- Browse All Articles
- Newsletter Sign-Up

TechnologicalInnovation →
No results found in working knowledge.
- Were any results found in one of the other content buckets on the left?
- Try removing some search filters.
- Use different search filters.
Suggestions or feedback?
MIT News | Massachusetts Institute of Technology
- Machine learning
- Social justice
- Black holes
- Classes and programs
Departments
- Aeronautics and Astronautics
- Brain and Cognitive Sciences
- Architecture
- Political Science
- Mechanical Engineering
Centers, Labs, & Programs
- Abdul Latif Jameel Poverty Action Lab (J-PAL)
- Picower Institute for Learning and Memory
- Lincoln Laboratory
- School of Architecture + Planning
- School of Engineering
- School of Humanities, Arts, and Social Sciences
- Sloan School of Management
- School of Science
- MIT Schwarzman College of Computing
Fostering ethical thinking in computing
Press contact :.

Previous image Next image
Traditional computer scientists and engineers are trained to develop solutions for specific needs, but aren’t always trained to consider their broader implications. Each new technology generation, and particularly the rise of artificial intelligence, leads to new kinds of systems, new ways of creating tools, and new forms of data, for which norms, rules, and laws frequently have yet to catch up. The kinds of impact that such innovations have in the world has often not been apparent until many years later.
As part of the efforts in Social and Ethical Responsibilities of Computing (SERC) within the MIT Stephen A. Schwarzman College of Computing, a new case studies series examines social, ethical, and policy challenges of present-day efforts in computing with the aim of facilitating the development of responsible “habits of mind and action” for those who create and deploy computing technologies.
“Advances in computing have undeniably changed much of how we live and work. Understanding and incorporating broader social context is becoming ever more critical,” says Daniel Huttenlocher, dean of the MIT Schwarzman College of Computing. “This case study series is designed to be a basis for discussions in the classroom and beyond, regarding social, ethical, economic, and other implications so that students and researchers can pursue the development of technology across domains in a holistic manner that addresses these important issues.”
A modular system
By design, the case studies are brief and modular to allow users to mix and match the content to fit a variety of pedagogical needs. Series editors David Kaiser and Julie Shah, who are the associate deans for SERC, structured the cases primarily to be appropriate for undergraduate instruction across a range of classes and fields of study.
“Our goal was to provide a seamless way for instructors to integrate cases into an existing course or cluster several cases together to support a broader module within a course. They might also use the cases as a starting point to design new courses that focus squarely on themes of social and ethical responsibilities of computing,” says Kaiser, the Germeshausen Professor of the History of Science and professor of physics.
Shah, an associate professor of aeronautics and astronautics and a roboticist who designs systems in which humans and machines operate side by side, expects that the cases will also be of interest to those outside of academia, including computing professionals, policy specialists, and general readers. In curating the series, Shah says that “we interpret ‘social and ethical responsibilities of computing’ broadly to focus on perspectives of people who are affected by various technologies, as well as focus on perspectives of designers and engineers.”
The cases are not limited to a particular format and can take shape in various forms — from a magazine-like feature article or Socratic dialogues to choose-your-own-adventure stories or role-playing games grounded in empirical research. Each case study is brief, but includes accompanying notes and references to facilitate more in-depth exploration of a given topic. Multimedia projects will also be considered. “The main goal is to present important material — based on original research — in engaging ways to broad audiences of non-specialists,” says Kaiser.
The SERC case studies are specially commissioned and written by scholars who conduct research centrally on the subject of the piece. Kaiser and Shah approached researchers from within MIT as well as from other academic institutions to bring in a mix of diverse voices on a spectrum of topics. Some cases focus on a particular technology or on trends across platforms, while others assess social, historical, philosophical, legal, and cultural facets that are relevant for thinking critically about current efforts in computing and data sciences.
The cases published in the inaugural issue place readers in various settings that challenge them to consider the social and ethical implications of computing technologies, such as how social media services and surveillance tools are built; the racial disparities that can arise from deploying facial recognition technology in unregulated, real-world settings; the biases of risk prediction algorithms in the criminal justice system; and the politicization of data collection.
"Most of us agree that we want computing to work for social good, but which good? Whose good? Whose needs and values and worldviews are prioritized and whose are overlooked?” says Catherine D’Ignazio, an assistant professor of urban science and planning and director of the Data + Feminism Lab at MIT.
D’Ignazio’s case for the series, co-authored with Lauren Klein, an associate professor in the English and Quantitative Theory and Methods departments at Emory University, introduces readers to the idea that while data are useful, they are not always neutral. “These case studies help us understand the unequal histories that shape our technological systems as well as study their disparate outcomes and effects. They are an exciting step towards holistic, sociotechnical thinking and making."
Rigorously reviewed
Kaiser and Shah formed an editorial board composed of 55 faculty members and senior researchers associated with 19 departments, labs, and centers at MIT, and instituted a rigorous peer-review policy model commonly adopted by specialized journals. Members of the editorial board will also help commission topics for new cases and help identify authors for a given topic.
For each submission, the series editors collect four to six peer reviews, with reviewers mostly drawn from the editorial board. For each case, half the reviewers come from fields in computing and data sciences and half from fields in the humanities, arts, and social sciences, to ensure balance of topics and presentation within a given case study and across the series.
“Over the past two decades I’ve become a bit jaded when it comes to the academic review process, and so I was particularly heartened to see such care and thought put into all of the reviews," says Hany Farid, a professor at the University of California at Berkeley with a joint appointment in the Department of Electrical Engineering and Computer Sciences and the School of Information. “The constructive review process made our case study significantly stronger.”
Farid’s case, “The Dangers of Risk Prediction in the Criminal Justice System,” which he penned with Julia Dressel, recently a student of computer science at Dartmouth College, is one of the four commissioned pieces featured in the inaugural issue.
Cases are additionally reviewed by undergraduate volunteers, who help the series editors gauge each submission for balance, accessibility for students in multiple fields of study, and possibilities for adoption in specific courses. The students also work with them to create original homework problems and active learning projects to accompany each case study, to further facilitate adoption of the original materials across a range of existing undergraduate subjects.
“I volunteered to work with this group because I believe that it's incredibly important for those working in computer science to include thinking about ethics not as an afterthought, but integrated into every step and decision that is made, says Annie Snyder, a mathematical economics sophomore and a member of the MIT Schwarzman College of Computing’s Undergraduate Advisory Group. “While this is a massive issue to take on, this project is an amazing opportunity to start building an ethical culture amongst the incredibly talented students at MIT who will hopefully carry it forward into their own projects and workplace.”
New sets of case studies, produced with support from the MIT Press’ Open Publishing Services program, will be published twice a year via the Knowledge Futures Group’s PubPub platform . The SERC case studies are made available for free on an open-access basis, under Creative Commons licensing terms. Authors retain copyright, enabling them to reuse and republish their work in more specialized scholarly publications.
“It was important to us to approach this project in an inclusive way and lower the barrier for people to be able to access this content. These are complex issues that we need to deal with, and we hope that by making the cases widely available, more people will engage in social and ethical considerations as they’re studying and developing computing technologies,” says Shah.
Share this news article on:
Related links.
- MIT Case Studies in Social and Ethical Responsibilities of Computing
- Program in Science, Technology, and Society
Related Topics
- Technology and society
- Education, teaching, academics
- Artificial intelligence
- Computer science and technology
- Diversity and inclusion
- Program in STS
- History of science
- Aeronautical and astronautical engineering
- Electrical Engineering & Computer Science (eecs)
- Urban studies and planning
- Human-computer interaction
- MIT Sloan School of Management
- School of Architecture and Planning
- School of Humanities Arts and Social Sciences
Related Articles

3 Questions: Marion Boulicault and Milo Phillips-Brown on ethics in a technical curriculum

A college for the computing age

Computing and artificial intelligence: Humanistic perspectives from MIT

3 Questions: The social implications and responsibilities of computing
Previous item Next item
More MIT News
A first-ever complete map for elastic strain engineering
Read full story →

Shining a light on oil fields to make them more sustainable

“Life is short, so aim high”

MIT launches Working Group on Generative AI and the Work of the Future

Atmospheric observations in China show rise in emissions of a potent greenhouse gas

Second round of seed grants awarded to MIT scholars studying the impact and applications of generative AI
- More news on MIT News homepage →
Massachusetts Institute of Technology 77 Massachusetts Avenue, Cambridge, MA, USA
- Map (opens in new window)
- Events (opens in new window)
- People (opens in new window)
- Careers (opens in new window)
- Accessibility
- Social Media Hub
- MIT on Facebook
- MIT on YouTube
- MIT on Instagram
Publications
- Analysis & Opinions
- News & Announcements
- Newsletters
- Policy Briefs & Testimonies
- Presentations & Speeches
- Reports & Papers
- Quarterly Journal: International Security
- Artificial Intelligence
- Conflict & Conflict Resolution
- Coronavirus
- Economics & Global Affairs
- Environment & Climate Change
- International Relations
- International Security & Defense
- Nuclear Issues
- Science & Technology
- Student Publications
- War in Ukraine
- Asia & the Pacific
- Middle East & North Africa
- North America
- South America
- Infographics & Charts

US-Russian Contention in Cyberspace
The overarching question imparting urgency to this exploration is: Can U.S.-Russian contention in cyberspace cause the two nuclear superpowers to stumble into war? In considering this question we were constantly reminded of recent comments by a prominent U.S. arms control expert: At least as dangerous as the risk of an actual cyberattack, he observed, is cyber operations’ “blurring of the line between peace and war.” Or, as Nye wrote, “in the cyber realm, the difference between a weapon and a non-weapon may come down to a single line of code, or simply the intent of a computer program’s user.”

The Geopolitics of Renewable Hydrogen
Renewables are widely perceived as an opportunity to shatter the hegemony of fossil fuel-rich states and democratize the energy landscape. Virtually all countries have access to some renewable energy resources (especially solar and wind power) and could thus substitute foreign supply with local resources. Our research shows, however, that the role countries are likely to assume in decarbonized energy systems will be based not only on their resource endowment but also on their policy choices.

What Comes After the Forever Wars
As the United States emerges from the era of so-called forever wars, it should abandon the regime change business for good. Then, Washington must understand why it failed, writes Stephen Walt.

Telling Black Stories: What We All Can Do
Full event video and after-event thoughts from the panelists.
- Defense, Emerging Technology, and Strategy
- Diplomacy and International Politics
- Environment and Natural Resources
- International Security
- Science, Technology, and Public Policy
- Africa Futures Project
- Applied History Project
- Arctic Initiative
- Asia-Pacific Initiative
- Cyber Project
- Defending Digital Democracy
- Defense Project
- Economic Diplomacy Initiative
- Future of Diplomacy Project
- Geopolitics of Energy Project
- Harvard Project on Climate Agreements
- Homeland Security Project
- Intelligence Project
- Korea Project
- Managing the Atom
- Middle East Initiative
- Project on Europe and the Transatlantic Relationship
- Security and Global Health
- Technology and Public Purpose
- US-Russia Initiative to Prevent Nuclear Terrorism
Special Initiatives
- American Secretaries of State
- An Economic View of the Environment
- Cuban Missile Crisis
- Russia Matters
- Thucydides's Trap
Analysis & Opinions - O'Reilly Media
- Mike Loukidos
- Hilary Mason
These studies provide a foundation for discussing ethical issues so we can better integrate data ethics in real life.
To help us think seriously about data ethics, we need case studies that we can discuss, argue about, and come to terms with as we engage with the real world. Good case studies give us the opportunity to think through problems before facing them in real life. And case studies show us that ethical problems aren't simple. They are multi-faceted, and frequently there's no single right answer. And they help us to recognize there are few situations that don't raise ethical questions.
Princeton's Center for Information Technology Policy and Center for Human Values have created four anonymized case studies to promote the discussion of ethics. The first of these studies, Automated Healthcare App , discusses a smartphone app designed to help adult onset diabetes patients. It raises issues like paternalism, consent, and even language choices. Is it OK to “nudge” patients toward more healthy behaviors? What about automatically moderating the users’ discussion groups to emphasize scientifically accurate information? And how do you deal with minorities who don’t respond to treatment as well? Could the problem be the language itself that is used to discuss treatment?
The next case study, Dynamic Sound Identification , covers an application that can identify voices, raising issues about privacy, language, and even gender. How far should developers go in identifying potential harm that can be caused by an application? What are acceptable error rates for an application that can potentially do harm? How can a voice application handle people with different accents or dialects? And what responsibility do developers have when a small experimental tool is bought by a large corporation that wants to commercialize it?
The Optimizing Schools case study deals with the problem of finding at-risk children in school systems. Privacy and language are again an issue; it also raises the issue of how decisions to use data are made. Who makes those decisions, and who needs to be informed about them? What are the consequences when people find out how their data has been used? And how do you interpret the results of an experiment? Under what conditions can you say that a data experiment has really yielded improved educational results?
The final case study, Law Enforcement Chatbots , raises issues about the tradeoff between liberty and security, entrapment, openness and accountability, and compliance with international law.
None of these issues are simple, and there are few (if any) "right answers." For example, it’s easy to react against perceived paternalism in a medical application, but the purpose of such an application is to encourage patients to comply with their treatment program. It’s easy to object to monitoring students in a public school, but students are minors, and schools by nature handle a lot of private personal data. Where is the boundary between what is, and isn’t, acceptable? What's important isn’t getting to the correct answer on any issue, but to make sure the issue is discussed and understood, and that we know what tradeoffs we are making. What is important is that we get practice in discussing ethical issues and put that practice to work in our jobs. That’s what these case studies give us.
Want to Read More?
The authors.

- Senior Fellow, Technology and Public Purpose Project
- Former Senior Fellow, Cyber Project
- Former U.S Chief Data Scientist
- Former CTO, Devoted Health
- Bio/Profile
- More by this author

Recommended
In the spotlight, most viewed.

Journal Article - Issues in Science and Technology
Nuclear Power Needs Leadership, but Not from the Military
- Michael J Ford
- Ahmed Abdulla
- M. Granger Morgan

Analysis & Opinions - The Washington Post
Don't Fear the TSA Cutting Airport Security. Be Glad That They're Talking about It.
- Bruce Schneier

Data's Day of Reckoning

The Aftermath of the Baltimore Bridge Collapse
- Juliette Kayyem

What Does Ukraine Need from NATO?
- Karen Donfired
- Ivo Daadler

Barham A. Salih Joins Belfer Center for Science and International Affairs and Middle East Initiative at Harvard Kennedy School as Senior Fellow
- Barham Salih

Paper - Belfer Center for Science and International Affairs, Harvard Kennedy School
The Great Tech Rivalry: China vs the U.S.
- Graham Allison
- Kevin Klyman
- Karina Barbesino
Press Release - Belfer Center for Science and International Affairs, Harvard Kennedy School

Attacking Artificial Intelligence: AI’s Security Vulnerability and What Policymakers Can Do About It
- Marcus Comiter
Belfer Center Email Updates
Belfer center of science and international affairs.
79 John F. Kennedy Street, Cambridge, MA 02138 (617) 495-1400
Exploration of Science and Technology Interaction: A Case Study on Taxol
Ieee account.
- Change Username/Password
- Update Address
Purchase Details
- Payment Options
- Order History
- View Purchased Documents
Profile Information
- Communications Preferences
- Profession and Education
- Technical Interests
- US & Canada: +1 800 678 4333
- Worldwide: +1 732 981 0060
- Contact & Support
- About IEEE Xplore
- Accessibility
- Terms of Use
- Nondiscrimination Policy
- Privacy & Opting Out of Cookies
A not-for-profit organization, IEEE is the world's largest technical professional organization dedicated to advancing technology for the benefit of humanity. © Copyright 2024 IEEE - All rights reserved. Use of this web site signifies your agreement to the terms and conditions.
- Open supplemental data
- Reference Manager
- Simple TEXT file
People also looked at
Original research article, knowledge transfer from science to technology—the case of nano medical device technologies.

- 1 United Nations University – Maastricht Economic and Social Research Institute on Innovation and Technology (UNU-MERIT), Maastricht University, Maastricht, Netherlands
- 2 National Science Library, Chinese Academy of Sciences, Beijing, China
This study explores to what extent scientific knowledge has contributed to the development of industrial technologies. Backward citation is used to track the contribution of scientific research to technologies, and forward citation is adopted to evaluate the impact of these technologies. Patents are classified in two different groups (citing and not citing scientific publications) and a special attention has been given to the comparisons between countries, different types of organizations and different subfields. Our result reveals that, in the field of nano medical device technologies, knowledge transfer from the academic domain to the industrial domain is on the rise. The forward citations received by science-based patents are 1.6 times higher than those received by non-science-based patents. Our results also show that interconnections between science and technology are especially important for patents invented by firms compared with those developed by universities. At country level, all the six studied countries (USA, Germany, UK, Japan, France, and China) have been applying more and more scientific knowledge to develop nano medical device technologies. The linkage between science and technology is strongest in the USA, while it is weakest in the latecomer country China.
Introduction
Public science has been regarded as an important driving force behind industrial technologies ( Mansfield, 1980 , 1991 ; Griliches, 1986 ; Rosenberg, 1990 ; Narin et al., 1997 ; McMillan et al., 2000 ). Exploring the linkage between science and technology is considered as an important subject which helps understand the nature of inventions ( Nelson and Winter, 1977 ). By tracing the scientific citations referenced by patents, a group of scholars find that scientific research contributes substantially in stimulating industrial innovations and that science-based patents receive more citations ( Malo and Geuna, 2000 ; Sorenson and Fleming, 2004 ). However, there are also studies showing that the interplay of science and technology does not always lead to impactful inventions ( Appio et al., 2017 ), in particular in some regions ( Acosta and Coronado, 2003 ).
We argue that the linkage between science and technology depends on the organizational, regional, and sectoral settings. It is crucial to keep several aspects in mind. First, the incentives and importance of patenting are subject to the ownership of the patents. University-owned patents are more related to scientific questions while corporate-owned patents are more connected with direct commercial goals ( Sterzi, 2013 ). Commercial patents from firms that “build upon the scientific and engineering base created by university research” are believed to be more economically important than those from generated directly by universities ( Henderson et al., 1998 ). Second, due to the heterogeneity of regional features in developing industrial technologies, the linkage between science and technology tends to vary across regions/countries ( Acosta and Coronado, 2003 ; Wong and Wang, 2015 ). Based on the studies of several autonomous regions in Spain, Acosta and Coronado (2003) show that the interconnection between science and technological systems depends on the regional setting, e.g., technological complexity and specialization. The degree of scientific contribution to innovation is higher in regions that are specialized in sectors using more intensive technologies (e.g., Madrid) than in regions with low technological complexity (e.g., Catalonia). Third, the scientific contribution to technology development also involves a sectoral dimension. The intensity of science–technology interrelation varies across sectors and there is a sector-specific characteristic in knowledge flows ( Meyer, 2000 ). McMillan et al. (2000) find that technologies in the biotechnology industry are more reliant on public science than those in the pharmaceutical industry, and Popp (2017) suggests that there is more scientific research applied in patenting in biofuels than in wind research.
Despite the increasing attention to the science–technology linkage, existing studies have mainly focused on the technologically leading countries, such as the USA and several other developed countries ( Narin et al., 1997 ; McMillan et al., 2000 ; Acosta and Coronado, 2003 ). This is largely due to the availability of patent data from the major patent offices, such as the United States Patent and Trademark Office (USPTO), the Japan Patent Office (JPO), the European Patent Office (EPO), etc.
However, little is known about the science–technology linkage in emerging economies, in particular the differences across countries and types of organizations. It is widely acknowledged that innovation is crucial in the catching up process ( Fu et al., 2011 ). We argue that, to fully understand the science–technology linkage, it is of great importance to include both advanced and less advanced countries. This study aims to fill this gap by exploring the interconnection between science and technology in developed and developing countries, while also comparing different types of organizations and different technology classes.
Nano medical device technology is chosen as a case study, in which numerous medical disciplines benefit from innovation enabled by nanotechnologies. 1 It is expected that the innovative medical applications of nanotechnologies will have a profound impact on health care in the near future ( Bleaker et al., 2014 ; RIVM, 2015 ). 2 In this paper, we examine what is the trend of science-based technology development in nano medical device, whether application of scientific knowledge is associated with a high value of such technologies, and whether there are differences across organizations, countries and subfields.
Data and Methods
Data collection.
In this study, patent data of nano medical devices were collected from the Derwent World Patents Index (DWPI) via the platform Derwent Innovation (previously known as Thomson Innovation). Derwent Innovation provides access to data from more than 50 patent issuing authorities, which were converted into a standard format and with English translations from 30 languages. 3 We used a keyword search method and applied the SSTO = (nano* and “medical device*”) query to the title and abstract of each patent. 4 Considering the time lag in forward citation (FC) data and the fact that there were too few filed patents in the earlier years, we limited our dataset to the period between 2003 and 2012. After extracting the matched patents (37,904 records), we expanded the list to the same patent families and obtained 330,022 patent applications. After removing all duplicate records, the total number of patent applications for the period 2003–2012 was 108,468. According to the information of assignees, we classified the patents into three organization types: corporate patents, university patents, and corporate–university collaborated patents. Country codes were extracted based on the addresses of inventors.
We extracted the International Patent Classification (IPC) code of each patent and summarized at the second hierarchical level of the classification, e.g., A61, B05, C07, etc. For the 108,468 patents, there are in total 118 two-digit class numbers. The 10 highest ranked patent types, which cover 98% of total patents, were selected for the backward and FC analysis.
Both backward and FCs for all harvested nano medical device patents were collected. Backward citations include both patent citations and non-patent citations (NPCs). NPCs consist of various types of references, including scientific articles, withdrawn patents, technical manuals, databases, web-based information, news, etc. By applying Automatic identification combined with the artificial recognition method, we managed to extract only the scientific articles as the valuable science-based citations.
Methodology and Indicators
Based on the content of the references made by patents, this study classifies the patents 5 into two groups. One is the group of patents which have cited scientific publications in their references ( P s ), the other is the group of patents which did not cite any scientific publications ( P non-s ). As illustrated in Figure 1 , the former group ( P s ) developed patents grounded on both technological and scientific bases, while the latter group ( P non-s ) developed patents with only a technological basis.
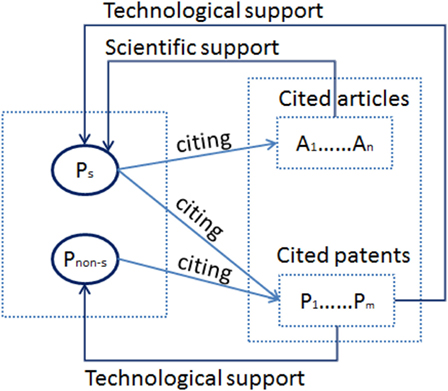
Figure 1 . Schematic diagram of science-based and non-science-based patents.
The degree to which science has contributed to the development of nano medical device technologies can be measured by the scientific knowledge application index (SKAI):
where P t,s is the number of patents filed at year t citing scientific publications and P t, non –s is the number of patents filed at year t without citing scientific publications. A higher level of SKAI indicates a higher influence from science to technological development.
Six countries (USA, Germany, UK, Japan, France, and China) are chosen to represent both advanced and emerging economies. The USA, Germany, UK, Japan, and France are technologically leading countries that represent the former group. For the emerging economies, the patent number of many countries (e.g., India, Russia, Brazil, etc.) is very low. Therefore, we choose China as a representative of the latter group. Given that each country has its own pattern in developing industrial technologies ( Wong and Wang, 2015 ), we normalize the SKAI by dividing one country’s SKAI value by the global average. In other words, we assume that the global average level is equal to 1 and the positions of all the studied countries will be compared with the average level. The normalized scientific knowledge application index (NSKAI) of country i at year t , NSKAI i,t , can be expressed as follows:
where P i,t,s is the number of patents in country i at year t citing scientific publications; P i,t, non- s is the number of patents in country i at year, t not citing scientific publications; P t,s is the number of patents in all countries at year t citing scientific publications; and P t, non -s is the number of patents in all countries at year t not citing scientific publications.
If one country’s NSKAI value is higher than 1, it means that nano medical device technologies in this country have a higher science base than that of the worldwide average. Similarly, a value less than 1 indicates that the linkage between science and technology 6 is weaker in this country.
Next, to examine the value or social impact of patents, we collect the information of FCs for each patent. The citation difference between the science-based and non-science-based patents can be captured by the forward citation differentiation index (FCDI). For instance, the FCDI for country i is defined as
The numerator is the number of FCs per patent (from country i ) which cited scientific publications, and the denominator presents the number of FCs per patent (from country i ) which did not cite scientific publications.
Similarly, we also calculate this index at organization level, namely for corporate patents, university patents, and corporate–university collaborated patents.
Trend of Scientific Bases in NMD Technologies
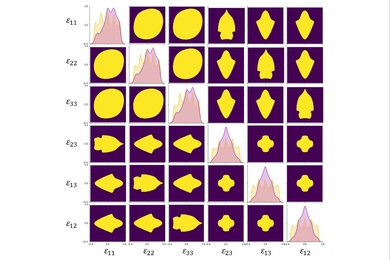
In the evolution of nano medical device technologies, the contribution of science has changed over the years. Figure 2 provides the ratio of patents citing non-patent publications. The y -axis represents for the SKAI, i.e., the degree to which science has contributed to the development of nano medical device technologies, which was explained in the earlier section.
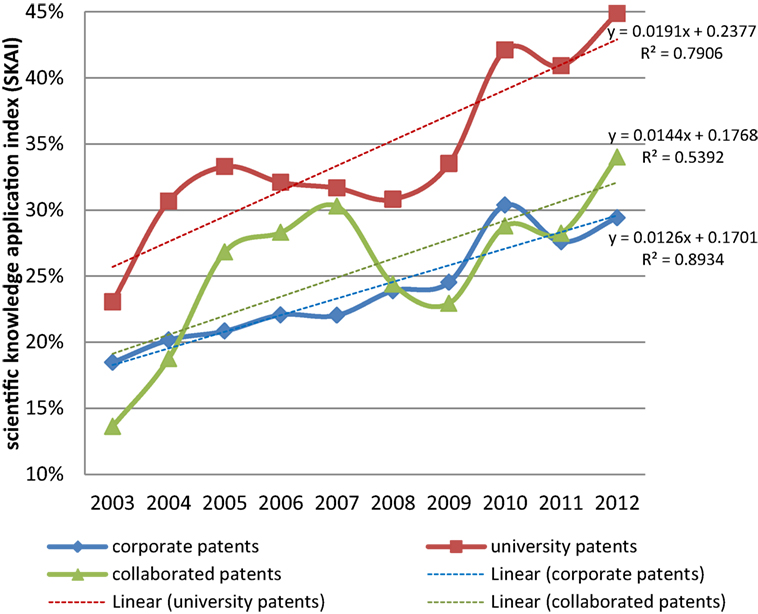
Figure 2 . Scientific knowledge application index (SKAI) by year. Note: See Eq. 1 for the calculation of this indicator.
Figure 2 shows that more and more inventions have been developed with a scientific knowledge basis. Among the three organizations, university patents have the highest share. This can be due to the fact that, in some cases, researchers from universities or research institutes publish and invent at the same time ( Van Looy et al., 2006 ). It is logical that they intend to cite their own scientific publications while patenting. Even if the cited papers are not from themselves, working in an academic environment, these researchers are more aware of the relevant scientific papers than inventors from firms. On the contrary, inventions from firms have a relatively low share in citing scientific publications. Such commercial patents have been developed with an industrial orientation rather than a scientific one. Corporate–university collaborated patents are located in the middle, with SKAI values lower than university patents and higher than corporate patents. In general, patents in all three types of organizations present an increasing value of scientific knowledge application over time.
Normalized Scientific Knowledge Application Index (NSKAI)
To explore the difference between countries in patenting activities, as explained in the previous section, we take the worldwide average into consideration and normalize the SKAI value for the studied countries. This assumes that the worldwide average SKAI value stays constant, at the level of 1. An NSKAI value >1 indicates a higher degree of applying scientific knowledge to develop patents in the country concerned. By contrast, an NSKAI value <1 suggests that less scientific knowledge has been applied into the studied technologies in this country.
The dynamic values of the NSKAI by country are presented in Figure 3 .
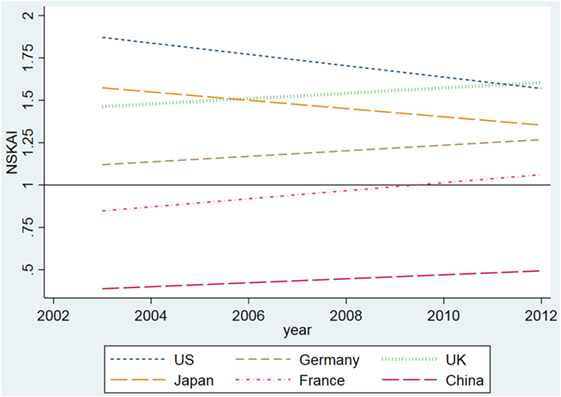
Figure 3 . Standardized scientific knowledge application index by country and by year. Note: The trend is plotted by lfit function in a linear regression. Original data for each year can be found in Figure A1 in Supplementary Material.
Figure 3 shows that the USA had the highest NSKAI value, suggesting that scientific knowledge contributed extensively to nano medical device patenting in the USA. In 2003, the NSKAI value in the USA was almost twice as high as the worldwide average. However, the NSKAI value decreased over time in the USA, which was mainly caused by an increase of the global average. In other words, other countries have increased their science application in patents more rapidly than the USA.
Similar to that of the USA, the NSKAI value of Japan was also higher than the average level, but with a decreasing trend moving closer to the average line. The UK and Germany both were above the average line, and still increased their values in the studied period. France had a relatively low starting point, but slowly moved upward.
Compared with other countries, China presented the lowest NSKAI value, which was far below the global average line 1. This indicates that patents filed by Chinese inventors were more industry-oriented than science-based. Nevertheless, in the long run, the NSKAI value in China has been improving steadily.
Effect of Scientific Bases for Patent Development
Acknowledging that more and more scientific knowledge has been applied to develop nano medical device technologies, one may wonder whether the application of science is associated with an improvement of patent quality.
Due to the time lag in FCs, patents filed in later years generally receive fewer citations than those filed earlier. Hence, we take patent age into consideration while investigating the value of patents (number of FCs). In our sample, the oldest patents were filed in 2003 (14 years old) and the youngest patents were filed in 2012 (5 years old). Because of the citation time lag, the number of FCs shows a decreasing trend in the Figure 4 .
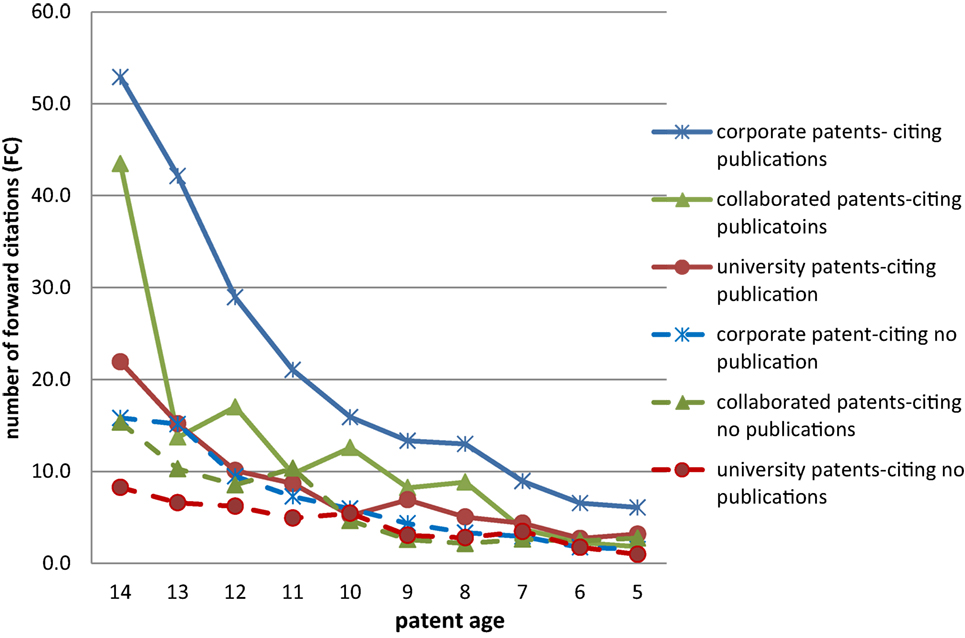
Figure 4 . The value of patent (forward citations) by patent age.
For patents from the same type of organization, the number of FC received by patents citing scientific publications was always higher than that of those received by patents not citing scientific publications. Taking the corporate patents filed in 2003 (14 years old) as an example, on average the number of FCs was 53 per patent in the group of patents citing publications, while it was 16 in the group of patents not citing publications. Namely, the FC in the former group was 2.3 times higher than the latter one.
The FCDI was less pronounced in university patents. For example, for the 14-year-old university patents, the average FC number was 22 for patents citing publications and 8 for patents not citing publications. The differentiation index for corporate–university collaborated patents was in the middle, lower than that of corporate patents, and higher than that of university patents.
On average, if all patents filed in the studied period (2003–2012) are included, the FCDI value was 2.73 for corporate patents, 1.62 for university patents, and 1.65 for corporate–university collaborated patents. This indicates that if commercial patents were developed by firms based on scientific knowledge, the value (or the social impact) of such patents can be amplified the most. For university patents, however, such added value was relatively low. Nevertheless, it is clear that, irrespective of the type of organization, it is valuable to apply scientific knowledge in developing technologies (see Table 1 ).
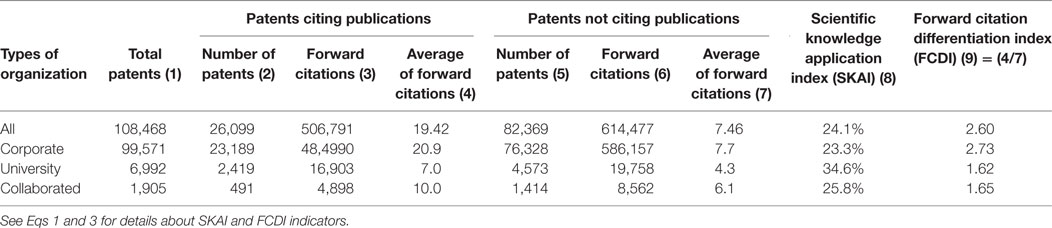
Table 1 . Comparison of scientific knowledge application by organization type, 2003–2012.
Effect of Scientific Bases for Patent Development––Comparison of Different Countries
Given that the quality of patents from different countries varies widely ( Hall et al., 2001 ), in this section we provide a comparison of scientific applications by country.
At the worldwide level in the period 2003–2012, the average number of FCs was 19.42 for patents citing scientific publications and 7.46 for patents without citing scientific publications (see Table 2 ). Thus, the differentiation index (FCDI) was 2.60, indicating that the value of the former patent group was 1.6 times higher than that of the latter patent group. In the studied six countries, the FCDI values in the UK, France, and the USA were relatively high, while Germany, Japan, and China presented relatively low FCDI values. However, it is worth noting that, in spite of having similar FCDI values, patents from these three countries received a different number of FCs. In China, the average FC received by science-based patents is merely 1.78, in contrast to 8.71 in Germany and 5.72 in Japan.
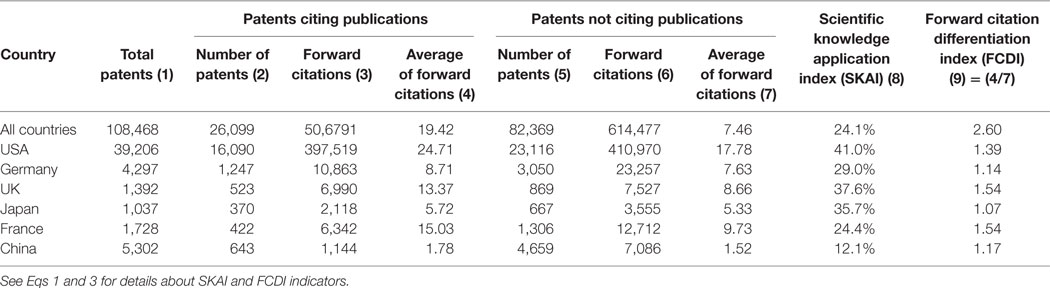
Table 2 . Comparison of scientific knowledge application in six studied countries, 2003–2012.
Table 2 also shows that the USA had the highest SKAI value (Col. 8), which indicates that a large share (i.e., 41%) of US patents cited academic research. Following that, the UK and Japan also exhibited a high value of SKAI, emphasizing the importance of scientific contribution in developing technologies in these countries. China, however, had the lowest value, merely 12.1%. This shows that inventions in China were not much grounded on scientific bases.
The low level of the SKAI value in China seems to be in line with regional features, as studied by Acosta and Coronado (2003) . From a geographical perspective, Acosta and Coronado (2003) find that the diffusion from scientific knowledge to innovations is stronger in regions using more intensive technologies than in regions with low technological complexity. Our results indicate that, compared with the studied five advanced countries, the interconnection between science and technology was low in latecomer countries such as China. This may be due to the fact that, on average, the technological complexity in nano medical devices is lower in China than in other developed countries.
Effect of Scientific Bases for Patent Development––Comparison of SubFields
As discussed in the section “Introduction,” the intensity of linkage between science and technology differs from area to area.
Table 3 documents the two-digit patent classes with the highest numbers in nano medical device patents. The 10 highest ranked patent types, which cover 98% of total patents, were selected for the backward and FC analysis. Explanations on the categories of these IPC classes and sections are provided in Table A1 in Supplementary Material.
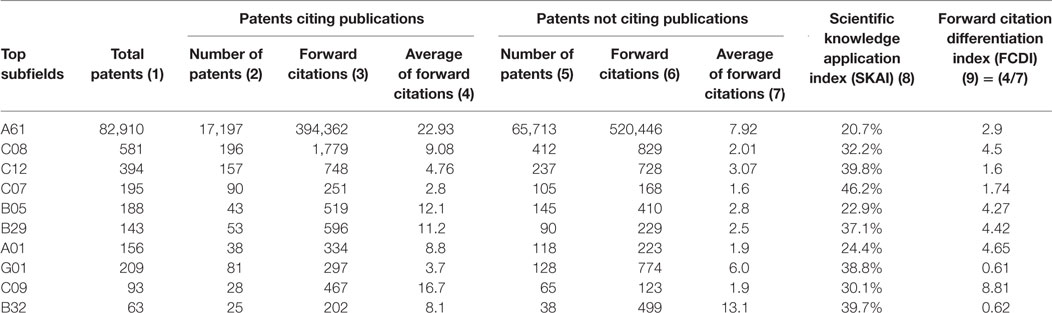
Table 3 . Comparison of scientific knowledge application in 10 subfields, 2003–2012.
The major type of nano medical device patents belongs to the A61 class, representing medical, veterinary science, and hygiene technologies in the subsection of Health, Life-saving, and Amusement category (see Table A1 in Supplementary Material). Although the patent numbers in other types (IPC codes of C08, C12, C07, B05, B29, A01, G01, C09, and B32) were relatively low, the scientific knowledge application indices were all higher than the index for the type A61. Technologies related to organic chemistry, IPC code C07, had the highest SKAI value (46.2%). Namely, this type of technology tended to cite scientific research more than others.
Patents in the C09 class, i.e., dyes, paints, polishes, etc., in the Chemistry section have the highest FCDI value (8.81), suggesting that science-based patents in this subfield received far more FCs (on average 16.7 per patent) than non-science-based patents (on average 1.9 per patent). On the contrary, technologies related to measuring and testing (G01) and technologies related to layered products (B32) exhibited an FCDI value <1. This shows that, in these two categories, non-science-based patents receive more FCs than science-based patents. Hence, a higher level of application of scientific knowledge in some types of technologies does not seem to be associated with higher patent impact.
Discussion and Conclusion
Using DWPI patent data between 2003 and 2012, this study explores whether (and to what extent) scientific knowledge has been contributing to the innovation activities in the field of nano medical device technologies. Our results show that there is an increasing link between science and technology in this field. That is, more and more nano medical device technologies have been developed based on science. By examining the FCs in two different patent groups (citing and not citing scientific publications), we find that knowledge transfer from science correlates with the impact of patents. This emphasizes that generally science has played an important role in stimulating technology.
On the other hand, this study underlines the multifaceted nature of the science–technology linkage, depending on the sectoral, organizational, and regional setting. In line with Meyer (2000) and McMillan et al. (2000) , this paper points out that there are sector-specific characteristics in technology transfer from science to technology. Certain types of technologies are more science-based than others. At organizational level, we find that the FCDI presents a higher value in corporate patents than in university patents. Namely, application of science has brought higher added value to patents developed by firms than those developed by universities.
At national level, our study shows that countries have different patterns in applying scientific knowledge to industrial technologies, at least in the nano medical device field. Among the six countries studied in this paper, the linkage between science and technology is strongest in the USA and weakest in the emerging country China. Although China’s nanoscience has developed rapidly over the past decades ( Zhou and Leydesdorff, 2006 ), such scientific knowledge has not been intensively transferred to the development of related industrial technologies. This reveals that latecomer countries may choose a different path from advanced countries. Due to the data limitation, unfortunately, we are unable to test the science–technology linkage in other emerging economies, such as India, Russia, and Brazil. Future studies on more developing countries would be encouraged.
Author Contributions
LW designed the project. ZL collected the data. LW and ZL conducted the analysis. LW and ZL wrote the paper.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at http://www.frontiersin.org/articles/10.3389/frma.2018.00011/full#supplementary-material .
- ^ See more discussions in ( RIVM, 2015 ).
- ^ Nano medical devices have contributed to the treatment of cardiovascular disease, cardiac arrhythmia, diagnostic tests in detection of cancer, and in the treatment in neurology, etc. ( RIVM, 2015 ).
- ^ See more at https://clarivate.com/products/derwent-innovation/ .
- ^ The data were extracted in July 2016.
- ^ Patents refer to nano medical device patents.
- ^ This refers only to nano medical device technology.
Acosta, M., and Coronado, D. (2003). Science-technology flows in Spanish regions: an analysis of scientific citations in patents. Res. Policy 32, 1783–1803. doi: 10.1016/S0048-7333(03)00064-7
CrossRef Full Text | Google Scholar
Appio, F. P., Martini, A., and Fantoni, G. (2017). The light and shade of knowledge recombination: insights from a general-purpose technology. Technol. Forecasting Soc. Change 125, 154–165. doi:10.1016/j.techfore.2017.07.018
Bleaker, E., Evertz, S., Geertsma, R., Peijnenburg, W., Westra, J., and Wijnhoven, S. (2014). Assessing Health & Environmental Risks of Nanoparticles: Current State of Affairs in Policy, Science and Areas of Application . Bilthhoven: National Institute for Public Health and the Environment. RIVIM Report 2014-0157.
Google Scholar
Fu, X., Pietrobelli, C., and Soete, L. (2011). The role of foreign technology and indigenous innovation in the emerging economies: technological change and catching-up. World Dev. 39, 1204–1212. doi:10.1016/j.worlddev.2010.05.009
Griliches, Z. (1986). Productivity, R&D, and basic research at the firm level in the 1970’s. Am. Econ. Rev. 76, 141–154. doi:10.3386/w1547
Hall, B. H., Jaffe, A. B., and Trajtenberg, M. (2001). The NBER patent citations data file: lessons, insights and methodological tools. NBER Work. Paper No. 8498. doi:10.1186/1471-2164-12-148
Henderson, R., Jaffe, A. B., and Trajtenberg, M. (1998). Universities as a source of commercial technology: a detailed analysis of university patenting, 1965–1988. Rev. Econ. Stat. 80, 119–127. doi:10.1162/003465398557221
Malo, S., and Geuna, A. (2000). Science-technology linkages in an emerging research platform: the case of combinatorial chemistry and biology. Scientometrics 47, 303–321. doi:10.1023/A:1005643127551
Mansfield, E. (1980). Basic research and productivity increase in manufacturing. Am. Econ. Rev. 70, 863–873.
Mansfield, E. (1991). Academic research and industrial innovation. Res. Policy 20, 1–12. doi:10.1016/0048-7333(91)90080-A
McMillan, G. S., Narin, F., and Deeds, D. L. (2000). An analysis of the critical role of public science in innovation: the case of biotechnology. Res. Policy 29, 1–8. doi:10.1016/S0048-7333(99)00030-X
Meyer, M. (2000). Does science push technology? Patents citing scientific literature. Res. Policy 29, 409–434. doi:10.1016/S0048-7333(99)00040-2
Narin, F., Hamilton, K. S., and Olivastro, D. (1997). The increasing linkage between U.S. technology and public science. Res. Policy 26, 317–330. doi:10.1016/S0048-7333(97)00013-9
Nelson, R. R., and Winter, S. (1977). In search of useful theory of innovation. Res. Policy 6, 36–76. doi:10.1016/0048-7333(77)90029-4
Popp, D. (2017). From science to technology: the value of knowledge from different energy research institutions. Res. Policy 46, 1580–1594. doi:10.3386/w22573
RIVM. (2015). Nanotechnologies in Medical Device . Bilthoven: National Institute for Public Health and the Environment. RIVIM Report 2015-0149.
Rosenberg, N. (1990). Why do firms do basic research (with their own money)? Res. Policy 19, 165–174. doi:10.1016/0048-7333(90)90046-9
Sorenson, O., and Fleming, L. (2004). Science and the diffusion of knowledge. Res. Policy 33, 1615–1634. doi:10.1016/j.respol.2007.02.023
Sterzi, V. (2013). Patent quality and ownership: an analysis of UK faculty patenting. Res. Policy 42, 564–576. doi:10.1016/j.respol.2012.07.010
Van Looy, B., Callaert, J., and Debackere, K. (2006). Publication and patent behavior of academic researchers: conflicting, reinforcing or merely co-existing? Res. Policy 35, 596–608. doi:10.1016/j.respol.2006.02.003
Wong, C.-Y., and Wang, L. (2015). Trajectories of science and technology and their co-evolution in BRICS: insights from publication and patent analysis. J. Informetrics 9, 90–101. doi:10.1016/j.joi.2014.11.006
Zhou, P., and Leydesdorff, L. (2006). The emergence of China as a leading nation in science. Res. Policy 35, 83–104. doi:10.1016/j.respol.2005.08.006
Keywords: knowledge transfer, science and technology, patent, citations, impact, countries, organizations
Citation: Wang L and Li Z (2018) Knowledge Transfer from Science to Technology—The Case of Nano Medical Device Technologies. Front. Res. Metr. Anal. 3:11. doi: 10.3389/frma.2018.00011
Received: 20 December 2017; Accepted: 20 February 2018; Published: 09 March 2018
Reviewed by:
Copyright: © 2018 Wang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zexia Li, lizexia@mail.las.ac.cn
Science and technology parks: an annotated and analytical literature review
- Published: 24 November 2016
- Volume 42 , pages 957–976, ( 2017 )
Cite this article
- Kelsi G. Hobbs 1 ,
- Albert N. Link 1 &
- John T. Scott 2
3555 Accesses
76 Citations
3 Altmetric
Explore all metrics
This paper summarizes the extant literature on science and technology parks in an effort to provide a foundation to stimulate additional research in this globally important topic. We find from our review of published scholarship over the past 30 years that attention to science and technology parks has indeed increased, but it has not yet exploded. We also find that the current distribution of the country focus of this research is skewed toward China, the United Kingdom, Spain, and the United States. Emphasis on studies related to UK and US parks has been primarily due to data availability; in China and Spain the emphasis has been primarily on case studies.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Similar content being viewed by others

What is Qualitative in Qualitative Research
Patrik Aspers & Ugo Corte

Research Methodology: An Introduction

Literature reviews as independent studies: guidelines for academic practice
Sascha Kraus, Matthias Breier, … João J. Ferreira
See, http://www.unesco.org/new/en/natural-sciences/science-technology/university-industry-partnerships/science-and-technology-park-governance/concept-and-definition/ .
See, http://www.iasp.ws/knowledge-bites .
See, http://www.ukspa.org.uk/our-sector .
See, http://www.aurp.net/what-is-a-research-park .
Our effort to summarize the works and findings of the scholars listed in the “ Appendix ” table in a few sentences is without question an over-simplification of their due diligence. Our sincere apology if we have overstated or understated any key findings. Such was completely unintentional.
Any omissions to the table in the “ Appendix ” are unintentional. We relied on references in other papers and on Internet searches to assemble the table. That approach to identifying the literature is biased against us finding all relevant book chapters and books. We hope that our review will stimulate others to expand on this literature review and to develop a taxonomy that characterizes it.
Future reviews might well construct alternative categories.
Some case studies relate to parks in different countries, some literature reviews are not country specific, and those publications related to evaluation methods are not country specific.
This finding holds for empirical as well as case study publication.
Albahari, A., Catalano, G., & Landoni, P. (2013a). Evaluation of national science park systems: A theoretical framework and its application to the Italian and Spanish systems. Technology Analysis & Strategic Management, 25 (5), 599–614.
Article Google Scholar
Albahari, A., Pérez-Canto, S., Barge-Gil, A., & Modrego, A. (2013b). Technology Parks versus Science Parks: Does the university make the difference? MPRA paper 49227.
Albahari, A., Pérez-Canto, S., & Landoni, P. (2010). Science and Technology Parks impacts on tenant organizations: A review of literature. MPRA paper 41914.
Bakouros, Y. L., Mardas, D. C., & Varsakelis, N. C. (2002). Science park, a high tech fantasy: An analysis of the science parks in Greece. Technovation, 22 (2), 123–128.
Carvalho, L. (2009). Four challenges for a new science park: AvePark in Guimaraes, Portugal. Urban Research and Practice, 2 (1), 103–108.
Chan, K. F., & Lau, T. (2005). Assessing technology incubator programs in the science park: The good, the bad, and the ugly. Technovation, 25 (10), 1215–1228.
Chan, K. A., Oerlemans, L. A. G., & Pretorius, M. W. (2010). Knowledge exchange behaviors of science park firms: The innovation hub scene. Technology Analysis & Strategic Management, 22 (2), 207–228.
Chen, C., Chien, C., & Lai, C. (2013). Cluster policies and industry development in the Hsinchu Science Park: A retrospective review after 30 years. Innovation: Management, Policy and Practice, 15 (4), 416–436.
Chou, T. (2007). The science park and the governance challenge of the movement of the high-tech urban region towards polycentricity: The Hsinchu science-based industrial park. Environment and Planning A, 39 (6), 1382–1402.
Dabrowska, J. (2011). Measuring the success of science parks: Performance monitoring and evaluation. Presented at the XXVIII IASP world conference on science and technology parks.
Díez-Vial, I., & Fernández-Olmos, M. (2015). Knowledge spillovers in science and technology parks: How can firms benefit most? Journal of Technology Transfer, 40 (1), 70–84.
Díez-Vial, I., & Montoro-Sánchez, A. (2016). How knowledge links with universities may foster innovation: The case of a science park. Technovation, 50–51 , 41–52.
Druilhe, C., & Garnsey, E. (2000). Emergence and growth of high-tech activity in Cambridge and Grenoble. Entrepreneurship & Regional Development, 12 (2), 163–177.
Edington, D. W. (2008). The Kyoto Research Park and innovation in Japanese cities. Urban Geography, 29 (5), 411–450.
Eto, H. (2005). Obstacles to emergence of high/new technology parks, ventures and clusters in Japan. Technological Forecasting and Social Change, 72 (3), 359–373.
Feldman, J. M. (2007). The managerial equation and innovation platforms: The case of Linkoping and Berzelius Science Park. European Planning Studies, 15 (8), 1027–1045.
Ferguson, R., & Olofsson, C. (2004). Science parks and the development of NTBFs: Location, survival and growth. Journal of Technology Transfer, 29 (1), 5–17.
Fikirkoca, A., & Saritas, O. (2012). Foresight for science parks: The case of Ankara University. Technology Analysis & Strategic Management, 24 (10), 1071–1085.
Fu, W. (2016). Industrial clusters as hothouses for nascent entrepreneurs? The Case of Tianhe Software Park in Guangzhou, China. Annals of Regional Science, 57 (1), 253–270.
Fukugawa, N. (2006). Science parks in Japan and their value-added contributions to new technology-based firms. International Journal of Industrial Organization, 24 (2), 381–400.
Fukugawa, N. (2015). Heterogeneity among science parks with incubators as intermediaries of research collaboration between startups and universities in Japan. International Journal of Technology Transfer and Commercialization, 12 (4), 231–262.
Gibson, L. J., Lim, J., & Pavlakovich-Kochi, V. (2012). The university research park as a micro-cluster: Mapping its development and anatomy. Studies in Regional Science, 43 (2), 177–189.
Gkypali, A., Kokkinos, V., Bouras, C., & Tsekouras, K. (2016). Science parks and regional innovation performance in fiscal austerity era: Less is more? Small Business Economics, 47 (2), 313–330.
Goldstein, H. A., & Luger, M. I. (1990). Science/technology parks and regional development theory. Economic Development Quarterly, 4 (1), 64–78.
Goldstein, H. A., & Luger, M. I. (1992). University-based research parks as a rural development strategy. Policy Studies Journal, 20 (2), 249–263.
Gower, S. M., & Harris, F. C. (1994). The funding of, and investment in, British science parks: A review. Journal of Property Finance, 5 (3), 7–18.
Guadix, J., Carrillo-Castrillo, J., Onieva, L., & Navascués, J. (2016). Success variables in science and technology parks. Journal of Business Research, 69 (11), 4870–4875.
Guo, Y., & Verdini, G. (2015). The role of geographical proximity in the establishment and development of science parks—evidence from Nanjing, China. Asian Geographer, 32 (2), 117–133.
Guy, I. (1996a). A look at Aston Science Park. Technovation, 16 (5), 217–218.
Guy, I. (1996b). New ventures on an ancient campus. Technovation, 16 (6), 269–270.
Hansson, F., Husted, K., & Vestergaard, J. (2005). Second generation science parks: From structural holes jockeys to social capital catalysts of the knowledge society. Technovation, 25 (9), 1039–1049.
Hommen, L., Doloreux, D., & Larsson, E. (2006). Emergence and growth of Mjardevi Science Park in Linkoping, Sweden. European Planning Studies, 14 (10), 1331–1361.
Huibing, X., & Nengli, S. (2005). Exploration of science parks. Chinese Journal of Population Resources and Environment, 3 (1), 55–59.
Jongwanich, J., Kohpaiboon, A., & Yang, C. (2014). Science park, triple helix, and regional innovative capacity: Province-level evidence from China. Journal of Asia Pacific Economy, 19 (2), 333–352.
Joseph, R. A. (1994). New ways to make technology parks more relevant. Prometheus, 12 (1), 46–61.
Kharabsheh, R. (2012). Critical success factors of technology parks in Australia. International Journal of Economics and Finance, 4 (7), 57–66.
Lai, H. C., & Shyu, J. Z. (2005). A comparison of innovation capacity at science parks across the Taiwan Strait: The case Zhangjiang High-Tech Park and Hsinchu Science-based Industrial Park. Technovation, 25 (7), 805–813.
Lamperti, F., Mavilia, R., & Castellini, S. (2015). The role of science parks: A puzzle of growth, innovation and R&D investments. Journal of Technology Transfer . doi: 10.1007/s10961-015-9455-2 .
Google Scholar
Larsen, K. (2004). Science and technology parks and the integration of environmental policy. Innovation: Management, Policy, and Practice, 6 (2), 294–305.
Leyden, D. P., Link, A. N., & Siegel, D. S. (2008). A theoretical and empirical analysis of the decision to locate on a university research park. IEEE Transactions on Engineering Management, 55 (1), 23–28.
Liberati, D., Marinucci, M., & Tanzi, G. M. (2016). Science and technology parks in Italy: Main features and analysis of their effects on the firms hosted. Journal of Technology Transfer, 41 (4), 694–729.
Lindelöf, P., & Löfsten, H. (2003). Science park location and new technology-based firms in Sweden: Implications for strategy and performance. Small Business Economics, 20 (3), 245–258.
Lindelöf, P., & Löfsten, H. (2004). Proximity as a resource base for competitive advantage: University–industry links for technology transfer. Journal of Technology Transfer, 29 (3–4), 311–326.
Link, A. N. (1995). A generosity of spirit: The early history of the Research Triangle Park . Research Triangle Park: The Research Triangle Foundation of North Carolina.
Link, A. N. (2002). From seed to harvest: The growth of the Research Triangle Park . Research Triangle Park: The Research Triangle Foundation of North Carolina.
Link, A. N., & Scott, J. T. (2003a). The growth of research Triangle Park. Small Business Economics, 20 (2), 167–175.
Link, A. N., & Scott, J. T. (2003b). US science parks: The diffusion of an innovation and its effects on the academic mission of universities. International Journal of Industrial Organization, 21 (9), 1323–1356.
Link, A. N., & Scott, J. T. (2005). Opening the Ivory tower’s door: An analysis of the determinants of the formation of US university spin-off companies. Research Policy, 34 (7), 1106–1112.
Link, A. N., & Scott, J. T. (2006). U. S. university research parks. Journal of Productivity Analysis, 25 (1), 43–55.
Link, A. N., & Scott, J. T. (2007). The economics of university research parks. Oxford Review of Economic Policy, 23 (4), 661–674.
Link, A. N., & Scott, J. T. (2015). Research, science, and technology parks: Vehicles for technology transfer. In A. N. Link, D. S. Siegel, & M. Wright (Eds.), The Chicago handbook of university technology transfer and academic entrepreneurship . Chicago: University of Chicago Press.
Chapter Google Scholar
Malairaja, C., & Zawdie, G. (2008). Science parks and university–industry collaboration in Malaysia. Technology Analysis & Strategic Management, 20 (6), 727–739.
Massey, D., & Wield, D. (1992). Evaluating science parks. Local Economy, 7 (1), 10–25.
Millar, C. C. J. M., Choi, C. J., & Chu, R. T. J. (2005). The state in science, technology, and innovation districts: Conceptual models for China. Technology Analysis & Strategic Management, 17 (3), 367–373.
Motohashi, K. (2013). The role of the science park in innovation performance of start-up firms: An empirical analysis of Tsinghua Science Park in Beijing. Asia Pacific Business Review, 19 (4), 578–599.
Nahm, K. (2000). The evolution of science parks and metropolitan development. International Journal of Urban Science, 4 (1), 81–95.
National Research Council. (2009). Understanding research, science and technology parks: Global best practices . Washington, DC: National Academy Press.
Phan, P. H., Siegel, D. S., & Wright, M. (2005). Science parks and incubators: Observations, synthesis and future research. Journal of Business Venturing, 20 (2), 165–182.
Phillimore, J. (1999). Beyond the linear view of innovation in science park evaluation: An analysis of Western Australian Technology Park. Technovation, 19 (11), 673–680.
Quéré, M. (1989). The Provence Alpes Cote d’ Azur high technology road: A technopolis network. Entrepreneurship & Regional Development, 1 (2), 155–166.
Quintas, P., Wield, D., & Massey, D. (1992). Academic-industry link and innovation: Questioning the science park model. Technovation, 12 (3), 161–175.
Robertson, M. (2007). Translating breakthroughs in genetics into biomedical innovation: The case of UK genetic knowledge parks. Technology Analysis & Strategic Management, 19 (2), 189–204.
Russel, M. G., & Moss, D. J. (1989). Science parks and economic development. Interdisciplinary Science Reviews, 14 (1), 54–63.
Rychev, M. V. (1993). Moscow University’s science park. Russian Education & Society, 35 (12), 75–80.
Salvador, E. (2011). Are science parks and incubators good ‘brand names’ for spin-offs? The case study of Turin. Journal of Technology Transfer, 36 (2), 203–232.
Shearmur, R., & Doloreux, D. (2000). Science parks: Actors or reactors? Canadian science parks in their urban context. Environment and Planning A, 32 (6), 1065–1082.
Shin, D. (2011). An alternative approach to developing science parks: A case study from Korea. Papers in Regional Science, 80 (1), 103–111.
Siegel, D. S., Westhead, P., & Wright, M. (2003a). Assessing the impact of science parks on research productivity: Exploratory firm-level evidence from the United Kingdom. International Journal of Industrial Organization, 21 (9), 1357–1369.
Siegel, D. S., Westhead, P., & Wright, M. (2003b). Science parks and the performance of new technology-based firms: A review of recent UK evidence and an agenda for future research. Small Business Economics, 20 (2), 177–184.
Simmie, J., & James, N. D. (1986). Will science parks generate the fifth wave? Planning Outlook, 29 (2), 54–57.
Sofouli, E., & Vonortas, N. S. (2007). S&T parks and business incubators in middle-sized countries: The case of Greece. Journal of Technology Transfer, 32 (5), 525–544.
Squicciarini, M. (2009). Science parks, knowledge spillovers, and firms’ innovative performance: Evidence from Finland. Economics , 32 , 1–28. http://www.economics-ejournal.org/economics/discussionpapers/2009-32 .
Sutherland, D. (2005). China’s science parks: Production bases or a tool for institutional reform? Asia Pacific Business Review, 11 (1), 83–104.
Vaidyanathan, G. (2008). Technology parks in a developing country: The case of India. Journal of Technology Transfer, 33 (3), 285–299.
Vásquez-Urriago, A. R., Barge-Gil, A., & Rico, A. M. (2016). Science and technology parks and cooperation for innovation: Empirical evidence from Spain. Research Policy, 45 (1), 137–147.
Vásquez-Urriago, A. R., Barge-Gil, A., Rico, A. M., & Paraskevopoulou, E. (2014). The impact of science and technology parks on firms’ product innovation: Empirical evidence from Spain. Journal of Evolutionary Economics, 24 (4), 835–873.
Vedovello, C. (1997). Science parks and university–industry interaction: Geographical proximity between the agents as a driving force. Technovation, 17 (9), 491–502.
Wang, K., & Liu, J. (2009). The dynamic effects of government-supported R&D subsidies: An empirical study on the Taiwan Science Park. Asian Journal of Technology Innovation, 17 (1), 1–12.
Watkins-Mathys, L., & Foster, M. J. (2006). Entrepreneurship: The missing ingredient in China’s STIPs? Entrepreneurship & Regional Development, 18 (3), 249–274.
Wessner, C. (1999). A review of the Sandia Science and Technology Park initiative . Washington, DC: National Academy Press.
Westhead, P. (1995). New owner-managed businesses in rural and urban areas in Great Britian: A matched pairs comparison. Regional Studies, 29 (4), 367–380.
Westhead, P. (1997). R&D ‘inputs’ and ‘outputs’ of technology-based firms located on and off science parks. R&D Management, 27 (1), 45–61.
Westhead, P., & Batstone, S. (1998). Independent technology-based firms: The perceived benefits of a science park location. Urban Studies, 35 (12), 2197–2219.
Westhead, P., & Batstone, S. (1999). Perceived benefits of a managed science park location. Entrepreneurship & Regional Development, 11 (2), 129–154.
Westhead, P., Batstone, S., & Martin, F. (2000). Technology-based firms located on science parks: The applicability of Bullock’ ‘soft-hard’ model. Enterprise Innovation and Management Studies, 1 (2), 107–139.
Westhead, P., & Cowling, M. (1995). Employment change in independent owner-managed high-technology firms in Great Britain. Small Business Economics, 7 (2), 111–140.
Westhead, P., & Storey, D. (1994). An assessment of firms located on and off science parks in the United Kingdom . London: HMSO.
Westhead, P., & Storey, D. (1997). Financial constraints on the growth of high-technology small firms in the UK. Applied Financial Economics, 7 (2), 197–201.
Westhead, P., Storey, D. J., & Cowling, M. (1995). An exploratory analysis of the factors associated with the survival of independent high-technology firms in Great Britain. In F. Chittenden, M. Robertson, & I. Marshall (Eds.), Small firms: Partnerships for growth . London: Paul Chapman.
Yang, W., & Lee, W. (2000). A study on management performance of Taiwan high technology industry—the Hsinchu Science Park experience. Journal of Information and Optimization Sciences, 21 (1), 19–44.
Zeng, S., Xie, X., & Tam, C. (2010). Evaluating innovation capabilities for science parks: A system model. Technological and Economic Development of Economy, 16 (3), 397–413.
Zhang, F., & Wu, F. (2012). Fostering indigenous innovation capacities: The development of biotechnology in Shanghai’s Zhangjiang High-Tech Park. Urban Geography, 33 (5), 728–755.
Zhou, Y. (2005). The making of an innovative region from a centrally planned economy: Institutional evolution in Zhongguancun Science Park in Beijing. Environment and Planning A, 37 (6), 1113–1134.
Zhu, D., & Tann, J. (2012). A regional innovation system in a small-sized region: A clustering model in Zhongguancun Science Park. Technology Analysis & Strategic Management, 17 (3), 375–390.
Zou, Y., & Zhao, W. (2014). Anatomy of Tsinghua University Science Park in China: Institutional evolution and assessment. Journal of Technology Transfer, 39 (5), 663–674.
Download references
Author information
Authors and affiliations.
University of North Carolina at Greensboro, Greensboro, NC, USA
Kelsi G. Hobbs & Albert N. Link
Dartmouth College, Hanover, NH, USA
John T. Scott
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Kelsi G. Hobbs .
Appendix: Literature related to science and technology parks (authors listed alphabetically)
Rights and permissions.
Reprints and permissions
About this article
Hobbs, K.G., Link, A.N. & Scott, J.T. Science and technology parks: an annotated and analytical literature review. J Technol Transf 42 , 957–976 (2017). https://doi.org/10.1007/s10961-016-9522-3
Download citation
Published : 24 November 2016
Issue Date : August 2017
DOI : https://doi.org/10.1007/s10961-016-9522-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Science park
- Technology park
JEL Classification
- Find a journal
- Publish with us
- Track your research

Global Dimensions of Intellectual Property Rights in Science and Technology (1993)
Chapter: 13 biotechnology case study, 13 biotechnology case study.
GEORGE B. RATHMANN
I want to describe a bit of the history of the biotechnology field to give you a strong sense of the importance of this field, not just in itself but as a prelude to a new technology as it develops over the next century. I then relate that history to some questions that have been raised and finally relate my conclusions with respect to biotechnology to the objectives of the conference.
As rocky as the road for biotechnology was in the United States, what we see coming up on the world scene is much more difficult, much more serious. We desperately need a legal system to solve the problems, and it is our hope that there are ways of dealing with these issues.
The biotech era really dawned when Watson and Crick defined the structure of deoxyribonucleic acid (DNA). As with many world-shattering discoveries, this was simple and concise—a publication of one page outlining the structure of DNA ( Nature, April 25, 1953, p. 737). They also had the vision to say it would affect not only how we looked at deoxyribonucleic acid, but how we looked at life itself and our ability to understand living systems. There would be products, there would be opportunities, and there would be new insights that would be most important. All that was recognized in a one-page article.
As important and earth shaking as that was, from the standpoint of the commercialization of biotechnology, something nearly as important occurred on June 17, 1980, when the Supreme Court ruled that live organisms could be patented. It was well recognized as important at the time, but I think few
people realized how important it was for launching the commercialization of biotechnology.
In that patent, Dr. Ananda Chakrabarty, who was at G.E. at the time, claimed an organism that would digest oil. The invention was never commercialized, but it told the world that this field was going to be important and there were going to be commercial opportunities. An investment in trying to understand the biochemistry of life would pay off in the sense that the intellectual property could be protected. Within four months (October 14, 1980), the biotechnology company Genentech went public and jolted Wall Street with a rise in its stock price from $35 to $71 1/4. So it is clear that as of that date, biotechnology assumed increasing commercial importance.
At that time, in October 1980, I was looking at the opportunity to start a biotech company called Amgen and we were putting out a document that we hoped would raise $15 million. Partly because of Genentech's success, we were able to raise $19 million—with only a scientific advisory board, one employee, and promises for two future hires. So it certainly had a profound effect on whether Amgen would ever be. As a matter of fact, within a year, Amgen, Genetics Institute, Immunex, Genetics Systems, Chiron, and many others companies were formed. Within two years, more than 100 companies were formed as this era was launched.
Now, the Chakrabarty decision made it look simple: life forms were patentable. Genentech, Cetus and many others afterwards launched public offerings, recognizing the commercial potential that biotechnology would lead to new discoveries of valuable intellectual property, which could be protected by patents. In reality, it was not quite that simple and the launchings were not that consistent.
Venture capital funds vacillated quite a bit, although after 1980 there was a very substantial influx of venture capital ( Figure 13-1 ). There were periods when it went down, and periods when it went up. Although these look like gigantic numbers, remember it takes about a quarter of a billion dollars to bring a pharmaceutical product to market. It probably takes more than that to commercialize something important in agriculture, food, or other areas. So this flow of venture capital was actually inadequate to keep it going. Of course, the public made the difference, but it can be seen that this was not exactly a consistent, reliable source of funds, either.
If we smooth everything out, the market value of biotechnology stocks moved dramatically from 1980, when it was literally zero, to 1991, when it was more than $35 billion ( Figure 13-2 ). Those of us in the industry saw some very serious bumps in that curve. In 1987 some biotech companies lost 30-40 percent of the value of the company in a matter of a few days. When you finally smooth everything out, it looks a lot simpler and surer than it felt.

FIGURE 13-1 Venture Capital Disbursements in Biotechnology
Source: Venture Economics and Ernst and Young
Figure 13-3 shows the amount of capital raised through public stock offerings. In 1991, more money was raised in six months than for many years, and as a matter of fact, when the total figures came in for the year they exceeded $4 billion—equal to all the money that had been raised in the previous years since the launching of commercial biotechnology. Of course, the big news is $550 million in initial public offerings. Those are new companies whose survival may mean wonderful improvements to our lives around the world. At the same time they will be facing some of the rocky roads that the earlier companies faced. So we can see that it is not a steady, easy trip.
Product sales in the industry today have reached about $8 billion and are expected to reach $20 billion by the year 2000. That may be a very conservative figure. The drug industry worldwide by that time will be well over $200 billion, and biotechnology is contributing roughly half of the most important products today. By the time the year 2000 comes around, biotechnology-derived products could be even more important. Of course there should be many other parts of the biotech industry that are commercially interesting by that time.

FIGURE 13-2 Market Value of Biotechnology Stocks

FIGURE 13-3 Amount of Capital Raised Through Initial Public Offerings and Other Public Offerings
Source: Paine Webber and Ernst and Young
So we are looking at something of great importance to the economy of the country and to international trade, which is discussed below.
I was asked by the National Research Council to address several questions. The first was, What adjustments in intellectual property rights have been made? Well, of course, the first is the allowance of claims to living organisms. The United States certainly led the way there. It was a very important opportunity that organisms that produced a pharmaceutical material could be claimed in patents. We had something tangible to claim even if the product being produced was already known or already had been defined.
One of the things that has been evolving over the last few years, and certainly in 1987 had a pretty dismal outlook, is referred to as In re Durden . This case implied that just because you have a novel starting material on which you carry out a process to produce another material, the process is not automatically patentable. That case was often interpreted much more severely to mean that unless the process is highly inventive, mere novelty because of novel starting materials does not make it patentable. So it was not possible in 1987 to get claims to the process that was going to produce, for example, in Amgen's case, erythropoietin by using a novel organism.
Because inventors could not claim the process, they had a very serious problem. They could not invoke any rights at all against companies who used their organism overseas, produced the product, and brought it in. They did not have a final product claim; they did not have a process claim; and there was no mechanism for protecting against the direct theft of the organism overseas—copying it, or following the teachings of the patent, and then just shipping the product to the United States.
However, an evolution has occurred since then. Certainly, a lot of process claims have now been granted. There is a bill authored by Congressman Boucher that would give guidance to the Patent Office to make sure it issues those claims. Without those claims, the organism patent is meaningless with respect to overseas competition. What if the overseas country does not issue the organism patent? The organism has only one purpose—to produce the protein, so the inventor is left with no protection against importation. Amazingly enough, the inventor is protected from infringement in the United States by U.S. companies but is unable to stop foreign infringement and U.S. importation. The trade implications are clear.
This has been a very serious problem that is now being addressed. Yet there are still concerns from people who wonder if it is really "fair" to keep foreign companies from bringing their products into the United States. They ask, "Isn't that protectionism?" This a very strange interpretation of fairness. I think these inventions are clearly being copied and misappropriated by foreign companies. Changes may or may not move smoothly, but these issues should be resolved in the next few years, and more and more compa-
nies are availing themselves of the process protection, though some opportunities have been abandoned after In re Durden objections.
There have been great differences in the interpretation of the scope of claims. My initial discussion is limited to the United States because global issues have really only come into play in the least five years. Even in the United States, the scope of claims has been quite a difficult issue with which to deal. The questions stated are, If the claims are too broad, doesn't it mean we are inhibiting the diffusion of technology? If the claims are too narrow, doesn't it mean that the inventor really is disadvantaged? I could say a lot about that, but in actual fact I will cite the record. A Boston court in the United States leaned toward a pretty narrow interpretation of the claims. In a Delaware court, a jury decided that the Genentech case should be very broadly interpreted and cover structures quite different from the ones that were defined in the patent simply because all the rest were straightforward once the patent teachings were available. So these are still issues, but I think we will move toward a pretty clear understanding over the next few years.
The effect on biotechnology advancement has not been smooth even in this country. Patent uncertainty has encouraged second entrants, who then plead that since they made such a significant investment, believing they were not going to be prevented from manufacturing the product, the terms of the claims of the patents should be relaxed. This has certainly been an expensive mistake in many cases.
Major delays in issuance of patents have prevented some innovators from pushing their products as rapidly as they could, because they feared that they might never have coverage and once they proved the success of the product, it could be duplicated relatively readily. I think many of us in the business got a lot of encouragement from the Orphan Drug Act, because that act suggested that we at least could get six years of protection if we were the first to have a product approved for an orphan indication. If we never received adequate patent protection, we still might be able to recoup our investments, which was very comforting. There has been a lot of controversy about the Orphan Drug Act and whether it should serve as a kind of substitute for the Patent Act. Nevertheless, it helped an embryonic biotechnology industry raise money and sustain its early critical momentum.
Finally, patents played a key role in attracting pharmaceutical companies' investments. These were very important for some companies in the early days. Even though the pharmaceutical companies were not the innovators, they certainly helped support many new biotechnology companies and they clearly needed the confidence of patent exclusivity.
As stated in congressional testimony by Dr. P. Roy Vagelos, Chairman of Merck & Co., "To sustain their ability to discover and develop products which form the basis of American competitiveness, U.S. pharmaceutical
companies count on renewed government support ... in strengthening international protection of intellectual property rights." We can illustrate that perhaps even more significantly in the biotech industry.
For example, in 1986 a pharmaceutical product would cost about $94 million and take somewhere between 10 and 20 years before entering the market. Some kind of protection is certainly required before that kind of investment is made. The figure today is $240 million. That number has been challenged by Congress and looked at many ways by the Office of Technology Assessment (OTA); the latest OTA study says that costs may often be that high, although sometimes they may be lower. However, it does not require a lot of arithmetic to figure this out. The pharmaceutical industry in this country alone spends about $10 billion on R&D per year, and about 30 new products—30 new molecular entities—are approved each year. That comes out to be more than $300 million invested for each success.
In fact, there are at most only four or five new therapeutic products approved each year that are important and if you divide by that, you arrive at astronomical figures for important new therapeutics. Also, all this investment is required years before you can enter the market and start to get a return. So this certainly fits the pattern of something that requires protection, and patents look like the way to do it.
In 1986 the average development time of a new pharmaceutical product was 10 years. The interesting thing is that biotechnology has compressed that time. Because of the rational design of these products, their remarkable efficiency and safety profile, and the understanding and cooperation of the U.S. Food and Drug Administration, the average development time is about four to seven years today for biotechnology products, which is a big help. However, it is still a long time and a large investment.
So let us review how biotechnology was commercialized. What happened is not particularly logical, not what anyone would have deduced sitting around a table trying to decide what was going to happen. When a biotech company decided it wanted to launch a product, it had to build a company to launch the product. All the different stages and structures had to be built—the vectors and expression systems, purifications, scale-up, manufacturing, clinical testing, regulatory submissions, and marketing. Surprisingly enough, almost all of these things were in place in major pharmaceutical companies, yet almost every single important invention was done by independent biotechnology companies. That is the fact; that is what we have to deal with. How were they able to do all this, why would they be the first to do it, and was it effective? Is it not terribly inefficient to have to create a company for each new product? That is what was done.
Small, start-up biotechnology companies were responsible for many miracle drugs. For example, Amgen developed erythropoietin, and we now know that 10 milligrams per year, one-fiftieth of an aspirin tablet, will
prevent 20 or more transfusions for people that are deficient in erythropoietin—and there are many more. Chiron produced the answer to hepatitis C, which is something that has plagued society and challenged scientists for more than 30 years—a well-defined disease about which nothing could be done. Cetus discovered ways of amplifying genes. Individual inventors, individual small companies, are pioneering and finding important new molecules and insights that are changing the way medicine is practiced today. This was done in a way that perhaps was hardly predictable—small, independent companies got started and did this all on their own—but this is exactly what happened. Sometimes it occurred with the support of large companies, but none of the key innovations and developments throughout the field were made by the large companies.
As I said, it was a fairly rocky road. I think that is important. The fragility of a new technology and the need for immediate action are more critical than making long-range plans to do wonderful things over long periods of time. These companies are fragile and their viability is always in question. Their survival is in jeopardy at all times. Take 1989 as an example. Headlines blared, "Clouds gather over the biotech field." Interestingly enough, firms were stumbling on regulation and patent problems. The patent situation looked very confused at that time. It was very difficult again to get financing, and the feeling was that many companies would go out of business and some did.
If we look at the number of financings, we see what has faced this emerging technology—and will probably apply to every new technology—big financing surges, dry spells, big surges. The dry spell in 1984 and 1985 seemed to last forever. We learned it can take eight quarters before you see another chance to raise money. When 1987 came along, the stock market wilted, and 1988, 1989, and 1990—one after another—were all very bad years. Of course, 1991 salvaged a lot of companies, but those were dangerous times for fragile, embryonic businesses.
So some protection is required. There is no question that patent protection fits the need in terms of the large investment required over a long period of time. The question is always asked, however, whether keeping the inventions secret would work. Well, it doesn't. Once the gene has been described, it is trivial to produce the product. Even if the gene is not described anywhere, once the structure is out, once the product is available even in clinical trials, the structure can be determined and often easily duplicated at a much lower cost. The cost is even lower because the copier only has to copy winners. He does not have to duplicate the losers. The copier avoids the major investments that the innovator had to make.
So international protection becomes the issue today. The problems in obtaining worldwide protection are difficult. There are many countries that do not honor the patent system. Surprisingly, countries that do not have
strong patent systems (e.g., China, India, Argentina, Brazil) are not troublesome to the biotech field, although the pharmaceutical industry has expressed concern. However, international trade competition with countries that purport to have a patent system is a very serious issue.
For example, Japan is a strong competitor. In Japan, patent flooding surrounds innovator's patents. The Japanese patent office grants narrow patents instead of broad ones. I think it is pretty obvious to those in this industry that small companies need broad patents. If you are going to try to compete in the marketplace with giants, you had better know that you have some reasonable protection against obvious duplication or partial duplication. The Japanese system has not produced many biotechnology innovations and has not produced biotechnology companies. Our problems with the Japanese system are narrow patents, sometimes taking 10 or more years to issue, and patent flooding, which surrounds the inventor's contribution and forces him to join up with a large, entrenched Japanese company to survive.
To summarize, developing countries have concerned some industries, but they have not been competitive in biotechnology. Europe has awarded strong patents that afford U.S. innovators reasonable protection. Japan has been a very serious issue. Today we see two companies in Japan enjoying the products of Amgen—two products approaching a billion dollars in sales, at prices two to four times that of the products in this country, guaranteeing high profits. It is very easy to see what is going to happen over the long term. Those companies are going to be able to invade other countries in the field of biotechnology and be very active participants in trade.
The question then is, Can the United States dictate or influence international patent practices? Well, somehow it has to. This sounds unfair to some, but it is equally unfair to have misappropriation of intellectual property.
We know the history of what happened: Japan behind, Japan even, Japan ahead. The outlook is very serious. If we think back about that 20year period around the 1960s when U.S. patents were not being upheld, that may have been why it was easy for the Japanese to move in and take over the territory.
Now, for future challenges: The federal government's patenting of the genome was a hypothetical question until a short time ago. Would this be serious? It has now become a very practical question. The U.S. Patent Office is currently examining the NIH's application for patents on certain gene sequences. In the meantime, the Industrial Biotechnology Association has held discussions with Reid Adler of the National Institutes of Health (NIH), biotech executives and administration officials who are examining this issue. What should the NIH do with respect to all of these gene patents? A good start is to provide a forum between industry, NIH and other inter-
ested parties to see if we can understand whether these patents should be applied for, whether they should be issued, and if issued, how they should be handled.
Finally, can patents be issued faster? The U.S. Patent and Trademark Office's numbers on the average time of application pendency are very strange and not helpful. The Patent Office has always figured out ways to say it is doing things in two years when, in fact, there has not been a useful biotech patent that has taken less than four years, and usually five. If we cannot get meaningful numbers, I don't think the problem can be solved. I think the Patent Office is misleading all of us.
In terms of the conference objectives, I would like to close with these thoughts concerning a few final issues: First, with respect to international perception of the importance of intellectual property rights, the world acknowledges that the United States was the pioneer in biotechnology, and that it was done by risk capital, as well as federal support of R&D, originally. The positive contribution to human welfare is acknowledged worldwide. That does not mean that all the countries in the world want to give strong patent protection for biotechnology, which is a very difficult issue.
Second, with respect to biotechnology patents, in the United States, the road has been rocky but reasonably satisfactory. Worldwide protection will ultimately be critical. It is sad that this did not occur long ago. Because of this lack, we are seeing companies in foreign countries appropriating U.S. technology to get started.
Finally, with respect to conflict resolution, the most precious resource of a budding new industry or budding new technology is time. The solutions have to be time sensitive. Grandiose solutions that involve 60 or 70 countries, and take years and years, will mean that a lot of the companies will fail before the solutions are in place. I think people should be aware of that.
I would remind you of one last thing. This is an industry of small companies. If you look at the profile of public biotechnology companies, only 13 percent have more than 300 employees, and none have more than 2,000 employees. If we look at all biotech companies (publicly and privately held), there are only 3 percent with more than 300 employees. We are dealing with a very, very broad-based, small-company business and my remarks apply as well to my firm, ICOS, which we started within the last year, as well as to the largest biotech companies, which are still relatively small. These are the companies seeking patent protection. Strong protection can hardly ''disadvantage small companies" as some critics suggest.
As technological developments multiply around the globe—even as the patenting of human genes comes under serious discussion—nations, companies, and researchers find themselves in conflict over intellectual property rights (IPRs). Now, an international group of experts presents the first multidisciplinary look at IPRs in an age of explosive growth in science and technology.
This thought-provoking volume offers an update on current international IPR negotiations and includes case studies on software, computer chips, optoelectronics, and biotechnology—areas characterized by high development cost and easy reproducibility. The volume covers these and other issues:
- Modern economic theory as a basis for approaching international IPRs.
- U.S. intellectual property practices versus those in Japan, India, the European Community, and the developing and newly industrializing countries.
- Trends in science and technology and how they affect IPRs.
- Pros and cons of a uniform international IPRs regime versus a system reflecting national differences.
Welcome to OpenBook!
You're looking at OpenBook, NAP.edu's online reading room since 1999. Based on feedback from you, our users, we've made some improvements that make it easier than ever to read thousands of publications on our website.
Do you want to take a quick tour of the OpenBook's features?
Show this book's table of contents , where you can jump to any chapter by name.
...or use these buttons to go back to the previous chapter or skip to the next one.
Jump up to the previous page or down to the next one. Also, you can type in a page number and press Enter to go directly to that page in the book.
Switch between the Original Pages , where you can read the report as it appeared in print, and Text Pages for the web version, where you can highlight and search the text.
To search the entire text of this book, type in your search term here and press Enter .
Share a link to this book page on your preferred social network or via email.
View our suggested citation for this chapter.
Ready to take your reading offline? Click here to buy this book in print or download it as a free PDF, if available.
Get Email Updates
Do you enjoy reading reports from the Academies online for free ? Sign up for email notifications and we'll let you know about new publications in your areas of interest when they're released.

- Technology Ethics Cases
- Markkula Center for Applied Ethics
- Focus Areas
- Technology Ethics
- Technology Ethics Resources
Find case studies illustrating dilemmas in technology and ethics.
Case studies are also available on Internet Ethics .
For permission to reprint cases, submit requests to [email protected] .
Looking to draft your own case studies? This template provides the basics for writing ethics case studies in technology (though with some modification it could be used in other fields as well).
AI-generated text, voices, and images used for entertainment productions and impersonation raise ethical questions.
Ethical questions arise in interactions among students, instructors, administrators, and providers of AI tools.
Which stakeholders might benefit from a new age of VR “travel”? Which stakeholders might be harmed?
Case study on the history making GameStop short and stock price surge that occurred during January 2021.
As PunkSpider is pending re-release, ethical issues are considered about a tool that is able to spot and share vulnerabilities on the web, opening those results to the public.
With URVR recipients can capture and share 360 3D moments and live them out together.
VR rage rooms may provide therapeutic and inexpensive benefits while also raising ethical questions.
A VR dating app intended to help ease the stress and awkwardness of early dating in a safe and comfortable way.
Ethical questions pertaining to the use of proctoring software in online teaching.
Concern is high over possible misuses of new AI technology which generates text without human intervention.
- More pages:
- MEMBER DIRECTORY
- Member Login
- Publications
- Clinician Well-Being
- Culture of Health and Health Equity
- Fellowships and Leadership Programs
- Future of Nursing
- U.S. Health Policy and System Improvement
- Healthy Longevity
- Human Gene Editing
- U.S. Opioid Epidemic
- Staff Directory
- Opportunities
- Action Collaborative on Decarbonizing the U.S. Health Sector
- Climate Communities Network
- Communicating About Climate Change & Health
- Research and Innovation
- Culture of Health
- Fellowships
- Emerging Leaders in Health & Medicine
- Culture & Inclusiveness
- Digital Health
- Evidence Mobilization
- Value Incentives & Systems
- Substance Use & Opioid Crises
- Reproductive Health, Equity, & Society
- Credible Sources of Health Information
- Emerging Science, Technology, & Innovation
- Pandemic & Seasonal Influenza Vaccine Preparedness and Response
- Preventing Firearm-Related Injuries and Deaths
- Vital Directions for Health & Health Care
- NAM Perspectives
- All Publications
- Upcoming Events
- Past Events
- MEMBER HOME

Regenerative Medicine: Case Study for Understanding and Anticipating Emerging Science and Technology

Introduction
This case study was developed as one of a set of three studies, focusing on somewhat mature but rapidly evolving technologies. These case studies are intended to draw out lessons for the development of a cross-sectoral governance framework for emerging technologies in health and medicine. The focus of the case studies is the governance ecosystem in the United States, though where appropriate, the international landscape is included to provide context. Each of these case studies:
- describes how governance of the technology has developed within and across sectors and how it has succeeded, created challenges, or fallen down;
- outlines ethical, legal, and social issues that arise within and across sectors;
- considers a multitude of factors (market incentives, intellectual property, etc.) that shape the evolution of emerging technologies; and
- identifies key stakeholders.
Each case study begins with two short vignettes designed to highlight and make concrete a subset of the ethical issues raised by the case (see Box 1 and Box 2 ). These vignettes are not intended to be comprehensive but rather to provide a sense of the kinds of ethical issues being raised today by the technology in question.

The cases are structured by a set of guiding questions, outlined subsequently. These questions are followed by the historical context for the case to allow for clearer understanding of the trajectory and impact of the technology over time and the current status (status quo) of the technology. The bulk of the case consists of a cross-sectoral analysis organized according to the following sectors: academia, health care/nonprofit, government, private sector, and volunteer/consumer. Of note, no system of dividing up the world will be perfect—there will inevitably be overlap and imperfect fits. For example, “government” could be broken into many categories, including international, national, tribal, sovereign, regional, state, city, civilian, or military. The sectoral analysis is further organized into the following domains: science and technology, governance and enforcement, affordability and reimbursement, private companies, and social and ethical considerations. Following the cross-sectoral analysis is a broad, nonsectoral list of additional questions regarding the ethical and societal implications raised by the technology.
The next section of the case is designed to broaden the lens beyond the history and current status of the technology at the center of the case. The “Beyond” section highlights additional technologies in the broad area the focal technology occupies (e.g., neurotechnology), as well as facilitating technologies that can expand the capacity or reach of the focal technology. The “Visioning” section is designed to stretch the imagination to envision the future development of the technology (and society), highlighting potential hopes and fears for one possible evolutionary trajectory that a governance framework should take into account.
Finally, lessons learned from the case are identified—including both the core case and the visioning exercise. These lessons will be used, along with the cases themselves, to help inform the development of a cross-sectoral governance framework, intended to be shaped and guided by a set of overarching principles. This governance framework will be created by a committee of the National Academies of Sciences, Engineering, and Medicine (https://www.nationalacademies.org/our-work/creating-a-framework-for-emerging-science-technology-and-innovation-in-health-and-medicine).
Case Study: Regenerative Medicine
Regenerative medicine as a field is quite broad but is generally understood to focus on the regeneration, repair, and replacement of cells, tissues, and organs to restore function (Mason and Dunnill, 2008). The aspect of regenerative medicine on which this case study focuses relates to the ability to treat—or cure—genetic hematologic disease safely and effectively, and the significant trade-offs that come with these novel therapies.
The story of this therapy begins in the history of bone marrow transplants. The medicinal value of bone marrow has long been recognized and was first discussed in the 1890s as a potential treatment (administered orally) of “diseases believed to be characterized by defective hemogenesis” (Quine, 1896).
While allogeneic bone marrow transplant (in which stem cells from a donor are collected and transplanted into the recipient) may be the most broadly known form of hematopoietic stem and progenitor cell (HSPC) transplant, a range of other cell types are also used. HSPCs used in transplant can be either allogeneic (i.e., from a donor) or autologous (i.e., from the person who will also receive the transplant). The cells used in transplant research and clinical care can come from bone marrow, peripheral blood stem cells (PBSCs), umbilical cord blood, and pluripotent stem cell-derived cells.
A major challenge throughout the history of HSPC transplantation has been the dire risks associated with these transplants, including the morbidity and mortality caused by immunological reactions between the transplanted cells and the tissues of the recipient. In particular, graft-versus-host disease (GVHD) is a serious response in which the transplanted stem cells view the recipient’s tissues as foreign and mount an immune response, attacking the recipient’s body. If an autologous transplant is not possible given the nature of the disease to be treated, an immunologically well-matched healthy donor for allogeneic transplant is critical. For genetic hematologic disease, a new approach that would not only treat but cure the condition is now being tested: genetic modification of the patient’s own HSPCs to correct or compensate for the defect, followed by transplantation of the corrected autologous cells.
This challenge of matching transplantable cells to patients has driven evolution within the field of regenerative medicine, including logistical fixes in the form of HSPC registries and banks to technological approaches including the use of pluripotent stem cell-derived cell sources and genome editing (e.g., clustered regularly interspaced short palindromic repeats [CRISPR]).
This challenge of immunological matching has also driven significant ethical challenges, even beyond the substantial risks of HSPC transplantation itself. In contrast to many novel technologies, where finances are a primary barrier to access, in the case of regenerative medicine, there is the additional barrier of biology. People who are not of European descent have a lower probability of finding well-matched donors than do people of European descent. Furthermore, genetic hematologic diseases like sickle cell disease (SCD) and thalassemia, for which HSPC transplant is the only established cure (and a fraught one, at that), have struggled to garner the financial and grant support needed to move research forward. This challenge persists despite SCD being three times more prevalent in the United States than cystic fibrosis, which has historically benefited from generous public and private funding (Farooq et al., 2020; Wailoo and Pemberton, 2006). All of this stands on a background of long-understood barriers even to standard of care (e.g., adequate pain management) for individuals with SCD in particular (Haywood et al., 2009). Together, these facts raise concerns related to equity and access at multiple stages of research, development, and clinical care.
Finally, advances in this science have also attracted the attention of those who are willing to take advantage of patients under the guise of cutting-edge therapy, creating a robust market of direct-to-consumer (DTC) cell-based services and interventions that at best waste time and money and at worst cause serious harm or death (Bauer et al., 2018).
Guiding Questions
(derived from global neuroethics summit delegates, 2018; mathews, 2017).
The following guiding questions were used to frame and develop this case study.
- Historical context: What are the key scientific antecedents and ethics touchstones?
- Status quo: What are the key questions, research areas, and products/applications today?
- Cross-sectoral footprint: Which individuals, groups, and institutions have an interest or role in emerging biomedical technology?
- Ethical and societal implications: What is morally at stake? What are the sources of ethical controversy? Does this technology or application raise different and unique equity concerns?
Additional guiding questions to consider include the following:
- Key assumptions around technology: What are the key assumptions of both the scientists around the technology and the other stakeholders that may impede communication and understanding or illuminate attitudes?
- International context and relevant international comparisons: How are the technology and associated ethics and governance landscape evolving internationally?
- Legal and regulatory landscape: What are the laws and policies that currently apply, and what are the holes or challenges in current oversight?
- Social goals of the research: What are the goals that are oriented toward improving the human condition? Are there other goals?
Historical Context
What are the key scientific antecedents and ethics touchstones, hspc transplant.
HSPC transplant was initially only attempted in terminally ill patients (Thomas, 1999). The first recorded bone marrow transfusion was given to a 19-year-old woman with aplastic anemia in 1939 (Osgood et al., 1939). This was long before the Nuremburg Code, the Declaration of Helsinki, or the Belmont Report and anything like current understandings of informed consent (NCPHSBBR, 1979; Rickham, 1964; International Military Tribunal, 1949). There was also little understanding of the factors associated with graft failure—no attempts at bone marrow transfusions succeeded, and all patients died. Despite this early experience, the consequences of World War II, particularly the need to improve radiation and burn injury treatment, propelled this work forward (de la Morena and Gatti, 2010).
As human transplant work continued, experiments in mice and dogs in the 1950s and 1960s showed that after lethal radiation, these animals would recover if given autologous bone marrow. However, if given allogeneic marrow, the animal would reject the graft and die or accept the graft but then die from “wasting syndrome,” which later came to be understood as GVHD (Mannick et al., 1960; Billingham and Brent, 1959; Barnes et al., 1956; Rekers et al., 1950). It became clear that close immunologic matching between donor and recipient and management of GVHD in the recipient would be vital to the success of allogeneic bone marrow transplants (de la Morena and Gatti, 2010).
A 1970 accounting of the reported experience with HSPC transplants to date described approximately 200 allogeneic stem cell graft attempts (six involving fetal tissue) in subjects aged less than 1 to over 80 years, most of which had taken place between 1959 and 1962 (Bortin, 1970). (Of note, there were likely scores of unreported cases; in fact, the author ended the article with a call for reporting of all HSPC transplant attempts to the newly established American College of Surgeons-National Institutes of Health Organ Transplant Registry.) Of the reported cases (which often included the subjects’ initials), only 11 individuals were “unequivocal” allogeneic chimeras, and of those, only five were still alive at the time their case was reported. Many of the reported subjects died of opportunistic infections or GVHD, the noting of which often did not capture the true human toll of these deaths. For many years, even “success” (i.e., engraftment of the transplanted marrow) ended in death due to these other causes (Mathé et al., 1965; Thomas et al., 1959). As Donnall Thomas, a pioneer and leader in the field who won the Nobel Prize for “discoveries concerning organ and cell transplantation in the treatment of human disease” in 1990, reflected years later, “the experience with allogeneic transplants had been so dismal that questions were raised about whether or not such studies should be continued” (Thomas, 2005; Nobel Prize, 1990). In fact, the dismal experience with HSPC transplant eventually led most investigators to discontinue this work in humans, the focus returning for a time to animal studies (Little and Storb, 2002).
However, the discovery of human leukocyte antigen (HLA) in 1958 by Jean Dausset, which helps the immune system differentiate between what is “self” and what is foreign, and subsequent advances in the understanding of HLA matching and immunosuppression during the 1960s and 1970s led to a resumption of human clinical trials (Nobel Prize, 1980). In 1971, the first successful use of HSPC transplant to treat leukemia was reported (Granot and Storb, 2020). The following decades saw additional developments in HSPC transplant, improving the safety of the intervention, thus enabling its consideration for treatment of a broader array of blood diseases, including the hemoglobinopathies (Granot and Storb, 2020; Apperley, 1993).
The first use of HSPC transplant to cure thalassemia was in 1981, in a 16-month-old child, with an HLA-identical sibling donor—this patient was alive and thalassemia-free more than 20 years later (Bhatia and Walters, 2008; Thomas et al., 1982). Thalassemia major (the most serious form of the disease) requires chronic blood transfusion and chelation for life, a process which leads to gradual iron buildup and related organ damage, including heart failure, which is a common cause of death. Life expectancy for treated patients has increased substantially and varies by thalassemia type and treatment compliance, but patients can now live into their 40s and beyond (Pinto et al., 2019).
The first cure of SCD via HSPC transplant was incidental. An 8-year-old girl with acute myeloid leukemia (AML) was successfully treated for her leukemia with a bone marrow transplant, curing her SCD in the process (Johnson et al., 1984). By this time, life expectancy for an individual with SCD had improved substantially, reaching the mid-20s due to advances in understanding and treatment of the disease (particularly the use of antibiotics to manage the frequent infections that plagued those with the disease) (Wailoo, 2017; Prabhakar et al., 2010). The first five patients, all children, in whom HSPC transplants were used intentionally to treat SCD were reported in 1988 (Vermylen et al., 1988). As Vermylen and colleagues reported, “In all cases there was complete cessation of vaso-occlusive episodes and haemolysis” (Vermylen et al., 1988).
Around this same time, there were also advancements in the sources of transplantable hematopoietic cells, expanding beyond bone marrow to include peripheral blood stem cells and umbilical cord blood (Gluckman et al., 1989; Kessinger et al., 1988). Cord blood was particularly appealing for a number of reasons, including that it is less immunogenic than the other cell sources, reducing the risk of GVHD.
The development of cord blood transplant has a very different origin story to that of bone marrow, beginning with a hypothesis and the founding of a company (Ballen et al., 2013). The company, Biocyte Corporation (later PharmaStem Therapeutics), funded the early work and held two short-lived patents over the isolation, preservation, and culture of umbilical cord blood (Shyntum and Kalkreuter, 2009). The longevity of the science has thankfully surpassed that of the company that launched it. The first cord blood–based HSPC transplant was conducted with the approval of the relevant institutional review boards (IRBs) and the French National Ethics Committee, to treat a 5-year-old boy with Fanconi anemia using cells from the birth of an unaffected, HLA-matched sister (Ballen et al., 2013; Gluckman et al., 1989). The success of the early cases (the 5-year-old boy was still alive and well 25 years later) led to the use of unrelated cord blood transplant and expansion of use beyond malignant disease (Ballen et al., 2013; Kurtzberg et al., 1996). Benefits of cord blood include noninvasive collection, ability to cryopreserve characterized tissue for ready use, reduced likelihood of transmitting infections, and lower immunogenicity relative to bone marrow, enabling imperfect HLA matching and expanding access, in particular for people not of European descent (Barker et al., 2010; Gluckman et al., 1997). Cord blood HSPC transplant was first used primarily in children, because it was thought that the relatively low number of cells in a cord blood unit would limit its use in adults, but over time, as techniques and supportive care have improved, so has success of cord blood transplant in adults (Eapen et al., 2010; Ballen et al., 2007). Today, cord blood is widely used for HSPC transplants in both children and adults, with outcomes as good as or better than with bone marrow. Despite these advancements, however, allogeneic HSPC transplant continued to depend on the availability of HLA-matched donors.
Public HSPC Banks
Unfortunately, only about 35 percent of patients have HLA-matched siblings, so patients have needed to look beyond their immediate family for matched donors. This need led to the creation of HLA-typed donor registries, starting with the founding of the Europdonor registry in the Netherlands in 1970 and the International Blood and Marrow Transplant Registry at the Medical College of Wisconsin in 1972 (McCann and Gale, 2018). In 1986, the National Marrow Donor Program (NMDP), which operates the Be the Match registry, was founded by the U.S. Navy. Other registries in the United States and Europe followed, and by 1988, there were eight active registries around the world with more than 150,000 donors (van Rood and Oudshoorn, 2008). The Bone Marrow Donors Worldwide network, which connected these registries, was formed in 1988 to facilitate the identification of potential donors, and in 2017 its activities were taken over by the World Marrow Donor Association (WMDA) (Oudshoorn et al., 1994). Today, the combined registry includes more than 37,600,000 donors and more than 800,000 cord blood units from 54 different countries (see Figure 1 ) (WMDA, 2021; Petersdorf, 2010).

However, even with tremendous global collaboration to identify and make available donor information, access is not equal. The NMDP estimates suggest that while approximately 90 percent of people of European descent will identify a well-matched unrelated marrow donor, the same will be true for only about 70 percent of people of Asian or Hispanic descent and 60 percent of those of African descent (Pidala et al., 2013). Causes for this disparity include higher HLA diversity among these populations compared to those of European descent and smaller numbers of racial and ethnic minority volunteers in donor registries and ultimately available for transplant (Sacchi et al., 2008; Kollman et al., 2004).
Private HSPC Banks
Alongside the public registries, trading on the success of cord blood HSPC transplants and playing on the fears of new parents, a thriving market of private cord blood banks has developed (Murdoch et al., 2020). These for-profit private banks market their services—collecting and storing cord blood for potential future personal use—as insurance policies for the health of one’s newborn, without much data to support the claim. While donation of cord blood to a public bank is free to the donor, costs associated with private banking include a collection fee (US$1,350–$2,300) and annual storage fees ($100–$175/year), which are unlikely to be covered by health insurance (Shearer et al., 2017). At the same time, public banks are held to transparent, rigorous storage and quality standards that do not apply to private banks, leading to lower overall quality of cord blood in private banks (Shearer et al., 2017; Sun et al., 2010; Committee on Obstetric Practice, 2008). Finally, cord blood stored in public banks is 30 times more likely to be accessed for clinical use than samples stored in private banks, and there is broad professional consensus, and associated professional guidance, that public banking is preferable to private banking (Shearer et al., 2017; Ballen et al., 2015). Despite these differences, in 2017, there were about 800,000 cord blood units in public banks, compared with more than 5 million in private banks (Kurtzberg, 2017).
New HSPC Sources
While adult stem cell sources (bone marrow, peripheral blood, and cord blood) have dominated research and clinical care for many decades, in the late 1990s and mid-2000s, new tools were added in the form of several pluripotent stem cell types, including embryonic stem cells, embryonic germ cells, nuclear transfer (NT)-derived stem cells, and most recently, induced pluripotent stem cells (iPSCs) (Tachibana et al., 2013; Yu et al., 2007; Takahashi and Yamanaka, 2006; Shamblott et al., 1998; Thomson et al., 1998). In contrast to the previous cell sources, which are restricted to repopulating blood cell types, these new pluripotent stem cells can turn into any of the approximately 220 cell types in the human body and have a correspondingly diverse array of potential applications. For the purposes of this case, the authors focus on the use of these cells in hematologic disease, but understanding some of the history of the development and use of these cells is helpful for the broader goals of the case. Importantly, these new cell types emerged in a very different regulatory and societal environment than the environment in which bone marrow transplants were first being developed.
The first derivations of human embryonic stem cells (ESCs) and embryonic germ cells (EGCs) were published in 1998 (Shamblott et al., 1998; Thomson et al., 1998). Both of these seminal papers concluded with discussion of the potential for the use of these cells in transplantation-based treatments and cures and emphasized the need to address the challenge of immune rejection, either through the development of cell banks, akin to the registries described previously, or through the genetic modification of the cells to create universal donor cells or to match the particular cellular therapy to the particular patient.
Unlike bone marrow or cord blood, however, the source of these cells was human embryos and fetal tissue, and at the time of these publications, there was already a notable history of governance of these tissues (Matthews and Yang, 2019; Green, 1995; NIH, 1994). In addition, the Dickey-Wicker Amendment had been in place for 3 years, prohibiting the use of federal funds to create human embryos for research or to conduct research in which human embryos are “destroyed, discarded, or knowingly subjected to risk of injury or death” (104th Congress, 1995). Within weeks of the papers’ publication, a legal opinion was issued from the Department of Health and Human Services (HHS) interpreting Dickey-Wicker with regard to the new research (Rabb, 1995). Though federal dollars could not be used to create ESCs or EGCs, it was determined that federal dollars could be used to conduct research with pluripotent stem cells thus derived. This interpretation was supported later that year by a report of the National Bioethics Advisory Commission (NBCA, 1999). This did not, however, settle the issue.
A year later, President George W. Bush was elected following a campaign in which he made clear his opposition to this research (Cimons, 2001). In August 2001, in his first address to the nation, President Bush announced that federal funding would be permitted for research using the approximately 60 ESC lines already in existence at the time of his announcement, but not for research with newly derived lines (CNN, 2001). The president seemed to be attempting to walk a fine line between allowing promising research to move forward and not causing the federal government (and taxpayers) to be complicit in the destruction of human embryos. Ultimately, many of these 60 approved “Bush lines” proved impossible to access or difficult to work with. Furthermore, the accounting required in institutions and laboratories working with both “Bush lines” and newer lines was daunting (Murugan, 2009).
As ethical and policy debates raged, states began passing their own legislation governing human ESC research, beginning with California, and creating over time a patchwork of state-level policy that ranged from providing government funding for ESC research, as in California, to classifying the work as a felony, such as in Arizona (CIRM.ca.gov, n.d.; Justia US Law, 2020). In 2005, Congress passed its own bill that would permit federal funding of research with an expanded number of human ESC lines, but the bill was subsequently vetoed by President Bush (109th Congress, 2005). The same year, the National Research Council and the Institute of Medicine published its tremendously influential report titled Guidelines for Human Embryonic Stem Cell Research (IOM and NRC, 2005). These guidelines led to highly effective self-regulation in the field, as the Guidelines were adopted across the United States at institutions conducting human ESC research (Robertson, 2010). The Guidelines recommended the creation of a new institutional oversight committee to review ESC research, similar to IRBs, among other recommendations. The Guidelines remained the primary source of governance for ESC research through the end of the Bush administration.
An additional scientific innovation during this time was the announcement of the creation of iPSCs in 2006 (Nobel Prize, 2012; Takahashi and Yamanaka, 2006). iPSCs are derived from somatic tissue, not embryonic or fetal tissue, through the introduction of a small set of transcription factors that effectively reset the mature cell back to a pluripotent state. This concept had actually been introduced as an alternative to ESCs by President Bush’s bioethics commission, though it had been met with skepticism, and Shinya Yamanaka’s announcement at the 2006 International Society for Stem Cell Research (ISSCR) annual meeting stunned the assembled scientists (Scudellari, 2016; The President’s Council on Bioethics, 2005). This scientific end-run around the destruction of human embryos led to a flood of new researchers, as scientists now needed only somatic cells, rather than highly regulated embryonic or fetal tissue, to participate in this new wave of regenerative medicine research.
By the end of President Bush’s second term, in addition to the National Academies’ Guidelines, guidelines were also issued from the ISSCR and a number of other academic groups (ISSCR, n.d.; The Hinxton Group, 2006). Internationally, as in the United States, a patchwork of policy responses had emerged, ranging from very restrictive to permissive to supportive, leading both domestically and internationally to a degree of “brain drain” as some scientists relocated to jurisdictions that permitted this research (Verginer and Riccaboni, 2021; Levine, 2012).
When President Barack Obama took office in 2009, he issued an Executive Order reversing former president Bush’s prior actions (White House, 2009). Rather than establishing the final rules himself, he permitted funding of ESC research “to the extent permitted by law” (a nod to the Dickey-Wicker Amendment) and charged the National Institutes of Health (NIH) with developing guidelines for such funding. The NIH guidelines, which largely followed the Guidelines, were finalized in July 2009 and were promptly tied up in a years-long battle in the courts until the Supreme Court declined to hear the final appeal in 2013, leaving the NIH guidelines intact (NIH, 2013, 2009).
Genetic Modification
The final piece of the regenerative medicine puzzle is the need to overcome immune rejection of transplanted cells. As noted in the initial HPSC papers, potential ways to overcome immune rejection (in the absence of iPSCs) included both banking of a large number of diverse cell lines and genetic modification of the cells intended for transplant, although at the time the technology to do so did not exist (Faden et al., 2003). Gene therapy of this sort had been contemplated for years, and gene transfer trials had begun in the 1990s using the tools scientists had at the time (IOM, 2014). Governance structures grew up around these trials, including the transition of the Recombinant DNA Advisory Committee (RAC) from reviewing NIH-funded research involving recombinant DNA (rDNA) to reviewing gene transfer protocols (IOM, 2014). Of note, though the RAC served as a model internationally for the governance of rDNA research, its mandate was repeatedly questioned and its work critiqued, even as its role evolved (IOM, 2014). As the pace and volume of gene transfer research picked up, the pace of review slowed. Responding not only to the resulting critiques but also the accumulated experience and data, the RAC relaxed restrictions and expedited reviews where possible, ultimately pivoting again to a focus on novel protocols, and leaving more straightforward protocols to the U.S. Food and Drug Administration (FDA) to approve or deny (IOM, 2014). But the original vision of genetically tailored cellular therapy articulated in the 1998 papers did not become possible until almost 15 years later.
In 2012, the publication of the paper that introduced clustered regularly interspaced short palindromic repeats-CRISPR associated protein 9 (CRISPR-Cas9) launched a new era of genetic modification (Jinek et al., 2012). This new tool dramatically improved upon prior gene editing tools with respect to technical ease, speed, and cost, putting the kind of editing imagined in the 1998 papers within reach.
What are the key questions, research areas, and products or applications today?
Hspc transplant access.
Today, median health care costs for HSPC (including the procedure and 3 months of follow-up) in the United States are approximately $140,000–$290,000, depending on the type of procedure (Broder et al., 2017). While 200-day nonremission mortality has decreased substantially since 2000, it remains high (11%) (McDonald et al., 2020). The risks of transplant remain a significant barrier to access, in particular for those with nonmalignant disease, such as SCD. Beyond this, and as noted previously, there are significant ethnic and racial disparities in access to HSPC transplant, largely due to the relatively lower probability of identifying a well-matched HSPC donor (Barker et al., 2019). A recent study demonstrated that while White patients of European descent have a 75 percent chance of finding a well-matched (8/8 HLA-matched) donor, for White Americans of Middle Eastern or North African descent, the probability is 46 percent (Gragert et al., 2014). For Hispanic, Asian, Pacific Islander, and Native American individuals, the probability of such a match ranges from 27 to 52 percent, and for Black Americans, the probability is 16–19 percent (Gragert et al., 2014). Contributing to these disparities for racial and ethnic minority groups are higher HLA diversity, smaller numbers of racial and ethnic minority volunteers in donor registries, and the higher rates at which matched minority volunteers become unavailable for donation (e.g., due to inability to reach the volunteer or medical deferral due to diabetes, asthma, infectious disease, or other identified condition) (Sacchi et al., 2008; Kollman et al., 2004). Giving preference to 8/8 HLA-matched pairs therefore benefits White patients and disadvantages patients of color, but removing this preference might result in higher rates of graft failure. Attempts to balance these competing considerations raise ethical questions about justice and beneficence.
Another ethical question in HSPC transplantation revolves around compensation or incentives for donation. Increasing the number and availability of HSCP donors would improve the probability of identifying an appropriate unrelated match for patients in need of a transplant, but the 1984 National Organ Transplant Act (NOTA) banned the sale of bone marrow and organs, making the provision of financial incentives to donate illegal (98th Congress, 1983). Nonetheless, debates over the ethics of providing incentives to encourage the donation of bone marrow and HSCs persist among bioethicists and health economists. In an effort to reduce disincentives to donate, the federal government offers up to 1 work week of leave for federal employees who donate bone marrow, and most states have followed suit for state employees (Lacetera et al., 2014). Some states also offer tax deductions for nonmedical donation-related costs, and there is some evidence that these types of legislation do lead to modest increases in donation rates (Lacetera et al., 2014).
Although removing disincentives to donation is generally considered ethically acceptable, there is more debate about whether offering financial incentives for donation equates to a morally problematic commodification of the human body. In 2011, the 9th Circuit held in Flynn v. Holder that compensation for the collection of PBSCs does not violate NOTA’s ban on compensation (Cohen, 2012). In response, a coalition of cell therapy organizations published a statement arguing that this decision would mean that donors would no longer be motivated by altruism, and that people seeking to sell PBSCs might withhold important health information (Be the Match, 2012). After a regulatory back-and-forth over the status of PBSCs, HHS withdrew a proposed rule that would have effectively reversed Flynn v. Holder, so the current state of the law allows compensation for PBSCs (Todd, 2017).
Genetic Hematologic Disease: The Case of Sickle Cell Disease
Although Linus Pauling declared sickle cell disease (SCD) to be the first “molecular disease” (i.e., the first disease understood at the molecular level) in 1949, and it has long been considered an ideal target for gene therapy given that it is predominantly caused by a single mutation in the HBB gene and its phenotypic consequences are in a circulating cell type, developing a cure has not been as straightforward as hoped (Pauling et al., 1949). Though the presentation of SCD can vary significantly, clinical effects include anemia, painful vaso-occlusive crises, acute chest syndrome, splenic sequestration, stroke, chronic pulmonary and renal dysfunction, growth retardation, and premature death (OMIM, n.d.a.).
Standard treatment for SCD consists primarily of preventative and supportive care, including prophylactic penicillin, opioids for severe chronic pain, hydroxyurea, and transfusion therapy (Yawn et al., 2014). Such care has dramatically increased the life expectancy of those living with SCD (median survival in the United States is in the mid- to late 40s) (Wailoo, 2017; Ballas et al., 2016; Prabhakar et al., 2010). At the same time, this care costs more than $35,000 annually, and many patients have difficulty accessing such high-quality care, particularly adequate pain management (Bergman and Diamond, 2013; Haywood, 2013; Haywood et al., 2009; Kauf et al., 2009; Smith et al., 2006). Until recently, the only evidence-based cure for SCD and beta-thalassemia major was allogeneic hematopoietic cell transplantation (HCT), which comes with significant costs and risks (Bhatia and Walters, 2008).
Despite the fact that SCD is one of the most common genetic diseases worldwide and it was the first genetic disease to be molecularly defined, it has received relatively little research funding over the years, an observation that has been a frequent subject of critique (Farooq et al., 2020; Demirci et al., 2019; Benjamin, 2011; Smith et al., 2006; Scott, 1970). In contrast to better-funded diseases, such as cystic fibrosis and Duchenne muscular dystrophy, which are more common in White individuals of European descent, in the United States, SCD predominantly affects non-Hispanic Black and Hispanic populations, including 1 in 365 Black individuals and 1 in 16,300 Hispanic individuals (OMIM, n.d.b., n.d.c.; CDC, 2022). This disparity in research funding despite disease prevalence is part of the larger story of the impacts of structural racism in the United States and on its medical system (The New York Times, 2019; IOM, 2003; HHS and AHRQ, 2003).
Furthermore, as noted previously, those of African and Hispanic ancestry are less likely to be able to identify a suitable match in the existing registries. Due to this difficulty, the improvements in treatment not focused on an HSPC transplant, and the risks of such a transplant, relatively few patients with SCD are treated with HSPC transplant (Yawn et al., 2014; Benjamin, 2011). Gene therapy delivered in the context of an autologous HSPC transplant offers the possibility not only of a safer cure but also broader access by eliminating the need to identify a matched donor.
Recently, the promise of regenerative medicine and gene therapy for genetic hematologic disease appears to be coming to fruition (Ledford, 2020; Stein, 2020; Kolata, 2019). While a number of approaches are currently in various stages of preclinical and clinical research, two promising clinical trials involve the induction of fetal hemoglobin (rather than direct correction of the disease-causing mutation in the HBB gene) (Demirci et al., 2019). Fetal hemoglobin is the predominant globin type in the second and third trimester fetus and for the first few months of life, at which point production shifts from fetal to adult hemoglobin. It has long been recognized that SCD does not present until after this shift occurs (Watson et al., 1948). Furthermore, some patients with the causative SCD mutation are nonetheless asymptomatic, due to also having inherited hereditary persistence of fetal hemoglobin mutations (Stamatoyannopoulos et al., 1975). These findings and others suggested that inducing fetal hemoglobin, even in the presence of a faulty HBB gene, could mitigate the disease.
The first trial uses a viral vector to introduce into autologous bone marrow a short hairpin RNA (shRNA) that inhibits the action of the BCL11A gene. BCL11A is an inhibitor of fetal hemoglobin, so when BCL11A is inhibited, fetal hemoglobin can be produced (Esrick et al., 2021). The second trial—the first published study to use CRISPR to treat a genetic disease—includes both patients with SCD and with transfusion-dependent ß-thalassemia (Frangoul et al., 2021). In this trial, CRISPR-Cas9 is used to target the BCL11A gene to affect the same de-repression of fetal hemoglobin as in the first trial. Both trials, which have collectively enrolled more than 15 patients, have reduced or eliminated the clinical manifestation of disease in all patients thus far, though it remains to be seen how long-lasting this effect will be. However, the first trial was recently suspended after participants in the first trial and a related trial developed acute myeloid leukemia (AML) or myelodysplastic syndrome (MDS) (Liu, 2021); an investigation is under way regarding the cause of the AML and MDS. Marketing of a treatment for transfusion-dependent ß -thalassemia currently approved and available in the European Union (EU) was also suspended, as that treatment is manufactured using the same vector (BB305 lentiviral vector) used in the current trials, and it is possible that the vector is the source of the serious adverse events in the research participants.
Further challenges remain, including technical challenges, such as the possibility that gene editing tools, as they are derived from bacterial systems, will provoke an immune response; and concerns about financial access, given the anticipated cost of such curative therapies (ICER, 2021; Kim et al., 2018). In addition, despite the technical ease of the technology and designing new nucleic acid targets, intellectual property protecting CRISPR has, to date, narrowed the number of developers actively pursuing CRISPR-based clinical trials (Sherkow, 2017). At the same time, this new technology might also solve a number of ethical issues around HSPC transplants, including by expanding biological access to HSPC transplant and mitigating the concerns raised by the creation of “savior siblings” for HLA-matched cord blood transplantation for older siblings (Kahn and Mastroianni, 2004).
Unproven Cell-Based Interventions
A long-standing challenge in the field of regenerative medicine is the DTC marketing of unproven cell-based interventions. Since at least the 2000s, unscrupulous scientists and health professionals in the United States and internationally have been offering “stem cell therapy” at significant cost, often to vulnerable individuals, and without a legitimate scientific or medical basis (Knoepfler and Turner, 2018; Murdoch et al., 2018; Regenberg et al., 2009; Enserink, 2006). From 2009 to 2016, the number of such clinics in the United States doubled annually (Knoepfler and Turner, 2018). While the clinics look legitimate, their claims are fantastical, promising to treat or cure everything from knee pain to Parkinson’s disease. Such clinics are often vague about the cell sources involved in the interventions offered, but sometimes they claim to use bone marrow, cord blood, embryonic stem cells, and iPSCs, as well as other types of autologous adult stem cells (e.g., adipose, olfactory) and a range of other cell types, cell sources, and cell mixtures (Murdoch et al., 2018). While such interventions launch from legitimate science and scientific potential, the claims exceed and diverge from what is proven. The interventions are at best very expensive placebos and at worst could cause serious harm or death (Bauer et al., 2018).
Over time, attempts have been made to rein in these clinics by the FDA, the Federal Trade Commission (FTC), the ISSCR, individual customers and their lawyers, and others, but these attempts have faced a number of challenges (Pearce, 2020). The ISSCR, the primary professional society for those engaged in regenerative medicine, has struggled for years against such clinics. Early on, they attempted to establish a mechanism to publicly vet these clinics, though the effort was abandoned in part due to push back from the clinics’ lawyers (Taylor et al., 2010; personal communication from ISSCR Leadership, n.d.). In part because the majority of US-based clinics offer autologous interventions (removing and then reintroducing the patient’s own cells), the FDA struggled to clarify the line between medical practice and their regulatory authority. The FDA began issuing occasional warning letters to these clinics starting in 2011, though the letters were issued infrequently (Knoepfler and Turner, 2018). Under this relatively weak enforcement, the market expanded dramatically, and pressure increased on the FDA to take meaningful action (Knoepfler, 2018; Turner and Knoepfler, 2016).
In late 2017, the FDA took several significant steps to curtail these clinics, including using U.S. marshals to seize product from a California clinic, bringing a lawsuit against a Florida clinic, and publishing largely celebrated finalized guidance outlining a risk-based approach to the regulation of regenerative medicine products (FDA, 2019; Pew Research Center, 2019). The following year, the FTC took independent action against clinics making false claims about their interventions, and Google banned advertising for “unproven or experimental medical techniques such as most stem cell therapy, cellular (non-stem) therapy, and gene therapy” (Biddings, 2019; Fair, 2018). In 2019, the FDA won their case against US Stem Cells in Florida, significantly strengthening their ability to regulate these clinics (Wan and McGinley, 2019). Following the establishment of clear regulatory authority over at least a subset of clinics, FDA has begun to step up its enforcement (Knoepfler, 2020; Wan and McGinley, 2019; FDA, 2018). Increased action is anticipated following the end of the 3-year grace period established in the 2017 guidance, though there is some concern about the capacity of the agency to make significant headway against the more than 600 clinics now in operation—a worry bolstered by a 2019 study suggesting that despite increased enforcement, the unproven stem cell market seems to have shifted rather than contracted (Knoepfler, 2019; Pew Research Center, 2019). What seems clear is that it will take a collective and multipronged approach to ensure that the cell-based interventions to which patients have access are safe and effective (Lomax et al., 2020; Pew Research Center, 2019; Master et al., 2017; Zarzeczny et al., 2014).
Cross-Sectoral Footprint
The cross-sectoral analysis is structured according to sectors (see Figure 2 ) and domains (science and technology, governance and enforcement, end-user affordability and insurance reimbursement [affordability and reimbursement], private companies, and social and ethical considerations). The sectors described subsequently are intended to be sufficiently broad to encompass a number of individuals, groups, and institutions that have an interest or role in regenerative medicine. Health care is the primary nonprofit actor of interest, and so in this structure, “health care” has replaced “nonprofit,” though other nonprofit actors may have a role in this and other emerging technologies, and, of course, not all health care institutions are nonprofits.

Today, many regenerative medicine technologies are researched, developed, and promoted by a scientific-industrial complex largely driven by market-oriented goals. The development of various components of regenerative medicine may be altered by differing intellectual property regimes. This larger ecosystem is also embedded in a broad geopolitical context, in which the political and the economic are deeply intertwined, shaping national and regional investment and regulation. The political economy of emerging technologies involves and affects not only global markets and regulatory systems across different levels of government but also nonstate actors and international governance bodies. Individuals and societies subsequently adopt emerging technologies, adjusting their own values, attitudes, and norms as necessary, even as these technologies begin to shape the environments where they are deployed or adopted. Furthermore, individual and collective interests may change as the “hype cycle” of an emerging technology evolves (Gartner, 2022). Stakeholders in this process may include scientific and technological researchers, business firms and industry associations, government officials, civil society groups, worker safety groups, privacy advocates, and environmental protection groups, as well as economic and social justice–focused stakeholders (Marchant et al., 2014).
This intricate ecosystem of stakeholders and interests may be further complicated by the simultaneous introduction of other technologies and platforms with different constellations of ethical issues, modes of governance, and political economy contexts. In the following sections, this ecosystem is disaggregated and organized for ease of presentation. It is important to keep in mind that there are entanglements and feedback loops between and among the different sectors, such that pulling on a single thread in one sector often affects multiple areas and actors across the broader ecosystem.
Cross-Sectoral Analysis
For the purposes of this case study, the primary actors within the academic sector are academic and clinical researchers and the professional societies that represent them.
Science and technology: This case involves a tremendous amount of research and development that has taken place in and grown out of academia, including preclinical and clinical HSPC transplant research; human ESC, EGC, and iPSC research; and genome editing.
Governance and enforcement: Current work at research institutions is governed by IRBs and REBs, stem cell research oversight committees, and institutional animal care and use committees, among other bodies. In addition, research funding bodies, academic publication standards, and scientific and professional societies (i.e., self-regulation) also have a role to play—in particular, the ISSCR and its role in the governance of pluripotent stem cell research and in addressing clinics offering unproven cell-based therapies. The National Academies of Sciences, Engineering, and Medicine played a critical role in the governance of ESC research, particularly from 2005 until 2010.
Affordability and reimbursement: While not strictly a matter of patient affordability, it is important to reiterate, as noted previously, that funding available for academic research has disproportionately benefited those with diseases such as cystic fibrosis and Duchenne muscular dystrophy, which are more common in White individuals of European descent, compared to SCD, which in the United States is more prevalent among non-Hispanic Black and Hispanic populations (Farooq et al., 2020; Demirci et al., 2019; Benjamin, 2011; Smith et al., 2006; Scott, 1970).
Private companies: Academic–industry research partnerships, including industry-funded clinical trials, are involved in this space; for example, the CRISPR-based clinical trial was funded by two biotechnology companies (Frangoul et al., 2021). Such partnerships are often predicated on exclusive intellectual property licenses to “surrogate licensors” (Contreras and Sherkow, 2017).
Social and ethical considerations: Extensive bioethics literature exists on the ethical, legal, and societal issues raised by human subjects research, first-in-human clinical trials, stem cell research, clinics offering unproven cell-based interventions, genome editing, health disparities, and structural racism. Much has also been written on the role of intellectual property and data and materials sharing in the context of human tissue research and genome editing.
Health Care
Given the focus of CESTI on health and medicine, for the purpose of this case study, the primary actors within the nonprofit sector are those involved in health care, including hematopoietic stem and progenitor cell registries, health insurance companies, and medical profession associations.
Science and technology: HSPC transplants have been clinically available for decades, but research and improvement in this space continue.
Governance and enforcement: Today, the WMDA serves as the accrediting body for registries and promulgates regulations and standards to which the registries adhere on issues like the organization of a registry, the recruitment of volunteer donors, and the collection and transportation of HPCs (WMDA, 2022; Hurley et al., 2010). These standards represent the minimum guidelines for registries, which “demonstrate their commitment to comply with WMDA Standards through the WMDA accreditation process” (Hurley et al., 2010). Other groups involved in the governance of aspects of HSPC transplant are included in Table 1.

It is important to note that the “nonprofit” label in this context is somewhat fraught. Many (perhaps most) health care organizations are very much in the business of making money. One of these is the NMDP, which operates Be the Match, and which has diversified its portfolio over time, including the launch in 2016 of Be the Match BioTherapies, which partners with dozens of cell and gene therapy companies, supplying cells and services to “advance the development of life-saving cell and gene therapies” (Be the Match, 2021a,b).
The FDA generally has authority to regulate bone marrow transplantation through its oversight of bone marrow itself as a human cellular tissue product (HCT/P) and, therefore, a “biologic” (U.S. Code § 262, n.d.). Typically, biologic products are required to submit to the FDA’s premarket review process, including the filing of an investigative new drug application and clinical trials. With that said, the FDA has exempted certain types of bone marrow transplantation procedures from such review: namely, bone marrow products that are used in a same-day surgical procedure and those that are only “minimally manipulated” (FDA, 2020). Importantly, while the FDA’s minimally manipulated exception broadly applies to autologous therapy, including the sort of therapy private cord blood banks are intended to plan for, it only applies to allogenic therapy if derived from a “first-degree or second-degree blood relative”; allogenic therapy using cells from more distant relatives requires the FDA’s premarket review (FDA, 2020).
Cord blood matching and donor priority is controlled by the NMDP and regulated by the FDA (CFR, 2012). However, because cord blood therapy is almost always allogenic and usually from anonymized donors unrelated to the patient, cord blood HSPC transplant generally does not fulfill the FDA’s “minimal manipulation” exemptions for HCT/P (FDA, 2020). As such, a total of eight public cord blood banks have applied for, and received, approval from the FDA for their cord blood products (FDA, 2022). Generally, public banks are held to transparent, rigorous storage and quality standards that do not apply to private banks, leading to lower overall quality of cord blood in private banks (Shearer et al., 2017; Sun et al., 2010; Committee on Obstetric Practice, 2008).
The American Academy of Pediatrics has taken a position on private versus public cord blood banks and supports public banking, as do the American Medical Association and the American Congress of Obstetricians and Gynecologists (AMA, n.d.; ACOG, 2019; Shearer et al., 2017).
Affordability and reimbursement: Both public and private insurers in the United States tend to distinguish autologous from allogenic bone marrow therapies, covering autologous transplantation for some indications and allogenic transplantation for others (CMS, 2016).
Leaving aside the broader issues of health insurance and health care affordability in the United States, annual and lifelong care costs for genetic hematologic diseases like SCD and thalassemia are considerable—the yearly cost of standard of care for a patient with SCD is more than $35,000 (Kauf et al., 2009). Novel therapies—both pharmacologic and those based on HSPC transplants—are anticipated to be extraordinarily expensive, if proven safe and effective. For example, the drugs Oxbryta and Adakveo, approved in 2019 for treating SCD, are estimated to cost $84,000 and $88,000 per year, respectively (ICER, 2021; Sagonowsky, 2020). CART-T cell therapy, which as another novel, genetically modified cell-based therapy may be a reasonable bellwether for the cost of the SCD therapies described previously, costs at least $373,000 for a single infusion before hospital and other associated costs (Beasley, 2019). Many patients suffering from these diseases are from historically marginalized and underserved populations that tend to have lower levels of income. In addition, as therapies become more bespoke, scaling will increasingly become a challenge, from both a regulatory and delivery perspective. However, these delivery challenges may also open new business opportunities.
While donation of cord blood to a public bank is free to the donor, costs associated with private banking include a collection fee ($1,350–$2,300) and annual storage fees ($100–$175 a year), which are unlikely to be covered by health insurance (Shearer et al., 2017).
Private companies: Many private companies advertise private cord blood banking to new parents as a form of biological insurance; however, the costs of collection and storage are not generally covered by medical insurance (private companies offering unproven cell-based interventions are included under the private sector rather than health care).
Social and ethical considerations: Significant literature exists on health disparities and racism in medicine, including their impact on patients with SCD in particular. As noted previously, the likely high costs of these therapies raise serious concerns about access. There is also literature on ethical issues raised by the private cord blood market.
Private Sector
For the purposes of this case study, the primary actors within the private sector are companies involved in basic and translational regenerative medicine research and clinics offering unproven cell-based interventions.
Science and technology: Many private biotechnology companies are involved in regenerative medicine and genome editing research and development. A recent analysis predicted that the global CRISPR genome editing market (including CRISPR products, applications, and end-users) could grow from about $850 million in 2019 to $10 billion by 2030 (BIS Research, n.d.). In the United States, there are more than 600 clinics offering unproven cell-based interventions.
Governance and enforcement: The ISSCR attempted to establish a mechanism to publicly vet clinics selling unproven cell-based interventions, though the effort was ended in part due to push back from the clinics’ lawyers (personal communication from ISSCR leadership, n.d.; Taylor et al., 2010). In part because the majority of U.S. clinics offer autologous interventions (removing and then reintroducing the patient’s own cells), the FDA has struggled to clarify the line between medical practice and their regulatory authority in this space. Under relatively weak enforcement, the market expanded dramatically.
In late 2017, the FDA took several significant steps to curtail these clinics, including using U.S. marshals to seize product from a California clinic, bringing a lawsuit against a Florida clinic, and publishing largely celebrated finalized guidance that outlined a risk-based approach to the regulation of regenerative medicine products (FDA, 2019; Pew Research Center, 2019). The following year, the FTC took independent action against clinics making false claims about their interventions, and Google banned advertising for “unproven or experimental medical techniques such as most stem cell therapy, cellular (non-stem) therapy, and gene therapy” (Biddings, 2019; Fair, 2018). In 2019, the FDA won their case against U.S. stem cells in Florida, significantly strengthening their ability to regulate these clinics (Wan and McGinley, 2019). Following the establishment of clear regulatory authority over at least a subset of clinics, the FDA has begun to step up its enforcement (Knoepfler, 2020; Wan and McGinley, 2019; FDA, 2018).
Affordability and reimbursement: Unproven cell-based interventions can cost anywhere from several thousand dollars to tens of thousands of dollars (Regenberg et al., 2009). These costs are not covered by insurance. Patients have engaged in public fundraising campaigns, including on crowdfunding sites, to raise the money necessary to access the unproven intervention.
Private companies: There are far too many companies offering unproven cell-based interventions to list, though a recent accounting can be found in a supplemental table to Turner and Knoepfler, 2016.
Social and ethical considerations: Many have written about the ethical and policy issues raised by DTC unproven cell-based interventions and private cord blood banks, including issues related to truth-telling, taking advantage of historically marginalized and underserved individuals, and significant financial costs and physical risk in the absence of demonstrable benefit, among other issues.
For the purposes of this case study, the primary actors within the government sector are the FDA, the FTC, the NIH, and other regulatory bodies.
Science and technology: The federal government, and especially the NIH, has funded a tremendous amount of the research outlined in this case and is a critical part of the biotechnology research and development ecosystem.
Governance and enforcement: NOTA banned the sale of bone marrow and organs (98th Congress, 1983). Nonetheless, debates over the ethics of providing incentives to encourage the donation of bone marrow and HSCs persist among bioethicists and health economists. In an effort to reduce disincentives to donate, the federal government offers up to 1 week of leave for federal employees who donate bone marrow, and most states have followed suit for state employees. Some states also offer tax deductions for nonmedical donation-related costs, and there is some evidence that these types of legislation do lead to modest increases in donation rates (Lacetera et al., 2014).
Regarding pluripotent stem cell research, current governance of federally funded research includes the Dickey-Wicker Amendment and NIH’s 2009 guidelines, which remain in effect.
A notable approach to governance of cell-based interventions in Japan and elsewhere is the implementation of a sunset provision for therapy approvals (Maeda et al., 2015). Combined with post-market surveillance, this mechanism creates a default that a provisionally approved therapy comes off the market after a defined period of time unless proven safe and effective. While this model has faced challenges in Japan due to the pressure to keep approved interventions on the market, it has been more successful than similar provisions implemented for drug approvals in Europe (Maeda et al., 2015).
A significant challenge of HSPC transplants, combined with CRISPR and other technologies going forward, will be monitoring for late effects and the governance structures associated with that process.
Affordability and reimbursement: Proven HSPC transplants may be covered by public funding schemes; unproven cell-based interventions are not.
Private companies: N/A
Social and ethical considerations: Concerns in this sector include the disproportionate lack of research funding available for genetic hematologic disease, such as SCD and thalassemia; public funding of embryonic stem cell research; and the role of the public in decision-making about research that bears on questions of human meaning (Frangoul et al., 2021).
Volunteer/Consumer
For the purposes of this case study, the primary actors within the volunteer/consumer sector are patients and consumers seeking regenerative medicine–based solutions to their medical concerns. It is important to keep in mind that many members of “the public” nationally and internationally never have the opportunity to be patients or consumers of emerging technologies, and so do not show up in the following analysis. These members of the public may nonetheless be affected by the development, deployment, and use of such technologies, and those impacts should be taken into account.
Science and technology: There are few approved regenerative medicine–based therapies in the United States or internationally beyond those described previously, though there are many clinical trials under way.
Governance and enforcement: The ISSCR attempted to establish a mechanism to publicly vet clinics selling unproven cell-based interventions, though the effort was abandoned in part due to push back from the clinics’ lawyers (Personal communication from ISSCR leadership, n.d.; Taylor et al., 2010). The ISSCR does have educational materials available for the public on this topic (A Closer Look at Stem Cells, 2022).
In 2018, the FTC took independent action against clinics making false claims about their interventions, and Google banned advertising for “unproven or experimental medical techniques such as most stem cell therapy, cellular (non-stem) therapy, and gene therapy” (Biddings, 2019; Fair, 2018). Reducing access to information about these clinics could lead to decreased use by customers. Direct action against the clinics by the FDA is described in the “Private Sector” section.
Affordability and reimbursement: As noted previously, unproven cell-based interventions can cost anywhere from several thousand dollars to tens of thousands of dollars (Regenberg et al., 2009). These costs are not covered by insurance. Patients have engaged in public fundraising campaigns, including on crowdfunding sites, to raise the money necessary to access the unproven intervention.
Private companies: Clinics offering unproven cell-based interventions and private cord blood banks are covered in the “Health Care” and “Private Sector” sections.
Social and ethical considerations: There are significant concerns about safety, therapeutic misconception among consumers, and use in children and other historically marginalized and underserved groups whose members lack the capacity to consent.
Ethical and Societal Implications
What is morally at stake what are the sources of ethical controversy does this technology/application raise different and unique equity concerns.
In outlining the concerns of the authors in terms of the use of this technology, we considered the following ethical dimensions, as outlined in the recent National Academies of Sciences, Engineering, and Medicine report, A Framework for Addressing Ethical Dimensions of Emerging and Innovative Biomedical Technologies: A Synthesis of Relevant National Academies Reports (NASEM, 2019).
- Promote societal value
- Minimize negative societal impact
- Protect the interests of research participants
- Advance the interests of patients
- Maximize scientific rigor and data quality
- Engage relevant communities
- Ensure oversight and accountability
- Recognize appropriate government and policy roles
It is important to keep in mind that different uses of this technology in different populations and contexts will raise different constellations of issues. For example, HSPC transplants for malignancies raise different issues than the same therapy for SCD; both of these are of course quite different than the many uses of unproven cell-based interventions in patients outside standard clinical care. Some of the specific concerns might include the following:
- How should the risks and benefits of first-in-human clinical trials be weighed?
- How should the risks/benefits of (ideal) existing standards of care be balanced against the risks/benefits of novel attempts at cures?
- What should be the role of the public in the governance of research and applications that bear on questions of human meaning?
- How can regulators more effectively address clinics offering DTC unproven cell-based interventions, including issues related to truth-telling, taking advantage of historically marginalized and underserved people, and significant risks in the absence of demonstrable benefit?
- In the DTC marketplace, how can the safety of interventions offered be ensured, and how can therapeutic misconception among consumers, including parents of sick children, be avoided?
- How can and should historical and ongoing health disparities, structural racism, and racism in medicine be taken into account in the assessment of new technologies?
- What are the benefits and challenges of intellectual property and data and materials sharing in the context of human tissue research and genome editing?
- What is the role of science and data in the governance of the private cord blood market?
- What is the appropriate governance response when the relevant regulatory authority lacks sufficient funds to execute its authority?
Beyond Regenerative Medicine
As noted at the beginning of this case, regenerative medicine, its applications, and its implications are very broad. The same work that enabled the development of iPSCs, and therefore the matching of cellular therapies to particular individuals, has also led to improved understanding of the processes of cellular aging and senescence (Svendsen, 2013). Despite the significant increase in average human lifespan, there has yet to be an equivalent increase in the human health span (Christensen et al., 2009). Diseases and conditions associated with age contribute to this discrepancy, causing older adults to spend more time in physiological deficiency, and have encouraged the scientific community to develop therapies that slow or even reverse the effects of aging (Beyret et al., 2018). The discovery of the ability to reverse cellular fate has encouraged researchers to better understand the biological process of aging, which could provide insight into the development of therapies to extend healthy longevity (Takahashi and Yamanaka, 2006). Several rejuvenation methods involving blood factors, metabolic changes, senescent cell ablation, and differing levels of cellular reprogramming are currently under investigation (Mahmoudi et al., 2019). Specific areas of interest include further research into the role of telomere shortening in cellular senescence and the ability of telomerase to counteract such shortening and extend cellular lifespan as well as applications of reprogramming aged stem cells into iPSCs or directly into tissue-specific stem cells (Spehar et al., 2020; Bernadotte et al., 2016; Nobel Prize, 2009; Bodnar et al., 1998). Moreover, genetic modifications to rejuvenate or extend the therapeutic effects of aged stem cells could enhance treatment capabilities for a multitude of diseases, including metabolic and neurodegenerative disorders (Navarro Negredo et al., 2020; Zhou et al., 2020; Ahmed et al., 2017).
Despite recent advances in the field of regenerative medicine, many challenges remain. Although the ability to reprogram cells in vitro is well documented, more work is needed to establish best practices for in vivo manipulation and to assess long-term outcomes in nonhuman animals before such therapies can be translated to the clinic (Beyret et al., 2018; Mertens et al., 2018). In addition, tampering with the natural safeguards that exist to prevent cellular reprogramming can lead to unintended consequences such as tumor growth (Brumbaugh et al., 2019; Abad et al., 2013). However, recent work to counteract the negative effects of aging shows promise that such challenges can be overcome. For example, Ocampo et al. explored partial cellular reprogramming by inducing temporary expression of the Yamanaka factors, Oct4, Sox2, Klf4, and c-Myc (OSKM) in vivo in mice (Ocampo et al., 2016). The results of their experiments demonstrated decreased cellular and physiological signs of aging; increased lifespan of progeroid mice; and shortened recovery time for older mice with metabolic diseases and muscle injury, all without the side effect of tumor growth. Continued investigation into the potential of regenerative medicine gives scientists the opportunity to better understand the aging process and to perhaps translate innovative therapies into the clinic to counteract the maladies that accompany old age.
As alluded to previously, it is possible to foresee numerous future scenarios regarding the evolution of regenerative medicine. In an effort to probe the kinds of worries that the authors have about the trajectories of emerging technologies, to expand the range of lessons learned from each case, and ultimately to “pressure test” the governance framework, the authors have developed a brief “visioning” narrative that pushes the technology presented in the core case 10–15 years into the future, playing out one plausible (but imagined) trajectory. The narrative was developed iteratively in collaboration with a case-specific working group, with additional feedback from all members of CESTI. All reviewers are acknowledged in the back matter of this paper. Each narrative is told from a particular perspective and is designed to highlight the social shifts that shape and are shaped by the evolving technology.
Regenerative Medicine Case Visioning Narrative
Perspective: Potential but conflicted off-label user
It is 2035. After the COVID-19 pandemic, mRNA delivery technology has expanded significantly. Scientists are now readily able to temporarily (and with some genome editing techniques, permanently) express synthetic proteins in a wide variety of cell types using lipid nanoparticle (LNP)-encased synthetic mRNA molecules. The mRNA mixture is delivered via simple intramuscular injection or intravenous infusion. In addition, researchers have made significant advancements in directing mRNA-LNPs to specific tissues.
Meanwhile, research on cellular rejuvenation has yielded dramatic insights into mechanisms of “turning back the cellular clock” via partial reprogramming. Researchers can now rejuvenate cellular function and growth. This can be accomplished by the transient (and careful) expression of the four Yamanaka factors—Oct3/4, Sox2, Klf4, and c-Myc—in a wide variety of human cell types. Researchers can now also provide safeguards to avoid the risk of tumor development. Early research in animals using a combination of mRNA-LNP and rejuvenation technology has produced startling insights. The technology appears to not only reverse aging in animals but also seems to extend youthful life—in some instances, for example, youthful life in treated mice was up to twice as long as in nontreated controls. Upon publication of these results, testing on extending the breeding life of racehorses quickly began. The implications for the technology are vast.
This marriage of mRNA delivery technology and cellular rejuvenation research has yielded two therapeutics, developed by LioRNA Therapeutics, which can dramatically reverse age-related conditions. A variant of this technology was first approved for use in pets. The first, an intramuscular shot delivered once every 5 to 10 years, rejuvenates T cell production to combat age-related deterioration; it is, essentially, an immune booster for aging. The second is a therapy designed to speed up healing of certain injuries in a variety of tissue types otherwise similarly affected by age-related deterioration. The results are astounding. Injuries that would have taken months to heal in older populations now take weeks; infections that would have claimed the lives of elderly patients are now easily surmountable with standard treatment. In addition, both therapies—as animal models indicated—seem to reverse the effects of aging. Whether they extend patients’ lifespans is, as of 2035, unclear but expected by many. Notably, however, LioRNA’s therapies do not cross the blood–brain barrier. The therapies are approved in the United States and the European Union in 2031.
The popular press—with help from LioRNA’s marketing team—hails the therapy as a “miracle” and a fulfillment of decades of promises of regenerative medicine. Scientists advocate the science behind the treatment through popular scientific outlets including TEDx talks and conferences, as well as YouTube and other forms of social media. The therapy is also immediately co-opted by professional athletes seeking to recover more quickly from their injuries. Amateur athletes in a variety of injury-inducing sports follow suit and post their accomplishments online. Bodybuilders have also adopted the therapy off-label to “naturally and undetectably” increase the benefits of strenuous exercise without the risk of injury. This extension of the therapy beyond its relatively narrow intended use raises its prestige, and the general public takes an interest. A number of adventurous younger people and wellness seekers get the shot off-label for enhancement purposes and brag about their newfound vigor on social media. This includes a number of celebrities and social media influencers.
In addition, because of a substantial global excess of idle mRNA LNP manufacturing facilities, left over from their dramatic expansion in 2022 to end the COVID-19 pandemic, a number of “wildcat” wellness clinics begin to attempt to copy LioRNA’s therapies to offer them as an “anti-aging cure.” Safety concerns related to these clinics abound.
By 2035, off-label use of LioRNA’s therapies (and copycats from various clinics) begin to take hold in various segments of the population. The treatment is especially popular in high-income areas where anti-aging interventions are popular (e.g., Los Angeles and South Korea). Some of the interest in the technology may also be related to public financial austerity programs around the world with respect to health care for the elderly. Seeing a tremendous increase in the cost of gerontological care, especially as the populations of high-income countries age, well-off governments around the world have begun to restrict a variety of health care interventions for the elderly. Not knowing whether they will have adequate care when they are older, taking LioRNA’s therapy (or getting it from a wildcat clinic) is, to many, a sensible “hedging of their bets.”
While the technology has not yet transformed society, it is on the cusp of doing so. Patients (and practitioners, some of whom are ardent advocates of the technology) are faced with a number of issues as they navigate a series of choices about whether to use LioRNA’s anti-aging therapy for purposes beyond its narrow label.
Scott Oliveri, a 59-year-old, healthy, widowed, middle-class heating, ventilation, and air conditioning engineer in Ohio with two sons, is faced with many of these issues. Scott has seen the results of numerous friends—his peer age group—taking LioRNA’s therapy, some on-label, others off. The increased physical activity in Scott’s peer group—largely, greater participation in recreational sports—has induced a form of peer pressure to obtain the therapy or be left out of these popular activities. In addition, Scott desires—but is conflicted about—receiving the therapy so he can continue to work and delay his retirement. Scott is both concerned about the longevity of a social safety net for the elderly (e.g., Medicare), and philosophically uncomfortable with the safety net. He finds assessing his insurance coverage prior to treatments to be complicated.
Gerontological Disease Management
The existence of LioRNA’s therapy has begun to revolutionize gerontological disease management. The ethics of its use in patients among the medical community is hotly contested. After watching patients senesce or succumb to accidents, many practitioners have now begun advocating patients get the rejuvenation shot. In particular, the unintended effect of LioRNA’s therapy on muscle production seems to miraculously stave off aging-related sarcopenia. As a consequence, in the United States, many physicians prescribe the treatment off-label or, even where indicated, prescribe it for the primary purpose of achieving rejuvenation benefits in their patients. In other instances, when physicians attempt to discuss healthy living and healthy aging with their patients, they are often cut short by discussions surrounding getting the shot, even for aging-related diseases for which the shot has little effect.
Use of the therapy, on- or off-label, is complicated by the fact that the therapies do not cross the blood–brain barrier. As a consequence, the effect of the technology on age-related dementia and other mental impairments is, as of 2035, entirely unclear. Early data suggest a risk of differential aging: the number of older, healthy, and physically able patients with declining mental acuity appears to be high. Alarmingly, in a subset of patients, the therapy has differential effects across tissues (e.g., it is shown to successfully rejuvenate muscle, but does not have the same effect on an injured tendon), which results in severe chronic pain. This is discounted by some physicians but is a topic of significant concern for others. Scott is vaguely aware of these concerns, but, as informed by his peer group, he believes these “side effects” to be small. In addition, Scott’s primary care physician, who he has seen for 20 years, is not a gerontological specialist and is not as up to date on these nuances of LioRNA’s therapy as other colleagues.
Insurance Practice
Insurers are initially hesitant to widely cover the LioRNA therapies, and they only partially reimburse or cover the therapy (and only where the primary indication—age-related immune deficiencies and injury recovery—is present). Some insurers, however, seeing the enormous benefit of the therapy beyond its label (and its cost-effectiveness), and begin to mandate the treatment as top-line therapy before covering others, especially where “injury” is present, using an intentionally broad definition. Less desirable interventions, some of which are, by clinical estimations, inferior to mRNA anti-aging treatment, become second-line therapy, if used at all. In addition, some insurers have negotiated value-based agreements with LioRNA, which have proven remarkably successful for both LioRNA and some payers, especially those covering aging populations. As a consequence, many patients, presented with their insurers’ directives, are induced to choose the therapy for a wide variety of conditions even where they would not otherwise choose the treatment. Scott, however, is 59 years old and healthy and is below the age and indication cutoff for many of these incentive programs. He has had difficulty getting an answer from his insurer as to whether and what extent he would be reimbursed for treatment. Scott has heard that friends his age who have injured themselves playing recreational sports or otherwise by accident were entitled to full reimbursement from their insurers. Scott has joked that one “needs to get hurt to get insurance to pay for the shot.”
Equity, Access, and Medical Tourism
Some uninsured patients, the “worried middle aged,” and LioRNA enthusiasts begin to visit wildcat clinics in the United States for either cheaper versions of the shot or for certain modifications, including tissue targeting for reproductive issues. Given the safety profile of these compounded treatments, many are injured as a result; it is also unclear if the modified forms of the technology work as advertised, as the evidence is mixed. Other patients resort to anti-aging medical tourism where mRNA-LNP manufacturing capacity is most available (notably India, following the increase in manufacturing capacity post COVID-19 pandemic). This has the effect of raising the therapy’s price globally and diminishing access to the poorest among a number of low- and middle-income countries, despite the increase in mRNA-LNP production capacity. This is lamented by a number of public health researchers who point out, correctly, that the world’s poorest are the most negatively affected by aging relative to other groups. In this sense, differential access will likely have a significant and negative impact on health equity.
The popularity of LioRNA’s therapy, and excess mRNA-LNP manufacturing capacity on a contract basis worldwide, has spurred a major biohacking movement. Biohackers are developing their own versions of the LioRNA therapies and also creating their modifications, both for disease-treatment and for enhancement purposes. Online, biohackers share mRNA sequences for synthesis, manufacture, and injection, including modifications pertaining to various age-related concerns (e.g., age-related vision loss). Some of these experiments appear to be successful. Others, however, are less so, including complications pertaining to cancer risk so studiously avoided in LioRNA’s commercial products. Scott—otherwise unsure as to whether his insurer will cover the therapy—has been encouraged by one of his sons to visit a wildcat clinic to receive the therapy, on the premise that “it’s cheaper” and that he doesn’t “need to worry about insurance.” Scott has also heard from his son that a hobbyist could make a hacked version for him. Scott is concerned about safety issues, some of which have been present in the news and on social media.

Social Context of Aging
LioRNA’s technology, and its popularity, has made aging-related infirmities, once a normal facet of life, increasingly viewed as treatable maladies. Among the elderly and aging, aging-related health conditions are increasingly viewed with skepticism, in the same manner as contracting a communicable but preventable illness. There is peer social pressure to “get the shot,” exacerbated by social and other electronic media. In addition, declining standards in elderly home care facilities is leveraged to encourage those who are aging to use LioRNA’s treatments. Children of aging parents, worried about their care, are also pressuring their parents to use the therapy. Beyond all of this, there is a popular fear (yet to be appreciably realized) that the widespread use of the therapy will result in the diminishment of social safety nets for the elderly, including social security and Medicare. Scott is worried about these same issues and is cognizant of not wanting to be a burden on his children. As uptake of the therapy in his peer group increases, and he becomes more aware of memories of his parents aging, Scott is leaning toward accepting the therapy.
One of the therapy’s first, as well as most public and prominent, uses is among professional athletes. Again, because one of the treatment’s primary indications is rapidly healing from injury, physicians routinely prescribe the therapy to injured athletes. Some team physicians are selected, in part, on their willingness to prescribe the treatment to aging but valuable franchise athletes. All major professional sports see significant uptake of the therapy among their athletes, with significant pressure placed on the organizations’ collective bargaining efforts regarding whether the therapy is properly characterized as an “enhancement.” The therapy also becomes popular among amateur athletes who see it as a way to ward off injury. Scott, a sports enthusiast, is similarly moved by these efforts and their popularization online.
Regulation and Liability
The LioRNA treatments also challenge several precepts regarding regulation and consumer safety. On regulation, after years of developing guidelines regarding modular therapies (e.g., CAR-T and CRISPR-based therapies), the almost limitless indications and ease of modification of the therapies have challenged the FDA’s ability to police the line between biologic and medical practice, and between a commercial manufacturer and a compounding laboratory. This is largely complicated by reluctance, both in the White House and in Congress, to allow the FDA to take a more active enforcement role in shutting down the wildcat clinics and biohackers dedicated to producing variants of LioRNA’s treatments. Beyond this, the popularity of the therapy, and its introduction outside typical commercial channels in many cases, has complicated litigation concerning consumer safety.
Regenerative Medicine Case Study: Lessons Learned
Following are some of the lessons drawn from the preceding core case and visioning exercise that can inform the development of a cross-sectoral governance framework for emerging technologies focused on societal benefit.
- It is important to consider and articulate the role of the public in decision making regarding research and technology development bearing on questions of human meaning.
- Particularly in the absence of existing binding law or guidance, the National Academies (and other nongovernmental organizations) can play a critical role in governance, even when guidance is voluntary and nonbinding.
- Underfunded/understaffed agencies cannot effectively regulate every technology that falls within their mandate.
- The private sector can play a role in governance gaps (e.g., Google’s action regarding stem cell clinic ads).
- The governance ecosystem around a technology will evolve with the technology.
- A state-by-state regulatory patchwork can stifle innovation and reduce or reshape the workforce in a field.
- Public perception of a technology may shift in response to positive clinical developments.
- Early, public success or failure can have an outsized impact on the development of a technology.
- Politics throws a spanner in the works.
- There is a critical need for trustworthy institutions at all stages and levels of technology governance.
- Special attention must be paid to research and technologies to which not all patients have access due to limits on knowledge or availability of genetic variation in the research, product, or patient (biological access).
- Special attention must be paid to the impact of compounding inequities (e.g., biological access and structural racism).
- Sometimes scientific and technological solutions can be found to ethical concerns.
- Special attention must be paid to technologies based on human tissues and data (i.e., human tissue or data as product).
- Japan’s governance approach involving sunset provisions for therapy approvals, combined with post-market surveillance is an interesting model that has met with some success.
- Well-timed public pressure can prompt oversight.
- Society’s response to a technology’s off-label uses (including for enhancement) can shape its evolution as much as uptake of its intended use.
- Social structures (and future expectation of social structures) can influence uptake and vary across the globe.
- Technology can change social structures themselves (e.g., views on aging, injury).
- Access to and distribution of technology by nonlegacy players can affect use cases and uptake (e.g., the role of biohackers or the do-it-yourself community).
- Insurance coverage shapes uptake.
- For modular technologies (e.g., mRNA-LNPs), excess manufacturing capacity may act as a driver of secondary use and associated innovation.
Join the conversation!
Download the graphics below and share on social media!

- 98th Congress. 1983. S. 2048—National Organ Transplant Act. Available at: https://www.congress.gov/bill/98th-congress/senate-bill/2048 (accessed September 6, 2022).
- 104th Congress. 1995. H.R. 2880—Balanced Budget Downpayment Act. Available at: https://www.congress.gov/bill/104th-congress/house-bill/2880 (accessed September 6, 2022).
- 109th Congress. 2005. H.R. 810—Stem Cell Research Enhancement Act of 2005. Available at: https://www.congress.gov/bill/109th-congress/house-bill/810 (accessed September 6, 2022).
- A Closer Look at Stem Cells. 2022. The ISSCR patient handbook on stem cell therapies. Available at: https://www.closerlookatstemcells.org/patient-resources (accessed September 7, 2022).
- Abad, M., L. Mosteiro, C. Pantoja, M. Cañamero, T. Rayon, I. Ors, O. Graña, D. Megías, O. Domínguez, D. Martínez, M. Manzanares, S. Ortega, and M. Serrano. 2013. Reprogramming in vivo produces teratomas and iPS cells with totipotency features. Nature 502(7471):340–345. https://doi.org/10.1038/nature12586.
- Ahmed, A. S., M. H. Sheng, S. Wasnik, D. J. Baylink, and K. W. Lau. 2017. Effect of aging on stem cells. World Journal of Experimental Medicine 7(1):1–10. https://doi.org/10.5493/wjem.v7.i1.1.
- American College of Obstetricians and Gynecologists (ACOG). 2019. Umbilical cord blood banking, committee opinion number 771. Available at: https://www.acog.org/clinical/clinical-guidance/committee-opinion/articles/2019/03/umbilical-cord-blood-banking (accessed September 7, 2022).
- American Medical Association (AMA). n.d. Code of medical ethics opinion 6.1.5. Available at: https://www.ama-assn.org/delivering-care/ethics/umbilical-cord-blood-banking (accessed September 7, 2022).
- Apperley, J. F. 1993. Bone marrow transplant for the haemoglobinopathies: Past, present and future. Bailliere’s Clinical Haematology 6(1):299–325. https://doi.org/10.1016/s0950-3536(05)80074-5.
- Ballas, S. K., E. D. Pulte, C. Lobo, and G. Riddick-Burden. 2016. Case series of octogenarians with sickle cell disease. Blood 128(19):2367–2369. https://doi.org/10.1182/blood-2016-05-715946.
- Ballen, K. K., F. Verter, and J. Kurtzberg. 2015. Umbilical cord blood donation: Public or private? Bone Marrow Transplantation 50(10):1271–1278. https://doi.org/10.1038/bmt.2015.124.
- Ballen, K. K., E. Gluckman, and H. E. Broxmeyer. 2013. Umbilical cord blood transplantation: The first 25 years and beyond. Blood 122(4):491–498. https://doi.org/10.1182/blood-2013-02-453175.
- Ballen, K. K., T. R. Spitzer, B. Y. Yeap, S. McAfee, B. R. Dey, E. Attar, R. Haspel, G. Kao, D. Liney, E. Alyea, S. Lee, C. Cutler, V. Ho, R. Soiffer, and J. H. Antin. 2007. Double unrelated reduced-intensity umbilical cord blood transplantation in adults. Biology of Blood and Marrow Transplantation 13(1):82–89. https://doi.org/10.1016/j.bbmt.2006.08.041.
- Barker, J. N., K. Boughan, P. B. Dahi, S. M. Devlin, M. A. Maloy, K. Naputo, C. M. Mazis, E. Davis, M. Nhaissi, D. Wells, C. Cooper, D. M. Ponce, N. Kernan, A. Scaradavou, S. A. Giralt, E. B. Papadopoulos, and I. Politikos. 2019. Racial disparities in access to HLA-matched unrelated donor transplants: A prospective 1312-patient analysis. Blood Advances 3(7):939–944. https://doi.org/10.1182/bloodadvances.2018028662.
- Barker, J. N., C. E. Byam, N. A. Kernan, S. S. Lee, R. M. Hawke, K. A. Doshi, D. S. Wells, G. Heller, E. B. Papadopoulos, A. Scaradavou, J. W. Young, and M. R. van den Brink. 2010. Availability of cord blood extends allogeneic hematopoietic stem cell transplant access to racial and ethnic minorities. Biology of Blood and Marrow Transplantation 16(11):1541–1548. https://doi.org/10.1016/j.bbmt.2010.08.011.
- Barnes, D. W. H., M. J. Corp, J. F. Loutit, and F. E. Neal. 1956. Treatment of murine leukaemia with x rays and homologous bone marrow. British Medical Journal 2(4993):626–627. https://doi.org/10.1136/bmj.2.4993.626.
- Bauer, G., M. Elsallab, and M. Abou-El-Enein. 2018. Concise review: A comprehensive analysis of reported adverse events in patients receiving unproven stem cell-based interventions. Stem Cells Translational Medicine 7(9):676–685. https://doi.org/10.1002/sctm.17-0282.
- Be the Match. 2021a. Be the Match BioTherapies History. Available at: https://bethematchbiotherapies.com/about-us/history (accessed September 7, 2022).
- Be the Match. 2021b. Our Cell and Gene Therapy Partners. Available at: https://bethematchbiotherapies.com/about-us/our-partners (accessed September 7, 2022).
- Be the Match. 2012. Coalition says PBSC donor compensation poses health risks to patients and donors. Available at: https://bethematch.org/templates/displaynewsrelease/?id=707 (accessed September 6, 2022).
- Beasley, D. 2019. Expensive Gilead, Novartis cancer therapies losing patients to experimental treatments. Reuters, July 30. Available at: https://www.reuters.com/article/us-gilead-novartis-trials-focus/expensive-gilead-novartis-cancer-therapies-losing-patients-to-experimental-treatments-idUSKCN1UP10O (accessed September 7, 2022).
- Benjamin, R. 2011. Organized ambivalence: When sickle cell disease and stem cell research converge. Ethnicity & Health 16(4–5):447–463. https://doi.org/10.1080/13557858.2011.552710.
- Bergman, E. J., and N. J. Diamond. 2013. Sickle cell disease and the “difficult patient” conundrum. The American Journal of Bioethics 13(4):3–10. https://doi.org/10.1080/15265161.2013.767954.
- Bernadotte, A., V. M. Mikhelson, and I. M. Spivak. 2016. Markers of cellular senescense. Telomere shortening as a marker of cellular senescence. Aging 8(1):3–11. https://doi.org/10.18632/aging.100871.
- Beyret, E., P. Martinez Redondo, A. Platero Luengo, and J. C. Izpisua Belmonte. 2018. Elixir of life: Thwarting aging with regenerative reprogramming. Circulation Research 122(1):128–141. https://doi.org/10.1161/CIRCRESAHA.117.311866.
- Bhatia, M., and M. C. Walters. 2008. Hematopoietic cell transplantation for thalassemia and sickle cell disease: Past, present and future. Bone Marrow Transplantation 41(2):109–117. https://doi.org/10.1038/sj.bmt.1705943.
- Biddings, A. 2019. A new policy on advertising for speculative and experimental medical treatments. Google Ads Help. Available at: https://support.google.com/google-ads/answer/9475042 (accessed September 6, 2022).
- Billingham, R. E., and L. Brent. 1959. Quantitative studies on tissue transplantation immunity. IV. Induction of tolerance in newborn mice and studies on the phenomenon of runt disease. Philosophical Transactions of the Royal Society of London B 242:439–477. https://doi.org/10.1098/rstb.1959.0008.
- BIS Research. n.d. Global CRISPR gene editing market. Available at: https://bisresearch.com/industry-report/crispr-gene-editing-market.html (accessed September 7, 2022).
- Bodnar, A. G., M. Ouellette, M. Frolkis, S. E. Holt, C. P. Chiu, G. B. Morin, C. B. Harley, J. W. Shay, S. Lichtsteiner, and W. E. Wright. 1998. Extension of life-span by introduction of telomerase into normal human cells. Science 279(5349):349–352. https://doi.org/10.1126/science.279.5349.349.
- Bortin, M. M. 1970. A compendium of reported human bone marrow transplants. Transplantation 9(6):571–587. https://doi.org/ 10.1097/00007890-197006000-00006.
- Broder, M. S., T. P. Quock, E. Chang, S. R. Reddy, R. Agarwal-Hashmi, S. Arai, and K. F. Villa. 2017. The cost of hematopoietic stem-cell transplantation in the United States. American Health & Drug Benefits 10(7):366–374. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5726064 (accessed September 6, 2022).
- Brumbaugh, J., B. Di Stefano, and K. Hochedlinger. 2019. Reprogramming: Identifying the mechanisms that safeguard cell identity. Development 146(23):dev182170. https://doi.org/10.1242/dev.182170.
- U.S. Centers for Disease Control and Prevention (CDC). 2022. Data & statistics on sickle cell disease. Available at: https://www.cdc.gov/ncbddd/sicklecell/data.html (accessed September 6, 2022).
- Centers for Medicare & Medicaid Services (CMS). 2016. Stem cell transplantation (formerly 110.8.1.). Available at: https://www.cms.gov/medicare-coverage-database/view/ncd.aspx?NCDId=366 (accessed September 7, 2022).
- Christensen, K., G. Doblhammer, R. Rau, and J. W. Vaupel. 2009. Ageing populations: The challenges ahead. Lancet 374(9696):1196–1208. https://doi.org/10.1016/S0140-6736(09)61460-4.
- Cimons, M. 2001. Bush is a threat to US stem-cell research. Nature Medicine 7(3):263–263. https://doi.org/10.1038/85369.
- CIRM.ca.gov. n.d. Text of proposed laws: Proposition 71. Available at: https://www.cirm.ca.gov/sites/default/files/files/about_cirm/prop71.pdf (accessed September 6, 2022).
- CNN. 2001. President George W. Bush’s address on stem cell research. Available at: https://edition.cnn.com/2001/ALLPOLITICS/08/09/bush.transcript (accessed September 6, 2022).
- Code of Federal Regulations (CFR). 2012. 21 CFR 1271—Human cells, tissues, and cellular and tissue-based products. Available at: https://www.govinfo.gov/app/details/CFR-2012-title21-vol8/CFR-2012-title21-vol8-part1271 (accessed September 7, 2022).
- Cohen, I. G. 2012. Selling bone marrow—Flynn v. Holder. New England Journal of Medicine 366:296–297. https://doi.org/10.1056/NEJMp1114288.
- Committee on Obstetric Practice and Committee on Genetics. 2008. ACOG committee opinion number 399, February 2008: Umbilical cord blood banking. Obstetrics and Gynecology 111(2 Pt 1):475–477. https://doi.org/10.1097/AOG.0b013e318166603c.
- Contreras, J. L., and J. S. Sherkow. 2017. CRISPR, surrogate licenses, and scientific discovery. Science 355(6326):698–700. https://doi.org/10.1126/science.aal4222.
- de la Morena, M. T., and R. A. Gatti. 2010. A history of bone marrow transplantation. Immunology and Allergy Clinics of North America 30(1):1–15. https://doi.org/10.1016/j.iac.2009.11.005.
- Demirci, S., A. Leonard, J. J. Haro-Mora, N. Uchida, and J. F. Tisdale. 2019. CRISPR/Cas9 for sickle cell disease: Applications, future possibilities, and challenges. Advances in Experimental Medicine and Biology 1144:37–52. https://doi.org/10.1007/5584_2018_331.
- Department of Health and Human Services and Agency for Healthcare Research and Quality (HHS and AHRQ). 2003. National Healthcare Disparities Report. Rockville, MD: Department of Health and Human Services and Agency for Healthcare Research and Quality. Available at: https://repository.library.georgetown.edu/bitstream/handle/10822/711771/NHDR2003.pdf?sequence=1&isAllowed=y (accessed September 6, 2022).
- Eapen, M., V. Rocha, G. Sanz, A. Scaradavou, M. J. Zhang, W. Arcese, A. Sirvent, R. E. Champlin, N. Chao, A. P. Gee, L. Isola, M. J. Laughlin, D. I. Marks, S. Nabhan, A. Ruggeri, R. Soiffer, M. M. Horowitz, E. Gluckman, J. E. Wagner, Center for International Blood and Marrow Transplant Research, Acute Leukemia Working Party and Eurocord, European Group for Blood and Marrow Transplantation, and National Cord Blood Program, New York Blood. 2010. Effect of graft source on unrelated donor haemopoietic stem-cell transplantation in adults with acute leukaemia: A retrospective analysis. Lancet Oncology 11(7):653–660. https://doi.org/10.1016/S1470-2045(10)70127-3.
- Enserink, M. 2006. Biomedicine. Selling the stem cell dream. Science 313(5784):160–163. https://doi.org/10.1126/science.313.5784.160.
- Esrick, E. B., L. E. Lehmann, A. Biffi, M. Achebe, C. Brendel, M. F. Ciuculescu, H. Daley, B. MacKinnon, E. Morris, A. Federico, D. Abriss, K. Boardman, R. Khelladi, K. Shaw, H. Negre, O. Negre, S. Nikiforow, J. Ritz, S.-Y. Pai, W. B. London, C. Dansereau, M. M. Heeney, M. Armant, J. P. Manis, and D. A. Williams. 2021. Post-transcriptional genetic silencing of BCL11A to treat sickle cell disease. New England Journal of Medicine 384:205–215. https://doi.org/10.1056/NEJMoa2029392.
- Faden, R. R., L. Dawson, A. S. Bateman-House, D. M. Agnew, H. Bok, D. W. Brock, A. Chakravarti, X. J. Gao, M. Greene, J. A. Hansen, P. A. King, S. J. O’Brien, D. H. Sachs, K. E. Schill, A. Siegel, D. Solter, S. M. Suter, C. M. Verfaillie, L. B. Walters, and J. D. Gearhart. 2003. Public stem cell banks: Considerations of justice in stem cell research and therapy. The Hastings Center Report 33(6):13–27. https://doi.org/10.2307/3527822.
- Fair, L. 2018. Stemming unproven stem cell therapy claims. Federal Trade Commission Business Blog, October 18. Available at: https://www.ftc.gov/business-guidance/blog/2018/10/stemming-unproven-stem-cell-therapy-claims (accessed September 6, 2022).
- Farooq, F., P. J. Mogayzel, S. Lanzkron, C. Haywood, and J. J. Strouse. 2020. Comparison of US federal and foundation funding of research for sickle cell disease and cystic fibrosis and factors associated with research productivity. JAMA Network Open 3(3):e201737. https://doi.org/ 10.1001/jamanetworkopen.2020.1737.
- U.S. Food and Drug Administration (FDA). 2022. Approved cellular and gene therapy products. Available at: https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/approved-cellular-and-gene-therapy-products (accessed September 7, 2022).
- FDA. 2020. Regulatory considerations for human cells, tissues, and cellular and tissue-based products: Minimal manipulation and homologous use. Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/regulatory-considerations-human-cells-tissues-and-cellular-and-tissue-based-products-minimal (accessed September 7, 2022).
- FDA. 2019. Framework for the regulation of regenerative medicine products. Available at: https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/framework-regulation-regenerative-medicine-products (accessed September 6, 2022).
- FDA. 2018. Letter regarding FDA’s regenerative medicine framework and enforcement discretion period for HCT/Ps. Available at: https://www.fda.gov/media/119936/download (accessed September 6, 2022).
- Frangoul, H., D. Altshuler, M. D. Cappellini, Y.-S. Chen, J. Domm, B. K. Eustace, J. Foell, J. de la Fuente, S. Grupp, R. Handgretinger, T. W. Ho, A. Kattamis, A. Kernytsky, J. Lekstrom-Himes, A. M. Li, F. Locatelli, M. Y. Mapara, M. de Montalembert, D. Rondelli, A. Sharma, S. Sheth, S. Soni, M. H. Steinberg, D. Wall, A. Yen, and S. Corbacioglu. 2021. CRISPR-Cas9 gene editing for sickle cell disease and ß-thalassemia. New England Journal of Medicine 384(3):252–260. https://doi.org/10.1056/NEJMoa2031054.
- Gartner. 2022. Gartner hype cycle. Available at: https://www.gartner.com/en/research/methodologies/gartner-hype-cycle (accessed September 6, 2022).
- Global Neuroethics Summit Delegates, K. S. Rommelfanger, S.-J. Jeong, A. Ema, T. Fukushi, K. Kasai, K. M. Ramos, A. Salles, and I. Singh. 2018. Neuroethics questions to guide ethical research in the international brain initiatives. Neuron 100(1):19–36. https://doi.org/10.1016/j.neuron.2018.09.021.
- Gluckman, E., V. Rocha, A. Boyer-Chammard, F. Locatelli, W. Arcese, R. Pasquini, J. Ortega, G. Souillet, E. Ferreira, J. P. Laporte, M. Fernandez, and C. Chastang. 1997. Outcome of cord-blood transplantation from related and unrelated donors. Eurocord transplant group and the European blood and marrow transplantation group. New England Journal of Medicine 337(6):373–381. https://doi.org/10.1056/NEJM199708073370602.
- Gluckman, E., H. A. Broxmeyer, A. D. Auerbach, H. S. Friedman, G. W. Douglas, A. Devergie, H. Esperou, D. Thierry, G. Socie, P. Lehn, S. Cooper, D. English, J. Kurtzberg, J. Bard, and E. A. Boyse. 1989. Hematopoietic reconstitution in a patient with Fanconi’s anemia by means of umbilical-cord blood from an HLA-identical sibling. New England Journal of Medicine 321(17):1174–1178. https://doi.org/10.1056/NEJM198910263211707.
- Gragert, L., M. Eapen, E. Williams, J. Freeman, S. Spellman, R. Baitty, R. Hartzman, J. D. Rizzo, M. Horowitz, D. Confer, and M. Maiers. 2014. HLA match likelihoods for hematopoietic stem-cell grafts in the U.S. Registry. New England Journal of Medicine 371(4):339–348. https://doi.org/10.1056/NEJMsa1311707.
- Granot, N. and R. Storb. 2020. History of hematopoietic cell transplantation: Challenges and progress. Haematologica 105(12). https://doi.org/10.3324/haematol.2019.245688.
- Green, R. M. 1995. Report of the Human Embryo Research Panel. Kennedy Institute of Ethics Journal 5(1):83–84.
- Haywood Jr., C. 2013. Disrespectful care in the treatment of sickle cell disease requires more than ethics consultation. The American Journal of Bioethics 13(4):12–14. https://doi.org/10.1080/15265161.2013.768857.
- Haywood, Jr., C., M. C. Beach, S. Lanzkron, J. J. Strouse, R. Wilson, H, Park, C. Witkop, E. B. Bass, and J. B. Segal. 2009. A systematic review of barriers and interventions to improve appropriate use of therapies for sickle cell disease. Journal of the National Medical Association 101(10):1022–1033. https://doi.org/ 10.1016/s0027-9684(15)31069-5.
- The Hinxton Group. 2006. An international consortium on stem cells, ethics, and law. February 24. Available at: http://www.hinxtongroup.org/au_trans_cs.html (accessed September 6, 2022).
- Hurley, C. K., L. Foeken, M. Horowitz, B. Lindberg, M. McGregor, N. Sacchi, and the WMDA Accreditation and Regulatory Committees. 2010. Standards, regulations and accreditation for registries involved in the worldwide exchange of hematopoietic stem cell donors and products. Bone Marrow Transplant 45(5):819–824. https://doi.org/10.1038/bmt.2010.8.
- Institute for Clinical and Economic Review (ICER). 2021. Crizanlizumab, voxelotor, and l-glutamine for sickle cell disease: Effectiveness and value: Evidence report. Available at: https://icer.org/wp-content/uploads/2021/02/ICER_SCD_Evidence-Report_031220-FOR-PUBLICATION.pdf (accessed September 6, 2022).
- Institute of Medicine (IOM). 2014. Oversight and review of clinical gene transfer protocols: Assessing the role of the Recombinant DNA Advisory Committee. Washington, DC: The National Academies Press. https://doi.org/10.17226/18577.
- Institute of Medicine (IOM). 2003. Unequal treatment: Confronting racial and ethnic disparities in health care. Washington, DC: The National Academies Press. https://doi.org/10.17226/12875.
- Institute of Medicine and National Research Council (IOM and NRC). 2005. Guidelines for human embryonic stem cell research. Washington, DC: The National Academies Press. https://doi.org/10.17226/11278.
- International Military Tribunal. 1949. Trials of war criminals before the Nuremberg Military Tribunals under Control Council law no. 10 Nurenberg, October 1946–April 1949. Washington, DC: Government Printing Office, 1950. Available at: https://lccn.loc.gov/2011525364 (accessed September 5, 2022).
- International Society for Stem Cell Research (ISSCR). n.d. Guidelines for stem cell research and clinical translation. Available at: https://www.isscr.org/guidelines (accessed September 6, 2022).
- Jinek, M., K. Chylinski, I. Fonfara, M. Hauer, J. A. Doudna, and E. Charpentier. 2012. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337(6096):816–821. https://doi.org/10.1126/science.1225829.
- Johnson, F. L., A. T. Look, J. Gockerman, M. R. Ruggiero, L. Dalla-Pozza, and F. T. Billings, 3rd. 1984. Bone-marrow transplantation in a patient with sickle-cell anemia. New England Journal of Medicine 311(12):780–783. https://doi.org/10.1056/NEJM198409203111207.
- Justia US Law. 2020. 2020 Arizona revised statutes title 36—Public health and safety. § 36-2313 Destructive human embryonic stem cell research; violation; classification. Available at: https://law.justia.com/codes/arizona/2020/title-36/section-36-2313 (accessed September 6, 2022).
- Kahn, J. P., and A. C. Mastroianni. 2004. Creating a stem cell donor: A case study in reproductive genetics. Kennedy Institute of Ethics Journal 14(1):81–96. https://doi.org/10.1353/ken.2004.0017.
- Kauf, T. L., T. D. Coates, L. Huazhi, N. Mody-Patel, and A. G. Hartzema. 2009. The cost of health care for children and adults with sickle cell disease. American Journal of Hematology 84(6):323–327. https://doi.org/10.1002/ajh.21408.
- Kessinger, A., J. O. Armitage, J. D. Landmark, D. M. Smith, and D. D. Weisenburger. 1988. Autologous peripheral hematopoietic stem cell transplantation restores hematopoietic function following marrow ablative therapy. Blood 71(3):723–727. https://doi.org/10.1182/blood.V71.3.723.723.
- Kim, S., T. Koo, H.-G. Jee, H.-Y. Cho, G. Lee, D.-G. Lim, H. S. Shin, and J.-S. Kim. 2018. CRISPR RNAs trigger innate immune responses in human cells. Genome Research 28(3):367–373. https://doi.org/10.1101/gr.231936.117.
- Knoepfler, P. 2020. Historic spring storm of FDA letters to stem cell clinic industry. The Niche, May 17. Available at: https://ipscell.com/2020/05/historic-spring-storm-of-fda-letters-to-stem-cell-clinic-industry (accessed September 6, 2022).
- Knoepfler, P. S. 2019. Rapid change of a cohort of 570 unproven stem cell clinics in the USA over 3 years. Regenerative Medicine 14(8):735–740. https://doi.org/10.2217/rme-2019-0064.
- Knoepfler, P. S. 2018. Too much carrot and not enough stick in new stem cell oversight trends. Cell Stem Cell 23(1):18–20. https://doi.org/10.1016/j.stem.2018.06.004.
- Knoepfler, P. S., and L. G. Turner. 2018. The FDA and the U.S. direct-to-consumer marketplace for stem cell interventions: A temporal analysis. Regenerative Medicine 13(1):19–27. https://doi.org/10.2217/rme-2017-0115.
- Kolata, G. 2019. These patients had sickle-cell disease. Experimental therapies might have cured them. The New York Times, January 27. Available at: https://www.nytimes.com/2019/01/27/health/sickle-cell-gene-therapy.html (accessed September 6, 2022).
- Kollman, C., E. Abella, R. L. Baitty, P. G. Beatty, R. Chakraborty, C. L. Christiansen, R. J. Hartzman, C. K. Hurley, E. Milford, J. A. Nyman, T. J. Smith, G. E. Switzer, R. K. Wada, and M. Setterholm. 2004. Assessment of optimal size and composition of the U.S. National Registry of Hematopoietic Stem Cell Donors. Transplantation 78(1):89–95. https://doi.org/10.1097/01.TP.0000132327.40702.97.
- Kurtzberg, J. 2017. A history of cord blood banking and transplantation. Stem Cells Translational Medicine 6(5):1309–1311. https://doi.org/10.1002/sctm.17-0075.
- Kurtzberg, J., M. Laughlin, M. L. Graham, C. Smith, J. F. Olson, E. C. Halperin, G. Ciocci, C. Carrier, C. E. Stevens, and P. Rubinstein. 1996. Placental blood as a source of hematopoietic stem cells for transplantation into unrelated recipients. New England Journal of Medicine 335(3):157–166. https://doi.org/10.1056/NEJM199607183350303.
- Lacetera, N., M. Macis, and S. S. Stith. 2014. Removing financial barriers to organ and bone marrow donation: The effect of leave and tax legislation in the U.S. Journal of Health Economics 33:43–56. https://doi.org/10.1016/j.jhealeco.2013.10.006.
- Ledford, H. 2020. CRISPR gene therapy shows promise against blood diseases. Nature 588(7838):383. https://doi.org/10.1038/d41586-020-03476-x.
- Levine, A. D. 2012. State stem cell policy and the geographic preferences of scientists in a contentious emerging field. Science and Public Policy 39(4):530–541. https://doi.org/10.1093/scipol/scs038.
- Little, M.-T., and R. Storb. 2002. History of haematopoietic stem-cell transplantation. Nature Reviews: Cancer 2(3):231–238. https://doi.org/10.1038/nrc748.
- Liu, A. 2021. Bluebird Bio hits pause on rollout for ill-fated gene therapy Zynteglo as trial flags 2 cancer cases. Fierce Pharma, February 16. Available at: https://www.fiercepharma.com/pharma/bluebird-bio-halts-selling-ill-fated-gene-therapy-zynteglo-as-trial-flags-2-cancer-cases (accessed January 25, 2023).
- Lomax, G. P., A. Torres, and M. T. Millan. 2020. Regulated, reliable, and reputable: Protect patients with uniform standards for stem cell treatments. Stem Cells Translational Medicine 9(5):547–553. https://doi.org/10.1002/sctm.19-0377.
- Mahmoudi, S., L. Xu, and A. Brunet. 2019. Turning back time with emerging rejuvenation strategies. Nature Cell Biology 21(1):32–43. https://doi.org/10.1038/s41556-018-0206-0.
- Maeda, D., T. Yamaguchi, T. Ishizuka, M. Hirata, K. Takekita, and D. Sato. 2015. Regulatory frameworks for gene and cell therapies in Japan. Advances in Experimental Medicine and Biology 871:147–162. https://doi.org/10.1007/978-3-319-18618-4_8.
- Mannick, J. A., H. L. Lochte Jr, C. A. Ashley, E. D. Thomas, and J. W. Ferrebee. 1960. Autografts of bone marrow in dogs after lethal total-body radiation. Blood 127(17):2047. https://doi.org/10.1182/blood-2016-01-696476.
- Marchant, G. E., K. W. Abbott, and B. Allenby. 2014. Innovative governance models for emerging technologies. Cheltenham, UK: Edward Elgar Pub.
- Mason, C., and P. Dunnill. 2008. A brief definition of regenerative medicine. Regenerative Medicine 3(1):1–5. https://doi.org/ 10.2217/17460751.3.1.1.
- Master, Z., W. Fu, D. Paciulli, and D. Sipp. 2017. Industry responsibilities in tackling direct-to-consumer marketing of unproven stem cell treatments. Clinical Pharmacology and Therapeutics 102(2):177–179. https://doi.org/10.1002/cpt.704.
- Mathé, G., J. L. Amiel, L. Schwarzenberg, A. Cattan, and M. Schneider. 1965. Adoptive immunotherapy of acute leukemia: Experimental and clinical results. Cancer Research 25(9):1525–1531.
- Mathews, D. J. H. 2017. When emerging biomedical technologies converge or collide. In J. Illes (ed.), Neuroethics: Anticipating the future. Oxford Academic: United Kingdom. https://doi.org/10.1093/oso/9780198786832.003.0001.
- Mathews, K. R. W., and E. H. Yang. 2019. Politics and policies guiding human embryo research in the United States. Available at: https://www.bakerinstitute.org/research/politics-and-policies-guiding-human-embryo-research-united-states (accessed January 25, 2023).
- Maus, M. V. and S. Nikiforow. 2017. The why, what, and how of the new FACT standards for immune effector cells. Journal for Immunotherapy of Cancer 5(36). https://doi.org/10.1186/s40425-017-0239-0.
- McCann, S., and R. P. Gale. 2018. A brief history of bone marrow transplantation. Advances in Cell and Gene Therapy 1(1):e8. https://doi.org/https://doi.org/10.1002/acg2.8.
- McDonald, G. B., B. M. Sandmaier, M. Mielcarek, M. Sorror, S. A. Pergam, G.-S. Cheng, S. Hingorani, M. Boeckh, M. D. Flowers, S. J. Lee, F. R. Appelbaum, R. Storb, P. J. Martin, H. J. Deeg, G. Schoch, and T. A. Gooley. 2020. Survival, nonrelapse mortality, and relapse-related mortality after allogeneic hematopoietic cell transplantation: Comparing 2003–2007 versus 2013–2017 cohorts. Annals of Internal Medicine 172(4):229–239. https://doi.org/10.7326/M19-2936.
- Mertens, J., D. Reid, S. Lau, Y. Kim, and F. H. Gage. 2018. Aging in a dish: iPSC-derived and directly induced neurons for studying brain aging and age-related neurodegenerative diseases. Annual Review of Genetics 52:271–293. https://doi.org/10.1146/annurev-genet-120417-031534.
- Murdoch, B., A. R. Marcon, and T. Caulfield. 2020. The law and problematic marketing by private umbilical cord blood banks. BMC Medical Ethics 21(1):52. https://doi.org/10.1186/s12910-020-00494-2.
- Murdoch, B., A. Zarzeczny, and T. Caulfield. 2018. Exploiting science? A systematic analysis of complementary and alternative medicine clinic websites’ marketing of stem cell therapies. BMJ Open 8(2):e019414. http://dx.doi.org/10.1136/bmjopen-2017-019414.
- Murugan, V. 2009. Embryonic stem cell research: A decade of debate from Bush to Obama. The Yale Journal of Biology and Medicine 82(3):101–103. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2744932 (accessed September 6, 2022).
- National Academies of Sciences, Engineering, and Medicine (NASEM). 2019. Framework for addressing ethical dimensions of emerging and innovative biomedical technologies: A synthesis of relevant National Academies reports. Washington, DC: The National Academies Press. https://doi.org/10.17226/25491.
- National Bioethics Advisory Commission (NBCA). 1999. Ethical issues in human stem cell research: Report and recommendations of the National Bioethics Advisory Commission. Rockville, MD. Available at: https://hdl.handle.net/1805/23 (accessed September 6, 2022).
- National Institutes of Health (NIH). 2013. Statement by NIH Director Francis S. Collins, M.D., Ph.D., on Supreme Court’s decision regarding stem cell case. Available at: https://www.nih.gov/about-nih/who-we-are/nih-director/statements/statement-nih-director-francis-s-collins-md-phd-supreme-courts-decision-regarding-stem-cell-case (accessed September 6, 2022).
- NIH. 2009. NIH guidelines for human stem cell research. Available at: https://stemcells.nih.gov/research-policy/guidelines-for-human-stem-cell-research (accessed September 6, 2022).
- NIH. 1994. Report of the Human Embryo Research Panel. National Institutes of Health, Bethesda, MD. Available at: https://repository.library.georgetown.edu/bitstream/handle/10822/559352/human_embryo_vol_1.pdf?sequence=1&isAllowed=y (accessed September 6, 2022).
- The National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research (NCPHSBBR). 1979. The Belmont Report: Ethical principles and guidelines for the protection of human subjects of research. Available at: https://www.hhs.gov/ohrp/regulations-and-policy/belmont-report/read-the-belmont-report/index.html (accessed September 5, 2022).
- Navarro Negredo, P., R. W. Yeo, and A. Brunet. 2020. Aging and rejuvenation of neural stem cells and their niches. Cell Stem Cell 27(2):202–223. https://doi.org/10.1016/j.stem.2020.07.002.
- The New York Times. 2019. The 1619 Project. Available at: https://www.nytimes.com/interactive/2019/08/14/magazine/1619-america-slavery.html (accessed September 6, 2022).
- The Nobel Prize. 2012. Shinya Yamanaka Nobel lecture. Available at: https://www.nobelprize.org/prizes/medicine/2012/yamanaka/lecture (accessed September 6, 2022).
- The Nobel Prize. 2009. Carol W. Greider biographical. Available at: https://www.nobelprize.org/prizes/medicine/2009/greider/biographical (accessed September 7, 2022).
- The Nobel Prize. 1990. E. Donnall Thomas, facts. Available at: https://www.nobelprize.org/prizes/medicine/1990/thomas/facts/ (accessed September 6, 2022).
- The Nobel Prize. 1980. Jean Dausset Nobel lecture. Available at: https://www.nobelprize.org/prizes/medicine/1980/dausset/lecture (accessed September 6, 2022).
- Ocampo, A., P. Reddy, P. Martinez-Redondo, A. Platero-Luengo, F. Hatanaka, T. Hishida, M. Li, D. Lam, M. Kurita, E. Beyret, T. Araoka, E. Vazquez-Ferrer, D. Donoso, J. L. Roman, J. Xu, C. Rodriguez Esteban, G. Nuñez, E. Nuñez Delicado, J. M. Campistol, I. Guillen, P. Guillen, and J. C. Izpisua Belmonte. 2016. In vivo amelioration of age-associated hallmarks by partial reprogramming. Cell 167(7):1719–1733. https://doi.org/10.1016/j.cell.2016.11.052.
- Online Catalog of Human Genes and Genetic Disorders (OMIM). n.d.a. #603903 sickle cell anemia. Available at: https://www.omim.org/entry/603903 (accessed September 6, 2022).
- OMIM. n.d.b. #219700 cystic fibrosis; CF. Available at: https://www.omim.org/entry/219700 (accessed September 6, 2022).
- OMIM. n.d.c. #310200 muscular dystrophy; Duchenne type; DMD. Available at: https://www.omim.org/entry/310200 (accessed September 6, 2022).
- Osgood, E. E., M. C. Riddle, and T. J. Mathews. 1939. Aplastic anemia treated with daily transfusions and intravenous marrow; case report. Annals of Internal Medicine 13(2):357–367. https://doi.org/10.7326/0003-4819-13-2-357.
- Oudshoorn, M., A. van Leeuwen, H. G. vd Zanden, and J. J. van Rood. 1994. Bone marrow donors worldwide: A successful exercise in international cooperation. Bone Marrow Transplant 14(1):3–8.
- Pauling, L., H. A. Itano, S. J. Singer, and I. C. Wells. 1949. Sickle cell anemia, a molecular disease. Science 110(2865):543–548. https://doi.org/10.1126/science.110.2865.543.
- Pearce, D. A. 2020. We need better regulation of stem cell therapies, especially rogue clinics. Stat, February 6. Available at: https://www.statnews.com/2020/02/06/rogue-stem-cell-clinics-better-regulation (accessed September 6, 2022).
- Personal communication from ISSCR Leadership. n.d.
- Petersdorf, E. W. 2010. The World Marrow Donor Association: 20 years of international collaboration for the support of unrelated donor and cord blood hematopoietic cell transplantation. Bone Marrow Transplantation 45(5):807–810. https://doi.org/10.1038/bmt.2010.10.
- Pew Research Center. 2019. FDA’s framework for regulating regenerative medicine will improve oversight: Further action needed to facilitate development of safe, effective treatments. Available at: https://www.pewtrusts.org/en/research-and-analysis/reports/2019/10/17/fdas-framework-for-regulating-regenerative-medicine-will-improve-oversight (accessed September 6, 2022).
- Pidala, J., J. Kim, M. Schell, S. J. Lee, R. Hillgruber, V. Nye, E. Ayala, M. Alsina, B. Betts, R. Bookout, H. F. Fernandez, T. Field, F. L. Locke, T. Nishihori, J. L. Ochoa, L. Perez, J. Perkins, J. Shapiro, C. Tate, M. Tomblyn, and C. Anasetti. 2013. Race/ethnicity affects the probability of finding an HLA-A, -B, -C and -DRB1 allele-matched unrelated donor and likelihood of subsequent transplant utilization. Bone Marrow Transplantation 48(3):346–350. https://doi.org/10.1038/bmt.2012.150.
- Pinto, V. M., M. Poggi, R. Russo, A. Giusti, and G. L. Forni. 2019. Management of the aging beta-thalassemia transfusion-dependent population—The Italian experience. Blood Reviews 38:100594. https://doi.org/10.1016/j.blre.2019.100594.
- Prabhakar, H., C. Haywood Jr., and R. Molokie. 2010. Sickle cell disease in the United States: Looking back and forward at 100 years of progress in management and survival. American Journal of Hematology 85(5):346–353. https://doi.org/10.1002/ajh.21676.
- President’s Council on Bioethics. 2005. White paper: Alternative sources of pluripotent stem cells. Available at: https://bioethicsarchive.georgetown.edu/pcbe/reports/white_paper/index.html (accessed September 6, 2022).
- Quine, W. E. 1896. Chairman’s address. JAMA 26(21):1012–1016. https://doi.org/10.1001/jama.1896.02430730014001c.
- Rabb, H. S. 1999. Letter to Harold Varmus, MD: Federal funding for research involving human pluripotent stem cells. Available at: https://profiles.nlm.nih.gov/spotlight/mv/catalog/nlm:nlmuid-101584926X407-doc (accessed September 6, 2022).
- Regenberg, A. C., L. A. Hutchinson, B. Schanker, and D. J. Mathews. 2009. Medicine on the fringe: Stem cell‐based interventions in advance of evidence. Stem Cells 27(9):2312–2319. https://doi.org/10.1002/stem.132.
- Rekers, P. E., M. P. Coulter, and S. L. Warren. 1950. Effect of transplantation of bone marrow into irradiated animals. Archives of Surgery 60(4):635–667. https://doi.org/10.1001/archsurg.1950.01250010656001.
- Rickham, P. P. 1964. Human experimentation. Code of ethics of the world medical association. Declaration of Helsinki. British Medical Journal 2(5402):177. https://doi.org/10.1136/bmj.2.5402.177.
- Robertson, J. A. 2010. Embryo stem cell research: Ten years of controversy. Journal of Law, Medicine & Ethics 38(2):191–203. https://doi.org/10.1111/j.1748-720X.2010.00479.x.
- Sacchi, N., P. Costeas, L. Hartwell, C. K. Hurley, C. Raffoux, A. Rosenmayr, H. Greinix, and the Quality Assurance and Clinical Working Groups of the World Marrow Donor Association. 2008. Haematopoietic stem cell donor registries: World Marrow Donor Association recommendations for evaluation of donor health. Bone Marrow Transplantation 42(1):9–14. https://doi.org/10.1038/bmt.2008.76.
- Sagonowsky, E. 2020. New sickle cell drugs from Novartis, GBT need big discounts: ICER draft. Fierce Pharma, January 27. Available at: https://www.fiercepharma.com/pharma/new-sickle-cell-disease-drugs-from-novartis-global-blood-therapeutics-need-big-discounts (accessed September 7, 2022).
- Scott, R. B. 1970. Health care priority and sickle cell anemia. JAMA 214(4):731–734. https://doi.org/10.1001/jama.1970.03180040039008.
- Scudellari, M. 2016. How iPS cells changed the world. Nature 534(7607):310–312. https://doi.org/10.1038/534310a.
- Shamblott, M. J., J. Axelman, S. Wang, E. M. Bugg, J. W. Littlefield, P. J. Donovan, P. D. Blumenthal, G. R. Huggins, and J. D. Gearhart. 1998. Derivation of pluripotent stem cells from cultured human primordial germ cells. Proceedings of the National Academy of Sciences 95(23):13726–13731. https://doi.org/10.1073/pnas.95.23.13726.
- Shearer, W. T., B. H. Lubin, M. S. Cairo, L. D. Notarangelo, Section on Hematology/Oncology, Section on Allergy and Immunology, J. Hord, G. Crouch, G. Hale, J. Harper, J. Lipton, Z. Rogers, E. Werner, E. C. Matsui, S. L. Abramson, C. Dinakar, A.-M. Irani, T. A. Mahr, J. S. Kim, M. Pistiner, and J. Wang. 2017. Cord blood banking for potential future transplantation. Pediatrics 140(5):e20172695. https://doi.org/10.1542/peds.2017-2695.
- Sherkow, J. S. 2017. CRISPR, patents, and the public health. Yale Journal of Biology and Medicine 90:667–672. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5733839/ (accessed September 6, 2022).
- Shyntum, Y., and E. Kalkreuter. 2009. Stem cell patents—reexamination/litigation—the last 5 years. Tissue Engineering: Part B Reviews 15(1):87–90. https://doi.org/10.1089/ten.teb.2008.0456.
- Smith, L. A., S. O. Oyeku, C. Homer, and B. Zuckerman. 2006. Sickle cell disease: A question of equity and quality. Pediatrics 117(5):1763–1770. https://doi.org/10.1542/peds.2005-1611.
- Spehar, K., A. Pan, and I. Beerman. 2020. Restoring aged stem cell functionality: Current progress and future directions. Stem Cells 38(9):1060–1077. https://doi.org/10.1002/stem.3234.
- Stamatoyannopoulos, G., W. G. Wood, T. Papayannopoulou, and P. E. Nute. 1975. A new form of hereditary persistence of fetal hemoglobin in blacks and its association with sickle cell trait. Blood 46(5):683–692. Available at: https://www.sciencedirect.com/science/article/pii/S0006497120742205?via%3Dihub (accessed September 6, 2022).
- Stein, R. 2020. 1st Patients to get CRISPR gene-editing treatment continue to thrive. NPR Morning Edition, December 15. Available at: https://www.npr.org/sections/health-shots/2020/12/15/944184405/1st-patients-to-get-crispr-gene-editing-treatment-continue-to-thrive (accessed September 6, 2022).
- Svendsen, C. N. 2013. Back to the future: How human induced pluripotent stem cells will transform regenerative medicine. Human Molecular Genetics 22(R1):R32–R38. https://doi.org/10.1093/hmg/ddt379.
- Sun, J., J. Allison, C. McLaughlin, L. Sledge, B. Waters-Pick, S. Wease, and J. Kurtzberg. 2010. Differences in quality between privately and publicly banked umbilical cord blood units: A pilot study of autologous cord blood infusion in children with acquired neurologic disorders. Transfusion 50(9):1980–1987. https://doi.org/10.1111/j.1537-2995.2010.02720.x.
- Tachibana, M., P. Amato, M. Sparman, N. M. Gutierrez, R. Tippner-Hedges, H. Ma, E. Kang, A. Fulati, H. S. Lee, H. Sritanaudomchai, K. Masterson, J. Larson, D. Eaton, K. Sadler-Fredd, D. Battaglia, D. Lee, D. Wu, J. Jensen, P. Patton, S. Gokhale, R. L. Stouffer, D. Wolf, and S. Mitalipov. 2013. Human embryonic stem cells derived by somatic cell nuclear transfer. Cell 153(6):1228–1238. https://doi.org/10.1016/j.cell.2013.05.006.
- Takahashi, K., and S. Yamanaka. 2006. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126(4):663–676. https://doi.org/10.1016/j.cell.2006.07.024.
- Taylor, P. L., R. A. Barker, K. G. Blume, E. Cattaneo, A. Colman, H. Deng, H. Edgar, I. J. Fox, C. Gerstle, L. S. Goldstein, K. A. High, A. Lyall, R. Parkman, F. J. Pitossi, E. D. Prentice, H. M. Rooke, D. A. Sipp, A. Srivastava, S. Stayn, G. K. Steinberg, A. J. Wagers, and I. L. Weissman. 2010. Patients beware: Commercialized stem cell treatments on the web. Cell Stem Cell 7(1):43–49. https://doi.org/10.1016/J.STEM.2010.06.001.
- Thomas, E. D. 2005. Bone marrow transplantation from the personal viewpoint. International Journal of Hematology 81(2):89–93. https://doi.org/10.1532/ijh97.04197.
- Thomas, E. D. 1999. A history of haemopoietic cell transplantation. British Journal of Haematology 105(2):330–339. https://doi.org/10.1111/j.1365-2141.1999.01337.x.
- Thomas, E. D., C. D. Buckner, J. E. Sanders, T. Papayannopoulou, C. Borgna-Pignatti, P. De Stefano, K. M. Sullivan, R. A. Clift, and R. Storb. 1982. Marrow transplantation for thalassaemia. The Lancet 320(8292): 227–229. https://doi.org/10.1016/s0140-6736(82)90319-1.
- Thomas, E. D., H. L. Lochte, Jr., J. H. Cannon, O. D. Sahler, and J. W. Ferrebee. 1959. Supralethal whole body irradiation and isologous marrow transplantation in man. Journal of Clinical Investigation 38(10 Pt 1–2):1709–1716. https://doi.org/10.1172/JCI103949.
- Thomson, J. A., J. Itskovitz-Eldor, S. S. Shapiro, M. A. Waknitz, J. J. Swiergiel, V. S. Marshall, and J. M. Jones. 1998. Embryonic stem cell lines derived from human blastocysts. Science 282(5391):1145–1147. https://doi.org/10.1126/science.282.5391.1145.
- Todd, K. 2017. Hope for to-marrow: The status of paid peripheral blood stem cell donation under the national organ transplant act. Journal of Law and the Biosciences 4(2):412–423. https://doi.org/10.1093/jlb/lsx027.
- Turner, L., and P. Knoepfler. 2016. Selling stem cells in the USA: Assessing the direct-to-consumer industry. Cell Stem Cell 19(2):154–157. https://doi.org/10.1016/j.stem.2016.06.007.
- U.S. Code § 262. Regulation of biological products. Available at: https://www.law.cornell.edu/uscode/text/42/262 (accessed September 7, 2022).
- Van Rood, J. J., and M. Oudshoorn. 2008. Eleven million donors in bone marrow donors worldwide! Time for reassessment? Bone Marrow Transplantation 41(1):1–9. https://doi.org/10.1038/sj.bmt.1705866.
- Verginer, L. and M. Riccaboni. 2021. Stem cell legislation and its impact on the geographic preferences of stem cell researchers. Eurasian Business Review 11:163–189. https://doi.org/10.1007/s40821-021-00182-0.
- Vermylen, C., E. Fernandez Robles, J. Ninane, and G. Cornu. 1988. Bone marrow transplantation in five children with sickle cell anaemia. Lancet 1(8600):1427–1428. https://doi.org/10.1016/s0140-6736(88)92239-8.
- Wailoo, K. 2017. Sickle cell disease—a history of progress and peril. New England Journal of Medicine 376(9):805–807. https://doi.org/10.1056/NEJMp1700101.
- Wailoo, K., and S. Pemberton. 2006. The troubled dream of genetic medicine: ethnicity and innovation in Tay-Sachs, cystic fibrosis, and sickle cell disease. Baltimore, MD: Johns Hopkins University Press.
- Wan, W., and L. McGinley. 2019. FDA wins groundbreaking case against for-profit stem cell company. The Washington Post, June 4. Available at: https://www.washingtonpost.com/health/fda-wins-groundbreaking-case-against-for-profit-stem-cell-company/2019/06/03/498373fa-864e-11e9-98c1-e945ae5db8fb_story.html (accessed September 6, 2022).
- Watson, J., A. W. Stahman, and F. P. Bilello. 1948. The significance of the paucity of sickle cells in newborn Negro infants. Obstetrical & Gynecological Survey 3(6):819–820. Available at: https://journals.lww.com/obgynsurvey/citation/1948/12000/the_significance_of_the_paucity_of_sickle_cells_in.22.aspx (accessed September 6, 2022).
- White House. 2009. Executive Order 13505. Removing barriers to responsible scientific research involving human stem cells. Available at: https://obamawhitehouse.archives.gov/the-press-office/removing-barriers-responsible-scientific-research-involving-human-stem-cells (accessed September 6, 2022).
- World Marrow Donor Association (WMDA). 2022. WMDA scientific publications & recommendations. Available at: https://share.wmda.info/pages/viewpage.action?pageId=322174994 (accessed September 7, 2022).
- WMDA. 2021. Total number of donors and cord blood units. Available at: https://statistics.wmda.info (accessed September 6, 2022).
- Yawn, B. P., G. R. Buchanan, A. N. Afenyi-Annan, S. K. Ballas, K. L. Hassell, A. H. James, L. Jordan, S. M. Lanzkron, R. Lottenberg, W. J. Savage, P. J. Tanabe, R. E. Ware, M. H. Murad, J. C. Goldsmith, E. Ortiz, R. Fulwood, A. Horton, and J. John-Sowah. 2014. Management of sickle cell disease: Summary of the 2014 evidence-based report by expert panel members. JAMA 312(10):1033–1048. https://doi.org/10.1001/jama.2014.10517.
- Yu, J., M. A. Vodyanik, K. Smuga-Otto, J. Antosiewicz-Bourget, J. L. Frane, S. Tian, J. Nie, G. A. Jonsdottir, V. Ruotti, R. Stewart, Slukvin, II, and J. A. Thomson. 2007. Induced pluripotent stem cell lines derived from human somatic cells. Science 318(5858):1917–1920. https://doi.org/10.1126/science.1151526.
- Zarzeczny, A., T. Caulfield, U. Ogbogu, P. Bell, V. A. Crooks, K. Kamenova, Z. Master, C. Rachul, J. Snyder, M. Toews, and S. Zoeller. 2014. Professional regulation: a potentially valuable tool in responding to “stem cell tourism.” Stem Cell Reports 3(3):379–384. https://doi.org/10.1016/j.stemcr.2014.06.016.
- Zhou, X., Y. Hong, H. Zhang, and X. Li. 2020. Mesenchymal stem cell senescence and rejuvenation: Current status and challenges. Frontiers of Cell Development and Biology 8:364. https://doi.org/10.3389/fcell.2020.00364.
https://doi.org/10.31478/202311d
Suggested Citation
Mathews, D., A. Abernethy, E. Chaikof, R. A. Charo, G. Q. Daley, J. Enriquez, S. Gottlieb, J. Kahn, R. D. Klausner, S. Tavazoie, R. Fabi, A. C. Offodile II, J. S. Sherkow, R. D. Sullenger, E. Freiling, and C. Balatbat. 2023. Regenerative Medicine: Case Study for Understanding and Anticipating Emerging Science and Technology. NAM Perspectives. Discussion Paper, National Academy of Medicine, Washington, DC. https://doi.org/10.31478/202311d .
Author Information
Debra Mathews, PhD, MA, is Associate Director for Research and Programs at the Johns Hopkins Berman Institute of Bioethics and Professor, Department of Genetic Medicine at the Johns Hopkins University School of Medicine. Amy Abernethy, MD, PhD, is President of Product Development and Chief Medical Officer at Verily. Elliot Chaikof, MD, PhD, is Chair, Department of Surgery and Surgeon-in-Chief at Beth Israel Deaconess Medical Center, and Johnson and Johnson Professor of Surgery at Harvard Medical School. R. Alta Charo, JD, is Principal, Alta Charo Consulting, LLC and Warren P. Knowles Professor Emerita of Law and Bioethics at University of Wisconsin-Madison. George Q. Daley, MD, PhD, is Dean of the Faculty of Medicine and Caroline Shields Walker Professor of Medicine at Harvard Medical School. Juan Enriquez, MBA, is Managing Director at Excel Venture Management. Scott Gottlieb, MD, is Senior Fellow at the American Enterprise Institute. Jeffrey Kahn, PhD, is Andreas C. Dracopoulos Director and Robert Henry Levi and Ryda Hecht Levi Professor of Bioethics and Public Policy at Johns Hopkins Berman Institute of Bioethics, and Professor, Department of Health Policy and Management, Johns Hopkins Bloomberg School of Public Health. Richard D. Klausner, MD, is Founder and Board Chair at Lyell Immunopharma. Sohail Tavazoie, MD, PhD, is Leon Hess Profesor and Senior Attending Physician at The Rockefeller University. Rachel Fabi, PhD, is Associate Professor, Center for Bioethics and Humanities at SUNY Upstate Medical University. Anaeze C. Offodile II, MD, MPH, is Chief Strategy Officer at Memorial Sloan Kettering Cancer Center. Jacob S. Sherkow, JD, MA, is Professor of Law at the Illinois College of Law, Professor of Medicine at the Carle Illinois College of Medicine, Professor at the European Union Center, and Affiliate of the Carl R. Woese Institute for Genomic Biology at the University of Illinois. Rebecca D. Sullenger, BSPH, is a medical student at the Duke University School of Medicine. Emma Freiling, BA, is a Research Associate at the National Academy of Medicine. Celynne Balatbat, BA, was the Special Assistant to the NAM President at the National Academy of Medicine while this paper was authored.
Acknowledgments
This manuscript benefitted from the thoughtful input of Guillermo Ameer, Northwestern University; and Kavita Shah Arora, University of North Carolina at Chapel Hill
Conflict-of-Interest Disclosures
Amy Abernethy reports personal fees from Verily/Alphabet, relationships with Georgiamune and EQRx, and personal investments in Iterative Health and One Health, outside the submitted work. Elliot Chaikof reports grants from the National Institutes of Health, outside the submitted work. George Q. Daley reports holding equity from Redona Therapeutics and from iTCells, outside the submitted work. Juan Enriquez reports investments with Excel Venture Management, outside the submitted work; investments in various life science technologies, including leading-edge brain technologies, and co-authoring a book on the impact of emerging brain technologies. Scott Gottlieb reports personal fees from Pfizer, Inc, Illumina, Inc, Aetion, Tempus Labs, National Resilience, Inc, Cell-Carta, Parker Institute for Cancer Immunotherapy, Mount Sinai Health System, New Enterprise Associates, and American Enterprise Institute outside the submitted work. Sohail Tavazoie reports personal fees from Inspirna, outside the submitted work. Jacob S. Sherkow reports employment with the University of Illinois, grants from National Institutes of Health, and personal fees from Expert Consulting services, outside the submitted work.
Correspondence
Questions or comments should be directed to Debra Mathews at [email protected].
The views expressed in this paper are those of the authors and not necessarily of the authors’ organizations, the National Academy of Medicine (NAM), or the National Academies of Sciences, Engineering, and Medicine (the National Academies). The paper is intended to help inform and stimulate discussion. It is not a report of the NAM or the National Academies. Copyright by the National Academy of Sciences. All rights reserved.
Join Our Community
Sign up for nam email updates.
Case study on adoption of new technology for innovation: Perspective of institutional and corporate entrepreneurship
Asia Pacific Journal of Innovation and Entrepreneurship
ISSN : 2398-7812
Article publication date: 7 August 2017
This paper aims at investigating the role of institutional entrepreneurship and corporate entrepreneurship to cope with firm’ impasses by adoption of the new technology ahead of other firms. Also, this paper elucidates the importance of own specific institutional and corporate entrepreneurship created from firm’s norm.
Design/methodology/approach
The utilized research frame is as follows: first, perspective of studies on institutional and corporate entrepreneurship are performed using prior literature and preliminary references; second, analytical research frame was proposed; finally, phase-based cases are conducted so as to identify research objective.
Kumho Tire was the first tire manufacturer in the world to exploit the utilization of radio-frequency identification for passenger carâ’s tire. Kumho Tire takes great satisfaction in lots of failures to develop the cutting edge technology using advanced information and communication technology cultivated by heterogeneous institution and corporate entrepreneurship.
Originality/value
The firm concentrated its resources into building the organization’s communication process and enhancing the quality of its human resources from the early stages of their birth so as to create distinguishable corporate entrepreneurship.
- Corporate entrepreneurship
- Institutional entrepreneurship
Han, J. and Park, C.-m. (2017), "Case study on adoption of new technology for innovation: Perspective of institutional and corporate entrepreneurship", Asia Pacific Journal of Innovation and Entrepreneurship , Vol. 11 No. 2, pp. 144-158. https://doi.org/10.1108/APJIE-08-2017-031
Emerald Publishing Limited
Copyright © 2017, Junghee Han and Chang-min Park.
Published in the Asia Pacific Journal of Innovation and Entrepreneurship . Published by Emerald Publishing Limited. This article is published under the Creative Commons Attribution (CC BY 4.0) licence. Anyone may reproduce, distribute, translate and create derivative works of this article (for both commercial & non-commercial purposes), subject to full attribution to the original publication and authors. The full terms of this licence may be seen at http://creativecommons.org/licenses/by/4.0/legalcode
1. Introduction
Without the entrepreneur, invention and new knowledge possibly have lain dormant in the memory of persons or in the pages of literature. There is a Korean saying, “Even if the beads are too much, they become treasure after sewn”. This implies importance of entrepreneurship. In general, innovativeness and risk-taking are associated with entrepreneurial activity and, more importantly, are considered to be important attributes that impact the implementation of new knowledge pursuing.
Implementation of cutting edge technology ahead of other firms is an important mechanism for firms to achieve competitive advantage ( Capon et al. , 1990 ; D’Aveni, 1994 ). Certainly, new product innovation continues to play a vital role in competitive business environment and is considered to be a key driver of firm performance, especially as a significant form of corporate entrepreneurship ( Srivastava and Lee, 2005 ). Corporate entrepreneurship is critical success factor for a firm’s survival, profitability and growth ( Phan et al. , 2009 ).
The first-mover has identified innovativeness and risk-taking as important attributes of first movers. Lumpkin and Dess (1996) argued that proactiveness is a key entrepreneurial characteristic related to new technology adoption and product. This study aims to investigate the importance of corporate and institutional entrepreneurship through analyzing the K Tire’s first adaptation of Radio-frequency identification (RFID) among the world tire manufactures. Also, this paper can contribute to start ups’ readiness for cultivating of corporate and institutional entrepreneurship from initial stage to grow and survive.
K Tire is the Korean company that, for the first time in the world, applied RFID to manufacturing passenger vehicle tires in 2013. Through such efforts, the company has built an innovation model that utilizes ICTs. The adoption of the technology distinguishes K Tire from other competitors, which usually rely on bar codes. None of the global tire manufacturers have applied the RFID technology to passenger vehicle tires. K Tire’s decision to apply RFID to passenger vehicle tires for the first time in the global tire industry, despite the uncertainties associated with the adoption of innovative technologies, is being lauded as a successful case of innovation. In the global tire market, K Tire belongs to the second tier, rather than the leader group consisting of manufacturers with large market shares. Then, what led K Tire to apply RFID technology to the innovation of its manufacturing process? A company that adopts innovative technologies ahead of others, even if the company is a latecomer, demonstrates its distinguishing characteristics in terms of innovation. As such, this study was motivated by the following questions. With regard to the factors that facilitate innovation, first, what kind of the corporate and institutional situations that make a company more pursue innovation? Second, what are the technological situations? Third, how do the environmental situations affect innovation? A case study offers the benefit of a closer insight into the entrepreneurship frame of a specific company. This study has its frame work rooted in corporate entrepreneurship ( Guth and Ginsberg, 1990 ; Shane and Venkataraman, 2000 ) and institutional entrepreneurship ( Battilana, 2006 ; Fligstein, 1997 ; Rojas, 2010 ). As mentioned, we utilized qualitative research method ( Yin, 2008 ). This paper is structured as follows. Section two presents the literature review, and section three present the methodology and a research case. Four and five presents discussion and conclusions and implications, respectively.
2. Theoretical review and analysis model
RFID technology is to be considered as not high technology; however, it is an entirely cutting edged skills when combined with automotive tire manufacturing. To examine why and how the firm behaves like the first movers, taking incomparable high risks to achieve aims unlike others, we review three kinds of prior literature. As firms move from stage to stage, they have to revamp innovative capabilities to survive and ceaseless stimulate growth.
2.1 Nature of corporate entrepreneurship
Before reviewing the corporate entrepreneurship, it is needed to understand what entrepreneurship is. To more understand the role that entrepreneurship plays in modern economy, one need refer to insights given by Schumpeter (1942) or Kirzner (1997) . Schumpeter suggests that entrepreneurship is an engine of economic growth by utilization of new technologies. He also insists potential for serving to discipline firms in their struggle to survive gale of creative destruction. While Schumper argued principle of entrepreneurship, Kirzner explains the importance of opportunities. The disruptions generated by creative destruction are exploited by individuals who are alert enough to exploit the opportunities that arise ( Kirzner, 1997 ; Shane and Venkataraman, 2000 ).
Commonly all these perspectives on entrepreneurship is an appreciation that the emergence of novelty is not an easy or predictable process. Based on literature review, we note that entrepreneurship is heterogeneous interests and seek “something new” associated with novel outcomes. Considering the literature review, we can observe that entrepreneurship is the belief in individual autonomy and discretion, and a mindset that locates agency in individuals for creating new activities ( Meyer et al. ,1994 ; Jepperson and Meyer, 2001 ).
the firm’s commitment to innovation (including creation and introduction of products, emphasis on R&D investments and commitment to patenting);
the firm’s venturing activities, such as entry into new business fields by sponsoring new ventures and creating new businesses; and
strategic renewal efforts aimed at revitalizing the firm’s ability to compete.
developing innovation an organizational tool;
allowing the employees to propose ideas; and
encouraging and nurturing the new knowledge ( Hisrich, 1986 ; Kuratko, 2007 ).
Consistent with the above stream of research, our paper focuses on a firm’s new adaptation of RFID as a significant form of corporate entrepreneurial activity. Thus, CE refers to the activities a firm undertakes to stimulate innovation and encourage calculated risk taking throughout its operations. Considering prior literature reviews, we propose that corporate entrepreneurship is the process by which individuals inside the organization pursuing opportunities without regards to the resources they control.
If a firm has corporate entrepreneurship, innovation (i.e. transformation of the existing firm, the birth of new business organization and innovation) happens. In sum, corporate entrepreneurship plays a role to pursue to be a first mover from a latecomer by encompassing the three phenomena.
2.2 Institution and institutional entrepreneurship
Most literature regarding entrepreneurship deals with the attribute of individual behavior. More recently, scholars have attended to the wider ecosystem that serves to reinforce risk-taking behavior. Institution and institutional entrepreneurship is one way to look at ecosystem that how individuals and groups attempt to try to become entrepreneurial activities and innovation.
Each organization has original norm and intangible rules. According to the suggestion by Scott (1995) , institutions constrain behavior as a result of processes associated with institutional pillars. The question how actors within the organizations become motivated and enabled to transform the taken-for-granted structures has attracted substantial attention for institutionalist. To understand why some firms are more likely to seek innovation activities despite numerous difficulties and obstacles, we should take look at the institutional entrepreneurship.
the regulative, which induces worker’s action through coercion and formal sanction;
the normative, which induces worker’s action through norms of acceptability and ethics; and
the cognitive, which induces worker’s action through categories and frames by which actors know and interpret their world.
North (1990) defines institutions as the humanly devised constraints that structure human action. Actors within some organization with sufficient resources have intend to look at them an opportunity to realize interests that they value highly ( DiMaggio, 1988 ).
It opened institutional arguments to ideas from the co-evolving entrepreneurship literature ( Aldrich and Fiol, 1994 ; Aldrich and Martinez, 2001 ). The core argument of the institutional entrepreneurship is mechanisms enabling force to motivate for actors to act difficult task based on norm, culture and shared value. The innovation, adopting RFID, a technology not verified in terms of its effectiveness for tires, can be influenced by the institution of the society.
A firm is the organizations. An organization is situated within an institution that has social and economic norms. Opportunity is important for entrepreneurship. The concept of institutional entrepreneurship refer to the activities of worker or actor who have new opportunity to realize interest that they values highly ( DiMaggio, 1988 ). DiMaggio (1988) argues that opportunity for institutional entrepreneurship will be “seen” and “exploited” by within workers and not others depending on their resources and interests respectively.
Despite that ambiguity for success was given, opportunity and motivation for entrepreneurs to act strategically, shape emerging institutional arrangements or standards to their interests ( Fligstein and Mara-Drita, 1996 ; Garud et al. , 2002 ; Hargadon and Douglas, 2001 ; Maguire et al. , 2004 ).
Resource related to opportunity within institutional entrepreneurship include formal or informal authority and power ( Battilana, 2006 ; Rojas, 2010 ). Maguire et al. (2004) suggest legitimacy as an important ingredient related to opportunity for institutional entrepreneurship. Some scholars suggest opportunity resources for institutional entrepreneurship as various aspects. For instance, Marquire and Hardy (2009) show that knowledge and expertise is more crucial resources. Social capital, including market leadership and social network, is importance resource related to opportunity ( Garud et al. , 2002 ; Lawrence et al. , 2005 ; Townley, 2002 ). From a sociological perspective, change associated with entrepreneurship implies deviations from some norm ( Garud and Karnøe, 2003 ).
Institutional entrepreneurship is therefore a concept that reintroduces agency, interests and power into institutional analyses of organizations. Based on the previous discussion, this study defines institution as three processes of network activity; coercion and formal sanction, normative and cognitive, to acquire the external knowledge from adopting common goals and rules inside an organization. It would be an interesting approach to look into a specific company to see whether it is proactive towards adopting ICTs (e.g. RFID) and innovation on the basis of such theoretical background.
2.3. Theoretical analysis frame
Companies innovate themselves in response to the challenges of the ever-changing markets and technologies, so as to ensure their survival and growth ( Tushman and Anderson, 1986 ; Tidd and Bessant, 2009 ; Teece, 2014 ). As illustrated above, to achieve the purpose of this study, the researcher provides the following frames of analyses based on the theoretical background discussed above ( Figure 1 ).
3. Case study
3.1 methodology.
It is a highly complicated and tough task to analyze the long process of innovation at a company. In this paper, we used analytical approach rather than the problem-oriented method because the case is examined to find and understand what has happened and why. It is not necessary to identify problems or suggest solutions. Namely, this paper analyzes that “why K Tire becomes a first mover from a late comer through first adoption of RFID technology for automotive tire manufacture with regards to process and production innovations”.
To study the organizational characteristics such as corporate entrepreneurship, institutional entrepreneurship, innovation process of companies, the qualitative case study is the suitable method. This is because a case study is a useful method when verifying or expanding well-known theories or challenging a specific theory ( Yin, 2008 ). This study seeks to state the frame of analysis established, based on previously established theories through a single case. K Tire was selected as the sample because it is the first global tire manufacturer, first mover to achieve innovation by developing and applying RFID.
The data for the case study were collected as follows. First, this study was conducted from April 2015 to the end of December 2015. Additional expanded data also were collected from September 12 to November 22, 2016, to pursue the goal of this paper. Coauthor worked for K Tire for more than 30 year, and currently serves as the CEO of an affiliate company. As such, we had the most hands-on knowledge and directed data in the process of adoption RFID. This makes this case study a form of participant observation ( Yin, 2008 ). To secure data on institutional entrepreneurship, in-depth interviews were conducted with the vice president of K Tire. The required data were secured using e-mail, and the researchers accepted the interviewees’ demand to keep certain sensitive matters confidential. The interviewees agreed to record the interview sessions. In this way, a 20-min interview data were secured for each interviewee. In addition, apart from the internal data of the subject company, other objective data were obtained by investigating various literatures published through the press.
3.2 Company overview
In September 1960, K Tire was established in South Korea as the name of Samyang Tire. In that time, the domestic automobile industry in Korea was at a primitive stage, as were auto motive parts industries like the tire industry. K Tire products 20 tires a day, depending on manual labor because of our backward technology and shortage of facilities.
The growth of K Tire was astonishment. Despite the 1974 oil shock and difficulties in procuring raw materials, K Tire managed to achieve remarkable growth. In 1976, K Tire became the leader in the tire sector and was listed on the Korea Stock Exchange. Songjung plant II was added in 1977. Receiving the grand prize of the Korea Quality Control Award in 1979, K Tire sharpened its corporate image with the public. The turmoil of political instability and feverish democratization in the 1980s worsened the business environment. K Tire also underwent labor-management struggles but succeeded in straightening out one issue after another. In the meantime, the company chalked up a total output of 50 million tires, broke ground for its Koksung plant and completed its proving ground in preparation for a new takeoff.
In the 1990s, K Tire expanded its research capability and founded technical research centers in the USA and the United Kingdom to establish a global R&D network. It also concentrated its capabilities in securing the foundation as a global brand, by building world-class R&D capabilities and production systems. Even in the 2000s, the company maintained its growth as a global company through continued R&D efforts by securing its production and quality capabilities, supplying tires for new models to Mercedes, Benz, Volkswagen and other global auto manufacturers.
3.3 Implementation of radio-frequency identification technology
RFID is radio-frequency identification technology to recognize stored information by using a magnetic carrier wave. RFID tags can be either passive, active or battery-assisted passive (BAP). An active tag has an on-board battery and periodically transmits its ID signal. A BAP has a small battery on board and is activated when in the presence of an RFID reader. A passive tag is cheaper and smaller because it has no battery; instead, the tag uses the radio energy transmitted by the reader. However, to operate a passive tag, it must be illuminated with a power level roughly a thousand times stronger than for signal transmission. That makes a difference in interference and in exposure to radiation.
an integrated circuit for storing and processing information, modulating and demodulating a radio frequency signal, collecting DC power from the incident reader signal, and other specialized functions; and
an antenna for receiving and transmitting the signal.
capable of recognizing information without contact;
capable of recognizing information regardless of the direction;
capable of reading and saving a large amount of data;
requires less time to recognize information;
can be designed or manufactured in accordance with the system or environmental requirements;
capable of recognizing data unaffected by contamination or the environment;
not easily damaged and cheaper to maintain, compared with the bar code system; and
tags are reusable.
3.3.1 Phase 1. Background of exploitation of radio-frequency identification (2005-2010).
Despite rapid growth of K Tire since 1960, K Tire ranked at the 13th place in the global market (around 2 per cent of the global market share) as of 2012. To enlarge global market share is desperate homework. K Tire was indispensable to develop the discriminated technologies. When bar code system commonly used by the competitors, and the industry leaders, K Tire had a decision for adoption of RFID technology instead of bar code system for tires as a first mover strategy instead of a late comer with regard to manufacture tires for personal vehicle. In fact, K Tire met two kinds of hardship. Among the top 20, the second-tier companies with market shares of 1-2 per cent are immersed in fiercer competitions to advance their ranks. The fierceness of the competition is reflected in the fact that of the companies ranked between the 11th and 20th place, only two maintained their rank from 2013.
With the demand for stricter product quality control and manufacture history tracking expanding among the auto manufacturers, tire manufacturers have come to face the need to change their way of production and logistics management. Furthermore, a tire manufacturer cannot survive if it does not properly respond to the ever stricter and exacting demand for safe passenger vehicle tires of higher quality from customers and auto manufacturers. As mentioned above, K Tire became one of the top 10 companies in the global markets, recording fast growth until the early 2000. During this period, K Tire drew the attention of the global markets with a series of new technologies and innovative technologies through active R&D efforts. Of those new products, innovative products – such as ultra-high-performance tires – led the global markets and spurred the company’s growth. However, into the 2010s, the propriety of the UHP tire technology was gradually lost, and the effect of the innovation grew weaker as the global leading companies stepped forward to take the reign in the markets. Subsequently, K Tire suffered from difficulties across its businesses, owing to the failure to develop follow-up innovative products or market-leading products, as well as the aggressive activities by the company’s hardline labor union. Such difficulties pushed K Tire down to the 13th position in 2014, which sparked the dire need to bring about innovative changes within the company.
3.3.2 Phase 2. Ceaseless endeavor and its failure (2011-2012).
It needs to be lightweight : An RFID tag attached inside a vehicle may adversely affect the weight balance of the tires. A heavier tag has greater adverse impact on the tire performance. Therefore, a tag needs to be as light as possible.
It needs to be durable : Passenger vehicle tires are exposed to extensive bending and stretching, as well as high levels of momentum, which may damage a tag, particularly causing damage to or even loss of the antenna section.
It needs to maintain adhesiveness : Tags are attached on the inner surface, which increase the possibility of the tags falling off from the surface while the vehicle is in motion.
It needs to be resistant to high temperature and high pressure : While going through the tire manufacture process, a tag is exposed to a high temperature of around 200°C and high pressure of around 30 bars. Therefore, a tag should maintain its physical integrity and function at such high pressure and temperature.
It needs to be less costly : A passenger vehicle tire is smaller, and therefore cheaper than truck/bus tires. As a result, an RFID tag places are greater burden on the production cost.
Uncountable tag prototypes, were applied to around 200 test tires in South Korea for actual driving tests. Around 150 prototypes were sent to extremely hot regions overseas for actual driving tests. However, the driving tests revealed damage to the antenna sections of the tags embedded in tires, as the tires reached the end of their wear life. Also, there was separation of the embedded tags from the rubber layers. This confirmed the risk of tire separation, resulting in the failure of the tag development attempt.
3.3.3 Phase 3. Success of adoption RFID (2013-2014).
Despite the numerous difficulties and failures in the course of development, the company ultimately emerged successful, owing to its institutional entrepreneurship and corporate entrepreneurship the government’s support. Owing to the government-led support project, K Tire resumed its RFID development efforts in 2011. This time, the company discarded the idea of the embedded-type tag, which was attempted during the first development. Instead, the company turned to attached-type tag. The initial stages were marked with numerous failures: the size of a tag was large at 20 × 70 mm, which had adverse impact on the rotation balance of the tires, and the attached area was too large, causing the attached sections to fall off as the tire stretched and bent. That was when all personnel from the technical, manufacturing, and logistics department participated in creating ideas to resolve the tag size and adhesiveness issues. Through cooperation across the different departments and repeated tests, K Tire successfully developed its RFID tag by coming up with new methods to minimize the tag size to its current size (9 × 45 mm), maintain adhesiveness and lower the tag price. Finally, K Tire was success the adoption RFID.
3.3.4 Phase 4. Establishment of the manufacture, logistics and marketing tracking system.
Whenever subtle and problematic innovation difficulties arise, every worker and board member moves forward through networking and knowledge sharing within intra and external.
While a bar code is only capable of storing the information on the nationality, manufacturer and category of a product, an RFID tag is capable of storing a far wider scope of information: nationality, manufacturer, category, manufacturing date, machines used, lot number, size, color, quantity, date and place of delivery and recipient. In addition, while the data stored in a bar code cannot be revised or expanded once the code is generated, an RFID tag allows for revisions, additions and removal of data. As for the recognition capability, a bar code recognizes 95per cent of the data at the maximum temperature of 70°C. An RFID tag, on the other hand, recognizes 99.9 per cent of the data at 120°C.
The manufacture and transportation information during the semi-finished product process before the shaping process is stored in the RFID tags, which is attached to the delivery equipment to be provided to the MLMTS;
Logistics Products released from the manufacture process are stored in the warehouses, to be released and transported again to logistics centers inside and outside of South Korea. The RFID tags record the warehousing information, as the products are stored into the warehouses, as well as the release information as the products are released. The information is instantly delivered to the MLMTS;
As a marketing, the RFID tags record the warehousing information of the products supplied and received by sales branches from the logistics centers, as well as the sales information of the products sold to consumers. The information is instantly delivered to the MLMTS; and
As a role of integrative Server, MLM Integrative Server manages the overall information transmitted from the infrastructures for each section (production information, inventory status and release information, product position and inventory information, consumer sales information, etc.).
The MLMTS provides the company with various systemic functions to integrate and manage such information: foolproof against manufacture process errors, manufacture history and quality tracking for each individual product, warehousing/releasing and inventory status control for each process, product position control between processes, real-time warehouse monitoring, release control and history information tracking across products of different sizes, as well as link/control of sales and customer information. To consumers, the system provides convenience services by providing production and quality information of the products, provision of the product history through full tracking in the case of a claim, as well as a tire pressure monitoring system:
“South korea’s K Tire Co. Inc. has begun applying radio-frequency identification (RFID) system tags on: half-finished” tire since June 16. We are now using an IoT based production and distribution integrated management system to apply RFID system on our “half-finished products” the tire maker said, claiming this is a world-first in the industry. The technology will enable K Tire to manage products more efficiently than its competitors, according to the company. RFID allows access to information about a product’s location, storage and release history, as well as its inventory management (London, 22, 2015 Tire Business).
4. Discussions
Originally, aims of RFID adoption for passenger car “half-finished product” is to chase the front runners, Hankook Tire in Korea including global leading companies like Bridgestone, Michaelin and Goodyear. In particular, Hankook Tire, established in 1941 has dominated domestic passenger tire market by using the first mover’s advantage. As a late comer, K Tire needs distinguishable innovation strategy which is RFID adoption for passenger car’s tire, “half-finished product” to overcome shortage of number of distribution channels. Adoption of RFID technology for passenger car’s tire has been known as infeasible methodologies according to explanation by Changmin Park, vice-CTO (chief technology officer) until K Tire’s success.
We lensed success factors as three perspectives; institutional entrepreneurship, corporate entrepreneurship and innovation. First, as a corporate entrepreneurship perspective, adopting innovative technologies having uncertainties accompanies by a certain risk of failure. Corporate entrepreneurship refers to firm’s effort that inculcate and promote innovation and risk taking throughout its operations ( Burgelman, 1983 ; Guth and Ginsberg, 1990 ). K Tire’s success was made possible by overcome the uncountable difficulties based on shared value and norms (e.g. Fligstein and Mara-Drita, 1996 ; Garud et al. , 2002 ; Hargadon and Douglas, 2001 ; Maguire et al. , 2004 ).
An unsuccessful attempt at developing innovative technologies causes direct loss, as well as loss of the opportunity costs. This is why many companies try to avoid risks by adopting or following the leading companies’ technologies or the dominant technologies. Stimulating corporate entrepreneurship requires firms to acquire and use new knowledge to exploit emerging opportunities. This knowledge could be obtained by joining alliances, selectively hiring key personnel, changing the composition or decision-making processes of a company’s board of directors or investing in R&D activities. When the firm uses multiple sources of knowledge ( Branzei and Vertinsky, 2006 ; Thornhill, 2006 ), some of these sources may complement one another, while others may substitute each other ( Zahra and George, 2002 ). Boards also provide managers with appropriate incentives that better align their interests with those of the firm. Given the findings, K Tire seeks new knowledge from external organizations through its discriminative corporate entrepreneurship.
When adopting the RFID system for its passenger vehicle tires, K Tire also had to develop new RFID tags suitable for the specific type of tire. The company’s capabilities were limited by the surrounding conditions, which prevented the application of existing tire RFID tag technologies, such as certain issues with the tire manufacturing process, the characteristic of its tires and the price of RFID tags per tire. Taking risks and confronting challenges are made from board member’s accountability. From the findings, we find that entrepreneurship leadership can be encouraged in case of within the accountability frame work.
Despite its status as a second-tier company, K Tire attempted to adopt the RFID system to its passenger vehicle tires, a feat not achieved even by the leading companies. Thus, the company ultimately built and settled the system through numerous trials and errors. Such success was made possible by the entrepreneurship of K Tire’s management, who took the risk of failure inherent in adopting innovative technologies and confronting challenges head on.
Second, institutional entrepreneurship not only involves the “capacity to imagine alternative possibilities”, it also requires the ability “to contextualize past habits and future projects within the contingencies of the moment” if existing institutions are to be transformed ( Emirbayer and Mische, 1998 ). New technologies, the technical infrastructure, network activities to acquire the new knowledge, learning capabilities, creating a new organization such as Pioneer Lab and new rules to create new technologies are the features. To qualify as institutional entrepreneurs, individuals must break with existing rules and practices associated with the dominant institutional logic(s) and institutionalize the alternative rules, practices or logics they are championing ( Garud and Karnøe, 2003 ; Battilana, 2006 ). K Tire established new organization, “Special lab” to obtain the know technology and information as CEO’s direct sub-committees. Institutional entrepreneurship arise when actors, through their filed position, recognize the opportunity circumstance so called “norms” ( Battilana et al. , 2009 ). To make up the deficit of technologies for RFID, knowledge stream among workers is more needed. Destruction of hierarch ranking system is proxy of the institutional entrepreneurship. Also, K Tire has peculiar norms. Namely, if one requires the further study such as degree course or non-degree course education services, grant systems operated via short screen process. Third, as innovation perspectives, before adopting the RFID system, the majority of K Tire’s researchers insisted that the company use the bar code technology, which had been widely used by the competitors. Such decision was predicated on the prediction that RFID technology would see wider use in the future, as well as the expected effect coming from taking the leading position, with regard to the technology.
Finally, K Tire’s adoption of the RFID technology cannot be understood without government support. The South Korean government has been implementing the “Verification and Dissemination Project for New u-IT Technologies” since 2008. Owing to policy support, K Tire can provide worker with educational service including oversea universities.
5. Conclusions and implications
To cope with various technological impasses, K Tire demonstrated the importance of institutional and corporate entrepreneurship. What a firm pursues more positive act for innovation is a research question.
Unlike firms, K Tire has strongly emphasized IT technology since establishment in 1960. To be promotion, every worker should get certification of IT sectors after recruiting. This has become the firm’s norm. This norm was spontaneously embedded for firm’s culture. K Tire has sought new ICT technology become a first mover. This norm can galvanize to take risk to catch up the first movers in view of institutional entrepreneurship.
That can be cultivated both by corporate entrepreneurship, referred to the activities a firm undertakes to stimulate innovation and encourage calculated risk taking throughout its operations within accountabilities and institutional entrepreneurship, referred to create its own peculiar norm. Contribution of our paper shows both importance of board members of directors in cultivating corporate entrepreneurship and importance of norm and rules in inducing institutional entrepreneurship.
In conclusion, many of them were skeptical about adopting RFID for its passenger vehicle tires at a time when even the global market and technology leaders were not risking such innovation, citing reasons such as risk of failure and development costs. However, enthusiasm and entrepreneurship across the organization towards technical innovation was achieved through the experience of developing leading technologies, as well as the resolve of the company’s management and its institutional entrepreneurship, which resulted in the company’s decision to adopt the RFID technology for small tires, a technology with unverified effects that had not been widely used in the markets. Introduction of new organization which “Special lab” is compelling example of institutional entrepreneurship. Also, to pursue RFID technology, board members unanimously agree to make new organization in the middle of failing and unpredictable success. This decision was possible since K Tire’s cultivated norm which was to boost ICT technologies. In addition, at that time, board of director’s behavior can be explained by corporate entrepreneurship.
From the findings, this paper also suggests importance of firms’ visions or culture from startup stage because they can become a peculiar norm and become firm’s institutional entrepreneurship. In much contemporary research, professionals and experts are identified as key institutional entrepreneurs, who rely on their legitimated claim to authoritative knowledge or particular issue domains. This case study shows that authoritative knowledge by using their peculiar norm, and culture as well as corporate entrepreneurship.
This paper has some limitations. Despite the fact that paper shows various fruitful findings, this study is not free from that our findings are limited to a single exploratory case study. Overcoming such limitation requires securing more samples, including the group of companies that attempt unprecedented innovations across various industries. In this paper, we can’t release all findings through in-depth interview and face-to-face meetings because of promise for preventing the secret tissues.
Nevertheless, the contribution of this study lies in that it shows the importance of corporate entrepreneurship and institutional entrepreneurship for firm’s innovative capabilities to grow ceaselessly.
Integrated frame of analysis
Aldrich , H.E. and Fiol , C.M. ( 1994 ), “ Fools rush in? The institutional context of industry creation ”, Academy of Management Review , Vol. 19 No. 4 , pp. 645 - 670 .
Aldrich , H.E. and Martinez , M.A. ( 2001 ), “ Many are called, but few are chosen: an evolutionary perspective for the study of entrepreneurship ”, Entrepreneurship Theory and Practice , Vol. 25 No. 4 , pp. 41 - 57 .
Battilana , J. ( 2006 ), “ Agency and institutions: the enabling role of individuals’ social position ”, Organization , Vol. 13 No. 5 , pp. 653 - 676 .
Battilana , J. , Leca , B. and Boxenbaum , E. ( 2009 ), “ How actors change institutions: towards a theory of institutional entrepreneurship ”, The Academy of Management Annals , Vol. 3 No. 1 , pp. 65 - 107 .
Branzei , O. and Vertinsky , I. ( 2006 ), “ Strategic pathways to product innovation capabilities in SMEs ”, Journal of Business Venturing , Vol. 21 No. 1 , pp. 75 - 105 .
Burgelman , R.A. ( 1983 ), “ A model of the interaction of strategic behavior, corporate context, and the context of strategy ”, Academy of Management Review , Vol. 8 No. 1 , pp. 61 - 70 .
Capon , N. , Farley , J.U. and Hoenig , S. ( 1990 ), “ Determinants of financial performance: a meta-analysis ”, Management Science , Vol. 36 , pp. 1143 - 1159 .
Covin , J. and Miles , M. ( 1999 ), “ Corporate entrepreneurship and the pursuit of competitive advantage ”, Entrepreneurship Theory and Practice , Vol. 23 , pp. 47 - 63 .
D’Aveni , R.A. ( 1994 ), Hyper-competition: Managing the Dynamics of Strategic Maneuvering . Free Press , New York, NY .
DiMaggio , P.J. ( 1988 ), “ Interest and agency in institutional theory ”, in Zucker , L.G. (Ed.), Institutional Patterns and Organizations , Ballinger , Cambridge, MA .
Emirbayer , M. and Mische , A. ( 1998 ), “ What is agency? ”, American Journal of Sociology , Vol. 103 , pp. 962 - 1023 .
Fligstein , N. ( 1997 ), “ Social skill and institutional theory ”, American Behavioral Scientist , Vol. 40 , pp. 397 - 405 .
Fligstein , N. and Mara-Drita , I. ( 1996 ), “ How to make a market: reflections on the attempt to create a single market in the European Union ”, American Journal of Sociology , Vol. 102 No. 1 , pp. 1 - 33 .
Garud , R. and Karnøe , P. ( 2003 ), “ Bricolage versusbreakthrough: distributed and embedded agency in technology entrepreneurship ”, Research Policy , Vol. 32 , pp. 277 - 300 .
Garud , R. , Jain , S. and Kumaraswamy , A. ( 2002 ), “ Institutional entrepreneurship in the sponsorship of common technological standards: the case of sun microsystems and Java ”, Academy of Management Journal , Vol. 45 No. 1 , pp. 196 - 214 .
Guth , W. and Ginsberg , A. ( 1990 ), “ Guest editors’ introduction: corporate entrepreneurship ”, Strategic Management Journal , Vol. 11 , pp. 5 - 15 .
Hargadon , A.B. and Douglas , Y. ( 2001 ), “ When innovations meet institutions: Edison and the design of the electric light ”, Administrative Science Quarterly , Vol. 46 No. 3 , pp. 476 - 502 .
Hisrich , R.D. ( 1986 ), Entrepreneurship, Intrapreneurship and Venture Capital , Lexington Books , MA .
Hoffman , A.J. ( 1999 ), “ Institutional evolution and change: environmentalism and the US chemical industry ”, Academy of Management Journal , Vol. 42 No. 4 , pp. 351 - 371 .
Jennings , D.F. and Lumpkin , J.R. ( 1989 ), “ Functioning modeling corporate entrepreneurship: an empirical integrative analysis ”, Journal of Management , Vol. 15 , pp. 485 - 502 .
Jepperson , R. and Meyer , J. ( 2001 ), “ The ‘actors’ of modern society: the cultural construction of social agency ”, Sociological Theory , Vol. 18 , pp. 100 - 120 .
Kirzner , I.M. ( 1997 ), “ Entrepreneurial discovery and the competitive market process: an Austrian approach ”, Journal of Economic Literature , Vol. 35 No. 1 , pp. 60 - 85 .
Kuratko , D. ( 2007 ), Corporate Entrepreneurship: Foundations and Trends in Entrepreneurship , Cambridge, MA .
Lawrence , T.B. , Hardy , C. and Phillips , N. ( 2002 ), “ Institutional effects of inter-organizational collaboration: the emergence of proto-institutions ”, Academy of Management Journal , Vol. 45 No. 1 , pp. 281 - 290 .
Lumpkin , G.T. and Dess , G.G. ( 1996 ), “ Clarifying the entrepreneurial orientation construct and linking it to performance ”, Academic. Management Review , Vol. 21 , pp. 135 - 172 .
Maguire , S. , Hardy , C. and Lawrence , T.B. ( 2004 ), “ Institutional entrepreneurship in emerging fields: HIV/AIDS treatment advocacy in Canada ”, The Academy of Management , Vol. 47 No. 5 , pp. 657 - 679 .
Meyer , J.W. , Boli , J. and Thomas , G.M. ( 1994 ), “ Ontology and rationalization in the western cultural account ”, in Richard Scott , W. and Meyer , J.W. (Eds), Institutional Environments and Organizations: Structural Complexity and Individualism , Sage Publications , Thousand Oaks, CA , pp. 9 - 27 .
North , D.C. ( 1990 ), Institutions, Institutional Change and Economic Performance , Cambridge University Press , Cambridge .
Phan , P.H. , Wright , M. , Ucbasaran , D. and Tan , W.L. ( 2009 ), “ Corporate entrepreneurship: current research and future directions ”, Journal of Business Venturing , Vol. 29 , pp. 197 - 205 .
Rojas , F. ( 2010 ), “ Power through institutional work: acquiring academic authority in the 1968 third world strike ”, Academy of Management Journal , Vol. 53 No. 6 , pp. 1263 - 1280 .
Srivastava , A. and Lee , H. ( 2005 ), “ Predicting order and timing of new product moves: the role of top management in corporate entrepreneurship ”, Journal of Business Venturing , Vol. 20 No. 1 , pp. 459 - 481 .
Schumpeter , J.A. ( 1942 ), Capitalism, Socialism, and Democracy , Harper and Brothers , New York, NY .
Scott , R. ( 1995 ), Institutions and Organizations , Sage , Thousand Oaks, CA .
Shane , S. and Venkataraman , S. ( 2000 ), “ The promise of entrepreneurship as a field of research ”, Academy of Management Review , Vol. 25 No. 1 , pp. 217 - 226 .
Teece , D.J. ( 2014 ), “ The foundations of enterprise performance: dynamic and ordinary capabilities in an (economic) theory of firms ”, Academy of Management Perspectives , Vol. 28 No. 4 , pp. 328 - 352 .
Thornhill , S. ( 2006 ), “ Knowledge, innovation and firm performance in high- and low-technology regimes ”, Journal of Business Venturing , Vol. 21 No. 5 , pp. 687 - 703 .
Tidd , J. and Bessant , J. ( 2009 ), Managing Innovation: Integrating Technological, Market and Organizational Change , 4th ed. , John Wiley & Sons , Sussex .
Tushman , M.L. and Anderson , P. ( 1986 ), “ Technological discontinuities and organizational environments ”, Administrative Science Quarterly , Vol. 31 No. 3 , pp. 439 - 465 .
Townley , B. ( 2002 ), “ The role of competing rationalities in institutional change ”, Academy of Management Journal , Vol. 45 No. 1 , pp. 163 - 179 .
Yin , R.K. ( 2008 ), Case Study Research: Design and Methods (Applied Social Research Methods) , 4th ed. , Sage, Thousand Oaks , CA .
Zahra , S.A. ( 1991 ), “ Predictors and financial outcomes of corporate entrepreneurship: an exploratory study ”, Journal of Business Venturing , Vol. 6 , pp. 259 - 285 .
Zahra , S.A. ( 1996 ), “ Governance, ownership, and corporate entrepreneurship: the moderating impact of industry technological opportunities ”, Academy of Management Journal , Vol. 39 No. 6 , pp. 1713 - 1735 .
Zahra , S.A. and George , G. ( 2002 ), “ Absorptive capacity: a review, reconceptualization, and extension ”, Academy of Management Review , Vol. 27 , pp. 185 - 203 .
Further reading
Bresnahan , T.F. , Brynjolfsson , E. and Hitt , L.M. ( 2002 ), “ Information technology, workplace organization, and the demand for skilled labor: firm-level evidence ”, The Quarterly Journal of Economics , Vol. 117 No. 1 , pp. 339 - 376 .
DiMaggio , P.J. ( 1984 ), “ Institutional isomorphism and structural conformity ”, paper presented at the 1984 American Sociological Association meetings, San Antonio, Texas .
Garud , R. ( 2008 ), “ Conferences as venues for the configuration of emerging organizational fields: the case of cochlear implants ”, Journal of Management Studies , Vol. 45 No. 6 , pp. 1061 - 1088 .
Maguire , S. and Hardy , C. ( 2009 ), “ Discourse and deinstitutionalization: the decline of DDT ”, Academy of Management Journal , Vol. 52 No. 1 , pp. 148 - 178 .
Acknowledgements
This work was supported by 2017 Hongik University Research Fund.
Corresponding author
Related articles, we’re listening — tell us what you think, something didn’t work….
Report bugs here
All feedback is valuable
Please share your general feedback
Join us on our journey
Platform update page.
Visit emeraldpublishing.com/platformupdate to discover the latest news and updates
Questions & More Information
Answers to the most commonly asked questions here

Raising a university's profile with distinctive curated research
Founded in 2009, King Abdullah University of Science and Technology aspires to be a key destination for scientific and technological education with high-impact research output. As a newer university, KAUST faces the challenge of building global recognition and visibility. By partnering with Nature Research, the university aimed to:
- Raise awareness of KAUST’s research and expertise across the global scientific community
- Promote KAUST’s impactful findings and build partnerships across industries
- Attract talented students and scientists to study, teach, or conduct research at KAUST
- Target policy makers in the fields of science and technology
To increase awareness of KAUST’s research excellence, the administration partnered with Nature Research to produce KAUST Discovery , a bi-annual print magazine and custom designed website. The content was curated by editors to highlight the university’s scientific findings. That research was then promoted to a global audience through a carefully targeted multi-channel campaign.
Custom site
Print magazine

A multi-channel approach was used to build traffic and drive engagement from the desired audience. Display ads were shown across different nature.com pages based on behavioral targeting. Print ads were placed in Nature and Nature branded journals. Email and social media marketing campaigns regularly promoted content that was developed for the site. Surveys were conducted to measure KAUST Discovery’s impact on the university’s reputation and recognition.
Awareness of KAUST increased by 9%
- 6 months after the launch of the KAUST Discovery, awareness of KAUST increased by 9%
- KAUST Discovery Magazine, Issue 3, won the PR Daily’s 2017 Content Marketing Award for Print Publication
- Consistent subscriber growth, including 40% after the third issue

Our global reach is greatly increased thanks to our custom publication, KAUST Discovery, created in partnership with Nature Research. The stories highlighted in KAUST Discovery are a snapshot of the research, discovery, and innovation ongoing at KAUST. Dr. Jean-Lou Chameau President King Abdullah University of Science and Technology

Academia.edu no longer supports Internet Explorer.
To browse Academia.edu and the wider internet faster and more securely, please take a few seconds to upgrade your browser .
Enter the email address you signed up with and we'll email you a reset link.
- We're Hiring!
- Help Center

Science, Technology and Society: a Case Study

1972, School Science and Mathematics
Related Papers
Steel is a critical material in our society and will remain an important one for a long time into the future [...]
Revue de Métallurgie
Jean-Pierre BIRAT
Melissa Indriani
Rochelle Forrester
The ultimate cause of much historical, social and cultural change is the gradual accumulation of human knowledge of the environment. Human beings use the materials in their environment, including fire and metals, to meet their needs and increased human knowledge of fire and metals enables human needs to be met in a more efficient manner. Fire and metals have particular properties and human knowledge of those properties increases over time in a particular order. Increasing human knowledge of how to create higher and higher temperatures enables the smelting and melting of a wider range of ores and metals. Those ores and metals that could be smelted and melted at lower temperatures were used before the ores and metals which had higher smelting and melting points. This meant that copper, and its alloy bronze, were used before iron and its alloy steel. Pure metals, like copper and iron, were used before alloys such as, bronze and steel, as the manufacture of alloys is more complicated than the manufacture of pure metals. The simplest knowledge is acquired first and more complex knowledge is acquired later. The order of discovery determines the course of human social and cultural history, as knowledge of new and more efficient means of smelting ores and melting metals, results in new technology, which contributes to the development of new social and ideological systems. This means human social and cultural history, had to follow a particular course, a course that was determined by the properties of the materials in the human environment.
Materials and Manufacturing Processes
Eva Hjärthner-Holdar , Christina Risberg
Advanced Engineering Materials
Alla Sergueeva
Benjamin Roberts
Jean-Pierre Birat
This paper reviews the relationship between the production of steel and the environment as it stands today. It deals with raw material issues (availability, scarcity), energy resources, and generation of by-products, i.e., the circular economy, the anthropogenic iron mine, and the energy transition. The paper also deals with emissions to air (dust, Particulate Matter, heavy metals, Persistant Organics Pollutants), water, and soil, i.e., with toxicity, ecotoxicity, epidemiology, and health issues, but also greenhouse gas emissions, i.e., climate change. The loss of biodiversity is also mentioned. All these topics are analyzed with historical hindsight and the present understanding of their physics and chemistry is discussed, stressing areas where knowledge is still lacking. In the face of all these issues, technological solutions were sought to alleviate their effects: many areas are presently satisfactorily handled (the circular economy—a historical’ practice in the case of steel, e...
Carol Johnson
Joseph Spoerl
This is a brief history of iron and steel production from its earliest origins through the 20th century, with a special focus on the career of Andrew Carnegie and on the economic impact of cheap and abundant steel.
RELATED PAPERS
alexandre m.da silva
Revista Facultad Nacional de Agronomía Medellín
Jesus Oswaldo Velasquez Restrepo
F1000posters
Noni Graham
Revista Colombiana de …
FRANCISCO DARIO RODRIGUEZ SAMAYOA
Miguel Carter
Comunicación y Sociedad
Eva Salgado
International Journal of Latest Trends in Finance and Economic Sciences
Manuel Ferreira
Medical Hypotheses
José Ferreira
Abstracts accepted for Publication
Sedat yılmaz
Iranian Red Crescent Medical Journal
omid garkaz
International Journal of Molecular Sciences
Memoona Khalid
Frontiers in Chemistry
Fareed Ullah
Felipe Marton dos Santos
Pest management science
muhammad hafeez
GLORIA ESTEFANY DURAND MATTA
Geoensenanza
Robert Kertz
Acta Médica Colombiana
Martha Viviana Alba Rodriguez
Diana Alarcón Castellanos
Journal of Natural Gas Science and Engineering
priambudi pujihatma
Journal of law and medicine
Barbara Pfeffer Billauer
The Pan African medical journal
Neeraj Agrawal
Journal of Animal Physiology and Animal Nutrition
Serena Calabrò
Psychological Science and Education
Marina Mdivani
Journal of Crystal Growth
Diana Loghin
See More Documents Like This
RELATED TOPICS
- We're Hiring!
- Help Center
- Find new research papers in:
- Health Sciences
- Earth Sciences
- Cognitive Science
- Mathematics
- Computer Science
- Academia ©2024

The Effects of Climate Change
The effects of human-caused global warming are happening now, are irreversible for people alive today, and will worsen as long as humans add greenhouse gases to the atmosphere.

- We already see effects scientists predicted, such as the loss of sea ice, melting glaciers and ice sheets, sea level rise, and more intense heat waves.
- Scientists predict global temperature increases from human-made greenhouse gases will continue. Severe weather damage will also increase and intensify.
Earth Will Continue to Warm and the Effects Will Be Profound

Global climate change is not a future problem. Changes to Earth’s climate driven by increased human emissions of heat-trapping greenhouse gases are already having widespread effects on the environment: glaciers and ice sheets are shrinking, river and lake ice is breaking up earlier, plant and animal geographic ranges are shifting, and plants and trees are blooming sooner.
Effects that scientists had long predicted would result from global climate change are now occurring, such as sea ice loss, accelerated sea level rise, and longer, more intense heat waves.
The magnitude and rate of climate change and associated risks depend strongly on near-term mitigation and adaptation actions, and projected adverse impacts and related losses and damages escalate with every increment of global warming.

Intergovernmental Panel on Climate Change
Some changes (such as droughts, wildfires, and extreme rainfall) are happening faster than scientists previously assessed. In fact, according to the Intergovernmental Panel on Climate Change (IPCC) — the United Nations body established to assess the science related to climate change — modern humans have never before seen the observed changes in our global climate, and some of these changes are irreversible over the next hundreds to thousands of years.
Scientists have high confidence that global temperatures will continue to rise for many decades, mainly due to greenhouse gases produced by human activities.
The IPCC’s Sixth Assessment report, published in 2021, found that human emissions of heat-trapping gases have already warmed the climate by nearly 2 degrees Fahrenheit (1.1 degrees Celsius) since 1850-1900. 1 The global average temperature is expected to reach or exceed 1.5 degrees C (about 3 degrees F) within the next few decades. These changes will affect all regions of Earth.
The severity of effects caused by climate change will depend on the path of future human activities. More greenhouse gas emissions will lead to more climate extremes and widespread damaging effects across our planet. However, those future effects depend on the total amount of carbon dioxide we emit. So, if we can reduce emissions, we may avoid some of the worst effects.
The scientific evidence is unequivocal: climate change is a threat to human wellbeing and the health of the planet. Any further delay in concerted global action will miss the brief, rapidly closing window to secure a liveable future.
Here are some of the expected effects of global climate change on the United States, according to the Third and Fourth National Climate Assessment Reports:
Future effects of global climate change in the United States:

U.S. Sea Level Likely to Rise 1 to 6.6 Feet by 2100
Global sea level has risen about 8 inches (0.2 meters) since reliable record-keeping began in 1880. By 2100, scientists project that it will rise at least another foot (0.3 meters), but possibly as high as 6.6 feet (2 meters) in a high-emissions scenario. Sea level is rising because of added water from melting land ice and the expansion of seawater as it warms. Image credit: Creative Commons Attribution-Share Alike 4.0

Climate Changes Will Continue Through This Century and Beyond
Global climate is projected to continue warming over this century and beyond. Image credit: Khagani Hasanov, Creative Commons Attribution-Share Alike 3.0

Hurricanes Will Become Stronger and More Intense
Scientists project that hurricane-associated storm intensity and rainfall rates will increase as the climate continues to warm. Image credit: NASA

More Droughts and Heat Waves
Droughts in the Southwest and heat waves (periods of abnormally hot weather lasting days to weeks) are projected to become more intense, and cold waves less intense and less frequent. Image credit: NOAA

Longer Wildfire Season
Warming temperatures have extended and intensified wildfire season in the West, where long-term drought in the region has heightened the risk of fires. Scientists estimate that human-caused climate change has already doubled the area of forest burned in recent decades. By around 2050, the amount of land consumed by wildfires in Western states is projected to further increase by two to six times. Even in traditionally rainy regions like the Southeast, wildfires are projected to increase by about 30%.
Changes in Precipitation Patterns
Climate change is having an uneven effect on precipitation (rain and snow) in the United States, with some locations experiencing increased precipitation and flooding, while others suffer from drought. On average, more winter and spring precipitation is projected for the northern United States, and less for the Southwest, over this century. Image credit: Marvin Nauman/FEMA

Frost-Free Season (and Growing Season) will Lengthen
The length of the frost-free season, and the corresponding growing season, has been increasing since the 1980s, with the largest increases occurring in the western United States. Across the United States, the growing season is projected to continue to lengthen, which will affect ecosystems and agriculture.
Global Temperatures Will Continue to Rise
Summer of 2023 was Earth's hottest summer on record, 0.41 degrees Fahrenheit (F) (0.23 degrees Celsius (C)) warmer than any other summer in NASA’s record and 2.1 degrees F (1.2 C) warmer than the average summer between 1951 and 1980. Image credit: NASA
Arctic Is Very Likely to Become Ice-Free
Sea ice cover in the Arctic Ocean is expected to continue decreasing, and the Arctic Ocean will very likely become essentially ice-free in late summer if current projections hold. This change is expected to occur before mid-century.
U.S. Regional Effects
Climate change is bringing different types of challenges to each region of the country. Some of the current and future impacts are summarized below. These findings are from the Third 3 and Fourth 4 National Climate Assessment Reports, released by the U.S. Global Change Research Program .
- Northeast. Heat waves, heavy downpours, and sea level rise pose increasing challenges to many aspects of life in the Northeast. Infrastructure, agriculture, fisheries, and ecosystems will be increasingly compromised. Farmers can explore new crop options, but these adaptations are not cost- or risk-free. Moreover, adaptive capacity , which varies throughout the region, could be overwhelmed by a changing climate. Many states and cities are beginning to incorporate climate change into their planning.
- Northwest. Changes in the timing of peak flows in rivers and streams are reducing water supplies and worsening competing demands for water. Sea level rise, erosion, flooding, risks to infrastructure, and increasing ocean acidity pose major threats. Increasing wildfire incidence and severity, heat waves, insect outbreaks, and tree diseases are causing widespread forest die-off.
- Southeast. Sea level rise poses widespread and continuing threats to the region’s economy and environment. Extreme heat will affect health, energy, agriculture, and more. Decreased water availability will have economic and environmental impacts.
- Midwest. Extreme heat, heavy downpours, and flooding will affect infrastructure, health, agriculture, forestry, transportation, air and water quality, and more. Climate change will also worsen a range of risks to the Great Lakes.
- Southwest. Climate change has caused increased heat, drought, and insect outbreaks. In turn, these changes have made wildfires more numerous and severe. The warming climate has also caused a decline in water supplies, reduced agricultural yields, and triggered heat-related health impacts in cities. In coastal areas, flooding and erosion are additional concerns.
1. IPCC 2021, Climate Change 2021: The Physical Science Basis , the Working Group I contribution to the Sixth Assessment Report, Cambridge University Press, Cambridge, UK.
2. IPCC, 2013: Summary for Policymakers. In: Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change [Stocker, T.F., D. Qin, G.-K. Plattner, M. Tignor, S.K. Allen, J. Boschung, A. Nauels, Y. Xia, V. Bex and P.M. Midgley (eds.)]. Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA.
3. USGCRP 2014, Third Climate Assessment .
4. USGCRP 2017, Fourth Climate Assessment .
Related Resources

A Degree of Difference
So, the Earth's average temperature has increased about 2 degrees Fahrenheit during the 20th century. What's the big deal?
What’s the difference between climate change and global warming?
“Global warming” refers to the long-term warming of the planet. “Climate change” encompasses global warming, but refers to the broader range of changes that are happening to our planet, including rising sea levels; shrinking mountain glaciers; accelerating ice melt in Greenland, Antarctica and the Arctic; and shifts in flower/plant blooming times.

Is it too late to prevent climate change?
Humans have caused major climate changes to happen already, and we have set in motion more changes still. However, if we stopped emitting greenhouse gases today, the rise in global temperatures would begin to flatten within a few years. Temperatures would then plateau but remain well-elevated for many, many centuries.
Discover More Topics From NASA
Explore Earth Science

Earth Science in Action

Earth Science Data
Facts About Earth

share this!
March 26, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
Two coral snakes recorded battling for prey in a scientific first
by Pensoft Publishers

Two red-tailed coral snakes have been observed competing over a caecilian in the first documented wild case of kleptoparasitism within the family Elapidae.
Kleptoparasitism, or food theft, is a well-documented behavior in many animal species , but is seldom reported among snakes in natural habitats.
The observation, detailed in a recent study published in Herpetozoa by Henrik Bringsøe and Niels Poul Dreyer, showcases the two Micrurus mipartitus snakes engaging in a tug-of-war over the limbless amphibian.
Elapid snakes are venomous and among the deadliest serpents in the world. There are more than 400 species comprising a very diverse group of snakes such as mambas, cobras, kraits, taipans, tiger snakes, death adders, sea snakes and coral snakes.
The battle occurred in the dense rainforests of Valle del Cauca, western Colombia. Surprisingly, in the tussle, one snake also bit the body of the other. However, the researchers suggest this was likely accidental.
After 17 minutes of observation, the losing coral snake released its bite hold on the caecilian. The winner then moved away from the losing snake, which did not follow.
The study suggests that while such behaviors may be more common in captivity due to controlled environments, their occurrence in nature has been largely underreported, likely due to the elusive nature of these reptiles and the challenges of observing them in their natural habitats .
"Snakes in captivity do that often when only one prey is offered in a terrarium with two or more snakes. But it is rather surprising that it has not been observed more frequently in the wild," says lead author Henrik Bringsøe.
This case sheds light on the coral snake interactions with prey species . Caecilians, such as the one in this study, have shown remarkable adaptations such as toxin resistance and increased mucus production.
Provided by Pensoft Publishers
Explore further
Feedback to editors

Romania center explores world's most powerful laser
10 hours ago

A cosmic 'speed camera' just revealed the staggering speed of neutron star jets in a world first
Mar 30, 2024
Saturday Citations: 100-year-old milk, hot qubits and another banger from the Event Horizon Telescope project

Curiosity rover searches for new clues about Mars' ancient water

Study says since 1979 climate change has made heat waves last longer, spike hotter, hurt more people

Scientist taps into lobsters' unusual habits to conquer the more than 120-year quest to farm them
Mar 29, 2024

Blind people can hear and feel April's total solar eclipse with new technology

Mapping the best route for a spacecraft traveling beyond the sun's sphere of influence

Researchers outline new approach in search for dark matter through future DUNE research project

Researchers reveal evolutionary path of important proteins
Relevant physicsforums posts, what do large moles on the body indicate, avian flu - a new study led by a team from the university of maryland.
Mar 27, 2024
Are all biological catabolic reactions exergonic?
Mar 20, 2024
A First of Its Kind: A Calcium-based signal in the Human Brain
Mar 18, 2024
Biological culture and cultural biology
Mar 17, 2024
Potentially fatal dog parasite found in the Colorado River
Mar 15, 2024
More from Biology and Medical
Related Stories

Unique feeding behavior of Asian kukri snakes gutting frogs and toads
Feb 18, 2021

Male sea snakes may have evolved bigger eyes to help them find a mate
Dec 13, 2023

Sea snakes may have evolved to see colors again
Jul 13, 2023

Can some snakes do cartwheels to escape or startle predators?
Apr 5, 2023
Family tree of major snake group rewritten and new branch of snakes found
Jan 23, 2023

How an unlikely amphibian survived its judgment day
Jul 24, 2023
Recommended for you

Chickadees have unique neural 'barcodes' for memories of stashing away food

Tiny orchid flowers pollinated by tiny flies

Discovering van Gogh in the wild: Scientists unveil a new gecko species
Mar 28, 2024

Supergene research solves the mystery of tiny ant queens

Small birds spice up the already diverse diet of spotted hyenas in Namibia
Let us know if there is a problem with our content.
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Phys.org in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter

IMAGES
VIDEO
COMMENTS
by Desmond Dodd. Many assume that major oil and gas companies adamantly oppose climate-friendly regulation, but that's not true. A study of 30 years of corporate advocacy by Jonas Meckling finds that energy companies have backed clean-energy efforts when it aligns with their business interests. 12 Mar 2024. HBS Case.
This science communication case study analyzes an online international co-taught course where students practiced blog article conceptualization and production covering a wide variety of science and technology related issues. Students had an international experience during the COVID-19 pandemic, and gained experience in communicating science and technology to intercultural audiences.
A case study by Andy Wu and David Yoffie lays out the key challenges immersive 3D technology must overcome to be truly transformative. ... Brian Trelstad discusses the importance of focusing on the use case when determining the value of adding a complex technology solution in his case, "SMART: AI and Machine Learning for Wildlife Conservation
Through this case study, the author analyzed, reported, and reflected on personal experience of a shift in technology use during the COVID-19 pandemic. There are two parts of this case study. The first part highlights the new technology used in five inside-of-school activities, including classroom learning, group projects, dance tutorials ...
A case studies series from the Social and Ethical Responsibilities of Computing program at MIT delves into a range of topics, from social and ethical implications of computing technologies and the racial disparities that can arise from deploying facial recognition technology in unregulated, real-world settings to the biases of risk prediction algorithms in the criminal justice system and the ...
Technology and Public Purpose. To help us think seriously about data ethics, we need case studies that we can discuss, argue about, and come to terms with as we engage with the real world. Good case studies give us the opportunity to think through problems before facing them in real life. And case studies show us that ethical problems aren't ...
Linkages between science and technology have been extensively studied using nonpatent literature citations or author-inventor matching. These methods suffer from limitations, such as the lack of citations to relevant documents or challenges with the disambiguation of author-inventor linkages. To mitigate these limitations, this paper uses Latent Dirichlet Allocation to create topic-based ...
The sociotechnical systems approach to energy research has emerged within the interdisciplinary academic field of "Science, Technology and Society" or "Science and Technology Studies" (STS), mentioned briefly in Section One, that has been defined as "the study of the processes by which scientific knowledge and technological artifacts ...
These case studies are intended to draw out lessons for the development of a cross-sectoral governance framework for emerging technologies in health and medicine. The focus of the case studies is the governance ecosystem in the United States, though where appropriate, the international landscape is included to provide context.
The integration of technology-based projects in the primary science curriculum has attracted recent international attention. Yet few case studies about what and how knowledge is constructed in this context have been reported. In this interpretive study of a Year Six classroom, we focused on how two contrasting groups of children designed ill-defined engineering structures. Of particular ...
On the other hand, this study underlines the multifaceted nature of the science-technology linkage, depending on the sectoral, organizational, and regional setting. In line with Meyer (2000) and McMillan et al. (2000) , this paper points out that there are sector-specific characteristics in technology transfer from science to technology.
Case study of Moscow University's Science Park and how the park has affected innovative activity in the country. Salvador . Case study. Italy. Case study of incubators and science and technology parks located in Turin, Italy. Concludes that lack of funding and lack of managerial experience are drivers for the lack of success in these parks
Suggested Citation:"13 Biotechnology Case Study." National Research Council. 1993. Global Dimensions of Intellectual Property Rights in Science and Technology. Washington, DC: The National Academies Press. doi: 10.17226/2054.
This template provides the basics for writing ethics case studies in technology (though with some modification it could be used in other fields as well). AI, Comedy, and Counterculture . An Ethics Case Study. AI-generated text, voices, and images used for entertainment productions and impersonation raise ethical questions.
These case studies are intended to draw out lessons for the development of a cross-sectoral governance framework for emerging technologies in health and medicine. The focus of the case studies is the governance ecosystem in the United States, though where appropriate, the international landscape is included to provide context.
To study the organizational characteristics such as corporate entrepreneurship, institutional entrepreneurship, innovation process of companies, the qualitative case study is the suitable method. This is because a case study is a useful method when verifying or expanding well-known theories or challenging a specific theory ( Yin, 2008 ).
Download Citation | On Mar 17, 2010, E. W. Jenkins published Science, Technology and Society: A Case Study | Find, read and cite all the research you need on ResearchGate
In summary, we performed a case study of a solar cell to develop a method of detecting gaps between science and technology via a combination of citation- and expert-based approaches. Scientific research tended to be more basic, especially the cell design, whereas patents focused on more applied technology around the basic research.
Related case studies. King Abdullah University of Science and Technology partnered with Nature Research to produce KAUST Discovery, a bi-annual print magazine and custom designed website. The content was curated by editors to highlight the university's scientific findings. That research was then promoted to a global audience.
Technology and Multiculturalism in the Classroom: Case Studies in Attitudes and Motivations.. ERIC Educational Resources Information Center. Chisholm, Ines Marquez; Wetzel, Keith. 2001-01-01. Uses a case study approach in examining the attitudes and motivations of five teacher educators who used technology in their classroom. Identifies and discusses six common elements of multicultural ...
The study method entails two main factors, purpose and the research synthesis process of the study. This study aims to explore the understanding and awareness of the teachers and students about STEM education; the challenges faced by them and their solutions in the light of literature review and research amalgamation and the abilities and skills to overcome these challenges.
See Full PDFDownload PDF. Science, Technology and Society: a Case Study E. W. Jenkins Department of Education^ The University of Leeds, Leeds, LS2f 9JT, United Kingdom INTRODUCTION In recent years, increasing attention has been focussed on the rela- tionships between scientific and technological developments and society in general.
Rather than relying on anecdotal-based assumptions about students and technology or single studies that validate our preexisting prejudices, this study supports these discussions by offering actual evidence for achieving these goals. Higher-education institutions can benefit from technology-based learning systems that improve student learning.
Global climate change is not a future problem. Changes to Earth's climate driven by increased human emissions of heat-trapping greenhouse gases are already having widespread effects on the environment: glaciers and ice sheets are shrinking, river and lake ice is breaking up earlier, plant and animal geographic ranges are shifting, and plants and trees are blooming sooner.
This case sheds light on the coral snake interactions with prey species. Caecilians, such as the one in this study, have shown remarkable adaptations such as toxin resistance and increased mucus ...