- - Google Chrome
Intended for healthcare professionals
- Access provided by Google Indexer
- My email alerts
- BMA member login
- Username * Password * Forgot your log in details? Need to activate BMA Member Log In Log in via OpenAthens Log in via your institution


Search form
- Advanced search
- Search responses
- Search blogs
- Advances in the care...
Advances in the care of breast cancer survivors
Linked editorial.
Understanding inequalities in breast cancer screening uptake
Linked research
Effect of invitation letter in language of origin on screening attendance
- Related content
- Peer review
- Elizabeth J Cathcart-Rake , medical oncologist ,
- Amye J Tevaarwerk , medical oncologist ,
- Tufia C Haddad , medical oncologist ,
- Stacy D D’Andre , medical oncologist ,
- Kathryn J Ruddy , medical oncologist
- Mayo Clinic, Department of Oncology, Rochester, MN, USA
- Correspondence to: E J Cathcart-Rake Cathcart-rake.elizabeth{at}mayo.edu
Breast cancer survivors may experience significant after effects from diagnoses of breast cancer and cancer directed therapies. This review synthesizes the evidence about optimal management of the sequelae of a diagnosis of breast cancer. It describes the side effects of chemotherapy and endocrine therapy and evidence based strategies for management of such effects, with particular attention to effects of therapies with curative intent. It includes strategies to promote health and wellness among breast cancer survivors, along with data to support the use of integrative oncology strategies. In addition, this review examines models of survivorship care and ways in which digital tools may facilitate communication between clinicians and patients. The strategies outlined in this review are paramount to supporting breast cancer survivors’ quality of life.
Introduction
Breast cancer is the most common cancer in the world, with 2.26 million diagnoses in 2020. 1 People with breast cancer are living longer as a result of improvements in screening and treatment, such that in the US alone the number of survivors of breast cancer is expected to grow by more than 2 million in the next decade. 2 3 Unfortunately, survivors may experience significant after effects from breast cancer diagnoses and cancer directed therapies. Symptoms related to surgery, radiation, and systemic therapies may persist lifelong and limit quality of life.
This review synthesizes the evidence base on optimal management of treatment sequelae in survivors of breast cancer. We describe the side effects of chemotherapy and endocrine therapy and evidence based strategies for management of such effects, with particular attention to effects of therapies with curative intent. We include strategies to promote health and wellness among breast cancer survivors, along with data to support the use of integrative oncology strategies. In addition, we examine models of survivorship care and ways in which digital tools may facilitate communication between clinicians and patients.
Sources and selection criteria
We searched PubMed for the term “breast cancer survivorship” (without restricting the date range) on 17 August 2022 and limited our search to papers designated as randomized controlled trials (RCTs) or meta-analyses. This identified 245 peer reviewed publications, some of which we excluded because they were focused more on disease outcomes than on management of toxicity and models of survivorship care. We supplemented this search strategy by a hand search of the references of key articles. We achieved inclusion of identified articles by assessing a study’s impact and its methods, with preference given to RCTs. When RCT evidence was not available for certain topics, we included other study types, focusing on the highest level of evidence available and excluding lower level evidence. To ensure that we covered all critical data relevant to management of endocrine therapy toxicity, management of long term effects of chemotherapy, new models of care and digital tools to facilitate communication and symptom management, integrative oncology for breast cancer survivors, general health maintenance (including screening for second cancers), genetic testing, and surveillance for recurrence, we also added select additional studies for inclusion on the basis of consensus among our author group (medical oncologists with specific expertise in symptom control (ECR, KR), survivorship (KR, AT), integrative medicine (SD), and digital oncologic tools (TH, AT)).
Epidemiology
Breast cancer is the most commonly diagnosed cancer, accounting for nearly a quarter of all cancer cases globally. 4 More than 2.3 million people worldwide are given a diagnosis of breast cancer annually, and more than 7.8 million women are breast cancer survivors with diagnoses over the past five years, according to 2020 statistics. 4 Although the incidence of and mortality from breast cancer vary from region to region, these numbers are steadily growing, such that more than 3 million new cases of breast cancer are projected to be diagnosed by 2040. 4
Burden and management of long term effects of systemic therapies
Despite recent advances that help us to tailor and de-escalate treatment on the basis of estimates of clinical benefit to limit toxicity, 5 6 many curatively treated breast cancer patients still receive chemotherapy. This is particularly true among patients with triple negative breast cancer, who may receive up to four chemotherapies (anthracycline, cyclophosphamide, taxane, platinum) and an immunotherapy (pembrolizumab) (known as the KEYNOTE-522 regimen), with a fifth chemotherapy agent added if residual disease is present at the time of surgery. 7 The persistent (chronic) and future (late) side effects of these systemic therapies are mediated by a variety of host factors, including age, menopausal status, and existing comorbidities such as diabetes and hypertension. 8 9 10 11 12 13 Moreover, some toxicities, such as cancer related cognitive impairment (also known as “chemo brain”), are broadly linked to the use of cancer therapy but not tied to a particular agent. 14 The robustness of evidence based screening and prevention strategies varies between different treatment sequelae. 15
“Traditional” chemotherapeutic agents
Three chemotherapeutic classes (that is, anthracyclines, alkylating agents, and taxanes) have relatively well understood toxicity profiles in the curative setting based on decades of use in breast cancer ( table 1 ). For instance, anthracyclines increase the risk of cardiovascular events in the short term (for example, arrhythmias) and can cause long term irreversible left ventricular dysfunction. 23 24 Anthracyclines and cyclophosphamide carry a risk of secondary leukemia, although this may be less true for cyclophosphamide at the doses used in breast cancer. 25 26 27 Likewise, anthracyclines and cyclophosphamide are gonadotoxic, 28 29 affecting reproduction, menopausal symptom burden, and potentially bone and cardiac health. Taxanes contribute to chemotherapy induced peripheral neuropathy (CIPN), a primarily sensory peripheral neuropathy involving the feet and hands in a sock and glove-like distribution, which may persist lifelong in a small subset of patients. Although duloxetine can assuage CIPN related burning pain, more information is needed to inform strategies used to prevent CIPN and methods to treat numbness/tingling. 13 30
Chronic and late effects after treatment with common chemotherapeutics, immunotherapeutics, and associated targeted therapeutics used in (neo)adjuvant breast cancer setting
- View inline
Capecitabine and platinums —Despite longer track records in other disease settings, capecitabine and platinums (namely, carboplatin) are relatively new additions to the adjuvant breast cancer armamentarium. The persistent and future toxicity profiles, particularly when used in younger patients, are less well characterized. For instance, platinums can cause gonadal failure, 31 although the effect on ovarian function is poorly studied for carboplatin in the breast cancer setting. 16 32 33 34 This is despite routine use of carboplatin in combination with docetaxel and trastuzumab (with or witout pertuzumab) in the TCH(+/−P) regimens for human epidermal growth factor receptor 2 (HER2) positive disease for more than a decade. 35 The effect of platinum dosing (for example, weekly versus every three weeks) on ovarian reserve remains unassessed. 27 Platinums have been shown to increase the risk of secondary leukemia in ovarian cancer 33 ; the additive risk when used with anthracyclines and cyclophosphamide as in the Keynote-522 regimen remains understudied. 33
Targeted therapy and immunotherapy agents
HER2 targeted agents —Trastuzumab has been joined by pertuzumab, ado-trastuzumab emtansine, and neratinib in the adjuvant setting. Studies of these newer agents report lower incidences of cardiotoxicity compared with trastuzumab, but ado-trastuzumab emtansine is more hepatotoxic and carries a risk of persistent neuropathy. 22 36 The degree to which the cardiac effects of HER2 targeted agents are confined to the treatment period is not well studied for the newer agents but is presumably similar to that for trastuzumab. 37 38 Patients who do experience cardiotoxicity during HER2 directed therapy do not seem to develop this later, in the absence of other risk factors such as receipt of anthracycline.
Immunotherapy, PARP inhibitors, and abemaciclib —Routine use of pembrolizumab in higher risk triple negative breast cancer has followed the Keynote-522 results. Most of the available data on chronic and late toxicity profiles come from other disease settings, in which long term immune related side effects occurred in 43.2% of patients and ranged from mild disease, such as hypothyroidism (14.0%), to severely debilitating diagnoses, such as hypophysitis (2.1%), which may necessitate lifelong hydrocortisone treatment. 39 Similarly, the poly ADP ribose polymerase (PARP) inhibitor olaparib has recently emerged as a tool for BRCA carriers with high risk disease or residual disease after neoadjuvant chemotherapy. 40 Most of what is known about the chronic and late toxicity profile comes from patients with ovarian cancer and short term follow-up. 41 Finally, abemaciclib is a recent adjuvant therapy for estrogen sensitive breast cancers with a high risk of recurrence; knowledge of toxicities is largely based on that seen in patients with metastatic breast cancer.
Although many targeted agents have fewer listed side effects than do traditional chemotherapeutic agents, their acute toxicities become subacute and longer lasting problems owing to a longer duration of therapy (for example, two years for abemaciclib in the adjuvant setting). 40 42 Certain toxicities, such as interstitial lung disease from abemaciclib, may even be fatal (albeit rarely). 43 Furthermore, owing to short term follow-up of studies with relatively small numbers supporting the efficacy of these agents, long term toxicities in the adjuvant setting have not yet been fully described.
Endocrine therapy
Endocrine therapies improve survival for people with hormone receptor positive breast cancer. 44 45 46 Endocrine therapy encompasses a range of therapeutics, including oral drugs, such as tamoxifen and the aromatase inhibitors (letrozole, anastrozole, and exemestane), as well as gonadotropin releasing hormone (GNRH) agonists, such as goserelin and leuprolide. Endocrine therapies may be recommended for up to 10 years in people with early stage disease. 47
Whereas some patients report manageable or no side effects from endocrine therapy, other patients experience debilitating prolonged symptoms. 48 49 Because of the duration of endocrine therapy, both on-therapy and long term effects of endocrine therapy are within the scope of this survivorship review. Side effects of endocrine therapy are particularly predominant among premenopausal patients taking GNRH agonists for ovarian suppression. 50 These may worsen quality of life, and severe symptoms may also contribute to non-adherence to and/or early discontinuation of endocrine therapy, in turn worsening breast cancer survival. 51 52 53 54 55 Therefore, control of side effects related to endocrine therapy is essential in people with estrogen receptor positive breast cancer.
Vasomotor symptoms
Vasomotor symptoms have been seen in up to 80% of patients receiving endocrine therapy in population based studies. 56 Patients may experience relief from vasomotor symptoms through use of a variety of strategies, as delineated in table 2 .
Side effects of endocrine therapy
As the efficacies of therapeutic strategies have not been directly compared, individualized evaluations of potential benefits and side effects should be completed on a patient-by-patient basis. For instance, although paroxetine is an effective agent for hot flashes, it has the potential to interact with tamoxifen metabolism contributing to a potential decrease in the efficacy of tamoxifen. 86 Patients treated with gabapentinoids for alternative diagnoses, such as diabetic neuropathy, might see improvements in their hot flashes, but in one randomized, crossover trial of 66 patients assigned to gabapentin or venlafaxine, 68% reported an overall preference for venlafaxine (P=0.01) over gabapentin. In this study, patients randomized to venlafaxine and to gabapentin both had a 66% reduction in hot flash scores, but patients receiving venlfaxine reported greated reduction in severity and frequency of hot flashes and fewer adverse effects. 87 Furthermore, patients who do not experience benefit from one drug may glean benefit from another, so trialing a second drug is worthwhile if the first is ineffective. 88
Finally, although estrogen based hormone replacement therapy should be avoided in this setting, data on the benefit-to-risk ratio of progestin monotherapy are limited. 89 Megestrol acetate was compared with placebo in an RCT including 97 women with breast cancer; 74% of the megestrol acetate group, compared with 20% of the placebo group, had a decrease of 50% or more in the frequency of hot flashes (P<0.001). 90 Medroxyprogesterone seems to be particularly effective; an RCT of 218 women found that a depot injection of medroxyprogesterone versus venlafaxine significantly increased the number of women with >50% reduction in hot flashes (74% v 46%; P<0.001). 91
Genitourinary symptoms
Genitourinary symptoms are related to low estrogenic states and are pervasive among patients receiving aromatase inhibitors and ovarian suppression. 66 67 However, although many patients wish to discuss such problems with clinicians, such symptoms are infrequently discussed in oncology clinics, which exacerbates non-use of safe and evidence based treatments. 92 93 94 Vaginal dryness is commonly experienced, and efficacious treatment options are available; however, dyspareunia, poor libido, and other sexual health concerns are frequently distressing to breast cancer survivors and lack data driven management options ( table 2 ).
Vaginal estrogen is commonly recommended for vaginal dryness and its sequelae in patients with no history of breast cancer, but this approach may carry some risks in patients with a history of estrogen sensitive breast cancers. Systemic estrogen concentrations may be very mildly increased after vaginal estrogen in a dose dependent fashion, but this may or may not be clinically significant. 95 One recent cohort study of 8461 breast cancer survivors in Denmark reported a 39% higher risk of recurrence of breast cancer at a median follow-up of 9.8 years after diagnosis, but no difference in mortality, among patients who received concurrent vaginal estrogen therapy and aromatase inhibitors. 96 This study was non-randomized and therefore different doses of vaginal estrogen may have been used; however, the risk-to-benefit ratio of vaginal estrogen remains controversial. Of note, a topical vaginal androgen, dehydroepiandrosterone, does not seem to raise estrogen concentrations in patients taking aromatase inhibitors. 70
Musculoskeletal symptoms
Approximately half of breast cancer survivors taking aromatase inhibitors experience “aromatase inhibitor associated musculoskeletal symptoms” or AIMSS. 76 Various plausible pathogenic explanations have been proposed as to why AIMSS occurs: aromatase inhibitors have pro-inflammatory properties, estrogen helps to maintain synovial fluid health, and pain sensitivity may be altered by aromatase inhibitors. 97 98 99 Of note, tamoxifen may also contribute to musculoskeletal symptoms, more commonly muscle cramping, but insufficient evidence exists about effective treatments. 100
Both duloxetine and acupuncture have been shown to reduce AIMSS in placebo controlled clinical trials ( table 2 ), 101 but one common first approach to AIMSS is a brief hiatus in treatment to ensure symptom improvement or resolution, followed by a switch from one aromatase inhibitor to another (or to tamoxifen). 77 80 102 One prospective, multicenter, non-randomized study of 179 patients reported that 74% had less severe AIMSS after switching from anastrozole to letrozole. Another open label randomized trial of exemestane versus letrozole in 503 women reported that 39% of patients were able to stay on aromatase inhibitor after switching to a second aromatase inhibitor, whereas all patients who did not switch chose to discontinue therapy; however, complete resolution of AIMSS after such a switch is uncommon. 80 102 This approach is safe and low risk, and it typically does not require additional time or cost. 103
More than a third of breast cancer survivors taking endocrine therapy report hair thinning and loss, described as frontal and parietal hairline recession. 104 105 Prospective investigations into the clinical characteristics and incidence of endocrine therapy related alopecia are ongoing. Minoxidil, a vasodilative agent that causes hair follicles to transition into growth phase and increases the size of hair follicles, offers a promising potential therapy for endocrine therapy related alopecia; the use of topical and low dose oral minoxidil has been supported by early studies. Although this may be offered as an off-label treatment, RCT results are eagerly awaited. 106 107
Osteoporosis
Aromatase inhibitors increase risks for bone thinning, osteoporosis, and fractures, which are major drivers of cost, morbidity, and mortality, particularly in breast cancer survivors. 108 109 Bisphosphonates and receptor activator of nuclear factor-κB ligand (RANKL) inhibitors lessen the risks for bone loss and fractures among both postmenopausal patients and premenopausal patients taking ovarian function suppression or with chemotherapy related ovarian failure. 110 111 112 113 114 115 116 117 118 119 Additionally, these agents decrease risk for bone recurrence and improve breast cancer-free survival. 120 The most recently published double blinded RCT of 3425 postmenoapusal patients compared denosumab with placebo; denosumab improved disease-free survival with a hazard ratio of 0.83 (95% confidence interval 0.71 to 0.97; P=0.016) and reduced fractures with a hazard ratio of 0.76 (0.63 to 0.92; P=0.004) at a median follow-up of eight years. 121
Other important components of survivorship care
Screening for recurrence.
Screening for recurrence relies on regular (usually at least annual) visits with a cancer specialist or primary care provider for physical examination and assessment of symptoms that might indicate that cancer has returned. 122 123 124 Female survivors who have residual breast tissue (because they have not undergone bilateral mastectomy) and no evidence of distant metastases are generally recommended to undergo annual mammography at least until age 75 (unless medical comorbidities substantially limit the projected benefit of early detection of local recurrences and new primaries). 125 Supplemental screening imaging (for example, magnetic resonance imaging of the breast, ultrasonography, or molecular breast imaging) may also be considered for female survivors with dense breast tissue (for whom mammography is less sensitive) or for those with an elevated risk of new primary cancer. 126 127 Male survivors should also consider unilateral surveillance mammography if they had breast conserving therapy (to detect local recurrences). 128
Routine imaging or blood tests to assess for distant recurrence is not recommended by current guidelines. 122 124 If a survivor develops a sign or symptom of possible recurrence on physical examination or history, distant imaging (for example, positron emission tomography, computed tomography, or bone scan) may be warranted.
Genetic testing
For patients who do not meet criteria for genetic testing at the time of the initial cancer diagnosis (or who undergo only limited genetic testing at that time), periodically asking about new cancers diagnosed in family members that might affect the projected yield of genetic testing is important. Survivors who are found to carry pathogenic variants predisposing to cancer may warrant additional screening or risk reducing surgeries.
Financial toxicity
Advances in breast cancer management have enabled more cures; however, they have also contributed to the unsustainable rise in the cost of care, 129 with the overall financial burden disproportionately affecting patients. Financial toxicity has been described as a combination of objective financial burden and subjective financial distress, and this adverse event has a spectrum of severity with 79% of patients with cancer reporting moderate to catastrophic financial burden. 130 131 High costs of cancer care, particularly out-of-pocket costs, are associated with worse patient reported outcomes, lower quality of life, and poor adherence to treatment. 131 132 Several factors have been associated with risk of financial toxicity, including younger age, female sex, non-white race, employment changes, low average household income, increasing distance from treatment centers, and increasing out-of-pocket costs. 133 Furthermore, significant geographic disparities exist in financial burden across the world. Large out-of-pocket costs may be required in some parts of the world (for example, the US, Africa, and Central and South America), whereas other healthcare systems (for example, Canada, the EU, and the UK) protect patients against out-of-pocket costs. 134 Defined risk factors for financial toxicity now exist, along with a validated assessment tool. 135 Globally, cancer practices need to systematically screen patients for financial toxicity or proactively discuss finances associated with care during or after cancer care, notably when options with variable costs are available.
Fear of recurrence
Fear of recurrence is experienced by up to 80% of women with breast cancer. 136 This can lead to worsened quality of life, depression, anxiety, and lack of proper follow-up surveillance. 137 138 139 Assessing patient safety is important, as is consideration of referral to mental health professionals. 140 Cognitive behavioral therapy (CBT), discussed further below, has been shown in multiple trials (and a recent systematic review to decrease fear of recurrence, which assessed the quality of the 17 RCTs). 121 One included study ranodmized 56 patients to a form of CBT, termed cognitively based compassion training, over eight weeks and found reduced fear of cancer recurrence and psychologic stress (significant time × group interaction of 3.521; P<0.05). 141 Cognitive behavioral therapy can be delivered via individual therapy, group therapy, or digitally. 140 An RCT of mindfulness based stress reduction (MBSR; also discussed further, below) included 322 patients with breast cancer who were given a six week MBSR course versus usual care. Patients assigned to the MBSR group had significantly reduced fear of recurrence and anxiety scores (P<0.01), as assessed at baseline/six weeks and 12 weeks. 142
Survivorship care plans
Survivorship care plans were proposed in the 2005 Institute of Medicine report “From Cancer Patient to Cancer Survivor: Lost in Transition” as a potential way to support primary care providers in the provision of survivorship care (in part to control costs and in part to assure that survivors receive all of the non-cancer related care that they need). 143 However, subsequent clinical trials assessing the impact of survivorship care plans have shown mixed effects on a variety of survivorship outcomes. 144 145 A persistent need remains for novel approaches to facilitate dynamic transmission of knowledge between survivors and clinicians. As noted below, digital tools are showing potential in this arena and allow for a shared tool for primary care physicians and sub-specialists to communicate and improve care coordination. However, scaling and disseminating interventions into the “real world” is challenging. When asked about survivorship care plans, breast cancer survivors surveyed in one qualitative study recommended better education and personalization with regard to nutrition, exercise, managing side effects, comorbidities, and provision of resources, such as health coaches. 146 Breast cancer support groups can also help patients to cope with their diagnosis and treatment related side effects.
General health maintenance
Screening for and treating new non-breast cancers and managing other medical conditions are critical components of breast cancer survivorship care. Cardiovascular disease is common, especially in this population, in part as a result of the toxicities of breast cancer therapy. 147 148
Screening for second cancers
Breast cancer survivors face elevated risks of colorectal, ovarian, lung, endometrial, and thyroid carcinomas, as well as sarcoma and non-lymphocytic leukemia. 149 150 151 In addition, they face standard age related risks of many other cancers. For people who are not known carriers of genetic mutations predisposing them to cancer or otherwise at high risk of a second malignancy, guidelines recommend continuing with age appropriate cancer screening as recommended for the general population. 122 152 The primary differences between recommendations in the US and Europe pertain to the age range for screening for colorectal cancer and to the fact that screening for Helicobacter pylori is recommended in some EU countries. 153
Cholesterol, diabetes, and blood pressure monitoring and management
Hypertension, diabetes, coronary artery disease, and cerebrovascular disease share the following risk factors with breast cancer: obesity, metabolic syndrome, age, and lack of physical activity. In addition, certain breast cancer therapies, such as anthracycline based chemotherapies and anti-HER2 therapies, are associated with various cardiovascular toxicities, including heart failure. 154 155 Interestingly, a recent case-control Kaiser Permanente study, including 3642 women with breast cancer and 68 202 matched controls, found that breast cancer survivors were more likely to develop diabetes (subdistribution hazard ratio 1.16, 1.07 to 1.26) but less likely to develop dyslipidemia (0.90, 0.86 to 0.95) than matched women without cancer. 156 Although some early studies suggested that aromatase inhibitors might lead to more cardiovascular disease than tamoxifen, a recent cross sectional evaluation of 569 breast cancer survivors, 40% of whom had used aromatase inhibitors, found no difference in carotid intima media thickness (median 0.63 (interquartile range 0.56-0.71) mm with low exposure to aromatase inhibitors, 0.66 (0.59-0.75) mm with intermediate exposure, 0.64 (0.59-0.73) mm with high exposure), advanced glycation end products (median 2.13 (1.90-2.40) arbitrary units with low exposure to aromatase inhibitors, 2.20 (1.90-2.51) with intermediate exposure, 2.11 (1.90-2.43) with high exposure), or hyperlipidemia (data not provided) by exposure to aromatase inhibitors. 157
Thus, for most breast cancer survivors, a reasonable approach is to follow the United States Preventive Service Task Force (USPSTF) guidelines, which include office blood pressure and weight measurement for all patients aged ≥18; fasting plasma glucose, glycated hemaglobin, or oral glucose tolerance test to screen for diabetes in those aged 35-70; lipid panel to screen for dyslipidemia for those aged 40-70 (plus those aged 21-39 on the basis of clinical judgment); and counseling about smoking cessation for all adults who smoke. Optimal screening intervals for these assessments are unknown, but the USPSTF suggests annual screening for hypertension and weight, screening every three years for diabetes, and screening every five years for dyslipidemia. 158 Guidelines from the European Society of Cardiology are similar.
A meta-analysis that included data from 10 RCTs and 1095 breast cancer survivors found that diet and exercise interventions improved anthropomorphic measurements, systolic blood pressure, and C reactive protein but did not affect cholesterol, glucose, insulin, or leptin. 159 Drugs are often needed to optimize cardiovascular health in survivors with abnormal cholesterol, blood pressure, and/or glucose. Unfortunately, evidence that screening and aggressive management of cardiovascular risk factors improve long term cardiac outcomes in breast cancer survivors is limited.
Integrative oncology approaches to breast cancer survivorship
Exercise is one of the most important lifestyle interventions patients can engage in to prevent recurrence and decrease symptoms associated with breast cancer treatments. Research has shown that exercise reduces both recurrence of and death from breast cancer; one meta-analysis reported that meeting recommended physical activity guidelines (at least eight hours of moderate intesnity aerobic exercise a week) after diagnosis was associated with lower risk of breast cancer related death (hazard ratio 0.67, 95% confidence interval 0.50 to 0.90) during average follow-up periods ranging from 4.3 to 12.7 years. 160 161 162 163 164 Exercise has also been shown to improve fatigue, anxiety, depression, quality of life, physical function, strength, sleep, and bone health. 165 166 167 168 169 170 171 172 173 Resistance training is safe in patients with lymphedema and should be encouraged. 174 Unfortunately, most breast cancer survivors do not meet the recommended goals of 150 minutes of moderate aerobic exercise a week and twice weekly strength training. 175 The American Cancer Society also recognizes that people should move about during the day: “move more and sit less.” 176
The American Society of Clinical Oncology (ASCO) recommends aerobic and strength exercises during active treatment to reduce the side effects of therapy. 177 Exercise in patients undergoing chemotherapy has been shown to improve fatigue and cognition, 168 169 177 178 179 quality of life, depression/anxiety, and sleep quality. 165 180 In people taking aromatase inhibitors, exercise, including aerobic and strength training for a 12 month program, reduced pain scores by 30%. 181 This is important given that many people will stop aromatase inhibitors because of side effects.
Patients with no comorbidities do not need medical clearance before starting an exercise program. However, patients with neuropathy, bone/arthritis problems, or lymphedema should be referred for evaluation by a rehabilitation specialist and can consider medical clearance. Those with serious comorbidities, such as coronary artery disease, chronic obstructive pulmonary disease, recent surgeries, severe nutritional deficiencies, fatigue, or bone metastasis, should undergo medical clearance and rehabilitation evaluation before starting a program. 182
Yoga has additional benefits and is recommended by the Society for Integrative Oncology (SIO)/ASCO. 183 Yoga has been shown to improve quality of life (grade B) and may improve depression/anxiety (grade C) and fatigue (grade C). 183 Gentle yoga can be considered to help with sleep problems (grade C). SIO/ASCO also recommends consideration of yoga for aromatase inhibitor induced joint pain and pain after breast cancer therapy (both low level evidence). Yoga can be adapted for patients with functional limitations and is available in person or online. Patients with limited mobility or serious comorbidities should be evaluated and work with a certified instructor to avoid injury. 184
Dietary factors
No one specific diet is known to improve prognosis or quality of life during or after cancer treatment. However, some general recommendations can be made. The Women’s Health Initiative trial was a dietary prevention trial that randomized 41 835 women to low fat (20%) plus increased fruits/vegetables and whole grains versus a standard diet. Women in the intervention arm who developed breast cancer had improved overall survival compared with the usual diet group (10 year overall survival 82% v 78%; hazard ratio 0.78, 0.65 to 0.94; P=0.01). 185 Whether the observed benefits were due to decreased fat intake or the increase in other healthy foods is unclear. In another trial, 2437 women with early stage breast cancer were randomized to a low fat (20%) diet versus a standard diet. The primary endpoint, relapse-free survival, was improved in those in the low fat diet group (9.8% v 12.4% with events; hazard ratio 0.78, 0.60 to 0.98; P=0.03). This was more pronounced in women with hormone receptor negative tumors. However, the low fat group lost weight, and the benefits may have been just due to weight loss. 186 Other studies have shown increased all cause mortality in breast cancer survivors consuming the highest amounts of saturated/trans fats. 187 188 Therefore, a plant heavy diet (which includes the Mediterranean diet and some Asian diets), incorporating healthy fats and whole grains while avoiding processed meats and carbohydrates, sugar sweetened drinks, and artificial sweeteners, is recommended. 176 A Mediterranean diet containing nuts and extra virgin olive oil has cardiovascular benefits, 189 190 191 and incorporating high fiber foods may also be beneficial. 192
The effects of meal timing has also been studied. For example, data collected from 2413 women from the Women’s Healthy Eating and Living study showed that women who fasted <13 hours per night had increased recurrences of breast cancer compared with those who extended overnight fasting periods (hazard ratio 1.36, 1.05 to 1.76). 193 Longer fasting was also associated with improvement in glycated hemoglobin. Further evidence is needed to know the effect of extended overnight fasting on risk for recurrence.
Recommendations for limiting alcohol consumption for primary and secondary breast cancer prevention vary; however, most evidence suggests that alcohol increases the risk of recurrence, especially after the menopause. A recent review suggests limiting alcohol to less than three drinks a week, 194 whereas the National Comprehensive Cancer Network’s survivorship guidelines take a more stringent view on alcohol and state that women with breast cancer should abstain.
Dietary soy is safe for people with a history of breast cancer and may be beneficial. 195 However, soy supplements are not recommended. 196
Acupuncture
Acupuncture is an ancient Chinese medicine practice of placing fine needles into various points in the body. Heat (moxibustion) or electrostimulation can be added to standard needling. 197 The mechanism of action is not well understood. Traditional Chinese medicine explains acupuncture as a technique for balancing the flow of energy or life force that flows through meridians in the body. 198 Acupuncture may also stimulate nerves, fascia, or muscles to affect its action. 199 200 Sessions are generally weekly and often include other evaluations of the patient’s constitution, lifestyle advice, and herbal therapies. These components of traditional acupuncture are not often included in clinical trials in which only the acupuncture needle part of the treatment is assessed. 201 Studies of acupuncture are challenging to perform and evaluate owing to heterogeneity of providers, difficulty in choosing a control group (waitlist versus sham versus standard of care), not including other components of acupuncture treatment (pragmatic study), and difficulty in double blinding. The placebo effect is well established as playing a significant role in these studies. 202 However, evidence has been mounting, and SIO/ASCO recommends electro-acupuncture for consideration for chemotherapy induced nausea on the basis of RCTs and a consensus conference (in addition to standard drug therapies) (grade B, SIO). 197 203 204 SIO/ASCO guidelines for managing pain recommend acupuncture for aromatase inhibitor related joint pain and general pain/musculoskeletal pain from cancer (both with intermediate level evidence quality) and consideration of acupuncture for CIPN and procedural or surgical pain (both with low quality level evidence). 205 Acupuncture is generally safe but should be avoided in patients with severe cytopenias, and needles should not be inserted into areas of tumors or infections. 206 207 208
Acupressure is another technique in which manual pressure is applied to different body parts to achieve an effect, using pressure from fingers, bands, or beads. This has been most well studied for nausea/vomiting, using a pressure point called Neiguan located on the inner arm near the wrist. 209 210 211 212 This treatment is a grade B recommendation from SIO/ASCO. 183 It may also be useful for other cancer related symptoms, including pain and fatigue. 205 213 214
Cognitive behavioral therapy
CBT is a psychological treatment that treats anxiety/depression and insomnia and improves relaxation and quality of life. 215 216 It may also help people with the fear of recurrence. 121 The techniques included in this form of talking therapy involve trying to change thinking patterns to improve a particular condition; for instance, a therapist treating a patient with anxiety will often identify a patient’s anxious/nervous thought patterns and help to reframe these thoughts so that they are less detrimental. CBT has been shown to improve sleep and anxiety/depressive symptoms and increase quality of life in cancer patients. For instance, among 11 RCTs of patients with breast cancer, the overall effect size of CBT on quality of life of breast cancer survivors was 0.39 (95% confidence interval 0.12 to 0.66; P<0.001, I 2 =83%). 215 216 217 218 219 220 221 222 223 224 225
Insomnia is a common problem for patients with breast cancer, both during and after treatment, owing to direct treatment effects (including tamoxifen or steroids prescribed with chemotherapy), stress of diagnosis and treatment, or other causes. Insomnia often coexists with other symptoms such as pain, anxiety/depression, and fatigue, leading to reduced quality of life. 226 CBT-insomnia is a form of CBT that has been shown to improve insomnia in breast cancer patients for up to 12 months post-intervention 217 223 ; in one systematic review and meta-analysis of 22 studies, CBT-insomnia significantly reduced severity of insomnia (g=0.78). 217 CBT-insomnia involves stimulus control, sleep hygiene, cognitive therapy, and relaxation therapy. 219 227
Mindfulness
Mindfulness practice is a technique used to train attention and awareness to focus on the present moment in a non-judgmental way. 228 Mindfulness practice is recommended by SIO/ASCO for anxiety, depression, and quality of life (grade A). 183 Patients may practice mindfulness in different ways, including meditation, yoga, Tai Chi, breathing techniques, and body scans. Mindfulness practices improve anxiety, depression, sleep, and quality of life in cancer patients. 229 230 231 232 233 234 235 236 MBSR is a more formal program that teaches a variety of meditation practices either in person or online, weekly over six to eight weeks, 228 and may also improve mood, sleep, fatigue, and quality of life. 226 234 235 237 Studies have been mixed regarding the effects of MBSR on cognitive function. 238 The duration of beneficial effects of MBSR may not be long lasting, and more studies are needed to determine whether ongoing practice or repeat courses are needed to maintain benefits. 237 239
Supplements
Guidelines state that dietary supplements are not recommended for the treatment of cancer or prevention of recurrence and are not recommended for cancer survivors in the absence of a documented nutritional deficiency, poor diet, or other medical indication. 183 240 241 However, some dietary supplements may be useful in symptom management. For example, Wisconsin ginseng has been shown to reduce fatigue in breast cancer patients undergoing chemotherapy, 242 and ginger may help with nausea. 243 244 245 Treating vitamin D deficiency may be associated with improved breast cancer outcomes and bone health. 246 247 Dietary soy is safe, but supplements are not recommended or useful for hot flashes. 248 249 250 Some supplements, however, can be harmful and should be avoided. For example, acetyl-L carnitine was shown to worsen taxane induced neuropathy. 251 Vitamin B 12 before/during chemotherapy and iron during chemotherapy were associated with increased recurrence of and death from breast cancer. 252 Patients should also avoid supplemental antioxidants during chemotherapy, as some studies suggest worse cancer outcomes. 253 Supplements/herbal medicines can interfere with cancer treatments, generally, through cytochrome P450/P-glycoprotein interactions, which may increase the toxicity of therapy or decrease its effectiveness. 254 255 Many patients are taking dietary supplements and do not inform their care teams. 256 257 Asking whether patients are taking dietary supplements and assessing for potential interactions are important.
Other integrative modalities
Other therapies that have shown promise in helping patients with breast cancer with a variety of problems are listed in table 3 (adapted from SIO guidelines). 205 240
Level of evidence for integrative medicine strategies used for symptom management.
Digital tools to enhance survivorship care
With the burgeoning population of breast cancer survivors, transformation of delivery models for cancer care has become a necessity. Telehealth and virtual care have been adopted globally, and they may offer solutions to enable the transition of some aspects of survivorship care from the clinic into the home or community environment. Communication with care team members, symptom management, and disease surveillance can be facilitated by digital products and platforms, as summarized in this section.
Electronic/mobile health services
Digital technologies leveraged for electronic health (eHealth) interventions are most commonly mediated through the web or internet, whereas mobile health (mHealth) interventions are mediated through smartphone or tablet devices, most commonly via applications. Digital delivery of educational content for patients and online peer support groups accessible through a web browser or mobile device are a few examples of eHealth/mHealth tools that foster self-management.
A systematic review with meta-analyses (random effects model) of RCTs evaluated the effectiveness of eHealth delivered interventions in patients during and after breast cancer treatment. 258 Most interventions were web based and included videos, forums, and electronic reminder systems. The 32 unique studies (4790 participants) included were conducted within health systems globally, showing the broad reach of these interventions and diversity within the populations represented. A significant effect of eHealth interventions on quality of life (standardized mean difference 0.20, 95% confidence interval 0.03 to 0.36), self-efficacy (0.45, 0.24 to 0.65), distress (–0.41, –0.63 to –0.20), and fatigue (–0.37, –0.61 to –0.13) was seen. They had no effect on anxiety or depression. Studies (78.1%) measuring patient reported experience measures (n=25) found that acceptability (n=9) was high, with high ratings for satisfaction (range 71-100%), usefulness (71-95%), and ease of use (73-92%).
A systematic review of mHealth applications used across the breast cancer continuum was conducted by searching four databases with an objective of providing an overview of available, research tested interventions. Ten of the 29 identified studies targeted breast cancer survivorship (846 patients). 259 All aimed to assess lifestyle changes, and nearly all interventions were mobile applications, some of which included email or SMS text messaging features, as well as video enabled sessions with a healthcare professional. Several of the included studies showed a significant association for weight loss and increased physical activity with the mHealth intervention. 260 261 More high quality research is needed to better understand the effect of mHealth applications on these and other clinical outcomes.
Telehealth services
The electronic health record (EHR) has become the standard for storing and viewing patients’ health data and clinical documentation. A shift has taken place from passively viewing health information to an interactive EHR, and patients report that this facilitates better communication, enables a more effective partnership with providers, and helps to track their health information more proactively. 262
Asynchronous telehealth services are a form of eHealth/mHealth, often mediated respectively through the web or application based EHR patient portals. They can facilitate secure messaging between survivors and their care teams, as well as store-and-forward transmission of data. 263 The latter can be used for electronic patient reported outcomes, such as self-reported symptom assessments, or for patients to send images. The submitted data can be reviewed by a healthcare provider and care recommendations can be returned to patients at a convenient time outside of an in-person assessment,.
Synchronous telehealth services require an interactive audio and/or video telecommunication system to permit real time communication between providers at the distant site and the patient at the originating site. 263 Emerging literature suggests that patients’ acceptance of and satisfaction with video telemedicine visits in the breast surgical oncology practice have been high. 264 265 High patient satisfaction scores were also observed in a multisite, multiregional medical oncology practice. In that study, rates of use of telehealth visits were lower for patients ≥65 years of age and those residing in rural communities than for younger patients and those in urban areas. 266 Breast cancer clinicians have also expressed satisfaction with telemedicine and recognize it as a valuable option to enhance outpatient care and routine follow-up. 267 When appropriate, moving some follow-up care to the home via telehealth visits may improve access to facility based care while still serving the needs of breast cancer survivors.
Remotely delivered rehabilitation, weight loss, and physical activity programs
Beyond episodic visits, entire programs supporting cancer survivors have been transformed for virtual care delivery. As an example, telerehabilitation has been developed and implemented in several cancer practices to facilitate physical therapy for complications of breast cancer treatments, including lymphedema, limited shoulder range of motion, pain, fatigue, and loss of muscle strength. 268 Although remotely delivered physical therapy is a viable model of care delivery for survivors, research is needed to understand patients’ acceptance of and compliance with the telerehabilitation program, as well as its efficacy compared with traditional in-person physical therapy.
Additionally, the feasibility and effectiveness of a remotely delivered weight loss program has been compared with usual care in an RCT of patients who have completed treatment for early stage breast cancer. 269 Compared with usual care, the virtual weight loss program, including telephone calls and optional text messaging, was associated with significantly greater improvements in weight, metabolic syndrome risk score, physical quality of life, musculoskeletal pain, and other variables. Notably, the effects on weight, adiposity, and metabolic syndrome risk scores were sustained at 18 months. Bringing these weight loss programs into the home environment with a goal of improving overall health and wellness may improve patients’ access and adherence to them.
Remotely delivered physical activity programs for breast cancer survivors have also emerged separately or in concert with weight loss programs, and early results have shown their feasibility and acceptance in both older rural and young breast cancer survivors. 270 271 Interventions supporting these programs include the use of video enabled exercise sessions, web based education, wearable devices/accelerometers, and certified instruction or peer coaching. In an RCT of a remotely delivered exercise program versus a waitlist wellness control group of breast cancer survivors, those receiving the intervention had significantly greater reductions in sedentary behavior, and the increase in moderate to vigorous physical activity was inversely proportional to sedentary behavior. As shown in a separate study, remotely delivered programs for cancer survivors, when combined with telecoaching, can improve program retention and adherence enabling improved outcomes for patients. 272
This growing body of evidence supports continued investment in the development and study of remotely delivered programs for breast cancer survivors that foster rehabilitation, weight loss, and increased physical activity. They have the potential to improve health related quality of life and clinical outcomes, and they can furthermore break down barriers to access and adherence to these services that traditionally have been offered at limited institutions through traditional in-person care models.
Cancer treatment delivery at home
Home hospital programs can provide supportive care to patients with cancer in the home environment, and studies have shown the feasibility, safety, and effectiveness of oncology specific home hospital programs. 273 274 Initially developed and adopted several decades ago in single payer health systems globally, 275 276 the programs have expanded to include delivery of systemic cancer treatment in the home. Studies have shown that this is safe, improves patient and care giver experience, and reduces treatment costs. 277 Many breast cancer drugs administered to breast cancer survivors have a low risk for reactions and would be amenable to administration in the home, such as GNRH analogs, biologics, and bone supportive care. This could be transformative for care of breast cancer survivors and warrants investigation.
Emerging treatments
Many emerging treatments for long term toxicities of systemic therapies exist. Two upcoming clinical trials will focus on therapies that might prevent the development of long term peripheral neuropathy, one testing a compound called GM-1 and another a device that provides both cryotherapy and compression therapy (NCT number not yet assigned). For therapy related alopecia, a clinical trial is under way to study the benefits of minoxidil in a randomized controlled fashion ( NCT05417308 ).
Various national and international organizations have issued breast cancer survivorship guidelines, as well as guidelines for the management of specific symptoms facing breast cancer survivors, for integrative medicine approaches, and for surveillance strategies. General survivorship guidelines from the American Cancer Society, American Society of Clinical Oncology, European Society of Clinical Oncology, and National Comprehensive Cancer Network guidelines were reviewed in detail to inform this review.
Conclusions
In general, the complexity of after effects from breast cancer are likely to increase as new therapies are added to our armamentarium and treatment becomes increasingly tailored. As the sections above highlight, survivors and clinicians face challenges in monitoring persistent and late after effects from cancer and its treatment. Following up for multiple aftereffects in trials of cancer directed therapy over the long term can be costly, and many clinical trials may be too small to capture rare side effects. Digital tools may help to engage survivors in long term tracking, but reporting bias will be a potential confounder and care must be exercised to prevent under-representation of populations with lower digital literacy.
Questions for future research
How can we prevent and/or treat many of the challenging long term side effects of systemic therapy, including peripheral neuropathy, therapy related alopecia, and vaginal dryness?
What is the optimal way to help patients to differentiate between evidence based integrative oncology approaches and approaches that may not help and may actually harm patients?
How can digital tools enhance the benefit of survivorship care plans?
How patients were involved in the creation of this article
All of the authors of this manuscript are clinicians who interact with breast cancer survivors regularly. All also engage with patient advocates as part of their research efforts. A team of breast cancer survivors and patient advocates provided feedback on the long term side effects and integrative oncology strategies that are covered in this manuscript; we would personally like to acknowledge the following breast cancer survivors and advocates: Lisa Halverson, Anne Mehnke, Tracee Cole, and Jody Koubsky .
Series explanation: State of the Art Reviews are commissioned on the basis of their relevance to academics and specialists in the US and internationally. For this reason they are written predominantly by US authors
Contributors: KJ was responsible for the original conception and design of the article, with input from all co-authors; all authors were responsible for the acquisition and interpretation of data for the work, drafting the work, revising it critically, and final approval of the version to be published. All authors agreed to be accountable for all aspects of the work and will assure that questions related to the accuracy or integrity of any part of the work will be appropriately investigated and resolved.
Competing interests: We have read and understood the BMJ policy on declaration of interests and declare the following interests: none.
Provenance and peer review: Commissioned; externally peer reviewed.
- ↵ World Health Organization, International Agency for Research on Cancer Statistics. Breast cancer. https://www.iarc.who.int/cancer-type/breast-cancer/ .
- Siegel RL ,
- Miller KD ,
- ↵ American Cancer Society. Cancer Treatment and Survivorship Facts and Figures 2019-2021. 2019. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/cancer-treatment-and-survivorship-facts-and-figures/cancer-treatment-and-survivorship-facts-and-figures-2019-2021.pdf .
- Sparano JA ,
- Makower DF ,
- Kalinsky K ,
- Barlow WE ,
- Gralow JR ,
- Salgado R ,
- Seretny M ,
- Currie GL ,
- Upshaw JN ,
- Ruthazer R ,
- Nieboer P ,
- Rodenhuis S ,
- Valcovici M ,
- Andrica F ,
- Loprinzi CL ,
- Lacchetti C ,
- Bleeker J ,
- Tevaarwerk A ,
- Denlinger CS ,
- Arteaga CL ,
- de la Torre-Montero JC ,
- López-Tarruella S ,
- Lambertini M ,
- Anderson RA ,
- Phillips KA ,
- POEMS/S0230 Investigators
- Tolaney SM ,
- Armenian SH ,
- Mertens L ,
- Slorach C ,
- Curigliano G ,
- Lenihan D ,
- Fradley M ,
- ESMO Guidelines Committee. Electronic address: [email protected]
- Curtis RE ,
- Boice JD Jr . ,
- Stovall M ,
- Overbeek A ,
- van den Berg MH ,
- van Leeuwen FE ,
- Kaspers GJ ,
- Lambalk CB ,
- van Dulmen-den Broeder E
- Peccatori FA ,
- Demeestere I ,
- Margulies A ,
- Cardoso F ,
- ESMO Guidelines Committee. Electronic address: [email protected] ,
- EONS Education Working Group. Electronic address: [email protected] ,
- EANO Guideline Committee. Electronic address: [email protected]
- Loncharich MF ,
- Anderson CW
- Travis LB ,
- Holowaty EJ ,
- Bergfeldt K ,
- Jensen KC ,
- Vinayak S ,
- Eiermann W ,
- Breast Cancer International Research Group
- von Minckwitz G ,
- KATHERINE Investigators
- Fosbøl EL ,
- Goldhar HA ,
- Patrinely JR Jr . ,
- Johnson R ,
- Lawless AR ,
- Garber JE ,
- Kaufman B ,
- OlympiA Clinical Trial Steering Committee and Investigators
- Morice PM ,
- Dolladille C ,
- Johnston SRD ,
- O’Shaughnessy J ,
- monarchE Committee Members
- Taguchi Y ,
- ↵ Surveillance, Epidemiology, and End Results (SEER) Program. Cancer Stat Facts: Female Breast Cancer. https://seer.cancer.gov/statfacts/html/breast.html .
- Budzar AU ,
- ATAC Trialists’ Group
- Adjuvant Tamoxifen: Longer Against Shorter (ATLAS) Collaborative Group
- Harris PF ,
- Remington PL ,
- Trentham-Dietz A ,
- ↵ Ribi K, Luo W, Bernhard J, et al. Patient-reported endocrine symptoms, sexual functioning and quality of life (QoL) in the IBCSG SOFT trial: adjuvant treatment with tamoxifen (T) alone versus T plus ovarian function suppression (OFS) in premenopausal women with hormone receptor-positive (HR+) early breast cancer (BC). San Antonio Breast Cancer Symposium. 2014.
- Bernhard J ,
- Kidwell KM ,
- Chirgwin JH ,
- Giobbie-Hurder A ,
- Coates AS ,
- Hershman DL ,
- Ferreira AR ,
- Di Meglio A ,
- Pistilli B ,
- Jotwani AC ,
- Chlebowski RT ,
- Mortimer JE ,
- Crandall CJ ,
- Barton DL ,
- LaVasseur BI ,
- Freeman EW ,
- Guthrie KA ,
- Kugler JW ,
- Leon-Ferre RA ,
- Novotny PJ ,
- Stearns V ,
- Program to Reduce Incontinence by Diet and Exercise Investigators
- Tanasijevic A ,
- Schover LR ,
- Brewster A ,
- Melhem-Bertrandt A
- Chlebowski RT
- van der Kaaij M ,
- van Dorst E ,
- Creutzberg C ,
- Faubion SS ,
- Larkin LC ,
- Stuenkel CA ,
- Shuster LT ,
- Dockter T ,
- Goetsch MF ,
- Juraskova I ,
- Goldfrank DJ ,
- Mathias C ,
- Cardeal Mendes CM ,
- Pondé de Sena E ,
- Greenlee H ,
- Capodice J ,
- Loprinzi CL
- Baglia ML ,
- Cartmel B ,
- Galantino ML ,
- Demichele A ,
- Stricker CT ,
- Juurlink DN ,
- Bordeleau L ,
- Pritchard KI ,
- Carpenter LA ,
- Holmberg L ,
- Anderson H ,
- HABITS steering and data monitoring committees
- Michalak JC ,
- Quella SK ,
- Cathcart-Rake E ,
- O’Connor J ,
- Ridgeway JL ,
- Iglehart EI ,
- Schover LL ,
- Kingsberg S ,
- Santen RJ ,
- Bernick B ,
- Constantine GD
- Jensen MB ,
- Cronin-Fenton D ,
- Christiansen P ,
- Ejlertsen B
- Dietrich W ,
- Tschugguel W
- Karatas F ,
- Babacan T ,
- Saunders C ,
- Partridge A ,
- Santoro N ,
- Tubiana-Hulin M ,
- Colleoni M ,
- Karlsson P ,
- SOLE Investigators
- Gallicchio L ,
- Calhoun C ,
- Helzlsouer KJ
- Moscetti L ,
- Agnese Fabbri M ,
- Sperduti I ,
- Freites-Martinez A ,
- Shapiro J ,
- ↵ Kuo AM, Reingold RE, Ketosugbo K, et al. Oral minoxidil fo rthe treatment of late alopecia in cancer survivors, Abstract #12022. American Society of Clinical Oncology Annual Meeting 2022.
- Wolinsky FD ,
- Fitzgerald JF ,
- Chlebowski R ,
- Vehmanen L ,
- Välimäki M ,
- Blomqvist C
- Delmas PD ,
- Confravreux E ,
- Hardouin C ,
- Brufsky A ,
- Harker WG ,
- Mlineritsch B ,
- Schippinger W ,
- ABCSG-12 Trial Investigators
- Luschin-Ebengreuth G ,
- Austrian Breast and Colorectal Cancer Study Group
- Shapiro CL ,
- Majithia N ,
- Atherton PJ ,
- Frantal S ,
- Pfeiler G ,
- all ABCSG-18 investigators
- Runowicz CD ,
- Grunfeld E ,
- Levine MN ,
- Julian JA ,
- Kyriakides S ,
- Freedman RA ,
- Minami CA ,
- Monticciolo DL ,
- Newell MS ,
- Monsees B ,
- Hassett MJ ,
- Somerfield MR ,
- Mariotto AB ,
- Yabroff KR ,
- Carrera PM ,
- Kantarjian HM ,
- Peppercorn JM ,
- Dusetzina SB ,
- Huskamp HA ,
- Parikh DA ,
- Ragavan M ,
- Papanicolas I ,
- Woskie LR ,
- de Souza JA ,
- Wroblewski K ,
- ↵ Syrjala KL, Yi J. Overview of psychosocial issues in the adult cancer survivor. 2023. https://www.uptodate.com/contents/overview-of-psychosocial-issues-in-the-adult-cancer-survivor .
- Bertram H ,
- Ploos van Amstel FK ,
- van den Berg SW ,
- van Laarhoven HW ,
- Gielissen MF ,
- Ottevanger PB
- FCR Study Advisory Committee
- ↵ National Comprehensive Cancer Network. NCCN survivorship guidelines. www.nccn.org/professionals/physician_gls/pdf/survivorship.pdf .
- Gonzalez-Hernandez E ,
- Lengacher CA ,
- Paterson CL ,
- Institute of Medicine ,
- National Research Council ,
- Greenfield S ,
- Wakefield CE ,
- Krok-Schoen JL ,
- Naughton MJ ,
- Noonan AM ,
- Pisegna J ,
- DeSalvo J ,
- Lustberg MB
- Hooning MJ ,
- Aleman BM ,
- Patnaik JL ,
- DiGuiseppi C ,
- Dabelea D ,
- Molina-Montes E ,
- Dávila-Arias C ,
- ↵ US Preventive Services Task Force. Recommendations. https://www.uspreventiveservicestaskforce.org/uspstf/search_results?searchterm=cancer%20screening%20 .
- ↵ European Commission Initiative on Colorectal Cancer. Colorectal cancer guidelines and quality assurance. 2022. https://healthcare-quality.jrc.ec.europa.eu/ecicc .
- Watson KE ,
- American Heart Association Cardiovascular Disease in Women and Special Populations Committee of the Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; and Council on Quality of Care and Outcomes Research
- Zamorano JL ,
- Lancellotti P ,
- Rodriguez Muñoz D ,
- Authors/Task Force Members ,
- ESC Committee for Practice Guidelines (CPG) ,
- Document Reviewers
- Iribarren C ,
- van Ommen-Nijhof A ,
- Jacobse JN ,
- Steggink LC ,
- Whelton PK ,
- Lahart IM ,
- Metsios GS ,
- Nevill AM ,
- Carmichael AR
- McNeely ML ,
- Campbell KL ,
- Klassen TP ,
- Mackey JR ,
- Courneya KS
- Rees-Punia E ,
- Schmidt MD ,
- La Vecchia C ,
- Spence RR ,
- Steele ML ,
- Sandler CX ,
- Elvsaas IKO ,
- Campbell R ,
- Vansteenkiste M ,
- Delesie L ,
- Hilfiker R ,
- Meichtry A ,
- Robinson PD ,
- Cataudella D ,
- Mustian KM ,
- Alfano CM ,
- Heckler C ,
- Rogers LQ ,
- Courneya KS ,
- Vitale JA ,
- Schmitz KH ,
- Troeschel AN ,
- Thomson CA ,
- Sullivan KR ,
- Ligibel JA ,
- Furmaniak AC ,
- Ehlers DK ,
- Schmidt ME ,
- Steindorf K
- Gradishar WJ ,
- Abraham J ,
- Krucoff C ,
- Aragaki AK ,
- Anderson GL ,
- Blackburn GL
- Beasley JM ,
- Newcomb PA ,
- Kroenke CH ,
- Sweeney C ,
- Castillo A ,
- Estruch R ,
- Salas-Salvadó J ,
- PREDIMED Study Investigators
- Gensini GF ,
- Willett WC ,
- Trichopoulou A ,
- Farvid MS ,
- Spence ND ,
- Holmes MD ,
- Marinac CR ,
- Nelson SH ,
- Haslam DE ,
- Touillaud M ,
- Mesrine S ,
- ↵ NIH Consensus Conference. Acupuncture . JAMA 1998 ; 280 : 1518 - 24 . pmid: 9809733 OpenUrl CrossRef PubMed Web of Science
- Kaptchuk TJ
- Langevin HM
- Garcia MK ,
- McQuade J ,
- Georgiade G
- Ismaila N ,
- Höxtermann MD ,
- Aboudamaah S ,
- World Health Organization
- Dibble SL ,
- Cooper BA ,
- Molassiotis A ,
- Dabbour R ,
- Hummerston S
- Russell W ,
- Murphy SL ,
- Arnedt JT ,
- Mboineki JF ,
- Squires LR ,
- Fawcett J ,
- Savard MH ,
- Garland SN ,
- Johnson JA ,
- Casault L ,
- Matthews EE ,
- Berger AM ,
- Schmiege SJ ,
- Fiorentino L ,
- Rissling M ,
- Ancoli-Israel S
- Kabat-Zinn J
- Potters L ,
- Wernicke AG ,
- Christodoulou G ,
- Witek Janusek L ,
- Witek-Janusek L ,
- Albuquerque K ,
- Chroniak KR ,
- Chroniak C ,
- Durazo-Arvizu R ,
- Schell LK ,
- Shomstein S ,
- Winkler MM ,
- DuPont-Reyes MJ ,
- Balneaves LG ,
- Frenkel M ,
- Society for Integrative Oncology
- Dakhil SR ,
- Sahebkar A ,
- Hashemian F ,
- Taghikhani M ,
- Abolhasani E
- Heckler CE ,
- Roscoe JA ,
- Hankinson SE ,
- Bertone-Johnson ER ,
- Madden JM ,
- MacGregor CA ,
- Canney PA ,
- Patterson G ,
- McDonald R ,
- Van Patten CL ,
- Olivotto IA ,
- Chambers GK ,
- Ambrosone CB ,
- Zirpoli GR ,
- Hutson AD ,
- Mooiman KD ,
- Beijnen JH ,
- Schellens JH ,
- Meijerman I
- Blumberg JB ,
- Mullan BA ,
- Singleton AC ,
- Raeside R ,
- Jongerius C ,
- Mazzocco K ,
- Pravettoni G
- Zickmund SL ,
- ↵ Matters MLN. CY2022 Telehealth Update Medicare Physician Fee Schedule. 2022. https://www.cms.gov/files/document/mm12549-cy2022-telehealth-update-medicare-physician-fee-schedule.pdf .
- DeGirolamo K ,
- Johnson BA ,
- Lindgren BR ,
- Pritchett J ,
- Dholakia R ,
- Hardy-Abeloos C ,
- de Rezende LF ,
- Francisco VE ,
- Reeves MM ,
- Terranova CO ,
- Winkler EAH ,
- Weiner LS ,
- Irene Su H ,
- Wagner LI ,
- Titchener K ,
- Haaland B ,
- Gaufberg E ,
- Titchener K
- Divorne N ,
- Trémolières P ,

- Center on Health Equity and Access
- Health Care Cost
- Health Care Delivery
- Value-Based Care
Use and impact of breast cancer survivorship care plans: a systematic review
- Original Article
- Published: 19 June 2021
- Volume 28 , pages 1292–1317, ( 2021 )
Cite this article
- Abhishek Joshi ORCID: orcid.org/0000-0002-7786-0039 1 ,
- Sarah Larkins 1 ,
- Rebecca Evans 1 ,
- Nishila Moodley 1 ,
- Amy Brown 1 &
- Sabe Sabesan 1
1215 Accesses
5 Citations
3 Altmetric
Explore all metrics
Survivorship care plan (SCP) comprising a treatment summary and plan for follow-up care is recommended by various organizations to address long-term needs of an increasing number of breast cancer survivors. Although there have been previous systematic reviews of SCPs in cancer, none has focused on breast cancer exclusively. This systematic review evaluates the use and impact of SCP in breast cancer survivors.
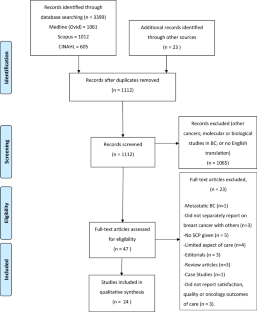
Randomized (RCTs) and non-randomized (non-RCT) studies evaluating health care and patient-related outcomes after implementation of SCPs for survivors were identified by searching databases (MEDLINE, EMBASE, CINHAL, and Scopus). Data were extracted, quality assessed, and summarized on the basis of qualitative synthesis.
Ten non-RCTs and 14 RCTs met the inclusion criteria. Although the overall quality of RCTs was superior to non-RCTs with mean quality score of 81.5% vs 64.3%, two mixed-methods non-RCTs which were individualized and included both provider and patient perspectives had comparable scores like RCTs. Several models of SCP were evaluated (paper based/online, oncologist/nurse/primary-care physician-delivered, and different templates). Descriptive information from non-RCTs suggests improvement in survivorship knowledge, satisfaction with care, and improved communication with providers. Findings from RCTs were variable. Potential gaps existed in content of SCP including unclear recommendation on frequency and ownership of follow-up. Levels of survivor satisfaction with, and self-reported understanding of, their SCP were high. Distal outcomes like health care delivery measures including costs and efficiency were mostly mixed, but heterogeneous study designs make interpretation difficult.
Conclusions
Existing research provides positive impact of SCPs on more proximal outcomes of patient experience and care delivery but mixed results for health outcomes in breast cancer survivors. Future research should focus on better defining SCP content and ensuring follow-up recommendations are acted upon, and provider feedback is included and use of novel tools to empower stakeholders.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Similar content being viewed by others
Survivorship care plans in cancer: a systematic review of care plan outcomes
M E Brennan, J F Gormally, … A J Spillane
Survivorship care plan preferences of cancer survivors and health care providers: a systematic review and quality appraisal of the evidence
Dori L. Klemanski, Kristine K. Browning & Jennifer Kue
Survivorship care plan outcomes for primary care physicians, cancer survivors, and systems: a scoping review
Weston LaGrandeur, Julie Armin, … Leila Ali-Akbarian
Li N, Deng Y, Zhou L, et al. Global burden of breast cancer and attributable risk factors in 195 countries and territories, from 1990 to 2017: results from the Global Burden of Disease Study 2017. J Hematol Oncol. 2019;12(1):1–12.
Article Google Scholar
Australian Institute of Health and Welfare. Cancer Data in Australia [online book]. Canberra, ACT: AIHW; 2020. [Available from: https://www.aihw.gov.au/reports/cancer/cancer-data-in-australia/contents/cancer-rankings-data-visualisation ].
Australian Institute of Health and Welfare. Cancer in Australia 2017. Canberra, ACT: AIHW; 2017. [Available from: https://www.aihw.gov.au/reports/cancer/cancer-in-australia-2017/contents/table-of-contents ].
Institute of Medicine. 2006. From cancer patient to cancer survivor: lost in transition: n American Society of Clinical Oncology and Institute of Medicine Symposium. The National Academies Press, Washington
Bettencourt BA, Schlegel RJ, Talley AE, et al. The breast cancer experience of rural women: a literature review. Psychooncology. 2007;16(10):875–87.
Article PubMed Google Scholar
D’Souza V, Daudt H, Kazanjian A. Survivorship care plans for breast cancer patients: understanding the quality of the available evidence. Curr Oncol. 2017;24(6):e446–65.
Article PubMed PubMed Central Google Scholar
Anbari AB, Wanchai A, Graves R. Breast cancer survivorship in rural settings: a systematic review. Support Care Cancer. 2020;28(8):3517–31.
Clinical Oncology Society of Australia (COSA) Model of Survivorship Care Working Group. Model of Survivorship Care: Critical Components of Cancer Survivorship Care in Australia Position Statement (Version 1.0). Sydney, NSW: COSA; November, 2016. [Available from: https://www.cosa.org.au/media/332340/cosa-model-of-survivorship-care-full-version-final-20161107.pdf ].
Hewitt ME, Bamundo A, Day R, et al. Perspectives on post-treatment cancer care: qualitative research with survivors, nurses, and physicians. J Clin Oncol. 2007;25(16):2270–3.
Australian Cancer Survivorship Centre (Peter McCallum Cancer Centre). Survivorship Care Plans: Toolkit. Melbourne, VIC: Peter MacCallum Cancer Centre; 2016; p. 37. [Available from: https://www.petermac.org/sites/default/files/media-uploads/ACSC_Survivorship_Care_Plan_toolkit_Jan_2016.pdf ].
Commission on Cancer (CoC). Cancer Program Standards: Ensuring Patient-Centered Care. Chicago, ILL: American College of Surgeons; 2016. [Available from: https://apos-society.org/wp-content/uploads/2016/06/CoCStandards.pdf ].
Mayer DK, Shapiro CL, Jacobson P et al. Assuring quality cancer survivorship care: we've only Just Begun: American Society of Clinical Oncology Educational Book 35. Alexandria, VA: American Society of Clinical Oncology; 2015. [Available from: https://ascopubs.org/doi/pdfdirect/ ]. Doi: https://doi.org/10.14694/EdBook_AM.2015.35.e583
Ganz PA, Casillas J, Hahn EE. Ensuring quality care for cancer survivors: implementing the survivorship care plan. Semin Oncol Nurs. 2008;24(3):208–17.
Cowens-Alvarado R, Sharpe K, Pratt-Chapman M, et al. Advancing survivorship care through the National Cancer Survivorship Resource Center: developing American Cancer Society guidelines for primary care providers. CA Cancer J Clin. 2013;63(3):147–50.
Faul LA, Rivers B, Shibata D, et al. Survivorship care planning in colorectal cancer: feedback from survivors and providers. J Psychosoc Oncol. 2012;30(2):198–216.
Brennan M, Gormally J, Butow P, et al. Survivorship care plans in cancer: a systematic review of care plan outcomes. Br J Cancer. 2014;111(10):1899–908.
Article CAS PubMed PubMed Central Google Scholar
Klemanski DL, Browning KK, Kue J. Survivorship care plan preferences of cancer survivors and health care providers: a systematic review and quality appraisal of the evidence. J Cancer Surviv. 2016;10(1):71–86.
Nicolaije KAH, Ezendam NPM, Vos MC, et al. Oncology providers’ evaluation of the use of an automatically generated cancer survivorship care plan: longitudinal results from the ROGY Care trial. J Cancer Surviv. 2014;8(2):248–59.
Birken SA, Raskin S, Zhang Y, et al. Correction to: survivorship care plan implementation in US cancer programs: a national survey of cancer care providers. J Cancer Educ. 2019;34(3):623–623.
Birken SA, Deal AM, Mayer DK, et al. Following through: the consistency of survivorship care plan use in United States cancer programs. J Cancer Educ. 2014;29(4):689–97.
Blanch-Hartigan D, Forsythe LP, Alfano CM, et al. Provision and discussion of survivorship care plans among cancer survivors: results of a nationally representative survey of oncologists and primary care physicians. J Clin Oncol. 2014;32(15):1578–85.
Parry C, Kent EE, Forsythe LP, et al. Can’t see the forest for the care plan: a call to revisit the context of care planning. J Clin Oncol. 2013;31(21):2651–3.
Runowicz CD, Leach CR, Henry NL, et al. American Cancer Society/American Society of Clinical Oncology breast cancer survivorship care guideline. CA Cancer J Clin. 2016;66(1):43–73.
Choi Y, Smith KC, Shukla A, et al. Breast cancer survivorship care plans: what are they covering and how well do they align with national guidelines? Breast Cancer Res Treat. 2020;179(2):415–24.
Coughlin SS, Caplan L, Stewart JL, et al. Do breast cancer survivorship care plans improve health outcomes? J Cancer Treatment Diagn. 2019;3(1):28–33.
Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1–34.
National Health and Medical Research Council. How to use the evidence: assessment and application of scientific evidence. Canberra, ACT: NHMRC; 2000. [Available from: https://www.nhmrc.gov.au/sites/default/files/documents/reports/clinical%20guidelines/how-to-use-evidence-cp69.pdf ].
Sirriyeh R, Lawton R, Gardner P, et al. Reviewing studies with diverse designs: the development and evaluation of a new tool. J Eval Clin Pract. 2012;18(4):746–52.
Su HI, Stark S, Kwan B, et al. Efficacy of a web-based women’s health survivorship care plan for young breast cancer survivors: a randomized controlled trial. Breast Cancer Res Treat. 2019;176(3):579–89.
Article PubMed Central CAS Google Scholar
Fang S-Y, Wang Y-L, Lu W-H, et al. Long-term effectiveness of an E-based survivorship care plan for breast cancer survivors: a quasi-experimental study. Patient Educ Couns. 2020;103(3):549–55.
Maly RC, Liang L-J, Liu Y, et al. Randomized controlled trial of survivorship care plans among low-income, predominantly Latina breast cancer survivors. J Clin Oncol. 2017;35(16):1814–21.
Kvale EA, Huang CHS, Meneses KM, et al. Patient-centered support in the survivorship care transition: outcomes from the Patient-Owned Survivorship Care Plan Intervention. Cancer. 2016;122(20):3232–42.
Ruddy KJ, Guo H, Baker EL, et al. Randomized phase 2 trial of a coordinated breast cancer follow-up care program. Cancer. 2016;122(22):3546–54.
Greenlee H, Molmenti CLS, Crew KD, et al. Survivorship care plans and adherence to lifestyle recommendations among breast cancer survivors. J Cancer Surviv. 2016;10(6):956–63.
Boekhout AH, Maunsell E, Pond GR, et al. A survivorship care plan for breast cancer survivors: extended results of a randomized clinical trial. J Cancer Surviv. 2015;9(4):683–91.
Coyle D, Grunfeld E, Coyle K, et al. Cost effectiveness of a survivorship care plan for breast cancer survivors. J Oncol Pract. 2014;10(2):e86–92.
Grunfeld E, Julian JA, Pond G, et al. Evaluating survivorship care plans: results of a randomized, clinical trial of patients with breast cancer. J Clin Oncol. 2011;29(36):4755–62.
Faul LA, Luta G, Sheppard V, et al. Associations among survivorship care plans, experiences of survivorship care, and functioning in older breast cancer survivors: CALGB/Alliance 369901. J Cancer Surviv. 2014;8(4):627–37.
Rocque GB, Wisinski KB, Buhr KA, et al. Development and evaluation of a survey to assess survivor knowledge change after survivorship care plans: WiSDOM-B (Wisconsin Survey of cancer DiagnOsis and Management in Breast cancer). J Cancer Educ. 2014;29(2):270–7.
Hershman DL, Greenlee H, Awad D, et al. Randomized controlled trial of a clinic-based survivorship intervention following adjuvant therapy in breast cancer survivors. Breast Cancer Res Treat. 2013;138(3):795–806.
Article CAS PubMed Google Scholar
Grunfeld E, Levine MN, Julian JA, et al. Randomized trial of long-term follow-up for early-stage breast cancer: a comparison of family physician versus specialist care. J Clin Oncol. 2006;24(6):848–55.
Grunfeld E, Mant D, Yudkin P, et al. Routine follow up of breast cancer in primary care: randomised trial. BMJ. 1996;313(7058):665–9.
Krok-Schoen JL, Naughton MJ, Noonan AM, et al. Perspectives of survivorship care plans among older breast cancer survivors: a Pilot Study. Cancer Control. 2020. https://doi.org/10.1177/1073274820917208 .
Nápoles AM, Santoyo-Olsson J, Chacón L, et al. Feasibility of a mobile phone app and telephone coaching survivorship care planning program among Spanish-speaking breast cancer survivors. JMIR Cancer. 2019;5:e13543. https://doi.org/10.12196/13543 .
Singh-Carlson S, Wong F, Oshan G. Evaluation of the delivery of survivorship care plans for South Asian female breast cancer survivors residing in Canada. Curr Oncol. 2018;25(4):e265–74.
Rohan EA, Townsend JS, Fleischmann A, et al. “When I needed it”: Evaluation of the use and timing of Sharsheret’s Thriving Again program for young breast cancer survivors. J Cancer Educ. 2018;33(5):976–82.
O’Hea E, Wu J, Dietzen L, et al. The Polaris Oncology Survivorship Transition (POST) System: a patient-and provider-driven cancer survivorship planning program. J Oncol Navig Surviv. 2016;7(10):11–24.
PubMed PubMed Central Google Scholar
Palmer SC, Stricker CT, Panzer SL, et al. Outcomes and satisfaction after delivery of a breast cancer survivorship care plan: results of a multicenter trial. J Oncol Pract. 2015;11(2):e222–9.
Tevaarwerk AJ, Wisinski KB, Buhr KA, et al. Leveraging electronic health record systems to create and provide electronic cancer survivorship care plans: a pilot study. J Oncol Pract. 2014;10(3):e150–9.
Blinder VS, Norris VW, Peacock NW, et al. Patient perspectives on breast cancer treatment plan and summary documents in community oncology care: a pilot program. Cancer. 2013;119(1):164–72.
Haq R, Heus L, Baker NA, et al. Designing a multifaceted survivorship care plan to meet the information and communication needs of breast cancer patients and their family physicians: results of a qualitative pilot study. BMC Med Inform Decis Mak. 2013;13(1):1–13.
Sprague BL, Dittus KL, Pace CM, et al. Patient satisfaction with breast and colorectal cancer survivorship care plans. Clin J Oncol Nurs. 2013;17(3):266–72.
Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–74.
Hill-Kayser CE, Vachani C, Hampshire MK, et al. High level use and satisfaction with internet-based breast cancer survivorship care plans. Breast J. 2012;18(1):97–9.
Hill RE, Wakefield CE, Cohn RJ, et al. Survivorship care plans in cancer: A meta-analysis and systematic review of care plan outcomes. Oncologist. 2020;25(2):e351–72.
Jacobsen PB, DeRosa AP, Henderson TO, et al. Systematic review of the impact of cancer survivorship care plans on health outcomes and health care delivery. J Clin Oncol. 2018;36(20):2088–100.
Birken SA, Urquhart R, Munoz-Plaza C, et al. Survivorship care plans: are randomized controlled trials assessing outcomes that are relevant to stakeholders? J Cancer Surviv. 2018;12(4):495–508.
Download references
No funding sources to declare.
Author information
Authors and affiliations.
Townsville Hospital, Townsville, Australia
Abhishek Joshi, Sarah Larkins, Rebecca Evans, Nishila Moodley, Amy Brown & Sabe Sabesan
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Abhishek Joshi .
Ethics declarations
Conflict of interest.
All participating authors declare no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Joshi, A., Larkins, S., Evans, R. et al. Use and impact of breast cancer survivorship care plans: a systematic review. Breast Cancer 28 , 1292–1317 (2021). https://doi.org/10.1007/s12282-021-01267-4
Download citation
Received : 10 May 2021
Accepted : 09 June 2021
Published : 19 June 2021
Issue Date : November 2021
DOI : https://doi.org/10.1007/s12282-021-01267-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Survivorship care plan
- Breast cancer
- Care pathway
- Find a journal
- Publish with us
- Track your research
- Research article
- Open access
- Published: 22 February 2021
High-intensity interval training in breast cancer survivors: a systematic review
- Katsunori Tsuji 1 ,
- Yutaka J. Matsuoka 1 &
- Eisuke Ochi ORCID: orcid.org/0000-0002-2497-0250 1 , 2
BMC Cancer volume 21 , Article number: 184 ( 2021 ) Cite this article
6376 Accesses
13 Citations
4 Altmetric
Metrics details
To review the settings and outcomes of high-intensity interval training (HIIT) interventions for breast cancer survivors, and to explore the feasibility of prescribing exercise for breast cancer survivors.
A systematic search of electronic databases was conducted for studies published up to May 31, 2020. Eligibility criteria included randomized controlled trials of HIIT intervention in breast cancer survivors. Studies were grouped by whether the intervention was conducted during or after breast cancer treatment, and intervention methods and outcomes were reviewed within each group.
Twenty-six studies were identified, and 13 satisfied the inclusion criteria. Intervention was conducted during treatment in 8 studies, and after treatment in 5. Intervention duration ranged from 3 to 16 weeks, with 2 or 3 sessions per week, for a total of 9 to 36 sessions. All interventions were supervised; 12 were lab-based, and 1 was community-based. One of most promising outcomes was improvement of cardiorespiratory fitness by HIIT.
This review found that all studies on HIIT for breast cancer survivors investigated lab-based, supervised interventions, but not home-based or unsupervised. HIIT is a time-efficient method for increasing cardiovascular function in breast cancer survivors, but further research is necessary to determine its effects on other outcomes.
Peer Review reports
Breast cancer survivors are suffering many problems with the complications of surgery and radiation therapy such as Axillary web syndrome, pain, limited range of motion and dysfunction of the upper limbs, posture imbalance, lymphedema, or psychological symptoms associated with those conditions. The rehabilitation approach is effective for these sequelas [ 1 , 2 , 3 , 4 , 5 , 6 , 7 ]. The strong association between physical activity and all-cause mortality risk in breast cancer survivors [ 8 ] has led experts to recommend that breast cancer survivors engage in physical activity and exercise [ 9 ] and prompted researchers to investigate exercise interventions for this population. Combination of aerobic training and resistance training is considered particularly effective [ 5 , 9 ].
High-intensity interval training (HIIT) interventions have recently been proposed as a promising method for quickly improving fitness. HIIT consists of repeated sets of short bursts of high-intensity exercise followed by a rest interval, and has been shown improve fitness in both athletes and the general population [ 10 , 11 ]. In recent years, research on the suitability of HIIT for cancer survivors has emerged as well. Systematic reviews and meta-analyses of HIIT for cardiorespiratory fitness in cancer survivors have already shown HIIT to have some degree of effectiveness [ 12 , 13 ]. Research on HIIT for breast cancer survivors was first published around 2016 [ 14 ], but no review article focusing exclusively on breast cancer survivors has been published to date.
Therefore, the purpose of this review is to determine whether there is a home-based HIIT intervention in breast cancer survivors. The specific characteristics of interest were (1) timing (during or after treatment), (2) setting (lab-based, community-based, or home-based), and (3) supervision (supervised or unsupervised). we thought that to devide the intervention timing is important to consider the rehabilitation approach (setting, exercise supervision etc..) of breast cancer survivors. In addition, in light of concerns that sheltering in place during the novel coronavirus (SARS-CoV-2) pandemic of 2020 will lead to inadequate physical activity and consequently increased risk of cardiovascular disease worldwide [ 15 ], this review will also explore the current landscape and future possibilities of home-based, unsupervised exercise interventions.
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist was used for this review [ 16 ], and was registered with the international database of prospectively registered systematic reviews in health and social care (PROSPERO Registration Number: CRD42020221206).
Information sources and search strategy
Electronic databases (PubMed, Cochran Library, Web of Science, and Igaku Chuo Zasshi) were searched for studies published using all available records up to May 31, 2020. The search expression used was as follows;
"breast cancer"[Title/Abstract] AND ("high intensity interval"[Title/Abstract] OR "high intensity intermittent"[Title/Abstract] OR "aerobic interval"[Title/Abstract]) AND (exercise OR training) AND (randomized controlled trial[pt] OR controlled clinical trial[pt] OR randomized[tiab] OR placebo[tiab] OR randomly[tiab] OR trial[tiab] OR groups[tiab] NOT (animals [mh] NOT humans [mh]))
All studies with keywords related to HIIT interventions for breast cancer survivors were included.
Inclusion criteria
Inclusion criteria were studies published in English and Japanese (only those with full text available) that included HIIT in the intervention and were conducted in breast cancer survivors. HIIT was defined as exercise consisting of multiple repetitions of short bursts (≤4 min) of high-intensity (≥90% of maximal oxygen uptake [VO 2 max], peak oxygen uptake [VO 2 peak] or rating of perceived exertion [RPE] ≥ 18) aerobic exercise (e.g., running or cycling) alternated with low-intensity exercise or passive rest. Studies of interventions that combined HIIT with resistance training or aerobic training were also included in the review. When multiple datasets were available from the same research group or follow-up data were available for the same cohort of participants, the earliest published dataset was used.
Study selection and data extraction
Irrelevant articles were excluded from the review by screening the titles and abstracts displayed in the search results (KT). Next, methods of intervention (exercise duration/frequency, exercise intensity, mode of exercise, HIIT intervals, and intervention setting) and outcomes (cardiorespiratory fitness, muscle strength, indicators of cardiotoxicity/cardiovascular function, health-related quality of life [HRQOL], fatigue, related biomarkers, adverse events, and compliance) were determined by reviewing the full text. The full text was independently reviewed by two of the authors (KT and EO). These outcomes were selected to investigate the effects of HIIT on physical function as the primary outcome of interest, as well as the effects of HIIT on areas of clinical concern for breast cancer survivors (HRQOL, fatigue, and cardiotoxicity/cardiovascular function) and safety of and compliance with HIIT among breast cancer survivors.
Risk of bias assessment
The Cochrane risk of bias tool was used to maintain internal validity [ 17 ]. All of the authors (KT, YM, EO) assessed selection bias, performance bias, detection bias, attrition bias, reporting bias, and other biases. The aim of this review is not assess the effect of HIIT on clinical outcome in breast cancer survivors, however, publication bias may affect the number of studies published. Any disagreements between reviewers were solved through discussion on a video conference.
Search outcome
A total of 93 search results were obtained from the four databases, but 26 were duplicates and were therefore excluded. After screening, 9 studies were excluded from the review based on their title and abstract, and 2 more studies were excluded because they were follow-up studies of the same cohort. All 15 articles extracted were available in full text. After the full text of the remaining studies was carefully reviewed, an additional 3 studies were excluded for not meeting the exercise intensity criteria described in the Methods section. Finally, a total of 12 studies satisfied the inclusion criteria (Fig. 1 ).
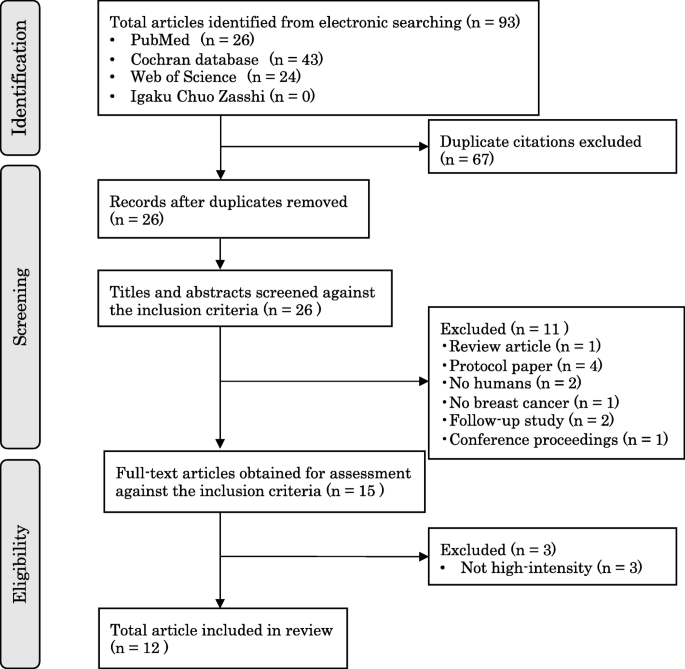
PRISMA flow diagram
Tables 1 and 2 summarize the studies included in the review. Table 1 lists interventions conducted during breast cancer treatment, and Table 2 lists interventions conducted after initial cancer treatment. Each table lists the authors, sample size, a summary of the HIIT program, outcomes, whether the intervention was supervised or unsupervised, and the intervention setting (lab-based, community-based, or home-based) for each study. Summaries of the HIIT programs include the duration of training, frequency, mode of exercise, intensity, and intervals.
The results of the methodological quality assessment of the studies included in this review are summarized in Fig. 2 . The proper procedure for randomly generated sequences has been fully described in ten studies [ 18 , 19 , 20 , 21 , 22 , 23 , 24 , 26 , 27 , 28 ], five of which hid the assignments [ 21 , 22 , 23 , 24 , 27 ]. Performance bias was found in all included trials. Blinding of participants is not possible due to the characteristics of exercise interventions. However, these do not pose a threat to internal validity. Only one study [ 27 ] blinded outcome assessors. Three trials found a high risk of having incomplete outcome data [ 21 , 22 , 23 ].
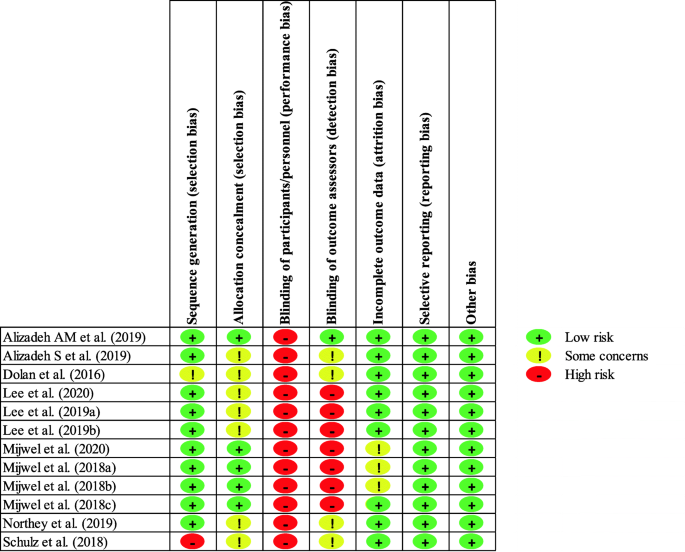
Summary of the Cochrane risk of bias tool
Intervention timing (during or after treatment)
Eight studies involved interventions during breast cancer treatment and 5 involved interventions after treatment. Of the 8 interventions conducted during treatment, 3 were conducted by Lee et al., 4 by Mijwel et al., and 1 by Schulz et al. The intervention was started at the same time as chemotherapy in 7 of those 8 studies. In the remaining study, the participants received chemotherapy before and during the intervention. Two of the studies of interventions conducted after treatment revealed the timing of the intervention: participants in the studies by Alizadeh AM et al. and Alizadeh S et al. started the intervention no earlier than 1 month after completing chemotherapy and/or radiotherapy. The studies by Northey et al., and Dolan et al. did not specify the timing of the intervention.

Setting (lab-based HIIT or community-based HIIT)
All of the 12 studies investigated lab-based interventions. All of the lab-based interventions conducted during breast cancer treatment used a cycle ergometer. Three of the interventions conducted after treatment used a treadmill, and one used a cycle ergometer.
Exercise supervision
No studies of unsupervised HIIT have been conducted to date, and thus all the studies in this review investigated supervised interventions. Two of the 12 studies did not specify who supervised the intervention. In those that did specify, the supervisor was an exercise trainer [ 18 , 19 , 20 ], an exercise physiologist [ 21 , 22 , 23 , 24 , 26 , 27 ], or an oncology nurse [ 21 , 22 , 23 , 24 ]. In Mijwel et al., the intervention was supervised by an exercise physiologist or oncology nurse. In Schulz et al., the intervention was supervised by a professional, but no further details were provided [ 25 ].
Exercise training protocols
Studies were sorted by HIIT protocol. The 12 studies included multiple studies conducted by the same research groups. In the group of studies on interventions during breast cancer treatment, Lee et al. (3 of 13 studies) had participants perform 7 sets consisting of 1 min of exercise at 90% peak power output determined by cardiopulmonary exercise testing, followed by 2 min of active rest, repeated 3 times per week for 8 weeks [ 18 , 19 , 20 ]. Mijwel et al. (4 of 13 studies) had participants perform 3 sets consisting of 3 min of exercise at RPE of 16 to 18 followed by 1 min of passive rest, repeated twice weekly for 16 weeks [ 21 , 22 , 23 , 24 ]. They also had participants perform resistance training (RT-HIIT group) or aerobic training (AT-HIIT group) 3 times a week on the days they did not perform HIIT. Resistance training consisted of 2 or 3 sets of 12 repetitions of resistance training exercises for 9 different muscle groups at 70 to 80% of their one-repetition maximum (1RM). Aerobic training consisted of 20 min of cycling at an RPE of 13 to 15. Schulz et al. had participants perform HIIT and resistance training as a group twice weekly for 6 weeks [ 25 ]. For HIIT, participants performed 3 sets consisting of 3 min of exercise on a cycle ergometer at an intensity of 85 to 100% VO 2 max followed by 1 min of active rest. For resistance training, they performed 8 to 12 repetitions of resistance training exercises for major muscle groups at 60 to 80% 1RM.
In the group of studies involving interventions after initial breast cancer treatment, Alizadeh AM et al. and Alizadeh S had participants perform 4 sets consisting of 4 min of inclined running at an intensity of 90 to 95% HRmax followed by 3 min of passive rest, repeated 3 times weekly for 12 weeks. Northey et al. had participants perform 4 sets consisting of 30 s of maximum-intensity pedaling followed by 2 min of rest, repeated 3 times weekly for 12 weeks [ 28 ]. Dolan et al. had participants perform an HIIT program on a treadmill that involved incrementally increasing exercise intensity over the intervention period in 3 weekly sessions for 6 weeks. The intervention started with 4 to 6 sets of 4-min running at 65% VO 2 max at 3-min intervals (50% VO 2 peak), but the intensity was increased to 90% VO 2 peak at the 13th session in Week 5, and ultimately to 4 to 6 sets of 2-min running at 95% VO 2 peak at 2-min intervals (< 60% VO 2 peak) in the final week (Week 6) [ 14 ].
Studies were also grouped by outcomes (cardiorespiratory fitness, muscle strength, indicators of cardiotoxicity/cardiovascular function, HRQOL, fatigue, related biomarkers, adverse events, and compliance). Five studies evaluated cardiorespiratory fitness, all using VO 2 peak. Three studies evaluated muscle strength, 2 using 1RM and 1 using maximum isometric contraction. One study evaluated cardiovascular function, and used endothelial function in terms of brachial artery flow mediated dilation (baFMD) and carotid intima-media thickness (cIMT) as an indicator. One study evaluated HRQOL, and used the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-Core 30 (EORTC QLQ-C30) [ 22 ]. One study evaluated fatigue, one using the 22-item Piper Fatigue Scale (PFS) [ 22 ].
Twelve studies evaluated HIIT compliance. The compliance rates in each study were as follows. Lee et al. reported an HIIT compliance rate of 82.3% across their studies. Mijwell et al. reported a compliance rate of 80 to 83% for HIIT plus resistance training and 57 to 75% for HIIT plus aerobic training across their studies. Schulz et al. and Alizadeh AM et al. reported HIIT compliance rates of 97 and 85%, respectively. Northey et al. reported an HIIT compliance rate of 78.7% across their studies. Dolan et al. reported a compliance rate of 99% for aerobic interval training. None of the studies that evaluated adverse events associated with HIIT reported any such events.
HIIT interventions during breast cancer treatment have been aimed at preventing or reducing cardiovascular effects of chemotherapy since Schulz et al. first investigated the feasibility of such interventions in 2018. HIIT interventions for breast cancer survivors have been investigated in only 3 studies since Dolan et al. published their study in 2016, and these studies have only investigated a narrow range of outcomes such as safety and cardiorespiratory fitness. Especially, there is no evidence to validate the efficacy of home-based HIIT, and future research results are awaited. The main findings of this review are that all HIIT interventions for breast cancer survivors to date were supervised, and nearly all were lab-based. Breast cancer survivors may face several challenges when trying to start exercising, including that resistance training and aerobic training are time-consuming, that gym memberships and exercise classes are expensive, and that access to exercise facilities may depend on where they live. In fact, the top responses in a survey that asked breast cancer survivors about barriers to exercising were lack of time and lack of access to facilities [ 29 ]. Therefore, home-based HIIT programs for breast cancer survivors will be necessary to overcome these barriers. In the following sections, individual aspects of the reviewed studies are discussed.
Setting of HIIT
All past studies of interventions during and after breast cancer treatment were lab-based. Possible reasons for this include that the purpose of these studies was to evaluate safety or feasibility, and that exercise intensity was exactly defined to validate the efficacy of HIIT. All past studies regardless timing of intervention were also lab-based, while there is no study of home-based or community-based. Recent review paper has shown that home-based exercise is an effective method for promoting exercise in cancer survivors [ 30 ]. Future studies will need to determine how to assist people in engaging in home-based or community-based HIIT exercise programs.
In all past studies of interventions during breast cancer treatment, the intervention was supervised by an exercise professionals or oncology nurse. In all past studies of interventions after breast cancer treatment, the intervention was also supervised by an exercise professional. A study comparing supervised and unsupervised HIIT interventions in healthy adults [ 31 ] showed that supervised interventions produced greater improvements in cardiorespiratory fitness, but unsupervised interventions still produced significant improvements. Another study of unsupervised HIIT in which participants exercised alone also showed improvements in cardiorespiratory fitness [ 32 ].
The following subsections discuss about frequency and period, type of exercise, intensity, and exercise and recovery intervals in the studies reviewed.
Frequency and intervention period
The period of HIIT interventions during breast cancer chemotherapy ranged from 6 [ 25 ] to 16 weeks [ 21 , 22 , 23 , 24 ]. The 16-week intervention was a combined intervention with resistance training or aerobic training. The longest HIIT-only interventions were 8 weeks [ 18 , 19 , 20 ]. The frequency of sessions during the intervention period was 3 times per week in 3 studies and twice per week in 5 studies. The smallest total number of sessions was 12, and the largest was 36.
The period of HIIT interventions for survivors in studies reviewed in this article ranged from 6 to 12 weeks. The frequency of sessions during the intervention period was 3 times per week in all 4 studies. The smallest total number of sessions was 18, and the largest was 36. In a study investigating the frequency and period of interval training programs, Edward Fox found that a 7-week HIIT program conducted 2 days per week produced comparable improvement in VO 2 max to a 7- or 13-week HIIT program conducted 4 days per week [ 33 ]. The study also found that training 2 days a week produced comparable improvement in cardiorespiratory fitness to training 4 days a week, and other studies reviewed in the present article also showed that a frequency of 2 to 3 times per week improves cardiorespiratory fitness [ 33 ]. Based on this evidence, a frequency of 2 to 3 times per week can be considered appropriate for HIIT interventions for breast cancer survivors. In this review, a significant improvement in cardiorespiratory function was found in the 6-week study, which was the shortest intervention period [ 14 ]. Therefore, it can be concluded that an intervention period of at least 6 weeks is necessary for HIIT to be effective.
Type of exercise
The mode of training was exercise on a cycle ergometer in all studies of interventions during cancer treatment. These studies likely selected a cycle ergometer because they decided to use VO 2 max as an indicator of exercise intensity during training in order to evaluate safety and feasibility of HIIT during breast cancer treatment, and a cycle ergometer allows for quantification of work. The mode of training in studies in cancer survivors was a treadmill in 3 studies [ 14 , 26 , 27 ] and cycling in 1 study [ 28 ].Almost all past studies of HIIT in subjects other than breast cancer survivors used equipment that allows for quantification of work (e.g., a cycle ergometer or treadmill) because VO 2 max was set as the indicator of exercise intensity. Exercise intensity is the most important factor in HIIT, and thus it is ideal to be able to quantify work. However, this requires exercise equipment, which makes such programs unfeasible for widespread implementation.
In all of the studies of interventions during treatment, the relative exercise intensity set at the start of the intervention was maintained until the end of the intervention, which would have resulted in the absolute intensity increasing over the duration of training. It is best to use a physiological index to calculate exercise intensity during HIIT, but Mijwel et al. used a rating of perceived exertion of 16 to 18 in their study. Past studies of home-based HIIT interventions that used the “talk test” (intensity should be great enough that talking is difficult) [ 32 ] or a modified Borg scale score of 6 to 8 (“very hard”) [ 34 ] as an indicator of exercise intensity showed significant improvement in the primary endpoint of cardiorespiratory fitness. Therefore, even though Mijwel et al. may have used a slightly lower or unclear exercise intensity for HIIT compared with other studies, that intensity may have been sufficient to increase VO 2 max.
In studies of interventions in survivors, the relative exercise intensity set at the start of the intervention was maintained until the end of the intervention in 3 of 4 studies, and the relative exercise intensity was increased incrementally from the start of the intervention in 1 study. Northey et al. [ 28 ] had participants pedal at maximum intensity for 30 s, which was likely the most intense burst of exercise out of all 4 studies (and also including interventions during cancer treatment).
Interval and recovery durations
In studies of interventions during treatment, the exercise and recovery intervals differed greatly depending on the HIIT exercise intensity. In the HIIT programs investigated in these studies, the exercise interval ranged from 1 to 3 min, the recovery interval from 1 to 2 min, the number of sets from 3 to 10, and the total exercise duration from 11 to 19 min.
In the HIIT programs used in studies of cancer survivors, the exercise interval ranged from 30 s to 4 min, the recovery interval from 2 to 3 min, number of sets from 4 to 6, and total exercise duration from 10 to 39 min. HIIT is currently attracting global interest, and there is ongoing debate about its methodology. As such, the optimal exercise interval, recovery interval, and number of sets have not yet been established, and studies on HIIT should consider these aspects alongside exercise intensity and feasibility. High intensity is most important to maximize the effects of HIIT. Northey et al., whose intervention used the most intense bursts of exercise of any study included in this review, had participants perform 4 sets consisting of 30 s of maximum-intensity pedaling followed by 2 min of rest. This method is similar to ones used for the healthy general population and athletes [ 35 ]. This indicates that exercise and recovery intervals in HIIT for breast cancer survivors can be investigated using methods similar to HIIT for the healthy general population. It will be necessary to develop a program with the most efficient exercise and recovery intervals optimized for breast cancer survivors on the basis of findings from studies on HIIT conducted to date.
Cardiorespiratory fitness
Of the 3 studies of interventions during treatment that evaluated cardiorespiratory fitness, 1 found that the HIIT intervention significantly increased cardiorespiratory fitness, and 2 found no difference. However, the 2 studies that found no difference did find that cardiorespiratory fitness decreased significantly at the end of the study in the control group, indicating that HIIT does prevent the reduction in cardiorespiratory fitness by cancer treatment. Of the 2 studies of interventions for cancer survivors that evaluated cardiorespiratory fitness, 2 found that the HIIT intervention significantly increased cardiorespiratory fitness. These findings suggest that HIIT has the effectiveness for improving cardiorespiratory fitness in breast cancer survivors.
Muscle strength and muscle mass
Both of the studies of interventions during cancer treatment that evaluated muscle strength showed significant improvements. Mijwell et al. and Schulz et al., who investigated interventions during breast cancer treatment, combined HIIT with resistance training. Mijwell et al. used back muscle strength (measured by isometric contraction) and grip strength to evaluate muscle strength. Schulz et al. used leg press 1RM to evaluate muscle strength. The effect of HIIT alone on muscle strength is not clear from these studies because resistance training had a strong effect. Mijwell et al. also evaluated muscle cross-sectional area (CSA) after the HIIT intervention. In that study, although it is unclear to what degree HIIT contributed to this result, they found that CSA of type II muscle fibers increased significantly and satellite cells increased after their HIIT plus resistance training intervention. Only 1 study of survivors evaluated muscle strength and found a significant increase. Dolan et al. used an intervention consisting solely of aerobic interval training and evaluated muscle strength by leg press 1RM. One study found that lower body muscle strength in breast cancer survivors is lower than or comparable to that in the general population [ 36 ], and HIIT has been shown to increase lower body muscle mass in healthy young men [ 37 ]. Although further evidence is necessary, HIIT shows promise for increasing muscle strength in breast cancer survivors, a population with reduced muscle strength deficit.
Cardiotoxicity and cardiovascular function
Two studies of interventions during treatment, both by Lee et al., evaluated the effects of HIIT on cardiotoxicity and vascular endothelial function. Breast cancer chemotherapy can be cardiotoxic, reduce cardiopulmonary function, and damage cardiac muscle tissue. Moderate-intensity exercise interventions added to chemotherapy have been investigated as a means to address these issues, and systematic reviews have shown the efficacy of such interventions [ 38 , 39 ]. However, the authors noted that research on exercise interventions to reduce cardiotoxicity is still initial stage, and further research into aspects such as intervention timing and intensity is necessary. Based on the findings of this review, Lee et al. conducted an HIIT program aimed at improving vascular endothelial function in patients undergoing chemotherapy for breast cancer. They found promising evidence that HIIT may reduce cardiotoxicity, including improvements in vascular endothelial function and cardiovascular biomarkers. Further research into the efficacy of high-intensity exercise such as HIIT for reducing cardiotoxicity is necessary to confirm its suitability in cancer survivors.
HRQOL, fatigue, and biomarkers
HIIT shows great potential for improving measures of physical function such as cardiorespiratory fitness and muscle strength. However, research on its effects on HRQOL and fatigue is lacking. Only 1 study evaluated HRQOL, and showed that HIIT improved HRQOL [ 22 ]. Only 1 study evaluated the effects of HIIT on fatigue. Mijwel et al., who investigated combination of HIIT plus resistance training or aerobic training, observed no change in fatigue evaluated by the PFS [ 22 ]. Alizadeh AM et al. found that HIIT significantly reduced levels of interleukin (IL)-6 [ 26 ]. In summary, there is insufficient evidence regarding the effects of HIIT on HRQOL and fatigue. Further research should be conducted to determine the efficacy of HIIT for these outcomes in breast cancer survivors.
Compliance rate and adverse events
All 8 studies of HIIT interventions during treatment reported compliance rates, and those rates ranged from 57 to 97%. The compliance rate was 82.3% for HIIT alone, 80 to 97% for HIIT plus resistance training, and 57 to 75% for HIIT plus aerobic training. Six of the 8 studies during chemotherapy reported about adverse events and all 6 reported no adverse events, thus demonstrating that prescription of HIIT is extremely safe. Four of the 5 studies of HIIT interventions after treatment reported compliance rates, which ranged from 78.7 to 99%. Two of the 4 studies evaluated adverse events, and all 2 reported no adverse events, thus demonstrating that prescription of HIIT for breast cancer survivors is also safe.
Perspective
Efficacy of hiit in cancer survivors.
The effects of HIIT on cardiorespiratory fitness were confirmed and comparable between interventions conducted during treatment (significant increase in 1 study, amelioration of treatment-related reduction in 2 studies) and after treatment (significant increase in 2 studies). HIIT compliance rates and incidence of adverse events also showed similar trends between interventions conducted during and after treatment, thus demonstrating the promising efficacy of HIIT. However, few studies examined muscle strength and mass or changes in cardiotoxicity or cardiovascular function after HIIT intervention in survivors. Therefore, further research on these outcomes is necessary. One of the limitations of this review is that the overall number of studies included was small. Also, this review did not focus on outcome differences. However, in assessing the risk of bias, the overall risk of bias is considered to be low, except for the higher risk of blindness due to the specificity of the exercise intervention.
Possibilities for home-based HIIT
A wide variety of basic and applied research has investigated HIIT in the general population. Given that HIIT is already known to improve cardiorespiratory fitness, more recent studies have investigated the feasibility of HIIT programs without specialized equipment or supervision. Blackwell et al. compared the effects of unsupervised bodyweight HIIT (home HIIT) and supervised HIIT using a treadmill (lab HIIT) on VO 2 max. They found that both lab HIIT (pre 26.50 ± 6.31, post 31.00 ± 6.69 mL/kg/min, p < 0.001) and home HIIT (pre 27.77 ± 4.75, post 29.98 ± 6.09 mL/kg/min, p < 0.05) significantly improved VO 2 max, but lab HIIT produced a significantly greater increase than home HIIT (p < 0.05) [ 31 ]. In contrast, Menz et al. found that home HIIT (pre 49.5 ± 6.6, post 54.4 ± 5.3 mL/kg/min, p < 0.001) produced comparable improvement in VO 2 max to lab HIIT(pre 47.8 ± 5.6, post 54.1 ± 5.6 mL/kg/min, p < 0.001) [ 40 ]. A systematic review of bodyweight HIIT methodology has also been conducted [ 41 ]. The findings of these studies suggest that bodyweight HIIT is beneficial for increasing cardiorespiratory fitness, and a home-based bodyweight HIIT program should be developed for breast cancer survivors. Home-based HIIT for breast cancer survivors has only ever been investigated in 1 study protocol [ 42 ]. In that study, participants performed bodyweight HIIT exercises at home, and their exercise was monitored with a wearable device [ 42 ].
All studies on HIIT for breast cancer survivors used lab-based, supervised interventions, while none of the home-based HIIT have reported so far. HIIT is a time-efficient method for increasing cardiorespiratory fitness in breast cancer survivors, but further research is necessary to determine its effects on other outcomes such as HRQOL, fatigue, muscle function, and cardiovascular function because few studies have evaluated those outcomes. Due to the lack of evidence of benefit from home-based HIIT for breast cancer survivors, additional studies should be conducted to confirm the effects of such programs.
Availability of data and materials
Not applicable.
Abbreviations
High-intensity interval training
Preferred reporting items for systematic reviews and meta-analyses
International prospective register of systematic reviews
Maximal oxygen uptake
Peak oxygen uptake
Rating of perceived exertion
Health-related quality of life
Resistance training
Aerobic training
One-repetition maximum
Brachial artery flow mediated dilation
Carotid intima-media thickness
european organization for research and treatment of cancer quality of life questionnaire-core 30
Piper fatigue scale
Cross-sectional area
Interleukin
Paolucci T, Bernetti A, Paoloni M, Capobianco SV, Bai AV, Lai C, et al. Therapeutic Alliance in a single versus group rehabilitative setting after breast Cancer surgery: psychological profile and performance rehabilitation. Biores Open Access. 2019;8(1):101–10. https://doi.org/10.1089/biores.2019.0011 .
Article PubMed PubMed Central Google Scholar
Mangone M, Bernetti A, Agostini F, Paoloni M, De Cicco FA, Capobianco SV, et al. Changes in spine alignment and postural balance after breast Cancer surgery: a rehabilitative point of view. Biores Open Access. 2019;8(1):121–8. https://doi.org/10.1089/biores.2018.0045 .
de Sire A, Losco L, Cisari C, Gennari A, Boldorini R, Fusco N, et al. Axillary web syndrome in women after breast cancer surgery referred to an Oncological Rehabilitation Unit: which are the main risk factors? A retrospective case-control study. Eur Rev Med Pharmacol Sci. 2020;24(15):8028–35. https://doi.org/10.26355/eurrev_202008_22486 .
Article PubMed Google Scholar
Michelotti A, Invernizzi M, Lopez G, Lorenzini D, Nesa F, De Sire A, et al. Tackling the diversity of breast cancer related lymphedema: perspectives on diagnosis, risk assessment, and clinical management. Breast. 2019;44:15–23. https://doi.org/10.1016/j.breast.2018.12.009 .
Paolucci T, Bernetti A, Bai AV, Segatori L, Monti M, Maggi G, et al. The sequelae of mastectomy and quadrantectomy with respect to the reaching movement in breast cancer survivors: evidence for an integrated rehabilitation protocol during oncological care. Support Care Cancer. 2020. https://doi.org/10.1007/s00520-020-05567-x .
Yang EJ, Kwon Y. Changes in shoulder muscle activity pattern on surface electromyography after breast cancer surgery. J Surg Oncol. 2018;117(2):116–23. https://doi.org/10.1002/jso.24800 .
Rizzi SK, Haddad CA, Giron PS, Pinheiro TL, Nazário AC, Facina G. Winged scapula incidence and upper limb morbidity after surgery for breast cancer with axillary dissection. Support Care Cancer. 2016;24(6):2707–15. https://doi.org/10.1007/s00520-016-3086-5 .
McTiernan A, Friedenreich CM, Katzmarzyk PT, Powell KE, Macko R, Buchner D, et al. Physical activity in Cancer prevention and survival: a systematic review. Med Sci Sports Exerc. 2019;51(6):1252–61. https://doi.org/10.1249/MSS.0000000000001937 .
Schmitz KH, Campbell AM, Stuiver MM, Pinto BM, Schwartz AL, Morris GS, et al. Exercise is medicine in oncology: engaging clinicians to help patients move through cancer. CA Cancer J Clin. 2019;69(6):468–84. https://doi.org/10.3322/caac.21579 .
Milanović Z, Sporiš G, Weston M. Effectiveness of High-Intensity Interval Training (HIT) and Continuous Endurance Training for VO2max Improvements: A Systematic Review and Meta-Analysis of Controlled Trials. Sports Med (Auckland, NZ). 2015;45(10):1469–81. https://doi.org/10.1007/s40279-015-0365-0 .
Article Google Scholar
Karlsen T, Aamot IL, Haykowsky M, Rognmo Ø. High intensity interval training for maximizing health outcomes. Prog Cardiovasc Dis. 2017;60(1):67–77. https://doi.org/10.1016/j.pcad.2017.03.006 .
Wallen MP, Hennessy D, Brown S, Evans L, Rawstorn JC, Wong Shee A, et al. High-intensity interval training improves cardiorespiratory fitness in cancer patients and survivors: A meta-analysis. Eur J Cancer Care. 2020:e13267. https://doi.org/10.1111/ecc.13267 .
Mugele H, Freitag N, Wilhelmi J, Yang Y, Cheng S, Bloch W, et al. High-intensity interval training in the therapy and aftercare of cancer patients: a systematic review with meta-analysis. J Cancer Surviv. 2019;13(2):205–23. https://doi.org/10.1007/s11764-019-00743-3 .
Dolan LB, Campbell K, Gelmon K, Neil-Sztramko S, Holmes D, McKenzie DC. Interval versus continuous aerobic exercise training in breast cancer survivors--a pilot RCT. Support Care Cancer. 2016;24(1):119–27. https://doi.org/10.1007/s00520-015-2749-y .
Peçanha T, Goessler KF, Roschel H, Gualano B. Social isolation during the COVID-19 pandemic can increase physical inactivity and the global burden of cardiovascular disease. Am J Physiol Heart Circ Physiol. 2020;318(6):H1441–h6. https://doi.org/10.1152/ajpheart.00268.2020 .
Article CAS PubMed PubMed Central Google Scholar
Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Ann Intern Med. 2009;151(4):264–9. https://doi.org/10.7326/0003-4819-151-4-200908180-00135 .
Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. https://doi.org/10.1136/bmj.d5928 .
Lee K, Kang I, Mack WJ, Mortimer J, Sattler F, Salem G, et al. Effect of high intensity interval training on matrix Metalloproteinases in women with breast Cancer receiving Anthracycline-based chemotherapy. Sci Rep. 2020;10(1):5839. https://doi.org/10.1038/s41598-020-61927-x .
Lee K, Kang I, Mack WJ, Mortimer J, Sattler F, Salem G, et al. Feasibility of high intensity interval training in patients with breast Cancer undergoing anthracycline chemotherapy: a randomized pilot trial. BMC Cancer. 2019;19(1):653. https://doi.org/10.1186/s12885-019-5887-7 .
Lee K, Kang I, Mack WJ, Mortimer J, Sattler F, Salem G, et al. Effects of high-intensity interval training on vascular endothelial function and vascular wall thickness in breast cancer patients receiving anthracycline-based chemotherapy: a randomized pilot study. Breast Cancer Res Treat. 2019;177(2):477–85. https://doi.org/10.1007/s10549-019-05332-7 .
Mijwel S, Bolam KA, Gerrevall J, Foukakis T, Wengström Y, Rundqvist H. Effects of exercise on chemotherapy completion and hospitalization rates: the OptiTrain breast Cancer trial. Oncologist. 2020;25(1):23–32. https://doi.org/10.1634/theoncologist.2019-0262 .
Article CAS PubMed Google Scholar
Mijwel S, Backman M, Bolam KA, Jervaeus A, Sundberg CJ, Margolin S, et al. Adding high-intensity interval training to conventional training modalities: optimizing health-related outcomes during chemotherapy for breast cancer: the OptiTrain randomized controlled trial. Breast Cancer Res Treat. 2018;168(1):79–93. https://doi.org/10.1007/s10549-017-4571-3 .
Mijwel S, Backman M, Bolam KA, Olofsson E, Norrbom J, Bergh J, et al. Highly favorable physiological responses to concurrent resistance and high-intensity interval training during chemotherapy: the OptiTrain breast cancer trial. Breast Cancer Res Treat. 2018;169(1):93–103. https://doi.org/10.1007/s10549-018-4663-8 .
Mijwel S, Cardinale DA, Norrbom J, Chapman M, Ivarsson N, Wengstrom Y, et al. Exercise training during chemotherapy preserves skeletal muscle fiber area, capillarization, and mitochondrial content in patients with breast cancer. FASEB J. 2018:fj201700968R. https://doi.org/10.1096/fj.201700968R .
Schulz SVW, Laszlo R, Otto S, Prokopchuk D, Schumann U, Ebner F, et al. Feasibility and effects of a combined adjuvant high-intensity interval/strength training in breast cancer patients: a single-center pilot study. Disabil Rehabil. 2018;40(13):1501–8. https://doi.org/10.1080/09638288.2017.1300688 .
Alizadeh AM, Isanejad A, Sadighi S, Mardani M, Kalaghchi B, Hassan ZM. High-intensity interval training can modulate the systemic inflammation and HSP70 in the breast cancer: a randomized control trial. J Cancer Res Clin Oncol. 2019;145(10):2583–93. https://doi.org/10.1007/s00432-019-02996-y .
Alizadeh S, Isanejad A, Sadighi S, Khalighfard S, Alizadeh AM. Effect of a high-intensity interval training on serum microRNA levels in women with breast cancer undergoing hormone therapy. A single-blind randomized trial. Ann Phys Rehabil Med. 2019;62(5):329–35. https://doi.org/10.1016/j.rehab.2019.07.001 .
Northey JM, Pumpa KL, Quinlan C, Ikin A, Toohey K, Smee DJ, et al. Cognition in breast cancer survivors: a pilot study of interval and continuous exercise. J Sci Med Sport. 2019;22(5):580–5. https://doi.org/10.1016/j.jsams.2018.11.026 .
Clifford BK, Mizrahi D, Sandler CX, Barry BK, Simar D, Wakefield CE, et al. Barriers and facilitators of exercise experienced by cancer survivors: a mixed methods systematic review. Support Care Cancer. 2018;26(3):685–700. https://doi.org/10.1007/s00520-017-3964-5 .
Turner RR, Steed L, Quirk H, Greasley RU, Saxton JM, Taylor SJ, et al. Interventions for promoting habitual exercise in people living with and beyond cancer. Cochrane Database Syst Rev. 2018;9:Cd010192. https://doi.org/10.1002/14651858.CD010192.pub3 .
Blackwell J, Atherton PJ, Smith K, Doleman B, Williams JP, Lund JN, et al. The efficacy of unsupervised home-based exercise regimens in comparison to supervised laboratory-based exercise training upon cardio-respiratory health facets. Physiol Rep. 2017;5(17). https://doi.org/10.14814/phy2.13390 .
Steen Krawcyk R, Vinther A, Petersen NC, Faber J, Iversen HK, Christensen T, et al. Effect of home-based high-intensity interval training in patients with lacunar stroke: a randomized controlled trial. Front Neurol. 2019;10:664. https://doi.org/10.3389/fneur.2019.00664 .
Fox EL, Bartels RL, Billings CE, O'Brien R, Bason R, Mathews DK. Frequency and duration of interval training programs and changes in aerobic power. J Appl Physiol. 1975;38(3):481–4. https://doi.org/10.1152/jappl.1975.38.3.481 .
Gauthier C, Brosseau R, Hicks AL, Gagnon DH. Feasibility, safety, and preliminary effectiveness of a home-based self-managed high-intensity interval training program offered to long-term manual wheelchair users. Rehabil Res Pract. 2018;2018:8209360. https://doi.org/10.1155/2018/8209360 .
Gist NH, Fedewa MV, Dishman RK, Cureton KJ. Sprint interval training effects on aerobic capacity: a systematic review and meta-analysis. Sports Med (Auckland, NZ). 2014;44(2):269–79. https://doi.org/10.1007/s40279-013-0115-0 .
Neil-Sztramko SE, Kirkham AA, Hung SH, Niksirat N, Nishikawa K, Campbell KL. Aerobic capacity and upper limb strength are reduced in women diagnosed with breast cancer: a systematic review. J Phys. 2014;60(4):189–200. https://doi.org/10.1016/j.jphys.2014.09.005 .
Miyamoto-Mikami E, Tsuji K, Horii N, Hasegawa N, Fujie S, Homma T, et al. Gene expression profile of muscle adaptation to high-intensity intermittent exercise training in young men. Sci Rep. 2018;8(1):16811. https://doi.org/10.1038/s41598-018-35115-x .
Chen JJ, Wu P-T, Middlekauff HR, Nguyen K-L. Aerobic exercise in anthracycline-induced cardiotoxicity: a systematic review of current evidence and future directions. Am J Physiol Heart Circ Physiol. 2017;312(2):H213–H22. https://doi.org/10.1152/ajpheart.00646.2016 .
Fraser S, Bigaran A, Selig S, LaGerche A. Exercise training during anthracycline-based chemotherapy for breast cancer. J Clin Oncol. 2017;35(15_suppl):e12110. https://doi.org/10.1200/JCO.2017.35.15_suppl.e12110 .
Menz V, Marterer N, Amin SB, Faulhaber M, Hansen AB, Lawley JS. Functional Vs. running low-volume high-intensity interval training: effects on VO(2)max and muscular endurance. J Sports Sci Med. 2019;18(3):497–504.
PubMed PubMed Central Google Scholar
Machado AF, Miranda MLJ, Rica RL, Figueira Junior A, Bocalini DS. Bodyweight high-intensity interval training: a systematic review. Rev Bras Med Esporte. 2018;24:234–7.
Tsuji K, Ochi E, Okubo R, Shimizu Y, Kuchiba A, Ueno T, et al. Effect of home-based high-intensity interval training and behavioural modification using information and communication technology on cardiorespiratory fitness and exercise habits among sedentary breast cancer survivors: habit-B study protocol for a randomised controlled trial. BMJ Open. 2019;9(8):e030911. https://doi.org/10.1136/bmjopen-2019-030911 .
Download references
Acknowledgements
This research was supported in-part by a Grant-in-Aid for Young Scientists (20 K18921) from the Japan Society for the Promotion of Science, and National Cancer Center Research and Development Fund (30-A-17).
Author information
Authors and affiliations.
Division of Health Care Research, Center for Public Health Sciences, National Cancer Center Japan, Tokyo, Japan
Katsunori Tsuji, Yutaka J. Matsuoka & Eisuke Ochi
Faculty of Bioscience and Applied Chemistry, Hosei University, Tokyo, Japan
Eisuke Ochi
You can also search for this author in PubMed Google Scholar
Contributions
KT contributed to data analysis, assembly of data, interpretation and drafting of the manuscript. YM contributed to data analysis, interpretation, and writing of the manuscript. EO contributed to conception and design, data analysis and interpretation, drafting and writing of the manuscript. All authors read and approved the final manuscript, and agree to be accountable for all aspects of the work.
Corresponding authors
Correspondence to Katsunori Tsuji or Eisuke Ochi .
Ethics declarations
Ethics approval and consent to participate.
This review article was a study that did not require ethical approval.
Consent for publication
Competing interests.
The authors declare that EO and KT have no conflicts of interest associated with this manuscript. YM has received speaker fees from Suntory, Pfizer, Mochida, Eli Lilly, and NTT Data, is conducting collaborative research with SUSMED, and has received a grant from SENSHIN Medical Research Foundation.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Tsuji, K., Matsuoka, Y.J. & Ochi, E. High-intensity interval training in breast cancer survivors: a systematic review. BMC Cancer 21 , 184 (2021). https://doi.org/10.1186/s12885-021-07804-w
Download citation
Received : 28 September 2020
Accepted : 11 January 2021
Published : 22 February 2021
DOI : https://doi.org/10.1186/s12885-021-07804-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Cancer survivor
- High intensity interval exercise
- Endurance performance
- Home-based exercise
ISSN: 1471-2407
- Submission enquiries: [email protected]
- General enquiries: [email protected]
SYSTEMATIC REVIEW article
Comparative efficacy and safety of targeted therapy and immunotherapy for her2-positive breast cancer: a systematic review and network meta-analyses.

- 1 Institute of Translational Medicine, Medical College, Yangzhou University, Yangzhou, China
- 2 Affiliated Hospital of Yangzhou University, Yangzhou University, Yangzhou, China
- 3 Department of Eighth Internal Medicine, Shenyang Traditional Chinese Medicine Hospital, Shenyang, China
Background: In recent years, novel therapies targeting specific molecular pathways and immunotherapies have exhibited promising outcomes for treating human epidermal growth factor receptor 2 (HER2)-positive breast cancer. Our work aimed to assess the effectiveness and safety of these emerging treatment regimens for this disease.
Material and methods: We systematically searched databases including PubMed, Embase, Web of Science, and the Cochrane Central Register of Controlled Trials their inception to August 2023 to identify relevant randomized controlled trials (RCTs). The quality of eligible RCTs was evaluated with the Cochrane risk-of-bias tool, version 2 (RoB2). Investigated outcomes encompassed progression-free survival (PFS), overall survival (OS), disease-free survival (DFS), pathologic complete remission (pCR), and adverse events (AEs). They were expressed as hazard ratio (HR) with 95% conference intervals (CI) or risk ratio (RR) with 95% CI.
Results: Our analysis identified a total of 28 RCTs suitable for inclusion in the NMA. Regarding the PFS, all these treatment regimens exhibited comparable effectiveness. In terms of OS, Capecitabine+Trastuzumab, Lapatinib+Trastuzumab and Pyrotinib+Capecitabine exhibited better effect compared to other treatments. Regarding pCR and AEs, all these treatment regimens exhibited comparable effectiveness, especially Lapatinib+Trastuzumab and Pyrotinib+Capecitabine.
Conclusion: Our study highlights the prominent role of targeted therapies and immunotherapies in treating HER2-positive breast cancer. The efficacy of trastuzumab-containing regimens was superior to other treatment options, while maintaining a comparable safety profile. Based on these findings, trastuzumab-containing regimens emerge as a preferable and recommended choice in clinical practice for managing HER2-positive breast cancer.
Systematic Review Registration: PROSPERO, identifier CRD42023414348.
1 Introduction
Breast cancer constitutes a significant threat to women’s health, ranking prominently among the leading causes of mortality in postmenopausal women. It accounts for a significant portion, up to 23%, of all cancer-related deaths, underscoring its profound global public health significance ( 1 ). Human epidermal growth factor receptor 2 (HER2), a member of the erythroblastic oncogene B (ErbB) family of receptor tyrosine kinases, underpins the development of breast cancer (BC) ( 2 ). Remarkably, around 25% of BC patients exhibit positive HER2 expression, signifying the clinical relevance of this molecular marker ( 3 ). Research by Slamon DJ, et al. has indicated that HER2 positivity correlated with heightened metastatic potential and a less favorable prognosis, further emphasizing the importance of HER2 as a prognostic factor ( 4 ). Consequently, the inhibition of HER2 has emerged as a promising avenue for therapeutic intervention, with the potential to curtail the growth of HER2-positive BC and offer new hope to affected individuals ( 2 ).
The preferred first-line therapy for HER2-positive BC currently is the combination of trastuzumab, pertuzumab, and paclitaxel (THP) ( 5 ). Recent decades have witnessed mounting evidence to support the significance of the immune system in regulating treatment response and survival outcomes among BC patients ( 6 ). The potential of immune checkpoint blockade therapy in managing BC has shown promise, highlighting its ability to leverage the immune system for clinical benefits. Consequently, targeted therapies and immunotherapy are anticipated to assume greater significance in standard treatment approaches, offering novel directions and renewed hope for the future management of breast cancer.
Network meta-analysis (NMA) presents a superior approach compared to traditional methods as it allows for simultaneous comparisons of multiple treatments while considering various potential sources of heterogeneity, potentially identifying the optimal treatment strategy more effectively. Randomized controlled trials (RCTs) have substantially aided in the integration of innovative therapeutic strategies into clinical practice over the past decade. This is especially true for the incorporation of new targeted therapies alongside immunotherapy for HER2-positive BC. As a result, leveraging RCT data serves as a robust foundation for supporting our current study.
Targeted therapies and immunotherapies have emerged as crucial components in the post-surgery, radiotherapy, and anti-tumor chemotherapy treatment landscape for malignancies ( 7 ). Given the ever-evolving interactions within immune signaling pathways and the immune microenvironment, there exists a wide array of immune-targeted drugs available. However, specific comparisons regarding their corresponding anti-tumor effects are often lacking. As a response to this gap, we performed this NMA to provide a comparison of the efficacy and safety of current immunotherapies and targeted therapies for HER2-positive BC, with the aim of providing valuable evidence to inform treatment decisions.
2 Materials and methods
This NMA was reported following the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) extension statement for systematic reviews incorporating NMAs ( 8 ). The prespecified protocol was registered on the International Prospective Register of Systematic Reviews (PROSPERO) with a registration number (CRD42023414348).
2.1 Literature search
Th literature search was performed in databases including PubMed, Embase, Web of Science, and the Cochrane Central Register of Controlled Trials. The search targeted RCTs related to targeted therapies and immunotherapies for HER2-positive BC, spanning from the inception of these databases to August 9, 2023. We imposed no restrictions regarding publication date or language.
2.2 Selection criteria
Studies should meet the following criteria for inclusion in this NMA: (1) study design: RCT; (2) Population: patients were diagnosed with HER2-positive BC; (3) Intervention: targeted therapies or immunotherapies (4) Outcomes: progression free survival (PFS), overall survival (OS), disease free survival (DFS), pathologic complete response (pCR), and adverse events.
2.3 Data extraction and quality assessment
Using a pilot-tested data extraction form, three investigators independently extracted data from included RCTs. The extracted information encompassed various aspects, such as the first author, publication year, sample size, treatment regimens, and outcomes (PFS, OS, DFS, and pCR), along with the number of adverse events. Any disparities were resolved through deliberation with the other three reviewers.
The Cochrane risk-of-bias tool was employed for assessing the quality of eligible RCTs ( 9 ), evaluating domains like randomization sequence generation; allocation concealment; blinding of participants and personnel, incomplete outcome data, selective outcome reporting, and other sources of bias. Each domain was assessed to rate each RCT as having a low, high, or unclear risk of bias.
2.4 Data analysis
We conducted the NMA considering both direct and indirect treatment comparisons to evaluate the effectiveness of the targeted treatment regimens. To gauge the extent of heterogeneity across included RCTs, we employed the I 2 statistic, with a value exceeding 50% indicating significant heterogeneity ( 10 ). The presence or absence of significant heterogeneity led to the application of two distinct models: a random-effects model ( 11 ) was selected in cases of substantial heterogeneity, while a fixed-effects model ( 12 ) was employed when heterogeneity was not pronounced. Our pooled estimates for PFS and DFS are presented alongside the corresponding hazard ratios (HRs) and corresponding 95% confidence intervals (CI). Dichotomous data, such as pCR and adverse events (AEs), were represented by risk ratios (RRs) and corresponding 95% CIs. Statistically significant findings were identified when both the upper and lower limits of the 95% CI were either greater than or less than one, equivalent to p-values < 0.05, the conventional threshold for statistical significance. We derived P scores as the frequentist equivalent of the Surface under the Cumulative Ranking Curve (SUCRA) ( 13 ). Data analyses was performed using the R package “netmeta” (version 1.2.0, R Foundation) ( 14 ), enabling us to conduct this extensive analysis of treatment effectiveness and rankings.
3.1 Study identification and characteristics
The initial search identified 7,261 studies, from which 1,855 duplicate records were excluded. Subsequently, the titles and abstracts of 5,406 papers were scrutinized, and 4,691 were excluded for various reasons, including case series (n=204), conference abstracts (n=1,515), reviews (n=795), protocols (n=24), and animal studies (n=2,153). Following this, 715 publications underwent a detailed eligibility assessment through full-text reading. However, 397 of them were excluded for various reasons, leaving 318 articles for further consideration. Among these, 290 were excluded for not meeting inclusion criteria. Finally, 28 RCTs were included in the NMA ( Figure 1 ).
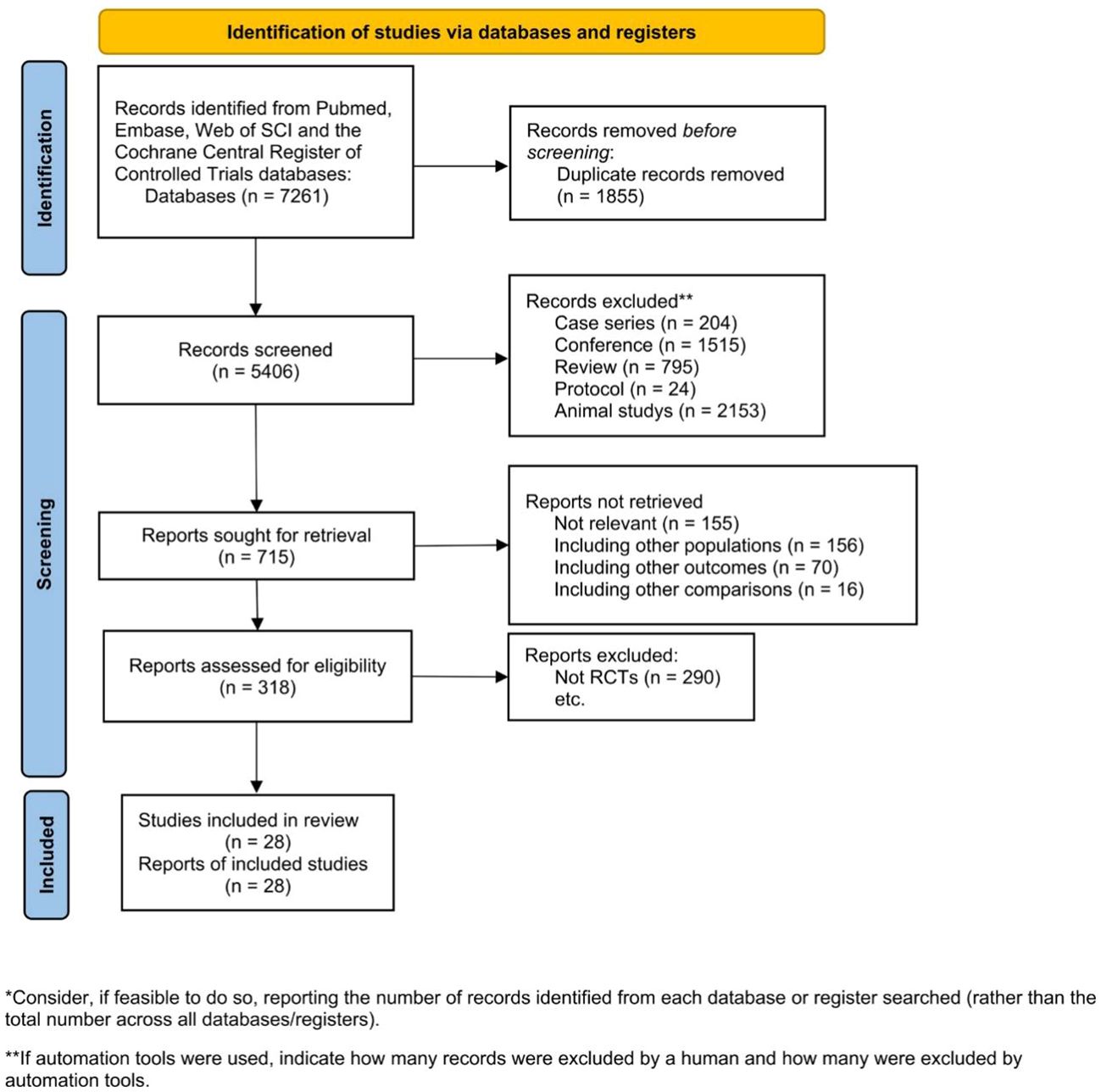
Figure 1 Eligibility of studies for inclusion in meta-analysis.
Study characteristics are outlined in Supplementary Table S1 . These RCTs spanned multiple regions, with 14 studies conducted in North America, 6 in China, and several encompassing a global scope. A comprehensive analysis involved 27,464 patients diagnosed with HER2-positive BC, facilitating assessments of OS and pCR. Targeted treatments and immunotherapy regimens were comprehensively compared across all included RCTs, incorporating lapatinib, afatinib, trastuzumab, pertuzumab, neratinib, atezolizumab, lapatinib, abemaciclib, T-DM1and margetuximab. These RCTs were published between 2013 and 2023, providing data on OS and pCR. Patient demographics revealed an age range of 19 to 88 years across all studies, while a median duration of follow-up ranging from 3 to 64 months.
3.2 Risk of bias
Figure 2 depicts the ROB assessment results for RCTs. Among the reviewed studies, 15 were rated as having a low ROB, while 13 were determined to have a high ROB, primarily due to at least one of the five ROB domains being assessed as a ‘high’. Regarding the randomization process, 20 studies reported information on the randomization sequence or concealment, resulting in a low ROB rating. Four studies were scored as having a high ROB in the ‘Deviations from Intended Interventions’ domain. Among these, two studies reported that both participants and implementers were aware of the assigned condition, disclosed deviations from the intended intervention, and conducted appropriate analyses, such as intention-to-treat, to estimate the effect of assignment to conditions. The assessment of incomplete outcome illustrated that all but one study was rated as having a low ROB. The exceptional study failed to provide a reason for the missing data, so it is difficult to determine whether the missing data correlate with the true value in all cases, or whether they might correlate with the true value of the results. All studies were rated as having a low ROB for the assessment of selective reporting.
Figure 2 Risk of bias summary.
3.3 Networks meta-analysis for outcomes
3.3.1 primary endpoints: overall survival.
Nineteen studies involving 21,243 patients and 21 interventional arms analyzed OS. The top three of SUCRA ranking for overall survival were Capecitabine+Trastuzumab (0.924), Lapatinib + Trastuzumab (0.901) and Pyrotinib + Capecitabine (0.900). The overall survival of Capecitabine+Trastuzumab was superior to the rest therapies. In terms of improvement for overall survival(OS), Capecitabine+Trastuzumab was significantly superior to Lapatinib+CT(HR=2.76 (95CI: 1.02 - 7.4)), Margetuximab+CT(HR=3.21 (95CI: 1.33 - 7.84)), Trastuzumab+ET (HR=3.49 (95CI: 1.45 - 8.46)), tdm1(HR=2.29 (95CI: 1.01 - 5.2)), tdm1+Pertuzumab(HR=2.46 (95CI: 1.02 - 6.01)), T-DXd(HR=4.17 (95CI: 1.65 - 10.59)), tpc(HR=2.98 (95CI: 1.28 - 6.95)). Lapatinib + Trastuzumab was significantly superior to Trastuzumab+ET(HR=2.03 (95CI: 1.4 - 2.93)), T-DXd(HR=2.42 (95CI: 1.53 - 3.85)). Pyrotinib + Capecitabine was significantly superior to Trastuzumab+CT(HR=2.41 (95CI: 1.3 - 4.45)), Trastuzumab+ET(HR=2.94 (95CI:1.53 - 5.68)), tdm1+Pertuzumab(HR=2.07 (95CI: 1.07 - 4.02)), T-DXd(HR=3.51 (95CI: 1.72 - 7.18)), tpc(HR=2.51 (95CI: 1.36 - 4.61)). Afatinib (SUCRA=0.143) and Trastuzumab deruxtecan(SUCRA=0.075) indicate poor performance in the results of overall survival. Based on their performance in the league tables, it can also be concluded that those two therapies are significantly less effective than other therapies. When those two drugs are used as necessary, it is important to exercise caution when determining the appropriate dosage ( Figure 3 , Supplementary Table S2 ).
Figure 3 Plots of overall survival (OS). (A) Network plot. (B) Forest plot. (C) Ranking plot.
3.3.2 Secondary endpoints: pathologic complete response
Two studies with a total of 1105 patients, containing 5 interventional arms that analyzed the pCR. The top one of SUCRA ranking for pathologic complete response (pCR) was Trastuzumab + chemical therapy. Trastuzumab+CT was significantly superior to Pertuzumab+Trastuzumab(HR=2.03 (95CI: 1.06 - 3.99)). However, Trastuzumab + chemical therapy didn’t have satisfactory performance in the results of overall survival, so it might not be a preferred choice ( Figure 4 , Supplementary Table S3 ).
Figure 4 Plots of pathologic complete response (pCR). (A) Network plot. (B) Ranking plot. (C) Forest plot.
3.3.3 Adverse events
There was a total of 27 RCTs with 25000 patients analyzing the endpoint of AEs. According to the SUCRA ranking, the safety of Pyrotinib + Capecitabine (0.144) and Atezolizumab+Trastuzumab emtansine (0.128) were relatively high. Pyrotinib + Capecitabine was significantly superior to Capecitabine + Trastuzumab (HR=6.91 (95CI: 2.14 - 23.92)). Atezolizumab+Trastuzumab emtansine was significantly superior to Capecitabine + Trastuzumab(HR=6.93 (95CI: 2.13 - 24.41)), Trastuzumab(HR= 3.57 (95CI: 1.71 - 7.68)), Trastuzumab+ET(95CI: 13.74 (5.19 - 38.56)). If patients are particularly concerned about adverse reactions, these drugs especially Pyrotinib+Capecitabine can be given priority. Besides, the safety of Lapatinib + Trastuzumab was also higher than placebo, in view of its excellent overall survival, it can also be a good choice. Although Capecitabine + Trastuzumab ranked high in the SUCRA ranking of overall survival, it didn’t perform well in the ranking of adverse events, which has a higher risk than placebo ( Figure 5 , Supplementary Table S4 ).
Figure 5 Plots of Adverse events (AEs). (A) Forest plot. (B) Ranking plot. (C) Network plot.
4 Discussion
4.1 major findings.
This NMA has comprehensively evaluated the effectiveness and safety of targeted therapies and immunotherapies available for the treatment of HER2-positive BC. The analysis result indicated that, concurrent use of targeted therapies and immunotherapies has demonstrated noteworthy enhancements in OS for individuals diagnosed with HER2-positive BC. For OS, Capecitabine + Trastuzumab, Lapatinib + Trastuzumab and Pyrotinib+Capecitabine exhibited better effect other treatments. For pCR and adverse event, all these treatments had no significant difference with each other.
4.2 Comparison with other reviews
In this meta-analysis, our findings underscore the significant superiority of trastuzumab plus capecitabine in OS when compared to various alternative treatments, including lapatinib + chemotherapy, margetuximab + chemotherapy, pertuzumab + trastuzumab, trastuzumab + chemotherapy, TDM1 + placebo, trastuzumab deruxtecan, and trastuzumab + endocrine therapy. These results underscore the superior survival benefits of trastuzumab for HER2-positive BC, aligning with prior studies ( 15 – 17 ), including the analysis by O’Sullivan CC et al. of five RCTs comparing the effectiveness of trastuzumab among individuals with small (≤ 2 cm) HER2-positive BC ( 15 ). Their findings, focusing on hormone receptor (HR)-positive patients with a median follow-up of 8 years, demonstrated considerable benefits in OS and DFS for trastuzumab-treated patients. The study highlighted that cumulative incidence rates favored trastuzumab over no trastuzumab, emphasizing its efficacy for DFS (17.3% versus 24.3%, P < 0.001) and OS (7.8% versus 11.6%, P = 0.005). Similar advantages were observed in patients with HR-negative disease ( 15 ). Furthermore, a meta-analysis published in 2023 highlighted the advantageous effects of combining trastuzumab with Tyrosine Kinase Inhibitors (TKIs) in HER2-positive BC ( 16 ). Systematically searching databases up to September 2022 and including sixteen RCTs, the authors reported that trastuzumab plus TKI was associated with significantly elevated OS (HR=0.77, 95%CI: 0.67-0.88, P<0.001), PFS (HR=0.52, 95%CI: 0.41-0.66, P<0.001), pCR (OR=1.90, 95%CI: 1.50-2.41, P=0.001), and ORR (OR=2.17, 95%CI: 1.34-3.50, P=0.002) compared to trastuzumab monotherapy ( 16 ). These results signify the enhanced efficacy of trastuzumab plus TKI across different stages of HER2-positive BC.
In another NMA focused on investigating the optimal neoadjuvant regimen for HER2-positive BC, researchers discovered that dual anti-HER2 therapy, involving pertuzumab or TKIs, combined with chemotherapy, exhibited significant superiority over trastuzumab and chemotherapy regarding pCR, OS, and Event-Free Survival (EFS) ( 18 ). This comprehensive study encompassed 46 RCTs with 11,049 patients diagnosed with HER2-positive BC, evaluating 32 different treatment modalities. The analysis of EFS (n=4919) demonstrated that dual HER2 blockade, either with the combination of pertuzumab + trastuzumab + chemotherapy or TKI + trastuzumab + chemotherapy, demonstrated improved EFS than chemotherapy + trastuzumab (HR=0.61, 95% CI: 0.43–0.85) and T-DM1 (HR=0.66, 95% CI: 0.34-1.26). Furthermore, the combination of dual anti-HER2 blockade and chemotherapy exhibited benefits in pCR, EFS, and OS compared to a single anti-HER2 agent combined with chemotherapy. Dual anti-HER2 combined with chemotherapy was superior to T-DM1 regarding EFS and pCR. Anti-HER2 + chemotherapy outperformed dual anti-HER2 alone regarding pCR and EFS, with a noticeable trend towards better OS. They concluded that dual HER2 blockade plus chemotherapy stands out as the preferred option for neoadjuvant therapy in HER2-positive BC. On one hand, our findings provide more evidence for previous studies. Those findings are in support of previous studies and are supported by newer evidence, adding to their credibility and relevance. On the other hand, the findings indicate that certain research methods require more attention, such as Lapatinib + Trastuzumab and Pyrotinib + Capecitabine. Regarding the safety profile of the various treatment regimens, this study found no significant differences among them. Notably, concerning cardiac safety, there was no increase in the incidence of cardiotoxicity or toxic deaths with trastuzumab-based regimens. It is essential to highlight that the ALTTO study reported more discontinuations due to increased toxicity linked to lapatinib plus trastuzumab compared to trastuzumab monotherapy ( 19 ). Specifically, compared to trastuzumab, therapy containing lapatinib exhibited a higher rate of grade ≥ 3 diarrhea (1% vs. 15%), predominantly contributing to treatment discontinuation. In contrast, Murthy et al. ( 20 ) found no significant differences in withdrawal rates between patients administered by tucatinib or trastuzumab plus capecitabine. This suggests that safety considerations vary across different treatment combinations, emphasizing the importance of carefully assessing safety profiles when determining treatment strategies.
4.3 Advantages and limitations
There are some limitations to our work. Firstly, the quality of the included literature requires improvement, as a majority of studies did not report the random allocation method. Additionally, a substantial number of studies were open-label, potentially introducing an increased risk of bias in the research outcomes. Secondly, the heterogeneity in outcome indicators across studies limits the comprehensiveness of drug effectiveness comparisons. Lastly, the relatively small number of included RCTs and the constraints in sample size may potentially exaggerate the efficacy of carious treatments. These limitations underscore the need for caution in interpreting the study findings and emphasize the importance of further research with improved methodological rigor and larger sample sizes to enhance the robustness of the conclusions drawn.
5 Conclusion
Our results demonstrated the prominent role of targeted therapies and immunotherapies for HER-2 breast cancer. Trastuzumab-based regimens demonstrated superior efficacy compared to other treatment options, while maintaining a comparable safety profile. Based on these findings, trastuzumab-containing regimens emerge as a preferable and recommended choice in clinical practice for managing HER2-positive breast cancer.
Data availability statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding authors.
Author contributions
SG: Conceptualization, Data curation, Writing – original draft. YL: Formal Analysis, Methodology, Software, Writing – original draft. YH: Formal Analysis, Investigation, Methodology, Writing – original draft. WL: Writing – review & editing, Conceptualization, Data curation, Validation, Visualization. KL: Visualization, Writing – review & editing, Project administration, Software.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
Thanks to all the authors who contributed to this work.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1331055/full#supplementary-material
1. Akram M, Iqbal M, Daniyal M, Khan AU. Awareness and current knowledge of breast cancer. Biol Res . (2017) 50:33. doi: 10.1186/s40659-017-0140-9
PubMed Abstract | CrossRef Full Text | Google Scholar
2. Cordo Russo RI, Chervo MF, Madera S, Charreau EH, Elizalde PV. Nuclear erbB-2: A novel therapeutic target in erbB-2-positive breast cancer? Hormones Cancer . (2019) 10:64–70. doi: 10.1007/s12672-018-0356-3
3. Asif HM, Sultana S, Ahmed S, Akhtar N, Tariq M. HER-2 positive breast cancer - a mini-review. Asian Pacific J Cancer Prev APJCP . (2016) 17:1609–15. doi: 10.7314/APJCP.2016.17.4.1609
CrossRef Full Text | Google Scholar
4. Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science . (1989) 244:707–12. doi: 10.1126/science.2470152
5. Nader-Marta G, Martins-Branco D, de Azambuja E. How we treat patients with metastatic HER2-positive breast cancer. ESMO Open . (2022) 7:100343. doi: 10.1016/j.esmoop.2021.100343
6. Emens LA. Breast cancer immunotherapy: Facts and hopes. Clin Cancer Res an Off J Am Assoc Cancer Res . (2018) 24:511–20. doi: 10.1158/1078-0432.CCR-16-3001
7. Goss PE, Smith IE, O'Shaughnessy J, Ejlertsen B, Kaufmann M, Boyle F, et al. Adjuvant lapatinib for women with early-stage HER2-positive breast cancer: a randomized, controlled, phase 3 trial. Lancet Oncol . (2013) 14:88–96. doi: 10.1016/S1470-2045(12)70508-9
8. Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Internal Med . (2015) 162:777–84. doi: 10.7326/M14-2385
9. Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomized trials. BMJ (Clinical Res ed) . (2019) 366:l4898. doi: 10.1136/bmj.l4898
10. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (Clinical Res ed) . (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
11. DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clin trials . (1986) 7:177–88. doi: 10.1016/0197-2456(86)90046-2
12. Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Institute . (1959) 22:719–48.
Google Scholar
13. Rücker G, Schwarzer G. Ranking treatments in frequentist network meta-analysis works without resampling methods. BMC Med Res Method . (2015) 15:58. doi: 10.1186/s12874-015-0060-8
14. Chaimani A, Salanti G. Using network meta-analysis to evaluate the existence of small-study effects in a network of interventions. Res synthesis Methods . (2012) 3:161–76. doi: 10.1002/jrsm.57
15. O'Sullivan CC, Bradbury I, Campbell C, Spielmann M, Perez EA, Joensuu H, et al. Efficacy of adjuvant trastuzumab for patients with human epidermal growth factor receptor 2-positive early breast cancer and tumors ≤ 2 cm: A meta-analysis of the randomized trastuzumab trials. J Clin Oncol Off J Am Soc Clin Oncol . (2015) 33:2600–8. doi: 10.1200/JCO.2015.60.8620
16. Li L, Zhang D, Wu Y, Wang J, Ma F. Efficacy and safety of trastuzumab with or without a tyrosine kinase inhibitor for HER2-positive breast cancer: A systematic review and meta-analysis. Biochim Biophys Acta Rev Cancer . (2023) 1878:188969. doi: 10.1016/j.bbcan.2023.188969
17. Wang Y, Xu H, Han Y, Wu Y, Sa Q, Wang J. Identifying the optimal therapeutics for patients with hormone receptor-positive, HER2-positive advanced breast cancer: a systematic review and network meta-analysis. ESMO Open . (2023) 8:101216. doi: 10.1016/j.esmoop.2023.101216
18. Villacampa G, Matikas A, Oliveira M, Prat A, Pascual T, Papakonstantinou A. Landscape of neoadjuvant therapy in HER2-positive breast cancer: a systematic review and network meta-analysis. Eur J Cancer (Oxford Engl 1990) . (2023) 190:112885. doi: 10.1016/j.ejca.2023.03.042
19. Mimura K, Kono K, Maruyama T, Watanabe M, Izawa S, Shiba S, et al. Lapatinib inhibits receptor phosphorylation and cell growth and enhances antibody-dependent cellular cytotoxicity of EGFR- and HER2-overexpressing esophageal cancer cell lines. Int J Cancer . (2011) 129:2408–16. doi: 10.1002/ijc.25896
20. Murthy RK, Loi S, Okines A, Paplomata E, Hamilton E, Hurvitz SA, et al. Tucatinib, trastuzumab, and capecitabine for HER2-positive metastatic breast cancer. New Engl J Med . (2020) 382:597–609. doi: 10.1056/NEJMoa1914609
Keywords: HER2-positive, breast cancer, immunotherapies, efficacy, safety, meta-analysis
Citation: Gu S, Liu Y, Huang Y, Lin W and Li K (2024) Comparative efficacy and safety of targeted therapy and immunotherapy for HER2-positive breast cancer: a systematic review and network meta-analyses. Front. Oncol. 14:1331055. doi: 10.3389/fonc.2024.1331055
Received: 04 December 2023; Accepted: 20 March 2024; Published: 03 April 2024.
Reviewed by:
Copyright © 2024 Gu, Liu, Huang, Lin and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenzheng Lin, [email protected] ; Ke Li, [email protected]
† These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
- Introduction
- Conclusions
- Article Information
eFigure. CONSORT Diagram
eTable 1. Characteristics of Study Participants
eTable 2. Sensitivity Analysis of Association of Race/Ethnicity With Odds of High-Risk Recurrence Score for Patients Diagnosed in 2010-2015
eTable 3. Mediational E Value
Data Sharing Statement
See More About
Sign up for emails based on your interests, select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
Get the latest research based on your areas of interest.
Others also liked.
- Download PDF
- X Facebook More LinkedIn
Parab AZ , Kong A , Lee TA, et al. Socioecologic Factors and Racial Differences in Breast Cancer Multigene Prognostic Scores in US Women. JAMA Netw Open. 2024;7(4):e244862. doi:10.1001/jamanetworkopen.2024.4862
Manage citations:
© 2024
- Permissions
Socioecologic Factors and Racial Differences in Breast Cancer Multigene Prognostic Scores in US Women
- 1 Department of Pharmacy Systems, Outcomes and Policy, University of Illinois, Chicago
- 2 Department of Pharmacy Practice, University of Illinois, Chicago
- 3 Center for Pharmacoepidemiology & Pharmacoeconomic Research, University of Illinois, Chicago
- 4 School of Public Health, University of Illinois, Chicago
- 5 University of Illinois Cancer Center, Chicago
- 6 Division of Hematology and Oncology, University of Illinois College of Medicine, Chicago
- 7 Titus Family Department of Clinical Pharmacy, University of Southern California, Los Angeles
Question To what extent are socioeconomic and residential factors associated with higher prevalence of estrogen receptor–positive breast tumors with high-risk recurrence scores (RSs) on the 21-gene assay among minoritized racial and ethnic groups?
Findings In this cohort study of 69 139 women with breast cancer, non-Hispanic Black and non-Hispanic American Indian and Alaska Native women were more likely to have tumors with high-risk RSs compared with non-Hispanic White women. For non-Hispanic Black women, area-level socioeconomic position, urban residence, and insurance status mediated 17% of the racial difference in the RSs, and racial differences between non-Hispanic Black and non-Hispanic White women in the RSs were observed only among urban women.
Meaning These findings suggest that disproportionately aggressive breast tumor biology among non-Hispanic Black women may be partially explained by socioecologic factors.
Importance Disproportionately aggressive tumor biology among non-Hispanic Black women with early-stage, estrogen receptor (ER)–positive breast cancer contributes to racial disparities in breast cancer mortality. It is unclear whether socioecologic factors underlie racial differences in breast tumor biology.
Objective To examine individual-level (insurance status) and contextual (area-level socioeconomic position and rural or urban residence) factors as possible mediators of racial and ethnic differences in the prevalence of ER-positive breast tumors with aggressive biology, as indicated by a high-risk gene expression profile.
Design, Setting, and Participants This retrospective cohort study included women 18 years or older diagnosed with stage I to II, ER-positive breast cancer between January 1, 2007, and December 31, 2015. All data analyses were conducted between December 2022 and April 2023.
Main Outcomes and Measures The primary outcome was the likelihood of a high-risk recurrence score (RS) (≥26) on the Oncotype DX 21-gene breast tumor prognostic genomic biomarker.
Results Among 69 139 women (mean [SD] age, 57.7 [10.5] years; 6310 Hispanic [9.1%], 274 non-Hispanic American Indian and Alaskan Native [0.4%], 6017 non-Hispanic Asian and Pacific Islander [8.7%], 5380 non-Hispanic Black [7.8%], and 51 158 non-Hispanic White [74.0%]) included in our analysis, non-Hispanic Black (odds ratio [OR], 1.33; 95% CI, 1.23-1.43) and non-Hispanic American Indian and Alaska Native women (OR, 1.38; 95% CI, 1.01-1.86) had greater likelihood of a high-risk RS compared with non-Hispanic White women. There were no significant differences among other racial and ethnic groups. Compared with non-Hispanic White patients, there were greater odds of a high-risk RS for non-Hispanic Black women residing in urban areas (OR, 1.35; 95% CI, 1.24-1.46), but not among rural residents (OR, 1.05; 95% CI, 0.77-1.41). Mediation analysis demonstrated that lack of insurance, county-level disadvantage, and urban vs rural residence partially explained the greater odds of a high-risk RS among non-Hispanic Black women (proportion mediated, 17%; P < .001).
Conclusions and Relevance The findings of this cohort study suggest that the consequences of structural racism extend beyond inequities in health care to drive disparities in breast cancer outcome. Additional research is needed with more comprehensive social and environmental measures to better understand the influence of social determinants on aggressive ER-positive tumor biology among racial and ethnic minoritized women from disadvantaged and historically marginalized communities.
Poor breast cancer outcomes disproportionately impact racial and ethnic minoritized groups despite concerted efforts to mitigate cancer health disparities. 1 - 3 Non-Hispanic Black, Hispanic, and non-Hispanic American Indian and Alaskan Native women with early-stage breast cancer have a significantly higher likelihood of disease recurrence and death 4 compared with non-Hispanic White women. Early-stage tumors that are estrogen receptor (ER) positive and ERBB2 (formerly HER2/neu ) (OMIM 164870 ) negative comprise 70% of breast cancer diagnoses, and a survival disparity according to race and ethnicity among patients with this disease subtype is well documented. 5
The Oncotype DX 21-gene breast recurrence score (RS) is the most commonly ordered multigene expression biomarker used to estimate the risk of distant metastatic recurrence in women with early-stage, ER-positive/ ERBB2 -negative breast cancer and to identify patients who may derive benefit from adjuvant chemotherapy. 6 , 7 Non-Hispanic Black women have a higher prevalence of tumors with a high-risk RS compared with non-Hispanic White women, indicating that biologically aggressive ER-positive tumors disproportionately afflict non-Hispanic Black women. 1 , 8 - 10 A recent study found that non-Hispanic Black women in the US are 30% more likely to have a high-risk RS (≥26) on the 21-gene assay compared with non-Hispanic White women. 10 Notably, the increased prevalence of biologically aggressive tumors among non-Hispanic Black women mediated 20% of the racial disparity in survival after a diagnosis of early-stage, ER-positive breast cancer in the US. 11
Racial disparity in breast cancer mortality is attributed to socially determined factors, such as greater exposure to social and environmental risk factors and inequitable access to health care. Although studies have found that neighborhood disadvantage, inadequate insurance coverage, and urban residence are associated with worse breast cancer outcomes, 12 - 15 whether these social and environmental factors influence tumor biology among non-Hispanic Black women is not known. Mediation analysis can quantify the relative contributions of factors on the causal pathway that mediate racial difference in the prevalence of high-risk RS and thereby provide insight into the complex and poorly understood interactions between socioecologic context and tumor biology. Previous mediation analyses have focused primarily on the mediating effects of socioeconomic and insurance status on racial disparities in breast cancer survival and prevalence of late-stage diagnoses. 10 , 16 - 22 Our aim is to provide an in-depth analysis of the complex association between the social and physical environment and breast tumor biology in racial minoritized women. The objective of this study was to examine the mediating influence of area-level socioeconomic position (SEP), insurance status, and urban residence on racial and ethnic differences in the prevalence of a high-risk 21-gene RS in a diverse cohort that is representative of the US population. Race is conceptualized as a social construct for this study.
This population-based, retrospective, observational cohort study used the Surveillance, Epidemiology, and End Results (SEER) Program Oncotype DX database 23 to obtain data on the 21-gene RS provided by the Genomic Health Clinical Laboratory through a linkage with invasive breast cancer cases in the SEER registry. We identified women 18 years or older diagnosed with first primary ER-positive/ ERBB2 -negative breast cancer between January 1, 2007, and December 31, 2015, that was stage I to II according to the American Joint Committee on Cancer’s (AJCC’s) Cancer Staging Manual , 6th edition, and was negative for axillary lymph node metastases. Information on ERBB2 status was only available for cases diagnosed in 2010 and later. Women diagnosed from 2010 to 2017 were excluded from the analysis if their tumors were ERBB2 positive or of borderline status. Our analytical cohort was a complete case analysis. Women whose race or ethnicity was unknown, those who did not receive RS testing, and those who were diagnosed with breast cancer at autopsy or by death certificate only were also excluded. This cohort study was approved by the institutional review board at the University of Illinois at Chicago, which granted a waiver of consent because the study did not involve human participants research, and all data were deidentified. This report follows the Strengthening the Reporting of Observational Studies in Epidemiology ( STROBE ) reporting guideline.
The primary outcome was the RS, which is measured as a continuous variable on a 0- to 100-point scale, with 0 indicating the lowest risk and 100 indicating the highest risk of distant recurrence. Study participants were categorized as having low- or intermediate-risk RSs (range, 0-25) or high-risk RSs (range, 26-100) based on the pivotal validation trial of the RS 24 and practice guidelines during the study period. 6 Race and ethnicity were categorized according to SEER recoding of race as non-Hispanic White, non-Hispanic Black, American Indian or Alaska Native, non-Hispanic Asian or Pacific Islander, and Hispanic. The choice of mediator variables and demographic factors adjusted as confounders was informed by the Institute of Medicine’s definition of health care disparities, treating social determinants of health as mediating measures of racial and ethnic inequities. Mediator variables included county-level SEP, insurance status, and rural vs urban residence. The SEP is a composite index derived from principal component analysis of 4 county-level variables extracted from SEER, including median household income (continuous), percentage of households below 150% of the federal poverty line (continuous), percentage of women with less than a high school education (continuous), and percentage of women unemployed (continuous). 11 The initial component, which captured 74% of the variance across these variables, was selected as the county-level SEP index. The calculated county-level SEP index was categorized into SEER breast cancer population–weighted quintiles (with 1 indicating advantage and 5 indicating disadvantage). Health insurance status was categorized as insured and uninsured or Medicaid. To categorize patients as either urban or rural, we used the 2013 Rural-Urban Continuum Codes. These codes categorize metropolitan counties based on population size and nonmetropolitan counties based on their level of urbanization and proximity to a metropolitan area. Following established rural-urban criteria, we considered patients in counties with codes 1 to 3 as urban, whereas those in counties with codes 4 to 9 were classified as rural. 25 Demographic and clinical variables were collected from SEER registry data. Individual-level demographic information included age at breast cancer diagnosis, insurance status, and marital status. Clinical information included tumor grade categorized as I, II, III, IV, or unknown; AJCC stage categorized as I or II; progesterone receptor (PR) status characterized as borderline, negative, positive, or unknown; and receipt of radiation therapy and chemotherapy (yes vs no or unknown).
Descriptive analysis of the study population was performed with the 2-tailed t test or Pearson χ 2 test for continuous and categorical variables, respectively. Nonparametric tests were used for variables with nonnormal distribution. Our approach to conducting mediation analyses followed the product method approach proposed by Baron and Kenny 26 and later extended for multiple mediators by VanderWeele and Vansteelandt. 27 There are 4 main assumptions of mediation analyses, including no unmeasured confounding in exposure and outcome association, mediator-outcome association, exposure-mediator association, and no mediator outcome confounder affected by the exposure. This approach avoids potential bias of considering each mediator separately, especially when they are interrelated, and accounts for their collective effect more efficiently. This method estimates the presence of mediation, direct effects, and indirect effects through a series of regression analyses. First, we conducted univariate and multivariable analyses regressing the outcome (high-risk RS) on the exposure (race and ethnicity) and a priori measured confounders that included age and marital status. Second, we conducted univariate and multivariate analyses separately, regressing the mediator of interest (ie, area-level socioeconomic status, insurance status, and rural vs urban residence) on race and ethnicity and a priori measured confounders. Using these estimates, we calculated the natural direct effects (NDE), natural indirect effects (NIE), and total effects (TE) as follows:
PM = ( NDE × [ NIE − 1]) / ( NDE × NIE − 1).
The direct effect describes the exposure-outcome association that does not include the mediator (θ), and the indirect effect describes the part of the exposure-outcome association that incorporates the mediator (1 − θ).
To assess how much the mediator influences the association between exposure and outcome, we performed a proportion-mediated analysis using the Mediation package in R, version 2022.12.0 + 353 (R Foundation for Statistical Computing). The formula provided represents proportion mediated, which quantifies the proportion of the total effect that can be attributed to the mediating variable. We used the calculated coefficients from the logistic regression model to characterize the outcome variables and the mediator variables. Additionally, we tested for statistical significance of exposure-mediator interaction terms and conducted stratified analyses by our socioecologic factors of interest to evaluate possible effect modification. We calculated the e-value for logistic regression of the exposure-outcome association as part of the sensitivity analysis to measure the minimum strength of association that an unmeasured confounder would need to have with both the exposure and the outcome to fully explain away the observed association. Another sensitivity analysis included only patients with confirmed ERBB2 -negative tumors who were diagnosed between January 1, 2010, and December 31, 2015. Data analysis was conducted between December 2022 and April 2023. All analyses were conducted using Stata, release 18 and R programming languages (StataCorp LLC). All P values were calculated from 2-sided tests, and results were deemed statistically significant at P < .05.
This study included a total of 69 139 women (mean [SD] age, 57.7 [10.5] years; 6310 Hispanic [9.1%], 274 non-Hispanic American Indian or Alaskan Native [0.4%], 6017 non-Hispanic Asian and Pacific Islander [8.7%], 5380 non-Hispanic Black [7.8%], and 51 158 non-Hispanic White [74.0%]) diagnosed with AJCC stage I to II, axillary node–negative, ER-positive breast cancer (eFigure in Supplement 1 ). Characteristics of study participants by racial and ethnic category are described in eTable 1 in Supplement 1 . More high-grade (grades III- IV) tumors were observed among non-Hispanic Black women (54 [19.4%]) and non-Hispanic American Indian and Alaskan Native women (1245 [19.7%]). Non-Hispanic Black women (1116 [20.7%]), non-Hispanic American Indian and Alaskan Native women (59 [21.5%]), and Hispanic women (1245 [19.7%]) were more likely to live in neighborhoods classified in the lowest SEP quintile compared with non-Hispanic White women (5629 [11.0%]) and non-Hispanic Asian and Pacific Islander (344 [5.7%]). More non-Hispanic Black women (5014 [93.2%]), non-Hispanic Asian and Pacific Islander women (5801 [96.4%]), and Hispanic women (6055 [96.0%]) included in this study tended to live in urban areas compared with non-Hispanic White women (45 377 [88.7%]). Additionally, more non-Hispanic Black women (982 [18.3%]), Hispanic women (1481 [23.5%]), and non-Hispanic American Indian and Alaskan Native women (60 [21.9%]) were enrolled in Medicaid or uninsured compared with non-Hispanic White women (3191 [6.2%]) and non-Hispanic Asian and Pacific Islander women (688 [11.4%]).
More non-Hispanic Black women (939[17.5%]) and non-Hispanic American Indian and Alaskan Native women (49 [17.9%]) had tumors with a high-risk RS compared with non-Hispanic White women (6973 [13.7%]), non-Hispanic Asian and Pacific Islander women (868 [14.5%]), and Hispanic women (874 [13.8%]) ( Table 1 ). Characteristics of study participants according to RS risk category are presented in Table 1 . Compared with women with a low- to intermediate-risk RS, the high-risk RS group comprised more high-grade tumors (4574 [47.1%] compared with 6322 [10.7%]), more stage II tumors (3058 [31.5%] compared with 13 305 [22.4%]), and more PR-negative tumors (2574 [26.5%] compared with 3727 [6.3%]). Most women diagnosed with tumors with a high-risk RS received chemotherapy (6353 [65.5%] vs 7038 [11.8%]), and more resided in neighborhoods with low SEP (5393 [55.6%]) compared with participants with low RSs (32 182 [54.1%]).
Compared with non-Hispanic White women, non-Hispanic Black women (odds ratio [OR], 1.33; 95% CI, 1.23-1.43) and non-Hispanic American Indian and Alaskan Native women (OR, 1.38; 95% CI, 1.00-1.86) were more likely to have high RSs after adjusting for age and marital status ( Table 2 ). The association of race with a high-risk RS among non-Hispanic Black women was attenuated and became nonsignificant after adjusting for multiple mediators (area-level SEP, insurance status, and urban residence) and their respective exposure mediator interaction terms (OR, 1.26; 95% CI, 0.92- 1.70). Sensitivity analyses that were restricted to patients with diagnosed in 2010 and later with confirmed ERBB2- negative tumors showed results consistent with the findings of the main analyses (eTable 2 in Supplement 1 ).
In multivariable analyses of the association between race and ethnicity and individual socioecologic factors ( Table 3 ), we found that non-Hispanic Black, Hispanic, and non-Hispanic American Indian and Alaskan Native women were 2-fold more likely to be uninsured or Medicaid covered and live in low-SEP areas, whereas non-Hispanic Black, non-Hispanic Asian and Pacific Islander, and Hispanic women were more likely to live in urban areas compared with non-Hispanic White women. Mediation analyses revealed that county-level SEP, insurance status, and rural vs urban residence taken together accounted for 7% to 20% of racial and ethnic differences in the prevalence of a high-risk RS ( Table 4 ). However, the contribution of these variables was significant only in non-Hispanic Black women (proportion mediated, 17%; P < .001). The magnitude of mediation in non-Hispanic Asian and Pacific Islander and non-Hispanic American Indian and Alaskan Native women who also had numerically higher odds of high-risk RSs was similar despite being not statistically significant. We observed an association with SEP, insurance status, and rural vs urban status; hence, we adjusted for the respective interaction terms with race in the mediation analysis.
Sensitivity analyses with e-values suggested that a potential unmeasured confounder would need to be associated with both the exposure and outcome with a risk ratio of 2 (or an OR of 2) to fully explain the observed association between race and high-risk RSs in non-Hispanic Black women in the absence of a true association (eTable 3 in Supplement 1 ). Sensitivity analysis restricted to patients diagnosed in 2010 to 2015 showed a similar crude association between race and high-risk RS, but no significant attenuation in the OR was seen after mediator adjustment in non-Hispanic Black women.
To better understand rural vs urban residence as a mediator of the association between non-Hispanic Black race and a high-risk RS, we performed analyses stratified by rurality status for non-Hispanic Black and non-Hispanic White women ( Table 5 ). Stratified models adjusted for age, marital status, SEP, and insurance status showed that non-Hispanic Black race was associated with high-risk RSs among women living in urban areas (OR, 1.35; 95% CI, 1.24-1.46), but no statistically significant difference was observed among women living in rural areas (OR, 1.05; 95% CI, 0.77-1.41). The association of non-Hispanic Black race with a high-risk RS was fully attenuated in an unstratified model adjusted only with an interaction term for race × rurality (OR, 1.09; 95% CI, 0.81-1.44). Taken together, these results suggest that the association of non-Hispanic Black race and a high-risk RS differs for urban and rural non-Hispanic Black women, although the interaction term (race × rurality) was not statistically significant ( P = .14 for interaction; OR, 1.27; 95% CI, 0.94-1.71).
To our knowledge, this is the first study to quantify the association between individual-level and contextual disadvantage and urban residence as mediators of racial differences in the prevalence of a high-risk gene expression profile in early-stage, ER-positive breast cancer. The results demonstrated that racial or ethnic differences in the RSs between non-Hispanic White and racial and ethnic minoritized women are not limited to non-Hispanic Black women; both non-Hispanic Black and non-Hispanic American Indian and Alaskan Native patients with breast cancer were 30% more likely to have a high-risk RS compared with non-Hispanic White individuals. Racial differences in the RS were partially mitigated after adjusting for health insurance status, neighborhood disadvantage, and urban residence among non-Hispanic Black women. The analysis found a modest degree of mediation (17%) of the racial difference in the prevalence of a high-risk RS by these socioeconomic and residential factors among non-Hispanic Black women. This finding indicates that socioeconomic deprivation and urban life exposures that are more pervasive in Black communities contribute to disproportionately aggressive tumor biology among non-Hispanic Black women. Notably, in the analysis stratified by rurality, non-Hispanic Black women living in urban areas had higher odds of developing tumors with high-risk RS, whereas that association was not seen in patients living in the rural areas. The study findings suggest that socioecologic context is the main factor underlying biologically aggressive ER-positive tumors in non-Hispanic Black women, although we were unable to examine whether ancestry-related risk alleles act as an effect modifier. Our findings have important implications for future research on the biological mechanisms underlying breast cancer disparities and support a social-genomic approach 28 to investigating this public health priority.
Socioeconomic position considers both social and economic factors as well as social status and is a well-established factor associated with health inequalities. 29 - 31 Residing in disinvested, underresourced areas can adversely affect health due to substandard housing, unfavorable social environments, and limited health care access. As results from this analysis suggest, persistent racial discrimination in the US has contributed to a higher proportion of Black, Hispanic, and American Indian populations living in communities with high disadvantage. Lower-economic and highly disadvantaged communities also have a greater number of social and environmental stressors, including higher environmental burden from inadequate housing, higher traffic density, increased industrial pollution, higher crime rates, and poorer access to resources such as healthy food, green space, and medical care. 31 , 32 Existing research underscores the notion that environmental and social factors can imprint modifications on the epigenome and subsequently influence gene expression, thus contributing to observed disparities. 33 , 34 To date, few studies have examined the potential role that social and environmental stressors from contextual disadvantage may have in contributing to more aggressive and deadly tumor biology.
Given the complexity and intricate nature of gene-environment interactions, it is critical to acknowledge even a modest level of mediation by a few socioecologic factors on disproportionately aggressive tumor biology among Black women. 35 - 38 It is plausible that using more comprehensive and nuanced measures of social determinants of health would explain a greater proportion of this racial difference. The SEP index used in this study was derived from county-level data. Perhaps more granular data at the census tract or block level would provide more insightful information. The SEER data set does not include data that would allow us to measure the effects of genetic ancestry, which prevents us from drawing any inferences about ancestry-related risk alleles that may act as effect modifiers. Previous literature 39 , 40 has hypothesized that one of the ways neighborhood socioeconomic disadvantages adversely affect tumor biology is through a correlation with increased obesity. A recent study by Gordon et al 41 offered evidence of this association by finding that a unit increase in insulin resistance increased the odds of a high-risk RS by 30%. Our study was constrained by the limited availability of measures for obesity and stress, which are presumed to be important factors through which the mediators influence the outcome.
There are several strengths of this study, including use of population-based data spanning multiple US states and geographic regions, which increases representation of diverse racial and ethnic groups and allows detailed analysis of residential and socioeconomic variables reflecting the US population. Moreover, we used a composite measure encompassing multiple domains comprising SEP. We also included an individual measure of SEP (insurance status), which increases our ability to measure disadvantage compared with area-level variables alone. 11 The linkage of SEER data with data from Genomic Health provides a genomic marker of tumor biology that is more robust than traditional histopathologic features. 11 This study uses mediation analysis to explore how race understood as a social construct can impact tumor biology. The multimediator analysis attempts to explain the disproportionate prevalence of high-risk RSs among Black women as the embodiment of social disadvantage through the accrual of a constellation of socioecologic factors.
This study also has some limitations. The study was restricted to individuals who underwent RS testing, potentially introducing selection bias. We calculated the weighted socioeconomic quintile for all patients with breast cancer, regardless of whether they received the test. We observed that patients with higher socioeconomic status were more likely to be included in the study sample, consistent with previous studies 42 , 43 finding testing disparities. It excludes tumors tested with genomic biomarkers other than the 21-gene RS. Additionally, this study only evaluates the exposure to social environments based on the patient’s residence at the cancer diagnosis, and we could not account for environmental exposures before breast cancer diagnosis. Hence, patients’ exposure at the time of cancer diagnosis captured in this study may not necessarily reflect their long-term exposures. Due to smaller sample sizes in non-Hispanic American Indian and Alaskan Native women and a less substantial amount of disparity in high-risk RSs among non-Hispanic Asian and Pacific Islander women, we were not able to evaluate the role of socioecologic factors in these disparities. Further research to evaluate these possible disparities is warranted. Furthermore, the SEER registry did not contain information on ERBB2 status before 2010. The SEER data set lacks information on insurance status before 2007; we had to exclude those cases from the analysis because insurance status was one of the mediators being studied. Finally, we cannot exclude the possibility of selection bias in the study cohort because RS data are only available for patients whose treating oncologist ordered the test.
This study demonstrates for the first time, to our knowledge, the influence of socioecologic context on a genomic biomarker that reflects the biology of ER-positive breast tumors. The study highlights the fact that the downstream effects of structural racism are not limited to inequities in health care; they also drive biologically aggressive tumor phenotypes. These effects are most clearly seen among non-Hispanic Black women, although the problem extends to other racial and ethnic minoritized groups (eg, non-Hispanic American Indian and Alaskan Native women). The finding that among non-Hispanic Black women only those with urban residence have a disproportionate prevalence of biologically aggressive tumors confirms the central role of contextual factors as the root cause of this disparity. However, future research should examine the possibility that ancestry-related genetic factors may act as effect modifiers. Additional research is needed with more comprehensive and nuanced measures of the social and environmental exposures that urban non-Hispanic Black and non-Hispanic American Indian and Alaskan Native women are subjected to. Mechanistic studies are also needed to identify potential targets for interventions that improve outcomes for racial and ethnic minoritized women with ER-positive breast cancer.
Accepted for Publication: February 6, 2024.
Published: April 3, 2024. doi:10.1001/jamanetworkopen.2024.4862
Open Access: This is an open access article distributed under the terms of the CC-BY License . © 2024 Parab AZ et al. JAMA Network Open .
Corresponding Author: Gregory S. Calip, PharmD, MPH, PhD, Titus Family Department of Clinical Pharmacy, Program on Medicines and Public Health, University of Southern California, 1989 Zonal Ave, Los Angeles, CA 90089 ( [email protected] ).
Author Contributions: Mr Parab and Dr Calip had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Parab, Kong, Hoskins, Calip.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Parab, Kong, Calip.
Critical review of the manuscript for important intellectual content: All authors.
Statistical analysis: Parab, Calip.
Administrative, technical, or material support: Kong, Calip.
Supervision: Kong, Lee, Kim, Nutescu, Hoskins, Calip.
Conflict of Interest Disclosures: Dr Kim reported receiving grants from Renalytix AI, AstraZeneca, Grail Bio, Institute for Clinical and Economic Review, and Takeda Pharmaceuticals outside the submitted work. Dr Hoskins reported receiving nonfinancial support from Agendia, Merck, Novartis, AbbVie, and Genentech outside the submitted work. Dr Calip reported receiving grants from Pfizer, receiving personal fees from AbbVie and Flatiron Health, and having stock ownership in Roche outside the submitted work. No other disclosures were reported.
Funding/Support: This work was funded by grants from the National Cancer Institute (Dr Hoskins).
Role of the Funder/Sponsor: The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Data Sharing Statement: See Supplement 2 .
Additional Contributions: We acknowledge the efforts of the National Cancer Institute, the Centers for Medicare & Medicaid Services, Genomic Health, Information Management Services Inc, and the SEER program tumor registries in the creation of the Oncotype DX database.
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
Physical activity in breast cancer survivors: A systematic review and meta-analysis on overall and breast cancer survival
Affiliations.
- 1 Department of Hygiene, Epidemiology, and Medical Statistics, School of Medicine, National and Kapodistrian University of Athens, 75 M. Asias Street, Goudi, 115 27, Athens, Greece. Electronic address: [email protected].
- 2 Department of Hygiene, Epidemiology, and Medical Statistics, School of Medicine, National and Kapodistrian University of Athens, 75 M. Asias Street, Goudi, 115 27, Athens, Greece. Electronic address: [email protected].
- 3 Department of Clinical Sciences and Community Health, University of Milan, 20133, Milan, Italy. Electronic address: [email protected].
- 4 Department of Clinical Sciences and Community Health, University of Milan, 20133, Milan, Italy. Electronic address: [email protected].
- 5 Department of Hygiene, Epidemiology, and Medical Statistics, School of Medicine, National and Kapodistrian University of Athens, 75 M. Asias Street, Goudi, 115 27, Athens, Greece. Electronic address: [email protected].
- 6 Department of Hygiene, Epidemiology, and Medical Statistics, School of Medicine, National and Kapodistrian University of Athens, 75 M. Asias Street, Goudi, 115 27, Athens, Greece. Electronic address: [email protected].
- PMID: 30780085
- DOI: 10.1016/j.breast.2019.02.001
Aim: To further quantify the association between physical activity (PA) after breast cancer diagnosis and all-cause mortality, breast cancer mortality and/or breast cancer recurrence.
Methods and results: PubMed was searched until November 2017 for observational studies investigating any type of PA in association with total mortality, breast cancer mortality and/or breast cancer recurrence among women with breast cancer diagnosis. Pooled hazard ratios (HRs) and 95% confidence intervals (CIs) were estimated using random-effects models for highest versus lowest categories of PA. Ten studies were included in the meta-analysis. During an average follow-up ranging from 3.5 to 12.7 years there were 23,041 breast cancer survivors, 2,522 deaths from all causes, 841 deaths from breast cancer and 1,398 recurrences/remissions. Compared to women in the lowest recreational PA level (lowest quintile/quartile), women in the highest level had a lower risk of all-cause mortality (HR = 0.58, 95% CIs: 0.45-0.75; 8 studies), of death from breast cancer (HR = 0.60, 95% CIs 0.36-0.99; 5 studies) and a lower, albeit non-significant, risk of recurrence (HR = 0.79, 95% CIs 0.60-1.05; 5 studies). There was evidence of heterogeneity between the studies evaluating recreational PA and total mortality (Ι 2 = 52.4%) and even higher for breast cancer mortality (Ι 2 = 77.7%) or recurrence (Ι 2 = 66.4%).
Conclusion: Highest recreational PA after breast cancer diagnosis was associated with lower all-cause and breast cancer mortality. This finding probably reflects the favorable impact of PA on cardiovascular mortality, and a possible favorable role on breast cancer survival, though reverse causation cannot be excluded.
Keywords: Breast cancer; Breast cancer survivors; Meta-analysis; Physical activity; Survival; Systematic review.
Copyright © 2019 Elsevier Ltd. All rights reserved.
Publication types
- Meta-Analysis
- Systematic Review
- Attitude to Health
- Breast Neoplasms / mortality
- Breast Neoplasms / therapy*
- Cancer Survivors / psychology*
- Health Behavior*
- Middle Aged
- Motor Activity
- Observational Studies as Topic

New Study Calls Efficacy of Clinical Breast Exams Into Question
Experts say these findings highlight the importance of follow-up screening and the value of patient communication among DCIS, or ductal carcinoma in situ, survivors.
A recent study, published December 28, 2023, sheds new light on how effectively mammograms identify second breast cancers among women with a history of ductal carcinoma in situ (DCIS) , an early stage of breast cancer. Over the study’s 10-year follow-up period, routine clinical examinations detected 2.2 percent of second breast cancers. Meanwhile, just over 20 percent were detected by patients themselves, and mammograms found 73.7 percent of second breast cancers.
Who Gets DCIS?
RELATED: Breast Cancer Stages: What Do They Mean?
- Family history of breast cancer
- Certain genetic mutations
- Previous breast disease
- Never being pregnant
- Hormone-related factors like an early start to menstruation or a later start to menopause.
RELATED: Closing the Gap in Breast Cancer Care and Support for Black Women
What Do These Findings Mean for Women With DCIS?
This recent study suggests that a stronger emphasis on educating patients to conduct self-examinations might be necessary. It also questions the efficacy of clinical breast exams in detecting these cancers when they recur.
According to Tari A. King, MD , the chief of breast surgery at Dana-Farber Brigham Cancer Center in Boston, “This study’s findings support current guidelines, with the caveat that educating patients on self-awareness of their breasts is important. [I]f a woman notes a change, having a [recent] normal mammogram should not dissuade her from bringing that change to the attention of her physician. [They also] clearly demonstrate the importance of patients being familiar with and aware of changes in their breasts, and they also need to be comfortable bringing these changes to their providers' attention.”
Dr. King says this study shows that mammography continues to be the most common method for diagnosing DCIS. Ninety-eight percent of patients in the study had their initial diagnosis of DCIS made by mammography, and 99 percent of patients who had recurrent disease detected on imaging had their diagnosis made by mammogram.
RELATED: Breast Cancer Screening 101: How to Navigate Through Your Options
King remains in favor of the guidelines as they are. “I support these guidelines for patients with DCIS who have received standard therapy to include surgery with or without radiation therapy. Although the authors suggest that clinical breast examinations may be less relevant, [these exams are] an opportunity to teach patients how to examine their own breasts and to educate them on what is normal and not normal.”
Irene Wapnir, MD , a surgical oncologist and breast specialist at Stanford Medicine in Palo Alto, California, agrees. “The other question is what kind of emotional reassurance do patients need in their follow-up?” she says. “Especially those who have conserved their breast.” A clinical breast exam, especially one done by a breast specialist, could offer patients peace of mind that they’re doing self-exams correctly and aren’t missing signs of a recurrence. “If you think you don’t feel anything in your breast but someone else reconfirms it, that may have some value.”
According to Dr. Wapnir, “What [the study authors] are saying is that the likelihood of a physical finding is low in this patient population, which is good news. The question is, if you ratchet down the clinical follow-up, what are you ratcheting it down to? I think [the study’s] value, its strength, is that it’s a real-world analysis of activity and surveillance rather than what academic centers publish. It’s thought-provoking, it’s interesting, but it leaves some questions unanswered.”
How Is DCIS Treated?
Breast-Conserving Surgery This procedure involves removing the tumor or area of malignant microcalcifications and a small amount of surrounding breast tissue. Usually, surgeons do not need to remove lymph nodes unless they find invasive cancer. After surgery, research shows that radiation therapy helps lower the chance of DCIS's return.
Mastectomy During this procedure, a surgeon removes the entire breast. Mastectomy might be necessary for large areas of DCIS or if breast-conserving surgery cannot or did not remove all the cancer. You may not need radiation after a mastectomy for DCIS.
Hormone Therapy After Breast Surgery If your DCIS is hormone receptor–positive, your care team might prescribe hormone therapy with medications like tamoxifen or aromatase inhibitors for five years after surgery. This helps lower the risk that DCIS will recur, or that invasive cancer will develop. Discuss the pros and cons of hormone therapy with your doctor if you have hormone receptor–positive DCIS.
Follow-Up Care: How and How Often Should I Get Screened?
The recent study’s authors suggest telehealth can be an effective alternative for monitoring DCIS survivors, with an in-person exam for someone who reports symptoms or has abnormal imaging results. They point out it can save time and money, and increase access for people who don’t have nearby healthcare options. But they also emphasize the importance of annual mammograms and education about self-detection.
RELATED: What to Do if You Feel a Breast Lump
The NCCN guidelines apply generally, and your specific circumstance may be different. For example, King points out, “Patients who choose non-operative management for DCIS may need more intensive surveillance — more frequent mammography — and patients with an inherited predisposition to cancer may also benefit from enhanced surveillance with MRI screening.”
Ultimately, you should do what your doctor recommends. People who have been treated for DCIS should stick to the recommended annual mammogram and get examined by a medical practitioner with expertise in breast health at least once each year. But you should also perform regular self-exams and communicate any changes to your care team.
Editorial Sources and Fact-Checking
Everyday Health follows strict sourcing guidelines to ensure the accuracy of its content, outlined in our editorial policy . We use only trustworthy sources, including peer-reviewed studies, board-certified medical experts, patients with lived experience, and information from top institutions.
- Waites BT et al. Mode of Detection of Second Breast Cancers in Patients Undergoing Surveillance After Treatment of Ductal Carcinoma In Situ. Journal of the National Comprehensive Cancer Network . December 28, 2023.
- Treatment of Ductal Carcinoma in Situ (DCIS). American Cancer Society. October 27, 2021.
- Key Statistics for Breast Cancer. American Cancer Society. January 17, 2024.
- Ductal Carcinoma in Situ (DCIS). Mayo Clinic. May 18, 2022.
- Timbres J et al. DCIS and LCIS: Are the Risk Factors for Developing In Situ Breast Cancer Different? Cancers . September 2, 2023.
- Kalwaniya D et al. Ductal Carcinoma in Situ: A Detailed Review of Current Practices. Cureus . April 15, 2023.
- Poelhekken K et al. The Natural History of Ductal Carcinoma in Situ (DCIS) in Simulation Models: A Systematic Review. The Breast . October 2023.
- Understanding Ductal Carcinoma in Situ (DCIS). Breast Cancer Research Foundation. March 9, 2023.
- Wapnir I et al. A Randomized Study Comparing Surgical Excision Versus Neoadjuvant Radiotherapy Followed by Delayed Surgical Excision of Ductal Carcinoma In Situ (NORDIS). Cancer Research . February 15, 2022.
Related Topics
- BARD1 Gene Mutation
- Lymphoma Survival Rate
- STK11 Gene Mutation
- TP53 Mutation

IMAGES
COMMENTS
Introduction. In 2020, there were estimated to be 19.3 million new cancer cases worldwide and almost 10 million cancer-related deaths. Female breast cancer was the most commonly diagnosed cancer, with 2.3 million new cases (11.7%), surpassing lung cancer. 1 Thanks to early diagnosis and improved treatment, the survival rate in patients with breast cancer has improved. 2 Almost 88% of patients ...
PURPOSE Despite a large volume of research, breast cancer survivors continue to experience high levels of unmet need. To better understand the breadth of evidence, we mapped systematic review-level evidence across cancer survivorship domains and outcomes and conducted network analyses of breast cancer survivorship care interventions. METHODS Umbrella review methodology was used to identify ...
Background Breast cancer is the most common type of cancer in women worldwide. Though improved treatments and prolonged overall survival, breast cancer survivors (BCSs) persistently suffer from various unmet supportive care needs (USCNs) throughout the disease. This scoping review aims to synthesize current literature regarding USCNs among BCSs. Methods This study followed a scoping review ...
Breast cancer is the most common type of cancer in women worldwide. Though improved treatments and prolonged overall survival, breast cancer survivors (BCSs) persistently suffer from various unmet supportive care needs (USCNs) throughout the disease. This scoping review aims to synthesize current literature regarding USCNs among BCSs.
Breast cancer survivors may experience significant after effects from diagnoses of breast cancer and cancer directed therapies. This review synthesizes the evidence about optimal management of the sequelae of a diagnosis of breast cancer. It describes the side effects of chemotherapy and endocrine therapy and evidence based strategies for management of such effects, with particular attention ...
This study aimed to identify supportive care needs from the point of view of breast cancer survivors. Methods Following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines for reporting systematic reviews, a comprehensive search of PubMed, Web of Science and Scopus was performed. The inclusion criteria were studies ...
This systematic review and meta-analysis aimed to assess the safety of systemic HRT on risk of disease recurrence in BC survivors. Methods: A systematic search of PubMed up to April 20, 2021 was conducted to identify randomized controlled trials (RCTs) that investigated the risk of disease recurrence with the use of HRT in BC survivors. A ...
Background Breast cancer (BC) is the most common cancer among women worldwide. We conducted a systematic review and meta-analysis to cover the existing research gap and contribute to existing knowledge to provide both researchers and clinicians with a better profile on the topic and consequently help improve the quality of life (QoL) of patients with BC. Methods A comprehensive review of ...
Purpose Advances in breast cancer care have led to a high rate of survivorship. This meta-review (systematic review of reviews) assesses and synthesises the voluminous qualitative survivorship evidence-base, providing a comprehensive overview of the main themes regarding breast cancer survivorship experiences, and areas requiring further investigation. Methods Sixteen breast cancer reviews ...
Methods: Umbrella review methodology was used to identify published systematic reviews reporting on survivorship care interventions for breast cancer survivors. Included reviews were mapped against domains and health care outcomes as specified by the Cancer Survivorship Quality Framework, and network analyses were conducted to determine the ...
Purpose Long-term breast cancer survivors are women surviving at least 5 years after diagnosis. This systematic review aimed to summarize the main characteristics and patterns of healthcare service use (frequency of visits, health providers visited, and preventive care performed) among long-term breast cancer survivors. Methods We used standard Cochrane Collaboration methods and searched the ...
Purpose: Advances in breast cancer care have led to a high rate of survivorship. This meta-review (systematic review of reviews) assesses and synthesises the voluminous qualitative survivorship evidence-base, providing a comprehensive overview of the main themes regarding breast cancer survivorship experiences, and areas requiring further investigation.
Breast cancer survivors face a complex landscape of challenges including lingering symptoms, anxieties about the future, identity shifts, and potential social isolation. ... Systematic reviews are ...
Background Survivorship care plan (SCP) comprising a treatment summary and plan for follow-up care is recommended by various organizations to address long-term needs of an increasing number of breast cancer survivors. Although there have been previous systematic reviews of SCPs in cancer, none has focused on breast cancer exclusively. This systematic review evaluates the use and impact of SCP ...
The Diet, Cancer, and Health cohort study by Ammitzbøll et al. with 959 breast cancer survivors and 144 deaths which found evidence that exercise was associated with longer survival after controlling for a wide range of important confounders including health behavior, comorbidity and cancer treatment [37] and the Tamoxifen Exemestane Adjuvant ...
The current systematic review with meta-analyses and RE-AIM framework revealed that eHealth interventions had broad reach, with high uptake among diverse (international and multilingual) patients with breast cancer and a significant positive impact on PROs QOL, health self-efficacy, psychologic distress, and fatigue compared with control ...
Multiple pharmacologic and behavioral treatments have been tested to improve sexual health after breast cancer. We present a systematic review of primary research on managing sexual dysfunction in breast cancer survivors to generate evidence-based content for improving knowledge on sexual health for BCS and their healthcare providers.
The small number of studies identified in this systematic review focused on breast cancer survivors in the extended or permanent survival stage (long-term survival), reflecting major deficiencies in the definition of cancer survivor and survival stage. 86 This is a great problem or difficulty because the late sequelae of breast cancer and its ...
WHAT THIS STUDY ADDS. ⇒ In this systematic study, we identified the most important supportive care needs of breast cancer survivors through an extensive search with no time limit. This study classified the needs of breast cancer survivors into main dimensions (10) and subdimensions (40). They are provided with supporting details.
To review the settings and outcomes of high-intensity interval training (HIIT) interventions for breast cancer survivors, and to explore the feasibility of prescribing exercise for breast cancer survivors. A systematic search of electronic databases was conducted for studies published up to May 31, 2020. Eligibility criteria included randomized controlled trials of HIIT intervention in breast ...
Pain and cancer-related fatigue (CRF) are impairments from breast cancer or the medical and surgical therapies for breast cancer. Aquatic therapy has been found to be effective for the problems of pain and CRF. The purpose of this systematic review was to determine whether aquatic therapy reduced pain and CRF among people with breast cancer and ...
Keywords: HER2-positive, breast cancer, immunotherapies, efficacy, safety, meta-analysis. Citation: Gu S, Liu Y, Huang Y, Lin W and Li K (2024) Comparative efficacy and safety of targeted therapy and immunotherapy for HER2-positive breast cancer: a systematic review and network meta-analyses. Front. Oncol. 14:1331055. doi: 10.3389/fonc.2024.1331055
Urban neighborhood and residential factors associated with breast cancer in African American women: a systematic review. Horm Cancer. 2018;9(2) ... both non-Hispanic Black and non-Hispanic American Indian and Alaskan Native patients with breast cancer were 30% more likely to have a high-risk RS compared with non-Hispanic White individuals ...
After neoadjuvant treatments, about 50%-65% of these patients achieve a pathologic complete response (pCR), defined as the absence of invasive cancer in breast tissue and lymph nodes at the time of surgery. pCR has shown a patient-level prognostic value, as having a positive association with disease-free survival (DFS) and overall survival (OS).
Physical activity in breast cancer survivors: A systematic review and meta-analysis on overall and breast cancer survival Breast. 2019 Apr;44:144-152. doi: 10.1016 ... During an average follow-up ranging from 3.5 to 12.7 years there were 23,041 breast cancer survivors, 2,522 deaths from all causes, 841 deaths from breast cancer and 1,398 ...
The Natural History of Ductal Carcinoma in Situ (DCIS) in Simulation Models: A Systematic Review. The Breast. ... breast cancer treatment can leave many survivors with lasting effects on their ...
Breast cancer was the most commonly diagnosed cancer (11.6% of the total cancer cases) and the leading cause of cancer death worldwide for females in 2018 [Citation 1].In the EU (EU-27), the incidence of breast cancer accounted for 28.7% of all female cancers in 2020 [Citation 2], and in the WHO EURO region alone, the number of breast cancer cases per year is expected to increase from 562,568 ...
Conclusion: This is the first systematic review and meta-analysis to summarize the literature on illness uncertainty among adult cancer survivors and family caregivers. Findings contribute to the growing literature on managing illness uncertainty among cancer survivors and family caregivers. Date of publication. 2023; Keyword. Cancer survivors ...
Many of the breast cancer patients do not adhere to the treatment that is associated with physical and psychological problems. Adherence to treatment is a key indicator of overall success in breast cancer treatment. ... anxiety and depression among cancer survivors: a systematic review. Ann Oncol. 2011; 22:761-772. doi: 10.1093/annonc/mdq413 ...
Purpose: To investigate the effectiveness of virtual reality (VR)-based interventions for functional rehabilitation of the upper limb in breast cancer patients through a systematic review and meta-analysis. Methods: The PubMed, Cochrane, Web of Science, CINAHL, Scopus, CNKI, Wanfang, and VIP databases were systematically searched for relevant literature published from the establishment of the ...