Enter search terms to find related medical topics, multimedia and more.

Advanced Search:
- Use “ “ for exact phrases.
- For example: “pediatric abdominal pain”
- Use – to remove results with certain keywords.
- For example: abdominal pain -pediatric
- Use OR to account for alternate keywords.
- For example: teenager OR adolescent
, MD, MPH, University of California San Diego
- Symptoms and Signs
More Information
- 3D Models (0)
- Calculators (0)

Gonorrhea is caused by the bacterium Neisseria gonorrhoeae . It typically infects epithelia of the urethra, cervix, rectum, pharynx, or conjunctivae, causing irritation or pain and purulent discharge. Dissemination to skin and joints, which is uncommon, causes sores on the skin, fever, and migratory polyarthritis or pauciarticular septic arthritis. Diagnosis is by microscopy, culture, or nucleic acid amplification tests (NAATs). Several oral or injectable antibiotics can be used, but drug resistance is an increasing problem.
(See also Overview of Sexually Transmitted Infections Overview of Sexually Transmitted Infections Sexually transmitted infection (STI) refers to infection with a pathogen that is transmitted through blood, semen, vaginal fluids, or other body fluids during oral, anal, or genital sex with... read more .)
N. gonorrhoeae is a gram-negative diplococcus that occurs only in humans and is almost always transmitted by sexual contact. Urethral and cervical infections are most common, but infection in the pharynx or rectum can occur after oral or anal intercourse, and conjunctivitis may follow contamination of the eye.
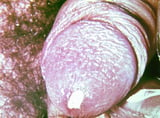
Disseminated gonococcal infection (DGI) due to hematogenous spread occurs in 1% of cases, predominantly in women. DGI typically affects the skin, tendon sheaths, and joints. Pericarditis, endocarditis, meningitis, and perihepatitis occur rarely.
1. Holmes KK, Johnson DW, Trostle HJ : An estimate of the risk of men acquiring gonorrhea by sexual contact with infected females. Am J Epidemiol 91(2):170-174, 1970. doi:10.1093/oxfordjournals.aje.a121125
Symptoms and Signs of Gonorrhea
About 10 to 20% of infected women and very few infected men are asymptomatic. About 25% of men have minimal symptoms.
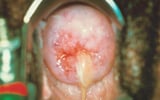
Male urethritis has an incubation period from 2 to 14 days. Onset is usually marked by mild discomfort in the urethra, followed by more severe penile tenderness and pain, dysuria, and a purulent discharge. Urinary frequency and urgency may develop as the infection spreads to the posterior urethra. Examination detects a purulent, yellow-green urethral discharge, and the meatus may be inflamed.
Pelvic inflammatory disease Pelvic Inflammatory Disease (PID) Pelvic inflammatory disease (PID) is a polymicrobial infection of the upper female genital tract: the cervix, uterus, fallopian tubes, and ovaries; abscess may occur. PID may be caused by sexually... read more occurs in 10 to 20% of infected women. PID may include salpingitis, pelvic peritonitis, and pelvic abscesses and may cause lower abdominal discomfort (typically bilateral), dyspareunia, and marked tenderness on palpation of the abdomen, adnexa, or cervix.
Fitz-Hugh-Curtis syndrome is gonococcal (or chlamydial) perihepatitis that occurs predominantly in women and causes right upper quadrant abdominal pain, fever, nausea, and vomiting, often mimicking biliary or hepatic disease.
Rectal gonorrhea is usually asymptomatic. It occurs predominantly in men practicing receptive anal intercourse and can occur in women who participate in anal sex. Symptoms include rectal itching, a cloudy rectal discharge, bleeding, and constipation—all of varying severity. Examination with a proctoscope may detect erythema or mucopurulent exudate on the rectal wall.
Gonococcal pharyngitis is usually asymptomatic but may cause sore throat. N. gonorrhoeae must be distinguished from N. meningitidis and other closely related organisms that are often present in the throat without causing symptoms or harm.

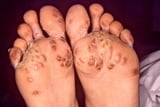
Gonococcal septic arthritis is a more localized form of DGI that results in a painful arthritis with effusion, usually of 1 or 2 large joints such as the knees, ankles, wrists, or elbows. Some patients present with or have a history of skin lesions of DGI. Onset is often acute, usually with fever, severe joint pain, and limitation of movement. Infected joints are swollen, and the overlying skin may be warm and red.
Diagnosis of Gonorrhea
Nucleic acid–based testing
Gram staining and culture
Gonorrhea is diagnosed when gonococci are detected via microscopic examination using a nucleic acid–based test, Gram stain, or culture of genital fluids, blood, or joint fluids (obtained by needle aspiration).
Nucleic acid amplification tests (NAATs) may be done on genital, rectal, or oral swabs and can detect both gonorrhea and chlamydial infection. NAATs further increase the sensitivity adequately to enable testing of urine samples in both sexes.
Gram stain is sensitive and specific for gonorrhea in men with urethral discharge; gram-negative intracellular diplococci typically are seen. Gram stain is much less accurate for infections of the cervix, pharynx, and rectum and is not recommended for diagnosis at these sites.

Culture is sensitive and specific, but because gonococci are fragile and fastidious, samples taken using a swab need to be rapidly plated on an appropriate medium (eg, modified Thayer-Martin) and transported to the laboratory in a carbon dioxide–containing environment. Blood and joint fluid samples should be sent to the laboratory with notification that gonococcal infection is suspected. Because NAATs have replaced culture in most laboratories, finding a laboratory that can provide culture and sensitivity testing may be difficult and require consultation with a public health or infectious disease specialist.

Men with urethritis
Men with obvious urethral discharge may be treated presumptively if likelihood of follow-up is questionable or if clinic-based diagnostic tools are not available.
Samples for Gram staining can be obtained by touching a swab or slide to the end of the penis to collect discharge. Gram stain does not identify chlamydiae, so urine or swab samples for NAAT are obtained.
Women with cervicitis or pelvic inflammatory disease
A cervical swab should be sent for culture or NAAT. If a pelvic examination is not possible, NAAT of a urine sample or self-collected vaginal swab can detect gonococcal (and chlamydial) infections rapidly and reliably.
Pharyngeal or rectal exposures
Swabs of the affected area are sent for culture or NAAT.
Arthritis, disseminated gonococcal infection (DGI), or both
An affected joint should be aspirated, and fluid should be sent for culture and routine analysis (see arthrocentesis Arthrocentesis Some musculoskeletal disorders affect primarily the joints, causing arthritis. Others affect primarily the bones (eg, fractures, Paget disease of bone, tumors), muscles (eg, myositis), peripheral... read more ). Patients with skin lesions, systemic symptoms, or both should have blood, urethral, cervical, and rectal cultures or NAAT. In about 30 to 40% of patients with DGI, blood cultures are positive during the first week of illness. With gonococcal arthritis, blood cultures are less often positive, but cultures of joint fluids are usually positive. Joint fluid is usually cloudy to purulent because of large numbers of white blood cells (typically > 20,000/microliter).
Diagnosis reference
1. Bazan JA, Peterson AS, Kirkcaldy RD, et al . Notes from the field. Increase in Neisseria meningitidis –associated urethritis among men at two sentinel clinics — Columbus, Ohio, and Oakland County, Michigan, 2015. MMWR Morb Mortal Wkly Rep 65:550–552, 2016. doi: 10.15585/mmwr.mm6521a5external icon
Screening for Gonorrhea
Asymptomatic patients considered at high risk of sexually transmitted infections (STIs) can be screened by NAAT of urine samples, thus not requiring invasive procedures to collect samples from genital sites. The following are based on CDC's Sexually Transmitted Infections (STI) Treatment Guidelines, 2021 .
Women are screened annually if they are sexually active and < 25 years of age or if they are ≥ 25 years of age, sexually active, and have one or more of the following risk factors:
Have a history of a prior STI
Engage in high-risk sexual behavior (eg, have a new sex partner or multiple sex partners; engage in sex work; or use condoms inconsistently when not in a mutually monogamous relationship)
Have a partner who has an STI or engages in high-risk behavior (eg, a sex partner who has concurrent partners)
Have a history of incarceration
Pregnant women who are < 25 years or who are ≥ 25 years with one or more of the risk factors are screened during their first prenatal visit and again during their 3rd trimester for women who are < 25 or at high risk.
There is insufficient evidence for screening heterosexual men who are at low risk for infection.
Men who have sex with men are screened at least annually if they have been sexually active within the previous year (for insertive intercourse, urine screen; for receptive intercourse, rectal swab; and for oral intercourse, pharyngeal swab), regardless of condom use. Those at increased risk (eg, with HIV infection, receive preexposure prophylaxis with antiretrovirals, have multiple sex partners, or whose partner has multiple partners) should be screened more frequently, at 3 to 6-month intervals.
Transgender and gender diverse people are screened if they are sexually active on the basis of sexual practices and anatomy (eg, annual screening for all people with a cervix who are < 25 years old; if ≥ 25 years old, people with a cervix should be screened annually if at increased risk; rectal swab based on reported sexual behaviors and exposure).
(See also the US Preventive Services Task Force’s summary of recommendations regarding screening for gonorrhea .)
Treatment of Gonorrhea
For uncomplicated infection, a single dose of ceftriaxone
Concomitant treatment for chlamydial infection
Treatment of sex partners
For disseminated gonococcal infection (DGI) with arthritis, a longer course of parenteral antibiotics
Uncomplicated gonococcal infection of the urethra, cervix, rectum, and pharynx is treated with the following:
A single dose of ceftriaxone 500 mg IM (1 g IM for patients weighing ≥ 150 kg)
If ceftriaxone is not available, use cefixime 800 mg orally in a single dose.
If chlamydial infection has not been excluded, treat for chlamydia with doxycycline 100 mg orally twice a day for 7 days. In patients who have a doxycycline allergy, treat for chlamydia with a single dose of azithromycin 1 g orally.
Patients who are allergic to cephalosporins (including ceftriaxone ) are treated with
Gentamicin 240 mg IM in a single dose plus azithromycin 2 g orally in a single dose

Gonococcal purulent arthritis usually requires repeated synovial fluid drainage either with repeated arthrocentesis or arthroscopically. Initially, the joint is immobilized in a functional position. Passive range-of-motion exercises should be started as soon as patients can tolerate them. Once pain subsides, more active exercises, with stretching and muscle strengthening, should begin. Over 95% of patients treated for gonococcal arthritis recover complete joint function. Because sterile joint fluid accumulations (effusions) may develop and persist for prolonged periods, an anti-inflammatory drug may be beneficial.
Posttreatment cultures are unnecessary if symptomatic response is adequate. However, for patients with symptoms for > 7 days, specimens should be obtained, cultured, and tested for antimicrobial sensitivity.
Patients should abstain from sexual activity until treatment is completed to avoid infecting sex partners.
Sex partners
All sex partners who have had sexual contact with the patient within 60 days should be tested for gonorrhea and other STIs and treated if results are positive. Sex partners with contact within 2 weeks should be treated presumptively for gonorrhea (epidemiologic treatment).
Treatment reference
1. Centers for Disease Control and Prevention: Sexually Transmitted Infections Treatment Guidelines, 2021 : Gonococcal Infections Among Adolescents and Adults. Accessed June 27, 2022.
Neisseria gonorrhoeae infection typically causes uncomplicated infection of the urethra, cervix, rectum, pharynx, and/or conjunctivae.
Sometimes gonorrhea spreads to the adnexa, causing salpingitis, or disseminates to skin and/or joints, causing skin lesions or septic arthritis.
Diagnose using NAAT, but culture and sensitivity testing should be done when needed to detect antimicrobial resistance.
Screen high-risk patients using NAAT.
Treat uncomplicated infection with a single dose of ceftriaxone 500 mg IM (1 g IM for patients weighing ≥ 150 kg); add oral doxycycline (100 mg twice a day for 7 days) when chlamydial infection has not been excluded.
The following English-language resource may be useful. Please note that THE MANUAL is not responsible for the content of this resource.
US Preventive Services Task Force: Chlamydia and Gonorrhea: Screening : A review of evidence that screening tests can accurately detect chlamydia and gonorrhea

Was This Page Helpful?

Test your knowledge
Brought to you by Merck & Co, Inc., Rahway, NJ, USA (known as MSD outside the US and Canada) — dedicated to using leading-edge science to save and improve lives around the world. Learn more about the MSD Manuals and our commitment to Global Medical Knowledge.
- Permissions
- Cookie Settings
- Terms of use
- Veterinary Manual
- IN THIS TOPIC
- Patient Care & Health Information
- Diseases & Conditions
To determine whether you have gonorrhea, your healthcare professional will analyze a sample of cells. Samples can be collected with:
- A urine test. This can help identify bacteria in your urethra.
- A swab of the affected area. A swab of your throat, urethra, vagina or rectum can collect bacteria that can be identified in a lab.
Testing for other sexually transmitted infections
Your healthcare professional may recommend tests for other sexually transmitted infections. Gonorrhea increases your risk of these infections, particularly chlamydia, which often accompanies gonorrhea.
Testing for HIV also is recommended for anyone diagnosed with a sexually transmitted infection. Depending on your risk factors, tests for other sexually transmitted infections could be beneficial as well.
Gonorrhea treatment in adults
Adults with gonorrhea are treated with antibiotics. Due to emerging strains of drug-resistant Neisseria gonorrhoeae, the bacterium that causes gonorrhea, the Centers for Disease Control and Prevention recommends that uncomplicated gonorrhea be treated with the antibiotic ceftriaxone. This antibiotic is given as a shot, also called an injection.
After getting the antibiotic, you can still spread the infection to others for up to seven days. So avoid sexual activity for at least seven days.
Three months after treatment, the CDC also recommends getting tested for gonorrhea again. This is to make sure people haven't been reinfected with the bacteria, which can happen if sex partners aren't treated, or new sex partners have the bacteria.
Gonorrhea treatment for partners
Your sexual partner or partners from the last 60 days also need to be screened and treated, even if they have no symptoms. If you are treated for gonorrhea and your sexual partners aren't treated, you can become infected again through sexual contact. Make sure to wait until seven days after a partner is treated before having any sexual contact.
Gonorrhea treatment for babies
Babies who develop gonorrhea after being born to someone with the infection can be treated with antibiotics.
There is a problem with information submitted for this request. Review/update the information highlighted below and resubmit the form.
From Mayo Clinic to your inbox
Sign up for free and stay up to date on research advancements, health tips, current health topics, and expertise on managing health. Click here for an email preview.
Error Email field is required
Error Include a valid email address
To provide you with the most relevant and helpful information, and understand which information is beneficial, we may combine your email and website usage information with other information we have about you. If you are a Mayo Clinic patient, this could include protected health information. If we combine this information with your protected health information, we will treat all of that information as protected health information and will only use or disclose that information as set forth in our notice of privacy practices. You may opt-out of email communications at any time by clicking on the unsubscribe link in the e-mail.
Thank you for subscribing!
You'll soon start receiving the latest Mayo Clinic health information you requested in your inbox.
Sorry something went wrong with your subscription
Please, try again in a couple of minutes
Preparing for your appointment
You'll likely see your primary healthcare professional. Here's some information to help you get ready for your appointment.
What you can do
When you make the appointment, ask if there's anything you need to do in advance, such as restrict your diet.
Make a list of:
- Your symptoms, if you have any, including those that may seem unrelated to the reason for which you scheduled the appointment, and when they began.
- All medicine, vitamins or other supplements you take, including doses.
- Questions to ask your healthcare professional.
For gonorrhea, questions to ask include:
- What tests do I need?
- Should I be tested for other sexually transmitted infections?
- Should my partner be tested for gonorrhea?
- How long should I wait before resuming sexual activity?
- How can I prevent gonorrhea in the future?
- What gonorrhea complications should I be alert for?
- Are there brochures or other printed material that I can have? What websites do you recommend?
- Will I need a follow-up visit?
Don't hesitate to ask other questions.
What to expect from your doctor
Questions your healthcare professional is likely to ask you include:
- Have your symptoms been continuous or occasional?
- How severe are your symptoms?
- Have you been exposed to sexually transmitted infections?
What you can do in the meantime
Avoid sexual activity until you see your healthcare professional. Alert your sex partners that you're having symptoms so that they can arrange to see a member of their healthcare teams for testing.
- Gonorrhea: CDC fact sheet (detailed version). Centers for Disease Control and Prevention. https://www.cdc.gov/std/gonorrhea/stdfact-gonorrhea-detailed.htm. Accessed Sept. 21, 2023.
- Ghanem KG. Clinical manifestations and diagnosis of Neisseria gonorrhoeae infection in adults and adolescents. https://www.uptodate.com/contents/search. Accessed Sept. 21, 2023.
- Gonorrhea. Office on Women's Health. https://www.womenshealth.gov/a-z-topics/gonorrhea. Accessed Sept. 21, 2023.
- Gonorrhea. Merck Manual Professional Version. https://www.merckmanuals.com/professional/infectious-diseases/sexually-transmitted-diseases-stds/gonorrhea. Accessed Sept. 21, 2023.
- AskMayoExpert. Chlamydia, gonorrhea, and nongonococcal urethritis. Mayo Clinic; 2023.
- Speer ME. Gonococcal infection in the newborn. https://www.uptodate.com/contents/search. Accessed Sept. 21, 2023.
- Workowski KA, et al. Sexually transmitted infections treatment guidelines, 2021. Morbidity and Mortality Weekly Reports. 2021; doi:10.15585/mmwr.rr7004a1.
Products & Services
- A Book: Mayo Clinic Family Health Book, 5th Edition
- Symptoms & causes
- Diagnosis & treatment
Mayo Clinic does not endorse companies or products. Advertising revenue supports our not-for-profit mission.
- Opportunities
Mayo Clinic Press
Check out these best-sellers and special offers on books and newsletters from Mayo Clinic Press .
- Mayo Clinic on Incontinence - Mayo Clinic Press Mayo Clinic on Incontinence
- The Essential Diabetes Book - Mayo Clinic Press The Essential Diabetes Book
- Mayo Clinic on Hearing and Balance - Mayo Clinic Press Mayo Clinic on Hearing and Balance
- FREE Mayo Clinic Diet Assessment - Mayo Clinic Press FREE Mayo Clinic Diet Assessment
- Mayo Clinic Health Letter - FREE book - Mayo Clinic Press Mayo Clinic Health Letter - FREE book
Your gift holds great power – donate today!
Make your tax-deductible gift and be a part of the cutting-edge research and care that's changing medicine.

NICOLE YONKE, MD, MPH, MIRANDA ARAGÓN, MD, AND JENNIFER K. PHILLIPS, MD
Am Fam Physician. 2022;105(4):388-396
Related Letter to the Editor: Doxycycline Preferred for the Treatment of Chlamydia
Patient information: See related handouts on chlamydia , written by the authors of this article, and on gonorrhea , which has been adapted from a previously published AFP article.
Author disclosure: No relevant financial relationships.
Infections caused by Chlamydia trachomatis and Neisseria gonorrhoeae are increasing in the United States. Because most infections are asymptomatic, screening is key to preventing complications such as pelvic inflammatory disease and infertility and decreasing community and vertical neonatal transmission. All sexually active people with a cervix who are younger than 25 years and older people with a cervix who have risk factors should be screened annually for chlamydial and gonococcal infections. Sexually active men who have sex with men should be screened at least annually. Physicians should obtain a sexual history free from assumptions about sex partners or practices. Acceptable specimen types for testing include vaginal, endocervical, rectal, pharyngeal, and urethral swabs, and first-stream urine samples. Uncomplicated gonococcal infection should be treated with a single 500-mg dose of intramuscular ceftriaxone in people weighing less than 331 lb (150 kg). Preferred chlamydia treatment is a seven-day course of doxycycline, 100 mg taken by mouth twice per day. All nonpregnant people should be tested for reinfection approximately three months after treatment or at the first visit in the 12 months after treatment. Pregnant patients diagnosed with chlamydia or gonorrhea should have a test of cure four weeks after treatment.
Chlamydia trachomatis and Neisseria gonorrhoeae are the most common sexually transmitted infections (STIs) in the United States and are required to be reported to state health departments. Between 2015 and 2019, reported chlamydial infections increased by 19%, and reported gonococcal infections increased by 53%. 1 These bacteria commonly infect the urogenital, anorectal, and pharyngeal sites but can become disseminated to affect multiple organ systems. Untreated infections may lead to pelvic inflammatory disease; scarring of fallopian tubes, which can increase the risk of ectopic pregnancy; infertility; easier transmission of new HIV infection; and vertical neonatal transmission. 2
Risk Factors
Young people 15 to 24 years of age account for 61% of all newly diagnosed STIs. 1 Racial and ethnic minorities, men who have sex with men (MSM), and transgender and gender diverse people are at higher risk of STIs. Inequitable access to health insurance and physicians, language barriers, and distrust of medical systems because of discrimination account for some of these disparities, independent of individual sexual behavior. 3 , 4 Other risk factors are reviewed in Table 1 . 2
Taking a thorough sexual history is important to identify overall risk of infection, as well as anatomic site-specific risk factors. Physicians should create supportive spaces where patients feel safe sharing information by using open-ended questions; avoiding assumptions regarding sexual preferences, practices, and gender/sex; and normalizing diverse sexual experiences. To obtain a complete sexual history, the five P’s (partners, practices, pregnancy attitudes, previous STIs, and protection from STIs) model can be used as outlined in Table 2 . 2 , 5
The U.S. Preventive Services Task Force (USPSTF) recommends behavioral counseling on condom use, communication strategies for safer sex, and problem solving with those at increased risk of STIs. 6 Adolescents and adults diagnosed with an STI in the past year, people reporting irregular condom use, and those with multiple partners or with partners belonging to a high-risk group are at increased risk. Physicians should emphasize barrier protection as the best way to prevent STIs. 2
The USPSTF and Centers for Disease Control and Prevention (CDC) recommend annual screening for chlamydial and gonococcal infections to prevent infertility and pelvic inflammatory disease in sexually active people 24 years and younger with a cervix and in older people with a cervix who have risk factors. 2 , 7 The CDC also recommends at least annual screening for MSM based on their risk factors. Screening should include the pharynx, urethra, and rectum based on reported anatomic sites of exposure. After discussion with the patient, it may be necessary to screen those sites even without reported exposure because of underreporting of sexual practices. 2 Table 3 summarizes screening recommendations for chlamydial and gonococcal infections. 2 , 8 There are significant gaps in research as it pertains to screening transgender and gender diverse patients. 9 The CDC recommends screening based on an individual’s current anatomy and sexual practices. 2
Screening for urogenital infections only and neglecting pharyngeal and rectal sites of exposure will miss a substantial proportion of chlamydial and gonococcal infections. 10 In one study of women who engaged in oral or anal sex with men, the prevalence of pharyngeal gonorrhea was 3.5%; rectal gonorrhea, 4.8%; and rectal chlamydia, 11.8%. 10 Pharyngeal and rectal screening may be offered to people with female anatomy based on sexual practices and shared decision-making. 2 Current evidence for screening extra-genital sites is strongest for MSM. Urine-only screening in an STI clinic misses 83% of infections among MSM. 11 They should be screened at each anatomic site of sexual exposure, regardless of condom use, at least annually. 2 Routine testing for chlamydial infections of the oropharynx is not recommended, but many laboratories will test for gonococcal and chlamydial infections simultaneously. 2 If oropharyngeal chlamydia is diagnosed, it should be treated to decrease the risk of transmission. 2
Presentation
Most chlamydial and gonococcal infections are asymptomatic. 8 Symptoms of infection are reviewed in Table 4 . 2 Because dysuria may be a symptom of chlamydial and gonococcal infections and causes leukocytes on urinalysis, women presenting with dysuria may be inaccurately diagnosed with a urinary tract infection if STI testing is not performed. 12 , 13 In women at risk for STIs or with a negative urine culture, physicians can consider STI testing in those presenting with dysuria. A pelvic examination is not required for diagnosis and may not improve the diagnosis of chlamydia and gonorrhea beyond history and diagnostic testing. 14 However, if pelvic inflammatory disease is suspected, a pelvic examination should be performed. The differential diagnosis of chlamydial and gonococcal infections is summarized in Table 5 . 2 , 15
Diagnostic Testing
The CDC recommends using nucleic acid amplification testing (NAAT) for the diagnosis of gonococcal or chlamydial infections because it is the most sensitive. 2 Specimens can be taken from a first-stream urine sample without urethral cleansing before collection. 2 Clinician- or patient-collected vaginal or endocervical swabs are also acceptable specimens. Self-collected vaginal swabs are as sensitive as clinician-collected swabs and are preferred by patients. 16 , 17 A recent meta-analysis showed that urine samples and vaginal and endocervical swabs have similar sensitivity. 17 For patients with male genitalia, a patient- or clinician-collected urethral swab may also be obtained, although a urine specimen is preferred. 2
In 2019, the U.S. Food and Drug Administration (FDA) approved the Aptima Combo 2 Assay and Xpert CT/NG, which use NAAT, for extragenital swabs of the throat and rectum. 18 The binx health io CT/NG assay, Visby Medical Sexual Health Test NAAT, and Cepheid Xpert CT/NG (not a waived test by the Clinical Laboratory Improvement Amendments) are point-of-care tests for the diagnosis of chlamydial and gonococcal infections. 19 Point-of-care testing provides same-day results, decreases loss to follow-up, and reduces overtreatment. 20 , 21
All people who test positive or report known exposure to C. trachomatis or N. gonorrhoeae should be treated. Patients and their partners should be advised to abstain from sex for seven days after completing a single-dose regimen or until the completion of a seven-day treatment course and resolution of symptoms. 2 Nonpregnant people should be tested for reinfection approximately three months after treatment or at the first visit in the 12 months after treatment. Follow-up care recommendations are reviewed in Table 6 . 2 , 22 , 23
Patients presenting clinically with nongonococcal urethritis can be treated empirically at the time of evaluation while diagnostic testing is pending. Cervicitis should be treated presumptively in those younger than 25 years or those at high risk of infection if NAAT is not available or follow-up is uncertain.
CHLAMYDIAL TREATMENT
Although spontaneous clearance of chlamydial infections is possible, people with positive test results should always be treated. 24 Because of increasing macrolide resistance, the recommended treatment for non-pregnant people is now doxycycline, 100 mg, twice per day for seven days. 2 Physicians may alternatively choose to treat patients with a single 1-g dose of azithromycin, especially when adherence to a multidose regimen may be a concern. 2 Treatment regimens are reviewed in Table 7 . 2 , 22 , 23
GONOCOCCAL TREATMENT
In 2018, more than one-half of cases of gonococcal infection were estimated to be resistant to at least one drug, leading the CDC to change treatment recommendations to higher doses of ceftriaxone 25 ( Table 8 2 , 15 , 25 ) . Azithromycin is no longer a recommended therapy for nonpregnant individuals because of an observed sevenfold increase in gonococcal resistance between 2013 and 2018. 25
UNRESOLVED SYMPTOMS
Most treatment failures are caused by reinfection from sex partners who have not received adequate treatment, rather than treatment failure from antimicrobial resistance. 2 If symptoms do not resolve or a test is persistently positive in a situation in which reinfection seems unlikely (i.e., the patient has reported no new sexual contact and is taking medication as prescribed), an infectious disease specialist and local health department should be consulted in case of possible antimicrobial resistance. 2
PARTNER EVALUATION AND EXPEDITED PARTNER THERAPY
If seeking care in person is not possible, expedited partner therapy is a strategy in which sex partners of a person diagnosed with a chlamydial or gonococcal infection within the past 60 days can be prescribed treatment without being seen by the physician. This strategy is supported by the American Academy of Family Physicians. 2 , 26 If the diagnosed person has not had a sex partner in the past 60 days, the most recent sex partner can be offered treatment. Sex partners with symptoms should be referred for evaluation and treatment. 2 Laws in 46 states permit expedited partner therapy. 27 Because recommended gonococcal treatment is based on intramuscular administration of medication, every effort should be made to see partners of infected patients in person for treatment and testing for other STIs. 28 If permissible by state law and the partner is highly unlikely to receive care, partners of those with gonococcal infections may be treated with a single dose of cefixime (Suprax), 800 mg orally, with the addition of 100 mg of oral doxycycline twice per day for seven days if chlamydial infection was not excluded. 28 Written instructions should be given to patients to convey to their partners how to take the medication, warnings about side effects and allergies, when to seek medical care, and STI education. 2 The best evidence for use of expedited partner therapy services is for male partners of women with gonococcal or chlamydial infections. 27 The risk of missing concomitant infections in MSM requires a more nuanced discussion, but these patients may be offered expedited partner therapy through shared decision-making. 28
LYMPHOGRANULOMA VENEREUM
Lymphogranuloma venereum is caused by a C. trachomatis serovar and can become invasive and cause colorectal fistulas and strictures. Treatment should be started presumptively at the initial visit to prevent complications if there is clinical suspicion for lymphogranuloma venereum. 2 Partners should be evaluated and treated empirically with a non–lymphogranuloma venereum chlamydial infection regimen. 2
Gonococcal and chlamydial infections in pregnancy are associated with increased risks, including preterm birth, premature rupture of membranes, stillbirth, low-birth-weight infants, and neonatal infection. 29 , 30 Pregnant patients should be screened as outlined in Table 3 . 2 , 8 Those at high risk of infection should be screened again in the third trimester. 2 Anyone diagnosed with a gonococcal or chlamydial infection during pregnancy should have a test of cure approximately four weeks after treatment, at three months after diagnosis, and in their third trimester. 2
NEONATAL INFECTIONS
The prevalence of perinatal gonococcal infections is 0.2 to 0.4 cases per 100,000 live births. 31 The USPSTF recommends universal prophylaxis with ocular erythromycin 0.5% ointment to prevent gonococcal ophthalmia neonatorum. The risk of infection without prophylaxis is 30% to 40%, and it can cause blindness as early as 24 hours after birth. 31 N. gonorrhoeae can also cause septic arthritis, meningitis, rhinitis, vaginitis, urethritis, pneumonia, and skin infections in neonates. 2 Asymptomatic newborns exposed to gonorrhea at birth from an untreated birthing parent should be swabbed for infection at the conjunctiva, oropharynx, vagina, and rectum and presumptively treated for gonorrhea. 2
Neonates are at high risk of contracting an infection if chlamydia is untreated in pregnancy. 2 , 32 Infants exposed during birth do not need to receive chlamydial-specific prophylactic antibiotics but should be monitored clinically for symptoms. 2 Ophthalmia neonatorum presents a few days to several weeks after birth with eyelid edema, discharge, and ocular congestion. 2 , 32 Chlamydial infections of the eye are not prevented by prophylactic erythromycin ointment. 32 Unlike trachoma, which is a chronic infection spread through close contact, clothes, and flies, ophthalmia neonatorum does not result in scarring and blindness. Diagnosis of ophthalmia neonatorum can be made by swabbing the conjunctiva for culture, direct fluorescence antibody testing, or NAAT. 2 The recommended treatment is oral erythromycin. 2 There should be close follow-up because a second course may be required. 2 C. trachomatis can also cause neonatal pneumonia. Infants present between two and 19 weeks of age with a staccato cough, tachypnea, rhinorrhea, and rales. 2 , 32 Exposed infants are at high risk; if they present with pneumonia, they should be treated empirically for chlamydial infection while awaiting test results from culture, direct fluorescence antibody testing (lower sensitivity), or NAAT (not FDA approved for the nasopharynx). 2 , 32
Any child diagnosed with gonococcal or chlamydial infections should be evaluated for sexual abuse. 2 , 32 Although perinatally transmitted chlamydial infections can be found in children up to three years of age, sexual abuse is the most common cause of infection in children. 2
MANAGEMENT DURING THE COVID-19 PANDEMIC
Disparities in STI testing have been more pronounced due to reallocation of resources for SARS-CoV-2 testing and decreased testing due to social distancing and stay-at-home orders. 3 , 19 However, telemedicine use has increased during the COVID-19 pandemic and is well-suited for STI screening because physical examination is not essential for diagnosis or treatment. 14 , 33 At-home C. trachomatis and N. gonorrhoeae self-testing kits are not FDA approved; however, multiple studies have found that when patients are instructed by a physician via telemedicine, self-collected swabs at home will diagnose cases similarly to office-collected samples, with increased volume of testing offsetting a slightly lower test sensitivity. 19 , 34 – 36 Physicians can safely incorporate home-based testing and treatment into telehealth practice.
This article updates previous articles on this topic by Mishori, et al. 23 ; Mayor, et al. 15 ; Miller 37 ; and Miller . 38
Data Sources: The U.S. Preventive Services Task Force, Cochrane Database of Systematic Reviews, Essential Evidence Plus, Centers for Disease Control and Prevention, the U.S. Food and Drug Administration, and American Academy of Family Physicians websites were reviewed for relevant publications. A PubMed search was conducted using the terms Neisseria gonorrhoeae , Chlamydia trachomatis , diagnosis, and treatment for the past 10 years including English language, meta-analysis, randomized controlled trials, reviews, and systematic reviews. Search dates: January 28, 2021; February 14, 2021; March 30, 2021; July 25, 2021; and November 27, 2021.
Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance 2019. U.S. Department of Health and Human Services; 2021. Accessed November 28, 2021. https://www.cdc.gov/std/statistics/2019/default.htm
- Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep. 2021;70(4):1-187.
Lieberman JA, Cannon CA, Bourassa LA. Laboratory perspective on racial disparities in sexually transmitted infections. J Appl Lab Med. 2021;6(1):264-273.
Hamilton DT, Morris M. The racial disparities in STI in the U.S.: concurrency, STI prevalence, and heterogeneity in partner selection. Epidemics. 2015;11:56-61.
Savoy M, O’Gurek D, Brown-James A. Sexual health history: techniques and tips. Am Fam Physician. 2020;101(5):286-293. Accessed November 28, 2021. https://www.aafp.org/afp/2020/0301/p286.html
- Krist AH, Davidson KW, Mangione CM, et al. Behavioral counseling interventions to prevent sexually transmitted infections: US Preventive Services Task Force recommendation statement. JAMA. 2020;324(7):674-681.
- Cantor A, Dana T, Griffin JC, et al. Screening for chlamydial and gonococcal infections: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2021;326(10):957-966.
Farley TA, Cohen DA, Elkins W. Asymptomatic sexually transmitted diseases: the case for screening. Prev Med. 2003;36(4):502-509.
- Van Gerwen OT, Jani A, Long DM, et al. Prevalence of sexually transmitted infections and human immunodeficiency virus in transgender persons: a systematic review. Transgend Health. 2020;5(2):90-103.
- Bamberger DM, Graham G, Dennis L, et al. Extragenital gonorrhea and chlamydia among men and women according to type of sexual exposure. Sex Transm Dis. 2019;46(5):329-334.
- Marcus JL, Bernstein KT, Kohn RP, et al. Infections missed by urethral-only screening for chlamydia or gonorrhea detection among men who have sex with men. Sex Transm Dis. 2011;38(10):922-924.
- Tomas ME, Getman D, Donskey CJ, et al. Overdiagnosis of urinary tract infection and underdiagnosis of sexually transmitted infection in adult women presenting to an emergency department. J Clin Microbiol. 2015;53(8):2686-2692.
- Shipman SB, Risinger CR, Evans CM, et al. High prevalence of sterile pyuria in the setting of sexually transmitted infection in women presenting to an emergency department. West J Emerg Med. 2018;19(2):282-286.
- Farrukh S, Sivitz AB, Onogul B, et al. The additive value of pelvic examinations to history in predicting sexually transmitted infections for young female patients with suspected cervicitis or pelvic inflammatory disease. Ann Emerg Med. 2018;72(6):703-712.e1.
Mayor MT, Roett MA, Uduhiri KA. Diagnosis and management of gonococcal infections [published correction appears in Am Fam Physician . 2013;87(3):163]. Am Fam Physician. 2012;86(10):931-938. Accessed September 22, 2021. https://www.aafp.org/afp/2012/1115/p931.html
- Lunny C, Taylor D, Hoang L, et al. Self-collected versus clinician-collected sampling for chlamydia and gonorrhea screening: a systemic review and meta-analysis. PLoS One. 2015;10(7):e0132776.
- Rönn MM, Mc Grath-Lone L, Davies B, et al. Evaluation of the performance of nucleic acid amplification tests (NAATs) in detection of chlamydia and gonorrhoea infection in vaginal specimens relative to patient infection status: a systematic review. BMJ Open. 2019;9(1):e022510.
U.S. Food and Drug Administration. FDA news release: FDA clears first diagnostic tests for extragenital testing for chlamydia and gonorrhea. May 23, 2019. Accessed March 10, 2021. https://www.fda.gov/news-events/press-announcements/fda-clears-first-diagnostic-tests-extragenital-testing-chlamydia-and-gonorrhea
- Kersh EN, Shukla M, Raphael BH, et al. At-home specimen self-collection and self-testing for sexually transmitted infection screening demand accelerated by the COVID-19 pandemic: a review of laboratory implementation issues. J Clin Microbiol. 2021;59(11):e0264620.
- Van Der Pol B, Taylor SN, Mena L, et al. Evaluation of the performance of a point-of-care test for chlamydia and gonorrhea. JAMA Netw Open. 2020;3(5):e204819.
- Gaydos CA, Ako MC, Lewis M, et al. Use of a rapid diagnostic for Chlamydia trachomatis and Neisseria gonorrhoeae for women in the emergency department can improve clinical management: report of a randomized clinical trial. Ann Emerg Med. 2019;74(1):36-44.
- Dombrowski JC, Wierzbicki MR, Newman LM, et al. Doxycycline versus azithromycin for the treatment of rectal chlamydia in men who have sex with men: a randomized controlled trial. Clin Infect Dis. 2021;73(5):824-831.
Mishori R, McClaskey EL, WinklerPrins VJ. Chlamydia trachomatis infections: screening, diagnosis, and management. Am Fam Physician. 2012;86(12):1127-1132. Accessed September 22, 2021. https://www.aafp.org/afp/2012/1215/p1127.html
- Geisler WM, Lensing SY, Press CG, et al. Spontaneous resolution of genital Chlamydia trachomatis infection in women and protection from reinfection. J Infect Dis. 2013;207(12):1850-1856.
- St Cyr S, Barbee L, Workowski KA, et al. Update to CDC’s treatment guidelines for gonococcal infection, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(50):1911-1916.
American Academy of Family Physicians. Expedited partner therapy. Accessed August 1, 2021. https://www.aafp.org/about/policies/all/expedited-partner-therapy.html
Centers for Disease Control and Prevention. Expedited partner therapy. U.S. Department of Health and Human Services; 2021. Accessed November 27, 2021. https://www.cdc.gov/std/ept/default.htm
Centers for Disease Control and Prevention. Guidance on the use of expedited partner therapy in the treatment of gonorrhea. U.S. Department of Health and Human Services; 2021. Accessed November 27, 2021. https://www.cdc.gov/std/ept/gc-guidance.htm
- He W, Jin Y, Zhu H, et al. Effect of Chlamydia trachomatis on adverse pregnancy outcomes: a meta-analysis. Arch Gynecol Obstet. 2020;302(3):553-567.
- Vallely LM, Egli-Gany D, Wand H, et al. Adverse pregnancy and neonatal outcomes associated with Neisseria gonorrhoeae: systematic review and meta-analysis. Sex Transm Infect. 2021;97(2):104-111.
- Curry SJ, Krist AH, Owens DK, et al. Ocular prophylaxis for gonococcal ophthalmia neonatorum: US Preventive Services Task Force reaffirmation recommendation statement. JAMA. 2019;321(4):394-398.
Baker CJ. Red Book: Atlas of Pediatric Infectious Diseases . 4th ed. American Academy of Pediatrics; 2020.
- Car J, Koh GCH, Foong PS, et al. Video consultations in primary and specialist care during the COVID-19 pandemic and beyond. BMJ. 2020;371:m3945.
- Fajardo-Bernal L, Aponte-Gonzalez J, Vigil P, et al. Home-based versus clinic-based specimen collection in the management of Chlamydia trachomatis and Neisseria gonorrhoeae infections. Cochrane Database Syst Rev. 2015;(9):CD011317.
- Carnevale C, Richards P, Cohall R, et al. At-home testing for sexually transmitted infections during the COVID-19 pandemic. Sex Transm Dis. 2021;48(1):e11-e14.
- Chow EPF, Bradshaw CS, Williamson DA, et al. Changing from clinician-collected to self-collected throat swabs for oropharyngeal gonorrhea and chlamydia screening among men who have sex with men. J Clin Microbiol. 2020;58(9):e01215-20.
Miller KE. Diagnosis and treatment of Neisseria gonorrhoeae infections. Am Fam Physician. 2006;73(10):1779-1784. Accessed September 22, 2021. https://www.aafp.org/afp/2006/0515/p1779.html
Miller KE. Diagnosis and treatment of Chlamydia trachomatis infection [published correction appears in Am Fam Physician . 2008;77(7) 920]. Am Fam Physician. 2006;73(8):1411-1416. Accessed September 22, 2021. https://www.aafp.org/afp/2006/0415/p1411.html
Continue Reading
More in AFP
More in pubmed.
Copyright © 2022 by the American Academy of Family Physicians.
This content is owned by the AAFP. A person viewing it online may make one printout of the material and may use that printout only for his or her personal, non-commercial reference. This material may not otherwise be downloaded, copied, printed, stored, transmitted or reproduced in any medium, whether now known or later invented, except as authorized in writing by the AAFP. See permissions for copyright questions and/or permission requests.
Copyright © 2024 American Academy of Family Physicians. All Rights Reserved.

- Mammary Glands
- Fallopian Tubes
- Supporting Ligaments
- Reproductive System
- Gametogenesis
- Placental Development
- Maternal Adaptations
- Menstrual Cycle
- Antenatal Care
- Small for Gestational Age
- Large for Gestational Age
- RBC Isoimmunisation
- Prematurity
- Prolonged Pregnancy
- Multiple Pregnancy
- Miscarriage
- Recurrent Miscarriage
- Ectopic Pregnancy
- Hyperemesis Gravidarum
- Gestational Trophoblastic Disease
- Breech Presentation
- Abnormal lie, Malpresentation and Malposition
- Oligohydramnios
- Polyhydramnios
- Placenta Praevia
- Placental Abruption
- Pre-Eclampsia
- Gestational Diabetes
- Headaches in Pregnancy
- Haematological
- Obstetric Cholestasis
- Thyroid Disease in Pregnancy
- Epilepsy in Pregnancy
- Induction of Labour
- Operative Vaginal Delivery
- Prelabour Rupture of Membranes
- Caesarean Section
- Shoulder Dystocia
- Cord Prolapse
- Uterine Rupture
- Amniotic Fluid Embolism
- Primary PPH
- Secondary PPH
- Psychiatric Disease
- Postpartum Contraception
- Breastfeeding Problems
- Primary Dysmenorrhoea
- Amenorrhoea and Oligomenorrhoea
- Heavy Menstrual Bleeding
- Endometriosis
- Endometrial Cancer
- Adenomyosis
- Cervical Polyps
- Cervical Ectropion
- Cervical Intraepithelial Neoplasia + Cervical Screening
- Cervical Cancer
- Polycystic Ovary Syndrome (PCOS)
- Ovarian Cysts & Tumours
- Urinary Incontinence
- Genitourinary Prolapses
- Bartholin's Cyst
- Lichen Sclerosus
- Vulval Carcinoma
- Introduction to Infertility
- Female Factor Infertility
- Male Factor Infertility
- Female Genital Mutilation
- Barrier Contraception
- Combined Hormonal
- Progesterone Only Hormonal
- Intrauterine System & Device
- Emergency Contraception
- Pelvic Inflammatory Disease
- Genital Warts
- Genital Herpes
- Trichomonas Vaginalis
- Bacterial Vaginosis
- Vulvovaginal Candidiasis
- Obstetric History
- Gynaecological History
- Sexual History
- Obstetric Examination
- Speculum Examination
- Bimanual Examination
- Amniocentesis
- Chorionic Villus Sampling
- Hysterectomy
- Endometrial Ablation
- Tension-Free Vaginal Tape
- Contraceptive Implant
- Fitting an IUS or IUD
Original Author(s): Grace Fitzgerald Last updated: 11th June 2019 Revisions: 6
- 1 Pathophysiology
- 2 Risk Factors
- 3.1 Genital infection
- 3.2 Rectal infection
- 3.3 Pharyngeal infection
- 4 Differential Diagnoses
- 5 Investigations
- 6 Management
- 7 Complications
- 8 Gonorrhoea in Pregnancy
Gonorrhoea is a curable sexually transmitted infection caused by the Gram-negative bacterium Neisseria gonorrhoeae . In the UK, gonorrhoea is the second most common bacterial STI (after chlamydia) and predominantly affects people under the age of 25 and men who have sex with men.
Throughout this article we will discuss the pathophysiology of gonorrhoea, its clinical features, management and how it may affect pregnancy and the neonate.
Pathophysiology
Gonorrhoea is transmitted through unprotected vaginal/oral/anal sex and can also be vertically transmitted from mother to child.
Neisseria gonorrhoeae is a Gram-negative diplococcus that has a strong affinity for mucous membranes. The organism can infect the uterus, urethra, cervix, fallopian tubes, ovaries, testicles, rectum, throat and less commonly the eyes. Once adhered to the mucous membrane, it invades the host cell and causes acute inflammation. N. gonorrhoea also has surface proteins that bind to the receptors of immune cells thus preventing an immune response.
Risk Factors
The following are risk factors associated with gonorrhoea, most of which are common to other STIs:
- Aged <25 years
- Men who have sex with men
- Living in high density urban areas
- Previous gonorrhoea infection
- Multiple sexual partners
Clinical Features
While gonorrhoea is often asymptomatic, as occurs in around 50% of female cases, symptoms can usually develop 2-5 days following infection:
Genital infection
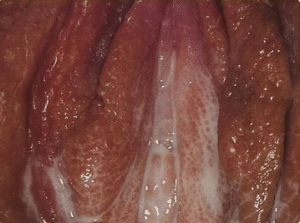
Figure 1. Vaginal discharge in female gonorrhoea infection
- Altered/increased vaginal discharge (commonly thin, watery, green or yellow)
- Dyspareunia
- Lower abdominal pain
- Rarely – intermenstrual and/or post-coital bleeding
- Mucopurulent endocervical discharge
- Easily induced cervical bleeding
- Pelvic tenderness
Often examination can be normal.
- Mucopurulent/purulent urethral discharge
- Epididymal tenderness
Rectal infection
- Usually asymptomatic
- Anal discharge
- Anal pain/discomfort
Pharyngeal infection
- Usually asymptomatic (>90%)
Differential Diagnoses
A full STI screen should be undertaken for a patient presenting with gonorrhoea due to the common presenting symptoms of various STIs.
In particular, it is often very difficult to clinically differentiate between gonorrhoea and chlamydia infection. These infections often co-exist and therefore empirical treatment for gonorrhoea has the aim of covering both the causative organisms.
Investigations
If someone has suspected gonorrhoea, they should be referred to a GUM clinic or other specialist sexual health service for specimens to be taken:
- Endocervical/vaginal swab – NAAT
- Endocervical/urethral swab – microscopy and culture
- First pass urine – NAAT
- Urethral/meatal swab – microscopy and culture
- Swabs for NAAT + microscopy & culture can be obtained from the throat, rectum or eye if indicated.
These swabs should then be sent for microscopy , culture or nucleic acid amplification testing (NAAT) . NAATs are the standard investigation for chlamydia and these tests often provide dual testing for both chlamydia and gonorrhea.
While waiting for these laboratory results the patient should be treated with empirical antibiotics if their signs and symptoms are indicative of gonorrhoea.
Following diagnosis of gonorrhoea, a patient should be treated with a single dose of intramuscular ceftriaxone 1g.
Patients should be offered screening for other STIs, especially chlamydia, as co-infections are common. People should be encouraged to contact previous sexual partners to advise them to be screened and treated empirically for gonorrhoea.
Future safe sex should also be encouraged and patients should abstain from sex until both partners have completed treatment. To ensure antibiotics have successfully treated a patient, a test of cure is recommended during a follow up appointment.
For full details please refer to the BASHH UK guidelines for the management of gonorrhoea .
Complications
If gonorrhoea is left untreated in females, it can lead to pelvic inflammatory disease (PID) , which can result in chronic pain, infertility and ectopic pregnancy. In males, gonorrhoea can spread from the urethra to the testes causing epididymo-orchitis which is painful but rarely leads to infertility. It can also lead to prostatitis . Disseminated gonococcal infection (DGI) is uncommon but can lead to joint pain and skin lesions.
A patient should be admitted to hospital if:
- Systemic symptoms are identified (e.g. malaise, joint pain, fever, rash) as this suggests disseminated gonorrhoea which can potentially develop into a life threatening infection such as gonococcal meningitis.
- Females show signs of complicated or severe pelvic inflammatory disease.
Gonorrhoea in Pregnancy
Having gonorrhoea during pregnancy may be associated with complications such as perinatal mortality, spontaneous abortion, premature labour and early fetal membrane rupture.
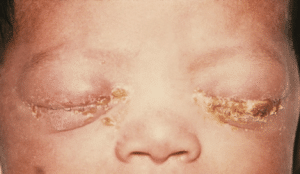
Figure 2. Neonatal conjunctivitis may develop if born to an untreated woman with gonorrhoea.
Gonorrhoea can be vertically transmitted during delivery from an untreated mother and this can cause the neonate to have gonococcal conjunctivitis, where the neonate will experience eye pain, redness and discharge. Prophylactic antibiotics can prevent this and treatment during pregnancy is the same as for uncomplicated gonorrhoea. For the infected neonate, urgent referal and appropriate treatment is necessary to prevent long term damage and blindness.
- Rarely - intermenstrual and/or post-coital bleeding
[start-clinical]
[end-clinical]
Found an error? Is our article missing some key information? Make the changes yourself here!
Once you've finished editing, click 'Submit for Review', and your changes will be reviewed by our team before publishing on the site.
We use cookies to improve your experience on our site and to show you relevant advertising. To find out more, read our privacy policy .
Privacy Overview
- Help & Feedback
- About epocrates
Gonorrhea infection
Highlights & basics, diagnostic approach, risk factors, history & exam, differential diagnosis.
- Tx Approach
Emerging Tx
Complications.
PATIENT RESOURCES
Patient Instructions
Gonorrhea infection is a common STI caused by Neisseria gonorrhoeae , a gram-negative diplococcus bacterium that is closely related to other human Neisseria species.
Men typically present with a urethral discharge; women are often asymptomatic, but may have vaginal discharge.
Risk factors include multiple sex partners in recent months, known partner with gonorrhea, drug use, prior STI, and men who have sex with men.
If left untreated, N gonorrhoeae can disseminate to areas of the body to cause skin and synovial infections; rarer complications include meningitis, endocarditis, and perihepatic abscesses.
High rates of antimicrobial resistance have been reported, and antibiotic treatment should be guided by local and national guidelines. The main treatment for uncomplicated gonorrhea is monotherapy with single-dose intramuscular ceftriaxone.
The treatment of N gonorrhoeae is important in the prevention of infertility, chronic pelvic pain, and ectopic pregnancy in women.
If acquired congenitally from an infected mother, the neonate can present with ophthalmia neonatorum, which left untreated can cause blindness.

Quick Reference
Key Factors
urethral discharge in men
Tenderness and/or swelling of the epididymis, mucopurulent or purulent exudate at the endocervix.
Other Factors
pelvic pain in women
Urethral irritation in men, dysuria in men, tenderness and/or swelling of testis, tenderness and/or swelling of prostate, anal pruritus, mucopurulent discharge from the rectum, rectal pain, rectal bleeding, vaginal discharge, cervical friability, uterine, adnexal, or cervical motion tenderness, uterine mass, anterior cervical lymphadenopathy, conjunctivitis, skin lesions (papules, bullae, petechiae, or necrotic) at extremities, polyarthritis, purpuric rash, positive brudzinski and kernig sign, focal cerebral signs, ophthalmia neonatorum, urethritis (infantile).
Diagnostics Tests
1st Tests to Order

nucleic acid amplification test (NAAT)
Urinalysis in men, gram stain of urine sediment, gram stain of urethral discharge, syphilis test.
Other Tests to consider
transvaginal ultrasound
Pelvic ct/mri, treatment options, nonpregnant >45 kg: urogenital/anorectal or pharyngeal infection (excluding complicated genitourinary infection).
with history of sexual assault
nonpregnant >45 kg: complicated genitourinary infection
with mild to moderate pelvic inflammatory disease (PID)
with severe pelvic inflammatory disease (PID)
with epididymitis (suspected sexually transmitted)
with conjunctivitis
Common Vignette 1
Common Vignette 2
Other Presentations
Epidemiology
Pathophysiology.
- Sheldon Morris, MD, MPH
- Vani Dandolu, MD, MPH
- Eva Jungmann, FRCP MSc

This gram-stained micrograph of a rectal smear specimen reveals the presence of diplococcal Neisseria gonorrhoeae bacteria

This patient presented with symptoms later diagnosed as due to gonococcal pharyngitis

Gonococcal conjunctivitis of the right eye

Gonococcal arthritic patient with inflammation of the skin of her right arm due to a disseminated Neisseria gonorrhoeae bacterial infection

Cutaneous lesions on the left ankle and calf due to a disseminated Neisseria gonorrhoeae infection

Gonococcal arthritis of the hand, which caused the hand and wrist to swell

A newborn with gonococcal ophthalmia neonatorum caused by a maternally transmitted gonococcal infection
Photomicrograph revealing the histopathology in an acute case of gonococcal urethritis using gram-stain technique
Partners (sex of partners, number of partners in prior 2 months/1 year)
Prevention of pregnancy - trying to conceive or contraceptive use
Protection from STIs/HIV - what does the patient do to protect him/herself from STIs and HIV?
Practices or types of sexual activities (oral/vaginal/anal and insertive/receptive), condom use
Past STIs/HIV - any prior diagnoses of STIs or HIV or viral hepatitis.
Physical exam
Inspection and palpation of the testis, epididymis, and spermatic cord should be performed. The penis shaft, glans, and meatus should also be examined and the presence of discharge assessed. A prostate exam is required if symptoms of prostatitis are present.
A swollen and/or tender testicle (usually one-sided) on palpation may indicate orchitis.
A swollen and/or tender epididymis on palpation may indicate epididymitis, which occurs in <5% of men with gonorrhea. [ 40 ] Unilateral testicular pain (without discharge or dysuria) and fever are also symptoms of epididymitis. [ 2 ]
The external genitalia (labia and clitoris) should be inspected before speculum exam of the cervix and vagina. It is recommended that lubricant jelly not be used as it can destroy Neisseria gonorrhoeae . Presence of mucopurulent or purulent exudate at the endocervix is looked for.
PID is the most important complication of gonorrhea in women. It may develop in up to one third of women with gonorrhea, and can lead to long-term sequelae even after resolution of infection. [ 41 ] [ 42 ] The most common sequelae of PID are chronic pelvic pain (40%), tubal infertility (10.8%), and ectopic pregnancy (9.1%). [ 43 ] [ 44 ] A bimanual exam for cervical motion tenderness, uterine tenderness, and adnexal tenderness is particularly important to assess possible ascending infection resulting in PID. Cervical motion tenderness is assessed using 1 or 2 fingertips to move the cervix and asking the patient if any pain results. Presence of a cervical mass suggests PID.
Bleeding that occurs with gentle passage of a cotton swab through the cervical os suggests cervical friability and cervicitis. [ 23 ]
Rectal gonorrhea may cause mucopurulent discharge from the anus.
The pharynx is examined for erythema and exudate. Image Anterior cervical lymphadenopathy may also be present. [ 2 ]
Gonococcal conjunctivitis can present with a thick white/yellow discharge. Examination of the eyes with a slit lamp is recommended so that infection of the cornea can be excluded.
Suspected disseminated gonococcal infection (DGI)
Pediatric gonococcal infection.
The most severe manifestations of pediatric gonococcal infection are ophthalmia neonatorum and sepsis, which can include arthritis and meningitis. Less severe manifestations include rhinitis, vaginitis, urethritis, and reinfection at site of fetal monitoring: for example, through scalp electrodes. Image
Infants at increased risk for gonococcal ophthalmia neonatorum are those who do not receive ophthalmia prophylaxis and those whose mothers have had no prenatal care, or have a history of STIs or substance misuse. [ 23 ] In the US, routine prophylactic eye drops are recommended for all newborns. [ 38 ] [ 46 ]
Sexual abuse is the most frequent cause of STIs (including gonococcal infection) in preadolescent children. [ 14 ] Anorectal and pharyngeal infection are common and frequently asymptomatic in sexually abused children. Vaginitis is the most common manifestation in preadolescent girls.
Laboratory evaluation: overview
Laboratory evaluation: men with urethral discharge, laboratory evaluation: men without urethral discharge, laboratory evaluation: women, laboratory evaluation: nongenital sites, laboratory evaluation: patients with suspected dgi, laboratory evaluation: infants with suspected gonococcal infection, laboratory evaluation for suspected gonococcal infection: children, adolescents, and adults with possible sexual assault, further investigations, age 20 to 24 years.
One of the strongest predictors of gonorrhea, with rates 4 to 5 times higher than the national average. According to US data, the highest rates in men and women are seen in the 20 to 24 years age group. [ 3 ]
men who have sex with men (MSM)
In the US, about one third of gonorrhea cases are reported in MSM. [ 3 ]
In 2016, the Centers for Disease Control and Prevention's MSM Prevalence Monitoring Project in several urban STI clinics in the US showed high median site-specific positivity for rectal gonorrhea (15.9%) and pharyngeal gonorrhea (8.8%) among MSM. [ 24 ]
black ancestry
In the US, people of black ancestry have a rate that remains higher than other races/ethnicities and is between 8 and 9 times higher than the rate in white people (652.9 vs. 78.9 cases per 100,000). [ 3 ] There is no biologic basis for this; rate differences by race/ethnicity may represent contextual factors such as geography, socioeconomic status, and social structure that affect sexual networks. [ 25 ] In the US-based National Longitudinal Study of Adolescent Health (Add Health) study the highest rate among those ages 18 to 26 years was seen in black people (2.13%). [ 26 ]
current or prior history of STI
This is consistently found to be a risk factor for repeat infections and therefore is a clear indication for screening. [ 27 ] [ 28 ] [ 29 ] In the Add Health study (a US-based cohort study of adults ages 18 to 26 years), chlamydia was found as a coinfection in 69% of those with gonorrhea. [ 26 ] In this study most gonorrhea was asymptomatic, which may be because symptomatic people had received treatment. Women with prior bacterial vaginitis had a 26% increased risk of having gonorrhea. Bacterial vaginosis is associated with an increased risk of subsequent gonorrhea infection. [ 30 ]
multiple recent sex partners
The definition of multiple sex partners is variable, but 2 or more partners in the most recent 2 months is a commonly accepted definition. [ 29 ] [ 31 ] [ 32 ] Working in the commercial sex industry qualifies as exposure to multiple partners.
inconsistent condom use
Sexual contact without a condom is a primary risk factor for gonorrhea infection. This includes any penetrative sex (usually referring to a penis) that involves a mucosa-lined orifice (oropharynx, vagina, and anus). [ 5 ] [ 6 ] [ 7 ] [ 8 ]
risk factors of partner
Unprotected sex is required for gonorrhea infection, but it does not constitute a high risk on its own if it is within a monogamous relationship. However, it is important to also consider the partner's risk factors because even if the patient has one partner, that partner may be linked to a high-risk sexual network by any of the same factors listed.
history of sexual or physical abuse
Reinfection of women with gonorrhea or chlamydia is associated with a history of physical or sexual abuse. [ 33 ]
substance use
Often linked to high-risk sexual networks and therefore in many circumstances can be reasonably considered a risk factor. [ 5 ] [ 6 ] [ 7 ] [ 8 ]
past incarceration
Some studies have demonstrated that people with a history of imprisonment may have higher rates of STIs (including gonorrhea) than those with no history of imprisonment. [ 23 ] In the US, 4.4% of females and 1.2% of males entering a juvenile correctional institution in 2011 were positive for gonorrhea. [ 34 ]
high-morbidity community
It is always important to consider the local epidemiologic factors in a decision to screen a person. Within the context of a local outbreak of gonorrhea the threshold to initiate screening may be different.
Early symptom of gonorrhea.
Suggests epididymitis. This requires specialized treatment and needs to be distinguished from testicular torsion.
Mucopurulent cervicitis is the classical sign of gonorrhea infection in women but as a sign it is not common enough nor specific enough for the predictive value to be sufficient to make a diagnosis without supportive laboratory tests.
Physical findings include frank mucopus on swab and cervical os friability.
Considered for an STI and needs a bimanual exam. A significant number of women may have endometritis without overt symptoms. [ 41 ] If no overt pelvic pain is reported it also important to elicit whether pain occurs with sex.
Early symptom of gonorrhea usually followed by discharge hours to days later. [ 18 ]
The most common symptom of gonorrhea in men and will precede discharge.
Orchitis is usually one-sided.
Prostatitis is an uncommon finding with gonorrhea but is suspected if urinary obstructive symptoms or pelvic pain is present.
Associated with rectal gonorrhea infection.
Associated with rectal gonorrhea infection. Usually occurs with a bowel movement.
Associated with rectal gonorrhea infection. More common in men who have sex with men.
Women with gonorrhea may have some vaginal discharge, but lack of a discharge does not exclude infection.
In most vaginal discharges, other types of vaginitis such as trichomonas, yeast, and bacterial vaginosis predominate.
The discharge should be sent for microscopy. Leukorrhea is defined as >10 white blood cell count on high-power field of a vaginal fluid smear. [ 23 ]
Bleeding that occurs with gentle passage of a cotton swab through the cervical os suggest cervicitis. [ 23 ]
Tenderness suggests pelvic inflammatory disease, which requires specialized treatment.
The presence of a mass suggests pelvic inflammatory disease, which requires specialized treatment.
May be present in pharyngeal gonorrhea infection.
Gonococcal conjunctivitis presents with thick/white yellow discharge. Image
Can be seen with ascending gonorrhea infection or disseminated gonorrhea infection.
Indication of disseminated gonococcal infection. Image
Indication of disseminated gonococcal infection. Most commonly affected joints are wrists, ankles, and small joints of hands and feet. Image
Manifestation of gonococcal meningitis.
Manifestation of gonococcal endocarditis
Neonatal conjunctivitis. One of the most severe manifestations of pediatric gonococcal infection. Image
Less severe manifestation of pediatric gonococcal infection.
Most common manifestation of gonococcal infection in preadolescent girls. May occur in infants with gonococcal infection.
positive for gonorrhea
Nonculture testing using NAATs is generally considered the most robust method for testing, but clinicians should use the approved local diagnostic method. [ 23 ] [ 48 ] The Association of Public Health Laboratories and the Centers for Disease Control and Prevention recommend NAATs for the detection of genital tract infections with gonorrhea without routine repeat testing for positive results. [ 47 ] [ 50 ]
Useful for urine, urethral, cervical, and vaginal specimens. However, it is not Food and Drug Administration-approved for use in nongenital sites (pharyngeal and rectum). Individual laboratories can perform NAATs at nongenital sites if they satisfy regulations for Clinical Laboratory Improvement Amendments compliance before reporting results. [ 47 ] [ 50 ]
Most sensitive method to detect gonorrhea but has less than 100% specificity, particularly with pharyngeal and rectum specimens.
Samples for NAATs can be collected by the clinician/healthcare provider or the patient (self-collected). [ 23 ] [ 49 ] Self-collected specimens sent for NAATs have been found to be noninferior to clinician-collected specimens, although local laboratory validation of this collection method should be conducted. [ 51 ] [ 52 ]
A NAAT for chlamydial infection is also recommended. [ 23 ]
positive chocolate agar culture
Urethral, endocervical, rectal, pharyngeal, blood, synovial fluid, cerebrospinal fluid, or conjunctival specimen can be used.
Definitive diagnostic test but has deficiencies in sensitivity with pharyngeal and rectal sites: sensitivity of culture for the pharynx is about 50%. [ 60 ]
It is the only available method to test for antimicrobial sensitivities.
Culture had been the only Food and Drug Administration-approved method for testing rectum and pharyngeal specimens, and it may be the only available option in some regions, but some NAAT platforms are now approved for these nongenital sites. [ 23 ]
Culture of swabs for chlamydial infection may also be requested.
positive leukocyte esterase
Useful if the patient has no urethral discharge.
Provides a presumptive diagnosis of urethritis and guides differential and further investigation. [ 23 ]
≥10 WBC per high-power field or ≥2 WBC per oil immersion field; intracellular gram-negative diplococci in polymorphonuclear leukocytes
Confirms urethritis and guides differential and further investigation.
Strongly suggests gonorrhea if organism seen. But does not rule out gonorrhea if organisms not seen. [ 61 ]
intracellular gram-negative diplococci in polymorphonuclear leukocytes
Confirms urethritis and guides differential and further investigation. Image
may be positive
Routine to rule out HIV. Time to HIV seropositivity with a third-generation enzyme immunoassay can be >21 days.
Routine to rule out syphilis. A Venereal Disease Research Laboratory test or serum rapid plasma reagin (RPR) test can take up to 3 months to become positive. Some laboratories may perform a reverse sequence screening algorithm that uses a serologic test before the RPR.
thickening of endometrium or tubes; fluid in the tubes or abscess
Highly specific for pelvic inflammatory disease. Useful in presence of chronic ascending infection resulting in tubo-ovarian abscess.
inflammatory changes of fallopian tubes and ovaries; abnormal fluid collection; thickened ligaments
Highly specific for pelvic inflammatory disease (PID). When diagnosis of PID is uncertain or ultrasound is equivocal, either a CT or MRI may be performed, if available.
Chlamydia infection
Differentiating Signs/Symptoms
There are no history or physical exam features that can distinguish between chlamydia and gonorrhea infection except for disseminated infection, which is unique to gonorrhea.
Chlamydia is around 10 times more common than gonorrhea in young populations. [ 26 ] Chlamydia does not seem to be efficient at colonizing the pharynx and is less likely to be found there. In men having sex with men, Chlamydia is the most common cause of rectal infections. [ 58 ]
A specific form of genital ulcers and proctitis (lymphogranuloma venereum) is also caused by Chlamydia from a less common strain of Chlamydia trachomatis .
Differentiating Tests
The absence of diplococcus on microscopic exam with sufficient WBC for a diagnosis of urethritis is suggestive of chlamydia. Commercial nucleic acid amplification test (NAAT) is usually a dual test combining both gonorrhea and chlamydia, therefore an ideal way to give a definite pathogenic diagnosis.
Diagnosis of chlamydia of the pharynx or rectum is by culture or with NAAT if available.
Diagnosis of lymphogranuloma venereum is suggested from high titers of chlamydial antibodies, NAAT positive for Chlamydia , and the typical clinical presentation.
Trichomonas
Trichomonas vaginalis is a common STI and is generally underreported. A survey of young Americans found an overall prevalence of 2.3%. [ 41 ] Common symptoms (e.g., vaginal discharge and itching) are not sufficient to distinguish gonorrhea from trichomonas.
T vaginalis is often diagnosed after failure of treatment for urethritis, in cases with negative tests for gonorrhea and Chlamydia .
Culture is the most efficacious test, but newer nucleic acid amplification tests are becoming available and will allow more rapid diagnosis. T vaginalis can be diagnosed by wet preparation from vaginal or urethral discharge but this technique has low sensitivity.
Other infectious causes of urethritis, cervicitis, pelvic inflammatory disease (PID), and epididymitis
Other microorganisms, which are sexually transmitted but are not easily diagnosed, may cause both cervicitis and urethritis. These include atypical herpes simplex recurrences, Mycoplasma genitalium , and Ureaplasma urealyticum .
PID may also be caused by a mixture of organisms.
Epididymitis may be caused by enteric gram-negative organism, especially when there is a history of insertive anal sex. It may also occur in older men (≥35 years), usually resulting from bladder outlet obstruction.
There are no specific differentiating features between these other infectious agents and gonorrhea.
No commercial tests are available for M genitalium or U urealyticum .
Suggestive symptoms with a positive antibody test to herpes simplex virus (HSV)-2 and repeated negative test results for other etiologies suggests HSV infection.
Urinary culture of gram-negative organisms may be positive in cases of epididymitis.
Candidal vaginitis or bacterial vaginosis
Does not usually involve the upper genital tract and is caused by yeast species or a disruption in normal bacterial flora (bacterial vaginosis). These types of vaginitis are not sexually transmitted.
Presents as vaginal discharge, odor, and irritation.
Wet mount microscopic examination, cultures, or smears may show Candida .
In bacterial vaginosis, clue cells may be seen on wet mount and amine whiff test may be positive.
Urinary tract infection, female
Symptoms include dysuria, hematuria, and urgency. Left untreated the ascending infection may result in pyelonephritis with flank pain and fever.
Mid-stream urine culture positive for causative infectious agent.
Urinary tract infection, male
Nonpregnant women, pregnant women, treatment approach, uncomplicated gonococcal infection.
First-line treatment is a single dose of intramuscular ceftriaxone. [ 23 ] One meta-analysis found that ceftriaxone had better efficacy for uncomplicated gonorrhea compared with other antibiotics. [ 67 ] If chlamydial infection has not been excluded, patients should receive oral doxycycline for 7 days in addition to the cephalosporin. [ 23 ]
In patients with urogenital or anorectal gonorrhea who have a cephalosporin allergy, a single dose of intramuscular gentamicin plus oral azithromycin may be considered; however, gastrointestinal adverse effects may limit the use of this regimen. [ 23 ] In an asymptomatic person, a single dose of ciprofloxacin could be used if the provider is able to perform gyrase A (gyrA) testing to identify ciprofloxacin susceptibility (wild type). [ 23 ] [ 68 ] An infectious disease specialist should be consulted if there is known penicillin/cephalosporin allergy.
A single dose of oral cefixime is a suitable alternative regimen if ceftriaxone is not available. [ 23 ] However, cefixime has a lower response rate and reduced susceptibility compared with ceftriaxone when used for nongenital sites. [ 23 ] If chlamydial infection has not been excluded, patients should receive oral doxycycline for 7 days in addition to the cephalosporin. [ 23 ]
Pharyngeal infections are more difficult to treat than urogenital or anorectal infections. Cefixime has limited efficacy against pharyngeal gonorrhea, and no reliable alternative treatments are available. An infectious disease specialist should be consulted for an alternative treatment recommendation if there is an anaphylactic reaction to ceftriaxone. If chlamydial infection is also identified, patients should receive oral doxycycline for 7 days in addition to ceftriaxone. [ 23 ] A test-of-cure is recommended 7-14 days after treatment regardless of the treatment regimen used for pharyngeal infections. [ 23 ] Use of an antiseptic mouthwash may help with clearance of pharyngeal infections. [ 69 ] In people previously treated for gonorrhea, reinfection within 12 months ranges from 7% to 12%, and so they should be retested 3 months after treatment regardless of whether they believe their sex partners were treated. [ 70 ] [ 71 ] If retesting at 3 months is not possible, retesting should be performed within 12 months of initial treatment. [ 23 ]
Persistent infection after treatment may be due to reinfection or resistance/treatment failure. Patients who have persistent symptoms after treatment should be retested by culture (preferably with simultaneous NAAT). If these cultures are positive for gonococcus, isolates should be submitted for resistance testing. [ 23 ]
Persistent infections should be retreated with a single dose of intramuscular ceftriaxone, and an infectious disease specialist should be consulted. If chlamydial infection has not been excluded, patients should receive oral doxycycline for 7 days in addition to the cephalosporin. [ 23 ]
A single-dose of intramuscular gentamicin plus oral azithromycin can be used as an alternative regimen for urogenital and rectal gonorrhea, particularly if resistance to cephalosporins is suspected. [ 23 ] No reliable alternative treatments are available for pharyngeal gonorrhea. [ 23 ]
Patients with treatment failure after receiving an alternative regimen (cefixime or gentamicin plus azithromycin) should be retreated with a single dose of ceftriaxone, with or without doxycycline if chlamydial infection has not been excluded. [ 23 ]
Sex partners from the preceding 60 days should be identified and treated with the same regimen. [ 23 ]
A test-of-cure should be done 7-14 days after retreatment. Treatment failures should be reported to the CDC through the local or state health department within 24 hours of diagnosis. [ 23 ]
Complicated gonococcal infection
The recommended regimen is dual therapy with single-dose intramuscular ceftriaxone plus oral doxycycline for 14 days. [ 23 ]
Cefoxitin (plus probenecid) may be used instead of ceftriaxone. Other parenteral third-generation cephalosporins may also be used. [ 23 ]
Metronidazole should be used in combination with doxycycline to provide extended coverage against anaerobic bacteria. [ 23 ]
Outpatient treatment with intramuscular and oral agents can be considered because they may be as efficacious as inpatient parenteral treatment in mild to moderate PID, but reassessment after 72 hours is recommended. [ 23 ] [ 79 ]
A Cochrane review assessing CDC-recommended antibiotic regimens for PID found no conclusive evidence that one antibiotic regimen is safer or more effective than another. [ 80 ]
Signs and symptoms of severe infection include: surgical abdomen; tubo-ovarian abscess; severe illness with nausea, vomiting, and fever; inability to take oral regimen; and no response from outpatient therapy.
Hospitalization and intravenous antibiotic therapy is required. Intravenous therapy with a cephalosporin (ceftriaxone, cefotetan, or cefoxitin) plus doxycycline is the recommended first-line regimen. [ 23 ] Ampicillin/sulbactam plus doxycycline or clindamycin plus gentamicin are suitable alternatives. [ 23 ]
If the patient can take oral medication, oral doxycycline may be preferred to intravenous doxycycline to minimize pain associated with intravenous infusion.
Metronidazole is added if there is a tubo-ovarian abscess, or suspicion of any anaerobic organism or trichomonas involvement. Metronidazole should be used with ceftriaxone as ceftriaxone is less active against anaerobic bacteria than cefotetan or cefoxitin.
Reassessment can be made at 24 to 48 hours as to whether to discontinue intravenous therapy and continue with suitable oral therapy to complete 14 days of treatment if there is clinical improvement. [ 23 ] Parenteral therapy can be discontinued 24-48 hours after clinical improvement; ongoing oral therapy after the parenteral cephalosporin regimen should consist of doxycycline plus metronidazole to complete a total of 14 days of therapy. Oral clindamycin or oral doxycycline can be used after the alternative parenteral clindamycin/gentamicin regimen. [ 23 ] If tubo-ovarian abscess is present, oral clindamycin or oral metronidazole should be used with doxycycline as this provides better anaerobic coverage. [ 23 ]
Due to the high rate of fluoroquinolone resistance, intramuscular ceftriaxone plus oral doxycycline is recommended for 10 days if epididymitis infection is suspected to be sexually transmitted (i.e., gonorrhea or chlamydia). [ 23 ] Chlamydia will be covered by doxycycline.
Reassessment should be made after 48 hours.
First-line treatment is intramuscular ceftriaxone. [ 23 ] Clinical studies have used a higher dose of ceftriaxone for gonococcal conjunctivitis than that used in other types of gonococcal infections. [ 81 ] There are no data for the use of oral cephalosporins in gonococcal conjunctivitis.
As gonococcal conjunctivitis is uncommon and data on treatment in adults are limited, an infectious disease specialist should be consulted.
The recommended first-line regimen is intravenous or intramuscular ceftriaxone. [ 23 ] Cefotaxime is a suitable alternative regimen.
Parenteral therapy should be continued for 24 to 48 hours after substantial clinical improvement, and then the patient switched to a suitable oral regimen for at least 7 days guided by antimicrobial sensitivity testing. [ 23 ]
If chlamydial infection has not been excluded, patients should receive oral doxycycline for 7 days in addition to the cephalosporin. [ 23 ]
The recommended first-line regimen is intravenous ceftriaxone. [ 23 ]
Treatment for meningitis is continued for 10 to 14 days, and for endocarditis treatment is continued for at least 4 weeks. [ 23 ]
Neonates, infants, and children
cephalosporin monotherapy or gentamicin plus azithromycin or ciprofloxacin monotherapy
Primary Options
body weight <150 kg: 500 mg intramuscularly as a single dose; body weight ≥150 kg: 1000 mg intramuscularly as a single dose
Secondary Options
240 mg intramuscularly as a single dose
2 g orally as a single dose
500 mg orally as a single dose
800 mg orally as a single dose
It is recommended that adult patients with a suspected or confirmed diagnosis of gonorrhea be treated with a single dose of ceftriaxone. [ 23 ] One meta-analysis found that ceftriaxone had better efficacy for uncomplicated gonorrhea compared with other antibiotics. [ 67 ]
A single dose of oral cefixime is a suitable alternative regimen if ceftriaxone is not available. [ 23 ] However, cefixime has a lower response rate and reduced susceptibility compared with ceftriaxone when used for nongenital sites. [ 23 ]
In patients with urogenital or anorectal gonorrhea who have a cephalosporin allergy, one option is a single dose of intramuscular gentamicin plus oral azithromycin may be considered; however, gastrointestinal adverse effects may limit the use of this regimen. [ 23 ] In an asymptomatic person, a single dose of ciprofloxacin could be used if the provider is able to perform gyrase A (gyrA) testing to identify ciprofloxacin susceptibility (wild type). [ 23 ] [ 68 ] An infectious disease specialist should be consulted for advice on management if there is known penicillin/cephalosporin allergy.
Pharyngeal infections are more difficult to treat than urogenital or anorectal infections. Cefixime has limited efficacy against pharyngeal gonorrhea, and no reliable alternative treatments are available. An infectious disease specialist should be consulted for an alternative treatment recommendation if there is an anaphylactic reaction to ceftriaxone. [ 23 ] The Centers for Disease Control and Prevention recommends a test-of-cure 7-14 days after treatment regardless of the treatment regimen used for pharyngeal infections. [ 23 ] Use of an antiseptic mouthwash may help with clearance of pharyngeal infections. [ 69 ] In people previously treated for gonorrhea, reinfection within 12 months ranges from 7% to 12%, and so they should be retested 3 months after treatment regardless of whether they believe their sex partners were treated. [ 70 ] [ 71 ] If retesting at 3 months is not possible, retesting should be performed within 12 months of initial treatment. [ 23 ]
The management of the patient's sex partners is an important consideration to prevent reinfection and further transmission. [ 23 ] CDC: expedited partner therapy
doxycycline
100 mg orally twice daily for 7 days
If chlamydial infection has not been excluded, patients should also receive oral doxycycline for 7 days (unless they are receiving the gentamicin plus azithromycin regimen). [ 23 ]
metronidazole
Metronidazole is added to the recommended drug regimen for women if there is a history of sexual abuse. [ 23 ]
cephalosporin plus doxycycline plus metronidazole
100 mg orally twice daily for 14 days
500 mg orally twice daily for 14 days
2 g intramuscularly as a single dose
1 g orally as a single dose
The Centers for Disease Control and Prevention recommends dual therapy with single-dose intramuscular ceftriaxone plus oral doxycycline for 14 days as first-line treatment. [ 23 ] Cefoxitin (plus probenecid) may be used instead of ceftriaxone. Other parenteral third-generation cephalosporins may also be used. [ 23 ]
PID is the most important complication of gonorrhea in women. It may develop in up to one third of women with gonorrhea and can lead to long-term sequelae even after resolution of infection. [ 41 ] [ 42 ] The most common sequelae of PID are chronic pelvic pain (40%), tubal infertility (10.8%), and ectopic pregnancy (9.1%). [ 43 ] [ 44 ]
The management of the patient's sex partners is an important consideration to prevent reinfection and further transmission. [ 23 ] CDC: expedited partner therapy
For further details of management, please see the BMJ Best Practice topic on Pelvic inflammatory disease.
hospitalization plus intravenous antibiotic therapy
1 g intravenously every 24 hours
100 mg intravenously/orally every 12 hours
500 mg orally/intravenously every 12 hours
2 g intravenously every 12 hours
2 g intravenously every 6 hours
3 g intravenously every 6 hours
100 mg orally/intravenously every 12 hours
900 mg intravenously every 8 hours
2 mg/kg intravenously/intramuscularly as a loading dose, followed by 1.5 mg/kg every 8 hours; or 3-5 mg/kg intravenously/intramuscularly every 24 hours
Severe PID requires intravenous antibiotic therapy.
The Centers for Disease Control and Prevention recommends dual therapy with a cephalosporin (ceftriaxone, cefotetan, or cefoxitin) plus doxycycline as first-line treatment. [ 23 ] If the patient can take oral medication, oral doxycycline may be preferred to intravenous doxycycline to minimize pain associated with intravenous infusion. However, ceftriaxone, cefotetan, or cefoxitin must be given intravenously. Metronidazole should be used with ceftriaxone as ceftriaxone is less active against anaerobic bacteria than cefotetan or cefoxitin. Alternative parenteral regimens include ampicillin/sulbactam plus doxycycline or clindamycin plus gentamicin. [ 23 ]
Reassessment can be made at 24 to 48 hours as to whether to discontinue intravenous therapy and continue with oral therapy (doxycycline) to complete 14 days of treatment if there is clinical improvement. [ 23 ]
switch to oral antibiotic therapy following clinical improvement
After parenteral cephalosporin regimen
100 mg orally twice daily to complete 14-day course
500 mg orally twice daily to complete 14-day course
After parenteral clindamycin/gentamicin regimen
450 mg orally four times daily to complete 14-day course
After parenteral clindamycin/gentamicin regimen with tubo-ovarian abscess
Patients should be reassessed 24 to 48 hours after treatment has begun and the decision about changing from parenteral to oral therapy, if appropriate, can be based on clinical improvement. [ 23 ]
Parenteral therapy can be discontinued 24 to 48 hours after clinical improvement; ongoing oral therapy after the parenteral cephalosporin regimen should consist of doxycycline plus metronidazole to complete a total of 14 days of therapy.
Oral clindamycin or oral doxycycline can be used after the alternative parenteral clindamycin/gentamicin regimen. [ 23 ] If tubo-ovarian abscess is present, oral clindamycin or oral metronidazole should be used with doxycycline as this provides better anaerobic coverage. [ 23 ]
ceftriaxone plus doxycycline
100 mg orally twice daily for 10 days
The Centers for Disease Control and Prevention recommends intramuscular ceftriaxone plus oral doxycycline as the first-line antibiotic regimen in patients with epididymitis in which the infection is suspected to be sexually transmitted (i.e., gonorrhea or chlamydia). [ 23 ] Chlamydia will be covered by doxycycline.
If the patient is suspected of having epididymitis due to enteric organisms, a fluoroquinolone could be used, but it is important to rule out gonorrhea and chlamydia first. [ 23 ]
Epididymitis occurs in <5% of men with gonorrhea. [ 40 ] Hospital admission is required for severe cases. Rarely epididymitis can lead to infertility or chronic inflammation. Diagnosis of the offending organism should be pursued because gram-negative rods can also be a causative agent.
For further details of management, please see the BMJ Best Practice topic on Acute epididymitis.
cephalosporin monotherapy
1 g intramuscularly as a single dose
The Centers for Disease Control and Prevention recommends intramuscular ceftriaxone as a first-line regimen. [ 23 ] Clinical studies have used a higher dose of ceftriaxone for gonococcal conjunctivitis than that used in other types of gonococcal infections. [ 81 ] There are no data for the use of oral cephalosporins in gonococcal conjunctivitis.
Providers should also consider one-time lavage of the infected eye with saline solution. [ 23 ]
As gonococcal conjunctivitis is uncommon and data on treatment in adults are limited, an infectious disease specialist should be consulted. [ 23 ]
nonpregnant >45 kg: disseminated gonococcal infection
excluding meningitis and endocarditis
1 g intramuscularly/intravenously every 24 hours
1 g intravenously every 8 hours
Disseminated gonococcal infection is a serious medical condition and it is recommended that the patient be hospitalized for initial therapy. [ 23 ] Treatment should be undertaken with an infectious disease specialist.
The Centers for Disease Control and Prevention recommends intramuscular or intravenous ceftriaxone as the first-line regimen. [ 23 ] Cefotaxime is a suitable alternative.
Parenteral therapy should be continued for 24 to 48 hours after substantial clinical improvement, and then the patient switched to a suitable oral regimen for at least 7 days guided by antimicrobial sensitivity testing. [ 23 ] Children with bacteremia or arthritis should continue parenteral therapy for 7 days. [ 23 ]
If chlamydial infection has not been excluded, patients should also receive oral doxycycline for 7 days. [ 23 ]
desensitization to penicillin/cephalosporin + interim fluoroquinolone
400 mg intravenously every 12 hours
Allergy to a specific antibiotic is a contraindication for that antibiotic. A much smaller number of patients than previously thought have cross-reactivity of penicillin antibiotics and cephalosporin as an allergy. [ 82 ] If the history of the penicillin allergy does not suggest immunoglobulin E-mediated allergy, then use of cephalosporin with close observation is warranted.
Desensitization to cephalosporins is an option if cephalosporin allergy is documented.
Fluoroquinolones can be used in the interim in adults, but should not be used in children.
with meningitis or endocarditis
1-2 g intravenously every 12-24 hours
The Centers for Disease Control and Prevention recommends intravenous ceftriaxone as the first-line regimen. [ 23 ]
Treatment for meningitis should be continued for 10 to 14 days; treatment for endocarditis should be continued for at least 4 weeks. [ 23 ]
pregnant: uncomplicated urogenital/anorectal or pharyngeal infection (excluding complicated genitourinary infection)
A single dose of intramuscular ceftriaxone is recommended as a first-line regimen in pregnant women, preferably given under direct observation. [ 23 ]
Consultation with an infectious disease specialist is recommended if the patient has a cephalosporin allergy or there are any other considerations that preclude treatment with this regimen.
Pharyngeal infections are more difficult to treat than urogenital or anorectal infections. The Centers for Disease Control and Prevention recommends a test-of-cure 7-14 days after treatment regardless of the treatment regimen used for pharyngeal infections. [ 23 ]
azithromycin or amoxicillin
500 mg orally three times daily for 7 days
If chlamydial infection has not been excluded, a single dose of azithromycin is also recommended in pregnant women. Amoxicillin is an alternative in pregnant women. [ 23 ]
pregnant: complicated infection
hospitalization and management by an experienced provider
Pregnant women with complicated infection (i.e., pelvic inflammatory disease, conjunctivitis, or disseminated gonococcal infection) require hospitalization and specialist management from an experienced provider.
born to a mother with gonococcal infection
ceftriaxone or cefotaxime
25-50 mg/kg intramuscularly/intravenously as a single dose, maximum 250 mg/dose
100 mg/kg intravenously/intramuscularly as a single dose
Neonates who are born to women with untreated gonococcal infections are at high risk of infections and should be treated presumptively in the absence of signs of gonococcal infection. [ 23 ] The Centers for Disease Control and Prevention recommends ceftriaxone as a first-line agent. [ 23 ] Ceftriaxone should be administered cautiously to neonates with hyperbilirubinemia, especially those born prematurely. [ 23 ] Cefotaxime can be given in neonates unable to receive ceftriaxone because of simultaneous administration of intravenous calcium. [ 23 ] An infectious disease specialist should be consulted for advice on management if there is known penicillin/cephalosporin allergy.
with ophthalmia neonatorum
25-50 mg/kg intravenously/intramuscularly as a single dose, maximum 250 mg/dose
The Centers for Disease Control and Prevention recommends ceftriaxone as a first-line agent. Ceftriaxone should be administered cautiously to neonates with hyperbilirubinemia, especially those born prematurely. [ 23 ]
Cefotaxime can be given in neonates unable to receive ceftriaxone because of simultaneous administration of intravenous calcium. [ 23 ]
An infectious disease specialist should be consulted for advice on management if there is known penicillin/cephalosporin allergy.
with scalp abscesses or disseminated gonococcal infection
25-50 mg/kg intravenously/intramuscularly every 24 hours
25 mg/kg intravenously/intramuscularly every 12 hours
The Centers for Disease Control and Prevention recommends ceftriaxone or cefotaxime as a first-line agent. [ 23 ]
Infants with scalp abscesses or disseminated gonococcal infection in the form of bacteremia or arthritis should receive treatment for 7 days. Infants with meningitis should receive treatment for 10 to 14 days.
child ≤45 kg
with uncomplicated vulvovaginitis, cervicitis, urethritis, pharyngitis, or proctitis
ceftriaxone
The Centers for Disease Control and Prevention recommends ceftriaxone as a first-line agent. [ 23 ]
It is important to consider the possibility of sexual abuse in children with gonorrhea. [ 14 ] If suspected it should be reported and child protection procedures should be followed accordingly.
with bacteremia, meningitis, endocarditis, or arthritis
50 mg/kg intravenously/intramuscularly every 24 hours, maximum 2000 mg/day
Meningitis should be treated for 10 to 14 days.
Endocarditis should be treated for at least 4 weeks.
Bacteremia and arthritis should be treated for 7 days.
recurrent/resistant: urogenital/anorectal infection or pharyngitis
repeat investigations and retreatment + report to health department
Persistent infection after treatment may be due to reinfection or resistance/treatment failure. Reinfection is a likely possibility, and partner treatment should be reinforced. [ 23 ]
Patients who have persistent symptoms after treatment should be retested by culture (preferably with simultaneous nucleic acid amplification test). If these cultures are positive for gonococcus, isolates should be submitted for resistance testing.
Persistent gonorrhea infections should be retreated with a single dose of intramuscular ceftriaxone, and an infectious disease specialist should be consulted. [ 23 ]
A single-dose of intramuscular gentamicin plus oral azithromycin can be used as an alternative regimen for urogenital and rectal gonorrhea, particularly if resistance to cephalosporins is suspected. [ 23 ] High-dose oral azithromycin is commonly accompanied by nausea and vomiting in patients. No reliable alternative treatments are available for pharyngeal gonorrhea. [ 23 ]
A test-of-cure should be done 7 to 14 days after retreatment.
Treatment failures should be reported to the Centers for Disease Control and Prevention through the local or state health department within 24 hours of diagnosis. [ 23 ]
doxycycline or azithromycin
If chlamydial infection has not been excluded, patients should also receive oral doxycycline for 7 days (unless they are receiving the gentamicin plus azithromycin regimen). Pregnant women should receive a single dose of azithromycin in place of doxycycline and in addition to the cephalosporin. [ 23 ]
Challenges for new antibiotic therapy development
Primary prevention, secondary prevention, follow-up overview, chronic pelvic pain (resulting from pelvic inflammatory disease).
Pelvic inflammatory disease (PID) complicating gonorrhea infection may develop in up to one third of women with gonorrhea. [ 41 ] [ 42 ] The most common sequelae of PID are chronic pelvic pain (40%), tubal infertility (10.8%), and ectopic pregnancy (9.1%). [ 43 ] [ 44 ]
ectopic pregnancy (resulting from pelvic inflammatory disease)
Infertility in women (resulting from pelvic inflammatory disease), fitz-hugh-curtis syndrome.
Gonococci may spread up toward the liver causing perihepatitis, which mimics acute cholecystitis in its presentation. It resolves with antibiotic therapy.
infertility in men
Rarely epididymitis, complicating gonorrhea infection, can lead to infertility or chronic inflammation.
May be a complication of ophthalmia neonatorum.
Key Articles
Centers for Disease Control and Prevention. Gonococcal isolate surveillance project (GISP). 30 August 2021 [internet publication]. [Full Text]
Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep. 2021 Jul 23;70(4):1-187. [Abstract] [Full Text]
Miller WC, Ford CA, Morris M, et al. Prevalence of chlamydial and gonococcal infections among young adults in the United States. JAMA. 2004 May 12;291(18):2229-36. [Abstract] [Full Text]
Centers for Disease Control and Prevention. Recommendations for the laboratory-based detection of Chlamydia trachomatis and Neisseria gonorrhoeae - 2014. MMWR Recomm Rep. 2014;63(RR-02):1-19. [Abstract] [Full Text]
Fifer H, Saunders J, Soni S, et al. 2018 UK national guideline for the management of infection with Neisseria gonorrhoeae. Int J STD AIDS. 2020 Jan;31(1):4-15. [Abstract] [Full Text]
US Preventive Services Task Force; Davidson KW, Barry MJ, Mangione CM, et al. Screening for chlamydia and gonorrhea: US Preventive Services Task Force recommendation statement. JAMA. 2021 Sep 14;326(10):949-56. [Abstract] [Full Text]
World Health Organization. WHO guidelines for the treatment of Neisseria gonorrhoeae. 2016 [internet publication]. [Full Text]
Centers for Disease Control and Prevention. Guidance on the use of expedited partner therapy in the treatment of gonorrhea. 18 August 2021 [internet publication]. [Full Text]
Other Online Resources
- CDC: expedited partner therapy
- CDC: gonorrhea fact sheet
Referenced Articles
1. Smith NH, Holmes EC, Donovan GM, et al. Networks and groups within the genus Neisseria: analysis of argF, recA, rho and 16S rRNA from human Neisseria species. Mol Biol Evol. 1999 Jun;16(6):773-83. [Abstract] [Full Text]
2. Miller KE. Diagnosis and treatment of Neisseria gonorrhoeae infections. Am Fam Physician. 2006 May 15;73(10):1779-84. [Abstract] [Full Text]
3. Centers for Disease Control and Prevention. Sexually transmitted disease surveillance, 2021: gonorrhea. Apr 2023 [internet publication]. [Full Text]
4. Centers for Disease Control and Prevention. Gonococcal isolate surveillance project (GISP). 30 August 2021 [internet publication]. [Full Text]
5. Ellen JM, Langer LM, Zimmerman RS, et al. The link between the use of crack cocaine and the sexually transmitted diseases of clinic population: comparison of adolescents with adults. Sex Transm Dis. 1996 Nov-Dec;23(6):511-6. [Abstract]
6. Liau A, Diclemente RJ, Wingood GM, et al. Associations between biologically confirmed marijuana use and laboratory-confirmed sexually transmitted diseases among African American adolescent females. Sex Transm Dis. 2002 Jul;29(7):387-90. [Abstract]
7. Mertz KJ, Finelli L, Levine WC, et al. Gonorrhea in male adolescents and young adults in Newark, New Jersey: implications of risk factors and patients preferences for prevention strategies. Sex Transm Dis. 2000 Apr;27(4):201-7. [Abstract]
8. Boyer CB, Shafer MA, Teitle E, et al. Sexually transmitted diseases in a health maintenance organization teen clinic: association of race, partner's age and marijuana use. Arch Pediatr Adolesc Med. 1999 Aug;153(8):838-44. [Abstract] [Full Text]
9. Platt R, Rice PA, McCormack WA. Risk of acquiring gonorrhea and prevalence of abnormal adnexal finding among women exposed to gonorrhea. J Am Med Assoc. 1983 Dec 16;250(23):3205-9. [Abstract]
10. Hooper RR, Reynolds GH, Jones OG, et al. Cohort study of venereal diseases: the risk of gonorrhea transmission from infected women to men. Am J Epidemiol. 1978 Aug;108(2):136-44. [Abstract]
11. Sadiq ST, Taylor S, Bennett SB, et al. The effects of antiretroviral therapy on HIV-1 RNA loads in seminal plasma in HIV-positive patients with and without urethritis. AIDS. 2002 Jan 25;16(2):219-25. [Abstract]
12. Røttingen JA, Cameron DW, Garnett GP. A systematic review of the epidemiologic interactions between classic sexually transmitted diseases and HIV: how much really is known? Sex Transm Dis. 2001 Oct;28(10):579-97. [Abstract]
13. Neinstein LS, Goldenring J, Carpenter S. Nonsexual transmission of sexually transmitted diseases: an infrequent occurrence. Pediatrics. 1984 Jul;74(1):67-76. [Abstract]
14. Rogstad KE, Wilkinson D, Robinson A. Sexually transmitted infections in children as a marker of child sexual abuse and direction of future research. Curr Opin Infect Dis. 2016 Feb;29(1):41-4. [Abstract]
15. Edwards JL, Apicella MA. The molecular mechanisms used by Neisseria gonorrhoeae to initiate infection differ between men and women. Clin Microbiol Rev. 2004 Oct;17(4):965-81. [Abstract] [Full Text]
16. Gibbs CP, Meyer TF. Genome plasticity in Neisseria gonorrhoeae. FEMS Microbiol Lett. 1996 Dec 1;145(2):173-9. [Abstract]
17. Fox KK, Knapp JS, Holmes KK, et al. Antimicrobial resistance in Neisseria gonorrhoeae in the United States, 1988-1994: the emergence of decreased susceptibility to the fluoroquinolones. J Infect Dis. 1997 Jun;175(6):1396-403. [Abstract]
18. Schneider H, Cross AS, Kuschner RA, et al. Experimental human gonococcal urethritis: 250 Neisseria gonorrhoeae MS11mkC are infective. J Infect Dis. 1995 Jul;172(1):180-5. [Abstract]
19. Harrison WO, Hooper RR, Wiesner PJ, et al. A trial of minocycline after exposure to prevent gonorrhea. N Eng J Med. 1979 May 10;300(19):1074-8. [Abstract]
20. Pariser H. Asymptomatic gonorrhea. Cutis. 1976 Apr;17(4):723-6. [Abstract]
21. Schmidt KA, Schneider H, Lindstrom JA, et al. Experimental gonococcal urethritis and infection in homologous gonococci in male volunteers. Sex Transm Dis. 2001 Oct;28(10):555-64. [Abstract]
22. Mehta SD, Moses S, Agot K, et al. Adult male circumcision does not reduce the risk of incident Neisseria gonorrhoeae, Chlamydia trachomatis, or Trichomonas vaginalis infection: results from a randomized, controlled trial in Kenya. J Infect Dis. 2009 Aug 1;200(3):370-8. [Abstract] [Full Text]
23. Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep. 2021 Jul 23;70(4):1-187. [Abstract] [Full Text]
24. Centers for Disease Control and Prevention. 2016 sexually transmitted diseases surveillance: STDs in men who have sex with men. Aug 2017 [internet publication]. [Full Text]
25. Aral SO, O'Leary A, Baker C. Sexually transmitted infections and HIV in the southern United States: an overview. Sex Transm Dis. 2006 Jul;33(suppl 7):S1-5. [Abstract]
26. Miller WC, Ford CA, Morris M, et al. Prevalence of chlamydial and gonococcal infections among young adults in the United States. JAMA. 2004 May 12;291(18):2229-36. [Abstract] [Full Text]
27. Cecil JA, Howell MR, Tawes JJ, Gaydos JC, McKee KT Jr, Quinn TC, Gaydos CA. Features of Chlamydia trachomatis and Neisseria gonorrhoeae infection in male army recruits. J Infect Dis. 2001 Nov 1;184(9):1216-9. [Abstract]
28. Klausner JD, Barrett DC, Dithmer D, et al. Risk factors for repeated gonococcal infections: San Francisco, 1990-1992. J Infect Dis. 1998 Jun;177(6):1766-9. [Abstract]
29. Mehta SD, Rothman RE, Kelen GD, et al. Unsuspected gonorrhea and chlamydia in patients of an urban emergency department: a critical population for STD control intervention. Sex Transm Dis. 2001 Jan;28(1):33-9. [Abstract]
30. Bautista CT, Wurapa EK, Sateren WB, et al. Association of bacterial vaginosis with chlamydia and gonorrhea among women in the US Army. Am J Prev Med. 2017 May;52(5):632-9. [Abstract] [Full Text]
31. Bachmann LH, Piggot D, Desmond R, et al. Prevalence and factors associated with gonorrhea and chlamydia infection in at-risk females presenting to an urban emergency department. Sex Transm Dis. 2003 Apr;30(4):335-9. [Abstract]
32. Marrazzo JM, Handsfield HH, Whittington WL, et al. Predicting chlamydia and gonococcal cervical infection: implications for management of cervicitis. Obstet Gynecol. 2002 Sep;100(3):579-84. [Abstract]
33. Champion JD. Behavioural interventions and abuse: secondary analysis of reinfection in minority women. Int J STD AIDS. 2007 Nov;18(11):748-53. [Abstract]
34. Centers for Disease Control and Prevention. 2011 sexually transmitted diseases surveillance: STDs in persons entering corrections facilities. Dec 2012 [internet publication]. [Full Text]
35. O'Connor EA, Lin JS, Burda BU, et al. Behavioral sexual risk-reduction counseling in primary care to prevent sexually transmitted infections: a systematic review for the US Preventive Services Task Force. Ann Intern Med. 2014 Dec 16;161(12):874-83. [Abstract] [Full Text]
36. National Institute for Health and Care Excellence. Reducing sexually transmitted infections. June 2022 [internet publication]. [Full Text]
37. Rietmeijer CA. Risk reduction counselling for prevention of sexually transmitted infections: how it works and how to make it work. Sex Transm Infect. 2007 Feb;83(1):2-9. [Abstract] [Full Text]
38. US Preventive Services Task Force. Ocular prophylaxis for gonococcal ophthalmia neonatorum: preventive medication. 29 January 2019 [internet publication]. [Full Text]
39. Petousis-Harris H, Paynter J, Morgan J, et al. Effectiveness of a group B outer membrane vesicle meningococcal vaccine against gonorrhoea in New Zealand: a retrospective case-control study. Lancet. 2017 Sep 30;390(10102):1603-10. [Abstract]
40. Gift TL, Owens CJ. The direct medical cost of epididymitis and orchitis: evidence from a study of insurance claims. Sex Transm Dis. 2006 Oct;33(suppl 10):S84-8. [Abstract]
41. Wiesenfeld HC, Hillier SL, Krohn MA, et al. Lower genital tract and endometritis: insight into subclinical pelvic inflammatory disease. Obstet Gynecol. 2002 Sep;100(3):456-63. [Abstract]
42. Bowie WR, Jones H. Acute pelvic inflammatory disease in outpatients: association with Chlamydia trachomatis and Neisseria gonorrhoeae. Ann Intern Med. 1981 Dec;95(6):685-8. [Abstract]
43. Ness RB, Trautmann G, Richter HE, et al. Effectiveness of treatment strategies of some women with pelvic inflammatory disease Obstet Gynecol. 2005 Sep;106(3):573-80. [Abstract]
44. Weström L, Joesoef R, Reynolds G, et al. Pelvic inflammatory disease and fertility. A cohort study of 1,844 women with laparoscopically verified disease and 657 control women with normal laparoscopic results. Sex Transm Dis. 1992 Jul-Aug;19(4):185-92. [Abstract]
45. Rice PA. Gonococcal arthritis (disseminated gonococcal infection). Infect Dis Clin North Am. 2005 Dec;19(4):853-61. [Abstract]
46. Darling EK, McDonald H. A meta-analysis of the efficacy of ocular prophylactic agents used for the prevention of gonococcal and chlamydial ophthalmia neonatorum. J Midwifery Womens Health. 2010 Jul-Aug;55(4):319-27. [Abstract]
47. Centers for Disease Control and Prevention. Recommendations for the laboratory-based detection of Chlamydia trachomatis and Neisseria gonorrhoeae - 2014. MMWR Recomm Rep. 2014;63(RR-02):1-19. [Abstract] [Full Text]
48. Fifer H, Saunders J, Soni S, et al. 2018 UK national guideline for the management of infection with Neisseria gonorrhoeae. Int J STD AIDS. 2020 Jan;31(1):4-15. [Abstract] [Full Text]
49. World Health Organization. WHO guideline on self-care interventions for health and well-being, 2022 revision. Jun 2022 [internet publication]. [Full Text]
50. Association of Public Health Laboratories; Center of Disease Control and Prevention. Expert consultation meeting summary report: laboratory diagnostic testing for Chlamydia trachomatis and Neisseria gonorrhoeae. Jan 2009 [internet publication]. [Full Text]
51. Sexton ME, Baker JJ, Nakagawa K, et al. How reliable is self-testing for gonorrhea and chlamydia among men who have sex with men? J Fam Pract. 2013 Feb;62(2):70-8. [Abstract]
52. Lunny C, Taylor D, Hoang L, et al. Self-collected versus clinician-collected sampling for chlamydia and gonorrhea screening: a systemic review and meta-analysis. PLoS One. 2015 Jul 13;10(7):e0132776. [Abstract] [Full Text]
53. Watchirs Smith LA, Hillman R, Ward J, et al. Point-of-care tests for the diagnosis of Neisseria gonorrhoeae infection: a systematic review of operational and performance characteristics. Sex Transm Infect. 2013 Jun;89(4):320-6. [Abstract]
54. Guy RJ, Causer LM, Klausner JD, et al. Performance and operational characteristics of point-of-care tests for the diagnosis of urogenital gonococcal infections. Sex Transm Infect. 2017 Dec;93(s4):S16-21. [Abstract] [Full Text]
55. May L, Ware CE, Jordan JA, et al. A randomized controlled trial comparing the treatment of patients tested for chlamydia and gonorrhea after a rapid polymerase chain reaction test versus standard of care testing. Sex Transm Dis. 2016 May;43(5):290-5. [Abstract]
56. Templeton DJ, Jin F, Imrie J, et al. Prevalence, incidence and risk factors for pharyngeal chlamydia in the community based Health in Men (HIM) cohort of homosexual men in Sydney, Australia. Sex Transm Infect. 2008 Oct;84(5):361-3. [Abstract]
57. Morris SR, Klausner JD, Buchbinder SP, et al. Prevalence and incidence of pharyngeal gonorrhea in a longitudinal sample of men who have sex with men: the EXPLORE study. Clin Infect Dis. 2006 Nov 15;43(10):1284-9. [Abstract] [Full Text]
58. Kent CK, Chaw JK, Wong W, et al. Prevalence of rectal, urethral, and pharyngeal chlamydia and gonorrhea detected in 2 clinical settings among men who have sex with men: San Francisco, California, 2003. Clin Infect Dis. 2005 Jul 1;41(1):67-74. [Abstract]
59. Barry PM, Kent CK, Philip SS, et al. Results of a program to test women for rectal chlamydia and gonorrhea. Obstet Gynecol. 2010 Apr;115(4):753-9. [Abstract]
60. Page-Shafer K, Graves A, Kent C, et al. Increased sensitivity of DNA amplification testing for the detection of pharyngeal gonorrhoea in men who have sex with men. Clin Infect Dis. 2002 Jan 15;34(2):173-6. [Abstract] [Full Text]
61. Geisler WM, Yu S, Hook EW. Chlamydial and gonococcal infection in men without polymorphonuclear leukocytes on Gram stain. Sex Transm Dis. 2005 Oct;32(10):630-4. [Abstract]
62. US Preventive Services Task Force; Davidson KW, Barry MJ, Mangione CM, et al. Screening for chlamydia and gonorrhea: US Preventive Services Task Force recommendation statement. JAMA. 2021 Sep 14;326(10):949-56. [Abstract] [Full Text]
63. Centers for Disease Control and Prevention (CDC). Recommendations for partner services programs for HIV infection, syphilis, gonorrhea, and chlamydial infection. MMWR Recomm Rep. 2008 Nov 7;57(RR-9):1-83. [Abstract] [Full Text]
64. World Health Organization. WHO guidelines for the treatment of Neisseria gonorrhoeae. 2016 [internet publication]. [Full Text]
65. British Association for Sexual Health and HIV. United Kingdom BASHH national guideline for the management of epididymo-orchitis, 2019. Sep 2020 [internet publication]. [Full Text]
66. British Association for Sexual Health and HIV. 2015 BASHH CEG guidance on tests for sexually transmitted infections. Dec 2015 [internet publication]. [Full Text]
67. Bai ZG, Bao XJ, Cheng WD, et al. Efficacy and safety of ceftriaxone for uncomplicated gonorrhoea: a meta-analysis of randomized controlled trials. Int J STD AIDS. 2012 Feb;23(2):126-32. [Abstract]
68. Klausner JD, Bristow CC, Soge OO, et al. Resistance-guided treatment of gonorrhea: a prospective clinical study. Clin Infect Dis. 2021 Jul 15;73(2):298-303. [Abstract] [Full Text]
69. Chow EP, Howden BP, Walker S, et al. Antiseptic mouthwash against pharyngeal Neisseria gonorrhoeae: a randomised controlled trial and an in vitro study. Sex Transm Infect. 2017 Mar;93(2):88-93. [Abstract]
70. Fung M, Scott KC, Kent CK, et al. Chlamydial and gonococcal reinfection among men: a systematic review of data to evaluate the need for retesting. Sex Transm Infect. 2007 Jul;83(4):304-9. [Abstract] [Full Text]
71. Hosenfeld CB, Workowski KA, Berman S, et al. Repeat infection with Chlamydia and gonorrhea among females: a systematic review of the literature. Sex Transm Dis. 2009 Aug;36(8):478-89. [Abstract]
72. Ostendorf W. Preparation for safe medication administration. In: Perry AG, Potter PA, Elkin MK, eds. Nursing interventions & clinical skills. 5th ed. St Louis, MO: Mosby; 2012:486-53.
73. Public Health England. Immunisation procedures: the green book, chapter 4. Mar 2013 [internet publication]. [Full Text]
74. Poland GA, Borrud A, Jacobson RM, et al. Determination of deltoid fat pad thickness. Implications for needle length in adult immunization. JAMA. 1997 Jun 4;277(21):1709-11. [Abstract]
75. Zaybak A, Güneş UY, Tamsel S, et al. Does obesity prevent the needle from reaching muscle in intramuscular injections? J Adv Nurs. 2007 Jun;58(6):552-6. [Abstract] [Full Text]
76. Nisbet AC. Intramuscular gluteal injections in the increasingly obese population: retrospective study. BMJ. 2006 Mar 18;332(7542):637-8. [Abstract] [Full Text]
77. Hutin Y, Hauri A, Chiarello L, et al. Best infection control practices for intradermal, subcutaneous, and intramuscular needle injections. Bull World Health Organ. 2003;81(7):491-500. [Abstract]
78. Jin JF, Zhu LL, Chen M, et al. The optimal choice of medication administration route regarding intravenous, intramuscular, and subcutaneous injection. Patient Prefer Adherence. 2015;9:923-42. [Abstract] [Full Text]
79. Ness RB, Soper DE, Holley RL, et al. Effectiveness of inpatient and outpatient treatment strategies for women with pelvic inflammatory disease: results from the Pelvic Inflammatory Disease Evaluation and Clinical Health (PEACH) randomized trial. Am J Obstet Gynecol. 2002 May;186(5):929-37. [Abstract]
80. Savaris RF, Fuhrich DG, Maissiat J, et al. Antibiotic therapy for pelvic inflammatory disease. Cochrane Database Syst Rev. 2020 Aug 20;8(8):CD010285. [Abstract] [Full Text]
81. Haimovici R, Roussel TJ. Treatment of gonococcal conjunctivitis with single-dose intramuscular ceftriaxone. Am J Ophthalmol. 1989 May 15;107(5):511-4. [Abstract]
82. Pichichero ME. A review of evidence supporting the American Academy of Pediatrics recommendation for prescribing cephalosporin antibiotics for penicillin-allergic patients. Pediatrics. 2005 Apr;115(4):1048-57. [Abstract]
83. Grad YH, Harris SR, Kirkcaldy RD, et al. Genomic epidemiology of gonococcal resistance to extended-spectrum cephalosporins, macrolides, and fluoroquinolones in the United States, 2000-2013. J Infect Dis. 2016 Nov 15;214(10):1579-87. [Abstract] [Full Text]
84. Taylor SN, Marrazzo J, Batteiger BE, et al. Single-dose zoliflodacin (ETX0914) for treatment of urogenital gonorrhea. N Engl J Med. 2018 Nov 8;379(19):1835-45. [Abstract] [Full Text]
85. Centers for Disease Control and Prevention. Guidance on the use of expedited partner therapy in the treatment of gonorrhea. 18 August 2021 [internet publication]. [Full Text]
86. Kerani RP, Fleming M, DeYoung B, et al. A randomized, controlled trial of inSPOT and patient-delivered partner therapy for gonorrhea and chlamydial infection among men who have sex with men. Sex Transm Dis. 2011 Oct;38(10):941-6. [Abstract] [Full Text]
Published by
Centers for Disease Control and Prevention
US Preventive Services Task Force
World Health Organization
British Association for Sexual Health and HIV
National Institute for Health and Care Excellence (UK)
Topic last updated: 2023-06-20
Sheldon Morris , MD, MPH
Assistant Professor
Division of Infectious Diseases
Department of Medicine
UCSD Antiviral Research Center
Division of Family Medicine
Department of Family and Preventive Medicine
UCSD La Jolla Family and Sports Medicine
Peer Reviewers
Vani Dandolu , MD, MPH
Associate Professor
Ob/Gyn and Urology
Division of Urogynecology
Associate Residency Program Director
Temple University Hospital
Philadelphia
Eva Jungmann , FRCP MSc
Consultant in Genitourinary and HIV Medicine
Archway Centre & Mortimer Market Centre
Learn how UpToDate can help you.
Select the option that best describes you
- Medical Professional
- Resident, Fellow, or Student
- Hospital or Institution
- Group Practice
- Patient or Caregiver
- Find in topic
RELATED TOPICS
INTRODUCTION
This topic will discuss the epidemiology, pathophysiology, clinical manifestations, diagnosis, and treatment of DGI. The clinical manifestations, diagnosis, and treatment of uncomplicated gonococcal infection (eg, cervicitis and urethritis) are discussed elsewhere. (See "Treatment of uncomplicated gonorrhea ( Neisseria gonorrhoeae infection) in adults and adolescents" and "Clinical manifestations and diagnosis of Neisseria gonorrhoeae infection in adults and adolescents" and "Epidemiology and pathogenesis of Neisseria gonorrhoeae infection" .)
EPIDEMIOLOGY
Most patients with DGI are younger than 40 years of age, although DGI can occur in any age group. Historically, DGI occurred more frequently in females than males; however, that sex ratio may be reversing, with increases in gonococcal infection in general among males, and because DGI might be more common among individuals with HIV (of whom males comprise the majority in North America and Western Europe) [ 1,4-11 ].
DGI is considered a common cause of acute polyarthralgias, polyarthritis, or oligoarthritis in young, otherwise healthy patients. Although Staphylococcus aureus is the most common cause of monomicrobial septic arthritis overall, among sexually active adults, N. gonorrhoeae is the most common causative organism [ 12 ]. Nevertheless, the overall proportion of septic arthritis cases that are due to N. gonorrhoeae is low. In case studies from Europe during the 1990s, N. gonorrhoeae was the causative organism in 1.7 percent in a series from France [ 13 ], 0.6 percent in a series from the United Kingdom [ 14 ], and 0 percent from a three-year prospective community-based study in the Netherlands [ 15 ]. In a retrospective study of 34 cases of musculoskeletal infections among intravenous drug users in Spain, N. gonorrhoeae was the causative agent in 2.9 percent [ 16 ]. The prevalence of gonococcal arthritis may be higher in the United States than in the United Kingdom [ 17 ]. Gonococcal arthritis appears to be more prevalent in more resource-limited settings, although there are limited epidemiologic studies from such regions [ 6 ].
Sexually transmitted infection rates have risen sharply among adults 55 and older, CDC data shows
Sexually transmitted infections are becoming more common in older adults.
Rates of chlamydia, gonorrhea and syphilis in people ages 55 and up more than doubled in the U.S. over the 10-year period from 2012 to 2022, according to data from the Centers for Disease Control and Prevention.
The number of syphilis cases among people ages 55 and up increased seven-fold during those 10 years, while gonorrhea cases increased nearly five-fold and chlamydia cases more than tripled during that time.
A presentation to be delivered Thursday — part of a lead-up event to the European Congress of Clinical Microbiology and Infectious Diseases next month — warns that both doctors and older adults are overlooking the risks of STIs in this age group.
“We talk about smoking, we talk about diet, exercise, so many things, and not about sex at all,” said Justyna Kowalska, the author of the presentation and a professor of medicine at the Medical University of Warsaw.
The issue is not limited to the U.S. In England, surveillance data published in 2022 suggested that STI diagnoses rose 22% from 2014 to 2019 among people ages 45 and up. Chlamydia was the most common, followed by gonorrhea.
Kowalska pointed to a few factors that may be driving up STI rates among older adults.
For one, people are living longer compared to past generations and enjoying more active lifestyles in their 60s, 70s and 80s. For many, that includes sex. A 2018 survey from AARP and the University of Michigan estimated that 40% of people ages 65 to 80 are sexually active, and nearly two-thirds are interested in sex.
Hormone replacement therapy, which can treat symptoms of menopause, can prolong sexual desire in older women, while erectile dysfunction drugs like Viagra can help older men remain sexually active.
But older adults may not have gotten the type of sex education provided to teenagers today, according to Matthew Lee Smith, an associate professor at the Texas A&M School of Public Health.
"Back in the '30s, the '40s, the '50s, traditional school wasn’t really doing sexual education very formally," said Smith, who studies behavioral health risks in older adults.
Smith's research has shown that older adults lack some knowledge about STI transmission, symptoms and prevention.
He said doctors can be sheepish about asking older patients about their sexual activity, and older people often aren’t inclined to discuss their sex lives with peers or family members.
“No one wants to think about grandma doing this,” Smith said. “You certainly aren’t going to ask grandma if she was wearing condoms — and that’s part of the problem, because every individual regardless of age has the right to intimacy.”
Some older men may struggle with condom use, Smith said, because of either a lack of dexterity or erectile dysfunction.
What's more, he added, many older adults married at a younger age than is typical now and only had one sexual partner until they divorced or were widowed. So some might not think to use a condom, Smith said — especially since pregnancy isn’t a concern.
Nursing homes also create opportunities for new sexual partners. The results of a U.S. survey of nursing home directors, published in 2016, found that sexual activity was common in these settings, which often have more female than male residents.
“In the heterosexual, older adult community, there’s a partner gap: Women live longer than men and there’s a larger proportion of females to men,” Smith said. “What it can lead to oftentimes is multiple partners and sharing of partners.”
Though STIs pose health risks to all age groups, older people may have a harder time clearing infections or be more susceptible to contracting them in the first place, medical experts said.
“The immune system is weaker, so you can get an infection easier, but there’s other physical things related to just sexual intimacy that make one more susceptible,” said Ethan Morgan, an assistant professor of epidemiology at The Ohio State University College of Nursing. Among women who are postmenopausal, for instance, the vaginal lining is more prone to tearing, which makes it easier for an infection to occur.
The experts stressed that doctors need to do a better job of discussing safe sex with older patients.
“We want them to have their best life," Smith said, "but we want them to have it safely."
Aria Bendix is the breaking health reporter for NBC News Digital.
- Mobile Site
- Staff Directory
- Advertise with Ars
Filter by topic
- Biz & IT
- Gaming & Culture
Front page layout
Alarming —
China has a big problem with super gonorrhea, study finds, drug-resistant gonorrhea is a growing problem—one that doesn't heed borders..
Beth Mole - Mar 28, 2024 6:12 pm UTC

Health officials have long warned that gonorrhea is becoming more and more resistant to all the antibiotic drugs we have to fight it. Last year, the US reached a grim landmark : For the first time, two unrelated people in Massachusetts were found to have gonorrhea infections with complete or reduced susceptibility to every drug in our arsenal, including the frontline drug ceftriaxone. Luckily, they were still able to be cured with high-dose injections of ceftriaxone. But, as the US Centers for Disease Control and Prevention bluntly notes: "Little now stands between us and untreatable gonorrhea."
Further Reading
While those single-digit percentages may seem low, compared to other countries they're extremely high. In the US, for instance, the prevalence of ceftriaxone-resistant strains never went above 0.2 percent between 2017 and 2021 , according to the CDC. In Canada, ceftriaxone-resistance was stable at 0.6 percent between 2017 and 2021. The United Kingdom had a prevalence of 0.21 percent in 2022.
Ceftriaxone is currently the first-line treatment for gonorrhea because Neisseria gonorrhoeae has spent the past several decades building up resistance to pretty much everything else. As the CDC notes , in the 1980s, the drugs of choice for gonorrhea infections were penicillin and tetracycline. But the bacteria developed resistance. By the 1990s, the CDC was forced to switch to a class of antibiotics called fluoroquinolones, including ciprofloxacin (Cipro). But fluoroquinolone-resistance developed, too, and resistance to Cipro is now widespread. In the early 2000s, the CDC began having to tweak the recommendations as resistance spread to new places and populations.
Resistance rising
By 2007, the agency switched to cephalosporins, including cefixime. In 2010, the CDC updated the treatment again, recommending that doctors combine cephalosporins with one of two other types of antibiotics—azithromycin or doxycycline—to try to thwart the development of resistance. But, it also was no use. Two years later, in 2012, the CDC updated recommendations when cefixime resistance developed. In 2020, azithromycin was also abandoned. The cephalosporin ceftriaxone is the last drug standing in the US to treat gonorrhea infections.
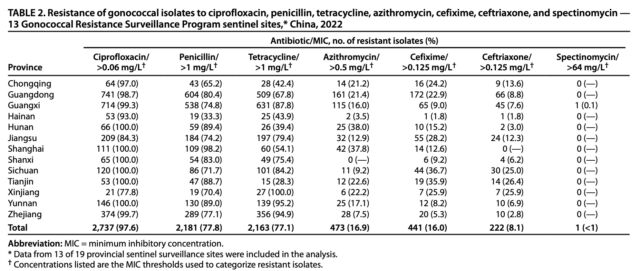
In China, the swift spread of ceftriaxone-resistance isolates is alarming. The data stems from 2,804 isolates, representing 2.9 percent of all cases reported in China during 2022. Those figures come from 13 of the country's 19 provinces. While the overall prevalence of ceftriaxone-resistance isolates was 8.1 percent among the 2,804 isolates, five of those 13 provinces had prevalence rates above 10 percent. Three provinces had prevalence rates above 25 percent. In all, 18 isolates were resistant to all the antibiotics tested except for a bygone antibiotic called spectinomycin, which is discontinued in the US and elsewhere.
The study has limitations. For one, the reported number of gonorrhea cases are very likely an undercount of actual cases. Beyond gaps in reporting, many people with gonorrhea have no symptoms and, as such, don't seek treatment. Additionally, the isolates the researchers did have represented less than 3 percent of reported cases, so it's possible the prevalence rates don't represent the isolates of the entire country. Also, the researchers didn’t have detailed case data that might help identify specific risk factors for resistance development, such as the antibiotic treatments patients had. The authors did note that antibiotics are only given by prescription in China.
"These findings underscore the urgent need for a comprehensive approach to address antibiotic-resistant N. gonorrhoeae in China, including identifying factors contributing to this high resistance rate, especially in provinces where the percentage of gonococcal isolates resistant to ceftriaxone is >10 percent," the authors write.
But they also note that this is not just an alarming finding for China but also a "pressing public health concern" for the entire world. "These resistant clones have spread internationally, and collaborative cross-border efforts will be essential to monitoring and mitigating its further spread," they write.
reader comments
Channel ars technica.
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.

StatPearls [Internet].
Gonococcal arthritis.
Raymund Li ; Jason D. Hatcher .
Affiliations
Last Update: May 29, 2023 .
- Continuing Education Activity
Gonococcal arthritis is arthritis that results from the bacteremic spread of Neisseria gonorrhoeae, a sexually transmitted pathogen. It comprises localized septic arthritis and arthritis-dermatitis syndrome. This activity reviews the evaluation and treatment of gonococcal arthritis and explains the role of the interprofessional team in managing patients with this condition.
- Outline the etiology of gonococcal arthritis.
- Review the pathophysiology of gonococcal arthritis.
- Describe the use of ceftriaxone in the treatment of gonococcal arthritis.
- Explain the importance of improving care coordination among the interprofessional team to improve outcomes for patients with gonococcal arthritis.
- Introduction
Gonococcal arthritis is a bacterial arthritis that results from the bacteremic spread of Neisseria gonorrhoeae, a sexually transmitted pathogen. It is a clinical manifestation of disseminated gonococcal infection. It comprises two major clinical forms: localized septic arthritis and arthritis-dermatitis syndrome. [1] [2] [3]
Neisseria gonorrhoeae , a gram-negative diplococcus, is the etiologic pathogen in gonococcal arthritis. It is usually transmitted sexually and is an important cause of bacterial arthritis in sexually active adolescents. However, it may also be acquired perinatally during childbirth and may cause gonococcal infection in newborns. [4] [5]
- Epidemiology
Approximately 0.5% to 3% of patients who are infected with N. gonorrhoeae develop disseminated gonococcal infection (DGI). There is a paucity of recent epidemiologic data that looks into the incidence of DGI in patients with arthritis, but historical data in the 1980s show that N. gonorrhoeae were associated with up to 14% of patients who have arthritis. Gonococcal arthritis is more commonly seen in young healthy individuals although it may occur in any age group. [6] [7] [8]
- Pathophysiology
Host risk factors that are associated with disseminated gonococcal infection (DGI) include complement deficiencies, menstruation, pregnancy, history of pelvic surgery, and intrauterine devices (IUD). Specific strains of N. gonorrhoeae have virulence factors that have been associated with dissemination. These include strains containing a specific outer membrane called the porin 1A serotype that promotes resistance, diminished host inflammatory response, and host cell invasion and strains that require specific substrates for growth. It is also hypothesized that the pathogenesis of DGI may be partly immune-mediated. This is supported by the fact that the pathogen is frequently not isolated from both the synovial fluid and blood of patients with clinical manifestations of dissemination. [9]
- History and Physical
Disseminated gonococcal infection (DGI) usually comprises two major clinical syndromes: the arthritis-dermatitis syndrome and localized purulent arthritis without associated skin lesions. There are, however, patients who present with symptoms that overlap between these two classic presentations. Although localized infection of the genitourinary tract, rectum, or pharynx by N. gonorrhoeae is a prerequisite for dissemination, patients with clinical manifestations of DGI often do not manifest symptoms of localized gonococcal infection.
- Arthritis-dermatitis syndrome: This is a form of DGI that usually comprises a triad of manifestations which include tenosynovitis, dermatitis, and polyarthralgia. It is also often associated with constitutional symptoms such as fever, chills, and body malaise. Approximately 60% developed a fever in one study in women with DGI. Tenosynovitis is a unique finding in gonococcal arthritis and is usually not seen in other forms of septic arthritis. It is demonstrated by tenderness along the flexor sheath and pain on a passive extension during a physical examination. It usually affects multiple tendons, more commonly the fingers, wrists, toes, and ankles. Polyarthralgia is typically asymmetric and may affect both large and small joints. Skin lesions occur in up to 75% of cases and are frequently seen in the trunk and extremities and usually spares the face. Although a large variety of skin lesions have been observed, DGI is most commonly associated with pustular or vesicular lesions. Other skin manifestations that occur less frequently include macules, papules, bullae, or nodules. Skin lesions are characteristically transient and may disappear after several days even without treatment.
- Localized septic arthritis: This syndrome usually presents as a monoarthritis or asymmetric oligo- or polyarthritis with pain and swelling of one or more joints. Most patients do not present with systemic symptoms such as fever or chills. Joints that are commonly affected include knees, ankles, wrist, and elbow.
A high clinical suspicion is necessary for patients who present with joint pain or swelling that is concerning for septic arthritis especially in those who are sexually active and younger than 40 years old and in certain at-risk populations such as men who have sex with men (MSM). A thorough history and physical examination including an emphasis on sexual history are needed in patients with suspected gonococcal arthritis. Menstrual history and pregnancy need to be assessed in premenopausal patients. Skin and joint examination may provide information that heightens clinical suspicion, especially in patients who present with the typical triad of tenosynovitis, arthralgia, and skin lesions. [1] [4] [10]
Definitive diagnosis of disseminated gonococcal infection (DGI) or gonococcal arthritis is made through the identification of the etiologic pathogen in a specimen taken from a non-mucosal site (such as blood, synovial fluid, or skin lesions). Microbiologic tests, however, are not always positive and in such cases, diagnosis is made clinically. A clinical diagnosis may be supported by evidence of N. gonorrhoeae infection from specimens obtained from mucosal sites. Screening for other sexually transmitted illnesses such as HIV, syphilis, and chlamydia is often recommended since these frequently co-exist with gonococcal infection.
Two sets of blood cultures should be obtained, and a positive result helps distinguish gonococcal arthritis from other pathogens that cause septic arthritis. Positive blood cultures, however, are only positive in less than one-third of cases and are more frequently seen in patients who present with arthritis-dermatitis syndrome. Specimens from mucosal sites should also be obtained and are frequently helpful in patients who have a high clinical suspicion of DGI but who have sterile synovial fluid or blood specimens. Nucleic acid amplification testing (NAAT) of either urine specimens in both men and women or vaginal swabs in women is preferred, if available. The culture of a urogenital specimen is an alternative.
In patients who demonstrate joint swelling and effusion, arthrocentesis and synovial fluid analysis should be performed. Typically, a synovial fluid analysis will show a white blood cell (WBC) count of 50,000 cells/mm3 or more; although, a cell count below 10,000 cells/mm3 is not uncommon. This may be associated with reduced glucose concentration and elevated LDH levels but these findings are non-diagnostic and of limited value. In patients who present with localized purulent arthritis, N. gonorrhoeae will be isolated in only about 50% (range of 25% to 75%) of synovial fluid specimens. This is even less in patients who present with the arthritis-dermatitis syndrome. NAAT of synovial fluid is more sensitive (greater than 75%) than culture and should be performed if available.
Obtaining skin lesion specimens is usually not done because of the difficulty of sampling and its very poor yield. The culture of skin lesions is often negative. NAAT of skin specimens may be slightly more sensitive, but this is not widely available.
- Treatment / Management
Initial management in the inpatient setting is recommended for patients with a presumptive diagnosis of disseminated gonococcal infection (DGI) or gonococcal arthritis. Ceftriaxone as a parenteral therapy, either given intravenously or intramuscularly, is the preferred initial antibiotic of choice. Intravenous administration of 1 gm every 24 hours is usually preferred in patients presenting with purulent arthritis. Doxycycline 100 mg twice a day for seven days is usually added to cover potential coinfection with Chlamydia trachomatis. Alternative antimicrobial agents include third-generation cephalosporin such as cefotaxime and ceftizoxime, which are given as 1 gm every 8 hours. A dose of azithromycin 1 g orally is an alternative to azithromycin. [11] [12] [13]
Widespread resistance to oral cephalosporins such as cefixime has been documented. Oral cephalosporins are therefore not recommended as initial therapy. Although not always possible, the microbiologic diagnosis should be attempted, and antimicrobial susceptibility testing should be performed to guide antibiotic therapy. After 1 to 2 days of clinical improvement with ceftriaxone as parenteral therapy, a seven-day course of antibiotics may be completed with daily intramuscular ceftriaxone given at 250 mg daily. Patients with septic arthritis or those who are immunocompromised or slower to respond to treatment may require a longer course of therapy (7 to 14 days). De-escalation to oral antibiotic therapy (such as cefixime, fluoroquinolones, or penicillin) may be done only in patients who do not have septic arthritis and only if culture and susceptibility results are available. However, not all laboratories are capable of performing N. gonorrhoeae culture and sensitivity testing. In cases wherein the microbiologic diagnosis is not established, the course of therapy should be completed with a parenteral cephalosporin.
Partners of patients diagnosed with DGI within 2 months should be contacted and treated whenever possible. Patients with recurrent DGI or gonococcal arthritis will require further evaluation for complement deficiency.
Patients with a history of beta-lactam allergy are often able to tolerate ceftriaxone. Due to the absence of effective alternative parenteral agents, patients who have a history of IgE mediated hypersensitivity reaction to beta-lactams may undergo either skin testing or graded challenge testing. Patients who test positive for these tests should undergo desensitization. Those with a history of severe, non-IgE mediated reaction will require consultation with an infectious disease specialist.
Patients presenting with localized purulent arthritis should undergo joint drainage, either arthroscopically or through repeated joint aspirations until there is evidence of response such as the resolution of fever, leukocytosis, joint pain, and effusions. Open surgical drainage may be necessary if aspiration is not adequate.
- Differential Diagnosis
- Septic arthritis: Acute purulent arthritis due to other bacteria may present similarly. They may be distinguished from gonococcal arthritis through microbiologic identification from synovial fluid culture.
- Poststreptococcal arthritis: Acute rheumatic arthritis may present with both polyarthritis and rash. The rash, however, is transient and is typically not vesicular or pustular.
- Crystal arthropathy: This may also present as mono- or oligo-arthritis and may be distinguished through synovial fluid analysis.
- Other inflammatory arthritis: Rheumatoid arthritis, reactive arthritis, and psoriatic arthritis may be mistaken for gonococcal arthritis, but these disease entities are usually associated with other clinical or radiologic manifestations not seen in disseminated gonococcal infection.
- Several other infections such as Lyme disease, infective endocarditis, and certain viruses may also present with some form of joint involvement.
Disseminated gonococcal arthritis has an excellent prognosis if appropriate therapy is given. Rare complications such as meningitis, perihepatitis, osteomyelitis, and endocarditis may occur if treatment is delayed or inadequate.
- Complications
- Joint damage
- Perihepatitis
- Endocarditis
- Osteomyelitis
- Postoperative and Rehabilitation Care
After the patient has completed treatment, re-evaluation is necessary. Patients also need to be screened for other sexually transmitted infections in 3 to 6 months. More importantly, the partners need to be contacted and treated.
- Consultations
- Orthopedic surgeon
- Infectious disease
- Enhancing Healthcare Team Outcomes
Gonococcal arthritis is ideally managed with an interprofessional team of healthcare professionals. While the acute joint problem is managed by a physician, many patients may require a short program in physical therapy to regain their joint mobility and muscle strength. The nurse should educate the patient on sexually transmitted infections and how to practice safe sex. Once a patient has been diagnosed with one STD, the nurse should inform the patient about getting tested for other STDs. The pharmacist should ensure compliance with antibiotics to ensure that full healing occurs. Finally, the social worker should recommend that the partner be examined and treated, otherwise the cycle of infection transmission will continue. [14] [15] (Level V)
When patients with gonococcal arthritis are treated with antibiotics, there is usually full recovery without any sequelae. For those who have other manifestations besides the arthritis, the prognosis may depend on the severity of the infection. For example, if a patient has gonococcal endocarditis, antibiotics may be required for 4 to 6 weeks and if the valve is damaged, valve replacement may be required. In general, complications following gonococcal arthritis are rare. [8] [16] [Level 5]
- Review Questions
- Access free multiple choice questions on this topic.
- Comment on this article.
Disclosure: Raymund Li declares no relevant financial relationships with ineligible companies.
Disclosure: Jason Hatcher declares no relevant financial relationships with ineligible companies.
This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) ( http://creativecommons.org/licenses/by-nc-nd/4.0/ ), which permits others to distribute the work, provided that the article is not altered or used commercially. You are not required to obtain permission to distribute this article, provided that you credit the author and journal.
- Cite this Page Li R, Hatcher JD. Gonococcal Arthritis. [Updated 2023 May 29]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.
In this Page
Bulk download.
- Bulk download StatPearls data from FTP
Related information
- PMC PubMed Central citations
- PubMed Links to PubMed
Similar articles in PubMed
- Gonococcal meningitis: An unusual presentation of disseminated gonococcal infection. [J Assoc Med Microbiol Infect D...] Gonococcal meningitis: An unusual presentation of disseminated gonococcal infection. Agabawi S, Tran V, McEachern J, Walkty A. J Assoc Med Microbiol Infect Dis Can. 2019 Jun; 4(2):116-120. Epub 2019 Jun 17.
- Disseminated Gonococcal Infection: A Case Report of Arthritis-Dermatitis Syndrome. [J Med Cases. 2019] Disseminated Gonococcal Infection: A Case Report of Arthritis-Dermatitis Syndrome. da Cruz M, Palma NZ, Ferraz RV, Oliveira M, Meireles R. J Med Cases. 2019 Oct; 10(10):312-314. Epub 2019 Oct 31.
- Gonococcal osteomyelitis in a pediatric patient with disseminated gonococcal infection: Implications for antimicrobial management. [IDCases. 2020] Gonococcal osteomyelitis in a pediatric patient with disseminated gonococcal infection: Implications for antimicrobial management. Liakos W, Schaffler B, Rajan S, Hagmann SHF. IDCases. 2020; 21:e00875. Epub 2020 Jun 18.
- Review Disseminated gonococcal disease in pediatrics: Case report and review of the literature. [Reumatol Clin (Engl Ed). 2024] Review Disseminated gonococcal disease in pediatrics: Case report and review of the literature. Leos-Leija AK, Calderón-Zamora RC, Villarreal-Treviño AV, García-Rodríguez F, de La O-Cavazos ME, Rubio-Pérez NE. Reumatol Clin (Engl Ed). 2024 Jan; 20(1):43-44. Epub 2023 Dec 20.
- Review Disseminated gonococcal infection (DGI) and gonococcal arthritis (GCA): II. Clinical manifestations, diagnosis, complications, treatment, and prevention. [Semin Arthritis Rheum. 1981] Review Disseminated gonococcal infection (DGI) and gonococcal arthritis (GCA): II. Clinical manifestations, diagnosis, complications, treatment, and prevention. Masi AT, Eisenstein BI. Semin Arthritis Rheum. 1981 Feb; 10(3):173-97.
Recent Activity
- Gonococcal Arthritis - StatPearls Gonococcal Arthritis - StatPearls
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers
Ceftriaxone-Resistant Gonorrhea — China, 2022
Weekly / March 28, 2024 / 73(12);255–259
Xiaoyu Zhu 1 ,2 ,* ; Yue Xi 1 ,2 ,* ; Xiangdong Gong, MD 1 ,2 ; Shaochun Chen, PhD 1 ,2 ,3 ( View author affiliations )
What is already known about this topic?
Gonorrhea is the fourth most reported notifiable infectious disease in China. Emergence and spread of ceftriaxone-resistant clones of Neisseria gonorrhoeae in China have posed a challenge to gonorrhea treatment.
What is added by this report?
During 2017−2022, the prevalence of antibiotic-resistant strains of N. gonorrhoeae increased in China, with resistance to ceftriaxone, the first-line treatment for gonorrhea, approximately tripling. Resistance varied by geographic region. Gonorrhea strains were resistant to other antibiotics at prevalences up to 97.6%, varying by antibiotic type.
What are the implications for public health practice?
Effective diagnosis and treatment are essential to protect the health of infected persons and prevent ongoing transmission of antibiotic-resistant gonorrhea. Identifying reasons for the spread of ceftriaxone-resistant N. gonorrhoeae in China could guide strategies, such as antibiotic stewardship, to curb the spread of resistant strains.
- Article PDF
- Full Issue PDF
Gonorrhea is a widespread sexually transmitted infection; in 2022, China reported 96,313 cases of gonorrhea, making it the fourth most common notifiable infectious disease in the country after viral hepatitis, pulmonary tuberculosis, and syphilis. The rise in prevalence in antimicrobial-resistant strains, particularly the international spread of ceftriaxone-resistant clones, poses a formidable challenge to gonorrhea control. The China Gonococcal Resistance Surveillance Program (China-GRSP), established in 1987 and covering 19 of 34 provincial-level administrative units, continuously monitors gonococcal antimicrobial resistance. In 2022, 13 China-GRSP sentinel sites collected 2,804 gonococcal isolates, representing 2.9% of all cases reported in China, and 4.1% of cases reported in the 13 participating provinces. The prevalence of Neisseria gonorrhoeae resistance to ceftriaxone was 8.1%, approximately three times the 2017 rate of 2.9%; five provinces reported >10% ceftriaxone resistance. Resistance prevalences to cefixime, azithromycin, tetracycline, penicillin, and ciprofloxacin were 16.0%, 16.9%, 77.1%, 77.8%, and 97.6%, respectively. Only one case of spectinomycin resistance was reported. These data highlight a substantial increase in ceftriaxone resistance from 2017 to 2022. Effective diagnosis and treatment and appropriate management of sex partners are essential to protect the health of infected persons and prevent ongoing transmission of gonorrhea, including transmission of resistant strains. Identifying reasons for the spread of ceftriaxone-resistant N. gonorrhoeae in China could guide strategies, such as antibiotic stewardship, to mitigate the rising resistance rate and curb the spread of resistant strains.
Introduction
Gonorrhea, a sexually transmitted bacterial infection caused by Neisseria gonorrhoeae , remains prevalent worldwide. The World Health Organization (WHO) estimated that approximately 82.4 million new gonorrhea cases were diagnosed among persons aged 15–49 years in 2020. † In China, a total of 96,313 gonorrhea cases were reported in 2022, representing a rate of 6.83 reported cases per 100,000 population, the fourth highest among class A and class B notifiable infectious diseases § in the country, ¶ after viral hepatitis, pulmonary tuberculosis, and syphilis. In the United States, in 2022, a total of 648,056 cases of gonorrhea were reported.**
In recent years, gonococcal resistance to multiple antibiotics has emerged ( 1 ). Ceftriaxone is recommended as the first-line treatment option for gonorrhea in China (single dose of 1 g, administered intramuscularly) †† as well as in the United States (single dose of 500 mg for persons weighing <150 kg, administered intramuscularly). §§ However, the emergence of ceftriaxone-resistant strains, particularly the ceftriaxone-resistant clone FC428 ( 2 ), has been identified worldwide. First identified in Beijing in 2016 ( 3 ), this resistant clone has become widely disseminated across various regions of China, with its proportion among all resistant clones steadily increasing since 2016, highlighting the challenge associated with addressing gonococcal resistance ( 4 ).
The China Gonococcal Resistance Surveillance Program (China-GRSP), established in 1987, monitors gonococcal resistance to azithromycin, cefixime, ceftriaxone, ciprofloxacin, penicillin, spectinomycin, and tetracycline in China ( 5 ). This report describes gonococcal resistance surveillance data from China for 2022, the most recent year for which data are available.
In 2022, China-GRSP conducted gonococcal resistance surveillance across 13 of the 19 provinces (among 34 national province-level administrative jurisdictions) that participate in the program, within six of seven regions of China (Supplementary Figure, https://stacks.cdc.gov/view/cdc/150923 ). N. gonorrhoeae isolates obtained from urethral (from males) or endocervical (from females) swab specimens were collected from the 2,804 identified cases included in the surveillance program from consecutively evaluated patients throughout the year. Consecutive evaluation involved specimen collection at each sentinel site from January through December, with some sites having larger sample sizes and sampling limitations that might result in data collection ending as early as September. Specimens were cultured on selective gonococcal culture media, and N. gonorrhoeae (an oxidase-positive, gram-negative diplococcus) was identified by microscopic examination of Gram-stained material, detection of a rapid oxidase reaction, and carbohydrate utilization test results. ¶¶ The susceptibility of isolates to azithromycin, cefixime, ceftriaxone, ciprofloxacin, penicillin, spectinomycin, and tetracycline was determined using the agar dilution method. Antibiotic resistance breakpoints were applied based on the European Committee on Antimicrobial Susceptibility Testing criteria,*** except for azithromycin, for which WHO criteria were used. WHO N. gonorrhoeae reference strains were used for quality assurance. The determination of antibiotic resistance was based on the minimum inhibitory concentration (MIC) values obtained through agar dilution. The antibiotic resistance breakpoints were as follows: azithromycin MIC >0.5 mg/L, cefixime MIC >0.125 mg/L, ceftriaxone MIC >0.125 mg/L, ciprofloxacin MIC >0.06 mg/L, penicillin MIC >1 mg/L, spectinomycin MIC >64 mg/L, and tetracycline MIC >1 mg/L. Resistance rate was expressed as the percentage of resistant isolates among the total number of isolates. This activity was reviewed and approved by the Medical Ethics Committee at the Institute of Dermatology, Chinese Academy of Medical Sciences & Peking Union Medical College, and the National Center for AIDS/STD Control and Prevention in China.
In 2022, a total of 2,804 isolates (4.1% of 68,217 gonococcal infection cases) from 13 provinces in China were tested for antimicrobial susceptibility. The largest numbers of cases were reported in Guangdong (22,171) and Zhejiang (13,460) provinces. Rates of reported cases ranged from 2.13 to 20.58 per 100,000 population, with highest rates reported in Zhejiang, Guangdong, Yunnan, Hainan, and Guangxi provinces ( Table 1 ).
Percentages of isolates tested by province ranged from 2.1% (Yunnan) to 18.1% (Tianjin). Among isolates submitted, resistance was identified to ciprofloxacin (97.6%), penicillin (77.8%), tetracycline (77.1%), azithromycin (16.9%), cefixime (16.0%), and ceftriaxone (8.1%) ( Table 2 ). Only one isolate was resistant to spectinomycin. Among 2,804 isolates, those from 18 cases were identified as resistant to all antibiotics except spectinomycin.
Antibiotic resistance rates differed among provinces. Whereas ceftriaxone resistance detected in most sentinel sites was ≤5% during the past decade, in 2022, five provinces (Chongqing, Jiangsu, Sichuan, Tianjin, and Xinjiang) reported >10% ceftriaxone resistance, with rates in Sichuan, Tianjin, and Xinjiang surpassing 20%; only Hainan, Hunan, Shanghai, and Zhejiang reported ≤5% ceftriaxone resistance ( Figure ). Among other antibiotics, overall resistance to cefixime was 16.0%, with rates in Jiangsu, Sichuan, Tianjin, and Xinjiang exceeding 25%. Azithromycin resistance was >35% in Hunan and Shanghai and >20% in Chongqing, Guangdong, Tianjin, and Xinjiang. Resistance to ciprofloxacin remained consistently high nationwide (97.6%), with Hunan, Shaanxi, Shanghai, Sichuan, Tianjin, and Yunnan reaching 100%. Overall resistance to tetracycline was 77.1%, ranging from 28.3% in Tianjin to 100% in Xinjiang. Penicillin resistance was 77.8% nationwide and was >70% in most provinces; the highest penicillin resistance rate (98.2%) was reported by Shanghai province.
The prevalence of ceftriaxone resistance among gonococcal isolates in China nearly tripled since 2017, increasing from 2.9% to 8.1% in 2022; this rate is relatively high compared with that in other countries ( 1 ). For example, in 2022, the percentage of strains with reduced susceptibility to ceftriaxone (MIC >0.03 mg/L) in the United Kingdom was 0.21%. ††† According to the U.S. CDC’s Gonococcal Isolate Surveillance Project report, the prevalence of isolates exhibiting elevated ceftriaxone MICs (≥0.125 μ g/mL) fluctuated at approximately 0.2% during 2016−2020. §§§ In Canada, prevalence of decreased susceptibility to ceftriaxone has remained relatively stable, at approximately 0.6% during 2017–2021 ( 6 ).
These findings underscore the urgent need for a comprehensive approach to address antibiotic-resistant N. gonorrhoeae in China, including identifying factors contributing to this high resistance rate, especially in provinces where the percentage of gonococcal isolates resistant to ceftriaxone is >10%. Factors that could contribute to ceftriaxone resistance include spread of the ceftriaxone-resistant FC428 strain, gaps in gonorrhea screening, treatment, and partner management, and nonrecommended prescribing or use of antibiotics (although antibiotics are only available by prescription in China). Understanding these factors is crucial to guiding the development and implementation of targeted interventions and preventive measures. The preliminary investigation revealed that the widespread dissemination of ceftriaxone-resistant FC428 clones might be the underlying reason for the high resistance rate in China ( 3 , 4 , 7 ), although whole-genome sequencing of isolates collected in 2022 is ongoing. These resistant clones have spread internationally ( 8 – 10 ), and collaborative cross-border efforts will be essential to monitoring and mitigating its further spread. These findings also reinforce the pivotal role of programs such as the China-GRSP in the ongoing monitoring and adapting of strategies to address evolving resistance patterns. The observed resistance rates for other antibiotics emphasize the complex landscape of gonococcal antimicrobial resistance, further highlighting the urgent need to develop alternative treatment strategies, including vaccines to counter this growing threat. ¶¶¶
Limitations
The findings in this report are subject to at least four limitations. First, relying on reported cases of gonorrhea might underestimate the actual incidence, because asymptomatic cases or those among patients not seeking medical attention might go unrecorded. Second, in 2022, China-GRSP only covered one third of the country, and fewer than 3% of isolates were available for testing, leading to potential bias, and results might not be representative of the entire country. Third, this analysis focused on antimicrobial resistance rates and did not address broader sociodemographic factors influencing gonorrhea transmission. Finally, the lack of detailed patient information hampers the identification of specific risk factors contributing to the observed resistance patterns. Future research should address these limitations for a more nuanced understanding of N. gonorrhoeae epidemiology in China.
Implications for Public Health Practice
The increasing prevalence of ceftriaxone resistance in N. gonorrhoeae in China highlights a pressing public health concern. Effective diagnosis and treatment and appropriate management of sex partners are essential to protect the health of infected persons and prevent ongoing transmission of gonorrhea, including transmission of resistant strains. Public health practitioners should prioritize assessment of screening practices, particularly in regions with higher reported rates of gonorrhea cases and resistance rates. Understanding the factors that could contribute to the spread of resistance, such the nonrecommended use of antimicrobials, is also crucial to guide prevention efforts. Collaborative efforts and ongoing surveillance to monitor the international spread of resistant strains, as exemplified by programs like China-GRSP, are vital for a global response. International collaboration and information sharing are critical to prevent the further cross-border spread of resistant strains and to identify alternative treatment options for gonorrhea. Given the identified limitations, future research should aim to broaden surveillance coverage, incorporate detailed patient information, and conduct a comprehensive analysis of sociodemographic factors. These efforts could improve understanding of gonococcal infections and antibiotic resistance in China. The findings underscore the dynamic nature of this public health issue, emphasizing the ongoing need for adaptive and collaborative approaches to address the growing threat of antibiotic-resistant N. gonorrhoeae effectively.
Acknowledgments
Members of China-Gonococcal Resistance Surveillance Program; Fundamental Research Operations of the Central-Level Public Welfare Research Institute of the Chinese Academy of Medical Sciences; Jiangsu Provincial Medical Key Laboratory for Jiangsu Province Capability Improvement Project Through Science, Technology, and Education.
Corresponding author: Shaochun Chen, [email protected] .
1 Hospital for Skin Diseases, Institute of Dermatology, Chinese Academy of Medical Sciences & Peking Union Medical College, Nanjing, China; 2 National Center for AIDS/STD Control and Prevention, Chinese CDC, Nanjing, China; 3 School of Public Health, Nanjing Medical University, Nanjing, China.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. All authors report support from the Chinese Academy of Medical Sciences for Fundamental Research Operations of the Central-Level Public Welfare Research, and from the Jiangsu Provincial Medical Key Laboratory for Jiangsu Province Capability Improvement Project through Science, Technology, and Education. No other potential conflicts of interest were disclosed.
* These authors contributed equally to this report.
† https://www.who.int/news-room/fact-sheets/detail/gonorrhoea-(neisseria-gonorrhoeae-infection)
§ Two class A and 28 class B notifiable infectious diseases are recognized in China; class A diseases include cholera and plague, and class B diseases include those associated with a high risk for outbreaks or that are likely to result in rapid spread once an outbreak occurs, such as AIDS, gonorrhea, measles, syphilis, and tuberculosis.
¶ http://www.nhc.gov.cn/guihuaxxs/s3585u/202309/6707c48f2a2b420fbfb739c393fcca92.shtml ; data on rates of reported cases of COVID-19 were not available; therefore, SARS-CoV-2 infections were not included in these statistics.
** https://www.cdc.gov/std/statistics/2022/overview.htm#Gonorrhea
†† https://doi.org/10.1097/JD9.0000000000000072
§§ https://www.cdc.gov/std/treatment-guidelines/gonorrhea-adults.htm
¶¶ https://www.who.int/publications/i/item/9789241505840
*** www.eucast.org/clinical_breakpoints/
††† https://www.gov.uk/government/publications/gonococcal-resistance-to-antimicrobials-surveillance-programme-grasp-report/grasp-report-data-to-june-2023
§§§ https://www.cdc.gov/std/statistics/gisp-profiles/default.htm
¶¶¶ https://www.who.int/publications-detail-redirect/9789240039827
- Unemo M, Lahra MM, Escher M, et al. WHO global antimicrobial resistance surveillance for Neisseria gonorrhoeae 2017–18: a retrospective observational study. Lancet Microbe 2021;2:e627–36. https://doi.org/10.1016/S2666-5247(21)00171-3 PMID:35544082
- Nakayama S, Shimuta K, Furubayashi K, Kawahata T, Unemo M, Ohnishi M. New ceftriaxone- and multidrug-resistant Neisseria gonorrhoeae strain with a novel mosaic penA gene isolated in Japan. Antimicrob Agents Chemother 2016;60:4339–41. https://doi.org/10.1128/AAC.00504-16 PMID:27067334
- Chen SC, Han Y, Yuan LF, Zhu XY, Yin YP. Identification of internationally disseminated ceftriaxone-resistant Neisseria gonorrhoeae strain FC428, China. Emerg Infect Dis 2019;25:1427–9. https://doi.org/10.3201/eid2507.190172 PMID:30900979
- Chen SC, Liu JW, Zhou K, Yin YP. Ceftriaxone-resistant Neisseria gonorrhoeae strain FC428: prevalence, resistance mechanisms and control strategies. Zhonghua Pifuke Zazhi 2022;55:1122–6. https://doi.org/10.35541/cjd.20200528
- Chen SC, Yin YP, Dai XQ, Unemo M, Chen XS. First nationwide study regarding ceftriaxone resistance and molecular epidemiology of Neisseria gonorrhoeae in China. J Antimicrob Chemother 2016;71:92–9. https://doi.org/10.1093/jac/dkv321 PMID:26472770
- Sawatzky P, Lefebvre B, Diggle M, et al. Antimicrobial susceptibilities of Neisseria gonorrhoeae in Canada, 2021. Can Commun Dis Rep 2023;49:388–97. https://doi.org/10.14745/ccdr.v49i09a05 PMID:38463902
- Chen SC, Yuan LF, Zhu XY, van der Veen S, Yin YP. Sustained transmission of the ceftriaxone-resistant Neisseria gonorrhoeae FC428 clone in China. J Antimicrob Chemother 2020;75:2499–502. https://doi.org/10.1093/jac/dkaa196 PMID:32473014
- Trinh TM, Nguyen TT, Le TV, et al. Neisseria gonorrhoeae FC428 subclone, Vietnam, 2019–2020. Emerg Infect Dis 2022;28:432–5. https://doi.org/10.3201/eid2802.211788 PMID:35076010
- Picker MA, Knoblock RJ, Hansen H, et al. Notes from the field: first case in the United States of Neisseria gonorrhoeae harboring emerging mosaic penA60 allele, conferring reduced susceptibility to cefixime and ceftriaxone. MMWR Morb Mortal Wkly Rep 2020;69:1876–7. https://doi.org/10.15585/mmwr.mm6949a5 PMID:33301430
- Day M, Pitt R, Mody N, et al. Detection of 10 cases of ceftriaxone-resistant Neisseria gonorrhoeae in the United Kingdom, December 2021 to June 2022. Euro Surveill 2022;27:2200803. https://doi.org/10.2807/1560-7917.ES.2022.27.46.2200803 PMID:36398578
* Data from 13 of 19 provincial sentinel surveillance sites were included in the analysis; only 2,804 isolates were tested for antimicrobial susceptibility, accounting for 4.1% of all reported cases in the 13 participating provinces. † Per 100,000 population.
Abbreviation: MIC = minimum inhibitory concentration. * Data from 13 of 19 provincial sentinel surveillance sites were included in the analysis. † Concentrations listed are the MIC thresholds used to categorize resistant isolates.
FIGURE . Reported rates of ceftriaxone resistance — 13 Gonococcal Resistance Surveillance Program sentinel sites,* China, 2022
Abbreviation: GRSP = Gonococcal Resistance Surveillance Program.
* Data from 13 of 19 provincial sentinel surveillance sites were included in the analysis.
Suggested citation for this article: Zhu X, Xi Y, Gong X, Chen S. Ceftriaxone-Resistant Gonorrhea — China, 2022. MMWR Morb Mortal Wkly Rep 2024;73:255–259. DOI: http://dx.doi.org/10.15585/mmwr.mm7312a2 .
MMWR and Morbidity and Mortality Weekly Report are service marks of the U.S. Department of Health and Human Services. Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of Health and Human Services. References to non-CDC sites on the Internet are provided as a service to MMWR readers and do not constitute or imply endorsement of these organizations or their programs by CDC or the U.S. Department of Health and Human Services. CDC is not responsible for the content of pages found at these sites. URL addresses listed in MMWR were current as of the date of publication.
All HTML versions of MMWR articles are generated from final proofs through an automated process. This conversion might result in character translation or format errors in the HTML version. Users are referred to the electronic PDF version ( https://www.cdc.gov/mmwr ) and/or the original MMWR paper copy for printable versions of official text, figures, and tables.
Exit Notification / Disclaimer Policy
- The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website.
- Linking to a non-federal website does not constitute an endorsement by CDC or any of its employees of the sponsors or the information and products presented on the website.
- You will be subject to the destination website's privacy policy when you follow the link.
- CDC is not responsible for Section 508 compliance (accessibility) on other federal or private website.

IMAGES
VIDEO
COMMENTS
Gonorrhea is most commonly spread during vaginal, oral or anal sexual activity. But babies can get the infection during childbirth. In babies, gonorrhea most commonly affects the eyes. Avoiding sexual activity and not having sex prevents the spread of gonorrhea. Using a condom during sexual activity can help prevent the spread of gonorrhea.
Gonorrhea is a sexually transmitted disease (STD) caused by infection with the Neisseria gonorrhoeae bacterium. N. gonorrhoeae infects the mucous membranes of the reproductive tract, including the cervix, uterus, and fallopian tubes in women, and the urethra in women and men. N. gonorrhoeae can also infect the mucous membranes of the mouth ...
Gonorrhea is a purulent infection of the mucous membrane surfaces caused by Neisseria gonorrhoeae. N gonorrhoeae is spread by sexual contact or through transmission during childbirth. ... The classic presentation of epididymitis is of unilateral pain and swelling localized posteriorly within the scrotum.
Neisseria gonorrhoeae, an obligate human pathogen, is a sexually transmitted disease that causes consequential worldwide morbidity both in resource-abundant and resource-limited nations, and its diagnosis and treatment require costly expenditures annually.[1][2] Like other sexually transmitted infections (STIs), gonorrhea disproportionately impacts young adult populations.[3]
Gonorrhea is a common sexually transmitted infection (STI) caused by a bacteria called Neisseria gonorrhoeae (N. gonorrhoeae). It's also sometimes called "the clap" or "drip.". Gonorrhea is spread through sexual fluids, including vaginal fluid and semen. You can get gonorrhea from intercourse, anal sex, oral sex, or sharing sex toys ...
INTRODUCTION. Gonorrhea, or infection with the gram-negative coccus Neisseria gonorrhoeae, is a major cause of morbidity among sexually active individuals worldwide.In the United States, it is the second most commonly reported communicable disease, with more than 600,000 cases reported annually [], with probably an equal number of cases that remain unreported [].
Gonorrhoea is a preventable and curable sexually transmitted infection caused by the bacterium Neisseria gonorrhoeae, which is primarily transmitted through vaginal, oral and anal sex. In 2020 there were an estimated 82.4 million new infections among adults globally. Most women with gonorrhoea do not have symptoms, and when they do, vaginal ...
Gonorrhea is caused by the bacterium Neisseria gonorrhoeae. It typically infects epithelia of the urethra, cervix, rectum, pharynx, or conjunctivae, causing irritation or pain and purulent discharge. Dissemination to skin and joints, which is uncommon, causes sores on the skin, fever, and migratory polyarthritis or pauciarticular septic arthritis.
Gonorrhea is a purulent infection of the mucous membrane surfaces caused by Neisseria gonorrhoeae.N gonorrhoeae is spread by sexual contact or through transmission during childbirth. The Centers for Disease Control (CDC) recommends that all patients with gonorrheal infection also be treated for presumed co-infection with Chlamydia trachomatis. []
Gonorrhea treatment in adults. Adults with gonorrhea are treated with antibiotics. Due to emerging strains of drug-resistant Neisseria gonorrhoeae, the bacterium that causes gonorrhea, the Centers for Disease Control and Prevention recommends that uncomplicated gonorrhea be treated with the antibiotic ceftriaxone.
Gonorrhea is a sexually transmitted disease (STD) that can infect both men and women. It can cause infections in the genitals, rectum, and throat. It is a very common infection, especially among young people ages 15-24 years. How is gonorrhea spread? You can get gonorrhea by having vaginal, anal, or oral sex with someone who has gonorrhea.
Pregnant patients diagnosed with chlamydia or gonorrhea should have a test of cure four weeks after treatment. Chlamydia trachomatis and Neisseria gonorrhoeae are the most common sexually ...
Gonorrhea is often asymptomatic in females and symptomatic in males. 1,5,11 When symptomatic, the clinical presentation in females includes vaginal discharge, dysuria, dyspareunia, abnormal uterine bleeding, lower abdominal and/or rectal pain. 5,11 In males, symptoms include urethral discharge and/or itch, dysuria and testicular or rectal pain.
Pathophysiology. Gonorrhoea is transmitted through unprotected vaginal/oral/anal sex and can also be vertically transmitted from mother to child. Neisseria gonorrhoeae is a Gram-negative diplococcus that has a strong affinity for mucous membranes. The organism can infect the uterus, urethra, cervix, fallopian tubes, ovaries, testicles, rectum ...
Gonorrhea Facts & Brochures. Basic fact sheets are presented in plain language for individuals with general questions about sexually transmitted diseases. Detailed fact sheets are intended for physicians and individuals with specific questions about sexually transmitted diseases. Detailed fact sheets include specific testing and treatment ...
1.Neisseria gonorrhoeae - drug therapy. 2.Gonorrhea - drug therapy. 3.Drug Resistance, Microbial. 4.Guideline. I.World Health Organization. ... Clinical presentation 10 Laboratory diagnosis 10 1.2 Rationale for new recommendations 11 1.3 Objectives 11 1.4 Target audience 11 1.5 Structure of the guidelines 11
Treatment of gonorrhea is also a means of secondary prevention to interrupt transmission within the community. To this end gonorrhea is a reportable disease to the local public health authority. For the health provider, partner management is an important aspect of treating the patient to prevent reinfection.
What are common clinical presentations? Most chlamydia and gonorrhea infections cause no symptoms. 12 If symptoms develop, the incubation period for gonorrhea is 2-7 days, compared with 2-6 weeks for chlamydia. 13 Chlamydia and gonorrhea may have genital or extragenital symptoms, which are generally reflective of the site of infection. The ...
INTRODUCTION. Disseminated gonococcal infection (DGI) results from bacteremic spread of the sexually transmitted pathogen, Neisseria gonorrhoeae, which can lead to a variety of clinical symptoms and signs, such as arthritis or arthralgias, tenosynovitis, and multiple skin lesions. This topic will discuss the epidemiology, pathophysiology, clinical manifestations, diagnosis, and treatment of DGI.
The host-adapted human pathogen Neisseria gonorrhoeae is the causative agent of gonorrhea. In this Review, Quillin and Seifert provide an overview of the bacterial factors that are important for the different stages of pathogenesis, including transmission, colonization and immune evasion, and discuss the problem of antibiotic resistance.
In England, surveillance data published in 2022 suggested that STI diagnoses rose 22% from 2014 to 2019 among people ages 45 and up. Chlamydia was the most common, followed by gonorrhea. Kowalska ...
The data stems from 2,804 isolates, representing 2.9 percent of all cases reported in China during 2022. Those figures come from 13 of the country's 19 provinces. While the overall prevalence of ...
Gonococcal arthritis is a bacterial arthritis that results from the bacteremic spread of Neisseria gonorrhoeae, a sexually transmitted pathogen. It is a clinical manifestation of disseminated gonococcal infection. It comprises two major clinical forms: localized septic arthritis and arthritis-dermatitis syndrome.[1][2][3]
Gonorrhea is a widespread sexually transmitted infection; in 2022, China reported 96,313 cases of gonorrhea, making it the fourth most common notifiable infectious disease in the country after viral hepatitis, pulmonary tuberculosis, and syphilis. The rise in prevalence in antimicrobial-resistant strains, particularly the international spread ...