjalainen T, Oikonen V, Alessandra B, Hannukainen JC, et al. Insulin resistance is associated with enhanced brain glucose uptake during euglycemic hyperinsulinemia: a large-scale PET cohort. Diabetes Care. 2021;44:1–7.
Dadson P, Landini L, Helmiö M, Hannukainen JC, Immonen H, Honka MJ, et al. Effect of bariatric surgery on adipose tissue glucose metabolism in different depots in patients with or without type 2 diabetes. Diabetes Care. 2016;39:292–9.
Immonen H, Hannukainen JC, Iozzo P, Soinio M, Salminen P, Saunavaara V, et al. Effect of bariatric surgery on liver glucose metabolism in morbidly obese diabetic and non-diabetic patients. J Hepatol Netherlands. 2014;60:377–83.
Need AG, O’Loughlin PD, Horowitz M, Nordin BEC. Relationship between fasting serum glucose, age, body mass index and serum 25 hydroxyvitamin D in postmenopausal women. Clin Endocrinol (Oxf) England. 2005;62:738–41.
Forouhi NG, Luan J, Cooper A, Boucher BJ, Wareham NJ. Baseline serum 25-hydroxy vitamin D is predictive of future glycemic status and insulin resistance: the Medical Research Council Ely Prospective Study 1990–2000. Diabetes. 2008;57:2619–25.
Hyppönen E, Power C. Vitamin D status and glucose homeostasis in the 1958 British birth cohort: the role of obesity. Diabetes Care United States. 2006;29:2244–6.
Scragg R, Sowers M, Bell C. Serum 25-hydroxyvitamin D, diabetes, and ethnicity in the Third National Health and Nutrition Examination Survey. Diabetes Care United States. 2004;27:2813–8.
Dawson-Hughes B, Staten MA, Knowler WC, Nelson J, Vickery EM, LeBlanc ES, et al. Intratrial exposure to vitamin d and new-onset diabetes among adults with prediabetes: a secondary analysis from the vitamin D and type 2 diabetes (D2d) study. Diabetes Care. 2020;43:2916–22.
Barbarawi M, Zayed Y, Barbarawi O, Bala A, Alabdouh A, Gakhal I, et al. Effect of vitamin D supplementation on the incidence of diabetes mellitus. J Clin Endocrinol Metab. United States. 2020;105:dgaa335.
Zhang Y, Tan H, Tang J, Li J, Chong W, Hai Y, et al. Effects of vitamin D supplementation on prevention of type 2 diabetes in patients with prediabetes: a systematic review and meta-analysis. Diabetes Care United States. 2020;43:1650–8.
Wu C, Qiu S, Zhu X, Li L. Vitamin D supplementation and glycemic control in type 2 diabetes patients: a systematic review and meta-analysis. Metabolism United States. 2017;73:67–76.
Li X, Liu Y, Zheng Y, Wang P, Zhang Y. The effect of vitamin D supplementation on glycemic control in type 2 diabetes patients: a systematic review and meta-analysis. Nutrients. 2018;10:375.
Poel YHM, Hummel P, Lips P, Stam F, van der Ploeg T, Simsek S. Vitamin D and gestational diabetes: a systematic review and meta-analysis. Eur J Intern Med Netherlands. 2012;23:465–9.
Irwinda R, Hiksas R, Lokeswara AW, Wibowo N. Vitamin D supplementation higher than 2000 IU/day compared to lower dose on maternal-fetal outcome: systematic review and meta-analysis. Womens Health (Lond Engl). United States; 2022;18:17455057221111066.
Faye PA, Poumeaud F, Miressi F, Lia AS, Demiot C, Magy L, et al. Focus on 1,25-dihydroxyvitamin D3 in the peripheral nervous system. Front Neurosci. Switzerland; 2019;13:348.
Tague SE, Smith PG. Vitamin D receptor and enzyme expression in dorsal root ganglia of adult female rats: modulation by ovarian hormones. J Chem Neuroanat Netherlands. 2011;41:1–12.
Shehab D, Al-Jarallah K, Abdella N, Mojiminiyi OA, Al MH. Prospective evaluation of the effect of short-term oral vitamin d supplementation on peripheral neuropathy in type 2 diabetes mellitus. Med Princ Pract Int J Kuwait Univ Heal Sci Cent Switzerland. 2015;24:250–6.
Costa PLF, França MM, Katayama ML, Carneiro ET, Martin RM, Folgueira MAK, et al. Transcriptomic response to 1,25-dihydroxyvitamin D in human fibroblasts with or without a functional vitamin D receptor (VDR): novel target genes and insights into VDR basal transcriptional activity. Cells. 2019;8:318.
Ding J, Kwan P, Ma Z, Iwashina T, Wang J, Shankowsky HA, et al. Synergistic effect of vitamin D and low concentration of transforming growth factor beta 1, a potential role in dermal wound healing. Burns Netherlands. 2016;42:1277–86.
Dobak J, Grzybowski J, Liu FT, Landon B, Dobke M. 1,25-Dihydroxyvitamin D3 increases collagen production in dermal fibroblasts. J Dermatol Sci Netherlands. 1994;8:18–24.
Gonzalez-Curiel I, Trujillo V, Montoya-Rosales A, Rincon K, Rivas-Calderon B, deHaro-Acosta J, et al. 1,25-dihydroxyvitamin D3 induces LL-37 and HBD-2 production in keratinocytes from diabetic foot ulcers promoting wound healing: an in vitro model. PLoS ONE. 2014;9: e111355.
Tian XQ, Chen TC, Holick MF. 1,25-dihydroxyvitamin D3: a novel agent for enhancing wound healing. J Cell Biochem United States. 1995;59:53–6.
Trujillo V, Marín-Luevano P, González-Curiel I, Rodríguez-Carlos A, Ramírez-Reyes M, Layseca-Espinosa E, et al. Calcitriol promotes proangiogenic molecules in keratinocytes in a diabetic foot ulcer model. J Steroid Biochem Mol Biol England. 2017;174:303–11.
Alam U, Petropoulos IN, Ponirakis G, Ferdousi M, Asghar O, Jeziorska M, et al. Vitamin D deficiency is associated with painful diabetic neuropathy. Diabetes Metab Res Rev England. 2021;37:e3361.
Zubair M, Malik A, Meerza D, Ahmad J. 25-Hydroxyvitamin D [25(OH)D] levels and diabetic foot ulcer: is there any relationship? Diabetes Metab Syndr Netherlands. 2013;7:148–53.
Tiwari S, Pratyush DD, Gupta SK, Singh SK. Vitamin D deficiency is associated with inflammatory cytokine concentrations in patients with diabetic foot infection. Br J Nutr England. 2014;112:1938–43.
Dai J, Jiang C, Chen H, Chai Y. Vitamin D and diabetic foot ulcer: a systematic review and meta-analysis. Nutr Diabetes. 2019;9:8.
Yammine K, Hayek F, Assi C. Is there an association between vitamin D and diabetic foot disease? A meta-analysis. Wound Repair Regener Off Publ Wound Heal Soc Eur Tissue Repair Soc. United States. 2020;28:90–6.
Razzaghi R, Pourbagheri H, Momen-Heravi M, Bahmani F, Shadi J, Soleimani Z, et al. The effects of vitamin D supplementation on wound healing and metabolic status in patients with diabetic foot ulcer: a randomized, double-blind, placebo-controlled trial. J Diabetes Complic United States. 2017;31:766–72.
Halschou-Jensen PM, Sauer J, Bouchelouche P, Fabrin J, Brorson S, Ohrt-Nissen S. Improved healing of diabetic foot ulcers after high-dose vitamin D: a randomized double-blinded clinical trial. Int J Low Extrem Wounds. United States. 2021;15347346211020268.
Eyles DW, Smith S, Kinobe R, Hewison M, McGrath JJ. Distribution of the vitamin D receptor and 1 alpha-hydroxylase in human brain. J Chem Neuroanat Netherlands. 2005;29:21–30.
Gezen-Ak D, Dursun E, Yilmazer S. Vitamin D inquiry in hippocampal neurons: consequences of vitamin D-VDR pathway disruption on calcium channel and the vitamin D requirement. Neurol Sci Off J Ital Neurol Soc Ital Soc Clin Neurophysiol Italy. 2013;34:1453–8.
Cui X, Eyles DW. Vitamin D and the central nervous system: causative and preventative mechanisms in brain disorders. Nutrients. Switzerland. 2022;14:4353.
Menéndez SG, Martín Giménez VM, Holick MF, Barrantes FJ, Manucha W. COVID-19 and neurological sequelae: vitamin D as a possible neuroprotective and/or neuroreparative agent. Life Sci Netherlands. 2022;297:120464.
AlJohri R, AlOkail M, Haq SH. Neuroprotective role of vitamin D in primary neuronal cortical culture. eNeurologicalSci. Netherlands. 2019;14:43–8.
Mizwicki MT, Liu G, Fiala M, Magpantay L, Sayre J, Siani A, et al. 1α,25-dihydroxyvitamin D3 and resolvin D1 retune the balance between amyloid-β phagocytosis and inflammation in Alzheimer’s disease patients. J Alzheimers Dis. 2013;34:155–70.
Knekt P, Kilkkinen A, Rissanen H, Marniemi J, Sääksjärvi K, Heliövaara M. Serum vitamin D and the risk of Parkinson disease. Arch Neurol. 2010;67:808–11.
Shrestha S, Lutsey PL, Alonso A, Huang X, Mosley THJ, Chen H. Serum 25-hydroxyvitamin D concentrations in mid-adulthood and Parkinson’s disease risk. Mov Disord. 2016;31:972–8.
Chitsaz A, Maracy M, Basiri K, Izadi Boroujeni M, Tanhaei AP, Rahimi M, et al. 25-hydroxyvitamin d and severity of Parkinson’s disease. Int J Endocrinol. 2013;2013: 689149.
The Parkinson progression marker initiative (PPMI). Prog Neurobiol. 2011;95:629–35.
Hiller AL, Murchison CF, Lobb BM, O’Connor S, O’Connor M, Quinn JF. A randomized, controlled pilot study of the effects of vitamin D supplementation on balance in Parkinson’s disease: Does age matter? PLoS ONE. 2018;13: e0203637.
Peterson A, Mattek N, Clemons A, Bowman GL, Buracchio T, Kaye J, et al. Serum vitamin D concentrations are associated with falling and cognitive function in older adults. J Nutr Health Aging. 2012;16:898–901.
Anastasiou CA, Yannakoulia M, Scarmeas N. Vitamin D and cognition: an update of the current evidence. J Alzheimers Dis Netherlands. 2014;42(Suppl 3):S71-80.
Abe E, Miyaura C, Sakagami H, Takeda M, Konno K, Yamazaki T, et al. Differentiation of mouse myeloid leukemia cells induced by 1 alpha,25-dihydroxyvitamin D3. Proc Natl Acad Sci USA. 1981;78:4990–4.
Deeb KK, Trump DL, Johnson CS. Vitamin D signalling pathways in cancer: potential for anticancer therapeutics. Nat Rev Cancer England. 2007;7:684–700.
Feldman D, Krishnan AV, Swami S, Giovannucci E, Feldman BJ. The role of vitamin D in reducing cancer risk and progression. Nat Rev Cancer England. 2014;14:342–57.
Estébanez N, Gómez-Acebo I, Palazuelos C, Llorca J, Dierssen-Sotos T. Vitamin D exposure and risk of breast cancer: a meta-analysis. Sci Rep. 2018;8:9039.
Maalmi H, Walter V, Jansen L, Boakye D, Schöttker B, Hoffmeister M, et al. Association between blood 25-hydroxyvitamin D levels and survival in colorectal cancer patients: an updated systematic review and meta-analysis. Nutrients. 2018;10:896.
Song Z-Y, Yao Q, Zhuo Z, Ma Z, Chen G. Circulating vitamin D level and mortality in prostate cancer patients: a dose-response meta-analysis. Endocr Connect. 2018;7:R294-303.
Wang W, Li G, He X, Gao J, Wang R, Wang Y, et al. Serum 25-hydroxyvitamin D levels and prognosis in hematological malignancies: a systematic review and meta-analysis. Cell Physiol Biochem Int J Exp Cell Physiol Biochem Pharmacol Germ. 2015;35:1999–2005.
Brunner RL, Wactawski-Wende J, Caan BJ, Cochrane BB, Chlebowski RT, Gass MLS, et al. The effect of calcium plus vitamin D on risk for invasive cancer: results of the Women’s Health Initiative (WHI) calcium plus vitamin D randomized clinical trial. Nutr Cancer. 2011;63:827–41.
Avenell A, MacLennan GS, Jenkinson DJ, McPherson GC, McDonald AM, Pant PR, et al. Long-term follow-up for mortality and cancer in a randomized placebo-controlled trial of vitamin D(3) and/or calcium (RECORD trial). J Clin Endocrinol Metab United States. 2012;97:614–22.
Trivedi DP, Doll R, Khaw KT. Effect of four monthly oral vitamin D3 (cholecalciferol) supplementation on fractures and mortality in men and women living in the community: randomised double blind controlled trial. BMJ. 2003;326:469.
Scragg R, Khaw K-T, Toop L, Sluyter J, Lawes CMM, Waayer D, et al. Monthly high-dose vitamin d supplementation and cancer risk: a post hoc analysis of the vitamin D assessment randomized clinical trial. JAMA Oncol. 2018;4: e182178.
Keum N, Lee DH, Greenwood DC, Manson JE, Giovannucci E. Vitamin D supplementation and total cancer incidence and mortality: a meta-analysis of randomized controlled trials. Ann Oncol Off J Eur Soc Med Oncol. 2019;30:733–43.
Li S, Jin Y, Fu W, Cox AD, Lee D, Reddivari L. Intermittent antibiotic treatment accelerated the development of colitis in IL-10 knockout mice. Biomed Pharmacother France. 2022;146:112486.
Kühn R, Löhler J, Rennick D, Rajewsky K, Müller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell United States. 1993;75:263–74.
Liu M, Yuan W, Park S. Association between IL-10 rs3024505 and susceptibility to inflammatory bowel disease: a systematic review and meta-analysis. Cytokine England. 2022;149:155721.
Cantorna MT, Munsick C, Bemiss C, Mahon BD. 1,25-Dihydroxycholecalciferol prevents and ameliorates symptoms of experimental murine inflammatory bowel disease. J Nutr United States. 2000;130:2648–52.
Lim W-C, Hanauer SB, Li YC. Mechanisms of disease: vitamin D and inflammatory bowel disease. Nat Clin Pract Gastroenterol Hepatol England. 2005;2:308–15.
Raftery T, Martineau AR, Greiller CL, Ghosh S, McNamara D, Bennett K, et al. Effects of vitamin D supplementation on intestinal permeability, cathelicidin and disease markers in Crohn’s disease: results from a randomised double-blind placebo-controlled study. United Eur Gastroenterol J. 2015;3:294–302.
Zhao H, Zhang H, Wu H, Li H, Liu L, Guo J, et al. Protective role of 1,25(OH)2 vitamin D3 in the mucosal injury and epithelial barrier disruption in DSS-induced acute colitis in mice. BMC Gastroenterol. 2012;12:57.
Del Pinto R, Pietropaoli D, Chandar AK, Ferri C, Cominelli F. Association between inflammatory bowel disease and vitamin D deficiency: a systematic review and meta-analysis. Inflamm Bowel Dis. 2015;21:2708–17.
Article PubMed Google Scholar
Gubatan J, Chou ND, Nielsen OH, Moss AC. Systematic review with meta-analysis: association of vitamin D status with clinical outcomes in adult patients with inflammatory bowel disease. Aliment Pharmacol Ther England. 2019;50:1146–58.
Narula N, Cooray M, Anglin R, Muqtadir Z, Narula A, Marshall JK. Impact of high-dose vitamin D3 supplementation in patients with Crohn’s disease in remission: a pilot randomized double-blind controlled study. Dig Dis Sci United States. 2017;62:448–55.
de Bruyn JR, Bossuyt P, Ferrante M, West RL, Dijkstra G, Witteman BJ, et al. High-dose vitamin D does not prevent postoperative recurrence of Crohn’s Disease in a randomized placebo-controlled trial. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc United States. 2021;19:1573–1582.e5.
Murdaca G, Tonacci A, Negrini S, Greco M, Borro M, Puppo F, et al. Emerging role of vitamin D in autoimmune diseases: an update on evidence and therapeutic implications. Autoimmun Rev Netherlands. 2019;18:102350.
May E, Asadullah K, Zügel U. Immunoregulation through 1,25-dihydroxyvitamin D3 and its analogs. Curr Drug Targets Inflamm Allergy Netherlands. 2004;3:377–93.
Slominski AT, Kim T-K, Takeda Y, Janjetovic Z, Brozyna AA, Skobowiat C, et al. RORα and ROR γ are expressed in human skin and serve as receptors for endogenously produced noncalcemic 20-hydroxy- and 20,23-dihydroxyvitamin D. FASEB. 2014;28:2775–89.
Slominski AT, Kim T-K, Hobrath JV, Oak ASW, Tang EKY, Tieu EW, et al. Endogenously produced nonclassical vitamin D hydroxy-metabolites act as “biased” agonists on VDR and inverse agonists on RORα and RORγ. J Steroid Biochem Mol Biol. 2017;173:42–56.
Lemke D, Klement RJ, Schweiger F, Schweiger B, Spitz J. Vitamin D resistance as a possible cause of autoimmune diseases: a hypothesis confirmed by a therapeutic high-dose vitamin D protocol. Front Immunol. 2021;12: 655739.
Harrison SR, Li D, Jeffery LE, Raza K, Hewison M. Vitamin D, autoimmune disease and rheumatoid arthritis. Calcif Tissue Int United States. 2020;106:58–75.
Filoni A, Vestita M, Congedo M, Giudice G, Tafuri S, Bonamonte D. Association between psoriasis and vitamin D: duration of disease correlates with decreased vitamin D serum levels: an observational case-control study. Medicine (Baltimore). United States. 2018;97:e11185.
Kurtul BE, Özer PA, Aydinli MS. The association of vitamin D deficiency with tear break-up time and Schirmer testing in non-Sjögren dry eye. Eye (Lond) England. 2015;29:1081–4.
Vieira IH, Rodrigues D, Paiva I. Vitamin D and autoimmune thyroid disease-cause, consequence, or a vicious cycle? Nutrients. Switzerland. 2020;12:2791.
Genome-wide association study identifies new multiple sclerosis susceptibility loci on chromosomes 12 and 20. Nat Genet. United States. 2009;41:824–8.
Jacobs BM, Noyce AJ, Giovannoni G, Dobson R. BMI and low vitamin D are causal factors for multiple sclerosis: a Mendelian randomization study. Neurol Neuroimmunol Neuroinflamm. 2020;7:e662.
Rhead B, Bäärnhielm M, Gianfrancesco M, Mok A, Shao X, Quach H, et al. Mendelian randomization shows a causal effect of low vitamin D on multiple sclerosis risk. Neurol Genet. 2016;2: e97.
Munger KL, Levin LI, Hollis BW, Howard NS, Ascherio A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA United States. 2006;296:2832–8.
Duan S, Lv Z, Fan X, Wang L, Han F, Wang H, et al. Vitamin D status and the risk of multiple sclerosis: a systematic review and meta-analysis. Neurosci Lett Irel. 2014;570:108–13.
Finamor DC, Sinigaglia-Coimbra R, Neves LCM, Gutierrez M, Silva JJ, Torres LD, et al. A pilot study assessing the effect of prolonged administration of high daily doses of vitamin D on the clinical course of vitiligo and psoriasis. Dermatoendocrinology. 2013;5:222–34.
McLaughlin L, Clarke L, Khalilidehkordi E, Butzkueven H, Taylor B, Broadley SA. Vitamin D for the treatment of multiple sclerosis: a meta-analysis. J Neurol Germ. 2018;265:2893–905.
Bouillon R, Manousaki D, Rosen C, Trajanoska K, Rivadeneira F, Richards JB. The health effects of vitamin D supplementation: evidence from human studies. Nat Rev Endocrinol. 2022;18:96–110.
Sutherland JP, Zhou A, Hyppönen E. Vitamin D deficiency increases mortality risk in the UK biobank: a nonlinear mendelian randomization study. Ann Intern Med United States. 2022;175:1552–9.
Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab United States. 2011;96:1911–30.
Recommendations abstracted from the American Geriatrics Society consensus statement on vitamin D for prevention of falls and their consequences. J Am Geriatr Soc. United States. 2014;62:147–52.
Excellence NI for H and CC (NICE). Vitamin D deficiency in adults - treatment and prevention. https//cks.nice.org.uk/topics/vitamin-d-deficiency-inadults-Treat. Accessed 10 Aug 2022
Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr United States. 2000;72:690–3.
Boonchaya-anant P, Holick MF, Apovian CM. Serum 25-hydroxyvitamin D levels and metabolic health status in extremely obese individuals. Obesity (Silver Spring) United States. 2014;22:2539–43.
Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium. Dietary reference intakes for calcium and vitamin D. Washington DC: The National Academies Press; 2011.
Kroll MH, Bi C, Garber CC, Kaufman HW, Liu D, Caston-Balderrama A, et al. Temporal relationship between vitamin D status and parathyroid hormone in the United States. PLoS ONE. 2015;10: e0118108.
Singh G, Bonham AJ. A predictive equation to guide vitamin D replacement dose in patients. J Am Board Fam Med United States. 2014;27:495–509.
Grant WB, Al Anouti F, Boucher BJ, Dursun E, Gezen-Ak D, Jude EB, et al. A narrative review of the evidence for variations in serum 25-hydroxyvitamin D concentration thresholds for optimal health. Nutrients. Switzerland. 2022;14:639.
Pludowski P, Holick MF, Grant WB, Konstantynowicz J, Mascarenhas MR, Haq A, et al. Vitamin D supplementation guidelines. J Steroid Biochem Mol Biol England. 2018;175:125–35.
Saksa N, Neme A, Ryynänen J, Uusitupa M, de Mello VDF, Voutilainen S, et al. Dissecting high from low responders in a vitamin D3 intervention study. J Steroid Biochem Mol Biol England. 2015;148:275–82.
Seuter S, Virtanen JK, Nurmi T, Pihlajamäki J, Mursu J, Voutilainen S, et al. Molecular evaluation of vitamin D responsiveness of healthy young adults. J Steroid Biochem Mol Biol England. 2017;174:314–21.
Download references
Author information
Authors and affiliations.
Turku PET Centre, University of Turku, Turku, Finland
Eleni Rebelos
Institute of Clinical Physiology, National Research Council (CNR), Pisa, Italy
1st Department of Propaedeutic and Internal Medicine, Medical School, National and Kapodistrian University of Athens, Laiko General Hospital, Athens, Greece
Nikolaos Tentolouris
Department of Medicine, Tameside and Glossop Integrated Care NHS Foundation Trust, Ashton-under-Lyne , England
Edward Jude
University of Manchester, Manchester, UK
Manchester Metropolitan University, Manchester, UK
You can also search for this author in PubMed Google Scholar
Contributions
ER and EJ searched the literature and drafted the manuscript. NT revised critically the text. All authors approved the final version of the text.
Corresponding author
Correspondence to Edward Jude .
Ethics declarations
Conflict of interest.
Edward Jude, Eleni Rebelos and Nikolaos Tentolouris declare that they have no conflicts of interest.
Ethical approval
Not applicable
Informed consent
Rights and permissions.
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Rebelos, E., Tentolouris, N. & Jude, E. The Role of Vitamin D in Health and Disease: A Narrative Review on the Mechanisms Linking Vitamin D with Disease and the Effects of Supplementation. Drugs 83 , 665–685 (2023). https://doi.org/10.1007/s40265-023-01875-8
Download citation
Accepted : 11 April 2023
Published : 06 May 2023
Issue Date : June 2023
DOI : https://doi.org/10.1007/s40265-023-01875-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Find a journal
- Publish with us
- Track your research
- Open access
- Published: 16 March 2024
Proportion of vitamin D deficiency in children/adolescents with type 1 diabetes: a systematic review and meta-analysis
- Xin Yang ORCID: orcid.org/0000-0002-1769-776X 1 , 2 ,
- Min Chai 1 , 2 &
- Meng Lin 1 , 2
BMC Pediatrics volume 24 , Article number: 192 ( 2024 ) Cite this article
334 Accesses
2 Altmetric
Metrics details
The impact of vitamin D on type 1 diabetes has been a controversial topic in public health. Furthermore, significant differences in the proportion of vitamin D have been noted. The purpose of this systematic review was to determine the overall proportion of vitamin D deficiency in children/adolescents with type 1 diabetes (T1D).
Based on six electronic databases (PubMed, Web of Science, Embase, Ovid Medline, ProQuest, and Cochrane Library), eligible studies since the databases’ inception up to April 2022 were searched. Reference lists were also manually searched to identify additional studies. Overall, studies with statistical information on vitamin D deficiency in children and adolescents with T1D were included, and a random effects model was applied for the meta-analysis. In addition, subgroup and sensitivity analyses were carried out to evaluate heterogeneity, and publication bias was evaluated by using Egger’s test.
A total of 45 studies involving 6,995 participants met the inclusion criteria; these included 25 countries covering Africa, Oceania, Europe, North America and Asia. The proportion of vitamin D deficiency in children/adolescents with T1D was 45% (95% confidence interval [CI] 37–54%, I 2 = 97.94%). Subgroup analysis further revealed that the publication year, study design, vitamin D classification, season and geographical region significantly contributed to the variation in the reported incidence of vitamin D deficiency.
Conclusions
The results of the meta-analysis showed that the proportion of vitamin D deficiency among T1D children/adolescents was 45%. In addition, the proportion remains higher, which has important implications for adapting health and social care systems.
Peer Review reports
Type 1 diabetes (T1D), an autoimmune disease that affects pancreatic beta cells, is one of the most common endocrine disorders affecting children and young adults worldwide [ 1 , 2 , 3 ]. According to statistics, 2.15 out of every 1,000 people that are 19 years or younger and from only 6 regions of the United States were diagnosed with T1D in 2017 [ 4 ]. Furthermore, a pooled analysis conducted in 26 European centers revealed a yearly increase of 3.4% in the incidence rate of T1D [ 5 ]. It is also referred to as a chronic autoimmune disease, and there is not current medical technology for its cure. This condition inflicts substantial lifetime morbidity, affecting patients both during their childhood and throughout their adult lives [ 6 ]. Therefore, we must determine an effective management strategy for children and adolescents with type 1 diabetes and their families. However, diabetic ketoacidosis (DKA) has a high incidence of recurrence and is a leading cause of mortality among patients with T1D, resulting in an elevated burden for patients, families, hospitals, and healthcare providers [ 7 ]. Therefore, it is important to find ways to prevent the prevalence of T1D. In this context, one potential factor, vitamin D (VD), has attracted the attention of many scholars. Indeed, vitamin D deficiency/insufficiency represents a substantial but modifiable public health risk that deserves increased attention [ 8 ], as the number of T1D patients suffering from vitamin D deficiency has been increasing rapidly [ 9 ].
Vitamin D deficiency seems to be a common issue even in the general population. Measurement of the circulating form of vitamin D that best describes total body stores, namely, 25-hydroxyvitamin D, can be unreliable despite the many sophisticated methodologies that have been proposed and implemented [ 10 ]. Similarly, evidence from clinical studies showing a beneficial role of vitamin D in different disease states has been controversial and at times speculative [ 11 ]. Additionally, significant differences in the proportion of vitamin D have been noted.
Vitamin D deficiency has been shown to be common in children/adolescents with T1D [ 12 ]. Vitamin D, also called calciferol, is an essential fat-soluble vitamin that plays a considerable role in the growth and strength of bones by controlling calcium and phosphorus homeostasis [ 13 ]. In addition to its role in calcium homeostasis, it has an antiproliferative and immunosuppressive properties that regulate cell proliferation and differentiation [ 14 , 15 ]. According to a review, vitamin D deficiency can potentially influence the incidence, comorbidity, and progression of T1D. Furthermore, in a cross-sectional study, 70% of children with T1D were reported to be vitamin D deficient [ 16 ].
However, epidemiological data based on various studies have shown that the prevalence of vitamin D deficiency among individuals with T1D varies greatly between 4% and 92% [ 17 , 18 ], indicating inconsistency and uncertainty in the currently available information.
Several factors could explain the above variations in the prevalence of vitamin D deficiency between the different sources of data. First, different criteria are used to assess vitamin D deficiency. In addition, the quality and number of examined studies as well as the sampling procedures used in recorded studies tend to be heterogeneous, thereby leading to variable and possibly imprecise estimates. These methodological challenges highlight the importance of assessing the prevalence of vitamin D deficiency in children/adolescents with T1D through a systematic approach.
Although different reviews on the subject are already available, to our knowledge, no systematic reviews and meta-analyses have been conducted to reliably establish the proportion of vitamin D deficiency in children/adolescents with T1D. Therefore, by synthesizing information from different sources, the current systematic review not only sought to address the above knowledge gap but also to evaluate how the characteristics of studies influence estimations of the prevalence of diabetes.
Protocol and registration
This study was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist [ 19 ]. The protocol was registered in the International Prospective Register of Systematic Reviews (CRD 42,022,301,690). This study did not include human research; therefore, no ethics approval was sought.
Search strategy
A thorough literature search was carried out to find published articles on the proportion of vitamin D deficiency in children and/or adolescents with T1D. Studies published from the inception of the database up to the end of April 2022 were considered. The following electronic databases were used for the search: PubMed, Web of Science, Cochrane Library, Ovid Medline, Embase and ProQuest. The following key terms were used: ‘diabetes mellitus insulin dependent’ or ‘diabetes mellitus juvenile onset’ or ‘juvenile onset diabetes mellitus’ or ‘IDDM’ or ‘diabetes juvenile onset’ or ‘diabetes mellitus sudden onset’ or ‘type 1 diabetes mellitus’ or ‘diabetes autoimmune’ or ‘diabetes mellitus brittle’ or ‘Ketosis-Prone’ or ‘ketosis prone diabetes mellitus’ or ‘Adolescen*’ or ‘Teen*’ or ‘Youth*’ or ‘Child*’ or ‘Vitamin D’ and Medical Subject Headings (MeSH) terms ‘diabetes mellitus, type 1’, ‘diabetes mellitus’, ‘Adolescent’, ‘Child’ and ‘Vitamin D’. The research team then created a search strategy based on the MeSH terms and free-text phrases. In this case, the team browsed through the references listed in the published research to discover additional potentially suitable studies, with no restrictions regarding the date or language of publication. The search strategies are shown in Appendix S1 .
Study selection and eligibility criteria
The following materials were selected: (1) observational studies (cross-sectional designs, longitudinal research baseline cross-sectional data, cohort studies, and case–control studies); (2) participants/subjects included children/adolescents (under 20 years of age) with T1D; (3) the proportion of vitamin D deficiency in children and/or adolescents with T1D was described in peer-reviewed literature; and (4) the primary outcome measured the proportion of vitamin D deficiency in children and/or adolescents with T1D while vitamin D insufficiency and vitamin D sufficiency were secondary outcome indicators. Studies were excluded if they were commentaries, reviews, posters, case reports or letters to the editor; if clear data were not provided; or if the article reported duplicated data.
Data extraction
Two independent reviewers (XY and MC) examined the publications’ titles and abstracts, followed by their entire texts to ensure that they met the inclusion criteria. Any discrepancies were settled through communication with a third reviewer (ML). Two separate researchers retrieved information from the selected papers, including the first author’s name, year, title, country, study design, and sample size and characteristics (sex, age, diagnostic criteria for diabetes, classification of vitamin D, etc.).
Quality assessment
The methodological quality of the included studies was independently evaluated by different reviewers (XY and MC) using appropriate instruments. The Newcastle–Ottawa Scale (NOS) [ 20 ] was used to assess the quality of the cohort and case–control studies. In this case, the NOS scores ranged from 0 to 9, with studies with NOS scores greater than 6 considered of reasonably high quality, scores 5–6 considered of medium quality and scores less than 5 deemed to be of low quality. In addition, using the “star system,” each included study was evaluated in three domains: representativeness of the study group during selection, group comparability and exposure or outcome ascertainment. Finally, the Agency for Healthcare Research and Quality (AHRQ) methodology checklist was used to measure the validity of the cross-sectional studies. Each study was evaluated based on 11 items from the checklist [ 21 ], with the quality rated as follows: decent quality = 8–11, moderate quality = 4–7, and poor quality = 0–3. If no agreement could be reached, a third researcher (ML) was recruited to settle the dispute.
Statistical analysis
The data analysis was carried out using the meta-analysis function in STATA software (Stata version 12.0; StataCorp, College Station, TX, USA). For the evaluation of the pooled effect, a 95% confidence interval (CI) was used, and P < 0.05 indicated statistical significance. Random effects were used to pool studies reporting the proportion of vitamin D deficiency in children and/or adolescents with T1D. The I 2 index was subsequently used to examine between-study heterogeneity. If the I 2 value was less than 50%, a nonsubstantial level of heterogeneity was assumed and the meta-analysis applied a fixed effects model. Conversely, an I 2 value greater than 50% was indicative of substantial heterogeneity, for which a random effects model was used. The impact of a single study on the overall estimate of proportion was also investigated by eliminating each study in turn during a sensitivity analysis. Additionally, when there was more than one study in a subgroup, subgroup analyses were performed based on overall study design, vitamin D classification, season (winter, summer, spring, and fall) and geographical location (Asia, Europe, Oceania, Africa, North America, and South America). Funnel plots and Egger’s test results were eventually combined to explore potential publication bias in this meta-analysis. The trim and fill method, developed by Duval and Tweedie, is employed to identify and correct funnel plot asymmetry potentially induced by publication bias. The presence of publication bias in the study findings was assessed using the nonparametric trim and fill method.
Search results and study characteristics
A total of 2,085 titles and abstracts were retrieved from the electronic database searches, and after removing 254 duplicates, 1,831 were screened based on their titles and abstracts. This process yielded 61 full-text studies that were subsequently evaluated for eligibility. Six supplementary articles were also found to be eligible from the reference lists of the included studies. After reviewing the full texts, 45 studies were ultimately included in the meta-analysis. A summary of the selection process for the studies is presented in Fig. 1 .
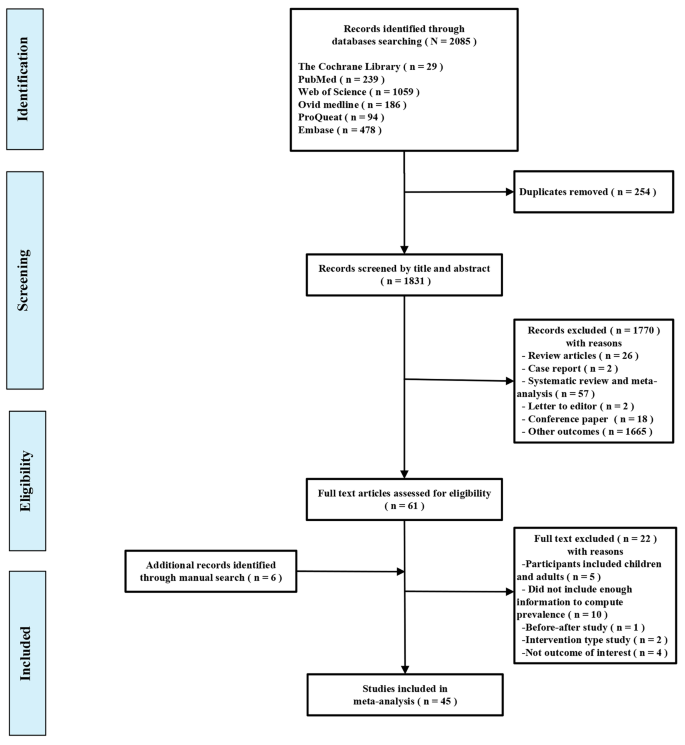
Flow diagram of the identification of eligible studies
Descriptions of the included studies
Out of the 45 studies, 19 had cross-sectional designs [ 16 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 ], 23 had case–control studies [ 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 ], 2 had baseline cross-sectional data from a longitudinal study [ 63 , 64 ] and one had baseline data from a cohort study [ 65 ]. The reported data also included 6,995 participants, mostly aged ≤ 18 years, 2,436 of whom were children/adolescents with T1D and vitamin D deficiency (sample size n = 13 ~ 1,426). Overall, T1D cases were mainly ascertained on the basis of criteria established by the World Health Organization (WHO) and the American Diabetes Association and the European Diabetes (EURODIAB) collaboration, while levels of 25-hydroxyvitamin D (25(OH)D) were measured using a radioimmunoassay kit or high-performance liquid chromatography (HPLC). Similarly, vitamin D status was ascertained mainly on the basis of the Endocrine Society Clinical Practice Guideline, the Institute of Medicine guidelines, the Australian Consensus Statement Criteria and the Central European Guidelines. Among the countries included in the studies, seven were conducted in America, four were conducted in Turkey, three were carried out each in Korea, Iran and India, two each were conducted in Australia, the United Kingdom, Egypt, Spain, Italy and the Kingdom of Saudi Arabia, and one was performed in China, Indonesia, Poland, Kuwait, Canada, Bangladesh, Slovakia, Switzerland, Boston, Ukraine, Tunisia, Iraq and Germany. The main characteristics of the 45 included studies are shown in Table 1 . In accordance with the recommended NOS and AHRQ criteria, only studies of acceptable quality were included in the present meta-analysis; eight studies received 9 stars [ 50 , 52 , 55 , 56 , 57 , 61 , 62 , 65 ], ten studies received 8 stars [ 42 , 47 , 48 , 49 , 51 , 53 , 54 , 58 , 59 , 60 ], five studies received 7 stars [ 41 , 43 , 44 , 45 , 46 ], and one study received 6 stars [ 40 ]. When using the quality assessment criteria from the AHRQ, three studies received a score of 11 [ 24 , 28 , 64 ], ten received a score of 10 [ 16 , 22 , 27 , 28 , 30 , 32 , 33 , 35 , 38 , 39 ], three received a score of 9 [ 23 , 31 , 34 ], one received a score of 8 [ 26 ], one received a score of 7 [ 36 ] and two received a score of 5 [ 25 , 37 ]; the quality assessment is shown in Appendix S2 . Therefore, no article from the meta-analysis was excluded for quality reasons.
Meta-analyses and data synthesis
For the whole sample of 6,995 individuals, the proportion of vitamin D deficiency in children and/or adolescents with T1D was 45% (95% CI; 37–54%; P < 0.01; Fig. 2 ). The analyses further indicated heterogeneity between studies (I-square [ I 2 ] = 97.94%, P < 0.001), and publication bias could be observed on the funnel plot. Publication bias in studies assessing the total proportion of vitamin D deficiency in T1D patients was analyzed using Begg’s test (z = 1.88; P = 0.060), Egger’s test ( P = 0.000) and a funnel plot (Fig. 3 ).
Forest plots for the total proportion of vitamin D deficiency in children/adolescents with type 1 diabetes. The diamond represents the pooled odds ratio and 95% confidence interval
The funnel plot of vitamin D deficiency in children/adolescents with type 1 diabetes
Subgroup analyses were carried out according to the publication year, study design, classification of vitamin D, season and geographical region of the studies, with Table 2 presenting the estimated proportion of patients with vitamin D deficiency after the analysis.
All the included studies were published between from 2009 to 2022. Twenty-one studies were published between 2009 and 2015, and 24 were published between 2016 and 2022. In contrast with the data from the previous six years (48%, 95% CI; 36–59%), more recent publications tended to yield a low proportion of vitamin D deficiency (43%, 95% CI; 31–56%). By comparing study designs, the subgroup analysis showed that a greater proportion of patients with vitamin D deficiency could be found in case‒control studies (58%, 95% CI; 45–72%), followed by one cohort study (51%, 95% CI; 45–58%) and 19 cross-sectional studies (31%, 95% CI; 22–40%), with the lowest proportion identified for 2 longitudinal studies (22%, 95% CI; 20–25%), but with significant heterogeneity. The proportion of vitamin D deficiency in children and/or adolescents with T1D was highest in Africa (65%, 95% CI; 42–85%), followed by Asia (54%, 95% CI; 40–68%), Europe (50%, 95% CI; 32–69%), North America (24%, 95% CI; 15–34%) and Oceania (15%, 95% CI; 12–18%), with significant differences among the five subgroups ( P < 0.01). The proportion of vitamin D deficiency in children and/or adolescents with T1D at low-mid latitudes was 56% (95% CI; 38–72%), followed by that in children at low latitudes (50%, 95% CI; 12–88%), at mid-high latitudes (42%, 95% CI; 37–47%) and at middle latitudes (39%, 95% CI; 29–50%). A higher proportion of patients with a vitamin D deficiency was detected at 30 ng/ml (87%, 95% CI; 82–92%), followed by 25 ng/ml (80%, 95% CI; 71–87%), 10 ng/ml (67%, 95% CI; 26–97%), 20 ng/ml (49%, 95% CI; 39–60%), and 15 ng/ml (24%, 95% CI; 11–41%), with the lowest proportion identified at 12 ng/ml (14%, 95% CI; 9–20%). Subgroup analyses for different seasons showed that the proportion of individuals with vitamin D deficiency in winter tended to be significantly greater than that in summer (50%, 95% CI; 37–64% vs. 17%, 95% CI; 8–27%). In addition, studies conducted in spring reported a greater proportion of individuals with vitamin D deficiency (28%, 95% CI; 23–33%) than did those conducted in autumn (20%, 95% CI; 12–29%), but these differences were not significant ( P > 0.01).
Sensitivity analysis was carried out to examine the influence of any particular study. To determine whether potential publication bias existed in the reviewed literature, Egger’s test was also carried out. The results of Egger’s test ( P < 0.05) did suggest the existence of publication bias. Thus the publication bias of this study was corrected using the trim-and-fill method. The results showed that publication bias had little effect on the combined amount of results, indicating that the robustness of the results of this study was high.
Thirty-five studies involving 5,862 participants were included in the meta-analysis of the rate of vitamin D insufficiency among children and/or adolescents with T1D. In this case, the random effects model indicated that the cumulative proportion was 33.0% (95% CI; 27–38%). Considerable heterogeneity was also observed across studies (I 2 = 94.27%, P < 0.01). Analyses of publication bias for studies estimating the total proportion of patients with vitamin D insufficiency were also conducted, with biases determined based on Begg’s test (z = 0.67; P = 0.504), Egger’s test ( P = 0.614) and the funnel plot.
Thirty-nine studies, grouping 6,490 individuals from Europe ( n = 11), Asia ( n = 17), Africa ( n = 1), North America ( n = 9), and Oceania ( n = 1), assessed the proportion of vitamin D sufficiency in children and/or adolescents with T1D. In this case, the proportion was estimated to be 27% (95% CI; 19–35%; I 2 = 97.87%). Analyses of publication bias for studies estimating the total proportion of patients with sufficient vitamin D concentrations were also performed, with biases determined as before (i.e., with Begg’s test (z = 0.11; P = 0.913), Egger’s test ( P = 0.007) and the funnel plot). Sensitivity analyses further revealed that 2 studies were off-center, and after omitting it [ 37 , 64 ], the biases were again determined by both Begg’s test (z = 0.29; P = 0.773) and Egger’s test ( P = 0.509).
This systematic review and meta-analysis comprehensively assessed the proportion of vitamin D deficiency in children and/or adolescents with T1D from a global perspective. The pooled estimate showed that vitamin D deficiency was prevalent among children and/or adolescents with T1D. As suggested by the present study, the rate of vitamin D deficiency in this particular group was high at 45%, which was high according to 45 studies involving 6,995 respondents. In addition, the proportions of patients with vitamin D insufficiency and vitamin D sufficiency were 33% and 27%, respectively. These findings may help to improve public health interventions for decreasing the proportion of vitamin D deficiency in children and/or adolescents with T1D. Moreover, these finding may serve as a reminder that greater attention should be given to vitamin D deficiency in clinical practice.
The high proportion of vitamin D deficiency in children and/or adolescents with T1D may be explained by the fact that vitamin D is lipophilic and is mainly absorbed in the small intestine before further processing in the skin, liver and kidneys to the biologically active compound 1,25-dihydroxyvitamin D. In addition, the absorption of lipophilic substances is dependent on a variety of intricate processes that require an intact epithelium in the small intestine but also on extraintestinal factors, such as the release of lipase from the pancreas and bile from the liver [ 66 ].
High heterogeneity was identified across the included studies. Subgroup analysis further revealed marked between-study variability in estimates of the proportion of patients with vitamin D deficiency. For instance, the results of subgroup analysis by publication year showed that more recent publications tended to yield low vitamin D deficiency proportion estimates. This discrepancy might be due to increasing awareness of the importance of vitamin D supplements and sun exposure. Furthermore, by comparing study designs, the present study revealed that the proportion of patients with vitamin D deficiency in case‒control studies tended to be greater than that in other studies. This inconsistency clearly indicated that different study designs could yield different estimates of the proportion of patients with vitamin D deficiency.
The other study-specific factor that we considered in the subgroup analysis was geographical region. Compared to those in other regions, we found that the proportion of vitamin D deficiency in children and/or adolescents with T1D in Africa tended to be greater than that in Asia (65% vs. 54%), followed by Europe (50%), North America (24%) and Oceania (15%), thus indicating that geographical regions could partly explain some of the variance. This could have been due to differences in culture, religion, ethnicity, dietary habits and forms of exercise. Indeed, low vitamin D levels in some populations are related to social customs such as the avoidance of sunlight or even breastfeeding without any vitamin D supplementation [ 67 ]. Due to differences in study design, only one study [ 16 ] statistically assessed dietary fortification as an influencing factor among the included studies, which is also one of the underlying reasons for the bias. Another important aspect to consider is that the recommended vitamin D intake for children and adolescents varies by country. For instance, the American Academy of Pediatrics recommends a minimum daily intake of 200 U/d of vitamin D beginning in the first 2 months after birth and continuing through adolescence [ 68 ]. In China, vitamin D supplementation is recommended to begin within a few days after birth, and at least 400 U/d is recommended during infancy to adolescence. Daily oral vitamin D supplementation is recommended. When compliance is poor, large doses of vitamin D can be administered orally. When gastrointestinal disease occurs, large doses of vitamin D can be administered intramuscularly [ 69 ]. According to global consensus recommendations on the prevention and management of nutritional rickets, at more than 12 months of age, all children need to meet their nutritional requirement for vitamin D through diet and/or supplementation, which is at least 600 U/d [ 70 ]. In addition to the fact that individuals originated from different territorial areas, participant characteristics such as age and ethnicity also varied among studies. Some participants could also have had higher vitamin D requirements for bone growth, especially during pubertal growth spurts [ 71 ], further contributing to the heterogeneity.
According to our subgroup analysis, one of the most important factors was the cutoff value for vitamin D deficiency. Compared with a cutoff value of < 25 ng/ml, a cutoff value of < 30 ng/ml was associated with a significantly greater incidence of vitamin D deficiency. This procedure was followed by a cutoff value of < 10 ng/ml, a cutoff value of < 20 ng/ml, and a cutoff value of < 15 ng/ml, with the lowest proportion identified for a cutoff value of < 12 ng/ml. This may be due to the small sample size. This variability could be partly attributed to the lack of standardized 25(OH)D measurements in vitamin D research. Beyond that, within a given methodology, there are several possible causes for differences, such as lot-to-lot variation in manufacturer reagents or differences in subjects included in different studies.
Subgroup analysis also revealed an interesting findings. The present study revealed that the proportion of vitamin D deficiency in children and/or adolescents with T1D in winter tended to be significantly greater than that in summer. In addition, these findings add weight to the conclusion that the proportion of vitamin D deficiency in children and/or adolescents with T1D at mid-low latitudes tends to be greater than that at low latitudes (56% vs. 50%), followed by at mid- to high latitudes (42%) and finally at middle latitudes (39%). This discrepancy might be because there is a longer sunlight duration in summer than in winter. While separating research into subgroups revealed numerous noteworthy differences, post hoc comparisons should be interpreted with caution. The heterogeneity in proportions between studies was not satisfactorily explained by any of the parameters examined, with I 2 values being greater than 65% for all subgroups.
The current research has some limitations. First, all the studies were clinic- or hospital-based, which could have affected the true prevalence in the general population. Second, the selected studies included cross-sectional, case‒control, cohort and longitudinal studies that were limited by study design and therefore had an inevitable risk of bias. Third, there is currently no internationally agreed upon classification standard for vitamin D deficiency, and as such, there may be significant variations during reporting. Finally, the possibility of publication bias could not be fully excluded by Egger’s test. Trim and fill analysis was also conducted, and the results did not change the estimate, indicating that the results are robust to the possibility of unpublished studies.
Vitamin D may have direct effects on β cells, including improving insulin secretion, enhancing the expression of the vitamin D receptor and improving islet morphology [ 72 ]. As vitamin D intake is a potentially important and modifiable behavioral target, clinical professionals need to screen for vitamin D deficiency in children and/or adolescents with T1D to guide appropriate supplementation.
This review demonstrated that vitamin D deficiency affects 45% of children and/or adolescents with T1D, and children and/or adolescents with T1D in winter had an increased susceptibility to vitamin D deficiency compared with those in other seasons. These results contribute to a better understanding of vitamin D deficiency in children and/or adolescents with T1D and demonstrate the importance of assessing vitamin D deficiency in children and/or adolescents with diabetes. Preventive strategies and interventions to address vitamin D deficiency in children and/or adolescents with T1D should be considered in healthcare settings. Future research should focus on increasing our understanding of the temporal relationship between diabetes and vitamin D deficiency.

Data availability
The datasets used and/or analysed during the current study are available from the corresponding/first author on reasonable request.
Abbreviations
- Type 1 diabetes
Diabetic Ketoacidosis
Confidence Interval
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses
Medical Subject Headings
The Newcastle–Ottawa Scale
The Agency for Healthcare Research and Quality
The World Health Organization
The American Diabetes Association
The European Diabetes collaboration
High-Performance Liquid Chromatography
Harvey JN, Hibbs R, Maguire MJ, et al. The changing incidence of childhood-onset type 1 diabetes in Wales: Effect of gender and season at diagnosis and birth. Diabetes Res Clin Pract. 2021;175:108739.
Article CAS PubMed Google Scholar
Esen I, Okdemir D. Trend of type 1 diabetes incidence in children between 2009 and 2019 in Elazig, Turkey. Pediatr Diabetes. 2020;21:460–5.
Article PubMed Google Scholar
Xia Y, Xie Z, Huang G, et al. Incidence and trend of type 1 diabetes and the underlying environmental determinants. Diabetes Metab Res Rev. 2019;35:e3075.
Lawrence JM, Divers J, Isom S, et al. Trends in Prevalence of Type 1 and type 2 diabetes in children and adolescents in the US, 2001–2017. JAMA. 2021;326:717–27.
Article PubMed PubMed Central Google Scholar
Patterson CC, Harjutsalo V, Rosenbauer J, et al. Trends and cyclical variation in the incidence of childhood type 1 diabetes in 26 European centres in the 25 year period 1989–2013: a multicentre prospective registration study. Diabetologia. 2019;62:408–17.
Vehik K, Dabelea D. The changing epidemiology of type 1 diabetes: why is it going through the roof? Diabetes Metab Res Rev. 2011;27:3–13.
Williams R, Karuranga S, Malanda B, et al. Global and regional estimates and projections of diabetes-related health expenditure: results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2020;162:108072.
Holick MF. The vitamin D deficiency pandemic: approaches for diagnosis, treatment and prevention. Rev Endocr Metab Disord. 2017;18:153–65.
Mansi QH, Aziz AR. Comparative study between diabetes mellitus and non-diabetic mellitus toward vitamin D deficiency in children under five year. Pakistan J Med Health Sci. 2021;15:818–22.
Google Scholar
Giustina A, Adler RA, Binkley N et al. Consensus statement from 2(nd) International Conference on Controversies in Vitamin D. Rev Endocr Metab Disord. 2020;21:89–116.
Dominguez-Riscart J, Buero-Fernandez N, Garcia-Zarzuela A, et al. Adherence to mediterranean diet is associated with better glycemic control in children with type 1 diabetes: a cross-sectional study. Front Nutr. 2022;9:813989.
Mihoubi E, Raache R, Amroun H, et al. Metabolic imbalance and vitamin d deficiency in type 1 diabetes in the Algerian population. Endocr Metab Immune Disord Drug Targets. 2019;19:1172–6.
Thacher TD, Clarke BL. Vitamin D insufficiency. Mayo Clin Proc. 2011;86:50–60.
Article CAS PubMed PubMed Central Google Scholar
Muscogiuri G, Mitri J, Mathieu C, et al. Mechanisms in endocrinology: vitamin D as a potential contributor in endocrine health and disease. Eur J Endocrinol. 2014;171:R101–10.
Tuomilehto J, Ogle GD, Lund-Blix NA, et al. Update on Worldwide trends in occurrence of Childhood Type 1 diabetes in 2020. Pediatr Endocrinol Rev. 2020;17:198–209.
PubMed Google Scholar
Alkharashi NA. Estimation of vitamin D deficiency prevalence among Saudi children in Armed Forces Hospital and Riyadh Care Hospital in Riyadh, Kingdom of Saudi Arabia and its relation to type 1 diabetes mellitus. Saudi Med J. 2019;40:1290–3.
Carakushansky M, Patel P, Ben KB, et al. Prevalence of vitamin d deficiency in children with type 1 diabetes mellitus. Cureus. 2020;12:e7836.
PubMed PubMed Central Google Scholar
Mithal A, Wahl DA, Bonjour JP, et al. Global vitamin D status and determinants of hypovitaminosis D. Osteoporos Int. 2009;20:1807–20.
Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8:336–41.
Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–5.
Zeng X, Zhang Y, Kwong JS, et al. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: a systematic review. J Evid Based Med. 2015;8:2–10.
Zabeen B, Nahar J, Ahmed B et al. Vitamin D status in children and adolescents with type 1 diabetes in a specialized diabetes care centre in Bangladesh. Endocrinology, Diabetes & Metabolism. 2022;5.
Segovia-Ortí R, Bennassar AB, de Sotto-Esteban D, et al. Vitamin D status is related to severity at onset of diabetes and worse glycemic control. J Pediatr Endocrinol Metab. 2020;33:1265–71.
Carakushansky M, Patel P, Ben Khallouq BA et al. Prevalence of vitamin d deficiency in children with type 1 diabetes mellitus. Cureus 2020;12.
Kör Y. The relationship between 25-Hydroxy vitamin d level and metabolic control in type 1 diabetes mellitus patients. Turkish J Pediatr Disease 2018.
Giri D, Pintus D, Burnside G, et al. Treating vitamin D deficiency in children with type I diabetes could improve their glycaemic control. BMC Res Notes. 2017;10:465.
Saki F, Omrani GR, Pouralborz Y, et al. Vitamin D deficiency and the associated factors in children with type 1 diabetes mellitus in southern Iran. Int J Diabetes Dev C. 2017;37:78–84.
Article CAS Google Scholar
Al-Zubeidi H, Leon-Chi L, Newfield RS. Low vitamin D level in pediatric patients with new onset type 1 diabetes is common, especially if in ketoacidosis. Pediatr Diabetes. 2016;17:592–8.
Al SA, Al ZA. Impact of vitamin D status on Cardiometabolic complications among children and adolescents with type 1 diabetes Mellitus. J Clin Res Pediatr Endocrinol. 2016;8:48–54.
Article Google Scholar
Zambrana-Calví GD, Palomo-Atance E, Gourdet ME, et al. Lipid changes and their relationship with vitamin D levels in children under 18 years with type 1 diabetes. Endocrinol Nutr. 2016;63:126–31.
Al Sawah S, Compher CW, Hanlon AL, et al. 25-Hydroxyvitamin D and glycemic control: a cross-sectional study of children and adolescents with type 1 diabetes. Diabetes Res Clin Pr. 2016;115:54–9.
Vojtkova J, Ciljakova M, Vojarova L, et al. Hypovitaminosis D in children with type 1 diabetes mellitus and its influence on biochemical and densitometric parameters. Acta Medica (Hradec Kralove). 2012;55:18–22.
Yeshayahu Y, Sochett EB, Deda L, et al. Type 1 diabetes as a risk factor for impaired vitamin d status in a multi-ethnic cohort of Canadian adolescents. Can J Diabetes. 2012;36:314–9.
Ataie-Jafari A, Rahmat AB, Abbasi F, et al. Vitamin D status and associated factors in recent-onset type 1 diabetic children in Iran. J Diabetes Metab Disord. 2012;11:12.
Mutlu A, Mutlu GY, özsu E, et al. Vitamin D deficiency in children and adolescents with type 1 diabetes. J Clin Res Pediatr Endocrinol. 2011;3:179–83.
Thnc O, Cetinkaya S, Kizilgün M, et al. Vitamin D status and insulin requirements in children and adolescent with type 1 diabetes. J Pediatr Endocrinol Metab. 2011;24:1037–41.
Kaur H, Donaghue KCPF, Chan AKM, et al. Vitamin d deficiency is associated with retinopathy in children and adolescents with type 1 diabetes. Diabetes Care. 2011;34:1400–2.
Janner M, Ballinari P, Mullis PE, et al. High prevalence of vitamin D deficiency in children and adolescents with type 1 diabetes. Swiss Med Wkly. 2010;140:w13091.
Svoren BM, Volkening LK, Wood JR, et al. Significant vitamin D deficiency in youth with type 1 diabetes mellitus. J Pediatr. 2009;154:132–4.
Biliaieva E, Vlasenko M. Diabetes mellitus type 1 in adolescents: impact of vitamin d status. Wiadomosci Lekarskie (Warsaw Poland: 1960). 2022;75:387–92.
Polat I, Can Yilmaz G, Dedeoglu O. Vitamin d and nerve conduction in pediatric type-1 diabetes mellitus. Brain Dev-Jpn. 2022;44:336–42.
Rochmah N, Faizi M, Triastuti IW, et al. Vitamin D level and early cow’s milk protein exposure in type 1 diabetes mellitus. Archives Hellenic Med. 2022;39:106–9.
Mansi QH, Aziz AR. Detect of vitamin-d deficiency in children under five years with type 1 diabetes mellitus at diabetes and endocrinology center. Indian J Forensic Med Toxicol. 2021;15:1285–91.
Ziaei-Kajbaf T, Aminzadeh M, Fatahinezhad E, et al. Vitamin D status in diabetic children and adolescents. Diabetes Metab Syndr. 2018;12:849–52.
Liu C, Wang J, Wan Y, et al. Serum vitamin D deficiency in children and adolescents is associated with type 1 diabetes mellitus. Endocr Connect. 2018;7:1275–9.
Federico G, Genoni A, Puggioni A, et al. Vitamin D status, enterovirus infection, and type 1 diabetes in Italian children/adolescents. Pediatr Diabetes. 2018;19:923–9.
Bae KN, Nam H, Rhie Y, et al. Low levels of 25-hydroxyvitamin D in children and adolescents with type 1 diabetes mellitus: a single center experience. Annals Pediatr Endocrinol Metabolism. 2018;23:21–7.
Kim HY, Lee YA, Jung HW, et al. A lack of association between vitamin D-binding protein and 25-hydroxyvitamin D concentrations in pediatric type 1 diabetes without microalbuminuria. Annals Pediatr Endocrinol Metabolism. 2017;22:247–52.
Wierzbicka E, Szalecki M, Pludowski P, et al. Vitamin D status, body composition and glycemic control in Polish adolescents with type 1 diabetes. Minerva Endocrinol. 2016;41:445–55.
Rasoul MA, Al-Mahdi M, Al-Kandari H, et al. Low serum vitamin-D status is associated with high prevalence and early onset of type-1 diabetes mellitus in Kuwaiti children. Bmc Pediatr. 2016;16:95.
Sonia H, Ali M, Yousr D et al. Serum vitamin d level in children with and without type 1 diabetes mellitus. J Diabetes Metabolism 2016;7.
Soliman GT, Ali BA, Mohamed AA et al. Assessment of vitamin d status in Egyptian children with type-1 diabetes mellitus. J Diabetes Metabolism 2015;6.
Franchi B, Piazza M, Sandri M, et al. Vitamin D at the onset of type 1 diabetes in Italian children. Eur J Pediatr. 2014;173:477–82.
Jung SS, Kim MS, Lee DY. Serum vitamin D status in children and adolescence with diabetes according to season and age. Annals Pediatr Endocrinol Metabolism. 2014;19:13–9.
Setty-Shah N, Maranda L, Benjamin UN. Increased risk for vitamin d deficiency in obese children with both celiac disease and type 1 diabetes. Gastroent Res Pract 2014;2014.
Azab SF, Saleh SH, Elsaeed WF, et al. Vitamin D status in diabetic Egyptian children and adolescents: a case-control study. Ital J Pediatr. 2013;39:73.
Lieberman R, Wadwa RP, Nguyen N, et al. The association between vitamin D and vascular stiffness in adolescents with and without type 1 diabetes. PLoS ONE. 2013;8:e77272.
Article ADS CAS PubMed PubMed Central Google Scholar
Greer RM, Portelli SL, Hung BS, et al. Serum vitamin D levels are lower in Australian children and adolescents with type 1 diabetes than in children without diabetes. Pediatr Diabetes. 2013;14:31–41.
Daga RA, Laway BA, Shah ZA, et al. High prevalence of vitamin D deficiency among newly diagnosed youth-onset diabetes mellitus in north India. Arq Bras Endocrinol. 2012;56:423–8.
Ghandchi Z, Neyestani TR, Yaraghi AA, et al. Vitamin D status and the predictors of circulating T helper 1-type immunoglobulin levels in Iranian subjects with type 1 diabetes and their siblings: a case-control study. J Hum Nutr Diet. 2012;25:365–72.
Borkar VV, Devidayal, Verma S, et al. Low levels of vitamin D in north Indian children with newly diagnosed type 1 diabetes. Pediatr Diabetes. 2010;11:345–50.
Bener A, Alsaied A, Al-Ali M, et al. High prevalence of vitamin D deficiency in type 1 diabetes mellitus and healthy children. Acta Diabetol. 2009;46:183–9.
Savastio S, Cadario F, Genoni G, et al. Vitamin d deficiency and glycemic status in children and adolescents with type 1 diabetes mellitus. PLoS ONE. 2016;11:e162554.
The NS, Crandell JL, Lawrence JM, et al. Vitamin D in youth with type 1 diabetes: prevalence of insufficiency and association with insulin resistance in the SEARCH Nutrition Ancillary Study. Diabet Med. 2013;30:1324–32.
Raab J, Giannopoulou EZ, Schneider S, et al. Prevalence of vitamin D deficiency in pre-type 1 diabetes and its association with disease progression. Diabetologia. 2014;57:902–8.
Holick MF, Vitamin D. A d-lightful solution for health. J Investig Med. 2011;59:872–80.
Mendes MM, Hart KH, Williams EL, et al. Vitamin D supplementation and sunlight exposure on serum vitamin D concentrations in 2 parallel, Double-Blind, randomized, placebo-controlled trials. J Nutr. 2021;151:3137–50.
Wagner CL, Greer FR. Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics. 2008;122:1142–52.
Zhang HF, Yu XD, Mao M, et al. Interpretation of practical guidelines for clinical issues related to vitamin D nutrition in Chinese children. Zhonghua Er Ke Za Zhi. 2022;60:408–12.
CAS PubMed Google Scholar
Munns CF, Shaw N, Kiely M, et al. Global consensus recommendations on prevention and management of nutritional rickets. J Clin Endocrinol Metab. 2016;101:394–415.
Taylor SN. Vitamin d in toddlers, preschool children, and adolescents. Ann Nutr Metab. 2020;76(Suppl 2):30–41.
Korsgren O. The role of vitamin D in the aetiology of type 1 diabetes. Diabetologia. 2020;63:1279–80.
Download references
Acknowledgements
Not applicable.
This research did not receive any funding.
Author information
Authors and affiliations.
Department of Pediatric Genetics, Metabolism and Endocrinology Nursing, West China Second University Hospital, Sichuan University, No. 1416, Section 1, Chenglong Avenue, Chengdu, Sichuan, China
Xin Yang, Min Chai & Meng Lin
Key Laboratory of Birth Defects and Related Diseases of Women and Children, Sichuan University, Ministry of Education, No. 1416, Section 1, Chenglong Avenue, Chengdu, Sichuan, China
You can also search for this author in PubMed Google Scholar
Contributions
Xin Yang. Contribution: Literature retrieval; Data collection and verification; Literature quality assessment; Statistical analysis; Prepared Figs. 1, 2 and 3; Wrote the main manuscript text. Min Chai. Contribution: Literature retrieval; Data collection and verification. Meng Lin. Contribution: Data collection and verification; Literature quality assessment; Revise the article.
Corresponding author
Correspondence to Meng Lin .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Supplementary material 2, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Yang, X., Chai, M. & Lin, M. Proportion of vitamin D deficiency in children/adolescents with type 1 diabetes: a systematic review and meta-analysis. BMC Pediatr 24 , 192 (2024). https://doi.org/10.1186/s12887-024-04683-5
Download citation
Received : 30 December 2022
Accepted : 01 March 2024
Published : 16 March 2024
DOI : https://doi.org/10.1186/s12887-024-04683-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Epidemiology
- Children/adolescents
BMC Pediatrics
ISSN: 1471-2431
- Submission enquiries: [email protected]
- General enquiries: [email protected]

Signs you have a vitamin D deficiency
H UNTINGTON, W.Va. (WSAZ) - During the gloom of winter, it’s hard to get all the nutrients you need -- one of those being vitamin D.
It comes from some fortified foods and sunshine. Without it, you could feel a difference in your body.
“It is most important in calcium regulation in your body,” said Dr. Cyrus Hajiran of Valley Health. “It helps maintain strong bones; it also has many roles in every system in your body.”
When you don’t have enough exposure to the sun, you can be at risk for vitamin D deficiency.
Potential symptoms include fatigue, bone and joint pain, cramping and issues with your mood.
“There’s some literature to suggest there could be a relation to mental health and depression,” Hajiran said.
You can get vitamin D from foods like fatty fish, mushrooms, fortified milks and cereals.
“As far as 18 years and older, there’s a daily recommended amount of about 600-800 international units daily as far as what people need to perform at baseline,” Hajiran said.
If you’re concerned you may be deficient, it’s important to get a blood test from your doctor before taking your own measures to supplement.
Keep checking the WSAZ app for the latest information.


Our wellness advice is expert-vetted . Our top picks are based on our editors’ independent research, analysis, and hands-on testing. If you buy through our links, we may get a commission. Reviews ethics statement
Best Vitamins for Hair Growth
Hair loss can be frightening. Be sure to include these vitamins that may help restore your hair and keep it healthy.

For many people, their hair is their crown or form of expression; losing it can be stressful. While experts say shedding between 50 to 100 hairs daily is normal, losing clumps of hair regularly can be a warning of a bigger issue at hand. Factors such as medical conditions , stress and vitamin deficiencies all can affect your hair health.
After consulting with a doctor or dermatologist to get to the root of your hair loss, one way to ensure you're doing the most to support your tresses is by evaluating your diet. A healthy lifestyle plays a major role in ensuring your hair stays in peak condition. Continue reading for the supplemental and natural ways for you to get the vitamins you need for healthy hair growth.
What vitamins are good for hair growth?
Vitamins do many amazing things for hair : They can aid in cell growth, prevent free radicals from damaging it, keep it from graying prematurely and nourish the follicles that stimulate growth.
Here are the best vitamins for hair growth and thickness.
Biotin, also known as vitamin B7 , stimulates the production of keratin to increase follicle growth. Biotin deficiencies tend to be rare, with those diagnosed with Biotinidase Deficiency being the most common. You can find this vitamin in many foods, including eggs, meat, fish, nuts, eggs, sweet potatoes and seeds.
The recommended intake is 30 micrograms for adults daily.
Vitamin A
Hair cells are the fastest-growing part of the body. It makes sense, then, that vitamin A is the perfect fuel for that growth. When your body absorbs vitamin A, it produces sebum. That's an oily substance that moisturizes your scalp, keeping it and your hair follicles healthy. Having a vitamin A deficiency could result in you experiencing hair loss.
If you're looking to consume more vitamin A, you'll want to consume foods high in beta-carotene , which turns into vitamin A. Foods high in beta-carotene include sweet potatoes, pumpkin, carrots, spinach and kale. You can also find it in cod liver oil, eggs, yogurt and milk.
The recommended daily intake for vitamin A is up to 900 mcg for men and 700 mcg for women.

Vitamin C
Oxidative stress is one of the main factors contributing to hair loss. This occurs when we have an imbalance of free radicals and antioxidants in our bodies which can lead to an electron imbalance that could result in hair loss.
The solution is to consume foods with vitamin C . Your body possesses antioxidants that curtail free radicals' hair damage by balancing their electrons when you do. Along with balancing the scales, Vitamin C aids your body in producing collagen (prevents hair from graying prematurely) and absorbing iron which can help hair grow. Smoking, drinking alcohol and having a poor diet can lead to a vitamin C deficiency.
You'll find vitamin C in citrus fruits , peppers, strawberries, tomatoes and guavas. Since your body doesn't produce it , you'll need to include these in your diet or have a supplement with vitamin C.
Daily intake for vitamin C is up to 90 milligrams per day for adult men and 75 milligrams for adult women. Taking too much Vitamin C could result in heartburn, muscle cramps, fatigue, skin flushing and possible kidney stones.
Vitamin D
Vitamin D deficiencies can lead to hair loss conditions like alopecia, female pattern hair loss and excessive shedding. You'll find these depletions more in people aged 65 and over.
To get more vitamin D intake, you can incorporate fatty fish, cod liver oil, fortified foods (cereal, eggs, bread, yogurt) and mushrooms into your diet. Alternatively, you can catch some midday sun rays .
600 IU of vitamin D is the recommended dosage for adults. Taking too much vitamin D could result in nausea, weight loss, disorientation, and heart rhythm issues.
Vitamin E
Vitamin E contains the same antioxidant prowess as its vitamin C counterpart possesses. It means it can curb oxidative stress by balancing out the electron level in free radicals. People more susceptible to vitamin E deficiencies include those with health conditions such as Crohn's or cystic fibrosis .
Vitamin E is an effective method for treating hair loss. A small study revealed that people taking vitamin E supplements for eight months experienced a 34.5% increase in hair growth . You can also find vitamin E in sunflower seeds, spinach, avocados and almonds.
If you plan to go the supplemental route, the recommended dietary allowance is 15 milligrams daily.

Iron fuels the production of hemoglobin , a protein found in your body's red blood cells. These cells distribute oxygen to cells throughout your body, aiding in their repair and growth. An iron deficiency can lead to hair loss, with women being the most susceptible.
You'll find iron in foods like eggs, red meat, lentils, spinach, oysters and clams. If your doctor recommends it, you can take an iron supplement.
The recommended daily iron intake is 45 mg . Keep in mind that taking too much iron could result in constipation, stomach pain and vomiting.

Zinc promotes hair growth and keeps the oil glands surrounding the follicles working well. If you have a Zinc deficiency , you could experience hair loss. Those most susceptible to zinc deficiencies are those who drink alcohol excessively, people with Crohn's, pregnant or breastfeeding women and those with chronic kidney ailments.
You can find zinc in many common foods like beef, spinach, wheat germ, pumpkin seeds, oysters and lentils. The recommended daily dosage of iron is 11 mg for men and 8 mg for women. Taking too much could result in loss of appetite, cramps and headaches. It can also lower your good cholesterol.

How long do hair growth vitamins take to work?
Hair supplements are not overnight solutions. It may take months before you'll notice small improvements. Remember that the success rate depends on the cause of the hair loss, your diet, genetics and other factors.

Bottom line
Vitamins can restore damaged hair, prevent it from aging prematurely, reduce hair loss, and improve growth and volume. They're also not a one-size-fits-all solution. You'll want to consult your doctor if you're losing a significant amount of hair, as it may stem from your environment, an underlying medical condition or another factor. They can work with you to create a targeted plan that may include vitamins.
Vitamins and Supplements Guides
- Best Multivitamins
- Best Multivitamins for Men
- Best Multivitamins for Women
- Best Creatine Supplements
- Best Probiotics
- Best Supplements to Gain Weight
- Best Vitamin Subscription
- Best Vitamins for Energy
- Best Vitamins and Supplements for Joint Health
- Best Vitamins for Healthy Hair, Skin and Nails
- CVS Coupons
- Walgreens Coupons
- iHerb Promo Codes
- Myprotein Coupons
- GNC Coupons
- Vitacost Coupons
- Prolon Life Coupons
- Swanson Vitamins Promo Codes
- FSA Store Coupons
- Life Extension Coupons
Autoimmune Thyroiditis and Vitamin D
Affiliations.
- 1 Department of Pediatrics, School of Medicine, University of Navarra, 431008 Pamplona, Spain.
- 2 Navarrabiomed (Biomedical Research Center), 31008 Pamplona, Spain.
- 3 Department of Pediatrics, Navarra Hospital Complex, 31008 Pamplona, Spain.
- PMID: 38542128
- DOI: 10.3390/ijms25063154
Hashimoto's thyroiditis (HT) is marked by self-tissue destruction as a consequence of an alteration in the adaptive immune response that entails the evasion of immune regulation. Vitamin D carries out an immunomodulatory role that appears to promote immune tolerance. The aim of this study is to elaborate a narrative review of the relationship between vitamin D status and HT and the role of vitamin D supplementation in reducing HT risk by modulating the immune system. There is extensive literature confirming that vitamin D levels are significantly lower in HT patients compared to healthy people. On the other hand, after the supplementation with cholecalciferol in patients with HT and vitamin D deficiency, thyroid autoantibody titers decreased significantly. Further knowledge of the beneficial effects of vitamin D in the prevention and treatment of autoimmune thyroid diseases requires the execution of additional randomized, double-blind, placebo-controlled trials and longer follow-up periods.
Keywords: Hashimoto thyroiditis; anti-thyroglobulin antibodies; anti-thyroid peroxidase; autoimmune thyroiditis; autoimmunity; immune cells; vitamin D; vitamin D deficiency; vitamin D supplementation.
Publication types
- Hashimoto Disease* / drug therapy
- Randomized Controlled Trials as Topic
- Vitamin D / therapeutic use
- Vitamin D Deficiency* / complications
- Vitamin D Deficiency* / drug therapy
- Vitamins / therapeutic use
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Food Nutr Res

Vitamin D – a systematic literature review for the 5th edition of the Nordic Nutrition Recommendations
Christel lamberg-allardt.
1 Department of Food and Environmental Sciences, University of Helsinki, Helsinki, Finland
Magritt Brustad
2 UIT-The Arctic University of Norway, Tromsø, Norway
Haakon E. Meyer
3 Department of Community Medicine, Institute of Health and Society, University of Oslo, Oslo, Norway
4 Norwegian Institute of Public Health, Oslo, Norway
Laufey Steingrimsdottir
5 Unit for Nutrition Research, Landspitali University Hospital & University of Iceland, Reykjavik, Iceland
The present literature review is part of the NNR5 project with the aim of reviewing and updating the scientific basis of the 4th edition of the Nordic Nutrition Recommendations (NNR) issued in 2004.
The overall aim was to review recent scientific data on the requirements and health effects of vitamin D and to report it to the NNR5 Working Group, who is responsible for updating the current dietary reference values valid in the Nordic countries.
The electronic databases MEDLINE and Swemed were searched. We formulated eight questions which were used for the search. The search terms related to vitamin D status and intake and different health outcomes as well as to the effect of different vitamin D sources on vitamin D status. The search was done in two batches, the first covering January 2000–March 2010 and the second March 2009–February 2011. In the first search, we focused only on systematic literature reviews (SLRs) and in the second on SLRs and randomized control trials (RCTs) published after March 2009. Furthermore, we used snowballing for SLRs and IRCTs published between February 2011 and May 2012. The abstracts as well as the selected full-text papers were evaluated in pairs.
We found 1,706 studies in the two searches of which 28 studies were included in our review. We found 7 more by snowballing, thus 35 papers were included in total. Of these studies, 31 were SLRs and 4 were RCTs. The SLRs were generally of good or fair quality, whereas that of the included studies varied from good to poor. The heterogeneity of the studies included in the SLRs was large which made it difficult to interpret the results and provide single summary statements. One factor increasing the heterogeneity is the large variation in the assays used for assessing 25-hydroxyvitamin D concentration [25(OH)D], the marker of vitamin D status. The SLRs we have reviewed conclude that the evidence for a protective effect of vitamin D is only conclusive concerning bone health, total mortality and the risk of falling. Moreover, the effect was often only seen in persons with low basal 25(OH)D concentrations. In addition, most intervention studies leading to these conclusions report that intervention with vitamin D combined with calcium and not vitamin D alone gives these benefits. It was difficult to establish an optimal 25(OH)D concentration or vitamin D intake based on the SLRs, but there are evidence that a concentration of ≥50 nmol/l could be optimal. The dose–response studies relating vitamin D intake (fortification and supplementation) to S-25(OH)D suggested that an intake of 1–2.5 µg/day will increase the serum concentration by 1–2 nmol/l but this is dependent on the basal concentration with a response being greater when the basal concentration is low.
Data show that a S-25(OH)D concentration of 50 nmol/l would reflect a sufficient vitamin D status. Results from this review support that the recommendation in NNR 2004 needs to be re-evaluated and increased for all age groups beyond 2 years of age. We refer to the total intake from food as well as supplements, given minimal sun exposure. Limited sunshine, however, does not reflect the situation for the majority of the Nordic population in the summertime. It should also be emphasized that there are large differences in results depending on assay methods and laboratories measuring 25(OH)D, adding to the uncertainty of determining an appropriate target concentration. Moreover, the dose–response of vitamin D on serum 25(OH)D-concentrations is not well established and is dependent on the basal concentrations, sunshine exposure and dietary intake. We advise that these uncertainties should be taken into account when setting the final Nordic recommendations.
This literature review is part of the NNR5 project with the aim of reviewing and updating the scientific basis of the 4th edition of the Nordic Nutrition Recommendations (NNR) issued in 2004 ( 1 ). The NNR5 project is mainly focused on a revision of those areas in which new scientific knowledge has emerged since the 4th edition with special relevance for the Nordic setting. A number of systematic literature reviews (SLRs) will form the basis for the establishment of dietary reference values in the 5th edition of NNR.
The dietary reference values for vitamin D in the 4th edition of the NNR are 10 µg/day for the age group 6–23 months, 7.5 µg/day for 2–60 years, 10 µg for 61 years and older, and for pregnant and lactating women 10 µg/day. The upper level of vitamin D intake for adults is 50 µg/day ( 1 ).
The overall aim was to review recent scientific data on requirements and health effects of vitamin D and to report it to the NNR5 Working Group, who is responsible for updating the current dietary reference values valid in the Nordic countries. The SLR followed the guidelines for conducting systematic reviews set by the working group ( 2 ).
The specific objectives of the review on health effects of vitamin D in human nutrition were to:
- review the scientific evidence to determine, based on a set of agreed criteria, dietary reference values for vitamin D for different life stages (infants, children, adolescents, adults, elderly and during pregnancy and lactation),
- assess the requirement for adequate growth, development and maintenance of health of vitamin D,
- assess the health effects of different intakes/exposures of vitamin D.
Scientific background
In humans, vitamin D is obtained from the diet and through cutaneous synthesis in the presence of ultra-violet irradiation supplied by sunlight. Vitamin D is converted to 25-hydroxy-vitamin D [25(OH)D] in the liver and is transported in the circulation by a vitamin-D-binding protein, DBP (also named Gc-protein or Gc-globulin). The 25(OH)D concentration measured in serum or plasma is considered to be the best marker of vitamin D status.
The biologically active form, 1,25-dihydroxy-vitamin D [1,25-(OH) 2 -D], is formed in the kidneys from 25(OH)D. 1,25-(OH) 2 -D stimulates bone resorption and intestinal calcium absorption, leading to an increase in serum calcium concentration. The synthesis and secretion of 1,25-(OH) 2 -D is mainly regulated by changes in serum parathyroid hormone (PTH) concentration, which is regulated by the serum calcium concentration, as well as by serum phosphate concentration and by itself. Fibroblast growth factor 23 (FGF23) is also involved in the regulation of 1,25-(OH) 2 -D ( 3 ). 1,25-(OH) 2 -D exerts its main biological effects via an intracellular vitamin D receptor (VDR). The VDR has been found in many cell types. Recent detailed analysis has not confirmed the presence of VDR in cardiac and skeletal muscle, but there is an ongoing debate on this issue, as 1,25-(OH) 2 -D has specific effects on, that is, muscle cells ( 4 , 5 ). The 1,25-(OH) 2 -D–VDR-complex acts as transcription factor in the target cells. The classical targets are the intestinal mucosa cells and the skeleton. In the intestine 1,25-(OH) 2 -D induces the calcium-binding protein (calbindin) and the calcium channel TRPV6 ( 6 ). In bone tissue, the role of 1,25-(OH) 2 -D is complex but it is a strong regulator of receptor activator of NF-κB ligand( RANKL) a key molecule in osteoclastogenesis ( 7 ). Recently, it has been shown that 1,25-(OH) 2 -D can be produced from circulating 25(OH)D locally in other cells than kidney cells, for example, in osteoblasts. In this way 1,25-(OH) 2 -D can exert its effects in an autocrine or paracrine manner (for review, see the study of Norman and Bouillon [8]).
1,25-(OH) 2 -D has important roles in many physiological systems beside calcium homeostasis: the immune system, the pancreatic beta-cells to name a few and has distinct biological responses in the related cells. Fig. 1 displays some of the roles of 1,25-(OH) 2 -D in physiological systems and the biological responses as well as diseases and health outcomes that could be related to vitamin D deficiency ( 8 ).

Overview of vitamin D and its role on physiological systems and the biological responses as well as possible vitamin D-related diseases. The three columns on the right side, respectively, indicate the following: physiological systems (the six physiological systems that the essential nutrient vitamin D 3 supports by its metabolism to 25(OH)D 3 and 1α,25(OH) 2 D 3 ); biological responses (examples of biological responses generated by 1α,25(OH) 2 D 3 in the six physiological systems); and vitamin D-deficient-related diseases (identifies for each system some of the disease states that are associated with an inadequate vitamin D nutritional status) ( 8 ).
Vitamin D status and vitamin D sources in the Nordic countries
The vitamin D status in Denmark has been evaluated in a few studies. Andersen et al. ( 9 ) performed a cross-sectional study in five different European countries, one of which was Denmark. They showed that 51% of teenage girls had 25(OH)D concentrations lower than 25 nmol/l and 93% had concentrations lower than 50 nmol/l in the winter. In addition, 17% of elderly women had concentrations lower than 25 nmol/l and 55% concentrations lower than 50 nmol/l. The median vitamin D intake from diet was 2.4 and 3.4 µg in the girls and women, respectively. Vitamin D supplements were used by 34% of the girls and 62% of the elderly women. In a recent study, Thuesen et al. ( 10 ) evaluated the vitamin D status in 6,146 randomly selected individuals aged 30–60 years that participated in a health examination in 1999–2001. The overall prevalence of vitamin D deficiency (25(OH)D <25 nmol/l) and insufficiency (25(OH)D < 50 nmol/l) was 13.8 and 52.2%, respectively. A marked seasonal variation was seen in the 25(OH)D concentrations, the median 25(OH)D concentrations were lowest in February and highest in August. Estimated dietary intake of vitamin D ranged between 0.2 and 22.5 µg/day (median 3.0 µg/day, n =6,224). Data on the use of supplements were not collected and the vitamin D sources were not explored in this study. Serum 25(OH)D concentrations were not associated with the estimated dietary intake of vitamin D. The Pakistanis are a large immigrant population in Denmark. In a study in girls, women and men of Pakistani origin, Andersen et al. ( 11 ) showed in a cross-sectional study spanning over January–November that the incidence of severe vitamin D deficiency was very common. Eighty-one percent of the girls and 84% of the women had 25(OH)D concentrations below 25 nmol/l and 46% of the girls and 40% of the women were below 10 nmol/l. Sixty-five percent of the men had 25(OH)D concentrations below 25 nmol/l and 13% were below 10 nmol/l. Almost all persons had 25(OH)D concentrations below 50 nmol/l. Use of vitamin-D-containing supplements had a positive association with S-25(OH)D for both men and women. Dietary vitamin D intake was 2.2 µg/day in men and 1.7 µg/day in women. Based on these reports, the vitamin D status in Denmark seems to be problem both in the native Danish population but especially in the Pakistanis.
In Iceland, cod liver oil is an important and traditional source of vitamin D, especially for children and the older generation, presently supplying 48% of total vitamin D from foods according to the National Nutrition Survey. Fatty fish and fortified fats are also important sources. Vitamin D intake varies considerably within the population, with 10% of adults having a habitual intake of ≤3.1 µg/day, while 10% have a habitual intake of ≥21.6 µg/day of vitamin D ( 12 ). Supplement use contributes greatly to this variation. Young adults, aged 18–30 years and not taking supplements, have a mean intake of 3.9 µg/day of vitamin D, while the same age group taking cod liver oil has a mean intake of 13.5 µg/day ( 12 ). The significance of supplement use, including cod liver oil, is also reflected in vitamin D status in Iceland, with serum 25 OHD concentrations averaging <28 nmol/l in February–March in adult men and women not taking supplements, compared with 48 nmol/l for those taking cod liver oil or other vitamin-D-containing supplements ( 13 ). The authors conclude that supplements are needed for adequate vitamin D status during winter in northern regions. Icelandic food and nutrition recommendations from 2004 advise the use of vitamin D supplements or cod liver oil ( 14 ) and pre-schools commonly supply cod liver oil to children throughout the year.
The vitamin D intake and vitamin D status has been low in Finland in all age groups. The authorities have, however, introduced fortifications schemes to broaden the sources of vitamin D in the population. In 2003, the Ministry for Trade and Affairs, based on simulations, recommended that all fluid milk products should be fortified with 0.5-µg vitamin D 3 /100 g, and all spreads with 10 µg/100 g (previously 7.5 µg/100 g). The effect of this fortification has been evaluated in a large population study of about 650 participants (aged 4–74 years) with blood samples and other data from 2002 and 2004. The median daily intake increased, for example, by 1.8 µg in 27–66 year olds and the increase in the 25(OH)D concentration was 7.0 nmol/l ( 15 ). In those using fluid milk products, the impact on intake and vitamin D status was considerable. The main sources were fish/fish products and fortified milk products, the importance of which is dependent on the age groups. The use of supplements was important as a source of vitamin D in all age groups ( 15 ). There were some groups that were still at risk – small children, pubescent girls, and young and middle-aged women. In the Findiet 2007 study, the mean daily dietary vitamin D intake of women aged 25–65 years was 5.2 µg and in 65 to 74-year-old women 6.5 µg, whereas it was 7.1 and 9.0 µg in the corresponding age groups for men ( 16 ).
In 2010, the National Board of Nutrition increased the recommendation for fortification to 1 µg/100 g fluid milk products and for spreads 20 µg/100 g. Moreover, the authorities (National Board of Nutrition; Institute of Welfare and Health; Finnish Paediatric Society) recommend since 2011 that children and youths aged 3–18 years should take a daily 7.5 µg vitamin D supplement all around the year, whereas children younger than 3 years should take a 10-µg daily supplement. Noteworthy is that vitamin D supplements have been recommended to children younger than 3 years for decades in Finland, but it has largely been given only to children during their first year of life ( 17 ) Currently, there are no published studies in Finland from 2010 to show what the actual intake and vitamin D status is in the Finnish population. Regarding ethnic groups, a recent study by Islam et al. ( 18 ) has shown that Bangladeshi women but especially Somali women has a very low vitamin D status in Finland.
The main dietary sources of vitamin D in the Norwegian population are fatty fish, fortified margarine and butter and cod liver oil supplements ( 19 ). In addition, it is common to take other vitamin D supplements. The use of cod liver oil supplements represents a long dietary tradition in Norway. A nationwide dietary survey found that 45% of middle-aged women reported cod liver oil supplement use ( 20 ). However, the use of this supplement has been found to be less among the younger population. The contribution of cod liver oil supplement to increase vitamin D intakes in Scandinavia compared to southern Europe has been described ( 21 ). A systematic review has been conducted by Holvik et al. ( 22 ) for available literature on vitamin D status in Norway. They concluded that the vitamin D status was sufficient for the majority in the general population (25(OH)D ≥50 nmol/l was considered as optimal) and that available data suggest that the vitamin D status in Norway is better than more southern locations in Europe. In spite of this, some have insufficient 25(OH)D concentrations, and that vitamin D status dropped in late winter, also in southern Norway. Some vulnerable groups were identified, that is, non-western immigrants and the elderly, especially those living in nursing homes. A working group on vitamin D in the Norwegian population, nominated by the National Council of Nutrition, recommended in their report ( 19 ) an increased fortification of foods, in particular milk, in order to improve the vitamin D status in the population including vulnerable groups.
The vitamin D intake of the adult Swedish population was reported in 1998 in the national survey, Riksmaten ( 23 ). The median daily vitamin D intake spanned from 4.0 µg/day in 17 to 24-year-old women to 5.6 µg in women aged 65 years and older. Correspondingly, the median daily vitamin D intake in 17 to 24–year-old men was 4.9 µg and 7.0 µg in men older than 65 years. The main sources were dietary fat, fish and fish products and fortified milk products. Serum 25(OH)D concentrations were not measured. A similar survey was performed in 2010–2011, but the results are not available. Vitamin D intake and status has been studied in children. In ‘Riksmaten–barn 2003’ ( 24 ), a nutrition survey in children, found that the mean intake was 6.6, 5.0 and 4.6 µg in 4-year-olds, 2nd grade and 5th grade, respectively. The higher intake in the youngest was due to the fact that 21% of them got vitamin D supplements and 28% ate fortified porridge. In a recent study, Eriksson and Strandvik ( 25 ) found that the mean 25(OH)D concentration was 76 and 68 nmol/l in 4- and 8-year olds, which could be considered satisfactory. However, a larger percentage (ca. 30%) of the older children had concentrations less than 50 nmol/l than the younger ones (<10%) and 65% of the older boys and 55% of the older girls had concentrations <75 nmol/l whereas the numbers were 50 and 40%, respectively in the younger age groups. The authors state that the comparably high 25(OH)D concentrations are due to the fact the children up to the age of five regularly get vitamin D supplementation.
Research/key questions for vitamin D
The selection of outcomes was based on our knowledge of the vitamin-D-related scientific literature. The NNR5 Working Group commented on and approved of the research questions.
The research questions for this systematic review were as follows:
- What is the effect of vitamin D from different sources on serum 25(OH)D concentrations?
- What is the relationship between 25(OH)D concentrations and different outcomes in different populations and age groups?
- What is the effect of dietary vitamin D intake on different outcomes in different populations and age groups?
- What is the effect of supplemental vitamin D on different outcomes in different populations and age groups?
- What is the effect of sun or UVB exposure on different outcomes in different populations and age groups?
- What is the UL (tolerable upper intake level) for vitamin D for different health outcomes in different populations and age groups?
- What are the interactions of vitamin D with calcium intake on different health outcomes in different populations and age groups?
- Which is the interaction of vitamin D intake or vitamin D status with vitamin A intake or vitamin A status on health outcomes in different populations and age groups?
Definitions
The exposures were:
For research question 1: diet/dose; sun exposure/season; supplements/dose/intervals; obesity; pregnancy/lactation.
For research questions 2–8: dietary vitamin D, fortified foods, supplementation and sunlight (natural UV irradiation) exposure, serum 25-hydroxy-vitamin D concentration, vitamin A intake.
Serum or plasma 25(OH)D-concentration was used as an indictor of exposure in research questions 2–8.
The following outcome measures were included:
For research question 1 : 25(OH)D. For research questions 2–5, 7 and 8 : Pregnancy outcomes and growth, bone health (all fractures, hip fractures, vertebral fractures, bone mineral density/osteoporosis, bone mass, bone quality, rickets, osteomalacia, dental health); muscle strength; falls; all cancers, breast cancer; colorectal cancer; prostate cancer; diabetes type I; diabetes type II; multiple sclerosis; obesity; total mortality; hypertension/blood pressure; cardiovascular disease (CVD) clinical outcomes; infections.
Research question 6: calcium metabolism: hypercalciuria, hypercalcemia; soft tissue calcification; renal outcomes vascular outcomes; mortality; adverse events reported in RCTs
Th e following life stages were included: infants, children, adolescents, adults, postmenopausal women, elderly, the very old.
Search methods and terms
Two expert reference librarians designed and conducted the electronic search strategy based on the research questions provided by the four investigators. The following electronic databases were searched: MEDLINE and Swemed. The search was conducted using medical subject heading terms (MESH) (see Appendix 1 ). The search was done in two batches, the first covering January 2000–March 2010 and the second March 2009–February 2011. In the first search, the investigators focused only on SLRs) and in the second on systematic reviews and randomized control trials (RCTs) published after March 2009. Furthermore, we used snowballing for SLRs and RCTs published after that and until May 2012.
Selection of articles/studies
The investigators screened all abstracts from both searches in pairs, and after that all four investigators made a common decision on the full-text articles to be acquired from the librarian. From the batches of full-text articles, we included those who met the criteria for SLRs. As regards RCT studies, only studies from Europe and North America were included. The full-text articles were examined in pairs and the four investigators made a common decision on which articles should be included and which to exclude. Eligible criteria for full-text articles were SLR, matching the research questions and healthy populations, not patients or medication, and not meta-analyses.
Quality assessment of studies
Results of systematic reviews and meta-analysis were quality assessed and evaluated using the NNR5-modified AMSTAR quality assessment tool and incorporated in the evidence tables. Quality assessment of the RCTs was made according to the NNR guidelines ( 2 ). The quality assessment methods of the studies included in the SLRs differed. The Jadad scale is one of the instruments used to assess the quality of RCTs and is referred to in some of the SLRs in this review ( 26 ).
Reporting of evidence
The evidence is reported in the evidence tables ( Appendix 2 ) and the summary tables ( Appendix 3 ).
Result of search
In total 1706 abstracts were screened ( Fig. 2 ). The search was done in two batches, the first covering January 2000–September 2010 and the second covering May 2009 to February 2011 In the first search, the investigators focused only on SLRs and in the second on SLRs and RCTs. Furthermore, the authors used snowballing for SLRs and RCTs published between March 2011 and May 2012. We primarily identified 108 studies for further consideration, whereas 1,598 studies were excluded. Finally, we included 28 studies based on the literature search and 7 by snowballing, 35 in total. The included studies are listed in the reference list and the excluded studies are listed in Appendix 4. The characteristics and quality of the SLRs and included RCTs are presented in Appendix 2, respectively. The results of the studies are presented in specific summary tables 1–23 (Appendix 3) .

Flowchart of study selection.
Noteworthy is, that two extensive SLRs, Cranney et al. ( 27 ), focusing on the effectiveness and safety of vitamin D, calcium in relation to bone health and Chung et al. ( 28 ) focusing on vitamin D, calcium and health outcomes, were performed for the North American vitamin D and calcium recommendations ( 29 ). Chung et al. ( 28 ) included material from Cranney et al. ( 27 ), and in some cases built their conclusions on this earlier evidence report.
The effect of vitamin D from different sources on serum 25-OHD concentrations (Research question 1)
Effect of dietary vitamin d on 25-hydroxy-vitamin d concentration.
We did not identify any SLR on the relationship on dietary vitamin D from natural sources and 25(OH)D-concentration.
Effect of fortified foods on 25-hydroxy-vitamin D concentration
We identified two SLRs on the effect of fortification ( 28 , 30 ). For more information, see summary table 1 . Moreover, O'Donnell et al. ( 31 ) published a paper based on part of the same material as Cranney et al. ( 27 ), which is included in this analysis. Chung et al. ( 28 ) did not perform a new SLR but based their conclusions on Cranney et al. ( 27 ).
Cranney et al. ( 27 ) included 13 RCTs that studied the effect of fortified dietary sources of vitamin D on circulating 25(OH)D-concentrations. Two of the 13 trials did not provide the vitamin D content of the dietary source and were excluded, thus 11 studies were included in the analyses. They studied a total of 1,281 subjects (697 interventions, 584 controls). All trials were carried out in adults. The quality of 6 out of 11 trials was scored ≥3 on the Jadad scale ( 26 ).
The vitamin D dietary interventions included fortified milk, nutrient dense fruit and dairy-based products, high vitamin D diet, fortified orange juice, fortified cheese and fortified bread. The only RCT with a factorial design had two other intervention groups that included an exercise program and a combined program of exercise and nutrient dense products. The type of vitamin D administered was vitamin D 3 in eight trials and was not specified in three. The vitamin D intake was 5–25 µg/day. Seven trials also specified the calcium content within the dietary intervention. The duration of the intervention ranged from 3 weeks to 24 months. Compliance was reported in four trials and was stated to be >85%.
Meta-analysis was conducted to quantify the effects of dietary sources with vitamin D with/without calcium versus placebo or calcium on serum 25(OH)D concentrations. Seven of the 11 included trials that reported (or provided sufficient data to calculate) the absolute change in total 25(OH)D or 25(OH)D 3 concentrations were included in the meta-analysis.
Combining all seven trials that investigated the effect of food fortification or dietary sources of vitamin D with or without calcium versus control was not possible due to heterogeneity of the treatment effect. However, the individual weighted mean differences demonstrated a clear trend toward a significantly higher absolute change in serum 25(OH)D concentration in the treatment group versus the control.
The positive direction of the treatment effect of dietary interventions with foods fortified with vitamin D was consistent. Those trials with low baseline 25(OH)D concentrations (i.e. <50 nmol/l) demonstrated a greater percent increase in 25(OH)D concentrations at the end of study compared to trials with higher baseline 25(OH)D concentrations (i.e. >50 nmol/l). The authors stated that observations from such indirect comparisons need to be interpreted cautiously due to differences in baseline characteristics of the study populations, the bioavailability of the vitamin D in the various food sources and the different measures of serum 25(OH)D used.
Cranney et al. ( 27 ) concluded that
Eleven of the thirteen identified trials on food fortification and circulating 25(OH)D provided the vitamin D content (5–25 µg) of the dietary source. Most trials used dairy products as the source of fortified foods. Food fortification with vitamin D resulted in significant increases in serum 25(OH)D concentrations with the treatment effect ranging from 15 to 40 nmol/L. The combined effect of fortified food from two trials with vitamin D 3 doses equivalent to 10–12 µg/d was 16 nmol/L (95% CI 12.9, 18.5). It was not possible from these trials to determine if the effect of food fortified with vitamin D on serum 25(OH)D concentrations varied by age, BMI or ethnicity.
Black et al. ( 30 ) performed an SLR based on 16 RCTs from 15 publications of which 8 were included in the Cranney et al. report ( 27 ). Five studies scored <3 on the Jadad scale and the rest scored ≥3( 26 ). Compliance rate was reported in 10 studies, which is important in food-based studies, but not included in the Jadad scale. The heterogeneity among the studies was high. The authors did not distinguish between vitamin D 3 and vitamin D 2 in the analyses. The authors concluded that
foods fortified with vitamin D increased circulating 25(OH)D concentrations in a dose-dependent manner. In addition they concluded that the treatment effect was higher in studies using doses ≥10 µg/d, in studies performed at latitudes 40 degrees and where baseline 25(OH)D concentrations were less than 50 nmol/l. Moreover, the authors calculated that a mean individual daily intake of about 11 µg vitamin D from fortified foods increased serum 25(OH)D concentrations by 19.4 nmol/l on an average corresponding to an average 1.2 nmol/l increase for each 1 µg vitamin D ingested.
Effect of supplementation on 25-hydroxy-vitamin D concentration
We identified two SLRs ( 27 , 32 ), for details see summary table 2 . Chung et al. ( 28 ) did not perform a new SLR but based their conclusions on Cranney et al. ( 27 ). They included further analyses of dose response.
Cranney et al. ( 27 ) analyzed the effect of vitamin D supplementation on circulating 25(OH)D concentrations in different age groups, and the the result are shown below.
Seven trials included term infants. Four trials used vitamin D 2 , vitamin D 3 was used in one and in three trials no information was given on the form of vitamin D. Most trials were of lower methodological quality. The authors concluded that
one trial suggested that 5 µg of vitamin D 2 may not be enough to prevent vitamin D deficiency, in some infants residing at northern latitudes. A dose-response was noted in this same trial (2.5, 5, 10 µg/day). Consistent responses to vitamin D supplementation were noted across the seven trials, and some trials suggested that infants, who are vitamin D deficient, may respond differently and require higher doses of vitamin D.
Pregnant women and lactating mothers
Six small trials of vitamin D supplementation in pregnant or lactating women were included. Three trials used vitamin D 2 and three used vitamin D 3 . All trials were of low methodological quality. The authors concluded that
25–90 µg/d of vitamin D 2 and 25 µg/d of vitamin D 3 resulted in significant increases in serum 25(OH)D concentrations in lactating mothers and in cord blood. One trial found that supplementation of lactating mothers with 25 µg of vitamin D 2 during winter months did not increase serum 25(OH)D concentrations in the infants.
Children and adolescent populations
The authors found four trials that examined the effect of vitamin D on 25(OH)D in children or adolescents with doses ranging from 5 to 50 µg of vitamin D 3 /day in three trials or 10 µg of vitamin D 2 in one trial. The study quality was rated ≥3 in three trials on the Jadad scale ( 26 ). The authors concluded that
there were consistent increases in 25(OH)D concentrations ranging from 8 nmol/L (with 5 µg of vitamin D 3 ), 16.5 (with 15 µg) to 60 nmol/L (50 µg).
Premenopausal women and younger men
Ten small trials included premenopausal women and younger males. Three trials compared vitamin D 2 to vitamin D 3 in healthy young adults. Doses of vitamin D 3 ranged from 15 to 250 µg/day and for vitamin D 2 the doses were 100 µg/day or 1,250–2,500 µg for one dose. The methodological quality of eight of the 10 trials was poor. The authors concluded that
Three trials found that vitamin D 2 and D 3 in healthy adults may have different effects on serum 25(OH)D concentrations. Vitamin D 2 appeared to have a smaller effect on serum 25(OH)D, which may have been due to more rapid clearance and/or different metabolism than vitamin D 3 . One trial compared 2500 µg vitamin D 2 orally versus injection and found a greater variability in response with the intramuscular preparation. A dose-response effect was noted in those trials that used multiple doses of vitamin D 3 .
Postmenopausal women, older men, and elderly populations
Forty-four trials were conducted exclusively in postmenopausal women and older men. Fourteen of these were performed in elderly populations living in long-term care or nursing homes. One trial was in early postmenopausal women. Doses ranged from 2.5 to 1,000 µg/day of vitamin D 3 and 225 µg vitamin D 2 /day. In three studies, single doses of 2,500–7,500 µg as injections were used. One trial was conducted in African American women. The methodological quality was ≥3 in 24 trials. One trial found that wintertime declines in 25(OH)D concentration were prevented with 12.5 µg of vitamin D 3 daily. A dose response with increasing doses of vitamin D 3 was noted. The authors also performed a meta-analysis of 16 of the 44 trials in postmenopausal women, older men, and elderly populations that investigated the effect of oral vitamin D supplementation with or without calcium versus no treatment, placebo or calcium on serum 25(OH)D concentrations. They concluded that
treatment effect of oral vitamin D 3 supplementation increases with increasing doses. Meta-regression results demonstrated a significant association between dose and serum 25(OH)D levels. The meta-regression results suggested that 2.5 µg/d of vitamin D 3 will increase the serum 25(OH)D concentrations by 1–2 nmol/L. This suggests that doses of 10–20 µg daily may be inadequate to prevent vitamin D deficiency in at-risk individuals. Vitamin D 3 doses of 17.5 µg daily or more significantly and consistently decreased serum concentrations of PTH in vitamin D deficient populations. Given the limitations in the measurement of 25(OH)D concentrations and the lack of standardization and calibration, it is difficult to suggest precise recommendations for adequate intakes, especially since optimal levels of serum 25(OH)D have not been defined.
Chung et al. ( 28 ) further analyzed the effect of vitamin D supplementation on changes in serum 25-OHD concentration based on the results from Cranney et al. ( 27 ). They plotted the net changes in serum 25(OH)D concentration against the doses of vitamin D supplementation using data from 26 RCTs with 28 comparisons in adults. Only RCTs of daily vitamin D 3 supplementation (doses ranged from 5 to 125 µg/day) alone or in combination with calcium supplementation (doses ranged from 500 to 1,550 mg/day) that provided sufficient data for the calculations were included in the plot. The studies had varied compliance rates in the vitamin D intake; limited or no adjustment for skin pigmentations, calcium intake, or background sun exposure; different vitamin D assay methodologies and measurement variability. They stated that these factors increased the heterogeneity and limited the usefulness of an overall summary estimate for an intake dose response in serum 25(OH)D concentration. Chung et al. ( 28 ) concluded that
a relationship between increasing doses of vitamin D 3 with increasing net change in 25(OH)D concentration was evident in both adults and children, that the dose-response relationships differed depending on study participants’ serum 25(OH)D status (≤40 vs. >40 nmol/L) at baseline, and depending on duration of supplementation (≤3 vs. >3 months). Vitamin D 2 supplementation was more commonly used in RCTs of infants and pregnant or lactating women, than vitamin D 3 supplementation. Results showed that supplementation of vitamin D 2 significantly increased 25(OH)D concentrations in infants, lactating mothers and in cord blood.
Cashman et al. ( 32 ) included 44 RCTs in their systematic review. In the analyses, priority was given to data from winter-based RCT ( n =12) performed at latitudes higher than 49.5 degrees N . Six of the 12 RCTs were included in Cranney et al. ( 27 ) and had a Jadad score ≥3 ( 26 ), the rest were not quality assessed but were included in the final IoM report ( 29 ). The authors concluded that
A combined weighted linear model meta-regression analyses of natural log total vitamin D intake (diet and supplemental vitamin D) versus achieved serum 25(OH)D-concentration in winter produced a curvilinear relationship. Use of non-transformed total vitamin D intake data (maximum 35 µg/d) provided for a more linear relationship. Although inputting an intake of 15 µg/d (i.e. the US RDA) into the 95% lower CI curvilinear and linear models predicted a serum 25(OH)D of 54.4 and 55.2 nmol/l, respectively, the total average vitamin D intake that would achieve 50 (and 40) nmol/l serum 25(OH)D was 8.9 µg (2.8) and 12 (6.5) µg/d, respectively. Inclusion of 95% range in the model to account for inter-individual variability increased the predicted intake of vitamin D needed to maintain serum 25(OH)D ≥50 nmol/l to 23.25 µg/d.
The authors also stated that
these results should be interpreted with caution because of the few data points in the analysis.
Vitamin D and different health outcomes (Research questions 2–4)
Vitamin d and pregnancy.
We found two SLRs on pregnancy-related outcomes and vitamin D that met our inclusion criteria ( 28 , 33 ). The reviews are presented in summary table 3 .
Chung et al. ( 28 ) evaluated one nested case-control study of healthy, nulliparous pregnant women ( n =274) that were followed from less than 16 weeks of pregnancy to delivery. Women who subsequently developed preeclampsia had lower adjusted mean 25(OH)D concentrations than controls. Early pregnancy maternal 25(OH)D concentrations below 37.5 nmol/l were associated with a fivefold increased risk of preeclampsia. Furthermore, babies of preeclamptic mothers were twice as likely to have serum concentrations below 37.5 nmol/l compared with controls. None of these associations varied with race or ethnicity. The study was rated B.
De-Regil et al. ( 33 ) reviewed six randomized trials including 1,023 pregnant women, in a report that updates a previous Cochrane report on vitamin D supplementation and maternal and neonatal outcomes. Intended maternal outcome measures were preeclampsia, gestational diabetes and vitamin D status at term. Infant outcome measures were preterm birth and low birth weight. In addition, there were a series of secondary intended outcome measures, including cesarian sections, maternal hypertension and Apgar score. Most of the studies were done in the 1980s while one was from 2008 and the dose of vitamin D given on a daily basis ranged from 20 to 30 µg. Three trials also included high doses in one of their arms: two of them used a single dose of 5,000 µg in the third trimester and one gave 15,000 µg twice during pregnancy. Five of the studies, including 623 women supplied vitamin D alone while one study of 400 women gave vitamin D in combination with calcium. None of the included studies reported on gestational diabetes or preterm birth. Preeclampsia was only reported in the one study giving both calcium and vitamin D, and found no difference in risk between the women receiving supplements compared with the placebo group.
The authors’ conclusions were as follows:
The use of vitamin D supplementation during pregnancy improves vitamin D concentrations as measured by 25-hydroxyvitamin D at term. However, the clinical significance of this finding is yet to be determined as there is currently insufficient high quality evidence relating to the clinical effects of vitamin D supplementation during pregnancy. Good quality studies are needed to determine the usefulness and feasibility of this intervention as a part of routine antenatal care.
Vitamin D and growth
One SLR ( 28 ) was identified evaluating seven interventions and two observational studies on vitamin D and growth in newborns, infants, or children. The review is presented in summary table 4 . Two interventions included in the review, where pregnant women in India received 15,000 µg in the 7th and 8th months of pregnancy, were the only intervention trials reporting statistically significant effects of vitamin D supplements on growth. The studies were rated C as important aspects of the methodology were not reported. Dietary vitamin D intakes of these mothers were estimated to be less than 0.7–0.9 µg/day. A British trial of 126 Asian women receiving 25 µg/day during the third trimester reported no effects on birth weight or length even though there was an insignificant reduction in the number of low birth-weight infants in the intervention group. Similarly, no significant effect was demonstrated by French, Chinese, or Australian trials. All of these trials were rated C for methodological quality except for the British trial which was rated B. Two cohort studies were also evaluated, one British and one Australian. Neither study showed significant associations between maternal serum 25(OH)D and growth of the offspring. The authors are cautious in their conclusions regarding the evidence on vitamin D related to growth, citing lack of methodologically solid studies . Chung et al. ( 28 ) also reviewed the relationship between vitamin D and calcium and growth. They found one C-rated study from India comparing vitamin D and calcium supplementation in women in their third trimester to no supplementation. Infants of the women receiving supplementation were significantly heavier.
Vitamin D and bone health
We identified two SLRs that met our inclusion criteria ( 28 , 34 ). For the results of the studies see summary table 5 .
Chung et al. ( 28 ) built on and updated the AHRQ-Ottawa evidence-based report of Cranney et al. ( 27 ), reviewing concentrations of 25(OH)D related to established vitamin-D-dependent rickets in infants and young children. As an updated search did not identify any new studies, they simply referred to Cranney et al. ( 27 ). In 6 of the 13 studies reviewed, mean or median 25(OH)D-concentration in children with rickets was <27.5 nmol/l, whereas it was between 30 and 50 nmol/l in the other studies. Most studies were conducted in developing countries with low calcium intake. Low calcium intake can influence the relationship between 25(OH)D and rickets, and the 25(OH)D threshold for rickets in populations with high calcium intake (e.g. North America) is unclear. The Cranney et al. ( 27 ) report thus concluded:
There is fair evidence for an association between low serum 25(OH)D and established rickets, regardless of assay type (RIA, CPBA, HPLC). There is inconsistent evidence to determine if there is a threshold concentration of serum 25(OH)D above which rickets does not occur.
In a Cochrane review by Lerch and Meissner ( 34 ), the aim was to evaluate the effects of interventions on the prevention of nutritional rickets in term-born children. The review was limited to studies performed in the last 50 years. Only four trials were included and in two of them no rickets occurred. The reference was placebo or no intervention. In a Turkish trial, vitamin D showed a reduced risk of rickets compared to no intervention. In a Chinese trial, a combined intervention of vitamin D and calcium supplementation and nutritional counseling reduced the risk of rickets compared to no intervention. They conclude:
There are only few studies on the prevention of nutritional rickets in term born children. Until new data become available, it appears sound to offer preventive measures (vitamin D or calcium) to groups of high risk, like infants and toddlers; children living in Africa, Asia or the Middle East or migrated children from these regions into areas where rickets is not frequent.
We identified three systematic reviews that met our inclusion criteria ( 27 , 35 , 36 ). For the results of the studies see summary table 6 .
The Cochrane review by Avenell et al. ( 35 ), comprising postmenopausal women and men over 65 years of age, concluded that based on available RCTs, it appears unlikely that vitamin D alone is effective in preventing hip fracture, vertebral fracture, or any new fracture. However, a significant reduction in the incidence of hip fracture in those receiving vitamin D (dose 10–20 µg/day) and calcium versus placebo or no treatment was found. Subgroup analysis showed a significant reduction in the subgroup of institutional residents but not in community dwellers. However, the difference between the two subgroups was not statistically significant. The reduction in incidence of non-vertebral fractures was not significant in those given vitamin D and calcium. However, in the subgroup analysis on residential status, a statistical significant effect was found in the institutional residents’ subgroup but not in community dwellers. There was no reported effect of vitamin D and calcium on clinical vertebral fracture. Avenell et al. ( 35 ) reported on the scientific quality on nine different items with scores from 0 to 2. No overall score was given.
The SRL by Vestergaard et al. ( 36 ) mainly refers to Avenell et al. ( 35 ) described above. In addition, the results from the DIPART study ( 37 ) are referred to. In this patient-based pooled analysis of seven major vitamin D fracture trials with 68,500 participants, no significant effect of vitamin D alone compared to placebo/no vitamin D was found for any fracture or hip fracture (doses of vitamin D 10–20 µg/day). However, the overall risk of fracture was reduced in those given combined supplementation with vitamin D and calcium compared to placebo/no vitamin D. The risk of hip fracture was hazard ratio (HR) 0.84, 95% CI 0.70–1.01, later corrected to HR 0.83, 95% CI 0.69–0.99 due to a coding error in the original publication, conf. BMJ 2010; 340:b5463). One of the studies included in DIPART included a drug review in those receiving vitamin D and calcium. Additional analysis excluding this study from the pooled analysis attenuated markedly the effect of vitamin D and calcium on hip fractures but not on all fractures. Vestergaard et al. ( 36 ) also reported on the results from an RCT published in 2010 ( 38 ) in 2,258 women, aged 70 years or older. A single high dose of vitamin D 3 (12,500 µg) µg or placebo was given orally once a year over a period of 3–5 years. Vitamin D 3 significantly increased the risk for any fracture compared with placebo. In addition, the incidence of falls was significantly increased in the vitamin D 3 group compared to placebo. The increased incidence of falls was most prominent in the first 3 months after dosing with vitamin D 3 . Vestergaard et al. ( 36 ) concluded that ‘concerning fracture prevention in postmenopausal women, vitamin D plus calcium is likely to be beneficial, whereas vitamin D alone is unlikely to be that’.
The SLR by Chung et al. ( 28 ) included and updated the Cranney et al. ( 27 ) evidence-based report and most of the results are presented/specified in the Cranney report. When we refer to the Chung et al. ( 28 ), this also includes the Cranney et al. report ( 27 ).
It was concluded that based on observational studies, the evidence was inconsistent for an association between serum 25(OH)D and the risk of fractures. Combining the results from 13 RCTs intervening with vitamin D 2 or D 3 (with or without additional calcium supplementation), a non-significant reduction in total fractures was found. Studies intervening with vitamin D alone showed no effect on fracture incidence by meta-analyses. However, meta-analyses of studies intervening with vitamin D 3 (10–20 µg/day) plus calcium, showed a reduction in the risk of total fractures and hip fractures. In a subgroup analysis, a significant effect was only present in institutionalized elderly. It was stated that one possible explanation for this was that the studies in institutionalized elderly achieved on average a higher 25(OH)D concentration at the end of the study than the studies in community dwellers. The combined result for studies with higher S-25(OH)D at follow-up (≥74 nmol/l) was a significant reduction in total fractures, which was not the case for studies achieving <74 nmol/l. Cranney et al. ( 27 ) stated that this should be interpreted with caution as 25(OH)D was only determined in subsamples and there was variability in measurement methods.
None of the trials in the meta-analysis were performed in premenopausal women.
Vitamin D 3 combined with calcium is effective in reducing fractures in institutionalized populations, whereas the evidence for community dwellers is less strong.
The Cranney et al. ( 27 ) report was updated in the Chung et al. ( 28 ) report by a new literature search, and a new RCT reporting on fracture showing no effect of an intervention with vitamin D 2 alone versus placebo was identified. They also identified an RCT quality rated B, performed in women aged 17–35 years, reporting that 20 µg vitamin D/day combined with a daily supplementation of 2-g calcium compared to placebo reduced the risk of stress fracture from military training.
Bone mineral density and bone mineral concentration
We identified two systematic reviews that met our inclusion criteria ( 28 , 39 ). For the results of the studies, see summary table 7 . In addition, we identified one RCT in the second search ( 40 ).
Chung et al. ( 28 ) included the Cranney et al. ( 27 ) evidence-based report, and most of the results are presented and specified in the Cranney et al. ( 27 ) report. When new data were identified in the update made by Chung et al. ( 28 ), this is mentioned in the text and/or in the summary in table 7 .
Cranney et al. ( 27 ) addressed whether specific concentrations of S-25(OH)D were associated with bone health outcomes in infants, older children and adolescents, pregnant and lactating women, and postmenopausal women and elderly men. They also addressed the evidence regarding the effect of vitamin D supplementation on bone density in women of reproductive age and postmenopausal women and elderly men. Moreover, they also reported on the association between S-25(OH)D and S-PTH. Details are given in the summary tables (summary table 7 ). They state the following:
There was fair evidence for an inverse relation between S-25(OH)D and S-PTH at low concentrations of 25(OH)D. A threshold may exist around 27 nmol/l. The evidence for an association between specific concentrations of 25(OH)D and bone mineral content (BMC) was inconsistent.
Older children and adolescents
No studies assessed the relation between 25(OH)D concentration and fracture. There was fair evidence for an inverse relation between 25(OH)D and s-PTH concentrations. The plateau of PTH concentration ranged from 25(OH)D concentrations of 30–83 nmol/l. They also concluded that there was fair evidence for 25(OH)D concentration being associated with a change in bone mineral density (BMD)/BMC. However, results from two RCTs did not consistently confirm that vitamin D supplementation had an effect. Moreover, they referred to a Finnish RCT ( 41 ) in 228 adolescent girls published after they had done their systematic search. The intervention was two doses of vitamin D3 (5 and 10 µg daily) compared to placebo. In per protocol analyses, they reported positive effects on BMC where mean S-25(OH)D >50 nmol/l was achieved in the intervention groups. The results were not statistically significant in the intention to treat analysis. In a cohort study, maternal vitamin D status was weakly related to whole body and spine BMC in children aged 9 years. In a Danish RCT among Pakistani immigrants with very low vitamin D status at baseline, BMD was unaffected by a one-year intervention with 10 or 20 µg/day vitamin D versus placebo.
Pregnant and lactating women
During pregnancy, there was fair evidence for a negative association between 25(OH)D and S-PTH concentrations, but insufficient evidence for a relation between 25(OH)D concentration and change in BMD. One good cohort study found no relationship between 25(OH)D concentration and BMD during lactation.
Postmenopausal women and older men
In five RCTs and three cohort studies, no association between 25(OH)D concentration and BMD or bone loss was found. A significant association between 25(OH)D concentration and bone loss was found in four cohort studies, most evident at the hip sites. The evidence for a relationship between 25(OH)D concentration and BMD in the lumbar spine was weak. An association between 25(OH)D concentration and BMD was suggested in six case-control studies, and the association was most consistent for femoral neck BMD. They conclude:
There was discordance between the results from RCTs and the majority of observational studies that may be due to the inability of observational studies to control for all relevant confounders. Based on results of the observational studies, there is fair evidence to support an association between serum 25(OH)D and BMD or changes in BMD at the femoral neck. Specific circulating concentrations of 25(OH)D below which bone loss at the hip was increased, ranged from 30–80 nmol/L.
Effect of vitamin D supplementation on bone density in women of reproductive age and postmenopausal women and elderly men
Cranney et al. ( 27 ) concluded that there was good evidence for vitamin D + calcium supplementation leading to a small increase in spine, femoral neck, total hip, and total body BMD. Based on available studies, it was less certain that vitamin D supplementation alone has an effect on BMD.
In a Cochrane review by Winzenberg et al. ( 39 ) including data up to autumn 2009 (6 RCTs, 541 subjects receiving vitamin D, and 343 placebo), the objective was to ‘determine the effectiveness of vitamin D supplementation for improving bone mineral density in children’. The dose administered ranged from 3.3 daily to 350 µg/week. Overall, they did not find any statistically significant effect of vitamin D supplementation on total body BMC, hip BMD, or forearm BMD, whereas a small effect on lumbar BMD was suggested. No statistically significant difference was found between studies using a high or low dose of vitamin D. The difference in effects between studies with high and low baseline S-25(OH)D studies was not statistically significant (total body BMC, p =0.09 for difference), although in studies with participants with low S-25(OH)D (≤ 35 nmol/l), a significant effect of supplementation was found for total body BMC and lumbar BMD.
They concluded that
These results do not support vitamin D supplementation to improve bone density in healthy children with normal vitamin D levels, but suggest that supplementation of deficient children may be clinically useful. Further RCTs in deficient children are needed to confirm this.
We also identified one new Danish RCT by Mølgaard et al. ( 40 ) with rating B. In this double-blinded RCT, 221 Danish girls aged 10–11 years were randomized to take vitamin D 3 (5 or 10 µg) or placebo over 1 year. Overall, the intervention had no effect on BMC or BMD (total body and lumbar spine). Compared to the somewhat similar study by Viljakainen et al. ( 41 ), which only included girls from September to March (and which found an effect in the compliance controlled analysis), the current study included girls throughout the year.
Vitamin D and dental health
We only found one, C-rated, SLR ( 42 ) (see summary table 8 ) including several nutrients with the endpoint being periodontal disease. Only one of the included original papers was on vitamin D. In this cross-sectional study, those in the lowest quartile of 25(OH)D concentration had higher clinical attachment loss compared to those in the highest quintile. The authors conclude that ‘the relationship between vitamin D and periodontal disease in elderly is unknown and not well researched’.
Vitamin D and falls
We identified seven SLRs ( 27 , 28 , 43 – 47 ), the results of which are presented in the summary table 9 . The definition of ‘falls’ and ‘falling’ varied among the included trials. It should be noted that the trials included in the different SLRs were mainly the same but with some variation due to inclusion and exclusion criteria and timeframes.
Chung et al. ( 28 ) included and updated the report by Cranney et al. ( 27 ), and most of the results are presented and specified in the Cranney report. This report included two additional RCTs related to vitamin D and falls. Chung et al. ( 28 ) concluded that these reports did not change the conclusion made by Cranney et al. ( 27 ).
Cranney et al. ( 27 ) evaluated the association of 25(OH)D concentrations with falls in postmenopausal women and elderly men. One RCT, three prospective cohorts and one case-control study were included in their analyses. The subjects included in the studies were elderly men and women. The RCT and the cohort studies were of good quality and the case-control of fair quality. The authors concluded that
There is fair evidence of an association between lower serum 25(OH)D concentrations and an increased risk of falls in institutionalized elderly. PTH may be an important confounder. One study suggested a specific serum 25-(OH)D concentration of 39 nmol (l below which fall risk is increased.
Cranney et al. ( 27 ) also asked ‘What is the evidence regarding the effect of supplemental vitamin D on falls in postmenopausal women and elderly men?’ A total of 14 trials in 16 publications were included, 12 of which were RCT with a parallel design and 4 using a factorial design. Eleven of the RCTs had a Jadad score ≥3 and the score of the factorial studies was less than three ( 26 ). Vitamin D was given by injection in two studies. Oral vitamin D was given as vitamin D 3 in all but one study. Oral vitamin D was given without calcium in three trials. Meta-analyses were conducted using data from the 12 RCTs. Oral vitamin D did not reduce the risk of falls in comparison to placebo or calcium. Oral vitamin D with calcium showed a reduction in falls as compared to placebo or calcium. Injectable vitamin D 2 did not reduce the risk of falls in comparison to placebo. The authors summarized that the combined results from 12 trials ( N =14,101) demonstrated a small reduction in falls with vitamin D 2 /D 3 (oral or injectable)±calcium. In the two factorial design trials, one demonstrated a significant fall reduction in postmenopausal women taking vitamin D 3 plus calcium (whereas the other trial did not show a reduction in falls in elderly individuals taking vitamin D 2 ). Moreover, the authors summarized that the results from trials examining the effect of supplemental vitamin D on falls are consistent, with 12 of the 14 trials demonstrating a non-significant reduction in falls. However, when combining RCTs (by an intervention method), there is inconsistent evidence regarding the effect of supplemental vitamin D on falls. The combination of 12 trials of either oral or injectable vitamin D 2 /D 3 ±calcium did demonstrate a small reduction in fall risk. Combination of eight RCTs of oral vitamin D 2 /D 3 supplementation with calcium showed a reduction in fall risk, whereas four RCTs of oral vitamin D 3 alone did not. Subgroup analyses showed a significant reduction in falls upon combining trials of postmenopausal women only. Sensitivity analyses showed a significant reduction in falls when combining: (i) RCTs that explicitly defined falls and the method of fall ascertainment; and (ii) those in which the allocation concealment was unclear. However, combining trials by degree of compliance and loss to follow-up did not result in significant reductions Cranney et al. ( 27 ) concluded that ‘there is inconsistent evidence that supplemental vitamin D reduces falls in postmenopausal women and older men’.
Kalyani et al. ( 43 ) included 10 RCTs performed in older adults for a systematic review on vitamin D treatment for the prevention of falls. Vitamin D 3 was used in six studies, vitamin D 2 in three studies and alfacalcidiol (a synthetic analog) in one study. The methodological quality of the studies was good in general. In pooled analysis, vitamin D (5– 25 µg/day) resulted in 14% fewer falls than calcium or placebo. According to this, SLR the following subgroups had significantly fewer falls: community-dwelling (aged <80), adjunctive calcium supplementation, no history of fractures or falls, duration longer than 6 months, vitamin D 3 , and a dose of 20 µg or greater. Meta-regression demonstrated no linear association between vitamin D dose or duration and treatment effect. Post-hoc analysis including seven additional studies (17 in total) without explicit fall definitions yielded smaller benefit and more heterogeneity but found significant intergroup differences favoring adjunctive calcium over none. The authors concluded that ‘vitamin D treatment effectively reduces the risk of falls in older adults’.
Cameron et al. ( 44 ) studied interventions for preventing falls in older people in nursing care facilities and hospitals and included 41 trials (25,422 participants). Five trials tested the effect of vitamin D supplementation on falls. The quality of the studies was generally good. Pooled data from the four studies with 4,512 participants that provided falls rate data show a statistically significant reduction in the rate of falls. Pooled data from all five studies with 5,095 participants did not show a reduction in the risk of falling. The authors stated that caution may be required with interpretation of these pooled data because of statistical and clinical heterogeneity. Two studies investigated vitamin D 3 and calcium and one vitamin D 2 in combination with calcium. Two studies compared vitamin D plus calcium to calcium and showed a significant reduction on rate of falls but no reduction in risk of falling. Generally, the baseline serum 25(OH)D concentrations were low in four of these studies. The authors did not distinguish between trials including or not including calcium. The authors concluded that 'vitamin D supplementation is effective in reducing the rate of falls in nursing care facilities’.
Gillespie et al. ( 45 ) included 13 RCTs focusing on the prevention of falls in older people living in the community. Thirteen studies (23,112 enrolled participants) evaluated the efficacy of vitamin D supplementation, either alone or with calcium co-supplementation for fall prevention. Two studies contained multiple intervention arms. The overall analysis of vitamin D versus control did not show a statistically significant difference in the rate of falls or risk of falling. A subgroup analysis showed no significant difference in either rate of falling or risk of falls in trials recruiting participants with higher falls risk or trials not so doing, and no significant difference in effect size between the subgroups in either analysis. The rate of falls was significantly reduced in trials with participants with lower 25(OH)D concentrations but not in participants not selected. There was a significant difference between these two subgroups with a greater reduction in rate of falls in the subgroup of trials only recruiting participants with lower 25(OH)D concentrations. The authors did not distinguish between trials including or not including calcium. The authors’ conclusion was
Overall, vitamin D does not appear to be an effective intervention for preventing falls in older people living in the community, but there is provisional evidence that it may reduce falls risk in people with low vitamin D levels [25(OHD)].
Michael et al. ( 46 ) published an SLR on primary-care-relevant interventions on prevention of falling in older adults. It included nine trial of vitamin D supplementation. Five of these included only women and the proportion of women in the others was 51–80%. Five trials were conducted in populations defined as high risk because of recent falls or vitamin D deficiency. The remaining four studies used populations that were unselected except for ages 65 years or older. All studies were rated as fair quality. The daily oral doses of vitamin D in the intervention ranged from 2.5 to 25 µg/day (median: 20 µg). One study provided a single intramuscular injection of 15,000 µg of vitamin D. Two studies evaluated vitamin D 2 and the remaining studies evaluated vitamin D 3 . Six trials included calcium supplements with vitamin D. The control groups ranged from no intervention to placebo or calcium supplements only. Vitamin D with or without calcium was associated with a 17% (CI: 11–23%) reduced risk of falling during 6–36 months of follow-up. Trials of vitamin D with calcium compared with no treatment or placebo did not support any added benefit of calcium.
The authors concluded that
There is strong evidence that several types of primary care applicable falls interventions (i.e. comprehensive multifactorial assessment and management, exercise/physical therapy interventions, and vitamin D supplementation) reduce falls among those selected to be at higher risk for falling.
Murad et al. ( 47 ) found 26 trials of moderate quality that enrolled 45,782 participants, the majority of which were elderly and female to evaluate the existing evidence on vitamin D use and the risk of falls. Eight studies used vitamin D 2 and 18 vitamin D 3 with or without calcium. In 24 studies, vitamin D was given orally and intramuscularly in the remaining two. The results indicated that vitamin D use was associated with a statistically significant reduction in the risk of falls. This effect was more prominent in subjects who were vitamin D deficient at baseline and in studies in which calcium was co-administered with vitamin D. The quality of evidence was low to moderate because of heterogeneity and publication bias, 19 studies were rated high and seven were low. The authors concluded that
vitamin D combined with calcium reduces the risk of falls. The reduction in studies without calcium co-administration did not reach statistical significance. The majority of the evidence is derived from trials enrolling elderly women.
Vitamin D and muscle strength or function
We identified two SLRs ( 48 , 49 ) that included the effects of vitamin D on muscle strength, which are presented in the summary table 10 .
Stockton et al. ( 48 ) included 17 studies. Inclusion criteria included randomized RCTs involving adults, who were older than 18 years of age. The quality of the studies was assessed on the PEDro scale and a median score of 8 out of 10 (range 4–10; mode 8) was found. The trials used a variety of vitamin D supplementation regimes. Six trials compared vitamin D alone with placebo, four of which used vitamin D 2 , and two used vitamin D 3 . One study compared 1,25(OH) 2 D with placebo. Treatment with a combination of vitamin D 3 and calcium supplements was used in nine studies. Five studies compared vitamin D and calcium with calcium alone, three studies investigated calcium and vitamin D versus placebo and one study used calcium and vitamin D versus nothing. Finally, one study investigated vitamin D via sunlight exposure (with a clearly defined exposed region and a documented daily exposure time) to usual care. Two studies did not state baseline 25(OH)D concentration, participants in four studies had baseline 25(OH)D >50 nmol/l, the mean baseline 25(OH)D level was 25–50 nmol/l in seven studies, and <25 nmol/l in four studies.
Meta-analysis showed no significant effect of vitamin D supplementation on grip strength or proximal lower limb strength in adults with 25(OH)D concentrations >25 nmol/l. Pooled data from two studies in vitamin D deficient participants (25(OH)D <25 nmol/l) demonstrated a large effect of vitamin D supplementation on hip muscle strength. The Authors′ conclusions were
vitamin D supplementation does not have a significant effect on muscle strength in adults with baseline 25(OH)D >25 nmol/L. However, a limited number of studies demonstrate an increase in proximal muscle strength in adults with vitamin D deficiency.
Muir et al. ( 49 ) included 13 RCTs, of which 8 were included in the Stockton analyses ( 48 ). The authors focused on the relation between vitamin D and balance, gait, and muscle strength as outcomes. The average age of the subjects in the studies was 78±4.1 years.
Statistically significant improvements in physical performance were noted in nine studies. Only one study demonstrated a beneficial effect on balance of a single large dose of vitamin D. All studies with daily doses of 20–25 µg demonstrated beneficial effects on balance and lower extremity muscle strength. The same vitamin D doses had beneficial effects in the two general populations of community-dwelling and older adults in institutional dwellings. Six of the eight studies that showed a beneficial neuromuscular effect included calcium supplementation in the regimens.
Twelve of the 13 RCTs included in this systematic review reported mean serum 25(OH)D concentration at baseline. Ten of these were in the deficiency range (<50 nmol/l) and two studies in the insufficiency range (50–75 nmol/l). Ten studies reported mean serum 25(OH)D concentrations at the end of the intervention period. In the intervention groups, three studies reached normal 25(OH)D concentrations with vitamin D supplementation and achieved improvements in muscle strength, gait, or balance function. Six studies showed an increase from <50 nmol to >50 nmol but <75 nmol/l after intervention, and four demonstrated a significant positive effect on physical function. One study was not able to improve the low 25(OH)D concentrations with treatment and did not demonstrate a positive effect on physical function outcomes. Statistically significant improvements in physical performance were noted in nine studies. Only one study demonstrated a beneficial effect on balance of a single large dose of vitamin D. All studies with doses of 20–25 µg/day demonstrated beneficial effects on balance and lower extremity muscle strength. Vitamin D doses of 20–25 µg had beneficial effects in the two general populations of community-dwelling and institutional-dwelling older adults. Six of the eight studies that showed a beneficial neuromuscular effect included calcium supplementation in the regimens.
Meta-analysis was performed for the outcomes of balance (body sway, Timed Up and Go (TUG) test), lower extremity muscle strength (knee extension), and grip strength without stratification according to dose or treatment regimen. The summary standardized mean difference, derived from studies with a total of 207 participants, on postural sway indicating a reduction in sway. Three studies with a total of 274 participants showed a decrease in time to complete the TUG test. A positive gain in knee extension strength was found.
Muir et al. ( 49 ) concluded that
vitamin D supplementation in doses of 20 µg to 25 µg/d have a beneficial effect on balance and muscle strength. An effect on gait was not found, although the studies that evaluated gait were of lower methodological quality and used low doses of vitamin D.
Vitamin D and cancer
We identified four SLRs that meet our inclusion criteria ( 28 , 50 – 52 ) regarding vitamin D and cancer.
Total cancer. Two of the identified SLRs presented data on the relationship between vitamin D and total cancer ( 28 , 51 ). Details of the SLRs are given in summary table 11 .
In the report by Chung al. ( 28 ), two B-graded RCTs in addition to two B- and C-graded cohort studies were included. The findings were sorted by some lifestage groups, that is, 19–50, 51–70, and ≥71 years, in addition to postmenopausal women. None of the included studies showed significant relationships between either total cancer and serum 25 (OH) D concentrations (the cohort studies) or supplement intakes (the RCTs, 25 µg/day or 2,500 µg/month). No gender interaction was found.
The IARC report ( 51 ) included three original papers on serum 25(OH)D concentrations and total cancer mortality. No scientific quality of the studies was included. These were all cohort studies of which one found no significant relationship between 25(OH)D concentration and total cancer whereas another study found a significant twofold increased risk for cancer deaths in subjects with 25(OH) D concentrations below 37.5 nmol/l. The third cohort found that an increment of 25 nmol/l was significantly associated with 17% reduction in total cancer incidence and 29% reduction in cancer mortality.
Colon/colorectal cancers
Colon or colorectal cancers were included in all four of the identified SLRs ( 28 , 50 – 52 ) and are summarized in summary table 12 . The report by the World Cancer Research Fund ( 50 ) concluded that
the evidence on vitamin D was inconsistent and stated that there is limited evidence suggesting that foods containing vitamin D, or better vitamin D status, protect against colorectal cancer.
The evidence for a protective effect of intakes of food containing vitamin D and colorectal cancer was therefore rated as limited suggestive .
The overall conclusion in the IARC report ( 51 ) on vitamin D and colorectal cancer was that the observational evidence for an inverse association between serum 25(OH)D concentrations was consistent and persuasive , however evidence for a causal link is limited due to possible confounding which is not controlled for. No scientific quality of the studies was included. The report states in the overall conclusion, RCTs have ‘not demonstrated an effect of vitamin D supplementation on colorectal cancer risk, but due to several issues (doses, interaction, duration), they cannot be judged as contradictory to the evidence from observational studies either’.
Chung et al. ( 28 ) identified one B-rated RCT, one B-rated cohort study, and five B-rated and two C-rated nested case-control studies on the relationship between vitamin D and colorectal cancers. The RCT was based on the relationship between vitamin D 3 supplementation and cancer and was conducted among the elderly. This study reported negative results for supplemental vitamin D 3 versus no supplements. The B-rated cohort study included in the Chung et al. report ( 28 ), found a reduced risk of colorectal cancer associated with higher concentrations of 25(OH)D, that is, concentrations <50 nmol/l as reference gave adjusted OR at 0.44 (0.20–0.95) and 0.28 (0.11–0.68) for levels 50–80 and ≥80 nmol/l, respectively. Chung et al. ( 28 ) reported that most nested case-control studies found no significant associations between 25(OH)D concentrations and risk of colorectal cancer incidence or mortality, except for two of the three B-rated nested case-control studies in women, where statistically significant trends between higher 25(OH)D concentrations and lower risk of colorectal cancer were found. However, no individual quartile of 25(OH)D concentration had a significantly increased risk of colorectal cancer when compared to the reference quartile.
In the SLR by Yin et al. ( 52 ), one cohort study was included and seven nested case-controls. No scientific quality of the studies was included. The authors’ conclusion was that
the results support that serum 25(OH)D concentration is inversely related to colorectal cancer risk.
Breast cancer. Breast cancer was included in three of the identified SLRs ( 28 , 50 ) ( 51 ). Details of the SLRs are presented in summary table 13 .
The overall conclusion in the IARC report ( 51 ) suggest
observational evidence of an inverse association between 25(OH)D and breast cancer, however, the overall evidence is weak when case-control are not included in the meta-analysis and the heterogeneity between studies are large.
No scientific quality of the studies was included.
The Chung et al. ( 28 ) report included one B-rated cohort study and two B-rated nested-case control studies on the relationship between vitamin D and breast cancer. The report concluded that studies on vitamin D intake and risk of breast cancer were generally negative and points out that studies on 25(OH)D concentrations and breast cancer risk were very heterogeneous. Chung et al. ( 28 ) concluded that meta-analysis showed a non-significant protective effect on 25(OH)D concentration in blood and breast cancer, but based on very heterogeneous results. One cohort study (NHANES III) assessed 25(OH)D concentrations and the risk of breast-cancer-specific mortality, and found a significant decrease in breast-cancer-specific mortality during 9 years of follow-up in those with 25(OH)D concentrations >62 nmol/l. However, the nested case-control studies did not find a relationship between 25(OH)D and risk for breast cancer (7–12 years follow-up time).
The World Cancer Research Fund report ( 50 ) evaluated both post- and pre-menopausal breast cancer in relation to vitamin D exposure. However, the data were either of too low quality, too inconsistent, or the number of studies too few to allow conclusions to be reached.
Prostate cancer. Prostate cancer was included in three of the identified SLRs ( 28 , 50 ) ( 51 ). Details of the SLRs are presented in summary table 14 .
The Chung et al. report ( 28 ) identified 12 studies, all nested-case control studies, on the relationship between vitamin D and prostate cancer in 14 publications. Three of these were rated B and the rest was rated C in scientific quality. Ten publications reported no relationship between 25(OH)D concentrations and prostate risk. One study, rated C, found an increased risk associated with the lowest quartile compared with the highest quartile (<30 compared to >55 nmol/l). This study also found an increased risk for prostate cancer in men <52 years but not for the ≥51 years old with ≤ 40 nmol/l 25(OH)D concentrations compared with >40 nmol/l. A later publication, rated C, based on men from the Nordic countries showed a U-shaped relationship between 25(OH)D concentrations and prostate cancer risk.
The IARC report ( 51 ) concluded that ‘ observational studies have provided evidence for little or no effect of vitamin D and prostate cancer’. Moreover, the World Cancer Research Fund report (WCRF, 50) evaluating vitamin D and prostate cancer concluded that
data were either of too low quality, too inconsistent, or the number of studies too few to allow conclusions to be reached.
Vitamin D and diabetes
Diabetes type 1. One SLR was identified on the relationship between vitamin D and diabetes type 1 ( 53 ). Five studies were included, that is, one cohort study rated B and four case-controls rated B. The overall conclusion in their work was that
supplementation with vitamin D in early childhood may offer protection against diabetes type 1, however, randomized controlled trials are needed to establish causality.
Details for this SLR are given in summary table 15 .
Diabetes type 2. We identified two systematic reviews ( 54 , 55 ) and one RCT ( 56 ) on the relationship between vitamin D and risk for diabetes type 2. The papers are summarized in summary table 16 .
Parker et al. ( 54 ) included nine studies in their SLR and meta-analysis. No grading of scientific quality of the included studies was given. They found an overall decrease in the prevalence of diabetes associated with higher 25-(OH)D concentrations. The conclusion in their work was that ‘high levels of vitamin D were associated with substantial decreased risk of diabetes type 2 and that further controlled trails are needed to evaluate causal associations’.
Pittas et al. ( 55 ) included four studies (two graded as fair quality and two as good) based on three cohorts. In addition, eight RCTs, three graded as good and five as fair, were included on this SLR. Pittas et al. ( 55 ) concluded that
the relationship between vitamin D and diabetes type 2 remains uncertain and that trials showed no clinical significant effect of vitamin D supplementation at the dosages given.
The RCT ( 56 ) did not support a protective effect of 20 µg vitamin D/day on diabetes type 2. We rated this as grade A in scientific quality. Diabetes was, however, not the primary outcome in this study.
Vitamin D and multiple sclerosis
No SLR on the relationship between vitamin D and multiple sclerosis for the general healthy population was identified in our search or in the additional search on recent RCTs.
Vitamin D and body weight
We only found one SLR ( 28 ) reviewing the relation between vitamin D and body weight. Three RCTs intervening with vitamin D alone were identified, and no effect on body weight was found. Chung et al. ( 28 ) also reviewed two RCTs intervening with vitamin D and calcium. In the Women's Health Initiative (methodological quality rate B) intervening with 10-µg vitamin D and 1,000-mg calcium (about 36,000 subjects) over 7 years lead to a statistical but not clinical significant effect (the weight change was 0.13 kg lower in the intervention group). In the other, and much smaller RCT ( n =63), the intervention group (10-µg vitamin D and 1,200-mg calcium daily) lost 1 kg more over 15 weeks than the control group, but the difference was not statistically significant (methodological quality rate C). For details see summary table 17 .
Vitamin D and total mortality
We identified three SLRs that met our inclusion criteria ( 27 , 35 , 54 ). Please refer to summary table 18 for details.
In the Cochrane review by Avenell on vitamin D and fractures ( 35 ), they also assessed the effect of interventions with vitamin D on deaths (total mortality) as a secondary endpoint. Based on 23 trials, the risk ratio (RR) of death was 0.97 (95% CI 0.93–1.01) in those given vitamin D with or without calcium compared to those given placebo or calcium. However, in those given vitamin D plus calcium versus placebo or control (14 trials, 54,203 participants), the RR of death was 0.94 (95% CI 0.89–0.99).
The Cranney et al. report ( 27 ) concluded that data from four cohorts suggest no association between baseline 25(OH)D concentrations and total mortality, but one cohort reported a statistically significant inverse trend. In meta-analyses including four RCTs, (13,899 participants, supplementation with vitamin D alone) had no significant effect on all-cause mortality (RR 0.97, 95% CI 0.92–1.02), and neither did supplementation with vitamin D and calcium (11 trials, 44,688 persons) result in a significant reduced mortality (RR 0.93, 95% CI 0.86–1.0) although the point estimate was very similar to that found in Avenell et al. ( 35 ).
A Cochrane review ( 57 ) reported that intervention with D 3 with or without calcium versus placebo or no intervention (32 studies, 74,789 participants) resulted in a 6% reduction in total mortality (RR 0.94, 95% CI 0.91–0.98). The effect was only significant in trials giving vitamin D 3 in combination with calcium. However, D 3 alone was merely tested out in a quarter of the trials, and the difference between trials intervening with vitamin D 3 alone and trials intervening with vitamin D 3 and calcium was not significant. Significant effects were found in trials including participants with low vitamin D status (<50 nmol/l) and in studies intervening with daily doses lower than 20 µg. However, the differences from the other trials (vitamin D adequacy (RR = 0.92, 95% CI 0.7–1.07) and dose ≥20 µg, respectively (RR = 0.96, 95% CI 0.92–1.01) were not statistically significant. Vitamin D 2 did not reduce mortality.
In the discussion, Bjelakovic et al. ( 57 ) also refer to a Swedish cohort study among elderly men. In this study, both low 25(OH)D-concentrations (<46 nmol/l, 10% of the men) and high concentrations (>98 nmol/l, 5% of the men) were associated with increased all-cause mortality ( 58 ).
Vitamin D and hypertension/blood pressure
Four SLRs ( 28 , 55 , 59 , 60 ) and one RCT ( 61 ) on blood pressure or hypertension outcomes fulfilled our selection criteria, see summary table 19 for details.
For hypertension, Chung et al. ( 28 ) assessed a combined nested case-control study of men from Health Professional follow-up study (HPFS) and women from Nurses’ Health study (NHS). This analysis showed a fivefold incidence of hypertension in men after 4 and 8 years who had 25(OH)D-concentrations below 37.5 nmol/l at baseline compared with those above 37.5, and sixfold higher than those above 75 nmol/l. Women with 25(OH)D below 37.5 at baseline had also higher incidence of hypertension after 4 years but not 8 years. A nested case-control study from the NHS2 showed that after 7 years, women in the three quartiles with baseline values below 80.5 nmol/l were 50–60% more likely to develop hypertension than those in the highest quartile. Chung et al. ( 28 ) also evaluated the relationship between combined vitamin D and calcium. The only study that was included was the Women's Health Initiative and they did not find any effect of vitamin D and calcium on the risk of hypertension.
For blood pressure, Chung et al. ( 28 ) included three trials, one British (grade A), one German (grade B), and one Indian study (grade B) with different doses of vitamin D (20 µg daily, a single dose of 2,500 or 3,000 µg every 2 weeks) compared with placebo None of the studies reported significant differences in diastolic blood pressure, while systolic blood pressure was decreased by 6 mm Hg in one study of older women who received both 20 µg vitamin D and calcium compared with calcium alone. The study of British older adults showed no effect of a single dose of 2,500 µg compared with placebo. In both study arms, systolic and diastolic blood pressure decreased to a similar extent. The Indian study of obese men (6 weeks of vitamin D, 3,000 µg every 2 weeks) reported a close to statistically significant increase in systolic blood pressure in the intervention group.
Witham et al. ( 59 ) included 11 RCTs, 3 of which used vitamin D 3 or vitamin D 2 as intervention in hypertensive adults. The studies were reported to be of variable quality. Two of the studies were included in Chung et al. ( 28 ). Four studies used either 1,25-(OH)2D or synthetic 1-α-calcidiol, and one UVB-irradiation. Eight studies were performed in hypertensive patients. Two studies used vitamin D 3 in normotensive subjects and no effect of supplementation was seen. The authors conclude: ‘We found weak evidence to support a small [lowering] effect of vitamin D on blood pressure in studies of hypertensive patients’.
Wu et al. ( 60 ) assessed four RCTs with changes in blood pressure as outcome, two of which were included in the Chung et al. review ( 28 ). The quality of the studies was assessed but not reported. One of the additional studies used 5-µg vitamin D 3 and the other 10-µg vitamin D 3 with calcium compared with placebo. They conclude:
Oral vitamin D supplementation may lead to a reduction in systolic blood pressure but not diastolic blood pressure. Given the small number of trials and small but statistically significant reduction in blood pressure, further studies and required to confirm the magnitude of the effect of vitamin D on blood pressure reduction and define optimum dose, dosing interval, and type of vitamin D to administer.
Pittas et al. ( 55 ) conducted a systematic review and meta-analysis on cardiometabolic outcomes and vitamin D, including 32 studies on diabetes, hypertension, and blood pressure. The three cohort studies assessing hypertension risk were all included in the Chung et al. report, showing significant associations between lower 25(OH)D concentrations and increased risk of hypertension. In addition, 10 RCTs of vitamin D supplementation were assessed in the review. Of these, three trials were considered of good quality, five of fair quality, and two of poor quality. Three studies not included in any of the above-mentioned systematic reviews, were assessed in this review, and two of those were rated as good quality. The Women‘s Health Initiative, rated good, combined a low-dose vitamin D supplement (10 µg/day) with calcium carbonate, the number of participants being about 36,000. This study found no effect on self-reported incident hypertension after 7 years of follow-up while a sub-group analysis found an increased risk of incident hypertension among black participants taking the supplements. However, vitamin D supplement alone was not assessed in this trial. The two additional trials included in this evaluation and not included in former reviews reported no significant effects of vitamin D supplements on blood pressure. Neither did the effect on systolic blood pressure differ between those trials providing higher (>25 µg vitamin D/day) or lower (<25 µg/day) doses. The authors concluded
A lower 25(OH)D concentration or vitamin D intake may be associated with higher risk of incident hypertension and cardiovascular disease.
We included one RCT ( 61 ), graded C, which was not included in the SLRs. The subjects were randomized to receive either 1,000 µg vitamin D 3 /week, 500 µg/week, or placebo. All subjects were given 500-mg calcium daily. No beneficial effects of the supplements were observed on blood pressure, while the group receiving 500 µg showed a slight but significant increase in systolic blood pressure compared with the placebo group. Mean baseline concentrations of 25(OH)D as well as blood pressure were within normal range in these subjects, and serum 25(OH)D concentration increased from 58 to 140 and 101 nmol/l in the two intervention groups. The authors’ conclusion was
Our results do not support a positive effect of vitamin D on hypertension. Further studies in subjects with low serum 25(OH)D levels combined with hypertension are needed.
Vitamin D and CVD clinical outcomes
Three SLRs of CVD outcomes and serum concentrations of 25(OH)D met our selection qualifications. These were Chung et al. ( 28 ), Parker et al. ( 54 ), and Grandi et al. ( 62 ). A fourth systematic review, Wang et al. ( 63 ), focused on vitamin D supplementation and CVD and one further review by Pittas et al. ( 55 ) analyzed cardiometabolic outcomes and vitamin D concentrations. See summary table 20 .
Chung et al. ( 28 ) focused on vitamin D and CVD. They included one RCT, one nested case-control study and one cohort study. The RCT, where almost 2,700 elderly British community-dwelling men and women received either placebo or 2,500-µg vitamin D every 4 months, reported no significant effects on total cardiovascular deaths, ischemic heart disease, myocardial infarction, or stroke after 5 years. Still there were fewer cardiovascular deaths (RR = 0.84; CI 0.56–1.10) as well as ischemic heart disease deaths (RR = 0.84; CI 0.56–1.27) in the intervention group receiving vitamin D than in the placebo group. In contrast, both cohort studies showed significantly lower risk associated with increased serum concentrations of 25(OH)D. In the Framingham Offspring Study (FOS), men and women with serum 25(OH)D concentrations below 37.5 nmol/l were 50–70% more likely to have a cardiovascular event within the 5.4-year study period, compared with those with levels between 25 and 37.5 nmol/l while a multivariate analysis suggested an increased likelihood of cardiovascular events in people with S-25(OH)D below approximately 50 nmol/l. Similarly, the nested case control Health Professional follow-up study (HPFS) reported over two-fold risk in men with 25(OH)D below 37.5 nmol/l and a 60% increased risk of cardiovascular events in those with concentrations between 56 and 75 nmol/l compared with those above 75 nmol/l or higher.
Grandi et al. ( 62 ) evaluated the prognostic value of 25-OH-D concentrations for CVD incidence and mortality. They reviewed seven prospective studies in addition to the two included in the Chung et al. ( 28 ) report. Three out of five mortality studies reported significant associations, with one showing a fivefold increase in risk in those with concentrations in the lowest quartile, below 30.7 nmol/l. However, two large population-based studies reported no significant effect on cardiovascular mortality. Mean age was lower in these two studies, or 44.8 and 49.4 years, respectively, compared with the studies reporting increased risk, where mean age ranged from 62 to 74 years. Grandi et al. included two additional incidence studies not included in the Chung et al. report, a New Zealand study of 1,471 postmenopausal women participating in a calcium supplementation trial, and a Finnish study of 689 men and women who were followed for up to 10 years. Neither study demonstrated a significant association of S-25(OH)D concentrations with cardiovascular events. Grandi et al. concluded
Data from prospective studies suggest an inverse relationship between 25(OH)D and cardiovascular risk. However, given the heterogeneity and small number of longitudinal studies, more research is needed to corroborate a potential prognostic value of 25(OH)D for cardiovascular disease incidence and mortality.
Parker et al. ( 54 ) evaluated the association between 25(OH)D and the presence of cardiometabolic disorders including CVD, diabetes, and metabolic syndrome. They reviewed and meta-analyzed 28 cross-sectional studies, case-control, cohort and RCTs on cardiometabolic disorders, including 16 studies with cardiovascular event outcomes and S-35(OH)D concentrations. Two of the seven additional studies of CVD outcomes not included in the review by Grandi et al. ( 62 ) did not report a significant association between CVD risk and vitamin D, four studies showed lower risk and one study of 216 people living in Southern India showed a 60% higher risk associated with higher S-25(OH)D concentrations. Meta-analysis of these 16 studies was consistent with a 33% reduction in the risk of having a cardiovascular disease . The authors conclude that
Our findings suggest that high levels of vitamin D, among adult populations, are associated with a substantial decrease in cardiovascular disease, type 2 diabetes and metabolic syndrome. Interventions targeting a positive modification of vitamin D deficiency in adult and elderly populations would substantially contribute to halting the current epidemics of cardio-metabolic disorders. Further controlled trials are needed to evaluate the causal association between vitamin D levels and cardio-metabolic disorders.
The systematic review of Wang et al. ( 63 ) assessed 17 studies in total out of which 6 were prospective studies and 4 were interventions on vitamin D with or without calcium supplementation for the risk of cardiovascular event outcomes. The quality was assessed but not reported in the article. None of the four RCTs on vitamin D supplements were specifically designed for CVD outcome, and five of the studies were on patients receiving dialysis. All five studies on haemodialysis patients showed a lower risk of cardiovascular events in those receiving vitamin D as did the single study on vitamin D in the general population. That study assessed the vitamin D intake and identified CVD endpoints. Supplemental intake greater than 20 µg/day was associated with a non-significant lower risk of CHD mortality RR= 0.80 (CI 0.57–1.13). The conclusions of Wang et al. ( 63 ) were:
Evidence from limited data suggests that vitamin D supplements at moderate to high doses may reduce CVD risk. Further research is needed to elucidate the role of these supplements in CVD prevention.
The systematic review of Pittas et al. ( 55 ) on vitamin D and cardiometabolic outcomes is an expansion of the evidence report for the Institute of Medicine decisions on vitamin D reference intakes ( 28 ). They included 32 studies on diabetes, hypertension, and blood pressure associated with either vitamin D status or intake of supplements. The quality was assessed but not reported in the article. These outcomes are evaluated separately below. Nine cohort studies analyzing CVD outcomes were also included in the review, seven of these were rated of good quality. Cardiovascular endpoints included myocardial infarction, cardiovascular-related death, a composite cardiovascular endpoint and stroke. All studies measured 25(OH)D concentrations. Overall, five out of the nine studies found that lower 25(OH)D concentrations were associated with increased risk for incident CVD. Five trials on vitamin D supplementation were included in the review. None of these reported a statistically significant effect of vitamin D supplementation on various cardiovascular outcomes, including myocardial infarction and stroke. The authors’ conclusion was:
The association between vitamin D status and cardiometabolic outcomes is uncertain. Trials showed no clinically significant effect of vitamin D in the dosages given. Adequate randomized controlled trials, conducted in well-defined populations, are needed to test the potential role of vitamin D in primary prevention or therapy. Vitamin D remains a promising, although unproven, new element in the prevention or management of cardiometabolic disease.
Vitamin D and infections
We identified three systematic reviews ( 28 , 64 ) ( 65 ) and one additional RCT ( 66 ) related to vitamin D and infections that met our selection criteria (see summary table 21 ).
Chung et al. ( 28 ) assessed a single cohort study from the NHANES III on infectious disease mortality, stratified by baseline 25(OH)D concentration. No differences in infectious disease mortality were detected between quartiles ranging from <44 to >80 nmol/l after 7–8 years of follow-up.
Yamshchikov et al. ( 64 ) assessed 13 trials, 10 of which were placebo controlled, studying vitamin D for the prevention or treatment of infectious disease (bacterial, viral, and parasitical). The included clinical trials demonstrated substantial heterogeneity in patient demographics and vitamin D interventions. On the basis of these heterogeneous studies, the authors conclude:
More rigorously designed clinical trials are needed for further evaluation of the relationship between vitamin D status and immune response to infection.
Another systematic review and meta-analysis on S-25(OH)D concentrations and tuberculosis assessed seven observational studies, only one of which was in a European setting ( 65 ). All but one of the studies reported a significant association between low S-25(OH)D and active tuberculosis. The authors conclude:
Low serum vitamin D levels are associated with higher risk of active tuberculosis. Although more prospective studies are needed to firmly establish the direction of this association, it is more likely that low body vitamin D levels increase the risk for active tuberculosis.
Urashima et al. ( 66 ) published a double-blinded RCT of vitamin D supplementation to prevent influenza A in Japanese school children. This 4-month trial where 430 children aged 6–15 years were randomized into two groups, placebo or supplements of 30 µg/day, had influenza A, diagnosed by medical doctors using a rapid influenza diagnostic test (RIDT), as primary outcome. A reduced risk of influenza was observed in the group receiving 30-µg vitamin D daily, and more prominent in those who had not been taking other vitamin D supplements (RR = 0.36; 0.17–0.78). Influenza A occurred in 18 out of 167 children taking vitamin D, but in 31 of 167 in the control group. No difference was observed in influenza B. The study was rated C, in part as 25(OH)D concentrations were not measured.
The effect of sun or UVB exposure on different outcomes in different population and age groups (Research question 5)
We identified one SLR on the relationship between both solar and artificial UVB radiation and 25(OH) in blood ( 27 ) (summary table 22 ). The report was based on eight RCTs. The overall quality of the trials was rated as low.
This SLR concluded that “there is fair evidence that solar and artificial UV-B exposure increase 25(OH)D levels. The included trials did not address the issue of whether serum 25(OH)D response is attenuated in heavily pigmented groups. It was also not possible, to evaluate the impact of effect modifiers such as age, ethnicity, seasonality and latitude.
The authors expressed that further research is needed to clarify the exact doses needed to maintain 25(OH)D concentrations over time, in the absence of supplementation.
The UL for vitamin D for different health outcomes in different population and age groups (Research question 6)
Both Cranney et al. ( 27 ) and Chung et al. ( 28 ) included research questions related to this question. See summary table 23 for details.
Cranney et al. ( 27 ) performed an SLR of a total of 22 RCTs to answer if vitamin D supplementation resulted in toxicity. A total of 22 trials reported data on toxicity-related outcomes, 21 of which used doses above 10 µg/day. Only 12 received a rating of >3 on the Jadad scale ( 26 ). An adequate description of allocation concealment was reported in three trials. Toxicity results from trials with intakes of vitamin D above current reference intakes varied and this may have been related to different doses, baseline characteristics of populations or exposure times. Most trials excluded subjects with renal insufficiency or hypercalcaemia, were of small sample size and had short durations of exposure to vitamin D. Event rates were low across trials in both the treatment and placebo arms. The Womens′ Health Initiative trial in women aged 50–79 years, examined the effect of vitamin D 3 10 µg in combination with 1,000-mg calcium carbonate versus placebo and found an increase in the risk of renal stones corresponding to 5.7 events per 10,000 person-years of exposure The results are complicated by the fact that the subjects (intervention and placebo) were allowed to take additional vitamin D supplements up to 15 µg and later 25 µg per day and also calcium supplements up to 1,000 mg.
The authors conclude
that overall, there is fair evidence that vitamin D supplementation above current reference intakes, with or without calcium supplementation, was well tolerated. A significant increase in kidney stones was observed in one large trial in postmenopausal women taking 10 µg vitamin D3 with calcium. The quality of reporting of toxicity outcomes was inadequate in a number of the trials, and most trials were not adequately powered to detect adverse events.
Chung et al. ( 28 ) considered all the RCTs included in their SLR focusing on a number of health outcomes. Only 16 out of 63 RCTs reported adverse effects and they were generally not powered to detect them. Eleven of these reported at least one adverse effect.
According to Chung et al. ( 28 ), RCTs of vitamin D (doses ranged from 10 to 143 µg, 5,714 IU/day vitamin D 3 or from 125 to 250 µg vitamin D 2 ) and/or calcium supplementations (doses ranged from 200 to 1,500 mg/day) reported few cases of gastrointestinal disruption such as constipation, diarrhea, upset stomach, musculoskeletal soreness, primary hyperparathyroidism, hypercalcemia, renal calculi, and craniotabes. However, comparisons between the intervention groups and the control groups were not usually reported. One RCT reported some adverse events that required hospital admission, including retrosternal pain, a non-ST elevation myocardial infarction and a transient ischemic attack (all three cases in vitamin D 20 µg/day plus exercise training group) and one case of acute cholecystitis (in calcium, vitamin D plus exercise training group). Another RCT reported that ‘there were no significant differences between the vitamin D and the control groups in the rate of incident cancer and vascular disease (ischemic heart disease and stroke)’ (actual data not provided), and one participant died during the study. However, these adverse events may or may not be associated with vitamin D and/or calcium supplementation in this study.
We have found the following harms reported in some of the SLRs included in our review. Michael et al. ( 46 ) concluded that on the basis of the nine fair-quality trials related to falls included in their review, they found no increase in falls, fallers, or other major adverse events. Only three trials specifically reported adverse effects – transient and asymptomatic hypercalciuria or hypercalcaemia in the intervention group – but no differences in adverse effects or clinically significant harms, such as incident kidney stones, cancer, ischemic heart disease, or stroke. Gillespie et al. ( 45 ) found that adverse effects (hypercalcaemia, renal disease, gastrointestinal effects) were reported in three trials but none were statistically significant. One RCT study ( 38 ) reported an increased risk of fracture and falls in those elderly that were given a single yearly dose of vitamin D corresponding to about 18 µg/day.
Bjelakovic et al. ( 57 ) reported that vitamin D 3 combined with calcium increased the risk of kidney stones (RR 1.17, 95% CI 1.02–1.34), whereas the effect of vitamin D was not significant on other side effects.
The interactions of vitamin D with calcium intake on different health outcomes in different population and age groups (Research question 7)
In general, we were not able to distinguish vitamin D and vitamin D together with calcium in our systematic reviews. Thus, this question has been handled within the other research questions.
The interaction of vitamin D intake or vitamin D status with vitamin A intake or vitamin A status on health outcomes in different population and age groups (Research question 8)
We did not find any SLRs on this topic.
The aim of this systematic review was to provide a scientific base for a Nordic recommendation for dietary intake of vitamin D. We analyzed the literature on the relationships between vitamin D, 25(OH)D concentration and different health outcomes. Moreover, we studied the relationship between vitamin D intake and 25(OH)D concentration. We focused on published systematic reviews but included a few RCTs which were published after the SLRs. Some of the SLRs included both observational studies as well as RCTs. In the result section, we did not include the recent American IoM report on vitamin D and calcium intake from 2010 ( 29 ) as it is not a systematic review. However, we included the SLRs forming the basis for the IOM report. We focused on populations in Europe and North America. However, if other populations were included in the SLRs, we were generally unable to separate them.
There are some general challenges when reviewing to establish evidence for the relationship between vitamin D and health. First, agreement has not yet been achieved for what is considered an optimal 25(OH)D concentration, second the relationship between 25(OH)D and health outcomes are likely to be confounded by diet, in particular fish intakes, but also physical activity, both of which are not easily adjusted for in observational studies. Third, in experimental studies vitamin D and calcium supplements are often combined, thus the separate effect of vitamin D supplements can be questioned.
Assessment of vitamin D status
The reliability of the assays for serum/plasma 25(OH)D measurement has been questioned.
It has been shown in a number of studies that different assays give different results (e.g. ( 67 – 70 )). In a recent Swedish study ( 71 ), the same samples were analyzed in three different laboratories. The results showed a large discrepancy in the concentrations. Thus, it seems fairly challenging to use the serum 25(OH)D-concentration as an outcome marker for assessing vitamin D deficiency and insufficiency as well as using it as an indicator of exposure.
Role of UV exposure
Vitamin D is produced in human skin when exposed to the sun. It is the ultraviolet (UV) radiation in the UV-B brand, that is, wavelengths between 290 and 315 nm that are needed for the photo conversion of provitamin D 3 to previtamin D 3 to occur in the skin.
At latitudes above ∼50°N, both the qualitative and quantitative properties of sunlight is not sufficient in parts of the year for vitamin D production to take place ( 72 ), leading to the so-called vitamin D winter. In Copenhagen, the vitamin D winter is estimated to start in mid-November and last until end of February whereas in Tromsø it is estimated to last 2.5 months longer. In Helsinki (60°N), the length of the vitamin D winter spans from mid-October to mid-March ( 73 ) and in Reykjavik (64°N) from early October to late March. In addition to latitude and season, the actual vitamin D production in skin in humans is affected by several individual and external factors. The ozone layer effectively absorbs UVB light, and clouds, when completely overcast, can attenuate the UVB radiation as much as 99%. Surface reflection, especially from snow can however reflect the UVB radiation up to 95%.
Time spent outdoors, the use of sunscreen, and clothing also affect the sun-induced vitamin D for individuals ( 74 ). In addition, individual vitamin D status has been shown to affect the effectiveness of cutaneous vitamin D 3 production, so that individuals with initial low levels of 25(OH)D seem to have a lower threshold concentration for vitamin D production in skin compared to individuals with higher concentrations ( 75 ). The sun-induced vitamin D production can be up to six times higher in people with pale skin compared to people with dark skin ( 76 ). The skins ability to produce vitamin D also decreases with age ( 77 ).
A down-regulating mechanism of vitamin D production in skin prevents vitamin D toxicity due to prolonged sun exposure by a photo-degradation of previtamin D 3 to biologically inert isomers ( 78 ).
Data available on seasonal variation in 25(OH)D concentrations in the general population in some Nordic countries have demonstrated less fluctuation between summer ( 79 , 80 ) and winter compared to other comparable populations ( 81 ). The vitamin D intake in the diet and common use of supplements is a possible explanation for this ( 21 ). However, in studies from Finland a marked seasonal variation in S-25(OH)D concentrations has been observed in adolescents, in adults and in the elderly ( 41 , 78 ) ( 82 ). A study from Sweden ( 83 ) among women aged 61–83 years, an increase from winter until summer in 25(OH)D concentrations was found to be 38%. In Iceland, adults who do not take vitamin D supplements show a marked seasonal variation ( 13 ). The magnitude of the difference in 25(OH)D concentrations between summer and winter decreases with increasing latitude ( 75 ).
Main findings in relation to the research questions
What is the effect of vitamin d from different sources on serum 25-ohd concentrations (research question 1).
Our first question was related to the effect of vitamin D from different sources on serum 25(OH)D concentrations. We did not find any SLR on the effect of natural vitamin D sources on 25(OH)D concentration. However, we are aware of one study in which the effect of edible wild mushrooms ( Cantharellus tubiformis ) on 25-OHD-concentration has been studied ( 84 ). In that study, a portion containing 15 µg vitamin D 2 /day increased the 25-OHD concentration to the same extent as a corresponding supplement of vitamin D 2 , from about 30 to about 45 nmol/l over 3 weeks.
Regarding fortified foods and supplementation, the SLRs indicated that there is a clear effect of fortified foods and supplementation on the S-25OHD concentration. However, it is not easy to conclude what doses are needed to achieve specific levels of 25-OHD. One SLR (Black et al., 30) estimated that 1 µg ingested from fortified foods increased the S-25(OH)D concentration by 1.2 nmol/l. Two SLRS focused on supplementation ( 27 , 32 ). In the SLR by Cashman et al. ( 32 ), the authors focused on studies performed at latitudes higher than 49°N, which is applicable for the Nordic countries. Both SLRs found a positive effect of vitamin D intake, including fortified foods ( 27 ) and supplements on 25-OHD concentrations. Cranney et al. ( 27 ) concluded that the meta-regression results suggested that 2.5 µg/day of vitamin D 3 will increase the serum 25(OH)D concentrations by 1–2 nmol/l. This suggested that doses of 10–20 µg daily may be inadequate to prevent vitamin D deficiency in at-risk individuals. Vitamin D 3 doses of 17.5 µg daily or more significantly and consistently decreased serum concentrations of PTH in vitamin-D-deficient populations. However, Cranney et al. ( 27 ) concluded that the increase in S-25(OH)D concentration was higher when the baseline concentration was low than when it was high, and also that the increase was larger if the duration of the study was longer. Cashman et al. ( 32 ) included age groups between 9 and 78 years. Using meta-regression analyses, they calculated that an average of 9 µg vitamin D was needed for a population to achieve 50 nmol/l. However, taking interindividual variation into account in the meta-regression analysis, 23.5-µg vitamin D/day was needed for 95% of the population to reach a serum level ≥50 nmol/l. However, the authors concluded that these latter results have to be treated with caution as the number of data points in the analysis is low.
Cranney et al. ( 27 ) considered different age groups. They concluded that 5-µg vitamin D/day may not be enough to prevent vitamin D deficiency in some infants at northern latitudes. A response in 25(OH)D concentration of vitamin D supplementation was seen in the trials included. Most of them used vitamin D 2 . Supplementation during pregnancy with either 25–90 µg/day vitamin D 2 or 25 µg vitamin D3 increased 25(OH)D concentration both in the mothers and cord blood. Cranney et al. ( 27 ) included one study on supplementation during pregnancy ( 85 ), in which they did not find an effect of 25-µg vitamin D given to the mother on the infant S-25(OH)D concentration. The authors actually performed another 15 week trial in the winter months after that ( 86 ) and found that giving 50-µg vitamin D 3 /day to the lactating mothers increased the infants’ S-25(OH)D to almost the same level as 10-µg vitamin D 2 /day to the infant – the level being ca. 70 and 83 nmol/l respectively. This study was excluded from the Chung analyses as it was regarded not to be an RCT. Cranney et al. ( 27 ) identified four trials in children and adolescents and found consistent increases in S-25(OH)D concentrations with different doses: 8 nmol/l with 5-µg vitamin D 3 /day, 16.5 nmol/l with 15 µg/day and 60 nmol/l with 50 µg/day. In premenopausal and younger males, a dose effect was noted in those trials that used multiple doses of vitamin D 3 . In this age group, Cranney et al. ( 27 ) also compared the effect of vitamin D 2 and D 3 on S-25(OH)D concentration and concluded that vitamin D 2 appeared to have a smaller effect than vitamin D 3 . A dose response was noted in trials in postmenopausal women, older men and in elderly populations in long-term care or nursing homes. The doses had a large range, the basal S-25(OH)D concentrations varied and the assays used were very heterogeneous.
What is the relationship between 25-OHD concentrations/dietary vitamin D intake/supplemental vitamin D and different outcomes in different population and age groups? (Research questions 2–4)
Three questions were related to the relationship between 25(OH)D-concentrations or dietary vitamin D or supplemental vitamin) and different health outcomes. In addition to RCTs, the SLRs included mostly cross-sectional, cohort or longitudinal studies. RCTs have been performed only for skeletal outcomes, falls, muscle function and weight and these have been included in the SLRs. In some cases, secondary analyses of the RCTs on some health outcomes have been performed. We found some evidence for a causal relationship with bone health, falls and muscle strength, and total mortality. We did not find evidence for establishing a causal relationship between vitamin D intake, vitamin D supplementation, or serum 25-OHD concentration and most other the health outcomes.
De-Regil et al. ( 33 ) reviewed six randomized trials including 1,023 pregnant women. They concluded that there is currently insufficient high-quality evidence relating to the clinical effects of vitamin D supplementation during pregnancy. The other SLR ( 28 ) included only one nested case-control study reporting that a 25(OH)D concentration lower than 37.5 nmol/l was associated with an increased risk of preeclampsia.
Seven intervention studies and two observational studies were included in one SLR ( 28 ). The authors concluded that due to the lack of methodologically solid studies, they were cautious in their conclusions about the effect of vitamin D in newborns, infants, and children.
Skeletal effects
Low 25(OH)D concentration increases the risk of rickets. The threshold is uncertain, but a number of the studies suggest increased risk at S-25(OH)D concentrations <27.5 nmol/l. Many studies were conducted in developing countries with low dietary calcium intake. Low calcium intake may influence the relationship between 25(OH)D and rickets, and the 25(OH)D threshold for rickets in populations with high calcium intake is unclear. It could be added that vitamin D has been used as a prophylaxis in the Nordic countries for decades, and the current recommended daily dose of 10 µg seems to be effective in preventing rickets if the supplement is given ( 87 ).
The data on the relationship between vitamin D and bone mineral content or bone mineral density are heterogeneous . In infants, there is fair evidence for an inverse relationship between 25(OH)D and PTH at low concentrations, whereas the relationship between 25(OH)D and BMC is inconsistent. In older children and adolescents, there is fair evidence for 25(OH)D to be associated with change in BMC/BMD, but RCTs have not consistently shown an effect of vitamin D supplementation. A Cochrane review ( 34 ) in children found no overall effect of vitamin D supplementation, but an effect was suggested in populations with low concentrations of 25(OH)D. There was insufficient evidence for a relationship between 25(OH)D and change in BMD in pregnant women, and one good cohort study did not find any relationship between 25(OH)D and BMD during lactation. Based on observational studies, there is fair evidence for a relationship between 25(OH)D and BMD or change in BMD at the femoral neck in the elderly. From intervention studies it is good evidence that supplementation with vitamin D combined with calcium leads to a small increase in spine, femoral neck, total hip, and total body BMD. Based on available studies, it is less certain that vitamin D supplementation alone has an effect on BMD.
It is challenging to describe optimal concentration of 25(OH)D for bone mineral density or bone mineral content based on available SLRs. In infants, a threshold around 27 nmol/l might exist for the relationship between 25(OH)D and PTH ( 27 ). This threshold was reported as 30–83 nmol/l in older children and adolescents ( 27 ). The SLR by Cranney et al. ( 27 ) also refers to one RCT among adolescent girls reporting an effect of vitamin D intervention to occur at mean levels of 25(OH)D >50 nmol/l, whereas the Cochrane review by Winzenberg et al. ( 39 ) only reported an effect of vitamin D supplementation in children with low mean 25(OH)D at baseline (≤ 35 nmol/l). In observational studies among the elderly, the bone loss at the hip was increased at concentrations of 25(OH)D ranging from 30 to 80 nmol/l in different studies.
Fracture is the primary skeletal health outcome in adults. Based on available data, the SLRs concluded that intervention with vitamin D (dose 10–20 µg/day) combined with calcium reduces the risk of total fracture and hip fracture , whereas intervention with vitamin D alone has not shown an effect in the doses tested out. Although a threshold of 74 nmol/l was considered to show a reduction in total fracture incidence, the variability in analytical methods and the fact that S-25(OH)D was assayed only in subsamples, make this threshold unreliable. To what extent an intervention with vitamin D and calcium prevent fracture in non-institutional elderly is debated. Both the Cochrane review by Avenell et al. ( 35 ) and the Cranney et al. ( 27 ) report only found a significant effect in studies performed in institutionalized elderly, although the difference between studies performed in institutionalized and community dwellers was not statistically significant. However, the DIPART study ( 37 ) reported that the effect was found across a wide range of age (mean age 70 years; range 47–107 years).
Although the overall conclusion is that intervention with vitamin D alone in the doses tested has not been proven effective in preventing fractures, it could be added that a British study by Trivedi et al. ( 88 ), Jadad score 3 ( 26 ) reported that 2,500-µg vitamin D every 4th months (corresponding to 20 µg/day) compared to placebo, reduced the risk for any new fracture (OR 0.78 [95% CI 0.61–0.99]). On the other hand, the Australian RCT referred to previously, reported an increased risk of fracture in those given a single yearly high dose of vitamin D ( 38 ).
Currently, there is also interest in studying the effect of higher doses of vitamin D: Vital (ClinicalTrials.gov; {"type":"clinical-trial","attrs":{"text":"NCT01169259","term_id":"NCT01169259"}} NCT01169259 ), FIND ( {"type":"clinical-trial","attrs":{"text":"NCT01463813","term_id":"NCT01463813"}} NCT01463813 ) and DO-Health(not registered as yet).
It could be added that a recent Swedish study referred to in the IOM report found increased risk of fracture in men with S-25(OH)D below 40 nmol/l ( 89 ).
Dental health
Lack of data precludes any conclusion concerning the relation between vitamin D and dental health.
Six SLRs were focused on vitamin D intake or 25(OH)D and falls. There was overall fair evidence that vitamin D with calcium is effective in preventing falls in the elderly especially in those with low baseline 25(OH)D concentrations, both community dwelling and in nursing care facilities. One SLR concluded that vitamin D was effective ( 43 ). Some but not all SLRs concluded that a dose greater than 20 µg was effective, in conjunction with calcium supplementation. One study suggested that 25(OH)D concentrations below 39 nmol/l were associated with an increased risk of falls.
Muscle function
Two SLRs focused on vitamin and outcomes related to muscle function in the elderly. Stockton et al. ( 48 ) concluded that vitamin D supplementation does not have an effect when basal 25(OH)D is greater than 25 nmol/l, but that vitamin D has an effect in adults with vitamin D deficiency. However, Muir et al. ( 49 ) concluded that vitamin D doses of 20– 25 µg/day have beneficial effects on balance and muscle without taking baseline S-25(OH)D into account. Thus, doses of 20–25 µg/day could be beneficial for muscle function in the elderly, but the information on the effect of lower doses was scarce.
Vitamin D and cancer have been studied in a number of cohort studies. Some RCTs have been performed but they are secondary analyses of supplemental studies for the prevention of fractures ( 88 , 90 ). There was not consistent evidence for an association between vitamin D status and total cancer in SLRs including cohort studies and RCTs. There is some observational evidence of an inverse association between vitamin D status and risk of colorectal cancer, however evidence for a causal relationship are lacking. The evidence for an inverse association between vitamin D status and breast cancer risk is weak due to lack of good quality studies and heterogeneity between studies. There is little or no evidence for a protective effect of vitamin D on prostate cancer.
Diabetes and multiple sclerosis
The evidence for a causal relationship or an association between vitamin D and type 1 and type 2 diabetes is limited and inconclusive. Lack of data precludes any conclusion concerning the relation between vitamin D and multiple sclerosis.
Body weight
There is no clear evidence for vitamin D to influence body weight development.
Total mortality
Based on the RCTs in the SLRs it is concluded that vitamin D3 (10–20 µg/day) combined with calcium significantly reduces total mortality. However, it is uncertain if co-supplementation with calcium is necessary to achieve this effect. It could be added that a recent a Swedish cohort study among elderly men followed for around 14 years reported increased all-cause mortality both in men at the low (<46 nmol/l) and the high end of 25(OH)D concentrations (>98 nmol/l) ( 58 ). Moreover, a recent Danish cohort study among subjects from the Copenhagen general practice sector including near to 250,000 subjects that were followed for 3 years found a reverse J-shaped relationship with the lowest mortality at 25(OH)D concentrations of 50–60 nmol/l ( 91 ).
Hypertension and blood pressure
Evidence from RCTs reviewed in four SLRs on blood pressure is inconclusive. Some RCTs detected a small reduction in diastolic blood pressure, particularly in people with higher baseline values, while another showed a small reduction in systolic pressure. All SLRs concluded that there was a need for further studies to explore this relationship for possible clinical significance. A recent RCT ( 61 ) which was not included in the SLRs did not support a positive effect of vitamin D supplementation in conjunction with calcium on hypertension, intervening with large doses (500 or 1,000 µg/week with 500-mg calcium) in overweight persons over 1 year.
However, low vitamin D status has repeatedly been associated with a higher incidence of hypertension as reviewed in two SLRs. Nested case control studies show marked reverse associations between incidence of hypertension and 25(OH)D in men and women with baseline <37.5 nmol/l compared with >37.5 nmol/l and also compared with those over 75 nmol/l.
Cardiovascular clinical outcomes
Systematic reviews based on cohorts or case-control studies have repeatedly found an association between low 25(OH)D concentrations, mostly below 37.5 or 50 nmol/l and an increased risk of CVD. However, a significant effect of supplementation on cardiovascular outcomes has not been reported. The trials in question were all designed for health outcomes other than CVD.
The evidence for an effect of vitamin D on infections is scarce and trials were very heterogeneous.
What is the effect of sun or UVB exposure on different outcomes in different population and age groups? (Research question 5)
The only SLR assessing this question concluded that there is fair evidence that both solar and artificial UV-B exposure increase 25(OH)D concentrations. We were not able to establish a dose response relationship. We did not find any SLR addressing the effect of sun or UVB exposure and other outcomes.
Which is the UL (Tolerable Upper Intake Level) for vitamin D for different health outcomes in different population and age groups? (Research question 6)
The SLRs did not give any definite answer to this question. Chung et al. ( 28 ), Cranney et al. ( 27 ), Vestergaard et al. ( 36 ), Avenell et al. ( 35 ) and Bjelakovic et al. ( 57 ) included adverse effects in their reviews of RCTs. Vitamin D given with calcium, but not vitamin D alone, moderately increased the risk of renal stones.
There are some observational studies suggesting that total mortality is increased at high 25(OH)D concentrations ( 58 , 91 ). Some studies have reported an increase in prostate cancer ( 92 ) or total cancer ( 58 ) at higher 25(OH)D-concentrations. A trial using large yearly doses of vitamin D reported increased incidence in fractures and falls in the elderly ( 38 ).
Which are the interactions of vitamin D with calcium intake on different health outcomes in different population and age groups? (Research question 7)
We were not able to distinguish between the effect of vitamin D alone and vitamin D together with calcium on most of the health outcomes. A combination of vitamin D and calcium seems to be important in the prevention of fractures, falls, and all-cause mortality (total mortality).
Which is the interaction of vitamin D intake or vitamin D status with vitamin A intake or vitamin A status on health outcomes in different population and age groups? (Research question 8)
Difference between vitamin d 2 and d 3 in increasing s-25-hydroxy-vitamin d concentration.
The difference between vitamin D 2 and D 3 was not one of our initial research questions. We, nevertheless, considered this to be an important topic that has to be included. Vitamin D 2 and D 3 supplementation has been reviewed in a recent SLR by Tripkovic et al. ( 93 ). Ten studies were included in the systematic review and seven studies in the meta-analysis. The doses, durations and age groups varied as well as the route of administration. The authors concluded that vitamin D 3 is more efficacious at raising 25(OH)D than vitamin D 2 . One of the studies ( 94 ) indicated that the effect of vitamin D 2 on S-PTH was very weak in comparison to the effect of vitamin D 3 .
Overall discussion
Vitamin D can influence numerous biological processes in the body. In addition to the effects on bone health, it has been claimed that vitamin D contributes in the prevention of many medical conditions including CVDs, type 1 and type 2 diabetes, some types of cancer, pregnancy outcome, and infections. And indeed, there is suggestive evidence for a number of health benefits of vitamin D and for plausible biological mechanism. For example, the observation that S-25(OH)D is inversely related to some types of cancer is supported by a new reanalysis of a subgroup of participants in the large Calcium and vitamin D trial in the Women's Health Initiative ( 95 ) and associations between lowered risk for cardiovascular outcomes and higher 25(OH)D concentrations has repeatedly been observed. Similarly, higher risk for preeclampsia has been reported in pregnant women with 25(OH)D below 37.5 nmol/l and the risk for low birth weight may be lowered in at-risk pregnant women by vitamin D supplementation.
However, the SLRs we have reviewed conclude that the evidence for a protective effect of vitamin D is only conclusive concerning bone health, total mortality and the risk of falling. In addition, most intervention studies leading to these conclusions report that intervention with vitamin D combined with calcium and not vitamin D alone gives these benefits.
Currently, there is a great interest and a high research activity concerning vitamin D. Although a large number of studies, including RCTs, have been performed, there are still many unanswered questions. For example, it is unclear why combined interventions with vitamin D and calcium and not interventions with vitamin D alone have shown an effect on fracture and mortality risk. The causes for the increased risk of fracture and falling in those given a large, annual dose of vitamin D are also unclear ( 38 ), and so is the increased risk of mortality related to high concentrations of 25(OH)D reported in some studies ( 58 , 91 ) Observational studies have rather consistently shown that low concentrations of 25(OH)D are related to increased risk of CVD. However, re-analyses of RCTs suggest that calcium supplementation (also with vitamin D) might increase the risk of myocardial infarction ( 95 ).
Although RCTs were emphasized in most of the SLRs, we would also point out some limitations. When interpreting the effect of doses given in RCTs, it is a challenge that the participants also receive vitamin D from other sources (diet and UVB-irradiation). In some studies, participants were also allowed to use personal supplements in addition to study medication. Basically, the RCTs give information on the effect of the difference in vitamin D exposure between the intervention group and the control group. A large difference in exposure may be difficult to obtain in RCTs. To test out the exposure of a moderate dose of vitamin D compared to very little vitamin D might therefore be difficult.
Many chronic diseases develop over many years, and it is also a challenge that RCTs in general are performed over shorter time frames. Whereas RCTs are feasible in testing out the effect and side effects of interventions with supplements, the feasibility of RCTs in establishing the relation between nutrition and disease has been debated ( 50 ).
It was difficult to establish an optimal 25(OH)D concentration or vitamin D intake based on the SLRs.
Other considerations
The IoM ( 29 ) also considered calcium absorption together with BMD, rickets and osteomalacia to find a totality of evidence for an optimal S-25(OH)D concentration. They found a congruence among these outcomes with no additional benefits of serum concentrations of 25(OH)D higher than 50 nmol/l.
The relationship between S-25(OH)D and S-PTH has been considered in numerous studies, and based on some of them the threshold for vitamin D sufficiency has varied between 25 and 125 nmol/l. Using S-PTH as an outcome is difficult as the variation is large and also other factors have an effect on S-PTH. Sai et al. ( 96 ) concluded in a systematic review that ‘vitamin D insufficiency should be defined as serum 25(OH)D less than 50 nmol/l as it relates to bone’.
A number of studies have shown an inverse relationship between S-25(OH)D and BMI or adiposity. Some supplementation studies, but not all, have shown a lower response in S-25(OH)D in obese persons than in normal weight subjects. Moreover, weight loss has led to an increase in S-25(OH)D in some studies. Thus, though no SLR addressed this subject, there are some indications that adiposity should be considered a determinant of S-25(OH)D-concentration ( 97 ). However, this does not suggest that there is evidence that higher intakes are needed in obese persons than in those with normal weight.
Limitations
We focused on SLRs and included only a few new RCTs. We were not able to perform quantitative analyses of the studies. The quality of the studies included in the SLRs varied and there was a large heterogeneity among them. All age groups were not covered and the study duration in the trials varied greatly. Different age groups were considered only in relation to bone health.
Study quality
Due to heterogeneity in the studies, it was difficult to interpret the results and provide single summary statements. The doses of vitamin D differed widely among the studies. Habitual vitamin D intake was seldom assessed and the methods for intake assessment varied. The assays used for the assessment of S-25(OH)D concentration varied among the studies. The study cohorts consisted mainly of Caucasians.
Publication bias
Publication bias cannot be ruled out, since relevant studies were searched in two electronic databases (MEDLINE and Swemed), and by snowballing for the last months. Unpublished or ongoing studies were not identified.
Authors’ conclusions
Implications for the nordic setting.
Cutaneous synthesis of vitamin D 3 is the physiological route for vitamin D supply. Due to our geographic situation, this way of supply is turned off for about 3–5 months during the year. Dietary vitamin D is thus needed to keep vitamin D status at an acceptable level. The SLRs that we have reviewed gave insufficient evidence for an optimal 25(OH)D concentration and corresponding vitamin D intake levels in relation to most health outcomes. However, the association between vitamin D status and skeletal outcomes and the effect of vitamin D supplementation on skeletal outcomes give some information, while the role of vitamin D without calcium supplementation on fracture incidence is unclear. Moreover, studies on the effect of vitamin D supplementation and vitamin D fortification on 25(OH)D concentrations gives some information on how to achieve specific concentrations of 25(OH)D. In this respect, the heterogeneity in the results by the 25(OH)D assays is a formidable problem.
Many studies suggest that there is an increased risk for rickets in infants and children when S-25(OHD) concentration is <27.5 nmol/l. A threshold for 25(OH)D at 40–50 nmol/l has been suggested in the SLRs for the prevention of falls and fractures in the elderly. Solid evidence for an optimal S-25(OH)D concentration (or optimal intake) in children, adolescents and adults was not found in the SLRs relating to the health outcomes. However, a S-25(OH)D concentration of 50 nmol/l could be a reasonable threshold in these age groups also.
The dose-response studies relating vitamin D intake (fortification and supplementation) to S-25(OH)D suggested that an intake of 1–2.5 µg/day will increase the serum concentration by 1–2 nmol/l but this is dependent on the basal concentration with response to being greater when the basal concentration is low. Chung et al. ( 28 ) concluded that doses of 10–20 µg/day may be inadequate to ensure concentrations of 25(OH)D at or above 50 nmol/l in the great majority of individuals in the population if the relationship above was used in the calculations. Cashman et al. ( 32 ) using meta-regression analysis concluded that 50 nmol/l 9 µg vitamin D was needed on average in the age groups between 9 and 78 years in the winter. However, 23.5 µg vitamin D/day was needed to reach a serum level ≥50 nmol/l if interindividual variation was taken into account by inclusion of the 95% range in the meta-regression analysis. These values have to be treated with caution as few studies (data points) were included. Moreover, as these results are based on group data, the final interpretation is difficult. An approach with primary data from the studies would probably have given other results. In the original studies, there are confounding factors affecting vitamin D status that have not always been taken into account in the studies, for instance dietary vitamin D intake and sunlight exposure. This affects the basal 25(OH) D concentrations which again affects the dose-response. Thus, it is not easy to make extrapolations regarding the actual dose/intake and a final 25(OH)D concentration. Compliance has not been taken into account in the analyses. This is important, as a low compliance in an RCT leads to ‘falsely’ low 25(OH)D concentrations in the non-complying subjects, therefore increasing the variance in the data.
For those older than 3 years, a 50 nmol/l target for S-25(OH)D concentration would probably require an average intake of 10 µg/day, that is, 50% of a population may need more, 50% may need less than this value. Adding 2 SDs to this average intake would cover 97.5% of the population. Given that 2SD equal 5 µg/day, this would result in an intake of 15 µg/day. It should be considered that these values are based on studies conducted in the winter without any sunlight exposure. We do not have any data or evidence on the dietary requirement during the summer months with sunlight exposure. It may be presumed that less is needed during this period for most people to reach 50 nmol/l. Vitamin D is stored for months after summer in the body. However, it can be debated to what extent dietary recommendations should assume dermal synthesis during summer, as outdoor activity with light clothing may not be universal, particularly not with the frail elderly and the institutionalized.
The vitamin D requirement for the elderly has to be given special consideration. The synthesis of vitamin D in the skin may be reduced and the intestinal absorption of vitamin D may be lower than in younger persons. Thus, older people may need more vitamin D than younger persons. The dose of vitamin D (10–20 µg/day) showed that to reduce the risk of fracture and total mortality is challenging to translate directly to recommended intake of vitamin D. The participants in these studies also got vitamin D from other sources (background intake and dermal synthesis), and additional calcium was given. However, it seems reasonable to recommend a somewhat higher intake in the elderly due to the above-mentioned reasons.
There was no evidence for a different intake requirement in pregnancy and lactation compared with the general population. There is some concern about the vitamin D status in obese persons. We did not find any evidence for different recommendation among ethnic groups.
An upper tolerable level (UL) was not possible to establish based on the SLRs. There is some concern that higher S-25(OH)D concentrations is associated with an increase in mortality. Notable is that the IoM as well as the European Food Safety Agency have set the Upper Tolerable Intake for adults at 100 µg/day ( 29 , 98 ).
In conclusion, if 97.5% of the population up to 75 years of age is to maintain the target 50 nmol/l concentration of 25(OH)D, the corresponding intake of vitamin D would be 15 µg/day. Higher intakes may be needed to cover this same percentage in an older population. Here, we refer to the total intake from food as well as supplements, given minimal sun exposure. Limited sunshine, however, does not reflect the situation for the majority of the Nordic population in the summertime. It should also be emphasized that there are large differences in results depending on assay methods and laboratories measuring 25(OH)D, adding to the uncertainty of determining an appropriate target concentration. Moreover, the dose response of vitamin D on serum 25(OH)D-concentrations is not well established and is dependent on the basal concentrations, sunshine exposure and dietary intake.
Implications for research
We have been able to identify some implications for the research:
- The role and dose response of sunshine
- Standardization of serum/plasma 25(OH)D assays
- Genes regulating the 25(OH)D concentration
- Bioavailability of vitamin D from different food sources
- Vitamin D status and adverse effects, including mechanisms
- Vitamin D's effects on various health outcomes
- Vitamin D dosing (including food-based), 25(OH)D, and health outcomes.
Acknowledgements
The authors thank Birgitta Järvinen and Jannes Engqvist for the literature search. Moreover, we want to thank Ulla-Kaisa Koivisto Hursti and Wulf Becker for their help and support during the process. The Nordic Council of Ministries has funded this work.
To access the summary tables and evidence tables to this article please see Supplementary files under Article Tools online
Authors’ Contributions
All authors contributed to methodological appraisal and data extraction. All authors decided independently and then by consensus which studies met inclusion criteria. All authors assessed quality and extracted data from included studies. All authors drafted the manuscript, commented on the draft review and suggested changes.
Conflict of interest and funding
Nordic Council of Ministries.
*indicates that the reference is included in the systematic review.

IMAGES
VIDEO
COMMENTS
Vitamin D physiology. Vitamin D was initially described as a substance that was able to cure rickets and was termed 'D' as it was the fourth in the sequence of vitamins discovered ().The main two isoforms are vitamin D3 (cholecalciferol) and vitamin D2 (ergocalciferol) that share a similar metabolism so that we will not differentiate between these isoforms unless otherwise stated.
In this narrative review, the current international literature on vitamin D deficiency, its relevance, and therapeutic options is discussed. Vitamin D testing and the use of vitamin D supplements ...
This Review highlights results from large randomized clinical trials performed during the period 2017-2020 and Mendelian randomization studies on vitamin D levels. Together, findings indicate ...
This systematic review to support the 2021 US Preventive Services Task Force Recommendation Statement on screening for vitamin D deficiency summarizes published evidence on the benefits and harms of screening and interventions for vitamin D deficiency in asymptomatic, community-dwelling adults.
Vitamin D insufficiency or deficiency (VDD) is a very prevalent condition in the general population. Vitamin D is necessary for optimal bone mineralization, but apart from the bone effects, preclinical and observational studies have suggested that vitamin D may have pleiotropic actions, whereas VDD has been linked to several diseases and higher all-cause mortality. Thus, supplementing vitamin ...
Background: Current studies suggest that vitamin D deficiency during pregnancy can produce a certain effect for preterm birth (PTB), but there is no research showing whether vitamin D deficiency has a consistent effect in different pregnancies; thus, we conducted a systematic review and meta-analysis of 24 observational studies, grouping them according to the gestational age at the time of ...
Vitamin D deficiency has been associated with numerous health outcomes, including risk of rickets in children or osteomalacia in adults, increased risk of fractures, falls, cancer, autoimmune ...
Vitamin D in health and disease: a literature review Br J Biomed Sci. 2013;70(4):161-72. doi: 10.1080/09674845. 2013. ... vitamin D may modify immune function, cell proliferation, differentiation and apoptosis. Vitamin D deficiency has been associated with numerous health outcomes, including risk of rickets in children or osteomalacia in adults ...
This systematic review and meta-analysis of the literature was conducted and reported according to the Preferred Reporting Items for Systematic Reviews and Meta ... was the only multicenter trial performed on HCWs. Vitamin D deficiency and insufficiency prevalence was consistent among the studies that included its measurement in their design ...
Results. One case-control study, ten cross-sectional studies and three cohort studies with a total of 31 424 participants were analysed. Lower vitamin D levels were found in people with depression compared with controls (SMD = 0.60,95% Cl 0.23-0.97) and there was an increased odds ratio of depression for the lowest v. highest vitamin D categories in the cross-sectional studies (OR = 1.31, 95 ...
The review only included literature published from 2012 until 2022, and 33 eligible studies met the inclusion criteria for this review. The included articles were critically appraised using the Mixed Method Appraisal Tool (MMAT). ... From our review, vitamin D deficiency led to decreased bone mineral density and contributed to the increased ...
The mean VHI-10 score of patients with vitamin D deficiency was 4.47 ± 5.79 in comparison to 1.58 ± 2.06 in the control group ( P = 0.052). Vitamin D deficiency was defined as a serum vitamin level <25 ng/dL. These findings were concurred in another study of 136 patients.
either vitamin D, calcium, or both nutrients and health outcomes in this heterogeneous body of literature proved challenging. KULIE T. (2009) Vitamin D: an evidence-based review. J. Am. Board Fam. Med., 22(6):698-706. Vitamin D is a fat-soluble vitamin that plays an important role in bone metabolism and seems to have
The objective of this review is to highlight the role of vitamin D as immunomodulatory, anti-inflammatory, and in improving maternal health and reducing the risk of developmental disorders in fetus. ... The literature published in the past two decades explaining the impact of maternal vitamin D level on mother and fetal health is reviewed ...
The impact of vitamin D on type 1 diabetes has been a controversial topic in public health. Furthermore, significant differences in the proportion of vitamin D have been noted. The purpose of this systematic review was to determine the overall proportion of vitamin D deficiency in children/adolescents with type 1 diabetes (T1D). Based on six electronic databases (PubMed, Web of Science, Embase ...
1 INTRODUCTION. Vitamin D deficiency is an important and growing public health problem and has a wide range of clinical manifestations. 1 Vitamin D deficiency is defined by serum levels of 25-dihydroxyvitamin D (25[OH] 2 Vitamin D) of <20 ng/mL. 2 Pseudotumor cerebri (PTC) syndrome encompasses a constellation of symptoms caused by elevated intracranial pressure of unclear etiology with normal ...
A Quick Review . Vitamin D is a vital nutrient for your bones, muscles, nerves, and immune system. Too little vitamin D can lead to fatigue, frequent illness or bone fractures, hair loss, and ...
You can get vitamin D from foods like fatty fish, mushrooms, fortified milks and cereals. "As far as 18 years and older, there's a daily recommended amount of about 600-800 international units ...
This review addresses general aspects of vitamin D deficiency and, in particular, the significance of vitamin D hypovitaminosis in the outcome of HBV- and HCV-related chronic liver diseases. Furthermore, current literature was reviewed in order to understand the effects of vitamin D supplementation in combination with IFN-based therapy on the ...
This review explores the association between vitamin D, CKD and periodontitis. The review summarises the current evidence base for the classical and non-classical vitamin D metabolic pathways, the biological mechanisms linking vitamin D deficiency, CKD and periodontitis, as well as the bidirectional relationship between the two chronic ...
Vitamin A Hair cells are the fastest-growing part of the body. It makes sense, then, that vitamin A is the perfect fuel for that growth. When your body absorbs vitamin A, it produces sebum. That's ...
The aim of this study is to elaborate a narrative review of the relationship between vitamin D status and HT and the role of vitamin D supplementation in reducing HT risk by modulating the immune system. There is extensive literature confirming that vitamin D levels are significantly lower in HT patients compared to healthy people.
This literature review is part of the NNR5 project with the aim of reviewing and updating the scientific basis of the 4th edition of the Nordic Nutrition ... The overall prevalence of vitamin D deficiency (25(OH)D <25 nmol/l) and insufficiency (25(OH)D < 50 nmol/l) was 13.8 and 52.2%, respectively. A marked seasonal variation was seen in the 25 ...