Call our 24 hours, seven days a week helpline at 800.272.3900

- Professionals

- Younger/Early-Onset Alzheimer's
- Is Alzheimer's Genetic?
- Women and Alzheimer's
- Creutzfeldt-Jakob Disease
- Dementia with Lewy Bodies
- Down Syndrome & Alzheimer's
- Frontotemporal Dementia
- Huntington's Disease
- Mixed Dementia
- Normal Pressure Hydrocephalus
- Posterior Cortical Atrophy
- Parkinson's Disease Dementia
- Vascular Dementia
- Korsakoff Syndrome
- Traumatic Brain Injury (TBI)
- Know the 10 Signs
- Difference Between Alzheimer's & Dementia
- 10 Steps to Approach Memory Concerns in Others
- Medical Tests for Diagnosing Alzheimer's
- Why Get Checked?
- Visiting Your Doctor
- Life After Diagnosis
- Stages of Alzheimer's
- Earlier Diagnosis
- Part the Cloud
- Research Momentum
- Our Commitment to Research
- TrialMatch: Find a Clinical Trial
- What Are Clinical Trials?
- How Clinical Trials Work
- When Clinical Trials End
- Why Participate?
- Talk to Your Doctor
- Clinical Trials: Myths vs. Facts
- Can Alzheimer's Disease Be Prevented?
- Brain Donation
- Navigating Treatment Options
- Lecanemab Approved for Treatment of Early Alzheimer's Disease
- Aducanumab Discontinued as Alzheimer's Treatment
- Medicare Treatment Coverage
- Donanemab for Treatment of Early Alzheimer's Disease — News Pending FDA Review
- Questions for Your Doctor
- Medications for Memory, Cognition and Dementia-Related Behaviors
- Treatments for Behavior
- Treatments for Sleep Changes
- Alternative Treatments
- Facts and Figures
- Assessing Symptoms and Seeking Help
- Now is the Best Time to Talk about Alzheimer's Together

- Daily Care Plan
- Communication and Alzheimer's
- Food and Eating
- Art and Music
- Incontinence
- Dressing and Grooming
- Dental Care
- Working With the Doctor
- Medication Safety
- Accepting the Diagnosis
- Early-Stage Caregiving
- Middle-Stage Caregiving
- Late-Stage Caregiving
- Aggression and Anger
- Anxiety and Agitation
- Hallucinations
- Memory Loss and Confusion
- Sleep Issues and Sundowning
- Suspicions and Delusions
- In-Home Care
- Adult Day Centers
- Long-Term Care
- Respite Care
- Hospice Care
- Choosing Care Providers
- Finding a Memory Care-Certified Nursing Home or Assisted Living Community
- Changing Care Providers
- Working with Care Providers
- Creating Your Care Team
- Long-Distance Caregiving
- Community Resource Finder
- Be a Healthy Caregiver
- Caregiver Stress
- Caregiver Stress Check
- Caregiver Depression
- Changes to Your Relationship
- Grief and Loss as Alzheimer's Progresses
- Home Safety
- Dementia and Driving
- Technology 101
- Preparing for Emergencies
- Managing Money Online Program
- Planning for Care Costs
- Paying for Care
- Health Care Appeals for People with Alzheimer's and Other Dementias
- Social Security Disability
- Medicare Part D Benefits
- Tax Deductions and Credits
- Planning Ahead for Legal Matters
- Legal Documents
- Get Educated
- Just Diagnosed
- Sharing Your Diagnosis
- Changes in Relationships
- If You Live Alone
- Treatments and Research
- Legal Planning
- Financial Planning
- Building a Care Team
- End-of-Life Planning
- Programs and Support
- Overcoming Stigma
- Younger-Onset Alzheimer's
- Taking Care of Yourself
- Reducing Stress
- Tips for Daily Life
- Helping Family and Friends
- Leaving Your Legacy
- Live Well Online Resources
- Make a Difference
- ALZ Talks Virtual Events
- ALZNavigator™
- Veterans and Dementia
- The Knight Family Dementia Care Coordination Initiative
- Online Tools
- Asian Americans and Pacific Islanders and Alzheimer's
- Native Americans and Alzheimer's
- Black Americans and Alzheimer's
- Hispanic Americans and Alzheimer's
- LGBTQ+ Community Resources for Dementia
- Educational Programs and Dementia Care Resources
- Brain Facts
- 50 Activities
- For Parents and Teachers
- Resolving Family Conflicts
- Holiday Gift Guide for Caregivers and People Living with Dementia
- Trajectory Report
- Resource Lists
- Search Databases
- Publications
- Favorite Links
- 10 Healthy Habits for Your Brain
- Stay Physically Active
- Adopt a Healthy Diet
- Stay Mentally and Socially Active
- Online Community
- Support Groups
- Find Your Local Chapter
- Any Given Moment
- New IDEAS Study
- RFI Amyloid PET Depletion Following Treatment
- Bruce T. Lamb, Ph.D., Chair
- Christopher van Dyck, M.D.
- Cynthia Lemere, Ph.D.
- David Knopman, M.D.
- Lee A. Jennings, M.D. MSHS
- Karen Bell, M.D.
- Lea Grinberg, M.D., Ph.D.
- Malú Tansey, Ph.D.
- Mary Sano, Ph.D.
- Oscar Lopez, M.D.
- Suzanne Craft, Ph.D.
- About Our Grants
- Andrew Kiselica, Ph.D., ABPP-CN
- Arjun Masurkar, M.D., Ph.D.
- Benjamin Combs, Ph.D.
- Charles DeCarli, M.D.
- Damian Holsinger, Ph.D.
- David Soleimani-Meigooni, Ph.D.
- Donna M. Wilcock, Ph.D.
- Elizabeth Head, M.A, Ph.D.
- Fan Fan, M.D.
- Fayron Epps, Ph.D., R.N.
- Ganesh Babulal, Ph.D., OTD
- Hui Zheng, Ph.D.
- Jason D. Flatt, Ph.D., MPH
- Jennifer Manly, Ph.D.
- Joanna Jankowsky, Ph.D.
- Luis Medina, Ph.D.
- Marcello D’Amelio, Ph.D.
- Marcia N. Gordon, Ph.D.
- Margaret Pericak-Vance, Ph.D.
- María Llorens-Martín, Ph.D.
- Nancy Hodgson, Ph.D.
- Shana D. Stites, Psy.D., M.A., M.S.
- Walter Swardfager, Ph.D.
- ALZ WW-FNFP Grant
- Capacity Building in International Dementia Research Program
- ISTAART IGPCC
- Alzheimer’s Disease Strategic Fund: Endolysosomal Activity in Alzheimer’s (E2A) Grant Program
- Imaging Research in Alzheimer’s and Other Neurodegenerative Diseases
- Zenith Fellow Awards
- National Academy of Neuropsychology & Alzheimer’s Association Funding Opportunity
- Part the Cloud-Gates Partnership Grant Program: Bioenergetics and Inflammation
- Pilot Awards for Global Brain Health Leaders (Invitation Only)
- Robert W. Katzman, M.D., Clinical Research Training Scholarship
- Funded Studies
- How to Apply
- Portfolio Summaries
- Supporting Research in Health Disparities, Policy and Ethics in Alzheimer’s Disease and Dementia Research (HPE-ADRD)
- Diagnostic Criteria & Guidelines
- Annual Conference: AAIC
- Professional Society: ISTAART
- Alzheimer's & Dementia
- Alzheimer's & Dementia: DADM
- Alzheimer's & Dementia: TRCI
- International Network to Study SARS-CoV-2 Impact on Behavior and Cognition
- Alzheimer’s Association Business Consortium (AABC)
- Global Biomarker Standardization Consortium (GBSC)
- Global Alzheimer’s Association Interactive Network
- International Alzheimer's Disease Research Portfolio
- Alzheimer’s Disease Neuroimaging Initiative Private Partner Scientific Board (ADNI-PPSB)
- Research Roundtable
- About WW-ADNI
- North American ADNI
- European ADNI
- Australia ADNI
- Taiwan ADNI
- Argentina ADNI
- WW-ADNI Meetings
- Submit Study
- RFI Inclusive Language Guide
- Scientific Conferences
- AUC for Amyloid and Tau PET Imaging
- Make a Donation
- Walk to End Alzheimer's
- The Longest Day
- RivALZ to End ALZ
- Ride to End ALZ
- Tribute Pages
- Gift Options to Meet Your Goals
- Founders Society
- Fred Bernhardt
- Anjanette Kichline
- Lori A. Jacobson
- Pam and Bill Russell
- Gina Adelman
- Franz and Christa Boetsch
- Adrienne Edelstein
- For Professional Advisors
- Free Planning Guides
- Contact the Planned Giving Staff
- Workplace Giving
- Do Good to End ALZ
- Donate a Vehicle
- Donate Stock
- Donate Cryptocurrency
- Donate Gold & Sterling Silver
- Donor-Advised Funds
- Use of Funds
- Giving Societies
- Why We Advocate
- Ambassador Program
- About the Alzheimer’s Impact Movement
- Research Funding
- Improving Care
- Support for People Living With Dementia
- Public Policy Victories
- Planned Giving
- Community Educator
- Community Representative
- Support Group Facilitator or Mentor
- Faith Outreach Representative
- Early Stage Social Engagement Leaders
- Data Entry Volunteer
- Tech Support Volunteer
- Other Local Opportunities
- Visit the Program Volunteer Community to Learn More
- Become a Corporate Partner
- A Family Affair
- A Message from Elizabeth
- The Belin Family
- The Eliashar Family
- The Fremont Family
- The Freund Family
- Jeff and Randi Gillman
- Harold Matzner
- The Mendelson Family
- Patty and Arthur Newman
- The Ozer Family
- Salon Series
- No Shave November
- Other Philanthropic Activities
- Still Alice
- The Judy Fund E-blast Archive
- The Judy Fund in the News
- The Judy Fund Newsletter Archives
- Sigma Kappa Foundation
- Alpha Delta Kappa
- Parrot Heads in Paradise
- Tau Kappa Epsilon (TKE)
- Sigma Alpha Mu
- Alois Society Member Levels and Benefits
- Alois Society Member Resources
- Zenith Society
- Founder's Society
- Joel Berman
- JR and Emily Paterakis
- Legal Industry Leadership Council
- Accounting Industry Leadership Council

Find Local Resources
Let us connect you to professionals and support options near you. Please select an option below:
Use Current Location Use Map Selector
Search Alzheimer’s Association

Double Match Challenge! Make 2x the Impact Today
- Models of Care Case Studies
The Alzheimer’s Association is committed to connecting clinicians to effective, evidence-based models of care that can be replicated in community settings. Two of these models — the UCLA Alzheimer’s and Dementia Care program and the Age-Friendly Health Systems initiative — are detailed below.
UCLA Alzheimer's and Dementia Care program Age-Friendly Health Systems initiative UCLA Alzheimer’s and Dementia Care program
A dementia-specific model of care that significantly improved the experience for caregivers and people living with the disease.
About the program
The Alzheimer’s Association has partnered with UCLA to replicate the UCLA Alzheimer’s and Dementia Care (ADC) program through a grant from the John A. Hartford Foundation. The program follows a co-management model within the UCLA health system and partners with community-based organizations (CBOs) to provide comprehensive, coordinated, individualized care for people living with Alzheimer’s disease and other dementias.
The goals of the program are to:
- Maximize function, independence and dignity for people living with dementia.
- Minimize caregiver strain and burnout.
- Reduce unnecessary costs through improved care.
To qualify for the program, participants must have a diagnosis of dementia and live outside of a nursing home. The mean age of the first program participants was 82 years old. Almost all of the caregivers were the children (59%) or spouses (41%) of individuals living with Alzheimer’s or other dementias.
Comprehensive care
The ADC program utilizes a co-management model in which a nurse practitioner Dementia Care Specialist (DCS) partners with the participant’s primary care doctor to develop and implement a personalized care plan. The DCS provides support via four key components:
- Conducting in-person needs assessments of individuals living with Alzheimer’s and their caregivers.
- Creating and implementing individualized dementia care plans.
- Monitoring and revising care plans, as needed.
- Providing access 24/7, 365 days a year for assistance and advice to help avoid Emergency Department (ED) visits and hospitalizations.
Community resources
The ADC program also connects caregivers with resources provided by CBOs, including:
- Adult day care.
- Counseling.
- Case management.
- Legal and financial advice.
- Workforce development focused on training families and caregivers.
Program effectiveness
At one year, the quality of care provided by the program as measured by nationally accepted quality measures for dementia was exceedingly high — 92% compared to a benchmark of 38%. As a result, the improvements experienced by both caregivers and patients were significant:
- Ninety-four percent of caregivers felt that their role was supported.
- Ninety-two percent would recommend the program to others.
- Confidence in handling problems and complications of Alzheimer’s and other dementias improved by 79%.
- Caregiver distress related to behavioral symptoms, depression scores and strain improved by 31%, 24% and 15%, respectively.
- Despite disease progression, behavioral symptoms like agitation, irritability, apathy and nighttime behaviors in people living with dementia improved by 22%.
- Depressive symptoms experienced by individuals living with the disease were reduced by 34%.
Cost benefits of the program
An external evaluator compared utilization and cost outcomes and determined that over the course of 3 1/2 years, participants in UCLA’s program had lower total Medicare costs of care ($2,404 per year) relative to those receiving usual care.
In addition to cost savings for individuals and their families, the ADC program reports several financial benefits for health systems, including:
- Hospitalizations: 12% reduction
- ED visits: 20% reduction
- ICU stays: 21% reduction
- Hospital days: 26% reduction
- Hospice in last six months: 60% increase
- Nursing home placement: 40% reduction
UCLA finds that a care program following the ADC model may be able to pay for itself depending on local labor costs, comprehensiveness of billing and local overhead applied to clinical revenue.
To learn more or to contact UCLA about training and replication of the program, visit the UCLA Alzheimer’s and Dementia Care Program website.
Age-Friendly Health Systems initiative
A model of care that incorporates person-centered dementia care into a broader framework for the care of older adults.
About the initiative
Age-Friendly Health Systems is an initiative of The John A. Hartford Foundation and the Institute for Healthcare Improvement (IHI) in partnership with the American Hospital Association (AHA) and the Catholic Health Association of the United States (CHA). Together in 2017, they set a bold vision to build a social movement so all care with older adults is age-friendly care, that:
- Follows an essential set of evidence-based practices.
- Causes no harm.
- Aligns with “What Matters” to the older adult and their family caregivers.
The Age-Friendly Health Systems initiative defines “What Matters” as knowing and aligning care with each older adult’s specific health outcome goals and care preferences including, but not limited to, end-of-life care, and across settings of care.
- Health outcome goals relate to the values and activities that matter most to an individual, help motivate the individual to sustain and improve health, and could be impacted by a decline in health — for example, babysitting a grandchild, walking with friends in the morning, or volunteering in the community. When identified in a specific, actionable, and reliable manner, patients’ health outcome goals can guide decision making.
- Care preferences include the health care activities (e.g., medications, self-management tasks, health care visits, testing, and procedures) that patients are willing and able (or not willing or able) to do or receive.
The 4Ms framework of an Age-Friendly Health System
The 4Ms are not a program, but a framework to guide how care is provided to older adults through every interaction with a health system’s care and services. The 4Ms — What Matters, Medication, Mentation, and Mobility — make the complex care of older adults more manageable because they:
- Identify the core issues that should drive all care and decision making with the care of older adults.
- Organize care and focus on the older adult’s wellness and strengths rather than solely on disease.
- Are relevant regardless of an older adult’s individual disease(s).
- Apply regardless of the number of functional problems an older adult may have, or that person’s cultural, ethnic or religious background.
The 4Ms framework is most effective when all 4Ms are implemented together and are practiced reliably (i.e., for all older adults, in all settings and across settings, in every interaction).
The intention is to incorporate the 4Ms into existing care — rather than layering them on top —to organize the efficient delivery of effective care. This is achieved primarily through redeploying existing health system resources. Many health systems have found they already provide care aligned with one or more of the 4Ms for many of their older adult patients. Much of the effort, then, is to incorporate the other elements and organize care so all 4Ms guide every encounter with an older adult and their family caregivers.
Cost benefits of the initiative
The business case for becoming an Age-Friendly Health System focuses on its financial returns and is stronger when:
- The financial benefits are captured by the health system that is making the investment.
- Utilization and associated expenses of “usual” care are especially burdensome.
- The health system is effective in mitigating those costs.
- The added expense of becoming age-friendly is lower.
See the IHI report, The Business Case for Becoming an Age-Friendly Health System , for guidance on how to make the business case for your health system.
To learn more or to contact IHI about joining the initiative, visit the IHI Age-Friendly Health Systems website.
Health Systems and Medical Professionals Menu
- Alzheimer’s Association Innovation Roundtable (AAIR)
- Prescribing Lecanemab
- Cognitive Screening and Assessment
- Dementia Care Navigation Guiding Principles
- Dementia Care Navigation Roundtable
- Differential Diagnosis of Alzheimer's Disease
- Differential Diagnosis of Creutzfeldt-Jakob Disease
- Differential Diagnosis of Lewy Body Dementia
- Differential Diagnosis of Frontotemporal Dementia
- Differential Diagnosis of Huntington's Disease
- Differential Diagnosis of Korsakoff Syndrome
- Differential Diagnosis of Mixed Dementia
- Differential Diagnosis of Normal Pressure Hydrocephalus
- Differential Diagnosis of Parkinson's Disease Dementia
- Differential Diagnosis of Vascular Dementia
- Disclosure of Diagnosis
- Advanced Imaging and Biomarkers
- Care Planning Visit
- Advanced Care Planning
- Alzheimer's Network (ALZ-NET)
- Guidelines Index
- Clinical Trials Recruiting
- Downloadable Resources for Patients and Caregivers
- I Have Alzheimer's
- Clinical Trials
- Instructional Videos
- Cognitive Assessment Tools
- ISTAART Membership
- Alzheimer's & Dementia Journal
- Continuing Education on Alzheimer's and Dementia
- The Alzheimer’s and Dementia Care ECHO® Program for Health Systems and Medical Professionals
Cookies on the NHS England website
We’ve put some small files called cookies on your device to make our site work.
We’d also like to use analytics cookies. These send information about how our site is used to a service called Google Analytics. We use this information to improve our site.
Let us know if this is OK. We’ll use a cookie to save your choice. You can read more about our cookies before you choose.
Change my preferences I'm OK with analytics cookies
Case studies
The below case studies are taken from the document ‘ Good care planning guide for dementia ‘
- Core elements of a care plan
- An example person centred care plan
- A care plan: system compatibility and configuration
- An example process for dementia annual review template
- Example QOF annual review templates
- A framework for annual review
- An example best practice care plan template
- An example dementia annual review template
- My advance care plan
- Caring for me: Advance care planning
- Caring for me: Advanced care plan – Supporting information for patients and their families/carers
- Planning for your future – Advance care planning
- My plan for making the most of my life
- Staying well check tool
- Sharing care plans
Best Practice Repository case studies
- Dementia Awareness Training
- Diagnosing Well: Stockton
- Diagnosing Well: Enfield Memory Service
- Improving harm from falls as part of the Patient safety initiative
- Diagnosing Well in Carlisle
- Diagnosing Well in Newcastle
- Diagnosing Well in Northallerton – Assessment and diagnosis in one day
- Dementia care improvements
- A Triangle of Care – Carers Included: A Guide to Best Practice for Dementia Care
- Getting Evidence into Admiral Nurse Services (GEANS)
- GEANS – ‘Getting Evidence into Admiral Nursing Services’
- Improving outcomes for people with dementia during a hospital stay
- The Dementia Assessment and Intervention: Striving for Innovative and Evidence-based Services (DAISIES
- A gesture speaks a thousand words
- Aging care commissioning strategy
- Factors associated with carer quality of life of people with dementia: A systematic review of non-interventional studies
- Dementia Friendly Hospices – Embedding a sustainable model to develop the skills, knowledge and confidence of Hospice staff across Yorkshire and the Humber
- Talking ‘Do Not Attempt Cardiopulmonary Resuscitation (DNACPR)’
Additional case studies
- Medications for treating people with dementia – summary of evidence on cost-effectiveness
- Dementia care mapping – Evidence Review
- Cognitive Stimulation Therapy (CST) – summary of evidence on cost-effectiveness
- A coping programme for family carers of people with dementia – summary of evidence on cost-effectiveness
- End of Life Care Service for People with Dementia Living in Care Homes in Walsall
Making Hospitals More Dementia Friendly: Case Studies
The following is an excerpt from the “Planning for your hospital” section of the Dementia Friendly Hospital Toolkit developed by CARE and clinical and research faculty at the University of Wisconsin–Madison School of Nursing.
As we developed CARE’s Dementia Friendly Hospital Toolkit, we learned from two Wisconsin hospitals with their own dementia friendly initiatives: the W.S. Middleton Memorial VA Hospital in Madison and Stoughton Hospital in Stoughton. One of the hospitals that piloted our training materials, Fort HealthCare in Fort Atkinson, shared how they were launching their dementia friendly initiative using our toolkit.
Getting input from others

“We need to involve more than just nursing,” realized Lisa Rudolph, MSN, RN, the Education Services Manager at Fort HealthCare, when she was asked to lead dementia friendly planning. “If you don’t get staff involved, then that buy-in is not there. I wanted to make a task force and make it interdisciplinary.”
Fort HealthCare’s interdisciplinary task force includes a wide range of staff, from a nurse practitioner to volunteer services to radiology. They began by educating themselves on dementia and will help develop the hospital’s training plan. Rudolph recruited task force members by “reaching out to leadership and saying, ‘I would like some people from your department.’ I also reached out more broadly and said, ‘If you have a loved one, or some interest, or a personal connection with dementia, then I want you to be part of this, too.’”
Don’t forget key stakeholders outside of hospital staff and leadership. “It’s really important to get patient and family perspectives,” said Mary Wyman, PhD, a clinical psychologist and research investigator at W.S. Middleton Memorial VA Hospital. “We need to hear those voices, though it can be hard.”
Connecting with community organizations
Fort HealthCare’s community connections have provided dementia education to staff. “I reached out to our ADRC [Aging and Disability Resource Center] dementia care specialist,” said Lisa Rudolph. “She did a training with our task force members. It was interactive and an eye-opening experience for us.”

Stoughton Hospital is part of Stoughton’s dementia friendly community coalition. Heather Kleinbrook, BSN, RN, PMH-C, CDP, the Department of Nursing’s Inpatient Services Manager, also chairs the community coalition.
“A lot of what we do is education, getting businesses engaged and trained in how to be dementia friendly,” Kleinbrook said. “We provide education at the hospital. We started a memory café which we hold at the hospital monthly. We’re doing music and memory with the library.” The hospital’s community involvement increases awareness of their dementia friendly commitment and offers meaningful volunteer opportunities to staff.
The Madison VA participates in a regional coalition of dementia friendly groups, organized by the county dementia care specialist. “It’s a good way to stay plugged into what’s happening,” said Mary Wyman. “It’s inspiring. So many creative ideas come from these groups getting together. It helps us be more effective.”
Advice for people launching dementia friendly hospital initiatives
“It’s important to have support from administration from the get-go,” said Lisa Rudolph at Fort HealthCare. “Also think about setting some dollars aside for equipment or training. This isn’t a one and done. Think about onboarding new employees and having staff practice some of these skills every month or so.”

“There are so many places to improve the experience and quality of care for people living with dementia,” said Mary Wyman with the Madison VA. “Start with one bite-sized project. Also think about how dementia friendly improvements can align with other goals of the organization. For example, we made big wayfinding changes early on. There were other things we could have done, but people had for years been raising concerns about how confusing it was to get around our facility. So we were able to partner with a larger group and build momentum because we had a shared goal of improving wayfinding.”
Stoughton Hospital’s Heather Kleinbrook stressed the importance of finding staff “who are passionate and have a story to tell. They will help get others on your side. All of my administrative team had stories. They had a mother or father or grandparents who were living with dementia. When people have a passion, it’s amazing what you can do.”

Case studies
These three case studies help you to consider different situations that people with dementia face. They are:
- Raj , a 52 year old with a job and family, who has early onset dementia
- Bob and Edith , an older married couple who both have dementia and are struggling to cope, along with their family
- Joan , an older woman, who lives alone and has just been diagnosed with dementia
Each case study contains:
- A vignette setting out the situation
- An ecogram showing who is involved
- An assessment which gives essential information about what is happening and the social worker’s conclusion
- A care and support plan which says what actions will be taken to achieve outcomes
You can use the practice guidance to think about how you would respond in these situations.

- Equal opportunities
- Complaints procedure
- Terms and conditions
- Privacy policy
- Cookie policy
- Accessibility

For care 0203 728 7577
For jobs 0203 728 7570
Looking for care? 0203 728 7577
Looking for a job? 0203 728 7570

Case Study: Dementia Care
See how we help Steve to live well with dementia in his own home
Case Study: Supporting Steve with dementia home care
Our vascular dementia client.
Steve is 88 years old and living with vascular dementia. He lives with his wife Anne who was his primary carer. Steve’s dementia meant that he was highly anxious, easily agitated and dependent on Anne. He would become paranoid and aggressive if she left his sight.
Steve had enjoyed a successful career in business, in the military and as a civilian pilot. Now the frustration he felt was showing itself as aggressive outbursts. He had been prescribed antipsychotic medication which was negatively affecting his physical and mental well being but sadly not making a difference to his behavioural symptoms. His care was putting an immense burden on his wife Anne.
When Anne’s daughter Carol contacted The Good Care Group, the challenge was to find a way of providing care that was acceptable to Steve, did not threaten his self-esteem or cause him undue agitation.
Our dementia home care service
Using the SPECAL® method*, we found Steve could still access many positive memories of his time as a pilot. When we referenced aeroplanes or flying, he would swing his arm in a wide arc and say with pride ‘I flew around the world, you know’.
Through accessing and cueing positive past memories we found we could allay Steve’s fears. Steve’s behaviour became calmer and he was less prone to fear, paranoia and aggressive outbursts.
However, he would still get upset and angry if he felt Anne had gone somewhere without him. Anne wished to take a break and the challenge was to facilitate this without causing Steve excessive anxiety.
Again, his dementia care team worked with Steve and his family to uncover old memories and found that Anne had owned and run a small florist. Steve was very proud of this but would not have been involved in the day to day running of the business. The next time Steve asked where Anne was, we used a phrase – ‘sorting out a problem at the shop’ – which was familiar to Steve, and which reassured him that Anne was tied up elsewhere without making him feel that he should be there with her. Once the twin themes of flying and Anne’s need to solve problems at the florist had been established, we managed to facilitate a holiday for Anne and her daughter without causing distress to Steve.
Our success
Steve continues to live with dementia in the comfort of his own home. He is off of his anti-psychotic medication and outbursts are rare events. The care and support offered by The Good Care Group has given back independence to Anne who can again be his loving wife, not solely his carer, and allow her to take days out and the occasional holiday abroad. For more information about the SPECAL® method, please visit www.contenteddementiatrust.org
See The Types of Home Care We Offer For Dementia

“The Good Care Group helped me to make time for myself so I can give Steve the love and care he deserves. The SPECAL approach really worked for Steve – his own doctor said nothing could be done and that the only option was a life on medication.”
Talk to us about your dementia care needs.
Our friendly and experienced team is here to help you and your family make sense of the options available to you. Call us today – we will help you every step of the way.
0203 728 7577
Enquiry Form
Enquiry – Floating Button
Enquire now

Dementia Caregiver Studies
Testing ways to minimize stress and promote health in family caregivers of people with dementia.
Welcome to the Dementia Caregiver Studies of Case Western Reserve University. For over a decade, we have learned from caregivers and for caregivers. Led by Dr. Jaclene Zauszniewski, an internationally recognized nurse-scientist and Distinguished Faculty Researcher Award recipient of CWRU, we provide opportunities for family caregivers to participate in a number of research projects that all share a common goal: helping caregivers to better manage stress and stay healthy.
Our current projects, both funded by the National Institutes of Health, are focused on adult family members of persons experiencing a progressive memory problem or dementia such as Alzheimer’s Disease. Based on both her research and her personal experience, Dr. Zauszniewski recognized that caregiving includes family members who provide support and supervision at home, those who partner with a care facility where an impaired family member now lives, and even bereaved former caregivers who have recently lost their loved one. All three of these types of family caregivers are welcome to participate in our research projects.
Study participants will learn one of a number of stress management methods that may help to better manage caregiver stress and promote a healthy body and mind. Participants complete 2 or 3 interviews during the course of the next year to assess whether the strategies were helpful. In addition to the possible benefit of the method learned, and the satisfaction of helping other caregivers, participants are compensated for their time.
We are enrolling new participants and invite you to fill out the form in the Contact Us section below to see if you are eligible to participate.
You can also visit the study's Facebook page here .
- Principal Investigator
- Faculty Associates
- Team Members

Jaclene Zauszniewski, PhD, RN-BC, FAAN , is the Kate Hanna Harvey Professor in Community Health Nursing. "Dr. Z" is known for her pioneering work on personal and social resourcefulness as it relates to managing stress, depression and chronic illness. As a psychiatric nurse scientist, Dr. Z has conducted five NIH-funded studies and has mentored countless students in the PhD nursing program at Case Western Reserve University.
Christopher Burant , PhD, MACTM, FGSA; Co-Investigator
Evanne Juratovac , PhD, RN, GCNS-BC, Intervention Supervisor
Eva Kahana , PhD, CPMA; Co-Investigator
Martha Sajatovic , MD, Co-Investigator
Evelina DiFranco, MPH Research Associate Email: [email protected]
Barbara Boveington-Molter, MS Research Assistant Email: [email protected]
Kari Colón-Zimmermann, MA Research Assistant Email: [email protected]
Catherine Larsen, BS Research Assistant Email: [email protected]
Hangying She, RN; PhD student Data Coordinator Email: [email protected]
John S. Sweetko, PhD, RN, CPEN, CNE, Robert Wood Johnson Foundation Future of Nursing Scholar Research Associate Email: [email protected]
Rayhanah Almutairi, PhD student, MSN, AGPCNP-BC Graduate Research Assistant
Teaching Resourcefulness to Women Caregivers of Elders with Dementia
Principle Investigator: Jaclene Zauzsniewski
Co-Investigators: Diana Morris , Amy Zhang
The goal of this R21 Exploratory Research Grant was to pilot test an adapted intervention that teaches personal and social resourcefulness skills to women caregivers of elders with dementia. This pilot study provided qualitative and quantitative data for examining the six parameters of Resourcefulness Training© (RT) that were believed to be essential for strengthening the scientific rationale for a subsequent full scale randomized controlled trial. Within the context of a modified partially randomized preference trial with 138 women dementia caregivers, the necessity, acceptability, feasibility, fidelity, safety , and effectiveness of two innovative methods of RT were investigated. The results suggested a substantial need for RT in women caregivers of elders with dementia and support moving forward with testing RT effectiveness for reducing caregiver stress and depressive symptoms.
- Dementia care case studies
Who we're supporting with specialist dementia care at home
Seeing how dementia is affecting someone close to you can be distressing. We’ve been supporting people with dementia nationwide for almost 30 years. Read some of the stories below from the families we’ve helped.
Whether you’re looking for a visiting care service or more permanent live-in care support , our care is completely flexible to suit yours and your family’s needs.
To learn more about how we can provide dementia care in your own home, call our friendly care team today.
Dorothy’s story – dementia case study
How home care helps dorothy live the life she wants.

Dorothy says, “There really is no place like home, and with Magda’s support I am able to keep in touch with all of my friends and neighbours. We visit church every week for the Sunday morning service, I can visit the shops and I also take part in a local knitting group. This really is one of the greatest joys of staying in my own home around people I know.”
By helping her to do the activities that mean most, Magda has made such a difference in Dorothy’s life. An experienced carer with plenty of care knowledge, what matters most to Magda is that Dorothy is happy.
Dorothy says, “Live-in care means friendship, a sense of security and feeling comfortable in my own home. Having previously spent a brief but unhappy period of time in a care home, I am able to recognise how perfect my situation is now. I feel very lucky and comfortable; I have a true friend. Magda is going nowhere! I want this to continue forever.”
Joan’s story – dementia case study

Switching from a care home to live-in care
For the families that we support, deciding that their loved one needs additional support can be a difficult choice to make. For Joan, whose husband, Peter, had vascular dementia, having a live-in carer helped to keep her family together.
After feeling let down by a care home, Joan came across our 24-hour care support. After talking through our services and her family’s needs, we provided her with profiles of some of our carers. Lazlo’s profile stood out immediately.
Lazlo and Peter built a close friendship and other members of Joan’s family also loved having Lazlo around. As Peter neared the end of his life, Lazlo helped to make sure he was as comfortable as possible. He also worked closely with other healthcare professionals involved in Peter’s care, and helped his loved ones in any way he could.
Sue’s story – dementia case study
A daughter’s peace of mind.
What matters most to the families we support is that their loved one is safe and comfortable. When Sue needed some more support for her mother, Barbara, she decided that a live-in carer would be the best option.
Barbara’s carer, Rosemary, quickly became part of the family. She cooks Barbara’s favourite meals, tends to her personal care needs, and even listens to her stories about her adventures in the Pembrokeshire countryside, where they have also taken short breaks together.
Sue says, “Live-in care has made such a difference. [Mum] is so much more contented, she doesn’t have to worry – and neither do I – about somebody different coming in.”
Andy's story – dementia case study
Expert care for a declining condition.
Andy’s mother was living with dementia and her condition suddenly started to worsen. The daycare she was receiving couldn’t stretch to overnight support, and Andy and his siblings all lived an hour or more away.
On the recommendation of a visiting nurse, Andy contacted Helping Hands. “Very quickly things started falling into place,” he said. “I never felt that I was talking to anyone other than professionals, and yet it was done with an empathy which was greatly appreciated.”
Andy explained how we found the right carer for his mum. “Mum’s needs were expertly assessed without coming across as intrusive. We were also invited to provide our thoughts as to the sort of person who would get on best with Mum, the village environment and the friends with whom she would become acquainted.”
After three months of live-in care, during which time Andy said that his mother’s decline “seemed to have slowed”, the family made the decision to move her to a care home so she would be closer to them.
Andy described their Helping Hands carer saying, “I have absolutely no doubt that on this member of staff’s ‘watch’ Mum had a great time… We would often call to speak to Mum and simply had to bail out because Mum and them were giggling too much!
“I have to say I don’t know where we would have been over these three months without their dedication and support,” added Andy. “Thanks to all involved in my mother’s care.”
Other people are interested in...
We’re here seven days a week to talk through your home care needs and find the best option for you. Call 03300376958 or request a callback and we will call you.

Live in care
Quality live-in care at home, across England and Wales

What makes our carers and support workers so special

Testimonials
What our customers say about our home care
Page reviewed by Deanna Lane , Senior Clinical Lead on March 24, 2022
Find your perfect carer
Find the right affordable care for you
- After Hospital Care
- Comfort Care
- Community Care
- Comprehensive Home Care Packages for the Elderly
- Convalescent Care
- Critical Care
- Disability Care
- Domestic Care
- Hire a Carer
- Hospice care at home
- Intensive Care
- Intermediate Care
- Long term care
- Medication Management
- Personal care
- Postoperative Care
- Preventative Care
- Reablement Care
- Supported Living
- The Advantages of Home Care
- What is home care?
- Domiciliary care
- Loneliness is a guest nobody wants this Christmas
- Respite care
- How Are Parkinson’s And Dementia Related?
- How Dementia Affects Day to Day Living
- How Is Dementia Diagnosed
- Types of dementia
- What Does Dementia Feel Like?
- What Is Dementia Care Mapping?
- What is dementia care?
- Dementia Life Story Book
- Dementia care questions
- Our dementia experts
- Live in care for dementia
- Dementia care guide
- Diet for dementia
- Overnight care
- Palliative care
- Elderly care
- Support for younger people
- Condition-led care
- End of life care
- Housekeeping services
- Emergency home care
- Mobility aids and home adaptations
- Carer supported holidays
- Home Care FAQs
- What Is Home Help?
- Alternative to care homes
- Preparing For Home Care
- What Is A Home Carer?
- Difficult conversations: How to start a conversation about care
- Emotional well-being
- Our independent life
- Home care for Christmas and New Year
Guide to dementia home care
Looking for local care?
Friendly, expert care is closer than you think

Show in maps
- Research article
- Open access
- Published: 26 March 2021
The full spectrum of ethical issues in dementia research: findings of a systematic qualitative review
- Tim G. Götzelmann ORCID: orcid.org/0000-0001-6998-0850 1 na1 ,
- Daniel Strech 2 &
- Hannes Kahrass 1 na1
BMC Medical Ethics volume 22 , Article number: 32 ( 2021 ) Cite this article
9812 Accesses
14 Citations
12 Altmetric
Metrics details
When including participants with dementia in research, various ethical issues arise. At present, there are only a few existing dementia-specific research guidelines (Committee for Medicinal Products for Human Use in Clinical investigation of medicines for the treatment Alzheimer’s disease (Internet). https://www.ema.europa.eu/en/clinical-investigation-medicines-treatment-alzheimers-disease ; Food and Drug Administration, Early Alzheimer’s Disease: Developing Drugs for Treatment Guidance for Industry [Internet]. http://www.fda.gov/regulatory-information/search-fda-guidance-documents/alzheimers-disease-developing-drugs-treatment-guidance-industy ), necessitating a more systematic and comprehensive approach to this topic to help researchers and stakeholders address dementia-specific ethical issues in research. A systematic literature review provides information on the ethical issues in dementia-related research and might therefore serve as a basis to improve the ethical conduct of this research. This systematic review aims to provide a broad and unbiased overview of ethical issues in dementia research by reviewing, analysing, and coding the latest literature on the topic.
We conducted a systematic review in PubMed and Google Scholar (publications in English between 2007 and 2020, no restrictions on the type of publication) of literature on research ethics in dementia research. Ethical issues in research were identified by qualitative text analysis and normative analysis.
The literature review retrieved 110 references that together mentioned 105 ethical issues in dementia research. This set of ethical issues was structured into a matrix based on the eight major principles from a pre-existing framework on biomedical ethics (Emanuel et al. An Ethical Framework for Biomedical Research. in The Oxford textbook of clinical research ethics, Oxford University Press, Oxford, 2008). Consequently, subcategories were created and further categorized into dementia stages and study phases.
Conclusions
The systematically derived matrix helps raise awareness and understanding of the complex topic of ethical issues in dementia research. The matrix can be used as a basis for researchers, policy makers and other stakeholders when planning, conducting and monitoring research, making decisions on the legal background of the topic, and creating research practice guidelines.
Peer Review reports
Dementia prevalence rates are estimated to quadruple by 2050 [ 1 , 2 ]. Though such forecasts must be interpreted carefully, the global community is likely to face several challenges concerning the individual and familial burdens, societal and political consequences, and economic impact of dementia. With the growing size of the population with dementia, the costs of care are expected to increase in the near future [ 1 ].
The need for research on risk factors [ 2 ], palliative care, and reducing individual psychological burden is therefore of global importance. Research conducted with participants living with dementia raises important ethical questions, such as how to protect cognitively impaired persons against exploitation, how to design informed consent (IC) procedures with proxies, how to disclose risk-factors for dementia given the lack of evidence for their reliability, and how to apply risk–benefit considerations in such cases [ 3 ].
Out of fear of not being able to fulfil the ethical obligations required when conducting research with incapacitated persons, some might suggest the overall exclusion of cognitively impaired persons, or even of all individuals affected by dementia, from research. This caution may lead to the abandonment of meaningful research on dementia and would exclude dementia research from medical progress, leaving affected persons and their relatives orphaned.
Several guidelines [ 4 , 5 ] provide some orientation as to what should be considered to ensure that research on humans is ethical. These guidelines cover the entire research process from planning, conducting, and monitoring the trial to post-trial. Furthermore, they claim specific protection for vulnerable groups and individuals but are not meant to provide details on what that means for dementia research or other patient groups. Many authors have discussed the ethical challenges of dementia research [ 3 , 6 , 7 , 8 , 12 , 14 , 15 , 16 ]. These publications are characterized by a rather narrow focus on certain issues, e.g., on alternatives for obtaining IC [ 3 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 ] or genetic testing [ 14 , 15 , 16 , 17 , 18 , 19 , 20 ]. Some even use a combination of a systematic and a narrative review approach, with the emphasis on identifying differences in the ways ethical issues are addressed [ 21 ]; however, a review of the full spectrum of ethical issues in dementia research is still missing in the current literature.
In our systematic review, we therefore aimed to identify the full and unbiased spectrum of research on ethical issues in dementia as discussed in the literature.
Literature search and selection
Three strategies were applied to the literature search: PubMed (database), Google scholar and hand searching methods. We included a publication only if it: (a) described a disease research-specific ethical issue (DREI) in dementia research, (b) did not only relate to ethics in dementia care, and (c) the publication was a peer reviewed journal article or a scientific book (monograph, textbook, edited volume). Methodological quality was no eligibility criteria because of the descriptive approach of our study.
The Flowchart (Fig. 1 ) presents further details on the search algorithm and the eligibility criteria. This approach has already been applied before and can be read in detail elsewhere [ 22 ]. For reference management, we used the programme “Zotero”.
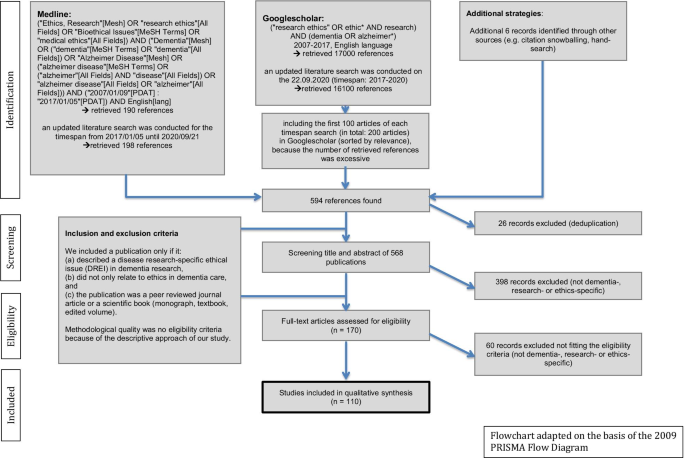
Literature search algorithm adapted on the basis of the 2009 “PRISMA Flow Diagram”
Definition and typology of dementia research-specific ethical issues (DREIs)
For the definition of DREI, we referred to the ethical theory of principlism. Emanuel et al. suggest eight principles that make clinical research ethical: respect for participants, independent review, fair participant selection/recruiting, favourable risk–benefit ratio, social value, scientific validity, collaborative partnership and IC [ 4 ]. These principles represent guiding norms that must be followed in a particular case unless there is a conflict with another obligation that is of equal or greater weight, e.g., alternatives to obtaining IC in special groups or situations. These principles provide only general ethical orientations that require further detail to give guidance in concrete cases. Thus, when applied, the principles must be specified and—if they conflict—balanced against one another.
There are two types of ethical issues that could arise: (a) inadequate consideration of one or more principles (e.g., “risk of insufficiently informing IRBs [institutional review boards] about adequate steps taken to fulfil the ethical obligations of dementia research”) or (b) conflicts between two or more principles (e.g., “challenge of balancing divergent statements in ARD [advance research directive] against current dementia patient wishes or proxy decisions (now vs. then)”). The terms "risk" (a) and "challenge" (b) used in the following refer to this conceptual consideration.
Analysis and synthesis of DREIs
For analysis, we used thematic content analysis [ 23 ] for all 110 included references. To identify and clarify potential ambiguities during content analysis as early as possible a first purposively sampled cluster of references (n = 10) was coded by two reviewers (TG, HK) independently. Another sample of detailed references (n = 9) was coded by one reviewer (TG) only. To capture as many ethical issues as possible this first cluster purposively included more detailed and comprehensive publications. The identified issues were then compared and grouped into the eight principles framework [ 4 ] in a consensus process using a programme for qualitative data analysis (“MAXQDA”). Because the consensus process revealed sufficient clarity for how to deal with ambiguous codings the remaining references (n = 45) were randomly split in half and analysed by one author (HK or TG) only. We updated the search in September 2020 and included another sample of 46 studies. These studies were coded by one author (TG). If further ambiguities during coding occurred they were discussed and clarified in the team.
For synthesis, we used a mixed deductive-inductive approach that takes into account the eight principles and the descriptions from the primary literature. We introduced subcategories if we found it reasonable to do so (for example if the number of DREIs was high). Finally, we used dementia stage and study phase to further categorize the identified issues (see Table 1 ). While we started with the established eight principles for clinical research ethics as a coding framework, our coding procedure was open for DREIs which could not be grouped under one of the eight principles.
References and journals
The literature search in PubMed and Google Scholar revealed a total set of 594 references, 110 of which were ultimately included in the analysis, published between 2007 and 2020 in 64 different journals. For more details, see the flowchart (Fig. 1 ).
Spectrum of dementia research ethical issues (DREIs)
The analysis of the 110 references revealed 105 DREIs. All identified issues could be grouped under one of the eight principles for ethical research, some having far more DREIs than others. In detail, “respect for participants” (n = 11 DREIs), “independent review” (n = 3), “fair participant selection/recruiting” (n = 5), “favourable risk–benefit ratio” (n = 16, 3 subcategories), “social value” (n = 2), “scientific validity” (n = 20, 5 subcategories), “collaborative partnership” (n = 5) and “informed consent (IC)” (n = 43, 12 subcategories). In the course of data analysis, we subsequently found fewer new codes, and the last 10 analysed papers raised no new issues. Thus, we appear to have achieved thematic saturation for the spectrum at least for the level of major groups and first-level subgroups. We updated the search in September 2020 which lead to the analysis of 46 references from the years 2017 until 2020. During the process of literature analysis, only one new subcategory was found in a paper from 2018 [ 24 ], hereafter no new sub-categories have been identified (for the years of 2019 and 2020).
All identified DREIs and subcategories are presented in Table 1 . This table also contains the categorization according to the dementia stage (based on the NIA-AA-2018-Framework) [ 25 ] and the phase of the research for each issue, symbolized by superscript numbers or characters. Additionally, the 105 DREIs are presented in separate tables for each category of dementia stage (Additional file 1 ) and the phase of the research (Additional file 2 ). A full list of the found issues together with the accompanying original text examples as well as the list of all references that were analysed during our systematic review are available in Additional file 3 : Table S3. The above listed tables are available at the supplemental data.
We used the nomenclature of the NIA-AA-2018-framework (“cognitively impaired”, “mild cognitive impairment (MCI)” and “dementia”) [ 25 ] for the first three categories in our dementia stages categorization. Most DREIs were related to more than one dementia stage (category IV, n = 60, Table 1 ). DREIs related to “cognitively unimpaired” (category I, n = 7) centre around the principle of favourable risk–benefit ratio, especially dealing with the sub-categories “determining risk adequately” and “considering risk adequately”, and the principle of respect for participants. No issues were found to fit “mild cognitive impairment” exclusively (category II), where people with dementia are not yet incapacitated. In category III = dementia (n = 11), issues mostly referred to “IC”, especially addressing the sub-category “proxy consent”. Finally, 27 DREIs could not be classified in that split spectrum.
Concerning the categorization due to study phase , we used a timeline approach in naming the different study phases (I = recruiting/pre-trial, II = conduction phase, III = post-trial, IV = general). For DREIs related to specific study phases, again, most DREIs were found to be of overarching relevance (category D, n = 45). In the recruiting/pre-trial phase, DREIs arise within “independent review”, “fair participant selection/recruiting”, “scientific validity”, and “informed consent” (category A, n = 32). While conducting the study (category B, n = 9), DREIs are related to “drop-outs” that endanger scientific validity and the “ongoing assessment” within the principle of informed consent. The post-trial phase is mostly concerned with the principle of respect for participants, communicating the results to the participants and the scientific community (“poor reporting quality”) and adequate follow-up of the volunteers (category C, n = 6). Thirteen issues could not be classified under the topic of study phase .
Specification of general principles for ethical dementia research
All principles for ethical research [ 4 ] were specified in the analysed literature. The references to general principles, such as “IC”, are rather implicit; however, authors elaborate on how the characteristics of dementia lead to specific ethical challenges, e.g., “However, a special ethical issue with regard to longitudinal studies that end in participants’ death is that participants are competent when first recruited, but have a significant likelihood of becoming incompetent while they are study subjects. […] [T]he gradual loss of the capacity to consent […] creates challenges for informed consent, the ethical bedrock of research with human subjects. […][Here], it may make sense to re-evaluate consent capacity […] at several intervals during the study" [ 6 ].
From this statement, the following DREI was paraphrased: “Risk that IC at the beginning of a dementia study alone is insufficient because of cognitive decline of participants”. This DREI is of general relevance for all dementia stages but has particular relevance to the study phase “recruiting/pre-trial”. The full spectrum of issues, including original text examples and all references, is presented in the online supplement (see Additional file 3 ).
Issues which were mentioned the most, are, for example, “Risk of excluding relevant subgroups, e.g. inhabitants of nursing homes, those lacking a proxy/spouse or patients with other psychiatric diseases, from dementia research” (n = 25 papers) and “Risk of excluding participants from research due to lack of capacity to consent” (n = 23). Examples of rarely mentioned DREI are “Risk that dementia patients experiencing stigmatization will lead to low follow-up rates or study withdrawal” (n = 1), “Risk of therapeutic misconception being higher in participants with MCI or mild dementia” (n = 1) and “Risk of RECs [research ethics committees] weighing opinions of physicians (protecting the participant) over patients’ willingness to participate and over nurse counsellors’ opinions” (n = 1).
Several DREIs were only addressed in an implicit manner; for example, “Risk that varying international regulations are a burden for international dementia research” is based on the following quotation: “However, only in Germany and Italy is the system of proxy determined by the courts—a procedure which is not necessarily required for the recognition of a proxy in other member states” [ 26 ].
This systematic literature review identified and synthesized the full spectrum of 105 ethical issues in dementia research (DREIs) based on 110 references published between 2007 and 2020 in 64 different journals.
Many ethical issues involved “IC” (n = 11) in incapacitated participants and “risk-information disclosure” (n = 8). However, this review shows that there are many more DREIs to consider when planning, reviewing, conducting, or monitoring research with this vulnerable group. We assume that the results will be of interest to different groups—clinical experts, researchers, policy makers, REC-members, lawyers, patient-organization representatives or even affected persons themselves—and that the different stakeholders will read and use the results differently.
Our review lists several ethical issues grouped under eight broadly established ethical principles for clinical research. These principles and the principlism approach in general are correlative to basic human rights [ 26 ]. The eight principles are not focused on capacity-based approaches but include approaches to express the right to participate in research via, for examples, advance directives. We would therefore argue, in line with many other ethical analyses based on a principlism approach, that human rights related ethical issues in dementia research are captured directly and indirectly by the many ethical issues addressed in our list of issues. The same applies to other overarching normative concepts such as “avoiding exploitation”. No specified ethical issue in our list mentions the risk of exploitation directly but more or less all specific ethical issues address this risk indirectly. Likewise, the wording “human rights” did not appear explicitly in the literature we analyzed.
Those looking for support or guidance on how to seek ethically appropriate dementia research might prefer detailed descriptions of very specific challenges. Articles such as “Seeking Assent and Respecting Dissent in Dementia Research” by Black et al. [ 9 ] serve this purpose. However, these publications often focus on particular aspects and do not aim to provide a detailed and systematic overview. Further, one also has to do thorough searching and read a large volume of material (we screened n = 594 and finally included n = 110 references) to be familiar with all the aspects discussed in the literature. In contrast to literature addressing very specific DREIs, there are also broad, theoretical frameworks for research ethics, such as that of Emanuel et al. [ 4 ]. However, if capacity building for ethics in dementia research is primarily informed by such general frameworks, it might overlook issues that only become apparent when specifying practice-related tasks. Our review is intended to bridge detailed specifications with a comprehensive and structured presentation of the DREIs at stake.
We illustrate the bridging character of our study by comparing one benchmark for the IC principle originating from Emanuel et al.’s framework [ 4 ] with one issue on our spectrum grouped under “IC” in the subcategory “proxy consent”. The benchmark is “Are there appropriate plans in place for obtaining permission from legally authorized representatives for individuals unable to consent for themselves?” [ 4 ]. A researcher with a specific trial in mind would, in order to conduct morally sound research, perhaps refer to that benchmark in a case where they plans to start a trial on incapacitated patients suffering from dementia. This person would then fulfil that benchmark by making it possible for legal representatives of the patients to fill out the IC document in place of the incapacitated participant. Thus, they would fulfil the benchmark and might not think about more specific ethical problems that might arise when one looks into the literature describing DREI. One such example is this quote stemming from an article on dementia research ethics: “Proxy consent, already an issue of debate in traditional research, was considered more problematic in genetic research, where children share the same genetic traits as their parents. On the one hand, this might be a motivation for the affected parent to participate in a research study to help their children. On the other hand, it was questioned that to what extent children still are able to make a decision in the best interest of their parents because they have an interest themselves. The more genetic research will be carried out, the higher the chance on a disease modifying or preventive therapy for them and their children” [ 14 ].
In that case, and if the researcher had a plan to conduct research in this field of genetic dementia research, the simple fulfilment of the abovementioned benchmark would be insufficient for the goal of morally acceptable research. The mentioned quotation informed the creation of the DREI “Risk of not considering that proxies have major self interest in dementia research, e.g., because they have same genetic traits, which could influence their proxy decision, and their manipulative behaviour may be difficult to detect”.
As we compared topics between both rounds of the literature analysing process, we noticed, that some topics were newly introduced in the scientific literature, in particular “deep brain stimulation” issues in dementia research. Other categories or sub-categories like “social value”, “qualified personnel” and “informed consent document” were not further discussed in scientific literature.
In addition, we found more and more text examples for issues which before the year of 2017 were only mentioned once, e.g. “Risk of over diagnosis in asymptomatic persons, if the diagnosis is derived from the risk marker status, since their corresponding validity regarding the occurrence and course of a disease is (still) limited”, which now was mentioned in six papers.
Also, in the course of the analysis of the studies between 2017 and 2020 we found 22 new issues, among them 17 issues which were only mentioned by one paper showing the rapid emergence of new issues in the dementia research ethics field.
Capturing this full spectrum of DREIs can serve multiple purposes. First, it can raise awareness of the ethical issues arising in the context of dementia research, highlighting issues that may be underrepresented in the published literature through the side-by-side presentation in our matrix. Second, it can serve as the basis for information or training materials for researchers and caregivers. Third, it can form the basis for discussions on the importance and/or relevance of the different ethical issues. Fourth, because our spectrum does not rank the difficult DREIs in order of importance, third parties can use it as a basis for exactly that purpose. Fifth, developers of specific research guidelines or policy papers may use this spectrum as an entry point to that topic.
At this point, it is important to state that our spectrum remains strictly descriptive. The qualitative and normative interpretation is therefore left to others, e.g., researchers, policy-makers, patient organizations, funding partners and the community as a whole. Those interpretations could further help in developing stakeholder-oriented guidelines for conducting ethically sound research in dementia. The list of ethical issues as presented in this paper, however, cannot directly serve as a checklist for review purposes. More conceptual work is needed to translate the in-depth results of this systematic review into effective and efficient normative or procedural guidance. Finally, existing guidelines, policy papers or new research articles on the topic of DREIs can be screened for completeness [ 27 ].
To make the results of the review more concise and accessible, we prepared overviews sorted by stage and phase (available as an online supplement). This is particularly suitable for readers who have a certain focus, e.g., because they are currently planning a study with people in an early stage of dementia (see Additional file 1 ) or are looking for an overview of DREIs in the phase of conducting the study (see Additional file 2 ). These tables show that ethical issues are situation-sensitive, e.g., certain questions on informed consent only arise at a later stage of the disease, while questions of reporting the status of risk factors are only relevant in early stage (pre-symptomatic) patients.
One limitation of this systematic review is that the search was limited to PubMed and Google Scholar. We do not consider this an overly disadvantageous factor and consider the approach to be appropriate for the following reasons: First, our search resulted in the identification of literature from different fields, not only from the bioethics and medicine field but also spanning nursing research [ 28 , 29 ], nursing ethics [ 30 , 31 ], a narrative review [ 3 ] and even one systematic review [ 21 ]. This systematic review by West et al. covered mostly literature concerning IC, advance directives and the role of proxies or surrogates. Second, thematic saturation for the first-level categories was achieved after analysing 54 of the 64 papers that were included after the first literature search in these two data sources. For the updated literature search, which only found one new first-level category, thematic saturation was achieved after analysing 25 of the 46 papers. Third, former systematic reviews [ 32 , 33 ] in the bioethics field, which based their research on additional databanks such as EMBASE, CINAHL or Euroethics, found few additional references. Another limitation is that we only reviewed the literature from the last 14 years. However, we included two (systematic) reviews, which included literature dating back to 1982 [ 3 ] and back to 1995 [ 21 ]. We further assume that an important ethical issue that was mentioned 15 years ago and that is still relevant nowadays would be addressed in some more recent references again.
Further, we only included references in the English language. Some culturally sensitive DREI might be preferably discussed in the respective language, and our review might have missed those discussions. Last but not least, we only included peer-reviewed literature and thus did not consider grey literature such as guidelines from advocacy organizations involved with dementia research [ 34 , 35 ]. As a future project, we aim to employ the results of our review to analyse whether and how guidelines for dementia research mention the identified issues. For a similar approach see the results of a systematic review of ethical issues in dementia care [ 22 ] that was followed-up by a content analysis of clinical practice guidelines for dementia care [ 27 ].
The authors of this review have different scientific backgrounds: medicine/psychiatry, physiotherapy, public health, ethics and philosophy. However, all authors are currently involved neither in clinical research nor in health care for people with dementia. However, we do not consider this to be a weakness of the review, as we have included these perspectives in the literature considered, e.g., expert opinions [ 9 , 10 , 14 , 36 , 37 , 38 , 39 ], views of patients, caregivers and proxies [ 11 , 28 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 ], papers focusing on legal and ethical guidelines [ 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 ], and the point of view of lay persons [ 13 ]. Our review found no papers on the opinions and views of relatives of people living with dementia. This might indicate the need for further research in that field.
This study has successfully shown that a systematic literature review leads to a wider spectrum of DREIs (n = 105) than other papers on the subject. The identified issues are specifications of eight general ethical principles for clinical research and could be categorized according to the dementia stage and study phase. Therefore, the spectrum can be used to raise awareness about the complexity of ethics in this field and can support different stakeholders in the implementation of ethically appropriate dementia research.
Availability of data and materials
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
Dementia research-specific ethical issue(s)
Informed consent
Institutional review board(s)
Advanced research directives
Tim Götzelmann
Hannes Kahrass
Mild cognitive impairment
Research ethics committee(s)
Daniel Strech
Randomized controlled trial(s)
European union
Positron emission tomography-computed tomography
Mini-Mental State Examination
Wimo A, Jönsson L, Bond J, Prince M, Winblad B, International AD. The worldwide economic impact of dementia 2010. Alzheimers Dement. 2013;9(1):1–11.
Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement. 2013;9(1):63–75.
Article Google Scholar
Johnson RA, Karlawish J. A review of ethical issues in dementia. Int Psychogeriatr. 2015;27(10):1635–47.
Emanuel EJ, Grady CC, Crouch RA, Lie RK, Miller FG, Wendler DD. An Ethical Framework for Biomedical Research. In: The Oxford textbook of clinical research ethics. Oxford University Press; 2008. p. 123–35.
WMA - The World Medical Association-WMA Declaration of Helsinki—Ethical Principles for Medical Research Involving Human Subjects [Internet]. [Cited 2019 Mar 19]. Available from: https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/ .
Davis DS. Ethical issues in Alzheimer’s disease research involving human subjects. J Med Ethics. 2017; medethics—2016.
Sherratt C, Soteriou T, Evans S. Ethical issues in social research involving people with dementia. Dementia. 2007;6(4):463–79.
van der Vorm A, Vernooij-Dassen MJFJ, Kehoe PG, Rikkert MGMO, van Leeuwen E, Dekkers WJM. Ethical aspects of research into Alzheimer disease. A European Delphi Study focused on genetic and non-genetic research. J Med Ethics. 2009;35(2):140–4.
Black BS, Rabins PV, Sugarman J, Karlawish JH. Seeking assent and respecting dissent in dementia research. Am J Geriatr Psychiatry. 2010;18(1):77–85.
Dubois M-F, Bravo G, Graham J, Wildeman S, Cohen C, Painter K, et al. Comfort with proxy consent to research involving decisionally impaired older adults: do type of proxy and risk–benefit profile matter? Int Psychogeriatr. 2011;23(09):1479–88.
Karlawish J, Kim SYH, Knopman D, van Dyck CH, James BD, Marson D. The views of alzheimer disease patients and their study partners on proxy consent for clinical trial enrollment. Am J Geriatr Psychiatry. 2008;16(3):240–7.
Kim SYH. The ethics of informed consent in Alzheimer disease research. Nat Rev Neurol. 2011;7(7):410–4.
Kim SYH, Kim HM, Knopman DS, De Vries R, Damschroder L, Appelbaum PS. Effect of public deliberation on attitudes toward surrogate consent for dementia research.—PubMed—NCBI [Internet]. [Cited 2017 Jan 4]. Available from: https://n.neurology.org/content/77/24/2097.short .
Olde Rikkert MG, van der Vorm A, Burns A, Dekkers W, Robert P, Sartorius N, et al. Consensus statement on genetic research in dementia. Am J Alzheimers Dis Other Demen. 2008;23(3):262–6.
SYH Kim, J Karlawish, BE Berkman. Ethics of genetic and biomarker test disclosures in neurodegenerative disease prevention trials.—PubMed—NCBI [Internet]. [Cited 2017 Jan 4]. Available from: https://n.neurology.org/content/84/14/1488.short .
Arribas-Ayllon M. The ethics of disclosing genetic diagnosis for Alzheimer’s disease: do we need a new paradigm? Br Med Bull. 2011;100(1):7–21.
Chao S, Roberts JS, Marteau TM, Silliman R, Cupples LA, Green RC. Health behavior changes after genetic risk assessment for Alzheimer disease: the REVEAL study. Alzheimer Dis Assoc Disord. 2008;22(1):94–7.
Christensen KD, Roberts JS, Uhlmann WR, Green RC. Changes to perceptions of the pros and cons of genetic susceptibility testing after APOE genotyping for Alzheimer disease risk. Genet Med. 2011;13(5):409–14.
Green RC, Roberts JS, Cupples LA, Relkin NR, Whitehouse PJ, Brown T, et al. Disclosure of APOE genotype for risk of Alzheimer’s disease. N Engl J Med. 2009;361(3):245–54.
Salmon D, Lineweaver T, Bondi M, Galasko D. Knowledge of APOE genotype affects subjective and objective memory performance in healthy older adults. Alzheimers Dement J Alzheimers Assoc. 2012;8(4):P123–4.
West E, Stuckelberger A, Pautex S, Staaks J, Gysels M. Operationalising ethical challenges in dementia research—a systematic review of current evidence. Age Ageing. 2017;1–10.
Strech D, Mertz M, Knuppel H, Neitzke G, Schmidhuber M. The full spectrum of ethical issues in dementia care: systematic qualitative review. Br J Psychiatry. 2013;202(6):400–6.
Mayring P. Qualitative content analysis. Companion Qual Res. 2004;1:159–76.
Google Scholar
Thorogood A, Mäki-Petäjä-Leinonen A, Brodaty H, Dalpé G, Gastmans C, Gauthier S, et al. Consent recommendations for research and international data sharing involving persons with dementia. Alzheimers Dement. 2018;14(10):1334–43.
Jack Cr, Da B, K B, Mc C, B D, Sb H, et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease [Internet]. Alzheimer’s & dementia: the journal of the Alzheimer’s Association. 2018 [cited 2020 Sep 24]. Available from: https://pubmed.ncbi.nlm.nih.gov/29653606/ .
Gainotti S, Imperatori SF, Spila-Alegiani S, Maggiore L, Galeotti F, Vanacore N, et al. How are the interests of incapacitated research participants protected through legislation? An Italian study on legal agency for dementia patients. PLoS ONE. 2010;5(6):e11150.
Knüppel H, Mertz M, Schmidhuber M, Neitzke G, Strech D. Inclusion of ethical issues in dementia guidelines: a thematic text analysis. PLoS Med. 2013;10(8):e1001498.
Hanson LC, Gilliam R, Lee TJ. Successful clinical trial research in nursing homes: the improving decision-making study. Clin Trials Lond Engl. 2010;7(6):735–43.
Garand L, Lingler JH, Conner KO, Dew MA. Diagnostic labels, stigma, and participation in research related to dementia and mild cognitive impairment. Res Gerontol Nurs. 2009;2(2):112–21.
Heggestad AKT, Nortvedt P, Slettebø Ashild. The importance of moral sensitivity when including persons with dementia in qualitative research. Nurs Ethics. 2012;0969733012455564.
Slaughter S, Cole D, Jennings E, Reimer MA. Consent and assent to participate in research from people with dementia. Nurs Ethics. 2007;14(1):27–40.
Sofaer N, Strech D. Reasons why post-trial access to trial drugs should, or need not be ensured to research participants: a systematic review. Public Health Ethics. 2011;4(2):160–84.
Strech D, Persad G, Marckmann G, Danis M. Are physicians willing to ration health care? Conflicting findings in a systematic review of survey research. Health Policy. 2009;90(2):113–24.
Europe A. Overcoming ethical challenges affecting the involvement of people with dementia in research: recognising diversity and promoting inclusive research. Luxemb Alzheimer Eur. 2019.
Alzheimer E. Alzheimer Europe Report: The ethics of dementia research: Alzheimer Europe; 2011. 2011.
Schicktanz S, Schweda M, Ballenger JF, Fox PJ, Halpern J, Kramer JH, et al. Before it is too late: professional responsibilities in late-onset Alzheimer’s research and pre-symptomatic prediction. Front Hum Neurosci. 2014;8:921.
Molinuevo JL, Cami J, Carné X, Carrillo MC, Georges J, Isaac MB, et al. Ethical challenges in preclinical Alzheimer’s disease observational studies and trials: Results of the Barcelona summit. Alzheimers Dement [Internet]. 2016 Mar [cited 2016 Apr 21]; Available from: http://linkinghub.elsevier.com/retrieve/pii/S1552526016000765 .
Harkins K, Sankar P, Sperling R, Grill JD, Green RC, Johnson KA, et al. Development of a process to disclose amyloid imaging results to cognitively normal older adult research participants. Alzheimers Res Ther [Internet]. 2015 Dec [cited 2017 Jan 6];7(1). Available from: http://alzres.com/content/7/1/26 .
Werner P, S S. Practical and Ethical Aspects of Advance Research Directives for Research on Healthy Aging: German and Israeli Professionals’ Perspectives [Internet]. Frontiers in medicine. 2018 [cited 2020 Sep 21]. Available from: https://pubmed.ncbi.nlm.nih.gov/29675415/ .
Cary MS, Rubright JD, Grill JD, Karlawish J. Why are spousal caregivers more prevalent than nonspousal caregivers as study partners in AD dementia clinical trials? Alzheimer Dis Assoc Disord. 2015;29(1):70–4.
Hellström I, Nolan M, Nordenfelt L, Lundh U. Ethical and Methodological Issues in Interviewing Persons With Dementia. Nurs Ethics. 2007;14(5):608–19.
Overton E, Appelbaum PS, Fisher SR, Dohan D, Roberts LW, Dunn LB. Alternative decision-makers’ perspectives on assent and dissent for dementia research. Am J Geriatr Psychiatry. 2013;21(4):346–54.
Grill Jd, Cg C, K H, J K. Reactions to learning a “not elevated” amyloid PET result in a preclinical Alzheimer’s disease trial [Internet]. Alzheimer’s research & therapy. 2018 [cited 2020 Sep 21]. Available from: https://pubmed.ncbi.nlm.nih.gov/30579361/ .
Hosie A, S K, N R, I G, D P, C S, et al. Older Persons’ and Their Caregivers’ Perspectives and Experiences of Research Participation With Impaired Decision-Making Capacity: A Scoping Review [Internet]. The Gerontologist. 2020 [cited 2020 Sep 21]. Available from: https://pubmed.ncbi.nlm.nih.gov/32866239/ .
Jongsma K, J P, S S, K R. Motivations for people with cognitive impairment to complete an advance research directive—a qualitative interview study [Internet]. BMC Psychiatry. 2020 [cited 2020 Sep 21]. Available from: https://pubmed.ncbi.nlm.nih.gov/32641010/ .
Mann J, Hung L. Co-research with people living with dementia for change. Action Res. 2019;17(4):573–90.
Morbey H, Harding AJ, Swarbrick C, Ahmed F, Elvish R, Keady J, et al. Involving people living with dementia in research: an accessible modified Delphi survey for core outcome set development. Trials. 2019;20(1):1–10.
Ries N, E M, R S-F. Planning Ahead for Dementia Research Participation: Insights from a Survey of Older Australians and Implications for Ethics, Law and Practice [Internet]. Journal of bioethical inquiry. 2019 [cited 2020 Sep 21]. Available from: https://pubmed.ncbi.nlm.nih.gov/31297689/ .
Robillard JM, Feng TL. When patient engagement and research ethics collide: lessons from a dementia forum. J Alzheimers Dis. 2017;59(1):1–10.
Jongsma K, Bos W, van de Vathorst S. Morally relevant similarities and differences between children and dementia patients as research subjects: representation in legal documents and ethical guidelines: children and dementia patients as research subjects. Bioethics. 2015;29(9):662–70.
Alpinar-Sencan Z, S S. Addressing ethical challenges of disclosure in dementia prediction: limitations of current guidelines and suggestions to proceed [Internet]. BMC Medical Ethics. 2020 [cited 2020 Sep 21]. Available from: https://pubmed.ncbi.nlm.nih.gov/32393330/ .
Fletcher JR, Lee K, Snowden S. Uncertainties when applying the mental capacity act in dementia research: a call for researcher experiences. Ethics Soc Welf. 2019;13(2):183–97.
Gove D, Diaz-Ponce A, Georges J, Moniz-Cook E, Mountain G, Chattat R, et al. Alzheimer Europe’s position on involving people with dementia in research through PPI (patient and public involvement). Aging Ment Health. 2018;22(6):723–9.
Ries NM, Thompson KA, Lowe M. Including people with dementia in research: an analysis of Australian ethical and legal rules and recommendations for reform. J Bioethical Inq. 2017;14(3):359–74.
Thorogood A, Dalpe G, McLauchlan D, Knoppers B. Canadian consent and capacity regulation: undermining dementia research and human rights. McGill J Law Health. 2018;12:67.
Committee for Medicinal Products for Human Use. Clinical investigation of medicines for the treatment Alzheimer’s disease [Internet]. European Medicines Agency. 2018 [cited 2019 Aug 1]. Available from: https://www.ema.europa.eu/en/clinical-investigation-medicines-treatment-alzheimers-disease .
Food and Drug Administration. Early Alzheimer’s Disease: Developing Drugs for Treatment Guidance for Industy [Internet]. U.S. Food and Drug Administration. 2019 [cited 2019 Aug 1]. Available from: http://www.fda.gov/regulatory-information/search-fda-guidance-documents/alzheimers-disease-developing-drugs-treatment-guidance-industy .
Download references
Acknowledgements
We would like to express our special gratitude to Marcel Mertz for his critical review of the categorization and paraphrasing of the issues. His expertise was used to confirm the validity of the final spectrum.
Not applicable.
Author information
Tim G. Götzelmann and Hannes Kahrass have contributed equally to this work
Authors and Affiliations
Institute for History, Ethics and Philosophy in Medicine, OE 5450, Hannover Medical School, Carl-Neuberg-Str. 1, 30625, Hannover, Germany
Tim G. Götzelmann & Hannes Kahrass
QUEST Center, Berlin Institute of Health, Anna-Louisa-Karsch-Straße 2, 10178, Berlin, Germany
You can also search for this author in PubMed Google Scholar
Contributions
TG had a major role in the acquisition of data, analysed the data and drafted the manuscript for intellectual content. DS had a major role in the design and conceptualization of the study and revised the manuscript for intellectual content. HK analysed the data and revised the manuscript for intellectual content. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Tim G. Götzelmann .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1. table s1.
: An overview of the 105 DREIs assigned to dementia stages.

Additional file 2. Table S2
: An overview of the 105 DREIs assigned to study phases.
Additional file 3. Table S3
: All principles, issues and text examples in one table.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Götzelmann, T.G., Strech, D. & Kahrass, H. The full spectrum of ethical issues in dementia research: findings of a systematic qualitative review. BMC Med Ethics 22 , 32 (2021). https://doi.org/10.1186/s12910-020-00572-5
Download citation
Received : 20 May 2020
Accepted : 21 December 2020
Published : 26 March 2021
DOI : https://doi.org/10.1186/s12910-020-00572-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Medical ethics
- Research ethics
- Dementia research
BMC Medical Ethics
ISSN: 1472-6939
- General enquiries: [email protected]

Hospice care for those with dementia falls far short of meeting people’s needs at the end of life
Associate Professor of Internal Medicine, University of Michigan
Disclosure statement
Maria J Silveira does not work for, consult, own shares in or receive funding from any company or organisation that would benefit from this article, and has disclosed no relevant affiliations beyond their academic appointment.
University of Michigan provides funding as a founding partner of The Conversation US.
View all partners
Jimmy Carter, who chose to forgo aggressive medical care for complications of cancer and frailty in February 2023 , recently reached his one-year anniversary since enrolling in hospice care. During this time, he celebrated his 99th birthday, received tributes far and wide and stood by the side of his beloved wife, Rosalynn, who died in November 2023.
In contrast to the former president, his wife, who had dementia, lived only nine days under hospice care .
Palliative care physicians like myself who treat both conditions are not surprised at all by this disparity.
Hospice brings a multidisciplinary team of providers to wherever a patient lives, be it their own home or a nursing home, to maintain their physical and psychological comfort so that they can avoid the hospital as they approach the end of life.
Hospice is not the same as palliative care , which is a multidisciplinary team that sees seriously ill patients in a clinic or hospital to help them and their families with symptoms, distress and advance care planning.
Strikingly, only 12% of Americans with dementia ever enroll in hospice . Among those who do, one-third are near death . This is in stark contrast to the cancer population: Patients over 60 with cancer enroll in hospice 70% of the time .
In my experience caring for dementia patients, the underuse of hospice by dementia patients has more to do with how hospice is structured and paid for in the U.S. than it does patient preference or differences between cancer and dementia.

The role of Medicare
In the U.S., most hospice stays are paid for by Medicare , which dictates what hospices look like, who qualifies for hospice and what services hospices provide. Medicare’s rules and regulations make it hard for dementia patients to qualify for hospice when they and their families need support the most – long before death.
In Canada, where hospice is structured entirely differently, 39% of dementia patients receive hospice care in the last year of life .
The benefits of hospice
The first hospice opened in the U.S. in 1974 .
Medicare began assessing the potential benefits of covering hospice care during Carter’s administration. The service gained popularity after Congress formalized a payment structure to reimburse hospice providers through Medicare in 1985.
At the time, lawmakers were responding to the realization that the cost of care at the end of life was the fastest-growing segment of Medicare’s budget. Most of those expenses covered costs for hospitalized patients with advanced incurable illnesses who died in the hospital after spending time in intensive care units.
Congress believed hospice would not only give seriously ill Americans an alternative to a medicalized death but would also help control costs. So it required hospices to provide holistic care to entice people to enroll in this new option.
The new Medicare coverage allowed hospice care to follow the patient wherever they lived, at home or in a nursing home. It would support families as well as patients.
These remain among hospices’ core values to this day.
Impossible trade-off
In exchange, people enrolling in hospice would have to forgo other kinds of care , such as seeing specialists or being admitted to a hospital.
But letting go of specialists is a nonstarter for many patients.
Researchers have found that people with life-limiting illnesses like dementia are half as likely to enroll in hospice when they want to continue treatments they cannot receive in hospice.
Psychiatrists and geriatricians who treat dementia, and the psychologists, nurses, social workers and others who support them, are invaluable to families struggling to manage a dementia patient’s disruptive and sometimes violent behavior. They adjust and rotate medications such as antidepressants, antipsychotics, anti-epileptics or sedatives to help their loved one experience less anxiety, agitation or depression as their memory fades.
These adjustments are the norm for many patients with dementia who are particularly prone to side effects such as agitation or drowsiness. Specialized dementia teams are difficult to give up in exchange for hospice clinicians who are generalists following a protocol and adhering to a short list of approved medications.
But that is what Medicare currently asks these families to do.
The best of both
Hospice advocates and palliative care providers like myself believe dementia patients should have access to both specialists to provide expert guidance and hospice providers to support the care at home .
So-called concurrent care is the standard of care for children facing life-ending illness , as well as for patients in the Veterans Health Administration . There is evidence that the concurrent care approach helps patients and, ironically, saves money .
For around a decade, the Centers for Medicare and Medicaid Services has been studying alternative models for hospice that include concurrent care for Americans covered by Medicare. The agency is proposing to study the issue a bit longer . However, more studies will not bring relief any time soon to the 7 million Americans with dementia and their families .
Criteria for hospice
Even when dementia patients and their families are willing to forgo specialists and hospitalization, they are unlikely to meet Medicare’s stringent criteria for hospice, which were designed to limit hospice to patients who are expected to die within six months.
It’s so difficult to qualify for hospice under a dementia diagnosis that one of my colleagues is known for saying, “If you want to get a dementia patient into hospice, find a cancer.”
Medicare’s criteria require that people with dementia not only depend upon others for help with toileting, transferring, bathing, walking and personal hygiene but also that they be bedridden, incontinent, minimally verbal and have a terminal complication of dementia such as aspiration pneumonia, recurrent urinary tract infections, significant weight loss, or bed sores.
These complications typically occur only in the most advanced stages of dementia and are less likely when people receive quality care at home .
More importantly, Medicare’s hospice benefits do not provide patients with dementia and their families the support they need most – hands-on care.
Most people are shocked to hear that, aside from providing a bath aide a couple of times a week and a home health aide for brief periods to give a family caregiver a break, hospice does not provide the hands-on care that dementia patients – and, frankly, anyone who is in hospice – requires.
Monitoring, toileting, hand-feeding, repositioning, ambulating, medication administration and wound care are left up to family caregivers .
Families of people with dementia must either sacrifice their personal well-being and livelihoods to care for a loved one at home, hire a professional home health aide, which costs from US$30 to $50 an hour, or place the loved one in a nursing home. The latter is paid out of pocket unless patients qualify for Medicaid. And hospice care, as currently structured, does nothing to help with that.
My heart breaks for people like the patient with early dementia I met recently. His daughter-in-law – his sole caregiver – requested he be enrolled in home hospice, only to find out that not only did he not qualify for hospice, but that hospice would not provide the hands-on support they needed.
“So, unless we can afford to pay for an aide or place him in memory care, I’m all the hands-on help he’s got?,” she asked. “I’m afraid so,” I answered.
Given this impossible choice, it’s no surprise that Rosalynn Carter only entered hospice near the end of her life.
- Palliative care
- Jimmy Carter
- Health care policy
- Gerontology
- Dementia care
- Health care reform
- Hospice care
- US health care

Biocloud Project Manager - Australian Biocommons

Director, Defence and Security

Opportunities with the new CIEHF

School of Social Sciences – Public Policy and International Relations opportunities

Deputy Editor - Technology

- Advanced Life Support
- Endocrinology
- Gastroenterology
- Infectious disease
- Intensive care
- Palliative Care
- Respiratory
- Rheumatology
- Haematology
- Endocrine surgery
- General surgery
- Neurosurgery
- Ophthalmology
- Plastic surgery
- Vascular surgery
- Abdo examination
- Cardio examination
- Neurolo examination
- Resp examination
- Rheum examination
- Vasc exacmination
- Other examinations
- Clinical Cases
- Communication skills
- Prescribing

Dementia case study with questions and answers

Dementia case study with questions and answers
Common dementia exam questions for medical finals, OSCEs and MRCP PACES
The case below illustrates the key features in the assessment of a patient with dementia or undiagnosed memory decline. It works through history, examination and investigations – click on the plus symbols to see the answers to each question
Part 1: Mavis
- Mavis is an 84-year old lady, referred to you in the memory clinic for assessment of memory impairment. She attends in the company of her son and daughter-in-law.
- On the pre-clinic questionnaire her son has reported a severe deterioration in all aspects of her cognition over the past 12 months.
- The patient herself acknowledges that there have been memory problems, but feels it is just her short term memory that is an issue.
Question 1.
- To begin the history, start broadly. Build rapport and establish both the patient’s view on memory impairment (if any) and the family’s (or other collateral history).
- Patient’s (and collateral) view of memory decline
- Biographical history
- Objective view of memory decline (e.g. knowledge of current affairs)
- Impact of memory decline on day-to-day living and hobbies
- Social history, including safety and driving
- General medical history (especially medications)
- See below for details on these…
Question 2.
- Is it for everything or are specific details missed out/glossed over?
- Try to pin down specific details (e.g. names of people/places).
- At what time in chronological order do things start to get hazy?
Question 3.
- If under 12 years this will lead to additional point being awarded on some cognitive tests
- Ask about long term memories, e.g. wedding day or different jobs
- Then move on to more recent memories, e.g. last holiday
Question 4.
- If your patient watches the news/read newspapers on a regular basis, ask them to recount the headlines from the past few days.
- Be sure to look for specifics to prevent your patient masking memory deficiencies with broad statements. For example: “The government are incompetent, aren’t they?!” should be clarified by pinning down exactly why they are incompetent, for example: “Jeremy Hunt”.
- If they like to read, can they recall plotlines from current books or items from magazines?
- If they watch TV, can they recount recent plot lines from soaps, or formats of quiz shows?
Question 5.
- Ask about hobbies and other daily activities, and whether or not these have declined recently.
- If your patient no longer participates in a particular hobby, find out why: is it as a result of a physical impairment (e.g. arthritis making cooking difficult), or as the result of a loss of interest/ability to complete tasks (e.g. no longer able to complete crosswords/puzzles).
- Once you have a good idea of the memory decline itself, begin to ask about other features. Including a social and general medical history.
Question 6.
- Review their social history and current set-up, and also subjective assessments from both patient and family over whether or not the current arrangements are safe and sustainable as they are.
- Previous and ongoing alcohol intake
- Smoking history
- Still driving (and if so, how safe that is considered to be from collateral history)
- Who else is at home
- Any package of care
- Upstairs/downstairs living
- Meal arrangements (and whether weight is being sustained).
- Of all these issues, that of driving is perhaps one of the most important, as any ultimate diagnosis of dementia must be informed (by law) to both the DVLA and also the patient’s insurers. If you feel they are still safe to drive despite the diagnosis, you may be asked to provide a report to the DVLA to support this viewpoint.
Now perform a more generalised history, to include past medical history and – more importantly – a drug history.
Question 7.
- Oxybutynin, commonly used in primary care for overactive bladder (anticholinergic side effects)
- Also see how the medications are given (e.g. Dossett box)
- Are lots of full packets found around the house?
Part 2: The History
On taking a history you have found:
- Mavis was able to give a moderately detailed biographical history, but struggled with details extending as far back as the location of her wedding, and also her main jobs throughout her life.
- After prompting from her family, she was able to supply more information, but it was not always entirely accurate.
- Her main hobby was knitting, and it was noted that she had been able to successfully knit a bobble hat for her great-grand child as recently as last month, although it had taken her considerably longer to complete than it might have done a few years previously, and it was a comparatively basic design compared to what she has been able to create previously.
- She has a few children living in the area, who would frequently pop in with shopping, but there had been times when they arrived to find that she was packed and in her coat, stating that she was “just getting ready to go home again”.
- She had been helping occasionally with the school run, but then a couple of weekends ago she had called up one of her sons – just before she was due to drive over for Sunday lunch – and said that she could not remember how to drive to his house.
- Ever since then, they had confiscated her keys to make sure she couldn’t drive. Although she liked to read the paper every day, she could not recall any recent major news events. Before proceeding to examine her, you note that the GP referral letter has stated that her dementia screen investigations have been completed.
Question 8.
- Raised WCC suggests infection as a cause of acute confusion
- Uraemia and other electrolyte disturbances can cause a persistent confusion.
- Again, to help rule out acute infection/inflammatory conditions
- Liver failure can cause hyperammonaemia, which can cause a persistent confusion.
- Hyper- or hypothyroidism can cause confusion.
- B12 deficiency is an easily missed and reversible cause of dementia.
- This looks for space occupying lesions/hydrocephalus which may cause confusion.
- This can also help to determine the degree of any vascular component of an ultimately diagnosed dementia.
Part 3: Examination
- With the exception of age-related involutional changes on the CT head (noted to have minimal white matter changes/small vessel disease), all the dementia screen bloods are reassuring.
- You next decide to perform a physical examination of Mavis.
Question 9.
- Important physical findings that are of particular relevance to dementia, are looking for other diseases that may have an effect on cognition.
- To look for evidence of stroke – unlikely in this case given the CT head
- Gait (shuffling) and limb movements (tremor, rigidity, bradykinesia)
- Affect is also important here and may also point to underlying depression
- Pay attention to vertical gaze palsy, as in the context of Parkinsonism this may represent a Parkinson plus condition (e.g. progressive supranuclear palsy).
- It is also useful to look at observations including blood pressure (may be overmedicated and at risk of falls from syncope) and postural blood pressure (again, may indicate overmedication but is also associated with Parkinson plus syndromes e.g. MSA)
Part 4: Cognitive Testing
- On examination she is alert and well, mobilising independently around the clinic waiting room area. A neurological examination was normal throughout, and there were no other major pathologies found on a general examination.
- You now proceed to cognitive testing:
Question 10.
- Click here for details on the MOCA
- Click here for details on the MMSE
- Click here for details on the CLOX test
Part 5: Diagnosis
- Mavis scores 14/30 on a MOCA, losing marks throughout multiple domains of cognition.
Question 11.
- Given the progressive nature of symptoms described by the family, the impairment over multiple domains on cognitive testing, and the impact on daily living that this is starting to have (e.g. packing and getting ready to leave her own home, mistakenly believing she is somewhere else), coupled with the results from her dementia screen, this is most likely an Alzheimer’s type dementia .
Question 12.
- You should proceed by establishing whether or not Mavis would like to be given a formal diagnosis, and if so, explain the above.
- You should review her lying and standing BP and ECG, and – if these give no contraindications – suggest a trial of treatment with an acetylcholinesterase inhibitor, such as donepezil.
- It is important to note the potential side effects – the most distressing of which are related to issues of incontinence.
- If available, put her in touch with support groups
- Given the history of forgetting routes before even getting into the care, advise the patient that she should stop driving and that they need to inform the DVLA of this (for now, we will skip over the depravation of liberty issues that the premature confiscation of keys performed by the family has caused…)
- The GP should be informed of the new diagnosis, and if there are concerns over safety, review by social services for potential support should be arranged.
- Follow-up is advisable over the next few months to see whether the trial of treatment has been beneficial, and whether side effects have been well-tolerated.
Now click here to learn more about dementia
Perfect revision for medical students, finals, osces and mrcp paces, …or click here to learn about the diagnosis and management of delirium.

How Black Mats Can Keep Dementia Patients Safe and Happy
D ementia is a condition that affects millions of people around the world, causing changes in their memory, thinking and behavior. One of the challenges that people with dementia and their caregivers face is wandering, which can lead to accidents, injuries or even death.
Wandering is often triggered by confusion, boredom, anxiety or curiosity. People with dementia may wander because they are looking for something familiar, trying to escape from a stressful situation, or following a habit or routine. Sometimes, they may not even realize that they are wandering.
One of the ways to prevent wandering is to use black mats in front of unsafe areas, such as outside exits. This technique is based on the observation that people with dementia may have visual perception problems, meaning that they may see things differently than they really are.
For example, they may see a dark space on the floor as a hole, and avoid stepping on it due to a fear of falling. This can create a natural barrier that discourages them from leaving the safe zone. This technique is considered an alternative to antipsychotic drugs and lockdown units, which may have negative effects on the person’s dignity, health and well-being.
However, this technique may also raise ethical concerns, as it uses fear and deception to control the person’s behavior. Some people may argue that this is disrespectful and manipulative, and that it may not work for everyone or in every situation.
Chuck Klosterman, a columnist for The New York Times Magazine, addressed this dilemma in his The Ethicist column. He wrote: “When dealing with ethical dilemmas involving those who have lost the ability to reasonably lobby on their own behalf, one must consider what they would most likely prefer if they were still in a position to do so.”
He added: “If a dementia patient were in a position to describe how she would want to be treated, I’d assume she would want the maximum level of independence, the highest degree of protection and the greatest potential for mental clarity. This concept comes closest to achieving those goals.”
The Alzheimer’s Society, a leading charity for people with dementia and their carers, also offers some advice on how to support a person experiencing changes in perception. They suggest: “By responding in a supportive way, you can keep up their confidence and help them to cope with the misunderstanding.”
They also recommend some ways to make the home environment safer and more comfortable for people with dementia, such as using bright colors, clear labels, familiar pictures and avoiding patterns or clutter.
The use of black mats is not the only solution for wandering, but it may be a helpful option for some people with dementia and their caregivers. As Psychiatric Times noted: “The third, a verbal interpretation of the visual deception created by a black mat, is a threat rather than a distraction. Demented patients’ avoidance of dark mats, tiles, or even lines on the floor has long been observed-and sometimes exploited clinically-although we could not locate any studies of the effectiveness and safety of this maneuver.”
Relevant articles:
– How to safeguard against visual pitfalls – Alzheimer’s and Dementia, Alzlive.com, December 12, 2012
– How can dementia change a person’s perception?, Alzheimer’s Society, no date
– A Dark Side of Dementia Care, Psychiatric Times, April 15, 2014
![Dementia is a condition that affects millions of people around the world, causing changes in their memory, thinking and behavior. One of the challenges that people with dementia and their caregivers face is wandering, which can lead to accidents, injuries or even death. Wandering is often triggered by confusion, boredom, anxiety or curiosity. People with […] Dementia is a condition that affects millions of people around the world, causing changes in their memory, thinking and behavior. One of the challenges that people with dementia and their caregivers face is wandering, which can lead to accidents, injuries or even death. Wandering is often triggered by confusion, boredom, anxiety or curiosity. People with […]](https://img-s-msn-com.akamaized.net/tenant/amp/entityid/AA1jvbgL.img?w=768&h=490&m=6&x=762&y=249&s=396&d=396)
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Psychiatry Investig
- v.14(4); 2017 Jul
A Case Report of a 37-Year-Old Alzheimer's Disease Patient with Prominent Striatum Amyloid Retention
Yoo hyun um.
1 Department of Psychiatry, Yeouido St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Republic of Korea.
Woo Hee Choi
2 Department of Radiology, Division of Nuclear Medicine, St. Vincent's Hospital, College of Medicine, The Catholic University of Korea, Suwon, Republic of Korea.
Won Sang Jung
3 Department of Radiology, St. Vincent's Hospital, College of Medicine, The Catholic University of Korea, Suwon, Republic of Korea.
Young Ha Park
Chang-uk lee.
4 Department of Psychiatry, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Republic of Korea.
Hyun Kook Lim
With recent advancement in amyloid imaging, diagnostic application of this new modality has become a great interest among researchers. New ligands, such as 18F- florbetaben, florbetapir and flutemetamol, have been discovered to overcome limitations of preexisting ligand Pittsburgh compound B. We report here a case of a 37-year-old male patient whose initial complaints comprised of gradual cognitive decline, apraxia, disorientation and sleep disturbances. 18F-Florbetaben amyloid imaging of the patient showed diffuse amyloid retention with prominent striatal uptake. This finding supports the clinical utility of amyloid imaging in diagnostic process of early-onset AD. Moreover, striatal dominant uptake pattern demonstrated in this patient include some meaningful clinical implications that warrant special attention among clinicians.
INTRODUCTION
Amyloid deposition has long been considered one of the pathognomonic markers of Alzheimer's disease (AD). Moreover, disruption in amyloid hypothesis has been frequently discussed as important targets of intervention for many years. 1 To date, most validated research results have been narrowed down to yield a model for biological trajectory of AD, where amyloid deposition far precedes clinical symptoms. 2 Thus, early detection of amyloid deposition has emerged a major target of intervention in AD patients. In this regard, amyloid imaging has emerged as an effective diagnostic tool that could enable early intervention in patients in AD trajectory, and the clinical utility of amyloid imaging has become a main topic of interest among researchers over the recent years. 3 If validated further, clinical usage of amyloid imaging is expected to extend beyond confirming AD pathology in patients with high risk factors, helping to differentiate AD from various types of dementia in those who present with atypical course or symptoms. 4
Pittsburgh compound B has been the first ligand used to detect amyloid deposition in AD patients. 5 However, its short half-life and resultant limitations in applying it to clinical setting have resulted in the development of new ligands for detecting amyloid deposition, such as 18F-florbetaben, florbetapir and flutemetamol. 6 Amyloid deposition usually initiates from temporal and orbitofrontal cortices, which later extends to frontal, parietal, precuneus, anterior and posterior cingulate cortices. 7 However, differential uptake patterns in autosomal dominant gene carriers have been noted that warrant special clinical attention. While typical amyloid deposition occurs from cortical structures, those with autosomal dominant gene carriers demonstrated initial amyloid deposition in striatum. 8 , 9
We report here a case where a case of early-onset AD patient who received a confirmatory diagnosis of AD by beta-amyloid imaging. There is relatively few evidence on the clinical application of beta-amyloid imaging in early-onset AD patients, and therefore, we expect our case can contribute to this line of inquiry. Moreover, validity of utilizing beta-amyloid imaging in differential diagnosis of dementias will be discussed.
A 37-year old male patient visited outpatient clinic, with complaints of gradual cognitive decline which had started 3 years earlier. Working as an industrial researcher, he started to make serious calculation mistakes that made him quit the job and began working as a manager in a company. However, his frequent forgetfulness, along with aggravation in recent memory impairments hampered him from fulfilling his duties, making him change jobs frequently. Apraxia and apathy had started 2 years before his visit to our clinic, and disorientation to time and person was worsened to a degree which it became impossible to commute daily between his workplace and home. At time of his visit to our clinic, not only he was fired from his recent job, but also he needed frequent reminder from his family to maintain hygiene. His sleep disturbance became prominent, frequently waking up middle of the night self-talking.
Before his visit to our clinic, he had visited two hospitals for evaluation and management of his symptoms, but to no avail. For a thorough examination of his symptoms, he was immediately admitted to our psychiatric ward. His laboratory findings did not reveal any abnormalities, and his tests for human immunodeficiency virus, syphilis all turned out to be negative. Upon his psychiatric admission, a neuropsychological test battery was implemented to evaluate the patient's cognitive status. He scored 22 in Mini-mental status examination, 1 in Clinical dementia rating scale (CDR), 10 and 4.5 in Clinical Dementia Rating-Sum of Box score(CDR-SB). 11 In his cognitive tests, in contrast to his relatively preserved language function, he displayed serious impairments in free recall, 20-minute delayed recall and recognition.
Brain magnetic resonance imaging demonstrated global cerebral atrophy of grade 1 by cortical atrophy scale 12 and notable medial temporal lobe atrophy of grade 2 by medial temporal lobe atrophy visual rating scale ( Figure 1A and B ). 13 Atypically early onset of dementia symptoms made the patient an eligible candidate for amyloid positron emission tomography (PET) imaging. 14 18-Florbetaben PET images revealed diffuse amyloid deposition with score 3 in brain beta-amyloid plaque load (BAPL), 15 with predominant amyloid deposition in the striatum ( Figure 1C and D ).

The patient's history, along with neuroimaging results and cognitive test results all satisfied the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association Alzheimer's (NINCDS-ADRDA) criteria16 for probable Alzheimer's disease with high level of evidence. 5 mg of donepezil was prescribed, and the patient was discharged on the 10th day of his admission. To control his persistent cognitive decline even after the discharge, donepezil was increased up to 23 mg with combination of memantine, which was also increased up to 20 mg. His cognitive decline has been relatively plateaued, but we advised the patient and his caregiver to regularly visit the clinic for monitoring of his symptoms.
This is one of the few case reports that demonstrated diagnosis of early-onset AD by 18F-florbetaben PET imaging. The patient demonstrated early onset of cognitive decline with accelerated deterioration. The fact that he meandered along various departments at different hospitals for confirmatory diagnosis reflect major role amyloid imaging played in the diagnostic process of the patient.
Amyloid imaging is usually indicated in patients with progressive MCI with dubious etiology, patients with atypical presentations and clinical course, and patients with early-onset progressive dementias. 14 Considering the patient in the case exhibited dementia symptoms at atypically early age, amyloid imaging was appropriately prescribed to diagnose the etiology of his cognitive decline. Integration of information attained from his history, clinical data indicated his diagnosis to be early-onset AD.
There have been relatively few reports utilizing 18F-labelled amyloid beta PET tracers that include clinical implications related to autosomal dominant AD. One study adopted 18F-florbetaben PET imaging in Down syndrome patients, suggesting potential role of amyloid imaging in identifying population at risk of dementia. 17 Similar study was conducted on patients with Down syndrome, but with 18F-florbetapir tracer. 18 An attempt to differentiate Down Syndrome pathology from AD has also been made with 18F-florbetapir tracer. 19 Future studies on autosomal dominant AD with 18F-labelled amyloid beta PET tracers could increase validity of adopting these new ligands in the diagnostic process.
Most notable test results in the case report arise from uptake patterns of 18F-florbetaben PET imaging. Unlike typical uptake patterns demonstrated by late-onset AD patients, where striatum is usually involved in the later course of illness, there was a dominant striatal uptake pattern in the patient. A previous study conducted on nondemented young adults with Down syndrome compared their results with that of studies conducted on autosomal dominant early-onset AD patients, where two groups of subjects concordantly showed predominant striatal uptake. 8 Indeed, previous studies on autosomal early-onset AD patients consistently showed high striatal amyloid deposition. 20 , 21 The aforementioned finding could explain 18F-florbetaben uptake patterns in the case.
The underlying mechanisms have been discussed in prior studies on the relatively early involvement of the striatum in autosomal dominant early-onset AD patients. Axonal mistrafficking induced by presenillin-1 gene mutation has been suggested as a potential culprit for striatal amyloid deposition in one animal study. 22 Such axonal mistrafficking is considered to stem from disruption in APP processing. 22 Indeed, APP processing patterns differed between autosomal dominant AD patients and sporadic AD patients. 23 Striatal vulnerability to early stages of tau protein accumulation in autosomal dominant AD has also been elucidated, and such phenomenon is considered more toxic to induce significant striatal neuronal injury. 24
The most prominent limitation of our case report is lack of genotype testing in the patient. If the genetic testing had been done, one missing puzzle in the diagnosis of patient would have been complete. Nevertheless, we believe our case report affirms diagnostic usefulness and clinical application of amyloid imaging in the differential diagnosis of early-onset dementia. We expect more prevalent use of amyloid imaging with accumulation of evidences and validation studies over time.
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (NRF-2015R1C1A1A02036578).

Create Free Account or
- Acute Coronary Syndromes
- Anticoagulation Management
- Arrhythmias and Clinical EP
- Cardiac Surgery
- Cardio-Oncology
- Cardiovascular Care Team
- Congenital Heart Disease and Pediatric Cardiology
- COVID-19 Hub
- Diabetes and Cardiometabolic Disease
- Dyslipidemia
- Geriatric Cardiology
- Heart Failure and Cardiomyopathies
- Invasive Cardiovascular Angiography and Intervention
- Noninvasive Imaging
- Pericardial Disease
- Pulmonary Hypertension and Venous Thromboembolism
- Sports and Exercise Cardiology
- Stable Ischemic Heart Disease
- Valvular Heart Disease
- Vascular Medicine
- Clinical Updates & Discoveries
- Advocacy & Policy
- Perspectives & Analysis
- Meeting Coverage
- ACC Member Publications
- ACC Podcasts
- View All Cardiology Updates
- Earn Credit
- View the Education Catalog
- ACC Anywhere: The Cardiology Video Library
- CardioSource Plus for Institutions and Practices
- ECG Drill and Practice
- Heart Songs
- Nuclear Cardiology
- Online Courses
- Collaborative Maintenance Pathway (CMP)
- Understanding MOC
- Image and Slide Gallery
- Annual Scientific Session and Related Events
- Chapter Meetings
- Live Meetings
- Live Meetings - International
- Webinars - Live
- Webinars - OnDemand
- Certificates and Certifications
- ACC Accreditation Services
- ACC Quality Improvement for Institutions Program
- CardioSmart
- National Cardiovascular Data Registry (NCDR)
- Advocacy at the ACC
- Cardiology as a Career Path
- Cardiology Careers
- Cardiovascular Buyers Guide
- Clinical Solutions
- Clinician Well-Being Portal
- Diversity and Inclusion
- Infographics
- Innovation Program
- Mobile and Web Apps
Risk of Dementia During Antihypertensive Drug Therapy
Quick Takes
- Exposure (or use) to antihypertensive medications was inversely associated with risk for dementia or Alzheimer’s disease.
- Among patients with the highest exposure (antihypertensive drugs available for >75% of the follow-up time), a 24% risk reduction of dementia or Alzheimer’s disease was observed compared to patients at the lowest exposure (antihypertensive drugs available for ≤25% of the follow-up time).
- This lower risk for dementia was observed across all age groups, including the very old. The association between antihypertensive exposure and reduced risk of dementia/Alzheimer’s disease was observed regardless of the patient’s sex and baseline clinical status.
Study Questions:
What is the effect of antihypertensive drug treatment on the risk of dementia or Alzheimer’s disease among new users of antihypertensive medications?
The investigators used a nested case-control study design with a cohort of 215,547 patients residing in Lombardy, Italy, who were aged ≥65 years and had started antihypertensive medications within the time frame of 2009–2012. Patients were identified through the health care databases of Lombardy, which account for 16% of Italy’s population (10 million individuals, predominantly of White race). Cases were defined as patients who developed dementia or Alzheimer’s disease during follow-up, with a date of onset defined as the date corresponding to the first event among: 1) hospitalization with a diagnosis of dementia or Alzheimer’s disease, 2) prescription of anti-dementia drugs (galantamine, rivastigmine, donepezil, and memantine), and 3) exemption from the copayment for drugs and other health services for dementia or Alzheimer’s disease.
For each case patient, five controls were identified from the cohort as individuals with the same sex, age at cohort entry (±3 years), clinical status, and index date (±30 days) of the corresponding case patient. Adherence to treatment, and thus exposure to antihypertensive drugs, was assessed by the ratio between the number of days in which the antihypertensive drug therapy was available and the days of follow-up, a measure defined as the “proportion of days covered” (PDC) by prescriptions. Four categories of adherence or exposure to antihypertensive drugs were considered: very low (PDC ≤25%), low (26%–50%), intermediate (51%–75%), and high (>75%).
A total of 215,547 patients ≥65 years of age (mean [standard deviation] age, 77.5 [6.6] years; 40% men) were included in the present analysis, of which 13,812 developed dementia or Alzheimer’s during the follow-up period (average, 7.3 years). About 80% of patients started treatment with one drug and the most frequent monotherapy was a renin-angiotensin system blocker, while the most frequent two-drug combination was that between a blocker of the renin-angiotensin system and a diuretic. Compared to patients with very low exposure, those with low, intermediate, and high exposure exhibited a risk reduction of 2% (95% confidence interval [CI], –4 to 7%), 12% (95% CI, 6–17%), and 24% (95% CI, 19–28%), respectively.
Antihypertensive drugs were associated with an inverse risk for dementia or Alzheimer’s across all age groups including the very old (≥85 years); however, the reduction in the risk of dementia was less pronounced in patients aged ≥85 years than in those aged 65-74 years. Among frail patients, the use of antihypertensive medications was also associated with a reduced risk.
Conclusions:
The authors conclude that in this cohort of older adults, antihypertensive medication treatment was associated with a lower risk of dementia. This inverse association was observed across all age groups and in frail patients.
Perspective:
Although not a randomized control design, this case-control study using a large cohort supports the use of blood pressure–lowering medications, presumably because associated lower blood pressure can lower the risk for dementia. Strengths of this study are that the population includes patients ≥85 years of age and those with frailty, as both groups appear to have had a lower risk for dementia among antihypertensive medication users. Examining blood pressure levels would have been interesting, and additional studies are warranted to further examine such measures and to examine these findings among diverse populations.
Clinical Topics: Sleep Apnea, Geriatric Cardiology, Prevention
Keywords: Antihypertensive Agents, Dementia
You must be logged in to save to your library.
Jacc journals on acc.org.
- JACC: Advances
- JACC: Basic to Translational Science
- JACC: CardioOncology
- JACC: Cardiovascular Imaging
- JACC: Cardiovascular Interventions
- JACC: Case Reports
- JACC: Clinical Electrophysiology
- JACC: Heart Failure
- Current Members
- Campaign for the Future
- Become a Member
- Renew Your Membership
- Member Benefits and Resources
- Member Sections
- ACC Member Directory
- ACC Innovation Program
- Our Strategic Direction
- Our History
- Our Bylaws and Code of Ethics
- Leadership and Governance
- Annual Report
- Industry Relations
- Support the ACC
- Jobs at the ACC
- Press Releases
- Social Media
- Book Our Conference Center
Clinical Topics
- Chronic Angina
- Congenital Heart Disease and Pediatric Cardiology
- Diabetes and Cardiometabolic Disease
- Hypertriglyceridemia
- Invasive Cardiovascular Angiography and Intervention
- Pulmonary Hypertension and Venous Thromboembolism
Latest in Cardiology
Education and meetings.
- Online Learning Catalog
- Products and Resources
- Annual Scientific Session
Tools and Practice Support
- Quality Improvement for Institutions
- Accreditation Services
- Practice Solutions
Heart House
- 2400 N St. NW
- Washington , DC 20037
- Email: [email protected]
- Phone: 1-202-375-6000
- Toll Free: 1-800-253-4636
- Fax: 1-202-375-6842
- Media Center
- ACC.org Quick Start Guide
- Advertising & Sponsorship Policy
- Clinical Content Disclaimer
- Editorial Board
- Privacy Policy
- Registered User Agreement
- Terms of Service
- Cookie Policy
© 2024 American College of Cardiology Foundation. All rights reserved.

COMMENTS
This approach focuses on the quality of life of the people supported and is based on four pillars: 1. Person-centered care and symptom control, 2. Interdisciplinary teamwork, 3. Family support, 4 ...
Early onset Alzheimer disease (EOAD) is a neurodegenerative dementing disorder that is relatively rare (<1% of all Alzheimer cases). Various genetic mutations of the presenilin 1 (PSEN1) and presenilin 2 (PSEN2) as well as the amyloid precursor protein (APP) gene have been implicated.Mutations of PSEN1 and PSEN2 alter γ-secretase enzyme that cleaves APP resulting in increase in the relative ...
Conducting in-person needs assessments of individuals living with Alzheimer's and their caregivers. Creating and implementing individualized dementia care plans. Monitoring and revising care plans, as needed. Providing access 24/7, 365 days a year for assistance and advice to help avoid Emergency Department (ED) visits and hospitalizations.
The US Reducing Disability in Dementia study 209 implemented an at-home multicomponent intervention including exercise education, training to increase pleasant events, and activator-behaviour-consequence problem-solving approach over 6 weeks by case managers in 255 community dwelling people with dementia older than 60 years and their family ...
The below case studies are taken from the document ' Good care planning guide for dementia '. Core elements of a care plan. An example person centred care plan. A care plan: system compatibility and configuration. An example process for dementia annual review template. Example QOF annual review templates. A framework for annual review.
The following is an excerpt from the "Planning for your hospital" section of the Dementia Friendly Hospital Toolkit developed by CARE and clinical and research faculty at the University of Wisconsin-Madison School of Nursing.. As we developed CARE's Dementia Friendly Hospital Toolkit, we learned from two Wisconsin hospitals with their own dementia friendly initiatives: the W.S ...
These three case studies help you to consider different situations that people with dementia face. They are: Raj, a 52 year old with a job and family, who has early onset dementia. Bob and Edith, an older married couple who both have dementia and are struggling to cope, along with their family. Joan, an older woman, who lives alone and has just ...
Integrated dementia care: A case study. July 2012; DOI:10.1016/j ... Join ResearchGate to discover and stay up-to-date with the latest research from leading experts in Dementia Care and many other ...
Steve is 88 years old and living with vascular dementia. He lives with his wife Anne who was his primary carer. Steve's dementia meant that he was highly anxious, easily agitated and dependent on Anne. He would become paranoid and aggressive if she left his sight. Steve had enjoyed a successful career in business, in the military and as a ...
One less-well-known type of dementia is frontotemporal dementia, which covers a spectrum of conditions that particularly affect behavior and language (Neary et al., 1998).A recent report published by the World Health Organization used figures from the Islington study (Stevens et al., 2002) and gave the rate of frontotemporal dementia within dementia in general as 3% of all cases (Dua et al ...
This paper reports a collective case study of the three social care case studies, which were all undertaken in care home settings. Aims The case studies aimed to understand the features and contextual factors associated with good practice regarding the design, delivery and implementation of dementia education and training and its impact on care ...
Keywords: systematic mixed studies review, dementia, case management, primary health care, implementation, ... improve the quality of dementia care, and develop cost-effective and efficient ways to coordinate services. 8 The Case Management Society of America defines CM as "a collaborative process of assessment, planning, ...
Teaching Resourcefulness to Women Caregivers of Elders with Dementia. Principle Investigator: Jaclene Zauzsniewski. Co-Investigators: Diana Morris, Amy Zhang. The goal of this R21 Exploratory Research Grant was to pilot test an adapted intervention that teaches personal and social resourcefulness skills to women caregivers of elders with dementia.
Summary. This chapter talks about an 85-year-old man who was enrolled in a longitudinal study of healthy aging and Alzheimer disease (AD) at the Washington University Alzheimer Disease Research Center (ADRC). He was diagnosed with dementia of the Alzheimer's type (DAT) and died shortly after his 91st birthday.
This article reports a study examining the creation of content for Portrait. Earlier work has reported on usability testing and feedback from care home managers. 9,10 Here we report case studies conducted with families of people with dementia who live in care home environments, by examining the processes involved in populating Portrait.
Case 2 - Care Planning and Decision-Making through the Stages of Dementia. pp 6-9. By Paige Moorehouse. Get access. Export citation. Case 3 - A Young Man with Memory and Walking Difficulties. pp 10-13.
Andy's story - dementia case study Expert care for a declining condition. Andy's mother was living with dementia and her condition suddenly started to worsen. The daycare she was receiving couldn't stretch to overnight support, and Andy and his siblings all lived an hour or more away.
The number of older people, including those living with dementia, is rising, as younger age mortality declines. However, the age-specific incidence of dementia has fallen in many countries, probably because of improvements in education, nutrition, health care, and lifestyle changes. Overall, a growing body of evidence supports the nine potentially modifiable risk factors for dementia modelled ...
Summary. An 85-year-old woman with hypertension and hyperlipidemia presented with gradual and progressive cognitive impairment for more than 2 years, involving cognitive domains of memory, executive function, visuospatial and mood. She has short-term memory loss such as forgetting whether she has eaten or showered.
Such services can easily reduce the burden of caregivers during the day time as stated in Table 1. The day-care centers also empower the caregivers on how to look after the person at home. After admitting the person to the day-care center, the primary caregiver could continue her studies. As mentioned in Table 1, the caregiver gained more ...
Access to local dementia care services and support groups is essential. These resources provide a network of assistance, information, and emotional support for both patients and caregivers. Dementia case study 2. John, a 68-year-old retired engineer, had always been known for his sharp wit and problem-solving abilities.
For a similar approach see the results of a systematic review of ethical issues in dementia care that was followed-up by a content analysis of clinical practice guidelines for dementia care . The authors of this review have different scientific backgrounds: medicine/psychiatry, physiotherapy, public health, ethics and philosophy.
Families of people with dementia must either sacrifice their personal well-being and livelihoods to care for a loved one at home, hire a professional home health aide, which costs from US$30 to ...
Part 1: Mavis. Mavis is an 84-year old lady, referred to you in the memory clinic for assessment of memory impairment. She attends in the company of her son and daughter-in-law. On the pre-clinic questionnaire her son has reported a severe deterioration in all aspects of her cognition over the past 12 months.
- A Dark Side of Dementia Care, Psychiatric Times, April 15, 2014 Dementia is a condition that affects millions of people around the world, causing changes in their memory, thinking and behavior.
During an average 5.1 years of follow-up, the cumulative prevalence of MCI was 26.6%; 12.2% of patients converted from MCI to dementia, and the cumulative prevalence of dementia was 18.5%.
We conducted this study using qualitative methods (interviews, focus groups, and observations) and qualitative case study analysis to understand approaches used by Care Team Navigators to address caregiver burden among caregivers of people with dementia (Figure 1). This study took place within the Care Ecosystem program at the University of ...
One study adopted 18F-florbetaben PET imaging in Down syndrome patients, suggesting potential role of amyloid imaging in identifying population at risk of dementia. 17 Similar study was conducted on patients with Down syndrome, but with 18F-florbetapir tracer. 18 An attempt to differentiate Down Syndrome pathology from AD has also been made ...
The investigators used a nested case-control study design with a cohort of 215,547 patients residing in Lombardy, Italy, who were aged ≥65 years and had started antihypertensive medications within the time frame of 2009-2012. ... Patients were identified through the health care databases of Lombardy, which account for 16% of Italy's ...