Primary Care Management of Asthma Exacerbations or Attacks: Impact of the COVID-19 Pandemic
- Open access
- Published: 14 February 2022
- Volume 39 , pages 1457–1473, ( 2022 )

Cite this article
You have full access to this open access article
- Monica Fletcher ORCID: orcid.org/0000-0002-9700-3552 1 ,
- Thys van der Molen 2 ,
- Warren Lenney 3 ,
- Isabelle Boucot 4 ,
- Bhumika Aggarwal 5 &
- Emilio Pizzichini 4
5034 Accesses
8 Citations
1 Altmetric
Explore all metrics
The COVID-19 pandemic has brought a renewed focus on appropriate management of chronic respiratory conditions with a heightened awareness of respiratory symptoms and the requirement for differential diagnosis between an asthma attack and COVID-19 infection. Despite early concerns in the pandemic, most studies suggest that well-managed asthma is not a risk factor for more severe COVID-related outcomes, and that asthma may even have a protective effect. Advice on the treatment of asthma and asthma attacks has remained unchanged. This article describes some challenges faced in primary care asthma management in adults and in teenagers, particularly their relevance during a pandemic, and provides practical advice on asthma attack recognition, classification, treatment and continuity of care. Acute attacks, characterised by increased symptoms and reduced lung function, are often referred to as exacerbations of asthma by doctors and nurses but are usually described by patients as asthma attacks. They carry a significant and underestimated morbidity and mortality burden. Many patients experiencing an asthma attack are assessed in primary care for treatment and continuing management. This may require remote assessment by telephone and home monitoring devices, where available, during a pandemic. Differentiation between an asthma attack and a COVID-19 infection requires a structured clinical assessment, taking account of previous medical and family history. Early separation into mild, moderate, severe or life-threatening attacks is helpful for continuing good management. Most attacks can be managed in primary care but when severe or unresponsive to initial treatment, the patient should be appropriately managed until transfer to an acute care facility can be arranged. Good quality care is important to prevent further attacks and must include a follow-up appointment in primary care, proactive regular dosing with daily controller therapy and an understanding of a patient’s beliefs and perceptions about asthma to maximise future self-management.
Avoid common mistakes on your manuscript.
Introduction
At the outset of the COVID-19 pandemic, there were concerns about its impact on patients with asthma and other chronic respiratory conditions [ 1 ], both in terms of its effect in triggering acute exacerbations or attacks, and as a risk factor for more severe disease and death [ 2 ]. As the pandemic continued, several studies showed a reduction in asthma attacks reported in primary care and in emergency departments [ 3 , 4 , 5 , 6 , 7 ], possibly related to social distancing, the wearing of face masks, less air pollution, and improved self-management [ 5 , 8 , 9 ], rather than pandemic-related healthcare avoidance [ 7 ]. In addition, reports from many countries have suggested that well-managed asthma is not a risk factor for more severe outcomes [ 2 , 10 ], and that asthma may offer some protection against the detrimental effects of COVID-19 infection [ 2 , 11 ]. Throughout the pandemic, asthma management guidelines have reinforced the importance of continuing treatment to maintain asthma control and reduce the risk of future attacks [ 12 , 13 , 14 ]. The advice on managing asthma attacks remains unchanged.
Asthma attacks are acute asthma episodes that comprise an increase in symptoms and a reduction in lung function needing increased reliever medication use, and perhaps a change in controller treatment [ 15 ]. They are also referred to as asthma exacerbations or flare-ups; however, a 2018 Lancet commission called for the term asthma attack to replace these terms in recognition of their importance as markers of a high risk of future attacks and even death, rather than mild episodes of inconvenience [ 16 ]. The term attack is also preferred by patients as it describes how they feel and is better understood by their friends and family [ 17 ]. For these reasons, we use the term attack in this article.
Asthma attacks are a major health burden to patients and a financial and staff burden to public health services [ 15 ]. They usually occur in those with an existing diagnosis of asthma but may be the first signs a patient seeks healthcare support [ 15 ]. Although greater asthma severity is associated with more attacks [ 18 ], all with asthma, irrespective of its severity, are at risk [ 18 , 19 , 20 , 21 ]. An asthma attack is a significant predictor of future events [ 18 , 22 , 23 , 24 ]. The severe attack rate (i.e. those requiring treatment with oral corticosteroids (OCS), an emergency department visit or hospitalisation) has been reported in epidemiological surveys as 0.1–0.2 per patient per year, with most treated with OCS only [ 18 , 25 ]. Although it is uncommon that patients with asthma have asthma-related hospitalisations or die (less than 1%) [ 26 ], prompt diagnosis of an attack with determination of its severity is important, as delay can be fatal [ 27 ].
In most countries, primary care is the first place of contact [ 28 , 29 ]. Primary care physicians (PCPs) deal with many other asthma challenges, including access to asthma medicines, spacer device availability, patient adherence to treatment regimens, implementation of a written asthma action plan, difficult access to care in rural areas and the demand of patients’ everyday work and life [ 29 , 30 , 31 , 32 ]. Many asthma-related deaths can be prevented by better education, use of asthma action plans, more accurate and timely diagnosis, rapid referral to a respiratory specialist when needed, better follow-up, and encouragement to continue taking regular preventative medicines [ 33 , 34 ]. PCPs have faced additional challenges in the COVID-19 pandemic, not only in the differential diagnosis between asthma attacks and COVID-19 infections but also in assessing the severity of an attack and asthma control via remote consultation to ensure the appropriate acute treatment and future care [ 35 ].
This article describes the challenges faced in primary care in managing asthma attacks in adults and teenagers, their relevance during a pandemic and offers practical advice about attack recognition, classification, treatment and continuing care (Fig. 1 ).
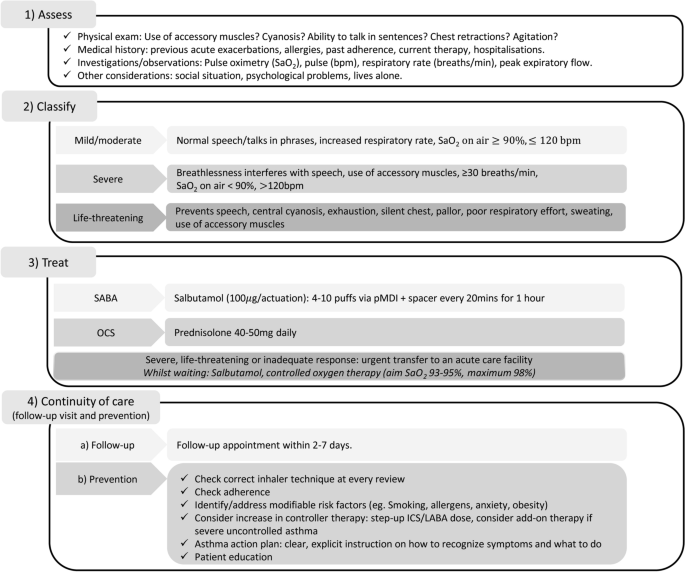
Overview of the management of acute attacks of asthma in primary care. bpm beats per minute, ICS inhaled corticosteroid, LABA long-acting β 2 -agonist, OCS oral corticosteroid, pMDI pressurised metered-dose inhaler, SaO 2 saturated oxygen, SABA short-acting β 2 -agonist
This article is based on previously conducted studies and does not contain new clinical studies involving human participants or animal studies by any of the authors.
What are the Key Indicators for Recognising an Asthma Attack and What is the Difference Between an Asthma Attack and a COVID-19 Infection?
Acute asthma attacks are characterised by a progressive increase in symptoms of breathlessness, cough, wheezing and/or chest tightness, plus a fall in lung function [ 15 ]. For differentiation between an attack and a COVID-19 infection, a structured clinical assessment is recommended, involving a detailed history and review of the patient’s clinical records including review of any investigations such as recent peak expiratory flow rate (PEFR), spirometry or blood eosinophil findings [ 35 , 36 ]. Whilst both may present with cough and breathlessness, patients experiencing an attack usually present with wheeze, a reduced PEFR and demonstrate symptoms which improve following use of a reliever inhaler [ 12 , 13 , 35 ]. A personal or family history of asthma is another criterion for a higher probability of asthma [ 36 ]. COVID-19 infection is more likely to be signalled by a high body temperature, a dry hacking continual cough, flu-like symptoms (fatigue, headache), loss of taste/smell, and symptoms unresponsive to the use of reliever treatment [ 12 , 13 , 35 ]. In many, differential diagnosis may be possible without PEFR and spirometry, which is preferable because of the potential transmission of viral infections [ 36 ]. As new variants of COVID-19 emerge, differences may become less distinct.
It is helpful to bear in mind that the most common trigger of an asthma attack is a viral respiratory infection or allergen exposure, both of which are subject to seasonal variation [ 15 , 37 , 38 ]. Pollutants (tobacco smoke, outdoor and indoor air pollutants) may also trigger an attack. Attacks are heterogeneous in their time course and severity, making them challenging to recognise and treat in primary care [ 39 ]. Several studies have shown variations in reliever use before and during the time course of an asthma attack [ 40 , 41 , 42 ], and such patterns can serve as a helpful indicator of an impending attack and its subsequent control.
What Are the Clinical Signs and Symptoms for Classifying the Severity of an Asthma Attack During a Pandemic?
Appropriate management depends on the asthma attack severity so it is important to immediately assess how severe it really is (Table 1 ) [ 15 , 27 , 43 , 44 ] and where best to manage it (Fig. 2 ).
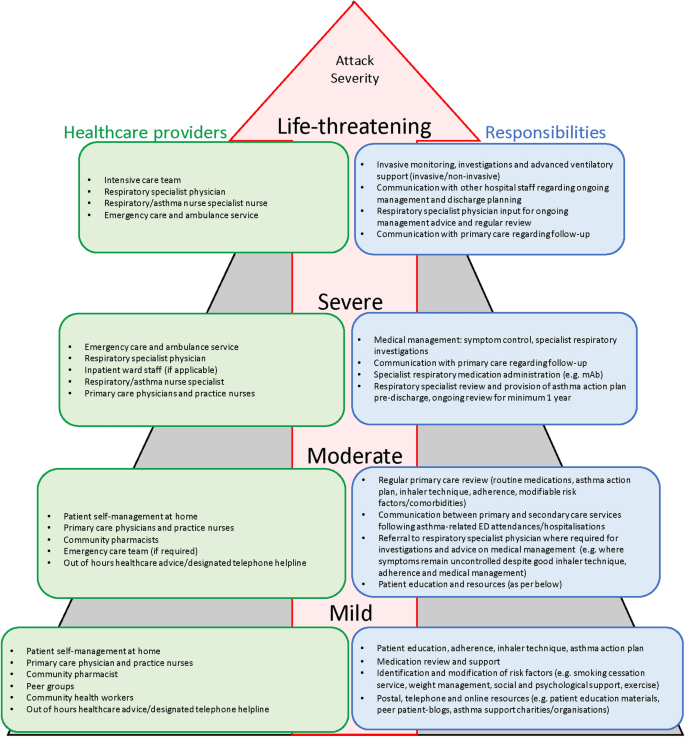
Stepwise healthcare provider model for acute attacks [ 15 , 27 , 94 ]. ED emergency department, mAb monoclonal antibody
Under conditions of normal clinical practice, a full clinical history and a careful physical examination are required to assess heart rate, the use of accessory muscles, any chest wall retraction, wheezing, level of breathlessness, respiratory rate, cyanosis of lips and tongue, any agitation or reduced consciousness. Also measurement of lung function (PEFR or forced expiratory volume in 1 s (FEV 1 )) are helpful to define an attack as mild, moderate, severe, or life-threatening (Table 1 ) [ 15 , 27 , 43 , 44 ]. Objective measures of lung function are more reliable indicators of attack severity than symptoms [ 15 , 44 ]. Pulse oximetry, if available, is also helpful to determine severity and has prognostic value [ 15 ].
During the COVID-19 pandemic the recommendations in Table 1 and Fig. 2 are suggested using a combination of patient self-assessment tools such as PEFR monitoring where available, an asthma action plan and ehealth strategies personalised for patients with asthma. This means leveraging the use of home monitoring devices assessing heart rate, breathing rate and oxygen saturation, handheld spirometers, and smart devices assessing adherence, and inhalation technique [ 35 , 36 , 45 , 46 ].
Remote clinical assessment, so often required during the COVID-19 pandemic, relies on a telephone call consultation, preferably augmented by a video link [ 35 ]. Some patients may still require face-to-face consultation. Patients’ key signs and symptoms should be determined in the context of their wider clinical history and through questioning them about their symptoms in relation to their normal health status such as their usual breathlessness and respiratory rate [ 35 , 36 , 47 ]. Try to establish any risk factors for asthma-related death (a history of near-fatal asthma, excessive use of reliever treatment (more than one cannister per month), not using or poor adherence to inhaled corticosteroid (ICS) preventative treatment, recent or current use of OCS, a recent emergency hospital visit or admission with asthma, a history of psychiatric disease or psychosocial problems, food allergy, comorbidities, including obesity and cardiovascular disease [ 15 , 27 , 44 ]). Patient medical records should also be checked for risk factors of poor outcome with COVID-19 infection. Patients who struggle to complete sentences, have a respiratory rate of 25 breaths/min or higher, a PEFR less than 50% of predicted or a heart rate of 120 bpm or higher, all suggest a severe attack, needing prompt transfer to an acute care facility (Table 1; Fig. 2 ) [ 15 , 27 , 43 , 44 ].
Self-assessment by patients to monitor their asthma using both objective and subjective measures is a pragmatic approach. The assessment of asthma control at home using asthma control questionnaires (e.g. Asthma Control Test or Asthma Control Questionnaire) provides one tool of assessment [ 35 , 36 ]. Remote monitoring through patients recording their daily PEFR readings, if available, and/or the presence of any asthma symptoms in a daily diary, may also help observe a cause-and-effect relationship between exposure to triggers and decrements in peak flow and/or exacerbations of asthma and to give early warning signs of a potential deterioration, for both PCPs and patients via their written action plan (Fig. S1, Supplementary Material). However, patients may be reluctant to continue this for a substantial length of time.
How Can Asthma Attacks Be Managed Effectively in Primary Care During a Pandemic?
The advice on managing asthma remains unchanged during the COVID-19 pandemic [ 12 , 13 , 48 ]. A shortage of asthma inhalers was observed during the pandemic; the shortage of short-acting β 2 -agonist (SABA) inhalers reinforced the importance and well-established role of SABA in the current management of asthma [ 49 ]. For most patients with asthma, the risk of inadequately treating an asthma attack is worse than the risk from COVID-19 infection [ 12 ].
Most attacks are classified as mild or moderate and can be entirely managed in primary care. Severe or life-threatening attacks usually require treatment in an acute care facility (Fig. 3 ) [ 15 ]. A comparative overview of treatment recommendations in primary care versus acute care is shown in Table S1 (Supplementary Material). There are some differences in recommendations across guidelines.
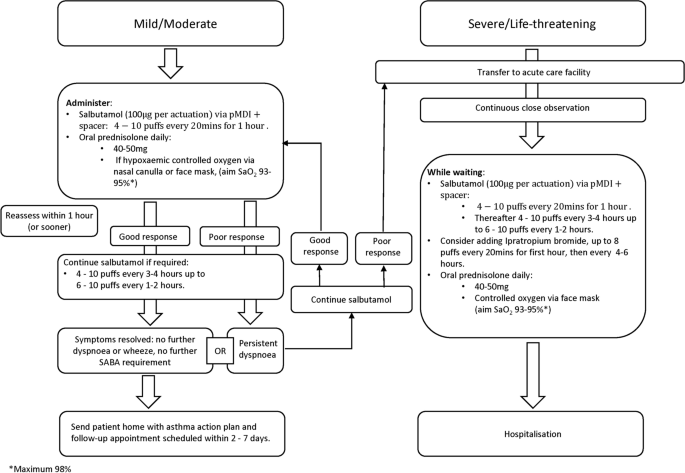
Treatment of acute attacks of asthma in adults/young people (> 12 years of age). pMDI pressurised metered-dose inhaler, SABA short-acting β 2 -agonist, SaO 2 saturated oxygen
Mild and Moderate Asthma Attacks
All patients need immediate treatment with inhaled SABA, usually salbutamol/albuterol, together with the prompt introduction of orally administered prednisolone [ 15 , 27 , 43 , 44 ]. Hypoxaemia requires supplemental oxygen. Patients should be closely observed and treatment adjusted according to response.
Inhaled SABAs have a rapid onset of action (less than 5 min), are well tolerated, and repeated administration achieves incremental bronchodilatation [ 43 ]. In adults and young people, the recommended dose is 4–10 puffs of inhaled SABA via a pressurised metered-dose inhaler (pMDI) plus a spacer (preferably a larger volume spacer, 750 mL) every 20 min for 1 h [ 15 ]. If symptoms rapidly resolve, no further SABA treatment may be needed; consider sending the patient home with appropriate controller therapy, an asthma management plan and a follow-up appointment [ 15 ]. Doubling the dose of ICS has been shown to improve asthma outcomes in self-management studies [ 50 ], although this strategy is not well supported by results of placebo-controlled trials [ 15 , 51 , 52 ]. It is hypothesised that in real life, patients may at this point be reintroducing the inhaled steroids as originally prescribed rather than actually doubling their medication—hence the incongruence.
OCS treat the underlying inflammation but take up to 4 h to show clinical benefit; therefore, they should be commenced as soon as possible (i.e. at the same time as initial SABA treatment). A dose of orally administered prednisolone 40–50 mg once daily is an accepted dose for teenagers and adults for 5–7 days [ 15 , 27 ]. To prevent an upset stomach, they should be taken with food, not on an empty stomach [ 53 , 54 ].
In the absence of pulse oximetry, if the patient appears cyanosed and/or distressed, oxygen should be given through nasal cannulae or using a face mask. Observe the patient closely for deterioration, drowsiness or fatigue [ 15 , 27 , 43 , 44 ]. If oximetry is available and oxygen saturations are below 94%, supplemental oxygen should be given to maintain saturations of 93–95% [ 15 , 43 ], with a maximum of 98% [ 27 ]. Excessive oxygenation can be harmful and may increase the risk of hypercapnia [ 15 , 27 , 43 , 55 ]. Where supplemental oxygen is not available, monitor the patient very closely, transferring as soon as possible to an acute care facility.
Reassess all patients after 1 h (sometimes sooner) [ 15 ]. If the response to initial SABA is suboptimal give further doses as needed (4–10 puffs every 3–4 h or even every 1–2 h if the clinical situation demands it) [ 15 ]. If symptoms fully resolve, consider sending the patient home with an asthma management plan, preventative and relief therapy together with a follow-up appointment date. Should symptoms fail to improve, or if the response to initial SABA administration is poor [ 56 ], start treatment as for a severe attack as described in the next section and arrange urgent transfer to an acute care setting [ 15 ]. Close observation is essential [ 44 ].
Severe or Life-Threatening Asthma Attacks
These patients need urgent transfer to an acute care facility preferably by ambulance [ 15 ]. Drowsiness, confusion, collapse, cyanosis, a silent chest, altered consciousness or oxygen saturations below 90% represent potentially life-threatening situations [ 15 , 43 ]. Whilst awaiting transfer, patients should remain under the direct supervision of a healthcare professional within the primary care facility and be continuously monitored for signs of hypoxaemia, worsening conscious levels, fatigue and/or somnolence [ 15 , 43 ]. Give salbutamol up to 10 puffs via a pMDI plus spacer every 20 min up to 1 h then up to 10 puffs every 1–2 or 3–4 h. Consider adding ipratropium bromide 8 puffs (every 20 min in the first hour and every 4–6 h subsequently) [ 15 , 43 , 44 ]. Patients with moderate to severe asthma attacks who were receiving both salbutamol and ipratropium bromide were at lower risk of needing hospital admission than those on salbutamol alone [ 57 ]. They did, however. experience more side effects. Give OCS as described in the previous section, and continue supplemental oxygen and SABA until transport arrives [ 27 ].
The aforementioned guidance is summarised in the algorithm for the treatment of acute attacks (Fig. 3 ).
Antibiotic Therapy
The use of antibiotics in the management of acute asthma is not recommended, unless there is strong evidence of a bacterial infection [ 15 , 58 ]. Most infections initiating an attack are likely to be viral in origin [ 27 ].
Device Choice for Managing Attacks
A nebuliser and pMDI plus large volume spacer are both options for SABA treatment [ 15 , 27 ]. Spacers reduce the requirement for patients to coordinate actuation and inhalation, thereby improving delivery of medicines into the airways where they are needed [ 59 ]. During acute attacks a pMDI and spacer is the preferred route of SABA delivery, and the most cost-effective [ 15 , 60 ]. While nebulisers are generally not recommended especially for inhaled steroids and in the current pandemic situation [ 12 , 13 , 14 ], they may be helpful in those who have life-threatening asthma or who are agitated, distressed or finding difficulty in using a pMDI and spacer [ 44 ], and should be administered with appropriate care [ 61 , 62 ].
Continuity of Care
The advice on patient continuity of care following an asthma attack remains unchanged [ 12 ] with requirement for a clear follow-up and review pathway in place, as described in the next section. Patients with asthma should be advised to look out for any worsening symptoms with clear advice on what to do and, with the potential for long waits for remote assessment, patients should be advised to have a low threshold to call the emergency services, in cases where symptoms deteriorate [ 35 ].
Follow-Up Appointment
A follow-up appointment in primary care, whether face to face or by remote monitoring, is an important next step for preventing future asthma attacks, both for attacks managed fully in primary care and those managed in acute care settings (Fig. 4 ) [ 15 ]. Following discharge from an acute care facility, a significant proportion of patients with asthma relapse within 4 weeks [ 63 ], highlighting the importance of an early follow-up in primary care to ensure that adequate, regular preventative therapy has been prescribed and is being taken. Depending on the asthma severity and the patient’s social circumstances, all patients should have a primary care follow-up appointment soon after an attack, preferably within 2–7 days but certainly within 2 weeks [ 15 , 27 ].
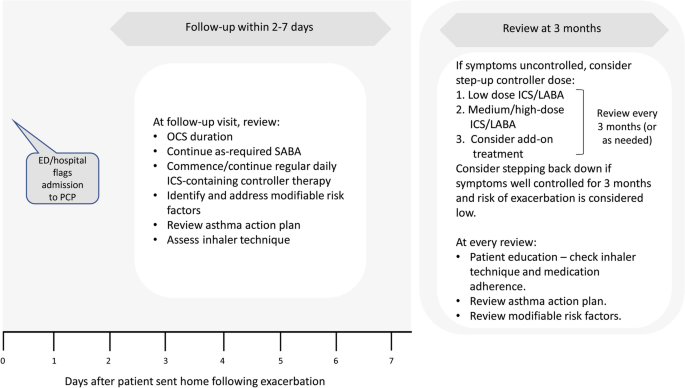
Primary care follow-up after an acute attack of asthma [ 15 ]. ED emergency department, GP general practitioner, ICS inhaled corticosteroid, LABA long-acting β 2 -agonist, OCS oral corticosteroid, SABA short-acting β 2 -agonist
At follow-up, it is important to establish the risk factors that led to the attack and the risk factors for further asthma attacks [ 15 , 27 ]. Comorbidities such as rhinitis are common and need managing [ 15 , 64 , 65 ]. Discussions should check that resources at home are adequate, including access to medications/spacer device, medication affordability and parent/carer awareness [ 15 , 31 , 65 ]. In low- and middle-income countries, patient affordability is a key consideration that influences treatment choice [ 65 ]. Inhaler technique and the patient’s asthma action plan should also be reviewed [ 15 , 27 ]. Subsequent reviews should be scheduled to assess the effectiveness of medication, and when asthma is well controlled, the frequency of reviews can be reduced [ 15 ]. These reviews could be either face to face or via social media platforms, the latter as used during the COVID-19 pandemic.
Overcoming the barriers to managing acute asthma attacks in primary care is essential to effectively treat existing symptoms, achieve long-term asthma control and help prevent future episodes. Every follow-up encounter should be viewed as an opportunity to review and extend patient knowledge and confidence with asthma management [ 27 ]. In addition to optimised controller medication, provision of patient education and an asthma action plan are integral components of effective follow-up [ 66 ].
Figure 5 provides an overview of the potential barriers to the management of acute attacks of asthma in primary care and summarises associated mitigating actions.
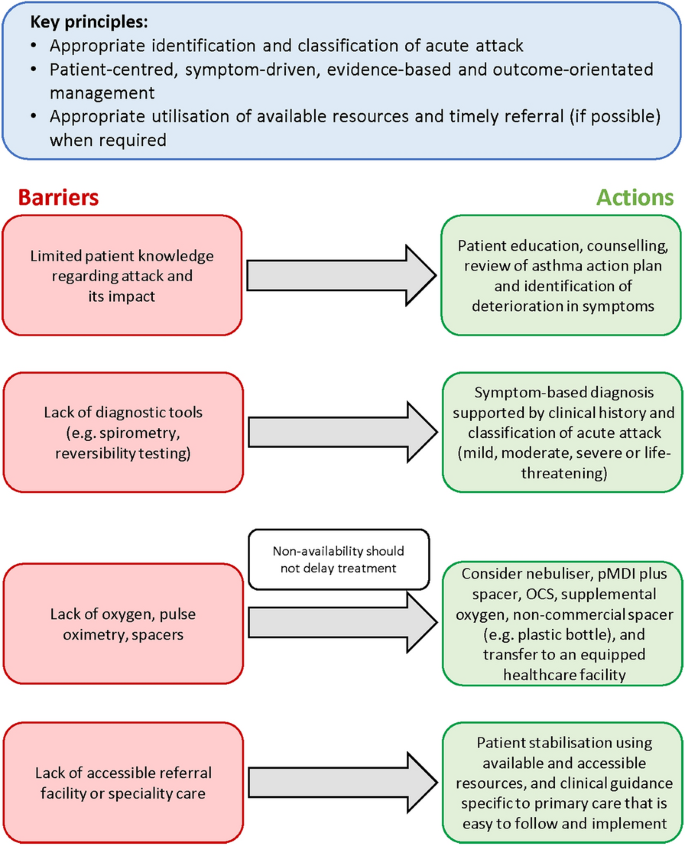
Overcoming barriers to the management of acute asthma in primary care. OCS oral corticosteroid, pMDI pressurised metered-dose inhaler
Prevention of Future Asthma Attacks
Inhaler technique.
Many patients with asthma are unable to use their inhaler correctly [ 67 , 68 ]. Common errors in the use of inhalers include exhalation during actuation, lack of hand–breath coordination, breath-hold too short, inspiratory flow too rapid, and inadequate shaking of inhaler [ 67 ]. It is a good idea to check a patient’s inhaler technique regularly, particularly following an asthma attack, and to determine causes of poor technique. Assessing inhaler technique remotely is challenging but can and should be done [ 45 , 46 ].
A review of inhaler technique critical errors indicated that 50% of patients with asthma fail to maintain correct inhaler technique over time and highlighted the importance of patient psychosocial factors for maintaining good inhaler skills [ 69 ]. In a qualitative, questionnaire-based study, Jahedi et al. reported that patients intrinsically linked inhalation device use, selection, and preference to medication effectiveness, overall views about asthma management, and belief and trust that their healthcare providers could make decisions about their medications for them [ 68 ]. Therefore, emphasizing that the correct inhalation technique can lead to better asthma control and fewer side effects may provide motivation for patients to learn and maintain a good inhaler technique [ 68 ].
Referral to an appropriately trained asthma nurse or pharmacist for education can be helpful [ 15 ]. Some patients may also benefit from the use of approved and reputable web-based inhaler training videos to supplement this [ 70 ].
Medications to Control Asthma
All patients who have had an asthma attack should be prescribed regular daily ICS-containing controller therapy [ 15 ], which should be started early to avoid the risk of repeated attacks [ 71 ]. Proactive regular dosing with ICS-containing therapies achieves symptom control and reduces attacks [ 15 , 72 ], both of which should be targeted for effective management of asthma [ 15 , 27 , 43 ]. In patients who are adherent and using their inhalers correctly, if symptoms remain uncontrolled after an attack, and/or the patient is at high risk of a future attack, treatment may be stepped up to a fixed-dose combination of ICS with a long-acting beta-agonist (LABA), plus ‘as-required’ use of SABA as a reliever [ 15 ]. Regular daily dosing with combined ICS and LABA is most effective in preventing severe attacks of asthma compared with other controller therapies, including ICS alone [ 73 , 74 ]. The AUSTRI study, a large randomised controlled trial of patients with a history of asthma attacks ( N = 11,751), demonstrated a 21% reduced risk of a severe attack with fluticasone propionate combined with salmeterol compared with fluticasone propionate alone [ 74 ].
In patients receiving regular maintenance therapy with either ICS or ICS-LABA plus SABA as a reliever, patterns of increased SABA use can serve as an indicator of an impending attack and should be heeded as a marker of uncontrolled asthma [ 75 , 76 ]. This should prompt patient evaluation by the physician and, if appropriate, changes to their treatment including close follow-up and reinforcing the use of an asthma action plan. In a database cohort study analysis, Nwaru et al. reported that, despite the increased risks associated with long-term high SABA use, these patterns of SABA use did not trigger any increases in maintenance ICS therapy, highlighting the need for paying closer attention to these patients to establish asthma control [ 76 ].
Adherence to Treatment
Many patients with asthma fail to take their medicines at least some of the time [ 77 ], with mean levels of adherence reported as ranging between 22% and 70% [ 78 , 79 , 80 , 81 , 82 , 83 ]. Factors contributing to poor adherence include a perceived burdensome medication regimen, cost, forgetfulness, poor health literacy, side effects, cultural issues or psychosocial factors [ 77 , 84 ]. Poor adherence to controller treatment is a risk factor for poor asthma outcomes including increased risk of asthma attacks [ 85 , 86 , 87 ]. A simple message for patients is that taking their regular controller medications as recommended is a good way to avoid attacks.
A lack of adherence may be intentional or non-intentional and establishing the rationale of non-adherence will help to find solutions to improve it [ 81 ]. Methods to encourage better adherence include simplifying treatment regimes, reducing the frequency of dosing, providing advice on medication reminders, and including patients in treatment decisions [ 15 ]. An understanding of a patient’s perceptions about their asthma may also help to identify and address their reasons for poor adherence [ 80 ]. The use of smart devices, where available, may also provide a useful tool for assessing adherence remotely [ 45 , 46 ].
Asthma Action Plan
Patient-led written asthma action plans have been recommended for many years [ 15 , 50 ], with the aim of reducing the likelihood of future attacks and of dying from asthma. They have been shown to improve health outcomes including fewer emergency department visits and hospitalisations [ 50 ]. An effective asthma action plan advises patients what to do in the event of worsening asthma symptoms, how to make short-term adjustments to their regular medications to manage their condition and provides clear instructions on how and when to access medical care [ 15 , 88 ]. Primary care physicians should bear in mind the available healthcare resources, patient literacy levels and cultural factors [ 15 ]. Figure S1 (Supplementary Material) provides an effective example of an asthma action plan, which has been developed by Asthma UK [ 88 ].
Patient Training and Education
Patient education forms the basis for encouraging good adherence and effective self-management of asthma [ 80 ]. Patient understanding of the purpose of adherence to medication, follow-up appointments and correct inhaler technique should be reviewed and its importance reiterated [ 15 , 43 ]. The value of educational interventions has been demonstrated in terms of both improved adherence and asthma outcomes [ 80 ]. Asthma attacks can represent a failure in management due to poor health literacy [ 43 ]; therefore, educational materials should be formatted and tailored to the needs of individual patients [ 80 ].
Patient education should also include a patient-centred discussion around minimising environmental attack triggers, cigarette smoke and avoidance of allergens [ 27 , 89 , 90 ].
What Has Been the Impact of COVID-19 on Asthma Attacks and What Are the Learnings from COVID-19?
During the COVID-19 pandemic, significant reductions in severe asthma attacks have been reported, in both primary care [ 3 ] and in those needing hospital admission [ 5 , 6 ]. The reasons for this are not totally understood but could be multifactorial and may include a decrease in air pollution, due to the reduction in the use of cars, a decrease in the circulation of respiratory viruses associated with distancing measures and wearing of masks, and improved self-management including increased adherence to preventative medications [ 8 , 9 , 14 ]. Understanding the reasons for such reductions could provide future learnings for managing patients with asthma [ 8 ]. Chalitsios et al. reported an increase in ICS prescriptions at the outbreak of the pandemic in the UK, with a decrease to baseline levels over the following months [ 91 ]. Analysis of digitally collected adherence data, based on electronic monitoring, demonstrated a 14.5% increase in adherence to controller medications during the first few months of the pandemic [ 9 ]. This suggests an increased recognition of asthma as a comorbidity and a heightened awareness of the importance of the disease. Understanding patients’ perceptions and concerns during this period could provide an opportunity to improve patient self-management and reduce avoidable attacks [ 8 ].
Several recent publications have highlighted that asthma outcomes, including mortality, are not worsened by COVID-19 infection, even suggesting some protective effects. In a systematic literature review of 62 studies, Hou et al. reported that asthma was associated with a reduced risk of COVID-19 mortality [ 11 ]. The mechanism for this is unclear but the authors suggested that it could be related to an increase in medical care received by patients with asthma and COVID-19 infection, or due to an anti-inflammatory protective effect associated with the type 2 immune response in patients with asthma or possibly from their treatment with ICS. Lombardi et al. also suggested that downregulation of angiotensin-converting enzyme 2 (ACE2) receptors, as well as chronic type 2 inflammation, younger age, absence of comorbidities and reduced viral exposure due to shielding in patients with asthma might be possible mechanisms by which asthma protects against COVID-19 infection and poor outcomes [ 2 ]. Another review and meta-analysis of 57 studies reported that patients with asthma have a lower risk of acquiring COVID-19, a possible explanation being the downregulation of ACE2 receptors observed in type 2-high asthma and with ICS treatment [ 92 ]. Asthmatics with confirmed COVID-19 based on positive reverse transcriptase-polymerase chain reaction (rt-PCR) test, however, have been reported to have a similar risk of hospitalisation, ICU admission, ventilator use and mortality as those without asthma [ 93 ]. Thus, maintaining asthma control with appropriate asthma maintenance therapy remains vital to avoid any deterioration and subsequent need for urgent healthcare visits.
Conclusions
The pandemic was an opportunity to increase the awareness of asthma as a chronic condition and the importance of managing asthma attacks. Well-managed asthma does not appear to worsen COVID-related outcomes, despite early concerns and may even offer some protection against COVID-related detrimental effects. Acute attacks of asthma can be well managed and, more importantly, prevented in primary care. During pandemics caused by respiratory viruses, such as in COVID-19, it is even more important for patients and PCPs to recognise asthma attacks and instigate early intervention, whether face to face or remotely. Remote assessment is more challenging but can be achieved with structural clinical assessment and the use of home monitoring devices where appropriate and available. The advice and algorithms provided in this article aim to simplify the appropriate and timely diagnosis, classification and treatment of acute attacks in adults and in teenagers. Continuity of care following an attack is crucial to establish its main cause and to agree the best preventative therapy that can help reduce further attacks. Proactive regular dosing with ICS-containing therapies achieves symptom control and reduces attacks, both of which should be targeted for effective management of asthma.
Jordan RE, Adab P, Cheng KK. Covid-19: risk factors for severe disease and death. BMJ. 2020;368:m1198.
PubMed Google Scholar
Lombardi C, Gani F, Berti A, Comberiati P, Peroni D, Cottini M. Asthma and COVID-19: a dangerous liaison? Asthma Res Pract. 2021;7(1):9.
PubMed PubMed Central Google Scholar
Shah SA, Quint JK, Nwaru BI, Sheikh A. Impact of COVID-19 national lockdown on asthma exacerbations: interrupted time-series analysis of English primary care data. Thorax. 2021;76:860–6.
de Boer G, Braunstahl G, Hendriks R, et al. Asthma exacerbation prevalence during the COVID-19 lockdown in a moderate-severe asthma cohort. BMJ Open Respir Res. 2021;8:e000758.
Davies GA, Alsallakh MA, Sivakumaran S, et al. Impact of COVID-19 lockdown on emergency asthma admissions and deaths: national interrupted time series analyses for Scotland and Wales. Thorax. 2021;76:867–73.
Huh K, Kim Y, Ji W, et al. Decrease in hospital admissions for respiratory diseases during the COVID-19 pandemic: a nationwide claims study. Thorax. 2021;76:939–41.
Salciccioli JD, She L, Tulchinsky A, Rockhold F, Cardet JC, Israel E. Effect of COVID-19 on asthma exacerbation. J Allergy Clin Immunol Pract. 2021;9(7):2896-2899.e1.
Skene IP, Pfeffer PE. Improved asthma control during the COVID-19 pandemic: are there lessons to be learnt? Thorax. 2021;76:852–3.
Kaye L, Theye B, Smeenk I, Gondalia R, Barrett MA, Stempel DA. Changes in medication adherence among patients with asthma and COPD during the COVID-19 pandemic. J Allergy Clin Immunol Pract. 2020;8:2384–5.
Wu X, Xu Y, Jin L, Wang X, Zhu H, Xie Y. Association of preexisting asthma and other allergic diseases with mortality in COVID-19 patients: a systematic review and meta-analysis. Front Med (Lausanne). 2021;8:670744.
Google Scholar
Hou H, Xu J, Li Y, Wang Y, Yang H. The association of asthma with COVID-19 mortality: an updated meta-analysis based on adjusted effect estimates. J Allergy Clin Immunol Pract. 2021;9(11):3944-3968.e5.
Primary Care Respiratory Society UK. PCRS Pragmatic Guidance. Diagnosing and managing asthma attacks and people with COPD presenting in crisis during the UK Covid 19 epidemic. 2020. https://www.pcrs-uk.org/sites/pcrs-uk.org/files/resources/COVID19/PCRS-Covid-19-Pragmatic-Guidance-v4-07-May-2020.pdf . Accessed 2 July 2021.
British Thoracic Society Advice for healthcare professionals treating people with asthma (adults) in relation to COVID-19. 2020. https://www.brit-thoracic.org.uk/document-library/quality-improvement/covid-19/bts-advice-for-healthcare-professionals-treating-patients-with-asthma/ . Accessed 2 July 2021.
Global Initiative for Asthma (GINA). GINA guidance about COVID-19 and asthma. https://ginasthma.org/wp-content/uploads/2021/03/21_03_30-GINA-COVID-19-and-asthma.pdf . Accessed 2 July 2021.
Global Initiative for Asthma (GINA). Global Strategy for Asthma Management and Prevention. 2021. https://ginasthma.org/gina-reports/ . Accessed 2 July 2021.
Pavord ID, Beasley R, Agusti A, et al. After asthma: redefining airways diseases. Lancet. 2018;391(10118):350–400.
Jones KA, Gibson PG, Yorke J, Niven R, Smith A, McDonald VM. Attack, flare-up, or exacerbation? The terminology preferences of patients with severe asthma. J Asthma. 2021;58(2):141–50.
CAS PubMed Google Scholar
Suruki RY, Daugherty JB, Boudiaf N, Albers FC. The frequency of asthma exacerbations and healthcare utilization in patients with asthma from the UK and USA. BMC Pulm Med. 2017;17:74.
Dennis RJ, Solarte I, Rodrigo G. Asthma in adults. BMJ Clin Evid. 2011;2011:1501.
Blakey JD, Woolnough K, Fellows J, Walker S, Thomas M, Pavord ID. Assessing the risk of attack in the management of asthma: a review and proposal for revision of the current control-centred paradigm. Prim Care Respir J. 2013;22:344–52.
Dusser D, Montani D, Chanez P, et al. Mild asthma: an expert review on epidemiology, clinical characteristics and treatment recommendations. Allergy. 2007;62:591–604.
Blakey JD, Price DB, Pizzichini E, et al. Identifying risk of future asthma attacks using UK medical record data: a respiratory effectiveness group initiative. J Allergy Clin Immunol Pract. 2017;5:1015–24.
Price D, Wilson AM, Chisholm A, et al. Predicting frequent asthma exacerbations using blood eosinophil count and other patient data routinely available in clinical practice. J Asthma Allergy. 2016;9:1–12.
CAS PubMed PubMed Central Google Scholar
Miller MK, Lee JH, Miller DP, Wenzel SE, TENOR Study Group. Recent asthma exacerbations: a key predictor of future exacerbations. Respir Med. 2007;101:481–9.
Bloom CI, Nissen F, Douglas IJ, Smeeth L, Cullinan P, Quint JK. Exacerbation risk and characterisation of the UK’s asthma population from infants to old age. Thorax. 2018;73:313–20.
Busse WW, Bateman ED, Caplan AL, et al. Combined analysis of asthma safety trials of long-acting β2-agonists. N Engl J Med. 2018;378:2497–505.
The British Thoracic Society. BTS/SIGN British guideline on the management of asthma. 2019. https://www.brit-thoracic.org.uk/standards-of-care/guidelines/btssign-british-guideline-on-the-management-of-asthma/ . Accessed 2 July 2021.
Small I. The majority of asthma cases can be managed in primary care. 2012. https://www.guidelinesinpractice.co.uk/respiratory/the-majority-of-asthma-cases-can-be-managed-in-primary-care/335790.article . Accessed 16 Sept 2021.
Chung L, Johnson P, Summers Q. Models of care for severe asthma: the role of primary care. Med J Aust. 2018;209:S34–40.
Szefler SJ, Fitzgerald DA, Adachi Y, et al. A worldwide charter for all children with asthma. Pediatr Pulmonol. 2020;55:1282–92.
Sánchez-Borges M, Capriles-Hulett A, Caballero-Fonseca F. Asthma care in resource-poor settings. World Allergy Organ J. 2011;4:68–72.
Gibbons DC, Aggarwal B, Fairburn-Beech J, et al. Treatment patterns among non-active users of maintenance asthma medication in the United Kingdom: a retrospective cohort study in the clinical practice research Datalink. J Asthma. 2021;58:793–804.
D’Amato G, Vitale C, Molino A, et al. Asthma-related deaths. Multidiscip Respir Med. 2016;11:37.
Royal College of Physicians. Why asthma kills. The national review of asthma deaths (NRAD). 2014. https://www.rcplondon.ac.uk/projects/outputs/why-asthma-still-kills . Accessed 2 July 2021.
Beaney T, Salman D, Samee T, Mak V. Assessment and management of adults with asthma during the COVID-19 pandemic. BMJ. 2020;369:m2092.
The Primary Care Respiratory Society (PCRS). PCRS Position Statement. Diagnostic work up of the patient presenting with respiratory symptoms during the COVID-19 pandemic. 2020. https://www.pcrs-uk.org/resource/diagnostic-work-patient-presenting-respiratory-symptoms-during-covid-19-pandemic . Accessed 4 Jan 2022.
Ramsahai JM, Hansbro PM, Wark PAB. Mechanisms and management of asthma exacerbations. Am J Respir Crit Care Med. 2019;199:423–32.
Castillo JR, Peters SP, Busse WW. Asthma exacerbations: pathogenesis, prevention, and treatment. J Allergy Clin Immunol Pract. 2017;5:918–27.
Martin MJ, Beasley R, Harrison TW. Towards a personalised treatment approach for asthma attacks. Thorax. 2020;75:1119–29.
Tattersfield AE, Postma DS, Barnes PJ, et al. Exacerbations of asthma: a descriptive study of 425 severe exacerbations. The FACET International Study Group. Am J Respir Crit Care Med. 1999;160:594–9.
Bateman ED, O’Byrne PM, Busse WW, et al. Once-daily fluticasone furoate (FF)/vilanterol reduces risk of severe exacerbations in asthma versus FF alone. Thorax. 2014;69:312–9.
Reddel HK, Busse W, Rabe KF, et al. Heterogeneity and time course of asthma exacerbations: data from AUSTRI. Eur Respir J. 2021;58:PA3716. https://doi.org/10.1183/13993003.congress-2021.PA3716 .
Article Google Scholar
National Asthma Council Australia. Australia's National Guidelines for Asthma Management. 2020. http://www.asthmahandbook.org.au/management/adults . Accessed 2 July 2021.
National Heart, Lung, and Blood Institute. National Asthma Education and Prevention Program. Expert Panel Report 3: guidelines for the diagnosis and management of asthma. 2007. https://www.nhlbi.nih.gov/sites/default/files/media/docs/asthgdln_1.pdf . Accessed 2 July 2021.
Asthma UK. Smart asthma: Real-world implementation of connected devices in the UK to reduce asthma attacks. https://www.asthma.org.uk/support-us/campaigns/publications/smartasthma/ . Accessed 7 Jan 2022.
GSK. How can we assess and improve inhaler technique virtually? https://offyourchest.gsk.com/detail/inhalertechnique . Accessed 7 Jan 2022.
Greenhalgh T, Koh GCH, Car J. COVID-19: a remote assessment in primary care. BMJ. 2020;368:1182.
Bakakos A, Krompa A. Asthma in the era of SARS CoV-2 virus. J Asthma. 2021. https://doi.org/10.1080/02770903.2021.1941093 .
Article PubMed Google Scholar
Amirav I, Newhouse MT. Asthma and COVID-19: In defense of evidence-based SABA. J Asthma Allergy. 2020;13:505–8.
Gibson PG. Written action plans for asthma: an evidence-based review of the key components. Thorax. 2004;59:94–9.
Kew KM, Quinn M, Quon BS, Ducharme FM. Increased versus stable doses of inhaled corticosteroids for exacerbations of chronic asthma in adults and children. Cochrane Database Syst Rev. 2016;6:CD007524.
Harrison TW, Oborne J, Newton S, Tattersfield AE. Doubling the dose of inhaled corticosteroid to prevent asthma exacerbations: randomised controlled trial. Lancet. 2004;363(9405):271–5.
Asthma UK. Steroids: Asthma steroids help to calm and prevent inflammation in the airways to keep symptoms under control. 2016. https://www.asthma.org.uk/advice/inhalers-medicines-treatments/steroids/ . Accessed 16 Sept 2021.
Richards R. Side-effects of short-term oral corticosteroids. J Cutan Med Surg. 2018;12:77–81.
Perrin K, Wijesinghe M, Healy B, et al. Randomised controlled trial of high concentration versus titrated oxygen therapy in severe exacerbations of asthma. Thorax. 2011;66:937–41.
Fergeson JE, Patel SS, Lockey RF. Acute asthma, prognosis, and treatment. J Allergy Clin Immunol. 2017;139:438–47.
Kirkland SW, Vandenberghe C, Voaklander B, Nikel T, Campbell S, Rowe BH. Combined inhaled beta-agonist and anticholinergic agents for emergency management in adults with asthma. Cochrane Database Syst Rev. 2017;1:CD001284.
Normansell R, Sayer B, Waterson S, Dennett EJ, Del Forno M, Dunleavy A. Antibiotics for exacerbations of asthma. Cochrane Database Syst Rev. 2018;6:CD002741.
Anderson G, Johnson N, Mulgirigama A, Aggarwal B. Use of spacers for patients treated with pressurized metered dose inhalers: focus on the VENTOLIN™ Mini Spacer. Expert Opin Drug Deliv. 2018;15:419–30.
Newman B, Milne S, Hamilston C, Hall KA. Comparison of albuterol administered by metered-dose inhaler and spacer with albuterol by nebulizer in adults presenting to an urban emergency department with acute asthma. Chest. 2002;121:1036–41.
Ari A. Practical strategies for a safe and effective delivery of aerosolized medications to patients with COVID-19. Respir Med. 2020;167:105987.
Levin M, Ansotegui IJ, Bernstein J, et al. Acute asthma management during SARS-CoV2-pandemic 2020. World Allergy Organ J. 2020;13(5):100125.
Hill J, Arrotta N, Villa-Roel C, Dennett L, Rowe BH. Factors associated with relapse in adult patients discharged from the emergency department following acute asthma: a systematic review. BMJ Open Resp Res. 2017;4:e000169.
Boulet LP. Influence of comorbid conditions on asthma. Eur Respir J. 2009;33:897–906.
Aggarwal B, Shantakumar S, Hinds D, Mulgirigama A. Asia-Pacific Survey of Physicians on Asthma and Allergic Rhinitis (ASPAIR): physician beliefs and practices about diagnosis, assessment, and treatment of coexistent disease. J Asthma Allergy. 2018;11:293–307.
FitzGerald JM, Gibson PG. Asthma exacerbations. 4: prevention. Thorax. 2006;61:992–9.
Price DB, Román-Rodríguez M, McQueen RB, et al. Inhaler errors in the CRITIKAL study: type, frequency, and association with asthma outcomes. J Allergy Clin Immunol Pract. 2017;5:1071-1081.e9.
Jahedi L, Downie SR, Saini B, Chan HK, Bosnic-Anticevich S. Inhaler technique in asthma: how does it relate to patients’ preferences and attitudes toward their inhalers? J Aerosol Med Pulm Drug Deliv. 2017;30:42–52.
Bosnic-Anticevich SZ, Cvetkovski B, Azzi EA. Identifying critical errors: addressing inhaler technique in the context of asthma management. Pulm Ther. 2018;4:1–12.
Müller T, Müller A, Hübel C, et al. Optimizing inhalation technique using web-based videos in obstructive lung diseases. Respir Med. 2017;129:140–4.
Stanford RH, Buikema AR, Riedel AA, Camargo CA Jr, Rey GG, Chapman KR. Asthma controller delay and recurrence risk after an emergency department visit or hospitalization. Respir Med. 2012;106:1631–8.
Bateman ED, Boushey HA, Bousquet J, et al. Can guideline-defined asthma control be achieved? The Gaining Optimal Asthma Control study. Am J Respir Crit Care Med. 2004;170:836–44.
Loymans RJB, Gemperli A, Cohen J, et al. Comparative effectiveness of long term drug treatment strategies to prevent asthma exacerbations: network meta-analysis. BMJ. 2014;348:g3009.
Stempel DA, Raphiou IH, Kral KM, et al. Serious asthma events with fluticasone plus salmeterol versus fluticasone alone. N Engl J Med. 2016;374:1822–30.
Stanford RH, Shah MB, D’Souza AO, Dhamane AD, Schatz M. Short-acting-agonist use and its ability to predict future asthma-related outcomes. Ann Allergy Asthma Immunol. 2012;109:403–7.
Nwaru BI, Ekström M, Hasvold P, Wiklund F, Telg G, Janson C. Overuse of short-acting β2-agonists in asthma is associated with increased risk of exacerbation and mortality: a nationwide cohort study of the global SABINA programme. Eur Respir J. 2020;55:1901872.
Boulet LP, Vervloet D, Magar Y, Foster JM. Adherence: the goal to control asthma. Clin Chest Med. 2012;33:405–17.
Cerveri I, Locatelli F, Zoia MC, Corsico A, Accordini S, de Marco R. International variations in asthma treatment compliance: the results of the European Community Respiratory Health Survey (ECRHS). Eur Respir J. 1999;14:288–94.
Bender B, Wamboldt FS, O’Connor SL, et al. Measurement of children’s asthma medication adherence by self report, mother report, canister weight, and Doser CT. Ann Allergy Asthma Immunol. 2000;85:416–21.
Eakin MN, Rand CS. Improving patient adherence with asthma self-management practices: what works? Ann Allergy Asthma Immunol. 2012;109:90–2.
Mäkelä MJ, Backer V, Hedegaard M, Larsson K. Adherence to inhaled therapies, health outcomes and costs in patients with asthma and COPD. Respir Med. 2013;107:1481–90.
Price D, Fletcher M, van der Molen T. Asthma control and management in 8000 European patients: the REcognise Asthma and LInk to Symptoms and Experience (REALISE) survey. NPJ Prim Care Respir Med. 2014;24:14009.
Bårnes CB, Ulrik CS. Asthma and adherence to inhaled corticosteroids: current status and future perspectives. Respir Care. 2015;60:455–68.
Foster JM, Aucott L, van der Werf RH, et al. Higher patient perceived side effects related to higher daily doses of inhaled corticosteroids in the community: a cross-sectional analysis. Respir Med. 2006;100:1318–36.
Engelkes M, Janssens HM, de Jongste JC, Sturkenboom MC, Verhamme KM. Medication adherence and the risk of severe asthma exacerbations: a systematic review. Eur Respir J. 2015;45:396–407.
Killane I, Sulaiman I, MacHale E, et al. Predicting asthma exacerbations employing remotely monitored adherence. Healthc Technol Lett. 2016;3:51–5.
Ismaila A, Corriveau D, Vaillancourt J, et al. Impact of adherence to treatment with fluticasone propionate/salmeterol in asthma patients. Curr Med Res Opin. 2014;30:1417–25.
Asthma UK. Filling in patient's asthma action plans. 2019 https://www.asthma.org.uk/globalassets/health-advice/resources/adults/adult-asthma-action-plan.pdf . Accessed 16 Sept 2021.
Eisner MD, Klein J, Hammond SK, Koren G, Lactao G, Iribarren C. Directly measured second hand smoke exposure and asthma health outcomes. Thorax. 2005;60:814–21.
Schatz M, Rachelefsky G, Krishnan JA. Follow-up after acute asthma episodes: what improves future outcomes? Proc Am Thorac Soc. 2009;6:386–93.
Chalitsios CV, Tricia MM, Langley TE, Shaw DE. Impact of COVID-19 on corticosteroids and antibiotics prescribing in England: an interrupted time series analysis. J Public Health. 2021. https://doi.org/10.1093/pubmed/fdab017 .
Sunjaya AP, Allida SM, Di Tanna GL, Jenkins C. Asthma and risk of infection, hospitalization, ICU admission and mortality from COVID-19: systematic review and meta-analysis. J Asthma. 2021;1:1–14.
Sunjaya AP, Allida SM, Di Tanna GL, Jenkins CR. Asthma and coronavirus disease 2019 risk: a systematic review and meta-analysis. Eur Respir J. 2021;24:2101209. https://doi.org/10.1183/13993003.01209-2021 .
Asthma UK. Meet your asthma healthcare team. 2019. https://www.asthma.org.uk/advice/nhs-care/healthcare-team/#asthmanurses . Accessed 16 Sept 2021.
Download references
Acknowledgements
Funding for this article was provided by GSK. GSK funded the journal’s Rapid Service and Open Access fees.
Medical Writing Assistance
Medical writing assistance (in the form of assistance with developing the initial draft of the manuscript, collating author comments, copyediting and compiling figures and tables) was provided by Gillian Wallace, MSc, at Fishawack Indicia Ltd, UK, and Kate Hollingworth of Continuous Improvement Ltd, UK, and was funded by GlaxoSmithKline (GSK).
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
All authors were involved in the conception/design of this manuscript, the acquisition of data, and data interpretation. All authors approved the final version prior to submission.
Disclosures
Monica Fletcher is a former employee of GSK. Warren Lenney is a former employee of GSK and holds GSK stocks/shares. Thys van der Molen is a former GSK employee and has provided advisory board consultancy for Chiesi. Bhumika Aggarwal, Isabelle Boucot and Emilio Pizzichini are employees of, and hold stocks/shares in, GSK.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Author information
Authors and affiliations.
The Usher Institute, University of Edinburgh, Edinburgh, UK
Monica Fletcher
Department of General Practice and GRIAG Research Institute, University Medical Center Groningen, University of Groningen, Groningen, The Netherlands
Thys van der Molen
Department of Pharmacy and Bioengineering, University of Keele, Keele, Staffordshire, UK
Warren Lenney
Respiratory, GlaxoSmithKline, Brentford, London, UK
Isabelle Boucot & Emilio Pizzichini
Respiratory, General Medicines Emerging Markets, GlaxoSmithKline, Singapore, 139234, Singapore
Bhumika Aggarwal
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Monica Fletcher .
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (PDF 1013 kb)
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/ .
Reprints and permissions
About this article
Fletcher, M., van der Molen, T., Lenney, W. et al. Primary Care Management of Asthma Exacerbations or Attacks: Impact of the COVID-19 Pandemic. Adv Ther 39 , 1457–1473 (2022). https://doi.org/10.1007/s12325-022-02056-x
Download citation
Received : 22 November 2021
Accepted : 21 January 2022
Published : 14 February 2022
Issue Date : April 2022
DOI : https://doi.org/10.1007/s12325-022-02056-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Exacerbations
- Primary care
- Find a journal
- Publish with us
- Track your research
- - Google Chrome
Intended for healthcare professionals
- Access provided by Google Indexer
- My email alerts
- BMA member login
- Username * Password * Forgot your log in details? Need to activate BMA Member Log In Log in via OpenAthens Log in via your institution

Search form
- Advanced search
- Search responses
- Search blogs
- Assessment and...
Assessment and management of adults with asthma during the covid-19 pandemic
Read our latest coverage of the coronavirus pandemic.
- Related content
- Peer review
- Thomas Beaney , academic clinical fellow in primary care 1 ,
- David Salman , academic clinical fellow in primary care 1 ,
- Tahseen Samee , specialist registrar in emergency medicine 2 ,
- Vincent Mak , consultant in respiratory community integrated care 3
- 1 Department of Primary Care and Public Health, Imperial College London, London, UK
- 2 Barts Health NHS Trust, London, UK
- 3 Imperial College Healthcare NHS Trust, London, UK
- Correspondence to: T Beaney Thomas.beaney{at}imperial.ac.uk
What you need to know
In patients with pre-existing asthma, a thorough history and structured review can help distinguish an asthma exacerbation from covid-19 and guide management
In those with symptoms of acute asthma, corticosteroids can and should be used if indicated and not withheld on the basis of suspected covid-19 as a trigger
Assessment can be carried out remotely, ideally via video, but have a low threshold for face-to-face assessment, according to local arrangements
A 35 year old man contacts his general practice reporting a dry cough and increased shortness of breath for the past three days. He has a history of asthma, for which he uses an inhaled corticosteroid twice daily and is now using his salbutamol four times a day. Because of the covid-19 outbreak, he is booked in for a telephone consultation with a general practitioner that morning.
Asthma is a condition commonly encountered in primary care, with over five million people in the UK prescribed active treatment. 1 While seemingly a routine part of general practice, asthma assessment is a particular challenge in the context of the covid-19 pandemic, given the overlap in respiratory symptoms between the two conditions and the need to minimise face-to-face assessment. Over 1400 people died from asthma in 2018 in England and Wales, 2 while analyses of non-covid-19 deaths during the covid-19 outbreak have shown an increase in deaths due to asthma, 31 highlighting the need to distinguish the symptoms of acute asthma from those of covid-19 and manage them accordingly.
This article outlines how to assess and manage adults with exacerbations of asthma in the context of the covid-19 outbreak ( box 1 ). We focus on the features differentiating acute asthma from covid-19, the challenges of remote assessment, and the importance of corticosteroids in patients with an asthma exacerbation.
Asthma and covid-19: what does the evidence tell us?
Are patients with asthma at higher risk from covid-19.
Some studies, mostly from China, found lower than expected numbers of patients with asthma admitted to hospital, suggesting they are not at increased risk of developing severe covid-19. 3 4 5 However, these reports should be viewed cautiously, as confounding by demographic, behavioural, or lifestyle factors may explain the lower than expected numbers. Recent pre-print data from the UK suggest that patients with asthma, and particularly severe asthma, are at higher risk of in-hospital mortality from covid-19. 6 In the absence of more conclusive evidence to indicate otherwise, those with asthma, particularly severe asthma, should be regarded as at higher risk of developing complications from covid-19. 7
Can SARS-CoV-2 virus cause asthma exacerbations?
Some mild seasonal coronaviruses are associated with exacerbations of asthma, but the coronaviruses causing the SARS and MERS outbreaks were not found to be. 8 9 In the case of SARS-CoV-2 virus, causing covid-19, data from hospitalised patients in China did not report symptoms of bronchospasm such as wheeze, but the number of patients with pre-existing asthma was not reported. 10 More recent pre-print data from hospitalised patients in the UK identified wheeze in a minority of patients with Covid-19. 11 Given the overlap of symptoms, such as cough and shortness of breath, until further published data emerges, SARS-CoV-2 may be considered as a possible viral trigger in patients with an asthma attack.
What you should cover
Challenges of remote consultations.
Primary care services have moved towards telephone triage and remote care wherever possible to minimise the risk of covid-19 transmission. This brings challenges to assessment as visual cues are missing, and, unless the patient has their own equipment, tests involving objective measurement, such as oxygen saturation and peak expiratory flow, are not possible. In mild cases, assessment via telephone may be adequate, but, whenever possible, we recommend augmenting the consultation with video for additional visual cues and examination. 12 However, many patients, particularly the elderly, may not have a phone with video capability. If you are relying on telephone consultation alone, a lower threshold may be needed for face-to-face assessment.
Presenting symptoms
Start by asking the patient to describe their symptoms in their own words. Note whether they sound breathless or struggle to complete sentences and, if so, determine whether immediate action is required. If not, explore what has changed, and why the patient has called now. The three questions recommended by the Royal College of Physicians—asking about impact on sleep, daytime symptoms, and impact on activity—are a useful screening tool for uncontrolled asthma. 13 Alternative validated scores, such as the Asthma Control Questionnaire and Asthma Control Test, which include reliever use, are also recommended. 14 In assessing breathlessness, the NHS 111 symptom checker contains three questions—the answers may arise organically from the consultation, but are a useful aide memoire:
Are you so breathless that you are unable to speak more than a few words?
Are you breathing harder or faster than usual when doing nothing at all?
Are you so ill that you’ve stopped doing all of your usual daily activities?
Consider whether an exacerbation of asthma or covid-19 is more likely. Both can present with cough and breathlessness, but specific features may indicate one over the other (see box 2 ). Do the patient’s current symptoms feel like an asthma attack they have had before? Do symptoms improve with their reliever inhaler? Do they also have symptoms of allergic rhinitis? Pollen may be a trigger for some people with asthma during hay fever season.
History and examination features helping distinguish asthma exacerbation from covid-19 10 11 14 15 16
Exacerbation of asthma*.
Improvement in symptoms with reliever inhaler
Diurnal variation
Absence of fever
Coexisting hay fever symptoms
Examination:
Reduced peak expiratory flow
Close contact of known or suspected case
Dry continuous cough
Onset of dyspnoea 4-8 days into illness
Flu-like symptoms including fatigue, myalgia, headache
Symptoms not relieved by inhaler
Absence of wheeze
Peak expiratory flow may be normal
*Note SARS-CoV-2 infection may be a trigger for an asthma exacerbation
Risk factors and medications
To assess the risk of deterioration, ask specifically about any previous hospital admissions for asthma and about oral corticosteroid use over the past 12 months. Does the patient have any other high risk conditions or are they taking immunosuppressive drugs? Ask the patient if they smoke and take the opportunity to offer support to quit.
Are they prescribed an inhaled corticosteroid (ICS) or a long acting β agonist (LABA) and ICS combination inhaler? Are they using this regularly? Are they using a spacer device, and do they have a personal asthma action plan to guide management?
Psychosocial factors
Taking a psychosocial history can be more challenging over the telephone, where cues are harder to spot. Lessons from asthma deaths have shown that adverse psychosocial factors are strongly associated with mortality. 14 17 These include a history of mental health problems, lack of engagement with healthcare services, and alcohol or drug misuse, along with employment and income problems. Social isolation is also a risk factor, which may be exacerbated during social distancing measures. 17 The covid-19 outbreak is an anxious time for many patients, and symptoms of anxiety can contribute to the overall presentation.
Examination
In remote assessment, video can help guide decision making, and we recommend its use in asthmatic patients presenting with acute symptoms. First, assess the general appearance of the patient. A fatigued patient sitting up in bed, visibly breathless, and anchoring their chest will raise immediate concerns, as opposed to someone who is walking around while talking. Vocal tone and behaviour may indicate any contributing anxiety. Observe if the patient can speak in complete sentences, listen for audible wheeze, and count the respiratory rate. Assess the work of breathing, including the use of accessory muscles, and consider the use of a chaperone where appropriate. The Roth score is not advocated for assessment of covid-19 or asthma. 18
Further objective assessment can be made, such as measuring peak expiratory flow (PEF). If the patient does not have a PEF device at home, one can be prescribed, though this may not be feasible in an acute scenario. We recommend that PEF technique be witnessed via video to assess reliability. Silent hypoxia may be a feature of covid-19, and oxygen saturations should be measured if this is a concern. 19 In some regions, oxygen saturation probe delivery services are being implemented, which may facilitate this. Heart rate can also be provided by the patient if they use conventional “wearable” technology, although, given the potential inaccuracies with different devices, the results should not be relied on. 20 If time allows, inhaler technique can also be checked.
What you should do
Determine the most likely diagnosis.
Decide on the most likely diagnosis on the basis of the history and clinical features (see box 2 and fig 1 ) or consider whether an alternative or coexisting diagnosis is likely, such as a bacterial pneumonia or pulmonary embolus. If you suspect covid-19 without asthmatic features, manage the patient as per local covid-19 guidance.

Assessment and management of patients with known asthma during the covid-19 outbreak 14
- Download figure
- Open in new tab
- Download powerpoint
Determine severity and decide if face-to-face assessment is necessary
If asthmatic features are predominant, determine severity and treat according to Scottish Intercollegiate Guidelines Network (SIGN) and British Thoracic Society (BTS) guidance ( fig 1 ). 14 If the patient cannot complete sentences or has a respiratory rate ≥25 breaths/min, treat the case as severe or life threatening asthma and organise emergency admission. A peak expiratory flow (PEF) <50% of best or predicted or a heart rate ≥110 beats/min also indicate severe or life threatening asthma. If the patient does not meet these criteria, treat as a moderate asthma attack—a PEF of 50-75% of best or predicted helps confirm this. If they do not have a PEF meter, or if you are unsure as to severity, brief face-to-face assessment to auscultate for wheeze and assess oxygen saturations can help confirm the degree of severity and determine if the patient may be suitable for treatment at home with follow-up. Do not rely solely on objective tests and use clinical judgment to decide on the need for face-to-face assessment, based on knowledge of the patient, risk factors, and any adverse psychosocial circumstances.
Wheeze has been reported as a presenting symptom in a minority of patients with confirmed covid-19, and it may be difficult to rule out the presence of SARS-CoV-2 via remote assessment. 11 We recommend that, when a face-to-face assessment is needed, it should take place via local pathways in place to safely assess patients with suspected or possible covid-19—for example, at a local “hot” clinic. At present, performing a peak flow test is not considered to be an aerosol generating procedure, but the cough it may produce could be, so individual risk assessment is advised. 21 Consider performing PEF in an open space or remotely in another room via video link. Any PEF meter should be single-patient use only and can be given to the patient for future use.
Initial management when face-to-face assessment is not required
For moderate asthma exacerbations, advise up to 10 puffs of a short acting β agonist (SABA) inhaler via a spacer, administered one puff at a time. There is no evidence that nebulisers are more effective: 4-6 puffs of salbutamol via a spacer is as effective as 2.5 mg via a nebuliser. 22 Alternatively, if the patient takes a combined inhaled corticosteroid and long acting β agonist (LABA) preparation, then maintenance and reliever therapy (MART) can be used according to their action plan. 14 Management of an acute exacerbation should not rely solely on SABA monotherapy, so advise patients to follow their personal asthma action plan and continue corticosteroid treatment (or start it if they were not taking it previously) unless advised otherwise ( box 3 ). Antibiotics are not routinely recommended in asthma exacerbations.
Risks and benefits of inhaled and oral corticosteroids in asthma and covid-19
There is substantial evidence for the benefits of steroids in asthma. Regular use of inhaled steroids reduces severe exacerbations of asthma 23 and the need for bronchodilators, 24 while the prompt use of systemic corticosteroids during an exacerbation reduces the need for hospital admissions, use of β agonists, 25 and relapses. 26
The evidence for corticosteroid use in early covid-19 is still emerging. A systematic review of steroid use in SARS reported on 29 studies, 25 of which were inconclusive and four of which suggested possible harm (diabetes, osteoporosis, and avascular necrosis) but no reported effects on mortality. 27 WHO have cautioned against the use of systemic corticosteroids for the treatment of covid-19 unless indicated for other diseases. 28
In light of the strong evidence of benefits in patients with asthma, inhaled and oral corticosteroids should be prescribed if indicated in patients with symptoms of bronchoconstriction. Steroids should not be withheld on the theoretical risk of covid-19 infection, in line with guidance from the Primary Care Respiratory Society (PCRS), British Thoracic Society (BTS), and Global Initiative for Asthma (GINA). 15 22 29
Response to initial SABA or MART treatment can be assessed with a follow-up call at 20 minutes. If there is no improvement, further treatment may be necessary at a local hot clinic for reviewing possible covid-19, emergency department, or direct admission to an acute medical or respiratory unit depending on local pathways. For those who do respond, BTS-SIGN and GINA advise starting oral corticosteroids in patients presenting with an acute asthma exacerbation (such as prednisolone 40-50 mg for 5-7 days). 14 15 There is an increasing move in personalised asthma action plans to early quadrupling of the inhaled corticosteroid dose in patients with deteriorating control for up to 14 days to reduce the risk of severe exacerbations and the need for oral steroids. 15 30 However, there may be a ceiling effect on those who are already on a high dose of inhaled corticosteroid (see BTS table 14 ), so quadrupling the dose may not be effective in this group of patients. A personalised asthma action plan is an extremely helpful guide to treatment and should be completed or updated for all patients.
Follow-up and safety-netting
We recommend that all patients with moderate symptoms are followed up via remote assessment within 24 hours. Asthma attacks requiring hospital admission tend to develop relatively slowly over 6-48 hours. 14 However, deterioration can be more rapid, and symptoms can worsen overnight. Patients should be advised to look out for any worsening breathing or wheeze, lack of response to their inhalers, or worsening PEF. They should receive clear advice on what to do, including use of their reliever, and who to contact (such as the local out-of-hours GP provider, 111, or 999). With potential long waits for remote assessment, particularly out of hours, they should be advised to have a low threshold to call 999 if their symptoms deteriorate. If covid-19 infection is also suspected, advise them to isolate for seven days from onset of symptoms and arrange testing, according to the latest guidance. 7
How this article was created
We performed a literature search using Ovid, Medline, and Global Health databases using the search terms (asthma OR lung disease OR respiratory disease) AND (coronavirus OR covid-19)). Articles from 2019-20 were screened. We also searched for specific guidelines, including NICE, British Thoracic Society, Scottish Intercollegiate Guidelines Network, Primary Care Respiratory Society, European Respiratory Society, International Primary Care Respiratory Group, Global Initiative for Asthma, and the American Academy of Allergy, Asthma and Immunology.
Education into practice
Do you feel confident in completing personalised asthma plans in collaboration with patients?
How often do you start or increase inhaled corticosteroids in patients at initial presentation with an exacerbation of asthma?
If you manage a patient with acute asthma remotely, what safety netting advice would you give and how could you check understanding?
How patients were involved in the creation of this article
No patients were involved in the creation of this article.
This is part of a series of occasional articles on common problems in primary care. The BMJ welcomes contributions from GPs.
Contributors: TB and TS conceived the article. TB, DS, and TS carried out the literature review and wrote the initial drafts. All four authors contributed to editing and revision, and VM provided expert advice as a respiratory specialist. All authors are guarantors of the work.
Competing interests: We have read and understood BMJ policy on declaration of interests and have no relevant interests to declare.
Provenance and peer review: Commissioned, based on an idea from the author; externally peer reviewed.
- Mukherjee M ,
- Stoddart A ,
- ↵ Asthma UK. Asthma facts and statistics. https://www.asthma.org.uk/about/media/facts-and-statistics/ .
- Scabini S ,
- Mornese Pinna S ,
- Di Perri G ,
- De Rosa FG ,
- Williamson E. ,
- Walker AJ ,
- Bhaskaran KJ ,
- ↵ Public Health England. Guidance on social distancing for everyone in the UK [Withdrawn]. 2020. https://www.gov.uk/government/publications/covid-19-guidance-on-social-distancing-and-for-vulnerable-people/guidance-on-social-distancing-for-everyone-in-the-uk-and-protecting-older-people-and-vulnerable-adults .
- Shaker MS ,
- Oppenheimer J ,
- Grayson M ,
- China Medical Treatment Expert Group for Covid-19
- Docherty AB ,
- Harrison EM ,
- Greenhalgh T ,
- Pinnock H ,
- Campbell S ,
- ↵ Scottish Intercollegiate Guidelines Network & British Thoracic Society. Sign 158 British guideline on the management of asthma. 2019. https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma .
- ↵ Primary Care Respiratory Society. PCRS Pragmatic Guidance: Diagnosing and managing asthma attacks and people with COPD presenting in crisis during the UK Covid 19 epidemic. 2020. https://www.pcrs-uk.org/sites/pcrs-uk.org/files/resources/COVID19/PCRS-Covid-19-Pragmatic-Guidance-v2-02-April-2020.pdf .
- Rapoport AB
- Royal College of Physicians
- ↵ Centre for Evidence-Based Medicine. Question: Should the Roth score be used in the remote assessment of patients with possible COVID-19? Answer: No. 2020. https://www.cebm.net/covid-19/roth-score-not-recommended-to-assess-breathlessness-over-the-phone/ .
- Goldstein BA ,
- ↵ Public Health England. Guidance: COVID-19 personal protective equipment (PPE). 2020. https://www.gov.uk/government/publications/wuhan-novel-coronavirus-infection-prevention-and-control/covid-19-personal-protective-equipment-ppe .
- ↵ British Thoracic Society. Advice for healthcare professionals treating people with asthma (adults) in relation to COVID-19. 2020. https://www.brit-thoracic.org.uk/about-us/covid-19-information-for-the-respiratory-community/ .
- Pauwels RA ,
- Pedersen S ,
- START Investigators Group
- Bestall JB ,
- Lasserson TJ ,
- Spooner C ,
- Ducharme FM ,
- Bretzlaff JA ,
- Spooner CH ,
- Stockman LJ ,
- Bellamy R ,
- ↵ World Health Organization. Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected: Interim guidance 13th March 2020. 2020. https://www.who.int/docs/default-source/coronaviruse/clinical-management-of-novel-cov.pdf .
- ↵ Global Initiative for Asthma (GINA). 2020 GINA report, global strategy for asthma management and prevention. 2020. https://ginasthma.org/gina-reports/ .
- McKeever T ,
- Mortimer K ,
- ↵ Office for National Statistics. Analysis of death registrations not involving coronavirus (COVID-19), England and Wales: 28 December 2019 to 1 May 2020. Release date: 5 June 2020. https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/articles/analysisofdeathregistrationsnotinvolvingcoronaviruscovid19englandandwales28december2019to1may2020/technicalannex .
- Join our Mailing List
Working locally in primary care and collaborating globally to improve respiratory health
Clinical case study - asthma, clinical case study - asthma, resource information.
- Disease management
Case Study: Managing Severe Asthma in an Adult
—he follows his treatment plan, but this 40-year-old male athlete has asthma that is not well-controlled. what’s the next step.
By Kirstin Bass, MD, PhD Reviewed by Michael E. Wechsler, MD, MMSc
This case presents a patient with poorly controlled asthma that remains refractory to treatment despite use of standard-of-care therapeutic options. For patients such as this, one needs to embark on an extensive work-up to confirm the diagnosis, assess for comorbidities, and finally, to consider different therapeutic options.

Case presentation and patient history
Mr. T is a 40-year-old recreational athlete with a medical history significant for asthma, for which he has been using an albuterol rescue inhaler approximately 3 times per week for the past year. During this time, he has also been waking up with asthma symptoms approximately twice a month, and has had three unscheduled asthma visits for mild flares. Based on the National Asthma Education and Prevention Program guidelines , Mr. T has asthma that is not well controlled. 1
As a result of these symptoms, spirometry was performed revealing a forced expiratory volume in the first second (FEV1) of 78% predicted. Mr. T then was prescribed treatment with a low-dose corticosteroid, fluticasone 44 mcg at two puffs twice per day. However, he remained symptomatic and continued to use his rescue inhaler 3 times per week. Therefore, he was switched to a combination inhaled steroid and long-acting beta-agonist (LABA) (fluticasone propionate 250 mcg and salmeterol 50 mcg, one puff twice a day) by his primary care doctor.
Initial pulmonary assessment Even with this step up in his medication, Mr. T continued to be symptomatic and require rescue inhaler use. Therefore, he was referred to a pulmonologist, who performed the initial work-up shown here:
- Spirometry, pre-albuterol: FEV1 79%, post-albuterol: 12% improvement
- Methacholine challenge: PC 20 : 1.0 mg/mL
- Chest X-ray: Within normal limits
Continued pulmonary assessment His dose of inhaled corticosteroid (ICS) and LABA was increased to fluticasone 500 mcg/salmeterol 50 mcg, one puff twice daily. However, he continued to have symptoms and returned to the pulmonologist for further work-up, shown here:
- Chest computed tomography (CT): Normal lung parenchyma with no scarring or bronchiectasis
- Sinus CT: Mild mucosal thickening
- Complete blood count (CBC): Within normal limits, white blood cells (WBC) 10.0 K/mcL, 3% eosinophils
- Immunoglobulin E (IgE): 25 IU/mL
- Allergy-skin test: Positive for dust, trees
- Exhaled NO: Fractional exhaled nitric oxide (FeNO) 53 parts per billion (pbb)
Assessment for comorbidities contributing to asthma symptoms After this work-up, tiotropium was added to his medication regimen. However, he remained symptomatic and had two more flares over the next 3 months. He was assessed for comorbid conditions that might be affecting his symptoms, and results showed:
- Esophagram/barium swallow: Negative
- Esophageal manometry: Negative
- Esophageal impedance: Within normal limits
- ECG: Within normal limits
- Genetic testing: Negative for cystic fibrosis, alpha1 anti-trypsin deficiency
The ear, nose, and throat specialist to whom he was referred recommended only nasal inhaled steroids for his mild sinus disease and noted that he had a normal vocal cord evaluation.
Following this extensive work-up that transpired over the course of a year, Mr. T continued to have symptoms. He returned to the pulmonologist to discuss further treatment options for his refractory asthma.
Diagnosis Mr. T has refractory asthma. Work-up for this condition should include consideration of other causes for the symptoms, including allergies, gastroesophageal reflux disease, cardiac disease, sinus disease, vocal cord dysfunction, or genetic diseases, such as cystic fibrosis or alpha1 antitrypsin deficiency, as was performed for Mr. T by his pulmonary team.
Treatment options When a patient has refractory asthma, treatment options to consider include anticholinergics (tiotropium, aclidinium), leukotriene modifiers (montelukast, zafirlukast), theophylline, anti-immunoglobulin E (IgE) antibody therapy with omalizumab, antibiotics, bronchial thermoplasty, or enrollment in a clinical trial evaluating the use of agents that modulate the cell signaling and immunologic responses seen in asthma.
Treatment outcome Mr. T underwent bronchial thermoplasty for his asthma. One year after the procedure, he reports feeling great. He has not taken systemic steroids for the past year, and his asthma remains controlled on a moderate dose of ICS and a LABA. He has also been able to resume exercising on a regular basis.
Approximately 10% to 15% of asthma patients have severe asthma refractory to the commonly available medications. 2 One key aspect of care for this patient population is a careful workup to exclude other comorbidities that could be contributing to their symptoms. Following this, there are several treatment options to consider, as in recent years there have been several advances in the development of asthma therapeutics. 2
Treatment options for refractory asthma There are a number of currently approved therapies for severe, refractory asthma. In addition to therapy with ICS or combination therapies with ICS and LABAs, leukotriene antagonists have good efficacy in asthma, especially in patients with prominent allergic or exercise symptoms. 2 The anticholinergics, such as tiotropium, which was approved for asthma in 2015, enhance bronchodilation and are useful adjuncts to ICS. 3-5 Omalizumab is a monoclonal antibody against IgE recommended for use in severe treatment-refractory allergic asthma in patients with atopy. 2 A nonmedication therapeutic option to consider is bronchial thermoplasty, a bronchoscopic procedure that uses thermal energy to disrupt bronchial smooth muscle. 6,7
Personalizing treatment for each patient It is important to personalize treatment based on individual characteristics or phenotypes that predict the patient's likely response to treatment, as well as the patient's preferences and practical issues, such as adherence and cost. 8
In this case, tiotropium had already been added to Mr. T's medications and his symptoms continued. Although addition of a leukotriene modifier was an option for him, he did not wish to add another medication to his care regimen. Omalizumab was not added partly for this reason, and also because of his low IgE level. As his bronchoscopy was negative, it was determined that a course of antibiotics would not be an effective treatment option for this patient. While vitamin D insufficiency has been associated with adverse outcomes in asthma, T's vitamin D level was tested and found to be sufficient.
We discussed the possibility of Mr. T's enrollment in a clinical trial. However, because this did not guarantee placement within a treatment arm and thus there was the possibility of receiving placebo, he opted to undergo bronchial thermoplasty.
Bronchial thermoplasty Bronchial thermoplasty is effective for many patients with severe persistent asthma, such as Mr. T. This procedure may provide additional benefits to, but does not replace, standard asthma medications. During the procedure, thermal energy is delivered to the airways via a bronchoscope to reduce excess airway smooth muscle and limit its ability to constrict the airways. It is an outpatient procedure performed over three sessions by a trained physician. 9
The effects of bronchial thermoplasty have been studied in several trials. The first large-scale multicenter randomized controlled study was the Asthma Intervention Research (AIR) Trial , which enrolled patients with moderate to severe asthma. 10 In this trial, patients who underwent the procedure had a significant improvement in asthma symptoms as measured by symptom-free days and scores on asthma control and quality of life questionnaires, as well as reductions in mild exacerbations and increases in morning peak expiratory flow. 10 Shortly after the AIR trial, the Research in Severe Asthma (RISA) trial was conducted to evaluate bronchial thermoplasty in patients with more severe, symptomatic asthma. 11 In this population, bronchial thermoplasty resulted in a transient worsening of asthma symptoms, with a higher rate of hospitalizations during the treatment period. 11 Hospitalization rate equalized between the treatment and control groups in the posttreatment period, however, and the treatment group showed significant improvements in rescue medication use, prebronchodilator forced expiratory volume in the first second (FEV1) % predicted, and asthma control questionnaire scores. 11
The AIR-2 trial followed, which was a multicenter, randomized, double-blind, sham-controlled study of 288 patients with severe asthma. 6 Similar to the RISA trial, patients in the treatment arm of this trial experienced an increase in adverse respiratory effects during the treatment period, the most common being airway irritation (including wheezing, chest discomfort, cough, and chest pain) and upper respiratory tract infections. 6
The majority of adverse effects occurred within 1 day of the procedure and resolved within 7 days. 6 In this study, bronchial thermoplasty was found to significantly improve quality of life, as well as reduce the rate of severe exacerbations by 32%. 6 Patients who underwent the procedure also reported fewer adverse respiratory effects, fewer days lost from work, school, or other activities due to asthma, and an 84% risk reduction in emergency department visits. 6
Long-term (5-year) follow-up studies have been conducted for patients in both the AIR and the AIR-2 trials. In patients who underwent bronchial thermoplasty in either study, the rate of adverse respiratory effects remained stable in years 2 to 5 following the procedure, with no increase in hospitalizations or emergency department visits. 7,12 Additionally, FEV1 remained stable throughout the 5-year follow-up period. 7,12 This finding was maintained in patients enrolled in the AIR-2 trial despite decreased use of daily ICS. 7
Bronchial thermoplasty is an important addition to the asthma treatment armamentarium. 7 This treatment is currently approved for individuals with severe persistent asthma who remain uncontrolled despite the use of an ICS and LABA. Several clinical trials with long-term follow-up have now demonstrated its safety and ability to improve quality of life in patients with severe asthma, such as Mr. T.
Severe asthma can be a challenge to manage. Patients with this condition require an extensive workup, but there are several treatments currently available to help manage these patients, and new treatments are continuing to emerge. Managing severe asthma thus requires knowledge of the options available as well as consideration of a patient's personal situation-both in terms of disease phenotype and individual preference. In this case, the patient expressed a strong desire to not add any additional medications to his asthma regimen, which explained the rationale for choosing to treat with bronchial thermoplasty. Personalized treatment necessitates exploring which of the available or emerging options is best for each individual patient.
Published: April 16, 2018
- 1. National Asthma Education and Prevention Program: Asthma Care Quick Reference.
- 2. Olin JT, Wechsler ME. Asthma: pathogenesis and novel drugs for treatment. BMJ . 2014;349:g5517.
- 3. Boehringer Ingelheim. Asthma: U.S. FDA approves new indication for SPIRIVA Respimat [press release]. September 16, 2015.
- 4. Peters SP, Kunselman SJ, Icitovic N, et al. Tiotropium bromide step-up therapy for adults with uncontrolled asthma. N Engl J Med . 2010;363:1715-1726.
- 5. Kerstjens HA, Engel M, Dahl R. Tiotropium in asthma poorly controlled with standard combination therapy. N Engl J Med . 2012;367:1198-1207.
- 6. Castro M, Rubin AS, Laviolette M, et al. Effectiveness and safety of bronchial thermoplasty in the treatment of severe asthma: a multicenter, randomized, double-blind, sham-controlled clinical trial. Am J Respir Crit Care Med . 2010;181:116-124.
- 7. Wechsler ME, Laviolette M, Rubin AS, et al. Bronchial thermoplasty: long-term safety and effectiveness in patients with severe persistent asthma. J Allergy Clin Immunol . 2013;132:1295-1302.
- 8. Global Initiative for Asthma: Pocket Guide for Asthma Management and Prevention (for Adults and Children Older than 5 Years).
- 10. Cox G, Thomson NC, Rubin AS, et al. Asthma control during the year after bronchial thermoplasty. N Engl J Med . 2007;356:1327-1337.
- 11. Pavord ID, Cox G, Thomson NC, et al. Safety and efficacy of bronchial thermoplasty in symptomatic, severe asthma. Am J Respir Crit Care Med . 2007;176:1185-1191.
- 12. Thomson NC, Rubin AS, Niven RM, et al. Long-term (5 year) safety of bronchial thermoplasty: Asthma Intervention Research (AIR) trial. BMC Pulm Med . 2011;11:8.
Treatable traits and future exacerbation risk in severe asthma, baker’s asthma, the long-term trajectory of mild asthma, age, gender, & systemic corticosteroid comorbidities, ask the expert: william busse, md, challenges the current definition of the atopic march, considering the curveballs in asthma treatment, do mucus plugs play a bigger role in chronic severe asthma than previously thought, an emerging subtype of copd is associated with early respiratory disease.

- Open access
- Published: 17 January 2023
Primary care asthma surveillance: a review of knowledge translation tools and strategies for quality improvement
- Max Moloney ORCID: orcid.org/0000-0003-2293-910X 1 , 2 ,
- Geneviève Digby 2 ,
- Madison MacKinnon 1 , 2 ,
- Alison Morra 1 , 2 ,
- David Barber 3 , 4 ,
- John Queenan 3 ,
- Samir Gupta 5 , 6 ,
- Teresa To 7 , 8 &
- M. Diane Lougheed 1 , 2
Allergy, Asthma & Clinical Immunology volume 19 , Article number: 3 ( 2023 ) Cite this article
2583 Accesses
1 Citations
3 Altmetric
Metrics details
Viable knowledge translation (KT) strategies are increasingly sought to improve asthma diagnosis, particularly in primary care. Despite this understanding, practical KT tools to support primary care practitioners are not widely available. Electronic medical records (EMRs) offer an opportunity to optimize the diagnosis and surveillance of chronic diseases such as asthma, and support quality improvement initiatives that increase adherence to guideline-recommended care. This review aims to describe the current state of electronic KT electronic tools (eTools) and surveillance systems for asthma and identify opportunities to increase adherence to asthma diagnostic guidelines by implementing digital KT eTools.
Systematic literature searches were conducted on Ovid MEDLINE that included the search terms: asthma, asthma diagnosis, asthma surveillance, electronic health records, translational medical research, quality improvement, professional practice gaps, and primary health care published in the previous 10 years. In total, the searches returned 971 articles, 163 of which were considered relevant and read in full. An additional 28 articles were considered after reviewing the references from selected articles. 75 articles were included in this narrative review.
Established KT eTools for asthma such as electronic questionnaires, computerized clinical decision support systems (CDSS), chronic disease surveillance networks, and asthma registries have been effective in improving the quality of asthma diagnosis and care. As well, chronic disease surveillance systems, severe asthma registries, and workplace asthma surveillance systems have demonstrated success in monitoring asthma outcomes. However, lack of use and/or documentation of objective measures of lung function, challenges in identifying asthma cases in EMRs, and limitations of data sources have created barriers in the development of KT eTools. Existing digital KT eTools that overcome these data quality limitations could provide an opportunity to improve adherence to best-practice guidelines for asthma diagnosis and management.
Future initiatives in the development of KT eTools for asthma care should focus on strategies that assist healthcare providers in accurately diagnosing and documenting cases of asthma. A digital asthma surveillance system could support adherence to best-practice guidelines of asthma diagnosis and surveillance by prompting use of objective methods of confirmation to confirm an asthma diagnosis within the EMR.
Globally, the number of people diagnosed with asthma is over 340 million and has continually increased over a 10-year period [ 1 ]. Asthma is diagnosed based on a combination of patient history, physical examination, and objective tests. Asthma poses a significant burden on individuals and the health care system at-large. As the prevalence of asthma increases, the burden of asthma on healthcare systems around the world will also increase given that individuals with asthma use significantly greater health care resources than those without asthma, have a poorer quality of life, and have a higher chance of suffering from mental illness [ 2 , 3 , 4 , 5 ].
A major contributor to the burden of asthma on individuals and the healthcare system is that gaps exist between the published guidelines for asthma diagnosis and actual strategies for diagnosis used in primary care [ 4 , 6 ]. Although standards for asthma diagnosis are well established, less than half of individuals diagnosed with asthma have a confirmed diagnosis through the use of objective measurements of pulmonary function within two years of their original diagnosis [ 7 ]. Other challenges in the diagnosis of asthma include differentiating asthma from other respiratory conditions given the vast differential diagnosis for characteristic asthma symptoms [ 8 ]. These issues are compounded by a limited number of validated knowledge translation (KT) initiatives that could potentially support practitioners in the diagnosis and surveillance of asthma patients in primary care [ 9 ]. Incorporation of data element standards outlined in the Pan-Canadian Respiratory Standards Initiative for Electronic Health Records (PRESTINE) and specific indicators for asthma in primary care have created the possibility for improved asthma KT eTools in primary care by adopting these data standards [ 10 , 11 ].
KT is a term that describes the process of implementing the results of research into practice [ 12 ]. The process of KT has been organized through the development of the Knowledge to Action (KTA) Framework put forward by Graham et al. [ 13 ]. KTA is broken down into two distinct phases: Knowledge Creation and Knowledge Action. The Knowledge Creation phase involves analysis of the available research on the topic of interest from primary studies to systematic reviews. The Knowledge Action phase occurs simultaneously or after Knowledge Creation. Knowledge Action involves synthesizing available research, identifying potential barriers, and implementing interventions [ 13 ].
KT has become a priority for many stakeholders with an interest in improving asthma diagnosis and care as its approach to integrating research from various fields provides an opportunity to create new tools to assist practitioners, particularly in primary care [ 14 , 15 ]. KT initiatives are required for quality improvement in asthma care as research findings must be translated into usable interventions that create actionable behaviour change in physicians. By following the KTA framework, researchers can ensure novel research findings are implemented effectively to reach more health care providers and improve decision making [ 13 ].
Despite this understanding, practical KT tools to support primary care practitioners are not widely available. Electronic medical records (EMRs) offer an opportunity to improve practitioner performance and support quality improvement efforts by accurately identifying patients with asthma. This review aims to describe the current state of KT initiatives for asthma, to assess the limitations of KT tools for asthma and identify opportunities for how to improve asthma diagnosis and surveillance through digital innovations.
Systematic literature searches were conducted on Ovid MEDLINE and Ovid MEDLINE Daily Epub Ahead of Print, In-Process & Other Non-Indexed Citations. The first search criteria included the key words: “asthma”, “validation study”, and “electronic health records”. Supplemental searches included the terms: “translational medical research”, “asthma surveillance system” “asthma definition”, “primary health care”, “quality improvement”, and “professional practice gaps”. The search criteria included original articles; had a date restriction limiting results from 2012 to 2022; and was limited to articles in English. Studies referenced in research articles from the original literature search were also identified. Articles were reviewed if they fit within one of four themes: (1) electronic KT tools for asthma; (2) electronic asthma surveillance; (3) quality improvement of asthma diagnosis in primary care; and (4) gaps in asthma diagnosis and surveillance in primary care.
In total, the search returned 971 articles, of which each title and abstract were reviewed. Following review of title and abstract, a total of 163 articles were considered sufficiently relevant to review and were subsequently read in full. Of the 163 articles read in full, 43 were met one of the themes identified. An additional 28 articles were included from references of the articles read in full. After exclusion of articles that had outdated information and were either not relevant to the topic or contained redundant information, 75 articles were included.
Electronic knowledge translation tools for asthma diagnosis
There are two primary categories of KT eTools for the diagnosis of asthma: electronic questionnaires and clinical decision support systems (CDSS).
Electronic questionnaires
Electronic questionnaires can be used in a variety of health care settings to gather information important to asthma diagnosis and surveillance [ 16 , 17 ]. In clinical practice, questionnaires are generally used as a standardized assessment tool prior to meeting with a clinician. Due to their ease of use and low cost, questionnaires have been used to estimate the prevalence of asthma in children and adults around the world [ 18 ]. These questionnaires collect information including symptoms, asthma control, and quality of life [ 19 , 20 ]. A limitation of electronic questionnaires is the reliance on patients to accurately self-report symptoms and exacerbations, which introduces recall and other biases that could impact validity [ 21 , 22 ]. Another challenge of implementation is the limited uptake of questionnaires by clinicians and patients who are provided electronic questionnaires [ 23 , 24 ]. Overall, questionnaires have been shown to be effective for improving the diagnosis of asthma by gaining additional insight into patient symptoms and history, however difficulties related to accuracy and uptake of these electronic questionnaires remain.
Clinical decision support systems
There are currently several ongoing initiatives utilizing clinical decision support systems (CDSS) to improve asthma diagnosis. A CDSS is a KT eTool designed to improve health care delivery by enhancing medical decisions with targeted clinical knowledge, patient information, and other health data [ 25 ]. A currently operational CDSS for asthma is the Electronic Asthma Management System (eAMS) in use in Toronto, Ontario. eAMS is a computerized CDSS aimed at addressing major care gaps for adult asthma and has demonstrated effectiveness in improving rates of assessment of asthma control levels and other metrics important to diagnosis of asthma in Canada [ 26 ]. The eAMS collects data through a pre-visit questionnaire that patients complete on a tablet in the office and includes questions on symptom control, medication usage, triggers, and allergies. This information is then inputted into a CDSS system unique to eAMS which then creates an output for the physician highlighting asthma control status, medication changes recommendations, and an asthma action plan. These outputs are integrated into the clinician facing EMR system for use during the patient consultation. eAMS is a KT eTool that has demonstrated effectiveness in improving asthma action plan delivery, assessments of asthma control, and prescription of asthma medications [ 26 ]. The findings of the eAMS study demonstrate the potential for KT eTools to support quality improvement of asthma diagnosis and management in primary care.
Other CDSSs have been created in various jurisdictions over the past decade and have demonstrated the potential for eTools to improve asthma diagnosis and outcomes [ 27 ]. For example, AsthmaCritic developed in the Netherlands is a guideline-based provider critiquing system that uses information from EMRs to monitor and change practitioner behavior [ 28 ]. AsthmaCritic has demonstrated the ability to improve the number of PFTs administered and improve adherence to best-practice guidelines a randomized controlled trial [ 28 ]. Another KT eTool to assist in diagnosis is the Severe Asthma Algorithm (SAA), which assists health care providers in diagnosing severe asthma by using standardized data elements and decision support that prompts adherence with best practice guidelines for severe asthma diagnosis within an EMR [ 29 ]. Other CDSS studies have incorporated the development of algorithms using machine learning principles, which have the potential to uncover new risk factors and triggers of asthma using EMR data in an effort to improve diagnosis [ 30 ]. Despite their potential, CDSS for asthma experience similar limitations to questionnaires, including limited practitioner uptake and lack of utilization by patients [ 31 , 32 ].
Potential of electronic knowledge translation tools from a quality improvement perspective
KT eTools for asthma diagnosis have demonstrated effectiveness as a tool for quality improvement. Considering these interventions within the Hierarchy of Intervention Effectiveness, a framework that rates interventions related to human behaviour lower on a scale of effectiveness compared with system-focused interventions, both electronic questionnaires and CDSS are best categorized as people-focused interventions [ 33 ]. People-focused interventions require individuals to make conscious decisions to both use the intervention and subsequently alter their behaviour based on the information provided by the intervention to impact quality of care. As such, KT eTools that are people-focused are likely best used as components within larger system-focused eTools to improve asthma diagnosis. An opportunity exists to build upon these interventions by using EMR data to support system-focused interventions for quality improvement of asthma diagnosis in primary care.
Electronic knowledge translation tools for asthma surveillance
There are four primary categories of KT eTools for the surveillance of asthma: chronic disease surveillance networks, asthma registries, asthma quality of care monitoring systems, and work-related asthma surveillance systems.
Chronic disease surveillance networks
Chronic disease surveillance networks are a category of asthma KT eTool that exist at international, national, and regional levels [ 34 ]. An example of a chronic disease network is the Canadian Primary Care Sentinel Surveillance Network (CPCSSN) [ 35 ]. CPCSSN gathers information from physician billing codes, emergency department visits, and administrative data to facilitate standardized estimates of the incidence, prevalence, and outcomes related to various chronic diseases and are used by governments and researchers. Although CPCSSN collects data on a variety of chronic diseases, it does not currently collect data on adult asthma [ 35 ]. An example of a chronic disease surveillance network for asthma is the Ontario Asthma Surveillance Information System (OASIS) [ 36 ]. OASIS uses a previously validated asthma case definition derived from hospital administrative data in Ontario and provides a population-based longitudinal surveillance system for asthma. OASIS data is used to provide estimates of asthma incidence and prevalence, measures of asthma-related morbidity, mortality, health services use, and provider practice patterns using hospital administrative data to track quality of care over time [ 36 ]. Chronic disease surveillance networks are often limited by their source of data and restricted criteria for defining a case of the condition [ 37 ]. While limited criteria from health administrative data such as billing codes are sufficient for chronic diseases such as diabetes, for more complex and heterogenous conditions such as asthma, health administrative data often do not accurately reflect when and how diagnoses were made [ 38 ]. These chronic disease surveillance networks that are based on health administrative data have limitations on the amount and quality of data they can leverage to improve asthma diagnosis and surveillance.
Asthma registries
Registries have been effective tools to collect uniform data to evaluate specific outcomes for a population defined by a particular disease. Registries differ from surveillance networks in that registries use data that is voluntarily provided and entered while surveillance networks make use of pre-existing data. The scope of asthma registries has been limited to severe asthma, which represents a minority of total asthma cases in Canada and worldwide [ 39 , 40 ]. Severe asthma registries gather anonymous, longitudinal, real-life data for patients with severe asthma. To date, there are over 25 severe asthma registries, the majority of which operate at a national level and contribute information to the International Severe Asthma Registry (ISAR), the Severe Heterogenous Asthma Research collaboration, Patient-centered (SHARP), or the Severe Asthma Research Program (SARP) [ 41 , 42 , 43 ].The availability of health information varies greatly between databases [ 41 ]. In addition to ISAR, SHARP, and SARP there are several local and regional initiatives aimed at creating registries for cases other than severe asthma however, to date there remains no asthma registry for general asthma patients, only severe asthma patients [ 44 ]. The result of this is a lack of centralized information worldwide for general asthma patients, which creates a significant challenge to monitor the state of asthma at a national or international level.
Asthma quality of care monitoring systems
Quality of care monitoring systems for asthma are emerging as useful eTools for KT. Audit and feedback systems for quality improvement of chronic disease can be effective in creating behaviour changes in health care providers when successfully implemented and can monitor quality of care over time [ 45 ]. There are currently several initiatives centered around creating quality of care monitoring systems for asthma in different forms. The Asthma Care Map (ACM) developed by the Lung Health Foundation (formerly the Ontario Lung Association) and used in the Primary Care Asthma Program (PCAP) is a paper KT tool for asthma quality of care [ 46 ] that is currently being adapted to integrate within primary care EMRs. Advancements in computing and data quality within EMRs have given rise to the potential for electronic systems to aid in the surveillance of chronic diseases, with the end goal of improving patient outcomes [ 47 ]. One example of a management and monitoring system that can be used for quality improvement is the Asthma Management and Outcomes Monitoring System (AMOMS) [ 48 ]. AMOMS is a point-of-care charting tool the prompts providers to document care in accordance with best practice guidelines. In doing so, AMOMS collects data from the patient and physician that can be extracted and used to support performance measurement, benchmarking, and quality improvement. Another quality of care monitoring system for asthma developed by Tomasallo et al. ( 49 ) found their EMR-based asthma surveillance system can be used to estimate the prevalence of asthma in both adults and children across time and was able to identify patients at risk of asthma in 50% more cases than traditional telephone surveys [ 49 ]. Despite the potential for quality of care monitoring systems to improve adherence to best-practice guidelines and patient outcomes, this literature review did not identify any quality of care monitoring systems for asthma that have achieved scale at a national or international level.
Work-related asthma surveillance systems
There are several surveillance systems dedicated to work-related asthma (WRA). WRA surveillance programs have been active for over 20 years in jurisdictions ranging from local to national in scale [ 50 , 51 ]. Similar to chronic disease surveillance networks, there are also occupational disease surveillance systems that record the incidence, prevalence, and outcomes of WRA [ 52 ]. WRA surveillance systems have been effective in supporting individuals with WRA; however, their scale is often limited by the source of the data and represents only a fraction of total asthma cases. In addition, previous efforts to develop workplace asthma surveillance systems have relied on practitioner reporting and have reported low uptake rates.[ 53 ] Due to their small scale and limited sources of data, WRA surveillance systems are unlikely to scale to the national or international level.
Potential of electronic knowledge translation tools for asthma surveillance from a quality improvement perspective
Within the Hierarchy of Intervention Effectiveness, system-focused interventions have been demonstrated to be more effective in producing behaviour change in health care providers.[ 33 ] Several of the described KT eTools for asthma surveillance bring a system-level component through standardized data collection, computerized registries, and automated data reporting. However, these eTools are primarily used for research purposes and population health analyses, and are not necessarily used at the point of care to drive change on an individual patient level. An opportunity exists to utilize improved EMR data and advances in computing to support surveillance interventions for quality improvement of asthma care at the patient level.
Opportunities for KT eTools in diagnosis and surveillance of asthma
To date, the majority of KT eTools for asthma have required users to make conscious changes to their behaviours in order to use the tools, requiring change in daily routines and practices. This serves as a significant barrier to the adoption of eTools for asthma diagnosis and surveillance. Future KT eTools should leverage improvements in EMRs to reduce cognitive load on physicians, automate decision making, and be embedded within the EMR to facilitate adherence to best-practice guidelines for asthma diagnosis and surveillance using a system-focused approach. The following is a summary of opportunities in the development of KT eTools to improve asthma diagnosis and surveillance.
An excellent opportunity exists for asthma KT eTool development by leveraging EMRs to support evidence-based diagnosis, surveillance, and quality improvement [ 48 , 54 ]. EMR-based tools also have the added benefit of potentially reducing friction between practitioners and the eTool through automation, a system-level intervention as per the Hierarchy of Intervention Effectiveness. This opportunity to improve asthma diagnosis and surveillance is most relevant to primary care practitioners, who face numerous challenges in keeping up with updated guidelines and effectively integrating them into their practice. As a result, KT eTools that involve decision support may improve adherence to evidence-based guidelines and improve outcomes [ 55 ].
To improve the accuracy of asthma diagnoses and the overall quality of asthma patient care, KT eTools within EMRs should be developed that reinforce evidence-based guidelines for asthma diagnosis, particularly in primary care. The symptoms of asthma are similar to several other obstructive lung diseases, in particular chronic obstructive pulmonary disease (COPD) and asthma-COPD overlap syndrome (ACOS). This adds another layer of difficulty in developing an EMR case definition that detects cases of asthma and is able to discriminate asthma from other respiratory conditions [ 56 ]. To account for these challenges, new KT eTools should support asthma diagnosis to include objective evidence of asthma confirmation through PFTs within the EMR [ 57 ]. Additionally, information to support quality improvement should be optimized to reduce the cognitive load on the health care provider, which has proven to increase the effectiveness of surveillance systems and EMR tools in practice [ 58 ]. Fully embedding a new KT eTool within the EMR is an excellent opportunity to facilitate adherence to best-practice guidelines for asthma diagnosis and surveillance.
Surveillance tools
KT eTools for asthma surveillance present a unique opportunity to improve provider diagnosis of asthma by promoting adherence to best-practice guidelines. Emphasis should be placed on addressing specific gaps in asthma diagnosis, such as the lack of objective measurements to confirm asthma. Surveillance eTools have the ability to provide a multitude of surveillance metrics that practitioners can track to improve their practices [ 59 ]. Surveillance tools also have the ability to prompt actionable, individualized feedback to facilitate adherence to best practice guidelines [ 60 ]. An optimal surveillance system to improve the quality of asthma care necessitates an accurate diagnosis of asthma to monitor patients over time and effectively change provider behaviour. With an accurate diagnosis of confirmed asthma, surveillance tools have the potential to greatly improve adherence to best-practice guidelines.
PRESTINE data elements
There are a variety of KT eTools that have been developed for diagnosis, education, and management of asthma that have been shown to improve outcomes for individuals with asthma despite data source limitations. These previous innovations or new KT eTools have the potential to be improved by utilizing data element standards outlined in the Pan-Canadian Respiratory Standards Initiative for Electronic Health Records (PRESTINE) [ 10 ]. PRESTINE is a set of data elements and definitions recommended by experts for inclusion in EMRs to support primary, secondary, and tertiary care for respiratory conditions, including asthma to enable monitoring, benchmarking, and performance evaluation. Adopting the PRESTINE data elements for asthma into primary care have created the possibility for new asthma eTools in primary care [ 11 ]. Adoption of these data standards into new KT eTools and EMRs could be beneficial in allowing eTools to distinguish between confirmed and suspected asthma.
Limitations of KT eTools for asthma diagnosis and surveillance
In order to consider the specific limitations of KT eTools in the diagnosis and surveillance of asthma, we need to understand the broader system factors that lead to suboptimal diagnosis of asthma in primary care. Previous publications, including a recent publication by Yamada et al. have identified several such barriers that can be categorized in the following themes: knowledge, skills, social/professional role and identity, beliefs about capabilities, reinforcement, intentions, goals, memory and decision processes, environmental context and resources, social influences, emotions, and behavioural regulation [ 61 ]. Figure 1 provides a root cause analysis for suboptimal asthma diagnosis in primary care, outlining several of these key factors. These barriers stem from a variety of sources, including availability of equipment and materials, culture, government policies, process and procedures for diagnosing patients, and people factors, all of which contribute to barriers to diagnosis of asthma in primary care (Fig. 1 ). We will consider these barriers in more detail below, in order to identify the potential for KT eTools to contribute to the necessary mitigating strategies to improve quality of asthma diagnosis and care.
Barriers to optimal asthma diagnosis in primary care
Lack of confirmation of asthma diagnosis
KT eTools for surveillance rely first on ensuring patients have an accurate diagnosis of their condition by a health care provider. The gold-standard definition of asthma, as outlined by the Canadian Thoracic Society and Global Initiative for Asthma, requires objective measurement of lung function or airway responsiveness using pulmonary function tests [ 57 , 62 , 63 ]. Reliance on clinical history without the support of objective measurements leads to misdiagnosis of asthma in 33% of cases [ 64 ]. While rates of use of pulmonary function tests vary widely based on jurisdiction, objective measurements are not widely utilized by primary care providers in asthma diagnosis [ 7 ]. Underutilization of objective measurements to diagnose asthma in primary care sites can lead to both underdiagnosis and overdiagnosis of asthma [ 65 ]. Individuals who suffer from asthma but have not received a diagnosis continue to struggle with symptom management and further contribute to the burden of asthma on the health care system through underdiagnosis [ 66 ]. Likewise, overdiagnosis of asthma also compounds the effects on the health care system through unnecessary patient visits and medication prescriptions [ 65 ].
In primary care, one of the greatest contributors to misdiagnosis stems from this lack of objective pulmonary function measurement in the process of diagnosing a patient with asthma [ 57 , 62 ]. The use of objective measurements such as spirometry, methacholine challenge tests, and exercise challenge tests are crucial to ensuring the confirmation of an asthma diagnosis. Without completion of objective measures confirming asthma diagnoses, the accuracy of EMR data for patients labelled with asthma may not be valid. This creates barriers for researchers in creating KT eTools for asthma surveillance as many charts billed for asthma are in fact suspected asthma and not confirmed asthma.
Gaps in case definitions of asthma in EMRs
The gold-standard definition for the diagnosis of asthma is well established and provides clear guidance for practitioners to make an accurate diagnosis of asthma for patients with a clinical suspicion of the condition. There have been several attempts to translate the evidence-based clinical definition of asthma into a case definition to incidences of asthma in EMRs, however, no consensus has been reached [ 67 ]. Al Sallakh et al. ( 67 ) conducted an extensive review of attempts to define asthma using electronic health record data [ 67 ]. The review analyzed a total of 76 case definitions to identify asthma in EMRs and found significant heterogeneity in the case definitions proposed. This review found that for case definitions to be effective, they must be tailored to the EMR environment in which they function and consider the charting techniques of the practitioners who use the EMR. In Canada, there have been recent attempts to create a case definition for asthma suitable for Canadian EMR vendors and primary care practitioners. Previous efforts to create and validate case definitions for asthma have come from a single EMR or restricted data environments [ 68 , 69 ]. Xi et al. ( 68 ) proposed a variety of case definitions using similar search fields with the addition of a search for asthma in the free text portion of the EMR and found a case definition of asthma that had a sensitivity of 90.2%, and a specificity of 83.9% [ 68 ]. Another recent publication from Cave et al. ( 69 ) conducted a study to validate a case definition for asthma using data from the Southern Alberta Primary Care Research Network, a node of the CPCSSN (SAPCReN-CPCSSN) [ 69 ]. The authors created a case-finding algorithm using a combination of search fields from the EMR including billing information, recorded encounter diagnosis information, and information inputted into a health condition field within the EMR. Cave et al. (2020) compared their algorithm against expert physician review of patient charts and found a sensitivity of 83.3% and specificity of 99.3%. Case definitions for asthma in EMRs have been proposed however, the limits of their generalizability remain unknown, as attempts have been limited to single EMRs in single jurisdictions and restricted data environments that do not have the ability to use all available data to make an accurate diagnosis.
Gaps in validation of case definitions for asthma
The reporting and validation of proposed case definitions within EMRs are critical to be able to draw reliable conclusions from the results of studies that derive their data from EMRs [ 70 ]. Determining the validity of the case definition of diseases such as asthma is more challenging than many other chronic conditions [ 71 ]. Asthma case definitions require a complex combination of symptom assessment, pulmonary function tests, and practitioner interpretation of the objective measurements. Meanwhile, other chronic conditions such as diabetes can be confirmed through a single blood test. Previous research has suggested several methods for creating and validating case definitions, including manual validation, comparison to external databases, comparison of rates in similar populations, and machine learning algorithms [ 72 ]. The preferred method for ensuring the accuracy of reference diagnoses when establishing cases of asthma is manual chart review, however manual reviews are labour-intensive and often involve additional methods to protect confidentiality that constitute barriers to this methodology [ 46 ]. Several studies assessed in this review contained minimal or no information on the methodology used in validating their proposed case definition for asthma. Overall, the information available on the methodology and results of previously published case definition validation studies for asthma is suboptimal. Thus, additional work is required to appropriately validate a case definition for asthma in which the methodology can be replicated.
Limitations of data sources
An important aspect of improving asthma outcomes is high quality datasets from which to derive health information to inform KT eTools for asthma diagnosis and surveillance [ 73 ]. A critical barrier to the scalability of asthma eTools are sources of accurate data. Most KT eTools described in this review originate from the local context in which they were created. This can limit the scalability of the eTools to other settings. The majority of KT eTools that have been developed are only functional in one EMR environment or derive information from health administrative data, which is limited in detail [ 38 , 67 , 68 , 69 ]. While these tools may be effective in a local context, the inability to scale tools across multiple EMRs and the limited information provided by health administrative data create limitations on the ability of these KT eTools to scale to the national and international level. Furthermore, the quality of data available for eTools within the data sources serves as another potential limitation in the development of eTools for asthma. Inconsistencies in practitioner charting behaviours for asthma, particularly in primary care, can have a negative impact on the quality of data that informs eTools [ 74 ]. Further studies have demonstrated the high variability and generally low quality of information inputted into various EMR fields, affecting the completeness of data available to these eTools [ 75 ]. Adopting PRESTINE data elements in EMRs could improve upon the data sources currently available KT eTools.
Setting of KT eTool implementation
Another barrier to the development of KT eTools for asthma is that proposed KT eTools are often designed for different purposes depending on the health care setting or the purposes of the original study from which the definition was derived [ 67 ]. Many studies designing case definitions for asthma to incorporate into eTools have a wide range of sensitivity, specificity, positive predictive value, and negative predictive value. This variation and how it affects the selection of a case definition is important because a case definition with a high sensitivity is key to identifying all cases of asthma within a database, but if excluding non-cases is the area of interest then a high specificity is more important. Likewise, there is a trade-off between PPV and NPV in which one statistic can be more important than the other depending on whether the aim of the study is to determine true positives or true negatives. As a result, testing multiple case definitions to determine the case definition for asthma that is best suited for KT intervention which it will be used for is crucial to ensure the best case definition for the purpose of the intervention is selected.
KT eTools from a quality improvement perspective
A quality improvement approach to optimizing asthma diagnosis in primary care requires tackling the numerous root causes identified above. If implemented at a system-level, in a standardized, automated, and computerized manner, the KT eTools outlined in this paper have the potential to target several of these root causes, particularly where decision making at the physician level is required. Sophisticated KT eTools could include automation and forced functioning to ensure spirometric confirmation of an asthma diagnosis. However, KT eTools alone will not be able to overcome all of the barriers to confirmatory testing with spirometry, such as availability of testing facilities or policy level factors that impact decision making. Yet, available data from existing KT eTools, registries, surveillance systems, and quality of care monitoring systems can be used to leverage policy level changes that could further alleviate barriers to optimal asthma diagnosis in primary care. Ultimately, future KT eTools and the strategies by which they are implemented, must be leveraged to address the identified barriers to improve patient outcomes in asthma.
This review identifies opportunities to improve the accuracy of asthma diagnosis and surveillance through the use of KT eTools to improve the quality of asthma care. The key barriers to effective KT for asthma using EMR data are lack of documentation of confirmation of an asthma diagnosis, challenges related to creating a valid EMR case definition for asthma in the absence of this documentation, and the limitations of data sources that can inform KT eTools. Limited access to and use of pulmonary function tests and specialist consultation contribute to misdiagnosis and suboptimal management. Existing KT tools for asthma have been limited in scope and many fail to address barriers and challenges in primary care, where the majority of asthma diagnoses are made. As a result, future research should focus on KT initiatives that integrate surveillance systems that can be used with multiple EMR vendors with system-level quality improvement strategies to improve health care provider adherence with guideline-recommended care on a national and international scale. By promoting and documenting accurate asthma diagnoses, KT tools and surveillance systems based on reliable EMR case definitions can be used for performance evaluation and optimization of asthma care.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
Asthma Care Map
Asthma Management and Outcomes Monitoring System
Asthma Research Unit
Clinical Decision Support System
Canadian Primary Care Sentinel Surveillance Network
Canadian Thoracic Society
Electronic Asthma Management System
Electronic Asthma Performance Indicator Reporting System
Electronic Asthma Quality of Life Questionnaires
Electronic medical record
Electronic tool
Knowledge translation
Knowledge to action
Ontario Asthma Surveillance Information System
Provider Asthma Assessment Form
Primary Care Asthma Performance Indicators
Pulmonary function test
Pan-Canadian Respiratory Standards Initiative for Electronic Health Records
Work-Related Asthma Screening Questionnaire-Long Version
Vos T, Abajobir AA, Abbafati C, Abbas KM, Abate KH, Abd-Allah F, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1211–59.
Article Google Scholar
Ivanova JI, Bergman R, Birnbaum HG, Colice GL, Silverman RA, McLaurin K. Effect of asthma exacerbations on health care costs among asthmatic patients with moderate and severe persistent asthma. J Allergy Clin Immunol. 2012;129(5):1229–35.
Gold LS, Thompson P, Salvi S, Faruqi RA, Sullivan SD. Level of asthma control and health care utilization in Asia-Pacific countries. Respir Med. 2014;108(2):271–7.
Klomp H, Lawson JA, Cockcroft DW, Chan BT, Cascagnette P, Gander L, et al. Examining asthma quality of care using a population-based approach. CMAJ. 2008;178(8):1013–21.
Becerra BJ, Banta JE, Ghamsary M, Martin LR, Safdar N. Burden of mental illness on hospital and patient outcomes among asthma hospitalizations. J Asthma. 2016;53(4):392–7.
Brigham EP, West NE. Diagnosis of asthma: diagnostic testing. Int Forum Allergy Rhinol. 2015;5(S1):S27-30.
Gershon AS, Victor JC, Guan J, Aaron SD, To T. Pulmonary function testing in the diagnosis of asthma: a population study. Chest. 2012;141(5):1190–6.
Miravitlles M, Andreu I, Romero Y, Sitjar S, Alteś A, Anton E. Difficulties in differential diagnosis of COPD and asthma in primary care. Br J Gen Pract. 2012;62(595):e68-75.
Licskai C, Sands T, Ong M, Paolatto L, Nicoletti I. Using a knowledge translation framework to implement asthma clinical practice guidelines in primary care. Int J Qual Heal Care. 2012;24(5):538–46.
Lougheed M, Minard J, Dworkin S, Juurlink M-A, Temple WJ, To T, et al. Pan-Canadian respiratory standards initiative for electronic health records (PRESTINE): 2011 National forum proceedings. Can Respir J. 2012;19(2):117–26.
Lougheed MD, Taite A, ten Hove J, Morra A, Van Dam A, Ducharme FM, et al. Pan-Canadian asthma and COPD standards for electronic health records: a Canadian thoracic society expert working group report. Can J Respir Crit Care, Sleep Med. 2018;2(4):244–50.
Google Scholar
Grimshaw JM, Eccles MP, Lavis JN, Hill SJ, Squires JE. Knowledge translation of research findings. Implement Sci. 2012;7(1):50.
Graham ID, Logan J, Harrison MB, Straus SE, Tetroe J, Caswell W, et al. Lost in knowledge translation: time for a map? J Contin Educ Health Prof. 2006;26(1):13–24.
Agache I, Annesi-Maesano I, Bonertz A, Branca F, Cant A, Fras Z, et al. Prioritizing research challenges and funding for allergy and asthma and the need for translational research—The European Strategic Forum on Allergic Diseases. Allergy Eur J Allergy Clin Immunol. 2019;74(11):2064–76.
Bisgaard H, Bonnelykke K. Long-term studies of the natural history of asthma in childhood. J Allergy Clin Immunol. 2010;126(2):187–97.
Olajos-Clow J, Minard J, Szpiro K, Juniper EF, Turcotte S, Jiang X, et al. Validation of an electronic version of the mini asthma quality of life questionnaire. Respir Med. 2010;104(5):658–67.
Article CAS Google Scholar
Killorn KR, Dostaler SM, Olajos-Clow J, Turcotte SE, Minard JP, Holness DL, et al. The development and test re-test reliability of a work-related asthma screening questionnaire. J Asthma. 2014;52(3):279–88.
Voorend-Van Bergen S, Vaessen-Verberne AA, De Jongste JC, Pijnenburg MW. Asthma control questionnaires in the management of asthma in children a review. Pediatr Pulmonol. 2015;50(2):202–8.
Juniper E, O’Byrne P, Guyatt G, Ferrie P, King D. Development and validation of a questionnaire to measure asthma control. Eur Respir J. 1999;14(4):902–7.
Pralong JA, Moullec G, Suarthana E, Gérin M, Gautrin D, L’Archevêque J, et al. Screening for occupational asthma by using a self-administered questionnaire in a clinical setting. J Occup Environ Med. 2013;55(5):527–31.
Carroll W. Limitations of asthma control questionnaires in the management and follow up of childhood asthma. Paediatr Respir Rev. 2013;14(4):229–31.
Kouri A, Yamada J, Sale JEM, Straus SE, Gupta S. Primary care pre-visit electronic patient questionnaire for asthma: uptake analysis and predictor modeling. J Med Internet Res. 2020;22(9):e19358.
Yamada J, Kouri A, Simard SN, Segovia SA, Gupta S. Barriers and enablers to using a patient-facing electronic questionnaire: a qualitative theoretical domains framework analysis. J Med Internet Res. 2020;22(10):e19474.
Sutton RT, Pincock D, Baumgart DC, Sadowski DC, Fedorak RN, Kroeker KI. An overview of clinical decision support systems: benefits, risks, and strategies for success. Digit Med. 2020;3(1):1–10.
Gupta S, Price C, Agarwal G, Chan D, Goel S, Boulet LP, et al. The Electronic Asthma Management System (eAMS) improves primary care asthma management. Eur Respir J. 2019;53(4):241–52.
Bell LM, Grundmeier R, Localio R, Zorc J, Fiks AG, Zhang X, et al. Electronic health record based decision support to improve asthma care: a cluster-randomized trial. Pediatrics. 2010;125(4):770–7.
Kuilboer MM, Van Wijk MAM, Mosseveld M, Van Der Does E, De Jongste JC, Overbeek SE, et al. Computed critiquing integrated into daily clinical practice affects physicians’ behavior: a randomized clinical trial with asthma critic. Methods Inf Med. 2006;45(4):447–54.
Lougheed MD, Morra A, Bullock E, Tregobov N, Barber D, Boulet LP, et al. Pan-Canadian standards for severe asthma in electronic medical records. Can J Respir Crit Care, Sleep Med. 2021;5(6):391–9.
Zhang W, Ram S. A comprehensive analysis of triggers and risk factors for asthma based on machine learning and large heterogeneous data sources. Manag Inf Syst. 2020;44(1):305–49.
Lam Shin Cheung J, Paolucci N, Price C, Sykes J, Gupta S. A system uptake analysis and guides checklist evaluation of the electronic asthma management system a point-of-care computerized clinical decision support system. J Am Med Informatics Assoc. 2020;27(5):726–37.
Bousquet J. Electronic clinical decision support system (eCDSS) in the management of asthma: from theory to practice. Eur Respir J. 2019;53(4):1900339.
Cafazzo JA, St-Cyr O. From discovery to design: the evolution of human factors in healthcare. Healthc Q. 2012;15(1):24–9.
Lix LM, Ayles J, Bartholomew S, Cooke CA, Ellison J, Emond V, et al. The Canadian chronic disease surveillance system: a model for collaborative surveillance. Int J Popul Data Sci J. 2018;3(3):433–45.
Garies S, Birtwhistle R, Drummond N, Queenan J, Williamson T. Data resource profile: national electronic medical record data from the Canadian primary care sentinel surveillance network (CPCSSN). Int J Epidemiol. 2017;46(4):1091–9.
Crighton EJ, Feng J, Gershon A, Guan J, To T. A spatial analysis of asthma prevalence in Ontario. Can J Public Heal. 2012;103(5):384–9.
Klompas M, Cocoros NM, Menchaca JT, Erani D, Hafer E, Herrick B, et al. State & local chronic disease surveillance using electronic health record systems. Am J Public Health. 2017;107(9):1406–12.
Gershon AS, Wang C, Guan J, Vasilevska-Ristovska J, Cicutto L, To T. Identifying patients with physician-diagnosed asthma in health administrative databases. Can Respir J. 2009;16(6):183–8.
Newby C, Heaney LG, Menzies-Gow A, Niven RM, Mansur A, Bucknall C, et al. Statistical cluster analysis of the British thoracic society severe refractory asthma registry: clinical outcomes and phenotype Stability. PLoS ONE. 2014;9(7):e102987.
Enilari O, Sinha S. The global impact of asthma in adult populations. Ann Glob Health. 2019;85(1):1–7.
Bulathsinhala L, Eleangovan N, Heaney LG, Menzies-Gow A, Gibson PG, Peters M, et al. Development of the international severe asthma registry (ISAR): a modified delphi study. J Allergy Clin Immunol Pract. 2019;7(2):578–88.
van Bragt JJMH, Hansen S, Djukanovic R, Bel EHD, ten Brinke A, Wagers SS, et al. SHARP: enabling generation of real-world evidence on a pan-European scale to improve the lives of individuals with severe asthma. ERJ Open Res. 2021;7(2):64–77.
Wenzel SE, Busse WW. Severe asthma: lessons from the severe asthma research program. J Allergy Clin Immunol. 2007;119(1):14–21.
Gliklich RE, Castro M, Leavy MB, Press VG, Barochia A, Carroll CL, et al. Harmonized outcome measures for use in asthma patient registries and clinical practice. J Allergy Clin Immunol. 2019;144(3):671–81.
Ivers N, Jamtvedt G, Flottorp S, Young JM, Odgaard-Jensen J, French SD, et al. Audit and feedback: effects on professional practice and healthcare outcomes. Cochrane Database Syst Rev. 2012;2012(6):59–65.
Minard JP, Dostaler SM, Taite AK, Olajos-Clow JG, Sands TW, Licskai CJ, et al. Development and implementation of an electronic asthma record for primary care: integrating guidelines into practice. J Asthma. 2014;51(1):58–68.
Williamson T, Green ME, Birtwhistle R, Khan S, Garies S, Wong ST, et al. Validating the 8 CPCSSN case definitions for chronic disease surveillance in a primary care database of electronic health records. Ann Fam Med. 2014;12(4):367–72.
Taite A, Podgers D, Olajos-Clow J, Patricia M, Schooley J, Day A, et al. 2017 Enabling asthma management and outcomes monitoring through standardized EMR data and eTools. Eur Respir J. 2017;50(61):PA2778
Tomasallo CD, Hanrahan LP, Tandias A, Chang TS, Cowan KJ, Guilbert TW. Estimating Wisconsin asthma prevalence using clinical electronic health records and public health data. Am J Public Health. 2014;104(1):65–73.
White GE, Seaman C, Filios MS, Mazurek JM, Flattery J, Harrison RJ, et al. Gender differences in work-related asthma: surveillance data from California, Massachusetts, Michigan, and New Jersey, 1993–2008. J Asthma. 2014;51(7):691–702.
Kwon SC, Song J, Kim Y, Calvert GM. Work-related asthma in Korea—findings from the korea work-related asthma surveillance (KOWAS) program, 2004–2009. Allergy Asthma Immunol Res. 2014;7(1):51–9.
Logar-Henderson C, MacLeod JS, Arrandale VH, Linn Holness D, McLeod CB, Peter A, et al. Adult asthma among Workers in Ontario: results from the occupational disease surveillance system. Ann Am Thorac Soc. 2019;16(5):563–71.
To T, Tarlo SM, McLimont S, Haines T, Holness DL, Lougheed MD, et al. Feasibility of a provincial voluntary reporting system for work-related asthma in Ontario. Can Respir J. 2011;18(5):275–7.
Fiks AG. Designing computerized decision support that works for clinicians and families. Curr Probl Pediatr Adolesc Health Care. 2011;41(3):60–88.
Cloutier MM, Schatz M, Castro M, Clark N, Kelly HW, Mangione-Smith R, et al. Asthma outcomes: composite scores of asthma control. J Allergy Clin Immunol. 2012;129(3):24-S33.
Van TN, Park HY, Nakano Y. Asthma–COPD overlap syndrome (ACOS): a diagnostic challenge. Respirology. 2016;21(3):410–8.
Yang CL, Hicks EA, Mitchell P, Reisman J, Podgers D, Hayward KM, et al. Canadian Thoracic Society 2021 Guideline update: diagnosis and management of asthma in preschoolers, children and adults. Can Respir J. 2021;5(6):348–61.
Brehaut JC, Colquhoun HL, Eva KW, Carroll K, Sales A, Michie S, et al. Practice peedback interventions: 15 suggestions for optimizing effectiveness. Ann Intern Med. 2016;164(6):15–22.
Straus SE, Tetroe J, Graham I. Defining knowledge translation. CMAJ. 2009;181(3):165–8.
Brehaut JC, Eva KW. Building theories of knowledge translation interventions: use the entire menu of constructs. Implement Sci. 2012;7(1):114–22.
Yamada J, Lam Shin Cheung J, Gagne M, Spiegel-Feld C, Aaron SD, FitzGerald JM, et al. Barriers and enablers to objective testing for asthma and COPD in primary care a systematic review using the theoretical domains framework. Chest. 2022;161(4):888–905.
Lougheed MD, Lemiere C, Ducharme FM, Licskai C, Dell SD, Rowe BH, et al. Canadian Thoracic Society 2012 guideline update: diagnosis and management of asthma in preschoolers, children and adults. Can Respir J. 2012;19(2):127–64.
Reddel HK, Bacharier LB, Bateman ED, Brightling CE, Brusselle GG, Buhl R, et al. Global Initiative for Asthma strategy 2021 executive summary and rationale for key changes. Am J Respir Crit Care Med. 2022;205(1):17–35.
Aaron SD, Vandemheen KL, Boulet LP, McIvor RA, FitzGerald JM, Hernandez P, et al. Overdiagnosis of asthma in obese and nonobese adults. CMAJ. 2008;179(11):1121–31.
Aaron SD, Boulet LP, Reddel HK, Gershon AS. Underdiagnosis and overdiagnosis of asthma. Am J Respir Crit Care Med. 2018;198(8):1012–20.
Gershon AS, Wang C, Guan J, To T. Burden of comorbidity in individuals with asthma. Thorax. 2010;65(7):612–8.
Al Sallakh MA, Vasileiou E, Rodgers SE, Lyons RA, Sheikh A, Davies GA. Defining asthma and assessing asthma outcomes using electronic health record data: a systematic scoping review. Eur Respir J. 2017;49(6):170–204.
Xi N, Wallace R, Agarwal G, Chan D, Gershon A, Gupta S. Identifying patients with asthma in primary care electronic medical record systems: Chart analysis-based electronic algorithm validation study. Can Fam Physician. 2015;61(10):474–83.
Cave AJ, Soos B, Gillies C, Drummond N, Pham ANQ, Williamson T. Validating a case definition for adult asthma in primary care electronic medical records. Prim Care Respir Med. 2020;30(1):1–4.
Nissen F, Quint JK, Wilkinson S, Mullerova H, Smeeth L, Douglas IJ. Validation of asthma recording in electronic health records: a systematic review. Clin Epidemiol. 2017;1(9):643–56.
Desai JR, Wu P, Nichols GA, Lieu TA, O’Connor PJ. Diabetes and asthma case identification, validation, and representativeness when using electronic health data to construct registries for comparative effectiveness and epidemiologic research. Med Care. 2012;50(1):30–5.
Nissen F, Quint JK, Morales DR, Douglas IJ. How to validate a diagnosis recorded in electronic health records. Breathe. 2019;15(1):64–8.
Minard JP, Turcotte SE, Lougheed MD. Asthma electronic medical records in primary care: an integrative review. J Asthma. 2010;47(8):895–912.
Singer A, Yakubovich S, Kroeker AL, Dufault B, Duarte R, Katz A. Data quality of electronic medical records in Manitoba: do problem lists accurately reflect chronic disease billing diagnoses? J Am Med Informatics Assoc. 2016;23(6):1107–12.
Evans RS. Electronic health records: then, now, and in the future. Yearb Med Inform. 2016;1(1):48–64.
Download references
Acknowledgements
Not applicable.
Funding for this work is supported by the Southeastern Ontario Academic Medical Organization’s Innovation Fund.
Author information
Authors and affiliations.
Asthma Research Unit, Kingston Health Sciences Centre, Kingston, ON, Canada
Max Moloney, Madison MacKinnon, Alison Morra & M. Diane Lougheed
Division of Respirology, Department of Medicine, Queen’s University, Kingston, ON, Canada
Max Moloney, Geneviève Digby, Madison MacKinnon, Alison Morra & M. Diane Lougheed
Department of Family Medicine, Queen’s University, Kingston, ON, Canada
David Barber & John Queenan
Canadian Primary Care Sentinel Surveillance Network (Eastern Ontario Network), Kingston, ON, Canada
David Barber
Division of Respirology, Department of Medicine, St. Michael’s Hospital, Toronto, ON, Canada
Samir Gupta
Li Ka Shing Knowledge Institute, St. Michael’s Hospital, University of Toronto, Toronto, ON, Canada
Child Health Evaluative Science, Research Institute, The Hospital for Sick Children, Toronto, ON, Canada
Dalla Lana School of Public Health, University of Toronto, Toronto, ON, Canada
You can also search for this author in PubMed Google Scholar
Contributions
MM and MDL conceived the premise of this literature review. MM wrote the first draft of the chapter with revisions from MDL. GD, MM, AM. DB, JQ, SG, TT, and provided guidance, suggestions, and revisions for this manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Max Moloney .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Moloney, M., Digby, G., MacKinnon, M. et al. Primary care asthma surveillance: a review of knowledge translation tools and strategies for quality improvement. Allergy Asthma Clin Immunol 19 , 3 (2023). https://doi.org/10.1186/s13223-022-00755-2
Download citation
Received : 03 August 2022
Accepted : 15 December 2022
Published : 17 January 2023
DOI : https://doi.org/10.1186/s13223-022-00755-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Allergy, Asthma & Clinical Immunology
ISSN: 1710-1492
- Submission enquiries: [email protected]
- General enquiries: [email protected]
- Open access
- Published: 15 July 2021
Asthma and COVID-19: a dangerous liaison?
- Carlo Lombardi 1 , 2 ,
- Federica Gani 3 ,
- Alvise Berti ORCID: orcid.org/0000-0002-7831-921X 4 , 5 ,
- Pasquale Comberiati 6 , 7 ,
- Diego Peroni 5 &
- Marcello Cottini 8
Asthma Research and Practice volume 7 , Article number: 9 ( 2021 ) Cite this article
27k Accesses
31 Citations
22 Altmetric
Metrics details
The coronavirus disease 2019 (COVID-19) pandemic, caused by the new severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), provoked the most striking international public health crisis of our time. COVID-19 can cause a range of breathing problems, from mild to critical, with potential evolution to respiratory failure and acute respiratory distress syndrome. Elderly adults and those affected with chronic cardiovascular, metabolic, and respiratory conditions carry a higher risk of severe COVID-19. Given the global burden of asthma, there are well-founded concerns that the relationship between COVID-19 and asthma could represent a “dangerous liaison”.
Here we aim to review the latest evidence on the links between asthma and COVID-19 and provide reasoned answers to current concerns, such as the risk of developing SARS-CoV-2 infection and/or severe COVID-19 stratified by asthmatic patients, the contribution of type-2 vs. non-type-2 asthma and asthma-COPD overlap to the risk of COVID-19 development. We also address the potential role of both standard anti-inflammatory asthma therapies and new biological agents for severe asthma, such as mepolizumab, reslizumab, and benralizumab, on the susceptibility to SARS-CoV-2 infection and severe COVID-19 outcomes.
Introduction
The coronavirus disease 2019 (COVID-19) pandemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), provoked the most striking global health crisis of the last century [ 1 , 2 ] .
COVID-19 can cause a range of breathing problems, from mild to critical, with older adults and people who have chronic comorbidities, such as hypertension, chronic obstructive pulmonary disease (COPD), obesity, heart disease, cancer, and diabetes, carrying a higher risk of severe symptoms [ 3 ] . Given the impact of SARS-CoV2 infection on the respiratory system on one side and the epidemiological burden of bronchial asthma worldwide on the other side, there are well-founded concerns that the relationship between COVID-19 and asthma could become a “dangerous liaison”.
In this regard, the relationship between asthma and COVID-19 needs to be better defined. This wide aspect could be further dissected in several questions that need to be addressed: 1) are asthmatics at increased risk of SARS-CoV-2 infection and/or severe COVID-19? 2) could different asthma endotypes (type 2 asthma vs. non-type 2 asthma) carry a different risk profile in terms of SARS-CoV-2 infection, COVID-19 development, and progression to severe disease outcomes? 3) if so, could type-2 asthma provide any protection against SARS-CoV-2 infection and/or severe COVID-19? 4) could smoking, asthma-COPD overlap (ACO), or obesity increase the risk of SARS-CoV-2 infection and/or severe COVID-19 in asthmatics? 5) could inhaled corticosteroid and bronchodilator therapy for asthma and new biological agents targeting type 2 inflammation, such as mepolizumab, reslizumab, and benralizumab, affect the susceptibility to SARS-CoV-2 infection and/or the risk of severe COVID-19? The ambitious aim of this review is to collect the latest evidence regarding the intricate relationship between asthma and COVID-19 and provide reasoned answers to the questions above.
Asthma and non-SARS CoV-2 viral infections
Several factors have been associated with increased risk for COVID-19 severity and mortality, such as older age, male sex, comorbidities, and metabolic abnormalities [ 3 , 4 ]. Early in the pandemic, asthma was also suggested as a risk factor for COVID-19 [ 5 ].
It seems plausible to think that a patient with asthma would be at increased risk of SARS-CoV-2 infection and more serious manifestations of COVID-19 because asthmatics normally carry an increased susceptibility to common viral respiratory infections [ 6 ], partly due to a deficient and delayed innate antiviral immune response. Asthmatic patients also show an increased frequency and severity of lower respiratory tract infections compared to healthy individuals [ 7 ]. Moreover, viral respiratory tract infections are a major trigger of asthma exacerbations in both children and adults. In particular, human rhinovirus is detected in 76% of wheezing children and 83% of adult exacerbations [ 8 , 9 , 10 ]. The influenza virus can also favor asthma exacerbations, while other viruses, such as coronavirus, adenovirus, parainfluenza virus, metapneumovirus and bocaviruses, seem to be potential triggers of acute asthma but to a lesser extent [ 11 ].
Environmental exposures and allergies can further boost the risk of virus-induced exacerbations [ 12 ].
Impaired innate immune responses have been observed in asthmatics. A high proportion of patients with asthma and atopic disease have a predisposition to produce lower levels of type I interferon (INF) ro other cytokines upon viral respiratory infections [ 13 , 14 , 15 ].
Through different mechanisms, Type 2 inflammation may have an inhibitory effect on the induction of type I interferon [ 16 ]. Intriguingly, defective production of IFNs by plasmacytoid dendritic cells (pDCs) and epithelial cells have been described in severe atopic patients [ 17 ] with a consequent delayed and inefficient antiviral defense. In this context, a cross-regulation mechanism between FceRI and TLRs in certain cell types such as pDCs has been described, which may explain why the crosslinking of IgE bound to FceRI by allergens may result in a reduced TLR expression and ultimately in a decreased capacity to secrete type I interferons for viral defense [ 16 , 18 ]. Asthmatic patients are known to be at greater risk of influenza-related complications as previous studies have shown that asthma is common among patients hospitalized with influenza [ 19 , 20 ]. During the Swine Flu pandemic, asthma was an undisputed risk factor associated with hospitalization, affecting 10–20% of the hospitalized populations worldwide [ 21 ] and, among patients hospitalized in the United States during April–June 2009, asthma was the most reported underlying chronic medical condition, affecting 28% of patients [ 22 ]. Asthmatic patients compared with non-asthmatic subjects were almost twice as likely to have pneumonia (50% vs. 27%) and required care in the intensive care unit (ICU) (33% vs. 19%) [ 23 ]. Finally, the risk for community-acquired bacterial and viral pneumonia has been estimated to be at least 2-fold in asthmatic patients compared with healthy control subjects [ 24 ].
Susceptibility of patients with asthma to COVID-19 infection
In the early stage of the pandemic, asthma was inconsistently mentioned among the significant clinical risk factors for SARS-CoV-2 infection in studies from China [ 4 , 25 , 26 , 27 ] and Italy [ 28 , 29 , 30 ]. Studies from Russia, Saudi Arabia, Brazil, and India also reported lower rates of asthma among patients with COVID-19 [ 31 , 32 , 33 ]. Instead, studies from the USA and the UK reported that comorbidity rates of asthma in patients with COVID-19 were similar or higher than those in the local population [ 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 ]. Recently, Broadhurst et al. performed a focused literature review among patients hospitalized for COVID-19 infection; their findings suggest that asthma prevalence appears to be similar to population asthma prevalence and significantly lower than asthma prevalence among patients hospitalized for influenza [ 42 ]. Kalyanaraman et al. reviewed the electronic health records (New York City’s public hospital system) of all patients who received a SARS-CoV-2 test and showed that asthma was not associated with testing positive [ 43 ]. A systematic review and meta-analysis of 131 studies from 39 countries (410,382 patients) reported asthma prevalence in adult or all-age-group patients with COVID-19. The regional asthma comorbidity rates were estimated as follows: East Asia and the Pacific, 2.2%; Europe, 6.4%; Latin America and the Caribbean, 3.5%; the Middle East and North Africa, 4.9%; North America, 10.2% [ 44 ]. Very recently, Terry et al. performed a systematic review and meta-analysis of 150 studies and did not find clear evidence of increased risk of COVID-19 diagnosis in asthmatics [ 45 ].
In conclusion, there is great variability in the prevalence of asthma among patients with COVID-19 in different countries; in most countries patients with asthma were not reported with higher, but rather similar or lower rates of COVID-19 infection, compared with the general population in the corresponding area, probably due to multiple factors including a low proportion of non-type 2 phenotypes [ 38 ]. Indeed, a Korean nationwide cohort showed that patients with non-allergic asthma had a greater risk of SARS-CoV-2 test positivity than patients with allergic asthma [ 46 ].
Risk of morbidity and mortality in patients with asthma and COVID-19
Results are heterogeneous when examining the association between asthma and severity of COVID-19. A study that analysed the UK Biobank data (493,000 patients) showed that adults with asthma had a higher risk of severe COVID-19 [ 41 ], and in a Korean nationwide cohort, asthma confers a greater risk of susceptibility to SARS-CoV-2 infection and severe clinical outcomes of COVID-19 [ 46 ]. An interesting aspect to note is that, in both studies, the higher risk of severe COVID-19 was driven by the increased risk in non-allergic asthma patients. In contrast, several studies found no statistically significant association between asthma and mortality or risk of intubation/mechanical ventilation in patients with COVID-19. In the International Severe Acute Respiratory and Emerging Infections Consortium (ISARIC) World Health Organization (WHO) Clinical Characterization Protocol UK study, despite a prevalence of 14.5%, asthma was not associated with an increased risk of ICU admission, mechanical ventilation, or death [ 39 ] . Broadhurst et al., using a cross-sectional analysis of patients with COVID-19 admitted to the University of Colorado Hospital, showed that asthma does not appear to be an independent risk factor for intubation among hospitalized patients with COVID-19, even after adjusting for well-known risk factors for severity [ 42 ]. Two independent studies [ 47 , 48 ] similarly demonstrated that patients with COVID-19 comorbid with chronic obstructive pulmonary disease or diabetes tended to be more severe, whereas those comorbid with asthma did not. In a recent matched cohort study conducted in Boston among patients hospitalized for COVID-19, asthma was not associated with an increased risk of ICU admission, hospitalization, mechanical ventilation, or death compared with inpatient comparators matched by age, sex, and date of positive SARS-CoV-2 test [ 49 , 50 ]. Moreover, in three studies from New York, among hospitalized patients with severe COVID-19, asthma diagnosis was not associated with worse outcomes and mortality [ 43 , 51 , 52 ]. Calmes et al. collected data from 596 adult patients hospitalized for SARS-CoV2 infection. The multivariate analysis showed that asthma was not an independent risk factor for ICU admission or death [ 53 ]. Patients with COPD, but not asthma, have a slightly increased risk of severe outcomes of COVID-19 compared with patients without obstructive lung disease [ 54 ]. Murillo-Zamora et al. conducted a nationwide, retrospective cohort study in Mexico in which data from 66,123 individuals were analyzed. Reduced risk of a fatal outcome was observed among patients with asthma history [ 55 ]. In a systematic review and meta-analysis of 131 studies from 39 countries (410,382 patients), no significant difference in asthma prevalence was found between hospitalized and non-hospitalized, severe and non-severe, ICU and non-ICU, dead and survived, intubated/mechanically ventilated and non-intubated/mechanically ventilated patients with COVID-19.
Patients with asthma have a lower risk of death compared with patients without asthma [ 44 ]. The overall findings of another recent meta-analysis (587,280 patients) suggest that people with asthma have a lower risk than those without asthma of acquiring COVID-19 and have similar clinical outcomes [ 56 ]. Finally, Terry et al. review the literature related to the role of asthma on COVID-19 outcomes: the results of this meta-analysis do not provide clear evidence of increased risk of COVID-19, hospitalization, or severity, due to asthma [ 45 ]. Moreover, the authors reported a significant 18% reduction in risk of mortality in asthma patients with COVID-19 compared to non-asthmatic patients. This finding is more robust due to the adjustment for major confounding factors, and provides reassurance to asthma sufferers, and those responsible for their care.
Using big data analytics and artificial intelligence through the SAVANA Manager® clinical platform (71,182 patients with asthma), Izquierdo et al. analyzed clinical data from patients with asthma [ 57 ]. The manifestation of the disease in this clinical population was not particularly severe, with a low rate of hospital admissions. The increased risk for hospitalization due to COVID-19 in patients with asthma was largely associated with age and related comorbidities. Moreover, in a recent study from France, among 768 hospitalized patients (37 (4.8%) with a history of asthma) worse outcomes were observed mainly in asthmatics with major comorbidities [ 58 ]. A study from the UK examined the association between different phenotypes of asthma and COVID-19 infection. Interestingly, the risk was mostly related to non-allergic asthma [ 59 ]. Unfortunately, there is still little information about asthma phenotypes in patients with COVID-19, but it is possible to speculate that different asthma endotypes may also have a differential impact on the progression and severity of COVID-19 [ 38 , 41 , 59 ]. These data need further examination in prospective large cohort studies.
SARS-CoV-2 and asthma exacerbations
Quite surprisingly, on April 8, 2020, Morbidity and Mortality Weekly Report (MMWR) published a report of 1482 patients hospitalized for COVID-19 in the USA, it was mentioned that wheezing was present in only about 7% of the patients for whom data were available on underlying conditions, which is less than the prevalence rate of about 10% of asthma in the general population [ 35 ]. This report suggests that SARS-CoV-2 rarely induces asthma exacerbations during hospitalization for COVID-19. Also in other studies, few patients were hospitalized for a COVID-19-related asthma exacerbation during the outbreak and very few developed an asthma attack during hospitalization [ 58 , 60 ]. These data thus reflect a striking difference from previous respiratory viral pandemics, most recently the H1N1/A outbreak in 2009, also because viral infections, including other types of non-SARS coronaviruses, are the main cause of asthma exacerbations.
Severe asthma and COVID-19
A minority of patients with asthma (5–10%) have uncontrolled or partially controlled asthma despite intensive treatment. One would expect increased vulnerability to SARS-CoV-2 infection in these patients, but scarce data are so far available to confirm this hypothesis. Despite the lack of sufficient evidence, the Center of Disease Control (CDC) in the USA issued warnings that patients with moderate-to-severe asthma may be at increased risk of contracting COVID-19 and suffer severe outcomes from the disease [ 61 ]. A database from the UK looking at electronic health records (EHR) of 17 million patients with COVID-19 showed an association between severe asthma and increased risk of death for hospitalized patients after adjustment for sex and race, but not for all comorbidities. The hazard ratio was higher for patients with asthma with documented recent oral corticosteroid use, which is a marker of the severity of asthma [ 62 ]. More recently, in the same cohort, Schultze et al. found that patients with asthma taking high-dose inhaled corticosteroids (ICSs) had a 55% increased risk of death from COVID-19, and which was interpreted as being due to confounding by disease severity [ 63 ]. Lee et al. selected 7272 adult COVID-19 patients (686 with asthma) from the Korean Health Insurance Review and Assessment COVID-19 database. Asthma was not a significant risk factor for respiratory failure or mortality among all COVID-19 patients. However, a history of acute exacerbation (OR = 2.63, p = 0.043) was a significant risk factor for death among COVID-19 patients with asthma [ 64 ]. In anotherstudy, asthma severity was not an independent factor for poor clinical outcomes of COVID-19, but patients with step 5 asthma had a prolonged in-hospital stay duration than those with step 1 asthma in both univariate and multivariate analyses [ 65 ]. A large COVID-19 hospitalized Italian population showed that patients with worse COVID-19 outcome (death/need for ventilation vs. discharge at home without requiring invasive procedures) suffered from more severe asthma (GINA 4/5, 43 vs. 14%, p = 0.040) [ 66 ]. Although biologic-treated patients with asthma typically present with the most severe manifestations of the disease, these studies showed that the number of COVID-19-related admissions and mortality in these patients was strikingly low [ 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 ]. Treatment with biologicals for severe asthma also seems to have no significant effect on the outcome of COVID-19.
In conclusion, it is now well-recognized that older age, obesity, cardiovascular diseases, and diabetes are risk factors of poor COVID-19 outcome. What is not yet clear is whether chronic respiratory diseases like asthma are also to be included as risk factors. The many studies that have addressed this question show discrepant results and point towards numerous factors that may play a role in the susceptibility and severity of COVID 19 in asthma patients. These include the severity of asthma itself, the asthma phenotypes/endotypes, asthma medications and co-morbidities. The reported incidences of severe COVID-19 cases among asthma patients are not determined by patient-related factors alone. Moreover, local factors (testing policies or shielding advice, such as the case of the older patients or those with co-morbidities like asthma better protected themselves) and the diagnostic methods to identify asthma and COVID-19 can play an important role [ 75 ].
Why might asthma protect against poor outcomes in COVID-19?
Several studies have suggested possible non-harmful or protective effects of asthma on the clinical outcomes of COVID-19. Asthma might protect against poor outcomes in COVID-19 due to several possible mechanisms (Fig. 1 ), including altered viral entry receptor expression, chronic type-2 inflammation, younger age and/or absence of comorbidities, reduced exposure due to shielding, increased adherence to therapy and ICS use [ 76 ].
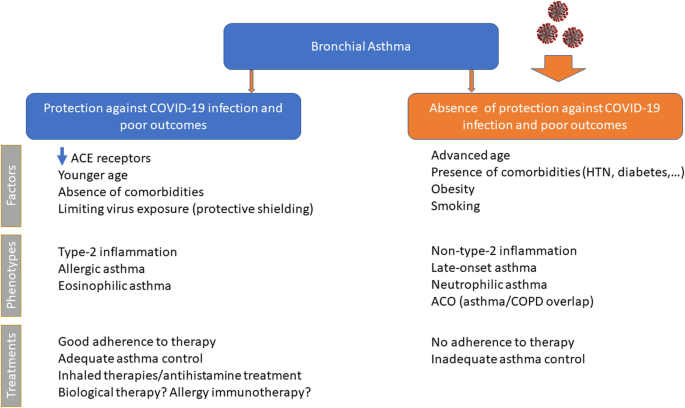
Possible mechanisms by which asthma might protect against poor outcomes of COVID-19
ACE2 receptor
The lack of susceptibility to COVID-19 in patients with pre-existing allergic asthma seems to be in contrast with the established link between these chronic respiratory conditions and susceptibility to common respiratory viruses, especially rhinoviruses [ 10 ]. However, rhinovirus uses the intercellular adhesion molecule 1 (ICAM-1) molecule as an entrance into respiratory epithelial cells, which is overexpressed in allergic airways as a marker of allergic inflammation [ 77 ]. In contrast, COVID-19 uses another host cell receptor abundantly present in the oral mucosa and within the (healthy) airways, i.e., the angiotensin-converting enzyme2 (ACE2) [ 78 ], which plays a crucial role in the disease development and associated lung injury [ 79 ]. Cofactors facilitating SARS-CoV-2 infectivity are transmembrane peptidase serine 2 (TMPRSS2), which cleaves the SARS-CoV-2 spike protein, and possibly protease furin [ 80 ]. Peters et al. [ 81 ] analyzed gene expression for ACE2 and TMPRSS2, and ICAM-1 (rhinovirus receptor as a comparator) in sputum cells from 330 participants in the Severe Asthma Research Program-3 and 79 healthy control subjects. Among patients with asthma, male sex, African American race, and history of diabetes mellitus were associated with higher expression of ACE2 and TMPRSS2. The use of ICSs was associated with lower expression of ACE2 and TMPRSS2. The asthma endotype is especially important, as cytokines can modify ACE expression. Song et al. [ 79 ] found that the mRNA expression levels of ACE2 in bronchial epithelial cells were significantly downregulated in allergic asthmatics compared to healthy controls. A lower expression of ACE2 has been described in airway cells of patients with respiratory allergy and/or asthma, while non-allergic asthma was not associated with ACE2 expression [ 82 ]. Furthermore, Kimura et al. reported that IL-13 exposure reduced ACE2 expression in airway epithelial cells from patients with asthma and atopy [ 83 ]. These findings suggest that patients with allergic asthma might be protected from COVID-19 because of the low expression of ACE2 in their epithelial cells [ 84 ]. By analyzing ACE2 gene expression in bronchial epithelial cells in asthmatic patients with different endotypes, Camiolo et al. identified a positive correlation between ACE2 expression and scores of T1 gene expression and a negative correlation between ACE2 expression and scores of Type-2 gene expression [ 85 ]. Kermani et al. [ 86 ] examined microarray mRNA expression of ACE2, TMPRSS2 and FURIN in sputum, bronchial brushing, and bronchial biopsies of the European U-BIOPRED cohort. Sputum FURIN expression levels were strongly associated with neutrophilic inflammation and with inflammasome activation. This might indicate the potential for a greater morbidity and mortality outcome from SARS-CoV-2 infection in non-type-2, neutrophilic severe asthma [ 79 ]. IL-17, which is produced by Th17 cells and type 3 ILCs, can stimulate neutrophilic airway inflammation and can upregulate ACE2 expression [ 83 ]. In a study from US observations in bronchial brush airway, epithelial cells identified a positive correlation between ACE2 gene expression and a previously described IL-17-dependent gene expression signature, with an inverse association with TH2 gene expression [ 87 ]. Smoking can also modulate ACE2 expression in the lungs of asthmatics. In an experimental model of smoke-induced acute respiratory distress, a Th17/neutrophilic syndrome, ACE2 was upregulated [ 88 ]. In addition, cytokine release from smoking-associated lung injury induces upregulation of ACE2 in the lungs [ 89 ]. In conclusion, these data strongly suggest an association between asthma endotypes and ACE2 gene expression.
Inflammatory endotypes and COVID-19
Asthma is a complex and very heterogeneous respiratory disease, with differences from patient to patient in causes and drivers, the severity of symptoms, type and degree of inflammation, and response to treatment. The identification of the different patient groups and the different underlying pathophysiological mechanisms in asthmatic patients appears to be very important in assessing the relationships between asthma and COVID-19. A type 2 inflammation is evident in more than 50% of those with a formal asthma diagnosis [ 90 ] and is typically characterized by activation of proinflammatory cytokines including interleukins (IL)-4, − 5, and − 13, manifesting as the type 2 endotype with raised levels of immunoglobulin E (IgE), eosinophils, and fractional exhaled nitric oxide (FeNO), and dysfunction of epithelial or epidermal barriers [ 91 ] . The Type 2-high endotype can have either allergic or non-allergic underpinnings and is typically characterized by some degree of eosinophilic airway inflammation, while the neutrophilic or pauci-granulocytic airway inflammation is associated with the Type-2-low endotype [ 92 ]. Several studies supporting the hypothesis that type 2 asthma does not represent a major risk factor for increased COVID-19 severity. A recent study showed that patients suffering from different asthma endotypes (type 2 asthma vs. non-type 2 asthma) present with a different risk profile in terms of SARS-CoV-2 infection, development of COVID-19, and progression to severe COVID-19 outcomes [ 38 ]. In a study from the USA, atopy was associated with significantly lower odds of hospitalization for COVID-19 [ 93 ]. Moreover, non-allergic asthma was associated with prolonged intubation time. A large population-based cohort study demonstrated that adults with asthma had a higher risk of severe COVID-19, which was driven by the increased risk in patients with non-allergic asthma [ 41 ] . Again, in a Korean nationwide cohort, patients with non-allergic asthma had a greater risk of SARS-CoV-2 test positivity and worse clinical outcomes of COVID-19 than patients with allergic asthma [ 46 ]. Finally, in a retrospective study on patients with SARS-CoV-2-induced pneumonia, hospitalized in several Italian hospitals, atopic subjects showed a much lower occurrence of severe or very severe COVID-19 pneumonia (33.3% vs. 67.7%, p < 0.0001) [ 94 ].
The second major subgroup of asthma is non-type 2 asthma, which contains a heterogeneous group of endotypes and phenotypes, such as obesity-induced asthma, smoking-related asthma, etc. Non-eosinophilic asthma is generally associated with the absence of eosinophils and activation of non-predominant type 2 immunological pathways [ 95 ]. Non-type 2 asthma involves greater Th1/Th17 activity than does atopic asthma, when bronchial epithelial cells release IL-33, IL-6, IL-23, IFNγ, and tumor necrosis factor-α in response to various irritants, resulting in neutrophilic airway inflammation [ 96 ]. The major mechanism leading to a non-type 2 response is thought to result from an irregular innate immune response, including intrinsic neutrophil abnormalities and activation of the IL-17-mediated pathway. IL-17, which is produced by Th17 cells and type 3 ILCs, can stimulate neutrophilic airway inflammation. Peters et al. reported that systemic IL-6 inflammation (a biomarker of non-type 2 asthma) occurs in a large subgroup of patients with asthma, most of whom are older and obese [ 97 ], and reported that systemic IL-6 inflammation as a biomarker for patients who have both metabolic dysfunction and severe asthma. Non-type 2 asthma is more frequent in women than in men, particularly those over 35 years of age [ 98 ]. Women with asthma have a combination of phenotypic heterogeneities, including a Th1 immunological skewness, a predisposition towards more severe asthma [ 99 ], structural lung parenchymal differences, and hormonal differences, which might increase their susceptibility to severe COVID-19 requiring hospitalization. The increased prevalence of non-atopic asthma in women might be related to distinct underlying causes of asthma, including obesity. Obese women have a disproportionate incidence and severity of asthma because of increased leptin concentrations, which promote inflammatory Th1 pathways [ 98 , 99 , 100 ]. Moreover, a study demonstrated an increase in neutrophilic airway inflammation in obese asthma, compared to obese healthy controls, and this relationship was significant only in females with asthma [ 101 ]. A study by Atkins and colleagues established female sex as an independent risk factor for SARS-COV2-related hospitalizations among patients with asthma in the UK [ 59 ]. This study and three others from Paris, France, Illinois, USA, and New York, USA, report that 56–71% of patients with asthma hospitalized for COVID-19 were women [ 50 , 51 , 58 ]. Besides obesity, pauci-granulocytic/neutrophilic asthma has been associated with environmental and/or host factors, in particular with smoking cigarettes. Cigarette smoke can damage the epithelium directly and has been associated with non-eosinophilic airway inflammation compared to never smokers with asthma. Through direct activation of macrophages, these cells produce inflammatory molecules, tissue proteases like MMP, IL-8, and other chemokines involved in the mobilization and prolonged survival of neutrophils in the lung tissue, while producing less IL-10, which leads to a non-type 2 pattern with a reduced B-cell number and lower levels of IL-4 and IL-5 [ 102 ]. Smokers and COPD patients presented an increase in COVID-19-associated inflammatory markers during the disease course in comparison to non-smokers and former smokers. Current reviews indicate that nicotine exposure is linked to cardiopulmonary vulnerability to COVID-19 and tobacco use can be a potential risk factor for not only getting the viral infection but also its severe manifestations [ 103 , 104 , 105 ]. Alberca et al. recently demonstrated that smoking and COPD are risk factors for severe COVID-19 with possible implications for the ongoing pandemic [ 106 ]. Together, the pulmonary and systemic effects of cigarette smoking could further potentiate SARS-CoV-2-induced endothelial dysfunction and systemic inflammation in asthmatic patients. Patients with COPD, but not asthma, have an increased risk of severe outcomes of COVID-19 compared with patients without obstructive lung disease [ 54 ]. Moreover, in the ISARIC WHO Clinical Characterization Protocol UK study, COPD was associated, unlike asthma, with an increased risk of ICU admission, mechanical ventilation, or death [ 39 ] . Pathological features of both asthma and COPD coexist in some patients and this is termed ACO [ 107 ]. Presently, this patient group is estimated to encompass 11.1–61.0% of the 339 million patients with asthma and 4.2–66.0% of the 252 million patients with COPD, worldwide [ 108 ]. Various cardinal features of asthma (reversible airflow limitation and eosinophilic/type-2 inflammation) and COPD (irreversible airflow limitation and neutrophilic/type-1 inflammation) frequently coalesce in patients with ACO [ 109 ]. A population-based prospective cohort study analyzed data from the UK Biobank. Participants with asthma, compared with those without, had a significantly higher risk of severe COVID-19 (odds ratio [OR], 1.44). These findings were driven by the significant association of non-allergic asthma with severe COVID-19 (adjusted OR, 1.48). In the stratified analysis by coexisting COPD ( n = 7815), the significant association persisted in both strata, with a larger magnitude in asthma with COPD (adjusted OR, 1.82) [ 41 ]. Therefore, it seems obvious to think that patients with asthma-COPD overlap would be at increased risk of SARS-CoV-2 infection and a more serious clinical picture of COVID-19. The Th17/neutrophilic endotype of asthma in smokers, ACO and obese patients might be exacerbated by the systemic inflammatory response of SARS-CoV-2 infection, which is similarly driven by Th1-related cytokines, including IFNγ, IL-6, MCP1, IP10, and IL-1β [ 110 ].
Eosinophilic inflammation
Further, the role of eosinophils, foes in asthma but possibly friends in COVID-19 infection, needs to be established [ 111 ]. Previous experimental studies indicated a potential role of eosinophils in promoting viral clearance and antiviral host defense [ 112 ]. The eosinophils are reduced in peripheral blood of SARS-CoV-2-infected patients [ 113 ]; therefore, it is tempting to speculate that increased numbers of eosinophils in the airways of asthmatic patients might be protective against the exaggerated inflammatory responses of the severe COVID-19 phenotype [ 111 ]. Patients with the type-2 low asthma endotype who have low eosinophils might be more prone to more severe COVID-19 outcomes, in the same way as in non-allergic asthma. Severe COVID-19 occurring in susceptible individuals may be associated with cytokine-mediated hyper-inflammation and associated coagulopathy with multisystem involvement and death [ 114 ]. Markers of worsening disease include hypoxemia, lymphopenia, thrombocytopenia, and raised levels of IL-6, C reactive protein, ferritin, lactate dehydrogenase, and D-dimers. Eosinopenia may also be part of the overall cytopenic process in the early phase of severe COVID-19, with the later resolution of eosinophil counts being associated with clinical recovery [ 115 ]. Peripheral blood eosinophil counts may, therefore, be an effective and efficient indicator in diagnosis, evaluation, monitoring and prognosis of COVID-19 patients [ 116 ]. Recently, Ferastraoaru et al. [ 117 ] retrospectively identified 737 asthma patients with COVID-19 seen in the emergency department (ED). In asthmatics, pre-existing eosinophilia (AEC ≥ 150 cells/mL) was protective from COVID-19 associated hospital-admission, and development of eosinophilia (AEC ≥150 cells/ mL) during hospitalization was associated with decreased mortality. According to the authors, having a Type 2-asthma endotype might be an important predictor for reduced COVID-19 morbidity and mortality which should be further explored in prospective and mechanistic studies. Based on the current evidence and clinical observations, it could be suggested that Type-2 airway disease associated with eosinophilic infiltration and down-regulation of ACE2 does not represent a risk factor for COVID19 infection [ 114 ]. Instead, Type 2-low asthmatics demonstrated characteristics corresponding to risk factors for severe COVID-19, including obesity and history of smoking and hypertension. This group of asthmatics has a different inflammatory profile, and due to the chronic sub-clinical inflammation associated with metabolic dysregulation, there is circumstantial evidence that the immune system is already (pre-) programmed to develop hyper-inflammation in the context of a cytokine storm in association with COVID-19. Taken together, although type-2 asthma appears to be a protective factor for COVID-19, the associations between different phenotypic and endotypic asthma and COVID-19 remain to be better defined.
Younger age and/or absence of comorbidities
Susceptibility and severity of COVID-19 infection increase with age [ 118 ], therefore, age is an important confounder in the assessment of the risk of contracting severe COVID-19. Izquierdo et al. showed that asthma patients without COVID-19 were younger and more likely to have eczema and rhinitis, while those with COVID-19 were older and more likely to have co-morbidities such as hypertension and diabetes [ 57 ]. In addition, children and young adults with asthma manifest Type-2 high airway inflammation that is driven predominantly by allergy, IL-4 and IL13. In comparison, non-type 2 asthma is more frequent in older adults, particularly in women over 35 [ 98 ] and presents with a different risk profile in terms of comorbidities, SARS-CoV-2 infection, development of COVID-19 and progression to severe COVID-19 outcomes [ 38 ]. Expression of ACE2, the co-receptor for SARS-CoV-2, varies with age [ 81 ]. Because Type-2 asthma sufferers tend to be younger than those with other comorbidities, the age factor probably explains why patients with asthma may not be at greater risk. However, to better address this question, age-adjusted models need to be formulated.
Protective shielding limiting virus exposure
Behavioral factors are likely to be important. Protective shielding for at-risk groups, including those with asthma, has been widely advocated by international guidelines. Reduced exposure to SARS-CoV-2 amongst patients with asthma may therefore be contributing to the low prevalence of asthma reported in hospitalized cohorts [ 76 ]. Government policies to limit the spread of the pandemic have also led to reductions in air pollution, which increases the severity of virus-induced asthma exacerbations [ 119 ]. Nationwide preventive measures for COVID-19 in Japan were associated with a sharp drop in hospitalizations for asthma as a secondary effect [ 120 ].
Adherence to therapy
As hospitalizations for asthma are considered a consequence of poor asthma control [ 121 ], these findings suggest that asthma was better controlled during this outbreak. Initial concerns that the COVID-19 spread might increase asthma attacks may have encouraged preventive behaviors among people with asthma and their families, including quitting smoking, cleaning their rooms more frequently to remove allergens, and better adherence to preventive medications. Patients with asthma are advised to closely adhere to their prescribed inhaler medication therapy due to the COVID-19 pandemic. In an interesting study, a cohort of 7000 patients had electronic monitoring of their controller and rescue inhaler use during the pandemic, and increased adherence of 15% was found in the controller medication use [ 122 ]. According to the results of a cross-sectional study from Mexico, male sex, active smoking, and the belief that COVID-19 was not more severe in asthma sufferers seemed to favor non-adherence to COVID-19 prevention measures [ 123 ]. It is therefore important that health professionals and patients with asthma maintain constant communication regarding the measures that patients must comply with to prevent COVID-19 and the timely use of medications to control their chronic disease.
Anti-asthmatic therapies in the context of COVID-19: protective or favorable role?
Inhaled antiasthmatic therapies and covid-19.
It is likely that maintenance of ICSs can also confer protection, but there is no evidence of the benefits or harm of ICSs in COVID-19 [ 124 ] . Several key questions arise. Are asthmatics, or some phenotypes, at increased risk of developing COVID-19? Do ICSs modify this risk, either increasing or decreasing it? Do ICSs influence the course of COVID-19? Epidemiological studies of COVID-19 must include detailed information on asthma comorbidity and prior medication to help answer these questions [ 125 ] . Today, there is no evidence of an association of increased risk of COVID-19 infection in asthmatic patients regularly taking ICSs [ 126 ]. Asthma exacerbations have been markedly reduced especially in children during the COVID-19 pandemic, which may not only be due to a decrease in exposure to triggers, i.e. air pollutants and aeroallergens, but may also be related to improved adherence to controller therapy [ 127 ] . To reduce inflammation in the lungs, such as in patients with asthma, therapeutic effects can be achieved with low doses of ICSs, which are associated with minimal detectable systemic effects. Immunosuppression at high doses of ICSs is weighted toward the lungs, but moderate systemic immunosuppression could also be expected because of its dual local and systemic bioactivity [ 128 ]. Delivering corticosteroids directly to the distal airways and alveoli by inhalation could effectively reduce inflammation in the lungs with fewer systemic side effects. Apart from their anti-inflammatory effects, some ICSs have been found to have antiviral effects. In vitro, corticosteroids inhibit rhinovirus and respiratory syncytial virus-induced cytokine release [ 129 ], but the timing of exposure to ICSs seems important with pre-treatment being less effective than administration at the time of infection [ 130 ]. Ciclesonide and mometasone suppressed the replication of SARS-CoV-2 and MERS-CoV in vitro, whereas dexamethasone, cortisone, prednisolone, and fluticasone did not exert antiviral effects [ 131 ] . In addition, an in vitro experimental study has shown that glycopyrronium, formoterol, and a combination of glycopyrronium, formoterol, and budesonide can reduce coronavirus HCoV-229E replication, partly by inhibiting receptor expression and/or endosomal function [ 132 ]. Considering the difference in the features of the viruses, the results for coronavirus HCoV-229E should be interpreted cautiously [ 133 ] . Many clinical trials utilizing ICSs for COVID-19 have been registered on ClinicalTrials.gov : four trials for ciclesonide and four for budesonide (one including formoterol). Further studies are needed to investigate the possible positive effect of ICSs on COVID-19 pneumonia as previously shown with dexamethasone in the RECOVERY trial [ 134 ]. A recent meta-analysis revealed no significant difference in the risk for the development of a severe or fatal course of COVID-19 with preadmission use of ICSs in patients with COVID-19 relative to non-use of ICSs [ 135 ]. These findings assured the safety of continued use of ICSs during the COVID-19 pandemic. Understanding the basis of differences in susceptibility to severe COVID-19 between asthmatic and non-asthmatic populations may ultimately offer important insights into therapeutically exploitable targets to reduce the overall burden of COVID-19. There are no current data that support or recommend a step-down of current treatments of patients. ICSs undoubtedly decrease the rate of exacerbations in asthmatic patients. If people with stable asthma stop or reduce their ICSs inappropriately in response to concerns about immunosuppression and worries about developing COVID-19, they may be at significant risk of experiencing exacerbation. The European Forum for Research and Education in Allergy and Airway Diseases (EUFOREA) concluded that proper treatment of allergic rhinitis and allergic asthma is important, and topical corticosteroids can be used in such cases [ 136 ] . In hospitals, the use of metered-dose inhalers (MDIs) and dry powder inhalers (DPI) is preferred to nebulizers if patients can perform the breathing maneuvers. If a nebulizer is used, a high-flow nasal cannula is preferable to a face mask and a mouthpiece should be used with a jet or mesh nebulizer, and viral filters or one-way valves should be attached to nebulizers to minimize the release of aerosols [ 126 ].
In conclusion, clinicians should be aware that there is no evidence to support the withdrawal of ICSs in patients treated with these drugs, and to do so is likely to be harmful. Patients with asthma who are stable while using ICSs should continue their treatment. If there is uncertainty about the diagnosis, physicians should be more careful about initiating ICSs or ICS/long-acting β-agonist in patients without clear objective evidence of asthma. Similarly, there is no evidence to suggest a change in the recommendation for asthma patients to increase the dose of ICSs at the onset of an exacerbation. Based on the above, the indications contained in the GINA document about asthma and COVID-19 are certainly acceptable [ 137 ]: a) advise patients to continue taking their prescribed asthma medications, particularly ICSs; b) make sure that all patients have a written asthma action plan; c) advising them to: increase controller and reliever medication when asthma worsens; d) avoid nebulizers where possible, to reduce the risk of spreading the virus; e) pressurized MDI via a spacer is preferred except for life-threatening exacerbations, and f) add a mouthpiece or mask to the spacer if required.
Biological therapies and COVID-19
Managing patients with severe asthma during the coronavirus pandemic and COVID-19 is a challenge. Unless relevant data during the pandemic is going to emerge soon, changing our understanding on the safety of biologic therapies, clinicians must follow the recommendations of current evidence-based guidelines for preventing asthma loss of control and exacerbations. Moreover, in the absence of data that would indicate any potential harm, the current advice is to continue the administration of biological therapies during the COVID-19 pandemic in patients with asthma for whom such therapies are indicated and have been effective [ 138 ] . For patients with severe asthma infected by SARS-CoV-2, the decision to maintain or postpone biological therapy until the patient recovers should be a case-by-case based decision supported by a multidisciplinary team [ 126 ]. Biologicals are indicated for severe asthma therapy in patients that are not controlled adequately with other treatments. The three anti-IL5/IL5r have their major effect in targeting eosinophils by producing either a reduction or depletion of tissue and peripheral blood eosinophils. The other two biologics (anti-IgE and anti-IL4/ IL13) have their primary effects by inhibiting Type 2 immunity. Thus, the primary question could be raised whether eosinophils and/or Type 2 mechanism immunomodulation have any role in modifying susceptibility, severity, immunity, or resistance to SARS-COV2 infection. Although the role of eosinophils in COVID-19 disease has yet to be elucidated, it has been shown that this viral infection can be associated with profound eosinopenia and that persistent eosinopenia may be associated with clinical deterioration and increased risk of mortality [ 116 ]. Several possible explanations for eosinopenia related to COVID-19 have been proposed: decreased eosinophilopoiesis, eosinophil apoptosis induced by type 1 IFN released during the acute infection, and increased eosinophil migration and retention within inflamed tissues [ 139 ]. There have been a few case reports in the literature of patients with COVID-19 who were on treatment with either omalizumab, mepolizumab, benralizumab, or dupilumab, but all had a favorable clinical course [ 140 , 141 , 142 , 143 , 144 , 145 , 146 ].
A multicenter Italian survey evaluated 473 consecutive patients with severe asthma treated with biological therapy (mepolizumab ( n = 200), omalizumab ( n = 145); benralizumab ( n = 124); dupilumab ( n = 4) [ 72 ] . Four of these patients contracted COVID-19 (3 were on omalizumab therapy and 1 on benralizumab); all of them were atopic patients. In contrast, no cases of COVID-19 were observed in the 200 patients treated with mepolizumab or the 4 patients treated with dupilumab. The four confirmed COVID-19 cases displayed good control of asthma symptoms before SARS-CoV-2 infection, without asthma exacerbations during the last 3 months before the illness. Three of them paused the therapy during illness. Two SARS-CoV-2 infected patients experienced a mild COVID-19, while two patients required admission to the ICU for severe and critical illness, respectively. All of them clinically recovered. Another observational study was carried out to evaluate the occurrence of COVID-19 in adult patients with severe asthma, based on data from the Belgian Severe Asthma Registry (BSAR), and to assess whether patients with severe asthma using biologics present an increased risk of severe COVID-19 compared to those who do not use these medications [ 68 ] . In this cohort of severe asthma patients, a small number of COVID-19 cases was found; none resulted in death or a very severe disease course. Of 676 participants, only 14 patients were identified with COVID-19 infection confirmed by either PCR and/or specific IgG. Of these 14 patients, only 5 had been hospitalized (with a hospital stay ranging from 2 to 8 days). None presented with a severe asthma exacerbation or required treatment with systemic corticosteroids, admission to the ICU, non-invasive ventilation, mechanical ventilation, or extracorporeal membrane oxygenation, and no deaths occurred. However, not all studies published so far are consistent with the previous one. Indeed, in a recent prospective ongoing survey from 15 hospitals of the Dutch Severe Asthma Registry (RAPSODI), 9 out of 634 (1.4%) severe asthma patients who received biologic therapy were diagnosed with COVID-19. Seven (1.1%) required hospitalization for oxygen therapy, 5 of whom were admitted to the ICU for intubation and mechanical ventilation. One patient died (0.16%). All intubated patients had ≥1 co-morbidity. Odds (95%CI) for COVID-19-related hospitalization and intubations were 14 (6.6–29.5) and 41 (16.9–98.5) times higher, respectively, compared to the Dutch population.
Although more data will need to be obtained in the future, the evidence currently available encourages the continuation of maintenance therapy and biologic treatment of patients with asthma in the context of this pandemic in non-infected individuals. A practical clinical algorithm has also recently been proposed on the use of biologicals for the treatment of allergic diseases in the context of COVID-19. Non-infected patients on biologicals for the treatment of asthma, AD, CRSwNP, or CSU should continue their biologicals targeting type-2 inflammation via self-application. In the case of an active SARS-CoV-2 infection and moderate-to-severe COVID-19, biological treatment needs to be stopped until clinical recovery and SARS-CoV-2 negativity are established. Thereafter, treatment with biologicals can be reinitiated [ 147 ] . Furthermore, maintenance of add-on therapy and a constant assessment of disease control, apart from acute management, are required. A consensus-based ad hoc expert panel of allergy/immunology specialists from the USA and Canada recommends continuing administration of biologicals in patients with proven efficacy and converting the patient to a prefilled syringe for potential home administration if this is available, otherwise in-office application is possible with a plan to transition to home administration [ 148 ] . Therefore, if available, it is recommended to prefer a formulation for self-application and to offer telemedical monitoring.
Allergen immunotherapy and COVID-19
Regarding allergen immunotherapy (AIT), this biological therapy has been used in allergic diseases for more than 100 years, and many new therapeutic advances have been introduced in recent years [ 149 ] . The AIT approach to patients with respiratory allergies may provide a possible theoretical advantage to patients during the COVID-19 pandemic and can be continued if patients are not diagnosed with COVID-19. An essential part of the complex mechanism of action of AIT is the generation and maintenance of functional allergen-specific regulatory T (Treg) cells and regulatory B (Breg) cells [ 150 ]. Treg cells play a role in preventing cytokine storm and limiting tissue damage [ 151 , 152 ] . It appears therefore conceivable that the immune tolerance induced by AIT might have a putative protective role in severe COVID-19 patients with cytokine storm. Furthermore, starting AIT in eligible patients, the sublingual route of administration (SLIT) is preferred to minimize in-person encounters for subcutaneous injections (SCIT); indeed, SLIT can be administered at home, thus avoiding the need to travel to or stay in an allergy clinic or doctor’s office, which would be associated with a risk of infection [ 153 , 154 ].
Finally, interrupting AIT is cautiously recommended in patients diagnosed with COVID-19, or suspected of SARS-CoV-2 infection, or symptomatic patients with exposure or contact with SARS-CoV-2 positive individuals, or patients with positive SARS-CoV-2 RT-PCR test results [ 155 ] .
Conclusions
Based on our review of the available literature on asthma and COVID-19, asthma does not confer an increased susceptibility to SARS CoV-2 infection or a worst clinical course in infected patients. Asthma per se does not appear to be a risk factor for COVID-19 overall; however, its actual contribution to the risk may depend on the presence of other environmental and behavioral factors (i.e. smoking, comorbidities) the type and severity of asthma (e.g. non-type 2 asthma phenotypes, uncontrolled asthma), asthma treatments and adherence to therapy (scarce adherence).
Asthma International Guidelines recommend complying with the basic measures of protection against COVID-19 and continuing asthma therapies including ICSs and biological agents in asthmatics during the COVID-19 pandemic. Maintaining current therapy with controller medications, including biological agents, is recommended in all patients with asthma, since the ongoing use of ICSs does not increase the risk of hospitalization in asthmatics with concomitant COVID-19 infection. Eventually, the decision to continue or postpone biologic therapy in patients already infected with SARS-CoV-2 should be individualized.
Further clinical and experimental studies will be necessary to confirm these preliminary data against a “dangerous liaison” between COVID-19 and asthma.
Availability of data and materials
Abbreviations.
Asthma-COPD overlap
Angiotensin-converting enzyme2
Allergen immunotherapy
Acute respiratory distress syndrome
Chronic obstructive pulmonary disease
Coronavirus disease 2019
Fractional exhaled nitric oxide
Intercellular adhesion molecule 1
Innate lymphoid cells
Intensive care unit
Inhaled corticosteroids
Interferons
Interleukin
Impulse oscillometry
International severe acute respiratory and emerging infections consortium
Plasmacytoid dendritic cells
Pulmonary function testing
Thymic stromal lymphopoietin
World health organization
Kolifarhood G, Aghaali M, Mozafar Saadati H, Taherpour N, Rahimi S, Izadi N, et al. Epidemiological and clinical aspects of COVID-19; a narrative review. Arch Acad Emerg Med. 2020;8(1):e41.
PubMed PubMed Central Google Scholar
Calabrese F, Pezzuto F, Fortarezza F, et al. Pulmonary pathology and COVID-19: lessons from autopsy. The experience of European pulmonary pathologists. Virchows Arch. 2020;477(3):359–72.
Article CAS PubMed PubMed Central Google Scholar
Ejaz H, Alsrhani A, Zafar A, et al. COVID-19 and comorbidities: deleterious impact on infected patients. J Infect Public Health. 2020;13(12):1833–9.
Article PubMed PubMed Central Google Scholar
Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese center for disease control and prevention. JAMA. 2020 Apr;323(13):1239–42.
Article CAS PubMed Google Scholar
Centers for Disease Control and Prevention. People with certain medical conditions. 2020. Available from: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html . Accessed January 31, 2021;
James KM. Stokes Peebles Jr R, Hartert T V. response to infections in patients with asthma and atopic disease: an epiphenomenon or reflection of host susceptibility? J Allergy Clin Immunol. 2012;130(2):343–51.
James KM, Peebles SR Jr, Hartert TV. Response to infections in patients with asthma and atopic disease: an epiphenomenon or reflection of host susceptibility? J Allergy Clin Immunol. 2012;130(2):343–51.
Jartti T, Gern JE. Role of viral infections in the development and exacerbation of asthma in children. J Allergy Clin Immunol. 2017;140(4):895–906. https://doi.org/10.1016/j.jaci.2017.08.003 .
Papadopoulos NG, Christodoulou I, Rohde G, et al. Viruses and bacteria in acute asthma exacerbations–a GA2 LENDARE systematic review. Allergy. 2011;66:458–68.
Jackson DJ, Makrinioti H, Rana BMJ, et al. IL-33-dependent type 2 inflammation during rhinovirus-induced asthma exacerbations in vivo. Am J Respir Crit Care Med. 2014;190(12):1373–82. https://doi.org/10.1164/rccm.201406-1039OC .
Fokkens WJ, Garcia-Garcia M, Gjomarkaj M, et al. Viruses and bacteria in acute asthma exacerbations–a GA2LEN-DARE systematic review. Allergy. 2011;66:458–68.
Article PubMed Google Scholar
Jackson DJ, Sykes A, Mallia P, Johnston SL. Asthma exacerbations: origin, effect, and prevention. J Allergy Clin Immunol. 2011;128(6):1165–74.
Channappanavar R, Fehr AR, Vijay R, et al. Dysregulated type I interferon and inflammatory monocyte-macrophage responses cause lethal pneumonia in SARS-CoV-infected mice. Cell Host Microbe. 2016;19(2):181–93.
Durrani SR, Montville DJ, Pratt AS, et al. Innate immune responses to rhinovirus are reduced by the high-affinity IgE receptor in allergic asthmatic children. J Allergy Clin Immunol. 2012;130:489–95.
Wark PA, Johnston SL, Bucchieri F, et al. Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. J Exp Med. 2005;201:937–47.
Novak N, Cabanillas B. Viruses and asthma: the role of common respiratory viruses in asthma and its potential meaning for SARS-CoV-2. Immunology. 2020;161(2):83–93. https://doi.org/10.1111/imm.13240 .
Lebre MC, van Capel TM, Bos JD, et al. Aberrant function of peripheral blood myeloid and plasmacytoid dendritic cells in atopic dermatitis patients. J Allergy Clin Immunol. 2008;122(5):969–76.
Gill MA, Bajwa G, George TA, et al. Counter-regulation between the fc epsilon RI pathway and antiviral responses in human plasmacytoid dendritic cells. J Immunol. 2010;184:5999–6006.
Hatchwell L, Collison A, Girkin J, Parsons K, Li J, Zhang J, et al. Toll-like receptor 7 governs interferon and inflammatory responses to rhinovirus and is suppressed by IL-5-induced lung eosinophilia. Thorax. 2015 Sep;70(9):854–61. https://doi.org/10.1136/thoraxjnl-2014-205465 .
Centers for Disease Control and Prevention. People at high risk of developing flu-related complications. Centers for Disease Control and Prevention 2010, Available at http://www.cdc.gov/flu/about/disease/ high_risk.htm (accessed 9 December 2010).
Van Kerkhove MD, Vandemaele KA, Shinde V, et al. Risk factors for severe outcomes following 2009 influenza a (H1N1) infection: a global pooled analysis. PLoS Med. 2011;8(7):e1001053. https://doi.org/10.1371/journal.pmed.1001053 .
Jain S, Kamimoto L, Bramley AM, Schmitz AM, Benoit SR, Louie J, et al. Hospitalized patients with 2009 H1N1 influenza in the United States, April–June 2009. N Engl J Med. 2009;361(20):1935–44. https://doi.org/10.1056/NEJMoa0906695 .
O’Riordan S, Barton M, Yau Y, et al. Risk factors and outcomes among children admitted to hospital with pandemic H1N1 influenza. CMAJ. 2010;182:39–44.
Almirall J, Bolibar I, Serra-Prat M, Roig J, et al. New evidence of risk factors for community-acquired pneumonia: a population-based study. Eur Respir J. 2008;31:1274–84.
Zhang JJ, Dong X, Cao YY, Yuan YD, Yang YB, Yan YQ, et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020;75(7):1730–41. https://doi.org/10.1111/all.14238 .
Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–13.
Guan W-J, Liang W-H, Zhao Y, Liang HR, Chen ZS, Li YM, et al. Comorbidity and its impact on 1590 patients with Covid-19 in China: a nationwide analysis. Eur Respir J. 2020;55(5):2000547. https://doi.org/10.1183/13993003.00547-2020 .
Grasselli G, Greco M, Zanella A, Albano G, Antonelli M, Bellani G, et al. Risk factors associated with mortality among patients with COVID-19 in intensive care units in Lombardy, Italy. JAMA Intern Med. 2020;180(10):1345–55. https://doi.org/10.1001/jamainternmed.2020.3539 .
Caminati M, Lombardi C, Micheletto C, et al. Asthmatic patients in COVID-19 outbreak: few cases despite many cases. J Allergy Clin Immunol. 2020;146:541–2.
Lombardi C, Roca E, Bigni B, Cottini M, Passalacqua G. Clinical course and outcomes of patients with asthma hospitalized for severe acute respiratory syndrome coronavirus 2 pneumonia: a single-center, retrospective study. Ann Allergy Asthma Immunol. 2020;125(6):707–9.
Avdeev S, Moiseev S, Brovko M, Yavorovskiy A, Umbetova K, Akulkina L, et al. Low prevalence of bronchial asthma and chronic obstructive lung disease among intensive care unit patients with COVID-19. Allergy. 2020;75(10):2703–4. https://doi.org/10.1111/all.14420 .
Shabrawishi M, Al-Gethamy MM, Naser AY et al. Clinical, radiological and therapeutic characteristics of patients with COVID-19 in Saudi Arabia. PLoS One. 2020;15(8):e0237130.
Aggarwal A, Shrivastava A, Kumar A, Ali A. Clinical and epidemiological features of SARS-CoV-2 patients in SARI ward of a tertiary care Centre in New Delhi. J Assoc Phys India. 2020;68:19–26.
Google Scholar
Petrilli CM, Jones SA, Yang J, Rajagopalan H, O'Donnell L, Chernyak Y, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in new York City: prospective cohort study. BMJ. 2020;369:m1966.
Garg S, Kim L, Whitaker M, et al. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019 - COVID-NET, 14 states, march 1-30, 2020. MWR Morb Mortal Wkly Rep. 2020;69(15):458–64.
Article CAS Google Scholar
Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the new York City area. JAMA. 2020;323:2052–9.
Goyal P, Choi JJ, Pinheiro LC, Schenck EJ, Chen R, Jabri A, et al. Clinical characteristics of Covid-19 in new York City. N Engl J Med. 2020;382(24):2372–4. https://doi.org/10.1056/NEJMc2010419 .
Skevaki C, Karsonova A, Karaulov A, Xie M, Renz H. Asthma-associated risk for COVID-19 development. J Allergy Clin Immunol. 2020 Dec;146(6):1295–301. https://doi.org/10.1016/j.jaci.2020.09.017 .
Docherty AB, Harrison EM, Green CA, et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO clinical characterisation protocol: prospective observational cohort study. BMJ. 2020;369:m1985.
Butler MW, O’Reilly A, Dunican EM, et al. Prevalence of comorbid asthma in COVID-19 patients. J Allergy Clin Immunol. 2020;146(2):334–5.
Zhu Z, Hasegawa K, Ma B, et al. Association of asthma and its genetic predisposition with the risk of severe COVID-19. J Allergy Clin Immunol. 2020;146(2):327–329.e4.
Broadhurst R, Peterson R, Wisnivesky JP, et al. Asthma in COVID-19 hospitalizations: an overestimated risk factor? Ann Am Thorac Soc. 2020;17(12):1645–8.
Kalyanaraman Marcello R, Dolle J, Grami S, Adule R, Li Z, Tatem K, et al. Characteristics and outcomes of COVID-19 patients in new York City's public hospital system. PLoS One. 2020;15(12):e0243027. https://doi.org/10.1371/journal.pone.0243027 .
Liu S, Cao Y, Du T, Zhi Y. Prevalence of comorbid asthma and related outcomes in COVID-19: a systematic review and meta-analysis. J Allergy Clin Immunol Pract. 2021 Feb;9(2):693–701. https://doi.org/10.1016/j.jaip.2020.11.054 .
Terry PD, Heidel RE, Dhand R. Asthma in adult patients with COVID-19: prevalence and risk of severe disease. Am J Respir Crit Care Med. 2021;203(7):893–905. https://doi.org/10.1164/rccm.202008-3266OC . Epub ahead of print.
Yang JM, Koh HY, Moon SY, Yoo IK, Ha EK, You S, et al. Allergic disorders and susceptibility to and severity of COVID-19: a nationwide cohort study. J Allergy Clin Immunol. 2020;146(4):790–8. https://doi.org/10.1016/j.jaci.2020.08.008 .
Li X, Xu S, Yu M, et al. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol. 2020;146(1):110–8.
Singer AJ, Morley EJ, Meyers K, et al. Cohort of 4404 persons under investigation for COVID-19 in a NY hospital and predictors of ICU care and ventilation. Ann Emerg Med. 2020;76(4):394–404.
Robinson LB, Fu X, Bassett IV, et al. COVID-19 severity in hospitalized patients with asthma: a matched cohort study. J Allergy Clin Immunol Prac. 2021;9(1):497–500.
Article Google Scholar
Chhiba KD, Patel GB, Huyen T, et al. Prevalence and characterization of asthma in hospitalized and nonhospitalized patients with COVID-19. J Allergy Clin Immunol. 2020;146(2):307–314.e.
Lovinsky-Desir S, Deshpande DR, De A, et al. Asthma among hospitalized patients with COVID-19 and related outcomes. J Allergy Clin Immunol. 2020;146(5):1027–1034.e4.
Lieberman-Cribbin W, Rapp J, Alpert N, et al. The impact of asthma on mortality in patients with COVID-19. Chest. 2020;158(6):2290–29.
Calmes D, Graff S, Maes N, et al. Asthma and COPD are not risk factors for ICU stay and death in case of SARS-CoV2 infection. J Allergy Clin Immunol Pract. 2021;9(1):160–9.
Hansen ESH, Moeller AL, Backer V, et al. Severe outcomes of COVID-19 among patients with COPD and asthma. ERJ Open Res. 2021;7:00594–2020.
Murillo-Zamora E. Hernandez- Suarez C M. survival in adult inpatients with COVID-19. Public Health. 2021 Jan;190:1–3. https://doi.org/10.1016/j.puhe.2020.10.029 .
Sunjaya AP, Allida SM, Di Tanna GL, Jenkins C. Asthma and risk of infection, hospitalisation, ICU admission and mortality from COVID-19: systematic review and meta-analysis. J Asthma. 2021;8:1–22.
CAS Google Scholar
Izquierdo JL, Almonacid C, González Y, et al. The impact of COVID-19 on patients with asthma. Eur Respir J 2020; in press (https://doi.org/ https://doi.org/10.1183/13993003.03142-2020
Beurnier A, Jutant E-M, Jevnikar M, et al. Characteristics and outcomes of asthmatic patients with COVID-19 pneumonia who require hospitalisation. Eur Respir J. 2020;56(5):2001875.
Atkins JL, Masoli JAH, Delgado J, Pilling LC, Kuo CL, Kuchel GA, et al. Preexisting comorbidities predicting COVID-19 and mortality in the UK biobank community cohort. J Gerontol A Biol Sci Med Sci. 2020;75(11):2224–30. https://doi.org/10.1093/gerona/glaa183 .
Grandbastien M, Piotin A, Godet J, et al. SARS-CoV-2 pneumonia in hospitalized asthmatic patients did not induce severe exacerbation. J Allergy Clin Immunol Pract. 2020;8(8):2600–7.
Coronavirus 19. Center for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/asthma.html . Updated Jan. 20, 2021.
Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, et al. Open SAFELY: factors associated with COVID-19 death in 17 million patients. Nature. 2020;584(7821):430–6. https://doi.org/10.1038/s41586-020-2521-4 .
Schultze A, Walker AJ, MacKenna B, Morton CE, Bhaskaran K, Brown JP, et al. Risk of COVID-19-related death among patients with chronic obstructive pulmonary disease or asthma prescribed inhaled corticosteroids: an observational cohort study using the open SAFELY platform. Lancet Respir Med. 2020;8:1106–20.
Lee SC, Son KJ, Han CH, Jung JY, Park SC. Impact of comorbid asthma on severity of coronavirus disease (COVID-19). Sci Rep. 2020;10(1):21805.
Choi YJ, Park J-Y, Lee HS, et al. Effect of asthma and asthma medication on the prognosis of patients with COVID-19. Eur Respir J. 2021;4:2002226.
Caminati M, Vultaggio A, Matucci A, Senna G, Almerigogna F, Bagnasco D, et al. Asthma in a large COVID-19 cohort: prevalence, features, and determinants of COVID-19 disease severity. Respir Med. 2021;176:106261. https://doi.org/10.1016/j.rmed.2020.106261 .
Izquierdo JL, Almonacid C, Gonzalez Y, et al. The impact of COVID 19 in patients with asthma. Eur Respir J. 2021;57(3):2003142.
Hanon S, Brusselle G, Deschampheleire M, et al. COVID-19 and biologics in severe asthma: data from the Belgian severe asthma registry. Eur Respir J. 2020;56:2002857.
Article CAS PubMed Central Google Scholar
Smith SJ, Busby J, Heaney LG, et al. The impact of the first COVID-19 surge on severe asthma patients in the UK. Which is worse: the virus or the lockdown? ERJ Open Res. 2021;7(1):00768–2020.
Heffler E, Detoraki A, Contoli M, Papi A, Paoletti G, Malipiero G, et al. COVID-19 in severe asthma network in Italy (SANI) patients: clinical features, impact of comorbidities and treatments. Allergy. 2020;76(3):887–92. https://doi.org/10.1111/all.14532 .
Antonicelli L, Tontini C, Manzotti G, et al. Severe asthma in adults does not significantly affect the outcome of COVID-19 disease: Results from the Italian Severe Asthma Registry. Allergy. 2020. https://doi.org/10.1111/all.14558 .
Matucci A, Caminati M, Vivarelli E, et al. COVID-19 in severe asthmatic patients during ongoing treatment with biologicals targeting type 2 inflammation: results from a multicenter Italian survey. Allergy. 2021;76(3):871–4.
Rial MJ, Valverde M, Del Pozo V, et al. Clinical characteristics in 545 patients with severe asthma on biological treatment during the COVID-19 outbreak. J Allergy Clin Immunol Pract. 2021;9(1):487–489.e1.
Eger K, Hashimoto S, Braunstahl GJ, et al. Poor outcome of SARS-CoV-2 infection in patients with severe asthma on biologic therapy. Respir Med. 2020;177:106287.
Eger K, Bel EH. Asthma and COVID-19: do we finally have answers? Eur Respir J. 2020 Dec;30:2004451.
Farne H, Singanayagam A. Why asthma might surprisingly protect against poor outcomes in COVID-19. Eur Respir J. 2020;56(6):2003045. https://doi.org/10.1183/13993003.03045-2020 .
Basnet S, Palmenberg AC, Gern JE. Rhinoviruses and their receptors. Chest. 2019;155(5):1018–25.
Walls AC, Park YJ, Tortorici MA, et al. Function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181(2):281–292.e6.
Song J, Zeng M, Wang H, et al. Distinct effects of asthma and COPD comorbidity on disease expression and outcome in patients with COVID-19. Allergy. 2020;00:1–14.
Lukassen S, Chua RL, Trefzer T, et al. SARSCoV-2 receptor ACE2 and TMPRSS2 are primarily expressed in bronchial transient secretory cells. EMBO J. 2020;39:e105114.
Peters MC, Sajuthi S, Deford P, et al. COVID-19-related genes in sputum cells in asthma. Relationship to demographic features and corticosteroids. Am J Respir Crit Care Med. 2020;202(1):83–90.
Jackson DJ, Busse W, Bacharier LB, et al. Association of respiratory allergy, asthma, and expression of the SARS-CoV-2 receptor ACE2. J Allergy Clin Immunol. 2020;146(1):203–206.e3.
Kimura H, Francisco D, Conway M, et al. Type 2 inflammation modulates ACE2 and TMPRSS2 in airway epithelial cells. J Allergy Clin Immunol. 2020;146(1):80–88.e8.
Matsumoto K, Saito H. Does asthma affect morbidity or severity of COVID-19? J Allergy Clin Immunol. 2020;146(1):55–7.
Camiolo MJ, Gauthier M, Kaminski N, et al. Expression of SARS-CoV-2 receptor ACE2 and coincident host response signature varies by asthma inflammatory phenotype. J Allergy Clin Immunol. 2020;S0091–6749(20):30828–9.
Kermani NZ, Song WJ, Badi Y, et al. U-BIOPRED Consortium. Sputum ACE2, TMPRSS2 and FURIN gene expression in severe neutrophilic asthma. Respir Res. 2021;22(1):10.
Bradding P, Richardson M, Hinks TSC, et al. ACE2, TMPRSS2, and furin gene expression in the airways of people with asthma-implications for COVID-19. J Allergy Clin Immunol. 2020;146(1):208–11.
Wösten-van Asperen RM, Lutter R, Specht PA, et al. Acute respiratory distress syndrome leads to reduced ratio of ACE/ACE2 activities and is prevented by angiotensin-(1-7) or an angiotensin II receptor antagonist. J Pathol. 2011;225(4):618–27. https://doi.org/10.1002/path.2987 .
Leung JM, Yang CX, Tam A, et al. ACE-2 expression in the small airway epithelia of smokers and COPD patients: implications for COVID-19. Eur Respir J. 2020;55(5).
Nelson RK, Bush A, Stokes J, et al. Eosinophilic asthma. J Allergy Clin Immunol Pract. 2020;8(2):465–73.
Busse W, Kraft M, Rabe KF, et al. Understanding the key issues in the treatment of uncontrolled persistent asthma with type 2 inflammation. Eur Respir J. 2021;4:2003393.
Fahy JV. Type 2 inflammation in asthma – present in most, absent in many. Nat Rev Immunol. 2015;15:57–65.
Keswani A, Dhana K, Rosenthal JA, et al. Atopy is predictive of a decreased need for hospitalization for coronavirus disease2019. Ann Allergy Asthma Immunol. 2020;125(4):479–81.
Scala E, Abeni D, Tedeschi A, et al. Atopic status protects from severe complications of COVID-19. Allergy. 2020. https://doi.org/10.1111/all.14551 .
Nair P, Prabhavalkar KS. Neutrophilic asthma and potentially related target therapies. Curr Drug Targets. 2020;21(4):374–88. https://doi.org/10.2174/1389450120666191011162526 .
Israel E, Reddel HK. Severe and difficult-to-treat asthma in adults. N Engl J Med. 2017;377:965–76.
Peters MC, McGrath KW, Hawkins GA, et al. Plasma interleukin-6 concentrations, metabolic dysfunction, and asthma severity: a cross-sectional analysis of two cohorts. Lancet Respir Med. 2016;4(7):574–84.
Leynaert B, Sunyer J, Garcia-Esteban R, et al. Gender differences in prevalence, diagnosis and incidence of allergic and non-allergic asthma: a population-based cohort. Thorax. 2012;67:625–31.
Zein JG, Erzurum SC. Asthma is different in women. Curr Allergy Asthma Rep. 2015;15:28.
Deng K, Zhang X, Liu Y, et al. Visceral obesity is associated with clinical and inflammatory features of asthma: a prospective cohort study. Allergy Asthma Proc. 2020;41(5):348–56.
Scott HA, Gibson PG, Garg ML, Wood LG. Airway inflammation is augmented by obesity and fatty acids in asthma. Eur Respir J. 2011;38(3):594–602.
Polosa R, Russo C, Caponnetto P, Bertino G, Sarvà M, Antic T, et al. Greater severity of new onset asthma in allergic subjects who smoke: a 10-year longitudinal study. Respir Res. 2011;12(1):16. https://doi.org/10.1186/1465-9921-12-16 .
Cerveri I, Cazzoletti L, Corsico AG. The impact of cigarette smoking on asthma: a population-based international cohort study. Int Arch Allergy Immunol. 2012;158:175–83.
Thomson NC, Chaudhuri R, Heaney LG, Bucknall C, Niven RM, Brightling CE, et al. Clinical outcomes and inflammatory biomarkers in current smokers and exsmokers with severe asthma. J Allergy Clin Immunol. 2013 Apr;131(4):1008–16. https://doi.org/10.1016/j.jaci.2012.12.1574 .
Gupta AK, Nethan ST, Mehrotra R. Tobacco use as a well-recognized cause of severe COVID-19 manifestations. Respir Med. 2021;176:106233.
Alberca RW, Lima JC, de Oliveira EA, et al. COVID-19 disease course in former smokers. Smokers and COPD Patients Front Physiol. 2021;11:637627.
Global Initiative for Asthma (GINA). Diagnosis and initial treatment of asthma, COPD and asthma-COPD overlap. 2017. https://ginasthma.org/asthma-copd-and-asthma-copd-overlap-syndrome-acos/
Uchida A, Sakaue K, Inoue H. Epidemiology of asthma-chronic obstructive pulmonary disease overlap (ACO). Allergol Int. 2018;67(2):165–71. https://doi.org/10.1016/j.alit.2018.02.002 .
Gibson PG, McDonald VM. Asthma-COPD overlap 2015: now we are six. Thorax. 2015;70:683–69.
Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20:363–74.
Carli G, Cecchi L, Stebbing J, et al. Is asthma protective against COVID-19? Allergy. 2020. https://doi.org/10.1111/all.14426 .
Rosenberg HF, Dyer KD, Domachowske JB. Respiratory viruses and eosinophils: exploring the connections. Antivir Res. 2009;83(1):1–9.
Munblit D, Nekliudov NA, Bugaeva P, et al. StopCOVID cohort: An observational study of 3,480 patients admitted to the Sechenov University hospital network in Moscow city for suspected COVID-19 infection. Clin Infect Dis. 2020:ciaa1535.
Lipworth B, Chan R, Kuo CR. Predicting severe outcomes in COVID-19. J Allergy Clin Immunol Pract. 2020;8:2582–4.
Lindsley AW, Schwartz JT, Rothenberg ME. Eosinophil responses during COVID-19 infections and coronavirus vaccination. J Allergy Clin Immunol. 2020;146:1–7.
Roca E, Ventura L, Zattra CM. C Lombardi. EOSINOPENIA: an early, effective, and relevant COVID-19 biomarker? QJM. 2021;114(1):68–9. https://doi.org/10.1093/qjmed/hcaa259 .
Ferastraoaru D, Hudes G, Jerschow E, et al. Eosinophilia in asthma patients is protective against severe COVID-19 illness. J Allergy Clin Immunol Pract. 2021;S2213–2198(20):31409–4.
Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–62.
Chauhan AJ, Inskip HM, Linaker CH, Smith S, Schreiber J, Johnston SL, et al. Personal exposure to nitrogen dioxide (NO2) and the severity of virus-induced asthma in children. Lancet. 2003;361(9373):1939–44. https://doi.org/10.1016/S0140-6736(03)13582-9 .
Abe K, Miyawaki A, Nakamura M, et al. Trends in hospitalizations for asthma during the COVID-19 outbreak in Japan. J Allergy Clin Immunol Pract. 2021;9(1):494–496.e1.
Castillo JR, Peters SP, Busse WW. Asthma exacerbations: pathogenesis, prevention, and treatment. J Allergy Clin Immunol Pract. 2017;5:918–27.
Kaye L, Theye B, Smeenk I, et al. Changes in medication adherence among patients with asthma and COPD during the COVID-19 pandemic. J Allergy Clin Immunol Pract. 2020;8(7):2384–5.
Vázquez-Nava F, Vazquez-Rodriguez EM, Vazquez-Rodriguez CF, et al. Risk factors of non-adherence to guidelines for the prevention of COVID-19 among young adults with asthma in a region with a high risk of a COVID-19 outbreak. J Asthma. 2020;17:1–7.
Halpin DMG, Singh D, Hadfield RM. Inhaled corticosteroids and COVID-19: a systematic review and clinical perspective. Eur Respir J. 2020 May 7;55(5):2001009. https://doi.org/10.1183/13993003.01009-2020 .
Miyazawa D, Kaneko G. Clinical trials of inhaled beclomethasone and mometasone for COVID-19 should be conducted. J Med Virol. 2021;93(2):637–8. https://doi.org/10.1002/jmv.26413 Epub 2020 Aug 16.PMID: 32776550.
Assaf SM, Tarasevych SP, Diamant Z, Hanania NA. Asthma and severe acute respiratory syndrome coronavirus 2019: current evidence and knowledge gaps. Curr Opin Pulm Med. 2021;27:45–53.
Kenyon CC, Hill DA, Henrickson SE, et al. Initial effects of the COVID-19 pandemic on pediatric asthma emergency department utilization. J Allergy Clin Immunol Pract. 2020;8:2774.e1–6.e1.
Lipworth BJ. Systemic adverse effects of inhaled corticosteroid therapy: a systematic review and meta-analysis. Arch Intern Med. 1999;159(9):941–55. https://doi.org/10.1001/archinte.159.9.941 .
Oliver BG, Robinson P, Peters M, et al. Viral infections and asthma: an inflammatory interface? Eur Respir J. 2014;44(6):1666–81. https://doi.org/10.1183/09031936.00047714 .
Bochkov YA, Busse WW, Brockman-Schneider RA, Evans MD, Jarjour NN, McCrae C, et al. Budesonide and formoterol effects on rhinovirus replication and epithelial cell cytokine responses. Respir Res. 2013;14(1):98. https://doi.org/10.1186/1465-9921-14-98 .
Matsuyama S, Kawase M, Nao N, et al. The inhaled corticosteroid ciclesonide blocks coronavirus RNA replication by targeting viral NSP15. bioRxiv. 2020. https://doi.org/10.1101/2020.03.11.987016 .
Yamaya M, Nishimura H, Deng X, Sugawara M, Watanabe O, Nomura K, et al. Inhibitory effects of glycopyrronium, formoterol, and budesonide on coronavirus HCoV-229E replication and cytokine production by primary cultures of human nasal and tracheal epithelial cells. Respir Investig. 2020;58(3):155–68. https://doi.org/10.1016/j.resinv.2019.12.005 .
Liu S, Zhi Y, Ying S. COVID-19 and asthma: reflection during the pandemic. Clin Rev Allergy Immunol. 2020;59(1):78–88. https://doi.org/10.1007/s12016-020-08797-3 .
Horby P, Lim WS, Emberson JR, et al. Dexamethasone in Hospitalized Patients with Covid-19 - Preliminary Report. RECOVERY Collaborative Group, N Engl J Med. 2020:NEJMoa2021436.
Kow CS, Syed Shahzad Hasan SS. Preadmission use of inhaled corticosteroids and risk of fatal or severe COVID-19: a meta-analysis. J Asthma. 2021;8:1–4.
Scadding GK, Hellings PW, Bachert C, et al. Allergic respiratory disease care in the COVID-19 era: a EUFOREA statement. World Allergy Organ J. 2020;13:100124.
GINA Global strategy for asthma: Management and prevention interim guidance about COVID-19 and asthma, updated 20 2020; www.ginaasthma.org
Morais-Almeida M, Aguiar R, Martin B, Ansotegui IJ, Ebisawa M, Arruda LK, et al. COVID-19, asthma, and biological therapies: what we need to know. World Allergy Organ J. 2020;13(5):100126. https://doi.org/10.1016/j.waojou.2020.100126 .
Riggioni C, Comberiati P, Giovannini M, et al. A compendium answering 150 questions on COVID-19 and SARS-CoV-2. Allergy. 2020;75:2503–41.
García-Moguel I, Campos RD, Charterina SA, et al. COVID-19, severe asthma, and biologics. Ann Allergy Asthma Immunol. 2020;125:341–60.
Renner A, Marth K, Patocka K, et al. COVID-19 in two severe asthmatics receiving benralizumab: busting the eosinophilia myth. ERJ Open Res. 2020;6:00457–2020.
Renner A, Marth K, Patocka K, Pohl W. COVID-19 in a severe eosinophilic asthmatic receiving benralizumab - a case study. J Asthma. 2020 Jun;18:1–3.
Aksu K, Yesilkaya S, Topel M, et al. COVID-19 in a patient with severe asthma using mepolizumab. Allergy Asthma Proc. 2021;42:1–3.
Azim A, Pini L, Khakwani Z, Kumar S, Howarth P, et al. Ann Allergy Asthma Immunol. 2021;S1081–1206(21):00014.
Lommatzsch M, Stoll P, Virchow JC. COVID-19 in a patient with severe asthma treated with Omalizumab. Allergy. 2020;75(10):2705–8.
Bhalla A, Mukherjee M, Radford K, et al. Dupilumab, severe asthma airway responses, and SARS-CoV-2 serology. Allergy. 2020. https://doi.org/10.1111/all.14534 .
Vultaggio A, Agache I, Akdis CA, Akdis M, Bavbek S, Bossios A, et al. Considerations on biologicals for patients with allergic disease in times of the COVID-19 pandemic: an EAACI statement. Allergy. 2020;75(11):2764–74. https://doi.org/10.1111/all.14407 .
Shaker MS, Oppenheimer J, Grayson M, et al. COVID-19: pandemic contingency planning for the allergy and immunology clinic. J Allergy Clin Immunol. 2020;8(5):1477–88.
Frati F, Incorvaia C, Lombardi C, Senna GE. Allergen immunotherapy: 100 years, but it does not look like. Eur Ann Allergy Clin Immunol. 2012 Jun;44(3):99–106.
CAS PubMed Google Scholar
Komlósi ZI, Kovács N, Sokolowska M, et al. Mechanisms of subcutaneous and sublingual aeroallergen immunotherapy: what is new? Immunol Allergy Clin N Am. 2020;40:1–14.
Tatura R, Zeschnigk M, Hansen W, Steinmann J, Goncalves Vidigal P, Hutzler M, et al. Relevance of Foxp3 + regulatory T cells for early and late phases of murine sepsis. Immunology. 2015;146(1):144–56. https://doi.org/10.1111/imm.12490 .
Qiu D, Chu X, Hua L, et al. Gpr174-deficient regulatory T cells decrease cytokine storm in septic mice. Cell Death Dis. 2019;10(3):233.
Article PubMed PubMed Central CAS Google Scholar
Klimek L, Pfaar O, Worm M, et al. Allergen immunotherapy in the current COVID-19 pandemic: a position paper of AeDA, ARIA, EAACI, DGAKI and GPA. Allergol Sel. 2020;4:44–52.
Larenas-Linnemann D, Rodrıguez-Perez N, Ortega-Martell JA, et al. Mexican immunotherapy working group. Coronavirus disease 2019 and allergen immunotherapy: theoretical benefits invite to adjustments in practice recommendations. Ann allergy. Asthma Immunol. 2020;125(3):247–9. https://doi.org/10.1016/j.anai.2020.06.009 .
Klimek L, Jutel M, Akdis C, et al. ARIA-MASK Study Group. Handling of allergen immunotherapy in the COVID-19 pandemic: an ARIA-EAACI statement. Allergy. 2020;75(7):1546–54.
Article PubMed CAS Google Scholar
Download references
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and affiliations.
Departmental Unit of Allergology, Immunology & Pulmonary Diseases, Fondazione Poliambulanza, Brescia, Italy
Carlo Lombardi
Departmental Unit of Pneumology & Allergology, Fondazione Poliambulanza Istituto Ospedaliero, Via Bissolati, 57, 25100, Brescia, Italy
Allergy Outpatients Clinic, Turin, Italy
Federica Gani
Ospedale Santa Chiara and Department of Cellular, Computational and Integrative Biology (CIBIO), University of Trento, Trento, Italy
Alvise Berti
Thoracic Disease Research Unit, Mayo Clinic, Rochester, MN, USA
Alvise Berti & Diego Peroni
Department of Clinical and Experimental Medicine, Section of Pediatrics, University of Pisa, Pisa, Italy
Pasquale Comberiati
Department of Clinical Immunology and Allergology, I.M. Sechenov First Moscow State Medical University, Moscow, Russia
Allergy and Pneumology Outpatient Clinic, Bergamo, Italy
Marcello Cottini
You can also search for this author in PubMed Google Scholar
Contributions
CL and MC conceived the study, MC drafted the first version of the manuscript, AB significanly recise the article and image. FG, DP, PC, AB, MC, CL were involved in the writing and editing of the manuscript, and approved the final version to be published. All authors declare that they have no financial and/or personal relationships with other individuals or organizations that could inappropriately influence their work. The author(s) read and approved the final manuscript.
Corresponding author
Correspondence to Carlo Lombardi .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors have no financial or non-financial potential conflicts of interest to declare related to this project. All authors confirm that there are no conflicts of interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Lombardi, C., Gani, F., Berti, A. et al. Asthma and COVID-19: a dangerous liaison?. asthma res and pract 7 , 9 (2021). https://doi.org/10.1186/s40733-021-00075-z
Download citation
Received : 29 March 2021
Accepted : 29 June 2021
Published : 15 July 2021
DOI : https://doi.org/10.1186/s40733-021-00075-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Biological agents
- Immunotherapy
Asthma Research and Practice
ISSN: 2054-7064
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
- Research article
- Open access
- Published: 27 March 2024
Comparing the clinical practice and prescribing safety of locum and permanent doctors: observational study of primary care consultations in England
- Christos Grigoroglou ORCID: orcid.org/0000-0003-1621-8648 1 ,
- Kieran Walshe 2 ,
- Evangelos Kontopantelis 3 , 4 ,
- Jane Ferguson 5 ,
- Gemma Stringer 2 ,
- Darren M. Ashcroft 3 , 6 , 7 &
- Thomas Allen 1 , 8
BMC Medicine volume 22 , Article number: 126 ( 2024 ) Cite this article
208 Accesses
21 Altmetric
Metrics details
Temporary doctors, known as locums, are a key component of the medical workforce in the NHS but evidence on differences in quality and safety between locum and permanent doctors is limited. We aimed to examine differences in the clinical practice, and prescribing safety for locum and permanent doctors working in primary care in England.
We accessed electronic health care records (EHRs) for 3.5 million patients from the CPRD GOLD database with linkage to Hospital Episode Statistics from 1st April 2010 to 31st March 2022. We used multi-level mixed effects logistic regression to compare consultations with locum and permanent GPs for several patient outcomes including general practice revisits; prescribing of antibiotics; strong opioids; hypnotics; A&E visits; emergency hospital admissions; admissions for ambulatory care sensitive conditions; test ordering; referrals; and prescribing safety indicators while controlling for patient and practice characteristics.
Consultations with locum GPs were 22% more likely to involve a prescription for an antibiotic (OR = 1.22 (1.21 to 1.22)), 8% more likely to involve a prescription for a strong opioid (OR = 1.08 (1.06 to 1.09)), 4% more likely to be followed by an A&E visit on the same day (OR = 1.04 (1.01 to 1.08)) and 5% more likely to be followed by an A&E visit within 1 to 7 days (OR = 1.05 (1.02 to 1.08)). Consultations with a locum were 12% less likely to lead to a practice revisit within 7 days (OR = 0.88 (0.87 to 0.88)), 4% less likely to involve a prescription for a hypnotic (OR = 0.96 (0.94 to 0.98)), 15% less likely to involve a referral (OR = 0.85 (0.84 to 0.86)) and 19% less likely to involve a test (OR = 0.81 (0.80 to 0.82)). We found no evidence that emergency admissions, ACSC admissions and eight out of the eleven prescribing safety indicators were different if patients were seen by a locum or a permanent GP.
Conclusions
Despite existing concerns, the clinical practice and performance of locum GPs did not appear to be systematically different from that of permanent GPs. The practice and performance of both locum and permanent GPs is likely shaped by the organisational setting and systems within which they work.
Peer Review reports
Staff shortages in the global health sector have been described as one of the most significant health issues of our time [ 1 ]. Recruitment difficulties, high vacancy rates, and low retention [ 2 ] have combined to result in increasing reliance on temporary staff [ 3 ]. Despite international growth in the number of temporary doctors (often known as locums or locum tenens) [ 4 , 5 , 6 ] there has been limited research on how the use of locum doctors might affect patient safety and quality of care [ 7 ].
Locums are a vital resource that enables healthcare organisations to deliver care; however, the way locums are recruited, employed and used by organisations may to have implications for quality and safety [ 8 ]. There have been some past high-profile examples of poor-quality care by locum doctors [ 9 , 10 , 11 ], the same could likely be said for permanent doctors, but comparative research between the two groups is lacking. However, previous research has shown that locums are often stigmatised, blamed for quality problems and treated with suspicion and even hostility by permanent doctors and other clinical staff [ 12 ]. The use of locum doctors in healthcare has also been associated with lower productivity [ 13 ] and higher costs [ 14 ]. But previous research is extremely limited and robust evidence about the quality and safety of locum doctors’ practice is lacking partly due to the poor availability of routinely collected data about locum doctors [ 8 ].
The aim of this research was to examine whether there exist differences in clinical practice and prescribing safety outcomes for locum and permanent doctors working in primary care in England using a unique database that allows for the distinction between locum and permanent general practitioners (GPs). This paper addresses a gap in the empirical evidence base by seeking to compare a range of quality and safety outcomes for locum and permanent general practitioners. These included practice revisits; prescribing, test ordering and referral rates; and subsequent accident and emergency attendances and emergency hospital admissions. It also compares permanent and locum doctors on a number of established indicators of prescribing safety.
Data sources and study population
We used the Clinical Practice Research Datalink (CPRD) GOLD, a large computerised database of anonymised primary care medical records. It contains complete electronic health records (EHRs) for over 14 million patients in general practices using the Vision system, with the healthcare events (diagnoses, treatments, referrals, tests and prescriptions) recorded using coding systems [ 15 ]. The database is broadly representative of the United Kingdom’s (UK) population in terms of age, gender and deprivation, and the data have been shown in numerous validation studies to be generally of high quality [ 16 , 17 ]. However, larger practices are slightly over-represented and the data are from practices using the Vision clinical system, and clinical system usage is geographically clustered in the UK [ 18 ]. Practices need to meet pre-specified data entry quality criteria to be defined as ‘up to research standard’, and for each study year, our main sample included all CPRD English practices that were classed as such for the whole year. We used all eligible patients in CPRD GOLD for the years 2010–2011 to 2021–2022.
We also obtained CPRD-linked Hospital Episode Statistics (HES) data. The national Hospital Episode Statistics (HES) data contain details of all admissions and A&E visits to NHS hospitals in England [ 19 ]. Area deprivation, as measured by the Index of Multiple Deprivation (IMD) 2015 was available at the 2011 Lower Super Output Area (LSOA) level, a level of English geography with approximately 1,500 residents. The IMD measures deprivation at the area level based on domains, such as income, employment, health, housing and general environment and is the most complete and widely used approach to quantify relative deprivation and affluence for small areas in England [ 20 ]. From CPRD, we obtained patient-level quintiles of deprivation.
Linkage between CPRD GOLD data and the IMD and HES data sources is available at the individual patient level for those patients registered at practices in England that have consented to data linkage. Linkage between data sets is undertaken by CPRD using a deterministic linkage algorithm, based on a patient's exact NHS identification number, sex, date of birth, and residential postcode and approximately 68.6% of patients were eligible for linkage with the majority of the remainder 31.4% living in the other constituent UK countries [ 21 ].
To compare the clinical practice and prescribing safety of locum and permanent doctors we used a range of outcome measures based on face-to-face consultation events with either a locum or permanent GP (consultations with other staff groups were not included). The clinical codes used to generate these outcomes are included in Additional file 1 (Tables S1–S15). These outcomes are widely used to compare clinical practice and prescribing safety and were selected on the basis of their relevance to patient quality and safety and the work locums undertake.
Return to practice for a revisit within 7 days, was selected as a general quality and safety measure under the assumption that a patient who revisits their practice within a week may not have been assured or satisfied by the initial consultation. Hospital visit outcomes are often used as performance indicators and good quality, accessible and continuous primary care may prevent the development of health problems that require an A&E visit or an emergency hospital admission, particularly admissions for ambulatory care sensitive conditions (ACSCs) [ 8 , 22 , 23 , 24 ]. Referral and test ordering rates were included to assess whether differences existed, though the interpretation of any such differences is complex [ 25 , 26 ]. Prescribing rates (including repeat prescriptions) for some drug groups were measured as, in the UK, national guidelines have stressed the need to control and reduce the use of antibiotics, [ 27 ] strong opioids, [ 28 ] and hypnotics [ 28 ]. Some well-established measures of prescribing safety were included based on validated indicators aimed at reducing rates of hazardous prescribing [ 29 ].

Practice revisit within 7 days
Our first outcome examined whether the patient revisited the general practice within 7 days of a consultation event. We identified consultation events within CPRD for each patient in each year, and we calculated the time in days between two consecutive consultation events including telephone and online consultations. We generated a binary variable indicating whether the patient revisited for a consultation within 7 days.
Antibiotic prescriptions
Antibiotic prescriptions were classified using the British National Formulary (BNF) sections (Additional file 1 : Table S1). For all consultation events, we created a binary variable indicating whether an antibiotic was prescribed during the consultation.
Strong opioid prescriptions
Strong opioid prescriptions (alfentanil, buprenorphine, cyclizine, diamorphine, methadone, morphine, naloxone, oxycodone, papaveretum, pentazocine, pethidine, tapentadol) were classified using the BNF sections (Additional file 1 : Table S2). For all consultation events, we created a binary variable indicating whether a strong opioid was prescribed during the consultation.
Hypnotics and anxiolytics prescriptions
Prescriptions for hypnotics were classified using the BNF sections (Additional file 1 : Table S3–S4). Benzodiazepines and z-drugs (zolpidem and zopiclone) were included in the analyses. For all consultation events, we created a binary variable indicating the prescription of a hypnotic during the consultation.
A&E visits
Using the HES A&E data, we identified all A&E visits within 7 days following a consultation event. Two binary variables were created indicating whether there was an A&E visit on the same day or within 1 to 7 days of the consultation event.
Emergency admissions
Emergency admissions are recorded in the HES Admitted Patient Care. We identified all emergency admissions within 7 days following a consultation event. Two binary variables were created indicating whether there was an emergency admission on the same day or within 1 to 7 days of the consultation event.
Ambulatory Care Sensitive Conditions (ACSC) admissions
Classification of ACSC hospital admissions for the study used the International Classification of Diseases, 10th edition (ICD-10) and included all hospital admissions with a primary diagnosis related to one of the nine ACSCs that are incentivised in the UK’s Quality and Outcomes Framework (QOF) [ 30 ]. We identified all ACSC admissions within 7 days following a consultation event. Two binary variables were created indicating whether there was an ACSC admission on the same day or within 1 to 7 days of the consultation event. The ICD-10 chapters used to define admissions for ambulatory care-sensitive conditions are provided in Table S16 in Additional file 2 .
We identified all consultation events and created a binary variable indicating whether any test was ordered during the consultation event.
We identified all consultation events and created a binary variable indicating whether a referral to any other service was made during the consultation event.
Prescribing safety indicators
We adapted 10 indicators of prescribing safety developed for PINCER, a pharmacist-led intervention to improve prescribing safety by identifying patients at risk of potentially hazardous prescribing events [ 31 , 32 ]. These indicators are associated with potentially harmful outcomes such as GI bleeding, asthma, heart failure and stroke. The code lists used to define product and medical codes for the potentially hazardous prescribing indicators are provided in Additional file 1 : Tables S5–S15.
Statistical analyses
We conducted an observational study of GP consultations of registered patients at 407 CPRD GOLD participating general practices in England, between 1st April 2010 to 31st March 2022.
Consultation information was extracted within each financial year, for each active patient (registered for at least 1 day during the respective year). Patients who had a recorded year of death before the beginning of the period of study were excluded from the analyses. Patients who had a consultation following their date of death as recorded within CPRD were excluded from the analyses. We restricted our sample to include only practices in England, as data on the IMD and hospital outcomes were only available for patients located in England.
Clinical practice
In the first set of models investigating various clinical practice indicators, we randomly selected one consultation event for each patient within each financial year, aligning all the patient outcomes and covariates to that specific event date. This allowed us to give equal weights to patients and limited the potential for confounding introduced by higher-need patients who may be visiting numerous times within a year. Our exposure was a binary variable indicating whether the consultation was by a permanent GP or a locum GP. We were able to identify permanent GPs and locum GP through the staff role field which is available for every consultation. This approach was used for practice revisits; prescribing of antibiotics, strong opioids and hypnotics; tests and referrals; and hospital outcomes.
In the second set of models investigating the PINCER prescribing safety indicators, for each indicator, we identified all consultation events with patients who could be exposed to potentially hazardous prescribing, because of a specific diagnosis or prescription on the day of the consultation (i.e. index event). These events were split into consultations by locum or permanent GPs. Second, for each index consultation event, we looked at consultation events during a pre-specified time window (which varied across indicators; Table S17 in Additional file 2 ) leading up to the index event, to identify pre-existing prescriptions or conditions that would trigger a potentially hazardous prescribing outcome when combined with the index event. For each index consultation event, a binary variable indicated whether potentially hazardous prescribing was triggered. This allowed us to operationalise rates of potentially hazardous prescribing events for both locum and permanent GPs. Our exposure was again a binary variable indicating whether the consultation involved a permanent GP or a locum GP and we aligned patient covariates to the index consultation event using unique patient IDs.
For example, for indicator A we identified consultations for patients who were over 65, at which they were prescribed an NSAID. We then identified those patients who were not also prescribed the recommended proton-pump inhibitor (PPI) or H2 receptor antagonist at the consultation or in the preceding 3 months. The operational definitions for the PINCER prescribing safety indicators are provided in Table S17 in Additional file 2 .
Model covariates
We used Read codes [ 33 ] to identify the presence of comorbidities and we calculated the validated Cambridge Multimorbidity Score [ 34 ] for each patient in our cohort in 2010, which was our baseline year. Additional information on patient age, gender, years registered with the practice, practice list size, patient urban/rural location, patient deprivation and region was used.
We employed multi-level mixed effects logistic regression models to quantify the association between the exposure of interest (locum/permanent GP) and the outcomes of interest, controlling for all available covariates over time. For the first set of models for clinical practice outcomes, our analyses used a random consultation per patient and accounted for the nested structure of the data: patients within general practices, within regions. For the prescribing safety indicators models, analyses were conducted on repeated consultations, with consultations nested within patients. We included random effects for practices and fixed effects for regions in both sets of models. In both sets of models, consultation events with missing information on age or gender were excluded from the analyses. We also performed a sensitivity analyses excluding the last 3 years of data, to evaluate whether the effect sizes of our exposure (ie locum consultations) on the outcomes were affected by the COVID-19 period in the UK.
Stata v17 was used for data cleaning, management and analyses and an α level of 1% was used throughout [ 35 ]. However, statistical significance is not very informative in analyses of datasets of this size and we focus on the clinical significance of the effect sizes rather than p values [ 35 ].
The number of practices in England participating in CPRD GOLD varied from 487 in 2010–2011, to 228 in 2015–2016, to 42 in 2021–2022. Of these, only 42 practices contributed data throughout the whole of the study period and 407 had complete data, including hospital admissions and deprivation for their patients. For the first set of models, our cohort consisted of 3,591,367 patients with 13,696,455 recorded consultations between 407 practices across all years. For the second set of models, our cohort consisted of 547,146 patients with 7,623,205 recorded consultations, which varied by indicator and included patients from 407 practices across all years. In Table 1 , we provide descriptive statistics for the consultation outcomes and some important practice and patient characteristics. In Table 2 , we summarise the numerators and denominators that allowed us to calculate the proportion of consultations that were exposed to potentially hazardous prescribing for each indicator. The numerator is the number of consultations that were exposed to each type of potentially hazardous prescribing, and the denominator, is the number of consultations of patients at risk of exposure to the hazardous prescribing indicator.
The results from our regression models are shown in Table 3 . We found mixed differences between permanent and locum GPs in both the clinical practice indicators and the prescribing safety indicators, with some rates being higher or lower for locums and some non-significant differences.
We find that a consultation with a locum was 12% less likely to lead to a practice revisit within 7 days (OR = 0.88, 95% CI 0.88 to 0.91). A consultation with a locum was 21% more likely to involve a prescription for an antibiotic (OR = 1.21, 95% CI 1.20 to 1.22), 8% more likely to involve a prescription for a strong opioid (OR = 1.08, 95% CI 1.06 to 1.09) and 3% less likely to involve a prescription for a hypnotic (OR = 0.97, 95% CI 0.94 to 0.99). Consultations with locums were also 15% less likely to involve a referral (OR = 0.85, 95% CI 0.84 to 0.86) and 20% less likely to involve a test being ordered (OR = 0.80, 95% CI 0.80 to 0.81). In terms of hospital-related outcomes, a consultation with a locum was 5% more likely to be followed by an A&E visit within 1 to 7 days (OR = 1.05, 95% CI 1.02 to 1.08) but there was no difference in rates of the same day A&E visits, emergency admissions or ACSC emergency admissions.
When comparing prescribing safety indicators for permanent and locum GPs, a consultation with a locum GP, was 11.2% (OR = 1.12, 95% CI 1.08 to 1.16) more likely to involve the prescription of an oral NSAID, without co-prescription of an ulcer healing drug, to a patient aged ≥ 65 years. But a consultation with a locum GP was 22.8% (OR = 0.77, 95% CI 0.64 to 0.93) less likely to involve the prescription of warfarin or a direct oral anticoagulant in combination with an oral NSAID, and 11.2% (OR = 0.89, 95% CI 0.85 to 0.93) less likely to involve the prescription of a long-acting beta-2 antagonist inhaler to a patient with asthma who is not also prescribed an inhaled corticosteroid. We didn’t find any significant differences between permanent and locum GPs across all other prescribing safety indicators. The full output from the multilevel regressions is presented in Table S18–S22 in Additional file 2 .
We plotted the effects and the confidence intervals of locum consultations on the patient outcomes in Fig. 1 and the effects and confidence intervals of locum consultation on the potentially hazardous prescribing indicators in Fig. 2 . The results from the sensitivity analyses, excluding the period 2020–2022, were effectively the same and we report them in Table S23 in Additional file 2 .
Coefficient plot for locum consultations across all outcomes. Note: Results are expressed as odd ratios (OR) and corresponding confidence intervals (CI). When the corresponding CIs cross the dashed vertical line coefficients are not statistically significant
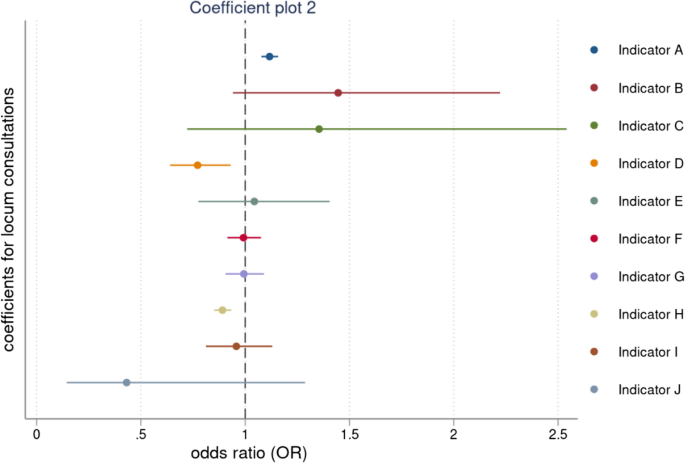
Coefficient plot for locum consultations across prescribing safety indicators. Note: Results are expressed as odd ratios (OR) and corresponding confidence intervals (CI). When the corresponding CIs cross the dashed vertical line coefficients are not statistically significant
This study set out to examine differences in the clinical practice and consultation outcomes for locum and permanent doctors working in primary care in England. Our findings suggest there are some differences, but their interpretation is complex and should be approached with caution.
For example, when considering a return to practice within 7 days, we find that this is 12% less likely for a locum GP than for a permanent GP. To some, this may seem counter-intuitive as they might have expected that patients seeing a locum GP could be less satisfied or assured by the consultation and so more likely to return within a week. But there are some important other considerations to take into account. Patients who prefer not to see a locum may have opted to wait to see a permanent GP, so the patient groups may not be comparable. Some practices may assign more straightforward cases to locums and although our regression models adjust for comorbidities, there are probably unmeasured differences in the case mix characteristics of patients seeing locum and permanent GPs. Moreover, our qualitative research suggests that some patients actually welcome the opportunity to see a locum GP because they get a fresh perspective on their condition [ 36 ].
However, we also find that locums consistently prescribed antibiotics and strong opioids more than permanent GPs, and we might speculate that locums could be less aware of or compliant with practice prescribing guidelines, or that locums may be more inclined to respond to patient requests for a prescription [ 36 ]. Other research has also suggested that locum doctors prescribe antibiotics more readily [ 37 ].
Perhaps our most striking finding is that locum GPs are markedly less likely to both order tests and refer patients to other services (such as hospital outpatient clinics) than permanent GPs. But here, we suspect this may in part be because practices set constraints on such decisions by locums, requiring them to be reviewed or approved by another GP in the practice [ 36 ].
To put these differences in outcomes into context, it can be helpful to consider how commonly they occur, which is reported in Table 1 . Several of the outcomes where differences are observed are also common: practice revisits (8%), antibiotic prescription (9%), referral (4%) and test (3%). However, other outcomes are comparatively rare: strong opioid prescription (2%), hypnotic prescription (< 1%), and A&E visit (0.15%). Therefore, our findings relating to revisits, antibiotics, referrals and tests are both statistically and clinically significant.
On the PINCER prescribing safety indicators, again the results are quite mixed. On most indicators, there is no significant difference between locum and permanent GPs — and the differences we do observe on three indicators are not large and move in different directions. There certainly seems to be no basis to argue that locum GPs differ significantly from permanent GPs on these indicators.
Past research largely in inpatient acute care has also found mixed differences in care between locum and permanent doctors. A US study investigating the impact of locum working on patient outcomes, including mortality, 30-day hospital readmissions and cost of care found significant differences in mortality rates for patients who were treated by locums who had worked for less than 60 days in the organisation but no significant differences for patients who were treated by locums who had worked in the organisation for 60 days or more [ 7 ]. Another study comparing locum and permanent doctors found locum doctors had shorter stays and lower treatment costs but there were no differences in mortality or readmissions [ 38 ].
Strengths and limitations of the study
This is the largest observational study of locum and permanent doctor consultations in primary care investigating differences in clinical practice indicators and prescribing safety indicators. However, there are important limitations. First, any study of this nature is limited by the reliability and accuracy of the data in the patient’s electronic record. We are confident about the reliability of the recorded patient contact data and patient characteristics as consultation events are central to how CPRD GOLD is organised but we could not assess the reliability with which the staff role field linked to each consultation is recorded. Moreover, we know from other research [ 39 ] that locum doctors working in primary care may undertake anything from very short placements of a few days in a practice to very much longer or regular placements as the preferred locum for a practice — and we could not distinguish between such short-term and long-term locums in our analysis. It may also be that some long-term and regular locum GPs working in practices get recorded on the Vision system as permanent GPs. If GP identifiers were made available this could be considered in future research.
Second, we were not able to assess the reasons why patients revisited their general practice within 7 days following a consultation with a locum GP and to distinguish for example between planned follow-ups from the first consultation and unplanned revisits, or between revisits which were clinically related to the first consultation and those which were unrelated and for another matter. Third, there may be other systemic differences between locum and permanent GPs which are not available within CPRD but which might be material to our analysis — such as gender, age, ethnicity, years of experience as a GP, where they qualified and trained, and so on. Additional information on locum doctor working arrangements as well as demographic information about doctors would enable more detailed comparisons between locum and permanent GPs [ 40 ]. Fourth, CPRD GOLD is representative of the UK population in terms of deprivation and population characteristics [ 15 ], but data is collected from a single clinical information system (Vision) and contributing practices are not uniformly distributed across English regions, while its market share is in decline [ 18 ]. Thus, generalisability to every English region could not be achieved.
We noted earlier that locum doctors are often regarded with some suspicion and portrayed by some as less clinically competent or professionally committed than permanent doctors [ 12 ] but there is little evidence in our findings to suggest that systemic differences exist in practice or performance between locum and permanent GPs. Rather, it seems likely that the performance of both locum and permanent GPs is shaped by the wider organisational context in which they practice — the quality of induction, supervision, communication, and practice management being obvious likely determinants [ 8 ]. Locums form a necessary component of the overall medical workforce and can enable practices to cope with staff shortages, planned or unplanned staff absences and variations in demand for appointments. Future research should focus on understanding how organisations can make the best use of locums as part of their wider medical workforce and how locum doctors can be enabled to practice and perform effectively as members of the clinical team.
Availability of data and materials
In this study, we used anonymised patient-level data from the CPRD that are not publicly available due to confidentiality considerations. However, researchers can access CPRD’s databases by contacting the CPRD. Details of the application process and conditions of access are available at https://www.cprd.com/Data-access .
Abbreviations
Accident and emergency
Ambulatory care sensitive conditions
British National Formulary
Confidence interval
Clinical Practice Research Datalink
Direct oral anticoagulants
- Electronic health records
General practitioner
Hospital Episode Statistics
International Classification of Disease
Index of Multiple Deprivation
Lower Layer Super Output Area
Medicines & Healthcare Products Regulatory Agency
National Health Service
No-steroidal anti-inflammatory drugs
Pharmacist-led information technology intervention
Proton-pump inhibitor
Quality and Outcomes Framework
United Kingdom
Aluttis C, Bishaw T, Frank MW. The workforce for health in a globalized context–global shortages and international migration. Glob Health Action. 2014;7(1):23611.
Article PubMed Google Scholar
Anderson M, O’Neill C, Macleod Clark J, Street A, Woods M, Johnston-Webber C, et al. Securing a sustainable and fit-for-purpose UK health and care workforce. Lancet. 2021;397(10288):1992–2011.
Article CAS PubMed PubMed Central Google Scholar
Sizmur S, Raleigh V. The risks to care quality and staff wellbeing of an NHS system under pressure. Oxford: The King's Fund; 2018. 24.
Salloch S, Apitzsch B, Wilkesmann M, Ruiner C. Locum physicians’ professional ethos: a qualitative interview study from Germany. BMC Health Serv Res. 2018;18(1):333.
Article PubMed PubMed Central Google Scholar
General Medical Council. What our data tells us about locum doctors. 2018.
Google Scholar
Staff Care. Survey of temporary physician staffing trends 2020. 2020. Available from: https://www.staffcare.com/uploadedFiles/staffcare2020surveyPDF.pdf .
Blumenthal DM, Olenski AR, Tsugawa Y, Jena AB. Association between treatment by locum tenens internal medicine physicians and 30-day mortality among hospitalized medicare beneficiaries. JAMA. 2017;318(21):2119–29.
Ferguson J, Walshe K. Quality, safety and locum doctors: a narrative review. J R Soc Med. 2019;112(11):462–71.
Dyer C. Locum histopathologist is struck off for clinical errors and lying. BMJ. 2014;349:g4741.
Dyer C. Locum surgeon is struck off for failing to call for help during botched appendectomies. BMJ. 2023;380:p507.
Article Google Scholar
Kirkup B. Reading the signals: maternity and neonatal services in East Kent–the report of the independent investigation. Department of Health and Social Care. London: House of Commons; 2022.
Ferguson J, Tazzyman A, Walshe K, Bryce M, Boyd A, Archer J, et al. “You’re just a locum”: professional identity and temporary workers in the medical profession. Sociol Health Illn. 2021;43(1):149–66.
Beech J, Bottery S, Charlesworth A, Evans H, Gershlick B, Hemmings N, et al. Closing the gap. Key Areas for Action on the Health and Care Workforce. The King's Fund. 2019.
Moberly T. Number of locums has doubled since 2009. BMJ. 2016;355:i6207.
Herrett E, Gallagher AM, Bhaskaran K, Forbes H, Mathur R, van Staa T, et al. Data Resource Profile: Clinical Practice Research Datalink (CPRD). Int J Epidemiol. 2015;44(3):827–36.
Herrett EL, Thomas SL, Smeeth L. Validity of diagnoses in the general practice research database. Br J Gen Pract. 2011;61(588):438–9.
Khan NF, Harrison SE, Rose PW. Validity of diagnostic coding within the General Practice Research Database: a systematic review. Br J Gen Pract. 2010;60(572):e128–36.
Kontopantelis E, Stevens RJ, Helms PJ, Edwards D, Doran T, Ashcroft DM. Spatial distribution of clinical computer systems in primary care in England in 2016 and implications for primary care electronic medical record databases: a cross-sectional population study. BMJ Open. 2018;8(2):e020738.
NHS Digital. Hospital Episode Statistics. 2020. Available from: https://digital.nhs.uk/data-and-information/data-tools-and-services/data-services/hospital-episode-statistics .
Ministry of Housing, Communities and Local Government. The English Indices of Deprivation. 2019.
Clinical Practice Research Datalink. Hospital Episode Statistics (HES) Admitted Patient Care and CPRD primary care data Documentation (set 22/January 2022). CPRD. 2021(Version 2.8).
Bankart M, Baker R, Rashid A, Habiba M, Banerjee J, Hsu R, et al. Characteristics of general practices associated with emergency admission rates to hospital: a cross-sectional study. Emerg Med J. 2011;28(7):558–63.
Article CAS PubMed Google Scholar
Cowling TE, Harris MJ, Watt HC, Gibbons DC, Majeed AJ. Access to general practice and visits to accident and emergency departments in England: cross-sectional analysis of a national patient survey. Br J Gen Pract. 2014;64(624):e434–9.
Steventon A, Friebel R, Deeny S, Gardner T, Thorlby R. Briefing: emergency hospital admissions in England: which may be avoidable and how? Health Foundation. 2018.
Lasserson D, Smith H, Garland S, Hunt H, Hayward G. Variation in referral rates to emergency departments and inpatient services from a GP out of hours service and the potential impact of alternative staffing models. Emerg Med J. 2021;38(10):784–8.
O’Sullivan JW, Stevens S, Oke J, Hobbs FR, Salisbury C, Little P, et al. Practice variation in the use of tests in UK primary care: a retrospective analysis of 16 million tests performed over 3.3 million patient years in 2015/16. BMC Med. 2018;16:1–9.
NHS England. The NHS Long Term Plan. 2019.
National Institure of Health Excellence. Medicines associated with dependence or withdrawal symptoms: safe prescribing and withdrawal management for adults. 2022.
Rodgers S, Taylor AC, Roberts SA, Allen T, Ashcroft DM, Barrett J, et al. Scaling-up a pharmacist-led information technology intervention (PINCER) to reduce hazardous prescribing in general practices: Multiple interrupted time series study. PLoS Med. 2022;19(11):e1004133.
Grigoroglou C, Munford L, Webb R, Kapur N, Doran T, Ashcroft D, et al. Impact of a national primary care pay-for-performance scheme on ambulatory care sensitive hospital admissions: a small-area analysis in England. BMJ Open. 2020;10(9):e036046.
Avery AJ, Rodgers S, Cantrill JA, Armstrong S, Cresswell K, Eden M, et al. A pharmacist-led information technology intervention for medication errors (PINCER): a multicentre, cluster randomised, controlled trial and cost-effectiveness analysis. Lancet. 2012;379(9823):1310–9.
Spencer R, Bell B, Avery A, Gookey G, Campbell S. Identification of an updated set of prescribing—safety indicators for GPs. Br J Gen Pract. 2014;64(621):e181–90.
Booth N. What are the read codes? Health Libr Rev. 1994;11(3):177–82.
Payne RA, Mendonca SC, Elliott MN, Saunders CL, Edwards DA, Marshall M, et al. Development and validation of the Cambridge Multimorbidity Score. CMAJ. 2020;192(5):E107–14.
Lin M, Lucas H, Shmueli G. Too big to fail: large samples and the p-value problem. Inf Syst Res. 2013;24(4):906–17.
Walshe K, Ferguson J, Allen T, Grigoroglou C, Stringer G, Kontopantelis E, et al. Locum doctors in the NHS: Understanding and improving the quality and safety of healthcare. Policy Report. The University of Manchester. 2023. Available from: https://documents.manchester.ac.uk/display.aspx?DocID=67075 .
Borek AJ, Pouwels KB, van Hecke O, Robotham JV, Butler CC, Tonkin-Crine S. Role of locum GPs in antibiotic prescribing and stewardship: a mixed-methods study. Br J Gen Pract. 2022;72(715):e118–27.
Mustafa Ali MK, Sabha MM, Mustafa SK, Banifadel M, Ghazaleh S, Aburayyan KM, et al. Hospitalization and post-hospitalization outcomes among teaching internal medicine, employed hospitalist, and locum tenens hospitalist services in a tertiary center: a prospective cohort study. J Gen Intern Med. 2021;36(10):3040–51.
Stringer G, Ferguson J, Walshe K, Grigoroglou C, Allen T, Kontopantelis E, et al. Locum doctors in English general practices: evidence from a national survey. Br J Gen Pract. https://doi.org/10.3399/BJGP.2023.0039 .
Grigoroglou C, Walshe K, Kontopantelis E, Ferguson J, Stringer G, Ashcroft DM, et al. Locum doctor use in English general practice: analysis of routinely collected workforce data 2017–2020. Br J Gen Pract. 2022;72(715):e108–17.
Download references
Acknowledgements
This study was funded by the National Institute for Health and Care Research (NIHR) Health Service and Delivery Research programme (project reference number: NIHR128349)and the NIHR Greater Manchester Patient Safety Research Collaboration (PSRC). The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care.
Author information
Authors and affiliations.
Manchester Centre for Health Economics, Division of Population Health, Health Services Research and Primary Care, University of Manchester, Manchester, UK
Christos Grigoroglou & Thomas Allen
Alliance Manchester Business School, University of Manchester, Manchester, UK
Kieran Walshe & Gemma Stringer
NIHR School for Primary Care Research, Centre for Primary Care, Division of Population Health, Health Services Research and Primary Care, University of Manchester, Manchester, UK
Evangelos Kontopantelis & Darren M. Ashcroft
Division of Informatics, Imaging and Data Sciences, University of Manchester, Manchester, UK
Evangelos Kontopantelis
Health Services Management Centre, University of Birmingham, Birmingham, UK
Jane Ferguson
NIHR Greater Manchester Patient Safety Research Collaboration, Division of Pharmacy and Optometry, University of Manchester, Manchester, UK
Darren M. Ashcroft
Centre for Pharmacoepidemiology and Drug Safety, School of Health Sciences, School of Health Sciences, Faculty of Biology, Medicine and Health, University of Manchester, Manchester, UK
Danish Centre for Health Economics, University of Southern Denmark, Odense, Denmark
Thomas Allen
You can also search for this author in PubMed Google Scholar
Contributions
CG, TA, EK, DA and KW designed the study. CG extracted the data from all sources, performed the analyses and drafted the manuscript. KW, EK, JF, GS, DA and TA critically revised the manuscript. CG is the guarantor of this work and, as such, has full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors read and approved the final manuscript.
Authors’ Twitter handles
Christos Grigoroglou @chris_grig; Kieran Walshe @kieran_walshe; Evangelos Kontopantelis @dataevan; Jane Ferguson @janefergo; Gemma Stringer @gemmakstringer; Darren Ashcroft @GM_PSRC, @NIHRPSRCs, @meds_safety; Thomas Allen @tommyallen87.
Corresponding author
Correspondence to Christos Grigoroglou .
Ethics declarations
Ethics approval and consent to participate.
This study is based on data from the Clinical Practice Research Datalink (CPRD) GOLD database obtained under license from the Medicines and Healthcare products Regulatory Agency (MHRA). The data are provided by patients and collected by the NHS as part of their care and support. Hospital Episode Statistics (HES) data are subject to Crown copyright (2023) protection, re-used with the permission of The Health and Social Care Information Centre, all rights reserved. The interpretation and conclusions contained in this study are those of the authors alone, and not necessarily those of the MHRA, the National Institute for Health and Care Research (NIHR), NHS, or the Department of Health. Approval to conduct this study using the CPRD was granted by the Independent Scientific Advisory Committee (ISAC) of the MHRA (protocol 20_000246). The authors thank the contributing patients and practices to the CPRD GOLD database who have allowed their data to be used for research purposes.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:.
Table S1. Codelist for antibiotics. Table S2. Codelist for opioids. Table S3. Codelist for benzodiazepines. Table S4. Codelist for z-drugs. Table S5. Codelist for conditions linked to potentially hazardous prescribing (READ codes). Table S6. Codelist for anticoagulants. Table S7. Codelist for antipsychotic drugs. Table S8. Codelist for aspirin products. Table S9. Codelist for antiplatelet drugs (non-aspirin). Table S10. Codelist for β-blockers. Table S11. Codelist for inhaler corticosteroids. Table S12. Codelist for long-acting beta-2 antagonists. Table S13. Codelist for non-steroidal anti-inflammatory drugs (NSAIDS). Table S14. Codelist for non-selective β-blockers. Table S15. Codelist for proton-pump inhibitors (PPIs) and H2 blockers.
Additional file 2:
Table S16. ICD-10 Codes for hospital admissions. Table S17. Definitions for PINCER Indicators. Table S18. Regression analyses for patient outcomes, pt.1. Table S19. Regression analyses for patient outcomes, pt.2. Table S20. Regression analyses for patient outcomes, pt.3. Table S21. Regression analyses for prescribing safety outcomes, pt.1. Table S22. Regression analyses for prescribing safety outcomes, pt.2. Table S23. Regression analyses for prescribing safety outcomes, excluding 2020–2022.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Grigoroglou, C., Walshe, K., Kontopantelis, E. et al. Comparing the clinical practice and prescribing safety of locum and permanent doctors: observational study of primary care consultations in England. BMC Med 22 , 126 (2024). https://doi.org/10.1186/s12916-024-03332-z
Download citation
Received : 22 November 2023
Accepted : 29 February 2024
Published : 27 March 2024
DOI : https://doi.org/10.1186/s12916-024-03332-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Locum doctors
- Patient safety
- Medical workforce
- General practice
BMC Medicine
ISSN: 1741-7015
- Submission enquiries: [email protected]
- General enquiries: [email protected]
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 05 September 2022
Diagnostic differentiation between asthma and COPD in primary care using lung function testing
- Jelle D. M. Bouwens 1 , 2 ,
- Erik W. M. A. Bischoff ORCID: orcid.org/0000-0002-3323-8475 1 ,
- Johannes C. C. M. in ’t Veen 3 , 4 &
- Tjard R. Schermer ORCID: orcid.org/0000-0002-1391-2995 1 , 5
npj Primary Care Respiratory Medicine volume 32 , Article number: 32 ( 2022 ) Cite this article
11k Accesses
7 Citations
217 Altmetric
Metrics details
- Chronic obstructive pulmonary disease
- Epidemiology
- Respiratory signs and symptoms
Asthma and COPD are defined as different disease entities, but in practice patients often show features of both diseases making it challenging for primary care clinicians to establish a correct diagnosis. We aimed to establish the added value of spirometry and more advanced lung function measurements to differentiate between asthma and COPD. A cross-sectional study in 10 Dutch general practices was performed. 532 subjects were extensively screened on respiratory symptoms and lung function. Two chest physicians assessed if asthma or COPD was present. Using multivariable logistic regression analysis we assessed the ability of three scenarios (i.e. only patient history; diagnostics available to primary care; diagnostics available only to secondary care) to differentiate between the two conditions. Receiver operator characteristics (ROC) curves and area under the curve (AUC) were calculated for each scenario, with the chest physicians’ assessment as golden standard. Results showed that 84 subjects were diagnosed with asthma, 138 with COPD, and 310 with no chronic respiratory disease. In the scenario including only patient history items, ROC characteristics of the model showed an AUC of 0.84 (95% CI 0.78–0.89) for differentiation between asthma and COPD. When adding diagnostics available to primary care (i.e., pre- and postbronchodilator spirometry) AUC increased to 0.89 (95% CI 0.84–0.93; p = 0.020). When adding more advanced secondary care diagnostic tests AUC remained 0.89 (95% CI 0.85–0.94; p = 0.967). We conclude that primary care clinicians’ ability to differentiate between asthma and COPD is enhanced by spirometry testing. More advanced diagnostic tests used in hospital care settings do not seem to provide a better overall diagnostic differentiation between asthma and COPD in primary care patients.
Similar content being viewed by others
An accurate prediction model to identify undiagnosed at-risk patients with COPD: a cross-sectional case-finding study
Kang-Cheng Su, Hsin-Kuo Ko, … Yu Ru Kou
Diagnostic spirometry in COPD is increasing, a comparison of two Swedish cohorts
Åsa Athlin, Karin Lisspers, … Josefin Sundh
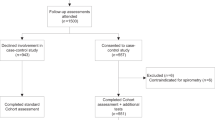
Accuracy of Vitalograph lung monitor as a screening test for COPD in primary care
A. P. Dickens, D. A. Fitzmaurice, … R. E. Jordan
Introduction
Asthma and chronic obstructive pulmonary disease (COPD) are both common chronic respiratory diseases affecting approximately 1 in 12 people worldwide 1 , 2 . The two conditions are defined as different disease entities with unique pathophysiological mechanisms and characteristic clinical features 1 , 2 . The underlying pathophysiology in COPD is characterized predominantly by neutrophilic inflammation, whereas in asthma the inflammatory pattern is mostly due to eosinophilic inflammation 3 . Asthma typically presents with intermittent respiratory symptoms caused by airflow obstruction predominantly due to bronchial hyperresponsiveness 4 . Asthma is often presented at younger age as part of an atopic constitution, but can also be diagnosed in adulthood 1 . In contrast, COPD is a slowly progressive lung disease with patients having persistent respiratory symptoms and airflow obstruction 2 . In high-income countries like the Netherlands COPD usually presents in patients older than forty who are generally current or former smokers 2 . Patients with asthma or COPD are mostly diagnosed and managed by primary care clinicians.
Looking at the classic pathophysiological and clinical presentations, the distinction between asthma and COPD seems clear, but in clinical practice patients often show features of both diseases 5 , 6 . These similarities make it difficult for clinicians to distinguish between asthma and COPD 7 , especially in older and more diverse patient populations encountered in primary care 8 , 9 , 10 . However, differentiating between the two respiratory conditions is important as they have different pharmacotherapeutic regimens. In patients with asthma, inhaled corticosteroids (ICS) are highly effective in reducing symptoms and reducing the risk of asthma-related mortality 1 . In contrast, patients with COPD respond poorly to ICS and are mainly treated with (long-acting) bronchodilators to relieve symptoms 2 . In addition to this, misdiagnosing asthma for COPD could lead to serious health risks considering that monotherapy with long-acting bronchodilators is contra-indicated in asthmatics since it increases the risk of severe exacerbation 11 , 12 , 13 . On the other hand, (unnecessary) treatment with ICS may cause pneumonia and increased risk of osteoporosis 14 , 15 , 16 , 17 .
Thus, establishing a correct diagnosis is essential for optimal treatment of asthma and COPD, but this can be challenging for primary care clinicians. Supporting them in the diagnostic process seems therefore essential, but this also depends on the availability of diagnostic tools. Although quality spirometry has shown to be feasible in primary care settings 18 there is substantial room for improvement of its use to accurately diagnose chronic respiratory diseases 19 , 20 . Thus, the first aim of our current study was to establish which patient characteristics distinguish between patients diagnosed with asthma or COPD. The second and main aim was to establish the added value of spirometry and more advanced lung function measurements to differentiate between these two chronic airways diseases.
Study design and population
In this observational multi-centre cross-sectional study, we compared patients diagnosed with asthma, patients diagnosed with COPD, and subjects without underlying chronic obstructive lung conditions using data from a previous study, i.e., the Detection, Intervention and Monitoring of COPD’ (DIMCA) program 21 . This program was originally set up to improve early detection of chronic airways disease in general practices. A random sample of 1,749 adult subjects (20–70 years) from ten general practices in The Netherlands were invited to participate 21 . At the start of the program, patients with pre-existing asthma, COPD or another airway disease were excluded. In 2007, ten years after the start of the initial DIMCA program, all subjects (now aged 30–80 years) received an invitation for a comprehensive respiratory assessment consisting of extensive lung function measurements and a myriad of medical history questions 22 . A total of 532 subjects agreed to participate in this follow-up study. The results of the respiratory assessment of these subjects were submitted to two experienced chest physicians who assessed if a chronic airways disease (i.e., COPD or asthma) was present or absent using a standardized protocol 22 that was based on the international clinical guideline criteria that applied at the time of the study (see below). The results of the chest physicians’ assessments were used as the golden standard in the current study.
The study was approved by the medical ethics review board CMO Regio Arnhem – Nijmegen ( https://www.radboudumc.nl/over-het-radboudumc/kwaliteit-en-veiligheid/commissie-mensgebonden-onderzoek ; file number: 2002/028). Participants provided written informed consent to take part in the study.
Measurements
Study participants were instructed to interrupt the use of any bronchodilators they might use for a specified number of hours before their visit to the pulmonary function laboratory. Lung function testing involved pre- and postbronchodilator spirometry (both static and dynamic) and measurement of carbon monoxide diffusion capacity (DLCO) and bronchial hyperresponsiveness (BHR) 22 . Aerosolized salbutamol 800 µg and/or ipratropium 160 µg were used as bronchodilators and were administered by volume spacer. Postbronchodilator forced expiratory volume in one second (FEV1) was measured 15 min after salbutamol and 45 min after ipratropium. Bronchodilator reversibility was defined as an increase in FEV1 after bronchodilation by at least 12% and 200 mL. BHR was assessed by histamine challenge test and considered positive in case of a >20% drop in FEV1 at a provocative dose histamine of ≤8 mg/mL (PC20) 1 , 23 . All lung function tests were conducted by certified lung function technicians in a hospital-based pulmonary function laboratory and were performed in accordance with the 1994 American Thoracic Society standards 24 . Predicted normal lung function values for FEV1 were calculated using European Community for Coal and Steel reference values 25 . Following lung function testing, subjects were interviewed by the lung function technician regarding respiratory symptoms, smoking behaviour, presence of allergies and eczema, respiratory problems triggered by environmental exposures, and family history of COPD or asthma 22 .
Diagnostic assessment
Based on the results of the respiratory assessment the chest physicians assessed if a chronic airways disease (i.e., asthma or COPD) was present or absent using guideline criteria, their expert knowledge, and their clinical expertise 22 . Study subjects were randomly assigned to the chest physicians in a 1:1 ratio. If a subject was diagnosed with a chronic airways disease by the assigned chest physician the subject’s data was also presented to the other chest physician and a final joint diagnosis was established. To standardize the diagnostic process, a decision tree (Fig. 1 ) was created based on international clinical guideline criteria for diagnosing asthma (GINA guideline, 2007 update 26 ) and COPD (GOLD guideline, 2006 update 27 ) that applied at the time, in co-operation with the two chest physicians. In case of uncertainty about the respiratory diagnosis the chest physicians could request additional diagnostic tests (i.e., allergy skin testing, peak expiratory flow (PEF) monitoring) in order to maximize their diagnostic certainty 22 . Because the concept of asthma-COPD overlap (ACO) was introduced after the current study was conducted, the chest physicians did not consider a diagnosis of ACO as a part of their assessment. They were instructed to, based on their systematic assessment of all diagnostic information available, assign one single preferred diagnosis (i.e., either asthma or COPD) that fitted best according to their expert opinion. Figure 2 illustrates the spectrum of chronic obstructive airways disease diagnoses and the parts of the spectrum on which the current study focuses. Strictly for the purpose of describing the study population and its diagnostic subgroups (see Table 1 ) the Global Lung function Initiative (GLI) reference equations were applied at the time of the data analysis for the current paper 28 .
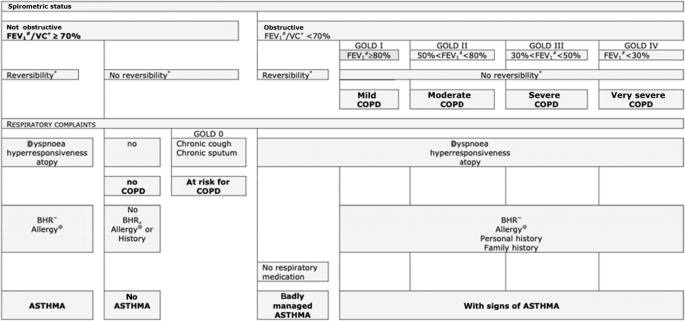
# Postbronchodilator forced expiratory volume. + Postbronchodilator vital capacity. *12% change in FEV1 (after bronchodilation), with a change of at least 200 mL. ~ Bronchial hyperresponsiveness (positive at a provocative histamine concentration ≤ 8mg/mL). @ Skin prick test.
The current study focusses on the parts to the left and right of the vertical dotted lines as indicated by the arrows. ACO asthma-COPD overlap, COPD chronic obstructive pulmonary disease.
Categorization of variables
In the present study, we categorized all items of the respiratory assessment in three subsections based on their availability in different healthcare settings, i.e., public health, primary care, and secondary care (Table 2 ). Subsection 1 consists of items that are available in any public health or healthcare setting since they require no measurements or testing equipment but only medical history questions (i.e., respiratory symptoms, smoking behaviour, body mass index (BMI)). Subsection 2 contains lung function test results that are available to primary care clinicians (i.e., spirometry and reversibility testing) in countries with well-developed healthcare systems 29 , 30 , 31 . Finally, Subsection 3 contains results from more advanced diagnostic tests as performed mainly in lung function laboratories in hospital care settings. These tests include measurement of static lung volumes, diffusion capacity, and histamine challenge testing.
Statistical analysis
Demographic characteristics, clinical features and lung function values were univariately compared between the subgroups of patients diagnosed with asthma and COPD using independent t -tests and Chi-square tests. The further analysis focussed on assessing the ability to differentiate between these chronic obstructive lung diseases in different healthcare settings. Since physicians are not limited to asking a single medical history question or to conducting a single diagnostic test, we used multivariable logistic regression analysis to construct predictive models based on the data of the subjects who were diagnosed with asthma or COPD by the chest physicians (i.e., the binary outcome measure for this analysis was to have a diagnosis of asthma or a diagnosis of COPD). As described above, the items from the patient assessment were categorized in three subsections based on diagnostic availability and multivariable logistic regression models were run for three ‘scenarios’ (Table 2 ). In the first scenario, we only used the medical history items from Subsection 1 in the model. In the second scenario, we added diagnostic items available to primary care clinicians (i.e., Subsections 1 plus 2) to the model. In the third scenario, we added diagnostic items available to secondary care clinicians to the model (i.e., Subsections 1 plus 2 plus 3). Only items with a p -value ≤0.20 in the univariate analysis were considered relevant as predictors and were included in the respective models. In each scenario, the item with the highest p -value was manually removed from the model after which the logistic model was re-run (‘backward selection’). This step was repeated until only variables with p -values < 0.10 remained in the model for each scenario. Odds ratios for diagnosing asthma were calculated with COPD as reference group and vice versa. For each scenario a receiver operator characteristics (ROC) curve was created and the percentage explained variance (Nagelkerke R square) determined. Area under the curve (AUC) values from the ROC curves of the three scenarios were statistically compared using a non-parametric approach for correlated ROC curves 32 . SPSS statistics version 25.0 and SAS version 9.4 were used for the analyses. Missing data were not imputed. Two-sided p -values < 0.05 were considered statistically significant, except for the testing of the AUC values between Scenarios 1 and 2 and Scenarios 2 and 3, respectively, in which multiple testing was taken into account by using p < 0.025 to define statistical significance (i.e., Bonferroni correction: p = 0.05/2 = 0.025).
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Study population
In the total sample of 532 study subjects (all Caucasians), 84 (16%) were diagnosed with asthma, 138 (26%) were diagnosed with COPD, and in 310 subjects (58%) no chronic airways disease was diagnosed (Table 1 ). Compared to patients with COPD the patients diagnosed with asthma were significantly younger (mean age 50.2 (SD 11.4) versus 57.8 (SD 10.0); p < 0.001) and more likely to be female (59.5% versus 44.2%; p = 0.027). There was no statistically significant difference in BMI between the two diagnostic subgroups ( p = 0.22).
Differences and similarities in clinical features and lung function
Table 1 gives an overview of the differences and similarities in demographic characteristics, clinical features and lung function values between patients with asthma and patients with COPD. Patients diagnosed with COPD were significantly more likely to be former or current smokers and had more packyears compared to patients with asthma (21.3 (SD 19.5) versus 9.1 (14.4); p < 0.001). Patients with asthma were significantly more likely to have allergies compared to patients with COPD ( p < 0.001) but there was no difference in the prevalence of eczema between the subgroups ( p = 0.99). Patients with asthma had significantly more often symptoms of wheezing ( p = 0.006) compared to patients with COPD. The prevalence of having chronic cough, phlegm or breathlessness was not significantly different between the groups. Patients with COPD had significantly lower % predicted postbronchodilator FEV1 values (88.2% versus 98.9%; p < 0.001) compared to patients with asthma. There were no differences in the presence of reversibility ( p = 0.75) or bronchial hyperresponsiveness ( p = 0.68) between the two subgroups. No additional diagnostic tests were requested by the two chest physicians.
Differentiating ability of diagnostic items
Demographic characteristics, clinical features and lung function tests yielded a total of 21 diagnostic variables (Table 1 ). Excluding items with p -values of >0.20 in the univariate analysis resulted in twelve items that were considered as relevant discriminants to be entered in the multivariable logistic models: age, gender, packyears, wheeze, phlegm, breathlessness, allergy, respiratory symptoms triggered by environmental exposures, postbronchodilator FEV1 % predicted, postbronchodilator FEV1/FVC < 0.70, RV/TLC and diffusion capacity.
Table 3 shows an overview of the differentiating ability of all relevant items. In Scenario 1 (only medical history questions), eight items were included in the model, four of which showed a statistically significant relationship when differentiating between asthma and COPD: packyears, wheeze, phlegm and allergy. In Scenario 2, ten items were included in the model, six of which showed a significant relation in differentiating between asthma and COPD: age, wheeze, breathlessness, allergy, FEV1 % predicted and FEV1/FVC. In Scenario 3, twelve items were included in the model, six showing statistical significance when differentiating between asthma and COPD: age, wheeze, breathlessness, allergy, FEV1 predicted and FEV1/FVC. Independent of the scenario, postbronchodilator FEV1/FVC was an important discriminant.
In Scenario 1 the logistic model showed a percentage explained variance of 41% and ROC characteristics showed an area under the curve (AUC) of 0.84 (95% confidence interval (CI): 0.78–0.89)) (Fig. 3 ). By adding diagnostic variables available to primary care (i.e., spirometry) in Scenario 2, the explained variance increased to 54% and AUC increased to 0.89 (95% CI 0.84–0.93). Finally, by adding more advanced diagnostic tests available to secondary care in Scenario 3, the explained variance increased to 56% but AUC remained 0.89 (95% CI 0.85–0.94). Statistical testing showed a statistically significant difference between the AUCs of Scenarios 2 and 1 ( p = 0.020) but no such difference between the AUCs of Scenarios 3 and 2 ( p = 0.967, see Table 3 ).
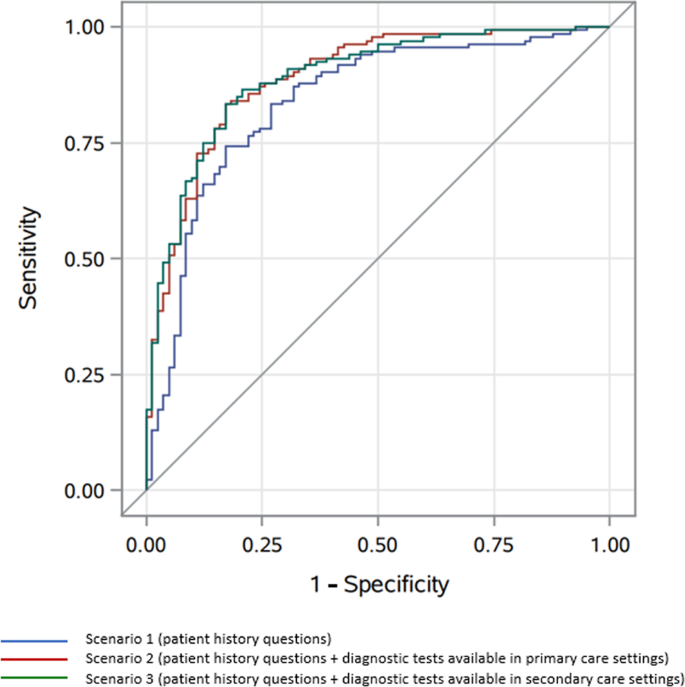
*Area under the curve (AUC) values and p -values of comparison between Scenario 1 and 2 and Scenario 2 and 3: see Table 3 .
In this study, we looked at which patient characteristics distinguish between patients diagnosed with asthma or COPD, and established the added value of spirometry and of more advanced lung function measurements when differentiating between the two chronic airways diseases. Although asthma and COPD are both heterogenous conditions with multiple overlapping features, there are important clinical differences as well. We observed that in the scenario using only medical history questions, it is already possible to reliably distinguish between asthma and COPD. The most important factors to aid differentiation are smoking behaviour, certain respiratory symptoms and the presence of allergies. The use of postbronchodilator spirometry provided important additional discriminative power in correctly labelling a patient as having asthma or COPD. More advanced diagnostic tests that are mainly used in secondary care, such as measuring bronchial hyperresponsiveness and diffusion capacity, did not provide a better differentiation in this primary care study population.
In the present study, both bronchodilator reversibility and bronchial hyperresponsiveness had a similar prevalence in patients diagnosed with asthma and COPD. This finding is noteworthy, as the current GINA guideline refers to reversibility testing and bronchial hyperresponsiveness as criteria supporting the diagnosis of asthma 1 . However, our finding is not unique as previous studies have concluded that solely the presence of reversibility or bronchial hyperresponsiveness does not distinguish between the two obstructive airways diseases 33 , 34 , 35 , 36 . Besides these similarities, there were several clinical features that were statistically different between the two diagnostic subgroups and for that reason, these features can aid primary care clinicians when differentiating between asthma and COPD. Using only medical history questions in the logistic model (Scenario 1) already showed rather good differentiating ability (AUC = 0.84). These findings are in line with other studies that assessed the ability of solely using medical history questionnaires to distinguish between asthma and COPD. Beeh et al. concluded that with only medical history questions, it is possible to distinguish between asthma and COPD for the majority of patients with suspected or established obstructive lung disease 37 . Likewise, in their study Tinkelman et al. reported that a simple self-administered questionnaire can facilitate differentiation between obstructive lung diseases 38 . However, these studies did not look at the additional use of spirometry or more advanced diagnostic tests to discriminate between asthma and COPD nor did they quantify this in, for instance, an area under the curve analysis like we did. In the present study we found that postbronchodilator spirometry was important when differentiating the two conditions and together with medical history questions, the discriminating ability of the model improved (from AUC = 0.84 in Scenario 1 to AUC = 0.89 in Scenario 2). In contrast, more advanced diagnostic tests did not provide a better diagnostic differentiation (AUC remained 0.89 in Scenario 3). This does not mean that these tests are useless, as they have an important role in evaluating the presence and severity of structural lung damage (like, for instance, in emphysema and bronchiectasis) and in differentiating obstructive lung disease from other aetiologies in selected patients 39 , 40 .
A particular strength of our study is that we used standardized methods to conduct the lung function testing and to obtain the respiratory diagnoses. All questionnaires and lung function tests were standardized and prospectively collected, were supervised by certified lung function technicians, and the lung function tests met established quality standards.
Given the central role of general practice in the Dutch healthcare system, nearly all inhabitants are registered in a general practice of their own choice. Therefore, the subjects who participated in the initial DIMCA program and provided for the sample in the current analysis can be seen as representative for the adult Dutch population. On top of this, our study is original in categorizing diagnostic variables based on their availability in different healthcare settings.
However, there were limitations as well. We only looked at the diagnosis itself and did not consider the severity of the diagnosed chronic airways diseases. Because each subject was initially assessed by only one of the chest physicians we were not able to look at the interobserver agreement. Subjects who were considered to have no asthma or COPD were not mutually discussed by the chest physicians to reach a maximum substantiated outcome. However, given that the aim of our study was to differentiate between asthma and COPD and not to distinguish between being ‘respiratory healthy’ or not, we do not consider this to be a relevant limitation of the study.
In some cases the chest physicians’ assessment may have led to false positive diagnoses of COPD, as some subjects who had a post-BD FEV1/FVC value >0.70 ( n = 3; see Table 2 ) or reported to never have smoked ( n = 27) were assigned a COPD diagnosis nonetheless. Unfortunately, we cannot in retrospect ascertain the chest physicians’ specific considerations for assigning this diagnosis in these cases.
Whereas the data collection and diagnostic approach in the DIMCA study by Albers et al. 22 were conducted in a prospective manner, our study was retrospective in design and we were limited to using a pre-existing list of diagnostic items. The data collection dates from more than a decade ago and therefore several more recent diagnostic tests were not included. For instance, several recent studies have shown that the underlying type of inflammation in patients with asthma and COPD is markedly different 3 , 41 . Tests like sputum cell count, peripheral eosinophil count, serum IgE and fractional exhaled nitric oxide (FeNO) provide relevant information about the underlying inflammatory process and could support differentiation between asthma and COPD, but were not assessed in our study. Besides their differentiating potential, these inflammatory markers could have taught us more about the pathogenesis of ‘Asthma-COPD Overlap’ (ACO), which is the subject of ongoing debate 42 , 43 . We call for researchers to perform a similar study as ours in a heterogenous sample of appropriate study subjects, with the addition of the aforementioned contemporary inflammatory markers to the study protocol.
By using the two distinct diagnoses (i.e., asthma and COPD) our study does not increase knowledge on how to identify patients with ACO. However, as the majority (i.e., two-thirds or more) 6 of patients with chronic obstructive airways disease do not concern ACO, our observations do add insight into how to discriminate between these two diagnoses in a substantial part of the overall group of patients with chronic obstructive airways disease. In other words, the study does not ‘solve’ the wider problem of how to distinguish patients with ACO from those with an ‘unambiguous’ diagnosis of asthma or COPD, but it does contribute to the issue of how to diagnose and distinguish the patients in which there is no overlap.
Lastly, it is important to note that the subgroups of patients labelled with asthma or COPD are defined by the diagnostic criteria used by Albers et al. 22 . These criteria were based on GOLD and GINA guidelines from 2006 and 2007, respectively 26 , 27 . But despite new pathophysiological insights, the definition, description and diagnostic criteria of asthma and COPD have not substantially changed ever since 1 , 2 . The fact that we did not apply the current Global Lung Function Initiative (GLI) references values nor the lower limit of normal definition of airway obstruction 44 will not have had a significant impact on our findings, as this mainly influences the interpretation of presence or absence of obstruction in elderly subjects 45 who were hardly present in our middle-aged study sample. Thus, in our view using the older guideline-based classification does not render the results of the present study obsolete or invalid.
A final limitation of the study that should be mentioned is that younger adults (i.e., those aged 18–30) were not included in the study. However, as the aim of the study was to differentiate between asthma and COPD and a diagnosis of COPD below the age of 30 is highly unlikely, we do not think this has had a relevant impact on the findings as reported.
Besides the good discriminating ability of solely using anamnestic questions, our results emphasize the importance of postbronchodilator spirometry in distinguishing asthma from COPD and vice versa. However, it is important to realise that the lung function tests in our study were conducted by well-trained staff in a pulmonary function laboratory and interpreted by experienced chest physicians. To translate these results to the real-life setting, it requires standardized procedures, quality assurance and trained clinicians to interpret the spirometry data accurately and this may be difficult to achieve in primary care 46 , 47 , 48 . However, previous studies have shown that it is feasible to conduct reproducible and clinically meaningful spirometry tests in primary care and that primary care clinicians can interpret spirometry test results correctly 29 , 49 , 50 . Even while in our study bronchial hyperresponsiveness testing did not improve diagnostic differentiation, it has been shown that bronchial challenge testing is safe and feasible in a suitably equipped primary care diagnostic centre 51 . Referral to secondary care is indicated in the few cases in which it is not possible to establish a diagnosis on the basis of thorough medical history taking and well-conducted spirometry alone, or to exclude other possible underlying conditions.
In conclusion, primary care clinicians should be able to reliably differentiate between asthma and COPD with the combination of relevant patient history questions and postbronchodilator spirometry tests for the majority of patients with suspected chronic airways disease. More advanced diagnostic tests used in hospital care settings do not seem to provide a better overall diagnostic differentiation between asthma and COPD in primary care patients. Given the important additional role of postbronchodilator spirometry in this process of differentiating, the implementation of quality-assured spirometry testing and sufficient training should be mandatory in primary care practices. Furthermore, the availability of inflammatory markers in primary care could potentially provide better discriminating diagnostic ability but we did not investigate this in the current study.
Data availability
The data from the DIMCA study are not made publicly accessible because the variable names, labels and codebook are all in the Dutch language. The dataset can be requested from the corresponding author without restrictions, in which case relevant variables and labels will be translated to English.
Code availability
Readers can access the code (i.e., SPSS syntax) of the statistical analyses by sending a request to the corresponding author.
Global Initiative for Asthma (GINA). Global Strategy for Asthma Management and Prevention. www.ginasthmaorg (2021).
Global Initiative for Chronic Obstructive Lung Disease (GOLD). The Global Strategy for the Diagnosis, Management and Prevention of COPD. www.goldcopdorg (2021).
Barnes, P. J. Immunology of asthma and chronic obstructive pulmonary disease. Nat. Rev. Immunol. 8 , 183–192 (2008).
Article CAS PubMed Google Scholar
Doeing, D. C. & Solway, J. Airway smooth muscle in the pathophysiology and treatment of asthma. J. Appl Physiol. 114 , 834–843 (2013).
Article CAS PubMed PubMed Central Google Scholar
Gibson, P. G. & Simpson, J. L. The overlap syndrome of asthma and COPD: what are its features and how important is it? Thorax 64 , 728–735 (2009).
Leung, J. M. & Sin, D. D. Asthma-COPD overlap syndrome: pathogenesis, clinical features, and therapeutic targets. BMJ 358 , j3772 (2017).
Article PubMed Google Scholar
Carolan, B. J. & Sutherland, E. R. Clinical phenotypes of chronic obstructive pulmonary disease and asthma: recent advances. J. Allergy Clin. Immunol. 131 , 627–634.quiz 635 (2013).
Tinkelman, D. G., Price, D. B., Nordyke, R. J. & Halbert, R. J. Misdiagnosis of COPD and asthma in primary care patients 40 years of age and over. J. Asthma 43 , 75–80 (2006).
Miravitlles, M. et al. Difficulties in differential diagnosis of COPD and asthma in primary care. Br. J. Gen. Pr. 62 , e68–e75 (2012).
Article Google Scholar
Jones, R. C., Dickson-Spillmann, M., Mather, M. J., Marks, D. & Shackell, B. S. Accuracy of diagnostic registers and management of chronic obstructive pulmonary disease: the Devon primary care audit. Respir. Res. 9 , 62 (2008).
Article PubMed PubMed Central Google Scholar
Nelson, H. S., Weiss, S. T., Bleecker, E. R., Yancey, S. W. & Dorinsky, P. M. The Salmeterol Multicenter Asthma Research Trial: a comparison of usual pharmacotherapy for asthma or usual pharmacotherapy plus salmeterol. Chest 129 , 15–26 (2006).
McMahon, A. W., Levenson, M. S., McEvoy, B. W., Mosholder, A. D. & Murphy, D. Age and risks of FDA-approved long-acting β 2 -adrenergic receptor agonists. Pediatrics 128 , e1147–e1154 (2011).
Rodrigo, G. J. & Castro-Rodríguez, J. A. Safety of long-acting β agonists for the treatment of asthma: clearing the air. Thorax 67 , 342–349 (2012).
Ernst, P., Gonzalez, A. V., Brassard, P. & Suissa, S. Inhaled corticosteroid use in chronic obstructive pulmonary disease and the risk of hospitalization for pneumonia. Am. J. Respir. Crit. Care Med. 176 , 162–166 (2007).
Crim, C. et al. Pneumonia risk in COPD patients receiving inhaled corticosteroids alone or in combination: TORCH study results. Eur. Respir. J. 34 , 641–647 (2009).
Chalitsios, C. V., Shaw, D. E. & McKeever, T. M. Corticosteroids and bone health in people with asthma: a systematic review and meta-analysis. Respir. Med. 181 , 106374 (2021).
Yang, I. A., Clarke, M. S., Sim, E. H. & Fong, K. M. Inhaled corticosteroids for stable chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 7 , CD002991 (2012).
Google Scholar
Derom, E. et al. Primary care spirometry. Eur. Respir. J. 31 , 197–203 (2008).
Casas Herrera, A. et al. COPD Underdiagnosis and misdiagnosis in a high-risk primary care population in four Latin American countries. A key to enhance disease diagnosis: The PUMA Study. PLoS ONE 11 , e0152266 (2016).
Article PubMed PubMed Central CAS Google Scholar
Yu, W. C. et al. Spirometry is underused in the diagnosis and monitoring of patients with chronic obstructive pulmonary disease (COPD). Int. J. Chron. Obstruct. Pulmon Dis. 8 , 389–395 (2013).
van den Boom, G. et al. Active detection of chronic obstructive pulmonary disease and asthma in the general population. Results and economic consequences of the DIMCA program. Am. J. Respir. Crit. Care Med. 158 , 1730–1738 (1998).
Albers, M. et al. Do family physicians’ records fit guideline diagnosed COPD? Fam. Pr. 26 , 81–87 (2009).
Sterk, P. J. et al. Standardized challenge testing with pharmacological, physical and sensitizing stimuli in adults. Eur. Respir. J. 6 (Suppl 16), 53–83 (1993).
American Thoracic Society. Standardization of Spirometry, 1994 Update. Am. J. Respir. Crit. Care Med. 152 , 1107–1136 (1995).
Quanjer, P. H. et al. Lung volumes and forced ventilatory flows. Report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal. Official Statement of the European Respiratory Society. Eur. Respir. J. Suppl. 16 , 5–40 (1993).
Global Initiative for Asthma (GINA). Global Strategy for Asthma Management and Prevention. www.ginasthmaorg . (2007).
Global Initiative for Chronic Obstructive Lung Disease (GOLD). The Global Strategy for the Diagnosis, Management and Prevention of COPD. www.goldcopdorg . (2006).
Quanjer, P. H. et al. Multi-ethnic reference values for spirometry for the 3-95 year age range: the global lung function 2012 equations. Eur. Respir. J. 40 , 1324–1343 (2012).
Yawn, B. P. et al. Spirometry can be done in family physicians’ offices and alters clinical decisions in management of asthma and COPD. Chest 132 , 1162–1168 (2007).
The Dutch College of General Practitioners (NHG) guideline Adult asthma, fifth version [NHG-Standaard Astma bij volwassenen, versie 5.0]. https://richtlijnen.nhg.org/ (2020).
The Dutch College of General Practitioners (NHG) guideline COPD, fifth version [NHG Standaard COPD, versie 5.0]. https://richtlijnen.nhg.org/ (2021).
DeLong, E. R., DeLong, D. M. & Clarke-Pearson, D. L. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44 , 837–845 (1988).
Tashkin, D. P. et al. Bronchodilator responsiveness in patients with COPD. Eur. Respir. J. 31 , 742–750 (2008).
Tashkin, D. P. et al. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N. Engl. J. Med. 359 , 1543–1554 (2008).
Grootendorst, D. C. & Rabe, K. F. Mechanisms of bronchial hyperreactivity in asthma and chronic obstructive pulmonary disease. Proc. Am. Thorac. Soc. 1 , 77–87 (2004).
van den Berge, M. et al. Clinical and inflammatory determinants of bronchial hyperresponsiveness in COPD. Eur. Respir. J. 40 , 1098–1105 (2012).
Beeh, K. M., Kornmann, O., Beier, J., Ksoll, M. & Buhl, R. Clinical application of a simple questionnaire for the differentiation of asthma and chronic obstructive pulmonary disease. Respir. Med. 98 , 591–597 (2004).
Tinkelman, D. G. et al. Symptom-based questionnaire for differentiating COPD and asthma. Respiration 73 , 296–305 (2006).
Hegewald, M. J. Diffusing capacity. Clin. Rev. Allergy Immunol. 37 , 159–166 (2009).
Lutfi, M. F. The physiological basis and clinical significance of lung volume measurements. Multidiscip. Respir. Med . 12 , 1–12 (2017).
Mauad, T. & Dolhnikoff, M. Pathologic similarities and differences between asthma and chronic obstructive pulmonary disease. Curr. Opin. Pulm. Med. 14 , 31–38 (2008).
Orie, N. G. M. & Sluiter, H. J. Bronchitis: an international symposium . (Royal van Gorcum, Assen, Netherlands, 1962).
Postma, D. S., Weiss, S. T., van den Berge, M., Kerstjens, H. A. & Koppelman, G. H. Revisiting the Dutch hypothesis. J. Allergy Clin. Immunol. 136 , 521–529 (2015).
Hall, G. L. & Stanojevic, S. Executive GLIN, Members of the GLINE. The Global Lung Function Initiative (GLI) Network ERS Clinical Research Collaboration: how international collaboration can shape clinical practice. Eur. Respir. J . 53 , 1–4 (2019).
Schermer, T. R. et al. Current clinical guideline definitions of airflow obstruction and COPD overdiagnosis in primary care. Eur. Respir. J. 32 , 945–952 (2008).
Enright, P. L. Should we keep pushing for a spirometer in every doctor’s office? Respir. Care 57 , 146–51. (2012).
Johns, D. P., Burton, D., Walters, J. A. & Wood-Baker, R. National survey of spirometer ownership and usage in general practice in Australia. Respirology 11 , 292–298 (2006).
Eaton, T. et al. Spirometry in primary care practice: the importance of quality assurance and the impact of spirometry workshops. Chest 116 , 416–423 (1999).
Schermer, T. R. et al. Validity of spirometric testing in a general practice population of patients with chronic obstructive pulmonary disease (COPD). Thorax 58 , 861–866 (2003).
Ruppel, G. L., Carlin, B. W., Hart, M. & Doherty, D. E. Office spirometry in primary care for the diagnosis and management of COPD: national lung health education program update. Respir. Care 63 , 242–252. (2018).
Bins, J. E., Metting, E. I., Muilwijk-Kroes, J. B., Kocks, J. W. H. & In ‘t Veen, J. The use of a direct bronchial challenge test in primary care to diagnose asthma. NPJ Prim. Care Respir. Med. 30 , 45 (2020).
Download references
Acknowledgements
The authors appreciate the statistical advice provided and analysis performed by Reinier Akkermans of the Department of Primary and Community of the Radboud University Medical Center regarding the comparison of the ROC curves for the three Scenarios. The study was funded by the Radboud University Medical Center, Nijmegen, The Netherlands.
Author information
Authors and affiliations.
Department of Primary and Community Care, Radboud Institute for Health Sciences, Radboud University Medical Center, Nijmegen, The Netherlands
Jelle D. M. Bouwens, Erik W. M. A. Bischoff & Tjard R. Schermer
Canisius Wilhelmina Hospital, Nijmegen, The Netherlands
Jelle D. M. Bouwens
Department of Pulmonary Medicine, ErasmusMC, Rotterdam, The Netherlands
Johannes C. C. M. in ’t Veen
Department of Pulmonary Diseases, Franciscus Gasthuis & Vlietland, Rotterdam, The Netherlands
Science Support Office, Gelre Hospitals, Apeldoorn, The Netherlands
- Tjard R. Schermer
You can also search for this author in PubMed Google Scholar
Contributions
J.D.M.B. and T.R.S. initiated the study, performed the data analysis, and wrote the initial draft version of the paper. E.W.M.A.B. and J.C.C.M.V. critically reviewed the paper. All authors approved the final version of the paper that was submitted.
Corresponding author
Correspondence to Tjard R. Schermer .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Reporting summary, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Bouwens, J.D.M., Bischoff, E.W.M.A., in ’t Veen, J.C.C.M. et al. Diagnostic differentiation between asthma and COPD in primary care using lung function testing. npj Prim. Care Respir. Med. 32 , 32 (2022). https://doi.org/10.1038/s41533-022-00298-4
Download citation
Received : 01 December 2021
Accepted : 16 August 2022
Published : 05 September 2022
DOI : https://doi.org/10.1038/s41533-022-00298-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Chronic comorbid conditions and asthma exacerbation occurrence in a general population sample.
- Emma Baljet
- Hilde Luijks
npj Primary Care Respiratory Medicine (2023)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Publication types
- Case Reports
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Allergy Asthma Clin Immunol
- PMC10644606

Validation of adult asthma case definitions for primary care sentinel surveillance
Max moloney.
1 Asthma Research Unit, Kingston General Hospital, Kingston Health Sciences Centre at Queen’s University, 72 Stuart Street, Kingston, ON K7L 2V7 Canada
2 Division of Respirology, Department of Medicine, Queen’s University, Kingston, ON Canada
Alison Morra
Rachael morkem.
4 Canadian Primary Care Sentinel Surveillance Network (Eastern Ontario Network), Kingston, ON Canada
John Queenan
3 Department of Family Medicine, Queen’s University, Kingston, ON Canada
Samir Gupta
5 Division of Respirology, Department of Medicine, St. Michael’s Hospital, Toronto, ON Canada
6 Department of Medicine, University of Toronto, Toronto, ON Canada
7 Child Health Evaluative Science, The Hospital for Sick Children, Toronto, ON Canada
8 Dalla Lana School of Public Health, University of Toronto, Toronto, ON Canada
Geneviève Digby
David barber, m. diane lougheed, associated data.
The datasets used during the study are available from the corresponding author upon reasonable request.
Most asthma diagnoses and patient care take place in primary care settings. Electronic medical records (EMRs) offer an opportunity to utilize technology to improve asthma diagnosis and care. The purpose of this study was to create and validate separate case definitions for suspected and confirmed asthma in primary care EMRs, to enable surveillance, benchmarking, and quality improvement in primary care settings. The objective of this study was to develop a case definition for suspected and confirmed asthma for use in a primary care sentinel surveillance system.
A single chart abstractor conducted a manual audit of 776 randomly selected patient charts from an academic primary care practice EMR in Kingston, Ontario. Following the single chart abstractor classification, a consensus on chart classification as “not asthma”, “suspected asthma”, or “confirmed asthma” was achieved between the abstractor, a family physician, and a respirologist using Canadian Thoracic Society (CTS) criteria. Case definition algorithms based on billing codes, clinical data elements and medications were applied to the site’s Canadian Primary Care Sentinel Surveillance Network (CPCSSN) data for the same charts and compared to abstractor classifications to determine each algorithm’s measurement properties.
The prevalence of suspected and confirmed asthma were 7.3% (n = 54) and 2.4% (n = 18), respectively. None of the proposed case definitions could differentiate between suspected and confirmed asthma. One algorithm consisting of billing, clinical, and medication elements had the highest Youden’s Index for either suspected or confirmed asthma. The algorithm had a sensitivity of 81%, a specificity of 96%, positive predictive value of 71%, negative predictive value of 98%, and a Youden’s Index of 0.77 for combined suspected or confirmed asthma cases.
An EMR case definition for suspected or confirmed adult asthma has been validated for use in CPCSSN. Implementation of this case definition will enable the development of a surveillance electronic tool (eTool) for adult asthma that can foster quality improvement.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13223-023-00854-8.
Asthma is diagnosed based on a combination of patient history, physical examination, and objective tests. There are over 3 million Canadians diagnosed with asthma and the prevalence of asthma in Canada is approximately 8.5% [ 1 ]. The Canadian Thoracic Society (CTS) defines asthma as the combination of a compatible clinical history of asthma and reversible airflow obstruction or airway hyperresponsiveness on lung function tests, or alternatively, a specialist diagnosis of asthma [ 2 , 3 ]. Accordingly, although confirmed asthma requires objective testing, a “suspected asthma” case is defined as a compatible clinical history without objective measures of lung function consistent with asthma or specialist diagnosis [ 2 , 3 ]. Most asthma diagnoses and care occur in primary care settings [ 4 ]. Despite available guidelines, less than half of patients diagnosed with asthma have undergone appropriate pulmonary function testing to confirm their diagnosis [ 5 ]. As such, the majority of real-world diagnoses in primary care are more accurately classified as cases of suspected asthma. This failure to use objective testing has led to a high degree of misdiagnosis of asthma [ 6 ].
Electronic medical records (EMRs) offer an opportunity to utilize technology to improve the process of asthma diagnosis. Potential benefits of using electronic tools (eTools) within EMRs include improved quality of care, outcome monitoring, and performance measurement [ 7 , 8 ]. The Canadian Primary Care Sentinel Surveillance Network (CPCSSN) is the first and only pan-Canadian chronic disease surveillance system based on primary care EMR data [ 9 ]. CPCSSN has validated case definitions for 13 chronic conditions, including COPD [ 10 ]. There have also been efforts to create case definitions for adult asthma using EMR data directly by Xi et al. [ 11 ], and using EMR data extracted into the CPCSSN database by Cave et al. [ 12 ]. To date, there remains no standardized case definition for diagnosis of asthma in primary care that can be applied to EMRs and databases across Canada. As primary care EMR data are increasingly being used for disease surveillance, validated case definitions are required [ 13 , 14 ]. A recent literature review on asthma case definitions identified the need to create a case definition that differentiates between suspected and confirmed asthma in primary care EMRs [ 15 ]. The purpose of this study was to create and validate separate case definitions for suspected and confirmed asthma in adults in primary care EMRs, and to determine the ability to distinguish between suspected and confirmed asthma using primary care EMR data.
Study design
A retrospective chart analysis was conducted at the Queen’s Family Health Team (QFHT) in Kingston, Ontario. The QFHT uses the open-source OSCAR EMR developed by McMaster University that is used across Canada, in the care of over 1 million patients [ 16 ]. CPCSSN collects de-identified patient data from source EMRs, including but not limited to demographics, visit dates, reason for encounter, medical conditions, billing history, procedure history, prescribed medications, laboratory results, and patient referrals. This study used coded and processed QFHT data in CPCSSN, stored at the Centre for Advanced Computing at Queen’s University. The random sample in this study was derived from a list of patient charts from CPCSSN data holdings generated by a computer algorithm. Patients that elected to opt-out of research from CPCSSN were removed from the list of patient charts for review.
QFHT patients were notified of the study and given the option to withdraw from the study. The study was approved by the Health Sciences and Affiliated Teaching Hospitals Research Ethics Board (HSERB) at Queen’s University. All data were recorded as non-identifiable information.
Chart abstraction and data collection
The criteria for generation of the patient list were age ≥ 18 years and currently registered at QFHT. Charts were excluded if there was no recorded visit to a physician in the previous 5 years or if a chart was marked as inactive by QFHT. A single chart abstractor conducted a manual audit of the 776 randomly selected patient charts for 776 patients. The sample of 776 patient charts was determined based on a power calculation assuming 8% prevalence of asthma in adults [ 17 , 18 ], along with a projected case definition algorithm with 80% sensitivity, 10% precision and 95% CIs. Given these parameters, the minimum sample size was determined to be 776. The abstractor collected 96 data points selected a priori by consensus of the lead investigators relevant to asthma diagnosis and management, and classified each chart as “not asthma”, “suspected asthma”, or “confirmed asthma”. The categories of data collected included patient demographics, prescribed medications, asthma symptom history, comorbidities, smoking status, documentation of asthma diagnosis, asthma exacerbation history, pulmonary function tests, and referral notes. Data collected included a lookback time frame of the entire patient’s history, with the exception of medication data, which was separated into expired (> 2 years) and current (≤ 2 years). The abstractor used a chart abstraction manual to ensure data entry accuracy and consistency. All data were collected using an online abstraction form created on Qualtrics™ software.
Asthma classification definition
The definition of “confirmed asthma” was based on the CTS guideline for asthma diagnosis [ 2 , 3 ]. Confirmed asthma was defined as a compatible clinical history plus pulmonary function tests (PFTs) confirming asthma, and/or a specialist diagnosis. “Suspected asthma” was defined as a compatible clinical history without PFTs consistent with asthma or a specialist diagnosis. All other patient charts were classified as “not asthma.” Following the single chart abstractor classification, each chart classified as suspected or confirmed asthma and 12 charts classified as not asthma which the abstractor thought required additional input for classification, were reviewed by a QFHT family physician and a respirologist to achieve consensus on the final classification. All chart classification reviews were completed on the original OSCAR EMR charts at QFHT.
Case definition development
Case definitions were developed and tested on the CPCSSN data holdings for the same charts reviewed and classified by the abstractor and expert physicians in the OSCAR EMR at QFHT. Case definitions tested in this study were developed by members of the study team. They included 3 case definitions for adult asthma previously published in the literature, in addition to new case definitions designed by the research team [ 11 , 12 ]. The proposed case definitions were developed based on all data fields possibly relevant for diagnosis in CPCSSN, including billing information, health condition, encounter diagnosis, and medication data. In total, 21 case definitions were developed and tested through an iterative process. Case definition criteria within the search fields used a combination of: text strings (in encounter diagnosis or health condition fields); International Classification of Disease, Ninth Revision (ICD-9) codes (used by QFHT) in billing diagnosis, encounter diagnosis, or health condition fields); and medication prescription information of the CPCSSN data. The complete list of case definitions and the specific search criteria tested are available in Table Table1 1 .
Sample Characteristics (n = 743)
ACOS Asthma-COPD Overlap Syndrome, COPD Chronic Obstructive Pulmonary Disease, GERD Gastroesophageal Reflux Disease
ICS Inhaled corticosteroid, LABA Long-acting β-agonist, LAMA Long-acting muscarinic antagonist, LTRA Leukotriene receptor antagonist, SABA Short-acting β-agonist, SAMA Short-acting muscarinic antagonist
Statistical analysis
The results of the proposed case definitions were compared to the confirmed asthma classification definition (reference standard). For each proposed case definition, sensitivity (SN), specificity (SP), positive predictive value (PPV), negative predictive value (NPV), and Youden’s Index (YI) [(sensitivity + specificity) – 1] were calculated in the (i) confirmed asthma subset, (ii) suspected asthma subset, and (iii) combined confirmed or suspected asthma subset. An ROC curve was plotted for combined confirmed or suspected asthma. Additionally, descriptive statistics were calculated for each of the 96 data points abstracted during the study. Statistical analysis was completed using Microsoft Excel™ and SAS™ software.
Sample characteristics
A total of 743 patient charts were included in the study's final analysis. Thirty-three charts were excluded (Fig. 1 ). The date cut off for chart inclusion was an entry into the EMR within the last two years.

Sample derivation
The characteristics of the sample are detailed in Table Table1. 1 . The estimated prevalence of suspected or confirmed asthma based on the 743 charts that met inclusion criteria was 9.7% (n = 72). Of the 72 charts determined to have suspected or confirmed asthma, 54 (7.4%) were classified as suspected and 18 (2.3%) were classified as confirmed.
In the study sample, 416 of 743 (56.0%) patients’ charts reviewed were female and 327 charts (44.0%) reviewed were male. The mean (± SD) age of patients reviewed was 50.3 (± 8.7). Additional patient characteristics are outlined in Additional file 1 : Table S2. In assessing rates of objective measures to confirm asthma diagnosis, spirometry was completed in 114 (15.3%) of charts reviewed. Completion of spirometry with bronchodilator testing was documented in 86 charts (11.6%). Documentation of a methacholine challenge test was completed in 15 charts (2.0%)., and evidence of a specialist diagnosis was present in 22 charts (3.0%).
Case definition results
The case definition algorithm determined to have the highest Youden’s Index for the combination of suspected or confirmed asthma was Case Definition 10, which used a combination of text strings and ICD-9 codes from the billing, encounter diagnosis, and health condition within CPCSSN (Table (Table2 2 and Fig. 2 ). This definition had a SN of 81%, a SP of 96%, PPV of 71%, NPV of 98%, and a Youden’s Index of 0.77. For suspected asthma, the case definition had a SN of 76%, a SP of 94%, PPV of 50%, NPV of 98%, and a Youden’s Index of 0.70. For confirmed asthma, the case definition had a SN of 94%, a SP of 91%, PPV of 21%, NPV of 99%, and a Youden’s Index of 0.85. None of the case definitions assessed in this study met the minimum standard (sensitivity and specificity > 70%) to differentiate between suspected and confirmed asthma. Complete results are available in Additional file 1 : Table S3–S5.
Case definition criteria and results
*Adapted from Xi et al. (2015)
†Adapted from Cave et al. (2020)
LABA Long-acting β-agonist, LAMA Long-acting muscarinic antagonist
TP True Positive, FP False Positive, FN False Negative, TN True Negative, SN Sensitivity, SP Specificity, PPV Positive Predictive Value, NPV Negative Predictive Value, YI Youden’s Index

ROC curve of proposed case definitions for suspected and confirmed asthma
We validated a case definition for combined suspected or confirmed asthma in primary care. This study's proposed case definitions had similar results for both suspected and confirmed asthma. Case definitions could not discriminate between suspected and confirmed asthma because the use of objective measures to confirm asthma diagnosis was either not completed or not documented. Our findings of a combined prevalence of suspected and confirmed asthma of 9,7% is comparable to current national statistics [ 18 ]. However, 75% of the cases in our study were suspected not confirmed. This highlights the importance of confirming and documenting the status of asthma diagnoses in EMRs. National statistics based on population surveys that rely on self-report of physician diagnosis or billing data may also be subject to considerable misclassification. Until EMR data elements are adopted that allow for the distinction between suspected and confirmed asthma [ 19 ], one case definition that can be used for combined suspected or confirmed asthma is recommended.
Our proposed case definitions had similar operating characteristics to those reported previously. However, in replicating case definition algorithms from both Xi et al. [ 11 ] and Cave et al. [ 12 ] (Table (Table1), 1 ), we found different results across all metrics calculated. For example, for Case Definition 1, Xi et al. (2015) report a SN of 78% and SP of 89%, compared to a SN of 35% and SP of 99% in our study. For Case Definition 3, Xi (2015) reported a SN of 7% and SP of 99%, compared to a SN of 4% and SP of 100% in our study [ 11 ]. Case Definitions 1 and 3 were attempts to replicate their algorithms and were considered approximated because the original case definition algorithms used information directly from the source EMR in OSCAR. For Cave et al., the metrics were similar, with a reported a SN of 83%, SP of 99%, PPV of 74%, NPV of 99%, and a Youden’s Index of 0.82, compared to a SN of 78%, SP of 97%, PPV of 75%, NPV of 98%, and YI of 0.73 in our study. [ 12 ]
This variability can likely be attributed to the variation in the data sources used for case definition analysis and the variation in charting behaviour between clinical sites. Xi et al. (2015) created a cohort with a high proportion of patients with asthma and COPD for analysis. In contrast, we used a population-based sample, thus having a lower asthma prevalence, reducing SP and PPV while improving SN and NPV. In Cave et al.’s (2020) study, the authors used data from the Southern Alberta Primary Care Research Network of CPCSSN (SAPCReN-CPCSSN) to classify cases of asthma. In this study, reviewers used the source EMR for classification, allowing for a complete review of the patient’s entire medical history.
The results of this study highlight the importance of having discrete data elements for asthma diagnostic tests in EMRs, particularly given that there were no searchable data elements that enabled us to differentiate between suspected and confirmed asthma. In addition, in EMRs, there is no requirement for confirming asthma diagnosis through objective measures such as spirometry or a methacholine challenge test. EMRs should incorporate data elements such as those proposed by the Pan-Canadian Respiratory Standards Initiative for Electronic Health Records (PRESTINE) so that providers are able to document whether asthma is suspected or confirmed, and if confirmed by what method [ 7 , 20 ]. Data elements that capture if asthma has been confirmed would enable case definition search strategies to differentiate between suspected and confirmed asthma [ 19 ]. By adopting these data elements, knowledge translation eTools could provide decision support to healthcare providers on cases of suspected asthma that require objective testing, while simultaneously improving asthma surveillance by ensuring cases of asthma are confirmed asthma [ 21 ].
In our study, although we included every medication combination presented in the CTS guidelines for asthma management [ 3 ] (Case Definitions M1-M7), medication data did not improve the operating characteristics of detection algorithms (Table (Table1). 1 ). The proposed case definitions that included medication data had a wide sensitivity range, from 0 to 76%. This result differs from previous literature on asthma case definitions, which discuss adding medications as an effective way to improve case definitions [ 22 ]. We believe that this may be because many medications are now being used for both asthma and COPD, and as could contribute to misdiagnosis of asthma and COPD if used as part of EMR algorithms. Additionally, this finding suggests that researchers creating asthma case definitions must be very specific in their inclusion or exclusion of medications in case definitions.
The findings of our study fit well within the existing literature on the validation of asthma diagnoses using EMRs. A recent study from Howell et al. [ 23 ] developed a case definition algorithm for asthma using EMR data from a pulmonary specialty clinic. This study’s best-case definition had a SN of 94% and a SP of 85%. These results are slightly higher than the results of our study. In this case, the slightly higher SN and SP can be attributed to using a specialty clinic, which would be more likely to have confirmed cases of asthma, improving specificity, and a higher relative proportion of patients with asthma, improving sensitivity. Another systematic review of literature on the validation of asthma diagnoses in electronic health records by Nissen et al. described 13 studies on the subject [ 22 ]. The authors found that most studies were able to demonstrate a high positive predictive value (PPV > 80%), with a high degree of variation based on methodology used. Our study builds upon the systematic review by using a national database that can utilize the case definition in primary care practices across Canada.
We were able to directly replicate the case definition proposed by Cave et al., given that it also used CPCSSN data holdings. For case definition 13, Cave et al. (2020) reported a SN of 83% (+ 5%), a SP of 99% (+ 2%), PPV of 74% (-1%), NPV of 99% (-1%), and a Youden’s Index of 0.82 (+ 0.09), which are nearly identical to our results. The discrepancy between the findings can be attributed to the data source used for classifying cases of asthma and the data source used for validating the case definition.
The clinical implications of using a combined case definition for asthma in primary care EMRs for suspected and confirmed asthma are important to consider. Until EMR data elements that document whether asthma has been confirmed by objective lung function tests are widely adopted, surveillance data utilizing an asthma EMR case definition that cannot differentiate between suspected and confirmed asthma may over-estimate true asthma prevalence. Separate case definitions would provide more accurate information on disease patterns, prevalence, and performance measurement for quality improvement. Future knowledge translation initiatives should focus on adoption of EMR data elements that would allow separate EMR case definitions for the suspected and confirmed asthma.
Strengths of this study include using the original EMR source data for chart abstraction and classification. By manually reviewing the patient chart, the abstractor and physicians had the entire medical record of a patient available to accurately classify the charts based on all information available. Another strength of this study is the use of CPCSSN data holdings for testing and validating case definitions. CPCSSN data is more granular than health administrative data that has been used for case definitions of asthma in the past. This is due to CPCSSN’s data being derived from primary care medical records which have more specific information than health administrative data. In addition to CPCSSN’s added specificity, CPCSSN remains more broadly applicable than data from a single EMR as it compiles data from multiple EMR platforms [ 24 ]. Another strength of utilizing CPCSSN as a database is to improve the generalizability of the study, as CPCSSN can analyze data from all major EMR providers in Canada. This allows the proposed case definition to be applied across the country to various primary care settings and EMR providers. Additional strengths of this study are the use of a single abstractor and experts for classification purposes, which ensured consistency in both data collection and final classification of cases.
Limitations
Limitations of this study include generalizability and the data source. This exercise was conducted at a single academic clinical site that is a member of CPCSSN. It may be difficult to generalize the findings at this academic primary care practice to community practices, as the case mix may differ, and this particular practice may have unique charting, billing, and data entry patterns. Additionally, this study used information from one EMR, OSCAR. As a result, the case definitions developed in this study may have different results when applied to other EMRs, although the criteria used in the CPCSSN database applies to sites across Canada.
An EMR case definition for confirmed or suspected adult asthma has been validated against original primary care EMR data for use in primary care, including CPCSSN. Implementation of this case definition will enable surveillance and quality improvement of adult asthma care in primary care sites across Canada. Currently, it is not possible to differentiate between suspected and confirmed asthma in primary care EMRs or CPCSSN datasets. As such, adoption of Pan-Canadian standards for EMR elements and algorithms, as proposed by PRESTINE, that identify suspected but unconfirmed asthma and prompt further investigations, is critical to improving the diagnostic accuracy of primary care surveillance and quality improvement systems. Incorporating these data elements into EMR platforms will enable validation of more specific asthma case definitions, and improve surveillance, and quality improvement opportunities for primary care practices.
Acknowledgements
Not applicable.
Abbreviations
Author contributions.
MDL conceived the premise of this study. MM, DB, and RM conducted the data analysis. MM wrote the first draft of the manuscript with revisions from MDL and AM. JQ, SG, TT, and GD provided guidance, suggestions, and revisions for this manuscript.
This study was funded by the Southeastern Ontario Academic Medical Organization Innovation Fund Grant.
Availability of data and materials
Declarations.
The study was reviewed for ethical compliance by the Queen’s University Health Sciences and Affiliated Teaching Hospitals Research Ethics Board (Reference #6029444).
The authors declare that they have no competing interests.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
March 19, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
Loneliness worse than smoking, alcoholism, obesity: Study suggests primary care clinicians can offer solutions
by Regenstrief Institute

Loneliness is a significant biopsychosocial stressor with a mortality risk comparable to smoking more than 15 cigarettes a day and more harmful than alcoholism, obesity, and lack of physical activity.
Despite its harmful effects , interventions to address the discrepancy between desired and actual social interaction are few and limited.
In a new study, Regenstrief Institute and Indiana University School of Medicine research scientists Monica Williams-Farrelly, Ph.D., Malaz Boustani, M.D., MPH, and Nicole Fowler, Ph.D., MHSA, identified evidence suggesting primary care clinicians can play an important role in developing and maintaining personal connections for patients experiencing loneliness .
The study found that 53 percent of older adults in the primary care population experience loneliness. Evidence also suggests that when older adults experience loneliness, their physical and mental health related to quality of life are reduced significantly.
"The first and obvious answer for loneliness is for primary care physicians to screen their patients," said Dr. Williams-Farrelly, the study's first author, a Regenstrief research scientist and an assistant research professor at IU School of Medicine.
"Based on the literature and research, loneliness has influences on health that are quite significant and quite strong, so in the same way that we ask older adults: Do you smoke? Or do you measure your blood sugar? We should be inquiring about and measuring loneliness and offering solutions."
Dr. Williams-Farrelly suggests that it is imperative for primary care physicians, nurse practitioners and other clinicians also to provide resources to patients to help address this significant issue.
"The topic of loneliness is more relevant now than ever given the May 2023 U.S. Surgeon General's call to action to tackle the loneliness epidemic," said Dr. Fowler, principal researcher and senior author.
"This research is important because it identifies and suggests evidence for interventions that are necessary for older adults in primary care who experience loneliness. Primary care clinicians should discuss loneliness with their older adult patients and provide resources to help them create meaningful social relationships." Dr. Fowler is also a Regenstrief research scientist, an associate professor, and a director of research at the IU School of Medicine.
An effective intervention, the researchers suggest, is the Circle of Friends concept, which consists of a three-month, group-based, psychosocial rehabilitation model aimed at enhancing interaction and friendships between participants. The model has shown effectiveness in both reducing loneliness and improving health outcomes , including subjective health, cognition, mortality, and lower health care costs.
"As older adults age, they have a lot of changes in their life due to a lot of circumstances—retirement, divorce, or the death of family and friends—making it a little more difficult for them to maintain social relationships. When connections are lost with coworkers or loved ones, it can be jarring," said Dr. Williams-Farrelly. "Older adults need their primary care physicians to screen and suggest effective resources that can allow them to maintain, foster and develop social relationships."
Data was gathered during the COVID-19 pandemic, but the researchers identified a steadily increasing trend in loneliness in this population prior to the global pandemic. The numbers are still increasing today.
"Loneliness may seem simple, but it can be complex to identify and address. It started to become a problem before COVID-19, and then with the national stay-at-home order caused by the pandemic, social contact was being prevented, which exacerbated the problem," said Dr. Williams-Farrelly.
This study used baseline data from the Caregiver Outcomes of Alzheimer's Disease Screening (COADS) clinical trial, supported by the National Institutes of Health's National Institute on Aging grant R01AG056325.
" Loneliness in older primary care patients and its relationship to physical and mental health-related quality of life ," is published in the Journal of the American Geriatrics Society .
Explore further
Feedback to editors
An infamous 'inflammasome'—a rogue protein complex—appears to underlie a rare and disabling autoimmune disorder
15 hours ago

Researchers discover skin biomarkers in infants that predict early development of food allergies
16 hours ago

Veterans help provide greater insight into Klinefelter and Jacobs syndromes
High-resolution images reveal similarities in protein structures between Alzheimer's disease and Down syndrome
How blocking a neural receptor responsible for addiction could reduce alcohol use

Study finds few hospitals promoting potentially predatory medical payment products

COVID-19 research: Study reveals new details about potentially deadly inflammation
17 hours ago
Enhanced melanoma vaccine offers improved survival for men

How music choices can affect productivity

Prescribing alcohol use disorder medications upon discharge from alcohol-related hospitalizations works
Related stories.

Loneliness found to increase the risk of health deterioration in older adults
Feb 29, 2024

Q&A: Meaningful social interactions are the only 'cure' for loneliness
Dec 19, 2023

Five things to know about loneliness in older adults
Apr 29, 2019

Multiple periods of loneliness may add up to higher mortality risk
Dec 13, 2023

Loneliness in later life lessens when older adults spend many hours volunteering, shows study
Feb 13, 2023

Study examines the devastating impact of loneliness on autistic people
Nov 2, 2023
Recommended for you

Psychological care delivered by phone can help combat loneliness and depression, study finds
19 hours ago

Steady rise in US suicides among adolescents, teens
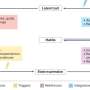
Research review suggests four key mechanisms are involved in changing one's personality
Mar 28, 2024

Positive associations found between premenstrual disorders and perinatal depression

Study suggests maintaining optimism contributes to better mobility in women as they grow older
Let us know if there is a problem with our content.
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter
- Search Menu
- Advance articles
- Editor's Choice
- Supplement Archive
- Cover Archive
- IDSA Guidelines
- IDSA Journals
- The Journal of Infectious Diseases
- Open Forum Infectious Diseases
- Photo Quizzes
- Author Guidelines
- Open Access
- Why Publish
- Advertising and Corporate Services
- Advertising
- Journals Career Network
- Reprints and ePrints
- Sponsored Supplements
- Branded Books
- About Clinical Infectious Diseases
- About the Infectious Diseases Society of America
- About the HIV Medicine Association
- IDSA COI Policy
- Editorial Board
- Self-Archiving Policy
- For Reviewers
- For Press Offices
- Journals on Oxford Academic
- Books on Oxford Academic

Article Contents
Supplementary data.
- < Previous
Stop COVID Cohort: An Observational Study of 3480 Patients Admitted to the Sechenov University Hospital Network in Moscow City for Suspected Coronavirus Disease 2019 (COVID-19) Infection
D. M., N. A. N., P. B., D. B., and P. G. contributed equally.
- Article contents
- Figures & tables
Daniel Munblit, Nikita A Nekliudov, Polina Bugaeva, Oleg Blyuss, Maria Kislova, Ekaterina Listovskaya, Aysylu Gamirova, Anastasia Shikhaleva, Vladimir Belyaev, Peter Timashev, John O Warner, Pasquale Comberiati, Christian Apfelbacher, Evgenii Bezrukov, Mikhail E Politov, Andrey Yavorovskiy, Ekaterina Bulanova, Natalya Tsareva, Sergey Avdeev, Valentina A Kapustina, Yuri I Pigolkin, Emmanuelle A Dankwa, Christiana Kartsonaki, Mark G Pritchard, Victor Fomin, Andrey A Svistunov, Denis Butnaru, Petr Glybochko, Sechenov StopCOVID Research Team , Stop COVID Cohort: An Observational Study of 3480 Patients Admitted to the Sechenov University Hospital Network in Moscow City for Suspected Coronavirus Disease 2019 (COVID-19) Infection, Clinical Infectious Diseases , Volume 73, Issue 1, 1 July 2021, Pages 1–11, https://doi.org/10.1093/cid/ciaa1535
- Permissions Icon Permissions
The epidemiology, clinical course, and outcomes of patients with coronavirus disease 2019 (COVID-19) in the Russian population are unknown. Information on the differences between laboratory-confirmed and clinically diagnosed COVID-19 in real-life settings is lacking.
We extracted data from the medical records of adult patients who were consecutively admitted for suspected COVID-19 infection in Moscow between 8 April and 28 May 2020.
Of the 4261 patients hospitalized for suspected COVID-19, outcomes were available for 3480 patients (median age, 56 years; interquartile range, 45–66). The most common comorbidities were hypertension, obesity, chronic cardiovascular disease, and diabetes. Half of the patients (n = 1728) had a positive reverse transcriptase–polymerase chain reaction (RT-PCR), while 1748 had a negative RT-PCR but had clinical symptoms and characteristic computed tomography signs suggestive of COVID-19. No significant differences in frequency of symptoms, laboratory test results, and risk factors for in-hospital mortality were found between those exclusively clinically diagnosed or with positive severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RT-PCR. In a multivariable logistic regression model the following were associated with in-hospital mortality: older age (per 1-year increase; odds ratio, 1.05; 95% confidence interval, 1.03–1.06), male sex (1.71; 1.24–2.37), chronic kidney disease (2.99; 1.89–4.64), diabetes (2.1; 1.46–2.99), chronic cardiovascular disease (1.78; 1.24–2.57), and dementia (2.73; 1.34–5.47).
Age, male sex, and chronic comorbidities were risk factors for in-hospital mortality. The combination of clinical features was sufficient to diagnose COVID-19 infection, indicating that laboratory testing is not critical in real-life clinical practice.
In Russia, the first confirmed cases of coronavirus disease 2019 (COVID-19) were reported by the state authorities in early March 2020 [ 1 ]. Since then, the Russian Federation climbed into the top 3 nations in the world affected by COVID-19, surpassing 400 000 cases by the end of May 2020.
The rate of infections in Moscow and the Moscow metropolitan area, with its high population density and number of inhabitants (20 million), has exceeded 180 000 confirmed cases, accounting for half of all the COVID-19 cases in Russia [ 2 ].
The clinical characteristics of COVID-19 have been described in studies from China [ 3 ], Italy [ 4 ], the United States [ 5–7 ], and the United Kingdom [ 8 ]. At present, no information on the clinical epidemiology, including clinical course, and outcomes of patients with COVID-19 in the Russian population is available. A recent editorial in The Lancet highlighted a surprisingly low mortality rate (~1%) in Russia [ 9 ]. With no academic data, perspectives on the COVID-19 pandemic in Russia are mainly based on media reports and briefs from Russian officials.
This study aimed to present demographic characteristics, symptoms, comorbidities, clinical test results, outcomes, and risk factors associated with mortality in a cohort of consecutively admitted patients with COVID-19 at the Sechenov University Hospital Network in Moscow. Secondarily, we aimed to test whether patients presenting with symptoms and radiological findings consistent with COVID-19 but without laboratory confirmation of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) have outcomes similar to those with positive reverse transcriptase–polymerase chain reaction (RT-PCR).
Study Design and Ethics
StopCOVID is an observational cohort study that took place at 4 large adult tertiary university hospitals in Moscow, Russia. All persons aged 18 years or olrder admitted to any of 4 Sechenov University Hospital Network hospitals between 8 April and 28 May 2020 with suspected COVID-19 infection were included in the study. RT-PCR to SARS-CoV-2 was the recommended mode of testing by the Russian Ministry of Health and was used throughout the study period in all the hospitals ( Supplementary Box 1 ). We enrolled all patients with confirmed or suspected COVID-19 infection, due to concerns of a high false-negative rate from RT-PCR results [ 10 ].
This study was approved by the Sechenov University Institutional Review Board on 22 April 2020 (protocol number 08–20).
Data Collection Process
The data were collected between 22 April and 6 June 2020. We reviewed electronic medical records for signs and symptoms on admission, baseline comorbidities, computed tomography (CT) imaging, and laboratory results for all admitted patients. Weight and height were self-reported by the patients to the clinical staff.
The data extraction was performed by a group of 40 medical students and resident doctors who went through personal protocol explanation webinars and data entry training prior to the beginning of the study. The team was supervised by senior academic staff members. The baseline characteristics were collected using the case report form (CRF) that was developed by the International Severe Acute Respiratory and Emerging Infection Consortium (ISARIC) and the World Health Organization (WHO) for use in outbreak investigations [ 11 ]. REDCap (Research Electronic Data Capture; Vanderbilt University, Nashville, TN, USA, hosted at Sechenov University) was used for data collection, storage, and management [ 12 , 13 ].
Study Definitions
Patients were defined as having confirmed COVID-19 if the diagnosis was confirmed by laboratory testing (at least 1 SARS-CoV-2 RT-PCR positive result).
Patients were defined as having “clinically diagnosed COVID-19” if laboratory confirmation was inconclusive or not available. Details of COVID-19 case definitions, criteria for hospitalization, grading of severity, and recommended treatment approaches are presented in Supplementary Box 1 .
We reviewed radiology reports of chest CT imaging during hospitalization. The data on the presence/absence of ground-glass opacities, consolidation, and severity of radiologic changes were retrieved. Incomplete reports containing no information on severity were excluded from the analysis. The severity of changes was graded by radiologists as per national COVID-19 guidelines using the modified visual assessment scale by Inui et al [ 14 ] ( Supplementary Table 1 ). The primary outcome in this study was in-hospital mortality.
Statistical Analysis
Descriptive statistics were calculated for baseline characteristics. Continuous variables were summarized as medians (interquartile range) and categorical variables as frequencies (percentage). The chi-square test or Fisher’s exact test was used for testing differences in proportions between individuals. The Wilcoxon rank-sum test was used to test for differences in laboratory test results between the groups.
We first ran univariate analysis to investigate associations between demographic characteristics and comorbidities with mortality. Then, we performed a multivariable logistic regression model, which included all statistically significant (at P = .001) potential predictors from the univariate analysis.
A Bonferroni correction was used to adjust for multiple comparisons, such that P values less than or equal to .001 were considered statistically significant for the analysis of symptoms and comorbidities and P values less than .001 were considered statistically significant for laboratory markers. All routine clinical laboratory measurements were used in the analysis, except the ones which were available for less than 10 deceased patients. Statistical analysis was performed using R version 3.5.1 (R Core Team).
A total of 4261 adults with suspected COVID-19 infection were admitted to the hospitals. Primary outcome data were available for 3535 patients who were discharged, died, or transferred to another hospital. The study primary endpoint was available for all but 55 individuals transferred to other hospitals; thus, 3480 (82%) individuals were included in the statistical analysis.
Half of the patients (n = 1728) had positive RT-PCR results, while the second half (n = 1748) were negative on RT-PCR but had clinical symptoms and CT signs suggestive of COVID-19. No differences were noted in the baseline demographic and clinical characteristics and laboratory and radiologic findings of those with RT-PCR–confirmed versus clinically diagnosed COVID-19 ( Table 1 , Supplementary Tables 2, 4, 5, 7 ).
Laboratory Test Results (Median [IQR]) in Patients With Clinically Diagnosed COVID-19 Infection (RT-PCR Negative) and Patients With RT-PCR–Confirmed COVID-19 Infection
Statistically significant results at P values <.001 are presented in bold. The number of patients is presented for each parameter.
Abbreviations: COVID-19, coronavirus disease 2019; IQR, interquartile range; RT-PCR, reverse transcriptase–polymerase chain reaction.
Baseline Characteristics
Table 2 and Supplementary Table 2 present an overview of baseline characteristics, stratified by the primary outcome and the RT-PCT result, respectively. The median age of all patients at admission was 56 years (interquartile range, 45–66; range, 18–100 years). Similar numbers of men (50.5%, n = 1758) and women (49.5%, n = 1722) were admitted to the hospitals ( P = .55). The median age of patients who died in the hospital was higher, 72 (61.5–81) years compared with 55 (44–65) years in survivors. Time from hospitalization to discharge/death was 14.5 (11.8–17.7) days, with shorter hospital stay in patients who died. Severity at admission was recorded as mild in 632 (18.2%), moderate in 2634 (75.7%), severe in 204 (5.9%), and critical in 7 (0.2%) patients, respectively.
Baseline Characteristics of Patients Admitted to Sechenov University Hospitals, Stratified by Outcome
Abbreviations: COVID-19, coronavirus disease 2019; ICU, intensive care unit; IQR, interquartile range; RT-PCR, reverse transcriptase–polymerase chain reaction; PT, Prothrombin.
a The proportion of patients in each subgroup is calculated from the total number of patients receiving a particular type of care (ICU, noninvasive ventilation, and invasive mechanical ventilation). Calculations were performed for each type of care, regardless of whether patients were discharged/died within the ICU facilities or were transferred to the ward and were discharged/died there.
Only 218 (6.3%) patients required admission and/or transfer to the intensive care unit (ICU), with some patients requiring noninvasive ventilation and/or invasive mechanical ventilation: 80 (2.3%) and 171 (5.0%), respectively. Although the proportion discharged alive from the ICU facilities was 42.5%, among all patients who received care in the ICU during the hospital stay, 57 (26.1%) were discharged from the hospital alive. Eight (4.7%) patients who received invasive mechanical ventilation during the hospital stay were discharged alive.
Data on symptoms and comorbidities at the time of hospital admission were available in 3382 (97%) patients. The most common symptoms in the medical records were fever (3157, 93.3%), fatigue/malaise (2684, 79.4%), cough (2476, 73.2%), and shortness of breath (2013, 59.5%). We also found a significant overlap between the top 3 most common symptoms, with 1912 (56.5%) patients having all 3 symptoms ( Figure 1 ). Shortness of breath, altered consciousness, and inability to walk were present significantly more often in patients who died, while anosmia, sore throat, fever, and muscle pain were found more frequently in those discharged alive ( Supplementary Table 3 ). Symptoms at admission did not differ significantly between the patients with laboratory-confirmed and clinically diagnosed COVID-19 ( Supplementary Table 4 ).

Stacked bar charts presenting the ( A ) top 10 most common symptoms and ( B ) most common comorbidities. Venn diagrams showing the coexistence of the ( C ) top 3 symptoms and ( D ) top 3 comorbidities at the time of hospital admission.
Detailed information on comorbidities in our cohort is presented in Table 3 , Supplementary Table 5 , and Figure 1 . The most common comorbidities were hypertension (1539, 45.5%), obesity (1129, 33.4%), chronic cardiovascular disease (621, 18.4%), and diabetes (predominantly type 2; 459, 13.6%). One in 10 patients reported current (139, 4.1%) or former (235, 6.9%) smoking. There was little overlap between the top 3 most common comorbidities, with only 145 (4%) patients having all 3, while 965 (28.5%) did not report any comorbidities.
Patient-reported Comorbidities at the Time of Hospital Admission and Chest Computed Tomography Imaging Stratified by Outcome
Statistically significant results at P values ≤.001 are presented in bold.
Abbreviations: ART, antiretroviral therapy; CT, computed tomography; HIV, human immunodeficiency virus.
aExcluding asthma.
b Obesity defined as body mass index based on electronic medical records data, and if data on height and weight were missing, records were screened for obesity definition by clinical staff.
Clinical Investigations
Most patients (71.6%) had significant changes on chest CT, equivalent to CT-2–CT-3 severity grade. Ground-glass opacity was found in over 95% of the patients and 77.95% had lung consolidation in accordance with the radiologist’s reports.
We reviewed routine clinical test measurements at admission and found abnormal changes to the coagulation profile, greater median levels of C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), aspartate aminotransferase (AST), and lactate dehydrogenase and decreased iron levels. Those patients who died in the hospital had more abnormal changes to their coagulation profile (D-dimer, international normalized ratio, prothrombin time, ferritin, fibrinogen), lymphocytopenia, and neutrophilia, and much higher levels of CRP and ESR, high blood urea nitrogen, AST, and γ-glutamyltransferase when compared with survivors ( Table 4 ). Platelet to lymphocyte ratio was associated with a higher in-hospital mortality odds ratio (1.003; 95% confidence interval, 1.002–1.004) adjusted for age and sex.
Laboratory Test Results (Median [IQR]), Stratified by Outcome
Statistically significant results at P values <.001 and parameters with levels higher/lower than the reference range are presented in bold. The number of patients is presented for each variable.
Abbreviations: IQR, interquartile range; PT, Prothrombin.
Results of the laboratory tests routinely performed in the clinical setting did not differ significantly between patients with confirmed and clinically diagnosed COVID-19 for 48 out of 51 parameters ( Table 1 ). Platelets, leukocytes, and neutrophil count were significantly lower in patients with confirmed COVID-19, but the differences were unlikely to be relevant, being within the normal reference ranges for both groups.
Patient Outcomes and Risk Factors
Among the 3480 patients who were discharged or died during hospitalization, the overall mortality was 5.5%, with a total number of 191 people who died.
In a univariate analysis, chronic cardiovascular disease, hypertension, chronic pulmonary disease, chronic kidney disease, chronic neurological disorder, malignant neoplasm, diabetes, and dementia significantly differed between survivors and patients who died ( Table 3 ). In multivariable analysis, older age was a predictor of in-hospital mortality with an odds ratio (per 1-year increase) of 1.05 (95% confidence interval, 1.03–1.06). Other predictors associated with in-hospital mortality were male sex (1.71; 1.24–2.37), chronic kidney disease (2.99; 1.89–4.64), diabetes (2.1; 1.46–2.99), chronic cardiovascular disease (1.78; 1.24–2.57), and dementia (2.73; 1.34–5.47) ( Figure 2 ). The same risk factors were significantly associated with the admission/transfer to the ICU, with only dementia not reaching statistical significance ( Supplementary Figure 1 ).

Odds ratios and 95% CIs for in-hospital mortality from a multivariable logistic regression model. Abbreviation: CI, confidence interval.
When including COVID-19 laboratory-confirmed/suspected status as a covariate in the multivariable logistic regression model we found no evidence that it was associated with mortality (odds ratio, 1.22; 95% confidence interval, .89–1.69) and it did not have major impact on the effect size and significance of other predictors ( Supplementary Figure 2 ).
We did not find any statistically significant association of CT severity grade with in-hospital mortality, adjusting for age and sex ( Supplementary Table 6 ). With respect to CT imaging, no evidence of difference was found between the patients with confirmed and clinically diagnosed COVID-19 ( Supplementary Table 7 ).
Hydroxychloroquine was the most frequently used (84%) medication, followed by antibiotics (azithromycin [77.7%] and ceftriaxone [30.3%]), heparin (56.4%), paracetamol (34.4%), mucolytics (25.4%), lopinavir/ritonavir (16.2%), and systemic corticosteroids (10.4%), respectively ( Supplementary Table 8 ). There was a significant overlap between the top 3 most commonly used medications, with hydroxychloroquine, azithromycin, and heparin used in 1322 patients ( Supplementary Figure 3 ).
To our knowledge, StopCOVID cohort is the first large-scale study of consecutively hospitalized patients with COVID-19 in Russia assessing clinical characteristics and risk factors for in-hospital mortality. This is also the first large cohort, including both RT-PCR–confirmed COVID-19 cases and patients, diagnosed with COVID-19 based on clinical and radiological presentation in the absence of the SARS-CoV-2 RT-PCR confirmation. We found that older age and male sex as well as existing comorbidities were associated with in-hospital mortality. We found no significant difference between patients with clinical COVID-19 and laboratory-confirmed COVID-19, either in clinical presentation or in clinical measurements and risk factors for in-hospital mortality. We feel it is entirely appropriate to treat patients with clinical and radiological signs of COVID-19 who do not have an alternative diagnosis to explain their symptoms equivalently to PCR-confirmed cases. Sequential RT-PCR testing can identify patients with COVID-19 whose initial result was false-negative [ 15 ]. In settings where repeat testing is not performed, it can also be appropriate to include patients with clinical and radiological COVID-19 alongside those with laboratory-confirmed disease.
Patients in our study were of an age very similar to the New York cohort [ 6 ] and of a much lower median age than similar cohorts in Italy [ 4 ] and the United Kingdom [ 8 ]. This may be partly explained by a lack of a clear message from the authorities to the public with regard to whom should present to a hospital. Healthcare-seeking behavior may further explain a younger age at admission, which differs between the countries. Russian people are known for active specialist-seeking behavior [ 16 ], particularly in the presence of distrust of media sources [ 17 ] and easy access to free healthcare. It is, however, more likely to be a reflection of varying approaches from health services in different countries.
Patients in Moscow typically presented with fever, fatigue, cough, and shortness of breath, which is in agreement with the previously reported symptom patterns in other countries [ 5 , 8 , 18 ]. Among symptoms, anosmia was associated with a more favorable outcome, which is similar to the data from Hopkins et al [ 19 ], which showed rapid improvement in patients with COVID-19 presenting with a loss of smell.
Similar to other cohorts, cardiological conditions, hypertension, obesity, and diabetes were common problems in the hospitalized population. The lower median age of the patients in our cohort may explain the lower comorbidity rate when compared with some other studies [ 6 , 8 ]. We recorded a much lower number of patients with chronic pulmonary diseases, which is in agreement with data from Richardson et al [ 7 ] but in contrast to other US [ 6 ] and particularly UK [ 8 ] cohorts. We also found low rates of asthma in our cohort, which did not exceed the prevalence in the general population, which has been reported previously [ 20 ].
Patient age, male sex, and the presence of major comorbidities were all predictors of in-hospital mortality. These findings are in line with other international cohorts [ 6 , 21 ], including a UK ISARIC study using a similar data-collection protocol [ 8 ]. We also found common changes in the coagulation profile [ 6 ] and previously reported clinical patterns, such as lymphocytopenia, neutrophilia, and very high levels of CRP and ESR in patients who subsequently died from COVID-19. The platelet to lymphocyte ratio has been previously reported to be associated with higher severity and mortality in patients with COVID-19 [ 22 ]. Our findings agree with previous research but require further validation.
The proportion of patients admitted to the ICU in our cohort study was much lower than in the similar cohorts from the United Kingdom (17%) [ 8 ] and the United States (14.2%) [ 7 ], but similar to published data from China [ 18 ]. The decision for ICU admission within the Sechenov University Hospital Network is normally based on a joint opinion of a multidisciplinary team of respiratory physicians and intensivists. Due to good access to high-flow oxygen and noninvasive ventilation within the COVID-19 wards, only critical patients were transferred into the ICU, which may explain the lesser need for ICU admission in our cohort. Active use of noninvasive ventilation on the wards may explain the low in-hospital mortality in this group of patients. As only the most severely unwell patients were admitted for invasive mechanical ventilation, this may explain the high mortality recorded in ICU patients. The overall mortality rate in our cohort was similar to the average worldwide estimate [ 23 ] but much lower than in other international cohorts of hospitalized individuals, which may be a direct reflection of their much younger age and moderate state of disease at the time of admission in most of the patients.
Half of the patients admitted to the Sechenov University Hospital Network did not have positive RT-PCR test results, despite having clinical features of COVID-19 infection. Our findings are similar to the US data, with 42% [ 5 ] to 51.8% [ 6 ] of individuals having negative RT-PCR test results. The false-negative rate of the RT-PCR tests varies between 20% and 66% depending on the day since symptom onset [ 10 ], meaning that results must be cautiously interpreted [ 24 ], which represents a major concern related to control of the pandemic [ 25 ]. Previous research suggests that a negative RT-PCR test result does not exclude the possibility of COVID-19. Repeated testing and sampling were shown to improve the sensitivity of RT-PCR [ 15 ]. To our knowledge, previous studies of patients with COVID-19 excluded those with suspected COVID-19 infection in the absence of a positive test result [ 3–8 ]. However, this approach differs from pragmatic clinical practice, in which, in the absence of an alternative diagnosis, patients with a clinical diagnosis of COVID-19 are treated equally to laboratory-confirmed cases. When evaluating radiological findings in COVID-19, it must be born in mind that some patients may present with clinical symptoms or extrapulmonary manifestations, such as hepatic, cardiovascular, or kidney injury, but initially will have normal CT findings [ 26 ]. In our study we did not solely rely on CT findings for clinical diagnosis of COVID-19. However, new approaches to minimize the exclusion of patients with false-negative RT-PCR results should be sought, as highlighted in a recent report suggesting real-time lung ultrasound as an auxiliary method to rule-in COVID-19 during screening [ 27 ].
Limitations
This cohort study has some limitations. First, the study population only included patients within Moscow. Second, the data were collected retrospectively from the electronic medical records with no access to additional information that could be potentially retrieved from the medical notes. Third, half of the patients in our cohort did not have RT-PCR–confirmed COVID-19 infection, although this is unlikely to affect the outcomes as we failed to find any significant differences between clinically diagnosed and laboratory-confirmed cases. Fourth, endpoint outcome data were available for 83% of admitted patients. Patients admitted and/or transferred to the ICU and receiving invasive mechanical ventilation can spend a significant amount of time attached to the machine [ 7 , 8 ]. The absence of data on patients (18%) who remained in the hospital at the time of data analysis completion may lead to bias and may influence overall mortality calculations. Fifth, morbidity related to invasive procedures or sequelae in clinically suspected and/or laboratory-confirmed cases has not been recorded. Sixth, the definition of “clinically diagnosed COVID-19” implies changes on chest CT and nonspecific signs and symptoms, which may be present in other respiratory viral illnesses. The scoring system used for radiological signs is able to differentiate between symptomatic and asymptomatic cases of COVID-19 but is not fully able to differentiate between COVID-19 from other similar conditions.
Conclusions
The clinical features, chest CT, and blood test results did not differ between test-confirmed and clinically diagnosed patients. Furthermore, clinical outcomes were also identical. Our study results suggest that in order to assess the full impact of this pandemic on populations, all clinically diagnosed patients should be included. Comorbidities associated with death were similar to other published studies on COVID-19. Mortality in our cohort was low, which may have been due to the mean age of patients being lower than in some other published studies. Anosmia was associated with milder disease while asthma did not appear to pose an increased risk of adverse outcome. As with other studies, manifestations of nonrespiratory problems including coagulopathy, immune deficiency, hyperinflammation and renal deficits were associated with higher risks of death. The data collection within StopCOVID cohort is continuing and further analysis focused on predictive models of adverse outcomes for routine clinical practice is in progress.
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Sechenov StopCOVID Research Team . Sechenov First Moscow State Medical University (Sechenov University), Moscow, Russia:Anna Berbenyuk, Polina Bobkova, Semyon Bordyugov, Aleksandra Borisenko, Ekaterina Bugaiskaya, Olesya Druzhkova, Dmitry Eliseev, Yasmin El-Taravi, Natalia Gorbova, Elizaveta Gribaleva, Rina Grigoryan, Shabnam Ibragimova, Khadizhat Kabieva, Alena Khrapkova, Natalia Kogut, Karina Kovygina, Margaret Kvaratskheliya, Maria Lobova, Anna Lunicheva, Anastasia Maystrenko, Daria Nikolaeva, Anna Pavlenko, Olga Perekosova, Olga Romanova, Olga Sokova, Veronika Solovieva, Olga Spasskaya, Ekaterina Spiridonova, Olga Sukhodolskaya, Shakir Suleimanov, Nailya Urmantaeva, Olga Usalka, Margarita Zaikina, Anastasia Zorina; 1C First Bit, Moscow, Russia:Nadezhda Khitrina.
Author contributions. D. M.: Conceptualization, methodology, validation, formal analysis, resources, data curation, writing (original draft, review, and editing), supervision, project administration. N. A. N.: Conceptualization, methodology, formal analysis, investigation, writing (original draft, review, and editing), visualization, project administration. P. B.: Conceptualization, methodology, investigation, writing (original draft, review, editing), project administration. O. B.: Conceptualization, methodology, software, validation, formal analysis, data curation, writing (original draft, review, and editing), visualization. M. K.: Formal analysis, investigation, writing (original draft, review, and editing), visualization. E. L.: Investigation, writing (original draft, review, and editing), project administration. A. G.: Investigation, writing (original draft, review, and editing), project administration. A. S.: Investigation, project administration. V. B.: Resources, writing (review and editing). P. T.: Resources, project administration, writing (review and editing). J. O. W., P. C., and C. A.: Writing (original draft, review, and editing). E. Bezrukov: Funding acquisition, writing (review and editing). M. E. P., A. Y., E. Bulanova, and N. T.: Writing (review and editing). S. A.: Writing (review and editing), investigation. V. K. and Y. P.: Writing (review and editing). E. A. D., C. K., and M. P.: Methodology, writing (review and editing). V. F.: Writing (review and editing). A. A. S.: Funding acquisition, writing (review and editing). D. B.: Conceptualization, methodology, resources, writing (review and editing), project administration, funding acquisition. P. G.: Project administration, funding acquisition, writing (review and editing), supervision. StopCOVID Research Team: Investigation, writing (review and editing).
Acknowledgments. The authors are very grateful to the Sechenov University Hospital Network clinical staff and to the patients, carers, and families for their kindness and understanding during these difficult times of the COVID-19 pandemic. We thank Dr Inna Tulina, Dr Yuri Kitsenko, Mrs Ekaterina Rebrova, and Mr Maksim Kholopov for providing technical support in data collection and database administration. We are grateful to Ms Olga Burencheva, Dr Daria Levina, Ms Olga Sokova, Ms Natalia Chepelova, and Ms Elizaveta Mikhsin for assistance in data extraction. We highly appreciate the kind expert advice from Professor Gareth Tudor-Williams, Dr Jethro Herberg, Dr Nikita Sushentsev, and Dr Anna Pokshubina for assistance in data interpretation. Finally, we extend our gratitude to Laura Merson and the entire ISARIC team for their continuous support and expertise and for providing access to the REDCap CRF module.
Financial support. This work was supported by the Russian Academic Excellence Project “5–100” and Russian Foundation for Basic Research (RFBR) (grant number 20-04-60063).
Potential conflicts of interest. J. W. reports grants and personal fees from Danone/Nutricia and Airsonnet, nonfinancial support from Anaphylaxis Campaign, and lecture fees from Friesland Campina, outside the submitted work. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Federal Service for Surveillance on Consumer Rights Protection and Human Wellbeing . About confirmed case of the novel coronavirus infection COVID-2019 in Russia. Available at: https://www.rospotrebnadzor.ru/about/info/news/news_details.php?ELEMENT_ID=13870 . Accessed 9 June 2020 .
Government of Russian Federation . Stopcoronavirus.rf—Official information about Covid-19 in Russia. Available at: https://xn--80aesfpebagmfblc0a.xn--p1ai/ . Accessed 10 June 2020 .
Zhou F , Yu T , Du R , et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study . Lancet 2020 ; 395 : 1054 – 62 .
Google Scholar
Grasselli G , Zangrillo A , Zanella A , et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy . JAM A 2020 ; 323 : 1574 – 81 . doi: 10.1001/jama.2020.5394 .
Argenziano MG , Bruce SL , Slater CL , et al. Characterization and clinical course of 1000 patients with COVID-19 in New York: retrospective case series . medRxiv 2020 . doi: 10.1101/2020.04.20.20072116 .
Petrilli CM , Jones SA , Yang J , et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study . BMJ 2020 ; 369 : m1966 .
Richardson S , Hirsch JS , Narasimhan M , et al. ; Northwell COVID-19 Research Consortium . Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area . JAMA 2020 ; 323 : 2052 – 9 .
Docherty AB , Harrison EM , Green CA , et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO clinical characterisation protocol: prospective observational cohort study . BMJ 2020 ; 369 : m1985 .
Salient lessons from Russia’s COVID-19 outbreak . Lancet 2020 ; 395 : 1739 .
Kucirka LM , Lauer SA , Laeyendecker O , Boon D , Lessler J . Variation in false-negative rate of reverse transcriptase polymerase chain reaction-based SARS-CoV-2 tests by time since exposure . Ann Intern Med 2020 ; 173 : 262 – 7 .
International Severe Acute Respiratory and Emerging Infection Consortium and World Health Organisation . Clinical data collection—the COVID-19 case report forms (CRFs). Available at: https://isaric.tghn.org/COVID-19-CRF/ . Accessed 22 June 2020 .
Harris PA , Taylor R , Thielke R , Payne J , Gonzalez N , Conde JG . Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support . J Biomed Inform 2009 ; 42 : 377 – 81 .
Harris PA , Taylor R , Minor BL , et al. ; REDCap Consortium . The REDCap consortium: building an international community of software platform partners . J Biomed Inform 2019 ; 95 : 103208 .
Inui S , Fujikawa A , Jitsu M , et al. Chest CT findings in cases from the cruise ship “Diamond Princess” with coronavirus disease 2019 (COVID-19) . Radiol Cardiothoracic Imaging 2020 ; 2 : e200110 .
Zhang JJ , Cao YY , Dong X , et al. Distinct characteristics of COVID-19 patients with initial rRT-PCR-positive and rRT-PCR-negative results for SARS-CoV-2 . Allergy 2020 ; 75 : 1809 – 12 . doi: 10.1111/all.14316 .
Ipsos . Global views on healthcare in 2018. Available at: https://www.ipsos.com/sites/default/files/ct/news/documents/2018-07/Global%20Views%20on%20Healthcare%202018%20Graphic%20Report.pdf . Accessed 17 June 2020 .
Benisovich SV , King AC . Meaning and knowledge of health among older adult immigrants from Russia: a phenomenological study . Health Educ Res 2003 ; 18 : 135 – 44 .
Guan WJ , Ni ZY , Hu Y , et al. Clinical characteristics of coronavirus disease 2019 in China . N Engl J Med 2020 ; 382 : 1708 – 20 .
Hopkins C , Surda P , Whitehead E , Kumar BN . Early recovery following new onset anosmia during the COVID-19 pandemic—an observational cohort study . J Otolaryngol Head Neck Surg 2020 ; 49 : 26 .
Avdeev S , Moiseev S , Brovko M , et al. Low prevalence of bronchial asthma and chronic obstructive lung disease among intensive care unit patients with COVID-19 . Allergy 2020 :1–3. doi: 10.1111/all.14420 .
Cummings MJ , Baldwin MR , Abrams D , et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study . Lancet 2020 ; 395 : 1763 – 70 .
Chan AS , Rout A . Use of neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios in COVID-19 . J Clin Med Res 2020 ; 12 : 448 – 53 .
World Health Organization . Coronavirus disease 2019 (COVID-19) situation report—46. Available at: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200306-sitrep-46-covid-19.pdf?sfvrsn=96b04adf_4 . Accessed 22 June 2020 .
Tahamtan A , Ardebili A . Real-time RT-PCR in COVID-19 detection: issues affecting the results . Expert Rev Mol Diagn 2020 ; 20 : 453 – 4 .
Woloshin S , Patel N , Kesselheim AS . False negative tests for SARS-CoV-2 infection—challenges and implications . N Engl J Med 2020 ; 383 : e38 .
Harmon SA , Sanford TH , Xu S , et al. Artificial intelligence for the detection of COVID-19 pneumonia on chest CT using multinational datasets . Nat Commun 2020 ; 11 : 4080 .
Smallwood N , Walden A , Parulekar P , Dachsel M . Should point-of-care ultrasound become part of healthcare worker testing for COVID? Clin Med (Lond) 2020 ; 20 : 486 – 7 .
Author notes
- comorbidity
- hospital mortality
- hospitals, university
- laboratory techniques and procedures
- reverse transcriptase polymerase chain reaction
- signs and symptoms
Supplementary data
Email alerts, more on this topic, related articles in pubmed, citing articles via, looking for your next opportunity.
- Recommend to your Library
Affiliations
- Online ISSN 1537-6591
- Print ISSN 1058-4838
- Copyright © 2024 Infectious Diseases Society of America
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.

IMAGES
VIDEO
COMMENTS
Asthma in the Primary Care Setting. Asthma is a chronic disease principally characterized by episodic wheeze, cough, and breathlessness resulting from airway hyperresponsiveness and inflammation. It is one of the most common chronic lung diseases in the United States, affecting approximately 8% of adults, or about 20 million individuals. 1, 2.
A number of studies have demonstrated the challenges for primary care physicians in providing ongoing support for people with asthma. 31,48,49 In some countries, nurses and other allied health ...
Introduction. Asthma is a common chronic condition that is estimated to affect 339 million people worldwide 1,2.Despite major advances in asthma treatment and the availability of both global 2 and national guidance, asthma continues to cause a substantial burden in terms of both direct and indirect costs 1.In 2016, estimated worldwide asthma deaths were 420,000 1 and although there have been ...
Primary Care Respiratory Update. The uncomfortable outputs of the NRAD1 report - why overuse of SABA can be indicative of poor asthma control. Understanding the signs and symptoms of asthma and early recognition of increasing symptoms - effective self-monitor-ing. Use of the Asthma Control Test and understanding of the scores.
Most patients with asthma are managed by primary care providers. Severe asthma is associated with substantial morbidity and health care resource use, and long-term sequelae of severe asthma include airway remodeling and a greater risk of developing chronic obstructive pulmonary disease. These consequences highlight the importance of early identification and improved management of patients with ...
A primary care asthma management quality improvement programme was developed with the support of the British Lung Foundation (now Asthma + Lung UK) and Optimum Patient Care (OPC) Limited.
One week later, she saw her primary care physician, who prescribed levofloxacin. ... Beaglehole R, Fenwick J, Sutherland DC. A case-control study of deaths from asthma. Thorax 1986;41: 833-839 ...
At the outset of the COVID-19 pandemic, there were concerns about its impact on patients with asthma and other chronic respiratory conditions [], both in terms of its effect in triggering acute exacerbations or attacks, and as a risk factor for more severe disease and death [].As the pandemic continued, several studies showed a reduction in asthma attacks reported in primary care and in ...
Asthma is a condition commonly encountered in primary care, with over five million people in the UK prescribed active treatment.1 While seemingly a routine part of general practice, asthma assessment is a particular challenge in the context of the covid-19 pandemic, given the overlap in respiratory symptoms between the two conditions and the ...
Background The COVID-19 pandemic dramatically affected asthma monitoring in primary care, but exploration of patients' views and their experiences of managing their asthma and seeking help from primary care during the pandemic has been limited. Aim To investigate patients' experiences of asthma management in the community during the COVID-19 pandemic. Design and setting A qualitative ...
Clinical case study - asthma . 2019 . Clinical Case Study - Asthma. pdf. Clinical Case Study - Asthma. 6.34 MB. Resource information. Respiratory conditions. Asthma; Respiratory topics. ... The International Primary Care Respiratory Group is a Scottish Charity (SC 035056) and a Scottish Company Limited by guarantee (Scottish Company Number ...
Background Most asthma diagnoses and patient care take place in primary care settings. Electronic medical records (EMRs) offer an opportunity to utilize technology to improve asthma diagnosis and care. The purpose of this study was to create and validate separate case definitions for suspected and confirmed asthma in primary care EMRs, to enable surveillance, benchmarking, and quality ...
Asthma imposes a substantial burden on individuals and societies. Patients with asthma need high-quality primary care management; however, evidence suggests the quality of this care can be highly ...
The majority of adverse effects occurred within 1 day of the procedure and resolved within 7 days. 6. In this study, bronchial thermoplasty was found to significantly improve quality of life, as ...
Introduction. Asthma is a common chronic disease in children and adults and is responsible for considerable healthcare use. 1 In 2020 there were 5.4 million people in the UK living with asthma. 2 Asthma accounts for 2%-3% of primary care consultations, 3 60 000 admissions to hospital with 200 000 bed-days per year, and is estimated to cost £1.1 billion a year to UK health services. 4 It is ...
We conducted a qualitative study based on the interviews of 30 asthma patients aged 18-40 years, recruited in French primary care. We performed a thematic analysis of the data collected, using ...
Another recent publication from Cave et al. conducted a study to validate a case definition for asthma using data from the Southern Alberta Primary Care Research Network, a node of the CPCSSN (SAPCReN-CPCSSN) . The authors created a case-finding algorithm using a combination of search fields from the EMR including billing information, recorded ...
The coronavirus disease 2019 (COVID-19) pandemic, caused by the new severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), provoked the most striking international public health crisis of our time. COVID-19 can cause a range of breathing problems, from mild to critical, with potential evolution to respiratory failure and acute respiratory distress syndrome. Elderly adults and those ...
Roshni Naik, MD, allergist at Mount Sinai Health System, discusses the history of present illness for a case of severe asthma with nasal polyps.
AHRQ's evidence-based tools and resources are used by organizations nationwide to improve the quality, safety, effectiveness, and efficiency of health care. The Agency's Impact Case Studies highlight these successes, describing the use and impact of AHRQ-funded tools by State and Federal policy makers, health systems, clinicians ...
Temporary doctors, known as locums, are a key component of the medical workforce in the NHS but evidence on differences in quality and safety between locum and permanent doctors is limited. We aimed to examine differences in the clinical practice, and prescribing safety for locum and permanent doctors working in primary care in England. We accessed electronic health care records (EHRs) for 3.5 ...
In the present study, we categorized all items of the respiratory assessment in three subsections based on their availability in different healthcare settings, i.e., public health, primary care ...
This is in agreement with adult studies from Russia and the UK reporting asthma to be associated with the development of long COVID. Recent data suggested that COVID-19 consequences may be linked with mast cell activation syndrome [ 24 ] and the T-helper type 2-biased immunological response in children with allergic diseases may be responsible ...
The progression of secondary pulmonary damage in SARS-COV-2 infection, associated with interstitial damage, inflammation and alveolar consolidation and eventually resulted in the development of pulmonary fibrosis (PF), remains one of the key clinical dilemmas for the treatment of patients in intensive care units (ICU).
As primary care EMR data are increasingly being used for disease surveillance, validated case definitions are required [13, 14]. A recent literature review on asthma case definitions identified the need to create a case definition that differentiates between suspected and confirmed asthma in primary care EMRs . The purpose of this study was to ...
Citation: Loneliness worse than smoking, alcoholism, obesity: Study suggests primary care clinicians can offer solutions (2024, March 19) retrieved 24 March 2024 from https://medicalxpress.com ...
The study primary endpoint was available for all but 55 individuals transferred to other hospitals; thus, 3480 (82%) individuals were included in the statistical analysis. Half of the patients (n = 1728) had positive RT-PCR results, while the second half (n = 1748) were negative on RT-PCR but had clinical symptoms and CT signs suggestive of ...