
One Platform for All Your Needs
Image-pro is now the most advanced 64-bit scientific image analysis platform available with capabilities evolved beyond its predecessors..
Newly designed base. New modular options.
AutoQuant Deconvolution
Autoquant is now available in image-pro, with image-pro 11 and its autoquant deconvolution module, you can quickly deconvolve and visualize multi-channel image sets in time and z—from right within image-pro..
- Get set-up faster with a streamlined dialog
- Save time with built-in GPU acceleration
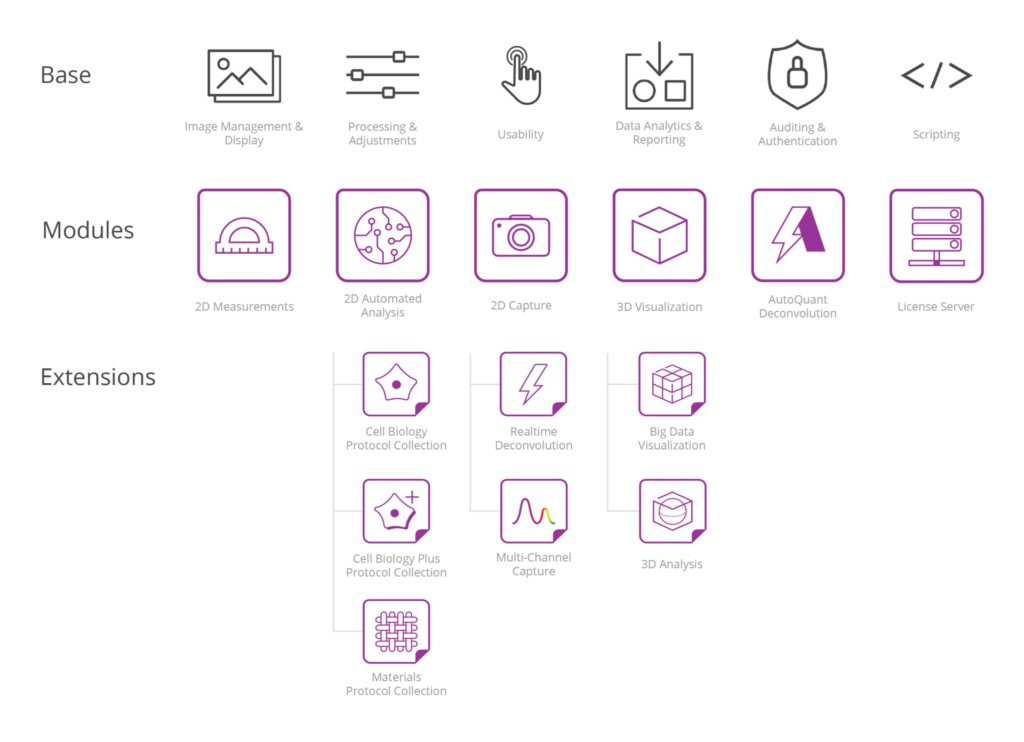
Analysis Protocols
Image-pro’s library of protocols is rapidly expanding and making image analysis easier than ever. each protocol is packed with powerful analysis tools designed to deliver flexible and intuitive options., powered by machine learning, multi-channel by design, customizable across similar routines, easy, repeatable performance, fast analysis in parallel, relevant data at your fingertips, analysis protocol collections.

Cell Biology
Cell Biology Plus
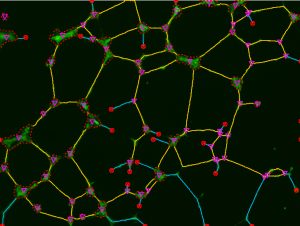
Fluorescent Capture Options
Supercharge your manual microscope, retrofit your existing microscope to support image capture with multi-channel capture and real-time deconvolution., multi-channel capture, whether in manual or auto mode, this extension to the 2d capture module will expand any microscope and make it capable of capturing multiple channels into an image set. whether capturing single snaps or movies, simply set up your channels, start the capture, and image-pro will prompt you to change the filter or light source when needed. finish the session and your set is combined, color-tinted, and ready to view or measure. a simple, yet powerful way to capture..
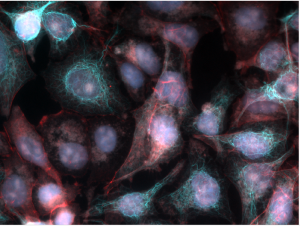
Real-Time Deconvolution
The optional real-time deconvolution extension leverages industry-leading autoquant technology to cost-effectively “upgrade” your existing widefield microscope to obtain images almost as clear as those from more expensive confocal microscopes. elevate your capture system to now deliver crisp, high fidelity, fluorescent images during preview and capture., improved usability, flexible licensing, manage all your licenses using a new online account, link any license to a computer without a usb dongle, optionally link to a license server or usb dongle as needed.
Search & Help
Search for help files & recent items with a click, dock the new help panel to keep guidance within reach, activate dynamic help to auto-display relevant topics, train users quickly with written & video documentation, looking for more information, give us a call today to speak to one of our media cybernetics specialists who can help answer questions and find the perfect solution for your application., (281) 579-0342.
- Houston, TX 77084
- 281-579-0342
- info@meyerinst.com
- Microscope Slide Art
- Custom Services
- Microscope Cleaning
- News & Press Releases
- ACCU-SCOPE RC500 Motorized Remote Control Microscope
- ACCU-SCOPE, Upright
- ACCU-SCOPE, Inverted
- Motic, Upright
- Motic, Inverted
- Panthera, Upright
- Labomed, Upright
- Labomed, Inverted
- Meiji, Upright
- ioLight Portable Microscopes
- Unitron, Upright
- Unitron, Inverted
- Motic, Panthera TEC MAT
- Labomed Pol
- Motic Panthera TEC POL
- Instec, Heating and Cooling Stages
- Linkam, Heating and Cooling Stages
- Bioptechs, Heating Stages
- Sutter Instruments
- Prior Scientific, XYZ Motorization
- C-Mount Adapters for Nikon ® & Olympus ® Microscopes
- C-Mount Adapters for Leica ® & Zeiss ® Microscopes
- X-Cite Fluorescence Illuminators – Coming Soon
- 89 North, PhotoFluor LM-75
- Labomed Prima Art
- Labomed Prima Trainer
- Promicra – Z Motorization
- C-Mount Adapters
- NIGHTSEA Fluorescence Adapter
- Sunflower LED Illuminator
- Littlite LED Illuminators
- Techniquip LED Illuminators
- Infinity Lenses
- PATHpix XLS
- Magnify2, Image Gallery
- Magnify2, Videos
- Ash OMNI 3 Core Macroscope
- Ash INSPEX 3 Macroscope
- Motic EasyStitch Pro – manual slide stitching software
- ACCU-Slide MS – manual slide stitching software
- Panoptiq – manual slide stitching software
- C-Mounts for Microscopes
- Kaiser Copy Stands
- DeepVision NIR-II/SWIR
- Heliosonix – OptoAcoustic Imaging Device
- HREM – 3D High Resolution Episcopic Microscope
- Image-Pro® v11
- AutoQuantX3, Deconvolution Software
- QuickPHOTO Micro
- QuickPHOTO Industrial
- QuickPHOTO Camera
- QuickPHOTO Deep Focus Module
- QuickPHOTO High Dynamic Range Module
- QuickPHOTO RECORD IT Module
- Figure Maker
- Simultaneous EDF and HDR Imaging
- StrataQuest
- PathScan Enabler 5
- Motic EasyScan One
- Motic EASYSCAN FL3
- Motic EasyScan FS-Live
- Motic EasyScan Pro 6
- Motic EasyScan Infinity
- MoticFlow Telepathology Platform
- Glissando POL
- TissueFAXS Fluo
- TissueFAXS i Fluo
- TissueFAXS Histo
- TissueFAXS i Histo
- TissueFAXS PLUS
- TissueFAXS SL
- TissueFAXS Q & SL Q Systems
- TissueFAXS SPECTRA
- OptraSCAN OS-Lite
- OptraSCAN OS-Ultra
- OptraSCAN OS-FS
- OptraSCAN OS-SiA
- OptraSCAN OS-FL
- OptraSCAN OS-FLi
- HemoLens 1, Oil Immersion Slide Scanner
- MorphoLens 1
- MorphoLens 6
- MorphoLens 240
- Accu-Slide MS – Slide Stitching
- EasyStitch Pro – Slide Stitching Software
- Panoptiq – Slide Stitching Software
- Microscope Slide Scanning Service
- Instec Heating and Cooling Stages
- Linkam Heating and Cooling Stages
- Metkon Servocut 602 Multi-Target Automatic Cutting Machine
- Metkon Servocut 402
- Metkon Servocut 302

Your Full Name (required)
Telephone Number
Your Email (required)
What can we help you with?
Microscope Stereomicroscope Slide Scanner Nightsea Fluoresence Kaiser Copy Stands GIGAmacro Illuminators C Mounts Sutter Pipette Pullers Motorized Stages Promicra Linkam Stages Media Cybernetics FREE Media Cybernetics Trial Digital Camera Rentals Other Training
Can you be more specific?
Toll Free: (855) BIO-IMAG (855) 246-4624
International: +1-416-826-4616

- $USD 0.00 0
- Customer Help
Want to chat?
Call us toll free +1 416 826 4616
- X (Twitter)

Image Pro Premier Software
$USD 5,645.00
2D IMAGE ANALYSIS SOFTWARE Capture, process, count, classify, measure and share. A powerful and customizable imaging platform driven by over 30 years of user feedback, the new Image-Pro Premier offers native 64-bit support, a user friendly interface and a suite of 2D measurement solutions. Rely on our worldwide network of Image-Pro partners, superb training tools, and technical support team to help you get started and keep your research moving forward.
Free worldwide shipping on all orders over $50
- 30 days easy returns
- Order yours before 2.30pm for same day dispatch

Product in question
Your Organization
Your Phone Number
Your message (optional)
- Introduction
- Description
- Specification
- Configuration
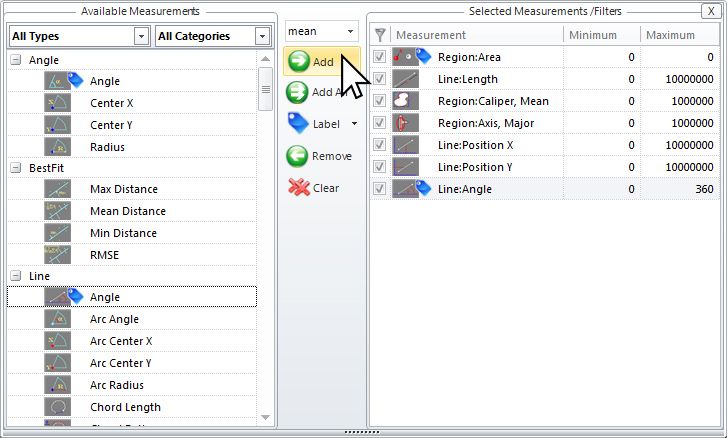
Automatic Measurements
Segment, count/size, classify, and analyze.
Image-Pro’s technique for performing Automatic Measurements is the foremost solution for gathering data from images by segmentation systems. Our simple step-wise approach to the problem is designed to provide the ultimate flexibility to analyze nearly any image type while remaining simple enough to quickly learn and teach to others. 1 Segment Identify what you want to measure in the image 2 Count/Size Create object outlines, instantly counted and sized 3 Classify Separate objects into custom groups by parameter 4 Analyze Generate data for making helpful comparisons
Identify what you want to measure
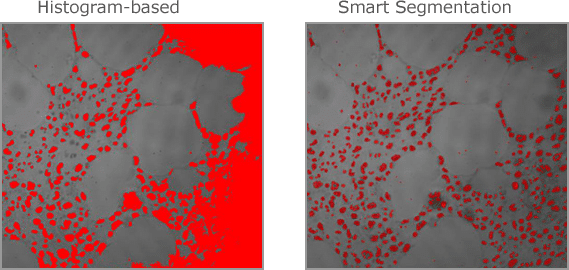
Histogram-based Segmentation
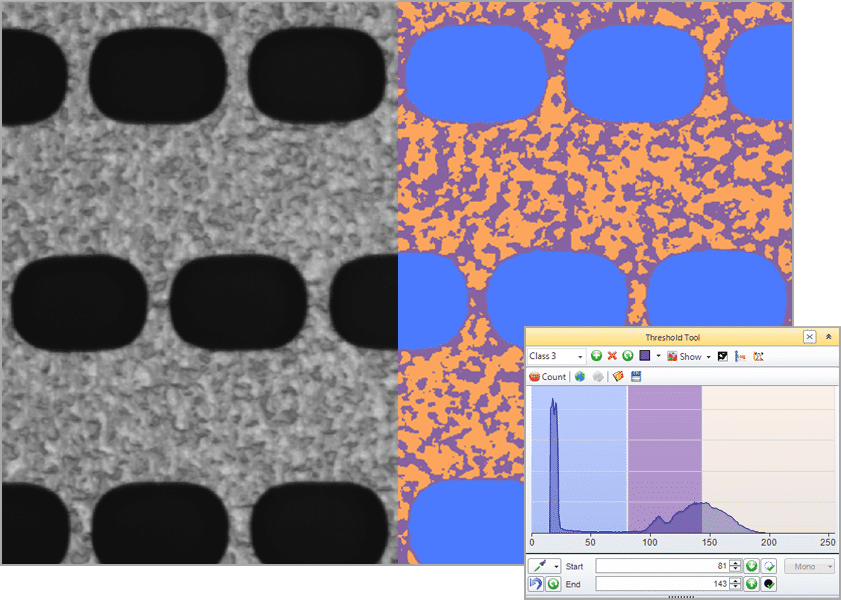
Select a single intensity range
Select multiple intensity ranges (classes), select with the picker, smart segmentation.
A revolutionary new method that employs a pixel classification algorithm able to identify hard to segment objects and regions. Use Smart Segmentation to identify faintly-colored objects, textured objects, and objects or regions on uneven backgrounds.
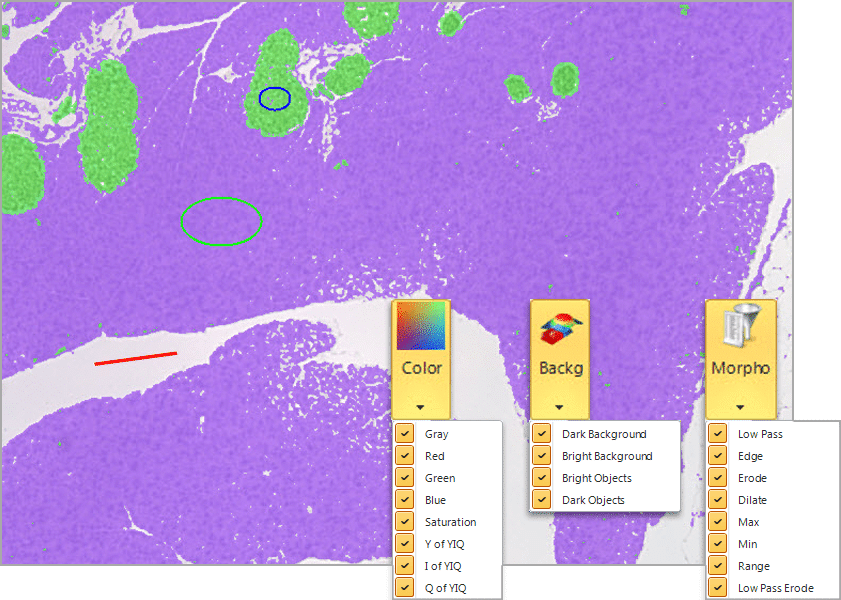
Select objects and background
Define the recipe parameters, train over multiple images, smart segmentation solves common problems..
Automatically compensate for uneven backgrounds
Select two regions, and Smart Segmentation will automatically calculate the difference in the unevenly illuminated background. This is normally very difficult with histogram-based methods as objects will typically be the same intensity as some areas of the background.
Select & Classify Objects by Color
Locate and segment objects based on their color. Create new classes to further characterize and streamline data collection and reporting. Creating classes is easy, and doesn’t have a limit to how many you can create, in order to make it easy to organize important objects.
2 COUNT/SIZE
Create object outlines, instantly counted and sized
Filter by Measurement
Choose from a wide number of measurements to filter the entire segmentation group from and apply specific range restrictions (graphically or numerically) that selectively leave your objects of interest for further classification and measurement.
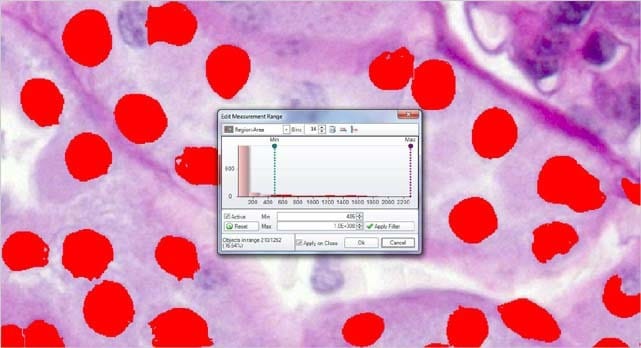
Area = ( 486 – Max ) Objects in range = 210
Filter out objects to be counted and sized by any number and combinations of parameters.
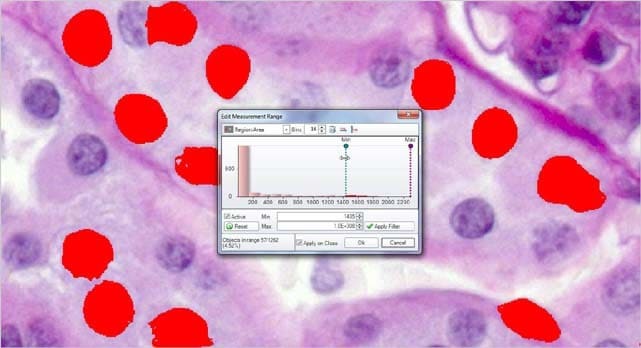
Area = ( 1486 – Max ) Objects in range = 57
Reduce the segmented objects down to the proper number and then press COUNT/SIZE.
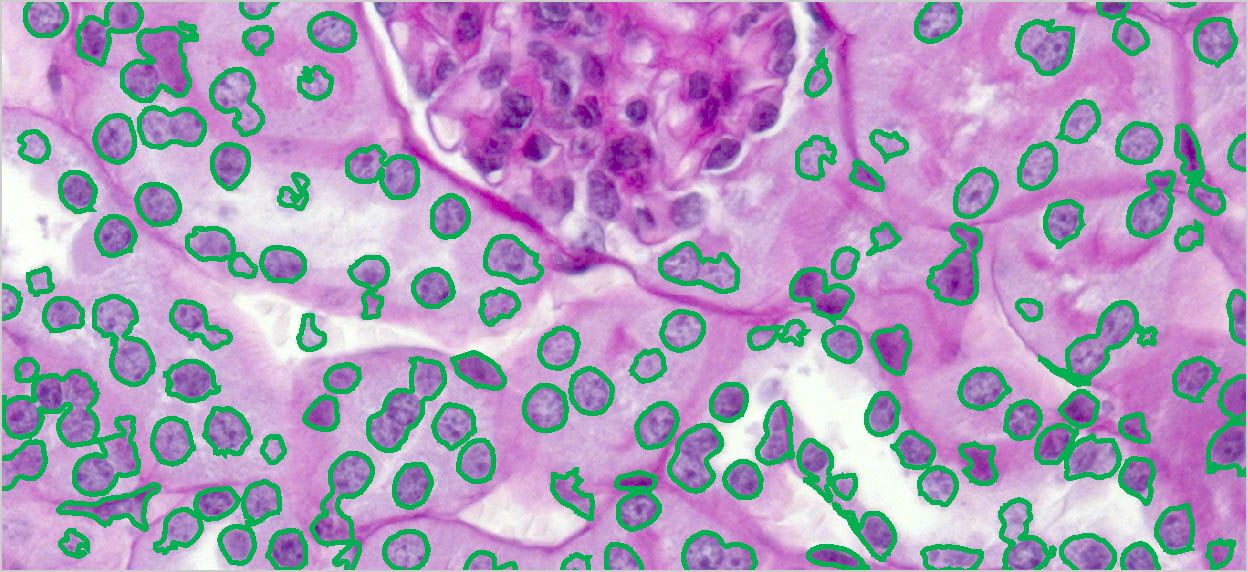
Create object outlines.
Once objects are identified and outlined you can count and measure areas, percent area, regions, intensity values, and more.
Count Number All discrete, separate objects can be automatically counted within the set range of intensities.
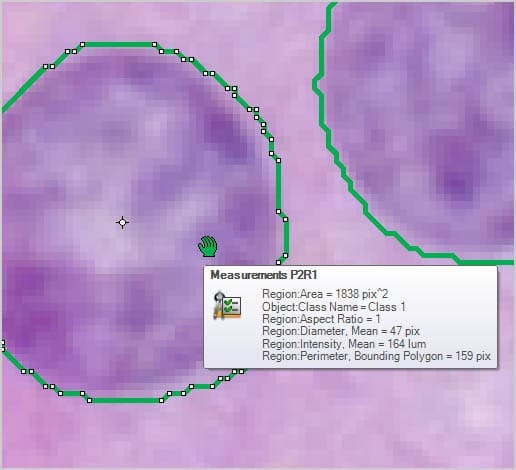
Size and Shape Automatically measure object area, percent area, perimeter, length & width, radii and feret ratio, etc.
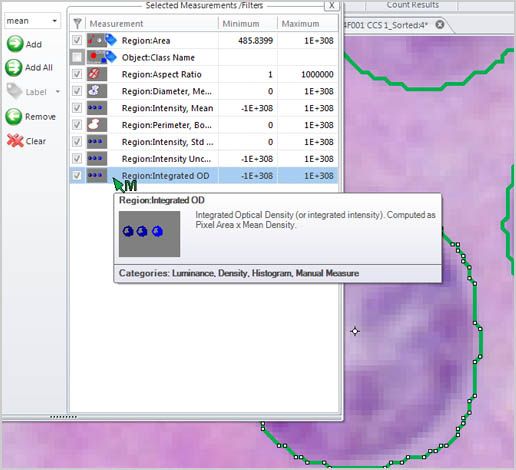
Intensity and Density Automatically measure object intensity, integrated optical density, density, and intensity over time.
Object Splitting & Merging It becomes necessary to split touching objects in many images so we’ve provided both automatic watershed and boundary shape-based splitting techniques as well as a manual point-to-point and polyline-based splitting methods to get the job done.
Object naming and coloring This makes it easier to keep track of what’s what by allowing each object to receive a unique name and color through the editing of the data table. Change a single object or select a large number of objects and change them as a group.
Eliminate objects touching image border In cases where you only want complete and intact volumes that are not cut off by the image stack’s borders you can enable a clean borders setting to ignore these objects.
Fill Holes The appearance of each object is very important to accurate visualization so parameters for each object are able to be edited including color, transparency, specularity.
Separate objects into custom groups by parameter
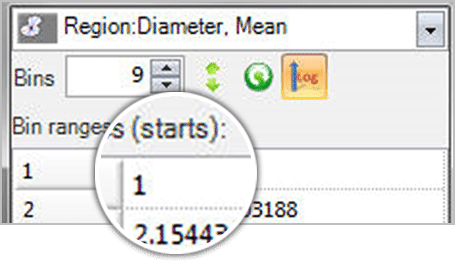
Single Variable Classification Define any number of classification bins, set the first and last values of each bin, and assign classes to objects according to the bin ranges. Use any parameter in the measurement list to classify the objects by that “single” variable.
Auto Classification Define the number of groups to be created, choose all the measurement parameters to be used for the classification, and then apply hierarchical clustering to the objects. Classes will be assigned based on the cluster created by cutting the clustering tree where the number of branches correspond to the number of requested classes.
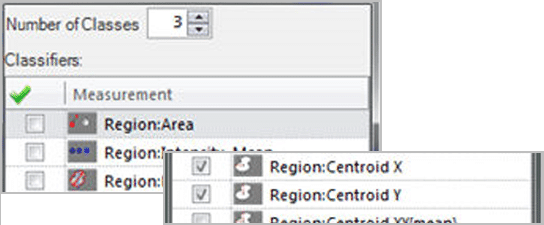
Learning Classification Classify objects using multiple parameters based on manually selected reference objects. This is an especially useful technique when you are not certain which parameters to use for the classification, but have some idea of how the objects should appear per class.
Generate data for making helpful comparisons
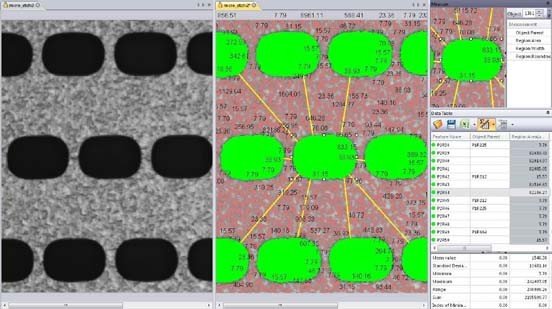
Measure Distances Between Objects Measure one-to-one and one-to-many distances between objects.
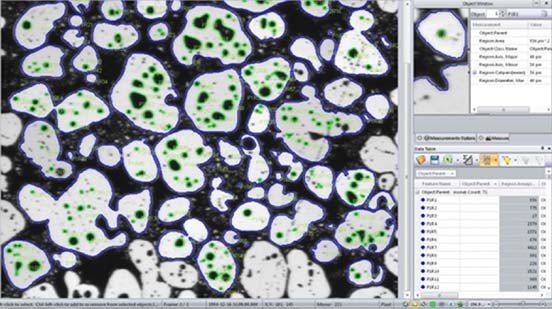
Measure Objects within Objects Analyze parent/child relationships with tools that allow you to automatically measure and group objects within objects.
Sort Counted Objects Create a new image displaying all counted objects arranged by size.
Analyze the Spatial Distribution of Objects
Measure and Analyze
Extract quantitative data with ease.
Image-Pro Premier has been designed for a diverse user base and a multitude of applications. Whether in the materials science research lab, the quality assurance room, or the manufacturing floor, Premier’s semi-automated measurement and editing tools enable accurate and expedient analysis.
Calibrate for accuracy
Calibration data is ideally read from the image, however is cases where it does not exist, we offer a variety of options to easily create and apply a calibration to an image
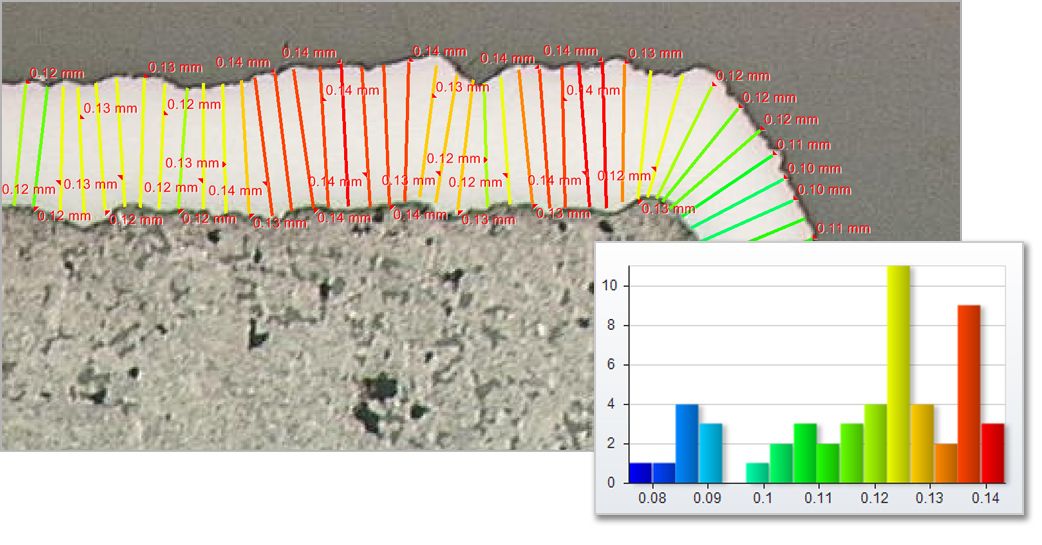
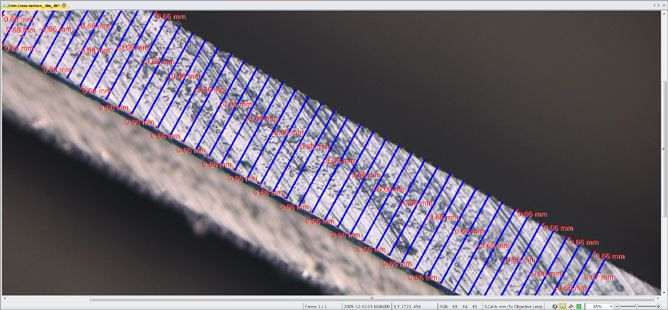
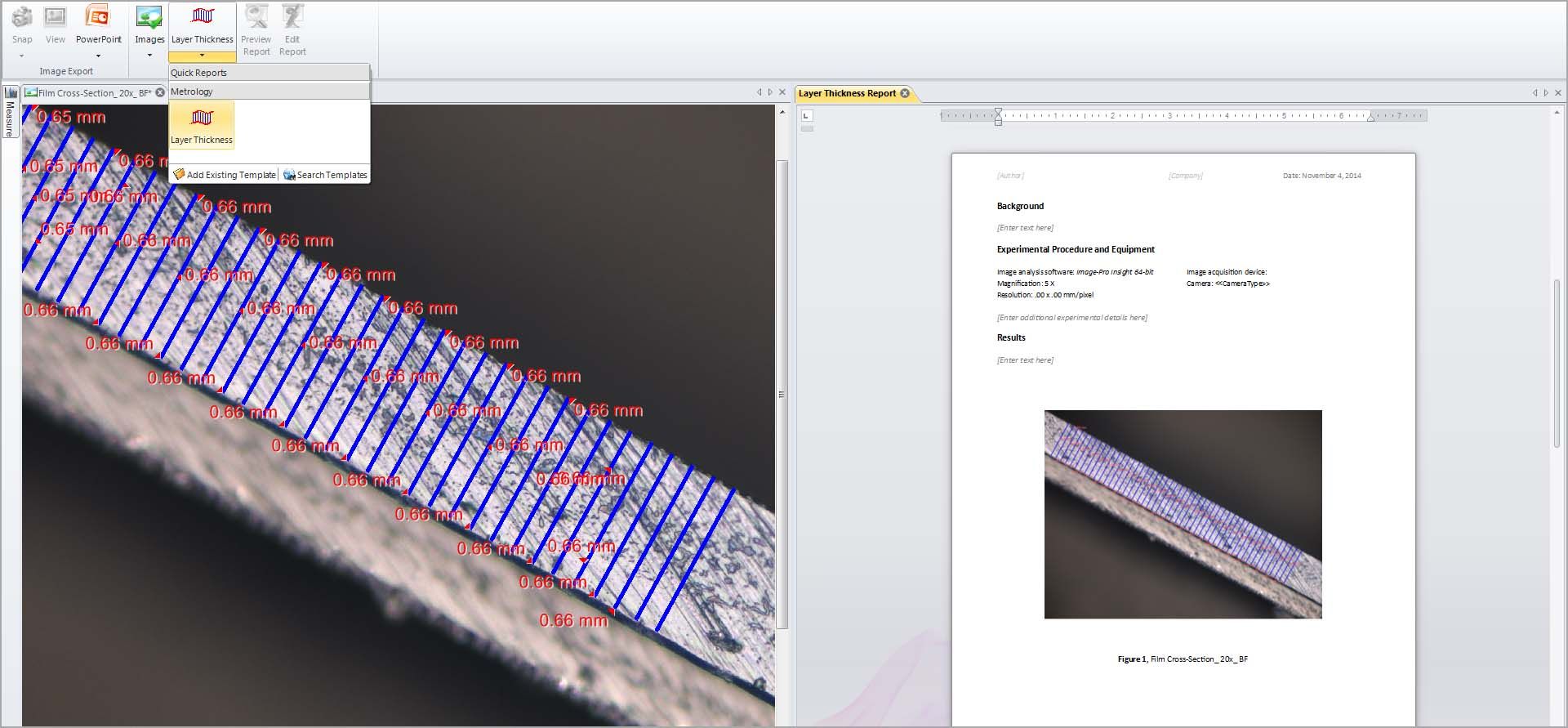
Measure Layer Thickness
Determine the top and bottom edges and allow the software to measure thickness and statistics about longest and shortest lengths.
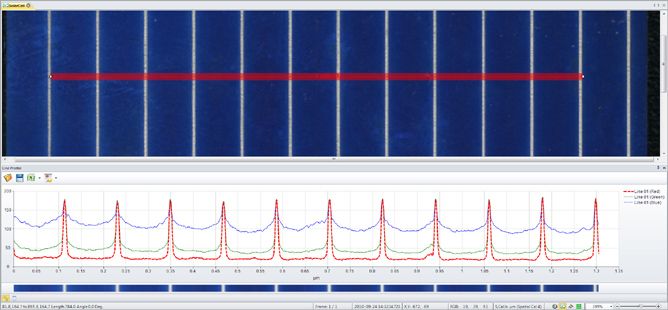
Measure Line Profile
Draw a variety of lines and shapes for displaying the intensity under the lines as Line Profiles. Apply any number of line profiles and export the graph and data out to Excel or to a custom report.
Measurement tools
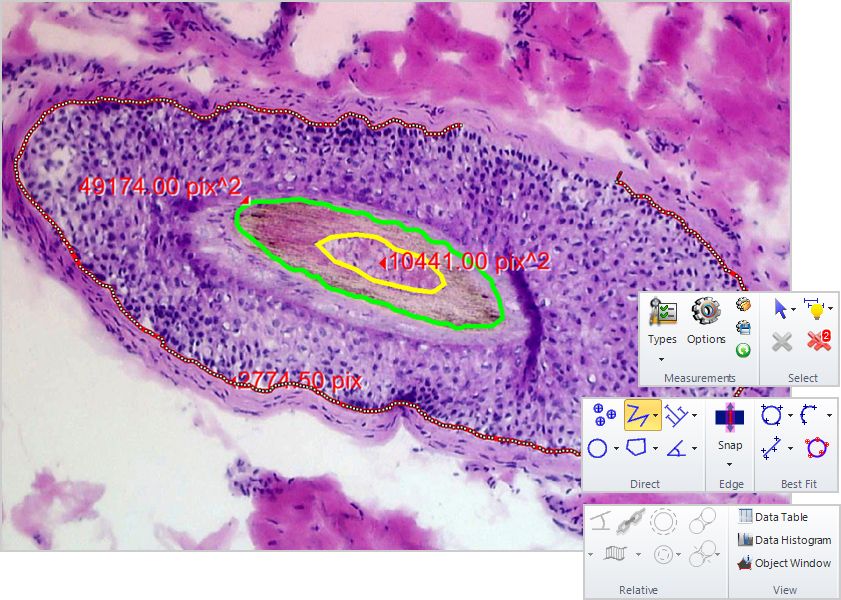
Direct and Relative
Manual and auto tracing, magic wand counting.
Magnetic Snap Measurements
Draw accurate line measurements every time with the Snap Measurement tools. Simply draw and the measurement will accurately snap along object edges.

Classify measurements
Use different colors, shapes, and custom names to classify and organize your measurement data. Use to organize measurements for greater clarity.
Data Management
Make the data work for you.
Rename and adjust
Rename objects by clicking and typing in the table. Also arrange all objects by color coded class to see relationships.
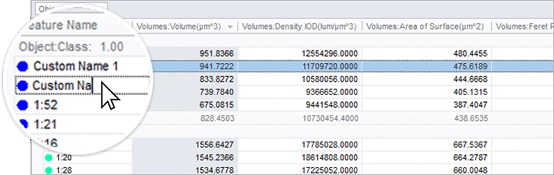
Sort and Condense
Sort by measurements and adjust tables to only show the relevant data.
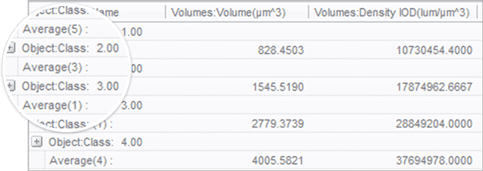
Group and view statistics
Combine classes of similar objects into a custom hierarchy and see statistics per measurement for each grouped class.
Data Collector
Gather data from multiple experiments.
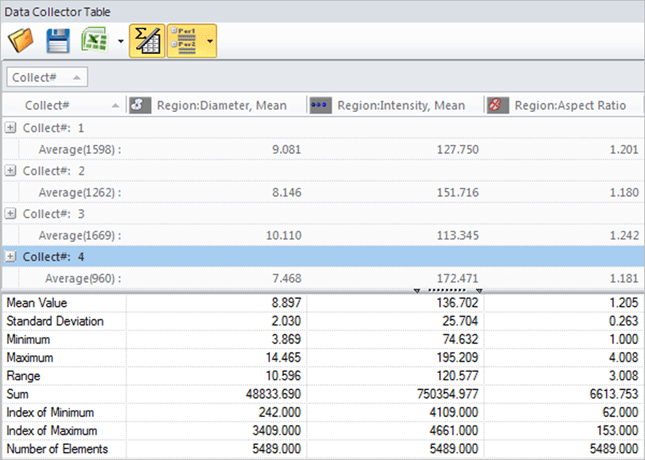
Collect data automatically
Keep data linked to images, graph the results.
Export Data
Export your data and statistics to Microsoft Excel, File, or the Clipboard for pasting anywhere on your PC.
Send data to reports
Send your data and screenshots to custom designed reports within Image-Pro for fast and easy creation of experiment results.
Process and Enhance
Prepare your images, one-click color composite.
Build composite color images by simply right-clicking on each grayscale image to select a color tint.
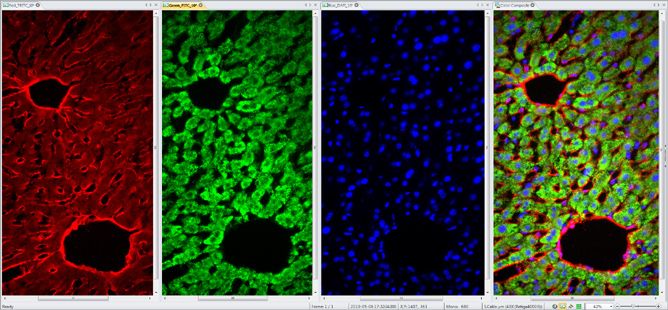
Extract and Merge Channels
Easily combine and separate images into RGB, HSI, and HSL color channels.
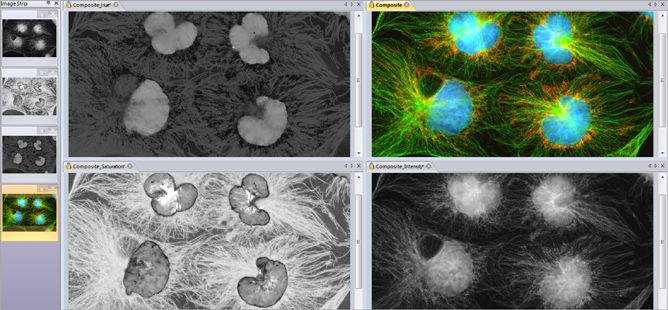
Image Correction
Correct your image background to better distinguish image objects, improving the downstream measurement operations and reducing false positives.
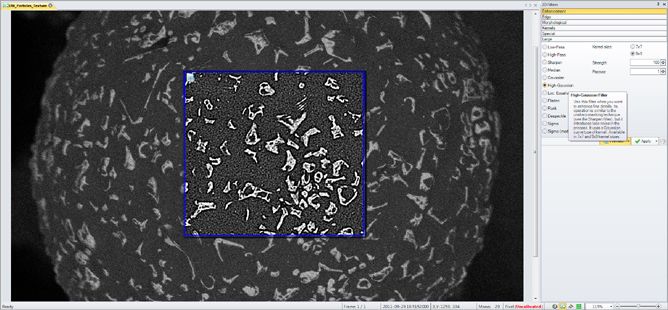
Image Filters
Remove background noise and reveal hidden details with an extensive set of image processing filters. Preview filters on your active image for instant results.
HDR Post process
Capture a sequence of images at different exposures, with variable options, and combine them into a single High Dynamic Range image. The resulting image is a compilation of image data using the widest possible range of dark to light pixels. No more black or saturated pixels.

Alignment & EDF
Correct for microscope shift with post-acquisition auto-alignment tools. Extended Depth of Focus (EDF) creates a focused image from a series of images captured at different z planes.
Report and Share
Prepare your images for what’s next, annotations.
Draw arrows, text annotations, meta-data overlays, Date/Time stamps, calibration marker bars and more.

Quick Reports
Send data and images to Premier’s report generator, able to create simple custom reports based on pre-defined templates. Print or export to Word or PDF.
1. Automatic Measurements:
COUNT/SIZE,
and ANALYZE
2.Measure and Analyze:
3. Measurement tools:
4.Data Management
5.Data Collector
6.Process and Enhance
7.Report and Share
Image-Pro’s technique for performing Automatic Measurements is the foremost solution for gathering data from images by segmentation systems. Our simple step-wise approach to the problem is designed to provide the ultimate flexibility to analyze nearly any image type while remaining simple enough to quickly learn and teach to others.
1 Identify what you want to measure in the image 2 Count/Size Create object outlines, instantly counted and sized 3 Classify Separate objects into custom groups by parameter 4 Analyze Generate data for making helpful comparisons
Use the method that best suites the image and identify what you want to measure
Export Data Export your data and statistics to Microsoft Excel, File, or the Clipboard for pasting anywhere on your PC.
Supported Devices
Image-Pro supports an ever growing list of scientific cameras to ensure a superior capture experience. Click the link below to browse our extensive database of supported devices.
Supported File Formats
System requirements.
Manufacturers change system specifications regularly so it is difficult to describe the exact configuration that is required to use Image-Pro Premier successfully. However, there are some important guidelines to consider before purchasing or upgrading your computer hardware for use with Image-Pro Premier.
Rule of Thumb:
Everything depends on memory and speed. For users who want the best possible experience and product performance we recommend that you review the requirements below and follow-up with a review of the hardware priorities to understand how the whole system plays a part in the applications performance.
Important Notes:
*This product may allow you to access certain features that are hosted online (“online services”), provided you have a high-speed Internet connection. The online services include but are not limited to, automatic updates, support links, access to video tutorials, and more.
Microsoft .NET Framework 4.0 (automatically installed by application).
Specifications are subject to change
Watch the Image-Pro Premier Introduction Video

Image-Pro Premier Intro Video
There are no reviews yet.
Only logged in customers who have purchased this product may leave a review.
Related products

Your order will be shipped ASAP
10 days money back guarantee
Offered in the country of usage
PayPal / MasterCard / Visa
- Early Access Forum
Access updates, System Requirements, Downloads & more
- Software Downloads
- Product * All Image-Pro AutoQuant Image-Pro Premier Image-Pro Premier 3D Image-Pro Insight Image-Pro Plus
- Product Version * All
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Front Endocrinol (Lausanne)

Combination Usage of AdipoCount and Image-Pro Plus/ImageJ Software for Quantification of Adipocyte Sizes
1 Department of Endocrine and Metabolic Diseases, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, China
2 Shanghai Key Laboratory of Regulatory Biology, Institute of Biomedical Sciences and School of Life Sciences, East China Normal University, Shanghai, China
Xiangdi Cui
Qianqian li, meiyao meng, mingwei guo.
3 Key Laboratory of Adolescent Health Assessment and Exercise Intervention of Ministry of Education, College of Physical Education and Health, East China Normal University, Shanghai, China
Dongmei Wang
Feixia shen, xuejiang gu, associated data.
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding authors.
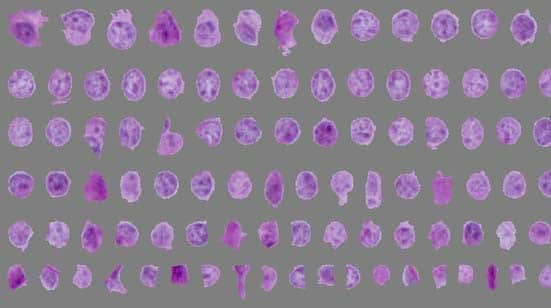
In recent decades, the prevalence of obesity has been rising. One of the major characteristics of obesity is fat accumulation, including hyperplasia (increase in number) and hypertrophy (increase in size). After histological staining, it is critical to accurately measure the number and size of adipocytes for assessing the severity of obesity in a timely fashion. Manual measurement is accurate but time-consuming. Although commercially available adipocyte counting tools, including AdipoCount, Image-Pro Plus, and ImageJ were helpful, limitations still exist in accuracy and time consuming. In the present study, we introduced the protocol of combined usage of these tools and illustrated the process with histological staining slides from adipose tissues of lean and obese mice. We found that the adipocyte sizes quantified by the tool combination were comparable as manual measurement, whereas the combined methods were more efficient. Besides, the recognition effect of monochrome segmentation image is better than that of color segmentation image. Overall, we developed a combination method to measure adipocyte sizes accurately and efficiently, which may be helpful for experimental process in laboratory and also for clinic diagnosis.
Introduction
Obesity results from energy intake in excess of energy expenditure, and surplus energy is stored in adipocytes as a form of triglycerides, which results in adipocyte hyperplasia and hypertrophy ( 1 , 2 ). When the capacity of adipocytes cannot meet the demand for lipid storage, excess lipids will be ectopically deposited in the muscles, liver, and pancreas, causing metabolic abnormalities, such as insulin resistance ( 3 , 4 ). Therefore, quantification of the number and size of adipocytes is essential for assessing the severity of obesity and providing the reference for the choice of treatment ( 5 ).
As technology advances, image processing software have been developed for the measurement of adipocytes ( 6 – 8 ). ImageJ and Image-Pro Plus (IPP) are both the open-software platform image processing tools for scientific image analysis and have been widely used in life sciences ( 9 , 10 ). Area of interest (AOI) and measurement threshold of adipose tissue slides can be set to screen out objects that meet the requirements automatically ( 11 , 12 ). However, because adipocytes are membrane-stained, and clear contrast or threshold is difficult to be set by these tools, the automatic counting of adipocyte sizes on adipose tissue slides may result in severe measurement errors. Meanwhile, drawing the adipocyte boundaries manually by IPP and ImageJ is accurate but time-consuming and intractable to large-scale investigations.
Researchers have been committed to developing software that can identify adipocytes accurately and efficiently ( 13 , 14 ). However, these automatic methods may require programming knowledge or plug-ins, and the counting results also rely on slide quality, software recognition, so the accuracy is often unsatisfactory ( 15 – 18 ). Recently, a freely available counting software for adipocyte numbers, AdipoCount, is reported to be convenient and has powerful capability to recognize adipocyte boundaries and segment images with simple operations for further analysis ( 19 ). During our operation, we found that adipocytes with empty holes, cell debris, and cells adhesion edges may also be mistakenly counted by AdipoCount software. Besides, AdipoCount software lacks threshold settings and one-step area measurement function, thus the counting mistake could not be adjusted, and final results have to be calculated.
In the present study, we combined AdipoCount with IPP/ImageJ to measure the size of adipocytes while considering the following reasons: 1) IPP and ImageJ could not identify contours of adipocytes clearly but they could provide accurate results with clear segmentation and perform threshold setting to screen out unreasonable data; 2) AdipoCount could recognize adipocyte boundaries and export segmentation images, although without area measurement and threshold setting functions. Therefore, we took advantage of AdipoCount and IPP/ImageJ and designed the combination protocol for adipocyte sizes counting. By comparison of the combined method to manual method, we confirmed its accuracy and time saving characteristic, which suggested the advantage of applying AdipoCount and IPP/ImageJ combination for adipocyte size measurement.
Materials and Methods
Animal studies.
Mouse studies were performed according to the guidelines of the East China Normal University Animal Care and Use Committee. C57BL/6J male mice, purchased from Shanghai Research Center for Model Organisms, were housed at 23 ± 1°C on a 12-h light/12-h dark cycle with free access to food and water. 8-week-old male mice (n=5 per group) were fed with normal chow diet (NCD) (Research Diet, D10012G) or high fat diet (HFD) (Research Diet, D12492). All mice were sacrificed 3 months later and epididymal fat (eWAT) and inguinal fat (iWAT) tissues were dissected for histological analysis.
Histology Processing and Image Capture
Adipose tissues were fixed in 10% buffered formalin for 12 h and dehydrated following a standard procedure. Then, the tissues were embedded in paraffin wax and sections (5-µm thick) were cut. Hematoxylin and eosin (H&E) staining was performed as previous report ( 17 ). Images were captured by a Nikon microscope instrument (MODEL ECLIPSE Ci-L) with a 20× objective using a Sony camera(DS-2000P, 20Mp 1” Color Sony Exmor CMOS SENSOR).
A 32/64-bit operating system (Windows7-10) was required for the established protocol. AdipoCount (version 1.0), a free open access software ( http://www.csbio.sjtu.edu.cn/bioinf/AdipoCount/ ), was used to segment the membranes of adipocytes. Image-Pro Plus (version 6.0.0.260, Media Cybernetics Corporation, USA) and ImageJ (version 1.52a, NIH, USA) were used as image analysis software. These softwares (AdipoCount, ImageJ, IPP) have good compatibility in Windows7-10 system.
Manual Measurement With IPP/ImageJ
Open base image.
The image of interest was imported through “File → Open” option on the tool bar.
An image with a known linear scale bar was opened firstly. Image was calibrated by clicking on “Measure → Calibration → Spatial Calibration”. In the pop-up window, “µm” was selected as unit, and “image” was clicked to show the “position line”, which was dragged to make it completely coincide with the known linear scale bar. Subsequently, the known length of scale bar was input in the box to finish the calibration.
Measurement
The measurement scale was selected firstly through “Measure →Calibration → Select Spatial Calibration”. The adipocyte areas were depicted manually using “Measure → Count/Size”, then clicking “Edit → Draw/Merge Objects” in the tool bar of pop-up window. During the measurement, the image should be enlarged by 50%, which would make the segmentation results more accurate. In this process, the following objects were excluded: objects whose sizes were not in the range of 240 to 15,000 μm 2 , cells adhesion edges, empty holes without clear structure, and cell debris.
“View → Measurement Data” was selected to show the measurement results.
The image of interest was imported through “File → Open” option in the tool bar.
An image with a known linear scale bar was opened firstly. The “Straight line” tool was chosen in the tool bar to draw a line, which was as long as the known linear scale bar. “Analyze → Set scale” option was clicked and the distance of known linear scale was displayed in the “Known distance” box of the “Set Scale” window. The pixel aspect ratio was set to 1. The known distance of the linear scale bar was entered into the “Known Distance” box. The unit was dependent on the scale bar (such as µm). The “Global” option was selected to maintain the calibration for all subsequent image analysis if all images were taken at the same magnification.
Subtract Background
To help clarify the image for subsequent analysis, the backgrounds of images were corrected. “Process → Subtract Background” was selected from the task bar. “Rolling Ball Radius” should be set to 50 pixels. “Light Background” and “Sliding Paraboloid” should be checked. When all of the parameters were set, “OK” was clicked.
The image should also be enlarged by 50%. The “Wand” (tracing tool) in the tool bar was chosen to select the individual adipocytes. However, this tool could not identify adipocytes accurately sometimes. For example, partial membranes of adipocytes were lost. Then, the “Polygon Selection” tool was select from the task bar to depict the adipocyte areas. Every time when a cell was depicted, “M” was pressed in keyboard, and the selected area would be measured. To avoid repeated measurements, “Backspace” was pressed to mark the cells which had been counted. Above operations were repeated until the last cell. The exclusion criteria were the same as IPP measurement.
General Measurement (Identifying Adipocytes by IPP/ImageJ Directly)
Open base image and set scale.
Same as described in “Manual measurement with IPP/ImageJ (2.4.1.)”.
Threshold Images
“Count/Size” option was selected under “File” drop-down menu. In the “Count/Size” window, the “Manual → Select Ranges” was clicked to show segmentation menu. “Histogram Based” option was selected in segmentation menu. The “HSI” was selected. In “H” and “S” channels, the first and second sliding bars were set to 0 and 255 respectively. As for “I” channel, the first sliding bar was set to 0, the second sliding bar was set to make the red highlight maximally cover the areas defined as adipocytes without impact on the membrane areas.
The threshold of area was set through “Measure → Select measurements” in the “Count/Size” window. The filter range of area was set starting from 240 µm 2 and ending at 15000 µm 2 . “Apply Filter Ranges” option was selected to apply the settings. Clicking “Count”, the software would measure the areas of objects.
Open Base Image, Set Scale, and Subtract Background
Same as described in “Manual measurement with IPP/ImageJ (2.4.2)”.
The images were converted into 8-bit using “Image → Type → 8-bit” option. Then the “Image → Adjust → Threshold” was selected from the tool bar. The first sliding bar was set to 0, the second sliding bar was set to make the red highlight maximally covering the areas defined as adipocytes without impact on the membrane areas.
The areas were measured through “Analyze → Analyze Particles”. In the pop-up window, the threshold of area, 240 to 15,000 µm 2 , was input into “Size” box. The options of “Display results”, “Exclude on edges” were selected. Clicking “OK”, the software would detect the areas.
Adipocyte Boundary Segmentation by AdipoCount
The adipocytes were segmented by AdipoCount as previously described ( 19 ). Briefly, the image was imported through “File → Input image”. The “Re-segment” option was selected to improve accuracy, and then “Process” button was clicked to get preliminary segmentation image ( Supplementary Figure 1B ). The segmentation results could be further corrected manually. Missing membrane segments were added and false membrane segments were deleted. Moreover, holes with large areas should be separated into several small holes by “Add line”, then these small holes could be excluded in further detection through area threshold. Finally, two different segmentation images (monochrome and color) were exported by clicking “Counting → Save” in “Counting” box ( Supplementary Figures 1C, D ).
Combined Methods for Measurement
Combination usage of adipocount and ipp, open images and select calibrated scale bar.
The monochrome and color segmentation images exported by AdipoCount were imported into IPP and the measure scale was selected as described above.
“Count/Size” option was selected under “File” drop-down menu. In the “Count/Size” window, the “Manual → Select Ranges” was clicked to show segmentation menu.
The next steps were slightly different about monochrome and color segmentation images. For monochrome segmentation images, the first sliding bar was set to 52 (this varied until the red highlight covered all the empty space defined as adipocytes), the second sliding bar was set to 255. For color segmentation images, “Histogram Based” option was selected in segmentation menu. The “HSI” was selected. In “H” and “S” channels, the first and second sliding bars were set to 0 and 255, respectively. As for “I” channel, the first sliding bar was set to 0, the second sliding bar was set to make the red highlight maximally covering the areas defined as adipocytes without impact on the membrane areas.
“Rectangular AOI” tool in task bar was selected to choose the area, which only contained intact cells. Then the “Options” was clicked, and “All Borders” was selected in “Clean Borders” box. In this way, the cells touching edge of the field would be excluded in next detections.
The threshold setting and the following operations were same as described in “General measurement (Identifying adipocytes by IPP/ImageJ directly) (2.5.1)”.
Combination Usage of AdipoCount and ImageJ
Before measurement, the quality of the slices needs to be manually checked: the cell boundaries in the slices are clear and there are enough cells for the measurement. In this study, we ensured that the number of cells in each field was not less than 25.
Open Images and Set Scale
The monochrome and color segmentation images were imported into ImageJ and the measure scale was set as described above.
The images were converted into 8-bit using “Image → Type → 8-bit” option. Then the “Image → Adjust → Threshold” was selected from the tool bar.
The next steps were slightly different about monochrome and color segmentation images. For monochrome segmentation images, the first sliding bar was set to 52 (this varied until the red highlight covered all the empty space defined as adipocytes), the second sliding bar was set to 255. For color segmentation images, the first sliding bar was set to 0, the second sliding bar was set to 240 (this varied to make the red highlight maximally covering the areas defined as adipocytes without impact on the membrane areas).
After these steps, membranes and adipocytes areas were filled by white and black respectively.
To exclude the cells touching edge of the field, “Rectangle” tool in task bar was used to select the area, which only contained intact cells (the area of rectangle would change to contain all intact cells). The areas were measured through “Analyze → Analyze Particles”. In the pop-up window, the threshold of area, 240-15000 µm 2 , was input into “Size” box. The options of “Display results” and “Exclude on edges” were selected. Clicking “OK”, the software would detect the areas.
The results would be presented in “Results” window.
Note: The Steps (2–3) could be automated by creating a macro. “Plugins → New → Macro” was selected and the computer script as following was pasted into a new screen. The new macro was saved by clicking “File → Save”. Then the subsequent image analysis could be performed easily using the created macro by clicking “Plugins → Macros → Run”.
The macro was different for monochrome and color segmentation images.
For monochrome segmentation images:
- run(“8-bit”);
- setAutoThreshold(“Default”);
- //run(“Threshold…”);
- setThreshold(52, 255);
- run(“Convert to Mask”);
- //setTool(“rectangle”);
- makeRectangle(48, 32, 5336, 3552);
- run(“Analyze Particles…,” “size=240-15000 show=[Bare Outlines] display exclude summarize add in_situ”);
- For color segmentation images:
- setThreshold(0, 240);
- setOption(“BlackBackground,” false);
- makeRectangle(48, 40, 5352, 3576);
Note: The area of “Rectangle” should be adjusted and recorded when size of image changes. Then this new area should be used in macro to replace the old one. By this way, the macro could work when the size of images changed.
Videos were made to describe the details of the combined methods ( Supplementary Video ).
Statistical Analysis
The number and size of adipocytes were measured by IPP or ImageJ. 2 slides were collected for each mouse, and 4 different fields were counted for each slide. Any objects with an area below 240 μm 2 or above 15000 μm 2 were excluded. In this study, the number of adipocytes for different tissues counted by each method was shown in Supplementary Tables 1 – 4 . The frequency distribution of size was calculated from 240 to 15000 μm 2 (this range might change for different animal model and tissue) in 500 increments. The number of adipocytes within the distribution was counted and the result was transformed to a percentage of total adipocytes in each group. A Student t test was performed to compare between two groups. Two-way ANOVA followed by a Bonferroni post hoc analysis was used for multiple comparisons in Prism 8 (GraphPad Software Inc.).
General Measurement Results of IPP and ImageJ Are Not Accurate
In this study, two eWAT histological slides were selected to evaluate whether IPP or ImageJ could detect the area of adipocytes accurately using general methods. H&E staining of eWAT was measured by manual and general methods respectively with IPP/ImageJ. As shown in Figures 1A–D , IPP and ImageJ could not identify and segment adipocytes accurately by themselves, compared with manual methods. In detail, there was no significant difference between manual measurement results of IPP and ImageJ ( Figure 1E ), whereas the area measured by general methods was significantly smaller than the manual measurement result ( Figures 1F, G ). These results indicated that the general methods of IPP and ImageJ were unable to measure the area of adipocytes accurately.

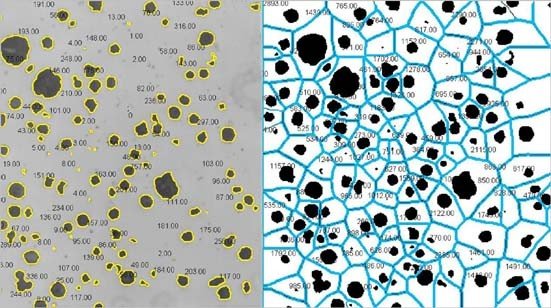
Adipocyte sizes measured by manual and general methods respectively with IPP/ImageJ. (A) Original image of adipocytes. (B) Manual segmentation results of IPP (left) and ImageJ (right). (C) General segmentation results of IPP, the image after threshold adjustment (left), and the output image, where the red circles represented identified adipocytes (right). (D) General segmentation results of ImageJ, the image after threshold adjustment (left), and the output image, where the identified adipocytes were filled with yellow (right). (E) Adipocyte sizes measured by manual methods with IPP and ImageJ. (F) Adipocyte sizes measured by manual and general methods with IPP. (G) Adipocyte sizes measured by manual and general methods with ImageJ. Data are presented as mean ± SEM and **P < 0.01 compared to IPP manual measurement group. ## P < 0.01 compared to ImageJ manual measurement group. n.s., not significant; IPP, Image-Pro Plus.
AdipoCount Cannot Recognize Empty Holes, Cell Debris, and Adhesion Edges
AdipoCount is a software designed for adipocyte counting, which is powerful to segment adipocyte and export the image, although it only gives numbers but not areas directly. Thus, we next examined whether dividing the total area of the eWAT image previously tested by the number of cells could get an accurate area. An original image was imported into AdipoCount ( Figure 2A ), and the segmentation image revealed that empty holes, cell debris, and adipocyte adhesion edges were counted mistakenly ( Figure 2B ). Because the software could not set the threshold and manual calibration could not eliminate the errors, the counting number result was significantly more than the actual number. So, the adipocyte areas achieved was significantly underestimated ( Figure 2C ). These results suggested that there was still improvement space for AdipoCount to better calculate the adipocyte area.

AdipoCount cannot recognize empty holes, cell debris, and adhesion edges. (A) Original image of adipocytes. (B) The segmentation image of AdipoCount (left), and the labeled image with cell numbers (right); what in the yellow circles were cell debris or empty holes (left), which were counted as cells mistakenly by AdipoCount (right). (C) Adipocyte sizes measured by manual methods with IPP and ImageJ, or calculated with AdipoCount. Data are presented as mean ± SEM and **P < 0.01 compared to IPP manual measurement group. ## P < 0.01 compared to ImageJ manual measurement group. n.s., not significant; IPP, Image-Pro Plus.
Combination of AdipoCount and IPP/ImageJ Identifies and Segments Adipocytes Accurately
Via the above tests, we found that IPP/ImageJ could set the threshold and had the area measurement function, although the ability to recognize adipocyte boundaries was poor during general measurement. On the other hand, AdipoCount had no measurement functions and threshold settings, although it could segment adipocytes with a high accuracy. Indeed, a preliminary segmentation image was generated by AdipoCount, which could be further corrected manually ( Supplementary Figure 1B ). Besides, from two different segmentation images (monochrome and color segmentation image) exported from AdipoCount, we could clearly see that adipocytes had clear boundaries in both two segmentation images ( Supplementary Figures 1C, D ). In addition, the size of segmentation images exported by AdipoCount was same as the original image. Images analyzed by the different software remained their original size of 5440 × 3648 pixels, suggesting that AdipoCount processing did not compress the image, and the calibration scale of original image could be used for segmentation image.
We next imported both monochrome and color segmentation images into IPP and ImageJ to measure the adipocyte size. Of note, adipocytes in segmentation images could be identified accurately using general methods of IPP/ImageJ. Moreover, empty holes, cell debris, and adhesion edges were eliminated by setting threshold to area of interest (AOI) ( Figures 3A–C ). These results suggested that combination of AdipoCount and IPP/ImageJ might be a better method to measure the adipocyte sizes accurately.

Combination of AdipoCount and IPP/Image identifies adipose cells accurately. (A) Original image of adipocytes. (B) Combination of AdipoCount and IPP, the identification results of monochrome segmentation image (left), and color segmentation image (right). (C) Combination of AdipoCount and ImageJ, the identification results of monochrome segmentation image (left), and color segmentation image (right). IPP, Image-Pro Plus.
The Combined Methods Can Measure the Area Accurately and Take Less Time
Next, manual methods and combined methods were performed to examine the adipocyte sizes in eWAT and iWAT of obese mice. The average adipocyte sizes and size distributions in eWAT measured by combined methods were comparable with the manual measurement results ( Figures 4A, C ) . Besides, there were no obvious differences between the measurement results of monochrome and color segmentation images ( Figures 4A, C ) . Of note, the combined methods could significantly save measurement time ( Figure 4B ). In addition, the measurement results in iWAT were similar as those in eWAT ( Figures 4D–F ).

The combined methods can measure the area accurately and take less time in eWAT and iWAT of obese mice. (A–C) The measurement results of eWAT, adipocyte sizes derived from manual and combined methods (A) , measurement time (B) , adipocyte size distributions measured by manual and combined methods (C) . (D–F) The measurement results of iWAT. Data are presented as mean ± SEM and **P < 0.01 compared to IPP manual measurement group. ## P < 0.01 compared to ImageJ manual measurement group. n.s., not significant; IPP, Image-Pro Plus; AC, AdipoCount.
Then, the combined methods were used to measure adipocyte sizes in eWAT and iWAT of lean mice. Similar to the results in obese mice, the combined methods could count the areas and size distributions accurately in lean mice, but saved time significantly ( Figures 5A–F ). Interestingly, we noticed that the manual measurement of ImageJ took more time than IPP, which was because of the different operating procedures of these two softwares ( Figures 5B, E ).

The combined methods can measure the area accurately and take less time in eWAT and iWAT of lean mice. (A–C) The measurement results of eWAT, adipocyte sizes derived from manual and combined methods (A) , measurement time (B) , adipocyte size distributions measured by manual and combined methods (C) . (D–F) The measurement results of iWAT. Data are presented as mean ± SEM and *P < 0.05, **P < 0.01 compared to IPP manual measurement group. ## P < 0.01 compared to ImageJ manual measurement group. n.s, no significant. IPP, Image-Pro Plus; AC, AdipoCount.
Very small adipocytes may lead to increased incidence of mis-counting, thus we further analyzed the size distributions in range of 0 to 1000 µm 2 . The results showed that the size distributions measured by the combined methods were comparable as the manual methods ( Supplementary Figures 2A–D ).
Limitation and Suggestion of the Combined Measurement
During the operation, we noticed that there were small adipocytes with the size close to the lower limit of threshold exclusion in iWAT of lean mice. The result of UCP-1 immunostaining indicated the small adipocytes might be caused by the process called “browning of white fat”, which was important for lipid mobilization and thermogenesis ( Supplementary Figure 3 ). These adipocytes were counted when using the manual measurement of IPP but excluded while the combined method was performed ( Figures 6A, B ), which might be due to the slight loss of area during segmentation by AdipoCount and the setting of the intensity thresholding. Of note, the combined measurement results were comparable to manual measurements, suggesting that individual cell misidentification had little effect on the overall result.

Limitation of the combined measurement. (A, B) The results of manual and combined measurement. The cell numbered 107 in yellow circle was counted when using manual measurement (A) ,whereas it was excluded when the combined method was performed (B) . (C, D) Comparison of measurement results of two different segmentation images. Two cells inside the red circle could be distinguished in monochrome segmentation image (C) , but not in color segmentation image (D) .
In a color segmentation image, if boundary between two adjacent adipocytes was thin and was filled with similar color, then the two adipocytes might be identified as one adipocyte in subsequent measurements. Although the area could be split in two manually, it would take more time. Thus, the monochrome segmentation image was better recognized and recommended ( Figures 6C, D ).
Adipocyte Counting by the Combined Methods Yields Comparable Data as Manual Methods But Is More Efficient
Finally, the combined methods using monochrome segmentation images were performed to measure the adipocyte sized in lean and obese mice. Both manual and combined methods identified a significant increase of adipocyte sizes and frequency of large adipocyte in fat tissues isolated from obese mice compared with lean mice ( Figures 7A–D ), with comparable area counting results, suggesting that the combined methods yielded the accurate results as manual methods with more efficiency.

Adipocyte counting by the combined methods yields comparable data as manual methods but saves more time. (A, B) Adipocyte sizes measured by manual methods with IPP and combined methods with IPP and AdipoCount, in eWAT (A) and iWAT (B) . (C, D) Adipocyte sizes measured by manual methods with ImageJ and combined methods with ImageJ and AdipoCount, in eWAT (C) and iWAT (D) . Data are presented as mean ± SEM and **P < 0.01 compared to manual methods group. ## P < 0.01 compared to combined methods group. n.s, no significant. IPP, Image-Pro Plus; AC, AdipoCount.
The measurement of adipocyte sizes is important for obesity research. In the present study, we provided an easy and semiautomated method by taking advantage of AdipoCount and IPP/ImageJ software to accurately and efficiently quantify adipocyte sizes. Briefly, adipocytes from H&E staining slides are segmented by AdipoCount, and clear segmentation images are exported. IPP or ImageJ is then performed to analyze segmentation images with threshold setting and one-step quantification. It has to be noticed that the monochrome image is more accurate than colored one. For the simple operation protocol, no programming experience is required. However, this method is not yet fully automated and still requires some manual manipulation, especially the manual correction of segmentation images from AdipoCount. The quality of slides, such as clear cell staining and complete membrane, is also important to get the accurate results, especially slides with small adipocytes. Further adjustment of the exclusion range and intensity threshold is required to improve the accuracy of the measurement. Theoretically, the combined method can be used for images with clear cell boundaries. More efforts will be made to explore whether this method can be applied besides adipocytes in the future.
Data Availability Statement
Ethics statement.
The animal study was reviewed and approved by the East China Normal University Animal Care and Use Committee.
Author Contributions
XG, FeixiaS and LX conceived the project and designed the experiments. YH and JY carried out most of the experiments. XCu, ZZ, QL, and WG assisted in histology processing and image capture. CZ, XCh, MM, and YL provided rodent biological samples for association analysis. MG, JQ, FeiS, DW, and XM assisted in software manipulation and technical support. XG, FeixiaS and LX wrote and edited the paper. All authors contributed to the article and approved the submitted version.
This project is supported by fundings from Key Research and Development Project of Zhejiang Province (2021C03069), Natural Science Foundation of Zhejiang Province (LY20H070003), National Key Research and Development Program of China (2019YFA0904500), National Natural Science Foundation of China (31800989, 32071148, 81902980), and ECNU public platform for Innovation (011), the Instruments Sharing Platform of School of Life Sciences, East China Normal University.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2021.642000/full#supplementary-material
Supplementary Figure 1
Two different segmentation images of AdipoCount. (A) Original image of adipocytes. (B) Preliminary segmentation image without correction. (C) Monochrome segmentation image after correction. (D) Color segmentation image after correction.
Supplementary Figure 2
The combined methods measured the size of very small adipocyte accurately. (A-D) The size distributions of adipocytes (range of 240-1000µm2, in 100 increments) measured by manual and combined methods, in eWAT of obese mice (A) , in iWAT of obese mice (B) , in eWAT of lean mice (C) , in iWAT of lean mice (D) IPP, Image-Pro Plus; AC, AdipoCount.
Supplementary Figure 3
UCP-1 immunostaining of iWAT in lean mice.
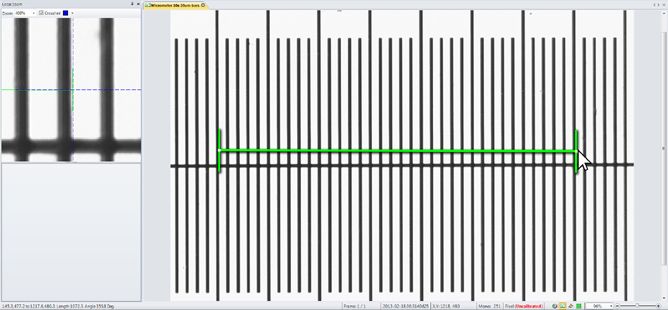
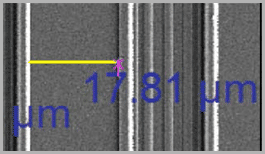
Using Spatial Calibrations
Using a spatial calibration, Image-Pro 's pixel-level measurements can be converted to any unit of measurement. Spatial calibrations can also correct for irregularities in the image’s vertical and horizontal spacing.
There are two primary components to a spatial calibration:
n Pixels per Unit
n Aspect Ratio.
Pixels per Unit
A spatial calibration tells Image-Pro the size to which a given image is scaled, just as the key of a roadmap tells us how many millimeters of image length represent one mile. Likewise, the spatial calibration tells Image-Pro how many pixels of image length represent some more meaningful unit of length in the image, for example, one micron, millimeter, or inch. To create a new spatial calibration, you must either know the number of pixels per unit to specify for the image, or there must be a feature pictured in the image that has a known length value in terms of the units of interest. If a feature of known length is pictured, Image-Pro can calculate the number of pixels per unit by determining the number of pixels it takes to represent the known length. (Companies often ‘plant’ an object of known length into images for this purpose.)
Aspect Ratio
Aspect ratio refers to the ratio of vertical to horizontal lengths. For example, when a television screen’s image appears flattened or squeezed, the ratio of horizontal length to vertical length is out of proportion. Similarly, cameras often inadvertently skew images in the process of capturing them and translating them to digital format.
Such skewing results in inaccurate lengths being ascribed to features that are measured in the image. When a skewed aspect ratio has a flattening effect, vertical lengths will be recorded as shorter than they actually are; when it has a squeezing effect, horizontal lengths will be recorded as shorter than they actually are.
If an image has a known aspect ratio problem, Image-Pro can compensate for the flattening or squeezing of vertical and horizontal lengths, and calculate the actual number of units that measurements of its features represent. To do this, you must be able to supply it with the value l / h (length divided by height) for a perfect square as represented in the image, or there must be a feature pictured in the image that represents a perfect square. If a square feature is pictured, Image-Pro can calculate the aspect ratio from that feature. (Companies often ‘plant’ a perfectly-square object into images for this purpose.)
- Sports Betting
- Sports Entertainment
- New York Giants
- New York Jets
- Transactions
Recommended
Breaking news, vontae davis, former nfl star, dead at 35.
- View Author Archive
- Email the Author
- Get author RSS feed
Contact The Author
Thanks for contacting us. We've received your submission.
Thanks for contacting us. We've received your submission.
Vontae Davis, a former NFL cornerback and the brother of Super Bowl champion tight end Vernon Davis, has died at the age of 35, according to WSVN 7 .
Davis’ body was found dead at a home belonging to his grandmother in Southwest Ranches, Fla.
No foul play was suspected, police told the outlet.

A former Illinois product, Davis was originally selected 25th overall by the Dolphins in the 2009 NFL Draft.
He spent the first three seasons of his NFL career in Miami before being traded to the Colts ahead of the 2012 NFL season.
Davis earned two Pro Bowl nods during his time in Indianapolis, where he played through the 2017 season.
He signed a one-year deal with the Bills in February 2018, only to retire midway through a game that September.

In February 2023, Davis was charged with driving under the influence in a Broward County, Fla. crash in which his Tesla allegedly hit a truck that was reportedly disabled on the side of the highway.
Davis appeared to be asleep on the side of the road and struggled to stay conscious after being placed into a patrol vehicle by responding officers.
Colts owner Jim Irsay mourned Davis’ death on Monday, sharing an emotional message on X .

“Extremely saddened to hear of the passing of Vontae Davis. A great guy, teammate, player. My prayers to Vontae’s family,” Irsay wrote.
Illinois assistant basketball coach Chester Frazier also addressed the former NFL pro’s passing on social media.
“#illinination we lost a great today!!! RIP TO @vontaedavis man nothing but good memories of that dude in school!!! Praying for the Davis family!!” the post on X read.
Share this article:

Advertisement

DJI Mavic 3 Pro review – three is the magic number
Two-minute review.
T he large three-camera gimbal dominating the front of the drone makes the DJI Mavic 3 Pro unmistakable and pushes the series towards increased creative potential, thanks to those three useful cameras for photography and video. This is the world’s first triple-camera drone, with all the impressive safety and flight features that Mavic 3 series drones are known for, and is easily one of the best drones available.
The main camera is the same Four Thirds 20MP Hasselblad as on the original Mavic 3/ Mavic 3 Cine and Mavic 3 Classic models. This camera offers the most functionality and the best image quality of the three, including an f/2.8-f/11 adjustable aperture and a dynamic range of up to 12.8 stops.
Video resolution is available up to 5.1K in Normal, HLG (hybrid log-gamma high dynamic range), and 10-bit D-Log M profiles at 200Mbps, while three Apple Pro Res options offer a significantly higher bitrate for professional workflows. 5.1K can be shot at up to 50fps, DCI 4K (4096 x 2160 17:9 cinematic 4K) up to 120fps, 4K up to 120fps and FHD up to 200fps. Plus, photos can be captured in 12-bit raw and JPEG.
The two telephoto cameras provide a 70mm equivalent with the same 48MP 1/1.3-inch sensor as the Mavic Mini 3 Pro and a 166mm camera with a 12MP 1/2-inch sensor. Both will shoot photos in raw and JPEG alongside a range of video options with manual control over both. This makes them incredibly useful in a range of situations, although the main 4/3 is likely to be the go-to option most of the time.
Moving beyond the cameras, which are undoubtedly the jewels in the Mavic 3 Pro’s crown, the drone offers excellent flight and safety features, including APAS 5.0 collision avoidance, ActiveTrack 5.0 for subject tracking, GEO 2.0 geofencing, Advanced Return to Home and Quickshots automated flight patterns. It’s not cheap, though, and launches at a similar price as the original Mavic 3 / Mavic 3 Cine, which it supersedes to become the flagship model in the Mavic 3 lineup.
DJI Mavic 3 Pro: release date and price
- Available in May 2023
- Two models available
- Two controller options
After months of speculation and internet rumors, the DJI Mavic 3 Pro was finally announced in April 2023 with availability in May 2023. And just like the DJI Mavic 3, there are two options available: the ‘standard’ model with 8GB of onboard storage and a Cine version with a 1TB SSD. There are also two smart controller options available with the standard model; the DJI RC, which is the same controller that’s available with the Mavic Mini 3/3 Pro, and the higher-spec DJI RC Pro, which has always been an option with Mavic 3 series drones.
In terms of pricing, the Mavic 3 Pro is the same launch price as the Mavic 3, which is now unavailable on the DJI website. Kit options include the Mavic 3 Pro with the DJI RC for $2,199 / £1,879 / AU$3,099, the DJI RC Fly More Bundle for $2,999 / £2,549 / AU$4,199, the DJI RC Pro Fly More Bundle for $3,889 / 3,169 GBP/ AU$5,329, and the Cine Premium Combo for $4,799 / £4,109 / AU$6,939. The Fly More bundles make sense for most people because they come with two additional batteries, a carry bag, a charging hub, ND filters, and other useful accessories.
DJI Mavic 3 Pro: Design and controller
- Similar design and build as other models
- Three cameras
- European C2 rating
The first thing that strikes you about the Mavic 3 Pro is the size of the camera and gimbal, which is naturally larger than those found on the Mavic 3 and Mavic 3 Classic models because it houses three cameras. In terms of design, there’s no mistaking its Mavic lineage thanks to excellent build quality and a folding design that takes the drone from 9.10 x 3.85 x 3.75 inches / 231.1 x 98 x 95.4mm when folded to 13.68 x 11.44 x 4.24 / 347.5 x 290.8 x 107.7mm unfolded (excluding propellers).
This makes the airframe marginally larger than other Mavic 3 models but the weight of the drone is the most significant difference, despite it being less than 2.4 oz / 70 g heavier than previous models at 33.79oz / 958g for the 3 Pro and 33.96oz / 963g for the 3 Pro Cine. This isn’t a huge leap, by any stretch, but it’s enough to place the Pro models into the European C2 classification. This means that pilots will have to use the drone 50m or more away from uninvolved people and at least 150m away from built-up areas in Europe.
In the UK, A2CofC certificate holders can fly in the A2 subcategory of the Open Category, which reduces the flight distances above. But this is, of course, not relevant to users in other parts of the world, so it’s worth checking local drone laws and regulations regarding usage of a drone of this weight in case of restrictions.
The Mavic 3 Pro uses the same 5000mAh batteries as previous models. Presumably as a result of the additional weight, advertised flight times have been reduced from 46 to 43 minutes. In testing, flight times weren’t noticeably different and still sit around the 30-minute mark of the other Mavic 3 models, so this is pretty much negligible.
When it comes to controllers, except for the Mavic 3 Pro Cine Premium Bundle, which is only available with the DJI RC Pro, you can choose between the DJI RC and DJI RC Pro. Both are smart controllers featuring 5.5-inch touchscreens – the RC Pro is slightly larger, 43% heavier and offers up to four hours of battery life compared to three and a brighter screen. Both are excellent overall. The Pro model offers many more features, including a stronger signal, but it’s much more expensive.
DJI Mavic 3 Pro: Features and flight
- Omnidirectional collision avoidance
- ActiveTrack 5.0 subject tracking
- Advanced Return to Home
The Mavic 3 Pro flies extremely well, and it’s impossible to differentiate between its performance and that of other Mavic 3 models. There are three flight modes available: Sport mode provides a top speed of 47 mph with collision avoidance switched off; Normal mode is slower with collision avoidance on and is the most commonly used mode; while Cine mode provides the slowest flight speed with reduced control sensitivity and is mostly used for capturing smooth cinematic video footage. Wind speed resistance is just under 27mph, which opens up many more possibilities than sub-250g models with lower wind resistance.
With the Pro moniker, you may be wondering if the Mavic 3 Pro is suitable for beginners, and the simple answer is absolutely; it’s incredibly easy to fly and offers excellent safety features that we’ll cover in a moment. You may be better suited to a more regulator-friendly sub-250g DJI Mavic Mini 3 because the Mavic 3 Pro is expensive and offers features aimed at professional photographers and videographers. If you’re a beginner with the cash to spend, though, it’s a great drone you’d no doubt enjoy.
GPS positioning is provided by connection to GPS, GLONASS, and BeiDou satellites and offers precise hovering. Omnidirectional Obstacle Sensing uses the Advanced Pilot Assistance System (APAS) 5.0 for collision avoidance, which collects data from six fisheye sensors and two wide-angle sensors to sense obstacles in all directions. When collision avoidance is turned on, the drone can be set to Brake or Bypass obstacles when they’re detected; the Bypass setting also offers a Nifty option designed to provide smoother flight when obstacles are detected in more complex environments. This allows you to fly in trickier environments, but it still comes with a greater risk of a collision, although during testing this mode proved extremely effective.
Additional safety features include Advanced Return to Home, which scans up to 200m to calculate the safest route back and AirSense ADS-B receivers, which provide notification of nearby planes and helicopters for additional safety. The Mavic 3 Pro uses the DJI 03+ transmission system that can transmit a 1080p/60fps live camera feed at a distance of up to 9.3 miles / 15km in the US and up to 5 miles / 8km in other regions.
Flight features include Cruise Control, Quickshots automated flight patterns for video, Mastershots, and Focus Track, which includes ActiveTrack 5.0. This is the subject tracking system that uses omnidirectional obstacle sensors to improve subject tracking. One point to note here is that ActiveTrack isn’t available when shooting above 4K or frame rates higher than 60fps.
The Mavic 3 Pro, alongside the Mavic 3 Classic and Mavic 3, is also compatible with the DJI Goggles Integra, DJI Goggles 2, and the DJI RC Motion 2, so it can also be flown in an FPV style for a more immersive flight experience and video footage. This is useful for those who can’t justify purchasing the DJI Avata but, inevitably, adds additional cost to the Mavic 3 Pro kits if you decide these accessories would be beneficial.
DJI Mavic 3 Pro: Image and video quality
- Hasselblad 20MP 4/3 main camera
- Fully functional telephoto cameras
- Up to 5.1K video with the main camera
I have to confess that when I first heard that the Mavic 3 Pro was likely to include three cameras, I was skeptical about how effective and useful the two telephoto cameras would be. This was simply down to the fact that the Mavic 3 telephoto camera was extremely limited at launch, to the point of being pretty much pointless. Then with the Mavic 3 Classic, which had a few reductions in video specs, including the removal of the Apple ProRes format for video, it felt like DJI might be giving up on the multi-camera approach.
The Mavic 3 Pro has, I’m happy to say, quelled my initial reservations about how useful the additional cameras would be. Having a 24mm 20MP Four Thirds main camera, a 70mm camera with the same 48MP 1/1.3-inch sensor as the Mavic Mini 3 Pro and a 166mm camera with a 12MP 1/2-inch sensor is incredibly useful. What’s more, except for aperture control for the telephoto lenses, which have fixed apertures, manual control is available with the additional cameras alongside shooting video in ApplePro Res and the Normal color profile. The medium telephoto can also shoot in HLG/D-Log M, although this is unavailable with the telephoto.
The Image quality of the main 24mm Four Thirds Hasselblad camera remains the best for both photos and videos. Plus, the adjustable f/2.8-f/11 aperture is incredibly useful for making exposure adjustments to video without having to land the drone to change ND filters. That said, image quality from all three cameras is excellent, and the ability to zoom in 3x and 7x is useful for situations where the drone needs to be further away from people and objects for safety and legal reasons. There’s also a digital zoom with each camera – the Hasselblad camera offers 1-3x, the medium telephoto 3-7x, and the telephoto 7-28x – but this feels superfluous with the two telephoto cameras.
All three cameras can capture photos in Raw and JPEG and offer photography modes, including Single Shot, Burst Shooting, AEB and Timed. The medium 70mm camera can shoot at both 12MP and 48MP. For video, the main camera holds the trump card in terms of resolution options, formats and framerates, but the two telephoto cameras are more than capable, just with a sliding scale of features and overall image quality. The medium telephoto remains useful in terms of what it offers, though. The Hasselblad camera can capture video in 5.1K up to 50fps, DCI 4K (4096 x 2160) 17:9 cinematic 4K up to 120fps, 4K up to 120fps and FHD up to 200fps. The medium telephoto and the telephoto cameras can capture 4K and FHD up to 60fps.
Should I buy the DJI Mavic 3 Pro?
Buy it if..., don't buy it if..., how i tested the dji mavic 3 pro.
The DJI Mavic 3 Pro was tested over several days of flying in a range of locations, environments and weather conditions (excluding rain) to test flight performance, flight features, overall handling and image quality for both photo and video capture. All testing is conducted in a way that meets local aviation laws and restrictions to ensure that all flights are safe and legal.
Drones are always tested using manual flight patterns for video that are typical of professional aerial video capture to capture visually interesting footage, as well as automated flight patterns and subject tracking features when available. Manual flight provides the opportunity to test aspects such as the connection between the drone and controller, latency between the two and the accuracy of the controls and flight in general.
With nearly 30 years of photographic experience and 15 years working as a photography journalist, I’ve been covering drones in terms of shooting and editing techniques, alongside writing drone reviews for a number of years. As well as flying most consumer and prosumer models, I’ve previously held a PfCO (Permission for Commercial Operations) issued by the Civil Aviation Authority in the UK, and now fly under an A2 CofC (A2 Certificate of Competency).
First reviewed May 2023


IMAGES
VIDEO
COMMENTS
Image-Pro, the world's most advanced scientific image analysis platform, lets you capture, process, measure, analyze, report, automate, and share images and insights.
REQUIRES: Valid Serial Number INCLUDES: Image-Pro - Basic Bundle (100-101), Optional: 2D Capture - Module (300-016) P -or- E $400 100-020 Image-Pro - Upgrade from Plus & Analyzer v6.3 and older Products Upgrade to the latest version of Image-Pro from Image-Pro "Plus" & "Analyzer" v6.3 and older to receive a product with similar features and
Serial number*. Get info. Read our warranty policy. Find support for ImagePRO-II series, all-in-one 4K video scaler, scan converter, and switcher.
Media Cybernetics is proud to release the all new Image-Pro® v11, our most advanced scientific image analysis platform for industrial and life science industries. This latest version goes beyond its predecessors, offering innovative capabilities and seamlessly connecting every aspect of the imaging workflow with a single solution. "Image-Pro version 11 is a significant leap forward […]
Media Cybernetics helps power your search for "what's next". For more than four decades, we've been advancing our scientific imaging platform by collaborating with researchers, scientists, and engineers like you—users that need to identify, strengthen and extract critical but often hidden information present in scientific digital images.
Image-Pro Plus 32-bit image analysis software for those who need support for Image-Pro Plus macros, microscope and stage control or 3D measurements.: Image-Pro Premier Image-Pro Premier (32 and 64-bit) software offers analysis tools for image acquisition, automated counting & sorting, automated measurements, object tracking, macro customization, filters and more.
Image-Pro Insight Brochure Catalog. Image-Pro Insight, the latest image analysis software from Media Cybernetics, offers a wide range of tools for capturing and analyzing images. Unlike most imaging systems, which require extensive training, Image-Pro Insight was designed for quick startup and ease of use. Utilizing more than 25 years of user ...
Image-Pro v11 is now the most advanced 64-bit scientific image analysis platform available with capabilities evolved beyond its predecessors. (281) 579-0342 [email protected]. Home. About Us; ... Telephone Number Your Email (required) What can we help you with?
Image Pro Premier Software. Capture, process, count, classify, measure and share. A powerful and customizable imaging platform driven by over 30 years of user feedback, the new Image-Pro Premier offers native 64-bit support, a user friendly interface and a suite of 2D measurement solutions. Rely on our worldwide network of Image-Pro partners ...
Software Downloads for Media Cybernetics Image-Pro imaging software.
Image Pro 3D® Sunlite is skin imaging reimagined, offering free software updates automatically & never charging annual licensing fees. Support With unlimited, free 1:1 training during the warranty period, we train you how to do effective consultations to promote and sell more products or treatments that you already offer.
Image-Pro Trial. Image-Pro can be installed and run with full capabilities for a 14-day trial period. After this, you must purchase and link a license to continue making use of Image-Pro.. Image-Pro can be downloaded for a trial from the My Products page of your myMediaCy account.. We are happy to support you during the trial period to make it as productive as possible.
number and dendrite length should be performed on objects which have been skeletonized, or "thinned" using the Thinning filter in Filters: Morphological. Dendrite measurements performed on non-thinned objects may return unacceptable results. 19. Density Blue: Reports the mean blue value for the measured object in a true color image. 20.
Image-Pro Plus (version 6.0.0.260, Media Cybernetics Corporation, USA) and ImageJ (version 1.52a, NIH, USA) were used as image analysis software. ... it only gives numbers but not areas directly. Thus, we next examined whether dividing the total area of the eWAT image previously tested by the number of cells could get an accurate area. An ...
ImagePRO-4K goes even further as it easily scales, converts and switches the highest quality signals without latency (4K, 4:4:4, 60p, 10-bit), effectively solving glitches before they even occur. The latest I/O connectors enable you to seamlessly convert HDMI 2.0, DP 1.2 and 3G-12G SDI signals while also enabling output image rotation.
Count. Click the Count button to initiate a counting and measuring operation on the active image.. When this button is clicked, Image-Pro creates ROIs around the currently highlighted objects in the image according to the current Segment settings. The Data Table is then opened and populated with the number and measurement data for each object.
Using a spatial calibration, Image-Pro 's pixel-level measurements can be converted to any unit of measurement. Spatial calibrations can also correct for irregularities in the image's vertical and horizontal spacing. ... Image-Pro can calculate the number of pixels per unit by determining the number of pixels it takes to represent the known ...
Rifampicin-induced injury in L02 cells is alleviated by 4-PBA via inhibition of the PERK-ATF4-CHOP pathway. October 2016. 1. 2.
By. Jaclyn Hendricks. Published April 1, 2024, 1:56 p.m. ET. Vontae Davis, a former NFL cornerback and the brother of Super Bowl champion tight end Vernon Davis, has died at the age of 35 ...
This camera offers the most functionality and the best image quality of the three, including an f/2.8-f/11 adjustable aperture and a dynamic range of up to 12.8 stops. Video resolution is ...
Kevin Breuninger @KevinWilliamB. Key Points. Donald Trump is promoting a line of pricey Bibles in a partnership with country music star Lee Greenwood. The "God Bless the U.S.A. Bible" costs ...
Get Started with Image-Pro. Compare current and past versions of Image-Pro and get inspired to do more with your Image-Pro advanced imaging analysis software.
A home that measures 2,000 square feet would require roughly 16 to 21 solar panels to generate an average level of energy consumption. This estimate is based on the premise that the home has an ...
Receive up to 80% off when you upgrade to Image-Pro Plus 7.1 🤑. Upgrade Now. Note: The new release of Image-Pro Plus 7.1 now supports the Windows 10 and 11 operating systems. However, many of the hardware devices supported by previous Image-Pro Plus versions such as cameras and microscopes may not be compatible with these operating systems.
Media Cybernetics is grounded in proactively supporting scientific and industrial business innovators with our advanced image analysis software. Talk to us. Skip links. Skip to content; Sign In; Support; Contact; Image-Pro ... Stay up on all things Image-Pro. Media Cybernetics 1700 Rockville Pike, Suite 240, Rockville, Maryland USA 20852 +1-301 ...
The optional Real-Time Deconvolution extension leverages industry-leading AutoQuant technology to cost-effectively "upgrade" your existing widefield microscope to obtain images almost as clear as those from more expensive confocal microscopes. Elevate your capture system to now deliver crisp, high fidelity, fluorescent images during preview ...
Image-Pro v10.0.14 is a free update for Image-Pro version 10 users and will update an existing installation of Image-Pro 10 through v10.0.13. You can apply this patch directly from Image-Pro by selecting "Check for Updates" in the Help Menu.