
# Type at least 1 character to search # Hit enter to search or ESC to close

No products in the cart.
Product Categories
- New Products
- Dissolved Oxygen
- Conductivity
- Temperature
- Oxygen (gas)
- Atlas Iot - Raspberry Pi Software
- AtlasDesktop
- Dosing Pumps
- Flow Meters
- Float Switches
- Probe Mounting
- EZO-Complete
- Waterproofing
- Carrier Boards
- Calibration Solutions
- Electrical Isolation
- Embedded Solutions
- EZO™ Accessories

Featured Products

- Distributors
- Calculators
- Privacy Policy
- Return Policy
What Is A Bioreactor And What Is It Used For?
- June 15, 2023

Share This Post
A bioreactor is a device or system that provides an optimal and controlled environment for biological and chemical reactions.
A bioreactor is a vessel or system that typically involves the growth and maintenance of microorganisms, mammalian, plant and stem cells, tissues, and algae.
Bioreactors have revolutionized various industries, including biotechnology, pharmaceuticals, food production, and wastewater treatment . They offer significant advantages in terms of cost-effectiveness, efficiency, and precise control of growth conditions.
What Are Bioreactors Used For?
Bioreactors are an absolute game changer in a wide range of fields and industries. From pharmaceuticals to food processing, bioreactors have an incredibly diverse range of applications that have advanced the way we approach a variety of processes. Whether you’re looking to grow cells or microorganisms, or produce biofuels or chemicals, bioreactors are the go-to tool for researchers and industry professionals alike.

One of the most common bioreactor applications is in the field of pharmaceuticals. Bioreactors are used to culture cells and microorganisms that are then used to produce a wide range of drugs and medications, or even vaccines. The ability to control and monitor the environment within a bioreactor allows for more consistent and reliable production of these medications, leading to greater efficiency and ultimately lower costs.
But it’s not just the pharmaceutical industry that’s benefiting from the use of bioreactors. Bioreactors are also being used in food processing, specifically in the production of fermented foods such as yogurt, cheese , and beer . The controlled environment within a bioreactor allows for precise control over the fermentation process, leading to higher-quality products that are both more consistent and flavorful.

In addition to these more traditional applications, bioreactors are also being used in cutting-edge research around biofuels and biomaterials. By using bioreactors to culture microorganisms that can produce biofuels or other useful materials, researchers can develop sustainable alternatives to traditional fossil fuels and materials. This has the potential to not only reduce our reliance on non-renewable resources but also to mitigate some of the negative environmental impacts associated with their use.
Advantages of Using Bioreactors
There are several advantages to using bioreactors for microbial and cell culture production:
- They are cost-effective
- They require less manual labor, reducing operational costs
- They are highly efficient and provide a high level of productivity, typically resulting in high yields and rapid production
- They provide precise control and monitoring , including pH, temperature, oxygen, and nutrient supply
However, there are also some disadvantages to bioreactors:
- They can be expensive to operate, resulting in high capital costs compared to traditional methods
- They must be sterilized properly to prevent contamination which is expensive and time-consuming
- They are complex systems therefore a skilled operator is required
- For any maintenance, highly-skilled maintenance personnel is needed
- They can have slower production methods than traditional processes
What is the Difference Between a Bioreactor and Fermentor?

Although the terms “bioreactor” and “fermentor” are often used interchangeably, there is a distinct difference between the two. A bioreactor typically refers to a device used for cultivating mammalian, plant, and stem cells, while a fermentor is specifically used for cultivating bacteria, yeast, and fungi. Bioreactors and fermentors share many similarities in terms of design and function, but their specific applications and cultivation requirements can vary depending on the type of organism being grown.
How Does A Bioreactor Work?
By providing a controlled environment, a bioreactor allows living organisms to grow, reproduce, and perform metabolic processes. This controlled environment is achieved through the regulation of various factors, including temperature, pH, oxygen concentration, and nutrient supply.

Bioreactor systems can be highly automated, using computerized processing systems to control environmental conditions, or they can be manually controlled.
Bioreactor Components
The specific design and application of the bioreactor will determine its exact components. However, there are some standard elements found in most types of bioreactors.
The main framework is known as the fermenter vessel . It’s the part that holds nutrients and biological material. The fermenter vessel is usually made from stainless steel or glass.

The thermal jacket is the outer layer that surrounds the inside of the bioreactor chamber. This is where biological reactions take place. Similar to the fermenter vessel, the thermal jacket is typically made from stainless steel. A cooling or heating fluid (like water) is found inside the thermal jacket to regulate and control the culture’s temperature.

Bioreactors require a component to mix and distribute the biological material and nutrients, and this is where the agitation system comes in. The agitation system has a motor that turns impellers or another mixing element.

Within the vessel are vertical plates/structures called baffles . Baffles prevent vortex formation or swirling within the liquid during the agitation process. This is important in reducing microbial colonies growing on the fermenter vessel walls. Baffles can also improve the flow of the culture medium and prevent sheer stress that could be harmful to microorganisms or cells.

Oxygen is supplied to the biological material via an oxygen delivery system , also known as an aeration system . The aeration system has a sparger that delivers sterile air.

Foam is a normal occurrence in bioreactors. To prevent the buildup of extra foam in the bioreactor vessel, a foam control system is in place. Excess foam in the vessel can cause overflow which not only contaminates the surroundings, but too much foam can also damage the bioreactor vessel.

The temperature inside the bioreactor vessel is controlled and regulated via a temperature control system .

Maintaining a specific pH is also important. The pH control system has sensors that monitor the pH level . The control system can also adjust the amount of acidic or alkaline buffer added to the bioreactor.

Sampling ports allow an entry point to the medium for easy monitoring and analysis.

Feed ports introduce additional materials the bioreactor needs for the bioprocess. The feed ports typically have sterile filters to sustain a sterile and controlled environment.
Finally, a cleaning/sterilizing system is used to clean the bioreactor. Depending on the type of bioreactor you are using, the system can either use heat, steam, or chemical sterilization methods.

How To Set Up A Bioreactor?
The first step in setting up a bioreactor is to choose the type of bioreactor that best suits your application. Next, you need to prepare the culture media, followed by sterilization of the bioreactor components.
Once you have sterilized the bioreactor, it is assembled according to the manufacturer’s guidelines.
During the bioreactor process various parameters, including temperature, pH, dissolved oxygen , and partial pressure of oxygen (pO2) are controlled and monitored.
Considerations in Bioreactor Design

There are some key factors to optimize the performance of your bioreactor.
Oxygenation for Aerobic Microorganisms
One of the critical factors to consider in bioreactor design is the method of oxygenation, particularly for aerobic microorganisms that require oxygen for their metabolic processes. Efficient oxygen transfer is essential for maintaining the growth and productivity of these organisms.
Oxygen Transfer Methods
There are several ways to transfer oxygen to the culture medium in a bioreactor:
Direct sparging: Air or oxygen is bubbled directly into the medium through a sparger or diffuser.
Surface aeration: Oxygen is transferred to the medium at the air-liquid interface through agitation or mixing.

Membrane aeration: Oxygen is supplied through a gas-permeable membrane ( membrane – aerated biofilm reactor) immersed in the medium. These offer a low-energy delivery of oxygen to the medium.
The choice of oxygen transfer method depends on the specific requirements of the application and the type of bioreactor being used.
Factors Influencing Oxygen Transfer
Several factors can influence the efficiency of oxygen transfer in a bioreactor, including:
Agitation speed: Faster agitation rates generally result in higher oxygen transfer rates, but excessive agitation can cause damage to sensitive cells or microorganisms.
Gas flow rate: Higher gas flow rates can improve oxygen transfer, but this can also lead to excessive foaming and increased energy consumption.
Bubble size: Smaller bubbles have a larger surface area and offer better oxygen transfer efficiency than larger bubbles. However, smaller bubbles can also cause foaming and may be more challenging to generate and maintain.
Medium viscosity: Higher viscosity media can reduce oxygen transfer rates, making it essential to consider the impact of medium properties on oxygenation.
Temperature Management
Temperature control is another crucial factor in bioreactor design, as it can significantly impact the growth and metabolism of the microorganisms or cells being cultured. Different organisms have specific temperature requirements for optimal growth, and maintaining a stable temperature within the bioreactor is essential for successful cultivation.

Temperature Control Methods
There are several ways to control the temperature within a bioreactor:
Heating or cooling jackets: A jacket surrounding the bioreactor vessel can contain heating or cooling fluids to regulate the temperature .
Internal heat exchangers: Coils or other heat exchange devices can be immersed in the culture medium to control temperature.
Direct heating or cooling: Electrical heaters or cooling elements can be placed directly in the bioreactor to manage the temperature.
The choice of temperature control method depends on the specific application and the type of bioreactor being used.
Factors Influencing Temperature Control
Several factors can influence the efficiency of temperature control in a bioreactor, including:
Heat generation: Microbial metabolism can generate heat, which may require additional cooling measures within the bioreactor.
Heat loss: Heat can be lost from the bioreactor through conduction, convection, and radiation, which needs to be accounted for in the temperature control strategy.
Temperature gradients: Temperature gradients can develop within the bioreactor, leading to uneven growth and metabolism. Efficient mixing and agitation can help minimize temperature gradients.
Methods for Monitoring the Culture
Monitoring the culture within the bioreactor is essential for ensuring optimal growth conditions and maintaining product quality. Various methods can be used to monitor different aspects of the culture, including cell density, nutrient levels, pH, and dissolved oxygen .

Photo courtesy of Eppendorf SE
Cell Density Measurement
Cell density can be measured using several techniques, such as:
Optical density: A spectrophotometer measures the amount of light absorbed by the culture at a specific wavelength, which is proportional to the cell density.
Viable cell counting: Cells can be counted using a hemocytometer or automated cell counter after staining with a viability dye.
Biomass measurement: The total mass of cells in the culture can be determined by filtering the culture and measuring the dry weight of the collected biomass.
Nutrient Monitoring
Nutrient levels in the bioreactor can be monitored using analytical techniques such as:
HPLC (high-performance liquid chromatography): Separates and quantifies individual components in the culture medium.
Spectrophotometry: Measures the absorbance of specific wavelengths of light by the culture medium to determine nutrient concentrations.
Ion-selective electrodes: Electrodes that respond selectively to specific ions in the culture medium, such as ammonium or nitrate.
pH and Dissolved Oxygen Monitoring
pH and dissolved oxygen levels can be monitored using sensors placed within the bioreactor. These sensors typically provide real-time measurements and can be connected to a control system to maintain the desired setpoints.
Maintaining sterility within the bioreactor is crucial for preventing contamination and ensuring product quality. Several strategies can be employed to maintain a sterile environment within the bioreactor:
Sterilization of the bioreactor and its components: Bioreactors and their components can be sterilized using steam, heat, or chemical methods.
Aseptic operation: Bioreactors can be operated under aseptic conditions, with sterile air or gases used for sparging and all inputs and outputs filtered to prevent contamination.
Single-use bioreactors: Disposable bioreactors eliminate the need for cleaning and sterilization between runs and reduce the risk of contamination.
Sterilization Methods
There are several methods for sterilizing bioreactors and their components:
Steam sterilization (autoclaving): High-pressure steam is used to sterilize bioreactors and their components at temperatures of 121°C or higher.
Dry heat sterilization: Bioreactors and components are heated in an oven at temperatures of 160°C or higher for a specific period.
Chemical sterilization: Bioreactors and components are exposed to chemical sterilants, such as hydrogen peroxide or ethylene oxide, to kill microorganisms.
The choice of sterilization method depends on the specific application and the materials used in the bioreactor and its components.
Mixing and Agitation
Efficient mixing and agitation within the bioreactor are essential for maintaining uniform growth conditions, promoting mass transfer, and preventing the formation of gradients within the culture medium. There are several factors to consider when designing the mixing and agitation system for a bioreactor:

Impeller type: Various impeller designs, such as Rushton turbines, pitched-blade impellers, or marine propellers, can be used to provide the desired level of mixing and agitation within the bioreactor.
Impeller speed: The speed of the impeller can be varied to achieve the desired level of mixing and agitation, with higher speeds typically resulting in better mixing but also higher shear forces that can be detrimental to sensitive cells or microorganisms.
Baffles: Baffles can be added to the bioreactor to increase turbulence and improve mixing.
Shear Sensitivity
Some microorganisms and cells, such as mammalian cells or shear-sensitive bacteria, can be damaged by excessive shear forces generated by mixing and agitation. In such cases, it is essential to design the mixing and agitation system to minimize shear forces while still providing adequate mixing and mass transfer.
Scale-up Considerations

When scaling up a bioreactor from a laboratory-scale system to a larger production-scale system, it is essential to maintain similar mixing and agitation conditions to ensure consistent growth and productivity. Factors such as impeller type, speed, and spacing, as well as the presence of baffles, should be considered when scaling up a bioreactor.
Bioreactor Materials
The choice of materials for constructing a bioreactor is essential for ensuring the durability, reliability, and sterility of the system. Some common materials used for bioreactor construction include:
Stainless steel: Widely used for large-scale bioreactors due to its durability, corrosion resistance, and compatibility with steam sterilization.
Glass: Commonly used for smaller-scale bioreactors due to its transparency, chemical resistance, and compatibility with steam sterilization.
Polymers and plastics: Used for disposable, single-use bioreactors and components, offering advantages in terms of cost, sterility, and disposal.
The choice of material depends on the specific application, the scale of the bioreactor, and the sterilization requirements.
Bioreactor Control Systems
Bioreactor control systems are essential for maintaining optimal growth conditions within the bioreactor and ensuring the quality and consistency of the product. These systems can be either manual or automated, depending on the level of control required and the complexity of the bioreactor system.

Types of Control Systems
Several types of control systems can be used for bioreactors:
Manual control: Operators manually adjust the bioreactor parameters, such as temperature, pH, and dissolved oxygen , based on measurements taken from the system.
PID (proportional-integral-derivative) control: An automated control system that uses feedback from sensors within the bioreactor to maintain the desired setpoints for temperature , pH, and dissolved oxygen.
Advanced control systems: More sophisticated control systems that use algorithms, models, or artificial intelligence to optimize the growth conditions within the bioreactor and adapt to changes in the culture or process.
Integration with Process Monitoring and Data Acquisition
Bioreactor control systems can be integrated with process monitoring and data acquisition systems to provide real-time feedback on the performance of the bioreactor and enable more precise control of growth conditions. This can help improve the efficiency, productivity, and quality of the bioprocess.
Bioreactor SCADA Software
The term ‘SCADA’ stands for Supervisory Control And Data Acquisition. It refers to a system that allows operators to remotely monitor and control various processes and equipment in a facility. The Bioreactor SCADA software is designed to provide real-time data on the bioreactor’s temperature, pH levels, nutrient levels, and other critical parameters. This information is essential for maintaining the optimal conditions for the growth and development of the microorganisms or cells in the bioreactor.

The Bioreactor SCADA software is a sophisticated system that uses a combination of hardware and software components. The hardware components include sensors that are used to measure the various parameters. These sensors are connected to a central control system that is responsible for collecting and processing the data. The software components include the user interface, which provides operators with an intuitive interface for monitoring and controlling the bioreactor.

One of the key benefits of using Bioreactor SCADA software is that it allows for greater process control and automation. This means that operators can remotely monitor and control the bioreactor from a central location, reducing the need for manual intervention. This can lead to significant improvements in process efficiency and reduced variability in product quality. In addition, the software can be configured to provide alerts when certain parameters fall outside of predefined limits. This enables operators to take corrective action before any damage is done to the bioreactor or the microorganisms or cells being grown within it.
Types Of Bioreactors
There are several types of bioreactors, each with their specific uses and benefits. Some common types include:
Photobioreactors
These are specialized bioreactors that cultivate photosynthetic microorganisms by providing artificial light conditions. This type of bioreactor offers precise temperature, pH, and light intensity control, and results in better growth rates and cleaner samples compared to algae , moss, and other photosynthetic microorganisms growing in natural environments.

Although closed systems are preferred as they have greater protection against contaminants and control of the environment, photobioreactors can have open or closed systems.
Photobioreactors are not like traditional bioreactors as they solely rely on the power of the sun, which makes them a more eco-friendly and efficient bioprocess method.
Continuous-Stirred Tank Bioreactors
CSTRs, otherwise known as continuous-stirred tank bioreactors, are the most popular bioreactors in use these days. The aspect ratio for these is generally between 3-5. The turbid static or chemo static principles are employed to regulate the flow rate with the help of a sparger device.

The sparger produces bubbles which are then broken down into smaller bubbles and dispersed throughout the medium. This even distribution of bubbles helps create a homogeneous and uniform environment within the bioreactor, allowing bioprocess reactions to take place.
Airlift Bioreactors
The airlift bioreactor is becoming more and more popular in recent years due to the way it works. It is akin to a bubble column reactor but contains a draft tube inside or outside the system. This tube is used to help circulation and oxygen transfer, as well as to equalize shear forces. Internal loop airlift bioreactors have a single container that allows liquid to circulate through an interior channel at a fixed rate. For external loop airlift bioreactors, the liquid is circulated through separate channels.

The utilization of renewable energy sources is becoming increasingly common, as they are considered to be more reliable and cost-effective than their non-renewable counterparts. The rise in popularity of these sources is due to their ability to be replenished and their eco-friendly properties. As a result, an increasing number of individuals and organizations are turning to renewable energy sources as a viable source for their energy needs.
Bubble Column Bioreactors

The bubble column bioreactor is an attractive option for many applications due to its straightforward design. The vessel is cylindrical and the height can be adjusted to meet any desired scale of production, between 4-6 in aspect ratio. Air or gas is added to the bioreactor via perforated plates or pipes, or microporous spargers, with plates typically preferred for improved performance. The flow rate of the gas is then affected by oxygen transfer and mixing. The bioreactor is used to produce products through a fermentation method, with reactants compacted using a finely dispersed catalyst.
Fluidized Bed Bioreactors (FBBRs)
FBBRs are like bubble column bioreactors, but the top part is enlarged and the column is narrowed down to reduce the flow velocity. This is to maintain the solids in the reactor while the liquid can exit. To achieve this, gas is supplied to form a ‘gas-liquid-solid’ bed and an acceptable suspended state. It is essential that the suspended particles are heavy enough to not float, and the same goes for dense particles. If there are too many dense ones, they might settle at the bottom. Fortunately, liquids can be recycled in FBBRs, which is essential to keep the biocatalysts in the bioreactor and the reaction ingredients in contact. This is an advantageous practice for bioprocessing and is necessary for good performance.

The use of digital technology is becoming increasingly prevalent in the workplace. It is becoming common to find that jobs now require a certain level of technological proficiency. As a result, workers must possess a knowledge of digital tools to remain competitive in the job market.
The ubiquity of digital technology in the workplace is growing. Consequently, many roles necessitate a certain understanding of technology to stay relevant in the employment sphere. It is thus becoming increasingly important for workers to possess the know-how to use digital tools to remain competitive.
Packed Bed Bioreactors

A packed bed bioreactor is an enclosed system of solid particles with biocatalysts. These solids are either porous or non-porous (rigid) gels. The biocatalyst is secured to the particles, and a nutrient broth perpetually circulates over them. The flow is typically directed downward due to the need to remain below the minimum fluidization velocity. The products and metabolites formed within the bioreactor are dispersed and eliminated as the fluid is drained.
Bioreactor Processes
The choice of bioreactor process for a particular organism and application will depend on the desired objectives, with each option having its benefits and drawbacks. These techniques may include batch, fed-batch, continuous, and perfusion.
Batch Processes
For batch processing, no more nutrients are provided during the bioprocess apart from elements for control such as gases, acids, and bases that are added at the start. This process continues until the added nutrients are all consumed by the cells. These processes are useful if the experiment needs to be conducted quickly, but the biomass and product yields are restricted. Oxygen transfer and carbon source are usually the main limitations, meaning that the microorganisms only grow exponentially for a short duration.

Fed-batch Processes
Fed-batch processing stands out from batch processing in that it involves adding nutrient feeds regularly during the process. This avoids nutrient depletion and encourages cell growth, leading to increased cell density and yield. The bioreactor is first stocked with media and inoculated with cells, after which fresh nutrient feeds are provided as required throughout the activity. Fed-batch processes are the most common and direct way to reach higher cell densities and better outcomes than with batch processing.

The utilization of technology has been increasing rapidly in recent years, with more and more people making use of it in their day-to-day lives. Technologies such as computers, the internet, and smartphones have become ubiquitous, and people are now relying on them for many tasks. This has led to a surge in the demand for technology and its applications. As a result, the development of innovative technologies and applications is now seen as an important part of modern life.
Perfusion Process
Using perfusion can facilitate a longer procedure than a fed-batch one, leading to a higher cell density and increased product yields. Tangential flow filtration eliminates unnecessary components and some medium is recycled.
This process provides a constant stream of a new medium into the bioreactor to guarantee that volume is retained, consequently augmenting cell densities and product yields. But, this is not a continuous process, since the cells will continue to grow until a point of no greater cell density is reached.
Continuous Processes
With continuous processing, bioreactors are kept stocked with a continuous supply of nutrients for the cells or microorganisms growing inside. In this type of process, a new medium is constantly being added to the bioreactor while the same amount of used media is taken away. This helps the culture remain steady in terms of growth and division, leading to a steady cell or microorganism density and a more predictable product output.

Single-use And Multi-use Bioreactors
Recently, the use of so-called disposable bioreactors has become increasingly popular due to their cost-effectiveness, flexibility, and ease of use. These bioreactors consist of a bag made of multiple polymer layers, each of which serves a specific purpose in applications such as clinical manufacturing and process development.

In contrast, multi-use bioreactors, which are usually made of either stainless steel or glass, can be employed for multiple production runs and are thus more suitable for large-scale production and extended use. However, they necessitate additional sterilization and cleaning between runs, which can be time-consuming and costly.
What Is The Future Of Bioreactors?
The future of bioreactors looks promising, with new technologies and applications emerging all the time. Some areas of growth for bioreactors include:
Synthetic biology: Bioreactors can produce synthetic organisms and genetic materials, paving the way for new advances in medicine and biotechnology.
Precision fermentation: Bioreactors can produce specific compounds or molecules through precision fermentation, allowing for more targeted production of pharmaceuticals and other products.
Sustainable agriculture: Bioreactors can produce sustainable fertilizers and pesticides, reducing the environmental impact of agriculture.
Bioreactors are fascinating pieces of technology that have revolutionized the world of science and engineering.

If you’re looking for more information on Bioreactors, please check out www.eppendorf.com/bioprocess
If you need assistance with setting up your bioreactor or what bioreactor will best suit your application needs, do not hesitate to contact the world-class team at Atlas Scientific .
Sensor & Probes for Bioreactors

Subscribe To Our Newsletter
Get product updates and learn from the best, more to explore.

How to calibrate the EZO Complete-TMP using the Atlas Desktop software
The Atlas Scientific line of EZO Complete circuits makes building a custom monitoring system easy, but if you don’t calibrate your sensors, then what good are your readings; And can you trust them? The accuracy of your readings is directly related to the quality of your calibration. Calibration is not difficult, and a little bit

How to calibrate the EZO Complete-Dissolved Oxygen using the Atlas Desktop software
Want to learn more about our products, atlas scientific | all rights reserved © 2024.
To track your order please enter your Order ID in the box below and press the "Track" button. This was given to you on your receipt and in the confirmation email you should have received.
Billing email


Bioreactor: Parameter, Parts, Types, and Application
Bioreactors are containers created and designed to offer an efficient environment for whole cells or enzymes to convert bio-chemicals into products. In other circumstances, such as water treatment, the bioreactor is used to inactivate or sterilize cells.
Numerous distinct bioreactors include stirred tank bioreactor, packed bed bioreactor, fluidized bed bioreactor, airlift bioreactor, and bubble column bioreactor. Depending upon the type of bioreactor, it holds various applications, such as those for cell growth, enzyme production, catalysis, biosensors, food production, milk processing, extrusion, tissue engineering, algae production, protein synthesis , anaerobic digestion, algae, and chitinolytic culture.
Table of Contents
Parameters to be Considered for Bioreactor
Bioreactor only works well when it meets specific parameters like temperature, mixing rate of microorganisms, uniform nutrient transfer, and gassing.
- Culture mixing: The culture for the bioreactor must be mixed constantly and carefully to ensure the proper mixing of nutrients and maintain the suitable pH and temperature among cells. The mixing rate varies according to the organisms used for culturing. For example, the rate for mixing fungi and yeast is 500-1500 per min, and for mammalian, insect, and plant cells is 30-300 per min
- Temperature and pH : Correct temperature and pH play crucial roles in the processes occurring in the bioreactor because culture cells and enzymes work efficiently in certain temperatures and pH. The specific temperature range for bacteria, fungi, and yeast cells is 20 °C to 60 °C, and the pH range for these organisms is 4.5-7.
- Nutrient required: Depending on the bioprocess strategy, nutrients such as water, energy sources like glucose, carbon, salt, and other trace elements (e.g., vitamins) are either added gradually over time (in a fed-batch or continuous process) or made available at the start of a bioprocess (batch bioprocess).
- Construction material: The materials (glass or stainless steel) used during construction should withstand the pressure and have to be anti-corrosive.
- Ideal foam collector : The ideal bioreactor must have a foam controller to prevent various side effects caused by it. The detector must sense when the foam touches it so that it adds the anti-foaming agent to a fermenter.
- Supplying air: The continuous stirring causes disturbance in nutrients and reduces bubbles’ size. So, it is essential to release the air molecules in the nutrient broth as air molecules are taken by the cell only when it dissolves in the nutrient solution. The demands of air molecules depend on the types of organisms or cells used (aerobic or anaerobic) and the fermentation phase. At the very beginning, only a few amounts of oxygen are needed, but the supply of oxygen is increased as there is faster growth of cells.
Parts of Bioreactor
The various parts of the bioreactor work together to meet the parameters like temperature, nutrients, aeration, and pH for the growth and development of the cells, enzymes, and many more. Some essential parts of a bioreactor are discussed below:
- Fermenter vessel: Most fermented containers are made of glass and stainless steel to reduce pressure and corrosion. It provides a workable environment for production.
- Heating and cooling apparatus: The cooling jacket and silicon in a reactor help to remove excess heat, while internal coils provide heat during fermentation.
- Feed ports: The silicon tubes are available for adding nutrients and acid/alkali for fermentation.
- Foam control: The foam produced during the fermentation process has many side effects like it decreases efficiency, and productivity, degrading product quality, and many more. So, the foam detector is placed in a reactor, adding some anti-foam is used to deform the fermenter.
- Valves: In the fermenter, valves regulate the liquid flow. Most of the reactor contains at least three valves in it.
- Sparger: It is used in introducing sterile air to a fermentation vessel. It also aids in providing the vessel with the correct aeration.
- Impeller: The role of an impeller in a fermenter is to distribute microbial cells in a nutrient media evenly and also to reduce the bubbles produced with the help of impeller blades.
- Computer: Modern automated and semi-automated software programs are used for collecting data, monitoring, and controlling the process, such as Ambr® Clone Selection software application used for cell line screening and Software called Biostat® T CHO introduces users to bioprocess engineering and controls a bioreactor as well.
- Baffles: Baffles are the metal stripes attached to the wall of the container to prevent vertex and improve aeration in the fermenter.
- Regulator: This device is used to control and maintain the temperature, pH, nutrients, oxygen concentration, and product concentration.

Types of Bioreactors
Different bioreactors have been constructed depending on their use. Some of them are listed below : (4)
- Continuous stirred tank bioreactor
- A Continuous stirred tank bioreactor is a cylindrical vessel with a motor-driven central shaft holding one or even more agitators (impellers).
- It consists of different features for proper agitation and baffles for aeration.
- It creates a homogeneous environment, is easy to operate, easy to clean, cheap, and which is why used to a great extent in industries.

- Airlift bioreactor
- An airlift bioreactor, also known as a tower reactor, contains a draft tube that boosts circulation and air transfer. The draft tube even increases the shear forces in a bioreactor.
- It consists of two zones, one is called riser, which is sparged with gas, and another one is known as downcomer, which does not contain sparged gas.
- Some products are temperature-dependent, and growing different types of cells in a wide range of temperatures (30-40℃) in the same instrument is complex. A two-stage reactor is necessary for producing such products where the cell growth occurs in one reactor, and the remaining process occurs in another reactor.

- Bubble column reactor
- It is a cylindrical vessel with an aspect ratio of 4:10 and contains a gas distributor at the bottom section of the vessel where gas is sparged in the form of bubbles.
- Like an airlift reactor, it doesn’t have a draft tube.
- It contains perforated pipes or porous metal spargers to sparge gas.
- Gas flow rate and fluid rheological characteristics significantly impact O2 transfer, mixing, and other operational aspects.
- The advantages of the bubble column reactor over other reactors are its high productivity in terms of volume and superior heat management, and self-regulation.
- It is specially used to produce aerobic products. (2)

- Fluidized-bed bioreactor
- This type of reactor consists of packed beds (solid-liquid material) where solid particles are retained, and liquid particles flow out.
- Except for the top position expanding to lower fluid velocity, a fluidized bed bioreactor is similar to a bubble column bioreactor.
- The merits of using this reactor are the uniform mixing of particles, uniform temperature gradients, and can operate in a continuous state.
- Inadequate fluidization occurs due to the accumulation of relatively massive or densely packed particles on the distributor plate, which is a significant issue with the steady functioning of fluidized beds.
- The major issue of fluidized bed bioreactors is inadequate fluidization due to the accumulation of relatively massive or densely packed particles on the distributor plates. (3)

- Packed bed bioreactor
- A packed bed bioreactor consists of a column of solid particles with biocatalysts on or within the solids matrix. It is also called a fixed-bed reactor.
- A nutrition broth is continuously applied on top of the immobilized biocatalyst.
- It is challenging to adjust the pH of the packed bed bioreactors because of improper adding of acid or alkali combined with poor mixing. The issues like undesired temperature gradient, difficulty in cleaning, hard-to-replace catalyst, and undesirable side effects are frequently observed.
- This type of reactor offers many advantages like a high catalyst conversion rate, simplicity to use, low construction time, affordable operating costs, better reactant-catalyst contact, and can operate at high temperatures and pressures.
- In addition, a packed bed bioreactor is used to create bio-artificial liver support systems and expanded bone marrow cells. (1)

- Photobioreactor
- A photobioreactor is a fermenter that uses either direct sunlight or artificial illumination.
- As the light must pass through a container, this reactor is constructed using glass, plastics, and flat panels.
- It operates efficiently at a temperature ranging from 25°C-40°C.
- This fermenter has limitations since it depends on light (requires maximum light); it needs frequent cleaning for light to pass through, and controlling temperature is also challenging.
Application of Bioreactor
A bioreactor’s application highly depends on the reactor type used.
- A continuous stirred tank reactor is suitable for the production of alcohol , antibiotics (like penicillin), certain enzymes (like a ligninolytic enzyme), tissue mass culture, citric acid, exopolysaccharides (like dextran), and amino acids (like glutamate).
- Bubble column fermenters are used for culturing algal and Chitinolytic enzyme cultures. It is also used for the bioconversion of glucose to gluconic acid using an enzyme called glucose oxidase.
- An airlift bioreactor is usually used to produce single-cell protein, methanol, cellulose, lactic acid, gibberellic acid, wastewater treatment, and pectinolytic enzymes like polygalacturonases.
- Fluidized bioreactors are suitable for producing enzymes like laccase, fluid-suspended biocatalysts (immobilized enzymes, cells, and microbial flocks), and also in bulk drying of materials.
- A packed bed reactor is mainly constructed for wastewater and sewage treatment and for cultivating anaerobic ammonium-oxidizing bacteria (ANAMMOX).
- Photobioreactors are usually preferred for producing photosynthetic cultures such as cyanobacteria, and microalgae, and for β-carotene.
- Chaudhuri, J., & Al-Rubeai, M. (2005). Bioreactors for tissue engineering: Principles, design, and operation. Bioreactors for Tissue Engineering: Principles, Design and Operation , (July), 1–372. https://doi.org/10.1007/1-4020-3741-4
- Kantarci, N., Borak, F., & Ulgen, K. O. (2005). Bubble column reactors. Process Biochemistry , 40 (7), 2263–2283. https://doi.org/10.1016/j.procbio.2004.10.004
- Seong, Y. S., Dong, H. L., & Sang, D. K. (2006). Effect of uniformity of gas distribution on fluidization characteristics in conical gas fluidized beds. Studies in Surface Science and Catalysis , 159 , 557–559. https://doi.org/10.1016/s0167-2991(06)81657-5
- พวงผกา มะเสนา และประณ. (2557). Bioreactors and their types. 4 (1), 88–100.
- Chisti, Y., & Moo-Young, M. (2003). Bioreactor.
Alisha Tripathi
Hello everyone. I am Alisha Tripathi. I have completed my Master's degree from National College. My teaching career counts for more than a year and have always been passionate about writing a blog and inclined toward research based on molecular science, immunology, and genetics.
We love to get your feedback. Share your queries or comments Cancel reply
This site uses Akismet to reduce spam. Learn how your comment data is processed .
Recent Posts
Restriction Fragment Length Polymorphism (RFLP): Steps
Restriction Fragment Length Polymorphism (RFLP), a method applicable in molecular biology to detect variations in DNA sequences among individuals. It relies on the fact that DNA sequences can vary...
Exons And Introns: Functions and Types
Exons are regions of a gene that contain coding information for the synthesis of proteins. During the process of gene expression exons are transcribed into mRNA and eventually translated into...
- What is Chemical and Biological Engineering?
- Engineering problem solving
- Error and uncertainty
- Process variables
- Process Fundamentals
- Material Balances
- Reacting systems
- Reaction kinetics
- Reactor design
- Bioreactors
- Fluids and fluid flow
- Mass transfer
- Energy balances
- Heat transfer
- Heat exchangers
- Mechanical energy balances
- Process safety
- Engineering ethics
- Sustainability
- Engineering in a global context
- What is a bioreactor
- Bioreactor basics
- Important biological issues
- Biological catalysts: enzymes
- Quantifying reactions rates for enzymes
- Reactor types
- Considerations in bioreactor design
- « Reactor Desig...
- Fluids and Fluid Flow »
Bioreactors ¶
Why the dinosaurs died out is not known, but it is supposed to be because they had minute brains and devoted themselves to the growth of weapons of offense in the shape of numerous horns. —Bertrand Russell
We have examined a number of conservation and rate equations that are applicable to all Chemical and Biological Engineering processes. However, it is useful to examine some processes specifically biological in nature.
We could cover biological heat transfer, biological mass transfer, biological reactors, etc.
Here we will focus on biological reactors, i.e., bioreactors.
What is a bioreactor ¶
An apparatus for growing organisms (yeast, bacteria, or animal cells) under controlled conditions. Used in industrial processes to produce pharmaceuticals, vaccines, or antibodies. Also used to convert raw materials into useful byproducts such as in the bioconversion of corn into ethanol.

Industrial bioreactor ¶

Glacial Lakes Energy in Watertown, South Dakota ¶
57+ million gallons per year ethanol production
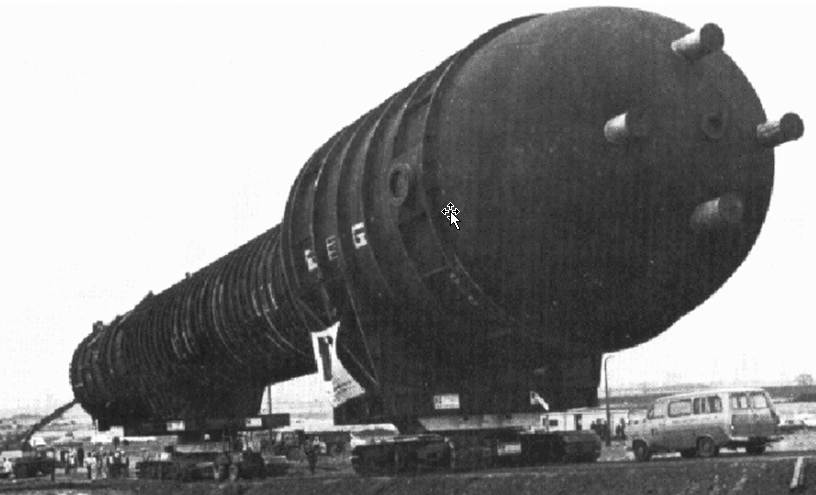
World’s largest industrial fermenter (as of 1978) ¶
The fermenter is 200 feet high and 25 feet in diameter
Bioreactor basics ¶
More about bioreactors:
They are systems or devices that supports a biologically active environment
They are vessels in which a chemical process is carried out which involves organisms or biochemically active substances derived from such organisms
They can be either aerobic or anaerobic
They are commonly cylindrical, ranging in size from liters to cubic meters, and are often made of stainless steel
They supply a homogeneous (same throughout) environment by constantly stirring the contents.
They give the cells a controlled environment by ensuring the same temperature, pH, and oxygen levels.
Important biological issues ¶
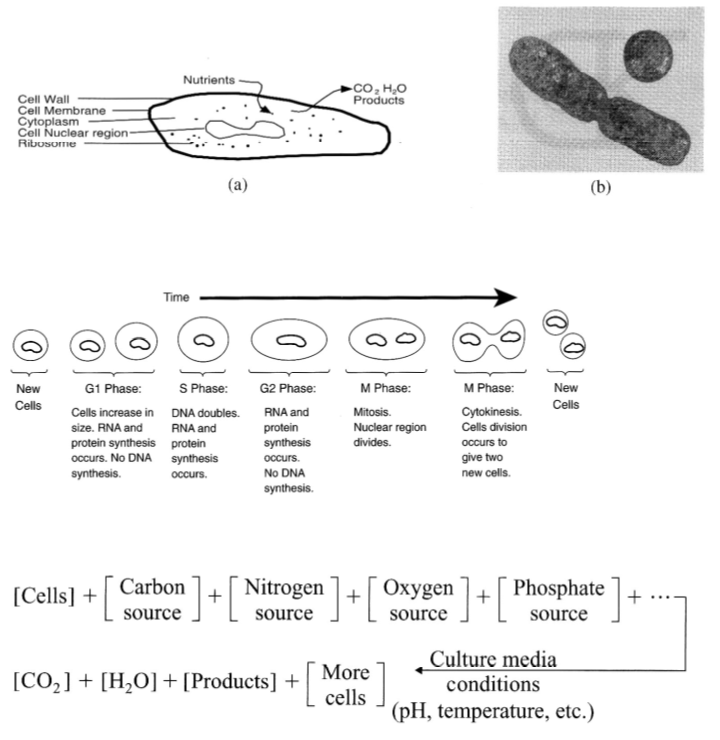
Limiting substrate : The substrate or substrate(s) (energy source, carbon source and/or nutrient source) which is/are first exhausted in batch growth. This substrate(s) has a direct influence on the kinetics of cell growth.
In excess : The substrate or substrate(s) (energy source, carbon source and/or nutrient source) which is/are not exhausted at the end of batch growth. This substrate(s) has no effect on the kinetics of cell growth.
Exercise: Cell numbers versus time in a bioreactor
Considering the above factors, make a sketch of the number of cells over time in a bioreactor.
Biological catalysts: enzymes ¶
Quantifying reactions rates for enzymes ¶
Maud Menten and Leonor Michaelis

Michaelis-Menten kinetics
where, \(v_{0}\) is the initial ‘reaction velocity’, \(r_{p}\) is the rate of production of the product, \(r_{s}\) is the rate of production of the substrate, \(S\) is substrate, \(P\) is product, and square brackets ( \([\ ]\) ) represent concentration.
This equation governs the initial reaction rates (i.e., not including the reversion of products).
Exercise: Michaelis-Menten kinetics
Consider the Michaelis-Menten equation.
What are the units of \(V_{\max}\) and \(K_{m}\) ?
Sketch out the rate of product formation as a function of substrate concentration.
What is the order of the reaction at ‘relatively low’ and ‘relatively high’ substrate concentrations?
Reactor types ¶
Chemostat (chemical environment is static)
A bioreactor to which fresh medium is continuously added, while culture liquid is continuously removed to keep the culture volume constant
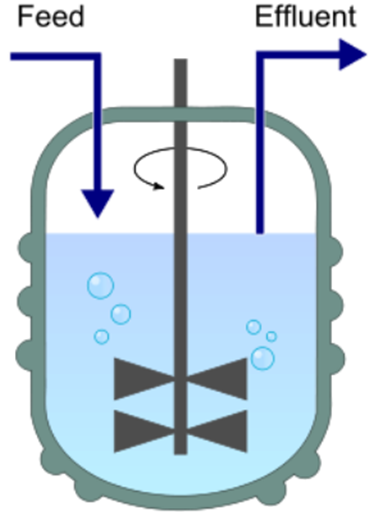
What type of reactor is this?
Let’s compare the nomenclature of chemical reactors (CSTRs) with that for bioreactors and show some typical units:
As for chemical reactors, we can construct our general material balance:
It is generally assumed that the biomass is not consumed in a bioreactor. With that in mind, we can use the appropriate symbols and relationships to arrive at the mathematical form of the material balance:
With a sterile feed (no biomass in), and recalling that for a well mixed reactor \(x_{out}=x\) , we have
Substituting in the expression for the growth and rearranging gives
Exercise: Chemostat analysis
What is the relationship between the dilution rate, \(D\) , and growth rate, \(\mu\) , at steady state?
What happens if \(D\) is very large?
Considerations in bioreactor design ¶
The design of a bioreactor can be different from that of a chemical reactor in many ways.
Exercise: Bioreactor features
What are some of the issues that must be considered to assure the health and productivity of microorganisms?
Based on part 1, make a list of the features that make a bioreactor different from a standard chemical reactor.
Based on part 2, sketch a bioreactor and include all of the equipment that you think is necessary.

What is a bioreactor?
A bioreactor is a vessel in which raw materials under controlled conditions are converted into products by activity of living cells (microorganisms, mammalian, plant and stem cells, tissues and algae) or by cellular components such as enzymes.
The difference between bioreactor and fermenter
Bioreactor and fermenter are similar terms, but with a distinct difference. The term bioreactor often relates to the cultivation of mammalian, plant and stem cells.
If the application is the cultivation of a bacteria, yeast or fungi, then the term fermenter is used. It would not be a mistake to use the term bioreactor in such cases as well, but in the case of cell cultivation only the term bioreacto r is used. The name fermenter is associated with reactors in which fermentations are carried out, i.e. metabolic processes that produce chemical transformations in organic substrates through the action of enzymes . The main difference between the bioreactors used for cell and microorganism cultivation is in the mixing and aeration requirements, as well as height and diameter ratio H/D. The mixing environment for microorganism cultivation is usually intensive, with effective dispergation of gas bubbles, and the aeration rate is between 0.5- 3 vvm (volume cultivation media/volume air flow per minute). However, cell cultures require gentle mixing and aeration rate is beween 0.01-0.1 vvm. In turn, the optimal H/D ratio of the vessel for microorganism cultivation is 3:1, but in the case of cell cultures it is 2:1.

The classification of bioreactors
Generally, bioreactors can be characterized in two ways:
The cultivation principle;
The operation mode.
Cultivation principles of microorganisms are further subdivided into: submerged , immobilisation and solid state .
The cultivation principles
Submerged cultivation
Inoculation of the microbial culture into the liquid medium for generation of the desired product is known as submerged cultivation or fermentation . Aerobic and anaerobic fermentation processes are the two separate fermentation processes.
In submerged fermentation (or cultivation) the cells of the producer (microorganism) are supplied with nutrient medium and oxygen (in the case of aerobic process) in the entire working volume of bioreactor by mixing and aeration. This makes the process highly economical. The bioreactor creates favorable conditions to accumulate a large amount of actively functioning producer biomass and in turn the target product.
As an example, relating to the history of biotechnology, it can be pointed out that replacing surface fermentation (in flasks and bottles) with submerged fermentation made it possible to increase the production of penicillin in a short time, especially urgently needed during the Second World War.
The submerged fermentation can be aerobic or anaerobic. For example, antibiotics and enzymes are produced through aerobic fermentation, which involves the incorporation of oxygen into the liquid medium, while butanol production proceeds under conditions of anaerobic fermentation, wherein the oxygen influence will have a inhibitory effect. Certain fermentation processes, such as ethanol production, use facultative anaerobic organisms like Saccharomyces cerevisiae, which may grow in the presence of oxygen and produce cell biomass before switching to anaerobic mode during the ethanol fermentation phase. Enzymes (amylases and proteases, amylases, and so on) are often made through aerobic submerged fermentation.
Submerged fermentation processes can be differentiated depending on the target product. The target product can be biomass, ferments or low-molecular compounds (for example, ethanol, methanol, acetates, oxalic and formic acids). Metabolites can be primary or secondary. A primary metabolite is a type of metabolite that plays a direct role in normal development, growth, and reproduction. Some common examples of primary metabolites are lactic acid , and certain amino acids . Secondary metabolites are generated toward or at the conclusion of the stationary phase of growth and do not play a role in growth, development, or reproduction. Atropine and antibiotics like erythromycin and bacitracin are examples of secondary metabolites.
Fermentation processes can finally be differentiated in terms of technology and the type of target product. The target product can be biomass, an individual high-molecular substance (for example, as an enzyme - constitutive or inducible) or low molecular weight metabolite. Metabolite, in turn, can be primary or secondary. Thus, the need for inductors and precursors, as well as the time of their introduction into the medium, depends on the target product. The biosynthesis of secondary metabolites is characteristic of certain stages in the development of the producer's culture and is stimulated in stressful situations. In this regard, the introduction of inducers and precursors is mandatory in the case when the target of the fermentation process is a secondary metabolite.
The biomass accumulation curve usually correlates with the accumulation curve of primary metabolites and does not coincide with the accumulation curve of secondary metabolites.
Submerged fermentation is best suited for microorganisms such as bacteria and yeasts that require high moisture. Another advantage of this method is that product purification is simplified. Submerged fermentation is most commonly employed to extract secondary metabolites that must be used in liquid form.
To carry out submerged fermentations are used as most typical stirred tank, bubble column, airlift type bioreactors, as well photobioreactors and membrane bioreactors in special applications.
The operation modes
Batch culture is a closed system in which the growth rate of biomass tends to zero due to depletion of the substrate and accumulation of inhibitors. Such systems are always in an unstable state.
There six growth phases of microorganisms during batch cultivation process.
1. The lag phase or induction period begins after inoculation into the nutrient medium of the microorganism and is the period of their adaptation.
During this phase, there is a reorganization of the micromolecular and macromolecular components of the microbial culture, the synthesis or suppression of enzymes or structural components of the cell. This phase, depending on external conditions, can be in ranges from 1 hour till 3 or more hours. During this phase, the cell mass can change without changing the number of cells.
2. The lag phase enters the exponential growth phase. This is a period of rapid accumulation of biomass and reaction products.
3. The phase of linear growth is characterized by balanced growth in the steady state, i. e. the growth rate remains constant throughout the entire cultivation process, and the chemical composition of the culture liquid changes, since nutrients are consumed and metabolic products are produced. As a consequence, the environment surrounding microorganisms is constantly changing, but the growth rate in a wide range of nutrient concentrations does not depend on them.
4. The phase of linear growth is replaced by a period during which the growth rate of the culture decreases to zero - this is the phase of growth retardation.
5. Further, the growth of the culture can pass into a fairly stable stationary phase, while the rate of death of microorganisms is compensated by the rate of increase in biomass.
6. With the complete depletion of the nutrient medium (substrate) and a significant accumulation of growth-inhibiting products, significant physiological changes in the culture (lysis) occur and the so-called phase of culture withering begins.

Solid state fermentation
Solid state fermentation (SSF) is carried out using solid phase substrate. The microorganisms are growing on a solid substrate in absence or near absence of free water.
The substrate must generate enough moisture to support growth and metabolism of the microorganism. SSF are applied for the production of fermented food products, for example, bread, meat cheese, pickles and yogurt. Using SSF can be recycled agro-industrial residues to obtain, for example enzymes, organic acids, food aroma compounds, biopesticides, mushrooms, pigments, xanthan gum and vegetable hormones. SSF requires less instrumentation and design of bioreactors are relative simple. However, scale-up is bothered, because it is difficult to ensure precise monitoring and control, and can not be controlled environmental conditions of the microorganisms. SSF are long, because the growth rate of microorganisms on solid substrate is slow. There are the processes, which successfully can realized only by SSF . For example, the sporulation of some fungi can attained only by SSF since these fungi do not sporulate in liquid media.
For SSF are used horizontal drum, tray-type, packed-bed and bench scale bioreactors.
Immobilization
Immobilization means the binding of an enzyme to an insoluble carrier while maintaining the functionality, i.e. the catalytic activity of the enzyme. The need of immobilization is determined by the fact that in many applications the end product must be completely free from enzyme resudues in order to avoid immune reactions.
The immobilization of enzymes not only significantly increases their stability, but allows the long-term use of one batch or series of industrial biocatalysts. The concept of " immobilization of a biological object" means the physical separation of a biocatalyst and a solvent, in which molecules of the substrate and reaction products can freely penetrate from a liquid to a solid medium, and vice versa. In other words, the substrate in the flow of the solvent is supplied to the bio-object associated with an insoluble carrier, and the reaction product in the flow of the solvent is removed from the bio-object and is used as the target product.
Enzymes, as well as entire cells, can be immobilized. The immobilization , i.e., the fixation of cells onto a carrier, for example, in ethanol production has several advantages. The cells could be reused and have extended lifespan. Some of the traditional purification procedures are not required because the desired final product is essentially free of biological substances and organisms. Inclusion of cells in gels, in which a cell solution is combined with gel-forming chemicals, is one of the most frequent immobilization procedures. Small molecules such as glucose can pass through the gel pores to reach the cells while their metabolic products (alcohol and carbon dioxide) can exit the beads. The living yeast cells so remain intact.
Various immobilization techniques such as the attaching of cells in stable porous gels (e.g., alginate , collagen , chitosan , agarose , cellulose , κ-carrageenan , or gel-matrix polymers such as polyacrylamide-hydrazide ) or hydrogels or immobilization in solid macroporous carriers has been established and is used on both laboratory and industrial sizes for a variety of applications, including the food, dairy, and beverage sector, medication production, wastewater treatment, agriculture, and biodiesel generation. In the case of the production of pharmaceutical preparations, the target substance will not contain components of the culture liquid (mycelium, products of partial lysis of cells, components of a complex nutrient medium, etc.), which greatly facilitates the task of isolating and purifying the target product, guarantees the absence of proteins and other harmful impurities.
The economic advantages of using immobilized biological objects in production conditions are undeniable. The use of immobilized systems makes it possible to make the conditions of biosynthesis more standard, and the entire production more compact. The resulting biological object works for a long time. At the same time, less raw materials are consumed per unit of production.
The application problem can be that the cells may contain numerous catalytically active enzymes, which can cause unwanted side reactions and the cell membrane itself may serve as a diffusion barrier, thus reducing productivity. It is difficult in immobilized cell bioreactors to control the physiological state of microorganisms, and due to the process variability and flexibility can not be ensured.
Immobilized cell bioreactors divide into stirred tank, fixed bed, fluidized bed, moving bed, packed bed and membrane reactors.
Fed-batch is based on feeding of a growth-limiting nutrient substrate to a culture. Cell growth and fermentation process can be controlled by varying the feeding strategy.
Usually fed-batch is started with batch fermentation phase until consumption of one or more substrates and/or inducers into a bioreactor. The fresh medium can be added by using different feeding regimes. Feeding can be added via a fixed volume or variable volume of a fresh medium or substrate only during the time course of the process. This feeding can be continuous or exponentially or pulses over a short or long period during the fed-batch phase.
When the target product is positively tied with microbial growth, fed-batch fermentation is highly advantageous for bioprocesses aiming for high biomass density or high product yield. A typical fed-batch process for the production of a product takes place in 3 stages. First, in the batch mode , the biomass is growth up to such a concentration that makes it possible to continue the process with a limit on the substrate (without the accumulation of the substrate in the medium). The second stage is the stage of biomass cultivation , when the media is fed with a substrate that promotes the rapid growth of the culture (glucose, sucrose or glycerin). The last stage is the stage of product synthesis (recombinant protein or other substances) when a biosynthesis inducer is introduced into the medium. This may be a substance that activates gene transcription (for example, IPTG) or a substrate that is an auto-inducer (for example, methanol for P. pastoris or lactose for E coli).
The simplest way of feeding strategy is calculation of time dependent adjusted feeding profile . This feeding profile usually is calculated using mathematical models. The feeding regime must be controlled by operator to prevent the repressive effects of high substrate concentrations and avoids catabolism repression. The control of fed-batch is problematic, because there are not available reliable biomass and substrate concentration on-line measurement methods.
By using adjusted feeding profiles in reality operator have to always these profiles during a fed-batch to correct. This is due to impossibility of providing fully repeatable fermentations. At certain stages of the fermentation process, it is possible to successfully perform the automatic feeding based on the dissolved oxygen DO sensor readings. But there are several problems here. Firstly, in this event it is impossible to control the DO concentration by rotation speed and/or oxygen-enriched air supplied to the aeration, and secondly, when relatively high biomass densities are reached, it is impossible to implement this control in an optimal version.
Aforementioned problem could be solved by model based fed-batch control . The ready solutions are yet not available in the market. Usually model based control systems are developed for specific applications. There are some attempts to develop systems available for wider applications (for example, https://www.bioreactors.net/model-based-fed-batch-control ).
Generally model based control functions in the following way: During the fermentation process, samples are taken in order to input the current results of the tests on biomass and substrates in the software. Performed the input of the results of the tests, the software performs automatic comparison of these results with the results of calculations according to the adopted mathematical model. If the deviations are above the set standards, the software performs calculation of the new feeding profile. The updated feeding profile is automatically uploaded into the process controller PLC, and the substrate feeding is further implemented according to the new profile (up to a following update). Mathematic model is implemented as PC program, and this program is connected to PLC with the help of OPC server.
Continuous cultivation is characterized by the constant addition of fresh nutrient medium to the bioreactor and the constant selection of either a suspension or a spent medium. Continuous culture is an open system that seeks to establish a dynamic balance. In this way, constant environmental conditions in the cultivation media can be ensured. Continuous cultivation is applicable if the produced product has the appropriate demand potential. The other typical application of continuous cultivation can be in wastewater treatments using wastewater as in-flow substrate.
Continuous cultivation is applied if is necessary regulary amount of product. structurally more complicated and requires additional automatic control, since it is associated with the introduction of additional devices into the bioreactor connection schema.
In a batch culture, conditions change all the time: the density of the culture increases, and the concentration of the substrate decreases. However, it is very often required that cells can be in the phase of exponential growth for a long time at a constant concentration of the substrate under unchanged other conditions. This can be achieved if a new nutrient solution is continuously introduced into a vessel containing a cell culture and at the same time an appropriate amount of cell suspension is removed from it.
In the practice of microbiological research, two types of open flow cultivation are widely used: chemostat and auxostats methods.
The chemostat method of cell cultivation is based on the use of a bioreactor, into which a nutrient medium is supplied at a constant rate and at the same time (for example, drainage according to the level) the cell suspension is taken. At the same time, the volume of the grown suspension remains constant. The growth of the culture in the chemostat is controlled by the concentration of the substrates. The stability of the system is based on this limitation of the growth rate by the concentration of one of the necessary substrates.
The auxostats are close-loop systems, which are controlled by feed-back regulation of some state variable, e.g. biomass or a substrate concentration or pH. Depending on the principle of operational control the auxostats can be classified as a turbidostat , a nutristat , or a pH-auxostat .
In a turbidostat the feed rate is adjusted by an optical density (turbidity) controller so that a constant biomass concentration is maintained over time. Under conditions of nutrient excess, the turbidostat provides the process close to maximal growth rate. The application problem of turbidostat is connected with some technical difficulties of sensor readings, e.g. fouling of the sensor due to microbial growth on its surface, disturbances in signal transfer by air bubbles or coloured and particulate media.
Nutristat operation is based on the measurement and control of substrate concentration by feeding of substrate. The use of nutristat is restricted due to the lack of suitable analytical tools for on-line measurement of most relevant substrate concentrations.
The pH-auxostat is based on measurement of the pH which is often correlated to the biomass production rate, but easier to measure and control as turbidity and substrate concentrations. The signal from the pH sensor is used to control the medium in-flow in a titration mode so that addition of the fresh medium brings the pH back to the setpoint, and the same amount media is taken away from bioreactor in out-flow. The pH-auxostat method is applicable for the microorganisms with growth that causes changes of the medium pH.
Application example of bioreactors
The cultivation for acquiring vaccines against the SARS-CoV-2
Recently, considerable effort has been put into the development of a vaccine against the SARS‑CoV‑2 The production process of all vaccines involves the use of bioreactors. For example, the approach for creating two types of coronavirus vaccines, which are already clinically confirmed, are the following:
RNA vaccines are made using a new technology that was previously used only in veterinary medicine. No RNA vaccine has yet been approved for use in humans. This vaccine contains a viral molecule similar in structure to the vaccine contains a viral messenger RNA (mRNA) molecule, which is similar in structure to human mRNA. Upon entering the human cells, the mRNA is used by the ribosomes in producing a viral protein. The mentioned protein stimulates an immune response in the human body, thus generating natural resistance to the virus. This method is used in the case of Pfizer-BioNTech and Moderna vaccine operation.
To manufacture this type of vaccines a host organism is required, which can produce large quantities of viral mRNA through cultivation. The most widely applied organism for such applications is the Escherichia coli bacterium. The process can be performed in stainless steel bioreactors, thus it is possible to cultivate the bacterium in up to 10 m3 (and even larger) working volume bioreactors. This means that the process is relatively easy scalable. Although, this type of cultivation process cannot be carried out in disposable (single-use) bioreactors, as they usually cannot provide sufficiently intensive mixing and aeration, which is necessary for E.coli growth and mRNA production. The other type of vaccines use a weakened type of adenovirus, which carries a specific viral protein for triggering an immune response.
These vaccines are examples of non-replicating viral vectors , using an adenovirus shell containing DNA that encodes a SARS‑CoV‑2 protein. The viral vector-based vaccines against the coronavirus are non-replicating, meaning that they are incapable of producing new viral cells, but rather produce only the antigen which elicits a systemic immune response. Vaccine of this type are the Oxford–AstraZeneca COVID-19 vaccine , Sputnik V (Russia), Convidicea (China) and Johnson & Johnson's Ad26.COV2.S .
For the manufacturing of these types of vaccines the cultivation of mammalian cells is used. In a such cultivations single-use bioreactors can be applied, allowing relatively easy production plant expansion in terms of simultaneously operating bioreactors. The maximum working volume of a stable single‑use bioreactors on the market today is about 2000 liters. Although, relatively recently the announcements of larger volume single-use bioreactors have been observed. For example, ABEC, a global provider of integrated solutions and services for biopharmaceutical manufacturing, recently announced the availability of single-use bioreactors with working volumes of up to 6000 liters.
Bench-Top Shakers
Incubator Shakers
- Multitron Standard
Shaker Accessories
- Test tube holder for shakers
- Box for Microtiter Plates
- Universal trays
- Fixed-configuration trays
- Trays for microtiter plates and deep-well plates
- Sticky Stuff
Bioreactors
Bench-Top Bioreactors
- Multifors 2
Pilot Bioreactors
Bioreactor Accessories
- Optek Turbidity Sensors
- Super Safe Sampler
Bioprocess Software
- eve – The Bioprocess Platform Software
Top Solutions
- High-throughput screening in 96-well-plates
- Cell cultivation in the incubator shaker
- Culturing stem cells in shakers
- High cell densities in the bioreactor
- Bioreactors for continuous cultivation with perfusion
- Culturing stem cells in a bioreactor
- Cell culture bioreactors
- Simultaneous saccharification and fermentation (SSF) in a bioreactor
- Chemostat bioreactors for continuous cultures
JavaScript seems to be disabled in your browser. You must have JavaScript enabled in your browser to utilize the functionality of this website.
What Is A Bioreactor And How Does It Work?
A bioreactor provides an ideal environment where cells can focus on what they are supposed to do: proliferate. Like lab workers, cells can only produce consistently good work if the conditions are right: it should not be too hot or too cold, and they have to have enough nutritous food and fresh air. In terms of a bioreactor, this means maintaining pH, temperature, ensuring sufficient gas supply and, depending on how the instrument has been configured, adding nutrients for successful maintenance of growth.
Tony Allman
04. Sep 2020
Given the abundance of functions that a bioreactor must perform, you might be wondering how it can do it all. What components does it need? How do you know what the conditions in the bioreactor currently are and how you can you correct them? And finally, just for perspective: how do you take the data captured during a batch bioprocess, display them in a meaningful way, and then save and evaluate them while keeping them organized? The most important process parameters and the mechanisms for regulating them are covered below:
Culture mixing
- Temperature control
- Feeding nutrients
- Pressure control
Preventing foam formation
The culture in a bioreactor needs to be mixed thoroughly at all times. If the nutrients in the bioreactor are not dispersed well enough, conditions in certain parts of the bioreactor will deviate significantly from the ideal. The pH could be too acidic, for example, or the supply of nutrients might be insufficient. Deviations like these not only reduce the efficiency of the planned bioprocess, but can also promote genetic modifications. Temperature distribution is an additional concern. Without uniform stirring, the microorganisms or cell cultures along the edge of the vessel will literally be boiled, while those in the middle get cold feet. If you have ever heated soup in the microwave and then eaten it with great anticipation without stirring it, you will know what we mean.
The typical stirring speed varies amongst other things depending on the cultivated organism:
Adjusting stirring speeds for cultivating either microorganisms, plant cells, animal cells or insect cells is very important, as these react differently to shear stress, i.e., the mechanical strain caused by stirring. Depending on the cell line, cell cultures can have a much more intense response to overly vigorous stirring, i.e., they simply die, a tendency that scientists describe more accurately (and diplomatically) as being “sensitive to shear stress.” Oxygen availability can be varied during the bioprocess by changing the stirring speed, thus ensuring optimum cell growth.
Measuring and controlling the temperature
Microorganisms and cell cultures alike have enzymes that work best within certain temperature and pH ranges. If conditions fall outside of these ranges, the desired bioprocess will proceed much more slowly, because growth and metabolic performance are highly dependent on these enzymes, i.e., catalytically active proteins. In the worst case scenario, unfavorable environmental conditions may even destroy them. Mammalian cell cultures are most comfortable within a very narrow range of temperatures – one that is only present from their perspective when the temperature inside the culture vessel is 37 °C.
Therefore, a platinum resistance sensor known as a Pt100 sensor is used in the bioreactor to determine the temperature. It has a resistance of 100 Ω at 0 °C, and covers the expected biologically relevant measurement range quite well when calibrated appropriately.
The typical temperature range varies amongst other things depending on the cultivated organism:
If working at temperatures near or below room temperature, you will need an active cooling system such as a recirculating chiller. For most bioprocesses, the temperature should remain constant during the entire cultivation. For some products, however, such as penicillin or recombinant proteins (i.e., bioengineered proteins using genetically modified organisms), changing the temperature at the end of the growth phase activates important genes for product formation and is therefore beneficial. For production methods involving cell cultures, the temperature is sometimes lowered at the end of the bioprocess as well (a technique known as “temperature shift” in biotechnology) so that the finished product will remain stable for later use. There are several ways of using a heating and/or cooling circuit to regulate temperature:
- An electric heating block with built-in cooling spiral
- A silicone heating pad wrapped around the culturing vessel after sterilization
- A double jacket in which water is circulated. The temperature is adjusted via an electric heater or steam and a solenoid valve for cooling water intake
Measuring and controlling the pH
Measuring and controlling pH is a very important aspect of bioprocesses, as changes in the pH can significantly alter growth conditions – usually with major consequences. Culture media commonly include buffers, i.e., substances that mitigate overly sudden changes to pH caused by the addition of an acid or base. Because an acid dripping into the culture medium can damage many cell lines, scientists often carefully enrich the gas mixture used in the cell culture with CO2 rather than adding a liquid acid. The gas then dissolves in the culture medium, allowing the carbon dioxide to influence the pH in combination with a buffer.
Typical pH ranges depending on the cultivated organism:
For measuring pH during the bioprocess, each bioreactor is equipped with a pH sensor known as a combination electrode for pH. The bioreactor can correct any deviations in the pH; for this purpose, an acid and/or an alkaline solution is made available and connected to the culture vessel via tubes and pumps. The concentration of the acid and base must be skilfully selected for this to work – if it is too high, the drops of concentrated acid or base may damage the microorganisms and cell cultures before they are distributed in the bioreactor. If, on the other hand, the concentration is too low, operators will have to add more acid or base, unnecessarily diluting the culture medium.
Adding nutrients
During a bioprocess, microorganisms usually consume a wide array of nutrients. The basic composition of a nutrient medium usually consists of water, a usable energy source for the organism (e.g. glucose), as well as the nutrients it needs (carbon, nitrogen, and phosphorus), salts and trace elements. Depending on the organism, other compounds are necessary that cannot be synthesized by yourself (vitamins, essential amino acids, etc.).
Depending on the bioprocess strategy these nutrients are either all made available at the beginning of a bioprocess (batch bioprocess) or added over time such as in a fed-batch or in a continuous process.
Schematic illustration of the correlations between living cell concentration, dissolved oxygen, and the limiting carbon source in batch operation. In the initial lag phase, the living cell count only increases slowly, which leads to a moderate but steady uptake of the carbon source. Oxygen consumption increases during the exponential growth phase until it exceeds possible oxygen input. Once the carbon source is depleted, the stationary phase starts and is followed by a dead phase, during which the living cell count drastically decreases.
If you’d like to find out more about the different feeding strategies have a look at our blog-post: The Difference Between Batch, Fed-batch and Continuous Processes.
During the bioprocess, the bioreactor feeds a sterile gas mixture such as air into the culture medium. Constant stirring not only distributes the nutrients – it also reduces the size of the gas bubbles that arise in the culture vessel, thus efficiently releasing oxygen into the nutrient solution. This is important, because microorganisms and cell cultures can only absorb the oxygen that has been dissolved in the nutrient solution.
Oxygen demands vary: aerobic bacteria need oxygen, whereas others prefer gas mixtures such as synthesis gas (“syngas”). Anaerobic organisms, however, can do without gassing entirely, feeding only on inorganic and organic substances from the culture medium such as nitrate or fumarate.
Unlike microorganisms, cell cultures are gassed with more than just air – the oxygen content of the gas mixture can also be influenced using pure nitrogen and pure oxygen. The exact composition depends on the cell culture application. In order to keep the gas atmosphere constant, the bioreactor needs precise control systems.
At the beginning of the bioprocess, for example, a culture often needs less oxygen – and thus a smaller gas feed – since growth is still progressing slowly. Later, however, faster growth requires much more oxygen. In addition to ensuring a constant supply of the desired gas or gas mixture, the bioreactor also delivers the right amount of gas at the right time. To do this, the bioreactor has gas ports connected to pressurized air from the building, a compressor, or a gas cylinder.
The gassing rate is usually measured in liters per minute. In order to have a generic parameter applicable to various bioreactors, the rate is also frequently indicated as the specific gassing rate, which refers to multiples of the working volume (vessel volumes per minute, vvm) and is expressed as min –1 . A typical value for microbial bioprocesses is 1 to 1.5 times the working volume per minute. For a bioreactor with 4 L working volume, the maximum gassing rate would therefore be 4 L * 2 L L –1 min –1 = 8 L min –1 . In cell cultures, by contrast, the maximum rate is often 10% to 15% of the working volume per minute as a way of keeping gas bubbles small and thus preventing foam formation and damage to cells from bursting bubbles.
Changing the gas rate, however, is not the only way of controlling the efficiency with which the bioreactor delivers oxygen and other gases to the culture medium. The greater the surface area of the total number of all gas bubbles in the bioreactor – i.e., the more finely distributed the gas bubbles themselves – the more efficiently oxygen will be transferred from the gas to the liquid phase. This means, for example, that increasing the stirring speed can improve oxygenation for microorganisms, since the stirrer makes the gas bubbles even smaller and thus increases the total surface area of all gas bubbles in the bioreactor.
Precise regulation of pO 2 – and thus precise control of the gassing rate and the gas composition – is very important since, normally, the pO 2 should not be the growth-inhibiting factor for the culture. If insufficiently controlled, however, the pO 2 does become the limiting factor.
Since the gas feed to the bioreactor is usually dry, moisture from the bioreactor can be collected in the exit stream during gassing. At a high gassing rate, not only would the fill level drop, but the moisture would also block the exhaust filter, preventing proper venting from taking place and allowing pressure to build up. To avoid this effect, bioreactors are equipped with an efficient exhaust cooler where the moisture in the exhaust condenses and can drip back into the bioreactor before it reaches the exhaust filter.
Measuring and controlling the pressure
The higher the pressure in the vessel, the more oxygen is dissolved. Culture vessels made of glass are frequently only approved for a pressure of up to 0.5 bar, which is not even half the pressure of a moderately filled bicycle tire. At a higher operating pressure, slightly damaged culture vessels made of glass can burst, which is a safety risk. That is why you should always ensure a free, non-pressurized exhaust line from the bioreactor by keeping the exhaust filter dry and regularly replacing it – in the process, you will also be ensuring the integrity of the culture vessel, of course. Unlike glass culture vessels, stainless steel bioreactors are designed for higher pressures and, even in their standard configuration, are suitable for pressures up to 2 bar (a well-filled bicycle tire). Plus, systems like these are often equipped with a pressure control mechanism based on a pressure sensor in the bioreactor and a proportional valve in the exhaust line. Not only can these measure the pressure in the bioreactor – they can actively control it as well.

Outside of bathtubs and beer glasses, foam is a rather unpopular side effect, especially in bioreactors. Foam forms at the interface between the liquid and gas phase in the culture vessel and can quickly find its way up under the top plate. In the worst-case scenario, it then blocks the exhaust filter, which in turn blocks the flow of gas. Most bioreactors are therefore equipped with a system for combatting foam formation. Mechanical foam breakers in the headspace are reserved for rather large stainless steel bioreactors, while antifoam control systems based on chemical agents (such as PPG, Struktol, or silicon-based defoamers) can be found in smaller bioreactors.
A typical antifoam control system consists of a sensor installed at a specific height in the culture vessel. If the foam height reaches the sensor, an antifoam agent is pumped from a reservoir into the culture vessel. These antifoam agents are active at the liquid-gas interface and increase the tendency of the foam bubbles to collapse. In particularly stubborn cases where the foam does not immediately dissolve, repeat the procedure after a preset time (a “shot & delay” strategy). Caution is advised when using an antifoam agent – if you dispense even slightly more than necessary, it can lie like a second skin on the surface of the liquid, which hinders the gas exchange. Antifoam agents also counteract efficient oxygen transfer, because the change in surface tension promotes the collapse of gas bubbles in the bioreactor, thus reducing the surface area available for gas exchange. The selection of the appropriate agent also depends on the bioprocess in question, because bacteria and cells react differently to certain chemicals.
Bonus: The benefits of using a SCADA software
Nowadays, the results generated in a bioreactor should be collected and evaluated as centrally as possible, as this is the only way of effectively implementing modern, big data algorithms in order to generate more information and to better understand how a process works. That is the job of the SCADA software. In the first step, all data from the bioreactor can be read out – with no major input on the part of the user – and stored centrally in order to evaluate it on its own or compare it with other batch data. This quickly triggers ideas for new experiments and possibly even complex batch strategies. A professional SCADA software lets you plan these easily and then control the bioreactor, which will ideally be a fully automated process. In addition, the SCADA software also integrates several components in the bioreactor environment. These include tools for process optimization using the design-of-experiment (DoE) technique or powerful software sensors, which can be used simultaneously to compute additional information directly from the batch process parameters and even to regulate those parameters. For example, the respiratory quotient (RQ) can be used to obtain an estimate of metabolic activity by means of the ratio of excreted carbon dioxide to absorbed oxygen.
There are so many possibilities – especially when the bioreactor and SCADA software are perfectly matched – that we could not possibly describe them all. If you are interested in learning more about all a modern SCADA software has to offer have look at the eve ® bioprocess platform software

Do you like this post?
Leave us a rating
Average rating 4.3/5
Great article - clear, concise and extremely informative Denis Coll Life Sciences Sector Training Manager - Watson Marlow Fluid Technology Group
Hi Tony, Great intro article. Nice to see you emphasize quality gas control, often underestimated. Maybe write an article dedicated to gas control? Paul de Waal Product Manager (Vogtlin.com)
Hi Paul, Thanks for the kind words and encouragement. I'm glad you liked the post and were able to focus on an important aspect out of this very basic introduction. I really like your idea of a specific post on the importance of effective gas strategies on bioprocesses as a stand-alone topic. I have an outline in mind for this already. If you have any specific ideas for content you would like to see included, please don't hesitate to get in contact. Tony
About us News Jobs Services Contact Blog Member Area
At AllTheScience, we're committed to delivering accurate, trustworthy information. Our expert-authored content is rigorously fact-checked and sourced from credible authorities. Discover how we uphold the highest standards in providing you with reliable knowledge.
Learn more...
What is a Bioreactor?
A bioreactor is a container which is used to hold organisms for the purpose of harnessing their natural biochemical processes. A simple and well known example of a bioreactor is a fermentation tank for beer, in which certain microorganisms are encouraged to thrive, causing the contents of the tank to ferment and creating a usable end product. There are a number of types of bioreactors, and they are used for a variety of purposes, from processing solid waste to manufacturing pharmaceuticals.
In a batch bioreactor, everything is added at once to a controlled and sealed environment, and the biochemical reactions are allowed to run their course before the reactor is opened so that the contents can be extracted and utilized, disposed of, or further processes. Others operate on a continuous flow method, in which materials constantly flow through the bioreactor. Waste treatment plants, for example, utilize continuous flow to process solid waste.
A number of criteria must be satisfied when a bioreactor is built. In order for the device to be effective, the conditions need to be tightly controlled, which means that there must be ways to moderate temperature, light levels, moisture, oxygen , and other components of the environment. It is also important to isolate the contents from contaminants so that the bioreactor will work properly, and so that adverse reactions do not occur. In beer, for example, the introduction of the wrong microorganisms can cause the beer to sour.
Conversion of organic waste such as compost or solid waste is a common application for bioreactors. When built properly, the reactor can greatly speed the breakdown process, which contributes to overall efficiency. Bioreactors are also used to promote growth, as for example in the production of tissue cultures, or the cultivation of specific fungi utilized in pharmaceuticals. In some cases, it may be necessary to devise a custom device to meet the specific needs of a particular application, in which case the skills of a biochemist are typically required.
Researchers are constantly devising new uses for bioreactor technology. For example, these devices could potentially be used to produce energy, or to grow tissue and bone grafts. Chemical production can rely heavily on bioreactors, depending on the compounds being manufactured, as can large-scale processing of compost and yard waste for municipalities. Many experiments have also been conducted with bioreactors in challenging environments such as space to learn more about biochemical processes and to generate useful scientific information.
Ever since she began contributing to the site several years ago, Mary has embraced the exciting challenge of being a AllTheScience researcher and writer. Mary has a liberal arts degree from Goddard College and spends her free time reading, cooking, and exploring the great outdoors.
AS FEATURED ON:

Related Articles
- What Is Aerobic Fermentation?
- What Are Batch Reactors?
- What is a Clinical Biochemist?
- What does a Biochemist do?
- What is Biochemical Research?
- What is Bioremediation?
- What is an Anaerobe?
Discussion Comments
Post your comments.
- By: Steve Lovegrove A simple and well known example of a bioreactor is a fermentation tank for beer in which certain microorganisms are encouraged to thrive, causing the contents of the tank to ferment and creating a usable end product.
- By: Lambros Kazan Bioreactors can be used in the manufacture of pharmaceuticals.
- To save this word, you'll need to log in. Log In
Definition of bioreactor
Examples of bioreactor in a sentence.
These examples are programmatically compiled from various online sources to illustrate current usage of the word 'bioreactor.' Any opinions expressed in the examples do not represent those of Merriam-Webster or its editors. Send us feedback about these examples.
Word History
1974, in the meaning defined above
Dictionary Entries Near bioreactor
biopyribole
biorefinery
Cite this Entry
“Bioreactor.” Merriam-Webster.com Dictionary , Merriam-Webster, https://www.merriam-webster.com/dictionary/bioreactor. Accessed 1 Apr. 2024.
Medical Definition
Medical definition of bioreactor.
Subscribe to America's largest dictionary and get thousands more definitions and advanced search—ad free!

Can you solve 4 words at once?
Word of the day.
See Definitions and Examples »
Get Word of the Day daily email!
Popular in Grammar & Usage
The tangled history of 'it's' and 'its', more commonly misspelled words, why does english have so many silent letters, your vs. you're: how to use them correctly, every letter is silent, sometimes: a-z list of examples, popular in wordplay, the words of the week - mar. 29, 10 scrabble words without any vowels, 12 more bird names that sound like insults (and sometimes are), 8 uncommon words related to love, 9 superb owl words, games & quizzes.

- Biology Article
- Bioreactor Obtaining Foreign Gene
Bioreactor - Obtaining The Foreign Gene Product
Table of Contents
Obtaining the Foreign Gene Product
Downstream processing.
Recombinant DNA technology is the process that involves the introduction of a foreign piece of DNA into the gene of interest and the new DNA thus formed is the host genome. This gene that is introduced is the recombinant DNA and the technique is called recombinant DNA technology. Inserting the desired gene into the genome of the host is not as easy as it sounds. The ultimate aim of this process is to obtain desirable protein.
Let’s learn how to obtain the foreign gene product using a bioreactor.
In recombinant technologies, the desired gene selected which is followed by selecting a perfect vector into which the desired gene has to be integrated and then recombinant DNA is formed by ligating the gene of interest with the vector. Once this foreign DNA is inserted, the host is multiplied and ultimately desirable protein is produced. The rDNA has to be maintained in the host and carried forward to the offspring. For the production of the desired protein, the gene encodes for it needs to be expressed. This happens only under optimized conditions. Not only the target protein has to be expressed but has to be produced on a large scale.
The recombinant cells can be multiplied on a large scale using a continuous culture system. Here the cells are cultured in a large vessel and the medium is refreshed on a regular interval to maintain the optimum conditions. This helps to culture a large mass of the desired protein. This can be achieved by using a bioreactor.
A bioreactor helps to produce a large volume of culture. The bioreactor is a large vessel where the different cells such as human or plant, or animal cells can be cultured to obtain new biological products. It provides optimum conditions like temperature, pH, substrate, oxygen, etc required for the culturing of cells producing desired products. Simple stirred-tank bioreactor and sparged stirred-tank bioreactor are the two types of bioreactors used for this purpose.

Downstream processing is a sequential step in which the isolation, purification and preservation of final products are done before it is marketed. In this stage, the final product is formulated with additives like preservatives, colours, etc., followed by clinical trials.
For more details on bioreactor and downstream processing, visit BYJU’S.
Frequently Asked Questions on Bioreactor – Obtaining The Foreign Gene Product
What is downstream processing in bioprocess engineering.
Downstream processing refers to the series of steps involved in the isolation and purification of products from the growth medium.
What is a bioreactor?
A bioreactor is a stainless steel vessel that provides a sterile environment for the growth of microbial or plant or animal cells. It contains the growth medium and dissolved oxygen along with temperature, pH, and pressure sensors.
Name any two types of bioreactor?
Simple stirred-tank bioreactor and sparged stirred-tank bioreactor are the two types of bioreactors.
How are proteins produced from foreign genes?
The foreign gene is inserted into a suitable vector to create rDNA. It is then inserted into the host cell where the foreign gene is expressed to produce proteins.
What is the importance of the agitation system in bioreactors?
The agitation system enables proper mixing of the contents inside the bioreactor. It helps in maintaining the uniform concentration of growth media inside the reactor.

Put your understanding of this concept to test by answering a few MCQs. Click ‘Start Quiz’ to begin!
Select the correct answer and click on the “Finish” button Check your score and answers at the end of the quiz
Visit BYJU’S for all Biology related queries and study materials
Your result is as below
Request OTP on Voice Call
Leave a Comment Cancel reply
Your Mobile number and Email id will not be published. Required fields are marked *
Post My Comment
- Share Share
Register with BYJU'S & Download Free PDFs
Register with byju's & watch live videos.


IMAGES
VIDEO
COMMENTS
Bioreactor. A bioreactor refers to any manufactured device or system that supports a biologically active environment. [1] In one case, a bioreactor is a vessel in which a chemical process is carried out which involves organisms or biochemically active substances derived from such organisms. This process can either be aerobic or anaerobic.
What is a bioreactor? Bioreactors are vessels used to culture cells or microorganisms under tightly controlled conditions to provide optimal productivity, efficiency, and product quality. These vessels can be used to culture various types of animal and human cells. However, they're also commonly used for the fermentation of microbes such as ...
A bioreactor is a device or system that provides an optimal and controlled environment for biological and chemical reactions. A bioreactor is a vessel or system that typically involves the growth and maintenance of microorganisms, mammalian, plant and stem cells, tissues, and algae.. Bioreactors have revolutionized various industries, including biotechnology, pharmaceuticals, food production ...
Bioreactor Definition. A bioreactor is a type of fermentation vessel that is used for the production of various chemicals and biological reactions. It is a closed container with adequate arrangement for aeration, agitation, temperature and pH control, and drain or overflow vent to remove the waste biomass of cultured microorganisms along with their products.
The various parts of the bioreactor work together to meet the parameters like temperature, nutrients, aeration, and pH for the growth and development of the cells, enzymes, and many more. Some essential parts of a bioreactor are discussed below: Fermenter vessel: Most fermented containers are made of glass and stainless steel to reduce pressure ...
What is a bioreactor? By Alex Williams, Cytiva. Bioreactors are used to culture cells in a controlled manner to produce biopharmaceuticals. They support the production of vaccines, therapeutics, gene therapies, and cell therapies. Biopharmaceuticals are very complex and sensitive and can only be synthesized by living cells. They are used in the ...
Essentially, a bioreactor is a system that provides an ideal environment for the production of biomolecules. Most bioreactors can be thought of as vessels with control units that help regulate cell growth conditions such as temperature, pH, oxygen/carbon dioxide levels, etc. Stirred-tank bioreactors are very commonly used for the industrial ...
What is a bioreactor ¶. An apparatus for growing organisms (yeast, bacteria, or animal cells) under controlled conditions. Used in industrial processes to produce pharmaceuticals, vaccines, or antibodies. Also used to convert raw materials into useful byproducts such as in the bioconversion of corn into ethanol.
A bioreactor is a vessel in which raw materials under controlled conditions are converted into products by activity of living cells (microorganisms, mammalian, plant and stem cells, tissues and algae) or by cellular components such as enzymes. The difference between bioreactor and fermenter . Bioreactor and fermenter are similar terms, but with ...
A bioreactor provides an ideal environment where cells can focus on what they are supposed to do: proliferate. Like lab workers, cells can only produce consistently good work if the conditions are right: it should not be too hot or too cold, and they have to have enough nutritous food and fresh air. In terms of a bioreactor, this means maintaining pH, temperature, ensuring sufficient gas ...
Bioreactors. Larry E. Erickson, in Encyclopedia of Microbiology (Fourth Edition), 2019 Abstract. Bioreactors are vessels or tanks in which whole cells or cell-free enzymes transform raw materials into biochemical products and/or less undesirable by-products. The microbial cell itself is a miniature bioreactor; other examples include shake flasks, Petri dishes, and industrial fermentors.
about bioreactors in this white paper, we usually mean systems for the cultivation of microbes or mammalian cells. Stirred-tank bioreactors Though many types of bioreactors exist, we will focus on stirred-tank bioreactors. The name is accurately descriptive. Cultivation takes place in the bioreactor tank—often called a vessel—and the
Bioreactors. Let's explore what bioreactors are and why we use them. We will talk about its basic structure and units like the control units and impellers. We will also explore the difference between the simple stirred tank bioreactors and the sparged stirred tank bioreactors. Created by Khadijatul Qubra.
This video gives a short introduction to bioreactors. As more chemical engineers are employed by the pharmaceutical industry, it is important to implement mo...
Bioreactors. Yusuf Chisti, Murray Moo-Young, in Encyclopedia of Physical Science and Technology (Third Edition), 2003. IV Concluding Remarks. A bioreactor is an indispensable part of any bioprocess irrespective of whether the process degrades pollutants or produces substances such as foods, feeds, chemicals and pharmaceuticals, and tissues and organs for use in biomedicine.
#bioreactor #fermenter #fermentation #biotechnology #microbiology101 #microbiology #microbiologylecturesonline #microbiologyclass #bioreactorapplication #bio...
Bioreactors. L.E. Erickson, in Encyclopedia of Microbiology (Third Edition), 2009 Bioreactors are vessels or tanks in which whole cells or cell-free enzymes transform raw materials into biochemical products and/or less undesirable by-products. The microbial cell itself is a miniature bioreactor; other examples include shake flasks, Petri dishes, and industrial fermentors.
Bioreactors must be operated aseptically; be able to control pH, dissolved oxygen (DO), and temperature, to optimize cell growth. They provide a medium that provides the nutrients for cells to grow in; and deliver sufficient mixing and aeration to the culture to encourage growth. On the surface bioreactors can appear to be complex pieces of kit ...
A bioreactor is a container which is used to hold organisms for the purpose of harnessing their natural biochemical processes. A simple and well known example of a bioreactor is a fermentation tank for beer, in which certain microorganisms are encouraged to thrive, causing the contents of the tank to ferment and creating a usable end product.
bioreactor: [noun] a device or apparatus in which living organisms and especially bacteria synthesize useful substances (such as interferon) or break down harmful ones (as in sewage).
An important operational variable for continuous flow bioreactors is the dilution rate D, or the volume flow rate of the feed medium divided by the constant volume of the broth in the reactor.The biomass productivity of a continuous culture is simply the dilution rate D multiplied by the biomass concentration X in the harvest stream. In a continuous flow well-mixed bioreactor, the biomass ...
A bioreactor is a large vessel where cells are used to culture new biological products. Explore the process and the various components
Dennis Vigil, Reginald R. Baxter Endowed Department Chair of Chemical and Biological Engineering and CoMFRE faculty researcher, alongside Zengyi Shao, associate professor of chemical and biological engineering and Hershel B. Whitney Professor, Global Initiatives, are leading the development of a continuous bioreactor that integrates product extraction and separation into the design, so less ...
The mean concentrations of the macrolide antibiotics in influent, grit chamber, rotating biological contactor, bacillus bio-reactor, second clarifier, sand filtering, and ultra-violet were 620 ng/L, 1100 ng/L, 1200 ng/L, 1300 ng/L, 1400 ng/L, 1100 ng/L, and 1200 ng/L, respectively (Table S3). Among the macrolide antibiotics, the highest ...