How to report a Covid-19 lateral flow result from the NHS or privately
What to do if your test came back positive, negative, or void
- 12:12, 21 APR 2022
- Updated 12:18, 21 APR 2022
Sign up to our free email newsletter to receive the latest breaking news and daily roundups
We have more newsletters
If you self-tested for Covid-19, you may be wondering whether you should report your result, and where to do so. The answer is - it depends on whether you used a free NHS test or on that you paid for.
From April, most people in England are not eligible for free PCR or lateral flow tests (LFTs). Free testing is only reserved for a few select groups, including NHS and care home staff and people with specific health conditions. Some free testing for the general population is still on in Scotland until the end of this month, and in Wales and Northern Ireland until the end of June.
You can report a free NHS rapid lateral flow test result on GOV.UK . This is different to registering a test kit, which you may be asked to do if you have a PCR test at home. In that case you will need to register your PCR test kit in order to get your result from the NHS.
READ MORE: The new full list of official COVID-19 symptoms including loss of appetite

Can I report a test I bought?
No, you can only use the gov.uk service to report free NHS lateral flow test results. You can't report tests you bought from private laboratories or retailers such as Boots and Tesco. The official advice says that if you paid for a test, you should check the test kit instructions to see if you should report your results to the private test provider.
Should I report a negative result?
The NHS says you should report your test result no matter what it is - positive, negative or void. You should do so every time you take a rapid lateral flow test, which shows you the result on a handheld device that comes with the kit.
This means that if you're testing daily because you are a contact of someone who has coronavirus, you should report your result every day. If you have a health condition that makes you eligible for Covid-19 treatments it's especially important to report a positive result so that the NHS can contact you about your treatment.
How to report a test result
You need to report your result within 24 hours of getting it. Remember to check the expiry date on the box the test came in to make sure your test is not out of date.
To use the online service, you will need the QR code or ID number printed on the test strip (the part of the kit that shows your result), and a mobile phone to receive a confirmation text your result was received. If you cannot use the online service you can call 119.
Get more health news from CambridgeshireLive straight to your inbox for free HERE .
- Coronavirus
- Most Recent

How do I enter a positive test result in the app?
You may get an 8-character long code from the testing service, by email or text, if you have taken a PCR test or reported your lateral flow test result online .
To input your test result to the app, select 'Enter test result' on the home screen of your app. Then, enter the 8-character code you received from the testing service.
By inputting a test result to the app, the app can give you the most relevant guidance. You can choose to anonymously share your result with others and help others to take steps to reduce the spread of COVID-19. Other app users you have been in ‘close contact’ with over the last few days will then receive an anonymous notification on their app . Read more about who gets an alert if you test positive .
- U.S. Department of Health & Human Services

- Virtual Tour
- Staff Directory
- En Español
You are here
News releases.
Media Advisory
Tuesday, November 22, 2022
NIH establishes website for self-reporting COVID-19 test results

Reporting a positive or negative test result just became easier through a new website from the National Institutes of Health. MakeMyTestCount.org, developed through NIH’s Rapid Acceleration of Diagnostics (RADx®) Tech program, allows users to anonymously report the results of any brand of at-home COVID-19 test.
COVID-19 testing remains an essential tool as the United States heads into the holiday season and people navigate respiratory viruses. While taking a rapid COVID-19 test has become commonplace, test results are not often reported. COVID-19 test results provide valuable data that public health departments can use to assess the needs and modify the responses in the local community, the state or the nation.
Lab tests have a well-established technology system for sharing test results. RADx Tech has been working on a system to standardize test reporting for at-home tests in a secure manner. The MakeMyTestCount.org website is built on this system for logging test results.
The National Institute of Biomedical Imaging and Bioengineering (NIBIB) supported development of MakeMyTestCount.org through the RADx Tech program.
NIBIB Director Bruce Tromberg, Ph.D., who leads the RADx Tech program, is available for comment.
About the Rapid Acceleration of Diagnostics (RADx ® ) initiative: The RADx initiative was launched on April 29, 2020, to speed innovation in the development, commercialization, and implementation of technologies for COVID-19 testing. The initiative has four programs: RADx Tech, RADx Advanced Technology Platforms, RADx Underserved Populations and RADx Radical. It leverages the existing NIH Point-of-Care Technology Research Network. The RADx initiative partners with federal agencies, including the Office of the Assistant Secretary of Health, Department of Defense, the Biomedical Advanced Research and Development Authority, and U.S. Food and Drug Administration. Learn more about the RADx initiative and its programs .
About the National Institute of Biomedical Imaging and Bioengineering (NIBIB): NIBIB’s mission is to improve health by leading the development and accelerating the application of biomedical technologies. The Institute is committed to integrating the physical and engineering sciences with the life sciences to advance basic research and medical care. NIBIB supports emerging technology research and development within its internal laboratories and through grants, collaborations, and training. More information is available at the NIBIB website .
About the National Institutes of Health (NIH): NIH, the nation's medical research agency, includes 27 Institutes and Centers and is a component of the U.S. Department of Health and Human Services. NIH is the primary federal agency conducting and supporting basic, clinical, and translational medical research, and is investigating the causes, treatments, and cures for both common and rare diseases. For more information about NIH and its programs, visit www.nih.gov .
NIH…Turning Discovery Into Health ®
Connect with Us
- More Social Media from NIH
How to report a positive Covid test: Where to register a lateral flow test result and rules explained
England scrapped free coronavirus tests for the majority of the population on 1 april, but still advises people to report their results.
Around seven in 10 people in England are likely to have had coronavirus since the early months of the pandemic , according to new figures, and rates continue to reman high.
England scrapped free coronavirus tests for the majority of the population on 1 April, while free testing continues in Scotland and Northern Ireland during April, and until the summer in Wales.
But what should you do if you get a positive lateral flow test ? How do you report your results and what do the current rules mean?
Here’s what you need to know.
Where to register a lateral flow test result
The Government advises people to report their result every time they take a lateral flow test.
You can report your test result to the Government here .
You will need the QR code or ID number printed on the test strip (the part of the kit that shows your result) and a mobile phone number so they can text you to confirm they have got your result.
If you cannot use the online service, call 119 (free from mobiles and landlines).
Lines are open every day, 7am to 11pm. 119 provides support in 200 languages.
SignVideo is a free online British Sign Language (BSL) interpreter service for 119.
More from Health
You cannot use this service service to report results from a test kit you’ve paid for. If you have paid for a test, check the test kit instructions to see if you should report your results to the private test provider.
You should report the result whether it is negative, positive or void. You cannot report a result after more than 24 hours, and can only report one result at a time.
What to do if you test positive
While self-isolation is no longer a legal requirement, the NHS advises that people infected with Covid “should stay at home and avoid contact with other people” to help reduce the spread of the virus.
In particular, you should avoid being in close contact with people at higher risk from Covid, for example if they are elderly or have a weakened immune system, even if they have had the vaccine.
Infected people should try to work from home if they can, Government advice states. “If you are unable to work from home, talk to your employer about options available to you,” it adds.
Positive cases should try to stay at home and avoid contact with other people for five days after the day of their test.
Authorities in Wales, Scotland and Northern Ireland also advise people who test positive to isolate if they test positive, but you can also order or book free testing in these countries.
Most Read By Subscribers

Report Covid-19 Home Test Results Through New NIH Website

Reporting a positive or negative test result just became easier through a new website from NIH, MakeMyTestCount.org . Developed through NIH’s Rapid Acceleration of Diagnostics (RADx®) Tech program, the new website allows users to anonymously report the results of any brand of at-home Covid-19 test.
Covid-19 testing remains an essential tool as the United States heads into the holiday season and people navigate respiratory viruses. While taking a rapid Covid-19 test has become commonplace, test results are not often reported. Covid-19 test results provide valuable data that public health departments can use to assess the needs and modify the responses in the local community, the state or the nation.
Lab tests have a well-established technology system for sharing test results. RADx Tech has been working on a system to standardize test reporting for at-home tests in a secure manner. The MakeMyTestCount.org website is built on this system for logging test results.
How to report a Covid test result and what to do if it's positive, negative or void
Coronavirus infections have been rising again in England, but more than a quarter say they would still go to work if they tested positive
- 12:44, 24 JUL 2022

Sign up for free to get the latest North East news and updates delivered straight to your inbox
We have more newsletters
Coronavirus infections and hospital admissions rose in England last week, but more than a quarter say they would still go to work if they tested positive.
Office for National Statistics data shows 3.8 million were estimated to have coronavirus in the week to July 13/14. That's a rise of seven per cent from the 3.5 million recorded the previous week.
This is the highest estimate for total infections since mid-April. But is still below the record high of 4.9 million seen at the peak of the Omicron BA.2 wave at the end of March.
Read more: You may have Covid-19 if you wake up with these symptoms
Kara Steel, ONS senior statistician for the Covid-19 infection survey, said: “Infections have, overall, continued to increase in England, reaching similar levels to those seen in April during the BA.2 wave.
“However, we are seeing some uncertain trends in the latest data across the other UK countries, some English regions, and among some age groups. It is too early to say if this most recent wave is starting to peak, but we will continue to closely monitor the data.”
Despite the latest wave, a poll suggests concern about Covid-19 has fallen to its lowest level since the start of the pandemic. Around two-thirds of people surveyed (65%) said they are concerned about the risk coronavirus poses to the country. That is down from 71% in March, according to data shared with the PA news agency by poll company Ipsos.
More than a quarter (28%) said they would go to work if they test positive for coronavirus. More than a third (38%) say they would leave the house. Nearly half (47%) would go for a walk outside.
In England, people are advised to isolate if they test positive. But they are not required to stay at home, and special Covid sick pay for NHS staff has been scrapped.
Keiran Pedley, research director at Ipsos, said: “Although concern about the virus is at its lowest level since the pandemic began, it should be noted that a majority of the public are still concerned. They are also prepared to avoid seeing others or spending time in public places should they test positive for the virus.
“This all shows that, whilst other issues, such as the cost of living, might be more foremost on people’s minds, the public are still prepared to take the virus seriously.”
How to report a Covid-19 test and what you should do if it is positive, negative, or void
With a rise in coronavirus levels, you may want to consider taking tests, especially if you develop symptoms or have been around a positive case. Below are reminders on how to report coronavirus tests, when you should report them and what you should do if it is positive, negative, or void.
How and when to report a lateral flow rapid coronavirus test
You may think you're supposed to only report a Covid test if it's positive. But strictly speaking, you're supposed to report them if they are negative or void too.
The NHS says: "Report your test result (positive, negative, or void) every time you do a free NHS rapid lateral flow test for coronavirus."
Gov.UK says reporting every result helps to reduce infections in communities, protects those at higher risk and prevents the spread of the virus.
You should report Covid tests results within 24 hours of taking the test. You will need the QR code or ID number printed on the test strip and a mobile phone number if reporting online. Those who can't use the internet can report their result via the 119 phone number.
Head here to report a lateral flow test result . You will need an NHS log-in, if you haven't got one, you can create one in two minutes.
Do I have to isolate if I test positive for Coronavirus?
There is no longer a legal requirement to isolate. But if you test positive you "should try to stay at home and avoid contact with other people for five days after the day you took the test," GOV.UK's rules for England have stated since April .
What do I do if my Covid test is negative? Should I stay home?
You don't have to do anything. However, there is a chance you still may be infectious. So you are advised to follow advice on how to avoid catching and spreading the virus, by hand washing, wearing a mask, keeping your distance and opening windows if you live with others.
If you have symptoms, you are advised - but not forced - by the NHS to "try to stay at home and avoid contact with other people".
What should I do if my Covid test is void?
A void test means the result is uncertain. You should do another test as soon as possible and are advised, but not required, to stay home and away from others as much as possible if you feel unwell or have symptoms.
- Long Covid risk for children who display at least four symptoms like this
European holiday destination to scrap Covid rules for UK travellers next week
Start wearing face masks again to avoid another Covid lockdown, expert warns
Advice for treating Covid symptoms at home and when you can seek NHS treatment
Train and ferry passengers 'asked to wear masks again'
- Coronavirus
- Most Recent

Cookies on the NHS England website
We’ve put some small files called cookies on your device to make our site work.
We’d also like to use analytics cookies. These send information about how our site is used to a service called Google Analytics. We use this information to improve our site.
Let us know if this is OK. We’ll use a cookie to save your choice. You can read more about our cookies before you choose.
Change my preferences I'm OK with analytics cookies
Lateral flow antigen test FAQs
Classification: Official
Publications approval reference: C0913
Second wave roll out trusts
Updated version 3, 28 January 2021 – Additional questions highlighted in yellow.
Frequently asked questions
These are the specific FAQs related to the lateral flow antigen tests. For all questions on HR processes following a positive test and related isolation questions, please refer to NHS Employers’ FAQs on asymptomatic staff testing .
Q. What type of test are we rolling out?
The Innova SARS-CoV-2 Antigen Rapid Qualitative Test uses a swab which has been in contact with the nostril of the person being tested. The swab is inserted into the extraction tube with the extraction fluid and then rotated and pressed to make sure that the sample from the swab is released into the extraction fluid (swab is then discarded at this point).
You then take the extraction tube with the nozzle cap and place 2 drops of extraction fluid into the sample well of the LFD testing device cartridge and wait for the results on the test device.
Q. What is the specificity and sensitivity of this particular test?
The government has published its latest research on these tests. This can be found here https://www.ox.ac.uk/news/2020-11-11-oxford-university-and-phe-confirm-high-sensitivity-lateral-flow-tests-following
Q. Is the test mandatory or voluntary?
Tests are voluntary, but staff should be encouraged to be involved in the testing to benefit their colleagues and patients.
Q. How frequently should staff be tested?
Staff should test themselves twice weekly every three to four days to fit with shift patterns and leave requirements; for example, Wednesday and Sunday, or Monday and Thursday.
Q. Should I continue testing after I’ve had the vaccine?
Yes, continue to test even though you have had the vaccine.
Q. If a staff member has a positive PCR COVID-19 test, when should they start the lateral flow antigen tests again?
A staff member who tested positive would recommence home testing 90 days after their positive test was taken. The staff member will need to liaise with their NHS organisations to track the date at which the retesting should start.
Q. What happens if staff get a positive result?
Staff should inform their manager of a positive result trust in the normal way. A confirmatory PCR test will be arranged. They and their household should isolate as set out in government guidance .
Q. What happens if my test is negative, but I have coronavirus symptoms?
If you have coronavirus (COVID-19) symptoms, please refer to NHS guidance online .
Q. Are we asking potentially positive staff to come to hospitals for a confirmatory PCR test?
Trusts should use their normal processes to access tests for staff members who have symptoms of COVID-19, whether that be through pillar 1 or 2. These processes assume that staff may be infected with COVID-19 and therefore suitable IPC and PPE will be in place. Staff should continue to isolate until they have the results of the PCR test.
Q. What should staff do with the used tests?
Staff can safely dispose of the test items in their normal household waste but should pour any residual buffer solution away first.
Q. What happens if the buffer solution is accidentally consumed?
As set out in the manufacturer’s safety instructions, the buffer solution is not hazardous; however, if accidentally ingested, a medical practitioner should be informed.
Q. What is the shelf life of the extraction (buffer) solution once opened?
The shelf life of extraction solution is 2 years, even after it is opened.
Q. How do I get additional bottles of extraction (buffer) solution as I don’t have any left (due to spillage etc.) but I still have kits left in my box of 25 kits?
Each region has identified one or more Trusts within the region who will hold a stock of extraction solution for onwards distribution.
For additional stock of extraction solution your Trust should contact their regional testing teams who will coordinate distribution.
Q. At what stage is Test and Trace informed of the result?
At the point the confirmatory PCR test result is known, and this is positive result, test results will, as normal, be referred to Test and Trace.
Q. If I am told to isolate by Test and Trace even though I have had the vaccine, do I need to do so?
Yes, continue to take advice and follow instructions given by Test and Trace.
Q. If staff are already regularly being tested through existing regimes – e.g. professionals visiting care homes, SIREN testing, PCR testing in Tier 3 areas, etc – should this be replaced by lateral flow tests?
If staff are already enrolled in another testing regime through their NHS organisation, this should not be replaced by the lateral flow tests unless agreed by your organisation.
If they are participating in research studies where the frequency of testing is not weekly (e.g. monthly) they should undertake twice-weekly LFD self-testing. For example, staff members participating in the SIREN study and having qRT PCR testing every two weeks should also be part of the twice-weekly LFD testing if they are a patient-facing member of staff.
Q. What about LAMP testing – I thought this was being used for asymptomatic staff testing?
LAMP testing has been piloted at a number of sites for consideration for asymptomatic staff testing. Further rollout will depend on the outcomes of the early adopter sites.
Q. How many tests will staff get?
The testing kits will arrive in boxes containing the following:
- 25 foil pouches containing the test cartridge and a desiccant
- two vials of 6 mls buffer solution
- 25 extraction tubes and 25 tube caps
- 25 sterilised swabs for sample collection
- The manufacturer’s instructions for use of the device (IFU). You will receive instructions for NHS staff separately from the box, and it is these NHS staff instructions that staff should follow.
Q. Can these tests be used for patients?
PCR tests should continue to be used for patients.
Q. Our trust would like to use these new faster tests to manage patient flow in the Emergency Department – are we okay to use in this way?
Yes. The tests can be used in accordance with the SOP at https://www.england.nhs.uk/coronavirus/publication/standard-operating-procedure-lateral-flow-device-testing-for-emergency-department-patient-pathways/ .
Q. Should patients who have been in direct care of a staff member who tests positive with lateral flow be tested while the confirmatory PCR test result is pending?
Your organisation’s protocols for tracing contacts should be followed.

Q. Will this testing regime remove the need for staff who have been exposed to a positive Covid-19 case to self-isolate?
Government self-solation advice should be followed at all times. This test does not remove the need to self-isolate should you otherwise need to.
Q. Can 14-day isolation following contact tracing be shortened through use of this testing?
No. Fourteen-day isolation following notification that a staff member has been in close contact with a COVID-19 case without relevant PPE should be followed as per Test and Trace advice. Testing with lateral flow antigen tests are being used in pilot sites to verify whether daily testing might lessen the need to isolate, but this is not currently the advice and isolation should be followed as per instructed by Test and Trace.
Q. Is there any prioritisation of which staff this should be rolled out to first?
Sufficient volumes of the lateral flow devices will be sent to organisations to enable all staff to be given the test asap.
Q. Can staff use the tests for their symptomatic family members?
Staff and family members who have symptoms should access tests in the normal way.
Q. Can tests be used as a response to Covid-19 outbreaks?
Should an outbreak be declared in your organisation, testing regimes should be discussed in line with your normal organisational response.
Q. Why is the testing method different from that described in the manufacturer’s original instructions for use?
We are recommending the swab is used and the sample taken in a different way to the instructions for use, with more rotation of the swab at a lower level of penetration, to enable easier self-administration of the test. This is based on advice from experts. The manufacturer has been informed of the planned use of the tests for self-administered asymptomatic staff testing within the NHS and trusts have been asked to provide a local support package to include staff access to a helpline/further training and, if deemed necessary, on-site training arrangements. It is recommended that staff are observed by a trained healthcare colleague the first time they administer the test.
Q. You say that it is recommended that the first test is observed. This presents logistical issues, so can staff be trained to take the test but not observed?
We advise that any staff member who needs support undertaking the test is provided with appropriate support and training and observed on the first occasion. Trusts should use their discretion as which staff may require additional support. Observation of the first test is not mandatory for all staff.
Q. Is there advice on giving staff time back from undertaking the test at home?
The test should take no longer than 5 minutes to undertake, with a 30 minute wait for results.
Q. When deliveries arrive what size of space should be allocated for them?
Tests will arrive on pallets, there are usually 12 boxes on a pallet that contain 27 smaller boxes that contain 25 tests in each – 8,100 tests in total.
Q. Should the tests be kept in specific conditions; will they require security like Tamiflu did?
Tests can be stored in typical warehouse conditions; they do not need refrigeration but should be kept out of direct sunlight and not be exposed to heat. They are not expected to require any additional security than other items of NHS deliveries.
Q. Can hospitals procure their own supply of lateral flow tests?
Lateral flow tests are purchased and provided centrally, and trusts should not purchase them directly from suppliers.
Q. What are the financial arrangements in place to be able to support this roll out of testing?
Providers were reimbursed for the rolling out of lateral flow testing for staff with the agreed fixed payment value based on the number of patient facing staff.
This payment was made on the same day (15 December) as the provider’s month 6 retrospective top up payment.
Q. When will we receive our delivery of tests?
We are rolling out the second wave of deliveries week commencing 25th January; you will be notified two days in advance of any scheduled delivery of tests.
Q. For trusts with multiple sites can you confirm they can have tests delivered to multiple sites?
Due to the extent of the logistics required, we can only have a single delivery for each organisation. Trusts will be notified in advance of the delivery schedule.
Q. Can you give guidance on whether Community Interest Companies and Independent Sector Providers providing patient facing care will be included in the testing?
Yes, staff delivering NHS services in Community Interest Companies and Independent Sector Providers are within scope
Q. Are 111 and 999 call handler staff included in the testing?
While 111 and 999 call handler staff are not patient facing, they are considered a high risk group because of the way in which they work and the impact of staff absence on the service should an outbreak occur. We are therefore including them in asymptomatic testing.
Q. How frequently should a contractor/temporary worker be working in a trust to be included in testing?
Staff who are patient facing and are regularly working in your trust should be included in the testing. This should include those patient-facing staff who are directly contracted by your trust.
Q. Doctors on their foundation programme move hospitals, how should their testing programme work?
All foundation year doctors in your trust when you roll out staff testing should be included as they are patient facing. If they move to another trust while they are still testing and have a supply of tests, they should keep these tests but report the test results to the trust they are now working at. The same instruction should be given to any new starters, if they don’t already have tests from a previous employer, they should be provided with tests to start testing with you.
Q. Do we treat two positive lateral flow antigen test results as an outbreak?
Lateral flow antigen positive test results should be confirmed through PCR testing; if the confirmatory tests are also positive, then normal outbreak protocols would apply.
Q. Should staff continue swabbing during annual leave?
Staff may continue to swab whilst on annual leave of longer than a week, but it is not a requirement.
Q. Do I have to share my result if I am going into a care home?
Yes, please share your result. If you are unable to do this you may be asked to undertake an LFD before entering the care home.
Q. Is confirmatory PCR testing accessible through pillar 2, and if yes what field should be filled to avoid symptomatic questions?
You should use whatever PCR route is in use by your organisation. If this is through pillar 2, tick the box that indicates you are a key worker but not part of a pilot, you will then see an option to say ‘I’ve been told to take a Coronavirus test’ on the form.
Q. Can you confirm the reporting requirements?
Trusts are asked to collate and report to PHE the positive and negative lateral flow results at least once a week. This fulfils the statutory reporting requirements for COVID-19 testing. Trusts are also required to report back on an NHSEI daily sitrep the number of tests distributed and number of staff who are absent from work having tested positive on a lateral flow device.
Q. How should organisations collate the positive and negative results from staff?
We are aware that trusts have developed a number of efficient solutions for capturing the data e.g. using webforms or apps. Examples of these will be shared with regional testing leads. NHS Digital is also developing a digital solution which will be shared when available.
Q. How do I submit the testing returns to Public Health England (PHE)?
All NHS organisations will be required to collate and submit returns to Public Health England via a data upload to its Point of Care Test (POCT) portal at least once a week. This is a statutory requirement as COVID-19 is a notifiable disease.
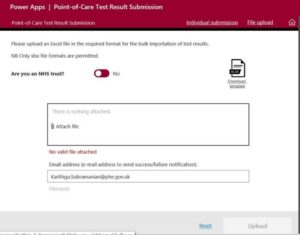
Organisations which do not currently have access to this portal should send an email to [email protected] . Users will then receive an email with a registration link. Once registered they can use the web app to upload the reporting template spreadsheet, which is available for download on the portal.
It is essential to use the reporting template provided by PHE to upload your Trust results.
On the portal the ‘Requesting Organisation Type’ field must be populated with the dropdown “Healthcare Worker Testing”. This is essential to ensure that test and trace activity is not triggered as a result of lateral flow testing.
Trusts must use the drop down in ‘Requesting Organisation’ to identify their organisation. This ensures that a consistent trust name is used so that reports are correctly linked.
The default is to have the toggle as ‘no’ for “Are you an NHS trust?” (see below). You will need to change this to “yes”. You may need to leave blank the email address section as this is defaulted to the person logged-in.
Q. Can we get the lateral flow guidance leaflet in other languages?
There are no plans to translate the lateral flow guidance leaflet.
Q. What about staff that form part of the local authority team e.g. school nurses, or those in care homes or hospices?
The DHSC are responsible for rolling out lateral flow antigen tests to local authorities and staff delivering care outside the NHS. Contact [email protected] for more information.
Q. Where should I direct any enquiries?
Email questions to [email protected] .
NHS Testing Programme NHS England and NHS Improvement Skipton House 80 London Road London SE1 6LH
This publication can be made available in a number of other formats on request. Publication approval reference: C0913
View your GP health record
Your GP health record includes information about the care you've had from your GP surgery.
When you view your record online, you may only see information that was added recently. If you need to see older information, you'll have to ask your GP surgery to make it visible.
What's not in your GP record
information kept by other services (such as hospitals, dentists and opticians) that has not been sent to your GP – to get this information, you'll need to contact these services directly
How to see all the information in your GP health record
When you view your GP health record online, you should be able to see any information that was added recently.
If you need to see older information in your record (historic record information), you'll have to ask your GP surgery to make it available online.
You can either:
- email or call your GP surgery and tell them you want to see historical information in your online GP health record
- mention it to a receptionist at the surgery when you go for your next appointment
Let your GP surgery know if you only need access to something specific in your record (such as previous test results). This can be quicker than getting access to your full historic record.
The surgery will consider your request and make sure there are no issues with making the information available to you (for example, if it could put you or someone else at risk of harm). This can take a few weeks.
If there are no issues with making this information available, you'll be able to see it when you view your GP record online.
View your GP health record using your NHS App or account
You can view your GP health record using the NHS App or by logging into your account on the NHS website.
You can create an account if you do not already have one.
To view your GP record online, you must be:
- registered with a GP surgery
- aged 16 or over
When you create an account, you'll need to prove your identity before you can view your GP health record. This helps keep your record secure.
Use other GP online services and apps
You may be able to use other GP online services and apps to view your GP record.
Some services and apps are only available in certain areas. Ask your GP surgery which you can use.
Contact your GP surgery
You can ask for your GP record at your GP surgery. They can give you a printed copy of your record or send you a digital version.
Viewing someone else's GP health record
Find out about accessing someone else's medical records
Non-urgent advice: Contact your GP surgery if:
- you're unable to see your record
- information is missing or has disappeared from your record
- you can only see very recent information in your record and you need to see older information
- there's incorrect information in your record
- you can see information that should not be there (for example, you can see information that's not yours)
- you do not want to be able to see your record
- someone is pressuring you for information in your record
Email or call the surgery, or speak to a receptionist at the surgery when you go for your next appointment.
Page last reviewed: 8 November 2023 Next review due: 8 November 2026
Cookies on GOV.UK
We use some essential cookies to make this website work.
We’d like to set additional cookies to understand how you use GOV.UK, remember your settings and improve government services.
We also use cookies set by other sites to help us deliver content from their services.
You have accepted additional cookies. You can change your cookie settings at any time.
You have rejected additional cookies. You can change your cookie settings at any time.
- Testing for COVID-19
COVID-19: testing from 1 April 2024
Explains testing from 1 April 2024 onwards and sets out the purpose of ongoing testing, who will be eligible to access testing and when tests should be used.
The role of testing
Throughout the coronavirus (COVID-19) pandemic, the government has prioritised protecting the most vulnerable and those in high-risk settings. Government-funded testing will continue to focus on these groups. In March 2023, the Government announced further changes towards managing COVID-19 like other respiratory illnesses. New changes from 1 April 2024 are the next stage in delivering this approach.
The ongoing success of the vaccination programme, increased access to treatments and high immunity among the population have allowed the government to scale back testing in England. From April onwards, testing will be provided to individuals at highest risk from COVID-19, continuing to support diagnosis for care and access to treatments.
From 1 April 2024, routine provision of free COVID-19 lateral flow device ( LFD ) tests for the management of outbreaks in higher risk settings will come to an end in England. However, free polymerase chain reaction ( PCR ) testing to determine the cause of an acute respiratory infection outbreak in higher risk settings, where deemed appropriate by a local UK Health Security Agency ( UKHSA ) health protection team ( HPT ), will remain to test for a wide range of respiratory viruses.
The cohort of people eligible for COVID-19 treatments can still access free COVID-19 LFDs from their local pharmacy. These people, who are at highest risk of getting seriously ill, are encouraged to test to gain timely access to treatments. A full list of those who are eligible, and information on how to access tests, is available on the NHS website: https://www.nhs.uk/COVIDtreatments
Routine asymptomatic COVID-19 LFD testing on discharge from hospital into care or hospice settings will also end to align with the approach for other respiratory illnesses, though NHS Trusts will have local discretion to re-introduce this or other forms of testing as clinically appropriate following risk assessment, involving local authority public health teams, UKHSA HPTs and care providers as necessary in decision making.
Within healthcare settings, limited testing, including symptomatic testing of staff working on inpatient wards focused on treating profoundly immunocompromised individuals, will continue in line with locally derived protocols to protect those most at risk. Symptomatic testing of patient-facing hospice staff who work closely with people who are severely immunocompromised will also continue as outlined in guidance, in line with similar NHS settings.
Where symptomatic testing is recommended, this should be based on the current list of COVID-19 symptoms .
All other residents, service users, patients and staff who are symptomatic should follow guidance for the general population on what to do if they have symptoms of a respiratory infection or a positive COVID-19 test , and the guidance on actions we can all take to help reduce the risk of catching COVID-19 and passing it on to others .
Testing recommended in NHS settings
In addition to the recommended testing identified, local healthcare organisations, with appropriate advice (including from Medical Directors, Nursing Directors or Directors of Infection Prevention and Control), may exercise local discretion to continue testing for specific individuals or cohorts in line with broader infection prevention and control measures. This includes emergency admission, elective pathway and transfer of care admissions, for example to a ward caring for patients who are severely immunosuppressed.
Together with the care home or hospice setting, hospitals should assess the risk in the period before planned discharge, seeking advice on proposed changes to testing arrangements from local authority public health teams or UKHSA HPTs , if needed. Following discussion with care home providers and any advice from public health teams or HPTs , hospitals may decide to undertake an LFD test, for example if there is a local outbreak within the hospital setting. This test should be provided and done by the hospital.
Testing recommended in care services (adult social care and hospices)
Guidance on a range of infection prevention and control measures in adult social care has now been combined with acute respiratory infection guidance in the guidance for infection prevention and control in adult social care: acute respiratory infection .
Testing recommended in other non-healthcare settings
These settings include prisons, immigration retention or removal centres, asylum reception centres, asylum hostel accommodation and reception centres, homelessness settings (including night shelters, hostels, hotels, and other temporary accommodation), domestic abuse refuges and respite rooms.
The risk of harm from COVID-19 for children and young people is very low.
Children and young people ( CYP ) settings do not require continued access to testing in residential special educational needs and disability ( SEND ) settings or the CYP secure estate ( CYPSE ).
Settings are encouraged to follow guidance on GOV.UK:
- Health protection in children and young people settings, including education
- Preventing and managing outbreaks of acute respiratory illness ( ARI ) in the CYPSE
Is this page useful?
- Yes this page is useful
- No this page is not useful
Help us improve GOV.UK
Don’t include personal or financial information like your National Insurance number or credit card details.
To help us improve GOV.UK, we’d like to know more about your visit today. We’ll send you a link to a feedback form. It will take only 2 minutes to fill in. Don’t worry we won’t send you spam or share your email address with anyone.

IMAGES
VIDEO
COMMENTS
You do not need to report the result from a COVID-19 rapid lateral flow test in England. Report a free NHS COVID-19 rapid lateral flow test result - GOV.UK Cookies on GOV.UK
View your test results using your NHS App or account. You can view test results in your GP health record using the NHS App or by logging into your account on the NHS website. You can create an account if you do not already have one. To view your test results online, you must be: registered with a GP surgery. aged 16 or over.
Negative test result. If you get a negative result, it means it's unlikely you have COVID-19. There's still a chance you could have the virus, so you should follow the advice on how to avoid catching and spreading COVID-19. If you're eligible for COVID-19 treatments and you get a negative result, you need to do a total of 3 rapid lateral flow ...
You can report a free NHS rapid lateral flow test result on GOV.UK. This is different to registering a test kit, which you may be asked to do if you have a PCR test at home. In that case you will ...
The Government is asking people who take coronavirus lateral flow tests to report their results to the NHS. These tests are being given to NHS and care home staff, and are also available to book ...
After taking the test each day, results must then be reported to NHS Test and Trace or gov.uk. This can be done online or by calling 119. If you do not test positive for seven days, you can ...
NHS PCR tests. Find out who can get free NHS tests, how to get tested, and what your test result means.
This includes reporting NHS lateral flow test results on GOV.UK. If you're eligible for COVID-19 treatment, you must report your result so the NHS can contact you about treatment. ... you must report your result so the NHS can contact you about treatment. Find out about your app data and privacy. From: UK Health Security Agency Published 13 ...
To input your test result to the app, select 'Enter test result' on the home screen of your app. Then, enter the 8-character code you received from the testing service. By inputting a test result to the app, the app can give you the most relevant guidance. You can choose to anonymously share your result with others and help others to take steps ...
Is it mandatory for staff to now report results to GOV.UK, or can trusts continue to use local reporting systems if these are working well? All test results must be reported whether they are positive, negative or invalid/void. It is a statutory requirement to report results every time a self-test is completed.
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site. The site is secure. ... Tech program to give people a secure place to report their at-home test results. The results are then submitted to the same public health systems ...
To report your result online you can visit the gov.co.uk website and follow the instructions. Before you start you will need the QR or ID number printed on the test strip and a mobile phone number so that you can receive a test confirming the NHS have your result. If you are having difficulty online, you can also report your result by phone by ...
COVID-19 test results provide valuable data that public health departments can use to assess the needs and modify the responses in the local community, the state or the nation. Lab tests have a well-established technology system for sharing test results. RADx Tech has been working on a system to standardize test reporting for at-home tests in a ...
Completing the test. put the end of the swab into the tube so it's in the liquid and swirl the swab around as directed in the test kit instructions, then close the lid. squeeze the liquid from the tube onto the test strip. check the waiting time in the instructions that came with your test kit. wait for the time shown in your test kit instructions.
The Government advises people to report their result every time they take a lateral flow test. You can report your test result to the ... 2024 Young people turning backs on NHS and going private ...
Covid-19 test results provide valuable data that public health departments can use to assess the needs and modify the responses in the local community, the state or the nation. Lab tests have a well-established technology system for sharing test results. RADx Tech has been working on a system to standardize test reporting for at-home tests in a ...
The NHS says: "Report your test result (positive, negative, or void) every time you do a free NHS rapid lateral flow test for coronavirus." Gov.UK says reporting every result helps to reduce ...
The Innova SARS-CoV-2 Antigen Rapid Qualitative Test uses a swab which has been in contact with the nostril of the person being tested. The swab is inserted into the extraction tube with the extraction fluid and then rotated and pressed to make sure that the sample from the swab is released into the extraction fluid (swab is then discarded at ...
View your GP health record using your NHS App or account. You can view your GP health record using the NHS App or by logging into your account on the NHS website. You can create an account if you do not already have one. To view your GP record online, you must be: registered with a GP surgery. aged 16 or over. When you create an account, you'll ...
You should count the day after you took the test as day 1. If a child or young person aged 18 or under has a positive coronavirus test result, they should try to stay at home and avoid contact with other people for 3 days after the day they took the test or from the day their symptoms started (whichever was earliest), if they can.
Attorney General Todd Rokita reveals faulty COVID-19 data in shocking report. On the four-year anniversary of Indiana's stay-at-home orders, Attorney General Todd Rokita unveiled the results of a report he commissioned to expose the real numbers associated with the coronavirus lockdowns. "The truth is our government produced and relied on severely flawed data, including inflated death ...
From April onwards, testing will be provided to individuals at highest risk from COVID-19, continuing to support diagnosis for care and access to treatments. From 1 April 2024, routine provision ...