Cytokine concentrations throughout pregnancy and risk for psychosis in adult offspring: a longitudinal case-control study
Affiliations.
- 1 Department of Psychology, Yale University, New Haven, CT, USA. Electronic address: [email protected].
- 2 Department of Pediatrics, Johns Hopkins School of Medicine, Baltimore, MD, USA.
- 3 Department of Epidemiology, School of Public Health, Brown University, Providence, RI, USA.
- 4 Department of Psychology, Yale University, New Haven, CT, USA; Department of Psychiatry, Yale University, New Haven, CT, USA.
- PMID: 32035031
- PMCID: PMC8287973
- DOI: 10.1016/S2215-0366(20)30006-7
Background: Schizophrenia has been associated with pregnancy and birth complications and fetal exposure to inflammation is thought to be a common underlying mechanism. However, whether the risk is specific to particular phases of pregnancy is unclear. The aim of this study was to characterise and compare longitudinal patterns of maternal serum concentrations of cytokines across pregnancy between offspring who were later ascertained to have a psychotic disorder, non-psychotic siblings of these cases, and unrelated, non-psychotic individuals who served as controls.
Methods: The National Collaborative Perinatal Project was a large-scale prospective longitudinal study that assessed the effects of perinatal factors on infant and child development. At sites across the USA, over 50 000 pregnant women were enrolled during prenatal clinical visits between 1959 and 1965. The present study draws from the Philadelphia cohort, which includes 9236 surviving offspring of 6753 pregnant women. Psychotic disorder diagnoses in adulthood were assessed with review of medical records and were confirmed with a validation study. Concentrations of TNFα, IL-1β, IL-5, IL-6, IL-8, IL-10, and IL-17a were assessed using a multiplex bead assay in archived maternal serum samples collected across prenatal visits and birth. We characterized cytokine patterns with linear mixed models.
Findings: Our final sample comprised 90 cases, 79 siblings (of 40 cases), and 273 matched controls. Concentrations of proinflammatory cytokines TNFα, IL-1β, and IL-6 were significantly higher in maternal serum of offspring who later developed psychosis compared with maternal serum of matched controls. These differences were greatest in the first half of pregnancy (7-20 weeks), with no difference observed during the second half of pregnancy.
Interpretation: Our results suggest that exposure to high maternal proinflammatory cytokine concentrations in early pregnancy might play a part in psychosis. These findings place the timing of risk associated with maternal inflammation much earlier in prenatal development than previously documented in humans and provide insight into a potential developmental pathway to the disorder.
Funding: National Institute of Mental Health (P50) Silvio O Conte Center at Johns Hopkins, Stanley Foundation, March of Dimes, Yale University, National Science Foundation, and National Institute of Child Health and Human Development/Division of Intramural Population Health Research.
Copyright © 2020 Elsevier Ltd. All rights reserved.

Publication types
- Research Support, N.I.H., Extramural
- Research Support, Non-U.S. Gov't
- Research Support, U.S. Gov't, Non-P.H.S.
- Adult Children* / psychology
- Adult Children* / statistics & numerical data
- Inflammation / blood
- Interleukin-1beta / blood*
- Interleukin-6 / blood*
- Longitudinal Studies
- Pregnancy Trimesters / blood
- Prenatal Exposure Delayed Effects / blood*
- Psychotic Disorders* / diagnosis
- Psychotic Disorders* / epidemiology
- Risk Factors
- Tumor Necrosis Factor-alpha / blood*
- United States / epidemiology
- Interleukin-1beta
- Interleukin-6
- Tumor Necrosis Factor-alpha
Grants and funding
- P50 MH094268/MH/NIMH NIH HHS/United States
- UL1 TR001863/TR/NCATS NIH HHS/United States
- DGE-1122492/National Science Foundation/International
- Study Protocol
- Open access
- Published: 21 November 2023
Neurophysiological explorations across the spectrum of psychosis, autism, and depression, during wakefulness and sleep: protocol of a prospective case–control transdiagnostic multimodal study (DEMETER)
- Valeria Lucarini 1 , 2 ,
- Anaëlle Alouit 3 ,
- Delphine Yeh 4 ,
- Jeanne Le Coq 2 ,
- Romane Savatte 2 ,
- Mylène Charre 2 ,
- Cécile Louveau 2 ,
- Meryem Benlaifa Houamri 2 ,
- Sylvain Penaud 4 ,
- Alexandre Gaston-Bellegarde 4 ,
- Stéphane Rio 5 ,
- Laurent Drouet 5 ,
- Maxime Elbaz 5 ,
- Jean Becchio 6 ,
- Sylvain Pourchet 6 ,
- Estelle Pruvost-Robieux 3 , 7 ,
- Angela Marchi 8 ,
- Mylène Moyal 1 , 2 ,
- Aline Lefebvre 9 ,
- Boris Chaumette 1 , 2 ,
- Martine Grice 10 ,
- Påvel G. Lindberg 3 ,
- Lucile Dupin 11 ,
- Pascale Piolino 4 ,
- Cédric Lemogne 12 ,
- Damien Léger 5 , 13 ,
- Martine Gavaret 3 , 7 ,
- Marie-Odile Krebs 1 , 2 &
- Anton Iftimovici 1 , 2
BMC Psychiatry volume 23 , Article number: 860 ( 2023 ) Cite this article
1235 Accesses
5 Altmetric
Metrics details
Quantitative electroencephalography (EEG) analysis offers the opportunity to study high-level cognitive processes across psychiatric disorders. In particular, EEG microstates translate the temporal dynamics of neuronal networks throughout the brain. Their alteration may reflect transdiagnostic anomalies in neurophysiological functions that are impaired in mood, psychosis, and autism spectrum disorders, such as sensorimotor integration, speech, sleep, and sense of self. The main questions this study aims to answer are as follows: 1) Are EEG microstate anomalies associated with clinical and functional prognosis, both in resting conditions and during sleep, across psychiatric disorders? 2) Are EEG microstate anomalies associated with differences in sensorimotor integration, speech, sense of self, and sleep? 3) Can the dynamic of EEG microstates be modulated by a non-drug intervention such as light hypnosis?
This prospective cohort will include a population of adolescents and young adults, aged 15 to 30 years old, with ultra-high-risk of psychosis (UHR), first-episode psychosis (FEP), schizophrenia (SCZ), autism spectrum disorder (ASD), and major depressive disorder (MDD), as well as healthy controls (CTRL) ( N = 21 × 6), who will be assessed at baseline and after one year of follow-up. Participants will undergo deep phenotyping based on psychopathology, neuropsychological assessments, 64-channel EEG recordings, and biological sampling at the two timepoints. At baseline, the EEG recording will also be coupled to a sensorimotor task and a recording of the characteristics of their speech (prosody and turn-taking), a one-night polysomnography, a self-reference effect task in virtual reality (only in UHR, FEP, and CTRL). An interventional ancillary study will involve only healthy controls, in order to assess whether light hypnosis can modify the EEG microstate architecture in a direction opposite to what is seen in disease.
This transdiagnostic longitudinal case–control study will provide a multimodal neurophysiological assessment of clinical dimensions (sensorimotor integration, speech, sleep, and sense of self) that are disrupted across mood, psychosis, and autism spectrum disorders. It will further test the relevance of EEG microstates as dimensional functional biomarkers.
Trial registration
ClinicalTrials.gov Identifier NCT06045897.
In light of the genetic and neuroanatomical continuum among psychiatric illnesses [ 1 , 2 ], transdiagnostic neurophysiological approaches have demonstrated shared neurofunctional abnormalities between schizophrenia, mood, and autism spectrum disorders [ 3 , 4 ]. High-level cognitive processes have long been shown to rely on synchronized neuronal oscillations, resulting from a balance between excitatory and inhibitory populations of neurons, which can be directly measured by electroencephalography (EEG). This balance is maintained by a network of GABAergic interneurons that regulate the activity of superficial pyramidal cells [ 5 ]. The topographically precise inhibitory activity of interneurons also allows for spatial sensory coding, which is key to a variety of memory processes [ 6 ]. Disruption in these systems may therefore explain a range of cognitive symptoms seen across the spectrum of psychiatric disorders. Moreover, the timing of the disruption may explain the neurodevelopmental continuum between autism spectrum on the one hand, and psychotic and mood disorders on the other. For instance, glutamatergic NMDA receptors, which regulate interneuron activity, can be affected by genetic mutations disrupting the function of subunits expressed either in early development or later on, leading respectively to neurodevelopmental phenotypes, such as ASD, or to schizophrenia spectrum-disorders [ 7 ]. In addition, interneurons are crucial to prefrontal maturation during adolescence and early adulthood [ 8 ], a timeframe when most psychiatric disorders occur [ 9 ]. EEG quantitative approaches therefore appear as accessible and promising tools to investigate the pathophysiology of psychiatric disorders, from autism to schizophrenia spectrum disorders.
Beyond frequential or oscillatory activities, EEG analyses now allow to study the temporal dynamics of neuronal networks throughout the brain [ 10 ]. At rest, brain activities alternate very rapidly, every 80 ms or so, between states of unstable equilibrium, called microstates, and characterized by a particular polarization of the entire cerebral electrical potential field. EEG coupled with functional MRI has suggested that these microstates may correspond to particular modes of spatial organization of information processing [ 11 ]. For instance, microstate classes have been associated with various functioning profiles: verbal (class A), visual (class B), self-oriented/self-referential processing (class C), cognition (class D), and interoception and sensorimotor processing (class E) [ 12 ]. Moreover the same microstate structures have been described from waking rest to deep sleep, confirming that they may reflect a robust large-scale resting-state network architecture, similar to the resting-state connectivity seen in fMRI that is also preserved in sleep [ 13 ]. Disruption in these microstate systems has been described across the spectrum of psychiatric disorders, in schizophrenia [ 14 ], autism spectrum disorder [ 15 ], or depression [ 16 ], but also in neurological disorders such as epilepsy [ 17 ]. Thus, preliminary results from our group suggested that a certain pattern of microstates could be associated with specific stages of disease progression in psychosis, but also translated a shared dimension on the schizophrenia-autism continuum [ 18 ]. They may therefore contribute to understanding the range of transdiagnostic endophenotypes shared between neuropsychiatric diseases, such as anomalies in sleep, sensorimotor integration, speech, and sense of self. Moreover, since microstates can be modulated under hypnotic conditions [ 19 ], and medical hypnosis has been associated with improved attentional and executive control over self-referential processes [ 20 ], EEG microstates may also provide a proxy for psychotherapeutic response. Thus, the effectiveness of hypnosis has been suggested in psychiatric disorders associated with overactivation of the default mode, such as depression [ 21 ].
Sleep disturbances are strongly linked to the pathophysiology of most neuropsychiatric disorders and may explain many of the cardiovascular, pneumologic, and neurologic comorbidities of psychiatric disorders [ 22 ]. Various mechanistic models have been described in bipolar disorder and depression, including circadian rhythm anomalies, internal desynchronization, or anomalies of sleep architecture [ 23 , 24 ]. Sleep dysregulation is also highly prevalent in autism spectrum disorders, leading to severe distress and impact on quality of life [ 25 ], while in the early stages of psychosis, there is a high prevalence of insomnia, nightmare disorder, sleep-related hallucinations, excessive sleepiness disorders or restless leg syndromes [ 26 ]. Moreover, having a sleep disorder exacerbates psychotic and mood symptoms among patients with psychosis [ 26 ]. From a neurophysiological perspective, the most replicated macroscopic EEG anomaly across the spectrum of psychosis, mood disorders, and autism, is the decrease in density of sleep spindles [ 27 , 28 , 29 ], which are determinant for cognitive processes such as memory consolidation [ 30 ]. However, more quantitative EEG analyses remain to be done to further explore brain connectivity during sleep.
- Sensorimotor integration
Sensorimotor abnormalities are a cross-cutting neuropsychiatric dimension [ 31 , 32 , 33 ], which can be robustly analyzed with quantitative EEG [ 34 ]. Motor deficits linked to alterations in cortical excitability/inhibition modulation of motor areas have been identified in various neurodevelopmental pathologies such as schizophrenia or autism [ 35 , 36 ], and in particular during adaptation to a probabilistic context [ 37 ].
Specifically, autism spectrum disorders have been shown to exhibit anormal context-sensitive processing mechanisms, sensorimotor gating deficits, as well as repetitive motor movements and atypical integration of sensory stimuli [ 38 , 39 , 40 ]. Recent behavioral and imaging studies investigating tactile processing in autism, suggested no difference in light touch detection and texture, but increased sensitivity in vibration [ 41 , 42 , 43 ]. Moreover, decreased connectivity in finger somatosensory areas and slower perceptual processing speed were shown [ 44 , 45 ]. Although sensory perturbations are well known, literature on sensory integration prior to motor movement is lacking. In schizophrenia, and more generally in psychotic disorders, it is still unclear how sensorimotor mechanisms are impaired. It has been hypothesized that a general disruption may cause a functional disintegration between sensory and cognitive processes [ 32 , 46 ], yet, further investigations are needed in order to shed light on precise sensorimotor integration. Only a handful of studies showed tactile perception accuracy deficits [ 47 , 48 , 49 ], and abnormal sensory predictions in a self- and non-self-elicited sensation discrimination task [ 50 ]. This indicates a failure of normal inhibitory regulation of sensory, motor, and attentional mechanisms, common in several neurodevelopmental disorders.
Another accessible neurophysiological function that reflects thought processing and also results from a complex integration of sensorimotor signals is represented by speech, considered in its quantitative dimensions, which has also been correlated with EEG microstate patterns [ 51 ]. Given that communication difficulties are key features of autistic and psychotic disorders [ 52 ], computational methods have recently been introduced to objectively quantify linguistic anomalies in the psychosis spectrum and to identify subtle and early linguistic peculiarities in UHR individuals [ 53 ]. Recent studies have shown that analyses in the semantic and syntactic areas could predict psychotic transition [ 54 ], but the predictive role of other linguistic domains, such as phonetics, has so far been poorly investigated. A main aspect of phonetic research is prosody, the tone of voice with which words are pronounced, crucial for communication [ 55 ]. Researchers from both the phonetic and psychiatric fields have invested significant effort into trying to precisely characterize the prosodic profile of patients with schizophrenia, generally finding reduced pitch variability and increased pause duration [ 56 ]. However, among the limitations of the existing research, are a weak generalizability of the results to languages other than English, a lack of comparisons with other clinical groups and scant attention devoted to voice quality [ 56 ]. Besides, prosodic cues have scarcely been explored in individuals with high risk of psychosis and more research is needed to clarify the potential predictive role of these features [ 57 , 58 ].
Alongside traditional approaches investigating communicative behavior in psychosis focusing only on the voice of the patient, it is also necessary to investigate what happens at the interactional level [ 59 ]. Turn-taking analysis specifically explores dialogical interactional behaviors. Turn-taking is the organization of the conversation into alternating speaking turns between different interlocutors and its main goal is to assure that no more than one person is speaking at any time. Another goal is to avoid long silent gaps between the end of one speaking turn and the beginning of the next one [ 60 ]. Turn-taking analysis has rarely been applied to individuals with psychosis and at-risk mental states so far [ 61 , 62 , 63 ].
It appears that in this group turn-taking patterns involving increased mutual silence are prevalent. Interestingly, voice atypicalities have also been quantified in individuals with autism spectrum disorders, both in childhood and adulthood [ 64 ]. Moreover, recent studies have found an increased number of silent gaps as compared to controls in the early stages of dialogues [ 65 , 66 ]. Of note, there is evidence suggesting that there are shared social cognition deficits between autism and schizophrenia spectrum disorders [ 67 ]. From this perspective, there is additional motivation for comparing prosodic and turn-taking patterns in individuals with ASD and along the psychosis spectrum.
Crucially, the possible link between prosodic and turn-taking variables and their neurophysiological substrate in microstates has never been studied in patients with these profiles.
Self-reference effect and disorders of the self
Neurophysiological measures may also shed light on the individual’s phenomenological experience, such as self-consciousness [ 68 ], and its alteration in patients with psychotic disorders [ 69 ]. The sense of self is multifaceted and can be examined through two main prisms: firstly, as knowledge about “Me”, object of a reflexive construct of the self-concept, stored in long-term memory (narrative self) [ 70 ], and secondly as an “I” subject of the pre-reflexive and embodied subjective experience in the here and now (minimal self) [ 71 ]. Self-disorders constitute a core feature of the schizophrenia spectrum, markers of vulnerability to psychosis and predictors of psychotic conversion in patients at ultra-high risk or who had a first episode of psychosis. One of the possible prisms for studying these self-disorders is based on the evaluation of the self-reference effect on memory, according to which processing information closely related to the self is the most effective strategy for remembering new material [ 72 ]. Indeed, the self is intimately linked to memory and acts as a processing bias that determines how and what information is encoded and retrieved [ 73 , 74 ], particularly in episodic memory, which refers to the memory of the past experiences of the self and contributes to one’s feeling of identity and temporal continuity. However, minimal or narrative self-disorders appear associated to an altered or even lack of self-reference effect on memory [ 75 , 76 ]. Studying the self-reference effect in early psychosis could therefore contribute to characterizing the extent and course of self-disorders in prodromal (ultra-high risk) and early (first episode of psychosis) stages of schizophrenia. An innovative task has been designed using immersive virtual reality to evaluate the self-reference effect on episodic memory via a naturalistic approach, relying on the encoding of multisensory daily life events rather than simplistic lists of words or objects.
Objectives of the DEMETER study
Building on our preliminary results, the DEMETER project (“Détermination Des Microétats EEG associés Aux Troubles Psychiques Dans Les États à Risque”—EEG Microstates Across At-Risk Mental States) is a prospective observational study that aims to characterize the EEG microstate signature with regard to underlying neurophysiological functions, including sensorimotor integration, speech, sleep, and sense of self, across a population of adolescents and young adults, with ultra-high-risk of psychosis (UHR), first-episode psychosis (FEP), schizophrenia (SCZ), autism spectrum disorder (ASD), and major depressive disorder (MDD), compared with healthy controls (CTRL) ( N = 21 × 6), between two timepoints one year apart.
Participants will undergo deep phenotyping based on psychopathology and neuropsychological assessments at baseline and after one year of follow-up, high-resolution EEG (64 electrodes) with a resting period and a sensorimotor task, a recording of the characteristics of their speech (prosody and turn-taking), a one-night polysomnography, and biological sampling for multi-omic analyses, and a self-reference effect task in virtual reality (the latter only in UHR, FEP, and CTRL).
The main questions it aims to answer are as follows. 1) Are EEG microstate anomalies associated with specific disorders, and clinical and functional prognosis, both in resting conditions and during sleep ? 2) Are EEG microstate anomalies associated with differences in sensorimotor integration, speech, and sense of self ? 3) An interventional ancillary study will involve only healthy controls, in order to assess whether light hypnosis conditions can modify the EEG microstate architecture in a direction opposite to what is seen in disease.
Participant recruitment
All participants will be included at the Clinical Research Centre (CRC), University Hospital Group Paris Psychiatry and Neurosciences (GHU). Inclusion criteria are: an age between 15 and 30 years old; French as the maternal language or spoken in the context of bilingualism; a DSM-5 diagnosis of schizophrenia or major depressive disorder or autism spectrum disorder; a diagnosis of ultra-high-risk of psychosis or first-episode psychosis based on the Comprehensive Assessment of at risk mental state (CAARMS) translated in its French version [ 77 ]; and healthy control subjects recruited from the general population. Exclusion criteria are: suicidal risk; severe or non-stabilized somatic and neurological disorders; epilepsy; head trauma; IQ below 70; presence of other psychiatric disorders (bipolar disorder, obsessive–compulsive disorder, or substance use disorders, except for tobacco or cannabis, tolerated up to 5 joints/day); for healthy control subjects, a family history of psychosis is an exclusion criterion. Pregnant or breast-feeding women will not be included.
Participants will be screened among the population of patients seen at an early psychosis outpatient clinic (Centre d’évaluation des jeunes adultes et adolescents—CJAAD, GHU). Healthy controls will be reached through the healthy volunteers database of the CRC. Participant assessment will be as follows (Fig. 1 , Table 1 ). 1) During the pre-inclusion visit, participants will be informed of all the details of the protocol, orally and in writing, and eligibility criteria will be verified. Then a two-week reflection period will be observed, before the signature of a written consent at the baseline inclusion. 2) The baseline visit will consist of a first visit of three half-day sessions including medical, psychopathological and neuropsychological assessments, biological sampling (described below), and speech recording. In the following two months, participants will undergo one night of polysomnography (everyone) and two half-day sessions for the sensorimotor task (everyone) followed by light hypnosis (only controls), and the self-reference effect task in virtual reality (only controls, UHR, and FEP). 3) The follow-up visit will consist of a second psychopathological and neuropsychological assessment, biological sampling, and shorter EEG recording (5–10 min).
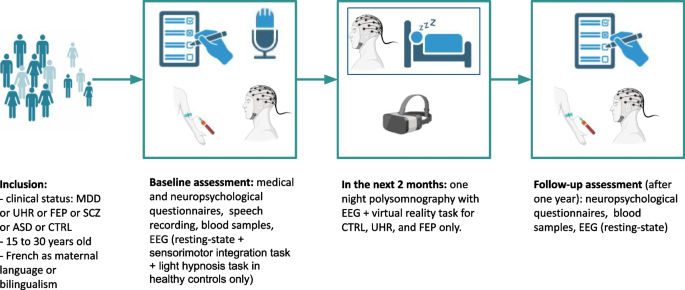
Protocol design. MDD: major depressive disorder. UHR: ultra-high-risk of psychosis. FEP: first-episode psychosis. SCZ: schizophrenia. ASD: autism spectrum disorder. CTRL: healthy controls
Healthy controls will receive a compensation of 120€, and participants with a psychiatric disorder will receive a financial compensation of 60€, as they will benefit to an access to more personalized care. This protocol has been approved by the ethics committee (Comité de protection des personnes) Ouest II (approval number: 2021-A01919-32).
Clinical, psychopathological and neuropsychological assessment
A clinical assessment will include anamnestic data collection (socio-demographic characteristics, treatment history, medical history, psychiatric history); a clinical examination with a physical examination, a psychiatric examination, the CAARMS, the positive and negative syndrome scale (PANSS), the Montgomery–Åsberg Depression Rating Scale (MADRS), Social and Occupational Functioning Assessment Scale (SOFAS), and neurological soft signs [ 78 ]. The neuropsychological assessment will include intellectual functioning (WAIS-IV), executive functioning (fluences, attentional capacities, episodic and working memory), and social cognition.
Speech assessment
Each participant will undergo a semi-structured interview with an experimenter. Interviews will focus on interests and passions, to elicit as free and spontaneous dialogues as possible. Topics related to the participants’ clinical symptomatology will not be approached, unless participants explicitly wish to do so. The recordings will not have a fixed duration, but an attempt will be made to obtain at least 15–20 min of dialogue. Both speakers will wear head-set AKG-C544L microphones, connected via AKG MPA VL phantom adaptors to a Zoom H4n Pro Handy recorder. Speech will be recorded at a sampling rating of 44,000 Hz (16-bit). The distance between the mouth and the microphone will be kept at 2 cm to ensure consistent levels of vocal loudness. Moreover, the two speakers will be placed as far as possible, to prevent crosstalk (i.e. speech of one interactant caught by the other interactant’s microphone). Finally, the recordings will be carried out in a quiet room to limit environmental noise. This is consistent with previous analyses on acoustic patterns in psychiatry [ 79 ]. The.wav files obtained will be annotated using Praat software and subsequently analyzed with Praat [ 80 ] and R. Prosodic features will be extracted using the Prosogram tool (a set of Praat scripts, open-source) [ 81 ] and voice quality features will be computed with a modified version of scripts from the Prosogram tool [ 82 ]. Turn-taking variables will be quantified with combined Praat and R scripts [ 62 , 66 , 83 ]. This task has been designed and will be supervised by an expert in Phonetics and by a psychiatrist trained in linguistic data extraction and analysis (MGr and VL).
Sensorimotor integration task
Sensorimotor integration is investigated using a visuo-tactile task. On each trial, the participant, seated in front of a screen, has a visual instruction: a point to the right or left of the screen. The task consists of pressing one of the two buttons positioned on each side of the body with the index finger of the corresponding hand according to the visual instruction. A vibrotactile stimulator (small bone conduction speakers wired to an Arduino electronic card modulated by an amplifier) is applied to the first dorsal interosseous muscle of both hands. 400 msec before the visual instruction, one of the two hands receives a tactile cue (vibration) on one hand for 100 msec. The tactile cue can be congruent or incongruent with the visual cue, both indicating or not the same hand. Depending on the block, the tactile cue can be more or less reliably coupled with the visual stimulus. In reliable blocks, 90% of the trials present the vibration and visual instruction congruently (indicating the same hand). In non-reliable blocks, only 50% of the trials are congruent, and in this case, the tactile cue is not reliable. Two blocks with 70% congruent cases are carried out intermediately. Finally, a baseline block which does not contain any tactile cues is presented at the beginning and the end of the task. The order of the 90% and 50% blocks is randomized. The tactile and visual stimuli are generated with a MATLAB script. Each block consists of 100 trials, in total 500 trials. EEG data is recorded throughout the task, using a 64-channel EEG cap (from Biosemi). The setup is coupled to an eye tracker in order to control that the participant is fixing the cross at the center of the screen during each block. At the end of the task, a five minute eyes closed resting-state EEG will also be recorded. In order to examine attentional modulation, measurement of alpha power band (in Hz) is computed. Cortical excitability and inhibition are analyzed with mu and theta bands (in Hz), and integration of sensory information as somatosensory evoked potentials (SEPs), where amplitudes (in µV) and latencies (in msec) are extracted. The adaptation of the reaction time (in msec) to the button press according to the probabilistic context of congruency is examined. Analysis is conducted with Python scripts with dedicated libraries such as MNE-Python [ 84 ]. This task has been designed and will be supervised by researchers trained in neurophysiology recording and analysis (AA, LD).
Hypnosis task
After the sensorimotor integration task, healthy controls will undergo a light hypnosis task coupled with two control tasks, in addition to the eyes-closed resting-state already recorded. First, participants will be asked to listen to a neutral text (a refrigerator manual) read by the investigator, with the instruction to listen attentively in order to be able to answer specific questions regarding the content of text. Second, participants will do a mental calculation test. Third, participants will undergo the light hypnosis task. Light hypnosis is based on Ericksonian hypnosis without inducing a trance state, and has been developed by the Collège International des Techniques par Activation de la Conscience (CITAC; Jean Becchio, Sylvain Pourchet) as part of the Paris-Saclay university training in clinical hypnosis. Participants will be asked to focus on any type of preoccupation they may have, and then to picture the first step towards resolving this preoccupation. They will then be asked to provide resources or qualities they have. The light hypnosis session will then start by asking the participant to assume a comfortable and at the same time tonic position, sitting straight, the back lifted from the chair. They will be asked to close their eyes, while being informed that they can open them at any time if needed. They will then be asked to picture their objective, and the first step toward its solution. Then, they will be asked to picture themselves in a situation where they learnt to do something. Proprioceptive, sensory (each of the five senses), and metaphorical suggestions based on their resources will be provided in order to guide the participant in this exercise. This task has been designed and will be performed by two psychiatrists trained in clinical hypnosis (AI, CLo).
Self-reference effect task in virtual reality
Virtual reality immersion will be achieved using the HTC VIVE Pro Eye (Taoyuan City, Taiwan: HTC corporation) virtual reality headset. The self-reference effect task will consist in a walk through a virtual city, where participants will encounter a total of 32 multisensory daily life events that aim to be incidentally encoded in episodic memory. Participants will embody a virtual avatar and navigate twice through two distinct parts of the city. Prior to each navigation, avatar embodiment will be induced using a visuomotor stimulation in front of a virtual mirror, asking participants to move their different body parts while looking at them directly or in the mirror. To manipulate the minimal self-reference, one navigation will be associated with a synchronous avatar to induce a high sense of embodiment, and therefore a stronger sense of minimal self. The other navigation will be associated with an asynchronous avatar with a 700 ms-delay between participants’ real performed movements and the seen movements of the avatar, to induce a low sense of embodiment, and thus a weaker sense of minimal self. To manipulate the narrative self-reference, in each navigation path, half of the events will be associated to the participants themselves (Self), and the other to someone else (Other). The association will be induced by asking participants to take a picture of each event and rate its personal significance for either Self or Other. All conditions will be counterbalanced.
Following each navigation, participants will be submitted to self-reported questionnaires assessing their sense of embodiment (Embodiment Questionnaire) [ 85 ], sense of presence (Igroup Presence Questionnaire) [ 86 ], cybersickness (Simulator Sickness Questionnaire) [ 87 ], and current emotional state (Mood Visual Analogue Scale) [ 88 ].
Finally, participants will undergo two episodic memory tests: a free recall task and a recognition task. The free recall will be based on a verbal interview of 20 min, during which participants will be asked to recall all the events that they remember encountering in the virtual city. The recognition test will be programmed using the Python module Neuropsydia [ 89 ] and consists of displaying on a computer screen all 32 encountered events mixed with 16 lures which were not encountered in a random order, and asking participants whether they encountered this event in the virtual city. For both memory tests, participants will be asked to provide systematically and the most precisely possible, for each recalled event: description of the event, spatiotemporal situation, source, referent for the personal significance rating, perceptive and phenomenological details, degree of reliving or familiarity of the event. This task has been designed by Delphine Yeh under the supervision of Pascale Piolino, and derived from Sylvain Penaud’s protocol for the procedures linked to the minimal self-reference [ 90 ]. The virtual environment has been developed by Alexandre Gaston-Bellegarde using Unity.
Polysomnography
An overnight polysomnography with 19 EEG channels and ventilatory polygraphy will be recorded for all participants within the first two months of inclusion, at the sleep medicine department of Hôtel-Dieu hospital, in Paris (Centre du Sommeil et de la Vigilance). Trained sleep technicians will set-up the head-sets in the evening and supervise the recording during the night. The recordings will be analyzed by trained sleep specialists (SR, LD).
Microstates analysis
Microstate analysis will be performed on eyes-closed resting-state, during the sensorimotor integration task, and during sleep. A minimal preprocessing will be done with the MNE EEG software on Python, which includes a bandpass filter between 0.5 and 40 Hz, rereferencing to the mean, and visual and automatic correction for artifacts using independent component analysis (ICA). Each recording will be visually reanalyzed by clinical neurophysiologists to check for any residual artifact. Microstate analysis will be done using the Pycrostates package [ 91 ]. Global field power (GFP) will be determined for each participant. Only EEG topographies at GFP peaks will be retained to determine microstates’ topographies, through a modified K-means clustering. For each subject the same number of GFP peaks will be extracted and concatenated into a single data set for clustering. A combined score will be used to compute the optimal number of clusters. The resulting clusters will be backfitted to each individual maps. Temporal smoothing will be used to ensure that periods of inter-peak noise, of low GFP, did not interrupt the sequences of quasi-stable segments. For each subject, three parameters will be computed for each microstate class: frequency of occurrence (“occurrence”), temporal coverage (“coverage”) and mean duration. Occurrence is the average number of times a given microstate occurs per second. Coverage (in %) is the percentage of total analysis time spent in a given microstate. Mean duration (in ms) is the average time during which a given microstate was present in an uninterrupted manner (after temporal smoothing).
Biological sampling
Peripheral blood samples will be collected for genetic, epigenetic, proteomic, and metabolomic studies.
Statistical power estimates
We considered a minimum expected effect size around 0.5, based on pairwise comparisons of EEG microstate parameters (mean duration, time coverage, occurrence) between chronic schizophrenia and relatives of subjects with schizophrenia [ 14 ]. Accepting an alpha risk of 0.05 and 95% power, we estimate the necessary number of subjects to be included in each of the 6 groups at 15 (calculated in R with the pwr.anova.test function) (Fig. 2 ). Given the risk of overestimating the minimum effect size associated with publications based on small cohorts, we estimate that a number of 21 subjects significantly increases the chances of obtaining a power greater than 95%. This represents a total of 126 subjects.
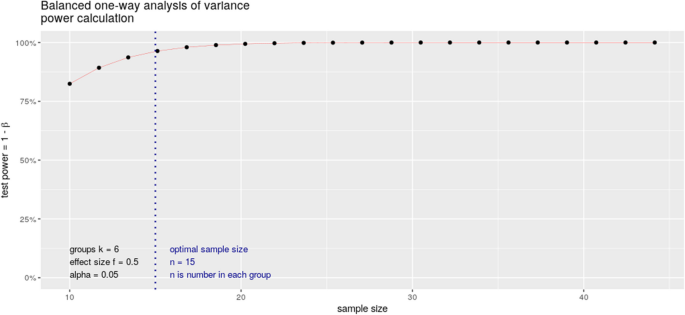
Power calculation
Statistical analysis design
For all neurophysiological variables, the investigators will apply a repeated measures ANOVA, and use the following contrasts:
“UHR, FEP, SCZ, ASD, MDD” vs. “Healthy subjects”, in order to test the variables as markers of general psychopathology;
“UHR, FEP, SCZ” vs. “ASD, MDD”, in order to test the variables as specific markers of psychosis; equivalently, their specificity in MDD and ASD will be tested);
“UHR” vs. “FEP" vs. “SCZ”, in order to test the variables as markers of state;
Finally, in a dimensional approach, the functional correlates of microstates will be studied across wakefulness and sleep, and during speech, regardless of diagnosis.
The Research Domain Criteria (RDoC) strategy has led to a paradigmatic change in psychiatric research, promoting the integration of dimensional constructs beyond current nosographic boundaries. In this context, sensorimotor integration, speech, sleep–wake rhythms, and sense of self appear as relevant phenotypes to understand transdiagnostic functional impairments that lead to a high burden at the individual level [ 92 , 93 ]. In a multimodal approach, the use of neurophysiological tools such as high-density EEG, polysomnography, or audio recorders offer an accessible means to study these dimensions at a very good temporal resolution. Hypnosis or virtual reality tools further give the opportunity to non-invasively modulate and test perceptions in relation with these neurophysiological assessments. Moreover, applying quantitative EEG analyses in this framework, such as microstates, may shed light on the connectivity networks underlying thought processing and provide clinically-relevant biomarkers of state that could be easily implemented in daily practice.
This protocol describes a transdiagnostic longitudinal case–control study that includes a multimodal neurophysiological assessment of sensorimotor integration, speech, sleep, and sense of self, in patients with major depressive disorder, ultra-high-risk of psychosis, first-episode psychosis, schizophrenia, and autism spectrum disorders, compared to healthy controls, in a population of adolescents and young adults aged 15 to 30 years old. Preliminary retrospective analyses from our group, with routine clinical low-resolution EEG recordings, have suggested that a variation in EEG microstates class D may be a marker of stage across psychotic disorders, as it decreases from UHR to FEP and schizophrenia. However, these changes were not specific to psychosis, and they appeared to reflect a shared dimension on the schizophrenia-autism spectrum. We also suggested that a microstate ratio imbalance between class C and class D may perhaps be more specific to schizophrenia, although it did not appear that EEG microstates were sufficient to differentiate between different groups of diseases [ 18 ]. Building on this preliminary data, we propose this prospective study with higher resolution EEG recordings to test whether anomalies in the EEG microstate architecture may be associated with diagnosis, clinical and functional prognosis, both in resting conditions and during sleep, across psychiatric disorders. We postulate that we may find EEG microstate anomalies associated with differences in sensorimotor integration, speech, sense of self, and sleep, and that the dynamic of EEG microstates may be modulated by a non-drug intervention such as light hypnosis, as a proof-of-concept of potential usefulness in psychotherapeutic approaches.
We further hypothesize that the attentional component of somatosensory integration in preparation for a motor response is modulated through visuo-tactile stimuli in healthy subjects, and is altered in patients with psychotic disorders, probably with abnormal inhibition mechanism responses. Specifically, we expect that primary sensory cortex activity, measured as alpha and beta oscillations, influences motor cortex excitability and would be desynchronized in psychosis. We also anticipate an impaired connectivity among the primary sensorimotor network, as well as altered synchrony states in an attentional context. Finally, at rest, we expect these anomalies to be associated with a C/D microstate imbalance and with microstate class E anomalies (postulated to be correlated with interoception and sensorimotor processing).
We also expect to find differences in prosodic and turn-taking patterns between patients and healthy controls. In particular, we predict all patients to display reduced pitch variability, reduced speech output and increased pause duration. Moreover, we expect patients’ voices to overlap more with the interlocutor’s. We also hypothesize that linguistic cues could be markers of the stage, with increasing levels of atypicality from UHR to SCZ. Finally, we speculate that patients with ASD and participants along the schizophrenia spectrum will present similar prosodic and turn-taking patterns.
FInally, we postulate a reduced self-reference effect on memory performance in UHR and FEP individuals, due to patients’ self disturbances. Specifically, we expect a preserved narrative self-reference effect but no minimal self-reference effect in UHR and FEP individuals, since minimal self disturbances are already present in early stages of psychosis whereas narrative disturbances of the self are less marked, as opposed to controls who are expected to exhibit both minimal and narrative self-reference effects.
This study will address several methodological challenges. Its transdiagnostic design will allow us to test the specificity of any relevant observed association. At the data collection level, the pipeline that integrates the sensorimotor task with the high-density EEG recording will follow stringent quality checks so that the EEG recordings are interpretable with regard to the underlying task. The one-night polysomnography recordings will require to anticipate all the risks associated with prolonged recordings, such as electrodes that come off during sleep due to participant movement. This implies time-consuming regular check-ups during the night from trained technicians. In order to allow reproducible results, the ancillary light hypnosis protocol will require the use of a simple standardized strategy from the two clinicians trained in hypnosis. Regarding the linguistic data collection, high-quality double-channel audio recordings are crucial to allow precise and reliable analyses with the Praat software. Regarding the self-reference effect, the innovative task using immersive virtual reality will enable to study this effect in a naturalistic and standardized context, which will capture the richness of episodic memory and its links with the self in everyday life better than the simplistic lists of words or objects that are traditionally used in self-reference effect studies. Moreover, the task will integrate both minimal and narrative self-reference, which will enable to examine under the same design the respective but also joint contributions of both facets of the self to the self-reference effect in patients with self-disorders.
In conclusion, this multimodal, transdiagnostic neurophysiological approach will help pave the way for personalized medicine through in-depth endophenotyping of sleep, speech, sensorimotor integration and self-perception, four dimensions that overlap in the spectrum of psychiatric disorders.
Availability of data and materials
Anonymized data will be stored at GHU Paris Psychiatrie et Neurosciences, and will be made available upon reasonable request to the authors.
Lee PH, et al. Genomic relationships, novel loci, and pleiotropic mechanisms across eight psychiatric disorders. Cell. 2019;179:1469-1482.e11.
Article Google Scholar
Writing Committee for the Attention-Deficit/Hyperactivity Disorder, et al. Virtual histology of cortical thickness and shared neurobiology in 6 psychiatric disorders. JAMA Psychiat. 2021;78:47.
Bellato A, et al. A systematic review and meta-analysis of altered electrophysiological markers of performance monitoring in Obsessive-Compulsive Disorder (OCD), Gilles de la Tourette Syndrome (GTS), Attention-Deficit/Hyperactivity disorder (ADHD) and Autism. Neurosci Biobehav Rev. 2021;131:964–87.
Article CAS PubMed Google Scholar
Newson JJ, Thiagarajan TC. EEG frequency bands in psychiatric disorders: a review of resting state studies. Front Hum Neurosci. 2019;12:521.
Article PubMed PubMed Central Google Scholar
Uhlhaas PJ, Singer W. Abnormal neural oscillations and synchrony in schizophrenia. Nat Rev Neurosci. 2010;11:100–13.
Harris KD, Mrsic-Flogel TD. Cortical connectivity and sensory coding. Nature. 2013;503:51–8.
Bar-Shira O, Maor R, Chechik G. Gene expression switching of receptor subunits in human brain development. PLOS Comput Biol. 2015;11:e1004559.
Caballero A, Tseng KY. GABAergic function as a limiting factor for prefrontal maturation during adolescence. Trends Neurosci. 2016;39:441–8.
Article CAS PubMed PubMed Central Google Scholar
Solmi M, et al. Age at onset of mental disorders worldwide: large-scale meta-analysis of 192 epidemiological studies. Mol Psychiatry. 2021;27:281–95.
Michel CM, Koenig T. EEG microstates as a tool for studying the temporal dynamics of whole-brain neuronal networks: a review. Neuroimage. 2018;180:577–93.
Article PubMed Google Scholar
Britz J, Van De Ville D, Michel CM. BOLD correlates of EEG topography reveal rapid resting-state network dynamics. Neuroimage. 2010;52:1162–70.
Tarailis P, Koenig T, Michel CM, Griškova-Bulanova I. The functional aspects of resting EEG microstates: a systematic review. Brain Topogr. 2023. https://doi.org/10.1007/s10548-023-00958-9 .
Brodbeck V, et al. EEG microstates of wakefulness and NREM sleep. Neuroimage. 2012;62:2129–39.
da Cruz JR, et al. EEG microstates are a candidate endophenotype for schizophrenia. Nat Commun. 2020;11:3089.
Takarae Y, et al. EEG microstates suggest atypical resting-state network activity in high-functioning children and adolescents with autism spectrum development. Dev Sci. 2022;25:e13231. https://doi.org/10.1111/desc.13231 .
Lei L, et al. EEG microstates as markers of major depressive disorder and predictors of response to SSRIs therapy. Prog Neuropsychopharmacol Biol Psychiatry. 2022;116:110514.
Gavaret M, Iftimovici A, Pruvost-Robieux E. EEG: Current relevance and promising quantitative analyses. Rev Neurol (Paris). 2023. https://doi.org/10.1016/j.neurol.2022.12.008 . S0035-3787(23)00869-X.
Iftimovici A, et al. Electroencephalography microstates imbalance across the spectrum of early psychosis, autism, and mood disorders. Eur Psychiatry. 2023;66:e41.
Katayama H, et al. Classes of multichannel EEG microstates in light and deep hypnotic conditions. Brain Topogr. 2007;20:7–14.
Landry M, Lifshitz M, Raz A. Brain correlates of hypnosis: a systematic review and meta-analytic exploration. Neurosci Biobehav Rev. 2017;81:75–98.
Fuhr K, et al. Efficacy of hypnotherapy compared to cognitive behavioral therapy for mild to moderate depression - Results of a randomized controlled rater-blind clinical trial. J Affect Disord. 2021;286:166–73.
Winkelman JW, Lecea LD. Sleep and neuropsychiatric illness. Neuropsychopharmacology. 2020;45:1–2.
Hühne A, Welsh DK, Landgraf D. Prospects for circadian treatment of mood disorders. Ann Med. 2018;50:637–54.
Pandi-Perumal SR, et al. Clarifying the role of sleep in depression: a narrative review. Psychiatry Res. 2020;291:113239.
Bernardi K, et al. Sleep disturbances in subjects with autism spectrum disorder: a parental perspective. Sleep Med. 2023;110:220–4.
Reeve S, Sheaves B, Freeman D. Sleep disorders in early psychosis: incidence, severity, and association with clinical symptoms. Schizophr Bull. 2019;45:287–95.
Lai M, et al. Investigating sleep spindle density and schizophrenia: a meta-analysis. Psychiatry Res. 2022;307:114265.
Ritter PS, et al. Sleep spindles in bipolar disorder - a comparison to healthy control subjects. Acta Psychiatr Scand. 2018;138:163–72.
Gorgoni M, Scarpelli S, Reda F, De Gennaro L. Sleep EEG oscillations in neurodevelopmental disorders without intellectual disabilities. Sleep Med Rev. 2020;49:101224.
Tamminen J, Payne JD, Stickgold R, Wamsley EJ, Gaskell MG. Sleep spindle activity is associated with the integration of new memories and existing knowledge. J Neurosci. 2010;30:14356–60.
Walther S, Mittal VA. Motor system pathology in psychosis. Curr Psychiatry Rep. 2017;19:97.
Walther S, et al. Movement disorder and sensorimotor abnormalities in schizophrenia and other psychoses - European consensus on assessment and perspectives. Eur Neuropsychopharmacol. 2020;38:25–39.
Le Boterff Q, et al. A tablet-based quantitative assessment of manual dexterity for detection of early psychosis. Front Psychiatry. 2023;14:1200864.
Böttcher A, et al. A dissociable functional relevance of theta- and beta-band activities during complex sensorimotor integration. Cereb Cortex N Y N. 2023;1991(33):9154–64.
Carment L, et al. Impaired attentional modulation of sensorimotor control and cortical excitability in schizophrenia. Brain. 2019;142:2149–64.
Carment L, et al. Common vs. distinct visuomotor control deficits in autism spectrum disorder and schizophrenia. Autism Res. 2020;13:885–96.
Dupin L, et al. Predictive modulation of corticospinal excitability and implicit encoding of movement probability in schizophrenia. Schizophr Bull. 2019;45:1358–66.
Arthur T, Vine S, Brosnan M, Buckingham G. Predictive sensorimotor control in autism. Brain. 2020;143:3151–63.
Perry W, Minassian A, Lopez B, Maron L, Lincoln A. Sensorimotor gating deficits in adults with autism. Biol Psychiatry. 2007;61:482–6.
Hannant P, Tavassoli T, Cassidy S. The role of sensorimotor difficulties in autism spectrum conditions. Front Neurol. 2016;7:124.
Cascio C, et al. Tactile perception in adults with autism: a multidimensional psychophysical study. J Autism Dev Disord. 2008;38:127–37.
Cascio CJ, Lorenzi J, Baranek GT. Self-reported pleasantness ratings and examiner-coded defensiveness in response to touch in children with ASD: effects of stimulus material and bodily location. J Autism Dev Disord. 2016;46:1528–37.
Mikkelsen M, Wodka EL, Mostofsky SH, Puts NAJ. Autism spectrum disorder in the scope of tactile processing. Dev Cogn Neurosci. 2018;29:140–50.
Coskun MA, Loveland KA, Pearson DA, Papanicolaou AC, Sheth BR. Functional assays of local connectivity in the somatosensory cortex of individuals with autism. Autism Res. 2013;6:190–200.
Espenhahn S, et al. Atypical tactile perception in early childhood autism. J Autism Dev Disord. 2023;53:2891–904.
Kaufmann T, et al. Disintegration of sensorimotor brain networks in schizophrenia. Schizophr Bull. 2015;41:1326–35.
Liu D, et al. Deficits of tactile passive perception acuity in patients with schizophrenia. Front Psychiatry. 2020;11:519248.
Noel JP, et al. Visual-tactile spatial multisensory interaction in adults with autism and schizophrenia. Front Psychiatry. 2020;11:578401.
Teale P, Pasko B, Collins D, Rojas D, Reite M. Somatosensory timing deficits in schizophrenia. Psychiatry Res Neuroimaging. 2013;212:73–8.
Shergill SS, et al. Functional magnetic resonance imaging of impaired sensory prediction in schizophrenia. JAMA Psychiat. 2014;71:28–35.
Jouen A-L, Lancheros M, Laganaro M. Microstate ERP analyses to pinpoint the articulatory onset in speech production. Brain Topogr. 2021;34:29–40.
Covington MA, et al. Schizophrenia and the structure of language: the linguist’s view. Schizophr Res. 2005;77:85–98.
Hitczenko K, Mittal VA, Goldrick M. Understanding language abnormalities and associated clinical markers in psychosis: the promise of computational methods. Schizophr Bull. 2021;47:344–62.
Bedi G, et al. Automated analysis of free speech predicts psychosis onset in high-risk youths. NPJ Schizophr. 2015;1:15030.
Lucarini V, et al. Speech prosody as a bridge between psychopathology and linguistics: the case of the schizophrenia spectrum. Front Psychiatry. 2020;11:531863.
Parola A, Simonsen A, Bliksted V, Fusaroli R. Voice patterns in schizophrenia: a systematic review and Bayesian meta-analysis. Schizophr Res. 2020;216:24–40.
Hitczenko K, Segal Y, Keshet J, Goldrick M, Mittal VA. Speech characteristics yield important clues about motor function: speech variability in individuals at clinical high-risk for psychosis. Schizophr Heidelb Ger. 2023;9:60.
Bianciardi B, et al. Investigating temporal and prosodic markers in clinical high-risk for psychosis participants using automated acoustic analysis. Early Interv Psychiatry. 2023;17:327–30.
Hamilton AFC, Holler J. Face2face: advancing the science of social interaction. Philos Trans R Soc Lond B Biol Sci. 2023;378:20210470.
Levinson SC, Torreira F. Timing in turn-taking and its implications for processing models of language. Front Psychol. 2015;6:731.
Tahir Y, et al. Non-verbal speech cues as objective measures for negative symptoms in patients with schizophrenia. PLoS ONE. 2019;14:e0214314.
Lucarini V, et al. Conversational metrics, psychopathological dimensions and self-disturbances in patients with schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2022;272:997–1005.
Sichlinger L, Cibelli E, Goldrick M, Mittal VA. Clinical correlates of aberrant conversational turn-taking in youth at clinical high-risk for psychosis. Schizophr Res. 2019;204:419–20.
Grice M, et al. Linguistic prosody in autism spectrum disorder—An overview. Lang Linguist Compass. 2023;17:e12498.
Wehrle S, Grice M, Vogeley K. Filled pauses produced by autistic adults differ in prosodic realisation, but not rate or lexical type. J Autism Dev Disord. 2023. https://doi.org/10.1007/s10803-023-06000-y .
Wehrle S, Cangemi F, Janz A, Vogeley K, Grice M. Turn-timing in conversations between autistic adults: typical short-gap transitions are preferred, but not achieved instantly. PLoS ONE. 2023;18:e0284029.
Martinez G, et al. ‘A circle and a triangle dancing together’: Alteration of social cognition in schizophrenia compared to autism spectrum disorders. Schizophr Res. 2019;210:94–100.
Bréchet L, Michel CM. EEG microstates in altered states of consciousness. Front Psychol. 2022;13:856697.
Vellante F, et al. Euthymic bipolar disorder patients and EEG microstates: a neural signature of their abnormal self experience? J Affect Disord. 2020;272:326–34.
Klein SB. Self, memory, and the self-reference effect: an examination of conceptual and methodological issues. Personal Soc Psychol Rev. 2012;16:283–300.
Gallagher S. Philosophical conceptions of the self: implications for cognitive science. Trends Cogn Sci. 2000;4:14–21.
Symons CS, Johnson BT. The self-reference effect in memory: a meta-analysis. Psychol Bull. 1997;121:371–94.
Prebble SC, Addis DR, Tippett LJ. Autobiographical memory and sense of self. Psychol Bull. 2013;139:815–40.
Conway MA. Memory and the self. J Mem Lang. 2005;53:594–628.
Bergouignan L, Nyberg L, Ehrsson HH. Out-of-body memory encoding causes third-person perspective at recall. J Cogn Psychol. 2022;34:160–78.
Compère L, et al. Self-reference recollection effect and its relation to theory of mind: an investigation in healthy controls and schizophrenia. Conscious Cogn. 2016;42:51–64.
Krebs M-O, et al. Évaluation des états mentaux à risque de transition psychotique : validation de la version française de la CAARMS. L’Encéphale. 2014;40:447–56.
Krebs MO, Gut-Fayand A, Bourdel M, Dischamp J, Olié J. Validation and factorial structure of a standardized neurological examination assessing neurological soft signs in schizophrenia. Schizophr Res. 2000;45:245–60.
de Boer JN, et al. Acoustic speech markers for schizophrenia-spectrum disorders: a diagnostic and symptom-recognition tool. Psychol Med. 2021:1–11. https://doi.org/10.1017/S0033291721002804 .
Boersma P, Weenink D. Praat: doing phonetics by computer. 2023.
Google Scholar
Mertens P. The Prosogram model for pitch stylization and its applications in intonation transcription. In: Barnes J, Shattuck-Hufnagel S, editors. Prosodic theory and practice. The MIT Press; 2022. p. 259–86. https://doi.org/10.7551/mitpress/10413.003.0010 .
JalalAl-Tamimi/Praat-VQ-Measurements: Praat VQ measurements. https://doi.org/10.5281/zenodo.7270191 .
Cangemi F, et al. Content-free speech activity records: interviews with people with schizophrenia. Lang Resour Eval. 2023. https://doi.org/10.1007/s10579-023-09666-z .
Gramfort A, et al. MEG and EEG data analysis with MNE-Python. Front Neurosci. 2013;7:267.
Gonzalez-Franco M, Peck TC. Avatar embodiment. Towards a standardized questionnaire. Front Robot AI. 2018;5:74.
Schubert T, Friedmann F, Regenbrecht H. The experience of presence: factor analytic insights. Presence Teleoperators Virtual Environ. 2001;10:266–81.
Kennedy RS, Lane NE, Berbaum KS, Lilienthal MG. Simulator sickness questionnaire: an enhanced method for quantifying simulator sickness. Int J Aviat Psychol. 1993;3:203–20.
Scoriels L, et al. Effects of modafinil on emotional processing in first episode psychosis. Biol Psychiatry. 2011;69:457–64.
Makowski D, Dutriaux L. Neuropsydia.py: a python module for creating experiments, tasks and questionnaires. J Open Source Softw. 2017;2:259.
Penaud S, Yeh D, Gaston-Bellegarde A, Piolino P. The role of bodily self-consciousness in episodic memory of naturalistic events: an immersive virtual reality study. Sci Rep. 2023;13:17013.
Férat V, Scheltienne M, Brunet D, Ros T, Michel C. Pycrostates: a python library to study EEGmicrostates. J Open Source Softw. 2022;7:4564.
Cuthbert BN, Insel TR. Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC Med. 2013;11:126.
Morris SE, et al. Revisiting the seven pillars of RDoC. BMC Med. 2022;20:220.
Download references
Acknowledgements
We thank the team of the Délégation à la Recherche Clinique et à l’Innovation (DRCI) of GHU Paris Psychiatrie et Neurosciences for providing administrative and legal support to build this protocol, with special thanks to Bernadette Lemercier, Thujin Yoharajah, Khaoussou Sylla, Didier André, and Kahina Belkhir Hadid. We thank the Centre de Recherche Clinique (CRC) of GHU Paris Psychiatrie et Neurosciences for providing the material support of subject inclusions. Figures were done with Biorender ( https://www.biorender.com/ ).
This study is funded by the DEMETER Starting Grant, GHU Paris Psychiatrie et Neurosciences (principal investigator: Anton Iftimovici). Valeria Lucarini was supported by the doctoral grant ‘Young Talents in Psychiatry 2021’ from the Fondation FondaMental—Fondation Bettencourt-Schueller. Anaëlle Alouit was also financed by the French government’s “Investissements d’Avenir” programme (ANR-18-RHUS-0014 PsyCARE).
Author information
Authors and affiliations.
Université Paris Cité, Institute of Psychiatry and Neuroscience of Paris (IPNP), INSERM U1266, Team “Pathophysiology of psychiatric disorders”, GDR 3557-Institut de Psychiatrie, 102-108 Rue de la Santé, Paris, 75014, France
Valeria Lucarini, Mylène Moyal, Boris Chaumette, Marie-Odile Krebs & Anton Iftimovici
GHU Paris Psychiatrie et Neurosciences, Pôle Hospitalo-Universitaire d’évaluation, Prévention, et Innovation Thérapeutique (PEPIT), Paris, France
Valeria Lucarini, Jeanne Le Coq, Romane Savatte, Mylène Charre, Cécile Louveau, Meryem Benlaifa Houamri, Mylène Moyal, Boris Chaumette, Marie-Odile Krebs & Anton Iftimovici
Université Paris Cité, Institute of Psychiatry and Neuroscience of Paris (IPNP), INSERM U1266, Team “Stroke: from prognostic determinants and translational research to personalized interventions”, Paris, 75014, France
Anaëlle Alouit, Estelle Pruvost-Robieux, Påvel G. Lindberg & Martine Gavaret
Laboratoire Mémoire, Cerveau et Cognition, UR7536, Université Paris Cité, Boulogne-Billancourt, F-92100, France
Delphine Yeh, Sylvain Penaud, Alexandre Gaston-Bellegarde & Pascale Piolino
Centre du Sommeil et de la Vigilance, AP-HP, Hôtel-Dieu, Paris, France
Stéphane Rio, Laurent Drouet, Maxime Elbaz & Damien Léger
Collège International de Thérapies d’orientation de l’Attention et de la Conscience (CITAC), Paris, France
Jean Becchio & Sylvain Pourchet
Service de Neurophysiologie Clinique, GHU Paris Psychiatrie et Neurosciences, Paris, France
Estelle Pruvost-Robieux & Martine Gavaret
Epileptology and Cerebral Rhythmology, APHM, Timone Hospital, Marseille, France
Angela Marchi
Department of Child and Adolescent Psychiatry, Fondation Vallee, UNIACT Neurospin CEA - INSERM UMR 1129, Universite Paris Saclay, Gentilly, France
Aline Lefebvre
IfL-Phonetics, University of Cologne, Cologne, Germany
Martine Grice
INCC UMR 8002, CNRS, Université Paris Cité, Paris, F-75006, France
Lucile Dupin
Inserm, INRAE, Center for Research in Epidemiology and StatisticS (CRESS), Service de Psychiatrie de l’adulte, AP-HP, Hôpital Hôtel-Dieu, Université Paris Cité and Université Sorbonne Paris Nord, Paris, France
Cédric Lemogne
VIFASOM, ERC 7330, Université Paris Cité, Paris, France
Damien Léger
You can also search for this author in PubMed Google Scholar
Contributions
This project is the fruit of a collaborative effort, including: project funding and overall coordination (AI), speech study design (VL, MGr), sensorimotor integration study design (AA), self-reference effect in virtual reality study design (DY, PP, SP, AGB), sleep study design (AI), polysomnography set-up and analysis (SR, LD, ME, DL), hypnosis study design (AI, CLo, JB, SP), participant screening, inclusion and clinical assessment (AI, VL, MC, MBH, MOK), neuropsychological assessment (JLC and RS), EEG preprocessing and analysis protocols (AI, EPR, AM, MM, AL, MGa), biological assessment (BC). AI, VL, AA, and DY drafted the initial manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Anton Iftimovici .
Ethics declarations
Ethics approval and consent to participate.
This study was approved by the ethics committee “Comité de protection des personnes” Ouest II (Approval Number: 2021-A01919-32), and is registered on ClinicalTrials.gov (ID: NCT06045897).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Lucarini, V., Alouit, A., Yeh, D. et al. Neurophysiological explorations across the spectrum of psychosis, autism, and depression, during wakefulness and sleep: protocol of a prospective case–control transdiagnostic multimodal study (DEMETER). BMC Psychiatry 23 , 860 (2023). https://doi.org/10.1186/s12888-023-05347-x
Download citation
Received : 31 October 2023
Accepted : 03 November 2023
Published : 21 November 2023
DOI : https://doi.org/10.1186/s12888-023-05347-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Neurophysiology
- EEG microstates
- Virtual reality
- Sense of self
BMC Psychiatry
ISSN: 1471-244X
- Submission enquiries: [email protected]
- General enquiries: [email protected]
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 28 November 2019
Cerebrospinal fluid oxidative stress metabolites in patients with bipolar disorder and healthy controls: a longitudinal case-control study
- Ulla Knorr ORCID: orcid.org/0000-0002-1222-1527 1 ,
- Anja Hviid Simonsen ORCID: orcid.org/0000-0002-5461-162X 2 ,
- Peter Roos 2 ,
- Allan Weimann 3 , 4 ,
- Trine Henriksen 3 , 4 ,
- Ellen-Margrethe Christensen 1 ,
- Maj Vinberg 1 ,
- Rie Lambæk Mikkelsen 1 ,
- Thomas Kirkegaard 1 ,
- Rasmus Nejst Jensen 1 ,
- Morten Akhøj 5 ,
- Julie Forman 5 ,
- Henrik Enghusen Poulsen 4 ,
- Steen Gregers Hasselbalch 2 &
- Lars Vedel Kessing 1
Translational Psychiatry volume 9 , Article number: 325 ( 2019 ) Cite this article
1740 Accesses
30 Citations
2 Altmetric
Metrics details
- Diagnostic markers
- Neuroscience
Bipolar disorder (BD) is a mental disorder characterized by recurrent relapses of affective episodes, cognitive impairment, illness progression, and reduced life expectancy. Increased systemic oxidatively generated nucleoside damage have been found in some neurodegenerative disorders and in BD. As the first, this naturalistic prospective, longitudinal follow-up case-control study investigated cerebrospinal fluid (CSF) oxidative stress markers 8-oxo-7,8-dihydroguanosine (8-oxoGuo) and 8-oxo-7,8-dihydro-2′-deoxyguanosine (8-oxodG) that relate to RNA and DNA damage, respectively. Patients with BD ( n = 86, 51% female) and gender-and-age-matched healthy control individuals (HC; n = 44, 44% female) were evaluated at baseline (T0), during (T1) and after a new affective episode (T2), if it occurred, and after a year (T3). Cerebrospinal and urine oxidative stress markers were analyzed using ultra-performance liquid chromatography–tandem mass spectrometry. CSF-8-oxoGuo was statistically significantly higher by 18% ( p = 0.003) in BD versus HC at T0, and by 22% ( p = 0) at T3. CSF-8-oxoGuo had increased by 15% ( p = 0.042) from T0 to T3, and by 14% ( p = 0.021) from T2 to T3 in patients, who experienced an episode during follow-up. CSF-8-oxodG had increased by 26% ( p = 0.054) from T0 to T2 and decreased by 19% ( p = 0.041) from T2 to T3 in patients, who experienced an episode during follow-up. CSF-8-oxoGuo did not show a statistically significant change in HC during the one-year follow-up. CSF and urine-8-oxoGuo levels correlated moderately. In conclusion, CSF oxidative stress marker of RNA damage 8-oxoGuo showed both state and trait dependence in BD and stability in HC. Central RNA damage may be a potential biomarker for BD.
Similar content being viewed by others
Biomarkers in the cerebrospinal fluid of patients with psychotic disorders compared to healthy controls: a systematic review and meta-analysis
Troels Boldt Rømer, Rose Jeppesen, … Michael Eriksen Benros

Altered levels of interleukins and neurotrophic growth factors in mood disorders and suicidality: an analysis from periphery to central nervous system
Bharathi S. Gadad, Javier Vargas-Medrano, … Peter M. Thompson
Neuroinflammation and neuroprogression produced by oxidative stress in euthymic bipolar patients with different onset disease times
Daniela Delwing-de Lima, Luiz Arthur Rangel Cyrino, … Heloiza Fiamoncini
Introduction
Bipolar disorder (BD) is a disabling mental illness with a prevalence of 1%, a high risk of recurrence of manic and depressive episodes, a lifelong elevated risk of suicide 1 and a heritability of 60–80% 2 . A vast body of literature evidence show clinical progression in BD with increasing risk of developing new mood episodes with every episode, progressive shortening of inter-episode intervals with each recurrence, and with increasing cognitive disabilities during the course of illness 1 , 3 , 4 , 5 , 6 , 7 , 8 . However, systematic research of the underlying neurobiology of illness progression is lacking.
Elevated levels of peripheral markers of oxidative stress have been found in psychiatric disorders, diabetes, and neurodegenerative disorders 9 , 10 , 11 . Oxidative stress reflects an increase in pro-oxidants, which subsequently leads to oxidative modifications of cellular components, such as RNA and DNA 12 . Oxidative stress markers 8-oxo-7,8-dihydroguanosine (8-oxoGuo), a marker of RNA oxidation, and 8-oxo-7,8dihydro-2′-deoxyguanosine (8-oxodG), a marker of DNA oxidation, can reliably be quantified in cerebrospinal fluid (CSF) 13 and urine 14 using a modified ultra-performance liquid chromatography and mass spectrometry assay, and are valid markers of central/whole-body RNA and DNA damage, respectively 14 .
We and other groups have found elevated levels of urine-8-oxoGuo and 8-oxodG in patients with BD compared to healthy control individuals (HC) 9 , 15 , 16 , 17 . Furthermore, in a longitudinal study our group has found increased oxidative stress in manic/hypomanic states versus remission 17 . Postmortem measurements indicate DNA as the main site of oxidative stress modifications in the central nervous system in severe mental illnesses and suggested that 8-oxoGuo may pass the blood–brain barrier more readily than 8-oxodG 18 . Data are largely missing on oxidative stress evolution during progression of BD 11 and as recently reviewed, CSF oxidative stress has not yet been investigated in either BD or HC 19 .
This study aimed, as the first, to investigate state-specific, intra-individual changes in repeated measures of cerebrospinal and urinary markers of oxidative stress in outpatients diagnosed with BD compared to HC individuals during a one-year prospective, longitudinal follow-up study.
The following hypotheses were tested: Cerebrospinal and urinary oxidative stress marker levels are: (1) higher in patients with BD compared to HC, (2) stable during a year in HC, (3) increased during and following an affective episode, and (4) correlated.
Participants and methods
The study was conducted at the Copenhagen Affective Disorder Research Center. Participants for the study were investigated from 1 April 2014 until 27 April 2017. All participants were assessed at baseline (T0) and after a follow-up of one year (T3). The mood states of patients with BD were evaluated by weekly contacts. In case of a new affective episode of depression, hypomania or mania patients were reassessed during the episode (T1) and at the time they had regained remission (T2; Table 1 ).
All participants provided written informed consent and were reimbursed regarding lumbar puncture.
Participants
Patients with bd.
Newly diagnosed patients aged 18–60 years with BD in remission were recruited from the Copenhagen Affective Disorder Clinic that receives patients from the Capital Region of Denmark covering 1.6 million people and all psychiatric centres in the region 20 . Diagnoses were initially provided by experienced psychiatrists in the Clinic. Exclusion criteria were significant physical illness, pregnancy or planned pregnancy within a year, substance abuse, expected noncompliance with the protocol, no informed consent, and finally practical reasons.
Healthy control individuals
Age-and-gender-matched HC with no personal or first-degree family history of psychiatric disorders were recruited among blood donors aged 18–60 years affiliated to the Blood Bank at Frederiksberg Hospital, Copenhagen as in prior studies from our group 21 . Exclusion criteria were the same as for the patients.
Clinical assessment
Baseline t0.
Written and oral information of the study was given to patients with BD at the Copenhagen Affective Disorder Clinic and at the Blood Bank for the HC followed up by a personal contact by e-mail or telephone. After giving informed consent, the participants were examined at baseline (T0). The clinical diagnosis was evaluated using the semistructured Schedules for Clinical Assessment in Neuropsychiatry (SCAN) interview 22 conducted by specialist in psychiatry (U.K.). The severity of mood symptoms was assessed using the 17-item Hamilton Depression Rating Scale (HAMD) 23 and the Young Mania Rating Scale (YMRS) 24 . Remission was defined as scores below 8 on both scales for at least two weeks. Furthermore, clinical characteristics were assessed, including weight, height, current medication, alcohol consumption, smoking habits, duration of illness from first hypomanic episode, and history of psychoses. Severity of illness was estimated using the Global Clinical Impression Scale 25 .
Follow-up T1, T2, and T3
All participants were followed prospectively for a year. The patients received treatment as usual and were instructed to daily self-monitoring of mood, sleep, alcohol, and medicine intake. Psychiatrist U.K. kept in weekly contact with the patients by their choices of either telephone, short message service, or e-mail. Patients who experienced a moderate to severe affective episode defined as scores above 13 points on either the HAMD or the YMRS for at least two weeks, had a repeated clinical assessment, including urine, blood, and CSF sampling during the episode (T1) and, also following the episode when being in stable remission for at least two weeks (T2). Finally, all participants were assessed at the one-year follow-up in remission, defined as at least eight weeks in a stable remission state (T3), see Flowchart, Fig. 1 . On the basis of prior data from the Copenhagen Affective Disorder Clinic 26 , we expected that 50% of the patients would experience an affective episode during the follow-up period.
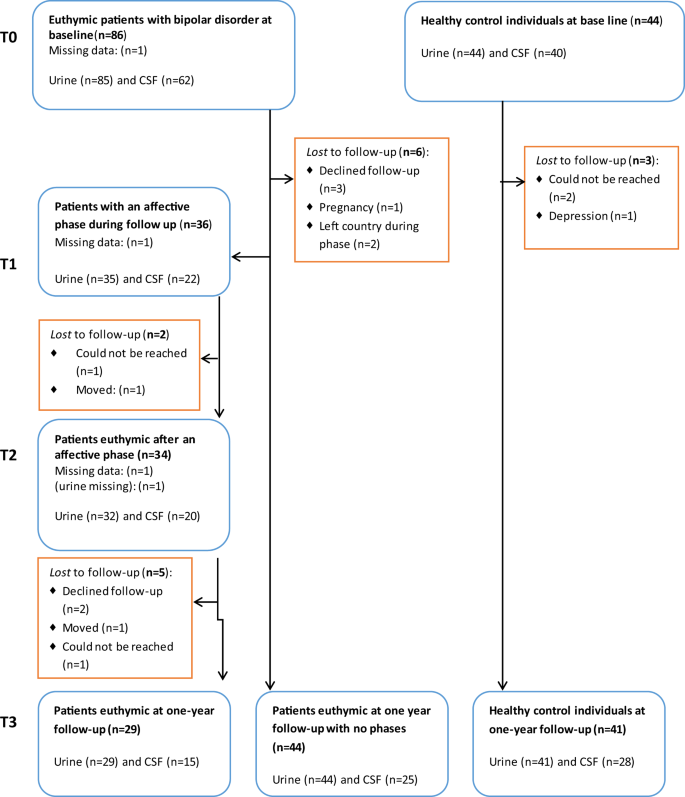
Flowchart for the Bipolar Oxidative Stress Follow-up Study.
Biological assessments
The participants (fasted overnight before the collection of CSF, blood, and urine samples between 0800 and 1000 h in the morning. At all timepoints (T0, T1, T2, and T3), the clinical assessments and the urine, blood, and CSF sampling from the participants were done on the same or the following day.
Sampling and handling of CSF
Specialists of neurology (S.G.H. and P.R.) performed lumbar puncture to collect CSF samples from patients with BD and HC individuals in the lateral decubitus position. The spinal needle was inserted into the L3/L4 or L4/L5 interspace, and a total volume of 10–12 ml of CSF was collected in polypropylene tubes, and gently inverted to avoid gradient effects. Samples where centrifuged on acquisition at 2,000 g for 10 min at +4 °C and stored in polypropylene tubes in 250 µL aliquots at −80 °C pending analysis. A general CSF screen was conducted, including albumin, immunoglobulin G (IgG), IgG index, erythrocytes, white blood cells, glucose, and protein.
Blood sampling
Board-certified laboratory technicians collected blood samples that were analyzed at the Clinical Biochemical Laboratory at Rigshospitalet, Denmark, regarding standard biochemical parameters, including hematological parameters, blood glucose, C-reactive protein, thyroid hormones, lipid status, ions, metabolites, liver enzymes, and lithium levels.
Urine sampling
A freshly voided spot urine was obtained using a standard sampling kit without any additives. The sample was kept on ice and centrifuged at 4 °C and 1590 g for 15 min, after which aliquots of 1.5 ml were transferred to Eppendorf tubes and stored at −80 °C pending analyses. The results for oxidative stress markers in urine were normalized for creatine 27 .
Analyses of 8-oxoGuo and 8-oxodG
The cerebrospinal and urinary oxidative stress markers 8-oxoGuo and 8-oxodG were analyzed at Laboratory of Clinical Pharmacology, Rigshospitalet using ultra-performance liquid chromatography–tandem mass spectrometry, as described in full detail elsewhere 13 , 27 .
Statistical analyses
Data were analyzed according to a preestablished protocol. All analyses were conducted with SAS software, version 9.4, (Copyright 2013, SAS Institute Inc., Cary, NC, USA). All p values were corrected for multiple testing using the Benjamini & Hochberg procedure 28 . We applied a conservative cutoff for the false discovery rate at 0.05, which limits the rate of false positives among the reported findings to one in 20, so that an adjusted p ≤ 0.05 was considered statistically significant. All biomarkers were found to have a skew distribution and were therefore log-transformed prior to analysis. Hence, estimated differences between groups and timepoints are expressed in relative terms as percent-wise differences.
Regarding sample size and power, the numbers of participants in this present study, including 86 patients with BD and 44 HC individuals are like the largest prior case-control studies, regarding peripheral oxidative stress markers in BD 15 , 16 , 17 .
Demographic and clinical data
Demographic and clinical data at timepoints T0, T2, and T3 were summarized in numbers and percentages (categorical data), means and s.d. (normally distributed continuous data), and medians and quartiles (non-normally distributed continuous data). Comparisons of BD and HC at T0 and T3 was made using Fisher’s exact test, Welch’ t -test or the Mann–Whitney U test, whichever was most appropriate.
Markers of oxidative stress in BD and HC at baseline and at the one-year follow-up
To compare biomarker levels of CSF-8-oxoGuo, CSF-8-oxodG, urine-8-oxoGuo, and urine-8-oxodG between BD and HC, a linear mixed model was applied with time (T0 or T3) and group (BD or HC) as fixed effects and with an unstructured covariance to account for correlation between the repeated measurements on the study participants. The analyses were performed in three versions, version 1: no adjustment for potential confounders; version 2: adjusted for gender, age, and body mass index (BMI); version 3: adjusted for gender, age, BMI, alcohol consumption, and smoking. Estimated differences between BD and HC are reported for biomarker levels at T0, biomarker levels at T3, and change in biomarker level from T0 to T3.
The analyses were repeated with further stratification of BD into the participants who either had or had not experienced an episode during follow-up.
Internal validity of the measured biomarkers was evaluated by comparing their levels at baseline and follow-up in HC.
Patients with BD, who had an affective episode during follow-up
A subgroup analysis was performed to evaluate changes in biomarker levels in patients with BD, who had experienced an affective episode during follow-up. To this end, a linear mixed model with timepoint (T0, T1, T2, T3) as fixed effect and an unstructured covariance was applied. Estimates were reported for changes between the timepoints. The analysis was performed in two versions, version 1: no adjustment for potential confounders; and version 2: adjusted for gender, age, BMI, and the three mood stabilizers lithium, quetiapine, and lamotrigine.
Correlations between CSF and urinary measures of oxidative stress
Spearman and Pearson correlations were estimated between CSF and urine 8-oxoGuo and 8-oxodG at timepoint T0 and T3, and in BP and HC separately.
Effect of dose of lithium, quetiapine, lamotrigine and smoking on CSF and urinary measures of oxidative stress
To investigate the effect of the three mood stabilizers, we applied a linear mixed model as previously described 29 , which distinguishes the cross-sectional effect (i.e., the effect of the average dose of the drug over time) from the longitudinal effect (i.e., the effect of changes in the dose of the drug over time) in order to address potential biases due to unmeasured confounders. In mixed models, the effect of smoking on each of the four outcomes was estimated with inclusion of data from all four timepoints.
Sensitivity analyses
All analyses were repeated including and excluding outliers. This did not alter the results to any significant extent. We report data including outliers.
Inclusion, demographics, clinical, and study characteristics
Out of a total of 497 eligible patients with BD, 86 patients were included in the study in remission. A total of 411 did not enter the study due to: not obtaining remission before the inclusion ended in January 2016 ( n = 57), significant physical illness ( n = 97), pregnancy or planned pregnancy ( n = 62), substance abuse ( n = 26), expected noncompliance with the protocol ( n = 90), not giving informed consent ( n = 49), and discharge from the clinic before an informed consent could be obtained ( n = 30).
Demographics and clinical characteristics of the participants of the study are presented in Table 1 . A total of 24 participants (BD = 15, HC = 9) received medical treatment for a stabilized physical disorder or as hormone anticonception: hypertension (BD = 1), diabetes mellitus type II (BD = 1), hypothyroidism (BD = 3, HC = 1), and hormonal contraceptives (BD = 10, HC = 9). Patients were most frequently treated with lithium, lamotrigine, and quetiapine, but three patients did not get any psychotropic medication at inclusion.
A total of 44 HC were included in the study. Patients with BD and HC individuals were well matched according to age, gender and, BMI at baseline and there were no differences either at follow-up. There were more smokers among patients with BD at baseline and at follow-up alcohol intake was higher in HC individuals.
The flow chart (Fig. 1 ) shows that 36 patients with BD developed a new affective episode during follow-up and of these 34 reached stable remission within the study period. The completion rates from baseline to follow-up for patients with BD and HC were 65% versus 86% regarding CSF, and 70% versus 93% regarding urine samples. All together 62 patients with BD and 40 HC gave samples of both CSF and urine at baseline. All participants, but one, provided a urine sample at baseline (BD = 85, HC = 44).
Levels of cerebrospinal and urinary oxidative stress marker levels in patients with BD compared to HC
CSF-8-oxoGuo was statistically significantly higher by 18% (95% confidence interval (CI) 8–28%, adj- p = 0.003) in patients with BD versus HC at baseline, and by 22% (95% CI 12–34%, adj- p = 0) at follow-up.
Urine-8-oxoGuo was statistically significantly higher by 17% (95% CI 8–27%, adj- p = 0.003) in patients with BD versus HC at baseline, and by 30% (95% CI 16–45%, adj- p = 0) at follow-up.
CSF-8-oxodG was statistically significantly higher by 29% (95% CI 6–55%, adj- p = 0.043) at baseline, and urine-8-oxodG was statistically significantly higher by 25% (95% CI 10–43%, adj- p = 0.005) at follow-up in BD versus HC. CSF-8-oxodG was higher by 13% at follow-up and urine-8-oxodG was higher by 12% at baseline in patients with BD versus HC, but these differences were not statistically significant in the adjusted models (Fig. 2 and Table 2 ).
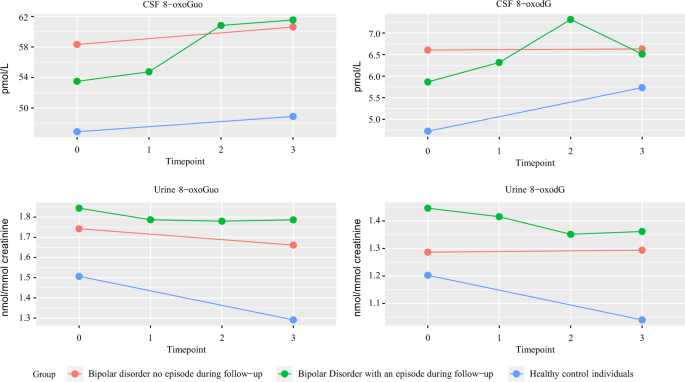
Cerebrospinal fluid and urine levels of 8-oxoGuo and 8-oxodG in patients with bipolar disorder with and without an episode during follow-up and healthy control individuals at baseline (T0), during (T1), and after (T2) an episode, did it occur and, at one-year follow-up.
When patients with BD were divided into subgroups with and without a new affective episode during follow-up, no statistically significant differences in the oxidative stress marker levels were found between the subgroups at the timepoints baseline (T0) and follow-up (T3) (Supplemental Table 1 ). Furthermore, when considering the relative changes from T0 to T3 in the subgroups with and without an episode during follow-up compared to HC, no differences were found in the relative changes between T0 and T3 (Supplemental Table 2 ).
Regarding the internal validity of the biomarkers only CSF-8-oxoGuo did not show a statistically significant change in HC during the one-year follow-up (Supplemental Table 3 ). On the contrary, CSF-8-oxodG in HC increased significantly by 22% while urine-8-oxoGuo and urine-8-oxodG decreased significantly by 15 and 14%, respectively (Supplemental Table 3 ).
Changes in levels of cerebrospinal and urinary oxidative stress marker levels in patients with BD during and following an affective episode
CSF-8-oxoGuo had increased by 15% (95% CI 4–27%, adj- p = 0.042) from T0 to T3, and by 14% (95% CI 6–23%, adj- p = 0.021) from T1 (during an episode) to T3 in patients who experienced an episode during follow-up (Table 3 ).
CSF-8-oxodG had increased by 26% (95% CI 5–51%, adj- p = 0.054) from T0 to T2 (after an episode) and decreased by 19% (95% CI −30–6%, adj- p = 0.041) from T2 to T3 in patients who experienced an episode during follow-up.
No statistically significant changes were found in the other CSF and urinary oxidative stress markers or between any of the remaining timepoints (Table 3 ).
Correlations between cerebrospinal and urinary oxidative stress markers
Measures of cerebrospinal and urinary oxidative stress markers of nucleoside damage correlated in separate analyses of all participants, patients with BD and HC (Supplemental Table 4 ).
Strong statistically significant correlations were found between CSF-8-oxoGuo and CSF-8-oxodG in patients, with BD and HC individuals at both baseline and follow-up. Weak to moderate statistically significant correlations were found between CSF-8-oxoGuo and urine-8-oxoGuo in patients, with BD and HC individuals at both baseline and follow-up. Furthermore, moderate statistically significant correlations were found between CSF-8-oxodG and urine-8-oxodG in patients, with BD and HC individuals at baseline and follow-up regarding HC individuals. However, a weak correlation between CSF-8-oxodG and urine-8-oxodG was not statistically significant in patients with BD at follow-up (Supplemental Table 4 ).
The influence of medication on oxidative stress markers
Measures of cerebrospinal and urinary oxidative stress markers of nucleoside damage tended to increase with increasing doses of lithium. The longitudinal effect, i.e., the effect of individual changes in dose of lithium was the strongest on CSF-8-oxoGuo (+0.9% per mmol increase in dose, 95% CI 0.3–1.6%, p -adj = 0.04) and remained statistically significant in multivariate analysis and after adjustment for multiple testing. A similar effect was only borderline statistically significant on CSF-8-oxodG (+1.2% per mmol increase in dose, 95% CI 0.3–2.2%, p -adj = 0.06). No significant effects of increasing doses of quetiapine and lamotrigine were found in 8oxoGuo and 8oxodG in either CSF or urine (Supplemental Table 5 ).
The influence of other covariates on oxidative stress markers in patients with BD
As predicted, measures of cerebrospinal and urinary oxidative stress markers of nucleoside damage depend on age and gender. However, there was no general effect of other covariates, including smoking and alcohol (Supplemental Table 6 ).
This study confirmed that levels of CSF-8-oxoGuo: (1) were statistically significantly higher at both baseline and follow-up in patients with BD compared to HC, (2) showed internal validity since the values in HC did not change from baseline to follow-up, (3) increased following an affective episode in patients with BD, and (4) correlated moderately with levels of urine-8-oxoGuo. Thus, cerebrospinal oxidative stress markers of RNA damage 8-oxoGuo showed both state and trait dependence in BD and stability in HC. In subgroup comparisons between patients with BD either with or without an episode during follow-up, a new affective episode was not predictable from baseline levels of oxidative stress markers.
Explorative analyses showed that increasing doses of lithium were associated with an increase of cerebrospinal and urinary oxidative stress markers, but this may likely be a result of confounding by indication (higher doses of lithium prescribed for more severe BDs). Furthermore, prior studies suggested that lithium was associated with decreased peripheral oxidative stress marker levels in euthymic patients with BD 30 , 31 , 32 .
This study found different pathophysiological characteristics in centrally and systemically generated oxidative stress. The results suggest that a possible pathogenic effect may be found down-stream from DNA, since no statistically significant differences between BD and HC were found regarding DNA damage measured by 8-oxodG, but merely in 8-oxoGuo that represents RNA damage. Also, the only weak to moderate correlation between centrally and systemically generated oxidative stress emphasize a role of the blood–brain barrier.
The findings regarding urinary oxidative stress from the present study are consistent with prior findings from our group, showing increased levels of urine markers of oxidative stress in unipolar depression 33 , BD rapid cycling 16 , BD type I 17 , and schizophrenia 34 . In these prior studies, state dependencies were found only in patients diagnosed with BD type I between the states of mania and stable remission 17 .
Increased levels of oxidative stress may predict mortality in patients with other chronic diseases such as diabetes 35 . Epidemiological studies have found that the life expectancy among patients with BD is decreased by 8–12 years compared to the general population 36 and that patients die due to natural causes of death already from adolescence 37 . It is a possibility that increased levels of oxidative stress seen in patients with BD may contribute to the decreased life expectancy by inducing accelerated ageing. The present results suggest that relapses of affective episodes may increase oxidative stress. This gives hope that effective long-term preventive treatment may contribute to normalized life expectancy in BD. Furthermore, our findings suggest that CSF oxidative stress may represent state (increased at T1) and trait markers (increased at T2 and T3) in BD, and may reflect neurobiological correlates of illness progression and sensitization 5 , 38 in BD.
Mitochondrial dysfunction reflected as increased oxidative stress may be a biological underpinning of BD 11 , 39 , and impaired autophagy has been suggested as the link between mitochondrial dysfunction and psychiatric disorders 11 .
Overall, our findings show that central RNA damage may be related to the pathogenesis of BD.
Limitations
Only a total of 36 patients (42%) experienced an affective episode during the follow-up period and the subjects had fewer repeated CSF samples than urine samples. Data were analyzed using linear mixed models, which implicitly imputes missing data from missed samples and drop outs, and provides unbiased results under the assumption that missing data is missing at random. However, results may still be biased if missingness depends on confounding factors that are not accounted for. Smoking was more prevalent in BD compared to HC. However, analyses of the effect of smoking showed no significant effect in uni- and mulitivariate analyses. Estimates and p values remained significant after adjusting for alcohol and smoking. Clinical researchers (U.K., S.G.H., and P.R.) were not blinded to the participant being BD or HC. However, they did not participate in the statistical analyses of the oxidative stress markers that for all practical reasons were blinded.
Kessing, L. V., Hansen, M. G., Andersen, P. K. & Angst, J. The predictive effect of episodes on the risk of recurrence in depressive and bipolar disorders - a life-long perspective. Acta Psychiatr. Scand. 109 , 339–344 (2004).
Article CAS Google Scholar
Lohoff F. W. B. W. Genetics of Bipolar disorder: Clinical and Neurobiological Foundations (ed Yatham, L. N. M. M.) (Wiley-Blackwell, Singapore, 2010).
Kessing, L. V., Andersen, P. K., Mortensen, P. B. & Bolwig, T. G. Recurrence in affective disorder. I. Case register study. Br. J. Psychiatry 172 , 23–28 (1998).
Kessing, L. V. & Andersen, P. K. Predictive effects of previous episodes on the risk of recurrence in depressive and bipolar disorders. Curr. Psychiatry Rep. 7 , 413–420 (2005).
Article Google Scholar
Post, R. M., Fleming, J. & Kapczinski, F. Neurobiological correlates of illness progression in the recurrent affective disorders. J. Psychiatr. Res. 46 , 561–573 (2012).
Schneider, M. R., DelBello, M. P., McNamara, R. K., Strakowski, S. M. & Adler, C. M. Neuroprogression in bipolar disorder. Bipolar Disord. 14 , 356–374 (2012).
Rizzo, L. B. et al. The theory of bipolar disorder as an illness of accelerated aging: implications for clinical care and research. Neurosci. Biobehav. Rev. 42 , 157–169 (2014).
Kessing, L. V. & Andersen, P. K. Evidence for clinical progression of unipolar and bipolar disorders. Acta Psychiatr. Scand. 135 , 51–64 (2017).
Raza, M. U., Tufan, T., Wang, Y., Hill, C. & Zhu, M. Y. DNA damage in major psychiatric diseases. Neurotox. Res. 30 , 251–267 (2016).
Cao, B. et al. Metabolic profiling for water-soluble metabolites in patients with schizophrenia and healthy controls in a Chinese population: a case-control study. World J. Biol. Psychiatry 1–11 (2019). [Epub ahead of publication].
Kim, Y. et al. Mitochondria, metabolism, and redox mechanisms in psychiatric disorders. Antioxid. Redox Signal. 31 , 275–317 (2019).
Poulsen, H. E. et al. RNA modifications by oxidation: a novel disease mechanism? Free Radic. Biol. Med. 52 , 1353–1361 (2012).
Weimann, A., Simonsen, A. H. & Poulsen, H. E. Measurement of 8-oxo-7,8-dihydro-2’-deoxyguanosine and 8-oxo-7,8-dihydro-guanosine in cerebrospinal fluid by ultra performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 1073 , 110–117 (2018).
Weimann, A., Belling, D. & Poulsen, H. E. Quantification of 8-oxo-guanine and guanine as the nucleobase, nucleoside and deoxynucleoside forms in human urine by high-performance liquid chromatography-electrospray tandem mass spectrometry. Nucleic acids Res. 30 , E7 (2002).
Brown, N. C., Andreazza, A. C. & Young, L. T. An updated meta-analysis of oxidative stress markers in bipolar disorder. Psychiatry Res. 218 , 61–68 (2014).
Munkholm, K., Poulsen, H. E., Kessing, L. V. & Vinberg, M. Elevated levels of urinary markers of oxidatively generated DNA and RNA damage in bipolar disorder. Bipolar Disord. 17 , 257–268 (2015).
Jacoby, A. S., Vinberg, M., Poulsen, H. E., Kessing, L. V. & Munkholm, K. Increased DNA and RNA damage by oxidation in patients with bipolar I disorder. Transl. Psychiatry 6 , e867 (2016).
Christensen, M. R. et al. Elevated levels of 8-oxoGuo and 8-oxodG in individuals with severe mental illness - an autopsy-based study. Free Radic. Biol. Med. 126 , 372–378 (2018).
Knorr, U. et al. Biomarkers in cerebrospinal fluid of patients with bipol ar disorder versus healthy individuals: a systematic review. Eur. Neuropsychopharmacol. 28 , 783–794 (2018).
Kessing, L. V. et al. The Bipolar Illness Onset study: research protocol for the BIO cohort study. BMJ Open 7 , e015462 (2017).
Knorr, U. et al. Increased blood BDNF in healthy individuals with a family history of depression. Psychiatry Res. 256 , 176–179 (2017).
Wing, J. K. et al. SCAN. Schedules for Clinical Assessment in Neuropsychiatry. Arch. Gen. Psychiatry 47 , 589–593 (1990).
Hamilton, M. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry 23 , 56–62 (1960).
Young, R. C., Biggs, J. T., Ziegler, V. E. & Meyer, D. A. A rating scale for mania: reliability, validity and sensitivity. Br. J. Psychiatry 133 , 429–435 (1978).
Beck, A. T., Ward, C. H., Mendelson, M., Mock, J. & Erbaugh, J. An inventory for measuring depression. Arch. Gen. Psychiatry 4 , 561–571 (1961).
Kessing, L. V. et al. Treatment in a specialised out-patient mood disorder clinic v. standard out-patient treatment in the early course of bipolar disorder: randomised clinical trial. Br. J. Psychiatry 202 , 212–219 (2013).
Rasmussen, S. T. et al. Simvastatin and oxidative stress in humans: a randomized, double-blinded, placebo-controlled clinical trial. Redox Biol. 9 , 32–38 (2016).
Benjamini, Y., Drai, D., Elmer, G., Kafkafi, N. & Golani, I. Controlling the false discovery rate in behavior genetics research. Behavi. Brain Res. 125 , 279–284 (2001).
Fitzmaurice, G. M., Laird, N. M. & Ware, J. M. Applied Longitudinal Analysis 2nd edn, ch. 9 (2011).
Bengesser, S. A. et al. Mood stabilizers, oxidative stress and antioxidative defense in Euthymia of bipolar disorder. CNS Neurol. Disord. Drug Targets 15 , 381–389 (2016).
Cui, J., Shao, L., Young, L. T. & Wang, J. F. Role of glutathione in neuroprotective effects of mood stabilizing drugs lithium and valproate. Neuroscience 144 , 1447–1453 (2007).
Machado-Vieira, R. et al. Oxidative stress parameters in unmedicated and treated bipolar subjects during initial manic episode: a possible role for lithium antioxidant effects. Neurosci. Lett. 421 , 33–36 (2007).
Jorgensen, A. et al. Systemic oxidatively generated DNA/RNA damage in clinical depression: associations to symptom severity and response to electroconvulsive therapy. J. Affect. Disord. 149 , 355–362 (2013).
Jorgensen, A. et al. Increased systemic oxidatively generated DNA and RNA damage in schizophrenia. Psychiatry Res. 209 , 417–423 (2013).
Kjaer, L. K. et al. Cardiovascular and all-cause mortality risk associated with urinary excretion of 8-oxoGuo, a biomarker for RNA oxidation, in patients with type 2 diabetes: a prospective cohort study. Diabetes Care 40 , 1771–1778 (2017).
Kessing, L. V., Vradi, E. & Andersen, P. K. Life expectancy in bipolar disorder. Bipolar Disord. 17 , 543–548 (2015).
Kessing, L. V., Vradi, E., McIntyre, R. S. & Andersen, P. K. Causes of decreased life expectancy over the life span in bipolar disorder. J. Affect. Disord. 180 , 142–147 (2015).
Post, R. M. Epigenetic basis of sensitization to stress, affective episodes, and stimulants: implications for illness progression and prevention. Bipolar Disord. 18 , 315–324 (2016).
Yoshimi, N. et al. Cerebrospinal fluid metabolomics identifies a key role of isocitrate dehydrogenase in bipolar disorder: evidence in support of mitochondrial dysfunction hypothesis. Mol. Psychiatry 21 , 1504–1510 (2016).
Download references
Acknowledgements
We thank Oda Jakobsen and Kathrine Bjarnø from the Danish Dementia Research Centre, Department of Neurology, Rigshospitalet University of Copenhagen, Jytte Rasmussen and Pia Weikop at the Neuropsychiatric Laboratory, Rigshospitalet University of Copenhagen, Allan Hansen and Anne Præstegaard, and Agnete Mehlsen and Katja Luntang Christensen from CADIC and Laboratory of Clinical Pharmacology for technical support. The study was supported by The Mental Health Services of Capital of Denmark Research Foundation, AP Møller Foundation for Promotion of Medical Science, The Beckett Foundation, The King Christian 10 th Foundation and the Max and Oda Wørzner Foundation (recipient author U.K.). The Danish Dementia Research Centre is supported by grants from the Danish Ministry of Health (J No. 2007-12143-112, project 59506/J No. 0901110, project 34501) and the Danish Health Foundation (J No. 2007B004). The funding sources of the study had no role in study design; in the collection, analysis and interpretation of data; in writing of the report; and in the decision to submit the paper for publication.
Author information
Authors and affiliations.
Copenhagen Affective Disorder Research Center (CADIC), Psychiatric Center Copenhagen, University of Copenhagen, Faculty of Health and Medical Sciences, Copenhagen, Denmark
Ulla Knorr, Ellen-Margrethe Christensen, Maj Vinberg, Rie Lambæk Mikkelsen, Thomas Kirkegaard, Rasmus Nejst Jensen & Lars Vedel Kessing
Danish Dementia Research Centre, section 6922, Rigshospitalet, University of Copenhagen, Faculty of Health and Medical Sciences, Copenhagen, Denmark
Anja Hviid Simonsen, Peter Roos & Steen Gregers Hasselbalch
Laboratory of Clinical Pharmacology, Rigshospitalet, University of Copenhagen, Faculty of Health and Medical Sciences, Copenhagen, Denmark
Allan Weimann & Trine Henriksen
Department of Clinical Pharmacology, Bispebjerg Hospital, University of Copenhagen, Faculty of Health and Medical Sciences, Copenhagen, Denmark
Allan Weimann, Trine Henriksen & Henrik Enghusen Poulsen
Section of Biostatistics, Department of Public Health, University of Copenhagen, Copenhagen, Denmark
Morten Akhøj & Julie Forman
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Ulla Knorr .
Ethics declarations
Conflict of interest.
Authors U.K., A.H.S., S.G.H., H.E.P., P.R., T.H., A.W., E.M.C., R.L.M., R.N.J., T.K., M.A., and J.F. reported no biomedical financial interests or potential conflicts of interest. E.M.C. reported having received a fee from Lundbeck for teaching a group of doctors within the last year. M.V. and L.V.K. reported having been a consultant for Lundbeck within the preceding three years.
Ethical approval
The study was approved by the Local Ethical Committee (H-6-2014-006) and the Danish Data Protection Agency, Capital Region of Copenhagen. The study complied with the latest Declaration of Helsinki.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplemetatry tables 1–6, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Knorr, U., Simonsen, A.H., Roos, P. et al. Cerebrospinal fluid oxidative stress metabolites in patients with bipolar disorder and healthy controls: a longitudinal case-control study. Transl Psychiatry 9 , 325 (2019). https://doi.org/10.1038/s41398-019-0664-6
Download citation
Received : 12 September 2019
Revised : 24 September 2019
Accepted : 01 November 2019
Published : 28 November 2019
DOI : https://doi.org/10.1038/s41398-019-0664-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative

This article is cited by
Biomarkers for neurodegeneration impact cognitive function: a longitudinal 1-year case–control study of patients with bipolar disorder and healthy control individuals.
- Anja Hviid Simonsen
- Lars Vedel Kessing
International Journal of Bipolar Disorders (2024)
Mania-related effects on structural brain changes in bipolar disorder – a narrative review of the evidence
- Christoph Abé
- Benny Liberg
- Mikael Landén
Molecular Psychiatry (2023)
Downregulation of long non-coding RNAs in patients with bipolar disorder
- Zahra Maloum
- Sahar Ramezani
- Zeinab Shirvani-Farsani
Scientific Reports (2022)
The interplay between oxidative stress and bioenergetic failure in neuropsychiatric illnesses: can we explain it and can we treat it?
- K. R. Walder
Molecular Biology Reports (2020)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
- Study protocol
- Open access
- Published: 30 January 2023
A prospective, longitudinal, case–control study to evaluate the neurodevelopment of children from birth to adolescence exposed to COVID-19 in utero
- Rachel A. Hill ORCID: orcid.org/0000-0001-6111-355X 1 , 2 ,
- Atul Malhotra 3 ,
- Vathana Sackett 3 ,
- Katrina Williams 3 , 4 , 5 ,
- Michael Fahey 3 ,
- Kirsten R. Palmer 6 , 7 ,
- Rod W. Hunt 3 , 8 ,
- Hayley Darke 1 ,
- Izaak Lim 1 , 9 ,
- Vesna Newman-Morris 1 , 9 ,
- Jeanie L. Y. Cheong 5 , 8 , 10 ,
- Clare Whitehead 5 , 11 ,
- Joanne Said 5 , 12 ,
- Paulo Bignardi 13 ,
- Evelin Muraguchi 13 ,
- Luiz Carlos C. Fernandes Jr 13 ,
- Carlos Oliveira 13 &
- Suresh Sundram 1 , 14
BMC Pediatrics volume 23 , Article number: 48 ( 2023 ) Cite this article
1906 Accesses
1 Citations
5 Altmetric
Metrics details
The Coronavirus disease (COVID-19) pandemic has created unprecedented acute global health challenges. However, it also presents a set of unquantified and poorly understood risks in the medium to long term, specifically, risks to children whose mothers were infected with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) during pregnancy. Infections during pregnancy can increase the risk of atypical neurodevelopment in the offspring, but the long-term neurodevelopmental impact of in utero COVID-19 exposure is unknown. Prospective, longitudinal studies are needed to evaluate children exposed in utero to SARS-CoV2 to define this risk.
We have designed a prospective, case-controlled study to investigate the long-term impacts of SARS-CoV2 exposure on children exposed in utero. Women infected with SARS-CoV-2 during pregnancy will be recruited from Monash Health, the Royal Women’s Hospital and Western Health (Melbourne, Australia) and Londrina Municipal Maternity Hospital Lucilla Ballalai and PUCPR Medical Clinical (Londrina, Brazil). A control group in a 2:1 ratio (2 non-exposed: 1 exposed mother infant dyad) comprising women who gave birth in the same month of delivery, are of similar age but did not contract SARS-CoV-2 during their pregnancy will also be recruited. We aim to recruit 170 exposed and 340 non-exposed mother-infant dyads. Clinical and socio-demographic data will be collected directly from the mother and medical records. Biospecimens and clinical and epidemiological data will be collected from the mothers and offspring at multiple time points from birth through to 15 years of age using standardised sample collection, and neurological and behavioural measures.
The mapped neurodevelopmental trajectories and comparisons between SARS-CoV-2 exposed and control children will indicate the potential for an increase in atypical neurodevelopment. This has significant implications for strategic planning in the mental health and paediatrics sectors and long-term monitoring of children globally.
Peer Review reports
Historically, it is well documented that infections during pregnancy increase the risk for atypical neurodevelopment in offspring such as intellectual disability, cerebral palsy, autism and schizophrenia [ 1 ]. This has been noted in large epidemiological studies following influenza and measles epidemics, with varying degrees of severity depending on the pathogen and the gestation at the time of exposure to the infection [ 2 ]. A plausible but unknown prospect are severe long-term neurodevelopmental impacts following in utero exposure to SARS-CoV-2. This highly concerning prospect must be tested to establish the absolute risk and enable early intervention.
Transplacental or vertical transmission of SARS-CoV-2 has been reported [ 3 ]. Several case reports have confirmed the presence of SARS-CoV-2 in the amniotic fluid and umbilical cord blood [ 3 , 4 , 5 ], although this appears to be rare. Limited case studies also report elevated anti-SARS-CoV-2 Immunoglobulin M (IgM) and IgG antibodies and positive nasopharyngeal swab tests in neonates born to SARS-CoV-2 infected mothers [ 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 ]. However, while vertical transmission is rare, of considerable concern is the maternal immune response to SARS-CoV-2 and the so called ‘cytokine storm’ that is a common occurrence following infection. Concern over this immune response is borne from previous ecological studies, birth cohort studies and animal models that have established key links between the activation of pro-inflammatory pathways in the mother with adverse neurodevelopment outcomes in the infant [ 2 , 12 , 13 , 14 , 15 , 16 , 17 , 18 ].
To this end, we have established a large-scale, multi-site international initiative to monitor the long-term neurodevelopmental outcomes of infants exposed to SARS-CoV-2 in utero. The aim of the study is to assess the neurodevelopmental outcomes for children exposed to SARS-CoV-2 in utero. We hypothesise that children of mothers who contracted SARS-CoV-2 infection during pregnancy will show a heightened risk for future neurodevelopmental disorders.
We describe here a prospective longitudinal protocol to assess children exposed to SARS-CoV-2 in utero at multiple key neurodevelopmental time points from birth to 15 years of age. This protocol was established at Monash University, Melbourne, Australia and adapted at the School of Medicine, Pontificia Universidade Catolica do Parana, Londrina, Brazil. We encourage international uptake of this protocol for standardised global monitoring of neurodevelopmental outcomes.
Methods / design
Aim and study setting.
The study is a case-controlled investigational assessment of the long-term impacts of SARS-CoV-2 in utero exposure on children from birth to 15 years old. Ethics approval has been obtained through Monash Health Human Research Ethics Committee RES-20–0000-801A (protocol #6, 17/03/2022) and the National Council of Research Ethics (CONEP, acronym in Portuguese) with protocol number 5.234.055. The study aligns with the SPIRIT guidelines. Women infected with SARS-CoV-2 during pregnancy are being recruited from Monash Health, the Royal Women’s Hospital and Sunshine Hospital (Melbourne sites), and Londrina Municipal Maternity Hospital Lucilla Ballalai and PUCPR Medical Clinic (Londrina, Brazil). A putative control group in a 2:1 ratio is also being recruited of women who gave birth in the same month of delivery, and are of similar age (within a 5-year age bracket) but who did not contract SARS-CoV-2 during their pregnancy. It is important to note here that there are currently no sufficiently specific or sensitive tests to differentiate past vaccination from past infection of COVID-19, and with vaccination rates over 95% in Australia we cannot definitively test if a mother has had a COVID-19 infection during their pregnancy. Therefore, this group is a putative control group based on whether the mother has reported infection with COVID-19 during the pregnancy or not. Demographic information is collected from the mother at the first visit. See Table 1 for the complete list of demographic data collected. Exclusion criteria are loss of pregnancy. Multiple births (twins) are included and matched to non-exposed multiple births. Assessments are planned at birth, 3 months, 1, 2, 3, 4, 5, 10 and 15 years (see Fig. 1 Timeline diagram).
Timeline of assessments
Maternal study specific data
For women who tested positive for COVID-19: timing of the illness (weeks of pregnancy), highest temperature recorded during illness, duration of illness and symptom severity (World Health Organisation (WHO), seven-point ordinal scale) [ 19 ], disease modifying treatments received are recorded. COVID-19 vaccination status at the first appointment is also recorded. If the participant is vaccinated, the date of each vaccination and brand of vaccine is recorded.
All mothers will complete the Edinburgh Postnatal Depression Scale (EPDS). The EPDS is a questionnaire designed to screen women for symptoms of emotional distress during pregnancy and the postnatal period [ 20 ]. This is a 10-question survey which takes approximately 5–10 min to complete. This test will be completed at the initial birth assessment as well as the 3 and 12-month follow up assessments and will be administered by the study coordinator.
All mothers will complete the Maternal Postnatal Attachment Scale (MPAS). The MPAS is 19-item self-report questionnaire to measure a mother’s subjective feelings of attachment to her infant [ 21 ]. The MPAS will be completed at birth, 3 month and 12-month time points and should take approximately 10–15 min to complete, and will be administered by the study coordinator.
Parent-completed questionnaires about the child
All parent-completed questionnaires are administered by the clinical trials coordinator through either Q Global web-based platform for test administration (Pearson Clinical Assessment, Sydney, NSW, Australia), ACER, Melbourne, Australia or Psychological Assessments Australia, NSW, Australia. A trained Allied health professional (psychologist / neuropsychologist) will score and interpret the data.
At birth, 3 and 12 months the following questionnaires will be administered:
1. The Vineland adaptive behaviour scale—Third Edition (VABS-3) is an assessment of the child’s adaptive functioning [ 22 ]. It assesses 4 domains: communication, daily living, socialisation and motor skills. The VABS takes approximately 15–20 min to complete and will be administered through Q Global web-based platform for test administration (Pearson Clinical Assessment, Sydney, NSW, Australia).
2. Sensory Profile-2 (SP-2 questionnaire) [ 23 ]: an assessment of the child’s sensory processing patterns to understand how they may be impacting their participation in home, school and community-based activities. It takes approximately 5–20 min to complete and will again be administered through the Q global web-based platform.
At 2 and 3 years of age the VABS-3 and SP-2 will be administered as well as the Child Behaviour Checklist (CBCL) and the Repetitive Behavior Scale-Revised (RBS-R).
The CBCL: Preschool Version assesses specific kinds of behavioural, emotional and social difficulties that can be experienced by pre-school and school-aged children [ 24 ]. The questionnaire is completed by parents and takes approximately 10–20 min to complete (ACER, Melbourne, Australia).
The RBS-R is a 43 item questionnaire that assesses presence and severity of stereotyped behaviour, self-injurious behaviour, compulsive behaviour, routine behaviour, sameness behaviour, and restricted behaviours, which are associated with autism [ 25 ]. The questionnaire takes approximately 5–15 min to complete and will be administered through the Q Global web-based platform.
At 4 years of age, the VABS-3, SP2, CBCL and RBS-R will be administered as well as the Children’s Communication Checklist – Second Edition (CCC-2). The CCC-2 screens children who are likely to have communication difficulties and pragmatic language impairments [ 26 ]. The questionnaire takes approximately 5–15 min to complete and will be administered through the Q global web-based platform.
At 5 years of age, the VABS-3, SP2, CBCL, RBS-R and CCC-2 will be administered as well as the Behaviour Rating Inventory of Executive Functioning (BRIEF) (child version). The BRIEF assesses aspects of executive functioning as observed in the home environment.
[ 27 ] (Psychological Assessments Australia, NSW, Australia).
At 10 and 15 years of age, the VABS-3, SP2, CBCL, RBS-R, CCC-2 and BRIEF (child version) will be administered as well as the Connors 3 rd Edition-Parent assessment of Attention Deficit / Hyperactivity disorder [ 28 ]. The Connors assessment is commonly used to assess for ADHD and its common comorbidities in children aged 6 to 18 years.
Study-specific data collected from the infant
Birth time point.
The following information will be collected at birth (or within gestational ages 40–44 weeks):
1. Anthropometry: weight, length, and head circumference.
2. Hammersmith Neonatal Neurological Examination (HNNE). The HNNE is a 34-item examination assessing tone, motor patterns, observation of spontaneous movements, reflexes, visual and auditory attention and behaviour [ 29 ]. This assessment will be scored by a health professional trained in the administration of the HNNE who is blinded of the maternal COVID-19 status, and takes approximately 10–15 min.
3. General movements assessment (GMA). The GMA is used to identify normal writhing, or abnormal cramped synchronised, poor repertoire or chaotic movements [ 30 ]. The assessment is scored from a 3–5 min video of the infant while they are lying on their back in a calm but alert state. This assessment will be scored by a health professional trained in the administration of GM’s who is blinded of the maternal COVID-19 status.
3 months (corrected age) time point
At 3 months of age (corrected for prematurity) anthropometry (weight, length, head circumference) will be recorded as well as the GMA and the Hammersmith Infant Neurological Examination (HINE). At 3 months of age the GMA is used to assess normal fidgety or absent or abnormal movement. The HINE is a neurological assessment for infants aged between 2 and 24 months. The assessment includes a neurological examination which is scored, developmental milestones and behaviour (which are not scored) [ 31 ]. The neurological examination consists of 26 items from 5 domains, including cranial nerve function, posture, quality and quantity of movements, muscle tone, and reflexes and reactions. The GMA and HINE at 3 months will be scored by a health professional trained to administer these assessments and who is blinded of the maternal COVID-19 status.
12 months (corrected age) time point
At 12 months of age, anthropometric data are collected. In addition, the following scales are administered by a trained allied health professional:
The Bayley’s Scale of Infant and Toddler Development Fourth Edition (BSID IV), which is a test of development quotient [ 32 ].
The Ages and Stages Questionnaire (ASQ-3) as well as the ASQ: social and emotional 2. This questionnaire is a developmental screening tool for children aged between one month to 5 1/2 years [ 33 ].
24 months (corrected age) time point
At 24 months of age anthropometric data will be collected and a medical examination for vision, hearing and cerebral palsy is conducted. In addition, the following scales are administered by trained health professionals (psychologist and speech pathologist) who are blinded of the maternal COVID-19 status:
1. Bayley’s Scale of Infant and Toddler Development Fourth Edition (BSID IV).
2. The Autism Diagnostic Observation Schedule-Second Edition (ADOS-2) [ 34 ]
3. Preschool Language Scales-Fourth Edition (PLS-4) [ 35 ], a test for communication skills.
3-year time point
At 3 years of age, the BSID IV, ADOS-2 and PLS-4 will be administered (as above at the 2-year time point).
4-year time point
At 4 years of age, the ADOS-2 and PLS-4 as well as the Stanford-Binet Intelligence Scale (SBIS) [ 36 ] – intelligence quotient, will be administered by trained health professionals.
5-year time point
At 5 years of age, the ADOS-2 and SBIS as well as the Clinical Evaluation of Language Fundamentals- Fourth Edition (CELF-4) [ 37 ] will be administered by trained health professionals. The CELF tests for communication and language skills for children 5 years and older.
10 and 15-year time point
At 10 years and at 15 years of age, the ADOS-2, SBIS and CELF-4 will be administered by trained health professionals.
Optional biospecimen collection
Maternal biospecimen collection.
For mothers who consent to biospecimen sample collection we will access their bio-banked samples collected during their infectious period. Blood samples and nasal mucosa will be assessed for viral load and inflammatory and cytokine marker analysis. If the mother has recovered prior to study participation biospecimens, including blood, saliva and buccal swabs, will be collected upon first visit. Blood samples will be collected by a health professional and assessed for levels of inflammatory markers [ 22 ]. Saliva will be collected to assess levels of cortisol [ 23 ]. Saliva samples are collected by the participant as soon as they wake, on the morning of their first assessment, in order to capture the waking cortisol response. Buccal swabs will be collected by a health professional and DNA will be extracted for epigenetic analysis.
Infant biospecimen collection
Parents may consent to provide a buccal swab sample from the infant. Biospecimens will be collected by a health professional at birth (or near the expected due date if born preterm). DNA will be extracted from buccal swabs for epigenetic analysis. At the time of birth, mothers who have a caesarean birth will also be given the option to consent to the collection of the umbilical cord blood and placental tissue. In cord blood and placental tissue, we will assess viral load (if infection was close to the time of birth), inflammatory and cytokine markers, mitochondrial function, and indices of mitochondrial structure and function. Additionally, placental morphology will be assessed using routine histopathological methods. Umbilical cord blood and stem and progenitor cell composition will be determined using flow cytometry.
Statistical analysis and power calculations
The data collected at each assessment will be compared between SARS-CoV-2 exposed and control groups longitudinally using a separate linear mixed effects analysis for each outcome measure. Given the number and frequency of measures there are likely to be missing datapoints, thus a mixed modelling approach will avoid the need for listwise deletion of incomplete data. Sociodemographic and clinical characteristics will be compared between groups using t-tests, Mann–Whitney U tests, or Chi-square tests as appropriate. If these confounders are statistically significant between the groups they will be included as covariates within the mixed modelling analysis. Machine learning approaches will be used to determine risk profiles based on demographic and biological data. Separate analysis will be done to split the COVID-19 group into those that scored higher than a 2 for illness severity (WHO ordinal scale) and those scoring under 2 (2 COVID-19 groups and 1 control group). Another analysis will split the COVID-19 group by those infected early in pregnancy (< 20 weeks) or late (> 20 weeks).
Power analysis using G*Power for an Analysis of Variance (ANOVA) repeated measure, between factor approach with 3 groups and 7 measures (7 assessment time points) suggests a sample size of 147 is required to have 95% power to detect a medium effect size of Cohen’s f = 0.25. To allow for potential dropouts we aim to recruit 170 mother-infant dyad cases and 340 mother-infant dyad controls to detect a medium effect.
Data management plan
Biospecimens will be stored and analysed in the laboratories at Monash Health, Monash Medical Centre, Monash University Clayton. Samples collected at Sunshine Hospital or at the Royal Women’s hospital will be stored short-term at these facilities before being transferred as a cohort to Monash Health (Behavioural neuroscience laboratory, Monash University). Samples collected will be de-identified at the time of collection and allocated a study code. This means that any information which could identify the participant, such as name, address, date of birth and hospital record number will be removed before the specimen is sent to the laboratory for analysis. We expect that all the blood, saliva and buccal swabs that we collect will be used for laboratory analysis. However, after the laboratory work has been completed, if there is any sample left over, it will either be stored at Monash Medical Centre (MMC) or discarded depending on the consent completed by the participant. Here we will give the participant the option (tick box) to either consent to immediate use, then any left over to be discarded, or to long-term storage of the samples for future unspecified use related to the study.
Maternal demographics and questionnaires will be stored in a password protected file, or in a locked cabinet held at MMC. Demographic and questionnaire data will be de-identified at the time of collection and allocated a study code. This means that any information which could identify the participant, such as name, address, date of birth and hospital record number will be removed prior to analysis. Data may only be accessed by researchers listed on the proposal.
Child developmental outcomes will be stored in a password protected file, or in a locked cabinet held at Monash Medical Centre. All assessment data will be de-identified at the time of collection and allocated a study code. This means that any information which could identify the participant, such as name, address, date of birth and hospital record number will be removed prior to analysis. Data may only be accessed by researchers listed on the proposal.
Plans for return of results of research to participants
We will generate a short summary report in lay terms following each assessment displayed as a ‘strengths and difficulties’ framework. Scores will not be shared with the parents/caregivers as assessment scores may be misinterpreted. We will ask for parent/caregivers’ permission to share data with professionals on an ‘as requested’ basis – as required for health, disability and/or education purposes.
The study is currently approved at Monash Health and Londrina participant health services. As Melbourne, Australia is currently experiencing a high prevalence of COVID-19 cases, thought to be attributed to the highly contagious Omicron strain, practical and operational issues to consider include hospital restrictions that discourage face to face participant involvement. Here, telehealth options have been explored, particularly for the 12-month assessments, which do not require neurological assessments, such as the Hammersmith neurological scales that must be done in person. For the birth and 3-month assessments, extra precautions have been planned, including personal protective equipment and social distancing compliance.
Another operational issue to consider is that with the high vaccination rates now in Victoria (~ 95% people aged 15 and over double vaccinated) and Brazil (~ 93% of the population have received 2 doses as at 09/03/2022), there is likely to be variation in that data, with some women having received 1, 2 or 3 doses and some being unvaccinated. This has been included in the study design as a question in the demographics; ‘Are you vaccinated? If so when? and How many doses?’. However, depending on the numbers this will need to be considered as a variable when analysing the data. We would anticipate that women who have been vaccinated will have a less severe course of illness, which will be reported through the WHO 7-point ordinal scale. These data will allow us to assess this anticipated hypothesis.
Our study design is such that biospecimen samples are collected at birth (or as close to birth as possible), then we will map our biomarker findings onto the neurodevelopmental trajectory of the child. For some women, the collection of biospecimens will be only shortly after they have been infected with COVID-19, while for others, they may have been infected early in their pregnancy. This variation in the time since infection is a limitation of the study. However, we have also linked this project to a COVID-19 biobank established through Monash Health, which collects mucosal swabs and serum samples at the time of infection. While not all participants will consent to both studies, these data will provide us with a unique opportunity to map biomarkers during infection to the neurodevelopmental trajectory of the child.
Overall, this established protocol will allow longitudinal, prospective analysis of the neurodevelopment of children exposed in utero to SARS-CoV-2 to determine the risk that COVID-19 infection during pregnancy poses to the infant. A secondary set of outcomes will be the biological findings from our biospecimen collections and consequent mapping of biological changes on the child’s neurodevelopmental trajectory. These data may provide valuable new knowledge on biomarkers or risk pathways of neurodevelopmental disturbances. The scales used in this study have been validated across multiple cultures, ensuring global uptake feasibility. With collaborations established in Londrina, Brazil, we call for international uptake of this protocol to inform health care professionals globally of the risk of COVID-19 infection during pregnancy to the neurodevelopment of the infant.
Availability of data and materials
The datasets generated during and/or analysed during the current study are not publicly available yet due to the majority of the data not collected yet but are available from the corresponding author on reasonable request.
Abbreviations
Severe acute respiratory syndrome coronavirus 2
Immunoglobulin M
Immunoglobulin G
Coronavirus disease of 2019
Edinburgh postnatal depression scale
Maternal postnatal attachment scale
Vineland adaptive behavior scale – 3 rd edition
Sensory profile-2
Child behavior checklist
Repetitive Behavior Scale-Revised
Children’s communication checklist – 2 nd edition
Behaviour Rating Inventory of Executive Functioning
Hammersmith neonatal neurological examination
Hammersmith infant neurological examination
General movement assessment
Bayley’s Scale of Infant and Toddler Development Fourth Edition
Ages and Stages Questionnaire 3 rd edition
Autism Diagnostic Observation Schedule-Second Edition
Preschool Language Scales-Fourth Edition
Stanford-Binet Intelligence Scale
Clinical Evaluation of Language Fundamentals- Fourth Edition
World health organisation
Analysis of variance
Brown AS. Epidemiologic studies of exposure to prenatal infection and risk of schizophrenia and autism. Dev Neurobiol. 2012;72(10):1272–6.
Article Google Scholar
Brown AS, Begg MD, Gravenstein S, Schaefer CA, Wyatt RJ, Bresnahan M, Babulas VP, Susser ES. Serologic evidence of prenatal influenza in the etiology of schizophrenia. Arch Gen Psychiatry. 2004;61(8):774–80.
Hosier H, Farhadian SF, Morotti RA, Deshmukh U, Lu-Culligan A, Campbell KH, Yasumoto Y, Vogels CB, Casanovas-Massana A, Vijayakumar P, et al. SARS-CoV-2 infection of the placenta. J Clin Invest. 2020;130(9):4947–53.
Article CAS Google Scholar
Dong L, Tian J, He S, Zhu C, Wang J, Liu C, Yang J. Possible vertical transmission of SARS-CoV-2 from an infected mother to her newborn. JAMA, J Am Med Assoc. 2020;323(18):1846–8.
CAS Google Scholar
Pulinx B, Kieffer D, Michiels I, Petermans S, Strybol D, Delvaux S, Baldewijns M, Raymaekers M, Cartuyvels R, Maurissen W. Vertical transmission of SARS-CoV-2 infection and preterm birth. Eur J Clin Microbiol Infect Dis. 2020;39(12):2441–5.
Patane L, Morotti D, Giunta MR, Sigismondi C, Piccoli MG, Frigerio L, Mangili G, Arosio M, Cornolti G. Vertical transmission of coronavirus disease 2019: severe acute respiratory syndrome coronavirus 2 RNA on the fetal side of the placenta in pregnancies with coronavirus disease 2019-positive mothers and neonates at birth. Am J Obstet Gynecol MFM. 2020;2(3):100145.
Tolu LB, Ezeh A, Feyissa GT. Vertical transmission of severe acute respiratory syndrome coronavirus 2: a scoping review. PLoS ONE. 2021;16(4):e0250196.
Zamaniyan M, Ebadi A, Aghajanpoor S, Rahmani Z, Haghshenas M, Azizi S. Preterm delivery, maternal death, and vertical transmission in a pregnant woman with COVID-19 infection. Prenat Diagn. 2020;40(13):1759–61.
Alzamora MC, Paredes T, Caceres D, Webb CM, Valdez LM, La Rosa M. Severe COVID-19 during pregnancy and possible vertical transmission. Am J Perinatol. 2020;37(8):861–5.
Kumar R, Yeni CM, Utami NA, Masand R, Asrani RK, Patel SK, Kumar A, Yatoo MI, Tiwari R, Natesan S, et al. SARS-CoV-2 infection during pregnancy and pregnancy-related conditions: concerns, challenges, management and mitigation strategies-a narrative review. J Infect Public Health. 2021;14(7):863–75.
Zeng H, Xu C, Fan J, Tang Y, Deng Q, Zhang W, Long X. Antibodies in infants born to mothers with COVID-19 pneumonia. JAMA J Am Med Assoc. 2020;323(18):1848–9.
Anderson G, Berk M, Dodd S, Bechter K, Altamura AC, Dell’osso B, Kanba S, Monji A, Fatemi SH, Buckley P, et al. Immuno-inflammatory, oxidative and nitrosative stress, and neuroprogressive pathways in the etiology, course and treatment of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2013;42:1–4.
Meyer U, Feldon J. Epidemiology-driven neurodevelopmental animal models of schizophrenia. Prog Neurobiol. 2010;90(3):285–326.
Atladottir HO, Thorsen P, Schendel DE, Ostergaard L, Lemcke S, Parner ET. Association of hospitalization for infection in childhood with diagnosis of autism spectrum disorders: a Danish cohort study. Arch Pediatr Adolesc Med. 2010;164(5):470–7.
Zerbo O, Qian Y, Yoshida C, Grether JK, Van de Water J, Croen LA. Maternal infection during pregnancy and autism spectrum disorders. J Autism Dev Disord. 2015;45(12):4015–25.
Patterson PH. Immune involvement in schizophrenia and autism: etiology, pathology and animal models. Behav Brain Res. 2009;204(2):313–21.
Atladottir HO, Thorsen P, Ostergaard L, Schendel DE, Lemcke S, Abdallah M, Parner ET. Maternal infection requiring hospitalization during pregnancy and autism spectrum disorders. J Autism Dev Disord. 2010;40(12):1423–30.
Mednick SA, Machon RA, Huttunen MO, Bonett D. Adult schizophrenia following prenatal exposure to an influenza epidemic. Arch Gen Psychiatry. 1988;45(2):189–92.
Coronavirus disease (COVID-2019) R&D. Geneva: World Health Organization; 2020. https://www.who.int/blueprint/prioritydiseases/key-action/novel-coronavirus/en/ .
Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh postnatal depression scale. Br J Psychiatry. 1987;150:782–6.
Condon JT, Corkindale CJ. The assessment of parent-to-infant attachment: development of a self-report questionnaire instrument. J Reprod Infant Psychol. 1998;16(1):57–76.
Sparrow SS, Cicchetti DV, Saulnier CA. Vineland adaptive behavior scales. 3rd ed. San Antonio TX: Psychological Corporation; 2016.
Google Scholar
Dunn W. Sensory profile 2. User’s manual. Bloomington: Pearson; 2014.
Achenback T, Edelbrock C. The child behavior checklist manual. Burlington, VT: The University of Vermont; 1991.
Bodfish JW, Symons FJ, Parker DE, Lewis MH. Varieties of repetitive behavior in autism: comparisons to mental retardation. J Autism Dev Disord. 2000;30(3):237–43.
Bishop DV. The children's communication checklist (CCC-2): CCC-2 manual (2nd ed.). London: Harcourt Assessment; 2003.
Gioia GA, Isquith PK, Guy SC, Kenworthy L. Test review behavior rating inventory of executive function. Child Neuropsychol. 2000;6(3):235–8.
Conners CK. Conners’ rating scales-revised technical manual. North Tonawanda, NY: Multi Health Systems; 1997.
Dubowitz L, Mercuri E, Dubowitz V. An optimality score for the neurologic examination of the term newborn. J Pediatr. 1998;133(3):406–16.
Morgan C, Crowle C, Goyen TA, Hardman C, Jackman M, Novak I, Badawi N. Sensitivity and specificity of General Movements Assessment for diagnostic accuracy of detecting cerebral palsy early in an Australian context. J Paediatr Child Health. 2016;52(1):54–9.
Novak I, Morgan C, Adde L, Blackman J, Boyd RN, Brunstrom-Hernandez J, Cioni G, Damiano D, Darrah J, Eliasson AC, et al. Early, accurate diagnosis and early intervention in cerebral palsy: advances in diagnosis and treatment. JAMA Pediatr. 2017;171(9):897–907.
Bayley N. Manual for the bayley scales of infant development. New York: Psychological Corportation; 1969.
Valleley RJR, B.M.: Review of Ages & Stages Questionnaires: A Parent-Completed Child Monitoring System, Third Edition. Lincoln, NE: Buros Institute of Mental Measurements; 2010.
Lord C, Rutter M, DiLavore P, Risi S, Gotham K, Biship S. Autism diagnostic observation schedule - 2nd edition (ADOS-2). Los Angeles, CA: Western Psychological Corporation; 2012.
Zimmerman IL, Steiner VG, Pond RE. Preschool Language Scale 4th. San Antonio, TX: The Psychological Corporation; 2002.
Roid GH. Stanford-Binet Intelligence Scales. 5. Rolling Meadows, IL: Riverside Publishing; 2003.
Semel E, Wiig EH, Secord WA. Clinical evaluation of language fundamentals (4). Amsterdam: Pearson; 2010.
Download references
Acknowledgements
We would like to thank the Clinical Trials Facility at Monash Health Translation Precinct for facilitating biospecimen collections.
This research is supported on a research grant awarded by the philanthropic organisation, One in Five, as well as internal departmental research funds. The external funding body has not played a role in the design, collection, analysis or interpretation of data or the writing of this manuscript or the decision to submit this manuscript.
Author information
Authors and affiliations.
Department of Psychiatry, School of Clinical Sciences at Monash Health, Monash Medical Centre, Monash University, Level 3, 27-31 Wright St, Clayton, VIC, 3168, Australia
Rachel A. Hill, Hayley Darke, Izaak Lim, Vesna Newman-Morris & Suresh Sundram
Florey Institute for Neuroscience and Mental Health, University of Melbourne, Parkville, VIC, Australia
Rachel A. Hill
Department of Paediatrics, Monash University, Clayton, VIC, Australia
Atul Malhotra, Vathana Sackett, Katrina Williams, Michael Fahey & Rod W. Hunt
Department of Paediatrics, The University of Melbourne, Parkville, VIC, Australia
Katrina Williams
Department of Obstetrics and Gynaecology, The University of Melbourne, Parkville, VIC, Australia
Katrina Williams, Jeanie L. Y. Cheong, Clare Whitehead & Joanne Said
Monash Women’s, Monash Health, Clayton, VIC, Australia
Kirsten R. Palmer
Department of Obstetrics and Gynaecology, Monash University, Clayton, VIC, Australia
Clinical Sciences, Murdoch Children’s Research Institute, Parkville, VIC, Australia
Rod W. Hunt & Jeanie L. Y. Cheong
Monash Medical Centre, Early in Life Mental Health Service, Monash Health, Clayton, VIC, Australia
Izaak Lim & Vesna Newman-Morris
Department of Neonatal Services, Royal Women’s Hospital, Parkville, VIC, Australia
Jeanie L. Y. Cheong
Department of Obstetrics and Gynaecology, Royal Women’s Hospital, Parkville, VIC, Australia
Clare Whitehead
Maternal Fetal Medicine, Joan Kirner Women’s & Children’s at Sunshine Hospital, Western Health, Sunshine, VIC, Australia
Joanne Said
School of Medicine, Pontifical Catholic University of Paraná, Londrina, Paraná, Brazil
Paulo Bignardi, Evelin Muraguchi, Luiz Carlos C. Fernandes Jr & Carlos Oliveira
Mental Health Program, Monash Health, Melbourne, VIC, Australia
Suresh Sundram
You can also search for this author in PubMed Google Scholar
Contributions
All authors have read and approved the manuscript. RH conceptualised and designed the study, wrote the study design and ethics submission, obtained philanthropic funding and will lead the data collection. AM assisted in the study design, ethics submission and the collection of neonatal data, and manuscript editing. VS assisted in the study design and the collection of neonatal data. KW assisted with study design and manuscript editing. MF assisted with study design and manuscript editing. KP assisted with study design and recruitment and manuscript editing. RH assisted with study design and manuscript editing. HD assisted with study design, ethics submission and manuscript editing. IL assisted in study design and manuscript editing. VNM assisted in study design and manuscript editing. JC assisted in study design, ethics submission and manuscript editing. CW assisted in study design, ethics submission and manuscript editing. JS assisted in study design, ethics submission and manuscript editing. PB adapted the protocol and coordinated the ethics submission for the Brazilian site, manuscript editing. EM adapted the protocol and assisted with the ethics submission for the Brazilian site. LF adapted the protocol and assisted with the ethics submission for the Brazilian site. CO adapted the protocol and assisted with the ethics submission for the Brazilian site. SS co-conceptualised the study and assisted in the study design, ethics submission and manuscript editing.
Corresponding author
Correspondence to Rachel A. Hill .
Ethics declarations
Ethics approval and consent to participate.
Ethics has been approved through Monash Health Human Research Ethics Committee RES-20–0000-801A. All participants sign a consent form prior to participation in the study. Upon signing the consent form, they consent to the collection of the above described information (methods/ design) for themselves and their child. Individual tick boxes have been included for all biospecimen collections (blood, saliva and buccal swab) as these are optional. Consent for long-term storage of biospecimen samples is also included as a tick box.
Ethics for Brazilian site: Ethics has been approved through the National Council of Research Ethics (CONEP, acronym in Portuguese) under protocol no. 5.234.055. All participants sign a consent form prior to participation in the study. Upon signing the consent form, they consent to the collection of the above-described information (methods/ design) for themselves and their child. Individual tick boxes have been included for all biospecimen collections (blood, saliva and buccal swab) as these are optional.
Consent for publication
Data will be published as population data and will not include any individual details, images or videos. Therefore, consent for publication is not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Hill, R.A., Malhotra, A., Sackett, V. et al. A prospective, longitudinal, case–control study to evaluate the neurodevelopment of children from birth to adolescence exposed to COVID-19 in utero. BMC Pediatr 23 , 48 (2023). https://doi.org/10.1186/s12887-023-03858-w
Download citation
Received : 13 April 2022
Accepted : 19 January 2023
Published : 30 January 2023
DOI : https://doi.org/10.1186/s12887-023-03858-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Neurodevelopment
- Prospective
BMC Pediatrics
ISSN: 1471-2431
- Submission enquiries: [email protected]
- General enquiries: [email protected]
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Mov Disord Clin Pract
- v.10(4); 2023 Apr
- PMC10105110

Levodopa Equivalent Dose of Safinamide: A Multicenter, Longitudinal, Case–Control Study
Roberto cilia.
1 Department of Clinical Neurosciences, Parkinson and Movement Disorders Unit, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milan Italy
Emanuele Cereda
2 Clinical Nutrition and Dietetics Unit, Fondazione IRCCS Policlinico San Matteo, Pavia Italy
Marco Piatti
3 Neurology Unit, Department of Neurology, Milan Center for Neuroscience, San Gerardo Hospital, Monza Italy
4 Centro Parkinson e Parkinsonismi, ASST Gaetano Pini‐CTO, Milan Italy
Andrea Pilotto
5 Neurology Unit, Department of Clinical and Experimental Sciences, University of Brescia, Brescia Italy
Luca Magistrelli
6 Department of Translational Medicine, Movement Disorders Centre, Neurology Unit, University of Piemonte Orientale, Novara Italy
Nico Golfrè Andreasi
Salvatore bonvegna, elena contaldi, francesca mancini.
7 IRCCS, Department of Neurology‐Stroke Unit and Laboratory of Neuroscience – Milan, Istituto Auxologico Italiano, Milan Italy
Gabriele Imbalzano
8 Department of Neuroscience "Rita Levi Montalcini", University of Torino, Turin Italy
9 SC Neurologia 2U, AOU Città della Salute e della Scienza, Turin Italy
Rosa De Micco
10 Department of Advanced Medical and Surgical Sciences, University of Campania “Luigi Vanvitelli”, Naples Italy
Fabiana Colucci
11 Azienda Ospedaliera Univerisitaria S. Anna, U.O. Neurologia, Ferrara Italy
12 University of Ferrara, Ferrara Italy
Arianna Braccia
Gabriele bellini.
13 Unit of Neurology, Department of Clinical and Experimental Medicine, University of Pisa, Pisa Italy
Francesco Brovelli
Roberta zangaglia.
14 Parkinson's Disease and Movement Disorders Unit, IRCCS Mondino Foundation, Pavia Italy
Giulia Lazzeri
15 Neurology Unit, Department of Neuroscience, Dino Ferrari Center, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milan Italy
Maria Claudia Russillo
16 Department of Medicine, Surgery and Dentistry, Scuola Medica Salernitana, Neuroscience Section, University of Salerno, Italy
Enrica Olivola
17 Parkinson and Movement Disorders Unit, IRCCS Neuromed, Pozzilli Italy
Chiara Sorbera
18 IRCCS Centro Neurolesi “Bonino‐Pulejo”, Messina Italy
Viviana Cereda
19 Department of Neurological Rehabilitation, Parkinson's Disease and Movement Disorders Center, Moriggia‐Pelascini Hospital, Gravedona ed Uniti, Gravedona Italy
Patrizia Pinto
20 Neurology Unit, ASST Papa Giovanni XXIII, Bergamo Italy
Patrizia Sucapane
21 Neurology Unit, San Salvatore Hospital, L'Aquila Italy
Giorgio Gelosa
22 Neurology Unit, ASST “Grande Ospedale Metropolitano” Niguarda, Milan Italy
Mario Meloni
23 IRCCS Fondazione Don Carlo Gnocchi ONLUS, Milan Italy
Francesca Pistoia
24 Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, L'Aquila Italy
Maria Sessa
Margherita canesi, nicola modugno, claudio pacchetti, laura brighina, maria teresa pellecchia, roberto ceravolo, mariachiara sensi, maurizio zibetti, cristoforo comi, alessandro padovani, anna l. zecchinelli, alessio di fonzo, alessandro tessitore, francesca morgante.
25 Neuroscience Research Centre, Molecular and Clinical Sciences Institute, St. George's, University of London, London UK
26 Department of Clinical and Experimental Medicine, University of Messina, Messina Italy
Roberto Eleopra
Associated data.
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Effects of dopaminergic medications used to treat Parkinson's disease (PD) may be compared with each other by using conversion factors, calculated as Levodopa equivalent dose (LED). However, current LED proposals on MAO‐B inhibitors (iMAO‐B) safinamide and rasagiline are still based on empirical approaches.
To estimate LED of safinamide 50 and 100 mg.
In this multicenter, longitudinal, case–control study, we retrospectively reviewed clinical charts of 500 consecutive PD patients with motor complications and treated with (i) safinamide 100 mg ( N = 130), safinamide 50 mg ( N = 144), or rasagiline 1 mg ( N = 97) for 9 ± 3 months and a control group of patients never treated with any iMAO‐B ( N = 129).
Major baseline features (age, sex, disease duration and stage, severity of motor signs and motor complications) were similar among the groups. Patients on rasagiline had lower UPDRS‐II scores and Levodopa dose than control subjects. After a mean follow‐up of 8.8‐to‐10.1 months, patients on Safinamide 50 mg and 100 mg had lower UPDRS‐III and OFF‐related UPDRS‐IV scores than control subjects, who in turn had larger increase in total LED than the three iMAO‐B groups. After adjusting for age, disease duration, duration of follow‐up, baseline values and taking change in UPDRS‐III scores into account (sensitivity analysis), safinamide 100 mg corresponded to 125 mg LED, whereas safinamide 50 mg and rasagiline 1 mg equally corresponded to 100 mg LED.
Conclusions
We used a rigorous approach to calculate LED of safinamide 50 and 100 mg. Large prospective pragmatic trials are needed to replicate our findings.
From 1990 to 2015, the prevalence of Parkinson's Disease (PD) doubled, and, keeping this similar growth rate, models of prediction estimate nearly 13 million people will be affected by 2040. 1 Although no effective disease‐modifying therapy is available yet, the best medical treatment of PD patients consists of a combination of multiple medications acting synergistically to compensate for motor disability and improve patients’ quality of life. 2
Although several drugs have been developed and marketed over the past two decades to provide better personalized therapy for PD patients, 3 Levodopa remains the gold standard of symptomatic treatment. Currently, the total dose of dopaminergic therapy taken by a PD patient can be obtained by summing the Levodopa equivalent dose (LED) of different types of anti‐PD drugs, such as dopamine agonists, monoamine oxidase‐B inhibitors (iMAO‐B) and catechol‐O‐methyl transferase inhibitors, (iCOMT). 4 LED conversion stemmed from the need to allow comparison of different treatment regimens in randomized clinical trials (RCTs) and has become increasingly useful in routine clinical practice to adjust patients’ therapy without inducing a negative effect on the overall clinical status. 4 , 5
Conversion factors may be used to switch from one dopaminergic drug to another within the same class (eg, dopamine agonists, iMAO‐B, iCOMT) or between different classes (eg, replacing a dopamine agonist with a iMAO‐B), or to allow compensatory increase of one drug while tapering another (eg, increasing Levodopa to reduce/withdraw dopamine agonist due to incident impulse control disorders or initiating device‐aided treatments). 5 This minimizes the risk for either overdosing and causing medication‐induced side effects or underdosing with subsequent increase of OFF‐related disability.
Safinamide is a novel effective reversible iMAO‐B with both dopaminergic and nondopaminergic (including glutamate release modulation) mechanisms of action, that indicated as add‐on treatment to levodopa in fluctuating PD patients. 6 , 7 To date, there is no reliable information on LED of Safinamide at both 50 and 100 mg/day. It has been recently proposed that both safinamide 50 mg and safinamide 100 mg should be converted into 100 mg LED. 8 , 9 However, it has been clearly acknowledged that the major limitation of these proposed LED calculations so far is that they are “based on clinical experience and empirical approaches” without scientific and objective data, inclusive of their own proposal on safinamide. 8
In the present study, we collected real‐life data on a large PD population to obtain a reliable calculation of LED of safinamide at a dose of 50 and 100 mg, as compared to control patients never treated with any iMAO‐B. In addition, we included a group of patients treated with rasagiline 1 mg, whose LED had been proposed to correspond to 100 mg despite the lack of data on dose equivalence, 4 aiming to either confirm or update this conversion.
Materials and Methods
Patient selection.
We included patients who had received a clinical diagnosis of idiopathic PD 10 and presented motor fluctuations and/or dyskinesias. We included subjects who received either (i) safinamide 100 mg, or (ii) safinamide 50 mg, or (iii) rasagiline 1 mg as add‐on therapy to levodopa for at least 6 months and had a follow‐up visit between 6 and 12 months (9 ± 3 months) after the initiation of iMAO‐B. As control group, we included (iv) patients with motor fluctuations and/or dyskinesias who had never been treated with any iMAO‐B. 11 We excluded: (i) PD patients without motor complications, (ii) those on treatment with any iMAO‐B at baseline, or (iii) device‐aided therapies (deep brain stimulation or infusion therapies), (iv) atypical or secondary parkinsonism.
Study Design
This retrospective, longitudinal, case–control study was conducted at 20 movement disorders centers throughout Italy. Movement disorders specialists at each participating center retrospectively reviewed demographic and clinical data from the electronic repositories from all consecutive PD patients visited between April 1, 2016, and October 31, 2019. Cases were excluded if the medical records did not contain well‐documented reports. General demographic (age, sex, body weight) and clinical data such as motor phenotype, 12 age of onset, disease duration, Unified Parkinson Disease Rating Scale (UPDRS) from part I to part IV, 13 and the Hoehn & Yahr stage that were already contained in clinical charts of patients were extracted and analyzed. In addition, items of UPDRS motor examination (Part III, collected in the ON‐medication state during the outpatient visit) were used to investigate dopaminergic and non‐dopaminergic deficiency scores, which indicate levodopa‐responsive features vs. axial impairment, respectively. 14 Data on all PD therapies were obtained to calculate the number of daily Levodopa intakes, total Levodopa daily dose (mg/day), Levodopa dose adjusted for body weight (mg/kg/day) and for iCOMT, total LED from dopamine agonists (DA, mg/day) and the final total‐LED excluding iMAO‐B (mg/day). 4 Data on amantadine and anticholinergics were collected. We additionally extracted from UPDRS parts I and II data on non‐levodopa‐responsive axial complications and UPDRS part IV subscores for dyskinesias and OFF periods. 15 Anonymized patient data were extracted from medical records and recorded into an electronic case report form.
The primary objective was to estimate LED of safinamide 100 mg by calculating the difference in change at follow‐up in total LED between patients on this regimen and the control group. Secondary objectives included (i) to estimate LED of safinamide 50 mg and (ii) LED of rasagiline 1 mg in comparison to the control group; (iii) to investigate whether the use of iMAO‐B was associated with a reduction of concomitant PD medications; (iv) to compare among groups the difference in change at follow‐up of motor clinical variables according to the UPDRS.
The study was approved by the ethics committee of each participating center (coordinating center ethics committee: Neurological Institute Carlo Besta, Milan; CE n.68/2019) and conducted in accordance with the declaration of Helsinki and local regulatory requirements, including written informed consent to the use of patient anonymized clinical data for research purposes.
Statistical Analysis
The sample size calculation was based on the primary endpoint (comparison of change in total LED from dopaminergic therapy between patients receiving safinamide 100 mg as add‐on therapy vs. patients receiving standard dopaminergic therapy without iMAO‐B medications). At baseline, it was expected a mean ± SD total LED of approximately 500 ± 350 mg/day. 11 It has been calculated that at least 86 patients in each group will be required to detect a meaningful difference in the change of total LED at follow‐up. This was based on a statistical power of 90% [Type II error], a medium effect size of 0.5 and a two‐tailed test with a 5% significance level [Type I error]. Two additional groups of 86 subjects each were included, the former including patients treated with safinamide 50 mg/day (to calculate its LED, as secondary objective) and the latter including patients treated with rasagiline 1 mg (as active control product). Therefore, the minimum sample size was planned to be 344 patients.
Analyses were performed with the software STATA 15 or subsequent versions (StataCorp, College Station, TX, USA). Two‐tailed p values <0.05 will indicate statistical significance. Descriptive statistics of categorical variables are presented as counts and percentages, while continuous variables are reported as mean and standard deviation or median and inter‐quartile range [25th–75th percentile (inter‐quartile range, IQR)] according to the normality of distribution (checked using the Kolmogorov–Smirnov test). To minimize selection bias, all eligible patients were included consecutively without matching a priori for baseline characteristics; between‐group changes from baseline in continuous variables were analyzed using repeated‐measure linear regression model adjusted for disease duration, age at assessment, duration of follow‐up and the baseline value of each parameter. Huber‐White robust standard errors were used to account for study center.
A sensitivity analysis was conducted on patients showing stability in UPDRS‐Part III, defined as a change between the 25th and the 75th percentile of its distribution at follow‐up visit.
We collected data on a total population of 509 PD patients. Of these, six were excluded due to incomplete clinical data and three because of exclusion criteria (one was on selegiline at baseline and two had early PD with neither fluctuations nor dyskinesias). A total cohort of 500 patients was suitable for statistical analysis, distributed as follows: Safinamide 100 mg ( N = 130), Safinamide 50 mg ( N = 144), Rasagiline 1 mg ( N = 97), and PD controls never treated with any iMAO‐B ( N = 129).
Demographic and clinical data of the study population are shown in Table 1 . At baseline, the four study groups had similar demographic (age, sex distribution, body weight) and clinical features (disease duration, severity of motor signs according to UPDRS‐III score and H&Y staging, motor phenotype, prevalence, and severity of motor complications according to UPDRS‐IV scores, namely OFF‐periods and Levodopa‐induced dyskinesias, LIDs). Compared to controls, patients on rasagiline had lower mean UPDRS‐II scores ( p = 0.005) and marginally lower total LED ( p = 0.046), mainly due to lower mean dose of Levodopa immediate release. There were no significant differences between the groups in non‐levodopa‐responsive motor complications.
Clinical and treatment characteristics of the study population by use of Monoamine Oxidase type B Inhibitors
Abbreviations: CR, Levodopa controlled release; COMT, catechol‐O‐methyltransferase; DA, dopamine agonists; iMAO‐B, MonoAmine Oxidase type B Inhibitors; IR, Levodopa immediate release; LED, levodopa equivalent dose; SD, standard deviation; UPDRS, Unified Parkinson's Disease Rating Scale.
Changes in clinical features and pharmacological treatment at follow‐up are shown in Tables 2 and and3, 3 , respectively. After a mean follow‐up of 8.8‐to‐10.1 months, patients on safinamide 50 mg and 100 mg (but not those on rasagiline 1 mg) had lower UPDRS‐III scores than controls ( p < 0.001), specifically concerning dopaminergic scores. This effect was greater with safinamide 100 mg compared to safinamide 50 mg, despite not reaching statistical significance. There were no significant changes in non‐dopaminergic motor features induced by iMAO‐B.
Follow‐up clinical data (adjusted change) of the study population by use of Monoamine Oxidase type B Inhibitors
Abbreviations: COMT, catechol‐O‐methyltransferase; DA, dopamine agonists; iMAO‐B, Monoamine Oxidase type B Inhibitors; SD, standard deviation; SE, standard error; UPDRS, Unified Parkinson's Disease Rating Scale.
Data on pharmacological treatment at the end of follow‐up data of the study population by use of Monoamine Oxidase type B Inhibitors
Abbreviations: COMT, catechol‐O‐methyltransferase; DA, dopamine agonists; iMAO‐B, Monoamine Oxidase type B Inhibitors; LED, levodopa equivalent dose; SD, standard deviation; SE, standard error; UPDRS, Unified Parkinson's Disease Rating Scale.
Concerning motor complications, the three iMAO‐B groups had lower mean UPDRS‐IV scores related to OFF‐periods than controls and similar dyskinesias scores. The prevalence of patients complaining about OFF‐periods and LIDs (UPDRS‐IV OFF‐related items and LIDs items ≠ 0, respectively) showed similar, albeit nonsignificant, trends for lower prevalence of OFF‐periods in all iMAO‐B groups (Fig. 1A ) and greater frequency of LIDs reported by control subjects (Fig. 1B ). During follow‐up, patients in the control group without iMAO‐B had larger increase in total LED ( p < 0.001) compared to the three iMAO‐B groups, particularly due to higher Levodopa dose. This was paralleled by a relative increase in new associations of iCOMT and amantadine in control subjects than the three iMAO‐B groups (Table 3 ). In particular, the use of safinamide 50 and 100 mg allowed to keep stable Levodopa dose adjusted for COMT inhibitors over time (significant difference compared to controls), whereas the rasagiline group did not differ from controls (Table 3 ). Direct comparison between safinamide 50 versus, 100 mg, safinamide 50 mg versus, rasagiline 1 mg, safinamide 50 mg versus, rasagiline 1 mg did not yield any significant difference.

Prevalence of motor fluctuations (panel A) and dyskinesias (panel B) in the study population at baseline (gray color) and follow‐up (white color). No iMAO indicates the control group of patients who never received iMAO‐B.
Sensitivity Analysis
Considering the significant effect played by the association of iMAO‐B on motor performance (as assessed by the UPDRS‐III, Table 2 ), a sensitivity analysis was conducted on patients reporting substantial stability in UPDRS‐Part III at follow‐up visit ( N = 271), which was performed on the following groups: safinamide 50 mg, N = 78; safinamide 100 mg, N = 67; rasagiline, N = 51; control subjects, N = 75 (Table 3 ). Although crude sensitivity analysis showed larger effects of safinamide 50 and 100 mg (but not rasagiline) than control subjects on the dose of Levodopa immediate release and total LED, the adjusted analysis confirmed that total LED remained significantly lower in the three iMAO‐B groups than in the control group ( p < 0.001, Table 3 ).
LED Calculation
According to our methodological approach to LED calculation using the mean difference in total LED change between each active group and the control group, data obtained from the primary analysis (after approximating by 6–8 mg/day) would be consistent with the conversion of all active groups to Levodopa 100 mg (Table 3 ). After adjusting for the effect of iMAO‐B on motor performance (sensitivity analysis), we obtained the following conversion factors (after approximating by 5–6 mg/day): Safinamide 100 mg = 1.25 (125 mg LED), Safinamide 50 mg = 2 (100 mg LED), Rasagiline 1 mg = 100 (100 mg LED) (Fig. 2 , Table 3 ).

Adjusted mean difference [95%CI] in total LED change between each active group versus the control group used to calculate LED of Safinamide 50 mg, Safinamide 100 mg, and Rasagiline 1 mg. Each column represents the mean change in total LED between baseline and follow‐up for each study group, adjusted for disease duration, age at assessment, duration of follow‐up and the baseline value. No iMAO indicates the control group of patients who never received iMAO‐B.
This multicenter study was specifically designed to calculate the conversion formula of LED of safinamide 50 and 100 mg on a large PD population, using a novel method that takes into account not only changes in dopaminergic medications (ie, total LED) but the clinical effects achieved. On the one hand, considering that total LED increases over time in PD patients with early fluctuations on medical therapy, 16 our calculation of LED was based on the comparison between the change in total LEDD between each iMAO‐B group versus, control group, thus including not only the reduction of dopaminergic medications at follow‐up (as previous studies on LED) but also the therapy adjustment over time. On the other hand, our effort to provide an objective measure of LED included the observation that motor performance in the ON state (change in UPDRS‐III score in the ON‐medication state between baseline and follow‐up) may differ between different iMAO‐B type and dosage, reflecting a change in the dopaminergic boost. Accordingly, a formula predicting the longitudinal changes of levodopa dose requirements using real‐world UPDRS‐III scores has been recently proposed, 17 confirming how LED and UPDRS‐III scores are closely related. It is worth mentioning that we designed a priori a short follow‐up observation period (9 ± 3 month) to minimize the confounding effect of disease progression on therapy adjustment and motor scores. In contrast with previous findings, 18 we found that safinamide 50 and 100 mg provided a significant improvement of UPDRS‐III scores ON‐medication, basically due to reduced dopaminergic score. 14 Why is this relevant? Let us consider an outpatient with suboptimal control of tremor and bradykinesia whose UPDRS‐III score is 18 with the drugs A + B and receives the add‐on drug C showing a 9‐point improvement of UPDRS‐III at follow‐up; the patient is satisfied and pharmacological therapy does not require any further change. How do we calculate the LED of C? Clearly, we cannot estimate it just by considering a reduction induced by C on total LED obtained from the A + B regimen, which did not occur in this case. As safinamide is an add‐on treatment for motor fluctuations, its association might not be followed by any change in concomitant medications at follow‐up. If we had not considered the change in UPDRS‐III by performing the sensitivity analysis, Safinamide 50 and 100 mg would have shared a similar 100 mg LED. Accordingly, we found a conversion factor of 1.25 for Safinamide 100 mg, which is 25% greater than the one currently used. 8 , 9 updating and overcoming the recent proposal to consider both safinamide 100 mg and 50 mg equal to Levodopa 100 mg despite the difference between the two dosages in terms of clinical effects, 7 , 19 , 20 including MDS‐UPDRS‐III scores. 19 Safinamide 50 mg and rasagiline 1 mg are equal to Levodopa 100 mg, which agrees with currently used estimates. 4 , 8 , 9
Providing reliable LED conversion factors aims to minimize patient discomfort whenever major therapy adjustments are needed. To our opinion, these conversion formulae represent a step forward in literature on LED. First, our study overcomes the “ pseudo‐validity ” of all existing LED proposals, which are based on personal experience of individual neurologists and approaches far from being evidence‐based. 8 Our study is an attempt to fill this gap and provide a framework for future studies aiming to provide objective measures of LED conversion formulae. Although previous RCTs on safinamide provided data on the relative changes in Levodopa dosage between the baseline and the end‐of‐study visits, none of them provided sufficient details on daily dose at baseline and/or on changes in other dopaminergic drugs (dopamine agonist and iCOMT) to allow any indirect inference on the conversion factor of safinamide. 7 , 19 , 20 , 21 , 22 , 23 Indeed, most studies limited their report on the relative number of patients on dopamine agonists and iCOMT neither reporting their LEDs at baseline nor their relative change at the end of the study. Second, it is worth highlighting that this is the first study supporting the conversion factor of 100 for rasagiline using an ad hoc study design. So far, rasagiline 1 mg has been considered equivalent to 100 mg Levodopa despite data on its dose equivalence had never been provided. 4 In a previous 3‐year retrospective case–control study, the use of rasagiline was associated with a levodopa dose reduction of about 100 mg/day compared with patients who had never been treated with any MAO‐B inhibitor, 11 indirectly supporting the present data.
Our findings provide useful information on the effects of iMAO‐B that are shared by safinamide and rasagiline. First, MAO‐B inhibition significantly reduced daily OFF periods without increasing LIDs, confirming data obtained from RCTs and meta‐analyses. 24 Second, iMAO‐B provided evidence supporting their effectiveness in routine clinical practice. Indeed, their use is associated with some significant changes at follow‐up, such as (i) lower dose of levodopa‐based medications, (ii) lower OFF‐state frequency and severity, and (ii) an overall simplification of the therapeutic scheme, as reflected by the lower prescription of iCOMT and amantadine at follow‐up compared to control subjects, thus reducing the cumulative risk and severity of motor complications as well as adverse events. It should be noted that the similar severity of dyskinesias between those on iMAO‐B and controls might have been masked by the relative increase of amantadine use in the control group.
There are limitations to acknowledge. The retrospective nature of the study intrinsically harbors potential prescription bias, such as the preference of clinicians to keep a simplified therapeutic regimen without iMAO‐B in patients with psychosis and the slightly lower UPDRS‐II scores and total LED at baseline in patients on rasagiline than control subjects. However, it is unlikely that these minor differences played a confounding effect of the results, because (i) the four groups had similar major demographic and clinical features (age, sex, motor phenotype, disease duration and severity) and (ii) all analyses were adjusted for several potential confounders, such as disease duration, age at assessment, duration of follow‐up and the baseline value of each parameter. Nevertheless, prospective pragmatic real‐world clinical trials on large cohorts of PD patients with early motor fluctuations are warranted to replicate our results. On the other hand, this design may also be considered a strength of the study as it allowed us to collect real‐life data on consecutive patients that is relatively less biased than the data obtained from more homogeneous but selected cohorts reported in clinical trials. Another strength is the large population of 500 patients recruited by neurologists with heterogeneous prescription patterns from 20 movement disorders clinics throughout Italy, which further increase the generalizability of our results.
In conclusion, according to the results of the present study, we propose that safinamide 100 mg corresponds to 125 mg of Levodopa, whereas safinamide 50 mg and rasagiline 1 mg equally correspond to 100 mg of Levodopa. Future studies aiming to define LED of dopaminergic drugs should apply rigorous methods and use real‐life data on a large PD population.
Author Roles
(1) Research project: A. Conception, B. Organization, C. Execution; (2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; (3) Manuscript Preparation: A. Writing of the first draft, B. Review and Critique.
R.C.: 1A, 1B, 1C, 2A, 2C, 3A, 3B.
E.C.: 1A, 2A, 2B, 3B.
A.P.: 1C, 3B.
N.G.A.:1C, 3B.
F.M.: 1C, 3B.
R.D.M.: 1C, 3B.
F.C.: 1C, 3A.
G.L.: 1C, 3B.
M.C.R.: 1C.
M.M.: 1C, 3B.
F.P.: 1C, 3B.
M.T.P: 1B, 3B.
R.C.: 1C, 3B.
M.C.S.: 1C, 3B.
M.Z.: 1B, 3B.
A.L.Z.: 1B.
A.D.F.: 1B, 3B.
A.T.: 1B, 3B.
F.M.: 1B, 3B.
R.E.: 1B, 3B.
Disclosures
Ethical Compliance Statement: We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines. Ethics Committee of the coordinating center: Fondazione IRCCS IStituto Neurologico Carlo Besta, Milano; reference number: CE n.68/2019. The study was approved by the ethics committee of each participating center and conducted in accordance with the declaration of Helsinki and local regulatory requirements, including written informed consent to the use of patient anonymized clinical data for research purposes.
Funding Sources and Conflicts of Interest: All the authors report no conflict of interest related to this manuscript.
Financial Disclosures for the Previous 12 Months: RCil has received speaking honoraria from Zambon Italia; Zambon SAU; Bial Italia Srl; Advisory board fees from Bial; Research support from the Italian Ministry of Health; Editor‐in‐chief of the neuromuscular and movement disorders section of Brain Sciences; Member of the editorial board of Parkinsonism and related disorders and Frontiers in Neurology. EC has received speaking honoraria from Zambon Italia. APil is supported by IMI H2020 initiative (IMI2‐2018‐15‐06) paid to the university of Brescia Italian Ministry ofHealth; he received lecture honoraria from Bial, Biomarin, Abbvie, Chiesi, Roche and Zambon Italia (payments made to AP as an individual); he received research support from Bial, Biomarin, Abbvie, Chiesi, and Zambon pharmaceuticals (payment made to the Institution University of Brescia). FP has received speaking honoraria from Novartis. MTP has received compensation for consultancies from Zambon Italia, Theravance, Teva, Orion. RCer has received speaking honoraria from Zambon Italia, Abbvie, Lusofarmaco, General Electric. MZ has received speaking honoraria from Medtronic, Bial, and AbbVie. APad is consultant and served on the scientific advisory board of GE Healthcare, Eli‐Lilly and Actelion Ltd. Pharmaceuticals and received speaker honoraria from Nutricia, PIAM, Langstone Technology, GE. Healthcare, Lilly, UCB Pharma and Chiesi Pharmaceuticals. He is funded by grant of the Ministry of University (MURST). FMor has received speaking honoraria from Abbvie, Medtronic, Zambon. SpA, Bial, Merz; Travel grants from the International Parkinson's disease and Movement Disorders. Society; Advisory board fees from Merz; Consultancies fees from Merz and Bial; Research support from Boston Scientific Merz and Global Kinetic; Royalties for the book “Disorders of Movement” from Springer; member of the editorial board of Movement Disorders, Movement Disorders. Clinical Practice, European Journal of Neurology. All other authors report no financial disclosures.
Acknowledgment
Open access funding provided by BIBLIOSAN.
Data Availability Statement

IMAGES
VIDEO
COMMENTS
Revised on June 22, 2023. A case-control study is an experimental design that compares a group of participants possessing a condition of interest to a very similar group lacking that condition. Here, the participants possessing the attribute of study, such as a disease, are called the "case," and those without it are the "control.".
Earlier articles in this series described classifications in research design, 1 prospective and retrospective studies, cross-sectional and longitudinal studies, 2 and cohort studies. 3 This article considers a research design that is often used in present-day research in medicine and psychiatry: the case-control study.
Case-control studies compare groups retrospectively, while longitudinal studies can compare groups either retrospectively or prospectively. In a longitudinal study, researchers monitor a population over an extended period of time, and they can be used to study developmental shifts and understand how certain things change as we age. In addition, case-control studies look at a single subject or ...
Case-control studies compare groups retrospectively and cannot be used to calculate relative risk. Longitudinal studies, though, can compare groups either retrospectively or prospectively. In case-control studies, researchers study one group of people who have developed a particular condition and compare them to a sample without the disease.
Revised on June 22, 2023. In a longitudinal study, researchers repeatedly examine the same individuals to detect any changes that might occur over a period of time. Longitudinal studies are a type of correlational research in which researchers observe and collect data on a number of variables without trying to influence those variables.
A classic example of longitudinal case study is the study of Middletown (a mid-sized city in Midwestern United States) by Lynd and Lynd , once in mid-1920s and again in mid-1930s. Though it is basically a descriptive case study, the primary focus of the authors was the transition from an agricultural to an industrial economy, which occurred in ...
Case-control studies are particularly useful for studying the cause of an outcome that is rare and for studying the effects of prolonged exposure. For example, a case-control study could be used ...
A case-control study is a type of observational study commonly used to look at factors associated with diseases or outcomes.[1] The case-control study starts with a group of cases, which are the individuals who have the outcome of interest. The researcher then tries to construct a second group of individuals called the controls, who are similar to the case individuals but do not have the ...
Case-control studies for longitudinal data are considered. Among repeated binary. measurements of disease status in each subject, the exposure levels of risk factors for all. diseased cases are identified and the exposure levels for only a small fraction of disease-free. cases, to be regarded as controls, are identified.
Cross sectional studies. A cross sectional study measures the prevalence of health outcomes or determinants of health, or both, in a population at a point in time or over a short period. Such information can be used to explore aetiology - for example, the relation between cataract and vitamin status has been examined in cross sectional surveys.
We detail study design options that generalize case-control sampling when longitudinal outcome data are already collected as part of a primary cohort study, but new exposure data must be retrospectively processed for a secondary analysis. Furthermore, we assume that cost will limit the size of the subsample that can be evaluated.
A longitudinal study (or longitudinal survey, or panel study) is a research design that involves repeated observations of the same variables (e.g., people) over long periods of time (i.e., uses longitudinal data).It is often a type of observational study, although it can also be structured as longitudinal randomized experiment. This research design assumes an alternative name 'panel studies ...
A case-control study (also known as case-referent study) is a type of observational study in which two existing groups differing in outcome are identified and compared on the basis of some supposed causal attribute. Case-control studies are often used to identify factors that may contribute to a medical condition by comparing subjects who have the condition with patients who do not have ...
Cohort Study: A longitudinal study that begins with the gathering of two groups of patients (the cohorts), one that received the exposure (e.g., to ... (prospective) to measure the development of different outcomes (diseases). Case-Control Study: A type of research that retrospectively compares characteristics of an individual who has a certain ...
The study is a case-controlled investigational assessment of the long-term impacts of SARS-CoV-2 in utero exposure on children from birth to 15 years old. Ethics approval has been obtained through Monash Health Human Research Ethics Committee RES-20-0000-801A (protocol #6, 17/03/2022) and the National Council of Research Ethics (CONEP ...
The aim of this longitudinal case-control study was to investigate variables associated with caries development from birth to 36 months. By 30 months (n = 608) there were 24 children (4%) who had caries and an additional 23 developed first caries at 36 months (n = 552), giving a total prevalence of 47 children with caries (9%) at 36 months ...
Cytokine concentrations throughout pregnancy and risk for psychosis in adult offspring: a longitudinal case-control study Lancet Psychiatry. 2020 Mar;7 ... Methods: The National Collaborative Perinatal Project was a large-scale prospective longitudinal study that assessed the effects of perinatal factors on infant and child development. At ...
This transdiagnostic longitudinal case-control study will provide a multimodal neurophysiological assessment of clinical dimensions (sensorimotor integration, speech, sleep, and sense of self) that are disrupted across mood, psychosis, and autism spectrum disorders. It will further test the relevance of EEG microstates as dimensional ...
Cohort studies and case-control studies are two primary types of observational studies that aid in evaluating associations between diseases and exposures. In this review article, we describe these study designs, methodological issues, and provide examples from the plastic surgery literature. Keywords: observational studies, case-control study ...
As the first, this naturalistic prospective, longitudinal follow-up case-control study investigated cerebrospinal fluid (CSF) oxidative stress markers 8-oxo-7,8-dihydroguanosine (8-oxoGuo) and 8 ...
Prospective, longitudinal studies are needed to evaluate children exposed in utero to SARS-CoV2 to define this risk. We have designed a prospective, case-controlled study to investigate the long-term impacts of SARS-CoV2 exposure on children exposed in utero. ... A prospective, longitudinal, case-control study to evaluate the neurodevelopment ...
In this multicenter, longitudinal, case-control study, we retrospectively reviewed clinical charts of 500 consecutive PD patients with motor complications and treated with (i) safinamide 100 mg (N = 130), safinamide 50 mg (N = 144), or rasagiline 1 mg (N = 97) for 9 ± 3 months and a control group of patients never treated with any iMAO-B (N ...
This retrospective, longitudinal, case-control study was conducted at 20 movement disorders centers throughout Italy. Movement disorders specialists at each participating center retrospectively reviewed demographic and clinical data from the electronic repositories from all consecutive PD patients visited between April 1, 2016, and October 31 ...